Sea ice cycle: chapter 4
Disintegration
After forming, sea ice changes shapes and dimensions, moves, and finally disintegrates. This final step in the sea-ice cycle is caused by various natural phenomena:

Melting
Disintegration of ice takes place primarily through melting. Melting occurs when the temperature of the ice is raised above its freezing point. The heat required to do this comes from two major sources:
- the absorption of the sun's radiation by the ice, and
- the conduction of heat from the surrounding air, water or land.
Solar Radiation
The incoming solar radiation is partially absorbed by the surface it strikes and partially reflected. The percentage that is reflected varies with the type of surface; this is called the albedo. The remainder of the incoming solar radiation is absorbed, causing a rise in the temperature of the surface.
| Surface | Albedo value |
|---|---|
| Sea water | 0.05-0.10 |
| Arable land | 0.10-0.25 |
| Snow-free sea ice | 0.30-0.40 |
| Melting snow | 0.40-0.50 |
| Fresh snow | 0.80-0.90 |
As you can see above, sea water absorbs almost all of the solar radiation that strikes it, whereas ice with a fresh snow cover absorbs only a small fraction.
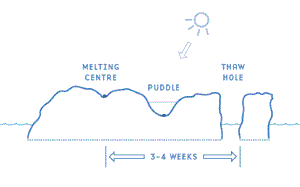
Because of this difference in albedo, dirt, dust, and brine cells on the surface of the ice act as centres of melting and puddles will form around them. Because of the lower albedo of water, these puddles will expand and deepen rapidly. Once there is open water, the water absorbs the radiation and warms up rapidly, so that nearby ice will absorb heat from the water by conduction. Once the disintegration of an ice sheet has proceeded to the point where free water surfaces appear, the rate of further disintegration is very much accelerated.
Conduction
Conduction is the process by which heat is transferred through the movement of particles. When warm air is in contact with the water (puddles), heat is transferred from the air to the water. Heat will be conducted down into the water, which in turn accelerates the melting of ice underneath.
Sea ice melts versus fresh ice melts
In addition to the lowering of the freezing temperature, salt in ice is responsible for other melting phenomena.
Since fresh water ice melts at 0°C, a large block of pure ice will remain solid at any temperature below zero. However, there is always some liquid brine present in salt ice, in what are called brine cells.
As the temperature falls, more and more pure ice is formed leaving a smaller volume of brine at a higher salinity.
Conversely with each temperature increase, the brine melts some of the pure ice around it, and this causes an increase in brine volume but at a lower salinity.
Simultaneous melting and freezing in polar ice
During summer months, the floes of polar ice are melting at the top and freezing below. Puddles form on the surface of the ice in summer, due to melting caused by solar radiation. This fresh water, which has a freezing point of 0°C, eventually seeps down through cracks or hole in the floe or over its sides, and collects around and under the floe. The temperature of the sea water below the floe is slightly below 0°C, and when the melt water comes in contact with it, the fresh water freezes to the bottom of the floe.