Screening assessment - sodium ortho-phenylphenate; SOPP
Official title: Screening Assessment - [1,1’-Biphenyl]-2-ol, sodium salt (Sodium ortho-phenylphenate; SOPP)
Chemical Abstracts Service Registry Number
132-27-4
Environment and Climate Change Canada
Health Canada
November 2022
Cat. No.: En84-320/2022E-PDF
ISBN 978-0-660-45512-9
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of [1,1’-biphenyl]-2-ol, sodium salt, hereinafter referred to as sodium ortho-phenylphenate (SOPP). The Chemical Abstracts Service Registry Number (CAS RNFootnote 1 ) for SOPP is 132-27-4.
SOPP does not occur naturally in the environment. According to information submitted in response to a CEPA section 71 survey, the manufactured quantity reported in Canada in 2008 was in a range of 10 000 kg to 100 000 kg, and the import quantities were reported in a range of 1000 kg to 10 000 kg.
SOPP is a material preservative agent. In Canada, SOPP is used in building or construction materials, in products available to consumers (such as cosmetics [bar soap] and tire and rubber lubricants). SOPP may be used as a component in the manufacture of food packaging materials and as a component in incidental additives used in food processing establishments or as a medicinal ingredient in disinfectants. It is also an active ingredient and a formulant in registered pest control products in Canada.
The ecological risk of SOPP was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, SOPP is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from SOPP. It is concluded that SOPP does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
SOPP has been reviewed internationally through the International Agency for Research on Cancer, the Food and Agriculture Organization/World Health Organization Joint Meeting on Pesticide Residues, the United States Environmental Protection Agency and the California Environmental Protection Agency. In 2008, SOPP was evaluated by Health Canada’s Pest Management Regulatory Agency. In laboratory studies, SOPP was found to be associated with an increased incidence of urinary bladder tumours. At lower doses, effects for SOPP and a structurally-related substance included decreased body weight gain and kidney effects.
The predominant source of exposure to SOPP from products available to consumers in Canada is from bar soap and tire and rubber lubricants. There is also potential for exposure to SOPP to the general population from food and its use in food packaging materials.
A comparison of the estimate of exposure to SOPP from the use of bar soap and tire and rubber lubricants with critical effect levels identified from laboratory studies results in margins of exposure which are considered adequate to address uncertainties in the health effects and exposure datasets. The risk to human health from exposure to SOPP from food packaging is considered to be very low and contributes negligibly to the overall dietary exposure of Canadians to SOPP.
Considering all the information presented in this screening assessment, it is concluded that SOPP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that SOPP does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of [1,1’-Biphenyl]-2-ol, sodium salt, hereinafter referred to as sodium ortho-phenylphenate (SOPP), to determine whether this substance presents or may present a risk to the environment or to human health. This substance was considered a priority on the basis ofother human health concerns (ECCC, HC [modified 2017]).
The substance currently being evaluated has been reviewed internationally by the International Agency for Research on Cancer (IARC) Monographs Programme (IARC 1999), the Food and Agriculture Organization/World Health Organization Joint Meeting on Pesticide Residues (JMPR 1999), the United States Environmental Protection Agency (US EPA 2006, 2019), and the California Environmental Protection Agency (Cal EPA 2007). SOPP was also reviewed by Health Canada’s Pest Management Regulatory Agency (PMRA) (Health Canada 2008a,b). These assessments undergo rigorous review and were used to inform the health effects characterization in this screening assessment.
The ecological risk of SOPP was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data for SOPP were identified up to October 2020. Empirical data from key studies as well as results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. The draft of this screening assessment (published February 29, 2020) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 2 . This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substance
The Chemical Abstracts Service Registry Number (CAS RNFootnote 3 ), Domestic Substances List (DSL) name, and molecular structure for SOPP are presented in Table 2‑1.
| CAS RN | DSL name (common name; abbreviation) |
Molecular structure and formula | Molecular weight (g/mol) |
|---|---|---|---|
| 132-27-4 | [1,1'-Biphenyl]-2-ol sodium salt (sodium ortho-phenylphenate; SOPP) | 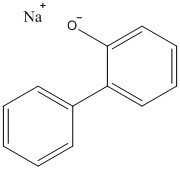 C12H10O.Na C12H10O.Na |
192.2 |
2.1 Selection of analogue
A read-across approach using data from an analogue was used to inform the human health assessment. An analogue was selected that was structurally and/or functionally similar to the substance in this assessment (similar physical-chemical properties, toxicokinetics), and that had relevant empirical data that could be used to read-across to limited empirical toxicity data for SOPP. SOPP is the sodium salt of ortho-phenylphenol (OPP). Results from toxicity studies conducted with OPP were used in a read-across approach to inform the health effects assessment of SOPP. Identity information on OPP is presented in Table 2‑2. Physical and chemical properties and the read-across summary of toxicological data on OPP and SOPP can be found in Appendix A.
| CAS RN | DSL name (common name; abbreviation) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 90-43-7 | [1,1'-Biphenyl]-2-ol (ortho-phenylphenol; OPP) | 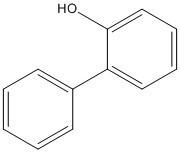 C12H10O C12H10O |
170.2 |
3. Physical and chemical properties
A summary of chemical properties of SOPP are presented in Table 3‑1. When experimental information was limited or not available for a property, data from analogues were used for read-across and/or (quantitative) structure-activity relationship ([Q]SAR) models were used to generate predicted values for the substance. Additional physical and chemical properties are reported in ECCC (2016b).
| Properties | SOPP | OPP | Reference |
|---|---|---|---|
| Vapour pressure (Pa) | 1.2 | 0.27 | ECHA c2007-2019 and HSDB 1983- |
| Water solubility (mg/L) | 1.00E+06 (pH 13.6) | 530 to 640 (pH 5-9) | ECHA c2007-2019 |
| log Kow (dimensionless) | 2.95 | 3.18 | ECHA c2007-2019 |
| Half-life in air (hours) | N/A | 14 | US EPA 2006 |
Abbreviations: Kow, octanol-water partition coefficient, N/A, not applicable.
4. Sources and uses
SOPP does not occur naturally in the environment (IARC 1999). SOPP was included in a survey issued pursuant to section 71 of CEPA (Canada 2009). In 2008, SOPP was reported to be manufactured (10 000 kg to 100 000 kg) and imported (1000 kg to 10 000 kg) into Canada (Environment Canada 2009).Footnote 4
On the basis of information submitted in response to a CEPA section 71 survey (Environment Canada 2009), SOPP was reported to be used commercially in building or construction materials, with no consumer uses reported. It is used as a material preservative agent (Health Canada 2008a). Additional uses identified in Canada are presented in Table 4‑1. SOPP was also identified in tire and rubber lubricants available to consumers in Canada (MSDS 2015).
| Use | SOPP |
|---|---|
| Incidental additivea,b | Y |
| Food packaging materialsa | Y |
| Medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug productsc | Y |
| Medicinal or non-medicinal ingredient in licensed natural health productsd | N |
| Formulant and active ingredient in registered pest control productse | Y |
| Notified to be present in cosmetics under the Cosmetic Regulationsf,g | Y |
Abbreviations: Y= yes, use was reported for this substance; N= no, use was not reported for this substance.
a Personal communication, email from the Food Directorate (FD), Health Canada (HC), to the Existing Substances Risk Assessment Bureau (ESRAB), HC, dated August 2018; unreferenced.
b While not defined under the Food and Drugs Act (FDA), incidental additives may be regarded, for administrative purposes, as those substances which are used in food processing plants and which may potentially become adventitious residues in foods (for example, cleaners, sanitizers).
c Medicinal ingredient in hospital/health Care facilities and institutional/industrial disinfectant (personal communication, email from the Pharmaceutical Drugs Directorate (PDD), HC, to the ESRAB, HC, dated August 2018; unreferenced).
d Personal communication, emails from the Natural and Non-prescription Health Products Directorate (NNHPD), HC, to the ESRAB, HC, dated August 2018, October 2020 and March 2022; unreferenced.
e Personal communication, emails from the Pest Management Regulatory Agency (PMRA), HC, to the ESRAB, HC, dated August 2018; unreferenced.
f Personal communication, email from Consumer and Hazardous Products Safety Directorate (CHPSD), HC, to the ESRAB, HC, dated September 2020; unreferenced.
g Internationally, SOPP is not permitted as a preservative in cosmetics in the European Union (EU) (EC 2020; personal communication, emails from CHPSD, HC to the ESRAB, HC, dated September 2020; unreferenced).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of SOPP was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (Q)SAR or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profile for SOPP, and the hazard, exposure and risk classification results are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, SOPP was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Environmental media and food
SOPP does not occur naturally in the environment. No data have been identified for SOPP in relevant environmental media in Canada or elsewhere. The dissociation constant (pKa) of OPP indicates that SOPP is unstable in the environment and dissociates to form its conjugate acid OPP, and sodium hydroxide in water (ECHA c2007-2019). The predominant degradation pathway for SOPP is the dissociation to OPP, which is not expected to persist in the environment (ECHA c2007-2019). OPP is reported to be photolytically unstable in a neutral aqueous medium and to degrade completely in 14 days when exposed to sunlight (Health Canada 2008a; US EPA 2006). In addition, due to its physical and chemical properties (see Table 3-1), SOPP is not expected to volatilize (US EPA 2014), is immobile in soils and is not likely to contaminate groundwater or surface water (Health Canada 2008a; US EPA 2006). Therefore, on the basis of the available information, it is expected that exposures of the general population to SOPP (and its environmentally relevant OPP form) via water, air or soil are negligible.
SOPP may be used as a component in the manufacture of food packaging materials with the potential for direct food contact. The probable daily intake for SOPP from its use in food packaging materials is estimated to be 6.2 ng/kg bw/day for an adult aged 19 years and older. It may also be used as a component in incidental additives (for example, cleaners, lubricants) used in food processing establishments. Exposure from its use in incidental additives is not expected, since there is no direct food contact with the incidental additive (personal communication, email from the Food Directorate (FD), HC to the Existing Substances Risk Assessment Bureau (ESRAB), HC, dated August 2018; unreferenced).
SOPP may be present as a residue on food from its use as a pest control product in Canada (Health Canada 2008a). In consideration of the contribution of the estimated dietary exposure to SOPP from its presence as pesticide residue in food, the estimated exposure to SOPP of the Canadian population from food packaging materials is considered to contribute negligibly to the overall dietary exposure to SOPP.
Products available to consumers
SOPP was found to be present in a limited number of products available to consumers (that is, bar soap and tire and rubber lubricants). SOPP has low vapour pressure and inhalation exposure to SOPP from the use of bar soap and tire and rubber lubricants is expected to be minimal. The primary route of exposure to SOPP from the use of these products is expected to be dermal.
Potential exposures from the use of bar soap and tire and rubber lubricants were estimated and are presented in Table 6.1. Details are presented in Appendix B (Table B-1).
| Product scenario | Concentration (%) | Route of exposure | Per event exposure (mg/kg bw) a | Daily exposure (mg/kg bw/day)a |
|---|---|---|---|---|
| Bar soap (0-5 months) | 0.1b | Dermal | N/A | 0.00031 |
| Tire and rubber lubricants (19+ years) | 0.2c | Dermal | 0.02 | N/A |
Abbreviation: N/A, not applicable
a Systemic exposure assuming 100% absorption through the dermal route.
b Personal communication, emails from the CHPSD, HC to the ESRAB, HC, dated September 2020; unreferenced.
c MSDS (2015).
Biomonitoring
Two metabolites of SOPP (ortho-phenylphenol-glucuronide and ortho-phenylphenol-sulfate) were measured in the urine of the Canadian Health Measures Survey (CHMS) participants aged 3 to 79 years in cycle 5 of the survey (2016-2017) (Health Canada 2019). The average 95th percentile concentrations in both sexes for ortho-phenylphenol-glucuronide and ortho-phenylphenol-sulfate were 0.38 µg/L (12.4% detection frequency [n=2620]) and 13 µg/L (99.8% detection frequency [n=2694]) respectively. However, CHMS biomonitoring data were not used in exposure estimation for risk characterization for several reasons. These metabolites are not unique to SOPP, since they represent exposures to OPP and its salts and therefore would not necessarily represent exposure from just SOPP. In addition, OPP has a short elimination half-life of 0.8 hours after absorption (Timchalk 1998), resulting in an inability to achieve steady-state urinary excretion levels without uniform and constant exposure (NRC 2006). Lastly, the fraction of urinary excretion for the OPP metabolites have not been defined. Therefore, these metabolites are not considered suitable for use as quantitative biomarkers of exposure to SOPP.
6.2 Health effects assessment
OPP and its sodium salt (SOPP) were reviewed by the JMPR (1999), the US EPA (2006, 2019), the CalEPA (2007), and Health Canada’s PMRA (Health Canada 2008a,b). Additionally, the CalEPA re-evaluated the reproductive and developmental toxicity (Kwok and Silva 2013). IARC classified SOPP as possibly carcinogenic to humans (Group 2B) (IARC 1999). The US EPA and Health Canada reviews were used as primary sources to inform the health effects characterization in this screening assessment, and supplemented with information from the other reviews mentioned above. OPP and its sodium salt were also reviewed by Australia’s National Industrial Chemicals Notification and Assessment Scheme (NICNAS 2016) and the European Commission Scientific Committee on Consumer Safety (SCCS 2015, 2018). The European Chemicals Agency has a registration dossier available for SOPP (ECHA c2007-2019). A literature search was conducted from January 1998 to October 2020. No studies which could result in a different health effects characterization were found.
Following administration of a single gavage dose of SOPP (250 to 500 mg/kg bw), approximately 85% was found to be absorbed in rats and excreted via urine within 24 hours (CalEPA 2007). Less than 8% of it was detected in tissue (including adipose, liver, kidneys, urinary bladder, stomach and intestine, brain and blood) at 24 hours and less than 1% detected at 7 days after dosing (IARC 1999; JMPR 1999; CalEPA 2007). It is rapidly excreted through the urine, but up to 26% and 4% of an oral dose in rats may also be excreted in bile and feces, respectively (Sato et al. 1988 cited in CalEPA 2007). Once absorbed, the renal clearance of OPP was rapid, with an average half-life of 0.8 hours in humans (Timchalk et al 1998). In mammals (including rodents and humans), orally absorbed SOPP produced metabolites such as sulfate and glucuronide conjugates of OPP, as well as the oxidative metabolites, such as unconjugated phenylhydroquinone (PHQ), phenylbenzoquinone (PBQ), and 2,5-dihydroxybiphenyl (IARC 1999; JMPR 1999). In rats, the urinary concentrations of metabolites were greater in males than females (Nakao et al. 1983; Morimoto et al. 1989 cited in IARC 1999; JMPR 1999).
F344 rats (10/sex/group) were administered SOPP in the diet at 0, 1250, 5000, 10 000, 20 000, or 40 000 ppm (equal to 0, 86, 180, 350, 700, 1350 or 2450 mg/kg bw/day, respectively) for 13 weeks. Body weight gains decreased 15% to 17% in both sexes at 350 mg/kg bw/day and higher (Iguchi et al. 1979 cited in JMPR 1999). In males at 700 mg/kg bw/day and higher, there were decreased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities, with increased relative liver weights observed at 1350 mg/kg bw/day and higher. Urinary bladder epithelium tumours (transitional papillomas and carcinomas in males, papillomas in females) were increased at 2450/1350 mg/kg bw/day and higher in males/females, with kidney inflammation (pyelonephritis) in both sexes at 2450 mg/kg bw/day and dose-related increase in urinary alkalinity (Iguchi et al. 1979; Hiraga and Fujii 1981 cited in JMPR 1999, CalEPA 2007; SCCS 2015). In this health effects assessment, the no observed adverse effect level (NOAEL) is considered to be 180 mg/kg bw/day based on decreased body weight gain at 350 mg/kg bw/day and higher, in alignment with the JMPR (1999).
F344 rats were administered SOPP via diet in two carcinogenicity studies. In the first study, animals (50/sex/group) were treated at 0, 7000 or 20 000 ppm SOPP in diet for males (equivalent to 0, 270 or 770 mg/kg bw/day, respectively) and 0, 5000 or 10 000 ppm in diet for females (equivalent to 0, 224 or 466 mg/kg bw/day, respectively) for 104 weeks with a 2-week recovery period. In the second study, F344 rats (25/sex/group) were administered SOPP as above, with an additional lower 2500 ppm dose group (equivalent to 95/113 mg/kg bw/day males/females) and a 56-week recovery period (Fujii and Hiraga 1985 cited in IARC 1999; Hiraga 1983 cited in CalEPA 2007). The incidences of interstitial nephritis of the kidney were increased in both sexes at 270/224 mg/kg bw/day and higher in the first study and at 270/466 mg/kg bw/day and higher in the second study (males/females). There was a dose-related increase in focal atrophy of the pancreas in females at 224 mg/kg bw/day and above in the first study only. Urinary bladder papillomas and/or carcinomas were observed in both sexes in both studies only at 224/270 mg/kg bw/day and above. In both studies, body weights were decreased in females at 466 mg/kg bw/day and in males at 770 mg/kg bw/day, although body weight data were only available in summary form in the first study, and no individual data were available in the second study (CalEPA 2007). The CalEPA (2007) identified a lowest observed effect level at the lowest tested dose of 224 mg/kg bw/day based on increased incidences of interstitial nephritis in both sexes and studies, and increased incidences of pancreatic focal atrophy in females in the first study. The CalEPA (2007) stated that the carcinogenicity component of this study was acceptable but not the chronic toxicity component due to insufficient data on hematology and ophthalmology. The US EPA (2019) considered that these were the two parts of the one study which did not satisfy either the chronic toxicity or carcinogenicity requirements due to study design and reporting deficiencies.
Mice were less sensitive than rats to dietary doses of SOPP in both a 13-week and a chronic/carcinogenicity study. In a 13-week study, a NOAEL of 730/1021 mg/kg bw/day (males/females) was identified based on decreased body weight gain in males at 1581 mg/kg bw/day and higher, and increased relative liver weights in females at 1926 mg/kg bw/day and higher (Shibata et al. 1985 cited in CalEPA 2007 and SCCS 2015; JMPR 1999). In a chronic/carcinogenicity study, at the lowest observed adverse effect level (LOAEL) of 480 mg/kg bw/day there was decreased body weight, increased alkaline phosphatase (ALP) activity, and decreased urine specific gravity in females (Hagiwara et al. 1984 cited in IARC 1999; Ito et al. 1983 cited in CalEPA 2007). Although there were increased liver tumours in both sexes at 3009 mg/kg bw/day, no urinary bladder tumours were observed.
SOPP was overall not genotoxic in vitro but the data was mixed in vivo (reviewed in IARC 1999; CalEPA 2007). While there were negative in vivo studies (including chromosome aberration, dominant lethal and Comet assays), cell transformation and DNA breaks were observed in the urinary bladder in male rats fed SOPP in the diet at 500 mg/kg bw/day and above for 1 week or 3 to 5 months, respectively. DNA breaks or adducts were also observed in mice orally and dermally exposed to single doses of 330 mg/kg bw and higher (IARC 1999; Sasaki et al. 2002; CalEPA 2007; De Boeck et al. 2015).
The mode of action for urinary bladder carcinogenicity from SOPP is unclear. At doses below 200 mg/kg bw/day, OPP is transformed primarily to the glucuronide and sulfate conjugates in both rats and mice. At doses greater than 200 mg/kg bw/day, there could be saturation of phase II detoxification pathways (that is, glurcuronide and sulfate conjugation), leading to increased levels of the metabolites PHQ or PBQ, which may induce carcinogenicity via non-genotoxic regenerative hyperplasia of the bladder, or by possible genotoxic mechanisms (IARC 1999; CalEPA 2007; US CDC 2017). An alternative mechanism proposed by the CalEPA (2007), is that there could be DNA damage mediated by reactive oxygen species during the conversion of PHQ to PBQ, and cell proliferation from chemically-induced cytotoxicity in the urothelium of the bladder.
The IARC has classified SOPP as possibly carcinogenic to humans (Group 2B) (IARC 1999). The US EPA (2006, 2019) and Health Canada (2008a) identified OPP and its salts as not likely to be carcinogenic below a specific dose range, without quantification of risk. They were considered “not likely to be carcinogenic to humans” below 200 mg/kg bw/day but “likely to be carcinogenic to humans” above 200 mg/kg bw/day (Health Canada 2008a; US EPA 2006, 2019).
In a developmental toxicity study, Jcl:ICR mice (20 pregnant females/group) were gavaged with SOPP in water at 0, 100, 200 or 400 mg/kg bw/day from gestation days (GDs) 7 to 15 and sacrificed on GD 18 (Ogata et al. 1978b cited in CalEPA 2007). The CalEPA (Kwok and Silva 2013) reported that at 100 mg/kg bw/day and higher, there were decreased fetal body weights in both sexes and increased litter incidences of cleft palate in fetuses and additional skeletal variations in the presence of decreased maternal body weight gain. At 200 mg/kg bw/day there was increased maternal death with vaginal bleeding in decedent animals. The CalEPA (2007) did not determine a maternal LOAEL because there were insufficient data to unambiguously distinguish the extent of decreased maternal body weight gain at 100 mg/kg bw/day (uterine weights were not measured). Although a developmental LOAEL was identified at 100 mg/kg bw/day by the CalEPA (2007), this study was considered unacceptable due to significant limitations in reporting, including lack of individual data (CalEPA 2007).
Ortho-phenylphenol (OPP)
This section focuses on the reproductive, developmental, chronic toxicity/carcinogenicity and genotoxicity studies for OPP. SOPP is the sodium salt of OPP which has similar physical-chemical properties. SOPP and OPP are in a pH-dependent equilibrium in aqueous solution (JMPR 1999; US EPA 2014). Their oral toxicokinetics are similar in mammalian species (JMPR 1999; CalEPA 2007; NICNAS 2016), although there is increased urine alkalinity with SOPP (Appendix A).
IARC (1999) classified OPP as Group 3 (not classifiable as to its carcinogenicity to humans). IARC (1999) stated that SOPP and OPP induce urinary bladder tumours mostly in male rats, with SOPP being more potent.
In a chronic/carcinogenicity study, mice (50/sex/group) were administered doses of OPP at 250 to 1000 mg/kg bw/day in diet for 2 years. A LOAEL of 250 mg/kg bw/day was identified by the US EPA (2014) based on increased absolute and relative liver weights and decreased absolute and relative spleen weights in both sexes, as well as increased liver masses or nodules at 500 mg/kg bw/day and higher (Quast and McGuirk 1995 cited in JMPR 1999 and US EPA 2006, 2019). The CalEPA (2007) also identified a LOAEL of 250 mg/kg bw/day from this study.
In a chronic oral toxicity/carcinogenicity study, F344 rats (46 to 50/sex/dose) were fed OPP in the diet at doses of 0, 800, 4000 or 8000/10 000 ppm (equivalent to 0, 39/49, 200/248 or 402/648 mg/kg bw/day in males/females, respectively) for 2 years, with an interim sacrifice at 1 year (20/sex/group in the control and high dose groups and 10/sex/group at low and mid-dose groups) (Wahle and Christenson 1996 cited in CalEPA 2007; US EPA 2006). The US EPA (2006) identified a NOAEL of 39 mg/kg bw/day based on decreased body weight, body weight gain, food consumption and food efficiency, increased clinical signs and gross pathological signs of toxicity at 200 mg OPP/kg bw/day and higher. According to the data evaluation record (DER) for this study (US EPA 1996), body weights at 200/248 mg/kg bw/day decreased with statistical signficance at 13, 52, and 78 weeks, but not at 104 weeks when the study was terminated, food efficiency decreased 6% to 7% at 13 weeks in both sexes (only timepoint measured), and there was brown ventrum staining in females and urinary bladder transitional cell carcinoma (4%, not statistically significant) in males.On the basis of the values in the DER, there was 5% or more decreased body weight only at 13 weeks in females (5.1%) and 78 weeks in males (5.3%). The US EPA (2019) re-evaluated the study and noted that the effects observed at 200/248 mg/kg bw/day were not considered adverse. The US EPA (2019) revised the NOAEL to 200 mg/kg bw/day (males) based on decreased body weight (more than 10% and persistent at 13, 52, 78 and 104 weeks), and an increased incidence of non-neoplastic findings of the urinary bladder (hyperplasia, mineralization, necrosis) and kidney (cyst, hyperplasia, infarct) at the LOAEL of 402 mg/kg bw/day. There was also increased urinary bladder papillomas and transitional cell carcinomas in males at the high dose (US EPA 2019).
Genotoxicity studies conducted with OPP showed mixed results in vitro and in vivo (CalEPA 2007; SCCS 2015). In contrast with the CalEPA (2007), the US EPA (2019) considered that OPP was not genotoxic at doses which did not result in cytotoxicity. The mode of action of OPP resulting in increased urinary bladder tumours is unclear but proposed possibilities are those previously described for SOPP (IARC 1999; CalEPA 2007; US CDC 2017). The CalEPA (2007) derived a benchmark dose (BMD10) of 222.8 mg/kg bw/day with 95% lower limit (BMDL10) of 185.2 mg/kg bw/day, based on the combined incidences of urinary bladder papillomas and carcinomas in male rats fed OPP (Wahle and Christenson 1996).
In a two-generation reproductive toxicity study, Sprague-Dawley rats (30/sex/group) received 0, 20, 100 or 500 mg/kg bw/day in the diet and there were two matings/generation to produce litters in the F1 and F2 generations (Eigenberg and Lake 1995 cited in Bomhard et al. 2002, Kwok and Silva 2013 and ECHA c2007-2019; US EPA 2006, 2019). The parental toxicity NOAEL was 100 mg/kg bw/day based on decreased body weights and body weight gain in both sexes, with kidney and urinary bladder related effects in males (including chronic inflammation in both and hyperplasia of ureters and urinary bladder), mortality (due to kidney failure in an adult male), and decreased body weight in 21-day old pups of both F1 and F2 generations at 500 mg/kg bw/day. The US EPA (2014) stated that the decreased pup weight was not due to lactational effects in the dams, but considered to be related to consumption of the treated food by pups. No reproductive adverse effects were noted in this study.
There were three developmental toxicity studies in mice, rats, and rabbits gavaged with OPP. In the rat developmental study (24 to 36 pregnant animals/group gavaged with OPP in cottonseed oil during GDs 6 to 15 and sacrificed on GD 21), the US EPA (2006, 2019) and Health Canada (2008a) selected a maternal NOAEL of 100 mg/kg bw/day for OPP based on decreased body weight gain, food consumption and food efficiency at 300 mg/kg bw/day OPP (John et al. 1978 cited in US EPA 2006, 2019 and CalEPA 2007; Health Canada 2008a). The US EPA (2006, 2019) and Health Canada (2008a) considered this to be a co-critical developmental study along with the rabbit developmental study.
In two phases of a rabbit developmental toxicity study, pregnant animals were gavaged with OPP (0, 25, 100, 250 mg/kg bw/day) in corn oil during GDs 7 to 19 and sacrificed on GD 28 (Zablotny et al. 1991 cited in US EPA 2006, 2019 and CalEPA 2007; Health Canada 2008a). Initially only 16 pregnant dams/group were assessed, so additional pregnant dams were gavaged (2 and 8 dams treated with 0 and 250 mg/kg bw/day OPP, respectively); this second phase was initiated 5 days after the last sacrifice of the first phase and completed 1 month later (Kwok and Silva 2013). At 250 mg/kg bw/day there was renal tubular degeneration and inflammation in dams. As cited in Kwok and Silva (2013), there was an increased number of litters with resorptions at 100 mg/kg bw/day and above without statistical significance (5/15 [33%], 8/14 [57%], 10/13 [77%], 13/18 [72%] for control, low, mid, and high doses, respectively, by combining phase I and phase II data; historical control range 11% to 67%), in the presence of clinical observations of blood in the cage. The CalEPA (Kwok and Silva 2013) reanalyzed the data and proposed a maternal NOAEL of 100 mg/kg day and a developmental NOAEL of 25 mg/kg bw/day based on a statistically significant increased percent post-implantation loss (sum of percent fetal resorptions per litter divided by the total number of litters) at 100 mg/kg bw/day and above. These points of departure were adopted by the European SCCS (2015) for assessing non-food consumer products. While there may be uncertainties regarding the significance of the resorptions (for example, assessing the fraction of fetuses resorbed per litter rather than the fraction of litters with resorptions) this assessment considered the rabbit developmental toxicity study to have a maternal NOAEL of 100 mg/kg bw/day based on effects in the kidneys (inflammation and tubular degeneration) at 250 mg/kg bw/day OPP and a developmental NOAEL of 250 mg/kg bw/day. These points of departure are consistent with those established in the re-registration evaluation performed by the US EPA (2006), which formed the basis for the Health Canada (2008a) assessment.
There were no other potential fetal effects (including fetal body weight, litter size, external, soft tissue, or skeletal anomalies or malformations) up to 700 mg/kg bw/day OPP in rats or up to 250 mg/kg bw/day OPP in rabbits. Mice (21 pregnant animals/group gavaged with OPP in olive oil during GDs 7 to 15 and sacrificed on GD 18) had severe fetal effects (including open eyelids, cleft palate, exencephalia) at the lowest tested dose of 1450 mg/kg bw/day (LOAEL), but only in the presence of maternal deaths (0, 4, 7 and 16 out of 21/group at 0, 1450, 1740 and 2100 mg/kg bw/day, respectively) (Ogata et al. 1978b cited in CalEPA 2007 and Kwok and Silva 2013). The CalEPA (2007) considered the developmental study in mice unacceptable because of doses selected which resulted in many maternal deaths and inadequate numbers of fetuses/group available for visceral and skeletal examinations. On the basis of endocrine disruptor screening program Tier 1 assays, there was either no potential interaction or no convincing potential interaction of OPP with effects on the mammalian estrogen, androgen and thyroid pathways (US EPA 2015, 2019). Examination of previous studies did not suggest that further investigation into immunotoxicity or neurotoxicity was warranted (US EPA 2016, 2019).
OPP is a dermal irritant but is not a skin sensitizer (US EPA 2019). In a 3-week dermal toxicity study in rats treated with OPP, dermal effects (erythema, edema, acanthosis, and hyperkeratosis) were observed at 500 mg/kg bw/day but no systemic effects were observed at doses up to 1000 mg/kg bw/day (Zempel 1993 cited in CalEPA 2007; US EPA 2006, 2019). However, this study was not considered in risk characterization for systemic effects in this health effects assessment because comparison of physical-chemical properties between OPP and SOPP suggests that OPP has a lower dermal absorption, and SOPP is more dermally corrosive (SCCS 2015).
6.3 Characterization of risk to human health
Table 6‑2 provides the relevant exposure estimates and critical effect levels as well as the resultant margins of exposure (MOEs) for the characterization of risk to human health from exposure to SOPP.
| Exposure scenario (age group with highest estimate) | Systemic exposurea | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily dermal exposure from use of bar soap (0-5 months) | 0.00031 mg/kg bw/day | Oral NOAEL = 39 mg/kg bw/day in a two-year chronic toxicity /carcinogenicity study in rats | Decreased body weights and body weight gain at 200 mg/kg bw/day (OPP, analogue) | 126 000 |
| Per event dermal exposure from use of tire and rubber lubricant (19+ years) | 0.02 mg/kg bw | Oral NOAEL (maternal) = 100 mg/kg bw/day in developmental toxicity studies in rats and rabbits | Maternal NOAEL of 100 mg/kg bw/day based on decreased body weight gain, food consumption and food efficiency at 300 mg/kg bw/day in rats and kidney effects in rabbits at 250 mg/kg bw/day (OPP, analogue) | 5000 |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effect level.
a Assumes the dermal absorption is equivalent to 100% oral absorption.
For assessment of the human health risk from daily dermal exposure to SOPP in bar soap, the NOAEL of 39 mg/kg bw/day was selected from the two-year dietary OPP chronic toxicity/carcinogenicity study in rats based on reduced body weight and body weight gain at 200 mg/kg bw/day. It was recognized that the US EPA (2019) subsequently revised their NOAEL for this chronic toxicity/carcinogenicity study to 200 mg/kw bw/day and consequently selected the NOAEL of 100 mg/ kg bw/day from the 2-generation reproductive toxicity study in rats for their chronic dietary assessment of OPP and its salts. In consideration of the aforementioned discussed uncertainty of potential developmental effects at 100 mg/kg bw/day (as previously discussed in the health effects section), the NOAEL of 39 mg/kg bw/day was used in the characterization of risk to human health. This is the same point of departure selected by Health Canada (2008a) in the re-evaluation of pesticidal uses of OPP and its salts for use to determine the acceptable daily intake of 0.39 mg/kg bw/day (as identified by the US EPA [2006]). The calculated MOE is considered adequate to address uncertainties in the health effects and exposure databases for SOPP.
For the per event dermal exposures from the use of tire and rubber lubricants, a NOAEL of 100 mg/kg bw/day was selected from developmental toxicity studies in rats and rabbits gavaged with the analogue OPP, for incidental oral short-term exposure to SOPP. This was based on maternal effects observed at higher doses in the absence of developmental toxicity. The calculated MOE is considered adequate to address uncertainties in the health effects and exposure databases for SOPP.
The use of SOPP in food packaging materials is considered to contribute negligibly to the overall dietary exposure of the general population to SOPP in consideration of the contribution of the estimated dietary exposure to SOPP from its presence as pesticide residue in food addressed by Health Canada (2008a). The risk to human health from exposure to SOPP from food packaging is considered to be very low.
While exposure of the general population to SOPP is not of concern at current levels, this substance is considered to have a health effect of concern on the basis of its potential hazard, due to its classification as an IARC Group 2B substance (possibly carcinogenic to humans) (IARC 1999).
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| No measured Canadian data in environmental media for OPP (the environmentally relevant form of SOPP). | +/- |
| No dermal absorption data for SOPP. | + |
| No repeated dose dermal study for SOPP. | +/- |
| No reproductive toxicity study or adequate developmental study by any route of exposure for SOPP and the health effects were, accordingly, addressed by read-across to OPP. | +/- |
+ = uncertainty with potential to cause over-estimation of risk; - = uncertainty with potential to cause under-estimation of risk; +/- = unknown potential to cause over- or under-estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from SOPP. It is concluded that SOPP does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that SOPP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that SOPP does not meet any of the criteria set out in section 64 of CEPA.
References
Bomhard EM, Brendler-Schwaab SY, Freyberger A, Herbold BA, Leser KH, Richter M. 2002. O-phenylphenol and its sodium and potassium salts: A toxicological assessment. Crit Rev Toxicol. 32(6):551-625.
[CalEPA] California Environmental Protection Agency. 2007. Ortho-phenylphenol (OPP) and sodium ortho-phenylphenate (SOPP) risk characterization document: Dietary exposure. Sacramento (CA): CalEPA. 225 pp.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. [PDF] Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2956.
ConsExpo Web [Consumer Exposure Web Model]. 2018. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
De Boeck M, van der Leede BJ, De Vlieger K, Geys H, Vynckier A, Van Gompel J 2015. Evaluation of p-phenylenediamine, o-phenylphenol sodium salt, and 2,4-diaminotoluene in the rat comet assay as part of the Japanese Center for the Validation of Alternative Methods (JaCVAM)-initiated international validation study of in vivo rat alkaline comet assay. Mutat Res Genet Toxicol Environ Mutagen. 786-788:151-157.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: eccc.substances.eccc@canada.ca
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2019 December 3].
[EC] European Comission. 2020. Cosmetic Ingredient Database (CosIng) Annex V: List of Preservatives Allowed in Cosmetic Products. Substance: sodium o-phenylphenate. [accessed 2020 October 1].
[ECHA] European Chemicals Agency. c2007-2019. Registered substances database; search results for CAS RN 132-27-4. Helsinki (FI): ECHA. [accessed 2019 January].
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC, 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food and Chemical Toxicology 78: 159-169. Health Canada. 2008a. Proposed Re-evaluation Decision: 2-Phenylphenol and salts. No. PRVD2008-04; 25 Jan. 2008. Pest Management Regulatory Agency. 23 pp.
Health Canada. 2008b. Re-evaluation Decision: 2-Phenylphenol and salts. No. RVD2008-13; 7 April 2008. Pest Management Regulatory Agency. 5 pp.
Health Canada. 2015. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa (ON): Health Canada.
Health Canada. 2019. Fifth Report on Human Biomonitoring of Environmental Chemicals in Canada. Results of the Canadian Health Measures Survey Cycle 5 (2016-2017). November 2019. Ottawa (ON): Health Canada. [accessed 2020 October].
Health Canada. 2020. Pest Management Regulatory Agency Re-evaluation and Special Review Work Plan 2020-2025. Re-evaluation Note REV2020-01. September 2020. Ottawa (ON): Health Canada. [accessed 2020 October].
[HSDB] Hazardous Substances Data Bank [database]. 1983- . Search results for CAS RN 90-43-7. Bethesda (MD): National Library of Medicine (US). [updated 2012 March 30; accessed 2020 July 7].
[IARC] International Agency for Research on Cancer. 1999. ortho-Phenylphenol and its sodium salt. [PDF] International Agency for Research on Cancer (IARC) Monograph. 73: 451-480.
[JMPR] Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group. 1999. Toxicological evaluations. 2-Phenylphenol and its sodium salt. Rome, 20-29 September 1999. International Programme on Chemical Safety. [accessed 2019 Feb].
Kwok ESC, Silva M. 2013. Re-evaluation of developmental and reproductive toxicity of ortho-phenylphenol (OPP) and sodium ortho-phenylphenate (SOPP). Cell Dev Biol. 2(3):123.
[MSDS] Material Safety Data Sheet. 2015. 12097 Tire & Rubber Lube. Dixon (IL): Plews and Edelmann. [accessed 2018 Sep 19].
[NRC] National Research Council of the National Academes 2006. Human Biomonitoring for Environmental Chemicals. Chapter: 5 Interpretation of Biomonitoring Results. The National Academies Press. Washington, DC.
[NICNAS] National Industrial Chemicals Notification and Assessments Scheme. 2016. Human health Tier II assessment for biphenylol and its sodium salt. Sydney (AU): NICNAS.
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K, Iwama K, Taniguchi K, Tsuda S. 2002. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res. 519(1-2):103-119.
[SCCS] Scientific Committee on Consumer Safety. 2015. Scientific opinion on o-phenylphenol, sodium o-phenylphenate and potassium o-phenylphenate. [PDF] Report No. SCCS/1555/15. Revised version of 15 December 2015. 122 pp.
[SCCS] Scientific Committee on Consumer Safety. 2018. Addendum to the scientific opinion on o-phenylphenol, sodium o-phenylphenate and potassium o-phenylphenate (SCCS/1555/15); Here: the use a preservative of sodium o-phenylphenate, potassium o-phenylphenate, MEA o-phenylphenate (CAS n. 132-27-4, 13707-65-8, 84145-04-0). [PDF] Report No. SCCS/1587/18. 36 pp.
Timchalk C, Selim S, Sangha G, Bartels MJ. The pharmacokinetics and metabolism of 14C/13C-labeled ortho-phenylphenol formation following dermal application to human volunteers. Hum Exp Toxicol 1998;17(8):411-7.
[US CDC] United States Centers for Disease Control and Prevention. 2017. Biomonitoring summary; ortho-Phenylphenol, CAS No. 90-43-7. Atlanta (GA): US CDC. 3 pp.
[US EPA] United States Environmental Protection Agency. 1996. Orthophenylphenol-Review of a carcinogenicity study in the rat, submitted under section 6(a)(2) of FIFRA. [PDF] Washington (DC): US EPA. 32 pp. Memorandum dated 30 July 1996.
[US EPA] United States Environmental Protection Agency. 2006. Reregistration eligibility decision for 2-phenylphenol and salts (orthophenylphenol or OPP). Washington (DC): US EPA. Report No.: 739-R-06-004; July 2006. [PDF] 146 pp.
[US EPA] United States Environmental Protection Agency. 2011. Exposure Factors Handbook 2011. [PDF] Final Report. Washington (DC): US EPA. Report No.: EPA/600/R-09/052F.
[US EPA] United States Environmental Protection Agency. 2014. ortho-Phenyl phenol (oPP) and salts final work plan; Registration Review: Initial Docket Case Number 2575. Docket Number EPA-HQ_OPP-2013-0524. Washington (DC): US EPA. 74 pp.
[US EPA] United States Environmental Protection Agency. 2015. EDSP Weight of Evidence Conclusions on the Tier 1 Screening Assays for the List 1 Chemicals: O-phenylphenol (o-PP). Washington (DC): US EPA. Memorandum dated 29 June 2015. 56 pp. Memorandum dated 29 June 2015.
[US EPA] United States Environmental Protection Agency. 2016. 2-Phenylphenol: Summary of Hazard and Science Policy Council (HASPOC) Meeting on July 21, 2016: Recommendations on the Requirements for Immunotoxicity Study (OCSPP 870.7800). Memorandum dated 30 August 2016. 6 pp.
[US EPA] United States Environmental Protection Agency. 2019. Registration Review Draft Risk Assessment for Ortho-Phenylphenol (O-PP) and Salts. Washington (DC): US EPA. 118 pp. Memorandum dated 30 September 2019.
Zempel JA, Szabo JR. 1993. Ortho-Phenylphenol: 21-Day Repeated Dermal Dose Study of Systemic Toxicity in Fischer 344 Rats. Health and Environmental Sciences- Texas, Freeport, Texas. Study ID K-001024-056.
Appendix A. Read-across from OPP to SOPP
| Chemical name | Ortho-phenylphenol (OPP) |
Sodium ortho-phenylphenate (SOPP) |
|---|---|---|
|
CAS RN |
90-43-7 |
132-27-4 |
|
Role |
Analogue |
Target substance |
|
Chemical structure |
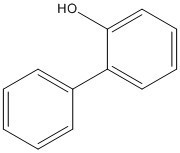 |
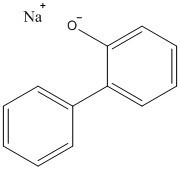 |
|
Molecular weight (g/mol) |
170.2 |
192.2 |
|
Vapour pressure (Pa at 25°C) |
0.27 |
1.2 |
|
Water solubility (mg/L) |
530-640 (pH 5-9) |
1.00E+06 (pH 13.6) |
|
Log Kow (dimensionless) |
3.18 |
2.95 |
|
pKa (dimensionless) |
9.55 |
N/A |
|
Absorption, distribution, metabolism, excretion |
Single oral dose of OPP is 80% to 90% of OPP absorbed in rats, mice and goats within 24 hours (for example, 85% to 86% absorption in male or female rats). A single gavage dose of radiolabelled OPP in rats resulted in less than 8% of it detected in tissue (including adipose, liver, kidneys, urinary bladder, stomach and intestine, brain and blood) at 24 hours, with less than 1% detected at 7 days after dosing. Urinary metabolites (rodents, humans): sulfate and glucuronide conjugates of OPP, unconjugated PHQ, PBQ, and 2,4'-dihydroxybiphenyl. In rats, the urinary concentrations of metabolites were greater in males than females. Excretion of OPP was rapid and complete (85-100%) in mice, rats, and humans and urine was the major excretion route in all species examined. In rats, up to 26 and 4% of an oral dose may also be excreted in bile and feces, respectively (IARC 1999; JMPR 1999; CalEPA 2007; NICNAS 2016). |
Single gavage dose of SOPP in rats is approximately 85% absorbed within 24 hours. A single gavage dose of radiolabelled SOPP in rats resulted in the same distribution results as those for OPP. Urinary metabolites (humans, rodents): sulfate and glucuronide conjugates of OPP, unconjugated PHQ, PBQ, and 2,5-dihydroxybiphenyl. In rats, the urinary concentrations of metabolites were greater in males than females. SOPP is primarily excreted in urine within 24 hours and the excretion results in bile and feces were the same as those for OPP, when SOPP was orally dosed in rats (IARC 1999; JMPR 1999; CalEPA 2007; NICNAS 2016). |
|
Eye and skin irritation, skin sensitization |
Eye irritant, strong skin irritant, not a skin sensitizer (Bomhard et al. 2002). |
Severe eye and skin corrosive, not a skin sensitizer (Bayer 1983 cited in ECHA c2007-2019; Toxicological Research Laboratory 1994 cited in ECHA c2007-2019; Bomhard et al. 2002). |
|
Subchronic repeat dose toxicity (oral) |
NR |
13-week rat study. Dose-related increase in urinary alkalinity at all doses. Decreased body-weight gain at 350 mg/kg bw/day and higher. Increased relative kidney weight, decreased AST and ALT activities in males at 700 mg/kg bw/day and higher. NOAEL of 180 mg/kg bw/day (JMPR 1999), 350 mg/kg bw/day (CalEPA 2007). 13-week mouse study. NOAEL = 730/1021 mg/kg bw/day based on decreased body weight gain in males at 1581 mg/kg bw/day and higher, and increased relative liver weights in females at 1926 mg/kg bw/day and higher (JMPR 1999; CalEPA 2007). |
|
Long-term repeat dose toxicity (oral, diet) |
2-year rat carcinogenicity study. Decreased body weight and body weight gain in both sexes, decreased food consumption in females and decreased food efficiency (sexes not specified), and increased clinical signs and gross pathological signs of toxicity in females at 200 mg OPP/kg bw/day. Increased incidence of urinary bladder papillomas and carcinomas in males (US EPA 2006,2019; CalEPA 2007); BMDL10 = 185.2 mg/kg bw/day (CalEPA 2007). NOAEL of 39 mg/kg bw/day (LTD) considered protective of precursor events leading to development of bladder and liver tumours that occur at doses above 200 mg/kg/day (US EPA 2006). This was reconsidered to be a NOAEL of 200 mg/kg bw/day (EPA 2019). |
2-year rat chronic toxicity/carcinogenicity studies. At 224 mg/kg bw/day and higher, there was increased incidences of interstitial nephritis and increased incidences of pancreatic focal atrophy in females (CalEPA 2007), with decreased body weights suggested at higher doses. Increased incidence of urinary bladder papillomas and/or carcinomas in both sexes at 224 mg/kg bw/day and higher (IARC 1999; CalEPA 2007). 2-year mouse chronic toxicity/carcinogenicity study. In females at 480 mg/kg bw/day and higher in females there was decreased body weight, increased ALP activity, and decreased urine specific gravity. Increased incidence of liver tumours in both sexes at 3009 mg/kg bw/day, decreased body weight and urine specific gravity in males (IARC 1999; CalEPA 2007). |
|
Reproductive Toxicity (oral) |
Rat 2-generation reproductive toxicity study. Parental NOAEL = 100 mg/kg bw/day based on decreased body weights and body weight gain in both sexes, with kidney and urinary bladder related effects in males (including chronic inflammation in both and hyperplasia of ureters and urinary bladder), mortality (due to kidney failure in an adult male), and decreased body weight in 21-day old pups at 500 mg/kg bw/day. Reproductive toxicity NOAEL = 500 mg/kg bw/day (HTD) (US EPA 2006, 2019). |
Read-across from OPP. |
|
Develop-mental Toxicity (oral) |
Rat developmental toxicity study. Maternal NOAEL of 100 mg/kg bw/day (LTD) based on decreased body weight gain, food consumption and food efficiency at 300 mg/kg bw/day. Developmental NOAEL = 700 mg/kg bw/day (HTD) (Health Canada 2008a; US EPA 2006, 2019; CalEPA 2007). Rabbit developmental toxicity study. Maternal NOAEL of 100 mg/kg bw/day (LTD) based on kidney effects (inflammation and tubular degeneration) at 250 mg/kg bw/day and above. Developmental NOAEL = 250 mg/kg bw/day (HTD) (Health Canada 2008a; US EPA 2006, 2019; CalEPA 2007). |
Read-across from rat and rabbit developmental toxicity studies using OPP. |
|
Genotoxicity |
Equivocal results in rodents in vivo and in mammalian cells in vitro (IARC 1999; CalEPA 2007; SCCS 2015). Evidence for genotoxic potential supported by CalEPA (2007), but not by US EPA (2006, 2 2019). |
Overall not genotoxic in vitro but the data was mixed in vivo (reviewed in IARC 1999; CalEPA 2007). |
|
Carcino-genicity |
Not classifiable (IARC Group 3) (IARC 1999). |
Possibly carcinogenic to humans (IARC Group 2B) (IARC 1999). |
Abbreviations: N/A, not applicable; ND, no data available; NR, read-across not required for risk characterization; NOAEL, no observed adverse effect level; LOAEL, lowest observed adverse effect level; HTD, highest tested dose; LTD, lowest tested dose; PHQ, phenylhydroquinone; PBQ, phenylbenzoquinone
Appendix B. Parameters used to estimate dermal exposures to humans from products available to consumers
Sentinel exposure scenario assumptions are summarized in Table B-1. Dermal absorption was assumed to be 100%.
| Exposure scenario | Assumptions |
|---|---|
|
Tire and rubber lubricants (19+ years) |
Concentration of SOPP: 0.2% (MSDS 2015). A thin-film approach as outlined in the EPA Exposure factors handbook (US EPA 2011) was used. Estimated Exposure = (Concentration × SA × T × DSY) / BW It was assumed that exposure from handling a cloth coated in the product can be described as a thin film. This approach characterizes the dermal deposition from a mineral oil substance following handling of a rag saturated with the oil material, that is, the mineral oil thickness ("thin film") estimated to remain on the skin (T) is 1.64 × 10−3 cm. This thickness was therefore assumed to apply to SOPP for characterizing dermal exposure for the application of the lubricant products. Assuming equal density of SOPP and the whole product (DSY) of 1302 mg/cm3 and an exposed skin surface area (SA) of 455 cm2 (half of both hands/palms), the dermal load was estimated to be 1.8 mg per 60-minute exposure event using a concentration of 0.2%. Using the selected body weight (BW) of 74 kg (considered to be representative of an average Canadian adult, 19+ years) (Health Canada 2015), dermal exposure was estimated to be 0.02 mg/kg-bw/event. |
|
Bar soap (0-5 months) |
Concentration of SOPP: 0.1%a Scenario from ConsExpo Web: Soap solid Dermal: Direct contact, instant application Frequency: 1.1/day (Ficheux et al. 2015) Exposed area: 2860 cm2 (US EPA 2011, surface area of body excluding the head) Product amount: 0.18 g (Ficheux et al. 2015 with surface area adjustment) Retention factor: 0.01 |
a Personal communication, email from the CHPSD, HC, to the ESRAB, HC, dated September 2020; unreferenced.
