Screening assessment - Certain organic flame retardants substance grouping - Phosphoric acid, tris(methylphenyl) ester (TCP)
Official title: Screening assessment - Certain organic flame retardants substance grouping - Phosphoric acid, tris(methylphenyl) ester (TCP)
Chemical abstracts service registry number 1330-78-5
Environment and Climate Change Canada Health Canada
May 2019
Cat. No.: En14-366/2019E-PDF
ISBN 978-0-660-29858-0
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of phosphoric acid, tris(methylphenyl) ester, commonly known as tricresyl phosphate or TCP (Chemical Abstracts Service Registry Number 1330-78-5). TCP is a substance within the Certain Organic Flame Retardants (OFR) Substance Grouping, which includes organic substances having similar function: application to materials to slow the ignition and spread of fire. This substance was identified as a priority for assessment as they met categorization criteria under subsection 73(1) of CEPA.
TCP does not occur naturally in the environment. Results from a 2011 industry survey indicated that TCP was not manufactured in Canada in 2011 but 1 000 to 10 000 kg of neat TCP substance and between 100 and 1 000 kg of TCP in mixtures and commercial products or products available to consumers were imported into Canada. In Canada, confirmed uses of TCP include adhesives and sealants, automobile parts, aircraft applications, fire-resistant lubricant and grease additive, and electrical and electronic applications. Internationally, TCP is used as a flame retardant and plasticizer in household applications such as furniture upholstery backcoating, adhesives and sealants, automobile parts, aircraft applications, electronic and electrical applications, various extruded manufactured items such as flexible polyvinyl chloride (PVC), vinyl tarpaulins, as well as extreme pressure additive in lubricants, and as a fire-resistant hydraulic fluid.
Current commercial products marketed as TCP consists primarily of a mixture of m-TCP and p-TCP isomers, with the o-TCP isomer at approximately 0.05%. The three isomers are considered to possess identical physical chemical properties for the purpose of this assessment. They are characterized by moderate water solubility and octanol-water partition coefficient, and a low vapour pressure and melting point.
TCP is not shown to be persistent in water, soil, sediment or air based on modelled and limited experimental data. Results from empirical and modelled hydrolysis data suggest a fast degradation rate which increases with increasing environmental pH. On the basis of TCP’s low modelled volatility, short half-life in air (18.74 hr) and estimated Characteristic Travel Distance of 363 km, TCP is not expected to reside in air long enough to be atmospherically transported a significant distance from its emission source.
TCP is considered to have low to moderate bioconcentration and bioaccumulation potentials on the basis of empirical fish bioconcentration studies and modelled data. TCP is considered to be rapidly metabolized in fish.
Based on the available empirical ecotoxicity studies and modelled data, TCP is considered to have a moderate to high level of toxicity to aquatic organisms with acute and chronic effects demonstrated from approximately 0.001 to 1 mg/L. There are no sediment, soil or wildlife toxicity data for TCP.
It is expected that TCP may be released to the Canadian environment as a result of industrial processing activities through wastewater. Although TCP can be found in commercial products and products available to consumers, information on release to the environment from this route is limited, and releases are expected to be diffuse and minimal, particularly when considering the low level of use for this substance identified in Canada. Exposure scenarios were developed for industrial releases, where release to water results in minor TCP partitioning to sediment. Although there are no soil toxicity data, exposure to soil-dwelling mammals from the application of biosolids containing TCP was estimated. To address the potential exposures to wildlife predators consuming fish with accumulated TCP, total daily intake modelling was performed for mink and river otter as representative wildlife species. Risk quotient analyses, integrating conservative estimates of exposure with the available toxicity information, were performed and showed a low potential for risk for aquatic organisms, soil-dwelling mammals, and fish-eating mammals.
Considering all available lines of evidence presented in this screening assessment, there is a low risk of harm to the environment from TCP. It is concluded that TCP does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
No classifications of the health effects of TCP (containing <0.1% of o-TCP) by national or international regulatory agencies were identified. On the basis of available information, TCP is not carcinogenic or genotoxic. On the basis of animal studies, the critical health effects of exposure to TCP are effects on the ovary and adrenal cortex. The main sources of exposure for the general population in Canada are expected to be from environmental media (air, dust, soil, and water), food, including breast milk, and from the use of products available to consumers such as furniture (with treated upholstery or foam) and lubricants.
The margins of exposure between estimates of intake from environmental media, food and from contact with products available to consumers, and effect levels are considered to be adequate to address uncertainties in the exposure and health effects databases. Therefore, it is concluded that TCP does not meet the criteria under paragraph 64(c) of CEPA, as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Overall conclusion
It is concluded that TCP does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to sections 68 and 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health conduct screening assessments of substances to determine whether these substances present or may present a risk to the environment or to human health.
The Substance Groupings Initiative is a key element of the Government of Canada’s Chemicals Management Plan. The Certain Organic Flame Retardants Substance Grouping consists of ten substances identified as priorities for action as they met the categorization criteria under section 73 (1) CEPA and/or were considered a priority on the basis of ecological and/or human health concerns (Environment Canada, Health Canada 2007). All of these substances have a similar function: the application to materials to slow the ignition and spread of fire. Also, these substances are potential alternatives for other flame retardants which are presently subject to regulatory controls or phase-out in Canada and/or globally.
This screening assessment focuses on the substance Phosphoric acid, tris(methylphenyl) ester, commonly known as Tricresyl Phosphate or TCP (CAS RN 1330-78-5). This substance was identified in the categorization of the Domestic Substances List (DSL) under subsection 73(1) of CEPA as meeting criteria for greatest potential for exposure of individuals in Canada. While the substance did not meet categorization criteria for persistence or bioaccumulation, it did meet criteria for inherent toxicity to non-human organisms.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure for TCP. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to January 2017 for the ecological and human health sections. Targeted literature searches were conducted up to July 2018 for human health components of this assessment. Empirical data from key studies as well as some results from models were used to reach the conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review and/or consultation. Comments on the technical portions relevant to the environment were received from Dr. Jon Arnot (Arnot Research and Consulting) and Mr. John A. Biesemeier (Chemtura Corporation). Comments on the technical portions relevant to human health were received from Raymond York, R.G. York & Associates; Donna Vorhees, The Science Collaborative; and Bernard Gadagbui, Toxicology Excellence for Risk Assessment (TERA). Additionally, the draft of this screening assessment was subjected to a 60-day public comment period. Some human health portions of this assessment have undergone an additional targeted external written peer consultation. Comments were received from Richard Manderville, University of Guelph; Mohamed Abou-Elwafa Abdallah, University of Birmingham; and Kebede K. Kefeni, Tshwane University of Technology. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 1 . This screening assessment presents the critical information and considerations on which the conclusion(s) is/are based.
2. Substance identity
This screening assessment focuses on the substance Phosphoric acid, tris(methylphenyl) ester (CAS RN 1330-78-5) within the Certain Organic Flame Retardants Substance Grouping. Phosphoric acid, tris(methylphenyl) ester is also known as Tricresyl Phosphate (TCP). A list of its additional chemical names (i.e. trade names) is available from the National Chemical Inventory (NCI 2013). For the purposes of this assessment, the substance will be referred to as TCP. Substance identity for TCP is shown in Table 2-1 and is described by a structure with undefined methyl group positions.
There are three isomers of TCP based on methyl group positions: tri-ortho-cresyl phosphate, tri-meta-cresyl phosphate, and tri-para-cresyl phosphate. They are referred to as o-TCP (CAS RN 78-30-8), p-TCP (CAS RN 78-32-0) and m-TCP (CAS RN 563-04-2), respectively, for this assessment. Substance identities of TCP and its isomers are presented in Table 2-1. Other names for TCP and its isomers are available (ECCC 2018a).
| CAS RN (acronym) | Chemical structure | Molecular mass | Chemical formula |
|---|---|---|---|
| 1330-78-5 (TCP) | 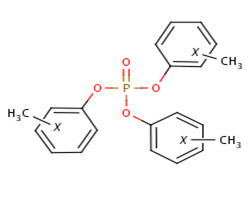 |
368.37 g/mol | C21H21O4P |
| 78-30-8 (o-TCP) | 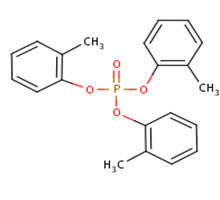 |
368.37 g/mol | C21H21O4P |
| 563-04-2 (m-TCP) | 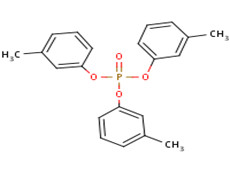 |
368.37 g/mol | C21H21O4P |
| 78-32-0 (p-TCP) | 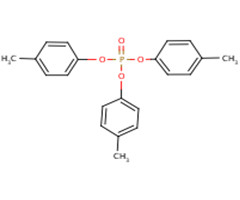 |
368.37 g/mol | C21H21O4P |
Most available studies on TCP involved the use of commercial products. Commercial TCP mixtures may contain 65-70% tricresyl phosphate (mixture of m-TCP and p-TCP isomers and 0.05% o-TCP) with 0.5% triphenyl phosphate and less than 0.05% free phenol and cresol (Bayer 2002). TCP is typically produced by the reaction of a synthetic mixture of m- and p-cresol with phosphorus oxychloride (Ashford 1994; UK EA 2009) to limit the formation of unwanted isomers (e.g., o-TCP) and contaminants (Sjögren et al. 2010). Early manufacturing practices in general used petroleum or coal tar derived cresols (naturally derived cresols) (Sjögren et al. 2010). While TCP manufacturing from naturally derived cresols has been reported to exist today (e.g., Great Lakes 2010; Chemnet 2014), manufacturing activities have changed over time, and the amount of o-TCP present has been minimized (UK EA 2009; Sjögren et al. 2010). However, it is difficult to obtain data on the amount of o-TCP and other orthocresyl isomers in commercially available materials containing TCP marketed worldwide (Sjögren et al. 2010). Conservative estimates of 0.1 to 1% of o-TCP have been reported (Sjögren et al. 2010; ACGIH 2012 cited in HSDB 2014); however, it has also been reported that the concentration is usually below 0.1% (Sjögren et al. 2010). Also, “new generation” materials typically claim to have an even lower o-TCP content (Sjögren et al. 2010). Based on the collective information, it is reasonable to consider that the TCP mixture contains less than 0.1% o-TCP for the purpose of this assessment.
3. Physical and chemical properties
Key physical and chemical property data for TCP and its isomers are presented in Table 3-1. Those which were reported from empirical studies were critically reviewed for data quality.
QSAR models were used to generate data for some of the physical and chemical properties of TCP. These models are mainly on the basis of fragment addition methods, i.e. they sum the contributions of sub-structural fragments of a molecule to make predictions for a property or endpoint. Most of these models rely on the Simplified Molecular-Input Line-Entry System (SMILES) notation (ECCC 2018a).
While the Least Square Adjustment (LSA) method was applied to harmonize physical chemical properties for TCP (Schenker et al. 2005), the original empirical data were used in preference as key physical and chemical properties for this assessment. TCP (the mixture of isomers) is a clear, colourless liquid with a very slight aromatic odour (IPCS 1990). Commercially marketed TCP is a clear colourless to slightly yellow liquid (UK EA 2009, Ashford 1994, Bayer 2002, Wildlife International Ltd. 2002, WHO 1990). TCP has a low melting point of -33 oC, while its three isomers have higher melting points, ranging from 11 to 78oC (UK EA 2009, WHO 1990). The boiling point of TCP is at 476.06oC at standard atmospheric pressure, while its isomers have lower values at various measured pressures. TCP has a density of 1160 kg/m3 at 20oC that is comparable among its three isomers.
TCP is characterized by moderate water solubility at room temperature and very low volatility based on its low vapour pressure and low Henry’s Law constant. This substance also has a moderate to high octanol/water partition coefficient (see Table 3-1). TCP can easily be hydrolyzed to dicresyl phosphate and cresol in an alkaline medium, but it is stable in neutral and acidic media (IPCS 1990; van der Veen and de Boer 2012; WHO 1990). Cresol was assessed by Environment Canada and was concluded not to meet the criteria under paragraphs 64(a) or (b) or (c) of CEPA in May 2016 (Environment Canada 2016). Therefore, cresol is not discussed in this assessment.
| Property | Value | Temperature (ºC) | Reference |
|---|---|---|---|
| Melting point (ºC) | -33 | NA | US EPA 2010 (Midwest Research Institute, 1977) UK EA 2009 (WHO 1990) |
| Boiling point (ºC) | 476.06 (standard atmospheric pressure at 101 325 Pa) | NA | MPBPVP v.1.43 |
| Density (kg/m3) | 1160 | 20 | UK EA 2009 (Ashford 1994) |
| Vapour pressure (Pa) | 6.6 x 10-5 | 25 | UK EA 2009 (extrapolated) |
| Henry’s Law constant (Pa·m3/mol) | 6.75 x 10-2 (6.843 x 10-7 atm.m3/mol) | 25 | Calculated from molecular weight, vapour pressure and water solubility listed in this table |
| Log Kow (dimensionless) | 5.11 | 25 (room temperature) | Saeger et al. 1979 |
| Log Koc (dimensionless) | 3.52 | NA | KOCWIN v.2.00 |
| Log Koa (dimensionless) | 9.59 | NA | KOAWIN v.1.10 |
| Log Kaw (dimensionless) | -4.564 | 25 | Calculated from Henry’s Law constant listed in this table |
| Water solubility (mg/L) | 0.36 | 25b | Saeger et al. 1979 |
| pKa (dimensionless) | NA | NA | NA |
Abbreviations: WS, water solubility; VP, vapour pressure; log Kow, octanol-water partition coefficient; log Koc, organic carbon-water partition coefficient; log Koa, octanol-air partition coefficient; pKa, acid dissociation constant; NA, not applicable; NS, not specified (likely experimental).
a Values in parentheses represent the original ones as reported by the authors or as estimated by the models.
4. Sources
TCP is a synthetic substance and does not occur naturally. On the basis of the responses to a more recent survey pursuant to Section 71 of CEPA (calendar year 2011), TCP was not manufactured in Canada; however 1 000 to 10 000 kg of TCP were imported into Canada during that year as a neat substance (1 000–10 000 kg), and in commercial products or products available to consumers (100–1 000 kg) (ECCC 2013-2014). While the survey included products, there is the possibility that the import volume of commercial products or products available to consumers is underestimated.
TCP is a high-production volume (HPV) chemical in the U.S., where TCP production is estimated to range from 1 to 10 million pounds (US EPA 2010). In 1998, 2002 and 2006, 412 to 4082 tonnes (454 to 4500 ton) of TCP were produced in the U.S. (van der Veen and de Boer 2012) and 0.73 tonnes, 0.54 tonnes, 3.3 tonnes, 4.5 tonnes were produced/used in Norway, Denmark, Finland and Sweden, respectively, in 2008 (van der Veen and de Boer 2012).There are two known European production sites; however information on production volume and market size is confidential (UK EA 2009).
5. Uses
According to submissions under section 71 of CEPA, in Canada, TCP has application in adhesives and sealants, automobile parts, aircrafts, electrical applications and electronics, and as a fire-resistant lubricant and grease additive (ECCC 2013-2014). In preliminary product testing conducted by Health Canada of children’s manufactured items purchased in Canada in 2014, TCP was detected in a foam chair above the limit of quantification at a concentration reported as ≥0.7%; however, TCP was not detected in the remaining 23 children’s manufactured items (e.g. nursing pillows, toys) (Health Canada 2014). According to manufacturer literature (ICL 2013c), TCP is marketed for flexible foam used in furniture.
TCP appears on the List of Prohibited and Restricted Cosmetic Ingredients (more commonly referred to as Health Canada’s Cosmetic Ingredient Hotlist or simply the Hotlist), an administrative tool that Health Canada uses to communicate to manufacturers and others that certain substances, when present in a cosmetic, may contravene (a) the general prohibition found in section 16 of the Food and Drugs Act or (b) a provision of the Cosmetic Regulations. The Hotlist prohibits the ingredient TCP (synonym: tritolyl phosphate) (Health Canada [modified 2015]).
TCP is not listed as an approved food additive in the Lists of Permitted Food Additives, which have been incorporated by reference into their respective Marketing Authorizations issued under the Food and Drugs Act (Health Canada [modified 2017]). TCP has not been identified as being used/present in formulations of food packaging materials, but is present in incidental additives used as a lubricant on equipment or machine parts where there is no contact with food (2013 email from Food Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). TCP is not listed in the Drug Products Database, the Therapeutic Products Directorate's internal Non-Medicinal Ingredient Database, the Natural Health Products Ingredients Database or the Licensed Natural Health Products Database as a medicinal or non-medicinal ingredient present in final pharmaceutical products, natural health products or veterinary drugs in Canada (DPD [modified 2017], NHPID [modified 2017], LNHPD modified 2016; 2013 email from the Therapeutic Products Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced).
The Commission for Environmental Cooperation (CEC) conducted a project in which furniture products from Canada, the U.S. and Mexico were analyzed for the presence of emerging flame retardants using X-ray fluorescence (XRF) screening followed by GC/MS analysis (CEC 2015). TCP was one of sixteen flame retardants evaluated in this study but it was not identified in any samples from the 132 products purchased between December 2014 and April 2015.
Internationally, TCP applications also include several manufactured items such as furniture and electronics. TCP was reported to be applied as a backcoating to furniture upholstery in the U.S. (US CPSC 1998; Piccirillo 1999; NRC 2000). TCP is used in synthetic leather furniture upholstery according to a report by the European Commission (2011) and in PVC seat covers (SinoHarvest 2011). Curtains, wallpaper, flooring and electronics in Japan have been reported to contain TCP (Kajiwara et al. 2011). TCP is used as a flame retardant in polystyrene plastics that are commonly used in computers, cabinets for display units and refrigerators. As well, TCP use as a flame retardant has been identified in printed circuit boards (GFEA 2001). TCP may also be used in various utility articles including footwear, raincoats and handbags (SinoHarvest 2011), in addition to PVC gloves (Siret-Alatrista et al. 2010) or garments that are made of imitation leather (IPCS 1990; Ash and Ash 2000; Ash and Ash 2003). Several other applications of TCP involve automobile interiors (IPCS 1990; ATSDR 1997). Minor uses have been reported for glasses frames (Siret-Alatrista et al. 2010) and a prosthetic leg (Grimalt et al. 2009). TCP is also used in various extruded products such as flexible polyvinyl chloride (PVC) cable insulation and films, hoses, mine conveyor belts, air ducts, as well as polystyrene and vinyl tarpaulins (Weil 2001; Ash and Ash 2003;UK EA 2009; SinoHarvest 2011).
Internationally, TCP is used as an additive flame retardant and plasticizer in a range of products. TCP finds use in cellulose nitrate, ethylcellulose, lacquers, adhesives, pigment dispersion, photographic film and clarifying agent in casein polymer production (IPCS 1990; Weil 1993, Ashford 1994; UK EA 2009). Owing to its anti-wear, anti-corrosion, anti-foaming, lubricating and flame retardant properties, TCP is used as an additive in engine turbine oils and hydraulic fluids in aircraft hydraulic systems (IPCS 1990; Okazaki et al. 2003).
6. Releases to the environment
Anthropogenic releases to the environment depend upon various losses occurring during the manufacture, industrial use, consumer or commercial use, service life and disposal of a substance. Releases of TCP to the Canadian environment, because of the substance’s use as a flame retardant, are expected to be diffuse, with some point sources (e.g. from processing facilities). Releases from commercial products and products available to consumers may occur in both indoor and outdoor environments.
According to submissions made under section 71 of CEPA, TCP is imported into Canada in neat form, and in commercial products and products available to consumers (ECCC 2013-2014). TCP is used in Canada as a flame retardant within several sectors; TCP use activities in Canada likely to result in point source TCP releases to the environment include blending into products and potentially from container cleaning.
TCP release to the environment is likely to occur during industrial activities. Releases to the environment are expected to occur primarily to the water compartment via wastewater. Release to the soil could also occur through the application of biosolids to agricultural and pasture lands. TCP is not a reportable substance in the National Pollutant Release Inventory (NPRI 2011; data from 1994–2009).
7. Measured environmental concentrations
Internationally, levels of TCP in air and water appear to be low, with somewhat higher levels in sediments and fish (Boethling and Cooper 1985). A detailed listing of the available concentrations of TCP and its isomers in air, water, sediment, soil, biota and other media from the open literature are available in supporting documentation (ECCC 2018b). Data concerning concentrations of TCP and its related isomers in the Canadian environment are limited, but are available for water and some biota.
7.1 Air
In Canada, no data are available for the concentration of TCP in air (ECCC 2018b).
Air samples collected near the facilities which produced aryl phosphates in Nitro and Gallipolis Ferry in West Virginia, USA were found to contain concentrations of TCP ranging from 0.01 to 2 ng/m3 (Boethling and Cooper 1985).
In Japan, three studies conducted from 1974 to 2000 reported TCP concentrations in air. Among them, concentrations of TCP ranging from non-detect to 70.3 ng/m3 were measured in 3 out of the 19 air samples collected from heavily-industrialized cities in Shikoku, Japan (IPCS 1990). Another two studies, however, reported lower concentrations of TCP ranging from non-detect to 21.4 ng/m3 (IPCS 1990; Kishi and Sekine 2003; Takimoto et al. 1999). Takimoto et al. (1999) concluded that in the Kurose River Basin area of Japan, the main source of TCP in air, likely sorbed onto particulate matter, were motorcycle and automobile exhausts.
In a more recent study out of Finland, TCP was not found above the detection limit of 0.0007 ng/m3 (a semi-quantitative value determined against another organophosphate flame retardant) in a sample of air collected in July 2004 from a rural area in Northern Finland—a site selected to verify potential long-range atmospheric transport (Marklund et al. 2005).
7.2 Water
There are limited studies characterizing concentrations of TCP in water (ECCC 2018b). In Canada, TCP was not detected in the open waters of the Great Lakes, but the tributaries of Lake Ontario and Lake Erie contained TCP ranging from 11 to 22 ng/L and from 2.5 to 10 ng/L, respectively in 2010 and 2011 (Lee et al. 2011).
In the United States, various studies have sought to determine levels of TCP in surface waters (Boethling and Cooper 1985). However, because many of these studies had high detection limits (e.g. ≥100 ng/L), only one surface water sample included a detected concentration of 20 ng/L which was reported downstream of a facility that produced aryl phosphates (Boethling and Cooper 1985).
In Europe, concentrations of TCP were reported below detection limit by three independent studies conducted in rivers in Denmark, Rome and Austria (Bacaloni et al. 2007; Martinez-Carballo et al. 2007; UK EA 2009).
In Japan, river and lake waters were tested for TCP in the 1970s and 1990s; however, only a limited number of studies reported measuring TCP concentrations above the detection limit. Concentrations of o-TCP, p-TCP and m-TCP were reported at below 500 ng/L (detection limits ranged from 5 to 2500 ng/L (UK EA 2009; Cho et al. 1994, 1996)). These studies also noted that concentrations of TCP above the detection limit were generally found in highly polluted areas and were often detected in water samples containing suspended sediments and sediments (UK EA 2009). In China, water samples were collected from 17 sites at Songhua River, and m-TCP was detected at concentrations between 5.2 and 45 ng/L (Wang et al. 2011).
7.3 Sediment
In Canada, a research project was conducted in 2012 and 2013 at Niagara Basin and Mississauga Basin of Lake Ontario, respectively (De Silva and Muir 2016). One sediment core was sampled at each location. The mean measured concentrations of TCP (total of all isomers) were 0.011 mg/kg dry weight (dw) at Niagara Basin and 0.0082 mg/kg dw in the Mississauga Basin.
Studies conducted elsewhere before 2005, mainly in the 1980s and 1990s, report detection of TCP in sediments near industrialized areas (ECCC 2018b).
In the United States, sediments from freshwater rivers and lakes were monitored for TCP. In the early 1980s, sediment concentrations of TCP in industrialized areas of the Delaware River, Kanawha River, Saginaw River, Baltimore Harbor and Detroit River ranged from non-detect (detection limit: 0.2 mg/kg) to 1.3 mg/kg (Boethling and Cooper 1985). In 1988-1990, sediment concentrations of TCP ranging from 0.05 to 3.40 mg/kg dw (mean level was 1.1 mg/kg dw) were measured in all 10 samples in the Grand Calumet River in Indiana, U.S. (Hoke et al. 1993).
In Europe, TCP in sediments from freshwater sources was measured at concentrations ranging from non-detect to 0.39 mg/kg dw in Denmark, Norway, Austria and Germany (Ricking et al. 2003; Martinez-Carballo et al. 2007; UK EA 2009; van der Veen and de Boer 2012). Concentrations in marine sediment ranged from non-detect to 0.37 mg/kg dw in Denmark (UK EA 2009).
Kawagoshi et al. (1999) reported TCP concentrations ranging from non-detect to 2.558 mg/kg dw over a 7-year period (1991–1997) at a solid waste disposal site in Japan. In 1992, sediment concentrations of TCP isomers ranged from non-detect to 0.08 mg/kg dw in Kurose River Basin, Japan (Cho et al. 1994). TCP was also measured in a marine sediment sample at a concentration of 0.004 mg/kg dw at Tokyo, Japan (Wakabayashi 1980).
7.4 Soil
The existing literature contains few references to soil concentrations of TCP (ECCC 2018b) and those identified are older (before 1996). No soil concentrations of TCP are available for Canada.
In the United States, concentrations of TCP were detected in soils at US air force bases contaminated with hydraulic fluids, ranging from 0.02 to 130 mg/kg (David and Seiber 1999b). Also, TCP was detected in soils near facilities that produce aryl phosphates in Gallipolis Ferry, West Virginia, with concentrations ranging from 1.0 to 4.0 mg/kg (Boethling and Cooper 1985).
In Japan, concentrations of TCP isomers measured in soils near a highway and in a forest ranged from non-detect to 1.52 mg/kg (Takimoto et al. 1999). Concentrations of m- isomer exceeded concentrations of o- and p-isomers (Takimoto et al. 1999).
7.5 Biota
Few studies report concentrations of TCP in biota (ECCC 2018b). In Canada, 0.12 ng/g wet weight (ww) of TCP has been detected in herring gull egg homogenate taken from the Great Lakes area (Chen et al. 2012). No other TCP concentrations are available for biota in Canada.
In the United States, concentrations of TCP in vegetation near facilities that produce aryl phosphates in Gallipolis Ferry, West Virginia, were between 1 and 20 mg/kg ww (Boethling and Cooper 1985). TCP was also detected in fish near a triaryl phosphate manufacturing plant in the United States at a concentration of 2–5 mg/kg ww (Muir 1984). A TCP concentration of 40 mg/kg ww was measured in sturgeon from the Columbia River, USA in an area downstream of several metal processing plants (Lombardo and Egry 1979; cited in UK EA 2009).
In Sweden, TCP has been measured in the muscle of various fish from lakes and coastal areas of Sweden (2003–2007) at concentrations ranging from 1.8 to 110 ng/g lipid weight (lw)(Sundkvist et al. 2010).
TCP at a concentration of 45.4 ng/g lw was detected in Epinephelus corallicola collected from Manila Bay, the Philippines (UK EA 2009; Kim et al. 2011).
7.6 Other media
Few data are available on the concentration of TCP in effluent, sludge or biosolids (ECCC 2018b). TCP was measured in wastewater treatment system (WWTS) effluent in Canada at a concentration of 1.14 ng/L (Woudneh et al. 2015),and from <0.50 to 24.1 ng/L (median = 4.01 ng/L) (De Silva et al 2017) and in Austria and Denmark at concentrations ranging from non-detect to 55 ng/L in 2005 (Martinez-Carballo et al. 2007) and non-detect to 530 ng/L (detection limit (DL)= 20 ng/L), respectively (UK EA 2009). TCP was measured in one wastewater biosolids sample and one biosolids at a concentration of 97.6 ng/g and 69.0 ng/L, respectively (Woudneh et al. 2015). TCP was also measured in 3 of the 15 wastewater biosolids samples collected in Denmark at a mean concentration of 613ng/g dw (UK EA 2009).
In Japan, TCP was measured in effluent from five industrial machinery complexes at concentrations of non-detect to 560 ng/L (Cho et al. 1994). It was also measured in effluent from five WWTPs in Kitakyushu City, at concentrations ranging from 400 to 580 ng/L (DL = 60 ng/L) (Ishikawa et al. 1985c, cited in UK EA 2009).
8. Environmental fate and behaviour
8.1 Environmental distribution
TCP is expected to be released primarily via wastewater from industrial facilities using the substance as an additive flame retardant. TCP is partially removed by adsorption to biosolids in wastewater treatment systems, which in turn can be applied to agricultural soils during biosolids amendment. Level III fugacity modelling (Table 8-1) using the EQC model (2012), was applied to describe the environmental distribution of TCP following release to air, water and soil.
Results from the HYDROWIN model suggest that the rate of hydrolysis for TCP increases with increasing pH of the water (half-life of 192.6 years at pH 5, 11.58 years at pH 6, 1.17 years at pH 7, 42.87 days at pH 8, 4.287 days at pH 9, and 10.29 hours at pH 10). The half-life for the more environmentally-relevant pH of 8 (pH 8.2 in natural water of Lake Ontario (Howard and Deo 1979)) of 42.87 days (or 1029 hours) was used in this assessment for making predictions about the fate of TCP in water. Half-lives of 1.5 years and 2 years for the acidic environment at less than pH 7 were also considered, however, the results showed similar modelling fate outcome of TCP in water using the half-life of 42.87 days at pH 8.
| TCP released to: | Percentage of TCP partitioning into air | Percentage of TCP partitioning into water | Percentage of TCP partitioning into soil | Percentage of TCP partitioning into sediment |
|---|---|---|---|---|
| Air (100%) | 3.32 | 4.54 | 91.6 | 0.6 |
| Water (100%) | negligible | 88.7 | 0.1 | 11.1 |
| Soil (100%) | negligible | 0.2 | 99.8 | negligible |
a Physical and chemical properties and environmental half-lives (t1/2) of TCP in environmental media are required for modelling and are listed in Appendix A
When TCP is released to air, about 3% of the substance is expected to reside in air in the gas phase while most is expected to partition to the particulate phase in air, given this substance’s moderate log Koa. This substance has a very low predicted half-life (≤1 day) because of its reaction with hydroxyl radicals, and therefore, in the gas phase, TCP is not expected to have long-range transport potential to remote regions in air. The particulate phase is deposited to land and water as wet and dry deposition. For the amount transferred from air to soil, the majority (~92%) will remain in soil while a smaller fraction can be further transported as surface runoff to aqueous systems and when combined with atmospheric inputs, results in approximately 0.6% of the mass fraction in sediment.
Further modelling was conducted to clarify the long-range atmospheric transport potential for TCP. Model estimates of the Characteristic Travel Distance (CTD) of 222 and 363 km in air, respectively, using the Transport and Persistence Level III Model (TaPL3) (TaPL3 2003) and the OECD POPs Screening Model (OECD QSAR Tool 2012; Scheringer et al. 2009) also support that TCP has a low potential for transport in air. In addition, the estimated persistence of TCP in air by TaPL3 and OECD POPs are 38.3 days and 71 days, respectively. Results from AEROWIN suggest that about 47% of TCP released to air will be associated with the particulate phase owing to its higher log Koa value (9.59) (AEROWIN 2010). While the percentage of the substance adsorbed to aerosols as predicted by the OECD POPs Screening Model is lower (4%); both AEROWIN and the OECD POPs Screening Model indicate that particle-associated long-range transport may be a factor for TCP.
The transfer efficiency (TE) is the percentage of emission flux to air that is deposited to the surface (water and soil) in a remote region (TE % = D/E x 100, where E is the emission flux to air and D is the deposition flux to surface media in a target region). The estimated TE for TCP at 0.0167% is well below the boundary of 2.248% established based on the model’s reference substance (PCB 28) meaning that TCP is unlikely to be deposited to Earth’s surface in a remote region.
When released to surface water, the vast majority (88.7%) of TCP is expected to stay in water. With a low to moderate water solubility (0.36 mg/L), a small fraction (11.1%) of TCP is expected to adsorb to suspended solids and/or sediments. Volatilization from surface water to air is very low. Thus, loss of TCP from aqueous systems is primarily a result of sediment burial (from the natural process of sedimentation) and from degradation (ECCC 2018c and Table 8-2).
When TCP is released to soil as a result of applying biosolids to agricultural lands, it is expected to become adsorbed to soil (~100%) because of its hydrophobic nature. Evaporation from soil into air is not expected because of an extremely low vapour pressure.
8.2 Environmental persistence
On the basis of likely TCP releases and partitioning characteristics, environmental persistence is most relevant for the water, sediment and soil compartments where the majority of the substance is expected to be found. Empirical and modelled data were considered in the weight-of-evidence for TCP persistence.
Modelled predictions for TCP in air suggest a half-life < 1 day (gas phase) and or an overall persistence (Pov) of 108 days (OECD POPs model). TCP testing under longer-term, environmentally-relevant conditions to determine the degradation pathways and transformation products is lacking.
Empirical and modelled degradation data for TCP are presented in Table 8-2.
8.2.1 Empirical data for persistence
TCP can easily be hydrolyzed to dicresyl phosphate and cresol in an alkaline medium, but is stable in neutral and acidic media (IPCS 1990; van der Veen and de Boer 2012; WHO 1990). Cresol was assessed by Environment Canada and was concluded not to meet the criteria under paragraphs 64(a) or (b) or (c) of CEPA in May 2016 (Environment Canada 2016). Therefore, cresol is not discussed in this assessment.
Hydrolysis of TCP, o-TCP and p-TCP at pH 10.3 resulted in half-lives of 70 minutes, 70 minutes and 27 minutes, respectively (David and Seiber 1999a). Hydrolysis of TCP, o-TCP and p-TCP at pH 10.7 resulted in half-lives of 200 minutes, 280 minutes and 670 minutes, respectively (David and Seiber 1999a). At these basic pHs, TCP is not stable in water. Although this study did not report the experimental temperature, heating the slurries will almost certainly increase hydrolysis (David and Seiber 1999a).
A hydrolysis study conducted using Lake Ontario water found that at 21oC TCP was 100% degraded within 5 to 6 days, and a mixture of m- and p-TCP degraded rapidly (< 4 days) after a two-day lag period (Howard and Deo 1979). Owing to the initial lag phase prior to degradation, microbial degradation instead of hydrolysis was probably the dominant degradation process occurring in these samples (UK EA 2009).
Several studies (Saeger et al. 1979; Ku and Alvarez 1982; Cho et al. 1996; David and Seiber 1999a; NITE 2002) have documented the aerobic biodegradation of TCP using tests of “inherent biodegradability” and the more stringent “ready biodegradability.” Although ready biodegradability was not shown, tests did show that TCP was inherently biodegradable (Saeger et al. 1979; US EPA 2010).
Muir et al. (1985) studied degradation of TCP in water and sediment of an artificial pond system, and in natural sediments. The half-life of m-TCP in natural river sediments was 10.1 days at 25°C. Using the Boethling et al. (1995) ratio of biodegradation in water to that in soil and sediment (1:1:4) yields half-lives of 2.5 days for both water and soil. These data are generally consistent with the half-life reported from other empirical studies, i.e. TCP has a short half-life in water, soil and sediment.
8.2.2 Modelling of persistence
A QSAR-based weight-of-evidence approach was also applied using the degradation models shown in Table 8-2. Given the ecological importance of the water, soil and sediment compartments and the fact that TCP is expected to partition mainly to these compartments, it is considered reasonable and relevant to examine biodegradation in water, soil and sediment.
The probability of biodegradration using the TOPKAT model (2004) suggests that TCP is 100% biodegradable within 28 days. Results are within the optimum prediction space (OPS), the structural domain of the model. The CATALOGIC model (2012) predicts that 72% of TCP biodegraded under aerobic conditions within 28 days reporting an ultimate half-life of 15.4 days. Both TOPKAT and CATALOGIC suggest moderate and high rates of mineralization from biodegradation consistent with empirical data. Biodegradation was predicted using BIOWIN 3 and BIOWIN 4 Expert-Survey models. These models also suggest that TCP is not persistent in water although BIOWIN 5 MITI Linear Probability, and BIOWIN 6 MITI Non-Linear Probability suggest otherwise. In addition, results from the HYDROWIN model suggest that the rate of hydrolysis for TCP increases with increasing pH of the water (11.58 years at pH 6, 1.17 years at pH 7, 42.87 days at pH 8 and 10.29 hours at pH 10) (Table 8-2).
In summary, results of empirical and modelled biodegradation data suggest that the half-life in water is likely in the order of several hours to less than 40 days. Applying a half-life extrapolation procedureusing a ratio of 1:1:4 for water:soil: sediment, it is expected that TCP will break down quickly in soil and sediment and not present long-term exposure in these media (Boething et al. 1995). Furthermore, results from empirical and modelled hydrolysis data suggest a faster degradation rate at an increasing pH in water, soil and sediment.
| Medium | Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|---|
| Air | Atmospheric oxidation | AOPWIN 2010a,b | t½ = 0.78 days | ≤ 2 |
| Air | Ozone reaction | AOPWIN 2010b | NAc | NA |
| Water | Hydrolysis | HYDROWIN 2010b | 11.58 years (pH 6) 1.17 years (pH 7) 42.87 days (pH 8) | NA |
| Primary Biodegradation | Biodegradation (aerobic) | BIOWIN 4.10b Sub-model 4 | Classification value of 3.58 “biodegrades fast” | < 182 |
| Ultimate Biodegradation | Biodegradation (aerobic) | BIOWIN 4.10b Sub-model 3 | Classification value of 2.31 “biodegrades fast” | < 182 |
Ultimate Biodegradation |
Biodegradation (aerobic) | BIOWIN 4.10b Sub-model 5: MITI linear probability | Probability value of -0.0061; “biodegrades very slowly” | > 182 |
| Ultimate Biodegradation | Biodegradation (aerobic) | BIOWIN 4.10b Sub-model 6: MITI non-linear probability | Probability value of 0.0098; “biodegrades very slowly” | > 182 |
| Ultimate Biodegradation | Biodegradation (aerobic) | TOPKAT 2004 (v. 6.1) Probability | 1.000 “biodegrades fast” | < 182 |
| Ultimate Biodegradation | Biodegradation (aerobic) | CATALOGIC 2012 % BOD (biological oxygen demand) | % BOD = 72 “biodegrades fast” Primary half-life: 11.613 days Ultimate half-life: 15.434 days | < 182 |
Abbreviations: NA, not applicable; t1/2, half-life; %, percent.
a On the basis of a day length of 12 hours, a hydroxyl radical concentration of 1.5×106 molecules/cm3 (12-hour annual average), and a system temperature of 25°C.
b EPI Suite (2012)
8.3 Bioaccumulation
The discussion on the potential for bioaccumulation examines several potential parameters, including properties of the substance (i.e. log Kow, log Koa, molecular size and cross-sectional diameters), bioconcentration factor (BCF), biomagnification factor (BMF), trophic magnification factor (TMF) and bioaccumulation factor (BAF). The potential derivation and role of metabolic biotransformation rate constants in determining bioaccumulation potential is also examined.
The empirical log Kow value of 5.11 for TCP suggests some potential to bioaccumulate or biomagnify in biota. Strong sorption to solids as indicated by a moderate log Koc suggests a potential for bound residues in the environment reducing the bioavailable fraction of TCP. There is uncertainty with estimates of the partition coefficients, but it is reasonable to assume that these will be high values, based on the chemical structure of TCP alone.
The bioconcentration of TCP has been examined in a number of studies on fish (ECCC 2018c). Low to moderate BCFs were reported in these studies. No empirical studies on BMFs, TMFs, or BAFs are available in the literature at the time of this assessment. Because publicly available TMF models are limited, TMF cannot currently be reliably modelled. Therefore, modelled bioconcentration and bioaccumulation data are considered to provide the best possible weight-of-evidence for the bioaccumulation potential of TCP.
8.3.1 Empirically determined bioaccumulation
8.3.1.1 Bioconcentration factor (BCF)
A few studies examining the bioconcentration of TCP on fish were discussed in this section and listed in Table 8-3. A detailed listing of the available bioconcentration of TCP (CAS RN 1330-78-5) on fish from the open literature is available in supporting documentation (ECCC 2018c).
In the first, a commercial product containing TCP was tested for uptake into Common Bleak (Alburnus alburnus) in natural brackish water (7% salinity) at 10°C for 14 days (Bengtsson et al. 1986). The steady state BCF was 800 L/kg. TCP was rapidly eliminated from the fish, with a depuration half-life of four days or less, and almost complete elimination within 14 days (Bengtsson et al. 1986). In the second study, the bioconcentration of TCP in Fathead Minnows (Pimephales promelas) was studied at 25°C over 32 days (Veith et al. 1979). A BCF of 165 L/kg was determined based on total radioactivity.
BCFs also have been measured in Rainbow Trout (Oncorhynchus mykiss) and Fathead Minnow (Pimephales promelas) for the p- and m-TCP isomers (Muir et al. 1983) (ECCC 2018c). Owing to the short test duration (24 hours), steady state was not reached for Rainbow Trout. Estimated BCFs based on total radioactivity varied from 165 to 2768 L/kg according to the method used to calculate or model the value (ECCC 2018c). A 4-week BCF study using Bluegill Sunfish (Lepomis macrochirus) for p-TCP determined a BCF of 1589 L/kg based on total radioactivity (Sitthichaikasem 1978). However, because of the possible uptake of TCP from food as well as water, this value may be more relevant for bioaccumulation (UK EA 2009).
| CAS RN | Test organism | BCF | Kinetic and/or steady-state value (L/kg)a | Reference |
|---|---|---|---|---|
| 1330-78-5 | Bleak, Alburnus alburnus |
BCF | 800 (14 d, 50 µg/L of the triaryl phosphate product) | Bengtsson et al. 1986 |
| 1330-78-5 | Fathead Minnow, Pimephales promelas | BCF | 165 (32 d; mean concentration 31.6 μg/L) | Veith et al. 1979 |
| 78-32-0 | Fathead Minnow, Pimephales promelas | BCF | 2199 ± 227 (initial rate method); 928 ± 8 (static test method); 588 ± 129 (BIOFAC model) (24 hr, 5 µg/L nominal) |
Muir et al. 1983 |
| 78-32-0 | Rainbow Trout, Oncorhynchus mykiss |
BCF | 2768 ± 641 (initial rate method); 1420 ± 42 (static test method); 1466 ± 138 (BIOFAC model); (24 hr, 5 µg/L nominal) *Steady-state concentration was not reached in 24 hours. |
Muir et al. 1983 |
| 563-04-2 | Fathead Minnow, Pimephales promelas | BCF | 1653 ± 232 (initial rate method); 596 ± 103 (static test method); 385 ± 92 (BIOFAC model) (24 hr, 5 µg/L nominal) |
Muir et al. 1983 |
| 563-04-2 | Rainbow Trout, Oncorhynchus mykiss |
BCF | 1162 ± 313 (initial rate method); 784 ± 82; (static test method); 1102 ± 137 (BIOFAC model); (24 hr, 5 µg/L nominal) *Steady-state concentration was not reached in 24 hours. |
Muir et al. 1983 |
| 78-32-0 | Bluegill Sunfish, Lepomis macrochirus | BCF | 1589 (4 wk, NS) | UK EA 2009 (Sitthichaikasem 1978) |
Abbreviations: BAF, bioaccumulation factor; BCF, bioconcentration factor; NA, not applicable; NS, not specified.
a Values in parentheses represent the duration and/or test concentrations at which the BAFs or BCFs were derived.
8.3.1.2 Biomagnification factor (BMF) and trophic magnification factor (TMF)
Biomagnification describes the process in which the concentration of a chemical in an organism reaches a level that is higher than that in the organism’s diet because of dietary absorption (Gobas and Morrison 2000). A BMF exceeding 1 indicates that biomagnification is potentially occurring. BMF data may be considered indicators of the potential for uptake and accumulation in biota and are considered in the overall weight-of-evidence. The TMF is a measure of the biomagnification potential of a substance within a studied food web under field conditions. It is estimated by correlating the normalized substance concentrations in biota at different trophic levels.
No experimental BMF or TMF studies were identified for TCP.
8.3.1.3 Bioaccumulation factor (BAF)
Bioaccumulation factors are measured under field conditions as the ratio of the whole body burden of chemical taken up from all exposures to that of the ambient water concentrations. Measures of BAF are a preferred metric for assessing the bioaccumulation potential of substances because it incorporates all chemical exposures including the diet, which predominates for substances with log Kow > ~4.0 (Arnot and Gobas 2003a).
One empirical study reported a rough estimation of maximum accumulation factor of 0.08–0.13% w/w, from food in Common Minnows (Phoxinus phoxinus) for TCP (Bengtsson et al. 1986; ECCC 2018c). No acceptable empirical BAF values were available for TCP at the time of this analysis and therefore metabolic biotransformation corrected kinetic mass-balance modelling was used to fill this data gap.
8.3.2 Modelling bioconcentration and bioaccumulation
Environment and Climate Change Canada estimated the BCF and BAF of TCP using both structure-based models, QSARS, and a three-trophic-level kinetic mass-balance model (Arnot and Gobas 2003b). All estimates of BCF and BAF, except sub-model 1 of the BCFBAF model in EPIWIN v4.0, were corrected for metabolism because it represents a fundamental elimination pathway for many chemicals like TCP. This correction was performed by deriving metabolic biotransformation rate constants (kM) using available empirical BCF or BMF study information or using a structure-based QSAR method.
The metabolic rate constant is a very sensitive input parameter for bioaccumulation modelling and can be highly variable (Arnot et al. 2008a, Arnot et al. 2008b). An empirical kM for TCP (CAS RN 1330-78-5) for a 10 g fish at 15oC of 0.20/ d has been estimated (Arnot et al. 2008a, Arnot et al. 2008b) from a reliable quality BCF study for Common Bleak (Alburnus alburnus) (Bengtsson et al. 1986). The metabolic rate constant for TCP was also estimated as 0.015 /d using the kM (Q)SAR sub-model in BCFBAF model v3.10 2010 in EPIWIN v4.0 2012 (based on Arnot et al. 2009), and the QSAR kM for a 10 g fish at 15oC. The empirical and QSAR estimates are in good agreement with each other and these rates are regarded as relatively “fast” (greater than 0.1) (Arnot and Gobas 2006; Nichols et al. 2007). The kM value of 0.15 /d was then normalized to model the BCF and BAF of a middle-trophic-level fish using a three-trophic-level modification of the fish bioaccumulation mass-balance model from Arnot and Gobas (2003a). The results of the BCF and BAF modelling for TCP are 1589 and 2043 L/kg wet weight, respectively.
The BCFmax model with Mitigating Factors (Dimitrov et al. 2005) was also applied and predicted a corrected BCF of 1251 (log BCF of 3.0974), correcting for the mitigating effects of acids, metabolic biotransformation, phenols, size, and water solubility. It predicts a metabolic biotransformation rate constant of 0.05 /day. However, these results are considered with caution since the substance is considered outside of the model’s structural domain.
With an empirical log Kow of 5.11, the predicted bioavailable fraction of TCP in the water column according to mass-balance fish models (BAF-QSAR v. 1.2) is 97%, which suggests that almost all of the chemical present in typical surface waters is bioavailable for chemical uptake at the gill surface.
In summary, with empirical BCFs ranging from 165 L/kg and 800 L/kg at TCP concentrations of 0.0316 mg/L and 0.050 mg/L (both less than the empirical water solubility value of 0.36 mg/L), respectively, the available empirical fish bioaccumulation studies collectively indicate a low bioconcentration potential. Both modelled BCF (1589 L/kg) and BAF (2043 L/kg) also support that TCP does not have a high bioaccumulation potential.
8.3.3 Bioaccumulation in plants
The uptake of p-TCP (CAS RN 78-32-0) from soil into plants was measured only in soybeans (Casterline et al. 1985). Seeds were planted in 2 cm of untreated soil overtop of the TCP-treated soil (10 mg/kg). The fresh weight BAF (plant shoot) was 0.17, where 1.72 µg/g and 10.14 µg/g p-TCP concentration were detected in shoot and soil, respectively (about 1:6 in shoot/soil ratio). The outcome of this study is uncertain because it was not a GLP study, it used sterilized soil, and the nominal concentrations were not verified.
8.4 Summary of environmental fate
TCP is expected to be predominantly released from industrial sources via wastewater. Once released to water, a high proportion (88.7%) of TCP is expected to remain in water. A strong tendency to sorb to the solid phase in various media (including particulate matter suspended in air) means that this chemical will reside in biosolids, soil and suspended air particles and be transferred to soil from dry deposition and application of biosolids to agricultural lands. In summary, high sorption characteristics indicate that TCP will reside in water, biosolids, sediments and soil.
9. Potential to cause ecological harm
9.1 Ecological effects assessment
Empirical ecotoxicity data for TCP was considered for assessing the ecological effects of TCP. TCP is expected to be predominantly released from industrial sources via wastewater. Exposure to aquatic organisms may be expected because, once released to water, a high proportion (88.7%) of TCP is expected to remain in water. A strong tendency to sorb to the solid phase in various media (including suspended air particles) indicates that TCP will reside in water, biosolids, sediments and soil. There are a number of available empirical aquatic toxicity data for TCP. Modelled data were used to support the empirical data. There are no available sediment toxicity data for TCP at the time of this assessment, and therefore, the focus of the assessment is for aquatic species. However, available terrestrial toxicity data for TCP are also considered in this assessment which are relevant for exposures to soil-dwelling and piscivorous mammals.
As detailed below, an empirical 35-day chronic study for early life stage of Three-spined Sticklebeak (Gasterosteus aculeatus) was selected for the derivation of the predicted no-effect concentration (PNEC) for water. When compared to empirical studies, the results from the QSAR toxicity models are in the same order of magnitude as empirical results. A soil exposure scenario was also developed to reflect potential concentrations of TCP in agricultural soil resulting from the possible application of biosolids originating from a wastewater treatment system receiving effluent from industrial activities. Soil concentrations and potential uptake rates and concentrations in a small mammal (i.e. shrew) were estimated using a fugacity-based model that involves equilibrium partitioning principles to estimate the overall fate of the substance in the soil and exposure to soil biota (BASL4 2011). An empirical study of a two-year oral feed study with male rats was used as critical toxicity value (CTV) for wildlife.
9.1.1 Empirical studies in water
A number of studies are available characterizing the toxicity of TCP to algae. One study on Scenedesmus pannonicus determined a chronic 96-hr EC50 and no observed effect concentration (NOEC) for TCP for growth of 1.5 and 0.32 mg/L, respectively (UK EA 2009). Additional chronic, 72-hr toxicity data for TCP for the green alga Pseudokirchneriella subcapitata were available from NITE (2008) and ECHA (c2007-2013). The lowest NOEC for growth rate was 0.088 mg/L. Other NOECs from these studies for growth rate, biomass and cell number were 4.7, >2.5, and 2.4 mg/L, respectively
Aquatic toxicity data for invertebrates are also available for TCP. A 48-hr unpublished acute study with Daphnia magna reported an EC50 for mortality/immobility of 0.27 mg/L and a NOEC of 0.1 mg/L. Two other 48-hr EC50s (Adema et al. 1983; ECHA c2007-2013) were much higher (5.6 mg/L and 146 mg/L).
Two chronic toxicity studies on Daphnia magna were identified in the open literature (Adema et al. 1981, 1983). A 21-d EC50 was reported to be between 0.1 and 0.3 mg/L (Adema et al. 1981) and a 21-d NOEC (mortality, reproduction) was determined to be 0.1 mg/L (Adema et al. 1983).
Van den Dikkenberg et al. reported embryo-larval studies on four fish species: Three-spined Stickleback (Gasterosteus aculeatus); Zebrafish (Brachydanio rerio); Medaka (Oryzias latipes); and Flagfish (Jordanella floridae). Among them, the NOEC and EC50 (early life stage (after 4-week-old fish stage) mortality, sublethal effects excluding growth), and the LC50, for Three-spined Stickleback exposed for 35 days were 0.001 mg/L, 0.0013 mg/L and 0.0017 mg/L, respectively (Van den Dikkenberg et al. 1989). The NOECs for growth and sublethal effects on the embryonic stage were lower at 0.00032 mg/L and 0.0032 mg/L, respectively (Van den Dikkenberg et al. 1989).
The NOECs from a six-week embryo-larval study on Zebrafish, Medaka and Flagfish were greater (0.0056 mg/L, 0.01 mg/L and 0.01 mg/L, respectively) than those for Three-spined Stickleback (Adema et al. 1983). The NOEC from a four-week chronic study of guppies was significantly greater at 1 mg/L (Adema et al. 1983).
Results from the ECOSAR model (v.1.00) are available in ECCC 2018d. These are generally consistent with the empirical data and support the calculated PNEC used for the risk analysis (see below). Although the log Kow of TCP (5.11) is greater than the model’s cut-off for acute toxicity estimation (~5), it is close, and thus, the ECOSAR predicted fish 96h-LC50 and Daphnia LC50 values (0.057 – 0.165 mg/L) are nevertheless considered but not used to estimate a PNEC for the risk analysis. Predictions for chronic toxicity, however, were below the model’s cut-off (log Kow ~8). These results ranged from 0.005 mg/L to 0.04 mg/L.
The lowest EC50 obtained from the more reliable studies is a 35-day EC50 of 0.0013 mg/L for early life stage (after 4-week-old fish stage) mortality and sublethal effects of embryo-larval study with Gasterosteus aculeatus (Three-spined Stickleback) (Van den Dikkenberg et al. 1989). This value is selected as the critical toxicity value (CTV) for pelagic organisms. The selection of this CTV is considered reasonable in comparison with modelled results from ECOSAR (0.005 mg/L chronic lethal toxicity; ECCC 2018d).
Overall, there is high confidence in the dataset on aquatic toxicity with data on several endpoints and organisms. To calculate the PNEC, an application factor of 3 is selected to account for differences in species sensitivity. Although there are a lot of data, the chronic toxicity data spans only three taxa: algae, vertebrates and invertebrates. The resulting PNEC is thus 0.00043 mg/L.
9.1.2 Empirical studies in sediment
There are no available data characterizing the toxicity of TCP to benthic organisms.
9.1.3 Empirical studies for soil organisms and terrestrial wildlife
One study is available using a commercial product (Durad 310M) with TCP present at <5% w/w. This study resulted in an LC50 (seedling emergence) of >100 mg/kg. This study cannot be interpreted to characterize the toxicity of TCP to plants given the low purity of the test substance.
There are also a few studies characterizing the effects to insects from contact with TCP (WHO 1990). These data, however, are not relevant for the derivation of a PNEC for terrestrial organisms.
A PNEC for small soil-dwelling mammals is derived from a review of mammalian toxicity. A thorough review of mammalian toxicity is presented in the human health component of this assessment and only the key study considered for the derivation of the toxicity reference value (TRV, considered equivalent to a PNEC) is summarized below.
Toxicity tests for TCP have been performed using 95 female rats (F344/N; up to 15 females per group) in a two-year oral feed study, also as described further in section 10.2.3 (NTP 1994). For this study, dietary levels of 0, 75, 150, and 300 mg/kg TCP were estimated to deliver average daily doses of 0, 4, 7, or 15 mg/kg to females. Over this two-year study, the body weights of female rats started with a mean of 91 g (91, 91, 92, 90 g, respectively, for four different concentrations of TCP in feeds) in the first week of the study, and ended with a mean of 320 g (315, 320, 332, 313 g, respectively, for four different concentrations of TCP in feeds) in the 104th week of the study. Endpoints of NOAEL and LOAEL for cytoplasmic vacuolization of the adrenal cortex were reported at 4 mg/kg/day and 7 mg/kg/day, respectively. This study is selected for the derivation of the toxicity reference value (TRV). The CTV of 5.3 mg/kg-bw/day is therefore calculated using a geometric mean of the NOAEL and the LOAEL values.
The rat toxicity endpoints (NOAEL of 4 mg/kg/day and LOAEL of 7 mg/kg/day) from this study and a body weight of 10 grams, derived from BASL 4, were used as input to estimate TRV of TCP for shrew. An assessment factor of 10 was applied to account for extrapolation from laboratory to field conditions. The resulting TRV of TCP for shrew is 1.259 mg/kg-bw/day (Appendix B).
The same rat toxicity endpoints used to calculate the TRV for shrew were also used to calculate TRVs of TCP for mink and river otter. The body weights of 1.08 kg and 7.98 kg were used to derive their corresponding TRVs. An assessment factor of 10 was applied to account for extrapolation from laboratory to field conditions. The resulting predicted TRVs of TCP for mink and river otter are 0.390 mg/kg-bw/day and 0.237 mg/kg-w/day, respectively (Appendix B).
9.2 Ecological exposure assessment
9.2.1 Industrial release
Limited data concerning concentrations of TCP in water in Canada have been identified. Therefore, environmental concentrations have been estimated from available information, including estimated substance quantities, estimated release rates, and characteristics of the receiving environment. Environmental concentrations have been estimated for industrial release scenarios, as described in the following sections.
9.2.1.1 Exposure scenarios and predicted environmental concentrations
Aquatic exposure:
Aquatic exposure to TCP is expected if the substance is released from industrial activities either to a wastewater system or directly to a receiving surface water body. The concentration of the substance in the receiving water near the discharge point of the wastewater system is used as the predicted environmental concentration (PEC) in evaluating the aquatic risk of the substance. It can be calculated using the equation:
Cwater-ind = (1000 x Q x L x (1-R)) /(N x F x D)
Where:
Cwater-ind: aquatic concentration resulting from industrial releases, mg/L
Q: total substance quantity used annually at an industrial site, kg/yr
L: loss to wastewater, fraction
R: wastewater system removal rate, fraction
N: number of annual release days, d/yr
F: wastewater system effluent flow, m3/d
D: receiving water dilution factor, dimensionless
These parameters are described in detail in ECCC 2018e.
As TCP is used by industrial facilities and is expected to be released to water, conservative aquatic industrial release scenarios were developed to cover a range of different potential industrial activities in Canada. For TCP, the relevant scenario includes blending of the substance at industrial facilities. The blending scenario estimates releases of TCP which is blended into various applications.
As TCP is imported in bulk in a pure liquid form or part of a liquid mixture which may generate residues in transport containers, container cleaning operations may lead to environmental releases of these substances. Although environmental concentrations of TCP resulting from these releases may be high, these releases would likely be episodic in nature and probably of short duration. Given these considerations and the current data gaps associated with container cleaning operations and practices, a quantitative exposure characterization was not developed for these releases.
Table 9-1 presents the range of inputs used to estimate resulting aquatic concentrations close to the industrial points of discharge. On the basis of these assumptions, these industrial scenarios yield total PECs of 7.3 x 10-8 to 2.7 x 10-6 mg/L (Table 9-2). These aquatic PEC values represent the total TCP concentrations (dissolved and particle associated) in the receiving water near the point of discharge at each site. It is noted that these calculated PECs are similar to water concentrations of 2.5x10-6 to 2.2x10-5 mg/L (11–22 ng/L and 2.5–10 ng/L) detected in Lake Ontario, the St. Lawrence River and Lake Erie in Canada in 2010 and 2011 studies.
| Input | Value | Justification and reference |
|---|---|---|
| Yearly quantity used at site (kg/yr) | <10 000 | As reported in ECCC (2013-2014) |
| Loss to wastewater (%) | 0.25 to 1.0 | Standard assumption based on OECD (2009) |
| Wastewater system removal efficiency (%) | 74.3 to 85.8 | ASTreat 1.0 model prediction for off-site primary level treatment, secondary level treatment |
| Number of annual release days (days) | 250 to 350 | Based on NPRI data, site specific information or professional assumption (NPRI 2011 and Environment and Climate Change Canada internal database) |
| Wastewater system effluent flow (m3/d) | 15 000 to 22 400 000 |
Site-specific data for wastewater treatment system |
| Dilution factor (–) | Up to 10 | Site-specific wastewater treatment system flow rate/ receiving environment flow rate. When a dilution factor was greater than 10, a maximum default value of 10 was used. |
| Use/Sector | PEC water (mg/L) | PEC sediment (4% OC) (mg/kg dw) | PEC soil (2% OC) (mg/kg dw) |
|---|---|---|---|
| Blending | 7.27 x 10-8 to 2.70 x 10-6 | 0.00001 to 0.00036 | 0.00001 to 0.00030 |
To estimate the concentration of TCP in the bottom sediment, an equilibrium sediment-water partition approach (ECHA 2010) was used. This involved estimating the substance’s concentration in the aqueous phase (dissolved) in the overlying water from its total concentration, according to studies by Gobas et al. (2003) and Gobas (2010). Then the substance’s concentration in bottom sediment from its concentration in the aqueous phase of the overlying water was estimated based on an equilibrium partitioning assumption between bottom sediment and overlying water described by the US EPA’s National Center for Environmental Assessment (US EPA 2003). At equilibrium, the PEC in bottom sediment can linearly correlate with the concentration in the aqueous phase of the overlying water. Sediment exposure scenarios were developed as an extension of the industrial aquatic release scenarios described above to determine equilibrium sediment PECs, standardized to 4% organic carbon (a typical organic carbon content in bottom sediment for rivers and lakes). The resulting sediment PEC value of TCP ranges from 0.00001 to 0.00036 mg/kg dw.
Soil exposure:
In the absence of suitable data, a soil exposure scenario was developed to reflect potential agricultural soil concentrations resulting from the possible application of biosolids originating from a wastewater treatment system. Soil concentrations and exposure to a small mammal (i.e. shrew or vole) were estimated using a fugacity-based model that involves equilibrium partitioning principles to estimate the overall fate of the substance (BASL4 2011). This analysis uses the maximum TCP concentration of 1.15 mg/kg measured in a biosolids sample taken from a Canadian WWTS (ECCC 2016).
A total daily intake (TDI) of 0.1185 mg/kg-bw/day (standardized to 2%OC) for TCP is thus estimated for shrew based on a soil food chain pathway. This value is considered conservative because the BASL4 model does not consider metabolism in its estimate.
Wildlife Exposure:
A wildlife TDI was derived for Mink (Mustela vison) and River Otter (Lontra canadensis) consuming fish following the approach of the US EPA (1993). In calculating TDI, the BAF of 2043 (log BAF of 3.31) (see Section 9.1.4 Empirical Studies for the Terrestrial Compartment (Wildlife)) was used in conjunction with the maximum water PEC value of 0.0000027 mg/L resulting in estimated TDIs of 0.0010 and 0.0008 mg/kg-bw/day for the Mink and River Otter, respectively.
9.2.2 Consumer or commercial release
Although TCP can be found in commercial products or products available to consumers, it is expected that these releases will be minimal. Additive use of TCP in products suggests diffuse emissions may occur from commercial products or products available to consumers, and although there are uncertainties, the rate is also expected to be very low in comparison to industrial point sources during incorporation of the substance into products. Emissions from industrial scenarios presented in this assessment would result in much higher environmental concentrations. Given that products made with TCP will generally not be in contact with water on a regular basis, leaching to water is likely to be minimal.
In the absence of information on the leaching of TCP from products, the UK EA (2009) estimated a potential of release of 0.25% over the lifetime of the TCP product if the product is for indoor use and 7.25% if it is for outside use. The potential release of OFRs from plastics during their service life is estimated at 0.05% to water if the substance is for indoor use or 0.16% over lifetime for outdoor use (OECD 2009). The large majority of products would be enclosed or used indoors; therefore, the release rate of 0.05% is most applicable and may likely be an overestimate since contact with water is not expected.
Therefore, the extreme worst-case scenario for the diffuse release of TCP throughout Canada (via WWTS and disperse release directly to the environment), using the indoor release rate of 0.05% over service life information from OECD 2009, was estimated at 10.5 kg. This scenario includes a number of assumptions: the maximum values from each range of import (1 000 kg for commercial products or products available to consumers; 10 000 kg for formulation; and 10 000 kg for neat substances); complete use of TCP in products; low exposure to water over the service lifetime and indoor use. This result suggests that significant release of TCP products is unlikely. The scenario result is considered to be highly uncertain.
Assessments of TCP in household dust and in dust from other microenvironments in Canada and abroad are discussed in the section on Exposure Assessment for Human Health of this assessment (10.1.1 Environmental Media and Food). TCP exposure from products available to consumers is described in the section on Exposure Assessment for Human Health of this assessment (10.1.2 Products Available to Consumers).
9.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine various sources of information and develop conclusions on the basis of a weight-of-evidence approach (Appendix C) and using precaution as required under CEPA. Lines of evidence considered include results from a conservative risk quotient calculation, as well as information on persistence, bioaccumulation, inherent or ecological toxicity, sources and fate of the substance and presence and distribution in the environment.
9.3.1 Risk Quotient Analysis
Risk quotient analysis was performed for aquatic, soil and wildlife scenarios (Table 9-3; Appendix B) to determine whether there is potential risk of harm in the vicinity resulting from industrial releases in Canada.
9.3.1.1 Water
The site-specific industrial release scenarios presented above yielded aquatic PEC of 7.27 x 10-8 to 2.70 x 10-6 mg/L. These PEC values represent the level of exposure in the receiving water near the point of the discharge of the wastewater treatment system at each site. A PNEC of 0.00043 mg/L was derived from the 35-day EC50 of 0.0013 mg/L (early life stage mortality) for Gasterosteus aculeatus (Three-spined Stickleback) (van den Dikkenberg et al. 1989) (see 9.1.1 Empirical studies in Water under the Ecological Effects Assessment section). The resulting risk quotients (PEC/PNEC) range from 0.0002 to 0.006 (Appendix B). Therefore, harm to pelagic organisms is unlikely at these sites.
9.3.1.2 Soil
BASL4 was employed to estimate exposure (TDI) to shrew via the soil food chain pathway. The estimated TDI of 0.1185 mg/kg-bw/day (see Section 9.2.1.1) is compared with the TRV of 1.259 mg/kg-bw/day (see Section 9.1.3). On the basis of these results, a risk quotient of 0.094 suggests that there is little concern for harm to soil organisms resulting from potential TCP exposure through a soil food chain.
9.3.1.3 Wildlife
TDIs for wildlife piscivores were estimated following the approach of US EPA (1993). In calculating TDI, the BAF of 2043 (see Section 8.3.2) was used in conjunction with the maximum water PEC value of 0.0000027 mg/L resulting in estimated TDIs of 0.0010 and 0.0008 mg/kg-bw/day for the Mink and River Otter, respectively. The derived TRVs for Mink and River Otter calculated in Section 9.1.3 are 0.390 and 0.237 mg/kg-bw/day, respectively. The resulting risk quotients (TDI/TRV) are 0.0026 (Mink) and 0.0032 (River Otter) (Appendix B). Therefore even with conservative assumptions, current use of TCP in Canada is unlikely to exceed the Mink and River Otter threshold level for effects.
| Media | Scenario | PNEC/TRV | PEC/TDI | RQ |
|---|---|---|---|---|
| Water | Industrial release to water | 0.00043 mg/L | 7.27 x 10-8 to 2.70 x 10-6 mg/L | 0.0002 to 0.006 |
| Soil | Biosolids application to soil | 1.259 mg/kg-bw/day (TRV) | 0.1185 mg/kg-bw/day (TDI) | 0.094 |
| Wildlife | Piscivore TDI (mink/fish) | 0.39 (mink) and 0.237 (otter) mg/kg- bw /day | 0.0010 (mink) and 0.0008 (otter) mg/kg- bw /day | 0.0026 (mink) 0.0032 (otter) |
Abbreviations: PNEC, predicted no-effect concentration; TRV, toxicity reference value; PEC, predicted environmental concentration; TDI, total daily intake; RQ, risk quotient.
9.3.2 Consideration of Lines of Evidence and Conclusion
The low import volumes of TCP into Canada along with information on its uses indicate low potential for widespread release into the Canadian environment. TCP has been detected at low levels in samples of air, water, sediment, soil and biota found near sources. Therefore, the main concern for TCP is for near-field exposure.
The empirical log Kow value of 5.11, the fast rate of metabolic biotransformation (kM) and the low to moderate BCF and BAF for TCP indicates a limited potential to bioaccumulate or biomagnify in biota. Weak sorption to solids as indicated by a relatively low log Koc and the moderate water solubility (0.36 mg/L) suggests a limited potential for bound residues in the environment increasing the bioavailable fraction of TCP in water. TCP is expected to have low potential for persistence in air, water, sediment and soil and is not likely to present long-term exposures in these media (Section 8.2 Environmental Persistence). TCP is not expected to have long-range transport potential in the air and is unlikely to be deposited to the earth’s surface in significant quantities in remote regions.
The site-specific industrial scenarios provided a level of exposure in the receiving water near the point of discharge of the wastewater treatment system at each site. The risk quotients showed that harm to pelagic organisms is unlikely at these sites. The importance of releases from products are an area of uncertainty due to the absence of data to inform accurate quantitation of environmental exposure because of the leaching of additive flame retardants from manufactured items; however, it is expected that releases to the environment via this route are minimal and diffuse.
TCP has a predicted short residence time in soil so the chemical is not expected to build up over time. Therefore, at potential current levels of use and based on low persistence and limited bioconcentration and bioaccumulation, TCP is not expected to have food chain effects and is not expected to result in harm to small soil-dwelling mammals. Even with conservative assumptions, current use of TCP in Canada is also unlikely to involve risk of harm to wildlife-consuming fish.
Considering all lines of evidence presented in this screening assessment, TCP does not have the potential to cause ecological harm in Canada.
9.3.3 Uncertainties in evaluation of ecological risk
The assessment recognizes that there is limited information characterizing potential releases from commercial products or products available to consumers in use and during dismantling or disposal at the end of their service life. While most landfills are expected to collect and treat their leachate in Canada, no Canadian TCP landfill leachate data have been reported to date which could help interpret end-of-life releases. However, on the basis of available information on low vapour pressure for TCP, releases are considered minimal. Environmental release of the substance from plastic polymers via leaching is considered possible, albeit low. Furthermore, many products identified with TCP will not be in contact with water on a regular basis, e.g. electronics. In this assessment, the low estimated extreme worst-case scenario for TCP release suggests that products of TCP are not likely to be a significant path of release. On the basis of available information, it is assumed that major TCP pathways of release from products in service are covered under the current industrial release scenarios. Generally, there is moderate confidence in the TCP exposure scenarios.
Finally, this assessment recognizes that there are information gaps on the toxicity of TCP to sediment and soil. However, TCP is not used in high quantities in Canada, and releases are likely low. Moreover, on the basis of the analysis in this assessment, most exposure would be to pelagic organisms and, thus, the risk assessment for water is assumed to be more relevant than potential risks for sediment-dwelling organisms. In addition, TCP is expected to have low potential for persistence in air, water, sediment and soil and is not likely to present long-term exposures to organisms via these media (Section 8.2 Environmental Persistence).
10. Potential to cause harm to human health
10.1 Exposure assessment
10.1.1 Environmental media and food
TCP has been monitored in various environmental compartments in Canada and elsewhere (see Section 7). When released to air, TCP is expected to degrade rapidly (t1/2 <1 day) or to partition to the particulate phase in air. Due to its moderate water solubility, when released to surface water, the majority of TCP is expected to stay in water and a small fraction is expected to adsorb to suspended solids and eventually sink to sediment. Volatilization from surface water to air is very low. When TCP is released to soil as a result of applying biosolids to agricultural lands, the mass fraction is expected to become adsorbed to soil (~100%) due to its hydrophobic nature. TCP is also expected to be stable in soil and resistant to degradation, and thus the loss process in soil will also mainly be driven by soil burial or surface runoff.
Upper-bounding estimates of daily intake of TCP from environmental media for the general population of Canada are presented in Appendix D. For most age groups, the main contribution to the estimated daily intake was from the ingestion of dust, followed by food. Exposure intakes from air and water were very low and considered negligible. The highest estimate of daily intake was derived for breastfed infants (0-6 months), with an estimated intake of 0.10 ug/kg-bw/d, predominantly from dust and breast milk.
10.1.1.1 Ambient air
TCP concentrations in ambient air in Canada were not identified (see Section 7.1). TCP was monitored in outdoor air samples collected in the U.S. in the late 1970s at a TCP production site in West Virginia, where concentrations ranged from 0.01 to 2 ng/m3 (Boethling and Cooper 1985; MRI 1979). In a recent study out of Finland, TCP was not detected (reported as <0.0007 ng/m3, a semi-quantitative value based on another organophosphate flame retardant) in a sample of air collected in July 2004 from a rural area in Northern Finland (Marklund et al. 2005). TCP was previously monitored in Japanese outdoor air studies, with TCP being detected in a limited number of samples (3 out of 19) collected in 1974 up to 70.3 ng/m3 from heavily-industrialized cities (IPCS 1990). TCP may be present in industrialized cities due to vehicular exhaust. o-TCP and m-TCP were found in exhaust gases from motor bikes and cars, where concentrations were approximately the same for both isomers in exhaust from motor bikes (150-300 ng/m3) and the proportion (o-TCP/m-TCP) was the same before and after combustion. The levels were somewhat lower in exhaust from cars (0.14 μg/m3) (Takimoto et al. 1999). Two more recent studies, however, reported lower concentrations of TCP, ranging from non-detected to 21.4 ng/m3 (Takimoto et al. 1999; Kishi and Sekine 2003).
10.1.1.2 Indoor air
Two Canadian studies that measured TCP in indoor air have been identified. Vykoukalová et al. (2017) monitored indoor air for two isomers of TCP, o-TCP and p-TCP (reported as o-TMPP and p-TMPP, respectively), in 23 homes in Toronto during a sampling period of 28 days in May to August 2013. Non-detects in this study were reported for samples where the blank level was >35% of the measured level. o-TCP was measured in the range of not detected to 0.05 ng/m3 with a median concentration of 0.005 ng/m3 and a detection frequency of 77%. p-TCP was measured over the range of not detected to 0.023 ng/m3 with a median concentration of 0.002 ng/m3 and a detection frequency of 71%. Indoor air was also monitored for the three isomers of TCP in 32 homes in the Greater Toronto Area and in 19 homes in Ottawa during a sampling period of 3 weeks from late February to late July 2015 (Yang et al. 2018). Air was sampled in bedrooms in all homes (n=51) and additionally in the “most used room” (MUR) in 26 of the 51 homes. TCP (reported as the sum of the TCP isomers, or TCPs) was detected in air in bedrooms at levels ranging from 0.00102 to 0.244 ng/m3 with a geometric mean of 0.00114 ng/m3 and a detection frequency of 2% (the method detection limit was 0.00034 ng/m3). TCP was not detected in the air of the most used rooms (n=26) in this study.
TCP has also been monitored in several indoor environments in the U.S., Europe and China (Appendix E, Table E-1).
In the U.S., o-TCP and p-TCP (reported as o-TMPP and p-TMPP, respectively) were measured in indoor air samples that were collected from 20 homes in Bloomington, Indiana during a sampling period of 28 days in May to August 2013. Non-detects in this study were reported for samples where the blank level was >35% of the measured level. o-TCP was measured in the range of not detected to 0.142 ng/m3 with a median concentration of 0.142 ng/m3 and a detection frequency of 3%. p-TCP was measured over the range of not detected to 0.256 ng/m3 with a median concentration of 0.256 ng/m3 and a detection frequency of 7% (Vykoukalová et al. 2017).
In studies in Europe, TCP has not been widely detected in indoor environments. In a recent study on 63 daycare centers in Germany, TCP was not detected in indoor air and dust although limits of detection (LODs) were not specified (Fromme et al. 2014). In a study by Tollbäck et al. (2006), air samples were collected in a kindergarten and a lecture room in Sweden. The concentration of TCP detected in the lecture room was 0.4 ng/m3, but TCP was not detected in the kindergarten classrooms. Cequier et al. (2014) investigated the occurrence of TCP in Norwegian households (n=48) and classrooms from two primary schools (n=6). TCP was not detected in classrooms and median concentrations of TCP in residential samples were below the method detection limit (MDL, ranging from 18 to 44 pg/m3 for organophosphate flame retardants), with a maximum of 0.644 ng/m3. TCP (reported as tritolyl phosphate) was not detected above the detection limit (1 ng/m3) in any of the samples collected in Swedish households (n=50) and non-residential environments (e.g. bakery, carpet store, garages) (n=55) (year unspecified) (Staaf and Östman 2005). In a separate study, o-TCP was not measured above the limit of quantification (4.1 ng/m3) in any samples (n=12) collected in various non-residential environments (e.g. electronics and furniture stores) in Zurich, Switzerland (Hartmann et al. 2004). However, in this same study, o-TCP was detected above the detection limit of 0.41 ng/m3 in the sample collected from a theatre (2.1 ng/m3) (year unspecified) (Hartmann et al. 2004).
A recent study reported particle size-specific concentrations of TCP (reported as TCrP) ranging in total from 0.03 to 0.2 ng/m3 in Chinese offices equipped with common office furniture and electronic products, away from industrial and high traffic zones (Yang et al. 2014). TCP concentrations peaked in the 0.7 to 1.1-µm particle size fraction, i.e. the ultrafine particle fraction (≤1 µm) (Yang et al. 2014).
Time spent in automobiles may also represent potential sources of exposure to TCP. TCP levels in vehicle air have been monitored in a few studies in the international literature. In Germany, a study of the emission of organophosphate esters to automobile interior air determined that TCP was not detected above the detection limit range of 10 to <50 ng/m3 in eight new vehicles, whether at room temperature or heated at 65°C (Wensing et al. 2005). TCP was not detected in any of the air samples collected from three cars in Zurich, Switzerland, including a new car, a one-year-old car and a nine-year-old car (Hartmann et al. 2004). TCP was not detected in air samples (n=5 for each vehicle) collected in a car (near the driver’s breathing zone), two buses and a subway car in Sweden, nor was it detected in any of the samples that were simultaneously collected from outside the vehicles in the parking garages (Staaf and Östman 2005).
Time spent in aircraft may also represent potential sources of exposure to TCP. Several studies have also investigated potential exposure in aircraft cabins due to the use of TCP in engine turbine oils and hydraulic fluids in the hydraulic system of aircrafts. van Netten (2008) reported that TCP was detected in cabin air during flight based on personal air monitoring. Two air samples were taken during two separate flights. The airline, country of departure or arrival, and sampling year were not specified. TCP concentrations adjusted for the sampler’s efficiency were found to be 108 ng/m3 and 36 ng/m3 for flights A and B, respectively. The authors noted that for flight A, the auxiliary power unit (APU) was used which was later associated with a failing oil seal (van Netten 2008). Another study showed that TCP, which was present in all turbine/engine oils in use in the aircraft included in this study, was detected in four out of 95 samples (4.2%) (Solbu et al. 2011). TCP concentrations in samples ranged from LOQ–0.29 µg/m3. TCP was however detected in 39% of the wipe samples (n=56; representing longer-term sampling) and in all HEPA-filters (n=6; spot samples), although for the latter no o-TCP was detected. It was also found that the TCP concentration during ground testing in an airplane that had experienced leakage of turbine oil with subsequent contamination of the cabin and cockpit air was an order of magnitude higher as compared to after engine replacement. A study of military aircraft found that TCP concentrations in cockpit air were below 5 μg/m3 with two exceptions (22 and 51 μg/m3). Ground engine starts at high power gave rise to the highest concentrations. All samples were collected under normal flight conditions (De Nola et al. 2011).
Daily intake estimates of exposure to TCP from air (both indoor and outdoor over a 24-hour period) for the general population of Canada were on the basis of the maximum concentration of 0.244 ng/m3 of TCP detected in the bedrooms of 51 homes in the Greater Toronto Area and Ottawa (Yang et al. 2018). While TCP outdoor air studies were identified, these were not considered as robust for characterizing exposure.
10.1.1.3 Dust
TCP has been monitored in household dust as well as in dust from other environmentsin Canada and elsewhere (Appendix E, Table E-1 and Table E-2).
TCP (reported as TCrP) was included in the Canadian baseline study of organophosphate flame retardants in archived house dust samples (n=818) collected in 2007–2008 from various Canadian cities within the Canadian House Dust Study (CHDS) (CHDS preliminary data; Kubwabo et al., manuscripts in preparation, Environmental Health Science and Research Bureau, Health Canada; unreferenced, dated December 13, 2013). The CHDS dust sampling technique was considered adequate for determining representative organophosphate flame retardant concentrations based on a comparison of dust sampling techniques (Fan et al. 2014). TCP was detected in 99.9% of the baseline study samples, and concentrations ranged from not detected (detection limit = 30 ng/g) to 295 000 ng/g. The median and 95th percentile found were 4 860 and 17 700 ng/g, respectively. The maximum concentration of TCP was the highest among all organophosphate flame retardants measured in this study.
TCP has also been measured in additional Canadian studies. Vykoukalová et al. (2017) measured two isomers of TCP, o-TCP and p-TCP (reported as o-TMPP and p-TMPP, respectively), in 23 homes in Toronto during a sampling period of 28 days in May to August 2013. Non-detects in this study were reported for samples where the blank level was >35% of the measured level. o-TCP was measured in the range of not detected to 7.02 ng/g with a median concentration of 0.712 ng/g and a detection frequency of 71%. p-TCP was measured in dust in the range of 0.871 to 116 ng/g with a median concentration of 6.18 ng/g and a detection frequency of 100%. Dust was collected from 32 homes in the Greater Toronto Area and 19 homes in Ottawa during a sampling period of 3 weeks in 2015. Dust was sampled from bedrooms in all homes (n=51) and additionally in the “most used room” (MUR) in 26 of the 51 homes. TCP (reported as the sum of the TCP isomers, TCPs) was detected in 74% of dust samples from bedrooms (n=51) at concentrations ranging from 0.75 to 1120 ng/g, a geometric mean of 23.2 ng/g, and a 95th percentile of 554 ng/g. TCP was detected in 81% of dust samples from most used rooms (n=26) at concentrations ranging from 0.75 to 699 ng/g, a geometric mean of 28.8 ng/g, and a 95th percentile of 217 ng/g (Yang et al. 2018).
A U.S. monitoring study of household dust carried out in California in 2011 and earlier, in 2006, showed relatively lower concentrations (Dodson et al. 2012). TCP median concentrations in samples collected from the living area surfaces of 16 homes in 2011 and 2006 were found to be 680 and 1000 ng/g, respectively, with concentrations ranging from 180 to 10 000 ng/g and 330 to 4 400 ng/g, respectively (Dodson et al. 2012). TCP concentrations reported in house dust studies in Europe and elsewhere were generally lower than those measured in the Canadian baseline study (Kersten and Reich 2003; Brommer et al. 2012; Dirtu et al. 2012; Van den Eede et al. 2011). A recent study in the Netherlands investigated electronic equipment (electronics) as a source of TCP (reported as tris(methylphenyl) phosphate or TMPP) in house dust. TCP levels in house dust on electronics (median of 330 ng/g) were significantly higher than those collected around electronics (110 ng/g) (Brandsma et al. 2014). The findings of higher TCP concentrations in dust on electronics is consistent with the results of Kajiwara et al. (2011) who observed TCP to be a major organophosphate flame retardant detected in electronic equipment (LCD televisions) from the Japanese market in 2008. In New Zealand, dust was collected from carpets and floors, as well as mattresses in rural and urban locations during 2008 (Ali et al. 2012). In carpet and floor dust, the median concentration (120 ng/g; n=34) was lower than that found in mattress dust (157 ng/g; n=16).
In a recent study in Germany, organophosphate substances were measured in paired indoor air and dust samples from 63 daycare centers (Fromme et al. 2014). TCP was not detected in indoor air and dust from the daycares although LODs were not specified (Fromme et al. 2014). In a separate study, dust samples (n=15) from a variety of retail outlets in Belgium, including an electronics store, a mattress store, pharmacies, a thrift shop and a furniture store, were collected in 2008 and analyzed for multiple flame retardants, including TCP (Van den Eede et al. 2011). TCP concentrations ranged from not detected (LOD =0.04) to 12.5 ng/g with a mean of 1.53 ng/g (Van den Eede et al. 2011)
TCP has been measured in dust from 12 automobile interiors in Germany by Brommer et al. (2012). Samples were collected between December 2010 and January 2011 using the vacuum cleaner typically used by the owner to clean the vehicle. TCP concentrations were much greater than those measured in household dust from the same study as concentrations ranged from not detected (detection limit = 40 ng/ng) to 150 000 ng/g with a mean of 24 000 ng/g (Brommer et al. 2012). Brandsma et al. (2014) recently reported TCP (reported as TMPP) concentrations in cars from the Netherlands. Eight cars were included in the study: two older cars (2003 and 2004) and four others manufactured between 2008 and 2012. Dashboard samples (n=8) and seat samples (n=8) showed TCP median concentrations of 750 ng/g (47 [detection limit] to-9500 ng/g) and 1400 ng/g (250-380 000 ng/g), respectively. The high concentration (particularly the maximum in the seat samples) is consistent with the use of TCP as a plasticizer in automobiles. The TCP levels in car seat dust were also significantly higher than those measured in house dust collected from electronics in the same study (Brandsma et al. 2014).
Daily intake estimates of TCP exposure related to the ingestion of dust for the general population of Canada were on the basis of the 95th percentile concentration of 17 700 ng/g from the Canadian House Dust Study (preliminary baseline data from the Canadian House Dust Study (personal communication from Environmental Health Science and Research Bureau, Health Canada, dated December 13, 2013). Evidence based on a limited number of studies in Europe indicate that time spent in a car may represent a source of exposure to TCP in dust; however the exposures derived from the house dust study in Canada were considered appropriately conservative for estimating exposure from dust for the general population in Canada.
10.1.1.4 Soil and sediment
No monitoring data on TCP in soil in Canada were identified (see Section 7.4). Available soil data in the U.S. (e.g. Boethling and Cooper 1985; David and Seiber 1999b) are older and are not deemed appropriate or relevant for deriving conservative present-day exposure intakes for the general population. A maximum TCP soil PEC of 300 ng/g dw (0.00030 mg/kg dw) was estimated for land application of biosolids on an agricultural field using conservative approaches, as detailed further in the Ecological Exposure Assessment section (see Section 9.2.1.1).
A recent Canadian study has examined one sediment core each from the Niagara Basin and Mississauga Basin, Ontario, sampled in 2012 and 2013, respectively (De Silva and Muir 2016, see Section 7.3). TCP (all isomers) was measured at mean levels of 0.011 mg/kg dw at Niagara Basin and 0.0082 mg/kg dw in the Mississauga Basin in this work.
In Europe, TCP in sediments from freshwater sources were measured at concentrations ranging from non-detect to 390 µg/kg in Denmark, Norway, Austria and Germany (Ricking et al. 2003; Martinez-Carballo et al. 2007; UK EA 2009; Van der Veen and de Boer 2012), with the most recent freshwater sediment monitoring (i.e. for 2005) associated with concentrations ranging from 1.5 to 39 ng/g dw (reported as <MQL - 39 ng/g dw) (Martinez-Carballo et al. 2007). Concentrations in marine sediment ranged from non-detect to 370 µg/kg in Denmark (UK EA 2009).
As no appropriate or relevant monitoring studies on TCP in soil were identified, the soil maximum PEC (0.00030 mg/kg dw) was selected for deriving intake estimates from the ingestion of soil for the general population in Canada.
10.1.1.5 Drinking water
Several studies have monitored TCP in drinking water in Canada (Appendix E, Table E-3). Individual TCP isomers were targeted in the City of Toronto municipal drinking water monitoring program from 2000-2003 and none were found to be present above the detection limit of 60 ng/L (e.g. City of Toronto 2003a, b,c).
TCP has also been monitored in several older studies of drinking water monitoring in Canada. TCP was not detected in the majority of drinking water samples collected in various municipalities (approximately 35) in Canada during the late 1970s, though where it was detected it was measured at levels ranging from 0.7 to 4.3 ng/L (LeBel et al. 1981; Wiliams and LeBel 1981). TCP was also detected in the drinking water of 5 of 12 Canadian Great Lakes municipalities (Thunder Bay, Owen Sound, Union-Leamington, St Catherines and Toronto) at concentrations of 0.6-1.8 ng/L during a 1980 monitoring study (Williams et al. 1982).
TCP has also been monitored in surface waters (see Section 7.2). In Canada, TCP was not detected in the open waters of the Great Lakes, but was measured in Lake Ontario and Lake Erie tributaries in 2010-11 at concentrations ranging from 2.5 to 22 ng/L (Lee et al. 2011). In recent surface water monitoring in Europe, TCP was not detected in three independent studies conducted in rivers in Denmark, Rome and Austria (Bacaloni et al. 2007; Martinez-Carballo et al. 2007; UK EA 2009).
Upper bounding daily intakes of TCP from drinking water exposure for the general population of Canada were derived using the 60 ng/L detection limit from the City of Toronto drinking water monitoring program for the years spanning from 2000 to 2003 (e.g. City of Toronto 2003a, b,c).
10.1.1.6 Food
No studies were identified that reported TCP in marketed foods in Canada; however, TCP was previously screened in the U.S. Food and Drug Administration (U.S. FDA) Total Diet Study (Appendix E, Table E-4). The Total Diet Study is an ongoing program conducted by the U.S. FDA to analyze contaminants in 357 different food types (ranging from dairy products to baby food) purchased throughout the U.S. (US FDA 2014). Market basket study results made available on the U.S. FDA website (US FDA 2014) showed that TCP was detected in one out of 44 samples of cracked wheat bread at a concentration of 90 ng/g (0.09 ppm) (resulting in a mean of 2.05 ng/g) in a market basket collected in 2003 (US FDA 2006). However, TCP was not detected in any other foods in all market baskets conducted from 1991 through to 2003, representing approximately 15 000 food items (US FDA 2006). As such, the detection of TCP in a single sample was considered to be an unrepresentative result, and therefore this data point was not included in the exposure assessment.
TCP has also been monitored in biota from the U.S., Europe, and Japan (Appendix E, Table E-5). TCP was monitored in the U.S. in several older studies of fish near industrial facilities (Lombardo and Egry 1979; Muir 1984); however, these data are not considered appropriate to characterize present day exposure. TCP was monitored in a wide range of freshwater and marine fish and shellfish collected in 2005 or 2007 across Sweden with a reported range of 1.8 to 110 ng/g lw (or maximum of 1.32 ng/g ww; Sundkvist et al. 2010). The maximum TCP value corresponded to a sample collected in a stream receiving water from an international airport in Marstaan, a suburb of Stockholm, which may explain the higher concentration measured (Sundkvist et al. 2010). This same sample was the only sample observed to have detectable levels of o-TCP of 2.5 ng/g lw. In environmental monitoring by the Ministry of Environment in Japan ([MOE Japan] 2003), TCP was not detected in archived fish collected from various locations in 1975 (n=96) and 1978 (n=93); however, TCP was detected in more recent samples collected in 1993 (n=75), and ranged from 63 to 82 ng/g lw.
On the basis of the available information, daily intake estimates of TCP from fish consumption for the general population of Canada were estimated using the maximum concentration (1.32 ng/g ww) of shellfish measured in Europe (Sundkvist et al. 2010). In the absence of recent North American monitoring, this concentration is considered appropriate for deriving chronic upper bound intakes for the general population of Canada. Also, the assumption that TCP is present at this concentration in the entire fish food group (i.e. in fish, shellfish and related food items) consumed by the general population is considered conservative. Although certain northern populations in Canada may, seasonally, consume larger quantities of seafood in their diet, this estimate is considered adequately conservative to account for this variability.
10.1.1.7 Breast milk
No studies were identified that reported TCP monitoring in breast milk in Canada; however, two international studies were identified. Sundkvist et al. (2010) reported TCP breast milk concentrations for samples originating from women in four Swedish towns between 1997 and 2007 (Appendix E, Table E-6). Milk samples were collected from mothers two to three weeks after the delivery of their first baby. Most of the samples per cohort (from a single town for a given year) were pooled, and a single value was reported. TCP concentrations ranged from not detected (detection limit = 0.1 ng/g lipid weight) in 2006 (50 pooled samples) to 3.7 ng/g lipid weight in 1997 (69 pooled samples), both from Uppsala (Sundkvist et al. 2010). The authors noted a similar trend (i.e. higher concentrations in older samples) for another organophosphate flame retardant, and noted the possibility that older samples may have been contaminated, for example, by the sampling equipment such as breast pumps.
TCP (reported as TMPP) was measured in human breast milk collected from Japan (n=20) in 2009–2011, the Philippines (n=41) in 2008, and Vietnam (n=26) in 2008 (Kim et al. 2014). While the overall study detection frequency was 59% (detection limit not specified), TCP was not detected in samples from Japan. The median concentrations in the Philippines (2.3 ng/g lw; range of ND to 11 ng/g lw) were higher than those measured in Vietnam (0.28 ng/g lw, range from ND to 7.9 ng/g lw), which was the same trend observed for total organophosphorus concentrations. The maximum concentrations in Vietnam and the Philippines were associated with samples collected from mothers residing near urban areas and e-waste recycling sites, respectively.
Daily intake estimates for TCP for nursing infants in the general population of Canada were estimated based on the concentration of 3.7 ng/g lipid weight (resulting in a concentration of 0.126 ng/g ww based on the reported lipid content of 3.4%) from the Swedish study (Sundkvist et al. 2010).
10.1.2 Products available to consumers
TCP is an additive flame retardant with a variety of uses and applications (see Section 5) which may result in general population exposure. Dermal exposure estimates were derived using conservative approaches for scenarios deemed relevant for the general population. TCP is characterized by very low volatility based on its low vapour pressure and low Henry’s Law constant (see Section 3). As such, releases to air from products available to consumers are expected to be accounted for through indoor air and dust intake estimates (see Sections 9.1.1.1 and 9.1.1.3).
10.1.2.1 Manufactured items
Flame retardants can be found in coatings applied to the inside face of the cover fabric as “backcoating”. The use of TCP as backcoating in furniture upholstery has not been reported in Canada, but has been documented in the U.S. Therefore, it is considered reasonable to assume that the general population of Canada can be exposed to TCP in furniture. According to a 2000 report by the U.S. National Research Council (NRC 2000), TCP was applied as a backcoating to furniture upholstery (Piccirillo 1999; NRC 2000). In a U.S. survey conducted by the Fire Retardant Chemicals Association prior to 1998, “aromatic phosphate esters” (such as TCP) were reported to be used in residential furniture on the basis of the information from U.S. flame retardant industries with marketing activities with the textiles industry (cited in US CPSC 1998). The Upholstered Furniture Action Council (UFAC), a voluntary coalition of furniture manufacturers, outlined the potential for dermal exposure to backcoating, either via direct contact with the substance from contamination of the outside surface of the fabric (from wet backcoating when textile is rolled up post-production), or from migration and degradation of the backcoating through the textile weave (cited in US CPSC 1998).
Other reports also document furniture-related uses of TCP including its use as a plasticizer in synthetic leather upholstery in Europe (European Commission 2011), and in PVC seat covers (SinoHarvest 2011). Also, according to manufacturer literature, TCP is marketed for flexible foam for furniture (ICL 2013c). In preliminary product testing conducted by Health Canada of children’s manufactured items purchased in retail stores in Ottawa, Ontario, in January and May 2014, TCP was detected in a foam chair above the limit of quantification at a concentration reported as ≥0.7%; however, TCP was not detected in the remaining 23 children’s manufactured items (e.g. nursing pillows, toys) (Health Canada 2014). In a study commissioned by the CEC, furniture products (e.g. office chairs, upholstered sofas and/or chairs) from Canada, the U.S. and Mexico were analyzed for the presence of 16 flame retardants using X-ray fluorescence (XRF) screening followed by GC/MS analysis. TCP was not identified in any of the 132 furniture products purchased for this study between December 2014 and April 2015 (CEC 2015).
Though TCP was not identified in most of the products available to consumers tested in recent studies (CEC 2015, Health Canada 2014), treated furniture was still considered the most likely source of exposure to TCP for Canadians in light of its reported use in such products internationally. As such, dermal exposure intakes were estimated for toddlers and adults in direct contact with adult furniture (with treated upholstery backcoating or foam) as a sentinel scenario (Table 10-1). No TCP-specific migration data were identified. As such, a range of migration values for other organophosphate flame retardants were used to account for the migration from both treated upholstery and foam, and the general variability in migration rates. These values were also considered conservative owing to their higher water solubilities relative to TCP since migration experiments measure the transfer of the substances from textile or foam into aqueous solutions (i.e., mimicking sweat-mediated migration). TCP has a water solubility of 0.36 mg/L (Saeger et al. 1979) while TCEP and TDCPP have water solubilities of 7820 mg/L (EU RAR 2009) and 18.1 mg/L (SafePharm Laboratories 2002), respectively. Migration rates ranged from 5.6 × 10-5 mg/cm2/hr for tris(1,3-dichloroisopropyl)phosphate (TDCPP) to 2.17 x 10-4 mg/cm2/h for tris(2-chloroethyl) phosphate (TCEP). The TCEP migration was based on textile backcoating described in the TCEP European Risk Assessment Report (EU RAR 2009), while the rate for TDCPP was based on treated upholstered foam as described by the U.S. Consumer Product Safety Commission (US CPSC 2005). Estimates of dermal exposure from contact with adult furniture ranged from 1.29×10-3 to 4.99×10-3 mg/kg-bw/d for toddlers, and from 6.61×10-3 to 2.56×10-2 mg/kg-bw/d for adults, where ranges reflect the different migration rates. These estimates were derived using conservative assumptions as described in Appendix F.
Other jurisdictions have conducted furniture-related assessments of TCP (NRC 2000; European Commission 2011); however, the approaches used varied substantially, as did intakes and route-specific conclusions due to differences in parameters considered, default values, conservatism, etc. The assessment report on flame retardants in consumer products published by the European Commission included several recommendations for refining scenarios (European Commission 2011), most of which were considered in this current assessment.
| Exposure route | Source | Product | Age group | Intake |
|---|---|---|---|---|
| Dermal | Upholstery backcoating or foam | Furniture | Toddler (15.5 kg) | 1.3×10-3 –5.0×10-3 mg/kg-bw/d |
| Dermal | Upholstery backcoating or foam | Furniture | Adult (70.9 kg) | 6.6×10-3 – 2.6×10-2 mg/kg-bw/d |
Other uses of TCP in manufactured items in Canada include automobile parts, such as the trunk and headliner (ECCC 2013-2014). TCP has been associated with dust in the interior of vehicles (Brommer et al. 2012; Brandsma et al. 2014; see section 10.1.1.3). As such, it is reasonable to assume that the general population of Canada can be exposed to TCP in the interior of vehicles. TCP uses in other manufactured items found in the international literature include home furnishings, electronics and other utility articles (i.e. footwear, raincoats, imitation leather and handbags) (Kajiwara et al. 2011; SinoHarvest 2011; Siret-Alatrista et al. 2010; IPCS 1990; Ash and Ash 2003) (Appendix F). While exposure to TCP can occur via these manufactured items, the overall exposure potential (frequency, duration and magnitude) for these scenarios are not expected to result in higher exposures than those quantitatively presented for the furniture scenario.
10.1.2.2 Products
TCP-containing products available to the general population in the area of hydraulic fluids, oils and lubricants may include automatic transmission fluid and automotive and motorcycle engine oils (Chemtura 2007; Takimoto et al. 1999; Solbu et al. 2010; ATSDR 1997; WHO 1990). The concentrations of TCP in various oil-based products are provided in Appendix G. Based on the Canadian industry survey, one product was identified for the general population with plausible direct contact, i.e. power steering fluid (ECCC 2013-2014). The dermal route was considered to be the only route of exposure due to the low vapour pressure of TCP. Using a thin film approach (Westat 1987), the highest short-term dermal exposure (tips of the fingers) to TCP from the use of power steering fluid was estimated to be 2.4 µg/kg-bw (Appendix G).
10.1.3 Biomonitoring
A Canadian study has been identified in which nine urinary metabolites of organophosphate flame retardants (OPFRs) were measured in urine samples (n=24) collected in 2010-12 from pregnant women in the Hamilton region of Ontario, Canada (Kosarac et al. 2016). Two metabolites of TCP were measured in this study: di-o-cresyl phosphate (DoCP) and di-p-cresyl phosphate (DpCP). These two urinary metabolites, with a combined detection frequency of 75%, were measured over a range of <0.13 to 4.38 ng/mL with a median of 0.69 ng/mL (MDL of 0.13 ng/mL) (Kosarac et al. 2016). TCP has also been measured in adipose tissue from 6 municipalities in Ontario in 1989, where TCP was not detected in any of the 82 samples analyzed with a detection limit of 1 ng/g (LeBel et al. 1989).
In the United States, spot urine samples from the 2013-2014 National Health and Nutrition Examination Survey (NHANES) were analyzed for DoCP and DpCP (Ospina et al. 2018). Of the 2666 samples, DoCP was only detected in 0.1% of the samples and DpCP was detected in 13% of the samples (LOD = 0.05 µg/L for both substances). As such, concentrations of DoCP were all considered to be below the LOD while for DpCP, 95th percentile values ranged from 0.090 µg/L for adults 60 years and older to 0.240 µg/L for children 6 to 11 years old (geometric means could not be calculated and 50th percentile values were below the LOD) (Ospina et al. 2018). A smaller study in the US measured nine urinary metabolites of target flame retardants. This included DoCP, which was not detected in any samples, and DpCP. DpCP was not detected above the LOD (0.05 ng/mL) in any samples collected from adults in Atlanta, Georgia (n=76) in 2015. It was, however, detected in 34% of samples collected from firefighters in 2010-2011 for a US National Institute for Occupational Safety and Health (NIOSH) study with a median level below the LOD (0.05 ng/mL) and over a range of <0.05 to 0.21 ng/mL (Jayatilaka et al. 2017).
In a study in Germany, the urine of 312 children between 22 and 80 months old who attend daycare centres was analyzed for the presence of eight OP metabolites, including those for TCP isomers (DoCP, DpCP and di-m-cresyl phosphate). All three isomeric metabolites of TCP were not found above the LOD (0.1 μg/L) (Fromme et al. 2014).
A marker of o-TCP exposure (butyrylcholinesterase adduct or phosphorylated butyrylcholinesterase), was found in 6 of 12 blood samples from twelve randomly selected jet airplane passengers likely as a result of worn or defective engine seals that may release engine oil into the cabin air supply (Liyasova et al. 2011). Blood samples were obtained 24 to 48 h after completing a flight. The authors described the levels of exposure as very low, averaging 0.05 to 1% phosphorylated butyrylcholinesterase. Four of the positive subjects were retested 3 to 7 months following their last airplane trip and were found to be negative for phosphorylated butyrylcholinesterase (Liyasova et al. 2011).
10.2 Health effects assessment
A summary of the available health effects information for TCP is presented in Appendix H.
No classifications of the health effects of TCP (containing <0.1% of o-TCP) by national or international regulatory agencies were identified.
10.2.1 Carcinogenicity and genotoxicity
The US NTP conducted a 2-year oral study in rats and mice to investigate the carcinogenicity of TCP. Fischer 344/N rats (95 per sex per group) were administered TCP in feed at 0, 75, 150 or 300 ppm (approximately 0, 3, 6 or 13 mg/kg-bw/day for males and 0, 4, 7 or 15 mg/kg-bw/day for females) for 2 years. An additional group of rats received 600 ppm TCP in feed for 22 weeks and then received control feed. B6C3F1 mice (95 per sex per dose) were administered TCP in feed at 0, 60, 125 or 250 ppm (approximately 0, 7, 13 or 27 mg/kg- bw/day in males and 0, 8, 18 or 37 mg/kg-bw/day in females) for 2 years. Rats and mice were examined at 3, 9 and 15 months and 2 years into the study. There was no evidence of carcinogenic activity of TCP found in the exposed rats and mice. Non-neoplasitic effects observed in the 2-year study included cytoplasmic vacuolization of the adrenal cortex in male and female rats, ovarian interstitial cell hyperplasia in female rats, ceroid pigmentation of the adrenal cortex in male and female mice, as well as increased incidence in clear cell foci, fatty change and ceroid pigmentation of the liver in male mice (NTP 1994). These effects are discussed in more detail in Section 9.2.3.
Studies on genotoxicity of TCP have been conducted in vitro and in vivo. TCP tested negative in bacteria mutation assays (Salmonella typhimurium TA 98, TA100, TA1535, TA1537, TA1538) with or without metabolic activation. It did not induce sister chromatid exchange in Chinese hamster ovary (CHO) cell with or without metabolic activation. In the chromosome aberration assays, TCP tested negative in CHO cells and in mouse lymphoma cell line, but ambiguous in mouse lymphoma L5178Y cells, and positive in BALB/3T3 cell line, with or without metabolic activation. In an in vivo assay, TCP did not induce unscheduled DNA synthesis in exposed rats. Therefore, TCP is unlikely to be genotoxic based on the available information.
10.2.2 Reproductive and developmental effects
The reproductive effects of TCP were examined in mice and rats of both sexes. In a study using continuous breeding protocol, male and female Swiss (CD-1) mice (20 per sex per dose for test group, 40 per sex for control group) were dosed at 0, 0.05, 0.1 or 0.2% of TCP (approximately 0, 63, 124, or 250 mg/kg-bw/day) in feed, for 7 days prior to breeding and 98 days during breeding. A significant (p<0.01) decrease in the number of litters per pair was observed in a dose-dependent manner. The proportion of pups born live and their weight was significantly decreased at 250 mg/kg-bw/day. In the crossover mating phase, impaired fertility was observed in both males and females treated with 250 mg/kg-bw/day, with a greater effect in females. There were dose-related changes in the adrenals for both sexes. Bodyweight was decreased in both males and females at the high dose. A significant (p<0.05) decrease in sperm motility was observed at 63 and 124 mg/kg-bw/day (males in the 250 mg/kg-bw/day dose group were not examined). Atrophy of seminiferous tubules, decreased testis and epididymal weights were observed in F0 males at the high dose, while no histopathological changes were found in the female reproductive tract. A LOAEL was identified at 63 mg/kg-bw/day based on decreased number of litters per pair. No NOAEL was identified (Chapin et al. 1988).
The reproductive effects of TCP were also examined in a one-generation study in Long Evans rats. TCP was administered to male rats (12 per dose) at 0, 100 or 200 mg/kg-bw/day, and to female rats (24 per dose) at 0, 200, or 400 mg/kg-bw/day in corn oil via gavage. Males were dosed for 56 days, and females for 14 days prior to breeding and throughout the 10-day breeding period. The low-dose (100 mg/kg-bw/day) males were mated with the low-dose (200 mg/kg-bw/day) females and the high-dose (200 mg/kgbw/day) males were mated with the high-dose (400 mg/kg-bw/day) females. A dose-dependent abnormal sperm morphology was observed at all doses. Sperm concentration, motility and progressive movement were decreased at 200 mg/kg-bw/day. The number of females delivering live pups was decreased at all doses. Decreased litter size and pup viability were observed at 400 mg/kg-day. Histopathological changes were observed in the testes and epididymides of male rats, and in the ovaries of female rats. A LOAEL was identified at 100 mg/kg-bw/day, the lowest dose tested, based on fetotoxicity, abnormal sperm morphology, histopathological changes in testis and epidymides (Carlton et al. 1987).
In a prenatal developmental toxicity study, pregnant SD rats were administered TCP via gavage at 0, 20, 100, 400 or 750 mg/kg-bw/day during gestation days 0 to 19. Clinical observations included increased salivation at 100 mg/kg-bw/day and higher dose, and alopecia and unkempt appearance at 400 mg/kg-bw/day and higher doses. Fetal body weights were reduced compared to control groups at all dose levels. Incomplete ossification was observed at 750 mg/kg-bw/day. A LOAEL was identified at 20 mg/kg-bw/day, the lowest dose tested, based on reduced fetal body weights while the LOAEL for maternal toxicity was 100 mg/kg-bw/day based on increased salivation. Also, a NOAEL for maternal toxicity was established at 20 mg/kg-bw/day. No NOAEL for developmental toxicity was identified (MPI Research Inc. 2004).
10.2.3 Repeated-dose toxicity
A number of repeated-dose studies have been identified, which are summarized in Appendix H. Some of these studies are presented below:
One short-term dermal study was identified. Female Sprague-Dawley rats (5 per dose) were administered TCP (3% in jet engine oil) via dermal application to the clipped (shaved) skin at 0, 500 or 1000 mg/kg-bw/day, for a total of 20 exposures over four weeks. Serum cholinesterase was significantly (p<0.05) reduced in all exposed rats. Clinical signs of hypersensitivity, such as increased motor activity were observed at 1000 mg/kg-bw/day (Mobil 1990).
The US NTP conducted a 13-week oral study in Fischer 344/N rats and B6C3F1 mice. Animals (10 per sex per dose) were administered TCP in corn oil by gavage up to 800 mg/kg-bw/day. No mortalities were observed in either species. Final mean body weights of male rats and mice were significantly lower than controls after exposure to doses of 200 mg/kg-bw/day and higher, whereas in female mice, mean body weights were significantly reduced after receiving doses of 400 mg/kg-bw/day and higher. No significant mean body weight changes were observed in female rats. Cytoplasmic vacuolization of the adrenal cortex was observed in rats and mice of both sexes at all dose groups in a dose-dependent manner. Ovarian interstitial cell hypertrophy was observed in all treated female rats and mice. In addition, atrophy of seminiferous tubules was observed in male rats at doses of 400 mg/kg-bw/day and higher. There were no biologically significant changes in neurobehavioral parameters in exposed rats. However, in mice, multifocal degeneration of the spinal cord was observed at 100 mg/kg-bw/day and higher, in both sexes; and multifocal degeneration of the sciatic nerve was observed at 200 mg/kg-bw/day and higher, in males, and at 100 mg/kg-bw/day and higher, in females. Significantly decreased forelimb grip strength was observed in males treated with doses of 200 and higher and in females treated with doses of 400 mg/kg-bw/day and higher. Significantly decreased hindlimb grip strength was observed at 200 mg/kg-bw/day and higher in both sexes. A LOAEL of 50 mg/kg-bw/day, the lowest dose tested, was identified both for rats and mice based on cytoplasmic vacuolization of the adrenal cortex, and ovarian interstitial cell hypertrophy (NTP 1994).
A similar 13-week feeding study was conducted in rats and mice., Fischer 344/N rats (10 per sex per dose) were treated with diets containing 0, 900, 1700, 3300, 6600 or 13,000 ppm TCP (approximately equal to 0, 55, 120, 220, 430 or 750 mg/kg-bw/day for males and 65, 120, 230, 430 or 770 mg/kg-bw/day for females), whereas B6C3F1 mice (10 per sex per dose) were treated with diets containing 0, 250, 500, 1000, 2100 or 4200 ppm TCP (approximately equivalent to 0, 45, 110, 180, 380 or 900 mg/kg-bw/day for males and 0, 65, 130, 230, 530 or 1050 mg/kg-bw/day for females). No mortalities were observed in either rats or mice of either sex. Final mean body weights were significantly decreased in male rats at 430 mg/kg-bw/day and higher, and in female rats at 230 mg/kg-bw/day and higher. In mice, mean body weights were decreased (significance not stated) at 900 mg/kg-bw/day in males, and at 530 mg/kg-bw/day and higher in females. Cytoplasmic vacuolization of the adrenal cortex was observed in all exposed rats and mice except for the male mice treated at 45 mg/kg-bw/day. In addition, ovarian interstitial cell hypertrophy and inflammation of the ovarian interstitium were observed in all female rats. Renal papillary edema and renal papillary necrosis were observed at 750 mg/kg-bw/day in male rats and at 430 mg/kg-bw/day and higher dose in female rats. Basophilic hypertrophy of the pituitary gland pars distalis and atrophy of the seminiferous tubules were observed at 430 mg/kg-bw/day and higher dose in male rats. No biologically significant changes in neurobehavioral parameters were observed in exposed rats. In mice, papillary hyperplasia of the gallbladder mucosa was observed in males which received doses of 110 mg/kg-bw/day and higher, and in females treated with doses of 230 mg/kg-bw/day and higher. Renal tubule degeneration was observed in all male mice at 900 mg/kg-bw/day. Axonal degeneration was observed in males dosed with 380 mg/kg-bw/day and higher, and in females treated with 230 mg/kg-bw/day and higher. Significant decreases in forelimb grip strength were observed in males and females treated at 380 and 530 mg/kg-bw/day, respectively (p ≤ 0.05) and in males and females treated at 900 and 1050 mg/kg-bw/day, respectively (p ≤ 0.01). Significant decreases in hindlimb grip strength were observed in males receiving 900 mg/kg- bw/day and in females receiving 530 mg/kg-bw/day and higher (p ≤ 0.01).
A LOAEL was identified for male rats at 55 mg/kg-bw/day based on cytoplasmic vacuolization of the adrenal cortex; and for females at 65 mg/kg-bw/day based on hyperplasia of ovarian interstitial cells. No NOAELs were identified in the rats study. In mice, a LOAEL was identified at 110 mg/kg-bw/day for males, and at 65 mg/kg-bw/day , the lowest dose tested, for females based on cytoplasmic vacuolization of the adrenal cortex; a NOAEL was identified at 45 mg/kg-bw/day for the males (NTP 1994).
TCP was further tested in a 2-year feeding study in rats and mice. Fischer 344/N rats (95 per sex per dose) were fed diets containing 0, 75, 150 or 300 ppm TCP (approximately 0, 3, 6 or 13 mg/kg-bw/day for males and 0, 4, 7 or 15 mg/kg-bw/day for females). An additional group of rats (95 per sex per dose) received diets containing 600 ppm TCP (approximately 26 mg/kg-bw/day for males and 30 mg/kg-bw/day for females) for 22 weeks and then received only control feed. No mortalities occurred. No effect was observed on the final mean body weights. Cytoplasmic vacuolization of the adrenal cortex was observed in males treated at 26 mg/kg-bw/day and in females treated at 7 mg/kg-bw/day and higher at 3 months. At 9 and 15 months, cytoplasmic vacuolization was observed primarily in females treated at 15 mg/kg-bw/day, with the incidence and severity significantly increased at the end of the study. Ovarian interstitial cell hyperplasia was observed in female rats at 7 mg/kg-bw/day and the incidence and severity were increased at the end of the study. A LOAEL was identified at 7 or 15 mg/kg-bw/day for ovary interstitium hyperplasia observed in females or cytoplasmic vacuolization of the adrenal cortex observed in males, respectively.. A NOAEL was identified at 4 or 7 mg/kg-bw/day, respectively (NTP 1994).
B6C3F1 mice (95 per sex per dose) were treated with diets containing 0, 60, 125 or 250 ppm TCP (approximately 0, 7, 13 or 27 mg/kg-bw/day for males and 0, 8, 18 or 37 mg/kg-bw/day for females). At 3, 9 and 15 months of exposure, up to15 mice/sex/dose were necropsied and evaluated for histopathological lesions. Body weights, survival and feed consumption of exposed groups were similar to that of controls. Ceroid pigmentation of the adrenal cortex was observed in all exposed groups including controls (with an incidence near/or at 100% at 9, 15 and 24 months) throughout most of the 2-year study, except in 8 and 18 mg/kg-bw/day females at the 3-month necropsy. The severity was markedly increased in females at 37 mg/kg-bw/day. Increased incidence in clear cell foci, fatty change and ceroid pigmentation of the liver were observed in males at 13 mg/kg-bw/day and higher doses. A LOAEL was identified at 7 or 37 mg/kg-bw/day for males or females, respectively, based on ceroid pigmentation of the adrenal cortex. A NOAEL was identified at 18 mg/kg-bw/day for the female mice, but no NOAEL was identified for the male mice (NTP 1994).
Since adrenal lesions were observed in all groups of male and female mice including controls, this suggests that in mice, adrenal lesions occur spontaneously and TCP accelerated its onset (ATSDR 2012). Therefore, the NOAEL of 4 mg/kg-bw/day based on cytoplasmic vacuolization of the adrenal cortex observed in female rats is identified as the lowest NOAEL for adrenal lesions.
Information from oral repeated-dose studies indicates that the target organs for TCP are the adrenal gland and ovary in rats. The mechanisms by which these effects occur have not been elucidated, but some studies have provided some insight. Among the possible mechanisms, a potential underlying mechanism of these effects seems to be an alteration in the storage pathway resulting from the inhibition by TCP of neutral cholesteryl ester hydrolase (nCEH), an enzyme that catalyzes the conversion of stored cholesteryl ester to free cholesterol. Such an action would result in accumulation of cholesteryl esters in adrenocortical and ovarian interstitial cells. TCP also inhibited A: cholesterol acyl transferase (ACAT) in the adrenals. ACAT is involved in the esterification of cholesterol to form cytoplasmic lipid droplets of cholesteryl ester, a mechanism by which the cells store and conserve cholesterol in excess of that required for steroidogenesis. Male B6C3F1 mice showed increased incidences of fatty change, clear foci, and ceroid pigmentation in hepatocytes after exposure to TCP for 2 years, which could indicate a disruption in lipid metabolism. However, since no such effects were reported in females, additional mechanisms are probably involved (ATSDR 2012).
10.2.4 Neurotoxicity
Exposure to TCP also induced neurotoxicity in mice and rats. In a 16-day gavage study in F344/N rats, a significant reduction of forelimb grip strengths was observed in females at 1450 mg/kg-bw/day and higher doses, and in males at 2900 and 5800 mg/kg-bw/day. A significant reduction in hindlimb grip strengths was observed in males at 2900 and 5800 mg/kg-bw/day, and in females at 5800 mg/kg-bw/day (NTP 1994). In a similar 16-day gavage study in B6C3F1 mice, a significant reduction of forelimb grip strengths was observed at 1450 mg/kg-bw/day in males and at 5800 mg/kg-bw/day in females while a significant reduction of hindlimb grip strengths was observed at 360 mg/kg-bw/day in males, at 730 mg/kg-bw/day in males and females, at 1450 in males and at 5800 mg/kg-bw/day in males and females, on day 14 of the study (NTP 1994).
Neurological effects of TCP were also observed in mice in the sub-chronic oral studies. In a 13-week gavage study in B6C3F1 mice, multifocal degeneration of the spinal cord was observed at doses of 100 mg/kg-bw/day and higher, both in males and females; multifocal degeneration of the sciatic nerve was observed in males at 200 mg/kg-bw/day and higher, and in females at 100 mg/kg-bw/day and higher. Decreased hindlimb grip strength was observed at doses of 200 mg/kg-bw/day and higher, in both sexes. Decreased forelimb grip strength was observed at 400 mg/kg-bw/day and higher doses in males, and at 200 mg/kg-bw/day and higher doses in females (NTP 1994).
In a similar 13-week feeding study in B6C3F1 mice, axonal degeneration was observed in males at 380 mg/kg-bw/day and higher doses, and in females at 230 mg/kg-bw/day and higher doses. Significant decreases in forelimb grip strength were observed at 380 mg/kg-bw/day and higher dose and at 530 mg/kg-bw/day and higher dose in males and females, respectively. Significant decreases in hindlimb grip strength were observed at 900 and 1050 mg/kg-bw/day in males and females, respectively (NTP 1994).
TCP can also induce neurological effects via dermal exposure. In the 4-week dermal study in female Sprague-Dawley rats, serum cholinesterase was significantly (p<0.05) reduced at 500 and 1000 mg/kg-bw/day, the only 2 doses tested. Clinical signs of hypersensitivity, such as increased motor activity, were observed at 1000 mg/kg-bw/day (Mobil 1990). This study was conducted in 1990, and the TCP formulation composition used for the study is unknown and may have been different from TCP formulations used commercially today.
10.2.5 Sensitization
In a local lymph node assay, twenty female CBA/J Rj mice were allocated to five groups of four animals each. TCP was applied to the surface of ears of mice at concentrations of 100% (undiluted), 50% and 25% (w/v). The negative control group received acetone olive oil (AOO) while the positive control group received 25% α-Hexylcinnamaldehyde (HCA) in AOO. No mortality, systemic toxicity or local irritation was observed during the study. No treatment-related effects were observed on animal body weights in any treated groups. Stimulation index values of the test item were 3.7, 3.4 and 5.7 at treatment concentrations of 100% (undiluted), 50% and 25% (w/v), respectively. A significant lymphoproliferative response (stimulation index value of 17.2) was noted for the positive control chemical and this result confirmed the validity of the assay. In conclusion, under the conditions of the present assay, Kronitex TCP, tested in a suitable vehicle, was shown to have sensitization potential (sensitizer) in the local lymph node assay (LAB Research Ltd. 2010).
10.2.6 Human studies
There are a number of generally poorly documented case reports of toxic effects caused by accidental ingestion or occupational exposure to TCP with unknown concentration of isomers. An outbreak of acute polyneuropathy in over 20 young females in Sri Lanka during 1977 to 1978 was reported. The cause of the neuropathy was TCP as a contaminant in special cooking oil (gingili oil). Contamination probably occurred during transport of the oil in containers previously used for storing mineral oils (Senanayake and Jeyaratnam 1981). In 1944, three cases of toxic polyneuropathy among workers who had worked for six to eight months in a plant manufacturing TCP in England were reported. Skin penetration and inhalation were deemed to be the main causes of the occupational poisoning (Hunter et al. 1944). A case of severe intoxication in a 4-year-old child following ingestion of a lubricant containing TCP was reported. The clinical findings were vomiting, diarrhea, weakness, drowsiness, delayed cholinergic crisis, and depressed nerve velocity. Full recovery occurred within four weeks (Goldstein et al. 1988). The concentration of the o-isomer, which is a potent neurotoxic agent (IPCS 1990), in the TCP mixture manufactured at the time of these human case reports is believed to have been higher than what is in current TCP formulations.
There was a case report about the allergic contact dermatitis induced by contacting Band-Aid brand adhesive bandages which contains TCP as an ingredient (Norris and Storrs 1990). Also, a maximization test conducted dermally on male human subjects showed that TCP was a moderately strong sensitizer (Dupont 1992).
10.2.7 Toxicokinetics
No information on toxicokinetics of the TCP mixture was identified. Kurebayashi et al (1985) conducted a study on the para-isomer, p-TCP, a component of TCP, in male Wistar rats. Rats were administered a single dose of 7.8 mg/kg 14C-p-TCP in corn oil by gavage. 41% of the administered dose was excreted in the urine in 7 days, indicating that at least that amount was absorbed.
The distribution of 14C -p-TCP-derived radioactivity was studied in rats for up to 168 hours after administration of a single dose of 89.6 mg/kg14C-p-TCP by gavage in corn oil. At 24 hours, radioactivity was widely distributed in the tissues. Relatively high concentrations of label were found in adipose tissue, liver and kidneys, in addition to the intestine and stomach, whereas the lungs, testes, spleen, thymus, and blood had intermediate amounts of label and the lowest concentrations of radioactivity were found in the heart, muscle, and brain. At 72 hours, the concentration of radioactivity in tissues had diminished to approximately 25% of that detected at 24 hours. At 168 hours, the radioactivity in tissues had further decreased to approximately 10% of the values reported at 24 hours.
Kurebayashi et al. (1985) also studied the metabolism of p-TCP in male Wistar rats. Metabolites were identified in blood, urine, feces and tissues of rats at various times (up to 72 hours) following administration of 7.8 or 89.6 mg/kg 14C-p-TCP by gavage in corn oil. Metabolism involved a series of successive oxidations (in the liver) and hydrolysis (in the intestine) that resulted in p-hydroxybenzoic acid, di-p-cresyl phosphate, and p-cresyl p-carboxyphenyl phosphate as the major urinary metabolites. In the bile, the major metabolites were di-p-cresyl phosphate, p-cresyl p-carboxyphenyl phosphate, and the oxidized triesters di-p-cresyl p-carboxyphenyl phosphate, and p-cresyl p-carboxyphenyl phosphate. Analysis of the feces revealed metabolites very similar to those monitored in the bile; at the high dose, the main fecal metabolite was the unchanged p-TCP, probably due to incomplete absorption. Analysis of expired air showed 14CO2 that appeared to be formed by decarboxylation of p-hydroxybenzoic acid by intestinal microbes.
The excretion of 14C-labeled p-TCP was found in urine and feces from male Wistar rats following the administration of a single dose of 7.8 or 89.6 mg/kg of the compound by gavage in corn oil. With both doses, most of the radioactivity was excreted within 24 hours. At the low dose, 41% of the radioactivity was excreted in the urine and 44% in the feces in 7 days. Expired air accounted for 19% of the administered dose. In rats with cannulated bile ducts, about 28% of the administered radioactivity was excreted into the bile during 24 hours. At the high dose, 12% of the radioactivity was excreted in the urine and 77% in the feces, and 6% in expired air in 7 days. The authors suggested that the enterohepatic circulation and intestinal microflora play an important role in the degradation of p-TCP biliary metabolites (Kurebayashi et al. 1985). Elimination via the bile has also been shown after intravenous injection into rabbits (Gross and Grosse 1932), and intraperitoneal injection into rats (Myers et al 1955).
10.3 Characterization of risk to human health
No classifications of the health effects of TCP (containing <0.1% o-TCP) by national and international regulatory agencies were identified. There was no evidence of carcinogenicity of TCP found in a two-year study in rats and mice administered TCP in diets. TCP is unlikely to be genotoxic based on available information.
Based principally on the weight of evidence of available information, the critical effects of exposure to TCP were identified to be cytoplasmic vacuolization of adrenal cortex and interstitial cell hyperplasia in the ovary observed in rats and mice. The lowest LOAEL was identified at 7 mg/kg-bw/day, based on interstitial cell hyperplasia in the ovary observed in female rats in a two-year feeding study; the corresponding NOAEL was 4 mg/kg-bw/day. Comparison of this NOAEL with the highest estimates of total daily intake via environmental media (0.10 µg/kg-bw/day; breastfed infants of 0-6 months) resulted in a margin of exposure of approximately 39,000. This margin is considered adequate to account for uncertainties in the exposure and effects databases. The selection of the lowest NOAEL as the point of departure is also considered protective of developmental and neurotoxicological effects observed in animals at higher dose levels.
With respect to exposure to TCP in certain manufactured items, contact with furniture (with treated upholstery or foam) was identified as a potential source of exposure (Table 10-2). Comparison of the NOAEL of 4 mg/kg-bw/day from the two-year oral study to the highest estimate of daily dermal exposure estimates of chronic dermal exposure (2.6×10-2 mg/kg-bw/day) for adults sitting on furniture resulted in a margin of exposure of 156. This approach is considered conservative as the estimates of exposure are based on migration rates for organophosphate flame retardants that are more water soluble, i.e. prone to migration, than TCP. Furthermore, these conservative estimates of exposure are compared to a level at which no effect was observed in animals treated for 2 years. Based on this information, the MOE presented is considered adequate to account for uncertainties in the exposure and effects databases.
Comparison of the LOAEL of 20 mg/kg-bw/day identified from the developmental study (short-term) to the highest estimate from handling TCP-containing products (power steering fluid) (0.002 mg/kg-bw, per event, infrequent) resulted in a margin of exposure of approximately 10,000. This margin is considered adequate to account for uncertainties in the exposure and effects databases.
| Exposure route and duration | Source | Age group (weight) | Intake (mg/kg-bw/day) | Critical effect level (mg/kg-bw/day) | MOE |
|---|---|---|---|---|---|
| Oral (primary route) | Environ-mental media and food | Breastfed infant (7.5 kg) | 1.0 × 10-4 | 4 (NOAEL) | 39 000 |
| Dermal (daily) | Furniture | Toddler (15.5 kg) | 1.3×10-3 to 5.0×10-3 | 4 (NOAEL) | 801 to 3104 |
| Dermal (daily) | Furniture | Adult (70.9 kg) | 6.6×10-3 to 2.6×10-2 | 4 (NOAEL) | 156 to 605 |
| Dermal (infrequent; per event) | Power steering fluid | Adult (70.9 kg) | 0.002 | 20 (LOAEL) | 10 000 |
10.4 Uncertainties in evaluation of risk to human health
This screening assessment acknowledges uncertainties regarding the exposure database and health effects database.
Intake estimates for air, drinking water and household dust were based on Canadian empirical data. European data were used to estimate TCP intakes from fish and breast milk given the absence of Canadian data. No data in the primary literature were available for marketed foods in North America and Europe; however, European environmental fish data was available. The assumption that fish is the only source of dietary exposure (with the exception of breast milk for nursing infants) based on levels measured in Europe is an uncertainty. To account for this uncertainty, the dietary assessment for the general population conservatively assumes that all food items in the fish food group (i.e. fish, shellfish and related foods) contain the substances at upper bounding levels. Monitoring data selected for several media were not recent, which is an uncertainty in this assessment.
No TCP-specific migration data were identified; however, migration was conservatively estimated based on migration studies of other organophosphate substances expected to result in higher migration due to their higher water solubilities. While the scenario for the furniture (with treated upholstery and foam) was considered to be appropriate for assessing upper bounding exposure from products available to consumers, there is uncertainty regarding the presence of TCP in other manufactured items or products available in the marketplace in Canada.
There is an uncertainty with regard to the concentration of the ortho- isomer of TCP, o-TCP, which can cause organophosphate-induced delayed neuropathy (OPIDN) in humans and experimental animals. However, studies on TCP containing less than 0.1% o-TCP did not show OPIDN in experimental animals. This assessment is based on the assumption that all current commercial products contain less than 0.1% of o-TCP (see Section 2). Although this assumption is deemed to be appropriate based on available information, it cannot be precluded that there might be products containing higher concentrations of o-TCP on the Canadian market.
There is an uncertainty associated with the results of one prenatal developmental toxicity study in which reduced fetal body weight was observed following in utero exposure at 20 mg/kg- bw/day, the lowest dose tested, a dose lower than the dose at which maternal effects were observed (MPI Research Inc. 2004). However, delayed fetal ossification occurred at a much higher dose than that associated with maternal toxicity. The point of departure (4 mg/kg-bw/day) selected for the characterization of risk to human health is lower than 20 mg/kg-bw/day. Information on effects via dermal route was limited. The margins of exposure derived for dermal exposure scenarios were based on the critical effect level identified from an oral study. With characterization of the risk associated with exposure to TCP from sitting on upholstered furniture, there is uncertainty in the assumption that use of a more realistic migration rate and dermal absorption specific to TCP would result in a higher MOE. However, this assumption is considered reasonable since exposure was estimated based on a) rates of migration from foam or textile for organophosphate flame retardants which are much more soluble in water than TCP, and b) on an equivalent dermal/oral rate that is likely an overestimate given the lower dermal absorption percentage for other organophosphate substances characterized with physical-chemical properties (such as a lower Kow and/or molecular weight) more amenable to dermal absorption than TCP.
11. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to organisms and the broader integrity of the environment from TCP. It is concluded that TCP does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the adequacy of the margin between the upper-bounding estimates of exposure from environmental media or products available to consumers and effect levels for chronic or sub-chronic exposure, it is concluded that TCP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that TCP does not meet any criteria set out in section 64 of CEPA.
References
Abou-Donia MB, Varga PA, Graham DG, Kinnes CG. 1980. Delayed neurotoxicity of a single dermal administration of o-ethyl o-4-nitro-phenyl phenylphosphonothioate and tri-o-cresyl phosphate to cats. Toxicol Lett. 1 (Special issue): 141.
Adipose Autopsy Samples from Six Ontario Municipalities. Bull Environ Contam Toxicol 43:225–230.
Adema DMM, Canton JH, Slooff W, Hanstveit AO. 1981. Onderzoek naar een geshikte combinatie toetsmethoden ter bepaling van de aquatische toxiciteit van milieugevaarlijke stoffen. Rapport nr RIV 627905001, Rijksinstituut Voor Volksgezondheid en Milieuhygiene, Biltoven, the Netherlands, 112. [cited in UK EA 2009].
Adema DMM, Kuiper J, Hanstveit AO, Canton HH. 1983. Consecutive system of tests for assessment of the effects of chemical agents in the aquatic environment. Pesticide Chemistry: Human Welfare Environmental Protection. International Congress of Pesticide Chemistry, 5th, Meeting Date 1982, 3, 537-544. [cited in UK EA 2009].
[AEROWIN] AEROWIN Program for Windows [Estimation Model]. 2010. Version 1.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014 January].
Ali N, Dirtu AC, Van den Eede N, Goosey E, Harrad S, Neels H, Mannetje A, Coakley J, Douwes J, Covaci A. 2012.Occurrence of alternative flame retardants in indoor dust from New Zealand: Indoor sources and human exposure assessment. Chemosphere 88:1276–1282.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2010. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014 January].
Arnot JA, Gobas FAPC. 2003a. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337–345.
Arnot JA, Gobas FAPC. 2003b. Categorization of organic substances on the Domestic Substances List for bioaccumulation potential. Report to Environment and Climate Change Canada, Existing Substances Branch. June 2003. Gatineau (QC): Environment and Climate Change Canada.
Arnot JA, Gobas FAPC. 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev 14:257–297.
Arnot JA, Mackay D, Parkerton TF, Bonnell M. 2008a. Database of fish biotransformation rate for organic chemicals. Environ Toxicol Chem 27(11): 2263–2270.
Arnot JA, Mackay D, Bonnell M. 2008b. Estimating metabolic biotransformation rates in fish from laboratory data. Environ Toxicol Chem 27 (2): 341–351.
Arnot JA, Meylan W, Tunkel J, Howard PH, Mackay D, Bonnell M, Boethling RS. 2009. A quantitative structure-activity relationship for predicting metabolic biotransformation rates for organic chemicals in fish. Environ Toxicol Chem 28(6): 1168–1177.
Ash M, Ash I. 2000. Handbook of Industrial Chemical Additives. Synapse Information Resources, Inc., Endicott, NY.
Ash M, Ash I. 2003. Handbook of Paint and Raw Coating Materials. Synapse Information Resources, Inc., Endicott, NY.
Ashford RD. 1994. Ashford’s Dictionary of Industrial Chemicals. Wavelength Publications Ltd., London, England. p. 908. [cited in US EPA 2010] and [cited in UK EA 2009].
ASTreat [sewage treatment plant removal model]. 2006. Version 1.0. Cincinnati (OH): Procter & Gamble Company. Available from Procter & Gamble Company, Cincinnati, OH, USA.
[ATSDR] Agency for Toxic Substances and Disease Registry. 1997. Toxicological profile for hydraulic fluid [PDF]. Washington (DC): US Department of Health and Human Services, Public Health Service. [cited June 2013].
[ATSDR] Agency for Toxic Substances and Disease Registry. 2012. Toxicological profile for phosphate ester flame retardants [PDF]. Atlanta (GA): US Department of Health and Human Services, Public Health Service. [cited June 2013].
Bacaloni A, Cavaliere C, Foglia P, Nazzari M, Samperi R, Lagana A. 2007. Liquid chromatography/tandem mass spectrometry determination of organophosphorus flame retardants and plasticizers in drinking and surface waters. Rapid Commun Mass Spectrom 21:1123–1130.
Banjeree, BD, Saha, S, Ghosh, KK and Nandy P. 1992. Effect of tricresyl phosphate on humoral and cell-mediated immune responses in albino rats. Bull Environ Contam Toxicol 49:312-317.
[BASL4] Biosolids-Amended Soil Level 4 Model. 2011. Version 2.00. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. [cited 2013 Nov 1]
Bayer. 2002. Technical information and safety data sheet for Disflamoll TKP. Bayer AG. [cited in UK EA 2009].
[BCFBAF] Bioaccumulation Program for Windows [Estimation Model]. 2010. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014 January].
Bengtsson BE, Tarkpea M, Sletten T, Carlberg GE, Kringstad A, Renberg L. 1986. Bioaccumulation and effects of some technical triaryl phosphate products in fish and Nitocra spinipes. Environ Toxicol Chem 5:853–861.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2010. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014 January].
Boethling GRS, Cooper JC. 1985. Environmental fate and effects of triaryl and trialkyl/aryl phosphate esters. Residue Reviews 94:49–99.
Boethling RS, Howard PH, Beauman JA, Larosche, ME. 1995. Factors for intermedia extrapolation in biodegradability assessment. Chemosphere 30:741–752.
Brandsma SH, de Boer J, van Velzen MJM, Leonards PEG. 2014. Organophosphorus flame retardants (PFRs) and plasticizers in house and car dust and the influence of electronic equipment. Chemosphere 116:3-9.
Brommer S, Harrad S, Van Den Eede N, Covaci A. 2012. Concentrations of organophosphate esters and brominated flame retardants in German indoor dust samples. J Environ Monit 14(9):2482–2487.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada Dept. of the Environment. 2013. Canadian Environmental Protection Act, 1999: Notice with respect to certain organic flame retardant substances [PDF]. Canada Gazette, Part I, vol. 147, no. 13, p. 613–633. [cited 2017 August 17]
Carlton BD, Hasaran AH, Mezza LE, Smith MK. 1987. Examination of the reproductive effects of tricresyl phosphate administered Long- Evans rats. Toxicology 46: 321-328. [cited in UK EA 2009, IPCS 1990, GLCC 2001].
Casterline JL Jr., Ku Y, Barnett NM. 1985. Uptake of tri-p-cresyl phosphate in soybean plants. Bulletin of Environ Contam Toxicol 35:209-212.
CATALOGIC [Computer Model]. 2012. Version 5.11.13. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry.
Cavalleri A and Cosi V. (1978) Polyneuritis incidence in shoe factory workers: Case report and etiological considerations. Arch. environ. Health, 33: 192-197.
CEC. 2015. Enhancing Trilateral Understanding of Flame Retardants and Their Use in Manufactured Items: Analysis of Select Flame Retardants Contained in Office and Household Furniture. Montreal, Canada: Commission for Environmental Cooperation. 16 pp.
Cequier E, Ionas AC, Covaci A, Marcé RM, Becher G, Thomsen C. 2014. Occurrence of a broad range of legacy and emerging flame retardants in indoor environments in Norway. Environ Sci Technol. 48: 6827-6835.
Chapin RE, George JD, Lamb JC. 1988. Reproductive toxicity of tricresyl phosphate in a continuous breeding protocol in Swiss CD-1 mice. Fund Appl Toxicol 10:344-354. [cited in IPCS 1990, UK EA 2009]
Chemtura Corporation. 2007. Chemtura Petroleum Additives: Components and Solutions [PDF]. West Lafayette (IN): Chemtura Corporation.
[ChemFate] Interactive ChemFate Database [database on the Internet]. 2012. Syracuse (NY): Syracuse Research Corporation. [cited 2014 June 11]
Chen D, Letcher RJ, Chu S. 2012. Determination of non-halogenated, chlorinated and brominated organophosphate flame retardants in herring gull eggs based on liquid chromatography–tandem quadrupole mass spectrometry. J Chromatogr A 1220:169–174.
Cho KJ, Kirakawa T, Mukai T, Takimoto K, Okada M. 1996. Origin and stormwater runoff of TCP (tricresyl phosphate) isomers. Water Research 30(6):1431–1438.
Cho KJ, Takimoto K, Okada M. 1994. Fate of tricresyl phosphate isomers in Kurose River (Japan). Water Science and Technology 30(10):189-197.
City of Toronto. 2003a. Water quality quarterly report (January–March 2003). Works and Emergency Services, Toronto.
City of Toronto. 2003b. Water quality quarterly report (April–June 2003). Works and Emergency Services, Toronto.
City of Toronto. 2003c. Water quality quarterly report (July–September 2003). Works and Emergency Services, Toronto.
Crockett AB. 1978 a. U.S. EPA: Letter to M.P. Halper, U.S. EPA, Oct. 26. [cited in Boethling and Cooper 1985].
Crockett AB. 1978b. U.S. EPA: Letter to P. Hilgard, U.S. EPA. [cited in Boethling and Cooper 1985].
Crockett AB. 1978c. U.S. EPA: Letter to G. B. Wiersma, U.S. EPA, June 2. [cited in Boethling and Cooper 1985].
Crockett A B. 1979. U.S. EPA: Letter to P. Hilgard, U.S. EPA, March 6. [cited in Boethling and Cooper 1985].
Daughtrey, W., Biles, R., Jortner, B, and Ehrich, M. (1996) Subchronic delayed neurotoxicity evaluation of jet engine lubricants containing phosphorus additives. Fundam Appl Toxicol 32:244–249
David MD, Seiber JN. 1999a. Accelerated hydrolysis of industrial organophosphates in water and soil using sodium perborate. Environmental Pollution 105:121-128.
David MD, Seiber JN. 1999b. Analysis of organophosphate hydraulic fluids in U.S. Air force base soils. Arch Environ Contam Toxicol 36:235–241.
De Nola G, Kibby J, Mazurek W. 2008. Determination of ortho-cresyl phosphate isomers of tricresyl phosphate used in aircraft turbine engine oils by gas chromatography and mass spectrometry.J Chromatogr A 1200:211–216.
DeNola G, Hanhela PJ, Mazurek W. 2011. Determination of tricresyl phosphate air contamination in aircraft. Ann Occup Hyg 55:710-722.
De Silva A and Muir D. 2016. Using remote environments to detect new persistent and bioaccumulative chemicals in commerce. Unpublished report. Gatineau (QC): Environment and Climate Change Canada.
De Silva A, Smyth S A, Derek M. 2017. Occurrence and fate of certain organic flame retardants in municipal wastewater treatment systems: organophosphorus. Unpublished report. Gatineau (QC): Environment and Climate Change Canada.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
Dirtu AC, Ali N, Van den Eede N, Neels H, Covaci A. 2012. Country specific comparison for profile of chlorinated, brominated and phosphate organic contaminants in indoor dust. Case study for Eastern Romania, 2010. Environ Int 49:1–8.
Dodson R, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. 2012. After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environ Sci Technol 46:13056–13066.
[DPD] Drug Product Database [database]. [modified 2017 Feb 17]. Ottawa (ON): Government of Canada. [cited 2014 April 24].
Dupont Chemical. 1992. Initial submission: study synopsis regarding tricresyl phosphate and 2,3-dibromopropyl phosphate with cover letter dated 101592. NTIS/OTS Fiche # 0571488.
[ECHA] European Chemicals Agency. 2010. Guidance on information requirements and chemical safety assessment. Chapter R.16:Environmental exposure estimation. May 2010. Guidance for the implementation of REACH. Helsinki (FI): European Chemicals Agency.
[ECHA] European Chemicals Agency. c2007-2013. Registered Substances database. Search results for CAS RN 1330-78-5. Helsinki (FI): ECHA. [updated 2013 July 26; cited 2013 July 17].
Environment Agency Japan. 1996. Chemicals in the environment. Report on environmental survey and wildlife monitoring of chemicals in F.Y. 1994. Environmental Health and Safety Division, Environment Agency Japan, May 1996. [Cited in UK EA 2009].
[EC] Environment Canada. 2001. Canadian Environmental Protection Act, 1999. Notice with respect to certain substances on the Domestic Substances List (DSL) [PDF]. Canada Gazette 135(46):4194–4211. [cited 2017 August 17]
Environment Canada, Health Canada. 2007. Chemical substances: Categorization. Ottawa (ON): Government of Canada. [updated 2007 April 20; cited 2014 June 10].
[ECCC] Environment and Climate Change Canada. 2013-2014. Data for certain organic flame retardants substance grouping collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain organic flame retardant substances. Data prepared by: Environment and Climate Change Canada, Health Canada; Existing Substances Program.
Environment Canada. 2016. Ecological screening assessment report on Cresol (phenol, methyl-) Substances, CEPA, May 2016. Environment and Climate Change Canada and Health Canada. Available from: JKuo(26Oct2017): revised based on page 5.
[ECCC 2018a] Environment and Climate Change Canada. 2018a. Supporting documentation: Summary tables of substance identity and physical chemical property information for Tricresyl phosphate. Gatineau (QC): Environment and Climate Change Canada. Information in support of the Screening Assessment for Phosphoric acid, tris(methylphenyl) ester. Available on request from: ec.substances.ec@canada.ca
[ECCC 2018b] Environment and Climate Change Canada. 2018b. Supporting documentation: Summary tables of environmental concentrations information for Tricresyl phosphate. Gatineau (QC): Environment and Climate Change Canada. Information in support of the Screening Assessment for Phosphoric acid, tris(methylphenyl) ester. Available on request from: ec.substances.ec@canada.ca
[ECCC 2018c] Environment and Climate Change Canada. 2018c. Supporting documentation: Summary tables of environmental fate and behaviour information for Tricresyl phosphate. Gatineau (QC): Environment and Climate Change Canada. Information in support of the Screening Assessment for Phosphoric acid, tris(methylphenyl) ester. Available on request from: ec.substances.ec@canada.ca
[ECCC 2018d] Environment and Climate Change Canada. 2018d. Supporting documentation: Summary tables of ecotoxicity data information for Tricresyl phosphate. Gatineau (QC): Environment and Climate Change Canada. Information in support of the Screening Assessment for Phosphoric acid, tris(methylphenyl) ester. Available on request from: ec.substances.ec@canada.ca
[ECCC 2018e] Environment and Climate Change Canada. 2018e. Supporting documentation: Summary tables of ecological exposure information for Tricresyl phosphate. Gatineau (QC): Environment and Climate Change Canada. Information in support of the Screening Assessment for Phosphoric acid, tris(methylphenyl) ester. Available on request from: ec.substances.ec@canada.ca
[ECOSAR] Ecological Structure Activity Relationships Class Program [Estimation Model]. 2012. Version 1.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited January 2014].
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2012. Version 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[EQC] Equilibrium Criterion Model. 2012. Version 1.00. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre.
European Commission. 2011. Identification and evaluation of data on flame retardants in consumer products. Final Report [PDF]. Brussels, Belgium: prepared for the European Commission, Health and Consumers Department, by ARCADIS Belgium, Contract no. 17.020200/09/549040.
[EU RAR] European Union. 2009. European Union Risk Assessment Report. Tris(2-chloroethyl)phosphate (TCEP) [PDF]. July 2009.
Evenset A, Leknes H, Christensen GN, Warner N, Remberger M, Gabrielsen GW. 2009. Screening of New Contaminants in Samples from Norwegian Arctic. NIVA Report 4351-1, SPFO-Report 1049/2009. TA-2510/2009. ISBN:978-82- 449-0065-2. [cited in Van der Veen and de Boer 2012].
Fan, X., Kubwabo, C., Rasmussen, P. E., Wu, F. (2014). Simultaneous determination of thirteen organophosphate esters in settled indoor house dust and a comparison between two sampling techniques. Sci. Tot. Environ. 491–492; 80–86.
FMC Corporation. 1995. Tricresyl phosphate/Durad 125L Acute delayed neurotoxicity study in hens (study # I94-1925). NTIS/OTS #0572164.
Food and Drug Research Laboratories, Inc. 1975a. Acute Dermal Toxicity Study in Rabbits. Conducted for FMC Corporation. Cited in IUCLID 2001
Food and Drug Research Laboratories, Inc. 1975b. Eye Irritation Test in Rabbits. Conducted for FMC Corporation. Cited in IUCLID 2001
Foster D. Snell, Inc. 1976. 28-Day Oral Administration in Rats. Conducted for FMC Corporation. Cited in IUCLID 2001
Freeman GB, Irwin R, Trejo R, Hejtmancik M, Ryan M, Peters AC. 1988. Reversibility and tolerance to tricresyl phosphate-induced neurotoxic effects in F344 rats. Toxicologist, 8, 76 (cited in IPCS, 1990).
Freudenthal RI, Rausch L, Gerhart JM, Barth ML, Mackerer, CR, Bisinger, EC. 1993. Subchronic neurotoxicity of oil formulations containing either tricresyl phosphate or tri-orthocresyl phosphate. J Am Coll Toxicol 12:409–416.
Fromme H, Lahrz T, Kraft M, Fembacher L, Mach C, Dietrich S, Burkardt R, Völkel W, Göen T. 2014. Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3). Environ. Int.71: 158-163.
Fukushima M, Kawai S, Yamaguchi Y. 1992. Behavior of organophosphoric acid triesters in Japanese riverine and coastal environment. Water Science and Technology 25:271–278. UK EA 2009 (Glob 1998). [cited in UK EA 2009].
[GFEA] German Federal Environmental Agency. 2001. Substituting Environmental Relevant Flame Retardants: Assessment Fundamentals–Results and summary overview [PDF]. Research report 204 08 542 (old) 297 44 542 (new) UBA-FB 000171/le. By Andre Leisewitz, Hermann Kruse, Engelbert Schramm. Commissioned by the German Federal Environmental Agency (Umweltbundesamt, UBA) June 2001. ISBN 0722-186X.
Glob E. 1998. Miljøfremmde stoffer i havbunden. Lillebætsamarbejdet. [cited in UK EA 2009].
Gobas FAPC, Kelly BC, Arnot JA. 2003. Quantitative Structure Activity Relationships for Predicting the Bioaccumulation of POPs in Terrestrial Food-Webs. QSAR Comb. Sci. 22: 329–336.
Gobas FAPC. 2010. Comments on approach to sediment exposure. Burnaby (BC): Simon Fraser University, School of Resource & Environmental Management, March 25.
Gobas FA, Morrison HA. 2000. Bioconcentration and biomagnification in the aquatic environment. In: Boethling RS, Mackay D, editors. Handbook of property estimation methods for chemicals, environmental and health sciences. Boca Raton (FL): CRC. p 189C.
Goldstein DA, Mcguigan MA, Ripley BD. 1988. Acutetricresyl phosphate intoxication in childhood. Hum Toxicol 7:179–182. Cited in IPCS 1990.
Grimalt R, Romaquera C, Vilaplana J. 2009. Allergic Contact Dermatitis from Tricresyl Phosphate. Dermatitis 20(5):297–298
Gross E, Grosse A. 1932. A contribution to the toxicology of ortho-tricresylphosphates. Naunyn-Shmiedeberg’s Archives of Pharmacology, 168:473–514 (in German). Cited in IPCS 1990.
Haggerty GJ, Hejtmancik M, Deskin R, Ryan MJ, Peters AC, Irwin R. 1986. Effects of subchronic exposure to tricresyl phosphate in F344 rats and B6C3F1 mice: behavioural and morphological correlates. Toxicologist 6:218 (cited in IPCS, 1990).
Hann RW Jr, Jensen PA. 1977. Water quality characteristics of hazardous materials [DOC]; NTIS-PB-285946. Texas A&M Univ., College Station Environmental Engineering Div. 1751 pp. 1977 [ cited in ChemFate 2012].
Hartmann PC, Burgi D, Giger W. 2004. Organophosphate flame retardants and plasticizers in indoor air. Chemosphere 57:781–787.
Haworth, S., Lawlor, T., Mortelmans, K., Speck, N., and Zeiger, E. (1983) Salmonella mutagenicity test results for 250 chemicals. Environ. Mutagen. Suppl., 1, 3-142 (cited in IPCS, 1990).
Health Canada. 1994 Canadian Environmental Protection Act: Human health risk assessment for priority substances. Ottawa, Ontario
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. [modified 2017 May 3]. Lists of Permitted Food Additives [internet lists]. Ottawa (ON): Health Canada Food Directorate. [cited 2014 April 24].
Health Canada. 2014. CMP Survey 2014-2015: Determination of flame retardants in consumer products. Unpublished. Health Canada. Ottawa, Ontario.
Health Canada. [modified 2015 Dec 14]. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products [database]. Ottawa (ON): Government of Canada [cited 2014 April 24].
Hine C, Rowe VK, White ER, Darmer KI Jr, Youngblood GT. 1981. In: Clayton, G.D. & Clayton, F.E., ed. Patty's industrial hygiene and toxicology, 3rd revised ed Vol. 2A. New York, Wiley-Interscience. p. 2362–2363. [cited in IPCS 1990].
Hoke RA, Giesy JP, Zabik M, Unger M.1993. Toxicity of sediments and sediment pore waters from the Grand Calumet River-Indiana Harbor area of concern. Ecotoxicity and Environmental Safety. 26(1):86–112. [cited in UK EA 2009].
Hollifield HC. 1979. Rapid nephelometric estimate of water solubility of highly insoluble organic chemicals of environmental interest. Bulletin of Environmental Contamination and Toxicology 23:579–586.
Houde M, Berryman D, de Lafontaine Y, Verrault J. 2014. Novel brominated flame retardants and dechloranes in three fish species from the St. Lawrence River, Canada. Sci Total Environ 479–480:48–56.
Howard PH, Deo PG. 1979. Degradation of aryl phosphates in aquatic environments. Bulletin of Environmental Contamination and Toxicology 22:337–344.
[HSDB] Hazardous Substances Data Bank [database]. 2012. Bethesda (MD): National Library of Medicine (US). [revised 2013 Mar 8; cited 2013 June 15].
[HSDB] Hazardous Substances Data Bank [database]. 2014. Bethesda (MD): National Library of Medicine (US). [cited 2018 Oct 29].
Hunter, D., Perry, KMA, Evans RB. 1944. Toxic polyneuritis arising during the manufacture of tricresyl phosphate. Br. J. Ind. Med. 1:227–231 (cited in IPCS, 1990).
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2010. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014 January].
[ICL] Israel Chemicals Ltd. Industrial Products, 2012. Flame Retardants [PDF]. Beer Sheva, Israel. [cited June 2013].
[ICL] Israel Chemicals Ltd. Industrial Products, 2013a. Application–Phenolic Resins. Tel Aviv, Israel. [cited June 2013].
[ICL] Israel Chemicals Ltd. Industrial Products, 2013b. Application–PVC. Tel Aviv, Israel. [cited June 2013].
[ICL] Israel Chemicals Ltd. Industrial Products, 2013c. Segment–Bedding and Seating [Internet]. Tel Aviv, Israel. [cited June 2013].
Imperial Oil. 1991. Male reproductive toxicity assessment following a fourteen-day oral exposure to phosphate esters in F344 rats. Study # 64412. Obtained from EC 2001.
[IPCS] International Programme on Chemical Safety. 1990. Tricresyl Phosphate. Geneva (CH): World Health Organization. (Environmental Health Criteria 110). Jointly sponsored by the United Nations Environment Programme, the International Labour Organization, and the World Health Organization.
Irwin R, Freeman GB, Trejo R, Hehtmancik M, Peters A. 1987. Effects of chronic exposure to tricresyl phosphate I F344 rats and B6C3F1 mice: behavioural and biochemical correlates. Soc Neurosci Abstr 13:90 (cited in IPCS 1990).
[IUCLID] 2001. IUCLID Data set for tris(methylphenyl) phosphate. Great Lakes Chemical Corporation. [cited in UK EA 2009].
Ishikawa S et al. 1985a. Suishitsu Odaku Kenkyu 8: 799–807. [cited in HSDB 2012] .
Ishikawa S, Taketomi M, Shinohara R. 1985b. Determination of trialkyl and triaryl phosphates in environmental samples. Water Research 19:119–125. [cited in UK EA 2009].
Ishikawa S, Taketomi M, Shinohara R. 1985c. Determination of organic phosphate esters in factory effluent and domestic effluent. Japanese Journal of Water Pollution Research 8:529–535. [cited in UK EA 2009].
Izmerov NF, Sanotsky IV, Sidorov KK. 1982. Toxicometric parameters of industrial toxic chemicals under single exposure, Moscow, Centre of International Projects, GKNT, p. 14. (Cited in IPCS 1990).
Jayatilaka NK, Restrepo P, Williams L, Ospina M, Valentin-Blasini L, Calafat AM. 2017. Quantification of three chlorinated dialkyl phosphates, diphenyl phosphate, 2,3,4,5-tetrabromobenzoic acid, and four other organophosphates in human urine by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 409:1323-1332.
Johanssen FR, Wright PL, Gordon DE, Levinskas GJ, Radue RW, Graham PR 1977. Evaluation of delayed neurotoxicity and dose-response relationships of phosphate esters in the adult hen. Toxicol Appl Pharmacol 41:291–304. (Cited in IPCS 1990).
Kajiwara N, Noma Y, Takigami H. 2011. Brominated and organophosphate flame retardants in selected consumer products on the Japanese market in 2008. J Hazard Mater 192: 1250–1259.
Kawagoshi Y, Fukunaga I, Itoh H. 1999. Distribution of organophosphoric acid triesters between water and sediment at a sea-based solid waste disposal site. J Mater Cycles Waste Manage 1:53–61.
Kenmotsu K, Matsunaga K, Ishida T. 1980 [Studies on the mechanisms of biological activities of various environmental pollutants. V. Environmental fate of organic phosphoric acid triesters.] Okayama-ken Kankyo Hoken Senta Nempo, 4:103–110 (in Japanese). [cited in IPCS 1990].
Kersten W, Reich T. 2003. [Non-volatile organic substances in Hamburg indoor dust.] Reinhalt Luft 63:85-91. [article in German with English abstract].
Kim JW, Ramaswamy BR, Chang KH, Isobe T, Tanabe S. 2011. Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry. J Chromatogr A. 1218:3511-3520.
Kim JW, Isobe T, Sudaryanto A, Malarvannan G, Chang KW, Muto M, Prudent M, Tanabe S. 2013. Organophosphorus flame retardants in house dust from the Philippines: occurrence and assessment of human exposure. Environ Sci Pollut Res 20:812–822.
Kim JW, Isobe T, Sudaryanto A, Malarvannan G, Chang KH, Muto M,Tanabe S. 2013. Organophosphorus flame retardants in house dust from the Philippines: occurrence and assessment of human exposure. Environ Sci Pollut Res20(2): 812–822.
Kinkead ER, Bunger SR, Wolfe RE Wall HG. 1990. The acute delayed neurotoxicity evaluation of two jet engine oil formulations. NTIS/OTS Fiche # 0516607-3.
Kinkead ER Wolfe RE, Salins SA, Godin CS, Flemming CD. 1992. Acute delayed neurotoxicity evaluation of two jet engine oils using a modified navy and EPA protocol. Govt Reports Announcements & Index, Issue 14, 1993; NTIS/AD-A262 580/4.
Kishi H, Sekine Y. 2003. Simple prediction of atmospheric concentration of hydrophilic compounds based on the classification of industrial uses. QSAR & Combinatorial Science 22:396–398.
Klimisch HJ, Andreae E, and Tillmann U. 1997. A systematic approach for evaluating the quality of experimental and ectoxicological data. Regulatory Toxicology and Pharmacology 25:1-5.
[KOAWIN] Octanol Air Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2010. Version 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014 January]. [
KOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2010. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014 January].
Kosarac I, Kubwabo C, Foster WG. 2016. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass sepectrometry (UPLC-MS/MS). J Chromatogr B 1014:24-30.
Ku Y, Alvarez GH. 1982. Biodegradation of 14C-tri-p-cresyl phosphate in a laboratory activated sludge system. Appl Environ Microbiol 43:619–622.
Kurebayashi H, Tanaka A, Yamaha T. 1985. Metabolism and disposition of the flame retardant plasticizer, tri-p-cresyl phosphate, in the rat. Toxicol Appl. Pharmacol 77: 395–404.
LAB Research Ltd. Final report: Skin sensitization: Local Lymph Node Assay with Kronitex TCP. Study code :10/160-037E. Date of Final Report: 01 October 2010. (section 71 submission 2014)
Latendresse J, Brooks C, Capen C. 1994a. Pathologic effects of butylated triphenyl phosphate-based hydraulic fluid and tricresyl phosphate on the adrenal gland, ovary, and testes in the Fischer 344 rat. Toxicol Pathol 22:341–352. [cited in UK EA 2009, IUCLID 2001].
Latendresse JR, Brooks CL, Capen CC. 1994a. Pathologic effects of butylated triphenyl phosphate-based hydraulic fluid and tricresyl phosphate on the adrenal gland, ovary, and testis in the Fischer-344 rat. Toxicol Pathol 22: 341–352.
Latendresse JR, Brooks CL, Flemming CD, Capen CC. 1994b. Reproductive Toxicity of Butylated Triphenyl Phosphate and Tricresyl Phosphate Fluids in F344 Rats. Fundam Appl Toxicol 22:392–399.
Latendresse JR, Brooks CL, Capen CC. 1995. Toxic effects of butylated triphenyl phosphate-based hydraulic fluid and tricresyl phosphate in female F344 rats. Vet Pathol. 32(4):394–402.
Latourette HK. 1978. FMC Corp.: Letter to U.S. Environmental Protection Agency, attn. 1. Urquhart. [cited in Boethling and Cooper 1985].
Latourette H.K.1980. FMC Corp.: Letter to D. W. Smith, U.S. EPA, July II. [cited in Boethling and Cooper 1985].
LeBel GL, Williams DT, Benoit FM. 1981. Gas Chromatographic Determination of Trialkyl/Aryl Phosphates in Drinking Water, Following Isolation Using Macroreticular Resin. J Assoc Off Anal Chem 64(4):991–998.
LeBel GL, Williams DT, Berard D. 1989. Triaryl/Alkyl Phosphate Residues in Human Adipose Autopsy Samples from Six Ontario Municipalities. Bull Environ Contam Toxicol 43:225 – 230.
Lee HB, Peart T, Muir D, Teixeria C, Sett A, Struger J, Backus S. 2011. Organophosphorus flame retardants and benzotriazoles in open waters and tributaries of the Great Lakes. Prepared by Environment and Climate Change Canada (poster).
Lefaux R. 1972. Industrial toxicology. Phosphoric esters. In: Practical toxicology of plastics, London, Iliffe Books Ltd, pp 121–127. [cited in IPCS 1990].
Leonards P, Steindal EH, van der Veen I, Berg V, Bustnes JO, van Leeuwen S. 2011. Screening of Organophosphorus Flame Retardants 2010. SPFO-Report 1091/ 2011. TA-2786/2011. [cited in Van der Veen and de Boer 2012].
Litton Bionetics Inc. 1979a. Evaluation of Phosflex 31 P in the in vitro transformation of BALB3T3 cells. Conducted for Stauffer Chemical Company. Cited in IUCLID 2001
Litton Bionetics Inc. 1979b. Mutagenicity evaluation of Phosflex 31 P in an in vitro cytogenetic assay measuring sister chromatid exchange and chromosomal aberrations. Conducted for Stauffer Chemical Company. Cited in IUCLID 2001
Litton Bionetics Inc. 1979c. Mutagenicity evaluation of phosflex 31 P in the Ames Salmonella microsome plate test. Conducted for Stauffer Chemical Company. Cited in IUCLID 2001
Litton Bionetics Inc. 1979d. Mutagenicity evaluation of Phosflex 31 P in the mouse
lymphoma forward mutation assay. Conducted for Stauffer Chemical Company. Cited in IUCLID 2001
Liu X, Ji K, Choi K. 2012. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol 114–115:173–181.
Liyasova M, Li B, Schopfer LM et al. 2011. Exposure to tri-o-cresyl phosphate
detected in jet airplane passengers. Toxicol Appl Pharmacol 256:337–347.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Government of Canada. [cited 2014 April 24]
Lombardo P, Egry IJ.1979. Identification and gas-liquid chromatographic determination of aryl phosphate residues in environmental samples. J AOAC Int 62:47–51. [cited in WHO 1990, UK EA 2009].
Mackerer CR, Barth ML, Kreuger AJ, Chawla B, Roy T.A. 1999. Comparison of neurotoxic effects and potential risks from oral administration of ingestion of jet engine lubricants. J Toxicol Environ Health 57:293–328.
Marhold, JV. 1972. Collection of results of toxicological assessments of compounds and formulations tested at the Department of Toxicology, Research Institute for Organic Synthesis, Prague, Institute for Management Training in the Chemical industry. (cited in IPCS 1990).
Marklund A, Andersson B, Haglund P. 2005. Traffic as a Source of Organophosphorus Flame Retardants in Snow. Environ Sci Technol 39:3555–3562.
Martínez-Carballo E, González-Barreiro C, Sitka A, Scharf S, Gans O. 2007. Determination of selected organophosphate esters in the aquatic environment of Austria. Sci Total Environ 388:290–299.
Marzulli FN, Callahan JF, Brown DW. 1965. Chemical structure and skin penetrating capacity of a short series of organic phosphates and phosphoric acid. J Invest Dermatol 44:339–344. (cited in Sjögren et al. 2010)
Mele JM, Jensh RP. 1977. Teratogenic effects of orally administered tri-ocresyl phosphate on Wistar albino rats. Teratology 15:32A. [cited in IPCS 1990, UK EA 2009]
Michaelis S. 2002. Aircraft cabin fumes: an aviation safety issue. J Occup Health Safety – Aust NZ 18(4):291–294.
Midwest Research Institute. 1977. Assessment of the Need for the Character of and the Impact Resulting from Limitations on Aryl Phosphates, MRI 4309-L. Midwest Research Institute, Kansas City, Mo., 279 p. [cited in ChemFate 2012, US EPA 2010].
Mirsalis, J. et al. 1985. Environmental Mutagenesis, 5:482. Cited in EA 2009
Mobil Oil Corporation. 1987. Determination of the effects of tritolyl phosphate (tricresyl phosphate, Stock 955) on rat serum, erythrocyte and brain cholinesterase activities. Study # 61739. Obtained from EC 2001
Mobil Oil Corporation (1990). Final Report: The Effect of a Generic Jet Engine Oil Formulation on Serum Cholinesterase (ChE) Activity in Female Sprague Dawley Rats Following Two Weeks of Dermal Administration: Range-Finding Study prepared by Mobil Environmental and Health Science Laboratory. Study # 63114.Obtained from EC 2001.
Mobil Oil Corporation (1991e). Male reproductive toxicity assessment following a fourteen-day oral exposure to Stock 955 in F344 and Long Evans Rats–Range Finding Study. Study # 64240. Obtained from EC 2001.
Mobil Oil Corporation (1992b). Reproductive toxicity assessment in male F344 rats following a 10-week oral exposure to generic jet engine oil formulations. Study # 64972. Obtained from EC 2001.
[MOE Japan] Japan Ministry of Environment. 2003. Surveyed Chemical Substances and their Detected Levels in the Environment (A Cumulative List for Fiscal Year 1974 – 1998) [PDF]. [cited 2013 June 14].
Morrissey RE, Schwetz BA, Lamb JC IV, Ross MD, Teague JL, Morris RW. 1988a. Evaluation of rodent sperm, vaginal cytology, and reproductive organ weight data from National Toxicology Program 13-week study. Fundam Appl Toxicol 11:343–-358 (cited in IPCS 1990).
Modern Plastics Encyclopedia. 1975. International Advertising Supplement 52(10A), New York, McGraw-Hill Inc. p 697. [cited in IPCS 1990].
[MPBPVP] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2010. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014 January].
MPI Research Inc. 2004. An oral prenatal developmental toxicity study of tricresyl phosphate in rats. Study# 1038-003. Obtained from ECCC 2013-2014 Muir DCG, Yarechewski AL, Grift NP.1983. Environmental dynamics of phosphate esters. III. Comparison of the bioconcentration of four triaryl phosphates by fish. Chemosphere 12:155–166.
Muir, DCG. 1984. Phosphate esters. In: Hutzinger, O., ed. The handbook of environmental chemistry, Berlin, Heidelberg, New York, Tokyo, Springer-Verlag, Vol. 3, Part C, pp. 41–66. [cited in UK EA 2009, WHO 1990]
Muir DCG, Lint D, Grift, NP. 1985. Fate of three phosphate ester flame retardants in small ponds. Environ Toxicol Chem 4:663–675.
Myers DK, Rebel JBJ, Veeger D, Kemp A, Simons EGL. 1955. Metabolism of triaryl phosphates in rodents. Nature (London), 176, 259–260. [cited in IPCS 1990]
[NCI] National Chemical Inventories [database on CD-ROM]. 2013. Issue 1. Columbus (OH): American Chemical Society.
New York University 1951. Bellevue Medical Centre April 10, 1951. Data from Exxon Research and Engineering Company, NTIS OTS 206104. [cited in EA 2009]
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2017 June 21]. Ottawa (ON): Government of Canada. [cited 2014 April 24].
[NITE] National Institute of Technology and Evaluation database [database on the Internet]. 2008. GHS Classification Guidance by the Japanese Government. [cited 2017 May 18].
[NRC] National Research Council (US). 2000. Toxicological Risks of Selected Flame-Retardant Chemicals. Washington, DC: National Academy Press.
Nichols JW, Fitzsimmons PN, Burkhard LP. 2007. In vitro – in vivo extrapolation of quantitative hepatic biotransformation data for fish. II. Modeled effects on chemical bioaccumulation. Environ Toxicol Chem 26:1304-1319.
NOMO5. 1987. Programs to Enhance PC-Gems Estimates of Physical Properties for Organic Compounds. The Mitre Corp. [cited in US EPA 2010].
Norris P, Storrs F J. 1990. Allergic contact dermatitis to adhesive bandages. Dermatologic Clinics 8:147–152. [cited in IUCLID 2001]
[NPRI]. National Pollutant Release Inventory. 2011.
NTP (National Toxicology Program). 1994. Toxicology and carcinogenesis studies of tricresyl phosphate (CAS No. 1330-78-5) in F344/N rats and B6C3F1 mice (gavage and feel studies). Research Triangle Park, North Carolina, National Toxicology Program (Technical Report Series No. 433, PB 95-227377).
[OECD] Organisation for Economic Co-operation and Development. 2009. Emission scenario document on plastics additives [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 3). Report No.: ENV/JM/MONO(2004)8/REV1. JT03267870. [cited 2014 October].
OECD QSAR Toolbox. [Read-across tool]. 2012. Version 3.0. Paris (FR): Organisation for Economic Co-operation and Development, Environment Directorate.
Okazaki ME, D’Souza A, Antika S. 2003. United States Patent No. 6,649,080 B2
Phosphate ester base stocks and aircraft hydraulic fluids comprising the same.[cited in Schindler et al. 2014]
Oishi H, Oishi S, Hiraga K. 1982. Toxicity of several phosphoric acid esters in rats. Toxicol Lett 13:29–34 (cited in IPCS 1990).
Ospina M, Jayatilaka NK, Wong L-Y, Restrepo P, Calafat AM. 2018. Exposure to organophosphate flame retardant chemicals in the U.S. general population: Data from the 2013-2014 National Health and Nutrition Examination Survey. Environment International. 110:32-41.
Piccirillo , VJ. 1999. Flame-retardant product chemistries, toxicology and hazard evaluations. NPC Inc., Sterling, VA. [cited in NRC 2000]
Repke VL, Shepherd RC. 1980.United States Patent 4193404. Stretchable and Comfortable pad. Johnson & Johnson (New Brunswick, NJ), Appl. No, 05/896,841. Filed April 17, 1978.
Ricking M, Schwarzbauer J, Franke S. 2003. Molecular markers of anthropogenic activity in sediments of the Havel and Spree Rivers (Germany). Water Research 37:2607–2617.
Saeger VW, Hicks O, Kaley RG, Michael PR, Mieure JP, Tucker ES. 1979. Environmental fate of selected phosphate esters. Environ Sci Technol 13(7):840-844.
SafePharm Laboratories. 2002. TDCP: Determination of general physicochemical properties, Report 1613/004, SafePharm Laboratories, PO Box 45, Derby, UK.
Saito C, Kato T, Taniguchi H, Fujita T, Wada H, Mori Y. 1974. Subacute toxicity of tricresylphosphate (TCP) in rats. Pharmacometrics, 8:107–118 (cited in IPCS 1990).
Sample BE, Opresko DM, Suter II GW. 1996. Toxicological Benchmarks for Wildlife: 1996 Revision. Health Sciences Research Division, Oak Ridge Tennessee. Submitted to United States Department of Energy. Contract No. DE-AC05-84OR21400.
Sax NI, Lewis RJ Sr. (eds.). 1987. Hawley's Condensed Chemical Dictionary. 11th ed. New York: Van Nostrand Reinhold Co. p. 1178. [cited in HSDB 2012]
Schenker U, MacLeod M, Scheringer M, Hungerbuhler K. 2005. Improving data quality for environmental fate models: A least-squares adjustment procedure for harmonizing physicochemical properties of organic compounds. Environ Sci Technol 39: 8434-8441.
Scheringer M, MacLeod M, Wegmann F. 2009. The OECD Pov and LRTP Screening Tool, Version 2.2, A manual for the OECD Pov and LRTP Screening Tool 2.2 that is a follow-up version of the software POPorNot 1.0 distributed at the OECD/UNEP Workshop on Application of Multimedia Models for Identification of Persistent Organic Pollutants. ETH Zurich, April 2009. Version 2.2 fixed a bug introduced by changes in Excel 2007.
Schindler BK, Weiss T, Schütze A, Koslitz S, Broding HC, Bünger J, Brüning T. 2013. Occupational exposure of air crews to tricresyl phosphate isomers and organophosphate flame retardants after fume events. Arch Toxicol87(4):645–648.
Schindler BK, Koslitz S, Weiss T, Broding HC, Brüning T, Bünger J. 2014. Exposure of aircraft maintenance technicians to organophosphates from hydraulic fluids and turbine oils: a pilot study. Int J Hyg Environ Health217(1):34–37.
Senanayake N, Jeyaratnam J. 1981. Toxic polyneuropathy due to gingili oil contaminated with tri-cresyl phosphate affecting adolescent girls in Sri Lanka. Lancet 10: 88–89 (cited in IPCS 1990).
SinoHarvest, 2011.Tricresyl phosphate (TCP) [Internet]. Shanghai, China. [cited June 2013].
Siret-Alatrista A, Zimerson E, Guillot B, Raison-Peyron N. 2010. Allergic contact dermatitis to tricresyl phosphate from safety goggles [abstract]. Contact Dermatitis. 63 (Suppl. 1):58-107.
Sitthichaikasem S. 1978. Some toxicological effects of phosphate esters on rainbow trout and bluegill. Dissertation, Iowa State University, University Microfilms 7813246 (cited in Zitko 1980). [cited in UK EA 2009]
Sjögren B, Iregren A, Järnberg J. 2010. The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals. 143. Phosphate triesters with flame retardant properties. Arbete och Hälsa 2010; 44(6):1–220.
Solbu K, Daae HL, Thorud S, Ellingsen DG, Lundanes E, Molander P. 2010. Exposure to airborne organophosphates originating from hydraulic and turbine oils among aviation technicians and loaders. J Environ Monit 12:2259–2268.
Somkuti SC, Lapadula DM, Chapin RE, Lamb JC IV, Aboudonia MB. 1987a. Reproductive tract lesions resulting from subchronic administration (63 days) of tri-o-cresyl phosphate in male rats. Toxicol Appl Pharmacol 89:49–63. [cited in UK EA 2009, IPCS 1990].
Somkuti SC, Lapadula DM, Chapin RE, Lamb JC IV, Aboudonia MB. 1987b. Time course of the tri-o-cresyl phosphate-induced testicular lesion in F-344 rats: enzymatic, hormonal, and sperm parameter studies. Toxicol Appl Pharmacol 89:64–72. [cited in UK EA 2009, IPCS 1990].
Staaf T, Östman C. 2005. Organophosphate triesters in indoor environments. J Environ Monit 7:883-887.
Stauffer Chemical Company. (1979). Acute inhalation toxicity of Phosflex 31 P in albino rats. Study No. T-6682. Cited in IUCLID 2001.
Stauffer Chemical Company. (1988) Test procedures and data summaries for t-butyl phenyl phosphate, tricresyl phosphate, trixylenyl phosphate, mixed triaryl phosphate and isopropyl phenyl diphenyl. (Personal communication as cited in IPCS 1990).
Sumitomo Chemical Company. 1974. Pharmaceuticals Division Report, Tests of the subacute toxicity of tricresylphosphate in rats. [cited in IUCLID 2001]
Sundkvist AM, Olofsson U, Haglund P. 2010. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monitor 12: 943–951.
Takimoto K, Hirakawa T, Ito K, Mukai T, Okada M. 1999. Source and transport of tricresyl phosphate (TCP) isomers in Kurose river basin. Atmos Environ 33:3191–3200.
[TaPL3] Long Range Transport and Persistence Level III model. 2003. Version 3.00. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre.
Tocco DR, Randall JL, York, RG, Smith MK.1987. Evaluation of the teratogenic effects of tri-ortho-cresyl phosphate in the Long-Evans hooded rat. Fund Appl Toxicol 8(3):291-297. [cited in UK EA 2009, IPCS 1990].
Tollbäck J, Tamburro D, Crescenzi C, Carlsson H. 2006. Air sampling with Empore solid phase extraction membranes and online single-channel desorption/liquid chromatography/mass spectrometry analysis: determination of volatile and semi-volatile organophosphate esters. J Chromatogr A 1129:1– 8. [cited in van der Veen and de Boer 2012].
[TOPKAT] Toxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc.
[UK EA] United Kingdom Environmental Agency. 2009. Environmental risk evaluation report: Tricresyl phosphate (CAS no. 1330-785) [PDF] [Internet]. Bristol (UK): UK Environmental Agency. [cited 2013 June 6] UNEP Publications. 1998. Triethyl Phosphate Case No.: 78-40-0. SIDS Initial Assessment Report. For SIAM 5, Organisation for Economic Co-operation and Development (OECD), Geneva, Switzerland, pp. 1–18 (October). [cited in van der Veen and de Boer 2012].
[US CPSC] US Consumer Product Safety Commission. 1998. Post-hearing comments of the Upholstered Furniture Action Council on the toxicity of flame retardants chemical treatments for upholstery fabrics [PDF]. Washington (DC).
[US CPSC] US Consumer Product Safety Commission, 2005. Migration of Flame Retardant Chemicals in Upholstered Furniture Foam [PDF]. Washington (DC): Division of Chemistry, US CPSC.
[US CPSC] US Consumer Product Safety Commission. 2006. CPSC Staff Preliminary risk assessment of flame retardant chemicals in upholstered furniture foam [PDF]. US Consumer Product Safety Commission.
[US EPA] US Environmental Protection Agency. 1993. Wildlife Exposure Factors Handbook. Washington (DC): US EPA, Office of Research and Development
[US EPA] US Environmental Protection Agency. 2010. HPV Chemical Hazard Characterizations, Screening-level hazard characterization, Phosphoric acid, trismethylphenyl ester. Washington (DC): U.S. EPA, Office of Pollution Prevention and Toxics. [cited 2013 June 6]
[US FDA] US Food and Drug Administration. 2006. Total Diet Study Market Baskets 1991-3 through 2003-4 [PDF]. College Park (MD): Center for Food Safety and Applied Nutrition.
[US FDA] US Food and Drug Administration. 2014. Total Diet Study. [Internet]. Silver Spring (MD): US Food and Drug Administration.
Van den Eede N, Dirtu AC, Neels H, Covaci A. 2011. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ Int 37(2):454 −461.
Van den Eede N, Dirtu AC, Ali N, Neels H, Covaci A. 2012.Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta 89:292 −300
van der Veen I, de Boer J. 2012. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere: 88:1119-1153.
van den Dikkenberg RP, Canton HH, Mathijssen-Spiekman LAM, Roghair CJ. 1989. Usefulness of Gasterosteus aculeatus – the three-spined stickleback – as a test organism in routine toxicity tests. Report 718625003, Rijksinstituut Voor Volksgezondheid en Milieuhygiene, Biltoven, the Netherlands, 28.
van Netten C. 2008. Design of a small personal air monitor and its application in aircraft. Sci Total Environ 407:1206–1210.
Veith GD, Defoe DL, Bergstedt BV. 1979. Measuring and estimating the bioconcentration factor of chemicals in fish. J Fish Res Board Can 36:1040–1048.
Vykoukalová M, Venier M, Vojta Š, Melymuk L, Bečanová J, Romanak K, Prokeš R, Okeme JO, Saini A, Diamond ML, Klánová J. 2017. Organophosphate esters flame retardants in the indoor environment. Environment International 106: 97-104.
Wakabayashi, A. 1980. Environmental pollution caused by organophosphoric fireproofing plasticizers. Annual Report Tokyo Metropolitan Research Institute of Environmental Protection 11:110–113 (in Japanese) [cited in IPCS 1990].
Wang X-W, Liu J-F, Yin Y-G. 2011. Development of an ultra-high-performance liquid chromatography–tandem mass spectrometry method for high throughput determination of organophosphorus flame retardants in environmental water. J Chromatogr A 1218:6705– 6711.
Weil, ED. 1993. Flame retardants (Phosphorus). Kirk-Othmer Encyclopedia of Chemical Technology, Volume 10, Fourth Edition, pp 976-998. John Wiley and Sons, Inc., 1993. [cited in UK EA 2009].
Wensing M, Uhde E, Salthammer T. 2005. Plastics additives in the indoor environment – flame retardants and plasticizers. Sci Total Environ 339:19 – 40.
Westat, Inc. 1987. National Usage Survey of Household Cleaning Products. Prepared for U.S. EPA, Office of Toxic Substances. EPA Contract No. 68-02-42431987. WHO. 1998. EHC 209: Flame Retardants: Tris-(Chloropropyl)Phosphate and Tris-(2- Chloroethyl)phosphate, Geneva, Switzerland. [cited in van der Veen and de Boer 2012].
WHO. 1990. Flame retardants: Tricresyl phosphate. Environmental Health Criteria 110. World Health Organization, Geneva.
Wildlife International Ltd. 2002. Technical information and safety data sheet for Kronitex TCP [cited in UK EA 2009].
Williams DT, LeBel GL. 1981. A National Survey of Tri(haloalkyl)-, Trialkyl-, and Triarylphosphates in Canadian Drinking Water. Bull Environ Contam Toxicol 27:450 – 457.
Williams DT, Nestmann ER, LeBel GL, Benoit FM, Otson R. 1982. Determination of mutagenic potential and organic contaminants of Great Lakes Drinking Water. Chemosphere 11(3):263 – 276.
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Human and Ecological Risk Assessment 19(1): 158-188.
Winder C, Balouet J-C. 2002. The toxicity of commercial jet oils. Environ Res. 89:146-164.
Wong PTS, Chau YK. 1984. Structure-toxicity of triaryl phosphates in freshwater algae. Sci Total Environ 32:157-165.
Woudneh MB, Benskin JP, Wang G, Grace R, Hamilton MC, Cosgrove JR. 2015. Quantitative determination of 13 organophosphorous flame retardants and plasticizers in a wastewater treatment system by high performance liquid chromatography tandem mass spectrometry. J Chromatography A 1400:149-155.
Yang F, Ding J, Huang W, Xie W, Liu W. 2014. Particle size-specific distributions and preliminary exposure assessments of organophosphate flame retardants in office air pariculate matter. Environ Sci Technol 48:63-70.
Yang C, Harris SA, Jantunen LM, Sidiqque S, Kubwabo C, Tsirlin D, Latifovic L, Fraser B, St-Jean M, De La Campa R, You H, Kulka R, Diamond ML. 2018. Are cell phones an indicator of personal exposure to organophosphate flame retardants and plasticizers? (Submitted for publication).
Yasuda H. 1980. Concentration of organic phosphorus pesticides in the atmosphere above the Dogo plain and Ozu basin. J Chem Soc Jpn 1980(4): 645–653 (in Japanese). [cited in WHO 1990]
Appendices
Appendix A. Input values for Level III fugacity modelling
| Environmental media | Half-life (hours) | Reference |
|---|---|---|
| Air | 18.74 | EPI Suite 4.10 2012 |
| Watera | 1029 | EPI Suite 4.10 2012 |
| Soila | 1029 | EPI Suite 4.10 2012 |
| Sediment | 4116 | EPI Suite 4.10 2012 |
| Molar mass | 368.37 g/mol | - |
| Data temperature | 25 oC | - |
| Water solubility | 0.36 mg/L | Saeger et al. 1979 |
| Vapour pressure | 6.6 x 10-5 Pa (at 25 oC) | UK EA 2009 (extrapolated) |
| Log Kow | 5.11 | Saeger et al. 1979 |
| Melting point | -33oC | US EPA 2010 (Midwest Research Institute, 1977) |
| Log Kaw | -4.564 | Calculated from the Henry’s Law to Kaw conversion and Scale.xlsb |
| Kaw | 2.729 x 10-5 | - |
| Henry’s Law Constant | 0.0675 Pa-m3/mol | Calculated from the Henry’s Law to Kaw conversion and Scale.xlsc |
| Log Koc | 3.5253 | KOCWIN v2.00 2010d |
| Koc | 3351.97 | - |
| Log Koa | 9.591 | KOAWIN v. 1.10 2010e |
| Koa | 3.90 x 109 | - |
a Half-life values for water, soil, and sediment are scaled 1:1:4, respectively (i.e. the sediment half-life is four times that of water) based on the Boethling extrapolation approach (Boethling et al. 1995).
b using empirical Ws = 0.36 mg/L and VP=6.6E-05 Pa at 25 oC as input
c using empirical Ws=0.36 mg/L and VP=6.6E-05 Pa at 25 oC as input
d with inputs of log Kow=5.11+MP=-33oC+Ws=0.36 mg/L
e log Kow=5.11+MP=-33oC+VP=3.5E+05 Pa+Ws=0.3 mg/L
Appendix B. Ecological risk quotient analysis of TCP
Parameters |
Pelagic organisms | Soil organisms | Fish-consuming wildlife (Mink)h | Fish-consuming wildlife (River Otter)h |
|---|---|---|---|---|
| Predicted exposure concentration (PEC) | 7.27 x 10-8 to 2.70 x 10-6 mg/L | 0.1185 mg/kg-bw/dayc (TDI; standardized to 2% OC) | 0.0010 mg/kg-bw/day (TDI) | 0.0008 mg/kg-bw/day (TDI) |
| Critical toxicity value (CTV) | 0.0013 mg/La | 5.3 mg/kg-bw/dayd | 5.3 mg/kg-bw/dayd | 5.3 mg/kg-bw/dayd |
| Application factor | 3b | 10e | 10e | 10e |
| Predicted no-effects concentration (PNEC) | 0.00043 mg/L | 1.259 mg/kg-bw/dayf (TRV) | 0.39 mg/kg-bw/dayf (TRV) | 0.237 mg/kg-bw/dayf (TRV) |
| Risk quotient (PEC/PNEC) | 0.0002 to 0.006 | 0.094g | 0.0026g | 0.0032g |
a Van den Dikkenberg et al. (1989) (also cited in UK EA 2009) 0.0013 mg/L for 35-day EC50 for early life stage (after 4-week-old fish stage) mortality.
b Application factor of 3 for species sensitivity would be 3 because, although there are a lot of data, the chronic data only covers two categories: vertebrates and invertebrates (not primary producers). Therefore, the AF = 1 x 3 = 3.
c BASL4
Due to the lack of measured data, PEC was calculated using the BASL4 model (BASL4 2011). To examine potential impacts from long-term application, an application time period of 10 consecutive years was considered. The calculation considered a maximum application rate of 8300 kg dw/ha per year (based on the highest existing provincial regulatory limit) with a mixing depth of 0.1 m (default value for BASL4) and a soil density of 1487 kg/m3 (default value for BASL4). The maximum biosolid concentration for TCP in Canada based on exposure analysis was 1.15 mg/kg dw was used as the concentration in biosolids in the calculation. Soil half-life = 42.87 days.
This generates a maximum mammallian uptake rate of 0.16 mg/kg-bw/day at 2.7% OC, which can be compared to a toxic reference intake value. After standardizing to 2% OC, the TDI is 0.1185 mg/kg-bw/day.
d Geomean of NOAEL and LOAEL values (geomean of 4 mg/kg-bw/day and 7 mg/kg-bw/day) from NTP (1994).
e To derive the wildlife PNEC for TCP, an application factor of 10 is applied to the TRV to account for extrapolation from laboratory to field conditions.
f TRV – total reference value
Due to the lack of data for wildlife species, the CTV is based on a Wildlife Toxicity Reference Value (TRV) (Sample et al. 1996), determined from the Wildlife Exposure Model, where potential effects in rodents for TCP (NTP 1994) are normalized to typical body weight of Mink, Mustela vison, a surrogate wildlife species:
TRV=s MATCts x (BWts/BWfs)
where:
TRVe = total reference value (mg/kg-bw/day)
MATCts = maximum allowable toxicant concentration for test species (mg/kg-bw/day), MATC= Geomean of NOAEL and LOAEL for test species. A LOAEL of 7 mg/kg-bw/d and NOAEL of 4 mg/kg-bw/d (NTP 1994), were selected to determine a TRV for the evaluation of potential effects in wildlife. This endpoint was considered relevant, based upon a 2-year feed study in female rats (see Health Assessment Section (NTP 1994))
BWts = mean body weight of test species (kg); BWts = 0.32 kg (mean for NOAEL and LOAEL rat weight, NTP 1994)
(the body weights of female rats started with a mean of 91 g (91, 91, 92, 90 g for four respective different concentrations of TCP in feeds) in the first week of the study, and ended with a mean of 320 g (315, 320, 332, 313 g for four respective different concentrations of TCP in feeds) in the 104th week of the study.)
BWfs = body weight of focal species (kg) = weight = density/volume = (1 g/cm3) x (10 cm3) = 10 g = 0.01 kg; = 0.01 (Shrew) (US EPA 1993).
g Risk Quotient = TDI/TRV
h Wildlife Exposure Model and Toxicity Equations
The wildlife exposure is based on Total Daily Intake (TDI) for Mink (Mustela vison) and River Otter (Lontra canadensis) consuming fish, determined from Wildlife Exposure Model (US EPA 1993), where
TDI = [FMR (Ci × ED × Pi / GEi × AEi) + (Cs × IRs) + (Cw × IRw)] × Pt
where:
- TDI = total daily intake (mg/kg-bw/day)
- FMR = free-living metabolic rate of wildlife receptor of interest; In this assessment, it is assumed FMR = 235 kcal/kg-bw/day for Mink and 179 kcal/kg-bw/day for River Otter.
- Ci = concentration of contaminant in the ith prey species (mg/kg) = Cw x BCF; determined as Ci =0.0056 mg/kg-bw (default), representing highest published TCP concentration in Canadian fish sample (northern pike liver) (Houde et al. 2014)
- Pi = proportion of the ith prey species in the diet (unitless); 0.85 for Mink and 0.84 for River Otter
- To be conservative it is assumed that approximately up to 100% of the diet of Mink and River Otter is fish: Pi = 1.0
- GEi = gross energy of the ith prey species (850 kcal/kg prey) 5th percentile used for conservative value; GEi=1177
- AEi = assimilation efficiency of the ith prey species by the wildlife receptor; AEi=0.94 (Mink and River Otter). Default value
- Cs = concentration of contaminant in the sediments (mg/kg dw); It is assumed that TCP exposure via sediment is negligible; Cs=0
- IRs = intake rate of sediments (kg dw/kg-bw/day); It is calculated that incidental sediment ingestion is zero IRs=0.006 (Mink), 0.001 (River Otter)
- Cw = concentration of contaminant in the water (mg/L); 2.7 ng/L, which is the highest measured total TCP concentration from exposure analysis, was selected to represent a potential regional water concentration (exposure section); Cw= 2.7 x 10-6 mg/L for both Mink and River Otter.
- IRw = intake rate of water (L/day); Assumed to be 10%; IRw=0.1064 (Mink), 0.642 (River Otter)
- Pt = proportion of the time the receptor spends in the contaminated area. Pt=1 (Mink and River Otter)
A description and definition of each variable in the above equation can be found in US EPA (1993).
Due to the lack of data for wildlife species, the CTV is based on a Wildlife Toxicity Reference Value (TRV) (Sample et al. 1996), determined from the Wildlife Exposure Model, where potential effects in rodents for TCP (NTP 1994) are normalized to typical body weight of Mink, Mustela vison, a surrogate wildlife species:
TRV=s MATCts x (BWts/BWfs)
where:
TRVe = total reference value (mg/kg-bw/day)
MATCts = maximum allowable toxicant concentration for test species (mg/kg-bw/day), MATC= Geomean of NOAEL and LOAEL for test species. A LOAEL of 7 mg/kg-bw/d and NOAEL of 4 mg/kg-bw/d (NTP 1994), were selected to determine a TRV for the evaluation of potential effects in wildlife. This endpoint was considered relevant, based upon a 2-year feed study in female rats (see Health Assessment Section (NTP 1994))
BWts = mean body weight of test species (kg); BWts = 0.32 kg (mean for NOAEL and LOAEL rat weight, NTP 1994)
(the body weights of female rats started with a mean of 91 g (91, 91, 92, 90 g for four respective different concentrations of TCP in feeds) in the first week of the study, and ended with a mean of 320 g (315, 320, 332, 313 g for four respective different concentrations of TCP in feeds) in the 104th week of the study)
BWfs = body weight of focal species (kg); =1.08 (Mink); 7.98 (River Otter) (US EPA 1993)
Risk Quotient = TDI/TRV
Appendix C. Weight of evidence in the ecological risk assessment
| Line of evidence | Level of confidencea | Relevance in assessmentb | Weight assignedc |
|---|---|---|---|
| Physical and chemical properties | Moderate | Moderate - High | Moderate |
| Persistence | High | Low | Low |
| Bio-accumulation | High | Moderate | Moderate |
| Effects (PNEC, TRV) | Moderate | High | Moderate - High |
| Exposure analysis (PEC, TDI) | Moderate | High | Moderate - High |
| Risk | Moderate | High | Moderate - High |
a Level of confidence is determined according to data quality, data variability, data gaps and if the data are fit for purpose.
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the combined level of confidence and relevance in the assessment.
Appendix D. Estimates of daily intake of TCP by various age groups within the general population of Canada
| Route of exposure | 0–6 moa Breastfedb | 0–6 mo Formula- fedc | 0–6 mo Not formula-fedd | 0.5–4 yre | 5–11 yrf | 12–19 yrg | 20–59 yrh | ≥60 yri |
|---|---|---|---|---|---|---|---|---|
| Ambient airj | 8.5E-06 | 8.5E-06 | 8.5E-06 | 1.8E-05 | 1.4E-05 | 8.1E-06 | 7.0E-06 | 6.1E-06 |
| Indoor airk | 6.0E-05 | 6.0E-05 | 6.0E-05 | 1.3E-04 | 1.0E-04 | 5.7E-05 | 4.9E-05 | 4.2E-05 |
| Drinking waterl | N/A | 6.4E-03 | 2.4E-03 | 2.7E-03 | 2.1E-03 | 1.2E-03 | 1.3E-03 | 1.3E-03 |
| Foodm | 1.3E-02 | NI | NI | 4.7E-03 | 3.8E-03 | 2.2E-03 | 2.1E-03 | 1.3E-03 |
| Dustn | 9.0E-02 | 9.0E-02 | 9.0E-02 | 4.7E-02 | 1.8E-02 | 6.6E-04 | 6.2E-04 | 6.1E-04 |
| Soilo | 0.0E+00 | 0.0E+00 | 0.0E+00 | 2.7E-07 | 2.0E-07 | 7.1E-09 | 6.8E-09 | 6.3E-09 |
| Total intake | 1.0E-01 | 9.6E-02 | 9.2E-02 | 5.4E-02 | 2.4E-02 | 4.1E-03 | 4.0E-03 | 3.3E-03 |
Abbreviations: N/A, not applicable; NI, data not identified in the literature; mo, months; yr, years
a Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day (Health Canada 1998), and to ingest 38 and 0 mg of dust and soil per day, respectively (Wilson et al. 2013).
b Exclusively for breastfed infants, assumed to consume 0.742 L of breast milk per day, and breast milk is assumed to be the only dietary source. No monitoring of TCP in breast milk in Canada was identified. The concentration for whole breast milk of 0.13 ug/L was based on a reported 3.7 ng/g lipid x 3.4% (lipid content of breast milk) x 1.03 g/mL (density of breast milk)] identified in 69 samples of human breast milk collected in 1997 in Sweden (Sundkvist et al. 2010).
c Exclusively for formula-fed infants, assumed to drink 0.8 L of water per day (Health Canada 1998), where water is used to reconstitute formula. No monitoring data on TBB and TBPH in formula were identified; therefore dietary intakes are only those from water. See footnote on drinking water for details.
d Exclusively for not-formula-fed infants, assumed to drink 0.7 L of water per day (Health Canada 1998), and approximately 50% of non-formula-fed infants are introduced to solid foods by 4 months of age, and 90% by 6 months of age (NHW 1990).
e Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day, to consume 54.7 g of fish per day (Health Canada 1998), and to ingest 41 and 14 mg of dust and soil per day, respectively (Wilson et al. 2013).
f Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day, to consume 89.8 g of fish per day (Health Canada 1998), and to ingest 31 and 21 mg of dust and soil per day, respectively (Wilson et al. 2013).
g Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day, to consume 97.3 g of fish per day (Health Canada 1998), and to ingest 2.2 and 1.4 mg of dust and soil per day, respectively (Wilson et al. 2013).
h Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day, to consume 111.7 g of fish per day (Health Canada 1998), and to ingest 2.5 and 1.6 mg of dust and soil per day, respectively (Wilson et al. 2013).
i Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day, to consume 72.9 g of fish per day (Health Canada 1998), and to ingest 2.5 and 1.5 mg of dust and soil per day, respectively (Wilson et al. 2013).
j No monitoring data of ambient air in Canada were identified. The maximum indoor air concentration of 0.244 ng/m3 for bedrooms in 51 homes in the Greater Toronto Area and Ottawa (Yang et al. 2018) was selected for deriving upper-bounding estimates of daily intake for ambient air exposure. Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
k The maximum indoor air concentration of 0.244 ng/m3 for bedrooms in 51 homes in the Greater Toronto Area and Ottawa (Yang et al. 2018) was selected for deriving upper-bounding estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
l The detection limit of 60 ng/L from the City of Toronto (2003) municipal monitoring program was selected for deriving upper-bounding estimates of daily intake for drinking water exposure.
m No monitoring data on marketed foods in Canada were identified; however environmental fish and shellfish data were available. The TCP concentration of 1.32 µg/kg wet weight (based on a reported maximum TCP concentration of 110 µg/kg lipid x 1.2% lipid content) in mussels collected in 2007 in Sweden (Sundkvist et al. 2010) was selected for deriving upper-bounding estimates of daily intake for exposure to all fish-related food items in the Fish food group. Amounts of foods consumed on a daily basis by each age group over 12 food groups were obtained from the 1970–1972 Nutrition Canada Survey (Health Canada 1998).
n The 95th percentile concentration of TCP (17 700 ng/g) from the baseline study based on samples collected from the Canadian House Dust Study (Kubwabo et al. 2014) was selected for deriving upper-bounding estimates of daily intake for dust exposure.
o No appropriate or relevant soil studies on TCP monitoring in North America were identified. The soil maximum predicted environmental concentration (PEC) of 300 ng/g dw (0.0003 mg/kg dw) was selected for deriving upper-bounding estimates of daily intake for soil exposure.
Appendix E. Environmental monitoring data
| Location | Country | n | DL (ng/m3) | Concentration (ng/m3) | Reference |
|---|---|---|---|---|---|
| Homes | Canada | 34 | ND reported for samples where blank level was >35% of measured level | ND – 0.050 (o-TCP) | Vykoukalová et al. 2017 |
| Homes | Canada | 34 | ND reported for samples where blank level was >35% of measured level | ND-0.023 (p-TCP) | Vykoukalová et al. 2017 |
| Homes (bedrooms) | Canada | 51 | 0.00034 | 0.00102 - 0.244 ng/m3 | Yang et al. 2018 |
| Homes (most used rooms) | Canada | 26 | 0.00034 | ND | Yang et al. 2018 |
| Homes | U.S. | 30 | ND reported for samples where blank level was >35% of measured level | ND-0.142 (o-TCP) | Vykoukalová et al. 2017 |
| Homes | U.S. | 30 | ND reported for samples where blank level was >35% of measured level | ND-0.256 (p-TCP) | Vykoukalová et al. 2017 |
| Electronics stores | Switzerland | 5 | 0.41 | ND to 0.21 | Hartmann et al. 2004 |
| Furniture stores | Switzerland | 2 | 0.41 | ND | Hartmann et al. 2004 |
| Offices | Switzerland | 4 | 0.41 | ND to 0.37 | Hartmann et al. 2004 |
| Theatre | Switzerland | 1 | 0.41 | 2.1 | Hartmann et al. 2004 |
| Homes | Sweden | 50 | 1 | <DL | Staaf and Östman 2005 |
| Various indoor environments (e.g. bicycle store, carpet store, clothing store, health care facilities, bakery, carpet store) | Sweden | 50 | 1 | <DL | Staaf and Östman 2005 |
| Car | Sweden | 5 | 1 | <DL | Staaf and Östman 2005 |
| Bus | Sweden | 10 | 1 | <DL | Staaf and Östman 2005 |
| Subway car | Sweden | 5 | 1 | <DL | Staaf and Östman 2005 |
| Car garage | Sweden | 5 | 1 | <DL | Staaf and Östman 2005 |
| Bus garage | Sweden | 5 | 1 | <DL | Staaf and Östman 2005 |
| Subway car garage | Sweden | 5 | 1 | <DL | Staaf and Östman 2005 |
| Aircraft | NA | - | 4.5 | Flight A:108 Flight B: 36 | Van Nette 2009 |
Abbreviations: n, sample size; ND, not detected; DL, detection limit; NA, not applicable
| Location | Sample type | Sampling year | n | Median (range) (ng/g) | Reference |
|---|---|---|---|---|---|
| Various locations, Canada | Vacuum Cleaner | 2007–08 | 818 | 4860 (<30–295 0000 | Kubwabo et al. 2014 |
| Various locations, Canada | Vacuum cleaner—Fresh dusta | 2007–2010 | 134 | 2600 (80–62000) | Fan et al. 2014 |
| Various locations, Canada | Vacuum cleaner—House dustb | 2007–2010 | 134 | 990 (50–75000) | Fan et al. 2014 |
| Toronto, Canada | Vacuum cleaner, floors | 2013 | 35 | 0.712 (ND–7.02) (o-TCP) | Vykoukalová et al. 2017 |
| Toronto, Canada | Vacuum cleaner, floors | 2013 | 35 | 6.18 (0.871–116) (p-TCP) | Vykoukalová et al. 2017 |
| Toronto and Ottawa, Canada | Homes (bedrooms) | 2015 | 51 | 23.2 (mean) (0.75–1120) | Yang et al. 2018 |
| Toronto and Ottawa, Canada | Homes (most used rooms) | 2015 | 26 | 28.8 (mean) (0.75–699) | Yang et al. 2018 |
| California, USA | Living area surfaces | 2006 | 16 | 1000 (330– 400) | Dodson et al. 2012 |
| California, USA | Living area surfaces | 2011 | 16 | 680 (180–10 000) | Dodson et al. 2012 |
| NS, Germany | Vacuum cleaner | NS | 65 | 2200 (NS–36 000) | Kersten and Reich 2003c |
| Urban area, Germany | Carpet and floor | 2010–2011 | 10 | 94 (mean) (<40 to 240) | Brommer et al. 2012 |
| Iasi, Romania | Carpet | 2010 | 47 | 500 (<50 to 5500) | Dirtu et al. 2012 |
| Urban area, Belgium | Carpet and floor | 2008 | 33 | 240 | Van den Eede et al. 2011 |
| NS, Belgium | Vacuum cleaner | 2006–2010 | 6 | 106 (29–1 110) | Van den Eede et al. 2012d |
| NS, Spain | Vacuum cleaner | 2006 | 1 | 89 (only value) | Van den Eede et al. 2012d |
| NS, Romania | Vacuum cleaner | 2007 | 3 | 430 (86–2 350) | Van den Eede et al. 2012d |
| Various locations, New Zealand | Carpet and floor | 2008 | 34 | 120 (<50 to 3 760) | Ali et al. 2012 |
| Various locations, New Zealand | Mattress | 2008 | 16 | 157 (<50 to 2 155) | Ali et al. 2012 |
| Malate, Philippines | Vacuum cleaner | 2008 | 17 | 18 (<0.27 to 25) | Kim et al. 2013 |
| Payatas, Philippines | Vacuum cleaner | 2008 | 20 | 7.7 (<0.27 to 140) | Kim et al. 2013 |
Abbreviations: n, sample size; NS, not specified
a Fresh dust refers to samples collected by trained technicians from living areas using a vacuum sampler; this excludes old house dust that has collected over time in areas not vacuumed on a regular basis.
b House dust refers to samples obtained from the vacuum systems used by the study participants.
c Cited in Wensing et al. 2005.
d Only raw data available. Calculated values.
| Location | Sample type | Sampling year | Sample size | Mean [range] (ng/L) | Reference |
|---|---|---|---|---|---|
| Toronto, ON, Canada | Drinking water | 2000 | 10 | : [ND] | City of Toronto 2000 |
| m-TCP: [ND] | |||||
| p-TCP: [ND] | |||||
| Toronto, ON, Canada | Drinking water | 2001 | 19 | : [ND] | City of Toronto 2001 |
| m-TCP: [ND] | |||||
| p-TCP: [ND] | |||||
| Toronto, ON, Canada | Drinking water | 2002 | 19 | : [ND] | City of Toronto 2002 |
| m-TCP: [ND] | |||||
| p-TCP: [ND] | |||||
| Toronto, ON, Canada | Drinking water | 2003 | 14 | : [ND] | City of Toronto 2003 |
| m-TCP: [ND] | |||||
| p-TCP: [ND] | |||||
| Ontario DWTPs, Canada | Drinking water | 1978 | 12 | [ND to 0.3] | Lebel et al. 1981 |
| Various locations, Canada | Drinking Water | 1979 | 60 | [ND to 4.3] | Williams and Lebel 1981 |
| Various locations, Canada | Drinking water | 1980 | 12 | [0.4–1.8] | Williams et al. 1982 |
| Rome, Italy | Drinking water | 2006 | 6 | [ND] | Bacaloni et al. 2007 |
| Food type | Location | Country | Sampling year | Sample size | Concentration (ng/g) | Reference |
|---|---|---|---|---|---|---|
| All other food types | Various locations | USA | 1991 to 2002 | >15 000 | ND | (US FDA (2006) |
| Bread | Various locations | USA | 2003 | 44 | 2.05 (mean) (ND-90) DF= 2% | US FDA (2013c) |
| All other food types | Various locations | USA | 2004 to 2005 | - | ND | US FDA (2013b) |
| Food type | Location | Sampling year | n | Conc. (range) (ng/g lw) | Conc. (range) (ng/g ww) | Reference |
|---|---|---|---|---|---|---|
| Sturgeon | Columbia River, USA | NS | NS | 40 | NR | Lombardo and Egry 1979 |
| NS | near TAP manufacturing site, USA | NS | NS | (2–5) | NR | Muir 1984 |
| Herring (marine) | Baltic Proper, Sweden | 2007 | 4 (72 P) | <0.4 | <0.010 | Sundkvist et al. 2010 |
| Perch (marine) | Holmon and Kvadofjarden, Sweden | 2007 | 2 (10 P) | 22 (20-23) | 0.095 (0.082-0.1) | Sundkvist et al. 2010 |
| Mussels (marine) | Sweden | 2007 | 30 | 110 | 1.32 | Sundkvist et al. 2010 |
| Mussels (marine) | Sweden | 2007 | 11 | 11 | 0.13 | Sundkvist et al. 2010 |
| Eelpout (marine) | Fjallbacka, Sweden | 2007 | 5 | 19 | 0.11 | Sundkvist et al. 2010 |
| Salmon (marine) | Bothnian Bay, Sweden | 2005 | 5 | 1.8 | 0.16 | Sundkvist et al. 2010 |
| Perch (fw) | Background locations, Sweden | 2007 | 7 (60 pooled) | 18 (<2.1 to 43) | 0.07 (0.01-0.13) | Sundkvist et al. 2010 |
| Perch (fw) | Near sources, Sweden | 2007 | 3 (27 P) | 24 (22–137) | 0.10 (0.10–0.20) | Sundkvist et al. 2010 |
| NS | Various locations, Japan | 1993 | 75 | (63 – 82) | NR | MOE Japan 2003 |
| NS | Various locations, Japan | 1978 | 93 | ND | NR | MOE Japan 2003 |
| NS | Various locations, Japan | 1975 | 96 | ND | NR | MOE Japan 2003 |
| Fish and shellfish | Seto Inland Sea, Japan | 1980 | 41 | (1–19) | NR | Kenmotsu et al. 1981 |
Abbreviations: n, sample size; ND, not detected; NR, not reported
| Food Type | Location | Spl year | n | Conc. (range) (ng/g lw) | Conc. (range) (ng/g ww) | Reference |
|---|---|---|---|---|---|---|
| Breast milk | Uppsala | 1997 | 69 (P) | 3.7 | 0.126 | Sundkvist et al. 2010 |
| Breast milk | Uppsala | 1998 | 90 (P) | 3.0 | 0.102 | Sundkvist et al. 2010 |
| Breast milk | Uppsala | 2006 | 50 (P) | <0.1 | <0.003 | Sundkvist et al. 2010 |
| Breast milk | Lycksele | 2003 | 39 (P) | <0.2 | <0.008 | Sundkvist et al. 2010 |
| Breast milk | Lund | 2003 | 37 (P) | <0.2 | <0.008 | Sundkvist et al. 2010 |
| Breast milk | Umeå | 2007 | 1 | 1.7 | 0.019 | Sundkvist et al. 2010 |
Abbreviations: n, sample size; ND, not detected; P, pooled.
Appendix F. Exposure estimates of TCP from manufactured items
Based on the available information, dermal exposure intakes were estimated for direct contact with furniture upholstery with TCP backcoating for young children and adults (Table F-1). The exposure estimates presented below are considered upper-bounding based on conservative assumptions. Note that these products are included based on the assumption of their use in Canada as these uses were only identified in U.S. or international information sources.
Dermal exposure intake estimates
Intake = [SA × SCF × M × ED] / BW
| Symbol | Description | Value |
|---|---|---|
| SAa | Surface area of skin contact | 357 cm2 (Toddler) 1395 cm2 (Adult) |
| SCFb | Skin contact factor | 1 |
| Mc | Migration rate | 2.17 x 10-4 mg/cm2/h (TCEP) 5.6 × 10-5 mg/cm2/h (TDCPP) |
| EDd | Exposure duration | 1 hr (Toddler) 6 hr (Adult) |
| BWe | Body weight | 15.5 kg (Toddler) 70.9 kg (Adult) |
| Intake | Intake estimate | 1.3×10-3 – 5.0×10-3 mg/kg-bw/d (Toddler) 6.6×10-3 – 2.6×10-2 mg/kg-bw/d (Adult) |
a For this scenario, it is assumed that an individual is wearing shorts and a t-shirt. The surface area of exposure is based on exposure to a fraction of the lower half of the limbs (arms and legs). The surface areas of the limbs (Health Canada 1995) were multiplied by one half to account for clothing coverage and then were multiplied by one third to account for the triangular shape of limbs, where only one side is directly in contact with upholstered furniture (US CPSC 2006).
b No TCP-specific skin contact factor (SCF), i.e. the fraction of substance on a surface adhering to skin, was identified in the literature. As such, a value of 1 was selected to assume that all of the chemical in contact with the skin is available for absorption.
c No TCP-specific migration rates were identified in the literature. Therefore a range in migration rates based on other organophosphate flame retardants were selected. The migration rate of 2.17 x 10-4 mg/cm2/h for TCEP from furniture textiles is based on an unpublished study by Bruckert et al. (1990) reported in the TCEP EU RAR (2009). The migration rate of 5.6 × 10-5 mg/cm2/hr for TDCPP were used to estimate dermal exposures based on migration studies of treated furniture foam by the US CPSC (US CPSC 2005). The US CPSC built a furniture miniseat mock-up consisting of a block of foam covered with cotton fabric and attached to plywood. The miniseat was wetted with a saline solution to mimic sweat, with pressure applied to imitate the action of lying down. The migration rate of 5.6 × 10-5 mg/cm2/hr for TDCPP was determined based on the reported maximum daily amount extracted (8 µg) onto the filter (5.5 cm diameter) over the course of the migration testing period (6 hours) (US CPSC 2005).
d Exposure duration for sitting was adjusted from durations reported in US CPSC (2006) for leisurely sitting.
e Health Canada (1998).
Appendix G. TCP in oils in engine oils, lubricants and fluids
| Product | Total TCP | o-TCP | m-TCP | p-TCP | Reference | Notes |
|---|---|---|---|---|---|---|
| Power steering fluid | 0.2% | NR | NR | NR | Environment Canada 2013 | NA |
| Automobile engine oil | NR | 2.2–2.9 mg/g | 1.9–2.3 mg/g | ND | Takimoto et al. 1999 | Measured in Japan |
| Motorcycle engine oil | NR | 1.7–7.3 mg/g | 1.5–6.8 mg/g | ND | Takimoto et al. 1999 | Measured in Japan |
| Aircraft turbine oil | NR | 13–150 mg/L | NR | NR | De Nola et al. 2008 | NA |
| Aircraft turbine oil | 2-3% | NR | NR | NR | De Nola et al. 2008 | From product MSDS |
| Aircraft turbine oil | NR | <50 mg/kg | NR | NR | De Nola et al. 2008 | NA |
| Aircraft Turbine oil | NR | <LOQ | ~ 2.5% | NR | De Nola et al. 2011 | NA |
| Aircraft turbine oil | NR | 20–40 μg/L | NR | NR | De Nola et al. 2011 | Manufactured after 2001 |
| Aircraft turbine oil | 1-5% | NR | NR | NR | Solbu et al. 2007 | From MSDS |
| Aircraft turbine oil | 32 mg/g | NR | NR | NR | Solbu et al. 2010 | New oil |
| Aircraft turbine oil | 21.8 mg/g | NR | NR | NR | Solbu et al. 2010 | 10 year-old oil |
| Aircraft turbine oil | 3% | NR | NR | NR | van Netten 2008 | NA |
| Aircraft turbine oil | 3% | NR | NR | NR | Winder and Balouet 2002; Michaelis 2002 | Amount in Mobil Jet Oil II |
| Hydraulic fluid | NR | 0.5% by weight | NR | NR | ATSDR 1997 | NA |
| Lubricating oil | NR | 2% | 42% | 31% | WHO 1990 | Measured in 1975 |
| Refrigeration lubricant | NR | 1–2% | NR | NR | HPD 2011 | Automotive air conditioning refrigerant |
Abbreviations: NR, not reported; ND, not detected; LOQ, limit of quantification; NA, not applicable
Dermal exposure from oils
Direct skin contact with oils can result in dermal exposure to TCP used in various oils and other fluids. Of all the potential uses of TCP in oils and other fluids, the selected product considered was power steering fluid based on confirmation of its use in Canada (Environment Canada 2013). The exposure event for an adult filling the power steering fluid reservoir of a vehicle is not expected to occur frequently (likely 2 to 4 times per year), and thus was estimated on a per event and acute or short-term basis. The exposure estimate presented below is based on conservative assumptions.
Intake = [SA × FT × ρ × WF] / BW
| Symbol | Description | Value | Reference |
|---|---|---|---|
| SA | Surface area of finger tips (cm2) | 6 | Versar handbook thin film, instant application scenario (Westat 1987) |
| FT | Thickness of oil film on hand (cm) | 1.59×10-2 | Versar handbook thin film, instant application scenario (Westat 1987) |
| r | Density of product (g/cm3) | 0.88 | Versar handbook thin film, instant application scenario (Westat 1987) |
| WF | TCP Weight fraction | 0.002 | Environment Canada (2013–2014) |
| BW | Body weight | 70.9 kg (Adult) | Health Canada (1998) |
| Intake | Intake (ug/kg-bw) | 2.4 | - |
Appendix H. Summary of health effects information for TCP
Endpoint |
Lowest effect levelsa/Results |
|---|---|
Acute toxicity |
Lowest oral LD50 (mouse) = 3900 mg/kg-bw (Izmerov, 1982 cited in IPCS 1990) Lowest dermal LC50 (cat) = 1500 mg/kg (Abou-Donia et al. 1980 cited in IPCS 1990) Lowest inhalation LC50 (rat) >5.2 mg/L (Stauffer Chemical Co. 1979 cited in ECB 2001) Oral LOAEL (rat) = 2000 mg/kg-bw based on significant inhibition of serum, erythrocyte and brain cholinesterase activity observed in female Sprague Dawley rats administered TCP (a mixture containing at least 20 organophosphates including 0.4%, 19.5% m-TCP and 2.4% p-TCP), via gavage, in a single dose of 2000 mg/kg-bw (Mobil 1987)) [Additional studies: FMC Corporation 1975 cited in IUCLID 2001; Johanssen et al. (1977); Marhold 1972 cited in IPCS 1990; Stauffer Chemical Co.1988 cited in IPCS 1990; EPA 2010. |
Irritation/ Sensitization |
Negative in Draize skin test (EPA guideline): Six albino rabbits had TCP (100%) applied on the intact left side of the back and the abraded right side of the back. The areas were wrapped in surgical gauze for 24 hours, after which the gauze was removed and the skin was observed for irritation. Erythema was observed in the abraded skin of one animal at 24 hours. The erythema was gone at the 72-hour observation. None of the animals showed edema at either the abraded or unabraded (intact) application areas. Thus TCP did not cause skin irritation in this test (Food and Drug Research Lab. Inc. 1975a cited in ECB 2001). Negative in Draize eye test (EPA guideline): Nine rabbits received 0.1 ml of undiluted TCP on their right eyes. The eyes of 6 rabbits remained unwashed during the observation period while the eyes of 3 rabbits were washed 4 seconds after application. All eyes were examined at 24, 48 and 72 hours after exposure and again after 7 days. The eyes were scored according to the Draize method. Conjunctival effects were observed at 24 hours in two of the six rabbits with unwashed eyes, which cleared by 48 hours. No ocular effects were observed in the eyes of rabbits whose eyes were washed 4 seconds after application. The laboratory reports that, on the basis of these results, TCP is not an eye irritant (Food and Drug Research Lab. Inc. 1975b cited in ECB 2001). Positive in Local Lymph Node Assay (OECD guideline): Twenty female CBA/J Rj mice were allocated to five groups of four animals each: – three groups received the appropriate formulation of Kronitex TCP at concentrations of 100% (undiluted), 50% and 25% (w/v), – the negative control group received acetone olive oil (AOO) and – the positive control group received 25% α-Hexylcinnamaldehyde (HCA) in AOO. The solutions of the test item were applied on the dorsal surface of ears of experimental animals (25 μl/ear) for three consecutive days (Days 1, 2 and 3). There was no treatment on Days 4, 5 and 6. On Day 6, the cell proliferation in the local lymph nodes was measured by incorporation of tritiated methyl thymidine (3HTdR) and the values obtained were used to calculate stimulation indices (SI). No mortality, systemic toxicity or local irritation was observed during the study. No treatment-related effects were observed on animal body weights in any treated groups. Stimulation index values of the test item were 3.7, 3.4 and 5.7 at treatment concentrations of 100% (undiluted), 50% and 25% (w/v), respectively. α-Hexylcinnamaldehyde (25% (w/v) dissolved in AOO) was used as a positive control to demonstrate the appropriate performance of the assay. A significant lymphoproliferative response (stimulation index value of 17.2) was noted for the positive control chemical and this result confirmed the validity of the assay. In conclusion, under the conditions of the present assay Kronitex TCP, tested in a suitable vehicle, was shown to have sensitization potential (sensitizer) in the Local Lymph Node Assay (LAB Research Ltd. 2010) |
Short-term toxicity |
Lowest oral LOAEL (mouse) = 360 mg/kg-bw /day based on decreased hindlimb grip strength observed in male mice. B6C3F1 mice (10 per sex per dose) were administered TCP (containing 79% tricresyl phosphate esters including 21% tri-m-cresyl phosphate, 4% tri-p-cresyl phosphate, < 0.1% tri-o-cresyl phosphate, and other unidentified tricresyl phosphate esters; plus 18% of dicresyl phosphate esters), via gavage, at 0, 360, 730, 1450, 2900 mg/kg-bw /day in corn oil or 5800 mg/kg-bw /day (neat) for 13 or 14 days in a 16-day period. A significant reduction of hindlimb grip strengths was also observed at 730 mg/kg-bw/day in males and females, at 1450 in males and at 5800 mg/kg-bw/day in males and females; a significant reduction of forelimb grip strengths was observed at 1450 mg/kg-bw/day in males and at 5800 mg/kg-bw/day in females on day 14 of the study. Five males and all females receiving 1450 mg/kg-bw/day, all mice receiving 2900 mg/kg-bw/day, and four males and one female receiving 5800 mg/kg-bw/day died before the end of the study. Final mean body weights of male mice receiving 1450 or 5800 mg/kg-bw/day were significantly lower than controls. Final mean bodyweight of female mice receiving 360, 730 or 5800 mg/kg-bw/day were significantly greater than controls. Multifocal necrosis of the mandibular lymph node, spleen and thymus were observed primarily in mice receiving 2900 and 5800 mg/kg-bw/day (NTP 1994). Oral LOAEL (rat) = 1450 mg/kg-bw /day based on reduced body weight in a dose-related manner in male and female F344/N rates (10 per sex per dose) administered TCP (containing 79% tricresyl phosphate esters including 21% tri-m-cresyl phosphate, 4% tri-p-cresyl phosphate, < 0.1% tri-o-cresyl phosphate, and other unidentified tricresyl phosphate esters; plus 18% of dicresyl phosphate esters), via gavage, at 0, 360, 730, 1450, 2900 or 5800 mg/kg-bw /day, for 13 or 14 doses in a 16-day period. One female receiving 1450 mg/kg-bw/day, and five males and eight females receiving 2900 mg/kg-bw/day died before the end of the study. Final mean bodyweight of rats receiving 1450 mg/kg-bw/day or higher doses were significantly lower than controls. Necrosis of the mandibular lymph node, spleen and thymus were observed primarily at 2900 and 5800 mg/kg-bw/day in males and females. Diffuse aspermatogenesis was observed in males at 2900 and 5800 mg/kg-bw/day. Significant reduction of forelimb grip strengths were observed in females at 1450 mg/kg-bw/day and higher doses, and in males at 2900 and 5800 mg/kg-bw/day. Significant reduction in hindlimb grib strengths were observed in males at 2900 and 5800 mg/kg-bw/day, and in females at 5800 mg/kg-bw/day (NTP 1994). Lowest dermal LOAEL (rat) = 500 mg/kg-bw /day based on reduced serum cholinesterase observed in female Sprague-Dawley rats (5/dose) administered TCP (3% in jet engine oil) via dermal application to the shaved skin at 0, 500 or 1000 mg/kg-bw /day, for a total of 20 exposures over four weeks. Clinical signs of hypersensitivity, such as increased motor activity, were observed at 1000 mg/kg-bw/day. Serum cholinesterase was significantly (p<0.05) reduced in all exposed rats (Mobil 1990a). [Additional studies: Foster D. Snell Inc. 1976 cited in ECB 2001; Oishi et al. 1982; Chapin et al. 1988; Kinkead et al. 1990, 1993; Freudenthal et al. 1993; NTP 1994; Mackerer et al. 1999] No inhalation studies were identified. |
Sub-chronic toxicity |
Lowest oral LOAEL (rat) = 50 mg/kg-bw /day based on cytoplasmic vacuolization of the adrenal cortex observed in Fisher 344/N rats (10 per sex per dose) administered TCP in a mixed isomer preparation (containing 79% tricresyl phosphate esters including 21% tri-m-cresyl phosphate, 4% tri-p-cresyl phosphate, < 0.1% tri-o-cresyl phosphate, and other unidentified tricresyl phosphate esters; plus 18% of dicresyl phosphate esters), via gavage, at 0, 50, 100, 200, 400 or 800 mg/kg-bw /day in corn oil, 5 days per week, for 13 weeks. No mortalities were observed. Final mean body weights of males were significantly lower than controls at 200 mg/kg-bw/day and higher doses. Cytoplasmic vacuolization of the adrenal cortex was observed in both sexes at all dose groups with severity increasing with dose. Ovarian interstitial cell hypertrophy was observed in all treated females. Atrophy of seminiferous tubules was observed in male rats at 400 mg/kg-day and higher doses. There were no biologically significant changes in neurobehavioral parameters. (NTP 1994). Lowest oral LOAEL (mouse) = 50 mg/kg-bw /day based on cytoplasmic vacuolization of the adrenal cortex observed in B6C3F1 mice (10 per sex per dose) administered TCP in a mixed isomer preparation (containing 79% tricresyl phosphate esters including 21% tri-m-cresyl phosphate, 4% tri-p-cresyl phosphate, < 0.1% tri-o-cresyl phosphate, and other unidentified tricresyl phosphate esters; plus 18% of dicresyl phosphate esters), via gavage, at 0, 50, 100, 200, 400 or 800 mg/kg-bw /day in corn oil, 5 days per week, for 13 weeks. No mortalities were observed. Final mean body weights were significantly decreased in males at 200 mg/kg-bw/day and higher doses and in females at 400 mg/kg-day and higher doses. Cytoplasmic vacuolization of the adrenal cortex was observed in both sexes of all treated groups in a dose-dependent manner. Ovarian interstitial cell hypertrophy was observed in all treated females. Multifocal degeneration of the spinal cord was observed at 100 mg/kg-day and higher doses in both sexes; and multifocal degeneration of the sciatic nerve was observed at 200 mg/kg-bw/day in males and at 100 mg/kg-bw/day in females. Significantly decreased forelimb grip strength was observed at 200 and higher doses in males and at 400 mg/kg-bw/day in females. Significantly decreased hindlimb grip strength was observed at 200 mg/kg-bw/day and higher doses in both sexes (NTP 1994). Other studies: Oral LOAEL (rat) = 55 or 65 mg/kg-bw /day (males or females males respectively) based on cytoplasmic vacuolization of the adrenal cortex observed in males, and hyperplasia of ovarian interstitial cells in females. Fisher 344/N rats (10 per sex per dose) were administered TCP (containing 79% tricresyl phosphate esters including 21% tri-m-cresyl phosphate, 4% tri-p-cresyl phosphate, < 0.1% tri-o-cresyl phosphate, and other unidentified tricresyl phosphate esters; plus 18% of dicresyl phosphate esters) in feed at 0, 900, 1700, 3300, 6600 or 13000 ppm (approximately 0, 55, 120, 220, 430, or 750 mg/kg-bw/day for males and 0, 65, 120, 230, 430, or 770 mg/kg-bw/day for females), 7 days per week, for 13 weeks. No mortalities were observed. Final mean body weights were significantly decreased at 430 mg/kg-bw/day and higher dose in males and at 230 mg/kg-bw/day and higher doses in females. There were no biologically significant changes in neurobehavioral parameters. Cytoplasmic vacuolization of the adrenal cortex was observed in all exposed groups of both sexes. Ovarian interstitial cell hypertrophy and inflammation of the ovarian interstitium were observed in all exposed female groups. Renal papillary edema and renal papillary necrosis were observed at 750 mg/kg-bw/day in males and at 430 mg/kg-bw/day and higher doses in females. Basophilic hypertrophy of the pituitary gland pars distalis and atrophy of the seminiferous tubules were observed at 430 mg/kg-bw/day and higher doses in males (NTP 1994). |
Other sub-chronic toxicity studies |
Oral LOAEL (mouse) = 65 or 110 mg/kg-bw /day (females or males respectively) based on cytoplasmic vacuolization of the adrenal cortex observed in B6C3F1 mice administered TCP (containing 79% tricresyl phosphate esters including 21% tri-m-cresyl phosphate, 4% tri-p-cresyl phosphate, < 0.1% tri-o-cresyl phosphate, and other unidentified tricresyl phosphate esters; plus 18% of dicresyl phosphate esters) in feed at 0, 250, 500, 1000, 2100 or 4200 ppm (approximately 45, 110, 180, 380, or 900 mg/kg b-w/day for males and 65, 130, 230, 530, or 1,050 mg/kg-bw/day for females), 7 days per week, for 13 weeks. No mortalities occurred. Mean body weights were decreased (significance not stated) at 900 mg/kg-bw/day in males, and at 530 mg/kg-bw/day and higher dose in females. Cytoplasmic vacuolization of the adrenal cortex was observed in all exposed groups except for the 45 mg/kg-bw/day males. Papillary hyperplasia of the gallbladder mucosa was observed at 110 mg/kg-bw/day and higher doses in males, and at 230 mg/kg-bw/day and higher doses in females. Renal tubule degeneration was observed in all males at 900 mg/kg-bw/day. Axonal degeneration was observed at 380 mg/kg-bw/day and higher doses in males, and at 230 mg/kg-bw/day and higher doses in females. Significant decreases in forelimb grip strength were observed at 380 and 530 mg/kg-bw/day in males and females, respectively (p ≤ 0.05), and at 900 and 1050 mg/kg-bw/day in males and females, respectively (p ≤ 0.01). Significant decreases in hindlimb grip strength were observed at 900 in males and at 530 mg/kg-bw/day and higher dose in females (p ≤ 0.01). A NOAEL was identified at 45 mg/kg-bw/day for males (NTP 1994). Oral LOAEL (rat) = 1000 mg/kg-bw /day based on hypertrophy of the adrenal cortex observed in Sprague-Dawley rats (5 per sex per dose) administered TCP in 5% gum arabic solution, via gavage, at 30, 100, 300 or 1000 mg/kg-bw/day, 6 days per week for 3 months. The control group received a 5% gum arabic solution. Excessive salivation was observed at all doses immediately following gavage. All treatment groups showed a slight increase in liver weights. Other observations at 1000 mg/kg-bw/day included significant decreases in body weight gain and slight decreases in spleen, heart, and lung weights in males, and increase in adrenal gland weight in females (Sumitomo Chemical Company 1974 cited in ECB 2001). [Additional studies: Saito et al. 1974,Haggerty et al. 1986 (abstract only), Irwin et al. (1987, abstract only), Freeman et al. (1988, abstract only), Daughtrey et al. (1996)–all cited in IPCS 1990] No dermal and inhalation studies were observed. |
Chronic toxicity/ carcinogenicity |
Oral carcinogenicity in rats: Fischer 344/N rats (95 per sex per group) were administered TCP (containing 79% tricresyl phosphate esters including 21% tri-m-cresyl phosphate, 4% tri-p-cresyl phosphate, < 0.1% tri-o-cresyl phosphate, and other unidentified tricresyl phosphate esters; plus 18% of dicresyl phosphate esters) in feed at 0, 75, 150 or 300 ppm (approximately 0, 3, 6 or 13 mg/kg-bw/day for males and 0, 4, 7 or 15 mg/kg-bw/day for females) for 2 years. An additional group of rats received 600 ppm TCP in feed for 22 weeks and then received control feed. Rats were examined at 3, 9 and 15 months and 2 years into the study. There was no evidence of carcinogenic activity of TCP found in the exposed male and female rats (NTP 1994). Oral carcinogenicity in mice: B6C3F1 mice (95 per sex per dose) were administered TCP (containing 79% tricresyl phosphate esters including 21% tri-m-cresyl phosphate, 4% tri-p-cresyl phosphate, < 0.1% tri-o-cresyl phosphate, and other unidentified tricresyl phosphate esters; plus 18% of dicresyl phosphate esters) in feed at 0, 60, 125 or 250 ppm (approximately 0, 7, 13 or 27 mg/kg-bw/day in males and 0, 8, 18 or 37 mg/kg-bw/day in females) for 2 years. Mice were examined at 3, 9 and 15 months and 2 years into the study. There was no evidence of carcinogenic activity of TCP found in the exposed male and female mice (NTP 1994). Non-neoplastic effect Lowest oral LOAEL (rat) = 7 (or 26) mg/kg-bw/day (females or males respectively) based on cytoplasmic vacuolization of the adrenal cortex observed in female rats. Fischer 344/N rats (95 per sex per group) were administered TCP (containing 79% tricresyl phosphate esters including 21% tri-m-cresyl phosphate, 4% tri-p-cresyl phosphate, < 0.1% tri-o-cresyl phosphate, and other unidentified tricresyl phosphate esters; plus 18% of dicresyl phosphate esters) in feed at 0, 75, 150 or 300 ppm (approximately 0, 3, 6 or 13 mg/kg-bw/day in males and 0, 4, 7 or 15 mg/kg-bw/day in females) in feed for 2 years. An additional group of rats (95 per sex per dose) received diets containing 600 ppm TCP for 22 weeks and then received only control feed (approximately 26 mg/kg-bw/day for males and 30 mg/kg-bw/day for females). At 3, 9 and 15 months of exposure, up to15 rats/sex/dose were necropsied and evaluated for histopathological lesions. No mortalities occurred. No effect on the final mean body weights was observed. Cytoplasmic vacuolization of the adrenal cortex was observed at 26 mg/kg-bw/day in males and at 7 mg/kg-bw/day and higher doses in females at 3 months. At 9 and 15 months, cytoplasmic vacuolization was observed primarily in females at 15 mg/kg-bw/day, with the incidence and severity significantly increased at the end of the study. Ovarian interstitial cell hyperplasia was observed in female rats at 15 mg/kg-bw/day and the incidence and severity were increased at the end of the study. A NOAEL was identified at 4 or 13 mg/kg-bw/day for females or males, respectively (NTP 1994). Oral LOAE L (mouse) = 7 (or 37) mg/kg-bw/day (males or females respectively) based on ceroid pigmentation of the adrenal cortex observed in male mice. B6C3F1 mice (95 per sex per dose) were administered TCP (21% m-TCP, 4% p-TCP, <0.1% and other unidentified tricresyl phosphate esters) in feed at 0, 60, 125 or 250 ppm (approximately 0, 7, 13 or 27 mg/kg-bw/day in males and 0, 8, 18 or 37 mg/kg-bw/day in females) for 2 years. Mice were examined at 3, 9 and 15 months and 2 years into the study. At 3, 9 and 15 months of exposure, up to15 mice/sex/concentration were necropsied and evaluated for histopathological lesions. Body weights, survival and feed consumption of exposed groups were similar to that of controls. Ceroid pigmentation of the adrenal cortex was observed in all exposed groups throughout most of the 2-year study, except in 8 and 18 mg/kg-bw/day females at the 3-month necropsy. The severity was markedly increased in females at 37 mg/kg-bw/day. Increased incidence in clear cell foci, fatty change and ceroid pigmentation of the liver were observed in males at 13 mg/kg-bw/day and higher doses. A NOAEL was identified at 18 mg/kg-bw/day for females (NTP 1994). No dermal and inhalation studies were identified. |
Reproductive toxicity
|
Lowest oral LOAEL (mouse) = 63 mg/kg-bw/day based on decreased number of litters per pair of mice. Swiss CD-1 mice (20 per sex per dose for test group, 40 per sex for control group) were administered 0, 0.05, 0.1 or 0.2% of TCP (with <0.1%) by weight (approximately 0, 63, 124, or 250 mg/kg-bw/day in feed, for 7 days prior to breeding and 98 days during the breeding. The study protocol comprised of a continuous breeding phase of the F0 generation, a cross-over mating to determine the affected sex in the F0 animals, and evaluation of the fertility and performance of the last litter (F1) from the continuous breeding. A significant (p<0.01) decrease in the number of litters per pair was observed in a dose-dependent manner. The proportion of pups born live was significantly decreased at 250 mg/kg/day. In the crossover mating phase, control males were mated with the treated females, and control females were mated with treated males. Impaired fertility was observed in both males and females at 250 mg/kg/day, with a greater effect in females. There were dose-related changes in the adrenals for both sexes; and body weight was decreased in both males and females at the high dose. A significant (p<0.05) decrease in sperm motility was observed at 62.5 and 124 mg/kg-bw/day (250 mg/kg-bw/day not examined). Atrophy of seminiferous tubules, decreased testis and epididymal weights in F0 males were observed at 250 mg/kg-bw/day. Changes in the adrenals in F0 of both sexes were significant at 250 mg/kg-bw/day, and the effect was dose-dependent. The last litter born in the 98-day breeding phase was reared to age 74 days and then mated within the control and two of the treatment groups (0.0., 0.05, and 0.1% TCP: there were too few offspring in the 0.2% group). There was a decrease in the fertility index in the 0.1% TCP group, and a decreased proportion of liveborn and number of liveborn pups per litter. In the FI males at necropsy, sperm concentration and morphology were normal at termination, although motility was decreased in both the 0.05% and the 0.1% groups compared to controls. These data show that TCP impaired fertility in both sexes of mice in the F0 generation and affected sperm motility at even the lowest dose in FI males. (Chapin et al. 1988). Other studies: LOAEL = 100 mg/kg-bw/day based on fetotoxicity, abnormal sperm morphology, histopathological changes in testes, epididymides. TCP (containing < 9%) was administered male Long Evans rats (12 per dose) at 0, 100 or 200 mg/kg-bw/day, and to females (24 per dose) and at 0, 200, or 400 mg/kg-bw/day in corn oil via gavage. Males were dosed for 56 days, and females for 14 days prior to breeding and throughout the 10-day breeding period. The 100 mg/kg-day males were mated with the 200 mg/kg-day females and the 200 mg/kg-day males were mated with the 400 mg/kgday females. Dose-dependent abnormal sperm morphology was observed at all doses. Sperm concentration, motility and progressive movement were decreased at 200 mg/kg-day. Reduced epididymal weights, necrosis and degeneration of seminiferous tubules, hypospermia in the epididymis, and increase in degeneration and immature spermatids were also observed in males at 200 mg/kg/day. In females, alteration of ovarian interstitial cells, increased number of follicles, and corpora lutea were observed at 200 mg/kg-bw/day and higher dose. Number of live-delivered pups was reduced at all doses. Reduced litter size was observed at 200 mg/kg-bw/d and higher dose in a dose-dependent manner (Carlton et al. 1987). [Additional studies: Morrissey et al. 1988b cited in IPCS 1990; Mobil (1991e, 1992b); Imperial Oil (1991); Latendresse et al. (1994a,b) No dermal and inhalation studies were identified. |
Developmental toxicity |
Lowest oral LOAEL (rat) = 20 mg/kg- bw/day based on significantly decreased mean fetal body weight. Female Sprauge Dawley rats were administered TCP (composition unknown), via gavage, at 0, 20, 100, 400 or 750 mg/kg-bw /day from day 0 to 19 of gestation. Clinical observations included increased salivation at ≥ 100 mg/kg-bw/day and alopecia and unkempt appearance at ≥ 400 mg/kg-day. Fetal body weights were reduced (significance not stated) compared to control groups at all dose levels. Incomplete ossification was observed at 750 mg/kg-bw/day (US EPA 2010). [Additional studies: Tocco et al. 1987 cited in IPCS 1990 and EA 2009; Mele and Jensh 1977 cited in IPCS 1990 and EA 2009] No dermal and inhalation studies were identified. |
Genotoxicity and related endpoints: in vivo |
Unscheduled DNA Synthesis Negative: in male rats administered TCP by gavage (Mirsalis 1985 cited in EA 2009). |
Genotoxicity and related endpoints: in vitro |
Mutagenicity in bacteria Negative: in Salmonella typhimurium TA 98, TA100, TA1535, TA1537, TA1538 with or without metabolic activation (Haworth et al. 1983 cited in NTP 1994 and EA 2009; Litto Bionetics Inc. 1979a cited in ECB 2001 and EA 2009). Chromosome aberration Negative: in Chinese hamster ovary cells (CHO) with or without metabolic activation (NTP 1994). Negative: in mouse lymphoma cell line with or without metabolic activation (Litto Bionetics Inc. 1979b cited in ECB 2001 and EA 2009). Ambiguous: in mouse lymphoma L5178Y cells with or without metabolic activation (Litto Bionetics Inc. 1979c cited in ECB 2001 and EA 2009). Positive: in BALB/3T3 cell line without metabolic activation (Litto Bionetics Inc. 1979d cited in ECB 2001 and EA 2009). Sister chromatid exchange Negative: in CHO with or without metabolic activation (NTP 1994 and EPA 2010). |
a LC50, median lethal concentration; LD50, median lethal dose; LOAEC, lowest-observed-adverse-effect concentration; LOAEL, lowest-observed-adverse-effect level; LOEL, lowest-observed-effect level
Epidemiology studies |
Results |
|---|---|
Polyneuropathy |
Several human studies were identified. An outbreak of acute polyneuropathy in over 20 young females occurred in Sri Lanka during 1977–1978. The cause of the neuropathy was traced to TCP found as a contaminant in special cooking oil (gingili oil). Contamination probably occurred during transport of the oil in containers previously used for storing mineral oils (Senanayake and Jeyaratnam 1981, cited in IPCS 1990). In 1944, three cases of toxic polyneuropathy among workers who had worked for six to eight months in a plant manufacturing TCP in England were reported. Skin penetration and inhalation were thought to be the main causes of the occupational poisoning (Hunter et al. 1944). A high prevalence of polyneuropathy among shoe factory workers has been reported in Italy since 1958. The cause may be attributed to TCP (Cavalleri and Cosi, 1978). However, the cause-effect relationship has not been established. This polyneuropathy might have various aetiological factors (including n-hexane) or be produced by a combination of them (Leveque 1983 cited in IPCS 1990). |
Irritation/ sensitization |
A case of severe intoxication in a 4-year-old child following ingestion of a lubricant containing TCP (substitution pattern not known) was reported. The clinical findings were vomiting, diarrhoea, weakness, drowsiness, delayed cholinergic crisis, and depressed nerve velocity. But full recovery occurred within four weeks (Goldstein et al. 1988). A human repeated patch test showed signs of irritation (New York University 1951, cited in EA 2009). In a study of 230 patients with possible occupational dermatitis from the metallurgical industry, 2.6 % of them showed positive patch test results with TCP (EA 2009). A case report described allergic contact dermatitis induced by contacting Band-Aid brand adhesive bandages which contains TCP as an ingredient (Norris and Storrs 1990, cited in IUCLID 2001, EA 2009). A maximization test conducted dermally on male human subjects showed that TCP is a moderately strong sensitizer (Dupont, 1992). (stimulation index value of 17.2) was noted for the positive control chemical and this result confirmed the validity of the assay. In conclusion, under the conditions of the present assay Kronitex TCP, tested in a suitable vehicle, was shown to have sensitization potential (sensitizer) in the Local Lymph Node Assay (LAB Research Ltd. 2010) |
