Louisiana waterthrush (Parkesia motacilla): COSEWIC assessment and status report 2015

Threatened
2015
Table of Contents
- Document Information
- Assessment Summary
- Executive Summary
- Technical Summary
- Preface
- Wildlife Species Description and Significance
- Distribution
- Habitat
- Biology
- Population Sizes and Trends
- Threats and Limiting Factors
- Protection, Status and Ranks
- Acknowledgements and Authorities Contacted
- Information Sources
- Biographical Summary of Report Writer(s)
- Collections Examined
List of Figures
- Figure 1. Adult Louisiana Waterthrush at nest with young (photo by Michael Patrikeev with permission)
- Figure 2. Global range of the Louisiana Waterthrush (map from Environment Canada 2012, based on Ridgely et al. 2007)
- Figure 3. Breeding distribution of the Louisiana Waterthrush in Ontario in three time periods: 1981-1985 (Cadman et al. 1987), 2001-2005 (Cadman et al. 2007) and 2006-2014 (compiled records)
- Figure 4. Breeding distribution of the Louisiana Waterthrush in Quebec in three time periods: 1984-1989 (Gauthier and Aubry 1996), 2005-2009 (compiled records; see Table 1 for details) and 2010-2014 (Québec Breeding Bird Atlas 2014).
- Figure 5. Annual indices of spring migration counts of Louisiana Waterthrushes at Long Point Bird Observatory, 1961-2012 (courtesy Bird Studies Canada).
List of Tables
- Table 1. Survey effort, survey results and other sources of information on the recent distribution and abundance of the Louisiana Waterthrush in Canada, 2005-2014.
- Table 2. Estimated size of Louisiana Waterthrush breeding population in various regions of Ontario and Quebec
- Table 3. NatureServe ranks, official status designations, and population estimates for Louisiana Waterthrush in Canadian provinces and in U.S. states adjacent to the Canadian range
List of Appendices
Document Information
COSEWIC
Committee on the Status
of Endangered Wildlife
in Canada

COSEPAC
Comité sur la situation
des espèces en péril
au Cananda
COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2015. COSEWIC assessment and status report on theLouisiana Waterthrush Parkesia motacilla in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 41 pp. (Species at Risk Public Registry website).
Previous report(s):
COSEWIC. 2006. COSEWIC assessment and update status report on the Louisiana Waterthrush Parkesia motacilla in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 26 pp.
Page, A.M. 1996. Update COSEWIC status report on the Louisiana Waterthrush Parkesia motacilla in Canada. Committee on the Status of Endangered Wildlife in Canada. 1-24 pp.
McCracken, J.D. 1991. COSEWIC status report on the Louisiana Waterthrush Parkesia motacilla in Canada. Committee on the Status of Endangered Wildlife in Canada. 1-26 pp.
Production note:
COSEWIC would like to acknowledge Audrey Heagy (Bird Studies Canada) for writing the status report on Louisiana Waterthrush (Parkesia motacilla) in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Jon McCracken, Co-chair of the COSEWIC Birds Specialist Subcommittee.
For additional copies contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment and Climate Change Canada
Ottawa, ON
K1A 0H3Tel.: 819-938-4125
Fax: 819-938-3984
COSEWIC E-mail
COSEWIC website
Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur L'hémileucin de Nuttall (Hemileuca nuttalli) au Canada.
Cover illustration/photo:
Louisiana Waterthrush -- Photo by © Michael Patrikeev.
COSEWIC Assessment Summary
Assessment Summary – November 2015
- Common name
- Louisiana Waterthrush
- Scientific name
- Parkesia motacilla
- Status
- Threatened
- Reason for designation
- During the breeding season in Canada, this songbird nests along clear, shaded, coldwater streams and forested wetlands in southern Ontario and southwestern Quebec. It occupies a similar habitat niche in Latin America during the winter. The Canadian population is small, probably consisting of fewer than 500 adults, but breeding pairs are difficult to detect. Population trends for the Canadian population are uncertain. Declines have been noted in some parts of the Canadian range, particularly in its stronghold in southwestern Ontario, while new pairs have been found in others. Immigration of individuals from the northeastern U.S. is thought to be important to maintaining the Canadian population. However, while the U.S. source population currently appears to be fairly stable, it may be subject to future population declines due to emerging threats to habitat.
- Occurrence
- Ontario, Quebec
- Status history
- Designated Special Concern in April 1991. Status re-examined and confirmed in April 1996 and April 2006. Status re-examined and designated Threatened in November 2015.
COSEWIC Executive Summary
Louisiana Waterthrush - Parkesia motacilla
Wildlife Species Description and Significance
The Louisiana Waterthrush (Parkesia motacilla) is a relatively large, drab wood-warbler that resembles a small thrush. Males and females are identical in appearance. The upper parts are dull brown. The lower parts are cream-coloured, with dark streaking on the breast and flanks. A bold, broad, white streak over the eye extends to the nape. The legs are bubble-gum pink, and the bill is rather long and heavy for a warbler.
Distribution
Most of the global breeding range (>99%) is within the eastern United States. In Canada, the Louisiana Waterthrush breeds in southern Ontario, where it is considered a rare, but regular local summer resident. It is also a rare, but sporadic breeder in southwestern Quebec. The bulk of the Canadian population is concentrated in two areas of Ontario: the Norfolk Sand Plain region bordering the north shore of Lake Erie, and the central Niagara Escarpment between Hamilton and Owen Sound.
Its wintering range extends from northern Mexico through Central America to extreme northwestern South America, and also throughout the West Indies.
Habitat
The Louisiana Waterthrush occupies specialized habitat, showing a strong preference for nesting and wintering along relatively pristine headwater streams and wetlands situated in large tracts of mature forest. Although it prefers running water (especially clear, coldwater streams), it also inhabits heavily wooded swamps with vernal or semi-permanent pools, where its territories can overlap with its sister species the Northern Waterthrush. It is often classified as both an area-sensitive forest species, and a riparian-obligate species. Louisiana Waterthrush nests are constructed within niches in steep stream banks, in the roots of uprooted trees, or in mossy logs and stumps, usually within a few metres of water.
Biology
The Louisiana Waterthrush is a long-distance migrant that typically arrives in southern Ontario much earlier in the spring than other neotropical songbirds. It displays annual fidelity to both breeding and wintering sites. Louisiana Waterthrush clutch size ranges from 4-6 eggs and incubation extends from 12-14 days. The species is generally single-brooded.
The Louisiana Waterthrush spends most of its time on or near the ground, along the margins of streams and pools. It has a specialized diet, feeding mostly on aquatic macro-invertebrates, especially insects, and sometimes eats small molluscs, fish, crustaceans, and amphibians.
Population Size and Trends
The Canadian population is estimated to be 235 to 575 adults. Population trends are poorly understood. The species has declined locally in parts of Canada in the past century and in the past few decades (related to habitat degradation and/or population fluctuations), but targeted surveys have found higher numbers in some parts of the Canadian range in recent years. Overall, populations in Canada and much of the U.S. currently appear to be relatively stable.
Threats and Limiting Factors
The Louisiana Waterthrush is a habitat specialist and its global population is limited by the supply of high-quality aquatic habitat on both its breeding and wintering grounds. There is no single imminent threat to the survival of the Canadian population; rather, it is the cumulative effects of many threats at different stages of its annual life cycle that are of particular concern. Habitat loss and changes in water quality/quantity due to agricultural intensification, and suburban residential development may have contributed to declines observed in parts of southern Ontario. Habitat conditions in Canada are expected to deteriorate due to the anticipated spread of Hemlock Woolly Adelgid, an exotic forest pest, into eastern Canada. Habitat fragmentation and degradation on its U.S. breeding grounds due to the combination of exotic forest pests and resource development could reduce immigration into the Canadian population. Habitat loss and degradation, including degraded water quality and deforestation due to agricultural and development activities, are ongoing threats in the wintering range. During migration, this species also experiences relatively high rates of mortality due to collisions with tall buildings and communication towers.
Protection, Status, and Ranks
The Migratory Birds Convention Act currently provides the most specific legislation protecting the Louisiana Waterthrush in Canada. A high proportion of known nesting sites are in protected areas. The specific habitats used by this species in Ontario are also provided some protection through various legislative policies. In addition, their physical characteristics generally preclude most kinds of agricultural and development activities.
Technical Summary
- Scientific Name:
- Parkesia motacilla
- English Name:
- Louisiana Waterthrush
- French Name:
- Paruline hochequeue
- Range of occurrence in Canada (province/territory/ocean): :
- Ontario, Quebec
Demographic Information
| Summary Items | Information |
|---|---|
| Generation time (usually average age of parents in the population; indicate if another method of estimating generation time indicated in the IUCN guidelines is being used) | 2 to 3 yrs |
| Is there an [observed, inferred, or projected] continuing decline in number of mature individuals? The outcome from the threats calculator suggests that a future decline could be projected. |
Unknown |
| Estimated percent of continuing decline in total number of mature individuals within [5 years or 2 generations] | N/A |
| [Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over the last 10 years. | Unknown, but overall population estimates have generally been stable |
| [Projected or suspected] percent [reduction or increase] in total number of mature individuals over the next 10 years. | Unknown |
| [Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over any 10 year period, over a time period including both the past and the future. | Unknown |
| Are the causes of the decline a. clearly reversible and b. understood and c. ceased? | N/A |
| Are there extreme fluctuations in number of mature individuals? | No |
Extent and Occupancy Information
| Summary Items | Information |
|---|---|
| Estimated extent of occurrence | 110,000 km2 |
| Index of area of occupancy (IAO) (Always report 2x2 grid value). | Estimated <500 km2(known 368 km2) |
| Is the population "severely fragmented" ie. is >50% of its total area of occupancy in habitat patches that are (a) smaller than would be required to support a viable population, and (b) separated from other habitat patches by a distance larger than the species can be expected to disperse? | No |
| Number of locations (use plausible range to reflect uncertainty if appropriate) (Note: See Definitions and Abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
Unknown, but more than the threshold of 10 locations |
| Is there an [observed, inferred, or projected] decline in extent of occurrence? | No |
| Is there an [observed, inferred, or projected] decline in index of area of occupancy? | No |
| Is there an [observed, inferred, or projected] decline in number of subpopulations? | N/A |
| Is there an [observed, inferred, or projected] decline in number of "locations"? (Note: See Definitions and Abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
Unknown |
| Is there an [observed, inferred, or projected] decline in [area, extent and/or quality] of habitat? | Yes, inferred and projected decline in habitat quality owing to loss of hemlock and effects of stream acidification. |
| Are there extreme fluctuations in number of subpopulations? | No |
| Are there extreme fluctuations in number of "locations"? (Note: See Definitions and Abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
No |
| Are there extreme fluctuations in extent of occurrence? | No |
| Are there extreme fluctuations in index of area of occupancy? | No |
Number of Mature Individuals (in each subpopulation)
| Population | N Clones (index of Mature Individuals) |
|---|---|
| Subpopulations/Regions: Based on estimated number of territories extrapolated from numbers of males detected during targeted surveys and assuming 75% of males are paired | N Mature Individuals |
| Southwestern Ontario | Estimated 116 to 254 adults (66 to 145 males and 50 to 109 females) |
| South-central Ontario | Estimated 93 to 234 adults (53 to 134 males and 40 to 100 females) |
| Southeastern Ontario (including Southern Shield region) | Estimated 26 to 70 adults (15 to 40 males and 11 to 30 females) |
| Southwestern Quebec | Estimated 0 to 17 adults (0 to 10 males and 0 to 7 females) |
| Total | Estimated 235 to 575 adults (134 to 329 males and 101 to 246 females) |
Quantitative Analysis
| Summary Items | Information |
|---|---|
| Probability of extinction in the wild is at least [20% within 20 years or 5 generations, or 10% within 100 years]. | No recent quantitative analysis is available. A very preliminary population modelling analysis in 2001 suggested that the Canadian population should persist for over 100 years (see Fluctuations and Trends section). |
Threats (actual or imminent, to populations or habitats, from highest impact to least)
Ongoing declines in water quality/quantity due to agricultural intensification, rural and suburban residential developments, and increased variability and severe weather due to climate change.
Habitat fragmentation and degradation on breeding grounds due to combination of new exotic forest pests (hemlock mortality due to Hemlock Woolly Adelgid ongoing or imminent on U.S. range, and emerging threat on Canadian range) and new resource developments (shale gas extraction, ridge-top wind turbine installations, mountaintop-removal coal mining ongoing or imminent in northern U.S. range). Habitat loss and degradation on the wintering grounds, including degraded water quality and deforestation.
Collisions with buildings and towers during migration.
Disturbance to nesting birds due to recreational activities (ATVs fording streams, trampling of streambanks by fishers and hikers, etc.)
- Was a threats calculator completed for this species and if so, by whom?
- Yes
- Dwayne Lepitzki, Jon McCracken, Audrey Heagy, Julie Perrault, Marcel Gahbauer, Lyle Friesen, Don Sutherland, François Shaffer, Ben Walters, Zoe Lebrun-Southcott, Brady Mattsson.
Rescue Effect (immigration from outside Canada)
| Summary Items | Information |
|---|---|
| Status of outside population(s) most likely to provide immigrants to Canada. See Population and Trends section. |
Populations in adjacent U.S. states have mostly been stable or increasing (Ohio, Pennsylvania, Vermont, New Hampshire), but recent declines in New York and Michigan. |
| Is immigration known or possible? | Yes |
| Would immigrants be adapted to survive in Canada? | Yes |
| Is there sufficient habitat for immigrants in Canada? | Yes |
| Are conditions deteriorating in Canada? See Table 3 (Guidelines for modifying status assessment based on rescue effect) |
Uncertain, but probably |
| Are conditions for the source population deteriorating See Table 3 (Guidelines for modifying status assessment based on rescue effect) |
Habitat conditions are currently deteriorating or are expected to deteriorate in large parts of the US range. |
| Is the Canadian population considered to be a sink? See Table 3 (Guidelines for modifying status assessment based on rescue effect) |
No |
| Is rescue from outside populations likely? | Yes (at least in the short term). |
Data-Sensitive Species
| Summary Items | Information |
|---|---|
| Is this a data sensitive species? | No |
Status History
COSEWIC: Designated Special Concern in April 1991. Status re-examined and confirmed in April 1996 and in April 2006. Status re-examined and designated Threatened in November 2015.
Status and Reasons for Designation:
- Status:
- Threatened
- Alpha-numeric code:
- Alpha-numeric codes: D1
- Reasons for designation:
- During the breeding season in Canada, this songbird nests along clear, shaded, coldwater streams and forested wetlands in southern Ontario and southwestern Quebec. It occupies a similar habitat niche in Latin America during the winter. The Canadian population is small, probably consisting of fewer than 500 adults, but breeding pairs are difficult to detect. Population trends for the Canadian population are uncertain. Declines have been noted in some parts of the Canadian range, particularly in its stronghold in southwestern Ontario, while new pairs have been found in others. Immigration of individuals from the northeastern U.S. is thought to be important to maintaining the Canadian population. However, while the U.S. source population currently appears to be fairly stable, it may be subject to future population declines due to emerging threats to habitat.
Applicability of Criteria
- Criterion A (Decline in Total Number of Mature Individuals):
- Not applicable. Rates of decline cannot be specified.
- Criterion B (Small Distribution Range and Decline or Fluctuation):
- Does not meet criteria. Potentially meets Endangered because IAO is < 500 km² and there is a continuing decline in habitat quality. However, the population is not severely fragmented, there are > 10 locations, and there are no extreme fluctuations.
- Criterion C (Small and Declining Number of Mature Individuals):
- Does not meet criteria. Could meet Threatened C2a(i) because there are < 1000 individuals, but there is insufficient evidence for a continuing population decline.
- Criterion D (Very Small or Restricted Population):
- Meets Threatened D1, because there are < 1000 mature individuals.
- Criterion E(Quantitative Analysis):
- Not applicable. Not done.
Preface
Since this species was last assessed by COSEWIC in 2006, new information on the distribution and abundance is available as a result of targeted surveys in southwestern Quebec and southern Ontario, as well as the completion of 5 years of fieldwork for the second Québec Breeding Bird Atlas. In addition, information on nesting productivity and parasitism rates in Canada is available as a result of nest monitoring efforts in southwestern Ontario. Some information on site fidelity, site turnover, and return rates in Ontario is also available as a result of a 4-year colour-banding project.
New and emerging threats on the breeding grounds are impacting breeding habitat in the northern United States range, which is considered an essential source of immigrants to sustain the small Canadian population. New forest pest species are also expected to affect forest habitat in the Canadian breeding range in the near future. Other threats to the population in southern Ontario are continuing.
COSEWIC History
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
COSEWIC Mandate
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC Membership
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
Definitions (2015)
- Wildlife Species
- A species, subspecies, variety, or geographically or genetically distinct population of animal, plant or other organism, other than a bacterium or virus, that is wild by nature and is either native to Canada or has extended its range into Canada without human intervention and has been present in Canada for at least 50 years.
- Extinct (X)
- A wildlife species that no longer exists.
- Extirpated (XT)
- A wildlife species no longer existing in the wild in Canada, but occurring elsewhere.
- Endangered (E)
- A wildlife species facing imminent extirpation or extinction.
- Threatened (T)
- A wildlife species likely to become endangered if limiting factors are not reversed.
-
Special Concern (SC)
(Note: Formerly described as "Vulnerable" from 1990 to 1999, or "Rare" prior to 1990.) - A wildlife species that may become a threatened or an endangered species because of a combination of biological characteristics and identified threats.
-
Not at Risk (NAR)
(Note: Formerly described as "Not In Any Category", or "No Designation Required.") - A wildlife species that has been evaluated and found to be not at risk of extinction given the current circumstances.
-
Data Deficient (DD)
(Note: Formerly described as "Indeterminate" from 1994 to 1999 or "ISIBD" [insufficient scientific information on which to base a designation] prior to 1994. Definition of the [DD] category revised in 2006.) - A category that applies when the available information is insufficient (a) to resolve a species' eligibility for assessment or (b) to permit an assessment of the species' risk of extinction.
The Canadian Wildlife Service, Environment and Climate Change Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Wildlife Species Description and Significance
Name and Classification
- Scientific Name:
- Parkesia motacilla (formerly Seiurus motacilla)
- English Name:
- Louisiana Waterthrush
- French Name;
- Paruline hochequeue
No subspecies have been recognized or described (American Ornithologist’s Union [AOU] 2013). This member of the New World wood-warbler (Parulidae) family was formerly classified in the genus Seiurus, along with Northern Waterthrush (now Parkesia noveboracensis) and Ovenbird (S. aurocapilla). Both waterthrush species were recently transferred to the new genus Parkesia based on genetic data indicating that the two waterthrushes are sister species (Sangster 2008; Chesser et al. 2010).
Morphological Description
The Louisiana Waterthrush is a relatively large, drab warbler that resembles a small thrush (Figure 1). Males and females are identical in appearance. The upper parts are dull brown. The lower parts are cream-coloured, with dark streaking on the breast and flanks, which fades out in the undertail coverts. A bold, broad, white supercilium (eye brow) extends to the nape. The legs are bubble-gum pink, and the bill is rather long and heavy for a warbler (Curson et al. 1994; Dunn and Garrett 1997).
The species is easily confused with the Northern Waterthrush (Parkesia noveboracensis), which is much more common and widespread in Canada. The most notable plumage difference between the two species is that the Northern Waterthrush’s supercilium (eye-brow) is cream-coloured or yellowish, relatively thin, and tapers behind the eye, whereas it is white and more extensive in the Lousiana Waterthrush. The Northern Waterthrush also has brown blotches on its undertail coverts, which are less distinct in the Louisiana Waterthrush. The two species are best separated in the field by song, and also by behaviour and habitat differences. The Louisiana Waterthrush’s distinctive song is preceded by a short series of very loud, down-slurred, piercing whistles, followed by a cascading series of jumbled whistles. Tail-bobbing is characteristic of both waterthrushes but, this behaviour is particularly exaggerated in this species (Dunn and Garrett 1997). Where their breeding ranges overlap, both species can sometimes be found occupying swamp forest, but the Louisiana Waterthrush is more apt to be found along coldwater streams (Craig 1985).
Figure 1. Adult Louisiana Waterthrush at nest with young.

Photo by Michael Patrikeev with permission.
Long description for Figure 1
Photo of an adult Louisiana Waterthrush (Parkesia motacilla) with young in the nest. The Louisiana Waterthrush is a relatively large, drab wood-warbler that resembles a small thrush. The upper parts are dull brown. The lower parts are cream-coloured, with dark streaking on the breast and flanks. A bold, broad, white streak over the eye extends to the nape. The legs are pink, and the bill is rather long and heavy for a warbler.
Population Spatial Structure and Variability
There is no evidence of population structuring within the small Canadian population of this species. Within the United States, eastern birds tend to be slightly larger than those in the west (Eaton 1958).
Designatable Units
There are no biological, genetic or geographic distinctions that warrant assessment below the species level. This report deals with a single designatable unit.
Special Significance
This species is considered a habitat specialist and is the only stream-dependent songbird in eastern North America (Mulvihillet al. 2008). Within its breeding and wintering ranges, the Louisiana Waterthrush is likely an excellent bio-indicator of the health of headwater, medium-gradient, coldwater streams and large, intact, mature deciduous forested swamps (Buffington et al. 1997; Prosser and Brooks 1998; Mulvihill et al. 2002; O’Connell et al. 2003; Mattsson and Cooper 2006; Mattsson and Cooper 2009). Due to its preference for clear, coldwater streams, the breeding habitat of this species often overlaps with trout streams (e.g., Stucker 2000).
It is also classified an area-sensitive forest bird that requires large tracts of contiguous, closed canopy forest for breeding (Robbins 1979; Freemark and Collins 1992). The breeding habitat requirements overlap with those of two other forest songbirds that are designated as Endangered in Canada: the Acadian Flycatcher (Empidonax virescens) and Prothonotary Warbler (Protonotaria citrea); these species co-occur at some sites in Ontario (COSEWIC 2007, 2010; Environment Canada 2012).
No Aboriginal traditional knowledge is currently available for this species.
Distribution
Global Range
Breeding
The Louisiana Waterthrush breeds from eastern Nebraska, north central Iowa, east central and southeastern Minnesota, central Wisconsin, southern Michigan, southern Ontario, central New York, central Vermont, central New Hampshire, and southern Maine south to eastern Kansas, eastern Oklahoma, eastern Texas, central Louisiana, southern Mississippi, southern Alabama, northern Florida, central and southwestern Georgia, central South Carolina, and central and northeastern North Carolina (Figure 2). The bulk of its 2,400,000 km2 breeding range (>99%) is within the eastern United States (Partners in Flight Science Committee [PIFSC] 2013).
Over the last century, its breeding range expanded slowly northward in the northeastern U.S. (Mattsson et al. 2009). This range expansion is probably attributed to re-colonization of formerly held territory that was heavily lumbered in the 1800s and is now largely reforested (Brewer et al. 1991). The northward expansion of the U.S. range seems to have halted (Mattsson et al. 2009). Since the 1980s, a reduction in distribution has been observed in northern New York (Rosenberg 2008) and southwestern Michigan (Hull 2011), but not in Vermont (Kibbe 2013) or Pennsylvania (Mulvihill 2012).
Figure 2. Global range of the Louisiana Waterthrush.
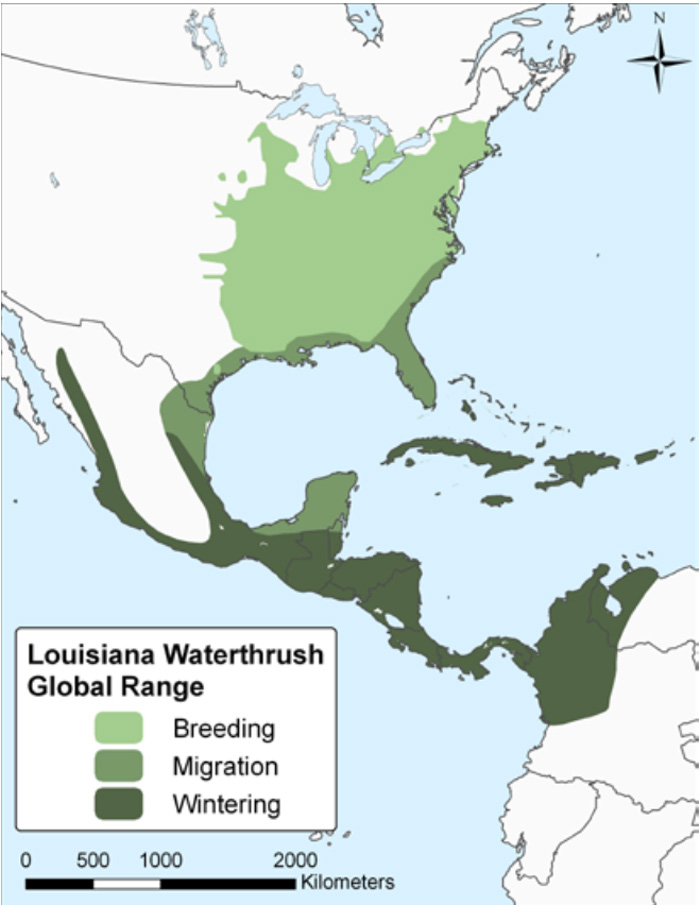
(Map from Environment Canada 2012, based on Ridgely et al. 2007).
Long description for Figure 2
Map of the global range of the Louisiana Waterthrush, indicating breeding, migration, and wintering areas. The species breeds from eastern Nebraska, north central Iowa, east central and southeastern Minnesota, central Wisconsin, southern Michigan, southern Ontario, central New York, central Vermont, central New Hampshire, and southern Maine south to eastern Kansas, eastern Oklahoma, eastern Texas, central Louisiana, southern Mississippi, southern Alabama, northern Florida, central and southwestern Georgia, central South Carolina, and central and northeastern North Carolina. It winters from northern Mexico, south through Central America to central Panama, northeastern Colombia and northwestern Venezuela, and throughout the West Indies.
Wintering
The Louisiana Waterthrush winters from northern Mexico, south through Central America (mainly at higher elevations; both slopes, although more commonly on the Gulf/Caribbean side) to central Panama, rarely to northeastern Colombia and northwestern Venezuela; also throughout the West Indies but progressively less numerous moving southward (Mattsson et al. 2009; Figure 2). Over 25% of the 1,729,000 km2 wintering range is in Mexico (Berlanga et al. 2010).
Canadian Range
Breeding
In Canada, the Louisiana Waterthrush breeds regularly in southern Ontario (Figure 3), where it is considered a rare, local summer resident (Eagles 1987; James 1991; McCracken 2007; Environment Canada 2011, 2012; Sandilands 2014). It is a rare and sporadic breeding bird in southwestern Quebec (Figure 4), where in 2006 it was confirmed nesting at one site (Savignac 2006) and has also been reported during the breeding season at several other sites (David 1996; St-Hilaire and Dauphin 1996; Savignac 2005, 2006, 2007, 2008; Les Oiseaux du Québec 2014; M. Robert, pers. comm. 2014; Québec BBA 2015).
Figure 3. Breeding distribution of the Louisiana Waterthrush in Ontario in three time periods: 1981-1985 (Cadman et al. 1987), 2001-2005 (Cadman et al. 2007) and 2006-2014 (compiled records).
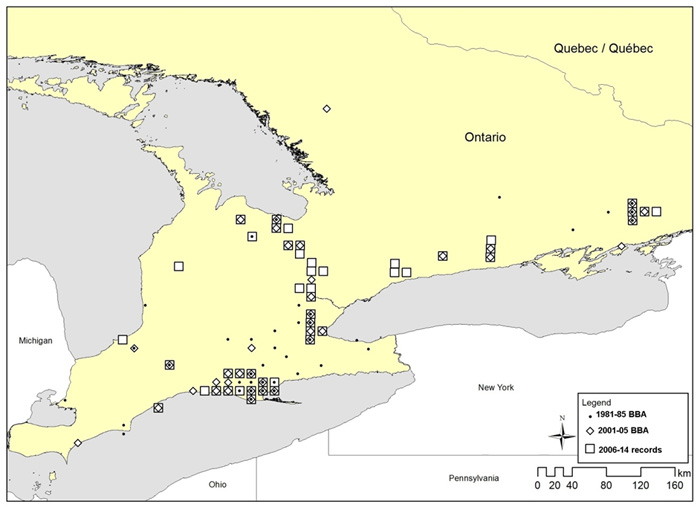
Long description for Figure 3
Map showing breeding distribution of the Louisiana Waterthrush in southern Ontario during three time periods: 1981 to 1985, 2001 to 2005, and 2006 to 2014.
Figure 4. Breeding distribution of the Louisiana Waterthrush in Quebec in three time periods: 1984-1989 (Gauthier and Aubry 1996), 2005-2009 (compiled records; see Table 1 for details) and 2010-2014 (Québec Breeding Bird Atlas 2014).
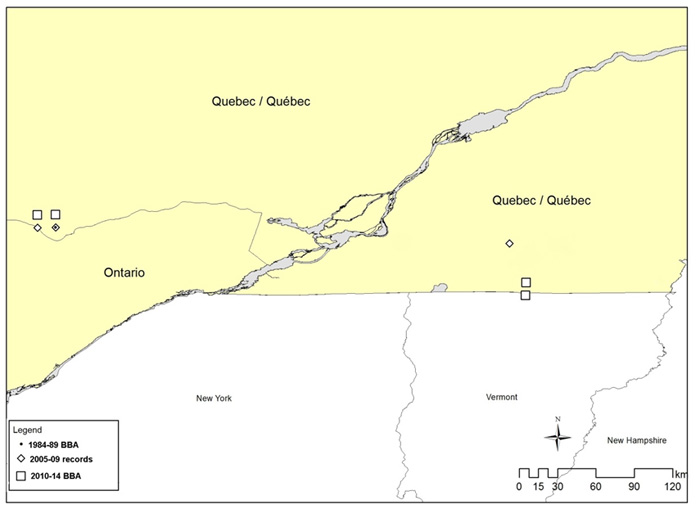
Long description for Figure 4
Map of the breeding distribution of the Louisiana Waterthrush in Quebec during three time periods: 1984 to 1989, 2005 to 2009, and 2010 to 2014.
In Canada, this species is highly localized, because it is associated with various physiographic features that provide the necessary combination of habitat and climatic conditions. The main concentration of Louisiana Waterthrush in Ontario is associated with the forested ravines and wetlands in the Norfolk Sand Plain (Norfolk, eastern Elgin, and southern Oxford counties). Other isolated breeding sites in southern Ontario are associated with various river systems (Ausable, Bayfield, Grand, Thames, and Maitland rivers), forested sites along the Great Lakes shoreline, and some inland forested wetlands. In south-central Ontario, this species breeds along relatively pristine headwater streams (and also some higher order streams with seepages and/or side streams) associated with the Niagara Escarpment, particularly in the central section of the escarpment from Hamilton to Collingwood. In southeastern Ontario, there is a small cluster of occurrences associated with small streams and wetlands near Frontenac Park in Ontario’s Frontenac Arch physiographic region. This species has also been found sporadically at other scattered sites in southeastern Ontario, including the eastern Oak Ridges Moraine, the Rice Lake Plains and the fringe of the Southern Shield
The only confirmed nesting records in Quebec are from a site on the Eardley Escarpment in Gatineau Park in the Outaouais region, an area where the species has been observed intermittently since at least 1974 (Savignac 2005, 2006, 2007, 2008). There are also several historical and recent breeding season reports from a cluster of sites in the Estrie region in southern Quebec, at the northern end of the Appalachian Mountain region (St-Hilaire and Dauphin 1996; Savignac 2005, 2008; Les Oiseaux du Québec 2014). During the 2010-2014 Québec Breeding Bird Atlas fieldwork (Figure 4), this species was reported from four 10 x 10 km squares, including two squares with probable breeding evidence in the Estrie region and two squares with possible breeding evidence in the Outaouais region (Québec BBA 2015).
A single singing male sighted in Welsford, New Brunswick was the only record in the first Maritimes Breeding Bird Atlas (1986-1990; Erskine 1992), and it likely represents a transient unpaired bird. This species was not recorded in the second Maritimes atlas (2006-2010; Maritimes BBA 2014).
The distribution of the Louisiana Waterthrush in Canada is governed by the availability of suitable habitat within climatic confines. In Ontario, it nests primarily in the extensively settled Carolinian ecoregion (Deciduous Forest region) and adjacent areas of the Great Lakes - St. Lawrence Forest region south of the Canadian Shield (McCracken 2007). The species’ distribution in Ontario is consistent with regions above the 6oC mean yearly isotherm and elevations less than 300 m (McCracken 1991). In Quebec, this species may be restricted to areas with relatively mild microclimates, such as stream valleys on south-facing hillsides and escarpments in the extreme southwestern part of the province.
The extent of the known breeding range of this species in Canada has increased gradually over the past century (Page 1996). However, this apparent northward range expansion may be largely attributable to improved knowledge of a previously under-reported species and/or resettlement of its pre-European settlement historical range.
The cluster of occurrences on the north shore of Lake Erie has long been recognized (MacClement 1915). The species became established as a regular breeder at a handful of sites in Frontenac County near Kingston in southeastern Ontario during the 1980s (Weir 2008). Targeted surveys in 2012 and 2013 found that this species is more widespread along the Niagara Escarpment (Hamilton, Halton, Peel, Dufferin, Simcoe and Grey counties) than previously known (Friesen and Lebrun-Southcott 2012; Lebrun-Southcott and Friesen 2013). There are a few other isolated sites that are occupied somewhat regularly (e.g., some sites in Middlesex and Lambton counties), but most other scattered occurrences in Ontario and the few occurrences in southwestern Quebec appear to be sites with sporadic occupancy. Moreover, this species has not been reported recently in some parts of its former Ontario range (e.g., Essex, Chatham-Kent, Niagara; see also Population Trends section).
As indicated by changes in distribution for the three time periods shown in Figures 3 and 4, there has been a relatively high turnover in occupied sites in Ontario and Quebec. For example, of 40 squares (10 km x 10 km) with breeding evidence during the 1981-1985 Ontario BBA, only 15 (37.5%) were still occupied during the 2001-05 BBA (McCracken 2007). Since 2005, breeding evidence has been reported for 15 additional squares, where no breeding evidence was found during either Ontario BBA. In Quebec, this species has not been reported since 2007 at the only confirmed breeding site in the province, but probable nesting evidence was reported at other sites (Savignac 2008; Québec BBA 2015).
Non-breeding
Being at the northern limits of its range in Canada, the Louisiana Waterthrush is rare on migration. At migration hotspots such as Point Pelee and Long Point, it is considered a regular but uncommon spring migrant, and is rarely reported in fall (McCracken 1991; Parks Canada Agency 2012; Long Point Bird Observatory 2014).
It is considered a “rare vagrant” in Nova Scotia (Tufts 1986), where 12 of 16 records since 1966 are from the fall period (McLaren 2012). This reflects a well-established pattern of southwest to northeast fall vagrancy for migrant passerine species in the province (McLaren 1981).
Extent of Occurrence and Area of Occupancy
The extent of occurrence (EOO) in Canada, as measured by a minimum convex polygon encompassing all occurrences with breeding evidence over the 2001-2014 period (as depicted in Figures 3 and 4) is about 110,000 km2. The EOO is about 20% smaller if outlying occurrences with only possible breeding evidence are excluded, or if only records for the past 10 years (2005-2014) are considered.
The index of area of occupancy (IAO) in Canada based on a 2 x 2 km grid and using all breeding occurrences for the 2005-2014 period is 356 km2. Given that new sites continue to be found, the actual IAO could approach 500 km2. However, not all sites are occupied every year, so this would clearly represent an upper limit.
Search Effort
Understanding of the breeding distribution of this species in Canada has improved greatly as a result of breeding bird atlas projects in Ontario during 1981-1985 and 2001-2005 (Eagles 1987; McCracken 2007) and in Quebec during 1984-89 and 2010-2014 (St-Hilaire and Dauphin 1996; Québec BBA 2015), as well as the accumulation of breeding season observations detected during regional and local ecological surveys and bird checklist programs. Systematic targeted searches of known and potential Louisiana Waterthrush habitat have also been conducted in most parts of the Canadian range over the past decade. See Table 1 and Sampling Effort and Methods section for additional details.
| Project | Protocol | Project Area | Years | Results | Reference |
|---|---|---|---|---|---|
| Ontario Breeding Bird Atlas | General surveys of breeding birds in 10 km x 10 km squares | Ontario | 2005 | 21 records from 11 atlas squares (2005 only). | Cadman et al. 2007 |
| Environment Canada Quebec Region: Louisiana Waterthrush surveys | Targeted surveys of known sites and suitable Louisiana Waterthrush habitat | Southwestern Quebec: Outaouais Region and Gatineau Park | 2005 | 1 single male at 1 of 18 sites | Savignac 2005 |
| Environment Canada Quebec Region: Louisiana Waterthrush surveys | Targeted surveys of known sites and suitable Louisiana Waterthrush habitat | Southwestern Quebec: Outaouais Region and Gatineau Park | 2006 | 1 pair and 1 single male at 2 of 21 sites (including first confirmed breeding for Quebec) | Savignac 2006 |
| Environment Canada Quebec Region: Louisiana Waterthrush surveys | Targeted surveys of known sites and suitable Louisiana Waterthrush habitat | Southwestern Quebec: Outaouais Region and Gatineau Park | 2007 | 1 pair at 1 of 6 sites | Savignac 2007 |
| Environment Canada Quebec Region: Louisiana Waterthrush surveys | Targeted surveys of known sites and suitable Louisiana Waterthrush habitat | Southwestern Quebec: Outaouais, Montérégie, Estrie Regions | 2008 | No birds at 20 sites | Savignac 2008 |
| Frontenac Bird Studies: Louisiana Waterthrush surveys | Targeted surveys | Southeastern Ontario: Frontenac Park study area (Frontenac County, Ontario) | 2010 | 1 pair, 4 single males at 5 of 17 sites | D. Derbyshire, pers. comm. 2014 |
| Frontenac Bird Studies: Louisiana Waterthrush surveys | Targeted surveys | Southeastern Ontario: Frontenac Park study area (Frontenac County, Ontario) | 2011 | 2 pairs, 2 single males at 4 of 28 sites | D. Derbyshire, pers. comm. 2014 |
| Frontenac Bird Studies: Louisiana Waterthrush surveys | Targeted surveys | Southeastern Ontario: Frontenac Park study area (Frontenac County, Ontario) | 2012 | 1 pairs , 2 single males at 3 of 14 sites | D. Derbyshire, pers. comm. 2014 |
| Bird Studies Canada: Forest Birds at Risk surveys | Targeted surveys | Southwestern Ontario: Norfolk Sand Plain (Norfolk Co. and Elgin Co.) | 2011 | 7 pairs, 6 single males, 7 nests at 11 of 31 sites | Allair et al. 2013 |
| Bird Studies Canada: Forest Birds at Risk surveys | Targeted surveys | Southwestern Ontario: Norfolk Sand Plain (Norfolk Co. and Elgin Co.) | 2012 | 17 pairs, 7 single males, 8 nests at 17 of 67 sites | Allair et al. 2013 |
| Bird Studies Canada: Forest Birds at Risk surveys | Targeted surveys | Southwestern Ontario: Carolinian ecoregion | 2013 | 11 pairs, 6 single males, 8 nests at 13 of 54 sites | Allair et al. 2014 |
| Bird Studies Canada: Forest Birds at Risk surveys | Targeted surveys | Southwestern Ontario: Carolinian ecoregion | 2014 | 13 pairs, 4 single males, 12 nests at 12 of 59 sites. | Allair et al. 2014 |
| Environment Canada: Louisiana Waterthrush surveys | Targeted surveys. | South-central Ontario: Niagara Escarpment | 2012 | 31 males, 8 females at 13 of 134 sites | Friesen and Lebrun-Southcott 2012 |
| Environment Canada: Louisiana Waterthrush surveys | Targeted surveys. | South-central Ontario: Niagara Escarpment, | 2013 | 34 males, 5 or 6 females at 13 of 94 sites | Lebrun-Southcott and Friesen 2013 |
| Environment Canada: Louisiana Waterthrush surveys | Targeted surveys. | South-central Ontario: Oak Ridges Moraine | 2014 | 4 males at 4 of 29 sites | Campomizzi et al. 2014 |
| Québec Breeding Bird Atlas, 2nd | General surveys of breeding birds in 10 km x 10 km squares | Quebec | 2010-2014 | 5 records from 4 atlas squares | QBBA 2015 |
| Ebird Canada | Casual birding observations | Ontario and Quebec | 2005-2013 | 414 records (mostly migrants, includes duplicate records) | Bird Studies Canada and Cornell Lab of Ornithology 2014 |
| Les Oiseaux du Québec | Summary of rare bird reports | Quebec | 2005-2014 | 7 records published in QuébecOiseaux | Les Oiseaux du Québec 2014 |
Habitat
Habitat Requirements
Breeding Habitat
The Louisiana Waterthrush nests amongst the roots of fallen trees, in niches of steep stream banks, and in and under mossy logs and stumps (Prosser and Brooks 1998; Mattsson et al. 2009; Ontario Nest Record Scheme [ONRS] 2014). Nests are generally well concealed by roots and hanging vegetation and are usually situated within a few metres of water (Mattsson et al. 2009; ONRS 2014). Mean canopy cover above 60 nests in Pennsylvania was 75% (Mattsson et al. 2009).
Of 40 nests found across several sites in Ontario in 2010-2014, 28 (79%) were found in ravines (Bird Studies Canada [BSC] 2014b). All of the ravine nests were situated in the stream bank, except for 2 in root tip-ups. Of 12 nests found in swamps, 9 were in root tip-ups and 3 in stumps. Almost all were located over water in mixed forests.
The Louisiana Waterthrush occupies specialized habitat, showing a strong preference for nesting along relatively pristine, headwater streams and associated wetlands situated in large tracts of mature forest (Mattsson et al. 2009). Breeding sites in the U.S. are typically along medium- to high-gradient, first- to third-order, perennial streams with gravel-bottoms in hilly areas (Mattsson et al. 2009). While the Louisiana Waterthrush favours running water, less frequently it inhabits heavily wooded swamps with vernal or semi-permanent pools, where its territories can overlap with Northern Waterthrush (Craig 1984, 1985). Habitat suitability models for parts of the U.S. range found that headwater streams with well-developed pools and riffles situated in large (>350 ha), late successional, deciduous or mixed forests with closed canopies and open understories in areas with high forest cover (>70%) are highly suitable habitat for this species (Prosser and Brooks 1998; Tirpak et al. 2009). Shrubby habitats near the nesting site may be important for young and adults during the post-breeding period (Vitz and Rodewald 2006, 2007).
Forests at occupied sites in Ontario and at Gatineau Park, Quebec are generally late-successional mixed or deciduous forest, typically with maple (Acer sp.) and Eastern Hemlock (Tsuga canadensis) components (unpubl. data from targeted surveys, see references in Table 1). Riparian territories in Ontario are typically associated with steep-walled forested ravines in sand plain areas, or rocky streams in incised valleys (Friesen and Lebrun-Southcott 2012; Lebrun-Southcott and Campomizzi 2014; J. Allair, pers. comm. 2014; J. Holdsworth, pers. comm. 2014).
Robbins (1979) regarded the Louisiana Waterthrush as an “area-sensitive” species -- one that requires large tracts of unbroken forest. Based upon studies in Maryland, he estimated that the minimum contiguous forest cover required to sustain a viable breeding population of Louisiana Waterthrushes was about 100 ha, although this species also regularly occurs in smaller forest patches (~25 ha) in some parts of its U.S. range (Sandilands 2014). Freemark and Collins (1992) also listed the Louisiana Waterthrush as an area-sensitive species, but did not suggest a minimum forest size, noting that area requirements are very much influenced by the regional pattern of forest cover. Although the area-sensitive classification of this species has not been rigorously tested (Parker et al. 2005), there is evidence that the species is sensitive to forest fragmentation (Prosser and Brooks 1998; Tirpak et al. 2009).
In its U.S. range, this species is generally encountered infrequently in streams having very narrow (<100 m) forest corridors (Keller et al. 1993; Mason et al. 2007). However, occupancy of riparian strips is also influenced by land cover and land use in the surrounding landscape matrix (Triquet et al. 1990; Peak and Thompson 2006; Rodewald and Bakerman 2006).
An analysis of land cover within 200 m at 110 sites along the Niagara Escarpment where Louisiana Waterthrush had been detected from 1981 to 2013 was recently completed (Lebrun-Southcott and Campomizzi 2014). This study found nearly total mixed and/or deciduous forest cover within 200 m of the majority of detection locations. Most (85%) detection points also had >50 ha of total forest cover within the 100 ha buffer. However, many detection points (45%) had between 20 and 60 ha of agricultural land cover within the 100 ha buffer, suggesting that in some parts of its Ontario range this species may not be as area-sensitive as previously reported (Lebrun-Southcott and Campomizzi 2014).
Migration Habitat
During migration, the Louisiana Waterthrush occurs in habitats similar to those in the breeding range and in a variety of non-typical habitats where flowing or standing water and sufficient canopy cover is available (Curson et al. 1994; Dunn and Garrett 1997; Mattsson et al. 2009).
Wintering Habitat
In winter, it is found in tropical evergreen forests and favours riparian woodland in hilly and montane areas (Mattsson et al. 2009; Berlanga et al. 2010). It is less common along streams in lowland areas and mangrove forests, which are favoured by Northern Waterthrushes (Mattsson et al. 2009). In Costa Rica, radio-tagged birds foraged mostly along streams, but also exploited food-rich ground substrates in off-stream habitats, including residential areas and wet pastures (Master et al. 2005; Hallworth et al. 2011).
Habitat Trends
Breeding Habitat
Many of southwestern Ontario’s historical wetland forests have disappeared, have been heavily fragmented, and/or have been drained for agricultural or development purposes (Snell 1987; Page 1996; Larson et al. 1999; Crins et al. 2007; Environment Canada 2014b). There are few, large intact blocks of deciduous swamp forest remaining in this region. Loss of Louisiana Waterthrush nesting habitat within forested ravines has occurred as well, but not to the same extent. Nonetheless, the quality of primary nesting habitat in forested ravines has undoubtedly declined appreciably in some regions owing to forest fragmentation, logging, stream pollution, and siltation. In contrast, the amount and quality of forested stream habitat in parts of southeastern Ontario, and along some sections of the Niagara Escarpment in south-central Ontario, have likely improved over the past century due to the replanting and regeneration of forests in protected areas and on marginal farmlands (Crins et al. 2007).
Forests and wetlands in much of southwestern Quebec have also been negatively impacted by agriculture, urbanization and forestry (Jobin et al. 2010). However, the Louisiana Waterthrush occurrences in this province are in areas with a relatively high proportion of forest cover (Environment Canada 2013a, b). Other than in areas affected by urban sprawl, there was little change in the extent of forest and wetland cover in southwestern Quebec between 1993 and 2001 (Jobin et al. 2010).
Despite significant historical and ongoing losses in the extent of Louisiana Waterthrush habitat in southern Ontario, there are areas of suitable habitat that are either not occupied or are occupied intermittently (COSEWIC 2006; J. Allair, pers. comm. 2014; Friesen and Lebrun-Southcott 2012; D. Derbyshire, pers. comm. 2014). There are also areas of apparently suitable habitat in southwestern Quebec that are not occupied (Savignac 2008). Failure to occupy all available habitat in Canada is likely because this is the northern periphery of the species’ range, and the population here is small and patchy.
Non-breeding Habitat
No specific recent information on habitat trends on migration stopover or wintering grounds is available. A recent threat assessment determined that this species faces elevated threats during the non-breeding period, due to deforestation and development pressures adversely affecting riparian forest habitat (PIFSC 2012). Since 2000, rates of deforestation in Central America and the Caribbean have slowed, and in some countries and regions reversed (Redo et al. 2012; Aide et al. 2013). However, degraded water quality is still a significant issue in some parts of the wintering range, including the Dominican Republic (Latta 2011) and Puerto Rico (Hallworth et al. 2011).
Biology
Life Cycle and Reproduction
Male Louisiana Waterthrushes sing profusely when they arrive on their breeding territories in April and early May. (Eaton 1958; Bent 1963; Mattsson et al. 2009; Bickerton and Walters 2011). Males aggressively defend their territories against conspecifics (Craig 1984; Mattsson et al. 2009). Along stream courses, territories are essentially linear (Mattsson et al. 2009). There is considerable local and regional variation in the length of riparian territories reported (range 90 to 1440 m), but most territories are in the 300 to 600 m range (Mattsson et al. 2009, 2011). Variation in territory size may reflect variable mating systems (polygnous males have large territories) and/or food availability (Mulvihill et al. 2002; Mulvihill et al. 2008; Mattsson and Cooper 2009). The average size of territories in Canada is not known, but the previous estimate of 2 ha (COSEWIC 2006) is likely reasonable (e.g., average territory length of 400 m and width of 50 m along streams, and 140 m diameter circle in forest swamps).
Mating is generally monogamous, but up to 2% of males may have two females (Mulvihill et al. 2002; Mattsson and Cooper 2009). Pairing success is generally high (e.g., in Pennsylvania 84% of males (n=55) on acidified streams and 92% (n=152) of males on circum-neutral streams (pH near 7) were paired; Mulvilhill et al. 2008). Many territorial males in Canada, however, appear to be unpaired (Savignac 2006; J. Allair, pers. comm. 2014; D. Derbyshire, pers. comm. 2014). Pairing rates reported during intensive targeted surveys in Canada range from 36% in Frontenac Park, Ontario (Frontenac Bird Studies 2014) to 70% in the Norfolk Sand Plain (BSC 2014b) (see Table 1). As females can be secretive, reported pairing rates may underestimate the actual proportion of territorial males that represent breeding pairs. Given the available information, pairing success in Canada is likely between 66% and 75%.
Egg dates in Ontario range from 1 May - 8 July, including re-nesting attempts after failed nests (Peck and James 1998; ONRS 2014). Depending on latitude, the nesting period may start anywhere between late April in southwestern Ontario to early May in the northern part of the breeding range and may end anywhere between mid-June to early July (Rousseu and Drolet 2015). Incubation extends from 12-14 days. Both parents assist with feeding the young, which remain in the nest for about 10 days (Bent 1963; Mattsson et al. 2009). Parents attend to fledglings for up to 4 weeks (Mattsson et al. 2009).
Clutch size ranges from 4-6 eggs (Bent 1963; ONRS 2014). The Louisiana Waterthrush is generally single-brooded, but second broods have been documented in the U.S. (Mattsson and Cooper 2007; Mulvilhill et al. 2009). Second (and even third) re-nestings are common if the first nest is destroyed early in the season (Mattsson et al. 2009).
Nests of the Louisiana Waterthrush are sometimes parasitized by Brown-headed Cowbirds (Molothrus ater). Parasitism rates are highly variable across the breeding range, but are generally higher in the U.S. midwest (e.g., 33% to 81% at two sites in Illinois, n=15 and n=11, respectively; Mattson et al. 2009) and low in the U.S. northeast (e.g., 4% in Pennsylvania, n=222, O’Connell et al. 2003), and southeast (0% in Georgia, n=190; Mattssonand Cooper 2009). In southern Ontario, a relatively high proportion of nests contain one or more cowbird eggs (e.g., 22.8% of nests with eggs reported to ONRS 2014). While Louisiana Waterthrushes can successfully fledge a brood containing cowbird and waterthrush young, the reduction in the number of host young fledged may approach 50% (Mattsson et al. 2009).
Mean daily nest survival is relatively high (0.97 in Georgia and Pennsylvania; Mattsson et al. 2011). Depredation was the main source of nest failure in U.S. studies (Mattsson and Cooper 2009; Mulvihill et al. 2009). Comparable productivity statistics are not available for the Canadian population, but nest monitoring in Norfolk and Elgin counties found that 63% of nests with known outcomes (n=38) fledged at least one Louisiana Waterthrush young (BSC 2014b). This is similar to nest success rates reported in U.S. studies (e.g., 59% of 190 nests in Georgia, Mattsson and Cooper 2009; 52% of 231 nests in Pennsylvania; Mulvihill et al. 2009).
Mean fecundity (number of young that reach fledgling age per female) in Georgia was 2.89 +/-1.86, n=130 (Mattsson and Cooper 2007), and 3.4 +/- 0.2, n=175 in Pennsylvania (Mulvihill et al. 2008). In the latter study, there was no change in nest success or annual fecundity of nests on acidified streams compared to circum-neutral streams despite smaller clutch sizes. However, the number of young produced was much lower on acidified streams due to larger territories resulting in lower nesting densities (Mulvihill et al. 2008). An analysis using a stochastic individual-based model and data from 418 nests in Georgia and Pennsylvania predicted that about half of all females should fledge at least 1 young (Mattsson et al. 2011).
Louisiana Waterthrushes mature in one year and, like most small birds, generally have a short life span. The longevity record is 11 years, 11 months (Mattsson et al. 2009; Lutmerding and Love 2014). The average age of breeding adults in the population is likely 2-3 years.
Mean apparent annual survival rates range from 0.53 (SE=0.05, n=379) in the southeast U.S., to 0.46 (SE=0.11, n=86) in south-central U.S., and 0.47 (SE=0.06, n=222) in the northeast U.S. (Michel et al. 2011). The proportion of females banded as adults re-sighted the following year later ranged from 26% in Georgia (n=58), to 47% and 55% in two Pennsylvania studies; whereas the proportion of males ranged from 34% in Georgia, to 50% and 39% in the Pennsylvania studies (see Mattsson et al. 2009). In Pennsylvania, birds nesting on acidified streams were less likely to return and the proportion of inexperienced first-time breeding birds was higher there than on circum-neutral streams (Mulvihill et al. 2008). Relatively few birds (5% to 10%) continue to return for 2 or more years after banding. In a 4-year study in southwestern Ontario, 43% of colour-banded adult females (n=14) and 29% of males (n=14) were re-sighted the following year (Allair et al. 2014). Based on this small sample, return rates (apparent survival rates) in southwestern Ontario appear to be similar to those reported in the U.S.
For young birds, fidelity to natal areas is low. In two U.S. studies, very few (0 of 49 in Tennessee and 1 of 240 in Pennsylvania, Mattson et al. 2009) colour-marked nestlings were re-sighted in subsequent years. Five of 73 birds banded as nestlings at sites in the Norfolk County area of southwestern Ontario between 2011 and 2014 were re-sighted the following year, up to 12 km (average 8 km) from their natal site (S. Dobney pers. comm. 2015).
Physiology and Adaptability
The Louisiana Waterthrush spends most of its time on or near the ground, along the margins of streams and pools, and even wading in shallow water (Bent 1963; Mattsson et al. 2009). Although able to tolerate moderate levels of direct human disturbance, the Louisiana Waterthrush is particularly susceptible to habitat perturbations, including deforestation, loss of canopy cover, fluctuating water levels, water pollution, and siltation (Mattsson et al. 2009).
Dispersal and Migration
The Louisiana Waterthrush is a long-distance migrant that arrives in southern Ontario much earlier than most other wood-warblers. Usual dates in the province range from late April to early September (James 1991). By mid-August, most birds from Canada have migrated south (Curson et al. 1994).
No important areas of concentration of migrant Louisiana Waterthrushes are known, and the species is believed to migrate solitarily or in small numbers across a broad front (Dunn and Garrett 1997; Curson et al. 2004; Mattsson et al. 2009). During spring migration, the species occurs fairly regularly in small numbers along the north shore of Lake Erie (e.g., Long Point, Point Pelee, Rondeau).
Fledged young remain along natal streams for about a month, then wander progressively farther (up to 5 km) away, unattended by parents (Eaton 1958). There is some evidence that young and adults move into dense shrubby areas in the post-fledging period, which is when adult birds undergo a complete moult (Vitz and Rodewald 2006, 2007; Mulvihill et al. 2009).
Annual fidelity to breeding areas has been recognized (see Life Cycle and Reproduction above, and Mattsson et al. 2009). In Pennsylvania, Mulvihill et al. (2002) reported that up to 50% of females reoccupied territories from the previous year, “not infrequently with the same mate.” Site fidelity and fidelity to previous mates have also been observed in Ontario (Allair et al. 2014). Site-tenacity has also been documented in the wintering grounds (Mattsson et al. 2009). Wintering birds appear to actively defend feeding territories ranging from 0.3 ha to 11 ha (Mattsson et al. 2009).
Diet and Foraging Behaviour
The Louisiana Waterthrush feeds mostly on aquatic macro-invertebrates, including mature and immature insects. It also sometimes eats small molluscs, fish, crustaceans, and amphibians (Eaton 1958; Bent 1963; Mattsson et al. 2009). The diet is quite atypical of other songbirds.
Aquatic foraging is commonplace, particularly early in the breeding season. Submerged and floating organisms are eaten (Eaton 1958; Craig 1984). The following types of aquatic organisms have been reported in the summer diet: Trichoptera, Ephemeroptera nymphs, and Diptera larvae (especially chironomids), Culicidae, Dytiscidae, isopods, and gastropods (Eaton 1958; Craig 1984; Mattsson et al. 2009). Terrestrial organisms included centipedes, caterpillars, adult mosquitoes, earthworms, spiders, and various emerging aquatic insects.
Important aquatic prey includes taxa that are sensitive to changes in water quality, particularly Ephemeroptera (mayflies), Plecoptera (stoneflies) and Trichoptera (caddisflies). The Louisiana Waterthrush’s occupancy of headwater streams is positively associated with the proportion of these taxa in the macrobenthic biomass (Stucker 2000; Mattsson and Cooper 2006; Mulvihill et al. 2008).
Although the two species of waterthrush have similar diets and foraging ecologies (Craig 1984, 1985), the Louisiana Waterthrush typically selects larger prey items than the Northern Waterthrush and has a greater preference for Trichoperta larvae (Craig 1987). Its selection of larger prey may be related to its larger bill size (Craig 1987).
Interspecific Interactions
Where sympatric, Louisiana Waterthrushes do not appear to interact much with Northern Waterthrushes, even when occupying the same breeding habitats and sharing overlapping territories (Craig 1984, 1985; Mattsson et al. 2009). This lack of interspecific aggression may stem from differences in diet (Craig 1987).
Adults are preyed upon by small raptors, while nest contents are preyed upon by a variety of snakes, small mammals, and jays (Mattsson et al. 2009).
Population Sizes and Trends
Sampling Effort and Methods
Specialized search effort is required for this species because of its early breeding season and its specialized breeding habitat, which is often hard to access (Mattsson et al. 2009; Bickerton and Walters 2011; Mordecai et al. 2011; Environment Canada 2012). In addition, Louisiana Waterthrushes have relatively large and/or linear breeding territories that contribute to low detectability during point count surveys (Buskirk and MacDonald 1995; Bickerton and Walters 2011; Reidy et al. 2011). Detectability is improved if surveys are conducted between late April and the first three weeks of May (Bickerton and Walters 2011; Friesen and Lebrun-Southcott 2012; L. Friesen, pers. comm. 2015a), if survey points arelocated along stream corridors (Mattson and Marshall 2009), and especially if conspecific playback of songs is used (Stucker 2000; Bickerton and Walters 2011). Linear territories in riparian areas can exceed 1 km, so individuals can be detected at multiple survey stations, which can lead to overestimates of population size (Mordecai et al. 2011).
Due to its rarity and localized distribution, standard, broad-scale bird monitoring programs like the Breeding Bird Survey do not detect sufficient numbers of birds to provide robust abundance estimates or trends for the Canadian Louisiana Waterthrush population (Environment Canada 2012). It is only in the past decade that systematic searches targeting this species have been conducted across most of its Canadian range.
Breeding Bird Survey (BBS)
The Breeding Bird Survey (BBS) is a volunteer survey designed to monitor trends in North American breeding bird populations (Environment Canada 2014c; Sauer et al. 2014). BBS routes consist of 50 roadside points along randomly selected, stratified routes throughout North America. Each point is surveyed (3-minute point count) once annually during the breeding season. BBS data are analyzed using a hierarchical Bayesian model (Sauer and Link 2011). While this species is not well-sampled by this roadside-based survey and the timing of BBS surveys is later than its main calling period, the BBS is still the best source of information on continental population trends (Mattsson et al. 2009; PIFSC 2013; Sauer et al. 2014). However, it is not an effective method for monitoring Canadian trends because of small sample sizes here.
Breeding Bird Atlases
The two Ontario Breeding Bird Atlases provide comparable information on changes in bird distribution in the province between 1981-1985 and 2001-2005 (Cadman et al. 1987, 2007). Atlas data were gathered based on searches for all bird species within 10 km x 10 km squares for at least 20 hours over each of the two atlas periods. Although effort was comparable between the two atlases, better information on known and suitable habitat for this species was available to surveyors during the second atlas. Similarly, the two Quebec Breeding Bird Atlases reflect changes in bird distribution in that province between 1984-1989 and 2010-2014 (Gauthier and Aubry 1996; Québec BBA 2015).
Targeted Surveys
From 2005-2014, there have been several targeted surveys for Louisiana Waterthrushes in Ontario and Quebec (see Table 1). These systematic surveys largely focused on sites where the species had been previously reported, but additional sites with suitable habitat were also searched. Surveys were conducted following a species-specific survey protocol (similar to Bickerton and Walters 2011). To maximize detectability, surveys were generally conducted early in the breeding season and made use of conspecific audio-playbacks to elicit responses. The intensity of these targeted surveys varied considerably, with many sites being visited only once, while others received multiple visits in multiple years. Bird Studies Canada also carried out nest monitoring and colour banding of adults and young in Norfolk County, Ontario between 2011 and 2014.
Migration Counts
Standardized migration count data are collected annually at more than 20 Canadian Migration Monitoring Network (CMMN) stations across Canada (Bird Studies Canada 2014), but only Long Point Bird Observatory has recorded this species in sufficient numbers for population trend analysis. Analysis of spring migration data for Louisiana Waterthrushes at Long Point for 1961-2012 was provided by T. Crewe (pers. comm., 2014), using a generalized additive model with Poisson distribution.
Other Information Sources
Breeding occurrences of this species are tracked by provincial conservation data centres in Ontario and Quebec. The Ontario database includes information on 62 sites (element occurrences), whereas the Quebec database includes 4. Additional occurrence information is available in the eBird Canada program, and the Étude des population d’oiseaux du Québec (ÉPOQ database).
Abundance
The global Louisiana Waterthrush population is currently estimated to be 360,000 mature individuals (180,000 pairs) based on BBS data from 1998-2007 (PIFSC 2013). Its population is small relative to most other wood-warbler species (e.g., the Northern Waterthrush population is estimated at 19 million individuals).The bulk of its population is within the eastern United States, particularly in the Appalachian Mountain region (46%, Mattsson et al. 2009; PIFSC 2013). The PIF database provides an estimate of 140 individuals for Canada, but this estimate is flagged as being of very poor quality (Blancher et al. 2007b, 2013; PIFSC 2013). Nevertheless, the Canadian population clearly represents <1% of the total population.
As in past status reports, a Canadian population estimate was developed for each municipality (Table 2). This approach provides flexibility in accounting for considerable regional variation in search effort and habitat availability. The estimates are based on the number of known occurrences with breeding evidence between 2005 and 2014. This 10-year period includes targeted surveys, the final year of fieldwork during the second Ontario BBA, and fieldwork for the second Québec BBA (see Table 1). Occurrences were defined by assigning records to 1 km x 1 km grid squares. Available information was used to determine the maximum number of territories reported for each 1 km x 1 km site in any single year.
| Region | County/ Regional Municipality | Estimated No. Pairs 2005a Min. |
Estimated No. Pairs 2005a Max. |
Known Territories (Max/year) 2005–2014b | Estimated No.Territories 2014c Min. |
Estimated No.Territories 2014c Max. |
|---|---|---|---|---|---|---|
| Southwestern Ontario | Essex, Chatham-Kent | 1 | 3 | 0 | 0 | 2 |
| Southwestern Ontario | Lambton | 5 | 8 | 1 | 2 | 5 |
| Southwestern Ontario | Middlesex | 5 | 11 | 1 | 2 | 5 |
| Southwestern Ontario | Huron, Perth, Bruce | 0 | 1 | 1 | 0 | 2 |
| Southwestern Ontario | Elgin | 30 | 45 | 14 (10) | 30 | 60 |
| Southwestern Ontario | Norfolk | 45 | 75 | 35 (14) | 30 | 60 |
| Southwestern Ontario | Oxford | 6 | 13 | 1 | 2 | 5 |
| Southwestern Ontario | Haldimand | 0 | 1 | - | 0 | 2 |
| Southwestern Ontario | Brant | 0 | 4 | 0 | 0 | 2 |
| Southwestern Ontario | Waterloo | 1 | 4 | 0 | 0 | 2 |
| Southwestern Ontario | Wellington | - | - | - | - | - |
| Southwestern Ontario | Sub-total | 93 | 165 | 53 | 66 | 145 |
| South-central Ontario | Niagara | 0 | 3 | 0 | 0 | 2 |
| South-central Ontario | Hamilton | 2 | 3 | 4 (2) | 2 | 5 |
| South-central Ontario | Halton | 1 | 4 | 20 (13) | 20 | 50 |
| South-central Ontario | Peel | - | - | 5 (3) | 5 | 12 |
| South-central Ontario | Dufferin | - | - | 7 (4) | 6 | 15 |
| South-central Ontario | Simcoe | 1 | 4 | 8 | 10 | 25 |
| South-central Ontario | Grey | 2 | 4 | 10 (6) | 10 | 25 |
| South-central Ontario | Sub-total | 6 | 18 | 54 | 53 | 134 |
| Southeastern Ontario | Durham | - | - | 3 (2) | 2 | 5 |
| Southeastern Ontario | Northumberland | 0 | 1 | 2 | 0 | 2 |
| Southeastern Ontario | Peterborough | 1 | 2 | 1 | 0 | 2 |
| Southeastern Ontario | Hastings | 0 | 1 | 0 | 0 | 2 |
| Southeastern Ontario | Lennox & Addington | - | - | 0 | 0 | 2 |
| Southeastern Ontario | Frontenac | 5 | 8 | 12 (5) | 8 | 15 |
| Southeastern Ontario | Leeds & Grenville | - | - | 1 | 0 | 2 |
| Southeastern Ontario | Sub-total | 6 | 12 | 19 | 10 | 30 |
| Central Ontario | Southern Shield | - | - | 0 | 5 | 10 |
| Southwestern Quebec | Estrie | - | - | 0 | 0 | 1 |
| Southwestern Quebec | Montérégie | - | - | 3 (2) | 0 | 3 |
| Southwestern Quebec | Outaouais | - | - | 4 (2) | 0 | 3 |
| Southwestern Quebec | Sub-total | - | - | 7 | 0 | 7 |
| Canada | Total | 105 | 195 | 133 | 134 | 326 |
a. COSEWIC 2006 estimates for Ontario based largely on information provided by regional coordinators of the second Ontario Breeding Bird Atlas and assuming an average annual site occupancy rate of about 75%.
b. Known territories 2005-2014: sum of the maximum number of males reported in any year at each site (1 km2) where breeding evidence was reported at least once during past 10 years; (Max/year): is the maximum count of males in that region in any one year in the past 10 years.
c. Estimated No. Territories 2014: estimated number of territorial males per region based on occurrence information for past decade, amount of habitat, and input from individuals with local knowledge.
In total, 126 Louisiana Waterthrushes at 99 sites in 49 10 x 10 km squares were reported in Ontario over the 2005-2014 period (Table 2). In Quebec, 7 Louisiana Waterthrushes at 7 sites in 7 atlas squares were reported.
As noted in the previous status report (COSEWIC 2006), most Louisiana Waterthrush sites in Canada are occupied intermittently. Sites may be occupied for several successive years before being abandoned, presumably due to the death of one or both members of the pair, or changes in habitat suitability (COSEWIC 2006). In developing the regional population estimates, the distribution and intensity of the recent survey effort relative to the amount of potential habitat in each region was also taken into consideration. A high proportion of the historical sites have been surveyed at least once in the past 10 years, but targeted surveys did not include all historical sites on private lands, or regions with few occurrences. The targeted surveys also included many areas with apparently suitable habitat, which resulted in the discovery of many “new” sites for this species.
Two other factors considered were the proportion of the “known” sites with possible breeding evidence that constituted bona fide breeding territories (versus non-breeding, transient males), and the detectability of the species. About 21% (22 of 106) of the “known” sites are based on very limited breeding evidence, which was typically a singing male observed in suitable habitat on one occasion over the past 10 years. Several of these possible breeding records could represent transient males. As it is also likely that some birds were also missed during single-visit surveys at other sites, the net effect of these two factors on the population estimate was considered negligible.
As in previous status reports, regional population estimates were reviewed by individuals with local knowledge (see Acknowledgements). Unlike the previous population estimates, the estimates in Table 2 refer to the number of territories (males), rather than the number of breeding pairs. Recent intensive fieldwork indicates that many males do not appear to be paired (see Biology section). The overall Louisiana Waterthrush pairing rate in Canada is likely less than 75% (see Biology section). Therefore, the estimate of 134 to 326 territorial males in Canada (Table 2) likely represents a reproductive population of no more than 100 to 245 pairs. If the actual pairing rate is closer to 66%, then the reproductive population would be somewhat less (88 to 215 pairs, or 176 to 430 mature individuals). Taking all factors into consideration, it is estimated that there are 235 to 575 adults in the Canadian population. This would consist of 134-329 males and 101-246 females.
The number of squares with breeding evidence reported during the Ontario and Québec Breeding Bird Atlas projects provides a reasonably consistent, albeit indirect, measure of abundance. A previous population estimate based on the first Ontario Atlas in 1981-85 was 50-100 pairs based on 40 occupied squares (Eagles 1987), and 105 to 195 pairs based on the occupancy of 39 atlas squares (McCracken 2007). These previous estimates suggest the average number of pairs per reported square is somewhere between 1.25 and 5. Over the past 10 years, breeding evidence has been reported in a total of 56 squares in Canada (49 in Ontario, 7 in Quebec), which provides an alternative population estimate of 70 to 280 pairs (140 to 560 birds), which is fairly consistent with the estimate provided above.
Fluctuations and Trends
Documenting population change for the Louisiana Waterthrush in Canada is difficult because it is not monitored well by any survey program. As noted previously, ascertaining the presence/absence of this species requires specialized search effort. Moreover, intermittent site occupancy is common. While patterns of local extirpations and persistence are apparent in some regions, population increases are more difficult to assess, particularly in regions with little baseline information.
U.S. Population Trends
The continental population is sampled by many BBS routes (n=941) across the U.S., but abundance is low and the roadside survey design does not sample all breeding habitat (Saueret al. 2014). The range-wide BBS data suggest that the continental population has been increasing modestly over both the long term (1966-2013: 0.55% per year; 95% credible intervals (CIs): 0.07, 1.01), and the past 10 years (2003-2013: 1.80% per year; 95% CIs: 0.75, 2.87; Saueret al. 2014).
BBS population trends for U.S. states bordering Canada are not statistically significant. While Breeding Bird Atlas data show declines in distribution in New York (Rosenberg 2008) and Michigan (Hull 2011), modest increases were reported in Ohio (Ohio BBA II 2014), Pennsylvania (Pennsylvania BBA 2014), and Vermont (Vermont BBA 2014a, b). These atlas results have not been adjusted for increased effort and coverage.
Historical Changes in Canada
This species has long been recognized as a rare breeder in Ontario (Baillie and Harrington 1937). It is generally assumed that historically this species was more widespread and numerous prior to European settlement, and then declined as forests and wetlands were cleared (McCracken 1991).
The first Ontario BBA (1981-1985) fieldwork provided the first comprehensive assessment of this species in Canada, resulting in a population estimate of 50-100 pairs (Eagles 1987). Higher estimates of 150 to over 300 pairs made in the 1990s were influenced by new information that suggested that as many as 100 pairs could be breeding in Norfolk and Elgin counties (McCracken 1991; Page 1996). However, these higher estimates did not consider intermittent site occupancy and incomplete saturation of available habitat (COSEWIC 2006).
During the second Ontario BBA (2001-2005), this species was reported from 39 squares, and the population was estimated to be about 105-195 pairs (COSEWIC 2006; McCracken 2007). The two-fold difference between 1987 and 2007 BBA population estimates was attributed to the species being under-recorded during the first atlas, as the Ontario population was “believed to have remained essential[ly] stable over the last two decades” (McCracken 2007).
Recent Population Changes in Canada
As indicated in Table 2, current population estimates are higher in some regions and lower in others compared to the 2005 estimates. Due to these regional differences, population trends are reviewed separately for the four geographic regions within the Canadian range.
Southwestern Ontario
Southwestern Ontario is generally considered the stronghold for this species in Canada (McCracken 1991; Page 1996). Previous population estimates suggested that this region supported about 85% of the Canadian population (COSEWIC 2006). The current population estimate (Table 2) for southwestern Ontario of 50 to 109 pairs (66 to 145 males based on 75% pairing success) is slightly lower than the 2005 estimate of 93 to 165 pairs (COSEWIC 2006).
Within this region, the population is still concentrated in the Norfolk Sand Plain region (Norfolk, eastern Elgin, and southern Oxford counties). However, the current estimate of 47 to 94 breeding pairs (62 to 125 males, 75% pairing success rate) is about two-thirds of the 2005 estimate of 81 to 133 pairs. This region (particularly Norfolk County) was the focus of intensive surveys between 2010 and 2014 (Table 1). Louisiana Waterthrushes were detected at 41 different sites (including 7 sites with multiple males) in these three counties. The maximum numbers of birds detected in any given year was: 11 pairs and 3 single males in Norfolk in 2012; 6 pairs and 4 single males in Elgin in 2012, and a single male in Oxford in 2005.
Migration count data collected during spring migration at Long Point Bird Observatory from 1961 through 2012 shows increased numbers in the 1980s and 1990s (Figure 5). However, migration counts since 2000 have returned to the low levels experienced earlier (Figure 5).
Previous population estimates for Norfolk and Elgin counties were strongly influenced by the large number of birds reported during local, intensive biological surveys conducted during the 1980s, which suggested that “there are probably over 100 pairs in these two regions alone” (McCracken 1991). The recent targeted surveys, which included most historical sites plus many other areas of suitable habitat, suggested that the current population may be lower. Surveys in Norfolk County over the past 5 years indicate that a population estimate of between 30 and 60 pairs is realistic (J. Allair, pers. comm. 2014). There is more uncertainty associated with the estimate of 30 to 60 pairs in Elgin County, as recent coverage there has been less extensive and there is lots of potential habitat that has not been surveyed (J. Allair, pers. comm. 2014). Oxford County has received relatively little survey coverage since the 1990s, but suitable habitat is quite limited and habitat in the area where up to 9 pairs were present about 25 years ago is now degraded (J. Holdsworth, pers. comm. 2014; J. Skevington, pers. comm. 2014). The Louisiana Waterthrush also occurs in small numbers locally in other parts of southwestern Ontario where suitable habitat is present (e.g., Middlesex and Lambton counties).
Figure 5. Annual indices of spring migration counts of Louisiana Waterthrushes at Long Point Bird Observatory, 1961-2012.
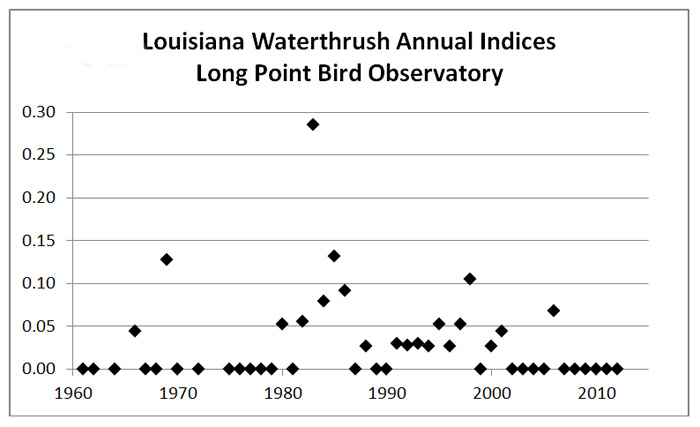
(courtesy Bird Studies Canada)
Long description for Figure 5
Graph presenting estimated sizes of Louisiana Waterthrush breeding populations in various regions of Ontario and Quebec. Information includes county or regional municipality, estimated number of pairs in 2005 (minimum and maximum), numbers of known territories from 2005 to 2014, and estimated numbers of territories in 2014 (minimum and maximum).
The species appears to be virtually extirpated from some areas where it was considered a regular breeder a century ago (see Page 1996). It has disappeared as a breeding bird in Chatham-Kent and Essex regions in extreme southwestern Ontario. It may also be gone from Waterloo Region (K. Burrell, pers. comm. 2014; Table 1). In 2005, these three regions supported an estimated 2 to 7 breeding pairs (COSEWIC 2006). These regions are subject to particularly high rates of agricultural intensification, human population growth, and suburban development (Larsonet al. 1999; Blancheret a l. 2007a) that have contributed to loss, fragmentation, and degradation of breeding habitat.
South-central Ontario
The current population estimate (Table 2) for south-central Ontario of 40 to 100 pairs (53 to 134 males assuming 75% pairing success) is over five times the 2005 estimate of only 6 to 18 pairs (COSEWIC 2006). The latest estimate is based largely on targeted surveys (Table 1), along with some casual reports. Targeted surveys in 2012 and 2013 found this species to be more common and widespread along the central Niagara Escarpment than previously known. However, only a few birds were reported elsewhere in south-central Ontario (Figure 3).
In total, 31 males were located at sites along the Niagara Escarpment in 2012, and 34 males were found in 2013 (Friesen and Lebrun-Southcott 2012; Lebrun-Southcott and Friesen 2013). Relatively few females were detected (see Table 1). All sites on the central Escarpment with multiple pairs in 2012 were re-surveyed in 2013, when birds were found at six of seven sites (Lebrun-Southcott and Friesen 2013). In contrast, only 4 single males were found in surveys of headwater streams along the Oak Ridges Moraine in 2014 (Campomizziet al. 2014).
The surveys focused on historical sites and potential habitat in public areas and along hiking trails. Occupancy at the historical sites was generally low (5 of 13 sites in 2012). Most birds were found at previously undocumented sites. It is unclear whether the presence of Louisiana Waterthrush at the previously undocumented sites along the Escarpment has simply gone unnoticed previously, or whether there has been an expansion in recent years (Lebrun-Southcott and Friesen 2013).
In stark contrast to the situation along the central Niagara Escarpment, the Louisiana Waterthrush appears to have abandoned most of its historical locales along the southern Niagara Escarpment, in Hamilton and Niagara regions. Birds were reported at only one of 15 historical sites along the Escarpment south of Hwy 401 during the 2013 surveys (Lebrun-Southcott and Friesen 2013). Many of these historical sites are forested stream valleys in small protected areas situated in a matrix of cropland and suburban development. In addition to habitat fragmentation, compromised water quality may be an issue at some of these unoccupied historical sites (Lebrun-Southcott and Friesen 2013).
Follow-up surveys along the central Niagara Escarpment in 2015 found only 8 singing males at 7 sites, where 20 to 23 singing males had been reported previously in 2012 and 2013, while three other sites with what appeared to prime habitat remained unoccupied (Friesen 2015b). This new information indicates that intermittent site occupancy is occurring in all parts of the Canadian range. It also suggests that current numbers in this region are nearer the lower end of the above population estimate.
Southeastern Ontario
The small population concentrated in the Frontenac Arch area north of Kingston has reportedly persisted since the early 1980s according to Weir (2008). However, targeted field work in 2010-2012 found few birds. In 2012, only 2 of 12 historical sites were occupied (Derbyshire 2012). These intensive studies indicated that fewer than 5 pairs were breeding in a large study area centred on Frontenac Park (D. Derbyshire, pers. comm. 2014). Elsewhere in this region, the species occurs only sporadically in Northumberland County (B. Walters, pers. comm. 2014).
Southwestern Quebec
The status of this species in Quebec has changed from being considered a rare vagrant to a rare and occasional breeding species. The only confirmed nesting was in 2006, at a site in Gatineau Park where the species had been reported occasionally for more than 25 years (Savignac 2006). In 2008, no birds were found at this site or during targeted surveys of the other historical sites with possible breeding evidence. During the second Québec BBA, this species was reported in 4 squares, compared to only 1 square in the 1984-1989 atlas (St-Hilaire and Dauphin 1996; Québec BBA 2015). Thus, available information suggests a small increase in numbers in southwestern Quebec, specifically in the Outaouais (Eardley Escarpment/Gatineau Hills) and Estrie regions. However, this apparent increase may be related to increased search effort and/or a short-lived fluctuation.
Summary
The current estimate of the size of the Canadian breeding population is similar to the 2005 estimate. Some local populations have declined or disappeared due to habitat loss and degradation. Recent surveys found that the population along the central Niagara Escarpment is larger than previously known, which could be related to greater search effort. This species seems to have established a small foothold in Quebec in the past decade, but its status there is still tenuous.
Rescue Effect
Although there is no direct evidence of immigration from the U.S., some immigration almost certainly takes place, particularly from the much larger breeding populations in Ohio, Pennsylvania and New York, and perhaps from smaller populations in Vermont, New Hampshire and Michigan (see population estimates for adjacent states in Table 3).
It has been suggested that the expansion of Louisiana Waterthrushes in the Kingston region of southeastern Ontario in the 1980s may have been driven by immigration from the nearby population in upper New York (COSEWIC 2006). Both of these populations have since contracted, providing further support for this linkage.
| Jurisdiction | Rank (NatureServe 2015) |
Designation (NatureServe 2015) |
Population Estimate (PIFSC 2013) |
Proportion of global population (PIFSC 2013) |
|---|---|---|---|---|
| Ontario | S3B | Special Concern | 140 | <0.1% |
| Quebec | S1B | Critically Imperilled | - | - |
| Michigan | S2S3 | Special Concern | - | - |
| New Hampshire | S4 | Not listed | 900 | 0.3% |
| New York | S5 | Not listed | 9,000 | 2.6% |
| Ohio | S5 | Not listed | 6,000 | 1.6% |
| Pennsylvania | S5 | Not listed | 20,000 | 6.3% |
| Vermont | S4 | Not listed | 1,100 | 0.3% |
Threats and Limiting Factors
The Louisiana Waterthrush is a habitat specialist and its population is limited by the supply of clear, clean, forested streams and forested wetland pools within its breeding and wintering ranges. In Canada, many areas of known or potential habitat are not regularly occupied owing to the small population. The persistence of the small, localized Canadian population may be dependent upon immigration from the U.S. (see Rescue Effect).
There is no single imminent threat to the survival of the Canadian population; rather, it is the cumulative effects of many different threats operating at various scales at different stages of its annual life cycle that are of concern. These threats are reviewed below, grouped geographically. Overall threat levels are considered to be high to medium (see Appendix 1 for details).
General Threats on Breeding Grounds in Canada
The nature and intensity of threats varies across its Canadian range. For example, impacts from agricultural activities are most intense in parts of extreme southwestern Ontario, pressures from increased development due to rapid human population growth and urban sprawl are most intense in the Niagara-Hamilton-Toronto corridor, and acid precipitation could be affecting habitats in the Frontenac Arch region of southeastern Ontario and southwestern Quebec.
i) Changes in water quantity
Due to its dependence on aquatic prey in streams and wetlands, the Louisiana Waterthrush is susceptible to changes in hydrology due to water-taking for irrigation (especially in the Norfolk Sand Plain), groundwater pumping for municipal and industrial water supplies (south-central Ontario), and construction and maintenance of agricultural drains through wetlands (across southern Ontario). The impact of these threats is particularly severe during drought conditions (Eaton 1958; COSEWIC 2006).
Extremes of water quantity can also affect habitat suitability. Habitat modifications that exacerbate storm run-off and result in “flashy” stream systems in southern Ontario include wetland drainage and forest clearing for development or agriculture, widespread use of tile drainage in croplands, and increased amounts of impervious surface areas associated with urban development and roads. High rainfall events that result in elevated water levels are also detrimental to Louisiana Waterthrush productivity due to direct loss of streamside nests and reduced access to prey (Stucker 2000; Mattsson and Cooper 2009; Mattsson et al. 2009).
ii) Changes in water quality
Anything that negatively affects the supply of aquatic insects in waterthrush habitat is likely to have a negative impact on breeding populations. Increased turbidity and acidification can reduce food availability (Mattsson and Cooper 2009). This species is absent from streams where water quality has been compromised by agricultural or urban activities (Prosser and Brooks 1998). This species may also bio-accumulate persistent chemical contaminants (Osborne et al. 2011; Latta et al. 2014).
Due to intensive land use and limited forest cover, water quality is poor in many watersheds in southern Ontario, which substantially limits the amount of suitable habitat for this species. Increased siltation and turbidity are commonly caused by agricultural run-off, road construction, forest clearing, and urban development (Kerr 1995). Other common pollutants include nutrients, bacteria, pesticides, road salt and contaminants from a range of agricultural, residential and industrial sources.
Acidification of aquatic habitats results in changes to benthic macroinvertebrate populations, which can negatively affect the food supply of Louisiana Waterthrushes (Mulvihill et al. 2008). Acidification also reduces the availability of calcium needed for eggshells (Graveland 1998), and amplifies the impacts of forest fragmentation and some other contaminants (e.g., mercury contamination, see below).
Louisiana Waterthrushes breeding density on acidified streams was significantly reduced compared to circum-neutral streams, indicating that acidification reduces habitat quality (Mulvihill et al. 2008). Despite improvements in treating airborne emissions since the 1980s, acid precipitation continues to affect aquatic systems in areas where the soil-buffering capacity is low, including the Frontenac Arch and Southern Shield regions in Ontario (Nantel et al. 2014). Acid-deposition rates do not exceed the soil-buffering capacity in most of the current Canadian range, but this is not the case in large parts of the U.S. range (Hames et al. 2002).
Uptake of mercury has been identified as a potential threat to this species (Evers et al. 2011; Osborne et al. 2011). Sampling from sites across the northeastern U.S. found that while blood-mercury levels were elevated compared to other songbirds, they are generally below the low risk threshold for adverse biological effects (<0.5 ppm Hg, n=20; Evers et al. 2011; Osborne et al. 2011). Evers et al. (2011) concluded that while this species appears to be at low risk from atmospheric mercury deposition, it is nonetheless at high risk of mercury exposure from contaminated river systems throughout its life cycle. There is circumstantial evidence that mercury may be affecting local populations in New York. Of 20 individuals sampled by Osborne et al. (2011), 2 birds from southern New York had the highest levels of this neurotoxin (maximum 0.62 ppm), exceeding the low risk threshold. Louisiana Waterthrush populations in southern New York have been in decline since the 1980s, particularly in southeastern New York, where the species was formerly abundant (Rosenberg 2008). No information is available on mercury levels in this species in Canada, but exposure on the breeding grounds is expected to diminish over time due to reduced emissions (e.g., recent closure of all coal-burning plants in Ontario), as shown by declining levels of mercury in fish in the Great Lakes Basin (Monson et al. 2011).
A potential emerging threat to this species is the indirect effect of neonicotinoid insecticides (Gibbons et al. 2015). Recent studies have shown that widespread use of neonicotinoid treatments on agricultural crops in southwestern Ontario and globally since the 1990s has resulted in contamination of surface waters at levels that pose a significant risk to aquatic invertebrates (Morrissey et al. 2015; Schaafsma et al. 2015). These persistent chemicals are especially toxic to Ephemeroptera and Trichoptera, aquatic insects that are important prey species for Louisiana Waterthrush (Morrissey et al. 2015).
iii) Effects of logging and invasive forest pests
This species exhibits a strong preference for mature, shaded forest cover and is absent from early-successional habitats (Noon et al. 1979; Buffington et al. 1997; Prosser and Brooks 1998; Tirpak et al. 2009). Burke et al. (2011) indicate that it responds negatively to clear-cutting, shelterwood, and diameter-limit forest harvests. Logging operations, particularly in stream valleys and hilly areas, can also lead to increased run-off, increased siltation and turbidity, and higher water temperatures, all of which are likely to negatively impact this species’ food supply (Eaton 1988).
Elevated mortality of canopy tree species due to invasive forest pests and diseases can have adverse effects on habitat conditions. Two non-native invasive forest insects are of particular concern as emerging threats. Due to the close association of the Louisiana Waterthrush with hemlock, the spread of the Hemlock Woolly Adelgid (Adelges tsugae)is seen as a significant emerging threat to breeding habitat in Ontario (Friesen, pers. comm. 2015). The insect has been detected at isolated locations in southern Ontario since 2012 (Ryan 2013). Widespread hemlock mortality is considered an ongoing threat to Louisiana Waterthrush habitat in its core breeding range in the Appalachian Mountains in the U.S. (Mattsson et al. 2009). The significance of this threat to the U.S. population is reviewed in more detail below, as declines in the U.S. would adversely impact Louisiana Waterthrush immigration into Canada.
The Asian Long-horned Beetle (Anoplophora glabripennis) is another exotic insect that could have a devastating impact on eastern deciduous forest ecosystems as it attacks and kills all broadleaved trees, with maple (Acer spp.) being its preferred host. In eastern Canada, this species has been detected in the Greater Toronto Area, where an infestation found in 2003 was successfully eradicated, but then a new infestation was discovered in 2013 (Ontario MNRF 2014).The majority of Canadian Louisiana Waterthrush occurrences are within 150 km of the current infestation. Local infestations are also present in the eastern U.S., including southern Ohio and southeastern New York (US Forest Service 2014).
Forest fragmentation and intensive logging have been linked to elevated levels of nest predation and parasitism in forest birds, which can suppress reproductive output (Burke et al. 2011). Despite relatively high rates of cowbird parasitism, there is no evidence of reduced productivity or elevated predation at Louisiana Waterthrush nests in southwestern Ontario (ONRS 2014). Moreover, Brown-headed Cowbird populations have undergone a steep decline in eastern Can ada since the 1980s and the threat of parasitism is likely diminishing (Environment Canada 2014d).
iv) Climate change and severe weather
The ability of this species to adapt to climate change is uncertain. Due to its early arrival on the breeding grounds, it may be particularly sensitive to mismatches in phenology of insect prey (Nantel et al. 2014). There is also evidence that it is vulnerable to bouts of severe weather, including high rainfall events and droughts (Eaton 1958; Mattsson et al. 2009). Such extreme weather events are predicted to become more frequent in Canada, although regional and seasonal variability is expected (Chiotti and Lavender 2008; Bush et al. 2014). Precipitation changes are particularly uncertain, but potential declines in southern Canada, combined with increased evaporation, could increase seasonal aridity and reduce water availability in some areas (Bush et al. 2014).
On the other hand, the Canadian population of Louisiana Waterthrush could benefit from warming temperatures. A recent study predicted that the bioclimatic envelope of this species will shift northeastwards (National Audubon Society 2014). Any easing of the climatic constraints on the northern limit of the Louisiana Waterthrush could enable the species’ range to expand into the Southern Shield region in Ontario and the Atlantic Forest Region in southeastern Quebec, regions that have considerably more forest cover and less pressure from human population growth than southern Ontario. However, climate warming will also promote the northward spread of forest pest species such as the Hemlock Woolly Adelgid, which currently appear to be somewhat constrained by cold winter temperatures (Paradis et al. 2008).
Specific Local Threats at Breeding Occurrences in Canada
Several specific threats to Louisiana Waterthrush occurrences in Canada have been observed recently (see references in Table 1):
- Residential developments situated in close proximity to Louisiana Waterthrush habitat are a local but serious and ongoing threat.
- Disturbance of nesting birds by recreational users (fishers, hikers, dog walkers) along streams. Disturbance and siltation due to fording of streams by off-road vehicles also continues to be a problem at some sites, particularly in the Norfolk Sand Plain.
- Forest clearing for new ski runs at resorts along the Niagara Escarpment. This threat was observed at only one site during recent surveys. Further expansion of ski resorts along the Niagara Escarpment is likely limited by current planning and land use restrictions (see Habitat Protection and Ownership below).
Threats on the Breeding Range in the United States
Until recently, threats to this species on its breeding grounds in the U.S. were generally considered localized or of low concern. Forest cover in the eastern U.S. is extensive, having rebounded from an historical low extent circa 1910 during a long-term cycle of deforestation for agriculture following European settlement, followed by an extended period of reforestation of abandoned agricultural lands (Drummond and Loveland 2010). Moreover, the numerous headwater streams occupied by this species are often within protected areas, public lands, or other sparsely populated areas. Stream acidification from acid precipitation and point-source discharge from abandoned coal mines was identified as the most significant and widespread threat to Louisiana Waterthrush habitat in the U.S. (Mulvihill et al. 2008).
Recently, a number of new threats have emerged which have the potential to seriously impact Louisiana Waterthrush populations in the northeastern United States, which could then impact resilience of the small Canadian population because of its reliance on continued immigration from U.S. source populations. A general concern is that the forest cover transition in the eastern United States has entered a new stage, with losses now exceeding gains (Drummond and Loveland 2010). Land use pressures driving the net forest conversion between 1973 and 2000 were largely forest harvest, development, and mining (Drummond and Loveland 2010).
Two emerging threats of particular importance to Louisiana Waterthrush habitat in the northeastern U.S. are described below.
i) Hemlock decline due to an invasive insect pest
There is considerable concern that widespread hemlock mortality due to Hemlock Woolly Adelgid will adversely impact forest and stream ecosystems in the eastern U.S. (Siderhurst et al. 2010; Trotter et al. 2013). This exotic insect causes severe defoliation and tree mortality within 4 to 15 years of infestation. Very few trees show any resistance and there are no known practical control measures that can be used at the landscape scale (Siderhurst et al. 2010; Trotter et al. 2013). First established in Virginia in 1951, this pest had gained a foothold in 45% of the hemlock’s range in the eastern U.S. by 2003 (Trotter et al. 2013). The rate of spread is generally slow (up to about 20 km/year) and has been even slower in northern regions, where the insect faces high overwinter mortality (Paradis et al. 2008). To date, hemlock mortality in the U.S. is apparent at the stand level but not at the landscape scale (Trotter et al. 2013). However, Trotter et al. (2013) suggested that the situation in the U.S. is now at a tipping point, beyond which the impact of this species on eastern U.S. forests will rapidly transition from negligible to significant.
Hemlock mortality results in increased light penetration of riparian systems that can impact Louisiana Waterthrush habitat by increasing water temperature and shifting benthic insect communities (Mattsson et al. 2009) that the species relies on, and also by changing forest structure (increase in shrub layer) and composition (shift from mixed forest to deciduous forest). Changes in forest composition and structure related to widespread hemlock decline could have long-lasting impacts on Louisiana Waterthrush populations. Local population declines have been found in other hemlock-associated forest bird species (e.g., 70% fewer Acadian Flycatcher pairs at heavily infested sites; Allen et al. 2009), but the response of Louisiana Waterthrush to hemlock mortality is still unknown and there is presently no evidence of population declines in those parts of the U.S. range where the adelgid has been established for many decades. Thus, while there remains considerable uncertainty as to the timing and severity of this threat to Louisiana Waterthrush populations, the scope will likely be widespread and could result in major shifts in ecological communities and interactions.
ii) Habitat loss, fragmentation and degradation due to resource development
Louisiana Waterthrush populations in Kentucky and West Virginia, which support a quarter of the global breeding population (PIFSC 2013), are impacted by coal mining, particularly by the newer practice of mountaintop removal, as well as by acid-mine drainage from conventional mining practices. In West Virginia, 35,000 ha of mature deciduous forest have been converted to grassland and over 483 km of streams have been directly impacted by mountaintop removal practices, and an additional 4830 km of riparian habitat has been degraded by acid mine drainage (West Virginia DNR 2005). West Virginia has the highest relative abundance of this species, and populations in this state have undergone a long-term decline (Sauer et al. 2014).
In the past decade, two new forms of energy development have emerged that geographically overlap with prime Louisiana Waterthrush habitat in parts of its U.S. range. Shale gas development (by “fracking” or hydraulic fracturing) and ridge-top wind turbine installations give rise to a suite of new threats to Louisiana Waterthrush breeding habitat. Both of these forms of energy development require construction of extensive road networks, resulting in forest fragmentation and increased siltation and turbidity. In addition, new utility corridors are needed to transport the electricity and natural gas produced by these projects.
Furthermore, shale gas extraction is water-intensive. The potential exists for shale gas development to impact groundwater and surface water quantity and quality at various scales, and this risk is highest in smaller streams (Abdalla and Drohan 2010). Development of extensive shale formations in the U.S. will affect a high proportion of the global Louisiana Waterthrush breeding range. For example, about 75% of its breeding range in Pennsylvania overlaps the Marcellus Shale formation (Mulvihill 2012). Latta et al. (2014) reported preliminary findings that Louisiana Waterthrushes in watersheds with fracking activity carry significantly higher loads of contaminants associated with drilling-fluids than individuals in watersheds without fracking. The initial results of a 5-year study by Franz et al. (2014) in an area of West Virginia with increasing fracking activity suggested that waterthrush habitat quality was lower near nests impacted by fracking activity, although changes in breeding density and nest survival were not apparent.
Habitat Loss and Degradation on the Wintering Grounds
This species faces threats during the non-breeding portion of its life cycle due to loss and degradation of wintering and migration habitat in Latin America (PIFSC 2012). These regions are all being impacted by extensive and rapid land-use change due to development pressures (NABCI 2002). The conversion of forest to farmland is of particular concern due to the direct loss of forested riparian habitats and forested wetlands, as well as reductions in water quality and quantity as a result of logging and agricultural activities. As noted in the Habitat Trends section of this report, recent studies of land-use change patterns across this region suggest that the threat of deforestation has eased somewhat, at least in some parts (Redo et al. 2012; Aide et al. 2013). However, degraded water quality is still a significant issue in parts of the wintering range, including the Dominican Republic (Latta 2011) and Puerto Rico (Hallworth et al. 2011).
Collisions with Buildings and Communication Towers During Migration
During migration, this species is at risk from collisions with communications towers (Longcore et al. 2013; see also Mattsson et al. 2009) and tall buildings (Loss et al. 2014). To date, there have been no published reports of Louisiana Waterthrush mortality at wind turbines. These sources of human-induced mortality are of particular concern, given the relatively small global population size (360,000 individuals).
Number of Locations
The number of locations of this species in Canada cannot be quantified. Given the dispersed distribution of this species in many sites across multiple jurisdictional boundaries, and the notion that the most serious threats are likely site-based and caused by individual landowners, then there are clearly more than 10 locations.
Protection, Status and Ranks
Legal Protection and Status
General protection for this species and its nest and eggs is afforded in Canada through the federal Migratory Birds Convention Act 1994 and parallel legislation in the United States and Mexico, and on public land in Quebec through the provincial Act Respecting the Conservation and Development of Wildlife. COSEWIC assessed this species as Threatened in 2015. It is currently listed on Schedule 1 of Canada’s Species at Risk Act as ‘Special Concern’, on Schedule 4 of Ontario’s Endangered Species Act, 2007; and on the list of species likely to be designated threatened or vulnerable according to Quebec’s Act Respecting Threatened or Vulnerable Species.
The management objective established by the federal Management Plan for this species is to maintain the current size and distribution of the Louisiana Waterthrush in Canada (Environment Canada 2012). Recent activities related to this Management Plan include the development of a draft monitoring protocol (Bickerton and Walters 2011) and increased survey effort.
Non-Legal Status and Ranks
At the global level, this species is considered Secure (G5) by NatureServe (2015) and of Least Concern according to the IUCN Red List (IUCN 2012). The species is also considered Secure (N5B) in the United States breeding range (NatureServe 2015).
Nationally, the Louisiana Waterthrush is ranked as N3B (Vulnerable, Breeding population) in Canada (last reviewed 15 January 2013), and N5B (Secure, Breeding population) in the United States (NatureServe 2015). At the subnational level, it is ranked as S3B (Vulnerable, Breeding population) in Ontario, and S1B (Critically Imperilled, Breeding population) in Quebec (NatureServe 2015). It is ranked as S2S3 in Michigan (Imperilled/ Vulnerable), where it has been designated “special concern”. It is considered secure (S5) or apparently secure (S4) in other U.S. states bordering the species’ Canadian breeding range (Table 3).
Within the Partners In Flight (PIF) North American Landbird Conservation Plan, the Louisiana Waterthrush is identified as one of 195 species of “continental importance” because it is a “stewardship species”, as 94% of its global population breeds within the Eastern avifaunal biome (Rich et al. 2004). It is not on the continental PIF Watch List (the list of species of greatest continental conservation concern).
Due to its Special Concern status in Canada, it is identified as a conservation priority in two Bird Conservation Regions (BCRs) in Ontario (BCRs 12 and 13; Environment Canada 2014a,b) and in three BCRs in Quebec (BCRs 12, 13, and 14; B. Drolet, pers. comm. 2015).
Habitat Protection and Ownership
About half of all recently occupied breeding sites are located on public lands, including some formally protected areas (provincial parks and nature reserves) and other conservation lands (e.g., Conservation Authority lands and managed forests). This statistic is particularly notable given that over 90% of land in southern Ontario and southwestern Quebec is privately held. While the high proportion of public ownership is partly a reflection of the biased search effort, which has targeted accessible public lands, to a large extent it is also because the typical breeding habitat is frequently conserved by public agencies due to its scenic and ecological values, and because these areas are not easily developed, logged or converted to agriculture.
The largest area of suitable nesting habitat on federal lands is Gatineau Park in the Outaouais region of Quebec. In Ontario, this species is considered a regular rare migrant at Point Pelee National Park (Parks Canada Agency 2012) and the Long Point National Wildlife Area (LPBO 2014). There is some historical breeding evidence from the Six Nations reserve in Brant County (Chamberlain et al. 1985) and Walpole Island First Nation (Eagles 1987), but the evidence is rather weak and there have been no recent reports from these areas. The only Louisiana Waterthrush record from Department of National Defence lands is a 2009 report from the former Camp Ipperwash site (now Stony Point First Nations; A. McIsaac, pers. comm. 2014). Several occurrences are in provincial parks and nature reserves, including multiple territories at Pretty River Valley, Devil’s Glen, and Frontenac parks in Ontario (Friesen and Lebrun-Southcott 2012; Lebrun-Southcott and Friesen 2013; D. Derbyshire, pers. comm. 2014). Several other occurrences with multiple pairs are in public conservation areas managed by local Conservation Authorities (Friesen and Lebrun-Southcott 2012; Lebrun-Southcott and Friesen 2013).
Louisiana Waterthrush breeding habitat along the Niagara Escarpment is afforded some protection from development under the Niagara Escarpment Planning and Development Act, 1973 and the associated Niagara Escarpment Plan (2005). Many of the breeding sites are in public lands within the Niagara Escarpment Parks and Open Space System, which includes extensive natural environment areas (Niagara Escarpment Commission 2014). Some additional habitat and breeding sites are protected by the Oak Ridges Moraine Conservation Action 2001, and the Greenbelt Act, 2005 and associated conservation and land use plans and natural heritage strategies. Several sites in Ontario occur in “Areas of Natural and Scientific Interest”, “Environmentally Sensitive Areas”, “Provincially Significant Wetlands”, or other natural heritage designations which are afforded various levels of protection under provincial or regional land use planning policies.
While the bulk of suitable breeding habitat in Canada occurs on private lands, these areas are apt to receive some protection through various policies and regulations. For example, ravines, floodplains, and wetlands are apt to be zoned “hazard land”. Headwater streams and associated groundwater recharge areas in southern Ontario are often included in Source Protection Areas under the Clean Water Act, 2006 . The Natural Heritage component of Ontario’s Provincial Policy Statementsupports conservation measures for various areas and habitats where Louisiana Waterthrushes may occur. Similarly, in Quebec, the Protection Policy for Lakeshores, Riverbanks, Littoral Zones and Floodplains protects habitat used by this species. There are also federal and provincial laws designed to protect streams and fish habitat. In Quebec, aquatic habitat and water quality for aquatic species are protected under the Act respecting the conservation and development of wildlife, and the Environmental Quality Act.
Several Important Bird Areas (IBAs) in Ontario have been identified based on nationally significant concentrations of breeding Louisiana Waterthrushes and other birds at risk, including the Little Otter Creek Complex, Norfolk Forest Complex, and Dundas Valley IBAs (IBA Canada 2014). The IBA designation does not provide any legal protection.
Acknowledgements and Authorities Contacted
The author extends thanks to the reviewers who commented on earlier drafts of this report, including Ruben Boles, Corina Brdar, Bruno Drolet, Richard Elliot, Gilles Falardeau, Lyle Friesen, Tony Gaston, Isabelle Gauthier, Greg Mitchell, Guy Morrison, Chris Risley, Michel Robert, Jim Saunders, François Shaffer, Josée Tardif, Junior Tremblay, and Scott Wilson.
Thanks from the author are offered to Environment Canada (Lyle Friesen, Gilles Falardeau, Russ Weeber), Bird Studies Canada (Sarah Dobney, Jody Allair and Catherine Jardine), and Frontenac Bird Studies (Daniel Derbyshire) for supplying unpublished reports and data from Louisiana Waterthrush field surveys in Ontario and Quebec.
The author also wishes to thank the Regroupement QuébecOiseaux, Environment Canada and Bird Studies Canada for supplying data from the Atlas of the Breeding Birds of Québec. Bird Studies Canada, Environment Canada, the Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature supplied data from the Atlas of the Breeding Birds of Ontario, with thanks to the thousands of participants who helped gather data.
Migration records were provided by Long Point Bird Observatory, Pelee Island Bird Observatory, and Haldimand Bird Observatory, as part of the Canadian Migration Monitoring Network (CMMN) program coordinated by Bird Studies Canada and Environment Canada. Bird Studies Canada and Cornell Lab of Ornithology supplied eBird Canada data, with thanks to all of the volunteer participants who gathered data for this project.
Information on the current status of the Louisiana Waterthrush in various parts of Ontario was provided by Dave Martin, Mike Burrell, Ken Burrell, Bill Wilson, Jeff Skevington, James Holdsworth, Ben Walters, Gregor Beck, Ted Maddeford, and Andrew McIsaac. Al Sandilands generously provided a manuscript of the Louisiana Waterthrush account from a forthcoming book. Information on historical occurrences in Ontario and Quebec were provided by the Ontario Natural Heritage Information Centre (Don Sutherland, Martina Furrer) and Centre de données sur le patrimoine naturel du Québec (Annie Paquet). Louisiana Waterthrush banding and recapture data were provided by the Canadian Bird Banding Office (Louise Laurin).
Information Sources
Abdalla, C.W and J.R. Drohan. 2010. Water Withdrawals for Development of Marcellus Shale Gas in Pennsylvania: Introduction to Pennsylvania’s Water Resources (PDF: 505KB. Marcellus Education Factsheet. Penn State Extension. 12 pp.
Aide, T. M, M.L, Clark, H.R. Grau, D. Lopez-Carr, M.A. Levy, D.Redo, M. Bonilla-Moehno, G. Riner, M.J. Andrade-Nunez, and M M. Muniz. 2013. Deforestation and Reforestation of Latin America and the Caribbean (2001–2010). Biotropica 45:262-271.
Allair, J. 2014. Pers. comm. to A. Heagy. October 2014. Biologist, Bird Studies Canada, Port Rowan, ON.
Allair, J., A. Heagy, S. Dobney, E. Bird, M. Falconer, and D. Bell. 2013. Forest birds at risk in the Norfolk Sand Plain region of southwestern Ontario: 2011/ 2012 summary report. Report produced for Environment Canada. Bird Studies Canada, Port Rowan, ON.
Allair, J., S. Dobney, H. Polowyk, J. Kloepfer, M. Falconer, and A. Heagy. 2014. Forest birds at risk in the Carolinian Forest region of southwestern Ontario: 2014 summary report. Report produced for Environment Canada. Bird Studies Canada, Port Rowan, ON.
Allen, M.C., J. Sheehan Jr., T.L. Master, and R. Mulvilhill. 2009. Responses of Acadian Flycatchers (Empidonax virescens) to Hemlock Woolly Adelgid (Adelges tsugae) infestation in Appalachian riparian forests. Auk 126:543-553.
AOU (American Ornithologists’ Union). 2013. AOU Check-list of North and Middle American birds, 7th edition, and its supplements. Online database, updated 13 September 2013. [accessed 6 October 2014].
Baillie, J.L. Jr. and P. Harrington. 1937. The distribution of breeding birds in Ontario. Part II. Transactions Royal Canadian Institute 21:199-283.
Bent, A.C. 1963. Life Histories of North American Wood Warblers. Part II. Dover Publ., New York.
Berlanga, H., J.A. Kennedy, T.D. Rich, M.C. Arizmendi, C.J. Beardmore, P.J. Blancher,G.S. Butcher, A.R. Couturier, A.A. Dayer, D.W. Demarest, W.E. Easton, M. Gustafson, E. Iñigo-Elias, E. A. Krebs, A. O. Panjabi, V. Rodriguez Contreras, K.V. Rosenberg, J.M. Ruth, E. Santana Castellón, R. Ma. Vidal, and T. Will. 2010. Saving Our Shared Birds: Partners in Flight Tri-National Vision for Landbird Conservation. Cornell Lab of Ornithology: Ithaca, NY.
Bickerton, H.J. and B.J. Walters. 2011. Louisiana Waterthrush (Parkesia motacilla) survey protocol. Final draft. Ontario Region, Canadian Wildlife Service, Environmental Conservation Branch.
Bird Studies Canada. 2014a. Canadian Migration Monitoring Network. Data accessed from NatureCounts, a node of the Avian Knowledge Network, Bird Studies Canada. . Accessed July 2014.
Bird Studies Canada. 2014b. Southern Ontario Forest Birds at Risk database. Unpublished database maintained by Bird Studies Canada, Ontario Region. Port Rowan, Ontario.
Bird Studies Canada and Cornell Lab of Ornithology. 2014. eBird Canada. Data accessed from NatureCounts, a node of the Avian Knowledge Network, Bird Studies Canada. Accessed July 2014.
Blancher, P., M.D. Cadman, B.A. Pond, A.C. Couturier, E.H. Dunn, C.M. Francis and R. S. Rempel. 2007a. Changes in bird distribution between atlases. Pp. 32-48 In Cadman, M.D., D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier (eds.). 2007. Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto. 706 pp.
Blancher, P.J., K.V. Rosenberg, A.O. Panjabi, B. Altman, J. Bart, C.J. Beardmore, G.S. Butcher, D. Demarest, R. Dettmers, E.H. Dunn, W. Easton, W.C. Hunter, E.E. Iñigo-Elias, D.N. Pashley, C.J. Ralph, T.D. Rich, C.M. Rustay, J.M. Ruth, and T.C. Will. 2007b. Guide to the Partners in Flight Population Estimates Database. Version: North American Landbird Conservation Plan 2004. Partners in Flight Technical Series No 5..
Blancher, P.J., K.V. Rosenberg, A.O. Panjabi, B. Altman, A.R. Couturier, W.E. Thogmartin and the Partners in Flight Science Committee. 2013. Handbook to the Partners in Flight Population Estimates Database, Version 2.0. PIF Technical Series No 6.
Brewer, R., G.A. McPeek, and R.A. Adams, Jr. 1991. The Atlas of Breeding Birds of Michigan. Mich. State Univ. Press, Lansing, MI.
Buffington, J.M., J.C. Kilgo, R.A. Sargent, K.V. Miller, and B.R. Chapman. 1997. Comparison of breeding bird communities in bottomland hardwood forests of different successional stages. Wilson Bulletin 109:314-319.
Burke,D., K. Elliott, K. Falk, and T. Piraino. 2011. A Land Manager’s Guide to Conserving Habitat for Forest Birds in Southern Ontario (PDF: 4.7 KB). Ontario Ministry of Natural Resources: Science and Information Resources Division, and Trent University. SIB SSI SR-02. 140 pp.
Burrell, K. 2014. Email correspondence to A. Heagy November 2014. Biologist and Waterloo Region Bird Records compiler, New Hamburg, ON.
Bush, E.J., J.W. Loder, T.S. James, L.D. Mortsch, and S.J. Cohen. 2014. An Overview of Canada’s Changing Climate. Pp. 23-64 in Warren, F.J. and D.S. Lemmen (eds.). Canada in a Changing Climate: Sector Perspectives on Impacts and Adaptation. Government of Canada, Ottawa, ON, p. 23-64.
Buskirk, W.H. and J.L. MacDonald. 1995. Comparison of point count sampling regimes for forest birds. Pp. 25-34 inRalph, C.J., J.R. Sauer, and S. Droege (eds.). Monitoring Bird Populations by Point Counts. USDA Forest Service General Report PSW-GTR-149.
Cadman, M.D., P.F.J. Eagles, and F.M. Helleiner (eds.). 1987. Atlas of the Breeding Birds of Ontario. University of Waterloo Press, Waterloo, ON. 617 pp.
Cadman, M.D., D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier (eds.). 2007. Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto, xxii + 706 pp.
Campomizzi, A.J., Z.M Lebrun-Southcott, and L. Friesen. 2014. Louisiana Waterthrush survey along the Oak Ridges Moraine, 2014 field report. Unpublished report for Canadian Wildlife Service, Ontario Region. 5 pp.
Chamberlain, D.A., R. Diebolt, and P.F.J. Eagles. 1985. A biological inventory of the natural environment of the Six Nations Indian Reserve. Dept. Recreation and Leisure Studies, Univ. Waterloo, Waterloo.
Chesser, R.T, R.C. Banks, F.K. Barker, C. Cicero, J.L. Dunn, A.W. Kratter, I.J. Lovette, P.C. Rasmussen, J.V. Remsen, Jr., J.D. Rising, D.F. Stotz, and K. Winker. 2010. Fifty-first supplement to the American Ornithologists’ Union Check-list of North American birds, 7th edition, and its supplements. Auk 127:726−744.
Chiotti, Q. and Lavender, B. 2008. Ontario; in From Impacts to Adaptation: Canada ina Changing Climate 2007, (ed.) D.S. Lemmen, F.J. Warren, J. Lacroix and E. Bush, 2008, Government of Canada, Ottawa, p. 227-274.
COSEWIC. 2006. COSEWIC assessment and update status report on the Louisiana Waterthrush (Seiurus motacilla) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 26 pp.
COSEWIC. 2007. COSEWIC assessment and update status report on the Prothonotary Warbler (Protonotaria citrea) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 31 pp.
COSEWIC. 2010. COSEWIC assessment and status report on the Acadian Flycatcher (Empidonax virescens) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 38 pp.
Craig, R.J. 1984. Comparative foraging ecology of Louisiana and Northern Waterthrushes. Wilson Bulletin 96:173-183.
Craig, R.J. 1985. Comparative habitat use by Louisiana and Northern Waterthrushes. Wilson Bulletin 97:347-355.
Craig, R.J. 1987. Divergent prey selection in two species of waterthrushes (Seiurus). Auk 104:180-187.
Crewe, T. 2014. Pers. comm. to A. Heagy, October 2014. Bird Population Biologist, Bird Studies Canada, Port Rowan, ON.
Crins, W.J., B.A. Pond, M.D. Cadman, and P.A. Gray. 2007. The biogeography of Ontario, with special reference to birds. Pp.11-22 in Cadman, M.D., D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier (eds.). Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto. 706 pp.
Curson, J., D. Quinn, and D. Beadle. 1994. Warblers of the Americas: an Identification Guide. Houghton Mifflin, Boston, MA. 252 pp.
David, N. 1996. Liste commentée des oiseaux du Québec. Association québécoise des groupes d’ornithologues, Montréal. 169 pp.
Derbyshire, D. pers. comm. 2014. Email correspondence with A. Heagy, October-November 2014. Biologist, Frontenac Bird Studies, Perth, ON.
Dobney, S. 2015. Pers. comm. to A. Heagy. June 2015. Contract Field Research Intern, Bird Studies Canada, Port Rowan, ON.
Drolet, B. 2015. Pers. comm. during Canadian Wildlife Service review of Louisiana Waterthrush 6-month Interim Report. September 2015. Biologist, Canadian Wildlife Service Québec Region.
Drummond, M.A. and T.R. Loveland. 2010. Land-use pressure and a transition to forest-cover loss in the eastern United States. BioScience 60:286-298.
Dunn, J.L. and K.L. Garrett. 1997. A field guide to warblers of North America. Peterson Field Guide Series, Houghton Mifflin Co., New York. 656 pp.
Eagles, P.F.J. 1987. Louisiana Waterthrush. Pp. 408-409 In: M.D. Cadman, P.F.J. Eagles, and F.M. Helleiner. Atlas of the Breeding Birds of Ontario. Univ. Waterloo Press, Waterloo.
Eaton, S.W. 1958. A life history study of the Louisiana Waterthrush. Wilson Bulletin 70:210-236.
Eaton, S.W. 1988. Louisiana Waterthrush. Pp. 410-411 In: Andrle, R.F. and J.R. Carroll. The Atlas of Breeding Birds of New York State. Cornell Univ. Press, Ithaca, New York.
Environment Canada. 2011. Louisiana Waterthrush in Status of Birds in Canada -- 2011. [accessed October 2014].
Environment Canada. 2012. Management Plan for the Louisiana Waterthrush (Seiurus motacilla) in Canada. Species at Risk Act Management Plan Series. Environment Canada, Ottawa. iii + 18 pp.
Environment Canada. 2013a. Bird Conservation Strategy for Bird Conservation Region 12 in Québec: Boreal Hardwood Transition – Abridged Version. Environment Canada, May 2013. 32 pp.
Environment Canada. 2013b. Bird Conservation Strategy for Bird Conservation Region 14 in Québec: Atlantic Northern Forest – Abridged Version. Environment Canada, October 2013. 32 pp.
Environment Canada. 2014a. Bird Conservation Strategy for Bird Conservation Region 12 in Ontario and Manitoba: Boreal Hardwood Transition – Abridged Version. Environment Canada, June 2014. 29 pp.
Environment Canada. 2014b. Bird Conservation Strategy for Bird Conservation Region 13 in Ontario: Lower Great Lakes/St. Lawrence Plain – Abridged Version. Environment Canada, July 2014. 34 pp.
Environment Canada. 2014c. Breeding Bird Survey – General Information. [accessed November 2014].
Environment Canada. 2014d. North American Breeding Bird Survey - Canadian Trends Website, Data-version 2012. [accessed November 2014].
Erskine, A.J. 1992. Atlas of Breeding Birds of the Maritime Provinces. Nova Scotia Museum.
Evers, D.C., A.K. Jackson, T.H. Tear and C.E. Osborne. 2011. Hidden Risk: Mercury in terrestrial ecosystems of the Northeast. Biodiversity Research Institute. Gorham, Maine. 33 pp.
Franz, M.W., P.B. Wood, J. Sheehan, and G. George. 2014. Response of Louisiana Waterthrush to shale gas development. Conference presentation at American Ornithological Congress – Coopers Ornithological Society – Society of Canadian Ornithologists Joint Meeting, Estes Park, Colorado, August 2014.
Freemark, K. and Collins, B. 1992. Landscape ecology of birds breeding in temperate forest fragments. Pp. 443-454 In Ecology and conservation of neotropical migrant landbirds (eds. J.M. Hagan and D.W. Johnston). Smithsonian Institution Press, Washington, D.C.
Friesen, L. and Z. Lebrun-Southcott. 2012. Louisiana Waterthrush Surveys: South-Central Ontario Sites, 2012. Unpublished report for Canadian Wildlife Service, Ontario Region.16 pp.
Friesen, L. 2015a. Pers. comm. to A. Heagy. March 2015. Landbird biologist, Canadian Wildlife Service, Ontario Region.
Friesen, L. 2015b. Louisiana Waterthrush Surveys 2015. Unpublished report for Canadian Wildlife Service, Ontario Region. 2 pp.
Frontenac Bird Studies. 2014. Unpublished bird survey data provided November 2014 to A. Heagy by D. Derbyshire, Frontenac Bird Studies, Perth, Ontario.
Gauthier, J. and Y. Aubry (eds.). 1996. The Breeding Birds of Southern Québec: Atlas of the Breeding Birds of Southern Québec. L’Association québécoise des groupes d’ornithologues, Province of Québec Society for the Protection of Birds, Canadian Wildlife Service, Environment Canada Québec Region. Montréal, Québec. 1302 pp.
Gibbons, D., C. Morrissey, and P. Mineau. 2015. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environmental Science and Pollution Research 22:103-118.
Graveland, J. 1998. Effects of acid rain on bird populations. Environmental Reviews 6(1): 41-54.
Hallworth, M.T., L.R. Reitsma, and K. Parent. 2011. Habitat use of the Louisiana Waterthrush during the non-breeding season in Puerto Rico. Wilson Journal of Ornithology 123:567–574.
Hames, R.S, K.V. Rosenberg, J.D. Lowe, S.E. Barker, A.A. Dhondt. 2002. Adverse effects of acid rain on the distribution of the Wood Thrush Hylocichla mustelina in North America. Proceedings of the National Academy of Sciences of the United States of America 99:11235-11240.
Holdsworth, J. 2014. Email correspondence with A. Heagy. October 2014. Biological Consultant, Woodstock, ON.
Hull, C.N. 2011. Louisiana Waterthrush (Seiurus motacilla). In A.T. Chartier, J.J. Baldy, and J.M. Brenneman (eds). The Second Michigan Breeding Bird Atlas (online) (PDF: 763KB. Kalamazoo Nature Center. Kalamazoo, Michigan, USA. Website:. [accessed October 2014].
IBA Canada. 2014. Important Bird Areas, Site Catalogue Query. [accessed November 2014].
IUCN 2012. The IUCN Red List of Threatened Species: Parkesia motacilla (Louisiana Waterthrush) account. Assessed 1 May 2012.[accessed 15 November 2014].
James, R.D. 1991. Annotated checklist of the birds of Ontario. Roy. Ont. Mus. Life Sci. Misc. Publ., Toronto. 128 pp.
Jobin, B., C. Latendresse, M. Grenier, C. Maisonneuve, A. Sebbane. 2010. Recent landscape change at the ecoregion scale in Southern Québec (Canada), 1993-2001. Environmental Monitoring and Assessment 164:631-647.
Keller, C.M.E., C.S. Robbins, and J.S. Hatfield. 1993. Avian communities in riparian forests of different widths in Maryland and Delaware. Wetlands 13(2), Special Issue: 137-144.
Kerr, S.J. 1995. Silt, turbidity and suspended sediments in the aquatic environment: an annotated bibliography and literature review. Ontario Ministry of Natural Resources, Southern Region Science & Technology Transfer Unit Technical Report TR-008. 277- pp.
Kibbe, D.P. 2013. Louisiana Waterthrush. Pp. 392-393 inRenfrew, R.B. (ed.). The Second Atlas of Breeding Birds of Vermont. University Press of New England. Hanover and London. 548 pp.
Larson, B.M., J.L. Riley, E.A. Snell, and H.G. Godschalk. 1999. The woodland heritage of southern Ontario: a study of ecological change, distribution and significance. Federation of Ontario Naturalists, Toronto, ON.
Latta, S.C. 2011. The Louisiana Waterthrush: fulfilling the Holy Grail of migration bird ecology. In Three perspectives on Louisiana Waterthrush research. Birding (May 2011): 5w1-5w6.
Latta, S.C, L. Marshall, M. Franz, and J. Toms. 2014. Widespread evidence indicates bioaccumulation of contaminants from hydraulic fracturing in a riparian-obiligate songbird. Conference presentation at American Ornithological Congress – Coopers Ornithological Society – Society of Canadian Ornithologists Joint Meeting, Estes Park, Colorado, August 2014.
Lebrun-Southcott, Z. and L. Friesen. 2013. Louisiana Waterthrush Surveys: Southern Ontario Sites, 2013. Unpublished report for Canadian Wildlife Service, Ontario Region. 19 pp.
Lebrun-Southcott, Z.M. and A.J. Campomizzi. 2014. Habitat metrics and water quality at Louisiana Waterthrush detections along the Niagara Escarpment. 31 March 2014. Unpublished report for Environment Canada, Ontario Region. 27 pp.
Les Oiseaux du Québec. 2014. Extreme migration dates and rare bird records (In French Only). [accessed November 2014].
Long Point Bird Observatory. 2014. CMMN (Canadian Migration Monitoring Network) Daily Estimated Totals: Long Point Bird Observatory. Data accessed from NatureCounts, a node of the Avian Knowledge Network, Bird Studies Canada. [accessed August 2014].
Longcore, T., C. Rich, P. Mineau, B. MacDonald, D.G. Bert, L.M. Sullivan, E. Mutrie, S.A. Gauthreaux Jr., M. L. Avery, R.L. Crawford, A.M. Manville II, E.R. Travis and D. Drake. 2013. Avian mortality at communication towers in the United States and Canada: which species, how many, and where? USDA National Wildlife Research Center - Staff Publications. Paper 1162.
Loss, S.R., T. Will, S. S. Loss, and P.P. Marra. 2014. Bird–building collisions in the United States: Estimates of annual mortality and species vulnerability. Condor 116: 8-23.
Lutmerding, J.A. and A.S. Love. 2014. Longevity Records of North American Birds. Version 2014.1. Patuxent Wildlife Research Center. Bird Banding Laboratory. Laurel, MD.
MacClement, W.T. 1915. The New Canadian Bird Book. Dominion, Toronto.
Maritimes BBA (Breeding Bird Atlas). 2014. Maritimes Breeding Bird Atlas [accessed October 2014].
Mason, J., C. Moorman, G. Hess, and K. Sinclair. 2007. Designing suburban greenways to provide habitat for forest-breeding birds. Landscape and Urban Planning 80:153-164.
Master, T.L., R.S. Mulvihill, R.C. Leberman, J. Sanchez, and E. Carman. 2005. A preliminary study of riparian songbirds in Costa Rica with emphasis on wintering Louisiana Waterthrushes. In Ralph, C. J. and T. D. Rich (eds.). Bird Conservation Implementation and Integration in the Americas: Proceedings of the Third International Partners in Flight Conference, Volume 1. Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture, General Technical Report PSW-GTR-191, Albany, CA.
Mattsson, B.J. and R.J. Cooper. 2006. Louisiana Waterthrushes (Seiurus motacilla) and habitat assessments as cost-effective indicators of instream biotic integrity. Freshwater Biology 51:1941-1958.
Mattsson, B.J. and R.J. Cooper. 2007. Which life-history components determine breeding productivity for individual songbirds? A case study of the Louisiana Waterthrush (Seiurus motacilla). Auk 124:1186-1200.
Mattsson, B.J. and M.R. Marshall. 2009. Occupancy modelling as a framework for designing avian monitoring programs: a case study along Appalachian streams in southern West Virginia. Proceedings of the Fourth International Partners in Flight Conference: Tundra to Tropics, MacAllen, Texas. Pp. 617-632.
Mattsson, B.J. and R.J. Cooper. 2009. Multiscale analysis of the effects of rainfall extremes on reproduction by an obligate riparian bird in urban and rural landscapes. Auk 126: 64-76.
Mattsson, B.J., T.L. Master, R.S. Mulvihill and W.D. Robinson. 2009. Louisiana Waterthrush (Parkesia motacilla) in The Birds of North America Online (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology; Retrieved from the Birds of North America Online. [Accessed November 2014].
Mattsson, B.J., S.C. Latta, R.J. Cooper and R.S. Mulvihill. 2011. Latitudinal variation in reproductive strategies by the migratory Louisiana Waterthrush. Condor 113:412-418.
McCracken, J.D. 1991. Status report on Louisiana Waterthrush (Seiurus motacilla) in Canada. Committee on the Status of Endangered Wildlife in Canada. 26 pp.
McCracken, J. 2007. Louisiana Waterthrush. Pp. 514-515 in Cadman, M.D., D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier, eds. Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto, xxii + 706 pp.
McIsaac, A., pers. comm. 2014. Email correspondence to A. Heagy. 28 August 2014. Environmental Policy Advisor, Directorate General Infrastructure and Environment, Department of National Defence, Ottawa, ON.
McLaren, I.A. 1981. The incidence of vagrant landbirds on Nova Scotian islands. Auk 98:243-257.
McLaren, I.A. 2012. All the Birds of Nova Scotia: Status and Critical Identification. Gaspereau Press, 247 pp.
Michel, N., D.F. DeSante, D.R. Kaschube, and M.P. Nott. 2011. The Monitoring Avian Productivity and Survivorship (MAPS) Program Annual Reports, 1989-2006. NBII/MAPS Avian Demographics Query Interface, January 2011. [accessed November 2014].
Monson, B.A., D.F. Staples, S.P. Bhavsar, T.M.Holsen, C.S.Schrank, S.K. Moses, D.J, McGoldrick, S.M. Backus, and K.A. Williams. 2011. Spatiotemporal trends of mercury in walleye and largemouth bass from the Laurentian Great Lakes Region. Ecotoxicol 20:1555-1567.
Morrissey, C.A., P. Mineau, J.H. Devries, F. Sanchez-Bayo, M. Liess, M.C. Cavallaro, and K. Liber. 2015. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environmental International 74:291-303.
Mordecai, R.S, B.J. Mattsson, C.J. Tzilkowski and R.J. Cooper. 2011. Addressing challenges when studying mobile or episodic species: hierarchical Bayes estimation of occupancy and use. Journal of Applied Ecology 48:56-66.
Mulvihill, R.S., A. Cunkelman, L. Quattrini, T.J. O’Connell, and T.L. Master. 2002. Opportunistic polygyny in the Louisiana Waterthrush. Wilson Bulletin 114:106-113.
Mulvihill, R. S., F. L. Newell, and S. C. Latta. 2008. Effects of acidification on the breeding ecology of a stream-dependent songbird, the Louisiana Waterthrush (Seiurus motacilla). Freshwater Biology 53:2158-2169.
Mulvihill, R.S., S.C. Latta, and F.L. Newell. 2009. Temporal constraints on the incidence of double brooding in the Louisiana Waterthrush. Condor 111:341-348.
Mulvihill, R.S. 2012. Louisiana Waterthrush. Pp. 346-347 in Wilson, A.M, D.W. Brauning and R.S. Mulvihill (eds.). Second Atlas of Breeding Birds in Pennsylvania. Pennsylvania State University Press. University Park, Pennsylvania. 586 pp.
Nantel, P., M.G. Pellatt, K. Keenleyside, and P.A. Gray. 2014. Biodiversity and Protected Areas. Pp. 159-190 in F.J. Warren and D.S. Lemmen (eds.). Canada in a Changing Climate: Sector Perspectives on Impacts and Adaptation. Government of Canada, Ottawa, ON, p. 159-190.
National Audubon Society. 2014. Audubon’s Birds and Climate Change Report: A Primer for Practitioners. National Audubon Society, New York. Version 1.2 (PDF: MB. [accessed November 2014].
NatureServe. 2015. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. [accessed 3 June 2015].
NABCI [North American Bird Conservation Initiative Canada]. 2012. The state of Canada’s birds, 2012 (PDF: 4.9MB); Environment Canada, Ottawa, Canada, 36 pp.
Niagara Escarpment Commission. 2014. Niagara Escarpment Plan, Part 3, Niagara Escarpment Parks and Open Space System. [accessed November 2014].
Noon, B.R., V.P. Bingman, and J.P. Noon. 1979. The effects of changes in habitat on northern hardwood forest bird communities. Pp. 33-48 In: Workshop proceedings: Management of northcentral and northeastern forests for nongame birds. Compiled by R.M. DeGraaf and K.E. Evans. USDA Forest Service General Technical Report NC-51.
O’Connell, T.J., R.P. Brooks, S.E. Laubscher, R.S. Mulvihill, and T.L. Master. 2003. Using bioindicators to develop a calibrated index of regional ecological integrity for forested headwater ecosystems. Final report to U.S. Environmental Protection Agency. Penn State Cooperative Wetlands Center, Report No. 2003-01. Pennsylvania State University, University Park, PA. 97 pp.
Ohio BBA (Breeding Bird Atlas) II. 2014. Louisiana Waterthrush summary table, Ohio first atlas (1982-87) and second atlas (2003-07)
Ontario MNR. 2000. A Silvicultural Guide to Managing Southern Ontario Forests. Version 1.1. Ontario Ministry of Natural Resources. Queen’s Printer for Ontario. Toronto. 648 pp.
Ontario MNRF (Ministry of Natural Resources and Forestry). 2014. Ontario Asian Long-horned Beetle. [accessed November 2014].
ONRS (Ontario Nest Records Scheme). 2014. Ontario Nest Records Scheme (electronic database). Royal Ontario Museum and Bird Studies Canada. [downloaded November 2014].
Osborne, C., D. Evers, M. Duron, N. Schoch, D. Yates, O. Lane, D. Buck, I. Johnson, and J. Franklin. 2011. Mercury contamination within terrestrial ecosystems in New England and Mid-Atlantic States: profiles of soil, invertebrates, songbirds, and bats. BRI Report 2011-09 (PDF: 5.9MB). Submitted to The Nature Conservancy.
Page, A.M. 1996. Update Status Report on the Louisiana Waterthrush, Seiurus motacilla, in Canada. Committee on the Status of Endangered Wildlife in Canada. 24 pp.
Paradis, A., J. Elkinton, K. Hayhoe, and J. Buonaccorsi. 2008. Role of winter temperature and climate change on the survival and future range expansion of the hemlock woolly adelgid (Adelges tsugae) in eastern North America. Mitigation and Adaptive Strategies for Global Change 13:541-554.
Parker, T.H, B.M Stansberry, C.D. Becker, and P.S. Gipson. 2005. Edge and area effects on the occurrence of migrant forest songbirds. Conservation Biology 19: 1157-1167.
Parks Canada Agency. 2012. Detailed Assessment for the Louisiana Waterthrush (Seiurus motacilla) in Point Pelee National Park of Canada. Species at Risk Detailed Assessments. Parks Canada Agency. Ottawa. 12 pp.
PIFSC (Partners in Flight Science Committee). 2012. Species Assessment Database, version 2012. [accessed November 2014].
PIFSC (Partners in Flight Science Committee). Population Estimates Database, version 2013. [accessed 6 October 2014].
Peak, R.G. and F.R. Thompson III. 2006. Factors affecting avian species richness and density in riparian areas. Journal of Wildlife Management 70:173-179.
Peck, G.K. and R.D. James. 1998. Breeding birds of Ontario: nidiology and distribution. Vol. 2: passerines (first revision - Part B: thrushes to warblers). Ontario Birds 16:11-25.
Pennsylvania BBA (Breeding Bird Atlas). 2014. Louisiana Waterthrush map and summary table, Pennsylvania first atlas (1983-89) and second atlas (2004-2009). [accessed November 2014].
Prosser, D.J. and R.P. Brooks. 1998. A verified habitat suitability model for the Louisiana Waterthrush. Journal of Field Ornithology 69:288-298.
Québec BBA (Québec Breeding Bird Atlas). 2015. Atlas of the Breeding Birds of Québec (In French Only). Regroupement QuébecOiseaux, Service canadien de la faune d’Environnement Canada et Études d’Oiseaux Canada. Québec, Québec, Canada. [accessed 2 June 2015].
Redo, D., H.R. Grau, T.M. Aide and M.L. Clark. 2012. Asymmetric forest transition driven by the interaction of socioeconomic development and environmental heterogeneity in Central America. Proceedings of the National Academy of Sciences of the United States of America 109:8839-8844.
Reidy, J.L., F.R. Thomson III, and J.W. Bailey. 2011. Comparision of methods of estimating density of forest birds from point counts. Journal of Wildlife Management 75:558-568.
Ridgely, R.S., T.F. Allnut, T. Brooks, D.K. McNicol, D.W. Mehlman, B.E. Young, and J.R. Zook. 2007. Digital Distribution Maps of the Birds of the Western Hemisphere, version 3.0. NatureServe, Arlington, Virginia, USA.
Rich, T.D., C.J. Beardmore, H. Berlanga, P.J. Blancher, M.S.W. Bradstreet, G.S. Butcher, D. Demarest, E.H. Dunn, W.C. Hunter, E. Inigo-Elias, J.A. Kennedy, A.M. Martell, A.O. Panjabi, D.N. Pashley, K.V. Rosenberg, C.M. Rustay, J.S. Wendt, and T.C. Will. 2004. Partners in Flight North American Landbird Conservation Plan. Cornell Lab of Ornithology. Ithaca, NY. 86 pp.
Robert, M., pers. comm. 2014. Email correspondence to A. Heagy. October 2014. Coordonnateur, Atlas des oiseaux du Québec, Environment Canada, Québec Québec .
Robbins, C.S. 1979. Effect of forest fragmentation on bird populations. Pp. 198-212 in R.M. DeGraaf and K.E. Evans (compilers). Workshop proceedings: Management of northcentral and northeastern forests for nongame birds. USDA Forest Service General Technical Report NC-51.
Rodewald, A.D. and M.H. Bakermans. 2006. What is the appropriate paradigm for riparian bird conservation? Biological Conservation 128: 193-200.
Rosenberg, K.R. 2008. Louisiana Waterthrush (Seiurus motacilla). Pp. 522-523 in McGowan, K.J. and K. Corwin (eds.). The Second atlas of the Breeding Birds of New York State. Cornell University Press, Ithaca, NY.
Rousseu, F. and B. Drolet. 2015. Prediction of the phenology of birds in Canada. In J. Hussell and D. Lepage. 2015. Bird Nesting Calendar Query Tool. Project NestWatch, Bird Studies Canada. Website: . [accessed18 August 2015].
Ryan, K. 2013. Hemlock Woolly Adelgid Factsheet. [accessed November 2014].
Sandilands, A. 2014. Louisiana Waterthrush account in Birds of Ontario - Habitat Requirements, Limiting Factors, and Status, Passerines. Unpublished manuscript. 21 pp.
Sangster, G. 2008. A new genus for the waterthrushes (Parulidae). Bulletin of the British Ornithological Club 128(3): 212-215.
Sauer, J.R., J.E. Hines, J.E. Fallon, K.L. Pardieck, D.J. Ziolkowski, Jr., and W.A. Link. 2014. The North American Breeding Bird Survey, Results and Analysis 1966 - 2013. Version 01.30.2015. 30 January 2015. USGS Patuxent Wildlife Research Center, Laurel, MD. [accessed 11 September 2015].
Sauer, J.R., and W.A. Link. 2011. Analysis of the North American Breeding Bird Survey using hierarchical models. Auk 128:87-98
Savignac, C. 2005. Inventaire de la Paruline hochequeue (Seiurus motacilla) en Outaouais, printemps et été 2005. Rapport final préparé pour le Service canadien de la faune, Environnement Canada. Sainte-Foy. Dendroica Environnement et Faune, Chelsea, 48 p.
Savignac, C. 2006. Inventaire de la Paruline hochequeue (Seiurus motacilla) et de la Paruline azurée (Dendroica cerulea) en Outaouais, printemps et été 2006. Rapport final préparé pour le Service canadien de la faune, Environnement Canada. Sainte-Foy. Dendroica Environnement et Faune, Chelsea, 35 p.
Savignac, C. 2007. Inventaire de sites historiques et potentiels pour la Paruline hochequeue (Seiurus motacilla), la Paruline azurée (Dendroica cerulea) ainsi que la Paruline à ailes dorées (Vermivora chrysoptera) en Outaouais et dans le parc de la Gatineau, 2007. Rapport final préparé pour Michel Robert et François Shaffer du Service canadien de la faune d’Environnement Canada, Dendroica Environnement et Faune, Val-des-Monts. 44 p.
Savignac, C. 2008. Inventaire de la Paruline hochequeue (Seiurus motacilla) et de la Paruline à ailes dorées (Vermivora chrysoptera) dans le sud de Québec, 2008. Rapport final préparé pour Michel Robert du Service canadien de la faune d’Environnement Canada, Dendroica Environnement et Faune, Val-des-Monts. 69 pp.
Schaafsma, A., V. Limay-Rios, T. Baute, J. Smith, and Y. Xue. 2015. Neonicotinoid insecticide residues in surface water and soil associated with commercial maize (corn) fields in southwestern Ontario. PLoS ONE 10(2): e0118139.
Siderhurst, L. A., H. P. Griscom, M. Hudy, and Z.J. Bortolot. 2010. Changes in light levels and stream temperatures with loss of eastern hemlock (Tsuga canadensis) at a southern Appalachian stream: Implications for brook trout. Forest Ecology and Management 260:1677-1688.
Skevington, J. 2014. Email correspondence to A. Heagy. October 2014. Biologist (and former Oxford County birder), Ottawa, ON.
Snell, E.A. 1987. Wetland distribution and conversion in southern Ontario. Canada Land Use Monitoring Program. Working Paper No. 48. Inland Waters and Lands Directorate, Environment Canada.
St-Hilaire, D. and D. Dauphin. 1996. Louisiana Waterthrush. Pp. 1180-1181 in Gauthier, J. and Y. Aubry (eds.). The Breeding Birds of Southern Québec: Atlas of the Breeding Birds of Southern Québec. L’Association québécoise des groupes d’ornithologues, Province of Québec Society for the Protection of Birds, Canadian Wildlife Service, Environment Canada Québec Region. Montréal, Québec. 1302 pp.
Stucker, J.H. 2000. Biodiversity of southeastern Minnesota forested streams: relationships between trout habitat improvement practices, riparian communities and the Louisiana Waterthrush. Report to Natural Heritage and Nongame Wildlife Program, Diversion of Ecological Services, Minnesota Department of Natural Resources. Department of Fisheries and Wildlife, University of Minnesota, St. Paul, MN. 158 pp.
Tirpak, J.M., D.T. Jones-Farrand, F.R. Thompson III, D.J. Twedt, W.B. Uihlein III. 2009. Multiscale Habitat Suitability Index Models for Priority Landbirds in the Central Hardwoods and West Gulf Coastal Plain/Ouachitas Bird Conservation Regions. General Technical Report NRS-49. U.S Department of Agriculture Forest Service, Northern Research Centre, Newton Square, PA. 195 pp.
Triquet, A.M., G.A. McPeek, and W.C. McComb. 1990. Songbird diversity in clearcuts with and without a riparian buffer strip. Journal of Soil and Water Conservation 45: 500-503.
Trotter III, R.T., R.S. Morin, S.N. Oswalt, and A. Liebhold. 2013. Changes in the regional abundance of hemlock associated with the invasion of hemlock woolly adelgid (Adelges tsugae Annand). Biological Invasions 15: 2667-2679.
Tufts, R.W. 1986. Birds of Nova Scotia. 3rd ed. Nimbus Publ. Ltd. & Nova Scotia Museum, Halifax. 478 pp.
U.S. Forest Service. 2014. Asian Long-horned Beetle. Forest Health and Economics, Northeast Region, United States Forest Service. [accessed November 2014].
Vermont BBA (Breeding Bird Atlas). 2014a. Louisiana Waterthrush map and summary table, Vermont Breeding Bird Atlas 1976-1981. In Breeding Bird Atlas Explorer (online resource), U.S. Geological Survey Patuxent Wildlife Research Center. [accessed November 2014].
Vermont BBA (Breeding Bird Atlas). 2014b. Louisiana Waterthrush map and summary table, Vermont Breeding Bird Atlas 2003-2007. In Breeding Bird Atlas Explorer (online resource), U.S. Geological Survey Patuxent Wildlife Research Center. [accessed November 2014].
Vitz, A.C. and A.D. Rodewald. 2006. Can regenerating clearcuts benefit mature-forest songbirds? An examination of post-breeding ecology. Biological Conservation 127: 477-486.
Vitz, A.C. and A.D. Rodewald. 2007. Vegetative and fruit resources as determinants of habitat use by mature-forest birds during the postbreeding period. Auk 124:494-507.
Walters, B. 2014. Email correspondence to A. Heagy October 2014. Forest Manager (and Bird Researcher), Northumberland County Forest, Cobourg, ON.
Weir, R.D. 2008. Birds of the Kingston Region, 2nd edition. Kingston Field Naturalists, Kingston, ON. 611 pp.
West Virginia DNR (Division of Natural Resources). 2005. It’s about habitat: West Virginia Wildlife Conservation Action Plan. West Virginia Division of Natural Resources, Wildlife Resources Section. 1057 pp.
Biographical Summary of Report Writer(s)
Audrey Heagy is a Bird Conservation Biologist with Bird Studies Canada (BSC), a non-profit, non-governmental bird research organization with headquarters in Port Rowan, Ontario. She has worked as a conservation biologist for environmental non-profit organizations and consulting firms for over 20 years, including more than 15 years’ experience working on landbird conservation and the recovery of bird species at risk in Ontario. She has been the lead writer on numerous technical reports and publications, including three COSEWIC status update reports, the Ontario Barn Swallow Recovery Strategy, and four Ontario Landbird Conservation Plans. She coordinated and conducted Louisiana Waterthrush field surveys in Norfolk County, Ontario in 2011 and 2012, including colour banding and nest monitoring.
Collections Examined
No collections were examined.
Appendix 1. Threat Calculator results for Louisiana Waterthrush.
Threats Assessment Worksheet
- Species or Ecosystem Scientific Name:
- Louisiana Waterthrush Larkesia motacilla
- Date:
- 17/03/2015
- Assessor(s):
- Dwayne Lepitzki, Jon McCracken, Audrey Heagy, Julie Perrault, Marcel Gahbauer, Lyle Friesen, Don Sutherland, François Shaffer, Ben Walters, Zoe Lebrun-Southcott, Brady Mattsson
| Threat Impact | Threat Impact (descriptions) | Level 1 Threat Impact Counts: high range |
Level 1 Threat Impact Counts: low range |
|---|---|---|---|
| A | Very High | 0 | 0 |
| B | High | 0 | 0 |
| C | Medium | 2 | 0 |
| D | Low | 3 | 5 |
| - | Calculated Overall Threat Impact: | High | Medium |
- Overall Threat Comments
- Threats on the wintering habitat and during migration will also be considered here and specified where applicable. For the most part, unless otherwise specified, the threat refers to the breeding habitat of the species.
| # | Threat | Impact (calculated) |
Scope (next 10 Yrs) |
Severity (10 Yrs or 3 Gen.) |
Timing | Comments |
|---|---|---|---|---|---|---|
| 1 | Residential and commercial development | D-Low | Small (1-10%) | Extreme (71-100%) | High (Continuing) | - |
| 1.1 | Housing and urban areas | D-Low | Small (1-10%) | Extreme (71-100%) | High (Continuing) | In the Collingwood area along the escarpment there is a lot of housing development ongoing. This bird also exists in many protected areas. Development can occur right up to stream borders. The number of birds affected is likely to be closer to 1% end for Scope. Restrictions are also present in Niagara escarpment. |
| 1.2 | Commercial and industrial areas | Negligible | Negligible (<1%) | Extreme (71-100%) | High (Continuing)) | Birds flying into office building windows during migration. |
| 1.3 | Tourism and recreation areas | Negligible | Negligible (<1%) | Extreme (71-100%) | Moderate (Possibly in the short term, < 10 yrs) | There are some ski resorts in some parts of Ontario (e.g., Osler Bluffs) and many have been in existence for 70 years. The impact will especially occur through drainage. Tree removal and resort expansion is ongoing. |
| 2 | Agriculture and aquaculture | Negligible | Negligible ( |