Screening assessment - protein derivatives and yeast extract
Official title: Screening assessment - protein derivatives and yeast extract
Chemical Abstracts Service Registry Numbers
- 8013-01-2
- 9015-54-7
- 92113-31-0
- 111174-63-1
Environment and Climate Change Canada
Health Canada
October 2022
Cat. No.: En84-308/2022E-PDF
ISBN 978-0-660-45039-1
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of four substances referred to collectively as the Protein Derivatives and Yeast Extract Group. The Chemical Abstracts Service Registry Numbers (CAS RN Footnote 1 ), their Domestic Substances List (DSL) names and their common names are listed in the table below.
| CAS RN | DSL name | Common name |
|---|---|---|
| 8013-01-2a | Yeast, ext. | Yeast extract |
| 9015-54-7a,b | Protein hydrolyzates | NA |
| 92113-31-0a,b | Collagens, hydrolyzates | Collagen hydrolyzates |
| 111174-63-1a | Protein hydrolyzates, leather, reaction products with isostearoyl chloride | Isostearoyl hydrolyzed collagen |
Abbreviations: NA, Not Available
The substances in the Protein Derivatives and Yeast Extract Group are derived from naturally occurring biological materials. The composition of the yeast extract, protein hydrolyzates, collagen hydrolyzates, and isostearoyl hydrolyzed collagen varies depending on the source of the material and manufacturing conditions. Data on all four substances were obtained in response to a CEPA section 71 survey. Between 100 000 kg and 1 000 000 kg of yeast extract, between 10 000 kg and 100 000 kg of protein hydrolyzates, and 67 702 kg of collagen hydrolyzates were imported to Canada for the reporting year of 2011. For the same year, no import volumes were reported for isostearoyl hydrolyzed collagen and no manufacture volumes were reported for any of the substances in the group in Canada above the reporting threshold of 100 kg. Reported uses include building or construction materials, food and beverage, nutritional product for animal feed, and personal care. More specifically, in Canada, substances in this group may be used in food as flavouring agents, for nutritional purposes in products such as protein supplements and infant formula, and in a number of products available to consumers including cosmetics and natural health products. In addition, collagen hydrolyzates and yeast extract are formulants in pest control products.
The ecological risks of the substances in the Protein Derivatives and Yeast Extract Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, substances in the Protein Derivatives and Yeast Extract Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from the four substances in the Protein Derivatives and Yeast Extract Group. It is concluded that yeast extract, protein hydrolyzates, collagen hydrolyzates and isostearoyl hydrolyzed collagen do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
With respect to human health, the hazard profiles of a variety of protein hydrolyzates of both plant and animal origin, which are representative of the substance protein hydrolyzates in the Protein Derivatives and Yeast Extract Group show that no adverse effects have been observed in laboratory studies at doses up to the limit dose of 1000 mg/kg bw/day. Similarly, for yeast extract and collagen hydrolyzates, there were no adverse effects reported in several laboratory studies. For isostearoyl hydrolyzed collagen, based on information on its individual reaction components isostearic acid and collagen hydrolyzates, and on other structurally similar amino acid alkyl amides, this substance is not expected to be associated with adverse health effects. Therefore, in consideration of the available toxicological information on a representative set of protein hydrolyzates (including those derived from yeast and collagen) and substances related to isostearoyl hydrolyzed collagen, the substances in the Protein Derivatives and Yeast Extract Group are considered to be of low hazard potential and therefore the risk to human health is considered to be low.
Considering all the information presented in this screening assessment, it is concluded that yeast extract, protein hydrolyzates, collagen hydrolyzates and isostearoyl hydrolyzed collagen do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that yeast extract, protein hydrolyzates, collagen hydrolyzates and isostearoyl hydrolyzed collagen do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of four substances referred to collectively as the Protein Derivatives and Yeast Extract Group to determine whether these substances present or may present a risk to the environment or to human health. The substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority through other mechanisms (ECCC, HC [modified 2017a]).
The ecological risks of the substances in the Protein Derivatives and Yeast Extract Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to September 2019. Empirical data from key studies as well as results from models were used to reach the conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portion of this assessment has undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Dr. Supratik Kar (Interdisciplinary Center for Nanotoxicity, Jackson State University), Dr. Mustafa Al-Zoughool (Kuwait University), Dr. Judy S. Lakind (LaKind Associates; University Maryland School of Medicine) and Dr. Joseph Caruso (Institute of Environmental Health Sciences, Wayne State University). The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this screening assessment (published on February 5, 2021) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution Footnote 2 . This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) names and common names for the individual substances are presented in Table 2-1.
| CAS RNa | DSL name | Common name |
|---|---|---|
| 8013-01-2 | Yeast, ext. | Yeast extract |
| 9015-54-7b | Protein hydrolyzates | Protein hydrolyzates |
| 92113-31-0b | Collagens, hydrolyzates | Collagen hydrolyzates |
| 111174-63-1 | Protein hydrolyzates, leather, reaction products with isostearoyl chloride | Isostearoyl hydrolyzed collagen |
a All substances in the Protein Derivatives and Yeast Extract Group are UVCBs (Unknown or Variable composition Complex reaction products or Biological materials). These materials are derived from natural sources or complex reactions and cannot practicably be synthesized by simply combining individual constituents A UVCB is not an intentional mixture of discrete substances and is considered a single substance. The complexity and variability of their compositions can make them difficult to fully and consistently characterize.
b This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority through other mechanisms
According to the United States Food and Drug Administration (US FDA) (1978), protein hydrolyzates represent a group of acid or enzymatically treated protein sources designed to provide a mixture of amino acids and/or peptides. There are four main types of protein hydrolyzates which are normally distinguished by the method of preparation: i) acid hydrolyzed proteins from plant or animal sources, ii) autolyzed yeast extracts from brewers or baker’s yeast, iii) soy sauces from enzymatically degraded wheat/soybean mixtures, and iv) enzymatically hydrolyzed casein (US FDA 1978).
Although the definition of yeast extract also falls under that of protein hydrolyzates, it will be addressed specifically (i.e., as a separate substance) as part of this assessment. The United States’ Toxic Substances Control Act (TSCA) 2018 defines CAS RN 8013-01-2 (yeast extract) as “Extractives and their physically modified derivatives such as tinctures, concretes, absolutes, essential oils, oleoresins, terpenes, terpene-free fractions, distillates, residues, etc., obtained from Saccharomyces” (US EPA 2018). Although additional information on substance identity are not available, further lines of evidence point to this organism being Saccharomyces cerevisiae; the US FDA lists this CAS RN as “baker’s yeast extract” which is known to be derived from S. cerevisiae (US FDA 2019) and Chemical Book/Sigma Aldrich also indicates that the extract associated to CAS RN 8013-01-2 originates from this species (Chemical Book 2017).
All four substances in the Protein Derivatives and Yeast Extract Group are considered to be UVCBs. The variation in composition is typically due to differences in the nature of the protein source and the manufacturing/processing conditions. For example, protein hydrolyzates can be prepared from various animal and/or plant sources (e.g., leather by-products, chicken feathers, vegetable by-products) and the starting materials may not consist of pure proteins. Mostly, the protein source also contains lipids, starch/other carbohydrates and potential contaminants. Contaminants are closely monitored to meet industry standards and are typically removed during heat treatment or purification stages of manufacturing (Petrova et al. 2018). Additional information on the identity of components in some representative protein hydrolyzates is presented in Appendix A.
The manufacturing process of protein hydrolyzates typically begins with a protein source (animal or plant) being subjected to a mechanical process of grinding or cutting followed by a water extraction and centrifugation stage where the protein concentrate is separated from other organic compounds (Colantoni et al. 2017). The resulting solution is then subjected to an enzymatic or acid hydrolysis step to break down the proteins into smaller peptides or free amino acids. Lastly, the water soluble compounds are subject to another centrifugation step followed by a concentration step where the protein is further purified (via distillation, spray drying, and/or lyophilisation). The differences in manufacturing and processing conditions (such as temperature, pH, centrifugation, extraction, purification) all have a significant impact on the composition of the subsequent hydrolysis product mixture.
Notably, obtaining isostearoyl hydrolyzed collagen requires additional manufacturing steps, which go beyond those that are required for protein hydrolyzates. This substance is produced by a condensation reaction between isostearic acid chloride and the amino acids that are present in collagen hydrolyzates (i.e., that are specifically derived from the hydrolysis of leather by-products) to form a corresponding mixture of amino acid isostearoyl amides. It is this resulting mixture of amino acid isostearoyl amides that is known as isostearoyl hydrolyzed collagen.
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship [(Q)SAR] models, where appropriate, has been used to inform the human health effects assessment for isostearoyl hydrolyzed collagen. As this substance is produced by a condensation reaction between isostearic acid chloride and the mixture of amino acids that are present in collagen hydrolyzates, health effects information for isostearic acid (which is formed by hydrolysis of isostearic acid chloride) and collagen hydrolyzates (part of the Protein Derivatives and Yeast Extract Group) informed its health effects characterization. In addition to using health effects data on those two components, consideration was given to data from a similar class of compounds known as amino acid alkyl amides. These substances are very similar mixtures to isostearoyl hydrolyzed collagen because they consist of a mix between mixture of acid chlorides and various amino acids. Information on the identities and chemical structures of the amino acid alkyl amides, as well as isostearic acid, which are used to inform this assessment, is presented in Table 2-2.
| CAS RN | DSL or other name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 2724-58-5 | Heptadecanoic acid, 16-methyl- (16-methylheptadecanoic acid; isostearic acid) |
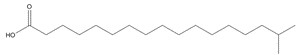 C18H36O2
C18H36O2 |
284.48 |
| 616-91-1 | 2-Cysteine, N-acetyl- (acetyl cysteine) |
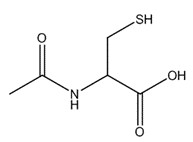 C5H9NO3S
C5H9NO3S |
163.20 |
| 1188-37-0 | Acetyl glutamic acid | 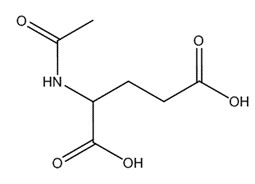 C7H11NO5
C7H11NO5 |
189.17 |
| 68-95-1 | Acetyl proline | 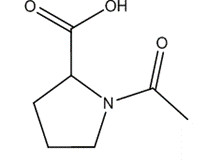 C7H11NO3
C7H11NO3 |
157.17 |
| 537-55-3 | 2-Tyrosine, N-acetyl- (acetyl tyrosine) |
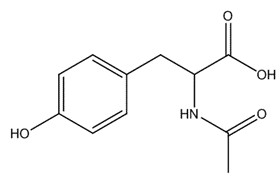 C11H13NO4
C11H13NO4 |
223.22 |
| 167888-81-5 | Disodium capryloyl glutamate | ![Representative chemical structure of disodium capryloyl glutamate, with SMILES notation: CCCCCCCC(=O)NC(CCC(=O)[O-])C(=O)[O-].[Na+].[Na+]](/content/dam/eccc/images/pded/protein-derivatives-yeast-extract/20210120-Table%202-2-6.jpg) C13H21NNa2O5
C13H21NNa2O5 |
317.29 |
| 68187-32-6 | Sodium cocoyl glutamate | ![Representative chemical structure of sodium cocoyl glutamate, with SMILES notation: C(CC(=O)[O-])C(C(=O)O)N.[Na+]](/content/dam/eccc/images/pded/protein-derivatives-yeast-extract/20210120-Table%202-2-7.jpg) C5H8NNaO4
C5H8NNaO4
|
169.11 |
| 29923-31-7 | Sodium lauroyl glutamate | ![Representative chemical structure of sodium lauroyl glutamate, with SMILES notation: CCCCCCCCCCCC(=O)NC(CCC(=O)O)C(=O)[O-].[Na+]](/content/dam/eccc/images/pded/protein-derivatives-yeast-extract/20210120-Table%202-2-8.jpg) C17H30NNaO5
C17H30NNaO5
|
351.42 |
3. Physical and chemical properties
Empirical data on the physical and chemical properties of substances in the Protein Derivatives and Yeast Extract Group were not available, but may generally be approximated using their major components (e.g., amino acids, isostearoyl chloride). The physical and chemical properties for representative components of these substances are reported in ECCC (2016b). However, due to the intrinsic variability in substance composition, and the approach taken to characterize risk to human health for substances in this group, derivation of approximate physical and chemical properties of these substances is not required for the human health portion of this assessment.
4. Sources and uses
All of the substances in the Protein Derivatives and Yeast Extract Group have been included in a survey issued pursuant to section 71 of CEPA (Canada 2012). Table 4-1 presents a summary of information reported on the total manufacture and import quantities for the Protein Derivatives and Yeast Extract Group for the 2011 reporting year (Environment Canada 2013).
| Common name | Total manufacturea (kg) | Total importsa (kg) |
|---|---|---|
| Yeast extract | NRb | 100 000 –1 000 000 |
| Protein hydrolyzates | NRb | 10 000 –100 000 |
| Collagen hydrolyzates | NRb | 67 702 |
| Isostearoyl hydrolyzed collagen | NRb | NRb |
Abbreviation: NR, Not Reported
a Values reflect quantities reported in response to a CEPA section 71 survey (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3). Exact values are provided when they are non-confidential. Confidential quantities are presented as a range of values.
b No manufacturing and/or import quantities were reported for the substance above the reporting threshold of 100 kg for the 2011 reporting year.
Table 4‑2 presents a summary of the major uses of the Protein Derivatives and Yeast Extract Group according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013).
| Major usesa | Yeast extract | Protein hydrolyzates | Collagen hydrolyzates | Isostearoyl hydrolyzed collagen |
|---|---|---|---|---|
| Building or construction materials | N | Y | N | N |
| Food and beverage | Y | N | Y | N |
| Intermediate | Y | N | N | N |
| Nutritional product for animal feed | N | Y | N | N |
| Personal care | N | N | Y | N |
Abbreviations: Y = yes this use was reported for this substance; N = no this use was not reported for this substance
a Non-confidential uses reported in response to a CEPA section 71 survey (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
Table 4‑3 presents additional uses for substances in the Protein Derivatives and Yeast Extract Group identified in Canada.
| Use | Yeast extract | Protein hydrolyzates | Collagen hydrolyzates | Isostearoyl hydrolyzed collagen |
|---|---|---|---|---|
| Medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug productsa | N | Yb | Yc | N |
| Medicinal or non-medicinal ingredients in natural health productsd | Y | Y | Y | N |
| Notified to be present in cosmetics under the Cosmetic Regulationse | Yf | Yg | Yh | Yg |
| Formulant in registered pest control productsi | Yj | N | Yk | N |
Abbreviations: Y = use was indicated for this substance; N = use was not indicated for this substance
a Personal communication, email from the Therapeutic Products Directorate (TPD) and the Natural and Non-prescription Health Products Directorate (NNHPD), Health Canada to the Existing Substances Risk Assessment Bureau (ESRAB), Health Canada, dated 2019, 2020; unreferenced
b Reported to be in one marketed veterinary drug as an active ingredient (personal communications, email from the TPD, Health Canada to ESRAB, Health Canada, dated 2019; unreferenced)
c Reported to be in multiple non-prescription drugs for human use as a non-medicinal ingredient (personal communications, email from the NNHPD, Health Canada to ESRAB, Health Canada, dated 2020; unreferenced)
d Personal communications, email from the NNHPD, Health Canada to ESRAB, Health Canada, dated 2019; unreferenced
e Personal communication, email from the Consumer and Hazardous Products Safety Directorate (CHPSD), Health Canada to ESRAB, Health Canada, dated 2019; unreferenced
f Reported to be present in products such as cleanser, conditioner, makeup, moisturizer, shampoos and other hair products (personal communication, email from the CHPSD, Health Canada to ESRAB, Health Canada, dated 2019; unreferenced)
g Reported to be present in products such as face moisturizers, cleansers, makeup removers, and nail polishes (personal communication, email from the CHPSD, Health Canada to ESRAB, Health Canada, dated 2019; unreferenced)
h Reported to be in products such as bath products, cleansers, exfoliants, hair colour, makeup, massage products, conditioners and nail polishes (personal communications, email from the CHPSD, Health Canada to ESRAB, Health Canada, dated 2019; unreferenced)
i Personal communications, emails from the Pest Management Regulatory Agency (PMRA), Health Canada to ESRAB, Health Canada, dated 2019; unreferenced; Health Canada 2010.
j Reported to be present as a formulant in insecticides, fungicides, and plant growth regulators (personal communications, email from the PMRA, Health Canada to ESRAB, Health Canada, dated 2020; unreferenced)
k Reported to be present as a formulant in acaricides, insecticides and insect growth regulators (personal communications, email from the PMRA, Health Canada to ESRAB, Health Canada, dated 2019; unreferenced)
Yeast extract is not known to be used as a component in the manufacture of food packaging material; however, it may be used as a component in incidental additives (e.g., a component in cleaners) which are subsequently used in food processing establishments. In Canada, yeast extracts would be considered non-additive food ingredients that could be added to any unstandardized food (e.g., marmite and vegemite), or any standardized food that permits the addition of yeast extracts. Yeast extracts may also be used as flavouring agents or flavour enhancers. While there is no definitive information on the use of yeast extract as a food flavouring agent in foods sold in Canada, this use is possible based on its permitted use as a food flavouring agent in the US (personal communications, emails from the Food Directorate (FD), Health Canada to Existing Substances Risk Assessment Bureau (ESRAB), Health Canada, dated 2019; unreferenced).
Protein hydrolyzates are not permitted food additives in Canada, nor are they known to be used in food packaging materials or in incidental additives. Protein hydrolyzates may be used as non-food additive ingredients in foods sold in Canada. The substance is considered a food ingredient when used in infant formulas based on isolated amino acids or protein hydrolyzates. These formulas are typically intended for infants who do not tolerate intact proteins, where the protein hydrolyzates in these specialized formulas may be the infant’s main source of dietary protein (personal communications, emails from FD, Health Canada to ESRAB, Health Canada, dated 2019; unreferenced). The Food Chemicals Codex (FCC) and US Food and Drug Administration (US FDA) indicate that protein hydrolyzates have several functions in food including as a flavouring agent and as a flavour enhancer (FCC USP 2016; US FDA 2019). While there is no definitive information on the use of protein hydrolyzates as a food flavouring agent in foods sold in Canada, this use is possible based on its permitted use as a food flavouring agent in the US (personal communications, emails from the FD, Health Canada to ESRAB, Health Canada, dated 2019; unreferenced).
The Canadian Food Inspection Agency (CFIA) lists protein hydrolyzates protein sources, such as animal bone, corn grain, animal hair and poultry feathers hydrolyzed, under Schedule IV Part I of the Feeds Regulations (Canada 1983) (which is a list of ingredients that do not require notification if used according to the definitions for feed ingredients). While this relates to animal feed, the CFIA assessment process also examines the impact on human health through potential transfer of these compounds to milk, eggs and meat for human consumption as well as any possible contaminants associated with these substances (CFIA 2019).
Regarding collagen hydrolyzates, this substance is indicated to be used in wound care products for animals (SDS 2014). Collagen hydrolyzates, as well as isostearoyl hydrolyzed collagen, are not permitted food additives, nor are they reported to be used as components in the manufacture of food packaging materials or incidental additives in Canada (personal communications, emails from the FD, Health Canada to ESRAB, Health Canada, dated 2019; unreferenced).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the substances in the Protein Derivatives and Yeast Extract Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Protein Derivatives and Yeast Extract Group, and the hazard, exposure and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the four substances in the Protein Derivatives and Yeast Extract Group are summarized in Table 5-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Yeast extract | low | Low | low |
| Protein hydrolyzates | low | Low | low |
| Collagen hydrolyzates | low | Low | low |
| Isostearoyl hydrolyzed collagen | high | Low | low |
On the basis of low hazard and low exposure classifications according to information considered under ERC, yeast extract, protein hydrolyzates and collagen hydrolyzates were classified as having a low potential for ecological risk. It is unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under ERC, isostearoyl hydrolyzed collagen was classified as having a low exposure potential. Isostearoyl hydrolyzed collagen was classified as having a high hazard potential on the basis of the agreement between reactive mode of action and elevated ecotoxicity ratio, both of which suggest that this chemical is likely of high potency. As well, structural alerts from OECD (Q)SAR toolbox (OECD 2014) identified this substance as being a potential DNA and/or protein binder. However, data used in hazard characterization under ERC was based on a single model for DNA flags, which is a conservative measure. Further assessment in Section 6.2 found that isostearic acid and the other identified substances related to isostearoyl hydrolyzed collagen were of low mutagenic potential. Isostearoyl hydrolyzed collagen was initially classified as having a moderate potential for ecological risk; however, the risk classification was decreased to low potential for ecological risk following the adjustment of risk classification based on current use quantities (see section 7.1.1 of the ERC approach document, ECCC 2016a). The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, this substance is unlikely to be resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
The Canadian general population may be exposed to substances in this group from the components of the substances in the Protein Derivatives and Yeast Extract Group as they occur naturally in the environment, and they may also be released to the environment through the use of products available to consumers (e.g., down-the-drain releases).
The Canadian general population may be exposed to substances in this group from food and from products available to consumers, including cosmetics. Exposures to these substances may occur from the oral route through food use, from the dermal route through use of cosmetics such as hair conditioners, and from the inhalation route through spray cosmetics such as face moisturizers and hair styling products.
As substances in the Protein Derivatives and Yeast Extract Group are considered to be of low hazard potential (Section 6.2), quantitative estimates of exposure for the general population were not derived.
6.2 Health effects assessment
The substances in the Protein Derivatives and Yeast Extract Group are complex mixtures and there is limited information available on the exact types (e.g., method of preparation) of substances in this group, and in the case of protein hydrolyzates, the sources (e.g., plant, animal, or yeast) that are used in Canada. Therefore, this assessment uses information on a variety of representative substances (e.g., animal and plant sourced hydrolyzates) to characterize the health effects profiles for the Protein Derivatives and Yeast Extract Group.
A review of the scientific literature of the four substances under assessment has shown that in the majority of studies, no adverse effects were observed at concentrations less than 1000 mg/kg bw/day. In the few cases where adverse effects were observed, it was attributed to improper diet formulation where certain amino acid deficiencies were unaccounted for. No effects related to potential carcinogenicity, genotoxicity, or reproductive/developmental toxicity were identified. Key details are provided below.
Yeast extract
A sub-chronic repeat dose toxicity study was conducted in Wistar rats via the oral route of administration. Test animals (10 animals/sex/dose) received 0, 200, 625 or 2000 mg/kg bw/day of MDA 11 (S. cerevisiae product consisting of 53% protein, 11% salt, 3.6% fat and other solids) for 13 weeks. A no observed adverse effect level (NOAEL) of 2000 mg/kg bw/day, which was the highest tested dose (HTD), was derived based on lack of adverse effects (ECHA c2007-2019c).
In another subchronic repeat dose toxicity study, Charles River rats (10 animals/sex/group) were exposed for 90 days to 0.2, 2 or 20 g/kg bw/day (200, 2000, or 20 000 mg/kg bw/day) of autolyzed yeast extract (40% protein, 16.7% ash and 15.8% nitrogen) via the oral route of exposure. A NOAEL of 2000 mg/kg bw/day was identified based on nephritis, mineral deposits in tubules of the inner cortex and corticomedullary junction and increased kidney-body weight ratios at the next dose (Hazleton Laboratories 1970).
A bacterial reverse mutation assay using S. cerevisiae and conducted with or without the use of metabolic activation for 72 h in Salmonella typhimurium strains tested negative in all 5 tested strains (ECHA c2007-2019c).
An in vivo micronucleus test using Maxarome powder (from primary grown yeast) and Gistex (powder, autolyzed yeast extract) determined that the test substances did not provide any indication of chromosomal damage and or damage to the mitotic apparatus in bone marrow cells of mice (ECHA c2007-2019c).
Based on the results of the above in vitro and in vivo assays, yeast extract is not expected to be genotoxic.
Several naturally occurring and genetically modified S. cerevisiae strains have also been granted a Generally Regarded as Safe (GRAS) notice by the US FDA (ECCC, HC 2017b). According to the European Food Safety Authority (EFSA), S. cerevisiae is presumed safe for use in animal feed and throughout the food chain with a few qualifications, as explained in their qualified presumption of safety (QPS) publications (EFSA 2007, 2010, 2011).
Protein hydrolyzates
Plant based sources
In a subchronic repeat dose toxicity study, Charles River rats (12 animals/group) were fed a diet consisting of 20% dietary protein hydrolyzates (approximately 3600 mg/day) from Lupinus albus, L. luteus or casein (control) for 112 days. The diets were supplemented with DL-methionine. No differences were observed in feed intakes, feed conversion ratio, organ to body-weight ratios of liver, spleen, heart or gross necropsy findings and microscopic examinations between the test group and the control (Ballester 1980).
A three-part chronic repeat dose toxicity study was conducted in Wistar rats and Colworth C57 BI mice via the oral route of administration. In the first part of the study, animals (19 rats/group), were fed diets consisting of acid hydrolyzed wheat gluten, modified with cysteine hydrochloride and either glucose or xylose at 2500 or 5000 mg/kg bw/dayfor 21 weeks. A NOAEL of 5000 mg/kg bw/day (HTD) was identified. In the second part of the study, the above formulations were fed to Wistar rats (40 animals/sex/group). for two years at 5000 or 9000 mg/kg bw/day A NOAEL of 5000 mg/kg bw/day was identified based on increased kidney and liver weights and increased incidence of telangiectasia at the next dose level of 9000 g/kg bw/day. In the third part of the study, the glucose-modified acid hydrolyzed wheat gluten product was fed to Colworth C57 BI mice (45 animals/sex/group) for 80 weeks at 5000 or 9000 mg/kg bw/day. A NOAEL of 9000 mg/kg bw/day (HTD) was identified. The NOAELs identified in this study were based on no treatment related effects observed in body weight gains, food intake, haematology, organ weights or histological findings. Although the incidence of hepatic nodules in treated animals at both dietary levels was higher than the equivalent controls, these differences were not considered statistically significant. There was also no significant effect on incidence, type, site or age of development of neoplasia (Unilever Research Laboratory 1976).
Another chronic repeated dose toxicity study was conducted in weanling ICR-JCL mice via the oral route of administration. Test animals (40 animals/sex/group) were exposed for 18 months to 0, 0.11, 1.1 or 11% (about 0 to 8000 mg/kg bw/day) soy sauce solids (which were derived from enzymatic hydrolysis) and were given a basal diet containing equivalent amounts of sodium chloride. A NOAEL of 8000 mg/kg bw/day (HTD) was identified based on no differences observed in body weight gain, mortality, organ to body weight ratios or histopathology of treated animals when compared to controls receiving sodium chloride. No evidence of carcinogenicity was observed (Ohshita 1977). Similar results were observed in Wistar rats (30 animals/sex/group) given diets of up to 7000 mg/kg bw/day of soy sauce solids during a 6-month feeding study (Ohshita 1977).
Acid hydrolyzed soy protein was found to be non-mutagenic in a series of in vitro microbial assays with Salmonella typhimurium strains (Litton Bionetics 1974). Hydrolyzed lupine protein (up to 26.7%), hydrolyzed pea protein (up to 25%), hydrolyzed sweet almond protein (up to 3.3%) and hydrolyzed vegetable protein (10.9%) were not mutagenic in various Ames tests (Personal Care Products Council 2016a,b).
A reproductive/developmental toxicity study was conducted in CD-1 mice and Wistar rats with acid hydrolyzed soy protein via the oral route of administration. Test animals were fed up to 1000 mg/kg bw/day (mice) and 1400 mg/kg bw/day(rats) for 10 days beginning on day 6 of gestation. An evaluation revealed no effects on fetal development or on maternal or fetal survival in mice and rats up to the highest tested dose (Morgareidge 1973).
Animal based sources
Based on information submitted to ECHA in a REACH dossier, a sub-chronic repeat dose toxicity study was conducted in Sprague-Dawley rats via oral route of administration. Test animals (40 animals/sex/dose). were exposed for 90 days to 0, 30, 300 or 1000 mg/kg bw/day of hydrolyzed chicken sternal cartilage (collagen). There was no mortality, adverse effects or clinical signs of toxicity in any of the treatment groups. One male from the intermediate dose group was euthanized on day 38 after the animal was found moribund. Post-mortem analysis revealed red discharge from animals’ eyes, crooked teeth and minimal stomach content being attributed to injury sustained in the cage and not treatment related. A NOAEL of 1000 mg/kg bw/day (HTD) was identified (ECHA c2007-2019a).
A bacterial reverse mutation assay using hydrolyzed casein concentrations up to 5000 μg/plate tested negative for all strains of Salmonella typhimurium (with or without the use of S9 metabolic activation). The same test was also performed on hydrolyzed keratin and hydrolyzed milk protein and tested negative, thus indicating that these substances were not considered mutagenic. An in vitro chromosome aberration assay using GlycoMacroPeptide fraction from cow’s milk at concentrations up to 5000 μg/plate also showed that the substance is not cytotoxic or genotoxic (ECHA c2007-2019b).
Protein hydrolyzates have been given the GRAS status in the United States. More specifically, hydrolyzed plant, vegetable, animal and milk proteins are considered GRAS substances (US FDA 1972, 1961). The US FDA also determined that the use of peptones (peptones are a variable mixture of polypeptides, oligopeptides and amino acids produced by partial hydrolysis of casein, animal tissue, soy protein, gelatin, egg albumin, whey protein) as direct food substances are GRAS under certain conditions of use (US eCFR 2019).
Collagen hydrolyzates
A sub-chronic repeat dose toxicity study was conducted in Sprague-Dawley rats via the gavage route of administration. Test animals were exposed for 90 days to 0, 30, 300 or 1000 mg/kg bw/day of BioCell Collagen II Footnote 3 (10 animals/sex/group) in 100 mL of water. A NOAEL of 1000 mg/kg bw/day was established (HTD) based on the lack of adverse effects in haematology, clinical chemistry and gross microscopic organ and tissue evaluations or clinical signs (US FDA 2017).
No adverse effects were observed in other 90-day feeding studies where Sprague Dawley rats were fed diets containing collagen, gelatin or casein in amounts between 2000 and 10 000 mg/kg bw/day) (Booth 1970; Whitmore 1975).
A short-term toxicity study was conducted in rabbits via the dermal route of administration. Test animals (2 animals/sex/group) received 100, 1000 or 3200 mg/kg bw/day of a marketed shampoo containing 2% hydrolyzed collagen once daily for 30 days. The skin of 1 male and 1 female in each group was abraded weekly. A NOAEL of 3200 mg/kg bw/day (HTD) was identified based on lack of gross or microscopic lesions, abnormal behaviour or deaths (CTFA 1979 as cited in Liebert M. 1985). Similar results were identified in another dermal toxicity study in Yorkshire pigs treated with 0.5, 1 or 2 mL/kg of a shampoo solution containing 2% hydrolyzed collagen (NOAEL = 2 mL/kg HTD) (TPS 1978).
A series of clinical studies were conducted in post-menopausal women, adults with joint pain, adults with osteoarthritis, type 2 diabetes, mild hypertension, or pressure ulcers and other healthy adults where patients consumed products composed primarily of isolated hydrolyzed collagen at doses up to 135 mg/kg bw/day Footnote 4 (alone or with low doses of other cartilage-derived compounds including hyaluronic acid and chondroitin sulfate) for up to 20 months. No compound-related adverse effects (e.g., wound healing, joint pain, inflammation) were reported up to the highest tested dose of 135 mg/kg bw/day (US FDA 2017).
Isostearoyl hydrolyzed collagen
As described in Section 2, isostearoyl hydrolyzed collagen is produced by a condensation reaction between isostearic acid chloride and the amino acids that are present in collagen hydrolyzates. Given that there is a lack of substance specific health effects data for this substance, the health effects information available for these two individual reaction components were used to inform the health effects of isostearoyl hydrolyzed collagen. In addition, health effects information for structurally similar amino acid alkyl amides was considered.
Isostearic acid
Rats given isostearic acid in three studies by gastric incubation had an acute oral LD50 between 32 and 64 mL/kg bw (Liebert 1983)
In an OECD Test Guideline 401 study, a group of five rats/sex was administered isooctadecanoic acid (also known as isostearic acid CAS RN 30399-84-9) at a dose of 2000 mg/kg bw. There were no clinical signs, deaths, or adverse findings at necropsy. The LD50 was > 2000 mg/kg bw (ECHA c2007-2019d).
A bacterial reverse mutation assay using the structural isomer stearic acid at concentrations up to 5000 μg/plate (in the presence or absence of metabolic activation) in Salmonella typhimurium strains tested negative for all cases (ECHA c2007-2019d).
Collagen hydrolyzates
Health effects information on this substance are provided in the section above on collagen hydrolyzates.
Amino acid alkyl amides
Various other amino acid alkyl amides that share some structural similarity to isostearoyl hydrolyzed collagen, to various degrees, have been reviewed by the Cosmetic Ingredient Review (CIR) Panel as part of the Amino Acid Alkyl Amides group, and a limited number of studies were identified.
In vitro studies using acetyl glutamic acid, acetyl proline, acetyl tyrosine, disodium capryloyl glutamate, sodium cocoyl glutamate, and sodium lauroyl glutamate were found to be negative for genotoxicity (Cosmetic Ingredient Review 2013).
A reproductive/developmental toxicity study was conducted in rats and rabbits with acetyl cysteine via the oral route of administration. Test animals were fed up to 2000 mg/kg bw/day (rats) and 1000 mg/kg bw/day (rabbits). An evaluation showed no evidence of impaired fertility or toxicity to foetuses in either animal up to the highest tested dose. In a separate reproductive toxicity study, male rats treated orally with 250 mg/kg bw/day acetyl cysteine for 15 weeks did not experience adverse effects to fertility or reproductive performance (US FDA 2006).
6.3 Characterization of risk to human health
No adverse effects have been observed in laboratory studies at concentrations up to 1000 mg/kg bw/day in a variety of structurally similar protein hydrolyzates of both plant and animal origin, representative of CAS RN 9015-54-7, the protein hydrolyzates. Similarly, for collagen hydrolyzates and for yeast extract, there were no adverse effects reported in several laboratory studies for doses up to 1000 mg/kg bw/day. For isostearoyl hydrolyzed collagen, there were no substance specific health effects data available. Based on the information on its individual reaction components, isostearic acid and collagen hydrolyzates, and on structurally similar amino acid alkyl amides, this substance is not expected to be associated with any adverse health effects. Therefore, in consideration of the available toxicological information presented in this section, the substances in the Protein Derivatives and Yeast Extract Group are considered to be of low hazard potential. As such, quantitative exposure estimates were not derived. The risk to human health from the four substances in the Protein Derivatives and Yeast Extract Group is considered to be low.
6.4 Uncertainties in evaluation of risk to human health
Although there are some limitations and uncertainties in the health effects database (e.g., with respect to using a representative dataset to address the variability in the complex protein hydrolyzate mixtures), they were not significant and unlikely to affect the outcome. Based on available information, a qualitative, hazard-based approach to risk characterization is considered appropriate for this assessment.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from the four substances in the Protein Derivatives and Yeast Extract Group. It is concluded that yeast extract, protein hydrolyzates, collagen hydrolyzates and isostearoyl hydrolyzed collagen do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that yeast extract, protein hydrolyzates, collagen hydrolyzates, and isostearoyl hydrolyzed collagen do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that yeast extract, protein hydrolyzates, collagen hydrolyzates and isostearoyl hydrolyzed collagen do not meet any of the criteria set out in section 64 of CEPA.
References
Ballester D, Yáñez E, García R, Erazo S, López F, Haardt E, Cornejo S, López A, Pokniak J, and Chichester C. et al 1980. Chemical composition, nutritive value, and toxicological evaluation of two species of sweet lupine (Lupinus albus and Lupinus luteus). J Agric Food Chem. 28:402-405.
Booth AL. 1970. U.S. Department of Agriculture, Berkeley, CA. Memorandum with attachments, dated July 21, to R.A. Whitmore, U.S. Department of Agriculture, Philadelphia.
Calabrese EJ, Kenyon EM. 1991. Air toxics and risk assessment. Chelsea (MI): Lewis Publishers, Inc.Canada. [1983]. Feeds Regulations. SOR/83-593.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
[CFIA] Canadian Food Inspection Agency. 2019. Chapter 2 – Data Requirements for Single Ingredient Approval and Feed Registration: 2.6 Guidelines for the Assessment of Novel Feeds: Plant Sources. Retrieved on October 31, 2019.
Chemical Book. 2017. Yeast Extract. Retrieved on September 6, 2019.
Colantoni A, Recchia L, Bernabei G, Cardarelli M, Rouphael Y, Colla G. 2017. Analyzing the Environmental Impact of Chemically-Produced Protein Hydrolysate from Leather Waste vs. Enzymatically-Produced Protein Hydrolysate from Legume Grains. Agriculture. 7(8):62.
Cosmetic Ingredient Review. 2013. Safety Assessment of Amino Acid Alkyl Amides as Used in Cosmetics [PDF]. Retrieved on September 6, 2019.
CTFA (Cosmetic, Toiletry and Fragrance Association). 1979. Submission of unpublished data by CTFA. Subchronic dermal toxicity test (as cited in Liebert M. 1985).
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017a Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2019 Sept 18].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017b. Final screening assessment saccharomyces cerevisiae strain F53. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. c2007-2019a. Registered substances database; search results for CAS RN 100085-61-8. Helsinki (FI): ECHA [updated 2006; accessed on 2019 Oct 7]
[ECHA] European Chemicals Agency. c2007-2019b. Registered substances database; search results for CAS RN 100085-61-8. Helsinki (FI): ECHA [updated 2008; accessed on 2019 Oct 7]
[ECHA] European Chemicals Agency. c2007-2019c. Registered substances database; search results for CAS RN 84604-16-0. Helsinki (FI): ECHA [updated 1999; accessed on 2019 Oct 7]
[ECHA] European Chemicals Agency. c2007-2019d. Registered substances database; search results for CAS RN 30399-84-9. Helsinki (FI): ECHA [updated 1999; accessed on 2022 Feb 15]
[EFSA] European Food Safety Authority. 2007. Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA Journal 587, 10-16.
[EFSA] European Food Safety Authority. 2010. Scientific Opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2010 update). EFSA Journal 8(12), 1-56.
[EFSA] European Food Safety Authority. 2011. Scientific Opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2011 update). EFSA Journal 9 (12), 1-82.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment and Climate Change Canada, Health Canada; Existing Substances Program.
[FCC USP] Food Chemicals Codex [database]. 016. Rockville (MD): US Pharmacopeial Convention. [accessed February 2019].
Hazleton Laboratories, Inc. 1970. 13 –week dietary administration - rats. Soup base mixture, compound FN; yeatex, yeast extract. Report submitted to Campbell Soup Company, Camden, N. J. Section II in comments by IHPC on SCOGS tentative report 37b "Protein hydrolyzates," Toxicology. 1977. International Hydrolyzed Protein Council, Washington, D.C
Health Canada. 2010. PMRA list of formulants [PDF]. Ottawa (ON): Government of Canada. HC Pub. No.: 100460, Cat. No.: H114-22/2010E. [accessed 2019 Oct 7].
Liebert MA. Inc Publishers. 1983. Final Report on the Safety Assessment of Isostearic Acid. J Am Coll Toxicol. 2(7):61-74.
Liebert MA. Inc Publishers. 1985. Final Report on the Safety Assessment of Hydrolyzed Collagen. J Am Coll Toxicol. 4(5):199-221.
Litton Bionetics Inc. 1974. Mutagenic evaluation of compound FDA 71-85, hydrolyzed vegetable protein (soy). Report prepared under DHEW Contract No. FDA 223-74-2104. Available from: NTIS, Springfield, VA; PB-245 432.
Morgareidge K. 1973. Food and Drug Research Laboratories, Inc. Teratologic evaluation of FDA 71-85 (hydrolyzed vegetable protein) in mice and rats. Final report prepared under DHEW Contract No. FDA 71-260. Waverly, NY. [27 p.]
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Ohshita K, Nakashima Y, Sugiyama S, Takahashi R. [1977]. Appendix 3. Safety evaluation of shoyu. In: Scientific data and information for the Select Committee on GRAS Substances relating to fermented soy sauce. Kikkoman Foods, Inc., Walworth, WI. p.36-109.
Personal Care Products Council. 2016a. Summary information Hydrolyzed Sweet Almond Protein and Hydrolyzed Lupine Protein. Unpublished data submitted by Personal Care Products Council.
Personal Care Products Council. 2016b. Summary information Hydrolyzed Pea Protein. Unpublished data submitted by Personal Care Products Council.
Petrova I, Tolstorebrov I, Eikevik TM. 2018. Production of fish protein hydrolysates step by step: technological aspects, equipment used, major energy costs and methods of their minimizing. Int Aquat Res. 10(3):223-241.
[SDS] Safety Data Sheet. 2014. Lambert Kay EMT Gel [PDF]. Missouri (US): Lambert Kay. [accessed 2019 Oct 16].
TPS (Toxicology and Pathology Services) of the Cosmetic Ingredient Review. 1978. Subchronic dermal toxicity test (2-19-10).
Unilever Research Laboratory. 1976. Biological evaluation of modified protein hydrolysates for use as meat flavors. Washington, DC. International Hydrolyzed Protein Council.
[US eCFR] United States Electronic Code of Federal Regulations. 2019. Title 21, vol. 3, c. I, part 184.1553: Direct Food Substances Affirmed as Generally Recognized as Safe. Washington (DC): National Archives and Records Administration’s Office of the Federal Register (OFR); Government Publishing Office. [accessed 2019 Sept 12].
[US EPA] United States Environmental Protection Agency. 2018. Substance Registry Services (SRS). Yeast, ext. [accessed 2019 Oct 25].
[US FDA] United States Food and Drug Administration. 1961. Approval as GRAS (to Snyder, ChadwelL Keck, Kayser, and Ruggles, Chicago, Ill.) of hydrolyzed milk protein based on a letter, dated October 20, 1961 from E. T. W., Food and Drug Administration.
[US FDA] United States Food and Drug Administration. 1972. Subcommittee on Review of the GRAS List (Phase II). 1972. A comprehensive survey of industry on the use of food chemicals generally recognized as safe (GRAS). Appendix A. Prepared under DHEW contract no. FDA 70-22, by Committee on Food Protection, Division of Biology and Agriculture, National Research Council. National Academy of Sciences, Washington, DC.
[US FDA] United States Food and Drug Administration. 1978. Evaluation of the Health Aspects of Protein Hydrolyzates as Food Ingredients. Life Sciences Research Office. [accessed 2019 Oct 7].
[US FDA] United States Food and Drug Administration. 2006. Acetadote® (acetylcysteine) Injection [package insert] NDA21-539/S-004 [PDF]. Nashville (TN): Cumberland Pharmaceuticals. [accessed 2019 Oct 7].
[US FDA] United States Food and Drug Administration. 2017. GRAS Notice 713 for Hydrolyzed Procine Trachea Cartilage. Submitted by Rousselot BVBA on May 3, 2017. Washington (DC): US FDA. [accessed 2019 Oct 7].
[US FDA] United States Food and Drug Administration. 2019. Protein Hydrolysate, unspecified. Washington (DC): US FDA. [accessed 2019 Oct 7].
Whitmore R, Booth A, Naghski J, Swift C. 1975. Digestibility and safety of limed hide collagen in rat feeding experiments. J Food Sci. 40:101-104.
Appendix A. Major components of certain protein hydrolyzates
| Major component (% w/w dry weight) | Wheat | Yeast | Casein |
|---|---|---|---|
| Protein | 43.2 | 32.4 | 91.9 |
| Ash | 41.1 | 54.2 | 0.9 |
| Carbohydrates | 0.12 | 0.14 | 0.04 |
| Organic acids | 6.45 | 7.63 | less than 0.4 |
| NH4Cl | 4.82 | 1.44 | 4.0 |
| Moisture | 2.4 | 2.2 | 2.9 |
| Total | 99.2 | 99.4 | 99.7 |
a Example compositions of commercial acid hydrolyzed protein products (on dry weight basis).
