Updated draft screening assessment - Certain organic flame retardants substance grouping - TCPP and TDCPP
Official title: Updated Draft Screening Assessment - Certain Organic Flame Retardants Substance Grouping - 2-Propanol, 1-chloro-, phosphate (3:1) (TCPP) and 2-Propanol, 1,3-dichloro-, phosphate (3:1) (TDCPP)
Chemical Abstracts Service Registry Numbers:
- 13674-84-5
- 13674-87-8
Environment and Climate Change Canada
Health Canada
October 2020
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment on 2-propanol, 1-chloro-, phosphate (3:1), hereinafter referred to as TCPP, Chemical Abstracts Service Registry Number (CAS RN) 13674-84-5, and 2-propanol, 1,3-dichloro-, phosphate (3:1), hereinafter referred as TDCPP, CAS RN 13674-87-8. TCPP and TDCPP are part of the Certain Organic Flame Retardants (OFR) Substance Grouping, which includes ten organic substances having similar function: application to materials to slow the ignition and spread of fire. These two substances were identified as a priority for assessment based on other human health concerns. A draft screening assessment for TCPP and TDCPP was published in October 2016. Significant new information subsequently became available regarding exposure to products available to consumers, specifically foam products containing flame retardants such as TCPP and TDCPP. As a result, the draft assessment was updated and is presented here.
TCPP and TDCPP are discrete organic chemicals that do not occur naturally in the environment. According to information identified from a survey issued under section 71 of CEPA, there is no manufacturing of either TCPP or TDCPP in Canada. Both substances were predominantly imported into Canada as pure substances or in manufactured items. The total import volumes in 2011 ranged from 1 000 000 to 10 000 000 kg of TCPP, and 100 000 to 1 000 000 kg of TDCPP.
TCPP is used as an additive flame retardant for manufacturing of building or construction materials in Canada (e.g., polyurethane spray foam insulation), and is contained in imported products of polyurethane spray foam insulation with the same functional use. TCPP is also imported into Canada in manufactured items containing flexible polyurethane foam (e.g., in upholstered furniture and mattresses) and as a textile waterproofing spray intended for consumer use. Available information indicates the potential for migration of flame retardants from foam objects.
TDCPP is used as an additive flame retardant in the manufacturing of flexible polyurethane foam in Canada (used in manufactured items such as upholstered furniture and mattresses). The substance is imported as a pure substance and in commercial products and products available to consumers with the same functional use.
TCPP is highly soluble in water and has a low octanol-water partition coefficient, while TDCPP possesses moderate water solubility and octanol-water partition coefficient. Both substances have a low vapour pressure and do not dissociate in water. Empirical studies indicate that neither substance is rapidly biodegradable. Both substances are considered to be very stable in water, sediment and soil, but not air (gas phase). Based on findings from environmental sampling studies, TCPP and TDCPP have been found to be associated with particulates in air where they are considered to be very persistent. Both substances have been detected in air samples over the Arctic areas in Canada and Europe and are considered to have potential for long-range transport when adsorbed to aerosols.
Potential environmental releases of TCPP and TDCPP are from industrial activities (during their blending with a polyol) and from use of commercial products and products available to consumers. Releases from industrial activities are expected to primarily enter water via wastewater treatment systems. Based on physical and chemical properties, TCPP will partition to water, with insignificant amounts partitioning to sediments. On the other hand, TDCPP may be found in both sediment and water to some extent. Unlike TCPP, which is expected to remain predominantly dissolved in effluents, TDCPP, given its greater propensity to adsorb to solids, is likely to be found adsorbed to wastewater treatment system biosolids, which ultimately may be applied to soils. Emissions from manufactured items, commercial products, and products available to consumers are expected to enter the environment through air or dust, and ultimately settle in surface water and soil. However, it is expected that releases to the environment via this route are minimal and diffuse.
As would be expected based on the physical and chemical properties of TCPP and TDCPP, laboratory studies have reported low bioconcentration factors and rapid metabolism for these two substances, indicating that both substances have a limited potential to accumulate in aquatic biota. Significant exposure in higher trophic level organisms through the food chain is not expected for TCPP and TDCPP. Rapid excretion of biotransformation products in the mammalian studies suggests that metabolites are also unlikely to bioaccumulate.
Empirical ecotoxicity data have been identified for both substances. TCPP has demonstrated moderate toxicity to aquatic organisms and terrestrial plants; while TDCPP has shown considerably higher toxicity to aquatic organisms including effects on the endocrine system in fish. Additional sub-lethal effects (i.e., neurotoxicity and genetic effects in birds) are also noted in both in vivo and in vitro studies. Data for endpoints from in vitro studies which show linkage to organism level effects have been considered in the risk assessment for these two substances.
Considering the environmental fate and available toxicity data for these two substances, risk quotient analyses were conducted in the aquatic compartment for TCPP and in the aquatic, sediment and soil compartments for TDCPP. Outcomes from the risk quotient analyses indicate that the risk associated with exposure of organisms to these two substances due to releases from industrial uses and products available to consumers is low at current predicted levels of release.
Considering all available lines of evidence presented in this updated draft screening assessment, there is low risk of harm to the environment from TCPP or TDCPP. It is therefore proposed to conclude that TCPP and TDCPP do not meet the criteria under paragraphs 64 (a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of available information on concentrations in environmental media and results from a survey under section 71 of CEPA, the general population is expected to be exposed to TCPP and TDCPP from environmental media (air, water, dust), from food and during the use of products available to consumers containing this substance (i.e., in products such as spray foam and waterproofing products and manufactured items such as foam-containing furniture and mattresses).
On the basis of the available information on health effects of TCPP, the critical effects for characterization of risk to human health are reproductive and developmental effects.
The margin of exposure between estimates of exposure to TCPP from environmental media (air, water, dust) and food (including breast milk) as well as use of spray insulation foam or sealant and waterproofing sprays, and the critical effect levels are considered to be adequate to address uncertainties in the health effects and exposure databases. The margins of exposure between estimates of exposure resulting from prolonged skin contact with certain manufactured items containing TCPP, such as foam-containing upholstered furniture and mattresses, and the critical effect levels are considered potentially inadequate to account for uncertainties in the exposure and health effect databases.
On the basis of available information and classifications by other international regulatory agencies, critical effects for characterization of the risk to human health from exposure to TDCPP are carcinogenicity and non-cancer effects on the kidneys and testes. Tumours were observed in multiple organ sites, including kidney and liver in both sexes, testes (in males) and adrenal gland (in females) in a two-year carcinogenicity study in rats. Results of genotoxicity tests were mixed in vitro and mostly negative in vivo.
The margins of exposure between estimates of exposure to TDCPP from environmental media (air, water, dust) and food (including breast milk), and the critical effect levels for cancer and non-cancer effects are considered to be adequate to address uncertainties in the health effects and exposure databases. The margins of exposure between estimates of exposure resulting from prolonged skin contact with manufactured items containing TDCPP, such as foam-containing upholstered furniture and mattresses, and the critical effect levels for cancer and non-cancer effects are considered potentially inadequate to account for uncertainties in the exposure and health effects databases.
On the basis of the information presented in this updated draft screening assessment, it is proposed to conclude that TCPP and TDCPP meet the criteria under paragraph 64 (c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that TCPP and TDCPP meet one or more of the criteria set out in section 64 of CEPA. It is also proposed that TCPP and TDCPP meet the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
List of abbreviations and acronyms
- 11-KT
- 11-ketotestosterone
- BAF
- Bioaccumulation Factor
- BCFs
- Bioconcentration Factors
- BCPP
- Bis(1-chloro-2-propyl) phosphate
- BDCPP
- Bis(1,3-dichloro-2-propyl) hydrogen phosphate
- BMD
- Benchmark Dose
- BMDL
- Benchmark Dose Limit
- BMDS
- Benchmark Dose Software
- BOD
- Biological Oxygen Demand
- CAS RN
- Chemical Abstracts Service Registry Number
- CDAT
- Chemical Data Access Tool
- CEPA
- Canadian Environmental Protection Act
- CHO-K1
- Chinese Hamster Ovary Cells
- CMP
- Chemicals Management Plan
- CTV
- Critical Toxicity Value
- DIY
- Do-It-Yourself
- DPD
- Drug Product Database
- Dpf
- Day Post Fertilization
- E2
- 17β-estradiol
- EC
- Effective Concentration
- ECCC
- Environment and Climate Change Canada
- ECHA
- European Chemicals Agency
- EU
- European Union
- F0
- Adult Fish
- F1
- First Generation
- HPT
- Hypothalamic-Pituitary-Thyroid
- HPT
- Hypothalmus-Pituitary-Testis
- i.v.
- Intravenous
- IARC
- International Agency for Research on Cancer
- Koc
- Organic Carbon–water partition coefficient
- Kow
- Octanol–Water partition coefficient
- LADD
- Lifetime Average Daily Dose
- LC
- Lethal Concentration
- LNHPD
- Licensed Natural Health Products Database
- LOD
- Limit of Detection
- LRTP
- Long-Range Transport Potential
- MDL
- Method Detection Limits
- MOE
- Margin of Exposure
- MUR
- Most Used Room
- NHANES
- National Health and Nutrition Examination Survey
- NHPID
- Natural Health Products Ingredients Database
- NOECs
- No Observed Effect Concentrations
- NRC
- National Research Council
- NTE
- Neurotoxic Esterase
- NTP
- National Toxicology Program
- OC
- Organic Carbon
- OECD
- Organisation for Economic Co-operation and Development
- OFR
- Organic Flame Retardants
- OPFRs
- Organophosphate Flame Retardants
- OPIDN
- Organophosphate-Induced Delayed Neurotoxicity
- P95
- 95th Percentile
- PEC
- Predicted Environmental Concentration
- PN
- Post-Natal
- PNEC
- Predicted No-Effects Concentration
- POD
- Point of Departure
- (Q)SAR
- (Quantitative) Structure-Activity Relationship
- RQ
- Risk Quotient
- SG
- Specific Gravity
- SMR
- Standardized Mortality Ratio
- SPF
- Spray Polyurethane Foam
- STP
- Sewage Treatment Plant
- T
- Testosterone
- T3
- Triiodothyronine
- T4
- Thyroxine
- TBP
- Tributyl Phosphate
- TCEP
- Tris(2-chloroethyl)phosphate
- TCPP
- 2-Propanol, 1-chloro-, phosphate (3:1)
- TDCPP
- 2-Propanol, 1,3-dichloro-, phosphate (3:1)
- TOC
- Total Organic Carbon
- TPF
- Textile Penetration Factor
- UDS
- Unscheduled DNA Synthesis
- UFAC
- Upholstered Furniture Action Council
- US EPA
- US Environmental Protection Agency
- VTG
- Vitellogenin
- WWTS
- Wastewater Treatment Systems
1. Introduction
Pursuant to sections 68 and 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health conduct screening assessments of substances to determine whether these substances present or may present a risk to the environment or to human health.
The Substance Groupings Initiative is a key element of the Government of Canada’s Chemicals Management Plan (CMP). The Certain Organic Flame Retardant Substance Grouping consists of ten substances identified as priorities for action as they met the categorization criteria under section 73 of CEPA or were considered as a priority on the basis of ecological or human health concerns (Environment Canada, Health Canada 2007). All of these substances have a similar function: the application to materials to slow the ignition and limit the spread of fire. Also, these substances are potential alternatives for other flame retardants which are presently subject to regulatory controls or phase-out globally or in Canada.
This draft screening assessment focuses on two substances in the Certain Organic Flame Retardants Substance Grouping: 2-propanol, 1-chloro-, phosphate (3:1) (CAS RN 13674-84-5) or TCPP and 2-propanol, 1,3-dichloro-, phosphate (3:1) (CAS RN 13674-87-8) or TDCPP. These substances are considered in one screening assessment on the basis of similarity in chemical structure and other assessment parameters. Both substances were identified as a priority for assessment on the basis of other human health concerns.
A draft screening assessment for TCPP and TDCPP was published in October 2016 (ECCC, HC 2016). It proposed that TCPP was harmful to human health and met the criteria under paragraph 64(c) but not harmful to the environment and that TDCPP was not harmful to human health or the environment. Significant new information on the dermal exposure to foam products subsequently became available as a result of consultations with the European Chemicals Agency (ECHA) on their “Screening report – An assessment of whether the use of TCEP, TCPP and TDCP in articles should be restricted” published in 2018. Following further consultation with other jurisdictions, the dermal exposure to foam products was re-examined and an updated scenario was adopted. On the basis of this information, an updated draft assessment was presented here.
This updated draft screening assessment includes consideration of information on physical and chemical properties, quantity, uses, exposure, hazards, including additional information submitted by stakeholders. Relevant data were identified until July 2018 for both the ecological and human health components of this assessment. Targeted literature searches were conducted up to February 2019 for human health component of this assessment. Key studies were critically evaluated and, along with the use of modelled results, were used to reach conclusions. When available and relevant, information presented in risk and hazard assessments from other jurisdictions was considered.
This updated draft screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment and Climate Change Canada (ECCC) and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review and consultation. Comments on the technical portions relevant to the environment were received from Jon Arnot at Arnot Research and Consulting, Miriam Diamond at University of Toronto, and Andy Wang at ICL IP. Comments on the technical portions relevant to human health were received from Cathy Petito Boyce, Leslie Beyer, Chris Long and David Mayfield from Gradient Corp and from Risk Assessment Division, Office of Pollution Prevention and Toxics, US Environmental Protection Agency (US EPA). Additionally, the initial draft of this screening assessment was subject to a 60-day public comment period. Some human health portions of this assessment have undergone an additional targeted external written peer consultation. Comments were received from Richard Manderville at the University of Guelph, Mohamed Abou-Elwafa Abdallah at the University of Birmingham, and Kebede K. Kefeni at the Tshwane University of Technology. While external comments were taken into consideration, the final content and outcome of the updated draft screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This updated draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 1. This updated draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Substance identity
The substance identities of TCPP and TDCPP, two chlorinated alkyl phosphate esters, are presented in Table 2‑1. A list of additional chemical names and trade names of these two substances can be found in the National Chemical Inventories (NCI 2013).
| CAS RN | 13674-84-5 (TCPP) | 13674-87-8 (TDCPP) |
|---|---|---|
| Chemical group(DSL Stream) | Organics | Organics |
| Chemical formula | C9H18Cl3O4P | C9H15Cl6O4P |
| Chemical structure | 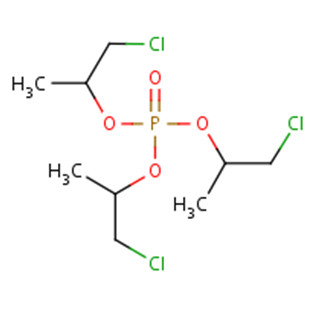 |
 |
| SMILESa string | O=P(OC(CCl)C)(OC(CCl)C)OC(CCl)C | O=P(OC(CCl)CCl)(OC(CCl)CCl)OC(CCl)CCl |
| Molecular mass | 327.57 g/mol | 430.91 g/mol |
a Simplified Molecular Input Line Entry System.
2.1 Isomers of TCPP and TDCPP
TCPP is manufactured from the reaction of phosphorous oxytrichloride with propylene oxide in the presence of a catalyst (UNEP 1999; WHO 1998). After removal of acidic impurities and residual catalyst, the final product, also referred to as TCPP, may consist of four chain isomers of TCPP (including another three CAS RNs 76025-08-6, 76649-15-5, 6145-73-9). The composition is dominated by TCPP (up to 85%), with the balance composed by the other three chain isomers in varying amounts based on commercial products provided by different suppliers.
The chemical names and structures of the three chain isomers of TCPP are illustrated in Table 2‑2.
| CAS RN | 76025-08-6 | 76649-15-5 | 6145-73-9 |
|---|---|---|---|
| Chemical name | Bis(1-chloro-2-propyl)-2-chloropropyl phosphate | Bis(2-chloropropyl)-1-chloro-2-propyl phosphate | Tris(2-chloropropyl) phosphate |
| Chemical structure | 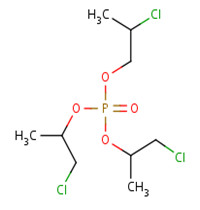 |
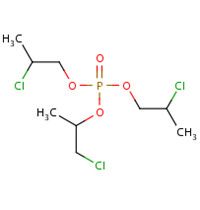 |
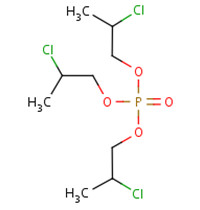 |
Studies cited in this assessment on TCPP were carried out using the commercial products of TCPP. TCPP and its chain isomers have demonstrated very similar chromatographic properties and are difficult to separate (EU RAR 2008a). Predicted physical and chemical properties by a (quantitative) structure-activity relationship ((Q)SAR) model (EPI Suite v4.1) differ only to a very small extent (ECCC 2019). For the purpose of this assessment, it is assumed that all these chain isomers have identical physical-chemical and hazard properties. Given that the differences in the isomer contents of the commercial products of TCPP would not affect the physical and chemical properties and the toxicity profile of TCPP, it is considered that data reported in studies that were carried out using the commercial products of TCPP (i.e., a mixture of chain isomers) are valid for assessing TCPP.
TDCPP is produced by the reaction of phosphorus oxychloride with epichlorohydrin (WHO 1998). Tris(2,3-dichloro-1-propyl) phosphate (CAS RN 78-43-3) is an isomer of TDCPP; however, there has been no report of this isomer identified in the commercial products of TDCPP.
2.2 Selection of analogues and use of (Q)SAR models
Guidance on the use of a read-across approach and (Q)SAR models for filling data gaps has been prepared by various organizations such as the Organisation for Economic Co-operation and Development (OECD). These methods have been applied in various regulatory programs including the European Union’s (EU) Existing Substances Programme. In this assessment, a read-across approach using data from analogues and the results of (Q)SAR models, where appropriate, have been used to inform the ecological and human health assessments. Analogues were selected that were structurally similar and/or functionally similar to substances within this grouping (e.g., based on physical-chemical properties, chemical structures, and toxicokinetics), and that had relevant empirical data that could be used to read-across to substances that were data poor. The applicability of (Q)SAR models was determined on a case-by-case basis.
Details of the read-across data and (Q)SAR models chosen to inform the ecological and human health assessments of TCPP and TDCPP are further discussed in the relevant sections of this report.
In general, for the ecological risk assessment, TCPP and TDCPP are used as analogues of each other when there is a lack of data for certain ecological endpoints. A read-across approach is applied where available empirical information for one substance is considered suitable to fill a data gap for the other substance. No additional analogues were used in the ecological risk assessment. (Q)SAR models are used for predicting environmental fate, persistence and bioaccumulation potential. Outcomes from these models are considered additional lines of evidence for assessing TCPP and TDCPP, with the relative weight assigned being dependent on reliability of the methods and results.
In the human health risk assessment, TDCPP and tris(2-chloroethyl)phosphate (TCEP) were considered qualitative analogues for assessing the carcinogenic potential of TCPP as no long-term or carcinogenicity studies of TCPP were identified (more details are available in Health Canada 2015a). The identity of TCEP is presented in Table 2‑3. In addition, several statistics-based (Q)SAR models were used to assess the carcinogenicity potential of TCPP (more details are available in Health Canada 2015a).
| Substance CAS RN | Substance name | Molecular Weight (g/mol) | Empirical Structure/Molecular Formula |
|---|---|---|---|
| 115-96-8 | tris(2-chloroethyl)phosphate (TCEP) | 285.49 | 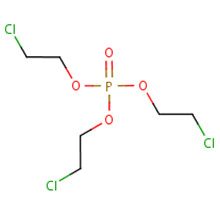 C6H12Cl3O4P |
3. Physical and chemical properties
Experimental data for physical and chemical properties of TCPP and TDCPP have been identified via literature searches and data submissions.
It is noted that there are multiple values reported for certain physical and chemical properties of TCPP and TDCPP (ECCC 2019). Upon data review and evaluation of experimental details and quality, one value was selected for characterizing each physical and chemical property (Table 3‑1 and Table 3‑2). These selected values are further applied in modelling in the assessment.
3.1 TCPP
Property |
Type |
Value |
Temperature (°C) |
Reference |
|---|---|---|---|---|
Melting point (ºC) |
Experimental |
<-20 |
- |
SafePharm Laboratories 2002a |
Boiling point (ºC) |
Experimental |
288 (boil with decomposition) |
- |
SafePharm Laboratories 2002a |
Density (kg/m3) |
Experimental |
1.29 × 103 |
20 |
SafePharm Laboratories 2002a |
Vapour pressure (Pa) |
Experimental |
0.0014 |
25 |
SafePharm Laboratories 2002b |
Henry’s Law constant (Pa·m3/mol) |
Calculated |
4.45 × 10-4 |
25 |
HENRYWIN 2011 |
Log Kow (dimensionless) |
Experimental |
2.68
|
Not specified |
SafePharm Laboratories 2002c |
Log Koc (dimensionless) |
Experimental |
2.76 |
- |
SafePharm Laboratories 2002c |
Log Koa (dimensionless) |
Modelled |
9.43 |
- |
KOAWIN 2010 |
Water solubility (mg/L) |
Experimental |
1080 |
20 |
SafePharm Laboratories 2002c |
Abbreviations: Koc, organic carbon-water partition coefficient; Kow, octanol-water partition coefficient; Koa, octanol-air partition coefficient.
A dissociation model (ACD/pKaDB c1997–2012) did not identify any dissociable functional group; TCPP is a neutral organic.
3.2 TDCPP
Property |
Type |
Value | Temperature (°C) |
Reference |
|---|---|---|---|---|
Melting point (ºC) |
Experimental |
<-20 |
- |
SafePharm Laboratories 2002d |
Boiling point (ºC) |
Experimental |
326 |
- |
SafePharm Laboratories 2002d |
Density (kg/m3) |
Experimental |
1513 |
20 |
SafePharm Laboratories 2002d |
Vapour pressure (Pa) |
Experimental |
5.6 × 10-6 |
25 |
SafePharm Laboratories 2002e |
|
(Pa·m3/mol) |
Calculated |
1.33 × 10-4 |
25 |
HENRYWIN 2011 |
Log Kow (dimensionless) |
Experimental |
3.69
|
20 |
SafePharm Laboratories 2002e |
Log Koc (dimensionless) |
Experimental |
3.25 |
Not specified |
Wildlife International 2006a |
Log Koa (dimensionless) |
Modelled |
10.96 |
- |
KOAWIN 2010 |
Water solubility (mg/L) |
Experimental |
18.1 |
20 |
SafePharm Laboratories 2002f |
Abbreviations: Koc, organic carbon–water partition coefficient; Kow, octanol–water partition coefficient; Koa, octanol-air partition coefficient.
A dissociation model (ACD/pKaDB c1997-2012) did not identify any dissociable functional group for this substance; TDCPP is a neutral organic.
4. Sources and uses
TCPP and TDCPP are not naturally occurring in the environment. Sources of TCPP and TDCPP include industrial activities and use of products containing TCPP or TDCPP.
In 2013, TCPP and TDCPP were included in a notice issued pursuant to section 71 of CEPA for the Certain Organic Flame Retardants Substance Grouping (Canada 2013), aiming to identify the current sources and uses of these substances in Canada. According to responses to this notice, there is no manufacture of either TCPP or TDCPP in Canada; however, imports to Canada totalled 1 000 000 to 10 000 000 kg for TCPP and 100 000 to 1 000 000 kg for TDCPP in 2011, with most as neat substances and a small portion in commercial products or products available to consumers (ECCC 2013-2014).
Both TCPP and TDCPP are included in the United States Environmental Protection Agency (US EPA) Chemical Data Access Tool (CDAT). The most recent data are available for 2012, reporting a national production volume of approximately 55 million lbs (approximately 25 000 000 kg) for TCPP, and 10 to 50 million lbs (approximately 4 500 000 to 22 500 000 kg) for TDCPP (US EPA 2012a). A couple of major international manufacturers of TDCPP recently discontinued their production of this substance (ECCC 2013-2014); however their reasons for doing so are unknown.
Information on use quantities of TCPP and TDCPP in Nordic countries is available up to year 2011 (SPIN 2013). TCPP has been used in all four Nordic countries (Denmark, Finland, Norway, and Sweden) in the latest 5 years and the total use quantities in these four countries ranged from 1 050 000 to 1 994 000 kg from 2007 to 2011. TDCPP has been used in some Nordic countries between 2007 and 2011; however, information on the use quantity remains confidential.
Information on the manufacturing and/or import quantity for both substances in Japan was available for recent years, 5 000 000 kg (reported as 5000 tonnes) in 2010 and at 7 000 000 kg (reported as 7000 tonnes) in 2011 for TCPP, and < 1 000 000 kg (reported as < 1000 tonnes) in 2010 and 2011 for TDCPP (J-CHECK c2010-).
According to data submissions in response to the notice issued pursuant to section 71 of CEPA for the Certain Organic Flame Retardants Substance Grouping (ECCC 2013-2014), TCPP is used as an additive flame retardant in Canada. It is mixed with other ingredients to manufacture building or construction materials (e.g., rigid polyurethane spray foam and foam board for insulation); it is also contained in imported products (e.g., rigid polyurethane spray foam) with the same functional use (ECCC 2013-2014). In addition, this substance is imported into Canada in manufactured items containing flexible polyurethane foam (e.g., furniture and mattresses) (ECCC 2013-2014; CEH 2013a,b; Stapleton et al. 2011); it has also been reported as a textile waterproofing spray intended for consumer use (SDS 2014).
TDCPP has been used as an additive flame retardant in Canada for manufacturing flexible polyurethane foam (used in furniture, mattresses and seating) (ECCC 2013-2014).
As additive flame retardants, these two substances are not chemically bound to the polymer in the finished products.
TCPP and TDCPP are not permitted for use as food additives, nor have they been identified as being used/present in formulations of food packaging materials or as incidental additives (2013 email from Food Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). TCPP and TDCPP are not listed in the Drug Product Database (DPD [modified 2017]), the Therapeutic Products Directorate's internal Non-Medicinal Ingredient Database, the Natural Health Products Ingredients Database (NHPID [modified 2017]) or the Licensed Natural Health Products Database (LNHPD [modified 2016]) as a medicinal or a non-medicinal ingredient present in final pharmaceutical products, natural health products or veterinary drugs (2013 email from Therapeutic Products Directorate, Natural Health Products Directorate and Veterinary Drugs Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). Based on notifications submitted under the Cosmetic Regulations to Health Canada, TCPP and TDCPP are not used in cosmetic products in Canada (2013 emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
Internationally, TCPP and TDCPP are used as flame retardants and plasticizers (Sundkvist et al. 2010). TCPP and TDCPP are also used in textile (i.e., upholstery) backcoating formulations in the Unites States and Europe (US CPSC 2005 a,b; EU RAR 2008b; Danish EPA 2014). While there is no confirmed textile use in Canada, TCPP and TDCPP have been measured in the upholstery of furniture purchased in Canada (CEC 2015). TDCPP is also used as a lacquer, paint and glue (Sundkvist et al. 2010).
5. Releases to the environment
Anthropogenic releases to the environment depend upon various losses occurring during the manufacture, industrial use, consumer/commercial use, service life and disposal of a substance. Releases of TCPP and TDCPP to the Canadian environment, due to the substance’s use as a flame retardant, are expected to be diffuse, with some point sources (e.g., from industrial facilities).
Direct emissions of TCPP and TDCPP to air are not expected. Releases of TCPP and TDCPP to the aquatic environment occur during the industrial use stages. According to information identified in Canada (ECCC 2013-2014), TCPP and TDCPP are used as additive ingredients in the process of manufacturing polyurethane foams. Releases from industrial activities are expected to happen via their blending with a polyol during manufacturing processes. Wastewater from the industrial manufacturing facilities may receive treatment on-site before it enters wastewater treatment systemsFootnote 2 (WWTS) nearby. From there, TCPP and TDCPP may be released to surface water and may partition to sediment. Having a high water solubility and moderate log KOC, TCPP is not expected to appreciably partition to biosolids during wastewater treatment, and the pathway leading to TCPP soil exposure due to the application of biosolids is therefore considered unlikely. On the other hand, TDCPP, with a higher log KOC and lower water solubility, has a greater propensity to partition to solids and may reside to some extent in sediment and biosolids from wastewater treatment systems. Due to partitioning to biosolids, exposure to TDCPP in soils would be expected due to land application of biosolids.
As additive flame retardants that are blended with the polymer product (rather than a reactive flame retardant chemically bonded to the polymer product), there is the possibility for some release of TCPP and TDCPP from products available to consumers to the environment (Guerra et al. 2011), likely to the air and directly to dust. Such emissions can result in atmospheric deposition to soil and water. When found in household dust, substances may end up going through the wastewater treatment systems via routine household cleaning activities. Overall, releases from products are expected to be geographically dispersed and spread out over the duration of the service life and end-of-life of these products.
Release information presented above is used to further develop exposure scenarios to estimate resulting environmental concentrations (see Section 7.2).
6. Environmental fate and behaviour
6.1 Environmental distribution
The environmental fate for a substance describes the processes by which it moves and is transformed in the environment. In this section, some general characteristics of TCPP and TDCPP will be discussed with respect to its environmental fate in different compartments in an effort to understand how organisms come into contact with the substances in a particular medium, the persistence of the two substances in the environmental compartments, degradation, and distribution among media.
TCPP and TDCPP are expected to be released from industrial activities to wastewater and undergo migration from use of products to the air and dust.
Based on physical and chemical properties (Table 3‑1 and Table 3‑2), the environmental fates of TCPP and TDCPP were predicted using Level III fugacity modelling (EQC 2011) assuming steady-state emissions to air, water and soil. The Level III EQC model assumes non-equilibrium conditions between environmental compartments, but equilibrium within compartments. The results (Table 6‑1 and Table 6‑2) represent the net effects of chemical partitioning, inter-media transport and loss by both advection (out of the modelled region) and degradation/transformation processes, i.e., relative steady-state distribution in the physical environmental compartments.
According to the EQC model, TCPP and TDCPP demonstrate similar environmental fate and distribution. Regardless of the environmental medium to which they are released, there is negligible distribution to air, but significant distribution to water and soil compartments, depending on the release scenario. Because of its considerably lower water solubility and higher KOC, partitioning to sediment is greater for TDCPP in comparison to TCPP. It is noted that the model is not able to account for the association with particulates in the atmospheric compartment; therefore the prediction of negligible partitioning in air differs from the environmental monitoring data identified for TCPP and TDCPP.
Substance released to |
Partitioning in air (%) |
Partitioning in water (%) |
Partitioning in soil (%) |
Partitioning in sediment (%) |
|---|---|---|---|---|
Air (100%) |
Negligible |
8.8 |
91.2 |
Negligible |
Water (100%) |
Negligible |
99.7 |
Negligible |
0.3 |
Soil (100%) |
Negligible |
7.9 |
92.0 |
Negligible |
Substance released to |
Partitioning in air (%) |
Partitioning in water (%) |
Partitioning in soil (%) |
Partitioning in sediment (%) |
|---|---|---|---|---|
Air (100%) |
Negligible |
1.6 |
98.3 |
0.1 |
Water (100%) |
Negligible |
94.6 |
Negligible |
5.4 |
Soil (100%) |
Negligible |
1.0 |
99.0 |
0.1 |
6.1.1 Long-range transport potential (LRTP)
The analysis of environmental fate by the EQC model has shown that, when TCPP and TDCPP are released to air, negligible fractions will remain in air, with most partitioning to soil. As a result, in air it is expected that both TCPP and TDCPP will mainly partition to suspended particulates. This has been confirmed by air monitoring in Ontario which has shown that TCPP and TDCPP are predominantly found in particle phase (Shoeib and Jantunen 2013; Shoeib et al. 2014). While there are a few models available for predicting LRTP (e.g., TaPL3, OECD Pov and LRTP Screening Tool), these models do not consider substance associated with the particle phase and would thus be considered to underestimate overall transport potential to remote locations.
In particulate phase, empirical evidence supports a high degree of persistence for TCPP and TDCPP. Liu et al. (2014a) examined hydroxyl- (OH-) initiated oxidation in air of (NH4)2SO4 particle-associated 3 organophosphate flame retardants. Keeping the OH radical concentration at a steady state in the experiment, pseudo first-order reactions are assumed. The half-life was estimated as 7.6–9.7 days for TDCPP, derived from the lifetime of 11–14 days reported in the study (Liu et al. 2014a). In another study using a different reference compound, Liu et al. (2014b) reported the half-lives of TDCPP in both gas phase and particle phase. The half-life of this substance in the particle phase was determined to be up to 5.6 days (extrapolated from the lifetime as 8.1 days), which is longer than that determined for the gas phase (the half-life at 1.3 days, extrapolated from the lifetime at 1.9 days) (Liu et al. 2014b).
Although TCPP was not included in the Liu et al. studies (2014a, b), based on structural similarity with TDCPP and other phosphate esters that were the subject of these studies, the particle phase half-life of TCPP for OH oxidation is expected to be similar to that of TDCPP.
Findings of Liu et al (2014a, b) are in agreement with the environmental data suggesting that particle-bound TCPP and TDCPP are highly persistent in the atmosphere with regard to OH radical oxidation. In particle phase, long-range atmospheric transport of these substances is supported based on measured air concentrations, particularly those from remote locations. Specifically TCPP and TDCPP were detected along with 4 other organophosphate flame retardants (OPFRs) in the North Sea air, predominantly adsorbed to airborne particles (Moller et al. 2011). Both were also found in airborne particles over the Northern Pacific and Indian Ocean toward the Polar Regions (Moller et al. 2012) and at a European Arctic site (Salamova et al. 2014a). In Canada, TCPP and TDCPP were found in air samples collected in the Great Lakes atmosphere (Salamova et al. 2014b) and the Canadian Arctic (Jantunen et al. 2013a) (ECCC 2019).
6.2 Environmental persistence
Based on monitoring studies, TCPP and TDCPP in air are predominantly associated with particles. When adsorbed to particles, both TCPP and TDCPP are expected to be highly persistent in air.
Empirical and modelled biodegradation data consistently indicate that both TCPP and TDCPP are stable in the environment and slow to degrade. Hydrolysis is not expected for either TCPP or TDCPP under the environmental conditions in Canada.
Several biodegradation studies were identified for TCPP and TDCPP. The findings suggest no rapid ready biodegradation of either substance in water; however, there is inherent biodegradation observed. The available (Q)SAR models predict slow biodegradation of both substances. Details are discussed below.
6.2.1 Degradation in air
AOPWIN (2010) has been used to estimate the half-life of an organic substance in the gaseous phase. For TCPP and TDCPP, the model predicts very short half-lives (i.e., 2.9 and 7.1 hours, respectively) based on atmospheric oxidation; however, as these substances are expected to be associated with suspended particulates in air, these predictions are not considered useful for the assessment. AOPWIN does not take into account the association of organophosphate esters with particulates in air, which demonstrate some resistance to oxidation of OH radicals. In addition, AOPWIN is not able to predict reaction of TCPP and TDCPP with photo-oxidative species like O3 in the atmosphere, and it cannot simulate the impact of direct photolysis (AOPWIN 2010). Due to these limitations, the AOPWIN model is not considered for use to assess degradation of these substances in air.
Weight of evidence is thus given to the measured half-lives obtained from the laboratory experiments of Liu et al. (2014a, b; half-life of 5.6 – 9.7 days for TDCPP), which is more relevant to their observed presence in the Canadian environment. Given the chemical similarity of TCPP to TDCPP and observed presence of TCPP adsorbed to atmospheric particulates, both TCPP and TDCPP are expected to demonstrate resistance to the OH-initialized oxidation, and therefore not degrade rapidly in air.
6.2.2 Hydrolysis
Hydrolysis of TCPP and TDCPP has been studied under a variety of pHs and temperatures (Akzo Nobel 2001a). Those findings are summarized in Table 6‑3 and Table 6‑4 below.
TCPP was tested at 50ºC and under three different pH conditions (pH=4, 7, and 9) (Akzo Nobel 2001a). At the end of this 5-day study, less than 1% of decrease in the concentration of the test substance was observed in all test groups. Results indicate that there is no significant hydrolysis of TCPP under the environmentally relevant pH conditions (6–9).
Percentage (%) of hydrolysis |
Test period (day) | Test condition (pH) | Test condition (temperature) | Extrapolated half-life |
|---|---|---|---|---|
<1 |
5 |
pH=4, 7 and 9 |
50ºC |
> 1 year |
Similarly to TCPP, TDCPP showed no loss to hydrolysis at 50oC and pH=4 and 7 over a 5-day study; however, at pH=9, a 6% and 16% hydrolysis of TDCPP were observed on days 2 and 4, respectively (Akzo Nobel 2001b). In a longer test of hydrolysis for 30 days, the substance was further tested at pH=9 at 20ºC and 40ºC (Akzo Nobel 2001b). Losses of 3.9% and 44.5% due to hydrolysis were observed at the end of the experiment. The findings suggest that hydrolysis of TDCPP is not significantly expected at typical environmental conditions (pH=6‒9 and temperature=5–25ºC).
| Test period (day) | Test condition (pH and temperature) | ercentage (%) of hydrolysis |
Extrapolated half-life |
|---|---|---|---|
5 |
pH=4, 50ºC |
No significant hydrolysis |
>1 yr |
5 |
pH=7, 50ºC |
No significant hydrolysis |
>1 yr |
5 |
pH=9, 50ºC |
16% |
14.7 d |
30 |
pH=9, 20ºC |
3.9% |
120 d |
30 |
pH=9, 40ºC |
44.5% |
28 d |
Considering the available empirical evidence, it is very unlikely that the rates of hydrolysis of both TCPP and TDCPP at environmentally relevant considerations are fast enough to have any influence on their environmental levels.
6.2.3 Biodegradation
Several studies have investigated the biodegradation of TCPP and TDCPP. The reported values of degradation endpoints are summarized in Table 6‑5 and Table 6‑6 below. (Q)SAR models were also used to provide additional lines of evidence for assessing degradation of these two substances.
6.2.3.1 TCPP
According to empirical data, TCPP does not rapidly biodegrade (Table 6‑5) and does not meet criteria for ready biodegradation (ECCC 2019).
Some inherent biodegradability has been shown for TCPP. In a prolonged closed bottle test under aerobic conditions, activated sludge was aerated for one week prior to the start of the test in which TCPP was present at 4 mg/L (Akzo Nobel 2002). Degradation started on day 21 based on measurement of oxygen consumption and reached 13% on day 28, indicating no rapid biodegradation of the test substance.
In a semi-continuous activated sludge study, TCPP was added to the activated sludge under aerobic conditions (Akzo Nobel 2001c). The substance was completely removed at the end of the 9-week study. Thus, TCPP was considered to be inherently biodegradable (Akzo Nobel 2001c).
In another study of inherent biodegradability, 21% degradation of TCPP was observed at the end of a 28-day exposure to activated sludge (SafePharm 1996). According to a study summary, there seems an acclimation period of around 13 days at the start of the test, followed by rapid degradation over three days (up to 13%) and then a period of slow degradation, although it had reached a total of 21% degradation by the end of the 28-day exposure. No details are available to further evaluate findings in this study (SafePharm 1996).
Fate Process |
Test inoculums |
Method |
Degradation result |
Reference |
|---|---|---|---|---|
| Biodegradation (ready biodegradability) | Activated sludge |
Equivalent to OECD 301C, MITI test |
28-day degradation=0%
|
MITI 1992
|
| Biodegradation (ready biodegradability) | Activated sludge |
Not specified |
28-day BOD=6% 28-day TOC=2%
|
J-CHECK c2010- |
| Biodegradation (ready biodegradability) | Activated sludge |
OECD 301E |
28-day degradation=14% (DOC removal) | Bayer 1991a |
| Biodegradation (ready biodegradability) | Activated sludge |
USEPA TSCA 796.3100 |
28-day CO2 O-day 28-day DOC≤18.3 %
|
ABC Laboratories 1993 |
| Biodegradation (inherent biodegradability) | Activated sludge |
EPA OPPTS 835.3200 |
28-day BOD=13% 50-day BOD=60% 84-day BOD=100% |
Akzo Nobel 2002 |
| Biodegradation (inherent biodegradability) | Activated sludge |
OECD 302A; EEC Directive 87/302; ISO TC 147 |
100% removal in the end of 9 weeks
|
Akzo Nobel 2001c |
| Biodegradation (inherent biodegradability) | Activated sludge |
Not specified |
28-day degradation=21% (O2 consumption) | SafePharm 1996 |
Abbreviations: BOD, biological oxygen demand; TOC, total organic carbon.
6.2.3.2 TDCPP
Empirical data suggest that TDCPP does not biodegrade rapidly (Table 6‑6). In general, the substance has a slower rate of biodegradation than TCPP, presumably due to chlorine replacing methyl groups. Reported values from the laboratory studies are all below the criteria for ready biodegradation (ECCC 2019).
Degradation of TDCPP was also studied in water collected from 2 rivers and 2 coastal areas in Japan (Hattori et al. 1981) under aerobic conditions. TDCPP was reported to degrade by 18.5% in water from Oh River and 22% in sea-water from Osaka Bay after 14 days.
In an inherent biodegradation study, no degradation was observed at the end of 28 days (SafePharm Laboratories 1996a). It is noted that there was no acclimation period used in the study, and therefore, the outcome is not considered suitable to draw any conclusion with respect to biodegradation.
Fate Process |
Test inoculums |
Method |
Degradation result |
Reference |
|---|---|---|---|---|
| Biodegradation (ready biodegradability) | Domestic sludge |
OECD 301B OECD 301D |
28-day degradation=0% (CO2 evolution) | Life Science Research 1990 |
Biodegradation (ready biodegradability) |
Activated sludge |
Not specified |
28-day BOD=1%
|
J-CHECK c2010- |
| Biodegradation (ready biodegradability) | Not specified |
OECD 301C OECD 302C |
28-day BOD = 0–4%
|
CITI 1992 |
| Biodegradation (inherent biodegradability) | Activated sludge |
OECD 302C |
28-day degradation=0% (O2 consumption) | SafePharm Laboratories 1996a |
| Biodegradation (anaerobic) | Anaerobic sludge |
Not specified |
60-day degradation=0% (chloride release) | van Ginkel 2005 |
Biodegradation |
Open water from Japan |
Molybdenum blue colorimetric method |
7-day degradation=0–12.5% 14-day degradation=0–22% |
Hattori et al. 1981 |
Biodegradation |
Natural soil |
OECD 307 |
122-day degradation=2.7–5.5% (CO2 evolution) | Wildlife International 2005a |
Abbreviation: BOD, biological oxygen demand.
(Q)SAR-based modelling (Environment Canada 2007) was also performed in order to provide additional lines of evidence for characterizing the biodegradation of TCPP and TDCPP. In summary, results for all of the BIOWIN biodegradation sub-models (BIOWIN Sub-models 3, 5, and 6) indicate no rapid biodegradation for TCPP and TDCPP; in addition, the ultimate degradation predictions from CPOPs (2012) indicate no rapid biodegradation (ECCC 2019).
6.2.4 Degradation in soil and sediment
One study was identified that investigated degradation of TDCPP in natural soil (Wildlife International 2005a). The substance was applied to the soil surface and the soil samples were incubated at 20 ± 2 oC for 17 weeks. At the end of the study, very little degradation (2.7-5.5% CO2 evolution) was reported (Wildlife International 2005a).
No additional experimental studies were found for the biodegradation of TCPP in soil or sediment or the biodegradation of TDCPP in sediment. Available modelling is limited for these two compartments. Therefore, an extrapolation ratio of 1:1:4 for water: soil: sediment biodegradation half-life based on Boethling et al. (1995) was applied. Given that the half-lives of TCPP and TDCPP in water are long and likely greater than 182 days (based on results in biodegradation studies summarized in Table 6‑6), it follows that the half-life of TCPP in soil is expected to be greater than 182 days and the half-lives of TCPP and TDCPP in sediments are expected to be greater than 365 days. Both TCPP and TDCPP are likely to persist in soil and sediment.
6.2.5 Metabolism of TCPP and TDCPP
TCPP and TDCPP have been reported to undergo rapid metabolism in organisms. While data are available indicating metabolic transformation pathways in rats, the pathway in aquatic organisms remains unclear.
In the training set of BCFBAF (2010), there are data for screening level whole body primary biotransformation half-lives (day) and rate constants (kM d-1) of discrete chemicals in fish calculated according to the method of Arnot et al. (2008a and 2008b). Empirical in vivo biotransformation half-life estimates for a 10 g fish are 0.05 and 0.30 days for TCPP and TDCPP, respectively. QSAR predicted half-lives in a 10 g fish are 0.14 and 0.41 days for TCPP and TDCPP, respectively (EPI Suite 2000–2012). The in vivo and in silico estimates are in very good agreement with each other for both chemicals. Available evidence suggests that primary biotransformations for TCPP and TDCPP are relatively fast in fish; however, the metabolic intermediates were not specified. In a recent avian study, bis(1,3-dichloro-2-propyl) hydrogen phosphate (BDCPP, CAS RN 72236-72-7) was confirmed to be a metabolic intermediate of TDCPP (Farhat et al. 2014).
Rapid metabolism of TCPP and TDCPP has also been reported in toxicokinetics studies using rodents (see sections 8.2.1.2 and 8.2.2.3 for details). One study on TCPP found that an average of 89% of the administration dose of this substance by oral or intravenous means were eliminated within 72 hours after the treatment. A major metabolite was identified as 0,0-[bis(1-chloro-2-propyl)]-0-(2,propionic acid)phosphate and was found to account for over 50% of the dose (Stauffer Chemical Co. 1984). In a toxicokinetic study on TDCPP, recovery of radioactivity 168 hours after administration was 43.2% in urine, 39.2% in feces, 16.24% in expired air (as carbon dioxide) and 2.51% in carcass (Minegishi et al. 1988). The major metabolite was a BDCPP, a diester of TDCPP (Lynn et al. 1981).
The rapid metabolism of TCPP and TDCPP suggests a low potential for accumulation in organisms (discussed further in next section). Concomitantly, this rapid metabolism results in the formation of potentially stable metabolites.
6.3 Potential for bioaccumulation
Based on measured bioconcentration factors (BCFs), empirical data suggest low bioconcentration potential for TCPP and TDCPP in aquatic biota. As there are no available empirical bioaccumulation factor (BAF) data for TCPP or TDCPP, (Q)SAR models were used to generate estimates and the resulting modelled BAFs are low. Considering these low BAFs and the rapid biotransformation rates for these substances, biomagnification of TCPP and TDCPP through the food web is unlikely, and exposure to higher trophic level organisms are expected to be lower than exposure to lower trophic level organisms.
6.3.1 Bioaccumulation in aquatic organisms
Empirical BCFs in aquatic organisms have been identified for TCPP and TDCPP and low BCFs have been reported for both substances (Table 6‑7).
In a study using a static water test system, absorption and elimination of 4 organophosphate flame retardants (including TDCPP) was investigated (Sasaki et al. 1981). Absorption of TDCPP was observed in the Killifish and Goldfish at a similar rate, according to measured concentrations in the test water; however the bioconcentration was reported to be much higher in Killifish than in Goldfish. Findings suggest a difference in metabolic activity for TDCPP in these two species (Sasaki et al. 1981). The half-life for elimination of TDCPP in killifish is 1.65 hours (WHO 1998).
Substance |
Test organism |
Exposure concentration and duration |
BCF (L/kg) |
Reference |
|---|---|---|---|---|
TCPP |
Carp Cyprinus carpio |
0.2 mg/L for 6 weeks |
0.8–2.8 |
CITI 1992 |
TCPP |
Carp Cyprinus carpio |
0.02 mg/L for 6 weeks |
<1.9–4.6 |
CITI 1992 |
TDCPP |
Carp Cyprinus carpio |
0.02 mg/L for 6 weeks |
0.3–3.3 |
CITI 1992 |
TDCPP |
Carp Cyprinus carpio |
0.002 mg/L for 6 weeks |
<2.2–22 |
CITI 1992 |
TDCPP |
Killifish Oryzias latipes |
0.3–1.2 mg/L for 96 hours (static) |
31–59 |
Sasaki et al. 1982 |
TDCPP |
Killifish Oryzias latipes |
0.04–0.4 mg/L for 72–144 hours (continuous) |
31–46 |
Sasaki et al. 1982 |
TDCPP |
Killifish Oryzias latipes |
0.04–0.08 mg/L for 30–32 days (continuous) |
49–59 |
Sasaki et al. 1982 |
MITI (Japan) also assessed bioconcentration for TCPP and TDCPP (J-CHECK c2010-). Both substances were determined as "not high bioconcentration" under the Chemical Substances Control Law in Japan (J-CHECK c2010-); however, no further details were provided in the database.
(Q)SAR models were used to provide additional lines of evidence for characterizing bioconcentration potential for TCPP and TDCPP. Outcomes from models (BCFBAF and CPOPs) have not indicated any high BCFs (see Table 6‑8).
Substance |
Test organism |
Endpoint and value |
Reference |
|---|---|---|---|
TCPP |
Fish | BCF=13.26 L/kg (middle trophic level fish) | BCFBAF 2010 |
TCPP |
Fish |
BCF=3.79 L/kg |
CPOPs 2012 |
TDCPP |
Fish
|
BCF=111.6 L/kg (middle trophic level fish) |
BCFBAF 2010 |
TDCPP |
Fish |
BCF=4.52 L/kg |
CPOPs 2012 |
BAF is also considered for assessing the bioaccumulation potential for TCPP and TDCPP.
BAF is measured under field conditions as the ratio of the whole body burden of a chemical taken up from all exposures to that of the ambient water concentrations. Measures of BAF are the preferred metric for assessing the bioaccumulation potential of substances because they incorporate chemical exposures from all routes including the diet, which predominates for substances with log KOW> ~4.0 (Arnot and Gobas 2003).
No empirical BAF data were found for either TCPP or TDCPP. Considering log KOW values of 2.68 for TCPP and 3.69 for TDCPP, accumulation through dietary uptake is not expected to be a relatively important process for these substances. The available (Q)SAR models were used to estimate this endpoint and estimates of BAFs are equivalent to BCFs for both substances (Table 6‑9).
Substance |
Test organism |
Endpoint and value |
Reference |
|---|---|---|---|
TCPP |
Fish | BAF=13.26 L/kg (middle trophic level fish) | BCFBAF 2010 |
TDCPP |
Fish | BAF=111.7 L/kg (middle trophic level fish) | BCFBAF 2010 |
The low bioaccumulation potential predicted for TCPP and TDCPP are in agreement with the low BCFs, the rapid biotransformation and low lipid (octanol) partitioning tendency of these two substances in aquatic organisms. As mentioned above, biotransformation half-lives of both substances are calculated to be shorter than 0.5 day in fish, based on empirical primary transformation rate constants (kM) (Table 6‑10). Hence, biomagnification through the food webs is unlikely, and exposure to higher trophic level organisms is expected to be lower than exposure to lower trophic level organisms.
Substance |
Experimental kM (/day) |
Biotransformation half-life(day) |
BCF (L/kg) |
|---|---|---|---|
TCPP |
14.12 |
0.05 |
8 |
TDCPP |
2.29 |
0.30 |
12 |
6.3.2 Bioaccumulation in terrestrial plants
Eggen et al. (2013) conducted a study to investigate uptake and translocation of chemicals (including TCPP and another two organophosphate esters, TCEP and tributyl phosphate (TBP)) in food and forage crops. Barley (Hordeum vulgare), Wheat (Triticum aestivum), Oilseed Rape (Brassica rapa), Meadow Fescue (Festuca pratense), and four cultivars of carrot (Daucus carota) were exposed to treated soil (TCPP at the measured concentration of 0.72 mg/kg dw) for 17 weeks. At the end of the study, higher concentrations of TCPP were found in leaves and roots, but lower concentrations in seeds, compared to the exposure concentration in the treated soil (ECCC 2019). The highest concentration factor was up to 25.6 (in leaves of Meadow Fescue), suggesting no significant accumulation of TCPP in plants.
6.3.3 Bioaccumulation potentials for metabolites
There has been no study identified to investigate bioaccumulation potentials for metabolites of TCPP and TDCPP in organisms; however, the findings in fish and mammalian studies on parent compounds have provided some indirect evidence (see sections 8.2.1.2 and 8.2.2.3 for details on metabolism in mammals). Regardless that the transformation products of TCPP and TDCPP may be somewhat different in fish and mammals, elimination of two substances and their transformation products from organisms is rapid, suggesting that metabolites of TCPP and TDCPP have low bioaccumulation potentials.
7. Potential to cause ecological harm
7.1 Ecological effects assessment
Empirical effects data suggest that TCPP possesses a considerably lower toxicity than TDCPP with respect to survival and growth of organisms. Effects on the endocrine system have been observed only for TDCPP in fish. Effects of both substances on enzyme activities and the transcription of genes associated with a variety of biological functions have been observed in cell assays but to different extents. The difference in the overall toxicity between the two substances may be due to the higher chlorination in TDCPP.
Key studies are discussed in the following sections, while details are presented in ECCC (2019). Data for endpoints of survival, growth or development of test organisms with relevance to the environmental exposure in Canada were considered in the risk characterization.
7.1.1 Toxicity to aquatic organisms
Acute toxicity data for TCPP and TDCPP are available for all three major taxa (fish, crustaceans and algae), while chronic toxicity data are available for crustaceans and algae (ECCC 2013-2014). In addition to in vivo studies, in vitro studies have examined effects on cells and gene transcription, in order to understand the mechanism of their effects on the endocrine system.
7.1.1.1 Effects of TCPP on aquatic organisms
TCPP has demonstrated moderate toxicity to aquatic organisms. The 24–96 hour EC50s/LC50s range from 13.5–180 mg/L for three major taxa (fish, crustaceans and algae). The chronic toxicity studies are only available for crustaceans and algae with no observed effect concentrations (NOECs) ranging from 6–32 mg/L. The substance is considered as a narcotic substance and not having strong potential for DNA or protein binding.
Regarding genotoxicity, the majority of in vitro data identified have reported negative findings in cell assays, while some assays were inconclusive or ambiguous with respect to genotoxicity of TCPP (ECHA c2007-2015). In addition, findings from rat studies suggest that this substance has not induced chromosomal or DNA damage (ECHA c2007-2015).
Liu et al. (2012) conducted a study to investigate effects of six organophosphate flame retardants, including TCPP and TDCPP, on the endocrine system in zebrafish (Danio rerio). The first part of the study measured: 1) the concentrations of sex hormones and the transcriptions of key genes involved in steriodogenesis; and, 2) the binding affinity to estrogen receptors. TCPP demonstrated a weaker effect in cell assays, as the lowest concentration (1 mg/L) of TCPP affecting 17β-estradiol (E2) and testosterone (T) in H295 cells were two orders of magnitude greater than the lowest concentration of TDCPP (0.01 mg/L) at which level comparable effects were observed. Therefore, TCPP was not further investigated for its potential effects on the endocrine system of zebrafish (Liu et al. 2012).
7.1.1.2 Effects of TDCPP on aquatic organisms
TDCPP exhibits considerably greater toxicity (exerting the same effect/response at lower exposure concentrations) to aquatic organisms than TCPP, likely due to the higher chlorination of this substance. For TDCPP, the 24–96 hour EC50s/LC50s range from 1.1–39 mg/L for all three taxa.
Effects on development and reproduction of test organisms have been observed after long-term exposure to TDCPP. In the second phase of Liu et al. (2012), after exposure to TDCPP for 14 days, plasma E2 and T concentrations in adult zebrafish significantly increased in both male and female fish exposed at a concentration of 1 mg/L. Plasma 11-ketotestosterone (11-KT) concentration significantly decreased at 0.04 mg/L and above in male fish; however, there was no significant change associated with female fish in any test concentration groups. Significant effects on related gene transcriptions (cytochrome P450 (CYP)17, CYP19A, and vitellogenin (VTG) 1) in fish gonads and liver were observed only in the 1 mg/L concentration group in both male and female fish, with an additional significant downregulation of VTG at 0.2 mg/L in female fish (Liu et al. 2012). However, there was no information on semen production and density in male fish reported in this study (Liu et al. 2012) (Table 7‑1).
Endpoint |
No effect concentration (mg/L) |
Lowest effect concentration (mg/L) |
|---|---|---|
E2 in plasma |
0.2 mg/L (both male and female) |
1 mg/L (both male and female) |
T in plasma |
0.2 mg/L (both male and female) |
1 mg/L (both male and female) |
11-KT in plasma |
0.04 mg/L (male) No effect at all test concentrations (female) |
0.04 mg/L (male) No effect at all test concentrations (female) |
Gene transcriptions in gonad and liver |
0.2 mg/L (male) 0.04 mg/L (female) |
1 mg/L (male) 0.2 mg/L (female) |
Abbreviations: E2, 17β-estradiol; T, testosterone; 11-KT, 11-ketotestosterone.
Wang et al. (2013) studied the effect of TDCPP on the thyroid endocrine system in zebrafish embryos. Test organisms were exposed to different concentrations of TDCPP (0.01 to 0.6 mg/L) from 2 hours post fertilization to 144 hours post fertilization. Developmental endpoints, whole-body concentrations of thyroid hormones and transcriptional profiles of genes in the hypothalamic-pituitary-thyroid (HPT) were examined (see Table 7‑2). A significant effect on hatching rate and survival rate was observed in the test organism with exposure at 0.6 mg/L. A significant incidence of malformation was observed at an even lower concentration at 0.3 mg/L. Besides its effects on development endpoints, the whole body thyroxine (T4) and triiodothyronine (T3) concentrations were significantly lower in fish exposed to the substance at concentrations of 0.05 and 0.3 mg/L, respectively. Ten genes involved in the HPT axis of zebrafish embryos/larvae were also studied; mRNA expression was affected in 8 of these genes by exposure to TDCPP at or above 0.1 mg/L (Wang et al. 2013).
Endpoint |
No effect concentration (mg/L) |
Lowest effect concentration (mg/L) |
|---|---|---|
Hatching rate |
0.3 |
0.6 (a significantly lower hatching rate) |
Survival rate |
0.3 |
0.6 (a significantly lower survival rate) |
Heart rate |
0.05 |
0.1 (a significantly lower heart rate) |
Body weight |
0.01 |
0.05 (a significantly lower body weight) |
Malformation |
0.01 |
0.3 (a significantly higher incidence of malformation rate, spinal curvature) |
T4 concentration |
0.01 |
0.05 (significantly lower) |
T3 concentration |
0.1 |
0.3 (significantly higher) |
mRNA expressions of 10 genes |
0.05 |
0.1 (a significant up-regulation) |
Abbreviations: T4, thyroxine; T3, triiodothyronine.
In a chronic study, thyroid hormone homeostasis and neuronal development was studied in the progeny of adult zebrafish exposed to TDCPP for 3 months (Wang et al. 2015a) (Table 7‑3). Effects on the overall development of the first generation (F1) zebrafish larvae, thyroid hormone levels, expression of genes associated with the nervous system were measured. In general, no significant effect has been observed at the concentration of 0.004 mg/L (Wang et al. 2015a).
Endpoint |
No effect concentration (mg/L) |
Lowest effect concentration (mg/L) |
|---|---|---|
Hatching rate |
0.004 |
0.02 (a significantly lower hatching rate) |
Malformation |
0.02 |
0.1 (a significantly higher incidence) |
Survival rate |
0.004 |
0.02 (a significantly lower survival rate) |
Body weight |
0.02 |
0.1 (a significantly lower body weight) |
F0 T4 concentration |
0.004 |
0.02 (significantly lower) |
F0 T3 concentration |
0.02 |
0.1 (significantly lower) |
Eggs T4 |
0.02 |
0.1 (significantly lower) |
Eggs T3 |
0.1 |
Not applicable |
F1 5-dpf T4 |
0.02 |
0.1 (significantly lower) |
F1 5-dpf T3 |
0.01 |
Not applicable |
F1 10-dpf T4 |
0.004 |
0.02 (significantly lower) |
F1 10-dpf T3 |
0.02 |
0.1 (significantly lower) |
5 genes associated with the nervous system |
0.004 |
0.02 (a significant downregulation) |
4 neurotransmitter concentrations in TDCPP-exposed F1 larvae |
0.004 |
0.02 (a significant lower concentration) |
Locomotor activity |
0.02 |
0.1 (a significantly slower swimming speed) |
Abbreviations: F0, adult fish; F1, first generation; T4, thyroxine; T3, triiodothyronine; dpf, day post fertilization.
In a longer term study, zebrafish larvae were exposed to TDCPP at 0, 0.004, 0.02, and 0.1 mg/L for 6 months (Wang et al. 2015b) (Table 7‑4). Developmental parameters were recorded at 5 days post fertilization (dpf) for the first generation (F1). The hatching, survival rates and growth were not significantly changed in the F1 derived from the exposed F0 fish; however, there was a significant increase in the incidence of malformation in F1 embryos derived from parents exposed to 0.02 and 0.1 mg/L TDCPP. In adult fish (F0), there were no significant differences in survival rates; however, decreased body weight was observed at the lowest concentration at 0.004 mg/L with additional growth parameters affected at or above 0.2 mg/L.
Endpoint |
No effect concentration (mg/L) |
Lowest effect concentration (mg/L) |
|---|---|---|
Body weight (F0) |
Not applicable |
0.004 (a significantly lower body weight) |
Length |
0.1 |
Not applicable |
Weight/length ratio (F0) |
0.004 |
0.2 (a significantly lower ratio) |
Gonad weight/body weight (F0) |
0.02 (male) 0.004 (female) |
0.1 (male) (a significantly lower ratio) 0.02 (female) (a significantly higher ratio) |
Hatching (F1) |
0.1 |
Not applicable |
Survival (F1) |
0.1 |
Not applicable |
Malformation (F1) |
0.004 |
0.02 (a significant incidence) |
Egg production |
0.004 |
0.02 (a significant lower production) |
Plasma estradiol (E2) and testosterone (T) in female fish |
0.004 |
0.02 (a significantly higher concentration) |
Plasma estradiol (E2) and testosterone (T) in male fish |
0.1 |
Not applicable |
4 gene transcription levels in brain |
Not applicable |
0.004 (1 within 4 gene expressions assessed) |
4 gene transcription levels in liver |
0.004 |
0.02 (1 within 4 gene expressions assessed) |
9 gene transcription levels in gonad |
Not applicable |
0.004 (1 within 9 gene expressions assessed) |
Abbreviations: F0, adult fish.
In another long term study on TDCPP with the similar experimental design, zebrafish larvae were exposed to TDCPP at 0, 0.004, 0.02, and 0.1 mg/L for 6 months (Wang et al. 2015c) (Table 7‑5). No effect on the overall development of the fish embryos/larvae was reported. In addition to effects on certain gene expression parameters, concentrations of two neurotransmitters were found much lower in female fish brain in all test groups; however, concentrations of those two neurotransmitters were not affected in male fish brain at any test concentration. The acetylcholinesterase activity (as a biomarker for the presence of neurotoxicants) and locomoter activity was not affected in all adult fish at any test concentration.
Endpoint |
No effect concentration (mg/L) |
Lowest effect concentration (mg/L) |
|---|---|---|
Development (hatching, malformation, survival, weight) |
0.1 |
Not applicable |
5 gene expression in the nervous system of zebrafish larvae |
0.02 |
0.1 (a significantly up-regulation for 1 within 5 genes assessed) |
5 gene expression in the nervous system of zebrafish adult fish |
0.004 |
0.02 (a significantly down-regulation) |
α1-tubulin in fish brain |
0.004 (female) 0.02 (male) |
0.02 (female) 0.1 (male) (a significantly lower production) |
Myelin basic protein in fish brain |
0.004 (female) 0.1 (male) |
0.02 (female) (a significantly lower production) Not applicable for male |
Dopamine and serotonin in female fish brain |
Not applicable |
0.004 (a significantly lower concentration) |
Dopamine and serotonin in male fish brain |
0.1 |
Not applicable |
Acetylcholinesterase activity in adult fish |
0.1 |
Not applicable |
Locomoter activity |
0.1 |
Not applicable |
In a study by Liu et al. (2013) investigating effects of TDCPP on zebrafish embryos/larvae, there was no change in either 72-hour post hatching rate or the 120-hour post hatching survival rate in test organisms with an exposure of the substance at 4 mg/L or lower. No malformation was observed when exposed to 2 mg/L TDCPP, which was the highest concentration used in the second part of this study (Liu et al. 2013). In addition, TDCPP was determined to affect expression of mRNAs involved in six receptor-centred gene networks at a very low concentration (0.02 mg/L).
In a study to assess overt toxicity and behavior in early life stage of zebrafish (Danio rerio), the test organisms were exposed to 0.033-100 µM TCPP and TDCPP from 0 to 5 days post fertilization (dpf) (Dishaw et al. 2014). Significant mortality and severe malformation were observed by 6 dpf in fish exposed to TDCPP at a concentration of 10 µM (equivalent to 4.3 mg/L); however, there was no mortality or teratogenicity in TCPP exposed fish. Larval swimming activity was used to evaluate neurobehavioral effects. Larvae exposed to TCPP (100 µM, equivalent to 33 mg/L) were hyperactive in light; although their swimming ability was not impaired as exhibited by normal activity during the dark period. TDCPP elicited hyperactivity during both the light (5.6 µM, equivalent to 2.41 mg/L) and dark periods (3.14 µM, equivalent to 1.35 mg/L) in the test organisms.
In a 28 day chronic Daphnia magna study, Li et al. (2015) found fewer offspring (34) at the highest treatment concentration (0.00645 mg/L) vs. the control group (39 offspring) (read from a figure). Effects on body length in both F0 and F1 generations of test organisms were also observed. A 28-day EC10 = 0.0065 mg/L was determined, based on mentioned toxicity endpoints. Expressions of 155 genes involved in 40 pathways were examined, showing that expressions of 57 genes involved in 30 pathways were significantly altered On the basis of expression change by at least 50% on at least three genes, 9 out of these 30 pathways were determined to be altered, including a pathway relevant to thyroid hormone synthesis. In the same study, effects on the reproduction of test organisms were reported.
In another chronic fish study (Zhu et al. 2015), total eggs per female were significantly fewer than those in the control group, when exposed to TDCPP at a concentration as 0.0063 mg/L after 21 days. This effect was correlated with expression of genes in the ovary. Effects on weight and body length were also observed, but only in females’ organisms after 120 days of exposure. There was no significant change in gonad histology or concentrations of 11-ketotestosterone and 17β-estradiol (Zhu et al. 2015).
Overall, the available studies show that chronic exposure to TDCPP impacts transcription of genes associated with a variety of biological functions (e.g., protein synthesis and the production of thyroid hormones). Further elucidation of the pathway of adverse outcomes is required to clarify what molecular initiation could trigger subsequent key events and ultimately cause organ- or organism-level changes.
7.1.1.3 Selection of critical toxicity value for aquatic organisms
Key aquatic toxicity studies are summarized in Table 7‑6 for consideration in choosing a critical toxicity value for both substances.
Substance |
Test Organism |
Endpoint |
Value (mg/L) |
Reference |
|---|---|---|---|---|
TCPP |
Rice fish Oryzias latipes |
48-hour LC50 |
54.2 |
ECHA c2007-2017 |
TCPP |
Fathead Minnow Pimephales promelas |
96-hour LC50 |
51 |
Mobil 1985 |
TCPP |
Zebrafish embryos Danio rerio |
96-hour LC50 |
13.5 |
Du et al. 2015 |
TCPP |
Bluegill Lepomis macrochirus |
96-hour LC50 |
84 |
ECHA c2007-2017 |
TCPP |
Rainbow fish Poecilia reticulata |
96-hour LC50 |
30 |
ECHA c2007-2017 |
TCPP |
Freshwater Algae Pseudokirchneriella subcapitata |
72-hour EC50 (growth rate) | 88 |
Wildlife International 2005b |
TCPP |
Freshwater Algae Desmodesmus subspicatus |
72-hour EC50 |
45 |
ECHA c2007-2017 |
TCPP |
Invertebrate Daphnia magna |
21-day NOEC (reproduction, parental mortality) |
32 |
SafePharm Laboratories 1995; UNEP 1999; WHO 1998 |
TCPP |
Freshwater algae Selenastrum capriconutum |
96-hour NOEC |
6 |
Kroon and Ginkel 1992 |
TDCPP |
Goldfish Carassius auratus |
24-hour LC50 |
1-5 |
ECHA c2007-2017 |
TDCPP |
Rainbow Trout Oncorhynchus mykiss |
96-hour LC50 |
1.1 |
SafePharm Laboratories 1993 |
TDCPP |
Rich fish Oryzias latipes |
96-hour LC50 |
3.6 |
ECHA c2007-2017 |
TDCPP |
Freshwater Algae Pseudokirchneriella subcapitata |
72-hour EC50 (growth rate) | 4.6 |
Wildlife International 2005c |
TDCPP |
Freshwater algae Desmodesmus subspicatus |
72-hour NOEC (growth rate and biomass) | > 10 |
ECHA c2007-2017 |
TDCPP |
Zebrafish Danio rerio |
6-day survival |
0.6a |
Wang et al. 2013 |
TDCPP |
Zebrafish Danio rerio |
6-day EC10b (malformation) | 0.3a |
Wang et al. 2013 |
TDCPP |
Zebrafish Danio rerio |
21-day EC50 (accumulated eggs) |
0.0063 |
Zhu et al. 2015 |
TDCPP |
Invertebrate Daphnia magna |
28-day EC10b (accumulated number of offspring, body length in F0 and F1) |
0.0065 |
Li et al. 2015 |
TDCPP |
Zebrafish Danio rerio |
90-day NOEC (survival and hatching rates) |
0.004 |
Wang et al. 2015a |
TDCPP |
Zebrafish Danio rerio |
180-day NOEC (malformation) |
0.004 |
Wang et al. 2015b |
TDCPP |
Zebrafish Danio rerio |
240-day EC10b (body mass) |
0.0075 |
Yu et al. 2017 |
Abbreviations: EC50, the concentration of a substance that is estimated to cause some effect on 50% of the test organisms; LC50, the concentration of a substance that is estimated to be lethal to 50% of the test organisms; NOEC, the no observed effect concentration is the highest concentration in a toxicity test not causing a statistically significant effect in comparison to the controls.
a The lowest test concentration at which a significant effect was observed.
b 10% difference for the reported endpoints in test organisms compared with the control.
For TCPP, the lowest identified acute toxicity value is 13.5 mg/L for zebrafish embryos determined from a 96-hour LC50 study. This result was selected as the critical toxicity value (CTV). An assessment factor of 50 was applied to extrapolate from the acute lethal effect concentration to a long-term no effect concentration (PNEC), with consideration of the number of species and categories of organisms represented in the available dataset (Table 7‑7).
For TDCPP, no effect on survival and hatching of zebrafish embryos and no incidence of malformation was reported in fish exposed to the substance at a concentration of 0.004 mg/L in long term studies (Wang et al. 2015a and 2015b). At the same time, in a few other studies (Zhu et al. 2015; Li et al. 2015; Yu et al. 2017), there were some developmental and reproductive effects on Daphnia magna and zebrafish. The 21-day EC50 (accumulated egg production) = 0.0063 mg/L was the lowest concentration causing the most significant effect in the chronic studies for TDCPP. Therefore, this value (21-day EC50 = 0.0063 mg/L) was selected as the CTV and used to calculate a PNEC for this substance (Table 7‑7).
Substance |
CTV (mg/L) |
AF |
PNEC (mg/L) |
|---|---|---|---|
TCPP |
96-hour LC50 = 13.5 |
50a |
0.27 |
TDCPP |
21-day EC50 = 0.0063 (accumulated egg production) |
5b |
0.0013 |
a An AF= 50 is applied to calculate a long term no-effect concentration (PNEC) from an acute lethal toxicity datapoint, with consideration of the number of species and categories of organisms in the available dataset.
b An AF=5 is applied to calculate a long term no-effect concentration (PNEC) from a chronic sub-lethal toxicity datapoint, with consideration of the number of species and categories of organisms in the available dataset.
7.1.2 Toxicity to sediment organisms
There are no sediment toxicity data identified for TCPP. Given that TCPP and TDCPP have demonstrated different levels of toxicity on aquatic organisms and effects on the endocrine system, it is not considered appropriate to determine the effects of TCPP on sediment organisms through read-across. Therefore, a PNEC is not calculated for TCPP for this compartment.
Sediment toxicity data have been identified for TDCPP. In a few studies investigating the chronic exposure of TDCPP to midges, the reported 28-day EC50s range from 16 to > 71 mg/kg dry weight (dw); the reported NOECs range from 3.9 to 71 mg/kg dw (Wildlife International 2006b, 2006c, 2006d).
Upon review of the empirical data, the 28-day EC50 (emergence of midge) of 16 mg/kg dw was selected as the CTV for TDCPP. Considering the organic carbon (OC) content as 5.3% was reported in this study, the CTV was adjusted to the standard 3% OC content prior to calculating the PNEC. Considering the available data points for the sediment compartment, an assessment factor of 50 was used to derive the PNEC, to extrapolate a chronic no-effect concentration from a chronic median-effect endpoint and to account for inter- and intra-species variability. This yields a sediment PNEC of 0.18 mg/kg dw for TDCPP.
7.1.3 Toxicity to soil organisms
Toxicity studies have been conducted on earthworms to investigate effects of TCPP and TDCPP in soil organisms. After earthworms (Eisenia foetida) were exposed to these substances for up to 8 weeks, EC50s/LC50s and NOECs were reported (SafePharm Laboratories 1996b and 1996c; Phytosafe 2003a and 2004a). These values are summarized in Table 7‑8.
Two studies investigated the toxicity of TCPP and TDCPP on terrestrial plants. In one study, Wheat (Triticum aestivum), Mustard (Sinapis alba), and Lettuce (Lactuca sativa) were exposed to TCPP for 21 days, and effects on the dry plant weight and the seeding emergence were assessed at the end of the experiment (Phytosafe 2003b). The lowest NOEC of the study was 17 mg/kg dw for the seeding emergence of Lettuce. In another study, Wheat (Triticum aestivum), Mustard (Sinapis alba) and Red Clover (Trifolium pratense) were exposed to TDCPP and effects on emergence and plant growth were assessed (Phytosafe 2004b). The lowest NOEC in this study was 19.3 mg/kg dw for seeding emergence.
Findings in key soil toxicity studies are summarized in Table 7‑8 below.
Substance |
Test organism |
Endpoint |
Value (mg/kg dw) |
Reference |
|---|---|---|---|---|
TCPP |
Earthworms Eisenia foetida |
14-day LC50 |
97 |
SafePharm Laboratories 1996b |
TCPP |
Earthworms Eisenia foetida |
14-day NOEC (mortality) | 32 |
SafePharm Laboratories 1996b |
TCPP |
Earthworms Eisenia foetida |
56-day EC50 (reproduction) | 71 |
Phytosafe 2003a |
TCPP |
Earthworms Eisenia foetida |
56-day NOEC (reproduction) | 53 |
Phytosafe 2003a |
TCPP |
Lettuce Lactuca sativa |
21-day NOEC (emergence) | 17 |
Phytosafe 2003b |
TDCPP |
Earthworms Eisenia foetida |
14-day LC50 |
130 |
SafePharm Laboratories 1996c |
TDCPP |
Earthworms Eisenia foetida |
14-day NOEC (mortality) | 100 |
SafePharm Laboratories 1996c |
TDCPP |
Earthworms Eisenia foetida |
57-day EC50 (reproduction) | 67 |
Phytosafe 2004a |
TDCPP |
Earthworms Eisenia foetida |
57-day NOEC (reproduction) | 9.6 |
Phytosafe 2004a |
TDCPP |
Mustard Sinapis alba |
19-day NOEC (emergence) | 19.3 |
Phytosafe 2004b |
The 56-day EC50 of 71 mg/kg dw and 57-day EC50 of 67 mg/kg dw were considered as CTVs for TCPP and TDCPP, respectively. Considering the organic carbon (OC) contents reported in these studies, the CTVs were adjusted to the standard 2% OC content prior to calculating PNECs.
Considering the available data points for the soil compartment, an assessment factor of 50 was used to derive PNECs for both substances, to extrapolate a chronic no effect concentration from a chronic sub-lethal median effect endpoint and to account for inter- and intra-species variation. Results are presented in Table 7‑9 below.
Substance |
CTV (mg/kg dw) |
OC content (%) |
AF |
PNEC (mg/kg dw) |
|---|---|---|---|---|
TCPP |
56-day EC50=71 |
1.4 |
50 |
2.03 |
TDCPP |
57-day EC50=67 |
10 |
50 |
0.27 |
7.1.4 Toxicity to birds and mammals
In vitro and in ovo studies on TCPP and TDCPP have been conducted to investigate their neurotoxicity, cytotoxicity and genetic effects and the findings provide evidence of chemical reactivity and mode of action. It is noted that exposure concentrations used in these studies are several orders of magnitude greater than concentrations of both substances measured in the Canadian environment (i.e., water and eggs). Therefore, effects observed in laboratory experiments are not expected in the wild according to their levels of environmental occurrence. As a result, the toxicity data reported from in vitro or in ovo studies were not used in the risk quotient analysis for these substances.
7.1.4.1 Neurotoxicity
Exposure to TCPP or TDCPP did not induce neurological effects in hens or mammals, respectively. Details are presented in sections 8.2.1.6 and 8.2.2.6.
7.1.4.2 Effects on the endocrine system
Effects on the endocrine system for these two substances were assessed in ovo and in vitro.
In a study using primary cultures of avian neuronal cells, Crump et al. (2012) reported a higher cytotoxicity of TDCPP than TCPP. Effects on mRNA expressions associated with a variety of biological functions were studied. Both substances may demonstrate an effect on the thyroid hormone pathway related gene transcription at or above 10 µM (equivalent to 3.3 mg/L and 4.3 mg/L, respectively) (Crump et al. 2012).
In an in ovo toxicity study (Farhat et al. 2013), chicken eggs were injected with TCPP and TDCPP separately, and the highest concentrations used were 51 600 and 45 000 ng/g wet weight (ww) of eggs, respectively. There was no lethal response to any treatment doses of either substance. Only TDCPP significantly reduced the plasma T4 levels at 7640 ng/g and higher. In addition, there was no effect on pipping success of chicken; however, a delay of pipping was observed in higher concentration groups of two substances, at 9240 ng/g and above for TCPP and at 7640 ng/g and above for TDCPP. Embryonic development (the tarsus length, the embryo mass, head and bill length, and the gallbladder length) was affected by both test substances at the highest concentration. It is noted that the exposure concentrations in the above studies are much higher than measured concentrations in avian eggs <6.7 ng/g for TCPP and up to 0.17 for TDCPP found in the environment (Chen et al. 2012; Leonards et al. 2011).
In a 21-day study, captive American Kestrels were grouped and fed with either TCPP or TDCPP at the same dose of 22 ng OPFR/g-bw per day (Fernie et al. 2015). The exposure to either substance had no significant effect on the body mass or temporal weight gain of the test organisms. Both substances were not detected in tissues, suggesting rapid metabolism. However, some biological effect, i.e., the plasma A:G ratio, was observed in kestrels exposed to TCPP and TDCPP. In addition, effects on the triiodothyronine (T3) and thyroxine (T4) concentrations in plasma and changes in thyroid gland structure and related enzyme activities were observed in the test organisms exposed to TCPP and TDCPP (Fernie et al. 2015).
There have been in vitro studies conducted to assess effects on the endocrine system for TCPP and TDCPP using mammalian cells (Follmann and Wober 2006; Kojima et al. 2013). Details are discussed in sections 8.2.1.4 and 8.2.2.5.
7.1.4.3 Genetic effects
Genetic effects were also assessed in the Farhat et al. study (2013). Among the 9 mRNA transcripts studied, effects of these two substances on some gene expressions were observed only at the highest concentrations of TCPP and TDCPP, at 51 600 and 45 000 ng/g wet weight (ww) of eggs, respectively (Farhat et al. 2013). Farhat et al. 2013 notes that low tissue residue concentrations relative to the injected doses may be due to rapid metabolism of TCPP and TDCPP in chicken embryos, which is in agreement with the observation in rat studies, showing that the majority of dosed TCPP and TDCPP was eliminated quickly within a few days of administration (Lynn et al. 1980; Minegishi et al. 1988).
In the same study on cytotoxicity and mRNA expression in cultures of avian cells (Crump et al. 2012), both substances affected transcription of genes associated with xenobiotic metabolism (CYP2H1), the thyroid hormone pathway (TTR), lipid metabolism (L-FABP and HRSP14-α), and growth (IGF-1). Both substances demonstrated upregulation of most genes studied at a concentration of 10 µM (equivalent to 3.3 mg/L and 4.3 mg/L for TCPP and TDCPP, respectively), except TDCPP which demonstrated an effect on mRNA expression of a lipid metabolism gene (L-FABP) at all test concentrations (0.01 µM and above, equivalent to 0.0043 mg/L and above).
7.2 Ecological exposure assessment
7.2.1 Measured environmental concentrations
7.2.1.1 Environmental monitoring data for Canada
There are several studies reporting environmental concentrations for TCPP and TDCPP in Canada. Some of these studies measured the individual TCPP isomers (i.e., those found in the commercial products). In these studies, measured concentrations were determined for TCPP itself and/or the sum of isomers in environmental samples. A few other studies did not discuss isomers in their reports. Given the predominance of TCPP and considerably lower proportion of its chain isomers in most of the commercial products as well as their similar environmental fates, it is considered that measured concentrations reported in the environmental monitoring studies are appropriate to characterize the presence of TCPP in the environment, even in the absence of information on the TCPP isomers.
Details are presented in ECCC (2019) and key findings are summarized as follows.
Concentrations of TCPP and TDCPP in air have been reported at high levels in samples collected over Lake Superior at 1.35 ng/m3 for TCPP and 0.034 ng/m3 for TDCPP during 2005 (Shoeib and Jantunen 2013) and more recently in the Canadian Arctic at 0.075-0.145 ng/m3 for TCPP and 0.005 ng/m3 for TDCPP (Jantunen et al. 2013a). In a recent study, TCPP and TDCPP were reported at 0.67 ng/m3 and 0.15 ng/m3, respectively, in air samples collected in Toronto during 2012 (Shoeib et al. 2014). Both substances have been found associated with air particles in these studies (Shoeib and Jantunen 2013; Jantunen et al. 2013a; Shoeib et al. 2014).
A number of environmental studies have reported both substances in effluents of WWTS, in rain samples, and in surface water (rivers and lakes) in Ontario (Andresen et al. 2007; Jantunen et al. 2013b; Venier et al. 2014; ECCC 2016a; Truong 2016; Truong et al. 2017; Hao et al. 2018). Both TCPP and TDCPP were found in samples collected at WWTS included in these projects.
For surface water, TCPP was detected in all rain samples and surface water samples collected in all locations in Great Lakes areas and tributaries in the Toronto area. However, the detection frequency of TDCPP in environmental samples was considerably less. TDCPP was not detected in all rain samples and in surface water samples from a few locations. In other locations where TDCPP was found, this substance was below the limit of detection (LOD) in some samples collected. Details of monitoring data for TCPP and TDCPP in the aquatic environment in Canada were summarized in Table 7‑10 and Table 7‑11as follows.
| Medium | Range of measured concentrations (mg/L) | Median measured concentration (mg/L) | Mean measured concentration (mg/L) | Detection frequency (%) (number of samples, if reported) | Reference |
|---|---|---|---|---|---|
| Effluent from WWTS1 | Not reported | Not reported | 7.8×10-5 | Not reported | Andresen et al. 2007 |
| Effluent from WWTS2 | Not reported | Not reported | 6.9×10-5 | Not reported | Andresen et al. 2007 |
| Effluents from 8 WWTS | 4.7×10-4 – 2.6×10-3 | 1.1×10-3 | Not reported | 100 (n=36) | ECCC 2016a |
| WWTS1 | 1.2×10-3 – 4.1×10-3 | 2.3×10-3 | 2.5×10-3 | 100 (n=8) | Truong 2016 |
| WWTS2 | 1.5×10-3 – 7.7×10-3 | 2.1×10-3 | 2.8×10-3 | 100 (n=7) | Truong 2016 |
| WWTS3 | 3.7×10-4 – 3.9×10-3 | 1.8×10-3 | 2.0×10-3 | 100 (n=10) | Truong 2016 |
| WWTS | 1.5×10-3 – 6.8×10-3 | Not reported | 3.8×10-3 | 100 (n=6) | Truong et al. 2017b |
| WWTS effluent | 1.3×10-3 – 2.4×10-3 | Not reported | Not reported | 100 (n=17) | Hao et al. 2018 |
| Biosolids from 8 WWTS | 7.6×10-5 – 1.5×10-3 | 2.5×10-4 | Not reported | 100 (n=36) | ECCC 2016a |
| Rain samples | LOD – 9.2×10-4 | 1.1×10-4 | 2.3×10-4 | 75 (n=16) | Truong 2016 |
| Rain samples | 5.0×10-5 – 9.2×10-4 | Not reported | 3.9×10-4 | 100 (n=8) | Truong et al. 2017 |
| 13 tributaries | 4.6×10-6 – 1.8×10-3 | Not reported | 2.4×10-5 – 8.4×10-4 | Not reported | Jantunen et al. 2013b |
| 4 sampling points at Lake Ontario | Not reported | Not reported | 3.4×10-6 – 4.9×10-5 | 100 (n=4) | Andresen et al. 2007 |
| Lake Ontario nearshore water | 6.6×10-5 – 3.6×10-4 | 6.6×10-5 | 1.2×10-4 | 67 (n=18) | Truong 2016 |
| Great Lake surface water | 2.6×10-6 – 1.2×10-5 | Not reported | Not reported | 100 | Venier et al. 2014 |
| Etobicoke Creek Low Flow | 3.4×10-4 – 1.4×10-3 | 1.0×10-3 | 9.2×10-4 | 100 (n=7) | Truong 2016 |
| Etobicoke Creek High Flow | 4.3×10-4 – 5.1×10-3 | 2.3×10-3 | 2.1×10-3 | 100 (n=26) | Truong 2016 |
| Don River Low Flow | 5.1×10-4 – 4.9×10-3 | 1.1×10-3 | 1.6×10-3 | 100 (n=7) | Truong 2016 |
| Don River High Flow | 7.9×10-4 – 4.2×10-3 | 1.5×10-3 | 1.8×10-3 | 100 (n=22) | Truong 2016 |
| Highland Creek Low Flow | 9.3×10-5 – 1.5×10-3 | 7.8×10-4 | 7.9×10-4 | 100 (n=7) | Truong 2016 |
| Highland Creek High Flow | 3.5×10-4 – 2.8×10-3 | 1.0×10-3 | 1.1×10-3 | 100 (n=24) | Truong 2016 |
| Streams | 1.1×10-3 – 4.4×10-3 | Not reported | 2.7×10-3 | 100 (n=14) | Truong et al. 2017 |
| Surface water | 2.9×10-4 – 2.0×10-3 | Not reported | Not reported | 60 (n=20) | Hao et al. 2018 |
Abbreviations: WWTS, wastewater treatment system; LOD, the limit of detection.
a Although wastewater system effluent and sludge/biosolids are not “environment,” they represent a direct source to the environment and are included in this table since they are the pathway via which TCPP and TDCPP from industrial inputs are expected to be released to the environment.
b In Truong et al. 2017, the laboratory analysis included each of isomers of TCPP. Measured concentrations presented in the table are the sum of 4 isomers of TCPP in samples.
| Medium | Range of measured concentrations (mg/L) | Median of measured concentration (mg/L) | Mean measured concentration (mg/L) | Detection frequency (%) | Reference |
|---|---|---|---|---|---|
| Effluent from WWTS1 | Not reported | Not reported | 3.5×10-5 | Not reported | Andresen et al. 2007 |
| Effluent from WWTS2 | Not reported | Not reported | 2.6×10-5 | Not reported | Andresen et al. 2007 |
| Effluents from 8 WWTS | 8.7×10-5 – 4.2×10-4 | 2.3×10-4 | Not reported | 100 (n=36) | ECCC 2016a |
| WWTS1 | 6.5×10-4 – 2.3×10-3 | 1.1×10-3 | 1.3×10-3 | 100 (n=8) | Truong 2016 |
| WWTS2 | 4.0×10-4 – 1.9×10-3 | 7.0×10-4 | 8.7×10-4 | 100 (n=7) | Truong 2016 |
| WWTS3 | LOD – 2.1×10-3 | 8.8×10-4 | 9.9×10-4 | ?? (n=10) | Truong 2016 |
| WWTS effluent | 2.1×10-4 – 4.0×10-4 | Not reported | Not reported | 100 (n=17) | Hao et al. 2018 |
| Biosolids from 8 WWTS | 6.6×10-5 – 3.6×10-4 | 1.4×10-4 | Not reported | 100 (n=36) | ECCC 2016a |
| Rain samples | LOD | Not applicable | Not applicable | Not applicable | Truong 2016 |
| 13 tributaries | – 1.4×10-3 | - | 2.0×10-6 – 1.3×10-5 | - | Jantunen et al. 2013b |
| 4 sampling points at Lake Ontario | Not reported | Not reported | 2.1×10-6 – 1.9×10-5 | Not reported | Andresen et al. 2007 |
| Lake Ontario nearshore water | LOD | Not applicable | Not applicable | 0 (n=18) | Truong 2016 |
| Great Lake surface water | 8.7×10-7 – 4.0×10-6 | Not reported | Not reported | 100 | Venier et al. 2014 |
| Etobicoke Creek Low Flow (n=7) | LOD | Not applicable | Not applicable | 0 (n=7) | Truong 2016 |
| Etobicoke Creek High Flow | LOD – 4.7×10-4 | 1.7×10-4 | 1.8×10-4 | 65 (n=26) | Truong 2016 |
| Don River Low Flow | LOD – 3.7×10-4 | LOD | 1.4×10-4 | 43 (n=7) | Truong 2016 |
| Don River High Flow | LOD – 3.7×10-4 | 1.6×10-4 | 1.6×10-4 | 64 (n=22) | Truong 2016 |
| Highland Creek Low Flow | LOD | - | - | 0 (n=7) | Truong 2016 |
| Highland Creek High Flow | LOD – 3.2×10-4 | 9.0×10-5 | 1.3×10-4 | 50 (n=24) | Truong 2016 |
| Wastewater effluent | 1.3×10-4 | Not reported | Not reported | 5 (n=20) | Hao et al. 2018 |
Abbreviation: WWTS, wastewater treatment system; LOD, the limit of detection.
a Although wastewater system effluent and sludge/biosolids are not “environment,” they represent a direct source to the environment and are included in this table since they are the pathway via which TCPP and TDCPP from industrial inputs are expected to be released to the environment.
No data have been identified for TCPP or TDCPP concentrations in soil or sediment in Canada.
McGoldrick et al. (2014) reported on the levels of 6 OPFRs in the homogenized whole Lake Trout and Walleye collected from 16 water bodies across Canada ranging from remote northern lakes with minimal human influence (e.g., Kusawa Lake) to lakes in heavily populated areas with intense agricultural and industrial activities (e.g., Lake Ontario). Both TCPP and TDCPP were found above their respective limits of quantification (0.23 ng/g ww for TCPP and 0.11 ng/g ww for TDCPP) in only one individual Lake Trout from Great Bear Lake in the Northwest Territories. The low to non-detectable concentrations of TCPP and TDCPP in fish are likely due to metabolic breakdown.
Chen et al. (2012) reported levels of OPFRs in herring gull eggs collected from the Channel-Shelter Island on Lake Huron in 2010. Concentrations of TCPP were found above the detection limit in 12 out of 13 samples and the highest concentration reported was 4.1 ng/g ww. Concentrations of TDCPP were found above the detection limit in 2 out 13 samples and the highest concentration reported was 0.17 ng/g ww (Chen et al. 2012).
In another study, 16 organophosphate esters were screened in female Herring Gulls (Larus argentatus) and their eggs from a Lake Huron colony site (Greaves and Letcher 2014). Both TCPP and TDCPP were found in the separated yolk and albumen at the same magnitudes reported by Chen et al. (2012). Among six body compartments (fat, muscle, red blood cells, blood plasma, liver, and brain), TCPP was detected only in fat and muscle tissues with a higher concentration in fat (2.31±1.64 ng/g ww); while TDCPP was detected in all six body compartments with the highest concentration in muscle (5.04±3.69 ng/g ww) (Greaves and Letcher 2014). Measurements of TCPP and TDCPP in eggs from these studies (Chen et al. 2012; Greaves and Letcher 2014) are indicators of breeding near highly populated urban areas.
Su et al. (2014) analyzed plasma samples from herring gulls that were collected from Chantry Island, Lake Huron. Considering that organophosphate triesters are expected to degrade to OP diesters, analysis of BCPP and BDCPP was also included in the study (Su et al. 2014). TCPP and BCPP were not detected in any of 6 plasma samples. However, TDCPP was found in half of the plasma samples and measured concentrations ranged from 0.11 to 0.41 ng/g ww. Meanwhile BDCPP was found in all 6 plasma samples and measured concentrations ranged from 0.72 to 3.49 ng/g ww, thus showing metabolism of TDCPP.
In an unpublished report, low plasma concentrations of TCPP (0.9−5.5 ng/g ww) and TDCPP (0.3−1.0 ng/g ww) were reported in nestling peregrine falcons (Falco peregrinus) (cited in Fernie et al. 2015).
7.2.1.2 Environmental monitoring data for other jurisdictions
Both TCPP and TDCPP have also been measured in ambient air, aquatic systems, soils, sediments, plants, and aquatic biota in other countries (Appendix B2, ECCC 2019). Variations in the reported concentrations of TCPP and TDCPP by country may result from different levels of use of products containing TCPP and TDCPP (Sundkvist et al. 2010).
Both of these two substances were also measured in soil in Europe and in China. Mihajlovic et al. (2011) measured a mean concentration of 0.0012 mg/kg of TCPP in six German soil samples; however, TDCPP was below the detection limit of 9 × 10-5 mg/kg.
Cui et al. (2017) measured TCPP and TDCPP in soil samples from urban soils of the subtropical city Guangzhou, China. Sampling was conducted in parks, paddy/vegetable fields, commercial areas, road greenbelts, and residential areas. Both TCPP and TDCPP were found in all of these sampling areas; the mean measured concentrations of TDCPP in these areas are higher than those of TCPP. Among these five sampling areas, there was 100% detection frequency reported for both substances in samples collected in parks (n=11), commercial areas (n=12), and road greenbelts (n=16); the highest mean concentrations of both substances were reported in commercial areas, at 0.006 mg/kg and 0.034 mg/kg, respectively (Cui et al. 2017).
Concentrations of OPFRs in influents and effluents of WWTS have been reported in a few environmental monitoring studies (van der Veen and de Boer 2012; Bendz et al. 2005). Results indicated poor wastewater treatment removal efficiency for both TCPP and TDCPP at the selected WWTS in Spain, Germany, Norway, Sweden, and Japan (ECCC 2019). Such information is considered to estimate releases of TCPP and TDCPP in the environment.
7.2.2 Exposure scenarios and predicted environmental concentrations (PECs) in Canada
There are some measured concentrations of TCPP and TDCPP reported in Canadian surface waters. For the purpose of this screening assessment, environmental concentrations of TCPP and TDCPP associated with industrial uses and use of products available to consumers are estimated based on available information, including the use quantities, estimated release rates, and characteristics of the receiving environment. Details are provided in the following sections.
7.2.2.1 PECs of TCPP and TDCPP in the aquatic compartment due to industrial uses
Industrial uses of TCPP and TDCPP include manufacturing of polyurethane and polyisocyanurate foams. Aquatic exposure to TCPP and TDCPP is estimated, assuming that both substances are released from industrial activities to a wastewater system that discharges its effluent to a receiving surface water body. Concentrations of substances in the receiving water near the discharge point of the wastewater system is used as the predicted environmental concentration (PEC), which are further used in characterizing the aquatic risk of substances.
The aquatic PEC due to releases from industrial activities (Cwater-ind) can be calculated using the equation as follows.
Cwater-ind: (1000 × Q × L × (1 - R)) / (N × F × D)
Where:
Cwater-ind: aquatic concentration resulting from industrial releases, mg/L
Q: total substance quantity used annually at an industrial site, kg/yr
L: loss to wastewater, fraction
R: wastewater system removal, fraction
N: number of annual release days, d/yr
F: wastewater system effluent flow, m3/d
D: receiving water dilution factor, dimensionless
As TCPP and TDCPP are used by industrial facilities and are expected to be released to water, several aquatic industrial release scenarios were developed to cover a range of different potential industrial activities in Canada. The scenarios include blending of polyol and manufacturing polyurethane foams. Information collected from different facilities were considered in developing exposure scenarios, aiming to best reflect practices and conditions, including type of wastewater treatment, direct or indirect releases to the receiving media and receiving environment.
Input values for estimating aquatic concentrations of TCPP and TDCPP from industrial activities are summarized in Table 7‑12.
Parameter |
Input Value for TCPP |
Input Value for TDCPP |
|---|---|---|
Quantity used per site (kg) |
100 000 to 2 000 000 (ECCC 2013-2014) | 10 000 to 200 000 (ECCC 2013-2014) |
Loss to wastewater (%)a |
0.0011 to 0.3
|
0.0011
|
On-site wastewater system removal efficiency (%)b |
0
|
0
|
Off-site wastewater system removal efficiency (%)c |
0 (van der Veen and de Boer 2012) | 10-13 (ASTreat 2006, STP-EX model (c2000-2013)) |
Number of annual release days (days)d |
200–250
|
250
|
Wastewater system effluent flow (m3/d) |
4210 to 2 100 000
|
2908 to 2 100 000 |
Dilution factor (–)e |
1 to 10 |
1 to 10 |
a 0.0011% accounts for 0.00006% from curing and storage at foam production sites and 0.0005% from further processing for both TCPP and TDCPP (EU RAR 2008a and 2008b); 0.3% is the standard assumption for high-volume blending vessel cleaning, only applicable for TCPP.
b No on-site wastewater treatment is assumed.
c The off-site wastewater system removal efficienty is determined, on the basis of different types of wastewater treatment, such as lagoons, primary and secondary treatment.
d Site-specific information available in the NPRI data is used, otherwise a standard 250 days is considered for HPV substances (European Commission 2003).
e In general, the dilution factor is the ratio between the receiving environment flow rate and the site-specific WWTS flow rate. When a dilution factor was greater than 10, a maximum default value of 10 was used.
Considering the above information, PECs in surface water are calculated as 2×10-6 – 0.12 mg/L for TCPP and 1×10-7 – 1.6×10-4 mg/L for TDCPP.
7.2.2.2 PECs of TCPP and TDCPP in the aquatic compartment via laundry wastewater due to use of products available to consumers
In addition to industrial sources, TCPP and TDCPP can be released to the environment from manufactured items and products available to consumers. For emissions from products available to consumers, the European Union reported that the total loss of either TCPP or TDCPP to air and wastewater over the product’s lifetime from indoor service is expected to be no more than 0.25%; loss to wastewater via outdoor service is anticipated to be at or below 0.75% per year for both substances; and the end-of-life foams will be deposited to landfills and releases are expected to be negligible (EU RAR 2008a and 2008b).
The presence of TCPP and TDCPP in indoor air and in dust samples in Canada and other countries strongly supports releases of both substances from products available to consumers to the Canadian environment (see Sections 8.1.1.2 and 8.1.1.3). Clothing and the dust collecting on it may create a pathway for TCPP and TDCPP released from household manufactured items to enter wastewater treatment systems via laundry activity (Schreder and La Guardia 2014; Saini et al. 2016).
Schreder and La Guardia (2014) measured the mean concentrations of TCPP and TDCPP in laundry wastewater sampled from 20 homes in the Northwestern United States between 2011 and 2012. The mean concentrations of TCPP and TDCPP in laundry wastewater were measured as 0.1 mg/L and 0.018 mg/L, respectively (Schreder and La Guardia 2014). It is noted that the measured concentrations of both substances in laundry wastewater are below their water solubility limits.
The influent and effluent concentrations of TCPP and TDCPP at two local wastewater treatment systems serving these homes were also reported in this study (Schreder and La Guardia 2014). These wastewater treatment systems receive over 80% of their input from households, with no known flame retardant discharges from the remaining industrial contribution. Using the proportion of influent expected from laundry wastewater and the proportion of influent from households, the authors determined that laundry wastewater may be the primary source of these flame retardants to the wastewater treatment systems (Schreder and La Guardia 2014).
Laundry wastewater data from the northwestern United States from the Schreder and La Guardia study (2014) is considered sufficiently representative to construct an exposure scenario relevant to Canada. The exposure concentrations of TCPP and TDCPP in the surface water (PECs) can therefore be estimated based on releases of these substances from consumer use of manufactured items via laundry wastewater.
Saini et al. (2016) conducted a study to measure releases of TCPP and TDCPP and a few other flame retardants from their accumulation by clothing. This study (Saini et al. 2016) reported the accumulation of these flame retardants on fabrics and their releases to wastewater via laundry activity. The authors noted study uncertainties, such as inconsistencies and human errors during manual washing and liquid extraction. Average concentrations in laundry wastewater of TCPP (total of three isomers) and TDCPP ranged up to 2,482 and 18 ng/L·dm2, respectively for cotton fabric. Based on various assumptions concerning laundry load composition, the authors estimated that the total organophosphate flame retardant wastewater concentrations from their study appeared higher than those determined by Schreder and La Guardia (2014). It is noted that this study (Saini et al. 2016) reflected a 30 day continuous fabric exposure period to an office environment, which is not a comparable scenario for Canada. Therefore, the data reported by Saini et al. (2016) are not considered in estimating releases of these substances in laundry wastewater.
Environment Canada indicates that average daily domestic water use is 343 L/day/Canadian and 20% of such usage is accounted for by laundry (Environment Canada 2013). This value, multiplied by 365 days/year, 35 540 400 Canadians (Statistics Canada 2014), and the mean concentrations of TCPP and TDCPP in laundry wastewater reported above (Schreder and La Guardia 2014), yielded national estimated releases of TCPP and TDCPP in laundry wastewater from use of products available to consumers as 88 929 kg/year and 15 918 kg/year, respectively.
Predicted environmental concentrations of TCPP and TDCPP near WWTS discharge points were calculated in a probabilistic manner using information from numerous municipalities in Canada, including local population, effluent flow of the WWTS and removal efficiencies of the WWTS. The same removal efficiencies employed above for the industrial scenarios were used. The 5th and 95th percentile PECs resulting from use of products available to consumers were 7.7×10-4 and 1.3×10-2 mg/L for TCPP and 1.2×10-4 and 2.2×10-3 mg/L for TDCPP.
For TCPP, the highest PEC associated with releases from industrial uses (0.12 mg/L) exceeds the highest value arising from releases from use of products available to consumers (1.3×10-2 mg/L), which is aligned with the assumption that industrial point sources may result in the largest localized concentrations in the environment.
Conversely for TDCPP, the highest PEC value associated with releases from industrial uses (1.6×10-4 mg/L) was approximately one order of magnitude lower than the 95th percentile PEC due to releases via laundry wastewater (2.2×10-3 mg/L), highlighting the importance of considering releases of this substance from the use of products available to consumers.
. Since the lowest WWTS removal reates were used, PECs associated with use of products available to consumers are considered conservative.
7.2.2.3 PECs of TCPP and TDCPP in the sediment compartment due to industrial uses and use of products available to consumers
An equilibrium sediment-water partition approach was used to estimate the concentration of TCPP and TDCPP in sediments. This approach is based on a partitioning principle described by the European Chemicals Agency (ECHA 2010) and incorporates two additional calculation methods. The first method is to estimate the substance’s concentration in the aqueous phase (dissolved) of the overlying water from its total concentration, according to studies by Gobas (2007 and 2010). The second method is to estimate a substance’s concentration in bottom sediment from its concentration in the aqueous phase of the overlying water based on an equilibrium partitioning assumption between bottom sediment and overlying water described by the US EPA’s National Center for Environmental Assessment (US EPA 2003). At equilibrium, the PEC in bottom sediment is assumed to be linearly correlated with the concentration in the aqueous phase of the overlying water. Sediment exposure scenarios were developed as an extension of the industrial aquatic release scenarios described above to determine equilibrium sediment PECs, standardized to 3% organic carbon (a typical organic carbon content in bottom sediment for rivers and lakes).
Considering all of the above, PECs in sediment associated with releases from industrial uses were calculated to range from 4.1×10-5 to 2.1 mg/kg dw for TCPP and 7.7×10-6 to 0.086 mg/kg dw for TDCPP.
For releases of these substances from use of products available to consumers, such emissions will be released to the surface water after the treatment of laundry wastewater. Considering their partitioning to sediment, PECs in the sediment compartment associated with releases from products available to consumers range from 1.4×10-2 to 0.22 mg/kg dw for TCPP and 6.4×10-3 to 0.12 mg/kg dw for TDCPP, based on the range of the 5th to 95th percentile probabilistic aquatic PECs.
7.2.2.4 PECs of TCPP and TDCPP in the soil compartment due to industrial uses and use of products available to consumers
As noted in sections 7.2.2.1 and 7.2.2.2, releases of TCPP to wastewater may happen at industrial manufacturing facilities and from use of products available to consumers; however, it is considered that there is no removal of this substance from wastewater treatment. Given that it is not being caught in the biosolids, minimal release to soil is expected via the application of biosolids or deposition in landfills. In addition, direct release to this compartment is not likely. Therefore, a PEC in soil is not calculated for TCPP.
For industrial uses of TDCPP, it is also assumed that there is no on-site wastewater treatment; however, there may be some removal during off-site wastewater treatment. To estimate releases of TDCPP in soil, an approach described by the European Chemicals Agency (ECHA 2010) was used to quantify TDCPP sorbed to biosolids and further estimate predicted environmental concentrations in soil (soil PECs) resulting from the land application of biosolids. This approach employed the quantity of biosolids accumulated within the top 20 cm layer (ploughing depth) of soil over 10 consecutive years as the basis for soil PECs. One underlying assumption of the approach was that substances were subject to no loss due to degradation, volatilization, leaching, and soil run-off upon their entry into soil. This assumption, therefore, yields conservative soil PECs. Soil exposure scenarios were developed as an extension of the aquatic release scenarios described above, using concentrations and production rates based on site specific wastewater treatment plants.
Standard assumptions/considerations are applied as follows:
- Removal from WWTS: According to site-specific information contained in the EC database (NPRI 1994–2009), a 13% removal rate for secondary treatment was considered at most of the off-site treatment plants; meanwhile primary treatment was considered at a few other off-site treatment plants and a 10% removal rate was applied. In some cases, if a lagoon was providing the applicable wastewater treatment, a 10% removal rate was applied.
- Biosolids application rate is 8.3 tonne/ha-yr.
- Biosolids application period is 10 consecutive years.
- Soil depth and density: 0.2 m and 1200 kg/m3.
For all industrial sites identified using TDCPP, soil PECs are estimated to range from 1.2 x 10-5 to 2.1 x 10-3 mg/kg dw.
TDCPP may also be released in laundry wastewater from use of products available to consumers. The sorption of this substance to the sludge is estimated from the total release quantity (15 918 kg per year estimated in section 7.2.2.2). Considering the same removal rate (13%) for the secondary treatment for most of the off-site wastewater treatment plants, the PEC in biosolids is calculated as 0.87 mg/kg. Applying the same standard assumptions on the biosolids application in soil as described above for the industrial uses, the resulting soil PECs are estimated as 0.03 mg/kg dw.
7.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA. Lines of evidence considered include results from a conservative risk quotient calculation, as well as information on physical and chemical properties, sources, fate of these substances and their presence in the Canadian environment, persistence, bioaccumulation potential and inherent toxicity to non-human organisms.
7.3.1 Risk quotient analysis for the aquatic environment
A risk quotient (RQ) analysis is conducted for selected scenarios by comparing the predicted environmental concentrations (PEC) to the selected predicted no-effects concentrations (PNEC) for organisms.
As discussed in Section 7.2, aquatic PECs were derived for TCPP and TDCPP to characterize their exposure to aquatic organisms resulting from releases associated with industrial uses and consumer laundry activity. Each scenario for industrial uses considered the quantity of TCPP and TDCPP used at each industrial site, the emission factor for releases to wastewater, the wastewater treatment system removal and effluent flow rates, and the dilution in the receiving water. For releases via consumer laundry activity, measured concentrations of both substances in laundry wastewater from the northwestern United States was used to represent such releases in Canada and further estimate the total releases of TCPP and TDCPP from use of products available to consumers. PECs of these two substances resulting from laundry wastewater were calculated in a probabilistic manner.
In addition, both substances have been found in Ontario surface water (Jantunen et al. 2013b); it is reasonable to expect that the environmental monitoring data would be reflective of releases from both industrial activities and the use of products available to consumers and their degradation in the environment. Given that, measured concentrations of these two substances in 13 tributaries in southern Ontario (Jantunen et al. 2013b) were also considered in risk quotient analysis.
Aquatic PNECs were extrapolated from the most sensitive effect endpoint for each substance (see Section 7.1).
Results of the risk quotient analysis are presented in Table 7‑13.
For TCPP, the RQ is below 1 for all industrial sites using this substance. Considering releases of this substance in laundry wastewater, the 95th percentile PEC resulting from laundry wastewater was also below the PNEC, yielding an RQ less than 1. Average measured concentrations of TCPP in rain samples, lake water in the Great Lakes, rivers and tributaries in Ontario range from 2.6×10-5 mg/L and 2.8×10-3 mg/L; the highest measured concentration as 1.8×10-3 mg/L was reported in samples collected from one river. All available measured concentrations in Canada are below the PNEC. These results indicate that risk in aquatic organisms associated with releases of TCPP from industrial uses or use of products available to consumers is low.
For TDCPP, the RQ is below 1 for all industrial sites using this substance. Based on the probabilistic PECs associated with releases from laundry wastewater, the 50th percentile PEC is well below PNEC and only the 87th percentile PEC is equal to the PNEC. The calculation of PECs using the probabilistic approach is considered conservative; the number of sites with estimated releases of TDCPP resulting in water concentrations above the PNEC is small.
According to the environmental monitoring data, TDCPP was not detected in rain water or surface water samples taken from two rivers at their low flows. Where this substance was detected in lake and river surface waters in Ontario, average measured concentrations ranged from 2.0×10-6 mg/L to 1.8×10-4 mg/L. These concentrations are below the PNEC for this substance. A maximum concentration of 1.4×10-3 mg/L was reported in samples collected in 1 river, while the second highest concentration was 8.4×10-4 mg/L and the average measured concentration was 1.3×10-4 mg/L for the same river. This suggests that the occasional high concentration (i.e., 1.4×10-3 mg/L) is not representative of the realistic existence of this substance in the environment. Based on all above evidence, it is considered that risk in aquatic organisms associated with releases of TDCPP from industrial uses or use of products available to consumers is low.
Substance |
PEC or range of measured concentrationsa (mg/L) |
PNEC (mg/L) |
RQ |
|---|---|---|---|
TCPP |
2×10-6–0.12 (PECs associated with releases from industrial uses) | 0.27 |
7.4×10-6–0.44 |
TCPP |
1.3×10-2 (the 95th percentile PECs due to use of products available to consumers) | 0.27 |
0.048 |
TCPP |
2.4×10-5–2.1×10-3 (average mean measured concentrations in river and lake water in Ontario; a concentration as 5.1×10-3 mg/L was reported as the maximum measured value in one tributary) | 0.27 |
8.9×10-5–7.8×10-3
|
TDCPP |
1×10-7 – 1.6×10-4 (PECs associated with releases from industrial uses) | 0.0013 |
7.7×10-5–0.12 |
TDCPP |
2.9×10-4, 1.3×10-3 and 2.2×10-3 (the 50th, 87th, and 95th percentile PECs due to use of products available to consumers) | 0.0013 |
0.22, 1, and 1.7 |
TDCPP |
2.0×10-6–1.8×10-4 (average mean measured concentrations in river and lake water in Ontario; a concentration as 1.4×10-3 mg/L was reported as the maximum measured value obtained in one river) | 0.0013 |
6.4×10-4–0.14 |
a Measured concentrations of TCPP and TDCPP in the surface water were reported in Jantunen et al. 2013b.
7.3.2 Risk quotient analysis for the sediment compartment
Due to minimal partitioning to sediments and the lack of effects data (read-across is not applicable for TCPP), a risk analysis for TCPP in sediment is not conducted.
For TDCPP, sediment PECs are calculated based on the aquatic PECs estimated for both scenarios of industrial manufacturing of polyurethane foams and releases from routine household cleaning in laundry wastewater. The comparison of sediment PECs with PNECs and the resulting risk quotients are presented in Table 7‑14. The results indicate low risk to sediment organisms associated with releases of TDCPP to this compartment from industrial activities or releases from products available to consumers.
Release sources |
PEC (mg/kg-dw) |
PNEC (mg/kg-dw) |
RQ |
|---|---|---|---|
Industrial uses |
7.7×10-6 – 0.0086 |
0.18 |
4.2×10-5 – 0.047 |
Products available to consumers |
6.4×10-3 – 0.12 |
0.18 |
0.035 – 0.67 |
7.3.3 Risk quotient analysis for the soil compartment
TCPP is not expected to be released to soil due to biosolids application, and thus, a risk quotient for soil was not derived for TCPP.
Results of risk analysis for TDCPP presented in Table 7‑15 indicate that there is low risk to soil organisms due to biosolids application, which are associated with releases of this substance from industrial uses or use of products available to consumers.
Release sources |
PEC (mg/kg-dw) |
PNEC (mg/kg-dw) |
RQ |
|---|---|---|---|
Industrial uses |
1.2×10-5 – 2.1×10-3 |
0.27 |
4.4×10-5 – 7.8×10-3 |
Products available to consumers |
0.03 |
0.27 |
0.11 |
7.4 Consideration of lines of evidence and conclusion
To characterize the ecological risk of TCPP and TDCPP, technical information for various lines of evidence was considered (as discussed in the relevant sections of this report) and qualitatively weighted. The key lines of evidence supporting the assessment conclusion are discussed below and summarized in Appendix B with respect to level of confidence, their relevance and overall weight assigned in the assessment.
TCPP and TDCPP have been used in significant volumes in Canada and internationally. Both have been found in the Canadian environment since 1970. Internationally, ICL-IP has been a major manufacturer of TDCPP. The company ceased supplying this substance for furniture and related polyurethane flexible foam applications in 2014 but still supplies it for flexible foam applications used in the auto industry (ECCC 2013-2014; ECCC 2016b). Another major manufacturer, Albemarle has also indicated it will cease manufacture of TDCPP (Albemarle 2012). There is a moderate level of confidence that no significant increase in manufacture, import or use of this substance is expected in the near future.
Releases of TCPP and TDCPP to air are expected from industrial manufacturing of polyurethane foams, building materials and products available for consumer use to which they have been added. However, direct emissions to air from industrial uses are expected to be low due to the low vapour pressures for these substances. While emissions from the use of products appears to be minimal and diffuse, their quantities in use are high and as such, overall emission volumes may not be insignificant. Releases of TCPP and TDCPP from products available to consumers have been measured in laundry wastewater, which enter the local wastewater treatment system. Therefore, it is reasonable to expect that measured concentrations in surface water would be reflective of releases from both industrial activities and the use of products available to consumers. After release to air, both TCPP and TDCPP are associated with particles, where they have demonstrated higher persistence. Along with measured air concentrations in the Arctic areas of Canada and Europe, there is sufficient evidence suggesting that both substances are very persistent in air and possess the potential for long-range atmospheric transport.
Releases of TCPP and TDCPP from both industrial activities and the use of products available to consumers will primarily enter surface water after wastewater treatment. TCPP remains in water and a very small percentage may partition to sediment. TDCPP also mainly stays in water with a small portion potentially partitioning to sediment. During wastewater treatment, it is expected that this substance will partition to biosolids to some extent, and these biosolids may in turn be applied to soils.
Both substances do not hydrolyze significantly under the environmental conditions and are not rapidly biodegradable. However, both TCPP and TDCPP have been shown to biotransform quickly in various vertebrate species (fish, mammals, and birds). Empirical and modelled (Q)SAR data indicate a low bioconcentration and bioaccumulation potential for both substances. Given that, there is a high level of confidence with data showing limited accumulation of TCPP and TDCPP in biota. Biomagnification via food chains is unlikely and exposure to higher trophic level organisms is expected to be lower than the exposure to lower trophic level organisms.
In the ecological effect assessment, empirical data have been identified, indicating moderate toxicity of TCPP and high toxicity of TDCPP to organisms based on survival, reproduction, and growth endpoints. While TCPP is not shown to elicit a significant effect on the endocrine system in fish, empirical data have confirmed that TDCPP could alter the thyroid concentrations in zebrafish, which may be a contributing factor to the lower survival rate and a higher incidence of malformation in the test organisms. TDCPP could also change sexual hormone concentrations in fish at low levels; however, there is no report of any effect on the filial generations following parental exposure. There is a moderate to high level of confidence with key toxicity data that have been chosen to extrapolate PNECs for these substances.
Data for neurotoxicity, cytotoxicity, genotoxicity, and effects on other biomarkers for these two substances have been identified in cell assays in fish and birds. These data are useful to clarify the chemical reactivity and the mechanism of toxic action. However, it is noted the highest estimated PECs in water (0.12 mg/L for TCPP and 0.0022 mg/L for TDCPP) are lower than exposure levels of TCPP (1 mg/L) and TDCPP (0.0033 mg/L) that can alter hormone concentrations or demonstrate effects on enzyme activities and mRNA expression in cell assays. Hypothetically speaking, the lowest exposure concentrations used in the cell assays (1 mg/L for TCPP and 0.0043 mg/L for TDCPP) extrapolates to tissue concentrations of TCPP and TDCPP in fish at approximately 3 mg/kg and 0.0043 mg/kg, respectively (assuming density at 1 kg/L for aquatic organisms). Such tissue concentrations are much higher than the measured concentrations reported in any of the fish samples collected from Canadian water bodies. Considering the measured concentrations of (and the high frequency of not detecting) TCPP and TDCPP and low bioacculumation in aquatic organisms, there is a high level of confidence that these two substances will not impact hormone pathways, gene transcription, or receptor-mediated endpoints at their current levels of occurrence in fish.
Both TCPP and TDCPP were found in herring gull eggs. These findings are in agreement with the protein binding-potentials associated with these two substances. In ovo toxicity data suggest that chicken embryos are also sensitive to TCPP and TDCPP. The timing of pipping and embryonic development were affected by both substances at magnitudes of 103 ng/g, while the hormonal effect was only associated with TDCPP at the same level. Such exposure concentrations associated with an effect in the toxicity study are three orders of magnitude higher than the measured concentrations of both substances in eggs.
Considering the above, a higher weight is applied to the effects data from in vitro and in vivo studies which are directly linked to organism level effects for the estimation of PNECs in this assessment.
The exposure assessment has focused on releases of both substances from the production of polyurethane foams and the use of products available to consumers via laundry activities. Environmental concentrations have been estimated for compartments with a high level of confidence where substances are most likely to be found based on the environmental fate analysis.
Risk characterization was conducted for the aquatic compartment for TCPP and the aquatic, sediment, and soil compartments for TDCPP. Outcomes from risk quotient analysis for TCPP in the aquatic compartment are below 1 indicating that risk associated with exposure to substance in the environment due to industrial uses or releases from the use of products available to consumers is low. This information indicates that TCPP has low potential to cause ecological harm in Canada.
For TDCPP, risk quotients determined for sediment and soil compartments are below 1. In the aquatic compartment, PECs associated with all industrial uses are also below the PNEC; and the probability of environmental concentrations exceeding the PNEC due to the use of products available to consumers is small. Given that, the risk associated with exposure to TDCPP in the environment due to industrial uses or releases from the use of products available to consumers is considered low. This information indicates that TDCPP has low potential to cause harm in Canada.
While exposure of the environment to TDCPP is not of concern at current levels, this substance is considered to have an environmental effect of concern on the basis of its high toxicity and potential effects on the endocrine system in fish. Therefore, there may be a concern for the environment if exposures were to increase.
7.5 Uncertainties in evaluation of ecological risk
The exposure assessment focuses on industrial point sources. From their use as additive flame retardants, both substances may migrate from products over time to the air and to dust, as evidenced by concentrations in air samples of both outdoor air and indoor dust. Diffuse emissions from the use of products are at a very low rate. Quantities of these substances imported in manufactured items have not been well captured in responses to the notice issued pursuant to section 71 of CEPA. TCPP and TDCPP quantity in products (considering all products imported and in use) could be high; however, it is assumed that major TCPP and TDCPP pathways of release from products are reflected in measured environmental concentrations. The environmental releases due to the use of products available to consumers have also been characterized by considering the measured concentrations of these substances in domestic laundry wastewater. Releases from industrial transport container cleaning were not considered in a quantitative manner due to a high degree of uncertainty. Conservative assumptions were made in the exposure assessment and there is a moderate level of confidence with the exposure scenarios used to calculate PECs.
According to its physical and chemical properties, TCPP is water soluble and the majority of this substance is expected to remain in the aquatic compartment. Outcomes from the Level III EQC only suggest 0.3% of the total release of this substance may reside in sediment, if released to water. Sediment PECs for TCPP were derived from the conservative PECs in the surface water and have been estimated at up to 2.8 mg/kg dw. There has been no empirical sediment toxicity data identified for this substance. Considering the difference in aquatic toxicity between TCPP and TDCPP and other phosphate esters, read-across is not considered applicable to determine the value of a toxicity endpoint; therefore, a PNEC for sediment is not calculated for TCPP and a risk quotient analysis is not conducted for this compartment. The lack of sediment effect data for TCPP is considered a data gap in this assessment and leaves the possibility for false negatives regarding risk to sediment-dwelling organisms.
8. Potential to cause harm to human health
8.1 Exposure assessment
8.1.1 Environmental media and food
Both TCPP and TDCPP are additive flame retardants and since they are not chemically bound to the polymer matrix, there is the potential for their release to the environment from products available to consumers.
Concentrations of TCPP and TDCPP reported in air, water, dust and food are further described below (see also Section 7.2.1, Appendix A; ECCC 2019). Based on monitoring data, the highest intake estimates of TCPP and TDCPP for the general population through environmental media and food are 2.5 and 0.86 µg/kg-bw per day for ages 6 months to 4 years and 0–6 months, respectively (Appendix C).
8.1.1.1 Ambient air
TCPP and TDCPP have been detected in outdoor air in Canada and elsewhere (Section 7.2.1). Both TCPP and TDCPP were measured via high-volume active air samplers around the Great Lakes and Toronto from 2011 to 2013. Mean concentrations from Lake Superior, Lake Ontario and Lake Huron ranged between 0.08 and 0.20 ng/m3 for TCPP and 4.3 × 10-3 and 6 × 10-3 ng/m3 for TDCPP (Shoeib et al. 2014, Jantunen 2014). As mentioned in Section 7.2.1, mean concentrations of 0.671 ng/m3 for TCPP and 0.158 ng/m3 for TDCPP with corresponding maximum concentrations of 1.52 ng/m3 for TCPP and 1.21 ng/m3 for TDCPP were measured in Toronto (n= 32) (Shoeib et al. 2014). TDCPP was also measured with a concentration of 9.5 × 10-3 ng/m3 in outdoor air samples (n=20) in another Toronto study from January 2011 to February 2012 using a high-volume active air sampler (Diamond et al. 2013). TCPP was not monitored by Diamond et al. (2013).
In Norway, 10 samples in an urban area were analyzed for TCPP with concentrations between 240 and 3700 ng/m3 (Green et al. 2008). Ambient TDCPP air levels were also measured in Chicago and Cleveland and mean concentrations were 0.079 ng/m3 (n=27) and 0.11 ng/m3 (n=22), respectively (Salamova et al. 2013). Ambient air samples for TCPP were collected in Chicago (n=27) and Cleveland (n=22) in 2012. Median concentrations were 0.41 ng/m3 (Chicago) and 0.32 ng/m3 (Cleveland) based on the sum of vapour and particle phases (Salamova et al. 2013). A study in Sweden reported a level of 0.81 ng/m3 of TCPP at a single location (Marklund et al. 2005a). Concentrations of TCPP and TDCPP are found in remote areas such as the Arctic and Antarctic, suggesting that both may undergo long-range atmospheric transport (Moller et al. 2012; Green et al. 2008). This is consistent with findings based on the physical chemical properties of these substances.
Maximum concentrations of 1.52 ng/m3 and 1.21 ng/m3 from the same Toronto study were selected to estimate daily intakes of TCPP and TDCPP, respectively, from exposure to ambient air (Shoeib et al. 2014).
8.1.1.2 Indoor air
Indoor air was monitored for TCPP and TDCPP in 23 homes in Toronto during a sampling period of 28 days from May to August 2013. TCPP was detected in air at levels ranging from 7.68 to 4190 ng/m3 with a median of 73.6 ng/m3 and a detection frequency of 97%. TDCPP was detected in air at levels ranging from 0.045 to 5.5 ng/m3 with a median of 0.525 ng/m3 and a detection frequency of 97% (Vykoukalová et al. 2017). Indoor air was also monitored for TCPP and TDCPP in 32 homes in the Greater Toronto Area and in 19 homes in Ottawa during a sampling period of 3 weeks from late February to late July 2015 (Yang et al. 2019). Air was sampled in bedrooms in all homes (n=51) and additionally in the “most used room” (MUR) in 26 of the 51 homes. TCPP (reported as the sum of the TCPP isomers, ƩTCPPs) was detected in indoor air at levels ranging from 0.005 to 313 ng/m3 in bedrooms with a 95th percentile of 71.5 ng/m3, a geometric mean of 2.58 ng/m3 and a detection frequency of 82%. TCPP was detected in indoor air at levels ranging from 0.005 to 127 ng/m3 in the MURs with a 95th percentile of 15.2 ng/m3, a geometric mean of 7.4 ng/m3 and a detection frequency of 96%. TDCPP was detected in 100% of indoor air samples collected in bedrooms over a range of 0.0173 to 0.672 ng/m3 with a 95th percentile of 0.418 ng/m3 and geometric mean of 0.113 ng/m3. TDCPP was also detected in 100% of the MUR samples over a range of 0.0243 to 1.55 ng/m3 with a 95th percentile of 0.730 ng/m3 and a geometric mean of 0.159 ng/m3 (Yang et al. 2019).
These substances have also been measured in homes in Sweden. In two studies of 10 homes, TCPP and TDCPP were detected in air at levels ranging from 2.4–160 ng/m3 and from below the detection limit (1 ng/m3) to 17 ng/m3, respectively (Staaf and Otsman 2005; Bergh et al. 2011). For TDCPP, the majority of the samples were below the detection limit of 1 ng/m3 (Bergh et al. 2011) (Appendix A).
Time spent indoors may occur in locations other than the home, such as an office for adults, or vehicle, daycare, school or gym for adults or children. Concentrations of TCPP and TDCPP in office environments in Sweden and Norway ranged from 10 to 240 ng/m3 and 0.2 to 150 ng/m3, respectively (Marklund et al. 2005b; Staaf and Otsman 2005; Green et al. 2008; Bergh et al. 2011; Hartmann et al. 2004). More recently, TCPP and TDCPP were measured in 10 offices in China with maximum concentrations of 81 and 14 ng/m3, respectively, much lower than those in offices in Europe (Yang et al. 2014). Indoor air from daycare centres and early childcare education centres has been sampled recently in North America. In a study of 40 early childcare education centres, Bradman et al. (2012) detected TDCPP with a mean concentration of 0.59 ng/m3 and 95th percentile of 1.25 ng/m3. TCPP was not measured in this study (Bradman et al. 2012). Levels in air in 10 Swedish daycares were measured; the mean concentration of TDCPP was 6.7 ng/m3. The mean concentration of TCPP in daycares in Stockholm was 19 ng/m3 (Bergh et al. 2011). Gymnasiums in Boston were monitored for TCPP and TDCPP given the prevalence of foam pits; concentrations were 2.68 ng/m3 and 12.5 ng/m3 for a single sample near the foam and 0.74 ng/m3 and 8.41 ng/m3 for a single sample away from the pit, for TCPP and TDCPP, respectively (Carignan et al. 2013a).
Time spent in automobiles and aircraft may also represent potential sources of exposure to TCPP or TDCPP. Indoor air measured in vehicles contained levels ranging from not detected (LOD = 0.12 ng/m3) to 2300 ng/m3 of TCPP (Hartmann et al. 2004; Staaf and Otsman 2005). Hartmann et al. (2004) sampled 4 cars of varying age in Switzerland, where the maximum TCPP concentration of 260 ng/m3 was measured in a 9 year-old car. Concentrations in the other cars, both less than 1 year old, were below 23 ng/m3. TDCPP was measured in cars, but not detected above the analytical detection limit of 0.11 ng/m3 (Hartmann et al. 2004). Staaf and Otsman (2005) measured a collection of personal and public vehicles (1 car, 2 public buses and 1 subway car) from Stockholm resulting in a TCPP concentration of 1800 ng/m3 in the car, 330 and 2300 ng/m3 in the buses and 2000 ng/m3 in the subway car. Only TDCPP was detected in the car at a concentration of 5 ng/m3 (concentrations were not detected in buses and subway [LOD = 1 ng/m3]) (Staaf and Otsman 2005). Given the potential variability of TCPP and TDCPP concentrations across various transportation vehicles, the limited number of study samples, and the increased air changes from opening doors frequently (and windows depending on the season), there is uncertainty in estimating exposures in vehicles.
Daily intakes of TCPP and TDCPP from exposure in indoor air for the Canadian general population were estimated based on the highest concentrations of 4190 and 5.5 ng/m3, respectively, in homes in Toronto (Vykoukalová et al. 2017). These air levels are considered to take into account the variability in environmental concentrations associated with different settings (e.g., daycare, office, gym, vehicles). These potential sources of exposure are expected to be lower than those conservatively estimated for homes based on lower frequency and duration of exposure and reported levels across these microenvironments are very similar.
8.1.1.3 Dust
TCPP and TDCPP have been measured in dust in several studies in homes, offices and other indoor environments in Canada and globally (Appendix A).
TCPP was measured in the Canadian House Dust Study (CHDS) during 2007–2008 in the dust of 818 homes in various Canadian cities resulting in median and 95th percentile concentrations of 1.62 mg/kg and 18.2 mg/kg, respectively. TCPP was measured in 95% of samples over a range of concentrations from <MDL (method detection limit, 0.11 mg/kg) to 120 mg/kg (personal communication, email from Environmental Health Science Bureau, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada, dated Dec. 13, 2013; unreferenced)). TCPP has also been measured in dust in additional Canadian studies. Dust from 23 homes in Toronto was collected during a sampling period of 28 days from May to August 2013. TCPP was detected in 100% of dust samples over a range of 0.270 to 39.3 mg/kg with a median of 1.470 mg/kg (Vykoukalová et al. 2017). Dust from 32 homes in the Greater Toronto Area and 19 homes in Ottawa was collected during a sampling period of 3 weeks in 2015. Dust was sampled from bedrooms in all homes (n=51) and additionally in the “most used room” (MUR) in 26 of the 51 homes. TCPP (reported as the sum of the TCPP isomers, ƩTCPPs) was detected in 96% of bedrooms over a range of 0.00375 to 299 mg/kg with a 95th percentile of 9.42 mg/kg and a geometric mean of 0.934 mg/kg. TCPP was detected in 100% of samples from the MURs over a range of 0.268 to 161 mg/kg with a 95th percentile of 10.84 mg/kg and a geometric mean of 1.33 mg/kg (Yang et al. 2019).
Results from studies from the United States and Europe are consistent with the Canadian data, where TCPP has been measured in household dust with mean concentrations ranging from 0.5 to 3.1 mg/kg (Van den Eede et al. 2011; Brommer et al. 2012; Dodson et al. 2012; Bergh et al. 2011; Stapleton et al. 2009; Marklund et al. 2003).
The 95th percentile concentration of TCPP (18.2 mg/kg) in household dust from the CHDS was used to estimate the daily intake from dust by the Canadian general population (personal communication from Environmental Health Science and Research Bureau, Health Canada, dated August 22, 2014).
TDCPP was reported in dust in several Canadian studies monitoring dust in Toronto and various Canadian cities In the Canadian House Dust Study (CHDS), TDCPP was measured in 99.4% of samples (n=818) at concentrations ranging from <MDL (0.08 mg/kg) to 139 mg/kg with a median and 95th percentile concentrations of 3.08 mg/kg and 12.7 mg/kg (personal communication, email from Environmental Health Science Bureau, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada, dated Dec. 13, 2013; unreferenced). TDCPP has also been measured in dust from 23 homes in Toronto during a sampling period of 28 days from May to August 2013. TDCPP was detected in 100% of dust samples over a range of 0.206 to 9.53 mg/kg with a median of 0.917 mg/kg (Vykoukalová et al. 2017). Dust from 32 homes in the Greater Toronto Area and 19 homes in Ottawa was collected during a sampling period of 3 weeks in 2015. Dust was sampled from bedrooms in all homes (n=51) and additionally in the MUR in 26 of the 51 homes. TDCPP was detected in 100% of bedrooms over a range of 0.430 to 9.28 mg/kg with a 95th percentile of 6.76 mg/kg and a geometric mean of 1.54 mg/kg. TDCPP was detected in 100% of samples from the MURs over a range of 0.337 to 130 mg/kg with a 95th percentile of 12.8 mg/kg and a geometric mean of 2.05 mg/kg (Yang et al. 2019). Another Canadian study measured TDCPP in dust in 35 homes and 10 offices in Toronto in 2012 (Abbasi et al. 2016). Concentrations of TDCPP in dust ranged from not detected to 46 mg/kg in homes (detection frequency 83%) and not detected to 190 mg/kg in offices (detection frequency 90%). Abbasi et al. (2016) also analyzed the association of TDCPP in dust from the 2012 study with dust on products (n=65) in the same locations. TDCPP was found mainly on the surface of large household appliances (not detected to 494 ng/wipe; detection frequency 43%, n=7) and flat screen TVs (not detected to 193.25 ng/wipe; detection frequency 36%, n=14). The authors showed that there was a positive correlation between the geometric mean concentrations of halogenated flame retardants (including TDCPP) in home and office dust with those in dust from the surfaces of electronic products (Abassi et al. 2016).
European and American studies monitoring TDCPP in household dust report similar mean concentrations as seen in Canada ranging from 0.08 to 2.8 mg/kg (Van den Eede et al. 2011; Brommer et al. 2012; Dodson et al. 2012; Stapleton et al. 2009; Ali et al. 2012).
Dust was also monitored for its TCCPP and TDCPP content in early childcare education environments. In Sweden, TCPP was measured in 10 daycare centres with a mean concentration of 4.5 mg/kg while TDCPP was found at a mean concentration of 28 mg/kg (Bergh et al. 2011). In California, USA, TDCPP in dust was detected in 39 early childcare education centres ranging from 0.76 mg/kg to 71 mg/kg, with a mean concentration of 6 mg/kg and 95th percentile of 37 mg/kg (Bradman et al. 2012). TCPP was not monitored as part of this study. Dust was also sampled in gymnasiums in Boston, Massachusetts (Carignan et al. 2013a). The median concentrations of TCPP and TDCPP inside the gymnastics facilities were found to be 2.48 mg/kg and 13 mg/kg, respectively, by Carignan et al. (2013a).
TCPP and TDCPP have also been frequently detected in offices and different vehicles. Webster et al. (2010) measured TDCPP in office dust and cars in the Boston area, USA, in 2009, where mean concentrations were found to be 9.8 and 26 mg/kg, respectively. In Germany, TCPP and TDCPP mean concentrations were reported in office dust (n=10; 3 mg/kg and 0.15 mg/kg, respectively) and cars (n=12; 3.1 mg/kg and 130 mg/kg, respectively) (Brommer et al. 2012). Concentrations in both offices and cars exceeded the concentrations of TCPP and TDCPP in the homes sampled in the same study. Other studies have also reported higher concentrations of TCPP and TDCPP in car dust compared to household dust in Europe and the Middle East (Ali et al. 2013; Brandsma et al. 2014). In addition, TDCPP was also measured in dust from aircraft cabins, with median levels in carpet of 2.1 mg/kg and of 5.6 mg/kg in air vents (Allen et al. 2013). TCPP was not measured in aircraft.
The maximum concentration of 139 mg/kg from the Canadian House Dust Study was used as a conservative level of TDCPP in dust for estimating intakes for the general population of Canada including potentially higher exposures from early childcare education facilities observed in the US.
8.1.1.4 Soil and sediment
There are no soil or sediment monitoring data for TCPP or TDCPP in Canada, however both substances were measured in soil in one study in Europe and one study in China. Mihajlovic et al. (2011) measured a mean concentration of 0.0012 mg/kg of TCPP in six German soil samples. TDCPP was below the detection limit of 9 × 10-5 mg/kg (Mihajlovic et al. 2011). Cui et al. (2017) measured TCPP and TDCPP in soil samples from urban soils of Guangzhou, China. Concentrations of TCPP ranged from less than the method detection limit (MDL=0.0002 mg/kg dry weight, dw) to 0.016 mg/kg dw with the highest mean concentration of 0.006±0.005 mg/kg dw for samples (n=12) from commercial areas (detection frequency = 100%). Concentrations of TDCPP ranged from <MDL (MDL=0.0002 mg/kg dw) to 0.091 mg/kg dw with the highest mean concentration of 0.034±0.026 mg/kg dw for samples (n=12) also from commercial areas (detection frequency = 100%) (Cui et al. 2017).
The mean soil concentration for TCPP (0.0012 mg/kg) and the detection limit for TDCPP (9 × 10-5 mg/kg) from the study by Mihajlovic et al. (2011) were used to estimate intakes of these substances from soil.
8.1.1.5 Drinking water
Levels of TCPP and TDCPP in water have been reported in Canada (Venier et al. 2014; Jantunen et al. 2013b; Truong 2016; Truong et al. 2017; Hao et al. 2018) (see Table 7‑10 and Table 7‑11). TCPP was measured in a pilot study on tap water in Barrie, Ontario (n=2) with a concentration of 11 ng/L (Jantunen 2014). Data on TDCPP concentrations in tap water were not identified.
TCPP and TDCPP were measured in surface water from rural and urban tributaries draining into Lake Ontario, with upper concentrations of 1839 ng/L and 1437 ng/L in urban areas, respectively. TCPP was reported at lower concentrations between 4.64 and 180 ng/L in rural areas (Jantunen et al. 2013b). Truong (2016) measured TCPP and TDCPP in three streams during high and low flow periods and in Toronto, Canada. The concentration of TCPP (based on the sum of two TCPP isomers) ranged from 3.4x10-4 to 5.1x10-3 ng/L. The concentration of TDCPP ranged from below the LOD (not reported) to 4.7x10-4 ng/L (Truong 2016). In another study, Venier et al. (2014) measured lower concentrations for both TCPP and TDCPP at different locations around the Great Lakes (ECCC 2019). Surface samples from three urban streams in Toronto were collected in 2014 to 2015 (n=14) and analyzed for the isomers of TCPP. A mean concentration of TCPP (reported as the sum of TCPP isomers, ƩTCPP) of 2700 ng/L and a range of 1100 to 4400 ng/L were reported (Truong et al. 2017). Another study measured both TCPP and TDCPP in urban stream water and nearshore lake water collected in the Toronto area in the summer and fall of 2014 and spring and summer of 2015. TCPP was detected in 12 of 20 surface water samples over a concentration range of 290 to 2010 ng/L and TDCPP was detected in 1 of 20 surface water samples at a concentration of 130 ng/L (Hao et al. 2018).
TCPP and TDCPP were measured in Europe from different deposition types, including snow and rain, in addition to surface water from rivers, estuaries and the ocean. TCPP concentrations in rain were found to be higher than in snow with mean concentrations of 372 ng/L and 233 ng/L in Germany, respectively (Mihajlovic and Fries 2012). TDCPP was detected at mean levels of 46 ng/L in rain and 100 ng/L in snow (Mihajlovic and Fries 2012). Both substances were found to be less concentrated further north with snow concentrations ranging between 68–210 ng/kg (TCPP) and 4–29 ng/kg (TDCPP) in Sweden (Marklund et al. 2005a).
TCPP was measured in river water at levels ranging from 24 to 570 ng/L in Germany, and which were higher than concentrations measured in the ocean or estuaries (Bollman et al. 2012). Measurements of waterways near wastewater treatment systems (WWTS) in Germany, Austria and Norway have shown that concentrations are approximately 10-fold higher downstream (Meyer and Bester 2004; Andreson et al. 2004; Green et al. 2008; Martinez-Carballo et al. 2007). Similar river concentrations of TCPP (100–310 ng/L) were detected in rural areas of South Korea, Asia (Yoon et al. 2010). TDCPP has been measured in rivers in Germany at concentrations ranging between 5 and 67 ng/L (Bollman et al. 2012). This is lower than water concentrations (20-740 ng/L) measured near WWTPs in Germany and Norway (Meyer and Bester 2004; Andreson et al. 2004; Green et al. 2008).
As the data on concentration of TCPP and TDCPP in drinking water was limited, the maximum surface water concentrations of 5100 ng/L (Truong 2016) and 1437 ng/L (Jantunen et al. 2013b), for TCPP and TDCPP, respectively, were considered to be the most relevant data to use in calculating an upper-bound estimate of daily intake from drinking water for the Canadian general population.
8.1.1.6 Food
No reports of studies monitoring TCPP or TDCPP in Canadian food were identified. TCPP and TDCPP have been monitored in food basket surveys by the US FDA since the 1980s. There are no reported amounts of TDCPP; TCPP was detected in fruit peels at concentrations ranging from 0.05 to 0.82 µg/kg (ATSDR 2012).
Monitoring of TCPP and TDCPP in fish and shellfish was conducted in four European studies (ECCC 2019). Levels in fish tissue (perch, cod, salmon and char) and bivalve shellfish (mussel) were reported from freshwater and marine locations in Sweden and Norway. Levels of TCPP and TDCPP were below the detection threshold of 10 µg/kg in mussels in Norway, but TCPP was detected in concentrations up to 15.6 µg/kg in mussels in Sweden (Green et al. 2008; Sundkvist et al. 2010). TCPP and TDCPP concentrations in finfish from Nordic countries ranged from below the detection limit of 0.1 µg/kg to 5.7 µg/kg and from 0.3 µg/kg to 8.1 µg/kg, respectively (Green et al. 2008; Evenset et al. 2009; Sundkvist et al. 2010). In a Swedish study, fish purchased from a grocer were sampled and neither substance was found above detection limits of 1 µg/kg and 9 µg/kg for TCPP and TDCPP, respectively (Campone et al. 2010).
Upper bound concentrations of 15.6 µg/kg from shellfish in Sweden and 8.1 µg/kg from arctic finfish in Norway were selected to estimate the daily intake of TCPP and TDCPP, respectively, from food for the general population (Sundkvist et al. 2010; Evenset et al. 2009). The upper bound concentration in fruit (0.82 µg/kg) was also used to estimate daily intake of TCPP from food. Upper-bounding estimates of daily intake from food for the Canadian general population were estimated to be 0.068 and 0.028 µg/kg-bw per day for TCPP and TDCPP, respectively (for children aged 0.5–4 yrs). These are conservative estimates because they were calculated assuming that all seafood and fish consumed would contain TCPP or TDCPP at the maximum levels measured in fish. Although certain northern populations or other subpopulations in Canada may consume larger quantities of seafood in their diet, this estimate is considered conservative enough to account for this higher consumption.
8.1.1.7 Breast milk
No data were identified on concentrations of TCPP and TDCPP in breast milk in Canada or the US. TCPP and TDCPP were measured in breast milk in six cohort studies in Sweden. TCPP concentration in breast milk was derived from the highest lipid weight concentration of 57 ng/g measured in breast milk from 50 women, and adjusted in the same manner to give a concentration of 1.99 µg/L. The concentration of 0.186 µg/L of TDCPP in breast milk is based on the highest lipid weight concentration (5.3 ng/g) measured in breast milk from 90 women, adjusted with the lipid content in breast milk (3.4%) and the density of breast milk. (Sundkvist et al. 2010).
TCPP and TDCPP were also measured in breast milk from three participants in Australia. Measured TCPP concentrations ranged from below the detection limit of 2.4 µg/L to 14 µg/L and measured TDCPP concentrations ranged from below the detection limit of 0.053 µg/L to 0.14 µg/L (He et al. 2018).
The maximum concentrations in breast milk of 1.99 µg/L and 0.186 µg/L of TCPP and TDCPP, respectively, from the Swedish cohort studies (Sundkvist et al. 2010) were selected as the most relevant data to estimate upper bound daily intakes to these substances by breast-feeding infants, due to the study’s larger sample size.
8.1.2 Products available to consumers
TCPP and TDCPP are additive flame retardants with a variety of uses and applications (see Section 4), some of which may result in general population exposure. Dermal and oral exposure estimates were derived using conservative approaches for scenarios considered relevant for the general population. TCPP and TDCPP are semivolatile substances; therefore, they are not expected to appear in their gaseous forms in high quantities under normal conditions. Inhalation exposure estimates were only derived for the do-it-yourself scenarios (i.e., application of spray foam insulation and waterproofing sprays) which could result in elevated levels in air for a short duration. Inhalation exposures resulting from daily releases to air (i.e., from off-gassing of products in the home such as furniture and mattresses or insulation that is present in the home behind walls) are expected to be accounted for through indoor air and dust exposure estimates (see Sections 8.1.1.2 and 88.1.1.3).
8.1.2.1 Manufactured items
TCPP and TDCPP are used in flexible foam products (for example in furniture such as seating or mattresses), nap mats, child restraint seats and in building construction in Canada (ECCC 2013-2014; CEH 2013a,b; Stapleton et al. 2011). TCPP and TDCPP can each be found in the foam of furniture at concentrations up to 9% w/w (Kemmlein et al. 2003; Stapleton et al. 2009; Stapleton et al. 2011; Stapleton et al. 2012; Ionas et al. 2014; US CPSC 2005a,b; ECCC 2013-2014). In addition, TCPP and TDCPP have also been measured in several children’s products containing foam in the U.S., including nap mats (CEH 2013b), foam chairs (including one containing TCPP, purchased in Canada) (CEH 2013a), child restraint seats, changing table pads, portable mattresses and rocking chairs, in concentrations ranging from 0.11 to 1.4% w/w for TCPP and 0.24 to 12.4% w/w for TDCPP (reported as 1.11 to 14.4 mg/g and 2.4 to 124 mg/g, respectively) (Stapleton et al. 2011). In product testing conducted by Health Canada on 23 children’s products (e.g., nursing pillows, polyurethane foam chair, toys purchased in retail stores in Ottawa, Ontario in 2014), TDCPP was detected in a foam toy at a mean concentration of approximately 7%; TCPP was not detected above the limit of quantification (LOQ of 0.3%) in any of the foam samples (Health Canada 2014). In an additional survey conducted in 2015 by Health Canada on 21 polyurethane children’s products (e.g., toys, cushions), concentrations of TCPP were found to range from 0.02 to 3.4% w/w (LOQ = 0.013% w/w) (highest concentrations found in bath toys) while concentrations of TDCPP ranged from 0.012 to 7.3% w/w (LOQ = 0.012% w/w) (Health Canada 2015b).
Flame retardants can also be found in coatings on the inside face of the cover fabric of furniture (e.g., couch) known as “backcoating”. The use of TCPP or TDCPP as backcoating in furniture upholstery has not been identified specifically for Canada but is a known use in the United States and Europe (US CPSC 1998 2005a,b; EU RAR 2008b; Danish EPA 2014), and it is considered reasonable to assume that the general population of Canada can be exposed to TCPP or TDCPP in furniture. The Upholstered Furniture Action Council (UFAC), a voluntary coalition of furniture manufacturers, outlined the potential for dermal exposure to backcoating, either via direct contact with the substance from contamination of the outside surface of the fabric (from wet backcoating when textile is rolled up post-production), or from degradation of the backcoating, or through the textile weave (cited in US CPSC 1998). A study by the Commission for Environmental Cooperation (CEC) tested 132 furniture products (chairs, sofas, ottoman) purchased between December 2014 and April 2015 in Canada, the U.S. and Mexico (CEC 2015). Sixteen flame retardants were measured and TCPP and TDCPP were among the six detected in 61 of the 132 products tested, at levels ranging from 0.01 to 8.9% w/w and 0.03 to 5.8% w/w, respectively. TCPP was the most frequently detected flame retardant across all three countries and was most frequently detected in samples from products purchased in Canada (45 of 193 samples or 23%). TDCPP was detected in 8 of 193 samples (4%) from products purchased in Canada. TCPP was found mostly in foam samples (91% of 54 products with TCPP), though it was also identified in fabric (24%), padding (11%) and stuffing samples (4%); it was also found in the upholstery of chairs, office chairs, ottomans and sofas. TDCPP was also measured predominantly in foam samples (73% of 15 products) and in fewer instances in upholstery (CEC 2015).
Although the European Union risk assessments for TCPP and TDCPP did not estimate dermal exposure from foam objects (EU RAR 2008a,b), they, as well as other organisations, reported that some flame retardants migrate from various foam objects (EU RAR 2009; US CPSC 2005b; Arcadis EBRC 2011; Danish EPA 2015). More specifically, the US CSPC has shown that TDCPP can migrate to the surface of foam covered with fabric under simulated perspiration and pressure conditions (i.e., to simulate sitting) (U.S. CPSC 2005b). More recently, ECHA considered children’s dermal exposure to TCEP, TCPP and TDCPP (i.e., TDCP) from children’s products, such as baby mattresses and child restraint seats (ECHA 2018).
In consideration of the collective information on flame retardant migration from foam objects and on models and algorithms developed by various jurisdictions to evaluate exposure from this source, it was considered appropriate to assess dermal exposure to TCPP and TDCPP from polyurethane flexible foam in mattresses or upholstered furniture (e.g., sofas).
Dermal exposure uptakes were estimated for children and adults in prolonged skin contact with foam-containing mattresses and furniture as a representative upper bounding scenario of potential exposure (Appendix D). Given the conservative nature of this scenario, the estimate would also address potential exposure from textile backcoating from furniture. This exposure was modelled using an algorithm that is consistent with that of models used by other jurisdictions to estimate dermal exposure to a substance migrating from foam or textile backcoating (US CPSC 2006a; ECHA 2018; NRC 2000; Danish EPA 2014, 2015; Arcadis EBRC 2011).
Extraction studies measuring migration of TCPP and TDCPP from furniture foam to aqueous solutions (e.g., artificial sweat) have been reported (US CPSC 2005b; TNO Quality of Life 2005 cited in EU RAR 2008a). TNO Quality of Life (2005) conducted a migration test on an uncovered foam block containing 10% TCPP, resulting in a migration rate of 4.6 × 10-3 mg/cm2/hr when pressure was applied to a wetted stack of 15 filter papers placed directly on top of the foam. The migration rate of 5.6 × 10-5 mg/cm2/hr for TDCPP used to estimate dermal exposures is based on a migration study performed by the U.S. CPSC using furniture foam containing 6.6% w/w TDCPP that was covered with fabric in a miniature furniture mock-up (U.S. CPSC 2005b). In this study, the furniture mock-up was wetted, a filter paper was placed on its surface, and pressure was applied. In both cases, the flame retardant that was measured in the filter paper was assumed to be available for dermal exposure via liquid-mediated migration from the foam. Since no data were identified on the relationship between the migration rate of TCPP or TDCPP from foam and their concentrations in the foam tested, this variable could not be included in the algorithm used to estimate dermal exposure.
Dermal absorption of TCPP and TDCPP through the skin has also been investigated. The EU RARs for TCPP and TDCPP (TNO Quality of Life 2005, 2006b cited in EU RAR 2008a,b) describe in vitro dermal absorption studies using human skin membranes with direct application of radiolabelled TCPP and TDCPP giving maximum total absorptions of 23% and 40% of TCPP and 15% of TDCPP. The two absorption values of 23 and 40% for TCPP were derived from studies testing different doses. The dermal absorption of 40% was based on testing with lower concentrations that were considered more representative of exposure from dermal contact with foam; this value was used in estimating dermal exposure from contact with furniture or mattreses containing TCPP (TNO Quality of Life 2005). The dermal absorption value of 15% for TDCPP derived from an in vitro study was based on a dose which was higher than that typically received from exposure to foam furniture (i.e., not considered representative) and therefore was adjusted in the EU RAR (2008b) based on the ratio of absorption values from the in vitro studies conducted on TCPP (i.e., multiplied by a factor of two). This resulted in an adjusted dermal absorption value of 30% for TDCPP (EU RAR 2008b); this value was used in estimating dermal exposure from contact with furniture or mattreses containing TDCPP.
The highest estimates of daily exposure to TCPP and TDCPP from prolonged dermal contact with mattresses or furniture containing polyurethane flexible foam were for infants and were estimated to be 0.54 mg/kg-bw/day and 5.0 × 10-2 mg/kg-bw/day, respectively. Further details on the assumptions used to derive the dermal exposure estimates are provided in Appendix D.
Estimates of exposure from mouthing a foam object were calculated based on migration rates for TCPP and TDCPP that were determined by the Danish EPA (2015) in experiments that were more representative of migration during mouthing than the surface migration studies used in the dermal exposure estimates. Foam from children’s products (i.e., child restraint seats, baby slings, baby mattresses) were submerged in sweat simulant and incubated at 37 °C for 3 hours (Danish EPA 2015). It is assumed that migration of TCPP and TDCPP to sweat simulant would be similar to migration in saliva. This resulted in average migration rates of 1.78 × 10-2 mg/cm2/hr and 2.97 × 10-3 mg/cm2/hr for TCPP and TDCPP, respectively (ECHA 2018). Assuming a foam object is mouthed over a surface area of 10 cm2 for infants and 20 cm2 for toddlers during a mouthing event of 24.5 min per day (Norris and Smith 2002 cited in US EPA 2011) results in intake estimates of 9.7 and 9.4 µg/kg-bw per day for TCPP for both infants and toddlers and intake estimates of 1.6 µg/kg-bw per day for TDCPP for infants and toddlers. Further details of assumptions used to derive the oral exposure estimates are provided in Appendix D.
Exposure Route and Duration |
Source |
Age Group |
Systemic exposure to TCPP |
Systemic exposure to TDCPP |
|---|---|---|---|---|
Dermal (daily) |
Foam in children’s mattresses or furniture |
Infant (0–6 mos; 7.5 kg) |
0.16 – 0.54 mg/kg-bw/day |
1.5 × 10-2 – 5.0 × 10-2 mg/kg-bw/day |
Dermal (daily) |
Foam in children’s mattresses or furniture |
Toddler (0.5–4 yr; 15.5 kg) |
0.11 – 0.41 mg/kg-bw/day |
1.0 × 10-2 – 3.8 ×10-2 mg/kg-bw/day |
Dermal (daily) |
Foam in mattresses or furniture |
Adult (70.9 kg) |
0.04 – 0.19 mg/kg-bw/day |
3.8 × 10-3 – 1.7 × 10-2 mg/kg-bw/day |
Mouthing (Intermittent) |
Foam in children’s products |
Infant (0–6 mos; 7.5 kg) |
9.7 × 10-3 mg/kg-bw/day |
1.6 × 10-3 mg/kg-bw/day |
Mouthing (Intermittent) |
Foam in children’s products |
Toddler (0.5–4 yr; 15.5 kg) |
9.4 × 10-3 mg/kg-bw/day |
1.6 × 10-3 mg/kg-bw/day |
TCPP and TDCPP are used in other manufactured items, such as specialized textiles for TDCPP (i.e., tent material) and various automotive parts found in the interior of vehicles for both substances (ECCC 2013-2014). One study detected TDCPP in the textile of three of 11 tents tested (Keller et al. 2014). While exposure to TCPP or TDCPP can occur via these products, the exposure potential from use of these products is not expected to be higher than that from flexible polyurethane foam due to the comparatively high frequency and duration of use of the foam products (i.e., mattresses, children’s products).
8.1.1.2 Other products available to consumers
In Canada, TCPP is reported to be used as an additive in several types of spray polyurethane foam (SPF) insulation (ECCC 2013-2014). According to the U.S. EPA’s website on SPF insulation, although two-component low pressure kits and one-component foam are used by professional applicators, these types of products are also available for do-it-yourself (DIY) applicators (U.S. EPA 2015). As such, two scenarios were developed for homeowners conducting DYI SPF insulation projects, i.e., a one-component can for smaller scale tasks and a two-component low-pressure kit for larger scale tasks. While SPF insulation product labels specify personal protection measures, such as the use of gloves or respiratory protection, exposure estimates derived do not consider that individuals are wearing personal protective equipment, given such equipment may not be readily accessible to consumers or may not be properly handled by consumers. Application of these product types is expected to occur infrequently (i.e., once every five years (RIVM 2007)).
For a one-component small scale project (i.e., sealing gaps around windows and doors), inhalation and dermal exposures to TCPP were estimated using ConsExpo Web (2016). The mean event air concentration of TCPP for the small scale project was estimated to be 180 µg/m3, equivalent to an intake of 0.88 µg/kg-bw/event through inhalation. Dermal uptake of TCPP during this same activity was estimated to be 6.8 µg/kg-bw/event. Details regarding this scenario are described further in Appendix E, Tables E1 and E3.
For a two-component large scale project (i.e., insulating an attic by a homeowner), inhalation and dermal exposures to TCPP were also estimated. One study is available in which air concentrations of TCPP were measured during the application of a two-component SPF for a large scale project completed by professionals (full wall spraying; ACC 2012). Monitoring results for the low-pressure system from this study were considered representative of a homeowner scenario. Concentrations of TCPP in the applicator’s breathing zone were reported to be between 477 and 2,940 µg/m3 (ACC 2012), resulting in an intake ranging from 2.3 and 14 µg/kg-bw/event (Appendix E, Table E4) through inhalation. This study also reported TCPP concentration in air (27 to 45 µg/m3), one to two orders of magnitude (approximately 10 to 100 times) lower during the post-spray testing (ACC 2012). TCPP dermal uptake for this scenario was estimated to be 635 µg/kg-bw/event during application of the two-component product based on the use of ConsExpo Web (2016). Details regarding this scenario are described further in Appendix E, Table E2.
TCPP can also be found in a waterproofing spray intended for use as a Do-It-Yourself spray for the exterior of tents (SDS 2014). This product is intended for outdoor application once the tent is assembled (SDS 2014). Given that this application is done outdoors, inhalation exposure from the sprayed product is expected to be minimal. The waterproofing spray may contact 50% of the hands (455 cm2) resulting in a dermal load of 0.071 mg/cm2 (equivalent to 4.7 µg/kg-bw/event) and a systemic exposure of 1.8 µg/kg-bw/event (Appendix E, Table E5).
8.1.2 Biomonitoring
Some biomonitoring studies have been identified that measure urinary concentrations of bis(1-chloro-2-propyl) phosphate (BCPP), a possible biomarker for TCPP (Kosarec et al. 2016; Yang et al. 2019; Ospina et al. 2018; Butt et al. 2014; Castorina et al. 2017; Petropoulou et al. 2016; He et al. 2018; Chen et al. 2018). However, the frequent low detection level of BCPP may be a result of the low formation yield of BCPP from TCPP (Van den Eede et al. 2013) and the authors suggested that more research was needed to determine a suitable biomarker for TCPP (Butt et al. 2014).
There have since been a number of studies on the metabolism of TCPP (Abdallah et al. 2015, Van den Eede et al. 2015b and 2016), but there is still uncertainty surrounding the most appropriate biomarker for this substance; therefore intakes based on the human biomonitoring data for TCPP were not derived.
TDCPP is extensively metabolized in the human body, resulting in a major metabolite: the diester of TDCPP, bis(1,3dichloro-2-propyl) hydrogen phosphate (BDCPP) (Van den Eede et al. 2013). BDCPP has been identified as the major metabolite (over 60% in rat urine) in in vivo rat toxicokinetic studies (Nomeir et al. 1981; Lynn et al. 1980, 1981). BDCPP has been monitored in human urine in Canada (Kosarac et al. 2016; Yang et al. 2019) and in several studies from the U.S., including at a national level by the National Health and Nutrition Examination Survey (NHANES), in pregnant women and in toddlers (Ospina et al. 2018; Hoffman et al. 2014; Butt et al. 2014). Since BDCPP is the diester form of TDCPP, it is considered to be a specific metabolite of TDCPP and an appropriate biomarker in urine samples for estimation of exposure to TDCPP. Additional information on this metabolite is provided in Section 8.2.2.3.
Two Canadian biomonitoring studies that measured BDCPP in urine samples were identified (Kosarac et al. 2016; Yang et al. 2019). Yang et al. (2018) detected BDCPP in 73% of spot urine samples from 44 premenopausal women from Toronto and Ottawa with unadjusted BDCPP concentrations in urine ranging from 0.104 to 13.8 ng/mL. The geometric mean and 95th percentile unadjusted concentrations of BDCPP in urine were reported to be 0.740 ng/mL and 4.32 ng/mL, respectively (Yang et al. 2019). Kosarac et al. (2016) collected urine samples from pregnant women (n=24) in the Hamilton region of Ontario, Canada from 2010 to 2012. BDCPP was detected in approximately 29% of samples at concentrations (unadjusted for hydration status) ranging from <0.25 to 1.77 ng/mL (MDL of 0.25 ng/mL) with a geometric mean of 0.27 ng/mL. Urine samples were also collected from 8 pregnant women (ages from 28 to 36 years old at conception) in Chapel Hill, North Carolina, U.S., between December 2011 and May 2012, during pregnancy and after birth (Hoffman et al. 2014). A total of 39 urine specimens were collected (3 samples during the 18th week of pregnancy (spot and 24 hr sample), 1 spot sample during the 28th week and a spot sample shortly after the child’s birth, one woman did not provide a sample shortly after birth). BDCPP was detected in 38 of 39 urine samples, and the maximum specific gravity (SG)-corrected concentration was 34.3 ng/mL, with a 95th percentile of 7.1 ng/mL and a geometric mean of 2.1 ng/mL (personal communication Hoffman to Health Canada, 2014 June). Based on analysis of the urine samples, the authors suggest that exposure to TDCPP is variable for pregnant women and that a single measure of BDCPP, taken in the second trimester, likely captures information on the rank order of exposure throughout pregnancy.
In the United States, spot urine samples from the 2013-2014 National Health and Nutrition Examination Survey (NHANES) were analyzed for BDCPP (Ospina et al. 2018). Of the 2666 samples, BDCPP was detected in 92% of the samples (LOD = 0.11 µg/L). The geometric mean concentration of BDCPP for all age groups (6 to 60 plus years) was 0.856 µg/L (0.913 µg/g creatinine corrected) with a 95th percentile of 7.23 µg/L (6.30 µg/g creatinine corrected). Children aged 6 to 11 years old had the highest urinary concentrations of BDCPP with a geometric mean of 2.25 µg/L (2.89 µg/g creatinine corrected) and a 95th percentile of 14.9 µg/L (14.8 µg/g creatinine corrected). Adults 60 years of age and older had the lowest BDCPP concentrations with a geometric mean of 0.497 µg/L (0.599 µg/g creatinine corrected) and a 95th percentile of 5.20 µg/L (4.03 µg/g creatinine corrected).
In a paired mother and toddler study, spot urine samples were collected from 22 mothers and 26 “paired” children (5 mothers were paired with 2 children; one mother did not provide child’s urine) from Princeton, New Jersey and analyzed for BDCPP (Butt et al. 2014). BDCPP was detected in 100% of samples for both mothers and toddlers. Maximum concentrations corrected for specific gravity (SG) were 11.0 ng/mL for mothers and 251 ng/mL for toddlers with geometric means of 2.4 ng/mL and 5.6 ng/mL for mothers and toddlers, respectively. The authors determined a positive correlation between the mothers and toddlers for BDCPP and this trend may indicate that a shared environment is an important determinant of TDCPP exposure (Butt et al. 2014). In Australia, BDCPP concentrations in infants were also found to be higher than adults (Van den Eede et al. 2015a). The widespread use of TDCPP in children’s products (Stapleton et al. 2011, CEH 2013b, Health Canada 2014, 2015b) as well as the unique behaviours of young children (i.e., mouthing and hand-to-mouth) could explain the higher BDCPP concentrations observed in children’s urine compared to adults.
Additional biomonitoring data has been identified in the U.S. (Cooper et al. 2011; Carignan et al. 2013b) as well as in Australia (Van den Eede et al. 2015a). Carignan et al. (2013b) reported a positive trend between urinary BDCPP and TDCPP in office dust, which was not observed in other environments (i.e., homes and vehicles). Another study in Boston measured BDCPP levels in urine and correlated those concentrations with dust levels in individual homes (Meeker and Stapleton 2010; Meeker et al. 2013). A statistical analysis concluded a weak (rS = 0.31) but statistically significant (p = 0.03) correlation between concentrations of BDCPP in urine (uncorrected for SG) and concentrations of TDCPP in house dust (Meeker et al. 2013).
Reverse dosimetry was used to derive estimates of daily intakes from the urine concentrations for a selection of the most relevant studies (i.e., Canadian studies, studies with large sample size from countries similar to Canada and studies that examined younger children not covered by other studies) to account for various age groups (children to adults) as well as for pregnant women, and results are shown in Table 8‑2. A correction factor for incomplete excretion of BDCPP of 21% was applied to the estimate. This was based on a combination of data from toxicokinetic studies conducted in rats (Minegishi et al. 1988; Nomeir et al. 1981; Lyn et al. 1980, 1981) (details in Appendix F). The metabolism of TDCPP and excretion in urine from rats is relatively rapid, with a urinary half-life of approximately 12 hours (Minegishi et al. 1988). In addition, the relatively rapid metabolism is consistent with the in vitro study in human cells by van den Eede et al. (2013). More details on the toxicokinetics of TDCPP are provided in Section 8.2.2.3. Details regarding the reverse dosimetry are provided in Appendix F.
Study |
Participants |
Location |
Geometric mean and [maximum] or [95th percentile] of urinary concentrations (ng/mL) |
Intake estimates (μg/kg-bw/day)a |
|---|---|---|---|---|
Kosarac et al., 2016 |
Pregnant Females (n=24) |
Hamilton, ON, Canada |
0.27b [1.77] |
0.07 [0.43] |
Yang et al., 2018 |
Premenopausal Females (n=44) |
Toronto and Ottawa, ON, Canada |
0.74b [P95=4.32] |
0.13 [0.79] |
Butt et al., 2014 |
Toddlers (2 to 5 years; paired with mothers) (n=23) |
New Jersey, U.S. |
5.6c [251] |
1.6 [72]d |
Ospina et al., 2018 |
Children (6 to 11 years) (n=421) |
U.S. |
2.89d [P95=14.8] |
0.39 [2.0] |
Ospina et al., 2018 |
Teens (12 to 19 years) (n=427) |
U.S. |
1.14d [P95=6.82] |
0.17 [1.0] |
Ospina et al., 2018 |
Adults (20 to 60 + years) (n=1818) |
U.S. |
0.850de [P95=4.33] |
0.13 [0.66] |
Abbreviations: P95, 95th percentile
a Intake estimates are based on the geometric mean concentration listed under previous column. Value in brackets refers to intake estimate derived from the maximum or P95 shown in previous column.
b Urinary concentrations are unadjusted.
c Concentrations are normalized to specific gravity (as reported in the studies).
d Concentrations are creatinine corrected (as reported in Ospina et al., 2018).
e Concentrations from 20 to 59 year olds are used (concentrations for older adults, 60 years and older, were lower).
8.2 Health effects assessment
8.2.1 TCPP
8.2.1.1 Carcinogenicity and genotoxicity
No chronic or carcinogenicity studies of TCPP were identified. The US National Toxicology Program (NTP) (2018) has conducted 90-day and two-year oral toxicity and carcinogenicity studies in both rats and mice, but a study report was not available at the time of assessment. The concern for potential carcinogenicity was due to structural similarity to other organophosphate esters that showed carcinogenic effects in two-year carcinogenicity studies in experimental animals.
Several other lines of evidence were investigated to characterize the carcinogenic potential of TCPP (more detail is available in Health Canada 2015a), including the analogue approach, the quantitative structural activity (QSAR) approach and the structural alerts approach. It was considered that TCEP and TDCPP may be used for qualitative read-across for carcinogenicity. The Government of Canada has published a final screening assessment for TCEP (Environment Canada, Health Canada 2009) and concluded that TCEP demonstrated carcinogenic potential, and that it could not be precluded that the induction of tumours could be via a mode of action involving direct interaction with genetic material. The human health effects assessment for TDCPP described in Section 8.2.2 shows that TDCPP is associated with carcinogenic potential. Overall, the evidence suggests that TCPP may be carcinogenic in rodents.
A number of in vitro and in vivo genotoxicity studies were identified.
Most of these studies are described in detail in the EU RAR for TCPP (EU RAR 2008a). In vitro genotoxicity studies (Ames assays) showed little evidence of mutagenic potential in bacteria and fungi (Stauffer Chemical Co. 1976, 1978d; Parmar 1977; Tenneco Chemicals Inc 1977a, b; Nakamura et al. 1979; Anon 1980; Mobil Environmental and Health Safety Laboratory 1980; SafePharm Laboratories Ltd 1992; Zeiger et al. 1992; Follmann and Wober 2006). In vitro studies tested in mammalian cells (mouse lymphoma assays, unscheduled DNA synthesis [UDS] assays, comet assays) generated equivocal results (Stauffer Chemical Co. 1978e,f,g, 1980a; Environmental Affairs and Toxicology Department 1981; Covance Laboratories Inc. 2005b; Bayer 1991b; Williams et al. 1989; Follmann and Wober 2006). One of the in vitro mouse lymphoma assays conducted according to OECD guidelines generated positive results in the presence of S9, suggesting clastogenicity potential of a metabolite. Results of some in vivo assays (micronucleus assays and comet assays) were negative (Bayer 1991c; Covance Laboratories Inc. 2006). Other in vivo studies (UDS assays, chromosomal aberration assays) generated equivocal results or negative results associated with limitations to the studies (Stauffer Chemical Co. 1978h; Bayer Healthcare 2005).
The US National Toxicology Program (NTP) recently conducted in vivo micronucleus assays in male and female B6C3F1 mice and Sprague-Dawley rats (NTP 2018). Cells were collected from peripheral blood of animals that were orally fed 0, 1250, 2500, 5000, 10000 or 20000 ppm TCPP in diet, 5 days a week for 90 days. The corresponding intakes were 0, 163, 325, 650, 1300 and 2600 mg/kg-bw/day, respectively. The final report is not yet available, but original data are presented on the NTP website. Positive results were observed in male mice. Results were negative in male and female rats and in female mice.
Overall, TCPP is not considered to be genotoxic in vivo (EU RAR 2008a).
8.2.1.2 Toxicokinetics
Several oral or intravenous dosing toxicokinetic studies conducted on experimental rats were identified (Minegishi et al. 1988; Stauffer Chemical Co. 1984). Based on these studies, oral absorption of TCPP appears to be at least 75%. TCPP is widely distributed in tissues including liver, kidney, lung and adipose tissue. The actual amount detected in these tissues was very low suggesting low bioaccumulation. The biliary/faecal excretion ratio suggested enterohepatic re-circulation from the GI tract after oral administration. TCPP is extensively metabolized prior to excretion. Urinary excretion is the primary route of elimination of TCPP, but both urinary and faecal excretions are dose-dependent and route dependent (oral and i.v.). One study (Stauffer Chemical Co. 1984) found that for the same dose level (20 mg/kg) administered orally or by i.v., the urine excretion was 49% for oral and 63% for i.v. routes. The faecal excretion was 40% for oral and 27% for i.v. routes. The total elimination via the two routes was rapid and constant, averaging 89% of the dose at 72h. In the same study, Stauffer Chemical Co. (1984) also administered a higher oral dose level (200 mg/kg). It was found that the urine excretion was 70% and faecal excretion was 22% suggesting dose-dependent excretion pattern. Approximately 2% of TCPP is excreted unchanged. A major metabolite identified in both urine and faeces, accounting for over 50% of the dose, is 0,0-[bis(1-chloro-2-propyl)]-0-(2,propionic acid)phosphate (triester form of TCPP with carboxylic acid substituting a chlorine atom). It was suggested that this major metabolite is responsible for the dose-dependent excretory pattern. At low doses, this metabolite was excreted approximately equally in the urine and faeces. At high doses, it was excreted predominantly in the urine. Other metabolites identified include possible products from hydrolysis reaction: diester form of TCPP, bis(1-chloro-2-propyl) monophosphoric acid and halo-alcohol, 1-chloro-2-propanol. 1-chloro-2-propanol, is a demonstrated mutagen in in vitro genotoxicity studies, but did not induce any tumours in two-year drinking water carcinogenicity studies conducted in both rats and mice (NTP 1998).
There are in vitro studies that have investigated TCPP metabolism (Study Submission 2013, Van den Eede et al. 2013, Abdallah et al. 2015, Van den Eede et al. 2015b and 2016) but uncertainty remains with respect to the toxicokinetics of this substance in humans.
Two in vitro dermal absorption studies, conducted according to OECD guidelines, were identified (TNO Quality of Life 2005, 2006b) (see section 8.1.2.1). The in vitro dermal absorption studies used human skin membranes with direct application of radiolabelled TCPP resulting in maximum total absorptions of 23% and 40%. The two absorption values of 23 and 40% for TCPP were derived from studies testing different doses (see section 8.1.2.1).
8.2.1.3 Repeated dose toxicity
In a 14-day study, Sprague-Dawley rats (10/sex/dose) were treated with 0, 4200, 6600, 10600 or 16600 ppm of TCPP in diet (Stauffer Chemical Co 1980b). The corresponding intakes were 0, 417, 648, 1015 and 1636 mg/kg-bw/day for males and 0, 382, 575, 904 and 1517 mg/kg-bw/day for females, respectively. For males, a NOAEL of 10 600 ppm (1015 mg/kg-bw/day) was identified based on reduced weight gain during the first week of treatment compared to controls at 16 600 ppm. Weight gain was not different from controls during week two. Food consumption was significantly reduced in the first 3 days of the study in male rats in the two highest treatment groups. For the remainder of the study, food consumption of all treated groups was similar to control groups. For females, the NOAEL was 16 600 ppm (1517 mg/kg-bw/day), the highest dose tested.
In an oral gavage study, male and female Wistar rats (6/sex/dose group) were treated with 0, 10, 100 or 1000 mg/kg-bw/day of TCPP for 28 days (Bayer 1991d). The study was conducted according to “EC guidelines” (EU RAR 2008a). A preliminary 7-day study was first conducted on male rats indicating no treatment-related effects observed when animals were dosed up to 1000 mg/kg-bw/day for 7 days. In the main study, a NOAEL of 100 mg/kg-bw/day was identified. Three animals died at 1000 mg/kg-bw/day (1 male rat died probably due to treatment error and 2 female rats died that could be treatment-related). Absolute and relative liver weights were significantly increased in both males and females at 1000 mg/kg-bw/day. All male rats in the 100 mg/kg-bw/day dose group except one exhibited minimal periacinary hepatocyte hypertrophy. One male rat at 100 mg/kg-bw/day and all male rats at 1000 mg/kg-bw/day dose group developed slight hypertrophy of the periacinary hepatocytes. TCPP treated female rats did not exhibit any hepatic alterations. Clinical chemistry indicated significant decrease in alanine aminotransferase activity in both male and female rats in the 1000 mg/kg-bw/day dose group.
The EU (EU RAR 2008a) described an unpublished 13-week study where Sprague-Dawley rats (20/sex/dose) were treated with 0, 800, 2500, 7500 or 20 000 ppm of TCPP in diet (Stauffer Chemical Co. 1981c cited in EU RAR 2008a). The corresponding intakes were 0, 52, 160, 481 and 1349 mg/kg-bw/day for males and 0, 62, 171, 570 and 1745 mg/kg-bw/day for females, respectively. For males, a LOAEL of 800 ppm (52 mg/kg-bw/day), the lowest dose level tested, was determined based on all treated males exhibiting a significant increase in absolute and relative liver weights, accompanied by mild thyroid follicular cell hyperplasia. The incidences of thyroid follicular cell hyperplasia were 0/20, 2/20, 2/20, 5/20 and 8/20 at 0, 800, 2500, 7500 or 20000 ppm, respectively. At 2500 ppm, a significant increase in relative kidney weight was observed, accompanied with mild renal cortical tubule degeneration (hyaline droplet formation). For females, a NOAEL of 2500 ppm (171 mg/kg-bw/day) was identified with a LOAEL of 7500 ppm based on significant increase in absolute and relative liver weights. At 20 000 ppm, mild renal cortical tubule vacuolative degeneration (4 animals, compared to 1 control animal) and mild thyroid follicular cell hyperplasia (5/20 treated vs 0/20 control) were observed in the female rats. Periportal hepatocyte swelling (hypertrophy) was observed at 20 000 ppm in both males (7/20 treated vs. 0/20 control) and females (8/20 treated vs. 5/20 control). The mean body weights of male and female rats in the high dose groups were significantly lower than the control animals. There were no significant alterations in clinical chemistry, haematology or urinalysis parameters. No treatment-related changes in plasma, erythrocyte or brain cholinesterase activity were observed.
Freudenthal and Henrich (1999) published a journal article on a subchronic toxicity study, in which the data were very similar to the Stauffer Chemical Co. study (1981c) described in the EU RAR (2008a), and are likely from the same study. Sprague-Dawley male and female rats (20/sex/dose) were treated with 0, 800, 2500, 7500 or 20 000 ppm of TCPP in diet. Similar changes in absolute and relative liver weights, relative kidney weights and mean body weights at the same dose levels as reported in the EU RAR (2008a) were described. However, the reported incidences of histopathology observations were not identical in those described in EU RAR (2008a). A LOAEL of 800 ppm was identified based on a significant increase in absolute and relative liver weights and mild thyroid follicular cell hyperplasia in males (EUR 2008a).
NTP (2018) has conducted a 90-day study where B6C3F1 male and female mice were orally fed with 0, 1250, 2500, 5000, 10 000 or 20 000 ppm of TCPP in diet. The corresponding intakes were 0, 163, 325, 650, 1300, 2600 mg/kg-bw/day respectively. A study report is not yet available. Preliminary results indicate male mice exhibited a significant decrease in body weight starting from 2500 ppm. A similar effect was observed in female mice, but at higher dose of 10 000 ppm. Histopathology observations reported incidences of liver hypertrophy in male mice starting from 2500 ppm, which was also observed in female mice starting from 5000 ppm. In male mice, but not in female mice, incidences of cytoplasmic alteration of the renal tubule in the kidney were observed starting from 2500 ppm. The rat study showed similar effects starting at dose of 10 000 ppm. Hematological effects were also observed in both sexes. Mortality in males receiving 20 000 ppm was high (NTP 2018).
8.2.1.4 Reproductive toxicity
An oral two-generation reproductive toxicity study in rats was conducted in accordance with OECD guidelines (TNO Quality of Life 2007 cited in EU RAR 2008a). This study included a preliminary range-finding study of a one-generation reproductive toxicity study. It was noted that there was a deviation from the study plan, the corpora lutea were not counted at scheduled sacrifice.
In the preliminary study, male and female rats were treated for 5 weeks prior to mating and during mating. Females were treated during gestation and lactation to post-natal day (PND) 21. Dams were sacrificed for necropsy at PND21. Males were sacrificed after at least 42 days of exposure. Rats (10/sex/dose) were orally fed 0, 1500, 5000 or 15 000 mg/kg diet containing TCPP. The administered doses were equivalent to 0, 95, 325 and 1000 mg/kg-bw/day, respectively, in male rats. In female rats, the administered doses were equivalent to 0, 108, 370 and 1176 mg/kg-bw/day, respectively, during premating; 0, 100, 314 and 963 mg/kg-bw/day, respectively, during gestation; and 0, 193, 680 and 1930 mg/kg-bw/day, respectively, during lactation. In parental (F0) females, the LOAEL was 1500 mg/kg diet (100-193 mg/kg-bw/day) based on a significant decrease in mean absolute and relative uterus weights at all treatment doses. This effect was independent of weight loss as a significant decrease in mean terminal body weight was observed in the high dose group only. In parental F0 males, the LOAEL was 95 mg/kg-bw/day based on a significant decrease in absolute prostate weight. Statistically significant decrease absolute prostate weight was observed in the low- and high- dose groups, with a non-significant decrease in mid-dose animals. No effects on motility or count of epididymal sperm or sperm morphology were observed. Changes in organ weights were not associated with any gross or histopathological changes. In terms of reproductive parameters, there were no effects on pre-coital time, mating index and male and female fertility index. The number of pups delivered and the sex ratio were not affected by treatment. Pup mortality was significantly higher in the high-dose group, including all 8 pups of one dam.
In the main study, the F0 parents, 28 Wistar rats per sex per group received TCPP in their daily diet for at least 10 weeks during premating and mating. Females were also treated throughout gestation (approximately 3 weeks) and lactation (3 weeks) until sacrifice. At weaning (PND21), F1 offspring (28 animals per sex per group, selected at random) were treated with TCPP for at least 10 weeks of exposure during their growth into adulthood and during mating. Female F1 animals continued to be treated during gestation and lactation until the F2 generation was weaned on PND21. The reported overall intakes were 0, 85, 293 or 925 TCPP mg/kg-bw/day in males and 0, 99, 330 or 988 mg/kg-bw/day in females.
In terms of effects on reproductive parameters, no treatment-related differences were observed in pre-coital time, mating index, female fecundity index, male and female fertility index and duration of gestation in both generations. A non-significant increase in post-implantation loss in the F1 generation was observed. All dams survived the delivery and there were no dams with stillborn pups in any of the groups. The mean number of pups delivered was decreased in the mid dose of F1 and in the high dose of F0 and F1, including the loss of one litter (10 pups) of a single dam in the high-dose group. In males, there were no treatment-related effects on epididymal sperm motility or sperm count, sperm morphology or mean testicular sperm count in F0 and F1 at necropsy.
The LOAEL for the F0 generation of females was the lowest dose tested of 99 mg/kg-bw/day based on a significant decrease in mean absolute and relative uterus weights and effects on oestrus cycle in females. Effects on oestrus cycle were observed, including a significant increase in the mean length of the longest oestrus cycle at all doses. At the high dose, a significant decrease in the number of oestrus cycle per animal and a significant increase in the number of acyclic animal were observed. In F1 females, a similar effect on uterus weights and oestrus cycles reached statistical significance at the high dose. The LOAEL for F1 females was 99 mg/kg-bw/day based on a significant decrease in absolute pituitary weight at all doses. In F0 males, the NOAEL was 85 mg/kg-bw/day based on a significant decrease in mean terminal body weight and mean absolute seminal vesicles at the next dose level of 293 mg/kg-bw/day. In F1 males, the LOAEL was 85 mg/kg-bw/day based on a significant decrease in mean absolute kidney weight observed at all treatment doses. No treatment-related macro- or microscopical changes were observed in the F0 or F1 animals.
Follmann and Wober (2006) conducted in vitro studies to examine potential estrogenic or anti-estrogenic effects of TCPP. No estrogenic or anti-estrogenic effect was observed in a recombinant yeast reporter gene assay tested in human endometrial cancer Ishikawa cells. Kojima et al. (2013) used cell-based transactivation in vitro assays to examine potential agonistic and/or antagonistic activities of TCPP against a number of human nuclear receptors. Overall, TCPP had no agonistic or antagonistic activities against nuclear receptors except a weak agonistic activity against PXR.
8.2.1.5 Developmental toxicity
In the preliminary range-finding study of the two-generation reproductive toxicity study described in Section 8.2.1.4 (TNO Quality of Life 2007 cited in EU RAR 2008a), it was reported that a significant number of runts was observed at all treatment doses on PND21. The EU RAR (2008a) did not provide their definition of runt. The OECD SIDS Initial Assessment Profile for TCPP (OECD 2009) defined runt as a pup with a weight less than the mean pup weight of the control group minus 2 standard deviations. In F0 animals, there was a significant decrease in body weight in the mid-dose group (293 mg/kg bw/day for males, 32.9 mg/kg bw/day for females) during premating. A similar body weight effect was observed in the mid- (293 mg/kg bw/day for males, 32.9 mg/kg bw/day for females) and high-dose (925 mg/kg bw/day for males, 988.2 mg/kg bw/day for females) groups during gestation and lactation. Overall, the LOAEL was 925 mg/kg bw/day for males and 988.2 mg/kg bw/day for females based on a significant increase in the number of runts.
In the main two-generation reproductive toxicity study described in Section 8.2.1.4 (TNO Quality of Life 2007), parameters related to developmental effects were examined in F0, F1 and F2 generations.
In F0 generation, a significant increase in the number of runts was observed at 99 mg/kg-bw/day or higher on PN1. The mean number of pups delivered was decreased in the high dose group. There was a significant increase in pup mortality during PN1-4 in the low- and high-dose groups, but it did not reach statistical significance in the mid-dose group. The mean pup weights were normal on PN1, but significantly decreased from PN14 onwards in the high-dose group. A significant decrease in absolute and relative spleen weights was observed in the mid- and high-dose groups. Maternal body weight was decreased in high dose females during gestation. Mean food consumption was decreased in F0 females in mid- and high-dose groups.
In the F1 generation, a significant increase in the number of runts was reported from F1 females treated with 99 mg/kg-bw/day and higher on PN21. The mean number of pups delivered and the mean number of live pups per litter were decreased in mid- and high-dose groups. In the high-dose group, there was a loss of all 10 pups in one litter from a dam on PND4. The mean pup weights were normal on PND1, but significantly decreased from PND7 onwards in the high-dose group and on PND21 in the mid-dose group. A significant decrease in absolute and relative spleen weights was observed in the mid- and high-dose groups. Maternal body weights were decreased in mid- and high-dose animals in F1 generation throughout premating, gestation and during lactation. Mean food consumption was decreased in F1 females of mid- and high-dose groups.
Anogenital distance was measured in all F2 pups on PND1, which was found to be comparable to controls. Sexual maturation parameters (vaginal opening and preputial separation) were assessed in 1 male and 1 female F2 pup per litter. A non-significant delay of the vaginal opening and a significantly delayed preputial separation were observed at the high dose. Body weights of the high-dose male and female F2 pups were significantly decreased from PND28 until PND42. “The effects observed in this dose group on vaginal opening and preputial separation is most likely secondary to toxicity” (EU RAR 2008a). These effects could be secondary to systemic toxicity as the body weights of the high-dose male and female F2 pups were significantly decreased from PND28 until PND42.
In both generations, the pups that were found dead showed no abnormalities. No treatment-related macroscopic findings were observed in the pups at necropsy.
Overall, a developmental LOAEL of 1500 mg TCPP/kg diet (99 mg/kg-bw/day) was identified in this study based on a significant increase in the number of runts observed in F0 generation on PND1. Similar effects were observed in F1 generation on PND21 but not on PND1. The EU (EU RAR 2008a) established the same LOAEL for this study based on a weight-of-evidence approach and considered this to be a relatively precautionary LOAEL as the effects on runts was not observed in both generations on PND1.
NTP (2018) has also conducted a teratology study (GD6-20) where SD female rats were treated with 0, 162.5, 325 or 650 mg/kg-bw/day of TCPP by gavage. A study report is not yet available. Preliminary results indicated increase in liver weight in all concentrations in dams. Decreased fetal weights were observed in the 650 mg/kg-bw/day group. No other significant effects were observed other than some skeletal variation in fetuses that was also present in the control group. Non-significant increases in body weight and food consumption in dams were also observed.
8.2.1.6 Neurotoxicity
In an acute neurotoxicity study, 4 female hens were orally administered 13 200 mg/kg-bw of TCPP (Sprague et al. 1981). No inhibition of plasma cholinesterase or brain neurotoxic esterase (NTE) was observed in the treated animals. In the second part of the study, 18 female hens were orally administered 13 200 mg/kg-bw of TCPP twice, 21 days apart. Animals showed signs of systemic toxicity (significant reduction in food consumption, decreased mean body weights, feather loss and cessation of laying). One out of 18 hens died on day 4. Histological examination showed 2 hens with minimal axonal degeneration in dorsal funiculi of the cervical, ventro-lateral funiculi of thoracic or ventromedial funiculi of the sacro-lumber spinal cord, tracts known to be sensitive to organophosphate-induced degeneration. One of these hens also showed impaired walking behaviour. The administered dose of 13 200 mg/kg-bw was excessively above the recommended dose limit of 2000 mg/kg-bw for acute OPIDN study in OECD guidelines (OECD 1995). Only isolated incidences of minimal axonal degeneration was observed in 2/18 hens and no plasma cholinesterase and NTE inhibitions were observed; overall, the EU (EU RAR 2008a) considered that there is no concern for acute delayed neurotoxicity.
The 13-week rat study (Stauffer Chemical Co. 1981c) described in Section 8.2.1.3, measured cholinesterase activities. No treatment-related changes in plasma, erythrocyte or brain cholinesterase activity were observed.
8.2.1.7 Sensitization
No skin sensitisation was observed in a Buehler’s test in guinea pigs and in a mouse local lymph node assay (SafePharm Laboratories 1979, 2005).
8.2.2 TDCPP
8.2.2.1 Carcinogenicity
The European Union has classified TDCPP as a Category 2 carcinogen (suspected human carcinogen) (European Union 1998-2017).
A two-year carcinogenicity study was conducted in Sprague Dawley rats (60/sex/group) where animals were fed diets (ad libitum) containing TDCPP (Stauffer Chemical Co. 1981a). The administered dose levels were 0, 5, 20 or 80 mg/kg-bw/day. Ten animals per sex per group were sacrificed after 12 months of treatment as an interim group.
Non-cancer effects are described in Section 8.2.2.5. The mortality rates were comparable between treatment groups and controls except for males in the high-dose group, for which mortality rate was significantly higher than controls after 12 months of treatment. Terminal body weights of male and female rats in the high-dose groups were significantly lower than the control animals (>20%).
For the 12-month interim group and rats found dead before 12 months, the incidence of tumours in kidney, testes, liver, brain, as well as thyroid and adrenal glands is presented in Table 8‑3 and Table 8‑4. The adrenal gland, brain and thyroid gland were not examined at the low and mid doses in both sexes. There was an increase in incidences of testicular interstitial cell tumours in the mid and high doses. There were similar incidences of neoplasms in all other tissues in control and treated animals.
| Dose levels (mg/kg-bw/day) | 0 | 5 | 20 | 80 |
|---|---|---|---|---|
| Testicular interstitial cell tumour | 0/14 | 0/12 | 3/13 | 3/11 |
| Hepatocellular adenoma | 0/15 | 0/12 | 0/13 | 3/14 |
| Adrenal cortical adenoma | 0/15 | N/A | N/A | 2/13 |
| Brain gliomas (astrocytoma/ oligodendrogloima) | 0/15 | N/A | N/A | 0/14 |
| Thyroid gland adenoma/parafollicular cell adenoma | 0/14 | N/A | N/A | 0/11 |
N/A-Not assessed in the study
a Includes tumor incidences from rats found dead prior to 12 months
| Dose levels (mg/kg-bw/day) | 0 | 5 | 20 | 80 |
|---|---|---|---|---|
| Hepatocellular adenoma | 0/11 | 0/13 | 0/9 | 1/10 |
| Adrenal cortical adenoma | 5/11 | N/A | N/A | 1/10 |
| Brain gliomas (astrocytoma/ oligodendrogloima) | 0/11 | N/A | N/A | 0/10 |
| Thyroid gland adenoma/parafollicular cell adenoma | 0/9 | N/A | N/A | 0/6 |
N/A-Not assessed in the study
a Includes tumor incidences from rats found dead prior to 12 months
In the 24-month group, cancer effects were observed (Stauffer Chemical Co. 1981a). The incidences of renal cortical adenomas (both sexes) and testicular interstitial cell tumours (males) were significantly increased in the mid- and high-dose groups compared to controls. At the high dose, significant increase incidences of hepatocellular adenomas (both sexes) and adrenal cortical adenomas (females) were observed. Tumour incidences for this group are presented in Table 8‑5.
| Dose levels (mg/kg-bw/day) | 0 | 5 | 20 | 80 |
|---|---|---|---|---|
| Renal cortical adenoma | 1/45 | 3/49 | 9/48* | 32/46* |
| Testicular interstitial cell tumour | 7/43 | 8/48 | 23/47* | 36/45* |
| Hepatocellular adenoma | 2/45 | 7/48 | 1/48 | 13/46* |
| Hepatocellular carcinoma | 1/45 | 2/48 | 3/48 | 7/46 |
| Brain gliomas (astrocytoma/ oligodendrogloima) | 0/44 | 0/4 | 1/1 | 5/46 |
| Thyroid gland adenoma/parafollicular cell adenoma | 0/40 | 2/2 | 1/2 | 5/41 |
* Significantly different when compared to control animals (p<0.05).
| Dose levels (mg/kg-bw/day) | 0 | 5 | 20 | 80 |
|---|---|---|---|---|
| Renal cortical adenoma | 0/49 | 1/48 | 8/48* | 29/50* |
| Adrenal cortical adenoma | 8/48 | 5/27 | 2/33 | 19/49* |
| Hepatocellular adenoma | 1/49 | 1/47 | 4/46 | 8/50* |
| Hepatocellular carcinoma | 0/49 | 2/47 | 2/46 | 4/50 |
| Brain gliomas (astrocytoma/ oligodendrogloima) | 1/46 | 1/4 | 2/5 | 1/48 |
| Thyroid gland adenoma/parafollicular cell adenoma | 3/42 | 0/2 | N/A | 9/49 |
* Significantly different when compared to control animals (p<0.05).
N/A-Not assessed in the study
The mode of action for the tumours observed in rodents has not been fully elucidated.
Two metabolites identified based on in vivo and in vitro studies were 1,3-DCP and 3-MCPD (Nomeir et al. 1981; Lynn et al. 1981; Ulsamer et al. 1980) (see Section 8.2.2.2 for details). 1,3-DCP and 3-MCPD were classified as Category 2B carcinogens by the International Agency for Research on Cancer (IARC) (IARC 2012a,b). As described in the IARC monographs for 1,3-DCP and 3-MCPD, proposed metabolic pathways suggested that 1,3-DCP can metabolize to 3-MCPD and the metabolism of 1,3-DCP and 3-MCPD can generate several known mutagens and genotoxic carcinogens (1,3-dichloroacetone, epichlorohydrin and glycidol). Based on the identified potential genotoxic metabolites and evidence of in vivo DNA binding in mice, the US California EPA (2011) concluded that TDCPP may be carcinogenic through a genotoxic mechanism. A recent in vitro metabolism study (Van den Eede et al. 2013) identified metabolites from another pathway (oxidative dehalogenation) which involves the generation of an aldehyde intermediate. Although aldehyde can further metabolize to carboxylic acid or alcohol, it has the potential to bind to DNA or protein.
The EU RAR (2008b) has not identified any mode of action, but stated that testicular interstitial cell tumours could be induced by chemicals via a non-genotoxic mode of action through alternations in the Hypothalmus-Pituitary-Testis (HPT) Axis. Also, hyperplasia is often considered to be a pre-cancer lesion and the EU RAR (2008b) hypothesized that kidney tumours could have developed through hyperplastic changes.
8.2.2.2 Genotoxicity
A number of in vitro and in vivo genotoxicity studies were identified.
Most of these studies are described in detail in the EU RAR for TDCPP (EU RAR 2008b). Results were mostly negative for in vitro gene mutation assays in bacteria and yeasts, in the presence or absence of metabolic activation (S9) (Mortelmans et al. 1986; Soderlund et al. 1985; Stauffer Chemical Co. 1981b, 1983b; Nakamura et al. 1979; Safepharm Laboratories Ltd 1984, 1985a; Ishidate 1983). Positive results were observed in particular strains of Salmonella typhimurium, TA97, TA100 and TA1535, in the presence of S9 (Stauffer Chemical Co. 1983b; Soderlund et al. 1985). In mammalian cells, results of some in vitro assays (point mutation assay, sister chromatid exchange assay and a limited unscheduled DNA synthesis assay) were negative (Stauffer Chemical Co. 1977; Soderlund et al. 1985). Other in vitro assays (mouse lymphoma assay, chromosomal aberration assay, transformation assay) generated equivocal results (Stauffer Chemical Co. 1977, 1981b; Ishidate 1983; Inveresk Research International 1985; Soderlund et al. 1985; Covance Laboratories Inc. 2004).
In in vivo testing, results were negative for a sex-linked recessive lethal mutations assay in Drosophila, an unscheduled DNA synthesis (UDS) assay in rats, a micronucleus assay in mice and a chromosomal aberration assay in mice (Stauffer Chemical Co. 1978a, 1981b; Brusick et al. 1980; Safepharm Laboratories Ltd 1985b; Covance Laboratories Inc. 2005a). Morales and Matthews (1980) examined covalent binding of TDCPP to macromolecules in mice intravenously treated with TDCPP. Animals were sacrificed 6 hours after treatment. It was found that TDCPP readily bound to DNA in the liver and kidney. TDCPP also bound to RNA and proteins in liver, kidney and muscle.
Overall, results from in vitro genotoxicity studies suggest there is some evidence of mutagenicity. However, results from a number of in vivo genotoxicity studies were negative.
8.2.2.3 Toxicokinetic
Three oral toxicokinetic studies conducted in rats were identified (Minegishi et al. 1988; Nomeir et al. 1981; Matthews and Anderson 1979). Overall, oral absorption from the gastrointestinal tract was greater than 90%. TDCPP was rapidly distributed in the body with high levels in kidneys, liver and lungs. The average Tmax was 9.6 h for TDCPP in blood and tissues in an oral toxicokinetic study (Minegishi et al. 1988). Metabolic degradation was extensive. Recovery of radioactivity 168 hours after administration was 43.2% in urine, 39.2% in faeces, 16.24% in expired air (as carbon dioxide) and 2.51% in carcass. Recovery of radioactivity 24 hours after administration was closer to 35% in urine (percentage was estimated in figure in reference) (Minegishi et al. 1988). Approximately 40% of the radioactivity was excreted via the bile. Bioaccumulation was expected to be low. The half-life of TDCPP clearance in tissues was between 1.5 and 5.4 hours depending on the tissue from a rat toxicokinetic study where TDCPP was intravenously administered (Nomeir et al. 1981).
TDCPP metabolites were recovered from rat urine in toxicokinetic studies where TDCPP was intravenously administered (Nomeir et al. 1981; Lynn et al. 1980, 1981). The major metabolite identified was a diester of TDCPP, BDCPP (>60%). Lynn et al. (1981) identified 1,3-dichloro-2-propanol (1,3-DCP), the halo-alcohol that would be generated from hydrolysis of TDCPP to diester BDCPP, 1,3-dichloro-2-propyl phosphate (monoester of TDCPP) and a trace amount of un-metabolized TDCPP. Nomeir et al. (1981) noted that an unidentified polar metabolite (32%) was found in the urine, in addition to trace amounts of 1,3-dichloro-2-propyl phosphate (0.29%) and 0.45% un-metabolized TDCPP. Ulsamer et al. (1980) reported 1,3-DCP as the only metabolite detected in the urine of TDCPP-treated animals (rats and rabbits) but did not provide further experimental details.
An in vitro metabolism study tested in liver samples resulted in identification of the following metabolites: BDCPP (64%), 3-monochloro-1,2-propanediol (3-MCPD) (20%), 1,3-DCP (5.7%) and an unknown metabolite (11%) (Nomeir et al. 1981). Nomeir et al. (1981) suggested that the absence of 3-MCPD and 1,3-DCP metabolites in urine or expired air in their in vivo study was probably due to further metabolism of these intermediate metabolites. Another in vitro metabolism study identified glutathione conjugate of TDCPP (substitution of Cl) and derived metabolites (Gly-Cys-adduct and Cys-adduct) (Study Submission 2013).
In vitro metabolism studies using rat liver fractions suggested that TDCPP was metabolized by a NADPH-dependent microsomal mixed-function oxidase system and a glutathione-dependent transferase system in the soluble fraction (Sasaki et al. 1984; Nomeir et al. 1981), In an in vitro metabolism study using human liver fractions (Van den Eede et al. 2013), consistent with other metabolism studies, BDCPP (45%) and the glutathione conjugate (20%) of TDCPP were identified. Another recent in vitro metabolism study (Abdallah et al. 2015) supports these results.
An in vitro dermal absorption study tested on human skin, conducted according to OECD guidelines (TNO Quality of Life 2006a), is described in Section 8.1.2.1. In an in vivo study, Nomeir et al. (1981) stated that TDCPP was readily absorbed through rat skin but the absorption rate was not stated. Distribution pattern showed the highest concentration in the liver, followed by lungs, skin, blood, kidneys, adipose tissue and muscle.
8.2.2.4 Repeated-dose toxicity
Kamata et al. (1989) conducted a 3-month oral subchronic study in mice (12/dose group) in which animals were administered a diet containing 0.01,0.04, 0.13, 0.42 and 1.33% of TDCPP. The no-observed-adverse-effect level (NOAEL) for female mice was 0.01%( 15.3 mg/kg-bw/day) based on a significant increase in absolute and relative kidney weights at the next dose level of 0.04% (61.5 mg/kg-bw/day). The NOAEL for male mice was 0.04% (47.3 mg/kg-bw/day) based on a significant increase in relative liver and kidney weights at the next dose level of 0.13% (171.0 mg/kg-bw/day).
In the two-year carcinogenicity study described earlier in Section 8.2.2.1 (administered dose levels of 0, 5, 20 or 80 mg/kg-bw/day), there were non-cancer effects observed in animals treated for both 12 or 24 months (Stauffer Chemical Co. 1981a; Freudenthal and Henrich 2000). The administered dose levels were 0, 5, 20 or 80 mg/kg-bw/day. In the 12-month interim group, the lowest-observed-adverse-effect level (LOAEL) was 80 mg/kg-bw/day based on a significant decrease in body weights (>20% lower than control group weights at termination of study), significant increase in absolute and relative liver weights and significant increase in absolute and relative kidney weights observed in both male and female rats. For the 24-month treated animals, the LOAEL was 5 mg/kg-bw/day, the lowest dose level tested, based on hyperplasia of the convoluted tubule epithelium in the kidneys, germinal epithelial atrophy with associated oligospermia in the testes, and atrophy of the seminal vesicles in male rats at 5 mg/kg-bw/day and higher. At the next dose level of 20 mg/kg-bw/day, a significant increase in absolute and relative kidney weights and relative liver weights were observed in both males and females. In males, there was also a significant increase in absolute liver weight, chronic nephropathy testicular enlargement at this mid-dose level. At the high dose of 80 mg/kg-bw/day, there was a significant decrease in body weight (>20%) and a significant increase in absolute and relative thyroid weights in both male and female rats compared to control animals. Macroscopic changes of the liver including various discolourations as well as masses/nodules/raised areas, erythroid/myeloid hyperplasia of the rib marrow and erythroid/myeloid metaplasia of the spleen were also observed.
8.2.2.5 Reproductive and developmental toxicity
A reproductive toxicity study was identified, which was conducted in male Dutch rabbits, where animals (10/dose group) were administrated 0, 2, 20 or 200 mg/kg-bw/day of TDCPP via oral gavage for 12 weeks (Stauffer Chemical Co. 1983c). The NOAEL was 20 mg/kg-bw/day based on a significant increase in absolute kidney and relative liver weights at the next dose level of 200 mg/kg-bw/day. There were no effects on mating behaviour, male fertility, sperm quality or quantity. No histological lesions were observed in kidneys, liver, pituitary, testes or epididymides.
In the two-year carcinogenicity study described in Sections 8.2.2.1 and 8.2.2.5 (Stauffer Chemical Co. 1981a; Freudenthal and Henrich 2000; NRC 2000), non-cancer effects in the male reproductive system were examined. Effects in the seminal vesicle (decreased secretory product and atrophy) reached statistical significance starting from 5 mg/kg-bw/day, the lowest dose level tested. According to the EU, “The effects observed on the testes described above may be secondary to an effect of the Leydig cell tumours” (EU RAR 2008b). Female reproductive organs were not analyzed in this study.
TDCPP, TCPP and TCEP, are closely related substances with similarities in chemical structures, physiochemical properties and toxicokinetics (more detail is available in Health Canada 2015a). However, the reproductive effects observed in female animals tested with TCPP and TCEP were not similar (Appendix G). The EU (EU RAR 2008b) considered it not appropriate to read-across from female fertility data on either TCPP or TCEP to address any possible effects on female fertility of TDCPP.
In a developmental toxicity study, pregnant Sprague-Dawley rats (20/dose group) were administered 0, 25, 100 or 400 mg/kg-bw/day of TDCPP by oral gavage during gestation day 6 to 15 (Stauffer Chemical Co. 1978b). The maternal NOAEL was 25 mg/kg-bw/day based on a significant decrease in food consumption and in body weights and clinical signs of toxicity such as alopecia, hunched appearance, rough hair coat and urine stains in some animals at the next dose level of 100 mg/kg-bw/day. At 400 mg/kg bw/day, in most animals there were clinical signs of toxicity consisting of urine stains, hunched appearance, salivation, alopecia as well as significant body weight loss. In a number of high dose animals, there were observations of rough coat, bloody crust around the nose and thinness. The developmental NOAEL was 100 mg/kg-bw/day based on a significant increase in the rate of resorption, a significant decrease in the fetal viability index and retarded skeletal development at the next dose level of 400 mg/kg-bw/day. These developmental effects could be secondary to maternal toxicity.
Another study was conducted with pregnant Wistar rats (15-24/dose group) administered 0, 25, 50, 100, 200 or 400 mg/kg-bw/day of TDCPP by oral gavage during gestation day 7–15 (Tanaka et al. 1981 cited in EU RAR 2008b, only abstract is in English). The maternal NOAEL was 100 mg/kg-bw/day based on a significant increase in absolute and relative kidney weights at the next dose level of 200 mg/kg-bw/day. Increased mortality (11/15 dams) and clinical signs of toxicity were observed at 400 mg/kg-bw/day. The developmental NOAEL was 200 mg/kg-bw/day based on a significant increase in foetal deaths at the next dose level of 400 mg/kg-bw/day. Performance of functional tests including open field, water maze, rota rod, inclined screen, pain reflex and auditory startle reflex were comparable to controls in postnatal examination performed at dose levels of 200 mg/kg/day and below.
A recent rat developmental study examined organ weights, serum thyroid hormone levels, acetylcholinesterase activities and developmental neurotoxicity endpoints (Moser et al. 2015). Pregnant Long-Evans rats (n=8-14/dose group) were administered TDCPP (0, 15, 50 or 150 mg/kg-bw/day) via oral gavage from gestation day 10 to weaning (postnatal day [PND] 22). In the dams, only liver, blood and thyroid were collected for analysis. The maternal NOAEL was 50 mg/kg-bw/day based on significant increase in relative and absolute liver weights at 150 mg/kg-bw/day. Serum acetylcholinesterase activity was not affected by TDCPP up to the highest dose level tested. For the offspring, neurobehavioural testing (locomotor activity, elevated zero maze, functional observational battery, Morris water maze) was conducted at several lifestages. Overall, slight changes were observed in certain behavioural testing, but the effects were either minimal, with no clear dose-response pattern or were highly variable (Moser et al. 2015). The developmental NOAEL was 50 mg/kg-bw/day based on significant decrease in body weight and absolute liver weight in both sexes at PND6 and PND22 at 150 mg/kg-bw/day. Serum acetylcholinesterase activity was significantly increased on PND22 at 150 mg/kg-bw/day, but brain acetylcholinesterase activity was not affected at all doses on PND 6 and PND22. Serum thyroid hormone levels (T3, T4) were not affected in both the dams and the pups (PND6, PND22).
8.2.2.6 Neurotoxicity
Certain organophosphorus substances have the potential to produce organophosphate-induced delayed neurotoxicity (OPIDN). This specific type of neurotoxicity might not be detected in standard toxicity studies. In regulatory protocols (US EPA and OECD guidelines), hens are the experimental animal of choice for this endpoint as other small laboratory animals (rats, guinea pigs, mice) are relatively non-susceptible (Weiner and Jortner 1999).
In three independent studies conducted in hens, animals treated orally with TDCPP for an acute duration, 5 consecutive days and 90 days did not show any significant signs of paralysis or neurotoxicity (Stauffer Chemical Co. 1978c, 1981b; US EPA 2008).
8.2.2.7 Sensitization
No skin sensitization was observed in guinea pig maximization tests (CIT 2001 cited in EU RAR 2008b).
8.2.2.8 Epidemiological studies
A retrospective cohort study examining mortality of workers employed in a TDCPP manufacturing plant was identified (Stauffer Chemical Co. 1983a). The study followed 289 workers, who were employed for a minimum of 3 months between 1956 and 1977, and were followed through to 1980. Over half of the workers worked fewer than 5 years and only 42 workers worked 15 or more years. The measured TDCPP levels, based on breathing zone samples measured between 1978 and 1981, were below the limit of detection of 8 ppb (140 µg/m3). The overall standardized mortality ratio (SMR) based on observed death versus expected death of all causes was 0.75. SMR for all malignant neoplasms was 1.31 based on 3 observed over 2.3 expected. The 3 observed cases were employees who died from lung cancers and who were known to be moderate to heavy cigarette smokers.
In an adjunct to the mortality study (Stauffer Chemical Co. 1983b), a total of 124 workers in the TDCPP manufacturing plant were part of a retrospective morbidity study (Stauffer Chemical Co. 1983d). Workers were classified as TDCPP-exposed (93 workers) or non-exposed (31 workers). Breathing zone sampling taken in the plant indicated that TDCPP levels in the air were always near or below the limit of detection of 8 ppb (140 μg/m3). A self-administered health questionnaire, a physical examination, a pulmonary function test, a chest x-ray and electrocardiogram and a spectrum of clinical and biochemical analyses were performed on these workers. Overall, there were no increased risk of adverse respiratory effects from exposure to TDCPP and no abnormal clinical findings. An excess of benign neoplasms (primarily lipomas) (5.4% vs. 0%), dermatitis (6.5% vs 3.2%) and gynaecomastia (3.3% vs 0%) were observed in the exposed group compared to the non-exposed group.
8.3 Characterization of risk to human health
8.3.1 TCPP
Based on the overall data available on health effects of TCPP, the critical effects for characterization of risk to human health associated with exposure to TCPP are reproductive and developmental toxicity. Although no chronic or carcinogenicity studies are available, there is evidence to suggest that TCPP may have carcinogenic potential.
The initial concern for carcinogenicity is due to the structural similarity of TCPP to other organophosphate esters (e.g., TCEP, TDCPP) that showed carcinogenic effects in carcinogenicity studies in experimental animals. Initiated in 2012, the NTP conducted 90-day and two-year oral toxicity and carcinogenicity studies in both rats and mice but a study report is not yet available. In the absence of a chronic study, several other lines of evidence were investigated to assess the carcinogenic potential of TCPP. Overall, the evidence suggests that TCPP may be carcinogenic in rodents; this is based on a qualitative read-across from TCEP and TDCPP which are considered to be structurally similar to TCPP, as well as QSAR and structural alerts analyses.
A two-generation reproductive toxicity study in rats was available. In this study, a LOAEL of 99 mg/kg-bw/day, the lowest dose level tested, was identified for both reproductive and developmental effects (TNO Quality of Life 2007 cited in EU RAR 2008a). For reproductive effects, the effect level was based on a significant decrease in uterus weights and effects on estrus cycle in F0 females. The effect on uterus weights was also observed in the preliminary one-generation reproductive toxicity study. At this effect level, there was also a significant decrease in absolute pituitary weight in F1 females in the two-generation study. In terms of developmental effects, a significant increase in the number of runts was observed at 99 mg/kg-bw/day or higher on PND1 in the F0 generation. Similar effects were observed in F1 generation and in the preliminary one-generation reproductive toxicity study on PND21 but not on PND1. It is not clear whether the developmental effects occurred in utero or from exposure after birth.
In a 13-week dietary study (Stauffer Chemical Co. 1981c cited in EU RAR 2008a and likely published by Freudenthal and Henrich 1999), a significant increase in liver weights in male rats was reported starting from the lowest dose tested of 52 mg/kg-bw/day. Although the same liver effects were not observed at higher doses in the two-generation study (TNO Quality of Life 2007), there are uncertainties in the purity of TCPP in the treatments used in each study and a range of critical effect levels based on both studies is used for the characterization of risk from exposure to TCPP.
A short-term critical NOAEL of 1015 mg/kg-bw/day was identified based on reduced body weight gain at the next dose level of 1636 mg/kg-bw/day in male rats that were treated with TCPP for 14 days (Stauffer Chemical Co. 1980b cited in EU RAR 2008a).
The principal sources of exposure to TCPP for the general population are expected to be environmental media (air, water, dust), food, including breast milk, and the use of products available to consumers.
Table 8‑7 and Table 8‑8 provide all of the relevant exposure and hazard values for TCPP as well as resultant MOEs, for determination of risk. Comparison of the estimate of total daily intake from environmental media and food to the range of subchronic critical effect levels, resulted in MOEs that are considered adequate to account for uncertainties in the exposure and health effect databases. The margins of exposure for infants and toddlers mouthing foam object (e.g., a toy) containing TCPP are also considered adequate to account for uncertainties in the exposure and health effect databases.
However, the margins of exposure for prolonged skin contact to TCPP from manufactured items, such as foam-containing upholstered furniture and mattresses, are considered to be potentially inadequate to account for uncertainties in the exposure and health effect databases. This is in line with ECHA’s recent assessment conclusion on the use of TCEP, TCPP and TDCPP in foam articles and residential upholstered furniture used by children (ECHA 2018).
Scenario |
Systemic exposure (mg/kg-bw/day) |
MOEs |
|---|---|---|
Environmental media and food (all age groups) |
8.5x10-4–2.5x10-3 |
20800–116470 (based on subchronic critical LOAELs of 52–99 mg/kg-bw/day) |
Infant dermal contact from lying on foam-containing mattresses or furniture |
0.16–0.54* |
96–620 (based on subchronic critical LOAELs of 52–99 mg/kg-bw/day) |
Adult dermal contact from lying on foam-containing mattresses or furniture |
0.04–0.19* |
275–2350 (based on subchronic critical LOAELs of 52–99 mg/kg-bw/day) |
Infant mouthing a foam object (e.g., toy) |
9.7×10-3 |
5400–10200 (based on subchronic critical LOAELs of 52–99 mg/kg-bw/day) |
Toddler mouthing a foam object (e.g., toy) |
9.4×10-3 |
5550–10500 (based on subchronic critical LOAELs of 52–99 mg/kg-bw/day) |
* Assumption that 40% of TCPP is absorbed based on doses considered most representative of exposure from dermal contact with foam (TNO Quality of Life 2005).
The use of spray foam insulation and waterproofing tent spray may result in inhalation and dermal exposures for an infrequent short-term duration. Margins of exposure were estimated based on upper-bounding inhalation and dermal estimates of exposure compared to the short-term critical NOAEL of 1015 mg/kg/day (Table 8‑8). These MOEs are considered adequate to account for uncertainties in the exposure and health effect databases.
Scenario |
Systemic exposure (μg/kg-bw/event) |
MOEs |
|---|---|---|
Applying spray foam insulation (small project) |
6.8* (dermal) |
1.5 ×105 (based on a short-term oral NOAEL of 1015 mg/kg-bw/day) |
Applying spray foam insulation (small project) |
0.88 (inhalation) | 1.2 ×106 (based on a short-term critical NOAEL of 1015 mg/kg-bw/day) |
Applying spray foam insulation (large project) |
635* (dermal) |
1600 (based on a short-term oral NOAEL of 1015 mg/kg-bw/day) |
Applying spray foam insulation (large project) |
2.3 to 14.0 (inhalation) | 7.25 x 104 to 4.4 x 105 (based on a short-term critical NOAEL of 1015 mg/kg-bw/day) |
Applying waterproofing tent spray |
1.8* (dermal) |
5.6 ×105 (based on a short-term oral NOAEL of 1015 mg/kg-bw/day) |
*assumption that 40% TCPP absorbed based on doses considered most representative of exposure from dermal contact with foam (TNO Quality Life 2005).
Overall, the MOEs for daily intake of TCPP from environmental media and food, exposure to TCPP by infant and toddlers mouthing foam products and the occasional use of spray insulation foams and waterproofing sprays are considered adequate to account for uncertainties in the exposure and health effect databases. However, the MOEs between critical effects and the estimated intakes of TCPP from prolonged skin contact with foam-containing upholstered furniture or mattresses for all age groups are considered potentially inadequate to account for uncertainties in the exposure and health effect databases.
8.3.2 TDCPP
Based on the classification from the European Union and on the available health effects data, the critical effect for characterization of risk to human health associated with exposure to TDCPP is carcinogenicity. A statistically significant increase in the incidence of tumours (adenomas) was observed in both male and female rats exposed to TDCPP for two years. Tumours were observed in multiple organ sites, including kidney and liver in both sexes, testes (in males) and adrenal gland (in females) (Stauffer Chemical Co. 1981a). Hyperplasia in the kidney and histological abnormalities in the testes were observed, which could be associated with the adenomas developed in these organs starting in the mid dose. In terms of genotoxicity, mixed results were observed in vitro and negative results were observed in vivo; however, there is evidence that TDCPP can covalently bind to DNA in mice (Morales and Matthews 1980).
Using the two-year carcinogenicity study where TDCPP was administered to male and female rats via the diet (Stauffer Chemical Co. 1981a; Freudenthal and Henrich 2000), benchmark dose (BMD) modelling was applied to derive a point of departure (POD) for critical cancer effects from oral exposure.
A BMDL10 was derived for each tumour type, and a model was selected on the basis of fit amongst the nine models available in the US EPA Benchmark Dose Software (BMDS v.24) (Appendix H). A dose-response analysis of each tumour site by BMDS shows that the testis (interstitial cell tumour in male rats) is the most sensitive organ with a BMDL10 of 6.74 mg/kg-bw per day. A similar BMDL10 of 6.84 mg/kg-bw per day was identified for renal cortical adenoma in males. In female rats, the BMDL10 for renal cortical adenoma was 8.29 mg/kg-bw per day. Given that the differences between the renal cortical adenoma BMDL10 levels for male and female rats were minimal, it is considered that TDCPP does not induce gender-specific effects in the kidney.
For non-cancer effects, a chronic critical LOAEL of 5 mg/kg-bw was identified, where hyperplasia of the epithelium of the convoluted tubule in the kidneys, and histological abnormalities in the testes, were observed in males at the lowest dose tested in a two-year chronic toxicity study in rats (Stauffer Chemical Co. 1981a).
Potential exposure to TDCPP for the general population is expected to be mainly from environmental media (air, water, dust), food, including breast milk, and from the use of manufactured items (foam mattresses and couches, furniture with upholstery backcoating) that may contain TDCPP. For the purpose of estimating the risk of cancer from exposure to TDCPP, lifetime average daily doses (LADDs) from environmental media and food as well as from dermal exposure to foam-containing furniture or mattresses were calculated (details in Appendix I).
Comparison of the LADD estimate of total daily intake from environmental media and food of 0.015 μg/kg-bw/day to the BMDL10 of 6.74 mg/kg-bw/day for cancer effects results in a lifetime margin of exposure (MOE) of 455 400. These MOEs are considered adequate to account for uncertainties in the exposure and health effect databases for cancer effects. Comparison of the LADD estimates from dermal exposure to foam-containing furniture or mattresses of 4.5 to 19.0 µg/kg-bw/day with the BMDL10 of 6.74 mg/kg-bw/day results in a lifetime MOE ranging from 355 to 1500 for cancer effects. These MOEs are considered potentially inadequate to account for uncertainties in the exposure and health effect databases for cancer effects.
Table 8‑9 provides all the relevant exposure and non-cancer hazard values for TDCPP as well as resultant MOEs, for determination of risk. The MOEs between critical effects and the estimate of daily intake of TDCPP from environmental media and food as well as from infant and toddlers mouthing foam products are considered adequate to account for uncertainties in the exposure and health effect databases for non-cancer effects. The MOEs between critical effects and the estimated dermal intakes of TDCPP through prolonged skin contact with foam-containing upholstered furniture or mattresses for all age groups are considered potentially inadequate to account for uncertainties in the exposure and health effect databases for non-cancer effects.
Scenario |
Systemic exposure (mg/kg-bw/day) |
MOE for non-cancer effects (based on a chronic oral LOAEL of 5 mg/kg-bw/day) |
|---|---|---|
Environmental media and food |
4.6x10-5 – 8.6x10-4 |
5 800 – 108 700 |
Infant dermal contact from lying on foam-containing mattresses or furniture |
1.5×10-2 – 5.0×10-2a |
100 – 333 |
Adult dermal contact from lying on foam-containing mattresses or furniture |
3.8×10-3 – 1.7×10-2a |
290 – 1300 |
Infant or toddler mouthing a foam object |
1.6×10-3 |
3 100 |
a uptake estimated based on TDCPP adjusted dermal absorption value of 30% obtained by using the ratio of the TCPP in vitro absorption values (23% and 40%) to the 15% TDCPP absorption rate (EU RAR 2008b).
Estimates of daily systemic exposure were also calculated using a reverse dosimetry approach from several biomonitoring studies (Kosarac et al. 2016; Yang et al. 2019; Ospina et al. 2018; Butt et al. 2014) in which concentrations of BDCPP, as a biomarker of TDCPP, were measured in urine spot samples. Although estimates of daily systemic exposure derived from biomonitoring data are associated with a number of uncertainties (see Section 8.1.3), biomonitoring intakes provide an approximation of the exposure estimates from all potential routes and sources of exposure (NRC 2006). The estimates of daily systemic exposure based on geometric means of BDCPP concentrations ranged from 7.0x10-5 mg/kg-bw/day for pregnant women to 1.6x10-3 mg/kg-bw/day for toddlers 2 to 5 years old. The high end estimates of daily systemic exposure based on maximum or 95th percentile BDCPP concentrations ranged from 4.3x10-4 mg/kg-bw/day for pregnant women to 0.072 mg/kg-bw/day for toddlers 2-5 years old. These intakes are consistent with estimates of exposure derived with the use of modelling and given the uncertainties associated with use of human biomonitoring, support the conclusions that MOEs may be inadequate in particular for young children.
Overall, the MOEs for daily intake of TDCPP from environmental media and food as well as from infant and toddlers mouthing foam products are considered adequate to account for uncertainties in the exposure and health effect databases for both cancer and non-cancer effects. However, the MOEs between critical effects and the estimated dermal intakes of TDCPP through prolonged skin contact with foam-containing upholstered furniture or mattresses for all age groups are considered potentially inadequate to account for uncertainties in the exposure and health effect databases for both cancer and non-cancer effects.
8.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty in the health assessments of TCPP and TDCPP are presented in the table below.
Key source of uncertainty |
Impact |
|---|---|
Absence of Canadian monitoring data in foods or breast milk for TCPP and TDCPP. Data from the US or Europe was used to derive exposure estimates from these sources. |
+/- |
Estimates of dermal exposure to TCPP and TDCPP only account for covered flexible polyurethane foam (PUF). Exposures to uncovered foam could result in higher dermal exposures. |
- |
The extent of the effect of textile covering on TCPP and TDCPP migration from flexible PUF are unknown. |
+/- |
Lack of empirical data on the relationship between the migration rate of TDCPP or TCPP from flexible PUF and their concentrations in the foam tested. |
+/- |
No TDCPP- or TCPP-specific skin contact factor (SCF) has been identified; a factor of 1 was assumed. |
+ |
For the dermal exposure scenario, it is assumed that constant liquid-mediated migration of TCPP and TDCPP occurs from flexible PUF in mattresses and furniture for the entire duration of the dermal contact (e.g., there could be different migration rates for dry and wet conditions). |
+ |
Use of passive migration rates determined using foam submerged in sweat simulant might not accurately portray migration for mouthing scenarios (i.e., migration might be higher due to sucking). |
- |
Limited Canadian biomonitoring data available for TCPP and TDCPP. |
+/- |
Determination of TDCPP intakes for various populations based primarily on spot urine samples, including assumption on routes of exposure, timing of exposure in relation to timing of sampling, and variability in daily urine volumes both between and within individuals. |
+/- |
Assumption that all BDCPP measured in urine is associated with direct exposure to TDCPP. |
+ |
Assumption that BDCPP levels in human urine represent an excretion of 21% of daily intake of TDCPP (due to lack of human toxicokinetic data) and that this remains constant across all age groups. |
+/- |
No dermal or inhalation toxicity studies available for TCPP and TDCPP, therefore route-to-route extrapolation was required. |
+/- |
No female animal reproductive studies were identified for TDCPP. |
- |
No available data on the mode of action for tumours associated with exposure to TDCPP or the possible contributions of two TDCPP metabolites (3-MCPD, 1,3-DCP) classified as IARC 2B carcinogens to the carcinogenic effect of TDCPP. |
+/- |
No chronic or carcinogenicity studies were available for TCPP. |
+/- |
No data available on the potential contribution of individual chain isomers to the overall toxicity of TCPP. |
+/- |
The BMD calculations for TDCPP used only the incidences of adenoma for analysis, as the information on the incidences of combined adenoma and carcinoma was not available from the original study. |
+/- |
The inclusion of the high-dose level may have had an impact on the dose response curve for the derivation of the BMD and BMDL for TDCPP. |
+/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
The US NTP has conducted two-year carcinogenicity studies for TCPP in both rats and mice but a study report is not yet available. The selection of TCEP and TDCPP as read-across analogues for assessing the carcinogenic potential of TCPP is associated with uncertainty as there are similarities but also differences between these chemicals. Overall, based on a qualitative read-across from analogues as well as QSAR and structural alerts analyses, the information suggests that TCPP may be carcinogenic in rodents.
There is uncertainty on the potential contribution of individual chain isomers to the overall toxicity of TCPP as well as levels of these isomers outside of this mixture; however, given that they are not commercially available nor are they typically isolated in pure form, further characterization of risk was not considered in this assessment.
9. Conclusion
Considering all available lines of evidence presented in this updated draft screening assessment, there is low risk of harm to the environment from TCPP and TDCPP. It is proposed to conclude that TCPP and TDCPP do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this updated draft screening assessment, it is proposed to conclude that TCPP and TDCPP meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that TCPP and TDCPP meet one or more of the criteria set out in section 64 of CEPA.
It is also proposed that TCPP and TDCPP meet the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
Abbasi G, Saini A, Goosey E, Diamond ML. 2016. Product screening for sources of halogenated flame retardants in Canadian house and office dust. Sci Total Environ 545–546:299–307.
ABC Laboratories. 1993. Aerobic aquatic biodegradation of Antiblaze 80 using a shake flask test system. Final Report #40822. Analytical Bio-Chemistry Laboratories, Inc.
Abdallah MA, Zhang J, Pawar G, Viant MR, Chipman JK, D’Silva K, Bromirski M, Harrad S. 2015. High-resolution mass spectrometry provides novel insights into products of human metabolism of organophosphate and brominated flame retardants. Anal Bioanal Chem 407:1871-1883.
Abdallah MA, Pawar G, Harrad S. 2016. Human dermal absorption of chlorinated organophosphate flame retardants; implications for human exposure. Toxicol Appl Pharmacol 291:28-37.
[ACC] American Chemistry Council. 2012. Spray polyurethane foam ventilation research project – phase 2, American Chemistry Council’s Center for the Polyurethanes Industry, Report dated December 19, 2012. Report submitted to Environment Canada on October 31, 2014.
ACD/pKaDB [Prediction Module]. c1997–2012. Version 9.04. Toronto (ON): Advanced Chemistry Development. [cited 2012]. [restricted access].
Addis T, Watanabe CK. 1961. The volume of urine in young healthy adults on a constant diet. J Biol Chem 27:267-272.
Ahrens VD, Maylin GA, Henion JD. 1979. Fabric release, fish toxicity, and water stability of the flame retardant Fyrol FR-2. Bull Environ Contam Toxicol 21:409–412.
Akzo Nobel. 2001a. Final research report. Hydrolysis as a function of pH of TCPP.CGS-ENV F01047 T 01007 H.
Akzo Nobel. 2001b. Test plan for FYROL FR-2 (Tris[1,3-dichloro-20propyl] Phosphate). CAS No. 13674-87-8. High Production Volume Challenge Plan. Akzo Nobel Functional Chemicals LLC, 5 Livingstone Avenue, Dobbs Ferry, NY 10522.
Akzo Nobel. 2001c. Final research report. Evaluation of the removal of TCPP in an aqueous medium – semi-continuous activated sludge method. CGS-ENV F01049 T01007 SC.
Akzo Nobel. 2002. Biodegradability of FYROL PCF in the prolonged closed bottle test. Akzo Nobel Report F02101. [cited in EU RAR 2008a].
Ali N, Dirtu A, Van den Eede N, Goosey E, Harrad S, Neels H, Mannetje A, Coakley J, Douwes J, Covaci A. 2012. Occurrence of alternative flame retardants in indoor dust from New Zealand: Indoor sources and human exposure assessment. Chemosphere 88: 1276–1282.
Ali N, Ali L, Medhi T, Dirtu A, Al-Shammari F, Neels H, Covaci A. 2013. Levels and profiles of organochlorines and flame retardants in car and house dust from Kuwait and Pakistan: Implication for human exposure via dust ingestion. Environ Int 55:62–70.
Allen JG, Stapleton HM, Vallarino J, McNeely E, McClean MD, Harrad SJ, Rauert CB, Spengler JD. 2013. Exposure to flame retardant chemicals on commercial airplanes. Environ Health 12:17.
Andresen JA, Muir D, Ueno D, Darling C, Theobald N, Bester K. 2007. Emerging pollutants in the North Sea in comparison to Lake Ontario, Canada, data. Environ Toxicol Chem 26(6): 1081–1089.
Anon. 1980. An Ames Salmonella/mammalian microsome mutagenesis assay for determination of potential mutagenicity of tris (2-chloropropyl) phosphate (Report No. 471-80). [cited in IPCS 1998].
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2010. Version 1.92. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Arcadis EBRC. 2011. Identification and evaluation of data on flame retardants in consumer products. Contract for European Commission [PDF].
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337–345.
Arnot JA, Mackay D, Parkerton TF, Bonnell M. 2008a. A database of fish biotransformation rates for organic chemicals. Environ Toxicol Chem 27(11): 2263–2270.
Arnot JA, Mackay D, Bonnell M. 2008b. Estimating metabolic biotransformation rates in fish from laboratory data. Environ Toxicol Chem 27: 341–351.
ASTreat Model [sewage treatment plant removal model]. 2006. Version 1.0. Cincinnati (US): Procter & Gamble Company. [cited 2014 March]. Available from Procter & Gamble Company, P.O. Box 538707, Cincinnati, OH 45253-8707, US.
[ATSDR] Agency for Toxic Substances and Disease Registry [PDF]. 2012. Toxicological profile for Phosphate Ester Flame Retardants. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
Bayer. 1991a. Biodegradation test on TCPP. Bayer AG. [cited in ECHA c2007-2015, EU RAR 2008a and UNEP 1999].
Bayer. 1991b. Tris-chlorisopropyl phosphate: Mutagenicity test on unscheduled DNA synthesis in rat liver primary cell cultures in vitro (Unpublished report). Bayer AG. [cited in EU RAR 2008a].
Bayer. 1991c. Tris-chloroisopropyl phosphate: Micronucleus test on the mouse (Unpublished report). Bayer AG. [cited in EU RAR 2008a].
Bayer. 1991d. 28-d study-full details needed. Bayer AG. [cited in EU RAR 2008a].
Bayer Healthcare. 2005. TCPP, Tris(2-chloro-1-methylethyl) phosphate Unscheduled DNA Synthesis test with rat liver cells in vivo (Unpublished report). Bayer AG. [cited in EU RAR 2008a].
[BCFBAF] Bioaccumulation Program for Windows [Estimation Model]. 2010. Version 3.01. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Bendz D, Paxeus N, Ginn TR, Loge FJ. 2005. Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Hoje River in Sweden. J Hazard Mater 122: 195–204.
Bergh C, Torgrip R, Emenius G, Ostman C. 2011. Organophosphate and phthalate esters in air and settled dust – a multi-location indoor study. Indoor Air 21:67–76.
Beyer A, Mackay D, Matthies M, Wania F, Webster E. 2000. Assessing long-range transport potential of persistent organic pollutants. Environ Sci Technol 34(4): 699–703.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2010. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741−752.
Bradman A, Gaspar F, Castorina R, Tong-Lin E, McKone T. 2012. Environmental exposures in early childhood education environments. For California Air Resources Board.
Brandsma S, de Boer J, van Velzen M, Leonards P. 2014. Organophosphorus flame retardants and plasticizers in house and car dust and the influence of electronic equipment. Chemosphere 116:3–9.
Brommer S, Harrad S, Van den Eede N, Covaci A. 2012. Concentrations of organophosphate esters and brominated flame retardants in German indoor dust samples. J Environ Monit 14: 2482.
Brusick D, Matheson D, Jagannath DR, Goode S, Lebowitz H, Reed M, Roy G, Benson S. 1980. A comparison of the genotoxic properties of tris(2,3-dibromopropyl)phosphate and tris(1,3-dichloro-2-propyl)phosphate in a battery of short-term bioassays. J Environ Pathol Toxicol 3:207–226.
Butt C, Congleton J, Hoffman K, Fang M, Stapleton H. 2014. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate (EH-TBB) in urine from paired mothers and toddlers. Environ Sci Technol 48:10432-10438.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette. Part III, vol. 22, no. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette, Part II, vol. 134, no. 7, p. 607−612.
Canada. 2013. CEPA section 71 Notice with respect to certain organic flame retardant substances. Canada Gazette, Part I: Vol. 147, No. 13. March 30, 2013.
Campone L, Piccinelli A, Ostman C, Rastrelli L. 2010. Determination of organophosphorus flame retardants in fish tissues by matrix solid-phase dispersion and gas chromatography. Anal Bioanal Chem 397:799–806.
Carignan CC, Heigher-Bernays W, McClean MD, Roberts SC, Stapleton HM, Sjödin A, Webster TF. 2013a. Flame Retardant Exposure among Collegiate United States Gymnasts. Environ Sci Technol 47:13848-13856.
Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, Stapleton HM, Webster TF. 2013b. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ Int 55:56–61.
[CEC] Commisison for Environmental Cooperation. 2015. Enhancing Trilateral Understanding of Flame Retardants and Their Use in Manufactured Items: Analysis of Select Flame Retardants Contained in Office and Household Furniture. Montreal, Canada: Commission for Environmental Cooperation. 16 pp.
[CEH] Center for Environmental Health. 2013a. Playing on poisons: harmful flame retardants in children’s furniture [PDF].
[CEH] Center for Environmental Health. 2013b. Naptime nightmares: toxic flame retardants in child care nap mats.
Chen D, Letcher RJ, Chu S. 2012. Determination of non-halogenated, chlorinated and brominated organophosphate flame retardants in herring gull eggs based on liquid chromatography–tandem quadrupole mass spectrometry. J of Chromatogr A 1220:169–174.
CIT. 2001. Skin sensitisation test in guinea pigs. Unpublished report [as cited in EU RAR 2008b].
CITI. 1992. Published by Japan Chemical Industry Ecology Toxicology and Information Centre. Report Number: 2-1914. [cited in ECHA c2007-2015, EU RAR 2008b, and HSDB 1983–].
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Cooper E, Covaci A, van Nuijs A, Webster T, Stapleton H. 2011. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 401: 2123–2132.
Covance Laboratories Inc. 2004. Chromosomal aberrations in chinese hamster ovary (CHO) cells. Unpublished report. [cited in EU RAR 2008b].
Covance Laboratories Inc. 2005a. In vivo/in vitro Unscheduled DNA Synthesis in rat primary hepatocyte cultures at two timepoints with a dose range finding assay. Unpublished report [cited in EU RAR 2008b].
Covance Laboratories Inc. 2005b. Tris (2-chloro-1-methylethyl) phosphate: Mutation at the Thymidine Kinase (tk) Locus of Mouse Lymphoma L5178Y Cells (MLA) using the MitrotitreÒ Fluctuation Technique (Unpublished report). [cited in EU RAR 2008a].
Covance Laboratories Inc. 2006. Detection of DNA damage in the liver of treated rats using the Comet assay (Unpublished report). [cited in EU RAR 2008a].
[CPOPs] Canadian Persistent Organic Pollutants Profiler Model. 2012. Version 1.1.18. Gatineau (QC): Environment Canada, Existing Substances Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005].
Crump D, Chiu S, Kennedy SW. 2012. Effect of TCPP and TDCPP on cytotoxicity and mRNA expression in primary cultures of avian hepatocytes and neuronal cells. Tox Sci 126(1):140–148.
Cui K, Wen J, Zeng F, Zhou X, Li S, Zeng Z. 2017. Determination of organophosphate ester flame retardants and plasticizers in soil samples by microwave-assisted extraction coupled with silica gel/alumina multilayer solid-phase extraction and gas chromatography-mass spectrometry. Anal Methods 9(6): 968-993.
Davison JM, Noble MCB. 1981. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. BJOG 88:10–17.
Danish EPA [Environmental Protection Agency]. 2014. Survey, health and environmental assessment of flame retardants in textiles. Survey of chemical substances in consumer products No. 126. Miljøstyrelsen.
Danish EPA [Environmental Protection Agency]. 2015. Chemical substances in car safety seats and other textile products for children. Survey of chemical substances in consumer products No. 135. Danish Ministry of the Environment.
Diamond M, Goosey E, Saini A, Chaudhuri S. 2013. Assessment of the prevalence and exposure to the new flame retardants (NFRs) in Canadian indoor environments. Contract prepared for Health Canada. Diamond Environmental Research Group, University of Toronto.
Dirtu A, Ali N, Van den Eede N, Neels H, Covaci A. 2012. Country specific comparison for profile of chlorinated, brominated and phosphate organic contaminants in indoor dust. Case study for Eastern Romania, 2010. Environ Int 49:1–8.
Dishaw LV, Hunter DL, Padnos B, Padilla S, Stapleton HM. 2014. Developmental exposure to organophosphate flame retardants elicits overt toxicity and alters behavior in early life stage zebrafish (Danio rerio). Toxicol Sci 142(2): 445-454.
[DPD] Drug Product Database [database]. [modified 2017 Feb 17]. Ottawa (ON): Government of Canada. [accessed 2014 April 24].
Driver J, Ross J, Mihlan G, Lunchick C, Landenberger B. 2007. Derivation of single layer clothing penetration factors from the pesticide handlers exposure database. Regul Toxicol Pharmacol 49(2): 125-37. [cited in ECHA 2018].
Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. 2012. After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environ Sci Technol 46(24):13056–13066.
Du Z, Wang G, Gao S, Wang Z. 2015. Aryl organophosphate flame retardants induced cardiotoxicity during zebrafish embryogenesis: By disturbing expression of the transcriptional regulators. Aqua Toxicol 161: 25-32.
[ECCC] Environment and Climate Change Canada. 2013-2014. Data collected pursuant to section 71 (CEPA) and in accordance with the published notice “Notice with respect to certain organic flame retardant substances” Canada Gazette, Vol. 147 no. 13”. Data prepared by: Environment Canada, Health Canada, Existing Substances Program.
[ECCC] Environment and Climate Change Canada. 2016a. Occurrence and Fate of Certain Organic Flame Retardants in Municipal Wastewater Treatment Systems: Organophosphorus. Environment and Climate Change Canada’s wastewater monitoring program under CMP. Unpublished internal report.
[ECCC] Environment and Climate Change Canada. 2016b. Data collected during the public comment period under the Canadian Environmental Protection Act, 1999: Publication after screening assessment of two substances — 2-propanol, 1-chloro-, phosphate (3:1) [TCPP], CAS RN 13674-84-5 and 2-propanol, 1,3-dichloro-, phosphate (3:1) [TDCPP], CAS RN 13674-87-8 — specified on the Domestic Substances List (paragraphs 68(b) and 68(c) of the Canadian Environmental Protection Act, 1999). Data prepared by: ECCC, Health Canada; Existing Substances Program.
[ECCC] Environment and Climate Change Canada. 2019. Supporting documentation: Information in support of the Updated Draft Screening Assessment for 2-Propanol, 1-chloro-, phosphate (3:1) (TCPP) and 2-Propanol, 1,3-dichloro-, phosphate (3:1) (TDCPP) in Certain Organic Flame Retardants Grouping. Ecological Supplemental Data. Gatineau (QC): Environment and Climate Change Canada, Ecological Assessment Division. Available from: substances@ec.gc.ca".
[ECCC, HC]. Environment and Climate Change Canada, Health Canada. 2016. Draft Screening Assessment for the Certain Organic Flame Retardants Grouping, 2-Propanol, 1-chloro-, phosphate (3:1), (TCPP), Chemical Abstracts Service Registry Number 13674-84-5, and 2-Propanol, 1,3-dichloro-, phosphate (3:1), (TDCPP), Chemical Abstracts Service Registry Number 13674-87-8.
[ECHA] European Chemicals Agency. 2010. Guidance on information requirements and chemical safety assessment. Chapter R.16: Environmental exposure estimation. May 2010. Guidance for the implementation of REACH. Helsinki (FI): European Chemicals Agency.
[ECHA] European Chemicals Agency. c2007-2015. Registered substances database; search results for CAS RNs 13674-84-5 and 13674-87-8. Helsinki (FI): ECHA. [accessed 2016 December].
[ECHA] European Chemicals Agency. 2018. Screening report: An assessment of whether the use of TCEP, TCPP and TDCP in articles should be restricted [PDF]. Version 3. Helsinki (FI): European Chemicals Agency.
Eggen T, Heimstad ES, Stuanes AO, Norli HR. 2013. Uptake and translocation of organophosphates and other emerging contaminants in food and forage crops. Environ Sci Poll Res 20:4520–4531.
Environmental Affairs and Toxicology Department. 1981. A murine lymphoma mutagenesis assay, heterozygous at the thymidine kinase locus for the determination of the potential mutagenicity of Antiblaze 80 (Unpublished report). [cited in EU RAR 2008a].
Environment Canada, Health Canada. 2009. Screening assessment for the Challenge: ethanol, 2-chloro, phosphate (3:1) (tris(2-chloroethyl)phosphate [TCEP]): Chemical Abstract Service Registry Number 115-96-8 [Internet]. Ottawa (ON): Environment Canada, Health Canada. [cited 2014 Jun 18].
Environment Canada. 2007. Guidance for conducting ecological assessments under CEPA, 1999. Science Resource Technical Series, Technical Guidance Module: QSARs. Reviewed Draft Working Document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2013. Wise water use. [accessed November 2014] .
Environment Canada, Health Canada. 2007. Chemical substances: Categorization [Internet]. Ottawa (ON): Government of Canada. [updated May 25, 2013; cited July 31, 2013.
Environment Canada, Health Canada. 2009. Screening Assessment for the Challenge. Ethanol, 2-chloro-, phosphate (3:1), (Tris(2-chloroethyl) phosphate [TCEP]), Chemical Abstracts Service Registry Number 115-96-8.
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2000–2012. Version 4.1. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014 January].
[EQC] Equilibrium Criterion Model. 2011. Version 1.0 (Beta). Peterborough (ON): Trent University, Canadian Environmental Modelling Centre.
Eulaers I, Jaspers VLB, Halley DJ, Lepoint G, Nygard T, Pinxten R, Covaci A, Eens M. 2014. Brominated and phosphorus blame retardants in White-tailed Eagle Haliaeetus albicilla nestlings: Bioaccumulation and associations with dietary proxies. SciTotal Environ 478:48–57.
EU RAR. 2008a. European Union Risk Assessment Report. Tris(2-chloro-1-methylethyl)phosphate (TCPP). Luxembourg: Office for Official Publications of the European Communities.
EU RAR. 2008b. European Union Risk Assessment Report. Tris[2-chloro-1-(chloromethyl)ethyl]phosphate (TDCP). Luxembourg: Office for Official Publications of the European Communities.
EU RAR. 2009. European Union Risk Assessment report. TRIS (2-CHLOROETHYL) PHOSPHATE (TCEP). Luzembourg: Office for Official Publications of the European Communities.
European Commission. 2003. Technical Guidance Document on Risk Assessment. Part II. European Chemicals Bureau.
European Union. 1998-2017. Commission Regulation (EU) No 618/2012 of 10 July 2012 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substance and mixtures. Luxembourg: The Publications Office of the European Union. [accessed 2017 October 26].
Evenset A, Leknes H, Christensen G, Waner N, Remberger M, Gabrielsen G. 2009. Screening of new contaminants in samples from the Norwegian arctic. Contract with Norwegian Pollution Control Authority.
Farhat A, Crump D, Chiu, S, Williams KL, Letcher RJ, Gauthier LT, Kennedy SW. 2013. In ovo effects of two organophosphate flame retardants – TCPP and TDCPP – on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol Sci 134(1):92–102.
Farhat A, Crump D, Porter E, Chiu S, Letcher RJ, Su G, Kennedy SW. 2014. Time-dependent effects of the flame retardant tris(1,3-dichloro-2-propyl) phosphate (TDCPP) on mRNA expression, in vitro and in ovo, reveal optimal sampling times for rapidly metabolized compounds. Environ Toxicol Chem 33(12):2842-2849.
Fernie KJ, Palace V, Peters LE, Basu N, Letcher RJ, Karouna-Renier NK, Schultz SL, Lazarus RS, Rattner BA. 2015. Investigating Endocrine and Physiological Parameters of Captive American Kestrels Exposed by Diet to Selected Organophosphate Flame Retardants. Environ Sci Technol 49(12): 7448-7455.
Follmann W and Wober J. 2006. Investigation of cytotoxic, genotoxic, mutagenic, and estrogenic effects of the flame retardants tris-(2-chloroethyl) -phosphate (TCEP) and tris-(2- chloropropyl) -phosphate (TCPP) in vitro. Toxicol Lett 161(2):124–134.
Francis WJA. 1960. Disturbances of bladder function in relation to pregnancy. J Obst Gyn Brit Empire LXVII(3):353–366.
Freudenthal RI and Henrich RT. 1999.A subchronic toxicity study of Fyrol PCF in Sprague-Dawley rats. Int J Toxicol 18:173–176.
Freudenthal RL and Henrich RT. 2000. Chronic toxicity and carcinogenic potential of tris-(1,3-dichloro-2-propyl) phosphate in Sprague-Dawley rat. Int J Toxicol 19:119–125.
Fries E, Mihajlovic I. 2011. Pollution of soils with organophosphorus flame retardants and plasticizers. J Environ Monit 13: 2692.
Gobas F. 2007. Development and review of a generic water–sediment modelling framework for organic chemicals. Report prepared for Environment Canada. Burnaby (BC): Simon Fraser University, Faculty of Environment. March 26, 2007.
Gobas F. 2010. Comments on approach to sediment exposure approach. Report prepared for Environment Canada. Burnaby (BC): Simon Fraser University, Faculty of Environment. March 25, 2010.
Greaves AK and Letcher RJ. 2014. Comparative body compartment composition and in ovo transfer of OPFRs in North American Great Lakes Herring Gulls. Environ Sci Technol 48:7942–7950.
Green N, Schlaback M, Bakke T, Brevik E, Dye C, Herzke D, Huber S, Plosz B, Remberger M, Schoyen M, Uggerud H, Vogelsang C. 2008. Screening of selected metals and new organic contaminants 2007. Norwegian Pollution Control Authority. SPFO-report: 1014/2008.
Guerra P, Alaee M, Eljarrat E, Barcelo D. 2011. Introduction to Brominated Flame Retardants: Commercial Products, Applications, and Physicochemical Properties. In Eljarrat E, Barceló D. editors. 2011. Brominated Flame Retardants. Series: The Handbook of Environmental Chemistry. Volume 16. Heidelberg Berlin:Springer. 290 pp.
Hao C, Helm PA, Morse D, Reiner EJ. 2018. Liquid chromatography-tandem mass spectrometry direct injection analysis of organophosphorus flame retardants in Ontario surface water and wastewater effluent. Chemosphere 191:288-295.
Hartmann P, Burgi D, Giger W. 2004. Organophosphate flame retardants and plasticizers in indoor air. Chemosphere 57:781–787.
Hattori Y, Ishitani H, Kuge Y, Nakamoto M. 1981. Environmental fate of organic phosphate esters. Suishitsu Odaku Kenkyu, 4(3):137–141. [cited in WHO 1998].
He C, Toms LL, Thai P, Van den Eede N, Wang X, Li Y, Baduel C, Harden FA, Heffernan AL, Hobson P, Covaci A, Mueller JF. 2018. Urinary metabolites of organophosphate esters: Concentrations and age trends in Australian children. Environ Int 111:124-130.
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations. Ottawa (ON): Great Lakes Health Effects Program, Health Protection Branch, Health Canada.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2013. Interim guidance on human health risk assessment for short-term exposure to carcinogens at contaminated sites [PDF]. Ottawa (ON): Health Canada. [accessed 2019 Jan].
Health Canada. 2014. CMP Survey 2014-2015: Determination of flame retardants in consumer products. Unpublished. Health Canada. Ottawa, Ontario.
Health Canada. 2015a. Supporting documentation: Carcinogenic potential information for 2-propanol, 1-chloro-, phosphate (3:1). Ottawa (ON): Health Canada. Available upon request from: substances@ec.gc.ca".
Health Canada. 2015b. Determination of TDCPP and TCPP in a Survey of Polyurethane Children’s Products. Unpublished. Health Canada, Ottawa, Ontario.
Health Canada. [modified 2017 May 3]. Lists of Permitted Food Additives. Ottawa (ON): Health Canada Food Directorate. [accessed 2014 April 24].
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2011. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014 January].
Higby K, Suiter CR, Phelps JY, Siler-Khodr T, Langer O. 1994.Normal values of urinary albumin and total protein excretion during pregnancy. Am J Obstet Gynecol 171:984–989.
Hoffman K. Daniels J. Stapleton HM. 2014. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int 63:169–172.
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1983–. Bethesda (MD): National Library of Medicine (US). Revised 2006 Apr 14; [cited 2013 December].
Hughes MF, Edwards BC, Mitchell CT, Bhooshan B. 2001. In vitro dermal absorption of flame retardant chemicals. Food Chem Toxicol 39:1263–1270.
[IARC] International Agency for Research on Cancer. 2012a. IARC monographs on the evaluation of the carcinogenic risks to humans: Some chemicals present in industrial and consumer products, food and drinking-water. 3-monochloro-1,2-propanediol [PDF]. IARC monograph 101:349–374. [Accessed 19 Feb 2014].
[IARC] International Agency for Research on Cancer. 2012b. IARC monographs on the evaluation of the carcinogenic risks to humans: Some chemicals present in industrial and consumer products, food and drinking-water. 1,3-dichloro-2-propanol [PDF]. IARC monograph 101:375–390. [Accessed 19 Feb 2014].
[ICRP].International Commission on Radiological Protection. 2003. Basic anatomical and physiological data for use in radiological protection: Reference values. ICRP Publication 89. Ann. ICRP 32(3-4).
Inveresk Research International. 1985. TDCP LV: Mouse lymphoma mutation assay, (Unpublished report). [cited in EU RAR 2008b].
Ionas A, Dirtu A, Anthonissen T, Neels H, Covaci A. 2014. Downside of the recycling process: harmful organic chemicals in children’s toys. Environ Int 65:54–62.
[IPCS] The International Programme on Chemical Safety. 1998. Environmental Health Criteria 209: Flame retardants: tris(chloropropyl)phosphate and tris(2-chloroethyl)phosphate [PDF]. World Health Organization, Geneva. [Internet]. [cited 2014 Jun 18].
Ishidate M. 1983. Application of chromosomal aberration tests in vitro to the primary screening for chemicals with carcinogenic and/or genetic hazards. From: Short-term tests for Carcinogenesis: Montpellier, 1981, 58–79. [cited in EU RAR 2008b].
[J-CHECK] Japan CHEmicals Collaborative Knowledge database [database]. c2010- . Tokyo (JP): National Institute of Technology and Evaluation (NITE). [accessed 2013 March].
Jantunen LM, Gawor A, Wong F, Bidleman T, Wania F, Stern G, Hung H. 2013a. Flame retardants, plasticizers and pesticides in the Canadian Arctic. A poster at Northern Contaminants Program 2013.
Jantunen L, Struger J, Backus S, Kraft J, Brommer S. 2013b. Organophosphate flame retardants in Southern Ontario tributaries and precipitation. A poster at the Sixth International Symposium of Flame Retardants (BFR 2013).
Jantunen L. 2014. TDCPP and TCPP concentrations in water from the Great Lakes area. Unpublished report. Personal communication to Health Canada: Ottawa, ON.
Juberg D, Alfano K, Coughlin R, Thompson K. 2001. An observational study of object mouthing behavior by young children. Pediatrics 107: 135.
Kajiwara N, Noma Y, Takigami H. 2011. Brominated and organophosphate flame retardants in selected consumer products on the Japanese market in 2008. J Hazard Mater 192:1250–1259.
Kamata E, Naito K, Nakaji Y, Ogawa Y, Suzuki S, Kaneko T, Takada K, Kurokawa Y, Tobe M. 1989. Acute and subacute toxicity studies of tris (1,3-dichloro-2-propyl) phosphate on mice. Eisei Shikenjo Hokoku 107:36–43 [abstract in English] [article in Japanese] [cited in IPCS 1998 and NRC 2000].
Keller AS, Raju NP, Webster TF, Stapleton HM. 2014. Flame retardant applications in camping tents and potential exposure. Environ Sci Technol Lett 1(2):152–155.
Kemmlein S, Hahn O, Jann O. 2003. Emissions of organophosphate and brominated flame retardants from selected consumer products and building materials. Atmos Environ 37:5485–5493.
[KOAWIN] Octanol Air Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2010. Version 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, Yoshida T. 2013. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 314:76–83.
Kosarac I, Kubwabo C, Foster WG. 2016. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). J Chromatogr B 1014:24-30.
Kroon AGM, van Ginkel CG. 1992. Toxicity of Fyrol PCF to the freshwater alga Selenastrum capricornutum. Report Number: CRL F92015. Akzo Research Laboratories, Arnhem. Cited in EU RAR 2008a.
Lakind JS, Naiman DQ. 2008. Bisphenol A (BPA) daily intakes in the United States: Estimates from the 2003-2004 NHANES urinary BPA data. J Expo Sci Environ Epidemiol 18: 608-615.
Lentner C, editor. 1981. Geigy scientific tables volume 1: Units of measurement, body fluids, composition of the body, nutrition. 8th ed. Basel (CH): Ciba-Geigy Ltd.
Leonards P, Steindal EH, van der Veen, I, Berg V, Bustnes JO, van Leeuwen S. 2011. Screening of organophosphorus flame retardants 2010.SPFO-Report 1091/2011.TA-2786/2011; 2011. [cited in Eulaers et al. 2014].
Li H, Su G, Zou M, Yu L, Letcher RJ, Yu H, Giesy JP, Zhou B, Liu C. 2015. Effects of Tris(1,3-dichloro-2-propyl) Phosphate on Growth, Reproduction, and Gene Transcription of Daphnia magna at Environmentally Relevant Concentrations. Environ Sci Technol 49: 12975-12983.
Life Science Research. 1990. FYROL FR-2: Assessment of its ready biodegradability. Modified sturm test. LSR Report No: 90/AKL029/0232. Life Science Research Limited, Eye, Suffolk IP23 7PX, England.
Liu X, Ji K, Chio K. 2012. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol 114-115:173–181.
Liu C, Wang Q, Liang K, Liu J, Zhou B, Zhang X, Liu H, Giesy JP, Yu H. 2013. Effects of tris(1,3-dichloro-2-propyl) phosphate and triphenyl phosphate on receptor-associated mRNA expression in zebrafish embryos/larvae. Aquat Toxicol 128-129:147–157.
Liu Y, Liggio J, Harner T, Jantunen L, Shoeib M, Li S-M. 2014a. Heterogeneous OH initiated oxidation: A possible explanation for the persistence of organophosphate flame retardants in air. Environ Sci Technol 48:1041–1048.
Liu Y, Huang L, Li S-M, Harner T, Liggio J. 2014b. OH initiated heterogeneous oxidation of tris-2-butoxyethyl phosphate: implications for its fate in the atmosphere. Atmos Chem Phys 14:19431–19468.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Government of Canada. [accessed 2014 April 24].
Lynn RK, Wong K, Dickinson RG, Gerber N, Kennish JM. 1980. Diester metabolites of the flame retardant chemicals tris(1,3-dichloro-2-propyl) phosphate and tris(2,3- dibromopropyl) phosphate in the rat: identification and quantification. Res Commun Chem Pathol Pharmacol 28(2):351–360. [cited in EU RAR 2008b].
Lynn RK, Wong K, Garvie-Gould C, Kennish JM. 1981. Deposition of the flame retardant, tris(1,3-dichloro-2-propyl) phosphate, in the rat. Drug Metab Dispos 9(5):434–41.
Marklund A, Andersson B, Haglund P. 2003. Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere 53:1137–1146.
Marklund A, Andersson B, Haglund P. 2005a. Traffic as a source of organophosphorus flame retardants and plasticizers in snow. Environ Sci Technol 39:3555–3562.
Marklund A, Andersson B, Haglund P. 2005b. Organophosphorus flame retardants and plasticizers in air from various indoor environments. J Environ Monit 7:814–819.
Martinez-Carballo E, Gonzalez-Barreiro C, Sitka A, Scharf S, Gans O. 2007.Dermination of selected organophosphate esters in the aquatic environment of Austria. Sci Total Environ 388: 290–299.
Matthews HB, Anderson MW. 1979. Disposition of tris-(1,3-dichloro-2-propyl) - phosphate in the rat. Toxicology and Applied Pharmacology. Abstracts Eighteenth Annual Meeting. A184. [cited in EU RAR 2008b].
McGoldrick DJ, Letcher RJ, Barresi E, Keir MJ, Small J, Clark MG, Sverko E, Backus SM. 2014.Organophosphate flame retardants and organosiloxanes in predatory freshwater fish from locations across Canada. Environ Pollut 193:254–261.
Meeker JD and Stapleton HM. 2010. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect 118(3): 318–323.
Meeker J, Cooper E, Stapleton H, Hauser R. 2013. Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ Health Perspect 121 (5):580.
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: A QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1–2):103−133.
Meyer J, Bester K. 2004.Organophosphate flame retardants and plasticizers in wastewater treatment plants. J Environ Monit 6:599–605.
Mihajlovic I, Miloradov MV, Fries E. 2011. Application to Twisselman extraction,SPME and GC-MS to assess input sources for organophosphate esters onto soil. Environ Sci Technol 45:2264–2269.
Mihajlovic I, Fries E. 2012. Atmospheric deposition of chlorinated organophosphate flame retardants (OFR) onto soils. Atmos Environ 56:177–183.
Miller RC, Brindle E, Holman DJ, Shofer J, Klein NA, Soules MR, O’Connor KA. 2004. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin Chem 50(5):924–932.
Minegishi KI, Kurebayashi H, Seiichi N, Morimoto K, Takahashi T, Yamaha T. 1988. Comparative studies on absorption, distribution, and excretion of flame retardants halogenated alkyl phosphate in rats. Eisei Kagaku 34(2):102–114 [cited in ATSDR 2012 and EU RAR 2008b].
MITI. 1992. Ministry of International Trade and Industry (Japan). Biodegradation in water: screening tests. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan, Tokyo (Japan): Japan Chemical Industry Ecology-Toxicology and Information Centre. [cited in EU RAR 2008a and ECHA c2007-2015].
Mobil. 1985. Static 96-hour acute toxicity of Antiblaze 80 to Fathead Minnows. Report of test number: 50593. Mobil Environmental Health Science Laboratory, Pennington, New Jersey 08534. [cited in EU RAR 2008a].
Mobil Environmental and Health Safety Laboratory. 1980. An Ames Salmonella/Mammalian Microsome Mutagenesis Assay for Determination of Potential Mutagenicity of Tris (2-chloropropyl) phosphate (Unpublished report). [cited in EU RAR 2008a].
Moller A, Xie Z, Caba A, Sturm R, Ebinghaus R. 2011. Organophosphorus flame retardants and plasticizers in the atmosphere of the North Sea. Environ Pollut 159:3660–3665.
Moller A, Sturm R, Xie Z, Cai M, He J, Ebinghaus R. 2012. Organophosphorus flame retardants and plasticizers in airborne particles over the Northern Pacific and Indian Ocean towards the polar regions: evidence for global occurrence. Environ Sci Tech 46(6):3127–3134.
Morales NM and Matthews HB. 1980. In vivo binding of the flame retardants tris(2,3-dibromopropyl)phosphate and tris(1,3-dichloro-2-propyl) phosphate to macromolecules of mouse liver, kidney and muscle. Bull Environ Contam Toxicol 25(1):34–8.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B and Zeiger E. 1986.Salmonella mutagenicity tests. 2. results from the testing of 270 chemicals. Environ Mutagen 8(suppl 7):1–119.
Moser VC, Phillips PM, Hedge JM, McDaniel KL. 2015. Neurotoxicological and thyroid evaluations of rats developmentally exposed to tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) and tris(2-chloro-2-ethyl)phosphate (TCEP). Neurotoxicol Teratol 52:236-247.
Nakamura A, Tateno N, Kojima S, Kaniwa M-A, Kawamura T. 1979. The mutagenicity of halogenated alkanols and their phosphoric acid esters for Salmonella typhimurium. Mutat Res 66:373–380. [cited in EU RAR 2008a,b].
[NCI] National Chemical Inventories [database on a CD-ROM]. 2013. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [cited 2013 October].
Neithardt AB, Dooley SL, Borensztajn J. 2002. Prediction of 24-hour protein excretion in pregnancy with a single voided urine protein-to-creatinine ratio. Am J Obstet Gynecol 186:883–886.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2017 June 21]. Ottawa (ON): Government of Canada. [accessed 2014 April 24].
Nomeir AA, Kato S, Matthews HB. 1981. The metabolism and disposition of tris(1,3-dichloro-2-propyl) phosphate (Fyrol FR-2) in the rat. Toxicol Appl Pharm 57:401–413.
Norris and Smith. 2002. Research into the mouthing behaviour of children up to 5 years old. London (UK): Consumer and Competition Policy Directorate, Department of Trade and Industry [cited in US EPA 2011].
[NPRI] National Pollutant Release Inventory. 1994–2009.
[NRC] National Research Council. 2000. Toxicological Risks of Selected Flame-Retardant Chemicals. Washington, DC: The National Academies Press. ( [accessed 16 Jun 2014].
[NRC] National Research Council. 2006. Human Biomonitoring for Environmental Chemicals. Washington, DC: The National Academies Press. ([accessed 5 Dec 2014].
[NTP].National Toxicology Program.1998. NTP technical report on the toxicology and carcinogenesis studies of 1-chloro-2-propanol (technical grade) (CAS No. 127-00-4) in F344/N rats and B6C3F1 mice (drinking water studies) [PDF]. NTP TR 477. NIH publication No. 98-3967. NC (Research Triangle Park): US Department of Health and Human Services, Public Health Service, National Institutes of Health. [cited 2014 April 4].
[NTP] National Toxicology Program. 2018. Link to data search for CAS RN 13674-84-5. Short-term Toxicity Studies (mice and rats) and Prenatal Developmental Toxicity Studies (completed, not report available). [cited 2018 June 29].
[OECD] The Organisation for Economic Co-operation and Development. 1995. Test No. 418. Delayed neurotoxicity of organophosphorus substances following acute exposure [PDF]. [cited 2014 Jan 6].
[OECD] The Organisation for Economic Co-operation and Development. 2002. SIDS Initial Assessment Report for tris(1-chloro-2-propyl)phosphate; CAS RN 13674-84-5. SIDS Initial Assessment Meeting 4; May 1996. [cited 2014 Jun 18].
[OECD] The Organisation for Economic Co-operation and Development. 2009. SIDS Initial Assessment Report for tris(1-chloro-2-propyl)phosphate; CAS RN 13674-84-5. SIDS Initial Assessment Meeting 28; April 2009. [cited 2014 Jun 18].
OECD Pov and LRTP Screening Tool. Version 2.0. Organisation for Economic Cooperation and Development; Zurich (CH).
Parboosingh J, Doig A. 1973. Studies of nocturia in normal pregnancy. J Obstet Gynaecol Br Commonw 80:888–895.
Parmar AS. 1977. Activity of trichloropropylene phosphate in the Salmonella/microsomal assay for bacterial mutagenicity. Bethesda, Maryland, Microbiological Associates (Project No. T1108). [cited in IPCS 1998].
Perucca J, Bouby N, Valeix P, Bankir L. 2007. Sex difference in urine concentration across differing ages, sodium intake, and level of kidney disease. Am J Physiol Regul Integr Comp Physiol. 292:R700-R705.
Phytosafe. 2003a. Laboratory determination of the long term toxicity of TCPP to earthworms (Eisenia fetida) using artificial soil substrate. PHYTOSAFE s.a.r.l., 2, rue Marx Dormoy, 64000 PAU, France.
Phytosafe. 2003b. Laboratory assessment of the side-effects of TCPP on plant growth. Report of Phytosafe Study Number: 03-69-012-ES. PHYTOSAFE s.a.r.l., 2, rue Marx Dormoy, 64000 Pau, France. [cited in ECHA c2007-2015 and EU RAR 2008a].
Phytosafe. 2004a. Laboratory determination of the long term toxicity of TDCP to earthworms (Eisenia foetida) using artificial soil. Study Number: 04-99-021-ES.PHYTOSAFE s.a.r.l. Pau, France. [cited in ECHA c2007-2015 and EU RAR 2008b].
Phytosafe. 2004b. Laboratory assessment of the side-effects of TDCP on plant growth. Study Number: 04-99-022-ES. PHYTOSAFE s.a.r.l. Pau, France. [cited in ECHA c2007-2015 and EU RAR 2008b].
[Red Devil] Red Devil Inc. 2004. Foam & Fill minimal expanding polyurethane sealant MSDS.
Revúsová V, Zvara V, Gratzlová J. 1971. Some Laboratory Findings in Patients with Urolithiasis. Int Urol Nephrol 3(3): 251-258.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007. Do-it-yourself products fact sheet: to assess the risks for the consumer [PDF]. Bilthoven (NL): RIVM. Report No.: 320104007/2007.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2008. Chemicals in Toys. A general methodology for assessment of chemical safety of toys with a focus on elements [PDF]. Bilthoven (NL): RIVM. Report No.:320003001/2008. [accessed 2018 July 9].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2014. General Fact Sheet: general default parameters for estimating consumer exposure – Updated version 2014 [PDF]. Bilthoven (NL): RIVM. Report No.: 090013003/2014.
SafePharm. 1996. Inherent biodegradation test. TCPP. SPL project number 0741457. [cited in EU RAR 2008a].
SafePharm Laboratories Ltd. 1979. Determination of the contact sensitisation potential of Tris Mono Chloropropyl Phosphate (unpublished report). [cited in EU RAR 2008a].
SafePharm Laboratories Ltd. 1984. Tolgard TDCP. LV: Ames test (Unpublished report). [cited in EU RAR 2008b].
SafePharm Laboratories Ltd. 1985a. Tolgard TDCP. LV: Ames test (Unpublished report). [cited in EU RAR 2008b].
SafePharm Laboratories Ltd. 1985b. Tolgard TDCP. LV: OECD 474 micronucleus study in the mouse. (Unpublished report). [cited in EU RAR 2008b].
SafePharm Laboratories Ltd. 1992. TMCPP: Reverse mutation assay “Ames Test” using Salmonella typhimurium and Escherichia coli (Unpublished report). [cited in EU RAR 2008a].
SafePharm Laboratories. 1993. Amgard TDCP. Acute toxicity to Rainbow Trout. Report reference JWH/AQ71/272. SafePharm Laboratories Limited, Derby, UK. [cited in ECHA c2007-2015 and EU RAR 2008b].
SafePharm Laboratories. 1995. TCPP: Daphnia magna reproduction test. SPL Project number: 071-386.
SafePharm Laboratories. 1996a. Assessment of inherent biodegradability. TDCPP. SPL Project Number 071/455.
SafePharm Laboratories. 1996b. Acute toxicity to earthworms. Report of SPL Project Number: 071/458. SafePharm Laboratories Ltd., Derby. [cited in ECHA c2007-2015 and EU RAR 2008a].
SafePharm Laboratories. 1996c. Amgard TDCP.Acute toxicity to earthworms (Eisenia foetida).Report of Project Number 071/456.SafePharm Laboratories Limited, Derby, UK.
SafePharm Laboratories. 2002a. TCPP: Determination of general physic-chemical properties. SPL Project Number: 1613/007. SafePharm Laboratories Limited, P.O. Box No. 45, Derby, DE1 2BT, UK.
SafePharm Laboratories. 2002b. TCPP: Determination of general physicochemical properties, Report 1613/001, SafePharm Laboratories, PO Box 45, Derby, UK. [cited in EU RAR 2008a].
SafePharm Laboratories. 2002c. TCPP: Determination of general physicochemical properties, Report 1613/002, SafePharm Laboratories, PO Box 45, Derby, UK. [cited in EU RAR 2008a].
SafePharm Laboratories. 2002d. TDCP: Determination of general physicochemical properties, Report 1613/008, SafePharm Laboratories, PO Box 45, Derby, UK.
SafePharm Laboratories. 2002e. TDCP: Determination of vapour pressure. Report 1613/003, SafePharm Laboratories, PO Box 45, Derby, UK.
SafePharm Laboratories. 2002f. TDCP: Determination of general physicochemical properties, Report 1613/004, SafePharm Laboratories, PO Box 45, Derby, UK.
SafePharm Laboratories 2005. TCPP: local lymph node assay in the mouse (unpublished report). [cited in EU RAR 2008a].
Saini A, Thaysen C, Jantunen L, McQueen RH, Diamond ML. 2016. From Clothing to Laundry Water: Investigating the Fate of Phthalates, Brominated Flame Retardants, and Organophosphate Esters. Environ Sci Technol 50: 9289-9297.
Salamova A, Ma Y, Venier M, Hites R. 2013. High levels of organophosphate flame retardants in the Great Lakes atmosphere. Environ Sci Technol Lett September 2013.
Salamova A, Hermanson MH, Hites RA. 2014a. Organophosphate and halogenated flame retardants in atmospheric particles from a European Arctic site. Environ Sci Technol 48(11):6133–6140.
Salamova A, Ma Y, Venier M, Hites RA. 2014b. High levels of organophosphate flame retardants in the Great Lakes atmosphere. Envi Sci Tech Lett 1(1):8–14.
Sasaki K, Suzuki T, Takeda M. 1982. Bioconcentration and excretion of phosphoric-acid triesters by killifish Oryzias-latipes. Bull Environl Contam Toxicol 28(6):752–759.
Sasaki K, Suzuki T, Uchiyama M. 1984. Metabolism of phosphoric acid trimesters by rat liver homegenate. Bull Environ Contam Toxicol 33:281–288.
Sasaki K, Takeda M, Uchiyama M. 1981. Toxicity, absorption, and elimination of phosphoric acid triesters by killifish and goldfish. Bull Environ Contam Toxicol 27:775–782.
Schreder ED, La Guardia MJ. 2014. Flame Retardant Transfers from U.S. Households (Dust and Laundry Wastewater) to the Aquatic Environment. Environ Sci Technol 48 (19): 11575–11583.
[SDS] Safety Data Sheet. 2014. Safe n Dry fire retardant and water repellent [PDF]. Brampton (ON): Empack Spraytech Inc. [accessed 2019 Feb].
[SDS] Safety Data Sheet. 2015a. LePage Quad Window and Door Foam [PDF]. Mississauga (ON): Henkel. [accessed 2019 Feb].
[SDS] Safety Data Sheet. 2015b. 1.75 Froth Polyol HFC INT [PDF]. Calgary (AB): Dow Chemical Canada ULC. [accessed 2019 Feb].
[SDS] Safety Data Sheet. 2015c. UltraSeal® PF-200/PF-600 “B” Component [PDF]. Guelph (ON): Nuco Inc. [accessed 2019 Feb].
[SDS] Safety Data Sheet. 2017. Large/small gap expanding polyurethane foam [PDF]. Pryor (OK): Red Devil, Inc. [accessed 2019 Feb].
Shoeib M, Jantunen L. 2013. Legacy and current use of flame retardants in Great Lakes atmosphere. A poster at the Sixth International Symposium of Flame Retardants (BFR 2013).
Shoeib M, Ahrens L, Jantunen L, Harner T. 2014. Concentrations in air of organobromine, organochlorine and organophosphate flame retardants in Toronto, Canada. Atmos Environ 99: 140-147.
Soderlund EJ, Dybing E, Holme JA, Hongslo JK, Rivedal E, Sanner T, Nelson SD. 1985. Comparative genotoxicity and nephrotoxicity and nephrotoxicity studies of the two halogenated flame retardants tris(1,3-dichloro-2-propyl) phosphate and tris(2,3- dibromopropyl) phosphate. Acta Pharm Toxicol 56:20–29. [cited in EU RAR 2008b].
[SPIN] Substances in Preparations in Nordic Countries [database on the Internet]. 2013. Copenhagen (DK): Nordic Council of Ministers. [cited 2013 October].
Sprague GL, Sandvik LL, Brookins-Hendricks MJ. 1981. Neurotoxicity of two organophosphorus ester flame retardants in hens. J Toxicol Environ Health 8(3):507–18.
Staaf T, Otsman C. 2005.Organophosphate trimesters in indoor environments. J Environ Monit 7:883–887.
Stapleton H, Klosterhaus S, Eagle S, Fuh J, Meeker J, Blum A, Webster T. 2009. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol 43:7490–7495.
Stapleton H, Klosterhaus S, Keller A, Ferguson P, van Bergen S, Cooper E, Webster T, Blum A. 2011. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol 45:5323–5331.
Stapleton H, Sharma S, Gerzinger G, Ferguson P, Gabriel M, Webster T, Blum A. 2012. Novel and high volume use flame retardants in U.S. couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol 46: 13432–13439.
Statistics Canada. 2014. Population estimates on July 1st, by age and sex. Table: 17-10-0005-01.
Stauffer Chemical Co. 1976. Mutagenicity evaluation of Fyrol PCF (Unpublished report). [cited in EU RAR 2008a].
Stauffer Chemical Co. 1977. Mutagenicity evaluation of Fyrol FR-2 in the mouse lymphoma multiple endpoint test (MET). (Unpublished report). [cited in EU RAR 2008b].
Stauffer Chemical Co. 1978a. Mutagenicity evaluation of Fyrol FR-2 in the mouse bone marrow cytogenetic analysis. (Unpublished report). [cited in EU RAR 2008b].
Stauffer Chemical Co. 1978b. Teratology study in rats: FR-2 (Fyrol). (Unpublished report). [cited in EU RAR 2008b].
Stauffer Chemical Co. 1978c. Toxicology reports on Fyrol FR-2. Vol I of II: Summary of in vitro delayed neurotoxicity evaluation. Report T-6303. USEPA TSCAT document OTS0204911.
Stauffer Chemical Co. 1978d. Mutagenicity evaluation of Fyrol PCF in the Ames Salmonella/microsome plate tests (Unpublished report). [cited in EU RAR 2008a].
Stauffer Chemical Co. 1978e. Mutagenicity evaluation of Fyrol PCF in the mouse lymphoma forward mutation assay (Unpublished report). [cited in EU RAR 2008a].
Stauffer Chemical Co. 1978f. Evaluation of Fyrol PCF in the unscheduled DNA synthesis in human WI-38 cells assay (Unpublished report). [cited in EU RAR 2008a].
Stauffer Chemical Co. 1978g. Mutagenicity evaluation of Fyrol PCF in the in vitro transformation of BALB/3T3 cells assay (Unpublished report). [cited in EU RAR 2008a].
Stauffer Chemical Co. 1978h. Mutagenicity evaluation of Fyrol PCF in the rat bone marrow cytogenetic analysis (Unpublished report). [cited in EU RAR 2008a].
Stauffer Chemical Co. 1979. A 90-day neurotoxicity study in hens. Unpublished Report. [cited in EU RAR 2008b and ECHA c2007-2015].
Stauffer Chemical Co. 1980a. Morphological transformation of BALB/3T3 cells (Unpublished report). [cited in EU RAR 2008a].
Stauffer Chemical Co. 1980b. Fyrol PCF: A two-week dietary acute toxicity range finding study in male and female Charles River Sprague-Dawley derived rats (Unpublished report). [cited in EURAR 2008a].
Stauffer Chemical Co. 1981a. A two-year oral toxicity/carcinogenicity study of Fyrol FR-2 in rats (volume I-IV) (final reports) with attachments, cover sheets and letter dated 093081. USEPA TSCAT document OTS0204911.
Stauffer Chemical Co. 1981b. Toxicology reports on Fyrol FR-2 (volume I - II) with attachments and cover letter dated 020381. EPA Doc No. 88-8100271. USEPA TSCAT document OTS0204911.
Stauffer Chemical Co. 1981c. Fyrol PCF 3-month dietary sub-chronic toxicity in rats (unpublished report). [cited in EU RAR 2008a].
Stauffer Chemical Co. 1983a. A mortality study of workers employed at a Fyrol FR-2 manufacturing plant. Report presented to TSCATS. EPA Doc No. 88-8400615 USEPA TSCAT document OTS0204911.
Stauffer Chemical Co. 1983b. Fyrol FR-2: Mutagenicity evaluation in Salmonella typhimurium. (Unpublished report). [cited in EU RAR2008b].
Stauffer Chemical Co. 1983c. Fyrol Fr-2 fertility study in male rabbits. Report presented to TSCATS. EPA Doc No. FYI-OTS-0183-0228 US EPA TSCAT document OTS0000228-0.
Stauffer Chemical Co. 1983d. A morbidity survey or workers employed at a Fyrol FR-2 manufacturing plant. Report presented to TSCATS. EPA Doc No. 88-8400602. US EPA TSCAT document OTS0204911.
Stauffer Chemical Co. 1984. Fyrol PCF metabolism/pharmacokinetic study in rats (Unpublished report). [cited in EU RAR 2008a].
[STP-EX] Sewage Treatment Plant Expanded Model. c2000-2013. Ver. 1.0. Windsor (ON): University of Windsor, Dept. of Civil and Environmental Engineering. [Model described in Seth R, Webster E, Mackay D. 2008. Continued development of a mass balance model of chemical fate in a sewage treatment plant. Water Res 42:595-604.].
Study Submission. 2013. Unpublished confidential studies submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Su G, Greaves AK, Gauthier LT, Letcher RJ. 2014. Liquid chromatography-electrospray-tandem mass spectrometry method for determination of organophosphate diesters in biotic samples including Great Lakes herring gull plasma. J Chromatogr A 1374: 85-92.
Sundkvist A, Olofsoon U, Haglund P. 2010. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monit 12:943–951.
Tanaka S, Nakaura S, Kawashima K, Nagao S, Endo T, Onoda KI, Kasuya Y, Omori Y. 1981. Effect of oral administration of tris(1,3-dichloroisopropyl) phosphate to pregnant rats on prenatal and postnatal developments. Eisei Shikenjo Hokoku 99:50–55. [cited in EU RAR 2008b].
[TaPL3] Long Range Transport and Persistence Level III model [Internet]. 2000. Version 2.10. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre.
Tenneco Chemical Inc. 1977a. Activity of trichloropropylene phosphate in the Salmonella/microsomal assay for bacterial mutagenicity (Unpublished report). [cited in EU RAR 2008a].
Tenneco Chemicals Inc. 1977b. Activity of TCPP in a test for differential inhibition of repair deficient and repair competent strains of Escherichia coli: Repair test (Unpublished report). [cited in EU RAR 2008a].
Thorp JM, Norton PA, Lewis Wall L, Kuller JA, Eucker B, Wells E. 1999. Urinary incontinence in pregnancy and the puerperium: A prospective study. Am J Obstet Gynecol 181:266–273.
TNO Quality of Life. 2005. In vitro percutaneous absorption of [14C]tris(2-chloro-1-methylethyl)phosphate (TCPP) through human skin membranes using flow-through diffusion cells (Unpublished report). [cited in EU RAR 2008a].
TNO Quality of Life. 2006a. In vitro percutaneous absorption of neat [1,3-14C]TDCP (Tris (1,3-chloro-2-propyl) phosphate) through human skin membranes using flow-through diffusion cells. Unpublished report [cited in EU RAR 2008b].
TNO Quality of Life. 2006b. In vitro percutaneous absorption of neat [14C]TCPP (Tris(2-chloro-1-methylethyl)phosphate) through human skin membranes using flow-through diffusion cells (Unpublished report). [cited in EU RAR 2008a].
TNO Quality of Life. 2007. Oral two-generation reproduction toxicity study (including a dose range finding study) with Tris(2-chloro-1-methylethyl) -phosphate in rats (Unpublished report). [cited in EU RAR 2008a].
Truong JW. 2016. Organophosphate Esters (OPEs) as Emerging Contaminants in the Environment: Indoor Sources and Transport to Receiving Waters. A thesis submitted in conformity with the requirements for the degree of Masters of Applied Science, Department of Chemical Engineering and Applied Chemistry University of Toronto.
Truong JW, Diamond ML, Helm PA, Jantunen LM. 2017. Isomers of tris(chloropropyl) phosphate (TCPP) in technical mixtures and environmental samples. Anal Bioanal Chem 409:6989-6997.
UNEP. 1999. United Nations Environmental Program. OECD Screening Information Data Sets (SIDS). SIDS Initial Assessment Profile. CAS RN 13674-84-5. UNEP Publications.
[US California EPA] California Environmental Protection Agency. 2011. Evidence on the carcinogenicity of tris(1,3-dichloro-2-propyl)phosphate [PDF]. Office of Environmental Health Hazard Assessment, Reproductive and Cancer Hazard Assessment Branch.
[US CPSC] US Consumer Product Safety Commission. 1998. Post-hearing comments of the Upholstered Furniture Action Council on the toxicity of flame retardants chemical treatments for upholstery fabrics [PDF]. Washington (DC).
[US CPSC] US Consumer Product Safety Commission. 2005a. Analysis of FR Chemicals Added to Foams, Fabrics, Batting, Loose Fill, and Barriers [PDF]. Washington (DC): Directorate for Laboratory Sciences, US CPSC. (uff6)
[US CPSC] US Consumer Product Safety Commission, 2005b. Migration of Flame Retardant Chemicals in Upholstered Furniture Foam [PDF]. Washington (DC): Division of Chemistry, US CPSC. (uhff2)
[US CPSC] US Consumer Product Safety Commission. 2006a. CPSC Staff Preliminary risk assessment of flame retardant chemicals in upholstered furniture foam [PDF]. Washington (DC): Directorate for Health Sciences, US CPSC.
[US CPSC] US Consumer Product Safety Commission. 2006b. Quantitative assessment of potential health effects from the use of fire retardant (FR) chemicals in mattresses [PDF]. Washington (DC): Directorate for Health Sciences, US CPSC.
[US EPA] US Environmental Protection Agency. 2003. Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds [PDF], Part I: Estimating exposure to dioxin-like compounds, Volume 3: Site-specific assessment procedures, Chapter 4: Estimating exposure media concentrations,” EPA/600/P-00/001Cb, U.S. Environmental Protection Agency, National Center for Environmental Assessment, Washington, DC, December 2003.
[US EPA] US Environmental Protection Agency. 2008. High Production Volume Information System (HPVIS): Unpublished study obtained from US EPA HPV Challenge Program. Neurotoxicity studies on CAS No. 13674-87-8. [Accessed 17 Jun 2014].
[US EPA] United States Environmental Protection Agency. 2011. Exposure Factors Handbook 2011. National Center for Environmental Assessment. Office of Research and Development. U.S. Environmental Protection Agency, Washington, DC 20460.
[US EPA] US Environmental Protection Agency. 2012a. Chemical Data Access Tool (CDAT).
[US EPA] U.S. Environmental Protection Agency. 2012b. Standard Operating Procedures for Residential Pesticide Exposure Assessment [PDF]. Office of Chemical Safety and Pollution Prevention, Washington (DC).
[US EPA] US Environmental Protection Agency. 2014. Benchmark dose software (BMDS). [accessed 20 Feb 2014].
[US EPA] US Environmental Protection Agency. 2015. Information on the various types of spray polyurethane foam products [PDF]. United States Environmental Protection Agency. [accessed 22 Jan 2015].
Ulsamer AG, Osterberg RE, McLaughlin J. 1980. Flame-retardant chemicals in textiles. Clin Toxicol 17(1):101–131.
Van den Eede N, Dirtu A, Neels H, Covaci A. 2011. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ Int 37:454–461.
Van den Eede N, Dirtu A, Ali N, Neels H, Covaci A. 2012. Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta 89:292–300.
Van den Eede N, Maho W, Erratico C, Neels H, Covaci A. 2013. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol Lett 223(1):9–15.
Van den Eede N, Heffernan AL, Alyward LL, Hobson P, Neels H, Mueller JF, Covaci A. 2015a. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ Int 74:1-8.
Van den Eede N, De Meester I, Maho W, Neels H, Covaci A. 2015b. Biotransformation of three phosphate flame retardants and plasticizers in primary human hepatocytes: untargeted metabolite screening and quantitative assessment. J Appl Toxicol 36 :1401-1408.
Van den Eede N, Tomy G, Tao F, Halldorson T, Harrad S, Neels H, Covaci A. 2016. Kinetics of tris (1-chloro-2-propyl) phosphate (TCPP) metabolism in human liver microsomes and serum. Chemosphere 144:1299-1305.
Van der Veen I, de Boer J. 2012. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88:1119–1153.
van Ginkel CG. 2005. Attempt to demonstrate anaerobic biodegradation of TDCP. Memorandum summarising the test. Reference number CER M05044, Akzo Nobel Research and Technology Chemicals, 28th November 2005. [cited in EU RAR 2008b].
Van Haarst EP, Heldeweg EA, Newling DW, Schlatmann TJ. 2004. The 24-h frequency-volume chart in adults reporting no voiding complaints: defining reference vales and analysing variables. BJU Int 93:1257–1261.
Venier M, Dove A, Romanak K, Backus S, Hites R. 2014. Flame retardants and legacy chemicals in Great Lakes’ water. Environ Sci Technol 48: 9563–9572.
Versar Inc. 1986. Standard Scenarios for Estimating Exposure to Chemical Substances During Use of Consumer Products. Prepared for the U.S. Environmental Protection Agency, Office of Toxic Substances, Washington, D.C.
Vykoukalová M, Venier M, Vojta Š, Melymuk L, Bečanová J, Romanak K, Prokeš R, Okeme JO, Saini A, Diamond ML, Klánová J. 2017. Organophosphate esters flame retardants in the indoor environment. Environ Int 106: 97-104.
Wang Q, Liang K, Liu J, Yang L, Guo Y, Liu C, Zhou B. 2013. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic–pituitary–thyroid axis. Aquat Toxicol 126: 207–213.
Wang Q, Lai NL-S, Wang X, Guo Y, Lam PK-S, Lam JW-H, Zhou B. 2015a. Bioconcentration and transfer of the organophorous flame retardant 1,3-dichloro-2-propyl phosphate causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish larvae. Environ Sci Technol 49: 5123-5132.
Wang Q, Lam JCW, Han J, Wang X, Guo Y, Lam PKS, Zhou B. 2015b. Developmental exposure to the organophosphorus flame retardant tris(1,3-dichloro-2-propyl) phosphate: Estrogenic activity, endocrine disruption and reproductive effects on zebrafish. Aquat toxicol 160: 163-171.
Wang Q, Lam JW-H, Man Y-C, Lai NL-S, Kwok KY, Guo Y, Lam PK-S, Zhou B. 2015c. Bioconcentration, metabolism and neurotoxicity of theorganophorous flame retardant 1,3-dichloro 2-propyl phosphate(TDCPP) to zebrafish. Aquat Toxicol 158: 108-115.
Webster T. Watkins D, Walker C. Fraser A, Heiger-Bernays W, Stapleton H, McClean M. 2010. PentaBDE alternatives in homes, offices and cars. Dioxin 2010: 30th International Symposium on Halogenated Persistent Organic Pollutants (San Antonio, Texas 12-17 September 2010).
Weiner ML and Jortner BS. 1999. Organophosphate-induced delayed neurotoxicity of triarylphosphates. Neurotoxicology 20(4):653–673.
WHO. 1998. Flame retardants: Tris(chloropropyl) phosphate and tris(2-chloroethyl) phosphate [PDF]. Environmental Health Criteria 209. World Health Organization, Geneva.
Wildlife International. 2005a. TDCP aerobic transformation in soil. Project No.: 497E-106. Wildlife International, Ltd., 8589 Commerce Drive, Easton, Maryland 21601.
Wildlife International. 2005b. TCPP: A 72-hour toxicity test with the freshwater alga (Pseudokirchneriella subcapitata). Project number: 583A-101. Wildlife International, Ltd., 8589 Commerce Drive, Easton, Maryland 21601.
Wildlife International. 2005c. TDCP: A 72-hour toxicity test with the freshwater alga (Pseudokirchneriella subcapitata). Project number: 583A-102. Wildlife International, Ltd., 8589 Commerce Drive, Easton, Maryland 21601.
Wildlife International. 2006a. Tris[2-chloro-1-chloromethyl)ethyl]-phosphate (TDCP): Adsorption/desorption characteristics in representative soils, sediment, and sludge solids in accordance with OECD Guideline for Testing of Chemicals, 106: Adsorption – Desorption Using a Batch Equilibrium Method. Wildlife International, Ltd. project no.: 584E-101. Draft report 2nd June 2006. [cited in EU RAR 2006b].
Wildlife International. 2006b. TDCP: A 28-day sediment toxicity test with Chironomus riparius using spiked sediment. Project number: 583A-104.
Wildlife International. 2006c. TDCP: A Prolonged Sediment Toxicity Test with Hyalella azteca Using Spiked Sediment. Project Number: 583A-105. Wildlife International, Ltd., Easton, Maryland 21601.
Wildlife International. 2006d. Tris[2-chloro-1-(chloromethyl)ethyl]-phosphate (TDCP): A Prolonged Sediment Toxicity Test with Lumbriculus variegatus using Spiked Sediment. Final Report Project Number: 583A-106. Wildlife International, Ltd., Easton, Maryland 21601, U.S.A. [cited in EU RAR 2008b].
Williams GM, Mori H, McQueen CA. 1989. Structure-activity relationships in the rat hepatocyte DNA-repair test for 300 chemicals. Mutat Res 221:263–286. [cited in EU RAR 2008a].
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Hum Ecol Risk Assess 19(1): 158–188.
Wu AHB. 2006. Tietz clinical guide to laboratory tests. 4th ed. St. Louis (MO): Saunders Elsevier. p. 1102–1104.
Yang F, Ding J, Huang W, Xie W, Liu W. 2014. Particle size-specific distributions and preliminary exposure assessments of organophosphate flame retardants in office air particulate matter. Environ Sci Technol 48(1):63–70.
Yang C, Harris SA, Jantunen LM, Sidiqque S, Kubwabo C, Tsirlin D, Latifovic L, Fraser B, St-Jean M, De La Campa R, You H, Kulka R, Diamond ML. 2018. Are Cell Phones an Indicator of Personal Exposure to Organophosphate Flame Retardants and Plasticizers? Environ Int 122: 104-116.
Yoon Y, Ryu J, Oh J, Choi B-G, Snyder S. 2010. Occurrence of endocrine disrupting compounds, pharmaceuticals and personal products in the Han River (Seoul, South Korea). Sci Total Environ 408:636–643.
Yu L, Jia Y, Su G, Sun Y, Letcher RJ, Giesy JP, Yu H, Han Z, Liu C. 2017. Parental transfer of tris(1,3-dichloro-2-propyl) phosphate and transgenerational inhibition of growth of zebrafish exposed to environmentally relevant concentrations. Environ Pollut 220: 196-203.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. 1992. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environ Mol Mutagen 19 (supplement 21):2-141. [cited in EU RAR 2008a].
Zhu Y, Ma X, Su G, Yu L, Letcher RJ, Hou J, Yu H, Giesy GP, Liu C. 2015. Environmentally Relevant Concentrations of the Flame Retardant Tris(1,3-dichloro-2-propyl) Phosphate Inhibit Growth of Female Zebrafish and Decrease Fecundity. Environ Sci Technol 49: 14579-14587.
Appendices
Appendix A: Environmental monitoring data for the indoor atmospheric compartment
Environmental monitoring data for TCPP and TDCPP for the atmospheric compartment (indoor air and dust) are summarized in the following tables, while information for the other compartments is presented in the supporting document (ECCC 2019).
A1. Environmental monitoring data for dust compartment in Canada
| Location | Sample typea |
Sampling year |
Sample size |
Median [range] (mg/kg) | P95 (mg/kg) |
Reference |
|---|---|---|---|---|---|---|
Canada |
Vacuum |
2007–2010 |
134 |
1.1 |
9.6 |
personal communication from Environmental Health Science and Research Bureau, Health Canada August 2014 |
Canada |
Fresh |
2007–2010 |
134 |
1.4 |
13.0 |
personal communication from Environmental Health Science and Research Bureau, Health Canada August 2014 |
Canada |
NS |
2007–2010 |
818 |
1.62 |
18.2 |
personal communication from Environmental Health Science and Research Bureau, Health Canada August 2014 |
Toronto, ON, Canada |
Fresh |
2013 |
23 |
1.470 [0.270 to 39.3] | NS |
Vykoukalová et al. 2017 |
Greater Toronto Area and Ottawa, ON, Canada |
NS |
2015 |
51 (bedrooms) | 0.934 (geometric mean) [0.00375 to 299] | 9.42 |
Yang et al. 2019 |
Greater Toronto Area and Ottawa, ON, Canada |
NS |
2015 |
26 (most used rooms) | 1.33 (geometric mean) [0.268 to 161] | 10.84 |
Yang et al. 2019 |
Abbreviations: P95 = 95th percentile; NS = not specified
a Fresh dust refers to samples collected by trained technicians from living areas using a vacuum sampler; this excludes old house dust that has collected over time in areas not vacuumed on a regular basis. Vacuum dust refers to samples obtained from the vacuum systems used by the study participants.
Location |
Sample Typea |
Sampling Year |
Sample Size |
Median [range] (mg/kg) | P95 (mg/kg) |
Reference |
|---|---|---|---|---|---|---|
Toronto, ON, Canada (TI) |
Vacuum |
2010 (fall) and 2011(summer) |
28 |
0.32 (mean) |
0.89 |
Diamond et al. 2013 |
Toronto, ON, Canada |
Vacuum |
2012 |
20 |
2.5 (mean) |
46 (max) |
Diamond et al. 2013 |
Canada |
Vacuum |
2007–2010 |
134 |
2.0 |
12 |
personal communication from Environmental Health Science and Research Bureau, Health Canada August 2014 |
Canada |
Fresh |
2007–2010 |
134 |
2.7 |
9 |
personal communication from Environmental Health Science and Research Bureau, Health Canada August 2014 |
Canada |
NS |
2007–2010 |
818 |
3.08 |
12.7 |
personal communication from Environmental Health Science and Research Bureau, Health Canada August 2014 |
Toronto, ON, Canada |
Fresh |
2012 |
35 homes |
3.463 (mean) [not detected to 46] | NS |
Abbasi et al. 2016 |
Toronto, ON, Canada |
Fresh |
2012 |
10 offices |
[not detected to 190] |
NS |
Abbasi et al. 2016 |
Toronto |
Fresh |
2013 |
23 |
0.917 [0.206 to 9.53] | NS |
Vykoukalová et al. 2017 |
Greater Toronto Area and Ottawa, ON, Canada |
NS |
2015 |
51 (bedrooms) | 1.54 (geometric mean) [0.430 to 9.28] | 6.76 |
Yang et al. 2019 |
Greater Toronto Area and Ottawa, ON, Canada |
NS |
2015 |
26 (most used rooms) | 2.05 (geometric mean) [0.337 to 130] | 12.8 |
Yang et al. 2019 |
Abbreviations: P95 = 95th percentile; ON = Ontario; NS = not specified; TI = Toronto Intensive pilot study
a Fresh dust refers to samples collected by trained technicians from living areas using a vacuum sampler; this excludes old house dust that has collected over time in areas not vacuumed on a regular basis. Vacuum dust refers to samples obtained from the vacuum systems used by the study participants.
A2. Environmental monitoring data for the atmospheric compartment in other jurisdictions
| Location (Sampling Year) | Area |
Sample size |
TCPP concentration (pg/m3) | TDCPP concentration (pg/m3) |
Reference |
|---|---|---|---|---|---|
Urban area, Sweden (NS) |
Home |
2 |
[38000–210000] |
<500 |
Marklund et al. 2005b |
Stockholm, Sweden (NS) |
Home |
10 |
[7000–160000] |
<DL (1 ng/m3) |
Staaf and Otsman 2005 |
Stockholm, Sweden (2009) |
Home |
10 |
15000 [2400–64000] |
3100 [ND–17000] |
Bergh et al. 2011 |
Urban area, Sweden (NS) |
Office |
7 |
[10000–160000] |
[200–150000] |
Marklund et al. 2005b |
Stockholm, Sweden (NS) |
Office |
3 |
[41000–120000] |
NM |
Staaf and Otsman 2005 |
Norway (2007) |
Office |
2 |
[10000–21000] |
[<40–7100] |
Green et al. 2008 |
Stockholm, Sweden (2009) |
Office |
10 |
110000 [16000–240000] |
24000 [NS–73000] |
Bergh et al. 2011 |
Zurich, Switzerland (NS) |
Office |
3 |
[ND–130000] |
ND |
Hartmann et al. 2004 |
Stockholm, Sweden (NS) |
public and personal vehicle |
4 |
[5000–2300000] |
NM |
Staaf and Otsman 2005 |
Zurich, Switzerland (NS) |
Personal vehicle |
4 |
[ND–260000] |
ND |
Hartmann et al. 2004 |
Abbreviations: NM = not measured; NS = not specified; ND = not detected; DL = detection limit
Location |
Sample type |
Sampling year |
Sample size |
Median [range] (mg/kg) | P95 (mg/kg) |
Reference |
|---|---|---|---|---|---|---|
USA |
Living area surfaces |
2006 |
16 |
2.1 |
120 (max) |
Dodson et al. 2012 |
USA |
Living area surfaces |
2011 |
16 |
2.2 (Max) |
2.2 |
Dodson et al. 2012 |
USA |
NS |
2002–2007 |
50 |
1.04 |
5.49 |
Stapleton et al. 2009 |
Germany |
Vacuum |
2010 |
6 |
0.74 (mean) |
0.96 (max) |
Brommer et al. 2012 |
Germany |
Cars |
2010–2011 |
12 |
3.1 [1.4–4.3] |
NS |
Brommer et al. 2012 |
Germany |
Homes |
2010–2011 |
6 |
0.74 [0.37–0.96] |
NS |
Brommer et al. 2012 |
Germany |
Offices |
2010–2011 |
10 |
3 [0.18–9.4] |
NS |
Brommer et al. 2012 |
Romania |
NS |
2010 |
47 |
0.86 (max) |
3.72 (75th%) |
Dirtu et al. 2012 |
Belgium |
NS |
2006–2010 |
41 |
[0.45–1.38] |
14.5 |
Van den Eede et al. 2012 |
Spain |
NS |
2006 |
1 |
0.185 |
0.185 |
Van den Eede et al. 2012 |
Stockholm, Sweden |
NS |
2009 |
10 |
3.1 [0.7–11] |
NS |
Bergh et al. 2011 |
Netherlands |
Near electronics |
NS |
8 |
1.3 [0.48–3.8] |
NS |
Brandsma et al. 2014 |
Netherlands |
On electronics |
NS |
8 |
1.3 [0.58–4.5] |
NS |
Brandsma et al. 2014 |
New Zealand |
Floor measurements |
2008 |
38 |
0.35 [0.02-7.6] |
2.93 |
Ali et al. 2012 |
New Zealand |
Mattress measurements |
2008 |
16 |
0.250 [0.133-1.92] |
1.34 |
Ali et al. 2012 |
Pakistan |
NS |
2011 |
15 |
<0.020 |
0.085 |
Ali et al. 2013 |
Kuwait |
NS |
2011 |
15 |
1.46 (max) |
7.06 |
Ali et al. 2013 |
Kuwait |
House dust |
2011 |
15 |
1.46 [0.12–7.065] |
NS |
Ali et al. 2013 |
Kuwait |
Car |
2011 |
15 |
30.73 [2.49–134] |
NS |
Ali et al. 2013 |
Pakistan |
House dust |
2011 |
15 |
<0.02 [<0.02–0.085] |
NS |
Ali et al. 2013 |
Pakistan |
Car |
2011 |
15 |
0.1 [<0.02–2.615] |
NS |
Ali et al. 2013 |
Abbreviations: P95 = 95th percentile; NS = not specified
Location |
Sample Type |
Sampling Year |
Sample Size |
Median [range] (mg/kg) | P95 (mg/kg) |
Reference |
|---|---|---|---|---|---|---|
USA |
Preschool, vacuum |
2010–2011 |
49 |
2.26 [0.76–70.9] |
36.9 |
Bradman et al. 2012 |
USA |
Living area surfaces |
2006 |
16 |
2.8 (max) |
24 |
Dodson et al. 2012 |
USA |
Living area surfaces |
2011 |
16 |
2.1 |
2.1 |
Dodson et al. 2012 |
USA |
NS |
2002–2007 |
50 |
1.88 (max) |
56.1 |
Stapleton et al. 2009 |
USA |
NS |
2009 |
30 |
6.3 (mean) |
NS |
Webster et al. 2010 |
Germany |
Vacuum |
2010 |
6 |
<0.08 (mean) |
0.11 |
Brommer et al. 2012 |
Germany |
Cars |
2010–2011 |
12 |
130 [<0.08–620] |
NS |
Brommer et al. 2012 |
Germany |
Homes |
2010–2011 |
6 |
<0.08 [<0.08–0.11] |
NS |
Brommer et al. 2012 |
Germany |
Offices |
2010–2011 |
10 |
0.15 [<0.08–0.29] |
NS |
Brommer et al. 2012 |
Romania |
NS |
2010 |
47 |
0.060 |
0.13 (75th%) |
Dirtu et al. 2012 |
Belgium |
NS |
2006–2010 |
41 |
[0.162–0.36] |
0.99 |
Van den Eede et al. 2012 |
Spain |
NS |
2006 |
1 |
0.124 |
0.124 |
Van den Eede et al. 2012 |
Stockholm, Sweden |
NS |
2009 |
10 |
12 [2.2–27] |
NS |
Bergh et al. 2011 |
Netherlands |
Near electronics |
NS |
8 |
0.28 [0.07–3.2] |
NS |
Brandsma et al. 2014 |
Netherlands |
On electronics |
NS |
8 |
0.68 [0.1–7.4] |
NS |
Brandsma et al. 2014 |
New Zealand |
Floor measurements |
2008 |
38 |
0.23 [0.02-1.7] |
1.89 |
Ali et al. 2012 |
New Zealand |
Mattress measurements |
2008 |
16 |
0.103 [0.02-6.5] |
0.303 |
Ali et al. 2012 |
Pakistan |
NS |
2011 |
15 |
<0.005 |
0.25 |
Ali et al. 2013 |
Kuwait |
NS |
2011 |
15 |
0.36 |
1.56 |
Ali et al. 2013 |
Kuwait |
House dust |
2011 |
15 |
0.36 [0.06–1.56] |
NS |
Ali et al. 2013 |
Kuwait |
Car |
2011 |
15 |
7.63 [0.6–166] |
NS |
Ali et al. 2013 |
Pakistan |
House dust |
2011 |
15 |
<0.005 [<0.005–0.255] |
NS |
Ali et al. 2013 |
Pakistan |
Car |
2011 |
15 |
0.029 [<0.005–1.24] |
NS |
Ali et al. 2013 |
Appendix B: Weight of evidence in the ecological risk assessment
Line of evidence |
Level of confidencea |
Relevance in assessmentb |
Weight assignedc |
|---|---|---|---|
Physical-chemical properties |
high |
high |
moderate to high |
Environmental fate and behaviour |
high |
high |
high |
Persistence in the environment |
high |
high |
high |
Long-range transport |
high |
high |
high |
Bioaccumulation in aquatic organisms |
high |
moderate |
high |
Potential decrease of the import quantity |
moderate |
moderate |
moderate |
Environmental concentrations data |
moderate |
high |
moderate to high |
Aquatic PECs of TCPP and TDCPP in release scenarios for the industrial activities and use of products available to consumers |
moderate |
high |
moderate to high |
Sediment PECs of TCPP and TDCPP in release scenarios for the industrial activities and use of products available to consumers |
low |
high |
moderate |
Soil PECs of TDCPP in release scenarios for industrial activities and use of products available to consumers |
low |
high |
moderate |
Risk quotient analysis for TCPP and TDCPP for water |
high |
high |
high |
Risk quotient analysis for TDCPP for sediment and soil |
low |
high |
moderate |
a Level of confidence is determined according to data quality, data variability, data gaps (i.e., are the data fit for purpose).
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the overall combined weights for level of confidence and relevance in the assessment.
Appendix C: Estimates of daily intake by various age groups within the general population of Canada
Route of exposure |
0–6 moa (breast milk-fed)b | 0–6 moa (formula fed)c | 0.5–4 yrd | 5–11 yre | 12–19 yrf |
20–59 yrg | ≥60+ yrh |
|---|---|---|---|---|---|---|---|
Ambient airi |
5.3E-05 |
5.3E-05 |
1.1E-04 |
8.9E-05 |
5.1E-05 |
4.3E-05 |
3.8E-05 |
Indoor airj |
1.0 |
1.0 |
2.2 |
1.7 |
9.8E-01 |
8.4E-01 |
7.3E-01 |
Drinking waterk |
N/A |
4.7E-01 |
2.0E-01 |
1.6E-01 |
8.9E-02 |
9.3E-02 |
9.8E-02 |
Foodl |
2.0E-01 |
NI |
6.8E-02 |
5.3E-02 |
2.9E-02 |
2.8E-02 |
1.9E-02 |
Dustm |
9.2E-02 |
9.2E-02 |
4.8E-02 |
1.8E-02 |
6.7E-04 |
6.4E-04 |
6.3E-04 |
Soiln |
N/A |
N/A |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
Total intake |
1.3 |
1.6 |
2.5 |
1.9 |
1.1 |
9.6E-01 |
8.5E-01 |
Abbreviations: N/A, not applicable; NI, data not identified in the literature; mo, months; yr, years.
a Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day (Health Canada 1998), and to ingest 38 and 0 mg of dust and soil per day, respectively (Wilson et al. 2013).
b Exclusively for breast milk-fed infants, assumed to consume 0.742 L of breast milk per day (Health Canada 1998), and breast milk is assumed to be the only dietary source. The concentration for whole (breast) milk of 1.99 µg/L was based on a reported TCPP of 57 ng/g lipid x 3.4% (lipid content of breast milk) x 1.03 g/mL (density of breast milk) identified in 50 samples of human breast milk collected in 2006 from subjects from Sweden (Sundkvist et al. 2010).
c Exclusively for not formula-fed infants, assumed to drink 0.7 L of water per day (Health Canada 1998), with approximately 50% of non-formula-fed infants introduced to solid foods by 4 months of age and 90% by 6 months of age (NHW 1990).
d Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day, to consume 54.7 g of fish and 249.7 g of fruit and fruit products per day (Health Canada 1998), and to ingest 41 and 14 mg of dust and soil per day, respectively (Wilson et al. 2013).
e Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day, to consume 89.8 g of fish and 276 g of fruit and fruit products per day (Health Canada 1998), and to ingest 31 and 21 mg of dust and soil per day, respectively (Wilson et al. 2013).
f Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day, to consume 97.3 g of fish and 251.6 g of fruit and fruit products per day (Health Canada 1998), and to ingest 2.2 and 1.4 mg of dust and soil per day, respectively (Wilson et al. 2013).
g Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day, to consume 111.7 g of fish and 281.2 g of fruit and fruit products per day (Health Canada 1998), and to ingest 2.5 and 1.6 mg of dust and soil per day, respectively (Wilson et al. 2013).
h Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day, to consume 72.9 g of fish and 242.9 g of fruit and fruit products per day (Health Canada 1998), and to ingest 2.5 and 1.5 mg of dust and soil per day, respectively (Wilson et al. 2013).
i The highest concentration of 1.52 ng/m3 from Toronto (Shoeib et al. 2014 Jantunen 2014) was selected for deriving upper-bounding estimates of daily intake for ambient air exposure. Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
j A maximum indoor air concentration of 4190 ng/m3 from homes in Toronto (Vykoukalová et al. 2017) was selected for deriving upper-bounding estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
k The TCPP maximum concentration of 4400 ng/L in water from urban streams in Toronto (Truong et al. 2017) was selected for deriving upper-bounding estimates of daily intake for drinking water exposure.
l No monitoring data on marketed foods in Canada were identified; however environmental fish and shellfish data were available. The TCPP concentration of 15.6 µg/kg (based on a reported maximum TCPP concentration of 1300 µg/kg lipid x 0.44 % lipid content in mussel) in mussels (n=30) collected in 2007 in Sweden (Sundkvist et al. 2010) was selected for deriving upper-bounding estimates of daily exposure to TCPP from all fish-related food items in the Fish food group. The maximum concentration in fruits with peels (0.82 µg/kg) reported in the US EPA food basket studies (ATSDR 2012) was selected for deriving upper-bounding estimates of daily exposure to TCPP from all fruit-related food items in the Fruits and Fruit Products food group. Amounts of foods consumed on a daily basis by each age group over 12 food groups were obtained from the 1970–1972 Nutrition Canada Survey (Health Canada 1998).
m For all age groups, the 95th percentile concentration of TCPP (18.2 mg/kg) in the Canadian baseline study (Canadian House Dust Study preliminary data; Kubwabo et al., manuscripts in preparation, Environmental Health Science and Research Bureau, Health Canada; unreferenced, dated December 13, 2013), measured in various Canadian cities, was selected for deriving upper-bounding estimates of daily intake for dust exposure.
n No monitoring data of soil in North America were identified. A mean concentration from a German soil study of 1.23x10-3 mg/kg was selected for deriving upper-bounding estimates of daily intake for soil exposure.
Route of exposure |
0–6 moa (breast milk-fed)b | 0–6 mo (formula fed)c | 0.5–4 yrd | 5–11 yre | 12–19 yrf |
20–59 yrg | ≥60 yrh |
|---|---|---|---|---|---|---|---|
Ambient airi |
4.2E-05 |
4.2E-05 |
9.1E-05 |
7.1E-05 |
4.0E-05 |
3.5E-05 |
3.0E-05 |
Indoor airj |
1.4E-03 |
1.4E-03 |
2.9E-03 |
2.2E-03 |
1.3E-03 |
1.1E-03 |
9.6E-04 |
Drinking waterk |
N/A |
1.5E-01 |
6.5E-02 |
5.1E-02 |
2.9E-02 |
3.0E-02 |
3.2E-02 |
Foodl |
1.8E-02 |
NI |
2.9E-02 |
2.4E-02 |
1.3E-02 |
1.3E-02 |
8.2E-03 |
Dustm |
7.0E-01 |
7.0E-01 |
3.7E-01 |
1.4E-01 |
5.2E-03 |
4.9E-03 |
4.8E-03 |
Soiln |
N/A |
N/A |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
Total intake |
7.2E-01 |
8.6E-01 |
4.6E-01 |
2.2E-01 |
4.9E-02 |
4.9E-02 |
4.6E-02 |
Abbreviations: N/A, not applicable; NI, data not identified in the literature; mo, months; yr, years.
a Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day (Health Canada 1998), and to ingest 38 and 0 mg of dust and soil per day, respectively (Wilson et al. 2013).
b Exclusively for breast milk-fed infants, assumed to consume 0.742 L of breast milk per day (Health Canada 1998), and breast milk is assumed to be the only dietary source. The concentration for whole (breast) milk of 0.186 µg/L was based on a reported TCPP of 5.3 ng/g lipid x 3.4% (lipid content of breast milk) x 1.03 g/mL (density of breast milk) identified in 90 samples of human breast milk collected in 2006 from subjects from Sweden (Sundkvist et al. 2010).
c Exclusively for formula-fed infants, assumed to drink 0.8 L of water per day (Health Canada 1998), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day, to consume 54.7 g of fish per day (Health Canada 1998), and to ingest 41 and 14 mg of dust and soil per day, respectively (Wilson et al. 2013).
e Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day, to consume 89.8 g of fish per day (Health Canada 1998), and to ingest 31 and 21 mg of dust and soil per day, respectively (Wilson et al. 2013).
f Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day, to consume 97.3 g of fish per day (Health Canada 1998), and to ingest 2.2 and 1.4 mg of dust and soil per day, respectively (Wilson et al. 2013).
g Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day, to consume 111.7 g of fish per day (Health Canada 1998), and to ingest 2.5 and 1.6 mg of dust and soil per day, respectively (Wilson et al. 2013).
h Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day, to consume 72.9 g of fish per day (Health Canada 1998), and to ingest 2.5 and 1.5 mg of dust and soil per day, respectively (Wilson et al. 2013).
i The highest concentration of TDCPP in outdoor air, (1.21 ng/m3, from Toronto, ON (Shoeib et al. 2014) was used for deriving upper-bounding estimates of daily intake for ambient air exposure. Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
j An indoor air concentration from Toronto of 5.5 ng/m3 (Vykoukalová et al. 2017) was selected for deriving upper-bounding estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
k The maximum concentration of TDCPP (1437 ng/L) in water from tributaries of urban and rural areas to Lake Ontario (Jantunen et al. 2013b) was selected for deriving upper-bounding estimates of daily intake for drinking water exposure.
l No monitoring data on marketed foods in Canada were identified; however environmental fish data in Europe were available. The TDCPP concentration for whole fish of 8.1 µg/kg wet weight (based on a reported maximum TDCPP concentration of 192 µg/kg lipid x 5.73% lipid content) (n= 23) of Atlantic cod, Polar cod and Arctic char collected in 2008 in Norway (Evenset et al. 2009) was selected for deriving upper-bounding estimates of daily exposure to TDCPP from all fish-related food items in the fish food group. Amounts of foods consumed on a daily basis by each age group over 12 food groups were obtained from the 1970–1972 Nutrition Canada Survey (Health Canada 1998).
m The maximum concentration of TDCPP (139 mg/kg) in the Canadian baseline study (Canadian House Dust Study preliminary data; Kubwabo et al., manuscripts in preparation, Environmental Health Science and Research Bureau, Health Canada; unreferenced, dated December 13, 2013), measured in various Canadian cities, was selected for deriving upper-bounding estimates of daily intake for dust exposure.
n No monitoring data of soil in North America were identified. The detection limit (LOD) (9 x10-5 mg/kg) from a German soil study was selected for deriving upper-bounding estimates of daily intake for soil exposure. This is considered a conservative upper bound to account for the reported samples all being below the LOD.
Appendix D: Exposure estimates of TCPP and TDCPP from manufactured items
Based on the available information, dermal exposure uptakes were estimated for contact with foam-containing mattresses and furniture for infants, toddlers, and adults. Dermal exposure to other age groups (i.e., children aged 5-18 years old) would fall within the range of these populations. Oral exposure estimates were also derived for infants and toddlers from mouthing (sucking) on foam-containing manufactured items intended for children. The exposure parameters and values used to estimate exposures are presented in Tables D1 and D2, and are based on conservative assumptions.
Dermal exposure uptake estimates
Uptake = [SA × SCF × TPF × M × ED × DA] / BW
Symbol |
Description |
Value |
|---|---|---|
SAa |
Surface area of skin contact |
545 - 1840 cm2 (Infant) 792 - 2890 cm2 (Toddler) 2033 - 9100 cm2 (Adult) |
SCFb |
Skin contact factor |
1 |
TPFc |
Textile penetration factor |
0.1 (TCPP) |
Md
|
Migration rate |
4.6 × 10-3 mg/cm2/hr (TCPP) 5.6 × 10-5 mg/cm2/hr (TDCPP) |
EDe |
Exposure duration |
12 hr/d (Infant) 12 hr/d (Toddler) 8 hr/d (Adult) |
DA |
Dermal absorption |
40% (TCPP)f 30% (TDCPP)g |
BWh |
Body weight |
7.5 kg (Infant) 15.5 kg (Toddler) 70.9 kg (Adult) |
Uptake |
TCPP Uptake (mg/kg-bw/day)
|
0.16 – 0.54 (Infant) 0.11 – 0.41 (Toddler) 0.04 – 0.19 (Adult) |
Uptake |
TDCPP Uptake (mg/kg-bw/day)
|
1.5 × 10-2 – 5.0 × 10-2 (Infant) 1.0 × 10-2 – 3.8 × 10-2 (Toddler) 3.8 × 10-3 – 1.7 × 10-2 (Adult) |
a For this scenario, a range in surface areas (SA) were used to represent dermal contact with a mattress. For the lower SA used, it is assumed that an individual is wearing shorts and a t-shirt that cover half of the limbs. The surface area of exposure is based on exposure to a fraction of the lower half of the limbs (arms and legs) and the back of the head. The surface areas of the limbs (Health Canada 1995) were multiplied by one half to account for clothing coverage and then were multiplied by one third to account for the triangular shape of limbs, where only one side is directly in contact with the mattress (US CPSC 2006a). The surface area of the head (Health Canada 1995) was multiplied by a factor of 0.5 to represent exposure to the back of the head only. For the higher SA used, it was assumed that half of the body was in dermal contact with the mattress (US EPA 2012b).
b No TCPP- or TDCPP-specific skin contact factor (SCF), i.e. the fraction of substance on a surface adhering to skin, was identified in the literature. As such, a value of 1 was selected to assume that all of the chemical in contact with the skin is available for absorption.
c A textile penetration factor (TPF) was applied for TCPP only to account for the migration rate of TCPP being determined using uncovered foam (EU RAR 2008a). No TCPP-specific textile penetration data was identified in the literature. As such, a value of 0.1 (Driver et al. 2007) was used for the TPF.
d The migration rates of 4.6 × 10-3 mg/cm2/hr for TCPP (uncovered foam) and 5.6 × 10-5 mg/cm2/hr for TDCPP (covered foam) used to estimate dermal exposures are based on migration studies of treated furniture foam conducted by TNO Quality of Life (EU RAR 2008a) and the U.S. CPSC (US CPSC 2005b), respectively. In the TNO Quality of Life (2005) study, filter papers wetted with artificial sweat were placed on top of an uncovered foam block containing 10% w/w TCPP and the foam was compressed for 2 hours; this resulted in a migration rate of 4.6 × 10-3 mg/cm2/hr. The US CPSC study built a furniture miniseat mock-up consisting of a block of foam covered with cotton-polyester fabric and attached to plywood. The miniseat was wetted with a saline solution, to mimic sweat, and pressure was applied to imitate the action of lying down. The migration rate of 5.6 × 10-5 mg/cm2/hr for TDCPP was determined based on the reported maximum daily amount extracted (8 µg) onto the filter (5.5 cm diameter) over the course of the migration testing period (6 hours) (US CPSC 2005b).
e Exposure durations for sleeping were adjusted from durations used by the US CPSC (2006a) for leisure sitting to account for longer sleeping durations relative to sitting. Consideration was also given to the exposure durations used by other jurisdictions for similar exposure scenarios (i.e., sleeping on a mattress treated with flame retardants) (US CPSC 2006b; ECHA 2018).
f EU RAR 2008a.
g EU RAR 2008b.
h Health Canada (1998).
Oral exposure intake estimates
Intakea = [SA × M × ED] / BW
Symbol |
Description |
Value |
|---|---|---|
SAb |
Surface area of direct mouthing |
10 cm2 (Infant) 20 cm2 (Toddler) |
Mc |
Migration rate |
1.78 × 10-2 mg/cm2/hr (TCPP) 2.97 × 10-3 mg/cm2/hr (TDCPP) |
EDd |
Exposure duration |
24.5 min/d |
BWe |
Body weight |
7.5 kg (Infant) 15.5 kg (Toddler) |
Intake |
Intake of TCPP calculated in mg/kg-bw/day |
9.7 × 10-3 (Infant) 9.4 × 10-3 (Toddler) |
Intake |
Intake of TDCPP calculated in mg/kg-bw/day |
1.6 × 10-3 (Infant) 1.6 × 10-3 (Toddler) |
a It is assumed that TCPP and TDCPP are completely absorbed through the oral route and that a textile covering on a foam object would not affect migration (i.e., no textile penetration factor, TPF, applied).
b Surface area for infants is based on multiple references (RIVM 2008). Surface area for toddlers is based on professional judgment reflecting twice the surface area of the opening of a toddler’s mouth.
c Migration rates of 1.78 × 10-2 mg/cm2/hr for TCPP and 2.97 × 10-3 mg/cm2/hr for TDCPP were used to estimate oral exposure. The Danish EPA determined the migration rates of TCPP and TDCPP from children’s products (i.e. car safety seats, baby slings, baby mattresses) by submerging pieces of foam from these products (usually with some of the fabric covering included in the samples) in sweat simulant and incubating them at 37°C for 3 hours (Danish EPA 2015). The particular values used here are the average of the rates found across all samples for each flame retardant (ECHA 2018). These migration rates are considered to be more representative of migration during mouthing than the surface migration studies used in dermal exposure estimates.
d The mouthing duration for children’s foam products such as nap mats, child restraint seats and small furniture was based on the duration for “other objects” in Norris and Smith (2002) [cited in US EPA (2011)].
e Health Canada (1998).
Appendix E: Exposure estimates of TCPP from products available to consumers
Exposure from polyurethane spray foam (PUF) products
Direct skin contact with PUF used in insulation spray and sealants can result in dermal exposure to TCPP. Inhalation exposure may also occur during application of the product from TCPP adhering to dust particles in the air. Both small-scale (sealants) and large-scale (insulation) products were considered based on confirmation of this use in Canada (ECCC 2013-2014). The exposure event for an adult using PUF products is not expected to occur frequently (likely every 5 years, RIVM 2007), and thus was estimated on a per event basis. The exposure estimates presented below are based on conservative assumptions.
Intake = [SA × FT × ρ × WF × DA] / BW
Symbol |
Description |
Value |
Reference |
|---|---|---|---|
SA |
Surface area of finger tips (cm2) |
10 |
Versar handbook thin film, instant application scenario (Westat 1987) |
FT |
Thickness of oil film on hand (cm) |
1.59×10-2 |
Versar handbook thin film, instant application scenario (Westat 1987) |
r |
Density of product (g/cm3) |
0.027 |
Versar handbook thin film, instant application scenario (Westat 1987) |
WF |
TCPP Weight fraction |
30% |
SDS 2015a; ; SDS 2015b; SDS 2017 |
DA |
Dermal absorption |
40% |
EU RAR 2008a |
BW |
Body weight |
70.9 kg (Adult) |
Health Canada 1998 |
Intake |
Intake (µg/kg-bw/event) |
6.8 |
- |
Intake = SA × PA × WF × DA / BW
Symbol |
Description |
Value |
Reference |
|---|---|---|---|
SA |
Surface area of back of hands and forearms (cm2) |
2185 |
Health Canada 1998 |
PA |
Product amount on skin (g) |
0.25 |
RIVM 2007 (insulation foams) |
WF |
TCPP Weight fraction |
0.45 |
SDS 2015ca ; |
DA |
Dermal absorption |
40% |
EU RAR 2008a |
BW |
Body weight |
70.9 kg (Adult) |
Health Canada 1998 |
Intake |
Intake (µg/kg-bw/event) |
635 |
- |
a Note that this product may only be available to professionals but was selected as it had the highest concentration identified and is considered a conservative approach.
Description |
Value |
Reference |
|---|---|---|
Room volume (m3) |
20 |
RIVM 2014 (unspecified room) |
Air exchange rate (/hr) |
0.6 |
RIVM 2014 (unspecified room) |
Exposure duration (min) |
30 |
RIVM 2007 (joint sealant) |
TCPP Weight fraction |
30% |
SDS 2015a; SDS 2015b; SDS 2017 |
Product amount (g) |
90a |
SDS 2017, RIVM 2007 |
Inhalation rate (m3/day) |
16.2 |
Health Canada 1998 |
Mean event concentration (µg/m3) |
180 |
ConsExpo Web 2016 |
a Adjusted product amount of 75 g (RIVM 2007) to reflect that some products come in larger sizes (e.g., 12 oz. for SDS 2017).
Description |
Value |
Reference |
|---|---|---|
Measured Air Concentration (Range; µg/m3) |
477 to 2 940 |
ACC 2012 |
Exposure duration (min) |
30 |
RIVM 2007 (insulation foams) |
Body Weight (Adult; kg) |
70.9 |
Health Canada 1998 |
Inhalation rate (m3/day) |
16.2 |
Health Canada 1998 |
Intake (µg/kg-bw/event) |
2.3 to 14 |
- |
Exposure from waterproofing spray
The exposure event for an adult using a waterproofing spray is not expected to occur frequently (likely once a year), and thus was estimated on a per event basis. Given that this scenario would take place outdoors (SDS 2014) resulting in a large air exchange rate, minimal inhalation exposure is expected. Direct skin contact with waterproofing spray can result in dermal exposure to TCPP. The dermal uptake estimates presented below are based on conservative assumptions.
Intake = [SA × PA × WF × DA] / BW
Symbol |
Description |
Value |
Reference |
|---|---|---|---|
SA |
Surface area of back of hands (cm2) |
455 |
Health Canada 1998 |
PA |
Product amount on skin (g) |
0.25 |
RIVM 2012 |
WF |
TCPP Weight fraction |
0.13 |
SDS 2014 |
DA |
Dermal absorption |
40% |
EU RAR 2008a |
BW |
Body weight |
70.9 kg (Adult) |
Health Canada 1998 |
Dermal load |
Dermal load (µg/kg-bw/event) |
4.7 |
- |
Uptake |
Uptake (µg/kg-bw/event) |
1.8 |
- |
Appendix F: TDCPP intake estimate from urinary BDCPP biomonitoring reverse dosimetry
Reverse dosimetry was used to derive estimates of daily intakes from urine concentrations for adults, pregnant women, toddlers (aged 2–5 yrs), children (aged 6 to 11 yrs), and teens (aged 12 to 19 yrs). The urine concentrations from the literature that were used to calculate intakes are presented in Section 8.1.3, with the maximum or 95th percentile concentrations for each age group shown in Table F1. All other parameters have been previously discussed and are also presented below. Daily intakes are calculated for reverse dosimetry as shown in the equation below.
Daily Intake = [[Urine] × Vurine OR CER × MWR] / [BW × FUE]
Symbol |
Description |
Value |
|---|---|---|
[Urine]SG |
Maximum urinary concentrations of metabolite corrected for specific gravity (ng/mL) |
251 (Toddlers)a |
[Urine] |
Maximum unadjusted urinary concentration of metabolite (ng/mL) |
1.77 (Pregnant women)b |
[Urine] |
95th Percentile unadjusted urinary concentration of metabolite (ng/mL) |
4.32 (Premenopausal women)c |
[Urine]CR |
95th Percentile urinary concentrations of metabolite creatinine corrected (µg/g) |
14.8 (Children)d 6.82 (Teens)d 4.33 (Adults)d |
Vurine |
Total daily urine volume (L/d) |
2.7 (Pregnant women)e 2.03 (Adult women)f 0.7 (Toddlers)g |
CERh |
Daily creatinine excretion rate (g Cr/day) |
0.65 (Children) 1.4 (Teens) 1.7 (Adults) |
BWi |
Body weight (kg) |
70.9 (Adult) 31.0 (Children) 59.4 (Teens) 15.5 (Toddlers) |
FUEj |
Fractional urine excretion (based on rat toxicokinetic study) | 21% (common to all age groups) |
MWR |
Molecular weight ratio between parent and metabolite, i.e. TDCPP and BDCPP |
1.34 (common to all age groups) |
Intake |
Intake (µg/kg-bw/day) |
0.43 (Pregnant women) 0.79 (Premenopausal women) 72 (Toddlers) 2.0 (Children) 1.0 (Teens) 0.66 (Adults) |
a Toddlers (n=23) in this study were from New Jersey, U.S., and were between 1–5 years of age (Butt et al. 2014).
b Pregnant females (n=24) in this study were from Hamilton, ON, Canada (Kosarac et al. 2016).
c Premenopausal females (n=44) in this study were from Toronto and Ottawa, ON, Canada (Yang et al. 2019).
d Total of 2666 samples, for children (n=421), teens (n=427) and adults (n=1818) from across the U.S. from the 2013-2014 NHANES data (Ospina et al. 2018). Data from NHANES is considered to be nationally representative.
e Mean total daily urinary void volumes were reported to range from 0.8–2.7 L/d for pregnant women (Davison and Nobel 1981; Francis 1960; Higby et al. 1994; Neithardt et al. 2002; Parboosingh and Doig 1973; Thorp et al. 1999). The upper bound value of 2.7 L/d was selected for conservatism for reverse dosimetry.
f Mean total daily urinary void volumes were reported to range from 0.6–2.03 L/d for adult women (Addis and Watanabe 1961; Davison and Nobel 1981; Francis 1960; ICRP 2003; Lakind and Naiman 2008; Lentner 1981; Parboosingh and Doig 1973; Perucca et al. 2007; Revúsová 1971; Wu 2006). The upper bound value of 2.03 L/d was selected for conservatism for reverse dosimetry.
g Mean total daily urinary void volumes were reported to range from 0.45–0.7 L/d for toddlers (3–5 yrs) (ICRP 2003; Lentner 1981; Wu 2006). The upper bound value of 0.7 L/d was selected for conservatism for reverse dosimetry.
h High end values from ICRP 2003.
i Health Canada 1998.
j Based on toxicokinetic studies conducted in rats, showing a recovery of TDCPP radioactivity of approximately 35% in the urine (estimated from figure in reference) 24 hours after administration (Minegishi et al. 1988), and a 60% urine recovery of metabolite BDCPP when TDCPP was administered to rats intravenously (Nomeir et al. 1981; Lynn et a. 1980, 1981). The FUE was calculated by determining the % BDCPP in urine to the original TDCPP dose, i.e., (0.35 moles total radiolabel in urine/ mole TDCPP administered radiolabel) × (0.6 moles of BDCPP in urine/moles total radiolabel in urine).
Appendix G: A summary of reproductive and developmental effects of experimental animals treated with TCPP, TCEP and TDCPP
Detailed information on TCPP and TDCPP is presented in other sections of this screening assessment report. Information on TCEP was obtained from the screening assessment for the Challenge report on TCEP (Environment Canada, Health Canada 2009).
TCPP
The major effects observed were decrease in uterus weights and effects on oestrus cycle in parental females and decrease in organ weights and terminal body weights in parental males. No other reproductive effects were observed. There was a significant increase in the number of runts born in both F1 and F2 generations, with no other abnormalities observed.
TCEP
Testicular toxicity was observed in male mice and rats in a number of studies via the oral route and the inhalation route. Decreased number of live pups per litter and decreased numbers of litters were observed in mice. In female mice, no effects on estrous cycle or cyclicity were observed. When pregnant rats and mice were treated with TCEP during gestation, no development toxicity or teratogenicity was observed.
TDCPP
No male reproductive effects were observed in rabbits. There is a data gap for the female reproductive health endpoint as no studies were identified. No developmental toxicity effect or neurodevelopmental toxicity effects were observed in pups at dose levels below which maternal toxicity was observed in pregnant rats that were treated with TDCPP during gestation.
Based on animal toxicity studies, it was found that treatment of TCPP, TCEP and TDCPP do not exhibit similar reproductive and developmental health effects.
Appendix H: Benchmark dose (BMD) modelling and identification of a point of departure for TDCPP cancer risk characterization
General Methodology
The US EPA Benchmark Dose Software (BMDS2.4) (US EPA 2014) was used to calculate the benchmark dose (BMD) and the corresponding lower limit of a one-sided 95% confidence interval (BMDL) for characterization of the cancer risk associated with chronic exposure to TDCPP. The BMD approach, which includes dose-response modelling, provides a quantitative alternative to the dose-response assessment which first defines the point of departure (POD), and then extrapolates the POD for relevance to human exposure. A dichotomous restricted model type is chosen for the BMD and BMDL analysis. A benchmark response of 10% extra risk above predicted background response for dichotomous data is chosen because 10% is at or near the limit of sensitivity in most cancer bioassays. In animal cancer studies, BMD10 refers to a dose of a substance that produces a 10% increase in the response rate of tumour relative to the background response rate of this tumour. BMDL10 refers to a lower one-sided 95% confidence limit on the corresponding benchmark dose (BMD10). BMD10 and BMDL10 levels are calculated for each tumour dataset from the nine models and a model is selected on the basis of best fit (see details in model section). The parameter of “restrict slope >=1” is applied. Then, the lowest BMDL10/BMD10 from various tumour types is chosen as a reasonable conservative estimate for subsequent risk characterization. For derivation of a BMD and BMDL for TDCPP, nine models were applied for analysis of each tumour type (described in Table H1) reported in the Stauffer Chemical Co. (1981a) study. These models included Gamma, Logistic, LogLogistic, LogProbit, Multistage, Multistage-Cancer, Probit, Weibull and Quantal-Linear (see Table H2).
Model Selection
The best-fit model is selected from nine models for each tumour type generally based on the highest P-value of goodness of fit; and the lowest Akaike's Information Criterion (AIC) value (a measure of information loss from a dose-response model that can be used to compare a set of models). A fit was judged adequate based on the goodness-of-fit P-value, scaled residual closest to the BMR (10% extra risk) and visual inspection of the model fit. A goodness-of-fit P-value > 0.1 and an absolute value of scaled residual of interest (SRI); represents observed minus predicted response divided by standard errors) <2, is considered to be indicative of an acceptable fit. If the models for a given tumour type were not accepted (e.g., P-values < 0.1), then the results from the high -dose group were omitted and remodelled.
The results for BMD10 and BMDL10 estimation (mg/kg-bw/day) for tumours induced by TDCPP in the Stauffer Chemical Co. (1981a) study are shown in Table H2.
Treatment dose (mg/kg-bw/day) |
0 |
5 |
20 |
80 |
|---|---|---|---|---|
Renal cortical adenoma, male |
1/45 |
3/49 |
9/48* |
32/46* |
Testes interstitial cell tumour, male |
7/43 |
8/48 |
23/47* |
36/45* |
Hepatocellular adenomas, male |
2/45 |
7/48 |
1/48 |
13/46* |
Renal cortical adenoma, female |
0/49 |
1/48 |
8/48* |
29/50* |
Adrenal cortical adenomas, female |
8/48 |
5/27 |
2/33 |
19/49* |
Hepatocellular adenomas, female |
1/49 |
1/47 |
4/46 |
8/50* |
*Statistically significantly different from control animals (p<0.05)
| Tumours | Model | Number of groups | AIC | P-value | SRI | BMR | BMD10 (mg/kg-bw/day) | BMDL10 (mg/kg-bw/day) |
|---|---|---|---|---|---|---|---|---|
| Renal cortical adenoma, male | Multistage 2 | 4 | 141.65 | 0.98 | 0.019 | 0.1 | 12.24 | 6.84 |
| Testes interstitial cell tumour, male | LogProbit | 4 | 197.24 | 0.436 | -0.364 | 0.1 | 9.07 | 6.74 |
| Hepatocellular adenomas, male | Multistage 3 | 4 | 131.24 | 0.037 | 0.048 | 0.1 | 59.64 | 33.87 |
| Renal cortical adenoma, female | LogLogistic | 4 | 125.06 | 0.972 | 0.149 | 0.1 | 13.87 | 8.29 |
| Adrenal cortical adenomas, female | Gamma | 4 | 156.47 | 0.289 | 0 | 0.1 | 66.45 | 27.89 |
| Hepatocellular adenomas, female | LogLogistic | 4 | 95.21 | 0.724 | 0.66 | 0.1 | 47.95 | 26.52 |
Abbreviations: AIC, Akaike’s Information Criterion; BMR, benchmark response; bw/day, body weight per day; SRI, scaled residual of interest
Appendix I: Lifetime average daily dose calculation
For the purpose of estimating the risk of cancer from exposure to TDCPP, a lifetime average daily dose (LADD) from environmental media and food and from dermal exposure to foam-containing furniture and mattresses was calculated using the following equation (Health Canada 2013):
LADD = exposure rate x exposure duration / Lifetime
where,
Exposure rate = daily intake in mg/kg bw/day
Exposure duration = exposure duration during lifestage (years)
Lifetime = years in a lifetime = 80 years
The estimates of daily intake from environmental media and food are shown in Table I-1. The low and high end estimates of systemic exposure to TDCPP from dermal exposure to foam-containing furniture or mattresses are shown in Table I-2.
Route of exposure |
0–6 moa (breast milk-fed)b | 0–6 mo (formula fed)c | 0.5–4 yrd | 5–11 yre | 12–19 yrf |
20–59 yrg | ≥60 yrh |
|---|---|---|---|---|---|---|---|
Ambient airi |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
Indoor airj |
1.3E-04 |
1.3E-04 |
2.8E-04 |
2.2E-04 |
1.2E-04 |
1.1E-04 |
9.1E-05 |
Drinking waterk |
N/A |
1.4E-03 |
5.9E-04 |
4.6E-04 |
2.6E-04 |
2.8E-04 |
2.9E-04 |
Foodl |
1.5E-02 |
NI |
2.9E-02 |
2.4E-02 |
1.3E-02 |
1.3E-02 |
8.2E-03 |
Dustm |
1.6E-02 |
1.6E-02 |
8.2E-03 |
3.1E-03 |
1.1E-04 |
1.1E-04 |
1.1E-04 |
Soiln |
N/A |
N/A |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
Total intake |
3.1E-02 |
1.7E-02 |
3.8E-02 |
2.7E-02 |
1.4E-02 |
1.3E-02 |
8.7E-03 |
Abbreviations: N/A, not applicable; NI, data not identified in the literature; mo, months; yr, years.
a Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day (Health Canada 1998), and to ingest 38 and 0 mg of dust and soil per day, respectively (Wilson et al. 2013).
b Exclusively for breast milk-fed infants, assumed to consume 0.742 L of breast milk per day (Health Canada 1998), and breast milk is assumed to be the only dietary source. The concentration for whole (breast) milk of o.151 µg/L was based on a reported mean TCPP of 4.3 ng/g lipid x 3.4% (lipid content of breast milk) x 1.03 g/mL (density of breast milk) identified in human breast milk collected in 2006 from subjects from Sweden (Sundkvist et al. 2010).
c Exclusively for formula-fed infants, assumed to drink 0.8 L of water per day (Health Canada 1998), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day, to consume 54.7 g of fish per day (Health Canada 1998), and to ingest 41 and 14 mg of dust and soil per day, respectively (Wilson et al. 2013).
e Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day, to consume 89.8 g of fish per day (Health Canada 1998), and to ingest 31 and 21 mg of dust and soil per day, respectively (Wilson et al. 2013).
f Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day, to consume 97.3 g of fish per day (Health Canada 1998), and to ingest 2.2 and 1.4 mg of dust and soil per day, respectively (Wilson et al. 2013).
g Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day, to consume 111.7 g of fish per day (Health Canada 1998), and to ingest 2.5 and 1.6 mg of dust and soil per day, respectively (Wilson et al. 2013).
h Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day, to consume 72.9 g of fish per day (Health Canada 1998), and to ingest 2.5 and 1.5 mg of dust and soil per day, respectively (Wilson et al. 2013).
i The mean concentration of TDCPP in outdoor air, (0.158 ng/m3, from Toronto, ON (Shoeib et al. 2014) was used for deriving upper-bounding estimates of daily intake for ambient air exposure. Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
j An indoor air mean concentration from Toronto of 0.525 ng/m3 (Vykoukalová et al. 2017) was selected for deriving mean estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
k The mean concentration of TDCPP (1.3E-02 µg/L) in water from tributaries of urban and rural areas to Lake Ontario (Shoeib et al. 2014; Jantunen et al. 2013b) was selected for deriving upper-bounding estimates of daily intake for drinking water exposure.
l No monitoring data on marketed foods in Canada were identified; however environmental fish data in Europe were available. The TDCPP concentration for whole fish of 8.1 µg/kg wet weight (based on a reported maximum TDCPP concentration of 192 µg/kg lipid x 5.73% lipid content) (n= 23) of Atlantic cod, Polar cod and Arctic char collected in 2008 in Norway (Evenset et al. 2009) was selected for deriving upper-bounding estimates of daily exposure to TDCPP from all fish-related food items in the fish food group. Amounts of foods consumed on a daily basis by each age group over 12 food groups were obtained from the 1970–1972 Nutrition Canada Survey (Health Canada 1998).
m The mean concentration of TDCPP (3.08 µg/kg) in the Canadian baseline study (Canadian House Dust Study preliminary data; Kubwabo et al., manuscripts in preparation, Environmental Health Science and Research Bureau, Health Canada; unreferenced, dated December 13, 2013), measured in various Canadian cities, was selected for deriving mean estimates of daily intake for dust exposure.
n No monitoring data of soil in North America were identified. The detection limit (LOD) (9 x10-5 mg/kg) from a German soil study was selected for deriving upper-bounding estimates of daily intake for soil exposure.
LADD from environmental media and food = (0.031 × 0.5/80) + (0.038 × 4.5/80) + (0.027 × 7/80) + (0.014 × 8/80) + (0.013 × 40/80) + (0.0087 × 20/80) = 0.0148 µg/kg-bw/day
Age Group |
0–0.5y |
0.5–4y |
5–11y |
12–19y |
20–59y |
≥60–71y |
|---|---|---|---|---|---|---|
Dermal uptake from lying on foam mattressesa (µg/kg-bw/day) |
14.6 – 49.5 |
10.3 – 37.6 |
5.5 – 20.9 |
4.5 – 18.3 |
3.9 – 17.3 |
3.8 – 17.0 |
a Uptake estimated based on the TDCPP adjusted dermal absorption value of 30%, obtained by applying the ratio of the TCPP in vitro absorption values (23% and 40%) to the 15% TDCPP absorption rate (EU RAR 2008b).
LADDlow = (14.6 × 0.5/80) + (10.3 × 4.5/80) + (5.5 × 7/80) + (4.5 × 8/71) + (3.9 × 40/80) + (3.8 × 20/80) = 4.50 µg/kg-bw/day
LADDhigh = (49.5 × 0.5/80) + (37.6 × 4.5/80) + (20.9 × 7/80) + (18.3 × 8/80) + (17.3 × 40/80) + (17.0 × 20/80) = 19.0 µg/kg-bw/day
