American hart’s-tongue fern (Asplenium scolopendrium ): COSEWIC assessment and status report 2016

Special Concern
2016
Table of contents
- Table of contents
- Assessment summary
- Executive summary
- Technical summary
- Preface
- Wildlife species description and significance
- Distribution
- Habitat
- Biology
- Population sizes and trends
- Threats and limiting factors
- 5.3 Logging and wood harvesting
- 3.2 Mining and quarrying
- 1.1 Residential
- 6.1 Recreational activities
- 8.1 Invasive species
- 8.2 Problematic native species
- 5.2 Collection of terrestrialplants
- 1.2 Development of tourism and recreational areas
- 11.1 Habitat shifting and alteration due to climate change
- Number of locations
- Protection, status and ranks
- Acknowledgements and authorities contacted
- Information sources
- Biographical summary of report writer
- Collections examined
List of figures
- Figure 1a. Figure 1a. American Hart's Tongue Fern from Upper Beaver Valley, Ontario. (Photo: © Judith Jones.)
- Figure 1b. Figure 1b. Fertile frond showing sori on the upper half of the underside of the leaf. (Photo: © Judith Jones.)
- Figure 2. Global range of American Hart's-tongue Fern. The Tennessee population was extant in 2011 but no plants were found in 2013. Mexican population in the state of Nuevo Leon not shown.
- Figure 3. Canadian range of American Hart's-tongue Fern. All subpopulations are in the province of Ontario.
List of tables
- Table 1. Extirpated and historical populations of American Hart's-tongue Fern. Changes in rank since 2000 shown in bold type.
- Table 1. Part A. Extirpated subpopulations.
- Table 1. Part B. Historical subpopulations probably not extant (but extirpation unconfirmed)
- Table 2. A comparison of numbers of subpopulations in each viability rank in 2000 and 2013. Percent of total is calculated using the number of subpopulations presumed extant: 109 in 2013 and 74 in 2000. Note: 7 subpopulations ranked H (historical) in 2013 are thought highly likely to be extant.
List of appendices
- Appendix 1.
- Appendix 2. Threats assessment for American Hart’s-tongue Fern
Document information
COSEWIC
Committee on the Status
of Endangered Wildlife
in Canada

COSEPAC
Comité sur la situation
des espèces en péril
au Canada
COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2016. COSEWIC assessment and status report on the American Hart’s-tongue Fern Asplenium scolopendrium var. americanum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 43 pp. (Species at Risk Public Registry website).
Previous report(s):
Austen, M.J.W. 2000. COSEWIC status report on the American hart’s-tongue fern Asplenium scolopendrium var. americanum in Canada, in COSEWIC assessment and status report on the American hart’s-tongue fern Asplenium scolopendrium var. americanum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-42 pp.
Production note:
COSEWIC acknowledges Judith Jones for writing the status report on the American Hart's-tongue Fern, Asplenium scolopendrium var. americanum, in Canada, prepared with the financial support of Environment and Climate Change Canada. This report was overseen and edited by Del Meidinger and Jeannette Whitton, Co-chairs of the COSEWIC Vascular Plants Specialist Subcommittee.
For additional copies contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment and Climate Change Canada
Ottawa, ON
K1A 0H3
Tel.: 819-938-4125
Fax: 819-938-3984
E-mail: COSEWIC E-mail
Website: COSEWIC
Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur la Scolopendre d’Amérique (Asplenium scolopendrium var. americanum) au Canada.
Cover illustration/photo:
American Hart’s-tongue Fern -- Photo by Judith Jones, with permission.
COSEWIC assessment summary
Assessment summary – November 2016
- Common name
- American Hart’s-tongue Fern
- Scientific name
- Asplenium scolopendrium var. americanum
- Status
- Special Concern
- Reason for designation
- This perennial evergreen fern occurs in some deeply shaded Sugar Maple woods on limestone and dolostone habitats of the Niagara Escarpment of southern Ontario. There are many individuals located within many subpopulations; however, they are restricted to a small geographic area, and some subpopulations are very small. Most of the global population occurs in Canada and ongoing threats, such as logging and quarrying, may place the species at heightened risk if the threats are not halted.
- Occurrence
- Ontario
- Status history
- Designated Special Concern in November 2000 and in November 2016.
COSEWIC executive summary
American Hart’s-tongue Fern
Asplenium scolopendrium var. americanum
Wildlife species description and significance
Globally, Hart’s-tongue Fern (Asplenium scolopendrium, sensu lato) is a perennial, evergreen fern that grows as a cluster of strap-shaped fronds (leaves). North American plants are part of variety americanum (herein called American Hart’s-tongue Fern). The Canadian population of American Hart's-tongue Fern accounts for 80% of global occurrences and 94% of global individuals of the variety.
Distribution
American Hart's-tongue Fern occurs in Ontario, upper Michigan, northern New York, Alabama, and Nuevo Leon, Mexico. In northern areas, it is associated with the limestone of the Niagara Escarpment. A population in Tennessee may be extirpated, and the population in Alabama is in serious decline. In Canada, 109 subpopulations are presumed extant. Since the last status assessment in 2000, 28 new subpopulations have been discovered, but all are assumed to have existed prior to 2000. Two subpopulations have been lost since 2000. With new discoveries, the total number of known subpopulations presumed to be extant has increased from 100 to 109.There is now a greater percentage of subpopulations with excellent and excellent or good estimated viability than there was in 2000 although this may be the result of better search efforts. The Canadian population is not severely fragmented.
Habitat
American Hart's-tongue Fern requires a specific microhabitat: moss-covered limestone or dolostone under deciduous trees in deep shade. The species can be found on boulders, blocks, ledges, talus, crevices, or level outcrops, at the top, middle, or bottom of the escarpment. It is most often found on north-facing aspects. The microhabitat is usually found within a rich, deciduous forest, dominated by Sugar Maple, with a highly diverse ground flora and continuous leaf litter. American Hart's-tongue Fern is mostly not found in younger successional forest, degraded forest, or in areas with weedy ground flora. The ferns usually grow in moss on a rock surface with very little protection from drying out. Thus, they are extremely sensitive to any opening of the canopy or other changes that lead to a reduction in humidity in the immediate habitat. American Hart's-tongue Fern sometimes persists after logging if the boulder on which it is growing remains well shaded.
Little information is available on habitat trends because few subpopulations have had more than one site visit. Habitat in protected areas may not have declined, and many other areas still show suitable forest cover on satellite imagery. However, more than half of the subpopulations are on privately owned lands, which may be subject to logging and other types of site alterations. Invasive species are now present at many sites including in some protected areas. Most threats to American Hart's-tongue Fern act by degrading or destroying habitat.
Biology
New fronds are produced at the start of each growing season and can become fertile in the second year. American Hart's-tongue Fern reproduces by spores, which germinate to produce gametophytes. The gametophytes are capable of self-fertilization, which may contribute to establishment of new sporophytes. American Hart's-tongue Ferns have been propagated from spores in the laboratory, but propagated plants transplanted into the wild have not survived the first season. In Canada, mature individuals appear to have been successfully transplanted from one locality to another.
Population size and trends
The size of the Canadian population is estimated to be greater than 110,000 individuals. There are four subpopulations with 10,000 to 20,000 individuals and 17 subpopulations with close to or more than 1,000 individuals. Abundance for the total Canadian population was not calculated in the past, so it is not possible to know if there has been any change. Declines or losses of entire subpopulations have been documented since the mid-1990s at several sites that have been logged, so it is inferred there has been some loss over the last 30 years. There is a small loss projected in the next 10 years from the possible loss of some subpopulations with fewer than 20 individuals.
Threats and limiting factors
Threats to American Hart's-tongue Fern include logging or other opening of the forest canopy; mineral resource extraction operations (quarrying); residential, commercial, and tourism development; recreational activities such as rock climbing and spelunking; invasive species and problematic native species, removal by fern collectors; and possibly increased temperature and reduced humidity from climate change. The number of locations has not been defined but may be very high due to a widely scattered distribution with numerous land owners.
Protection, status, and ranks
American Hart's-tongue Fern is listed as Special Concern on Schedule 1 of the federal Species at Risk Act and on Schedule 5 of the Ontario Endangered Species Act 2007. However, these laws confer no legal protection to special concern species and no critical or significant habitat is required to be protected. At four sites where land is owned by the Ontario Ministry of Natural Resources and Forestry the species is protected by the Crown Forest Sustainability Act, 1994. Nearly half of Canadian subpopulations are entirely on privately owned land and less than a third are in protected areas. The habitat of American Hart's-tongue Fern is considered significant wildlife habitat, and most privately owned lands with American Hart's-tongue Fern are designated areas of natural and scientific interest (ANSIs). Both designations confer some protection under the Ontario Provincial Policy Statement. The most protective designation of the Niagara Escarpment Plan also limits certain types of development in the habitat. Nevertheless, these designations do not prevent landowners from making many types of site alterations that do not require applications or permits. In the U. S., American Hart's-tongue Fern is legally listed Threatened under the federal Endangered Species Act. In Ontario, American Hart's-tongue Fern is ranked S3 or vulnerable.
Technical summary
- Scientific name:
- Asplenium scolopendrium var. americanum
- English name:
- American Hart's-tongue Fern
- French name:
- Scolopendre d’Amérique
- Anishnaabemowin name:
- Waawaashkeshi Denioo Waagaak
- Range of occurrence in Canada:
- Ontario
Demographic information
| Summary items | Information |
|---|---|
Generation time (age of mature plants or age at which plants can become mature) Plants can become fertile in 2nd year; actual average time to fertility is unknown; percentage that are fertile unknown but >50%; some other species of Asplenium may live 30 to 50 years, and many older plants have been observed in Canada; shift towards older demographic reported in NY and MI. |
Unknown but plants may be very long-lived and some very old plants are present |
Is there a continuing decline in number of mature individuals? Declines observed in some subpopulations due to logging and other threats; documented loss of some subpopulations since mid-1990s or before; new information shows a few subpopulations are very large; unknown how climate change will affect species in the future. |
Unknown but inferred small net loss; projected ongoing, long-term loss from logging and other threats. |
| Estimated percent of continuing decline in total number of mature individuals within 5 years | Unknown |
[Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over the last [10 years, or 3 generations] Most sites with abundance data have had only one observation |
Unknown |
| [Projected or suspected] percent [reduction or increase] in total number of mature individuals over the next [10 years, or 3 generations]. | Unknown |
| [Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over any [10 years, or 3 generations] period, over a time period including both the past and the future. | Unknown |
Are the causes of the decline clearly reversible (a) and understood (b) and ceased (c)? Slight declines are inferred and projected |
a. Some threats yes; climate change probably no; b. Partially, yes; c. No |
| Are there extreme fluctuations in number of mature individuals? | No |
Extent and occupancy information
| Summary items | information |
|---|---|
| Estimated extent of occurrence | 10,375 km2 |
| Index of area of occupancy (IAO) | 520 km2 |
Is the population severely fragmented? >50% of its total area of occupancy in habitat patches that are (a) smaller than would be required to support a viable population, and (b) separated from other habitat patches by a distance larger than the species can be expected to disperse? |
No a. No b. Probably yes |
Number of locations 109 subpopulations with many different types of ownership and a highly patchy, discontinuous distribution. Number of locations potentially could be very high. |
Not defined, but possibly greater than 109 because even parts of subpopulations may be locations |
Is there an observed, inferred, or projected continuing decline in extent of occurrence? Observed decline since 1970s but little change since 2000; projected decline if two subpopulations on west side of range (not visited since 1990s) are not extant or if small southernmost subpopulation is lost. Projected loss could be ~38% or greater. Some suitable habitat still apparently present at western subpopulation sites; southernmost site still extant. |
Uncertain |
Is there an observed, inferred, or projected continuing decline in index of area of occupancy? Observed decline of 32 sq. km or ~6% since mid-1990s; inferred loss is greater because some of the 17 sites ranked historical are probably no longer extant; loss projected to continue due to increased demand for development and aggregate extraction. |
Yes--observed, inferred, and projected |
Is there an observed, inferred, or projected continuing decline in number of subpopulations? 8 lost since mid-1990s and of those 2 lost since 2000; other recent losses of portions but not entire subpopulations; 7 subpopulations have <20 plants; projected that some of these could be lost in the next 10 years |
Yes, observed and projected |
Is there an observed, inferred, or projected continuing decline in number of locations? Projected if each subpopulation or patch constitutes a location; projected if southernmost subpopulations are lost. |
Yes, projected (locations not defined but number may be high) |
Is there an observed, inferred, or projected continuing decline in area, extent and/or quality of habitat? Decline in quality observed at some sites due to logging; projected decline due to threats; declines observed even in some protected areas. |
Yes--observed, inferred, and projected |
| Are there extreme fluctuations in number of subpopulations? | No |
| Are there extreme fluctuations in number of locations? | No |
| Are there extreme fluctuations in extent of occurrence? | No |
| Are there extreme fluctuations in index of area of occupancy? | No |
Number of mature individuals (in each subpopulation)
| Population | N clones (index of mature individuals) |
|---|---|
Total Population: 109 subpopulations Unknown what percentage are mature but at least >50% have fronds large enough to produce spores (vegetative reproduction is not known). |
>110,000 individuals |
| Probability of extinction in the wild is at least [20% within 20 years or 5 generations, or 10% within 100 years]. | Not done |
Quantitative analysis
| Summary items | Information |
|---|---|
| Probability of extinction in the wild is at least [20% within 20 years or 5 generations, or 10% within 100 years]. | Not done |
Threats (direct, from highest impact to least, as per IUCN Threats Calculator)
| Summary items | Information |
|---|---|
| Was a threats calculator completed for this species? | Yes (April 2015) |
| 5.3 Logging and wood harvesting | medium impact |
| 3.2 Mining and quarrying | medium impact |
| 1.1 Residential | low impact |
| 6.1 Recreational activities | low impact |
| 8.1 Invasive species | low impact |
| 8.2 Problematic native species | low impact |
| 5.2 Collection of terrestrial plants | negligible impact |
| 1.3 Development of tourism and recreational areas | negligible impact |
| 11.1 Habitat shifting and alteration due to climate change | negligible impact |
| What additional limiting factors are relevant? | Recent studies show genetic isolation and increased temperatures from climate change may be affecting U.S. populations; effects in Canada are unknown at this time. |
Rescue effect (immigration from outside Canada)
| Summary items | Information |
|---|---|
Status of outside population(s) most likely to provide immigrants to Canada. U.S. population overall is listed as Threatened State-listed as Endangered in MI and AL Tennessee possibly extirpated New York: S2 |
Small, declining, or extirpated |
| Is immigration known or possible? | No |
| Would immigrants be adapted to survive in Canada? | Unknown |
| Is there sufficient habitat for immigrants in Canada? | Yes |
Are conditions deteriorating in Canada? Some sites have had recent logging or have invasive species present |
Yes in some places |
Are conditions for the source population deteriorating? U.S. populations are small and declining; climate change may be a factor. |
Unknown but probable |
Is the Canadian population considered to be a sink? 80% of occurrences (subpopulations) and 94% of individuals of var. americanum occur in Canada |
No |
| Is rescue from outside populations likely? | No |
Data-sensitive species
| Summary items | Information |
|---|---|
| Is this a data sensitive species? | No |
Status history
Designated Special Concern in November 2000 and in November 2016.
Status and reasons for designation:
| Summary items | Information |
|---|---|
| Status: | Special Concern |
| Alpha-numeric code: | Not applicable |
| Reasons for designation: | This perennial evergreen fern occurs in some deeply shaded Sugar Maple woods on limestone and dolostone habitats of the Niagara Escarpment of southern Ontario. There are many individuals located within many subpopulations; however, they are restricted to a small geographic area, and some subpopulations are very small. Most of the global population occurs in Canada and ongoing threats, such as logging and quarrying, may place the species at heightened risk if the threats are not halted. |
Applicability of criteria:
| Summary items | Information |
|---|---|
| Criterion A (Decline in total number of mature individuals): |
Not applicable; changes in mature individuals not monitored for subpopulations. |
| Criterion B (Small distribution range and decline or fluctuation): |
Not applicable. Meets Threatened B1 and nearly meets Endangered B2 for size of EOO and IAO, respectively. Observed and projected declines are evident in IAO, number of subpopulations, and area, extent and quality of habitat; projected decline in number of locations. However, there are more than 10 locations, the species is not severely fragmented, and it does not exhibit extreme fluctuations. |
| Criterion C (Small and declining number of mature individuals): |
Not applicable; estimate of mature individuals exceeds thresholds. |
| Criterion D (Very small or restricted population): |
Not applicable; estimate of mature individuals, IAO, and number of locations exceeds thresholds. |
| Criterion E (Quantitative analysis): |
Not applicable. Lack of population data to conduct analysis. |
Preface
American Hart's-tongue Fern (Asplenium scolopendrium var. americanum) was last assessed in 2000. At that time, total abundance was not calculated, and extent of occurrence and area of occupancy were reported only as very broad ranges. As of 2015, most subpopulations still have had only one census. As a result, it is nearly impossible to assess changes in abundance. Since 2000, 28 new subpopulations have been discovered, and a few subpopulations have been split into two or more because they were found to consist of patches more than 1 km apart or had patches mapped incorrectly. Six subpopulations previously listed as historical have been confirmed to be extant. Ten subpopulations have been confirmed as extirpated (although only two of these have been extirpated since 2000). The use of mapping software has also allowed more precise calculation of extent of occurrence and area of occupancy. Information in this updated status report should enable better detection of change in this species in the future.
COSEWIC history
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
COSEWIC mandate
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC membership
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
Definitions (2015)
- Wildlife species
- A species, subspecies, variety, or geographically or genetically distinct population of animal, plant or other organism, other than a bacterium or virus, that is wild by nature and is either native to Canada or has extended its range into Canada without human intervention and has been present in Canada for at least 50 years.
- Extinct (X)
- A wildlife species that no longer exists.
- Extirpated (XT)
- A wildlife species no longer existing in the wild in Canada, but occurring elsewhere.
- Endangered (E)
- A wildlife species facing imminent extirpation or extinction.
- Threatened (T)
- A wildlife species likely to become endangered if limiting factors are not reversed.
-
Special concern (SC)
(Note: Formerly described as “Vulnerable” from 1990 to 1999, or “Rare” prior to 1990.) - A wildlife species that may become a threatened or an endangered species because of a combination of biological characteristics and identified threats.
-
Not at risk (NAR)
(Note: Formerly described as “Not In Any Category”, or “No Designation Required.”) - A wildlife species that has been evaluated and found to be not at risk of extinction given the current circumstances.
-
Data deficient (DD)
(Note: Formerly described as “Indeterminate” from 1994 to 1999 or “ISIBD” [insufficient scientific information on which to base a designation] prior to 1994. Definition of the [DD] category revised in 2006.) - A category that applies when the available information is insufficient (a) to resolve a species’ eligibility for assessment or (b) to permit an assessment of the species’ risk of extinction.
The Canadian Wildlife Service, Environment and Climate Change Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Wildlife species description and significance
Name and classification
- Scientific name:
- Asplenium scolopendrium L.
- Synonyms:
-
Phyllitis scolopendrium (L.) Newman
Phyllitis scolopendrium (L.) Newman var. americanum Fernald
P. fernaldiana A. Löve
P. japonica Komarov var. americana (Fernald) A. Löve & D. Löve
Common names:
- English:
- American Hart's-tongue Fern
- French:
- Scolopendre d’Amérique
- Anishnaabemowin:
- Waawaashkeshi Denioo Waagaak
- Spanish:
- Lengua de Cervina
- Varieties occurring in the wild in Canada:
- Asplenium scolopendrium L. var. americanum (Fernald) Kartesz & Gandhi [Phytologia 70: 196. 1991]
- Family:
- Aspleniaceae (Spleenwort Family)
- Major plant group:
- Pteridophyta, the Ferns and Fern-allies
- Type specimen:
- collected by Fernald, Thompson, and Wright 19 June, 1934 from Inglis Falls, in Grey County, near Owen Sound, Ontario. Deposited in Gray Herbarium (Fernald 1935).
This taxon is listed under the Canadian Species at Risk Act (SARA) at the species level as Asplenium scolopendrium rather than at the varietal level as A. scolopendrium var. americanum, and in English as Hart's-tongue Fern, rather than as American Hart's-tongue Fern. The federal management plan also names the taxon in the same way. However, this COSEWIC status report discusses characteristics of the European and Mesoamerican varieties of the species and compares information about other species of Asplenium with A. scolopendrium var. americanum. To make it clearer exactly which taxa are being discussed, this report uses varietal names throughout unless referring to the species as a whole.
Hart's-tongue Fern is a member of the large, diverse genus Asplenium, which includes 700 species worldwide and 28 species in North America north of Mexico (Wagner et al. 1993). Hart's-tongue Fern was originally assigned to the genus Phyllitis. However, species of Phyllitis were found to hybridize freely with some species of the genus Asplenium, so Phyllitis is now included within Asplenium (Kartesz and Gandhi 1991). The North American var. americanum was separated from the European var. scolopendrium by Fernald (1935) based on the former’s smaller leaves, narrower scales with longer, drawn-out tips, and blades tending to bear the sori in the distal half (towards the tip) rather than over the entire length. Also, the American variety is reported to be tetraploid while the European variety is diploid (Britton 1953). There are also differences in perispore morphology between the varieties (Arreguin-Sanchez and Aguirre-Claveran 1986).
An additional variety, A. scolopendrium var. lindenii (Hooker) Viane, Rasbach, & Reichstein occurs in southern Mexico, in the states of Oaxaca and Chiapas, and in the West Indies (CONABIO 2015). This variety is tetraploid (Viane and Reichenstein 1991) and has a pubescent stipe and rachis (Arreguin-Sanchez and Aguirre-Claveran 1986). Wagner et al. (1993) point out that further work is needed to determine if var. lindenii is actually distinct from var. americanum. If var. lindenii is found to be the same taxon as var. americanum, the global range for American Hart's-tongue Fern could be much bigger than is currently recognized. Synonyms for this variety include:
- Asplenium scolopendrium ssp. japonicum var. lindenii (Hook.) Viane, Rasbach & Reichstein
- Phyllitis japonica Kom.
- Phyllitis lindenii (Hook.) Maxon
- Phyllitis scolopendrium (L.) Newman.
- Scolopendrium lindenii Hook.
Morphological description
Hart's-tongue Fern is a perennial, evergreen fern that grows as clusters of undissected, smooth-margined fronds (leaves) from a short caudex (upright woody stem at or below ground surface) (Figure 1a). The blades of the fronds are oblong to strap-shaped, 8 to 35 cm long by 2.0 to 4.5 cm wide, with a notch at the base where they connect to the stipe (stalk). The stipes are light brown, 3 to 12 cm long, and covered with narrow, curling, cinnamon-coloured scales. The sporangia (spore cases) are clustered into linear sori that are covered by thin, translucent indusia and borne on the backs of the fronds at nearly right-angles to the midrib (Cody and Britton 1989; Wagner et al. 1993) (Figure 1b).

Long description for figure 1a
The image shows clusters of undissected, smooth-margined fronds (leaves) from a short caudex. The blades of the fronds are oblong to strap-shaped, with a notch at the base where they connect to the stipe.

Long description for figure 1b
The image shows the linear sori that occur on the upper half of the underside of the frond, almost at right-angles to the midrib.
American Hart's-tongue Fern is a very distinctive plant and is unlikely to be confused with other North American ferns. However, it may be confused with the European var. scolopendrium, which is sometimes cultivated as an ornamental and has occasionally escaped or been intentionally introduced into the wild. American Hart's-tongue Fern can be distinguished from the European variety by the location of the sori, which in var. americanum are situated in the half of the lamina closest to the tip, rather than spaced over the total length, and by the shape of the scales, all of which are long, narrow, and curling, rather than having some that are flat and more broadly triangular.
Population spatial structure and variability
The Canadian population of American Hart's-tongue Fern consists of numerous, scattered but separated subpopulationsFootnote1 along the exposed limestone of the Niagara Escarpment. Although the Niagara Escarpment is a more or less continuous corridor, the population of American Hart’s-tongue Fern is not continuous throughout due to narrow habitat requirements and also due to extirpation of subpopulations likely from human activities. Several subpopulations are isolated from the next nearest ones by as much as 30 km. Subpopulation size varies greatly from tens of thousands of plants in more than 2 km of linear habitat to fewer than 10 plants growing on a single boulder.
Many subpopulations consist of more than one patch of ferns, and these patches are sometimes separated by several hundred metres. For the purpose of analysis in this report, the standard separation distance of 1 km (NatureServe 2002; NHIC 2015) has been used to define subpopulations (or occurrences). However, it is recognized that a separation distance of 1 km may be meaningless for American Hart's-tongue Fern if, for example, the linear habitat is running north-south, and the 1 km radius includes patches of the ferns on non-contiguous outcrops across farm fields to the east and west. There is also evidence that this species has a very short dispersal distance (Fernando et al. 2015). On the other hand, with more than 250 patches of American Hart's-tongue Fern documented in Canada, the standard 1 km framework is a useful tool for analysis of data and comparison to previous reports. Therefore, the 1 km separation distance has been used in this report despite some limitations.
A genetic study of American Hart's-tongue Fern subpopulations in Michigan and New York (Fernando et al. 2015) found that the genetic diversity of the species in these states was higher than that for other rare ferns and rare plants, but that this diversity was mainly due to the diversity within just one subpopulation and that overall genetic diversity was low. There was no correlation between diversity and subpopulation size, or between diversity and geographic distance, showing that even closely located subpopulations may be genetically isolated. Genetic work has not been done on the Canadian population, so the diversity of the Canadian American Hart's-tongue Fern gene pool is unknown. However, it can be hypothesized that many of the smaller Canadian subpopulations may be similarly genetically isolated with very low or no genetic diversity.
Designatable units
The Canadian population of American Hart's-tongue Fern is considered one designatable unit, occurring in similar habitats with little disjunction over the entire Canadian range. The entire Canadian range falls within the Great Lakes Plains Ecological Area as defined by COSEWIC (2013). The European variety is excluded from the assessment.
Special significance
The vast majority of the global population of American Hart's-tongue Fern occurs in Canada. There are only 28 subpopulations in the U.S (U.S.F.W.S. 2012; Watkins pers. comm. 2013) compared to 109 in Canada, and many U.S. subpopulations are small and in severe decline. It is unknown how many subpopulations there are in Mexico. There are an estimated 6500 American Hart's-tongue Fern individuals in the U.S. (USFWS 2012) compared to the approximately 110,000 present in Canada. Thus, the Canadian population accounts for roughly 80% of North American occurrences and 94% of North American individuals of variety americanum, making the Canadian population of the utmost significance for the persistence of the North American variety of the species.
Although the European variety appears to have been used medicinally for many ailments (Grieve 1971), American Hart's-tongue Fern does not appear to have been used medicinally or for other reasons. This may be due to its relative rarity or to the remoteness of the habitat. Several people were contacted at Cape Croker, Beausoleil, and Wikwemikong First Nations (including both elders and species at risk specialists) as well as many non-Indigenous private landowners with familiarity with the habitat and the escarpment, but no traditional knowledge specific to the American Hart's-tongue Fern was found.
Distribution
Global range
The European variety of Hart's-tongue Fern (A. scolopendrium var. scolopendrium) has a large distribution ranging in the west from Iceland and the British Isles, through Scandinavia to central Asia, and south to the northern part of the Mediterranean (Birks 1976). In North America, American Hart's-tongue Fern (A. scolopendrium var. americanum) occurs naturally in small, widely separated, localized groups of subpopulations. In the northern part of the range, subpopulations are associated with the limestone of the Niagara Escarpment and are found in five counties or regions in southern Ontario, two counties in the upper peninsula of Michigan, and three counties in northern New York (MNFI 2013; NHIC 2013; NYDEC 2013) (Figure 2). A population was introduced in New Jersey in 1936 as a rescue operation from New York (Austen 2000; NatureServe 2015). The current status of this population is unknown and it was not found in a search in 2012 (Snyder pers. comm. 2013). The total extent of occurrence of the northern range from Michigan, Ontario, and New York is approximately 73,000 km2.
Historically, disjunct subpopulations occurred in Alabama and Tennessee associated with limestone caves and sinkholes (NatureServe 2015). However, these subpopulations are now in serious decline or extirpated. In 2012, the Tennessee subpopulation consisted of only two individuals, neither of which was found in 2013 (Watkins pers. comm. 2013). The Alabama subpopulation has one site which has declined from 33 individuals (Tennessee Natural Heritage Program 2012; U.S.F.W.S. 2012) to about half that number (Watkins pers. comm. 2013). Thus, the global range may soon become significantly smaller. A population of American Hart's Tongue Fern (A. scolopendrium var. americanum) in Mexico in the northern the state of Nuevo Leon is presumed extant and is listed in the current catalogue of Mexican pteridophytes (CONABIO 2015). The species is not listed as at risk in Mexico (Biodiversidad Mexicana 2015) and is not on the proposed list of priority species for conservation (CONABIO 2013).
Canadian range
The Canadian range of American Hart's-tongue Fern (Figure 3) falls entirely within Ontario and is restricted to exposed limestone and dolostone of the Niagara Escarpment (see Riley et al. (1996) for maps of the Niagara Escarpment). American Hart's-tongue Fern is found in five counties or regions in Ontario (Bruce, Grey, Simcoe, Dufferin and Halton) and is apparently extirpated from Peel Region. Appendix 1 lists all known Canadian subpopulations with abundance and date of most recent observation. There are 109 subpopulations presumed extant in Canada, the majority of which are found in Grey County. There are 2 subpopulations in Halton Region, 3 in Dufferin County, 6 in Simcoe County, 17 in Bruce County (15 of which are on the Bruce Peninsula north of Wiarton), and 81 in Grey County.
The northernmost Canadian subpopulation is near Clarke’s Corners on the Bruce Peninsula, and the southernmost subpopulation is at Mt. Nemo in Halton Region. The northern and southern extremes of the Canadian range are very similar to those mentioned by Soper (1954) and Austin (2000).
Since 2000, 28 new subpopulations have been discovered, and a few subpopulations have been split into two or more because they were found to consist of patches more than 1 km apart or had patches mapped incorrectly (Jones 2013). Six subpopulations previously listed as historical (not seen in more than 20 years) or extirpated have been confirmed to be extant. Ten subpopulations have been confirmed as extirpated, but eight of these were historical and already not counted as extant in 2000. Four other subpopulations not seen since the 1980s or 1990s are now considered historical and it is unknown if they remain extant. Several other reports (previously considered subpopulations), which had been ranked historical or extirpated, are now part of larger extant subpopulations. Some of these patches are in fact extant, while others remain with status unknown. Information on extirpated and historical subpopulations is given in Table 1.
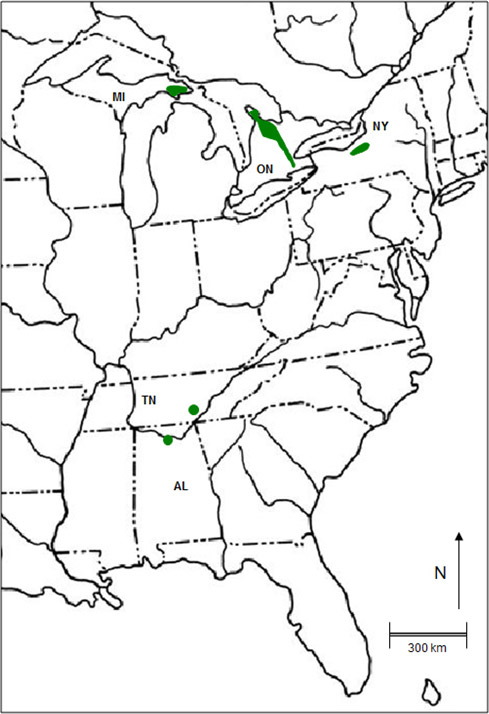
Long description for figure 2
Map of the global range of the American Hart's-tongue Fern, which occurs naturally in small, widely separated, localized groups of subpopulations. In the northern part of the range, subpopulations are associated with the limestone of the Niagara Escarpment and are found in five counties or regions in southern Ontario, two counties in the upper peninsula of Michigan, and three counties in northern New York. Historically, disjunct subpopulations occurred in Alabama and Tennessee associated with limestone caves and sinkholes; however, these subpopulations are now in serious decline or extirpated.
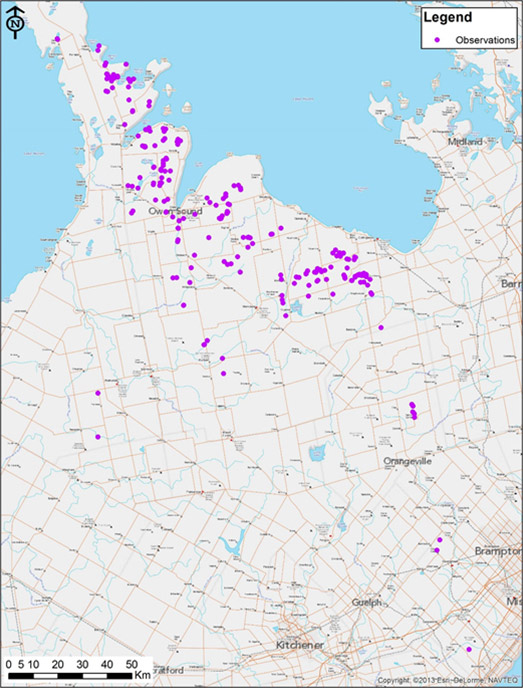
Long description for figure 3
Map of the Canadian range of the American Hart's-tongue Fern, which falls entirely within Ontario and is restricted to exposed limestone and dolostone of the Niagara Escarpment. American Hart's-tongue Fern is found in five counties or regions in Ontario (Bruce, Grey, Simcoe, Dufferin, and Halton) and is apparently extirpated from Peel Region.
Table 1. Extirpated and historical populations of American Hart's-tongue Fern. Changes in rank since 2000 shown in bold type.
| EO ID | Subpopulation name | Last observation | Last checked | EO Rank | Comments |
|---|---|---|---|---|---|
| blank | Red Wing | Specimen 1932-06-12 | No other observation known | X (Extirpated) | Listed by Austen 2000 but no obs data; no obvious suitable habitat present; likely refers to another location. |
| 22663 | 3.2 KM S Barrow Bay Village | 1975 | blank | X | Extirpated by heavy logging in the mid-1970s |
| 22658 | Traverston Creek Forest | 1981 | Not found 2001 | X? | Negative search by Maher, but possibly not in the right area (Brownell pers. comm. 2013). County forest heavily impacted by logging but fern could possibly still be present on private land. |
| 21659 | 7 Miles S Mulock | 1966-07-18 | Not found 2013 | H (Historical)→X | No remaining habitat. |
| 21676 | Inglewood Vicinity =Inglewood Slope |
1958-08-18 | Not found 2013 | H→X | Habitat lost due to quarry and logging |
| 21643 | Credit Forks | 1976-PRE | Not found 2001 by Maher; not found 2013 by Jones |
H→X | 1955 specimen actually said "Caledon". This specimen DID NOT refer to Credit Forks Provincial Park. Appears to come from north of Grange Rd off Caledon Mountain Rd. Negative search 2013 over large area off Grange Rd and near Varga’s escarpment study plots. |
| 21667 | Near Scenic Caves, E Banks | 1937-08-23 | Not found 2001 by Maher; not found 2013 by Jones |
H→X | Not found by Maher but many other ferns found, so location probably valid; negative search in 2013 in tourist area and off Bruce Trail. No suitable habitat seen; area heavily logged. |
| 21683 | Stayner | 1970-09-19 | Not found 2013; no suitable habitat found | H→X | Reznicek (pers. comm. 2013) thinks location is probably erroneous and name was meant to refer to sites just west. |
| 23008 | Milton Heights | 1977 | Not found 2013 | H→X | Habitat degraded and weedy; logging, quarrying |
| blank | Halton Forest North | Specimen 1981 |
Not found in 1995 or 2013 | Extant [no other information] E?→X | Habitat degraded and weedy; past logging |
| 21674 | Mckinney's Hill Includes Nottawasaga Bluffs CA |
1983 | Not found 2013 | H→X | Habitat degraded and weedy; recent logging |
| 21646 | Caledon Mountain Slope Forest | 1993 | Negative but incomplete searches in 2000 and 2013 | D (Poor Viability)→probably X | Presence very unlikely but still remotely possible |
| 22659 | Between boat and spry lake (Bluewater Outdoor Centre) | 1996? | Dates unknown | C (Fair Viability)→X | Never seen by Outdoor Centre staff despite several searches |
| EO ID | Subpopulation name | Last observation | Last checked | EO Rank | Comments |
|---|---|---|---|---|---|
| 21658 | Jones’s Falls | Macoun 1901-09-02? |
No other observation known; "No recent reports" in 2000; never found by Maher | H? | Now part of Pottawatomi CA, but >1 km from northern subpopulations.; this is a popular hiking area, and Maher would certainly have searched here over the years although no negative searches were recorded in his notes from 1998-2004. |
| 21651 | Wiarton area | Specimens 1909 and 1919 | ? | H | Location too vague; could refer to many subopulations; Not useful as an EO name |
| 21662 | Saugeen River | Specimen 1960-05-27 |
No other observation known | H | Location too vague; could refer to many subopulations; Not useful as an EO name |
| 21649 | 1 - 1.5 Miles E Stokes Bay | 1952-08-24 | Location uncertain | H | Location too vague; could also refer to Clarke’s Corners |
| 21661 | Hayward Falls, Rocky Saugeen River |
1962-09-08 | Not found 2001 | H | Unsure whether search was in correct area; unsure if suitable habitat still exists |
| blank | 5 KM S Singhampton | Reznicek 1971-12-21 |
blank | H | Suitable habitat unlikely but possible |
| blank | East Of Edward Lake, Simcoe County |
Reznicek 1976-07-18 |
blank | H | Suitable habitat unlikely but possible |
| 21668 | 1 Mile E Eugenia | Bobbette 1976-05-18 |
No new searches | H | Suitable habitat unlikely but possible |
| 21669 | 3 Miles E Eugenia | Bobbette 1976-05-26 |
No new searches | H | Suitable habitat unlikely but possible |
| 22660 | 6 - 7 KM NE Hepworth | 1980 | No new searches | H | Location vague; could refer to a lot of area; suitable habitat probably still exists |
| 21665 | 3.5 KM W Goring (Not Lily Oak Forest) | 1984 | No new searches | H | Suitable habitat may still exist; location data are mixed up with Lily Oak |
a EO rank legend: C: Fair Viability, D: Poor Viability, E: Extant (no other information), H: Historical, X: Extirpated
If 28 newly discovered subpopulations are added to the 72 that were presumed extant in 2000, the number of known subpopulations has increased slightly (109 now vs. 100 in 2000), showing that the Canadian population is slightly bigger than was previously known. This does not indicate an increase in the size of the Canadian population because the newly discovered subpopulations are presumed to have existed in 2000. In addition, the confirmation of eight historical subpopulations as extirpated does not constitute a new loss because Austen (2000) did not count any historical subpopulations as extant. The timeframe over which these extirpations have occurred is unknown. The last observations for these subpopulations range from 1958 to 1996.
Two subpopulations that were considered extant in 2000 have been confirmed as extirpated (Table 1). One was last seen in 1993 and the other in 1996. Both were listed as consisting of only a few fronds at the last observation. The cause of the extirpation for one is likely habitat degradation as the site was found to be disturbed and weedy in 2013. The cause for the loss of the other occurrence is unknown. Although there are few losses of entire subpopulations, there are partial losses within at least six subpopulations, and that number may be higher because only about a third of sites have been surveyed for more than just presence/absence of the species.
Table 2 shows the number of subpopulations listed by Austen (2000) with viability rankFootnote2, compared with known subpopulations and viability ranks in 2013 (from NHIC 2013). Interestingly, there is now a greater percentage of subpopulations ranked excellent estimated viability (A) and excellent or good estimated viability (AB) than there were in 2000. This may be the result of increased recent search efforts, but nevertheless it shows a better outlook for overall viability than was previously known. The Canadian population of American Hart's-tongue Fern is not considered severely fragmented because more than 50% of subpopulations have habitat patches large enough or number of individuals great enough to have a viability rank of C or better. Still, there are seven small subpopulations documented to have ≤20 individuals. It is projected that some of these subpopulations could be lost in the next ten years.
| Viability rank | Number in 2013 | number in 2000 | % of 2013 total | % of 2000 total |
|---|---|---|---|---|
| A | 18 | 9 | 17 | 12 |
| AB | 13 | 6 | 12 | 8 |
| B | 24 | 15 | 22 | 20 |
| BC | 8 | 10 | 7 | 9 |
| C | 15 | 25 | 14 | 19 |
| CD | 3 | 1 | 3 | 4 |
| D | 6 | 2 | 5 | 7 |
| E | 15 | 6 | 14 | 19 |
| H | 17 | 23 | blank | blank |
| X | 13 | 4 | blank | blank |
| Total | 132 | 101 | blank | blank |
| Presumed extant | 109 | 74 | blank | blank |
b Legend: A: Excellent Viability, B: Good Viability, C: Fair Viability, D: Poor Viability, E: Extant (no other information), H: Historical, X: Extirpated
American Hart's-tongue Fern has not been found on Manitoulin Island, despite much fieldwork (Bell 1870; Soper 1963; Morton and Venn 2000; Jones 2001). There is very little habitat on Manitoulin with suitable deciduous canopy (Jones 2001), perhaps due to an extensive history of fire (Fassett 1933). A subpopulation from the Niagara Falls area, documented by a specimen in 1895, may have been entirely introduced, as it is known that a patch was planted in the gorge sometime before 1882 (Soper 1954). Regardless of the provenance, this subpopulation has not been seen since 1945, despite several searches targeting the species (Soper 1954).
In New Brunswick, a subpopulation first reported in 1881 from a ravine in the Meduxnakeag Valley near Woodstock (Fernald 1935; Hagenah 1954) was later determined to be the European variety of Hart’s-tongue Fern and most likely from an introduction (Hinds 1986; Cody and Britton 1989). Other historical and discounted Canadian records for American Hart's-tongue Fern are detailed in Austen (2000).
Extent of occurrence and area of occupancy
The extent of occurrence (EOO) for American Hart's-tongue Fern in Canada is 10,375 km2. This is calculated by plotting all known reports of American Hart's-tongue Fern and drawing a polygon with no concave sides around the outside of all of them.
On the western side of the range, two disjunct subpopulations occur some 30 km away from the face of the Niagara Escarpment. These subpopulations were last visited in 1994. If the subpopulation near Teeswater is no longer extant, EOO would be 9,652 km2. If neither the Teeswater nor the subpopulation south of Greenock is extant, EOO would be 6,403 km2. Thus, approximately 38 percent of EOO is the result of these two disjunct subpopulations. On the eastern side of the range, a subpopulation was historically reported from Stayner in Simcoe County but has not been seen since 1970 (NHIC 2013). This locality was searched in 2013, but no plants were found. Thus, at some time between 1970 and 2000 (Austen 2000), there was a loss of EOO at the eastern side of the range.
The index of area of occupancy (IAO) for American Hart's-tongue Fern is 520 km2, based on the number of 2 x 2 km squares that are occupied by the species. Austen (2000) reported IAO as <500 km2. New discoveries increase the former IAO, but it is assumed that these subpopulations were actually extant in 2000. Two subpopulations have been extirpated since 2000 (Table 1), representing a loss of IAO of <1%.
Search effort
With 109 subpopulations and more than 250 reported observations (see Appendix 1), it was not possible to visit all known sitesFootnote3 to inform this report. In 2013, 27 sites were surveyed by Jones (2013), but some sites were visited to confirm extirpation. Between 2005 and 2013, more than a third of the 109 subpopulations were visited by Jones (Morton and Jones 2005-2013; Jones 2013). As well, in 2011-2012 she visited more than 50 sites on the Niagara Escarpment (Southern Science and Information Section 2012). Between 2000 and 2004, at least 19 sites were visited by the late fern enthusiast Nels Maher (Maher unpubl. notes; NHIC 2013), and another 42 sites have been visited by other workers since 2000. Roughly half of the subpopulations have been confirmed extant since 2000, but most of these reports give no abundance information. Search effort and area covered vary greatly. Several reports that come from environmental impact studies have high search effort despite reporting only presence/absence.
Of 109 subpopulations, 30 have not been visited since before 2000, and six of these have not been visited since the 1980s. A portion of the Violet Hill/Mono Cliffs North subpopulation that had not been visited since 1973 (NHIC 2013) was still extant in 2013. Several other subpopulations not visited since the 1990s were also extant in 2013 (Jones 2013). Therefore, subpopulations are not presumed extirpated unless searches have been done with an adequate level of effort and no plants have been found, or unless the habitat is severely altered or completely destroyed. Apparently suitable habitat is visible on satellite imagery of the sites of at least seven historical subpopulations. Thus, these are still presumed to be extant.
Habitat
Information in this section is based on observations of Jones (pers. obs. 2013-2016) unless another citation is given.
Habitat requirements
American Hart's-tongue Fern requires a very specific microhabitat and with few exceptions is rarely found in conditions outside very narrow parameters. The species requires moss-covered limestone or dolostone under deciduous trees in deep shade (Cinquemani Kuehn and Leopold 1993; Austen 2000; Stantec 2008; Jones 2013).
The species can be found in a variety of limestone exposures. For example, it is found: on boulders or blocks that have separated from any part of the escarpment; on low limestone walls or ledges exposed on level, mid-slope terraces; in talus of rubble or blocks; in crevices and holes in exposed limestone outcrops; infrequently on the main escarpment wall or cliff face itself; and occasionally away from the escarpment face altogether if there are exposed outcrops or erratic limestone blocks present. In Canada, suitable habitat can be found at the bottom of cliffs, mid-slope, or on the top level of the escarpment. The species requires deep shade, so the ferns are most often found on north-facing aspects unless they are well down in a crevice or on the shaded side of a large block. Unlike the situation in New York (Cinquemani Kuehn and Leopold 1993; Brumbelow pers. comm. 2013), suitable habitat in Canada is not restricted to slopes.
Individuals of American Hart's-tongue Fern are often found growing in a deep layer of moss over a rock surface. Without the moisture-retaining capacity of soil, the ferns have very little protection from drying out. However, the mosses in which the ferns grow are often moist from dew and condensation, which in turn may allow the ferns to stay moist. Presumably, there must be sufficient humidity in the air to supply moisture to the moss so that the ferns may survive periods without precipitation. This requirement makes American Hart's-tongue Fern extremely sensitive to any opening of the canopy or to any other changes that cause increased light, heat, or air movements that lead to a reduction in humidity. Presumably, changes that cause a loss of leaf litter and soil moisture retention may also affect humidity levels. Studies are underway to determine correlations between humidity levels and relative predicted extinction risks for American Hart's-tongue Fern in New York (Brumbelow pers. comm. 2013).
The depth and duration of snow cover is also an important habitat factor for American Hart's-tongue Fern. Snow insulates the upper layers of soil, can prevent frost heaving, and contributes to soil moisture. The depth of snow cover and its persistence into spring months also affect ambient temperatures (Stantec 2008). Occurrences of American Hart's-tongue Fern appear to correlate with microhabitat that has greater and longer duration of snow cover into the spring (Cinquemani Kuehn and Leopold 1992; Austen 2000; Stantec 2008).
The surrounding habitat that supports the specific required microhabitat is a rich, deciduous forest, usually dominated by Sugar Maple (Acer saccharum), with a highly diverse ground flora and continuous leaf litter on the ground. Ground flora typically includes Large-flowered Trillium (Trillium grandiflorum), Blue Cohosh (Caulophyllum thalictroides), Wild Leek (Allium tricoccum), Maidenhair Fern (Adiantum pedatum), and many other species. Wild Ginger (Asarum canadense) is usually present within metres of American Hart's-tongue Fern. Common moss associates found growing on rock surfaces under American Hart's-tongue Fern in 2013 include: Brachythecium salebrosum, Entodon cladorrhizans, Fissidens taxifolius, Anomodon attenuatus, and Tortella tortuosa.
American Hart's-tongue Fern is generally not found in younger successional forest, degraded forest, or with weedy ground flora, but a few exceptions are known. Reznicek (pers. comm. 2015) collected American Hart's-tongue Fern in older successional Balsam Poplar (Populus balsamifera) forest in Simcoe County in 1976 (University of Michigan specimen #1283816). Futyma (1980) reported on this collection, and this paper has been cited in several places as evidence that American Hart's-tongue Fern grows under poplar in Canada. However, this type of habitat is very atypical. None of the Canadian subpopulations observed by Jones grew under Balsam Poplar, and Austen (2000) reported poplar trees only at one atypical site dominated by White Cedar (Thuja occidentalis). Reznicek (pers. comm. 2013) also agrees that typical habitat is mesic maple woods. It is not known if American Hart's-tongue Fern persisted after the collection in 1976 or whether that subpopulation is extant.
Poplar and other earlier successional species usually indicate an opening of the canopy in the recent past, which usually causes a drying out of the habitat and leads to the loss of American Hart's-tongue Fern. The absence of American Hart's-tongue Fern from early successional forest or even from apparently suitable habitat may not be entirely due to intolerance of current conditions or species in these types of forests. Rather, it may be the result of intolerance to former conditions, such as those that would have occurred at the time of the disturbance that created the early successional forest.
Somewhat paradoxically, American Hart's-tongue Fern can occasionally persist in sites that have been disturbed if the boulder on which it is growing just happens to remain well shaded. This was observed a few times (Jones 2013), for example, at Lily Oak Forest, at the Krug Tract at Kinghurst, and at the Klondike Forest east of Desboro (where ferns were growing on a single, isolated boulder in an area logged in 2012). At Lily Oak Forest, it was evident that other parts of the forest had been logged in the past, but that the ferns had either been able to remain in place while the forest regenerated or had moved back in after suitable conditions returned. There was no indication of the time frame over which this happened, but one can speculate that it would take at least 30 to 50 years for the forest canopy to close again. It is also possible that there may be a maximum amount of tree or canopy removal through which American Hart's-tongue Fern can survive, and survival may also depend on conditions favouring tree regeneration.
The intolerance to drying out and the ability for small patches of ferns to persist on isolated boulders is likely the dynamic that has created the current scattered demographic pattern of the species. Because there is almost no old-growth deciduous forest anywhere on the Niagara Escarpment (Riley 2013), all forests have had some degree of logging, and the species has somehow survived or recovered. Currently, suitable habitat is much more widespread on the Niagara Escarpment than is the American Hart's-tongue Fern, so it is probable that in many places the ferns did not survive past disturbance, although today it looks like much of the forest has recovered.
American Hart's-tongue Fern usually does not grow on rock surfaces that have much cover of other vascular plants and only very rarely grows underneath other herbs (Cinquemani Kuehn and Leopold 1993; Jones pers. obs. 2013). However, in rubble talus or on lightly disturbed surfaces it may occur with a few individuals of Bulblet Fern (Cystopteris bulbifera), Herb Robert (Geranium robertianum), or Pale Touch-Me-Not (Impatiens pallida). Futyma (1980) reported Northern Holly Fern (Polystichum lonchitis) and Walking Fern (Asplenium rhizophyllum) as frequent associates, and these species do grow in similar mossy limestone habitats. However, both species are able to grow in drier conditions and are not always good indicators of suitable habitat for American Hart's-tongue Fern.
Suitable habitat in New York was studied in detail by Cinquemani Kuehn and Leopold (1993). They observed that American Hart's-tongue Fern was most vigorous under deciduous canopies on north-facing slopes, similar to habitat in Canada. However, they found that in New York, mature plants were associated with an accumulation of organic matter, a low cover of bryophytes, and deep humus-filled crevices. They concluded that older ferns outcompete bryophytes and eventually replace them. This does not match observations from Canada, where the majority of American Hart's-tongue Ferns, regardless of life stage, are growing in a bryophyte mat. In addition, the New York study found the ferns predominantly at upper and mid-slope positions but absent from lower slope and level areas. In Canada, many American Hart's-tongue Fern subpopulations are found on blocks at the bottom of the escarpment or on level outcrops.
Habitat trends
Most threats to American Hart's-tongue Fern act by degrading or destroying habitat, which has already caused the extirpation of ten subpopulations (Jones 2013). However, these subpopulations were probably already gone prior to 2000, so there has been habitat lost over a long term. More recently, logging continues to be a frequent activity on private lands and in county forests. Of 27 sites visited in 2013, six had been logged in the last five years and three others evidently had heavy historical logging in at least part of the habitat. In addition, in the last 5 years two sites have been turned into quarries, also resulting in habitat loss.
In addition, invasive species, such as Garlic Mustard (Alliaria petiolata) and Common Buckthorn (Rhamnus cathartica) are now present at some sites, and workers in New York report that subpopulations there are severely threatened by Pale Swallow-wort (Cynanchum rossicum) (Brumbelow pers. comm. 2013). Based on these and other threats, it appears likely that there has been a slight decline in habitat suitability, which will likely continue in the future.
Very little information is available on habitat trends because very few subpopulations have had more than one site visit. It is possible there has not been a great decline in habitat where subpopulations are in protected areas, and many other areas still show good forest canopy cover present on satellite imagery. However, more than half of subpopulations are on privately owned lands which may be subject to logging and other types of site alterations, and invasive species are present in some protected areas.
Thus, although the trends in habitat loss are not quantifiable at this time, sources of degradation are so widespread that a decline in habitat is inferred.
Biology
Life cycle and reproduction
Fronds of American Hart's-tongue Fern remain green throughout the winter and subsequent growing season. New fronds are produced at the start of each growing season, and spores are produced from May to August on year-old fronds (NatureServe 2015; Jones pers. obs.). Maher (unpubl. notes) counted upwards of 50 fronds on some unusually large individuals. These may be very old individuals. The average life span for American Hart's-tongue Fern plants is not known, but three European species of Asplenium are known to have life spans in the range of 30-50 years (Bucharová et al. 2010).
Vegetative reproduction, such as by rhizomes or runners, is not known to occur in this species. Like all ferns, Hart's-tongue Fern reproduces by spores, which germinate to produce gametophytes. Bryophytes probably enhance reproduction by providing a favourable site for spore germination and gametophyte growth. In addition, bryophytes may be crucial to the survival of sporelings as over 80% of sporelings observed at sites in New York were on bryophyte beds (Cinquemani Kuehn and Leopold 1993).
American Hart's-tongue Fern is capable of producing a sporophyte from a single spore through self-fertilization of the gametophyte (Vogel et al. 1999). Testo and Watkins (2011) found that among gametophytes of the American variety, few male (antheridiate) gametophytes and no bisexual (antheridiate and archegoniate) gametophytes are produced. However, female (archegoniate) gametophytes produced copious outgrowths that could become functional asexual propagules able to develop into sporophytes. Fernando et al. (2015) found genetic work on New York and Michigan subpopulations supported the existence of a mixed mating system where gametophytes preferentially out-cross, but may undergo self-fertilization in the absence of genetically different mates. Fernando et al. (2015) point out this system may explain the presence of many genetically identical individuals and overall low diversity within many subpopulations.
Testo and Watkins (2011) found gametophytes of the American variety germinated earlier, grew significantly slower, and produced sporophytes much later (166 days vs. 119 days) than the Old World variety. In a second study (Testo and Watkins 2013), they found American Hart's-tongue Fern had lower rates of spore germination and sporophyte production than five other common fern species and that the gametophyte phase of American Hart's-tongue Fern is extremely sensitive to increases in temperature, to desiccation, and to competition from other species. Specifically, gametophytes grown at 25°C were 84.6% smaller than those grown at 20°C, and only 1.5% produced sporophytes. Similar reactions to temperature were not observed in five other fern species studied. These results underscore the sensitivity of American Hart's-tongue Fern to environmental conditions and may partly explain the rarity of this fern, in the absence of obvious threats.
Physiology and adaptability
In the laboratory, American Hart's-tongue Ferns have been successfully propagated from spores to produce plants large enough to bear fertile fronds. However, when transplanted into the wild, none of the propagated plants have survived the first season, perhaps due to lack of water. (U.S.F.W.S. 2012). Transplantation of American Hart's-tongue Fern is apparently possible. In Canada, a pilot project was undertaken in 2008 to transplant mature individuals as possible mitigation for the expansion of an aggregate operationFootnote4. Monitoring was done in 2013 and 2015, so presumably many transplants were still alive after seven years. Long-term success of the project was not evaluated (Stantec 2008; Leslie pers. comm. 2013). The aggregate operation was given approval without a requirement for mitigation (Grbinicek pers. comm. 2016), so apparently no further transplantation or monitoring was done. In another experiment, a small number of plants transplanted in 2013 and in 2015 were all still alive in the spring of 2016, and some had produced fertile leaves (Jones unpubl. data). Failure to select correct habitat with suitable environmental characteristics may be one reason for a lack of transplantation success.
Although Hart's-tongue Fern requires shade to remain moist, light may be a naturally limiting factor. Ferns that grow deep in crevices do not produce large fronds. The smaller surface area, in turn, means fewer sori and sporangia are produced. For example, at Mount Nemo most ferns had only small, sterile fronds (3-4 cm long; no sori formed) and were growing nearly in the dark. At Skinner's Bluff and Clarke's Corner, plants that were down in narrow crevices were also small and sterile. Thus, there may be a trade-off between the need for shade for humidity and the need for light for growth and reproduction.
Dispersal and migration
It has been estimated that a single frond of American Hart's-tongue Fern may produce up to 18,000,000 spores (Billington 1952). The light-weight spores of most ferns are known to have substantial capacity for long-distance dispersal (Kato 1993; Vogel et al. 1999). However, many Canadian sites have American Hart's-tongue Ferns on some boulders but not on others that are adjacent and appear equally suitable. It is possible that the sheltered nature of the microhabitat in which this species grows, such as in crevices, on the sides of blocks that face each other, and at the bottom of escarpment slopes, limits the movement of spores by air currents or wind. In addition, dry conditions are required for spore release and dispersal, yet American Hart's-tongue Fern habitat is generally humid, and plants of this species grow nearly prostrate on the surfaces of rocks, possibly limiting wind-assisted movement of spores. Fernando et al. (2015) found a lack of gene flow and lack of shared genotypes among New York subpopulations of American Hart's-tongue Fern, implying an absence of long-distance dispersal for this species. They speculated that the vast majority of this species’ spores fall close to the parent plants.
Interspecific interactions
American Hart's-tongue Fern occurs primarily on rock surfaces and requires a substrate of mosses for growth. It rarely grows in close proximity to or covered by other herbaceous plants.
Population sizes and trends
Sampling effort and methods
Appendix 1 lists abundance and date of most recent observation for all known subpopulations. Details on 27 sites surveyed in 2013 are found in Jones (2013).
Abundance
The size of the Canadian population is estimated to be greater than 110,000 individuals. There are four subpopulations with 10,000 to 20,000 individuals and 17 subpopulations with close to or more than 1,000 individuals. Many other subpopulations have hundreds of individuals. Twenty-six subpopulations have no abundance information, but if a conservative number of 50 individuals is assigned to these, a total of approximately 110,000 is obtained. The actual total abundance is certainly higher because most counts have covered only portions of subpopulations and many sites with no information are very likely to have more than 50 individuals. It is not known what percentage of the population is fertile, but the majority of plants at any given site are usually fertile.
Fluctuations and trends
Austen (2000) did not give an estimate of abundance for the total Canadian population, so it is not possible to know if there has been any change. Furthermore, very few subpopulations have had more than one observation where abundance was recorded. Thus, it is nearly impossible to assess trends in population or subpopulation size.
Some counts by Jones in 2013 turned up greater numbers than counts by Maher in 1998-2004, but it is likely that Jones covered more habitat so the greater numbers do not represent an increase in abundance. In addition, when there are several observations from different parts of a subpopulation, it can be confusing to determine trends. For example, Maher counted 30 plants at Purple Valley in 2001, but Jones counted 700-1000 on the next lot to the north in 2013. This is also not an increase but rather a survey of a more abundant part of the same subpopulation. A subpopulation near Shallow Lake was reported by Maher to have declined to only 12 plants in the period between the late 1970s and 1998 (Austen 2000). However, Maher counted this subpopulation again in 2001 and found 62 plants (Maher unpubl. notes). Most likely he searched a larger area in the second survey because an increase from 12 to 62 plants in three years seems improbable. This problem of survey coverage occurs frequently in the abundance data, especially in larger subpopulations with multiple observations.
There are only a few subpopulations where changes in abundance can be detected. At Mt. Nemo, there were at 12 plants in 1994, 4 plants in 2008, and 13 in 2013. At Kemble Mountain in 1998, Maher and Gumby counted 6,804 plants in only one section of the habitat and estimated the area to contain >10,000 plants (Austen 2000). In 2010, Bowles counted 4,147 in the entire habitat and estimated a total subpopulation of ~8,150 plants for the site (Bowles 2011). Given the actual numbers counted, it seems likely there has been some decline in this subpopulation.
A part of the Kolapore Southwest ANSI subpopulation was counted in 1998 and in 2001 by Maher (Maher unpubl. notes; NHIC 2013). Presumably, he covered the same area of habitat in each count. In 1998, he counted >200 plants but in 2001 only 111 plants. However, there are now several more recent observations (with no abundance data) from other patches within this subpopulation, so it is not really possible to know if there has been a decline in the overall subpopulation.
Declines have certainly occurred on sites that have been logged in the last five years although the magnitude is unknown. These include Lily Oak Forest, Klondike east of Desboro, Osler Bluff, and on the private lands within the Mud Creek South, Cape Croker-Malcolm Bluff, and Devil's Glen subpopulations. There are probably other sites that have not been visited recently that have been logged by private landowners and may have unreported declines.
It would be useful to know how subpopulations are faring in protected areas where anthropogenic threats are relatively low. Unfortunately, there is no information on this. However, most U.S. subpopulations are in protected areas yet are experiencing continued, long-term declines. Some of the decline has been attributed to climate change (U.S.F.W.S. 2012; Testo and Watkins 2013), which presumably also affects the Canadian population. In Canada, a documented decline at Kemble (a managed area with >4000 individuals in 2010) may point to the possibility of declines even in larger populations. No reason for the decline at Kemble was provided by Bowles (2011).
Given the documented long-term presence of many subpopulations, the Canadian population of American Hart's-tongue Fern is not expected to undergo any type of extreme fluctuations (numbers of mature individuals, number of subpopulations, number of locations, EOO or IAO). A study of two endangered European species of Asplenium that are restricted to a highly specialized habitat and occur in small isolated populations found that even small populations with only 10 individuals had a reasonable (>55%) probability of surviving over the next 50 years (Bucharová et al. 2010). These findings could also apply to American Hart's-tongue Fern.
In summary, limited information makes net trends difficult to determine. Overall, there has been a small net gain in number of subpopulations since 2000 due to new discoveries, so the total abundance of the population may be greater than previously known due to new information. However, there are also indications that declines are occurring in some subpopulations due to threats including climate change. Some habitat is protected and is of good quality, but more than half of the subpopulations are on private lands, which may be more subject to threats. Some habitat has been observed to have declined or become degraded.
Rescue effect
A rescue effect from the Michigan or New York populations is nearly impossible as these populations are hundreds of kilometres from the nearest Canadian suitable habitat and have very small population sizes. Furthermore, genetic work has shown a lack of gene flow in New York and Michigan populations, indicating that long-distance dispersal does not occur (Fernando et al. 2015). Finally, there are large areas of Great Lakes water and urbanization between most of the Canadian and U.S. populations, making long-distance dispersal very unlikely.
Threats and limiting factors
The overall threat impact to American Hart's-tongue Fern has been calculated as High, based on the IUCN-CMP (World Conservation Union-Conservation Measures Partnership) unified threats classification system (Master et al. 2009). Threats are defined as the proximate activities or processes that directly and negatively affect the Hart’s-tongue Fern population. The impact, scope, severity, and timing of threats are presented in tabular form in Appendix 2.
There are 109 subpopulations, some of which are very large spatially and in numbers of individuals, and an estimated abundance of >110,000 plants. Approximately one third of the total Canadian population abundance is in two subpopulations. Given this, it is unlikely that the scope of any threat could ever be greater than 50 percent, other than for climate change.
Threats to American Hart's-tongue Fern include:
- 5.3 Logging and wood harvesting
- Medium impact
- 3.2 Mining and quarrying
- Medium impact
- 1.1 Residential
- Low impact
- 6.1 Recreational activities
- Low impact
- 8.1 Invasive species
- Low impact
- 8.2 Problematic native species
- Low impact
- 5.2 Collection of terrestrial plants
- Negligible impact
- 1.3 Development of tourism and recreational areas
- Negligible impact
- 11.1 Habitat shifting and alteration due to climate change
- Negligible impact
5.3 Logging and wood harvesting
Logging is a serious and current threat because it results in opening of the forest canopy and drying of the microclimate. Historically, logging likely caused extirpation of the species from Milton Heights, Halton Forest North, McKinney’s Hill, Barrow Bay Village, East of Banks, and probably from Caledon Slope Forest (extirpation still needs full confirmation) and caused declines at Purple Valley and Malcom Bluff. Logging in 2010-12 Lily Oak Forest, Klondike east of Desboro, and part of Mud Creek South resulted in the loss of large portions of these subpopulations, and at the Klondike site only a few ferns remain on a single boulder. Logging remains a current threat, especially on private lands where landowners may not be aware of the ferns or where there is no enforcement of protective legislation.
3.2 Mining and quarrying
Mineral resource extraction (quarrying) completely removes suitable habitat needed by American Hart's-tongue Fern. Where habitats are adjacent to quarries, dust and changes in hydrology may also be threats. In addition, even if American Hart's-tongue Fern is not immediately present, removal of forested habitat may affect the adjacent microclimate where American Hart's-tongue Fern is present. In the past 5 years at least, American Hart's-tongue Fern has been discovered in at least five areas proposed for aggregates extraction. Historically, quarrying was likely responsible for extirpation of the species from Inglewood and from part of Milton Heights. Theoretically, the current application review process for new aggregate operations should prevent the loss of American Hart's-tongue Fern plants. However, it is still unknown whether the protection measures being put in place will ensure the long-term health of subpopulations and habitats that are adjacent to quarries.
1.1 Residential
A great deal of development is underway in the range of American Hart's-tongue Fern. Residential and commercial development threatens the species in the same way as quarrying and logging, by causing a total loss of habitat or by causing a loss of forest canopy and drying of microclimate. Again, the land use planning process requires protection measures be put in place when American Hart's-tongue Fern is found in a development application. However, there is no proof that these measures are adequate. Even though seemingly suitable, shaded, mossy blocks can be seen in some recently built subdivisions, the loss of the surrounding forest habitat may result in a loss of required humidity. Residential development is likely the cause of extirpation of subpopulations from Credit Forks and Stayner, and probably from two sites east of Eugenia (extirpation still needs final confirmation). American Hart's-tongue Fern has been found in more than 25 development applications in Grey County since 2008 (Grey-Sauble Conservation Authority unpubl. data; Morton and Jones unpubl. data). The long-term viability of these mitigated subpopulations is unknown.
6.1 Recreational activities
Recreational activities such as rock climbing, spelunking (cave exploration), and off-trail hiking may trample and destroy American Hart's-tongue Fern plants and can dislodge the fragile moss mats that cover rocks. These threats are sometimes greater in protected areas where human usage is more frequent. Private land accessible from the Bruce Trail is also subject to this threat. The small subpopulation at Mount Nemo is seriously threatened because it occurs at the entrance to a cave that is used recreationally. A person entering the cave carelessly could wipe out the entire subpopulation. At Mono Cliffs and Pottawatomi Conservation Area in 2013, Inglis Falls in 2011, and Mud Creek East in 2010, trampling and dislodged patches of moss from off-trail hiking were evident near American Hart's-tongue Fern (Jones pers. obs.).
8.1 Invasive species
Invasive species were observed in many places in the habitat of American Hart's-tongue Fern in 2013 (Jones unpubl. data). Large patches of Garlic Mustard (Alliaria petiolata) and Common Buckthorn (Rhamnus cathartica) were noted in field verifications, and Garlic Mustard may have been involved in the extirpation of some subpopulations. In addition, in degraded habitat, some native species may become overabundant on the same rock faces as American Hart's-tongue Fern. Subpopulations at Lion's Head, Clarke's Corners, and Devil's Glen are on disturbed outcrops, and the plants are currently surrounded by an overgrowth of Blue Cohosh, Herb Robert, and some Eurasian weeds. At the sites of extirpated subpopulations at Halton Forest North, Milton Heights, Inglewood, Caledon Slope Forest, McKinney's Hill, and East of Banks, some habitat still appeared suitable, but there was a lot of herbaceous growth of Garlic Mustard, Herb Robert, and Blue Cohosh on the rocks. Garlic Mustard is sporadically present in the habitat at Mono Cliffs (the largest Canadian subpopulation), as well as surrounding the subpopulations at Mount Nemo and Inglis Falls, and potentially at some of the more than 60 sites not visited prior to this report. Invasive species take over habitat by spreading quickly, and many (including Garlic Mustard) secrete toxins from their roots (Vaughn and Berhow 1999) which make habitat unsuitable for American Hart's-tongue Fern.
8.2 Problematic native species
Other factors that open the canopy, such as the Forest Tent Caterpillar (Malacosoma disstria), may have a similar effect (Jones pers. obs.). In addition, other factors that affect forest health and soil moisture retention, such as the presence of exotic earthworms, may be potential threats if they lead to a change in humidity. American Hart's-tongue Fern appears able to recover into logged habitat once suitable conditions return; however, this takes decades, and it is not known whether recovered subpopulations ever match their former size and health.
5.2 Collection of terrestrial plants
Removal of American Hart's-tongue Fern by collectors is an ongoing threat where subpopulations are accessible. Collection was observed in 2013 at Mono Cliffs but likely also occurs at other locations. The severity of this threat is negligible.
1.2 Development of tourism and recreational areas
Recreational development of ski hills and upgrades to the Bruce Trail are occurring in two sites in the habitat of American Hart's-tongue Fern. The development approvals process will require mitigation for the species, and the portions of subpopulations that would be affected are very small. This threat’s impact is currently thought to be negligible but is mentioned here so that it may be monitored for future change.
11.1 Habitat shifting and alteration due to climate change
Climate change will likely be a threat to American Hart's-tongue Fern in Canada in the next 10 to 30 years. Increases in temperature and loss of humidity have been shown to cause significant negative effects on spore germination and gametophyte survival (Testo and Watkins 2013). The overall effect of climate change on the Canadian population is not yet known, but Testo and Watkins (2013) speculate that the recent and rapid declines in U.S. populations and the observed demographic shift in the New York populations towards older plants may be linked to the impact of climate change on gametophytes and spore germination.
Number of locations
The exact number of locations has not been determined for this species but is probably quite high and is not expected to fluctuate. The most serious plausible threats to American Hart's-tongue Fern are logging and mineral resource extraction (quarrying). These activities occur in diverse and scattered localities based on ownership and resource needs, and it would be unlikely for these threats to affect more than one subpopulation at a time. Similarly, invasive species and development also occur in widely separated places. Given this and the distribution of this species in numerous scattered patches with many different owners, the number of locations may be very high. Climate change may affect the entire population as one location, but it is unlikely that this threat would act quickly as a stochastic event.
Protection, status and ranks
Legal protection and status
In Canada, Hart's-tongue Fern (Asplenium scolopendrium) is legally listed as Special Concern on Schedule 1 of the federal Species at Risk Act and on Schedule 5 of the Ontario Endangered Species Act 2007. However, these laws confer no legal protection to species listed as special concern, and no critical or significant habitat is required to be protected. At four sites where land is owned by the Ontario Ministry of Natural Resources and Forestry (OMNRF), Hart's-tongue Fern would be protected as a special concern species by the Crown Forest Sustainability Act, 1994. A federal management plan has been prepared for Hart's-tongue Fern (Environment Canada 2013). The objective of the management plan is to maintain extant populations at their current abundance and distribution by reducing the threats that act upon the species across its range in Canada. Ten recovery steps are suggested to achieve the objective by managing and conserving habitat, monitoring and doing research, and providing outreach and communication to the public. Little progress has been made in accomplishing these steps, although the work for this current report may help to provide a better understanding of population size and species abundance (step 2.2). In the United States, American Hart's-tongue Fern is legally listed as Threatened under the federal Endangered Species Act.
Non-legal status and ranks
Globally, American Hart's-tongue Fern has a conservation rank of G4T3, indicating that the American variety is vulnerable and of global conservation concern. Subnationally, American Hart's-tongue Fern is ranked S3 or vulnerable in Ontario (NHIC 2015). In New York the species is ranked S2 or imperiled. In Alabama, Tennessee, and Michigan, it is ranked S1 or critically imperiled. Nationally, American Hart's-tongue Fern is ranked N3 or vulnerable in Canada and N2 or imperiled in the U.S. (NatureServe 2015). The taxon has not been assessed by the IUCN (IUCN 2016).
Habitat protection and ownership
Of the 109 Canadian subpopulations, 59 are on land that is entirely privately owned, 32 are in protected areas (either in public ownership or owned by conservation non-governmental organizations), 13 have a mix of protected and private ownership, 2 are in county-owned forests, 2 are on First Nation lands, and 1 has mixed county forest and private ownership. Owners or managers of protected areas include Ontario Parks, OMNRF, Grey-Sauble Conservation Authority, Conservation Halton, Nature Conservancy Canada, the Bruce Trail Association, and the Ontario Heritage Trust.
The habitat of special concern species and cliffs and talus slopes are considered significant wildlife habitat, and some habitats of American Hart’s-tongue Fern may also be considered significant woodland, depending on the size of the habitat and its geographic location (OMNRF 2000, 2010, 2015). In addition, most privately owned lands with American Hart's-tongue Fern are designated as areas of natural and scientific interest (ANSI) (Riley et al. 1996). The natural heritage section of the Ontario Provincial Policy Statement (PPS) requires there be no development or site alteration in significant wildlife habitat or in ANSI designations unless it has been demonstrated that there will be no negative impacts to the natural features or their ecological functions. As well, many private lands fall within the escarpment natural area or escarpment protection area land use designations of the Niagara Escarpment Plan (Niagara Escarpment Commission 2005). Within these designations certain types of development are restricted. The Niagara Escarpment Plan also contains policies that require that impacts to the habitat of special concern species must be minimized. Nevertheless, these designations do not prevent a landowner from making many types of site alterations that do not require applications or permits. Thus, clear cutting, on-site road construction, and extraction of stone or aggregate for on-site private use may still occur on these lands. In addition, in somewhat circular reasoning, the presence of many individuals or subpopulations elsewhere has at times been used to show no negative impacts to a subpopulation of a special concern species (Grbinicek pers. comm. 2016).
Acknowledgements and authorities contacted
The report writer wishes to thank Katherine Lindsay, John Riley, and Anthony Chegahno for providing expertise, field assistance, and support.. The report writer is grateful to David J. Bradley (OMNRF), the Bruce Trail Association, Malcolm Campbell, Danilo Fernando (SUNY), Lisa Grbinicek (Niagara Escarpment Commission), Joe Johnson, Jean Maher, John Morton (AWS Environmental), the Natural Heritage Information Centre (Ontario Ministry of Natural Resources and Forestry), A.A. Reznicek (University of Michigan Herbarium), and Andrew Sorenson (Grey-Sauble Conservation Authority) for sharing information. The report writer also wishes to acknowledge the many private landowners who generously allowed access to their properties for survey work.
Information sources
Arreguin-Sanchez, M de la L, and R. Aguirre-Claveran. 1986 Una nueva localidad para norteamericana de Phyllitis scolopendrium (L.) Newm. var. americana Fernald. Phytologia, 60(6):399-403.
Austen, M.J.W. 2000. COSEWIC status report on the American hart’s-tongue fern Asplenium scolopendrium var. americanum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-42 pp.
Bell, J. 1870. List of plants of the Manitoulin Islands, Lake Huron. Geological Survey of Canada Report for 1867-1869. Pp. 1-23.
Billington, C. 1952. Ferns of Michigan. Cranbrook Institute of Science. Bulletin No. 32. p. 4.
Biodiversidad Mexicana 2015. Especies en riesgo. [accessed November 13, 2015].
Bowles, R. 2011. Field research results for American Hart's Tongue Fern colonies in Ontario. Unpublished presentation to the Clearview Community Coalition, http://clearviewcommunitycoalition.com, 26 pp.
Britton, D.M. 1953. Chromosome studies in ferns. American Journal of Botany 40:575-583.
Brumbelow, T.R., pers. comm. 2013. Email to J. Jones, October 18, 2013. M.S. student doing thesis research on Hart's-tongue Fern at State University of New York, Syracuse.
Bucharová, A., Z. Münzbergová, and P. Tájak. 2010. Population biology of two rare fern species: long life and long-lasting stability. American Journal of Botany 97(8): 1260-1271.
Cinquemani Kuehn, D.M., and D.J. Leopold. 1992. Long-term demography of Phyllitis scolopendrium (L.) Newm. var. americana Fern. in central New York, Bulletin of the Torrey Botanical Club 119(1):65-76.
Cinquemani Kuehn, D.M., and D.J. Leopold. 1993. Habitat characteristics associated with Phyllitis scolopendrium (L.) Newm. var. americana Fern. (Aspleniaceae) in central New York. Bulletin of the Torrey Botanical Club 120(3):310-318.
Cody, W.J., and D.M. Britton. 1989. Ferns and Fern Allies of Canada. Agriculture Canada Publication 1829/E, Canadian Government Publishing Centre, Ottawa, Ontario.
CONABIO 2015. Asplenium scolopendrium var. americanum in Catálogos de CONABIO [accessed November 13, 2015].
CONABIO. 2013. Propuesta de lista de especies prioritarias para la conservación de la biodiversidad de México (PDF; 914 KB) [accessed November 3, 2015].
COSEWIC. 2013. National Ecological Areas Map. http://www.cosewic.gc.ca/images/Fig1-TerrestrialEcologicalAreas_Eng.jpg [accessed June 3, 2013].
Environment Canada. 2013. Management Plan for the Hart’s-tongue Fern (Asplenium scolopendrium) in Canada. Species at Risk Act Management Plan Series. Environment Canada, Ottawa. iii + 16 pp.
Fassett, N.C. 1933. Notes from the herbarium of the University of Wisconsin – X. Rhodora 35(420):387-391.
Fernald, M.L. 1935. Critical plants of the upper Great Lakes region of Ontario and Michigan. Rhodora 37:197-222.
Fernando, D.D. pers. comm. 2013. Email to J. Jones October 17-21, 2013. Associate professor, State University of New York, Syracuse.
Fernando, D.D., J.J. Discenza, J.R. Bouchard and D.J. Leopold. 2015. Genetic analysis of the threatened American hart's-tongue fern (Asplenium scolopendrium var. americanum [Fernald] Kartesz and Ganhi): Insights into its mating system and implications for conservation. Biochemical Systematics and Ecology 62: 25-35.
Futyma, R.P. 1980. The distribution and ecology of Phyllitis scolopendrium in Michigan. American Fern Journal 70(3):81-87.
Grbinicek, L. 2016. Personal communication to D. Meidinger by email June 14, 2016. Senior strategic advisor, Niagara Escarpment Commission, Georgetown, Ontario.
Grieve, M. 1971. A Modern Herbal, vol. 1. Dover Publications, New York, 427 pp.
Hagenah, D.J. 1954. The Hart’s-tongue in Michigan. American Fern Journal 44:2-7.
Hinds, H.R. 1986. Flora of New Brunswick. Primrose Press, Fredericton, New Brunswick. 460 pp.
International Union of Conservation of Nature and Natural Resources (IUCN). 2016. The IUCN Red List of Threatened Species. Version 2015-4. [accessed April 19, 2016].
Jones, J. 2001. Natural Features of the Niagara Escarpment on Manitoulin Island. Unpublished report prepared for the Escarpment Biosphere Conservancy, Toronto, Ontario
Jones, J. 2013. American Hart's-tongue Fern field work summary. Unpublished report to COSEWIC, Environment Canada, Ottawa, Ontario.
Kartesz, J.T. and N. Gandhi. 1991. Nomenclatural notes for the North American Flora –V. Phytologia 70(3):194-208.
Kato, M., 1993. Biogeography of ferns: dispersal and vicariance. Journal of Biogeography 20 (3) 265-274.
Leslie, J., pers. comm. 2013. Email to J. Jones, October 31, 2013. Stantec, Guelph, Ontario.
Maher, W.V.N. 1998-2004. Unpublished field notes. Field naturalist with extensive expertise in ferns. Mr. Maher died in 2005. Notes courtesy of Jean Maher, Owen Sound.
Master, L., D. Faber-Langendoen, R. Bittman, G.A. Hammerson, B. Heidel, J. Nichols, L. Ramsay, and A. Tomaino. 2009. NatureServe conservation status assessments: factors for assessing extinction risk. NatureServe, Arlington, VA. <http://www.natureserve.org/publications/ConsStatusAssess_StatusFactors.pdf> [accessed November 2015].
Michigan Natural Features Inventory. 2013. Rare Species Explorer (Web Application). http://mnfi.anr.msu.edu/explorer [accessed June 4, 2013].
Morton, J.K., and J.M. Venn. 2000. The Flora of Manitoulin Island, 3rd ed. University of Waterloo Biology Series Number 40. Waterloo, Ontario.
Morton, J.D., and J. Jones. 2005-2013. Unpublished environmental impact studies (EIS) and natural environment technical report (NETR) contributions by Aquatic and Wildlife Services, Shallow Lake, Ontario.
NatureServe. 2002. Element occurrence data standard. NatureServe, Arlington, Virginia. http://www.natureserve.org/conservation-tools/standards-methods/element-occurrence-data-standard [accessed November 20, 2016]
NatureServe. 2015. NatureServe Explorer: Version 7.1. NatureServe, Arlington, Virginia. [accessed November 20, 2015].
Niagara Escarpment Commission. 2005. The Niagara Escarpment Plan. http://www.escarpment.org/_files/file.php?fileid=filewbdNbckeSV&filename=file_2_NEP_Office_Consolidation_Updated_April_3__2013___Final.pdf [accessed October 15, 2013].
Natural Heritage Information Centre (NHIC). 2013. Biotics database information provided to J. Jones. Natural Heritage Information Centre, Ontario Ministry of Natural Resources and Forestry, Peterborough.
New York Department of Environmental Conservation (NYDEC). 2013. Nature Explorer on-line database. [accessed June 4, 2013].
Ontario Ministry of Natural Resources. 2000. Significant wildlife habitat technical guide. Toronto: Queen’s Printer for Ontario. 151p. http://www.mnr.gov.on.ca/stdprodconsume/groups/lr/@mnr/@fw/documents/document/mnr_e001285.pdf
Ontario Ministry of Natural Resources. 2010. Natural Heritage Reference Manual for Natural Heritage Policies of the Provincial Policy Statement, 2005. 2nd Ed. Toronto: Queen’s Printer for Ontario. 248 pp. http://publicdocs.mnr.gov.on.ca/View.asp?Document_ID=12714&Attachment_ID=32290
Ontario Ministry of Natural Resources and Forestry. 2015. Significant wildlife habitat criteria schedules for Ecoregion 6E. OMNRF Regional Operations Division, Peterborough, Ontario. 38 pp. [accessed November 15, 2015].
Reznicek, A. A., pers. comm. 2013. Email to J. Jones, October 15 and 29, 2013. Assistant director and curator of vascular plants, University of Michigan Herbarium, Ann Arbor.
Riley, J.L. 2013. The Once and Future Great Lakes Country. McGill Queens University Press, Montreal. 488 pp.
Riley, J.L., J.V. Jalava, and S. Varga. 1996. Ecological Survey of the Niagara Escarpment Biosphere Reserve. Volume I. Significant Natural Areas. Volume II. Technical Appendices. OMNRF, Southcentral Region, Peterborough, Ontario. Open File Site Report SR 9601. v + 629 pp., vii + 310 pp.
Soper, J.H. 1954. The Hart’s-tongue Fern in Ontario. American Fern Journal 44(4):129-147.
Soper, J.H. 1963. Ferns of Manitoulin Island. American Fern Journal 53:113-132.
Southern Science and Information Section. 2012. Niagara Escarpment Biodiversity Re-inventory Project. Ontario Ministry of Natural Resources, Peterborough. 40 pp.
Stantec. 2008. Memos pertaining to "Preliminary Off-site AHTF Habitat Survey" presented as part of public record for Ontario Municipal Board case no. 08-094 on Walker Aggregates - Duntroon Quarry. Stantec, Guelph, Ontario.
Snyder, D., pers. comm. 2013. Email to Steve Young, New York Department of Environmental Conservation by email on April 25, 2013. Message forwarded to J. Jones by email on November 19, 2015. Snyder is New Jersey Heritage Botanist, New Jersey Department of Environmental Protection.
Tennessee Natural Heritage Program. 2012. Rare species by county. [accessed June 4, 2013].
Testo, W.L. and J.E. Watkins, Jr. 2011. Comparative development and gametophyte morphology of the hart's-tongue fern, Asplenium scolopendrium L. Journal of the Torrey Botanical Society 138(4) 400-408.
Testo, W.L. and J.E. Watkins, Jr. 2013. Understanding mechanisms of rarity in pteridophytes: Competition and climate change threaten the rare fern Asplenium scolopendrium var. americanum (Aspleniaceae). American Journal of Botany 100(11):2261-2270.
U.S.F.W.S. 2012. American Hart's-tongue Fern (Asplenium scolopendrium var. americanum) 5 year review: summary and evaluation. U.S. Fish and Wildlife Service Ecological Services Field Office Cookeville, TN. 23 pp. [accessed October 20, 2013].
U.S.F.W.S. 2013. Species Profile, American Hart's-tongue Fern (PDF; 399 KB). Environmental Online System. [accessed June 4, 2013].
VASCAN. 2013. Data base of vascular plants of Canada. [accessed June 4, 2013].
Vaughn, S.F. and M.A. Berhow. 1999. Allelochemicals isolated from tissues of the invasive weed garlic mustard (Alliaria petiolata). Journal of Chemical Ecology 25(11):2495-2504.
Viane, R.L.L., and T. Reichstein. 1991. Note about Asplenium II: Some new names and combinations in Asplenium L. (Aspleniaceae, Pteridophyta). Biologisch Jaaboek Dodonaea 59: 157-165.
Vogel, J.C., F. Rumsey, J. Jakob Schneller, J.A. Barrett, and M. Gibby. 1999. Where are the glacial refugia in Europe? Evidence from pteridophytes. Biological Journal of the Linnean Society 66:23-37.
Wagner, W.H., R.C. Morran, and C.R. Werth. 1993. 19. Aspleniaceae; pp. 228-245. in Flora of North America North of Mexico, vol. 2 Pteridophytes and Gymnosperms, Oxford University Press, New York.
Watkins, J.E., Jr., pers. comm. 2013. Email to J. Jones, October 17, 2013. Assistant Professor of Biology, Colgate University, Hamilton, NY.
Biographical summary of report writer
Judith Jones, B.S., M.S., has been a biological consultant since 1995. In 2011-12 she participated in a study to re-survey 110 forest inventory plots on the Niagara Escarpment, working on 50 sites between Orangeville and Tobermory. Her consulting experience also includes inventories of natural areas, preparation of management plans and conservation recommendations, assisting First Nations with land use planning, surveys for environmental impact studies for the private sector, and teaching. She has been involved with surveys and recovery planning for more than 15 species at risk and is the author of four other COSEWIC status reports and more than 20 recovery strategies, action plans, and management plans.
Collections examined
Digital images of two specimens from the University of Michigan Herbarium were examined for label data.
Appendix 1. threats assessment worksheet.
List of all known Canadian subpopulations of American Hart's-tongue Fern with most recent dates of observation, abundance where known, and ownership.
Legend: EO--element occurrence; /--separate observations; &--two observers together or two types of ownership; *--new discovery since 2000; CA--conservation authority; PP--provincial park; NCC--Nature Conservancy Canada; OHT--Ontario Heritage Trust.
| EO ID | Subpopulation name | Last observation | Abundance date | Abundance | Abundance observer | Ownership |
|---|---|---|---|---|---|---|
| 22657 | Aberdeen | 2001 | 2001 | 330 | Maher | private |
| new | Adamsville 2.5 km NNW* | 2004 | 2004 | 350-500 | Morton | private |
| 22681 | Annan 2.4 km SE | 1999 | 1999 | 300 | Maher | private |
| 21602 | Barrow Bay South ANSI | 1993 | 1993 | no info | Jalava | private |
| 21617 | Bass Lake Escarpment | 1993 | 1987 | "abundant" | Piercey & Bradley | CA |
| 21625 | Bayview Escarpment | 2013 | 2013 | >15,000 | Gould | PP |
| 21629 | Beaver Valley - Upper | 2013 | 2013 | 250-300 | Jones | OMNRF |
| 21628 | Beaver Valley West Slope | 1993 | 1993 | no info | Varga | OMNRF, CA 70% private 30% |
| needs #-not 21623 | Bognor NE | 2012 | 1998 | >5000 | Austen & Maher | CA & private |
| 21623 | Bognor SW | 2010 | 2010 | >8000 | Bowles/Maher | 85% CA & private |
| new | Boundary Bluffs Bruce Trail* | 2009 | none | no info | Brylowski | Private |
| 22666 | Cape Croker - Malcolm Bluff | 2011 | 2001 | >100 (2011: "scattered clumps") | Maher (Brylowski) | First Nation |
| 22667 & 6191 | Cape Croker - Sydney Bluff | 2013 | 2013 | >300 | Jones | First Nation |
| 21607 | Cape Dundas | 1996 | 1996 | "common" | Austen | private |
| new | Castle Glen | 2006 | 2006 | 300 | Brinker | private |
| new | Castle Glen SE* | 2012 | 2012 | "several clumps" | Brylowski | private |
| new | Chatsworth 3 km South* | 2007 | 2007 | ~10 | Copeland | NCC |
| 34319 | Chatsworth L9 Con2 | 2000 | 2000 | 30 | Maher | private |
| 21600 | Clark's Corner | 2013 | 2013 | 500 | Jones | OMNRF |
| 22668 | Colpoys Bay | 1997 | 1997 | "very small population" | Johnson | private |
| 21655 | Cruikshank 2 km NE | 1994 | 1994 | no info | Johnson | private |
| 21597 | Devil's Glen | 2013 | 2013 | ~300 | Jones | 50% PP/50% private |
| 21632 | Duncan Escarpment PNR | 1993 | 1966 | 300 | Knox | PP & private |
| needs #-not 22671 | Duncan Lake South | 1985 | 1975/1985 | local but abundant/ ~150 | Cuddy/Johnson | private |
| 21640 | Duntroon W Escarpment | 2003 | 2003 | 30 | Zoladeski | private |
| 22676 | Durham 3.6 km ENE | 2001 | 2001 | 20 | Maher | private |
| new | Durham 5 km SE* | 2002 | 2002 | 384 | Maher | private |
| new | E of Desboro* | 2012 | 2012 | <100 | Jones | private |
| 21657 | E of Desboro - Klondike | 2013 | 2013 | 46 | Jones | county forest |
| 21608 | East Wiarton Upland Woods | 2001 | 2001/2009 | 442/100's - 1000's | Maher/GSCA | 75% CA & private |
| 21636 | Feversham Gorge | 1999 | 1999 | 100 | Maher | CA |
| 21671 | Gibraltar | 1983 | 1983 | no info | Johnson | private |
| 23281 | Greenock 2 km SW | 1994 | 1994 | no info | Monet | private |
| new | Griersville SE* | 2012 | 2012 | no info | Brylowski | private |
| 22662 | Hepworth 1.5 km NE | 2001 | 2001 | 200 | Maher | private |
| new | Hope Bay 1.7 km SW* | 2010 | 2010 | ~100 | Jones | private |
| 35618 | Hope Bay Cathedral Woods | 2001 | 2001 | 2020 | Maher | private? |
| 63557 | Hope Bay PNR/ANSI | 2008 | 1998 | >15,000 | Austen | PP & private |
| 22674 | Indian Creek Mgt Area 2.5 km SE Lindenwood |
1998 | 1998 | 700 | Maher | private |
| 21620 | Inglis Falls | 2011 | 2003/1998 | 100's/915 | Oldham/Maher | CA |
| 34305 | Irish Block* | 2001 | 2001 | 20 | Maher | private |
| new | Irish Block 2 km NW* | 2012 | 2012 | <50 | Brylowski | private |
| 21618 | Kemble Mountain | 2010 | 2010 | 4143 | Bowles | CA |
| 67528 | Kimberley Bruce Trail* | 2002 | 2002 | "3 colonies" | Steinacker | private |
| 21630 | Kimberley Creek | 1993 | 1993 | 100's | Jalava & Varga | private |
| 22678 | Kinghurst (should be EAST of not west of) |
2013 | 2002/2004 | 1978/~100 | Maher/Winters | Ontario Nature |
| 21635 & 21634 | Kolapore Escarpment | 2010 | 1998+2001 | >1600 | Maher | OMNRF |
| new | Kolapore Swamp East | 2010 | 2010 | no info | Gormaly | CA |
| 21633 | Kolapore Uplands Mgt Area (SE) | 2010 | 2010 | >111 | Maher | CA |
| new | Lady Bank 2 km WSW* | 2008 | 2008 | no info | GSCA | private |
| new | Lake Charles 1 km S* | 2012 | 2012 | "upland bush full of HTF" (100s?) | GSCA | private |
| new | Lake Charles 2 km E* | 2008-2011 | 2008-2011 | no info | GSCA | private |
| needs #-not 21655 | Lily Oak Forest | 2013 | 2013 | 600-800 | Jones | county forest |
| new | Lindenwood SW | 2011 | 2011 | 50-100 | Brylowski | Bruce Trail & CA |
| 21601 | Lion's Head North | 2001 | 2001 | 7 | Maher | PP |
| new | Lion's Head South* | 2013 | 2013 | 16 | Jones | Bruce Trail |
| new | Little Germany Management Area* | 2010 | 2010 | 100s | Gormaly | CA |
| 21621 | Massie Hills Management Area | 1984 | 1984 | various locations | Johnson | CA & private |
| 21653 & 64085 & 64086 | McIver Side Road | 2004 | 2004 | 200 | Maher | private |
| 23150 | McNab Lake | 2001 | 2001 | 807 | Maher | CA |
| new | Minniehill SSE* | 2012 | 2012 | <50 | Brylowski | private |
| 21641 | Mono Cliffs PP | 2013 | 2013 | 18,000 | Jones & Lindsey | PP |
| 21609 | Mountain Lake Fen | 2001 | 1998 | 3460 | Austen & Maher | county & private |
| 21599 | Mt. Nemo | 2013 | 2013 | 13 | Jones | CA |
| needs #-not 21616 | Mud Creek East | 2010 | 1993 | no info | Jalava | private |
| needs #-not 21616 | Mud Creek North | 2002 | 2002 | 200 | Maher | CA |
| 21616 | Mud Creek South/Shouldice ANSI | 2006 | 2006 | 500 - 1000 | Jones | CA & private |
| 21598 | Noisy River PP | 2013 | 2013 | 1500 | Jones | PP |
| 21592 | Nottawasaga Lookout | 2010 | 2010 | 164/41 | Bowles/Maher | private & PP |
| new | Nottawasaga South* | 2008-2011 | 2008-2011 | no info | GSCA | private |
| 21671 | Osler Bluff | 2012 | 2012 | ~500 | Jones | private |
| 22669 | Owen Sound 4.7 km NW | 1980s | 1980s | no info | Johnson | private |
| 21654 | Owen Sound Rifle Range | 1998 | 1998 | ~500 | Maher | private |
| new | Owen Sound South* | 2010 | 2010 | no info | Brylowski | private |
| new | Owen Sound Southeast* | 2003 | 2003 | 10 to 20 | GSCA | private |
| new | Park Head* | 2012 | 2012 | 750 | Morton | private |
| 22684 | Pottawatomi CA | 2013 | 2013 | 147 | Jones | CA |
| 21639 | Pretty River PP* | 2002 | 2002 | 25 | Korol | PP |
| 6190 | Purple Valley | 2013 | 2013 | 700-1000 | Jones | private |
| 21637 | Rob Roy Management Area | 1993 | 1990 | "considerable populations" (100s?) | Johnson | CA |
| needs #-not 21637 | Rob Roy NE | 1993 | 1993 | no info | Varga | private |
| 21624 | Robson Lakes | 1993 | 1993 | no info | Jalava & Varga | 90% CA & private |
| 22680 | Rockford 3.5 km SW | 1998 | 1998 | 25-50 | Maher | private |
| 21627 | Rocklyn Creek Valley East | 2001 | 2001 | "lush and healthy pop" + 215 | Maher | CA |
| needs #-not 21627 | Rocklyn Creek Valley West | 1993 | 1993 | no info | Jalava | CA |
| needs #-not 21622 | Rocky Saugeen East | 2001 | 2001 | 116 | Maher | private |
| 21622 | Rocky Saugeen South | 2001 | 2001 | 282 | Maher | private |
| 22664 | Rush Cove Corner | 2001 | 2001 | 1100 | Maher | private |
| new | Scotsdale Farm* | 2010? | 2010? | no info | OHT | OHT |
| 23281 | SE Greenock | 1994 | 1994 | no info | S. Monet; Malcolm Campbell | private |
| 22661 | Shallow Lake 2.8 km NNW | 2001 | 2001 | 62 | Maher | private |
| 21610 | Shouldice Forest/The Glen | 2002 | 2002 | 1488 | Maher | CA & private |
| 6189 | Skinner's Bluff | 2013 | 2013 | ~1000 | Jones | CA & private |
| 21619 | Slough of Despond | 2010 | 1992 | ~200 | Johnson | CA |
| new | Talisman Ski Area W of Kimberley* | 2007 | 2007 | 30-50 | Clark & Hodges | private |
| 22673 | Teeswater SE on Teeswater River | 1994 | 1994 | no info | Johnson | private |
| 22679 | Telfer Creek | 1999 | 1999 | >200 | Maher | Bruce Trail |
| new | Vandeleur* | 2011 | 2011 | >100 | Morton & Jones | private |
| Austen EO 214 | Violet Hill – Mono Cliffs North | 2013 | 2013 | ~300 | Jones & Lindsey | 70% PP/30% private |
| new | Walker Quarry SW of Duntroon* | 2013 | 2008 | 10,500 | Stantec | private |
| 21626 | Walter's Creek Headwaters Area NE | 1984 | 1984 | no info | Johnson | private |
| needs separate # | Walter's Creek Headwaters Area SW | 1984 | 1984 | no info | Johnson | private |
| 21664 | Walter's Falls 3.5 km ESE | 2008-2012 | 2008-2012 | no info | GSCA | private |
| 22675 | West Rocks Management Area | 2004 | 1998 | 275 | Maher | CA |
| 34317 | Wildwood Manor Ranch* | 2001 | 2001 | ~12 | Milne | private |
| 22672 | Williams Lake 1 km E | 1983 | 1983 | no info | Johnson | private |
| 22670 | Williamsford Bridge 2.2 km | 1990 | 1990 | no info | Johnson | private |
| 22682 & 22663 | Woodford 2.3 km NNW | 2010 | 2010 | >3000 | GSCA | private |
| new | Woodford W Bruce Trail* | 2012 | 2008 | 100's - 1000's | GSCA | private |
Appendix 2: Threats assessment for American Hart’s-tongue Fern
- Species or ecosystem scientific name:
- American Hart's-tongue Fern (Asplenium scolopendrium var. americanum)
- Element ID
- -
- Elcode
- -
- Date:
- 01/04/2015
- Assessor(s):
- Judith Jones (report writer), Dave Fraser (facilitator), Del Meidinger, Bruce Bennett, Jim Pojar, Vivian Brownell, John Wiley (USFWS), Steve Young (NY Natural Heritage Program), Karen Timm (Secretariat)
- References:
- -
| Threat impact | Threat impact (descriptions) | Level 1 threat impact counts: high range |
Level 1 threat impact counts: low range |
|---|---|---|---|
| A | Very high | 0 | 0 |
| B | High | 0 | 0 |
| C | Medium | 2 | 2 |
| D | Low | 3 | 3 |
| - | Calculated overall threat impact: | High | High |
- Assigned overall threat impact:
- High
- Impact adjustment reasons:
- Although most threats are at medium or low levels, there are a great number of threats. Also, new research (Fernando et al. 2015) indicates this fern may be sensitive to the effects of climate change.
- Overall threat comments
- Generation time estimated at 10 years. It is helpful to remember there were 109 EOs documented in 2013, some of which are very large spatially and in numbers of individuals. There were a total of >110,000 plants, of which approximately 1/3 were in two EOs. Given this, it is unlikely that that scope could ever be greater than 50%, other than by something like climate change.
| # | Threat | Impact (calculated) | Impact (description) | Scope (next 10 yrs) | Severity (10 yrs or 3 gen.) | Timing | Comments |
|---|---|---|---|---|---|---|---|
| 1 | Residential & commercial development | D | Low | Restricted (11-30%) | Moderate (11-30%) | High (Continuing) | blank |
| 1.1 | Housing & urban areas | D | Low | Restricted (11-30%) | Moderate (11-30%) | High (Continuing) | Niagara Escarpment Development plan (under review) does not currently appear to provide adequate buffering (as part of mitigation). Hart's-tongue Fern has been found in more than 25 development applications in Grey County since 2008 (Grey-Sauble Conservation Authority 651 unpubl. data; Morton and Jones unpubl. data). Several protected areas within this range. |
| 1.2 | Commercial & industrial areas | blank | blank | blank | blank | blank | blank |
| 1.3 | Tourism & recreation areas | blank | Negligible | Negligible (<1%) | Moderate (11-30%) | High (Continuing) | Ski hills and trail development (potential upgrades to Bruce Trail) were considered. |
| 2 | Agriculture & aquaculture | blank | blank | blank | blank | blank | blank |
| 2.1 | Annual & perennial non-timber crops | blank | blank | blank | blank | blank | blank |
| 2.2 | Wood & pulp plantations | blank | blank | blank | blank | blank | blank |
| 2.3 | Livestock farming & ranching | blank | blank | blank | blank | blank | blank |
| 2.4 | Marine & freshwater aquaculture | blank | blank | blank | blank | blank | blank |
| 3 | Energy production & mining | C | Medium | Restricted (11-30%) | Serious (31-70%) | High (Continuing) | blank |
| 3.1 | Oil & gas drilling | blank | blank | blank | blank | blank | blank |
| 3.2 | Mining & quarrying | C | Medium | Restricted (11-30%) | Serious (31-70%) | High (Continuing) | Note one quarry site (Walker's) has 10,000 plants. |
| 3.3 | Renewable energy | blank | blank | blank | blank | blank | blank |
| 4 | Transportation & service corridors | blank | blank | blank | blank | blank | blank |
| 4.1 | Roads & railroads | blank | blank | blank | blank | blank | blank |
| 4.2 | Utility & service lines | blank | blank | blank | blank | blank | blank |
| 4.3 | Shipping lanes | blank | blank | blank | blank | blank | blank |
| 4.4 | Flight paths | blank | blank | blank | blank | blank | blank |
| 5 | Biological resource use | C | Medium | Restricted (11-30%) | Serious (31-70%) | High (Continuing) | blank |
| 5.1 | Hunting & collecting terrestrial animals | blank | blank | blank | blank | blank | blank |
| 5.2 | Gathering terrestrial plants | blank | Negligible | Pervasive (71-100%) | Negligible (<1%) | High (Continuing) | Attractive fern prized by collectors at accessible sites. |
| 5.3 | Logging & wood harvesting | C | Medium | Restricted (11-30%) | Serious (31-70%) | High (Continuing) | Mainly on private land where mitigation, management, protections are not in place. |
| 5.4 | Fishing & harvesting aquatic resources | blank | blank | blank | blank | blank | blank |
| 6 | Human intrusions & disturbance | D | Low | Large (31-70%) | Slight (1-10%) | High (Continuing) | blank |
| 6.1 | Recreational activities | D | Low | Large (31-70%) | Slight (1-10%) | High (Continuing) | Includes rock-climbing, spelunking, off-trail hiking. Some on private or public sites. |
| 6.2 | War, civil unrest & military exercises | blank | blank | blank | blank | blank | blank |
| 6.3 | Work & other activities | blank | blank | blank | blank | blank | blank |
| 7 | Natural system modifications | blank | blank | blank | blank | blank | blank |
| 7.1 | Fire & fire suppression | blank | blank | blank | blank | blank | blank |
| 7.2 | Dams & water management/use | blank | blank | blank | blank | blank | blank |
| 7.3 | Other ecosystem modifications | blank | blank | blank | blank | blank | blank |
| 8 | Invasive & other problematic species & genes | D | Low | Restricted (11-30%) | Moderate (11-30%) | High (Continuing) | blank |
| 8.1 | Invasive non-native/alien species | D | Low | Restricted (11-30%) | Moderate (11-30%) | High (Continuing) | Huge patches of Garlic Mustard (has already wiped out sites), as well as Common Buckthorn, noted in field verifications. |
| 8.2 | Problematic native species | D | Low | Restricted (11-30%) | Moderate (11-30%) | High (Continuing) | Huge patches of Blue Cohosh and Impatiens pallida noted in field verifications. Potentially higher impacts here than the non-native species. Note that the severity would be at the low end of this range. |
| 8.3 | Introduced genetic material | blank | blank | blank | blank | blank | blank |
| 9 | Pollution | blank | blank | blank | blank | blank | blank |
| 9.1 | Household sewage & urban waste water | blank | blank | blank | blank | blank | blank |
| 9.2 | Industrial & military effluents | blank | blank | blank | blank | blank | blank |
| 9.3 | Agricultural & forestry effluents | blank | blank | blank | blank | blank | blank |
| 9.4 | Garbage & solid waste | blank | blank | blank | blank | blank | blank |
| 9.5 | Air-borne pollutants | blank | blank | blank | blank | blank | blank |
| 9.6 | Excess energy | blank | blank | blank | blank | blank | blank |
| 10 | Geological events | blank | blank | blank | blank | blank | blank |
| 10.1 | Volcanoes | blank | blank | blank | blank | blank | blank |
| 10.2 | Earthquakes/tsunamis | blank | blank | blank | blank | blank | blank |
| 10.3 | Avalanches/landslides | blank | blank | blank | blank | blank | blank |
| 11 | Climate change & severe weather | blank | Negligible | Pervasive (71-100%) | Negligible (<1%) | High (Continuing) | blank |
| 11.1 | Habitat shifting & alteration | blank | Negligible | Pervasive (71-100%) | Negligible (<1%) | High (Continuing) | Increased temperature and loss of humidity affect this species as summers get hotter. Microclimate specialist. Overall magnitude of this threat is unknown. Potential for some temperature increase in next 10 years, and certainly in the next 30 years. |
| 11.2 | Droughts | blank | blank | blank | blank | blank | blank |
| 11.3 | Temperature extremes | blank | blank | blank | blank | blank | blank |
| 11.4 | Storms & flooding | blank | blank | blank | blank | blank | blank |
c Classification of Threats adopted from IUCN-CMP, Salafsky et al. (2008).