Screening assessment - Sucrose acetate isobutyrate
Official title: Screening assessment
α-D-Glucopyranoside, 6-O-acetyl-1,3,4-tris-O-(2-methyl-1-oxopropyl)-β-D-fructofuranosyl, 6-acetate 2,3,4-tris(2-methylpropanoate)
(Sucrose acetate isobutyrate)
Chemical Abstracts Service Registry Number
126-13-6
Environment and Climate Change Canada
Health Canada
June 2022
Cat. No.: En84-293/2022E-PDF
ISBN 978-0-660-43468-1
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of α-D-Glucopyranoside, 6-O-acetyl-1,3,4-tris-O-(2-methyl-1-oxopropyl)-β-D-fructofuranosyl, 6-acetate 2,3,4-tris(2-methylpropanoate), hereinafter referred to as sucrose acetate isobutyrate (SAIB). The Chemical Abstracts Service Registry Number (CAS RNFootnote 1 ) for SAIB is 126-13-6.
SAIB does not occur naturally in the environment. According to information submitted in response to a CEPA section 71 survey in 2011, there were no reports of manufacture of SAIB in Canada, but 0.1 kg was imported. SAIB is used as an adhesive and film-forming agent in cosmetics, and is a permitted food additive on the List of Permitted Food Additives with Other Accepted Uses (Lists of Permitted Food Additives) as a density adjusting agent in beverages containing citrus or spruce oils.
The ecological risks of SAIB were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, SAIB is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from SAIB. It is concluded that SAIB does not meet the criteria under paragraphs 64 (a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Exposure of the general population to SAIB from environmental media is not expected to be significant. For the general population in Canada, potential exposure to SAIB can result from the consumption of certain flavoured alcoholic and non-alcoholic beverages, use of cosmetic products such as nail polish, lipsticks, eye shadows, face stickers, body tattoos, and artificial skin products in spray formulations.
In 1993, the toxicological profile of SAIB was reviewed internationally by the Joint Food and Agriculture Organization of the United Nations/World Health Organization Expert Committee on Food Additives, in which Health Canada had actively participated. SAIB is considered to have low hazard potential. In short-term and chronic toxicity studies (in relevant animal species through oral route), it did not cause genotoxic, carcinogenic, reproductive, developmental or any other adverse effects relevant to human health up to a dose of 2000 mg/kg bw/day. No adverse effects were observed in humans when administered a daily dose of up to 20 mg/kg bw/day. Exposure estimates for SAIB from the consumption of food or use of cosmetics are not presented as the risk to human health is considered to be low.
Considering all the information presented in this screening assessment, it is concluded that SAIB does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that SAIB does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of α-D-Glucopyranoside, 6-O-acetyl-1,3,4-tris-O-(2-methyl-1-oxopropyl)-β-D-fructofuranosyl, 6-acetate 2,3,4-tris(2-methylpropanoate), hereinafter referred to as sucrose acetate isobutyrate (SAIB), to determine whether this substance presents or may present a risk to the environment or to human health. This substance was considered a priority on the basis of human health concerns (ECCC, HC [modified 2017]).
The ecological risk of SAIB was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
The substance currently being evaluated has been reviewed internationally through the Joint Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) Expert Committee on Food Additives (JECFA) in which Health Canada was a contributing member. These assessments undergo rigorous review (including peer-review). In addition, the European Scientific Committee for Food (SCF), and the United States Food and Drug Administration (US FDA) evaluated the toxicity profile of SAIB. The JECFA assessment was used to inform the health effects characterization in this screening assessment (WHO 1993).
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to September 2019. Empirical data from key studies, as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the Consumer and Hazardous Products Safety Directorate with technical support from the Existence Substances Risk Assessment Bureau at Health Canada and the CEPA Risk Assessment Program at Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portion of this assessment has undergone external review. Comments on the technical portions relevant to human health were received from Dr. Mustafa Al-Zoughool from Kuwait University, Dr. Muhammad Sajid Arshad from the Government College University of Faisalabad, Pakistan, and Dr. Katalin Csáki from the Directorate for Food Safety Risk Assessment of Budapest, Hungary. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this screening assessment (published in November 14, 2020) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Substance identity
The Chemical Abstracts Service Registry Number (CAS RN), Domestic Substances List (DSL) name, common name, and acronym for SAIB are presented in Table 2‑1.
| CAS RN (abbreviation) |
DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
|
126-13-6 (SAIB) |
α-D-Glucopyranoside, 6-O-acetyl-1,3,4-tris-O-(2-methyl-1-oxopropyl)-β-D-fructofuranosyl, 6-acetate 2,3,4-tris(2-methylpropanoate)
(sucrose acetate isobutyrate) |
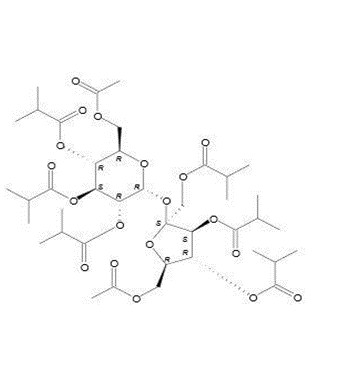 C40H62O19
C40H62O19
|
846.92 |
3. Physical and chemical properties
SAIB is a highly viscous liquid at 20°C with a slightly yellow colour. A summary of physical and chemical property data of SAIB is presented in Table 3-1. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Value | Key references |
|---|---|---|
| Physical state | Liquid | PubChem 2022 |
| Boiling point (°C) | 758.6b | SciFinder 2022 |
| Density (g/mL; 20°C) | 1.12a | ECHA 2019 |
| Vapour pressure (Pa, 25°C) | 8.77E-21b | SciFinder 2022 |
| Water solubility (mg/L, 25°C) | 5.5b | SciFinder 2022 |
| Log Kow (dimensionless; 25°C) | 6.62b | SciFinder 2022 |
| Log Koc (dimensionless; 25°C) | 4.98 (pH 1 to 10)b | SciFinder 2022 |
Abbreviations: Kow, octanol-water partition coefficient; Koc, organic carbon-water partition coefficient
a Measured value
b Modelled
4. Sources and uses
SAIB does not occur naturally. It was included in a survey issued pursuant to section 71 of CEPA (Canada 2012). There were no reports of manufacture of SAIB in Canada for the calendar year of 2011. Approximately 0.1 kg was reported to be imported into Canada for the same year (Environment Canada 2013).
SAIB is used as an adhesive and film-forming agent in cosmetics. Based on notifications submitted under the Cosmetic Regulations to Health Canada from 2016 to 2019, SAIB is used in 1262 cosmetic products including facial stickers, nail polish, lipsticks, and body tattoos in Canada (internal data, Consumer and Hazardous Products Safety Directorate, Health Canada, dated July 22, 2019; unreferenced). Currently, there are no specific conditions for the use of SAIB in cosmetics in Canada (Health Canada 2018). SAIB may be used as a density adjusting agent in flavours used in alcoholic, and non-alcoholic beverages containing citrus or spruce oils at a maximum permitted level of 300 parts per million (ppm) in the beverage as consumed (personal communication from Food Directorate, Health Canada to Consumer and Hazardous Product Safety Directorate, Health Canada, dated September 26, 2019; unreferenced). Internationally, SAIB is also used in the production of inks, coatings, and paper (Reynolds and Chappel 1998; Eastman 2019).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of SAIB was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization.
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for SAIB, and the hazard, exposure and risk classification results are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, SAIB was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
As SAIB is considered to be of low hazard potential (see health effects assessment below), quantitative estimates of exposure to the general population were not derived. This section provides general information on exposure to SAIB.
Environmental media and food
No measured concentrations of SAIB in environmental media were identified in Canada. Due to its high Kow and high KOC values, SAIB is expected to partition into soils and sediments. With its low vapour pressure, it is not expected to volatize from water or soil surfaces.
In Canada, SAIB may be used as a density adjusting agent in flavours for use in beverages (including non-alcoholic and alcoholic beverages) containing citrus or spruce oils (personal communication from Food Directorate, Health Canada to Consumer and Hazardous Products Safety Directorate, Health Canada, dated September 26, 2019, unreferenced).
Products available to consumers
Dermal exposure is expected as the primary route of exposure to SAIB for the general population of Canada, from the use of cosmetic products. Among cosmetics, SAIB is used in face stickers, body tattoos, nail polish, lipsticks and eye shadows. SAIB is also notified in Canada in a simulated skin product which could be used with a spray gun or airbrush (internal data, Consumer and Hazardous Products Safety Directorate, Health Canada; unreferenced). Due to its poor volatility and low concentration, the per event inhalation and dermal exposures from the use of such products to the general population would be expected to be low.
6.2 Health effects assessment
SAIB has been reviewed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (WHO 1975, 1978, 1982, 1993, 1997). Based on its forty-first meeting, JECFA published a complete toxicity profile of SAIB (WHO 1993). The committee derived an acceptable daily intake (ADI) of 0 to 20 mg/kg bw/day (WHO 1997). Consistent with JECFA, the European Union’s Scientific Committee on Food (SCF) and US FDA have evaluated SAIB and issued an ADI of 20 mg/kg bw/day (SCF 2016; US FDA 1999).
The toxicological profiles listed above were used to inform the hazard section of this assessment, including the selection of effect levels for critical endpoints. A literature search was conducted up to September 2019, and no new health effect studies which could result in points of departure lower than those indicated in previous international assessments were identified.
Toxicokinetics studies conducted in rats, dogs and humans indicate that fate and the disposition of SAIB in dogs were substantially different than that of rats and humans. Dogs absorbed about 50% of the orally administered SAIB-14C, whereas rats and humans absorbed about 73% and more than 88% respectively (Reynolds et al. 1974; WHO 1993). In rats and humans, elimination of absorbed SAIB was primarily through exhaled 14CO2 (between 40 and 66%), followed by urine (between 11 and 21%) and feces (between 10 and 27%). Dogs eliminated SAIB primarily in feces (between 46 and 53%), followed by exhalation as 14CO2 (about 28%) and urine (about 7%). Moreover, paper chromatographic profiles of urine extracts indicated the presence of higher acylated sucrose as primary metabolites in dogs whereas in humans and rats these metabolites were absent (SCF 2016; SCF 1994; WHO 1993; US FDA 1999). No data on the absorption of SAIB through dermal and inhalation routes were identified.
Acute oral toxicity studies carried out in rats, mice, dogs, and monkeys indicate that SAIB has very low toxicity. No treatment-related mortality was observed in these studies and the median lethal dose in all reported studies corresponded to the highest dose tested, which typically was above 5000 mg/kg bw (WHO 1993). No acute toxicity study via inhalation route was identified.
SAIB was well-tolerated by rats, dogs and monkeys in short-term and sub-chronic toxicity studies at oral dose levels well above 2000 mg/kg bw/d. Effects related to suppressed liver function such as decreased glucose-6-phosphate activity, increased serum alkaline phosphatase (SAP) levels, increased retention of bromosulfophthalein (BSP) and indocyanine green (ICG) were observed in some studies in rats and monkeys while these effects were consistently observed in dogs even at dose levels as low as 130 mg/kg bw/day. Results from these studies indicated that liver is a target organ of toxicity (WHO 1993; US FDA 1999; SCF 2016).
JECFA concluded that the effects on liver functions observed in dogs are not relevant in humans due to substantial differences in the fate and disposition of SAIB between these species. Since some effects related to the liver function were observed from short-term rats and monkey studies, data from long-term studies were evaluated to ascertain the effects of SAIB on the liver.
JECFA considered a chronic toxicity study in rats as the key study to evaluate systemic toxicity of SAIB (MacKenzie et al. 1998a; WHO 1993). In this study, F344 rats (20 animals per sex per group) were fed daily 0, 500, 1000, or 2000 mg/kg bw/day of SAIB-containing diet for one year. No consistent decrease in body weights, body weight gain and food consumption were noted. No treatment-related changes in hematological (red blood cells [RBC], white blood cells [WBC], haemoglobin, haematocrit, platelet counts, and nucleated RBC counts) and clinical chemistry parameters (such as glucose, bilirubin, cholesterol, aspartate aminotransferase, SAP, alanine aminotransferase, blood urea nitrogen [BUN], creatinine, and electrolytes) were observed. The BSP clearance rates were measured in animals in control and highest dose groups and were found to be similar. No differences in organ weights or changes at microscopic and macroscopic levels, particularly in liver, were observed in animals in control and SAIB-treated groups. The highest dose (2000 mg/kg bw/day) was concluded as the no observed adverse effect level (NOAEL) for SAIB (MacKenzie et al. 1998a).
Blair and Chappel (1998) reported the results of a one-year oral chronic toxicity study in cynomolgus monkeys. Four animals per sex per group were fed daily a diet containing 0, 500, 1450 or 2400 mg/kg bw/day of SAIB. There were no mortality, behaviour, physical, or ophthalmoscopic changes observed in any animals. No statistically significant differences in clinical chemistry parameters including those related to liver functions, namely, serum proteins, transaminase enzymes, ornithine carbamoyltransferase [OCT], SAP, bilirubin, bile acids or BSP retention, or haematological parameters between animals from treatment and control groups were noted. Electron microscopic analysis did not show any changes in the liver sections of animals from high dose group compared to those from the control group. The highest tested dose of 2400 mg/kg bw/day was considered to be the NOAEL (US FDA 1999). Furthermore, these chronic toxicity studies demonstrated that SAIB does not affect the liver function in rats or monkeys.
In-vitro assays performed using Salmonella typhimurium, Chinese hamster ovary cells, and rat primary hepatocyte cells indicated that SAIB is neither mutagenic nor clastogenic (Myhr et al. 1998; CIR 2016). In two studies on carcinogenicity conducted in F344 rats and B6C3F1 mice fifty animals per sex per group were fed daily a SAIB-containing diet for two years (Mackenzie et al. 1998a). Dose levels for rats and mice studies were 500, 1000 or 2000 mg/kg bw/day and 1250, 2500, or 5000 mg/kg bw/day respectively. Two sets of control animals, each for rats and mice studies, were maintained on a SAIB-free diet for the duration of the study period. No treatment-related differences in the survival rates, physical and behavioural changes, food consumption, and body weight between control and treatment groups were noted in both studies. No statistically significant changes in the haematological and clinical chemistry parameters in animals in control and treatment groups were observed in rat or mice study. Incidence of multiple-organ mononuclear cell leukaemia, and interstitial cell tumors were similar in rats in both control and treatment groups and were not considered to be treatment-related. Likewise, there were no statistically significant differences in the incidence of alveolar/bronchiolar adenomas in mice between treatment and control groups. The NOAEL was considered to be the highest dose, 2000 mg/kg bw/day for the rat study and 5000 mg/kg bw/day for the mice study.
In a three-generation reproductive toxicity study in rats, thirty F344 rats per sex per dose group were exposed to 0, 500, 1000 or 2000 mg/kg bw/day of SAIB in diet (MacKenzie et al. 1998b). Prior to mating, males and females (F0 generation) were treated with SAIB-containing diet for 10 and 2 weeks respectively. The SAIB treatment for F0 females continued during mating, pregnancy, and lactation. F1 litters, produced by mating F0 males with females, were exposure to SAIB in utero and continued during mating, gestation, and lactation. F1 litters were mated twice to produce F2a and F2b litters. F2 litters followed the same treatment regime as F1 throughout mating, gestation and lactation periods until F3 litters were produced. F2b litters were used for teratology examinations.
No treatment-related ante-mortem toxicity or mortality was noted in F0, F1 or F2 generations. Occasional and inconsistent differences in food consumptions and body weight changes in animals from F0, F1 and F2 generations were observed at different dose levels. No significant differences in the fertility index or other reproductive, developmental parameters between animals in the control and the treatment groups were observed. Examination of pubs of F2b generation did not reveal any soft tissue or skeletal abnormalities. It was concluded that the NOAEL was 2000 mg/kg bw/day (MacKenzie et al. 1998b).
In a teratology study, 16 pregnant female New Zealand White rabbits per dose were exposed to SAIB at doses of 500, 850 and 1200 mg/kg bw/day in corn oil by gavage on days 7 through 19 of gestation (MacKenzie et al. 1998b). A control group (32 animals) received only the vehicle. No mortality or treatment-related general signs of toxicity was observed in any animal. Examination of the offspring for visceral malformations did not indicate any treatment-related abnormalities. The highest tested dose (1200 mg/kg bw/day) was concluded as the NOAEL.
Results from skin irritation and sensitization studies in guinea pigs and humans indicated that 20% SAIB solution is not a skin sensitizer and causes only transient skin irritation (Krasavage et al. 1973). The Cosmetic Ingredient Review (CIR) Expert Panel has reviewed these data and concluded that SAIB is neither a skin irritant nor a skin sensitizer in humans (CIR 2016).
The effects of single daily dose of SAIB up to 20 mg/kg bw/day on the hepatobiliary functions in humans were evaluated in three 14-days clinical studies (US FDA 1999). No differences were noted in the hematological and clinical chemistry parameters as well as BSP clearance rates between pre- and post-SAIB treatment time points. It was concluded that SAIB does not induce liver toxicity in humans up to a dose of 20 mg/kg bw/day for 14 days (US FDA 1999).
6.3 Characterization of risk to human health
Based on the available toxicological information, SAIB is considered to have low hazard potential. SAIB is neither mutagenic nor carcinogenic. Similarly, SAIB did not induce any reproductive and developmental abnormalities up to an oral dose of 2000 mg/kg bw/day in a three-generation reproductive and developmental rat study (MacKenzie et al. 1998b). A NOAEL of 2000 mg/kg bw/day was derived from the oral chronic oral toxicity study in rats (MacKenzie et al. 1998a). All existing international assessments consistently concluded SAIB as having a low toxicity and used a NOAEL of 2000 mg/kg bw/day for risk characterization.
Quantitative exposure estimates for SAIB in the general Canadian population from food, cosmetics, and environmental media are not presented due to its low hazard potential. The risk to human health from exposure to SAIB from various sources in the general Canadian population is expected to be low.
6.4 Uncertainties in evaluation of risk to human health
No significant uncertainties were identified. Some data gaps exist in the health effects database pertaining to dermal and inhalation exposure; however, availability of additional health effects data for these routes of exposures is not likely to impact the determination of low hazard potential of SAIB.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from SAIB. It is concluded that SAIB does not meet the criteria under paragraphs 64 (a) or (b) of CEPA, as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that SAIB does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that SAIB does not meet any of the criteria set out in section 64 of CEPA.
References
Blair M, Chappel, CI. 1998. 4-week range-finding and 1-year oral toxicity studies of sucrose acetate isobutyrate (SAIB) in the cynomolgus monkey. Food Chem Toxicol. 36(2):121-126.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
[CIR] Cosmetic Ingredient Review Expert Panel. 2016 [PDF]. Safety assessment of saccharide esters as used in cosmetics. [accessed 2019 Oct 9].
Eastman. 2019. SAIB-100 (Sucrose Acetate Isobutyrate). [accessed 2019 Oct 11].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2020 Jan 10].
[ECHA] European Chemicals Agency. C2007-2019. Registered substances database; search results for CAS RN 126-13-6. Helsinki (FI): ECHA. [updated 2019 July 9; accessed 2019 Oct 9].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental
Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances
List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Health Canada. [modified 2018]. Cosmetic Ingredient Hotlist: list of ingredients that are prohibited or restricted for use in cosmetic products. Ottawa (ON): Government of Canada. [accessed 2019 Oct 16].
Krasavage WJ, DiVicenzo GD, Astill BD, Roudabush RL, Terhaar CJ. 1973. Biological effects of sucrose acetate isobutyrate in rodents and dogs. J Agric Food Chem. 21(3):473-479.
Mackenzie KM, Tisdel PJ, Hall RL, Boysen BG, Field WE, Chappel CI, 1998a. Oral toxicity and carcinogenicity studies of sucrose acetate isobutyrate (SAIB) in the Fischer 344 rat and B6C3F1 mouse. Food Chem Toxicol. 36(2):111-120.
Mackenzie KM, Henwood SM, Tisdel PJ, Boysen BG, Palmer TE, Schardein JL, West AJ, Chappel CI, 1998b. Sucrose acetate isobutyrate (SAIB): three-generation reproduction study in the rat and teratology studies in the rat and rabbit. Food Chem Toxicol. 36(2):135-140.
Myhr BC, Cifone MA, Ivett JL, Lawlor TE, Young RR. 1998. Lack of genotoxic effects of sucrose acetate isobutyrate (SAIB). Food Chem Toxicol. 36(2):127-134.
OECD QSAR Toolbox. 2014. Ver. 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
PubChem [database]. 2004- . Bethesda (MD): US National Library of Medicine, National Center for Biotechnology Information. PubChem Compound Summary for CID 31339, Sucrose acetate isobutyrate [accessed 2022 February 15]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Sucrose-acetate-isobutyrate.
Reynolds RC, Chappel CI. 1998. Sucrose acetate isobutyrate (SAIB): historical aspects of its use in beverages and a review of toxicity studies prior to 1988. Food Chem Toxicol. 36(2):81-93.
Reynolds RC, Astill BD, Terhaar CJ, Fassett DW. 1974. Fate and disposition of sucrose-U-14C acetate isobutyrate in humans, rats, and dogs. J Agric Food Chem. 22(6):1084-1088.
SciFinder [database]. 2022. Columbus (OH): Chemical Abstract Services. [accessed February 2022].
[SCF] European Commission Scientific Committee for Food, 32nd series. 1994. The evaluation of sucrose acetate isobutyrate (SAIB), Luxembourg, Brussels.
[SCF] European Commission Scientific Committee for Food. 2016. Re-evaluation of sucrose acetate isobutyrate (E 444) as a food additive, EFSA Panel on Food additives and Nutrient Sources added to Food (ANS). EFSA Journal. 14(5):4489.
[US FDA] United States Food and Drug Administration, 1999. Food Additives Permitted for Direct Addition to Food for Human Consumption; Sucrose Acetate Isobutyrate, Federal Register, 64 (107):29949.
[WHO] Joint FAO/WHO Expert Committee on Food Additives. 1975. Evaluation of certain food additives: some food colours, thickening agents, smoke condensates, and certain other substances. (Nineteenth report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series, No. 576.
[WHO] Joint FAO/WHO Expert Committee on Food Additives. 1978. Evaluation of certain food additives (Twenty-first report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series, No. 617.
[WHO] Joint FAO/WHO Expert Committee on Food Additives. 1982. Evaluation of certain food additives and contaminants (Twenty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series, No. 683.
[WHO] Joint FAO/WHO Expert Committee on Food Additives. 1993. Toxicological evaluation of certain food additives and contaminants. WHO Food Additives Series, No. 32.
[WHO] Joint FAO/WHO Expert Committee on Food Additives. 1997. Evaluation of certain food additives and contaminants (Forty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series, No. 868.
