Screening assessment phenol, 2-methoxy-5-(2-propenyl)- (eugenol) and rose, rosa canina, ext.
Screening assessment phenol, 2-methoxy-5-(2-propenyl)- (eugenol) and rose, rosa canina, ext.
Chemical abstracts service registry numbers
97-53-0 and 84696-47-9
Environment and Climate Change Canada Health Canada
December 2018
Cat. No.: En14-347/2018E-PDF
ISBN 978-0-660-28823-9
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment on two of four substances referred to collectively under the Chemicals Management Plan as the Eugenol and Isoeugenol Derivatives Group. Phenol, 2-methoxy-4-(2-propenyl)- (commonly referred to as eugenol) was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA. Rose, Rosa canina, ext. (commonly referred to as Rosa canina extract) was considered a priority on the basis of other ecological concerns. Two of the four substances were subsequently determined to be of low concern through other approaches, and decisions for these substances are provided in a separate report.Footnote 1 Accordingly, this screening assessment addresses the two substances listed in the table below.
Domestic Substances List namea |
Common name(s) |
|
97-53-0 |
Phenol, 2-methoxy-4-(2-propenyl)- |
eugenol |
84696-47-9b,c |
Rose, Rosa canina, ext. |
Rosa canina extract |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
b This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other concerns.
c This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
Eugenol is a naturally occurring substance. It is a major component of the bud, leaf and stem of clove (Syzygium aromaticum) and is also reported as a constituent in other volatile oils (e.g., laurel and cinnamon leaf oils). According to information submitted pursuant to CEPA section 71 surveys, between 100 and 1000 kg of eugenol was manufactured in Canada and between 100 and 1000 kg was imported into Canada in 2011. Eugenol is used as an odour agent or fragrance ingredient in a range of products, including personal care products, cleaning products and air care products. Eugenol and clove oil (a source of exposure to eugenol) are ingredients in cosmetic and natural health products. Eugenol is naturally occurring in food, and eugenol and clove oils (bud, leaf and stem) are used as food flavouring agents.
Rosa canina extract is obtained from the Rosa canina plant, a wild rose species widely distributed in Europe, Asia, the Middle East and North America. In 2011, no Canadian manufacturing activities were reported for Rosa canina extract above the reporting threshold of 100 kg; 1200 kg of Rosa canina extract was imported into Canada. Rosa canina extract is used in a wide variety of cosmetic and natural health products in Canada, with medicinal and non-medicinal roles.
The ecological risks of eugenol and Rosa canina extract were characterized using the ecological risk characterization of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity are established. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. The ERC identified eugenol and Rosa canina extract as having low potential to cause ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from eugenol and Rosa canina extract. It is concluded that eugenol and Rosa canina extract do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
With respect to human health, exposure of the general population to eugenol in Canada may occur through the use of a range of products including cosmetics, natural health products, air fresheners and cleaning products (as a fragrance ingredient). Eugenol is also naturally present in food, which is expected to be the predominant source of dietary exposure, and is also used as a food flavouring agent. The International Agency for Research on Cancer has classified eugenol as a Group 3 carcinogen (not classifiable as to its carcinogenicity to humans) based on limited evidence of carcinogenicity in laboratory studies. Available information indicates that eugenol is not likely to be genotoxic. The only health effect associated with repeated exposure to eugenol in laboratory studies is a decrease in body weight gain, observed only at high doses, with no associated adverse effects. Accordingly, this substance is considered to be of low hazard potential. Given the low hazard potential of this substance, the risk for human health is considered to be low.
Exposure of the general population in Canada to Rosa canina extract may occur primarily through use of cosmetics and natural health products by the oral and dermal routes. Based on composition information, eugenol and 2-phenethyl alcohol (CAS RN 60-12-8) were identified as primary constituents of Rosa canina flower extract. On the basis of a comparison of estimates of exposure to 2-phenethyl alcohol and levels of 2-phenethyl alcohol associated with adverse effects in laboratory studies, margins of exposure are considered adequate to address uncertainty in health effects and exposure databases.
On the basis of the information presented in this screening assessment, it is concluded that eugenol and Rosa canina extract do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that eugenol and Rosa canina extract do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment on two of four substances referred to collectively under the Chemicals Management Plan as the Eugenol and Isoeugenol Derivatives Group, to determine whether these two substances present or may present a risk to the environment or to human health. The two substances addressed in this screening assessment report are phenol, 2-methoxy-4-(2-propenyl)- (commonly referred to as eugenol) and rose, Rosa canina, ext. (commonly referred to as Rosa canina extract). Eugenol was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]). Rosa canina extract was considered as a priority on the basis of other ecological concerns (ECCC [modified 2013]).
Two other substances [(CAS RNFootnote 2 120-11-6 (benzene, 2-methoxy-1-(phenylmethoxy)-4-(1-propenyl)) and CAS RN 120-24-1 (benzeneacetic acid, 2-methoxy-4-(1-propenyl)phenyl ester)] were considered in the Ecological Risk Classification of Organic Substances (ERC) and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances science approach documents (ECCC 2016a; Health Canada 2016) and were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this report. Conclusions for these two substances are provided in the Substances Identified as Being of Low Concern based on the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances [ACSD1] Screening Assessment (ECCC, HC 2017), which addresses a range of substances in this situation.
Although eugenol and Rosa canina extract were grouped together because of their similar use patterns and because of the potential for Rosa canina extract to contain eugenol, they are considered individually in this screening assessment.
The ecological risks of eugenol and Rosa canina extract were characterized using the ERC (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to October 2016. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada. This screening assessment also incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 20, 2016), which was peer-reviewed and subject to a 60-day public comment period. The human health portions of this screening assessment have undergone external peer review and/or consultation. Comments on the technical portions relevant to human health were received from Bernard Gadagbui (University of Cincinnati), Jennifer Sahmel (Insight Exposure and Risk Sciences Inc.) and John Urban (ToxStrategies). Additionally, the draft of this screening assessment published September 30, 2017, was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 3 This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Number (CAS RN), DSL name and common name for eugenol is presented in Table 2-1.
CAS RN |
DSL name |
Chemical structure and molecular formula |
Molecular weight (g/mol) |
97-53-0 |
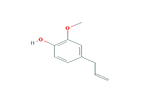
Phenol, 2-methoxy-4-(2-propenyl)- (eugenol) |
|
164.20 |
CAS RN |
DSL name |
Representative chemical structuresa |
84696-47-9 |
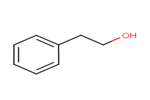
Rose, Rosa canina, ext. (Rosa canina extract) |
45.1%: eugenol 13.6%: 2-phenethyl alcohol |
a Concentrations based on the n-pentane extraction of the aromatic water obtained from the flowers of Rosa canina by hydrodistillation (Hosni et al. 2010).
The CAS RN assigned to Rosa canina extract (i.e., 84696-47-9) has been found to apply to a variety of parts, extracts or preparations of the Rosa canina plant, including rose extract, Rosa canina extract, rose hips extract, Rosa canina callus extract, Rosa canina flower, Rosa canina flower extract, Rosa canina flower oil, Rosa canina flower powder, Rosa canina fruit, Rosa canina fruit extract, Rosa canina fruit oil, Rosa canina fruit juice, Rosa canina seed extract, Rosa canina leaf extract, Rosa canina seed, Rosa canina seed oil and Rosa canina seed powder (ChemIDplus 1993, CIR 2016, CIR 2011, PCPC 2017 as cited in personal communication, email from the Non-Prescription and Natural Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, January 2017; unreferenced).
Varying results for compositional information for Rosa canina exist (Nadpal et al. 2016; Ozyurt et al. 2016; Winther et al. 2016; Ghazghazi et al. 2010; Hosni et al. 2010; Ozel and Clifford 2004; Ozcan 2002). The wide variety in composition information may be attributed to many factors, including the part(s) of plant used for extraction, differences in extraction methods, regional variation in plant source(s), seasonal variation, and plant maturity.
Extracts or oils of Rosa canina may be derived from fruits (also known as rosehips), flowers or leaves. Analyses of species related to Rosa canina, specifically Rosa x alba, indicate that there are marked differences in the chemical composition between fruits and flowers (Hosni 2011).
Rosehips (or fruits) are a common source of Rosa canina extracts or oils. The entire rosehip may be used or separated into specific elements (e.g., fruit without seeds, seeds alone). In products available to consumers, the ingredient identification may be specific (e.g., “Rosa canina seed extract”) or unspecific (e.g., “Rosa canina”).
Investigations of the composition of the fruit of Rosa canina have identified a large number of chemicals, with very few of the identified volatile chemicals found to be present at levels comprising more than 10% (Ozyurt et al. 2016). Known constituents of the Rosa canina fruits include vitamins, carotenoids (these substances impart colour to fruits), flavonoids, triterpene acids and fatty acids (Winther et al. 2016; Ercisli 2007; CIR 2016).
2-Phenethyl alcohol (CAS RN 60-12-8) and eugenol were noted as primary substances resulting from hydrodistillation of Rosa canina flowers (Hosni et al. 2010), who found that hydrodistillation is the most usual method for the extraction of the aromatic water. Representative concentrations and chemical structures of these two primary substances of Rosa canina flowers are outlined in Table 2-2.
In the absence of health effects information on the mixture, the focus of the human health assessment for Rosa canina extract was based on these primary substances of Rosa canina flower extract (i.e., eugenol and 2-phenethyl alcohol).
3. Physical and chemical properties
A summary of physical and chemical properties for eugenol are presented in Table 3-1. Limited physical and chemical property information was available for Rosa canina extract. However physical and chemical properties for 2-phenethyl alcohol, a primary component of Rosa canina flower extract, are presented in Table 3-2. When experimental information was limited or not available for a property, models were used to generate predicted values for the substance. Additional physical and chemical properties are presented in ECCC (2016b).
Property |
Value or range |
Type of data |
Key reference(s) |
Physical state |
Liquid |
Experimental |
Merck Index 2006, cited in HSDB 1983- |
Melting point (°C) |
-9 |
Experimental |
Merck Index 2006, cited in HSDB 1983- |
Vapour pressure (mm Hg) |
2.2 x10-2 |
Experimental |
Van Roon et al. 2005, cited in HSDB 1983- |
Henry’s law constant (atm·m3/mol) |
1.92 x10-6 |
Modelled |
Epi Suite c2000-2012 |
Water solubility (mg/L) |
2460 |
Experimental |
Yalkowsky et al. 2010 |
log Kow (dimensionless) |
2.49 |
Experimental |
Dias et al. 2003 |
Abbreviations: Kow, octanol–water partition coefficient
Property |
Value or range |
Type of data |
Key reference(s) |
Physical state |
Liquid |
|
Fenaroli’s Handbook 1975, cited in HSDB 1983- |
Melting point (°C) |
-27 |
Experimental |
Merck Index 2006, cited in HSDB 1983- |
Vapour pressure (mm Hg) |
8.7 x10-2 |
Modelled |
Daubert et al. 1989, cited in HSDB 1983- |
Water solubility (mg/L) |
16000 |
Experimental |
Valvani et al. 1981, cited in HSDB 1983- |
log Kow (dimensionless) |
1.36 |
Experimental |
Hansch and Leo 1987, cited in HSDB 1983- |
4. Sources and uses
Eugenol is a naturally occurring substance present in plants; it is mainly found as a component in essential oils (IARC 1985). Eugenol is a major component (75% to 88%) of the bud, leaf and stem oils of clove (Syzygium aromaticum; Lis-Balchin 2006). Eugenol is also reported as a constituent in volatile oils, including laurel and cinnamon leaf oils (Burdock 2010).
Rosa canina is a wild rose species and is widely distributed in Europe, Asia, the Middle East and North America (Neilsson 1997).
Eugenol and Rosa canina extract were included in surveys issued pursuant to section 71 of CEPA (Canada 2012). Table 4‑1 presents a summary of the total manufacture and total import quantities for eugenol and Rosa canina extract in 2011.
Common name |
Total manufacture (kg) |
Total imports (kg) |
Eugenol |
100–1000 |
100–1000 |
Rosa canina extract |
NRb |
1200 |
a Values reflect quantities reported in response to surveys conducted under section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (Schedules 2 and 3).
bNR: not reported above reporting threshold of 100 kg
Clove oils (CAS RNs 8000-34-8, 8015-97-2 and 8015-98-3) are found in products available to consumers in Canada, including cosmetics, natural health products and pest control products. Products containing clove oils were therefore considered in this screening assessment as possible sources of exposure to eugenol.
According to notifications submitted to Health Canada under the Cosmetic Regulations, eugenol is used as an ingredient in over 1900 cosmetic products in Canada, including moisturizers, cleansers and hair products (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, June/September 2016; unreferenced) and clove oils are used in over 700 cosmetic products in Canada, including cleansers, moisturizers, massage products and perfumes (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, June/September 2016; unreferenced).
Eugenol is listed in the Natural Health Products Ingredients Database (NHPID) with both a medicinal role as classified as a natural health product (NHP) substance under item 2 (an isolate) of Schedule 1 to the Natural Health Products Regulations (NHPR) and a non-medicinal role as a flavour enhancer or fragrance ingredient in NHPs. It is associated with an upper limit of 2.5 mg/kg bw/day, based on the acceptable daily intake (ADI) established by the Joint FAO/WHO Expert Committee on Food Additives (WHO 2006a, 2006b; NHPID [modified 2018]). Eugenol is listed in the Licensed Natural Health Products Database (LHNPD) as being present as both a medicinal and non-medicinal ingredient in currently licensed natural health products in Canada (LNHPD [modified 2018]). Eugenol is also found as a non-medicinal ingredient in a small number of non-prescription drugs approved for use in Canada (personal communication, emails from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, January 2016; unreferenced).
Clove essential oil is listed in the NHPID with a medicinal role as an NHP under item 2 (an extract) of Schedule 1 to the NHPR. It is also listed in the Natural and Non-prescription Health Products Directorate (NNHPD)’s Counterirritants monograph as a medicinal ingredient with a dose range of 0.1 to 2.0% and in the NNHPD’s Aromatherapy – Essential Oils monograph with concentration ranges of 1 to 4% (bud and stem) and 1-2% (leaf) (NNHPD 2015a, 2013). Clove flower bud, leaf and stem oils are also identified in the NHPID with a non-medicinal role for use as a fragrance ingredient, skin-conditioning agent and/or flavour enhancer in natural health products (NHPID [modified 2018]). Clove oils are listed in the LNHPD as being present as both a medicinal and non-medicinal ingredient in a number of currently licensed natural health products in Canada (LNHPD [modified 2018]).
Eugenol and clove oils are formulants found in a range of pesticide products registered in Canada (personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, March 2016; unreferenced).
Eugenol is used as a fragrance ingredient in products available to consumers in Canada, including air fresheners and scented candles (MSDS 2016). Individual fragrance ingredients (such as eugenol) may not be identified as part of the fragrance mixture; therefore, it is assumed that eugenol may also be present in other products (e.g., cleaning products). Eugenol and clove oils are internationally recognized as fragrance ingredients and are on the International Fragrance Association (IFRA) Ingredient List (IFRA 2013).
Eugenol occurs naturally as a constituent in several volatile oils (e.g., clove oils, as well as laurel and cinnamon leaf oils) as well as in some foods (Burdock 2010). In Canada, eugenol and clove oils (bud, leaf and stem) may be used as food flavouring ingredients. In addition, the Food and Drug Regulations identifies that flavour preparations of Ceylon cinnamon may contain up to 10 percent eugenol (Canada [1978]). The Food Chemicals Codex (FCC) indicates that eugenol and clove oils have a function as flavouring agents [KV1] (FCC USP 2016, cited in personal communication as part of comments from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, January 2017; unreferenced). They are also listed in Fenaroli’s Handbook of Flavor Ingredients (Burdock 2010). The European Union permits eugenol to be used as a flavouring in food (EC Food Flavourings Database). The United States Food and Drug Administration has affirmed clove and its derivatives (including eugenol) to be “generally recognized as safe” (GRAS) as food flavouring agents, provided they meet conditions set out in Section 184.1257, including that they be used in foods at levels not to exceed good manufacturing practice in accordance with 184.1(b) (1) (US CFR 2016a). Eugenol is also permitted for use as a component in the production of or added to textiles or fibres of textiles for food contact surfaces (US CFR Section 177.2800) (US CFR 2016b).
In Canada, eugenol was identified as an ingredient of an incidental additive (i.e., in hand soaps for use in food processing establishments). Clove oil was also identified for use in the manufacture of a limited number of food packaging materials as well as in a number of incidental additives, such as hand cleaners and disinfectants for use in food processing establishments (personal communication, Health Products and Food Branch, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, January 2016; unreferenced).
Rosa canina fruits have a long history of use in traditional medicine, such as in the treatment of the common cold and eczema (Winther et al. 2016). The fruits have also been traditionally used for making marmalades and soups and have been reported as having a high vitamin C content (Winther et al. 2016).
According to notifications submitted to Health Canada under the Cosmetic Regulations, Rosa canina is used in over 2000 cosmetic products in Canada. In cosmetics, Rosa canina extract may be identified in several ways including: Rosa canina, Rosa canina extract, Rosa canina flower, Rosa canina fruit, Rosa canina fruit extract, Rosa canina fruit oil, Rosa canina seed extract, Rosa canina seed powder, and rose hips seed oil. The most common product types include moisturizers, cleansers, conditioners, hair colour and bath products (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, June/September 2016; unreferenced).
Rosa canina is listed on the NHPID with a medicinal role (Canada 2003). Rosa canina fruit is listed as medicinal ingredient in the NNHPD’s Antioxidants monograph with a maximum daily dose of 100 g fresh or 45 g dry (NNHPD 2015c). Rosa canina, its ripe fruits, flowering twigs with leaves and flowers on stem with the leaves are listed in the NHPID with a homeopathic role. Rosa canina flower extract, Rosa canina fruit extract, Rosa canina fruit oil, Rosa canina leaf extract and Rosa canina seed extract are listed in the NHPID with a non-medicinal role for use as cosmetic astringent, flavour enhancer, skin-conditioning agent, and/or skin-conditioning agent – occlusive (NHPID [modified 2016]). Rosa canina is listed in the LNHPD as being present in various preparations as listed above, and as both a medicinal and non-medicinal ingredient, in a number of currently licensed natural health products in Canada (LNHPD [modified 2018]).
Rosa canina extract is not commonly considered to function as a fragrance ingredient in products and is not listed on the IFRA Ingredient List (IFRA 2011). Rosa canina extract was also not found in other types of products available to consumers.
Although Rosa canina extract (rose hips extract) is listed in Fenaroli’s Handbook of Flavor Ingredients (Burdock 2010), it is not referenced in any other flavouring compendia, including JECFA. Rose fruit (hips) are listed as “generally recognized as safe” under the United States Code of Federal Regulations (Section 182.20 - Essential Oils, oleoresins (solvent-free, and natural extractives (including distillates)) (US CFR 2016c).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of eugenol and Rosa canina extract were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., LC50) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were either collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox), and from responses to CEPA section 71 notices or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were established principally on the basis of metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also established using multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error in empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. The impact of this error is mitigated, however, by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error in underestimation of acute toxicity will be mitigated through the use of other hazard metrics, such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is believed to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for eugenol and Rosa canina extract, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
The hazard and exposure classifications for eugenol and Rosa canina extract are summarized in Table 5-1.
Substance |
ERC hazard classification |
ERC exposure classification |
ERC risk classification |
Eugenol |
low |
low |
low |
Rosa canina extract |
low |
low |
low |
On the basis of low hazard and low exposure classifications according to information considered under ERC for eugenol and Rosa canina extract, these substances were classified as having a low potential for ecological risk. It is therefore unlikely that they result in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Environmental Media
Measured concentrations of eugenol and Rosa canina extract in ambient air and water were not identified in Canada. These substances were reported to be imported and/or manufactured in low quantities in Canada; therefore releases from industrial sources are expected to be low. Accordingly, exposure of the general population from levels in environmental media is not expected.
Food
No Canadian data were identified on the levels of eugenol that may be present in foods. Eugenol occurs naturally as a constituent in several volatile oils (e.g., clove oils, as well as laurel and cinnamon leaf oils) as well as in some foods (Burdock 2010). It is also identified as a food flavouring agent. The predominant source of dietary exposure to eugenol is expected to result from its natural occurrence in foods (Stofberg and Grundschober 1987).
The JECFA evaluated a flavouring group of hydroxyallylbenzene derivatives, including eugenol, at its 65th meeting (WHO 2006a, 2006b). As part of that evaluation, the Committee estimated the per capita intake of eugenol from its use as a food flavouring agent to be 3.4 mg per day.
In this evaluation, the Committee concluded that the use of eugenol as a flavouring agent would not present a safety concern at the estimated exposure levels. EFSA (2009) agreed with the JECFA conclusion of no safety concern at estimated levels of intake as a flavouring substance.
Dietary exposure, if any, from uses of eugenol or clove oil in food packaging applications or for use as components in cleaners or disinfectants for possible use in food processing establishments is expected to be negligible (personal communication, Health Products and Food Branch, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, January 2016; unreferenced).
Products Available to Consumers
Eugenol
Eugenol and clove oils are used in a wide range of cosmetic products in Canada. Most cosmetic products (>90%) contain less than 1% eugenol or clove oil. Higher concentrations of eugenol (up to 10%) were reported in products such as spray perfumes, conditioners, cleansers and body moisturizers. Massage products (30% to 100% clove oil) as well as lubricants and perfumes (up to 30% clove oil) contained the highest concentration of clove oil (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, June/September 2016; unreferenced) and represent a significant source of exposure to eugenol from products available for use by the general population, including infants (e.g., products used as baby oil or moisturizer).
Many natural health products containing eugenol or clove oil are for topical use and therefore result in dermal exposure (e.g., acne treatments, antiperspirants, aromatherapy/essential oils, counterirritants, medicated skin care products and sunscreens). Information on quantity of eugenol or clove oil was not available for most products (personal communication, emails from the Natural and Non-Prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, January 2016; unreferenced). However, dermal exposure to eugenol from these natural health products is expected to be comparable to or less than that from use of cosmetics.
Dermal exposure can also occur through the use of cleaning products in which eugenol is a component of the fragrance mixture.
Of note, eugenol is predicted to be relatively well absorbed through the skin. Results of various in vitro studies conducted with rat and human skin suggest approximately 65% of the applied dose may be considered to be absorbed (Schmitt et al. 2009, 2010; Lui and Hotchkiss 1997). Key limitations associated with these studies include a low number of replicates and limited recovery information. Eugenol has also been identified for its potential in enhancing dermal permeation (Zhao and Singh 2000).
Cosmetics resulting in oral exposure to eugenol include mouthwashes and toothpastes. In addition, many natural health products containing eugenol or clove oil as non-medicinal ingredients are for buccal, dental or oral use and therefore may result in oral exposure (e.g., capsules, kits, liquids, gels, lozenges, mouthwashes, and toothpastes, including toothpastes for toddlers).
Inhalation exposure to eugenol may result from the use of certain cosmetics (e.g., spray perfume, nail polish) and natural health products (e.g., topical spray). Eugenol has also been found in other products available to consumers that may also result in inhalation exposure, specifically solid air fresheners and refillable plug-in air fresheners, with concentrations up to 5% (MSDS 2016).
Given the low hazard potential of eugenol (as noted in section 6.3, Characterization of Risk to Human Health), further exposure characterization (i.e., derivation of estimates of exposure) was not warranted.
Rosa canina extract
Rosa canina flower, flower extract and flower oil have been reported in more than 100 cosmetics. Cosmetics with the highest exposure potential were identified as those containing Rosa canina flower, flower oil or flower extract at concentrations of up to 30% and consisted of make-up removers, hand creams and face moisturizers (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, June/September 2016; unreferenced). Estimates of exposure to the general population from 2-phenethyl alcohol in these cosmetics were derived assuming a phenethyl alcohol concentration of up to 4% (based on results from Hosni et al. 2010 indicating that Rosa canina flower extract contains 13.6% phenethyl alcohol when hydrodistillation was used as the method of extraction). Dermal exposure estimates for 2-phenethyl alcohol are outlined in Table 6-1.
Product scenarioa |
Maximum concentration of Rosa canina flower (2‑phenethyl alcohol)b,c (% w/w) |
Estimated daily systemic exposure (mg/kg bw/day) |
Hand cream |
30 (4) |
2 |
Body cream |
10 (1.4) |
0.96 |
Facial cream |
30 (4) |
1.2 |
Make-up remover |
30 (4) |
0.28 |
a All scenarios are based on adult body weight (70.9 kg).
b Concentrations are based on notifications submitted to Health Canada under the Cosmetic Regulations (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June/Sept. 2016; unreferenced).
c 2-Phenethyl alcohol concentrations were calculated using the concentration of 13.6% reported by Hosni et al. (2010), based on the n-pentane extraction of the aromatic water obtained from the flowers of Rosa canina by hydrodistillation.
Oral exposure to 2-phenethyl alcohol associated with Rosa canina flower in cosmetics was estimated to be 1.4 x 10-2 mg/kg bw/day for a lip balm scenario with a product containing 30% Rosa canina flower extract (i.e., 4% 2-phenethyl alcohol).
A limited number of natural health products associated with oral exposure were noted to contain Rosa canina flower extract as non-medicinal ingredient. A representative product was used to estimate exposure using the following information: 133 mg Rosa canina flower extract per tablespoon (tbsp) of product, a recommended intake of 2 tbsp of product per day and 13.6% 2-phenethyl alcohol in Rosa canina flower extract. The resulting oral exposure estimate for 2-phenethyl alcohol was 0.5 mg/kg bw/day. Label instructions for products resulting in these exposure estimates indicate that they would result in short-term exposure (e.g., “for occasional use only” or “consult a health care practitioner if using beyond 7 days”).
6.2 Health effects assessment
Eugenol
The International Agency for Research on Cancer (IARC) has classified eugenol as a Group 3 carcinogen (not classifiable as to its carcinogenicity to humans). This classification was based on limited evidence of carcinogenicity in experimental animals (IARC 1985, 1987). Eugenol has been previously evaluated by JECFA (WHO 2006a, 2006b).
Oral carcinogenicity studies for eugenol have been conducted on both rats and mice. Eugenol was not carcinogenic to rats; in B6C3F1 mice, an increased incidence of liver tumours was observed. The US National Toxicology Program (NTP) judged this increase in mice tumour incidence to be equivocal evidence for carcinogenicity (NTP 1983). In a study in CDI mice, eugenol was negative for carcinogenicity. Overall, the available data indicates that eugenol is likely not carcinogenic. The studies are described below.
In a chronic lifetime study, groups of Fischer 344/N rats (50/sex/test group; 40/sex/control group) were fed diets containing 0, 3000 or 6000 ppm eugenol (males) and 0, 6000 or 12 500 ppm eugenol (females) (equivalent to 0, 150 and 300 mg/kg bw/day and 0, 300 and 625 mg/kg bw/day, respectively, using a dose conversion generated by Health Canada 1994) for 2 years (103 weeks). Increases in alveolar/bronchiolar adenomas or carcinomas (combined) were observed in males exposed at the lowest dose only (0/40, 5/49 and 2/50 at 0, 150 and 300 mg/kg bw/day, respectively). An increase in C-cell adenomas of the thyroid gland was also observed in female rats exposed at the lowest dose (3/40, 11/49 and 2/50 at 0, 300 and 625 mg/kg bw/day, respectively), and a non-significant increase in incidence of endometrial stromal polyps of the uterus was noted in female rats exposed at the highest dose (6/40, 6/50 and 16/50 at 0, 300 and 625 mg/kg bw/day, respectively). Fibroadenomas of the mammary gland were decreased in treated groups of female rats compared to controls (14/40, 8/50 and 6/50 at 0, 300 and 625 mg/kg bw/day, respectively). None of these differences were considered to be associated with the dietary administration of eugenol by the NTP. Under the experimental conditions, there was no evidence of carcinogenicity observed for male or female rats exposed to eugenol (NTP 1983).
In the lifetime study in mice, male and female B6C3F1 mice (50/sex/group) were fed diets containing 0, 3000 or 6000 ppm eugenol (equivalent to 0, 390 or 780 mg/kg bw/day, using a dose conversion by Health Canada 1994) for 2 years (103 weeks). A statistically significant increase in the incidence of liver tumours was noted in males at the lowest dose tested (hepatocellular adenomas 4/50, 13/50 and 10/49 at 0, 390 and 780 mg/kg bw/day, respectively; hepatocellular carcinomas 10/50, 20/50 and 9/49, respectively; hepatocellular adenomas and carcinomas combined 14/50, 28/50 and 18/49, respectively). The authors therefore concluded that no dose-response relationship was observed in the study (NTP 1983). Liver adenomas and carcinomas are common neoplasms in male B6C3F1 mice in NTP studies; the background incidence of hepatocellular tumours is 42.2% in male mice and 23.6% in female mice in control groups of dietary studies (Haseman et al. 1998). Of note, the incidence of hepatocellular tumours in the male control group of the eugenol lifetime study was significantly lower than the background incidence for this type of tumour in NTP studies (28 vs 42.2%, respectively). In male mice exposed to the lowest dose, incidence of liver tumours was higher than the background incidence in NTP studies (56%), but the incidence of the highest dose group was below the background incidence (37%). In female mice, the increases in incidence of hepatocellular adenomas and carcinomas were not statistically significant at either dose tested. The increase in incidence of hepatocellular adenomas and hepatocellular carcinomas combined followed a dose-related trend and was statistically significant at the highest dose (hepatocellular adenomas 0/50, 4/49 and 3/49 at 0, 390 and 780 mg/kg bw/day, respectively; hepatocellular carcinomas 2/50, 3/49 and 6/49, respectively; hepatocellular adenomas and carcinomas combined 2/50, 7/49 and 9/49, respectively) (NTP 1983). However, the incidence of hepatocellular tumours (combined) in females was below the background incidence in female mice for these neoplasms in NTP studies (18 vs 23.6%, respectively). No other statistically significant dose-dependent increases in tumour incidences were noted in any other tissues and/or organs examined.
Under these experimental conditions, the NTP concluded that there was equivocal evidence of carcinogenicity for mice, and the rationale used was that although an increased incidence of both carcinomas and adenomas of the liver was observed in male mice at the 3 000 ppm dietary level (the lowest dose tested), this increase was not observed at the highest dose tested. Also, eugenol treatment was found to be associated with an increase in incidences of hepatocellular carcinomas or adenomas combined in female mice exposed to the highest dose, but there was no increase in incidences when adenomas or carcinomas were counted separately (NTP 1983).
In a study designed to investigate the potential for development of hepatomas in mice, young female CD-1 mice (30/group) were fed diets containing 0 or 0.5% eugenol (5 000 ppm; equivalent to 650 mg/kg bw/day, using a dose conversion by Health Canada 1994), with or without 0.05% phenobarbital in the drinking water, for 12 months. The animals received a grain diet without eugenol for an additional 6 months following administration of the test diets. Groups administered phenobarbital received it from the beginning of the study until its termination (total of 18 months). Groups fed a diet containing eugenol and diet controls did not develop hepatic tumours at study termination. Two of the phenobarbital controls developed hepatomas. In this study, methyl eugenol and the structurally-related carcinogens safrole and estragole were also tested in order to compare their carcinogenic potential. The authors concluded that while methyl eugenol appears to be carcinogenic in the mouse liver similarly to the two related carcinogens, eugenol did not cause any hepatogenic responses in mice (Miller et al. 1983).
Two dermal carcinogenicity studies have also been identified in the literature for eugenol. The ability of eugenol to promote skin tumours was studied using groups of ICR/Ha Swiss mice (20 females/group). One group was given a single cutaneous initiating dose of 7,12-dimethylbenz(a)anthracene (DMBA) to the back. This group and another group not initiated with DMBA received cutaneous applications of 5 mg of eugenol, 3 times per week for 63 weeks. No carcinomas were found in either group, and no papillomas were found in the animals receiving only eugenol, while three animals developed papillomas in the group initiated with DMBA and also treated with eugenol. Two papillomas and 1 carcinoma developed in control animals initiated with DMBA and then treated 3 times weekly with DMSO, the solvent control (Van Duuren et al. 1966). In another study carried out in the same laboratory, eugenol was reported to have a partial inhibitory action on the carcinogenicity of benzo(a)pyrene when the compounds were applied together in a carcinogenic skin painting study (Van Duuren and Goldschmidt 1976).
In in vitro assays, eugenol was not mutagenic in bacterial mutation assays using Salmonella typhimurium and Escherichia coli, with and without metabolic activation (Green and Savage 1978; Sekizawa and Shibamoto 1982; To et al. 1982; Haworth et al. 1983; Nestmann et al. 1983; NTP 1983; Amonkar et al. 1986; Azizan and Blevins 1995). Positive results were observed in mouse lymphoma cell mutation assays with and without metabolic activation (Myhr and Caspary 1991; Honma et al. 1999). An assay for chromosome aberration in Syrian hamster embryo cells in the presence and absence of metabolic activation showed a positive response (Hikiba et al. 2005). Similarly, positive results were also reported in chromosomal aberration assays in Chinese hamster ovary (CHO) cells in the presence of metabolic activation (NTP 1983; Galloway et al. 1987; Armstrong et al. 1992), but negative results were reported in the absence of metabolic activation (NTP 1983; Galloway et al. 1987). A positive result was observed in a sister chromatid exchange assay in CHO cells in the presence and absence of metabolic activation (NTP 1983), but assays for unscheduled deoxyribonucleic acid synthesis (UDS) in cultured hepatocytes from male Fischer 344 rats and female B6C3F1 mice showed negative results (Howes et al. 1990; Burkey et al. 2000).
In in vivo assays, mostly negative results for genotoxicity were observed. Negative results were noted for micronucleus induction and chromosomal aberration in peripheral blood cells of 10 healthy male volunteers who ingested 150 mg/day eugenol for 7 days (Rompelberg et al. 1996). In micronuclei assays in experimental animals, eugenol produced mixed results. No increase in the number of polychromatic erythrocytes containing micronuclei was seen in mice exposed to eugenol via the diet or intraperitoneal injection at doses up to 680 and 800 mg/kg bw (Hayashi et al. 1988; Shelby et al. 1993; Rompelberg et al. 1995) or in rats exposed via oral gavage at doses up to 2680 mg/kg bw (Maura et al. 1989; Allavena et al. 1992) for exposure durations going from a single administration to daily for up to 15 days. However, positive results were observed in one assay conducted in male mice exposed to up to 600 mg eugenol/kg bw via intraperitoneal injection (single administration) (Ellahuene et al. 1994) and in bone marrow of mice exposed via gavage and the intraperitoneal route (up to 14 794 and 740 mg/kg bw, respectively, daily for 2 days) (Woolverton et al. 1986). No DNA adduct formation was detected in the liver of newborn male B6C3F1 mice treated by intraperitoneal injection with 0.25 to 3.0 µmol eugenol on days 1, 8, 15, 22 after birth (Phillips et al. 1984). Eugenol did not induce UDS in hepatocyte primary cultures prepared from liver of male Sprague-Dawley rats administered 0, 1340, 2680 mg/kg bw by gavage, daily for 2 days (Allavena et al. 1992). No genotoxic potential was found for eugenol in a host-mediated gene mutation assay in male C3H/HeJ mice administered eugenol (0 or 200 mg/kg) as a single intramuscular dose (Green and Savage 1978). Finally, ambiguous results were observed in a Drosophila wing somatic mutation and recombination test (SMART). In this assay, eugenol was administered to male and female Drosophila melanogaster (mwh/flr3) larva in feed for approximately 48 hours at doses of 0, 1.0, 5.0, 10.0, 20.0 mM (standard cross) or 0, 1.0, 5.0, 7.5, 10.0, 15.0 mM (improved cross). No statistically significant induction of any categories of spots was observed at any concentration levels for eugenol in the standard cross version of the SMART. The absence of a correlation between genotoxic effect and dose applied was observed for eugenol in the improved cross; although the substance was able to induce a significant increase, it was limited to small single and total spots in three concentrations only: 1.0, 7.5 and 15 mM (Munerato et al. 2005).
While mixed results were observed for eugenol in mutagenicity assays, most in vitro assays in mammalian cells with positive results were found to be performed at concentrations that resulted in cytotoxicity or severe cell cycle delay (WHO 2006a, 2006b). Also, in vivo assays mainly gave negative results, even at very high doses of eugenol. On the basis of the collective evidence on genotoxicity, it is considered that eugenol is likely not mutagenic.
In humans and rodents, eugenol administered by the oral route is rapidly absorbed from the gastrointestinal tract and efficiently extracted by the liver, where it mainly undergoes phase II conjugation. The resulting glucuronide and sulfate conjugates are subsequently excreted quickly in the urine. In humans, 95% of ingested eugenol is excreted in conjugated form in the urine within 24 hours. Eugenol is also metabolized to a lesser extent to polar products, which are in some cases more reactive than the parent molecule. These metabolites are also conjugated and readily eliminated, mainly in the urine. Less than 1% of eugenol is excreted unchanged (WHO 2006a, 2006b). Eugenol is structurally similar to methyl eugenol, a carcinogen assessed during Batch 9 of the Challenge under the Chemicals Management Plan (Environment and Health Canada 2010), which differs from eugenol only by having a methoxy group at the 4-position rather than a phenolic OH. The reasons for the difference in activity of the two compounds could be related to the presence of the phenolic OH group in eugenol, which makes the molecule a better candidate for conjugating enzymes and thus more readily detoxified than methyl eugenol and other carcinogenic alkenylbenzenes (Phillips et al. 1984).
Non-cancer effects have been examined in several toxicity studies. The studies critical to the risk characterization are reported below.
Chronic non-cancer effects have been examined in the NTP carcinogenicity studies described above. In the rat study, female rats exposed at the highest dose (625 mg/kg bw/day) had mean body weights lower than controls throughout most of the study. Body weights were decreased by up to 15% from weeks 51 to 102 of treatment and from week 28, the decrease in body weight gain was consistently 10% or more until the end of the study. Mean body weights for low-dose females and for male rats (the highest exposure dose was 300 mg/kg bw/day) were comparable among groups. Food consumption among groups was not different in comparison with controls and no significant difference in survival was observed for any of the exposed groups (NTP 1983).
In the chronic study conducted in mice, body weight gain decrease was noted in females at the highest dose tested (780 mg/kg bw/day). Mean body weights were 14% and 11% lower than controls in high-dose females at weeks 101 and 103, respectively. Body weight gain decreased up to 21% within the entire study (after 103 weeks of exposure) and the decrease was higher than 10% from week 49. Food consumption among groups was not different in comparison with controls and again no significant differences in survival were seen between any of the groups of either sex (NTP 1983). The oral no-observed-adverse-effect level (NOAEL) for chronic non-cancer effects, as determined by the NTP on the basis of reduction in mean body weights and body weight gain in female rats and mice at the next dose tested, was 300 to 390 mg/kg bw/day.
In range-finding studies conducted by the NTP, male and female Fischer 344/N rats and B6C3F1 mice (5/sex/group) were fed diets containing 6000, 12 500, 25 000, 50 000, or 100 000 ppm eugenol (0.6, 1.25, 2.5, 5 or 10%; equivalent to 300, 625, 1250, 2500 or 5000 mg/kg bw/day for rats, and 780, 1625, 3250, 6500 or 13 000 mg/kg bw/day for mice using a dose conversion by Health Canada 1994) for 14 days. There was no concurrent control group used in these studies and no histopathological analysis were conducted. In the rat study, a dose-associated decrease in mean body weight gain was observed for both males and females at or above 1250 mg/kg bw/day. One of five male rats and all females that received the highest dose died. In the mice study, a dose-associated decrease in mean body weight gain was observed for both male and female mice (at 1625 mg/kg bw/day in male mice and in all mice that received 3250 or 6500 mg/kg bw/day). Deaths occurred in three of five male mice that received 6500 mg/kg bw/day eugenol and in all males and females that received the highest dose (NTP 1983).
In a 13-week study, male and female Fischer 344/N rats (10/sex/group) were fed diets containing 0, 800, 1500, 3000, 6000, or 12 500 ppm eugenol (equivalent to 40, 75, 150, 300, and 625 mg/kg bw/day, using a dose conversion by Health Canada 1994). A NOAEL was identified at 300 mg/kg bw/day based on reduced final body weight of 10% together with reduction in body weight gain of 12% observed in male rats administered 625 mg/kg bw/day eugenol compared to control males. In female rats, final body weights were only 6% lower at the highest dose compared to final body weights in control females. No chemically-related gross or histopathologic effects were observed among the rats (NTP 1983). When male and female B6C3F1 mice (10/sex/group) were fed diets containing 0, 400, 800, 1500, 3000, or 6000 ppm eugenol (equivalent to 52, 104, 195, 390, and 780 mg/kg bw/day, using a dose conversion by Health Canada 1994) for 13 weeks, a NOAEL of 780 mg/kg bw/day was identified on the basis of no mortality and no significant changes in body weight, body weight gain, gross pathology and histopathology noted in exposed mice of both sexes (all doses) (NTP 1983).
No reproductive toxicity study has been identified in the literature. Developmental toxicity has been investigated in a study where pregnant NMRI mice (5-6 females/group) were exposed daily by oral gavage to 0 or 100 mg eugenol/kg bw/day on gestation days (GD) 5 to 15. Teratogenicity was not observed (i.e., there were no effects on crown rump length and no defects of the craniofacial region, hands, feet, or tail of fetuses) and there were no significant changes in number of live fetuses. The NOEL for developmental toxicity was set by the authors at 100 mg/kg bw/day, the highest dose tested (Anonymous 2003, as cited in ECHA 2016).
Eugenol was a mild skin sensitizer in a maximization test conducted in guinea pigs exposed to 5% eugenol (Takeyoshi et al. 2004). In another guinea pigs maximization test, eugenol (at concentrations of 0.1% for intradermal induction, 100% for induction patch, and 25% for challenge patch) had positive results for skin sensitizing potential (Hilton et al. 1996). Positive results were observed in 4/4 local lymph node assays (EC3 [effective concentration for a stimulation index of 3] values of 5.4 and 25.1%) (Hilton et al. 1996; Bertrand et al. 1997; Takeyoshi et al. 2004; Lalko et Api 2006). In an assay in mice, eugenol did not have a significant potential to cause sensitization of the respiratory tract (Hilton et al. 1996). In humans, a patch test study was conducted in 1754 patients with suspected allergic contact dermatitis. A total of 21 patients (1.2%)–13 females and 8 males–were positive to eugenol. However, positive reactions were considered “relevant” by the authors in only 6 patients (Giusti et al. 2001). A few case report studies also reported evidence of sensitizing activity for eugenol (Bhalla and Thami 2003; Tammannavar et al. 2013; Behzad et al. 2014). In its opinion concerning fragrance allergy in consumers published in 1999, the Scientific Committee on Cosmetic Products and Non-food Products Intended for Consumers (SCCNP) has identified eugenol as a fragrance chemical which, according to existing knowledge, is among the most frequently reported and well-recognized consumer allergens (SCCNP 1999). In the Research Institute of Fragrance Materials (RIFM) fragrance ingredient safety assessment for eugenol, it is reported that based on the existing data, this substance is considered a weak skin sensitizer with a weight-of-evidence no-expected-sensitization induction level of 5900 µg/cm2 (Api et al. 2015).
Rosa canina extract
Limited information is available for Rosa canina extract.
Rosa canina fruit juice, Rosa canina leaves and Rosa canina seeds (concentration of each not stated) were not mutagenic to Salmonella typhimurium TA 100 without metabolic activation (Karakaya and Kavas 1999). No mutagenicity was also reported in an Ames test with Salmonella typhimurium strains YG1041 and YG1042 in the presence and absence of metabolic activation (Health Canada 2017). Negative results were observed in the Ames assay and in a chromosome aberration test using the Chinese hamster lung cell line for Rosa canina fruit extract, but the details of these studies were not provided (CIR 2016; Personal Care Products Council 2016).
No carcinogenicity or chronic toxicity studies were identified in the literature for the different forms of Rosa canina extract. However, in one short-term study looking at the effect of Rosa canina fruit extract on skin pigmentation, no evidence of toxicity was observed. In this study, 12 female brown guinea pigs were orally administered Rosa canina fruit daily for 35 days at 500 mg/kg bw/day through an aqueous extract diluted to 10% w/v. The control group received water. The general condition and behaviour of all animals were described as normal, and no difference in body weights and food consumption were observed between the treated and control groups. No histopathological analyses were conducted in the different organs. In this study, UVB-induced skin pigmentation was reduced after dosing with Rosa canina fruit (Fujii et al. 2011).
In humans, no adverse effects were seen in two clinical trials conducted with Rosa canina fruit (extract and powder) (Willich et al. 2010; Nagatomo et al. 2015).
In the 2016 Cosmetic Ingredient Review Expert Panel review of the safety of Rosa canina-derived ingredients, many unpublished dermal sensitization studies submitted by the Personal Care Products Council were summarized. Overall, negative results were reported in sensitization tests conducted in animal and humans with solutions or cosmetics containing Rosa canina fruit extract or Rosa canina flower extract at concentrations ranging between 0.018% and 0.3% (Consumer Product Testing Co 2010; TKL Research 2012; Anonymous 2016; CIR 2016; Personal Care Products Council 2016).
To inform the risk assessment, the hazard information available for the two main components of Rosa canina flower, eugenol and 2-phenethyl alcohol, has been considered. Available studies for eugenol have been described earlier in this section. A literature review has been completed for 2-phenethyl alcohol. The critical studies identified are summarized below.
No chronic studies have been identified for 2-phenethyl alcohol. Negative results were observed in in vitro and in vivo genotoxicity assays (US EPA 2002; Belsito et al. 2012).
In a 90-day study, 2-phenethyl alcohol was administered dermally to shaved dorsa of male and female Sprague-Dawley rats (15/sex/group) at 0, 0.25, 0.5, 1.0, or 2.0 ml/kg (pure substance, no occlusion; equivalent to 0, 250, 500, 1000 or 2000 mg/kg bw/day). A NOAEL of 500 mg/kg bw/day was identified on the basis of significant decrease in body weights and body weight gains in animals treated with the two highest doses, starting after 1 week of treatment. Body weights remained significantly decreased for the duration of the study. No decrease in food consumption was noted in treated animals. In males exposed to 2000 mg/kg bw/day, hemoglobin concentrations and leukocyte counts were significantly decreased. Significant increases in relative brain, kidney, and gonad weights were also observed in rats of both sexes at the highest dose tested. Microscopic examination of tissues taken from the control and high-dose animals did not show any treatment-related differences between the two groups (Owston et al. 1981; Belsito et al. 2012).
Oral and dermal developmental studies on this substance have been sponsored by RIFM. In an oral developmental study, pregnant Sprague-Dawley rats (groups of 28 females) were fed diets containing 0, 1000, 3000, or 10 000 ppm microencapsulated 2-phenethyl alcohol (calculated intake: 0, 83, 266, or 799 mg/kg bw/day) on GD 6 to 15. A NOEL of 799 mg/kg bw/day is identified from this study. No treatment-related maternal effect was observed in any of the exposed animals at any dose tested. Also, no developmental effects were noted. Litter parameters (embryo-fetal loss, litter sizes, sex ratios, and litter and mean fetal weights), and the incidence, type, and distribution of fetal malformations, anomalies, and skeletal variants were equivalent in control and exposed groups (Politano et al. 2013a).
In dermal studies conducted in rats, 2-phenethyl alcohol was applied to the skin under occlusion on GD 6 to 15 at doses of 0, 140, 430, or 1400 mg/kg bw/day (Palmer et al. 1986; Belsito et al. 2012; Politano et al. 2013a) and at 0, 70, 140, 280, 430, or 700 mg/kg bw/day in a corroborative study (Christian and Hoberman 1988; Belsito et al. 2012; Politano et al. 2013a). In the first study, 4 groups of 25 to 35 time-mated female rats were used. A NOAEL of 140 mg/kg bw/day for developmental toxicity was identified on the basis of an increase in incidence of fetuses with rudimentary cervical ribs (RCR) and thoracic vertebrae changes (non-statistically significant) at the dose level of 430 mg/kg bw/day. At the highest dose tested (1400 mg/kg bw/day), dose-related increase in soft tissue and skeletal abnormalities (morphological changes in 160/161 fetuses) and statistically significant increases in post-implantation loss (resorption of 5/23 litters), reduction in mean litter size, and depression of mean fetal body weight were noted. High incidences of the following types of defects were noted in more than 40% of the fetuses and 70% of the litters at the highest dose: anophthalmia or microphthalmia, ventricular septal defects, defects or irregularities affecting thoracic, lumbar, and sacrocaudal vertebrae (associated with short or kinky tail), defects of the thoracic ribs, and presence of RCR. A NOAEL of 430 mg/kg bw/day for maternal toxicity was identified on the basis of marked decreases in mean feed consumption and body weight gains and clinical signs of toxicity in animals exposed at 1400 mg/kg bw/day. Local irritation was located at the application site in exposed dams, especially in those exposed to the highest dose. While a reversal of these effects was noted upon cessation of treatment, mean body weights were still statistically decreased at study termination (Palmer et al. 1986; Belsito et al. 2012; Politano et al. 2013a).
The second study was conducted to corroborate the findings observed in the previous dermal study and to define more accurately the dosage associated with observed effects. In this study, 10 female rats per dose were used, and all fetuses (rather than half in the first study) were examined for skeletal defects. The previous doses of 140 and 430 mg/kg bw/day were repeated and additional doses were tested (70, 280 and 700 mg/kg bw/day). In this study, a NOAEL of 70 mg/kg bw/day for developmental toxicity was identified by the authors on the basis of dose-dependent reductions in live fetal body weights observed from 140 mg/kg bw/day. However, the reductions were non-biologically significant (less than 10% vs. control). The decreases in fetal body weights were found to be biologically and statistically significant in animals treated with doses of 430 mg/kg bw/day and higher. A statistically significant increase in incidence of RCR was noted at the highest dose tested (700 mg/kg bw/day) and minimal increases of reversible delays in ossification were observed at all dose levels. Signs of toxicity such as ptosis and/or urine-strained abdominal fur were noted in dams exposed to the highest dose. Dermal irritation (low level of erythema and/or desquamation) was noted in all dosed groups. The severity of the reactions was dose-related. Reduced feed intake and body weight gains did not occur in a consistent manner. A NOAEL for maternal toxicity of 70 mg/kg bw/day was established by the authors on the basis of dermal irritation in exposed female rats. The authors of the corroborative study acknowledged that the decrease in fetal body weight and consequent reversible delays in ossification were likely associated with maternal stress produced by the dermal irritation at the application site (Christian and Hoberman 1988; Belsito et al. 2012; Politano et al. 2013a). In its review of the study, conducted under the High Production Volume Challenge Program, the United States Environmental Protection Agency (US EPA) found that the study was compromised due to the dermal irritation seen at all dose levels and indicated that the decreases in fetal body weights and incomplete ossification, possibly an indirect result of the maternal irritation, were considered reversible effects (US EPA 2002).
Recently, a similar study was conducted by RIFM, with additional parameters evaluated such as the reversibility of the effects in pups euthanized on postnatal day 21 (RIFM 2010; Belsito et al. 2012). In this study, female rats (40/dose) were exposed dermally at doses of 0, 140, 430, or 1400 mg/kg bw/day, under occlusion, on GD 7 to 20. Twenty rats/group were Cesarean-sectioned on GD 21 and the remaining rats/group were allowed to naturally deliver their litters and were euthanized on postnatal day 21. Dermal irritation, including flaking and/or erythema, was observed in female rats administered 430 and 1400 mg/kg bw/day during the gestation period. The onset and severity of skin reactions were generally dose-dependent. A developmental NOAEL of 140 mg/kg bw/day was determined on the basis of reductions in fetal weight with corresponding delays in fetal skeletal ossification and an increase in the incidence of cervical ribs in fetuses from the rats selected for Caesarean delivery at maternal dosages of 430 and 1400 mg/kg bw/day. However, all apparent delays in ossification and increases in incidence of cervical ribs that were observed in the fetuses of Caesarean-sectioned dams exposed to 430 mg/kg bw/day were resolved in pups from rats exposed to the same dose for the same duration and selected for natural delivery by postnatal day 21. A maternal NOAEL of 430 mg/kg bw/day was identified on the basis of mortality and decrease in body weight and feed consumption in female rats exposed at 1400 mg/kg bw/day (RIFM 2010; Belsito et al. 2012; Scognamiglio et al. 2012).
Overall, taking into consideration the different developmental toxicity studies available for 2-phenethyl alcohol, clear evidence of adverse developmental effects associated with maternal toxicity was observed at 1400 mg/kg bw/day. While developmental toxicity was observed from 140 mg/kg bw/day in one study (Christian and Hoberman 1988; Belsito et al. 2012; Politano et al. 2013a), the effects observed (decrease in fetal body weight and reversible delays in ossification) are likely associated with maternal stress caused by the irritation noted in females at all dose levels. This was noted by the authors of the study and by the US EPA in its review of the study (US EPA 2002). Dermal irritation was also observed in other dermal developmental studies available for 2-phenethyl alcohol and likely accounts for similar fetal effects observed at doses below 1400 mg/kg bw/day. A recent study has also shown that fetal effects observed at a dose of 430 mg/kg bw/day in previous dermal developmental studies are likely reversible (RIFM 2010; Belsito et al. 2012). The lack of clear evidence of adverse developmental effects at doses below 1400 mg/kg bw/day in dermal studies is also supported by the absence of treatment-related maternal or developmental effects (no decrease in fetal body weight, no malformations, skeletal defects) observed in animals exposed to up to 799 mg/kg bw/day in an oral developmental study (Politano et al. 2013a).
6.3 Characterization of risk to human health
Eugenol
Exposure of the general population to eugenol may occur through a wide range of products, including cosmetics, natural health products, air fresheners, and cleaning products. Dietary exposure occurs as a result of its natural occurrence in food, as well as through its possible use as a food flavouring agent. Exposures are expected to occur via the oral, dermal and inhalation routes. Eugenol has a moderate vapour pressure and is soluble in water. It is considered to be well absorbed through the oral and dermal routes of exposure.
The IARC has classified eugenol as a Group 3 carcinogen (not classifiable as to its carcinogenicity to humans). This classification was based on limited evidence of carcinogenicity in chronic studies conducted in experimental animals (IARC 1985, 1987). In the available lifetime studies, eugenol was not carcinogenic to rats. In mice, an increased incidence of liver tumours was observed at low dose in males and at high dose in females (only when adenomas and carcinomas were combined). The NTP (1983) concluded that this increased incidence in mice represented equivocal evidence for carcinogenicity. In another study in mice, eugenol was negative for carcinogenic potential. No carcinogenicity was reported for eugenol in dermal chronic studies. The available information on genotoxicity indicates that eugenol is not likely to be genotoxic.
As a result of rapid absorption, metabolism and excretion, only low toxicity has been observed for eugenol in experimental studies. The only health effect associated with long-term exposure to eugenol in laboratory studies is a decrease in mean body weight associated with a decrease in body weight gain which is observed only at high doses of eugenol (with LOAELs of 625 to 780 mg/kg bw/day and NOAELs of 300 to 390 mg/kg bw/day). No chemically-related gross or histopathologic effects were observed in any study.
This substance is considered to be of low hazard potential, and characterization of exposure potential (i.e., derivation of exposure estimates) was not considered to be warranted. The risk for human health is considered low.
Eugenol has been recognized in the literature as a weak skin sensitizer with a defined weight-of-evidence no-expected-sensitization induction level of 5900 µg/cm2 (Api et al. 2015). The type of products, ingredient concentrations, skin surface areas and the uncertainty associated with interindividual variability, product and use considerations were examined along with the weight-of-evidence no-expected-sensitization induction level. Given these considerations, dermal sensitization is not expected to be a concern for this substance based on current uses.
Rosa canina extract
On the basis of composition information, eugenol and 2-phenethyl alcohol were identified as the primary constituents of Rosa canina flower extract. In light of the information described above, exposure to eugenol from products containing Rosa canina extract is not considered to be of concern.
No chronic studies have been identified for 2-phenethyl alcohol. Negative results were observed in in vitro and in vivo genotoxicity assays. The critical effect level noted in the animal database for the dermal route was a NOAEL of 500 mg/kg bw/day identified in a 90-day study on the basis of significant decrease in body weights and body weight gain in animals treated with 1000 mg 2-phenethyl alcohol/kg bw/day and above. Hemoglobin concentrations and leukocyte counts were also significantly decreased at 2000 mg/kg bw/day (Owston et al. 1981). The use of this dermal NOAEL of 500 mg/kg bw/day as the critical effect level to characterize risk for dermal scenarios is considered a conservative approach, despite the limitations of the study (no occlusion, exposition for 90 days only). Developmental effects were observed in dermal developmental studies at doses lower than 500 mg/kg bw/day but were likely attributable to irritation effects of the mothers. It should be noted that irritation effects were observed in dermal studies at levels as low as 70 mg/kg bw/day in animal studies.
The critical effect level identified following oral exposure to 2-phenethyl alcohol was 799 mg/kg bw/day, as determined on the basis of no treatment-related maternal or developmental effect observed in any of the exposed animals at any dose tested in a developmental study (Politano et al. 2013a). The absence of effect reported for 2-phenethyl alcohol in this study is consistent with the absence of adverse effect observed for Rosa canina extract in one oral short-term study conducted in guinea pigs and in clinical trials conducted in human subjects.
Exposure of the general population to Rosa canina extract occurs primarily through use of cosmetics and natural health products. The margins of exposure (MOEs) associated with the highest exposure estimates to 2-phenethyl alcohol from dermal contact with cosmetics containing Rosa canina flower extract and oral ingestion of natural health products, also containing Rosa canina flower extract, were characterized. Table 6-2 provides relevant exposure and critical effect levels for 2-phenethyl alcohol estimated on the basis of Rosa canina flower extract, as well as resultant MOEs, for determination of risk.
Exposure scenario |
Exposure (mg/kg bw/day)a,b |
MOEs (based on a dermal NOAEL of 500 mg/kg bw/day)c |
MOEs (based on an oral NOEL of 799 mg/kg bw/day)d |
Hand cream: Rosa canina flower at 30% (4% 2-phenethyl alcohol) |
2 |
250 |
400e |
Body cream: Rosa canina flower at 10% (1.4% 2-phenethyl alcohol) |
0.96 |
521 |
832e |
Face cream: Rosa canina flower oil at 30% (4% 2-phenethyl alcohol) |
1.2 |
417 |
666e |
Make-up remover: Rosa canina flower at 30% (4% 2-phenethyl alcohol) |
0.28 |
1785 |
2853 e |
a Exposure estimate does not incorporate dermal absorption corrections.
b 2-Phenethyl alcohol exposure estimates were calculated on the basis of the concentration of 13.6% reported by Hosni et al. (2010), using the n-pentane extraction of the aromatic water obtained from the flowers of Rosa canina by hydrodistillation.
c Critical dermal hazard endpoint is reduction in mean body weights and body weight gain at 1000 mg/kg bw/day in a dermal 90-day study (2-phenethyl alcohol).
d Critical effect level is the highest dose tested in a developmental study in which no treatment-related maternal or developmental effects were observed in animals at any dose of 2-phenethyl alcohol.
e Assuming that oral and dermal absorption are equivalent.
Exposure scenario |
Exposure (mg/kg bw/day)a |
MOEs (based on an oral NOEL of 799 mg/kg bw/day)b |
|
Oral exposure to natural health products containing Rosa canina flower |
0.5 |
1598 |
|
a 2-Phenethyl alcohol exposure estimates were calculated on the basis of the concentration of 13.6% reported by Hosni et al. (2010), using the n-pentane extraction of the aromatic water obtained from the flowers of Rosa canina by hydrodistillation.
b Critical effect level is the highest dose tested in a developmental study in which no treatment-related maternal or developmental effects were observed in animals at any dose of 2-phenethyl alcohol.
Overall, the MOEs are considered adequate to address uncertainties in the health effects and exposure databases. Although a 90-day dermal study was used to derive MOEs for daily dermal scenarios (hand cream, face cream, make-up remover and body cream), the MOEs were considered adequate given the conservative nature of the exposure assessments (e.g., a concentration of 30% Rosa canina flower represents the maximum value in the range of concentrations between 10% and 30% in the case of the hand cream scenarios).
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below:
Key sources of uncertainty |
Impacta |
Rosa canina |
|
Exposure |
|
The composition of Rosa canina is variable and uncertain; the use of the composition information reported by Hosni et al. (2010) to estimate risk on the basis of 2-phenethyl alcohol is considered conservative as many other identified compounds of Rosa canina have been noted to be found in low proportions (relative to eugenol and 2-phenethyl alcohol) and many are considered to be of low priority for risk assessment. |
+ |
There is uncertainty with regards to identification of Rosa canina components in some products; the ingredient identified as Rosa canina was assumed to be a fruit extract, on the basis that it is more common than flower extract. |
- |
Hazard |
|
There is no or limited information on repeated-dose effects via all relevant routes of exposure (oral, dermal) and all durations for Rosa canina extract. |
+/- |
There is uncertainty associated with the use of available toxicological information for 2-phenethyl alcohol to characterize risk of Rosa canina extract to human health. |
+/- |
a + = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure/risk; +/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from eugenol and Rosa canina extract. It is concluded that eugenol and Rosa canina extract do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that eugenol and Rosa canina extract do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that eugenol and Rosa canina extract do not meet any of the criteria set out in section 64 of CEPA.
References
Allavena A, Martelli A, Robbiano L, Brambilla G. 1992. Evaluation in a battery of in vivo assays of four in vitro genotoxins proved to be noncarcinogens in rodents. Teratog Carcinog Mutagen. 12:31-41.
Amonkar AJ, Nagabhushan M, D'Souza AV, Bhide SV. 1986. Hydroxychavicol: a new phenolic antimutagen from betel leaf. Food Chem Toxicol. 24:1321-1324.
Api AM, Belsito D, Bhatia S, Bruze M, Calow P, Dagli ML, Dekant W, Fryer AD, Krominas L, La Cava S, Lalko JF, Lapczynski A, Liebler DC, Miyachi Y, Politano VT, Ritacco G, Salvito D, Schultz TW, Shen J, Sipes IG, Wall B, Wilcox DK. 2015. RIFM fragrance ingredient safety assessment, Eugenol, CAS Registry Number 97-53-0. Food Chem Toxicol. 17:S0278-6915.
Armstrong MJ, Bean CL, Galloway SM. 1992. A quantitative assessment of the cytotoxicity associated with chromosomal aberration detection in Chinese hamster ovary cells. Mutat Res Fundam Mol Mech Mutagen. 265(1):45-60.
Azizan A, Blevins RD. 1995. Mutagenicity and antimutagenicity testing of six chemicals associated with the pungent properties of specific spices as revealed by the Ames Salmonella microsomal assay. Arch Environ Contam Toxicol. 28:248-258.
Behzad M, Michl C, Arweiler N, Pfützner W. 2014. Lichenoid contact reaction to eugenol presenting as oral lichen planus. Allergo J Int. 23(7):242-245.
Belsito D, Bickers D, Bruze M, Calow P, Dagli ML, Fryer AD, Greim H, MIyachi Y, Saurat JH, Sipes IG. 2012. A toxicological and dermatological assessment or aryl alkyl alcohols when used as fragrance ingredients. Food Chem Toxicol. 50:S52-S99.
Bertrand F, Basketter DA, Roberts DW, Lepoittevin JP. 1997. Skin sensitization to eugenol and isoeugenol in mice: possible metabolic pathways involving ortho-quinone and quinone methide intermediates. Chem Res Toxicol. 10(3):335-43.
Bhalla M, Thami GP. 2003. Acute urticaria due to dental eugenol. Allergy 58(2):158.
Burdock, GA. 2010. Fenaroli’s handbook of flavor ingredients. 6th ed. Boca Raton (FL): CRC Press.
Burkey JL, Sauer JM, McQueen CA, Sipes IG. 2000. Cytotoxicity and genotoxicity of methyleugenol and related congeners-a mechanism of activation for methyleugenol. Mutat Res. 453:25-33.
Canada. [1978]. Food and Drug Regulations, C.R.C., c. 870, s.B.10.013. [RC1]
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
Canada. 2003. Food and Drugs Act: Natural Health Products Regulations [PDF].. P.C. 2003-847, 2003 June 5, SOR 2003/196.
Canada. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement. ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [updated 2016 Jul 20; accessed 2016 Nov 28].
Christian MS, Hoberman AM. 1988. Dosage-range developmental toxicity (embryo/fetal toxicity and teratogenicity) study of 2-phenylethylalcohol (PEA) administered dermally to presumed pregnant mice. Unpublished report to RIFM [as cited in US EPA 2002].
[CIR] Cosmetic Ingredient Review. 2011. Plant-derived fatty acid oils as used in cosmetics. Final Report. Washington (DC), Cosmetic Ingredient Review. 100 p.
[CIR] Cosmetic Ingredient Review. 2016. Safety assessment of Rosa canina-derived ingredients as used in cosmetics. Tentative Report for Public Comment. October 7, 2016 (Panel date: Dec 5-6, 2016).
[ConsExpo] Consumer Exposure Model Ver. 4 Cosmetics Fact Sheet. 2006. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. Report 320104001/2006.
Consumer Product Testing Co. 2010. Repeated insult patch test of a lip balm containing 0.04% Rosa canina Flower Extract. Unpublished data submitted by the Personal Care Products Council on April 4, 2016. Pp. 1-13 [as cited in CIR 2016].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1983. Summary of the results of surveys of the amount and frequency of use of cosmetic products by women. Report prepared by Pitkin B, Rodericks JV, Turnbull D. Environ Corporation, Washington (DC).
[EC] European Commission. 2003. Technical guidance document on risk assessment [PDF], Part I. 1998. Institute for Health and Consumer Protection, European Chemicals Bureau.
[EC] European Commission Database of flavouring substances [accessed 2017 Feb. 13]
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2016 Dec 5].
[ECCC] Environment and Climate Change Canada. [modified 2013 Jul 3]. Status of Prioritized Substances: Government of Canada. [accessed 2016 Dec 5]..
[ECCC] Environment and Climate Change Canada 2016a. Ecological Science Approach: Ecological Risk Classification of Organic Substances.
[ECCC] Environment and Climate Change Canada. 2016b. Gatineau (QC): Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances . Available from: eccc. substances.eccc@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017. Draft [ACSD2]Screening Assessment: Substances Identified as Being of Low Concern based on the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. 2016. Registration dossier for eugenol.
[EFSA] European Food Safety Authority. 2009. Flavouring Group Evaluation 60 (FGE.60): Consideration of eugenol and related hydroxyallylbenzene derivatives evaluated by JECFA (65th meeting) structurally related to ring-substituted phenolic substances evaluated by EFSA in FGE.22 (2006). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC). The EFSA Journal ON-965, 1-53.
Ellahuene MF, Perez-Alzola LP, Orellana-Vandebenito M, Munoz C, Lafuente-Indo N. 1994. Genotoxic evaluation of eugenol using the bone marrow micronucleus assay. Mutat Res. 320:175-180.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada and Health Canada. 2010. Screening Assessment for the Challenge: Benzene, 1,2-dimethoxy-4-(2-propenyl)- (Methyl eugenol) [PDF]. Chemical Abstracts Service Registry Number 93-15-2.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Ercisli S. 2007. Chemical composition of fruits in some rose (Rosa spp.) species. Food chemistry 104:1379-1384.
[FCC] Food Chemicals Codex. 1996. Food Chemicals Codex, 5th Edition. Washington (DC): National Academy Press.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC. 2016. Consumption of cosmetic products by the French population second part: amount data. Food Chem Toxicol. 90:130-141.
Fujii T, Katsumi I, Saito M. 2011. Inhibitory effect of rose hip (Rosa canina L.) on melanogenesis in mouse melanoma cells and on pigmentation in brown guinea pigs. Biosci Biotechnol Biochem. 75(3):489-495.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C, Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick MA, Anderson B, Zeiger E. 1987. Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluations of 108 chemicals. Environ Mol Mutagen. 10:1-175.
Ghazghazi H, Miguel MG, Hasnaoui B, Sebei H, Ksontini M, Figueiredo AC, Pedro LG, Barroso JG. 2010. Phenols, essential oils and carotenoids of Rosa canina from Tunisia and their antioxidant activities. Af J Biotechnol. 9(18):2709-2716.
Giusti F, Porcaro V, Seidenari S. 2001. Evaluation of eugenol allergy in a patch-test population. Contact Dermatitis 44(1):37-8.
Green NR, Savage JR. 1978. Screening of safrole, eugenol, their ninhydrin positive metabolites and selected secondary amines for potential mutagenicity. Mutat Res. 57:115-121.
Hagan EC, Hansen WH, Fitzhugh OG, Jenner PM, Jones WI, Taylor JM. 1967. Food flavourings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet Toxicol. 5:141-157.
Haseman JK, Hailey JR, Morris RW. 1998. Spontaneous neoplasm incidences in Fischer 344 rats and B6C3F1 mice in two-year carcinogenicity studies: a National Toxicology Program update. Toxicol Pathol. 26(3):428-441.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E. 1983. Salmonella mutagenicity test results for 250 chemicals. Environ Mutagen. 5 (Suppl. 1):3-142.
Hayashi M, Kishi M, Sofuni T, Ishidate M Jr. Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. 1988. Food Chem Toxicol. 26(6):487-500.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2016. Science Approach Document. Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances. September 2016.
Health Canada. 2017. Assessment of mutagenicity for selected Chemical Management Plan-directed needs compounds with the plate incorporation Ames mutagenicity assay. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Sciences and Research Bureau.
Hikiba H, Watanabe E, Barrett JC, Tsutsui T. 2005. Ability of fourteen chemical agents used in dental practice to induce chromosome aberrations in Syrian hamster embryo cells. J Pharmacol Sci. 97(1):146-152.
Hilton I, Dearman RJ, Fielding I, Basketter DA, Kimber I. 1996. Evaluation of the sensitizing potential of eugenol and isoeugenol in mice and guinea pigs. J Appl Toxicol. 16(5):459-64.
Honma M, Hayashi M, Shimada H, Tanaka N, Wakuri S, Awogi T, Yamamoto KI, Kodani N, Nishi Y, Nakadate M, Sofuni T. 1999. Evaluation of the mouse lymphoma tk assay (microwell method) as an alternative to the in vitro chromosomal aberration test. Mutagenesis 14(1):5-22.
Hosni K, Kerkenni A, Medfei W, Ben Brahim N, Sebei H. 2010. Volatile oil constituents of Rosa canina L.: Quality as affected by the distillation method. Org Chem Int 2010: article ID 621967 (7 pages).
Hosni K. 2011. Rosa x alba: source of essential minerals and volatile oils. Nat Prod Bioprospect. 1:57-61.
Howes AJ, Chan VSW, Caldwell J. 1990. Structure-specificity of the genotoxicity of some naturally occurring alkenylbenzenes determined by the unscheduled DNA synthesis assay in rat hepatocytes. Food Chem Toxicol. 28:537-542.
[HSDB] Hazardous Substances Data Bank [database]. 1983- . Bethesda (MD): National Library of Medicine (US). [updated 2013 April; accessed 2016 Nov 28].
[IARC] International Agency for Research on Cancer [PDF]. 1985. Allyl Compounds, Aldehydes, Epoxides and Peroxides. IARC Monogr Eval Carcinog Risks Hum. 36.
[IARC] International Agency for Research on Cancer. 1987. List of Classifications. IARC Monogr Eval Carcinog Risks Hum. 1-116.
[IFRA] International Fragrance Association Standard. 2009. IFRA Standard – Eugenol, October 14, 2009.
[IFRA] International Fragrance Association Ingredient List [database]. [modified 2013]. International Fragrance Association. [accessed 2016 Sep 16]..
Karakaya S, Kavas A. 1999. Adsorption of direct-acting and indirect-acting mutagens by various dietary fibers. Int J Food Sci Nutr. 50(5):319-23.
Lalko J, Api AM. 2006. Investigation of the dermal sensitization potential of various essential oils in the local lymph node assay. Food Chem Toxicol. 44(5):739-46.
Lis-Balchin M. 2006. Aromatherapy science: A guide for healthcare professionals. London (UK): Pharmaceutical Press. 462 p.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 06]. Ottawa (ON): Health Canada. [accessed 2017 Apr].
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43:279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, Re T, Renskers K, Scrafford C, Vater S. 2006. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44:2008-2018.
Lui, FC, Hotchkiss SAM. 1997a. Comparative disposition of the cutaneous sensitizers eugenol and isoeugenol in rat and human skin. Hum Exp Toxicol. 16(1):50.
Maura A, Pino A, Ricci R. 1989. Negative evidence in vivo of DNA-damaging, mutagenic and chromosomal effects of eugenol. Mutat Res. 227:125-129.
Miller EC, Swanson AB, Phillips D, Fletcher LT, Liem A, Miller JA. 1983. Structure activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 43:1124-1134.
Munerato MC, Sinigaglia M, Reguly ML, de Andrade HH. 2005. Genotoxic effects of eugenol, isoeugenol and safrole in the wing spot test of Drosophila melanogaster. Mutat Res. 582(12):87-94.
MSDS (2016) Febreze Noticeables Air Freshener - Apple Delish [PDF]. Procter & Gamble, OH [accessed 2017 Feb 10]
Myhr BC, Caspary WJ. 1991. Chemical mutagenesis at the thymidine kinase locus in L5178Y mouse lymphoma cells: Results for 31 coded compounds in the National Toxicology Program. Environ Mol Mutagen. 18:51-83.
Nadpal J, Lesjak M, Sibul F, Anackov G, Cetojevic-Simin D, Mimica-Dukic N, Beara I. 2016. Comparative study of biological activities and phytochemical composition of two rose hips and their preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 192:907-14.
Nagatomo A, Nishida N, Fukuhara I, Noro A, Kozai Y, Sato H, Matsuura Y. 2015. Daily intake of rosehip extract decreases abdominal visceral fat in preobese subjects: a randomized, double-blind, placebo-controlled clinical trial. Diabetes Metab Syndr Obes. 8:147-156.
Neilsson O. 1997. Rosa. In: Davis PH, editor. The Flora of Turkey and the East Aegean Islands, vol. 4, p. 106-128. Edinburgh (UK): Edinburgh University Press [cited in Ghazghazi et al. 2010].
Nestmann ER, Lee EGH, Matula TI, Douglas GR, Mueller JC. 1980. Mutagenicity of constituents identified in pulp and paper mill effluents using the Salmonella/mammalian-microsome assay. Mutat Res. 79:203-212.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2018 May 07]. Ottawa (ON): Health Canada. [accessed 2017 Apr].
[NNHPD] Natural and Non-prescription Health Products Directorate. 2013. Counterirritants monograph. March7, 2017.
[NNHPD] Natural and Non-prescription Health Products Directorate. December 8 2015a. Aromatherapy – Essential Oils monograph.
[NNHPD] Natural and Non-prescription Health Products Directorate. August 1, 2017. Antioxidants monograph.
[NTP] National Toxicology Program. 1983. Carcinogenesis Studies of Eugenol (CAS No. 97-53-0) in F344/N Rats and B6C3F1 Mice (Feed Studies). Natl Toxicol Program Tech Rep Ser. 223:1-159.
Owston E, Lough R, Opdyke DL. 1981. A 90-day toxicity study of phenethyl alcohol in the rat. Food Cosmet Toxicol. 19(6):713-715.
Ozcan M. 2002. Nutrient composition of Ros (Rosa canina L) seed and oil. J Med Food. 5(3):137-140.
Ozel M, Clifford AA. 2004. Superheated water extraction of fragrance compounds from Rosa canina. Flavour Fragr J. 19:354-359
Ozyurt D, Demirata B, Apak R, Hamilton JF, Lewis A, Ozel M. 2016. GCxGC-TOF/MS chromatographic analysis, antioxidant capacity and phenolic content of Rosa canina L. at different maturities. Rec Nat Prod. 10(4):407-425.
Palmer AK, Bottomley AM, Ratcliffe HE, Clark R, John DM. 1986. Effect of phenethyl alcohol (PEA) on pregnancy of the rat. Huntingdon Research Center. Unpublished report to RIFM [as cited in US EPA 2002].
[PCPC] Personal Care Products Council. 2016. Summary information. Rosa canina fruit extract (ethanol and butylene glycol extracts). Unpublished data submitted by the Personal Care Products Council on 2-29-2016. p.1-12 [as cited in CIR 2016].
[PCPC]. Personal Care Products Council. 2017. Cosmetic Ingredient Identification Database [database]. [accessed 2017 Jan].
Phillips DH, Vijayaraj Reddy M, Randerath K. 32P-post-labelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occurring alkenylbenzenes. II. Newborn male B6C3F1 mice. Carcinogenesis. 5:1623-1628.
Politano VT, Diener RM, Christian MS, Hoberman AM, Palmer A, Ritacco G, Adams TB, Api AM. 2013a. Oral and dermal developmental toxicity studies of phenethyl alcohol in rats. Int J Toxicol. 32(1):32-38.
Politano VT, Diener RM, Christian MS, Hawkins DR, Ritacco G, Api AM. 2013b. The pharmacokinetics of phenethyl alcohol (PEA): safety evaluation comparisons in rats, rabbits, and humans. Int J Toxicol. 32(1):39-47.
Polzin GM, Stanfill SB, Brown CR, Ashley DL, Watson CH. 2007. Determination of eugenol, anethole, and coumarin in the mainstream cigarette smoke of Indonesian clove cigarettes. Food Chem Toxicol. 45(10):1948-1953.
[RIFM] Research Institute for Fragrance Materials, Inc. 2010. Combined dermal developmental and perinatal/postnatal reproduction toxicity study of PEA (phenethyl alcohol) in rats. RIFM report number 58462, March 22. RIFM, Woodcliff Lake (NJ).
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2006a. Cosmetics fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No.: 320104001/2006.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006b. Cleaning products fact sheet: to assess the risks for the consumer [PDF]. Bilthoven (NL): RIVM. Report No.: 320104003/2006. [accessed 2016 Nov 3].
Rompelberg CJM, Stenhuis WH, de Vogel N, van Osenbruggen V, Schouten A, Verhagen H. 1995. Antimutagenicity of eugenol in the rodent bone marrow micronucleus test. Mutat Res. 346:69-75.
Rompelberg CJM, Vogels JTWE, de Vogel N, Bruijntjes-Rozier GCDM, Stenhuis WH, Bogaards JJP, Verhagen H. 1996. Effect of short-term dietary administration of eugenol in humans. Hum Exp Toxicol. 15:129-135.
Schmitt S, Schaefer U, Doebler L, Reichling J. 2009. Cooperative interaction of monoterpenes and phenylpropanoids on the in vitro human skin permeation of complex composed essential oils. Planta Med. 75:1381-1385.
Schmitt S, Schaefer U, Sporer F, Reichling J. 2010. Comparative study on the in vitro human skin permeation of monoterpenes and phenylpropanoids applied in rose oil and in form of neat single compounds. Pharmazie. 65:102-105.
Scognamiglio J, Jones L, Letizia CS, Api AM. 2012. Fragrance material review on phenethyl alcohol. Food Chem Toxicol. 50:S224-S239.
Sekizawa J, Shibamoto T. 1982. Genotoxicity of safrole-related chemicals in microbial test systems. Mutat Res. 101:127-140.
Shelby MD, Erexson GL, Hook GL, Tice RR. 1993. Evaluation of a three-exposure mouse bone marrow micronucleus protocol: results with 49 chemicals. Environ Mol Mutagen. 21:160-179.
Stofberg J, Grundschober F. 1987. Consumption ratio and food predominance of flavouring materials. Perfum Flavor. 12(4):27.
Strittholt CA, McMillan DA, He T, Baker RA, Barker ML 2016. A randomized clinical study to assess ingestion of dentifrice by children. Regul Toxicol Pharmacol. 75:66-71.
Takeyoshi M, Noda S, Yamazaki S, Kakishima H, Yamasaki K, Kimber I. 2004. Assessment of the skin sensitization potency of eugenol and its dimers using a non-radioisotopic modification of the local lymph node assay. J Appl Toxicol. 24(1):77-81.
Tammannavar P, Pushpalatha C, Jain S, Sowmya SV. 2013. An unexpected positive hypersensitive reaction to eugenol. BMJ Case Rep pii: bcr2013009464. doi: 10.1136/bcr-2013-009464.
ter Burg W, Bouma K, Schakel D, Wijnhoven S, van Engelen J, van Loveren H, Ezendam J. 2014. Assessment of the risk of respiratory sensitization from fragrance allergens released by air fresheners. Inhal Toxicol. 26(5):310-318.
TKL Research. 2012. Repeated insult patch test of a lip liner containing 0.018% Rosa canina flower extract. Unpublished data submitted by the Personal Care Products Council on April 21, 2016. p. 1-10 [as cited in CIR 2016].
To LP, Hunt TP, Andersen ME. 1982. Mutagenicity of trans-anethole, estragole, eugenol, and safrole in the Ames Salmonella typhimurium assay. Bull Environ Contam Toxicol. 28:647-654.
Tumbas B, Čanadović-Brunet J, Četojević-Simin D, Ćetković G, Đilas S, Gille L. 2012. Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J Sci Food Agr. 92:1273-1281.
[US CFR] US Code of Federal Regulations. 2016a. 21CFR Section 184.1257 Clove and its derivatives. [accessed 2016 Nov].
[US CFR] US Code of Federal Regulations. 2016b. 21CFR Section 177.2800 Textiles and textile fibers. [accessed 2016 Nov]. .
[US CFR] US Code of Federal Regulations. 2016c. 21CFR Section 182.20 Essential oils, oleoresins (solvent-free, and natural extractives (including distillates). [accessed 2016 Nov].
[US EPA] United States Environmental Protection Agency. 2002. Robust Summaries for phenethyl alcohol. Submitted to the EPA under the HPV Challenge Program by: The Flavor and Fragrance High Production Volume Chemical Consortia.
Van Duuren BL, Sivak A, Segal A, Orris L, Langseth L. 1966. The tumor-promoting agents of tobacco leaf and tobacco smoke condensate. J Natl Cancer Inst 37(4):519-26.
Van Duuren BL, Goldschmidt BM. 1976. Cocarcinogenic and tumor-promoting agents in tobacco carcinogenesis. J Natl Cancer Inst. 56(6):1237-42.
[WHO] World Health Organization. 2006a. Evaluation of certain food additives: sixty-fifth report of the Joint FAO/WHO Expert Committee on Food Additives (WHO technical report series, 934) [PDF].
[WHO] World Health Organization. 2006b. Safety evaluation of certain food additives / prepared by the Sixty-fifth meeting of the joint FAO/WHO Expert Committee on Food Additives (JECFA) [PDF]. WHO Food Additives Series, No. 56.
Winther K, Hansen A, Campbell-tofte J. 2016. Bioactive ingredients of rose hips (Rosa canina L) with special reference to antioxidative and anti-inflammatory properties: in vitro studies. Botanics. 6:11-23.
Willich SN, Rossnagel K, Roll S, Wagner A, Mune O, Erlendson J, Kharazmi A, Sörensen H, Winther K. 2010. Rose hip herbal remedy in patients with rheumatoid arthritis – a randomised controlled trial. Phytomedicine. 17:87-83.
Woolverton C, Fotos P, Mokas M, Mermigas M. 1986. Evaluation of eugenol for mutagenicity by the mouse micronucleus test. J Oral Pathol. 15:450-453.
Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. 2006. What are the sources of exposure to eight frequently used pthalic acid esters in Europeans? Risk Anal. 26(3):803-824.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48:3109-3119.
Yalkowsky HS, Yan H, Jain P. 2010. Handbook of aqueous solubility data, 2nd ed. Boca Raton (FL): CRC Press, p. 687. [cited in HSDB 2013, accessed 2016 Nov 28].
Zhao K, Singh J. 2000. Mechanism(s) of in vitro percutaneous absorption enhancement of tamoxifen by enhancers. J Pharm Sci. 89(6):771-780.
Appendices
Appendix A. Exposure parameters used to estimate exposure to 2-phenethyl alcohol (as a primary component of Rosa canina flower extract)
The adult body weight of 70.9 kg for an adult is from Health Canada (1998). Dermal and oral exposure parameters were based on RIVM Fact Sheets (RIVM 2006a,b); in some cases, frequency of use or product amount values were based on professional judgement (these values are based on an in-house review of Personal Care Product information). Scenario-specific assumptions are provided in Tables A-1 and A-2.
Scenario |
Assumptions |
Cosmetic products |
1) Hand cream/adult:
3) Facial cream/adult:
4) Make-up remover/adult:
|
Scenario |
Assumptions |
Oral exposure to cosmetics |
Lip balm/adult:
|