Screening assessment nitrilotriacetic acid trisodium salt
Official title: Screening Assessment - Glycine, N,N-bis(carboxymethyl)-, trisodium salt (Na3NTA)
Chemical Abstracts Service Registry Number: 5064-31-3
Environment Canada
Health Canada
November 2022
Cat. No.: En84-318/2022E-PDF
ISBN 978-0-660-45382-8
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of glycine, N,N-bis(carboxymethyl)-, trisodium salt, hereinafter referred to as Na3NTA, derived from its more commonly used name nitrilotriacetic acid trisodium salt. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1) for Na3NTA is 5064-31-3.
According to the information submitted in response to a CEPA section 71 survey, 932 414 kg of Na3NTA were imported into Canada in 2011 and there were no reports of manufacture of Na3NTA in Canada above the reporting threshold of 100 kg. Reported uses in Canada include water treatment, cleaning and furnishing care, as a component in the manufacture of food packaging materials, paper products, fabric, textile and leather articles, personal care, photographic supplies, film and photochemicals, agricultural products, and metal chelation. Na3NTA is used in products available to consumers, which mainly include cleaning products and cosmetics. Na3NTA was also identified as an ingredient in disinfectant products. Additionally, Na3NTA may be a used as a component in cleaners, sanitizers, and hand treatments used in food processing establishments, and has been identified as a formulant in pest control products registered in Canada.
The ecological risk of Na3NTA was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, Na3NTA is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from Na3NTA. It is concluded that Na3NTA does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends.
The predominant sources of exposure to Na3NTA from products available to consumers in Canada are cleaning products (for example, wood floor cleaning liquid, wood floor polishing spray, boat cleaner) and cosmetics (for example, hair dye, face moisturizer). There is also potential for exposure to Na3NTA for the general population from drinking water.
Based on the occurrence of urinary tract tumours in studies on laboratory animals, carcinogenicity is the critical effect for the characterization of risk to human health from exposure to Na3NTA. Na3NTA is not considered to be genotoxic. Although the mode of induction of tumours has not been fully elucidated, the tumours observed in experimental animals are unlikely to have resulted from direct interaction with genetic material and therefore a threshold approach is used to assess the risk to human health. A comparison of the estimates of exposure and the critical effect levels resulted in margins of exposure that are considered adequate to address uncertainties in the health effects and exposure datasets.
Considering all the information presented in this screening assessment, it is concluded that Na3NTA does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that Na3NTA does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of glycine, N,N-bis(carboxymethyl)-, trisodium salt, hereinafter referred to as Na3NTA, derived from its more commonly used name nitrilotriacetic acid trisodium salt. The Chemical Abstracts Service Registry Number (CAS RN) for Na3NTA is 5064-31-3. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA.
The ecological risk of Na3NTA was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
Na3NTA was previously assessed internationally through the European Union and there is a European Union Risk Assessment Report (EU RAR) available (EC 2008). In addition, there is an existing Canadian Drinking Water Quality Guideline for nitrilotriacetic acid (NTA) , which considers health effects information for Na3NTA (Health Canada 1990).
The health effects data on this substance were previously included as supporting information in the screening assessment for NTA (glycine, N,N-bis(carboxymethyl)-) (CAS RN 139-13-9) conducted under the Challenge initiative of the Chemicals Management Plan (CMP) (Environment Canada, Health Canada 2010). With respect to the exposure assessment, it was noted in the screening assessment for NTA that NTA and Na3NTA cannot be distinguished analytically in environmental media and, accordingly, exposure estimates for environmental media encompassed NTA and its salts. This approach, along with updated use and monitoring data, is used in the current assessment.
The EU RAR and screening assessment for NTA will be used to inform the human health effects section in this assessment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to June 2022. Empirical data from key studies as well as results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this screening assessment (published December 19, 2020) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This screening assessment presents the critical information and considerations on which the conclusion is based.
2. Identity of substances
The CAS RN, Domestic Substances List (DSL) name, common names, chemical structure, molecular formula and molecular weight for the individual substance are presented in Table 2‑1.
| CAS RN | DSL name(common names) | Chemical structure and molecular formulaa | Molecular weight (g/mol)a |
|---|---|---|---|
| 5064-31-3 | Glycine, N,N-bis(carboxymethyl)-, trisodium salt(Nitrilotriacetic acid trisodium salt; Trisodium NTA; Na3NTA) | 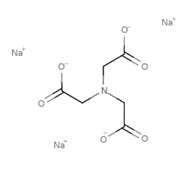 C6H9NO6•Na3 C6H9NO6•Na3 |
257.08 |
a ChemID Plus 1993
NTA and its sodium salts, including Na2NTA, Na3NTA•H2O, and Na3NTA, can dissociate to nitrilotriacetate ions in water, which is the common moiety (Figure 2-1).

Long description
Dissociation of nitrilotriacetic acid trisodium salt, nitrilotriacetic acid, and trisodium nitrilotriacetate monohydrate into the common moiety nitrilotriacetate.
3. Physical and chemical properties
A summary of physical and chemical properties of Na3NTA are presented in Table 3-1, with the range in values indicated for each property. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Value or range | Type of data | Key reference(s) |
|---|---|---|---|
| Physical state | Solid | Experimental | ECHA c2007-2019 |
| Vapour pressure (Pa) | 1.0 × 10−7 | Estimated | ECHA c2007-2019 |
| Water solubility (mg/L) | 6.4 × 105 to 4.6 × 107 | Experimental | EC 2008; ECHA c2007-2019 |
| Log Kow (dimensionless) | -13.2 to -2.62 | Estimated | ECHA c2007-2019; EC 2008 |
| pKa (dimensionless) | 1.2 | Estimated | ECHA c2007-2019 |
Abbreviations: Kow, octanol-water partition coefficient; pKa, acid dissociation constant
4. Sources and uses
Following the publication of the screening assessment for NTA, Na3NTA was included in a survey issued pursuant to section 71 of CEPA (Canada 2012). For the calendar year of 2011, Na3NTA was reported to be imported into Canada at a volumeFootnote 3 of 932 414 kg. There were no reports of manufacture of Na3NTA in Canada above the reporting threshold of 100 kg (Environment Canada 2013).
Uses of Na3NTA in Canada were reported to include water treatment, cleaning and furnishing care, food packaging, paper products, fabric, textile and leather articles, personal care, photographic supplies, film and photochemicals, agricultural products, and in metal chelation (Environment Canada 2013).
Na3NTA is also used in automotive manufacturing in non-production cleaners, as well as in the production process (pre-treatment process) (personal communication, email from the Canadian Vehicle Manufacturers’ Association [CVMA] to Products Division, ECCC, dated January 27th, 2020).
Na3NTA can be used in Canada in various cleaning products available to consumers, such as a boat cleaner, wood spray polish, wood floor cleaner, and automotive cleaner (SDS 2014; SDS 2015a; SDS 2015b; SDS 2016). In addition, according to notifications submitted under the Cosmetic Regulations to Health Canada, Na3NTA is found in various cosmetic products, such as facial moisturizers, hair conditioners, hair shampoos, hair styling products, face exfoliants, body washes, and permanent hair dyes (personal communication, email from the Consumer and Hazardous Products Safety Directorate [CHPSD], Health Canada [HC], to the Existing Substances Risk Assessment Bureau [ESRAB], HC, dated Feb. 4, 2019; unreferenced). Na3NTA is listed with a non-medicinal role for topical use as a chelating agent in the Natural Health Products Ingredients Database (NHPID [modified 2022]); however, it is not listed in the Licensed Natural Health Products Database as being present in natural health products in Canada (LNHPD [modified 2021]).
Na3NTA may be used as a component of cleaners and sanitizers, and hand treatments used in Canadian food processing facilities. Na3NTA may also be used as a boiler water additive, not to exceed 5 parts per million in boiler feedwater, and not to be used where steam will be in contact with milk and milk products (personal communication, email communication from Food Directorate [FD], HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced). In addition, Na3NTA may be used in food packaging materials as a component in adhesives, also in products used in the manufacture of paper or paperboard, PVC-based film products, and printing inks (personal communication, email communication from FD, HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced).
In Canada, Na3NTA is listed in 6 marketed disinfectant products for hospital and health care facilities, domestic use, and use in food premises (personal communication, email from the Therapeutic Products Directorate [TPD], HC, to the ESRAB, HC, dated Jan. 18, 2019; unreferenced).
Na3NTA was historically used as an active ingredient in registered pest control products. Currently, it is used as a formulant in various registered pest control products in Canada (personal communication, email from the Pest Management Regulatory Agency [PMRA], HC, to the ESRAB, HC, dated Jan. 10, 2019; unreferenced).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of Na3NTA was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (for example, OECD QSAR Toolbox 2014), from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (for example, classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for Na3NTA, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, Na3NTA was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Environmental media
To assess potential exposure to Na3NTA from environmental media, the same approach as outlined in the screening assessment for NTA was taken, where measured concentrations of [NTA]3- in the environment were attributed solely to the release of Na3NTA (Environment Canada, Health Canada 2010). Both NTA and Na3NTA are converted into the common nitrilotriacetate form, and the measured concentrations of [NTA]3- represent the contributions from all forms of NTA (neutral compounds or salts) in the environment.
The screening assessment for NTA considered historical release and disposal data for NTA and its salts obtained from the National Pollutant Release Inventory (NPRI), for 1994 to 2007 (Environment Canada, Health Canada 2010). In 2012, it was identified that NTA and its salts had reported releases to air of 1.8 tonnes by one facility in Canada based on the NPRI (NPRI 2019). The US EPA SCREEN3 modelling tool (SCREEN3 2011) was used to estimate ambient air concentrations of Na3NTA from the source facility based on NPRI reported releases of 1.8 tonnes and facility information (details on the SCREEN3 model and parameters are found in Appendix A). The modelled air concentration in the vicinity of the facility was used to estimate exposures of the general population to Na3NTA from outdoor air. These air concentrations resulted in negligible human exposures (< 2.5 ng/kg bw/day). In addition, due to the very high water solubility of this substance, the predominant exposures from the environment are expected to be through drinking water.
There are a number of studies that have measured concentrations of NTA in Canadian municipally treated drinking water, surface water, groundwater and industrial water. The maximum concentration of NTA measured in treated drinking water in Canadian municipalities was 30.4 µg/L from a 1976-1977 national survey of NTA in drinking water (Malaiyandi et al. 1979). This value was used to determine exposure to drinking water in the screening assessment for NTA, and is also considered in this screening assessment as no new data in Canadian drinking water have been identified. The value of 30.4 µg/L results in a daily intake from drinking water of 4.0 x 10-3 mg/kg bw/day in formula fed 0 to 5 month olds, which are the most exposed age group. Details of parameters for estimating drinking water intakes can be found in Appendix B. Currently, the maximum acceptable concentration (MAC) of NTA in drinking water is 400 µg/L, as defined in the Guidelines for Canadian Drinking Water Quality (Health Canada 1990).
The Canadian market use of Na3NTA as reported in 2011 (932 414 kg) (Environment Canada 2013) is approximately 29 times lower than that reported in 1977 (>27 million kg), which suggests that NTA levels in various water sources may have decreased since the 1970s (Environment Canada, Health Canada 2010). However, no additional information on current Canadian reporting volumes or concentrations of NTA in drinking water were identified.
Food
In Canada, Na3NTA may be used as a component in the manufacture of various food packaging materials. Na3NTA may be a component in adhesive products used in food packaging applications, and in printing inks, however, no exposure is expected as there is no potential for direct food contact from these uses. Na3NTA may also be used as a component of products used in the manufacture of paper or paperboard, and in polyvinyl chloride (PVC)-based film products, for food packaging materials. These applications have potential for direct food contact; however, exposure is considered to be negligible as Na3NTA is present at less than 0.01% in the finished paper products and finished products of PVC film packaging (personal communication, email from the FD, HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced).
Na3NTA may also be a component in cleaners that are used for surfaces in contact with food, in hand cleaners, and in sanitizers, including dish and laundry detergents, used in food processing facilities in Canada. The use of the aforementioned cleaning products is followed by a potable water rinse, which is expected to remove any residual Na3NTA, therefore, exposure through food is not expected (personal communication, email from the FD, HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced). Na3NTA may also be used as a boiler water additive, where it is not to exceed 5 parts per million in boiler feedwater, and not to be used where steam will be in contact with milk and milk products. While there is potential for direct food contact, exposure from this source is expected to be negligible (personal communication, email from the FD, HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced).
Products available to consumers
Cosmetics
Na3NTA has a reported function in cosmetics as a chelating agent (EC 2019) and is found in body wash products, hair conditioners, hair shampoos, hair styling products, facial moisturizers, face exfoliants, and permanent hair dyes in Canada (personal communication, email from the CHPSD, HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced). A facial makeup, fragrance, hair shampoo, skin cleanser (body and face), hair dye, foot lotion and nail polish containing Na3NTA had been previously assessed in the screening assessment for NTA; however, additional sentinel exposure scenarios from use of cosmetics, specifically a facial moisturizer and permanent hair dye have since been identified, and will be considered for risk characterization in the current assessment.
Dermal exposures were estimated from the use of these products as the dermal route is expected to be the primary route of exposure due to the low vapour pressure of the substance.
Dermal absorption
To estimate systemic exposure from the dermal route of exposure, a 10% dermal absorption value from the screening assessment for NTA was used (Environment Canada, Health Canada 2010).
The sentinel scenarios for dermal exposure to cosmetics are presented in Table 6-1 below. Additional dermal scenarios for the other cosmetic product types listed, as well as other relevant age groups, were considered, but exposure estimates were lower than those presented in Table 6-1. Details on the method and parameters used to derive estimates of dermal exposure to Na3NTA are available in Appendix C.
| Product scenario | Maximum concentrationa | Exposure estimate (mg/kg bw/day)b |
|---|---|---|
| Face moisturizer (19+ years) | 0.1% | 4.1 x 10-3 |
| Permanent hair dye (14-18 years) | 0.1% | 2.1 x 10-2 |
a Personal communication, email from the CHPSD, HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced
b Systemic exposure assuming 10% absorption through the dermal route
Other products
Na3NTA has been identified as an ingredient in various cleaning products at a maximum concentration of 1%; these products include a spray boat cleaner, a wood furniture polish spray, concentrated liquid wood floor cleaner, and a spray automotive cleaner (SDS 2014; SDS 2015a; SDS 2015b; SDS 2016). In addition, Na3NTA is found in a liquid concentrate disinfectant at a concentration of 0.04%, and a spray disinfectant at 2.0 x 10-4% with identified domestic uses (personal communication, email from the TPD, HC, to the ESRAB, HC, dated Jan. 18, 2019; unreferenced). Exposure estimates from use of a bathroom cleaner containing Na3NTA were derived in the screening assessment for NTA; however, additional cleaning products (for example, spray boat cleaner, a wood furniture polish spray, concentrated liquid wood floor cleaner, and a spray automotive cleaner) have since been identified, and will be considered for risk characterization.
Inhalation, oral and dermal exposures from the use of cleaning products were estimated using ConsExpo Web (ConsExpo Web 2019). Although the substance has a low vapour pressure, the inhalation route is considered due to exposures to aerosols resulting from the use of spray cleaning products. For dermal exposures, the 10% dermal absorption is applied to these scenarios. The sentinel exposure estimates for Na3NTA for these various cleaning products are presented in Table 6-2. Additional scenarios for other cleaning and disinfectant products (i.e., spray automotive cleaner, spray disinfectant product, and liquid concentrated disinfectant product) were evaluated, but exposure estimates were lower than those presented in Table 6-2, and are not presented. Details on the method and parameters used to derive exposure estimates to Na3NTA are available in Appendix C.
| Product scenario | Maximum concentration | Dermal exposure (mg/kg bw/day)a | Inhalation exposuree (mg/kg bw/day) | Oral exposure (mg/kw bw/day) |
|---|---|---|---|---|
| Spraying and polishing with wood furniture polish spray (19+ years) | 1%b | 2.0 x 10-2 | 2.8 x 10-2 | N/A |
| Spray boat cleaner (19+ years) | 1%c | 1.3 x 10-2 | N/Af | N/A |
| Mixing and applying wood floor cleaning liquid (19+ years) | 0.1%d | 5.0 x 10-4 | N/A | N/A |
| Crawling on floor treated with floor cleaner (1 year) | 0.1%d | 1.8 x 10-4 | N/A | 1.4 x 10-5 |
Abbreviation: N/A, Not Applicable
a Systemic exposure assuming 10% absorption through the dermal route
b SDS 2015a
c SDS 2014
d SDS 2015b
e Systemic exposures assuming 100% absorption through the inhalation routef
This product is expected to be used outdoors. The predicted concentration in outdoor air was not estimated. Weather conditions, which can be highly variable and affect ventilation rate as well as temperature, and an undefined room volume (infinitely large) prevent the quantification of reasonable outdoor inhalation exposures (RIVM 2007).
6.2 Health effects assessment
The health effects assessment for Na3NTA is based on the previous NTA screening assessment (Environment Canada, Health Canada 2010) since toxicological studies using both NTA and Na3NTA were considered in the assessment. As stated in the NTA screening assessment, given that both Na3NTA and NTA dissociate to a common moiety, all toxicological studies for the various forms of NTA were taken into consideration in the NTA and Na3NTA health effects assessment. A literature search conducted from the year prior to the NTA screening assessment to May 2019 identified no new health effects studies, which could impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated in the screening assessment for NTA [Environment Canada, Health Canada 2010]).
Na3NTA has been classified as a carcinogen by several national and international agencies. It is considered a Group 2B carcinogen by the International Agency for Research on Cancer (IARC 1999), a Category 3 carcinogen by the European Commission (ECHA c2007-2019; EU 2008), “reasonably anticipated to be a human carcinogen” by the US National Toxicology Program (NTP 2005); and a Group IIIB carcinogen (“possibly carcinogenic to humans”) by Health Canada (Health Canada 2008).
Details on the hazard studies for NTA and Na3NTA are provided in the NTA screening assessment (Environment Canada, Health Canada 2010)
6.3 Characterization of risk to human health
The main sources of exposure of the general population to Na3NTA are through the consumption of drinking water and from the use of products available to consumers, including cosmetics.
Based on the available information and based on the NTA screening assessment (Environment Canada, Health Canada, 2010), a critical effect for the characterization of risk to human health from exposure to Na3NTA is carcinogenicity. As indicated in the NTA assessment, the organ system mainly affected by repeated oral treatment with NTA or Na3NTA is the urinary system and lesions were observed in the kidneys, ureters and urinary bladders in rodents within weeks of dosing and increased in severity with duration of dosing. Na3NTA is not considered to be genotoxic and although the mode of induction of tumours has not been fully elucidated, the tumours observed in experimental animals are unlikely to have resulted from direct interaction with genetic material and a threshold approach is used to assess risk to human health (Environment Canada, Health Canada, 2010).
A lowest observed effect level (LOEL) of 10 mg/kg bw/day was used to characterize the effects of oral, dermal and inhalation short term and chronic exposure based on the observation of marginally increased incidences of transitional cell hyperplasia in the renal pelvis, ureters and and urinary bladders of rats exposed to Na3NTA through diet for 2 years (NCI 1977 as cited in Environment Canada, Health Canada 2010).
Table 6-3 provides key exposure and hazard values for Na3NTA. The resulting margins of exposure (MOEs) for determination of risk are also presented.
| Exposure scenario | Exposure (mg/kg bw/day) | Critical effect levela | MOE |
|---|---|---|---|
| Drinking water | 4.0 x 10-3 | 10 mg/kg bw/day | 2500 |
| Face moisturizer | 4.1 x 10-3 | 10 mg/kg bw/day | 2439 |
| Permanent hair dye | 2.1 x 10-2 | 10 mg/kg bw/day | 476 |
| Wood furniture polishing spray | 2.8 x 10-2 | 10 mg/kg bw/day | 357 |
| Wood floor cleaning liquid | 5.0 x 10-4 | 10 mg/kg bw/day | 20000 |
| Crawling on floor treated with wood floor cleaning liquid | 2.0x 10-4 | 10 mg/kg bw/day | 50000 |
| Spray boat cleaner | 1.3 x 10-2 | 10 mg/kg bw/day | 769 |
a Oral LOEL is based on marginally increased incidences of transitional cell hyperplasia in the renal pelvis, ureters and and urinary bladders of rats exposed to Na3NTA through diet for 2 years
With respect to the use of cosmetic products and various cleaning products containing Na3NTA, comparison of the critical effect levels to the estimated exposures resulted in MOEs ranging from 357 to 50 000. These MOEs are considered adequate to address uncertainties in the health effects and exposure databases.
While exposure to the general population to Na3NTA is not of concern at current levels, the common moiety, nitrilotriacetate, is considered to have a health effect of concern on the basis of its potential for carcinogenicity of the urinary tract. Therefore, there may be a concern for human health if exposures were to increase.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below:
| Key source of uncertainty | Impact |
|---|---|
| Limited information is available concerning the potential toxicity of Na3NTA following inhalation and dermal exposures. | +/- |
| Exposure estimates from drinking water were derived from Canadian monitoring data in municipally treated drinking water from the 1970s. | + |
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from Na3NTA. It is concluded that Na3NTA does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity of concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that Na3NTA does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that Na3NTA does not meet any of the criteria set out in section 64 of CEPA.
References
Bernard A, Houssin A, Ficheux AS, Wesolek N, Nedelec AS, Bourgeois P, Hornez N, Batardière A, Misery L, Roudot AC. 2016. Consumption of hair dye products by the French women population: Usage pattern and exposure assessment. Food Chem Toxicol. 88: 123-132.
Brat SV, Williams GM. 1984. Nitrilotriacetic acid does not induce sister-chromatid exchanges in hamster or human cells. Food Chem Toxicol 22: 211–215.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [accessed 2019 May 03].
[ConsExpo Web] Consumer Exposure Web Model. 2019. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Curry P, Kramer G, Newhook R, Sitwell J, Somers D, Tracy B, Oostdam JV. 1993. Reference values for Canadian populations. Prepared by the Environmental Health Directorate Working Group on reference values. Health Canada. 1988 (updated in 1993).
Delmaar, JE, and Schuur, AG. 2016. ConsExpo Web Consumer exposure models – Model documentation [PDF]. Bilthoven (NL): RIVM. Report No.: 2016-0171. [accessed 2022 Apr 21].
[EC] European Commission. 2008. European Union risk assessment report: trisodium nitrilotriacetate: CAS No. 5064-31-3 [PDF]. Draft dated August 20, 2008. [accessed 2019 May 03].
[EC] European Commission. 2019. Cosmetic Ingredients and Substances (CosIng) Simple Search Database [Internet].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECHA] European Chemicals Agency. c2007-2019. Registered substances database; search results for CAS RN 5064-31-3. Helsinki (FI): ECHA. [updated 2019 Apr 08; accessed 2019 May 03].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice amending the Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2010. Screening assessment for the Challenge: Glycine, N,N-bis(carboxymethyl)- (Nitrilotriacetic acid): Chemical Abstracts Service Registry Number 139-13-9 [PDF]. Ottawa (ON): Government of Canada. [accessed 2019 May 03].
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC, 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food Chem Toxicol. 78: 159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, et al. 2016. Consumption of cosmetic products by the French population second part: Amount data. Food Chem Toxicol. 90: 130-141.
Gomez-Berrada MP, Gautier F, Parent-Massin D, Ferret PJ. 2013. Retrospective exposure data for baby and children care products: An analysis of 48 clinical studies. Food Chem Toxicol. 57: 185–194.
Goyer RA, Falk HL, Hogan M, Feldman DD, Richter W. 1981. Renal tumours in rats given trisodium nitrilotriacetic acid in drinking water for two years. J Natl Cancer Inst. 66: 869–880.
Health Canada. 2008. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Nitrilotriacetic Acid (NTA). Ottawa (ON): Government of Canada. [accessed 2019 May 03].
Health Canada. 2015. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa (ON): Government of Canada.
Health Canada. 2019. Canadian exposure factors used in human health risk assessment. Unpublished report. Ottawa (ON): Government of Canada.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1990. Nitrilotriacetic acid and its salts. IARC Monogr Eval Carcinog Risks Hum. 48: 181–212.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1999. Nitrilotriacetic acid and its salts. IARC Monogr Eval Carcinog Risks Hum. 73: 385–399.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2021 Sept 08]. Ottawa (ON): Government of Canada. [accessed 2022 Feb 13].
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, et al. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43: 279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, et al. 2006. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44: 2008-2018.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, et al. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol. 46: 1516-1524.
Mahaffey KR, Goyer RA. 1972. Trisodium nitrilotriacetate in drinking water. Metabolic and renal effects in rats. Arch Environ Health. 25: 271–275.
Malaiyandi M, Williams DT, O’Grady R. 1979. A national survey of nitrilotriacetic acid in Canadian drinking water. Environ Sci Technol. 13(1): 59–62.
Montaldi A, Zentilin L, Venier P, Gola I, Bianchi V, Paglialunga S, Levis AG. 1985. Interaction of nitrilotriacetic acid with heavy metals in the induction of sister chromatid exchanges in cultured mammalian cells. Environ Mutagen. 7: 381–390.
[NCI] National Cancer Institute (US). 1977. Bioassays of nitrilotriacetic acid (NTA) and nitrilotriacetic acid, trisodium salt, monohydrate (Na3NTA ·H2O) for possible carcinogenicity. Bethesda (MD): US Department of Health, Education and Welfare, Public Health Service, National Institutes of Health, National Cancer Institute.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2022 Apr 09]. Ottawa (ON): Government of Canada. [accessed 2022 Jun 22].
Nixon GA. 1971. Toxicity evaluation of trisodium nitrilotriacetate. Toxicol Appl Pharmacol. 18: 398–406.
Nolen GA, Klusman LW, Back DL, Buehler EV. 1971. Reproduction and teratology studies of trisodium nitrilotriacetate in rats and rabbits. Food Cosmet Toxicol. 9: 509–518.
[NPRI] National Pollutant Release Inventory. 2019. NPRI Datasets: NPRI On-line Facility Data Search. Ottawa (ON): Government of Canada. Search results from 2012 for CAS RN 139-13-9. [accessed 2019 May 04].
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Ramirez-Martinez A, Granda-Torres P, Wesolek N, Ficheux AS, Roudot AC. 2015. Exposure of hairdressers to the main cosmetics used in hairdressing salons from France by means of a preliminary study. Arch Environ Occup Health 71: 247-258.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007. Do-It-Yourself Products Fact Sheet: To assess the risks for the consumer [PDF]. Bilthoven (NL): RIVM. Report No.: 320104007. [accessed 2019 May 1].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2018. Cleaning products fact sheet: default parameters for estimating consumer exposure – updated version 2018 [PDF]. Bilthoven (NL): RIVM. Report No.: 2016-0179. [accessed 2019 May 1].
SCREEN3 [computer model]. 2011. Ver. 3.5.0. Research Triangle Park (NC): US Environmental Protection Agency, Office of Air Quality Planning and Standards, Emissions, Monitoring, and Analysis Division.
[SDS] Safety Data Sheet. 2014. Starbrite Instant Black Streak Remover. [accessed 2019 May 03].
[SDS] Safety Data Sheet. 2015a. Murphy oil soap wood cleaner spray multiuses. New York (NY): Colgate-Palmolive Co. [accessed 2019 May 03] [restricted access].
[SDS] Safety Data Sheet. 2015b. Murphy oil soap wood cleaner liquid – original. New York (NY): Colgate-Palmolive Co. [accessed 2019 May 03] [restricted access].
[SDS] Safety Data Sheet. 2016. Armor all Quicksilver Aerosol [PDF]. Danbury (CT): The Armor All/STP Products Company. [accessed 2019 May 03].
Tjälve H. 1972. A study of the distribution and teratogenicity of nitrilotriacetic acid (NTA) in mice. Toxicol Appl Pharmacol. 23: 216–221.
[US EPA] US Environmental Protection Agency. 1980. Final report: NTA. Unpublished report. Washington (DC): US EPA, Office of Pesticides and Toxic Substances, Assessment Division.
[US EPA] United States Environmental Protection Agency. 1992. Screening procedures for estimating the air quality impact of stationary sources, revised. Washington (DC): US EPA, Office of Air Quality. p 102.
[US EPA] United States Environmental Protection Agency. 2011. Exposure Factors Handbook. 2011 Edition. Washington (DC): US EPA, National Centre for Environmental Assessment.
[US EPA] United States Environmental Protection Agency. 2012. Standard Operating Procedures for Residential Pesticide Exposure Assessment [PDF]. US Environmental Protection Agency, Office of pollution prevention and toxics; Syracuse research corporation.
Versar, Inc. 1985. Methods for assessing exposure to chemical substances. Vol. 7. Methods for assessing consumer exposure to chemical substances. Washington (DC): Versar, Inc. Prepared for US Environmental Protection Agency.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48: 3109-3119.
Appendices
Appendix A. SCREEN3: model and inputs
SCREEN3 is a screening-level Gaussian air dispersion model based on the Industrial Source Complex (ISC) model (for assessing pollutant concentrations from various sources in an industry complex) (SCREEN3 2011). The driver for air dispersion in the SCREEN3 model is wind. The maximum calculated exposure concentration is selected based on a built-in meteorological data matrix of different combinations of meteorological conditions, including wind speed, turbulence and humidity. This model directly predicts concentrations resulting from point, area and volume source releases. SCREEN3 gives the maximum concentrations of a substance at chosen receptor heights and at various distances from a release source in the direction downwind from the prevalent wind within one hour of a given release event. During a 24-hour period, for point emission sources, the maximum 1-hour exposure (as assessed by the ISC Version 3) is multiplied by a factor of 0.4 to account for variable wind direction. This gives an estimate of the air concentration over a 24-hour exposure (US EPA 1992; SCREEN3 2011). Similarly, for exposure events happening over the span of a year, it can be expected that the direction of the prevalent winds will be more variable and uncorrelated to the wind direction for a single event; thus, the maximum amortized exposure concentration for one year is determined by multiplying the maximum 1-hour exposure by a factor of 0.08 (US EPA 1992; SCREEN3 2011). Parameters used to estimate ambient air concentrations using the SCREEN3 model are presented in Table A-1 below.
| Source Type | Area |
|---|---|
| Effective emission Areaa | 100 m x 250 m |
| Receptor Heightb | 1.74 m |
| Source release heighta | 30 m |
| Adjustment Factorc | 0.4 |
| Urban-rural optiond | Urban |
| Meterologyd | 1 (full meteorology) |
| Minimum and maximum distancea | 0-3000 m |
a Site specific; based on aerial photograph analysis and professional judgement
b Average adult height (Curry et al. 1993)
c Factor to account for variable wind direction over a 24-hour period (US EPA 1992)
d Default in SCREEN3
Appendix B. Estimates of daily intakes of drinking water
The concentration of 30.4 µg/L identified from a 1976-1977 national survey of NTA in drinking water (Malaiyandi et al. 1979) was used to derive intake estimates from drinking water. Estimates were calculated based on default body weights of 74 kg for 19+ year olds, 62 kg for 14-18 year olds, 42 kg for 9-13 year olds, 23 kg for 4-8 year olds, 15 kg for 2-3 year olds, 11 kg for 1 year olds, 9.1 kg for 6-11 month olds, 6.3 kg for 0-5 month olds (Health Canada 2015). Drinking water intakes used to determine intake estimates are presented in Table B-1 below. The estimates of daily intake from drinking water are presented in Table B-2 below.
| Age group | Drinking water intake (L/day)a | Infant formula intake (L/day)a |
|---|---|---|
| 0-5 months | N/Ab | 0.826b |
| 6-11 months | N/Ab | 0.764b |
| 1 year old | 0.36 | N/A |
| 2-3 year old | 0.43 | N/A |
| 4-8 year old | 0.53 | N/A |
| 9-13 year old | 0.74 | N/A |
| 14-18 year old | 1.09 | N/A |
| 19+ year old | 1.53 | N/A |
Abbreviation: N/A, Not Applicable
a Health Canada 2019
b It is assumed that infants younger than 1 year old consume drinking water through formula intake
| Age Group | Estimated intake (mg/kg bw/day) |
|---|---|
| 0-5 months | 4.0 x 10-3 |
| 6-11 months | 2.6 x 10-3 |
| 1 year old | 9.9 x 10-4 |
| 2-3 year old | 8.7 x 10-4 |
| 4-8 year old | 7.0 x 10-4 |
| 9-13 year old | 5.4 x 10-4 |
| 14-18 year old | 5.3 x 10-4 |
| 19+ year old | 6.3 x 10-4 |
Appendix C. Parameters to estimate human exposures from use of products available to consumers in Canada
Exposure estimates were calculated based on default body weights of 74 kg for 19+ year olds, 62 kg for 14-18 year olds, 42 kg for 9-13 year olds, 23 kg for 4-8 year olds, 15 kg for 2-3 year olds, 11 kg for 1 year olds, 9.1 kg for 6-11 month olds, 6.3 kg for 0-5 month olds (Health Canada 2015). The estimated inhalation, oral, and dermal exposure parameters for cosmetics and other products available to consumers are described in Tables C-1 and C-2, respectively. Exposures for products available to consumers were estimating using ConsExpo Web (ConsExpo Web 2019), unless stated otherwise. Defaults parameters from ConsExpo Web were used unless otherwise specified in Tables C-1 and C-2. For dermal exposures, a 10% dermal absorption value was applied.
| Product (substance) | Assumptions |
|---|---|
| Face moisturizer |
Concentration in product: 0.1%a Dermal – Direct contact, instant application Frequency of use: 2.0 per day (19+ year old) (Loretz et al. 2005), 1.0 times per day (9-13 year old, and 14-18 year old) (Ficheux et al. 2015) |
| Permanent hair dye |
Concentration in product: 0.1%a Dermal – Direct contact, instant application |
a Personal communication, email from the CHPSD, HC, to the ESRAB, HC, dated Feb. 4, 2019; unreferenced
| Exposure scenario | Assumptions |
|---|---|
| Wood furniture polish spray, spraying and polishing |
Concentration in product: 1% (SDS 2015a) Scenario: spraying and polishing with wood furniture polish spray (trigger spray) in Cleaning Products Fact Sheet (RIVM 2018) Spraying (non-volatile substance) |
| Wood floor cleaning liquid, mixing and applying (adults) |
Concentration in product: 0.1% (SDS 2015b) Scenario: mixing and applying wood floor cleaning liquid in Cleaning Products Fact Sheet (RIVM 2018) Mixing and loading Application – cleaning |
| Crawling on floor treated with wood floor cleaner (1 year old) |
Concentration in product: 0.1% (SDS 2015b) Scenario: post-application (rubbing-off) of wood floor cleaning liquid Dermal (post-application – rubbing off) Deposited residue (mg/cm2): Calculated assuming 14.4 g of product per 22 m2 of floor (ConsExpo Cleaning Fact Sheet (RIVM 2018)) * 1000 mg/g * 1 m2/10000 cm2 Incidental oral (hand to mouth contact) HR: hand residue loading (mg/cm2); calculated using the following algorithm: HR = [Faihands * Dermal exposure (mg) (calculated above)] / (SAH * 2) Faihands: 0.15 (unitless); fraction of active ingredient on hands compared to total surface residue from jazzercise study |
| Spray boat cleaner, spraying |
Product scenario: boat hull cleaner Dermal C (concentration in product):1% (SDS 2014) |
