Pacific water shrew (Sorex bendirii) COSEWIC assessment and status report: chapter 6
Except for diet and habitat, the basic biology of this species has been little studied. Information on life history traits is limited to a study of captive animals (Pattie 1969), a single study of food habits (Whitaker and Maser 1976), observations in various regional mammal synopses (e.g., Maser and Franklin 1974; Maser et al. 1984; Nagorsen 1996; Verts and Carraway 1998) and data associated with museum specimens. Some aspects of its life history can also be inferred from studies done on the common water shrew (Conaway 1952; Calder 1962), an aquatic shrew of comparable body size with similar ecological requirements. Although an aquatic shrew with several anatomical adaptations for an aquatic life style, the Pacific water shrew would be expected to demonstrate the typical life history traits of temperate shrews -- a high metabolic rate and short life span.
Life cycle and reproduction
The Pacific water shrew is primarily insectivorous, although it also consumes some vertebrate animals and plant material. Pattie (1969) observed this shrew foraging under water. Several recent museum records are from animals that drowned in submerged minnow traps, additional evidence for underwater foraging. A study in coastal Oregon (Whitaker et al. 1976) identified 26 food types with insect larvae, slugs, snails, mayfly naiads, and earthworms the dominant prey. At least 25% of the food items were aquatic. Of the five shrew species studied by Whitaker et al. (1976), the Pacific water shrew was the only species with mayfly naiads or earthworms as major prey. Other data on diet are limited to anecdotal observations. Lampman (1947) reported a water shrew (identified as S. bendirii from location; see Verts and Carraway 1998) capturing salmon parr in a small shallow pool. Maser and Franklin (1974) noted that on the Oregon coast a terrestrial snail (Haplotrema vancouverense) is commonly eaten by Pacific water shrews. H. vancouverense is found in forested habitats in the Fraser River valley of British Columbia (Forsyth 2004). Dietary data on the Canadian population are scanty. A museum specimen taken 8 July 1929 at Peardonville had the notation “stomach contained water beetles, worms, insects” on its tag. Glen Ryder (pers. comm.) has observed Pacific water shrews feeding on salamander and dragonfly larvae in British Columbia.
No breeding data exist for the Canadian population. In Oregon the breeding season extends from February to August (Maser et al. 1984). Pregnant females have been observed in April and May and nestlings found in March. Litter size for three females from Oregon ranged from 5 to 7 (Verts and Carraway 1998). The numbers of litters produced by a female in the breeding season has not been documented but it would be expected to be two or three, similar to the common water shrew(Conaway 1952). According to Pattie (1969), males do not reach sexual maturity in their first summer and first breed at about 10 months of age. Conaway (1952) reported a similar pattern of sexual maturity for male common water shrews. Age at sexual maturity is unknown for female Pacific water shrews but in the common water shrew successful pregnancy is uncommon for females in their first summer (Conaway 1952). The maximum life span would be expected to be about 18 months similar to that of the common water shrew (Conaway 1952). Assuming that most females do not breed in their first summer, the generation time is about one year.
Nothing is known about population structure or mortality rates.
Predation
Maser et al. (1984) speculated that Pacific water shrews are eaten by owls, domestic cats, fish, and Pacific giant salamanders (Dicamptodon tenebrosus). A Pacific water shrew skull was recovered in a Barn Owl (Tyto alba) pellet found near Burns Bog, British Columbia. Galindo-Leal and Runciman (1994) suggested that domestic cats were a major predator.
Physiology
No research has been done on physiological requirements. Typical of all species of the genus Sorex, it would be expected to have a high metabolic rate and high energy demands. Captivecommon water shrews, for example, consumed 10.3 grams of food per day (Conaway 1952). Although associated with aquatic habitats, the physical and chemical characteristics (e.g., temperature, pH, salinity, turbidity, water quality) of aquatic habitats tolerated by the Pacific water shrew have not been documented.
Dispersal/migration
Nothing is known about dispersal patterns or movements. Maser et al. (1984) noted that during winter rains there was a tendency for young-of-the-year to disperse, and Maser and Franklin (1974) reported that this shrew was “caught at great distances from water during the wettest season” but gave no specific details. Craig and Vennessland (2004a) speculated that most movements would be expected to be linear bordering riparian or water edge habitats. However, McComb et al. (1993), captured this shrew up to 350 m from streams in mature upland forests suggesting that it will disperse through forests that lack standing water. To what extent it disperses through disturbed forests or anthropogenic habitats such as cultivated fields, drainage ditches that border agricultural lands, and urban areas is unknown. However, paved roads, cultivated agricultural lands, and urban areas presumably would represent formidable barriers to movements.
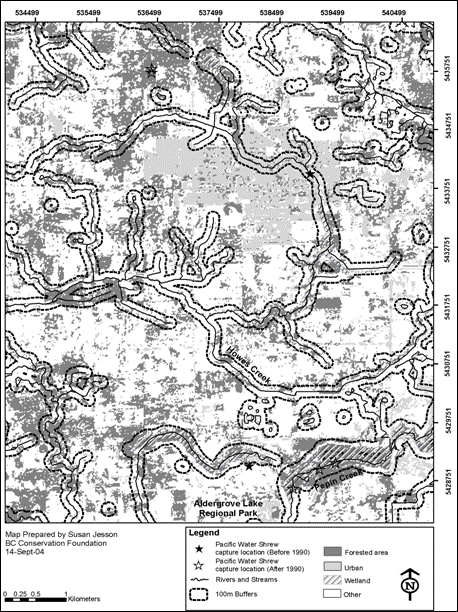
Despite the lack of information on dispersal, this species’ Canadian range appears to be fragmented. Because of intense urban and agricultural development, fragmentation is most pronounced in the municipality of Vancouver and in areas on the south side of the Fraser River. Zuleta (1993) estimated that of 270 forest fragments (most < 50 ha) in the Langley area, only 61 (23%) had suitable riparian forest for the Pacific water shrew. A recent GIS analysis for the Aldergrove area (Figure 4) demonstrates the scale of fragmentation. Remaining forest fragments are small and widely scattered separated by large urban and agricultural areas. Streams and wetlands even with a 100-m buffer as recommended by the province’s Best Management Practices Guidelines for Pacific water shrew in Urban and Rural Areas (Craig and Vennesland 2004b) are also disconnected.
Figure 4. Map derived from a GIS analysis illustrating habitat fragmentation in the Aldergrove area of southwestern British Columbia.
Interspecific interactions
Four other shrew species are sympatric with the Pacific water shrew in southwestern British Columbia: common shrew (Sorex cinereus), dusky shrew (Sorex monticolus), Trowbridge’s shrew (Sorex trowbridgii), and vagrant shrew (Sorex vagrans). Because of differences in body size, diet, behaviour, and microhabitat (Nagorsen 1996), competition with the Pacific water shrew is likely minimal. Competition with the common water shrew, an aquatic shrew similar in body size to the Pacific water shrew, is a possibility. The two species are generally allopatric in southwestern British Columbia segregated by elevation (Nagorsen 1996), but their distributions are parapatric in parts of the southern Coast Mountains on the north side of the Fraser River and in the Chilliwack Valley. To what extent this distributional pattern is maintained by competitive exclusion is unknown.
Adaptability
Semi-aquatic, the Pacific water shrew is capable of swimming and diving in water. Air trapped under the fur provides buoyancy and possibly insulation while in water. Swimming movements are derived from alternate strokes of the hind feet. Pattie (1969) observed swimming periods up to 3.5 minutes in captive animals. Dives probably do not exceed 60 seconds in duration (Calder 1969). Churchfield (1990) noted that despite their semi-aquatic life-style, the European water shrew (Neomys fodiens) and North American water shrews (S. bendirii, S. palustris) have no physiological adaptations for diving. Their specializations for swimming are rudimentary consisting of fimbriated hind feet, water-resistant pelage, and a large body size that reduces heat loss. Evidently the Pacific water shrew can run on top of the water without submerging for 3 to 5 seconds. Presumably this species frequently grooms and dries its fur after swimming similar to the common water shrew (Calder 1969) and European water shrew (Vogel 1990) to reduce thermal conductance and heat loss. Pattie (1969) observed captive animals caching excess earthworms after immobilizing them by a series of bites. The Pacific water shrew is unable to enter daily torpor (McNab 1991) and food caching would be an important foraging strategy for coping with periodic food shortages.
The only available data on home range size is Harris’ (1984) estimate of 1.09 ha. However, Harris (1984) gave no information about the source of his estimate. Based on Harris’ estimate and an assumption that a Pacific water shrew’s home range would be 25-m wide on a streamside, Craig and Vennesland (2004a) calculated that the home range would extend about 400 m along a water body. Nothing is known about the social structure of this shrew or the extent that home ranges overlap among individuals. Radio-tracking studies are required to determine the home range size and social structure.