COSEWIC Assessment and Status Report on the Colicroot aletris Farinosa in Canada - 2015

- Document Information
- COSEWIC Assessment Summary
- COSEWIC Executive Summary
- Technical Summary
- Preface
- COSEWIC History
- COSEWIC Mandate
- COSEWIC Membership
- Definitions (2015)
- Wildlife Species Description and Significance
- Distribution
- Habitat
- Biology
- Population Sizes and Trends
- Threats and Limiting Factors
- Protection, Status and Ranks
- Acknowledgements and Authorities Contacted
- Information Sources
- Biographical Summary of Report Writer
- Collections Examined
- Figure 1. Flowers of Colicroot showing the mealy texture of the petals.
- Figure 2. Global range of Colicroot (after Kartesz 2013).
- Figure 3. Range of Colicroot in Canada. Black circles: regions with extant subpopulations. Windsor-LaSalle: 4 subpopulations, 30 patches. Walpole Island: 2 subpopulations, 4 patches. Eagle: 1 subpopulation, 1 patch. Turkey Point: 1 subpopulation, 1 patch with status unknown but presumed extirpated. Historical subpopulations are not shown.
- Table 1. Status of Colicroot patches in Canada with previous and current abundance and ownership.
- 1. Walpole Island A
- 2. Walpole Island B
- Walpole Island C
- 3. Windsor - OJIBWAY (EO 3186)
- 4. Windsor - Todd Lane & Reddock St East (EO 3192)
- 5. Lasalle - Normandy S. & Huron Church Rd (EO--needs #)
- 6. Lasalle - Reaume Street And South (EO 3187)
- 7. West Elgin - Eagle (EO 5952)
- 8. Haldiman - Norfolk - Turkey Point Area (EO 3184)
- West Elgin (EO 11374)
- Northern Essex County (EO 66551)
- Total
- Table 2. Loss of abundance at patches where quantitative data are available.
- Appendix 1. Threats Assessment Worksheet.
COSEWIC
Committee on the Status
of Endangered Wildlife
in Canada

COSEPAC
Comité sur la situation
des espèces en péril
au Canada
COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2015. COSEWIC assessment and status report on the Colicroot Aletris farinosa in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 39 pp. (Species at Risk Public Registry website).
Previous report(s):
COSEWIC 2000. COSEWIC assessment and update status report on the colicroot Alextris farinosa in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 8 pp.
White, D.J., and M.J. Oldham. 2000. Update COSEWIC status report on the colicroot Aletris farinosa in Canada in COSEWIC assessment and update status report on the colicroot Aletris farinosa in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-8 pp.
Kirk, D.A. 1988. COSEWIC status report on the colicroot Aletris farinosa in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 39 pp.
COSEWIC would like to acknowledge Judith Jones (Winter Spider Eco-Consulting) for writing the status report on Colicroot, Aletris farinosa, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Bruce Bennett, Co-chair of the COSEWIC Vascular Plants Specialist Subcommittee.
For additional copies contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment Canada
Ottawa, ON
K1A 0H3
Tél. : 819-938-4125
Fax : 819-938-3984
COSEWIC email and COSEWIC website
Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur L'alétris farineux (Aletris farinosa) au Canada.
Cover illustration/photo:
Colicroot -- Photo credit: Mira Jones. This image may not be reproduced separately from this document without permission of the photographer.)
Colicroot (Aletris farinosa) is a herbaceous perennial in the Bog Asphodel Family (Nartheciaceae). It has a basal rosette of yellowy-green, lance-shaped leaves. In early summer, it produces an upright flowering stalk about 40 - 100 cm tall, with a spike of small, white flowers with a mealy texture. After flowering, the dried petals remain on the fruit capsules. Colicroot has been used to treat menstrual and uterine problems and contains active chemicals that may have hormonal properties.
In Canada, Colicroot is restricted to four geographic regions in southwestern Ontario: the City of Windsor-Town of LaSalle; Walpole Island; near Eagle (Municipality of West Elgin); and is inferred to be extirpated near Turkey Point (Haldimand-Norfolk County).
Colicroot grows in open, moist, sandy ground associated with tallgrass prairie habitats and damp sandy meadows. It is currently found in prairie remnants, old fields, utility corridors, and woodland edges. It is intolerant of shading by surrounding vegetation. For habitat to remain suitable, some type of disturbance must occur to keep vegetation open, short, and sparse. Historically, fire probably maintained habitat but more recently, human activities, such as periodic mowing, cultivation, and the use of walking and bicycling trails, create disturbance in Colicroot habitat but keep habitat only marginally suitable. Loss of habitat due to succession is the number one cause of the decline of Colicroot and is an urgent threat. Habitat has also been lost to urban development, to construction of the Right Honourable Herb Gray Parkway (Parkway), and to conversion to agricultural use.
Habitat in Parkway restoration sites and at some sites on Walpole Island is currently maintained by controlled burning and manual removal of woody and invasive species. However, habitat has been lost in Natural Heritage Areas and a provincial nature reserve, showing that Colicroot is not protected if management is not adequate. It is unknown whether habitat can be restored from a completely wooded state.
Colicroot is perennial and some plants probably live for decades. The time required to reach maturity from seed is unknown but is likely more than one year and probably depends on site conditions. It is unknown how long seeds remain viable or if there is a seed bank in the soil. In addition to sexual reproduction, vegetative reproduction is possible but infrequent from buds on the rhizome. Thus, some plants in a patch may not be genetically distinct individuals. Flowers are insect-pollinated, mainly by bumblebees and solitary bees. It is unknown whether the flowers are self-fertile. It has been suggested that Colicroot may have mycorrhizal requirements because, until recently, most attempts to transplant the species were unsuccessful. However, greenhouse tests found no evidence that mycorrhizal fungi confer an advantage. Colicroot has no specialized structures to assist dispersal. Flowering stalks are frequently eaten by deer or other herbivores, and the leaves are sometimes eaten by insects. It is unknown whether herbivores can disperse seeds through the gut.
Total abundance in 2014 was between 14,000 to 15,000 plants, with ~14,600 the best available estimate. Over half of the individuals in the Canadian population are the results of transplants and those propagated to allow for the construction of the Parkway. There are 35 patches of Colicroot in seven subpopulations confirmed extant and one patch in one subpopulation presumed extant with status unknown. Approximately 93% of all individuals occur within 12 km2 in Windsor-LaSalle, and 82% of individuals (~12,000) are in the Parkway restoration sites. Only about 18% (~2700 plants) are present elsewhere. All Colicroot planted in Parkway restoration sites were originally naturally occurring plants, so plants in restoration sites are considered natural individuals.
Discoveries of new sites and increases of mature individuals constitute ~14,000 plants, but the total 2014 abundance is around 14,600: most of the population known when it was first assessed in 1987 has been lost. Assuming newly discovered plants existed previously and including the plants remaining from the previously known population, there may have been a base population of at least 18,330 in 1986. If the transplanted 7,680 individuals are removed from the total, a population of 10,650 remains. Since then, there has been a measurable loss of more than 5000 plants or >47% of the population, with the actual decline well upwards of that.
Threats to Colicroot include:
- Lack of Disturbance
- Invasive Species
- Herbivory, and
- Development.
To maintain Colicroot, its habitat must be actively and frequently managed to arrest succession; most of the habitat isn't managed this way, even in protected areas. Recreational activities may cause trampling but sometimes also provide necessary disturbance. It is unknown whether the net result is beneficial or detrimental.
COSEWIC most recently assessed this species as Endangered in November 2015. Colicroot is currently listed as Threatened on Schedule 1 of the Canadian Species at Risk Act (SARA) and as Threatened under the Ontario Endangered Species Act 2007. As of November 2014, no habitat has been regulated under provincial law. Sixteen Colicroot patches are in publicly owned "protected" areas, yet Colicroot remains highly threatened with significant declines on these lands. Ten patches are in private ownership, four patches are on First Nation lands, five are in Parkway restoration sites, and one has corporate ownership.
| Summary Items | Information |
|---|---|
| Generation time (usually average age of parents in the population; indicate if another method of estimating generation time indicated in the IUCN guidelines (2011) is being used) | Estimated to be 7-15 years (Generation time could be longer if seedbank half-life is considered). |
| Is there an observed, inferred, or projected continuing decline in number of mature individuals? | Yes. Inferred projected decline of 22-70% (median 40%) in the next 10 years based on a calculated threat impact of High. Observed and measurable loss of >47% since 1986, with actual loss much greater from an additional 20 patches lost that have no abundance data. Losses are evident since 2008, showing that declines continue. |
| Estimated percent of continuing decline in total number of mature individuals within 5 years | Unknown but projected that patches with fewer than 20 plants may not last 5 years, based on number of patches that have disappeared since 2008. Projected loss of at least 11 patches with a total of 67 plants, which is only a small percent of total individuals but is a 31% loss of total patches. |
| [Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over the last [10 years, or 3 generations]. | Yes. Measurable loss (observed and estimated) of at least (47% of total) since 1986. Actual loss is much greater due to 20 additional patches lost where number of plants lost is unknown. |
| Projected or suspected] percent [reduction or increase] in total number of mature individuals over the next 10 years. | Projected decline of 22-70% (median 40%) in the next 10 years based on a calculated threat impact of High. Unknown but suspected that patches with <30 plants may not last 10 years based on losses of some small patches since 2008. |
| [Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over any [10 years, or 3 generations] period, over a time period including both the past and the future. | Projected decline of 22-70% (median 40%) in the next 10 years based on a calculated threat impact of High. Observed loss of >47% of individuals and inferred loss of 30 to 35% in last 28 years. |
Are the causes of the decline:
|
|
| Are there extreme fluctuations in number of mature individuals? | No. |
| Summary Items | Information |
|---|---|
| Estimated extent of occurrence (EOO) | 1967 km2 A decline from 3678 km2 with the loss of Turkey Point. |
| Index of area of occupancy (IAO) (number of occupied 2x2 km grid squares). |
32 km2 |
| Is the population "severely fragmented" i.e. is >50% of its total area of occupancy in habitat patches that are (a) smaller than would be required to support a viable population, and (b) separated from other habitat patches by a distance larger than the species can be expected to disperse? | a. possibly (due to management actions, it is not known if >50% of suitable habitat is smaller than required to support a viable population). b. Yes--seeds have no active dispersal mechanisms. Patches are separated by significant barriers to dispersal such as buildings, lawns, streets, and asphalted areas, as well as by forest. |
| Number of locations (Note: See Definitions and Abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
Ranges between 6-14, based on property ownership. |
| Is there an [observed, inferred, or projected] decline in extent of occurrence? | Yes--inferred loss of Turkey Point subpopulation, resulting in a loss of 1711 km2 or 47% of previous EOO. |
| Is there an [observed, inferred, or projected] decline in index of area of occupancy? | Yes--observed loss of some Walpole Island patches, and several Windsor patches, and inferred loss of Turkey Point, resulting in a loss of 20 km2 or 36% of previous IAO. |
| Is there an [observed, inferred, or projected] decline in number of subpopulations? Is there an [observed, inferred, or projected] decline in number of patches? | Yes--inferred loss of Turkey Point subpopulation. Yes--observed: loss of at least 24 patches since 1986, or approximately 30% of those present in 1986. See text for justification for analysis with a small separation distance. |
| Is there an [observed, inferred, or projected] decline in number of "locations"? | Yes--inferred loss of Turkey Point |
| Is there an [observed, inferred, or projected] decline in [area, extent and/or quality] of habitat? | Yes--observed: complete loss of habitat, due to succession, development, or conversion to agriculture, of at least 20 patches. Vegetation succession continues to fill in habitat. |
| Are there extreme fluctuations in number of subpopulations? | No |
| Are there extreme fluctuations in number of "locations"? | No |
| Are there extreme fluctuations in extent of occurrence? | No |
| Are there extreme fluctuations in index of area of occupancy? | No |
| Summary Items | Information |
|---|---|
| 1. Walpole Island A - 2 patches | Unknown (potentially up to 100?) |
| 2. Walpole Island B - 2 patches | Unknown (potentially up to 500?) |
| 3. Windsor Ojibway - 21 patches | ~3300 (+4473 transplants in restoration sites) |
| 4. Windsor Todd Lane - 3 patches | ~2700 (+3207 transplants in restoration sites) |
| 5. LaSalle Normandy St. - 4 patches | ~104 |
| 6. LaSalle Reaume St. - 2 patches | 22 |
| 7. West Elgin - Eagle - 1 patch | 420 |
| 8. Turkey Point - 1 patch | inferred extirpated |
| Totals: 8 subpopulations; 36 patches | 6800+ (plus 7680 transplants in restoration sites) |
| Summary Items | Information |
|---|---|
| Probability of extinction in the wild is at least [20% within 20 years or 5 generations, or 10% within 100 years]. | Not done. |
- Lack of Disturbance (High impact)
- Invasive Species (High impact)
- Problematic Native Species (Medium-low impact)
- Urban Development (Low impact)
Was a threats calculator completed for this species and if so, by whom?
Yes
February 25, 2015 by Bruce Bennett, Judith Jones, Ruben Boles, Del Meidinger, Jim Pojar, Stephanie Pellerin, Michael Oldham, Dan Benoit, and Karen Timm.
Overall threat level was calculated to be very high to high.
| Summary Items | Information |
|---|---|
| Status of outside population(s) most likely to provide immigrants to Canada. | Species is Imperilled (S2) in New York, Vulnerable (S3) in Indiana, Vulnerable to Apparently Secure (S3S4) in Michigan, and Apparently Secure (S4) in Ohio. |
| Is immigration known or possible? | Not likely as Colicroot has no long-distance dispersal mechanisms. |
| Would immigrants be adapted to survive in Canada? | Probably yes |
| Is there sufficient habitat for immigrants in Canada? | No |
| Are conditions deteriorating in Canada? | Yes |
| Are conditions for the outside population deteriorating? | No |
| Is the Canadian population considered to be a sink? | No |
| Is rescue from outside populations likely? | No |
| Summary Items | Information |
|---|---|
| Is this a data sensitive species? | Possibly--plants have medicinal value. |
COSEWIC: Designated Threatened in April 1988. Status re-examined and confirmed in November 2000. Status re-examined and designated Endangered in November 2015.
Additional considerations:
Occurrence within protected areas has not protected Colicroot from threats (succession, invasive species, and deer browse). Also, 93% of Canadian population occurs in one location in 12 km2 in urban area within City of Windsor - Town of LaSalle.
Colicroot (Aletris farinosa) was first assessed as Threatened in 1988. The Threatened status was reconfirmed in 2000. For the 2000 assessment, an update status report was prepared, but no new fieldwork was done at that time. The update reorganized existing Colicroot records into subpopulations using a 1 km separation distance and reported one new subpopulation. Major new fieldwork has now been done as background for the construction of the Right Honourable Herb Gray Parkway in Windsor. As well, the Walpole Island First Nation community is actively working to protect species at risk and has done recent survey work. However, urban development and ecological succession of remnant prairie vegetation have changed Colicroot habitat greatly over the past 30 years.
As part of mitigation work for the construction of the Parkway, thousands of previously unknown Colicroot plants were discovered. These were removed before construction began and transplanted into restoration sites containing suitable habitat and pre-existing, naturally established Colicroot plants. The restoration sites are currently owned by the Ontario Ministry of Transportation and monitoring and management activities are ongoing.
For this report, more than 46 sites where Colicroot was reported since 1975 were visited to update information. Despite discoveries of new sites, the results show a loss of 22 patches (38% of total patches), a measurable loss of more than 27% of total number of mature individuals, and an inferred loss much greater than that. Based on guideline 3 of the COSEWIC Guidelines on Manipulated Populations, the subpopulations manipulated during the construction of the Parkway are not included in the application of quantitative criteria until it can be demonstrated that the transplants are having a net positive impact on Colicroot. Furthermore, it is unknown whether one subpopulation present in 1992 is extant. If extirpated, the loss of this subpopulation would cause a decline of 47% in the extent of occurrence (EOO). Habitat for Colicroot is extremely limited, and protective management has not prevented losses.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
The Canadian Wildlife Service, Environment and Climate Change Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Scientific name: Aletris farinosa L.
Synonyms: Aletris alba Michaux
Common names: Colicroot, Alétris Farineux
Other common names:
White-tubed Colicroot, White Star-grass, Unicorn Root
White Colicroot, Licorne Vraie,
Aakshkodewa' Jiibig (Anishnaabemowin)
Family: Nartheciaceae (Bog Asphodel Family)
Major plant group: Monocotyledoneae
The genus Aletris has traditionally been placed in the Liliaceae (Fernald 1950; Gleason 1968; Gleason and Cronquist 1991; Reveal and Pires 2002). NatureServe (2015) currently places the genus there. Some authors have placed Aletris in the Melanthiaceae based on the half-inferior ovary position (Reznicek et al. 2011; Voss and Reznicek 2012) or in the Nartheciaceae based on the presence of air spaces in the root cortex and a single style (Zomlefer 1997). Utech (2002) in his introduction to the Lily Family in the Flora of North America recognizes that Aletris may be a member of the segregate family Nartheciaceae. Recent taxonomic studies that include phylogenetic work (Mercx et al. 2008; Fuse et al. 2012; Zhao et al. 2012) all place Aletris in the Nartheciaceae. The North American databases BONAP (Kartesz 2013) and VASCAN (Brouillet et al. 2014) also now place Aletris in the Nartheciaceae. The genus Aletris is made up of 25 species, five of which are found in North America (Sullivan 2002).
The name Aletris is said to come from the Greek name for the female slave who ground grain, alluding to the mealy texture of the flower petals (Sullivan 2002). The specific epithet farinosa, which means floury, also refers to this texture.
Colicroot is a herbaceous perennial that arises from a short, thick rhizome. The plant produces a cluster or "basal rosette" of pale yellowy-green, lance-shaped leaves 4 - 20 cm long and 0.5 - 2.6 cm wide. In late June and early July, an upright flowering stalk (or scape) about 40 - 100 cm tall, bearing only a few bracts and no leaves, arises from the leaf rosette. The flowering stalk supports a narrow spike of small (7-10 mm), mealy, white, tubular-shaped flowers (Figure 1). A single rosette may produce several flowering stalks. After flowering, the dried petals remain on the developing fruit, which is a many-seeded, three-parted capsule (Sullivan 2002). The dried fruits and flowering stalks as well as the basal rosettes persist through the winter, and some seed is retained in the capsules until spring (Kirk 1987).

Long description for Figure 1
Close-up photo of two flowering stalks of the Colicroot, showing the mealy, white, tubular-shaped flowers.
NOTE: In this document Population refers to the sum total of all Colicroot plants in Canada. Patch refers to a group of Colicroot plants that are not separated from each other by barriers taller than their flowering stalks (~ 1 m) or by non-natural features such as streets or buildings. Subpopulation refers to all patches that are within 1 km of each other, which also corresponds to an element occurrence or EO (NatureServe 2004). Site refers to a physical place where Colicroot occurs or has occurred. Location refers to a geographically or ecologically distinct area in which a single threatening event can rapidly affect all plants of Colicroot.
Approximately 93% of all Canadian individuals are in the Windsor-LaSalle region in an area of 12 km2, and two of those subpopulations contain 85% of all Canadian individuals. The subpopulations in Windsor-LaSalle occur as numerous, small groups of plants (usually fewer than 40) in very small (often 5 to 30 m2) patches of habitat, separated from each other by urban development. Of the three confirmed extant subpopulations not in Windsor-LaSalle, one subpopulation is separated from Windsor by more than 120 km, and two other subpopulations are each approximately 50 km from Windsor.
The current spatial structure of the Colicroot population likely reflects remnants of its historical range in Canada, rather than recent dispersal patterns. Across its global range, Colicroot is associated with prairies and meadows (Zomlefer 1997), but almost all natural prairie in Ontario has now been converted to agriculture or urban development. Bakowsky and Riley (1994) estimate that only about 2100 ha or 0.5% of the tallgrass prairie and savannah present in the 19th century remains in Ontario. The current spatial distribution of Colicroot corresponds to places where Bakowsky and Riley (1994) show "large intact remnants" or "large remnants" of prairie still persist.
There are no recognized subspecies or varieties within the species. Although some subpopulations are widely separated, there are no genetic studies that might support separate designatable units (DU). Reports from fieldwork (Kirk 1987; Oldham 2000; Bowles 2005; Bowles pers. comm. 2010; Pratt pers. comm. 2010; Woodliffe pers. comm. 2010; R. Jones pers. comm. 2014; Waldron pers. comm. 2014) have not indicated that any subpopulation appeared different or distinct.
Tallgrass prairie habitats have been used by Native people for centuries as a place to find food, medicinal plants and crafting materials, and as hunting grounds (Walpole Island Heritage Centre 2006). The historical distribution of grasslands is closely tied to the ancestral activities of Native people, especially intentional burning to maintain open land (Riley 2013). Thus, the current distribution of Colicroot habitat is closely linked to ancestral and current Native traditions.
Colicroot is reported to have medicinal value both in historical and current literature. The rhizome and roots have been used as remedies for the treatment of menstrual and uterine problems, as well as abdominal pain, constipation, flatulence, joint pain, and rheumatism (Butler and Costello 1944; Hutchens 1973; Kirk 1987; WebMD 2014). The species was listed as an accepted remedy in the Dispensatory of the United States from at least 1869 until 1947 (Wood and Bache 1869; Osol and Farrar 1955) and may have been one of the first applications of progesterone treatment for premenstrual syndrome (Norris 1987). Colicroot contains several active chemicals that may have medicinal properties including diosgenin-based steroids (Marker et al. 1940) that can be converted to progesterone and thus have hormonal properties (Norris 1987). Challinor et al. (2013) reported the isolation of a sesterterpine derivative, which may have medicinal value, from the roots of Colicroot. Thus, the medicinal use of Colicroot continues to be of contemporary interest.
Colicroot is part of a group of species that only occur in prairie remnants or related habitats. Many of these species occur in Canada only in southwestern Ontario, and many are legally listed as at risk or have a high sub-national rank in Ontario.
Colicroot is indigenous to North America (Figure 2). In the United States, it occurs in 27 states from New York and Wisconsin, south to Florida and eastern Texas (Sullivan 2002; NatureServe 2015). It is most frequent in the southeastern states of Virginia, North Carolina, and South Carolina (Kartesz 2013).
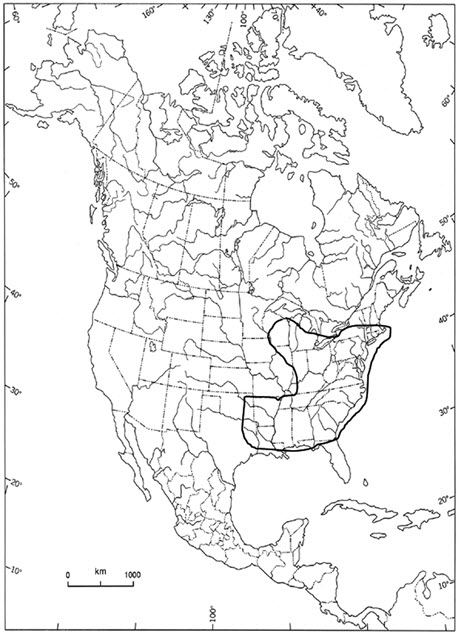
Long description for Figure 2
Map of the global distribution of the Colicroot in North America. In the United States, it occurs in 27 states from New York and Wisconsin, south to Florida and eastern Texas. In Canada it occurs only in southwestern Ontario.
Colicroot has an extremely restricted geographic range in Canada and has never been observed outside of southwestern Ontario. Current confirmed occurrences are the City of Windsor and the adjacent Town of LaSalle; Walpole Island (in Lake St. Clair); and near Eagle, in the municipality of West Elgin, Ontario (Figure 3). Seven subpopulations with 35 patches are confirmed extant, and one patch in one subpopulation is presumed extirpated (Table 1). Detailed locality data for all recent observations (Jones 2014) are on file with the Natural Heritage Information Centre (NHIC) of the Ontario Ministry of Natural Resources and Forestry. Colicroot occurs within the Great Lakes Plains Ecological Area.
Historically, Colicroot was collected in:
- 1886 from sandy thickets near Leamington, Essex County, by T.J. Burgess;
- 1891 from Caradoc Township, Middlesex County, by J. Dearness;
- 1896 near Sarnia, Lambton County, by C.K. Dodge;
- 1901 also near Leamington by John Macoun;
- 1958 from Squirrel Island, Lambton County by L.O. Gaiser;
- 1979 near West Lorne, Elgin County, by W.G. Stewart.
It was observed but not collected in:
- 1956 from Concession 6 in Charlotteville Township, Norfolk County, by M. Landon.
All of these subpopulations have never been found again and are now considered extirpated (Kirk 1987; NHIC 2014).
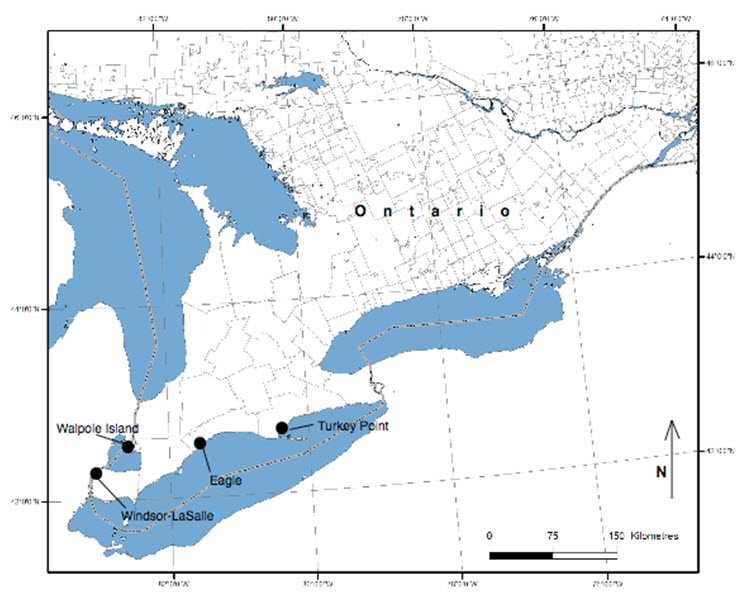
Long description for Figure 3
Map of the distribution of the Colicroot in Canada, indicating regions with extant subpopulations. Historical subpopulations are not shown.
Sources: Kirk (1987); LGL and URS (2010); Woodliffe (pers. comm. 2010); LGL (2013); Jacobs (pers. comm. 2014); Jones (2014); R. Jones (pers. comm. 2014); NHIC (2014); Town of LaSalle (2014); Waldron (pers. comm. 2014).
Legend: Extant patches in black; extirpated patches in grey italics. EO number = element occurrence in NHIC (2014); FN = First Nation; FRS = Parkway final restoration site; PNR = provincial nature reserve; NV = not viable; V = Viable; VU = Viability unknown
Note: Abundance numbers are mature individuals unless indicated. Abundance of sterile rosettes is reported if known.
Note: At the request of Walpole Island First Nation, locality data are not presented.
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| Walpole #1 | Extant | NV | ~100 Kirk 1986 |
2014 | 3 | FN |
| Walpole #2 | Presumed Extant (burned regularly) |
VU | 13 Kirk 1986 |
unknown | unknown | FN |
| Walpole #3 | Extirpated (by agriculture?) |
- | Allen 1986 | - | - | FN |
| walpole #4 | extirpated by Succession |
- | 4+numerous rosettes Kirk 1986 |
1987? | - | FN |
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| Walpole #5 | Presumed extant; prairie species still present | VU | ~ 5 Allen 1986 |
unknown | unknown | FN |
| Walpole #6 | Extant | V | 90+numerous rosettes Kirk 1986 |
2014 | 312 2012: 236 2006: 310 2004: 325 |
FN |
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| Walpole #7 | Extirpated probably by agriculture, 1985 |
- | allen 1984 | - | - | FN |
| Squirrel Island | Extirpated Restoration ongoing |
- | Geiser 1958 | unknown | - | FN |
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| Ojibway Prairie PNR #s 1, 8, 9, 10 | Extant | V | Kirk 1986 pt. 4 | 2014 | 69 + additional rosettes | Ontario Parks |
| Ojibway Prairie PNR #s 2 & 3 | Extant | V | Kirk 1986 pt. 3 | 2014 | 189 | Ontario Parks |
| Ojibway Prairie PNR #4 | Extant | V | - | 2014 | 23 + 12 rosettes |
Ontario Parks |
| Ojibway Prairie PNR #5 | Extant | NV | Kirk 1986 pt. 2 | 2014 | 4 | Ontario Parks |
| Ojibway Prairie PNR #6 | Extant | NV | Kirk 1986 pt. 6 | 2014 | 3 | Ontario Parks |
| Ojibway Prairie PNR #7 | Extant | V | - | 2014 | >300 | Ontario Parks |
| Ojibway Prairie PNR - Ball Diamond | Extant | V | 600 Woodliffe 2009 E of Kirk pt. 7 |
2014 | >500 +additional rosettes |
Ontario Parks |
| Ojibway Prairie PNR | Presumed Extant | VU | Kirk 1986 pt. 8 | unknown | unknown | Ontario Parks |
| Tallgrass Prairie Heritage Park Hydro corridor (Not ,Kirk's Essex #6) |
Extant | NV | - | 2014 | 6 | City of Windsor |
| Spring Garden Natural Area Part of Kirk's Essex #7 |
Extant | NV | Part of 190 in 1986 Kirk's Essex #7; Oldham 1994 |
2014 | 22 | City of Windsor |
| Spring Garden Natural Area Part of Kirk's Essex #7 |
Extant | NV | Part of 190 in 1986 Kirk's Essex #7 |
2014 | 8 | City of Windsor |
| Kent Street at SGNA Part of Kirk's Essex #7 |
Extant | V | Part of 190 in 1986 Kirk's Essex #7 |
2014 | ~300 + ~50 rosettes |
Private |
| Spring Garden Natural Area | Extant | NV | Oldham 1994 | 2014 | 3 | City of Windsor |
| Lansing Street at SGNA | Extant | V | Oldham 1994 | 2014 | 120 | Private |
| SGNA east side between Lamont & Lambton Streets | Extant | VU | - | 2014 | unknown | Private |
| Reddock Avenue West hydro station | Extant | V | - | 2014 by R. Jones | ~50 | Private |
| Sandwich West Public School hydro line | Extant | NV | - | 2014 | 116 + ~20 rosettes |
Town of LaSalle |
| Herb Gray Parkway Chappus St. Restoration site FRS #7 | Extant | V | 1526 naturally occurring plants present prior to transplantation in these four restoration sites) | 2009 | 373 transplanted plus naturally occurring plants (left) | MTO |
| Herb Gray Parkway Chappus St Restoration site FRS #15 |
Extant | V | 1526 naturally occurring plants present prior to transplantation in these four restoration sites) | 2009 | 920 transplanted plus naturally occurring plants (left) | MTO |
| Herb Gray Parkway Chappus St Restoration site FRS #25 |
Extant | V | 1526 naturally occurring plants present prior to transplantation in these four restoration sites) | 2009 | 0 transplanted but naturally occurring plants present (left) |
MTO |
| Herb Gray Parkway Chappus St Restoration site FRS #23 |
Extant | V | 1526 naturally occurring plants present prior to transplantation in these four restoration sites) | 2009 | 3180 transplanted | MTO |
| North of Raceway - Kirk's Essex #10 | Extirpated | - | Oldham 1984 | 2014 | not present | Corporate |
| Ojibway Prairie PNR | Extirpated | - | Kirk 1986 pt. 1 | 2014 | not present | Ontario Parks |
| Ojibway Prairie PNR | Extirpated | - | Kirk 1986 pt. 5 | 2014 | not present | Ontario Parks |
| Spring Garden Natural Area | Extirpated | - | Oldham 1994 #1 pt. #1 |
2014 | not present | City of Windsor |
| Spring Garden Natural Area | Extirpated | - | Oldham 1994 #2 | 2014 | not present | City of Windsor |
| Spring Garden Natural Area | Extirpated | - | Oldham 1994 #3 | 2014 | not present | City of Windsor |
| Spring Garden Natural Area | Extirpated | - | Oldham 1994 #4 | 2014 | not present | City of Windsor |
| Spring Garden Natural Area | Extirpated | - | Oldham 1994 #5 | 2014 | not present | City of Windsor |
| Spring Garden Natural Area | Extirpated | - | Oldham 1994 #6 | 2014 | not present | City of Windsor |
| Tallgrass Prairie Heritage Park Kirk's Essex #6 |
Extirpated by succession & Phragmites |
- | 10 Kirk 1987 |
2014 | not present | City of Windsor |
| MicMac Park area Kirk's Essex #8 |
Extirpated by succession (& industrial development?) |
- | Pratt 1976 | 2014 | not present | City of Windsor |
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| Herb Gray Parkway -Todd Lane Restoration site FRS #5 (includes South of Reddock Ave) |
Extant | V | 2700 naturally occurring plants present prior to transplantation | 2009 by MTO | 3207 transplanted plus naturally occurring plants (left) | MTO |
| Reddock Avenue East | Extant | VU | - | 2009 by MTO | unknown | Private |
| North of Reddock Avenue East | Extant | VU | - | 2009 by MTO | unknown | Private |
| Oakwood Park Kirk's Essex #9 | Extirpated by succession |
- | Pratt 1984 | 2014 | not present | City of Windsor |
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| Westbrook at Parkway | Extant | V | - | 2009 by MTO | 30 | Private |
| Normandy & Huron Church Not the same as Kirk Essex # 4 |
Extant | NV | - | 2014 | 31 | Private |
| Villa Maria Blvd NE of Huron Church | Extant | VU | - | 2009 by MTO | 18 | Private |
| LaSalle Woodlot west of Brunet Park (Washington Street Prairie) | Extant | V | ~1000 Bakowsky 1994 |
2014 by R. Jones |
~25 Loss of ~1000 to succession |
Town of LaSalle |
| Brunet Park, Kirk's Essex #3 | Extirpated by succession |
- | Pratt 1987 | 2014 | not present | Town of LaSalle |
| Normandy & Huron Church - Kirk's Essex #4 | Extirpated by succession |
- | Allen 1985 | - | not present | Private |
| St. Clair College grounds | Extirpated by succession |
- | Waldron 2008 | 2014 | not present | St. Clair College |
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| Reaume Prairie, Kirk Essex #1 | Extant | NV | 1000s Kirk 1986 |
2014 | 15 | Town of LaSalle |
| Bouffard Road west of railway | Extant | NV | - | 2014 | 7 | Town of LaSalle |
| West Reaume St., Kirk's Essex #2 | Extirpated by development |
- | 12 Kirk 1986 |
1990s | not present | Private |
| Stanton Street, CNHS TC4 | Extirpated by development |
- | present; no census Waldron 2008 |
2014 | not present | Town of LaSalle |
| Deerview Crescent west end of CNHS TC6 | Extirpated by development |
- | 30 Waldron 2004 |
2014 | not present | Private |
| Malden and Bouffard west end CNHS TC6 | Extirpated by succession |
- | Waldron 2004 | 2014 | not present | Town of LaSalle |
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| Eagle | Extant | V | 60 Oldham 1993 |
2014 | 420 | Private |
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| North of Turkey Point | Unknown Likely extirpated | VU | 50 in 1986 10-20 in 1996 |
1996 | not found in searches in 2002 | Corporate |
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| West Lorne Woods | Extirpated by succession |
- | 6 Stewart 1979 |
2014 | not present | NGO |
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| Ruscom Shores Conservation Area | Erroneous report from 1983 | - | - | 2014 | not present | Conservation Authority |
| Subpopulation name and component patches |
Status | Viability | Previous abundance (if known), observer, date of observation | Date most recent observation |
Abundance | Ownership |
|---|---|---|---|---|---|---|
| Totals: 35 extant patches, 1 patch status unknown, in 8 subpopulations. | - | NV - 11 VU - 7 V - 17 |
- | - | ~14,600 (based on totals above 14,480+rosettes) 6800+ 7680 in restoration sites |
- |
There is also a record of another subpopulation north of Turkey Point in Haldimand-Norfolk County (not the same as the 1956 site, above) where the continued presence of Colicroot is considered extirpated but perhaps still possible. In 1996, a small cluster of 10 to 20 plants was observed at this site, but the site was searched extensively in 2002 (see Search Effort) and no plants were found. Habitat encroachment by shrubs and disturbance from logging activities were noted at that time (NHIC 2014). Permission to access the property was not granted in 2014 (Jones 2014), but the site may still have suitable habitat present, based on its appearance on satellite imagery. As well, Colicroot requires disturbance in its habitat and has persisted or recolonized at other sites after disturbance (Jones 2014; R. Jones pers. comm. 2014). Therefore, although unlikely, the continued presence of Colicroot at this site cannot yet be ruled out.
In Windsor, thousands of previously unknown Colicroot plants were discovered mostly near the current Todd Lane and Windsor-Ojibway subpopulations when environmental work began in 2004 for the construction of the Right Honourable Herb Gray Parkway (the Parkway) leading to the new Detroit River International Crossing (URS 2009; LGL and URS 2010; Waldron pers. comm. 2010; Woodliffe pers. comm. 2010). These individuals make up the vast majority of the Canadian population of Colicroot. Extensive mitigation work has been undertaken to offset impacts from Parkway construction. All Colicroot plants have been removed from the Parkway corridor and most have been transplanted into restoration sites in Windsor-LaSalle on lands currently owned by the Ontario Ministry of Transportation (MTO). Prior to any transplantation, the areas chosen as restoration sites already contained natural habitat and naturally occurring patches of Colicroot (LGL and URS 2010; LGL 2013). Data from mitigation work for the Parkway are included in this report. These occurrences are considered an intra-limital introduction that were established for conservation and were locally sourced and as such are considered part of the wildlife species. However, following Manipulated Populations Guideline #3 (COSEWIC 2010) COSEWIC will generally only include such subpopulations in the application of quantitative criteria to establish status where the population is predicted or demonstrated to have a net positive impact on the wildlife species being assessed. A net positive impact would result in an increase in the average fitness of individuals of the wildlife species. The loss of some of the best remaining habitat and the removal of the largest Canadian subpopulations for the construction of the Parkway was not considered to have a net positive impact on Colicroot, and as such the transplanted individuals are not included in the application of quantitative criteria.
Transplanted individuals have been shown producing viable seed (MNRF 2014), although it is unknown whether this has resulted in an overall increase to the Canadian population.
The extent of occurrence (EOO) is 1967 km2, which includes Walpole Island to the north, Windsor-Lasalle to the west, and the site near Eagle to the southeast. If the Turkey Point site is included, the EOO would be 3678 km2. The loss of Turkey Point is a 47% reduction of the previous EOO. The index of area of occupancy (IAO) is 32 km2. Of that total, 20 km2 is in Windsor-Lasalle, 8 km2 is at Walpole Island, and 4 km2 is at Eagle. Roughly 93% of all individuals are in 12 km2 (actual polygon area) in Windsor-LaSalle.
The majority of the Canadian population is in restoration sites from the Herb Gray Parkway (Parkway) construction project in Windsor-LaSalle.
The population of Colicroot may be severely fragmented. Patches with fewer than 20 individuals or in habitat of only a few square metres are considered not viable. Of 35 total patches, 31% (11) are considered not viable and 20% have viability unknown (7; Table 1 ).
Approximately 93% of mature individuals are scattered in small, disconnected patches in Windsor-LaSalle. Although some of these patches are within 1 km of each other, they are separated by significant barriers to dispersal such as residential developments. Colicroot has no particular dispersal mechanism to overcome these barriers, and most suitable habitat now consists of just a few square metres of open ground surrounded by unsuitable habitat. The probability of seeds successfully arriving at suitable habitat is very low.
In 2014, all records of Colicroot in the database of the Natural Heritage Information Centre (NHIC 2014), in previous COSEWIC reports (Kirk 1987; White and Oldham 2000), and known to the MTO from the Parkway were tabulated. In addition, a number of people knowledgeable about Colicroot were consulted (see Acknowledgements).
In July, 2014, the report writer surveyed 46 sites where Colicroot had been reported previously (Jones 2014). Some of these were small patches within greater subpopulations, but patches were surveyed individually to provide data that could be compared to previous reports to discern trends. Judith Jones visited sites in Windsor-LaSalle, greater Essex County, and the Municipality of West Elgin but did not visit Walpole Island First Nation at the request of the community. At Walpole Island, surveys were done for some patches by Clint Jacobs in 2014 (Jacobs pers. comm. 2014). In Windsor, monitoring data from the Parkway sites were obtained. Incidental observations and estimated abundance were provided for two additional Windsor patches by Russ Jones of AMEC (R. Jones pers. comm. 2014).
In the Turkey Point area, there were three separate searches in 2002 of one hour each at the only known site and the species was not found (NHIC 2014). It is only a seven acre area to search, the observers were very experienced, and the species is distinctive even vegetatively (Oldham pers. comm. 2015). Turkey Point Provincial Park is nearby and there have been periodic prescribed burns. However, the areas that have been burned have been visited by botanists (Foster pers. comm. 2015; Oldham pers. comm. 2015). It is quite unlikely that Colicroot would be in the park and not seen. There are no historical records from the park. However, there is a lot of private land which is difficult to access so it is not possible to categorically say Colicroot is extirpated from the area.
Fieldwork was conducted when Colicroot was in flower and the tall stalks of white flowers are easy to see. At all patches, presence or absence of Colicroot was noted, and where present, abundance was tallied (see Sampling Methods). All patches were georeferenced, either as a point or as a polygon, with a hand-held GPS unit usually with accuracy of ±2 or 3 m (Jones 2014).
Characteristics of habitat were documented at each site. In most cases, Colicroot was present only in a few square metres of open ground within a larger matrix of unsuitable vegetation, so the features of the immediate microhabitat surrounding the plants were documented in detail, including dominant cover and common associate plant species. As well, some general characteristics of the matrix vegetation were noted along with the presence of any other distinguishing features, such as obvious disturbance or past land use history, evidence of moisture regimes, or presence of threats such as invasive species. At sites where Colicroot was no longer present, an attempt was made to discern the reason, in case there was a chance the plants might be able to return.
At two places where Colicroot was surrounded by a larger area of suitable habitat, Jones (2014) documented the Ecological Land Classification (ELC) vegetation type including soil characteristics. This has also been done for the Ojibway Prairie Provincial Nature Reserve (PNR) (Chambers 2010) and for the Parkway restoration sites (LGL and URS 2010).
Colicroot grows in open, moist, sandy ground associated with tallgrass prairie habitats and damp sandy meadows. The species is currently found in prairie and savannah remnants, old fields, utility corridors, and woodland edges. It is intolerant of shading by herbaceous or woody vegetation. High quality habitat has natural prairie soil of coarse sand, loamy sand, or sandy loam, shallow to no surface organic layer, fresh-moist soil moisture, full sun exposure, the presence of prairie-associated plant species, and few invasive species (Kirk 1987; LGL and URS 2010; Jones 2014; J. Jones unpubl. data 2014). A key factor is that the surrounding vegetation is either short or sparse enough to allow high levels of light to reach the basal rosettes at ground level.
Colicroot colonizes and thrives in bare, sandy ground and seems to do well in places where the topsoil has been exposed (Kirk 1987; Woodliffe pers. comm. 2010; J. Jones unpubl. data 2014). After disturbance, the species is able to grow for an unknown number of years as vegetation gradually becomes denser and taller, until conditions become unsuitable. If new disturbance occurs, Colicroot has been known to recur or to return after an apparent absence (Woodliffe pers. comm. 2010; J. Jones unpubl. data 2014; R. Jones pers. comm. 2014).
Thus, for habitat to remain suitable, some type of disturbance must either expose new ground or keep vegetation in a suitably open, short, sparse state. In historical times, fire probably removed tall vegetation and burnt off the litter or thatch. At Walpole Island, wild horses also once aided in maintaining the prairie habitat (Walpole Island Heritage Centre 2006). More recently, human activities, such as periodic mowing, cultivation, and the use of walking and bicycling trails, are involved in creating and maintaining habitat. However, such activities seem to result in only one or two of the three required habitat characteristics (open ground; short vegetation; sparse vegetation), so most habitat is currently only marginally suitable (J. Jones pers. obs. 2014). There is currently almost no possibility of wildfire or natural disturbance at any Colicroot site, so the only source of required disturbance is from human actions, whether intended as management or merely incidental.
Colicroot habitat occurs on coarse and fine lacustrine sands with a depth of 38-61 m (Kirk 1987). At Windsor-LaSalle and Walpole Island lacustrine sands are underlain by the St. Clair Clay Plain (Chapman and Putnam 1984) which prevents full drainage of surface sands and helps to maintain a fresh-moist soil moisture regime (Kirk 1987; Chambers 2010; LGL and URS 2010; Jones 2014). In these areas, the ground may be saturated during the early spring but may become very dry in mid-summer when the water table drops to the sand-clay interface (Chambers 2010). Ideally, if there is an organic horizon, it should show signs of being burnt in the recent past and should contain little nitrogen (LGL and URS 2010), a condition which favours prairie plants over non-native species (Morgan 1994). Additional information on soils at Colicroot sites is given in Kirk (1987).
Suitable habitat generally occurs as small areas of short, sparse microhabitat patches within larger areas of unsuitable tall, dense vegetation. Dominant species in the immediate microhabitat of Colicroot may include: Broom-sedge Bluestem (Andropogon virginicus), Hanging Bulrush (Scirpus pendulus), Rough-stemmed Goldenrod (Solidago rugosa), Virginia Mountain Mint (Pycnanthemum virginianum), Old-field Cinquefoil (Potentilla simplex), Prickly Raspberry (Rubus flagellaris), or Grass-leaved Goldenrod (Euthamia graminifolia). The greater vegetation polygon in which suitable microhabitat occurs may be dominated by Indian Nutgrass (Sorghastrum nutans), Little Bluestem (Schizachyrium scoparium), or Big Bluestem (Andropogon gerardii), or by common old field species such as Canada Goldenrod (Solidago canadensis), Canada Tick-trefoil (Desmodium canadensis), or Eurasian grasses such as Canada Bluegrass (Poa compressa) and Kentucky Bluegrass (P. pratensis) (J. Jones unpubl. data 2014).
Frequent associates include: Canada Cinquefoil (Potentilla canadensis), Bushy Seedbox (Ludwigia alternifolia), Short-fruited Rush (Juncus brachycarpus), Greene's Rush (Juncus greenei), Blood-red Milkwort (Polygala sanguinea), Whorled Milkwort (P. verticillata), Slender Fragrant Goldenrod (Euthamia caroliniana), Flowering Spurge (Euphorbia corollata), Arrow-leaved Violet (Viola sagittata), Wild Strawberry (Fragaria virginiana), Large Purple Agalinis (Agalinis purpurea), Three-awned Grass (Aristida purpurascens var. purpurascens), Virginia Anemone (Anemone virginiana var. virginiana), Field Thistle (Cirsium discolor), Poverty Oat Grass (Danthonia spicata), Grass-leaved Rush (Juncus marginatus), Round-headed Bush Clover (Lespedeza capitata), Gray-stemmed Goldenrod (Solidago nemoralis), White Heath Aster (Symphyotrichum ericoides), Smooth Aster (S. laeve), Tall Coreopsis (Coreopsis tripteris), and Dense Blazing Star (Liatris spicata) (LGL and URS 2010; J. Jones unpubl. data 2014; Waldron pers. comm. 2014). Many of these associate species are rare or at-risk (NatureServe 2014). More than 60 species at risk are known from the Ojibway Prairie Remnants Area of Natural and Scientific Interest (ANSI) in Windsor-LaSalle (Chambers 2010).
Vegetation at the Ojibway PNR, at Spring Garden Natural Area, and at Eagle, has been classified and mapped (Chambers 2010; Jones 2014) according to the Ecological Land Classification (ELC) of Southern Ontario (Lee 2008). Vegetation in the Parkway restoration sites was mapped and classified (LGL 2013) according to the older ELC system (Lee et al. 1998). Based on this work, Colicroot is found in the following vegetation community types:
Ojibway Prairie PNR:
- Dry Little Bluestem Graminoid Tallgrass Prairie Type
- Dry Indian Grass Graminoid Tallgrass Prairie Type
- Dry-Fresh Goldenrod Forb Meadow Type
- Dry-Fresh Forb Tallgrass Prairie Type
- Dry-Fresh Black Oak Deciduous Savanna Type
- Cultural - recreational
In addition, in 2014, the vegetation in the old baseball ball diamond area on the PNR had regenerated enough to be classified as Fresh-Moist Mixed Tallgrass Prairie Ecosite (Jones 2014), rather than as Cultural-Recreational.
Parkway Sites:
- Fresh-Moist Tallgrass Prairie Type
- Fresh-Moist Tallgrass Prairie / Mineral Meadow Marsh Type
- Gray Dogwood Mineral Cultural Thicket Type
- Dry Old Field Meadow Type
- Pin Oak Mineral Deciduous Swamp Type
- Openings within Fresh-Moist Poplar Deciduous Forest Type
Eagle:
- Dry-Fresh Mixed Meadow Ecosite or Dry-Moist Old Field Meadow Type
At Ojibway Prairie PNR Colicroot is most abundant in Dry-Fresh Goldenrod Forb Meadow and in Cultural-Recreational vegetation, both of which contained the greatest amount of microhabitat with short, sparse vegetation. Historically, the area of Dry-Fresh Goldenrod Forb Meadow was a cultivated farm field (Chambers 2010), and the Cultural-Recreational site was a baseball diamond that was mowed periodically (Woodliffe pers. comm. 2010). One of the more abundant Colicroot patches occurs at Eagle, where the habitat is open sandy ground that was formerly a cultivated field. The habitat at the Reaume Prairie was quarried for top soil and sand about 50 years ago (Waldron pers. comm. 2014). These examples demonstrate the history of disturbance in the habitat of Colicroot and show that fire is not necessarily the only source of acceptable disturbance.
A lack of suitable disturbance, leading to a loss of habitat, is the number one cause of the decline of Colicroot and is a most urgent threat. Without suitable disturbance, succession proceeds and vegetation height and density increase to levels unsuitable for Colicroot. Since 1986, eleven patches of Colicroot have been lost due to succession ( Table 1 ) to taller and denser vegetation, and nine additional patches have been reduced in size. Historically, an additional three subpopulations (not seen since before 1979) were lost to succession. In Windsor-LaSalle, all patches are smaller than they were in the past (Waldron pers. comm. 2014; J. Jones unpubl. data 2014), and most are now rapidly shrinking from the encroachment of tall vegetation, especially woody shrubs such as dogwoods (Cornus spp.), willows (Salix spp.), and Autumn Olive (Elaeagnus umbellata) (Jones 2014).
At Ojibway Prairie PNR and Spring Garden Natural Area, controlled burning is done periodically, but most of the formerly burned areas are now very densely covered with tall, native grasses. While still classified as prairie vegetation, this does not provide suitable habitat for Colicroot. It is possible that burning is not frequent or intense enough to expose ground for Colicroot to colonize or to release a potential seed bank. At Walpole Island First Nation, some habitat is actively maintained and burned on a regular basis by the community (Walpole Island Heritage Centre 2006; Jacobs pers. comm. 2014).
Succession can cause habitat loss in as little as 10 years. Several patches of habitat in LaSalle Natural Heritage Areas where plants were present in 2004 or 2008 (Waldron pers. comm. 2014) were no longer visibly open and no plants remained in 2014 (Jones 2014; Table 1 ). More than an acre (0.4 ha) of habitat in the LaSalle Woodlot west of Brunet Park, which supported more than 1000 plants in 1994 (NHIC 2014), was almost eliminated in 20 years. In 2014, this area had become tall, dense thickets of dogwood, raspberries (Rubus spp.), and tree saplings with almost no Colicroot habitat remaining. The habitat near Reaume Street (Essex #1 of Kirk (1987) supported thousands of Colicroot in 1986, but in 2014 no suitable habitat remained except a few square metres of ground kept open by a walking trail. Thus, the complete loss of habitat may happen in as little as 6 to 10 years, and almost certainly will occur in 20 years if no disturbance occurs. Successional degradation of Colicroot habitat has not been effectively managed.
Habitat has also been lost to urban development ( Table 1 ). Three patches have been turned into residential streets and houses (Jones 2014), including Essex #2 (Kirk 1987) and two patches documented in LaSalle in 2004 (Waldron pers. comm. 2014). Whether this trend will continue is unknown. Much of the undeveloped lands in the Town of LaSalle have been delineated as Natural Heritage Areas that will not be developed (Town of LaSalle 2014), so perhaps it can be speculated that the trend will stop. However, one patch of Colicroot has been lost since the Natural Heritage Areas were identified.
In addition, habitat has been lost due to the construction of the Parkway. The area occupied by Colicroot plants impacted in the Parkway footprint was approximately 5.65 ha, but the amount of habitat created, enhanced, and restored for Colicroot as mitigation is approximately 3.85 ha (LGL and URS 2010) as part of the Todd Lane and Ojibway-Windsor subpopulations. However, despite the loss of area, management of the restoration sites ensures the habitat remains high-quality. Actions include controlled burning and manual removal of woody and invasive species and monitoring to ensure effective results for the targeted species at risk. For the immediate future, the habitat at these sites is expected to remain suitable. However, the long-term management of these sites and the agency to be responsible for it is yet to be determined. Based on experience in LaSalle Natural Heritage Areas and in the Ojibway Prairie PNR, it is apparent that without appropriate management, Colicroot habitat can be lost to succession even in protected areas.
It is unknown whether habitat for Colicroot can be restored, from the seedbank, after a complete loss of mature individuals, (e.g., full coverage of woody plants), or whether Colicroot can re-establish from new dispersal. The length of time seeds may be viable in soil and the length of time the site has been woody may be factors. On Walpole Island, other rare prairie species are beginning to reappear at a site being restored to prairie from a full woody canopy (Bowles pers. comm. 2010; Jacobs pers. comm. 2014).
Historically, most natural prairie in southwestern Ontario was converted to agricultural fields (Bakowsky and Riley 1994). At Walpole Island, conversion of Colicroot habitat to agriculture continues to be a possibility, as exceptionally high rental fees are offered for prairie land because it has never been sprayed and thus can be used for certified organic crops (Jacobs pers. comm. 2014). Since 1986, at least two patches of Colicroot have been lost from conversion of habitat to agriculture.
The generation time for Colicroot in Canada is estimated to be 7-15 years. The time it takes for Colicroot plants to reach maturity from seed is unknown but is likely more than one year. Greenhouse-raised plants do not flower in their first year (Bernyk pers. comm. 2014). Other species of Aletris in the southeastern United States have been reported to reach maturity in two years, from seed in the wild (Sullivan 1973). The time to maturity for Colicroot in Canada probably depends on local site conditions, especially light levels and availability of open ground (J. Jones pers. obs. 2014). Sterile rosettes are frequently seen in a variety of situations, so there must be time periods, sizes, or conditions where plants do not flower. Colicroot plants are perennial and can be very long-lived. Based on a comparison of sizes between new seedlings raised in the greenhouse and some very large plants removed from the Parkway footprint, some Colicroot plants probably live for decades (Bernyk pers. comm. 2014), but habitat dynamics may make older plants unusual. Due to younger individuals in Parkway sites, average age could be 3 to 5 years, although plants elsewhere may be quite old.
The length of time seeds remain viable or whether Colicroot maintains a seed bank is unknown. Colicroot has been known to re-emerge to sites after mowing has ceased (Woodliffe pers. comm. 2010; Jones 2014) and after sod containing prairie species is newly disturbed (R. Jones pers. comm. 2014), but it is unknown whether this occurred from a seed bank or from unseen small rosettes. Colicroot flowers are perfect (having both male and female parts) on a single flowering stalk. It is unknown whether they are self-fertile. Sullivan (1973) estimated that individual flowers of two Aletris species, closely related to Colicroot, can produce approximately 1000 seeds with germination rates for Yellow Colicroot (A. lutea) and White Colicroot (A. obovata) at 44% and 70%, respectively.
In addition to sexual reproduction, vegetative reproduction is also possible from buds on the rhizome, but in greenhouse trials of rhizome cuttings, vegetative reproduction was found to occur only infrequently (Bernyk pers. comm. 2014). Still, it may occasionally create a few plants that are genetically identical (clones), potentially resulting in a census where the number of plants is slightly greater than the number of genetic individuals. However, clones can become mature reproducing plants, and their presence in small patches is likely beneficial. Therefore, the undetected presence of a small number of clones within a census is likely not significant.
Until recently, most attempts to transplant Colicroot were unsuccessful (Waldron, pers. comm. 2010; Woodliffe pers. comm. 2010). However, as part of efforts to mitigate Parkway construction, extensive trials were done to find effective protocols for germinating, transplanting, seed germination, and greenhouse production of plants (LGL and URS 2010; LGL 2012; Native Trees and Plants 2012; LGL 2013; WEMG 2013). Greenhouse trials found that seeds from Colicroot in Windsor required at least three months of cold-moist stratification to break dormancy, and seedlings grew best in a Berger BM2 mix of peat moss, perlite, and vermiculite (Bernyk pers. comm. 2014). In field trials, the most successful method was to transplant Colicroot plants surrounded by large, intact mats of sod (LGL 2013; R. Jones pers. comm. 2014). Thousands of Colicroot plants have now been transplanted successfully or raised from seed and planted out (Snyder pers. comm. 2014).
Neither seeds nor fruits of Colicroot have specialized structures to assist dispersal or to enable long-distance migration. Seeds likely fall from the plants by gravity and by movement of the stalks in the wind.
Colicroot is insect-pollinated. MacPhail (2013) investigated pollinators of Colicroot at two Parkway sites. A total of 25 species of insects were observed. Colicroot was visited primarily by bumblebees (Bombus bimaculatus, Bombus sp.) and solitary bees (especially Agapostemon virescens and Anthophora terminalis). Flowering stalks are frequently eaten by deer or other herbivores, and the leaves are sometimes eaten by insects (Jones 2014).
Kirk (1987) suggested Colicroot may have an obligate symbiotic relationship with mycorrhizal fungi, which seemed to be corroborated by the fact that, until recently, most attempts to transplant the species were unsuccessful (Waldron, pers. comm. 2010; Woodliffe pers. comm. 2010). However, greenhouse tests found that most seeds were viable and were able to germinate without mycorrhizae; that the addition of a mix of mycorrhizal species did not appear to confer any advantage to seedlings; and that seedlings were able to grow in sterilized media without any fungi (Bernyk pers. comm. 2014). Field trials found that the most successful transplantation methods involved moving Colicroot plants surrounded by large, intact blocks of sod (LGL 2013; R. Jones pers. comm. 2014), which would preserve mycorrhizal relationships. Thus, Colicroot could be facultatively mycotrophic perhaps forming beneficial associations as the plants grow.
For the 2014 survey work (Jones 2014), abundance of Colicroot (where present) was determined by tallying the number of flowering stalks (see Search Effort). Sterile (non-flowering) rosettes were tallied if visible. Although individual plants can have more than one flowering stalk, the number of flowering stalks is the primary measure of abundance that has been used previously, so in order to provide comparable data for trends analysis, the same method was used. Although counting flowering stalks could potentially overestimate abundance by counting extra stems on a single plant, the overestimate is probably balanced somewhat by the fact that non-flowering rosettes, hidden under taller vegetation, are not often counted and are certainly underestimated. When entire plants were visible, those with more than one flowering stalk were counted as a single individuals, but due to time constraints it was usually not possible to check every plant to see which ones had more than one stalk or to search for all rosettes through all adjacent tall grass, and this would certainly have resulted in a lot of site disturbance. There is no estimate of immature individuals.
Analysis of trends in population size was done by comparing data at the patch level rather than at the subpopulation level. The four subpopulations in Windsor-LaSalle contain 93% of all individuals. The Windsor Ojibway subpopulation in particular is made up of 21 patches including some Parkway restoration patches. It is unlikely that this entire subpopulation will be extirpated in the near future, but individual patches may easily decline or be lost. Thus trends for the majority of the Canadian population will not be very apparent by just considering the persistence of the subpopulation. Furthermore, given the limiting factors of the biology of Colicroot (i.e., lack of dispersal adaptations, possible mycorrhizal requirements), its specific habitat requirements, and the fragmented habitat, subpopulation trends are not very informative because patches may be physically quite close to one another but may be isolated by barriers such as urban infrastructure or tall vegetation.
Abundance of the Canadian population of Colicroot in 2014 was 14,000 to 15,000 mature individuals ( Table 1 ). The range reflects unknown abundance in two patches on Walpole Island and in two patches in Windsor-LaSalle. However, three of those patches are expected to contain fewer than 100 plants each, based on the size of the habitat. Thus, from the known abundance in 2014 of approximately 14,600 plants, the maximum expected for the Canadian population is approximately 15,000 plants. There are 35 patches of Colicroot in seven subpopulations that are extant and one patch in one subpopulation that is presumed extant with status unknown. Whether some of the population may be present in a seed bank is unknown. Currently, 82% of total population (around 12,000 plants) is in the Parkway restoration sites and only about 18% (around 2600 plants) is present elsewhere.
In 2004, thousands of plants of Colicroot were discovered in the footprint of the Parkway (Woodliffe pers. comm. 2010). All Colicroot transplanted out of the Parkway were originally naturally occurring plants, and all plugs (green-house raised) were grown from the seeds of plants naturally occurring in the immediate Parkway area. Therefore, for the purpose of this report, all plants in the restoration sites are considered natural plants of the Canadian population.
In 2012, in preparation for mitigation, an estimated 6,515 to 9,728 Colicroot plants were identified in the Parkway footprint, with recognition that the actual number was closer to the lower end of the range (LGL 2013). As well, more than 4000 plants were also found on MTO lands adjacent to the Parkway (LGL and URS 2010). In 2014, all Colicroot in the Parkway footprint had been transplanted to restoration sites, and some augmentation of restoration patches with plugs had been done. Additional plugs were expected to be planted in September 2014 (WEMG 2013). In 2013, there were approximately 11,900 plants in Parkway restoration sites ( Table 1 ). Of these, at least 4226 were naturally occurring plants already present and (as of 2013) at least 7680 were transplants (WEMG 2013). The transplanted plants and plugs are not included in the Canadian population when applying quantitative criteria to determine status (see Canadian Range).
There are very few baseline data from which to evaluate trends. The following comparisons are based on data from Kirk (1987), Jacobs (pers. comm. 2014), Jones (2014), and NHIC (2014).
Among the few previous abundance counts that exist (Table 2), there is a measurable loss of at least 4000 - 5000 plants since 1986. In addition, there are 20 previously documented Colicroot patches with no abundance data that are no longer present. Thus, the exact magnitude of the total loss is unknown but is well over 5000 plants. Extirpated patches are shown in grey italic type in Table 1 .
| Patch Name | Previous abundance and year | 2014 abundance |
|---|---|---|
| Walpole 1 | ~100 in 1986 | 3 |
| Reaume Street | "several thousand" in 1986 | 15 |
| West of Reaume Street | 12 in 1986 | 0 (site gone) |
| Tallgrass Kirk #6 | 10 in 1986 | 0 |
| Deerview Crescent | 30 in 2004 | 0 |
| LaSalle Woodlot (West of Brunet Park) | ~1000 in 1994 | ~25 |
A few increases have added approximately 1270 plants to the total population since 1986. Oldham reported 60 plants at Eagle in 1993: in 2014, there were 420 plants. Kirk reported 190 plants in a patch in the Spring Garden Natural Area. In 2014 there were three patches near his coordinates (which have at least a 50 m error factor) with a combined abundance of ~380 plants. This may be an increase or may be new discoveries. In 2009, approximately 600 plants were discovered in an old baseball diamond after mowing had ceased for several years. In 2013, Colicroot was found when a private landowner stopped mowing his backyard. There were 120 plants there in 2014. At Walpole Island, a loss of ~100 plants at one site was offset by an increase of ~100 at another site. In total, increases plus new discoveries in the Parkway and in Windsor-LaSalle constitute ~14,000 plants. Given the current population estimate of 14,600 plants, it appears that most of the current population was unknown in 1986.
New discoveries and increases constitute ~14,000 plants, but total abundance in 2014 is 14,600. If only 600 plants remain of the previously known population, and there is a measurable loss of more than 5000, this would constitute a reduction of more than 89%. However, assuming that newly discovered plants existed previously and subtracting the increase of 1270 plants, this translates to a base population of at least ~13,330 plants, and at least 18,330 present in 1986. If the transplanted 7,680 individuals are removed from the total, a population of 10,650 remains. A loss of >5000 would constitute a decline of >47% since 1986 with the actual decline upwards of that. Of the 58 patches that have been documented since 1979, 41% (24 patches) have been lost. There are no abundance data for 20 of the lost patches, but if 41% have been lost, the loss of individuals is probably in the range of 30 to 35% of the population since 1986.
It is extremely unlikely that additional Colicroot plants could become established from dispersal from populations outside Canada. Colicroot has no known long distance dispersal mechanisms. Furthermore, at isolated subpopulations such as Eagle, Colicroot has not dispersed to nearby suitable areas. Therefore, the likelihood of rescue from outside populations is very low. It is unknown whether individuals from outside populations would be adapted to survive in Canada. However, individuals in the northernmost part of the range, such as Michigan, Wisconsin or New York, may grow in conditions similar to those of southwestern Ontario in terms of the length of the growing season, average temperatures, and amount of rainfall. Perhaps these individuals could survive in Canadian conditions as well.
Possible threats were assessed using the IUCN threats calculator (Salafsky et al. 2008; Master et al. 2012; Appendix 1) and only the most plausible ones are summarized below. The important threats are discussed below in order of threat impact score. The most serious threats are those that lead to habitat degradation and loss. Information in this section comes from Jacobs (pers. comm. 2014), J. Jones (2014), and R. Jones (pers. comm. 2014). Titles in italics correspond to entries in the Threats Assessment Worksheet (Salafsky et al. 2008). The overall threat impact was calculated as Very High but was adjusted to Very High to High (which is estimated to result in an overall decline in the Canadian population of 22-100%). The adjustment was made due to two high threats (7.3 and 8.1) that may be additive or have a feedback interaction.
A lack of suitable disturbance, which enables succession to progress to taller, denser vegetation, is the most widespread and urgent threat and the cause of most of the observed declines. Many patches of Colicroot that were present in 1986, and even as recently as 2008, have been eliminated by encroachment of surrounding vegetation. Furthermore, most extant patches of Colicroot were larger and more abundant historically and most plants now persist in small remnants of open ground amongst shrubs. Succession is a widespread and urgent threat even in protected areas.
Invasive plants, especially European Common Reed (Phragmites australis ssp. australis), are present to some extent throughout the range of Colicroot. European Common Reed has taken over many wet or damp places, filled in drains, and formed large walls of tall vegetation that separate and isolate some Colicroot patches from others. European Common Reed dominates all former habitat in one part of Tallgrass Park and threatens what little remains in another part. It has filled in habitat in the hydro corridor near Brunet Park and is present in many areas of the Ojibway Prairie PNR. At the Parkway restoration sites, MTO is actively managing European Common Reed and other invasives, but elsewhere invasive species are a serious threat. At several other sites including Sandwich West Public School and some parts of the Spring Garden Natural Area, Autumn Olive is filling in the open ground.
Jones (2014) observed that most patches of Colicroot had at least one or two flowering stalks that were browsed. In Windsor, the browsing was mainly by deer, based on the height where stalks are nipped and daily sightings of deer during fieldwork. Jacobs (pers. comm. 2014) counted 27 nipped stalks in a patch of 312 plants on Walpole Island. Given the very small number of plants in many patches, the loss of even one or two flowering stalks could have a serious impact. Even in protected areas including the Ojibway Prairie PNR and the Parkway sites, deer browsing is a problem. In the Parkway sites, staff have attempted to reduce deer browse of Colicroot by applying carnivore or human urine or hair, Bitrex, rotten eggs, or blood meal near the plants, with limited success. In 2013, browsing continued to be a serious threat in the Parkway, with browsing detected in 54% of monitoring quadrats at one restoration site (WEMG 2013). Insect damage to leaves of Colicroot has also been observed at several sites (WEMG 2013; J. Jones pers. obs. 2014).
Since the mid-1990s, several sites have been lost to development, including new residential and industrial construction and public infrastructure (not including the Parkway). This continues to be a current threat with at least two sites lost since 2008. Many Colicroot patches are within protected areas where this threat should be restricted. On the Walpole Island First Nation there is an urgent need for additional housing, but with few areas available for development within the small land base, land that appears unused may be in demand.
In the recent threat assessment for the recovery strategy, "incidental harm" (examples given are mowing, off-road vehicles, and trail use) is considered a medium threat (Environment Canada 2014). In 2014, there was some danger of trampling from foot or mountain bike traffic on Windsor trails, but such traffic was also keeping some ground open and suitable for Colicroot. Whether recreational activities are a net threat or net benefit is unknown.
In the recent threat assessment for the recovery strategy, conversion of habitat to agriculture (agricultural expansion) is considered a high impact threat (Environment Canada 2014) and is still a threat at Walpole Island (Jacobs pers. comm. 2014). Other than this locality, no evidence of agricultural expansion was observed (Jones 2014).
In the recent threat assessment for the recovery strategy, dumping is considered a medium threat (Environment Canada 2014). Dumping was reported in the 1990s at the Turkey Point site and cited as a possible reason for the loss of Colicroot there in 2002 (NHIC 2014). However, in 2014 the driveway at the Turkey Point site was very overgrown, and lacked evidence of any current dumping activity (Jones 2014).
Limiting factors include the biology of Colicroot (i.e., lack of dispersal adaptations, possible mycorrhizal requirements), its specific habitat requirements, and the fragmented habitat. Potential limitations such as lack of dispersal potential, mycorrhizal associations, genetic inbreeding in small patches, and other factors have not been investigated.
In terms of vulnerability to a threat that would rapidly affect all individuals of Colicroot present, the most plausible (and rapid) threat would be invasive species. Because reduction or elimination of the effects of invasive species depends on how landowners manage their lands, all patches in the same ownership or management regime would be considered a single location. Under this scheme, five locations would be defined:
- Parkway Sites: active reduction of invasive species;
- Walpole Island: occasional actions to reduce invasives;
- Ojibway Prairie PNR: occasional actions to reduce invasives;
- Windsor-LaSalle outside Parkway sites and Ojibway Prairie PNR: no actions to reduce invasives;
- Eagle: no invasives seen so far but protective private owners.
If location is assessed by number of landowners, the number is estimated to be between 6-14 considering if some of the private owners are the same at multiple sites ( Table 1 ; Appendix 2).
COSEWIC most recently assessed this species as Endangered in November 2015. Colicroot is listed as Threatened in Canada on Schedule 1 of the federal Species at Risk Act (SARA). A recovery strategy that identifies the critical habitat to be protected has recently been prepared (Environment Canada 2014) but actions have not yet been implemented. In Ontario, the species is listed as Threatened under the Ontario Endangered Species Act,2007. As of November 2014, no habitat had been regulated under this law. Mitigation for impacts to Colicroot from construction of the Parkway was specified in Permit No. AY-D-001-09 issued under Clause 17(2)(d) of the Ontario Endangered Species Act, 2007 (LGL and URS 2010). Colicroot is legally listed in New York as Threatened (New York Department of Environmental Conservation 2014), and in Rhode Island as a species of conservation concern (Rhode Island Department of Environmental Management 2007). Colicroot is not listed at risk in the states closest to southwestern Ontario including Michigan (Michigan Natural Features Inventory 2014), Ohio (Ohio Department of Natural Resources 2013), or Indiana (Indiana Department of Natural Resources 2013).
Globally, Colicroot is ranked Secure (G5-last reviewed 2001). Subnationally, Colicroot is Extirpated (SX) in Maine; Historical (SH) in New Hampshire; Critically Imperilled (S1) in Pennsylvania; Critically Imperilled to Imperilled (S1S2) in Oklahoma, Imperilled (S2) in Ontario, New York, and Rhode Island, Vulnerable (S3) in Delaware, West Virginia, Indiana, and Illinois; Vulnerable to Apparently Secure (S3S4) in Michigan and Kentucky; Apparently Secure (S4) in Wisconsin, New Jersey, Ohio, and Kentucky; Secure (S5) in Georgia, North Carolina, and Virginia; and Not Ranked (SNR) in 7 other states (Homoya pers. comm. 2014; Slaughter pers. comm. 2014; NatureServe 2015).
Ownership of all patches is shown in Table 1 . Sixteen patches of Colicroot are on lands in public ownership, (a provincial nature reserve, municipal Natural Heritage Areas, city parks) on land protected from development. Yet Colicroot remains highly threatened by succession, invasive species, and deer browse despite being in "protected" areas. The fact that fourteen previously known patches of Colicroot have been lost from these areas in the last 10 to 20 years demonstrates the continued threat.
The five Parkway restoration patches are owned by the Ontario MTO and are currently carefully protected, managed, and monitored. However, the ownership and management of these sites may change in the future, and final ownership has yet to be determined. Once the mandatory monitoring period ends, these sites may face the same threats profile as other patches.
Ten patches are on privately owned lands. At least two of these patches are owned by interested and engaged landowners who are actively managing or protecting species. One patch is corporately owned.
Four patches are on the Walpole Island First Nation. The Walpole Island Land Trust has acquired at least one site where Colicroot is present and will protect the land from development. Some controlled burning of prairie sites is done by the community (Jacobs pers. comm. 2014). The adequacy of burning has not been evaluated but may not be frequent enough for some prairie species (Jacobs pers. comm. 2014).
Thus, other than at Walpole Island, almost no habitat is adequately protected for the long term because maintaining suitability of Colicroot habitat requires active and frequent management to prevent succession. Land that is protected to remain natural is often given mainly "hands-off" management (e.g., no human interference). However, it is unlikely that a major wildfire or other natural disturbance will maintain habitat in the urban area of Windsor-LaSalle, so unfortunately the current land protection system does not ensure the survival of Colicroot.
The following people are gratefully acknowledged by the author for sharing information on Colicroot and for providing field assistance to Judith Jones: Melody Cairns (Ontario Parks, Aylmer), Clint Jacobs (Walpole Island Heritage Centre), Mira Jones (Winter Spider Eco-Consulting), Russ Jones (AMEC, Windsor), Don Kirk (Ontario Ministry of Natural Resources and Forestry, Guelph), Dan Lebedyk (Essex Region Conservation Authority), Paul Pratt (Ojibway Nature Centre), Elizabeth Reimer (Ontario Ministry of Natural Resources and Forestry, Windsor), and Gerry Waldron (consulting biologist, Amherstburg). Additional advice was provided by Bruce Bennett. Historical data on Colicroot were provided by the Natural Heritage Information Centre of the Ontario Ministry of Natural Resources and Forestry. Thanks are extended by the author to the Ontario Ministry of Transportation for allowing access to Parkway restoration sites and to Mira Jones for the use of her photos.
Bakowsky, W.D., and J.L. Riley. 1994. A survey of the prairies and savannas of southern Ontario, pp. 7-16 in R.G. Wickett, P.D. Lewis, P.A. Woodliffe, and P. Pratt, (eds.). Proceedings of the Thirteenth North America Prairie Conference, Windsor, Ontario.
Bernyk, V. pers. comm. 2014. Telephone correspondence to Judith Jones on October 30, 2014. Greenhouse and nursery operator, Native Trees and Plants, Amherstberg, Ontario.
Bowles, J.M. 2005. Draft Walpole Island ecosystem recovery strategy prepared for the Walpole Island Heritage Centre, Environment Canada, and the Walpole Island Recovery Team. 50 pp.
Bowles, J.M. pers. comm. 2010. Email correspondence to Judith Jones on January 10 and December 20, 2010. Curator of Herbarium, University of Western Ontario, London; and consulting ecologist [Deceased 2013].
Brouillet, L., F. Coursol, S.J. Meades, M. Favreau, M. Anions, P. Bélisle and P. Desmet. 2014. Aletris farinosa L. in VASCAN, the Database of Vascular Plants of Canada [accessed October 15, 2014].
Butler, C.L., and C.H. Costello. 1944. Pharmacological Studies. I. Aletris farinosa. Journal of the American Pharmaceutical Association 33(6):177-183.
Challinor, V.L., S. Chap, R.P. Lehmann, P.V. Bernhardt, and J.J. De Voss. 2013. Structure and Absolute Configuration of Methyl (3 R)-Malonyl-(13 S)-hydroxycheilanth-17-en-19-oate, a sesterterpene derivative from the roots of Aletris farinosa. Journal of Natural Products 76(4):485-488.
Chambers, J. 2010. Ecological Land Classification of Ojibway Prairie Provincial Nature Reserve. Ontario Parks, Ontario Ministry of Natural Resources, Aylmer. 36 pp.
Chapman, L.J., and D.F. Putnam. 1984. The Physiography of Southern Ontario. 3rd Edition. Ontario Geological Survey. 270 pp.
Committee on the Status of Endangered Wildlife in Canada (COSEWIC). 2010. COSEWIC Guidelines on Manipulated Populations. Approved by COSEWIC in April 2010. Website: http://www.COSEWIC.gc.ca/eng/sct2/sct2_8_e.cfm [accessed August 2015].
Doubt, J. pers. comm. 2010. Email correspondence to Judith Jones on October 6, 2014. Canadian Museum of Nature, Ottawa.
Environment Canada. 2014. Recovery Strategy for the Colicroot (Aletris farinosa) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. v + 29 p.
Faber-Langendoen, D., L.L. Master, J. Nichols, K. Snow, A. Tomaino, R. Bittman, G. Hammerson, B. Heidel, L. Ramsay, and B. Young. 2009. NatureServe Conservation Status Assessment: Methodology for Assigning Ranks. NatureServe, Arlington, VA.
Fernald, M.L. 1950.Gray's Manual of Botany, 8th ed. Van Nostrum Reinhold Co., New York. 1632 pp.
Foster, J. pers. comm. 2015. Email correspondence to Bruce Bennett, 29 August 2015. Park Superintendent, Turkey Point Provincial Park.
Fuse, S., N.S. Lee, and M.N. Tamura. 2012. Biosystematic studies on the family Nartheciaceae (Dioscoreales) I. Phylogenetic relationships, character evolution and taxonomic re-examination. Plant Systematics and Evolution 298(8):1575-1584.
Gleason, H.A. 1968. The New Britton and Brown Illustrated Flora of the Northeastern United States and Adjacent Canada vol. 1. New York Botanical Garden and Hafner Publishing Company, New York. 482 pp.
Gleason, H.A., and A. Cronquist. 1991. Manual of Vascular Plants of Northeastern United States and Adjacent Canada, 2nd ed. New York Botanical Garden, 910 pp.
Homoya, M.A. pers. comm. 2014. Email correspondence to Bruce Bennett on November 17, 2014. Botanist/Plant Ecologist, Indiana Department of Natural Resources, Indianapolis, Indiana.
Hutchens, A.R. 1973. Indian Herbology of North America. Merco, Windsor, Ontario. 382 pp.
Indiana Department of Natural Resources. 2013. Endangered, Threatened, Rare and Extirpated Plants of Indiana, PDF [accessed November 10, 2014].
Jacobs, C. pers. comm. 2014. Telephone correspondence to Judith Jones on October 7, 2014. Walpole Island Heritage Centre and Walpole Island Land Trust.
Jones, J. 2014. Report from field work on Colicroot (Aletris farinosa). Unpublished report prepared for the Committee on the Status of Endangered Wildlife in Canada, Ottawa, Ontario. 9 pp.
Jones, R. pers. comm. 2014. In person communication with Judith Jones on July 7, 2014 and telephone correspondence with Judith Jones on October 28, 2014. Species at Risk Specialist, AMEC, Windsor, Ontario.
Kartesz, J.T. 2013. The Biota of North America Program (BONAP). North American Plant Atlas. Chapel Hill, N.C. [accessed October 15, 2014]
Kirk, D.A. 1987. COSEWIC status report on the Colicroot, Aletris farinosa, in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa. 37 pp.
Lee, H.T. 2008. Southern Ontario Ecosystem Table, Conservation Ontario. [accessed August 5, 2014]
Lee, H.T., W.D. Bakowsky, J.L. Riley, J. Bowles, M. Puddister, P. Uhlig, and S. McMurray. 1998. Ecological Land Classification for Southern Ontario: First Approximation and Its Application. OMNR, Southcentral Science Section, Science Development and Transfer Branch. SCSS Field Guide FG-02. 225 pp.
LGL. 2012. Colicroot (Aletris farinosa) Trials 2011, Annual Monitoring Report for the Windsor-Essex Parkway, prepared for Ontario Ministry of Transportation, Windsor, Ontario. 104 pp.
LGL. 2013. Amendment No.1 to Colicroot (Aletris farinosa) Management, Monitoring, and Habitat Restoration Plan Created to Meet Conditions of Permit Number: AY-D-001-09, Issued Under s. 17 (2) (d) of the Endangered Species Act, 2007. Prepared for Ontario Ministry of Transportation, Windsor, Ontario. 38 pp.
LGL, and URS. 2010. Colicroot (Aletris farinosa) Management, Monitoring, and Habitat Restoration Plan Created to Meet Conditions of Permit Number: AY-D-001-09, Issued Under s. 17 (2) (d) of the Endangered Species Act, 2007. Prepared for Ontario Ministry of Transportation, Windsor, Ontario. 266 pp.
MacPhail, V.J. 2013. Investigating the pollination biology of Species-At-Risk plants in southern Ontario: results from 2013. Unpublished report prepared for Wildlife Preservation Canada, Guelph Ontario. 36 pp.
Marker, R.E., D.L. Turner, A.C. Shabica, E.M. Jones, J. Krueger, and J.D. Surmatis. 1940. Sterols. CVII. Steroidal sapogenins of Aletris, Asparagus and Lilium. Journal of the American Chemical Society, 62(10):2620-2621.
Master, L.L., D. Faber-Langendoen, R. Bittman, G.A. Hammerson, B. Heidel, L. Ramsay, K. Snow, A. Teucher, and A. Tomaino. 2012. NatureServe Conservation Status Assessment: Factors for Evaluating Extinction Risk. NatureServe, Arlington, VA.
Merckx, V., P. Schols, K. Geuten, S. Huysmans, and E. Smets. 2008. Phylogenetic relationships in Nartheciaceae (Dioscoreales) with focus on pollen and orbicule morphology. Belgian Journal of Botany 141(1):64-77.
Michigan Natural Features Inventory. 2014. Michigan's Special Plants [accessed November 10, 2014].
Ministry of Natural Resources and Forestry (MNRF). 2014. 2014 Annual monitoring report for plant species at risk: The Rt. Hon. Herb Gray Parkway: Volume 1 mitigation and monitoring. Report #PIC-83-119-0155. 158 pp. + appendices.
Morgan, J.P. 1994. Soil impoverishment a little-known technique holds potential for establishing prairie. Ecological Restoration 12(1):55-56.
Native Trees and Plants. 2012. Final Seed Viability Report. Unpublished report to the Ontario Ministry of Transportation, Windsor. 4 pp.
NatureServe. 2004. Habitat-based Plant Element Occurrence Delimitation Guidelines [accessed October 30, 2014].
NatureServe. 2014. Aletris farinosa in NatureServe Explorer: An online encyclopedia of life. NatureServe, Arlington, Virginia. [accessed: October 30, 2014]
NatureServe. 2015. Aletris farinosa in NatureServe Explorer: An online encyclopedia of life. NatureServe, Arlington, Virginia. [accessed: March 24, 2014].
Natural Heritage Information Centre (NHIC). 2014. Database data for Aletris farinosa. Natural Heritage Information Centre, Ontario Ministry of Natural Resources, Peterborough, Ontario.
New York Department of Environmental Conservation. 2014. New York Protected Native Plants. http://www.dec.ny.gov/regs/15522.html [accessed October 30, 2014].
Norris, R.V. 1987. Historical development of progesterone therapy. pp. 273-285 in Ginsburg, B.E. and B.F. Carter (eds.), Premenstrual Syndrome, Springer-Verlag, US.
Ohio Department of Natural Resources. 2013. 2012-2013 Rare Native Ohio Plants Status List [accessed November 10, 2014].
Oldham, M.J. 2000. Element Occurrence records of White-tubed Colic-root (Aletris farinosa) from the database of the Natural Heritage Information Centre, Ontario Ministry of Natural Resources, Peterborough. 30 pp.
Oldham, M.J. pers. comm. 2015. Email correspondence to Bruce Bennett, 29 August 2015. Botanist. Ontario Natural Heritage Information Centre, Peterborough, Ontario.
Osol, A., and G.E. Farrar Jr. 1955. The Dispensatory of the United States of America, 25th ed. J.B. Lippincott, Philadelphia, PA. 1535 pp.
Pratt, P. pers. comm. 2010. Telephone correspondence to Judith Jones on December 9, 2010. Retired; Naturalist, Ojibway Nature Centre, City of Windsor, Ontario.
Reveal, J.L., and J.C. Pires. 2002. Phylogeny and classification of the Monocotyledons: an update, pp. 3-36 in Flora of North America v. 26. Flora of North America Editorial Committee, Oxford University Press, New York.
Reznicek, A.A., E.G. Voss, and B.S. Walters 2011. Aletris farinosa. Michigan Flora Online. University of Michigan[accessed October 15, 2014].
Riley, J.L. 2013. The Once and Future Great Lakes County: An Ecological History. McGill-Queens University Press, Montreal, Quebec. 488 pp.
Rhode Island Department of Environmental Management 2007. Rare native plants of Rhode Island. PDF [accessed October 30, 2014].
Salafsky, N., D. Salzer, A. J. Stattersfield, C. Hilton-Taylor, R. Neugarten, S. H. M. Butchart, B. Collen, N. Cox, L. L. Master, S. O'Connor, and D. Wilkie. 2008. A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conservation Biology 22:897-911. Classification online at http://conservationmeasures.org/CMP/IUCN/browse.cfm?TaxID=DirectThreats
Snyder, S. pers. comm. 2014. Email correspondence to Judith Jones on October 31, 2014. Senior plant ecologist, AMEC, Windsor, Ontario.
Sullivan, V.I. 1973. Biosystematics of Aletris lutea Small, Aletris obovata Nash, and natural hybrids (Liliaceae). Brittonia 25:294-303.
Sullivan, V.I. 2002. Aletris, pp. 64-66 in Flora of North America v. 26. Flora of North America Editorial Committee, Oxford University Press, New York.
Town of LaSalle. 2014. Publicly owned natural areas in the Town of LaSalle. PDF [accessed October 29, 2014].
URS 2009. Canadian Environmental Assessment Act Screening Report CEAR No. 06-01-1870: Detroit River International Crossing. 55 pp + appendices.
Utech, F.H. 2002. Liliaceae, pp. 50-58 in Flora of North America v. 26. Flora of North America Editorial Committee, Oxford University Press, New York.
Voss, E.G., and A.A. Reznicek. 2012. Field Manual of Michigan Flora, University of Michigan Press, Ann Arbor, MI. 990 pp.
Waldron, G. pers. comm. 2010 and 2014. Telephone, email, and in person correspondence with Judith Jones (in person July 9, 2014). Consulting Ecologist, Amherstberg, Ontario.
Walpole Island Heritage Centre. 2006. E-niizaanag Wii-Ngoshkaag Maampii Bkejwanong: Species at Risk on the Walpole Island First Nation. Bkejwanong Natural Heritage Program, Wallaceburg, Ontario.130 pp.
WebMD 2014. Aletris.[accessed October 28, 2014].
WEMG 2013. 2013 Plant annual monitoring report. The Windsor-Essex Parkway. Windsor-Essex Mobility Group and Parkway Infrastructure Constructors document no. PIC-83-119-0130. 149 pp.
White, D.J., and M.J. Oldham 2000. Update COSEWIC status report on the Colicroot Aletris farinosa in Canada, Committee on the Status of Endangered Wildlife in Canada, Ottawa, Ontario. 8 pp.
Wood, G.B., and F. Baches. 1869. Dispensatory of the United States, 12th ed. Lippincott and Co., Philadelphia, PA. 1699 pp. Google Book [accessed October 21, 2014].
Woodliffe, P.A. pers. comm. 2010. Email correspondence to Judith Jones on December 6, 2010. Retired; District Ecologist, Ontario Ministry of Natural Resources, Aylmer District, Chatham, Ontario.
Zhao, Y.M., W. Wang, and S.R. Zhang. 2012. Delimitation and phylogeny of Aletris (Nartheciaceae) with implications for perianth evolution. Journal of Systematics and Evolution 50(2):135-145.
Zomlefer, W.B. 1997. The genera of Nartheciaceae in the southeastern United States. Harvard Papers in Botany 2:195-211.
Judith Jones, B.S., M.S, has been an independent biological consultant since 1995. Her work gives input both to conservation and to intelligent development and resource use. She works on a broad range of issues, including alvar ecosystems, inventories of natural areas, and environmental impact studies (EIS) of proposed developments in Southern Ontario. Judith has also been involved with surveys and recovery planning for more than 22 different species at risk (SAR). She is the author of a number of recovery strategies, action plans, management plans, and COSEWIC reports.
No collections were examined. A list of specimens housed at the Canadian Museum of Nature was provided by the museum (Doubt pers. comm. 2014).
| Threat Impact | Threat Impact (descriptions) | Level 1 Threat Impact Counts: high range |
Level 1 Threat Impact Counts: low range |
|---|---|---|---|
| A | Very High | 0 | 0 |
| B | High | 2 | 2 |
| C | Medium | 0 | 0 |
| D | Low | 1 | 1 |
| - | Calculated Overall Threat Impact: | Very High | Very High |
| Threat | Threat | Impact (calculated) | Impact (calculated) | Scope (next 10 Yrs) | Severity (10 Yrs or 3 Gen.) | Timing | Comments |
|---|---|---|---|---|---|---|---|
| 1 | Residential & commercial development | D | Low | Small (1-10%) | Extreme (71-100%) | High (Continuing) | - |
| 1.1 | Housing & urban areas | D | Low | Small (1-10%) | Extreme (71-100%) | High (Continuing) | Few patches and few individuals will be affected, but the result will be a complete loss. Likelihood of occurring is very high. Note that >80% of plants are in Parkway restoration sites (only 18 % are not in Parkway sites). |
| 1.2 | Commercial & industrial areas | D | Low | Small (1-10%) | Extreme (71-100%) | High (Continuing) | Few patches and few individuals will be affected, but the result will be complete loss. Likelihood of occurring is very high. Note that >80% of plants are in Parkway restoration sites (only 18 % are not in Parkway sites). |
| 1.3 | Tourism & recreation areas | - | - | - | - | - | - |
| 2 | Agriculture & aquaculture | - | Unknown | Small (1-10%) | Unknown | Unknown | - |
| 2.1 | Annual & perennial non-timber crops | - | Unknown | Small (1-10%) | Unknown | Unknown | Threat only on Walpole Island (small proportion): Conversion to agriculture has caused total loss of habitat and individuals in some areas but has also created needed (favourable) disturbance in one area. |
| 2.2 | Wood & pulp plantations | - | - | - | - | - | - |
| 2.3 | Livestock farming & ranching | - | - | - | - | - | - |
| 2.4 | Marine & freshwater aquaculture | - | - | - | - | - | - |
| 3 | Energy production & mining | - | - | - | - | - | - |
| 3.1 | Oil & gas drilling | - | - | - | - | - | - |
| 3.2 | Mining & quarrying | - | - | - | - | - | - |
| 3.3 | Renewable energy | - | - | - | - | - | - |
| 4 | Transportation & service corridors | - | - | - | - | - | - |
| 4.1 | Roads & railroads | - | - | - | - | - | Colicroot exists here but does not appear to be a threat. |
| 4.2 | Utility & service lines | - | - | - | - | - | Colicroot exists here but does not appear to be a threat. |
| 4.3 | Shipping lanes | - | - | - | - | - | - |
| 4.4 | Flight paths | - | - | - | - | - | - |
| 5 | Biological resource use | - | - | - | - | - | - |
| 5.1 | Hunting & collecting terrestrial animals | - | - | - | - | - | - |
| 5.2 | Gathering terrestrial plants | - | - | - | - | - | Collected for medicinal use but likely not enough to cause an impact. |
| 5.3 | Logging & wood harvesting | - | - | - | - | - | - |
| 5.4 | Fishing & harvesting aquatic resources | - | - | - | - | - | - |
| 6 | Human intrusions & disturbance | - | Unknown | Restricted (11-30%) | Unknown | High (Continuing) | - |
| 6.1 | Recreational activities | - | Unknown | Restricted (11-30%) | Unknown | High (Continuing) | Threat could be beneficial at some sites but negative at others, overall may be of an unknown impact. |
| 6.2 | War, civil unrest & military exercises | - | - | - | - | - | - |
| 6.3 | Work & other activities | - | - | - | - | - | - |
| 7 | Natural system modifications | B | High | Pervasive (71-100%) | Serious (31-70%) | High (Continuing) | - |
| 7.1 | Fire & fire suppression | - | - | - | - | - | - |
| 7.2 | Dams & water management/use | - | - | - | - | - | - |
| 7.3 | Other ecosystem modifications | B | High | Pervasive (71-100%) | Serious (31-70%) | High (Continuing) | Lack of disturbance--either natural or anthropogenic. Many current subpopulations are in areas that historically were ploughed fields, not necessarily in burned areas. |
| 8 | Invasive & other problematic species & genes | B | High | Pervasive (71-100%) | Serious (31-70%) | High (Continuing) | - |
| 8.1 | Invasive non-native/alien species | B | High | Pervasive (71-100%) | Serious (31-70%) | High (Continuing) | Risk of plants being smothered by encroaching vegetation. May be due to lack of disturbance, or that the lack of disturbance is allowing for increased spread of invasive species. Interaction of 7.3 and 8.1 is possible. |
| 8.2 | Problematic native species | CD | Medium - Low | Pervasive (71-100%) | Moderate - Slight (1-30%) | High (Continuing) | Issue mainly of deer browse in city of Windsor. May impact all subpopulations but severity may be variable. Very widespread. |
| 8.3 | Introduced genetic material | - | - | - | - | - | - |
| 9 | Pollution | - | Unknown | Small (1-10%) | Unknown | Unknown | - |
| 9.1 | Household sewage & urban waste water | - | - | - | - | - | - |
| 9.2 | Industrial & military effluents | - | - | - | - | - | - |
| 9.3 | Agricultural & forestry effluents | - | - | - | - | - | - |
| 9.4 | Garbage & solid waste | - | Unknown | Small (1-10%) | Unknown | Unknown | Reported in the past but currently may be of unknown threat. |
| 9.5 | Air-borne pollutants | - | - | - | - | - | - |
| 9.6 | Excess energy | - | - | - | - | - | - |
| 10 | Geological events | - | - | - | - | - | - |
| 10.1 | Volcanoes | - | - | - | - | - | - |
| 10.2 | Earthquakes/tsunamis | - | - | - | - | - | - |
| 10.3 | Avalanches/landslides | - | - | - | - | - | - |
| 11 | Climate change & severe weather | - | - | - | - | - | - |
| 11.1 | Habitat shifting & alteration | - | - | - | - | - | - |
| 11.2 | Droughts | - | - | - | - | - | - |
| 11.3 | Temperature extremes | - | - | - | - | - | - |
| 11.4 | Storms & flooding | - | - | - | - | - | - |
Classification of Threats adopted from IUCN-CMP, Salafsky et al. (2008).