Screening assessment - Thiocarbamates Group
Official title: Screening assessment - Thiocarbamates Group
Chemical Abstracts Service Registry Numbers
120-54-7
137-26-8
Environment and Climate Change Canada
Health Canada
January 2021
Cat. No.: En84-158/2020E-PDF
ISBN 978-0-660-36658-6
Synopsis
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of Environment and the Minister of Health have conducted a screening assessment of two substances referred to collectively under the Chemicals Management Plan as the Thiocarbamates Group. Substances in this group were identified as priorities for assessment as they met the categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1 ), their Domestic Substances List (DSL) names and their acronyms are listed in the table below.
| CAS RN | DSL name | Acronyms and common names |
|---|---|---|
| 120-54-7a | Piperidine, 1,1'-(tetrathiodicarbonothioyl)bis- | DPTT |
| 137-26-8 | Thioperoxydicarbonic diamide ([(H2N)C(S)] 2S2), tetramethyl- | TMTD, thiram |
a This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered as a priority on the basis of other human health concerns.
TMTD and DPTT do not occur naturally in the environment. Information obtained from CEPA section 71 surveys indicate that no company manufactured either of them in Canada above the 100 kg reporting threshold; however, between 170 300 and 403 100 kg of TMTD was imported into Canada in 2008 and 150 000 kg of DPTT was imported in 2011.
TMTD is primarily used as a process regulator for manufacturing solid rubber products in Canada. It is used as a component in automotive parts, in sealants and adhesives, and in the manufacture of a limited number of food packaging materials. This substance is also registered as an active ingredient in pest control products in Canada (under the name of thiram). Health Canada’s Pest Management Regulatory Agency (PMRA)’s final re-evaluation decision for thiram includes a risk assessment and the required risk mitigation measures to protect human health and the environment from pesticidal uses of TMTD under the authority of the Pest Control Products Act. As such, exposures to TMTD resulting from pesticidal sources and uses of thiram are not characterized further in this screening assessment.
DPTT is only used in Canada as a process regulator for manufacturing solid rubber products.
Releases of TMTD and DPTT to surface water are expected to mainly occur as a result of discharges from wastewater treatment systems associated with solid rubber products manufacturing facilities.
TMTD and DPTT are expected to degrade rapidly in the environment and their potential for long-range transport is low. They are not expected to bioaccumulate; empirical bioconcentration factors are low for both substances and mammalian data suggest that they could undergo rapid metabolism and elimination. Current uses of these substances may result in exposures to aquatic organisms near points of release.
Empirical data suggest that TMTD is highly toxic to aquatic organisms. DPTT does not demonstrate any effect on aquatic organisms at water solubility limits.
The ecological risk characterization for TMTD indicates that releases from uses of this substance in the manufacturing of solid rubber products may pose a risk to aquatic organisms. The risk to aquatic organisms associated with current uses of DPTT in the manufacturing of solid rubber products is considered to be low.
Considering all lines of evidence presented in this screening assessment, there is risk of harm to the environment from TMTD. It is concluded that TMTD meets the criteria under paragraph 64(a) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is concluded that TMTD does not meet the criteria under paragraph 64(b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends.
Considering all lines of evidence presented in this screening assessment, there is low risk of harm to the environment from DPTT. It is concluded that DPTT does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
From a human health perspective, TMTD has been reviewed by Health Canada’s PMRA and the identified effects of concern included developmental neurotoxicity and carcinogenicity. TMTD was also reviewed internationally through the Cooperative Chemicals Assessment Programme of the Organization for Economic Cooperation and Development (OECD), the European Chemicals Agency (ECHA), the European Food Safety Authority (EFSA), the United States Environmental Protection Agency (US EPA); these agencies identified the same effects of concern.
For the general population of Canada, exposures to TMTD through environmental media from non-pesticidal uses are not expected to be a significant source of exposure due to rapid photodegradation and hydrolysis in water, low persistence in soil, and low volatility. In Canada, TMTD is not a permitted food additive, nor is it currently used in any prescription or non-prescription drug, natural health product, or cosmetics. Regarding its possible use in the manufacture of a limited number of food packaging materials, dietary exposure from this use, if any, is expected to be negligible. Exposure to TMTD is not expected from its uses in the manufacture of automobiles or from rubber products since residual TMTD is not expected in the final products. In products available to the general population, exposure to TMTD from use of adhesive tape products is expected to be minimal.
DPTT was evaluated using the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances, which is based on the potential hazard of similar chemical structures, as well as available chemical-specific genotoxicity data, when available. The estimate of exposure generated for DPTT was lower than the TTC value, indicating a low probability of risk to human health. Therefore, DPTT is considered to be a low concern for human health at current levels of exposure.
On the basis of the information presented in this screening assessment, it is concluded that TMTD and DPTT do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that TMTD meets one or more of the criteria set out in section 64 of CEPA. It is concluded that DPTT does not meet any of the criteria set out in section 64 of CEPA.
It has also been determined that TMTD does not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and Minister of Health have conducted a screening assessment of two substances referred to collectively under the Chemicals Management Plan as the Thiocarbamates Group to determine whether these substances present or may present a risk to the environment or to human health. The substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
One of these two substances, thioperoxydicarbonic diamide ([(H2N)C(S)]2S2), tetramethyl- (TMTD), was reviewed internationally through the Organisation for Economic Cooperation and Development (OECD) Cooperative Chemicals Assessment Programme (OECD 2010). These assessments undergo rigorous review (including peer-review) and endorsement by international governmental authorities. Environment and Climate Change Canada and Health Canada are active participants in these processes and consider these assessments as reliable. In addition, the ecological and human health effects of TMTD were evaluated by the European Chemicals Agency (ECHA c2007-2017), the United States Environmental Protection Agency (US EPA 2004a and 2004b) and the European Food Safety Authority (EFSA 2016), whose evaluations were also used to inform the ecological and human health effects assessment in this screening assessment. In Canada, Health Canada’s Pest Management Regulatory Agency (PMRA) reviewed TMTD as an active ingredient in pesticides (Health Canada 2016a and 2018). The PMRA Proposed Re-evaluation Decision and Re-evaluation Decision documents were used to inform the health effects characterization of TMTD in this screening assessment.
Piperidine, 1,1'-(tetrathiodicarbonothioyl)bis- (DPTT), was included in the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Science Approach Document (Health Canada 2016b). In the approach, Health Canada used a structure-based decision tree and chemical-specific data on genotoxicity (e.g., Ames test), when available, to assign a human exposure threshold value for a chemical, below which there is a low probability of risk to human health (i.e., TTC value). For each substance in the TTC-based approach, potential exposure of the Canadian general population was characterized and compared to the TTC value assigned to the substance. DPTT was associated with exposure lower than its assigned TTC value. Therefore, DPTT is considered to be a low concern for human health at current levels of exposure.
This screening assessment includes consideration of information on physical-chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data for the ecological risk assessment were identified up to October 2016 and relevant data for the human health risk assessment were identified up to December 2018. Empirical data from key studies as well as some results from models were used to reach conclusions.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Environment and Climate Change Canada and Health Canada and incorporates input from other programs within these departments. The ecological portion of this assessment has undergone a peer review by the PMRA and an external peer review by Anne Kim from the US EPA. The health portion of the assessment on DPTT is based on the TTC document (published October 1, 2016), which was peer-reviewed and subject to a 60-day public comment period. Additionally, the draft of this screening assessment (published February 3, 2018) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Environment and Climate Change Canada and Health Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 3 ), Domestic Substances List (DSL) names and acronyms for the two substances in the Thiocarbamates Group are presented in Table 2‑1. A list of additional chemical names (e.g., trade names) is available from the National Chemical Inventories (NCI 2015). For the purpose of this screening assessment report, CAS RNs 120-54-7 and 137-26-8 are referred to as DPTT and TMTD, respectively.
| CAS RN | DSL name(acronyms and common names) | Chemical structure, molecular formula and SMILESa string | Molecular weight (g/mol) |
|---|---|---|---|
| 120-54-7 | Piperidine, 1,1'-(tetrathiodicarbonothioyl)bis-(DPTT) | 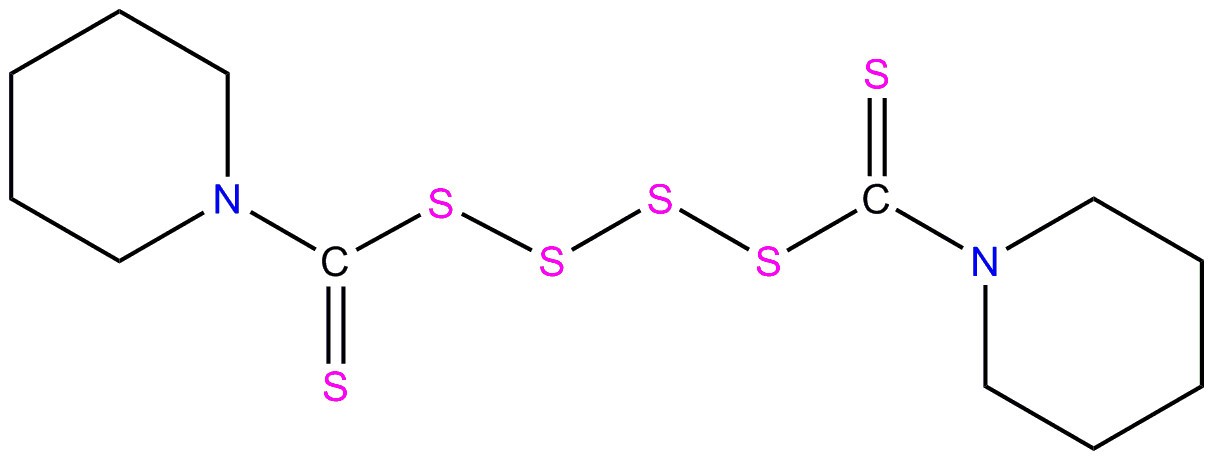 C12H20N2S6 N(C(=S)SSSSC(N(CCCC1)C1)=S)(CCCC2)C2 C12H20N2S6 N(C(=S)SSSSC(N(CCCC1)C1)=S)(CCCC2)C2 | 384.7 |
| 137-26-8 | Thioperoxydicarbonic diamide ([(H2N)C(S)]2S2), tetramethyl-(TMTD, thiram) | 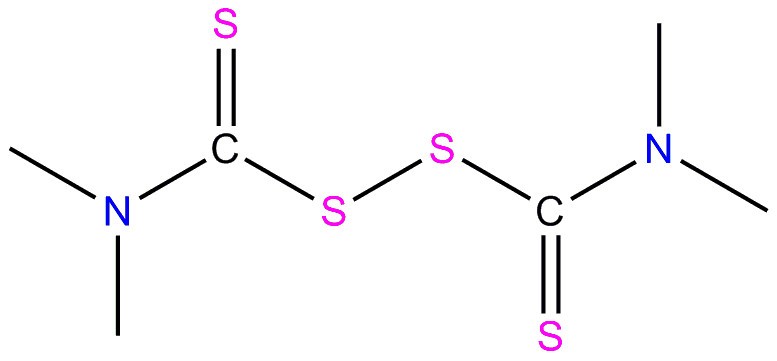 C6H12N2S4 N(C(=S)SSC(N(C)C)=S)(C)C C6H12N2S4 N(C(=S)SSC(N(C)C)=S)(C)C | 240.4 |
a SMILES, Simplified Molecular Input Line Entry System. The SMILES strings were cited from EPI Suite v4.11.
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from an analogue and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, have been used to inform the ecological assessment. An analogue was selected that was structurally similar and/or functionally similar to a substance within this group (e.g., on the basis of physical-chemical properties and/or toxicokinetics) and that had relevant empirical data that could be used to read across to substances with limited empirical data. The applicability of (Q)SAR models was determined on a case-by-case basis. Details of the read-across data and (Q)SAR models chosen to inform the ecological assessment of DPTT are further discussed in the relevant sections of this report.
Information on the identity and the chemical structure of the analogue (CAS RN 971-15-3) used to inform the ecological assessment of DPTT is presented in Table 2-2.
| CAS RN | DSL name | Chemical structure, molecular formula, and SMILESa string | Molecular weight (g/mol) |
|---|---|---|---|
| 971-15-3 | Piperidine, 1,1'-(hexathiodicarbonothioyl)bis- | 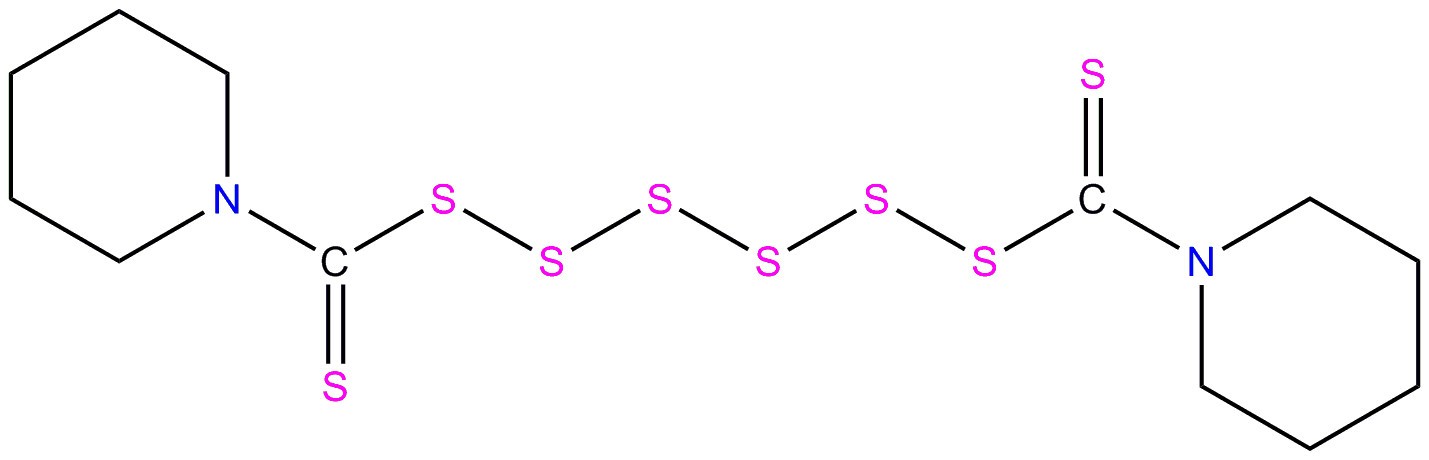 C12H20N2S8 N(C(=S)SSSSSSC(N(CCCC1)C1)=S)(CCCC2)C2 C12H20N2S8 N(C(=S)SSSSSSC(N(CCCC1)C1)=S)(CCCC2)C2 | 448.8 |
a SMILES, Simplified Molecular Input Line Entry System. The SMILES string was cited from EPI Suite v4.11.
3. Physical and chemical properties
TMTD and DPTT are both solids at room temperature and will not volatilize. TMTD is soluble in water while DPTT is slightly soluble in water. Both substances do not dissociate under environmental conditions (pH= 6-9) and remain as neutral compounds.
A summary of key physical and chemical properties of TMTD, DPTT and the analogue of DPTT (CAS RN 971-15-3) is presented in Tables 3-1, 3-2, and 3-3. When experimental information was limited or not available for a property for DPTT, data from the analogue were used for read-across or (Q)SAR models were used to generate predicted values for such properties.
| Property | Value or rangea | Reference |
|---|---|---|
| Melting point (ºC) | 144-156 | ECHA c2007-2017; KEMI 2015; UH PPDB 2015; Kidd and James 1991 |
| Boiling point (ºC) | 129 at 20 mmHg | Lide 2003 |
| Vapour pressure (Pa) | 2×10-5 – 2.4×10-3 at 25 ºC | J-CHECK c2010-; ECHA c2007-2017 |
| Henry’s law constant (Pa·m3/mol) | 0.035 (calculatedb) | Not applicable |
| Water solubility (mg/L) | 16.5-30 at 25 ºC | J-CHECK c2010-; HSDB 1983- |
| Log Kow (dimensionless) | 1.73-2.1 | Tomlin 2003; ECHA c2007-2017; KEMI 2015; OECD 2010 |
| Log Koc (dimensionless) | 2.83 | Schuurmann et al. 2006; OECD 2010 |
| Log Koa (dimensionless) | 6.90 (modelled) | EPI Suite v4.11 |
| pKa (dimensionless) | 8.19 at 25 ºC | EC 2003 |
Abbreviations: Kow, octanol-water partition coefficient; Koc, organic carbon-water partition coefficient; Koa, octanol-air partition coefficient; pKa, acid dissociation constant.
a Reported values are empirical data, unless otherwise specified.
b Henry’s Law Constant was calculated on the basis of the empirical water solubility (16.5 mg/L at 25 ºC) and vapour pressure (0.0024 Pa at 25 ºC).
| Property | Value or rangea | Reference |
|---|---|---|
| Melting point (ºC) | 96-98 | J-CHECK c2010- |
| Boiling point (ºC) | 510 at 760 mmHg | Chemnet 2015 |
| Vapour pressure (Pa) | 2.13×10-8 at 25 ºC | Chemnet 2015 |
| Henry’s law constant (Pa·m3/mol) | 8.19×10-4 (calculatedb) | Not applicable |
| Water solubility (mg/L) | Not availablec | Not applicable |
| Log Kow (dimensionless) | 2.8 | CITI 1991 |
| Log Kow (dimensionless) | 4.33 (modelled) | EPI Suite v4.11 |
| Log Koc (dimensionless) | 3.66 (modelledd) | EPI Suite v4.11 |
| Log Koa (dimensionless) | 5.36 (modelledd) | EPI Suite v4.11 |
| pKa (dimensionless) | 0.2-0.8 (modelled) | ACD/Percepta 2015 |
Abbreviations: Kow, octanol-water partition coefficient; Koc, organic carbon-water partition coefficient; Koa, octanol-air partition coefficient; pKa, acid dissociation constant.
a Reported values are empirical data, unless otherwise specified.
b Henry’s Law Constant was calculated on the basis of the read-across water solubility (0.01 mg/L at 20 ºC) and the empirical vapour pressure (2.13×10-8 Pa at 25 ºC).
c The read-across data for CAS RN 971-15-3 (0.01 mg/L) was used to characterize this physical-chemical property for DPTT.
d Calculated on the basis of the empirical log Kow= 2.8.
| Property | Value or rangea | Reference |
|---|---|---|
| Melting point (ºC) | 121 | ECHA c2007-2017 |
| Boiling point (ºC) | >250 | ECHA c2007-2017 |
| Vapour pressure (Pa) | < 1×10-7 at 25 ºC | ECHA c2007-2017 |
| Henry’s law constant (Pa·m3/mol) | < 4.48×10-3 (calculatedb) | Not applicable |
| Water solubility (mg/L) | 0.01 at 20 ºC | ECHA c2007-2017 |
| Log Kow (dimensionless) | 4.33 (modelled) | EPI Suite v4.11 |
| Log Koc (dimensionless) | 5.56 (modelled) | EPI Suite v4.11 |
| Log Koa (dimensionless) | 6.61 (modelled) | EPI Suite v4.11 |
| pKa (dimensionless) | 0.2-0.8 (modelled) | ACD/Percepta 2015 |
Abbreviations: Kow, octanol-water partition coefficient; Koc, organic carbon-water partition coefficient; Koa, octanol-air partition coefficient; pKa, acid dissociation constant.
a Reported values are empirical data, unless specified.
b Henry’s Law Constant was calculated on the basis of the water solubility (0.01 mg/L at 20 ºC) and the vapour pressure (1×10-7 Pa at 25 ºC).
4. Sources and uses
TMTD and DPTT do not occur naturally in the environment. Both substances have been included in surveys issued pursuant to CEPA section 71 notices (Environment Canada 2009 and 2013). Follow-ups with stakeholders were conducted in 2016 and 2018 (ECCC 2016a and 2018) to confirm the current uses and the recent import volumes of these substances in Canada. There was no report of manufacturing of DPTT above the 100 kg reporting threshold; there were two reports of manufacturing TMTD but neither were above the 100 kg reporting threshold. Both substances were reported to be imported into Canada at the quantities summarized in Table 4-1.
| Acronym | Total imports (kg)a | Reporting year | Survey reference |
|---|---|---|---|
| DPTT | 150 000 | 2011 | Environment Canada 2013 |
| TMTD | 170 300 - 403 100 | 2008 | Environment Canada 2009 |
a Values reflect quantities reported in response to CEPA section 71 surveys (Environment Canada 2009, 2013). See surveys for specific inclusions and exclusions (schedules 2 and 3).
In Canada, TMTD is primarily used as a process regulator (accelerator and curing agent) (Environment Canada 2009) by the Rubber Products Manufacturing sector (defined by the North American Industry Classification System (NAICS) under code 3262). This sector comprises three sub-sectors: tire manufacturing (NAICS 326210), plastic and rubber hose and belting manufacturing (NAICS 326220), and other rubber manufacturing (NAICS 326290) (Cheminfo 2013).
Information reported to a CEPA section 71 survey in Environment Canada (2009) indicates that TMTD can potentially be used as a process regulator within these subsectors to manufacture various solid rubber products including automotive gaskets and seals, pipe, manhole and box culvert gaskets, pipe and tank linings, tracks, conveyor belts, and tires. In addition to solid rubber, TMTD can also be used to manufacture latex products (Namazie International c2014); however, no facilities that use TMTD for latex products manufacturing have been identified in Canada (ECCC 2018).
Other uses of TMTD in Canada include as a component in automotive sealants and adhesives, and in adhesive tape products available to consumers (Environment Canada 2009). Global uses of TMTD (OECD 2010) include industrial uses in Europe as a matrix in general rubber goods and the tire industries and as a biocide (KEMI 2015). Moreover, TMTD may also be used in the manufacture of a limited number of food packaging materials (personal communication, email from Health Product Food Branch, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada, August 2016; unreferenced).
TMTD is listed in the Natural Health Products Ingredients Database (NHPID) (NHPID [modified 2018]) with a non-natural health product role as it is not a naturally occurring substance falling under Schedule 1 of the Natural Health Products Regulations. As such, it is not listed in the Licensed Natural Health Products Database (LNHPD) (LNHPD [modified 2018]) as being present in currently licensed natural health products in Canada. TMTD is not listed as a product in Health Canada's Drug Product Database or Internal Non-medicinal Ingredient Database as medicinal or non-medicinal ingredients present in final pharmaceutical products or veterinary drugs in Canada (personal communication, email from Health Product Food Branch, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada, August 2016; unreferenced). Health Canada’s Cosmetic Ingredient Hotlist is an administrative tool that Health Canada uses to communicate to manufacturers and others that certain substances may contravene the general prohibition found in section 16 of the Food and Drugs Act (FDA) or may contravene one or more provisions of the Cosmetic Regulations. TMTD (thiram, CAS RN 137-26-8) is described as being a restricted ingredient on Health Canada’s Cosmetic Ingredient Hotlist, to be used only in latex products to a maximum concentration of 14% (Health Canada [modified 2019]). However, TMTD is currently not notified to be present in cosmetics in Canada, based on notifications submitted under the Cosmetic Regulations to Health Canada (personal communication, email from Consumer and Hazardous Product Safety Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada, May 2019; unreferenced).
It is noted that TMTD (known as thiram) is registered as an active ingredient in pest control products in Canada (Health Canada 2018). On the basis of a final re-evaluation decision by the PMRA, pest control products registered for animal repellent use and seed treatment use (except for seed treatment of grasses, dry bulb onion, and alfalfa grown for forage) are acceptable for continued registration with the implementation of new mitigation measures and label amendments. The following registered uses will be cancelled: all foliar and dip applications; seed treatment of grasses, dry bulb onion, and alfalfa grown for forage in Canada and importation of these treated seeds into Canada; on-farm hopperbox/seed drill seed treatment; and commercial seed treatment (wheat, barley, oats, canola, mustard, rapeseed, rye, triticale, corn). All maximum residue limits (MRLs) for thiram, including those established for imports, will be revoked (Health Canada 2018). As a result of the registered use cancellations, revocation of the MRLs, and the required risk mitigation measures described in the re-evaluation decision, the exposure potential is reduced and risks to the environment and human health associated with registered pest control products containing TMTD were considered acceptable by the PMRA (Health Canada 2018). Potential exposures to TMTD as a result of pesticidal uses are therefore not characterized further in this screening assessment.
Presently, the only use of DPTT in Canada is as a process regulator (accelerator and curing agent) in the manufacturing of rubber products such as gaskets and seals for the automotive industry (Environment Canada 2013). It is not used in any pest control products, drug or natural health products, cosmetic, or food/food-related products (food processing, manufacturing, or packaging), or other products available to consumers in Canada.
5. Releases to the environment
5.1 TMTD
The primary use of TMTD in Canada is as a process regulator (accelerator and curing agent) in the manufacture of solid rubber compounds by merchant and captive compounders (Environment Canada 2009). The solid rubber compounds are then further processed (molded) to final solid rubber products, either by a different processor (merchant) or by the same company (captive compounding).
Compounding is the first step in the production of a rubber product. This industrial process involves the blending of one or more types of rubber with fillers and functional additives, such as carbon black, oils, antioxidants, catalysts, plasticizers, pigments, accelerators and vulcanizing agents. Solid rubber compounding is a dry blending process (Cheminfo 2013).
Most solid rubber used by rubber processors in the Canadian Rubber Products Manufacturing sector is pre-compounded by companies that specialize in rubber compounding. Since rubber compounding requires considerable investment in capital equipment and organizational design, this process is more commonly found at larger, high-volume rubber operations (Cheminfo 2013). Merchant compound and captive compound facilities are the sites where TMTD is handled in raw form and in larger daily use quantities in comparison with rubber processing sites.
Merchant compounders sell rubber compounds to other facilities that do not have compounding operations. Captive compounding facilities may mix more compounds than they require internally and transfer the remainder for processing to other facilities of the same company. An example of captive compounding is the tire industry with its compounding sites producing compounds for tires only.
At the solid rubber compounding stage, potential releases of TMTD to the environment are expected mainly from the loss of raw TMTD powders during weighing and compounding mixer loading operations. Wastewater generated from equipment and floor cleaning in raw material weighing/handling and compounding areas is considered to be the main source of the release of this substance to the aquatic environment. Additionally, the laundering of workers’ overalls or clothing and showering of workers at the site at the end of their shifts are potential routes of TMTD powder residues to enter wastewater treatment systemsFootnote 4 . After the mixing step, unvulcanised rubber compounds (sheets) pass through a soap and water bath in order to provide a lubrication/anti-tack layer (milling process). For the anti-tack solution application, the common industrial practice is to have a recirculating loop and to discharge the solution at service end through a third party as a hazardous waste. The anti-tack solution can be also applied through surface spraying to rubber sheets, where most of the water is evaporated on the surface of heated rubber (Cheminfo 2013). Milling operations are employed by rubber compounders and processors.
At the solid rubber processing stage, the rubber compounds (sheets) are formed into the desired finished shape, combined with other resins or materials (e.g. during automotive tire building) and cured (vulcanized). During vulcanization, TMTD releases may occur from substance leaching to autoclave steam and condensate that ultimately enter the wastewater. However, no information is available to quantify releases from the vulcanizing process.
In general, TMTD releases to wastewater are expected to be much higher during compounding operations in comparison to processing operations (i.e. curing/vulcanizing step). Only small quantities of unreacted residues of TMTD are expected to be present in cured finished solid rubber products.
TMTD is also used as a component in automotive sealants and adhesives and in various other automotive parts. The substance is expected to be transformed during these applications and, therefore, releases of the unreacted substance are not expected. On the basis of information provided by stakeholders (Environment Canada 2009 and 2016a), there is a potential for the uncured sealants and adhesives to have contact with water during automotive manufacturing that can result in minor releases of TMTD to wastewater at their sites. However, insufficient information is available to quantify such releases.
5.2 DPTT
For DPTT, the only use identified in Canada is as a process regulator (accelerator and curing agent) in rubber products manufacturing (e.g. seals, gaskets, hoses) for the automotive industry (Environment Canada 2013). Releases of DPTT to the environment are similar to what are expected for TMTD, as described in section 5.1. Loss of this substance to wastewater generated from rubber compounding processes is considered to be the main source of potential releases to the environment.
6. Environmental fate and behaviour
6.1 Environmental distribution
A Level III fugacity model (EQC 2011) was used to characterize partitioning of TMTD and DPTT into the various environmental media. Results are presented in Tables 6-1 and 6-2 below.
| Substance released to | Partitioning in air (%) | Partitioning in water (%) | Partitioning in soil (%) | Partitioning in sediment (%) |
|---|---|---|---|---|
| Air (100%) | 3.6 | 5.1 | 91.2 | 0.2 |
| Water (100%) | Negligible | 96.3 | Negligible | 3.7 |
| Soil (100%) | Negligible | 1.3 | 98.7 | Negligible |
| Substance released to | Partitioning in air (%) | Partitioning in water (%) | Partitioning in soil (%) | Partitioning in sediment (%) |
|---|---|---|---|---|
| Air (100%) | Negligible | 1.7 | 97.8 | 0.5 |
| Water (100%) | Negligible | 88.6 | Negligible | 11.4 |
| Soil (100%) | Negligible | 0.2 | 99.7 | 0.1 |
If released to air, both substances are expected to mainly partition into soil.
If released to water, both substances are expected to remain mainly in the aquatic compartment, with only a small fraction partitioning into sediment. Volatilization of the substances from surface water to air is unlikely. Due to its lower water solubility and higher potential for adsorbing to particles, the partitioning of DPTT into sediment is higher than that of TMTD.
If released to soil, the majority of both substances is expected to remain in this compartment.
6.2 Environmental persistence
6.2.1 TMTD
6.2.1.1 Degradation
TMTD undergoes rapid abiotic degradation in water via photodegradation and hydrolysis. A few studies reported a photodegradation half-life in water ranging from 4.1 to 8.8 hours (ECHA c2007-2017 and OECD 2010). Using spiked river water, the photodegradation half-life was reported to be 28 minutes, suggesting that organic matter and other natural river components may increase the photodegradation rate of this substance (Filipe el al. 2013).
TMTD transforms relatively rapidly via hydrolysis in both aerobic and anaerobic aquatic environments and alkaline and neutral conditions favour such degradation (Health Canada 2016a). The half-lives of hydrolysis range from 1.2 and 4.2 days (Health Canada 2016a). This substance degrades in the water-sediment environment with a reported half-life of 1.6 days (UH PPDB 2015).
TMTD also transforms rapidly in soil. Under aerobic conditions, the transformation half-life ranges from 1.5 to 15 days (Health Canada 2016a; UH PPDB 2015). This substance also degrades rapidly in plants with half-lives ranging from 5.8 to 11.3 days (Gupta et al. 2012).
There has been only one biodegradation study identified for this substance, reporting a slow biodegradation in activated sludge over a 14-day test period (J-CHECK c2010-). This suggests that biodegradation is not a major degradation pathway for TMTD.
TMTD is not expected to significantly partition in the atmospheric compartment. Rapid hydrolysis in the aquatic environment suggest that this substance is not persistent in the environment. Give that, the potential for long-range transport in either the atmospheric or aquatic media is low. Releases of this substance associated with uses considered in the assessment may cause exposures to aquatic organisms near points of release; exposures far from sources are not expected.
6.2.1.2 Degradation products from environmental pathways
Degradation products of TMTD have been studied in different environmental media. Gupta et al. (2012) examined the degradation of this substance in water, soil, and plants. Regardless of the test medium, the immediate degradants are primary products resulting from hydrolysis of the disulphide bond (-S-S-) of TMTD. TMTD and its primary degradants undergo further degradation via oxidation or cleavage at the -C-S- bond and form other intermediate compounds (Gupta et al. 2012).
In soil, degradation products include dimethyldithiocarbamate, dithiocarbamate, dimethylamine and carbon disulfide (HSDB 1983-). The ultimate transformation products of TMTD in the environment are CO2 and CS2, which are both volatile and, therefore, not expected to remain in soil or water (Health Canada 2018).
6.2.2 DPTT
Only one biodegradation study has been identified for DPTT, reporting a slow biodegradation in activated sludge over a 14-day test period (J-CHECK c2010-). This suggests that biodegradation is not a major degradation pathway for DPTT.
The model EPI Suite (c2000-2012) considers thiocarbamates as hydrolysable compounds as they contain a -((S-C)=S)-N- structural substitute. Findings in Gupta et al. (2012) reported that metabolite formation in both aquatic and soil media is initiated by breakdown of the S-S bond via hydrolysis, suggesting that DPTT may undergo this degradation pathway. Catalogic v5.11.13 (2015) predicted hydrolysis products for DPTT via breakdown of the S-S bond; the model also predicted metabolites via other reactions, such as oxidative thio desulfuration, oxidative deamination and N-dealkylation, and oxidation of piperidine. Given the above, DPTT is anticipated to undergo rapid degradation and not persist in the environment.
DPTT is not expected to significantly partition in the atmospheric compartment. Rapid hydrolysis in the aquatic environment suggest that this substance is not persistent in the environment. Given that, the potential for long-range transport in either the atmospheric or aquatic media is low. Releases of this substance associated with uses considered in the assessment may cause exposures to aquatic organisms near points of release; exposures far from sources are not expected.
6.3 Potential for bioaccumulation
Empirical bioaccumulation data are available for both substances. Measured bioconcentration factors (BCF) are 4.4 and 32 L/kg or less for TMTD and DPTT, respectively (Table 6‑3).
| Substance | Test organism | Experimental duration | BCF(L/kg) | Reference |
|---|---|---|---|---|
| TMTD | FishCarp | 6 weeks | 1.1-4.4 | OECD 2010 |
| TMTD | Not specified | Not specified | 3.39 | EPI Suite v4.11 (training set) |
| DPTT | Not specified | Not specified | 3.89 | Catalogic v5.11.13 (training set) |
| DPTT | FishCyprinus carpio | 6 weeks | 1.9-32 | J-CHECK c2010- |
| DPTT | Not specified | Not specified | 17.1 | EPI Suite v4.11 (training set) |
The metabolism of TMTD has been studied in birds and mammals (Health Canada 2016a; Gay et al. 1992; Gay 1987; Norris 1993a and 1993b, as cited in FAO 1997). Findings in these studies suggest that the majority of this substance undergoes rapid elimination and metabolism.
The empirical BCFs for TMTD and DPTT, and the rapid metabolism/elimination of TMTD reported in bird and mammal studies, suggest little accumulation of these two substances in organisms.
Given the above, it is considered that TMTD and DPTT do not bioaccumulate in organisms to significant levels.
7. Potential to cause ecological harm
7.1 TMTD
7.1.1 Ecological effects assessment
7.1.1.1 Mode/mechanism of action
TMTD is a dithiocarbamate fungicide considered to have a multi-site mode of action (Health Canada 2016a; Yang et al. 2011). Broad biological activity, involving a variety of endpoints related to growth and development, neurological, and immunological effects across taxa, has been reported for TMTD. Although the mechanisms through which TMTD acts are not fully known, the structural profile of TMTD suggests the thiolate groups in this molecule can undergo covalent reactions (binding) with biological macromolecules such as RNA (and DNA) and other proteins via SN2 (nucleophilic substitution) resulting in the formation of disulfide bridges (Chipinda et al 2007; Hermens 1990). These interactions can also interfere with protein transcription and synthesis resulting in structural deformations within organisms. The molecule was also profiled to have a high reactivity (above 21% peptide depletion) via the Direct Peptide Reactivity Assay (DPRA) which evaluates the ability of chemicals to react with proteins to reduce glutathione production (a detoxification mechanism) and may disrupt protein synthesis and metabolism (Nollet 2000). TMTD also demonstrated effects on mitochondrial functions, by inducing irreversible oxidation of NAD(P)H and glutathione (GSH) pools; the collapse of transmembrane potential; and the uncoupling of oxidative phosphorylation (Balakirev and Zimmer 2001) leading to cell death.
TMTD has also been reported to affect the endocrine systems of mammals. For example, it interferes with corticosteroid hormones which are involved in the regulation of energy, immune reactions, and stress responses (Atanasov et al. 2003; Garbrecht et al. 2006; DCED 2012). In the rat, this substance was observed to delay or block ovulation and inhibit spermatogenesis affecting fecundity (HCN 2003; Stoker et al. 1993 and 2003; Mishra et al. 1998). TMTD may also inhibit thyroid hormone synthesis, similarly to thyroid peroxidase inhibitors (DCED 2012). It may also act as a neuroendocrine disruptor, by inhibiting conversion of dopamine to norepinephrine (Lopez-Antia et al. 2015).
In summary, TMTD is known to interact with biomolecular targets resulting in adverse effects. There are in vivo data indicating that the substance is capable of causing lethal and developmental effects within 24 hours of exposure; effects data on aquatic, sediment, and soil organisms, as well as birds, are discussed in the following sections.
7.1.1.2 Effects on aquatic organisms
The toxicity of TMTD to aquatic organisms has been well characterized. The available empirical toxicity data identified for this substance cover more than ten species in three major groups of aquatic organisms (fish, invertebrates, and algae) from ECHA (c2007-2017) and reviews by other jurisdiction (US EPA 2004a and 2004b; EC 2003; Health Canada 2016a). However, due to the limited number of species of invertebrates, a Species Sensitivity Distribution analysis was not performed. These data indicate that this substance is highly toxic to aquatic organisms (Table 7-1).
| Test duration | Organism | Endpointa | Range of values (mg/L) |
|---|---|---|---|
| Short-term | Fish | EC50/LC50 | 0.0017 – 0.79 |
| Short-term | Invertebrates | EC50/LC50 | 0.0033 – 0.38 |
| Short-term | Algae | EC50/LC50 | 0.06 – 0.19 |
| Long-term | Fish | NOEC | 0.0011 – 0.02 |
| Long-term | Invertebrates | NOEC | 0.002 – 0.04 |
Acronyms: EC50, concentration of a substance that is estimated to cause some toxic sub-lethal effect on 50% of the test organisms; LC50, concentration of a substance that is estimated to be lethal to 50% of the test organisms; NOEC, the highest concentration in a toxicity test not causing a statistically significant effect in comparison with the controls.
a Endpoints for the short-term toxicity studies include survival, growth and mobility. Endpoints for the long-term toxicity studies include survival, growth and reproduction.
The lowest acute endpoint comes from a study on zebrafish (Teraoka et al. 2006), in which fish embryos within 3-hours post fertilization (hpf) were exposed to TMTD for 24 hours. A 24-hpf EC50 of 0.0017 mg/L and a NOEC 0.0012 mg/L were reported on the basis of observations of distorted notochords, disorganized somites, and shortened yolk sac extensions. The Teraoka et al. 2006 study was reviewed and found to be reliable; upon endpoint standardization, the 24-hpf EC50 of 0.0017 mg/L was selected as the critical toxicity value (CTV). A predicted no-effect concentration (PNEC) for the relevant environmental compartment is then calculated from the CTV through the application of an assessment factor.
An assessment factor (AF) is derived as the product of endpoint standardization (FES), species variation (FSV), and mode of action (MoA) (FMOA) factors (i.e., AF = FES × FSV × FMOA). An endpoint standardization factor (FES) is used to account for extrapolations from the toxicity endpoint reported in a study to a long-term, sub-lethal no-effect endpoint. A species variation factor (FSV) factor is determined on the basis of the number of different species in three major groups of aquatic organisms (fish, invertebrate, and algae) for which empirical data are available in the dataset. A mode of action factor (FMOA) is applied to address a known or suspected non-narcotic MoA.
Considering the toxicity endpoint associated with the CTV reported in Teraoka et al. 2006, the number of different species in three major groups of aquatic organisms (fish, invertebrate, and algae) available in the dataset, as well as the mode of action factor, an AF of 9 was used in the calculation, yielding an aquatic PNEC of 0.00019 mg/L, as presented in Table 7-2.
| CTV (mg/L) | AF (FES×FSV×FMOA) | FES | FSV | FMOA | Aquatic PNEC (mg/L) |
|---|---|---|---|---|---|
| Zebrafish embryos 24hpf EC50 = 0.0017 | 9 | 3 | 1(empirical data identified for more than 7 species in 3 major aquatic organisms) | 3(specifically acting and chronic non-narcotic MoA expected) | 0.00019 |
7.1.1.3 Effects on sediment organisms
Limited sediment and soil toxicity data were identified for TMTD. In a sediment toxicity study using Chironomus larvae, a chronic NOEC of 1 mg/L was reported without specifying the test period (EC 2003). This substance inhibited the growth of denitrifying bacteria and an EC50 was reported to be above 3 mg/L (Milenkovski et al. 2010).
A sediment study has been conducted using mayflies (Hexagenia spp.) and fresh water amphipods (Hyalella azteca) (ECCC 2016c). In this range-finding experiment, both organisms were exposed to TMTD in sediment for up to 3 weeks at 5 concentrations (nominal) ranging from 0.1 to 1000 mg/kg-dry weight (dw) sediment (ECCC 2016c). The LC50 and EC50 (growth) were determined to be 230 and 61 mg/kg-dw sediment, respectively, for mayflies and 190 and 140 mg/kg-dw sediment, respectively, for amphipods. With longer exposure periods of up to 6 weeks as part of the same study (ECCC 2016c), LC50 values of 110 and 86 mg/kg-dw sediment for mayflies and amphipods were determined, respectively. The 6-week EC50 (growth) in mayflies was determined to be 69 mg/kg-dw sediment, similar to the 3-week exposure EC50 (growth) of 61 mg/kg-dw sediment. With amphipods, there was no effect on growth after 6-weeks of exposure to TMTD at any test concentrations.
On the basis of the above results, TMTD is expected to have low toxicity to sediment organisms.
7.1.1.4 Effects on soil organisms
A 14-day LC50 of 540 mg/kg was reported for earthworms (OECD 2010; UH PPDB 2015; EC 2003). A 7-day EC50 of 32-100 mg/kg and a 14-day EC50 of 54 mg/kg were reported in lettuce (Lactuca sativa) (OECD 2010); however, the effect endpoint was not specified. When testing the effect of this substance via a water solution to which the plant was exposed, an EC50 of 1.6 mg/L for biomass was reported after a 7-day period (UH PPDB 2015).
These limited results indicate that TMTD has low toxicity to soil organisms.
7.1.1.5 Effects on birds
Effects of TMTD on birds have been reported in dietary studies. In mallard ducks, effects were noted in avian reproduction studies with this substance and included abnormal egg production, reductions in eggs laid, abnormal embryos, and hatchlings (US EPA 2004a). The no-observable-adverse-effect concentration was reported as 9.6 mg/kg-diet (US EPA 2004a). In two studies using Japanese quail (Coturnix japonica) and mallard duck (Anas platyrhynchos), the 14-day LC50 was reported to be greater than 805.2 mg/kg bodyweight (bw) (ECHA c2007-2017). After a 23-week exposure to this substance, the NOEC (for mortality, body weight, feed consumption and reproductive parameter) was determined to be 500 mg/kg-diet for a ground-dwelling bird (Colinus virginianus) (ECHA c2007-2017).
These results indicate that TMTD has low-to-moderate dietary toxicity to birds; it can cause reproductive and development effects, likely associated with underlying specific mode(s) of action.
7.1.2 Exposure assessment
7.1.2.1 Environmental monitoring data
In 2017-2018, limited surveillance was conducted in surface water at eight sites upstream and downstream of wastewater treatment plant discharge points, with some sites receiving wastewater from rubber products manufacturers. No samples contained TMTD above the method detection limit. There are very limited monitoring data reported by other countries. This substance was not detected in ground or surface water as recorded in certain US databases (US EPA 2004a). In Japan, this substance was included in a few environmental monitoring projects (J-CHECK c2010-). The limit of detection was 0.9-1 μg/L for the water sample and 0.02 μg/g-dw for the sediment sample. TMTD was not found in any sample collected in sediment or surface water; however, no information on the sampling locations was available (J-CHECK c2010-).
7.1.2.2 Exposure scenario
Rubber products manufacturing facilities can be categorized according to two attributes: whether the facility has compounding and/or processing operations, and the predominant form of rubber resin used (solid or latex) (Cheminfo 2013). TMTD is not known to be used in the manufacture of latex products in Canada at the time of assessment. To develop exposure scenarios for TMTD in rubber products manufacturing, the process of compounding to manufacture solid rubber products is considered in a quantitative scenario based on consideration of raw material handling (section 7.1.2.2.1). Releases to the environment may also occur as a result of processing operations in rubber manufacturing such as vulcanization; however, sufficient information is not available to estimate environmental exposure from processing steps. Exposure from the use of TMTD in automotive, and adhesive and sealants manufacturing sectors is addressed qualitatively in section 7.1.2.2.2.
7.1.2.2.1 Industrial local exposure scenario: solid rubber compounding
The solid rubber compounding process is expected to have a higher potential for release; therefore, it was chosen for estimating releases of TMTD to the aquatic environment in the quantitative exposure assessment. This scenario simulates releases of this substance from raw material weighing/handling and compounding areas (see Section 5). Such releases will go through wastewater treatment systems prior to entering surface water.
The exposure of this substance in the aquatic environment is estimated in the form of a Predicted Environmental Concentration (PEC), as follows:
The values selected for each of the parameters included in this equation are described in Table 7-3. Additional explanations are also provided later in this section.
| Symbol | Input | Value | Justification and reference |
|---|---|---|---|
Q | Quantity used per site per day (kg/day) | 509 | Quantity was determined on the basis of daily rubber compounding production capacities for the identified users, and an assumed concentration of 0.45% of the substance in the produced rubber compounds (based on the range of reported concentrations in rubber compounds, and below the highest reported concentration of 0.9% (ECCC 2016a)). It is noted that this value is below the concentration of 0.65% recommended by the OECD ESD on additives in rubber industry (OECD 2004). Overall potential daily use quantities of TMTD among these sites ranged from 100 to 1,000 kg/day; the average (509 kg) was used as the representative daily use quantity. See also discussion below on daily use quantity. |
L | Losses to wastewater | 0.0021 0.0003 | According to the OECD Emission Scenario Document for Plastic Additives (OECD 2009), releases from raw material handling and compounding for powders of particle size greater than 40 μm is 0.21%. In the Tyre and General Rubber Goods Generic Exposure Scenario guidance document (ChemRisk 2010), the upper bound emission factor for small or moderate scale use (on the basis of the total annual quantity) with no pre-treatment is 0.03%. Further discussion on the application of emission factors recommended by OECD (2009) and ChemRisk (2010) is provided below. |
R | Wastewater treatment system removal efficiency | 0.16 | The available information suggests that wastewater from the majority of rubber compounding facilities is discharged to wastewater treatment systems that use a secondary treatment. The removal efficiency associated with secondary wastewater treatment was estimated using the SimpleTreat (4.0) model as 16%. Hydrolysis, as the major degradation mechanism, was taken into account in the modelling. |
D | Daily dilution volumea (L/day) | 40 846 000 | This representative dilution volume represents the median of the dilution volumes associated with confirmed users of the substance. (This value corresponds to the 25th percentile of the distribution of the dilution volumes associated with the 46 known compounding and processing sites in the rubber sector in Canada.) |
Not applicable | Not applicable | 1 000 000 | Convert kg to mg. |
a The term “dilution volume” is used to express the potential dilution capacity of the receiving water body in relation to the effluent flow of the wastewater treatment system. It is calculated as the effluent flow (L/day) times the dilution factor of the receiving waterbody. Dilution factor is capped at 10. The 2.5th percentile low flow of receiving water body is used (to account for the occasional releases resulting in short-term exposure).
For the purpose of estimating environmental exposure of TMTD, two emission estimation documents, namely the OECD Emission Scenario Document (ESD) for Plastic Additives (OECD 2009) and the Tyre and General Rubber Goods Generic Exposure Scenario guidance document (ChemRisk 2010), have been considered for determining the losses of a substance to wastewater.
OECD ESD for Plastic Additives (OECD 2009) is one in a series of documents developed under the auspices of the OECD. These ESDs are developed by regulatory agencies in collaboration with industry, are peer-reviewed by other Exposure Assessment Task Force members (now known as the Working Party on Exposure Assessment) and are approved for declassification by OECD member countries prior to publication. These documents typically estimate ‘high end’ or ‘realistic worst case’ releases. The emission estimation approach of OECD ESD on Additives in Rubber Industry (OECD 2004) is not applied in this exposure assessment as it only addresses the fraction of chemicals remaining in vulcanised rubber, and does not take into account rubber additives released during raw material handling and compounding stages. It is considered that raw material handling and compounding releases of dry powders during manufacturing of plastic compounds resemble dry powder releases at rubber compounding sites; therefore, the OECD ESD on Plastic Additives (OECD 2009) was used to estimate these releases.
The ChemRisk guidance document (2010) was developed by ChemRisk for the European Tyre & Rubber Manufacturers’ Association (ETRMA). The emission factor estimates in this document incorporate risk management measures, and facilities employing a number of different types of practices and wastewater treatment were included in the analysis. However, the specific data for facilities are not provided in the report, making it difficult to evaluate the variability of releases between practices and treatment types, and to extrapolate the results to the Canadian context. The concerns associated with the lack of detail in Specific Environmental Release Categories (SpERC) documents developed for use under European REACH legislation are in line with reservations expressed by other jurisdictions regarding reliability and interpretation of these values (Ahrens et al. 2011). For these reasons, a lower weight is given to this source of information when estimating releases to wastewater.
The PECs in the receiving water near discharge sites (i.e., close to wastewater treatment systems discharge points) are calculated to be 0.022 mg/L and 0.0031 mg/L, based on the two emission estimation documents OECD 2009 and ChemRisk 2010, respectively.
In order to derive a daily TMTD use quantity for the representative rubber compounding site under this exposure scenario (Table 7-2), several known users of TMTD in Canada, both merchant and captive compounders, were considered. Their rubber compound production limit per day and their reported concentrations of the substance in rubber compounds were applied for estimation of possible daily use quantities of TMTD at solid rubber compounding facilities. In addition, the reported import quantities of TMTD by these companies were compared to the estimated daily substance use quantities in order to derive number of use days per year. The results indicated a range from 50 to 135 days of use of TMTD per year, which suggests both batch and continuous production. Facilities examined to develop this scenario are not necessarily dedicated to using only TMTD as their process regulator, since they can produce various types of solid rubber compounds for different industrial sectors. Therefore, in this scenario, TMTD daily use quantity is not assumed to be continuous, given the lower number of use days for some facilities (occasional releases). Consequently, this quantitative exposure scenario is representative of short-term rather than long-term exposure.
According to ChemRisk (2010) measured data for three chemical substances, where two of them were rubber accelerators, annual tonnage at monitored facilities ranged between 0.25 and 94 tonnes/year for small/moderate scale facilities, and between 111 and 1337 tonnes/year for large scale facilities. Based on emission days per year provided in Table 5 of ChemRisk (2010), the daily use quantity for the three substances is estimated to vary from 42 to 264 kg/day for small/moderate scale facilities, and from 572 to 3663 kg/day for large scale facilities. ChemRisk (2010) does not provide many details on the facilities where the measurements were taken; it is only known that the data for emission factor calculation were available from 19 facilities consisting of 13 tire facilities and 6 general rubber goods facilities. ChemRisk (2010) data also support available Canadian data that some of the users of rubber accelerators are direct dischargers with oil/water separation being the only on-site wastewater treatment. The calculation of critical daily use quantity (i.e., the minimum daily use quantity resulting in risk quotient =1) was performed with the assumptions presented in Table 7-3 (i.e., L, R, D). The critical daily use quantity is calculated (using the above equation) to be 4 kg/day and 28 kg/day with the assumed losses to wastewater (with consideration of emission factors being 0.0021 and 0.0003, respectively).
Considering the environmental fate anticipated for this substance, TMTD is expected to mainly remain in the aquatic compartment in surface water after being discharged from wastewater treatment systems. Partitioning into sediment is not expected to be significant. The low removal efficiency (16%) via wastewater treatment reflects the fact that a very small quantity of this substance is expected to sorb to biosolids. Application of biosolids to agricultural land or disposal in landfill is not expected to cause significant release to terrestrial environments. Given the above, exposure of TMTD to organisms in sediment or soil is expected to be minor and is not quantified in this exposure assessment.
7.1.2.2.2 Automotive and adhesive & sealants manufacturing sectors
Data obtained in 2016 in a follow-up to the CEPA section 71 survey (Environment Canada 2009), including information from the Canadian Vehicle Manufacturing Association (CVMA), were analyzed and indicate that TMTD has also been imported to Canada as an ingredient in ready-to-use sealant products and as a part of finished vehicles, as well as in different rubber products. In these rubber products or finished vehicles imported in Canada, TMTD would be vulcanized or cured, so only traces of TMTD are expected to remain in these products.
It may be possible for TMTD to enter a wastewater stream at a vehicle assembly facility associated with the use of sealants. When applying sealants on automobiles at vehicle assembly plants, the vehicle frame is subject to a cleaning process prior to curing under high temperature and painting; there may be releases of TMTD during this cleaning process. The TMTD-containing cleaning water is transferred to the on-site wastewater treatment system and the effluent from this treatment facility is subsequently sent to a publicly-owned wastewater treatment plant. Releases from this industrial use would eventually enter surface water via the publicly-owned wastewater treatment plant. Data for the automotive industry also suggest that if some sealants come off from the automobile body during the cleaning process, these sealants are collected in a sludge tank and treated as hazardous waste. Given that the quantity of TMTD applied as sealants in vehicle assembly plants is expected to be small, this source of release is considered to result in low exposure to TMTD in the aquatic environment.
A few companies reported, via CEPA section 71 survey (Environment Canada 2009), using TMTD to manufacture adhesive and sealant products (i.e., adhesive and sealant tapes). Although limited quantities were reported, there may be some releases of this substance via raw material handling and cleaning of formulation vessels. Given the limited volumes, this source of release is unlikely to be of concern and is not quantified in the assessment.
7.1.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine assessment information and develop conclusions using a weight-of-evidence approach and precaution. Evidence was gathered to determine the potential for TMTD in the Thiocarbamates Group to cause harm in the Canadian environment. Lines of evidence considered include those evaluated in this assessment that support the characterization of ecological risk in the Canadian environment. Secondary or indirect lines of evidence are considered when available, including regulatory decisions and classification of hazard or fate characteristics made by other regulatory agencies.
7.1.3.1 Risk quotient analysis
Risk quotient analyses were performed by comparing estimates of exposure (PECs; see the Ecological Exposure Assessment section) with ecotoxicity information (PNEC; see the Ecological Effects Assessment section) to determine whether there is potential for ecological harm in Canada.
While there is variability in the duration of environmental releases of TMTD from facilities across Canada, the representative quantitative exposure scenario focused on the shorter-term releases from solid rubber compounding processes. The resulting aquatic PECs were estimated to be 0.022 mg/L and 0.0031 mg/L, on the basis of two emission estimation documents (OECD 2009; ChemRisk 2010), respectively.
Structural evidence and in vivo and in vitro data confirm the high potency mode of action for TMTD and its high toxicity to aquatic organisms. This substance can have sub-lethal effects during a short-term exposure at low concentrations. On the basis of findings from a short-term developmental study with fish embryos, a PNEC of 0.00019 mg/L was derived.
Risk quotients (RQs) were calculated by dividing the PEC by the PNEC for relevant environmental compartments and associated exposure scenarios. Outcomes from the RQ analysis are presented in Table 7-4, suggest that releases of TMTD from its use in manufacturing of rubber products are expected to pose a risk to aquatic organisms in the environment.
| Scenario | PEC (mg/L) | PNEC (mg/L) | RQ |
|---|---|---|---|
| Industrial local exposure scenario: rubber compounding (using emission factor from OECD 2009) | 0.022 | 0.00019 | 116 |
| Industrial local exposure scenario: rubber compounding (using emission factor from ChemRisk 2010) | 0.0031 | 0.00019 | 16 |
As this substance is not expected to significantly partition into sediment or be released to soil, it is not expected to result in exposure to organisms in these two compartments. Furthermore, TMTD does not accumulate in organisms, hence birds and wildlife are not expected to be exposed to it via the food chain. Given the above, PNECs in sediment, soil or wildlife were not calculated.
7.1.3.2 Consideration of the lines of evidence
To characterize the ecological risk of TMTD, technical information for various lines of evidence was considered (as discussed in the relevant sections of this report) and qualitatively weighted. The key lines of evidence supporting the assessment conclusion are presented in Table 7-5, with an overall discussion of the weight of evidence provided in section 7.1.3. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence. The relevance refers to the impact the line of evidence has when determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis ranged from low to high, with the assigned weight having five possible outcomes.
The scope of this screening assessment is limited to TMTD. It can be noted that there are other rubber accelerators belonging to the chemical class of thiocarbamates. Some of these other rubber accelerators have similar physical-chemical properties to TMTD and, hence, could be present in similar environmental compartments. Available empirical data suggest that these substances may also be highly toxic to organisms (ECHA c2007-2017).
| Line of evidence | Level of confidencea | Relevance in assessmentb | Weight assignedc |
|---|---|---|---|
| Persistence in the environment | high | low | moderate |
| Long-range transport potential | high | low | moderate |
| Bioaccumulation in aquatic organisms | high | low | moderate |
| Mode of action and/or other non-apical data | moderate | moderate | moderate |
| PNEC for aquatic organisms | high | high | high |
| Model estimates for concentrations in wastewater effluents and surface water | moderate | high | moderate to high |
| Risk quotient for water | moderate | high | moderate to high |
Abbreviations: NA, Not Available; N/A, Not Applicable.
a Level of confidence is determined according to data quality, data variability, data gaps and whether the data are fit for purpose.
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the combined level of confidence and relevance in the assessment.
7.2 DPTT
7.2.1 Ecological effects assessment
7.2.1.1 Mode of action
A few structural alerts profiled using the OECD Toolbox (2015) for DPTT suggest some potential for a reactive mode of action via protein and DNA binding. However, no studies addressing the mode of action of DPTT have been identified. A few mutagenicity tests were conducted for the analogue CAS RN 971-15-3, which has the same structural profiling results as DPTT (ECHA c2007-2017). Positive results have been reported in an in vitro assay; however, negative results have been reported in a different in vitro assay and an in vivo study. The DPTT molecule is sterically hindered which can limit reactivity. Metabolism and elimination may also play a role in attenuating adverse effects. For further characterisation of effects, narcosis is considered as the mode of action and critical body residues are calculated.
7.2.1.2 Effects data
There is a lack of empirical ecotoxicity data for DPTT, and available QSAR models are considered not applicable to estimate toxicity for this substance. A Material Safety Data Sheet reported a 48-hour LC50 of 500 mg/L for a fish study (Americas International 2016). This value is much higher than the water solubility of this substance; no further details were available.
In an algal study conducted with the analogue CAS RN 971-15-3, no effects were observed on test organisms at an average measured concentration of 0.0079 mg/L after a 72-hour exposure (ECHA c2007-2017). This measured concentration is very close to its water solubility (0.01 mg/L), suggesting that the analogous substance hasn’t demonstrated any effect on the test organism at its saturation level in water.
A reproductive toxicity study on the analogue (CAS RN 971-15-3) has been identified (ECHA c2007-2017). Both F0 and F1 generations of the test animals (Sprague-Dawley rat) were administered the test substance at a dosage of 1000 mg/kg-bw/day during the test period. The No Observed Effect Level (NOEL) for parental toxicity, reproductive performance (mating and fertility), or toxic effects on progeny was considered to be 1000 mg/kg/day. These findings for the analogue have suggested that DPTT possesses low reproductive toxicity.
For sediment, a study was conducted on mayflies (Hexagenia spp.) and fresh water amphipods (Hyalella azteca) (ECCC 2016b). After a 3-week exposure to DPTT, there were no lethal effects observed on any test species at test concentrations up to 1000 mg/kg-dw (nominal) sediment. For growth effects, an EC25 of 980 mg/kg-dw sediment was reported for mayflies; while on fresh water amphipods, there were no effects on growth at any test concentration up to 1000 mg/kg-dw sediment. These data suggest that DPTT possesses low toxicity to sediment organisms.
For soil organisms as well as for wildlife, no empirical data were identified for either DPTT or its analogue. No estrogen receptor binding or protein binding alerts were predicted by the OECD Toolbox (v3.3.5), mainly due to a lack of phenol or aromatic amine in the molecular structure of this substance.
7.2.1.3 Lethal Body Burden and the external effect concentration
Due to a lack of empirical ecotoxicity data, the Lethal Body Burden (LBB) approach is considered to provide an additional line of evidence for characterizing the effects of DPTT on aquatic organisms. In the LBB approach, the body burden associated with a lethal narcotic effect is assumed to be fairly constant; such an internal effect concentration is the result of the product of the bioconcentration factor (BCF) and the external effect concentration (median lethal concentration, or LC50). To account for any specifically-acting Mode of Action, an additional assessment factor may be applied to account for the uncertainty associated with the extrapolation.
Based on the internal toxicity thresholds (to cause an acute or chronic effect on organisms) and the BCF, the external effect concentration (i.e., in surface water) can be calculated as:
The tissue residues associated with acute lethality for narcotic substances range from 2 to 8 mmol/kg (median of 3 mmol/kg); applying an acute-to-chronic ratio of 10, the median chronic lethality is 0.3 mmol/kg. However, considering structural alerts predicted by the OECD Toolbox (2006), the OASIS component (v1.3) suggests that this substance may be bioactive. To account for the potential bioactivity, an assessment factor of 30 was applied to estimate the tissue residues (ECCC 2016a). The tissue residues for effect thresholds from acute and chronic exposures are calculated to be 0.1 mmol/kg and 0.01 mmol/kg, respectively, for DPTT.
Among the few empirical BCFs reported for DPTT (Table 6-3), the highest value of 32 L/kg (CHRIP c2008) was selected to calculate the LC50. This BCF value was normalized to a standard 5% lipid content for mid-trophic level fish to yield a value of 32.65 L/kg. Considering the acute and chronic internal toxicity thresholds as determined above, the external concentrations required to cause an acute or a chronic lethal effect on aquatic organisms are calculated as follows.
The resulting acute and chronic LC50 values are 1.18 and 0.12 mg/L, respectively. Both values are above the water solubility of DPTT (0.01 mg/L based on analogue data). However, doing the reverse calculation using the water solubility (0.01 mg/L) and the bioaccumulation potential (BCF=32.65 L/kg) of this substance, results in an internal residue in fish of approximately 0.00085 mmol/kg. This value is much lower than the acute internal lethal effect threshold (0.1 mmol/kg) that has been established for DPTT as a specifically active substance. There is a wide margin between the maximum exposure to aquatic organisms and the minimal external concentration to cause an acute effect.
7.2.2 Exposure assessment
7.2.2.1 Environmental monitoring data
There have been no environmental monitoring data identified for DPTT in surface water or any other environmental medium in Canada. There are very limited monitoring data reported in other countries. In Japan, this substance was included in a few environmental monitoring projects in 1980 (J-CHECK c2010-). The limit of detection was 0.002-0.07 μg/L for water samples and 0.2 μg/g-dw for sediment samples. DPTT was not found in any sample collected in sediment or surface water; however, no information on the sampling locations was available (J-CHECK c2010-).
7.2.2.2 Exposure scenarios
DPTT is used in manufacturing rubber products in Canada. Similarly to TMTD, loss of this substance to wastewater generated from solid rubber products manufacturing facilities is considered to be the main source of potential releases to the environment.
On the basis of information from the CEPA section 71 survey (Environment Canada 2013) and follow-ups conducted in 2016, there are fewer than four industrial sites using this substance to manufacture solid rubber products. This substance is not expected to have as broad a use as TMTD. The quantitative exposure scenario was developed based on parameters from a selected Canadian solid rubber compounding facility reported to be using DPTT. The environmental exposure is estimated and presented in the form of a Predicted Environmental Concentration (PEC), as follows.
The values selected for each of the parameters included in this equation are described in Table 7-6.
| Symbol | Input | Value | Justification and reference |
|---|---|---|---|
| Q | Quantity used per site per day (kg/day) | 581 | Quantity was estimated on the basis of daily rubber compounding production capacity for the selected industrial site and the known concentration of DPTT in rubber compounds. |
| L | Losses to wastewater | 0.0021 0.0003 | According to the OECD Emission Scenario Document for Plastic Additives (OECD 2009), releases from raw material handling and compounding for powders of particle size greater than 40 μm is 0.21%. In the Tyre and General Rubber Goods Generic Exposure Scenario guidance document (ChemRisk 2010), the upper bound emission factor for small or moderate scale use (on the basis of the total annual quantity) with no pre-treatment is 0.03%. |
| R | Wastewater treatment system removal efficiency | 0.41 | The available information suggests that wastewater from this facility is discharged to a wastewater treatment plant that uses a secondary treatment. The removal efficiency associated with secondary wastewater treatment was estimated using the SimpleTreat (v4.0) model as 41%. Biodegradation, as the major degradation mechanism, was taken into account in the modelling. |
| D | Daily dilution volumea (L/day) | 25 776 000 | This value represents the daily dilution volume of the selected facility. |
| Not applicable | Not applicable | 1 000 000 | Convert kg to mg. |
a The term “daily dilution volume” is used to express the potential dilution capacity of the receiving water body in relation to the effluent flow of the wastewater treatment system. It is calculated as the effluent flow (L/day) times the dilution factor of the receiving waterbody. Dilution factor is capped at 10. The 2.5th percentile low flow of the receiving water body is used (to account for the occasional releases resulting in short-term exposure).
Considering all the above, the PECs in the receiving water nearby discharge sites are estimated to be 0.028 mg/L and 0.004 mg/L, respectively, on the basis of two emission estimation documents (OECD 2009; ChemRisk 2010).
After being discharged from wastewater treatment systems, DPTT is expected to mainly remain in the aquatic compartment in surface water and to undergo degradation; partitioning into sediment is not expected to be significant. During the wastewater treatment process, there is some sorption to biosolids. However, application of biosolids to agricultural land or disposal in landfill is not expected to cause significant release to terrestrial environments. Given the above, exposure of DPTT to organisms in sediment or soil is expected to be minor and is not quantified in the exposure assessment.
7.2.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine assessment information and develop conclusions using a weight-of-evidence approach and precaution. Evidence was gathered to determine the potential for DPTT in the Thiocarbamates Group to cause harm in the Canadian environment. Lines of evidence considered include those evaluated in this assessment that support the characterization of ecological risk in the Canadian environment. Secondary or indirect lines of evidence are considered when available, including regulatory decisions and classification of hazard or fate characteristics made by other regulatory agencies.
7.2.3.1 Risk quotient analysis
On the basis of the available toxicity data identified for DPTT and the analogue, this substance is not expected to demonstrate any effect on aquatic organisms at its water saturation level. It also possesses low toxicity to sediment organisms. Given that, a quantitative risk quotient analysis is not conducted for the aquatic or sediment compartments for this substance.
7.2.3.2 Considerations of all lines of evidence and their weights for determining potential to cause harm to the Canadian environment
To characterize the ecological risk of DPTT, technical information for various lines of evidence was considered (as discussed in the relevant sections of this report) and qualitatively weighted. The key lines of evidence supporting the assessment conclusion are presented in Table 7-7, with an overall discussion of the weight of evidence provided in section 7.2.2.2. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence. The relevance refers to the impact the line of evidence has when determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis ranged from low to high, with the assigned weight having five possible outcomes.
There is a general lack of empirical data for DPTT; available data for the analogous substance, CAS RN 971-15-3, have been considered when assessing this substance. DPTT possesses low vapour pressure and low solubility in water (0.01 mg/L). It is expected to undergo rapid degradation and not persist in the environment. It does not accumulate in organisms.
Available information suggests that this substance is not widely used in Canada, as only a few companies have been reported to use DPTT in manufacturing rubber products. Releases from industrial uses are expected to be occasional and to enter surface water via a wastewater treatment system. In the aquatic environment, the substance is expected to undergo fairly rapid degradation and the potential for long-range transport in water is low. Therefore, the current uses of this substance are expected to result only in short-term exposures to organisms in surface water in the vicinity of the discharge site. The PECs in the receiving water near discharge sites are estimated to be 0.004 and 0.028 mg/L, which is slightly higher than, but of the same order of magnitude as, the water solubility of DPTT (0.01 mg/L on the basis of analogue).
Although structural evidence suggests that DPTT is bioactive, there is a lack of in vivo and in vitro studies to further characterize its mode of action. Some QSAR structural alerts suggest the potential for DNA binding for this substance; there is also a mutagenic effect reported in an in vitro assay for the analogue, which has the same structural profiling results as DPTT. However, in vivo, no genetic effect was observed in test animals with exposure to the analogous substance at fairly high dosage.
There is a lack of empirical toxicity data for DPTT on organisms. On the basis of the acute toxicity data for the analogue, this substance is not expected to cause any effect at its water saturation concentration following short-term exposure. On the basis of the LBB approach, there is also a wide margin between the estimated maximum exposure to aquatic organisms (0.00085 mmol/kg) and the minimal external concentration to cause an acute effect (0.1 mmol/kg), suggesting that the environmental exposure to this substance is not expected to reach the internal concentration to cause an acute affect.
As discussed above, the weighting of key lines of evidence are presented in Table 7‑7 below. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence. The relevance refers to the impact the line of evidence has when determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis ranged from low to high.
| Line of evidence | Level of confidencea | Relevance in assessmentb | Weight assignedc |
|---|---|---|---|
| Persistence in the environment | high | low | moderate |
| Long-range transport potential | high | low | moderate |
| Bioaccumulation in aquatic organisms | high | low | moderate |
| Mode of action and/or other non-apical data | low | moderate | low to moderate |
| CBR for aquatic organisms | moderate | moderate | moderate |
Abbreviations: NA, Not Available; N/A, Not Applicable.
a Level of confidence is determined according to data quality, data variability, data gaps and if the data are fit for purpose.
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the combined level of confidence and relevance in the assessment.
d The quantitative risk analysis is not conducted for DPTT.
7.3 Sensitivity of conclusion to key uncertainties
It is noted that wastewater generated from solid rubber manufacturing is expected to be the main source of releases of TMTD and DPTT; however, such releases may vary from site to site depending on specific practices. There is uncertainty due to limited information and inherent variability in the frequency of usage of TMTD in the manufacturing of solid rubber products in Canada. Consequently, daily production capacities of solid rubber compounding facilities associated with TMTD were used to calculate the representative daily use quantity of TMTD as 509 kg/day in the quantitative exposure scenario. In contrast, the critical daily use quantity, one that results in RQ = 1, was calculated as 4 kg/day and 28 kg/day using the assumed losses to wastewater of 0.0021 and 0.0003, respectively. It suggests that daily use quantities of TMTD much lower than those noted in the quantitative exposure scenario have the potential to cause ecological harm.
8. Potential to cause harm to human health
8.1 DPTT
DPTT was included in the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Science Approach Document (Health Canada 2016b). In the approach, a decision tree that considers chemical structural features and chemical-specific data on genotoxicity (e.g., Ames test), when available, was used to assign a human exposure threshold value for a chemical, below which there is a low probability of risk to human health (i.e., TTC value). Structural representations of substances were retrieved and used to derive TTC values. Substances were examined against exclusion criteria. Then, for each substance in the TTC-based approach, conservative estimates of exposure were generated. Environmental exposures were estimated as a result of potential releases into the environment from commercial activities. Exposure was estimated for substances that may be used in products available to consumers, such as fragrance ingredients in cosmetics, food (e.g., possible food flavouring agents and substances used in the manufacture of food packaging materials), and in products available to consumers such as lubricants and adhesives. For each substance, exposure estimates were compared to their assigned TTC value, and substances that had exposure estimates below TTC values were considered to be of low concern to human health, as based on current levels of exposure. Results of the TTC-based approach specific to DPTT are presented in Table 8-1. Additional details with regards to data and considerations used to in the TTC-based approach are presented in the science approach document (Health Canada 2016b).
| CAS RN | TTC value (µg/kg bw/day) | Environmental intake estimate (µg/kg bw/day) | Exposure estimate from use of products (µg/kg bw/day) |
|---|---|---|---|
| 120-54-7 | 1.5 | 3.42E-1 | n/a |
On the basis of these results, DPTT was considered not to be a concern for human health at current levels of exposure.
8.2 TMTD
8.2.1 Exposure assessment
TMTD does not occur naturally in the environment. According to ECHA (2010), TMTD is of moderate water solubility but degrades quickly in water. It has low vapour pressure so is not expected to be in air. It is also not expected to bioaccumulate nor is it persistent. TMTD has also not been detected in freshwater, marine water, rain water, or groundwater in Europe (ECHA c2007-2017). There are no Canadian data for substances in the Thiocarbamates Group in biomonitoring studies, dust, indoor or outdoor air, or drinking water. Distribution to the environment was estimated by ChemCAN, based on the average concentrations over broad regions. This model predicted low TMTD concentrations (nanogram levels) primarily to surface water, with less than 0.001% partitioning to soil, air, or sediment (ChemCAN 2003). The estimated concentration in surface water was used as a surrogate for drinking water data, with resultant drinking water intakes of up to 1.3×10-6 mg/kg bw/day, corresponding to formula fed infants (zero to six months), where a drinking water intake rate of 0.8 L/day and a body weight of 7.5 kg were used (Health Canada 1998). Hence, the environment is not expected to be a significant source of human exposure to TMTD.
According to data collected from the follow-up with stakeholders in 2016 (ECCC 2016a), no residual TMTD is expected from its use in automobile manufacturing. Because TMTD is desulfurated within minutes in the rubber vulcanization process, no TMTD residues are expected from vulcanized rubber products; it is also not detected in final products (Bergendorff et al. 2007).
Regarding exposure from products available to consumers, minimal exposure is expected from adhesive tape products based on the low concentration of TMTD in the adhesives (personal communication from submitters in response to CEPA section 71 surveys; Health Canada 2016) and the limited dermal contact area.
Exposure, if any, to TMTD from its possible use in the manufacture of food packaging materials is expected to be negligible (personal communication, email from Health Product Food Branch, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada, August 2016; unreferenced).
To summarize, exposure of the general population to non-pesticidal uses of TMTD is not expected or exposure is expected to be low or negligible.
8.2.2 Health effects assessment
On the basis of a PMRA review on thiram (Health Canada 2016a), this substance is quickly absorbed, distributed, and metabolized after oral administration. It is primarily eliminated through respiration, and to a minor extent in feces, with low distribution to tissues. It is of slight acute oral toxicity, low acute dermal and inhalation toxicity, moderately irritating to the eyes but not the skin, and is a skin sensitizer. The no observed adverse effect level (NOAEL) of 1.86 mg/kg bw/day was used to characterize risks of all exposures and durations, based on developmental neurotoxicity at 4.36 mg/kg bw/day. Young rats demonstrated altered motor activity, decreased motor activity habituation, and effects on learning in memory, in the absence of maternal toxicity. Young rats also exhibited decreased body weight in the absence of parental effects in a two-generation reproductive toxicity study in rats. Reproductive toxicity was observed in another reproductive toxicity study in rats, based on sperm abnormalities, testicular effects, and decreased fertility at higher doses. Upon provision of new data, the PMRA re-affirmed this NOAEL in an updated review on thiram and its associated end-use products (Health Canada 2018); this review concluded that thiram may have some mutagenic and clastogenic activity, but there is uncertainty concerning its potential for other types of genotoxicity. Additionally, increased incidences of thyroid C-cell adenomas and hepatocellular adenomas in a two-year carcinogenicity study in rats were considered treatment-related in the high-dose group. The incidence of each tumour type lacked pairwise statistical significance, although there was a statistically significant trend. These tumours were benign and there was no evidence of progression to malignant tumours. The margin from the acceptable daily intake (ADI) to the NOAEL for these tumours was considered sufficiently health protective (Health Canada 2018). Health Canada is aware of the research on the endocrine and reproductive effects of thiram in mammals and the toxicology reference doses selected by the PMRA are protective of these effects for pesticidal uses of thiram (personal communication, email from the Pest Management Regulatory Agency, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada, February 2019; unreferenced).
8.2.3 Characterization of risk to human health
In terms of non-pesticidal uses, TMTD is a chemical that is used primarily as an additive to manufacture materials and products. The highest estimated intake from drinking water (1.3×10-6 mg/kg bw/day) is well below the ADI (0.002 mg/kg bw/day) established by the PMRA, and the risk to human health is expected to be less because of the conservative nature of the exposure estimate (e.g., rapid hydrolysis in water, low vapour pressure, and low potential for bioaccumulation or persistence). As such, the health risk from environmental media is not of concern. No residues are expected from its use as an additive in the vulcanized rubber products. Exposure, if any, from use of TMTD to manufacture food packaging materials is expected to be negligible. There may be potential for minimal exposure to residual TMTD from use of certain adhesive products.
While exposure of the general population from non-pesticidal uses of TMTD is not of concern at current levels, this substance is considered to have health effects of concern including developmental neurotoxicity and carcinogenicity. Therefore, there may be a concern for human health if exposures were to increase.
8.2.4 Uncertainties in evaluation of risk to human health
There is uncertainty associated with the predicted concentrations of TMTD in the environment, but given the volumes being used and rapid degradation, exposures via industrial releases of TMTD are not expected to be of concern to humans. There is no short-term inhalation toxicity study that assesses neurotoxicity parameters, and provision of this data to the PMRA could have an impact on the toxicology reference doses selected by the PMRA.
9. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is risk of harm to the environment from TMTD. It is concluded that TMTD meets the criteria under paragraph 64(a) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity; however, it is concluded that this substance does not meet the criteria under paragraph 64(b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from DPTT. It is concluded that DPTT does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that TMTD and DPTT do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that TMTD meets one or more of the criteria set out in section 64 of CEPA and that DPTT does not meet any of the criteria set out in section 64 of CEPA.
It has also been determined that TMTD does not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
ACD/Percepta [prediction module]. 2015. Version 14.0.0. Toronto (ON): Advanced Chemistry Development, Inc.
Ahrens A, Aust N, Bögi C, Heezen L, Schöder F, Tolls J, Traas T. 2011. SPERCs: refined emission estimation under REACH. [unpublished workshop report, April 4, 2011, Brussels]. Poster presented at SETAC Europe, Milan, May 2011.
Atanasov AG, Tam S, Rocken J M, Baker M E, Odermatt A. 2003. Inhibition of 11
beta-hydroxysteroid dehydrogenase type 2 by dithiocarbamates. Biochemical and Biophysical Research Communications. Vol. 308, pp. 257-262.
Balakirev MY, Zimmer G. 2001. Mitochondrial injury by disulfiram: two different mechanisms of the mitochondrial permeability transition. Chemico-Biological Interactions 138(3): 299-311.
Bergendorff O, Persson C, Ludtk A, Hansson C. 2007. Chemical changes in rubber allergens during vulcanization. Contact Dermatitis, 57: 152–157.
Canada. [1978]. Food and Drug Regulations. C.R.C., c.870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
CATALOGIC [environmental fate and ecotoxicity model]. 2015. Ver. 5.11.13. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Ver. 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [updated 2012 Nov 26; accessed 2016 Nov 2].
Cheminfo. 2013. Rubber Products Manufacturing Sector Study: Sector Overview, Pollution Prevention Practices and Pollution Control Measures. Prepared by Cheminfo Service Inc.
Chemnet. 2015. CAS RN 120-54-7.
ChemRisk 2010. Tyre and general rubber goods generic exposure scenario. Emission factor guidance for formulation and industrial use. Prepared by ChemRisk LLC, Pittsburgh, PA.
Chipinda I, Hettick JM, Simoyi RH, Siegel PD. 2007. Oxidation of 2-Mercaptobenzothiazole in Latex Gloves and Its Possible Haptenation Pathway. Chem. Res. Toxicol., 20 (8): 1084–1092.
CITI. 1991. Data of existing chemicals based on the CSCL Japan. Chemicals Inspection and Testing Institute, Japan.
DCED. 2012. Evaluation of 22 SIN List 2.0 substances according to the Danish proposal on criteria for endocrine disruptors. Danish Centre on Endocrine Disrupters.
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Government of Canada. [accessed 2016 June 30].
[EC] European Commission. 2003. Review report for the active substance thiram [PDF]. EC, Directorate-General for Health and Consumer Protection.
[ECCC] Environment and Climate Change Canada. 2016a. Data collected from a targeted information gathering initiative for assessments under the Chemicals Management Plan (June 2016). Data prepared by ECCC, Health Canada; Existing Substances Program.
[ECCC] Environment and Climate Change Canada. 2016b. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016c. Preliminary results from a research project under CMP. Environment and Climate Change Canada, unpublished.
[ECCC] Environment and Climate Change Canada. 2018. Data collected from a targeted information gathering initiative for assessments under the Chemicals Management Plan (June 2016) and subsequent follow up in 2018. Data prepared by ECCC, Health Canada; Existing Substances Program.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2016 July 19].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of risk assessment priorities: results of the 2015 review. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. c2007-2017. Registered substances database; search results for CAS RNs 120-54-7, 137-26-8, 971-15-3, 97-74-5, 97-77-8. Helsinki (FI): ECHA. [updated 2018 May 24; accessed 2016 August].
[ECHA] European Chemicals Agency. 2010. Annex 14: Summary profiles of chemicals with information on use, production, mission, monitoring and legal status [PDF]. Helsinki (FI): ECHA.
[EFSA] European Food Safety Authority. 2016. Public consultation on the active substance thiram. Parma (IT): EFSA.
Environment Canada. 2009. DSL Inventory Update Phase 1 data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2013. DSL Inventory Update Phase 2 data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[EQC] Equilibrium Criterion Model. 2011. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre.
FAO. 1997. Pesticide Residues in Food. Report sponsored jointly by FAO and WHO. 4.24, Thiram (Dithiocarbamates, 105) [accessed 2016 July 12].
Filipe OMS, Santos SAO, Domingues MRM, Vidal MM, Silvestre AJD, Neto CP, Santos EBH. 2013. Photodegradation of the fungicide thiram in aqueous solutions.Kinetic studies and identification of the photodegradation products by HPLC–MS/MS. Chemosphere 91: 993-1001.
Garbrecht MR, Krozowski ZS, Snyder JM, Schmidt TJ. 2006. Reduction of glucocorticoid receptor ligand binding by the 11-beta hydroxysteroid dehydrogenase type 2 inhibitor, Thiram. Steroids.71: 895-901.
Gay MH. 1987. Rat metabolism of 14C thiram single dose study. Project 87003B. Biotek Inc, USA. Unpublished.
Gay MH, Norris KJ, Nomeir AA, Markham P, McManus JP. 1992. Metabolism of orally administered 14C-thiram in rats. Project 8767, 8833, 8839, 8926A, 9049. Biotek, Inc. Analytical Development Corp. Arthur D. Little, Inc. Uniroyal Chemical Co Inc. Unpublished.
Gupta B, Rani M, Kumar R, Dureja P. 2012. Identification of degradation products of thiram in water, soil and plants using LC-MS technique. Journal of Environmental Science and Health Part B, 47(8): 823-831.
[HCN]. Health Council of the Netherlands. 2003. Health-based reassessment of administrative occupational exposure limits. Thiram. CAS RN 137-26-8 [PDF]. The Hague (NE): HCN, Committee on Updating of Occupational Exposure Limits.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. 2016a. Proposed re-evaluation decision. Thiram. PRVD2016-07. Ottawa (ON): Government of Canada.
Health Canada. 2016b. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
Health Canada. 2018. Re-evaluation decision. Thiram and Its Associated End-use Products. RVD2018-38. Ottawa (ON): Government of Canada.
Health Canada. [modified 2019 Dec 3]. Cosmetic Ingredient Hotlist: The List of Prohibited and Restricted Cosmetic Ingredients. Ottawa (ON): Health Canada, Consumer Product Safety Directorate. [accessed 2020 Jan 16].
Hermens JLM. 1990. Electrophiles and Acute Toxicity to Fish. Environmental Health Perspectives, 87: 219-225.
[HSDB] Hazardous Substances Data Bank [database]. 1983- . Bethesda (MD): National Library of Medicine (US). [accessed 2015 November 26].
[J-CHECK] Japan CHEmicals Collaborative Knowledge database [database]. c2010- . Tokyo (JP): National Institute of Technology and Evaluation (NITE). [accessed 2016 August].
[KEMI] Swedish Chemicals Agency. 2015. Substance evaluation conclusion as required by REACH Article 48 and evaluation report for thiram: EC No 205-286-2: CAS RN 137-26-8 [PDF]. Sundbyberg (SE): KEMI.
Kidd H and James DR, Eds. The Agrochemicals Handbook, Third Edition. Royal Society of Chemistry Information Services, Cambridge, UK, 1991, 3-11.
Lide DR. 2003. CRC Handbook of chemistry and physics. 84th edition.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 6]. Ottawa (ON): Government of Canada.
Lopez-Antia A, Ortiz-Santaliestra ME, Blas EG, Camarero PR, Mougeot F, Mateo R. 2015. Adverse effects of thiram-treated seed ingestion on the reproductive performance and the offspring immune function of the red-legged partridge. Environmental Toxicology and Chemistry 34(6): 1320-1329.
Milenkovski S, Bååth E, Lindgren PE, Berglund O, 2010. Toxicity of fungicides to natural bacterial communities in wetland water and sediment measured using leucine incorporation and potential denitrification. Ecotoxicology, 19(2): 285–294.
Mishra VK, Srivastava MK, Raizada RB. 1998. Testicular toxicity in rat to repeated oral administration of tetramethylthiuram disulfide (Thiram). Indian J Exp Biol 36(4): 390-394.
Namazie International. c2014. Latex: concentrated latex. [accessed 2019 April 15].
[NCI] National Chemical Inventories [database on a CD-ROM]. 2015. Issue 2. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [accessed 2016 December 1].
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2018 May 7]. Ottawa (ON): Government of Canada.
Nollet. 2000. Handbook of water analysis. Editor, Marcel Dekker, Inc. ISBN 0-8247-8433-2, page 619.
Norris KJ. 1993a. Determination of the metabolic fate of 14C-thiram orally administered to lactating goats. Project 1057. ADC report 1057-1. Analytical Development Corporation (ADC), USA. Unpublished. Cited in FAO 1997.
Norris KJ. 1993b. Determination of the metabolic fate of 14C-thiram orally administered to laying hens. Project 1058. ADC report 1058-1, including addendum no. 1. Analytical Development Corporation (ADC), USA. Unpublished. Cited in FAO 1997.
[OECD] Organisation for Economic Co-operation and Development. 2004. Emission scenario document on additives in rubber industry [PDF]. Paris (France): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 6; Report No.: ENV/JM/MONO(2004)11, JT00166668).
[OECD] Organisation for Economic Co-operation and Development. 2006. The OECD Pov and LRTP Screening Tool 2.2. Software and Manual, OECD, Paris.
[OECD] Organisation for Economic Co-operation and Development. 2009. Emission scenario document on plastic additives [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 3; Report No.: ENV/JM/MONO(2004)8/REV1, JT03267870).
[OECD] Organisation for Economic Co-operation and Development. 2010. SIDS initial assessment report: bis(dimethylthiocarbamoyl)disulfide; thiram: CAS No. 137-26-8. SIAM [SIDS Initial Assessment Meeting] 31; 2010 October; Paris, France.
OECD QSAR Toolbox. [read across tool]. 2015. Ver. 3.3.5. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Schuurmann G, Ebert RU, Kuhne R. 2006. Prediction of the Sorption of Organic Compounds into Soil Organic Matter from Molecular Structure. Environ. Sci. Technol, 40: 7005-7011.
Stoker TE, Goldman JM, Cooper RL. 1993. The dithiocarbamate fungicide thiram disrupts the hormonal control of ovulation in the female rat. Reprod Toxicol 7(3): 211-218.
Stoker TE, Jeffay SC, Zucker RM, Cooper RL, Perreault SD. 2003. Abnormal fertilization is responsible for reduced fecundity following thiram-induced ovulatory delay in the rat. Biology of Reproduction 68: 2142-2149.
Teraoka H, Urakawa S, Nanba S, Nagai Y, Dong W, Imagawa T, Tanguay RL, Svoboda K, Handley-Goldstone HM, Stegeman JJ, Hiraga T. 2006. Muscular contractions in the zebrafish embryo are necessary to reveal thiuram-induced notochord distortions. Toxicology and Applied Pharmacology 212: 24-34.
Tomlin CDS. 2003. The e-Pesticide Manual, version 3.0. Thiram. 13th editon. Editor. Surrey (UK): British Crop Protection Council.
[UH PPDB] University of Hertfordshire Pesticide Properties DataBase [database]. 2015. Hertfordshire (UK): University of Hertfordshire. [accessed 28 Oct 2016].
[US EPA]. US Environmental Protection Agency. 2001. The grouping of a series of dithiocarbamate pesticides based on a common mechanism of toxicity [PDF]. Washington (DC): US EPA, Office of Pesticide Programs.
[US EPA]. US Environmental Protection Agency. 2004a. Environmental fate and ecological risk assessment for the registration of Thiram. Washington (DC): US EPA, Office of Prevention, Pesticides and Toxic Substances.
[US EPA]. US Environmental Protection Agency. 2004b. Reregistration Eligibility Decision for Thiram [PDF]. Washington (DC): US EPA, Office of Prevention, Pesticides and Toxic Substances. [accessed 2019 July 2].
[US EPA] US Environmental Protection Agency. 2010. TSCA New Chemicals Program (NCP) chemical categories [PDF]. Washington (DC): US EPA, Office of Pollution Prevention and toxics.
Yang C, Hamel C, Vujanovic V, Gan Y. 2011. Fungicide: Modes of action and possible impact on nontarget microorganisms. International Scholarly Research Network. Volume 2011, Article ID 130289.
