Screening assessment - Heterocycles group
Official title: Screening assessment - Heterocycles group
Chemical Abstracts Service Registry Numbers
100-97-0, 110-91-8, 4174-09-8
Environment and Climate Change Canada
Health Canada
June 2019
Cat. No.: En14-353/2019E-PDF
ISBN: 978-0-660-29216-8
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment on three of seven substances referred to collectively under the Chemicals Management Plan as the Heterocycles Group. Four substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA; the three noted in the table below and the substance 2-imidazolidinethione (commonly known as ethylene thiourea (ETU)). ETU was included in the draft screening assessment for the Heterocycles Group published on November 11, 2017 however, activities associated with the presence of ETU as a metabolite and a residual in select pesticides are ongoing, therefore the CEPA conclusion on this substance will be provided in a separate screening assessment. Three of the seven substances were subsequently determined to be of low concern through other approaches, and decisions for these substances are provided in a separate reportFootnote 1 . Accordingly, this screening assessment addresses the three substances listed in the table below. The three substances addressed in this screening assessment will hereinafter be referred to as the Heterocycles Group.
| CAS RNa | Domestic Substances List (DSL) name | Common name |
|---|---|---|
| 100-97-0 | 1,3,5,7-tetraazatricyclo[3.3.1.13,7] decane | methenamine |
| 110-91-8 | tetrahydro-1,4-oxazine | morpholine |
| 4174-09-8 | 3H-pyrazol-3-one, 2,4-dihydro-4-[(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-5-methyl-2-phenyl- | N/A |
N/A Not applicable
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
In 2011, between 100 000 and 1 000 000 kg of methenamine were reported to be manufactured in Canada, and between 100,000 and 1 000 000 kg were reported to be imported into Canada during the same calendar year according to information submitted pursuant to a CEPA section 71 notice. The largest reported use of methenamine is as a cross-linking agent in phenolic and urea formaldehyde resins and in rubber. Methenamine is consumed during this process. Another reported use is as a chemical intermediate in nitration reactions for explosives production and in the production of fuel tablets. Cosmetics may also contain methenamine at low levels as a preservative. Food packaging materials may also contain methenamine.
In 2011, between 1 000 and 10 000 kg of morpholine were reported to be manufactured in Canada, and between 100 000 and 1 000 000 kg were reported to be imported into Canada during the same calendar year according to information submitted pursuant to a CEPA section 71 notice. Reported primary uses of morpholine include use as an intermediate in the production of rubber accelerators, pharmaceuticals, pesticides, optical brighteners, antioxidants and as an industrial solvent. It is also used in closed water or steam systems to prevent corrosion, as an oil field production chemical and as a solvent and emulsifier in the preparation of wax coatings for fruits and vegetables. Morpholine has also been identified as a component in the manufacture of some food packaging materials (e.g. interior coatings).
In 2011, between 1 000 and 10 000 kg of CAS RN 4174-09-8 were reported to be imported into Canada according to information submitted pursuant to a CEPA section 71 notice. CAS RN 4174-09-8 was reported to be used as a colourant for plastic materials and articles, varnishes and coatings. It has been identified for use as colourants in polystyrene, polycarbonate and polyethylene terephthalate food packaging materials.
The ecological risks of the substances in the Heterocycles Group were characterized using the ecological risk classification of organic substances (ERC) approach, which is a risk-based approach that employs multiple metrics for both hazard and exposure with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are established primarily on the basis of mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. The ERC identified the three substances in the Heterocycles Group as having low potential to cause ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from methenamine, morpholine and CAS RN 4174-09-8. It is concluded that methenamine, morpholine and CAS RN 4174-09-8 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
General population exposure to methenamine can occur from use of cosmetics when it is used as a preservative, when it is used in products available to consumers, and from its use in food packaging materials. For the general population, margins of exposures comparing effect levels for the critical health effects and the estimates of methanamine exposures are considered adequate to address uncertainties in the health effects and exposure databases.
Exposure of the general population to morpholine is expected to be limited to the use of a small number of products available to consumers, primarily home and auto polishes/waxes and related auto care products. Morpholine may also be added to some wax coating compounds used on fresh produce, such as apples. As such, there is the potential for dietary exposure to trace levels of morpholine when coated produce is consumed. In the case of food packaging use, morpholine is not a significant source of dietary exposure. There is also the potential for exposure from disinfectant sprays. Health Canada (2002) previously conducted a safety assessment of the use of morpholine in wax coatings used on apples and determined that such use did not present a risk to humans. For the general population, the margins of exposure between morpholine exposures and the critical effect levels identified from laboratory studies are considered adequate to address uncertainties in the health effects and exposure databases.
CAS RN 4174-09-8 may be used as a colourant in food packaging materials though it is not expected to migrate from the packaging material. As exposure is considered to be negligible, risk to human health is considered to be low.
On the basis of the information presented in this screening assessment, it is concluded that methenamine, morpholine and CAS RN 4174-09-8 do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that methenamine, morpholine and CAS RN 4174-09-8 do not meet the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of three of seven substances, referred to collectively under the Chemicals Management Plan as the Heterocycles Group, to determine whether they present or may present a risk to the environment or to human health. Four substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA; (ECCC, HC [modified 2017]) the three described in Section 2 of this report and the substance 2-imidazolidinethione (commonly known as ethylene thiourea (ETU)). ETU was included in the draft screening assessment for the Heterocycles Group published on November 11, 2017 however, activities associated with the presence of ETU as a metabolite and a residual in select pesticides are ongoing, therefore the CEPA conclusion on this substance will be provided in a separate screening assessment.
The other three substances (listed in Table 1‑1 below) were considered in the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances science approach documents (ECCC 2016a; Health Canada 2016), and were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this report. Conclusions for these three substances are provided in the Substances Identified as Being of Low Concern Using the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Screening Assessment (ECCC, HC 2018).
| CAS RNa | Domestic Substances List (DSL) ethylene name | Approach under which the substance was addressed | References |
|---|---|---|---|
| 132-65-0 | Dibenzothiophene | ERC/TTC | ECCC, HC 2017 |
| 28984-69-2 | 4,4(5H)-Oxazoledimethanol, 2-(heptadecenyl)- | ERC/TTC | ECCC, HC 2017 |
| 68909-18-2 | Pyridinium, 1-(phenylmethyl)-, Et Me derivs., chlorides | ERC/TTC | ECCC, HC 2017 |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
The other three substances will be addressed directly in this screening assessment.
The ecological risks of substances in the Heterocycles Group were characterized using the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of factors including potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
While the three substances considered in this screening assessment are collectively referred to as the Heterocycles Group, they lack sufficient similarities that would support a group approach to exposure, hazard and risk characterization; thus, their use and/or hazard profiles were independently assessed for risk to the environment and human health. The assessment of each substance forms its own chapter below.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure. Relevant data were identified up to July 2016. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered. The approach includes the use of previously established points of departure for health effects for the substance morpholine from Health Canada’s Food Directorate. In addition, international assessment work and points of departure were adopted for methenamine from European assessment activities under the European Chemicals Agency (EChA).
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was peer-reviewed and subject to a 60-day public comment period. Additionally, the draft of this screening assessment published November 11, 2017, was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Environment and Climate Change Canada and Health Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA, by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 2 The screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The substance 1,3,5,7-tetraazatricyclo[3.3.1.13,7] decane, commonly known as methenamine, herein referred to as methenamine, is an organic chemical belonging to a substance group known as heterocycles (PubChem 2015). Information regarding the substance identity of methenamine is summarized in Table 2-1.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 100-97-0 | 1,3,5,7-tetraazatricyclo[3.3.1.13,7]decane (methenamine) | 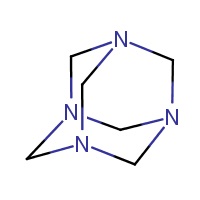 |
140.2 |
The substance tetrahydro-1,4-oxazine, commonly known as morpholine, herein referred to as morpholine, is an organic chemical belonging to a substance group known as heterocycles (PubChem 2015). Information regarding the substance identity of morpholine is summarized in Table 2-2.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 110-91-8 | tetrahydro-1,4-oxazine (morpholine) | 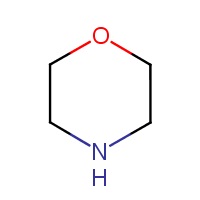 |
87.1 |
The substance 3H-pyrazol-3-one, 2,4-dihydro-4-[(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-5-methyl-2-phenyl- , herein referred to as CAS RN 4174-09-8, is an organic chemical belonging to a substance group known as heterocycles (PubChem 2015). Information regarding the substance identity of CAS RN 4174-09-8 is summarized in Table 2-3.
| CAS RN | DSL name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 4174-09-8 | 3H-pyrazol-3-one, 2,4-dihydro-4-[(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-5-methyl-2-phenyl- | 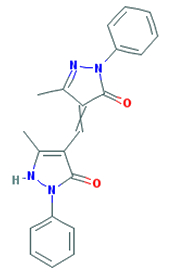 C21H18N4O2 C21H18N4O2 |
358.4 |
Physical and chemical properties of the three heterocycle substances are summarized in Appendix A. Additional physical and chemical properties are presented in ECCC (2016b).
3. Characterization of ecological risk
The ecological risks of substances in the Heterocycles Group were characterized using the ERC approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure with weighted considerations of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization, in contrast to an approach that relies on a single metric in a single medium (e.g., LC50) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties fate (chemical half-lives in various media and biota, partition coefficients, fish bioconcentration), acute fish ecotoxicity, and chemical import and manufacture volumes in Canada were either collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox), and from responses to CEPA section 71 notices or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substances hazard and exposure profiles.
Hazard profiles were established primarily on the basis of metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error in empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error in underestimation of acute toxicity will be mitigated through the use of other hazard metrics, such as structural profiling of mode of action, reactivity and/or estrogen-binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada considering what is believed to be the current use quantity, and may not reflect future trends.
4. Methenamine
4.1 Sources and uses
Methenamine does not occur naturally in the environment. It is produced by the reaction of formaldehyde and ammonia in water at low pressure. According to information submitted pursuant to a CEPA section 71 notice (Canada 2012), between 100 000 and 1,000 000 kg of methenamine were manufactured in Canada in 2011 and between 100 000 and 1 000 000 kg were imported into Canada that same year.Footnote 3 In the United States, the national production volume (production and import) for methenamine was approximately 42.2 million kg (approximately 93 million pounds) for the year 2012 (CDAT 2015).
The main reported use of methenamine is as a cross-linking agent resins and in rubber production. The substance is consumed in this use, which accounts for approximately 95% of the substance in commerce. These resins are used primarily for wood materials and wood adhesives (ECHA 2008; OECD 2007; AGDH 2016a). They are also used in coatings, binders/refractories, auto parts, and manufactured items available to consumers, including toys, sporting goods, seals and tubing adhesives (ECHA 2008; AGDH 2016a). About 3% is used as a chemical intermediate in nitration reactions for explosives production (ECHA 2008; AGDH 2016a). The production of fuel tablets accounts for 2% of methenamine volumes (ECHA 2008; AGDH 2016a). Additional information on uses in Canada is presented in Table 5-1.
| Use | Methenamine |
|---|---|
| Food additivea | No |
| Food packaging materialsa | Yes |
| Internal Drug Product Database as a medicinal or non-medicinal ingredient in disinfectant, human or veterinary drug products in Canadab | No |
| Natural Health Products Ingredients Databasec | Yes |
| Licensed Natural Health Products Database as a medicinal or non-medicinal ingredient in natural health products in Canadac | Yes |
| List of Prohibited and Restricted Cosmetic Ingredientsd | No |
| Notified to be present in cosmetics, on the basis of notifications submitted under the Cosmetic Regulations to Health Canadad | Yes |
| Formulant in pest control products registered in Canadae | Yes |
a Email communication from Food Directorate to Existing Substances Risk Assessment Bureau; unreferenced.
b Email communication from Therapeutic Products Directorate to Existing Substances Risk Assessment Bureau; unreferenced.
c Email communication from Natural and Non-prescription Health Products Directorate to Existing Substances Risk Assessment Bureau; unreferenced.
d Email communication from Consumer Product Safety Directorate to Existing Substances Risk Assessment Bureau; unreferenced.
e Email communication from Pest Management Regulatory Agency to Existing Substances Risk Assessment Bureau; unreferenced.
Notifications submitted under the Cosmetic Regulations to Health Canada indicate that methenamine is used in certain cosmetic products in Canada with an upper concentration of 0.3% (personal communication, November 2015 email from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Methenamine is listed in the Natural Health Products Ingredients Database (NHPID) with a medicinal role as it is classified as a natural health product (NHP) substance falling under item 2 (an isolate) of Schedule 1 to the Natural Health Products Regulations (NHPR), as well as with a non-medicinal role for topical use only as preservative antimicrobial in NHPs, up to 0.16% according to CIR (1992) (NHPID [modified 2018]). Methenamine is listed in the Licensed Natural Health Products Database (LNHPD) as a medicinal or non-medicinal ingredient in a limited number of currently licensed NHPs(LNHPD [modified 2018]). It is not used in any other currently registered drugs (DPD 2015).
Methenamine has been identified as a component in the manufacture of a variety of food packaging materials, including paint, ink and adhesives that do not come in contact with food. It has also been identified for use as a component in preservatives and fungicides used in the manufacture of some food contact materials, including paper and paperboard and resins (personal communication, November 2015 email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
Information from the American Cleaning Institute’s (ACI) website suggested use of methenamine in household cleaning products, including all-purpose liquid cleaners, dish detergents, laundry detergents and laundry pre-treatment products, and in laundry fabric conditioners. Follow up with ACI on this confirmed that as of 2012, ACI found only three products (wrinkle and stain removers) in the United States that contained morpholine. For methenamine, ACI found that methenamine was not an ingredient in cleaning products but it may be found as a by-product of some preservatives that may be used in cleaning products at low levels. In those cases, methenamine is not expected to be present in cleaning products at greater than 0.05%. To determine the Canadian disposition for this product class, information was sought from the Canadian Consumer Specialty Products Association (CCSPA). CCSPA surveyed its members and reported back that methenamine and morpholine have very limited use in household cleaning products in Canada.
4.2 Potential to cause ecological harm
Critical data and considerations used to develop the substance-specific profiles for methenamine and the hazard, exposure and risk classification results are presented in ECCC (2016b).
Methenamine was classified as having a high exposure potential due to its estimated long overall persistence and large use quantities reported under section 71 of CEPA. This substance has been classified as presenting a low ecological hazard and a low potential for ecological risk. It is unlikely that it results in concerns for the environment in Canada.
4.3 Potential to cause harm to human health
4.3.1 Exposure assessment
In considering environmental media, according to the Mackay model (level 1), water is the target compartment for methenamine (100%) in the environment (ECHA 2008). Under atmospheric conditions, methenamine has a half-life of 45 minutes because of reaction with the OH radical (ECHA 2008). It is highly water soluble and, upon reaching the aqueous environment, is degraded hydrolytically to ammonium and formaldehyde (ECHA 2008). Thus, exposure to methenamine from environmental media is expected to be low.
Dietary exposure to methenamine from its use as a component in the manufacture of food packaging results in an estimated probable daily intake of 0.00188 mg/kg-bw (personal communication, November 2015 email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
General population exposure to methenamine may occur from the daily use of cosmetics, in which it is used as a preservative, with reported concentrations in six products of less than 0.3%. Product testing for methenamine in 24 household cleaning products, including laundry soaps/softeners, all-purpose cleaners, stain removers, soaps and shampoos, found low amounts in a shampoo and a laundry softener, with percentages of less than 0.08% (CPSD 2016).
The highest repeated dermal exposure to methenamine is expected to occur from the daily use of cosmetics. This exposure was estimated on the basis of the use of body cream with a reported upper-limit of 0.3% methenamine. The mean use frequency considered was 1.1 times per day with 4.4 grams per application. Dermal exposure was determined to be 0.205 mg/kg-bw/day (1.1 events/day × 4.4 g body cream/event × 0.3% methenamine in body cream) (Lorentz 2005; Health Canada 1998).
4.3.2 Health effects assessment
Methenamine was reviewed internationally (ECHA 2008; OECD 2007), and these reviews were used to inform the health effects characterization in this screening assessment. On the basis of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity and reproductive toxicity, methenamine was not identified as posing a high hazard to human health nor is it identified on the ECHA’s Candidate List of Substances of Very High Concern for Authorisation (ECHA [modified 2015]).
Critical effects for methenamine are potential for dermal sensitization at high doses (ECHA 2008; OECD 2007). In a local lymph node assay, methenamine had a positive effective concentration (EC3) of 30.6%, whereas the same study showed an EC3 of 0.96% for formaldehyde. It was suggested that formaldehyde is responsible for the potential sensitizing properties of methenamine (ECHA 2008). Developmental effects have been observed in beagle dogs (LOAEL = 31 mg/kg-bw/day in food; NOAEL = 15 mg/kg-bw/ day), including lower pup birth weights and increased mortality by the first month (Hurni and Ohder 1973; ECHA 2008). However, this study is of limited utility as one dog had to be sacrificed after a fight, and pups were treated differently (some were fed cow’s milk while others were not). Also, much higher doses (1000 mg/kg-bw/day) are required to produce similar developmental effects in rats, and doses of 1500 to 2500 mg/kg-bw/day are considered to have no effect on rat fertility (OECD 2007). Women administered methenamine as a therapeutic treatment for urinary infections at approximately 13 and 27 mg/kg-bw/day during pregnancy showed no apparent adverse effects on fetal development or birth outcomes (ECHA 2008). Noting the deficiencies in the beagle dog study, ECHA 2008 recommended the use of 27 mg/kg-bw/day as a human-derived NOAEL for risk assessment of developmental toxicity.
Methenamine is also used as an oral therapeutic for its urinary antibacterial effect. Maintenance doses up to 57 mg/kg-bw/day for weeks or months did not give rise to adverse effects in humans other than a low rate of gastrointestinal disturbances. Higher oral doses of methenamine of approximately 114 mg/kg-bw have been administered to humans for therapeutic purposes for 3 to 4 weeks; at this level of exposure, bladder irritation, painful and frequent micturition, albuminuria and haematuria have been noted. ECHA (2008) recommended the use of 57 mg/kg-bw/day as a human-derived NOAEL for use in repeated dose risk characterization. The systemic availability of methenamine after oral administration was assumed to be 100% by ECHA (2008).
4.3.3 Characterization of risk to human health
The risk from environmental media is expected to be low because of short atmospheric half-lives and hydrolytic degradation to ammonium and formaldehyde in aqueous media, which limit environmental concentrations.
The general population may be dermally exposed to methenamine from a variety of products including cosmetics and some cleaning products. Dermal exposure to methenamine from cosmetic products was estimated using body cream with an external applied dose determined to be 0.205 mg/kg-bw/day. This dermal exposure scenario is expected to cover any other product available to consumers, as well as to the topical NHP listed in the LNHPD as containing methenamine as a non-medicinal ingredient. Oral and topical NHPs listed in the LNHPD as containing methenamine as a medicinal ingredient were subject to safety and efficacy assessment on the basis of their recommended conditions of use prior to licensing in accordance with the NHPR. On the basis of the 50% dermal absorption rate reported in ECHA (2008), the systemic exposure from use of cosmetics was determined to be 0.102 mg/kg-bw/day. Comparison of this exposure to the human-derived developmental oral NOAEL of 27 mg/kg-bw/day results in a MOE of 265. This MOE is considered adequate to address uncertainties in health effects and exposure databases. It is noted that some individuals may be sensitive to methenamine (and/or its breakdown products formaldehyde and ammonia), and sensitization reactions may occur from certain topical products. A safety assessment conducted on cosmetic uses found methenamine to be safe at concentrations up to 0.16%, as less than 0.2% formaldehyde would be released (CIR 1992). However, the maximum concentration noted in cosmetics of 0.3% is approximately 100-fold lower than the EC3 of 30.6%.
Potential daily dietary intake of methenamine from its use in the manufacture of food packaging materials was determined to be 0.00188 mg/kg-bw/day. Comparison with the human-derived developmental NOAEL of 27 mg/kg-bw/day results in a MOE of 14400. Oral exposures from food packaging are therefore not considered to be a risk to human health.
Exposure of the general population to methenamine is therefore considered to be of low risk to human health.
5. Morpholine
5.1 Sources and uses
Morpholine does not occur naturally in the environment. It is typically produced by the reaction of diethylene glycol with ammonia in the presence of hydrogen and catalysts (IPS 1995). According to information submitted pursuant to a CEPA section 71 notice (Canada 2012), between 1 000 and 10 000 kg of morpholine were manufactured in Canada in 2011 and between 100 000 and 1 000 000 kg were imported into Canadathat same year.Footnote 4 In the United States, the national production volume (production and import) for methenamine was between 4.5 and 45 million kg (10 and 100 93 million pounds) for the year 2012 (CDAT 2015).
A large amount of morpholine is used as an intermediate in the production of rubber accelerators, pharmaceuticals, pesticides, optical brighteners, and antioxidants and as an industrial solvent (Huntsman 2015; BASF 2009). Morpholine is also used in closed-water or steam systems to prevent corrosion and as a petroleum production chemical (IARC 1999). A variety of other industrial uses include adhesive and binding agents, tanning agents, surface treatments, emulsifiers, solvents for resins and waxes, reducing agents, process regulators, lubricants, hydraulic fluids, cutting fluids, colouring and anti-condensation agents (AGDH 2016b). Additional information on uses in Canada is presented in Table 6-1.
| Use | Morpholine |
|---|---|
| Food (other)a | Yes |
| Food packaging materialsa | Yes |
| Internal Drug Product Database as a medicinal or non-medicinal ingredient in disinfectant, human or veterinary drug products in Canadab | Yes |
| Natural Health Products Ingredients Databasec | Yes |
| Licensed Natural Health Products Database as a medicinal or non-medicinal ingredient in natural health products in Canadac | No |
| List of Prohibited and Restricted Cosmetic Ingredientsd | No |
| Notified to be present in cosmetics, on the basis of notifications submitted under the Cosmetic Regulations to Health Canadad | No |
| Formulant in pest control products registered in Canadae | Yes |
a Email communication from Food Directorate to Existing Substances Risk Assessment Bureau; unreferenced.
b Email communication from Therapeutic Products Directorate to Existing Substances Risk Assessment Bureau; unreferenced.
c Email communication from Natural and Non-prescription Health Products Directorate to Existing Substances Risk Assessment Bureau; unreferenced.
d Email communication from Consumer Product Safety Directorate to Existing Substances Risk Assessment Bureau; unreferenced.
e Email communication from Pest Management Regulatory Agency to Existing Substances Risk Assessment Bureau; unreferenced.
Morpholine is listed as a formulant by PMRA in pest control products (personal communication, November 2015 email from the Risk Management Bureau, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Morpholine has been identified for use as an emulsifier in the preparation of wax coatings for fresh produce, such as apples (Health Canada 2002). Morpholine can be used as a component in the manufacture of certain food packaging materials, such as interior coatings. Incidental additives, such as boiler water additives, can also contain morpholine (personal communication, November 2015 email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Morpholine is a non-medicinal ingredient in disinfectant drugs which are typically, but not exclusively, marketed to commercial facilities, such as hospitals or food premises (DPD 2015). It is listed without any role in the NHPID, and it is not listed in the LNHPD as being present in currently licensed NHPs (NHPID [modified 2018]; LNHPD [modified 2018]).
Morpholine is used in some products available to consumers, such as floor waxes and polishes. Additionally there are several auto care products to which the general population could be exposed, such as car waxes, wax-based surface cleaners and tire products (CPID 2016).
5.2 Potential to cause ecological harm
Critical data and considerations used to develop the substance-specific profiles for morpholine and the hazard, exposure and risk classification results are presented in ECCC (2016b).
On the basis of the low hazard and low exposure classifications according to information considered under ERC, morpholine was classified as having a low potential for ecological risk. It is therefore unlikely that this substance results in concerns for the environment in Canada.
5.3 Potential to cause harm to human health
5.3.1 Exposure assessment
Environmental media exposures to morpholine could occur through fugitive air emissions and on-site land disposal (OECD 2013). Level III fugacity modelling, using loading rates of 1 000 kg/h each for air, soil and water, shows the following percent distribution when morpholine is released simultaneously to all three compartments: 0.4% to air; 41.7% to water; 57.9% to soil; and 0.09% to sediment (OECD 2013). After evaporation or exposure to the atmosphere, indirect photo-oxidation of morpholine is expected to occur by reaction with OH-radicals with a half-life of 0.9 hours (OECD 2013). Morpholine is considered resistant to hydrolysis because it does not contain labile functional groups. Therefore, hydrolysis is not expected under environmental conditions (OECD 2013). Multi-site environmental monitoring in lake water near an Ontario nuclear plant that uses morpholine in its cooling water system was considered (OPG 2014). Fifteen water samples were collected at various distances and depths in the vicinity of Darlington Nuclear Station, and all were below the detection limit of 1.0 ug/L and met the Ontario Ministry of the Environment interim provincial water quality objective of 4 ug/L (OPG 2014; MOEE 1994). Morpholine is considered readily biodegradable (92.6% in 22 days) after a lag phase of 15 days and will assist in limiting soil and water concentration (OECD 2013).
Exposure to morpholine by the general population is expected to be limited to a small number of products available to consumers, primarily home and auto polishes/waxes and related auto care products. Other products with incidental direct exposure include radiator leak stop, corrosion inhibitors as well as disinfectant drugs in which morpholine is used as a non-medicinal ingredient.
Several auto care products that could reasonably be expected to be used by the general public contain morpholine, including car waxes (<5%), surface cleaners (<3%), and tire care products (<1%). The primary route of exposure is expected to be dermal as it is assumed that these products are used outdoors and/or in a garage. Inhalation is expected to contribute a very small fraction of the overall exposure because of the high rate of air flow, the viscous matrices of these products, and the moderate vapour pressure of morpholine. To assess the potential for harm to human health from dermal exposure to these products during use, a thin-film approach as outlined in the EPA-Versar document (US EPA 2011) was used. It was assumed that exposure from handling a cloth coated in the product can be described as a thin film. This approach characterizes the dermal deposition from a mineral oil substance following handling of a rag saturated with the oil material, i.e., the mineral oil thickness (“thin film”) estimated to remain on the skin after wiping is 1.64 × 10-3 cm. This thickness was therefore assumed to apply to morpholine for characterizing dermal exposure for the application of the auto care products. Assuming equal density of morpholine and the whole product of 1010 mg/cm3 and an exposed skin surface area of 455 cm2 (half of both hands/palms), the dermal load was estimated to be 75.4 mg per 60-minute exposure event using car wax, the product with the highest reported morpholine concentration of 5%. Using the selected body weight of 70.9 kg (considered to be representative of an average Canadian adult) (Health Canada 1998), dermal exposure was estimated to be 0.53 mg/kg-bw/event.
Exposure to morpholine can occur from use of floor waxes formulated with morpholine at 1 to 2%, from over-the-counter disinfectant sprays with morpholine concentrations ranging from 0.15% to 0.64%, or from glass cleaners formulated with less than 0.5%. Dermal exposures from these products are expected to be covered by the previously described auto care scenario. Inhalation exposure to morpholine from floor wax and aerosol disinfectant sprays was estimated using algorithms and recommended defaults developed by RIVM (2006) and presented in the Cleaning Products Fact Sheet. The highest exposure scenario identified was for the use of floor wax. Using the default parameters for a water-based floor polish with evaporation occurring from an increasing area, the largest mean event concentration was determined to be 0.078 mg/m3 for 90 minutes of exposure.
Health Canada assessed the safety of morpholine for its use in wax coating compounds for apples (Health Canada 2002). In that assessment, exposure of children and adults to morpholine was estimated to be approximately 8% and 5%, respectively, of Health Canada’s acceptable daily intake (ADI) of 0.48 mg/kg bw/day. Compared to these uses, food packaging applications are not considered to be a significant source of dietary exposure to morpholine (personal communication, November 2015 and October 2016 emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
5.3.2 Health effects assessment
The Organization for Economic Cooperation and Development (OECD 2013), IARC (1999) and Health Canada’s Food Directorate (Health Canada 2002; and supplemental report available upon request) summarized the health effects literature and characterized hazard for morpholine. These reports were used to inform the health effects characterization in this screening assessment, and the critical endpoints and corresponding effect levels for morpholine, are summarized below. A literature search was conducted from the year prior to the OECD Cooperative Chemicals Assessment Programme (CoCAM 5) assessment (OECD 2013) to July 2016. No health effects studies, which could impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated in OECD 2013), were identified.
A short-term, repeated inhalation exposure study was conducted in rats via whole body exposure. Animals were exposed to 80 mg/m3 morpholine for 4 hours a day for 2, 4 or 8 days. This exposure level, based upon 4 days of exposure, was established as the critical effect level on the basis of thyroid gland hypersecretion, as indicated by histological analysis and by increased 131I (radioisotope of iodine) uptake (OECD 2013).
A chronic oral toxicity study was conducted in mice via drinking water. Animals had access to water containing 0, 2 500 or 10 000 mg/L of morpholine oleic acid salt for 96 weeks, and an absence of adverse effects were noted (Shibata et al. 1987). In setting an ADI for morpholine, Health Canada (2002) identified this as a key study, and a NOAEL of 96 mg/kg-bw/day was determined assuming complete dissociation of the morpholine salt in animals at the 2 500 mg/L exposure level. Using this NOAEL and several safety factors, an acceptable daily intake (ADI) of 0.48 mg/kg-bw per day was established. Absorption is assumed to be 100%, as indicated by animal studies showing that high levels (80-93%) of the orally administered radioactivity from 14C-morpholine-HCl were excreted in the urine with minimal excretion in the feces (<2%) (Health Canada 2002).
In reproductive studies, morpholine did not cause developmental effects at the highest dose tested (225 mg/kg-bw/day). An unpublished prenatal developmental study was conducted in rat dams via the oral route of exposure. Animals were exposed to morpholine for 14 days (GD 6-19) at 75, 250 or 1000 mg/kg-bw/day. A maternal LOAEL of 250 mg/kg-bw/day was identified on the basis of mild regenerative anemia and increased liver weights. At 75 mg/kg-bw/day, there were fetal skeletal variations in the absence of maternal toxicity, including statistically significant increases in the mean number of fetuses with incomplete ossification of parietal bone and skull. The percentage of fetuses with the effect positively correlated with dose, and at the next dose level (250 mg/kg-bw/day), the prevalence of incomplete ossification was higher than the maximum observed in historical control data. This increase in skeletal variations (above the maximum observed in historical controls) therefore occurred in the presence of maternal toxicity. As an outcome of the review, delayed ossification was not considered to be an adverse effect in this study (OECD 2013).
IARC considers morpholine a Group 3 carcinogen (‘not classifiable as to its carcinogenicity to humans’) as there was inadequate evidence to support carcinogenicity in animals, and no human data was available for review (IARC 1999). Additionally, Health Canada’s HPFB (Health Canada 2002; supplemental data) described the low likelihood of formation of relevant quantities of N-nitrosomorpholine (possible human carcinogen) from the ingestion of low levels of morpholine, including considerations of pH and physiological and metabolic differences between rats and humans.
Morpholine was weakly positive in in vitro genotoxicity assays (in yeast and mammalian cells) but not in bacterial assays (IARC 1999; OECD 2013). Considering the weight of evidence from in vitro and in vivo studies, it was concluded that morpholine is not mutagenic (OECD 2013).
5.3.3 Characterization of risk to human health
Exposure to morpholine from environmental media could occur through fugitive air emissions, releases to water and on-site land disposal. Indirect photo-oxidation of morpholine is expected to occur by reaction with OH-radicals with a half-life of 0.9 hours (OECD 2013). Limited water samples in Ontario near a large industrial were all below the detection limit of 1.0 ug/L and met the Ontario Ministry of the Environment interim provincial water quality objective of 4 ug/L (OPG 2014; MOEE 1994). Additionally, morpholine is considered to be readily biodegradable and this will assist in limiting soil and water concentration (OECD 2013). Given these considerations and that there were not measurements above the limit of detection for morpholine in raw lake water the risk from environmental media is considered to be low.
In order to characterize risk to human health from products available to consumers, inhalation and dermal exposure estimates were developed for the use of various products that contain morpholine. The highest per event inhalation exposure of the general population to morpholine from product use was estimated to be 0.078 mg/m3 from floor wax application. In rats, short-term inhalation of 80 mg/m3 morpholine resulted in thyroid hypersecretion. Comparing this critical effect level with the exposure estimates for the general population results in an MOE of 1025. This margin is considered adequate to address uncertainties in health effects and exposure databases.
The highest per event estimate of dermal exposure of the general population to morpholine from product use was estimated to be 0.53 mg/kg-bw/event from car wax application. A 50% dermal absorption factor was applied considering the short duration (less than 1 hour) as was previously described in the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances science approach document (Health Canada 2016), resulting in an estimated systemic exposure of 0.26 mg/kg-bw/event. Comparison of this exposure to an oral NOAEL of 96 mg/kg-bw per day results in an MOE of 370. This is considered adequate to address uncertainties in health effects and exposure databases. Therefore, risk is considered to be low.
Health Canada’s previous health hazard assessment of morpholine use in wax coating compounds on apples concluded that dietary exposure from those uses did not pose a risk to human health.
Risk to human health from exposure to morpholine is therefore considered to be low.
6. CAS RN 4174-09-8
6.1 Sources and uses
CAS RN 4174-09-8 does not occur naturally in the environment. According to information submitted pursuant to a CEPA section 71 notice (Canada 2012), between 1 000 and 10 000 kg of this substance were imported into Canada in 2011, with none manufactured in Canada.Footnote 5 No information was available on quantities used internationally.
There is limited information on the uses of CAS RN 4174-09-8. It has approved uses in Europe, and information from European Union legislation indicates it is used to colour plastic materials and articles, varnishes and coatings. This substance is also reported to be closely related to CI Class Solvent Yellow 93 (EC 2004). Similar uses as a colouring agent in some food packaging materials, such as polystyrene, polycarbonate and polyethylene terephthalate (PET) plastics, were identified by Health Canada’s Food Directorate. Examples include polystyrene lids and polycarbonate bottles (personal communication, November 2015 email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). No other uses were identified.
This substance is not listed in the NHPID, nor in currently licensed NHPs (NHPID [modified 2018]; LNHPD [modified 2018]).
6.2 Potential to cause ecological harm
Critical data and considerations used to develop the substance-specific profiles for CAS RN 4174-09-8 and the hazard, exposure and risk classification results are presented in ECCC (2016b).
According to information considered under ERC, CAS RN 4174-09-8 was classified as having a high hazard potential on the basis of agreement between a reactive mode of action and elevated toxicity ratio, both of which suggest that this chemical is likely of high potency, and moderate potential to cause adverse effects in aquatic food webs given its bioaccumulation potential. CAS RN 4174-09-8 was initially classified as having moderate potential for ecological risk; however, the risk classification was decreased to low potential for ecological risk following the adjustment of risk classification on the basis of current use quantities (see section 7.1.1. of the ERC approach document ECCC 2016a). The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, this substance is unlikely to result in concerns for the environment in Canada.
6.3 Potential to cause harm to human health
6.3.1 Exposure assessment
Exposure from environmental media is expected to be very low for air and water given the vapour pressure (2.4 × 10-14 at 25 °C) and limited water solubility (0.1814 g/L). Level III fugacity modelling suggests the substance will be found in sediment and soil (EPI Suite 2012). No data was found on concentrations of this chemical substance in the environment.
The probable daily intake of CAS RN 4174-09-8 resulting from its use in food packaging applications is estimated to be 0.0077 µg/kg-bw. However, migration from food packaging material (e.g. hard plastic matrix) is expected to be limited. Therefore, dietary exposure from uses of CAS RN 4174-09- in food packaging materials is expected to be negligible. No other exposures are expected given its limited use profile (email dated October 2016 from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
6.3.2 Health effects assessment
No health effects data were identified for CAS RN 4174-09-8. This substance is related to CI Class Solvent Yellow 93 (CAS RN 4702-90-3) (EC 2004)—which also has no health effects data registered with ECHA (2016)—in both chemical structure and use pattern.
6.3.3 Characterization of risk to human health
The general population may be exposed to CAS RN 4174-09-8 through food packaging material, but dietary exposure, if any, is expected to be negligible (email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Therefore, risk to human health is considered to be negligible.
7. Uncertainties in evaluation of risk to human health
Overall confidence in the exposure and hazard databases for the three substances is moderate.
There is uncertainty for the durations and frequencies of exposure for all substances. However, given the conservative nature of the exposure scenarios, the risk characterization is not expected to underestimate risk.
There is uncertainty associated with systemic exposures to methenamine and morpholine from the dermal route due to the lack of dermal absorption studies.
There is uncertainty associated with CAS RN 4174-09-8 due to the lack of health effects data for this substance.
8. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from methenamine, morpholine and CAS RN 4174-09-8. It is concluded that methenamine, morpholine and CAS RN 4174-09-8 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that methenamine, morpholine and CAS RN 4174-09-8 do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that methenamine, morpholine and CAS RN 4174-09-8 do not meet the criteria set out in section 64 of CEPA.
Reference
[ACI] American Cleaning Institute. 2015. Exposure Assessment for Methenamine (CAS RN: 100-97-0). Washington (DC): American Cleaning Institute.
[AGDH] Australian Government Department of Health. 2016a. Inventory Multi-tiered Assessment and Prioritisation, Human Health Tier II Assessment for 1,3,5,7-Tetraazatricyclo[3.3.1.13,7]decane. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme (NICNAS).
[AGDH] Australian Government Department of Health. 2016b. Inventory Multi-tiered Assessment and Prioritisation, Human Health Tier II Assessment for Morpholine. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme (NICNAS).
[BASF] BASF. 2009. Morpholine Technical Data Sheet [PDF]. BASF Chemical Company, Florham Park (NJ). [accessed 2018 April 30].
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette. Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2956.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
[CDAT] Chemical Data Access Tool. 2015. Non-confidential 2012 Chemical Data Reporting Information: search results for CAS RN 108-24-7. Washington (DC): US Environmental Protection Agency. [updated 2014 Jun; accessed 2015 Sep 3].
[CIR] Cosmetic Ingredient Review. 1992. Final Report on the Safety Assessment of Methenamine. J Am Coll Toxicol. 11(4):531-558.
[CPID] Consumer Product Information Database [database]. 2016. Products containing Morpholine. Providence Terrace (VA): DeLima Associates.
[CPSD] 2016. Consumer Product Safety Directorate. Methenamine Content in Consumer Products: Testing Samples with Various Matrix Types. Product Safety Laboratory. Ottawa (ON): Government of Canada.
[DPD] Drug Product Database [database]. [modified 2015 July 17]. Ottawa (ON): Government of Canada. [accessed 2015 Nov].
[EC] European Commission. 2004. Order on the colouring of plastic materials and articles, varnishes and coatings intended to come into contact with foodstuffs, food products and drinks for human and animal consumption. Brussels (BE): European Commission.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2015 Sep 25].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018.Screening assessment: substances identified as being of low concern using the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. 2008. Risk Assessment: Methenamine. Final Approved Version, 27.05.2008. Dortmund (DE). pp 1-134.
[ECHA] European Chemicals Agency. [modified 2015 Jun 15]. Candidate List of Substances of Very High Concern for Authorisation [Internet]. Helsinki (FI): European Chemicals Agency. [accessed 2016 Jun 1]..
[ECHA] European Chemicals Agency. 2016. Registered substances database. Helsinki (FI): European Chemicals Agency.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. 2012. Version 4.1. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Health Canada. 1998. Exposure Factors for Assessing Total Daily Intake of Priority Substances by the General Population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. 2002. A summary of the health hazard assessment of morpholine in wax coatings of apples. Ottawa (ON): Government of Canada.
Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
[Huntsman] Huntsman International. 2015. Morpholine Product Information Brochure [PDF]. Salt Lake City (UT) Huntsman Corporation.
Hurni H and Ohder H. 1973. Reproduction study with formaldehyde and hexamethylenetetramine in beagle dogs. Food Cosmet Toxicol. 11:459-462.
[IARC] International Agency for Research on Cancer. 1999. Morpholine. IARC Monogr Eval Carcinog Risks Hum. 71:1511-1514.
[IPCS] International Programme on Chemical Safety. 1995. Morpholine Health and Safety Guide. Geneva (CH): United Nations Environment Programme; International Labour Organization; World Health Organization.
[Look Chem] Look Chem. 2016. Product Page for 4174-09-8. Hangzhou (CN).
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 06]. Ottawa (ON): Government of Canada. [accessed 2017 Apr].
[MOEE] Ontario Ministry of Environment and Energy. 1994. Water Management: Policies, Guidelines, Provincial Water Quality Objectives. Toronto (ON): Ontario Ministry of Environment and Energy.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2018 May 07]. Ottawa (ON): Government of Canada. [accessed 2017 Apr].
[OECD] Organisation for Economic Cooperation and Development. 2007. SIDS Initial Assessment Profile Methenamine. SIAM 24. Paris (FR): Organisation for Economic Cooperation and Development.
[OECD] Organisation for Economic Cooperation and Development. 2013. SIDS Initial Assessment Report for Morpholine. CoCAM 5. Paris (FR): Organisation for Economic Cooperation and Development. pp 1-47.
[OPG] Ontario Power Generation. 2014. 2014 Results of Environmental Monitoring Programs. Report Number: N-REP-03443-10014. Toronto (ON): Ontario Power Generation.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006. Cleaning products fact sheet: to assess the risks for the consumer [PDF]. Bilthoven (NL): RIVM. Report No.: 32104003/2006.
Shibata M A, Kurata Y, Ogiso T, Tamano S, Fukushima S, Ito N. 1987. Combined chronic toxicity and carcinogenicity studies of morpholine oleic acid salt in B6C3F1 mice. Food Chem. Toxicol. 25: 569-574.
[US EPA] US Environmental Protection Agency. 2011. Exposure factors handbook. Washington (DC): US EPA, National Center for Environmental Assessment, Office of Research and Development.
Appendices
Appendix A. Physical and chemical properties
| Property | Value | Type of data | Reference |
|---|---|---|---|
| Melting point (oC) | 65 | experimental | EPI Suite 2012 |
| Boiling point (oC) | 209 | experimental | EPI Suite 2012 |
| Water solubility (g/L) | 449 at 12 °C | experimental | EPI Suite 2012 |
| Density (g/mL) | 1.27 | experimental | EPI Suite 2012 |
| Vapour pressure (Pa) | 12.1 @ 25 °C | experimental | EPI Suite 2012 |
| Henry’s law constant (Pa m3/mol) | 1.65 × 104 | modelled (bond method) | EPI Suite 2012 |
| Henry’s law constant (Pa m3/mol) | 1.66 × 10-4 | experimental | EPI Suite 2012 |
| log Kow (dimensionless) | -4.15 | modelled | EPI Suite 2012 |
| log Koc (dimensionless) | 1.000 | modelled (MCI method) | EPI Suite 2012 |
| log Koc (dimensionless) | -1.632 | modelled (Kow method) | EPI Suite 2012 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient
| Property | Value | Type of data | Reference |
|---|---|---|---|
| Melting point (°C) | -4.9 | experimental | EPI Suite 2012 |
| Boiling point (°C) | 128 | experimental | EPI Suite 2012 |
| Water solubility (g/L) | 1000 | experimental | EPI Suite 2012 |
| Density (g/mL) | 1.007 @ 20 °C | experimental | EPI Suite 2012 |
| Vapour pressure (Pa) | 1060 @ 20 °C | experimental | EPI Suite 2012 |
| Henry’s law constant (Pa m3/mol) | 0.118 | experimental | EPI Suite 2012 |
| log Kow (dimensionless) | 0.86 | modelled | EPI Suite 2012 |
| log Koc (dimensionless) | 7.36 | modelled (MCI method) | EPI Suite 2012 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient
| Property | Value | Type of data | Reference |
|---|---|---|---|
| Melting point (oC) | 282.3 | modelled | EPI Suite 2012 |
| Boiling point (oC) | 562.6 | modelled | EPI Suite 2012 |
| Water solubility (mg/L) | 0.1814 | modelled | EPI Suite 2012 |
| Density (g/cm3) | 1.27 | unknown | LookChem 2016 |
| Vapour pressure (Pa) | 2.4 × 10-14@ 25 °C | modelled | EPI Suite 2012 |
| Henry’s law constant (Pa m3/mol) | 7.19 × 10-19 | experimental | EPI Suite 2012 |
| log Kow (dimensionless) | 5.25 | modelled | EPI Suite 2012 |
| log Koc (dimensionless) | 5.16 | modelled (MCI) | EPI Suite 2012 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient
