Screening assessment - sulfurized isobutylene
Official title: Screening assessment - 1-Propene, 2-methyl-, sulfurized (Sulfurized isobutylene)
Chemical Abstracts Service Registry Number
68511-50-2
Environment and Climate Change Canada
Health Canada
May 2022
Cat. No.: En84-291/2022E-PDF
ISBN 978-0-660-42828-4
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 1-propene, 2-methyl-, sulfurized (CAS RNFootnote 1 68511-50-2), hereinafter referred to as sulfurized isobutylene. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA.
Sulfurized isobutylene is a substance of unknown or variable composition, complex reaction products or biological materials (UVCB), and does not occur naturally in the environment. In 2011, results from a survey issued pursuant to section 71 of CEPA indicated that it was not manufactured in Canada above the reporting threshold of 100 kg, and that it was imported in quantities between 10 000 kg to 100 000 kg. Its primary use in Canada was reported to be as a lubricant and lubricant additive in lubricants and greases.
The ecological risk of sulfurized isobutylene was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, sulfurized isobutylene is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from sulfurized isobutylene. It is concluded that sulfurized isobutylene does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on available information, the general population may be exposed to sulfurized isobutylene from the use of products available to consumers (lubricants and greases) and from drinking water, due to industrial releases.
Based on observations in laboratory studies, the critical effects following dermal exposure to sulfurized isobutylene were decreased bodyweight gain and hematological effects. On the basis of the effects of a similar substance observed in laboratory studies, the critical health effect identified for chronic oral exposure was decreased pup weight in rats.
Comparison of levels of exposure to the general population with levels associated with critical health effects resulted in margins considered adequate to address uncertainties in the health effects and exposure databases.
Considering all the information presented in this screening assessment, it is concluded that sulfurized isobutylene does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that sulfurized isobutylene does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of sulfurized isobutylene to determine whether this substance presents or may present a risk to the environment or to human health. Sulfurized isobutylene was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA.
The ecological risk of sulfurized isobutylene was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to May 2019. Empirical data from key studies as well as results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered, namely evaluations of the United States Environmental Protection Agency and of the Australian Government Department of Health.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Jennifer Flippin, Theresa Lopez, and Dr. Joan Garey, all affiliates of Tetra Tech. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this screening assessment (published July 4, 2020) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This screening assessment presents the critical information and considerations on which the conclusion is based.
2. Substance identity
The Chemical Abstracts Service Registry Number (CAS RNFootnote 3 ) and Domestic Substances List (DSL) name for the individual substance are presented in Table 2-1.
| CAS RN | DSL name (common name) | Representative chemical structurea | Molecular weight (g/mol)a |
|---|---|---|---|
|
b |
1-Propene, 2-methyl-, sulfurizedb (Sulfurized isobutylene) |
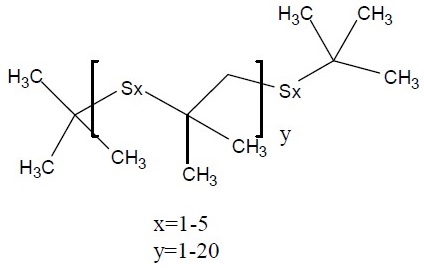 |
160 – 1600 (mean of 480) |
a US EPA 2009
b UVCB, which is an Unknown or Variable composition Complex reaction products or Biological material. These materials are derived from natural sources or complex reactions and cannot practicably be synthesized by simply combining individual constituents. A UVCB is not an intentional mixture of discrete substances and is considered a single substance.
2.1 Selection of analogues
Where appropriate, a read-across approach using data from analogues informed the human health assessment. Analogues were selected that were structurally and/or functionally similar to sulfurized isobutylene (e.g., based on physical-chemical properties, reactivity, metabolism) and that had relevant empirical data. Appendix A provides further details on the factors considered in the identification of analogues. Details on the read-across data chosen to inform the human health assessment of sulfurized isobutylene are further discussed below.
The US EPA (2009) reviewed sulfurized isobutylene by grouping it with other similar substances as part of the Alkyl polysulfides category, along with alkenes C15-18 α-, sulfurized. This grouping was based on structural similarity, the limited reactivity, low biological activity, very low water solubility and low vapour pressure of its components.
A list of the analogues used to inform this assessment is presented in Table 2-2. For further information on the physical-chemical properties of the analogues, refer to Appendix A.
| CAS RN (acronym/abbreviation) | DSL name (common name) | Chemical structure | Molecular weight (g/mol) |
|---|---|---|---|
|
67124-09-8 |
2-Propanol, 1-(tert-dodecylthio)- (1-(tert-Dodecylthio)propan-2-ol) |
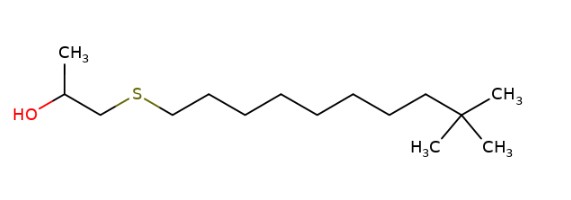 |
160 – 1 600 (mean of 480) |
|
68425-16-1a |
Polysulfides, di-tert-nonyl |
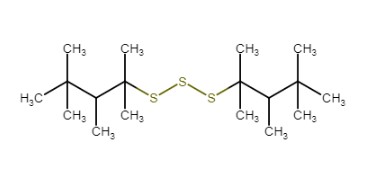 |
Unspecified |
|
67762-55-4a |
Alkenes, C15-18 α-, sulfurized |
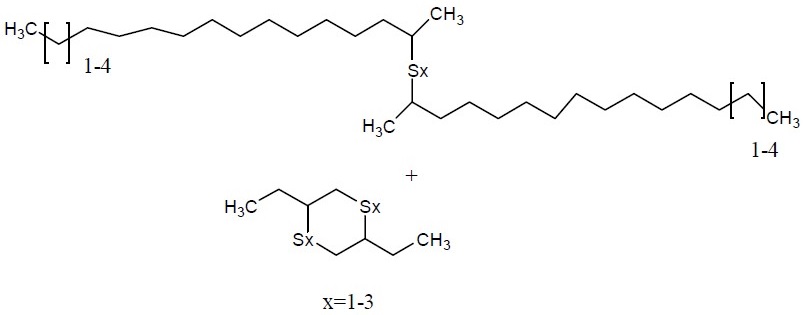 |
Unspecified |
a UVCB, which is an Unknown or Variable composition Complex reaction products or Biological material. These materials are derived from natural sources or complex reactions and cannot practicably be synthesized by simply combining individual constituents. A UVCB is not an intentional mixture of discrete substances and is considered a single substance.
3. Physical and chemical properties
A summary of the physical and chemical properties for sulfurized isobutylene is presented in Table 3-1. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Value or range | Key reference(s) |
|---|---|---|
|
Physical state |
Liquid |
US EPA 2009 |
|
Vapour pressure (Pa) |
1.0 × 10−6 – 2.7 |
US EPA 2009 |
|
Water solubility (mg/L) |
6.3 × 10−6 – 2.7 |
US EPA 2009 |
|
Log Kow (dimensionless) |
5.1- >6 a |
US EPA 2009 |
|
Log Koc (dimensionless) |
11.98 |
US EPA 2009 |
Abbreviations: Kow, octanol-water partition coefficient; Koc, organic carbon-water partition coefficient
a At temperature of 25°C and pH of 6.8
4. Sources and uses
Sulfurized isobutylene has been included in a survey issued pursuant to section 71 of CEPA (Canada 2012). For the calendar year 2011, there were no reports of manufacture in Canada above the reporting threshold of 100 kg, and 10 000 kg to 100 000 kg were reported to be imported into CanadaFootnote 4 (Environment Canada 2013). The reported use of sulfurized isobutylene in Canada is as a lubricant and lubricant additive in lubricants and greases, including products available to consumers (Environment Canada 2013; ECCC 2016c; SDS 2019).
Other potential uses in Canada include use as a lubricant for equipment and machine parts in food processing facilities, which is not expected to come into contact with food (personal communication, email from the Food Directorate [FD], Health Canada [HC], to the Existing Substances Risk Assessment Bureau [ESRAB], HC, dated February 1, 2019; unreferenced).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of sulfurized isobutylene was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of sulfurized isobutylene, hazard and exposure could not be fully profiled because of the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed by examining the UVCB constituents, analyzing information submitted in response to a CEPA section 71 survey, and making decisions on the basis of consideration of similar substances and/or application of expert judgement.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in (ECCC 2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for sulfurized isobutylene, and the hazard, exposure and risk classification results are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, sulfurized isobutylene was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Products available to consumers
Sulfurized isobutylene was identified as an ingredient in automotive lubricant and grease products available to consumers at a concentration of up to 5% (SDS 2019). Use of these products may result in exposure of the general population (e.g., for those who perform their own vehicle maintenance). Inhalation exposure is not expected due to the low vapour pressure of the substance. Exposure is expected to be mainly via the dermal route and is estimated to be 0.12 mg/kg bw per event for adults. Details on the method and parameters used to derive estimates of dermal exposure to sulfurized isobutylene are found in Appendix B.
Food
Sulfurized isobutylene has potential use in food processing facilities in Canada, where it may be used as a lubricant for equipment and machine, which is not expected to result in contact with food (personal communication, e-mail from FD, HC to ESRAB, HC, dated February 1, 2019; unreferenced).
Environmental media
Measured concentrations of sulfurized isobutylene in environmental media were not identified in Canada or elsewhere. Using total import volumes of sulfurized isobutylene in Canada (Environment Canada 2013), concentrations in environmental media were modelled using ChemCAN (ChemCAN 2003). Based on this, exposure to sulfurized isobutylene from air and soil is expected to be negligible.
Drinking water intakes resulting from potential industrial releases were modelled using the Environmental Assessment Unit (EAU) Drinking Water Spreadsheet (Health Canada 2015a) and total import quantities reported in Canadian commerce in 2011 (Environment Canada 2013). Information on model parameters can be found in Table C-1 (Appendix C). The modelled maximum 50th percentile surface water concentration among the 10 receiving water bodies was 1.1 µg/L. This surface water concentration resulted in estimated daily intakes ranging from 2.0 x 10-5 to 1.4 x 10-4 mg/kg bw/day (for 19+ year olds and formula fed 0-5 month olds, respectively). A drinking water intake table for various age groups is presented in Table C-2 (Appendix C). The use of a modelled surface water concentration to estimate drinking water intake may be conservative as water treatment is likely to occur prior to distribution for consumption.
6.2 Health effects assessment
Sulfurized isobutylene has been reviewed by the US EPA as part of the Alkyl Sulfides group in a Screening-Level Hazard Characterization (US EPA 2009). The US EPA review as well as any available data on sulfurized isobutylene and its analogues were used to inform the health effects characterization.
Repeated-dose toxicity
A short-term repeated-dose study was conducted in rabbits via the dermal route of exposure onto intact or abraded skin. Test animals were exposed for 4 weeks to 0, 200 or 2000 mg/kg bw/day of sulfurized isobutylene (6 animals/sex/dose). A no observed adverse effect level (NOAEL) of 200 mg/kg bw/day was established on the basis of haematological effects (increased monocytes, chloride and globulin as well as decreased alkaline phosphatase activity) at the next dose level of 2000 mg/kg bw/day. Severe irritation was observed at both dose levels; however, at the lower dose, it was accentuated and attributed to skin abrasion (US EPA 2009).
Another short-term repeated-dose toxicity study was conducted in New Zealand White rabbits via the dermal route of exposure. Rabbits were exposed for 3 weeks to 140, 560 or 2240 mg/kg/day of sulfurized isobutylene (10 animals/dose). The authors identified a lowest observed effect level (LOEL) of 140 mg/kg bw/day based on clinical signs of toxicity (moderate to severe erythema, edema, epithelial hyperplasia, cracked skin, bleeding and discoloration) and systemic toxicity (AGDH 2006). These effects were progressive in severity over the study duration. Urinalysis values were normal in all groups and sporadic occurrences of dark lungs and liver, red and bloated intestines, pale kidney or small or gray spleen at necropsy were not considered treatment-related. For this study, the US EPA 2009 identified a NOAEL of 2240 mg/kg bw/day (highest tested dose) based on a lack of a dose-dependent response in the tested groups and noted that the observed clinical signs were related to handling and not the test substance.
A sub-chronic repeated-dose study was conducted in Sprague-Dawley rats via the dermal route of exposure. Test animals were exposed for 13 weeks to 0, 500 or 2000 mg/kg bw/day of undiluted sulfurized isobutylene and to 0, 10, 50, 100, 250 or 500 mg/kg bw/day of sulfurized isobutylene diluted in mineral oil (10 animals/sex/dose). A NOAEL of 100 mg/kg bw/day was established by the author on the basis of decreased bodyweight gain in males, increased neutrophil production, decreased red blood cell production and local effects (i.e., moderate to severe reactions in the skin such as erythema and edema) observed in both sexes at the next dose level of 250 mg/kg bw/day. While an increase in white blood cells was also noted at 100 mg/kg bw/day, the US EPA did not consider it to be adverse. At the highest diluted concentration of 500 mg/kg bw/day, the above effects increased in severity. In addition, male rats treated with undiluted doses at 500 or 2000 mg/kg bw/day had increased kidney weights correlating with dose-related increases in hyaline droplet formation. The author indicated that this was indicative of hyaline droplet nephropathy (US EPA 2009).
No oral repeated-dose studies were identified for sulfurized isobutylene. The analogues alkenes, C15-C18 α-, sulfurized (CAS RN 67762-55-4) and polysulfides, di-tert-nonyl (CAS RN 68425-16-1) were taken into consideration based on data availability. The US EPA and the Australian Industrial Chemicals Introduction Scheme (AICIS) have also identified alkenes, C15-C18 α-, sulfurized as an adequate analogue to sulfurized isobutylene, belonging to the same class of alkyl polysulfides (AGDH 2006; US EPA 2009). Polysulfides, di-tert-nonyl was not included in the aforementioned reports, but was considered as an adequate analogue and its associated data was used to inform this assessment. Despite differences in structure, physical and chemical properties and reactivity, most structural alerts were similar.
Based on information submitted in a REACH registration dossier, a combined repeated-dose and reproductive/developmental toxicity screening study was conducted with alkenes, C15-C18 α-, sulfurized. Wistar Han rats (10 animals/sex/dose) were administered 0, 100, 300 or 1000 mg/kg bw/day via the oral route for 29 days in males and 45 days in females. No observable clinical signs, changes in haematology, clinical biochemistry, microscopic and macroscopic changes were noted. A NOAEL of 1000 mg/kg bw/day was identified by the authors, representing the highest dose tested (ECHA 2013).
Based on information submitted in a REACH registration dossier, a repeated-dose and developmental toxicity screening study was conducted with polysulfides di-tert-nonyl. Sprague-Dawley rats (6-12 animals/sex/dose) were administered 0, 50, 250 or 1000 mg/kg bw/day by gavage for 29 days. A satellite group in the control and high-dose groups were monitored for two weeks for recovery. Two deaths were seen in females possibly as a result of gavage error and were not considered to be test substance related. No toxicologically significant changes were seen in bodyweight, food consumption, haematology, clinical chemistry, urinalysis, gross pathology and histopathology in any group. No clinical signs were observed in the highest dose group during the dosing and recovery phase of the study. A NOAEL of 1000 mg/kg bw/day was identified by the authors, representing the highest dose tested (ECHA 1995).
Reproductive and developmental toxicity
Studies examining the effects of sulfurized isobutylene on reproduction and development were not identified. Available data on the analogues 1-(tert-dodecylthio) propan-2-ol (CAS RN 67124-09-8), alkenes, C15-C18 α-, sulfurized (CAS RN 67762-55-4) and polysulfides, di-tert-nonyl (CAS RN 68425-16-1) were taken into consideration.
As described in the repeated-dose toxicity section, a combined repeated-dose and reproductive/developmental toxicity screening study was conducted with alkenes, C15-C18 α-, sulfurized in Wistar Han rats via the oral route of exposure. Test animals were exposed for 29 days for males and 45 days for females to 0, 100, 300 or 1000 mg/kg bw/day (10 animals/sex/dose). No treatment-related effects on reproductive parameters, gestation index and duration, parturition, maternal care and early postnatal pup developmental were observed. Body weight of pups was considered to be unaffected by treatment and was within normal historical values. A NOAEL of 1000 mg/kg bw/day was identified, representing the highest dose tested (ECHA 2013).
As described in the repeated-dose toxicity section, a combined repeated-dose and developmental toxicity experimental study was conducted with polysulfides, di-tert-nonyl in Sprague-Dawley rats via the oral route of exposure. Test animals were exposed to 50, 250 or 1000 mg/kg bw/day (25 animals/dose) up to gestation day 20. No treatment-related effects in terms of maternal toxicity or developmental effects were observed. At 1000 mg/kg bw/day, a slight increase in post-implantation loss was noted in one female; however, it was not considered treatment-related. A NOAEL of 1000 mg/kg bw/day was identified, representing the highest dose tested (ECHA 1997).
1-(tert-Dodecylthio)propan-2-ol (CAS RN 67124-09-8) was also identified as an analogue based on data availability and similar physical-chemical properties and reactivity as the evaluated substance. It was also assessed by AICIS (formerly NICNAS) and in the Screening-Level Hazard Characterization for Alkyl Sulfides conducted by the US EPA along with sulfurized isobutylene.
Based on information submitted in a REACH registration dossier, reproductive effects of 1-(tert-dodecylthio)propan-2-ol were tested in a one-generation toxicity study conducted on Sprague-Dawley rats via the oral route of exposure. Test animals were exposed for up to 3 months to 50, 167 or 500 mg/kg bw/day (28 animals/sex/group). A NOAEL of 167 mg/kg bw/day was established by the authors on the basis of decreased pup weight at the next dose level of 500 mg/kg bw/day, in the absence of parental toxicity (ECHA 2002). Other parameters examined in the study which did not show any significant treatment-related changes included clinical signs, food consumption, reproductive function (estrous cycle, sperm measures, reproductive performance), gross pathology and histopathology for the parents. No adverse treatment-related effects on viability, clinical signs and gross pathology for the offspring were observed (ECHA 2002).
Genetic toxicity
In an in vitro bacterial reverse mutation study, Salmonella typhimurium TA98, TA100, TA1535, TA1537 and TA1538 were exposed to sulfurized isobutylene in dimethylsulfoxide at 0.01, 0.05, 0.1, 0.5 or 1 μL/plate in the presence and absence of metabolic activation. Sulfurized isobutylene was not mutagenic in this assay (US EPA 2009).
In an in vivo micronucleus test, B63CF1 mice (5 animals/sex/dose) were administered 3500 mg/kg bw/day sulfurized isobutylene via intraperitoneal injection. Sulfurized isobutylene did not induce micronuclei in the assay (US EPA 2009).
Another in vivo micronucleus test was conducted in rats via the dermal route of exposure. Animals were exposed for 13 weeks to sulfurized isobutylene (5 animals/sex/dose). Micronuclei were analyzed from femoral bone marrow samples taken 24 hours following the final dermal administration. Sulfurized isobutylene did not induce micronuclei in the assay (US EPA 2009).
Based on these results, sulfurized isobutylene is not expected to be genotoxic.
Carcinogenicity
No carcinogenicity studies have been identified for sulfurized isobutylene or for any analogue substances. An analysis of structural alerts using the OECD QSAR Toolbox (2016) did not identify structural alerts for carcinogenicity for sulfurized isobutylene or its analogue substances.
6.3 Characterization of risk to human health
Table 6-1 provides all relevant exposure and hazard values for sulfurized isobutylene, as well as resulting margins of exposure (MOEs), for determination of risk.
| Exposure scenario | Exposure estimate | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
|
Automotive lubricant product; dermal; adult; acute |
0.12 mg/kg bw per event |
100 mg/kg bw/day (NOAEL) |
On the basis of decreased bodyweight gain in males, increased neutrophil production, decreased red blood cell production, as well as local effects observed at the next dose of 250 mg/kg bw/day in a 13-week dermal study. |
833 |
|
Industrial releases; Drinking water intake; oral; 0-5 month old; daily |
1.4 x 10-4 mg/kg bw/day |
167 mg/kg bw/day |
On the basis of decreased pup weight observed at the next dose of 500 mg/kg bw/day in an oral one generation reproductive 3 months study (conducted with the analogue 1-(tert-dodecylthio)propan-2-ol). |
> 1 000 000 |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effect level
These margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
|
Lack of measured concentrations of sulfurized isobutylene in environmental media. |
+/- |
|
No carcinogenicity or chronic studies identified for sulfurized isobutylene or any of the analogues. |
+/- |
|
No reproductive or developmental studies were identified for sulfurized isobutylene. |
+/- |
+ = uncertainty with potential to cause over-estimation of risk; - = uncertainty with potential to cause under-estimation of risk; +/- = unknown potential to cause over- or under- estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from sulfurized isobutylene. It is concluded that sulfurized isobutylene does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that sulfurized isobutylene does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that sulfurized isobutylene does not meet any of the criteria set out in section 64 of CEPA.
References
[AGDH] Australian Government Department of Health. 2006. 1-Decene, sulfurized. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme (NICNAS). Full Public Report. [accessed 2019 Feb 27].
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
ChemIDplus [database]. 1993-. Bethesda (MD): US National Library of Medicine. [accessed 2019 Mar 4].
ChemSpider [database]. 2015. Royal Society of Chemistry. [accessed 2019 May 5].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2016c. Data collected from a targeted information gathering initiative for assessments under the Chemicals Management Plan (Fall 2016). Data prepared by ECCC, Health Canada; Existing Substances Program.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. 1995. Guidance on the safe use of the substance: Polysulfides, di-tert-dodecyl; 68425-15-0. Helsinki (FI): ECHA. [accessed 2019 Feb 27].
[ECHA] European Chemicals Agency. 1997. Guidance on the safe use of the substance: Polysulfides, di-tert-dodecyl; 68425-15-0. Helsinki (FI): ECHA. [accessed 2019 Feb 27].
[ECHA] European Chemicals Agency. 2002. Guidance on the safe use of the substance: 1-(tert-dodecylthio)propan-2-ol; 67124-09-8. Helsinki (FI): ECHA. [accessed 2019 Feb 27].
[ECHA] European Chemicals Agency. 2013. Guidance on the safe use of the substance: Alkenes, C15-18 α-, sulfurized; 67762-55-4. Helsinki (FI): ECHA. [accessed 2019 Feb 27].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Health Canada. 2015a. Environmental Assessment Unit Drinking Water Spreadsheets [Excel format]. Ottawa (ON): Health Canada. [cited 2016 Apr 8].
Health Canada. 2015b. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa.
Health Canada. 2017. Water Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa.
Health Canada. 2018. Draft backgrounder document on default values for breast milk and formula intakes. Unpublished report. Ottawa (ON): Government of Canada.
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
OECD QSAR Toolbox. [Read-across tool]. 2016. Version 4.1. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[SDS] Safety Data Sheet. 2019. Valvoline™ ALL CLIMATE 10/400 G GREASE. Valvoline LLC. [accessed 2019 May 1].
[US EPA] US Environmental Protection Agency. 2009. Screening-Level Hazard Characterization: Alkyl Sulfides Category. Washington (DC): US EPA.
Versar, Inc. 1986. Standard scenarios for estimating exposure to chemical substances during use of consumer products. Vol. II. Consumer use of used motor oil. Springfield (VA): Versar, Inc. Prepared for US Environmental Protection Agency.
Appendices
Appendix A. Read-across approach
| Consideration | Rationale |
|---|---|
|
1) Chemical structure. Emphasis was placed on analogues with similar branching patterns (e.g., isobutyl, di-tert-butyl, isopropyl, etc.) and were considered to be mono- or polysulfides. Chemical structures containing cyclicity or complex branching patterns were not considered during the read-across approach. In addition, structurally similar chemicals containing additional reactive groups (e.g., nitrogen, aluminum, oxygen) were not considered. |
Analogues that have similar chemical structure are expected to have similar toxicity profiles. |
|
2) Common structural alerts |
Analogues with similar structural alerts are expected to share greater similarity in terms of toxicity. |
|
3) Similar physical-chemical properties. Emphasis was placed on chemical structures with similar molecular weight, water solubility, vapour pressure, and log Kow. |
Analogues with similar physical chemical properties may potentially share similar toxicological profiles. |
|
4) Metabolism (an analysis of metabolism was conducted using OECD toolbox and OASIS TIMES) |
Analogues undergo similar metabolic pathways. Main reactions involve hydroxylations or conversion to acids. Given that all of the substances with the exception of CAS RN 67124-09-8 represent UVCBs, the modelling exercises were limited to SMILES available on databases such as QSAR Toolbox or ChemID. |
| CAS RN | 68511-50-2 | 67762-55-4 | 67124-09-8 | 68425-16-1 |
|---|---|---|---|---|
|
Name |
Sulfurized isobutylene (target substance) |
Alkenes, C15-18 α-, sulfurized |
1-(tert-Dodecylthio)propan-2-ol |
Polysulfides, di-tert-nonyl |
|
Structure |
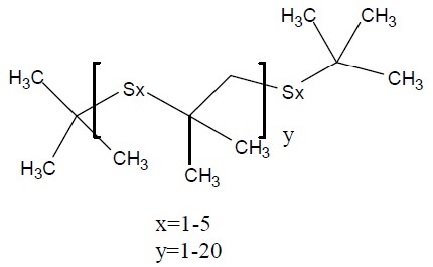 |
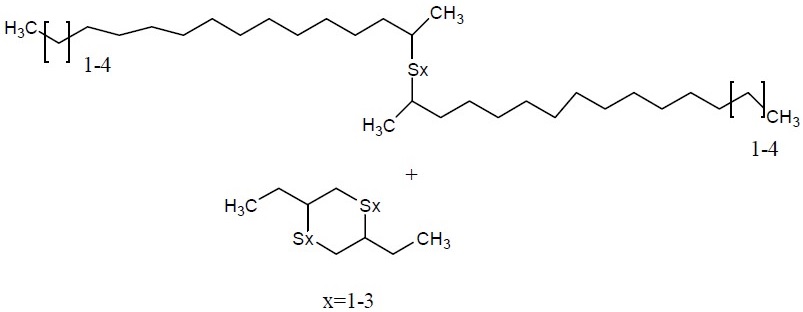 |
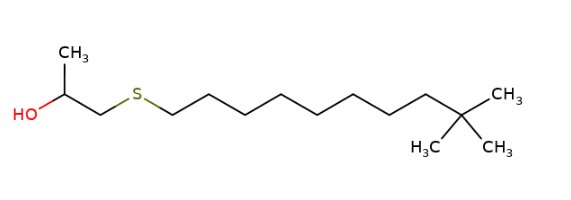 |
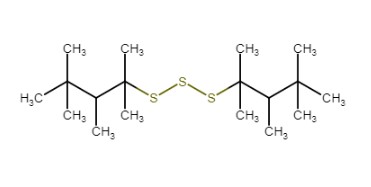 |
|
MW (g/mol) |
210b |
Unspecified |
260c |
Unspecified |
|
Boiling point (°C) |
140d |
NA |
164a |
236a |
|
Vapour pressure (Pa @ 25°C) |
1.0 × 10−6 – 2.7d |
0.052a |
0.63a |
0a |
|
Water solubility (mg/L) @ 25°C |
6.3 × 10−6 – 2.7d |
0.0056a |
4.84a |
2.6 x10-4a |
|
Log Kow (unitless) |
5.1- >6d |
9.4a |
5.7a |
5.2a |
|
Repeat Dose Toxicity (mg/kg-bw/day) |
Rats - 13 week dermal Doses: 10, 50, 100, 250 and 500 NOAEL = 100 LOAEL = 250 on the basis of decreased body weight gain in males and various haematological effects Rabbits - 4-week– dermal study Doses: 200 and 2000 NOAEL = 200 LOAEL = 2000 on the basis of haematological and clinical chemistry effects Rabbits 3-week– dermal study Doses: 140, 560 and 2240 LOAEL = 140 (lowest tested dose) on the basis of clinical signs and systemic toxicity (Epithelial hyperplasia, severe erythema) |
Rats - 29-45 day oral study Doses: 0, 100, 300 and 1000 NOAEL = 1000 |
- |
Rats - 29 days oral Doses: 50, 250 and 1000 NOAEL = 1000 |
|
Reproductive/Developmental Toxicity (mg/kg-bw/day) |
- |
Developmental Toxicity (4 months) Doses: 100, 300 and 1000 NOAEL = 1000 No observed parental/developmental toxicity |
One-generation reproductive toxicity (9 months) Doses: 50, 167 and 500 NOAEL = 167 LOAEL = 500 on the basis of significant decreases in pup weight |
Developmental Toxicity Doses: 50,250 and 1000 NOAEL = 1000 No treatment-related effects in terms of maternotoxicity, embryofetotoxicity or teratogenic effects were observed |
|
Genotoxicity |
Negative Not mutagenic, not cytotoxic |
N/A |
N/A |
N/A |
|
Carcinogenicity (mg/kg-bw/day) |
N/A |
N/A |
N/A |
N/A |
Abbreviations: MW, molecular weight; Kow, octanol-water partition coefficient; NA, not available; N/A, not applicable; NOAEL, no observed adverse effect level; LOAEL, lowest observed adverse effect level
a ECHA
b QSAR Tools 2004-
c ChemIDplus 1993-
d US EPA
Appendix B. Parameters to estimate exposures to products available to consumers in Canada
Exposure estimates were calculated based on default body weights of 74 kg for 19+ year olds (Health Canada 2015b). The estimated dermal exposure parameters for products available to consumers are described in Table B-1.
| Product (substance) | Assumptions |
|---|---|
| Automotive lubricant product |
Product scenario: application of lubricant – small job (tips of fingers, 2 hands) (Versar, Inc. 1986) Dermal C (concentration of sulfurized isobutylene) = 5% (SDS 2019) |
Appendix C. Parameters to estimate drinking water exposure
| Parameter | Input |
|---|---|
|
Scenario |
Industrial release |
|
Wastewater treatment system (WWTS) removal rate (%) |
62.89a |
|
Number of industrial sites |
1b |
|
Release days (per year) |
250b |
|
Daily release to wastewater (%) |
4c |
|
Flow rate for the receiving water body (m3/s) |
21b |
a ECCC 2016a
b EAU Drinking Water Spreadsheet default for the receiving water body that has the maximum surface water concentration
c Based on 3% as container residues and 1% as transfer line/process vessel residues for liquid substance
| Age group | Drinking water exposure (mg/kg bw/day)a,b |
|---|---|
|
0-5 months (breast fed)c, d |
N/A |
|
0-5 months (formula fed)c, e |
1.4 x 10-4 |
|
6-11 monthsf |
9.0 x 10-5 |
|
1 yearg |
4.0 x 10-5 |
|
2-3 yearsh |
3.0 x 10-5 |
|
4-8 yearsi |
2.0 x 10-5 |
|
9-13 yearsj |
2.0 x 10-5 |
|
14-18 yearsk |
2.0 x 10-5 |
|
19+ yearsl |
2.0 x 10-5 |
Abbreviations: N/A, not applicable.
a Based on an estimated surface water concentration as modelled from the EAU Drinking Water Spreadsheet using parameters from Table C-1.
b The use of a modeled surface water concentration to estimate drinking water intake may be conservative as water treatment is likely to occur prior to distribution for consumption.
c Assumed to weigh 6.3 kg (Health Canada 2015b).
d Exclusively for breast milk-fed infants, assumed to consume 0.744 L of breast milk per day (Health Canada 2018), and breast milk is assumed to be the only dietary source.
e Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula.
f Assumed to weigh 9.1 kg (Health Canada 2015b). For breast milk-fed infants, assumed to consume 0.632 L of breast milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula.
g Assumed to weigh 11.0 kg (Health Canada 2015b), and to drink 0.36 L of water per day (Health Canada 2017).
h Assumed to weigh 15 kg (Health Canada 2015b), and drink 0.43 L of water per day (Health Canada 2017)
i Assumed to weigh 23 kg (Health Canada 2015b), and drink 0.53 L of water per day (Health Canada 2017).
j Assumed to weigh 42 kg (Health Canada 2015b) and drink 0.74 L of water per day (Health Canada 2017).
k Assumed to weigh 62 kg (Health Canada 2015b), and drink 1.09 L of water per day (Health Canada 2017).
l Assumed to weigh 74 kg (Health Canada 2015b), and drink 1.53 L of water per day (Health Canada 2017).
