Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
2-Propenoic acid, ethyl ester
(Ethyl acrylate)
Chemical Abstracts Service Registry Number
140-88-5
Environment Canada
Health Canada
September 2011
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential for Bioaccumulation
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix 1: Upper-Bounding Estimates of Exposure to the General Population of Canada from Environmental Media (µg/kg-bw/d)
- Appendix 2: Upper-Bounding Estimates of Exposure to Ethyl Acrylate from Consumer Products
- Appendix 3: Summary of health effects information for ethyl acrylate
- Appendix 4: Robust Study Summary Aquatic Toxicity
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment of 2-Propenoic acid, ethyl ester (ethyl acrylate), Chemical Abstracts Service Registry Number 140-88-5[1]. The substance ethyl acrylate was identified in the categorization of the Domestic Substances List as a high priority for action under the Challenge initiative under the Chemicals Management Plan. Ethyl acrylate was identified as a high priority as it was considered to pose the greatest potential for exposure of individuals in Canada and it is classified by other agencies on the basis of carcinogenicity. The substance did not meet the ecological categorization criteria for persistence, bioaccumulation potential or inherent toxicity to aquatic organisms.
According to information submitted under section 71 of CEPA 1999, ethyl acrylate was not manufactured by any company in Canada in the calendar year 2006 above the 100 kg reporting threshold. However between 1 000 000 and 10 000 000 kg of ethyl acrylate was reported to have been imported in 2006. The major use of ethyl acrylate is in the manufacture of polymers and copolymers. Releases of ethyl acrylate to the environment from these sources do occur. However, exposure of the general population of Canada to ethyl acrylate is not expected to occur at any appreciable level.
As ethyl acrylate was classified on the basis of carcinogenicity by international regulatory agencies, carcinogenicity was a key focus for this screening assessment. Induction of forestomach tumours were observed in rats and mice administered ethyl acrylate by oral gavage for 2 years. However, no induction of tumours was observed by other routes of administration including oral drinking water, inhalation and dermal. Collective evidence from genotoxicity studies suggests that ethyl acrylate is not likely to be mutagenic but may exert some clastogenic effects in vitro. While the mode of induction of tumours has not been fully elucidated, sustained forestomach hyperplasia has been suggested to be a precursor event. Therefore a threshold approach is used to characterize risk to human health.
Margins between upper-bounding estimates of exposure to ethyl acrylate from environmental media, food and the use of consumer products and levels associated with effects in experimental animals are considered to be adequate to address uncertainties in the health effects and exposure databases.
Based on the information presented in this screening assessment, it is concluded that ethyl acrylate is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Based on available empirical data and modelling results, ethyl acrylate is not expected to be persistent or to bioaccumulate in the environment. The substance therefore does not meet the persistence criteria or the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations. In addition, available empirical data suggest that the substance has a moderate to high potential to be toxic to aquatic organisms. However, based on a comparison of predicted no-effect concentrations with estimated reasonable worst-case environmental exposure concentrations, it is considered unlikely that ethyl acrylate is causing ecological harm in Canada.
Based on the information available, it is concluded that ethyl acrylate is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on the information available, it is concluded that ethyl acrylate does not meet any of the criteria set out in section 64 of CEPA 1999.
This substance will be considered for inclusion in theDomestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE) and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance 2-propenoic acid, ethyl ester (ethyl acrylate) was identified as a high priority for assessment of human health risk because it was considered to present the greatest potential for exposure and had been classified by other agencies on the basis of carcinogenicity. The Challenge for this substance was published in the Canada Gazette on September 26, 2009 (Canada 2009). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the substance were received.
Although ethyl acrylate was determined to be a high priority for assessment with respect to human health, it did not meet the categorization criteria for persistence or bioaccumulation in thePersistence and Bioaccumulation Regulations and it did not meet the criteria for toxicity to aquatic organisms.
Screening assessments focus on information critical to determining whether a substance meets the criteria for defining a substance as toxic as set out in section 64 of CEPA 1999. Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution[2].
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to April 2010 for human and ecological sections of the document. Key studies were critically evaluated; modelling results may have been used to reach conclusions.
Evaluation of risk to human health involves consideration of data relevant to estimation of exposure (non-occupational) of the general population, as well as information on health hazards (based principally on the weight of evidence assessments of other agencies that were used for prioritization of the substance). Decisions for human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the critical information upon which the concluded conclusion is based.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments.
The ecological and human health portions of this assessment have undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from scientific experts selected and directed by Toxicology Excellence for Risk Assessment (TERA), including Dr. Bernard Gadagbui (Toxicology Excellence for Risk Assessment [TERA]), Dr. Michael Jayjock (The LifeLine Group) and Dr. Chris Bevans (CJB Consulting). Although external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada.
Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada. Approaches used in the screening assessments under the Challenge have been reviewed by an independent Challenge Advisory Panel.
The critical information and considerations upon which the assessment is based are summarized below.
For the purposes of this document, this substance will be referred to as ethyl acrylate, the common chemical name for the substance.
Table 1. Substance identity for ethyl acrylate
| CAS Registry Number | 140-88-5 |
| DSL name | 2-propenoic acid, ethyl ester |
| National Chemical Inventories(NCI) names[1] | Ethyl acrylate (EINECS, ENCS) 2-Propenoic acid, ethyl ester (TSCA, AICS, SWISS, PICCS, ASIA-PAC, NZIoC) 2-Propenoic acid ethyl ester (ECL) Acrylic acid, ethyl ester (PICCS) Acrylate, ethyl (PICCS) |
| Other names | Ethyl 2-propenoate; Acrylic acid ethyl ester; Ethyl 2-propenoate; Ethyl acrylic ester; Ethyl propenoate; NSC 8263; UN 1917 |
| Chemical group (DSL Stream) | Discrete organics |
| Major chemical class or use | Esters |
| Major chemical sub-class | Acrylates |
| Chemical formula | C5H8O2 |
| Chemical structure | 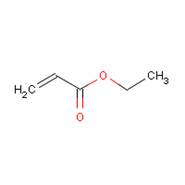 |
| SMILES[2] | O=C(OCC)C=C |
| Molecular mass | 100.116 g/mol |
[2] Simplified Molecular Input Line Entry Specification
Table 2 contains experimental and modelled physical and chemical properties of ethyl acrylate that are relevant to its environmental fate.
The models based on quantitative structure-activity relationships (QSAR) were used to generate data for some of the physical and chemical properties of ethyl acrylate.
Table 2. Physical and chemical properties for ethyl acrylate
| Property | Type | Value[1] | Temperature (°C) |
Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Melting point (ºC) |
Experimental | -72[2] | BASF AG 2000 | ||||||
| Modelled | -70.73 | MPBPWIN 2008 | |||||||
| Boiling point (ºC) |
Experimental | 100 | BASF AG 2000 | ||||||
| Experimental | 99.4[2] | O’Neil et al. 2001 | |||||||
| Modelled | 100.51 | MPBPWIN 2008 | |||||||
| Density (kg/m3) |
Experimental | 922 (0.922 g/cm3) |
20 | BASF AG 2000 | |||||
| Vapour pressure (Pa) |
Experimental | 3800 | 20 | BASF AG 2000 | |||||
| Experimental | 3900 (29.3 mmHg) |
20 | IPCS 2004 | ||||||
| 3800[2] (28.5 mm Hg) |
CEDRE 2006 | ||||||||
| 5147 (38.6 mm Hg) |
25 | Daubert and Danner 1989 | |||||||
| Modelled | 5120 (38.6 mm Hg) |
20 | MPBPWIN 2008 | ||||||
| Henry’s Law constant (Pa·m3/mol) |
Experimental | 25.3[2] | BASF AG 2000 | ||||||
| Calculated | 25.3 | 20 | ECETOC 1994 | ||||||
| Modelled | 6.37 (6.29x10-5 atm m3/mol) |
25 | HENRYWIN 2008 (Group) | ||||||
| 12.46 (1.23x10-4 atm m3/mol) |
25 | HENRYWIN 2008 (Bond) | |||||||
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
Experimental | 1.18[2] | 25 | BASF AG 2000 | |||||
| Modelled | 1.22 | 25 | KOWWIN 2008 | ||||||
| Log Koc (Organic carbon-water partition coefficient) (dimensionless) |
Experimental | 1.34 | IUCLID 2000 | ||||||
| Modelled | 1.07 | PCKOCWIN 2008 | |||||||
| Log KOA (Organic –air partition coefficient) |
Modelled | 3.5 | KOAWIN 2008 | ||||||
| Water solubility (mg/L) |
Experimental | 15 000[2] | 25 | BASF AG 2000 | |||||
| 20 000 | Tyler and Smock 1993 | ||||||||
| 20 000 | 20 | O’Neil et al 2001 | |||||||
| 15 000 | Riddick et al 1986 | ||||||||
| Modelled | 17 630 (KOW method) |
25 | WSKOWWIN 2008 | ||||||
| pKa (Acid dissociation constant) (dimensionless) |
Modelled | Does not ionize in water | ACD/pKaDB 2005 | ||||||
[1] Values in parentheses represent the original ones as reported by the authors or as estimated by the models.
[2] Value used for modelling.
Ethyl acrylate occurs naturally in blackberries, raspberries, pineapples, yellow passion fruit and durian (NTP 1998; Burdock 1997).
Sources of human exposure to ethyl acrylate in Canada, in addition to those occurring naturally, may either be from point source releases such as those associated with industrial sites of processing, and non-point sources such as from food, food packaging release of residual ethyl acrylate, and commercial or industrial products within the Canadian marketplace, e.g., by off-gassing or migration.
Based on information collected through a survey conducted pursuant to section 71 of CEPA 1999, between 1 000 000 and 10 000 000 kg of ethyl acrylate were imported into Canada in 2006. The substance was not reported to be manufactured in Canada (Environment Canada 2010a).
Previously received information from the Domestic Substances List nomination (1984–1986) showed that the quantity reported to be manufactured, imported or in commerce in Canada during the calendar year 1986 was 12 100 000 kg (Environment Canada 1988). Outside of Canada, ethyl acrylate has been identified as a high production volume (HPV) chemical by the Organisation for Economic Co-operation and Development (OECD) (OECD 2005). The production volume of ethyl acrylate is estimated to be 50 000 to 100 000 tonnes per year in Europe and 250 000 to 500 000 tonnes per year in North America (OECD 2005).
Process for Industrial Production of Ethyl Acrylate: The majority of ethyl acrylate is prepared commercially by the catalyzed esterification of acrylic acid with ethanol (McLaughlin et al. 1993). Ethyl acrylate will polymerize readily under the influence of heat, light or peroxides (O’Neil 2006). To prevent premature polymerization, ethyl acrylate is usually inhibited with 10 to 20 ppm of monomethyl ether of hydroquinone (OECD 2005). Some dissolved oxygen should be present in the liquid for the inhibitor to be effective (OECD 2005). Pure ethyl acrylate monomer can be stored below 10oC without incurring polymerization (O’Neil 2006).
Ethyl acrylate is primarily used in closed systems during manufacture and transport. This is due to its volatility and flammability (McLaughlin et al. 1993). Ethyl acrylate has an unpleasant irritating odour and a low odour-detection threshold (0.0012 ppm) (Amoore 1983). Air concentrations of greater than 0.05 ppm would be intolerable to most individuals (McLaughlin et al. 1993).
In Canada, ethyl acrylate is used for synthesis of polymers and copolymers for use in formulations of industrial and consumer products. There are no direct consumer end-use products of ethyl acrylate itself; ethyl acrylate may be found in products as a residual from the polymerization process. Ethyl acrylate imparts flexibility to hard films (O’Neil 2006).
Ethyl acrylate emulsion (water-based) polymers are used in latex paints, coatings, caulks and construction products; in pigment binders and overvarnishes for gravure printing inks; in basecoats for treating natural leather; and in adhesives (McLaughlin et al. 1993). Emulsion polymers using ethyl acrylate are found in floor polishes, sealants, and in textile-treatment processes such as binding fibrefill and non-wovens, laminating, flocking, back coating and fabric finishing (McLaughlin et al. 1993). Solvent-based ethyl acrylate polymer products include lacquer, enamels, and viscosity-index improvers for oils (McLaughlin et al. 1993). Solid-grade polymers include acrylic plastic sheet goods and plastic impact modifiers (McLaughlin et al. 1993).
According to submissions made under section 71 of CEPA 1999 and from the Challenge questionnaire submissions, 1 000 000 - 10 000 000 kg of ethyl acrylate were used in Canada in 2006 (Environment Canada 2010a). Not all ethyl acrylate used remains within Canada, as some is exported from Canada in finished products (Environment Canada 2010b).
Ethyl acrylate is not listed as an approved food additive in the Canadian Food and Drug Regulations (Canada 1978). However it is possible that ethyl acrylate is used as a flavour in foods that are offered for sale in Canada. Food flavours are not regulated as a food additive. The Flavor and Extract Manufacturers Association (FEMA) of the United States reported that there is a decreasing trend in the amount of ethyl acrylate being used as a food flavour in the United States. They reported a total poundage used of 11 lbs (4.98 kg) in 1995 (Lucas et al. 1999) and 3 lbs (1.36 kg) in 2005 (Gavin et al. 2008).
Ethyl acrylate is also used in food packaging materials as a starting material monomer used in the manufacturing of acrylic polymers and copolymers (2010 emails from Food Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). Ethyl acrylate is listed in the Natural Health Products Ingredients Database as an acceptable non-medicinal ingredient to be used as a flavour enhancer in natural health products (NHPID 2010). As ethyl acrylate is listed in the Licensed Natural Health Products Database, it is present in currently licensed natural health products (LNHPD 2010).
In Canada, ethyl acrylate is not listed in the Drug Products Database nor the Therapeutic Product Directorate's internal Non-Medicinal Ingredients Database as a medicinal or non-medicinal ingredient in pharmaceutical products or veterinary drugs (DPD 2010; 2010 e-mails from Therapeutic Products Directorate, Health Canada to Risk Management Bureau, Health Canada; unreferenced).
Based on information collected through a survey conducted pursuant to section 71 of CEPA 1999, 306 kg of ethyl acrylate was released to air and 1,954 kg was reported as transferred to off-site waste management facilities in Canada in 2006 (Environment Canada 2010a). No releases to water or land were reported (Environment Canada 2010a). According to releases reported under the National Pollutant Release Inventory (NPRI), 476 kg of ethyl acrylate was released to the environment in 2006 by point sources in Ontario and Quebec. A total of 72 kg was reported as released to ambient air and 3 kg reported as off-site disposal (NPRI 2008). The remaining 401 kg was not specified (air, water, land). The reported releases to NPRI went down to 133 kg to air and 8 kg to off-site disposal in 2008 (NPRI 2008). A total of 31 000 kg of ethyl acrylate was reported under TRI as released in the United States (TRI 2008).
Based on reported releases to the NPRI, the majority of ethyl acrylate releases to the environment occur to ambient air (Environment Canada 2008). It should be noted that there is a possibility of incomplete reporting to the NPRI including some industrial release to water. This assessment assumes some release of ethyl acrylate to surface water in estimating predicted environmental concentrations in water.
Table 3. NPRI Data for Ethyl Acrylate (Environment Canada 2008)
| Year | On-Site Releases (tonnes) | Disposa (tonnes) | ||||
|---|---|---|---|---|---|---|
| Air | Water | Land | Total | On-Site | Off-Site | |
| 2008 | 0.133 | 0.133 | 0.008 | |||
| 2007 | 0.555 | 0.555 | 0.006 | |||
| 2006 | 0.072 | 0.476[1] | 0.003 | |||
| 2005 | 0.059 | 0.115[2] | 0.024 | |||
| 2004 | 0.044 | 0.116[3] | ||||
| 2003 | 0.015 | 0.114[4] | ||||
| 2002 | 0.048 | 0.156[5] | 0.004 | |||
| 2001 | 1.5 | 1.7[6] | 9.2 | |||
| 2000 | 1.8 | 1.9[7] | 15 | |||
| 1999 | 0.322 | |||||
[2] 0.056 tonnes as total release but no indication of to which media
[3] 0.073 tonnes reported as total but no indication of to which media
[4] 0.099 tonnes reported as total but no indication of to which media
[5] 0.108 tonnes reported as total but no indication of to which media
[6] 0.21 tonnes reported as total but no indication of to which media
[7] 0.128 tonnes reported as total but no indication of to which media
Based on its physical and chemical properties (Table 2), the results of Level III fugacity modelling (Table 4) suggest that ethyl acrylate will predominantly reside in air, water or soil, depending on the compartment of release. The modelling program pKadB (ACD 2005) indicates that the substance does not ionize in water (there are no ionizable groups present).
Table 4. Results of the Level III fugacity modelling (EQC 2003)
| Substance released to: | Percentage of substance partitioning into each compartment | |||
|---|---|---|---|---|
| Air | Water | Soil | Sediment | |
| Air (100%) | 93.8 | 5.6 | 0.7 | 0.0 |
| Water (100%) | 1.8 | 97.9 | 0.0 | 0.2 |
| Soil (100%) | 3.2 | 11.4 | 85.3 | 0.0 |
If released to air, high amounts of the substance are expected to reside in air (see Table 4 above). Based on the high experimental vapour pressure of 3900 Pa and moderate to high Henry's Law constant of 25.3 Pa·m3/mol, ethyl acrylate is considered volatile. Therefore, if released solely to air, it will tend to remain in this compartment although a small amount of the substance will also be deposited to water (~6%, see Table 4).
If released into water, ethyl acrylate is expected to weakly adsorb to suspended solids and sediment based upon a low log Koc value of 1.34. Volatilization from water surfaces is expected to be an unimportant fate process based upon this compound's Henry's Law constant and due to the rapid degradation in water that limits transport to air. There is a possibility that polymerization of ethyl acrylate may be initiated via ultraviolet radiation in the river system, however, polymerization will only result in low molecular weight oligomers (<4 units in length) which are expected to biodegrade. Thus, if water is a receiving medium, ethyl acrylate is expected to mainly reside in water and to a very limited extent partition to air (see Table 4).
If released to soil, ethyl acrylate is not expected to have high adsorptivity to soil (i.e., expected to be mobile based upon its low log Koc). Volatilization from moist soil surfaces seems to be an unimportant fate process based upon its Henry's Law constant. This chemical may slightly volatilize from dry soil surfaces based upon its vapour pressure. Therefore, if released to soil, ethyl acrylate is expected to mostly remain in this environmental compartment, although some will likely be transported to surface water from run-off, diffuse to groundwater and volatilize to air.
Environmental Persistence
Table 5a presents the empirical biodegradation data that indicates that for ethyl acrylate, the half-life in air is likely to be shorter than two days and the half-life in water is likely to be shorter than 182 days (6 months) and that the substance is therefore likely to not persist in those environmental compartments.
Table 5a presents empirical biodegradation data (MITI 1992) that show 52% ultimate biodegradation of ethyl acrylate over 14 days in a ready biodegradation test. This test result suggests that the half-life in water would be about 13 days (assuming first order kinetics) – which is much shorter than 182 days (6 months) indicating that the substance is unlikely to persist in that environmental compartment. Staples et al. (2000) reported 57% degradation in 28 days using OECD methods (OECD 301D) (OECD 1992), indicating that ethyl acrylate is inherently biodegradable. The authors also tested the substance in the five day biochemical oxygen demand (BOD5) test, and found that ethyl acrylate degrades easily (77%). Ethyl acrylate was shown to be readily biodegradable (80-90% after 28 days) using the ISO (14593) method, identical to OECD 310 (OECD 2005; OECD 2003).
Table 5a. Empirical data for degradation of ethyl acrylate
| Medium | Fate Process | Degradation Value | Degradation Endpoint | Reference |
|---|---|---|---|---|
| Air | Ozone reaction | 2.01 | Half-life, days | Atkinson 1989 |
| Air | Atm. Oxidation | 0.67 | Half-life, days | Atkinson 1989 |
| Water | Hydrolysis | 1277.5 | Half-life, days (pH 7) |
Mabey and Mill 1978 |
| Water | Biodegradation | 52 | Biodegradation, % after 14 days (BOD) | MITI 1992 |
| Water | Biodegradation | 92.6 - 100 | Biodegradation, % after 14 days (analysis for parent compound) | MITI 1992 |
| Water | Biodegradation | 57 | Biodegradation, % after 28 days | Staples et al. 2000 |
| Water | Biodegradation | 80-90 | Biodegradation, % after 28 days | OECD 2005 |
Although experimental data on the degradation of ethyl acrylate are available, a QSAR-based weight-of-evidence approach (Environment Canada 2007) was also applied using the degradation models shown in Table 5b below. Given the ecological importance of the water compartment, the fact that most of the available models apply to water and the fact that ethyl acrylate is expected to be released to this compartment, biodegradation in water was primarily examined.
Table 5b summarizes the results of available QSAR models for degradation in water and air.
Table 5b. Modelled data for degradation of ethyl acrylate
| Fate Process | Model and model basis |
Model Result and Prediction | Extrapolated Half-life (days) |
|---|---|---|---|
| Air | |||
| Atmospheric oxidation | AOPWIN 2008[1] | t1/2 = 11.8 hours | ≤ 2 |
| Ozone reaction | AOPWIN 2008[1] | t1/2 = 6.5 days | ≥ 2 |
| Water | |||
| Hydrolysis | HYDROWIN 2008[1] | t1/2 = 9 years (pH7) t1/2 = 329 days (pH8) |
≥ 182 |
| Primary biodegradation | |||
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 4: Expert Survey (qualitative results) |
3.9[2] (biodegrades fast) |
≤ 182 |
| Ultimate biodegradation | |||
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 3: Expert Survey (qualitative results) |
3.1[2] (biodegrades fast) |
≤ 182 |
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 5: MITI linear probability |
0.8[3] (biodegrades fast) |
≤ 182 |
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 6: MITI non-linear probability |
0.9[3] (biodegrades fast) |
≤ 182 |
| Biodegradation (aerobic) | TOPKAT 2004 Probability |
1.0[3] (biodegrades very fast) |
≤ 182 |
| Biodegradation (aerobic) | CATABOL c2004-2008 |
% BOD = 52 (biodegrades fast) |
≤ 182 |
[2] Model does not provide an estimate for this type of structure.
[3] Output is a numerical score from 0 to 5.
[4] Output is a probability score.
In air, a predicted atmospheric oxidation half-life value of 11.8 hours (see Table 5b) demonstrates that this substance is likely to be rapidly oxidized. The substance is expected to react with other photo-oxidative species in the atmosphere such as O3 , but at a significantly slower rate. In addition, the atmospheric half-life as a result of indirect photolysis was estimated to be 6.5 hours (OECD 2005). Therefore, it is expected that reactions with hydroxyl radicals will be the most important fate process in the atmosphere for ethyl acrylate. With an empirically based half-life of 0.67 days (~16 hours; Table 5a) and an estimated half-life of 11.8 hours via reactions with hydroxyl radicals, ethyl acrylate is considered not persistent in air.
In water, a predicted hydrolysis half-life value of 9 years at pH 7 (see Table 5b) demonstrates that this chemical is likely to be slowly hydrolysed. However, other fate processes in water need to be considered to determine overall persistence in this medium.
The five ultimate biodegradation models suggest that biodegradation is fast and that the half-life in water would be significantly less than 182 days. The result of the BIOWIN Sub-model 4 (primary survey model) would suggest the substance has a primary half-life of much less than 182 days and the ultimate biodegradation sub-models of BIOWIN indicate that complete mineralization would occur within 182 days. Also, the predictions for CATABOL and TOPKAT are in the domains of both models. Thus, they are considered to be reliable and suggest a fast rate of biodegradation.
Using an extrapolation ratio of 1:1:4 for water: soil: sediment biodegradation half-life (Boethling et al. 1995), and an ultimate biodegradation half-life of < 90 days in water the ultimate biodegradation half-life in soil is also < 90 days and the half-life in sediments is ≤365 days. This indicates that ethyl acrylate is not expected to be persistent in soil and sediment.
Based on the empirical and modelled data (see Tables 5a and 5b) ethyl acrylate does not meet the persistence criteria in air, soil, water or sediment (half-life in air ≥ 2 days, half-lives in soil and water ≥ 182 days and half-life in sediment ≥ 365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Experimental log Kow values for ethyl acrylate suggest that this chemical has low potential to bioaccumulate in biota (see Table 2). Although the log Kow focuses primarily on aquatic based criteria and does not evaluate the potential impacts in the terrestrial environment and organisms, terrestrial organisms will not be exposed to this chemical through the diet and there will be low biomagnification potential in terrestrial animals.
Table 6: Modelled data for bioaccumulation for ethyl acrylate
| Test Organism | Log Kow | Endpoint | Value Wet Weight (L/kg) |
Reference |
|---|---|---|---|---|
| Fish | 1.18 | BAF | 1.59 | Gobas BAF T2MTL (Arnot and Gobas 2003) |
| Fish | 1.18 | BCF | 1.59 | Gobas BCF T2LTL (Arnot and Gobas 2003) |
| Fish | 1.22 | BCF | 0.91 | OASIS Forecast 2005 |
| Fish | 1.18 | BCF | 2.79 | BCFWIN 2000 |
| Fish | 1.33 | BCF | 6.0 | Tyler and Smock 1993[1] |
The available evidence indicates that ethyl acrylate is expected to have a low bioaccumulation potential due to its low experimental log Kow value. Model-estimated BCF and BAF values are much less than 5000 (Table 6). A BAF and BCF of 1.593 L/kg resulted from using an estimated metabolic rate constant to correct bioaccumulation predictions (Arnot and Gobas 2003). Based on the available kinetic-based and other modelled values, ethyl acrylate does not meet the bioaccumulation criterion (BCF or BAF ≥ 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
As indicated earlier, ethyl acrylate does not meet the persistence or bioaccumulation criteria as set out in thePersistence and Bioaccumulation Regulations (Canada 2000).
Ecological Effects Assessment
A study on the chronic aquatic toxicity of ethyl acrylate to Daphnia magna resulted in a LOEC of 0.45 mg/L and an EC50 of 0.5 mg/L (Table 7a). These values are considered to be indicative of potential for moderate to high chronic toxicity to aquatic organisms. The authors concluded that the low measured test concentration results may have been as a result of a number of factors, including the volatility, adsorption to the glass aquaria, adsorption to particulate matter and biodegradability of ethyl acrylate.
Table 7a. Empirical data for aquatic toxicity
| Test Organism | Test Type | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Algae (Selenastrum capricornutum) | Acute (96 hours) |
EC50 | 5.5 | OECD 2005 |
| Daphnia magna | Acute (48 hours) |
EC50[1] | 7.9 | OECD 2005 |
| Daphnia magna | Chronic (21 days) | NOEC[2] | 0.19 | OECD 2005 |
| Daphnia magna | Chronic (21 days) | LOEC[3] | 0.45 | OECD 2005 |
| Daphnia magna | Chronic (21 days) | EC50 | 0.5 | OECD 2005 |
| Fish (Cyprinodon variegatus) | Acute (96 hours) |
LC50[4] | 2.0 | IUCLID 2000 |
| Fish (Pimephales promelas) | Acute (96 hours) |
LC50[4] | 2.5 | Geiger et al. 1990 |
| Fish (Oncorhynchus mykiss) | Acute (96 hours) |
LC50[4] | 4.6 | OECD 2005 |
[2] NOEC – No observed Effect Concentration.
[3] LOEC – Lowest Observed Effect Concentration.
[4] LC50 – The concentration of a substance that is estimated to be lethal to 50% of the test organisms.
Table 7b. Modelled data for aquatic toxicity.
| Test organism | Type of test |
Endpoint | Value (mg/L) |
Reference |
|---|---|---|---|---|
| Fish | Acute (96 hours) |
LC50[1] | 2.2 | ECOSAR 2008 |
| 2.06 | OASIS Forecast 2005 | |||
| 18.79 | AIES 2003-2005 | |||
| 8.0 | TOPKAT 2004 | |||
| Fish | Chronic (30 day) |
ChV | 0.16 | ECOSAR 2008 |
| Daphnia | Acute (96 hours) |
EC50[2] | 6.8 | ECOSAR 2008 |
| Acute | 36.4 | TOPKAT 2004 | ||
| Acute (48 hours) | <221.9 | OASIS 2004 | ||
| Algae | Acute (96 hours) |
EC50[2] | 1.07 | ECOSAR 2008 |
[2] LC50 – The concentration of a substance that is estimated to be lethal to 50% of the test organisms.
The experimental and modeled toxicity results are comparable and indicate that ethyl acrylate has a moderate to high potential to be harmful to aquatic organisms. Ethyl acrylate is a reactive chemical, however, the mode of action is not known.
No suitable ecological effects studies were found for ethyl acrylate in media other than in water.
Ecological Exposure Assessment
No data concerning concentrations of this substance in water in Canada have been identified; therefore, environmental concentrations are estimated from available information, including estimated substance quantities, release rates, and size of receiving water bodies.
A – Industrial Release
As ethyl acrylate is used industrially (see Uses section) and is expected to be released to water, a reasonable worst-case industrial release scenario is used to estimate the aquatic concentration of the substance with the help of Environment Canada's (2009a) Industrial Generic Exposure Tool – Aquatic (IGETA). A site-specific exposure analysis was conducted for the aquatic compartment at two separate industrial sites where ethyl acrylate is used (Environment Canada 2010c). These sites were identified based on responses to the CEPA Section 71 Survey (Environment Canada 2010a). Each user reported an annual consumption quantity of ethyl acrylate in the range of 10 000 to 200 000 kg. The selection of these sites is therefore expected to represent a realistic worst case release scenario across Canada based on a general assumption that the quantity released is proportional to the quantity consumed.
In this site-specific exposure analysis, each site includes one facility, one wastewater treatment plant and one receiving water body. The predicted environmental concentration (PEC) in the receiving water was estimated based on the concentration in the wastewater treatment effluent and applying a dilution factor of 10. The concentration in the wastewater treatment effluent was estimated based on a fraction of the substance assumed to be lost from the facility to a local municipal wastewater treatment plant, an assumed wastewater treatment plant removal rate and the effluent flow rate of the treatment plant. The loss fraction was conservatively estimated to be 5% resulting from the chemical container handling operations and the industrial processes relevant to the facilities under consideration. It should be noted that this value is expected to represent the upper bound of the losses to wastewater and the release from an actual facility is expected to be below this upper bound. The removal at the local wastewater treatment plant was conservatively assumed to be zero. The effluent flow of the local wastewater treatment plant is proportional to the population served and was in the range of 100 000 to greater than 1 000 000 m3 per day for the sites considered. An assumption for the frequency of release was also used in the estimation which is 250 days/year for the industrial users (small or medium sized facilities).
Based on the above assumptions, the PECs at the 2 separate industrial sites where ethyl acrylate is used, were estimated to be 0.0002 mg/L and 0.0305 mg/L, respectively. The PEC values obtained are considered to represent the level of exposure under a realistic worst case release scenario in the receiving water near the point of the discharge from wastewater treatment plants at industrial sites in Canada.
B – Consumer Release
As ethyl acrylate is found in consumer products and can be released to water, Mega Flush, Environment Canada’s spreadsheet tool was employed to estimate the substance concentration in multiple water bodies receiving sewage treatment plant effluents to which consumer products containing the substance may have been released (Environment Canada 2009b). The spreadsheet tool provides these estimates for approximately 1000 release sites across Canada based on realistic assumptions. Although ethyl acrylate is found in consumer products, the amount of the substance that is released to sewers is estimated to be very small (1%) as the substance is transformed at the industrial stage.
The realistic assumptions include:
- loss to sewer at 100%;
- sewage treatment plant removal rate estimated at 0.0 % in case of no treatment;
- 3.1 % for primary only treatment and 68 % for primary-secondary combined treatment;
- number of annual release days at 365 days/year; and
- receiving water dilution factor in the range of 1 to 10.
The predicted environmental concentration (PEC) of ethyl acrylate in the receiving water bodies was estimated to be 0.0065 mg/L. The estimate is based on a range of 10 000 to 100 000 kg/year for the quantity of the substance used by consumers. The equation and inputs used to calculate the PEC are described in Environment Canada (2010d).
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA 1999. Lines of evidence considered include results from conservative risk quotient calculations, as well as information on persistence, bioaccumulation, toxicity, sources and fate of the substance.
Ethyl acrylate is not expected to be persistent in air, water, soil and sediment; it is also expected to have a low bioaccumulation potential. Although there are high importation volumes of ethyl acrylate into Canada, once released into the environment, it will be found mainly in water, although based on the substance’s volatility and emission pattern, there will be release to the atmosphere. It has also been demonstrated to have moderate to high potential for toxicity to aquatic organisms.
A risk quotient analysis, integrating conservative estimates of exposure with toxicity information, was performed for the aquatic medium to determine whether there is potential for ecological harm in Canada. The two separate site-specific industrial scenarios (considering the actual receiving water bodies) presented above yielded predicted environmental concentrations (PECs) of 0.0002 mg/L and 0.0305 mg/L (Environment Canada 2010c). By dividing the chronic toxicity value (LOEC) of 0.45mg/L (the most sensitive valid experimental value) for Daphnia magna, by an assessment factor of 10 (to account for interspecies and intraspecies variability in sensitivity) a predicted no effect concentration (PNEC) of 0.045 mg/L is derived. Conservative risk quotients (PEC/PNEC) for the two industrial scenarios are thus determined to be 0.0047 and 0.68. Therefore harm to aquatic organisms is unlikely.
When the PEC of 0.0065 mg/L for ethyl acrylate predicted for the consumer release scenario is divided by the PNEC of 0.045 mg/L, the resulting conservative risk quotient (PEC/PNEC) = 0.14. As with the industrial release scenario, harm to aquatic organisms from consumer use is unlikely.
This information suggests that ethyl acrylate is unlikely to cause ecological harm in Canada.
Uncertainties in Evaluation of Ecological Risk
There is uncertainty present from the use of QSAR predictions but due to the small relatively simple structure of this chemical, the majority of the modelled results are judged to be reliable and within the domain of the models and therefore the uncertainty is deemed to be lower overall.
There is uncertainty in the results of the aquatic effects to ethyl acrylate as the substance is expected to degrade rapidly in water, however polymerization is also possible. Only valid aquatic effects measured data were considered.
Although there is some uncertainty in the estimation of environmental exposure levels, as no Canadian monitoring data were identified, two conservative release scenarios were used to fill the data gap.
Exposure Assessment
Environmental Media and Food
The upper-bounding estimate of intake of ethyl acrylate from ambient air, indoor air, soil, drinking water, and food and beverages in Canada is 0.00581 mg/kg-bw per day (Appendix 1). Although the principal source of environmental exposure is identified as indoor air; this is considered highly conservative as ethyl acrylate was not detected in any of 757 homes monitored in a Canadian study and the limit of detection (10 µg/m3 ) was assumed in deriving indoor air concentrations. (Otson et al. 1994). Indoor air concentrations of 0.04-2.1 mg/m3 ; ethyl acrylate were reported in a US office building (no location or sample information reported, BUA 1992).
Ethyl acrylate was reported as naturally occurring in fresh pineapple (IARC 1986), raspberries, blackberries, yellow passion fruit, and durian (NTP 1998; Burdock 1997). The Joint FAO/WHO Expert Committee on Food Additives (JECFA) reports an estimated daily intake of ethyl acrylate from food flavouring based on annual production volumes of ethyl acrylate used as a flavouring agent (JECFA 2006). The intake was estimated to be 1.1 x 10-5and 2.6 x 10-5 mg/kg bw/day in the United States and Europe, respectively (JECFA 2006). Based on the information available, foods are not the primary contributors to overall exposure to ethyl acrylate (Appendix 1).
Although ethyl acrylate-based materials are used in food packaging applications, residual ethyl acrylate in food packaging materials is low and migration into food is negligible (2010 emails from Food Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). The potential daily intake resulting from residual ethyl acrylate in food packaging applications is insignificant compared to exposures from its natural occurrence in some fruits and its use as a food flavour.
Ethyl acrylate may also be present as a residual in packaging for pharmaceutical products. This is not considered to be a significant source of exposure to the general population.
The ambient air concentration is based on an air monitoring study conducted in Houston and Boston, US, with 22 samples collected at each location between August 1990 and August 1991 (Kelly et al. 1993). Ethyl acrylate was not detected in any samples, so the reported limit of detection of 0.2 ppb (0.82 µg/m3) for the concentration of ethyl acrylate in ambient air was used as a conservative input for modelling. No Canadian data were available. Ethyl acrylate was also detected in ambient air (0.6-1.8 ng/m3) in 3 of 15 samples at 1 of 5 locations collected in Japan in 2001 (NITE 2010).
Ethyl acrylate was also qualitatively detected in the ambient air at a US landfill (no location or sample information reported) (BUA 1992) and in the exhaust air of production plants in the US (12 500 and 25 000 mg/m3), New Zealand (11-622 mg/m3) and Japan (qualitatively) (BUA 1992), however these point sources would not represent general population exposure.
Ethyl acrylate was not detected in any of the more than 250 groundwater samples taken from or around five industrial plants which produce ethyl acrylate, therefore the drinking water concentration used for modelling exposure was based on the reported limit of detection of between 1 and 10 ppb (McLaughlin et al. 1993). Since no soil monitoring studies were identified, a Canadian-specific environmental exposure model was used to predict conservative concentrations in soil based on the amount of the substance released in Canada (ChemCAN 2003).
Other reported monitoring studies of ethyl acrylate in water and sediment include: Ethyl acrylate non-detects in 51 surface water and bottom sediment samples collected from 17 locations in Japan in 1980 (the limit of detection was 0.3-50 µg/L, NITE 2010). In 100 samples of soil, wastewater and surface water analysed, only two sediment samples showed detectable levels of ethyl acrylate at 2.1 and 2.2 mg/kg (no limit of detection was reported, McLaughlin et al. 1993). Ethyl acrylate was not detected at a wastewater treatment plant in Germany (the limit of detection was 0.02 mg/L; BUA 1992). Ethyl acrylate was detected, but not quantified, in one of 17 ground water samples collected in the USA (location not reported, BUA 1992).
Consumer Products
Based on information received under the s. 71 survey, consumer exposure modeling was performed using RIVM’s ConsExpo v.4.1 software (ConsExpo 2006) of the Netherlands for consumer product and personal care product scenarios (Table 3, Appendix 2).
Ethyl acrylate may be found as a residual in consumer products. For modelling purposes, 100% uptake by the inhalation and dermal routes was used for ConsExpo v.4.1. Due to the types of products where ethyl acrylate is used, the oral route of exposure was not relevant. These conservative uptakes would result in slightly higher modelled exposures compared to those that may be occurring.
In addition to use of ConsExpo (ConsExpo 2006), the US EPA’s Wall Paint Exposure Model (WPEM) software (US EPA 2001) was used to model consumer exposure during household painting. The WPEM results were consistently lower; therefore, the ConsExpo model outputs were considered to provide a more conservative estimate of exposure.
Acrylic polymers that could contain ethyl acrylate are typically found in more expensive indoor paints, since the addition of an acrylic polymer to paint provides increased durability and scrub resistance, as well as in semi-gloss paints that are used to paint interior wood trim (Frederick 1998). In a small market basket study (Frederick 1998), 30 samples of a variety of consumer paints were analysed for ethyl acrylate. They reported between 1.26 and 4.49 ppm (mean 1.28 ppm) ethyl acrylate in flat latex wall paint, and between 1.61 and 13.02 ppm (mean 4.06 ppm) in indoor trim paint (Frederick 1998).
In a laboratory study, two samples of paints with residual concentrations of ethyl acrylate (940 ppm and 2000 ppm w/w) were prepared and used to paint a test room (McLaughlin et al. 1993). There was no ventilation in the test room. Maximum concentrations of ethyl acrylate reported in the air in the room were 2.5 ppm and 8.0 ppm from the 940 and 2000 ppm paint samples, respectively (McLaughlin et al. 1993). The report also indicated that when the painting scenarios were repeated with proper ventilation, no ethyl acrylate was detected (limit of detection 0.2 ppm) (McLaughlin et al. 1993).
Ethyl acrylate may be present as a residual in a variety of do-it-yourself products. An estimate of exposure to individuals using caulk is provided in Table 3. A concentration of <0.1% was assumed based on a reference to paintable joint sealant on the Household Products Database (Household Products Database 2009).
Ethyl acrylate can be used in the manufacture of non-woven personal wipes and other non-woven materials for medical and hospital applications at an estimated maximum ethyl acrylate concentration of 28 ppm[3],[4] (Environment Canada 2010b).
Other products that may contain residual ethyl acrylate were identified: Roofing products and other industrial binding and coating applications containing a maximum estimated concentration of 29 ppm[4] (Environment Canada 2010b). Ethyl Acrylate may also be present in wood glue at a maximum concentration of 40 ppm[3](Environment Canada 2010b). Since these products are used infrequently, they are not considered a significant source of exposure to the general population and exposure estimates were not derived.
Cosmetics that previously contained acrylic polymers that may have included ethyl acrylate have been re-formulated and ethyl acrylate is no longer used. These products are no longer available on the Canadian market (2010 emails from Consumer Product Safety Bureau, Health Canada, to Existing Substances Division, Health Canada; unreferenced).
In the literature, ethyl acrylate has been described as an additive to adhesives (McLaughlin et al. 1993), such as in self-adhesive bandages. The amount of residual ethyl acrylate in self-adhesive bandages is considered to be very low. Since self-adhesive bandages are used infrequently, it is not considered a significant source of exposure to the general population and exposure estimates were not, accordingly, derived.
Ethyl acrylate is used in various parts of automotive vehicles including: the exterior paint, sealants, and in the plastic components. Based on information received under the s71 survey, the estimated total concentration of ethyl acrylate in a finished vehicle is less than 0.031 kg/vehicle (Environment Canada 2010a). These components are not in the passenger area of the vehicle and are therefore not considered a significant source of exposure to the general population.
Ethyl acrylate may be present as a residual in additional products[4] (Environment Canada 2010b) used in industrial settings only, and are therefore not considered a source of exposure to the general population.
Table 3. ConsExpo v.4.1 models (See Appendix 2)
| Modelled consumer product at specified level | Dermal acute exposure (mg/kg) |
Dermal chronic exposure (mg/kg/day) | Inhalation mean event concentration (mg/m3) |
|---|---|---|---|
| Paint – flat latex (4.5 ppm) |
2.54 x 10-4 | 1.39 x 10-6 | 0.225 |
| Paint – semi-gloss trim (13.2 ppm) | 3.6 x 10-4 | 1.99 x 10-6 | 0.108 |
| Caulk (<0.1%) | 2.12 x 10-3 | 1.74 x 10-5 | 0.427 |
| Personal wipes - infants (28 ppm) |
7.47 x 10-5 | 3.73 x 10-4 | - |
| Personal wipes - adults (28 ppm) |
7.9 x 10-6 | 7.89 x 10-6 | - |
Health Effects Assessment
A summary of the available health effects information for ethyl acrylate is provided in Appendix 3.
The International Agency for Research on Cancer (IARC) has classified ethyl acrylate as a Group 2B carcinogen (possibly carcinogenic to humans) because of sufficient evidence for carcinogenicity in experimental animals and no adequate epidemiological data relevant to the carcinogenicity in humans was available (IARC 1986, 1999). US EPA has classified ethyl acrylate as a possible human carcinogen (Group B2) (US EPA 2005, 2009). Ethyl acrylate was first listed in the National Toxicology Program (NTP) Fifth Annual Report on Carcinogens as “reasonably anticipated to be a carcinogen” but later delisted from the NTP Ninth Annual Report on Carcinogens after reassessment indicating no carcinogenic potential (NTP 1989, 2000).
In experimental animal toxicity studies, increased incidences of forestomach tumours were observed in rats and mice orally administered ethyl acrylate by gavage. Fischer 344 rats and B6C3F1 mice treated with ethyl acrylate by oral gavage at 0, 100 or 200 mg/kg-bw per day, 5 days/week for 103 weeks had a significantly increased incidence of forestomach tumours (NTP 1986a). Forestomach tumours (squamous cell papillomas and squamous cell carcinomas) were observed at 100 and 200 mg/kg-bw per day in both sexes in both species. In a follow-up study, male Fischer 344 rats were orally administered ethyl acrylate by gavage at 0 or 200 mg/kg-bw per day, 5 days/week for 6 or 12 months followed by recovery periods of up to 15 months (Ghanayem et al. 1993, 1994). No neoplastic effects were observed in animals treated 200 mg/kg-bw per day ethyl acrylate for 6 months with 0, 2 or 15 months of recovery and animals sacrificed immediately after 12 months. Development of forestomach tumours was observed during 2-9 month recovery periods in animals treated with ethyl acrylate for 12 months.
In contrast to the gavage studies, no neoplastic effects were observed in experimental animals administered ethyl acrylate by other routes. In a drinking water study, Wistar rats were administered ethyl acrylate at 0, 6-7, 60-70 or 2000 ppm (approximately 0, 0.84-0.98, 9.4-9.8 and 280 mg/kg-bw respectively) for 2 years (Borzelleca et al. 1964). Histopathological findings showed no treatment-related lesions. Borzelleca et al. (1964) also conducted a chronic toxicity study in dogs. Beagle dogs were orally administered ethyl acrylate in gelatin capsules at 0, 10, 100 or 1000 ppm (approximately 0, 0.3, 3 and 30 mg/kg-bw per day respectively) for 2 years and no neoplastic effects were observed.
In inhalation carcinogenicity studies, no treatment-related neoplastic lesions were observed in Fischer 344 rats and B6C3F1 mice exposed to ethyl acrylate at 0, 25 or 75 ppm (approximately 0, 100 and 310 mg/m3 respectively), 6 hour per day, 5 day per week for 27 months (Miller et al. 1985). In another study conducted by Miller et al. (1985), Fischer 344 rats and B6C3F1 mice were exposed to ethyl acrylate at 0 or 5 ppm (approximately 20 mg/m3) for 24 months and no neoplastic effects were observed.
In a dermal carcinogenicity study, male C3H/HeJ mice were administered 800 mg/kg-bw per day of ethyl acrylate, 3 times per week for life (DePass et al. 1984). No epidermal tumours were observed.
In epidemiological studies, results from workplace cohort studies were inconsistent. Ethyl acrylate exposures were not well quantified and workers were exposed to other chemicals as well. In a cohort study in 1933-1945, workers in an acrylic sheet manufacturing facility at Bristol, Pennsylvania, were exposed to ethyl acrylate, methyl methacrylate, lead, ethylene dichloride, methylene chloride and acrylonitrile. Excess mortality from colon cancer and rectal cancer were observed in exposed workers 20 years after the equivalent of 3 years of employment (Walker et al. 1991). However, two other similar cohort studies described by Walker et al. (1991) reported no excess mortality from any cause.
Limited mutagenic potential was observed in in vitromutation assays. A number of Ames tests in different strains ofSalmonella typhimurium were conducted in the presence and/or in the absence of metabolic activation and the results were negative (Rohm and Haas 1977, 1981; Ishidate et al. 1981; Haworth et al. 1983; Waegemaekers and Bensink 1984; NTP 1986a; Brusick 1977; Zeiger et al. 1992; ECETOC 1994; Emmert et al. 2006). Result from a umu test conducted using Salmonella typhimurium was negative (Yasunaga et al. 2004) whereas induction of mitotic recombination in Saccharomyces cerevisiae was positive (Zimmermann and Mohr 1992). In mammalian cells, induction of gene mutations was observed in mouse lymphoma L5178Y TK+/- cells (Myhr 1980; McGregor et al. 1988; Moore et al. 1988; Dearfield et al. 1991) but not in HGPRT assays in Chinese hamster ovary (CHO) cells (Moore et al. 1989). In terms of clastogenic effects, chromosome aberration assays were mostly positive in mouse lymphoma cells, CHO cells, mouse splenocytes and Chinese hamster lung (CHL) cells (NTP 1986b; Moore et al. 1988; Ishidate et al. 1981; Loveday et al. 1990; Kligerman et al. 1991). Mixed results were available for sister chromatid exchange assays in CHO cells and in mouse splenocytes (NTP 1986b; Loveday et al. 1990; Kligerman et al. 1991).
In in vivo assays, genotoxicity results were mostly negative. No induction of chromosome aberrations was observed in mice administered ethyl acrylate intraperitoneally (Kligerman et al. 1991). Micronucleus induction assays conducted in different strains of mice administered ethyl acrylate intraperitoneally, orally or dermally were mostly negative (Przybojewska et al. 1984; Basler and van der Hude 1987; Ashby et al. 1989; Kligerman et al. 1991; Hara et al. 1994; Morita et al. 1997; Tice et al. 1997). Sister chromatid exchange assays conducted in mice administered ethyl acrylate intraperitoneally were negative (Kligerman et al. 1991). In germ cells, results from sex-linked recessive lethal assays conducted in Drosophilia melanogaster by feeding or by injection were negative (Valencia et al. 1985).
The Organisation for Economic Co-operation and Development (OECD) Screening Information Dataset Initial Assessment Meeting (SIAM) in 2004 concluded that ethyl acrylate posed “no mutagenic hazard” concerns (OECD 2004).
The proposed mode of induction of rat forestomach tumours by ethyl acrylate has not been fully elucidated. In rodents, forestomach is a non-glandular food storage organ that connects to the oesophagus and is lined by keratinized, stratified squamous epithelium; whereas, the glandular stomach, which empties into the duodenum, is lined by a specialized glandular epithelium. In contrast, in humans, the entire stomach is glandular; however, comparable squamous epithelial tissues are present in the oral cavity and the upper two-thirds of the oesophagus. Unlike rodents, neither location, plays a role in food storage. IARC (2003) published a report that discussed the predictive value of rodent forestomach tumours in evaluating carcinogenic risks to humans of several chemicals including ethyl acrylate. Although IARC (2003) did not propose a mode of action for ethyl acrylate induced forestomach tumour, the working group suggested that a certain time of sustained hyperplasia is required for effective tumourigenesis (Boorman and Sills 2003). Oral gavage studies in rodents indicted that ethyl acrylate induced forestomach hyperplasia is sustained as long as ethyl acrylate is administered. Full recovery of forestomach hyperplasia was observed in animals treated for 6 months, but persistence of forestomach hyperplasia was observed in rats treated for 12 months (Ghanayem et al. 1993, 1994). In addition, development of forestomach tumours was not observed immediately, but observed during recovery periods in rats treated for 12 months.
Williams and Iatropoulos (2009) proposed that the induction of forestomach tumours by ethyl acrylate is route-specific. The authors proposed that a long transit time ranging from half a day to 2 or 3 days and the less acidic pH in the forestomach allows for the presence of proliferating bacteria, and that the progression from local tissue irritation, inflammation and hyperkeratosis to sustained hyperplasia are major precursor events leading toward ethyl acrylate induced forestomach neoplasia. Hyperplasia or cell proliferation has long been recognized as an important factor in carcinogenesis. It has been suggested that sustained cell proliferation, rather than just cell proliferation is required for carcinogenesis (Preston-Martin et al. 1990; Melnick et al 1993; Huff 1995). Proctor et al. (2007) further suggested that forestomach tumours associated with chronic irritation of the forestomach epithelium, particularly those induced by repeated oral gavage dosing might result in a tissue dose that is not representative of human exposure.
No classifications for reproductive or developmental toxicity were available from national and international regulatory agencies. The lowest-observed-effect level (LOEL) identified for oral developmental toxicity was 25 mg/kg-bw per day based on delayed ossification when rats were administered ethyl acrylate by gavage at 0, 25, 50, 100, 200 or 400 mg/kg-bw per day on gestation days 7-16 (Pietrowicz et al. 1980). At all doses, a reduction in maternal body weight gain was also observed. For the inhalation route, the LOEC was 200 ppm (800 mg/m3) based on a significant decrease in fetal body weight in Sprague-Dawley rats exposed to ethyl acrylate at 0, 25, 50, 100 or 200 ppm (approximately 0, 100, 200, 400 and 800 mg/m3respectively), 6 hours per day on gestation days 6-20 (Saillenfait et al. 1999). A significant decrease in maternal body weight gain and absolute weight gain were also observed at 200 ppm. In a limited case study, a mother who was exposed to polymers containing ethyl acrylate reported to have a child diagnosed with congenital anomalies; however, further details of the study were not available (Sherman 1985). No reproductive toxicity studies and no dermal developmental toxicity studies were identified.
In rodents, oral repeated dosing of ethyl acrylate generally affects the forestomach. The lowest LOAEL identified was 20 mg/kg-bw per day based on an increase in stomach weight and a dose-related increase in epithelial hyperplasia and hyperkeratosis of the forestomach in male Fischer 344 rats administered ethyl acrylate orally by gavage in several studies conducted from 14 days to 13 weeks (Rohm and Haas 1986b, 1987; Frederick et al. 1990). In a similar study conducted by Frederick et al. (1990), a LOAEL of 1000 ppm (99 mg/kg-bw per day) was identified based on minimal irritation and diffuse epithelial hyperplasia of the forestomach when male Fischer 344 rats were administered ethyl acrylate in drinking water at 0, 200, 1000, 2000 or 4000 ppm (corresponding to 0, 23, 99, 197 and 369 mg/kg-bw per day respectively), 5 days/week for 14 days. However, in a 2-year study in rats administered ethyl acrylate by drinking water, no histopathological changes were observed (Borzelleca et al. 1964).
Rodent inhalation toxicity studies generally resulted in nasal effects. The lowest LOAEC for acute exposure was 25 ppm (100 mg/m3) based on reversible olfactory epithelium lesions in male rats exposed nose-only to ethyl acrylate for 3 hours (Frederick et al. 2002). It should be noted that rodents have substantive differences in nasal anatomy and nasal air flow compared to human (Frederick et al. 1998, 2002). Computational inhalation model simulations suggested that human olfactory epithelium is expected to have at least two- to threefold lower tissue concentrations of inhaled organic acid vapour such as ethyl acrylate than that of rodents (Frederick et al. 1998, 2002). A higher LOAEC for acute exposure was 75 ppm (310 mg/m3) based on olfactory epithelium lesions (focal degeneration, necrosis, exfoliation and mild inflammation) in monkeys exposed to ethyl acrylate for 3 hours (Rohm and Haas 1994; Harkema et al. 1997). The US EPA used both the Frederick et al. (2002) rat study and the Rohm and Haas (1994), Harkema et al. (1997) monkey study as points of departure for derivation of the interim Acute Exposure Guideline Level-1 (AEGL-1) and Acute Exposure Guideline Level-2 (AEGL-2) respectively (US EPA 2007). The critical LOAEC for short term exposure was 300 ppm (1200 mg/m3) based on inflammation, degeneration, focal necrosis and squamous metaplasia of the nasal turbinates in rats and mice exposed to ethyl acrylate for 30 days (Miller et al. 1979). The critical LOAEC for long term inhalation exposure was 25 ppm (100 mg/m3) based on non-neoplastic lesions of the olfactory mucosa which included degeneration, inflammation and hyperplasia in rats and mice exposed to ethyl acrylate from 6 to 27 months (Jersey et al. 1978; Dow Chemical 1979a; Miller et al. 1985).
For the dermal route, the lowest LOAEL identified was 800 mg/kg-bw per day based on dermatitis, dermal fibrosis, epidermal necrosis and hyperkeratosis in male C3H/HeJ mice administered approximately 800 mg/kg-bw per day ethyl acrylate, 3 times per week for life (DePass et al. 1984).
In humans, a workplace cohort study suggested a correlation of ethyl acrylate exposure to central nervous system disturbance. However, this study is of limited utility in characterizing health effects associated with ethyl acrylate exposure as individuals were also concurrently exposed to significant levels of other chemicals (n-butyl acrylate and acrylonitrile) (Kuzelova et al. 1981).
Ethyl acrylate is a strong skin irritant and eye irritant in experimental animals (Haskell Laboratories 1945; Pozzani et al. 1949; Treon et al 1949; Oettel and Zeller 1958; Celanese Chem Co. 1972; BASF AG 1978; Lomonova and Klimova 1979; Poole 1980; Potokar et al. 1985; Rohm and Haas 1986a, c, d, 1991; Union Carbide Corp 1989; BAMM 1994; BASF 2005). In a human case study, accidental exposure to ethyl acrylate resulted in severe eye irritation (Dow Chemical 1964). In addition, there is some evidence suggesting that ethyl acrylate is a sensitizer and exposure to ethyl acrylate may result in cross-sensitization with other acrylates in animals (Van der Walle et al. 1982). However, results from ear swelling tests and local lymph node assays were negative (Kimber 1992; NTP 1994; Hayes and Meade 1999). In humans, sensitization was observed in volunteers (Epstein 1974) and in patients who had suspected allergic contact dermatitis (Foulger and Fleming 1945; Jordan 1975; Fregert 1978; Bjorkner and Dahlquist 1979; Malten et al. 1984; Conde-Salazar et al. 1988; Kanerva et al. 1988; 1989, 1992, 1995; Stenman and Bergman 1989; Skoglund and Egelrud 1991; Koppula et al. 1995; Marks et al. 1995; Rustemeyer and Frosch 1996; Tucker and Beck 1999; Lazarov 2007; Aalto-Korte et al. 2007). Some of these dermatitis patients had a history of exposure to acrylates or had developed occupational dermatitis in response to dental materials, acrylic sealants or artificial nails.
Toxicokinetic studies in experimental animals show that ethyl acrylate is absorbed and metabolized rapidly following oral and inhalation exposure (Stott and McKenna 1984; Ghanayem et al. 1987). The two major routes of metabolism are hydrolysis of the ester linkage and conjugation with glutathione (GSH). Hydrolysis of ethyl acrylate is catalyzed by the carboxylesterases, resulting in the production of ethanol and acrylic acid (Miller et al. 1981; Frederick et al. 1994a). Ethanol is further metabolized under catabolic process and acrylic acid goes through the propionate degradative pathway of cellular metabolism, resulting in the formation of carbon dioxide in both cases. Conjugation with GSH can occur either spontaneously by a Michael addition or can be mediated by GSH transferase (Ghanayem et al. 1987; Potter and Tran 1992). Inhalation of ethyl acrylate in rats resulted in nonprotein sulfhydryl (NPSH) depletion most pronounced in liver followed by blood, brain and lungs (Vodicka et al. 1990). In oral gavage toxicity studies in rodents, significant reduction of NPSH in the forestomach and the glandular stomach was observed suggesting that conjugation at the site of contact might be an important detoxification process (De Bethizy et al. 1987). Following GSH conjugation, ethyl acrylate is rapidly eliminated by urinary excretion (De Bethizy et al. 1987). Although theoretically possible, no evidence was available for the generation of epoxidation products in ethyl acrylate metabolism (Delbressine et al. 1982; De Bethizy et al. 1987). A physiologically based pharmacokinetic and pharmacodynamic model of rats was developed and described the absorption, distribution and metabolism of orally dosed ethyl acrylate (Frederick and Chang-Mateu 1990; Frederick et al. 1992). Similar biologically based interspecies dosimetry models for inhalation of ethyl acrylate were also developed (Frederick et al. 1994b, 2002; Sweeney et al. 2004).
The confidence in the toxicity database for ethyl acrylate is considered to be moderate to high. Data were identified for carcinogenicity, genotoxicity, developmental toxicity, acute and repeated–dose toxicity in experimental animals. However, reproductive toxicity studies were not identified and conclusive epidemiology studies were not available.
Characterization of Risk to Human Health
Based on the classifications by other national and international regulatory agencies, a critical effect for characterization of risk to human health for ethyl acrylate is carcinogenicity. Forestomach tumours were induced in both sexes in rats and mice when ethyl acrylate was administered orally by gavage for 2 years. However, no induction of tumours was observed when ethyl acrylate was administered by other routes including orally in drinking water, inhalation and dermal. The collective evidence from genotoxicity studies indicates that ethyl acrylate is not likely to be mutagenic but may exhibit some clastogenicity in in vitro assays. The OECD Screening Information Data Set Initial Assessment Meeting 18 (meeting concluded that ethyl acrylate posed “no mutagenic hazard” concerns (OECD 2004). Although the mode of induction of rodent specific forestomach tumours is not fully elucidated, it is proposed that chronic ethyl acrylate bolus gavage dosing into the forestomach (an organ not found in humans), induces sustained irritation and hyperplasia as a precursor for the development of tumors (OECD 2004, NTP 2000, Williams and Iatropoulos (2009)).
Accordingly, a threshold approach is used to characterize risk to human health.
In terms of non-cancer effects, no reproductive toxicity studies were identified. Developmental effects were accompanied by maternal toxicity suggesting a secondary effect. The margins of exposure are based on conservative upper-bounding estimates of general population exposure and the critical LOAELs and LOAEC for non-cancer effects. The critical LOAEL for the oral route is 20 mg/kg-bw per day based on an increase in stomach weight and forestomach hyperplasia and hyperkeratosis in rats treated from 14 days to 13 weeks by gavage (Rohm and Haas 1986b, 1987; Frederick et al. 1990). A higher LOAEL of 99 mg/kg-bw per day with similar health effects via drinking water was identified; however, the more conservative LOAEL from gavage studies was used to derive margins of exposure.
The critical LOAECs for acute inhalation exposure were 25 ppm (100 mg/m3) and 75 ppm (310 mg/m3) based on olfactory epithelium lesions in rats (Frederick et al. 2002) and in monkeys (Rohm and Haas 1994; Harkema et al. 1997), respectively, exposed to ethyl acrylate for 3 hours. Based on substantive differences in nasal anatomy and nasal air flow, human olfactory epithelium is expected to have at least two- to threefold lower tissues concentration than that of rodents (Frederick et al. 1998, 2002). The US EPA adopted these studies for derivation of AEGL-1 and AEGL-2 levels (US EPA 2007). The critical LOAEC for short term inhalation exposure is based on inflammation, degeneration, focal necrosis and squamous metaplasia of the nasal turbinates observed in rats and mice exposed to ethyl acrylate at 300 ppm (1200 mg/m3) for 30 days (Miller et al. 1979). The critical LOAEC for long term inhalation is 25 ppm (100 mg/m3) based on olfactory mucosa lesions in rats and mice exposed for 6 to 27 months. The critical LOAEL for the dermal route is 800 mg/kg-bw per day based on induction of dermatitis in a chronic mouse study.
The principal routes of exposure to ethyl acrylate to the general population in Canada is expected to be from short term inhalation of indoor air and from the use of consumer products (such as paint and other do-it-yourself products). Spontaneous polymerization, photodegradation and volatilization properties of ethyl acrylate (OECD 2004) are expected to minimize long term inhalation exposure for the general population.
Comparison of the critical effect dose level for repeated dosing via the oral route (20 mg/kg-bw per day) and the upper-bounding estimate of total daily intake from environmental media and food by the general population in Canada (0.00199 - 0.00581 mg/kg-bw per day) results in margins of exposure of 3500 - 10 000.
Based on consumer product scenario modelling, the air concentration of ethyl acrylate resulting from using do-it-yourself caulking is 0.427 mg/m3. Comparison of this upper-bounding acute estimate with the range of critical effect concentrations for acute inhalation of 100-310 mg/m3results in margins of exposure of 230-700. The dermal exposure of ethyl acrylate resulting from use of personal wipes (infants) was 3.73 x 10-4 mg/kg-bw per day. Comparison of this exposure level with the critical effect level for the dermal route from a chronic mouse study (800 mg/kg-bw per day) results in a margin of exposure of 2.14 x106. This is a conservative margin of exposure because exposure from use of personal wipes is compared to the critical effect level from a chronic dermal study.
This margin of exposure is considered adequate for both skin sensitization and skin irritation.
It is also noteworthy that 14 of 24 human volunteers dermally exposed to ethyl acrylate (4% ethyl acrylate in petrolatum) in a maximization test for 48 hours showed no sensitization and no skin irritation (Epstein 1974 cited in Opdyke 1975).
The margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
Uncertainties in Evaluation of Risk to Human Health
The determination of margins of exposure within the scope of this screening assessment does not take into account possible differences between humans and experimental animals in terms of sensitivity to effects induced by ethyl acrylate. The rodent forestomach tumours are not considered relevant to human health risk assessment as humans lack a forestomach, although histologically similar organs are present. In addition, the tissue dose in rodent forestomach via gavage administration is not representative of the nature of human exposures. Developmental toxicity is accompanied by maternal toxicity suggesting a secondary effect. No information for reproductive toxicity was available. The purity of ethyl acrylate is generally >99% and some studies indicated that the ethyl acrylate used contained a polymerization inhibitor such as monomethyl ether of t-butylhydroquinone or 4-methoxyphenol. The presence of a polymerization inhibitor, though the quantity is minimal, might confound the experimental results. Spontaneous polymerization, photodegradation and volatilization of ethyl acrylate might also affect the actual dosage in the experimental studies.
Inhalation was identified as the primary exposure route for the general population for both environmental media and consumer products, as one could expect from ethyl acrylate’s vapour pressure and volatile nature. Canadian environmental data available for indoor air showed no quantifiable levels; however, the LOD of the analytical method was used for derivation of the upper-bounding estimates of exposure. There is a moderate degree of confidence in upper-bounding estimates of exposure from use of consumer products, because there is ome uncertainty around the concentrations of residual ethyl acrylate remaining in consumer products in the marketplace. However, ethyl acrylate polymerizes when exposed to light and is therefore not expected to be available as a monomer.
On the basis of the adequacy of the margins between upper-bounding estimates of exposure to ethyl acrylate and critical effect levels, it is concluded that ethyl acrylate is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Based on the information presented in this screening assessment, it is concluded that ethyl acrylate is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. Additionally, ethyl acrylate does not meet the criteria for persistence and bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
It is therefore concluded that ethyl acrylate does not meet any of the criteria under section 64 of CEPA 1999.
This substance will be considered for inclusion in the Domestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
Aalto-Korte K, Alanko K, Kuuliala O, Jolanki R. 2007. Methacrylate and acrylate allergy in dental personnel. Contact Derm 57(5):324-330.
ACD/pKaDB [Prediction Module]. 2005. Version 9.04. Toronto (ON): Advanced Chemistry Development. [cited 2010 May 18]. ACD/Labs. [restricted access]
[AIES] Artificial Intelligence Expert System. 2003-2005. Version 1.25. Ottawa (ON): Environment Canada. Model developed by Stephen Niculescu. Available from: Environment Canada, Existing Substances Division, New Substances Division.
Amoore JE, Hautala E. 1983. Odor as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. Journal of Applied Toxicology 3:272-290.
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337–345.
Ashby J, Richardson CR, Tinwell H. 1989. Inactivity of ethyl acrylate in the mouse bone marrow micronucleus assay. Mutagenesis 4:283-285.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2008. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models
Atkinson R. 1989. Kinetics and Mechanisms of the Gas-Phase Reactions of the Hydroxyl Radical with Organic Compounds. Journal of Physical and Chemical Reference Data. Monograph No. 1.
BASF AG. 1958. Department of Toxicology, Report of toxicity studies with different acrylates, unpublished studies (VII/309). [cited in OECD 2004]
BASF AG. 1978. Department of Toxicology, Report on the acute skin irritation of methyl acrylate, ethyl acrylate, butyl acrylate and ethyl hexyl acrylate, unpublished study, (XXV/218), Jan 20, 1978. [cited in OECD 2004]
BASF AG. 2000. Ethyl Acrylate. In: IUCLID Dataset for ethyl acrylate. European Commission – European Chemicals Bureau. 18-Feb-2000.
BASF. 2005. Acute dermal irritation / corrosion in rabbits. M. Remmele (Study Director). BASF Aktiengesellschaft. Laboratory Project No.: 18H0651/042267. August 15. [cited in OECD 2004].
[BAMM] Basic Acrylic Monomer Manufacturers. 1994. Japanese Acrylic Ester Manufacturers: “Japanese Studies on Acrylic Esters and Acrylic Acid”, 11-15-94. [cited in European Commission 2000].
Basler A, van der Hude W. 1987. Erbgutveraendernde Gefahrstoffe bga-Schriften 3/87, MMV Muenchen. [cited in OECD 2004].
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 2.15. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2008. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models
Bjorkner B, Dahlquist I. 1979. Contact allergy caused by UV-cured acrylates. Contact Dermatitis 5:403-404.
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741-752.
Boorman GA, Sills RC. 2003. Ethyl acrylate. In: IARC monographs on the predictive value of rodent forestomach and gastric neuroendocrine tumours in evaluating carcinogenic risks to humans. IARC Technical Publication 39:57-64.
Borzelleca JF, Larson PS, Hennigar GR Jr., Huf EG, Crawford EM, Smith RB Jr. 1964. Studies on the chronic oral toxicity of monomeric ethyl acrylate and methyl methacrylate. Toxicology and Applied Pharmacology 6:29-36.
Brusick D. 1977. Ethyl acrylate. Mutagenicity evaluation. Litton Bionetics. BAMM, Washington, DC. [cited in ECETOC 1994].
BUA. 1992. Ethyl acrylate. The Advisory Committee on Existing Chemicals of Environmental Relevance, Germany. BUA report 128.
Burdock, GA. 1997. Fenaroli’s handbook of flavor ingredients. 6th ed. New York (NY): CRC Press.
Canada. 1978. Food and Drug Regulations, C.R.C., c. 870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette, Part III, Vol. 22, No. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette, Part II, Vol. 134, No. 7.
Canada, Dept. of the Environment, Dept. of Health. 2006. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, Vol. 140, No. 49.
Canada, Dept of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances identified in the Challenge, published in the September 26, 2009 Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, Vol. 143, No. 39.
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. CATALOGIC.
[Cedre] Centre for Documentation, Research and Experimentation on Accidental Water Pollution. 2006. Ethyl acrylate. Chemical response guide.
Celanese Chem. Co. 1972. Primary Skin Irritation Tests with Eighteen Materials in Albino Rabbits with Cover Letter dated July 28, 1972. OTS0206028 Doc ID 878212151. [cited in European Commission 2000].
[ChemCAN]. 2003. ChemCAN: level III. fugacity model of regional fate of chemicals [Internet]. Version 6.00. Peterborough (ON): Canadian Environmental Modelling Centre, Trent University. The Canadian Centre for Environmental Modelling and Chemistry.
Conde-Salazar L, Guimaraens D, Romero LV. 1988. Occupational allergic contact dermatitis from anaerobic acrylic sealants. Contact Dermatitis 18:129-132.
[ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment). ConsExpo 4.1.
Daubert TE, RP Danner. 1991. Physical and thermodynamic properties of pure chemicals: Data compilation. Design Inst. Phys. Prop. Data., Amer. Inst. Chem. Eng. NY. NY: Hemisphere Pub. Corp. 5 Vol.
Dearfield KL, Harrington-Brock K, Doerr CL, Rabinowitz JR, Moore MM. 1991. Genotoxicity in mouse lymphoma cells of chemicals capable of Michael addition. Mutagenesis 6(6):519-525.
De Bethizy JD, Udinsky JR, Scribner HE, Frederick CB. 1987. The disposition and metabolism of acrylic acid in male Sprague-Dawley rats. Fund Appl Toxicol 8:549-561.
Delbressine LPC, Van Balen HCJG, Seutter-Berlage F. 1982. Isolation and identification of mercapturic acid metabolites of phenyl substituted acrylate esters from urine of female rats. Arch Toxicol 49:321-330.
De Ceaurriz JC, Micillino JC, Bonnet P, Guenier JP. 1981. Sensory irritation caused by various industrial airborne chemicals. Toxicol Lett 9(2):137-143.
DePass LR, Fowler EH, Meckley DR, Weil CS. 1984. Dermal oncogenicity bioassays of acrylic acid, ethyl acrylate, and butyl acrylate. Journal of Toxicology and Environmental Health 14:115-120.
Dow Chemical Co. 1957. Results of range finding toxicological tests on ethyl acrylate. TSCATS, Doc. I.D. 90001181S (1989). [cited in BUA 1992].
Dow Chemical Co. 1964. Preliminary toxicology information on ethyl acrylate. TSCATS, Doc. I.D. 86-90001182S (1989). [cited in BUA 1992].
Dow Chemical Co. 1979a. Pathologic findings in rats exposed by inhalation to vapors for 12 months including a group which was exposed to 225 ppm for 6 months & then held for a recovery period of 6 months. OTS0520802, Doc ID 86-890001298.
Dow Chemical Co. 1979b. Pathologic findings in mice exposed by inhalation to 225 ppm ethyl acrylate for 6 months and subsequently held for a recovery period of three months with cover letter dated 061589. OTS0520788, Doc ID 86-890001282.
DPD 2010 - Drug Product Database.
[ECETOC] European Centre for Ecotoxicology and Toxicology of Chemicals. 1994. Ethyl acrylate (CAS No. 140-88-5). Joint Assessment of Commodity Chemicals (JACC) Report No. 28
[ECOSAR] Ecological Structural Activity Relationships [Internet]. 2008. Version 1.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models
Emmert B, Bunger J, Keuch K, Muller M, Emmert S, Hallier Ernst, Westphal GA. 2006. Mutagenicity of cytochrome P450 2E1 substrates in the Ames test with the metabolic competent S. typhimurium strain YG7108pin3ERb5. Toxicology 228:66-76.
Environment Canada. 1988. Data relating to the Domestic Substances List (DSL) 1984–1986, collected under CEPA, 1988, s. 25(1). Based on Reporting for the Domestic Substances List [guide] 1988. Data prepared by: Environment Canada.
Environment Canada. 2007. Guidance for Conducting Ecological Assessments under CEPA, 1999, Science Resource Technical Series, Technical Guidance Module: QSARs. Reviewed Draft Working Document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2008. National Pollutant Release Inventory [database on the Internet]. Gatineau (QC): Environment Canada. [cited 2010 06 20]. National Pollutant Release Inventory.
Environment Canada. 2009a. Guidance for Conducting Ecological Assessments under CEPA, 1999, Science Resource Technical Series, Technical Guidance Module: The Industrial Generic Exposure Tool – Aquatic (IGETA). Working document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2009b. Guidance for conducting ecological assessments under CEPA 1999: science resource technical series, technical guidance module: Mega Flush consumer release scenario. Working document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available on request.
Environment Canada. 2010a. Data for Batch 11 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to Batch 11 Challenge substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2010b. Voluntary submission of data for Batch 11 substances collected under the Chemical Management Plan Challenge initiative. Data prepared by: Environment Canada, Existing Substances Division.
Environment Canada. 2010c. IGETA report: CAS RN140-88-5,[2010 03 11). Unpublished report. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2010d. Mega Flush report: CAS RN140-88-5, 2010 4 9. Version1. Unpublished report. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
[EPIsuite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2008. Version 4.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Epstein WL. 1974. Report to Research Institute for Fragrance Materials (RIFM), 7 October, 1974. [cited in Opdyke 1975].
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, The Canadian Environmental Modelling Centre.
European Commission. 2000. IUCLID Dataset: Ethyl acrylate, CAS No. 140-88-5 [Internet]. Ispra (IT): European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. [cited 2010 Jan 18].
Foulger JH, Fleming AJ. 1945. Toxicity of ethyl acrylate monomer. TSCATS, Doc. I.D. 86-890000837, DuPont de Nemours and Co. [cited in BUA 1992].
Frederick CB, Hazelton GA, Frantz JD. 1990. The histopathological and biochemical response of the stomach of male F344/N rats following two weeks of oral dosing with ethyl acrylate. Toxicol Pathol 18(2):247-56.
Frederick CB, Chang-Mateu LM. 1990. Contact site carcinogenicity: estimation of an upper limit for risk of dermal dosing site tumors based on oral dosing site carcinogenicity. In: Gerrity TR and Henry CJ eds. Principles of Route-to-Route Extrapolation for Risk Assessment . New York (NY): Elsevier. p. 237-270.
Frederick CB, Potter DW, Chang-Mateu MJ, Andersen ME. 1992. A physiologically based pharmacokinetic and pharmacodynamic model to describe oral dosing of rats with ethyl acrylate and its implications for risk assessment. Toxicol Appl Pharmacol 114:246-260.
Frederick CB, Udinsky JR, Finch L. 1994a. The regional hydrolysis of ethyl acrylate to acrylic acid in the rat nasal cavity. Toxicol Lett 70:49-56.
Frederick CB, Morris JR, Kimbell JS, Morgan KT, Scherer PW. 1994b. Comparison of four biologically-based dosimetry models for the deposition of rapidly metabolized vapors in the rodent nasal cavity. Inhalation Toxicology 6 (supp.): 135-157.
Frederick CB. 1998. Potential for inhalation exposure to residual ethyl acrylate monomer from latex paints. Rohm and Haas Company, Toxicology Department, Report Number 98R-1107.
Frederick CB, Bush ML, Lomax LG, Black KA, Finch L, Kimbell JS, Morgan KT, Subramaniam RP, Morris JB, Ultman JS. 1998. Application of a hybrid computational fluid dynamics and physiologically based inhalation model for interspecies dosimetry extrapolation of acidic vapors in the upper airways. Toxicol Appl Pharmacol. 152(1):211-31.
Frederick CB, Lomax LG, Black KA, Finch L, Scribner HE, Kimbell JS, Morgan KT, Subramaniam RP, Morris JB. 2002. Use of a hybrid computational fluid dynamics and physiologically based inhalation model for interspecies dosimetry comparisons of ester vapors. Toxicol Appl Pharmacol 183(1):23-40.
Fregert S. 1978. Allergic contact dermatitis from ethylacrylate in a window sealant. Contact dermatitis 4, 56.
Gabor S, Anca L, Ivanov L, Borda M, Bozac L. 1965. [The chronic effect of inhalation of low methyl methacrylate and ethyl acrylate concentrations as shown by experiments on animals]. Igiena 14: 593-600 [Roumanian]. [cited in ECETOC 1994].
Gavin, C.L, Williams, M.C. and Hallagan, J.B. (2008) Flavor and Extract Manufacturers Association of the United States 2005 Poundage and Technical Effects Update Survey, Washington DC: Flavor and Extract Manufacturers Association of the United States.
Geiger DL, Brooke LT, Call DJ. 1990. Acute Toxicities of Organic Chemicals to Fathead Minnows (Pimephales promelas). Vol. 5. Center for Lake Superior Environmental Studies, University of Wisconsin-Superior, Superior, Wisconsin. 332 p.
Ghanayem BI, Maronpot RR, Matthews HB. 1985. Ethyl acrylate-induced gastric toxicity I. Effect of single and repetitive dosing. Tox Appl Pharmacol 80:323-335.
Ghanayem BI, Maronpot RR, Matthews HB. 1986. Ethyl acrylate-induced gastric toxicity III. Development and recovery of lesions. Toxicol. Appl. Pharm. 83:576-583.
Ghanayem BI, Burka LT, Matthews HB. 1987. Ethyl acrylate distribution, macromolecular binding, excretion, and metabolism in male Fisher 344 rats. Fund Appl Toxicol 9:389-397.
Ghanayem BI, Matthews HB, Maronpot RR. 1991. Sustainability of forestomach hyperplasia in rats treated with ethyl acrylate for 13 weeks and regression after cessation of dosing. Tox Pathol 19:273-279.
Ghanayem BI, Sanchez IM, Maronpot RR, Elwell MR, Matthews HB. 1993. Relationship between the time of sustained ethyl acrylate forestomach hyperplasia and carcinogenicity. Environ Health Perspect 101:277-280.
Ghanayem BI, Sanchez IM, Matthews HB, Elwell MR. 1994. Demonstration of a temporal relationship between ethyl acrylate-induced forestomach cell proliferation and carcinogenicity. Toxicol Pathol 22(5):497-509.
Harkema JR, Lee JK, Morgan KT, Frederick CB. 1997. Olfactory epithelial injury in monkeys after acute inhalation exposure to acrylic monomers. Toxicologist 36:113 [cited in US EPA 2007]
Haskell Laboratories for Dupont de Nemours. 1945. Toxicity of ethyl acrylate monomer with cover sheet dated 061289. OTS0520953 Doc ID 86-890000837. [cited in European Commission 2000].
Hara T, Katoh M, Horiya N, Shibuya T. 1994. Ethyl acrylate is negative in the bone marrow micronucleus test using BDF1 male mice. Environ Mut Res Commun 16:211-215.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E. 1983. Salmonella mutagenicity test results for 250 chemicals Environ. Mutagen. Vol. 5 (Suppl 1):3-142.
Hayes BB and Meade BJ. 1999. Contact sensitivity to selected acrylate compounds in B6C3F1 mice: relative potency, cross reactivity, and comparison of test methods. Drug Chem Toxicol 22:491-506.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2008. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models
Household products database [database on the internet]. 2009. Sept 2009. Bethesda, MD: US Department of Health & Human Services. Household products database
Huff J. 1995. Mechanisms, chemical carcinogenesis, and risk assessment: cell proliferation and cancer. Am J Ind Med 27(2):293-300.
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1986. Ethyl acrylate. IARC Monogr Eval Carcinog Risks Hum 39:81-98.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1999. Ethyl acrylate. IARC Monogr Eval Carcinog Risks Hum 71(3):1447-57.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2003. Predictive value of rodent forestomach and gastric neuroendocrine tumours in evaluating carcinogenic risks to humans. Views and expert opinions of an IARC working group, Lyon, 29 November-1 December 1999. IARC Technical Publication No. 39.
[IPCS]. International Programme on Chemical Safety. 2004. Ethyl acrylate. ICSC: 0267.
Institute of Biological Research. 1983. Comparative histopathologic evaluation of changes in the nasal cavity of the rat after exposure to methyl, ethyl and butyl acrylate with cover letter dated 071289. OTS0520801, Doc ID 86-890001297.
Ishidate M, Sofuni T Jr., Yoshikawa K. 1981. Chromosomal aberration tests in vitro as a primary screening tool for environmental mutagens and/or carcinogens. Gann Monograph on Cancer Research 27:95-108.
[IUCLID]. International Uniform Chemical Information Database. 2000. IUCLID Dataset. Ethyl acrylate. European Commission – European Chemicals Bureau. 18 February 2000.
Japan. Summary of initial risk assesment report. Ethyl acrylate. CAS No. 140-88-5. PRTR No. 4. R0074.
[JECFA] The Joint FAO/WHO Expert Committee on Food Additives. 2006. Safety and evaluation of certain food additives, WHO food additives series: 54 [Internet]. Geneva Switzerland. [2010-07-07].
Jersey GC, Keyes DG, Battjes JE. 1978. Pathologic findings in rats exposed by inhalation to vapors of ethyl acrylate for 6 months. Midland (MI): Health and Environmental Research, Toxicology Research Laboratory, Dow Chemical. TSCATS: OTS0520180, Doc-ID: 86-890001037
Jordan WP Jr. 1975. Cross-sensitization patterns in acrylate. allergies. Contact Dermatitis 1(1):13-5.
Kanerva L, Estlander T, Jolanki R. 1988. Sensitization to patch test acrylates. Contact Dermatitis. 18(1):10-15.
Kanerva L, Estlander T, Jolanki R. 1989. Allergic contact dermatitis from dental composite resins due to aromatic epoxy acrylates and aliphatic acrylates. Contact Dermatitis 20(3):201-11.
Kanerva L, Estlander T, Jolanki R 1992. Double active sensitization caused by acrylics. Am J Contact Derm 3:23-26.
Kanerva L, Estlander T, Jolanki R, Tarvainen K. 1995. Statistics on allergic patch test reactions caused by acrylate compounds, including data on ethyl methacrylate. Am J Contact Derm 6:75.
Kelly TJ, Callahan PJ, Pleil J, Evans GF. 1993. Method development and field measurements for polar volatile organic compounds in ambient air. Environ. Sci, Technol. 27: 1146-1153.
Kimber I. 1992. Skin sensitization [EA negative in murine local lymph node assay]. Pers comm. To Mackay JM, 12 Nov 92. ICI CTL, Macclesfield. [cited in ECETOC 1994].
Kligerman AD, Atwater AL, Bryant MF, Erexson GL, Kwanyuen PK, Dearfield KL. 1991. Cytogenetic studies of ethyl acrylate using C57BL/6 mice. Mutagenesis 6(2):137-141.
[KOAWIN] Octanol Air Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models
Koppula SV, Fellman JH, Storrs FJ. 1995. Screening allergens for acrylate dermatitis associated with artificial nails. Am J Contact Derm 6:78-85.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models
Kuzelova M, Kovarik J, Fiedlerova D, Popler A. 1981. [Acrylic compounds and general health of the exposed persons.] Pracov Lék 33:95-99. [Czech; Doc Occup Health 8, 1612; Chem Abstr 96, 24169v]. [cited in ECETOC 1994].
Lazarov A. 2007. Sensitization to acrylates is a common adverse reaction to artificial fingernails. Journal of the European Academy of Dermatology and Venereology 21(2):169-174.
[LNHPD] Licensed Natural Health Products Database. 2010.Licensed Natural Health Products Database.
Lomonova GV and Klimova EI. 1979. [Data on the toxicology of acrylic acid methyl and ethyl esters]. Gig Tr Prof Zabol 9: 55-56 [cited in ECETOC 1994, European Commission 2000].
Loveday KS, Anderson BE, Resnick MA, Zeiger E. 1990. Chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells in vitro. V: results with 46 chemicals. Environ Mol Mutagen 16:272-303.
Lucas CD, Putnam JM, Hallagan JB. 1999. 1995 Poundage and Technical Effects Update Survey. Flavor and Extract Manufacturers Association (FEMA) of the United States, Washington DC, USA.
Mabey W,T Mill. 1978. Critical Review of Hydrolysis of Organic Compounds in Water Under Environmental Conditions. J. Phys. Chem. Ref. Data. 7: 383-415.
Malten KE, van Ketel WG, Nater JP, Liem DH. 1984. Reactions in selected patients to 22 fragrance materials. Contact Dermatitis 11:1-10.
Marks JG, Belsito DV Jr., DeLeo VA, Fowler JF, Fransway AF Jr., Maibach HI, Mathias CGT, Nethercott JR, Rietschel RL, Rosenthal LE, Sherertz EF, Storrs FJ, Taylor JS. 1995. North American Contact Dermatitis Group standard tray patch test results (1992 to 1994). Am J Contact Derm 6:160-165.
Matthews EJ, Spalding JW, Tennant RW. 1993. Transformation of BALB/c-3T3 cells: V. Transformation responses of 168 chemicals compared with mutagenicity in Salmonella and carcinogenicity in rodent bioassays. Environ Health Perspect 101(Suppl 2):347-482.
McCarthy TJ, Hayes EP, Schwartz CS, Witz G. 1994. The reactivity of selected acrylate esters toward glutathione and deoxyribonucleosides in vitro: structure-activity relationships. Fund Appl Toxic 22:543-548.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride DM, Riach C, Caspary WJ. 1988. Responses of the L5178Y tk+/tk- mouse lymphoma cell forward mutation assay: III. 72 coded chemicals. Environ Molec Mutagen 12:85-154.
McLaughlin JE, Baldwin RC, Smith JM. 1993. Ethyl Acrylate Health Effects Overview. In Health Effect Assessments of the Basic Acrylates. Tyler, T.R., Reiss Murphy, S., Hunt, E.K. (eds). CRC Press, Ann Arbor, 1993.
Melnick RL, Huff J, Barrett JC, Maronpot RR, Lucier G, Portier CJ. 1993. Cell proliferation and chemical carcinogenesis: symposium overview. Environ Health Perspect 101(Suppl 5):3-7.
Miller RR, Jersey GA, Betso JE, Rampy LW. 1979. 30-day ethyl acrylate vapor inhalation study with rats and mice, final report. Midland (MI): Dow Chemical USA. [cited in ECETOC 1994].
Miller RR, Ayres JA, Rampy LW, McKenna MJ. 1981. Metabolism of acrylate esters in rat tissue homogenates. Fundam Appl Toxicol 1:410-414.
Miller RR, Young JT, Kociba RJ, Keyes DG, Bodner KM, Calhoun LL, Ayres JA. 1985. Chronic toxicity and oncogenicity bioassay of inhaled ethyl acrylate in Fischer 344 rats and B6C3F1 mice. Drug and Chemical Toxicology 8:1-42.
[MITI] Ministry of International Trade & Industry (JP), Basic Industries Bureau, Chemical Products Safety Division. 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (JP): Japan Chemical Industry Ecology-Toxicology & Information Center.
Moore MM, Amtower A, Doerr CL, Brock KH, Dearfield KL. 1988. Genotoxicity of acrylic acid, methyl acrylate, ethyl acrylate, methyl methacrylate, and ethyl methacrylate in L5178Y mouse lymphoma cells. Environmental and Molecular Mutagenesis 11:49-63.
Moore MM, Harrington-Brock K, Doerr CL, Dearfield KL. 1989. Differential mutant quantitation at the mouse lymphoma tk and CHO hgprt loci. Mutagenesis 4:394-403.
Morita T, Asano N, Awogi T, Sasaki YF, Sato S, Shimada H, Sutou S, Suzuki T, Wakata A, Sofuni T, Hayashi M. 1997. Evaluation of the rodent micronucleus assay in the screening of IARC carcinogens (groups 1, 2A and 2B) the summary report of the 6thcollaborative study by CSGMT/JEMS MMS. Collaborative Study of the Micronucleus Group Test. Mammalian Mutagenicity Study Group (published erratum). Mutat Res 391(3):259-67, 389:3-122.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2008. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models
Murray JS, Miller RR, Deacon MM, Hanley TR, Hayes WC, Rao KS, John JA. 1981. Teratological evaluation of inhaled ethyl acrylate in rats. Tox Appl Pharmacol 60:106-111.
Myhr BC. 1980. Mutagenicity evaluation of ethyl acrylate monomer in the mouse lymphoma forward mutation assay. Washington(DC): Litton Bionetics, Basic Acrylic Monomer Manufacturers, Inc (BAMM). [cited in ECETOC 1994].
[NCI] National Chemical Inventories [database on CD-ROM]. 2006. Issue 1. Columbus (OH): American Chemical Society. [cited2010 Jan]. National Chemical Inventories.
NHPID 2010 - Natural Health Products Ingredients Database.
[NITE] National Institute of Technology and Evaluation [Internet]. c2008. Japan: Ethyl Acrylate; [cited 2010 Mar 19]. Chemical Management Field.
[NPRI] National Pollutant Release Inventory [database on the Internet]. 2008. Gatineau (QC): Environment Canada. [cited 2010 Mar 21]. National Pollutant Release Inventory.
[NTP] National Toxicology Program. 1986a. Carcinogenesis studies of ethyl acrylate (CAS # 140-88-5) in F344/N rats and B6C3F1 mice (gavage studies). Research Triangle Park (NC): U.S. Department of Health and Human Services, National Toxicology Program. Report No.: TR 259.
[NTP] National Toxicology Program. 1986b. In vitro cytogenetics chromosome aberration and sister chromatid exchange studies of ethyl acrylate (CAS # 140-88-5). Research Triangle Park (NC): U.S. Department of Health and Human Services, National Toxicology Program. Study ID: 550589.
[NTP] National Toxicology Program. 1989. Report on Carcinogens, 5th ed., Carcinogen Profiles. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program. 1994. Assessment of contact hypersensitivity to ethyl acrylate in female B6C3F1 mice. Report to National Toxicology Program, Medical College of Virginia. Protocol No. EAC-3-1-TO [cited in OECD 2004].
[NTP] National Toxicology Program. 1998. Report on Carcinogens background document for ethyl acrylate. Research Triangle Park (NC): US Department of Health Services, National Toxicology Program.
[NTP] National Toxicology Program. 2000. Report on Carcinogens, 9th ed., Carcinogen Profiles. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
Nylander-French LA and French JE. 1998. Tripropylene glycol diacrylate but not ethyl acrylate induces skin tumors in a twenty-week short-term tumorigenesis study in Tg.AC (v-Ha-ras) mice. Toxicologic Pathology 26(4):476-483.
[OASIS Forecast] Optimized Approach based on Structural Indices Set [Internet]. 2005. Version 1.20. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. OASIS Software.
Oberly R and Tansy MF. 1985. LC50 Values for rats acutely exposed to vapors of acrylic and methacrylic acid esters. Journal of Toxicology and Environmental Health 16:811-822.
[OECD] Organization for Economic Cooperation and Development. 1992. Guidelines for testing of chemicals, method 301D ready biodegradability: closed bottle test.
[OECD] Organization for Economic Cooperation and Development. 2003. Guidelines for testing of chemicals. Ready biodegradability – CO2 in sealed vessels (Headspace Test).
[OECD] Organisation for Economic Co-operation and Development. 2004. Ethyl acrylate, CAS n. 140-88-5. SIDS initial assessment report for SIAM 18 [Internet]. Geneva: United Nations Environment Programme. [2010 Feb 03]. OECD Existing Chemicals Database.
[OECD] Organisation for Economic Co-operation and Development. 2005. Ethyl Acrylate, CAS n. 140-88-5. SIDS Dossier [Internet]. Geneva: United Nations Environment Programme. [2009 Aug 19]. OECD Existing Chemicals Database.
Oettel H and Zeller H. 1958. Bericht über die Prüfung der Haut- und Schleimhautreizwirkung verschiedener Acrylsäureester. BASF, Ludwigshafen. [cited in ECETOC 1994].
Oettel H and Hofmann HT. 1960. Bericht über die toxikologische Prüfung verschiedener Acrylsäureester an Kaninchen and Katzen. BASF, Ludwigshafen. [cited in ECETOC 1994].
O’Neil MJ, editor. 2006. The Merck index--An encyclopedia of chemicals, drugs and biologicals. 14th ed. Whitehouse Station (NJ): Merck & Co.
O’Neil M.J, A. Smith, PE Heckleman, JR Obenchain Jr, JR Gallipeau, MA D’Arecca, S Budavari (eds). 2001. The Merck Index.Ethyl acrylate.
Opdyke DLJ. 1975. Ethyl acrylate, monograph on raw materials 17. Food Cosmet Toxicol suppl 13:801-802.
Otson, R., Fellin, P., Tran, Q. 1994. VOCs in representative Canadian residences. Atmospheric Environment 28:3563-3569
Paulet G and Vidal M. 1975. De la toxicité de quelques esters acryliques et méthacryliques, de l’acrylamide et des polyacrylamdies. Arch Mal Prof Med Trav Secur Soc 36:58-60. [cited in OECD 2004].
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2008. Version 1.66. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models
Pietrowicz D., Owecka A, Baranski B. 1980. Disturbances in rats embryonal development due to ethylacrylate. Zwierzeta Laboratoryine 17:67-72. [cited in IARC 1986, OECD 2004].
Poole LJ. 1980. Acrylate D’Ethyle (AE): Irritation studies in the rabbit. Report Cl80:66:2031. Consultox Laboratories Limited. Report prepared for Norsolor. [cited in OECD 2004].
Potokar M, Grundler OJ, Heusener A, Jung R, Mürmann P, Schöbel C, Suberg H, Zechel HJ. 1985. Studies on the design of animal tests for the corrosiveness of industrial chemicals. Fd Chem Toxic 23:615-617.
Potter DW and Tran TB. 1992. Rates of ethyl acrylate binding to glutathione and protein. Toxicol Lett 62:275-285.
Pozzani UC, Weil CS and Carpenter CP. 1949. Subacute vapor toxicity and range-finding data for ethyl acrylate. J Industrial Hyg Toxicol 31(6):311-316.
Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE. 1990. Increased cell division as a cause of human cancer. Cancer Res 50(23):7415-21.
Proctor DM, Gatto NM, Hong SJ, Allamneni KP. 2007. Mode-of-action framework for evaluating the relevance of rodent forestomach tumors in cancer risk assessment. Toxicol Sci 98(2):313-326.
Przybojewska B, Dziubaltowska E, Kowalski Z. 1984. Genotoxic effects of ethyl acrylate and methyl acrylate in the mouse evaluated by the micronucleus test. Mutation Research 135:189-191.
Riddick JA, WB Bunger, TK Sakano. 1986. Ethyl acrylate. Techniques of Chemistry. Volume II. Organic solvents. Physical properties and methods of purification. Fourth edition. John wiley & Sons. Toronto.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2006. Cosmetics fact sheet: To assess the risks for the consumer. Updated version for ConsExpo 4 [Internet]. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). RIVM Report No.: 320104001/2006.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2007a. Do-it-yourself products fact sheet: To assess the risks for the consumer [Internet]. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). RIVM Report No.: 320104007/2007.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2007b. Paint products fact sheet: To assess the risks for the consumer. Updated version for ConsExpo 4 [Internet]. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). RIVM Report No: 320104008/2007.
Rohm and Haas Company. 1950a. Munch Research Laboratories, Inc. (Testing Facility). Acute oral toxicity in mice. Spring House(PA): Rohm and Haas Toxicology Department, Report No. 50RC-1003. Unpublished report. [cited in OECD 2004].
Rohm and Haas Company. 1950b. Acute toxicity studies, group III: methyl acrylate and ethyl acrylate. Prepared by Munch Res. Lab. Spring House(PA): Rohm and Haas. [cited in ECETOC 1994].
Rohm and Haas Company. 1977. Mutagenicity evaluation of ethyl acrylate. Litton Bionetics, Inc. (Testing Facility). LBI Project No. 2683. Spring House(PA): Rohm and Haas Toxicology Department Report No. 77RC-1024. Unpublished report. [cited in OECD 2004].
Rohm and Haas Company. 1981. Ethyl acrylate: microbial mutagen test. Spring House(PA): Rohm and Haas Toxicology Department. Report No. 80R-212. Unpublished report. [cited in OECD 2004].
Rohm and Haas Company. 1983. Situation and results of recent investigations into the toxicity of acrylic acid and its esters and a NTP technical report on the carcinogenesis bioassay of ethyl acrylate W-attachments. OTS0520798, Doc ID 86-890001294. [cited in European Commission 2000].
Rohm and Haas.Company. 1984. Acute definitive oral LD50 in rats- ethyl acrylate monomer. Spring House(PA): Rohm and Haas Toxicology Department. Report No. 84R-0208. Unpublished report. [cited in OECD 2004].
Rohm and Haas Company. 1986a. Acute definitive dermal LD50 in rats (occluded) - ethyl acrylate monomer. Spring House(PA): Rohm and Haas Toxicology Department. Report No. 86R-017A. Unpublished report. [cited in OECD 2004].
Rohm and Haas Company. 1986b. Ethyl acrylate: 2-week oral (gavage) study in rats. Spring House(PA): Rohm and Haas Toxicology Department. Report No. 86R-063. Unpublished report. [cited in OECD 2004].
Rohm and Haas Company. 1986c. Toxicity report, acute dermal LD50 definitive, rats (unoccluded). Prepared by Morrison RD and Hazelton GA. Spring House(PA): Rohm and Haas. [cited in ECETOC 1994].
Rohm and Haas 1986d. Toxicity report, acute dermal LD50 definitive, mice (occluded). Prepared by Morrison RD and Hazelton GA. Rohm and Haas, Spring House, PA. [cited in ECETOC 1994].
Rohm and Haas Company. 1987. Ethyl acrylate: three month oral gavage study in rats. Spring House(PA): Rohm and Haas. Report No. 86R-153. Unpublished report. [cited in OECD 2004].
Rohm and Hass Company. 1991. Ethyl acrylate (15 ppm MEHQ) skin irritation study in rabbits. Spring House(PA): Rohm and Hass. Report No. 90R-268. Unpublished report. [cited in OECD 2004].
Rohm and Haas Company. 1994. Single dose inhalation toxicity study of ethyl acrylate (EA) and acrylic acid (AA) in monkeys, interim report from pharmacokinetic study, with cover letter dated 12/08/94. Doc. ED 86-950000051 OTS0557564.
Rustemeyer T and Frosch PJ. 1996. Occupational skin diseases in dental laboratory technicians: (I). Clinical picture and causative factors (p 125-133) Contact Dermatitis 34:125-133.
Saillenfait A, Bonnet P, Gallissot F, Protois JC, Peltier A, Fabries JF. 1999. Relative developmental toxicities of acrylates in rats following inhalation exposure. Toxicological Sciences 48:240-245.
Schwartz BS, Doty RL, Monroe C, Frye R, Barker S. 1989. Olfactory function in chemical workers exposed to acrylate and methacrylate vapors. Am J Public Health 79:613-618.
Sherman J. 1985. Case study re: birth defects reported in a baby of mother exposed to SK-131-A and SK-131-B with attachments and cover letter dated 081685. TSCATS, FYI-OTS-0885-0402, submitted by: Sherman J, Michigan. [cited in BUA 1992].
Silver EH and Murphy SD. 1981. Potentiation of acrylate ester toxicity by prior treatment with the carboxylesterase inhibitor triorthotolyl phosphate (TOTP). Tox Appl Pharmacol 57:208-219.
Shimizu M et al. 1994. Annual Report of Osaka City Institute of Public Health and Environmental Sciences 51, 11-17 (1988); cited in Basic Acrylic Monomer Manufacturers, Japanese Acrylic Ester Manufacturers: “Japanese Studies on Acrylic Esters and Acrylic Acid”, 11-15-94. [cited in European Commission 2000].
Skoglund A and Egelrud T. 1991. Hypersensitivity reactions to dental materials in patients with lichenoid oral mucosal lesions and in patients with burning mouth syndrome. Scand J dent Res 99:320.
Staples, CA, SR Murphy, JE McLaughlin, H-W Leung, TC Cascieri, CH Farr. 2000. Determination of selected fate and aquatic toxicity charactericstics of acrylic acid and a series of acrylic ester. Chemosphere, 40: 29-38.
Steele VE, Arnold JT, Van Arnold J, Mass MJ. 1989. Evaluation of a rat tracheal epithelial cell culture assay system to identify respiratory carcinogens. Environ Mol Mutagen 14:48-54.
Stenman E and Bergman M. 1989. Hypersensitivity reactions to dental materials in a referred group of patients. Scand J dent Res 97:76.
Stott WT and McKenna MJ. 1984. The comparative absorption and excretion of chemical vapors by the upper, lower and intact respiratory tract of rats. Fund Applied Tox 4:594-602.
Sweeney LM, Andersen ME, Gargas ML. 2004. Ethyl acrylate risk assessment with a hybrid computational fluid dynamics and physiologically based nasal dosimetry model. Toxicol Sci. 9(2):394-403.
Tanii H and Hashimoto K. 1982. Structure-toxicity relationship of acrylates and methacrylates. Toxicol Letters 11:125-129.
Tennant RW, Spaldinig J, French JE. 1996. Evaluation of transgenic mouse bioassays for identifying carcinogens and noncarcinogens. Mutat Res 365:119-127.
Tice RR, Nylander-French LA, French JE 1997. Absence of Systemic In Vivo Genotoxicity after Dermal Exposure to Ethyl Acrylate and Tripropylene Glycol Diacrylate in Tg.AC (v-Ha-ras) Mice. Environ Mol Mutagen 29:240-249.
[TOPKAT] TOxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc.
Treon JF, Sigmon H, Wright H, Kitzmiller K. 1949. The toxicity of methyl and ethyl acrylate. J Ind Hyg Tox 31:317-325.
[TRI] Toxics Release Inventory Program [Internet]. 2008. TRI Explorer version 4.7. Washington (DC): US Environmental Protection Agency. [cited 2010 Mar 21]. TRI Explorer.
Tucker SC and Beck MH. 1999. A 15-year study of patch testing to (meth)acrylates. Contact Dermatitis 40(5):278-279.
Tyler and Smock. 1993. Overview. In: Tyler, T.R., Murphy, S.R., Hunt, E.K., (Eds), Health Effect Assessments of the Basic Acrylates. CRC Press, Boca Raton, Florida, pp. 1-31.
Union Carbide Corp. 1971. Toxicology studies-ethyl acrylate. New York(NY): Industrial Medicine and Toxicology Department. [cited in IARC 1986].
Union Carbide Corp. 1989. Attachments: letter from US EPA to Union Carbide Corporation, listing of items in attachment Iii, part i - Brc studies & part Ii external toxicology reports with cover letter 071489. TSCATS: OTS0520180, Doc-ID: 86-890001037 [cited in European Commission 2000].
[US EPA] United States Environmental Protection Agency. 2005. Health Effects Information Used In Cancer and Noncancer Risk Characterization For the 1999 National-Scale Assessment.
[US EPA] United States Environmental Protection Agency 2007. Acute Exposure Guideline levels (AEGLs) for ethyl acrylate (CAS Reg. No. 140-88-5) (interim). Washington(DC): National advisory Committee for AEGLs for Hazardous Substances, US EPA.
[US EPA] United States Environmental Protection Agency. 2009. Ethyl acrylate. [Internet] Washington(DC): Air Toxics, Technology Transfer Network. [2010 Feb 03]
Valencia R, Mason JM, Woodruff RC, Zimmering S. 1985. Chemical mutagenesis testing in drosophila. III. Results of 48 coded compounds tested for the National Toxicology Program. Environmental Mutagenesis 7:325-348.
Van der Walle HB, Klecak G, Geleick H, Bensink T. 1982. Sensitizing potential of 14 mono-(meth)acrylates in the guinea pig. Contact Dermatitis 8:223-235.
Vodicka P, Gut I, Frantik E. 1990. Effects of inhaled acrylic acid derivatives in rats. Toxicology 65(1-2):209-21.
Waegemaekers THJM and Bensink MPM. 1984. Non-mutagenicity of 27 aliphatic acrylate esters in the Salmonella-microsome test. Mutation Res 137:95-102.
Walker AM, Cohen AJ, Loughlin JE, Rothman KJ, DeFonso LR. 1991. Mortality from cancer of the colon or rectum among workers exposed to ethyl acrylate and methyl methacrylate. Scand J Work Environ Health 17:7-19.
[WPEM] Wall Paint Expoure Assessment Model [Internet]. 2001. Version 3.2. Washington, DC: US EPA Office of Pollution Prevention and Toxics and National Paint and Coatings Association. Exposure Assessment Tools and Models.
Williams GM and Iatropoulos MJ. 2009. Evaluation of potential human carcinogenicity of the synthetic monomer ethyl acrylate. Regul Toxicol Pharmacol 53(1):6-15.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2008. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models
Yasunaga K, Kiyonari A, Oikawa T, Abe N, Yoshikawa K. 2004. Evaluation of the Salmonella umu test with 83 NTP chemicals. Environmental and Molecular Mutagenesis 44:329-345.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. 1992. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environ Mol Mutagen 19 (Supplement 21):2-141.
Zimmermann FK and Mohr A. 1992. Formaldehyde, glyoxal, urethane, methyl carbamate, 2,3-butanedione, 2,3-hexanedione, ethyl acrylate, dibromoacetonitrile and 2-hydroxypropionitrile induce chromosome loss in Saccharomyces cerevisiae. Mutat Res 270:151-166.
Appendix 1: Upper-Bounding Estimates of Exposure to the General Population of Canada from Environmental Media (µg/kg-bw/d)
| Route of exposure | 0–6 months[1], [2], [3] | 0.5–4 years[4] | 5–11 years[5] | 12–19 years[6] | 20–59 years[7] | 60+ years[8] | ||
|---|---|---|---|---|---|---|---|---|
| breast fed | formula fed | not formula fed | ||||||
| Ambient air[9] | 0-2.87x10-2 | 0-6.15x10-2 | 0-4.79x10-2 | 0-2.73x10-2 | 0-2.34x10-2 | 0-2.04x10-2 | ||
| Indoor air[10] | 0-2.45 | 0-5.25 | 0-4.09 | 0-2.33 | 0-2.00 | 0-1.74 | ||
| Drinking water[11] | 0.00 | 0-1.07 | 0-0.400 | 0-0.452 | 0-0.355 | 0-0.202 | 0.212 | 0-0.222 |
| Food and beverages[12] | 0.00 | 0.00 | 0.00 | 4.93 x10-2 | 1.82x10-2 | 1.26x10-2 | 9.12x10-3 | 1.35x10-2 |
| Soil[13] | 3.22x10-10 | 5.19x10-10 | 1.69x10-10 | 4.07x10-11 | 3.41x10-11 | 3.35x10-11 | ||
| Total intake | 2.48 | 3.55 | 2.88 | 5.81 | 4.51 | 2.57 | 2.24 | 1.99 |
[2] Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, to drink 0.8 L of water per day (formula fed) or 0.3 L/day (not formula fed) and to ingest 30 mg of soil per day (Health Canada 1998).
[3] For exclusively formula-fed infants, intake from water is synonymous with intake from food. No data on concentrations of ethyl acrylate in formulae were identified for Canada.
[4] Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day and to ingest 100 mg of soil per day (Health Canada 1998).
[5] Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day and to ingest 65 mg of soil per day (Health Canada 1998).
[6] Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
[7] Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
[8] Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
[9] No Canadian data were identified. As a surrogate, the LOD of 0.2 ppb (0.82 µg/m3) for the concentration of ethyl acrylate in ambient (outdoor) air reported by Kelly et al., 1993 was used to calculate the upper bounding limit of exposure estimate. Air monitoring was conducted in Houston and Boston, US with 22 samples taken at each location between August 1990 and August 1991. Ethyl Acrylate was not detected in any samples. This had no impact on the upper bounding estimates of daily intake.
[10] Estimates of intake from indoor air are based on use of the estimated LOD of 10 µg/m3of ethyl acrylate in indoor air reported by Otson et al., 1994. 757 Canadian dwellings were monitored and ethyl acrylate was not detected in any samples. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
[11] No Canadian data were identified. As a surrogate, the highest reported LOD of 10 ppb for the concentration of ethyl acrylate in drinking water was used (McLaughlin et al., 1993). They reported that ethyl acrylate was not detected in any of the more than 250 groundwater samples taken from or around five plants which produce ethyl acrylate. The limit of detection was reported to be between 1 and 10 ppb (McLaughlin et al., 1993).
[12] No data were identified for the concentration of ethyl acrylate in foods in Canada. The only reported concentration of ethyl acrylate in fruit was in fresh pineapple in a study from Europe (0.77 mg/kg; IARC 1986). In the absence of any Canadian data, this value was used as a surrogate for the other fruits containing ethyl acrylate in studies from the United States and Europe (blackberries, raspberries, yellow passion fruit, and durian) in the calculations for estimating upper-bounding exposure from food.
Amounts of foods consumed on a daily basis by each age group are described by Health Canada (1998). The Joint FAO/WHO Expert Committee on Food Additives (JECFA) reported an estimated daily intake of ethyl acrylate from food flavouring based on annual production volumes of ethyl acrylate used as a flavouring agent in the United States and Europe (JECFA 2006). These estimates were not included in the calculation for estimating upper bounding exposure from food.
[13] No reported data for the concentration of ethyl acrylate in soil were identified. ChemCan Version 6.0.0. (2003) modeling was run for the 306 kg of releases to air reported under section 71 in Quebec (Environment Canada 2010a). The estimated modeled concentration was 8.05 x 10-5 ug/L. This had no impact on the upper bounding estimates of daily intake.
| No. | Consumer Product Type | Model Parameters | Estimated Exposure |
|---|---|---|---|
| 1a | Paint – flat latex | Molecular weight of 100 g/mol Vapour pressure of 28.5 mm Hg KOW of 1.18 10Log Exposure frequency of 2 x / year (RIVM 2006, 2007b) Adult body weight of 70.9 kg (Health Canada 1998) Weight fraction of 4.5 x 10-6 or 4.5 ppm (Frederick 1998) Exposure duration of 132 min (RIVM 2006, 2007b) Room volume of 20 m3 (RIVM 2006, 2007b) Ventilation rate of 0.6 x /hr (RIVM 2006, 2007b) Applied amount of 3.75 x 103 g (RIVM 2006, 2007b) Release area of 15 m2 (RIVM 2006, 2007b) Application duration of 120 min (RIVM 2006, 2007b) Mol weight matrix of 120 g/mol (RIVM 2006, 2007b) Mass transfer rate of 0.282 m/min (RIVM 2006, 2007b) Uptake fraction of 100%[1] Inhalation rate of 16.2 m3/day (Health Canada, 1998) Inhalation model: exposure to vapour by evaporation where area of release increases over time. Scenario describes the brushing or rolling of two walls in a small room with low ventilation. Density of waterborne wall paint of 1.5 g/cm3 |
Inhalation mean event concentration: 0.225 mg/m3 |
Molecular weight of 100 g/mol Vapour pressure of 28.5 mm Hg KOW of 1.18 10Log Adult body weight of 70.9 kg (Health Canada 1998) Weight fraction of 4.5 x 10-6 or 4.5 ppm (Frederick 1998) Exposed area of 0.367 m2 (RIVM 2006, 2007b general fact sheet area for hands and arms) Contact rate of 30 mg/min (RIVM 2006, 2007b) Release duration of 120 min (RIVM 2006, 2007b) Dermal uptake of 100%[1] Dermal model: direct dermal contact with product: constant rate |
Dermal acute (internal) dose: 2.54 x 10-4 mg/kg Dermal chronic (internal) dose: 1.39 x 10-6 mg/kg/day |
||
| 1b | Paint – semi-gloss trim | Molecular weight of 100 g/mol Vapour pressure of 28.5 mm Hg KOW of 1.18 10Log Exposure frequency of 2 x / year (RIVM 2006, 2007b) Adult body weight of 70.9 kg (Health Canada 1998) Weight fraction of 1.3 x 10-5 or 13.2 ppm (Frederick 1998) Exposure duration of 66 min Room volume of 20 m3 (RIVM 2006, 2007b) Ventilation rate of 0.6 x /hr (RIVM 2006, 2007b) Applied amount of 450 g Release area of 1.82 m2 Application duration of 60 min Mol weight matrix of 120 g/mol (RIVM 2006, 2007b) Mass transfer rate of 0.282 m/min (RIVM 2006, 2007b) Uptake fraction of 100%[1] Inhalation rate of 16.2 m3/day (Health Canada 1998) Inhalation model: exposure to vapour by evaporation where area of release increases over time. Scenario describes brushing or rolling of surface area of baseboard trim in a small room with low ventilation. Density of waterborne wall paint of 1.5 g/cm3 |
Inhalation mean event concentration: 0.108 mg/m3 |
Molecular weight of 100 g/mol Vapour pressure of 28.5 mm Hg KOW of 1.18 10Log Adult body weight of 70.9 kg (Health Canada 1998) Weight fraction of 1.32 x 10-5 or 13.2 ppm (Frederick 1998) Exposed area of 0.367 m2 (RIVM 2006, 2007 general fact sheet area for hands and arms) Contact rate of 30 mg/min (RIVM 2006, 2007b) Release duration of 120 min (RIVM 2006, 2007b) Dermal uptake of 100%[1] Dermal model: direct dermal contact with product: constant rate |
Dermal acute (internal) dose: 6.6 x 10-4 mg/kg Dermal chronic (internal) dose: 3.61 x 10-6 mg/kg/day |
||
| 2 | Caulk | Molecular weight of 100 g/mol Vapour pressure of 28.5 mm Hg KOW of 1.18 10Log Caulk exposure frequency of 3 x / year (RIVM 2006, 2007a) Adult body weight of 70.9 kg (Health Canada 1998) Weight fraction of 0.01% (Household products database 2009) Exposure duration of 45 min (RIVM 2006, 2007a) Room volume of 10 m3 (RIVM 2006, 2007a) Ventilation rate of 0.6 x / hr (RIVM 2006, 2007a) Applied amount of 75 g (RIVM 2006, 2007a) Release area of 250 cm2 (RIVM 2006, 2007a) Application duration of 30 min (RIVM 2006, 2007a) Mol weight matrix of 3 x 103 g/mol (RIVM 2006, 2007a) Mass transfer rate of 3.73 x 103 m/min (RIVM 2006, 2007a) Uptake fraction of 100%[1] Inhalation rate of 16.2 m3/day (Health Canada, 1998) Inhalation model: exposure to vapour by evaporation where area of release increases over time. |
Inhalation mean event concentration of: 0.427 mg/m3 |
Molecular weight of 100 g/mol Vapour pressure of 28.5 mm Hg KOW of 1.18 10Log Adult body weight of 70.9 kg (Health Canada 1998) Weight fraction of 0.01% (Household products database 2009) Exposed area of 2 cm2 (RIVM 2006, 2007a) Contact rate of 50 mg/min Release duration of 30 min Dermal model: direct dermal contact with product at a constant rate. |
Dermal acute (internal) dose: 0.00212 mg/kg Dermal chronic (internal) dose : 1.74 x 10-5 mg/kg/day |
||
| 3a | Personal wipes - infants | Molecular weight of 100 g/mol Vapour pressure of 28.5 mm Hg KOW of 1.18 10Log Exposure frequency of 5 x / day Infant body weight of 7.5 kg (Health Canada 1998) Weight fraction of 2.8 x 10-5 or 28 ppm (Environment Canada 2010b) Exposed area of 199 cm2 (RIVM 2006, 2006) Applied amount of 0.02 g (RIVM 2006, 2006) Uptake fraction of 100%[1] Dermal model: direct dermal contact with product- instant application |
Dermal acute (internal) dose: 7.47 x 10-5 mg/kg Dermal chronic (internal) dose: 3.73 x 10-4 mg/kg/day |
| 3b | Personal wipes - adults | Molecular weight of 100 g/mol Vapour pressure of 28.5 mm Hg KOW of 1.18 10Log Exposure frequency of 365 x / year (RIVM 2006, 2007) Adult body weight of 70.9 kg (Health Canada 1998) Weight fraction of 2.8 x 10-5 or 28 ppm (Environment Canada 2010b) Exposed area of 215 cm2 (RIVM 2006, 2006) Applied amount of 0.02 g (RIVM 2006, 2006) Uptake fraction of 100%[1] Dermal model: direct dermal contact with product- instant application |
Dermal acute (internal) dose: 7.9 x 10-6 mg/kg Dermal chronic (internal) dose: 7.89 x 10-6 mg/kg/day |
[1] uptake fraction of 100% was assumed.
| Endpoint | Lowest effect levels[i]/ results | |||
|---|---|---|---|---|
| Experimental animals and in vitro | ||||
| Acute toxicity | Oral LD50(rat) = 550-2000 mg/kg-bw (Pozzani et al. 1949; BASF AG 1958; Oettel and Hofmann 1960; Union Carbide Corp. 1971, 1989; Paulet and Vidal 1975; Rohm and Haas 1984; Ghanayem et al. 1985). Oral LD50(mouse) = 1300-1800 mg/kg-bw (Rohm and Haas 1950a,b; Tanii and Hashimoto 1982). Oral LD50 (rabbit) = 280-370 mg/kg-bw (Treon et al. 1949; Oettel and Hofmann 1960). Inhalation LC50 (rat) = 1414-2180 ppm (5790-8930 mg/m3) (Pozzani et al. 1949; Lomonova and Klimova 1979; Silver and Murphy 1981; Oberly and Tansy 1985; Union Carbide Corp. 1989). Inhalation LC50 (mouse) = 3950 ppm (16200 mg/m3) (Lomonova and Klimova 1979). Inhalation LC50 (rabbit) = <1204 ppm (4930 mg/m3) (Treon et al. 1949). Acute inhalation toxicity: Lowest inhalation LOAEC: 25 ppm (100 mg/m3) based on lesions in the olfactory epithelium in male F344/N rats (5 per group) exposed (nose-only) with ethyl acrylate at 0, 5, 25 or 75 ppm (approximately 0, 20, 100, 310 mg/m3 respectively) for 1, 3 or 6 hours. No effects were observed at 5 ppm exposed for up to 6h and no effects were observed for 1 hour exposure at all concentrations. Lesions in the olfactory epithelium (unilateral sustentacular cell necrosis and olfactory neuron degeneration and desquamation) were observed at 25 and 75 ppm exposed for 3 and 6 hours with nearly complete recovery following a 6-week recovery period (Frederick et al. 2002) Other inhalation LOAEC: 75 ppm (310 mg/m3) based on olfactory lesions in Cynomolgus monkeys (male and female randomly distributed, 3 per group) exposed (head-only) with ethyl acrylate at 0 or 75 ppm (approximately 0 and 310 mg/m3 respectively) for 3 or 6 hours. Examination of the nasal cavity showed lesions consisting of focal degeneration, necrosis and exfoliation with mild inflammation limited to the olfactory epithelium. Approximately 15% and 50% of the olfactory epithelium had damage after 3 and 6 hours ethyl acrylate exposure respectively (Rohm and Haas 1994; Harkema et al. 1997). RD50 (mouse) = 315 ppm (approximately 1290 mg/m3) in Swiss mice (n=6) exposed (head-only) with ethyl acrylate (De Ceaurriz et al. 1981). Dermal LD50(rat) = 470-5000 mg/kg-bw (Rohm and Haas 1986a, c, d; Union Carbide Corp. 1971, 1989). Dermal LD50 (mouse) = 2000-5000 mg/kg-bw (Rohm and Haas 1986d, e). Dermal LD50 (rabbit) = 126-1950 mg/kg-bw (Pozzani et al. 1949; Dow Chemical Co. 1957). |
|||
| Short-term repeated-dose toxicity | Lowest oral LOAEL: 20 mg/kg-bw per day based on increased stomach weight and dose-related hyperplasia and hyperkeratosis of squamous epithelium of the stomach in male Fischer 344 rats (10 per group) orally administered ethyl acrylate by gavage at 0, 20, 100 or 200 mg/kg-bw per day, 5 days/week for 28 days. Increased stomach weight was observed at all dose levels compared to control. An additional 200 mg/kg-bw per day treated group (10 rats) was held for a 9-week recovery period and showed full recovery (Rohm and Haas 1987). Lowest oral LOAEL: 20 mg/kg-bw per day based on dose-related increase in epithelial hyperplasia and hyperkeratosis of the forestomach in male Fischer 344 rats (10 per group) orally administered ethyl acrylate by gavage at 0, 2, 10, 20, 50, 100 or 200 mg/kg-bw per day, 5 days/week for 5 or 12 days. Increased in absolute and relative stomach weight were observed at 20 mg/kg-bw or higher after the 5-day period and at 50 mg/kg-bw or higher after the 12-day period. Manifestation of irritation, consisting of gastritis, submucosal edema and ulcer/erosion/eschar formation of the forestomach occurred in a dose related manner at 50 mg/kg-bw or higher after the 5-day period and at 100 mg/kg-bw or higher after the 12-day period. Epithelial hyperplasia and hyperkeratosis of the forestomach occurred in a dose-related manner at 20 mg/kg-bw or higher after 5 or 12-day period (Rohm and Haas 1986b). Lowest oral LOAEL: 20 mg/kg-bw per day based on hyperkeratosis of the forestomach in male Fischer 344 rats (10 per group) orally administered ethyl acrylate (>99% purity) by gavage at 0, 2, 10, 20, 50, 100 or 200 mg/kg-bw per day, 5 days/week for 14 days. Histopathological changes of the forestomach included hyperkeratosis at 20 mg/kg-bw or higher, subacute to chronic submucosal inflammation at 100 mg/kg-bw or higher, focal epithelial cell hyperplasia, submucosal oedema and ulceration/erosion at 200 mg/kg-bw. Histopathological changes of the glandular stomach included submucosal inflammation and edema at 100 mg/kg-bw or higher (Frederick et al. 1990). Other oral LOAEL: 1000 ppm (99mg/kg-bw per day) based on minimal irritation and diffuse epithelial hyperplasia of the forestomach in male Fischer 344 rats (10 per group) orally administered ethyl acrylate (>99% purity) in drinking water at 0, 200, 1000, 2000 or 4000 ppm (corresponding to 0, 23, 99, 197 and 369 mg/kg-bw per day respectively), 5 days/week for 14 days. Diffuse epithelial hyperplasia of the forestomach was observed at 1000 ppm or higher. Mild chronic submucosal inflammation and/or focal epithelial hemorrhage were observed at 2000 ppm or higher. To prevent degradation of ethyl acrylate in water, the author noted water bottles were changed twice a week with no significant loss of the parent compound (greater than 1%) detected (Frederick et al. 1990). [additional studies: Treon et al. 1949; Ghanayem et al. 1985; Ghanayem et al. 1986; NTP 1986a] |
|||
Lowest inhalation LOAEC: 70 ppm (290 mg/m3) based on an increase in relative kidney weight in male rats when Sherman albino rats (15/sex/group) were treated with ethyl acrylate at 0, 70 or 300 ppm (approximately 0, 290 and 1200 mg/m3 respectively), 7 h/day, 5 days/week for 30 days. At 70 ppm, an increase in kidney weight was observed in male rats and no other effects or histopathological abnormalities were observed. A significant increase in relative kidney weight in both sexes was observed in the 300 ppm exposed group. Two animals died of lung infection in each of the 70 ppm and its control groups. A total of 18/30 animals exposed to 300 ppm died during exposure compared to no deaths in its control group. Most deaths showed “catarrhal pneumonic involvement” where 10/18 animals had pulmonary, kidney and liver damage. (Pozzani et al. 1949). Other inhalation LOAEC: 300 ppm (1200 mg/m3) based on inflammation, degeneration, focal necrosis and squamous metaplasia of the nasal turbinates in males in F344 rats and B6C3F1 mice (10/sex/group/species) exposed with ethyl acrylate at 0, 75, 150 or 300 ppm (approximately 0, 310, 610 and 1200 mg/m3 respectively), 6h/day, 5 days/week for 30 days. Only the 0 and 300 ppm groups were subjected to histopathological examination. Decreased body weight gain was observed in both sexes and both species at 150 and 300 ppm. Inflammation, degeneration, focal necrosis and squamous metaplasia of the nasal turbinate were observed in males of both species at 300 ppm. Alternation in relative kidney and liver weights showed no corresponding histological findings according to ECETOC (1994) (Miller et al. 1979). [additional studies: Pozzani et al. 1949; Treon et al. 1949] No dermal studies were identified. |
||||
| Subchronic toxicity | Lowest oral LOAEL: 20 mg/kg-bw per day based on increased stomach weight and dose-related hyperplasia and hyperkeratosis of the squamous epithelium of the stomach in male Fischer 344 rats (10 per group) treated with ethyl acrylate by gavage at 0, 20, 100 or 200 mg/kg-bw per day, 5days/week for 13 weeks. Increased stomach weight was observed in all dosed groups compared to controls. Dose-related hyperplasia and hyperkeratosis of the squamous epithelium of the forestomach were observed at 20 mg/kg-bw or higher. Decreased body weight was observed at 200 mg/kg-bw (Rohm and Haas 1987). | |||
Other LOAEL: 100 mg/kg-bw per day based on severe epithelial hyperplasia of the forestomach in male Fischer 344 rats (25 per group) administered ethyl acrylate (99% purity) by gavage at 0, 100 or 200 mg/kg-bw per day, 5 days/week for 13 weeks. Severe epithelial hyperplasia of the forestomach was observed at 100 mg/kg-bw or higher. No lesions in the glandular stomach or the liver were observed. Significant decline in the incidence and severity of forestomach mucosal hyperplasia were observed after 8 weeks and 19 months of recovery. No increased incidence of papillomas or carcinomas of the forestomach was observed (Ghanayem et al. 1991). [additional studies: NTP 1986a] Lowest inhalation LOAEC: 100 mg/m3(25 ppm) based on histopathological lesions of the nasal mucosa in Fischer 344 rats (5-10/sex/group) exposed to ethyl acrylate (purity not specified) at 0, 25, 75 or 225 ppm (approximately 0, 100, 310 and 920 mg/m3), 6hours/day, 5days/week for 6 months. Histopathological examinations were limited to nasal mucosa at 25 and 75 ppm whereas an extensive list of tissues was examined in controls and 225 ppm exposed groups. Very slight focal degeneration of the olfactory portion of the nasal mucosa at 25 ppm, slight degeneration and inflammation of the nasal mucosa at 75 ppm and moderate severity of nasal mucosa effects including degeneration, inflammation and necrosis at 225 ppm were observed. No gross pathological lesions were observed in any exposed group. At 225 ppm, decreased amounts of adipose tissue were reported in both males and females and the author noted that this observation was consistent with the body weight data; however, body weight data was not reported (Jersey et al. 1978). Lowest inhalation LOAEC: 100 mg/m3(25 ppm) based on histopathological lesions of the nasal mucosa in Fischer 344 rats (5/sex/group) exposed to ethyl acrylate (purity not specified) at 0, 25 or 75 ppm (approximately 0, 100 and 310 mg/m3), 6h/day, 5days/week for 12 months. No grossly visible treatment-related alterations were observed. Histopathological examination revealed lesions of the olfactory region of the nasal mucosa in all treated groups. Lesions included focal degeneration of the olfactory epithelium, focal hyperplasia of the basal cells of the olfactory epithelium and inflammation of the mucosa and submucosa with severity from very slight at 25 ppm to slight to moderate at 75 ppm (Dow Chemical 1979a). [additional studies: Treon et al. 1949; Gabor et al. 1965; Dow Chemical 1979a,b; Institute of Biological Research 1983; Miller et al. 1985] Lowest dermal LOEL: 2000 mg/kg-bw per day based on decreased in body weight observed in female transgenic Tg.AC (v-Ha-ras) mice (10 per group) dermally administered ethyl acrylate (99% purity) at 0, 60, 300 or 600 µmoles/dose (approximately 0, 200, 1000 and 2000 mg/kg-bw/dose respectively), 3 times/week for 20 weeks. No induction of papillomas was observed at the site of application (Tennant et al. 1996; Nylander-French and French 1998). NTP (1998) commented that the use of transgenic models for carcinogen identification is in developmental stages and results from these studies are suggestive but not to be taken as conclusive evidence. [No additional studies identified.] |
||||
| Chronic toxicity/ carcinogenicity | Oral carcinogenicity in rats: Fischer 344 rats (50 per sex per group) were orally administered ethyl acrylate (99-99.5% purity) by gavage at 0, 100 or 200 mg/kg-bw per day, 5 days/week for 103 weeks. There was a significant increase in the incidence of squamous cell papillomas and squamous cell carcinomas in males at 100 and 200 mg/kg-bw and a significant increase in the incidence of squamous cell papillomas in females at 100 and 200 mg/kg-bw. In males, the incidence of squamous cell papillomas was 1/50, 15/50, 29/50; the incidence of squamous cell carcinomas was 0/50, 5/50, 12/50; the incidence of squamous cell papillomas and carcinomas was 1/50, 18/50, 36/50 for 0, 100, 200 mg/kg-bw per day respectively. In females, the incidence of squamous cell papillomas was 1/50, 6/50, 9/50; the incidence of squamous cell carcinomas was 0/50, 0/50, 2/50; the incidence of squamous cell papillomas and carcinomas was 1/50, 6/50, 11/50 for 0, 100, 200 mg/kg-bw per day respectively. The LOAEC was 100 mg/kg-bw per day based on an increase in hyperkeratosis, hyperplasia and inflammation of the forestomach observed at 100 mg/kg-bw and higher. No effects on mean body weights, clinical signs and survival were observed (NTP 1986a). Male Fischer 344 rats approximately 3 months of age (5-16/group) were orally administered ethyl acrylate (99% purity) by gavage at 0 or 200 mg/kg-bw per day, 5 days/week for 6 or 12 months followed by up to 15 months of recovery. Forestomach tumours developed during 2-9 months of recovery in rats treated for 12 months. Squamous cell papillomas (2/5) of the forestomach were observed after 2 months of recovery. Squamous cell carcinomas (3/13) and squamous cell papillomas (1/13) of the forestomach were observed after 9 months of recovery. No neoplasms were observed in the controls, the 6 months treated groups with or without recovery periods and in animals immediately sacrificed after 12 months of treatment. No neoplastic lesions were grossly observed in the liver or other organs in any treatment group. The LOAEC was 200 mg/kg-bw per day based on forestomach hyperplasia. In animals treated for 6 months, forestomach hyperplasia (5/5) developed, with recovery observed after 2-months (0/5) and 15-months (1/18). In animals treated for 12 months, forestomach hyperplasia (5/5) was observed and persisted after 2 months (5/5) and 9 months (10/13) of recovery. (Ghanayem et al. 1993, 1994). IARC (1999) noted that the histopathological evaluation in Ghanayem (1993) was limited to the stomach. Wistar rats (25/sex/group) were orally administered ethyl acrylate (purity not specified) by drinking water at 0, 6-7, 60-70 or 2000 ppm (approximately 0, 0.84-0.98, 9.4-9.8 and 280 mg/kg-bw respectively) for 2 years (number of days per week not specified). No neoplastic effects were observed. The LOEL was 2000 ppm (280 mg/kg-bw per day) based on significant reduction in fluid consumption in both sexes and significant reduction in body weight and food consumption in females. No changes in mortality, hematology, clinical pathology measurement or histopathology were observed. No significant loss of ethyl acrylate in water when tested after 72hr and water bottles were changed twice a week (Borzelleca et al. 1964). Oral carcinogenicity in mice: B6C3F1 mice (50/sex/group) were orally administered ethyl acrylate (99-99.5% purity) by gavage at 0, 100 or 200 mg/kg-bw per day, 5 days/week for 103 weeks. There was a significant increase in the incidence of squamous cell carcinomas and papillomas of the forestomach at 200 mg/kg-bw. In males, the incidence of squamous cell papillomas was 0/48, 4/47, 9/50; the incidence of squamous cell carcinomas was 0/48, 2/47, 5/50; the incidence of squamous cell papillomas and carcinomas was 0/48, 5/47, 12/50 for 0, 100, 200 mg/kg-bw per day respectively. In females, the incidence of squamous cell papillomas was 1/50, 4/49, 5/48; the incidence of squamous cell carcinomas was 0/50, 1/49, 2/48; the incidence of squamous cell papillomas and carcinomas was 1/50, 5/49, 7/48 for 0, 100, 200 mg/kg-bw per day respectively. The LOAEC was 100 mg/kg-bw per day based on an increase in hyperkeratosis, hyperplasia and inflammation of the forestomach observed at 100 mg/kg-bw and higher. No effects on mean body weights, clinical signs and survival were observed (NTP 1986a). Oral carcinogenicity in dogs: Beagle dogs (2/sex/group) were orally (in gelatin capsules dissolved in corn oil) administered ethyl acrylate at 0, 10, 100 or 1000 ppm (approximately 0, 0.3, 3 and 30 mg/kg-bw per day respectively) for 2 years (number of days per week not specified). ANOEL of 1000 ppm was identified. Animals at 1000 ppm (30 mg/kg-bw per day) exhibited emesis initially where dose was reduced to 300 ppm (9 mg/kg-bw per day) and gradually increased to 1000 ppm (30 mg/kg-bw per day) over a 16 week period. No systemic toxic effects were observed. A decrease in weight gain and food consumption at 1000 ppm (30 mg/kg-bw per day) compared to control was not statistically significant. No changes in histopathology, hematologic values, urine concentrations of protein and reducing substances were observed (Borzelleca et al. 1964). Inhalation carcinogenicity in rats and mice: Fischer 344 rats (75/sex/exposed group and 60/sex/control group) and B6C3F1 mice were exposed to ethyl acrylate (>99.5% purity) at 0, 25 or 75 ppm (approximately 0, 100 and 310 mg/m3respectively), 6 hours/day, 5 days/week for 27 months. Subgroups of animals were sacrificed after 3, 6, 12 and 18 months of exposure. No treatment-related neoplastic lesions were observed. The incidence of thyroid follicular adenomas in male mice at 225 ppm (7/69) was significantly higher than the concurrent controls (2/121) but not the historical controls. The LOAEC was 25 ppm (100 mg/m3) based on non-neoplastic lesions of the olfactory mucosa (glandular and basal-cell hyperplasia, increase in intraepithelial glands and respiratory metaplasia). A significant decrease in mean body weight gain was observed in both sexes at 310 mg/m3. No effects on survival, organ weights, hematology, clinical chemistry and urinalyses were observed (Miller et al. 1985). Fischer 344 rats and B6C3F1 mice (90/sex/exposed group and 80/sex/control group) were exposed to ethyl acrylate (>99.5% purity) at 0 or 5 ppm (approximately 20 mg/m3), 6h/day, 5d/week for 24 months. Subgroups of animals were sacrificed after 6, 12 and 18 months of exposure. Histopathologic examination was limited to nasal turbinate and showed no treatment related effects. The NOEC was 5 ppm (20 mg/m3) (Miller et al. 1985). Dermal carcinogenicity in mice: Male C3H/HeJ mice (40/group) were dermally administered acetone (control) or 25 µl ethyl acrylate (99% purity) per animal (approximately 23 mg/application, 800 mg/kg-bw per day), 3 times per week for life. Animals were initially housed as groups of 5. According to the authors, due to an increase in early mortality, animals in the ethyl acrylate group were housed individually after 13 months of study to minimize the spread of infectious disease that may have contributed to the early mortality. No epidermal tumours were observed. The LOAEL was 800 mg/kg-bw based on the development of dermatitis, dermal fibrosis, epidermal necrosis and hyperkeratosis. No statistically significant effect on survival was observed; the mean survival time for ethyl acrylate was 408 days and for acetone control was 484 days (DePass et al. 1984). IARC (1986) noted that no mention was made of control for possible losses of the parent compound by volatilization or polymerization. No additional studies were identified |
|||
| Reproductive toxicity | No studies were identified. | |||
| Developmental toxicity | Lowest oral LOEL for developmental toxicity in rats: 25 mg/kg-bw per day based on delayed ossification in fetuses when female Wistar rats (10-23/group) were administrated ethyl acrylate (purity not specified in IARC 1986) by gavage at 0, 25, 50, 100, 200 or 400 mg/kg-bw per day on gestation days 7-16. A reduction in maternal body weight gain was observed at 25 mg/kg-bw or higher. The total number of resorptions was significantly increased at 100 mg/kg-bw or higher but there was no effect on the number of live fetuses per litter. Fifty percent of the fetuses were examined for skeletal defects and it was found that the overall incidence of delayed ossification was increased in all treated groups (Pietrowicz et al. 1980). Other oral NOEL for developmental toxicity in rats: 400 mg/kg-bw per day in female Wistar rats (number not specified in European Commission 2000) orally administered ethyl acrylate (purity not specified) at 44, 133 or 400 mg/kg-bw per day on gestation day 0-19. No data specified for the inclusion of a control group. A slight but not statistically significant increase in the number of dead or absorbed fetuses was observed. No induction of external, skeletal and visceral anomalies were observed. No adverse changes were found in postnatal development in offspring (Shimizu et al. 1994). Lowest inhalation LOAEC for developmental toxicity in rats: 200 ppm (800 mg/m3) based on significant decrease in fetal body weight in female Sprague-Dawley rats (17-19/group) exposed to ethyl acrylate at 0, 25, 50, 100 or 200 ppm (approximately 0, 100, 200, 400 and 800 mg/m3respectively) 6 hours/day on gestation days 6-20. A significant decrease in maternal body weight gain and absolute weight gain were observed at 200 ppm. No significant differences in the number of implantation sites and live fetuses, in the incidence of non-live implants and resorptions or in the fetal sex ratio were observed. Fetal body weight was significantly decreased by 7-8% compared to controls at 200 ppm. No treatment-related increase in embryo/fetal lethality or fetal malformations were observed at any dose level. The incidences of external, visceral and skeletal variations were similar to controls (Saillenfait et al. 1999). Other inhalation NOEC for developmental toxicity in rats: 150 ppm (610 mg/m3) in female Sprague-Dawley rats (33/group) exposed to ethyl acrylate at 0, 50 or 150 ppm (approximately 0, 200, 610 mg/m3respectively), 6 hours/day on gestation days 6-15. A reduction in maternal body weight and food consumption and an increase in water consumption were observed at 150 ppm. No effects on mean litter size, corpora lutea, implantation sites, live fetuses, incidence of resorptions, fetal sex ratio, fetal crown-rump length were observed. A significant increase in mean fetal body weight at 150 ppm was considered not to be toxicologically significant by the authors. 3/308 pups at 150 ppm showed fetal malformations (hypoplastic tail, small anal orifice) or skeletal variations (delayed ossification, missing ribs or vertebrae, or fused ribs) where the incidences were not statistically significant compared to concurrent and historical controls. No adverse effects on fetal body weight or survival were observed (Murray et al. 1981). No dermal studies were identified. |
|||
| Genotoxicity and related endpoints: in vivo | Chromosomal aberrations: Negative: Male C57BL/6 mice (5/group) administered intraperitoneally ethyl acrylate at 0, 125, 250, 500 or 1000 mg/kg-bw (acute) (Kligerman et al. 1991). Sex-linked recessive lethal: Negative: Drosophilia melanogaster, up to 40000 ppm ethyl acrylate (99.7% purity) feeding in a solution of 5% aqueous sucrose (Valencia et al. 1985). Negative: Drosophilia melanogaster, up to 20000 ppm ethyl acrylate (99.7% purity) injection in 0.7% aqueous NaCl (Valencia et al. 1985). DNA binding: Negative: Male Fischer 344 rats (3/group) orally administered 2,3-[14C]ethyl acrylate (90-92% purity) by gavage at 0, 100, 200, or 400 mg/kg-bw (Ghanayem et al. 1987). IARC (1999) commented that the method for determining DNA binding was inadequate. Micronucleus induction: Negative: C57B16J mice (5-10/sex/group) administered ethyl acrylate intraperitoneally (98.5% purity) at 0, 461 or 738 mg/kg-bw (acute) and 0 or 738 mg/kg-bw per day (2 consecutive days) (Ashby et al. 1989). Negative: Male BDF1 mice (6-9/group) administered ethyl acrylate intraperitoneally (>99.0% purity) at 0, 375, 500, 750, 1000 or 1500 mg/kg-bw (acute). All animals at 1000 and 1500 mg/kg-bw and 1 animal at 750 mg/kg-bw died (Hara et al. 1994). Negative: Male C57BL/6 mice (5/group) administered ethyl acrylate intraperitoneally (99% purity) at 0, 125, 250, 500 or 1000 mg/kg-bw (acute) (Kligerman et al. 1991). Negative: Male BDF1 mice (6/group) administered ethyl acrylate intraperitoneally at 0, 375, 500, 750 or 1000 mg/kg-bw (acute) (Morita et al. 1997). Negative: Male BDF1 mice (6/group) administered orally ethyl acrylate (>99.0% purity) at 0, 188, 375 or 750 mg/kg-bw per day for acute and for 2 consecutive days (Hara et al. 1994). Negative: Male BDF1 mice (6/group) administered ethyl acrylate orally at 0, 188, 375, 750 or 1000 mg/kg-bw (acute) (Morita et al. 1997). Negative: Tg.AC mice (7-9/group) administered ethyl acrylate (99% purity) dermally at 0, 200, 1000 or 2000 mg/kg-bw per day, 3 times/week for 20 weeks (Tice et al. 1997). NTP (1998) commented that the use of transgenic models for carcinogen identification is in developmental stages and results from these studies are suggestive but not to be taken as conclusive evidence. Positive: Male Balb/c mice (4/group) administered ethyl acrylate intraperitoneally for 2 consecutive days at 0, 112.5, 225, 450, 900 or 1800 mg/kg-bw per day. Two animals at 1800 mg/kg-bw died (Przybojewska et al. 1984; Basler and van der Hude 1987). Uncertain/Variable: C57B16J mice (5-10/sex/group) administered ethyl acrylate intraperitoneally for 2 consecutive days at 0 or 812 mg/kg-bw per day. Identical experiments were repeated twice with conflicting results (Ashby 1989). Sister chromatid exchange: Negative: Male C57BL/6 mice (5/group) administered ethyl acrylate intraperitoneally at 0, 125, 250, 500 or 1000 mg/kg-bw (acute) (Kligerman et al. 1991). |
|||
| Endpoint | Mutagenicity | |||
|---|---|---|---|---|
| Assay, species, strain | Result | Metabolic activation | References | |
| Genotoxicity and related endpoints: in vitro | Mouse lymphoma assay, L51784TK+/- cells | Positive | +/- | Dearfield et al. 1991 |
| Positive | - | Myhr 1980; McGregor et al. 1988; Moore et al. 1988 | ||
| HGPRT assay, Chinese hamster ovary (CHO) cells | Negative | - | Moore et al. 1989 | |
| Ames assay, Salmonella typhimurium TA98 | Negative | +/- | Rohm and Hass 1977, 1981; Ishidate et al. 1981; Haworth et al. 1983; Waegemaekers and Bensink 1984; NTP 1986a | |
| Negative | - | Brusick 1977; ECETOC 1994 | ||
| Negative | + | Zeiger et al. 1992 | ||
| Ames assay, Salmonella typhimurium TA100 | Negative | +/- | Rohm and Hass 1977, 1981; Ishidate et al. 1981; Haworth et al. 1983; Waegermaekers and Bensink 1984; NTP 1986a | |
| Negative | - | Brunsick 1977; ECETOC 1994 | ||
| Negative | + | Zeiger et al. 1992 | ||
| Ames assay, Salmonella typhimurium TA1535 | Negative | +/- | Rohm and Hass 1977, 1981; Haworth et al. 1983; Waegemaekers and Bensink 1984; NTP 1986a | |
| Negative | - | Brusick 1977; ECETOC 1994 | ||
| Negative | + | Zeiger et al. 1992 | ||
| Ames assay, Salmonella typhimurium TA1537 | Negative | +/- | Rohm and Hass 1977, 1981; Ishidate et al. 1981; Haworth et al. 1983; Waegemaekers and Bensink 1984; NTP 1986a | |
| Negative | - | Brusick 1977; ECETOC 1994 | ||
| Ames assay, Salmonella typhimurium TA1538 | Negative | +/- | Rohm and Hass 1977; Waegemaekers and Bensink 1984 | |
| Negative | - | Brusick 1977 | ||
| Ames assay, Salmonella typhimuriumYG7108pin3ERb5 | Negative | - | Emmert et al. 2006 | |
| Umu test, Salmonella typhimurium TA1535/pSK1002 | Negative | +/- | Yasunaga et al. 2004 | |
| Yeast cytogenic assay, Saccharomyces cerevisiaeD61.M | Positive | _ | Zimmermann and Mohr 1992 | |
| Yeast cytogenic assay, Saccharomyces cerevisiae, Strain D4 | Negative | +/- | Rohm and Haas 1983 | |
| Endpoint | Lowest effect levels[i]/ results | |||
|---|---|---|---|---|
| Genotoxicity and related endpoints: in vitro | Chromosome aberration: Positive: CHO cells in the presence and absence of metabolic activation S9 (NTP 1986b). Positive: CHO cells in the presence of metabolic activation S9 (Loveday et al. 1990). Positive: L5178Y/TK+/- mouse lymphoma cells in the absence of metabolic activation S9 (Moore et al. 1988). Positive: Chinese hamster lung (CHL-Zellen) cells in the presence and absence of metabolic activation S9 (Ishidate et al. 1981). Positive: Isolated mouse splenocytes in the absence of S9 at G1-S phase (Kligerman et al. 1991). Negative: CHO cells in the absence of metabolic activation S9 (Loveday et al. 1990). Negative: Isolated mouse splenocytes treated in the absence of S9 at G0 phase (Kligerman et al. 1991). Sister chromatid exchange: Positive: CHO cells in the presence of metabolic activation S9 (Loveday et al. 1990). Positive: CHO cells in the absence of metabolic activation S9 (NTP 1986b). Negative: CHO cells in the absence of metabolic activation S9 (Loveday et al. 1990). Negative: Isolated mouse splenocytes in the absence of S9 at G0 and G1-S phases (Kligerman et al. 1991). DNA alkylation: Negative: Ethyl acrylate incubated with deoxyribonucleosides for up to 24hr at pH 6.7 or 7.4 at 37oC or up to 8hr at 50oC (McCarthy et al. 1994). Cell transformation assay: Positive: A-31-1-13 BALB/c-3T3 cells (Matthews et al. 1993). Positive: Fischer 344 rat tracheal epithelial cells (Steele et al. 1989). BUA (1992) commented that the method is unvalidated. |
|||
| Sensitization | Positive: Freund’s complete adjuvant test in guinea pig and cross-sensitization to challenges with several other acrylates (Van der Walle et al. 1982). Negative: Maximization test in guinea pig (van der Walle et al. 1982). Negative: Mouse Ear Swelling test for contact hypersensitivity (NTP 1994; Hayes and Meade 1999). Negative; Murine local lymph node assay (Kimber 1992; NTP 1994; Hayes and Meade 1999). |
|||
| Irritation | Skin irritation: Skin inflammation, moderate to severe erythema and edema when tested in rabbits (Pozzani et al. 1949; Treon et al. 1949; Oettel and Zeller 1958; BASF AG 1978; Poole 1980; Potokar et al. 1985; Rohm and Haas 1991; BASF 2005). Reported as irritating in rabbits where severity was not specified (Celanese Chem Co. 1972; Lomonova and Klimova 1979; Union Carbide Corp 1989; BAMM 1994). Reported as no irritation in rabbits (no further information) (Union Carbide Corp 1989). Slight transient erythema under unocclusive conditions and severe erythema and oedema under occlusive conditions in rats (Rohm and Haas 1986a, c). No irritation under unocclusive conditions and severe erythema and moderate oedema under occlusive conditions in mice (Rohm and Haas 1986d). |
|||
Eye irritation: Reddening and inflammation of the conjunctiva and cloudiness of the cornea when tested in rabbits (Pozzani et al. 1949; BASF AG 1958; Oettel and Zeller 1958; Poole 1980). Irritating in guinea pigs, no further information (Haskell Laboratories 1945). |
||||
| Humans studies | ||||
| Sensitization studies | Twenty-four volunteers (no further information specified in Opdyke 1975) were in a maximization test treated with ethyl acrylate for 48 hours with 4% ethyl acrylate in petrolatum. Ten volunteers showed sensitization. No irritation was observed (Epstein 1974). Five men who had reacted to 2-ethylhexyl-acrylate (2 EHA) and/or n-tert-butyl maleamic acid (NTBM) previously were closed patch tested with 5% ethyl acrylate in olive oil for 48 hours. One person showed irritation. Three people developed cross sensitization to ethyl acrylate (Jordan 1975). 124 dermatitis patients with a history of acrylate exposure (no further information) were closed patch tested with 0.1-0.5% ethyl acrylate in petrolatum for 24 or 48 hours. 9/124 patients were positive for sensitization (Kanerva et al. 1995). 55 dental technicians (no further details) with suspected occupational dermatitis were patch tested with 0.1% ethyl acrylate in petrolatum for 24hr. 3/55 showed sensitization (Rustemeyer and Frosch 1996). 23 patients with allergic contact dermatitis to acrylates (17 women and 6 men ranging from age 30-69) were patch tested with 0.1% ethyl acrylate. 72% of the patients were sensitized to ethyl acrylate. 10/11 patients allergic to artificial nails were sensitized to ethyl acrylate (Koppula et al. 1995). [additional studies: Foulger and Fleming 1945; Fregert 1978; Bjorkner and Dahlquist 1979; Malten et al. 1984; Conde-Salazar et al. 1988; Kanerva et al. 1988, 1989, 1992; Stenman and Bergman 1989; Skoglund and Egelrud 1991; Marks et al. 1995; Tucker and Beck 1999; Aalto-Korte et al. 2007; Lazarov 2007] |
|||
| Epidemiological studies | ||||
| Case studies | One man with accidental exposure to ethyl acrylate showed serious eye irritation. No details were available (Dow Chemical 1964). One pregnant woman was exposed to polymers SK-131-A (polymer from ethyl acrylate, acrylonitrile and methylenesuccinic acid) and SK-131-B (polymer from ethyl acrylate, methylolacrylamide and methylenesuccinic acid) and her child was diagnosed with congenital anomalies. No further details were available (Sherman 1985). |
|||
| Cohort studies | In an early Bistol cohort study (1933-1945), 3924 men employed in an acrylic sheet manufacturing facility at Bristol, Pennsylvania were exposed to ethyl acrylate, methyl methacrylate, lead, ethylene dichloride, methylene chloride and acrylonitrile. Concentrations of exposure were not measured and exposure scores were based on job task reconstructed from production records and interviews with plant personnel. Excess mortality from colon cancer (14 observed vs. 7.53 expected) were observed in workers exposed to ethyl acrylate and methyl methacrylate compared to local rates 20 years after the equivalent of 3 years employment in jobs producing the highest exposure to ethyl acrylate and/or methyl methacrylate. Excess mortality from rectal cancer (10 observed vs. 5.23 expected) were observed in exposed workers compared to local rates 20 years after the equivalent of 3 years of employment (Walker et al. 1991). In a late Bistol cohort study (1946-1986), 6548 men employed in an acrylic sheet manufacturing facility at Bristol, Pennsylvania, were exposed to ethyl acrylate, methyl methacrylate, lead, ethylene dichloride, methylene chloride and acrylonitrile. Exposure levels were semi-quantitative in a four-level ordinal exposure scale for methyl methacrylate and/or ethyl acrylate (level 1 = no routine exposure, level 2 = 1-5 ppm, level 3 = 5-24 ppm, level 4 = > 25 ppm). Exposure levels for methyl methacrylate and ethyl acrylate were not distinguished. Exposure values were monitored starting from 1972. Previous exposure values were reconstructed from production records and interviews with plant personnel. No excess mortality from any cause was observed (Walker et al. 1991). In a Knoxville plant cohort study (1943-1982), 3381 men employed in an acrylic sheet manufacturing facility at Knoxville, Tennessee, were exposed to ethyl acrylate, methyl methacrylate, lead, ethylene dichloride, methylene chloride and acrylonitrile. Exposure levels were semi-quantitative in a 4-level ethyl acrylate/methyl methacrylate vapour exposure scale based on job task. Exposure levels for methyl methacrylate and ethyl acrylate were not distinguished. Exposure values were monitored starting from 1972. Previous exposure values were reconstructed from production records and interviews with plant personnel. No excess mortality from any cause was observed (Walker et al. 1991). Workers employed for an average of 5 years (20 women, 13 men) were exposed to 4-58 mg/m3 (1-14 ppm) ethyl acrylate, up to 50 mg/m3 (9.4 ppm) n-butyl acrylate and 0.11-2 mg/m3 (0.05-0.9 ppm) acrylonitrile. 14/33 individuals were diagnosed with neuroautonomic or neurotic disturbances of the central nervous system and electroencephalographic examination showed no organic dysfunction (Kuzelova et al. 1981). Workers in a chemical facility manufacturing acrylates and methacrylates (618 men, 113 females, 17-69 years old, mean age 42.9) were exposed to a mixture of ethyl and butyl acrylate, acrylic acid and methyl methacrylate. Ethyl acrylate and acrylic acid level varied from 0.01-56 ppm. Reduction of olfactory function was observed but the association was not statistically significant (Schwartz et al. 1989). |
|||
| No | OECD, EU, national, or other standard method? | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: OECD 2005. Ethyl acrylate, CAS 140-88-5. SIDS Dossier. Geneva: United Nations Enironmental Programme. | |||
| 2 | Substance identity: CAS RN | n/a | y | |
| 3 | Substance identity: chemical name(s) | n/a | y | |
| 4 | Chemical composition of the substance | 2 | y | |
| 5 | Chemical purity | 1 | y | |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | n | |
| Method | ||||
| 7 | Reference | 1 | y | |
| 8 | OECD, EU, national, or other standard method? | 3 | y | |
| 9 | Justification of the method/protocol if not a standard method was used | 2 | y | |
| 10 | GLP (Good Laboratory Practice) | 3 | y | |
| Test organism | ||||
| 11 | Organism identity: name | n/a | y | |
| 12 | Latin or both Latin & common names reported? | 1 | y | |
| 13 | Life cycle age / stage of test organism | 1 | n | |
| 14 | Length and/or weight | 1 | n | |
| 15 | Sex | 1 | n | |
| 16 | Number of organisms per replicate | 1 | ||
| 17 | Organism loading rate | 1 | n | |
| 18 | Food type and feeding periods during the acclimation period | 1 | y | |
| Test design / conditions | ||||
| 19 | Test type (acute or chronic) | n/a | y | Chronic |
| 20 | Experiment type (laboratory or field) | n/a | y | laboratory |
| 21 | Exposure pathways (food, water, both) | n/a | ||
| 22 | Exposure duration | n/a | y | 21 days |
| 23 | Negative or positive controls (specify) | 1 | y | |
| 24 | Number of replicates (including controls) | 1 | y | five |
| 25 | Nominal concentrations reported? | 1 | y | five |
| 26 | Measured concentrations reported? | 3 | y | |
| 27 | Food type and feeding periods during the long-term tests | 1 | y | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | y | five |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | y | |
| 30 | Photoperiod and light intensity | 1 | n | |
| 31 | Stock and test solution preparation | 1 | n | |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | n | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | n | |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | n | |
| 35 | Monitoring intervals (including observations and water quality parameters) reported? | 1 | y | |
| 36 | Statistical methods used | 1 | n | |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism’s health (e.g. when mortality in the control >10%) or physical effects (e.g. 'shading effect')? | n/a | y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | y | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | y | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | y | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | y | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | y | |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | |
| 45 | Other endpoints reported - e.g. BCF/BAF, LOEC/NOEC (specify)? | n/a | y | LOEC/NOEC/MATC/EC50 |
| 46 | Other adverse effects (e.g. carcinogenicity, mutagenicity) reported? | n/a | n | |
| 47 | Score: ... % | 77.1 | ||
| 48 | EC Reliability code: | 2 | ||
| 49 | Reliability category (high, satisfactory, low): | Satisfactory Confidence | ||
| 50 | Comments | |||
Footnotes
[2] A determination of whether one or more of the criteria of section 64 are met is based upon an assessment of potential risks to the environment and/or to human health associated with exposures in the general environment. For humans, this includes, but is not limited to, exposures from ambient and indoor air, drinking water, foodstuffs, and the use of consumer products A conclusion under CEPA 1999 on the substances in the Chemicals Management Plan (CMP) Challenge Batches 1-12 is not relevant to, nor does it preclude, an assessment against the hazard criteria specified in the Controlled Products Regulations, which is part of regulatory framework for the Workplace Hazardous Materials Information System [WHMIS] for products intended for workplace use.
[3] Voluntary submission provided by the Basic Acrylic Monomer Manufactures association (BAMM).
[4] Voluntary submission provided by Rohm and Haas Canada