Screening assessment resins and rosins group
Official title: Screening Assessment - Resins and Rosins Group
Environment and Climate Change Canada
Health Canada
July 2022
Cat. No.: En84-300/2022E-PDF
ISBN 978-0-660-43856-6
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 12 substances collectively referred to as the Resins and Rosins GroupFootnote 1 Footnote 2 . The Chemical Abstracts Service Registry Numbers (CAS RN)Footnote 3 , their Domestic Substances List (DSL) names and their abbreviations or common names are listed in the table below.
| CAS RN | Domestic Substances List (DSL) name | Abbreviation or common name |
|---|---|---|
| 1740-19-8 | 1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-, [1R-(1α,4aβ,10aα)] | DHAA |
| 8002-26-4a | Tall oil | CTOb or DTOb |
| 8016-81-7a,c | Tall-oil pitch | TOP |
| 8046-19-3a,c | Storax (balsam) | Storax |
| 8050-09-7a,d | Rosin | Rosind |
| 8050-15-5a,c | Resin acids and Rosin acids, hydrogenated, Me esters | RHME |
| 8050-28-0a | Rosin, maleated | RMa |
| 8052-10-6a,d | Tall-oil rosin | Rosind |
| 9007-13-0a | Resin acids and Rosin acids, calcium salts | RCa |
| 61790-51-0a | Resin acids and Rosin acids, sodium salts | RNa |
| 68186-14-1a | Resin acids and Rosin acids, Me esters | RME |
| 73138-82-6a,d | Resin acids and Rosin acids | Rosind |
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
b Crude tall oil (CTO) and distilled tall oil (DTO) are both covered under this DSL name and CAS RN although they may have different properties, compositions and uses.
c This substance was not identified under subsection 73(1) of CEPA, but was included in this screening assessment as it was considered a priority on the basis of other human health concerns.
d These substances may be used interchangeably by industry and are referred to under the same name (rosin).
Resins and Rosins Group substances may be imported or manufactured in Canada and are naturally present in the environment. Variability in composition of the Resins and Rosins Group substances may be due to source material variability and/or the production process conditions.
All of the substances in the Resins and Rosins Group have been included in a survey issued pursuant to section 71 of CEPA and subsequent voluntary surveys. All 12 substances were reported to be imported into Canada in quantities for each substance ranging from <100 kg to 1 000 000 kg, for the 2011 reporting year. CTO was incidentally co-produced in Canada at 10 000 000 kg to 100 000 000 kg for the 2011 reporting year. RCa and RNa were manufactured in Canada at 10 000 kg to 100 000 kg and 100 kg to 1 000 kg respectively, for the 2011 reporting year.
Commercial and industrial uses of the substances in this group include processing aids, electronics solder, concrete production, rubber compounding, steelmaking, and formulation of paints and coatings, as well as products available to consumers, such as adhesives, binding agents, cosmetics, natural health products and non-prescription drugs.
The major sources of emissions of substances in the Resins and Rosins Group to the environment in Canada are related to manufacturing and industrial uses. Potential releases of concern occur primarily to surface water.
Most components of CTO, DTO, rosin, RCa and RNa are moderately persistent in water and are expected to be moderately to highly persistent in sediments. Components of TOP, RHME and RMa are predicted to have a moderate to high persistence in water and a high persistence in sediments.
Most substances in the Resins and Rosins Group have components with a low to moderate bioconcentration potential. The bioconcentration factors of components of RHME show a moderate to high bioconcentration potential. Certain CTO, DTO and TOP representative chemicals are predicted to have a high bioaccumulation potential based on modelled bioaccumulation factor results.
CTO, DTO, TOP, rosin, RCa, RNa and RMa all consist of components that could have non-specific (i.e., narcotic) or compound-specific effects to organisms in the environment at low concentrations of exposure. RHME consists of only narcotic components with effects at low concentrations. Exposure scenarios were developed for the manufacturing and industrial use of the Resins and Rosins Group substances. Risk quotient analyses were conducted to compare estimated aquatic concentrations with adverse effect concentrations, assuming a concentration addition of the components of the UVCBs in aquatic organisms for different exposure scenarios. Scenarios for the manufacturing of CTO indicate that there is a risk to aquatic organisms; however, no risk was identified for the other scenarios for the Resins and Rosins Group substances at levels of exposure based on reported quantities.
The ecological risks of four substances in the Resins and Rosins Group (DHAA, storax, RME, and rosin CAS RN 73138-82-6) were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The ERC identified DHAA, storax, RME, and rosin CAS RN 73138-82-6 as having low potential to cause ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is risk of harm to the environment from tall oil (CAS RN 8002-26-4), specifically due to CTO. It is concluded that tall oil meets the criteria under paragraph 64(a) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is concluded that tall oil does not meet the criteria under paragraph 64(b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends. It is also concluded that the other 11 Resins and Rosins Group substances do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
RMa and rosin (CAS RN 8052-10-6) were previously evaluated using the approach applied in the Rapid Screening of Substances with Limited General Population Exposure , which determined that the substances required further assessment. The potential for exposure of the general population to RMa and rosin (CAS RN 8052-10-6) was considered in this assessment to be negligible, indicating a low probability of risk to human health. Therefore, RMa and rosin (CAS RN 8052-10-6) are considered to be of low concern for human health at current levels of exposure.
TOP was evaluated using the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances, which is based on the potential hazard of similar chemical structures, as well as chemical-specific genotoxicity data, when available. The estimate of exposure generated for TOP was lower than the TTC value, indicating a low probability of risk to human health. Therefore, TOP is considered to be of low concern for human health at current levels of exposure.
Substances in the Resins and Rosins Group have not been identified as carcinogenic. Limited toxicological effects have been reported in repeated-dose studies with resins and rosins substances with effects such as decreased body weights. Some histopathological changes were noted in target organs.
Exposure to the Resins and Rosins Group substances is expected to be predominantly via the dermal route and can occur from use of rosin as a gripping agent by athletes and violinists, as a non-medicinal ingredient in sunscreens, and in cosmetic products, such as moisturizers and cleansers. There is the potential for oral ingestion from uses such as non-medicinal ingredient in dental varnishes, as well as from dental sealants and lipsticks. On the basis of a comparison of estimates of exposure to substances in the Resins and Rosins Group and levels associated with effects observed in laboratory studies, margins of exposure are considered adequate to address uncertainities in the health effects and exposure datasets.
Considering all the information presented in this screening assessment, it is concluded that the 12 Resins and Rosins Group substances do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that tall oil meets one or more of the criteria set out in section 64 of CEPA, specifically on the basis of risk presented by CTO and that the other 11 Resins and Rosins Group substances do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 12 of 14 substances collectively referred to under the Chemicals Management Plan as the Resins and Rosins Group, to determine whether these 12 substances present or may present a risk to the environment or to human health. These 14 substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
Two substances (CAS RN 26266-77-3, 1-phenanthrenemethanol, dodecahydro-1,4a-dimethyl-7-(1-methylethyl)-, and CAS RN 91081-53-7, rosin, reaction products with formaldehyde) were both considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a) and in either the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Science Approach Document (Health Canada 2016) or via the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018a), and both were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this screening assessment. The conclusion for the substance bearing CAS RN 26266-77-3 is provided in the Substances Identified as Being of Low Concern based on the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Screening Assessment (ECCC, HC 2018b). The conclusion for the substance bearing CAS RN 91081-53-7 is provided in the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018a).The 12 substances addressed in this draft screening assessment will hereinafter be referred to as the Resins and Rosins Group.
Four of the 12 substances in the Resins and Rosins Group (DHAA, storax, RME, and rosin CAS RN 73138-82-6) were identified as having a low potential to cause ecological harm using the ERC approach (ECCC 2016a; Appendix A). A further three substances were identified as having a low potential to cause harm to human health: RMa and rosin (CAS RN 8052-10-6), as determined on the basis of the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018a), and tall-oil pitch (TOP), as determined on the basis of the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Science Approach Document (Health Canada 2016). These results, in conjunction with any other relevant information that became available after the publication of these documents, are considered in support of the conclusions made under section 64 of CEPA in this screening assessment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders and collected by Environment and Climate Change Canada (ECCC) staff during site visits of Canadian kraft pulp mills. Relevant data and observations were identified up to March 2020. Empirical data from key studies as well as some results from models are used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Environment and Climate Change Canada and Health Canada and incorporates input from other programs within these departments. The ecological and human health portions of this screening assessment have undergone external review and/or consultation. Comments on the technical portions relevant to the environment were received from Dr. Pamela M. Campbell at ToxEcology Environmental Consulting Ltd., Dr. Bjarne Holmbom at Separation Research Inc. and Dr. Vickie Tatum at the United States (U.S) National Council for Air and Stream Improvement (NCASI). Comments on the technical portions relevant to human health were coordinated and received from Tetratech. Additionally, the draft of this screening assessment (published June 22, 2019) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and ECCC.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 4 This screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The CAS RN, Domestic Substances List (DSL) names and common names and/or abbreviations of the individual substances along with (if applicable) their representative chemical structures and percentage of the substance represented in the Resins and Rosins Group are presented in Tables 2-1 and 2-2.
|
CAS RN (abbreviation) |
DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 1740-19-8 (DHAA) |
1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-, [1R-(1α,4aβ,10aα)]- (Dehydroabietic acid) |
![Chemical structure of dehydroabietic acid (DHAA), with SMILES notation: C[C@@]1([C@]2([C@@](CCC1)(C)C3=C(CC2)C=C(C(C)C)C=C3)[H])C(O)=O](/content/dam/eccc/images/pded/resins-rosins/20190524Figure2.1.1.jpg) C20H28O2
C20H28O2
|
300.44 |
|
CAS RN (abbreviation) |
DSL name (common name) |
Representative chemical structure Molecular formula, molecular weight Chemical Name, %wt./wt. represented (in bold) |
|---|---|---|
|
8002-26-4 (CTO and DTOa) |
Tall oil | 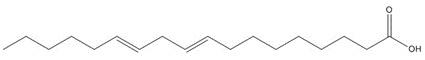 C18H32O2, 280.45 g/mol
C18H32O2, 280.45 g/mollinoleic acidb, 41%/65a% 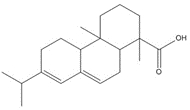 C20H30O2, 302.46 g/mol
C20H30O2, 302.46 g/molabietic acidc, 20%/15a%  C29H50O, 414.72 g/mol
C29H50O, 414.72 g/molβ-sitosterold,e, 7%/0a%  C20H32O, 288.25 g/mol
C20H32O, 288.25 g/molabietinolf,e, 10%/2a% 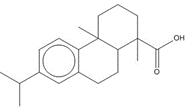 C20H28O2, 300.44 g/mol
C20H28O2, 300.44 g/moldehydroabietic acid (DHAA)c, 5%/8a% 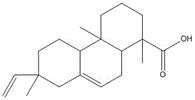 C20H30O2, 302.46 g/mol
C20H30O2, 302.46 g/molisopimaric acid (IPA)c, 5%/10a% 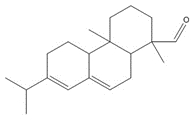 C20H30O, 286.46 g/mol
C20H30O, 286.46 g/molabietinalg,e, 2%/0a% [No Structure] polymeric and esters > 750 g/mol and/or log Kow >9, 10%/0% |
| 8016-81-7 (TOP) | Tall oil pitch | 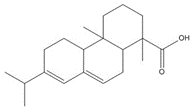 C20H30O2, 302.46 g/mol
C20H30O2, 302.46 g/molabietic acidc, 15%  C29H50O, 414.72 g/mol
C29H50O, 414.72 g/molβ-sitosterolh,e, 15%  C18H32O2, 280.45 g/mol
C18H32O2, 280.45 g/mollinoleic acidb, 5% [No Structure] polymeric and esters > 750 g/mol and/or log Kow >9, 65% |
| 8050-09-7 / 8052-10-6 / 73138-82-6 |
Rosin / tall-oil rosin / resin acids and rosin acids (rosin) |
 C20H30O2, 302.46 g/mol
C20H30O2, 302.46 g/molabietic acidh,c, 60%  C20H28O2, 300.44 g/mol
C20H28O2, 300.44 g/moldehydroabietic acid (DHAA)c, 15% 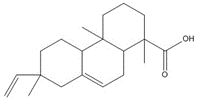 C20H30O2, 302.46 g/mol
C20H30O2, 302.46 g/molisopimaric acid (IPA)c, 25% |
| 9007-13-0 (RCa) | Resin acids and rosin acids, calcium salts | 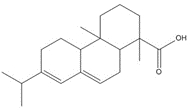 C20H30O2, 302.46 g/mol
C20H30O2, 302.46 g/molabietic acidh,c, 58% 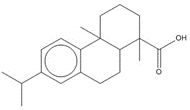 C20H28O2, 300.44 g/mol
C20H28O2, 300.44 g/moldehydroabietic acid (DHAA)c, 13% 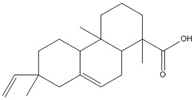 C20H30O2, 302.46 g/mol
C20H30O2, 302.46 g/molisopimaric acid (IPA)c, 23% Ca2+, 40.08 g/mol calcium counter ion, 6% |
| 61790-51-0 (RNa) | Resin acids and rosin acids, sodium salts | 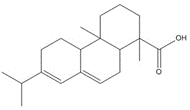 C20H30O2, 302.46 g/mol
C20H30O2, 302.46 g/molabietic acidh,c, 57% 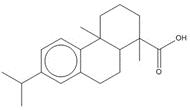 C20H28O2, 300.44 g/mol
C20H28O2, 300.44 g/moldehydroabietic acid (DHAA)c, 13% 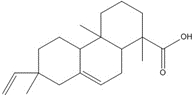 C20H30O2, 302.46 g/mol
C20H30O2, 302.46 g/molisopimaric acid (IPA)c, 23% Na1+, 22.99 g/mol sodium counter ion, 7% |
| 8050-15-5 (RHME) | Resin acids and rosin acids, hydrogenated, methyl esters | 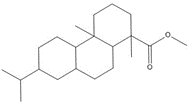 C21H36O2, 320.27 g/mol
C21H36O2, 320.27 g/moltetrahydroabietic acid methyl ester (THAME)h,i, 75%  C21H30O2, 314.47 g/mol
C21H30O2, 314.47 g/moldehydroabietic acid methyl ester (DHAME)i, 20%  C20H28O2, 300.44 g/mol
C20H28O2, 300.44 g/moldehydroabietic acid (DHAA)c, 5% |
| 68186-14-1 (RME) | Resin acids and rosin acids, methyl esters |  C21H34O2, 318.50 g/mol
C21H34O2, 318.50 g/molabietic acid methyl esterh,i, 100% |
| 8050-28-0 (RMa) | Rosin, maleated |  parent hydrolysis product parent hydrolysis productC24H32O5, 400.52 g/mol maleopimaric acid (MPA)h15% → C24H34O6, 418.53 g/mol MPA hydrolysis product, 15% 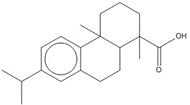 C20H28O2, 300.44 g/mol
C20H28O2, 300.44 g/moldehydroabietic acid (DHAA)c, 25% 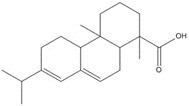 C20H30O2, 302.46 g/mol
C20H30O2, 302.46 g/molabietic acidc, 60% |
| 8046-19-3 |
Storax (balsam) |
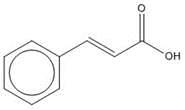 C9H8O2, 148.16 g/mol
C9H8O2, 148.16 g/molcinnamic acidh, 100% |
Abbreviations: Kow, octanol–water partition coefficient; % wt./wt., weight percentage
a Distilled tall oil (DTO) has a distinct composition, compared to crude tall oil (CTO) and thus, the associated percentage representation of each representative chemical will differ accordingly. However, both CTO and DTO bear the CAS‑RN 8002-26-4.
Chemical Classes: b Fatty acid; c Resin acid; d Phytosterol; e;Estimated component as the neutrals fraction may not have been fully characterized and uncertainty exists f Alcohol; g Aldehyde; i Ester.
h Key representative chemical (used for initial tier profiling of this substance during categorization, 2006, and more recently in Ecological Risk Classification of Organic Substances (ECCC 2016b).
In the ecological assessment, representative chemicals are used to represent the UVCB (unknown or variable composition, complex reaction products, or biological materials) substances in the Resins and Rosins Group for the purposes of estimating the properties of the many components or of the whole substance. Whole substance-based empirical data (if available) is also considered together with this component-based information in a weight-of-evidence approach. However, in many instances, there are significant deficiencies in the whole substance testing of these UVCBs, such that relevant and reliable whole substance data are generally not available. The principal factors taken into account to select the representative chemicals (shown for each UVCB in Table 2-2) were bioavailability, persistence, and toxicity and/or reactivity. The availability of empirical data for each representative chemical was also considered in the selection process. In general, components with a higher bioavailability, persistence and toxicity and known presence in the environment were selected to represent the respective sub-classes. However, given the high degree of variability in the types and amounts of components present, there may be some uncertainty respecting the degree of the representation of the subclass.
The proportions allocated for each representative chemical in Table 2-2 are determined on the basis of information available from the published literature along with information obtained from industry. As a conservative approach, a higher proportion of those representative chemicals that are more hazardous were allocated. In most cases, more than one representative chemical is used to describe the composition of the substance rather than selecting a single “worst case” component, which could result in a less realistic assessment. Each representative structure represents a number of components within a fraction of the substance (not just the proportion known for that specific representative chemical), and a distinction must therefore be made between a representative chemical and a component of the substance for the purposes of this screening assessment.
Most of the Resins and Rosins Group substances are derivatives of CTO, which is a co-product of kraft pulping of coniferous wood formed by acidifying black liquor soap skimmings with sulfuric acid. CTO is a dark oily liquid with 26% to 42% resin acids (represented by abietic acid, isopimaric acid (IPA), and DHAA), 36% to 48% fatty acids (e.g., linoleic acid), and 10% to 38% neutral compounds (represented by β-sitosterol, abietinol and abietinal) (Huibers 2000). Variability in composition may be due to both the pulpwood variability (e.g., tree species used) and the process or operational conditions.
CTO may serve as a source material for several downstream products manufactured through extensive fractional distillation. This process is aimed at separating out desirable fatty and resin acid components while minimizing the proportion of neutral compounds. The first step in CTO distillation is the removal of the TOP fraction (see Figure 2-1). The composition of TOP can be highly variable, and since the commercial uses are limited, this fraction is often burned as fuel at the distillation plants. Recent data submitted to ECCC suggests that a large percentage (~65%) of TOP may be a polymeric material with molecular weights greater than 750 g/mol (Study Submission 2017a). Thus, the remaining and more bioavailable fraction of TOP includes 2% to 8% fatty acids represented by linoleic acid, 5% to 15% resin acids represented by abietic acid, along with ~15% phytosterols represented by β-sitosterol (Table 2-2) (Zinkel and Russell 1989; Study Submission 2017a). While esterified neutral compounds including polymeric and esterified phytosterols (e.g., sitosterol linoleate with a molecular weight of 677 g/mol) may also be present in CTO (10%), these are not considered significantly bioavailable due to their large molecular size, and thus, are not considered further as representative chemicals for the purpose of this screening assessment.
After the TOP has been removed, the depitched CTO is fed into a distillation column to produce a heads (i.e.,. low boiling point or volatile components), rosin and crude fatty acid fractions. Rosin (which in this screening assessment includes CAS RNs 8050-09-7, 8052-10-6 and 73138-82-6) has a higher boiling point than the fatty acid components and is taken from the bottom of the column. Rosin is largely (~90% w/w) made up of specific resin acids (also named rosin acids) along with smaller amounts of fatty acids (1% to 5%) and neutrals (1% to 7%), the latter of which are mainly diterpenoids (US EPA 2004; Holmbom 2011). Two other rosin production methods exist: extraction from live pine trees (gum rosin) and, to a lesser degree, extraction from wood stumps (wood rosin). Depending on the production method, the relative proportion of these resin acids may vary as shown in Table 2-3 (Zinkel and Russell 1989).
| Components (ID) | Tall oil rosinc | Gum rosinc | Wood rosinc |
|---|---|---|---|
| Abietic acida | 38 | 24 | 51 |
| Palustric acida | 8 | 21 | 8 |
| Isopimaric acidb | 11 | 17 | 16 |
| Dehydroabietic acida | 18 | 5 | 8 |
| Neoabietic acida | 3 | 19 | 5 |
| Pimaric acidb | 4 | 5 | 7 |
a Abietic-type.
b Pimaric-type.
c Percent proportion of total acids fraction.
Three types of rosin derivatives included in this screening assessment are also shown in Figure 2-1. The first includes sodium (Na) or calcium (Ca) salts of unmodified rosin (i.e., CAS RNs 9007-13-0 and 61790-51-0), made by treating rosin with the appropriate alkali earth or alkali metal (US EPA 2004). The second type, resin acid methyl esters, is produced through methylation (CAS RN 68186-14-1) or methylation preceded by hydrogenation (CAS RN 8050-15-5). Hydrogenated methyl esters of resin acids are produced by catalyzed hydrogenation to saturate one or more of the conjugated double bonds and create di- or tetra-hydro products (Zinkel and Russell 1989), a process that typically achieves a 75% hydrogenation level (Panda 2005). The hydrogenated product is then methylated. The processes typically have high (85% to 95%) yields of methylated resin acid or methylated hydrogenated resin acid derivatives (Study Submission 2016a, 2016b). The third type is maleated rosin (CAS RN 8050-28-0), which is produced when abietic-type resin acids (e.g., abietic, neoabietic, palustric and levopimaric acids) in rosin react with maleic anhydride under conditions that favour the Diels-Alder reaction and formation of maleopimaric acid (MPA). Yield of MPA varies depending on reaction conditions, relative proportions of reactants, and types of solvents and acids used. Gonis et al. (1973) reported 32% to 42% (weight basis) after refluxing rosin, maleic anhydride, and glacial acetic acid under nitrogen at elevated temperatures in a laboratory study. However, MPA content is much lower, i.e., 12%-16%, in certain commercial products (Study Submission 2017b).
Distilled tall oil (DTO) is produced as a product of CTO distillation (Zinkel and Russell 1989). DTO is obtained from the crude fatty acid fraction, which is distilled into a heads fraction, a purified tall oil fatty acids (TOFAs) fraction and a DTO fraction. DTO consists of 25% to 30% resin acids (represented by abietic acid, IPA, and DHAA), 60% to 70% fatty acids (represented by linoleic acid), and 2% to 6% neutrals (represented by abietinol. However, the neutrals fraction of DTO has not been characterized) (Holmbom et al. 2010). In 1994, CAS RN 8002-26-4 was added to the DSL with the DSL name “tall oil”. The substance, as nominated to the DSL. may refer to both CTO and DTO. Where possible distinction is made in the screening assessment between these two substances. However, given that this distinction was not made during the original DSL nomination and CTO and DTO share a common CAS RN, instances may remain where it is not possible to accurately distinguish between CTO and DTO.

Long description
CTO is distilled on distillation columns to produce, in decreasing boiling point, TOP, rosin, DTO, tall oil fatty acids and tall oil heads, the latter two of which are not being assessed. Rosin may be further chemically reacted to form: rosin, maleated; resin acids and rosin acids, hydrogenated, methyl esters; resin acids and rosin acids, methyl ester; resin acids and rosin acids, calcium salts; and resin acids and rosin acids, sodium salts.
This screening assessment also includes the essential oil storax produced from steam or water distillation of resins from the deciduous tree species sweetgum (Liquidambar spp.). This substance is a type of resin (thus, grouped together with rosin in this group). However, unlike rosin from North American coniferous wood species (e.g., CAS RNs 8050-09-7, 8052-10-6, and 73138-82-6), storax is comprised predominantly of esters of cinnamic acid and benzoic acid (Baser and Demirci 2011).
3. Physical and chemical properties
A summary of physical and chemical property data for the substances in the Resins and Rosins Group is presented in Tables 3-1 to 3-9. For the UVCBs, a range of empirical or modelled physical and chemical property values are provided for each UVCB substance based on either the whole substance or its representative chemicals. Most standard tests for physical-chemical properties were originally developed for application to discrete organic substances, although they have been applied to UVCBs. Thus, results of applying such tests to a whole UVCB substance are interpreted with caution. For example, while empirical whole substance data are available for certain UVCB substances on melting point, vapour pressure and water solubility, these data do not accurately reflect the properties of the individual components or the range present for all components within the UVCB substance. Component-based information is therefore used for modelling purposes, and empirical whole-substance data is included as an added line of evidence where possible. In several cases, a large range of values is shown, reflecting the large range in individual representative chemical physical-chemical properties.
When experimental data are limited or not available for a property, quantitative structure-activity relationship (QSAR) models are used to generate predicted values for the substance and/or analogues are used for read-across. Component specific physical-chemical information is provided in ECCC (2021b).
Where more than one valid modelled or empirical value is available for a given property for a specific component, the mean or geometric mean is taken as the key value for that parameter. The selected key values for the estimation of vapour pressure, water solubility and log Kow or log Dow (log Dow is used in place of log Kow when the component ionizes more than 50% within the range of pH 6-8) are adjusted using the least-squares adjustment procedure (Cole and Mackay 2000; Schenker et al. 2005) and represent internally consistent partitioning properties considering thermodynamic constraints.
| Property | Value | Key reference(s) |
|---|---|---|
| Physical state | Liquida,b (w) | ECHA c2007-2017 |
| Melting point (°C) |
-8.5–171a,b (c)
-3.15c/-20d (w) |
Liss et al. 1997 US EPA 2017 ECHA c2007-2017 |
| Vapour pressure (Pa) |
2.5 × 10−5c–0.19c,a (c) 4.6 x 10-5c–0.19c,b (c) |
MPBPWIN 2010 |
| Henry’s law constant (Pa·m3/mol) | 1.79 × 10−2–84a,b (c) | HENRYWIN 2008 |
| Water solubility (mg/L) |
7.62 × 10−7c–153a,b,c, (c) 73a / 8–42b (w) |
Meylan et al. 1996 Nyren and Back 1958 WATERNT 2010 WSKOWWIN 2010 ACD/Percepta c1997-2012 ECHA c2007-2017 |
| log Kow or log D (dimensionless) at pH 7 unless otherwise specified |
3.15–7.02a (c) 3.15–5.78b (c) 3.2–6.8a,b,d (w) |
ACD/Percepta c1997-2012 Meylan and Howard 1995 VCCLab 2005 ECHA c2007-2017 |
| log Koc (dimensionless) | 1.88——5.08a (c) 1.88–3.52b (c) | KOCWIN 2010 |
| pKa (dimensionless) | 4.8–15a,b (c) | ACD/Percepta c1997-2012 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient; pKa, acid dissociation constant; D, distribution coefficient.
a CTO (EC#931-433-1).
b DTO (EC#232-304-6).
c Sub-cooled corrected for solids at standard temperature.
d pH = 5-6.
| Property | Value | Key reference(s) |
|---|---|---|
| Physical state | Tacky thermoplastic | Zinkel and Russell 1989 |
|
Softening / Pour point (°C)
Melting point (°C) |
40 (w) 20.9 (w) -8.5–171 (c) |
Zinkel and Russell 1989 ECHA c2007-2017 US EPA 2017 |
| Vapour pressure (Pa) | 2.5 × 10-5a–0.19a (c) | MPBPWIN 2010 |
| Henry’s law constant (Pa·m3/mol) | 0.16–30 (c) |
US EPA 2017 HENRYWIN 2008 |
| Water solubility (mg/L) |
7.6 × 10-7a–153a (c)
<1–20 mg/L (w) |
Nyren and Back 1958 Meylan et al. 1996 WATERNT 2010 WSKOWWIN 2010 ACD/Percepta c1997-2012 ECHA c2007-2017 |
| log Kow or log D (dimensionless) at pH 7 unless otherwise specified |
3.34–8.26 (c)
2.8–4.4b (w) |
ACD/Percepta c1997-2012 Meylan and Howard 1995 VCCLab 2005 ECHA c2007-2017 |
| log Koc (dimensionless) | 2.00–5.08 (c) | KOCWIN 2010 |
| pKa (dimensionless) | 4.8–15 (c) | PhysProp c2013, ACD/Percepta c1997-2012 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient; N/A, not applicable; pKa, acid dissociation constant; D, distribution coefficient.
a Sub-cooled corrected for solids at standard temperature.
b pH = 7.5.
| Property | Range | Key reference(s) |
|---|---|---|
| Physical state | Solid (w) | ECHA c2007-2017 |
| Melting point (°C) |
160–171 (c)
67–93 (w) |
US EPA 2017 Liss et al. 1997 ECHA c2007-2017 |
| Vapour pressure (Pa) |
2.2×10-3b–0.19b(c) 6 (w) |
MPBPWIN 2010 ECHA c2007-2017 |
| Henry’s law constant (Pa·m3/mol) | 1.8×10-2–0.68 (c) | HENRYWIN 2008 |
| Water solubility (mg/L) |
18b–153b (c)
0.6–0.9 (w) |
Meylan et al. 1996 WATERNT 2010 WSKOWWIN 2010 ACD/Percepta c1997-2012 Liss et al. 1997 ECHA c2007-2017 |
| log Kow or log D (dimensionless) at pH 7 unless otherwise specified |
3.2–5.8 (c)
3.0–6.2c (w) 1.9–7.7d (w) |
ACD/Percepta c1997-2012 ECHA c2007-2017 |
| log Koc (dimensionless) | 1.9–2.3 (c) | KOCWIN 2010 |
| pKa (dimensionless) | 4.8–6.4 (c) |
ACD/Percepta c1997-2012 Nyren and Back 1958 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient; pKa, acid dissociation constant; D, distribution coefficient.
a Considered the principal CAS RN for rosin in this assessment.
b Sub-cooled corrected for solids at standard temperature.
c pH = 6-7.
d pH = 2.
| Property | Range | Key reference(s) |
|---|---|---|
| Physical state | Viscous liquid | ECHA c2007-2017 |
| Melting point (°C) |
113–171 (c)
-5.5 (w) |
Liss et al. 1997 US EPA 2017 MPBPWIN 2010 ECHA c2007-2017 |
| Vapour pressure (Pa) |
2.2 x 10-3a–7.8 x 10-3a (c)
2.6 × 10-2 (w) |
MPBPWIN 2010
ECHA c2007-2017 |
| Henry’s law constant (Pa·m3/mol) | 1.8 × 10-2–250 (c) | HENRYWIN 2008 |
| Water solubility (mg/L) |
0.2a–124a (c)
0.42–6 (w) |
Liss et al. 1997 WSKOWWIN 2010 WATERNT 2010 ACD/Percepta c1997-2012 ECHA c2007-2017 |
| log Kow or log D (dimensionless) at pH 7 unless otherwise specified |
4.8–6.6 (c)
6.4–7.6b; >6.5c (w) |
KOWWIN 2010 VCCLab 2005 ACD/Percepta c1997-2012 ECHA c2007-2017 |
| log Koc (dimensionless) | 1.9–4.5 (c) | KOCWIN 2010 |
| pKa (dimensionless) | 4.8–5.7 (DHAA only) |
Liss et al. 1997 ACD/Percepta c1997-2012 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient; pKa, acid dissociation constant; D, distribution coefficient.
a Sub-cooled corrected for solids at standard temperature.
b pH = 6.
c pH = 7.
| Property | Range | Key reference(s) |
|---|---|---|
| Physical state | Solid | |
| Melting point (°C) |
171–207 (c)
94–116 (w) |
Liss et al. 1997 US EPA 2017 MPBPWIN 2010 Zinkel and Russell 1989 |
| Vapour pressure (Pa) |
1.33 × 10-7a—0.19a (c) 4 (w) |
MPBPWIN 2010
ECHA c2007-2017 |
| Henry’s law constant (Pa·m3/mol) | 1 x 10-4--0.68 (c) |
HENRYWIN 2008 PhysProp c2013 |
| Water solubility (mg/L) |
25a–153 (c)
1.4 (w) |
Nyren and Back 1958;
Liss et al. 1997 WSKOWWIN 2010 WATERNT 2010 ACD/Percepta c1997-2012 ECHA c2007-2017 |
| log Kow or log D (dimensionless) at pH 7 unless otherwise specified |
3.1515–3.6 (c)
1.5b–7.6b (w) 2.2c–5.9c (w) |
ACD/Percepta c1997-2012
Study Submission 2016d ECHA c2007-2017 |
| log Koc (dimensionless) | 1.72 (c) | KOCWIN 2010 |
| pKa (dimensionless) | 4.8–6.4 |
Liss et al. 1997 ACD/Percepta c1997-2012 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient; pKa, acid dissociation constant; D, distribution coefficient.
a Sub-cooled corrected for solids at standard temperature.
b pH = 2.
c pH > 2.
| Property |
Range RCa / RNa |
Key reference(s) |
|---|---|---|
| Physical state | Solid (w) | ECHA c2007-2017 |
| Melting point (°C) |
160–171a (c)
>300b / >255 (w) |
US EPA 2017 Liss et al. 1997 ECHA c2007-2017 |
| Vapour pressure (Pa) | 2.2×10-3ac–0.19ac (c) | MPBPWIN 2010 |
| Henry’s law constant (Pa·m3/mol) | 1.8×10-2a–0.68a (c) | HENRYWIN 2008 |
| Water solubility (mg/L) |
18a–153a (c)
43 / miscibled (w) |
Meylan et al. 1996 WATERNET 2010 WSKOWWIN 2010 ACD/Percepta c1997-2012 Liss et al. 1997 ECHA c2007-2017 |
| log Kow or log D (dimensionless) at pH 7 unless otherwise specified |
3.2a–5.8a (c)
3.01e / 0.9–6.6f (w) |
ACD/Percepta c1997-2012
ECHA c2007-2017 |
| log Koc (dimensionless) | 1.9a–2.3a (c) | KOCWIN 2010 |
| pKa (dimensionless) | 4.8a–6.4a (c) |
ACD/Percepta c1997-2012 Nyren and Back 1958 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient; pKa, acid dissociation constant; D, distribution coefficient.
a Values are for organic components of rosin (CAS RNs 8050-09-7, 8052-10-6, 73138-82-6); does not account for properties of Na or Ca salts.
b Decomposes at > 115°C (ECHA c2007-2017).
c Sub-cooled corrected for solids at standard temperature.
d Concentration tested was 0.25 to 4 g/mL of water.
e pH = 6.8 - 7.3.
f pH = 7.
| Property | RME | Key reference |
|---|---|---|
| Physical state | Viscous liquid | ECHA c2007-2015 |
| Molecular Weight (g/mol) | ~318.5 | n/a |
| Boiling point (°C) | 360 – 430 (decomposition) | ECHA c2007-2015 |
| Vapour pressure (Pa) | 3.1 ×10-3 | ECHA c2007-2015 |
| Henry’s law constant (Pa·m3/mol) | 2.16 × 10-3 | HENRYWIN 2008 |
| Log Kaw (dimensionless) | -6.155 | HENRYWIN 2008 |
| Water solubility (mg/L) | <0.22 to <32.3 | ECHA c2007-2015 |
| log Kow | 2.44 to >6.5 2.13 | ECHA c2007-2015 |
| log Koc (dimensionless) | 1.334–1.731 | KOCWIN 2010 |
| log Koa (dimensionless) | 8.285 | KOAWIN v 1.10 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient; Kaw, air-water partition coefficient; Koa, octanol-air partition coefficient.
| Property | Storax (balsam) | Key reference(s) |
|---|---|---|
| Physical state | solid | ECHA c2007-2015 |
| Molecular Weight (g/mol) | ~212.3 | n/a |
| Boiling point (°C) | 300–343.9 | Pubchem |
| Vapour pressure (Pa) | 6.67 × 10-3 | ECHA c2007-2015 |
| Henry’s law constant (Pa·m3/mol) | 1.71 × 10-3 | HENRYWIN 2008 |
| Log Kaw (dimensionless) | -6.155 | HENRYWIN 2008 |
| Water solubility (mg/L) | 0.1 (at 18°C) | ECHA c2007-2015 |
| log Kow | 2.13 | ECHA c2007-2015 |
| log Koc (dimensionless) | 1.73 | KOCWIN 2010 |
| log Koa (dimensionless) | 8.28 | KOAWIN v 1.10 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient; Kaw, air-water partition coefficient; Koa, octanol-air partition coefficient.
| Property | DHAA | Key reference(s) |
|---|---|---|
| Physical state | solid | ECHA c2007-2015 |
| Molecular Weight (g/mol) | 300.4 | n/a |
| Boiling point (°C) | 326–425 | US EPA Chem Dashboard |
| Vapour pressure (Pa) | 7.37 × 10-6–6.13 × 10-5 | US EPA Chem Dashboard |
| Henry’s law constant (Pa·m3/mol) | 4.78 × 10-3 | HENRYWIN 2008 |
| Log Kaw (dimensionless) | -5.14 | HENRYWIN 2008 |
| Water solubility (mg/L) | 2.4 | WSKOWWIN 2008 |
| log Kow | 4.80 | KOAWIN 2008 |
| log Koc (dimensionless) | 2.81–4.34 | KOCWIN 2010 |
| log Koa (dimensionless) | 9.94 | AEROWIN v 1.10 |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient; Kaw, air-water partition coefficient; Koa, octanol-air partition coefficient.
4. Sources and uses
All of the substances in the Resins and Rosins Group have been included in a survey issued pursuant to section 71 of CEPA (Canada 2012) and subsequent voluntary surveys (ECCC 2016c, 2016d; 2017). Table 4-1 presents a summary of information reported on the total manufacture and total import quantities for the Resins and Rosins Group.
| Abbreviation or Common name | Number of companies or facilities manufacturing | Total manufacturea (kg) | Number of companies or facilities importing | Total importsa (kg) |
|---|---|---|---|---|
| DHAA | 0 | NR | <4 | 100–1 000 |
| CTO | 4 | 10 000 000–100 000 000b | <4 | 10 000–100 000 |
| DTO | 0 | NR | 18 | 100 000–1 000 000 |
| TOP | 0 | NR | 4 | 100 000–1 000 000 |
| Storax | 0 | NR | <4 | <100 |
| Rosin (CAS RNs 8050-09-7, 8052-10-6, 73138-82-6c) | 0 | NR | 31 | 100 000–1 000 000 |
| RCa | <4 | 10 000–100 000 | 10 | 10 000–100 000 |
| RNa | <4 | 100–1 000 | 13 | 10 000–100 000 |
| RHME | 0 | NR | 6 | 10 000–100 000 |
| RME | 0 | NR | <4 | 100–1 000 |
| RMa | 0 | NR | 4 | 1 000–10 000 |
Abbreviations: NR, not reported above the 100 kg reporting threshold.
a Values reflect quantities reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2013). See surveys for specific inclusions and exclusions (Schedules 2 and 3). Values also reflect quantities reported from voluntary surveys (ECCC 2016c, 2016d and 2017).
b CTO manufacture is a result of incidental co-production.
c 4 companies reported importing 100-1 000 kg under this CAS RN only.
| Major usesa | CTO | DTO | TOP |
Rosin (CAS RNs 8050-09-7, 8052-10-6) |
|---|---|---|---|---|
| Lubricants and greases | N | Y | N | Y |
| Plastic and rubber | N | N | Y | Y |
| Agriculture | N | N | N | Y |
| Adhesives and sealants | N | Y | N | Y |
| Building or construction materials | N | Y | N | Y |
| Oil and natural gas extraction | N | Y | Y | N |
| Explosives | N | N | N | Y |
| Intermediate | Nb | Y | N | Y |
| Pigments | N | N | N | Y |
| Processing aids | N | Y | Y | Y |
| Plasticizer | N | Y | N | Y |
| Paints and coatings | N | Y | N | Y |
| Solvents | N | N | N | Y |
| Propellant | N | N | N | Y |
| Solder flux | N | N | N | Y |
| Water treatment | N | Y | N | N |
| Fabrics and textiles | N | Y | N | N |
| Pharmaceuticals | N | N | N | Y |
| Personal care | N | N | N | Y |
| Toys, playground and sporting equipment | N | N | N | Y |
| Food packaging | N | Y | N | N |
| Metal manufacturing | N | Y | N | N |
Abbreviations: N, this use was not reported for this substance; Y, this use was reported for this substance in 2011.
a Non-confidential uses reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2013). See surveys for specific inclusions and exclusions (Schedules 2 and 3). Also reflects uses quantities reported from voluntary surveys (ECCC 2016c, 2016d, 2017).
b CTO may be refined to produce other products including DTO, TOP and rosin which are also listed here (this activity is not known to occur currently in Canada).
| Major usesa | RCa | RNa | RHME | RMa |
|---|---|---|---|---|
| Lubricants and greases | N | N | Y | N |
| Plastics and rubber | Y | N | N | N |
| Adhesives and sealants | Y | Y | N | N |
| Building or construction materials | N | Y | N | Y |
| Pigments | Y | Y | N | N |
| Processing aids | N | N | Y | N |
| Plasticizers | N | Y | N | Y |
| Paints and coatings | Y | Y | N | Y |
| Odour agents | N | N | Y | N |
| Surface active agents | N | Y | N | N |
| Pest control | Y | N | N | N |
| Automotive care | N | N | Y | N |
| Laundry and dishwashing | N | N | Y | N |
| Cleaning and furnishing care | N | N | Y | N |
| Personal care | N | N | Y | N |
| Air care | N | N | Y | N |
| Apparel and footwear care | N | N | Y | N |
| Pet care | N | N | Y | N |
| Agricultural products | Y | N | N | N |
| Floor coverings | Y | N | N | N |
| Arts, crafts and hobby materials | Y | N | N | N |
Abbreviations: N, this use was not reported for this substance; Y, this use was reported for this substance in 2011.
a Non-confidential uses reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2013). See surveys for specific inclusions and exclusions (Schedules 2 and 3) Also reflects uses reported from voluntary surveys (ECCC 2016c, 2016d and 2017).
As described in section 2, CTO is a co-product of kraft pulping, which is an industrial activity in Canada. CTO produced as a co-product of kraft pulping is typically burned in a recovery boiler (Wising and Stuart 2006). In addition, the intermediate use of CTO is as a feedstock for refining into various downstream products including TOP, rosin and DTO. CTO refining is not known to occur currently in Canada, but downstream products of CTO are imported into Canada. CTO that is imported into Canada may have various industrial applications, including use as a raw material for oil and gas drilling applications (Georgia-Pacific 2018). In addition to the uses outlined in Table 4-1, TOP (CAS RN 8016-81-7) has been known to be used in corrosion inhibitors, coatings, as a rubber modifier, in cement and asphalt and minerals processing, as well as burned as a fuel (Zinkel and Russell 1989; Lesokhimik Trade House 2018). Products formulated with CTO or DTO that are available to consumers include cosmetics (up to 30%), adhesives and sealants (<10%), paints and coatings (5% to 30%), kitchen cleaners (1% to 10%) and degreasers (>5%) (COSING c2009-2017a; MSDS 2007, 2009, 2010, 2015a, 2015b, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 26, 2017; unreferenced).
DTO, rosin (primarily CAS RNs 8050-09-7 and 8052-10) and its derivatives RCa, RNa, RHME and RMa have a diverse number of industrial and consumer/commercial uses, as specified in Tables 4-2 and 4-3. In addition, some commonly known applications for DTO include use in the manufacture of certain materials and use in agricultural products, drilling muds, cement additive, washing fluids, metal working fluids, oilfield chemicals, soaps, cleaners, and alkyd resins (Pine Chemicals Group 2018; UCY Energy 2018). Rosin salts, RCa and RNa, and rosin derivative RHME were also reported to be manufactured in Canada at over 100 kg in 2011, as shown in Table 4-1. RCa and RNa are also used in paints and coatings.
Other reported Canadian uses for substances in the Resins and Rosins Group are presented in Tables 4-4 and 4-5.
| Use | DHAA | DTO | Storax |
Rosin (CAS RNs 8050-09-7, 73138-82-6) |
|---|---|---|---|---|
| Food Flavouring Agenta | N | N | Y | N |
| Food packaging materialsa | N | N | N | Y |
| Incidental Additivesa | N | N | N | Yf |
| Internal Drug Product Database as medicinal or non-medicinal ingredients in pharmaceutical, disinfectant or veterinary drug products in Canadab | N | N | Y | Y |
| Natural Health Products Ingredients Databasec | N | Y | Y | Y |
| Licensed Natural Health Products Database as medicinal or non-medicinal ingredients in natural health products (NHPs) in Canadac | N | N | Y | Y |
| List of Prohibited and Restricted Cosmetic Ingredientsd | N | N | N | N |
| Notified to be present in cosmetics, based on notifications submitted under the Cosmetic Regulations to Health Canadad | N | Y | Y | Y |
| Formulant in pest control products registered in Canadae | N | Y | Y | Y |
Abbreviations: Y = this use was reported for this substance; N = this use was not reported for this substance.
a Personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 4, 2017; unreferenced. While not defined under the Food and Drugs Act (FDA), incidental additives may be regarded, for administrative purposes, as those substances which are used in food processing plants and which may potentially become adventitious residues in foods (e.g.,. cleaners, sanitizers).
b Personal communication, email from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated May 31, 2017; unreferenced.
c Personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 1, 2017; unreferenced.
d Personal communication, email fromthe Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 26, 2017; unreferenced.
e Personal communication, email from the Pest Management Regulatory Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 29, 2017; unreferenced.
f Rosin (CAS RN 8050-09-7) may be used as a component in incidental additives used in food processing establishments with no direct food contact, therefore exposure is not expected. Rosin (CAS RN 73138-82-6) was not identified to be used as a component in incidental additives.
| Use | RHME | RCa | RNa | RME |
|---|---|---|---|---|
| Food packaging materialsa | Y | Y | Y | N |
| Internal Drug Product Database as medicinal or non-medicinal ingredients in pharmaceutical, disinfectant or veterinary drug products in Canadab | Y | N | N | N |
| Natural Health Products Ingredients Databasec | Y | N | N | Y |
| Licensed Natural Health Products Database as medicinal or non-medicinal ingredients in NHPs in Canadac | Y | N | N | Y |
| List of Prohibited and Restricted Cosmetic Ingredientsd | N | N | N | N |
| Notified to be present in cosmetics, based on notifications submitted under the Cosmetic Regulations to Health Canadad | Y | N | N | N |
| Formulant in pest control products registered in Canadae | Y | Y | Y | Y |
Abbreviations: Y = this use was reported for this substance; N = this use was not reported for this substance.
a Personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 4, 2017; unreferenced.
b Personal communication, email from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated May 31, 2017; unreferenced.
c Personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 1, 2017; unreferenced.
d Personal communication, email fromthe Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 26, 2017; unreferenced.
e Personal communication, email from the Pest Management Regulatory Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 29, 2017; unreferenced.
5. Releases to the environment
According to an analysis of sources and use information along with relevant monitoring data (ECCC 2021a), the major sources of emissions for substances in the Resins and Rosins Group are related to industrial activities in Canada. Releases of concern occur primarily to surface water where certain components may transfer to sediments via partitioning from overlying water, along with releases to soils via wastewater treatment systemFootnote 5 biosolids amendment. However, soil amendment using biosolids from pulp and paper mills is not a common practice, and there are limited contributions to collective biosolids from a small number of facilities in other sectors, which suggests that soils are less important to the evaluation of environmental risk than the aquatic environment for the Resins and Rosins Group. Significant components found in CTO, DTO, TOP, rosin, RCa, RNa, RMa and RHME are also naturally occurring in terrestrial (e.g., plants and soil) and aquatic environments (e.g., lakes, streams) due to natural processes. Releases to air or transfers to air from other environmental media are not considered significant for Resins and Rosins Group substances based on the evaluation of the physical-chemical properties of the representative chemicals together with consideration of the major industrial uses and use volumes of these substances.
Despite recovery measures in place, CTO may be released to water from kraft pulping facilities in Canada, such as through spills. Releases of other substances in the Resins and Rosins Group to water may occur from RCa manufacturing and industrial uses of CTO, DTO, rosin, RNa, TOP and RMa.
6. Environmental fate and behaviour
The fate, persistence and bioaccumulation potential of the Resins and Rosins Group is characterized using empirical and/or modelled data for the suite of 12 representative chemicals (see Table 2-2) along with some available empirical whole substance biodegradation data where applicable. Given the natural occurrence of a number of the components of the UVCBs in this screening assessment it is important to note that fate, persistence, and bioaccumulation must be interpreted in the context of these components occurring naturally and being released via natural processes (e.g., from the decomposition of vegetation), resulting in near continuous background exposure in many aquatic and terrestrial environments.
6.1 Environmental distribution
Level III fugacity-based Equilibrium Criterion Model (New EQC 2011) results based on modelling of representative chemicals are presented in Table 6-1 for each of the substances. The detailed representative chemical-based media-partitioning information is available in a supporting document (ECCC 2021b). Once released to the environment, substances in the Resins and Rosins Group will tend to partition to water and soil (depending on the compartment in which they are released), with lesser amounts also partitioning to sediment from water and negligible amounts to air. No significant direct releases to air are expected for the substances in this group (see section 5). This, along with the relatively low log Kaw values for most representative chemicals in this group, suggest that exposure in this medium is not significant.
Given their pKa values of ≥4.8 (see Tables 3-1 to 3-6), it is expected that certain substances and representative chemicals in the Resins and Rosins Group, especially the resin acids, will ionize within an environmentally relevant pH range (6 to 9). Components containing carboxylic acid functional groups (pKa ≤6) will be present primarily in ionized form, while components containing alcohol functional groups (pKa >9) will be present primarily in the neutral form at an environmentally relevant pH range (6 to 9). Although the input parameters that account for this ionization (e.g., log D vs. log Kow) are used in the New EQC modelling, some of the potential interactions with solids (suspended solids or sediment) may not be predictable. Many solid particles, including sediment in the environment, may be negatively charged so the freely available fraction of a chemical to which organisms are exposed could be greater for anionic chemicals (charge repulsion), such as carboxylate ion groups, than for neutral chemicals. However, there are many empirical studies that show that resin acids (e.g., representative chemicals abietic acid, DHAA, IPA, etc.) may accumulate in sediments of waters receiving industrial (largely pulp and paper) effluent (Meriläinen et al. 2006; Leppänen et al. 2000; Leppänen and Oikari 2001) despite the relatively low percentages (≤1%) of these components predicted to partition to sediments (ECCC 2021b). Some uncertainty therefore exists despite EQC results and additional ionization considerations.
| CTO Substance released to: |
Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | 1–80 | 2–15 | 17–96 | 0–2 |
| Water (100%) | 0 | 51–99 | 0–1 | 1–48 |
| Soil (100%) | 0 | 0–6 | 94–100 | 0 |
| DTO Substance released to: |
Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | 3-48 | 4-15 | 37-92 | 0–2 |
| Water (100%) | 0 | 84–99 | 0 | 1–16 |
| Soil (100%) | 0 | 0–6 | 94–100 | 0 |
| TOP Substance released to: |
Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | 1–48 | 2–15 | 37–96 | 0–1 |
| Water (100%) | 0 | 51–99 | 0–1 | 1–48 |
| Soil (100%) | 0 | 0–5 | 95–100 | 0 |
| Rosin (CAS RNs 8050-09-7 / 8052-10-6 / 73138-82-6) and RCa and RNa Substance released to: |
Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | 3–48a | 9–15a | 37–87a | 0a |
| Water (100%) | 0a | 99a | 0a | 1a |
| Soil (100%) | 0a | 0–6a | 94–97a | 0a |
| RHME Substance released to: |
Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | 3–35 | 1–9 | 59–87 | 0–4 |
| Water (100%) | 0–1 | 31–99 | 0–1 | 1–67 |
| Soil (100%) | 0 | 0–6 | 94–100 | 0 |
a Values do not account for dissociation of salts (RCa and RNa).
| RMa Substance released to: |
Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | 0-48a | 9–28a | 37–87a | 0a |
| Water (100%) | 0a | 99–100a | 0a | 0–1a |
| Soil (100%) | 0a | 5–25a | 75–95a | 0a |
a Takes into account hydrolysis product of MPA.
Although no significant releases to soil are expected, most of the substances in the Resins and Rosins Group will stay in soil if released to this medium. However, some of the RMa components will also partition into water from soil.
6.2 Environmental persistence
Abiotic degradation
Given the importance of aqueous media, the hydrolysis rates for the substances that may have representative chemicals with hydrolysable groups, such as methyl esters (e.g., components of RHME) and succinic anhydride functional groups of MPA (e.g., component of RMa), are estimated using HYDROWIN 2010. Representative chemicals of THAME and DHAME are expected to show hydrolysis half-lives of >10 years based on estimation for cyclohexyl methyl ester available in HYDROWIN 2010. Hydrolysis rates for the succinic anhydride functional group associated with the MPA representative chemical of RMa are estimated to be 4.3 min. Considering such quick hydrolysis, it is assumed that the dicarboxylic acid hydrolysis products shown in Table 2-2 for MPA would be the main component associated with MPA in the environment.
Biodegradation
CTO and DTO have a complex composition, and some components are known to be easily biodegraded, such as linoleic acid (representing 41% and 65% of CTO and DTO, respectively), which has been shown to pass the criteria for ready biodegradation (70% empirical biodegradation in 28-days) in water via the modified MITI test I protocol (TG 301 C)(J-CHECK c2010-). No empirical biodegradation data exist for any other representative chemicals used in the assessment of the UVCB substances in the Resins and Rosins Group. However, aerobic biological wastewater treatment systems have been shown to reduce resin acid concentrations (notably for representative chemicals abietic acid, IPA and DHAA) from pulp and paper mill effluent (MacLeay and Associates Ltd. 1986; Liss et al. 1997; Sturthridge et al. 1991; Kostamo et al. 2004), although a considerable amount of this removal may be attributed to sorption to sludge. The performance of aerobic biological treatment systems in degrading resin acids is greatly influenced by the variation in effluent component composition, nutrient availability, and the status of the microbial community. Given the inhibiting properties of some resin acid components, biodegradation of resin acid mixtures can experience a lag period of variable duration (Hemingway and Greaves 1973). Pimaric-type resin acids such as isopimaric acid (IPA) are observed to be less readily removed than abietic-type resin acids such as abietic acid or dehydroabietic acid because of the presence of the vinyl group (Liss et al. 1997). While biodegradation occurs in the natural environment, these rates are often slow, and only a few bacteria are able to use resin acids as a sole carbon source (Liss et al. 1997). Lastly, it is important to note that a well-characterized stable resin acid metabolite, called retene, is known to be the major product of biodegradation from resin acids in an anaerobic environment (Tavendale et al. 1997; Leppänen et al. 2000), such as those found in certain benthic environments.
Dykstra et al. (2014) showed that biodegradation rates of phytosterols treated with a mixed culture developed from a pulp and paper wastewater treatment system are limited (i.e., <20% decrease in chemical oxygen demand or COD in 26 days) by their limited solubility (e.g., β-sitosterol water solubility estimated to be 7.6 x 10-7 mg/L). In addition, this study also suggests that when the solubility of phytosterols is enhanced, there may still be a significantly slower degradation period of about 7 days, followed by a period of more rapid degradation, suggesting that the induction of enzymes may be required for microorganisms to biodegrade this component.
Table 6-2 summarizes the key data regarding the biodegradation of substances in the Resins and Rosins Group based on the available whole substance empirical data and/or empirical or modelled representative chemical data in ranges. Detailed representative chemical-based modelled biodegradation information may be found in a supporting document (ECCC 2021b). Given the paucity of empirical biodegradation data for RCa, empirical biodegradation results for RNa, rosin calcium/zinc and rosin magnesium (CAS RNs 68334-35-0 and 68440-56-2), are used as read-across data to RCa. All of these analogues showed a similar water solubility to that of RCa (43 mg/L; see Table 3-6), as rosin calcium/zinc has a water solubility of 18 mg/L and rosin magnesium has a water solubility of 65 mg/L (ECHA c2007-2017).
| Abbreviation or Common name | Test conditions | Degradation endpoint or prediction (28 days) | t1/2 (days) | Reference |
|---|---|---|---|---|
| CTO | Empirical OECD 301 F of whole substance | 79%–83% | NA | ECHA c2007-2017 |
| CTO | Modelled OECD 301 B, C of components | 0%–97% | 6–960a | CATALOGIC 2016 |
| DTO | Empirical OECD 301 F, E and D of whole substance | 60%-73% | NA | ECHA c2007-2017 |
| DTO | Modelled OECD 301 B, C of components | 0%–97% | 6–960a | CATALOGIC 2016 |
| TOP | Empirical OECD 301 B, D of whole substance | 9%–36% | NA | ECHA c2007-2017 |
| TOP | Modelled OECD 301 B, C of components | 5%–97% | 6–233a | CATALOGIC 2016 |
| Rosin | Empirical OECD 301 B | 14%–64% | NA | ECHA c2007-2017 |
| Rosin | Modelled OECD 301 B, C of components | 0%–22% | 78–960a | CATALOGIC 2016 |
| RCa | Empirical OECD 301 B, D of whole substance | 71%–89%c | NA | ECHA c2007-2017 |
| RCa | Modelled OECD 301 B, C of componentsd | 0%–22% | 78–960a | CATALOGIC 2016 |
| RNa | Empirical OECD 301 D of whole substance | 71% | NA | ECHA c2007-2017 |
| RNa | Modelled OECD 301 B, C of componentsd | 0%–22% | 78–960a | CATALOGIC 2016 |
| RHME | Empirical OECD 301 B, D of whole substance | 18%–40% | NA | ECHA c2007-2017 |
| RHME | Modelled OECD 301 B, C of components | 0%–8% | 233–960a | CATALOGIC 2016 |
| RMab | Empirical OECD 301 B of whole substance | 0.34% | NA | ECHA c2007-2017 |
| RMab | Modelled OECD 301 B, C of components | 0%–19% | 233–960a | CATALOGIC 2016 |
Abbreviations: NA, not available.
a Greater than 182 days, suggesting a high environmental persistence is likely.
b Including hydrolysis products.
c Analogue information for Na, Ca/Zn and Mg salts.
d Components of rosin (does not take salt into account).
The results of standard biodegradation tests (e.g., OECD Test Guideline 301 and 302 series) are considered in a weight-of-evidence approach along with modelled or empirical results available for representative chemicals of each UVCB to determine the environmental persistence of each substance in this group. CTO and DTO have a range of highly biodegradable components (such as fatty acids which are represented by linoleic acid), while still containing some moderate to highly persistent components such as resin acids (e.g., DHAA, abietic acid), alcohols (e.g., abietinol) and aldehydes (e.g., abietinal). Empirical evidence suggests that CTO component phytosterols (e.g., β-sitosterol) may be poorly degraded because of a low solubility, despite estimated biodegradation half-lives of <182 days (see Table 6-2). The more recalcitrant components of CTO and DTO will largely reside in water and sediment.
TOP has shown a lower potential for biodegradation and contains a high proportion of highly persistent components, including resin acids (e.g., abietic acid) and phystosterols (e.g., β-sitosterol). The more recalcitrant components of TOP will largely reside in water and sediment.
Rosin has moderate persistence, and recalcitrant components of rosin will largely reside in water and sediment. Whole substance empirical information shows that calcium and sodium salts of rosin (RCa and RNa) have low to moderate persistence, likely due to higher solubility and thus enhanced bioavailability associated with the salt forms of rosin compared with the neutral form. Components with higher predicted persistence in water, such as resin acids, are also present in these substances. However, there is additional uncertainty about the persistence of components of RCa and RNa in water as water solubility, bioavailability and biodegradability may vary depending on the pH of the receiving environment for resin acids. Lastly, the more recalcitrant components of RCa and RNa are predicted to largely reside in water and sediment. Given the higher persistence of these substances, the long-range transport distance of abietic acid (the major representative chemical in rosin, RNa and RCa) in water was estimated using TaPL3 (TaPL3, 2003) as an extra line of evidence. TaPL3 uses a multi-media fugacity based model to evaluate a chemical’s potential for long-range transport (LRT) in a mobile medium (either air or water). The LRT value calculated for abietic acid in water was 4 500 km, suggesting an increased potential for the spatial distribution of exposure for rosin, RNa and RCa.
Components of CTO, DTO, TOP, rosin, RCa and RNa are known to partition to sediment from water. As mentioned in section 5, soil is expected to be less important as a medium for exposure for the Resins and Rosins Group. Given their persistence in water, as already discussed and using an extrapolation ratio of 1:1:4 for a water:soil:sediment biodegradation (Boethling et al. 1995), components of CTO, DTO, TOP, rosin, RCa and RNa are expected to be moderately to highly persistent in sediments (and soil).
The representative chemicals of RHME are predicted to have high persistence in both water (e.g., 0% to 8% degradation of components based on modelled data) and sediment based on modelled evidence. However, whole substance empirical data shows that a significant fraction (18% to 40%) of this substance is available for ready biodegradation in a 28-day test, suggesting that the modelling results may be over-predicting environmental persistence in this case.
RMa is very persistent in water based on empirical biodegradation data (28-day biodegradation of 0.34%). Modelling of RMa representative chemicals supports this given that all representative chemicals, including the hydrolysis productss of MPA, show biodegradation half-lives >182 days in water (ECCC 2021b).
Since no direct releases to air or transfers to air from other environmental media are expected for this group (see section 5), metrics for persistence in air have not been evaluated.
6.3 Potential for bioaccumulation
Experimental data on the bioconcentration of representative chemical resin acids (e.g., abietic acid, IPA and DHAA) in rainbow trout showed steady-state bioconcentration factors (BCFs) ranging from <25 to 130 L/kg (wet weight) at exposure concentrations of 0.7 to 3 µg/L at ~15°C and pH ~8 for 20 days (Niimi and Lee 1992). In addition, the metabolic half-lives (t1/2) of these acids are reported as less than 4 days. There is empirical evidence showing that resin acids are taken up in fish primarily via the gills into the blood stream, are converted to glucuronide conjugates in the liver, and are then excreted through the bile (Oikari et al. 1984; Oikari and Holmbom 1985). In addition, a decarboxylated resin acid degradation product called fichtelite (CAS RN 2221-95-6) was shown in a freshwater mussel study to have a BCF of at least an order of magnitude greater than that of the parent resin acids (Burggraaf et al. 1996). The results of this study suggest that metabolites of resin acids may increase the overall body burden and associated effect of narcosis of resin acid exposed organisms.
Table 6-3 summarizes the modelled component based data regarding the bioconcentration and bioaccumulation of the substances in the Resins and Rosins Group in aquatic organisms. Representative chemical specific modelled bioaccumulation information can be found in a supporting document (ECCC 2021b).
| Common name | log Dowa/Kow | Metabolic t1/2 (days)b | BCF/BAF (L/kg) | Reference |
|---|---|---|---|---|
| CTO | 3.2–7.00 | 2–111 | 3–3210 (BCF) | BCFBAF 2010 (regression-based estimate) |
| DTO | 3.2-5.8 | 2-10 | 3-1780 (BCF) | BCFBAF 2010 (regression-based estimate) |
| CTO | 3.2–7.00 | 2–111 | 13–1585 (BCF) | CATALOGIC 2016 |
| DTO | 3.2-5.8 | 2-10 | 447-1585 (BCF) | CATALOGIC 2016 |
| CTO | 3.2–7.00 | 2–111 | 125–1.6 x 106 (BAF) | BCFBAF 2010 (Arnot-Gobas upper trophic) |
| DTO | 3.2-5.8 | 2-10 | 125-6270 (BAF) | BCFBAF 2010 (Arnot-Gobas upper trophic) |
| TOP | 3.3–8.3 | 5–111 | 3–3210 (BCF) | BCFBAF 2010 (regression-based estimate) |
| TOP | 3.3–8.3 | 5–111 | 13–1318 (BCF) | CATALOGIC 2016 |
| TOP | 3.3–8.3 | 5–111 | 212–1.6 x 106 (BAF) | BCFBAF 2010 (Arnot-Gobas upper trophic) |
| Rosin, RCa and RNa | 3.2–5.8 | 2–6 | 3–132 (BCF) | BCFBAF 2010 (regression-based estimate) |
| Rosin, RCa and RNa | 3.2–5.8 | 2–6 | 447–1585 (BCF) | CATALOGIC 2016 |
| Rosin, RCa and RNa | 3.2–5.8 | 2–6 | 125–519 (BAF) | BCFBAF 2010 (Arnot-Gobas upper trophic) |
| RHME | 4.8–6.6 | 1–7 | 132–1.1 x 104 (BCF) | BCFBAF 2010 (regression-based estimate) |
| RHME | 4.8–6.6 | 1–7 | 447–5754 (BCF) | CATALOGIC 2016 |
| RHME | 4.8–6.6 | 1–7 | 125–1.1x104 (BAF) | BCFBAF 2010 (Arnot-Gobas upper trophic) |
| RMac | 1.7–3.6 | 2–12 | 3–132 (BCF) | BCFBAF 2010 (regression-based estimate) |
| RMac | 1.7–3.6 | 2–12 | 10–1318 (BCF) | CATALOGIC 2016 |
| RMac | 1.7–3.6 | 2–12 | 3–413 (BAF) | BCFBAF 2010 (Arnot-Gobas upper trophic) |
Abbreviations: Dow, octanol-water partitioning coefficient for ionized organic chemicals; Kow, octanol-water partitioning coefficient for neutral chemicals. Metabolic T1/2, metabolic half-lives; BCF, bioconcentration factor; BAF, Bioaccumulation Factor.
a log Dow is used for BCF/BAF estimate if greater than 50% of representative chemical is predicted to be ionized at pH 6-8.
b Estimated using BCFBAF 2010 normalized to 10 gram fish.
c Including hydrolysis products.
Generally, Table 6-3 shows that CTO, DTO, TOP, rosin, RCa, RNa and RMa have representative chemicals with low to moderate bioconcentration potential based on modelling of their representative chemicals. The BCFs of components of RHME show moderate to high bioconcentration potential, with THAME having the highest predicted BCF.
With respect to bioaccumulation, the THAME representative chemical, which represents 75% of the RHME substance, is predicted to have high bioaccumulation potential by all three models (see Table 6-3). Representative chemical-specific modelled bioaccumulation information can be found in a supporting document (ECCC 2021b). Given its high bioavailability, the uptake rate would likely be rapid for THAME, with a log Kow of 6.6 being quite close to optimal for bioavailability. However, in general, esters are known to be quite quickly hydrolyzed into their corresponding acids (THA in this case), which, in turn, are known to have a much lower bioaccumulation potential. The estimated metabolic half-life for THAME was approximately 7 days, corresponding to a metabolic rate of (Km) 0.1 / day. The metabolic rate of most classes of esters, including a number of benzenedicarboxylic esters among several other classes, are empirically known to be even faster than this prediction indicates (Arnot et al. 2008). However, resin acid esters, including those in RHME are known to be recalcitrant to abiotic hydrolysis under even strong alkaline conditions (Holmbom and Ekman 1978). Thus, some uncertainty exists in the overall bioaccumulation prediction for THAME due the uncertainty in metabolic rate (Km).
Certain CTO and DTO representative chemicals, including β-sitosterol (CTO only), abietinal and to some extent abietinol, are predicted to have a high bioaccumulation potential based on modelled BAF results. These components are predicted to have relatively slow biotransformation half-lives (111, 17 and 10 days, for β-sitosterol, abietinal and abietinol respectively). Given the large discrepancy in BCFs between the BCFBAF (2010) estimate and that by CATALOGIC (2016) for abietinal (1290 vs 17 L/kg) and β-sitosterol (3210 vs 13 L/kg), there is some uncertainty and possible overestimation in the bioconcentration and bioaccumulation estimates (e.g., 1.6 x 106 L/kg for β-sitosterol) using BCFBAF (2010). In this regard, it is important to note that mitigating factors such as metabolic rate and effect of molecular size (Deff = 10.3 nm for β-sitosterol) are taken into account in the BCF estimate from CATALOGIC (2016). Nevertheless, it is expected that the bioaccumulation potential for β-sitosterol may still be high. In addition, a recent study has shown that certain diterpene components (sclarene, abieta-7,13-diene, dehydroabietane, norabietatetraene, tetrahydroretene, simonellite and retene), some of which may be present in the neutrals fraction of CTO and possibly also in DTO (uncertainy exists in the characterization of the neutrals fraction in DTO), may show a high biota-sediment accumulation based on empirical field studies with intertidal clams (Yunker et al. 2011).
Representative chemical resin acids (e.g., abietic acid, IPA and DHAA) show a limited bioaccumulation potential based on both modelled BCF and BAF values and empirical BCF studies in mussels and fish at an environmentally relevant pH range (6 to 8). This includes the representative chemical resin acid esters THAME and DHAME once they are hydrolyzed within the organism. Lastly, the representative chemical linoleic acid shows a moderate bioaccumulation potential (BAF ~ 2500) and makes up a significant proportion of CTO and DTO (41% and 65%).
7. Potential to cause ecological harm
7.1 Ecological effects assessment
7.1.1 Mode/mechanism of action
The analysis of their representative chemicals shows that CTO, DTO, TOP, rosin, RCa, RNa and RMa all consist of components that could have non-specific (e.g., narcotic) or compound-specific effects (ECCC 2021b). Because of the significant presence of specifically acting components that would likely dominate the effects profile of the whole substance, CTO, DTO, TOP, rosin, RCa, RNa and RMa are considered specifically acting substances as a whole. The analysis of the representative chemicals of RHME shows that it consists of only narcotic components and is thus considered a narcotic substance (ECCC 2021b). Determination of mode of action (MoA) is based on predictions from the United States Environmental Protection Agency (US EPA)’s ASTER, TEST and the OASIS MoA profiler in OECD toolbox (ASTER 1999; TEST 2016; OECD QSAR Toolbox 2016). In addition, for the representative chemical β-sitosterol, a number of low dose sub-lethal effects related to reproduction and endocrine function have been documented, including atrophy of the albumen gland in a European snail at a no observed effect concentration (NOEC) of 0.0001 mg/L (Czech et al. 2001), reductions in plasma sex steroids,an increase in vitellogenin in rainbow trout at NOEC of <0.075 mg/L (Tremblay and Van Der Kraak 1999), and reduction in plasma male and female sex steroid levels with a NOEC of 0.01 mg/L (MacLatchy and Van Der Kraak 1995) among others. Despite not being directly tied to adverse outcomes in test organisms, these low-level effects support the prediction that β-sitosterol is a specifically acting component.
7.1.2 Effects on aquatic organisms
Water-accommodated fraction (WAF) studies are sometimes used for toxicity testing for poorly soluble UVCBs (OECD 2000) and are available for many of the UVCBs subject to this assessment. A WAF is an aqueous fraction containing the dissolved, suspended and/or emulsified fraction of a poorly water soluble UVCB that can be used in aquatic toxicity testing with fish, daphnia, or algae. The main advantage of this test is that the observed aquatic toxicity reflects the multi-component dissolution behaviour of the components for a given substance-to-water loading. Ultimately, the interpretation of the results should consider both the reliability of the test based on relevant OECD 23 guidance (OECD 2018) and how closely the exposure conditions in the WAF study match the exposure scenario of the risk assessment. Often, there is concern about exposure to a substance in the environment after wastewater treatment. In such cases, a WAF value may be of lower relevance, since components may degrade or partition to solids once released in the environment, and the composition of the original UVCB to which organisms may ultimately be exposed in the environment would very likely not match that of the same substance in the WAF test. Due primarily to this limitation, the analysis of ecological effects, exposure and risk for substances in the Resins and Rosins Group is based on empirical and modelled component toxicity data, with WAF data used as an additional supporting line of evidence in certain cases.
A detailed summary of the available modelled and empirical aquatic toxicity data for components of substances in the Resins and Rosins Group, along with available WAF data, is summarized in ECCC (2021b). The lowest reliable endpoint standardized value for all categories of indicator organisms are used as the critical toxicity values (CTVs) for predicted no/effects concentration (PNEC) derivation (Table 7-1). Empirical data are available for use as CTVs for abietic acid, β-sitosterol, DHAA and IPA from the peer-reviewed literature (Peng and Roberts 2000; Lehtinen et al. 1999). Despite the availability of empirical data on sub-lethal effects data for β-sitosterol illustrating effects at lower concentrations than those seen in Lehtinen et al. (1999), the reported endpoint were not conclusively linked to an adverse outcome in the organism (e.g., Czech et al. 2001). The reliabilities of the empirical endpoint values selected as CTVs are considered in robust study summaries (ECCC 2021b).
A number of studies have investigated the impact of pulp and paper discharges, including components of CTO, on the receiving environment and fish (e.g., Borton et al. 2004; Liss et al. 1997; Oikari et al. 1984; Rogers et al. 1975). Many of these studies show adverse effects focusing on endocrine and reproductive effects. However, the exposures in these studies were due to whole effluent discharges and not confined to the impact of CTO co-production from tall oil soap and thus are not considered directly in the evaluation of the effects data for Resins and Rosins Group substances.
Various modelled endpoint are used as CTVs when reliable empirical data are not available. ACD/Percepta c1997-2012 uses GALAS (Global, Adjusted Locally According to Similarity) modelling methodology to predict LC50 values and associated reliability indices (RI) that provide an estimate of the prediction accuracy (all values cited in Table 7-1 showed a RI > 0.3 and thus are reliable based on the evaluation of prediction confidence recommended by the model developers). Those endpoints that showed borderline reliability (RI = 0.3-0.5) are selected as CTVs if these are also supported by consensus with at least one other model. US EPA’s TEST results are based on a sub-model consensus method which takes the average of predicted toxicities from 5 different QSAR methods (e.g., group contribution, nearest neighbour, etc.), provided that the individual predictions are within the respective applicability domains. Lastly, the Artificial Intelligence Expert Predictive System (AIEPS) model for acute fish (fathead minnow) toxicity is used in CTV co-selection for the representative chemical THAME (AIEPs c2010-2012).
| Common name | Test organism | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Abietic acid | Water flea (Daphnia magna) | 48 h LC50 empirical | 0.68 | Peng and Roberts 2000 |
| Abietinol | Water flea (Daphnia magna) | Acute LC50 modelled | 0.21 | ACD/Percepta c1997-2012 |
| Abietinal | Fathead minnow (Pimephales promelas) | Acute LC50 modelled | 0.20 |
ACD/Percepta c1997-2012 TEST 2016 |
| β-Sitosterol | Brown trout (Salmo trutta lacustris L.) | LOEC mortality of eggs | 0.02 | Lehtinen et al. 1999 |
| DHAA | Water flea (Daphnia magna) | 48 h LC50 empirical | 1.3 | Peng and Roberts 2000 |
| DHAME | Water flea (Daphnia magna) | Acute LC50 modelled | 0.26 | ACD/Percepta c1997-2012 |
| IPA | Water flea (Daphnia magna) | 48 h LC50 empirical | 0.07 | Peng and Roberts 2000 |
| Linoleic acid | Water flea (Daphnia magna) | 48 h LC50 modelled | 0.87 | TEST 2016 |
| THAME | Water flea (Daphnia magna) | Acute LC50 modelled | 0.50 |
ACD/Percepta c1997-2012 AIEPs c2010-2012 |
| MPA hydrolysis product | Fathead minnow (Pimephales promelas) | 96 h LC50 modelled | 0.24 | TEST 2016 |
Abbreviations: LC50, median lethal concentration; LOEC, lowest observed effects concentration.
The lowest reliable WAF endpoint for substances in the Resins and Rosins Group for CTO are included as a line of evidence supporting data on components showing a high level of toxicity. Studies completed using CTO showed a range of reported acute median lethal loading (LL50) values (ECCC 2021b). The lowest value (LL50 = 20 mg/L) is determined for zebrafish (Danio rerio) from two studies using CTO (CAS RN 8002-26-4) in a semi-static renewal exposure system (based on OECD Test No. 203: Fish, Acute Toxicity Test). Linoleic acid and abietic acid are two of the representative chemicals used in this assessment. They were measured in test solution of these WAF tests at 0 h, 24 h, 72 h and 96 h for all CTO loadings (i.e., control, 12.5, 50 and 100 mg/L). Abietic acid was the only representative chemical detected and was found at 0.2 to 0.5 mg/L, with a slight increase with increased loading (ECHA c2007-2017). Considering that abietic acid may be present at 20% in CTO, these concentrations are much lower than expected based on CTO loading values (i.e., at CTO loading of 100 mg/L, abietic acid concentrations of up to 10 mg/L might be expected, which is still lower than the adjusted subcooled water solubility limit of 153 mg/L). Given that abietic acid is one of many potential components of CTO (i.e., several others remained unmonitored or undetected during this WAF test), these WAF results are difficult to interpret from a quantitative environmental fate and risk perspective.
As mentioned previously the stable resin acid degradation product retene (CAS RN 483-65-8), a polycyclic aromatic hydrocarbon (PAH), may accumulate in sediments. Retene is known to cause teratogenic effects in fish larvae and induction of cytochrome P450 enzymes (Oikari et al. 2002). This may add to the ecological effects caused by resin acids.
Assessment factors (AFs) are used to derive PNECs by dividing CTVs by appropriate AFs. The total AF for each representative structure is then calculated as the product of an applicable endpoint standardization factor (FES), mode of action factor (FMoA) and species variation factor (FSV). Specific assessment factors used in each case may be found in ECCC (2021b).
PNECs (summarized below in Table 7-2) for representative chemicals of CTO, DTO, TOP, Rosin, RHME, RMa, RCa and RNa ranged from 0.0007 to 0.13 mg/L.
| Common name | Component of | PNEC (mg/L) |
|---|---|---|
| Abietic acid | CTO, DTO, TOP, Rosin, RNa, RCa, RMa | 0.03 |
| Abietinol | CTO, DTO | 0.01 |
| Abietinal | CTO | 0.002 |
| β-Sitosterol | CTO, TOP | 0.002 |
| DHAA | CTO, DTO, Rosin, RNa, RCa, RMa, RHME | 0.13 |
| DHAME | RHME | 0.01 |
| IPA | CTO, DTO, Rosin, RNa, RCa | 0.0007 |
| Linoleic acid | CTO, DTO, TOP | 0.04 |
| THAME | RHME | 0.01 |
| MPA hydrolysis product | RMa | 0.001 |
7.2 Ecological exposure assessment
Major scenarios considered in the exposure analysis of substances in the Resins and Rosins Group include co-production of CTO at kraft pulp mills and other facilities, manufacture of RCa, and various industrial uses of all the substances. Important functions of the Resins and Rosins Group in these uses include as a plasticizer, surfactant, viscosity adjustor, solvent, filler, and odour agent. The environmental releases of the substances in the Resins and Rosins Group are expected to occur mainly in the form of wastewater treatment system effluents according to their use patterns. Their predicted environmental concentrations (PECs) in receiving waters are estimated for the major scenarios identified. These PECs are used for risk characterization by comparing them with PNECs.
7.2.1 Calculation of aquatic PECs and general assumptions
Wastewater treatment system removal is a key parameter in all exposure calculations presented in sections 7.2.3 to 7.2.6. The estimation results for representative chemicals of the Resins and Rosins Group ranged from 15% for MPA hydrolysis product (a representative chemical for RMa) to 92% for β-sitosterol (a representative chemical for CTO and TOP).
Three quantitative scenario categories are described below in sections 7.2.3 to 7.2.5 and the remaining qualitative scenarios are discussed in section 7.2.6.
7.2.2 CTO co-production scenario
The PEC of each representative chemical of CTO in the receiving water is estimated from the amount of each representative chemical released to the wastewater treatment effluent, the effluent volume and the receiving water’s dilution of kraft pulping mills in Canada. The PECs of each CTO representative chemical near the discharge point are calculated by:
Where,
PECx: predicted environmental concentration of representative structure X in receiving water near discharge point, µg/L
X: mass fraction of a representative structure in crude tall oil, unitless
m: crude tall oil yield, kg/tonne of pulp
E: emission factor to wastewater treatment, unitless
Rx: wastewater treatment removal for representative chemical X, unitless
f: wastewater treatment effluent generation rate, L/tonne of pulp
D: receiving water dilution factor near discharge point, unitless
109: conversion factor from kg to µg, µg, mg/kg
The emission factor (E) was estimated based on a CTO release pathway identified in two site visits conducted by ECCC in 2019. The first visit found that: 1) the CTO-containing spent acid from CTO production as a co-product was concentrated in evaporators before being combusted for chemical recovery; 2) evaporators’ condensates were grouped in two categories: clean and foul; and 3) clean condensates were used in a wash cycle before being sent to the on-site wastewater treatment system, and foul condensates were collected and treated in a steam stripper to remove volatile compounds and then used in a wash cycle before being sent to the on-site wastewater treatment system (personal communications, from a Canadian forest product company to the Ecological Assessment Division, ECCC, December 2019 and January 2021, unreferenced). The information gathered from the second visit was essentially the same as the first one with respect to the treatment of the CTO-containing spent acid, the use of clean condensates, and the fate of foul condensates except for a minor difference. The difference was that foul condensates were either pumped back to weak black liquor tanks (which feed to evaporators) or treated in a steam stripper and the steam-stripped foul condensates were sent to the on-site wastewater treatment system (personal communications, from Cariboo Pulp & Paper Co., to the Ecological Assessment Division, ECCC, December 2019 and January 2021, unreferenced).Based on the information collected from the two visits, a CTO release pathway was identified as spent acid – clean and steam-stripped foul condensates with or without subsequent use in a wash cycle – on-site wastewater treatment – receiving water. The following exposure analysis was focused on foul condensates because relevant data is available while clean condensates were not considered.
Resin acids are key CTO components. They were reported in foul condensates at 28-148 mg/L (Blackwell 1978). The minimum concentration in the range (28 mg/L resin acids) was selected to provide a baseline for the resulting PECs. The concentration of CTO in foul condensates was estimated to be 100 mg/L by dividing the resin acids concentration of foul condensates by their proportion (~28%) reported in Canadian CTO (Huibers 2000). The 28% proportion is approximately equivalent to the sum (30%) allocated for CTO’s three representative resin acids (abietic acid, DHAA and IPA) (Table 2-2). Mill condensate data obtained for a representative Canadian kraft pulping mill showed that mills generate approximately 11.6 m3 (11 600 L) of total condensates per tonne of pulp while foul condensates accounted for 10% of the total generated condensate (Berube and Hall 1999). Thus, the amount of CTO in foul condensates was estimated as 0.116 kg of CTO per tonne of pulp by multiplying the estimated CTO concentration in foul condensates (100 mg/L) by the generation rate of foul condensates (10% of 11 600 L or 1 160 L/tonne of pulp). This per tonne of pulp based amount was assumed to enter an on-site wastewater treatment system. In other words, the CTO loss is assumed to be negligible when foul condensates are treated in a steam stripper or used in a wash cycle before entering an on-site wastewater treatment system. The rationale for this assumption is that no particular removal mechanism is available for CTO during steam stripping or washing operations.
The emission factor was then calculated as the ratio of the CTO in foul condensates to the CTO production yield. CTO yields for Canadian kraft pulping mills were reported to ECCC in the range of 2-25 kg CTO/tonne of pulp (personal communication, from FPAC/NCASI, to the Ecological Assessment Division, ECCC, August 2019, unreferenced). Their average was 13 kg CTO/tonne of pulp. The same average was also reported for Canadian mills in literature (Foran 2006). The emission factor (E) was determined to be 0.89% by dividing the CTO in foul condensates by the average CTO yield.
Several kraft pulping mills are confirmed to produce CTO as a co-product in Canada (NCASI 2018).). A range of PECs were estimated for each component because the effluent generation rate (f) among these mills varied from 80 000 to 200 000 L/tonne of pulp.. The other parameter values used to calculate the PECs (X, m, E, Rx, and D) were assumed to be the same across those mills. The average CTO production yield (m) is 13 kg/tonne of pulp based on the data provided (personal communication, from FPAC/NCASI, to the Ecological Assessment Division, ECCC, August 2019, unreferenced). The receiving water bodies for Canadian kraft pulping mills are considered sufficiently large to have a more than 10-fold dilution capacity for wastewater treatment effluent discharges. However, 10-fold dilution (D) was used to account for the limited dilution near the discharge point. The wastewater treatment removal for each CTO component (RX) was based on modeled or measured (if available) data. It ranged between 77% and 92%, depending upon individual representative chemicals (see Table 2-2). The PECs were estimated as follows: abietic acid 1.7-4.5 µg/L, abietinol 1.3-3.5 µg/L, abietinal 0.2-0.6 µg/L, β-sitosterol 0.3-0.9 µg/L, DHAA 0.5-1.2 µg/L, IPA 0.4-1.1 µg/L, and linoleic acid 3.5-9.3 µg/L.
7.2.3 RCa manufacture scenario
The PEC of each representative chemical of RCa is estimated on the basis of the releases of representative structures to receiving water via wastewater treatment systems.
Where,
PEC: predicted environmental concentration in receiving water near discharge point, µg/L
q: daily quantity of a substance manufactured at a facility, kg/d
X: proportion of a representative structure in a substance, unitless
E: emission factor to liquid waste (solvent + water), unitless
y: mass fraction of a representative structure in aqueous phase of liquid waste, unitless
R: wastewater treatment removal, unitless
F: daily wastewater treatment effluent flow, L/d
D: receiving water dilution factor near discharge point, unitless
109: conversion factor from kg to mg, mg/kg
The mass fraction y is a parameter to account for a representative structure in the liquid waste’s aqueous phase because the solvent phase is commonly burned for energy recovery. The parameter is derived as a function of the apparent octanol-water partition coefficient (P) and the volume fraction of solvent in liquid waste according to the definition of P.
Where,
P: apparent octanol-water partition coefficient, unitless
Vsol: solvent fraction in liquid waste, unitless
The aquatic PEC is estimated for a facility with the highest RCa quantity manufactured, namely between 10 000 and 100 000 kg/yor between 100 and 1 000 kg/day (personal communication, from an RCa manufacturing company, to the Ecological Assessment Division, ECCC, November 2017, unreferenced). The upper bound (1 000 kg/d) of the daily quantity (q) is selected for the calculation. The emission factor to liquid waste is approximated at 2% for the daily quantity of 1 000 kg/d (European Chemicals Bureau 2003). The liquid waste generated from the facility is disposed of by a waste management company (personal communication, from a waste management company, to the Ecological Assessment Division, ECCC, December 2017, unreferenced). The liquid waste contains a solvent (used as a fuel) fraction between 10% and 90%, with the balance being water. The solvent fraction in the liquid waste is conservatively assumed to be at the lower bound (10%) in order to estimate the maximum amount of RCa in the aqueous phase. The value of FxD (wastewater treatment effluent flow x receiving water dilution) is 21 ML/d for the wastewater treatment system at the liquid waste disposal site. Aquatic PECs for RCa representative chemicals are 0.36 µg/L, 0.19 µg/L and 0.05 µg/L for abietic acid, DHAA and IPA, respectively.
7.2.4 Industrial use scenarios
As discussed previously, the major industrial use scenarios identified are concrete production, rubber compounding, steelmaking, and formulation. The PEC of representative chemicals for these scenarios for CTO, DTO, TOP, Rosin, RNa, RHME and RMa is estimated on the basis of its release to receiving water via off-site wastewater treatment systems.
Where,
PEC: predicted environmental concentration in receiving water near discharge point, µg/L
Q: annual quantity of a substance used at a facility, kg/y
X: proportion of a representative chemical in a substance, unitless
E: emission factor to wastewater, unitless
R: wastewater treatment removal, unitless
N: number of annual release days, d/y
F: daily wastewater treatment effluent flow, L/d
D: receiving water dilution factor near discharge point, unitless
109: conversion factor from kg to µg, µg/kg
Table 7-3 summarizes parameter values used in the calculation. According to information submitted in response to a CEPA section 71 survey (Environment Canada 2013) the use quantity of each CAS RN is within the range of 1 000 to 10 000, 10 000 to 100 000, or 100 000 to 1 000 000 kg in 2008. The logarithmic means of these ranges are selected to represent typical use quantities and are included in PEC calculations. The number of annual release days is assumed to be the same as the number of annual operation days, which is typically 250 days a year or more, because each CAS RN is expected to be used in all products produced. The emission factor to wastewater is 2% for an annual use quantity below 1 million kg/y (European Chemicals Bureau 2003). The value of FxD (wastewater treatment effluent flow times receiving water dilution factor) depends on the location of a facility. It is 657 ML/d or higher for locations using CAS RN 8016-81-7 and 181 ML/d or higher for locations using all other CAS RNs. The lower end values are used in the calculations. For minor industrial users, annual use quantities are substantially lower than the logarithmic means given in Table 7-3, and their releases and subsequent exposure are also expected to be lower.
| Substance abbreviation |
Annual use quantity range per substance at a facility (kg/y) (logarithmic mean) Q |
Number of annual release days (d/y) N |
Emission factor to wastewater (%) E |
Wastewater flow x Receiving water dilution factor (ML/d) FxD |
|---|---|---|---|---|
| RMa RHME | 1 000–10 000 (3 162) | 250 | 2 | 181 |
| CTO/DTO RNa Rosina | 10 000–100 000 (31 623) | 250 | 2 | 181 |
| TOP | 100 000–1 000 000 (316 228) | 250 | 2 | 657 |
a Based on information available for CAS RN 8050-09-7.
Aquatic PEC estimates are summarized in Table 7-5. These estimates are considered to be conservative because values for dilution (FxD) are conservatively selected and the likelihood of on-site wastewater treatment at a facility is not accounted for. The PECs of CAS RN 8002-26-4 are determined for both CTO and DTO given the possibility that either or both may be used.
| Representative chemical | RMa | RHME | CTO | DTO | RNa | Rosina | TOP |
|---|---|---|---|---|---|---|---|
| Abietic acid | 0.14 | NA | 0.48 | 0.36 | 1.35 | 1.43 | 0.98 |
| β-sitosterol | NA | NA | 0.09 | NA | NA | NA | 0.52 |
| Linoleic acid | NA | NA | 1.09 | 1.73 | NA | NA | 0.37 |
| THAME | NA | 0.11 | NA | NA | NA | NA | NA |
| DHAME | NA | 0.04 | NA | NA | NA | NA | NA |
| DHAA | 0.08 | 0.02 | 0.17 | 0.27 | 0.44 | 0.50 | NA |
| MPA hydrolysis product | 0.18 | NA | NA | NA | NA | NA | NA |
| SAPA hydrolysis product | NA | NA | NA | NA | NA | NA | NA |
| Isopimaric acid (IPA) | NA | NA | 0.11 | 0.22 | 0.51 | 0.56 | NA |
| Abietinol | NA | NA | 0.32 | 0.06 | NA | NA | NA |
| Abietinal | NA | NA | 0.06 | NA | NA | NA | NA |
Abbreviation: NA, not applicable.
a Based on information available for CAS RN 8050-09-7 for which the majority of rosin was reported. Only minor use volumes were reported under the other rosin CAS RN 8052-10-6, and the estimate based on CAS RN 8050-09-7 would therefore encompass that associated with CAS RN 8052-10-6.
7.2.5 Other unquantified exposures
According to information submitted in response to a CEPA section 71 survey (Environment Canada 2013), RCa (CAS RN 9007-13-0) and rosin (CAS RN 8052-10-6) are used either in low quantities (less than 1 000 kg/y) or in products that are unlikely to involve water during their production or application. Because of the low quantities in use and little involvement with water, the aquatic releases of and exposure to the two CAS RNs are expected to be low and no quantitative exposure analysis was pursued.
7.3 Characterization of ecological risk
The approach taken in this ecological screening assessment is to examine assessment information and develop proposed conclusions using a weight-of-evidence approach and precaution. Evidence is gathered to determine the potential for the Resins and Rosins Group substances to cause harm in the Canadian environment. Lines of evidence considered include those evaluated in this assessment that support the characterization of ecological risk in the Canadian environment. Reliable secondary or indirect lines of evidence are considered when available, including classifications of hazard or fate characteristics by other regulatory agencies.
7.3.1 Risk quotient analysis
Risk quotient analyses are performed by integrating estimates of exposure (PECs; see section 7.2) with ecological toxicity information (PNECs; section 7.1) to determine whether there is potential for ecological harm in Canada. Risk quotients (RQs) are calculated by dividing each representative chemical PEC by the corresponding PNEC for the relevant environmental compartments and associated exposure scenarios. RQs are then totalled for each representative chemical within each Resins and Rosins Group substance following a concentration addition (CA) approach methodology to determine a RQCA for each substance. This is the recommended approach by Backhaus and Faust (2012), irrespective of the mode/mechanism of action of the mixture components. RQs for all major releases to the environment for the substances in the Resins and Rosins Group are summarized in Table 7-5.
| Substance | Exposure scenario | Risk quotient | Type of estimate |
|---|---|---|---|
| CTO | Co-production | 1.1 – 3.1 | Refined range |
| CTO | Industrial use | 0.3 | Upper bound |
| DTO | Industrial use | 0.4 | Upper bound |
| TOP | Industrial use | 0.3 | Upper bound |
| Rosin | Industrial use | 0.8 | Upper bound |
| RMa | Industrial use | 0.2 | Upper bound |
| RHME | Industrial use | 0.01 | Upper bound |
| RCa | Manufacture | 0.05 | Refined |
| RNa | Industrial use | 0.8 | Upper bound |
7.3.2 Consideration of the lines of evidence
To characterize the ecological risk of the Resins and Rosins Group substances tall oil (including CTO and DTO under CAS RN 8002-26-4), TOP (CAS RN 8016-81-7), rosin (CAS RN 8050-09-7 and 8052-10-6 only), RHME (CAS RN 8050-15-5), RMa (CAS RN 8050-28-0), RCa (CAS RN 9007-13-0) and RNa (CAS RN 61790-51-0), technical information for various lines of evidence is considered (as discussed in the relevant sections of this screening assessment) and qualitatively weighted. The approach taken to assess these substances is to consider data and associated lines of evidence on representative chemicals, and, if and when applicable, any data and associated lines of evidence available on the UVCB substance as a whole (i.e., whole substance). The key lines of evidence supporting the assessment conclusion are presented in Table 7-6, with discussion of the lines of evidence and associated weighting contributing to an overall strength of evidence in section 7.3.3. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence. The relevance refers to the impact of the line of evidence when determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis ranged from low to high, with the assigned weight having five possible outcomes.
| Line of evidence for substances listed | Level of confidencea | Relevance in assessmentb | Weight assignedc |
|---|---|---|---|
|
Representative chemical persistence (and LRT) in the environment 1. CTO, DTO, TOP, Rosind, RCad, RNad 2. RMa 3. RHME |
1. Moderate 2. Moderate 3. Low |
1. Moderate 2. High 3. High |
1. Moderate 2. Moderate to high 3. Moderate |
|
Whole substance persistence in the environment 1. CTO, DTO, TOP, Rosin 2. RCa and RNa 3. RMa 4. RHME |
1. Low 2. Moderate 3. Moderate 4. Low |
1. Low 2. Low 3. High 4. Moderate |
1. Low 2. Low to moderate 3. Moderate to high 4. Low to moderate |
|
Representative chemical bioaccumulation in aquatic organisms 1. CTO, DTO, TOP, Rosin, RCa and RNa 2. RHME, RMa |
1. Moderate 2. Moderate |
1. Moderate 2. High |
1. Moderate 2. Moderate to high |
|
Representative chemical mode-of-action and/or other non-apical data 1. CTO, DTO, TOP, Rosin, RCa and RNa 2. RHME, RMa |
1. Moderate 2. Low |
1. High 2. High |
1. Moderate to high 2. Moderate |
|
Representative chemical PNECs for aquatic organisms 1. CTO, DTO, TOP, Rosin, RCa and RNa 2. RHME, RMa |
1. High 2. Moderate |
1. High 2. High |
1. High 2. Moderate to high |
|
Whole substance / WAF LL50 and EL50 for aquatic organisms 1. CTO and DTO 2. TOP, Rosin, RHME, RMa, RCa and RNa |
1. Low 2. NAe |
1. Low 2. NAe |
1. Low 2. NAe |
| Representative chemicalmonitoring data for concentrations in surface water; wastewater effluents; sediments; biota [all substances] | Moderate | Low | Low to moderate |
|
Representative chemical PECs in water 1. CTOf, RCaf 2. CTOg, DTOg, TOPg, Rosing,h, RHMEg, RMag, RNag, RCah |
1. Moderate 2. Low |
1. High 2. High |
1. Moderate to high 2. Moderate |
Abbreviations: LRT, long range transport; PNEC, predicted no-effect concentration; WAF LL50, water accommodated fraction median lethal loading concentration; WAF EL50, water accommodated fraction median effects-loading concentration; PEC, predicted effects concentration.
a Level of confidence is determined according to data quality, data variability, data gaps and if the data are fit for purpose (i.e., plausible and show causality).
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the combined level of confidence and relevance in the assessment.
d Includes consideration of LRT potential for abietic acid representative chemical.
e WAF data summarized in ECCC (2021b), however, did not use WAF to derive PNECs.
f Manufacturing scenario.
g Industrial use scenario(s).
h Use in products (qualitative).
7.3.3 Weight of evidence for determining potential to cause harm to the Canadian environment
CTO and DTO have very complex compositions with a large range of components, including highly biodegradable components such as fatty acids (e.g., linoleic acid). However, a significant proportion of the components (44% in CTO and 25% in DTO) show moderate to high persistence, including resin acids (e.g., DHAA, abietic acid), alcohols (e.g., abietinol), aldehydes (e.g., abietinal) and phytosterols (e.g., β-sitosterol; CTO only). CTO and DTO representative chemicals show low to moderate bioconcentration overall, but certain components representing almost 20% of CTO but only ~2% of DTO show a high, but somewhat uncertain bioaccumulation potential. However, components of CTO and DTO also occur naturally (e.g., from the decomposition of vegetation), and near continuous background exposure is therefore likely present in many aquatic environments. For that reason, elevated persistence and bioaccumulation potential for these components was interpreted with some reduced weight compared with that of a non-naturally occurring chemical. CTO and DTO are found to contain a significant proportion of specifically acting components, with PNEC values ranging from 0.7 to 130 µg/L based on modelled and empirical data available for representative chemicals. PEC values estimated on the basis of a CTO co-production scenario at Canadian kraft pulp and paper mills are 0.2 – 9.3 µg/L (based on each representative chemical). Risk quotients ranged from 1.1 – 3.1 for the component-based PNEC. These calculation were supported by WAF tests. Upper bound RQs (based on conservative PECs) for CTO and DTO industrial use activities are approximately 0.3 to 0.4. Overall, the strength of evidence suggests that there is a risk posed by CTO, but not DTO, in the aquatic environment in Canada. However, considering the potential but uncertain presence of isopimaric acid (a specifically acting component with elevated toxicity, PNEC = 0.7 µg/L) and related components, there may be a concern for the environment if exposures to DTO were to increase.
TOP (CAS RN 8016-81-7) is predominantly (95%) made up of components shown to have a lower potential for biodegradation and thus higher persistence, including resin acids (e.g., abietic acid), phystosterols (e.g., β-sitosterol) and large molecular size and poorly bioavailable polymeric and esterified material. TOP representative chemicals had a low to moderate bioconcentration potential overall, but β-sitosterol which represents roughly 15% of TOP showed a high but somewhat uncertain bioaccumulation potential. However, components of TOP also occur naturally via natural processes (e.g., from the decomposition of vegetation), and near continuous background exposure is therefore likely present in many aquatic environments. For that reason, elevated persistence and bioaccumulation potential for these components was interpreted with some reduced weight compared with that of a non-naturally occurring chemical. TOP is found to contain a significant proportion of specifically acting components, and PNEC values were estimated to range from 2 to 44 µg/L on the basis of modelled and empirical data available for representative chemicals. Conservative PEC values estimated on the basis of TOP industrial uses in Canada are 0.4 to 1.0 µg/L (based on each representative chemical), yielding upper bound RQs of 0.3. Overall, the strength of evidence suggests there is no significant risk posed by TOP in the aquatic environment in Canada.
Rosin (CAS RNs 8050-09-7, 8052-10-6), RNa (CAS RN 61790-51-0) and RCa (CAS RN 9007-13-0) are predominantly made up of components (e.g., resin acids) that show a higher persistence. However, whole substance empirical data show that RNa and RCa appear to have a slightly lower persistence compared to rosin, likely due to the fact that the organic moieties are more soluble in salt form than in neutral form. Rosin, RNa and RCa representative chemicals had a low to moderate bioaccumulation potential. Rosin, RCa and RNa contain a significant proportion of specifically acting components (e.g., those represented by IPA) where PNEC values ranged from 0.7 to 130 µg/L based on modelled and empirical data available for representative chemicals. Conservative PEC values estimated on the basis of rosin and RNa industrial uses in Canada are 0.4 to 1.4 µg/L (based on each representative chemical), yielding upper bound RQs of 0.8. Refined PEC values based on RCa manufacturing in Canada are 0.03 to 0.36 µg/L, yielding a refined RQ of 0.05. Overall, the strength of evidence suggests that there is no significant risk posed by rosin, RNa or RCa in the aquatic environment in Canada. However considering the significant presence of isopimaric acid (a specifically acting component with elevated toxicity, PNEC = 0.7 µg/L) and related components, there may be a concern for the environment if exposures to rosin (CAS RNs 8050-09-7, 8052-10-6), RNa and RCa were to increase.
RMa (CAS RN 8050-28-0) maleopimaric acid (MPA; 15% of composition) will rapidly hydrolyze (half-lives ~<5 min) into its dicarboxylic acid derivative. All components of RMa, including the hydrolysis product / dicarboxylic acid derivative of MPA, show higher persistence, which is corroborated by the very low empirical ready biodegradation seen for the whole substance of only 0.34% over 28 days. RMa has components with low bioaccumulation potential based on modeling of their representative chemicals. RMa is found to contain some specifically acting components (e.g., hydrolysis product of MPA) and PNECs are estimated (using modelled and empirical data) to be 1 to 130 µg/L. Conservative PEC values estimated on the basis of RMa industrial uses in Canada are 0.1 to 0.2 µg/L (based on each representative chemical), yielding an upper bound RQ of 0.2. Overall, the strength of evidence suggests there is no significant risk posed by RMa in the aquatic environment in Canada.
RHME (CAS RN 8050-15-5) components show a very low potential for biodegradation, and thus high persistence is predicted. However, available empirical ready biodegradation data suggest that there are some biodegradable components (i.e., biodegradation of the whole substance is 18% to 40% over 28 days) suggesting some uncertainty in the modelled component based results that may lead to a potential overestimation of risk. Components of RHME (75% of composition) have a high bioaccumulation potential. RHME contains narcotic components and PNECs are estimated (using modelled and empirical data) to range from 10 to 128 µg/L. Conservative PEC values estimated on the basis of RHME industrial use in Canada are 0.02 to 0.1 µg/L (based on each representative chemical), yielding an upper bound RQ of 0.01). Overall, the strength of evidence suggests there is no significant risk posed by RHME in the aquatic environment in Canada.
7.3.4 Sensitivity of conclusion to key uncertainties
Although distinction is made between CTO and DTO in this screening assessment where possible, it should be noted that they both share the same DSL name and CAS RN (tall oil, CAS RN 8002-26-4). This is because the distinction between CTO and DTO was not established during the original DSL nomination, and consequently they share the same DSL name and CAS RN.
The 28-day biodegradation data for UVCBs in the Resins and Rosins Group are uncertain as the protocol followed for these tests was designed for single component/discrete substances and no measurement of UVCB components was performed to know which components were degraded. Thus, a biodegradation result that might indicate that the pass level of a ready biodegradation test was attained (e.g., >60%) may overpredict the true biodegradation potential if the components remaining are recalcitrant. This uncertainty may lead to a potential underestimation of risk. Also, the modelling of component-based persistence for RCa and RNa components did not account for the ionization characteristics and thus are uncertain and may lead to a potential overestimation of risk.
As there are no empirical bioaccumulation data for the components of RHME, including the representative chemical THAME, modelled results are used. They suggest a very high potential for certain components of RHME to bioaccumulate in aquatic organisms. Esters are generally known to be quite quickly hydrolyzed into corresponding resin acids (e.g., THA), which have a much lower bioaccumulation potential. However, resin acid esters in particular are known to be recalcitrant to abiotic hydrolysis under even strong alkaline conditions and thus some uncertainty exists in the bioaccumulation prediction for components of RHME that may lead to a potential overestimation of risk.
The potential for Resins and Rosins Group substances to cause adverse effects in aquatic organisms is estimated on the basis of the respective toxicity of individual representative chemicals, with consideration given to their proportion in the substances in question. However, the majority of RHME and some RMa representative chemicals lacked a reliable base set of empirical toxicity information for fish, invertebrates and plants, and no suitable analogue data were available. Thus, purely modelled toxicity information is considered for the selection of CTVs leading to some uncertainty in the resultant PNECs and associated RQs. Although WAF information is available, the lack of measured concentrations in test solutions and a general lack of relevance to the specific exposure scenarios involved with these substances resulted in the decision not to use these results to derive the PNECs. Empirical toxicity studies for the major representative chemicals or fractions they represent (if they could be fractionated/isolated and tested) would help improve the accuracy of the estimated risk for these substances.
There was also uncertainty regarding the bioaccumulation potential of CTO, DTO, and TOP as indicated by their selected representative chemicals. Although representative chemicals for these substances had low to moderate bioconcentration overall, certain components including β-sitosterol (CTO and TOP), abietinal (CTO), and abietinol (CTO and DTO) indicated high but uncertain bioaccumulation potential. Uncertainty in the proportions of these components along with a large discrepancy in BCFs between the BCFBAF (2010) estimate and that by CATALOGIC (2016) for abietinal and β-sitosterol indicates that this uncertainty may lead to a potential overestimation of risk.
Several sites were confirmed to produce crude tall oil as a co-product according to information submitted in response to a CEPA section 71 survey(Environment Canada 2013) and subsequent voluntary follow-up with the relevant industry in Canada (NCASI 2018). The number of sites of CTO co-production in Canada has previously been estimated to range from 12 to 16 (Uloth et al. 2009; Wong 2010). Despite the fact that some of these latter estimates may be theoretical in nature and that the total number of sites may have decreased since these estimates were made, some uncertainty still remains in the total number of sites where CTO is co-produced in Canada. Thus, the potential environmental exposure resulting from CTO co-production in Canada may be greater than that determined strictly on the basis of the confirmed sites considered in this assessment.
Upper bound RQs for CTO, DTO, TOP, Rosin, RNa, RMa, and RHME industrial use activities were derived using conservative PECs. Conservative selection of values for dilution (FxD) that factored into the PEC calculations and not accounting for on-site wastewater treatment at a facility may lead to uncertainty and a potential overestimation of risk.
Given the uncertainty and variability in both the composition and identity of the components within a UVCB, the assumptions made in assigning the fixed proportions of the various representative chemicals leads to uncertainty in the resultant conclusions. The representative chemicals that had the greatest influence in the estimated risk of the UVCB substances in this assessment included MPA and MPA hydrolysis product, IPA, THAME, β-sitosterol, and DHAME. For that reason, accurate percent proportion data, along with the accurate representation of the properties of their respective fractions for these representative chemicals in particular, will have a larger impact on uncertainty of the estimated risk.
8. Potential to cause harm to human health
8.1 Exposure assessment
Substances in the Resins and Rosins Group are naturally occurring substances derived from coniferous trees such as pine. Unprocessed resins and rosins substances, and the resin acids which comprise them, can be found in the natural environment (Zinkel and Russel 1989). Resins and rosins substances and their components will be released into the environment from forest fires and natural degradation of coniferous trees. Drinking water treatment is expected to remove resins and rosins components during processing of surface waters. Thus, exposures from drinking water are expected to be low.
Substances in the Resins and Rosins Group are present in products available to consumers. On the basis of the physical and chemical properties of these substances, inhalation exposure is expected to be low from use of products available to consumers (EC, HC 2011). However, the use of some products available to consumers containing resins and rosins substances can result in exposure through spray droplet form.
There is limited data on the dermal absorption of substances in the Resins and Rosins Group or their components. The absorption of linoleic acid, a major component of tall oil, was determined in a 95-hour dermal absorption study (Hoelgaard 1982), which provided the basis for deriving estimates of systemic exposure following dermal exposure. The total skin uptake using human abdominal skin was determined to be 15.8 µg/cm2, with an absorption value of 8.6%. The reported value of 15.8 µg/cm2 was adjusted considering the standard deviation of 3.4 µg/cm2 to result in an amount absorbed of 19 µg/cm2 and an adjusted absorption value of approximately 11%. It was determined that the percent absorbed (11%) would be used in scenarios where the dermal loading exceeds the amount in the study (i.e., > 185 µg/cm2); whereas if the dermal loading was low (<185 µg/cm2), the amount absorbed (up to a maximum of 19 µg/cm2) would be used. The results of the study were considered for all substances in the Resins and Rosins Group, with the exception of storax (balsam), given the similarities in their molecular weights and physical-chemical properties.
The dermal absorption value for storax (balsam) was based on dermal absorption of one of its major components, cinnamic acid. Bronaugh et al. (1985) studied dermal absorption in vivo in monkeys and in vitro in human skin. A dermal load of 4 µg/cm2 was applied to the abdominal region of monkeys for 24 hours. An excretion-corrected absorption of 39% (standard error mean (SEM) 8.3) under non-occlusive conditions was reported. In vitro dermatomed human abdominal skin loaded with 4 µg/cm2 for 24 hours resulted in 18% (SEM 4.9) absorption under non-occlusive conditions. Adjusting these values for the standard deviation resulted in 42% absorption or an amount absorbed of 2 µg/cm2. These values were used to determine estimated systemic exposure of the general population to storax (balsam) via the dermal route.
Details on the parameters used in the determination of estimates of exposure are provided in Appendix B, Table B-1.
8.1.1 Dehydroabietic acid
Dehydroabietic acid (DHAA; CAS RN 1740-19-8) is a major component of rosin. Available information, including safety data sheets (SDS), did not identify any products directly formulated with this substance but rather as a resin or rosins mixture (Mitani 2007). Intermittent dermal exposure to products which contain DHAA as a portion of the resin or rosin substance may result in brief dermal contact, which is expected to result in minimal exposure and would also be captured in the exposure characterization for rosin (section 8.1.3).
DHAA is regarded as a major marker compound associated with the burning of conifer wood. Long-range transport of smoke from this activity is apparent through the detection of DHAA in oceanic samples at concentrations ranging from 0.0001 to 0.4 ng/m3 (Bai 2013). In terrestrial aerosol particulate matter, this substance appears at much higher concentrations, ranging from 0.23 to 440 ng/m3 (Bai 2013). DHAA is commonly found in house dust, which is considered to be the predominant source of exposure for the general population (Bai 2013). Field studies in Quebec City conducted by the National Research Council (NRC) found that concentrations in house dust ranged from 1.80 to 114.08 µg/g, with an arithmetic mean of 22.77 µg/g (NRC 2011). Using soil and dust ingestion rates from Wilson (2013) with the assumption that the concentration in soil and dust is the same, the largest oral intakes occur for infants aged 0 to 0.5 years, with an average daily intake of 0.12 µg/kg bw/day.
8.1.2 Tall oil
Available information indicates that tall oil (CTO or DTO; CAS RN 8002-26-4) may be used in a variety of products available to consumers. Industry submitted information suggests that products available to consumers are made solely with DTO; however, many labels and safety data sheets (SDS) list only “tall oil”. Sentinel scenarios for inhalation and dermal exposure were identified for two products: a kitchen cleaner and a facial cleanser. Potential inhalation for tall oil was considered for a kitchen cleaner containing 10% tall oil from a hand-spray kitchen cleaner (MSDS 2007). ConsExpo modelling using a spray application scenario for a non-volatile chemical with scenario defaults resulted in a mean event air concentration of 0.3 mg/m3, with an internal dose on the day of exposure determined to be 0.0064 mg/kg bw/day based on daily exposure (ConsExpo Web 2016). The associated dermal dose during spraying and rinse/wiping were determined to be 0.003 mg/kg bw/day and 0.0489 mg/kg bw/day respectively (ConsExpo Web 2016, ConsExpo Web 2020). A sentinel scenario for dermal exposure was developed for a cosmetic facial cleanser formulated with 30% tall oil that is expected to be used daily (1.6 times per day, 637 cm2 exposed area, 2.58 g/application, 0.01% retained) and actively applied to the face (personal communication, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 26, 2017; unreferenced). The resulting applied daily dose was estimated to be 0.18 mg/kg bw/day. This exposure is expected to cover incidental exposure resulting from infrequent uses of products such as adhesives, sealants, paints, coatings and/or degreasers, which are also expected to be limited to the hands only. Dermal absorption data for tall oil was read across to linoleic acid data, its most prevalent component. Considering that the dermal loading in the above scenario at 12.2 µg/cm2 is smaller than the dermal loading from the study (Hoelgaard 1982), this results in a daily dose of 0.18 mg/kg bw/day.
8.1.3 Rosin
Rosin (CAS RN 8050-09-7) and the related substance, resin acids and rosin acids (CAS RN 73138-82-6), are considered a single substance on the basis of information provided by industry and will be evaluated together (personal communication from Pine Chemicals Association to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). A search of SDS for CAS RN 73128-82-6 revealed no products, which is consistent with the information received pursuant to a CEPA section 71 survey.
Rosin (CAS RN 8050-09-7) is a component that may be used in the manufacture of food packaging materials for which there may be direct contact with food; however dietary exposure is considered to be low (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Resin acids and rosin acids (CAS RN 73138-82-6) may be used as components in the manufacture of printing inks with no direct food contact; therefore dietary exposure from these uses is not expected (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
According to notifications submitted under the Cosmetic Regulations to Health Canada, rosin is used in certain cosmetic products in Canada, but CAS RN 73138-82-6 (resin acids and rosin acids) is not. For rosin, the categories of adhesive, depilator, epilator, makeup and nail polish account for over 94% of products. Adhesives and depilator and epilator products can contain a high concentration, up to 100%, of rosin (personal communication, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). These solid or semi-solid gels or cakes are applied to large areas of the body (legs) for hair removal. The rosin is not expected to absorb through the skin as the material is designed to be applied and physically removed from the skin surface to facilitate hair removal. Systemic exposure is therefore expected to be minimal.
Exposures to rosin can occur from nail polishes, makeup/adhesives for the face and eyes, and lipsticks. In adults, exposure through dermal deposition from the use of nail polish containing up to 10% rosin, assuming that two coats are applied to fingernails and toenails, was estimated at 0.23 mg/kg bw/event. The internal dose, estimated on the basis of 11% dermal absorption, is 0.025 mg/kg bw/event (Hoelgaard 1982). Eye makeup can contain up to 10% by weight of rosin and may be applied daily to the eyelid area. The internal dose of 0.0077 mg/kg bw/day based upon the absorption of 19 µg/cm2 from 24 cm2 use area. Lipsticks contain between 0.1% and 3% of rosin by weight, with ingestion from the lip area during the day. An upper-bounding estimate of daily oral exposure to lipstick was determined to be 0.01 mg/kg bw/day.
Rosin is listed in the Natural Health Products Ingredient Database (NHPID) with a non-medicinal role for use as a base, binder, coating agent, emulsifying agent, encapsulating agent, or film former (NHPID [modified 2021]). Rosin is also listed as being present as a non-medicinal ingredient NHPS in the Licensed Natural Health Products Database (LNHPD [modified 2021]) (personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Rosin appears in many dental varnishes to treat sensitive teeth in both adults and children at concentrations of up to 59% rosin. The suggested frequency of application to the teeth is very infrequent, i.e., a few times per year. At the suggested amount of 1.6 mL, this gives a potential oral ingestion of 0.944 mL of rosin or 1.05 grams (0.944 mL × density of 1.115 g/mL) per application. This results in a per event dose of 33.9 mg/kg bw for children and 14.8 mg/kg bw for adults. This is expected to be slowly released from the tooth surface over a period of months. Assuming release over a period of 90 days followed by re-application, this would result in an average daily dose of 0.38 mg/kg bw/day for children and 0.164 mg/kg bw/day for adults.
Rosin also appears in non-perscription drugs as a non-medicinal ingredient. Topical treatments, typically marketed for pain relief, in the form of a patch or plaster can contain rosin likely formulated as an adhesive. Little information is available on the amount of rosin present in these products, which have a suggested use of 3 to 4 times daily, with warnings to limit use beyond 7 days (personal communication, email from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Given the suggested short duration of use, exposure is not expected to be greater than exposures previously determined for cosmetics.
A limited number of over-the-counter allergy and cold relief products contain rosin as a non-medicinal ingredient, with an upper limit of 7 mg per tablet. Assuming 2 tablets are taken per day, this would result in an average daily dose of 0.20 mg/kg bw/day for adults (personal communication, email from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
Other products available to consumers containing rosin include various sealants and construction materials, electronics solder or flux, adhesives, paint, printing inks and various manufacturing process aids for other products available to consumers (AGDH 2017). International work completed by Australia’s National Industrial Chemicals Notification and Assessment Scheme (NICNAS) estimated concentrations in these products to be up to 30% (AGDH 2017). Inhalation exposures from solder/flux as it is heated are not expected to be from the rosin itself but rather from the combustion products, which are beyond the scope of this assessment.
Some resin products, containing nearly pure rosin, are used by amateur sports players, dancers and violinists. Rosin is used for improved grip in tennis and baseball and is liberally applied to the hands. Inhalation and ingestion of this solid material is not expected, but dermal exposure of the palms to pure powdered rosin can routinely occur. The deposition on both hands for this scenario was based on the US EPA high-end soil adhesion factor for adults in a residential setting (0.07 mg/cm2), due to the similarities in powdered rosin and particulate soil (US EPA 2007). The estimated applied mass for the palms of both hands (455 cm2) was 31.45 mg, resulting in internal dose of 0.122 mg/kg bw/event assuming 19 µg/cm2 dermal absorption. It is considered that up to 10 such exposure events could occur in a given day, but that exposure would not occur at all on other days, leading to a combined internal dose of 1.22 mg/kg bw/day on days of exposure. It is noted that the thickness of the palm may further limit dermal uptake; the dose presented is therefore likely an overestimation.This exposure is expected to cover incidental dermal exposures resulting from any infrequent uses of adhesives, inks or paints.
8.1.4 Storax (balsam)
Storax (CAS RN 8046-19-3) is reported as a food flavouring agent in the United States, where it is listed in the Everything Added to Food in the United States (EAFUS 2017, CFR 2017). Storax is classified as “generally regarded as safe” by the Flavor and Extract Manufacturers Association (FEMA) for use in various beverages, candy, baked and frozen goods (FEMA 1965). It is also listed in Fenaroli’s Handbook of Flavor Ingredients as a flavouring agent in similar foods identified by FEMA with an estimated individual consumption intake of 0.00090 mg/kg bw/day for the general population (Burdock 2010). There is no definitive information available concerning the potential use of storax as a food flavouring agent in Canada; however, given its known use in the United States, it is possible that the substance is present as a food flavouring agent in foods sold in Canada (personal communication from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
According to notifications submitted under the Cosmetic Regulations to Health Canada, storax is used in a small number of cosmetic products in Canada, such as cleansers and moisturizers, at up to 0.3%. In addition, storax can be found in medical adhesives to assist in bandage or wound dressing adhesion at up to 10% (personal communication, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 26, 2017; unreferenced; MSDS 2010). This exposure is expected to be limited to the area adjacent to a cut or wound from s the tacky or sticky part of the covering. The duration of exposure and frequency of exposure is expected to be limited. A sentinel dermal exposure scenario for a face moisturizer containing 0.3% storax resulted in an internal dose of 0.038 mg/kg bw/day, based on 42% dermal absorption of cinnamic acid, a main component of storax (Bronaugh et al. 1985).
Storax, as well as Liquidambar formosana, Liquidambar orientalis, Liquidambar styraciflua, and Resina Liquidambaris, are listed in the NHPID with a medicinal role as classified as NHP substances falling under Schedule 1 to the Natural Health Products Regulations. Liquidambar orientalis balsam essential oil and Liquidambar styraciflua oil are also listed in the NHPID with a non-medicinal role, for topical use only as fragrance ingredient and as flavour enhancer - natural or fragrance ingredient, respectively. (NHPID [modified 2021). Such ingredients are also listed as being present as medicinal or non-medicinal in NHPs in the LNHPD ([modified 2021]). No information was available on the quantity or concentration of Liquidambar orientalis balsam essential oi and Liquidambar styraciflua oil when used as non-medicinal ingredients; however, products available include balms, medicated plasters, and liquids for short-term conditions, such as the relief of skin or mouth irritation and muscle pain, to be applied on specific areas of the body (i.e., not intended for general use over large areas and/or long duration of use) (personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Given the physical-chemical properties of storax with low vapour pressure and its designation as a fixative, it is expected that inhalation from a topical product would result in minor inhalation exposures from dermal exposure.
8.1.5 Resin acid and rosin acids, calcium salts
RCa (CAS RN 9007-13-0) may be used as a component in the manufacture of printing inks used in food packaging materials, with no direct food contact, and in colour concentrates for which there is potential food contact. However, exposre to RCa from uses in food packaging applications is not expected to be a significant source of exposure (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
Searches of SDS for products containing RCa revealed a number of paints and coatings that contain RCa at concentrations up to 20% (MSDS 2009). Its use as a colouring agent in such products was identified by NICNAS (AGDH 2017). Dermal exposure from paint from incidental drips and spills is expected to be limited to a thin film on the palms of the hands (455 cm2) as characterized by the US EPA (2011). A film thickness of 0.62 × 10-3 cm was selected based on a mineral oil with a partial wiping with an assumed density of 1 g/mL and an upper-bounding percentage of 20% RCa. The resulting dermal deposition was determined to be 0.80 mg/kg bw/event with an internal dose of 0.12 mg/kg bw/event (19 µg/cm2 × 455 cm2).
8.1.6 Resin acid and rosin acids, sodium salts
RNa (CAS RN 61790-51-0) may be used as a component in the manufacture of gloves (as a rubber production emulsifier), which could be used in handling food during processing. Dietary exposure to RNa as a result of handling food with gloves potentially containing residual RNa is not expected to be a significant source of exposure (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
Based on notifications submitted under the Cosmetic Regulations to Health Canada, RNa is used in a small number of cosmetic products in Canada. It is used at concentrations ranging from >0.1% to 10% in body cleanser products (personal communication, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Dermal deposition was estimated for the upper-bounding concentration in cleanser products and resulted in a daily internal dose of 0.0186 mg/kg bw/day based on a body surface of 16 925 cm2 leading to the complete absorption of the applied dose.
Searches of SDS for products containing RNa revealed a number of adhesive/sealant products and concrete surface coatings or cleaners that contain RNa at concentrations up to 20% (SDS 2003). It is noted that some of these products are designated as professional use, and general population exposure is not expected. Dermal exposures to these products are expected to be limited to the palms of the hands. Dermal deposition was determined by thin-film method from an adhesive containing 20% RNa. A film thickness of 0.62 × 10-3 cm was selected based on a mineral oil with partial wiping with an assumed density of 1 g/mL and an upper-bounding percentage of 20% RNa. The resulting dermal deposition was determined to be 0.80 mg/kg bw/event with an internal dose was 0.12 mg/kg bw/event (19 µg/cm2 × 455 cm2). RNa has also been identified as a production aid in the generation of styrene-butadiene and acrylonitrile-butadiene rubbers with a residual weight percent of 2.35% to 5% as reported on SDS (2010, 2016). These types of rubber materials are typically used in the manufacture of items such as tires, auto parts, belt materials and some gloves; exposures would be covered by the above scenario. Oral exposures from mouthing/leaching are not expected given the product types.
8.1.7 Resin acids and rosin acids, Me esters
RME (CAS RN 68186-14-1) is listed as methyl rosinate in the NHPID with a non-medicinal role for topical use only as a fragrance ingredient, skin-conditioning agent - emollient, or viscosity increasing agent – non-aqueous (NHPID [modified 2021]). It is also listed as being present as non-medicinal ingredient in NHPs in the LNHPD, with no information available on the quantity or concentration of RME when used as such in these acne therapy products (LNHPD [modified 2021]; personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
A small number of products were identified through SDS searches, including specialized gasket sealants (30% RME) (MSDS 2015b). Exposure to such products is expected to result in dermal deposition of RME on the surface of the skin. Assuming that the use of a gasket sealant containing 30% RME can result in a dermal exposure to the part of the palms of the hands (455 cm2) with a thin-film (0.62 × 10-3 cm) of the sealant with an assumed density of 1 g/mL, the internal dose of 0.131 mg/kg bw/event was based on a dermal absorption of 11%.
Specialized dental materials can also contain RME at up to 25% to 50% for sealer bases and 1% to 5% for dental cements, which could lead to acute oral exposures (SDS 2015a,b). Assuming 2 grams of the sealer base containing 50% RME was used in dental maintenance and orally extractable which was assumed to be leached completely over a period of 90 days resulting in a daily oral dose of 0.16 mg/kg bw/day for an adult or 0.36 mg/kg bw/day for a child.
8.1.8 Resin acids and rosin acids, hydrogenated, Me esters
RHME (CAS RN 8050-15-5) is reported to be used as a component in the manufacture of printing inks used in food packaging for which there is no direct food contact; therefore, exposure from food is not expected (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). The US Food and Drug Administration (FDA) permits the use of RHME as a food additive in the manufacture of chewing gum. In Canada, “chewing gum base” is considered a food ingredient, and certain components of the base are exempt from declaration. There is no definitive information available concerning the potential use of RHME as a food ingredient in chewing gum in Canada. However, given its known use in the United States, it is possible that the substance is present in chewing gum in Canada (personal communication from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
Information from the internal Drug Product Database indicates that RHME is reported to be present in non-prescription drugs as non-medicinal ingredient at concentrations below 1%. These products are limited to sunscreens as lotions or sprays and are expected to result in dermal and potentially inhalation exposures. Dermal exposure from a lotion containing 0.01% RHME would result in an internal dose 0.036 mg/kg bw/day based on a dermal absorption of 0.13 µg/cm2. Inhalation exposure from a spray containing 0.01% RHME was estimated using the ConsExpo spray exposure scenario (ConsExpo Web 2016). Assuming indoor spraying for 5 minutes directed towards the user, the mean event air concentration is estimated to be 0.19 mg/m3 with an internal dose of 0.0018 mg/kg bw/day based on an inhalation rate of 16.2 m3/day.
RHME is also listed in the NHPID with a non-medicinal role for topical and dental use only as skin-conditioning agent - emollient or viscosity increasing agent – non-aqueous (NHPID [modified 2021]). It is also listed as being present as non-medicinal ingredient in NHPs in the LNHPD, with no information available on the quantity or concentration of RHME when used as such in these acne therapy and sunscreen products (LNHPD [modified 2021]; personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
A variety of skin and hair products containing from 0.1% to 10% RHME could result in dermal exposures. The majority of these products are formulated with approximately 3% RHME and are cosmetic face and body moisturizers (personal communication, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). A sentinel dermal scenario for a face moisturizer containing 10% RHME was considered as an upper-bounding dermal exposure and is expected to cover off any exposures from drug or NHPsgiven that they are believed to be formulated with less, or result in lower exposure to, RHME. The dermal deposition estimated for the upper-bounding concentration of 10% in face moisturizer resulted in an external daily dose of 3 mg/kg bw/day with an internal dose of 0.34 mg/kg bw/day was based upon 11% dermal absorption.
According to notifications submitted under the Cosmetic Regulations to Health Canada, RHME is also used in certain cosmetic products in Canada at concentrations from less than 0.1% up to a range of 30% to 100% in lipstick products, though typically at 10%, which could result in oral exposures (personal communication, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). The estimated oral dose from lipstick comprised almost entirely of RHME would result in a daily oral dose of 0.33 mg/kg bw/day. This is expected to be an overestimation based on the 100% RHME composition assumption.
A search of SDS revealed a handful of products available to consumers to which exposure may occur. Specialized dental varnishes may contain up to 10% RHME; however, any oral exposures from this product use are expected to be covered by the lipstick scenario as it would provide a daily source of exposure over a longer duration (MSDS 2013). Several solid air freshener products contain RHME at concentrations ranging from 1% to 10% (SDS 2017). Given the low vapour pressure associated with RHME, inhalation of the substance is expected to be negligible, with the RHME in these products used as a fixative to slow release of the fragrance by lowering the overall vapour pressure of the mixture. Any dermal exposures from a fragrance product are expected to be covered by the face moisturizer scenario which has a larger use amount.
8.2 Health effects assessment
On the basis of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity, the Resins and Rosins Group substances in this assessment were not identified as posing a high hazard to human health.
8.2.1 Dehydroabietic acid
There are a limited number of health effect studies on dehydroabietic acid (CAS RN 1740-19-8). In repeated oral dose testing over 28 days in Sprague-Dawley rats, there were no pathological or histological changes associated with exposures of up to 250 mg/kg bw/day DHAA in the diet (Villeneuve 1977). The study authors determined a no-observed-adverse-effect level (NOAEL) of 25 mg/kg bw/day and a lowest-observed-adverse-effect level (LOAEL) of 250 mg/kg bw/day based on an increase in liver aniline hydroxylase activity and serum alkaline phosphatase activity, which was only observed at 28 days (Villeneuve 1977).
8.2.2 Tall oil
There are a limited number of health effect studies for CTO or DTO (CAS RN 8002-26-4). As there were no studies by the dermal route, oral studies were used to characterize the hazard associated with this substance. It was determined that the substance was not a dermal sensitizer (ECHA c2007-2017). Tall oil produced generally negative results in tests for genotoxicity and mutagenicity, with the lone positive response in a chromosomal aberration assay only at cytotoxic concentrations (ECHA c2007-2017). Acute oral toxicity was determined to be low in two rat assays (US EPA 2008b).
In repeated-dose oral reproduction/developmental toxicity testing conducted according to OECD Guideline 422, male and female Sprague-Dawley (SD) rats (10/sex/dose) were administered tall oil at 0, 1 000, 5 000 or 20 000 ppm in the died (approximately 0, 80, 414 and 1 600 mg/kg bw/day respectively) (US EPA 2008b). Males were dosed for a total of four weeks (beginning two weeks prior to mating) and females were dosed for two weeks prior to mating, through mating, until study termination on the sixth day of lactation. A decrease in implantation sites was observed at 1 600 mg/kg bw/day. There were no effects on development as a result of prenatal exposure and possible postnatal exposure (via lactation) during days 0 to 4. At 1 600 mg/kg bw/day, decreased food consumption, decreases in body and adrenal gland weights, and changes in clinical chemistry parameters (increases in bilirubin and alkaline phosphatase levels) were observed in both sexes. There were increases in liver weight, spleen weight and cholesterol levels in males and decreases in white blood cell count and ovary weight in females. At 414 mg/kg bw/day, increased liver weights in males, decreased adrenal gland weights in females, and changes in clinical chemistry parameters (increase in alkaline phosphatase levels) in both sexes were observed. The only effect observed in the low dose group was an increase in alkaline phosphatase levels (females only). Histopathological data did not reveal any findings in the high-dose group, which was the only group examined. The LOAEL for systemic toxicity was considered to be 414 mg/kg bw/day based on decreased adrenal gland weight in females and changes in clinical chemistry in both sexes with a NOAEL for systemic toxicity of 80 mg/kg bw/day. The reproductive toxicity endpoints were considered to be a LOAEL of 1 600 mg/kg bw/day based on a decrease in implantation sites at the NOAEL of 414 mg/kg bw/day. The developmental toxicity NOAEL was considered to be 1 600 mg/kg/day (US EPA 2008b). However, these effects were noted only at excessive doses and in the presence of maternal toxicity and are not considered to be indicative of frank developmental toxicity.
8.2.3 Rosin
No hazard information was identified for resin acids and rosin acids (CAS RN 73138-82-6) and therefore the hazard characterization relies on information from rosin (CAS RN 8050-09-7). Gum rosin, wood rosin, and tall oil rosin are of low acute oral toxicity in rats, mice and/or guinea pigs (Kay 1961), while rosin was also determined to be of low dermal toxicity (REACH 2022a). Rosin was determined to be a skin sensitizer (ECHA c2007-2017; Machovcova 2010; Sadhra et al. 1994, EC 2000); however, a later evaluation suggested that only the oxidized form of rosin should be considered a skin sensitizer (REACH 2022b).
In a developmental study, pregnant rats were fed 0, 2 500, 5 000 or 7 500 ppm rosin (equivalent to 0, 199, 387, and 561 mg/kg bw/day, respectively) from gestation days 3 to 19 (REACH 2022c). At 561 mg/kg bw/day it was noted that there was decreased fetal weight for both sexes. There were significant reductions reported in the number of fetuses/litters showing incomplete ossification of the squamosal bone of the skull and no ossification of the hyoid in the skull. An increase in the number of fetuses/litters showing ossification center associated with the first lumber vertebra were also observed (REACH 2022c). At 387 mg/kg bw/day, a reduction in the number of fetuses/litters showing incomplete ossification of the squamosal bone of the skull and an increase in the number of fetuses/litters showing dumb-bell shaped thoracic centrum and ossification center associated with the first lumber vertebra were observed (REACH 2022c). Observations at 199 mg/kg bw/day showed a reduction in the number of fetuses/litters with no ossification of the hyoid in the skull. This same dosing level was also associated with an increase in the number of fetuses/litters showing an ossification center associated with the first lumbarar vertebra (REACH 2022c). No information was provided regarding the historical laboratory controls to determine if the skeletal observations represent an adverse effect and so the data are presented without further analysis (REACH 2022c).
In a combined oral reproductive/developmental toxicity study using rosin, no treatment-related effects on mating performance, fertility or duration of gestation were observed in SD rats at doses of 0, 105, 275, or 825 mg/kg bw/day via diet. No obvious external abnormalities were noted in the pups at any dose level. Testes and epididymides weights were similar in all groups. Litter survival, as indicated by the birth index and viability index, was similar in all groups. The reproductive/developmental effect reported was the reduction in the litter size and fetal weight as the result of reduced food intake in dams at 825 mg/kg bw/day (Clubb and Sutherland 2002). The study authors reported a NOAEL of 275 mg/kg bw/day based on the high dose effects noted above. Similar to the review conducted by the US EPA, the NOAEL was considered to be 825 mg/kg bw/day as none of the effects were considered to be significantly different than the controls (US EPA 2008a).
In a two-year chronic/carcinogenicity study, no significant differences were observed between treated groups and controls with respect to tumour rate, haematology, urinalysis, or gross or microscopic pathology in SD rats orally exposed to rosin in the diet up to 1 000 mg/kg bw per day (US EPA 2008a). Increased relative liver weight and decreased mean body weight gain, associated with a decrease in food consumption, were observed at the high dose level (1 000 mg bw/kg/day) (US EPA 2008a; Kay 1962). Similar results were obtained in 90-day dietary oral toxicity studies with rosin (Calandra 1960) and hydrogenated rosin in the same strain of rat (US EPA 2008a).
8.2.4 Storax (balsam)
Storax (CAS RN 8046-19-3) was positive for allergic reactivity in human patch tests (Fregert 1962). Storax extracted with ethanol has been shown to be genotoxic only at cytotoxic concentrations in human lymphocytes in vitro (Karadeniz et al. 2013). Genotoxic effects of the storax extract were studied using a sister chromatid exchange system. High concentrations of storax extract caused inhibition of the cell cycle and sister chromatid exchange was higher than in the positive control group treated with CCl4 (Karadeniz et al. 2013). The frequency was found to increase with concentration of the storax administered. Since the sister chromatid exchange was only noted at cytotoxic concentrations, it is not considered to be a frank genotoxicant (Karadeniz et al. 2013).
In a World Health Organization Food Additive monograph cinnamyl alcohol and related substances were evaluated for safety and found no safety concern at current levels of intake when used as a flavouring agent (WHO 2001). As cinnamic acid is the major constituent in storax, the health effects studies conducted with cinnamic acid and related compounds were used as surrogate data in the absence of relevant studies conducted with storax. Cinnamic acid was noted to be negative for developmental effects in rats fed 50 mg/kg bw/day cinnamic acid throughout gestation while a systemic NOAEL was calculated to be 54 mg/kg bw/day following 4 months of dietary exposure (Zaitsev and Maganova 1975 as reported in WHO 2001). In this study developmetal effects were not analysed in all tested animals. In a 16 week feeding study in rats, a NOAEL of 120 mg/kg bw/day was determined based on mild cellular swelling in the liver at a dose of 500 mg/kg bw/day.
8.2.5 Resin acid and rosin acids, calcium salts and resin acid and rosin acids, sodium salts
No hazard data were identified for RCa (CAS RN 9007-13-0) or RNa (CAS RN 61790-51-0). Hazard identification in the REACH dossier was completed by read-across to related substances, primarily rosin, which has the most robust hazard profile (ECHA c2007-2017). RNa and RCa were determined to not be skin sensitizers in local lymph node assays (AGDH 2017).
Given the lack of sufficient hazard data, read-across to other Resins and Rosins Group substances will be done for the most sensitive endpoint for the exposure route.
8.2.6 Resin acids and rosin acids, Me esters
Limited hazard testing has been completed for RME (CAS RN 68186-14-1); in acute oral studies both RME and rosin, partially hydrogenated methyl ester were noted to be of low toxicity (US EPA 2008c).
Repeated dose reproductive / developmental oral toxicity testing in Sprague-Dawley (SD) rats was completed for RME. Four groups of 10 male and 10 female SD rats received RME via the diet at concentrations of 0, 5 000, 10 000 or 20 000 ppm (approximate doses of 400, 760 and 1 530 mg/kg bw/day, respectively) from two weeks prior to mating to two weeks after mating (males) or sixth day of lactation (females) (Clubb 2003). Effects of treatment included reduced body weight gain and food consumption at all levels. In male animals, there was a reduction in mean food consumption and body weight gain in the first week of treatment at all dietary levels with the greatest reduction at 1 530 mg/kg bw/day. After one week of treatment, food consumption and mean body weight gains were similar to those of controls. In females at all treatment levels, group mean body weight gain prior to mating was lower than that of the controls, with further reductions during gestation and lactation. The extent of the reduction during gestation/lactation was dependent on the concentration of the diet. In females at 760 mg/kg bw/day and 1 530 mg/kg bw/day there was a reduction in food consumption on commencement of treatment which persisted for the remainder of the study (Clubb 2003).
There was a dose related increase in liver weight in both sexes at all levels. In females, mean heart, kidney, lung, spleen and salivary gland weights were all lower than controls and were considered secondary to the low body weights. Histological examination of the liver revealed hepatocellular hypertrophy in all animals treated at 760 mg/kg bw/day and 1 530 mg/kg bw/day. Thymic atrophy was observed in 4/8 females examined at 1 530 mg/kg bw/day. Effects of treatment with RHME included reduced body weight gain and food consumption at all dose levels. The authors considered this to be a palatability issue. A dose related increase in liver weights in both sexes was associated with an increase in the incidence of hepatocellular hypertrophy across all groups. There was no evidence of cell damage, cholestasis or changes to lipid metabolism revealed by histological examination (Clubb 2003).
The authors considered the parental NOAEL to be < 400 mg/kg bw/day based on the observed liver effects which are attributed to the reduced food consumption and body weight effects from severe palatability issues (Clubb 2003). However, as these effects are considered compensatory and/or related to palatability, health effects were not extracted from this study for risk characterization.
8.2.7 Resin acids and rosin acids, hydrogenated, Me esters
Acute oral toxicity to RHME (CAS RN 8050-15-5) via oral and dermal routes was considered to be low (Riebeek 1990; ECHA c2007-2017). RHME was negative for mutagenic activity in bacterial reverse mutation assay (Stevenson 2001). RHME was also negative in Chinese hamster ovary (CHO) cells for clastogenic activity both with and without metabolic activation (Murie 2001).
8.3 Characterization of risk to human health
Exposure estimates, critical effects levels, characterization of risk and resulting margins of exposure (MOEs) are provided in Table 8-1. Considering products available to consumers, there is potential for daily oral, dermal and inhalation exposure to uses and products such as sports grips, dental sealants and varnishes, and cosmetics. Oral exposure to storax is possible based on its potential use as a food flavouring agent. Oral ingestion is also possible from house dust, which contains DHAA. Inhalation exposures to Resins and Rosins Group substances are expected to be low due to their low vapour pressures across the substance group. For Resins and Rosins Group substances internal dermal doses ranged from 0.00012 to 1.22 mg/kg bw/day.
Broadly, the hazard studies across the Resins and Rosins Group substances in this assessment demonstrate no evidence of carcinogenicity or genotoxicity in experimental animals or cell lines. The acute toxicity of Resins and Rosins Group substances is low across both dermal and oral routes of exposure (US EPA 2008a). Limited toxicological effects occurred in repeated dose studies with Resins and Rosins Group substances for doses between 760 to 825 mg/kg bw/day.
The endpoints selected for repeated exposures were substance specific in the case of DHAA, tall oil and rosin and were the NOAELs of 25, 80 and 275 mg/kg bw/day, respectively. The NOAEL of 275 mg/kg bw/day for rosin was based on reduction in litter size and pup weight at the next dose level (825 mg/kg bw/day) following exposure of pregnant rats; these effects are likely the result of reduced food intake in dams and are not considered to be substance specific, butthis endpoint was selected as a conservative approach nevertheless (US EPA 2008a). For repeated oral and dermal exposure to storax (balsam) read-across to its major constituent, cinnamic acid, was considered in absence of substance-specific data. A NOAEL of 54 mg/kg bw/day (highest dose tested) was identified in a 4-month dietary study in rats (Zaitsev and Maganova 1975 as reported in WHO 2001). For repeated oral and dermal exposure to RCa, RNa, RME and RHME, read-across to rosin data was chosen given the compositional similarity of the substances. These substances are UVCBs so the oral endpoint for DHAA was excluded as it is a discrete substance lacking the complex compositional nature reflected in the four substances. The composition of rosin is more similar to the four substances than the composition of tall oil, which is composed primarily of linoleic acid, such that it is not considered to be the most representative substance (see section 2 for details). The oral point of departure for repeated exposures selected was the endpoint for rosin of 275 mg/kg bw/day (NOAEL) on the basis of reduced litter size and pup weight at 825 mg/kg bw/day (US EPA 2008a). However, this endpoint is considered highly conservative as the effects noted are the result of maternal food restriction due to poor palatability and are unlikely to be a primary toxicological effect of the test material.
The margins of exposure for the oral scenarios in Resins and Rosins Group substances are summarized in Table 8-1.
| Exposure scenario | Internal exposure | Critical effect level for characterization of risk | Corresponding adverse health effect | MOE |
|---|---|---|---|---|
| DHAA oral ingestion of house dust | 0.00012 mg/kg bw/day for infants | 25 mg/kg bw/day (NOAEL for DHAA) | 250 mg/kg bw/day (LOAEL) based on increased liver and serum enzymes | 208 000 |
| Tall oil dermal exposure from a facial cleanser | 0.18 mg/kg bw/day | 80 mg/kg bw/day (NOAEL for tall oil) | 414 mg/kg bw/day (LOAEL for tall oil) increased liver weight and decreased adrenal gland weight | 440 |
| Tall oil dermal and inhalation exposure from a kitchen spray cleaner | 0.0583 mg/kg bw/day | 80 mg/kg bw/day (NOAEL for tall oil) | 414 mg/kg bw/day (LOAEL for tall oil) increased liver weight and decreased adrenal gland weight | 1 372 |
| Rosin dermal exposure from a nail polish | 0.023 mg/kg bw/event | 275 mg/kg bw/day (NOAEL for rosin) | 825 mg/kg bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 11 960 |
| Rosin dermal exposure from eye shadow | 0.0077 mg/kg bw/day | 275 mg/kg bw/day (NOAEL for rosin) | 825 mg/kg bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 35 710 |
| Rosin oral ingestion from lipstick | 0.01 mg/kg bw per day | 275 mg/kg bw/day (NOAEL for rosin) | 825 mg/kg bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 27 500 |
| Rosin oral ingestion from its use as non-medicinal ingredient in dental varnishes | 0.38 mg/kg bw/day for a child and 0.164 mg/kg bw/day for an adult | 275 mg/kg bw/day (NOAEL for rosin) | 825 mg/kg bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 725 to 1 680 |
| Rosin oral ingestion from its use as non-medicinal ingredient in tablets | 0.20 mg/kg bw/day | 275 mg/kg bw/day (NOAEL for rosin) | 825 mg/kg bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 1 375 |
| Rosin dermal exposure from sports grip materials, dancers or violinists rosin | 1.22 mg/kg bw/day | 275 mg/kg- bw/day (NOAEL for rosin) | 825 mg/kg -bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 225 |
| Storax oral ingestion as a food flavouring agent | 0.00090 mg/kg bw/day | 54 mg/kg bw/day (NOAEL for cinnamic acid; highest dose tested) | 500 mg/kg bw/day (LOAEL for cinnamaldehyde) based on mild cellular swelling in the liver | 59 700 |
| Storax dermal exposure from a face moisturizer | 0.038 mg/kg bw/day | 54 mg/kg bw/day (NOAEL for cinnamic acid; highest dose tested) | 500 mg/kg bw/day (LOAEL for cinnamaldehyde) based on mild cellular swelling in the liver | 1 420 |
| RCa dermal exposure from paint | 0.12 mg/kg bw/event | 275 mg/kg -bw/day (NOAEL for rosin) | 825 mg/kg -bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 2 290 |
| RNa dermal exposure from body cleanser | 0.0186 mg/kg bw/day | 275 mg/kg bw/day (NOAEL for rosin) | 825 mg/kg bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 14 780 |
| RNa dermal exposure from surface coatings and an adhesive/sealants | 0.12 mg/kg bw/event | 275 mg/kg -bw/day (NOAEL for rosin) | 825 mg/kg -bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 2 290 |
| RME oral ingestion from dental sealer base | 0.36 mg/kg bw/day for a child and 0.16 mg/kg-bw/day for an adult | 275 mg/kg bw/day (NOAEL for rosin) | 825 mg/kg bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 760 to 1 720 |
| RME dermal exposure from a gasket sealant | 0.131 mg/kg bw/event | 275 mg/kg -bw/day (NOAEL for rosin) | 825 mg/kg -bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 2 100 |
| RHME dermal exposure sunscreen lotion | 0.036 mg/kg bw/day | 275 mg/kg bw/day (NOAEL for rosin) | 825 mg/kg bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 7 640 |
| RHME inhalation and exposure sunscreen spray | 0.0018 mg/kg bw/day | 275 mg/kg bw/day (NOAEL for rosin) | 825 mg/kg bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 152 780 |
| RHME oral ingestion from lipstick | 0.33 mg/kg bw/day | 275 mg/kg bw/day (NOAEL for rosin) | 825 mg/kg bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 830 |
| RHME dermal exposure from face moisturizer | 0.34 mg/kg bw/day | 275 mg/kg bw/day (NOAEL for rosin) | 825 mg/kg bw/day (LOAEL) based on reduction in the litter size and pup weight as the result of reduced food intake in dams | 810 |
These margins of exposure are considered adequate to address uncertainties in the health effects and exposure datasets. Thus, the risk to the general population from resins and rosins substances is considered to be low and is not of concern.
8.4 Uncertainties in evaluation of risk to human health
There is uncertainty in the assumptions made on the composition of the UVCB substances in this assessment and the relative percentages of each substance in the mixture. There is uncertainty introduced by the lack of route-specific repeat-dose dermal toxicity studies and in the lack of substance specific hazard data.
9. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is risk of harm to the environment from tall oil (CAS RN 8002-26-4, specifically due to CTO. It is concluded that tall oil meets the criterion set out in paragraph 64(a) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is concluded that tall oil does not meet the criterion set out inparagraph 64(b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends.
It is also concluded that the other 11 substances in the Resins and Rosins Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that the 12 substances in the Resins and Rosins Group do not meet the criterion set out inparagraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that tall oil meets one or more of the criteria set out in section 64 of CEPA, specifically on the basis of risk presented by CTO and that the other 11 substances in the Resins and Rosins Group do not meet any of the criteria set out in section 64 of CEPA.
References
ACD/Percepta [prediction module]. c1996-2019. Toronto (ON): Advanced Chemistry Development, Inc.
[AIEPS] Artificial Intelligence Expert Predictive System. c2010-2012. Ver. 3.0. Gatineau (QC): Environment Canada. Model developed by Stephen Niculescu.
[ASTER] Assessment Tools for the Evaluation of Risk. 1999. Duluth (MN): US Environmental Protection Agency, Mid-Continent Ecology Division. [restricted access].
Arnot JA, Mackay D, Parkerton TF, Bonnell M. 2008. A database of fish biotransformation rates for organic chemicals. Environ Toxicol Chem. 27(11):2263-2270.
[AGDH] Australian Government Department of Health. 2017. Inventory Multi-tiered Assessment and Prioritisation, Human Health Tier II Assessment for Rosin, hydrogenated rosin and salts. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme (NICNAS).
Backhaus T, Faust M. 2012. Predictive environmental risk assessment of chemical mixtures: a conceptual framework. Environ Sci Technol. 46(5):2564-2573.
Bai J, Sun X, Zhang C, Zhao Y, Gong C. 2013. The atmospheric degradation reaction of dehydroabietic acid (DHHA) initiated by OH radicals and O3. Chemosphere. 92:933-940.
Baser KHC, Demirci F. 2011. Essential Oils. Online version. John Wiley & Sons, Inc. [accessed 2017 Nov 23]. [restricted access].
[BCFBAF] Bioaccumulation Program for Microsoft Windows [estimation model]. 2010. Ver. 3.01. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Berube P, Hall E. 1999. Treatment of evaporator condensate using a high temperature membrane bioreactor: determination of maximum operating temperature and system costs. A report for sustainable forest management network project – membrane bioreactors for contaminant control in closed pulp and paper mills.[PDF] Vancouver (BC): The University of British Columbia. [accessed 2020 Mar 10].
[BIOWIN] Biodegradation Probability Program for Microsoft Windows [estimation model]. 2008. Ver. 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Blackwell BR. 1978. Air stripping of kraft foul condensates to remove volatile impurities including methanol. Ph. D. thesis. University of British Columbia, Vancouver, British Columbia, Canada.
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741−752.
Borton DL, Hall TJ, Fisher RP, Thomas JF. 2004. Pulp & Paper Mill Effluent Environmental Fate & Effects. DEStech Publications, Inc. ISBN No. 1-932078-37-1.
Bronaugh RL, Stewart RF, Wester RC, Bucks D, Mailbach HI, Anderson J. 1985. Comparison of percutaneous absorption of fragrances by humans and monkeys. Food Chem Toxicol. 23(1):111-114.
Burdock GA. 2010. Fenaroli’s Handbook of Flavor Ingredients. 6th ed. Boca Raton (FL): CRC Press.
Burggraaf S, Langdon AG, Alistair LW, Roper DS. 1996. Accumulation and depuration of resin acids and fichtelite by the freshwater mussel Hyridella menziesi. Environ Toxicol Chem. 15(3):369–375.Calandra JC. 1960. Ninety-day subacute oral toxicity of rosin [trade name deleted]. Industrial Bio-Test Laboratories, Inc., Northbrook (IL). [Cited in the High Production Volume Information System, US EPA].
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Cite>Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
CATALOGIC [environmental fate and ecotoxicity model]. 2016. Ver. 5.12.1. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
[CFR] Code of Federal Regulations. 2017. Food Additives Permitted for Direct Addition to Food for Human Consumption. Title 21, Volume 3. Sec 172,510. Washington (DC): Government of the United States of America.
Clubb S, Sutherland JR. 2002. Rosin (CAS No. 8050-09-7) reproduction/developmental toxicity screening test. Report No. 21491. Tranent (UK): Inveresk Research. [Cited in the High Production Volume Information System, US EPA].
Clubb S. 2003. Rosin, PHME (CAS No. 8015-15-5) Combined Repeated Dose Toxicity Study with Reproduction/Developmental Toxicity Screening Test. Report No. 22143. Tranent (UK): Inveresk Research. [Cited in the High Production Volume Information System, US EPA].
Cole JG, Mackay D. 2000. Correlating environmental partitioning properties of organic compounds: the three solubilities approach. Environ Toxicol Chem. 19(2):265-270.
[ConsExpo Web] Consumer Exposure Web Model. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment).
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[ConsExpo Web] Consumer Exposure Web Model. 2020. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[COSING] Cosmetic Ingredients and Substances [database]. c2009-2017a. CAS RN 8002-26-4; CAS RN 61790-51-1 Brussels(BE): European Commission. [accessed 12 Sept 2017].
Czech P, Weber K, Dietrich DR. 2001. Effects of endocrine modulating substances on reproduction in the hermaphroditic snail Lymnaea stagnalis L. Aquat Toxicol. 53(2):103-114.
[DPD] Drug Product Database [database] [modified 2015 July 17] Ottawa (ON): Health Canada. [accessed 2015 Nov].
[EAFUS] Everything added to Food in the United States. Storax. [modified 2013 Apr 4].Silver Spring (MD): US Food and Drug Administration. [updated 2013 Apr 4; accessed 2017 Nov 10].
[EC] European Commission. 2000. IUCLID Dataset [Resin, acid and rosin acids], CAS No. 8050-09-7 [Internet]. Year 2000. Ispra [IT]: European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. [accessed 2010 Feb 9].
[EC] Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EC, HC] Environment Canada, Health Canada. 2011. Screening assessment for the Challenge: Rosin, hydrogenated Chemical Abstracts Service Registry Number 65997-06-0, Resin acids and Rosin acids, hydrogenated, esters with pentaerythritol Chemical Abstracts Service Registry Number 64365-17-9, Resin acids and Rosin acids, hydrogenated, esters with glycerol Chemical Abstracts Service Registry Number 65997-13-9,Resin acids and Rosin acids, hydrogenated, esters with triethylene glycol Chemical Abstracts Service Registry Number 68648-53-3. Ottawa (ON): Government of Canada. [accessed 2017 Oct 12].
[ECCC] Environment and Climate Change Canada. 2017. Data collected from a targeted information gathering initiative for assessments under the Chemicals Management Plan (July 14th, 2017). Data prepared by ECCC.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Gatineau (QC): Data used to create substance-specific hazard and exposure profiles and assign risk classifications. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2016cData collected from a targeted information gathering initiative for assessments under the Chemicals Management Plan (June 2016). Data prepared by ECCC, Health Canada; Existing Substances Program.
[ECCC] Environment and Climate Change Canada. 2016d. Data collected from a targeted information gathering initiative for assessments under the Chemicals Management Plan (Fall 2016). Data prepared by ECCC, Health Canada; Existing Substances Program.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017. Categorization of Chemical Substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2021a. Supporting documentation: summary tables of environmental monitoring data for representative components of the Resins and Rosins Group. Gatineau (QC): ECCC. Information in support of the draft screening assessment for the [Resins and Rosins]. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2021b. Supporting documentation: Summary tables of data on physical-chemical properties, bioaccumulation, persistence, fate and toxicity, as well as robust study summaries for representative components of the Resins and Rosins Group. Gatineau (QC): ECCC. Information in support of the draft screening assessment for the [Resins and Rosins]. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018a. Rapid screening of substances with limited general population exposure. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018b. Screening assessment: substances identified as being of low concern using the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. [modified 2015 Jun 15]. Candidate List of Substances of Very High Concern for Authorisation. Helsinki (FI): European Chemicals Agency. [accessed 2016 Jun 1].
[ECHA] European Chemicals Agency. c2007-2017. Registered substances [database]; search results for CAS RNs 8002-26-4, 8016-81-7, 8050-09-7, 8050-15-5, 8050-28-0, 9007-13-0, 61790-51-0 and EC / List number 931-433-1. Helsinki (FI): ECHA. [accessed 2017 Nov 30].
[ECOSAR] ECOlogical Structure Activity Relationships Class Program [estimation model]. 2012. Ver. 1.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
European Chemicals Bureau. 2003. Technical guidance document on risk assessment in support of Commission Directive 93/67/EEC on risk assessment for new notified substances and Commission Regulation (EC) No 1488/94 on risk assessment for existing substances. Luxembourg City (LU): European Chemicals Bureau.
Fregert S, Rorsman H.1963. Hypersensitivity to balsam of pine and spruce. Arch Dermatol. 87:693-6957.
Fernandez MP, Ikonomou MG, Buchanan I. 2007. An assessment of estrogenic organic contaminants in Canadian wastewaters. Sci Total Environ. 373:250-269.
[FEMA] Flavoring and Extract Manufacturers’ Association. 1965. Recent Progress in the Consideration of Flavoring ingredients Under the Food Additives Amendment. III GRAS Substances. Washington (DC): Flavoring and Extract Manufacturers’ Association.Ficheux AS, Morriset T, Chevillotte G, Postic C, Roudot AC. 2014. Probabilistic assessment of exposure to nail cosmetics in French consumers. Food and Chemical Toxicology. 66:36-43.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC, 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food and Chemical Toxicology 78: 159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC. 2016. Consumption of cosmetic products by the French population. Second part: Amount data. Food and Chemical Toxicology 90: 130-141.
Foran CD. 2006. Tall oil soap recovery [PDF]. Arizona Chemical Company, Savannah, GA, USA. [accessed 2017 Dec 6].
[FPAC, NCASI] Forest Products Association of Canada, National Council for Air and Stream Improvement. 2019. Pulp and paper sector’s response to the draft screening assessment for the resins and rosins group of substances and the proposed risk management scope for crude tall oil (CTO). Submitted to Program Development and Engagement Division, Environment and Climate Change Canada.
Georgia-Pacific. 2018. Products & Technologies: Crude Tall Oil. [accessed 2018 Mar 6].
Gonis G, Slezak FB, Lawson NE. 1973. Preparation of maleopimaric acid. Ind. Eng. Chem. Prod. Res. Dev. 12(4):326-327.
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations.Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances [PDF]. Ottawa (ON) Government of Canada.
Hemingway RW, Greaves H. 1973. Biodegradation of resin acid sodium salts. TAPPI J 56 (12); 189-192.
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [estimation model]. 2008. Ver. 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Hoelgaard A, Mollgaard B. 1982. Permeation of linoleic acid through skin in vitro. J. Pharm. Pharmacol. 34:610-611.
Holmbom B, Ekman R. 1978. Tall oil precursors of scots pine and comment spruce and their change during sulphate pulping. ACTA Academiae Aboensis, Ser. B, vol. 38 no 3. pp. 1 – 11.
Holmbom B, Sundberg A, Strand A. 2010. Surface-active compounds as forest-industry by-products. In: Kjellin M, Johansson I, editors. Surfactants from Renewable Resources. New York (NY): Wiley. p. 45-62.
Holmbom B. 2011. Extraction and utilisation of non-structural wood and bark components. In: Alén R, editor. Biorefining of Forest Resources. Helsinki (FI): Paperi ja Puu Oy. p. 178-224.
Huibers DTA. 2000. Tall oil. In: Kirk-Othmer encyclopedia of chemical technology. Online version. [accessed 2016 Oct 5]. [restricted access].
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [estimation model]. 2010. Ver. 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[J-CHECK] Japan CHEmicals Collaborative Knowledge database [database]. c2010- . Tokyo (JP): National Institute of Technology and Evaluation (NITE). [accessed 2017 Nov 9].
Karadeniz B, Ulker Z, Alpsoy L. 2013. Genotoxic and cytotoxic effects of storax in vitro. Toxicol Ind Health. 29(2):181-186.
Karadeniz B, Ulker Z, Alpsoy L. 2011. Genotoxic and cytotoxic effects of storax in vitro. Toxicol Ind Health. 29(2):181-186.
Kay JH. 1961. Acute toxicity of rosins. Northbrook (IL): Industrial Bio-Test Laboratories Inc. [Cited in the High Production Volume Information System, US EPA].
Kay JH. 1962. Two-year chronic oral toxicity of b-wood resin – albino rats. Northbrook (IL): Industrial Bio-Test Laboratories, Inc. [cited in the High Production Volume Information System, US EPA].
[KOAWIN] Octanol-Air Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[KOCWIN] Organic Carbon Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Kostamo A, Holmbom B, Kukkonen J. 2004. Fate of wood extractives in wastewater treatment plants at Kraft pulp mills and mechanical pulp mills. Water Res. 38(4):972-982.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Lehtinen KJ, Mattsson K, Tana J, Engström C, Lerche O, Hemming J. 1999. Effects of wood-related sterols on the reproduction, egg survival, and offspring of Brown Trout (Salmo trutta lacustris L.). Ecotoxicol Environ Saf. 42(1):40-49.
Leppänen H, Kukkonnen JVK, Oikari AOJ. 2000. Concentration of retene and resin acids in sedimenting particles collected from a bleached kraft mill effluent receiving lake. Water Res 34(5):1604-1610.
Leppänen H, Oikari A. 2001. Retene and resin acid concentrations in sediment profiles of a lake recovering from exposure to pulp mill effluents. J Paleolimnol. 25:367-374.
Lesokhimik Trade House 2018. Russia, Saint Petersburg, 197342, Russia [accessed 2018 Mar 6].
Liss SN, Bicho PA, Saddler JN. 1997. Microbiology and biodegradation of resin acids in pulp mill effluents: a minireview. Can J Microbiol. 75:599-611.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2021 April 4]. Ottawa (ON): Health Canada. [accessed 2021 July].
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol 43: 279-291.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol 46: 1516-1524.
Machovcova A. 2010. Colophony, a hidden allergen on ECG electrodes in a boy after cardiovascular surgery. Pediatr Dermatol. 28(3):345-347.
MacLatchy DL, Van Der Kraak GJ. 1995. The phytoestrogen β-sitosterol alters the reproductive endocrine status of goldfish. Toxicol Appl Pharmacol. 134(2):305-312.
MacLeay and Associates Ltd. 1986. Aquatic toxicity of pulp and paper mill effluent: a review. Prepared for Environment Canada, Fisheries and Oceans Canada, Canadian Pulp and Paper Association, and Ontario Ministry of the Environment. Rep. EPS 4/pf/1.
Meriläinen P, Lahdelma I, Oikari L, Hyötyläinen, Oikari A. 2006. Dissolution of resin acids, retene and wood sterols from contaminated lake sediments. Chemosphere 65(5):840-846.
Meylan WM, Howard PH. 1995. Atom/fragment contribution method for estimating octanol-water partition coefficients. J Pharm Sci. 84:83-92.
Meylan WM, Howard PH, Boethling RS. 1996. Improved method for estimating water solubility from octanol/water partition coefficient. Environ Toxicol Chem. 15:100-106.
Mitani K, Fujioka M, Uchida A, Kataoka H. 2007. Analysis of abietic acid and dehydroabietic acid in food smaples by in-tube solid-phase microextraction coupled with liquid chromatography –mass spectrometry. J Chromatogr A. 1146:61-66.
Moreno OM. 1972. Acute oral toxicity in rats of [methyl ester of rosin]. Toxicological Resources, East Millstone, New Jersey.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [estimation model]. 2010. Ver. 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[MSDS] Material Safety Data Sheet. 2007. King Pine Dark 32oz. Bayamon (PR): Luis Garration Inc. [accessed 2017 Sept 7].
[MSDS] Material Safety Data Sheet. 2009. 3M Body Schutz [PDF] P.N. 08864. St. Paul (MN):3M Company [accessed 2017 Dec 1].
[MSDS] Material Safety Data Sheet. 2010. 3M Steri-Strip. St. Paul (MN): 3M Company [accessed 2017 Oct 10].
[MSDS] Material Safety Data Sheet. 2013. PreviDent Varnish-Raspberry. New York (NY): Colgate-Pamolive Company [accessed 2018 Jan 1].
[MSDS] Material Safety Data Sheet. 2015a. Biorenewables Industrial Degreaser [PDF]. Maumee (OH): Spartan Chemical Company Inc. [accessed 2017 SeptSep 7].
[MSDS] Material Safety Data Sheet. 2015b. Hi-Tack Gasket Sealant [PDF]. Mississauga (ON): Henkel [accessed 2017 SeptSep 7].
Murie E. 2001. Rosin, partially hydrogenated methyl ester, CAS No. 8050-15-5 Chromosomal Aberration Assay with Chinese Hamster Ovary Cells in vitro (Complying with EC (Annex V) and OECD 473 Guidelines). Report Number 20718. Tranent (UK): Inveresk Research. [Cited in the High Production Volume Information System, US EPA].
[NCASI] National Council for Air and Stream Improvement. 2018. Tall oil production in the pulp & paper sector in Canada. A memorandum from NCASI staff to Environment and Climate Change Canada. Montreal (Quebec): NCASI.
[New EQC] New Equilibrium Criterion Model. 2011. Ver. 1.00 (Beta). Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2021 July 01]. Ottawa: (ON): Health Canada. [accessed 2021 July].
Niimi AJ, Lee HB. 1992. Free and conjugated concentrations of nine resin acids in rainbow trout (Oncorhynchus mykiss) following waterborne exposure. Environ Toxicol Chem. 11:1403-1407.
[NRC] National Research Council. 2011. Data gathering on chemicals released to indoor air of residences from building materials and furnishings. Ottawa (ON): Health Canada, National Research Council. Internal report.
Nyren V, Back E. 1958. The ionization constant, solubility product and solubility of abietic and dehydroabietic acid. Acta Chemica Scandinavica 12(7):1516-1520.
[OECD] Organisation for Economic Co-operation and Development. 2018. Guidance document on aquatic toxicity testing of difficult substances and mixtures. OECD Series on Testing and Assessment Number 23 (Second Edition). ENV/JM/MONO(2000)6/REV1. Paris (FR): OECD, Environment Directorate.
OECD QSAR Toolbox. 2016. Ver. 3.4. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Oikari A, Fragoso N, Leppänen H, Chan T, Hodson P. 2002. Bioavailability to juvenile rainbow trout (Oncorhynchus mykiss) of retene and other mixed-function oxygenase-active compounds from sediments. Environ Toxicol Chem. 21(1):121-128.
Oikari A, Ånäs E, Kruzynki Kruzynski G, Holmbom B. 1984. Free and conjugated resin acids in the bile of rainbow trout, Salmo gairdneri. Bull Environ Contam Toxicol. 33(2):233-240.
Oikari A, Holmbom B. 1986. Assessment of water contamination by chlorophenolics and resin acids with the aid of fish bile metabolites. In: Poston TM, Purdy R. editors. Aquatic Toxicology and Environmental Fate. ASTM, Philadelphia. p. 252-267.
Panda H. 2005. Handbook on speciality gums, adhesives, oils, rosin and derivatives, resins, oleoresins, katha, chemicals with other natural products. Delhi (IN): Asia Pacific Business Press, Inc. 792 p.
Panda H. 2013. Handbook on tall oil rosin production, processing and utilization. Asia Pacific Business Press Inc. Chapter 24: Tall Oil in Rubber. p. 391-395.
Peng G, Roberts JC. 2000. Solubility and toxicity of resin acids, Water Res. 34(10):2779-2785.
[PhysProp] Interactive PhysProp Database [database]. c2013. Syracuse (NY): SRC, Inc. [accessed 2017 Nov 30].
Pine Chemical Group. Distilled Tall Oil (DTO) 2018. Helsinki, Finland.
Pipeline Profiler. 2016. V.1.0.52.13. Developed by LMC Oasis for ECCC.
Quinn BP, Booth MM, Delfino JJ, Holm SE, Gross TS. 2003. Selected resin acids in effluent and receiving waters derived from a bleached and unbleached Kraft pulp and paper mill. Environ Toxicol Chem. 22(1):214-218.
REACH dossier [Registration, Evaluation, Authorisation and Restriction of Chemicals]. 2022a. Registration (REACH) dossier for Rosin (CASRN 8050-09-7). First published: 2017. Last modified: 30-Mar-2022. [accessed 2022 April].
REACH dossier [Registration, Evaluation, Authorisation and Restriction of Chemicals]. 2022b. Registration (REACH) dossier for Rosin (CASRN 8050-09-7). First published: 2018. Last modified: 30-Mar-2022. [accessed 2022 April].
REACH dossier [Registration, Evaluation, Authorisation and Restriction of Chemicals]. 2022c. Registration (REACH) dossier for Rosin (CASRN 8050-09-7). First published: 2016. Last modified: 30-Mar-2022. [accessed 2022 April].
Riebeek, WM. 1990. Determination of the acute oral toxicity of methyl ester of partially hydrogenated rosin in rats. Report No. V.90203. Amsterdman (NE): TNO-CIVO Institutes.
Robinson V, Bergfeld WF, Belsito DV, Klaassen CD, Marks JG, Shank RC, Slaga TJ, Snyder PW, Anderson FA. 2009. Amended safety assessment of tall oil acid, sodium tallate, potassium tallate, and ammonium tallate. Int J Toxicol. 28(Suppl 3):222S-258S.
Rogers IH, Davis JC, Kruzynski G, Mahood HW, Servizi JA, Gordon RW. 1975. Fish toxicants in Kraft effluents. TAPPI. 58:136-140.
Sadhra S, Foulds IS, Gray CN, Koh D, Gardiner K. 1994. Colophony--uses, health effects, airborne measurement and analysis. Ann Occup Hyg. 38:385-396.
Sagdic O, Ozkan G, Ozcan M, Ozcelik S. 2005. A study of inhibitory effects of Sigla tree (Liquidambar orientalis Mill. var. orientalis) storax against several bacteria. Phytother Res. 19(6):549-551.
Schenker URS, Macleod M, Scheringer M, Hungerbϋhler K. 2005. Improving data quality for environmental fate models: a least-squares adjustment procedure for harmonizing physicochemical properties of organic compounds. Environ Sci Technol. 39(21):8434-8441.
[SDS] Safety Data Sheet. 2003. Loctite Pipe Joint Compound [PDF]. Rocky Hill (CN): Henkel Loctite Corporation. [accessed 2017 Dec 2].
[SDS] Safety Data Sheet. 2007. Altex Sea Barrier Alloy 100 Plus Antifouling [PDF]. Bay of Plenty (NZ): Altex Coatings Inc. [accessed 2017 Dec 7].
[SDS] Safety Data Sheet. 2010. HIPREN EM 1723T [PDF]. Pancevo (SB): Pencevo Petrochem [accessed 2017 Dec 3].
[SDS] Safety Data Sheet. 2015a. Temporary Cement-Base [PDF]. Alsip (IL): GC America Inc. [accessed 2017 Dec 8].
[SDS] Safety Data Sheet. 2015b. Root Canal Sealer-Base[PDF]. Alsip (IL): GC America Inc. [accessed 2017 Dec 8].
[SDS] Safety Data Sheet. 2016. Baymod N XL 33.61 VP [PDF]. Pittsburg (PA): Arlanxeo LLC. [accessed 2017 Dec 3].
[SDS] Safety Data Sheet. 2017. Summer Breez Vent Air Freshener [PDF]. Mississauga (ON): CPS Products Canada Ltd. [accessed 2018 Jan 2].
Simoneit BRT, Elias VO. 2000. Organic tracers for biomass burning in atmospheric particulate matter over the ocean. Marine Chemistry. 69:301-312.
Stevenson, FM. 2001. Rosin, partially hydrogenated methyl ester, CAS No. 8050-15-5 Testing for Mutagenic Activity with Salmonella Typhimurium TA 1535, TA 1537, TA 98 and TA 100 and Escherichia coli WP2uvrA. Report No. 20337. Tranent (UK): Inveresk Research. [cited in the High Production Volume Information System, US EPA].
Study Submission. 2016a. Unpublished confidential studies submitted to Environment and Climate Change Canada (ECCC) under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division. Internal reference ID No. 702.
Study Submission. 2016b. Unpublished confidential studies submitted to Environment and Climate Change Canada (ECCC) under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division. Internal reference ID No. 703.
Study Submission. 2016c. Unpublished confidential studies submitted to Environment and Climate Change Canada (ECCC) under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division. Internal reference ID No. 705.
Study Submission. 2016d. Unpublished confidential studies submitted to Environment and Climate Change Canada (ECCC) under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division. Internal reference ID No. 706.
Study Submission. 2017a. Unpublished confidential studies submitted to Environment and Climate Change Canada (ECCC) under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division. Internal reference ID No. 701.
Study Submission. 2017b. Unpublished confidential studies submitted to Environment and Climate Change Canada (ECCC) under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division. Internal reference ID No. 704.
Sturthridge TR, Campin DN, Langdon AG, Mackie KL, McFarlane PN, Wilkins AL. 1991. Treatability of bleached Kraft pulp and paper mill wastewaters in a New Zealand aerated lagoon treatment system. Water Sci Technol 24(3/4):309-317.
[TaPL3] Long Range Transport and Persistence Level III Model. 2003. Ver. 3.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
Tavendale MH, McFarlane PN, Mackie KL, Wilkins AL, Langdon AG. 1997. The fate of resin acids-1. The biotransformation and degradation of deuterium labelled dehydroabietic acid in anaerobic sediments. Chemosphere 35(10):2137–2151.
[TEST] Toxicity Estimation Software Tool. 2016. Ver. 4.2. Washington (DC): US Environmental Protection Agency.
Tremblay L, Van Der Kraak G. 1999. Comparison between the effects of the phytosterol β-sitosterol and pulp and paper mill effluents on sexually immature rainbow trout. Environ Toxicol Chem. 18(2):329-336.
Tse TJ, Codling G, Jones PD, Thoms K, Liber K, Giesy JP, Wheater H, Doig LE. 2014. Reconstructing long-term trends in municipal sewage discharge into a small lake in northern Manitoba, Canada. Chemosphere. 103:299-305.
UCY Energy 2007. UCY business services & trading (Germany) GmbH. Product Datasheet Item number 10-011 [PDF]. Am Villepohl 4 D-53347 Alfter, Germany. [accessed 2018 Mar 6].
Uloth, V, Shewchuk D, Guy E, Heek RV 2009. Waste fatty acid addition to black liquor to decrease tall oil soap solubility and increase skimming efficiency in Kraft mills pulping mountain pine beetle-infested wood. Mountain Pine Beetle Working Paper 2009-26. Natural Resources Canada, Victoria, BC.
[US EPA] United States Environmental Protection Agency. 2004. HPV Final Submission for Rosins and Rosin Salts September 2004. 201-15573A. Washington (DC): US EPA, Office of Pollution Prevention and Toxics.
[US EPA] United States Environmental Protection Agency. 2007. Dermal Exposure Assessment: A Summary of Approaches. Washington (DC): National Center for Environmental Assessment, Office of Research and Development. EPA/600/R-07/040F.
[US EPA] United States Environmental Protection Agency. 2008a. Supporting Documents for Initial Risk-Based Prioritization of High Production Volume Chemicals. Rosin and Rosin Salts Category. Washington (DC): US EPA.
[US EPA] United States Environmental Protection Agency. 2008b. Supporting Documents for Initial Risk-Based Prioritization of High Production Volume Chemicals. Tall Oil and Related Substances Category. Washington (DC): US EPA.
[US EPA] United States Environmental Protection Agency. 2008c. Supporting Documents for Initial Risk-Based Prioritization of High Production Volume Chemicals. Rosin Esters Category. Washington (DC): US EPA.
[US EPA] United States Environmental Protection Agency. 2011. Exposure Factors Handbook. 2011 Edition. Washington (DC): US Environmental Protection Agency. EPA/600/R-09/052F, 2011.
[US EPA] United States Environmental Protection Agency. 2016. Chemical Data Reporting (CDR) data for 2016. Washington (DC): US EPA, Office.
[US EPA] United States Environmental Protection Agency. 2017. The Chemistry Dashboard App and Database [database]. US Environmental Protection Agency's Chemical Safety for Sustainability Research Program. National Center for Computational Toxicology, NC, USA.
[VCCLab] Virtual Computational Chemistry Laboratory . ALOGPS [non-Java interface]. 2005. Ver. 2.1. Munich (DE): VCCLab. [Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, Palyulin VA, Radchenko EV, Zefirov NS, Makarenko AS, et al. 2005. Virtual computational chemistry laboratory - design and description. J Comput Aid Mol Des. 19:453-463.].
Villeneuve DC, Yagminas AP, Marino IA, Becking GC. 1977. Toxicity studies on dehydroabietic acid. Bull Environ Contam Toxicol. 18:42-47.
[WATERNT] Water Solubility Program [estimation model]. 2010. Ver. 1.01. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[WHO] World Health Organization. 2001. Safety evaluation of certain food additives: fifty-fifth meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additive Series, No. 46. Geneva (CH): World Health Organization, International Programme on Chemical Safety. [accessed 2018 Jun 6].
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Hum Ecol Risk Assess 19(1):158-188.
Wising U, Stuart P. 2006. Identifying the Canadian forest biorefinery. Pulp and Paper Canada 107(6):25-30.
Wong, A. 2010. Shrinking source: A 2010 assessment of the potential production of tall oil in Canada. Forest Chemicals Review. March-April 2010. 16-19.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [estimation model]. 2010. Ver. 1.42. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Yunker MB, Lachmuth CL, Cretney WJ, Folwer BR, Dangerfield N, White L, Ross PS. 2011. Biota: sediment partitioning of aluminium smelter related PAHs and pulp mill related diterpenes by intertidal clams at Kitimat, British Columbia. Mar Environ Res. 72(3):105-126.
Zinkel DF, Russell J. 1989. Naval stores: Production, chemistry, utilization. Pulp Chemicals Association, Inc. New York.
Appendix A. The ecological risk classification of organic substances
The ecological risk classification of organic substances (ERC) is a risk-based approach that considers multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., LC50) for characterization. Since some of the substances are UVCB substances and could not be suitably represented by single chemical structures, a manual judgement-based approach to classification was used. The following paragraphs summarize the approach, which is described in detail in ECCC (2016a).
Hazard profiles were established based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also developed consisting of multiple metrics, including potential emission rate, overall persistence and long-range transport potential. The hazard and exposure profiles were compared to decision criteria to classify the hazard and exposure potentials for each organic substance as low, moderate or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of storax (balsam), RME and rosin, hazard and exposure could not be fully profiled due to the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed by examining the UVCB constituents and DSL Inventory Update information and making decisions by considering similar substances and applying expert judgement.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance based on its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error in empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error of underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure, as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is believed to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for 4 substances in the Resins and Rosins Group, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
The hazard and exposure classifications for 4 substances from the Resins and Rosins Group are summarized in Table A-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| DHAA | moderate | low | low |
| Storax (balsam) | low | low | low |
| RME | low | low | low |
| Rosin (CAS RN 73138-82-6) | high | low | low |
According to information considered under ERC, DHAA was classified as having a low exposure potential. DHAA was classified as having a moderate hazard potential on the basis of reactive mode of action and potential to cause adverse effects in aquatic food webs given its bioaccumulation potential. DHAA was classified as having a low potential for ecological risk. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, this substance is unlikely to be resulting in concerns for the environment in Canada.
On the basis of low hazard and low exposure classifications according to information considered under ERC, storax (balsam) and RME were classified as having a low potential for ecological risk. It is unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under ERC, rosin (CAS RN 73138-82-6) was classified as having a low exposure potential. Rosin (CAS RN 73138-82-6) was classified as having a high hazard potential on the basis of reactive mode of action, potential to cause adverse effects in aquatic food webs given its bioaccumulation potential, and structural alerts from the OECD toolbox identified rosin (CAS RN 73138-82-6) as being potential DNA binder. Rosin (CAS RN 73138-82-6) was classified as having a moderate potential for ecological risk; however, the risk classification was decreased to low potential for ecological risk following the adjustment of risk classification based on a low potential for local-scale exposures (see section 7.1.1 of the ERC approach document [ECCC 2016a]). The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of rosin (CAS RN 73138-82-6). On the basis of current use patterns, it is unlikely that this substance is resulting in concerns for the environment in Canada.
Appendix B. Human health exposure parameters
| Substance | Products available to consumers | Assumptions | Exposure estimate |
|---|---|---|---|
| Tall oil | Facial cleanser cosmetic | Frequency of use: 1.6/day (PCP II, Loretz 2008) Product amount: 2.58 g/application (PCP II, Loretz 2008) Surface area- half of the head (adult): 637 cm2 (PCP II, Health Canada 1995) Rosin assumption: weight fraction: 0.3 (cosmetic notifications submitted to Health Canada) Retention factor: 0.01 (PCP II ) Dermal absorption: 12.2 μg/cm2 (from product amount * weight fraction / surface area) |
Internal daily dose: 0.18 mg/kg bw/day |
| Tall oil | Kitchen cleaner | Inhalation exposure during spraying
ConsExpo Web (2016) Fact Sheet: Cleaning and washing, All-purpose cleaners, All purpose cleaning spray, application – spraying (non-volatile substances)
Frequency of use: 1/day Spray duration: 0.23 min Exposure duration: 60 min Weight fraction: 10% (MSDS 2007) Room volume: 15m3 Room height: 2.5 m Ventilation rate: 2.5/hr Inhalation rate: 25.5 L/min (mild exercise) Mass generation rate: 1.6 g/s Airborne fraction: 0.006 Density non volatile: 1 g/cm3 Inhalation cut off diameter: 15 µm Aerosol diameter distribution: lognormal Median diameter (CV): 2.4 (0.37) µm Dermal exposure during spraying ConsExpo Web (2016) Fact Sheet: Cleaning and washing, All-purpose cleaners, All purpose cleaning spray, Application – spraying (non-volatile substances) Dermal absorption 11% Exposed area: 2200 cm2 Contact rate 46 mg/min Release Duration 0.46 min Dermal load: 0.96 μg/cm2 (from contact rate * release duration * weight fraction / surface area) Dermal exposure during rinse/wipe ConsExpo Web (2020) Fact Sheet: Cleaning and washing, All-purpose cleaners, All purpose cleaning spray, Application – rinsing Population: Adult Model: Dermal, direct product contact – instant application Exposed area (ConsExpo default): 225 cm2 Weight fraction substance: 0.57% Product amount: 0.31 g (default) Note: inhalation exposure to during rising/wiping during rinsing/wiping is expected to be negligible. |
Mean event air concentration: 0.3 mg/m3 Internal inhalation daily dose: 0.0064 mg/kg bw/day External dermal dose (during spraying): 0.96 µg/cm2 Internal dermal dose: 0.003 mg/kg bw/day Internal dermal dose (during rinse/wiping): 0.0489 mg/kg bw/day Combined internal daily dose: 0.0583 mg/kg bw/day |
| Rosin / resin acids and rosins acids | Nail polish |
Concentration: 10% rosin in nail polish Product amount: 0.16 g (PCP IV Ficheux et al. (2014) Dermal absorption: 11% |
Internal daily dose: 0.23 mg/kg bw/eventevent |
| Rosin / resin acids and rosins acids | Eye shadow |
Concentration: 10% rosin in eye shadow (cosmetic notifications submitted to Health Canada) Frequency of use: 1.2/day (PCP II, Loretz et al. 2008) Product amount: 0.009 g (PCP II, Loretz et al. 2008) Exposed area: 24 cm2 (PCP II, ConsExpo 2006) Dermal absorption: 19 μg/cm2 |
Internal daily dose: 0.0077 mg/kg bw/day |
| Rosin / resin acids and rosins acids | Lipstick |
Maximum weight fraction: 3% (cosmetic notifications submitted to Health Canada) Frequency of use: 2.35/day (PCP II, Loretz et al. 2005) Amount applied/ingested: 0.01 g (PCP II, Loretz et al. 2005) Uptake fraction: 1 |
Internal daily dose: 0.01 mg/kg bw/day |
| Rosin / resin acids and rosins acids | Non-medicinal ingredient in dental varnish |
59% rosin Application volume: 1.6 mL Application mass: 1.05 g of rosin (0.944 mL × 1.115 g/mL) Released duration: 90 days Child mass: 31 kg Adult mass: 70.9 kg |
Internal daily dose: Adult: 0.164 mg/kg bw/day Child 0.38 mg/kg bw/day |
| Rosin / resin acids and rosins acids | Non-medicinal ingredient in tablet |
7 mg/tablet (TPD data) 2 tablets/day (professional judgement) |
Internal daily dose: 0.2 mg/kg bw/day |
| Rosin / resin acids and rosins acids | Violinists rosin/ sports grip agent |
US EPA high-end soil adhesion factor for adults in a residential setting: 0.07 mg/cm2 (professional judgement due to the similarities in powdered rosin and particulate soil) Surface area (half of both hands/palms: 455 cm2 (Health Canada 1995) Surface area: 455 cm2 (palms of both hands) Frequency: 10 events/day (professional judgement) Dermal absorption: 19 μg/cm2 |
Internal daily dose: 1.22 mg/kg bw/day |
| Storax | Face moisturizer |
Concentration: 0.3% storax (cosmetic notifications submitted to Health Canada) Frequency of use: 1.8/day (PCP I, Loretz et al. 2005) Product amount: 1.2 g/ application (PCP I, Loretz et al. 2005) Surface area- half of the head (adult): 637.5 cm2 (PCP I, Health Canada 1995) Retention factor: 1 (PCP I) Dermal absorption: 42% |
Internal daily dose: 0.038 mg/kg bw/day |
| Resin acids and rosin acids, calcium salts (RCa) | Paints and coatings |
Concentration: 20% RCa (from MSDS sheet, SDS 2007) EPA Thin Film (mineral oil with partial wipe) 0.62 × 10-3 cm (mineral oil with partial wipe) (US EPA 2011) Density: 1 g/mL Surface area (half of both hands/palms: 455 cm2 (Health Canada 1995) Dermal absorption: 19 μg/cm2 |
Internal event dose: 0.12 mg/kg bw/event |
| Resin acids and rosin acids, sodium salts (RNa) | Body cleanser |
Concentration: 10% RNa (cosmetic notifications submitted to Health Canada) Frequency of use: 1.2/day (PCP IV, Loretz et al. 2005) Product amount: 1.1 g (PCP IV, Loretz et al. 2005) Exposed area: 16 925 cm2 (PCP IV, Health Canada 1995) Retention factor: 0.01 (PCP IV) Dermal absorption: 0.065 μg/cm2 (from product amount * concentration / surface area) |
Internal daily dose: 0.0186 mg/kg bw/day |
| Resin acids and rosin acids, sodium salts (RNa) | Surface coatings and adhesives/sealants |
Concentration: 20% RNa (SDS 2003) from MSDS sheet. Thin film (mineral oil with partial wipe): 0.62 × 10-3 cm (mineral oil with partial wipe (US EPA 2011) Density: 1 g/mL Surface area (half of both hands/palms:palms of hands adult) 455 cm2 (Health Canada 1995) Dermal absorption: 19 μg/cm2 |
Internal dose: 0.12 mg/kg bw/event |
| Resin acids and rosin acids, me esters (RME) | Gasket sealant |
Concentration: 30% RMe (MSDS 2015b) from MSDS sheet. Thin film ( mineral oil with partial wipe) 0.62 × 10-3 cm (mineral oil with partial wipe (US EPA 2011) Surface area (half of both hands/palms: 455 cm2 (Health Canada 1995) Surface area (palms of hands adult): 455 cm2 Assumed product density: 1 g/mL Dermal absorption: 11% |
Internal dose: 0.131 mg/kg bw/event |
| Resin acids and rosin acids, me esters (RME) | Dental sealer base |
Concentration: 25% to 50% (SDS 2015a,b) Application mass: 2.0 g of sealer base Released duration: 90 days Child mass: 31 kg Adult mass: 70.9 kg |
Internal daily dose: Adult: 0.16 mg/kg bw/day Child: 0.36 mg/kg bw/day |
| Resin acids and rosin acids, hydrogenated, me esters (RHME) | Sunscreen (spray) |
Concentration: 1% RHME from NNHPD Frequency of use: 1.4/day (PCP V, Ficheux et al. 2015) Product amount: 5.2 g/day (PCP V, Ficheux et al. 2016) Surface area (adult): >14000 cm2 (PCP V, Ficheux et al. 2016) Retention factor: 1 (PCP V) 5 minute spray duration with 60 minutes of exposure Room volume: 15 m3 Ventilation rate: 2.5/hr Inhalation : 16.2 m3/day Spray towards user Cloud volume: 0.5 m3 Mass generation rate: 1.72 g/min Airborne fraction: 0.1 Density non-volatile: 1 g/cm3 Inhalation cutcut-off: 15 µm Aerosol distribution: log normal |
Mean event air concentration: 0.19 mg/m3 Internal dose: 0.0018 mg/kg bw/day |
| Resin acids and rosin acids, hydrogenated, me esters (RHME) | Sunscreen (lotion) |
Concentration: 0.01% RHME from DPD Frequency of use: 1.4/day (PCP V, Ficheux et al. 2015) Product amount: 18.2 g /application (PCP V, Ficheux et al. 2016) (Surface area (adult): >14000 cm2 PCP V, Ficheux et al. 2015) Retention factor: 1 (PCP I) Dermal absorption: 0.13 μg/cm2 (from product amount * concentrationconcentration / surface area) |
Internal daily dose: 0.036 mg/kg bw/day |
| Resin acids and rosin acids, hydrogenated, me esters (RHME) | Lipstick |
Maximum weight fraction: 100% (cosmetic notifications submitted to Health Canada) Frequency of use: 2.35/day (PCP II, Loretz et al. 2005) Amount applied/ingested: 0.01g (PCP II, Loretz et al. 2005) Uptake fraction: 1 |
Internal daily dose: 0.33 mg/kg bw/day |
| Resin acids and rosin acids, hydrogenated, me esters (RHME) | Face moisturizer |
Concentration: 10% RHME (cosmetic notifications submitted to Health Canada) Frequency of use: 1.8/day (PCP I, Loretz et al. 2005) Product amount: 1.2 g /application (PCP I, Loretz et al. 2005) Surface area (adult): 637.5 cm2 (PCP I, Health Canada 1998) Retention factor: 1 (PCP I) Dermal absorption: 11% |
Internal daily dose: 0.34 mg/kg bw/day |
PCP I, II: Health Canada. 2013. Recommended default values for personal care product exposure scenarios. Unpublished report. Ottawa (ON): Government of Canada.
PCP III: Health Canada. 2015. Recommended default values for personal care product exposure scenarios. Unpublished report. Ottawa (ON): Government of Canada.
PCP IV, V: Health Canada. 2018. Recommended default values for personal care product exposure scenarios. Unpublished report. Ottawa (ON): Government of Canada.
