Screening Assessment
Ethanamine, 2-[4-[(1Z)-1,2-diphenyl-1-butenyl]phenoxy]-N,N-dimethyl-
(Tamoxifen)
Chemical Abstracts Service Registry Number
10540-29-1
Environment Canada
Health Canada
February 2015
Table of Contents
- Synopsis
- 1. Introduction
- 2. Substance Identity
- 3. Physical and Chemical Properties
- 4. Sources and Uses
- 5. Releases to the Environment
- 6. Measured Environmental Concentrations
- 7. Environmental Fate
- 8. Potential to Cause Ecological Harm
- 9. Potential to Cause Harm to Human Health
- 10. Uncertainties in Evaluation of Risk to Human Health
- 11. Conclusion
- 12. References
Tables
- Table 1a. Substance identity: tamoxifen and tamoxifen citrate
- Table 1b. Substance identity for 4-hydroxytamoxifen (4-HT), a metabolite of tamoxifen
- Table 1c. Substance identity: endoxifen, a metabolite of tamoxifen
- Table 2a. Physical and chemical properties of neutral form of tamoxifena
- Table 2b. Physical and chemical properties of 4-hydroxytamoxifen (4-HT), a metabolite of tamoxifen
- Table 2c. Physical and chemical properties of endoxifen, a metabolite of tamoxifen
- Table 3. Results of Level III fugacity modelling for tamoxifen (EQC 2003), indicating the percentage of tamoxifen partitioning into each compartment
- Table 4a. Modelled data for degradation of tamoxifen
- Table 4b. Modelled data for degradation of 4-hydroxytamoxifen (4-HT), a metabolite of tamoxifen
- Table 4c. Modelled data for degradation of endoxifen, a metabolite of tamoxifen
- Table 5a. Summary of modelled data for bioaccumulation of tamoxifen (neutral and ionized forms)
- Table 5b. Summary of modelled data for bioaccumulation of 4-HT, a metabolite of tamoxifen
- Table 5c. Summary of modelled data for bioaccumulation of endoxifen, a metabolite of tamoxifen
- Table 6a. Empirical data for aquatic toxicity of tamoxifen..
- Table 6b. In vitro empirical data for the toxicity of 4-HT, a metabolite of tamoxifen
- Table 7a. Summary of input values used for estimating aquatic concentrations resulting from industrial releases of tamoxifen
- Table 7b. Summary of input values used for estimating aquatic concentrations resulting from prescribed use of tamoxifen
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment of the substance ethanamine, 2-[4-[(1Z)-1,2-diphenyl-1-butenyl]phenoxy]-N,N-dimethyl-, Chemical Abstracts Service Registry NumberFootnote[1]10540-29-1, also known as tamoxifen. This substance was identified as a priority for assessment because it had been found to meet the categorization criteria for bioaccumulation and inherent toxicity to non-human organisms and is known to be in commerce in Canada. Tamoxifen was also identified as a priority for assessment because it had been identified as posing a potential high hazard to human health based on classifications by other national or international agencies for carcinogenicity.
Drugs containing tamoxifen as an ingredient are assessed under the Food and Drugs Act (F&DA) with respect to their safety, effectiveness and quality. This assessment focused on uses and exposures that were not covered as part of the F&DA assessment, specifically the risks posed by the residues resulting from manufacture, formulation and disposal after use.
Tamoxifen is an organic substance that does not occur naturally in the environment. It can be manufactured as chemical-grade tamoxifen (CAS RN 10540-29-1) or as pharmaceutical- grade tamoxifen citrate (CAS RN 54965-24-1). Tamoxifen with CAS RN 10540-29-1 is the only substance of the two that is listed on the Domestic Substances List. In Canada, tamoxifen citrate is primarily used as a human pharmaceutical, and chemical-grade tamoxifen can also be used as a laboratory research tool. Specifically, tamoxifen is used as a therapeutic substance to treat estrogen-responsive breast cancer; in research, its properties as a selective estrogen receptor modulator are used to examine mechanisms of endocrine function. The citrate moiety associated with tamoxifen is expected to have negligible ecotoxicological effects.
Tamoxifen is highly metabolized in the liver, and both the parent compound and its metabolites are excreted from the body when ingested. The hydroxylated metabolites of tamoxifen, 4-hydroxytamoxifen and endoxifen, which are structurally very similar to the parent compound, can be released to the environment together with unmetabolized tamoxifen and remain biologically active. Therefore, their properties are considered concurrently with the properties of tamoxifen in this screening assessment.
Commercially available data on pharmaceutical sales in Canada for 2011 and 2012 indicate that over 300 kg of tamoxifen citrate was purchased by hospitals and pharmacies for prescription in each of those years. Similar data were also available to estimate that 250 kg of the substance was purchased by hospitals and pharmacies for prescription across Canada in 2007. There are several pharmaceutical companies that are licensed to market tamoxifen in Canada for human use. Chemical-grade tamoxifen can also be purchased from major chemical manufacturers.
Based on its physical and chemical properties, if released to the environment, tamoxifen is expected to reside in water, soil and sediment, depending on the compartment of release. Based on the modelled data and empirical evidence, tamoxifen, 4-hydroxytamoxifen and endoxifen are expected to persist in the water, soil and sediment. Tamoxifen is not expected to bioaccumulate in organisms due to its low water solubility, relatively large cross-sectional diameter (resulting in restricted uptake across the gill as a result of steric hindrance) and the high potential for fish to metabolize it. Modelled data also indicated that 4-hydroxytamoxifen and endoxifen have a limited bioaccumulation potential.
Tamoxifen is registered for pharmaceutical use in Canada. Tamoxifen can potentially make its way to surface waters through release from manufacturing or formulation sites. Tamoxifen and its metabolites, 4-hydroxytamoxifen and endoxifen, can be found in surface water as a result of releases of these substances in feces or urine from the therapeutic drug use of tamoxifen. Given these potential releases, the main source of ecological exposure to tamoxifen is through water. Because no information was available regarding actual releases of this substance in Canada, realistic conservative exposure scenarios, selected for a site-specific industrial operation and for down-the-drain releases through prescribed use of tamoxifen were developed to estimate discharges of the substance into the aquatic environment. Tamoxifen and its metabolites, 4-hydroxytamoxifen and endoxifen, are considered to be highly toxic to aquatic organisms, and have potential for endocrine disruption. The predicted environmental concentrations in water were below the predicted no-effect concentration calculated for aquatic organisms.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to organisms or the broader integrity of the environment from tamoxifen. It is concluded that tamoxifen does not meet the criteria under paragraph 64(a) or (b) of CEPA 1999, as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
In terms of general population exposure to tamoxifen, the principal source of exposure is drinking water containing the drug. The exposure to tamoxifen present in drinking water is significantly smaller than the exposure to tamoxifen through its use as a pharmaceutical.
For this assessment, conservative assumptions were used when estimating the potential indirect exposure of the general population to tamoxifen. Limited data on tamoxifen in Canadian waters are currently available. Very low concentrations of tamoxifen were measured in the influent, effluent, and biosolids samples collected from select Canadian waste water treatment plants, and leachate samples from Canadian landfills. Therefore, for the purposes of this assessment, modelled data in surface water in Canada and the reporting limits for the samples collected from Canadian wastewater effluent were used as conservative proxies for Canadian drinking water concentrations. Upper-bounding estimated intakes from environmental media were very low (less than 0.1 ng/kg body weight per day). Based on these low exposures, risks from this substance are not expected. To further support this risk characterization, the upper-bounding estimated indirect exposures of the general population were compared with the lowest therapeutic dose. The margins of exposure ranged from greater than 82 000 to greater than 4 000 000.
Based on the information presented in this screening assessment, it is concluded that tamoxifen does not meet the criteria under paragraph 64(c) of CEPA 1999, as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Conclusion
It is concluded that tamoxifen does not meet any of the criteria set out in section 64 of CEPA 1999.
1. Introduction
The Canadian Environmental Protection Act, 1999(CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health
Screening assessments focus on information critical to determining whether a substance meets the criteria as set out in section 64 of the Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999). Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and precautionFootnote[2].
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure. Relevant data were identified up to March 2013. Key studies were critically evaluated, along with modelled results, to reach conclusions. When available and relevant, information presented in risk and hazard assessments from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the critical studies and lines of evidence most pertinent to the conclusion.
Drugs containing tamoxifen as an ingredient are assessed under the Food and Drugs Act (F&DA) (Canada 1985) with respect to their safety, effectiveness and quality. This assessment focused on uses and exposures that were not covered as part of the F&DA assessment, specifically the risks posed by the residues resulting from manufacture, formulation and disposal after use.
This screening assessment was prepared by staff in the Existing Substances programs at Health Canada and Environment Canada and incorporates input from other programs within these departments and comments from the 60-day public comment period following the publication of the draft screening assessment. The ecological and human health portions of this assessment have undergone external written peer review and/or consultation. Comments on the technical portions relevant to the environment were received from Chris Metcalfe, Trent University and Vance Trudeau, University of Ottawa. Comments on the approach used to assess the substance with respect to human health were received from Warren Foster, McMaster University, Sam Kacew, McLaughlin Centre for Population Health Risk Assessment, and Beate Escher, University of Queensland. While comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada.
The critical information and considerations upon which the screening assessment is based are summarized below.
2. Substance Identity
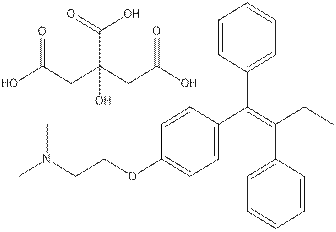
For the purposes of this document, the substance ethanamine, 2-[4-[(1Z)-1,2-diphenyl-1-butenyl]phenoxy]-N,N-dimethyl- will be referred to by its common name, tamoxifen. Tamoxifen can be manufactured as chemical-grade tamoxifen (CAS RN 10540-29-1) or as pharmaceutical- grade tamoxifen citrate (CAS RN 54965-24-1). Tamoxifen with CAS RN 10540-29-1 is the only substance of the two that is listed on the Domestic Substances List (DSL). Tamoxifen citrate is available as a pharmaceutical for humans in products marketed under different names, such as Novaldex-D, Apo-Tamox and Mylan-Tamoxifen (DPD 2010)(see Table 1a).; however, both forms of tamoxifen (i.e., chemical and pharmaceutical grade), have been used in a variety of toxicity studies. For the purpose of the present screening assessment, both the pharmaceutical-grade tamoxifen citrate and the chemical-grade tamoxifen are treated equally and interchangeably. The presence of citrate salt is generally omitted from discussion, given that its function is predominantly pharmacokinetic and it is not expected to contribute to the toxicity of tamoxifen itself. Therefore, tamoxifen is the primary subject of this screening assessment.
2.1.1 Metabolites
Tamoxifen is a pharmaceutical substance, known as a selective estrogen receptor modulator (SERM) substance (Williams et al. 2007). When ingested by humans, tamoxifen is extensively metabolized in the liver by cytochrome p450 enzymes, and both tamoxifen and its metabolites are known to be excreted from the body (Kisanga et al. 2005). Hydroxylated metabolites, 4-hydroxytamoxifen (4-HT) and endoxifen, are the major active metabolites of tamoxifen; they exhibit properties similar to those of the parent compound and are both very powerful antiestrogens (Williams et al. 2007; Lim et al. 2005; Kisanga et al. 2005). Moreover, tamoxifen has been referred to as a pro-drug, in that the effectiveness of the therapeutic treatment depends on its adequate conversion to the hydroxylated metabolites (Jaremko et al. 2010).
Metabolism of tamoxifen to 4-HT and endoxifen occurs in steps that include hydroxylation and demethylation. Consequently, tamoxifen and its hydroxylated metabolites are very similar structurally, differing mainly by the addition of the hydroxyl (OH) group to one of the phenyl rings in both metabolites and the replacement of the dimethylamino group by the methylamino group in one of the metabolites, endoxifen (Goetz and Loprinzi 2003; Kisanga et al. 2005). Therefore, since both hydroxylated metabolites of tamoxifen, 4-HT and endoxifen, remain biologically active, are excreted from the human body upon ingestion, are structurally very similar to the parent compound and can co-occur with unmetabolized tamoxifen in the environment, their toxicity properties are evaluated concurrently with those of tamoxifen in this screening assessment. Substance identity information for tamoxifen, 4-HT and endoxifen is provided in Tables 1a, 1b and 1c, respectively.
| Substance name | Tamoxifen | Tamoxifen citrate |
|---|---|---|
| CAS RN | 10540-29-1 | 54965-24-1 |
| DSL name | Ethanamine, 2-[4-[(1Z)-1,2-diphenyl-1-butenyl]phenoxy]-N,N-dimethyl- | n/a |
| NCI namesFootnote Table 1a[a] | Ethanamine, 2-[4-[(1Z)-1,2-diphenyl-1-butenyl]phenoxy]-N,N-dimethyl- (ASIA-PAC, DSL, NZIoC); Ethanamine, 2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-N,N-dimethyl-, (Z)- (AICS); Tamoxifen (English, German) (REACH, EINECS); Tamoxifene (French) (EINECS); Tamoxifeno (Spanish) (EINECS) | |
| Other names | (Z)-2-[4-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethanamine; Ethanamine, 2-[4-[(1Z)-1,2-diphenyl-1-buten-1-yl]phenoxy]-N,N-dimethyl-; Ethylamine, 2-[p-(1,2-diphenyl-1-butenyl)phenoxy]-N,N-dimethyl-, (Z)-; ICI 47699; Mammaton; Novaldex; Tamoxifen and its salts; trans-Tamoxifen; Z-Tamoxifen |
(Z)-(2-(4-(1,2-Diphenylbut-1-enyl)phenoxy)ethyl)dimethylammonium dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate; Ethanamine, 2-(4-((1Z)-1,2-diphenyl-1-butenyl)phenoxy)-N,N-dimethyl-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1); Ethanamine, 2-(4-(1,2-diphenyl-1-butenyl)phenoxy)-N,N-dimethyl, (Z)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1) Novaldex-D, Apo-Tamox, Mylan-TamoxifenFootnote Table 1a[b] Caditam; Apo-Tamox, Caditam, Farmifeno, Genox, Ginarsan, Emblon, Jenoxifen, Kessar, Ledertam, Nolgen, Noltam, Nourytan, Noxitem, Oncotam, Soltamox, Tafoxen, Tamofen, Tamoplex, Tamox-Puren, Tamoxasta, Taxus, Terimon, TMX, Zemide, Zitazonium, ZynoplexFootnote Table 1a[c] |
| Chemical group (DSL stream) |
Discrete organics | Discrete organics |
| Major chemical class or use | Aliphatic amines | Aliphatic amines |
| Major chemical subclass | Phenols | Phenols |
| Chemical formula | C26H29NO | C26H29NO.C6H8O7 |
| Chemical structure | 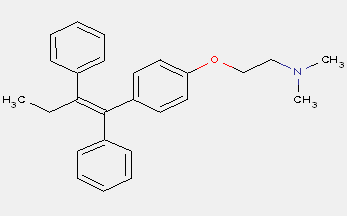 |
 |
| SMILES | C(=C(/c1ccccc1)CC)(\c1ccc(OCCN(C)C)cc1)c1ccccc1 | C(=C(/c1ccccc1)CC)(\c1ccc(cc1)OCCN(C)C)cc1)c1ccccc1.C(CC(O)=O)(CC(O)=O)(C(O)=O)O |
| Molecular weight (g/mol) | 371.52 | 563.62 |
| CAS RN | 68047-06-3 |
|---|---|
| Other registry numbers | 65213-48-1, 72732-26-4, 76276-99-8 |
| Systematic nameFootnote Table 1b[a].1 | Phenol, 4-((1Z)-1-(4-(2-(dimethylamino)ethoxy)phenyl)-2- phenyl-1-butenyl)-; Phenol, 4-(1-(4-(2-(dimethylamino)ethoxy)phenyl)-2-phenyl-1-butenyl)-, (Z)- |
| Synonyms | (Z)-4-Hydroxytamoxifen (US NLM); 4-((1Z)-1-(4-(2-(dimethylamino)ethoxy)phenyl)-2-phenyl-1- butenyl)phenol (US NLM); BRN 4910749 (RTECS); Hydroxytamoxifen (RTECS); ICI 79,280 (RTECS); ICI 79280 (US NLM); trans-4-(1-(4-(2-(Dimethylamino)ethoxy)phenyl)-2-phenyl-1-(butenyl)phenol (RTECS); trans-4-Hydroxytamoxifen (US NLM) |
| Chemical group (DSL stream) | Discrete organics |
| Major chemical class or use | Aliphatic amines |
| Major chemical subclass | Phenols |
| Chemical formula | C26H29NO2 |
| Chemical structure |  |
| SMILES Footnote Table 1b[b].1 | c1cccc(\C(CC)=C(\c2ccc(cc2)O)c2ccc(OCCN(C)C)cc2)c1 |
| Molecular mass (g/mol) | 387.52 |
| Chemical Abstracts Service Registry Number(CAS RN) | 110025-28-0 |
|---|---|
| Systematic nameFootnote Table 1c[a].2 | Phenol, 4-(1-(4-(2-(methylamino)ethoxy)phenyl)-2-phenyl-1-butenyl)- |
| Synonyms | 4-Hydroxy-N-demethyltamoxifen (MeSH); Endoxifen (MeSH, US NLM) |
| Other names | 4-Hydroxy-N-desmethyltamoxifen (MeSH) |
| Chemical group (DSL stream) | Discrete organics |
| Major chemical class or use | Aliphatic amines |
| Major chemical sub-class | Phenols |
| Chemical formula | C25H27NO2 |
| Chemical structure |  |
| SMILESFootnote Table 1c[b].2 | c1(ccc(cc1)C(=C(/CC)c1ccccc1)\c1ccc(cc1)OCCNC)O |
| Molecular mass (g/mol) | 373.49 |
3. Physical and Chemical Properties
Table 2a contains the available experimental and modelled physical and chemical properties of tamoxifen that are relevant to its environmental fate. In addition, physical and chemical properties for the hydroxylated tamoxifen metabolites, 4-HT and endoxifen, were modelled and are presented in Tables 2b and 2c, respectively. Experimental physical and chemical properties of 4-HT and endoxifen were not found in the published literature.
Models based on quantitative structure–activity relationships (QSARs) were used to generate data for some of the physical and chemical properties of tamoxifen and its hydroxylated metabolites, 4-HT and endoxifen. These models (except WSKOWWIN) are mainly based on fragment addition methods, i.e., they rely on the structure of a chemical. Since these models accept only the neutral form of a chemical as input (in SMILES form), the modelled values shown in Tables 2a, 2b and 2c are for the neutral forms of tamoxifen, 4-HT and endoxifen. However, the relatively high acid dissociation constant (pKa) of 8.69 for the basic functional group indicates that 50% of tamoxifen will be found in its ionized form at pH 8.69 (ACD/pKaDB 2005). In water bodies at environmentally relevant pH values (pH 6–9), about 33% (pH 9) to 100% (pH 6) of tamoxifen will be in its neutral form, indicating that aquatic exposure to tamoxifen can be to both the neutral and ionized forms. The proportion of dissociated tamoxifen [0.2% (pH 6) to 67% (pH 9)] (ACD/pKaDB 2005) indicates that partitioning behaviour predicted using the log D is appropriate for the ionized form of the substance. Therefore, the physical and chemical property predictions likely do not fully represent the properties and environmental behaviour of tamoxifen as an ionized compound. However, as the neutral form of tamoxifen is also present in the environment, the predicted physical and chemical properties remain relevant.
| Property | Type | Value[a],Footnote Table 2a[b].3 | Temperature (°C) | Reference |
|---|---|---|---|---|
| Melting point (°C) | Modelled | 180.85* | MPBPWIN 2008 | |
| Boiling point (°C) | Modelled | 468.20 | MPBPWIN 2008 | |
| Density (kg/m3) | Experimental | N/A | ||
| Vapour pressure (Pa) | Modelled | 4.62 × 10−6* | 25 | EPI Suite 2008 |
| Henry’s Law constant (Pa·m3/mol) |
Modelled | 4.55 × 10−5* | 25 | MPBPWIN 2008 |
| Log Kow (dimensionless) | Modelled | 6.3 | KOWWIN 2008 | |
| Log D (dimensionless) | ExperimentalFootnote Table 2a[c] | Log Doct = 4.51* | CEREP c2010a | |
| Log D (dimensionless) | Modelled | Log Dcyc = 4.60 | ACD/pKaDB 2005 | |
| Log Koc (dimensionless) | Modelled | 6.42 | KOCWIN 2008 | |
| Water solubility (mg/L) | Experimental[c] | 0.4829* (1.3 µM) |
CEREP c2010b | |
| Water solubility (mg/L) | Modelled (estimated from log Kow) |
0.1936 | 25 | WSKOWWIN 2008 |
| Water solubility (mg/L) | Modelled (estimated from fragments) |
0.0246 | 25 | WSKOWWIN 2008 |
| pKa (dimensionless) | Experimental | 9.24 | Flexser et al. 1935 | |
| pKa (dimensionless) | Modelled | 8.69 | ACD/pKaDB 2005 |
| Property | Type | ValueFootnote Table 2b[a].4 | Temperature (°C) | Reference |
|---|---|---|---|---|
| Melting point (°C) | Modelled | 211.01* | MPBPWIN 2008 | |
| Boiling point (°C) | Modelled | 503.04 | MPBPWIN 2008 | |
| Density (kg/m3) | Experimental | N/A | ||
| Vapour pressure (Pa) | Modelled | 4.14 × 10−9* | 25 | EPI Suite 2008 |
| Henry’s Law constant (Pa·m3/mol) | Modelled | 4.73 × 10−9* | 25 | MPBPWIN 2008 |
| Log Kow (dimensionless) | Modelled | 5.82 | KOWWIN 2008 | |
| Log Koc (dimensionless) | Modelled | 6.53 | KOCWIN 2008 | |
| Water solubility (mg/L) | Modelled (estimated from log Kow) |
1.51 | 25 | WSKOWWIN 2008 |
| Water solubility (mg/L) | Modelled (estimated from fragments) |
0.73* | 25 | WSKOWWIN 2008 |
| pKa (dimensionless) | Modelled | 10.35 (acid) 8.7 (base) |
ACD/pKaDB 2005 | |
| Log D (dimensionless) | Modelled | 4.25* | ACD/pKaDB 2005 |
| Property | Type | ValueFootnote Table 2c[a].5 | Temperature (°C) | Reference |
|---|---|---|---|---|
| Melting point (°C) | Modelled | 212.24* | MPBPWIN 2008 | |
| Boiling point (°C) | Modelled | 501.85 | MPBPWIN 2008 | |
| Density (kg/m3) | Experimental | N/A | ||
| Vapour pressure (Pa) | Modelled | 4.32 × 10−9* | 25 | EPI Suite 2008 |
| Henry’s Law constant (Pa·m3/mol) | Modelled | 2.16 × 10−9* | 25 | MPBPWIN 2008 |
| Log Kow (dimensionless) | Modelled | 5.61 | KOWWIN 2008 | |
| Log Koc (dimensionless) | Modelled | 6.56 | KOCWIN 2008 | |
| Water solubility (mg/L) | Modelled (estimated from log Kow) | 2.79 | 25 | WSKOWWIN 2008 |
| Water solubility (mg/L) | Modelled (estimated from fragments) | 2.19* | 25 | WSKOWWIN 2008 |
| pKa (dimensionless) | Modelled | 10.36 (acid) 9.4 (base) |
ACD/pKaDB 2005 | |
| Log D (dimensionless) | Modelled | 3.74* | ACD/pKaDB 2005 |
4. Sources and Uses
The substance tamoxifen is not produced naturally in the environment. Tamoxifen is a pharmaceutical that has been used as an antiestrogen to treat estrogen-responsive human breast cancers.
Tamoxifen is known as a selective estrogen receptor modulator (SERM) substance, able to act as both an estrogen antagonist and an estrogen agonist in different types of tissues (Williams et al. 2007; Isidori et al. 2009; Marty et al. 2011). It has become a research tool in the field of fish endocrinology and also has applications in aquaculture and ecotoxicology (Williams et al. 2007). Moreover, to improve understanding of the potential impacts of selective estrogen receptor modulators in aquatic life, tamoxifen was recommended for use as a reference compound in fish chronic testing in a 2003 Society of Environmental Toxicology and Chemistry Pellston Workshop (Williams et al. 2007).
To date, a survey pursuant to section 71 of CEPA 1999 has not been issued for this substance. Entry characterization for tamoxifen in Canada consisted of searching for information on sources and uses of the substance in relevant databases to identify potential for exposure of the general population from all sources, including pharmaceutical use (Canada [1978]; HSDB 1983– ; Household Products Database 1993– ; LNHPD 2008;DPD 2010; EAFUS 2011; NHPID 2011). Based on notifications submitted under the Cosmetic Regulations to Health Canada, tamoxifen is not used in cosmetic products in Canada (2012 email from the Consumer Product Safety Directorate, Health Canada, to the Existing Substance Risk Assessment Bureau, Health Canada; unreferenced).
Tamoxifen is not an approved drug for use in livestock feed in Canada (personal communication, email from the Canadian Food Inspection Agency [CFIA] to Health Canada, dated October 29, 2010; unreferenced). It is not listed in the CFIA’s Compendium of Medicating Ingredient Brochures, which lists drugs approved for administration through feed for specific purposes, with detailed claims and use levels (CFIA 2007). Tamoxifen also does not have any licensed Drug Identification Numbers (DINs) for use in veterinary applications in Canada (DPD 2010). However, there is still the possibility that veterinarians may be prescribing the drug for uses that have not been approved (i.e., off-label uses). Outside of Canada, applications of tamoxifen in the food industry have been identified, including its use in promoting growth in poultry (Park et al. 2004) and as a potential tool in aquaculture to mass produce sterile or monosex fish populations (Singh 2013). Information available for this substance indicates that in Canada, its use is limited to pharmaceuticals and research. Searches for this substance were conducted up to March 2013, and no information was found regarding alternative uses or releases of this substance in Canada.
For the years 2011 and 2012, data on pharmaceutical sales in Canada were obtained from IMS Health (IMS 2013). According to this source, in 2011, 54.9 kg of tamoxifen was sold to hospitals and 250.9 kg was sold to pharmacies, for a total of 305.8 kg. In 2012, the total amount of tamoxifen sold in Canada was higher than in 2011, at 320.2 kg, where 58.8 kg originated from hospital sales, and 261.4 kg from pharmacies (IMS 2013). Similar data were available to estimate that 250 kg of the substance was purchased by hospitals and pharmacies for prescription across Canada for the year 2007 (McLaughlin and Belknap 2008). There are several pharmaceutical companies that are licensed to market tamoxifen in Canada, including two innovator companies and five generic companies (DPD 2010). Pharmaceutical-grade tamoxifen (tamoxifen citrate) is sold in either 10 mg or 20 mg tablets (DPD 2010). The annual quantities of chemical-grade tamoxifen imported, manufactured or sold in Canada remain undetermined. Chemical-grade free base tamoxifen and tamoxifen citrate salt can be purchased from major chemical manufacturers; the typical format is 1 g or 5 g vials, as well as radiolabelled tamoxifen in 1 mL vials (Sigma-Aldrich c2010). For research applications (i.e., animal testing), tamoxifen is used in solution in concentrations ranging between micrograms and milligrams per kilogram body weight or in parts per million (ppm, equivalent to mg/L). Therefore, the total quantity stemming from laboratory research use in Canada is not expected to exceed, and is likely to be significantly less than that used for human therapeutic purposes.
Although there is the possibility that drug products containing the substance may be imported into Canada, no information is available on the quantity of such imports.
5. Releases to the Environment
The production and use of tamoxifen as a pharmaceutical may result in its release to the environment through various waste streams. In general, wastewater is a common point of entry of a substance into water through wastewater systemFootnote[3]effluent . The potential for exposure to tamoxifen from indirect sources (i.e., down-the-drain releases from patients using the substance for cancer therapy) and direct sources (i.e., released during manufacture or formulation) was considered in this assessment. Given these potential releases, the main compartment of concern for exposure to this substance is water. Pharmaceutical substances used in human and veterinary medicine can enter the aquatic environment following manufacture, use or excretion following administration of the drug in the form of non-metabolized parent compounds and as metabolites (Ashton et al. 2004). An additional source of the pharmaceuticals in water is the incorrect disposal of unused drugs into household wastewater. No information was available regarding actual releases of this substance from manufacturing or formulation. There is also insufficient information available to estimate potential down-the-drain releases of tamoxifen from research facilities as a result of tamoxifen use in basic research. It is expected, based on concentrations typically used for research purposes, that this is not a significant source of tamoxifen in water.
6. Measured Environmental Concentrations
In Canada, data are available for concentrations of tamoxifen in samples collected from a variety of wastewater treatment plants representing typical Canadian treatment systems and geographic variations. Tamoxifen was detected in the range of 5.97 × 10−7–1.04 × 10−6 mg/L (0.597–1.04 ng/L) in influent samples, in the range of 1.30 × 10−6–1.73 × 10−6 mg/L (1.30–1.73 ng/L) in effluent samples, and in the range of 6.66 × 10−3–5.08 × 10−3 mg/kg (0.666–5.08 ng/g) in biosolids. Municipal landfill leachate discharged into the wastewater treatment system was also sampled, and tamoxifen was detected in the range of 1.3 × 10−3–4.54 × 10−3 mg/kg (1.33–4.54 ng/g) (Teslic and Smyth 2013). No data on Canadian environmental concentrations of the hydroxylated metabolites of tamoxifen were identified.
Given the limited scope of information regarding the presence of tamoxifen in Canadian waters, environmental concentrations of the substance were estimated from available information, including estimated substance quantities, release rates and size of receiving water bodies, for the purpose of ecological risk assessment (see “Ecological Exposure Assessment” section).
Concentrations of tamoxifen in surface waters and wastewater have been reported in several locations in Europe. In addition, in a recent study, the presence of the tamoxifen metabolites endoxifen and hydroxy-tamoxifen (it is not clear whether it is 4-HT, therefore the metabolite name as it appears in the publication is used) was also identified in wastewater samples in Europe (Ferrando-Climent et al. 2013).
Ferrando-Climent et al. (2013) collected wastewater samples from hospitals in the towns of Coimbra (Portugal), Valencia and Girona (both in Spain), as well as from municipal water treatment plants located in Girona and Toulouse (Spain). The sizes of the hospitals included in the study ranged from 400 to over 1400 beds. The population sizes of the towns featured in the study fell between 145 000 (Girona) and nearly 800 000 (Valencia) inhabitants.
Tamoxifen was detected in all hospital effluent samples collected, at concentrations ranging from 2.6 × 10−5 to 1.33 × 10−4 mg/L (26.3 to 133.4 ng/L). The substance was also detected in all wastewater treatment plant influent samples, at concentrations ranging from 3.0 × 10−5 to 5.83 × 10−5 mg/L (30 to 58.3 ng/L). The tamoxifen metabolites endoxifen and hydroxy-tamoxifen were also identified in some of the hospital effluent samples using the information-dependent acquisition tool (IDA), a method based on searching IDA-generated chromatographs for the theoretical molecular ions of the target metabolites. Characteristics of the metabolite retention time were used to confirm the IDA results in the absence of metabolite standards. The presence of hydroxy-tamoxifen and endoxifen was identified in two out of four hospital influent samples, even though tamoxifen was detected in all samples.
Tamoxifen has been detected in surface water samples collected in the UK from some estuaries (sampling sites were located at the lower reaches of the rivers Tyne, Tees, Mersey, Thames and Belfast Lough) at concentrations ranging from less than 4 × 10−6 to 7.1 × 10−5 mg/L (less than 4 to 71 ng/L) (Thomas and Hilton 2004). It was detected in 2 of 45 samples collected from sewage treatment plants (at Corby, Great Billing, East Hyde, Harpenden and Ryemeads, located in the southeastern UK) at concentrations of 2.0 × 10−5 to 4.0-5 mg/L (20 and 40 ng/L), but was not detected in samples of surface water collected upstream or downstream from the plants, at a detection limit of 1.0 × 10−5 mg/L (10 ng/L) (Ashton et al. 2004). Slightly higher, but comparable, environmental concentrations of tamoxifen in the wastewater effluent and surface waters of the lower river Tyne in the UK have been reported by Roberts and Thomas (2006). In this study, tamoxifen concentrations ranged from 2.7 × 10−5 to 2.12×0-4 mg/L (27 to 212 ng/L) in the surface waters [with a median concentration of 5.3 × 10−5 mg/L (53 ng/L)] and from 1.46×0-4 mg/L to 3.69×0-4 mg/L (146 to 369 ng/L) in the final effluent from the wastewater treatment works at Howdon. Zhou et al. (2009) took samples from three wastewater treatment plants located near the river Ouse in the UK. The concentrations of tamoxifen measured in the effluents from these plants ranged from 2.0 × 10−7 to 7.0 × 10−7 mg/L (0.2 to 0.7 ng/L), although concentrations were below the detection limit in samples of river water taken both upstream and downstream of the site.
In France, discharge from a conventional wastewater treatment plant located in Alés, in the Languedoc Roussillon region in the south, into the Gardon River has been studied (Coetsier et al. 2009). Tamoxifen concentrations in the surface waters in the Gardon River ranged from less than 5.8 × 10−6 to 2.5 × 10−5 mg/L (less than 5.8 to 25 ng/L), whereas measured concentrations in the effluent samples ranged from less than 5.8 × 10−6 to 1.02 × 10−4 mg/L (less than 5.8 to 102 ng/L). It is noted that tamoxifen consumption in France for the year 2006 was estimated to be 335 kg, based on quantities of medicine paid for by the French social health care system and distributed throughout the French population of 62.9 million inhabitants (Coetsier et al. 2009).
Tauxe-Wuersch et al. (2006) measured the concentrations of tamoxifen in 37 samples of hospital and urban wastewaters in Lausanne and Morges, Switzerland. Tamoxifen was detected in almost all samples of raw sewage between the limit of detection and limit of quantification of the method used [1.0 × 10−6 and 4.0 × 10−6 mg/L (1 and 4 ng/L)], but was not detected in any samples of treated effluent. It was reported that 156 kg of tamoxifen is sold in Switzerland annually.
In Spain, Bueno et al. (2010) sampled 10 different rivers across the country for tamoxifen. With a detection limit of 1.1 × 10−5 mg/L (11 ng/L), tamoxifen was not detected in any samples.
7. Environmental Fate
Level III fugacity modelling (EQC 2003) simulates the distribution of a substance in a hypothetical environment according to chemical partitioning, reactivity and inter-media transport processes. The mass fraction values shown in Table 3 for tamoxifen represent the net effect of these processes under conditions of continuous release when a non-equilibrium “steady state” has been achieved. Given that the EQC model results for tamoxifen metabolites were very similar, to avoid duplication, only a brief summary is provided for these substances. Model inputs to EQC (2003) are summarized in Environment Canada (2014) and noted with an asterisk in Tables 2a, 2b and 2c.
Based on the physical and chemical properties of tamoxifen (Table 2a), the results of Level III fugacity modelling, presented in Table 3, suggest that the neutral form of tamoxifen is expected to reside in water, soil and sediment, depending on the compartment of release. However, the relatively high pKa of 8.69 for the basic functional group indicates that 50% of tamoxifen will be found in its ionized form at pH 8.69 (ACD/pKaDB 2005). In water bodies at environmentally relevant pH values (pH 6–9), about 33% (pH 9) to 100% (pH 6) of tamoxifen will be in its neutral form, indicating that aquatic exposure to tamoxifen can be from both the neutral and ionized forms. The proportion of dissociated tamoxifen [0.2% (pH 6) to 67% (pH 9)] indicates that partitioning behaviour predicted using the log D is appropriate to the ionized form of the substance (ACD 2011). However, the level III EQC model cannot address the potential for tamoxifen to ionize in the aquatic environment as a salt or the likelihood that the salt form will be more soluble than the free acid form (i.e., non-salt form). Nor can the model address the potential for binding in soil from electrostatic interactions (cation exchange) or binding to clays which are negatively surface charged. Therefore, the model cannot fully simulate the fate distribution of tamoxifen in the environment.
| Substance released to: | Air | Water | Soil | Sediment |
|---|---|---|---|---|
| Air (100%) | 2 | 2 | 94 | 2 |
| Water (100%) | Negligible | 50 | Negligible | 50 |
| Soil (100%) | Negligible | Negligible | 100 | Negligible |
If released to air, a very small amount of the substance is expected to reside in air (Table 3). Based on its negligible modelled vapour pressure of 4.62 × 10−6 Pa and Henry’s Law constantof 4.55 × 10−5Pa·m3/mol, tamoxifenis non-volatile. Therefore, if released solely to air, it will tend to be deposited to soil from wet and dry deposition (approximately 94%; see Table 3).
If released into water, tamoxifen is expected to strongly adsorb to suspended solids and sediment, based on the very high estimated log Koc value of 6.42 for the neutral form. Volatilization from water surfaces is expected to be an unimportant fate process based upon this compound’s estimated Henry’s Law constant. Thus, if water is a receiving medium, tamoxifen is expected to reside in water and sediment in approximately equal proportions (see Table 3).
If released to soil, tamoxifen is expected to have high adsorptivity to soil (i.e., expected to be immobile) based on its estimated log Koc for the neutral form. Volatilization from moist soil surfaces seems to be an unimportant fate process based on its estimated Henry’s Law constant. This chemical is not expected to volatilize from dry soil surfaces based on its vapour pressure. Therefore, if released to soil, tamoxifen will reside mainly in this environmental compartment, with less than 1% advected to water and sediments and negligible amounts partitioning to air, as illustrated by the results of the Level III fugacity modelling (see Table 3).
EQC model results for the hydroxylated metabolites of tamoxifen generated using their respective modelled physical and chemical properties indicated that the metabolites 4-HT and endoxifen would also likely be distributed in a manner similar to the parent compound, tamoxifen.
7.1 Environmental Persistence
In order to provide the best possible weight of evidence for determination of the persistence of tamoxifen, both empirical and modelled data were considered. Model estimates of the persistence of tamoxifen are strictly structure based and not expected to be influenced by chemical speciation. Chemical speciation, however, may affect bioavailability for biodegradation. This is not accounted for in the model estimates of biodegradation.
7.1.1 Empirical Data
Although pharmaceuticals can be degraded by biotic or abiotic processes, they may act as quasi-persistent compounds (also called pseudo-persistent) simply because of their continual release into surface waters via wastewater treatment plant (WWTP) effluents, which may result in multi-generational exposure for the resident organisms (Daughton and Ternes 1999; Ferrari et al. 2003). Therefore, the aquatic compartment is of key importance for evaluating the environmental persistence of pharmaceutical substances such as tamoxifen. Knowledge of physical, biological and chemical processes, such as adsorption, degradation, photolysis and hydrolysis, as well as the presence of possible transformation products in the environment (including metabolites), is needed to understand the fate, effects and risk associated with the presence of tamoxifen in the aquatic environment.
To date, few studies have addressed the degradation potential of tamoxifen in water. Degradation processes studied in water include photolysis (DellaGreca et al. 2007) and radiolytic oxidation (Leguéné et al. 2001). In addition, studies of the degradation of tamoxifen by microbes have also been performed (El-Sharkawy and Abul-Hajj 1987; El-Sharkawy 1991).
Tamoxifen is photosensitive. It exhibits a strong ultraviolet (UV) absorption band at a wavelength of 277 nm and a tail at wavelengths greater than 310 nm (DellaGreca et al. 2007). Moreover, product monographs indicate that pharmaceutical products containing tamoxifen should be protected from light during storage (DPD 2010). Irradiation of tamoxifen in water by sunlight, a solar simulator and a UV lamp under close to natural conditions was studied by DellaGreca et al. (2007). The effects of pH as well as of natural photosensitizers, including humic acid and nitrate, on the rate of degradation of tamoxifen were also investigated using irradiation by a solar stimulator (DellaGreca et al. 2007). Methylene blue–sensitized photo-oxygenation of tamoxifen was also carried out (Foote et al. 1995). In all experimental scenarios, the main products derived from photolysis of tamoxifen in water were identified following evaporation of water from the test samples and analysis of the residue composition by thin-layer chromatography and nuclear magnetic resonance spectroscopy.
Experiments showed that tamoxifen was recovered unchanged by keeping it in the dark in an aqueous solution for 30 days. Irradiation of tamoxifen by a solar simulator for 80 hours produced the following stable degradation products: trace amounts of cis-isomer of tamoxifen and two phenanthrenes at a yield of about 2% and 90% of the unchanged parent compound tamoxifen. Similar 80-hour experiments were carried out in the presence of nitrate and humic acid, at pH 4 and 9. Tamoxifen remained unchanged in the presence of nitrate and humic acid at pH 9. At pH 4, 70% of tamoxifen remained unchanged, cis-isomer was identified at about 4% and a mixture of two phenanthrenes was identified at about 8%. The increasing degradation rate at acidic pH was likely due to the greater solubility of the protonated drug in water compared with the neutral form. Dispersions of tamoxifen were exposed to solar light for 1 month at pH 4. Photoproducts of tamoxifen were identified as 50% of the parent compound tamoxifen, 10% of a complex polar fraction that contained benzoic acid, 4% cis-isomer of tamoxifen and trace amounts of two phenanthrenes and a ketone. Tamoxifen solutions at pH 2 saturated by either argon or oxygen were irradiated by a UV lamp for 7 hours. The residues obtained from irradiation under an oxygen atmosphere were 23% parent compound tamoxifen, two phenanthrenes at 23%, 9% ketone and cis-isomer of tamoxifen at 2%. Following irradiation under an argon atmosphere, photoproducts were 47% parent compound tamoxifen, 36% cis-isomer and trace amounts of two phenanthrenes (DellaGreca et al. 2007).
Solar simulator irradiation of tamoxifen aqueous dispersions carried out for 57 hours in the presence of methylene blue under an oxygen atmosphere produced 85% of the parent compound tamoxifen, 6% of a ketone and minor unidentified products. Methylene blue is known to be an efficient sensitizer for singlet oxygen, which adds to the C=C double bond, leading to dioxetanes, and, in the presence of allylic hydrogens, gives an ene-type reaction, leading to allylic hydroperoxides (Foote et al. 1995). The authors noted that this reaction proceeded very slowly (Foote et al. 1995).
Overall, the degradation products of tamoxifen formed by photo-induced reactions included the cis-isomer, phenanthrenes and ketones. Isomerization, cyclization and, to some degree, photo-oxygenation were the main photo-induced reactions of tamoxifen. However, in general, these reactions proceeded very slowly when experimental settings closely mimicked natural conditions. At most, approximately 50% of tamoxifen was converted to photoproducts over the exposure period of up to 1 month. It should be noted that if water turbidity and water depth are considered, then photodegradation would be limited.
In another study, the antioxidant properties of tamoxifen were investigated in vitro by Leguéné et al. (2001). Specifically, the ability of tamoxifen to scavenge OH and HO2 free radicals that are produced by water radiolysis was investigated. Aqueous solutions of tamoxifen were gamma-irradiated in aerated acidic conditions. Tamoxifen reacted quantitatively with the OH free radicals, but not with the HO2 free radicals, under the experimental conditions (Leguéné et al. 2001). In addition, tamoxifen metabolites resulting from both fungal and microbial transformation were identified (El-Sharkawy and Abul-Hajj 1987; El-Sharkawy 1991). It was noted that some microbes display the full range of drug metabolism observed in mammals (El-Sharkawy and Abul-Hajj 1987). It was found that tamoxifen was generally resistant to microbial and fungal metabolism, and only a few species exhibited the ability to metabolize this substance. In the El-Sharkawy (1991) study, 48 microbial species representing 20 genera were screened. Tamoxifen was metabolized to desmethyltamoxifen and tamoxifen-N-oxide by eight species (Cunninghamella blakesleeana, C. bainieri, C. echinulata, Caenorhabditis elegans, Mucor ramannianus, Beauveria bassiana, Curvularia lunata, Rhizopus stolonifer), while only one species (Streptomyces rimosus) was able to biotransform tamoxifen to 4-HT. In the El-Sharkawy and Abul-Hajj (1987) study, 96 fungal species were screened, and only 1 species (Gliocladium roseum) was able to metabolize tamoxifen. The resulting biotransformation products obtained were N-oxide and N-desmethyl metabolites. The results of microbial and fungal transformation studies (El-Sharkawy and Abul-Hajj 1987; El-Sharkawy 1991) indicated that tamoxifen was very resistant to metabolic transformation by a multitude of fungi and microbes.
7.1.2 Modelling Results
Since few experimental data on the degradation of tamoxifen are available, a QSAR-based weight of evidence approach (Environment Canada 2007) was also applied using the degradation models shown in Table 4a. Given the ecological importance of the water compartment, the fact that most of the available models apply to water and the fact that tamoxifen is expected to be released exclusively to this compartment, biodegradation in water was primarily examined. Tamoxifen does contain functional groups expected to undergo hydrolysis. Table 4a summarizes the results of available QSAR models for degradation in various environmental media.
A QSAR-based weight of evidence approach was also applied to the tamoxifen metabolites 4-HT and endoxifen. Results using the available degradation models are shown in Tables 4b and 4c for 4-HT and endoxifen, respectively.
Tamoxifen and its hydroxylated metabolites 4-HT and endoxifen exhibit short predicted atmospheric oxidation half-lives of 0.04 day and ozone reaction half-lives of 0.001 day. Therefore, with a half-life of less than 2 days via reactions with hydroxyl radicals, photolysis and potentially with ozone, tamoxifen and its hydroxylated metabolites 4-HT are not considered persistent in air.
In water, hydrolysis half-lives could not be predicted for tamoxifen, 4-HT or endoxifen, as the model HYDROWIN (2008) does not estimate hydrolysis rate constants for these types of structures.
Ultimate biodegradation model results for tamoxifen collectively suggest a very slow biodegradation rate in water. In contrast, the primary biodegradation (BIOWIN Sub-model 4) result of 3.1 for tamoxifen falls just above the recommended conservative threshold of 3.0, adapted for indication of a faster rate of biodegradation (Aronson et al. 2006). However, based on the overall modelled evidence and the weight of inference given to results obtained from the ultimate biodegradation models (BIOWIN Sub-models 3, 5 and 6; TOPKAT and CATABOL), it is considered that the model evidence for biodegradation of tamoxifen indicates slow biodegradation rates in water. Based on the ultimate biodegradation model results, the half-life of tamoxifen is expected to be greater than or equal to 182 days in this compartment (see Table 4a).
Similarly, for 4-HT and endoxifen, ultimate biodegradation model results also suggest that these substances biodegrade slowly in water, but the primary biodegradation results (from BIOWIN Sub-model 4) indicate a faster rate of biodegradation. Considering the consistency of biodegradation results from ultimate models suggesting a slow rate of biodegradation (especially TOPKAT, CATABOL and BIOWIN Sub-models 5 and 6) and the weight of evidence obtained from these results, it is considered that 4-HT and endoxifen biodegrade slowly in water. As a result, the predicted half-lives of 4-HT and endoxifen in water are expected to be greater than or equal to 182 days (see Tables 4b and c).
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation | AOPWIN 2008Footnote Table 4a[a] | t½ = 0.04 day | less than 2 |
| Ozone reaction | AOPWIN 2008[a] | t½ = 0.001 day | less than 2 |
| Hydrolysis | HYDROWIN 2008[a] | n/aFootnote Table 4a[b] | n/a |
| Primary biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 4: Expert Survey (qualitative results) |
3.1Footnote Table 4a[c] “biodegrades fast” |
less than 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 3: Expert Survey (qualitative results) |
2.1[c] “biodegrades slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 5: MITI linear probability |
−0.018Footnote Table 4a[d] “biodegrades very slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 6: MITI non-linear probability |
0.009[d] “biodegrades very slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | TOPKAT 2004 Probability |
0[d] “biodegrades very slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | CATABOL c2004-2008 % BOD |
% BOD = 0.07 “biodegrades very slowly” |
greater than or equal to 182 |
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation | AOPWIN 2008Footnote Table 4b[a] | t½ = 0.04 day | less than 2 |
| Ozone reaction | AOPWIN 2008[a] | t½ = 0.001 day | less than 2 |
| Hydrolysis | HYDROWIN 2008[a] | n/aFootnote Table 4b[b] | n/a |
| Primary biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 4: Expert Survey (qualitative results) |
3.1Footnote Table 4b[c] “biodegrades fast” |
less than 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 3: Expert Survey (qualitative results) |
2.1[c] “biodegrades slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 5: MITI linear probability |
−0.0098Footnote Table 4b[d] “biodegrades very slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 6: MITI non-linear probability |
0.008[d] “biodegrades very slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | TOPKAT 2004 Probability |
0[d] “biodegrades very slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | CATABOL c2004-2008 % BOD |
% BOD = 0.07 “biodegrades very slowly” |
greater than or equal to 182 |
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation | AOPWIN 2008Footnote Table 4c[a] | t½ = 0.04 day | less than 2 |
| Ozone reaction | AOPWIN 2008[a] | t½ = 0.001 day | less than 2 |
| Hydrolysis | HYDROWIN 2008[a] | n/aFootnote Table 4c[b] | n/a |
| Primary biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 4: Expert Survey (qualitative results) |
3.5Footnote Table 4c[c] “biodegrades fast” |
less than 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 3: Expert Survey (qualitative results) |
2.4[c] “may biodegrade fast” |
less than 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 5: MITI linear probability |
0.15Footnote Table 4c[d] “biodegrades slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 6: MITI non-linear probability |
0.021[d] “biodegrades very slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | TOPKAT 2004 Probability |
0[d] “biodegrades very slowly |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | CATABOL c2004-2008 % BOD |
% BOD = 0.08 “biodegrades very slowly” |
greater than or equal to 182 |
Finally, using an extrapolation ratio of 1:1:4 for water:soil:sediment biodegradation half-lives (Boethling et al. 1995), the half-life in soil is also greater than or equal to 182 days, and the half-life in sediments is greater than or equal to 365 days. This indicates that tamoxifen as well as 4-HT and endoxifen are expected to be persistent in soil and sediment.
Based on the empirical data, and in particular evidence for the slow photodegradation of tamoxifen in aqueous systems described by DellaGreca et al. (2007) as well as the modelled data for both tamoxifen and its hydroxylated metabolites, 4-HT and endoxifen, it is concluded that tamoxifen, 4-HT and endoxifen is persistent in water, soil and sediment (half-lives in soil and water greater than or equal to 182 days and half-life in sediment greater than or equal to 365 days), but not in air (half-life in air less than or equal to 2 days).
7.2 Potential for Bioaccumulation
The high modelled log Kow of 6.3 for tamoxifen suggests that the neutral form of tamoxifen may have potential to bioaccumulate in biota (see Table 2a). The experimental log D value of 4.51 for the ionized fraction of tamoxifen suggests a lower potential for bioaccumulation in biota. However, the partition coefficient in isolation is not considered sufficient evidence to determine bioaccumulation potential, as it cannot account for physiological parameters such as metabolism.
7.2.1 Metabolism
Tamoxifen is extensively metabolized by the cytochrome p450 enzyme system (Kisanga et al. 2005), a highly conserved system of enzymes among vertebrate and invertebrate organisms that catalyzes oxidation of organic substances. Small differences in p450 enzymes exist among species, and result in differences in drug metabolism, including substrate specificity and catalytic activity (Martignoni 2006). For example, uptake rates of tamoxifen from oral dosing were observed to be higher in fish than in rats, and 4-HT was noted to be the dominant metabolite of tamoxifen in fish, whereas in rats, the metabolite N-desmethyltamoxifen was prevalent (Mills et al. [date unknown]).
7.2.2 Estimating bioconcentration factor (BCF) and bioconcentration factor (BAF)
Since no experimental bioaccumulation factor (BAF) or bioconcentration factor (BCF) data for tamoxifen were available, a predictive approach was applied using available BAF and BCF models, as shown in Tables 5a, 5b and 5c.
Kinetic mass balance modelling is in principle considered to be the most reliable prediction method for determining bioaccumulation potential because it allows for metabolism correction as long as the log Kow of the substance is within the log Kow domain of the model. For this reason, BAF are the preferred metric for assessing the bioaccumulation potential of substances.
Given that tamoxifen has the potential to ionize, both the log D (for the ionized form) and the log Kow (for the neutral form) values were used as model inputs into the BCFBAF (2008) model. BCF and BAF estimates, corrected for potential biotransformation, were generated using the BCFBAF (2008) model (in EPI Suite 2008) and CPOPs (2008). Metabolic rate constants (kM) were derived using structure–activity relationships described further in Arnot et al. (2008a; 2008b; 2009). Modelled BCF and BAF values corrected for metabolism are presented in Table 5a for tamoxifen. Modelled BCF and BAF values corrected for metabolism are presented in Tables 5b and 5c for the tamoxifen metabolites 4-HT and endoxifen, respectively.
| Test organism | Model and model basis | Endpoint | Value (L/kg wet weight) | kM (/day) | Reference |
|---|---|---|---|---|---|
| Fish | BCFBAF Sub-model 2 (mass balance) Log D = 4.51 |
BCFFootnote Table 5a [a] | 538 | 0.16 | BCFBAF 2008 |
| Fish | BCFBAF Sub-model 3 (Gobas mass balance) Log D = 4.51 |
BAF[a} | 552 | 0.16 | BCFBAF 2008 |
| Fish | BCFmax with mitigating factors Log D = 4.51 |
BCFFootnote Table 5a[b] | 61 | 0.03 | CPOPs 2008 |
| Fish | BCFBAF Sub-model 2 (mass balance) Log Kow = 6.3 |
BCF[a] | 1 695 | 0.16 | BCFBAF 2008 |
| Fish | BCFBAF Sub-model 3 (Gobas mass balance) Log Kow = 6.3 |
BAF[a] | 10 640 | 0.16 | BCFBAF 2008 |
| Fish | BCFmax with mitigating factors Log Kow = 6.3 |
BCF[b] | 587 | 0.03 | CPOPs 2008 |
| Test organism | Model and model basis | Endpoint | Value (L/kg wet weight) | kM (/day) | Reference |
|---|---|---|---|---|---|
| Fish | BCFBAF Sub-model 2: mass balance |
BCF | 299 | 1.01 | BCFBAF 2008 |
| Fish | BCFBAF Sub-model 3: Gobas mass balance |
BAF | 302 | 1.01 | BCFBAF 2008 |
| Test organism | Model and model basis | Endpoint | Value (L/kg wet weight) | kM (/day) | Reference |
|---|---|---|---|---|---|
| Fish | BCFBAF Sub-model 2: mass balance |
BCF | 328 | 0.15 | BCFBAF 2008 |
| Fish | BCFBAF Sub-model 3: Gobas mass balance |
BAF | 333 | 0.15 | BCFBAF 2008 |
The modelled results indicate that the ionized form of tamoxifen is not expected to have high bioaccumulation potential. The predicted BCF and BAF values obtained from the BCFBAF (2008) and the CPOPs (2008) models and corrected for metabolism were less than 5000. For the neutral form of tamoxifen, the predicted BCF values indicate a low potential for bioconcentration, and the predicted BAF value from the BCFBAF (2008) model indicates a high potential for bioaccumulation. There is, however, high uncertainty in the predicted values for kM for both the neutral and ionized forms, given the lack of chemical structures similar to pharmaceutical substances such as tamoxifen in the training sets of models such as BCFBAF (2008).
The modelled bioaccumulation results for 4-HT and endoxifen also indicate that these substances do not have a high potential to bioaccumulate in fish. The predicted BCF and BAF values, corrected for metabolism, were slightly lower than the BCF and BAF values predicted for tamoxifen by the same models (i.e., BCFBAF 2008 Sub-models 2 and 3) and well below 5000.
Information regarding molecular size and cross-sectional diameters is also useful to consider as weight of evidence for bioaccumulation potential. Analysis relating fish BCF data to molecular size parameters (Dimitrov et al. 2002, 2005; Sakuratani et al. 2008) suggests that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing Dmax. The probability of passive diffusion decreases appreciably when the maximum diameter is greater than approximately 1.5 nm and much more so for molecules having a maximum diameter of greater than 1.7 nm. It was observed that substances that do not have a very high bioconcentration potential often have a Dmax of greater than 2.0 nm and an effective diameter (Deff) greater than 1.1 nm. Based on three-dimensional analysis of conformers, calculated using the BCFmax Model with Mitigating Factors (Dimitrov et al. 2005), the maximum diameters (Dmax) for the ionized form of tamoxifen range from 1.51 to 1.91 nm, and the Dmax values for the neutral form of tamoxifen range from 1.62 to 1.91 nm. This suggests that both forms of tamoxifen may experience restricted uptake from steric effects at the gill surface.
The available weight of evidence indicates that tamoxifen as well as its metabolites 4-HT and endoxifen are not expected to significantly bioaccumulate in biota. Modelled results suggest that these substances, especially in ionized form, are characterized by low BCF and BAF values. In addition, these substances have low water solubilities, which may limit their availability for uptake from water. It was determined that tamoxifen has a relatively large cross-sectional diameter, and this can further restrict its uptake across the gills as a result of steric hindrance. It is also expected that tamoxifen has a high potential to be metabolized by fish. Therefore, based on the available data, it is concluded that tamoxifen and its hydroxylated metabolites 4-HT and endoxifen have low bioaccumulation potential.
8. Potential to Cause Ecological Harm
8.1 Ecological Effects Assessment
Tamoxifen as well as its hydroxylated metabolites are not expected to be released to any degree into environmental compartments other than water. However, based on their modelled physical and chemical properties (see Tables 2a, 2b and 2c) and the results of fugacity modelling (see Table 3), tamoxifen, 4-HT and endoxifen may partition to sediments. No ecological effects studies in sediment were found for these compounds.
In order to provide the best possible weight of evidence for assessing the ecological effects of tamoxifen and its metabolites, empirical data were considered. Since waterborne tamoxifen mainly originates from tamoxifen citrate pharmaceutical formulations as they are ingested and excreted into wastewater by humans, it was relevant to evaluate the effects of tamoxifen citrate in fish. It is expected, based on the toxicity profile of citric acid (OECD 2001), that the citrate moiety associated with tamoxifen would have negligible ecotoxicological effects on aquatic organisms.
It has been indicated that 15.2 mg of tamoxifen citrate in tablets is equivalent to 10 mg of tamoxifen (Drug Infonet c1996–2005). Therefore, to illustrate exposure concentrations and effects from the tamoxifen active ingredient, a factor of 0.66 is applied to exposure concentrations and ecological endpoints obtained when tamoxifen citrate was used [i.e., endpoints from the unpublished AstraZeneca studies reported in Williams et al. 2007; Knacker et al. 2010].
8.1.1 Aquatic Toxicity of Tamoxifen
Studies evaluating the toxicity of tamoxifen to aquatic invertebrates and vertebrates, including crustaceans, rotifer species, fish and frogs, as well as in vitro cytotoxic assays in fish cell lines are presented in Table 6a and discussed below.
Since tamoxifen is not readily soluble in water (solubility is less than 0.5 mg/L; CEREP c2010b), carrier solvents such as acetone, triethylene glycol (trigol) or dimethyl sulfoxide (DMSO) are typically used to make experimental stock solutions. Appropriate solvent controls were incorporated into study designs.
Tamoxifen is an endocrine-active substance; hence effects observed in pelagic organisms are endocrine disrupting in nature, particularly in studies with vertebrates where the underlying estrogenic mode of action of tamoxifen becomes apparent. Of note are the shifts in sex ratios observed in tamoxifen-exposed fish populations (e.g., Van der Ven et al. 2007; Knacker et al. 2010; Liu et al. 2010; Singh 2013), and alteration in gonadotropin expression levels in female frogs (Urbatzka et al. 2006, 2007).
The toxicity of tamoxifen and its photochemical derivatives to aquatic invertebrates was investigated by DellaGreca et al. (2007). Products of tamoxifen derived in water from exposure to sunlight were identified, and their chronic and acute toxicities were evaluated using a rotifer species, Brachionus calyciflorus, and three crustaceans, Thamnocephalus platyurus, Daphnia magna and Ceriodaphnia dubia. Irradiation of tamoxifen by a solar simulator and sunlight produced five stable products: a cis-isomer (Product 1), two phenanthrenes (Products 2 and 3), a ketone (Product 4) and benzoic acid.
Acute 24-hour bioassays were performed on B. calyciflorus and T. platyurus to evaluate mortality (i.e., LC50) and on D. magna to evaluate immobilization (i.e., EC50) from exposure to tamoxifen and its photochemical derivatives. As a result of exposure to tamoxifen, the acute LC50 values for B. calyciflorus and T. platyurus were 0.97 and 0.40 mg/L, respectively, and the acute EC50 for D. magna was 1.53 mg/L. Exposure to photodegradates of tamoxifen (i.e., Products 1–4) resulted in LC50 values in the range of 0.95–1.31 mg/L for B. calyciflorus and 0.47–1.59 mg/L for T. platyurus and an EC50in the range of 1.74–3.27 mg/L for Products 1–3 in D. magna, with no effects noted for Product 4 at exposure concentrations up to 5 mg/L.
Chronic tests to establish EC50 values for tamoxifen and its photodegradates were also carried out in B. calyciflorus and C. dubia for 48 hours and 7 days, respectively. EC50 values resulting from exposure to tamoxifen were 0.25 mg/L for B. calyciflorus and 8.1 × 10−4 mg/L for C. dubia. Moreover, EC50 values established from exposure to products 1–4 were in the range of 0.123–0.26 mg/L for B. calyciflorusand in the range of 4.1 × 10−4 – 9.6 × 10−3 mg/L for C. dubia, indicating an equivalent or higher toxicity of photodegradation products compared with that of the parent compound. Photodegradation products of tamoxifen investigated in the chronic tests revealed greatest effects on C. dubia, with a toxic potential 3 orders of magnitude higher than that established in acute tests for the related species D. magna. C. dubia was also the most sensitive to undegraded tamoxifen in chronic exposure tests. Overall, the findings by DellaGreca et al. (2007) indicated that exposure to tamoxifen and its photodegradates in water posed a hazard to aquatic invertebrates, particularly in longer-term or chronic exposure scenarios.
The toxicity of tamoxifen was also investigated in a marine crustacean species, Acartia tonsa,during early developmental stages of the larvae known as napular stages (Andersen et al. 200; Hilton et al. 2003). A. tonsa larvae with juvenile morphology are named nauplii, whereas larvae possessing the adult morphology are referred to as copepodites. These differences in morphology make it feasible to test effects of chemicals on growth and development, with the test endpoint being the fraction of juveniles that develop into copepodites in a given time frame. The effect of tamoxifen was assessed in a semistatic test, covering the period of development from egg until approximately 50% of larvae in the control had reached the copepodite stage. It was observed that tamoxifen inhibited development of A. tonsa at low exposure concentrations. Reported EC50 and EC10 values following a chronic 5-day test were 0.049 and 0.0087 mg/L, respectively.
Reproductive effects of tamoxifen citrate in the fathead minnow (Pimephales promelas) in partial and full cycle studies were undertaken by AstraZeneca PLC to define potential adverse effects of chronic waterborne tamoxifen exposure using established regulatory endpoints, and to generate biomarker data to improve current understanding of tamoxifen influence on the endocrine homeostasis in fish (Williams et al. 2007). In the partial life cycle study, adverse effect endpoints were examined in fathead minnows during the F0 (first breeding pair) reproduction phase and F1 (first generation from first breeding pair) embryo-larval phase following exposure to tamoxifen at mean measured concentrations ranging between 7.3 × 10−5 and 0.012 mg/L (1.1 × 10−4 – 0.018 mg/L tamoxifen citrate) over 42 days (Williams et al. 2007). Endpoints studied were F0 fecundity, fish weight and length, F0 vitellogenin levels and, for the first generation, F1 hatching success at 4 days, larval length and weight at 28 days and vitellogenin levels at 42 days. In the full life cycle study, similar adverse effect endpoints were examined for F0 and F1 fish generations following exposure to tamoxifen at mean measured concentrations ranging from 5 × 10−6 to 2.7 × 10−3 mg/L (7 × 10−6 – 4.1 × 10−3mg/L tamoxifen citrate). For F0, measurements were taken for survival during the spawning phase, fish lengths and weights at 112 and 211 days post-hatch (dph), vitellogenin levels at 211 dph and, for F1, lengths and weights at 28 and 112 dph and vitellogenin levels at 112 dph in both males and females.
Data from partial and full life cycle studies were analyzed statistically to identify significant differences between control and treatment groups. The full life cycle study showed no statistically significant reduction in F0 and F1 hatching success after exposure to tamoxifen at concentrations up to the maximum mean measured concentration of 2.7 × 10−3 mg/L (4.1 × 10−3 mg/L tamoxifen citrate). Moreover, F0 fecundity was not reduced significantly by exposure concentrations up to the maximum mean measured concentration of 2.7 × 10−3 mg/L (4.1 × 10−3 mg/L tamoxifen citrate). However, in the partial life cycle study, a 42-day exposure to tamoxifen at 0.012 mg/L (0.018 mg/L tamoxifen citrate) caused a 70% reduction in spawning (p less than 0.01). Survival was unaffected by tamoxifen at exposure concentrations up to the maximum mean measured concentration of 2.7 × 10−3 mg/L (4.1 × 10−3 mg/L tamoxifen citrate) over 211 days in the full life cycle study and at exposure concentrations of less than or equal to 0.012 mg/L after 42 days in the partial life cycle study. The no-observed-effect concentration (NOEC) and the lowest-observed-effect concentration (LOEC) were calculated based on the chronic data from the partial and full life cycle studies based on adverse effect endpoints such as altered development, growth and reproduction, excluding the 28 dph larval growth data. The NOEC and LOEC values for the fathead minnow established in Williams et al. (2007) were 0.0034 mg/L (0.0051 mg/L tamoxifen citrate) and 0.0037 mg/L (0.0056 mg/L tamoxifen citrate), respectively. Acute (96-hour) LC50 values for other fish species previously determined in unpublished studies by AstraZeneca were also mentioned in Williams et al. (2007). The LC50values were 0.15 mg/L (0.23 mg/L tamoxifen citrate) for the bluegill sunfish and 0.27 mg/L (0.41 mg/L tamoxifen citrate) for rainbow trout (Williams et al. 2007).
Williams et al. (2007) expressed the biomarker responses (i.e., vitellogenin levels) as biomarkerNOEC and biomarkerLOEC. However, the authors advised that biomarker responses alone should not be used for calculating PNECs. Measured vitellogenin levels appeared to be gender and life stage specific. Exposure to tamoxifen citrate had no effect on plasma vitellogenin levels in adult fish in a 42-day study. However, there was a 50% reduction (p less than 0.01) in whole-body vitellogenin levels in F1 fish larvae at exposure concentrations of less than or equal to 0.012 mg/L ( less than or equal to 0.018 mg/L tamoxifen citrate) after 42 days, and in the full life cycle study, there was a significant increase in plasma vitellogenin levels in F0 males at 211 dph and also in F1 females at 112 dph at an exposure concentration of 0.0034 mg/L (0.0051 mg/L tamoxifen citrate). Discrete values for biomarkerNOEC and biomarkerLOEC were not provided.
Toxicological effects of tamoxifen were also studied long term, life cycle studies using zebrafish (Danio rerio) (Knacker et al. 2010; Van der Ven et al. 2007).
Van der Ven et al. (2007) studied the effects of tamoxifen in a partial life cycle assay with zebrafish (Danio rerio), where parental zebrafish (P) and their progeny (F1) were exposed to the substance during reproduction, sexual differentiation and development (Van der Ven et al. 2007). Reproductive parameters (fertility, fecundity), mortality and growth, as well as vitellogenin expression and histology, were evaluated. Knacker et al. 2011 evaluated effects of tamoxifen citrate in a two-generation study, encompassing the P generation, the filial F1 generation (early life stages, juvenile growth and reproduction), and the early life stage phase of the F2 generation. For P and F1 generations, vitellogenin and sex steroid 11-keto testosterone were also measured.
In Van der Ven et al. (2007), general toxicity was observed at higher, range-finding exposure concentrations of 0.01–10 mg/L (27–27 000 nM) over a 10-day period. At an exposure concentration of 1 mg/L (2 700 nM), mortality was observed in fish larvae, whereas juvenile fish showed increased mortality at 0.1 mg/L, and no progeny were present at an exposure concentration of 0.3 mg/L. At exposure concentrations of 1 mg/L and higher, hemorrhage, disturbed locomotion and disturbed respiration in both juvenile and adult fish were observed. At lower tamoxifen exposure concentrations of 0.003, 0.01, 0.03, 0.1 and 0.3 mg/L (8.6, 27, 86, 270 and 860 nM), female zebrafish in all tamoxifen exposure groups had oviducts filled with degenerated eggs.
In Knacker et al. (2010), observations in the P generation included a reduced rate of egg fertilization rate at the highest tested concentration of 0.009 mg/L (0.014 mg/L tamoxifen citrate), significantly decreased vitellogenin levels in both male and female fish at the two highest tamoxifen concentration levels of 0.0026 mg/L and 0.009 mg/L (0.004 mg/L and 0.014 mg/L tamoxifen citrate, respectively), and a reduced concentration of of 11-keto testosterone in male fish at exposure concentration of 0.009 mg/L (0.014 mg/L tamoxifen citrate). For F1 generation, both the hatching rate and growth were significantly reduced at tamoxifen concentration of 0.007 mg/L (0.011 mg/L tamoxifen citrate). There were no effects on the hatching success and post-hatch survival in the F2 generation, but a slight increase in length was observed at the highest concentration tested of 0.0015 mg/L (0.0023 mg/L tamoxifen citrate).
Significant alternations in the male to female population sex ratio in the F1 generation were observed by both Van der Ven et al. (2007) and Knacker et al. (2010). In Van der Ven et al. (2007), an exposure concentration of 0.03 mg/L significantly increased proportion of males to females, and also caused a higher proportion of individuals with undifferentiated gonads. Knakcer et al (2010) observed changes in the population sex ratio at a much lower tamoxifen exposure concentration of 0.00051 mg/L (0.00077mg/L). A complete sex-reversal where no females were found was observed at the highest concentration tested of 0.007 mg/L (0.011 mg/L tamoxifen citrate).
Tamoxifen was also shown to affect the population sex ratio in other fish species (Singh 2013; Liu et al. 2010). In a study using carp (Cyprinus carpio), exposure to tamoxifen through diet significantly affected sex differentiation and gonadal maturity (Singh 2013). Carp fingerlings were exposed to tamoxifen mixed in feed at concentrations of 100 and 200 mg/kg feed for 60 days and fed twice daily. Exposure to the higher tamoxifen concentration of 200 mg/kg feed brought about 82.5% masculinization of the fish population (Singh 2013). Elsewhere, it was also reported that treatment with tamoxifen is effective in inducing female to male sex reversal in the southern catfish (Silurus meridionalis) (Liu et al. 2010).
Other relevant information identified, comprising several ecotoxicological endpoints from unpublished studies by AstraZeneca and including LOEC, NOEC and LC50 values for algal species, D. magna and fish species, was summarized in the Swedish Drug Database (2011). These values, determined by exposing organisms to tamoxifen citrate, are presented in Table 6a. For the algae Selenastrum capricornutum and the blue-green algae Microcystis aeruginosa, LOEC and NOEC values for growth rate were determined in chronic tests. Tamoxifen was highly toxic to both species: for S. capricornutum, the LOEC and NOEC were 0.008 mg/L (0.012 mg/L tamoxifen citrate) and 0.003 mg/L (0.0049 mg/L tamoxifen citrate), respectively, and for M. aeruginosa, the LOEC and NOEC were 0.13 mg/L (0.2 mg/L tamoxifen citrate) and 0.065 mg/L (0.098 mg/L tamoxifen citrate), respectively. Tamoxifen was also highly toxic to D. magnain chronic tests addressing reproduction and growth rate; the LOEC for reproduction was determined to be 0.09 mg/L (0.14 mg/L tamoxifen citrate), whereas NOEC values for reproduction and length were 0.05 mg/L (0.078 mg/L tamoxifen citrate) and 0.03 mg/L (0.043 mg/L tamoxifen citrate), respectively. Lastly, in addition to the LC50 values for rainbow trout and bluegill sunfish reported by Williams et al. (2007), endpoints from another unpublished rainbow trout study were also summarized in the Swedish Drug Database (2011). For Salmo gairdneri, the LC50 and NOEC in an acute 96-hour study were determined to be 0.21 mg/L (0.32 mg/L tamoxifen citrate) and 0.18 mg/L (0.27 mg/L tamoxifen citrate), respectively.
Endocrine disrupting effects of tamoxifen were studied in adult frogs, Xenopus laevis, at an exposure concentration of 0.0037 mg/L (10−8 M) over a period of 4 weeks (Urbatzka et al. 2006, 2007). The chosen exposure concentration was regarded to be within the physiological range of circulating sex steroids in an adult X. laevis, but higher than environmental concentrations, measured in the range of 1 × 10−6– 2 × 10−5 mg/L (1–20 ng/L). In general, reproduction in vertebrates is under endocrine control of the hypothalamus–pituitary–gonad axis, which governs circulating sex steroids that exert effects in several peripheral target organs, including the liver (Urbatzka et al. 2006). Therefore, biomarker genes were selected for the detection of endocrine disrupting activity, including three transport proteins in the plasma: retinol binding protein (RBP), involved in the transport of the vitamin A precursor, retinol; transferrin (TF), an iron transporter; and transthyretin (TTR), involved in transporting thyroid hormones (Urbatzka et al. 2007). The messenger ribonucleic acid (mRNA) expression of the three transporter genes in the liver of male and female X. laevis was compared to that of vitellogenin at the mRNA and plasma protein level. mRNA expression of vitellogenin in the liver was decreased in female frogs, but plasma vitellogenin levels were not changed following exposure to tamoxifen. TF mRNA expression was increased in the female frogs; however, the RBP basal expression level as well as the TTR mRNA expression level were not altered following tamoxifen treatment. Hence, the TF and vitellogenin mRNA expression pattern in the liver indicated an anti-estrogenic response that may impact processes involved in protein homeostasis. In addition, measured plasma concentrations of testosterone were not changed in either male or female frogs, and plasma concentrations of 17β-estradiol were increased in the females following exposure to tamoxifen, reflecting modification in a negative feedback mechanism on the hypothalamus–pituitary–gonad axis.
As well, brain (including the pituitary gland) mRNA expression patterns of hypophyseal gonadotropins--follicle stimulating hormone (FSH) and luteinizing hormone (LH), key reproductive hormones involved in gonadal development--and gonadotropin-releasing hormone (GnRH) were investigated to determine potential disturbance of reproductive processes in response to exposure to tamoxifen (Urbatzka et al. 2006). Effects were noted in female frogs only, in which LH and FSH mRNA levels were increased by approximately 1.5- and 2.5-fold, respectively. Levels of GnRH mRNA remained unchanged in both male and female frogs. Overall, these results indicated that expression of gonadotropins in amphibians can be significantly altered in a gender-specific pattern by endocrine disrupting compounds, including tamoxifen.
Tamoxifen was also tested in vitro in several cytotoxic assays, such as the thiazolyl blue tetrazolium bromide (MTT), neutral red (NR), lactate dehydrogenase (LDH), alamarin-blue (AB) and 5-carboxyfluorescein diacetate acetoxymethyl ester (CFDA-AM) assays (see footnote below Table 6a for brief assay descriptions), in fish cell lines (Caminada et al. 2006; Bopp and Lettieri 2008). These quantitative colorimetric or fluorometric assays employ different cellular modes of action to indicate cytotoxicity of the test compound, such as disruption of membrane integrity and detection of enzyme or metabolic activity (Caminada et al. 2006; Bopp and Lettieri 2008). Cytotoxicity assays in fish cell lines can be a valuable tool in the risk assessment to estimate and rank the acute toxicity of compounds in order to minimize in vivoacute toxicity testing.
In the Caminada et al. (2006) study, it was noted that both the NR and MTT assays produced equal cytotoxicity results; that is, experimental EC50 values from both tests were in the same order of magnitude in the two fish cell lines tested, PLHC-1 (Poeciliopsis lucida hepatoma cell line) and RTG-2 (rainbow trout gonadal cell line). The reported EC50values resulting from exposure to tamoxifen using the MTT assay were 7.4 and 7.09 mg/L in the PLHC-1 and RTG-2 cell lines, respectively. The EC50 value from the NR assay using the RTG-2 cell line was 7.2 mg/L. In addition, there was a clear correlation (p less than 0.0001) between the EC50 values and the tamoxifen log D value (at pH 7), indicating that cytotoxicity is due to non-specific toxicity or narcosis. Log D considers the partitioning of a compound at a specific pH; therefore, it tends to reflect the situation occurring in the cytotoxicity assay, such that compounds that are more lipophilic generally tend to be more toxic.
Four cytotoxic assays were performed in a zebrafish (Danio rerio) liver cell line, ZFL (Bopp and Lettieri 2008). Two assays were colorimetric, the MTT assay and the LDH assay, and two assays were fluorometric, the AB assay and the CFDA-AM assay. There were no significant differences in the EC10 or EC50 values established from the four assays; however, the authors indicated that, overall, the fluorometric assays were more precise, more robust and consequently better suited for cytotoxicity assessment.
EC10 and EC50 values were established for tamoxifen and its metabolite 4-HT in the ZFL cell line (see Table 6b for 4-HT results). For tamoxifen, EC10 values ranged between 0.23 and 0.94 mg/L, whereas EC50 values ranged between 0.70 and 1.28 mg/L (see Table 6a). It should be noted that the MTT assay was performed in both the Caminada et al. (2006) and Bopp and Lettieri (2008) studies, and the EC50 values for tamoxifen were approximately 10 times less in the ZFL cell line than those established by Caminada et al. (2006) in the RTG-2 and PLHC-1 fish cell lines, indicating that there may be cell line–specific differences in sensitivity.
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Blue-green algae (Microcystis aeruginosa) | Chronic (21 days) | LOEC (growth rate) | 0.13 (0.2 mg/L tamoxifen citrate) |
Unpublished AstraZeneca study in Swedish Drug Database 2011Footnote Table 6a[a] |
| Blue-green algae (Microcystis aeruginosa) | Chronic (21 days) | NOEC (growth rate) | 0.065 (0.098 mg/L tamoxifen citrate) |
Unpublished AstraZeneca study in Swedish Drug Database 2011[a] |
| Green algae (Selenastrum capricornutum) |
Chronic (14 days) | LOEC (growth rate) | 0.008 (0.012 mg/L tamoxifen citrate) |
Unpublished AstraZeneca study in Swedish Drug Database 2011[a] |
| Green algae (Selenastrum capricornutum) |
Chronic (14 days) | NOEC (growth rate) | 0.003 (0.0049 mg/L tamoxifen citrate) |
Unpublished AstraZeneca study in Swedish Drug Database 2011[a] |
| Thamnocephalus platyurus |
Acute (24 h) | LC50 | 0.40 | DellaGreca et al. 2007Footnote Table 6a[b] |
| Water flea (Daphnia magna) |
Acute (24 h) | LC50 | 1.53 | DellaGreca et al. 2007[b] |
| Ceriodaphnia dubia | Chronic (7 days) | EC50 (population growth inhibition) | 0.00081 | DellaGreca et al. 2007[b] |
| Water flea (Daphnia magna) |
Chronic (21 days) | LOEC (reproduction) | 0.09 (0.14 mg/L tamoxifen citrate) |
Unpublished AstraZeneca study in Swedish Drug Database 2011 |
| Water flea (Daphnia magna) |
Chronic (21 days) | NOEC (length) | 0.03 (0.043 mg/L tamoxifen citrate) |
Unpublished AstraZeneca study in Swedish Drug Database 2011 |
| Water flea (Daphnia magna) |
Chronic (21 days) | NOEC (reproduction) |
0.05 (0.078 mg/L tamoxifen citrate) |
Unpublished AstraZeneca study in Swedish Drug Database 2011 |
| Marine copepod (Acartia tonsa) |
Chronic (5 days) | EC50 (inhibition of napular development) | 0.049 | Andersen et al. 2001 |
| Marine copepod (Acartia tonsa) |
Chronic (5 days) | EC10 (inhibition of napular development) | 0.0087 | Andersen et al. 2001 |
| Brachionus calyciflorus | Chronic (48 h) | EC50 (population growth inhibition) | 0.25 | DellaGreca et al. 2007 |
| Zebrafish (Danio rerio) | Chronic or full life cycle (filial generation | LOEC(sex reversal) | 0.00051 (0.00077 mg/L tamoxifen citrate) |
Knacker et al. 2010[a] |
| Brachionus calyciflorus | Acute (24 h) | LC50 | 0.97 | DellaGreca et al. 2007 |
| Fathead minnow (Pimephales promelas) |
Chronic or full life cycle (284 days) | NOEC | 0.0034 (0.0051 mg/L tamoxifen citrate) |
Williams et al. 2007[a] |
| Fathead minnow (Pimephales promelas) |
Chronic or full life cycle (284 days) | LOEC | 0.0037 (0.0056 mg/L tamoxifen citrate) |
Williams et al. 2007[a] |
| Fathead minnow (Pimephales promelas) |
Partial life cycle (42 days) |
EC70 (spawning) | 0.012 (0.018 mg/L tamoxifen citrate) |
Williams et al. 2007[a] |
| Bluegill sunfish (Lepomis macrochirus) |
Acute (96 h) | LC50 | 0.15 (0.23 mg/L tamoxifen citrate) |
Unpublished AstraZeneca study in Williams et al. 2007 and Swedish Drug Database 2011a |
| Rainbow trout (Oncorhynchus mykiss) |
Acute (96 h) | LC50 | 0.27 (0.41 mg/L tamoxifen citrate) |
Unpublished AstraZeneca study in Williams et al. 2007 and Swedish Drug Database 2011a |
| Rainbow trout (Salmo gairdneri) |
Acute (96 h) | LC50 | 0.21 (0.32 mg/L tamoxifen citrate) |
Unpublished AstraZeneca study in Swedish Drug Database 2011[a] |
| Rainbow trout (Salmo gairdneri) |
Acute (96 h) | NOEC | 0.18 (0.27 mg/L tamoxifen citrate) |
Unpublished AstraZeneca study in Swedish Drug Database 2011[a] |
| Poeciliopsis lucida (desert topminnow) hepatoma fish cell line PLHC-1 | MTT assayFootnote Table 6a[c] | EC50 (damage to cell membranes) | 7.4 (0.02 mM) |
Caminada et al. 2006Footnote Table 6a[d] |
| Poeciliopsis lucida (desert topminnow) hepatoma fish cell line PLHC-1 | NR assayFootnote Table 6a[e] | EC50 (damage to cell membranes) | 7.2 (0.0194 mM) |
Caminada et al. 2006[d] |
| Rainbow trout gonadal cell line RTG-2 | MTT assay[c] | EC50 (damage to cell membranes) | 7.09 (0.0191 mM) |
Caminada et al. 2006[d] |
| Zebrafish (Danio rerio) liver cell line ZFL | LDH assayFootnote Table 6a[f] | EC10 | 0.23 (0.61 µM) |
Bopp and Lettieri 2008 |
| Zebrafish (Danio rerio) liver cell line ZFL | LDH assay[f] | EC50 | 0.70 (1.88 µM) |
Bopp and Lettieri 2008 |
| Zebrafish (Danio rerio) liver cell line ZFL | MTT assay[c] | EC10 | 0.52 (1.39 µM) |
Bopp and Lettieri 2008 |
| Zebrafish (Danio rerio) liver cell line ZFL | MTT assay[c] | EC50 | 1.28 (3.46 µM) |
Bopp and Lettieri 2008 |
| Zebrafish (Danio rerio) liver cell line ZFL | AB assayFootnote Table 6a[g] | EC10 | 0.51 (1.37 µM) |
Bopp and Lettieri 2008 |
| Zebrafish (Danio rerio) liver cell line ZFL | AB assay[g] | EC50 | 1.12 (3.12 µM) |
Bopp and Lettieri 2008 |
| Zebrafish (Danio rerio) liver cell line ZFL | CFDA-AM assayFootnote Table 6a[h] | EC10 | 0.94 (2.52 µM) |
Bopp and Lettieri 2008 |
| Zebrafish (Danio rerio) liver cell line ZFL | CFDA-AM assay[h] | EC50 | 1.24 (3.35 µM) |
Bopp and Lettieri 2008 |
8.1.2 Aquatic Toxicity of Tamoxifen Metabolites
In vivo studies addressing the aquatic toxicity of the hydroxylated tamoxifen metabolites, 4-HT and endoxifen, were not identified in the published literature. Similarly to tamoxifen, 4-HT was tested in vitro in several cytotoxic assays performed in fish cell lines (Caminada et al. 2006; Bopp and Lettieri 2008). EC10 and EC50 values from these studies are presented in Table 6b. Similar studies were not identified for endoxifen.
EC50 values for 4-HT established from the MTT assays were 5.3 and 5.6 mg/L in the PLHC-1 and RTG-2 cell lines, respectively, and an EC50 of 1.8 mg/L was established from the NR assay in the PLHC-1 cell line (Caminada et al. 2006). In the ZFL cell line, EC50 values ranged from 0.45 to 0.69 mg/L, and EC10 values ranged from 0.28 to 0.58 mg/L, as established in four cytotoxicity assays (Bopp and Lettieri 2008). It should be noted that the EC50 results from the MTT assay were approximately 10 times higher in the PLHC-1 cell line than in the ZFL cell line, at 5.3 mg/L versus 0.69 mg/L, indicating that there may be cell line–specific differences in sensitivity (Table 6b). An analogous trend was observed when the parent compound tamoxifen was tested using the MTT assay in these cell lines (see Table 6a).
| Test cell line | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Poeciliopsis lucida (desert topminnow) hepatoma fish cell line PLHC-1 | MTT assayFootnote Table 6b[a] | EC50 (damage to cell membranes) | 5.3 (0.0138 mM) |
Caminada et al. 2006Footnote Table 6b[b] |
| Poeciliopsis lucida (desert topminnow) hepatoma fish cell line PLHC-1 | NR assayFootnote Table 6b[c] | EC50 (damage to cell membranes) | 1.8 (0.004 64 mM) |
Caminada et al. 2006[b] |
| Rainbow trout gonadal cell line RTG-2 | MTT assay[a] | EC50 (damage to cell membranes) | 5.6 (0.0145 mM) |
Caminada et al. 2006[b] |
| Zebrafish (Danio rerio) liver cell line ZFL | LDH assayFootnote Table 6b[d] | EC10 | 0.28 (0.73 µM) |
Bopp and Lettieri 2008[b] |
| Zebrafish (Danio rerio) liver cell line ZFL | LDH assay[d] | EC50 | 0.63 (1.62 µM) |
Bopp and Lettieri 2008[b] |
| Zebrafish (Danio rerio) liver cell line ZFL | MTT assay[a] | EC10 | 0.58 (1.49 µM) |
Bopp and Lettieri 2008[b] |
| Zebrafish (Danio rerio) liver cell line ZFL | MTT assay[a] | EC50 | 0.69 (1.78 µM) |
Bopp and Lettieri 2008[b] |
| Zebrafish (Danio rerio) liver cell line ZFL | AB assayFootnote Table 6b[e] | EC10 | 0.31 (0.79 µM) |
Bopp and Lettieri 2008[b] |
| Zebrafish (Danio rerio) liver cell line ZFL | AB assay[e] | EC50 | 0.45 (1.17 µM) |
Bopp and Lettieri 2008[b] |
| Zebrafish (Danio rerio) liver cell line ZFL | CFDA-AM assayFootnote Table 6b[f] | EC10 | 0.42 (1.08 µM) |
Bopp and Lettieri 2008[b] |
| Zebrafish (Danio rerio) liver cell line ZFL | CFDA-AM assay[f] | EC50 | 0.67 (1.73 µM) |
Bopp and Lettieri 2008[b] |
8.1.3 Derivation of the predicted no-effect concentration (PNEC)
A conservative predicted no effect concentration (PNEC) for tamoxifen in the aquatic environment was derived from the critical toxicity value (CTV) of 0.00051 mg/L for zebrafish (Danio rerio) (see Table 6a; the CTV is indicated in bold). This CTV is considered to be the most sensitive, population-relevant endpoint, and it is also several orders of magnitude lower than the cytotoxicity values for tamoxifen metabolite 4-HT (see Tables 6a and 6b). Based on a robust study summary (RSS), the study from which the value originated (Supplement A in Knacker et al. 2010) was found to be reliable with satisfactory confidence (Environment Canada 2014).
The CTV of 0.00051 mg/L, a LOEC for the sex reversal effect on the filial generation of fish, was divided by an assessment factor of 10 (to consider inter-species and intra-species variability in sensitivity, and to account for potential higher toxicity, including the more potent anti-estrogenic properties of the tamoxifen metabolites, 4-HT and potentially endoxifen) to derive a PNEC value of 5.1 × 10−5 mg/L. The value of the assessment factor also reflects the fact that the critical study is based on chronic exposure (a two-generation study), that there is a relatively large set of toxicity data, and that the CTV is approximately 10-fold lower than most other measured or calculated chronic toxicity data. The aquatic PNEC for tamoxifen of 5.1 × 10−5 mg/L is also considered applicable to both 4-HT and endoxifen.
8.2 Ecological Exposure Assessment
Limited data on concentrations of tamoxifen in water in Canada have been identified. Therefore, environmental concentrations have been estimated from available information, including estimated substance quantities, estimated release rates and characteristics of the receiving environment. Environmental concentrations have been estimated for an industrial release scenario and a down-the-drain release scenario.
8.2.1 Industrial Release
It is currently unknown whether tamoxifen is manufactured in Canada. However, releases from potential manufacturing activities into water are estimated below, based on the total quantity of the substance sold in Canada in the year 2012. Aquatic exposure to tamoxifen is expected if the substance is released during its manufacture at a pharmaceutical production facility to a wastewater treatment plant and the treatment plant discharges its effluent to a receiving water body. The concentration of the substance in the receiving water near the discharge point of the wastewater treatment plant is used as the predicted environmental concentration (PEC) in evaluating the aquatic risk of the substance. It is calculated using the equation:
PECaq = (1000 × Q × L) × (1 − R) / (N × F × D)
where:
- PEC aq:
- Aquatic concentration resulting from industrial releases (mg/L)
- 1000:
- Conversion factor (g/kg)
- Q:
- Total substance quantity produced annually at an industrial site (kg/year)
- L:
- Loss to wastewater (fraction)
- R:
- Wastewater treatment plant removal rate (fraction)
- N:
- Number of annual release days (days/year)
- F:
- Wastewater treatment plant effluent flow (m 3/day)
- D:
- Receiving water dilution factor (dimensionless).
If produced in Canada, some tamoxifen would be expected to be released to water during production, and a conservative industrial release scenario is used to estimate the aquatic concentration of the substance. The scenario is made conservative by assuming that the total quantity of the substance used as a pharmaceutical in Canada is produced at a single production facility. The facility is assumed to be located in Mississauga (Ontario), a typical Canadian manufacturing site for pharmaceuticals. The loss of the substance (L), resulting from the cleaning of process equipment, to the local WWTP (i.e., located in Mississauga), is estimated to be low, at 0.5% (L) of the total quantity produced,. The WWTP is a secondary system, has an effluent flow (F) of 160 244 m3/day and discharges to Lake Ontario. The scenario also assumes that the release occurs 21 days/year (N), the WWTP removes 83.5% (R) of the substance, as predicted by a model (ASTreat 2006), and the receiving water (Lake Ontario) dilution factor is 10 (D). Based on the above assumptions, the substance at a total industrial production quantity (Q) of approximately 320 kg/year yields an aquatic concentration of 7.9 × 10−6 mg/L in the aquatic receiving water near the discharge point of the wastewater treatment plant (Environment Canada 2013a).
| Input | Value | Justification and reference |
|---|---|---|
| Quantity (kg/year) | 320 | Estimated quantity as prescribed at hospitals and pharmacies across Canada for the year 2012, as the most conservative quantity in comparison with estimates for years 2007 and 2011 (McLaughlin and Belknap 2008; IMS 2013) |
| Loss to wastewater (%) | 0.5 | Personal communication, Technical Support Document for Pharmaceutical Spreadsheets, from Environmental Assessment Unit, New Substances [Health Canada to Exposure Unit, Existing Substances Environment Canada, dated 2007 (unreferenced) |
| Wastewater system removal efficiency (%) | 83.5 | Based on estimation from ASTreat (2006) |
| Number of annual release days (days/year) | 21 | Assumed to be manufactured or processed in small batches over 1 month, due to the assumption of the low substance quantity manufactured or processed per industrial site |
| Wastewater system effluent flow (m3/day) | 160 244 | Effluent flow of a large wastewater treatment plant (that uses 2ry treatment) located in Mississauga, Ontario (a typical Canadian pharmaceuticals manufacturing site, assumed to be located in Mississauga) |
| Receiving water dilution factor (dimensionless) | 10 | Environment Canada’s default assumption for large lakes, the WWTP in the scenario discharges to Lake Ontario |
8.2.2 Down-the-Drain Releases from Pharmaceutical Use
As tamoxifen can be released into water as a result of its prescribed use (i.e., patients ingesting and subsequently excreting the pharmaceutical substance), an aquatic exposure scenario resulting from down-the-drain releases was developed. It has been shown that in humans approximately 65% of the administered tamoxifen is excreted in feces (24.7%), bile (11.5%) and urine (26.7%) (Kisanga et al. 2005). Releases of tamoxifen metabolites, 4-HT and endoxifen, were also considered. Some quantities of tamoxifen can also be released down the drain as a result of tamoxifen use in laboratory research. Few data are available to confirm the exact quantities of tamoxifen used by research facilities. However, it is expected, based on the methodologies and concentrations described in research papers, that these use quantities are low, considerably lower than the quantities of tamoxifen in commerce for human consumption. Based on these considerations, the down-the-drain release scenario is limited to exposure from tamoxifen and its metabolites stemming from human pharmaceutical use.
A down-the-drain release from pharmaceutical use scenario was employed to estimate the tamoxifen concentration in multiple water bodies receiving wastewater treatment system effluents to which the substance may have been released (Environment Canada 2009). This calculation also includes consideration of the tamoxifen metabolites 4-HT and endoxifen.
The loss to wastewater resulting from the prescribed use of tamoxifen was assumed to be 100%. This assumption included losses due to the metabolized tamoxifen, i.e., its metabolites 4-HT and endoxifen, and unmetabolized tamoxifen. It is noted that model input parameters that affect model calculations, such as the wastewater removal efficiencies, were very similar for tamoxifen, 4-HT and endoxifen [i.e., 84% for tamoxifen, and more than 85% for both 4-HT and endoxifen (ASTreat 2006)]. Therefore, it was considered that capturing the percent loss of the unmetabolized tamoxifen and tamoxifen metabolites as 100% was a simplified and appropriate approach.
The realistic assumptions include:
- loss to sewer at 100% (i.e., includes unmetabolized tamoxifen and tamoxifen metabolites, 4-HT and endoxifen);
- WWTP removal rate estimated at 0.0% in case of no treatment, 55% for primary treatment and 84% for primary–secondary combined treatment;
- number of annual release days at 365 days/year;
- receiving water dilution factor in the range of 1–10.
The number of annual release days was assumed to be 365 to account for the variable use of the drug throughout the year as well as the variability between locations (i.e., hospitals where the drug is administered). As distribution of use across Canada is unknown, a variability factor of 2 was applied on every location to account for uneven distribution.
Given the above assumptions, the maximum PEC of tamoxifen in receiving water bodies was estimated to be 3.2 × 10−5 mg/L. The estimate is based on a total of 320 kg/year for the quantity of the substance used (estimated amount of tamoxifen purchased by hospitals and pharmacies for prescription pharmaceutical needs for the year 2012). The equation and inputs used to calculate the PEC are also described in Environment Canada (2013b).
| Input | Value(s) | Justification and reference |
|---|---|---|
| Quantity (kg/year) | 320 | Estimated quantity as prescribed at hospitals and pharmacies across Canada for the year 2012, as the most conservative quantity in comparison with estimates for years 2007 and 2011 (McLaughlin and Belknap 2008; IMS 2013) |
| Loss to wastewater (%) | 100% (includes tamoxifen, 4-HT and endoxifen) | It was determined that model parameters for tamoxifen, 4-HT- and endoxifen were very similar, therefore it was considered appropriate to capture % loss with one value. |
| Variability factorFootnote Table 7b[a] | 2 | Default |
| Wastewater system removal efficiency (%) | 84 | Based on estimation from ASTreat (2006) |
| Number of annual release days (days/year) | 365 | Assumes that the drug is taken daily |
| Receiving water dilution factor (dimensionless) | 1–10 | Environment Canada Existing Substances default assumption |
8.3 Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight of evidence approach and using precaution, as required under CEPA 1999. Lines of evidence considered include results from a conservative risk quotient calculation as well as information on persistence, bioaccumulation, toxicity, sources and fate of the substance.
Tamoxifen is a pharmaceutical approved for the market in Canada, and it also has applications as an investigative tool in research laboratories. Based on its uses, it has potential for dispersive releases into the Canadian environment. Once released into the environment, it will be found mainly in water in both the ionized and neutral forms. Upon ingestion, tamoxifen is metabolized to active metabolites, most notably 4-HT and endoxifen. Tamoxifen metabolites are expected to be excreted into water systems along with the parent compound. From the water compartment, tamoxifen and its metabolites may also partition to the sediments. Tamoxifen as well as its hydroxylated metabolites are expected to be persistent in water, soil and sediment. Tamoxifen, 4-HT and endoxifen have potential to harm aquatic organisms at low concentrations. They also have anti-estrogenic properties, and the hydroxylated metabolites are known to have a much greater affinity for estrogen receptor α than the parent compound tamoxifen.
A risk quotient analysis, integrating conservative estimates of exposure with toxicity information, was performed for the aquatic medium to determine whether there is potential for ecological harm in Canada. The conservative industrial release scenario presented above yielded a PEC of 7.9 × 10−6 mg/L. A PNEC of 5.1 × 10−5 mg/L was derived based on the most sensitive, chronic experimental toxicity value, and dividing this value by an assessment factor of 10 that accounts for inter- and intra-species variability in sensitivity and the potential higher toxicity associated with the more potent anti-estrogenic properties of the tamoxifen metabolites. This resulted in a risk quotient (PEC/PNEC) of 0.15 for releases from industry. Therefore, harm to aquatic organisms is unlikely for industrial releases, even under conservative (protective) assumptions.
For exposure resulting from down-the-drain releases from pharmaceutical use, the PEC (the maximum PEC was determined to be 3.2 × 10−5 mg/L) did not exceed the PNEC (5.1 × 10−5 mg/L) at any site across Canada (Environment Canada 2010b). Therefore, based on the estimated number of receiving water bodies that will not be negatively affected by the use of tamoxifen, coupled with the magnitude of the risk quotient and the more realistic scenario run, it is proposed that tamoxifen is unlikely to cause harm to aquatic organisms from down-the-drain releases.
When tamoxifen is released into a water body, it may partition into suspended particulate matter and to bottom sediments, where sediment-dwelling organisms would be exposed to the substance. However, no environmental monitoring data or toxicity data specific to sediment-dwelling organisms are available for this substance. For this substance, a risk quotient based on exposure in sediment pore water may be calculated based on the aquatic compartment PEC and PNEC values presented above and used for sediment risk characterization. In the calculation, bottom sediment and its pore water are assumed to be in equilibrium with the overlying water, and benthic and pelagic organisms are assumed to have similar sensitivities to the substance. Therefore, the PEC and PNEC for sediment pore water are considered to be the same as for the aquatic compartment. This equilibrium approach would result in a risk quotient (PEC/PNEC) for the sediment compartment that is the same as for the aquatic compartment. Therefore, harm to sediment-dwelling organisms from tamoxifen and its metabolites, 4-HT and endoxifen, in Canada is unlikely.
Together, the information available suggests that there is low risk of harm to organisms or the broader integrity of the environment from these substances. It is therefore concluded that tamoxifen does not meet the criteria set out in paragraph 64(a) or 64(b) of CEPA 1999, as it is not entering the environment in a quantity or concentration or under conditions that have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
8.4 Uncertainties in Evaluation of Ecological Risk
There are uncertainties regarding tamoxifen use quantities in research laboratories and associated releases into the environment. The available information, including tamoxifen concentrations used for research purposes and quantity formats available for sale from chemical companies, is very limited and insufficient to derive a quantitative estimate that would help determine the importance of this source. For estimation of the releases stemming from potential manufacturing of tamoxifen is Canada, the proportion of the substance manufactured and released from each individual industrial facility is unknown. Therefore, it was conservatively assumed that all tamoxifen used in Canada was manufactured at a single location. Similarly, as the distribution of use across Canada is unknown, a variability factor of 2 was applied on every location in Mega Flush to account for uneven distribution. Due to the limited information regarding the environmental presence of tamoxifen metabolites and the difficulty in discerning their overall hazard contribution based on tamoxifen metabolism, characterization of exposure from tamoxifen metabolites was based on assumptions of no metabolism of tamoxifen and incorporated a PNEC value that accounted for the known increased endocrine potency of these substances.
The partitioning and physical and chemical property models cannot address the potential for tamoxifen to ionize in the aquatic environment or the potential for binding in soil from electrostatic interactions (cation exchange) or binding to clays which are negatively surface charged. Therefore, these model predictions likely do not represent the properties and environmental behaviour of tamoxifen both as an ionized and neutral compound.
The bioaccumulation assessment is limited by the absence of empirical bioaccumulation data. Modelled bioaccumulation and bioconcentration factors were derived and all predictions using models have some degree of error. There is some uncertainty, as tamoxifen may not be in their training sets; many of the structural classes of pharmaceuticals are not amenable to model prediction because they are considered “out of the model domain of applicability” (e.g., structural and water solubility domains). In addition, there is concern that the lack of metabolic transformation data for tamoxifen may provide results that could be interpreted as a false positive.
Also, regarding ecotoxicity, based on the predicted partitioning behaviour of this chemical, the significance of sediment as an important medium of exposure is not well addressed by the available effects data. Indeed, the only effects data identified apply primarily to pelagic aquatic exposures, although the water column may not be the medium of primary concern based on partitioning estimates.
9. Potential to Cause Harm to Human Health
Tamoxifen has been classified as carcinogenic to humans (Group 1) by the International Agency for Research on Cancer (IARC 1996, 2012) and as a known human carcinogen by the National Toxicology Program in the United States (NTP 2011).
Drugs containing tamoxifen as an ingredient are assessed under the F&DA (Canada 1985) with respect to their safety, effectiveness and quality. This assessment focused on uses and exposures that were not covered as part of the F&DA assessment, specifically the risks posed by the residues resulting from manufacture, formulation and disposal after use.
Releases of tamoxifen could occur during its manufacture from a pharmaceutical production facility to a wastewater treatment plant and the subsequent discharge of effluent from the treatment plant to a receiving water body. A conservative industrial release scenario is used to estimate the aquatic concentration of the substance and yields a concentration of 7.9 × 10−6mg/L (7.9 ng/L) in the receiving water near the discharge point of the wastewater treatment plant (see section 8.2.1).
When patients use pharmaceuticals, some of the drug may not be absorbed or metabolized, and even drugs that are metabolized may have active metabolites or may revert to the parent form in environmental media. This may lead to the excretion of active drug residues into the wastewater system and release of the wastewater effluent containing these residues into surface water (i.e., lakes, rivers), and this surface water has the potential to be used as drinking water. Additionally, the drug may be released to wastewater during the manufacturing process or via incorrect disposal of the excess pharmaceutical. Therefore, a focus of this assessment is on the potential for indirect exposure of humans to these pharmaceuticals through drinking water.
Only a portion of the pharmaceuticals used in Canada would be released into the wastewater system. When ingested, metabolism of a pharmaceutical results in a smaller portion of the pharmaceutical being excreted by the patient in the urine and feces. This amount can be further reduced as a result of wastewater treatment, environmental biodegradation and/or drinking water treatment prior to consumption. The concentration in the water source is also significantly reduced via dilution as the waste is released into waterways.
For this assessment, conservative assumptions were used when estimating the potential indirect exposure of humans to tamoxifen. Releases to surface water were modelled using the down-the-drain releases from pharmaceutical use scenario, as described above. For the purposes of modelling, it was assumed that 100% of the pharmaceutical that was purchased by hospitals and pharmacies was prescribed and administered to patients and excreted into wastewater after administration (i.e., no absorption or metabolism of the drug). It was also assumed that removal of tamoxifen during wastewater treatment was dependent on the treatment process applied (see above section on down-the-drain releases).
This scenario estimates concentrations in approximately 1000 waterways across Canada. The highest values estimated by this model are typically in small waterways with low dilution capacity, which are unlikely sources of drinking water. As a result, this scenario would be expected to highly overestimate actual concentrations in drinking water. The maximum PEC was 3.2 × 10−5 mg/L (32 ng/L).
Limited measured concentration data for tamoxifen were identified in Canada and other countries of the world and are summarized in the “Measured Environmental Concentrations” section above. Concentrations measured in wastewater effluent, surface water, groundwater and drinking water were examined when available. Overall, the studies indicated that the amount of pharmaceutical measured decreases significantly as the substance moves from the wastewater treatment plant and is released into surface water. As there is variability in the use of pharmaceuticals in different countries (due to different population levels, prescription preferences, drug registrations, etc.), the measured concentrations in other countries are not necessarily representative of concentrations in Canadian waters. They do, however, account for releases from all potential sources and for potential reductions in drug concentrations resulting from metabolism, environmental degradation, removal via wastewater, drinking water treatment, etc. For these reasons, measured concentrations are preferable to modelled concentrations for characterizing human exposure, even if measurements were taken in other countries.
Limited measured concentration data for tamoxifen were identified. In a study of tamoxifen in samples collected from a variety of wastewater treatment plants representing typical Canadian treatment systems and geographic variations, tamoxifen was detected in the range of 1.30 × 10−6–1.73 × 10−6 mg/L (1.30–1.73 ng/L) in effluent samples (Teslic and Smyth 2013). It is recognized that this concentration would not be expected to be found in drinking water, as it would be further reduced via dilution after the effluent was released to surface water and possibly reduced during the drinking water treatment process prior to consumption. However, this value can be used as a conservative estimate of exposure of Canadians.
The estimated intakes of tamoxifen by humans can be represented by formula-fed infants 0–6 months of age, which is estimated to be the most highly exposed age class, on a body weight basis, of those examined. The equation for deriving the estimated intake is given below:
Intake = (PEC × IR) / bw
where:
- Intake:
- Estimated intake of the substance from drinking water (mg/kg bw per day)
- PEC:
- Predicted environmental concentration in receiving water from modelled or measured data (mg/L)
- IR:
- Ingestion rate of drinking water for formula-fed infants (0.8 L/day) (Health Canada 1998)
- bw:
- Default body weight for infants 0–6 months of age (7.5 kg) (Health Canada 1998)
The maximum estimated intake of tamoxifen, based on the maximum value detected in samples of wastewater effluent of 1.73 × 10−6 mg/L (1.7 ng/L), is 0.18 ng/kg bw per day. Based on the modelled concentration of 32 ng/L in surface water, the estimated intake would be 3.4 ng/kg bw per day.
Given the low levels of estimated exposure, the potential risk of indirect exposure to tamoxifen is expected to be low.
To further characterize potential risks associated with the intake of tamoxifen via drinking water, the lowest therapeutic dose (LTD) for tamoxifen was identified, and a margin of exposure (MOE) was calculated to determine the ratio between the upper-bounding estimate of intake by the general population and the dose that would be expected to produce a pharmacological effect. This approach is consistent with methodology described elsewhere (Webb et al. 2003; Schwab et al. 2005; Watts et al. 2007; Bull et al. 2011; WHO 2011). The LTD is the lowest concentration that evokes a desired therapeutic effect among target populations and is equivalent to the lowest dose prescribed or recommended, taking into account the number of doses per day (WHO 2011). These values are derived from an assessment of the balance between safety and efficacy.
The tamoxifen products currently registered for use in Canada by humans are all tablets for oral ingestion (DPD 2010). Dosage information for these products indicates a recommended dose of 20–40 mg/day (Pharmascience Inc. 2003; Pharmel Inc. 2003; Apotex Inc. 2004; Teva Canada Limited 2011; AstraZeneca Canada Inc. 2012; Mylan Pharmaceuticals ULC 2012). Using an adult body weight of 70.9 kg (Health Canada 1998) for conversion, an LTD of 20 mg/day is equivalent to a dose of 0.28 mg/kg bw per day.
MOEs were derived using the equation below:
MOE = LTD/Intake
where:
- MOE:
- Margin of exposure (dimensionless)
- LTD:
- Lowest therapeutic dose (mg/kg bw per day)
- Intake:
- Maximum estimated intake for drinking water derived from modelled or measured concentrations (mg/kg bw per day)
For tamoxifen, this results in an MOE greater than 1 000 000, based on an intake calculated using the maximum value measures in effluent samples taken from wastewater effluent in Canada. The MOE calculated using the maximum modelled PEC would be greater than 82 000. Given the highly conservative nature of the exposure inputs and the use of human data to derive a point of departure for risk characterization, these MOEs support the determination that risks from indirect exposure to tamoxifen are low.
10. Uncertainties in Evaluation of Risk to Human Health
There is uncertainty regarding the estimation of exposure due to the lack of representative measured concentrations of tamoxifen in Canadian surface water or drinking water and the use of models for estimating risk to human health. However, confidence is high that actual exposures to tamoxifen in Canadian drinking water would be lower than the exposures estimated using both the model and the maximum concentrations measured in surface water outside of Canada. This is supported by data available from other countries and the highly conservative default assumptions used. The uncertainty in the human risk estimates could be reduced significantly by the use of measured concentration data from Canadian surface water and/or drinking water for this substance.
Potential exposures to tamoxifen could occur via other sources, such as ingestion of fish or swimming in waters where the pharmaceutical is present, but these exposures are expected to be much less than the exposure through drinking water and so are not considered in this assessment.
Tamoxifen may also be used for additional off-label or veterinary uses that are not considered in this assessment. The quantity of the substance being used for these purposes is unknown, and so estimation of releases is not possible at this time. These potential releases may be accounted for in the measured concentrations if they are occurring in the area of study.
It is recognized that the LTD represents an exposure level at which a desired pharmacological response is achieved and further that at this exposure level, adverse effects, in addition to intended effects, may occur in some patients. For certain indications and certain classes of drugs, the nature of these unintended effects may be significant. However, the LTD is developed for patients who require treatment for a particular illness and therefore are likely to be more susceptible to potential effects than a healthy individual. Although the use of the LTD provides a tier 1 type of assessment that does not utilize all the toxicity data that may be available for the substance, the highly conservative exposure defaults that have been used still lead to significant MOEs between the LTD and the estimated intakes. The LTD also allows for derivation of an MOE based on a human dose as the point of departure, which is preferable to using a point of departure developed using experimental animals.
11. Conclusion
Considering all lines of evidence presented in this screening assessment, there is low risk of harm to organisms or the broader integrity of the environment from this substance. It is therefore concluded that tamoxifen does not meet the criteria under paragraph 64(a) or 64(b) of CEPA 1999, as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on information presented in this screening assessment, it is concluded that tamoxifen does not meet the criteria set out in paragraph 64(c) of CEPA 1999, as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that tamoxifen does not meet any of the criteria set out in section 64 of CEPA 1999.
11.1.1 Considerations for Follow up
Monitoring of future use quantities of tamoxifen is considered important given its ecotoxicological and hazard properties. Estimates of use quantities between 2007 and 2012 indicate an increase in demand of approximately 20%. Although no ecological risk in the Canadian environment due to exposure to tamoxifen was determined, a rising demand may lead to increases in its use quantities and consequently to increases in environmental releases and exposure, potentially leading to ecological harm. It is noted that tamoxifen (CAS RN 10540-29-1) is listed on the DSL, while tamoxifen citrate (CAS RN 54965-24-1) is neither on the DSL nor Non-Domestic Substance List (NDSL). Tamoxifen citrate is the prevalent form available as a prescribed pharmaceutical; tamoxifen is the active ingredient in the medicinal products. Options on how best to monitor changes in the use profile of this substance such as monitoring of international activities or surveillance of the Canadian marketplace will be investigated. Tamoxifen may be considered for inclusion in the Domestic Substances List inventory update initiative.
12. References
ACD/pKaDB [Prediction Module]. 2005. Version 9.04. Toronto (ON): Advanced Chemistry Development. Available from: http://www.acdlabs.com/products/phys_chem_lab/pka/. [restricted access]
Andersen HR, Wollenberger L, Halling-Sorensen B, Kusk KO. 2001. Development of copepod napulii to copepodites--a parameter for chronic toxicity including endocrine disruption. Environ Toxicol Chem 20(12):2821–2829.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2008. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Apotex Inc. 2004. Product monograph for Apo-Tamox. [revised 2004 Jan 8]. [cited in DPD 2010].
Arnot JA, Gobas FA. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337–345.
Arnot JA, Mackay D, Bonnell M. 2008a. Estimating metabolic biotransformation rates in fish from laboratory data. Environ Toxicol Chem 27(2):341–351.
Arnot JA, Mackay D, Parkerton TF, Bonnell M. 2008b. A database of fish biotransformation rates for organic chemicals. Environ Toxicol Chem 27(11):2263–2270.
Arnot JA, Meylan W, Tunkel J, Howard PH, Mackay D, Bonnell M, Boethling RS. 2009. A quantitative structure–activity relationship for predicting metabolic biotransformation rates for organic chemicals in fish. Environ Toxicol Chem 28(6):1168–1177.
Arnot JA, Arnot MI, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P. 2010. Molecular size cutoff criteria for screening bioaccumulation potential: fact or fiction? Integr Environ Assess Manag 6(2):210–224.
Aronson D, Boethling R, Howard P, Stiteler W. 2006. Estimating biodegradation half-lives for use in chemical screening. Chemosphere 63:1953–1960.
Ashton D, Hilton M, Thomas KV. 2004. Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom. Sci Total Environ 333:167–184.
AstraZeneca Canada Inc. 2012. Product monograph for Nolvadex-D. [revised 2012 Nov 14]. [cited in DPD 2010].
ASTreat Model [sewage treatment plant removal model]. 2006. Version 1.0. Cincinnati (US): Procter & Gamble Company. Available from Procter & Gamble Company, P.O. Box 538707, Cincinnati, OH 45253-8707, USA.
[BCFBAF] Bioaccumulation Program for Windows [Estimation Model]. 2010. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2010. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741–752.
Bopp SK, Lettieri T. 2008. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol 8:8.
Bueno MJM, Hernando MD, Herrera S, Gomez MJ, Fernandez-Alba AR, Bustamante I, Garcia-Calvo E. 2010. Pilot survey of chemical contaminants from industrial and human activities in river waters of Spain. Int J Environ Anal Chem 90(3–6):321–343.
Bull RJ, Crook J, Whittaker M, Cotruvo JA. 2011. Therapeutic dose as the point of departure in assessing potential health hazards from drugs in drinking water and recycled municipal wastewater. Regul Toxicol Pharmacol 60:1–19.
Caminada D, Escher C, Fent K. 2006. Cytotoxicity of pharmaceuticals found in aquatic systems: comparison of PLHC-1 and RTG-2 fish cell lines. Aquat Toxicol 79(2):114–123.
Canada. [1978]. Food and Drug Regulations, C.R.C., c. 870. Available from: www.canlii.org/en/ca/laws/regu/crc-c-870/latest/crc-c-870.html
Canada. 1985. Food and Drugs Act, R.S.C. 1985, c. F-27. Available from: www.canlii.org/ca/sta/f-27/whole.html
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. Canada Gazette, Part III, vol. 22, no. 3. Available from: publications.gc.ca/gazette/archives/p3/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 29 March 2000, SOR/2000-107. Available from: http://gazette.gc.ca/archives/p2/2000/index-eng.html
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004−2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. Available from: http://oasis-lmc.org/?section=software&swid=1
CEREP. c2010a. ADME-Tox Application note: Partition coefficient Log D. Paris (FR): Cerep. [cited 2010 Sept]. Available from: www.cerep.com
CEREP. c2010b. ADME-Tox Application note: Aqueous solubility. Paris (FR): Cerep. [cited 2010 Sept]. Available from: www.cerep.com
[CFIA] Canadian Food Inspection Agency. 2007. Compendium of Medicating Ingredient Brochures. 8th ed. Ottawa (ON): CFIA. Available from: www.inspection.gc.ca/english/anima/feebet/mib/cmibe.shtml
[ChemIDplus] Internet chemicals search system. 1993– . Bethesda (MD): National Library of Medicine (US). [cited 2014 Sept 8]. Available from: www.chem.sis.nlm.nih.gov/chemidplus/
Coetsier CM, Spinelli S, Lin L, Roig B, Touraud E. 2009. Discharge of pharmaceutical products (PPs) through a conventional biological sewage treatment plant: MECs vs PECs? Environ Int 35:787–792.
[CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada, Ecological Assessment Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available from: Environment Canada, Ecological Assessment Division.
Daughton CG, Ternes TA. 1999. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107(Suppl 6):907–938.
DellaGreca M, Iesce MR, Isidori M, Nardelli A, Previtera L, Rubino M. 2007. Phototransformation products of tamoxifen by sunlight in water. Toxicity of the drug and its derivatives on aquatic organisms. Chemosphere 67:1933–1939.
Dimitrov S, Dimitrova N, Walker J, Veith G, Mekenyan O. 2002. Predicting bioconcentration potential of highly hydrophobic chemicals. Effect of molecular size. Pure Appl Chem 74(10):1823–1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
[DPD] Drug Product Database [database on the Internet]. 2010. Ottawa (ON): Health Canada. [cited 2013 Mar]. Available from: www.hc-sc.gc.ca/dhp-mps/prodpharma/databasdon/index-eng.php
Drug Infonet [Internet]. c1996–2005. Tamoxifen citrate. [cited 2010 Jun 7]. Available from: www.druginfonet.com/
[EAFUS] Everything Added to Food in the United States [database on the Internet]. 2011. Silver Spring (MD): US Food and Drug Administration. [cited 2013 Mar]. Available from: www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm115326.htm
El-Sharkawy SH. 1991. Microbial conversion of tamoxifen.Appl Microbiol Biotechnol 35:436–439.
El-Sharkawy SH, Abul-Hajj YJ. 1987. Microbial transformation of tamoxifen. Pharm Res 4(4):353–354.
Environment Canada. 2007. Guidance for conducting ecological assessments under CEPA, 1999--Science Resource Technical Series. Technical guidance module: QSARs. Reviewed draft working document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2009. Guidance for conducting ecological assessments under CEPA, 1999--Science Resource Technical Series. Technical guidance module: Mega Flush consumer release scenario. Working document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2013a. IGETA report: CAS RN 10540-29-1, 2014-08-12. Unpublished report. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2013b. Mega Flush v.3.1.4 report: CAS RN 10540-29-1, 2014-08-12. Unpublished report. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2014. Supporting documentation: robust study summary and model inputs for tamoxifen. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available on request from: substances@ec.gc.ca
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2012. Version 4.1. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
Ferrando-Climent L, Rodriguez-Mozaz S, Barceló D. 2013. Development of a UPLC-MS/MS method for the determination of ten anticancer drugs in hospital and urban wastewaters, and its application for the screening of human metabolites assisted by information-dependent acquisition tool (IDA) in sewage samples. Anal Bioanal Chem 405(18):5937–5952.
Ferrari B, Paxéus N, Lo Giudice R, Pollio A, Garric J. 2003. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol Environ Safe 55(3):359–370.
Flexser LA, Hammett LP, Dingwall A. 1935. The determination of ionization by ultraviolet spectrophotometry: its validity and its application to the measurement of the strength of very weak bases. J Am Chem Soc 57:2103–2115.
Foote CS, Valentine JS, Greenberg A, Liebman JF, editors. 1995. Active oxygen in chemistry. London (GB): Chapman & Hall; p. 105–140. [cited in DellaGreca et al. 2007].
Goetz MP, Loprinzi CL. 2003. A hot flash on tamoxifen metabolism. J Natl Cancer Inst 95(23):1734–1735.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Hilton MJ, Thomas KV. 2003. Determination of selected human pharmaceutical compounds in effluent and surface water samples by high-performance liquid chromatography–electrospray tandem mass spectrometry. J Chromatogr A 1015:129–141.
Hilton MJ, Thomas KV, Ashton D. 2003. Targeted monitoring programme for pharmaceuticals in the aquatic environment [Internet]. R&D Technical Report P6-012/06/TR. Bristol (GB): Environment Agency; 57 p. [cited 2010 Jun 4]. Available from: a0768b4a8a31e106d8b0-50dc802554eb38a24458b98ff72d550b.r19.cf3.rackcdn.com/sp6012-06-tr-e-e.pdf
Household Products Database [database on the Internet]. 1993– . Bethesda (MD): National Library of Medicine (US). [updated 2013 Jan; cited 2013 Mar]. Available from: www.householdproducts.nlm.nih.gov/
Howse JR, Malayannan Subramaniam M, Cicek M, Wu X, Gingery A, Grygo SA, Sun Z, Pitel KS, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC. 2013. Endoxifen’s molecular mechanisms of action are concentration dependent and different than that of other anti-estrogens. PLoS One 8(1):e54613.
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1983– . Bethesda (MD): National Library of Medicine (US). [revised 2009 Aug12; cited 2013 Mar]. Available from: www.toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1996. Some pharmaceutical drugs. IARC Monogr Eval Carcinog Risks Hum 66:1–514. Available from: monographs.iarc.fr/ENG/Monographs/vol66/index.php
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2012. A review of human carcinogens: pharmaceuticals. IARC Monogr Eval Carcinog Risks Hum 100A:1–469. Available from: monographs.iarc.fr/ENG/Monographs/vol100A/index.php
[IMS] Intercontinental Marketing Services. 2013. Health Canada Sales Database 2011 & 2012 [MIDAS database on CD]. IMS Brogan,Toronto (ON), IMS Brogan
Isidori M, Bellotta M, Cangiano M, Parrella A. 2009. Estrogenic activity of pharmaceuticals in the aquatic environment. Environ Int 35:826–829.
Jaremko M, Kasai Y, Barginear MF, Raptis G, Desnick RJ, Yu C. 2010. Tamoxifen metabolite isomer separation and quantification by liquid chromatography–tandem mass spectrometry. Anal Chem 82(24):10186–101893.
Kisanga ER, Mellgren G, Len EA. 2005. Excretion of hydroxylated metabolites of tamoxifen in human bile and urine. Anticancer Res 25:4487–4492.
Klimisch HJ, Andreae M, Tillmann U. 1997. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol 25:1–5.
Knacker T, Boetcher M, Frische T, Rufli H, Stolzenberg H-C, Teigeler M, Zok S, Braunbeck T, Schäfers C. 2010. Environmental effect assessment for sexual endocrine disrupting chemicals: fish testing strategy. Intergr Environ Assess Manag. 6(4 Supp A): 653–662
[KOCWIN] The Soil Adsorption Coefficient Program [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[KOWWIN] Octanol–Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Leguéné C, Clavère D, Jore D, Gardès-Albert M. 2001. Oxydation radiolytique du tamoxifène par les radicaux libres OH et (ou) HO2. Can J Physiol Pharmacol 79:184–188.
Lim YC , Skaar TC . 2005. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxytamoxifen. Cancer Chemother Pharmacol 55(5):471–478.
Liu ZH, Zhang YG, Wang DS. 2010. Studies on feminization, sex determination, and differentiation of the southern catfish, Silurus meridionalis--a review. Fish Physiol Biochem 36:223–235.
[LNHPD] Licensed Natural Health Products Database [database on the Internet]. 2008. Version 1.0. Ottawa (ON): Health Canada. [cited 2013 Mar]. Available from: webprod3.hc-sc.gc.ca/lnhpd-bdpsnh/index-eng.jsp
Martigoni M. 2006. Species and strain differences in drug metabolism in liver and intestine [dissertation]. Groningen (NL): University of Groningen. Available from: http://dissertations.ub.rug.nl/faculties/science/2006/m.martignoni/
Marty MS, Carney EW, Rowlands JC. 2011. Endocrine disruption: historical perspectives and its impact on the future of toxicology testing. Toxicol Sci 120(Suppl 1):S93–S108.
McLaughlin A, Belknap A. 2008. Annual kg quantity of medicinal ingredients distributed and dispensed in Canada: analysis of intercontinental medical statistics (IMS) data for 2007. [Excel format data summary]. Ottawa (ON): Health Canada, Health Products and Food Branch, Environmental Impact Initiative.
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: a QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1–2):103–133.
Mills JL, S. Jayaraman S, Simoneau MM, Gutjahr-Gobell R, Zaroogian G, Riffle BW, Laws SC. [date unkown]. Differences in uptake, metabolism and clearance of atrazine and tamoxifen in a fish and a rat Species. Research Triangle Park (NC): US Environmental Protection Agency. Available from:
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2008. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[MSDSonline] Material Safety Data Sheets [Internet]. c1998 − . Chicago (IL): MSDSonline. Available from: www.msdsonline.com. [restricted access].
Mylan Pharmaceuticals ULC. 2012. Product monograph for Mylan-Tamoxifen. [revised 2012 Aug 8]. [cited in DPD 2010].
[NCI] National Chemical Inventories [database on CD-ROM]. 2009. Issue 1. Columbus (OH): American Chemical Society. [cited 2010 Sept]. Available from: www.cas.org/products/other-cas-products/nci-on-cd
[NHPID] Natural Health Products Ingredients Database [database on the Internet]. 2011. Version 2.1. Ottawa (ON): Health Canada. [cited 2013 Mar]. Available from: webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do
[NTP] National Toxicology Program (US). 2011. Tamoxifen. In: Report on carcinogens. 12th ed. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Available from: ntp.niehs.nih.gov/go/roc12
[OECD] Organisation for Economic Co-operation and Development. 2001. SIDS Initial Assessment Report for: Citric acid; CAS RN 77-92-9. SIDS Initial Assessment Meeting 11; January, 2001. Available from: www.inchem.org/documents/sids/sids/77929.pdf
Park I, Kim J, Cho SH, Kim DS. 2004. Sex differentiation and hormonal sex reversal in the bagrid catfish Pseudobagrus fulvidraco (Richardson). Aquaculture 232:183–193.
Pharmascience Inc. 2003. Product monograph for pms-Tamoxifen. [revised 2003 Sep 11]. [cited in DPD 2010].
Pharmel Inc. 2003. Product monograph for Tamoxifen. [revised 2003 Sep 11]. [cited in DPD 2010].
Roberts PH, Thomas KV. 2006. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci Total Environ 356:143–153.
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol 29(1):89–92.
Schwab BW, Hayes EP, Fiori JM, Mastrocco FJ, Roden NM, Cragin D, Meyerhoff RD, D’Aco VJ, Anderson PD. 2005. Human pharmaceuticals in US surface waters: a human health risk assessment. Regul Toxicol Pharmacol 42:296–312.
Sigma-Aldrich. ©2010. Product search: tamoxifen. St. Louis (MO): Sigma-Aldrich. [cited 2010 Sep 29]. Available from: www.sigmaaldrich.com/
Singh AK. 2013. Introduction of modern endocrine techniques for the production of monosex population of fishes. Gen Comp Endocrinol 181:146–155.
Swedish Drug Database [database on the Internet]. 2011. Stockholm (SE): Swedish Drug Database. [cited 2011 Feb 15]. Available from: www.fass.se/LIF/produktfakta/artikel_produkt.jsp?NplID=19860829000074&DocTypeID=78#IDE4POEFUA6FBVERT1
Tauxe-Wuersch A, De Alencastro F, Grandjean D, Tarradellas J. 2006. Trace determination of tamoxifen and 5-fluorouracil in hospital and urban wastewaters. Int J Environ Anal Chem 86(7):473–485.
Teslic S, Smyth SA. 2013. Occurrence and fate of pharmaceuticals and personal care products in municipal wastewater treatment systems – year 3. Unpublished final report, Sept 24, 2013. Ottawa (ON): Health Canada, New Substances Assessment and Control Bureau; 18 p.
Teva Canada Limited. 2011. Product monograph: Teva-Tamoxifen (tamoxifen citrate): 10 and 20 mg tablets, BP; antineoplastic agent. Date of Preperation: May 19, 2011. Available from: http://webprod5.hc-sc.gc.ca/dpd-bdpp/info.do?code=10790&lang=eng
Thomas KV, Hilton MJ. 2004. The occurrence of selected human pharmaceutical compounds in UK estuaries. Mar Pollut Bull 49:436–444.
[TOPKAT] TOxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. Available from: http://www.accelrys.com/products/topkat/index.html
Urbatzka R, Lutz I, Opitz R, Kloas W. 2006. Luteinizing hormone, follicle stimulating hormone, and gonadotropin releasing hormone mRNA expression of Xenopus laevis in response to endocrine disrupting compounds affecting reproductive biology. Gen Comp Endocrinol 146:119–125.
Urbatzka R, Bottero S, Mandich A, Lutz I, Kloas W. 2007. Endocrine disrupters with (anti)estrogenic and (anti)androgenic modes of action affecting reproductive biology of Xenopus laevis: I. Effects on sex steroid levels and biomarker expression. Comp Biochem Physiol C 144:310–318.
Van der Ven LTM, van den Brandhof EJ, Vos JH, Wester PW. 2007. Effects of the estrogen agonist 17β-estradiol and antagonist tamoxifen in a partial life-cycle assay with zebrafish (Danio rerio). Environ Toxicol Chem 26(1):92–99.
Watts C, Maycock D, Crane M, Fawell J, Goslan E. 2007. Desk based review of current knowledge on pharmaceuticals in drinking water and estimation of potential levels. Final report prepared by Watts and Crane Associates for Drinking Water Inspectorate, Department for Food, Environment and Rural Affairs (Defra Project Code: CSA 7184/WT02046/DWI70/2/213). Available from: dwi.defra.gov.uk/research/completed-research/reports/dwi70-2-213.pdf
Webb S, Ternes T, Gibert M, Olejniczak K. 2003. Indirect human exposure to pharmaceuticals via drinking water. Toxicol Lett 142:157–167.
[WHO] World Health Organization. 2011. Pharmaceuticals in drinking-water. Geneva (CH): World Health Organization, Public Health and Environment, Water, Sanitation, Hygiene and Health. Report No.: WHO/HSE/WSH/11.05.
Williams TD, Caunter JE, Lilicrap AD, Hutchinson TH, Gillings EG, Duffell S. 2007. Evaluation of the reproductive effects of tamoxifen citrate in partial and full life-cycle studies using fathead minnows (Pimephales promelas). Environ Toxicol Chem 26(4):695–707.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2008. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Zhou JL, Zhang ZL, Bank E, Grover D, Jiang JQ. 2009. Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. J Hazard Mater 166:655–661.