State of the Science Report - Part 1
Phthalate Substance Grouping
Medium-Chain Phthalate Esters
Chemical Abstracts Service Registry Numbers
84-61-7; 84-64-0; 84-69-5; 523-31-9; 5334-09-8; 16883-83-3; 27215-22-1; 27987-25-3; 68515-40-2; 71888-89-6
Environment Canada
Health Canada
August 2015
Table of Contents
- Tables and Figures
- Synopsis
- 1. Introduction
- 2. Identity of Substances
- 3. Physical and Chemical Properties
- 4. Sources
- 5. Uses
- 6. Releases to the Environment
- 7. Environmental Fate and Behaviour
- 8. Potential to Cause Ecological Harm
- 9. Potential to Cause Harm to Human Health
- 10. References
- Appendices
Tables and Figures
- Table 1. Substances in the medium-chain phthalates subgroup
- Table 2-1. Summary of substance identity information for the medium-chain phthalates subgroup
- Table 2-2. Analogue identities for the medium-chain phthalates subgroup
- Table 2-3. Substances in the medium-chain phthalates subgroup identified for read-across approach
- Table 2-4. Read-across data used to inform various parameters evaluated in this assessment
- Table 2-5. Information on identity, chemical structure and branching of analogues used for read across
- Table 3-1. Range of experimental and predicted physical and chemical properties (at standard temperature) for phthalate esters in the medium-chain subgroup
- Table 4-1. Summary of Canadian manufacturing, imports and exports of substances in the medium-chain phthalates subgroup for 2012, based on CEPA 1999, section 71 industry survey
- Table 4-2. Summary of international production and use of substances in the medium-chain phthalates subgroup
- Table 5-1. Summary of Canadian uses of five medium-chain phthalates (based on consumer and commercial Domestic Substances List [DSL] codes that were reported in response to Section 8 of the section 71 industry survey)
- Table 5-2. Summary of major uses of medium-chain phthalates identified internationallya
- Table 7-1. Summary of Level III fugacity modelling (New EQC 2011) for DIBP, BCHP and CHIBP, showing percent partitioning into each medium for three release scenarios
- Table 7-2. Summary of Level III fugacity modelling (New EQC 2011) for DCHP, DBzP, BIOP, B79P, DIHepP and DMCHP, showing percent partitioning into each medium for three release scenarios
- Table 7-21. (Q)SAR model predictions for degradation of the medium-chain phthalates subgroup in air by hydroxyl radicals and through hydrolysis
- Table 7-22. Summary of key empirical data for biodegradation of medium-chain phthalates and analogue substances in water
- Table 7-23. Summary of modelled primary and ultimate biodegradation data for DIBP, BCHP, CHIBP, DCHP, DMCHP and DIHepP
- Table 7-24. Summary of modelled primary and ultimate biodegradation data for DBzP, B84P, B79P and BIOP
- Table 7-3. Summary of Level III fugacity modelling (New EQC 2011) for B84P, showing percent partitioning into each medium for three release scenarios
- Table 7-4. Summary of bioaccumulation factors for medium-chain phthalates
- Table 8-1. Key aquatic toxicity studies for medium-chain phthalates considered in choosing a critical toxicity value for water
- Table 8-2. Key aquatic toxicity studies for analogues of BCHP, CHIBP and DBzP considered in choosing a critical toxicity value for water
- Table 8-3. Ranges of predicted aquatic toxicity endpoints generated by ECOSAR v1.00 for medium-chain phthalates
- Table 8-4. Summary of model results for B79P and BIOP
- Table 8-5. PNECs derived for medium-chain phthalates
- Table 8-6. CBR equation input and output values for DIHepP and B84P
- Table 8-7. Maximum saturation in sediment input and output values for medium-chain phthalates
- Table 8-8. Sediment PNEC input and output values for DCHP, BIOP, B79P and DMCHP
- Table 8-9. Predicted environmental concentrations (PECs) based on measured environmental concentrations considered in ecological risk characterization
- Table 8-10. Summary of risk quotients obtained for different environmental media and exposure scenarios for DIBP and DCHP
- Table 8-11. Summary of data needs for the ecological assessment of medium-chain phthalates
Synopsis
The Minister of the Environment and the Minister of Health have prepared a state of the science report on ten phthalate esters part of the Phthalate Substance Grouping. The purpose of this report is to review the currently available science on medium-chain phthalates, so that the public has an opportunity to review, comment, and/or provide additional information for consideration prior to proposing conclusions through the publication of a draft screening assessment. A proposed approach for considering the cumulative risk of phthalates has also been prepared for public review and comment, and will be used in the development of the draft screening assessment. Key selection considerations for this group were based on similar potential health effects of concern; potential ecological effects of concern for some phthalates; potential exposure of consumers and children; potential to leverage/align with international activity; and potential risk assessment and risk management efficiencies and effectiveness.
While many phthalate substances have common structural features and similar functional uses, differences in the potential health hazard, as well as environmental fate and behaviour, have been taken into account through the establishment of subgroups. The primary basis for the subgroups from a health hazard perspective is a structure activity relationship (SAR) analysis using studies related to important events in the mode of action for phthalate-induced androgen insufficiency during male reproductive development in the rat. The effects of phthalate esters for these important events appear to be structure-dependent and highly related to the length and nature of their alkyl chain. Further information on the approach by which the substances in the Phthalate Substance Grouping were divided into three subgroupings from a health hazard perspective is provided in Health Canada (2015a). From an ecological perspective, subgrouping was based primarily on differences in log Kow and water solubility, and their resulting effects on bioaccumulation and ecotoxicity. Further information on the ecological rationale for the subgroups is provided in an appendix to the draft approach for considering the cumulative risk of phthalates (Environment Canada and Health Canada 2015a).
The Chemical Abstracts Service Registry Number (CAS RNFootnote[1]), their Domestic Substances List (DSL) names and their common names and acronyms are listed in the table below.
| CAS RN | Domestic Substances List name | Common name (acronym) |
|---|---|---|
| 84-61-7 | 1,2-Benzenedicarboxylic acid, dicyclohexyl ester | Dicyclohexyl phthalate (DCHP) |
| 84-64-0 | 1,2-Benzenedicarboxylic acid, butyl cyclohexyl ester | Butyl cyclohexyl phthalate (BCHP) |
| 84-69-5 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | Diisobutyl phthalate (DIBP) |
| 523-31-9 | 1,2-Benzenedicarboxylic acid, bis(phenylmethyl) ester | Dibenzyl phthalate (DBzP) |
| 5334-09-8 | 1,2-Benzenedicarboxylic acid, cyclohexyl 2-methylpropyl ester | Cyclohexyl isobutyl phthalate (CHIBP) |
| 16883-83-3 | 1,2-Benzenedicarboxylic acid, 2,2-dimethyl-1-(1-methylethyl)-3-(2-methyl-1-oxopropoxy)propyl phenylmethyl ester | Benzyl 3-isobutyryloxy-1-isopropyl-2,2-dimethylpropyl phthalate (B84P) |
| 27215-22-1 | 1,2-Benzenedicarboxylic acid, isooctyl phenylmethyl ester | Benzyl isooctyl phthalate (BIOP) |
| 27987-25-3 | 1,2-Benzenedicarboxylic acid, bis(methylcyclohexyl) ester | Bis(methylcyclohexyl) phthalate (DMCHP) |
| 68515-40-2 | 1,2-Benzenedicarboxylic acid, benzyl C7-9-branched and linear alkyl esters | Benzyl octyl phthalate (B79P) |
| 71888-89-6 | 1,2-Benzenedicarboxylic acid, di-C6-8-branched alkyl esters, C7-rich | Diisoheptyl phthalate (DIHepP) |
The ten substances in the medium-chain phthalates subgroup do not occur naturally in the environment. Five substances, DIBP, DCHP, DIHepP, B79P and B84P, are known to be imported into Canada; import quantities for 2012 were less than 10 000 kg for DCHP and DIHepP, between 10,000 and 100,000 kg for DIBP, and between 100,000 and 1,000,000 kg for both B79P and B84P (Environment Canada 2014). The other five substances, CHIBP, BCHP, DMCHP, BIOP and DBzP, were not reported to be imported into Canada above the reporting threshold of 100 kg in 2012. None of the medium-chain phthalates are known to be manufactured in Canada above the reporting threshold of 100 kg. Major uses identified for DIBP, DCHP, DIHepP, B79P and B84P are in adhesives and sealants used in construction and/or in the automotive sector. Other applications of medium-chain phthalates in the automotive industry include their addition to automotive paints and coatings or to resins that are then molded into automobile parts. Most of these substances are also used as plasticizers in the production of plastic, and used in manufactured items such as electrical and electronics, and children's toys. Import of B79P in raw material form for use in various applications was also reported.
Medium-chain phthalates are expected to be released primarily to water through wastewater effluents from industrial sources and through disperse releases from consumer products. In products, medium-chain phthalates are not bound within the matrix and are therefore subject to migration and environmental release. Consumer products disposed to wastewater treatment systemsFootnote[2] are another potential source of environmental releases. When released to water, these substances are predicted to remain in water and to distribute to sediments, with the degree of partitioning driven by their molecular size and water solubility. Medium-chain phthalates are hydrophobic, capable of adsorption to soil particulates, and have limited potential for volatilization from water. Certain medium-chain phthalates were detected and measured in all environmental media (i.e., air, water, sediment and soil), including remote locations, wastewater and in biota.
These substances undergo relatively rapid biodegradation, particularly in aerobic conditions. However, at very low concentrations, biodegradation rates are slower. Abiotic degradation processes, such as hydrolysis, are slow. However, none of these substances are expected to persist in the environment.
Empirical and modelled data indicate that medium-chain phthalates have low to moderate bioaccumulation and biomagnification potential. However, DIBP and DIHepP have been measured in a variety of aquatic species, which confirms that these substances are bioavailable.
Based on high partition coefficients and low to moderate water solubilities, exposure of medium-chain phthalates to organisms will occur primarily through the diet. Results from standard laboratory tests suggest that most medium-chain phthalates have moderate to high hazard potential in aquatic species. DIHepP and B84P were not found to have adverse effects at concentrations up to and exceeding their water solubility limits. Results from an analysis of critical body residues (CBRs) conducted for aquatic organisms based on the solubility limit, determined that the maximum tissue concentration of DIHepP and B84P will be much lower than levels associated with adverse acute or chronic lethality effects due to neutral narcosis.
Based on results from laboratory tests for DIBP and BBP, BCHP, CHIBP and DBzP are also expected to have low hazard potential in sediment dwelling organisms. A CBR analysis conducted for sediment organisms indicated that the maximum tissue concentration calculated from the saturation limit of DIHepP and its biota-sediment accumulation factor (BSAF) does not exceed minimum concentrations estimated to cause narcotic effects. Toxicity values for sediment-dwelling organisms were derived from aquatic toxicity results for DCHP, BIOP, B79P and DMCHP using the Equilibrium Partitioning method, generating moderate sediment toxicity values.
It should be noted that the CBR analysis does not consider the potential for adverse effects resulting from modes of action other than baseline narcosis. Secondary endpoints that may be mediated by endocrine activity and mechanisms of action other than narcosis are not well studied for medium-chain phthalates. Studies suggest that certain phthalates (such as BBP) have the potential for endocrine disruption; however, such studies have not been conducted for any of the substances in the medium-chain phthalates subgroup. The limited information on the estrogenic activity of medium-chain phthalates in aquatic organisms has not been demonstrated to result in population-level effects (such as growth, reproduction or survival).
Qualitative exposure scenarios were developed for B79P and B84P for the automotive sector to describe releases of these substances to water from facilities where they are used in applications such as automotive sealants and coatings. Calculations of the predicted environmental concentrations (PECs) were highly uncertain for B79P and B84P; therefore, monitoring or surveillance data were used for the purpose of developing PECs. Monitoring data were used to estimate potential exposure concentrations for DIBP and DCHP, whereas a critical body residue analysis was done for DIHepP, from the disperse uses of these substances. The calculated risk quotients (RQs) indicated that harm to aquatic organisms is unlikely. Given that CHIBP, BCHP, DMCHP, BIOP and DBzP were not reported to be imported into Canada above the reporting threshold of 100 kg, exposure scenarios were not developed for these substances.
For the general population in Canada, sources of exposure for medium-chain phthalates are from indoor air, dust, food and breast milk. Due to the information received indicating that a portion of these substances in manufactured items may come in contact with skin, exposure scenarios were identified to characterize dermal exposure for adults and infants. Finally, DIBP may also be present in children's toys and articles; therefore, oral exposure from mouthing these products was also evaluated.
With regard to human health, the health effects data for medium-chain phthalates shows that there is evidence of effects in animal studies that include developmental, reproductive and systemic effects related to the liver and kidneys. Of these, depending on the phthalate in question, the critical effect for risk characterization is developmental effects on males, as the available evidence is strongest for effects on the development of the reproductive system, such as alterations of feminization parameters and reproductive tract malformations, and effects on fertility related to a relatively well studied mode of action called the "rat phthalate syndrome" (RPS). This syndrome has been associated with the lowest levels of exposure to this subgroup of phthalates examined to date in animal studies.
Comparisons of estimates for exposure to seven of the medium-chain phthalates from various sources, such as environmental media, food, contact with plastic articles (PVC, polyurethane, polyester, etc.), toys and/or personal care productsFootnote[3] as well as biomonitoring levels (if available) for all age groups with the appropriate critical effect levels, result in margins of exposures (MOEs) that are considered adequate to address uncertainties in the exposure and health effects databases. Further, these margins are also considered protective of potential reproductive effects not only in males at older life stages but also in females, in addition to effects in other organ systems.
Results of the section 71 industry survey indicate that CHIBP, BCHP and BIOP are not currently in use above the reporting threshold of 100 kg, and the likelihood of exposure to the general population in Canada is considered to be low. Hence, the potential risk to human health is considered to be low for these three substances.
Based on the information available, there is evidence that phthalates in the medium-chain subgrouping have a common mode of action, as they elicit effects on the developing male reproductive system indicative of RPS. Although the MOEs associated with the ten phthalates included in this report are currently considered adequate on an individual substance basis, these MOEs do not address potential risk from concurrent exposure to these phthalates.
Accordingly, a proposed cumulative risk assessment approach for certain phthalates is provided in a separate report (Environment Canada and Health Canada 2015a).
1. Introduction
Pursuant to sections 68 and 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999), the Minister of the Environment and the Minister of Health conduct evaluations of substances to determine whether these substances present or may present a risk to the environment or to human health.
The Substance Groupings Initiative is a key element of the Government of Canada/s Chemicals Management Plan (CMP). The Phthalates Substance Grouping consists of 14 substances that were identified as priorities for assessment, as they met the categorization criteria under section 73 of CEPA 1999 and/or were considered as a priority based on human health concerns (Environment Canada, Health Canada 2007). Certain substances within this Substance Grouping have been identified by other jurisdictions as a concern due to potential reproductive and developmental effects in humans. There are also potential ecological effects of concern for some phthalates. A survey conducted for phase 1 of the Domestic Substances List (DSL) Inventory Update identified that a subset of phthalates have a wide range of consumer applications that could result in exposure to humans, including children (Environment Canada 2012). Addressing these substances as a group allows for consideration of cumulative risk, where warranted.
This state of the science (SOS) report provides a summary and evaluation of the current available science intended to form the basis for a draft screening assessment scheduled for publication in 2016. The Government of Canada developed a series of SOS reports for the Phthalate Substance Grouping to provide an opportunity for early public comment on a proposed cumulative assessment approach for certain phthalates (Environment Canada and Health Canada 2015a), prior to that approach being used to propose conclusions on the substances in Phthalate Substance Grouping through publication of a draft screening assessment report.
This SOS report focuses on ten phthalate esters, listed in Table 1, that are referred to as the medium-chain phthalates subgroup based on the intermediate size of their functional side groups. These substances were identified in the categorization of the DSL under subsection 73(1) of CEPA 1999 as priority for assessment. These substances also met the categorization criteria for persistence but not for inherent toxicity of non-human organisms or bioaccumulation.
| CAS RN | Substance name | Acronym |
|---|---|---|
| 84-69-5 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | DIBP |
| 84-64-0 | 1,2-Benzenedicarboxylic acid, butyl cyclohexyl ester | BCHP |
| 5334-09-8 | 1,2-Benzenedicarboxylic acid, cyclohexyl 2-methylpropyl ester | CHIBP |
| 84-61-7 | 1,2-Benzenedicarboxylic acid, dicyclohexyl ester | DCHP |
| 27987-25-3 | 1,2-Benzenedicarboxylic acid, bis(methylcyclohexyl) ester | DMCHP |
| 71888-89-6 | 1,2-Benzenedicarboxylic acid, di-C6-8-branched alkyl esters, C7-rich | DIHepP |
| 523-31-9 | 1,2-Benzenedicarboxylic acid, bis(phenylmethyl) ester | DBzP |
| 16883-83-3 | 1,2-Benzenedicarboxylic acid, 2,2-dimethyl-1-(1-methylethyl)-3-(2-methyl-1-oxopropoxy)propyl phenylmethyl ester | B84P |
| 27215-22-1 | 1,2-Benzenedicarboxylic acid, isooctyl phenylmethyl ester | BIOP |
| 68515-40-2 | 1,2-Benzenedicarboxylic acid, benzyl C7-9-branched and linear alkyl ester | B79P |
While phthalates have common structural features and similar functional uses, differences in their potential health hazard, environmental fate and behaviour have been taken into account through the establishment of subgroups. The primary basis for the subgroups from a health hazard perspective is a structure activity relationship (SAR) analysis using studies related to important events in the mode of action for phthalate-induced androgen insufficiency during male reproductive development in the rat. The effects of phthalate esters for these important events appear to be structure-dependent and highly related to the length and nature of their alkyl chain (Health Canada 2015a). From an ecological perspective, subgrouping was based primarily on differences in log Kow and water solubility and their resulting effects on bioaccumulation and ecotoxicity (Environment Canada and Health Canada 2015a).
This SOS report includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to December 2014 for the ecological portion and up to August 2014 for the health portion of the assessment. New hazard-related information was submitted after the literature cut-off date and will be incorporated in the next phase of the assessment process. Empirical data from key studies as well as some results from models were used. When available and relevant, information presented in assessments from other jurisdictions was considered.
The SOS report does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical and reliable studies and lines of evidence pertinent to development of a screening assessment in the future.
This SOS report was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada, and incorporates input from other programs within these departments. The ecological and human health portions of this report have undergone external written peer review and/or consultation. Comments on the technical portions relevant to the environment were received from Dr. Frank Gobas (Frank Gobas Environmental Consulting), Dr. Chris Metcalfe (Ambient Environmental Consulting, Inc.), Dr. Thomas Parkerton (ExxonMobil Biomedical Sciences, Inc.), and Dr. Charles Staples (Assessment Technologies, Inc.). Comments on the technical portions relevant to human health were received from Dr. Andreas Kortenkamp (Brunel University), Donna Vorhees (The Science Collaborative), Dr. Michael Dourson (Toxicology Excellence for Risk Assessment), and Dr. Raymond York (York & Associates). While external comments were taken into consideration, the final content and outcome of the report remain the responsibility of Health Canada and Environment Canada.
2. Identity of Substances
Phthalate esters are synthesized through the esterification of phthalic anhydride (1,2-Benzenedicarboxylic acid anhydride; CAS RN 85-44-9) with various alcohols (ACC 2001). The resulting phthalate esters are diesters of benzenedicarboxylic acid comprised of a benzene ring with two side-chain ester groups. Phthalates have the general structure outlined in Figure 1, where R1 and R2 represent ester side chains that can vary in length and structure (ACC 2001). The ester side groups can be the same or different and the nature of the side groups determines both the identity of the particular phthalate and its physical and toxicological properties. All substances in the Phthalate Substances Grouping are ortho-phthalates (o-phthalates), with their ester side chains situated adjacent to each other at the 1 and 2 positions of the benzene ring (refer to Figure 1; US EPA 2012).
The structural formula for phthalate esters is derived from the isomeric composition of the alcohol used in their manufacture (Parkerton and Winkelmann 2004). Dialkyl phthalates have ester groups of linear or branched alkyl chains containing from one to thirteen carbons, while benzyl phthalates generally contain a phenylmethyl group and an alkyl chain as ester side groups, and cyclohexyl phthalates contain a saturated benzene ring as an ester group (Parkerton and Winkelmann 2004).
Figure 1. General structure of ortho-phthalates

Long description for figure 1
A two-dimensional representation of the general molecular structure for the phthalates of interest.
The depiction has two elements.
1) Starting on the left is the general molecular structure for the phthalates of interest. The general molecular structure consists of a benzene ring with ester substitutions at the 1 and 2 positions. The chains on the ester linkage are represented by “R1” and “R2”.
2) On the right of the figure are the definitions of the R groups. R1 and R2 may be saturated linear or branched alkyl chains. R1 and R2 may also be a phenyl group or a cyclohexyl ring.
The ten substances of the Phthalate Substance Grouping that are the focus of this SOS report belong to the medium-chain phthalates subgroup and are characterized by ester side groups that mainly contain between 3 and 7 carbons, and do not exceed 9 carbons. Molecular weights of these substances range from 278.4 to 454.6 g/mol. The ester side groups, always in the ortho-position, occur in one of three side group combinations: as dialkyl phthalates, which are linear and/or branched alkyl chains; as phenyl or benzyl phthalates that have both an alkyl chain and a cyclic group; or as dicyclic phthalates. Substance identity information for the medium-chain phthalates subgroup is summarized in Table 2-1.
Two substances, DIHepP (CAS RN 71888-89-6) and BIOP (CAS RN 27215-22-1), are isomeric mixtures with alkyl chains that have a defined number of carbons, but can vary in branching. One substance, B79P (CAS RN 68515-40-2), is considered to be a substance of Unknown or Variable Composition, Complex Reaction Products or Biological Materials (UVCB), and it has alkyl chains that can vary in length from 7 to 9 carbons and in the degree of branching. The other seven substances are single constituent, discrete chemicals.
| CAS RN acronym |
DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
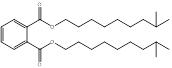
| 84-69-5 DIBP |
1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester (Diisobutyl phthalate) |
 C16H22O4 |
278.35 |
| 84-64-0 BCHP |
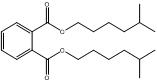
1,2-Benzenedicarboxylic acid, butyl cyclohexyl ester |  C18H25O4 |
304.39 |
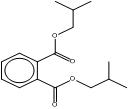
| 5334-09-8 CHIBP |
1,2-Benzenedicarboxylic acid, cyclohexyl 2-methylpropyl ester | 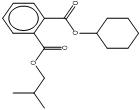 C18H25O4 |
304.39 |
| 84-61-7 DCHP |
1,2-Benzenedicarboxylic acid, dicyclohexyl ester | 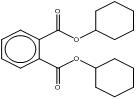 C20H26O4 |
330.43 |
| 523-31-9 DBzP |
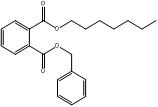
1,2-Benzenedicarboxylic acid, bis(phenylmethyl) ester |  C22H18O4 |
346.39 |
| 27987-25-3 DMCHP |
1,2-Benzenedicarboxylic acid, bis(methylcyclohexyl) ester |  C22H30O4 |
358.48 |
| 71888-89-6 DIHepPFootnote Table 2-1[a] |
1,2-Benzenedicarboxylic acid, di-C6-8-branched alkyl esters, C7-rich |
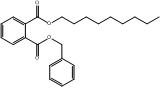
C22H34O4 |
362.51 |
| 27215-22-1 BIOPa |
1,2-Benzenedicarboxylic acid, isooctyl phenylmethyl ester |
C23H28O4 |
368.48 |
| 68515-40-2 B79PFootnote Table 2-1[b] |
1,2-Benzenedicarboxylic acid, benzyl C7-9-branched and linear alkyl esters |
C22H28O4 |
368.48 |
| 16883-83-3 B84P |
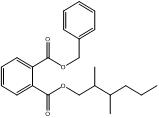
1,2-Benzenedicarboxylic acid, 2,2-dimethyl-1-(1-methylethyl)-3-(2-methyl-1-oxopropoxy)propyl phenylmethyl ester |  C27H34O6 |
454.57 |
2.1 Selection of analogues and use of (Q)SAR models
Guidance on the use of a read-across approach and Quantitative Structure-Activity Relationships or (Q)SAR models for filling data gaps has been prepared by various organizations, such as the Organisation for Economic Co-operation and Development (OECD). These methods have been applied in various regulatory programs, including the European Union's (EU) Existing Substances Programme. In this assessment, a read-across approach using data from analogues and the results of (Q)SAR models, where appropriate, has been used to inform the ecological and human health assessments. Analogues were selected that were structurally and/or functionally similar to substances within this subgroup (e.g., based on physical-chemical properties and toxicokinetics) and that had relevant empirical data that could be used to inform target substances for which limited empirical data was available. The applicability of (Q)SAR models was determined on a case-by-case basis.
2.1.1 Selection of Analogues for Ecological Assessment
For the read-across approach to the ecological assessment, candidate analogues were selected using the OECD (Q)SAR Toolbox software (2012). Substances that were both structurally and functionally similar to the substances in the medium-chain phthalates subgroup with similarity indices of 80% and above were considered as a starting point. The selected analogues are phthalate esters with comparable molecular size and side groups, known to act through narcosis, and characterized by comparable physical-chemical properties, particularly water solubility and partition coefficients such as the Kow, which influence the potential for environmental bioavailability.
The following substances in the medium-chain phthalates subgroup have limited experimental fate and effects data: BCHP, CHIBP, DBzP, DIHepP and BIOP. A well-studied substance, butyl benzyl phthalate (BBP), characterized by a benzyl ester side group and a straight four-carbon alkyl side chain, is used as an analogue for BCHP, CHIBP and DBzP-each one containing at least one cyclic group and/or an alkyl chain. The substance diphenyl phthalate (DPhP), with two phenyl ester side groups, is used as an analogue for DBzP, which has two benzyl ester side groups. Diisooctyl phthalate (DIOP) and diethylhexyl phthalate (DEHP), characterized by two branched alkyl side chains of up to eight carbons, are used as analogues for DIHepP, which is predominantly composed of two seven-carbon branched alkyl side chains. In addition, the data-rich dibutyl phthalate (DBP), with its straight four-carbon alkyl side chains, can be used to fill data gaps for DIBP, which has branched 4-carbon alkyl side chains, although, it is noted that the available dataset for DIBP is extensive.
Analogues and their substance identity information are presented in Table 2-2. Additional substance identity information for the analogues is provided in Appendix A-1, and their physical chemical properties are summarized in Appendix A-2 and A-3. Table 2-4 provides a summary of the types of data sourced from the analogues.
Also, when appropriate, based on structural and functional similarities, information for data-rich substances in the subgroup is used to read-across for similar substances with limited or no data. Information available for DCHP is used to evaluate properties of DMCHP, and information available for B79P is used for BIOP. Structurally, these substances are quite similar. DCHP features two cyclohexanes as part of its ester side groups, and DMCHP has two methylcyclohexanes instead. B79P, a UVCB substance, has a benzyl ester side group, and a variable eight-carbon straight or branched alkyl ester side chain, whereas BIOP has an eight-carbon branched alkyl ester side chain and a methylphenyl ester side group. Substance names and structures are summarized in Table 2-3.
| Systematic name of analogue (CAS RN) | Common name or acronym of analogue | Analogue chemical structure, molecular formula and molecular weight (g/mol) | Target medium-chain subgroup substance |
|---|---|---|---|
| Butyl benzyl phthalate (85-68-7) |
BBP | 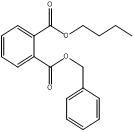 C19H20O4 312.35 |
1) BCHP (84-64-0) 2) CHIBP (5334-09-8) 3) DBzP (523-31-9) |
| Diphenyl phthalate (84-62-8) |
DPhP | 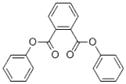 C20H14O4 318.33 |
DBzP (523-31-9) |
| Dibutyl phthalate (84-74-2) |
DBP | 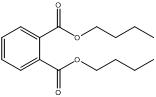 C16H22O4 278.34 |
DIBP (84-69-5) |
| Diisooctyl phthalate (27554-26-3) | DIOP |  C24H38O4 390.56 |
DIHepP (71888-89-6)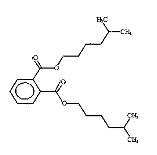 |
| Diethylhexyl phthalate (117-81-7) |
DEHP |  C24H38O4 390.56 |
DIHepP (71888-89-6) |
| Common name or acronym of subgroup analogue (CAS RN) | Analogue chemical structure | Common name or acronym of subgroup target substance (CAS RN) | Chemical structure of target substance |
|---|---|---|---|
| DCHP (84-61-7) |  |
DMCHP (27987-25-3) |  |
| B79P (68515-40-2) | 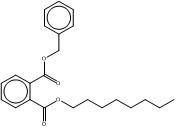 |
BIOP (27215-22-1) | 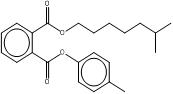 |
| Analogue common name or acronym (CAS RN) | Persistence | Bioaccumulation | Ecotoxicity |
|---|---|---|---|
| BBP (85-68-7) | abiotic and biotic degradation studies | BCF, BAF, BSAF, BMF data | aquatic, soil and sediment toxicity studies |
| DPhP (84-62-8) | N/A | N/A | aquatic toxicity studies |
| DIOP (27554-26-3) | biotic degradation | N/A | aquatic toxicity studies |
| DEHP(117-81-7) | abiotic and biotic degradation studies | BAF, BSAF data | aquatic and sediment toxicity studies |
| DBP (84-74-2) | abiotic and biotic degradation studies | N/A | aquatic and sediment toxicity studies |
2.1.2 Selection of Analogues for Human Health Assessment
Based on the consideration of similarities in length and nature of the ester chains, several phthalates were identified as the "closest analogue(s)" for the phthalates of interest within its subgroup (Health Canada 2015a). See Table 2-5 for information on analogues within the medium-chain phthalates subgroup.
| Analogue CAS RN | Analogue DSL name | Analogue common name (acronym) | Analogue chemical structure and molecular formula | Analogue branching (number of carbons in longest chain) | CMP2 phthalate (s) where analogue was used |
|---|---|---|---|---|---|
| 84-74-2 | 1,2-Benzenedicarboxylic acid, dibutyl ester | Dibutyl phthalate (DBP) |  |
Linear (4) | DIBP BCHP |
| 84-61-7 | 1,2-Benzenedicarboxylic acid, dicyclohexyl ester | Dicyclohexyl phthalate (DCHP) |  |
Cyclo (6) | DMCHP CHIBP BCHP |
| 84-69-5 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | Diisobutyl phthalate (DIBP) | 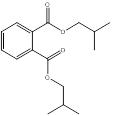 |
Branched (3) | CHIBP B84P |
| 2528-16-7 | 1,2-Benzenedicarboxylic acid, mono(phenylmethyl)ester | Monobenzyl phthalate (MBzP) | 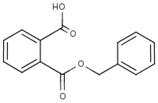 |
Mono (4) | DBzP B84P BIOP B79P |
| 85-68-7 | 1,2-Benzenedicarboxylic acid, butyl phenylmethyl ester | Butyl benzyl phthalate (BBP) |  |
Linear/Benzyl (4 benzyl) | B84P |
| 27554-26-3 | 1,2-Benzenedicarboxylic acid, diisooctyl esters | Diisooctyl phthalate (DIOP) | dimethyl hexyl ester groups (mixed isomers) methyl heptyl ester groups (mixed isomers) |
Branched (6-7) | BIOP |
| 28553-12-0 | 1,2-Benzenedicarboxylic acid, diisononyl ester | diisononyl phthalate (DINP-1) | methylethyl hexyl ester groups dimethyl heptyl ester groups methyl octyl ester groups isodecyl ester groups |
Branched (6*-9) | B79P |
3. Physical and Chemical Properties
Physical and chemical properties determine the overall characteristics of a substance and are used to determine the suitability of different substances for different types of applications. Such properties also play a critical role in determining the environmental fate of substances (including their potential for long-range transport), as well as their toxicity to humans and non-human organisms.
Where experimental information was limited or not available, models based on quantitative structure-activity relationships (QSARs) were used to generate data. These models are mainly based on fragment addition methods and rely on the neutral form of a chemical as input. Phthalate esters in the medium-chain subgroup are considered amenable to model prediction using QSARs, as they are within the model domain of applicability (i.e., structural and/or property parameter domains are represented in the training set used for the models). These substances also occur as neutral (non-ionized) substances in the environment.
Experimental and modelled physical and chemical properties for the substances in the medium-chain subgroup are presented in ranges in Table 3-1. Key physical-chemical property data identified for the individual substances are presented in Appendix B. Median values were calculated for water solubility, log Kow and log Koc based on the estimates generated from various (Q)SAR models (see Appendix B-2). Representative experimental and/or modelled values chosen for key physical-chemical properties were checked for internal consistency using the three solubility approach described by Cole and Mackay (2000) and Schenker et al. (2005). Based on the results, the log Kow values calculated from the model VCCLab (2005), rather than median values, were considered for DIHepP, BIOP and B84P.
| Property | Range | Type of data | Reference |
|---|---|---|---|
| Physical state | Liquid | Experimental | US EPA 2010 |
| Melting point (°C) | -64-66 | Experimental | HSDB 1983-; Phys-Prop 2006; European Commission 2000 |
| Boiling point (°C) | ~205-390 | Experimental | Haynes and Lide 2010; European Commission 2000 |
| Boiling point (°C) | 323-474 | Modelled | EpiSuite 2012 |
| Density (kg/m3) | 787-1076 | Experimental | Haynes and Lide 2010; ECHA c2007-2014 |
| Vapour pressure (Pa) | 3.8 × 10-6-6.3 × 10-3 | Experimental | Daubert and Danner 1989; Werner 1952; European Commission 2000; Cousins and Mackay 2000; ECHA c2007-2014 |
| Vapour pressure (Pa) | 8.48 × 10-7-0.322 | Modelled | EPI Suite 2012 |
| Water solubility (mg/L) | 0.02-20.3 | Experimental | Leyder and Boulanger 1983; Yalkowsky et al. 2010; HSDB 1983-; European Commission 2000; Letinksi et al. 2002; ECHA c2007-2014 |
| Water solubility (mg/L) | 0.001-5.0 | Modelled | EPI Suite 2012; ACD/Percepta c1997-2012; VCCLab 2005 |
| Henry's Law constant (Pa·m3/mol)Footnote Table 3-1[a] | 1.03 × 10-6- 1.60 × 102 |
Modelled (bond estimate) |
EPI Suite 2012 |
| Log Kow (dimensionless)Footnote Table 3-1[b] | 4.11-5.5 | Experimental | Leyder and Boulanger 1983; ECHA c2007-2014 |
| Log Kow (dimensionless) | 4.46-7.41 | Modelled | EPISuite 2012; ACD/Percepta c1997-2012; VCCLab 2005; ppLFER |
| Log Koc (dimensionless)a | 2.91-6.10 | Modelled (average of MCI and log Kow methods |
EPI Suite 2012 |
| Log Koa (dimensionless)a | 8.41-14.65 | Modelled | EPI Suite 2012 |
Substances in the medium-chain phthalates subgroup are oily liquids at room temperature (US EPA 2010); however, some of the phthalates have the potential to exist as solids at low environmental temperatures (Cousins et al. 2003). They do not contain functional groups with ionizing potential and are therefore expected to exist as neutral chemicals at environmentally relevant pH (6-9).Their experimental melting points range from -64°C to 66°C. It is noted that melting point values and information regarding the physical state are inconsistent for BCHP and DBzP, and unknown for DMCHP. This introduces some uncertainty with respect the evaluation of their properties, in particular ecotoxicity, using the analogue BBP or the read-across approach using DCHP. Melting points of phthalates cannot be predicted reliably using (Q)SAR models; therefore, these modelled values are not reported herein. Experimental boiling points ranged from approximately 205°C to 390°C, and modelled boiling points were in the range of 323°C to 474°C.
In general, both the vapour pressure and water solubility display an overall trend of decreasing values with increasing alkyl chain length, although this pattern is more pronounced for water solubility values (Appendix B-2). Medium-chain phthalates have low to moderate water solubilities and very low to low vapour pressures. The empirical and modelled log Kow values were determined to be high, and the modelled log Kocvalues were high to very high.
4. Sources
There is limited evidence showing that certain phthalate esters, DEHP, DBP and DIOP, can be synthesized by algae species, including red algae (Bangia atropurpurea) and brown algae (Sargassum wightii) (Chen 2004; Sastry and Rao 1995). The production process and the physiological role of phthalate esters in algae have not been defined. Similar studies have not been identified for the substances in the medium-chain phthalates subgroup; it is uncertain if they can occur naturally in the environment.
An industry survey, issued pursuant to section 71 of CEPA 1999, was conducted in 2013 to obtain information on quantities in commerce for substances in the Phthalate Substance Grouping in Canada (Canada 2013). Table 4-1 presents a summary of the total manufacture, import and export quantities reported for 2012 for the medium-chain phthalates subgroup. Due to the targeted nature of the survey, reported use quantities may not fully reflect all uses in Canada.
Results of a section 71 industry survey for the year 2012 (Environment Canada 2014) indicated that none of the ten substances in the medium-chain phthalates subgroup were manufactured in Canada, and that five of the substances, CHIBP, BCHP, DMCHP, BIOP and DBzP, were not imported into Canada above the reporting threshold of 100 kg. Five substances, DCHP, DIHepP, DIBP, B79P and B84P, were imported. Import quantities were less than 10,000 kg for DCHP and DIHepP, between 10,000 and 100,000 kg for DIBP, and between 100,000 and 1,000,000 kg for each of B79P and B84P (Environment Canada 2014; see Table 4-1).
| Common name | Total manufacture (kg)Footnote Table 4-1[a] | Total imports (kg)a | Total exports (kg)a |
|---|---|---|---|
| DIBP | 0 | 10,000 - 100,000 | 0 |
| BCHP | 0 | 0 | 0 |
| CHIBP | 0 | 0 | 0 |
| DCHP | 0 | less than 10,000 | 0 |
| DBzP | 0 | 0 | 0 |
| DMCHP | 0 | 0 | 0 |
| DIHepP | 0 | less than 10 000 | 0 |
| BIOP | 0 | 0 | 0 |
| B79P | 0 | 100,000 - 1,000,000 | greater than 100,000 |
| B84P | 0 | 100,000 - 1,000,000 | greater than 100,000 |
Four of the medium-chain phthalates, DMCHP, B84P, DIHepP and DBzP, were previously included in phase 1 of the Domestic Substances List Inventory Update (DSL IU) initiative (Canada 2009), and their quantities in commerce were reported for 2008. Similar to the results of the section 71 industry survey for 2012 (Environment Canada 2014), DMCHP was not manufactured or imported in either 2008 or 2012. DIHepP was imported at higher quantities in 2008 in the range of 100,000 to 1,000,000 kg, compared to less than 10,000 kg in 2012. In 2008, less than 100,000 kg of DBzP was imported, and the substance was not imported above the reporting threshold of 100 kg in 2012. B84P was imported in the same range of 100,000 to 1,000,000 kg in 2008 as in 2012, and manufacturing below the reporting threshold of 100 kg was reported by three companies in 2008.
A summary of the combined production and use quantities for the medium-chain phthalates in the United States and the European Union is presented in Table 4-2. There have been no recent submissions under the United States Inventory Update Reporting (US EPA 2014a, b) or the European Union's Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Initiative (ECHA c2007-2014a) for CHIBP, DBzP, DMCHP and BIOP. For BCHP, a low-use quantity was reported in the United States in 2002 but not in 2006, and there were no submissions through REACH. This suggests a similar commercial status to that in Canada, where these five substances do not appear to be in commerce above the reporting threshold of 100 kg. Furthermore, CHIBP, DBzP and BIOP were not identified as high- or low-production-volume substances by the European Union Industry (ESIS 2014). DMPCHP, however, has been identified as a low-production-volume chemical in Europe (ESIS 2014).
In the United States, high quantities of DIHepP in production and use were reported both in 2002 and 2006, in the range of approximately 22 to 45 million kg (US EPA 2014ab). There were no submissions for DIHepP under REACH (ECHA c2007-2014a); however, this substance was previously identified as a high-production-volume chemical in Europe (ESIS 2014). Recent information indicates that DIHepP is no longer manufactured in North America and Europe (ECHA c2007-2014a; BASF Corporation 2011a; BASF Corporation 2011b).
Internationally, production and use have been reported for DIBP, DCHP, B79P and B84P in the United States and the European Union (US EPA 2014ab; ECHA c2007-2014a; refer to Table 4-2). In addition, these four substances have all been identified as high-production-volume chemicals in Europe (ESIS 2014).
| Common name | United States 2002 (kg)Footnote Table 4-2[a] |
United States 2006 (kg)a |
European Union (kg)Footnote Table 4-2[b] |
|---|---|---|---|
| DIBP | greater than 227,000 - 454,000 | 227,000 - less than 454,000 | 1,000,000 - 10,000,000 |
| BCHP | less than 5,000 - less than 227,000 | NS | NS |
| CHIBP | NS | NS | NS |
| DCHP | greater than 227,000 - 454,000 | less than 227,000 | 100,000 - 1,000,000 |
| DBzP | NS | NS | NS |
| DMCHP | NS | NS | NS |
| DIHepP | greater than 22,680,000 - 45,359,000 | 22,680 000 - less than 45,359,000 | NS |
| BIOP | NS | NS | NS |
| B79P | greater than 453,000 - less than 4,536,000 | greater than 453,000 - less than 4,536,000 | 10 000,000 - 100,000,000 |
| B84P | greater than 453,000 - less than 4,536,000 | greater than 453,000 - less than 4,536,000 | 1 000,000 - 10,000,000 |
5. Uses
The results of a section 71 industry survey (Environment Canada 2014) included information on the uses for DIBP, DCHP, DIHepP, B79P and B84P for 2012 (Environment Canada 2014).
Major uses identified are summarized in Table 5-1, based on responses to Section 8 of the industry survey (Environment Canada 2014). All five substances are used in adhesives and sealants that are used in construction and/or the automotive sector. Most of the substances are also used as plasticizers for applications in electrical and electronics, and children's toys. Import of B79P in raw material form for use in various applications was also reported (Environment Canada 2014).
| Major usesFootnote Table 5-1[a] | DIBP | DCHP | DIHepP | B79P | B84P |
|---|---|---|---|---|---|
| Adhesives and sealants | X | X | X | X | X |
| Paints and coatings | X | ||||
| Electrical/electronics | X | X | |||
| Automotive and transportation products | X | X | X | ||
| Printing inks | X | ||||
| Children's toys and articles | X | ||||
| Plastic and rubber materials | X |
None of the ten substances included in this SOS report are listed in the Drug Products Database, the Therapeutic Product Directorate's internal Non-Medicinal Ingredients Database, the Natural Health Products Ingredients Database or the Licensed Natural Health Products Database as medicinal or non-medicinal ingredients present in final pharmaceutical products, veterinary drugs or natural health products in Canada (DPD 2014; NHPID 2014; LNHPD 2014; September 2014 email from the Therapeutic Products Directorate, Health Canada, to the Risk Management Bureau, Health Canada, unreferenced).
With the exception of DIBP, none of phthalates in this report are identified to be present in food packaging materials or incidental additives. DIBP has been identified as a plasticizer in polypropylene films used to package all types of food (September 2014 email from the Food Directorate, Health Canada, to the Risk Management Bureau, Health Canada, unreferenced). However, DBzP and DCHP are present on the FDA's List of Indirect Additives Used in Food Contact Substances (US FDA 2014).
None of the ten phthalates are included on the List of Prohibited and Restricted Cosmetic Ingredients (more commonly referred to as the Cosmetic Ingredient Hotlist or simply the Hotlist), an administrative tool that Health Canada uses to communicate to manufacturers and others that certain substances, when present in a cosmetic, may contravene the general prohibition found in section 16 of the Food and Drugs Act or a provision of the Cosmetic Regulations (Health Canada 2007a). Based on notifications submitted under the Cosmetic Regulations to Health Canada none of the ten medium chain phthalates were notified to be present in (September 2014 email from the Consumer Product Safety Directorate (CPSD), Health Canada to Existing substances Risk Assessment Bureau (ESRAB), Health Canada).
A search of uses internationally was also conducted. No use information was identified for either CHIBP or BIOP, and while general uses were reported for BCHP, no specific product or article types were identified. Table 5-2 provides a summary of the general use information that was available for the remaining medium-chain phthalates in the subgroup.
| Major uses | Examples of uses | Substances |
|---|---|---|
| Automotive and transportation products | Caulks and sealants, glass insulation units, automotive paint, lacquers, varnishes, adhesive for automotive manufacture or repair, plastic articles, car mats, steering wheel covers | DIBP, DCHP, DIHepP, B79P, B84P |
| Coatings / adhesives / DIY products | Adhesives and binding agents; sealants, coatings, paints, thinners, paint removers, finger paints; acrylic coatings, lacquers and varnishes; fillers and filling agents, caulk, putties, plasters, modelling clay; process regulators, hardeners; polyvinyl acetate adhesives, polysulphide and castable polyurethane sealants, coatings and caulks; corrosion inhibitors; product used to detect surface flaws or cracks in vehicles parts, farm equipment, pipelines, non-metal surface treatment products, polishes, wax blends | DIBP, DCHP, DBzP, DIHepP, B79P, B84P |
| Inks / printing products | Screen printing inks; paper products, inks, toners; colouring agents; solvent for pressure-sensitive copying paper; plasticizer in printing inks; serigraphic printing paper/cardboard/paperboard; reprographic agents for publishing, printing and reproduction of recorded media | DCHP, DMCHP, DIHepP, B79P, B84P |
| Cables / wires / appliances / construction materials | Wire and cable insulation; electrical batteries and accumulators; vinyl flooring, tile and carpet backing; construction materials and specialized construction activities; artificial turfs, PVC with foam backing and cushioned PVC, PVC air mattresses | DIBP, DCHP, DMCHP, DIHepP, B79P, B84P |
| Clothing articles / furniture | Rubber and plastic articles such as belts, head phones, furniture, shoes, plastic sandals, balance balls | DIBP |
| Children's articles and toys | Nursing pillows, baby carriers, aprons for perambulators, baby mattresses, toys produced from foam plastic, erasers, plastic sandals and in childcare articles and children's toys, crayons | DIBP |
| Textiles | Urethane fabric coatings, fabrics, textiles, apparel; leather articles; textiles with decorative printings on the outer side of the fabric; textile dyes, and finishing and impregnating products, including bleaches and other processing aids; screen printing inks for textiles; plastisols in articles such as fabrics, textiles and apparel | DIBP, DCHP, B79P, B84P |
| Food packaging and processing | Food wrappers and labels; acrylic plastics when products are intended for food or drink contact; as a component in coated or uncoated food-contact surface of paper and paperboard used for all aspects of handling aqueous or fatty foods; as a component of adhesives for packaging in contact with food; in polymeric substances used in all aspects of food handling; in plastic film, foil, cellophane. | DIBP, DCHP, DBzP |
6. Releases to the Environment
The presence of the substances in the medium-chain phthalates subgroup in the environment is mainly from anthropogenic sources.
Based on the use information regarding the medium-chain phthalates gathered from the section 71 industry survey and the related follow-up (Environment Canada 2014), aquatic systems are thought to be the major recipient of phthalate releases. Medium-chain phthalates are expected to be released primarily to the aquatic medium through wastewater effluents from industrial sources and through disperse releases from consumer products. Nonetheless, the degree of release into water and other environmental compartments is uncertain or largely unknown. At industrial sites, washing of phthalate-containing floors and wall-coverings may result in environmental releases. The general transport of phthalates can result in releases through reconditioning of transport containers and trucks. Consumer products disposed to wastewater treatment systems are another potential source of release.
In some cases, these phthalates can be transported to off-site facilities for disposal, where releases are possible via effluents. Releases to air were reported in some cases (Environment Canada 2014). The medium-chain phthalates are not reported on the National Pollutant Release Inventory (NPRI) Substance List (NPRI 1995-).
Phthalates are not chemically bound to the polymer matrix and therefore can migrate from plastic products, including those disposed of in landfills. Landfills that do not collect and treat their leachate may potentially release substances to soil and groundwater via leachate.
7. Environmental Fate and Behaviour
7.1 Environmental distribution
A summary of the mass-fraction distribution for substances in the medium-chain phthalates grouping based on individual steady-state emissions to air, water and soil is provided in tables 7-1, 7-2 and 7-3 below. Due to the range of physical-chemical properties and molecular weights of the substances in the medium-chain phthalates grouping, a wide range of distributions resulted, particularly in the air and water release scenarios. Substances with similar mass-fraction distribution predictions have been grouped together. The groups generally align with the molecular weight of the phthalates. The results in tables 7-1 to 7-3 represent the net effect of chemical partitioning, inter-media transport and loss by both advection (out of the modelled region) and degradation/transformation processes. The results of Level III fugacity modelling suggest that medium-chain phthalates can be expected to distribute into any of the four environmental compartments (air, water, soil and sediment), depending on the physical-chemical properties of the phthalate and the compartment of release. Fugacity modelling results for individual substances in the grouping are presented in Appendix C.
| Substances released to | Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | 21-40 | 810 | 50-70 | negligible |
| Water (100%) | negligible | 93-98 | negligible | 2-7 |
| Soil (100%) | negligible | negligible | 100 | negligible |
| Substances released to | Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | 0-10 | 4-7 | 79-94 | 2-8 |
| Water (100%) | negligible | 47-70 | negligible | 30-53 |
| Soil (100%) | negligible | negligible | 100 | negligible |
| Substances released to | Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | negligible | 3 | 85 | 12 |
| Water (100%) | negligible | 18 | negligible | 83 |
| Soil (100%) | negligible | negligible | 100 | negligible |
When released to air, medium-chain phthalates exhibit a trend of increasing partitioning to solid matrices as hydrophobicity increases, with a corresponding trend in decreasing partitioning to air. These trends align with the physical-chemical properties of the phthalates and the increasing capacity to adsorb to organic carbon and decreasing volatility as molecular weight increases.
When released to water, the lower-molecular-weight medium-chain phthalates are predicted to remain primarily in water, with a small proportion distributing into sediment. Medium-chain phthalates with an intermediate molecular weight are predicted to distribute more evenly between water and sediment, while B84P is predicted to distribute mainly into sediment with a lesser proportion remaining in the water.
The moderate to very high hydrophobicity of the medium-chain phthalates also influences their movement through soil. The hydrophobic nature of these substances results in their adsorption to soil particulates, thereby substantially reducing soil mobility and delaying entry into groundwater and aquatic systems (CCME 1999). When medium-chain phthalates are released into soil, essentially all of the substance is predicted to remain within this environmental compartment.
The low Henry's Law constant values generated from models (Table 3-1) indicate that phthalates have little tendency to volatilize from water. In their trend analysis of Henry's Law constants for the phthalates, Cousins et al. (2003) noted that while lower-molecular-weight phthalates have fairly high vapour pressures and are therefore expected to volatilize readily in the pure state, their high solubility in water results in very low Henry's Law constants and therefore only slow volatilization from aqueous solution. For the higher-molecular-weight phthalates, water solubility has been observed to decrease more rapidly with increasing alkyl chain length than does vapour pressure (Staples et al. 1997; Cousins et al. 2003), leading to an apparent increase in the Henry's law constant. Therefore, higher-molecular-weight phthalates should evaporate more rapidly from water; however, this tendency is mitigated by an increase in sorption potential to suspended matter in the water column. The combined effects determine the overall distribution characteristics, although, in general, higher-molecular-weight phthalates volatilize only slowly from water (Cousins et al. 2003).
7.1.1 Long-range transport potential
Long-range transport (LRT) refers to the ability of a substance to be transported from its point of release in a mobile medium (usually air or water) over long distances. Following this movement, the substance can undergo a variety of fate processes, such as deposition from air into water and uptake in biota. Concentrations of DIBP have been measured far from any expected sources of release in sediment and biota along the eastern coast of Hudson's Bay (Morin 2003) and in air in the Norwegian Arctic (Xie et al. 2007). To investigate LRT as a potential explanation for these detected concentrations, the Transport and Persistence Level III Model (TaPL3 2000), developed by the Canadian Environmental Modelling Centre, and the OECD POPs Screening Tool, developed by the OECD Expert Group for Follow-up to the OECD/UNEP Workshop on Multimedia Models, were run for DIBP. Model inputs are available in Environment Canada (2015). The calculated Critical Travel Distance (CTD) in both models was very similar: 246 km and 269 km, respectively. This indicates that relatively little long-range atmospheric transport is expected.. This is consistent with what would be expected from the limited releases to air (described in section 6), the predicted partitioning (Table 7.1) and lack of persistence of DIBP (see section 7.2). Discussion of the concentrations of DIBP detected in the Arctic and possible explanations can be found in section 8.2.1. Some of the medium-chain phthalates have quite high log Koa (i.e., 12), suggesting that they could sorb to fine particles and be transported though air; however, given their low persistence and limited releases to air, long-range transport is not expected.
7.2 Environmental persistence
Studies addressing environmental persistence of substances in the medium-chain subgroup are not available for most of these substances. However, degradation of phthalate esters has been well characterized through studies focusing on a few phthalate ester substances with short, medium and long chain or cyclic side groups. Numerous studies have been conducted for these few substances. In general, these studies provide a relatively good understanding of biotic and, to a lesser extent, abiotic degradation pathways of phthalate esters, their typical behaviour in environmental media, and degradation rates. These studies can be used to characterize environmental persistence of the less studied substances, including those in the medium-chain phthalates subgroup. Biotic and abiotic degradation was best characterized for the analogue substances BBP and DEHP (Peterson and Staples 2003).
Phthalates can be degraded by abiotic and biotic processes. Abiotically, they undergo hydrolysis and photolysis, but these processes tend to be slow (Peterson and Staples 2003). It is the biodegradation by both micro-organisms and fungi in aerobic conditions, and less so, in anaerobic conditions, that contributes most to the breakdown of these substances in the environment. Studies have demonstrated that phthalates with shorter ester chains (such as BBP) can be readily biodegraded and mineralized, whereas phthalates with longer side chains (e.g., DEHP) tend to be less biodegradable (Liang et al. 2008). Moreover, the biodegradability differences among phthalates are attributed to the steric effects of the ester chains, where binding of hydrolytic enzymes is hindered, resulting in limited hydrolysis. Differences in phthalate isomers can also influence rates of degradation, as phthalate-hydrolyzing enzymes are structurally specific (Liang et al. 2008). In contrast, the degree of branching of the ester chains is thought to not play a significant role in limiting degradation (Ejlertsson et al. 1997). The medium-chain phthalates subgroup contains substances with diverse side chains of different lengths; therefore, their biodegradation rates are expected to be varied.
Empirical biodegradation data on persistence were available for three substances in the medium-chain phthalates subgroup, DIBP, DCHP and B79P. In contrast, such data and information on abiotic pathways were not found in the open literature and from unpublished sources for the rest of the phthalates in the medium-chain subgroup.
To characterize degradation potential for the data-poor medium-chain phthalates, data for the analogue substance BBP were used to inform BCHP, CHIBP and DBzP, and data for the analogue DEHP were used for DIHepP. Data for DBP were also used to fill data gaps for DIBP. Within the subgroup, data for DCHP were used to evaluate the properties of DMCHP, and data for B79P were used to evaluate BIOP and B84P. The medium-chain phthalates were found to be amenable to (Q)SAR model predictions. Therefore, hydrolysis rates, degradation by hydroxyl radicals, and primary and ultimate biodegradation were predicted using models EPI Suite 2012 (specifically HYDROWIN 2010, AOPWIN 2010 and the BIOWIN 2010 submodels) and CATALOGIC 2012.
Empirical biodegradation results for medium-chain phthalates and analogues are summarized in Table 7-2-2. Model results are summarized in tables 7-2-1, 7-2-3 and 7-2-4.
7.2.1 Abiotic degradation
Phthalate esters, including the medium-chain phthalates, tend to be relatively stable in the abiotic environment. Abiotic degradation processes, including hydrolysis and photolysis, occur very slowly and appear to be influenced by pH levels. Biodegradation studies that included controls in which organisms had been inactivated by sterilization indicate that losses of phthalate esters are limited to only a few percent of the initial concentration (Cheung et al. 2007; Kickham et al. 2012; Hashizume et al. 2002; Peng and Li 2012). The size and complexity of the phthalate ester side chains also impact rates of abiotic degradation, for example, DEHP degradation rates are observed to be much longer than those for BBP (Lertsirispon et al. 2009).
Hydrolysis rates of phthalate esters have been observed to decrease and the corresponding half-lives to increase with the length of the side chains (Staples et al. 1997), and to proceed at faster rates at higher pH levels (Wolfe et al. 1980). At pH 8, half-lives determined from second order kinetics varied from approximately months to a couple of years for a shorter-chain phthalate DBP, and 100 years for the medium-chain phthalate DEHP (Wolfe et al. 1980). Gledhill et al. (1980) estimated a hydrolysis half-life of greater than 100 days for BBP (an analogue to BCHP, CHIBP and DBzP). In 140-day tests at pH 5-9, degradation of DBP, BBP and DEHP by hydrolysis was found not to exceed 20%. DEHP did not hydrolyze at the neutral pH of 7 (Lertsirisopon et al. 2009). Modelled hydrolysis half-lives for the medium-chain phthalate subgroup ranged from 263 days for BIOP to 11.6 years for DMCHP at pH 7, and were considerably lower at pH 8, in the range of 26 days for BIOP to 1.1 years for DMCHP. Based on these observations, it can be concluded that hydrolysis is slow and unlikely to be an important fate process for phthalate esters under typical environmental conditions (Staples et al. 1997).
Photolysis is a more significant degradation pathway for phthalates, although it can also be a slow process in the aquatic environment at neutral pH (pH 7). Also, certain phthalates, such as DEHP, are less susceptible to photolysis. Exposure to sunlight conditions resulted in about 20% degradation of BBP and DBP over 140 days (Lertsirisopon et al. 2009). Photolysis was found to be considerably enhanced by acid- and alkali-catalyzed conditions (Lertsirisopon et al. 2009). Degradation half-lives of the phthalate esters DBP, BBP and DEHP were observed to decrease with pH and were fastest at the most extreme pH test conditions of 5 and 9, resulting in four- to eight-fold faster half-lives than those established at pH 7 (Lertsirisopon et al. 2009).
Degradation of medium-chain phthalates by hydroxyl radicals in air was investigated by using (Q)SAR models. Modelled half-lives ranged from 5.3 hours for DMCHP to 13.8 hours for DIBP (AOPWIN 2010), suggesting that when in air, these substances may be degraded relatively quickly by hydroxyl radicals.
(Q)SAR model predictions for atmospheric oxidation (AOPWIN 2010) and hydrolysis (HYDROWIN 2010) are summarized in Table 7-2-1 below. Degradation by ozone reaction could not be estimated for these substances.
| Substance name | Extrapolated half-life in air (hours) | Estimated hydrolysis half-life | Reference |
|---|---|---|---|
| DIBP | 13.8 | 194 days (pH 8); 5.3 years (pH 7) | AOPWIN 2010; HYDROWIN 2010 |
| BCHP | 7.6 | 193 days (pH 8); 5.3 years (pH 7) | AOPWIN 2010; HYDROWIN 2010 |
| CHIBP | 7.7 | 267 days (pH 8); 7.3 years (pH 7) | AOPWIN 2010; HYDROWIN 2010 |
| DCHP | 5.3 | 1.1 years (pH 8); 11.6 years (pH 7) | AOPWIN 2010; HYDROWIN 2010 |
| DMCHP | 5.3 | 1.1 years (pH 8); 11.6 years (pH 7) | AOPWIN 2010; HYDROWIN 2010 |
| DIHepP | 7.2 | 125 days (pH 8); 3.4 years (pH 7) | AOPWIN 2010; HYDROWIN 2010 |
| DBzP | 10 | 32 days (pH 8); 317 days (pH 7) | AOPWIN 2010; HYDROWIN 2010 |
| B84P | 7.4 | 57 days (pH 8); 1.5 years (pH 7) | AOPWIN 2010; HYDROWIN 2010 |
| BIOP | 8.8 | 26 days (pH 8); 263 days (pH 7) | AOPWIN 2010; HYDROWIN 2010 |
| B79P | 7.4 | 55 days (pH 8); 1.4 years (pH7) | AOPWIN 2010; HYDROWIN 2010 |
7.2.2 Biodegradation
Biodegradation is the main route through which phthalate esters break down in the environment. It has been demonstrated that medium-chain phthalate esters with shorter ester chains, such as DBP or BBP, can be more easily biodegraded and mineralized, whereas those with longer ester chains, such as DEHP, tend to be less susceptible to biodegradation (Wang et al. 2000; Chang et al. 2004). This is likely due to the steric effect of ester side groups that hinder the hydrolytic enzymes from binding, thereby inhibiting hydrolysis. Also, phthalate-hydrolyzing enzymes are known to be structure-specific, with unique abilities to degrade phthalate isomers (Gu et al. 2005).
Phthalate esters can be biodegraded by aerobic and facultative anaerobic bacteria. However, fewer strains that are capable of degrading anaerobically have been isolated (Chang et al. 2005). In addition to bacteria, a few fungi species (Ganji et al. 1995; Sivamurthy et al. 1991; Engelhardt et al. 1977; Kim and Lee 2005; Lee et al. 2007; Kim et al. 2002, 2003, 2007) and green microalgae species (Yan and Pan 2004; Yan et al. 2002) can also degrade phthalate esters.
Microbial mineralization of phthalic acid esters in the environment involves a sequence of reactions common to all phthalates (Hashizume et al. 2002; Staples et al. 1997; Yuan et al. 2002). This process requires diverse metabolic enzymes, such as esterases, dehydrogenases, decarboxylases and dioxygenases, and therefore a single organism is unlikely to be able to completely mineralize these complex organics (Staples et al. 1997; Liang et al. 2008). So far, only mixed cultures have been shown to completely mineralize phthalates (Chatterjee and Dutta 2008; Wang et al. 2004; Vega and Bastide 2003). Initially, the ester linkages between alkyl chains and the aromatic ring are hydrolyzed to form monoesters and then phthalic acid, while forming alcohols simultaneously (Amir et al. 2005). Secondary oxidation steps via the 3-oxoadipate pathway cleave the phthalic acid aromatic ring (Chatterjee and Karlovsky 2010). Biodegradation of phthalate esters may be preceded by a lag phase, and is thought to be related to the abundance of organisms with the specific ability to degrade phthalate isomers (Kleerebezem et al. 1999). Fungal degradation pathways of phthalates differ from bacterial degradation and are attributed to strong extracellular ligninolytic enzyme peroxidases and laccases (Kim et al. 2002; Liang et al. 2008). In addition, phytoplankton has also been shown to biodegrade phthalate esters, including DEHP, under sufficient nutrient and illumination conditions (Li et al. 2007).
The biodegradation of phthalate esters releases monoalkyl phthalate esters (MPEs) into the environment (McConnell 2007). Most studies suggest that biodegradation rates of MPEs proceed faster than those of phthalate esters (Peterson and Staples 2003). Moreover, studies on the biodegradation of short- and longer-chain MPEs, including mono-n-butyl-, mono-isobutyl-, mono-2-ethylhexyl phthalate-, mono-isononyl, mono-n-hexyl/n-octyl/n-decyl-, and mono-n-octyl/n-decyl- side chains, show that all these substances are readily biodegradable (Scholz 2003). The environmental fate of MPEs is largely unknown, but according to Peterson and Staples (2003), based on model evidence, MPEs partition more strongly to water than to solids in wastewater treatment systems given the differences in hydrophobicity. In sediments, MPE biodegradation rates are not affected by sorption because monoesters are largely ionized at environmental pH levels; sediment half-lives of various MPEs, including MEHP and MBP, were determined to be in the range of 0.34 to 2 days (Otton et al. 2008; Kickham et al. 2012). McConnell (2007) investigated the distribution of MPEs in a marine ecosystem, and levels of MPEs were detected in both water and sediment samples. It has been observed that MPEs degrade at a common rate, that is to say, the structure of the functional side group does not affect the rate of biodegradation.
At low concentrations, biodegradation rates of phthalate esters have been observed to be very slow, in other words, biodegradation occurs until levels fall to the order of parts per billion (ppb) and then biodegradation ceases, resulting in ubiquitous background levels of phthalates (Rubin et al. 1982; Boethling and Alexander 1979). This phenomenon has been attributed to the general inability of bacteria to produce metabolic enzymes at low concentrations (Peterson and Staples 2003) and to the characteristics of the bacteria capable of chemical biodegradation. This is true of the eutrophs, capable of growing at a high concentration of a chemical but with low capacity for its degradation at low concentrations, and the oligotrophs, which can degrade chemicals at low concentrations but are less specific to the target chemical (Rubin et al. 1982). Boethling and Alexander (1979) hypothesized that the energy obtained from oxidizing chemicals at low concentrations may be insufficient to meet the energy demands of the microorganisms, limiting the proliferation of the organisms to levels needed to cause appreciable loss of the chemical. Other explanations included the lack of bioavailability for biodegradation due to particle adsorption and, simply, contamination of laboratory equipment (Peterson and Staples 2003). Despite their inherent biodegradability, phthalate esters exhibit long half-lives in sediments due to the high degree of sorpion driven by their hydrophobicity (Kickham et al. 2012).
7.2.2.1 Inherent and ready biodegradation by sludge microorganisms
Most phthalate esters are biodegradable by micoorganisms, and first-order kinetics is frequently used to describe their biodegradation (Liang et al. 2008). Numerous inherent and ready biodegradation studies have been conducted using microorganisms found in activated sludge and, in some studies, pre-adapted sludge and varying concentrations of the test substances, to determine the biodegradation potential of phthalate esters in water.
In addition to published studies, the unpublished industry studies summarized for the EU Regulation on the Registration, Evaluation, Authorisation and Restriction of Chemical Substances (REACH) were considered; study summaries were available from ECHA (c2007-2014a).
| Common name | Fate process | Degradation value | Degradation endpoint/units | Test method; date | Reference |
|---|---|---|---|---|---|
| DIBP | Aerobic biodegradation 28 days (activated sludge) |
98 | % BOD | OECD Guideline 302C (Inherent Biodegradability: Modified MITI Test (II)); study report dated 2002 | ECHA c2007-2014b |
| DIBP | Aerobic biodegradation 28 days (non-adapted sludge) |
66-70 | % BOD | OECD Guideline 301D (Inherent Biodegradability: Closed Bottle Test); study report dated 2007 | ECHA c2007-2014b |
| DIBP | Aerobic biodegradation (activated sludge) 28 days |
98 | % BOD | OECD Guideline 301C (Ready Biodegradability: Modified MITI Test (I)); study report dated 2002 | ECHA c2007-2014b |
| DIBP | Aerobic biodegradation 14 or 28 days | 42 | % degradation measured as CO2 evolution | OECD Guideline 301B (Ready Biodegradability: CO2 Evolution Test); study report dated 2010 | ECHA c2007-2014b |
| DIBP | Aerobic biodegradation (activated sludge) 14 days; 28 days |
60-70 70-80 |
% CO2 evolution | OECD Guideline 301B (Ready Biodegradability: CO2 Evolution Test); report dated 2007 | ECHA c2007-2014b |
| DCHP | Aerobic biodegradation 28 days | 68.5; 91 |
% BOD; % degradation |
Not specified; study performed from 1976 to 1977 | ECHA c2007-2014c |
| B79P | Aerobic biodegradation 24 hours (activated sludge) |
57-81 | % degradation | OECD Guideline 302A (Inherent Biodegradability: Modified SCAS Test); 1981 | ECHA c2007-2014d |
| B79P | Aerobic biodegradation 28 days (pre-adapted activated sludge) |
83 | % degradation | ASTM shake flask procedure; 1979 | ECHA c2007-2014d |
| BBP (analogue to BCHP, CHIBP, DBzP) |
Aerobic biodegradation 14 days (activated sludge) |
81 | % BOD | OECD Guideline 301 C (Ready Biodegradability: Modified MITI Test (I)); 1992 | ECHA c2007-2014e |
| BBP (analogue to BCHP, CHIBP, DBzP) |
Aerobic biodegradation 27 days (activated sludge) |
93 | % CO2 evolution | Analytical Chemistry Method 71-42 (SCAS test); 1976 | ECHA c2007-2014e |
| BBP (analogue to BCHP, CHIBP, DBzP) |
Aerobic biodegradation 27 days (acclimated bacteria) |
96 | % CO2 evolution | Thompson-Duthie-Sturm procedure; 1976 | ECHA c2007-2014e |
| BBP (analogue to BCHP, CHIBP, DBzP) |
Aerobic biodegradation 2 days (activated sludge) |
50 | % degradation | River Die-Away Procedure; 1976 | ECHA c2007-2014e |
| BBP (analogue to BCHP, CHIBP, DBzP) |
Aerobic biodegradation (activated sludge) 24 hours; 48 days |
99; 93-99 |
% degradation | Primary biodegradation | Graham 1973; Saeger and Tucker 1976 |
| DIOP | Aerobic biodegradation (activated sludge) 24 hours; 4 days |
84.5; greater than 90 |
% degradation | Combined SCAS and activated sludge die-away procedure | O'Grady et al. 1985 |
| DEHP (analogue to DIHepP) |
Aerobic biodegradation 29 days (activated sludge from a region where DEHP is produced) |
82 | % CO2 evolution | OECD Guideline 301 B (Ready Biodegradability: CO2 Evolution Test); 1994 | ECHA c2007-2014f |
| DEHP (analogue to DIHepP) |
Aerobic biodegradation 28 days (activated sludge) |
4-5 | % CO2 evolution | OECD Guideline 301 B (Ready Biodegradability: CO2 Evolution Test); 1990 | Struijs and Stoltenkamp 1990; ECHA c2007-2014f |
| DEHP (analogue to DIHepP) |
Aerobic biodegradation 28 days (activated sludge from industrial source) |
60-70 | % BOD | EU Method C.5 (Degradation: Biochemical Oxygen Demand) from EG-guideline 79/831; 1984 | ECHA c2007-2014f |
| DEHP (analogue to DIHepP) |
Aerobic biodegradation 28 days (activated sludge) |
63 | % BOD | OECD Guideline 301 F (Ready Biodegradability: Manometric Respirometry Test); 1995 | ECHA c2007-2014f |
Data from biodegradation studies using activated sludge were available for DCHP, DIOP, DIBP, B79P, BBP and DEHP. Inconsistent results were reported in some cases, for example for DEHP. These differences in biodegradation rates can be attributed to differences in experimental protocols and concentrations of the test substance and the substrate.
For DCHP, a single biodegradation study was summarized on ECHA (c2007-2014c). The study was performed between August 1976 and January 1977. Degradation of 25 µg/L of DCHP was followed for 28 days. The percentage of biological oxygen demand (BOD) was measured as 68.5, and the substance was found to be 91% degraded, as measured by gas chromatography. Results indicated that under the test conditions, DCHP was readily degradable. Given the structural similarities between DCHP and DMCHP, it is expected that DMCHP is also readily degradable.
Several mineralization biodegradation studies for DIBP, conducted between 2002 and 2010 according to various OECD protocols, were summarized on ECHA (c2007-2014b). In three studies, biodegradation of DIBP was determined by percent BOD over 28 days. The test results ranged between 66 and 98% BOD, indicating that DIBP is readily biodegradable. Similar results suggesting ready biodegradation of DIBP were obtained in two studies that measured CO2 evolution as an indication of biodegradation. In the 2007 study, at least 60% biodegradation was observed in 14 days and up to 80% in 28 days, whereas the 2010 study indicated only 40% biodegradation of DIBP. The 2010 study summary is unclear with respect to the length of the study, as the final percent degradation is provided based on a 28-day duration. However, it is also indicated that the study was terminated after 14 days. This implies that 40% degradation occurred over 14 days, rather than 28 days. Overall, the majority of the available studies suggest that DIBP is readily biodegradable.
Two studies, a 24-hour study and a 28-day study, to determine biodegradation of B79P were summarized in ECHA (c2007-2014d). Study details were limited, and the results were provided only as percent degradation. B79P was found to be both inherently and readily biodegradable under the test conditions of both the 24-hour study and the 28-day study, respectively. The percent degradation reported was as high as 83% in 28 days. It is noted that in the 28-day study (ECHA c2007-2014d), pre-adapted activated sludge was used, whereas the condition of the activated sludge was not specified in the 24-hour study. Given that up to 80% biodegradation of B79P was observed in 24 hours, it is possible that pre-adapted sludge was also used in this study.
Three biodegradation studies for BBP, conducted in the 1970s and in 1992, were summarized on ECHA (c2007-2013e). The substance was observed to be readily biodegradable, based on percent BOD (81% BOD in 14 days) (ECHA c2007-2013e) and percent CO2 evolution (over 90% CO2 evolution in 27 days) (ECHA c2007-2013 e, f). In another study (ECHA c2007-2013e), also measuring percent CO2 evolution as an indication of biodegradation, it was found that BBP degraded by 50% in 2 days. Graham (1973) reported 99% biodegradation in 48 hours in activated sludge, with similar results (93-99% biodegradation in 24 hours) also reported by Saeger and Tucker (1976).
Several biodegradation studies were also available for DEHP (ECHA c2007-2014f). Two ready CO2 evolution biodegradation studies, conducted according to the same protocol (i.e., OCED 301B) in the 1990s, showed contradictory results. In one study from 1992, DEHP was shown to be readily biodegradable in 28 days, with observed 82% CO2 evolution (ECHA c2007-2014f). In contrast, in the 1990 study, only 4-5% CO2 evolution was observed after 28 days (Struijs and Stoltenkamp 1990; ECHA c2007-2014f). Both studies used activated sludge, but in the 1994 study, sludge from a sewage treatment plant located in a region with major producers of DEHP was used. Therefore, it is possible that microorganisms used in the 1994 study may have been pre-adapted to DEHP from local manufacturing activities. In another earlier study from 1984, using sludge from an industrial source, also likely pre-adapted to DEHP, between 60 and 70% BOD was observed after 28 days (ECHA c2007-2014f). Ready biodegradation, measured as 63% BOD, was observed in a 1994 manometric respirometry study (ECHA c2007-2013l). In this study, an eight-day lag-phase that preceded biodegradation of DEHP was reported. Rapid primary biodegradation of DEHP in activated sludge was reported by Graham (1973) and O'Grady et al. (1985), were 91% biodegradation in 48 hours and 81.5% biodegradation in 24 hours were observed in each study, respectively, and over 90% biodegradation was achieved between 2 and 5 days (O'Grady et al.1985). O'Grady (1995) also reported rapid primary biodegradation for DIOP, where nearly 85% of the substance was found to biodegrade in 24 hours and over 90% in 4 days.
In summary, medium-chain phthalates are readily biodegradable by microorganisms found in activated sludge. Rates of biodegradation for DCHP, DIOP, DIBP, B79P, BBP and DEHP were generally rapid, but some variability was observed, likely due to non-standard experimental conditions. For example, the use of pre-adapted sludge may have accelerated or aided biodegradation of B79P. However, these results are similar to the majority of degradation values observed for other medium-chain phthalates, where activated sludge was used as substrate rather that pre-adapted sludge. Given that the medium-chain phthalates are generally structurally similar, they are considered both inherently and readily degradable.
Modelled primary and ready biodegradation
(Q)SAR models EPI Suite 2012 and CATALOGIC 2012 were also used to evaluate inherent and ultimate biodegradation potential of the medium-chain phthalates. SMILES and, when available, physical chemical properties, such as water solubility and log Kow, were used as model inputs. Medium-chain phthalates were found to be amenable to model predictions, with the exception of two substances, B84P and BIOP, determined to be outside of the model domain of the model CATALOGIC (2012). However, since the CATALOGIC (2012) results for B84P and BIOP were consistent with the results generated for all substances, they were considered to be acceptable and were included in the assessment.
The (Q)SAR-determined biodegradation rates for substances in the medium-chain subgroup were relatively rapid. Results for DIBP, BCHP, CHIBP, DCHP, DMCHP, DIHepP and BIOP based on BIOWIN submodels 3, 4, 5 and 6 universally showed fast biodegradation rates; primary biodegradation based on submodel 4 was estimated to be in the range of days to weeks, and the ultimate degradation half-lives calculated based on CATALOGIC (2012) were between 11 to 37 days. It is noted that the calculated half-life for DIHepP was the longest among the medium-chain phthalates, which was expected for a phthalate ester of a larger molecular size. However, its nearly linear side chains may be amenable to fast biodegradation as suggested by BIOWIN submodels. Model results are summarized in Table 7-2-3.
Slightly slower biodegradation rates were determined for DBzP, B84P and B79P. These results are consistent with the observation that phthalates with longer and more complex ester side groups are less biodegradable. B84P is the largest phthalate in the medium-chain phthalate subgroup. The primary biodegradation for DBzP, B84P and B79P was determined to be in the order of weeks, whereas results from the ultimate biodegradation models varied, with BIOWIN submodel 3 suggesting biodegradation rates in the order of weeks to months, and BIOWIN submodel 6 indicating that these substances were not readily biodegradable. Half-lives calculated based on CATALOGIC (2012) results ranged from 14 to 21 days. Model results are summarized in Table 7-2-4.
Overall, the model results indicate that substances in the medium-chain phthalate subgroup are readily biodegradable and are in agreement with the experimental biodegradation data, including that for the analogue phthalate esters BBP and DEHP. Based on the model results, the following ranking for rates of biodegradation of the medium-chain phthalates is proposed:
DIBP greater than BCHP, CHIBP, DBzP, DCHP, DMCHP greater than B79P, BIOP, B84P greater than DIHepP.
DIBP, the smallest and simplest phthalate in the medium-chain subgroup, is expected to be most easily degradable. DIHepP is expected to be the least degradable, which is consistent with the experimental data available for its analogue substance DEHP showing lowest biodegradation rates.
| Fate process | Degradation endpoint or prediction | Test method or model basis | Model-assigned or calculated half-life (t1/2 = days) | Reference |
|---|---|---|---|---|
| Primary aerobic degradation | 3.1-4.1 (biodegrades fast; "days-weeks") |
Submodel 4: Expert Survey (qualitative) | n/a | BIOWIN 2010 |
| Ultimate aerobic degradation | 2.7-3.1Footnote Table 7-2-3[a] (biodegrades fast; "weeks to months") |
Submodel 3: Expert Survey (qualitative) | n/a | BIOWIN 2010 |
| Ultimate aerobic degradation | 0.5-0.7Footnote Table 7-2-3[b] (biodegrades fast) |
Submodel 5: MITI linear probability | n/a | BIOWIN 2010 |
| Ultimate aerobic degradation | 0.3-0.8b (biodegrades fast) |
Submodel 6: MITI non-linear probability | n/a | BIOWIN 2010 |
| Ultimate aerobic degradation | 40-80 (biodegrades fast) |
% BOD | 11-37Footnote Table 7-2-3[c] | CATALOGIC 2012 |
| Fate process | Degradation endpoint or prediction | Test method or model basis | Model-assigned or calculated half-life (t1/2 = days) | Reference |
|---|---|---|---|---|
| Primary aerobic degradation | 3.7-3.8 (biodegrades fast, "weeks") |
Submodel 4: Expert Survey (qualitative) | n/a | BIOWIN 2010 |
| Ultimate aerobic degradation | 2.4-2.7Footnote Table 7-2-4[a] (biodegrades fast, "months") |
Submodel 3: Expert Survey (qualitative) | n/a | BIOWIN 2010 |
| Ultimate aerobic degradation | 0.3-0.6Footnote Table 7-2-4[b] (biodegrades fast) |
Submodel 5: MITI linear probability | n/a | BIOWIN 2010 |
| Ultimate aerobic degradation | 0.1-0.2b (biodegrades slowly) |
Submodel 6: MITI non-linear probability | n/a | BIOWIN 2010 |
| Ultimate aerobic degradation | 50-70 (biodegrades fast) |
% BOD | t1/2 = 14 - 21Footnote Table 7-2-4[c] | CATALOGIC 2012 |
7.2.2.2 Biodegradation in surface waters and sediments
Phthalate esters have been detected in freshwater worldwide and tend to adsorb to sediments (Chang et al. 2005). Most phthalate esters are readily biodegradable in surface waters (Furtmann 1994). Both aerobic and anaerobic microorganisms found in sediments can degrade phthalate esters (Hashizume et al. 2002; Chang et al. 2004; Kim et al. 2008), although they are not as abundant as isolates from activated sludge or soil. Abiotic biodegradation rates for phthalate esters with longer side chains tend to be slow (Lertsirisopon et al. 2006).
Furtmann (1994) reported that DIBP and DCHP in samples of Rhine, Ruhr and Emscher river water from Germany were subject to rapid primary degradation at 20°C. At 4°C, the degradation was delayed by as much as 3 to 4 days. The addition of sodium azide (bacteriostatic poison of cytochrome oxidase in mitochondria) stopped the degradation processes. Hashizume et al. (2002) observed complete biodegradation of DIBP over 7 days in river water samples collected from urban Japan.
Both phthalate esters and their primary metabolites, monoalkyl phthalate esters, have the capacity to be quickly degraded by microbes in sediments. However, their biodegradation rates decrease with increasing sorption, and this can result in long sediment half-lives for those phthalates with higher Koc values (Kickham et al. 2012).
Monoalkyl phthalate esters were shown to be quickly degraded in natural sediments (Otton et al. 2008). In this study, biodegradation rates measured at 22ºC did not differ between marine and freshwater sediments, and no apparent relationship between the degradation half-life and the length or extent of branching of the monoester alkyl-chain was observed. Half-lives of eight monoalkyl esters tested (characterized by ethyl-, butyl-; benzyl-; iso-hexyl-; ethylhexyl-; n-octyl; iso-nonyl-; iso-decyl- alkyl side chain) ranged from 16 to 39 hours at 22ºC in freshwater and marine sediments, and were somewhat longer, up to 200 hours at 5ºC in freshwater sediments (Otton et al. 2008). Lower temperatures cause a lower microbial activity, thereby limiting biodegradation.
Yuan et al. (2002) studied biodegradation of eight phthalates, including DEHP, in river sediment in Taiwan. Average aerobic half-lives ranged from 2.5 to 14.8 days, with anaerobic half-lives being longer, in the range of 14.4 days to 34.7 days. Yuwatini et al. (2006) estimated the biodegradation half-life of DEHP in sediment to be about 14 days.
Anaerobic biodegradation in sediments of DBP (similar to DIBP), BBP and DEHP was studied by Lertsirisopon et al. (2006). DBP and BBP were degraded in a few days (average half-lives for DBP and BBP were 1.4 and 1.8 days, respectively) and were preceded by short lag phases (up to 0.7 days for DBP and 1.4 days for BBP). DEHP, characterized by longer alkyl chains, was degraded with a 5-day lag phase and an average half-life of 252 days. Kickham et al. (2012) determined sediment half-lives for BBP and for DEHP and DINP to be 2.9 and 347 days, respectively.
7.2.2.3 Biodegradation in soils
The pathways and rates of biodegradation for phthalate esters in different types of soils were studied. Soil biodegradation was identified for DIBP (Ferreira and Morita 2012), but not for the remaining phthalates in the medium-chain subgroup. In contrast, numerous data were available for the analogue substance DEHP (Gejlsberg et al. 2001; Scheunert et al. 1987; Wang et al. 2009; Schmitzer et al. 1988; Madsen et al. 1999). DEHP is considered to be a good analogue to DIHepP. However, the findings from the numerous studies on DEHP can also be applied generally to the medium-chain phthalate subgroup. DEHP tends to be most slowly biodegraded of the medium-chain phthalates and, therefore, biodegradation rates for the substances in the medium-chain phthalates subgroup are expected to not exceed or to be equal to those of DEHP.
The patterns for biodegradation rates of phthalate esters in soil are very similar to those in water (Peterson and Staples 2003). Both the length and the branching of the ester side group affect biodegradation rates. Short and linear phthalates tend to be degraded faster, whereas slower biodegradation rates were observed for phthalates with longer and branched side groups (Zeng et al. 2004). Increased temperature appears to speed up soil biodegradation rates (Rüdel et al. 1993; Chen et al. 1997; Chang et al. 2009). Madsen et al. (1999) noted that doubling temperature resulted in the doubling of the degradation rate for DEHP. Soil moisture also affects the degradation rates of phthalate esters, where higher soil moisture contributes to greater rates of biodegradation and mineralization (Peterson and Staples 2003). Finally, aerobic conditions are expected to result in faster biodegradation rates in soil. For DEHP, slower biodegradation rates were observed in anaerobic conditions (Madsen et al. 1999; Sheunert et al. 1987).
Biodegradation of DIBP in bioremediated soil from an industrial site in São Paulo, Brazil, was investigated by Ferreira and Morita (2012). Using a first-order kinetics equation (half-life = ln2/k) and the degradation rate constant (k) of 0.01 provided in Ferreira and Morita (2012), the half-life of DIBP can be calculated as 69.3 days. Reported half-lives (calculated using first order kinetics) for DEHP in different types of soil range from 2 days in loam soil to 69.3 days in sand (Rüdel et al. 1993; Shanker et al. 1985; Roslev et al. 1998; Peterson and Staples 2003) and up to 77 days in bioremediated soil from the industrial site in Brazil (Ferreira and Morita 2012).
The mineralization of DEHP in soil starts with a lag phase (observed in some studies) and then follows two distinct kinetic phases: the initial phase over approximately 30 days that follows first-order kinetics, followed by a slower late phase (Dörfler et al. 1996; Peterson and Staples 2003). The phenomenon known as "sequestration", whereby a hydrophobic chemical penetrates into solid particles and is no longer surface-adsorbed and available to extraction solvents, was put forward by Peterson and Staples (2003) as a possible explanation for the slow degradation phase of DEHP. The DEHP half-life for the initial phase was calculated as 58 days, and 147 days for the late phase (Dörfler et al. 1996). Similarly, in a soil study by Wang et al. (2009), there was a lag phase of approximately 5 days for degradation of DEHP at all concentrations tested (ranging from 10 to 1000 mg/kg), followed by rapid degradation in the first 30 days to as low as 10% of the initial concentration at the lowest test concentration of 10 mg/kg. In this study, degradation past 30 days remained stable with no further appreciable decreases in concentration until the end of experiments at 55 days. The extent of DEHP biodegradation was shown to be dependent on the initial substance concentration, as at 55 days DEHP biodegraded to less than 10% in the lowest 10 mg/kg treatment, and to over 20 to 35% in the 50 to 1000 mg/kg treatments, respectively. The dependence of DEHP biodegradation rates on the initial concentrations was also shown by Fairbanks et al. (1985), where half-lives were two- to four-fold longer at higher concentrations, and by Dörfler et al. (1996), where higher initial substance concentrations resulted in slower biodegradation.
Environmental conditions, such as temperature, soil moisture and oxygen levels, as well as initial substance concentration and the type of soil all have an impact on the biodegradation rate of phthalate esters. Biodegradation of phthalate esters is also known to be affected by the length and complexity of the ester side groups. Biodegradation of DEHP seems to proceeds in two phases, a rapid biodegradation phase and a slow phase, which can be preceded by a lag phase. This may or may not be the case for the substances in the medium-chain phthalate subgroup, in particular the initial lag phase, since similar degradation studies have not been conducted for these substances. It is considered, however, that the soil half-lives determined for DEHP, ranging from 2 to up to approximately 77 days, can be representative of the medium-chain phthalate grouping. The soil half-life for DIBP was determined to be 69.3 days.
7.3 Potential for bioaccumulation
The discussion on the potential for bioaccumulation considers several potential parameters, including the substance properties (i.e., log Kow, log Koa), bioconcentration factor (BCF), biomagnification factor (BMF), food web magnification factor (FWMF) and bioaccumulation factor (BAF). The role of metabolism in determining bioaccumulation potential is also examined.
Empirical bioaccumulation data were available for two substances in the medium-chain phthalate subgroup, DIBP and DIHepP. Data-rich BBP will be used as an analogue for BCHP, CHIBP and DBzP, and DEHP will be used as an analogue for DIHepP where data on the target compounds are not available (i.e., BCF). Refer to Section 2.1 for the analogue selection rationale. Modelling data are used to inform the bioaccumulation evaluation of the remaining medium-chain phthalates.
The log Kow values for the medium-chain phthalates range from 4.11 to 6.92, suggesting that they can partition to biota from water and other media. Results from the laboratory studies discussed below indicate that other factors come into play that influence the bioaccumulation of phthalates, such as metabolism.
7.3.1 Bioconcentration factor (BCF)
Bioconcentration data are only available for the analogue substances BBP and DEHP, which are being used as read-across for BCHP, CHIBP, DBzP and DIHepP (refer to Section 2.1 for analogue selection rationale). Studies show that BCFs range from 188 to 1890, with a median value of 663, using calculations based on total and dissolved concentrations (Table D-1 in Appendix D).
Ratzlaff (2004) performed bioconcentration studies on several phthalates to determine how BCF values are affected when different analytical methods are used to measure phthalate concentrations in water. With their moderate to high Kow, it is expected that some of the medium-chain phthalates would be associated with organic matter, thereby reducing their bioavailability. Measured water concentrations that include the fraction sorbed to organic matter would be expected to overestimate the amount of dissolved phthalates in the system and underestimate the BCF. Measuring the freely dissolved concentrations would include only this bioavailable fraction that can be absorbed via the respiratory surface of fish and, therefore, bioconcentrated. Ratzlaff (2004) exposed rainbow trout to BBP for 61 days, and BCFs were calculated based on both the total water concentrations (including fraction associated with organic matter) and the operationally defined freely dissolved water concentrations. As some phthalates were sorbed to small-diameter (less than 0.45 µm) particulate matter and were still measured in the operationally defined freely dissolved concentration, a model was also used to predict the freely dissolved concentrations based on the three-phase sorption model using the Kow, Koc, organic carbon content, concentration of small suspended matter and degree of chemical disequilibrium between the small-diameter suspended matter and the water. Generally, BCFs increased over the duration of the uptake period and then reached a maximum value by day 21, indicating that steady state was reached. As expected, the freely dissolved water concentrations of BBP were lower than total water concentrations. The corresponding BCFs using the operational freely dissolved water concentration were higher (BCF 1890) than those calculated using the total water concentration (BCF 918). The BCF based on the predicted freely dissolved concentration was much higher, with a predicted value of 11500.
Carr et al. (1997) exposed bluegill sunfish to 0.034 mg/L of BBP over 3 days and measured BBP in the fish. An exposure duration of 3 days was selected based on a previous study (Monsanto, unpublished) that had shown that equilibrium in bluegill tissues was reached after 3 days (Carr et al. 1997). This is significantly less than the 21 days identified by Ratzlaff (2004) required to achieve steady state in rainbow trout. Experiments that use short exposure times have a tendency to underestimate the BCF, since steady state conditions may not be achieved (Staples 2003). In fact, by measuring BBP in bluegill sunfish tissues, Carr et al. (1997) calculated a BCFwhole fish of 9.4, which is lower than the BCF of 918 obtained by Ratzlaff with the longer exposure, based on total BBP concentration. Carr et al. (1997) also calculated the BCF using radiolabelled BBP and obtained a BBP equivalent BCFwhole fish of 194, which is in line with results from earlier tests that measured total radioactivity and would have included metabolites in the measurement.
Studies that measure C-14 labelled BBP are available. However, it has been noted by Carr et al. (1997) that these calculations overestimate bioconcentration, since metabolites cannot be distinguished from the parent substance. An unpublished BCF study is summarized in detail in the ECHA database (ECHA c2007-2013), which involved a flow-through test using radiolabelled BBP at exposure concentrations of 0.002 mg/L over 21 days. It was found that BBP has a low potential to bioconcentrate in bluegill sunfish, with a BCF of 188 for the whole body. Concentrations were also measured in the muscle and viscera, and were found to concentrate more in the viscera than the muscle (BCF values of 1693 compared to 29).
Barrows et al. (1980) exposed bluegill sunfish to C-14 labelled BBP in flow-through conditions. Fish and water samples were collected throughout the test for 21 days, when equilibrium was reached. With an average water concentration of 0.0097 mg/L, a whole body BCF of 663 was calculated. Following the exposure phase, the fish were placed in a clean aquarium so that depuration could be monitored.
The empirical bioconcentration data indicate that BBP is quickly metabolized by fish. Carr et al. (1997) compared intact BBP measurements to radioactivity in tissue, and calculated that more than 90% of the radiolabelled compounds in the fish tissue were metabolites of BBP. The reduction of intact BBP in fish tissue was determined to be influenced by metabolism of BBP and the exposure of BBP degradation products directly from water and subsequent metabolism, as the concentration of BBP in solution was observed to decrease over the 3-day exposure period.
Ratzlaff (2004) measured the BBP monoester metabolite monobenzyl phthalate in fish that were exposed to BBP; however, it was not detected. It was noted that the MDL was quite high (31.3 ng/g) due to the relative insensitivity of the new analytical technique.
McConnell (2007) measured phthalate ester metabolites in biota in False Creek Harbour, British Columbia, in 2005. The metabolite of DIHepP, monoheptyl phthalate (referred to as MC7P), was detected in samples of blue mussel, softshell Cclam, dungeness crab and juvenile shiner perch in concentrations ranging from 6.9 to 350 ng/g lipid equivalent, but was not detected in the higher trophic level white spotted greenling or spiny dogfish (where the MDL ranged from 0.018 to 41 ng/g wet weight [ww]). In most cases, the DIHepP concentration was greater than the metabolite concentration. The metabolite of the analogue BBP, monobenzyl phthalate (MBzP), was found only in blue mussel and juvenile Shiner Perch, and BBP was detected in higher concentrations than MBzP.
A study by Mayer (1976) calculated BCFs for DEHP using both C-14 labelled DEHP and concentrations of the parent compound measured by gas chromatography. Reported BCF values in the fathead minnow based on total reactivity ranged from 155 to 886 over a 56-day exposure period, while the BCF values based on actual DEHP concentrations ranged from 91 to 569. Fish were exposed to concentrations of C-14 labelled DEHP ranging from 0.0019 to 0.062 mg/L, the lower concentrations being within the range of solubility for DEHP (0.003 mg/L). The BCFs were found to decrease with increasing exposure concentrations, and the authors suggest that this may be due to the induction of detoxification enzymes in the liver and an increase in the DEHP degradation and elimination. These values suggest that DIHepP would also have a low potential for bioaccumulation.
BCF estimates were generated using a modified three-trophic-level version of the Arnot-Gobas mass balance model (Arnot and Gobas 2003) for a middle-trophic-evel fish weighing 184 g. The modelled data are considered to be reliable, as the medium-chain phthalates fall within the parametric and metabolism domains of the model. Table D-2 in Appendix D lists the BCF predictions for each of the medium-chain phthalates, which range from 29 to 237 L/kg ww and include biotransformation rate estimates (kM). The experimental BCF data for BBP, the analogue for BCHP, CHIBP and DBzP, ranges from 9.4 to 1890. The BCFBAF models predict a BCF in the range of 17 to 112 L/kg for these substances, indicating that the kM in the model is perhaps overestimated at 3.4, 3.8 and 25. The metabolic biotransformation rate constant database from Arnot et al. (2008a) contains kMs for higher-molecular-weight phthalates, with values for diisoheptyl phthalate of 1.17/day and diisononyl phthalate of 3.52/day, which suggest that particularly the kMs of 25 for DBzP and B84P are overestimated. Using the kM of 3.52/day generates a BCF estimate of 96 L/kg and 45 L/kg for DBzP and B84P, respectively (Arnot et al. 2008b). Nevertheless, the model and experimental bioconcentration data and measurement of metabolites in aquatic organisms suggest that the medium-chain phthalates are subject to metabolism and thus do not tend to significantly bioconcentrate.
7.3.2 Bioaccumulation factor (BAF)
Bioaccumulation factors are measured under field conditions as the ratio of the whole body burden of chemical taken up from all exposures to that of the ambient water concentrations. Measures of BAF are a preferred metric for assessing the bioaccumulation potential of substances because it incorporates all chemical exposures, including diet, which predominates for substances with log Kow greater than ~4.0 (Arnot and Gobas 2003). BAF studies were not found in the published literature for the medium-chain phthalates; however, a thesis by Mackintosh (2002) calculated BAFs for DIBP, DIHepP, DEHP and BBP, among other phthalates, in 18 organisms in a marine food web. The mean BAF values for DIBP, DIHepP, DEHP and BBP in green algae, sculpin and dogfish muscle can be found in Table D-3, in Appendix D, and are expected to represent three levels of the False Creek food web (1st, 3rd and 4th trophic levels, respectively). Ranges and medians obtained by Mackintosh (2002) are presented in Table 7-4.
| Substance | Endpoint | Range of values (L/kg) | Median (L/kg) | Reference |
|---|---|---|---|---|
| DIBP | BAF, wwFootnote Table 7-4[a] | 34-776 | 143 | Mackintosh 2002 |
| DIHepP | BAF, wwa | 12-3236 | 125 | Mackintosh 2002 |
| BBP | BAF, wwa | 186-8709 | 717 | Mackintosh 2002 |
| DEHP | BAF, wwa | 4.9-1097 | 38 | Mackintosh 2002 |
Mean BAF values calculated by Mackintosh (2002) indicate that medium-chain phthalates have a low potential for bioaccumulation, with most BAFs reported below 1000 and almost all BAFs below 3000. An exception was the BAF of 8709 L/kg that was calculated for BBP in the surf scoter, based on liver sample analysis only. The location of the samples and differences in foraging area may also contribute to these differences in BAF, as the surf scoter occupies a larger foraging area and is more mobile than the other organisms in the study.
The steady-state middle-trophic-level bioaccumulation model published by Arnot and Gobas (2003) predicts BAFs for the medium-chain phthalates in the range of 1.48 to 2.6 L/kg ww using the same kMs as the BCF predictions and a dietary uptake efficiency of 1% for each phthalate to account for gut metabolism (Table D-2 in Appendix D). Studies with DBP, BBP and DEHP in staghorn sculpin have demonstrated that these substances are very effectively transformed in the gut with no significant accumulation from dietary exposure, indicating very low (less than 0.01) assimilation efficiencies (Webster et al. 2003). All BCF and BAF predictions are below 1000, suggesting that the medium-chain phthalates have a low potential to bioaccumulate. Experimental BAF values in sculpin for DIBP and BBP of 78 and 631 L/kg are higher than the modelled BAF values, with predictions of 34.31 for DIBP and 117, 104 and 17 for BCHP, CHIBP and DBzP, respectively. Differences could be due to the assumptions used in the model.
7.3.3 Biota-sediment accumulation factor (BSAF)
BSAF is a parameter describing bioaccumulation of sediment-associated compounds into tissues of ecological receptors (Burkhard 2009). Because of the different sorptive capacities of lipid and organic carbon, equilibrium is represented by a value of three, as the sorptive capacity of organic carbon is 0.35 times that of octanol (lipid). A BSAF greater than three is therefore an indication of more chemical in biota compared to sediment (Morin 2003). Alternatively, ASTM (1997) recommends a "cut-off" value of 1.7 to represent equilibrium conditions. BSAFs that exceed approximately 1.7 to 3 (on a normalized basis) indicate more uptake than can be explained by partitioning theory alone (bioaccumulation is occurring).
In a review of the bioaccumulation of phthalate esters in aquatic food webs, Gobas et al. (2003) describe a disequilibrium that occurs between sediment pore water and overlying water for all phthalate esters, to varying degrees. They found that sediment pore water concentrations were higher than overlying water concentrations, which would result in a higher degree of direct exposure to a sediment-burying invertebrate than to epibenthic organisms that inhabit the epilimnion. Mackintosh (2002) calculated the BSAF values of phthalate esters in 18 organisms in the marine food web by dividing the mean lipid normalized biota concentration by the mean organic carbon normalized sediment concentration. Calculated BSAFs for DIBP, DIHepP, BBP and DEHP were below 1.7 (values can be found in Table D-3 in Appendix D), even for sediment-burying invertebrates like the Geoduck Clam and the Manila Clam.
In a study on the distribution of phthalate esters (including DIBP and BBP) in mammals, fish, sediment and air in Hudson's Bay, Morin (2003) calculated BSAF values of phthalate esters in the beluga whale and Arctic Cod. Sediment is considered a source of dietary exposure for the beluga whale, as it uses suction while scavenging for benthic organisms and could ingest sediment (Morin 2003). In Arctic cod, BSAF values are reported as 2.75 and 3.45 kg OC/kg lipid, for DIBP and BBP, respectively. Beluga whale BSAFs were 4.19 and 3.71 kg OC/kg lipid for DIBP and BBP, respectively (Morin 2003). Beluga whale BSAFs are similar to Arctic cod BSAFs and are close to unity, suggesting that sediment is not a major source of DIBP for the beluga whale. These BSAF values are higher than those measured in False Creek Harbour by Mackintosh (2002). Morin (2003) measured higher biota concentrations and lower sediment concentrations of DIBP in the Arctic. A discussion on possible explanations for higher concentrations in biota in the Arctic can be found in Section 8.2.1.5.
7.3.4 Biomagnification
Biomagnification describes the process in which the concentration of a chemical in an organism reaches a level that is higher than that in the organism's diet due to dietary absorption (Gobas and Morrison 2000). A biomagnification factor (BMF) greater than 1 indicates that biomagnification is potentially occurring. Food web magnification factors (FWMFs) are another measure of the degree of biomagnification in the food web, representing the average increase in lipid-equivalent chemical concentration for a unit increase in trophic position (Mackintosh et al. 2004). An FWMF greater than 1 indicates chemical biomagnification in the food web, while an FWMF less than 1 indicates trophic dilution. Few studies measured biomagnification of medium-chain phthalates and the analogue BBP.
Morin (2003) examined DIBP and BBP in adult beluga whale and Arctic cod tissues, and calculated lipid-equivalent biomagnification factors of 1.52 (cod to beluga; DIBP) and 1.07 (cod to beluga; BBP). These values suggest limited ability of these phthalates to biomagnify, as the values are close to unity (Morin 2003).
Field studies were conducted to determine the occurrence of biomagnification or trophic dilution for a variety of phthalate esters, including DIBP, BBP and DIHepP (identified as di-iso-heptyl, and referred to as C7 in the studies) (Mackintosh et al. 2004; McConnell 2007). The studies calculated FWMFs in a marine food web in False Creek Harbour, in British Columbia. Samples were taken from four trophic levels, and FWMFs were calculated using the phthalate concentrations in biota and the ratio of 15N/14N, which has been shown to increase with trophic level (Mackintosh et al. 2004). Mackintosh et al. (2004) and McConnell (2007) calculated FWMFs for DIBP, BBP and DIHepP in the range of 0.38 to 0.94, indicating that biomagnification is not taking place (see tables D-4 and D-5 in Appendix D for all values). DIHepP was not detected in the muscle of the top predator, dogfish, which may reduce the power to detect statistically significant trends in the food web. Lipid-equivalent concentrations of DIBP, BBP and DIHepP appeared to decline slightly with increasing trophic position in the food web; however, the correlation was not statistically significant, suggesting that a small amount of trophic dilution may take place (Mackintosh et al. 2004).
McConnell (2007) also calculated FWMFs for the metabolite of DIHepP, MC7P, which was found to be 0.22 (McConnell 2007). It would be expected that the metabolites of the medium-chain phthalates would not biomagnify as they are more water soluble and more rapidly metabolized in the organisms.
The available biomagnification data are limited to just a few of the medium-chain phthalates and suggest that dietary exposure may not significantly contribute to trophic transfer and food web accumulation in the environment. This is consistent with the low to moderate BCF/BAF calculations and the observation that the medium-chain phthalates are metabolized by fish.
7.4 Summary of environmental fate
The medium-chain phthalates are expected to be released primarily to the aquatic medium through wastewatereffluents originating from industrial sources and through disperse releases from consumer products .Within the medium-chain phthalates subgroup, as the molecular weight of the phthalates increases, there is an increasing tendency to sorb to the solid phase in various media. Therefore, some medium-chain phthalates will tend to reside in sediments and in sludges, whereby they could also be transferred to soil from their application to agricultural lands. Medium-chain phthalates are not expected to undergo long-range transport based on modelled results. Nonetheless, DIBP, characterized by the lowest molecular weight in the subgroup, has been detected far from release sources, likely due to particle transport, a phenomenon that is not presently captured by (Q)SAR modelling. Abiotc and biotic degradation processes have been relatively well characterized for phthalate esters. Medium-chain phthalates are susceptible to degradation through abiotic processes, although these processes are very slow in the environment and are influenced by environmental conditions, such as pH and the presence of oxygen. In contrast, biodegradation processes can be relatively fast; however, substance-specific biodegradation rates and the degree of mineralization vary depending on the size and complexity of ester side groups, with the more complex and larger molecules having longer residence time in the environment. Biodegradation rates in soils and sediments are similar to those in water. Lower-molecular-weight medium-chain phthalates are more water soluble and are therefore more bioavailable to aquatic organisms. Bioaccumulation data were only available for a small subset of the subgroup, primarily for the substances that have lower molecular weights (i.e., DIBP and the analogue BBP) and suggest that the medium-chain phthalates have low to moderate potential for bioaccumulation. Bioconcentration studies suggest that these substances can be metabolized. Food web field studies indicate that bioaccumulation potential is not significant in the food web, as the medium-chain phthalates are not found to biomagnify in the food web.
8. Potential to Cause Ecological Harm
8.1 Ecological effects
Empirical aquatic toxicity data were available for the following medium-chain phthalates: DIBP, DCHP, B84P and B79P. These data were sourced from both published and unpublished studies. The unpublished industry studies were summarized for the EU Regulation on the Registration, Evaluation, Authorisation and Restriction of Chemical Substances (REACH), and study summaries were available from the European Chemicals Agency website (ECHA c2007-2014a). Since limited study details were provided in some of the study summaries, multiple studies were used to compare the results.
Empirical data for DIBP, DCHP, B84P and B79P as well as information available for analogue substances BBP, DPhP and DIOP, along with (Q)SAR model data, were used to evaluate BCHP, CHIBP, DBzP, DMCHP and DIHepP.
It has been proposed that the lower-molecular-weight phthalates, including DBP and BBP (with molecular weights ranging from 278.34 to 312.35 g/mol), likely act through non-polar narcosis, based on the positive correlation of toxicity data to the Kocvalues (Oehlmann et al. 2009). Adams et al. (1995) also described that lower-molecular-weight phthalates have higher toxicity relative to neutral organic non-specific narcotics, which would suggest that they would be classified as either polar narcotics or compounds having an "unspecified reactivity" mode of action. For higher-molecular-weight compounds (molecular weights greater than 312.35 g/mol), Rhodes et al. (1995) suggest that their data support a "solubility cutoff" mode of action, wherein aqueous solubility declines with increasing molecular weight, such that critical body burdens eliciting adverse effects cannot be achieved.
The potential for the medium-chain phthalates to cause effects on the endocrine system is well studied in mammals, but data on aquatic organisms is scarce. The ability to extrapolate the data obtained from these mammalian studies to other vertebrates or invertebrates is uncertain. Christiansen et al. (2000) point out that teleost fish, as compared with mammalian species, have lower xenobiotic metabolizing abilities, different hormonal control and sexual differentiation, and certain steroid hormone receptors that are specific to teleosts. The section on ecological effects is limited to the evaluation of aquatic toxicity studies, while the section on human health (Section 9.2) provides an evaluation of the mammalian data. Limited data on secondary endpoints (measured at the molecular, biochemical, cellular, tissue, blood or organ level) suggest that certain phthalates (such as BBP) may have the potential to cause endocrine effects; however, studies on primary endpoints (including development and reproduction) show no evidence of effects on the endocrine system . Studies on secondary endpoints that may be mediated by endocrine activity have not been conducted for any of the substances in the medium-chain phthalates subgroup. The use of an analogue for the evaluation of these effects, which are substance-specific and structure-dependent, is highly uncertain for aquatic organisms. They have therefore not been used as read-across or in the calculation of assessment factors for the medium-chain phthalates.
The ecological effects of the medium-chain phthalates are discussed below in the sections for each environmental compartment, namely water, sediment and soil.
In brief, available empirical toxicity information indicates that the medium-chain phthalates are highly to moderately toxic to aquatic organisms (with median lethal concentration [LC50] values ranging from less than 1 to 10 mg/L). Aquatic toxicity data are compiled in Appendix E, in Tables E-1 and E-2.
Most aquatic toxicity studies with medium-chain phthalates measure primary endpoints, including survival, growth, development and reproduction. Secondary endpoints that may be mediated by endocrine activity and mechanisms of action other than narcosis are discussed in subsection 8.1.1.3 of the water compartment section. Available results are summarized in Appendix E in Table E-4.
While there is no information on potential effects in wildlife species, a number of studies examine toxicity in rodents (see Section 9.2). Chronic oral exposure to low levels of medium-chain phthalates, for example, through the ingestion of food, is a possible scenario of concern for wildlife. A detailed consideration of potential impacts in mammalian species is provided in the Health Effects Assessment (Section 9.2).
Monoester metabolites appear to exhibit less toxicity to aquatic organisms than their parent compounds. The effects are discussed in subsection 8.1.1.4.
Sediment and soil toxicity data are extremely limited for the medium-chain phthalates. The limited data available on analogues are presented in subsections 8.1.2 and 8.1.3, and suggest that the medium-chain phthalates would have low toxicity to sediment and soil-dwelling organisms (LC50 greater than 100 mg/kg dry weight [dw]).
8.1.1 Water
Empirically determined aquatic toxicity
Ecotoxicological experiments on medium-chain phthalates have been conducted using a variety of aquatic organisms, with several toxicological responses observed depending on the organisms and the particular substances that were studied. Available empirical toxicity information for the medium-chain phthalates indicates that these substances are highly to moderately toxic to aquatic organisms (median lethal concentration [LC50] values ranging from less than 1 to 10 mg/L]) (refer to tables E-1 and E-2 in Appendix E).
Bradlee and Thomas (2003) describe a solubility cut-off (threshold) for phthalates, where solubility decreases below 1 mg/L when the carbon chain length on the alkyl side chains increases above 6 carbons. They suggest that in terms of aquatic toxicological properties, phthalates can be classified as "lower phthalates", with ester chains of less than C6, and "higher phthalates," with ester chains of C6 or greater. They indicate that higher phthalates do not pose intrinsic toxicity to aquatic organisms, as the rapid metabolism and low water solubility prevent their critical body burden for toxicity from being reached (Bradlee and Thomas 2003). They also note that the higher phthalates form stable emulsions that lead to artificial toxicity in laboratory tests, where daphnids become entrapped. DBzP, DCHP, DMCHP, B84P, B79P, BIOP and DIHepP are all phthalates in the medium-chain subgroup where both ester chains have 6 or more carbons. Many of the ecotoxicological studies described below for these substances were conducted above the water solubility limit of the substance and comment on the presence of a surface layer. When solubility is exceeded in a toxicity test, a stressor (i.e., undissolved test chemical) is present in the test that under normal circumstances (i.e., in the absence of spills) cannot occur in the environment. Hence, the toxicity results are not applicable to normal environmental situations. This includes the results from toxicity tests, where part of the exposure concentrations is above and the other part is below the solubility. In these cases, it is possible to create an apparent but "false" dose-response curve, as the dosing concentrations above the solubility include the undissolved chemical stressor, while the dosing concentrations below the solubility do not include the undissolved chemical stressor (personal communication, external science review to Ecological Assessment Division (Environment Canada), dated November 5, 2014, unreferenced). In most cases, studies were carried out within the water solubility limit.
DIBP is the most studied phthalate in the medium-chain phthalate group, with studies available for fish, invertebrates and algae. It also has the lowest molecular weight and, therefore, is the most water-soluble and bioavailable of the medium-chain phthalates. In a well-documented acute toxicity study summary on the fathead minnow, a non-guideline study that was not specified as conforming to good laboratory practices (GLP) resulted in behavioural abnormalities at low concentrations of DIBP, generating a 96-hour EC50 of 0.73 mg/L (ECHA c2007-2014b). The study was conducted within the range of solubility of DIBP. Daphnia magna were also found to exhibit chronic reproductive effects, with a 21-day NOEC of 0.27 mg/L. However, details of the guidelines followed for this study were not available (ECHA c2007-2014b). A very well-documented summary of a study on algae indicated that DIBP is highly toxic to algae, with a 72-hour EC50 of 0.56 mg/L, based on reductions in biomass (ECHA c2007-2014b). In this study, concentrations of test solutions were based on measurements made at the beginning of the test. The empirical data indicate that DIBP is highly toxic to fish, invertebrates and algae, with effects observed between 0.27 and 4.8 mg/L (see Table E-1 in Appendix E).
Most aquatic toxicity studies on B84P were conducted at concentrations exceeding the water solubility limit (0.81 mg/L). B84P is a substance with ester chains that exceed 6 carbons in length. A confidential study of acute toxicity on Daphniawas conducted using nominal concentrations in the range of 1 to 10 mg/L, and generated a 48-hour LC50 of 7.5 mg/L (Study Submission 2014a). Acetone was used as a vehicle in this test up to a concentration of 1 mL per 200 mL of water in the beaker. There was no indication if a surface film was present or if Daphnia were entrapped. Summaries of fish studies consistently show no effects at concentrations near the water solubility limit or exceeding the water solubility limit. LC50 is reported to exceed 0.3, 5 and 1000 mg/L. None of the summaries of the fish studies provide sufficient detail to evaluate the study. A 96-hour fathead minnow test conducted according to OECD Guideline 203 and following GLP resulted in an LC50 greater than 0.3 mg/L, although no further details are provided (ECHA c2007-2014g). These data indicate that Daphnia are more sensitive to B84P than fish, as Daphnia mortality is observed at 7.5 mg/L and no effects have been observed in fish studies.
DCHP toxicity has been studied in fish, invertebrates, algae and amphibians. Due to its structural similarity and similar physical chemical properties to DMCHP, it will also be used as read-across for DMCHP, which is data-poor. DCHP and DMCHP have ester chains equal to or exceeding a length of 6 carbons (C6 and C7, respectively), which has been described as the limit where emulsions start to be formed in water when solubility is exceeded. Water solubility measurements of DCHP range from 0.2 to 4 mg/L and are estimated to be 0.275 mg/L for DMCHP. Two Daphniastudies were available where testing occurred at concentrations near the limits of water solubility so that emulsion effects would not be expected. An acute Daphnia study with nominal concentrations ranging from 0.2 to 2 mg/L resulted in no loss of mobility over 48 hours at any concentration (ECHA c2007-2014c). However, a 21-day study, with measured concentrations (nominal concentrations were not reported), generated an EC50 of 0.679 mg/L and a NOEC of 0.181 mg/L (ECHA c2007-2014c). The summary provided very little details, but was considered reliable given that it was reported to be carried out following OECD guidelines. Available fish and algae studies were not considered reliable as both tests were conducted at concentrations well above the water solubility limits (10-100mg/L and 2 mg/L, respectively) (ECHA c2007-2014c).
De Solla and Langlois (2014) used the Frog Embryo Teratogenesis Assay - Xenopus (ASTM, 1998) as a rapid test to identify potential developmental toxicity. In a progress report on ongoing work, they reported that in 72-hour acute toxicity tests, exposure to DCHP was observed to lead to mortality, malformations and developmental delays in western clawed frog tadpoles in concentrations above the water solubility limit. DCHP was observed to significantly induce mortality relative to controls in western clawed frog tadpoles at measured concentrations above 4.1 mg/L (corresponding to nominal concentrations of 23 mg/L). In the same lethal range, DCHP increased the presence of malformations in tadpoles, including edemas and heart and gill abnormalities. All DCHP concentrations augmented the rate of underdeveloped tadpoles when compared to the control, although this was significant only at 4.1 and 19 mg/L. Below 4.1 mg/L, sublethal effects, such as alterations to gene expression, were observed, suggesting that DCHP-induced cellular stress perhaps contributes to the malformations and developmental effects observed at concentrations above 4.1 mg/L. These results indicate that DCHP and DMCHP are highly toxic to aquatic invertebrates and moderately toxic to amphibians.
Aquatic toxicity of B79P has been determined in algae, Daphnia and fish (see Table E-1 in Appendix E). Based on the structural similarities between B79P and BIOP and similar aquatic bioavailability, and given the lack of data for BIOP, effects data for B79P can be used to evaluate BIOP. Several summaries for studies on B79P using fish, Daphnia and algae studies were available (ECHA c2007-2014d). The algae studies were conducted above the water solubility limit and with a solvent; EC50 in the range of 521 to 674 mg/L was observed, although details were lacking on the study (ExxonMobil 2006). Two studies for D. magna are available (Study Submission 2014b; ECHA c2007-2014d). A study exposing daphnidsto nominal concentrations of 1 to 10 mg/L of B79P in acetone generated a 48-hour LC50 of 4.5 mg/L. There are limitations to this study, however, as the submitting company notes that the composition of B79P at the time of testing was different than that of the product currently on the market. The test was carried out on a substance that was largely formed from C8 alcohols, while it is currently predominantly made from C9-rich alcohol. Furthermore, entrapment of Daphnia was observed at the 1 and 1.8 mg/L concentrations of B79P (Study Submission 2014b). A study summary provided a 48-hour EC50 for B79P of 0.3 mg/L, which was determined according to OECD Guideline 202, although further study details, including the nominal concentrations used in the test, are lacking (ECHA c2007-2014d). Fish studies were conducted at concentrations exceeding water solubility (0.3 mg/L), and effects were not observed (ExxonMobil 2006). Brown et al. (1998) tested the chronic effects of di-heptyl/nonyl phthalate and other phthalate esters on Daphnia magna in the presence of a chemical dispersant. The study followed OECD test guidelines and included analytical confirmation. At a nominal concentration of 1 mg/L, no chronic effects were observed on daphnid survival or reproduction. The study summary indicates that BIOP and B79P exhibited high to moderate toxicity to Daphnia at concentrations within the water solubility limit.
Key studies that were available for the medium-chain phthalates and considered in choosing a critical toxicity value are summarized in Table 8-1. The key studies were critically reviewed and found to be reliable for use as a critical toxicity value (Environment Canada 2015).
| Substance name | Test organism | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| DIBP | Fathead minnow, Pimephales promelas | 96 h EC50 | 0.73 | ECHA c2007-2014b |
| DIBP | Water flea, Daphnia magna | 21 d NOEC Reproduction |
0.27 | ECHA c2007-2014b |
| DIBP | Green algae, Pseudokirchneriella subcapitata | 72 h EC50 Biomass |
0.56 | ECHA c2007-2014b |
| DCHP | Water flea, Daphnia magna | 21 d NOEC | 0.181 | ECHA c2007-2014c |
B79P and BIOP did not have any aquatic toxicity studies available that were considered reliable for use as a critical toxicity value, therefore modelled results were used for the critical toxicity value, as discussed in Section 8.1.1.2.
BCHP and CHIBP did not have empirical data available, but BBP is considered a close structural analogue with similar physical-chemical properties and bioavailability, as shown in Appendix A, and will be used as read-across for these two substances. Fish, invertebrate and algal studies indicate that these substances have a high acute toxicity, with toxicity values less than 1 mg/L (see Table E-2 in Appendix E for all values). Adams et al. (1995) conducted a study on 14 phthalate esters and found that BBP was acutely toxic to both fish and algae. In the study by Adams et al. (1995), many aquatic organisms were tested, and algae was the most sensitive species to the acute effects of BBP, with a 96-hour EC50 of 0.21 mg/L and a NOEC below the lowest tested concentration (less than 0.1 mg/L). Fish were less sensitive, but still exhibited LC50 between 0.82 and 1.7 mg/L. In many of the species examined, insufficient mortality was observed at the highest BBP concentration tested to calculate an LC50. The values reported by Adams et al. (1995) are based on concentrations measured on the first and last days of the study. The authors also make reference to BBP values reported by Gledhill et al. (1980), where a 96-hour NOEC for the bluegill was observed at 0.36 mg/L. While EC50 values for Daphnia could not be calculated in the Adams et al. (1995) study, toxicity to Daphnia was observed by Rhodes et al. (1995), where a 21-day study resulted in a LOEC of 1.4 mg/L and a NOEC of 0.28 mg/L. The latter study also showed that fish were less sensitive than daphnids, with no effects observed in fish at the highest concentration tested (0.2 mg/L). The Adams et al. (1995) study and the Rhodes et al. (1995) study both tested 14 phthalates and noted that many of the tests had to be repeated due to the presence of a microlayer of test chemical on the surface of the water. In both studies, BBP was the exception, showing no signs of the test chemical floating on the surface, as the test was conducted at measured concentrations below the water solubility limit (measured exposure concentrations reported by Rhodes et al. [1995] indicate that all exposure concentrations were below the water solubility limit of 2.96 mg/L). A 28-day flow-through test was conducted with mysid shrimp in saltwater with mean measured exposure concentrations of radiolabelled BBP ranging between 0.024 and 0.75 mg/L (corresponding to nominal concentrations ranging from 0.031 to 0.5 mg/L; mean measured concentrations were 62-68% of nominal in most treatment levels). NOECs calculated based on survival, reproduction and growth were found to be 0.17, 0.075 and 0.075 mg/L, respectively (Study Submission 2014c).
A 2-generation fathead minnow study was conducted to measure the potential effects of BBP on development, growth and reproduction (Study Submission 2014d). The study was conducted in a flow-through system according to GLP and multiple guidelines. The study involved two phases, where phase 1 lasted 21 days and assessed the survival and reproductive performance of adults, and phase 2 lasted 126 days and assessed the performance of the embryos from phase 1. BBP was tested at measured concentrations of 0.018 and 0.064-0.067 mg/L (from 0.025 and 0.1 mg/L nominal, respectively) with 100 µL/L of the vehicle triethylene glycol. In phase 1, effects on adult survival, number of eggs, number of spawns, number of eggs per spawn, percent fertility and hatchability were not found to be significantly affected by BBP. In phase 2, effects on fry survival, adult survival, female length and weight, male length and weight, and female and male vitellogenin were not found to be significantly affected by BBP (Study Submission 2014d).
The toxicity of BBP to sediment-dwelling organisms in water-only tests has been examined in two studies. Santicizer 160 (BBP) was tested on the sediment organism Chironomus tentans over 48 hours at nominal concentrations ranging from 1.25 to 20 mg/L and generated a 48-hour LC50 of 1.64 mg/L. A NOEC of 1.25 mg/L was observed (ECHA c2007-2013). The study was conducted prior to the implementation of GLP and was described as "closely following" a guideline from Mosher et al. (1982) and a US EPA guideline (1975). Call et al. (2001a) studied the effects of BBP on H. azteca, C. tentans and L. variegatus over 10 days in a water-only toxicity test with mean measured concentrations ranging from 0.036 to 1.76 mg/L and a 90% spike recovery from water. LC50 values were 0.46, greater than 1.76 and 1.23 mg/L, respectively. These data indicate that BCHP and CHIBP can also be moderately to highly toxic to aquatic organisms and sediment-dwelling organisms presumably exposed to the test substances via pore water.
There are no empirical toxicity data for DBzP, but an acute toxicity study on DPhP will be used as read-across. This is because these two substances differ by only two carbon atoms and have very comparable water solubility and log Kow estimates (Appendix A), with DBzP expected to be slightly less bioavailable due to lower water solubility (0.51 mg/L) and higher log Kow (5.1) compared to DPhP (2.47 mg/L and 4.1). A study by Geiger et al. (1985) generated an acute LC50 of 0.08 mg/L to fathead minnow, which is below the water solubility limit of DBzP (0.51 mg/L) and DPhP (3.039 mg/L). The study tested a range of nominal concentrations from 0.1 to 0.48 mg/L (corresponding to measured concentrations in the range of 0.069 to 0.48 mg/L), which were all below the water solubility limit of DPhP. This can be considered a worst case toxicity estimate for DBzP, which is expected to be less bioavailable than the analogue.
Since only one aquatic toxicity study was available for DIHepP, toxicity data for DIOP was also used as read-across. DIOP has the same basic structure as DIHepP, with one extra carbon in each of the alkyl side chains. Brown et al. (1998) tested the chronic effects of DIHepP (identified as di-iso heptyl phthalate) and other phthalate esters on Daphnia magna in the presence of a chemical dispersant. The study followed OECD test guidelines and included analytical confirmation. At a nominal concentration of 1 mg/L, no chronic effects were observed on daphnid survival or reproduction. DIOP has been studied in two well-documented articles. Since phthalates with side chains exceeding 6 carbons tend to exist as surface film in concentrations above their water solubility, Adams et al. (1995) conducted an acute toxicity study using a bottom drain in a flask to transfer the stock solution to the test beakers so that any floating test chemical remained in the flask. Even with these precautions, the DIOP study had to be repeated, as Daphnia were entrapped at the surface in the first test. The test solutions were analyzed at the beginning and end of the study. Of the14 chemicals studied, the final test concentrations in the static tests were frequently 50% of the initial concentration, and mean measured concentrations were used in the EC50 calculation. It was hypothesized that this is likely due to adsorption to the test vessel. The findings are based on daphnid immobility and not entrapment. The 48-hour EC50 for Daphnia to DIOP was greater than the highest concentration tested (0.16 mg/L), which is also above its water solubility limit (0.09 mg/L). Adams et al. (1995) tested other aquatic organisms (fish, other invertebrates and algae) and, in all cases, the LC50 and EC50 values were above the highest concentration tested (in both static and flow-through tests). Rhodes et al. (1995) examined the chronic toxicity of 14 phthalates to Daphnia magna. Test concentrations were measured at 7-day intervals throughout the 21-day test. While no visible film of DIOP was observed on the surface, test organisms were observed floating on the surface at the LOEC of 0.14 mg/L. A NOEC of 0.062 mg/L was observed. Rhodes et al. (1995) looked at the most sensitive endpoints of the Daphnia studies and observed that survival and reproduction were equally sensitive endpoints for DIOP. They note that it is common in daphnid chronic toxicity studies for reproduction to be the most sensitive endpoint. However, of the 14 phthalates tested, reproduction was never the most sensitive endpoint. They postulated that this was because mortality was due to physical effects. It appears that immobilization of Daphnia is the primary mechanism of toxicity for DIOP and DIHepP, even at concentrations near the water solubility limit (DIHepP water solubility is 0.02 mg/L and DIOP water solubility is 0.09 mg/L [HSDB 2014]).
Key studies for analogues of BCHP, CHIBP and DBzP considered in choosing a critical toxicity value in water are summarized in Table 8-2. Key studies were critically reviewed and found to be reliable for use as a critical toxicity value (Environment Canada 2015).
| Substance name | Test organism | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| BBP | Rainbow trout, Salmo mykiss | 96 h LC50 | 0.82 | Adams et al. 1995 |
| BBP | Mysid shrimp, Mysidopsis bahia | 28 d NOEC | 0.075 | Study Submission 2014c |
| BBP | Water flea, Daphnia magna | 21 d NOEC | 0.28 | Rhodes et al. 1995 |
| BBP | Green algae, Pseudokirchneriella subcapitata | 96 h EC50 | 0.21 | Adams et al. 1995 |
| BBP | Green algae, Pseudokirchneriella subcapitata | 96 h NOEC | less than 0.1 | Adams et al. 1995 |
| DPhP | Fathead minnow, Pimephales promelas | 96 h LC50 | 0.08 | Geiger et al. 1985 |
Modelling aquatic toxicity
For many of the medium-chain phthalates, there are no experimental toxicity data available, and the assessments are being informed by read-across data. The (Q)SAR program (ECOSAR 2012) was run using the ester structure activity relationships to build on the weight of evidence. Some of the predictions for CHIBP, DMCHP, DIHepP and BIOP included flags that the chemical may not be soluble at the predicted effect concentration for acute exposures. Upon further examination, the predicted effect concentrations are above the water solubility values for only DBzP and DIHepP; therefore, the modelled effect concentrations will only be retained for the evaluation of toxicity for the remaining medium-chain phthalates. The ranges predicted by ECOSAR (2012) (Table 8-3) are in agreement with the experimental data, which suggest that the medium-chain phthalates are highly to moderately toxic to aquatic organisms. The complete predictions are summarized in Table E-3 of Appendix E.
| Organism | Duration (hr) | Endpoint | Range of predictions (mg/L) |
|---|---|---|---|
| Fish | 96 | LC50 | 0.049-1.48 |
| Daphnid | 48 | LC50 | 0.05-2.2 |
| Green algae | 96 | EC50 | 0.012-0.72 |
Key aquatic toxicity studies could not be identified for B79P or BIOP, therefore modelled predictions were considered in choosing a critical toxicity value in water. In addition to ECOSAR v1.00, the models AIEPS v2.05 and the OASIS sub-model CPOPs (2008) were run for B79P and BIOP. The model results are summarized in Table 8-4 and the complete predictions are provided in Table E-3 of Appendix E. It was found that AIEPS v2.05, which uses fragments to generate predictions, had good structural coverage in the training set (greater than 70%), but produced results that exceeded the water solubility limits in almost all cases. The OASIS-CPOPs (2008) model predictions were higher than the ECOSAR v1.00 predictions by approximately one order of magnitude, and in both cases, algae was the most sensitive species. These substances were considered out of the domain for the OASIS-CPOPs (2008) model as their log Kow's exceed 5. Therefore, the algal 96 hr EC50 values calculated by the esters SAR in ECOSAR (2012) were selected as the critical values for both B79P and BIOP (with values of 0.012 and 0.032 mg/L, respectively). These modelled critical toxicity values are within an order of magnitude of the experimental critical toxicity values for the other medium-chain phthalates (shown in Table 8-5).
| Substance name | Organism | Endpoint | Prediction (mg/L) | Reference |
|---|---|---|---|---|
| B79P | Fish | 96hr LC50 | 0.0045 - 0.763 | ECOSAR 2012; TOPKAT 2001; AIEPS 2003-2007; CPOPs 2008 |
| B79P | Daphnid | 48hr LC50 | 0.05 - 31.11 | ECOSAR 2012; AIEPS 2003-2007; CPOPs 2008 |
| B79P | Algae | 72hr and 96hr EC50 | 0.012 - 1.36 | ECOSAR 2012; AIEPS 2003-2007; CPOPs 2008 |
| BIOP | Fish | 96hr LC50 | 0.108 - 0.504 | ECOSAR 2012; AIEPS 2003-2007; CPOPs 2008 |
| BIOP | Daphnid | 48hr LC50 | 0.122 - 13.89 | ECOSAR 2012; AIEPS 2003-2007; CPOPs 2008 |
| BIOP | Algae | 72hr and 96hr EC50 | 0.032 - 1.75 | ECOSAR 2012; AIEPS 2003-2007; CPOPs 2008 |
Derivation of the predicted no-effect concentration (PNEC)
In order to determine a predicted no-effect concentration (PNEC) for the medium-chain phthalates, the most sensitive (reliable) endpoint was chosen as a critical toxicity value (CTV) in considering the acceptability of available studies. The CTV was then divided by an assessment factor to account for such factors as inter- and intra-species variability, short-term to long-term effects, and the extent of species covered by the dataset to give a PNEC value for each of the substances in the medium-chain phthalate subgroup. The CTVs, assessment factors and corresponding PNECs are presented in Table 8-5. Although there is uncertainty caused by the limited coverage of species in the dataset, inter- and intra-species variability and extrapolation of short-term to long-term effects, an assessment factor of 30 was used in the case of DBzP because the analogue used to derive the CTV is expected to be more bioavailable than DBzP.
| Substance name | Test organism | Endpoint | CTV (mg/L) | Reference | AF | PNEC (mg/L) |
|---|---|---|---|---|---|---|
| DCHP | Water flea, Daphnia magna | 21 d NOEC | 0.181 | ECHA c2007-2014c | 3 | 0.06 |
| DIBP | Green algae, Pseudokirchneriella subcapitata | 72 h EC50 Biomass |
0.56 | ECHA c2007-2014b | 3 | 0.19 |
| BIOP | Algae | 96 h EC50 | 0.032 | ECOSAR v1.00 | 10 | 0.0032 |
| B79P | Algae | 96 h EC50 | 0.012 | ECOSAR v1.00 | 10 | 0.0012 |
| BCHPFootnote Table 8-5[a] | Mysid Shrimp, Mysidopsis bahia | 28 d NOEC | 0.075 | Study Submission 2014c | 3 | 0.025 |
| DBzPFootnote Table 8-5[b] | Fathead Minnow, Pimephales promelas | 96 h LC50 | 0.08 | Geiger et al. 1985 | 30 | 0.003 |
| CHIBPa | Mysid Shrimp, Mysidopsis bahia | 28 d NOEC | 0.075 | Study Submission 2014c | 3 | 0.025 |
| DMCHPFootnote Table 8-5[c] | Water Flea, Daphnia magna | 21 d NOEC | 0.181 | ECHA c2007-2014c | 3 | 0.06 |
No evidence of chemical toxicity was seen in standard aquatic toxicity testing with DIHepP or B84P up to their water solubility limits, although mortality was observed in Daphnia at 0.14 and 7.5 mg/L, respectively. In the absence of a definitive lowest observed adverse effect concentration (LOAEC), a PNEC cannot be derived.
The low water solubility (ranging from 0.001 to 0.81 mg/L) and high hydrophobicity (log Koc 6.15 to 6.76) of DIHepP and B84P suggest that dietary exposure could be a more relevant route of uptake for organisms, rather than uptake from the surrounding medium. For this reason, endpoint values derived from water concentrations may not fully describe the potential for effects. The toxic potential of substances that are taken up primarily through diet is better captured by examining whole-body residues (internal concentrations) of the substance in an organism. Critical body residues (CBRs) can then be calculated in order to estimate the potential for the substance to reach internal concentrations that are sufficiently high to cause effects through baseline narcosis (McCarty and Mackay 1993; McCarty et al. 2013).
CBRs were calculated for DIHepP and B84P using the McCarty and Mackay (1993) equation:
CBR = BAF × WS / MW
where:
- CBR =
- the critical body residue (mmol/kg)
- BAF =
- fish bioaccumulation factor (L/kg); normalized to 5% body lipid
- WS =
- water solubility of the substance (mg/L)
- MW =
- molecular weight of the substance (g/mol)
Input values to the equation for DIHepP and B84P and the outputs are summarized in Table 8-5 below. Using maximum water solubility in the calculation of CBR represents a conservative but realistic scenario. Experimental BAF data are not available for the calculation of the CBR for B84P; however, a general trend was noticed with the other medium-chain phthalates that the BAF predictions obtained using the Arnot and Gobas (2003) approach with a 1% dietary uptake efficiency were lower than the experimental values. Therefore, CBR calculations were also completed using the higher BAF prediction obtained using the BCFBAF model (v3.01) (Table 8-6).
| Substance name | BAF (L/kg) | Water solubility (mg/L) | Molecular weight (g/mol) | CBR (mmol/kg) |
|---|---|---|---|---|
| DIHepP | 115 (fish; Mackintosh 2002) |
0.017 (Letinski et al. 2002) |
362.51 | 5.39 × 10-3 |
| DIHepP | 427 (mussel; Mackintosh 2002) |
0.017 (Letinski et al. 2002) |
362.51 | 0.02 |
| B84P | 54 (fish; Arnot and Gobas 2003) |
0.81 (European Commission 2000) |
454.57 | 0.1 |
| B84P | 71 (fish; BCFBAF v3.01) |
0.81 (European Commission 2000) |
454.57 | 0.13 |
McCarty and Mackay (1993) determined that CBRs associated with acutely lethal baseline neutral narcosis in small aquatic organisms typically range from about 2 to 8 mmol/kg, while those for chronic exposures range from 0.2 to 0.8 mmol/kg. The CBR values calculated for DIHepP and B84P are lower than these, indicating that internal concentrations are unlikely to reach levels sufficient to elicit acute or chronic effects through a neutral narcosis mode of toxic action.
Secondary endpoints in aquatic organisms
Secondary endpoints at lower levels of organization (e.g., the molecular, biochemical, cellular, tissue or organ level) are useful measurements to support the assessment of mode of toxicological action (Staples et al. 2011). A search of the literature revealed that none of the medium-chain phthalates have data on secondary endpoints that would suggest effects on the endocrine system in fish. Limited data for DCHP and DIHepP are available on the amphibian thyroid system, where DCHP and DIHepP have been studied for their potential to alter genes involved in reproduction and thyroid-hormone homeostasis in frogs (De Solla and Langlois 2014; Sugiyama et al. 2005). Numerous secondary endpoints, both in vivo an in vitro, have been determined for BBP and DEHP - substances used as analogues for certain medium chain phthalates (see Table2-4 for the summary of the read-across approach).
DCHP and DIHepP are being studied for their potential to alter gene expression in the Western Clawed Frog, particularly looking at genes involved in reproduction, thyroid-hormone (TH) homeostasis and cellular stress (De Solla and Langlois 2014). While DIHepP was not found to alter the expression of the targeted genes in frog embryos, DCHP disrupted gene expression from the sex steroid, TH and cellular axes. DCHP increased the expression of reproduction-related genes and TH-related genes in frogs exposed to concentrations ranging from 0.3 to 4.1 mg/L. Disruption of sex steroid-related gene expression may affect later life events, such as sex differentiation and reproduction, while effects on active TH levels could potentially delay tadpole development (De Solla and Langlois 2014). Data are presented in Table E-4 of Appendix E.
Sugiyama et al. (2005) conducted in vitro tests to screen phthalates, including DCHP, BBP and DEHP, for potential interference with the amphibian thyroid system and compared those with results from in vivo tests with tadpoles. They observed that DCHP was the most potent antagonist to the TH, triiodothyronine (T3), with an IC50 of 0.43 mg/L, followed by BBP and DEHP (IC50 = 12.5 mg/L and greater than 19.53 mg/L, respectively). The phthalates were also found to inhibit the expression of the TH nuclear receptor β by greater than 50% for BBP (at 1.25 mg/L), 42% for DCHP (at 6.6 mg/L) and 29% for DEHP (at 19.53 mg/L). In a 5-day in vivo test with tadpoles, only BBP was found to inhibit the increase in the amount of TRβ transcript by 48% (at 1.25 mg/L).
For BBP, secondary endpoints have been examined in vivoin the fathead minnow (Study Submission 2014d; Harries et al. 2000; Christiansen et al. 2000) and in vitro in the rainbow trout (Chen et al. 2014; Jobling et al. 1995; Knudsen and Pottinger 1999; Tollefsen 2002).The effects on the endocrine system of DEHP has been extensively studied in vivo (Kim et al. 2002; Caunter et al. 2004; Carnevali et al. 2010; Wang et al. 2013; Ye et al. 2014) and in vitro (Sugiyama et al. 2005), although many of these studies are performed at concentrations above the water solubility limit of DEHP.
In the rainbow trout, BBP has been observed to displace estradiol (E2) from the hepatic estrogen receptor (ER) (effects observed at 0.3 mg/L, Jobling et al. 1995; 51.5 mg/L, Knudsen and Pottinger 1999) and from the sex steroid binding protein (1124 mg/L, Tollefsen 2002). BBP has also been observed to inhibit binding to the African clawed frog ER by 50% at 7.4 mg/L (Suzuki et al. 2004). Upon binding to the ERs, phthalates can alter the production of vitellogenin (VTG) in aquatic species (Mathieu-Denoncourt et al. 2015). In one study, BBP has been found to increase VTG in the rainbow trout following intra-peritoneal (IP) injection (500 mg/kg, Christiansen et al. 2000), while in studies on the fathead minnow, no changes were observed (0.0675 mg/L, Study Submission 2014d; 0.071 mg/L, Harries et al. 2000). At IP injection levels of 50 mg/kg, Knudsen et al. (1998) observed no effects on the induction of zona radiata proteins in the rainbow trout, which would be induced at lower concentrations of estrogen than necessary to induce VTG. In a study on the adult male clawed frog, VTG was not found to increase after exposure to concentrations of BBP as high as 31 mg/L (Nomura et al. 2006).
Effects were observed in what appears to be a novel test for estrogen activity, where Chen et al. (2014) used transgenic medaka (Oryzias melastigma) eleutheroembryos and their green fluorescence reporter gene signal to test estrogen responsiveness to BBP and other phthalates. At a concentration of 1.5 mg/L, BBP induced a fluorescent signal in the liver of the exposed eleutheroembryos, with an intensity reading close to that of medaka exposed to 17β-estradiol (E2) at 0.002 mg/L (2 ppb). Chen et al. (2014) concluded that BBP possesses estrogenic activity.
Qualitative histopathologic observations were made on male and female fathead minnow tissues exposed to BBP at 0.018 and 0.067 mg/L (Study Submission 2014d). At both concentrations, the only effects observed were impacts on the gonadal histology of the fish, including increased incidence and severity of spermatogonia (minimal to moderate) in the testes of males, characterized by the visual impression of the proportion of spermatogonia relative to spermatocytes and spermatids expected for a given stage. In females, a slight increased incidence of oocyte atresia (minimal to severe) was observed. In the high treatment group, altered gonadal stage scores were observed in both males and females (Study Submission 2014d).
Mankidy et al. (2013) investigated the molecular mechanisms of action of BBP and DEHP by assessing transcriptional changes in developing fathead minnow embryos exposed to the phthalates until 96 hours post fertilization. The concentrations used in the tests were above the water solubility limit of DEHP. Exposure to 1 mg/L BBP and 1 mg/L DEHP caused two-fold greater lipid peroxidation in membranes of developing embryos. Neither phthalate altered the expression of mRNA of the ER (α or β); however, BBP exhibited a small, yet significant increase in the expression of mRNA of the androgen receptor (AR) in developing fish embryos at 1 mg/L, while DEHP caused only a slight increase in expression of mRNA of the AR at 1 mg/L. Fathead minnow embryotoxicity tests showed that DEHP was more potent than BBP, with 30% mortality observed at 1 mg/L. Given the significantly greater products of lipid peroxidation observed in membranes of the developing embryos exposed to DEHP and the lack of up-regulation in the expression of mRNA of the enzymes involved in mitigating oxidative stress, the authors concluded that oxidative stress is the critical mechanism of toxic action for DEHP (Mankidy et al. 2013).
DEHP has been found to increase VTG in the zebrafish (effects observed at 5000 mg/kg, Uren-Webster et al. 2010; 2 × 10-5 mg/L, Carnevali et al. 2010), the Chinese rare minnow (0.0128 mg/L in females and 0.0394 mg/L in males, Wang et al. 2013) and the marine medaka (0.1 mg/L, Ye et al. 2013). In a study on juvenile Atlantic salmon, VTG was not detected in blood plasma after IP injection with DEHP (160 mg/kg, Norrgren et al. 1999). VTG levels were not found to statistically increase in male fathead minnow exposed to DEHP via both water (0.005 mg/L) and food (125 or 500 mg/kg), but increased in females at the high dose (ECHA c2007-2014f).
Changes in sex hormone levels in fish following exposure to DEHP have been reported in several papers. In male fish, T levels have been found to increase at 0.039 mg/L DEHP (Wang et al. 2013), while E2 levels have been found to significantly increase after exposure to 0.039 and 0.1 mg/L (Wang et al. 2013; Ye et al. 2013). T levels were not found to change significantly in male fish from two studies (at DEHP concentrations of 0.5 mg/L, Ye et al. 2013; 0.012 mg/L, Crago and Klaper 2012). Crago and Klaper (2012) observed a significant decrease in plasma E2 concentrations at exposures of 0.012 mg/L DEHP. In female fish, T levels were observed to increase at exposures of 0.1 mg/L DEHP (Wang et al. 2013; Ye et al. 2013), while E2 levels have been found to both decrease (0.013 mg/L, Wang et al. 2013) and increase (0.1 mg/L, Ye et al. 2013). In all cases, the exposure concentrations were above the water solubility limit of DEHP.
The gonadal-somatic index (GSI) is usually used as a biomarker in aquatic wildlife to assess exposure to environmental estrogens (Wang et al. 2013). The GSI of DEHP has been calculated by several authors, with mixed results. Increased GSI was found in male Chinese rare minnow exposed to 0.118 mg/L DEHP (Wang et al. 2013), and in female fish exposed to 2 × 10-5 mg/L and 0.118 mg/L DEHP (Carnevali et al. 2010; Wang et al. 2013). GSI was found to decrease in female Japanese medaka following exposure to 0.01 and 0.05 mg/L (Kim et al. 2002), while no effects were observed in males at 0.012 and 0.05 mg/L (Crago and Klaper 2012; Kim et al. 2002). The hepatosomatic index (HSI) of the male Chinese rare minnow was increased significantly at 0.013 mg/L, but no differences were observed for the females (Wang et al. 2013). Uren-Webster et al. (2010) also observed an increase in the HSI in male zebrafish exposed to 5000 mg/kg DEHP via IP-injection.
Reports of histological changes following exposure to DEHP have also been reported. Exposure of DEHP to marine medaka at 0.1 and 0.5 mg/L resulted in a reduced number of spermatozoa in the testes and an increased number of atretic follicles in the ovaries (Ye et al. 2013). In female Japanese medaka, retardation of oocyte development has been observed, with 37, 0 and 22% of female fish having mature oocytes in their ovaries at 0.001, 0.01 and 0.05 mg/L, compared to 54% of female fish in the control. No deformation of the testes in males was observed (Kim et al. 2002). Histological examination of the gonads from Atlantic salmon exposed to 1500 mg/kg DEHP via diet showed a small but significant incidence (3%) of intersex fish, but no complete sex reversal resulting in skewed sex ratios were found (Norman et al. 2007). Uren-Webster et al. (2010) observed that after ten days of exposure to 0.5, 50 and 5000 mg/kg DEHP via IP injection, there was no evidence of DEHP-induced sperm DNA damage in male Zebrafish; however, at 50 mg/kg, there was a decrease in the proportion of spermatozoa and an increase in the proportion of spermatocytes.
Staples et al. (2011) assessed if primary endpoints at the whole-body level and the population level were integrating secondary endpoints for phthalate esters by comparing the primary and secondary endpoints that were both measured within studies. They found that for the low-molecular-weight phthalate esters (C1 to C4), primary and secondary NOECs did not span the same ranges, and concluded that secondary endpoints provided limited benefit in practical ecological risk assessment to aquatic species. However, the number of secondary endpoints available to compare to primary endpoints was quite limited. An analysis of medium-chain phthalates from C5 to C7 was not provided. A comparison between the primary and secondary endpoints for DEHP could not be completed, as Staples et al. (2011) indicate that DEHP was not reported to have adverse effects for either primary or secondary endpoints consistent with solubility constraints. Indeed, many of the studies on DEHP are conducted at concentrations above the water solubility limit; however, two studies that looked at primary and secondary endpoints have recently been conducted within the water solubility limits of DEHP. Carnevali et al. (2010) found a significant reduction in fecundity of female Zebrafish exposed to nominal DEHP concentrations ranging from 2 × 10-5 to 0.40 mg/L. By measuring several key regulators of oocyte maturation and ovulation, they concluded that DEHP affected signals involved in oocyte growth, maturation and ovulation, which impaired ovarian functions and embryo production. Furthermore, Corradetti et al. (2013) found that exposure to 2 × 10-4 mg/L DEHP impaired reproduction in Zebrafish by inducing a mitotic arrest during spermatogenesis, increasing DNA fragmentation in sperm cells and reducing embryo production (up to 90%).
Mammalian data, however, point to androgen insufficiency as a mode of action, which is discussed in detail in the Health Effects Assessment (Section 9.2). This has not been studied in aquatic organisms for the medium-chain phthalates or the analogue BBP, and is considered a data gap, although a multi-generational fathead minnow study on BBP shows no effects on phase 2 embryos (Study Submission 2014d). The data available for the analogue DEHP suggest an estrogenic mode of action (Norrgren et al. 1999; Norman et al. 2007; Carnevali et al. 2010; Corradetti et al. 2013), while some data indicate the anti-androgenic mode of action that occurs in mammals may also be involved in aquatic species (Wang et al. 2013, Ye et al. 2014).
Aquatic toxicity of phthalate ester metabolites
Given the rapid degradation of the medium-chain phthalate esters to monoesters and phthalic acid, the aquatic toxicity of these degradation products was examined. While not all metabolites of the medium-chain phthalates have been studied, Scholz (2003) found that the short-chain monoesters are considerably less toxic to aquatic organisms than the short-chain diesters. Acute toxicity tests on mono-isobutyl phthalate, the degradation product of DIBP, with fish and Daphnia result in LC50 values of 125 and 141 mg/L, respectively (Scholz 2003). A study by Jonsson and Baun (2003) examined the toxicity of the analogue BBP and its metabolite, monobenzyl phthalate (MBzP), to algae and Daphnia magna. They noted that the monoesters can be expected to be more water soluble, more hydrophilic and less volatile from aqueous solution than their corresponding diesters. In a 72-hour algal toxicity test, they found that BBP had an EC50 of 0.96 mg/L, which is consistent with what is found in the literature, and that its degradation product MBzP had an EC50 of 28.6 mg/L and phthalic acid had an EC50 of 2270 mg/L. Similarly, the 48-hour Daphnia magna test resulted in a BBP EC50 of 2.43 mg/L, a MBzP EC50 greater than 274 mg/L and a phthalic acid EC50 of 103 mg/L. This trend was consistent in all of the tested diester phthalates and their monoesters, where the diester showed greater toxicity than the monoester. It is therefore expected that the diesters would be of primary concern in the aquatic compartment.
8.1.2 Sediment
No published data on sediment toxicity have been identified for the medium-chain phthalates. Two studies were found that examined sediment toxicity to DBP and DEHP, which are phthalate esters that also have chain lengths below 6 carbons (Call et al. 2001b; Brown et al. 1996).
Call et al. (2001b) indicate that previous benthic invertebrate testing with other chemicals has shown that benthic invertebrates exhibit a similar range of species sensitivities as pelagic or planktonic invertebrates and that water-only test data for benthic species can be used with equilibrium partitioning (EqP) to predict the effects of phthalates in spiked-sediment laboratory tests. Call et al. (2001b) conducted sediment toxicity tests with DBP (the straight chain isomer of DIBP) to compare with toxicity predictions derived from EqP theory. The overlying water concentrations were found to be much lower than the pore water concentrations and are therefore expected to have negligible impact on the LC50determination. The empirical sediment toxicity results showed that DBP exposures reduced survival and weight of C. tentans at all sediment organic carbon levels, with 10-day LC50ranging from 826 to 4730 mg/kg dw. H. azteca was less sensitive, with LC50 greater than the highest exposure concentration of 71,900 mg/kg dw, perhaps due to the avoidance of the higher concentrations in sediment and migration to the overlying water (Call et al. 2001b). Call et al. (2001b) used the EqP theory with the aqueous toxicity values for DBP from their previous study (Call et al. 2001a) to predict its acute toxicity in spiked sediment laboratory tests. They found that it accurately predicts the acute toxicity of phthalate esters in sediment to benthic invertebrates when the concentrations are stable in the sediment and pore water, and when animal behaviour patterns are such that they receive a continuous exposure. Call et al. (2001a) calculated aqueous LC50 for BBP. Given the similar aqueous toxicity values of DBP and BBP obtained in the 2001a study and their similar Koc values, it would be expected that BBP would also have sediment LC50 in the range of 826 to 4730 mg/kg dw. No adverse effects on either survival or growth based on dry weight were observed at pore water concentrations between 0.273 and 0.382 mg/L or bulk sediment concentrations between 3070 and 3170 mg/kg dw for the analogue DEHP.
Brown et al. (1996) examined the toxicity of DEHP and DIDP on Chironomus riparius at 100, 1000 and 10000 mg/kg dw over 28 days. Test concentrations were measured at the start and finish of the study and were consistent with the nominal concentrations. From the results on emergence and sex distribution, they concluded that there were no effects from either phthalate on survival, development or emergence of C. riparius at any of the concentrations tested. The study also measured the tissue concentration from 14C activity in the midges, and demonstrated quite high body burdens (from 70 to 14,000 mg/kg), which did not appear to be affecting the health of the midges.
The maximum saturation of phthalates in sediment can be determined using the following relationship:
Cs = Cw × Koc × foc
where:
- C s =
- maximum saturation of the substance in sediment (mg/kg dw)
- C w =
- water solubility of the substance (mg/L)
- K oc =
- organic carbon-water partition coefficient of the substance (L/kg OC)
- f oc =
- fraction of organic carbon (OC) in the sediment (kg OC/kg)
Maximum saturation reflects the theoretical maximum thermodynamic saturation of a compound in a given medium at equilibrium. It cannot be exceeded according to thermodynamic principles. In surface waters, the presence of co-solvents or surfactants can create conditions that allow for an "apparent solubility" that exceeds the maximum solubility. In solid phases, such as sediments and soils, maximum saturation is a direct function of the amount of organic carbon present in the matrix if it is assumed that only hydrophobic interactions with organic matter occur. Sediment organic carbon content can vary from location to location, and often average carbon contents are used for calculating maximum saturation in sediments. The apparent solubility in water, and saturation in sediment or soil, can increase or decrease the bioavailability of a compound. The values calculated using the above equation therefore represent the theoretical saturation limit, which, for the purposes of bioavailability, may be exceeded under some circumstances. For example, it is difficult to be certain that only hydrophobic interactions are responsible for defining the maximum theoretical saturation limit in solid phases. These circumstances cannot be easily predicted without specific information regarding the nature of release and the characteristics of the receiving environment. Given the very high hydrophobicity of the long-chain phthalates, hydrophobic interactions are likely to be the major factor influencing the maximum saturation limit.
The maximum saturation of the medium-chain phthalates is summarized in Table 8-7, using a default foc value of 0.04 (default value for average Canadian sediment OC content).
| Substance name | Cw (mg/L) | Koc (L/kg OC) | Calculated Cs (mg/kg dw) |
|---|---|---|---|
| DIBP | 20.3 | 977 | 793.52 |
| BCHP | 3.76 | 4898 | 736.63 |
| CHIBP | 4.82 | 4266 | 822.45 |
| DCHP | 0.2 | 6166 | 49.33 |
| DBzP | 0.51 | 13490 | 275.19 |
| BIOP | 0.22 | 45709 | 402.24 |
| B79P | 0.3 | 15849 | 190.19 |
| DMCHP | 0.275 | 40738 | 448.12 |
| DIHepP | 0.017 | 48978 | 33.30 |
| B84P | 0.81 | 239883 | 7772.22 |
Derivation of the predicted no-effect concentration (PNEC)
There is a significant lack of data available on sediment toxicity relating to the medium-chain phthalates. Based on the available data, the 10-day LC50 of 1664 mg/kg dw in C. tentans will be selected as a conservative CTV, based on the effects of DBP (and used as read-across for DIBP and BBP) in sediments with an organic carbon content of 4.8%. The CTV was then divided by an assessment factor of 100 to account for inter- and intra-species variability and to extrapolate to long-term effects in order to give a PNEC value of 16.64 mg/kg dw. This PNEC applies to DIBP and the analogues of BBP, BCHP, CHIBP and DBzP. This PNEC value is below the calculated maximum saturation in sediment for each of the substances shown in Table 8-7. Since no effects were observed in sediment toxicity testing with DEHP, a PNEC cannot be calculated for DIHepP. The BSAF value of 0.526 kg OC/kg lipid that was reported for DIHepP in the Pacific staghorn sculpin (Mackintosh 2002) can be used in a CBR analysis to determine the potential for adverse effects.
Applying the CBR relationship to DIHepP in sediment,
CBR = BSAF × SS / MW
where:
- CBR =
- the critical body residue (mmol/kg)
- BSAF =
- biota-sediment accumulation factor (kg/kg); normalized to 5% body lipid
- SS =
- saturation limit of the substance in sediment (mg/kg)
- MW =
- molecular weight of the substance (g/mol)
Input values to the equation were: BSAF 0.526 kg/kg (Mackintosh 2002 for Pacific staghorn sculpin; see Appendix D, Table D-3), saturation limit of DIHepP in sediment 33.3 mg/kg (assuming 4% OC content for average Canadian sediment; see Table 8-7) and molecular weight 362.51 g/mol (Table 2-1).
Using the maximum saturation in the calculation of CBR represents a conservative but realistic scenario.
Based on these input values, the calculated CBR is 0.05 mmol/kg. This suggests that tissue levels of DIHepP in sediment-dwelling organisms will remain below those predicted to result in acute or chronic effects due to baseline narcosis.
PNECs for DCHP, DMCHP, BIOP and B79P in sediment can be derived using the Equilibrium Partitioning method (Redman et al. 2014) by multiplying the aquatic PNEC by the substances' Kocvalues and using a default sediment organic carbon content to present the values on a dry weight basis. Using the equation from Call et al. (2001b):
Cs/foc = Koc × Cd
where:
- C s =
- bulk sediment concentration (mg/kg dw)
- f oc =
- organic carbon content of the sediment (kg OC/kg dry)
- K oc =
- sediment organic carbon-water partition coefficient (L/kg OC)
- C d =
- free dissolved concentration (mg/L)
An organic carbon content of 4% was assumed in the calculation. The resulting calculated PNECs in sediment (PNECsediment) range from 0.76 to 97.8 mg/kg dw as shown in Table 8-8. These PNECs are all below the maximum saturation in sediment values calculated in Table 8-7.
| Substance | Koc (L/kg OC) | Cd (mg/L) (from PNECaquatic) |
Calculated Cs (mg/kg dw) (PNECsediment) |
|---|---|---|---|
| DCHP | 6,166 | 0.06 | 14.8 |
| BIOP | 45,709 | 0.0032 | 5.85 |
| B79P | 15,849 | 0.0012 | 0.76 |
| DMCHP | 40,738 | 0.06 | 97.8 |
A PNEC cannot be determined for B84P, as sediment studies have not been conducted and aquatic PNECs could not be calculated. BSAFs have not been reported for B84P; therefore, a CBR analysis cannot be conducted.
8.1.3 Soil
No published data on soil toxicity have been identified for the medium-chain phthalates. One study summary is available on the analogue BBP.
In a summary of an unpublished study, earthworms (Eisenia fetida) were exposed to nominal concentrations of 95 to 1000 mg/kg dw of BBP in artificial soil, following OECD Guideline 207. The weight and survival was recorded after 7 and 14 days. After 14 days, no effects were observed at the highest concentration (1000 mg/kg dw). There were no marked differences in weight changes between the test and control groups (ECHA c2007-2013).
Due to the lack of data, and the lack of effects observed in the study summary that is available, a PNEC for soil cannot be calculated.
8.2 Ecological exposure
8.2.1 Measured concentrations in environmental media and wastewater
The discussion on the measured environmental concentrations of the medium-chain phthalate esters considers several media, including air, water, sediment, soil and biota. Measured data are primarily available for DIBP, and to a lesser extent DCHP and DIHepP in water, sediment and biota in urban areas. Results can be found in Environment Canada (2015). Measured environmental concentrations are not available for B79P or B84P, the two medium-chain phthalates that have been reported to be in commerce in the highest quantities in Canada. Measured concentrations are also not available for the medium-chain phthalates that were not reported to be in commerce (CHIBP, DMCHP, BIOP, DBzP and BCHP).
Phthalate esters are commonly found as background concentrations in both sampling and analytical equipment, as well as in laboratory air and reagents (McConnell 2007). Reducing and determining the background contamination of samples and properly cleaning field equipment is crucial for ensuring that environmental measurements on phthalate esters are acceptable, accurate and of high quality (Lin et al. 2003).
Measured concentrations in air
No monitoring data for concentrations of the medium-chain phthalates in air could be found in Canada. Internationally, DIBP and DCHP have been detected in outdoor air, the results for which can be found in Environment Canada (2015). Rudel et al. (2010) measured DIBP in more than 90% of air samples at concentrations ranging from 1.4 to 18 ng/m3. Lower concentrations have been measured in Sweden, with the maximum air concentration of 2.6 ng/m3 measured near industrial sites (Cousins et al. 2007). Concentrations have also been measured in the Norwegian Arctic, ranging from 0.096 to 0.549 ng/m3 (Xie et al. 2007). DCHP has been monitored at industrial and rural sites in California, where the method reporting limit was 1 ng/m3, with no concentrations detected in most samples (Rudel et al. 2010).
Measured concentrations in water
A range of concentrations of medium-chain phthalates have been measured in surface waters in Canada (Environment Canada 2015). Data were available for surface waters in Alberta and British Columbia, predominantly for DIBP. In urbanized areas such as False Creek Harbour in British Columbia, mean concentrations of DIBP of approximately 5 ng/L have been detected (McConnell 2007; Mackintosh et al. 2006), although these concentrations are near the detection limits (6.4-7.9 ng/L). Sosiak and Hebben (2005) measured median concentrations of 2.85 ng/L downstream of wastewater treatment plants in Alberta using detection limits ranging from 0.01 to 5.7 ng/L. DIHepP has been detected in higher concentrations in False Creek Harbour, ranging from 2.91 to 153 ng/L, with an average of 21.1 ng/L (Mackintosh et al. 2006).
DIBP concentrations in urban environments in other countries have been measured at higher concentrations than those reported in Canada. Concentrations are listed in Environment Canada (2015). In Germany and China, median concentrations were as high as 56 ng/L in the early 1990s and 430 ng/L in 2005, respectively (Furtman 1994 and Zeng 2008).
Measured concentrations of DCHP in the environment are only available for other countries. A monitoring study in the Netherlands found a median surface water concentration of 8 ng/L, while the median concentration in wastewater effluents was slightly higher at 15 ng/L (Vethaak et al. 2005). DCHP has also been measured in Germany and China, with median concentrations below 0.03 and 76 ng/L (Furtman 1994 and Zeng 2008). It would be expected that DCHP concentrations in Canada would be far below those found in China, based on DIBP measured concentrations, which were found to be much higher in China than in Canada.
Degradation products of DIBP and DIHepP were measured by Sosiak and Hebben (2005) in wastewater treatment plant effluents and receiving rivers in Alberta. In all cases, mono-butyl phthalate and the mixture of mono C7 isomers were not detected. This was consistent with what has been measured in Japan, where MIBP was not detected in the Tama River (with a method detection limit of 12 ng/L) (Suzuki et al. 2001). Blair et al. (2009) measured the concentration of the isomeric mixture mono-iso-heptyl phthalate in False Creek Harbour, and measured between 2.71 and 6.61 ng/L in all ten samples.
BBP and DBP have been measured in biosolids in Vancouver, British Columbia, in the range of less than 0.02 to 1.3 µg/g (Bright and Healy 2003). Biosolids monitoring data are not available for substances in the medium-chain phthalate subgroup.
Measured concentrations in sediment
DIBP and DIHepP have been detected in sediment in False Creek Harbour, with mean concentrations ranging from 4 to 5.6 ng/g dw, and 27 to 60.8 ng/g, respectively (Mackintosh et al. 2006; McConnell 2007). DIHepP is known to have a significantly lower commercial involvement than DIBP (Environment Canada 2014). Its higher concentrations in sediment could therefore be attributed to its more hydrophobic nature. Mackintosh et al. (2006) found that concentrations of the phthalates in bottom sediments were significantly lower than those in suspended sediments. For example, the concentration of DIBP in suspended sediments was 1190 ng/g dw compared to 4 ng/g dw in bottom sediment. DIBP has been measured in more remote areas of Hudson's Bay and found to have a lower mean concentration (0.22 ng/g) (Morin 2003). A complete list of concentrations in sediment can be found in Environment Canada (2015).
Concentrations in the Netherlands have been measured at a maximum of 11 ng/g, but most samples are below the detection limit of 2 ng/g (Vethaak et al. 2005).
Measured concentrations in soil
No monitoring data for concentrations of the medium-chain phthalates in soil could be found in Canada. DIBP and DCHP were detected in soils in China (Environment Canada 2015). Residential areas in China had DIBP concentrations ranging from 40 to 1420 ng/g (Zeng et al. 2009; Hongjun et al. 2013). DCHP levels in the soil of residential areas were lower, with concentrations of approximately 71 ng/g and a greater number of samples with concentrations below the detection limits (Zeng et al. 2009). Based on trends found in comparing water and sediment samples from Canada to those from China, it would be expected that soil in Canada would also have lower concentrations.
Measured concentrations in biota
A series of studies on the distribution of dialkyl phthalate esters in a marine environment have been conducted in False Creek Harbour. Environment Canada (2015) lists the lipid equivalent concentrations found in biota in False Creek Harbour. Samples from 1999 and 2005 show that DIBP was detected in at least four levels of the aquatic food chain: algae, bivalves, fish and the Surf Scoter (Mackintosh et al. 2004; McConnell 2007). Bivalves and fish exhibited the highest concentrations, ranging from 32.4 to 160 ng/g lipid weight (lw) and 7 to 162 ng/g lw, respectively. While DIBP and other medium-chain phthalates are expected to be metabolized by aquatic organisms, a continuous exposure in these urban areas could be responsible for the concentrations measured in biota.
A study in Hudson's Bay measured the concentrations of DIBP in the Arctic cod and beluga whale, and found concentrations in both organisms. Morin (2003) compared the measured fish concentrations in the Arctic with those measured by Mackintosh et al. (2004) in urban areas. Comparisons were made between the Pacific staghorn sculpin (Leptocottus armatus) and the Arctic cod, as they hold a similar trophic level in the food web. Mackintosh et al. (2004) measured approximately 145 ng/g lw of DIBP in the sculpin in False Creek Harbour, while Morin (2003) measured a significantly higher concentration (p less than 0.05) of 413 ng/g lw in the Arctic. Morin noted DIBP was the only phthalate that exhibited this significant increase in the Arctic. Median concentrations in the beluga whale were higher, at approximately 544 ng/g lw.
Long range transport is not expected to occur with the medium-chain phthalates, as discussed in section 7.1.1, and the high measurements of DIBP in biota in the Arctic are unexpected. The low measured concentration of 0.22 ng/g in sediment in the Arctic (Morin 2003), as compared to the higher measurements of 27 to 60.8 ng/g DIBP in False Creek Harbour (Mackintosh et al. 2006 and McConnell 2007), are consistent with the long-range transport predictions. DIBP concentrations in water were not measured in the Arctic. Nevertheless, Morin (2003) noted that the results indicate that phthalate esters are being transported northwards from mid-latitudes, as concentrations in the different ecosystems are similar, and emissions from sources in the Arctic would be minimal (e.g., small landfills). The source of the exposure is a key uncertainty of the data. While DIBP sources in the Arctic are expected to be minimal, it is possible that the Arctic cod and beluga whale were exposed to DIBP in another area of their migration. The Eastern Hudson Bay population of beluga whales are described by Morin (2003) as being permanent residents of the North. They have been found to leave Eastern Hudson Bay in the fall and go into Ungava Bay and as far as Nain, Newfoundland and Labrador, in the winter (COSEWIC 2004). Another possible source is from prey that could have been exposed to DIBP in their migration. The migration patterns of Arctic cod could not be found in the literature.
Contamination during field sampling or laboratory analysis is another possible reason for the high biota concentrations measured in the Arctic, although this is unlikely, as steps were taken to reduce the potential for contamination. The Arctic study was carried out by the same research group at Simon Fraser University that conducted the False Creek Harbour studies reported by McConnell (2007) and Mackintosh et al. (2004).
The measurements from the Morin study (2003) are the only data available in the literature for medium-chain phthalate concentrations in biota in the Arctic. Further work is needed in this area to determine if these measurements can be repeated, given the unexpected nature of the results.
8.2.2 Exposure scenario for B79P and B84P
Measured environmental concentrations are not available for B79P or B84P, the two medium-chain phthalates that have been reported to be in commerce in the highest quantities per industrial site in Canada (Environment Canada 2014). These substances are used in applications of automotive sealants and coatings in automobile and light-duty motor vehicle manufacturing. Through section 71 submissions and related communication with the automotive industry (Environment Canada 2014), it was learned that B84P and B79P are contained within automotive adhesives and sealers, which are applied in the body shop, in the paint shop and in general assembly at automobile and light-duty motor vehicle manufacturing plants. Body shop adhesives are applied between vehicle body panels, whereas body shop automotive sealers are applied as a weld sealer when vehicle bodies are assembled in the plant's body shop from sheet metal parts. The weld sealers are typically used for seam sealing, waterproofing and dust-proofing, and are applied to seams and joints directly on the metal. In the body shop, adhesives and sealers are pumped directly from their containers (typically lined 55 gallons drums) and applied to the body frame and panels using robots.
In the paint shop, sealers are supplied in lined 55 gallon drums and 300 gallon magna drums, and are pumped directly to the point of use. Most paint shop sealers are applied by robots directly to the vehicle body, depending on where the materials are needed. For example, some paint shop sealers are used for sound deadening and are applied to the inside of the vehicle floor and other inside panels where acoustical controls are needed. Other sealers are typically applied along the floor boards, the inside of the trunk and other interior cavities of the frame of the vehicle. These are not typically visible after vehicle assembly is complete. For certain areas in the vehicle, sealers may be applied manually by an operator, or by using a wand. In general assembly, urethane adhesive is used for windshield installation and is contained in lined 55 gallon drums. The material is applied by a robot.
In addition, some repair paints contain phthalates. Repair paints are used for quality control and are applied in very limited quantities (typically a few millilitres per vehicle) manually in a controlled area with air filtration system.
Among these applications, there are some possibilities for B84P and B79P to enter a wastewater stream at the facility of use. For example, the automotive industry specified that after the body shop weld sealant application, prior to painting, the vehicle frame known as the "Body in White" enters the wash and rinse stages to remove any oil, grease and dirt from the metal surfaces for quality control (Environment Canada 2014). There is therefore a theoretical potential for some sealer and phthalates to be transferred to an on-site wastewater treatment system during the cleaning process (phosphating). However, no information is available on the amounts of weld sealants and their constituents that could potentially be lost to wastewater during the phosphating or cleaning process. Also, the automotive industry pointed out that if some sealers are removed during the wash and rinse stages, they will settle as solids at the bottom of the sludge tank and are removed during a prescribed cleaning schedule (at least once per year) (Environment Canada 2014). Phthalates are not chemically bound to the polymer, meaning that a migration of phthalates from the sealant solids at the bottom of the sludge tank is theoretically possible over the course of one year. This is a large uncertainty for this exposure assessment. Consequently, the quantitative exposure analysis cannot be performed at this time due to a lack of information to assess. The sampling of on-site effluent could be recommended in this case to obtain the predited environmental concentration for use in risk characterization.
With respect to the sealers and adhesives applied in the paint shop or in general assembly, they are all applied after the rinse and wash stages that occur prior to painting and are therefore not rinsed after application. Consequently, no releases to on-site wastewater are expected.
8.3 Characterization of ecological risk
8.3.1 Consideration of lines of evidence
The substances in the medium-chain phthalate subgroup biodegrade relatively quickly in the environment and are therefore expected to have a relatively short residence time in water, soil and sediment. However, at very low concentrations, biodegradation processes have been observed to slow down, resulting in background levels. With low to moderate water solubilities and high log Kocvalues, these substances are expected to be found in water, soils and sediments. Levels of medium-chain phthalates have been measured globally in these media, including Canadian locations. The analogues BBP and DBP have been measured in biosolids in Vancouver. There is limited evidence for long-range transport, based on a study of the smallest of the medium-chain phthalates, DIBP, which can potentially be attributed to fine particle transport. Medium-chain phthalates have low to moderate bioaccumulation potential. They are moderately toxic to organisms, acting through polar narcosis. Data related to endocrine effects of the medium-chain phthalates are limited. While in vitrostudies suggest that the analogue phthalate BBP could be weakly estrogenic, the limited in vivo studies suggest lack of endocrine activity in fish. The analogue DEHP has been found to act as a weak estrogen agonist in fish in both in vitro and in vivo studies. DCHP was found to disrupt gene expression of the thyroid hormone, and cellular axes in amphibians.
Based on industry submissions for the medium-chain phthalates in response to a section 71 survey (Environment Canada 2014), the reported uses of these phthalates in Canada are in automotive sealants and coating applications, in high-temperature coating manufacturing and applications, in adhesive and sealant manufacturing and applications, and in printing ink manufacturing and applications. Also, the medium-chain phthalates are imported to Canada as solvents for manufacture of thermoplastic and thermosetting resins or as a part of plastic resins and PVC cables for electronic equipment. However, only B84P and B79P were identified with high-use volumes in Canada. Five of the substances, CHIBP, BCHP, DMCHP, BIOP and DBzP, do not appear to be imported or used in Canada above the reporting thresholds, and three of the substances, DIBP, DCHP and DIHepP, are imported to Canada either in small quantities and distributed among different customers or imported to Canada as part of the final articles (e.g., PVC cables, computer parts and farm, lawn and garden machinery) (Environment Canada 2014). Therefore, for these eight phthalates, releases to the aquatic environment from industrial activities are expected to be dispersed and low.
CHIBP, BCHP, DMCHP, BIOP and DBzP
Given that CHIBP, BCHP, DMCHP, BIOP and DBzP are either not used or used in very limited quantities in Canada (Environment Canada 2014), exposure and risk characterization will not be further defined.
DIBP and DCHP
Releases to the aquatic environment from industrial activities and consumer uses of DIBP and DCHP are expected to be dispersed and low. Due to the limited information on the potential industrial process and releases from products of DIBP and DCHP, the predicted environmental concentrations (PECs) were not calculated using estimates or calculations for parameters such as substance loss to environmental media and removal rates. Instead, measured environmental concentrations in Canadian locations were used to define the PECs and to characterize risk. In selecting the PECs based on measured concentrations, consideration was given to the analytical precision of the reported values (i.e., proximity to detection limits) and the number of samples included in the estimate. For the medium-chain phthalates, the available measured concentrations that were selected as PECs are listed in Table 8-6. As monitoring data were not available for DCHP, the measured value for DIBP has been used to calculate the PEC for DCHP. This is expected to be a conservative assumption, given that DIBP quantities reported in Canada are higher than DCHP (Table 4-2), and the reported uses are similar (Table 5-1). This is further supported by a comparison of the DCHP concentrations reported in wastewater effluent in other countries (in the range of 5.43 to 15 ng/L), which are lower than those reported for DIBP in Canada (17.65 ng/L). A sediment PEC could only be calculated for DIBP (reported in Table 8-9), as other medium-chain phthalates have not been measured in sediment in Canada.
PEC values based on the measured substance concentration in the environment are presented in Table 8-9.
| Common name | Location | Sampling period | Predicted environmental concentration | Reference |
|---|---|---|---|---|
| DCHP | Alberta, Canada Downstream of MWWTP |
2002-2003 | 2.85 × 10-6 mg/L (2.85 ng/L)Footnote Table 8-9[a] |
Sosiak and Hebben 2005 |
| DIBP | Alberta, Canada Downstream of MWWTP |
2002-2003 | 2.85 × 10-6 mg/L (2.85 ng/L) |
Sosiak and Hebben 2005 |
| DIBP | British Columbia, Canada False Creek Harbour sediment |
1999 | 5.6 ng/g dw | McConnell 2007 |
A risk quotient analysis for DIBP and DCHP, based on a qualitative analysis of exposure, predicted environmental concentrations (PEC) (based on measured quantities in the environment) and toxicity information, was performed. Table 8-10 provides a summary of this information.
| Common name | Media | PNEC | PEC | RQ |
|---|---|---|---|---|
| DCHP | Water | 6.0 × 10-2 mg/L | 2.85 × 10-6 mg/L | 4.75 × 10-5 |
| DIBP | Water | 0.19 mg/L | 2.85 × 10-6 mg/L | 1.5 × 10-5 |
| DIBP | Sediment | 16.64 mg/kg dw | 5.6 mg/kg dw | 0.34 |
Based on the information available, DIBP and DCHP are unlikely to cause harm in the Canadian environment. It is noted that the use of PECs derived from measured environmental concentrations may contribute to the underestimation of environmental risk. Sampling locations away from the actual sources of release, delayed timing of sampling allowing for environmental degradation, errors in detection, or degradation of samples may be some of the reasons that could influence the measurement of chemicals in the environment. For DIBP and DCHP, there is a five-order-of-magnitude margin of safety in the calculated aquatic RQs that reduces the significance of this uncertainty.
DIHepP
Releases to the aquatic environment from industrial activities and consumer uses of DIHepP are expected to be dispersed and low.
Results from an analysis of critical body residues (CBRs) derived using the water solubility limit of DIHepP indicated that maximum tissue concentrations of DIHepP based on solubility limits will be much lower than levels associated with adverse acute or chronic effects in organisms due to neutral narcosis. Similar analyses conducted for DIHepP in sediment organisms indicated that maximum tissue concentrations calculated from the saturation limit of DIHepP in a 4% OC sediment do not exceed minimum concentrations estimated to cause narcotic effects. Therefore, while DIHepP has been measured in Canadian surface waters and sediment (no soil monitoring data are available), it is unlikely that internal body concentrations in exposed organisms will reach levels that are sufficiently high to cause adverse effects. For example, a maximum freshwater concentration of 68.7 ng/L was reported for DIHepP upstream of a municipal wastewater treatment plant (Sosiak and Hebben 2005). This corresponds to a CBR in aquatic organisms of 0.085 mmol/kg (see CBR calculation in the ecological effects assessment section). As this value falls below the ranges of 2 to 8 mmol/kg and 0.2 to 0.8 mmol/kg for acute and chronic effects, respectively, aquatic organisms exposed to this concentration in the environment are unlikely to exhibit adverse effects resulting from baseline narcosis. Similarly, Mackintosh et al. (2006) reported a highest sediment concentration of 60.8 ng/g dw for DIHepP in an estuarine sediment collected in Vancouver, British Columbia. The CBR in sediment organisms is 8.8 × 10-5mmol/kg (see CBR calculation in the ecological effects assessment section), indicating that adverse effects due to neutral narcosis are unlikely to occur.
Several studies report the presence of DIHepP in a number of Canadian aquatic species. A mean concentration in fish of 44 ng/g ww was measured in juvenile Shiner Perch (McConnell 2007). This was converted to CBR units in order to investigate whether tissue levels in the fish were high enough to potentially result in adverse effects attributable to baseline narcosis. The CBR for this tissue concentration is 1.2 × 10-4 mmol/kg (0.044 mg/kg / MW 362.51 g/mol). This value is below the ranges of 2 to 8 mmol/kg and 0.2 to 0.8 mmol/kg attributed to acute and chronic narcotic effects, respectively, suggesting that the Pacific staghorn sculpin in the study are not likely to be experiencing adverse narcotic effects due to the presence of DIHepP in their tissues.
B84P and B79P
Due to the limited information on the potential industrial process and releases from products of B84P and B79P and the lack of measured environmental concentrations, no Predicted Environmental Concentrations (PECs) for B84P and B79P are proposed at this time. When available, monitoring data may be considered for estimating potential exposure and in risk characterization.
8.3.2 Uncertainties in Evaluation of Ecological Risk
There is limited knowledge of industrial processes for the medium-chain phthalates, which has a direct impact on an accurate evaluation of their potential releases and risk characterization. For most substances, a derivation of a PEC based on modelled scenarios was not possible. PECs were therefore derived from measured environmental concentrations. Lack of monitoring data and limitations in the availability of information on the potential industrial processes and releases from products of B84 and B79P do not allow for the calculation of a PEC.
Although monitoring data were available at numerous locations, they may not be ideal for characterizing the risk of the medium-chain phthalates, as the locations may not correspond to areas of environmental releases. A monitoring campaign of the ten medium-chain phthalates will be undertaken by Environment Canada from 2014 to 2015. It will include wastewater treatment plants situated across Canada.
Limited monitoring data for DIBP in the Arctic that show the presence of this substance in biota were presented by Morin (2003). It is thought that these findings require further investigation, as they point to an unexpected presence of these substances in locations distant from sources of exposure. Long-range transport predictions suggested that medium-chain phthalates do not have the potential to travel long distances. Fine particle transport is considered as a plausible explanation.
The monitoring data provide clear evidence that the medium-chain phthalates are found in the aquatic environment. Studies suggest that certain phthalates (such as BBP) have the potential to cause effects on the endocrine system; however, such studies have not been conducted for any of the substances in the medium-chain phthalates subgroup. The use of the analogue BBP for the evaluation of the potential for endocrine effects of the subgroup in aquatic organisms is uncertain. Such effects are substance-specific and structure-dependent and can affect organisms at different life stages through different mechanisms of action. Therefore, medium-chain phthalates should be evaluated independently for their potential to cause effects on the endocrine system.
For numerous medium-chain phthalates, there were no experimental data on physical-chemical properties, degradation, bioaccumulation and ecological effects. Consequently, the analogue and read-across approaches were heavily used throughout the assessment of these substances. These approaches were considered appropriate; analogues and (Q)SAR models were thoughtfully considered. However, because the data were not specific to the medium-chain phthalates, there is a level of uncertainty associated with the application of read-across and modelled data that, in effect, may translate into over- or underestimation of overall risk associated with the environmental presence of these substances.
There was uncertainty in the analysis of biodegradation of medium-chain phthalates. Although biodegradation studies are available for numerous substances, they follow different protocols of varied duration and degree of inoculum acclimation. This leads to difficulty in assessing biodegradation rates and comparing results across the studies.
To be more certain in the evaluation of risk, the data outlined in Table 8-11 are needed for the substances in the medium-chain phthalate subgroup.
| Data gaps | Details |
|---|---|
| Sediment and soil effects | Toxicity studies in soil and sediment species conducted according to OECD or other internationally recognized protocols. |
| Effects studies addressing endocrine activity and effects on reproduction in aquatic and terrestrial organisms | Studies or assays addressing endocrine activity and effects on reproduction (including testicular dystrophy) in aquatic species, such as fish, and in terrestrial organisms, such as earthworms, according to OECD or other internationally recognized protocols. |
| Monitoring in urban areas and in the Arctic | Work to be undertaken by Environment Canada in 2014-2015 in urban areas. Additional studies in the Arctic are needed. |
| Industrial processes and applications | Information specific to industrial application in the automotive sector for B79P and B84P. General knowledge of processes and applications allowing evaluation of industrial releases of medium-chain phthalates. |
9. Potential to Cause Harm to Human Health
10. References
Appendices
- Appendix A: Structural identity and physical chemical properties of analogue substances
- Appendix B: Physical and chemical properties for the substances in the medium-chain phthalate subgroup
- Appendix C: Results of Level III fugacity modelling (EQC 2011) for the medium-chain phthalate esters in the phthalate substance grouping
- Appendix D: Bioaccumulation
- Appendix E: Toxicity values
- Appendix F-1: Estimates of daily intake
- Appendix F-2: Methodology - Dietary intakes
- Appendix G: Methodology - Biomonitoring intakes
- Appendix H: Summary of toxicokinetics of medium-chain phthalates (MCPs)
- Appendix I: Supporting information of the chronic toxicity and carcinogenicity of BBP
- Appendix J: Description and Application of the Downs and Black Scoring System and Guidance for Level of Evidence of An Association