COSEWIC Assessment and Status Report on the Peary Caribou Rangifer tarandus pearyi in Canada - 2015

- Document Information
- COSEWIC Assessment Summary
- COSEWIC Executive Summary
- Technical Summary
- Préface
- COSEWIC History
- COSEWIC Mandate
- COSEWIC Membership
- Definitions (2015)
- Wildlife Species Description and Significance
- Distribution
- Habitat
- Biology
- Population Sizes and Trends
- Threats and Limiting Factors
- Protection, Status and Ranks
- Acknowledgements and Authorities Contacted
- Information Sources
- Biographical Summary of Report Writer
- Collections Examined
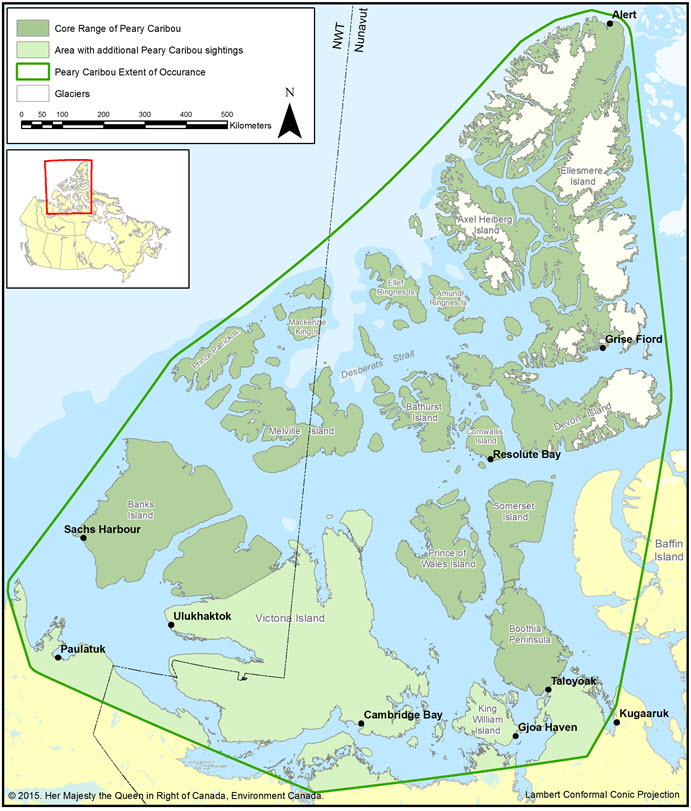
- Figure 1. Subpopulations of Peary Caribou (Johnson et al. in prep.; see Subpopulation Structure; Table 1). Light green and light purple shading denotes areas of additional sightings of Peary Caribou outside core range for the Banks-Victoria and Prince of Wales-Somerset-Boothia subpopulations, respectively.
- Figure 2. Community information on location of important habitat and movement routes for Peary Caribou.
- Figure 3. Peary Caribou distribution (with extent of occurrence polygon) based on most recent surveys and community information.
- Figure 4. Terrestrial ecozones in the Arctic Archipelago (based on Olsen et al., 2001).
- Figure 5. Abundance estimates from various island surveys for four Peary Caribou subpopulations: (A) Banks-Victoria; (B) Prince of Wales-Somerset-Boothia; (C) Eastern Queen Elizabeth Islands; (D) Western Queen Elizabeth Islands. Estimates are extrapolated from study areas to whole islands to aid in comparison across years and some earlier estimates (especially from WQEI) include calves. Totals were computed only when abundance estimates were available for each island in a group within a particular year. Standard errors are available for some surveys in Appendix 1.
- Figure 6. Resource development potential (including roads and shipping lanes) in the Canadian Arctic.
- Figure 7. National parks and other protected areas (e.g., Wildlife Management Areas and Migratory Bird Sanctuaries).
- Table 1. Island groups and their associated islands included for each subpopulation of Peary Caribou (modified from Johnsonet al., in prep.). See Figure 1 for corresponding map.
- Table 2. Summary of the number of surveys by subpopulation of Peary Caribou, from 1961-2014. Source: Gunn and Poole (2014).
- Table 3. Area-corrected abundance and trend (3-generation [27y] and 2-generation [18y]) estimates for four Peary Caribou subpopulations. Complete survey data can be found in Appendix 1.
- Appendix 1. Survey Estimates and Area-corrected Population Estimates
- Appendix 1a.Survey Estimates and Area-corrected Population Estimates for Surveys of Banks-victoria Island Subpopulation (adapted From Johnson Et Al. in Prep.).
- Appendix 1b.Survey Estimates and Area-corrected Population Estimates for Surveys of Prince of Wales-somerset-boothia Subpopulation (adapted From Johnsonet Al.in Prep.).
- Appendix 1c.Survey Estimates and Area-corrected Population Estimates for Surveys of Eastern Queen Elizabeth Islands Subpopulation (adapted From Johnsonet Al.in Prep.).
- Appendix 1d.Survey Estimates and Area-corrected Population Estimates for Surveys of Western Queen Elizabeth Islands Subpopulation (adapted From Johnsonet Al.in Prep.).
- Appendix 2. IUCN Threats Calculator for Peary Caribou (DU1).
COSEWIC
Committee on the Status
of Endangered Wildlife
in Canada

COSEPAC
Comité sur la situation
des espèces en péril
au Canada
COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2015. COSEWIC assessment and status report on the Peary Caribou Rangifer tarandus pearyi in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 92 pp. (Species at Risk Public Registry website).
Previous report(s):
COSEWIC. 2004. COSEWIC assessment and update status report on the Peary caribou Rangifer tarandus pearyi and the barren-ground caribou Rangifer tarandus groenlandicus (Dolphin and Union population) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 91 pp.
Gunn, A., F.L. Miller and D.C. Thomas. 1979. COSEWIC status report on the Peary caribou Rangifer tarandus pearyi in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 40 pp.
Miller, F.L. 1991. Update COSEWIC status report on the Peary caribou Rangifer tarandus pearyi In Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 124 pp.
COSEWIC would like to acknowledge Lee Harding (SciWrite Environmental Services) for writing the status report on the Peary Caribou, Rangifer tarandus pearyi, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Justina Ray, Co-chair of the COSEWIC Terrestrial Mammals Specialist Subcommittee.
For additional copies contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment Canada
Ottawa, ON
K1A 0H3
Tel.: 819-938-4125
Fax: 819-938-3984
Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Caribou de Peary (Rangifer tarandus pearyi) au Canada
Cover illustration/photo:
Peary Caribou -- Photo (Peary Caribou in Svartfjeld Peninsula, Ellesmere Island, 2015). Photo credit: Morgan Anderson, Government of Nunavut.
Peary Caribou
Rangifer tarandus pearyi
Peary Caribou are the smallest North American caribou. They are mostly white with a slate back and a grey stripe down the front of the legs. In winter, the slate back may turn a dingy brown, and some individuals appear almost entirely white. Antler velvet is slate-coloured instead of brown like deer and other caribou. The antlers tend not to spread as wide as those of other caribou but otherwise they are similar. The skull has a short rostrum and high cranium. The hooves are short and wide. They are genetically distinct from other caribou in Canada.
Peary Caribou are integral components of Inuit and Inuvialuit culture and economy. As the only source of caribou meat for several Arctic communities, they are important in the subsistence economy of local communities, and represented in traditional crafts that are marketed and collected throughout Canada and internationally. Persisting at the limits of plant and animal existence, Peary Caribou are an integral part of Arctic biodiversity and increasingly important in the scientific study of ecosystem response to climate change.
Peary Caribou are endemic to Canada in the Northwest Territories and Nunavut. They have the northernmost distribution of all caribou in North America, situated almost entirely within the Canadian Arctic Archipelago, with the exception of Baffin Island. Peary Caribou move relatively long distances, including annual migrations across sea ice, regular movements within multi-island home ranges and erratic large-scale movements among islands during severe winters. Four subpopulations are recognized, based on genetic evidence, extent of inter-island movements, and scientific and local expertise:
- Banks-Victoria islands
- Prince of Wales-Somerset-Boothia
- Eastern Queen Elizabeth Islands, and
- Western Queen Elizabeth Islands.
The habitat of Peary Caribou is treeless Arctic tundra primarily within High and Middle Arctic tundra ecoregions. Most of the range can be characterized as a polar desert with short, cool summers and long, cold winters. The growing season is brief (50-60 days) and variable. Snow cover is generally present from September to May (Banks Island) or mid-late June (Melville Island). Land dominated by dry vegetation covers about 36% of the ice-free area within Peary Caribou range while the terrain ranges from relatively flat (south and west) to mountainous (north and east). The climate is also strongly regionalized with east-west and north-south gradients in precipitation and temperature, affecting primary productivity and forage availability. Above-ground plant biomass ranges from < 100 g/m2 (Queen Elizabeth Islands and parts of the Prince of Wales-Somerset group) to some areas (Banks Island and Prince of Wales Island) having up to 500–2000 g/m2. Peary Caribou have a broad/varied diet and are versatile feeders with diet varying seasonally in relation to available forage and corresponding nutritional content. Essentially all historical Peary Caribou habitat is available and has not been lost or fragmented by industrial or other anthropogenic developments.
Peary Caribou have several adaptations to their Arctic environment such as compact body size for conserving heat, hooves that allow them to walk on and dig through wind-driven snow, and pelage that provides camouflage. They are adapted to limited plant growth with a highly compressed growing season and long periods of snow-covered frozen standing vegetation.
Peary Caribou are polygynous, living in small groups and maintaining a wide dispersion across the landscape, even during calving and rutting. They are thought to live approximately 15 years in the wild, and have widely variable vital rates. Cows usually produce their first offspring by 3 years of age; under conditions of high forage availability cows can calve every year but this is rare. Peary Caribou cows cope with occasional years of restricted forage access either by not becoming pregnant, or by weaning a calf prematurely. The intergeneration period (the average age of parents of the current year's cohort) cannot be precisely calculated, but is estimated at 9 years.
Evaluating trends in abundance for Peary Caribou since the first surveys were conducted in the 1960s is made difficult by irregular frequency in surveys (in time and space), as well as changes in survey design and methodology. From 1961 to 2014, government agencies conducted a total of 154 aerial surveys to estimate Peary Caribou abundance throughout the Canadian Arctic. There has been no single year when the entire range has been surveyed.
The current population of Peary Caribou is estimated at about 13,200 mature individuals. In the early 1960s, when the first population counts were made, there were ca. 50,000 Peary Caribou. The population in 1987 was ca. 22,000 mature individuals. It reached its lowest known point in 1996 at ca. 5,400 animals following die-offs related to icing events that affected the Western Queen Elizabeth Islands subpopulation in particular. Numbers have increased since that time, but have not fully recovered. The Prince of Wales-Somerset-Boothia subpopulation, which comprised almost half of the known Peary Caribou population in 1987, began to decline in the 1980s, for reasons that remain ill-understood. Although the last survey was in 2006, there is no evidence for any recovery today. Banks-Victoria numbers have been increasing in the past decade, but not on Victoria Island. The two northern subpopulations (Western and Eastern Queen Elizabeth Islands) have increased overall since the mid-1990s, although baseline levels are not well known. The overall three-generation population (27 years) decline for Peary Caribou is estimated at 35%, while the two-generation trend is positive (ca. 142%).
The overall calculated and assigned threat impact is Very High-Medium for Peary Caribou. This wide range rank of threat impacts is due to the combined effect of the high number of mostly low-impact threats, and the considerable uncertainty, unpredictability, and potential overlap and interaction of most individual threats.
The highest-impact threat to Peary Caribou arises from the myriad effects of a changing climate, including increased intensity and frequency of severe weather events negatively affecting forage accessibility in the winters, and decreased extent and thickness of sea ice causing shifts in migration and movement patterns. The extent to which such negative effects could be offset by increases in plant productivity is uncertain. Other threats that are known, suspected, or predicted to have negative impacts on reproductive success or survival of Peary Caribou under a warming climate include pathogens (especially Brucella and Erysipelothrix) and increased shipping. Lower-impact direct threats include hunting, energy production and mining, human intrusions from work (non-tourist) activities, year-round military exercises, increases in traffic from snowmobiles, helicopters, and airplanes, competition with Muskoxen and airborne pollution.
COSEWIC most recently assessed this species as Threatened in 2015. Peary Caribou are currently listed under Schedule 1 as Endangered under the federal Species at Risk Act (2011) and were listed as Threatened under NWT's Species at Risk Act (NWT) in 2013. Peary Caribou are co-managed in Nunavut according to the Nunavut Land Claims Agreement and in NWT according to the Inuvialuit Final Agreement, which confer primary wildlife management authority on the Nunavut Wildlife Management Board and the Wildlife Management Advisory Council, respectively.
| Summary Items | Information |
|---|---|
| Generation time | 9 years |
| Is there an [observed, inferred, or projected] continuing decline in number of mature individuals? | No |
| Estimated percent of continuing decline in total number of mature individuals within 2 generations | Overall increase ca. 142% |
| [Observed, estimated, inferred or suspected] percent [reduction or increase] in total number of mature individuals over the last 3 generations. | Overall decline ca. 35% |
| [Projected or suspected] percent [reduction or increase] in total number of mature individuals over the next 3 generations. | Unknown |
| [Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over any [3 generations] period, over a time period including both the past and the future. | Unknown |
| Are the causes of the decline clearly reversible and understood and ceased? | No, for the 2 subpopulations in decline |
| Are there extreme fluctuations in number of mature individuals? | No |
| Summary Items | Information |
|---|---|
| Estimated extent of occurrence | 1 914 910 km2 |
| Index of area of occupancy (IAO, 2x2 grid) | 366 384 km2 |
| Is the population severely fragmented? | No |
| Number of locations (Note: See Definitions and Abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
Unknown, but > 10 |
| Is there an [observed, inferred, or projected] continuing decline in extent of occurrence? | No |
| Is there an [observed, inferred, or projected] continuing decline in index of area of occupancy? Past area of occupancy decline based on virtual extirpation of Prince of Wales-Somerset-Boothia subpopulation. | No |
| Is there an [observed, inferred, or projected] continuing decline in number of (sub) populations? Number of subpopulations is stable unless Prince of Wales-Somerset-Boothia subpopulation is confirmed extirpated. | Possibly |
| Is there an [observed, inferred, or projected] continuing decline in number of locations? | Unknown |
| Is there an [observed, inferred, or projected] continuing decline in [area, extent and/or quality] of habitat? Sea ice is projected to decline and extreme weather events (projected to increase in frequency and perhaps severity in some places) may lead to decreases in habitat quality. On the other hand, habitat productivity may increase, especially for the two northern subpopulations. | Possibly |
| Are there extreme fluctuations in number of populations? | No |
| Are there extreme fluctuations in number of locations? | No |
| Are there extreme fluctuations in extent of occurrence? | No |
| Are there extreme fluctuations in index of area of occupancy? | No |
| Summary Items | Information |
|---|---|
| Subpopulations (at time of last survey) | - |
| Banks-Victoria | ~2,250 |
| Prince of Wales-Somerset-Boothia | < 10 |
| Eastern Queen Elizabeth Islands | ~3,000 |
| Western Queen Elizabeth Islands | ~8,000 |
| Total (sum of most recent surveys) | ~13,200 |
| Summary Items | Information |
|---|---|
| Probability of extinction in the wild is at least [20% within 5 generations (=54 years), or 10% within 100 years]. | N/A |
Was a threat calculator completed for this species: Yes
Members: Justina Ray (TM SSC Co-chair, moderator), Dave Fraser (BC, moderator), Dan Benoit (ATK SC Co-chair), Suzanne Carrière (NT), Nic Larter (NT)
External Experts: Tracy Davison (NT), Marsha Branigan (NT), Joanna Wilson (NT), Morgan Anderson (NU), Lisa-Marie LeClerc (NU), Andrew Maher (PCA), Renee Wissink (PCA), Peter Sinkins (PCA), David Lee (NTI), Cheryl Johnson (EC), Agnes Richards (EC), Donna Bigelow (CWS), Dawn Andrews (CWS), Lisa Pirie (CWS), Anne Gunn (Status Report writer for Barren-ground Caribou (DU3)), Karla Letto (NWMB), John Lucas (WMAC), Phillip Manik, Sr. (Resolute Bay HTO), Peter Qayutinuak Sr. (Spence Bay HTA - Taloyoak), Issiac Elanik (Sachs Harbour HTC), Bradley Carpenter (Olohaktomiut HTC - Uluhaktok) Overall threat impact: Very High-Medium. High-Medium Impact: Climate change: a) terrestrial habitat changes, sea ice loss, sea level rise and b) severe weather (rain on snow) events (icing). Medium-Low Impact: Pathogens, shipping lanes Low impact: hunting, competition (Muskoxen) and predation (Wolves), energy production and mining, human intrusions from work (non-tourist) activities and year-round military exercises, traffic from snowmobiles, helicopters, and airplanes, and airborne pollutants.
| Summary Items | Information |
|---|---|
| Status of outside population(s)? | None |
| Is immigration known or possible? | No |
| Would immigrants be adapted to survive in Canada? | N/A |
| Is there sufficient habitat for immigrants in Canada? | N/A |
| Is rescue from outside populations likely? | N/A |
| Summary Items | Information |
|---|---|
| Is this a data sensitive species? | No |
COSEWIC: The original designation considered a single unit that included Peary Caribou, Rangifer tarandus pearyi, and what is now known as the Dolphin and Union Caribou, Rangifer tarandus groenlandicus. It was assigned a status of Threatened in April 1979. Split to allow designation of three separate populations in 1991: Banks Island (Endangered), High Arctic (Endangered) and Low Arctic (Threatened) populations. In May 2004 all three population designations were de-activated, and the Peary Caribou, Rangifer tarandus pearyi, was assessed separately from the Dolphin and Union Caribou, Rangifer tarandus groenlandicus. The subspecies pearyi is comprised of a portion of the former “Low Arctic population”, and all of the former “High Arctic” and “Banks Island” populations, and it was designated Endangered in May 2004. Peary Caribou was recognized as one of 12 caribou designatable units in Canada by COSEWIC (2011).
This report incorporates information that became available after the last COSEWIC Status Update (COSEWIC 2004) for Peary Caribou Rangifer tarandus pearyi. In 1991, prior to the enactment of the Species at Risk Act (SARA), caribou throughout the Canadian Arctic Archipelago except for Baffin Island were considered to be Peary Caribou (Miller 1991). In 2004, COSEWIC assessed two entities:
- Peary Caribou, which included all caribou in the Arctic Archipelago except for Baffin Island and central and southern Victoria Island and
- Dolphin and Union Caribou, a genetically distinct population that occupies the remainder of Victoria Island, and migrates to the mainland in winter across the Dolphin and Union Strait.
COSEWIC undertook an analysis of designatable unit (DU) structure of caribou in Canada as a special project (COSEWIC 2011) to define the units for future status assessments and reassessments of this species according to the latest guidelines. Recognition of Peary Caribou and Dolphin and Union Caribou as two of 12 DUs in Canada was affirmed by this special project.
Unlike COSEWIC (2004), this report considers Peary Caribou only. Since the last assessment, surveys have been conducted in all four Peary Caribou subpopulation ranges to provide updated information on abundance and trends. The most important of these took place in the eastern High Arctic where populations had not been surveyed since 1961. Other aerial surveys clarified trends or updated trends. Recent genetic analyses (McFarlane et al. 2014) based on nuclear (microsatellite) DNA has confirmed the genetic distinctiveness of Peary Caribou from other caribou, particularly their isolation and divergence from Barren-ground Caribou in the relatively recent past (end of Pleistocene/early Holocene).
Other significant contributions to this update include:
- an assessment of the conservation status of Peary Caribou (SARC 2012), including Aboriginal Traditional Knowledge, undertaken by the Government of Northwest Territories; and
- updates from traditional ecological knowledge on caribou collected and summarized from Aboriginal sources by the COSEWIC Aboriginal Traditional Knowledge (ATK) Subcommittee.
In 2011, Peary Caribou was listed under SARA as Endangered, following the results of the last COSEWIC assessment in 2004. Environment Canada is in the process of developing a recovery strategy for Peary Caribou (Environment Canada, in prep.). This report has benefited from ATK (including Inuit Qaujimajatuqangit [IQ; Inuit traditional knowledge]), compilation of population data, various maps, and additional scientific information gathered through this process.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
The Canadian Wildlife Service, Environment and Climate Change Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Class: Mammalia; Order: Artiodactyla; Family: Cervidae; Subfamily: Capreolinae
Scientific name: Rangifer tarandus pearyi Allen, 1902.
Common names: Peary Caribou (English), Caribou de Peary (French), Tuktu (Plural: Tuktuk; Inuvialuktun), Tuktuinak (Inuinnaqtun), Tuktuaraaluit (Siglitun), Tuttunguluurat (Ummarmiutun).
The Peary Caribou (see cover), is a subspecies of caribou (Rangifer tarandus) that is primarily restricted to the Arctic Archipelago of Canada. It was first described by Allen (1902) as Rangifer pearyi, but Flerov (1952) later reduced it to subspecies rank. This designation was retained by Banfield (1961), who conducted the last formal taxonomic revision of Rangifer, relying on the account of Manning (1960) for Peary Caribou that was based on an examination of 60 skulls, hides and leg bones.
In comparison with other caribou DUs in Canada, Peary Caribou have a whiter to greyer pelage in all seasons. They have smaller bodies with shorter legs and faces, blunter and wider hooves, and grey antler velvet (Manning 1960, Geist 1998; Ekaluktutiak HTA 2013; Gjoa Haven HTA 2013; Spence Bay HTO 2013). The pelage is long, silky and creamy-white in early winter, becoming shaggy and brown-tinged on the back by spring when dark brown eye and neck patches appear as a result of shedding. The summer coat is slate grey above, sometimes lacking a pronounced flank stripe, and white below; legs are white except for a narrow frontal stripe (see Designatable Units).
Peary Caribou was formally described in 1902 from skulls and skins collected on Ellesmere Island and nearby islands (Allen 1902, 1908). The skull has a short pointed rostrum but the molar tooth row is proportionally long (Banfield 1961; Manning and Macpherson 1961). Manning (1960) described a cline in skull size and proportions with increasing size from the southern islands (Banks, Prince of Wales) to the northern Queen Elizabeth Islands (QEI). Within the latter, size tends to increase from east to west and from north to south (Manning 1960; Thomas and Everson 1982). Inuit of Resolute Bay reported that the features that are unique to Peary Caribou become more pronounced on the islands north of Bathurst Island Complex (Taylor 2005).
Thomas and Everson (1982) worked with Inuit hunters to collect caribou measurements across the western QEI (WQEI) and Prince of Wales, Somerset and Boothia Peninsula and samples were later used for DNA analyses (McFarlane et al. 2009; 2014). The body measurements supported the cline in skull size noted by Manning (1960). Mean body length ranged from 146.1 ± SE 1.3 cm (n=27) for females from Prince Patrick Island, the western-most large island in the QEI, to 152.9 ± SE 1.1 cm (n=25) for Prince of Wales Island females (Thomas and Everson 1982; the series did not include animals from the eastern Queen Elizabeth Islands [EQEI], or Banks, or northwest Victoria islands). Unusually large-bodied caribou that were otherwise similar to Peary Caribou were collected on Prince of Wales Island in August 1958 and 1978 (Manning and Macpherson 1961; Thomas and Everson 1982), termed “ultra pearyi” (Manning and Macpherson 1961) or “super pearyi” (Banfield 1961). The measurements of those seven 1958 bulls were similar to five exceptionally large-bodied bulls collected on Prince of Wales Island (Thomas and Everson 1982).
North American caribou have been divided into two lineages using genetic analysis of mitochondrial DNA (mtDNA) sequences. The Beringian-Eurasian and the North American Lineages were each named for their ancestral sources in presumed Pleistocene refugia (COSEWIC, 2011; Klütsch et al. 2012; Yannic et al. 2014). Barren-ground, Peary, and Dolphin and Union Caribou are part of the Beringian-Eurasian Lineage. After the last ice age, as populations expanded and colonized (or re-colonized) northern lands, hybridization resulted in introgression of haplotypes from each group into the other at a low enough frequency to leave each lineage distinct and clearly separable (Klütschet al.2012). Eger et al. (2009) suggested that mtDNA analyses supported two refugia during the last ice age: Banks Island and High Arctic. The High-Arctic refugium was represented by caribou from Bathurst Island, which was isolated from other Peary Caribou. Within the Beringian-Eurasian Lineage, mtDNA patterns have not distinguished among subspecies (Eger et al. 2009).
Genetic analysis based on nuclear (microsatellite) DNA, on the other hand, supports the contention that Peary Caribou are genetically distinct from other caribou DUs, including the Dolphin and Union and Barren-ground DUs (COSEWIC 2011; McFarlane et al. 2014). Serrouya et al. (2012) used Peary Caribou from Bathurst Island (n=20) and Dolphin-Union Caribou (n=43), and two Barren-ground Caribou herds as outgroups in their examination of mountain caribou. They observed that Peary formed a distinct clade with significant differentiation (FST= 0.07) from their nearest neighbour (Dolphin and Union). McFarlane et al. (2009) analysed nuclear DNA for specimens from Melville, Banks, NW Victoria, Bathurst, and Prince of Wales islands. McFarlane et al. (2014) also included the earliest available specimens of Peary Caribou (1914-1958) as well as the contemporary samples to examine, in particular, the relationship of the ‘ultra-pearyi’ collected from Prince of Wales Island in 1958. The ‘ultra-pearyi’ bulls were not genetically distinct from other Peary Caribou, suggesting that they were not an intergraded form between Barren-ground and Peary Caribou, and that their large body size was most likely due to environmental conditions.
The overall allele frequencies significantly differed among the sample locations supporting subpopulation structure. The lowest diversity (heterozygosity and allele diversity) was from caribou inhabiting Melville Island, Bathurst Island complex, and Prince of Wales–Somerset islands, including the 1958 Prince of Wales samples. Variability was less than those from Banks Island and Boothia Peninsula, or Dolphin and Union and Barren-ground Caribou (McFarlane et al. 2009; 2014). The lower genetic diversity likely reflects periodic reductions in abundance, although the historical and contemporary samples were not distinct from each other. Peary Caribou from northern Ellesmere also had low variability, often an indication of a past genetic bottleneck (Petersen et al. 2010).
The wide distribution of Peary Caribou across multiple islands and habitats has led to various iterations of units being proposed for management purposes. COSEWIC (Miller 1991) gave separate status designations for four island groups within Peary Caribou, while COSEWIC (2004) separated Peary from Dolphin and Union for status designation purposes, while recognizing the same subpopulation structure within Peary Caribou. This structure has not been completely supported by subsequent genetic analyses. Early work identified significant genetic differentiation among samples from various islands (McFarlane et al. 2009), but wider sampling and the use of Bayesian analysis that does not rely on sampling location to cluster animals supported two clusters:
- Prince of Wales, Somerset, and QEI and
- Boothia Peninsula, Dolphin and Union and Barren-ground Caribou.
Specimens from Banks and northwest Victoria islands did not strongly assign to either cluster. However, pair-wise comparisons revealed significant differences between sample localities (McFarlane et al. 2014). The analyses also revealed a genetic basis to the latitudinal cline in morphological measurements.
An examination of scientific and community information derived from the SARA recovery planning process (Johnson et al., in prep.) used three lines of evidence to define four Peary Caribou subpopulations:
- genetic analyses;
- extent of inter-island movements, based on local knowledge and limited telemetry data; and
- scientific and local expert input.
The spatial structure used in this report refers to subpopulations inhabiting islands or island complexes that have defined locations of surveys and life history information ( Table 1 ).
There likely is restricted gene flow between caribou on Banks and Victoria islands and the rest of the range of Peary Caribou. Zittlau et al. (2009) found that samples from Banks Island and Minto Inlet (northwest Victoria Island) were not significantly different and cross-assigned a high proportion of the time (58% and 33%, respectively). These samples had low assignment to other samples suggesting some degree of isolation (Zittlau et al. 2009).
| Subpopulation | Island Group | Islands |
|---|---|---|
| Banks-Victoria | Banks and Victoria islands | Banks and Victoria islands |
| Prince of Wales-Somerset-Boothia | Prince of Wales-Somerset islands, Boothia Peninsula | Prince of Wales, Somerset, Russell, King William, Pandora, Prescott, Vivian, and Lock islands, Boothia Peninsula |
| Western Queen Elizabeth Islands | Bathurst Island Group | Bathurst Island complex (Cameron, Ile Vanier, Marc, Massey, Alexander, Bathurst islands), Cornwallis, Little Cornwallis, and Helena islands |
| Western Queen Elizabeth Islands | Melville Island Group | Melville, Prince Patrick, Eglinton, Emerald, and Byam Martin islands |
| Western Queen Elizabeth Islands | Devon Island Group | Devon, Baillie Hamilton, Coburg, Dundas/Margaret, and North Kent islands |
| Western Queen Elizabeth Islands | Prime Minister Island Group | Mackenzie King, Brock, and Borden islands |
| Western Queen Elizabeth Islands | Ringnes Island Group | Ellef Ringnes, Amund Ringnes, Cornwall, King Christian, Meighen, and Lougheed islands |
| Eastern Queen Elizabeth Islands | Ellesmere Island | Ellesmere, Graham, and Buckingham islands |
| Eastern Queen Elizabeth Islands | Axel Heiberg Island | Axel Heiberg, Stor, and Hevod islands |
Scientific evidence and Inuvialuit ATK agree that before about 1980 when abundance was still relatively high, Peary Caribou made seasonal movements between Banks and northwestern Victoria islands, and so caribou residing on these two islands were recognized as a subpopulation by COSEWIC (2004). Notably, several aerial surveys since 1982 along with more recent satellite-tracking have failed to detect evidence of such travel, and Inuit hunters reported no evidence of movement in the past decade (Paulatuk HTC 2013).
Movements of satellite-collared cows during 1987–1989 (Gunn and Fournier 2000) and 1996–2006 (Poole et al. 2010; ENR unpubl. data 2011, cited in SARC 2012) showed a spatial and temporal separation of the northwestern Victoria Island subpopulation of Peary Caribou from Dolphin and Union Caribou. Although telemetry studies indicated that Peary Caribou cows have been mainly limited to the area north and west of a line between Minto Inlet and Wynniatt Bay, Inuvialuit ATK reveals that they can (albeit rarely) occur south to Admiralty Inlet and east to the Kagloryuak River (ATK in Poole et al. 2010;SARC 2012; Figure 1). Inuvialuit from Ulukhaktok and Inuit from Cambridge Bay recognize two kinds of caribou on Victoria Island that are different in size, colour and taste: those in the northwest (Peary Caribou) and others that summer on the central, southern and eastern parts (Dolphin and Union Caribou; Elias 1993; Gunn et al. 2011). Inuit from Victoria Island recalled both migratory and non-migratory caribou on Victoria Island before the 1920s (Manning, 1960; SARC 2013).

Long description for Figure 1
Map outlining the range of each of the four Peary Caribou subpopulations (Banks-Victoria; Prince of Wales-Somerset-Boothia; Western Queen Elizabeth Islands, and Eastern Queen Elizabeth Islands). Areas of additional sightings of Peary Caribou outside the core range for the Banks-Victoria and Prince of Wales-Somerset-Boothia subpopulations are also indicated.

Long description for Figure 2
Map illustrating the locations of important habitat and movement routes, including sea crossings, for the Peary Caribou based on information from northern communities.
COSEWIC (2011) recognized the subspecies of Peary Caribou with all of its subpopulations as one of 11 extant caribou DUs. Measures of genetic divergence among Peary and Barren-ground Caribou on the mainland, and also between Peary Caribou and the Dolphin and Union Caribou, support the discrete nature of Peary Caribou regardless of occasional overlap in annual distribution. New genetic information since the DU report was published reaffirms the unique nature of Peary Caribou (McFarlane et al., 2014). Morphological specializations reflect adaptations for Arctic environments (e.g., shorter face and legs) (Banfield 1961). Unique behaviours include the use of several islands as part of their home range by some subpopulations (see Population Spatial Structure and Variability), and not forming large post-calving aggregations, in contrast to Barren-ground Caribou (Festa-Bianchet et al. 2011).
Peoples of the Canadian Arctic have hunted caribou for > 4,000 years (Manseau et al. 2004). Peary Caribou are important in the subsistence economy of communities where they occur and are integral to the cultures of Inuit and Inuvialuit. They are the only source of caribou meat for several arctic communities. They are frequently represented in the art of Inuit and Inuvialuit and their shed antlers are carved to produce traditional crafts. Persisting at the limits of plant and animal existence, Peary Caribou are an integral part of Arctic ecology and biodiversity. They can be an important prey for Wolves (Canis lupus) and are increasingly important in the scientific study of ecosystem response to climate change. Peary Caribou are an important symbol of the Canadian Arctic islands.
Peary Caribou range is entirely within Canada, with the possible exception of animals on Greenland. Anderson (1946) suggested that caribou from northwestern Greenland north of Kane Basin may be Peary Caribou, and Banfield (1961) agreed. Miller (1991), citing Meldgaard (1986) who summarized reports of Greenland Inuit, confirmed that small caribou, possibly migrants from Canada, were regularly seen and taken by hunters there. The Inuit reported that normally up to 10 (but occasionally > 100 individuals) were taken annually and that caribou tracks were often seen crossing from Ellesmere Island to Greenland. Roby et al. (1984) surveyed the Inglefield Bay-Kane Basin area and did not see any live caribou, but found a caribou mandible in northwest Greenland (Renssalaer Bay, north of Cape Inglefield and on the southern edge of Kane Basin) that was 178 mm long, “…outside the range of [i.e., smaller than] Canadian Barren-ground Caribou... the mandible probably belonged to a specimen of Peary Caribou.” They also reviewed the history of caribou declines from this area as a result of severe weather and excessive hunting. It seems probable, therefore, that the Kane Basin caribou were R. t. pearyi, but are now extirpated from Greenland, although a few may rarely cross from Ellesmere Island (Taylor 2005).
Peary Caribou have the northernmost distribution of all caribou in North America (Figure 1; Festa-Bianchet et al., 2011). They are found across the Arctic Archipelago except for Baffin Island (which is occupied by Barren-ground Caribou). Peary Caribou also occur on northwestern Victoria Island with some evidence of movements to other parts of that island. A small number occur (or occurred) on Boothia Peninsula and possibly on King William Island (see Subpopulation Structure). Peary Caribou disperse across sea ice, either occasionally or as part of seasonal movements, and may be found on any island, although not all of the small islands have year-round inhabitants.
Because population surveys are usually conducted in spring and summer due to day length, winter distribution is less well documented. However, recent information collected in the context of recovery planning led by Environment Canada has indicated a broader-scale distribution than reported in COSEWIC (2004). Cambridge Bay members reported that Peary Caribou have been observed year-round all over Victoria Island, albeit in small numbers (Ekaluktutiak HTA 2013). They have been occasionally spotted on the mainland in two main areas: Pearce Point and Parry Peninsula (Paulatuk HTC 2013). They have been seen near Cambridge Bay, and on the mainland near Kugluktuk (Ekaluktutiak HTA 2013). There were reports (Banfield 1961; Manning and Macpherson 1958; Youngman 1975) of Peary Caribou as far west on the mainland as Old Crow (Yukon), Herschel Island (Yukon), Baillie Island (Northwest Territories), and Cape Dalhousie (Northwest Territories) in the early 1950s, which were linked with years with icing on Banks Island.
The extent of occurrence for Peary Caribou is 1,914,910 km2 based on the minimum convex polygon within Canada’s extent of jurisdiction as shown in Figure 3 (map and area calculations by D. Andrews, Environment Canada). The index of area occupancy (based on 2 km x 2 km grid cells) as defined by survey observation data only (Johnson et al. in prep.) is 91,465 cells or 366,384 km2 (D. Andrews, Environment Canada, in litt.).
The extent of occurrence polygon encloses all caribou observations, based on the most recent survey for each island (Appendix 1) combined with community information (see Population Status and Trends).
Long description for Figure 3
Map outlining the range of the Peary Caribou within an extent of occurrence polygon, based on recent scientific surveys and information from northern communities.
Banks Island is the westernmost island of the Canadian Arctic Archipelago and covers an area of ca. 71,000 km2. Historical records indicate that Peary Caribou occupy virtually all of the island, at least seasonally (Nagy et al. 1996). Based on summer survey distribution during the 1980s, Peary Caribou were most numerous in the northwest and the eastern side of the island with some caribou in the southern end (Nagy et al. 1996, Figure 4.). During the 1990s, caribou numbers were at their lowest. The summer 1998 survey showed that caribou were most numerous in the northwest and along the west coast; no caribou were found at the southern end and few on the eastern side (Nagy et al. 2013a). Caribou numbers have increased since the 1990s with the most recent survey showing a more widespread distribution on the island, although most occurrences remain concentrated in the northwest (Davison et al., 2014).
Peary Caribou occupy an approximate 36,000 km2 area of northwestern Victoria Island to the north of Minto Inlet (Nagy et al. 2009b). Although Peary Caribou numbers have fluctuated, they have always occupied the northwestern area of the island which, based upon satellite telemetry, remains separated from the area inhabited by Dolphin and Union caribou (Davison and Williams 2013).
Prince of Wales and Somerset islands cover more than 58,000 km2 in area and, based on historical records (Gunn and Decker 1984; Miller and Kiliaan 1981; Gunn and Dragon 1998), were virtually all occupied, at least seasonally, when populations were high in the 1960s and 1970s. Annual migrations within this subpopulation are well documented by communities (Gjoa Haven HTA 2013; Resolute HTO 2013; Sachs Harbour HTC 2013; Spence Bay HTO 2013). For example, during 1977–1980, caribou trails across the sea ice effectively joined these two main islands, several satellite islands and the northern part of the Boothia Peninsula (see below) for most of each year, making this complex essentially a single range of >93,000 km2 (Miller et al. 2005b).
After caribou essentially vanished by the 1940s (summarized in Gunn and Ashevak 1990), Boothia Peninsula was re-occupied by caribou based on data from the first aerial survey in 1973 (Fischer and Duncan 1976) through the 1980s. Although both Peary and Barren-ground Caribou occurred there, the proportion of each was not quantified during the aerial surveys. Most Peary Caribou were resident on the Boothia Peninsula north of Taloyoak, but some seasonally migrated from Somerset Island or Prince of Wales Island in the fall and back in the spring (Gunn and Ashevak 1990). Caribou in this subpopulation have declined again to very low numbers (see Fluctuations and Trends).
The WQEI cover an area of about 180,000 km2; the largest islands are Melville (42,776 km2) and Devon (38,764 km2), followed by Prince Patrick (16,316 km2) and Bathurst Island (16,042 km2). Much of the land area (with the exception of Devon Island) lies below 300 m elevation (Miller et al. 2005a), and most is usable habitat, not covered by glaciers. The sporadic nature of surveys and little-documented ATK restrict known distribution patterns mostly to the summer There is some evidence that smaller islands tend not to be used by Peary Caribou during times of reduced abundance (Miller et al. 1977a). For example, although Peary Caribou had been consistently recorded on Brock, Eglinton and Emerald islands in 1961, 1972-74 and 1987-88, they were not seen in 1997 (Gunn and Dragon, 2002) when population numbers were very low in the region. They were once again confirmed present in 2012 (Davison and Williams 2012), corresponding with a population increase (Appendix 1).
The Bathurst Island complex and surrounding islands have been subjected to the most significant survey effort within the WQEI, with available data spanning a 50-year period. This provides a window into caribou spatial distribution across seasons and over periods of both high and low population abundance (Poole et al. 2015).
The two largest islands that make up this subpopulation are ca. 240,000 km2 in area. In contrast to WQEI, a majority of the area is above 300m elevation and covered by glaciers and ice caps, and hence unusable for Peary Caribou. Recent surveys (Jenkins et al. 2011; Anderson et al. 2014; Anderson and Kingsley 2015) have recorded Peary Caribou on Ellesmere and Axel Heiberg islands on all non-glacier-covered areas of both.
Peary Caribou distribution is known from aerial surveys that have covered most islands and the experience of local and traditional knowledge, mostly through hunting.
In areas accessible from the eight settled Inuit and Inuvialuit communities within Peary Caribou range (Figures 1-3), many families and individual hunters, trappers and fishers from Inuit and Inuvialuit communities spend weeks or months at all seasons out on the land. The widespread adoption of snow machines since the 1970s or use of bush planes to reach remote camp sites has made it possible for individual hunters to cover a greater distance searching for caribou or Muskoxen (Ovibos moschatus) (Condon, 1996). In areas that people visit regularly, the specific skills required to pursue cultural traditions results in a high overall level of awareness of caribou and other wildlife distribution, density, and condition (c.f. Dumond 2007; SARC 2012, 2013).
Information particular to wildlife management is also shared in meetings of local hunters and trappers associations, and between them and regional wildlife management boards. In this way, knowledge of status, movements, and condition of wildlife is accumulated and disseminated within and among villages. People in remote villages are, therefore, aware of wildlife events throughout the territories and beyond. Such knowledge may be variously understood, interpreted, or communicated by different individuals, but nevertheless becomes shared community knowledge.
The distribution patterns and trends of Peary Caribou are less known in areas that are remote from communities. Most incidental observations of Peary Caribou are derived from hunting trips (SARC 2012; CWS 2013). Frequency of individual hunting expeditions is also declining. For example, fewer hunters in Sachs Harbour and Ulukhaktok hunt for caribou than in the past (Condon 1996; Collings and Condon 1996; Nagy 1999; Pearce et al. 2011), and unreliability of snow and ice conditions has families preferring to travel along the coast rather than inland (Riedlinger 2001). Cambridge Bay residents remarked in community meetings that travel to the northern part of Victoria Island is uncommon (Ekaluktutiak HTA 2013). Similarly, Gjoa Haven residents travel too infrequently to Prince of Wales, Matty and Tennet islands to know when caribou are there or how numbers have changed over time (Gjoa Haven HTA 2013). Sachs Harbour members indicated that due to changes in hunting practices, people no longer spend long periods travelling on the land on Banks Island following caribou, and now seldom venture further than 50 miles north of town (Sachs Harbour HTC 2013).
Search effort to measure spatial distribution within each of the four subpopulations has also been based on aerial surveys of each island. The frequency and coverage of these surveys has been highly variable since the first systematic surveys in 1961 (see Sampling Effort and Methods; Table 2 ). It is, however, unlikely that there are unexplored areas within Peary Caribou range, given the nature of the systematic effort and extent of coverage in an overall sense. Nevertheless, distribution and abundance through time in most subpopulations is not well known, and even current distribution is unknown in parts of the range.
Peary Caribou live primarily in High Arctic and Middle Arctic tundra (Olson et al. 2001; Figure 4).
The climate of Peary Caribou range is unpredictably variable and severe, with short, cool summers and long, cold winters. The growing season (breaking dormancy to 50% leaf colouration) is relatively fixed within 50-60 days for plant species (Svoboda 1977). Snow cover is generally present from September to May (Banks Island) or mid-late June (Melville Island) (SARC 2012).
Climate data are available from only eight meteorological stations across the Peary Caribou range, and these are all coastal. Hence, they are more representative of conditions on QEI, and not the large continental island areas of Banks and Victoria islands. For example, summer temperatures in interior Banks Island can be as much as 10oC higher than those recorded by the Sachs Harbour weather station (N. Larter, pers. comm. 2015).

Long description for Figure 4
Map illustrating the location of terrestrial ecozones in the Arctic Archipelago in relation to the ranges of the Peary Caribou and the Dolphin and Union Caribou. Peary Caribou live primarily in the High Arctic and Middle Arctic tundra.
Since 1980, spatial climate data have become available at the scale of 1/2 degree latitude by 2/3 degree longitude from the Modern Era Retrospective Analysis for Research and Applications (MERRA) dataset. MERRA data from 1980 - 2014 for island or island groupings for Peary Caribou demonstrate how climate variables vary across Peary Caribou range with east-west and north-south gradients; there is also a high degree of annual variability, which itself varies regionally (Russell et al., 2013). For example compared to Banks Island, Bathurst Island has fewer cumulative growing degree days (GDD) (the base temperature below which plant growth is zero) > 0 in June and July (230 ± 20.0 SE vs. 557 ± 34.0 SE). This result is best explained by its location further north, but also by its smaller landmass with an incised coastline. It also has a later onset of plant growth (up to a 10-fold mean difference on 15 June), which is characterized by higher annual variability than Banks Island.
The climate across the Arctic islands is strongly regionalized with east-west and north-south gradients in precipitation and temperature due to the influence of Pacific air masses in the west and Atlantic air masses in the east (Maxwell 1981). It is these intrusions that periodically cause warmer temperatures during snowstorms leading to icing and dense, deep snow (Rennert et al. 2009). Decadal-scale atmospheric pressure oscillations in the north Atlantic and north Pacific complicate trend analysis of weather patterns. Spatial diversity of climate regimes across the range of Peary Caribou creates a great diversity of vegetation types, with implications for how each subpopulation responds to climate variation.
Land dominated by dry vegetation covers about 36% of the ice-free area within Peary Caribou range. Above-ground plant biomass ranges from < 100 g/m2 in much of the QEI and parts of the Prince of Wales-Somerset group with some areas having up to 500–2000 g/m2 on Banks Island and Prince of Wales Island (Gouldet al. 2003). Net primary productivity is 0–50 g/m2/yr over most of the range of Peary Caribou, with 150–250 g/m2/yr on parts of Banks Island and Victoria Island (Gouldet al. 2003). Banks Island has the greatest extent of area with high plant biomass (>1000 g/m2), shrub cover and primary productivity of all Peary Caribou subpopulation ranges (Gouldet al. 2003).
Permafrost is continuous throughout and only a thin (~40 cm--Callaghanet al. 2005) active layer thaws during summer, limiting dominant vegetation to flowering perennials such as saxifrage (Saxifraga spp.), Arctic Poppy (Papaver radicatum), Moss Campion (Silene acaulis), louseworts (Pedicularis spp.), and Mountain Sorrel (Oxyria digyna), as well as mosses, rushes, grasses, sedges, and dwarf shrubs (e.g., Salix spp., Dryas spp.).
Peary Caribou use a wide variety of habitats and are most commonly found on upland polar desert and tundra habitat types that are mesic-xeric with sparse-moderate vegetation cover at intermediate-high elevations (Parker and Ross 1976; Wilkinson et al. 1976; Miller et al. 1977a, b; Russell et al. 1978; SARC 2012). In the WQEI, Thomas et al. (1999) showed that the Peary Caribou did not use or select habitat types with the greatest vegetation cover and standing crop. The latter study demonstrated that caribou pellet densities in summer were greatest in sparsely vegetated upland ridges where lichens, willow, wood rushes (Luzula spp.), Arctic Poppy and Long-stalked Starwort (Stellaria longipes) were relatively abundant. Winter forage sites were typically characterized by high densities of Luzula spp. and lichens.
Studies have been conducted during snow-free periods on forage availability, plant standing crop, biomass, above-ground primary productivity, and abundance of plant species or groups (Larter and Nagy 2001a; Gould et al. 2003, Larter and Nagy 2003). Generally, these studies showed that there was more forage or available plant biomass than was necessary for adequate nutrition, although it may not be accessible during winter due to snow conditions.
The low densities of Peary Caribou, their relatively small group size and their mobility while foraging usually prevent overuse of forage sites despite the characteristically low productivity of such ranges (e.g., Parker 1978; Miller and Kiliaan 1981). Unfortunately, as noted by Miller et al. (1977a:46), “…we have no quantitative measures of range condition” associated with declines of Peary Caribou and this knowledge gap persists. Overall, studies have suggested that, while forage availability may not limit Peary Caribou populations, high densities could in theory affect vegetation and there is potential for competition among herbivores under certain conditions. Only limited research has been conducted on linkages between foraging and snow conditions in relation to subpopulation dynamics (Larter and Nagy 2000a; 2001b) and this research has not been conducted during all phases of high and low populations for all subpopulations (Tyler 2010; but see below for Banks Island).
Of importance to Peary Caribou is energy accumulation during the short plant growing season, which can drive fitness for the rest of the year. This implies some degree of behavioural plasticity to allow animals to respond to the variation in forage availability. Most evidence for such plasticity comes from Svalbard, a high arctic island group north of Norway where Svalbard reindeer (Rangifer platyrhynchus) increase movements when ground-fast icing restricts forage (Meland 2014). The Svalbard reindeer switch between selecting forage quality versus quantity depending on changes in abundance of lichen, moss/graminoids, and parasite avoidance strategies (Van der Wal 2006).
Peary Caribou diet has been relatively well studied in the western Arctic (Shank et al. 1978; Thomas and Kroeger 1980; Thomas and Edmonds 1983; Larter and Nagy 1997; Lenart et al. 2002). Peary Caribou have a broad/varied diet and are versatile feeders with diet varying seasonally in relation to available forage and corresponding nutritional content.
Diet on Banks Island has been described when Peary Caribou numbers were increasing (Shank et al. 1978) and decreasing (Larter and Nagy 1997) in the context of overlap with Muskox diet. Thomas and Kroeger (1980) examined the summer and winter digestibility of forage using caribou from Prince of Wales Island. Digestibility was greater for sedges in winter than summer; the digestibility of the White Worm Lichen Thamnolia vermicularis was 18% in summer in contrast to 62% in winter, but the digestibility of mosses was higher in summer than winter. Thomas and Edmonds (1983) reported on late winter diet from across the WQEI to Prince of Wales and Somerset islands. In that study, lichens comprised 2-15%, while sedges and mosses provided 15-57% and 13-58%, respectively. In summer, caribou select forage high in digestible protein by foraging on flowers especially Purple Saxifrage (Saxifraga oppositifolia), lousewort, and Arctic Poppies (Parker and Ross 1976; Parker 1978) and made high use of willow leaves on Melville and Axel Heiberg islands. During unusually severe winters caribou are restricted to a diet with highly indigestible forage such as willow twigs, which can result in malnutrition (Parker 1978).
Measurements of diet have shown that lichens comprise a relatively low proportion of winter and summer diet for Peary Caribou compared to Barren-ground (reviewed by Wilkinson and Shank 1974; Miller 1998; Larter and Nagy 2004). For example, in a study on Banks Island, lichen was of minor dietary importance, likely because of its low availability (standing crop 2.96 g/m2), whereas sedges, willows, legumes (Astragalus spp., Oxytropis spp.), and Dryas integrifolia dominated the diet (Larter and Nagy 1997; Larter and Nagy 2004). Inuvialuit TK reveals that Peary Caribou eat lichens (genera Cladina and Cladonia), known broadly as “tuktut niqait” (“tuttut niqingi” in Uummarmiutun), or ‘caribou food’; Snow Lichen (Flavocetraria nivalis) and White Worm Lichen known as “aqiarungat” or “akeagonak”; and various kinds of rock lichens, known generally as “qaviut” (Bandringa 2010). Caribou winter range is often correlated with the abundance of lichens Cetraria delisei and Thamnolia vermicularis, crustose lichens, and grasses (e.g., Alpine Foxtail [Alopecurus alpinus]) and rushes (e.g., Two-glumed Rush [Juncus biglumis]). On eastern Melville Island, Thomas et al. (1999) found that the amount of lichens in the winter diet of Peary Caribou depended on snow conditions, with lower occurrence of lichen in the diet in years with deeper, harder snow.
The low proportion of lichens in the diet measured either from rumen or fecal pellet samples may reflect that lichens are scarcer in Peary Caribou range than on the ranges of other caribou (Thomas et al. 1999, Russell et al. 1978). A likely reason is the underlying substrates are mostly alkaline and unfavourable to lichens. A possible parallel might be the low occurrence of lichens on Svalbard where the vegetation following reindeer grazing from 1978 to 2013 shifted from lichens to more productive and resilient moss-graminoids (van der Waal et al. 2001, Ronning 2014). However, where reindeer declined, fruticose lichens have recovered after 100-200 years (van der Waal et al. 2001).
Peary Caribou usually forage while walking, rather than by feeding in place as Muskoxen do (COSEWIC 2004 and references therein). Caribou can average 3-4 km of travel per hour while actively foraging (Miller et al. 1982). Under ideal conditions when the snow is soft and relatively shallow, caribou forage by simply pushing the snow off the vegetation with their noses. As snow density increases, they dig small individually scattered craters, unlike the large cratered areas often used by groups of Muskoxen and groups of Barren-ground Caribou. When snow cover becomes too hard and dense, Peary Caribou seek forage on snow-free sites or sites with only shallow snow cover (e.g., exposed wind-swept areas). On Banks Island, they often feed in winter by cratering in the snow of upland habitats (upland barrens, hummock tundra, and stony barrens) where it is softer and shallower than in wet meadows (Larter and Nagy 2001b).
Essentially all historical Peary Caribou habitat is available and has not been lost or fragmented by industrial or other anthropogenic developments. There is little potential habitat that is currently unoccupied, other than Prince of Wales-Somerset group of islands and Boothia Peninsula.
At community information meetings conducted during Environment Canada-led recovery meetings, members of the Cambridge Bay HTO (2013) expressed concerns that past activities have affected caribou habitat. There were also multiple comments about past exploration activities leaving contaminated sites and fuel drums from Gjoa Haven, Grise Fiord, and Resolute Bay community members (Gjoa Haven HTA 2013; Iviq HTA 2013; Resolute Bay HTO 2013).
Under a changing climate, habitat changes (e.g., vegetation changes [productivity and shrub growth] and snow conditions) for Peary Caribou have already occurred (SARC 2012) and the rate of these changes is projected to increase (see Threats-Climate Change).
Caribou and reindeer are polygynous (c.f. Holand et al. 2007), but little is known of the Peary Caribou mating system (Petersen et al. 2010). The small group size typical of Peary Caribou (Tener 1963; Miller et al. 1982; Nagy et al. 1996) suggests a harem-guarding mating system.
Peary Caribou have widely variable vital rates. Productivity (the proportion of females with calves) in the WQEI has varied from 0 to 88%, and on Banks Island from 3 to 33% between 1970 and 2010 (SARC 2012). Overwinter calf survival on Banks Island from 1991-1999 varied from 23 to 86% (SARC 2012). Information on adult sex ratios is generally lacking, as are data on longevity and age at last reproduction. ATK indicates that Peary Caribou females in good condition can calve every year after sexual maturity is reached at 2 to 4 years of age, but hunters report finding no fetuses in harvested caribou after harsh winters (SARC 2012 and references therein).
Information regarding generation time is lacking for Peary Caribou. COSEWIC (2004) estimated the intergeneration time for Peary Caribou at 7 years, although no rationale was provided; this was also adopted by SARC (2012) for the NWT assessment. Females may live to 15 years in the wild (SARC 2012). They presumably are fecund for their whole adult lives (at least 13 years, the maximum age sampled--Thomas et al. 1976), although senescence has been observed in reindeer between the ages of 7 and 11.5 years (e.g., Weladji et al. 2010). Hence, the median age of Peary Caribou parents could be up to 8.5 to 9.5 years. Given the IUCN definition of generation length as the average age of parents of the current cohort, and reflecting the turnover rate of breeding individuals in a population (IUCN 2014), Peary Caribou generation time was established as 9 years for the purposes of this assessment.
Peary Caribou are adapted to limited plant growth with a highly compressed growing season and long periods of snow-covered frozen standing vegetation (see Habitat).
Despite their modest genetic differentiation, behavioural and morphological differences between Peary and Barren-ground Caribou are assumed to result from strong selection pressure in their high Arctic environment (Manning 1961). Given that shorter body extremities minimize external surface area and heat loss, it may be that the adaptive value of a shorter broader muzzle of Peary Caribou also prevents heat loss while maintaining a long enough molariform tooth row to forage effectively.
Tener (1963) and others noted the small group size of Peary Caribou (typically a dozen or fewer) and widely dispersed aggregations relative to Barren-ground Caribou (often in herds of 1,000 or more). Group size increases slightly prior to calving, stabilizes or decreases during calving and then increases into post-calving aggregations as they move inland from coastal areas (Nagy et al. 1996). However, the post-calving aggregation is a relative term as the group sizes are tens of individuals not the hundreds to thousands typical of Barren-ground Caribou. The underlying mechanisms may differ; small group size and dispersion may be an adaptation to an environment with thin and patchy forage (relative, to mainland caribou ranges), avoidance of predation, and/or lack of insect harassment.
The forage biomass of some Peary Caribou habitats (e.g., Banks Island--Larter and Nagy 2001a), and the relatively low prevalence of mosquitoes and warble flies, which allows for uninterrupted foraging (Gunn and Skogland 1997), can lead to accumulation of substantial fat stores. The accumulation of fat reserves in the summer and autumn is critical to survival and reproduction in severe winters (Thomas 1982; Nagy et al. 1996).
Peary Caribou move relatively long distances, including annual migrations across sea ice, regular movements within multi-island home ranges and erratic large-scale movements among islands during severe winters (see Population Spatial Structure and Variability; Figure 3).
The islands of the Canadian Arctic Archipelago are surrounded by ice for ≥ 9 months each year (Miller et al. 2005b); most inter-island crossings by Peary Caribou occur during the period of highest quality and concentration of fast ice, corresponding with travel to winter and spring/summer ranges (Jenkins and Lecomte 2012). However, there are also observations of Peary Caribou swimming between islands during seasonal movements (Miller 1995a).
There are many records of Peary Caribou crossing the sea ice in seasonal migrations among the islands and between the mainland and Arctic Islands. These are not necessarily fixed migration routes that are used habitually, but rather broad migration zones that individuals use to travel from winter ranges to calving areas and summer ranges (Miller et al. 2005b). For example, Miller et al. (2005b) documented 73 crossing sites representing 850 Peary Caribou trails on northeastern Franklin Strait (between Boothia Peninsula and Prince of Wales Island) and Peel Sound (between Somerset and Prince of Wales Islands) in three years (1977-1980). These crossings were also relatively evenly distributed, regardless of the length of the sea-ice crossing site or the elevation at its origin or terminus. There is also some evidence to support forced dispersal during winters characterized by icing events or above average snow fall (see SARC 2012).
Little is known about dispersal except that mtDNA analyses showed a low frequency of recent (“within the last several generations”) unidirectional dispersal from WQEI into Banks Island, Northwest Victoria Island, and the Prince of Wales-Somerset islands; and from the latter to Banks Island and the Boothia Peninsula (McFarlane et al. 2014).
There has been substantial concern, particularly at the community level, about interspecific interactions between Muskoxen and Peary Caribou. ATK and community knowledge has emphasized this issue (see SARC 2012). Inuit from Resolute Bay and Grise Fiord reported that “a large abundance of Muskoxen is often followed by the decline in the population of caribou in a specific area” (Taylor 2005). In Environment Canada recovery meetings, community participants have identified competition with Muskoxen as a major threat to Peary Caribou, as would be suggested by evidence of displacement of the latter by the former, or contrasting population trends (Olohaktomiut HTC 2013; Paulatuk HTC 2013; Spence Bay HTO 2013).
Historically, on Banks Island, northwestern Victoria Island, and Prince of Wales-Somerset islands, Peary Caribou and Muskoxen have had opposite trajectories in abundance (Gunn et al. 1991; Gunn and Dragon 1998; Nagyet al. 2009e; Davisonet al. 2013). By the late 1980s, concurrent with a major decline of Peary Caribou on Somerset Island, hunters noted that areas previously occupied by caribou were now occupied by Muskoxen (cited in Taylor 2005). Recent disease-associated declines of Muskoxen on Banks and Victoria islands (Kutz et al., 2015) have not been accompanied by as rapid an increase in Peary Caribou as historically observed (see Threats and Limiting Factors). The bacteria isolated from Muskoxen as a disease-causing agent is a generalist and also able to infect caribou; however, its role in the current Peary Caribou population dynamics is uninvestigated. Concurrent declines in both Muskoxen and Peary Caribou have also been observed, for example, on WQEI, although there were differences in the rates of recovery (Miller et al. 1977b; Gunn and Dragon 1998; Anderson 2014). Weather-related events are often implicated in these concurrent declines.
The frequent comments in recorded Inuvialuit ATK (e.g., Peter Esau quoted by Berger 1976) suggest that Peary Caribou and Muskoxen are competitors for forage. On the other hand, Parker (1978) concluded that in winters with average snow conditions on Bathurst Island, there is no interspecific competition with Peary Caribou and Muskoxen. However, he suggested that in severe winters there could be competition as both species sought willows on exposed slopes and ridges. During the 1973-1974 severe winter when many individuals of both species died on Bathurst Island, a retrospective analysis suggested there was no interspecific competition between them because the fecal pellet densities were negatively associated with one another and relationships with certain forage species contrasted significantly (Thomas et al. 1999).
Investigators have largely compared habitat use or forage overlap between the two species as a means of indirectly assessing competition. On Banks Island, Wilkinson and Shank (1974) and Vincent and Gunn (1981) found no evidence to suggest competition between Peary Caribou for forage or space. As abundance of Muskoxen increased during the 1990s, studies did, however, reveal that diets overlapped (Larter and Nagy 1997; 2004). The potential for apparent competition under certain conditions cannot be ruled out. Jenkins (2006) suggested that caribou may avoid Muskoxen to avoid predation by Wolves. Gunn et al. (2011) also speculated that “…the increasing Muskox abundance supported increased Wolf numbers which, in turn, could increase predation rates on Peary caribou.”
Several observers have noted that the spatial segregation between Peary Caribou and Muskoxen may have a deeper, behavioural basis than habitat preferences. Segregation has been reported on Banks Island (Kevan 1974 and others; Wilkinson and Shank 1974), Melville Island (Thomas et al. 1999), Axel Heiberg (Tener 1963), Bathurst Island (Ferguson 1987) and Ellesmere Island (Jenkins 2006; Manseau et al. 2004; Tener 1963). People in Ulukhaktok suggested that the caribou had moved toward Cambridge Bay to escape the Muskoxen at Minto Inlet (Gunn 2005). Inuvialuit and Inuit ATK has many references to caribou avoidance of Muskoxen because they dislike their smell, or simply because “caribou don’t like Muskox” (Ulukhaktok residents quoted by Kassam 2009; Ekaluktutiak HTO 2013; Iviq HTA 2013; Palaulatuk HTC 2013). ATK suggests that caribou may avoid areas of high Muskox use because they trample the vegetation and pack the snow, which impedes feeding by caribou (SARC 2012).
Sachs Harbour residents have previously linked the high Wolf numbers with the increasing Muskox numbers and declining Peary Caribou on Banks Island (Sachs Harbour Community Conservation Plan 1998 cited in SARC 2012). On Banks and northwestern Victoria islands, Muskox populations greatly increased in the 1960s after a 1955–1959 poisoning program reduced the number of Wolves (Heard 1984). Nagy et al. (1996) noted that Wolf populations had increased “dramatically” on Banks Island during a period of Muskox increase/caribou decline, that Wolf predation on caribou had been observed, and that “Peary caribou on Banks Island may be in a situation … where a high bio-mass of Muskoxen supports an increasing Wolf population… Even if predation rates on caribou are low, the impact may be significant especially given their recent low numbers.” Nagy et al. (2013) noted that 1998 was the first time in 20 years that the Muskox population on Banks Island showed signs of decreasing while the number of Wolves seen during ungulate surveys continued to increase.
Similarly, on northwestern Victoria Island, a survey of local knowledge showed that Wolves had increased from the 1970s through the 1990s, coincident with the increase of Muskoxen and decline of Peary Caribou (Gunn 2005). Gunn (2005) suggested that higher numbers of Muskoxen could maintain high numbers of Wolves and lead to relatively high predation on the remaining caribou.
Other predators include Grizzly Bears (Ursus arctos) and Wolverines (Gulo gulo). Arctic Foxes (Vulpes lagopus) sometimes attack juvenile caribou (SARC 2013). Community members within the two southern Peary Caribou subpopulations report increasing numbers of recent sightings of Grizzly Bears and/or Wolverines (Ekaluktutiak HTA 2013; Gjoa Haven HTA 2013; Sachs Harbour HTC 2013; Spence Bay HTO 2013).
The prevalence and intensity of parasite infections and diseases in Peary Caribou is little known. One caribou parasite that is relatively easily tracked is the warble fly but the prevalence of warbles parasitizing caribou on Banks or northwestern Victoria islands is not known. On Melville and Prince Patrick islands, 11 and 16% of Peary Caribou, respectively, collected in 1974-79 had warbles (Thomas and Kiliaan 1990). Almost the only information on other parasites and diseases is from Banks Island where Inuvialuit report tapeworm cysts in the muscle of Peary Caribou: the primary hosts of the tapeworms are wolves or foxes (Vulpes spp); numbers of cysts in the caribou vary and may be related to fox cycles (Nagy et al. 1998).
More is known about diseases in Muskoxen on Banks Island, but it is unknown whether Muskox diseases and parasites are a threat for Peary Caribou. Some parasites and diseases recorded for Muskoxen have not been found in Caribou, including Yersiniosis, which is prevalent among muskoxen (Larter and Nagy 1999). Giardia is found in Muskoxen but not in caribou although another protozoan parasite, Cryptosporidium, was in 22% of Peary Caribou fecal samples from Banks Island in the 1990s (Nagy et al. 1998).
Barren-ground Caribou and Muskoxen share several parasites, including gastrointestinal helminths and a species of lungworm (Kutz et al. 2012), and are susceptible to a number of the same pathogens, including the bacteria Brucella suis and Erysipelothrix rhusiopathiae (see Threats and Limiting Factors). Parasite-mediated competition between caribou and Muskoxen has been postulated with respect to the abomasal nematodes (Hughes et al. 2009). The abomasal nematodes, Teladorsagia boreoarcticus and Marshallagia marshalli, are associated with poorer body condition (both) or protein indices in Muskoxen and caribou, respectively (Steele 2013; Kutz et al. unpubl. data). These species are common in Muskoxen, and the relative abundance in caribou appears to increase where they are sympatric with Muskoxen (Hughes et al. 2009; Kutz et al. 2012; Steele et al. 2013). In the Kangerlussuaq area, west Greenland, Barren-ground Caribou have a parasite fauna dominated by parasites also found in the introduced Muskoxen. Marshallagia marshalli is associated with lower protein and kidney fat indices in barren-ground caribou in Greenland (Steele et al., 2013). Studies to date have been inadequately designed to assess the effect of T. boreoarcticus on caribou; however, this parasite negatively impacts body condition in Muskoxen (Kutz, Nagy, Checkley unpubl. data) and the related nematode of caribou, Ostertagia gruehneri, negatively impacts body condition and pregnancy in caribou and reindeer (Irvine et al., 2001; Steele 2013).
A parallel with Peary Caribou may be the documented sub-clinical effects of parasitic nematodes on Svalbard reindeer. In Svalbard reindeer, gastro-intestinal nematodes affected body weight sufficiently to reduce pregnancy rates (Irvine et al., 2001), which does suggest that parasites may have sub-clinical effects. Those effects include changes in foraging behaviour to avoid the risk of infection (Van der Waal et al. 2000).
Survey design in the Arctic Archipelago has to account for low densities and a widespread distribution of animals (Gunn and Poole, 2014). The enormous size (7% of the total area of Canada) and remoteness of the area, which has few operational bases, are logistical constraints. As a result, surveys have been infrequent, with each covering only one or a subset of islands at a time. Evaluating trends in abundance for Peary Caribou since the first surveys were conducted in the 1960s is made difficult by irregular frequency in surveys (in time and space), as well as changes in survey design and methodology (Gunn and Poole, 2014).
Most surveys were aerial strip transects and extrapolated densities observed within the strips to off-transect areas, under the assumption that Peary Caribou are evenly distributed within strata. Most surveys have been stratified, applying higher effort in areas of known or suspected high relative densities, and less effort spent in other areas. Not all investigators have differentiated age classes; those who did have reported “non-calves” or yearlings plus adults, or “short yearlings” (the previous summer’s calf crop at about 10 months old) plus adults, depending on the time of the survey. Increasing survey accuracy (i.e., by reducing survey altitude and transect width) with the same survey effort results in decreases in precision, because coverage is less (Gunn and Poole, 2014). Precision is usually, but not always (especially in earlier years), a measure of variance (i.e., 95% confidence interval [CI] or standard error [SE]). Otherwise, population numbers are minimum counts, which are also sometimes generated from unsystematic aerial searches or surveys for other species (e.g., Muskoxen). Telemetry by VHF radio or satellite transmitters was applied on Banks, Bathurst and Ellesmere islands, which increased description of seasonal movements for Bathurst Island (Poole et al.2015) but elsewhere the telemetry remains unreported.
Bias through sightability of animals (pelage relative to background, lighting conditions, etc.) and observer experience is likely high and typically unmeasured (Gunn and Poole, 2014).
The first systematic aerial surveys for Peary Caribou (and Muskoxen) were led by J.S. Tener in 1961 across the QEI (Tener 1963). The researchers applied stratification but did not allocate survey effort by caribou density as prior information was unavailable. Bias was likely similar to other surveys given the narrow strip width and survey altitude. While Tener did not calculate the variance of the estimate, a subsequent recalculation of the estimates conducted by Miller et al. (2005b) included confidence limits. Consequently, the coefficient of variation (CV) for western and eastern portions of the study area was 8% and 22%, respectively, which reflects the coverage and is similar to the precision of subsequent estimates. Tener’s (1963) surveys resulted in a provisional Peary Caribou abundance estimate of 25,845 individuals on the QEI (two of four subpopulations recognized in this assessment). This included 12,799 caribou on Melville Island alone (Tener 1963).
Concerns were raised by Inuit in Grise Fiord and Resolute Bay that the Peary Caribou population could not have been as high as reported by Tener (Ferguson et al. 2001), and these doubts have persisted in recent Environment Canada-led technical community meetings during recovery planning for this species (e.g., Iviq HTA 2013; Resolute Bay HTO 2013). On the other hand, Tener’s (1963) estimated abundance for Bathurst Island in 1961 was similar to the estimates recorded in 1993 (Miller 1995b) and 2013 (Anderson, 2014). The recent surveys since the last status report include Jenkins et al. (2011) who reported population numbers in Nunavut (with the exception of Byam Martin, eastern Melville, eastern Mackenzie King, and Borden islands) during 2001-2008. They used a combination of spring aerial and winter snowmobile surveys and distance sampling (Buckland 2001), using line-transect methods to estimate density and abundance of adults and short yearlings.
Most surveys used transects on individual islands or groups of islands, which is advantageous for comparing estimates between years. In other areas, as has been shown with reindeer on Svalbard (Norway), even slight differences in consecutive survey areas can lead to underestimates and inter-annual variations in abundance (Lee et al. 2015). Because most recent aerial surveys have been conducted during summer, only summer surveys are presented here for those islands that had multiple surveys in a single year so as to maintain consistency across years. Densities (number of caribou per area surveyed) were calculated from caribou counts along transects, and in turn were used to estimate caribou abundance for a given survey area (usually island).
Abundances reported from various surveys were not consistently extrapolated to the same area for all the surveys over the past several decades. To ensure consistency, Johnson et al. (in prep.) recalculated island areas (after Nagy et al. 2009) using a land mask that was generated from the CanVec dataset, an open source digital cartographic reference product produced by Natural Resources Canada (Government of Canada 2015). They used the Canada Albers Equal Area Conic projection to generate area estimates, which are used consistently in this assessment to establish area-corrected abundance estimates. Area-adjusted estimates assume uniform density within each surveyed island, which although unlikely, facilitates comparisons across years (Johnson et al. in prep.). Precision was not accounted for in those area-corrected estimates (Appendix 1).
From 1961 to 2014, government agencies conducted a total of 154 aerial surveys to estimate Peary Caribou abundance throughout the Canadian Arctic ( Table 2 ; Appendix 1). Survey frequency and spatial extent have been highly variable across this geography over these 53 years. The most frequently surveyed islands have been Banks Island (Banks-Victoria subpopulation) and Bathurst Island (Western QEI subpopulation). Gunn and Poole (2014) calculated coverage (the percentage of the total area that was surveyed) and precision (Coefficient of Variation; CV) on an island-by-island basis. On average, across the four subpopulations, coverage was between 14-33% and precision 17-33% ( Table 2 ).
| Subpopulation | Precision (CV) (%) | Coverage (%) | Number of Surveys | Time Period |
|---|---|---|---|---|
| Banks-Victoria | 31 | 18 | 39 | 1970 to 2014 |
| Prince of Wales-Somerset-Boothia | 17 | 15.5 | 26 | 1974 to 2006 |
| Western QEI | 26 | 33 | 79 | 1961 to 2013 |
| Eastern QEI | 22 | 14 | 10 | 1961 to 2007 |
Where possible, number of adults (> 1 year) was used to approximate number of mature individuals. Some surveys did not report calf estimates. The number of mature individuals was estimated for each subpopulation by summing the abundances across major islands with relatively frequent surveys during the same time period; a rough estimate of total abundance was derived from summed abundances across the four subpopulations.
There has been no single year where the entire range has received full coverage, nor has this been attempted since Tener's 1961 survey (Tener 1963). Overall three-generation and two-generation trends for Peary Caribou and those for each of the main subpopulations are estimated here through comparisons of area-corrected survey estimates for each of the main islands in each subpopulation (see Abundance).
These abundance and trends estimates have much compounded uncertainty owing to factors ranging from errors in survey estimates (discussed above), later onset of reproductive capability for Peary Caribou yielding overestimates of mature individuals (see Life Cycle and Reproduction), variable survey methods, variable ranges of the time span among islands to approximate 3-generation or 2-generation population trends, lack of precision in the land area, and unmet assumptions associated with the area-corrected estimates (see above).
The most recent surveys for Peary Caribou across the subspecies' High Arctic range yield an estimated total of about 13,700 adult and yearling Peary Caribou ( Table 3 ). However, this estimate is derived from a subset of all islands, some of which were not surveyed within the last decade. Hence, the certainty associated with this estimated population is low.
The summed abundances across islands serve as average estimates of Peary Caribou population size through time ( Table 3 ; Figure 5). Periodic stochastic (and unpredictable) die-offs are a feature of Peary Caribou ecology as described in following subpopulation sections (Miller et al. 1977a; Parker 1978; Harding 2004; Festa-Bianchet et al. 2011). These events may not all be known, because the long periods between surveys may have resulted in missing some abrupt declines and subsequent recoveries. Neither die-offs nor periods of increase appear to be synchronous across Peary Caribou range based on available information. The following section describes abundance patterns derived from scattered surveys within each subpopulation over the past five decades.
The most recent surveys from Banks Island (2014; Davison et al., 2014) and northwestern Victoria Island (2015; Davison and Williams 2013), respectively, indicated a total of about 2,252 mature individuals for this subpopulation ( Table 3 ; Appendix 1). Surveys from the late 1980s point to a considerably higher population (> 8,000), with an overall decline in three generations (27 years) of approximately 68% for both Banks and Victoria Islands combined. The latest surveys have indicated a modest increasing trend in numbers of mature individuals on Banks Island, whereas numbers on Victoria Island may have declined again more recently (Figure 5; Appendix 1).
According to local community knowledge (cited by Usher 1971), caribou numbers had fluctuated with severe winters in the early 1950s, causing deaths and desperation movements off Banks Island. Early estimates by quantitative surveys on Banks Island were 4,000 adults and calves in 1952–1953 (Manning and Macpherson 1958), 2,351 caribou in 1959 (MacPherson 1960), 5,000-8,000 in 1970 (Kevan 1974), and 12,098 in 1972 (Urquhart 1973). The 1970 and 1972 estimates were from systematic aerial surveys although Kevan (1974) only surveyed the northern half of Banks. Before 1972, observers said that most or all caribou were concentrated on the north end of the island. By 1972 the subpopulation had spread throughout the island (Urquhart 1973). Urquhart (1973) commented that an unusually heavy snowfall in the fall of 1970 had caused some caribou to leave Banks Island for the mainland, while others died from malnutrition. Hunters reported that many caribou died during that winter (cited in Gunn and Dragon 1998) and Urquhart (1973) extrapolated from 39 carcasses counted in June 1971 to estimate that 879 caribou died.
| Subpopulation | Island (group) |
Earliest survey within 3 generations | Earliest 3-gen area-corrected estimate | Earliest survey within 2 generations | Earliest 2-gen area-corrected estimate | Most recent survey | Most recent area-corrected estimate | Years of monitoring data (3-gen) | Approx. % 3-gen change | Years of monitoring data (2-gen) | Approx. % 2-gen change |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Banks-Victoria | Banks | 1987 | 4296 | 1998 | 454 | 2014 | 2248 | 27 | -47.67% | 16 | 395.15% |
| Banks-Victoria | NW Victoria | 1987 | 2790 | 1998 | 137 | 2015 | 4 Table Footnotea | 28 | -99.86% | 17 | -97.08% |
| - | - | - | - | - | - | - | - | - | - | - | - |
| Prince-Of-Waales-Somerset Boothia | Boothia | 1985 | 4738 | 1995 | 3265 | 2006 | 1 Table Footnotea | 21 | -99.98% | 11 | -99.97% |
| Prince-Of-Waales-Somerset Boothia | Prince of Wales | 1980 | 4212 | 1995 | 5 Table Footnotea | 2004 | 1 Table Footnotea | 24 | -99.98% | 9 | -80.00% |
| Prince-Of-Waales-Somerset Boothia | Somerset | 1980 | 577 | 1995 | 115 | 2005 | 4 Table Footnotea | 25 | -99.31% | 10 | -96.52% |
| Prince-Of-Waales-Somerset Boothia | Russell | 1980 | 605 | 1995 | 0 | 2004 | 0 | 24 | -100.00% | 9 | |
| - | - | - | - | - | - | - | - | - | - | - | - |
| Eastern QEI | Axel Heiberg | 1995 | 94 Table Footnoteb | 1995 | 94 Table Footnoteb | 2007 | 2255 | 12 | - | 12 | 2298.94% |
| Eastern QEI | Ellesmere | 1989 | 396 Table Footnoteb | 1995 | 149 Table Footnoteb | 2015 | 918 | 26 | 132.81% | 20 | 516.11% |
| - | - | - | - | - | - | - | - | - | - | - | - |
| Western QEI | Melville | 1987 | 955 Table Footnoteb | 1997 | 797 | 2012 | 2740 | 25 | 186.91% | 15 | 243.79% |
| Western QEI | Prince Patrick | 1986 | 156 Table Footnoteb | 1997 | 87 | 2012 | 2746 | 26 | 1660.26% | 15 | 3056.32% |
| Western QEI | Eglinton | 1986 | 79 Table Footnoteb | 1997 | 0 | 2012 | 181 | 26 | 129.11% | 15 | - |
| Western QEI | Emerald | 1986 | 14 Table Footnoteb | 1997 | 0 | 2012 | 45 | 26 | 221.43% | 15 | - |
| Western QEI | Byam-Martin | 1987 | 100 Table Footnoteb | 1997 | 0 | 2012 | 121 | 25 | 21.00% | 15 | - |
| Western QEI | McKenzie King | 1974 | 60 Table Footnoteb | 1997 | 36 | 1997 | 36 | 23 | - | 0 | - |
| Western QEI | Borden | 1973 | 16 Table Footnoteb | 1973 | 16 Table Footnoteb | 1973 | 16 | 0 | - | 0 | - |
| Western QEI | Brock | 1973 | 24 Table Footnoteb | 1997 | 0 | 1997 | 0 | 24 | - | 0 | - |
| Western QEI | Devon | 2002 | 110 Table Footnoteb | 2002 | 110 Table Footnoteb | 2008 | 17 | 6 | -84.55% | 6 | -84.55% |
| Western QEI | Lougheed | 1985 | 0 | 1997 | 103 | 2007 | 375 | 22 | - | 10 | 264.08% |
| Western QEI | Bathurst Is. Complex | 1988 | 1070 Table Footnoteb | 1997 | 81 | 2013 | 14631 | 25 | 36.73% | 16 | 1706.17% |
| Western QEI | Cornwallis | 1988 | 52 Table Footnoteb | 2002 | 2 | 2013 | 4 Table Footnote a | 25 | -92.31% | 11 | 100.00% |
| Western QEI | Little Cornwallis | 1988 | 0 | 2002 | 0 | 2013 | 1 Table Footnote a | 25 | - | 11 | - |
| Western QEI | Helena | 1988 | 26 Table Footnoteb | 1997 | 0 | 2013 | 2 Table Footnote a | 25 | - | 16 | - |
| - | - | - | - | - | - | - | - | - | - | - | - |
| Overall (approx.) | - | - | 21,637 | - | 5,451 | - | 13,178 | - | -35.31% | - | 141.75% |

Long description for Figure 5
Four chart panels presenting abundance estimates from various island surveys for each of the four Peary Caribou subpopulations. Estimates are extrapolated from study areas to whole islands to aid in comparison across years.
Available estimates from aerial surveys on Banks Island suggest steady declines from 1982 and relative stability at a low level from 1992 to 2010 (Gunn 2005; Davison and Williams 2013). The increase from 2,351 in 1959 (MacPherson 1960) to 12,098 in 1972 (Urquhart 1973) implies an average finite rate of increase (λ) of 1.14, or 14% per year. It declined more or less consistently, reaching a low of 451 ± CI 60 in 1998 (Nagy et al. 2013a). However, Nagy et al. (2006) suggested that the 1998 estimate was low for unspecified reasons. Abundance then increased to an estimated 1,142 ± CI 324 in 2001 (Nagy et al. 2006; a finite rate of increase of 30% for 3 years) and increased again to 2,234 in 2014 (Davison et al. 2014), the most recent estimate (Appendix 1).
The overall trend of Peary Caribou on northwestern Victoria appears more variable than Banks Island although survey frequency has been less. Historical information gathered for the Olokhaktomiut Community Conservation Plan (Anonymous 2008) related to northwestern Victoria Island stated that from 1900 to around 1920, Peary Caribou were increasing; however, a freezing rain event in about 1920 caused extensive mortality. Numbers fluctuated from then through the 1970s. Hunters from Ulukhaktok had difficulty finding Peary Caribou in the winters of 1991-1992 and 1992-1993 (Ulukhaktok, Wildlife Management Advisory Council (NWT), and Joint Secretariat 2008). Between 1980 and 1993, Peary Caribou from northwestern Victoria Island were surveyed five times, revealing a rapid decline from a high of 4,512 caribou in July-August 1980 (Jakimchuk and Carruthers 1980) to an estimated 159 in 1993 (Gunn 2005). A 2015 survey (April-May) recorded only one group of two individual Peary Caribou, while the most recent survey prior to that (July-August, 2010) yielded an estimate of 150 ± 104 adults. Reasons for the continued decline on northwestern Victoria Island are unknown, but are not thought to be related to disease and/or hunting (Davison and Williams 2013).
Current numbers of Peary Caribou in the Prince of Wales-Somerset-Boothia subpopulation are suspected to be close to zero at present, although the most recent survey was conducted almost 10 years ago. Surveys flown in 1980 and 1985 for this subpopulation yielded estimates of as many as 10,000 mature individuals, which plunged to a handful of individuals in the most recent surveys, suggesting close to 100% decline. Local hunters continue to observe occasional Peary Caribou or their tracks on the islands (Ekaluktutiak HTA 2013; Resolute Bay HTO 2013), but only at very low densities, and predicted a long slow recovery for the subpopulation (Campbell 2006).
An Inuk elder remembered his father saying that caribou were present in large numbers in the 1920s on Somerset Island and were hunted there until 1928–1930 when many caribou died; caribou persisted in small numbers there and on Prince of Wales Island until the late 1960s when they began to increase (Taylor 2005). Hunters from Taloyoak also reported that caribou numbers on Prince of Wales, Somerset, and Russell islands and Boothia Peninsula were low from the 1940s to the early 1970s and then increased (Gunn et al. 2006 and references therein). By the late 1970s there were “…lots of caribou, enough for winter clothing” on both islands (ATK in Taylor 2005).
The peak abundance recorded for Prince of Wales-Somerset islands was 5,682 total caribou in 1974 (Fischer and Duncan 1976), and 4,831 ± 543 on Boothia Peninsula in 1985 (Gunn and Ashevak 1990; Gunn and Dragon 1998). In the 1980s, during a period with high caribou numbers on Somerset Island and the small islands surrounding it, Inuit began seeing evidence of disease or parasites in caribou. Some caribou found dead had not died of old age or Wolf predation and caribou numbers began declining (ATK in Taylor 2005). The Resolute Bay hunters also said that by the early 1990s, the decline was so severe that they stopped hunting on Somerset and Prince of Wales islands. A 1995 survey, using the same methods and survey coverage as in 1980, found only 7 caribou on the three islands (Gunn and Dragon 1998). Because only two of those seen in 1995 were “on-transect”, no quantitative estimate was possible. A non-systematic survey looking for caribou and tracks in April-May 1996 reported two caribou on Somerset Island (Miller, 1997). In 2004 no caribou were seen during aerial surveys of the islands, and only four were seen on Somerset Island by ground crews (Jenkins et al., 2011). There have been no surveys conducted in the area since 2006, when Dumond (2006) spotted one caribou during a Muskox survey. Although tracks and individuals are spotted on occasion (Ekaluktutiak HTA 2013; Resolute HTO 2013), there is no evidence that numbers have recovered.
Gunn et al. (2006) examined factors explaining the near-total loss of Peary Caribou on Prince of Wales, Somerset, and Russell islands, and concluded that the decline from the mid-1980s to the mid-1990s resulted from long-term reduction in survival rates of calves and reproductive females associated with continued hunting and increased Wolf predation. Caribou declines in this subpopulation also coincided with an increase and range expansion of Muskoxen (Campbell 2006; Gunn et al. 2006), although there was no scientific evidence for or against deteriorating range condition. Miller et al. (2007a) put forward a combination of factors could limit population growth rates including Wolf predation, extreme weather, hunting, and disease.
Despite scientific uncertainty, the decline of Peary Caribou in the Prince of Wales-Somerset-Boothia subpopulation had been foretold: Simon Idlout recalled his father, Timothy Idlout, predicting in the early 1980s that the caribou would drastically decline, based on a die-off under similar conditions that the elder Idlout had observed in the 1920s (cited in Taylor 2005). Hunters in Gjoa Haven have reported that some caribou came from Prince of Wales Island to King William Island in the early or mid-1970s (J. Keanik pers. comm. cited by Gunn and Dragon 1998). Campbell (2006) also stated: “IQ indicates that the decline was a natural and predicted occurrence caused by the impacts of overabundance in the 1970s and early 1980s. According to IQ the major mechanism of the decline was emigration.” Gunn et al. (2006) examined this factor, concluding that there was no known severe and prolonged environmental stimulus sufficient to cause so many caribou to abandon their ranges, nor was there any evidence of population increases on neighbouring islands to make up for these losses.
In 1974, 1975 and 1976, Thompson and Fischer (1980) estimated Peary Caribou on the Boothia Peninsula to number 561-626 (June and August surveys), 1,109-1,739 (March and June surveys), and 1,120 (a March survey), respectively; they interpreted the sudden increase from 1974 to 1975 as a large-scale immigration from Prince of Wales Island. They pointed out (citing Fischer and Duncan 1976) that Prince of Wales Island experienced a concurrent population decrease of similar magnitude, and suggested that because the Prince of Wales population did not increase in 1976, while the Boothia population stayed the same or increased that year, the large number of immigrants from Prince of Wales had stayed on Boothia. Gunn and Dragon (1998) estimated 6,658 ± 1,728 (SE) on the Boothia Peninsula in 1995, but did not distinguish between Peary and Barren-ground Caribou, although both types were seen. The migration of Peary Caribou from Somerset Island apparently stopped with their near-extirpation by the mid-1990s.
Two Peary Caribou subpopulations are recognized in the QEI, with the majority of islands belonging to the WQEI ( Table 1 ; Figure 1). Most of the largest islands were last surveyed in 2012 and 2013 (Anderson, 2014), together comprising almost half the total area of WQEI (179,648 km2). Bathurst Island has received the most regular attention with ten estimates over a 41-year interval (Gunn and Poole, 2014). Surveys have recorded two die-offs and recoveries during this period. Miller and Barry (2009) examined population data during the 20 years between crashes on the southcentral QEI, where Peary Caribou experienced an average annual rate of increase of 13.2% from 1974 to 1994, which accelerated to 20.5% for the last six years from 1988 to 1994. Following the first crash, Miller et al. (1975) calculated subpopulation declines of 92% on Bathurst Island, 87% on Melville Island and 72% on Prince Patrick Island. Aerial surveys in spring 1975 confirmed that the decline continued (or a second decline occurred) during 1974–1975 (Gunn et al. 1981). Surveys confirmed another “catastrophic die-off” (or two, if individual years are counted) in the WQEI: in 1994–1995, when the south-central subpopulation (Bathurst and adjacent islands) crashed from 3,155 (based on another recalculation--Miller and Barry 2009) to 542 and again in 1996–1997 (Gunn and Dragon 2002), leaving only 78 caribou (no calves were seen) in the seven main islands of the subpopulation.
Some islands have received relatively little survey attention; the most recent survey in the Prime Minister Group was in 1997 (Mackenzie King, Brock) with Borden Island having been surveyed only in 1973 ( Table 3 ; Appendix 1).
The most current combined population estimate (2012-2013) from Melville, Prince Patrick, Eglinton, Emerald, Byam-Martin, Bathurst Island complex, Cornwallis, Little Cornwallis, and Helena islands is about 7,300 adults. Surveys that were conducted in the same areas in 1986-1988 totalled 2,500 individuals (including calves). This implies a 232% increase in the overall population over the past three generations.
Miller and Barry (2009) asserted that the primary factor controlling Peary Caribou numbers on the QEI has been infrequent, isolated, stochastic weather events, namely exceptionally severe snow or ice conditions, causing reduced or failed reproduction, poor early calf survival, and/or high adult mortality. They found no evidence of range deterioration or limits to the abundance of aboveground annual plant production to suggest any direct density-dependent responses.
Bathurst Island complex: The earliest surveys (Tener 1963) estimated 3,509 individuals, including calves (recalculated by Miller et al. 2005) on the Bathurst Island complex in 1961. Subsequent surveys in 1973-1974 recorded precipitous declines, after which the population increased by ca. 4% per year over the first seven years after the crash (1974–75 to 1980–81; Miller and Barry2009). By 1994, it had recovered to just about the same level as in the early 1960s (Appendix 1). Having suspended hunting after the 70s crash, hunters began returning to Bathurst Island in the late 1980s until another crash in mid-1990s that followed a fall rain/icing event, after which they again saw many carcasses of Peary Caribou and Muskoxen (ATK in Taylor 2005). Three successive single-year winter crashes from 1994–95 to 1996–97 resulted in a population of ca. 2–3% of its 1961 or 1994 size (Miller and Barry 2009; Appendix 1). Only two surveys have been conducted since that time, with the latest (2013) demonstrating an increase to 1,482 ± 387 (SE) individuals (including calves; Anderson, 2014).
As discussed in detail in COSEWIC (2004), available evidence clearly implicates density-independent weather events as the cause of both population crashes, with the chief cause of death being starvation as a result of prolonged snow or ice conditions hindering access to forage on a prolonged basis. Reproductive success and calf survival was poor during these periods; emigration was ruled out because of the number of carcasses. Resolute Bay elders recall similar die-offs in the 1930s (Resolute Bay HTO 2013).
Regarding recovery from population crashes, the infrequent nature of systematic surveys makes comparing and interpreting increases difficult, even in the relatively well-studied Bathurst Island complex. From 1975 to 1994, caribou on Bathurst and adjacent islands increased at an average finite rate of increase of about 13% per year (λ=1.13; Miller and Gunn 2003b), although from 1988 to 1993 it was 20% per year and from 1998 to 2001, after the mid-1990s die-offs, 36% per year. After 2001 through 2013 they grew at a more modest rate of λ = 1.18, or 18% per year. High levels of annual reproduction, early calf survival, and low mortality among adults was evident from 1988 to 1994, when the population tripled in size and weather was favourable (Miller and Barry 2009).
Caribou appear never to have been numerous on Cornwallis Island and surrounding, smaller islands which are mostly calcareous rock with very little vegetation cover.
Melville-Prince Patrick Group and Prime Minister Group: While not nearly as frequently monitored as the Bathurst Island Group, the islands of the Melville-Prince Patrick group and the Prime Minister Group do not appear to have had as many or as severe die-offs. Surveys in 1973 (4,323 caribou) and 1974 (2,418 caribou) documented a decline or die-off previous to 1973 and a die-off during 1973–1974, based on carcass counts and low (almost zero) percentage of calves (Miller et al. 1975). However, the severity was “…dissimilar between islands and [was] most marked on north-western islands”; declines were also less severe than on Bathurst and adjacent islands (Miller et al. 1975:20).
Long-term trends for the Melville-Prince Patrick-Prime Minister Group of islands show a decline from the 1970s to 1997 (although Borden Island was not surveyed), and an increase to ca. 6,000 adults and yearlings reported by Davison and Williams (2012) for July 2012 (although the Prime Minister Group was not surveyed). The 2012 survey also documents re-colonization of formerly occupied islands.
The infrequent surveys may conceal abrupt population crashes, as in the winter of 1996–1997, when numerous caribou carcasses were observed (Gunn and Dragon 2002). Because the subpopulation estimates were similar in 1986–1987 compared to 1997 (see above), Gunn and Dragon (2002) suggested that this also implied an undocumented increase between 1987 and 1996.
Early explorers commented on the abundance of caribou and other wildlife in the two westernmost groups of islands (e.g., Parry 1821; M’Dougall 1857; Henessey cited in Bernier 1910; Stefansson 1921). In 1958–1959, MacPherson (1961) surveyed Emerald Isle, Eglinton Island, Melville and Prince Patrick islands, and the Prime Minister Group and estimated a total population of 6,898 (there were none on Brock or Eglinton islands). Tener’s (1963) 1961 estimate was 12,799 total caribou for Melville Island, extrapolated from his counts of 769 caribou in 3 strata on Melville Island; he noted that they were distributed widely across the island, as opposed to the clumped coastal distribution he had seen on Bathurst Island. While admitting uncertainty in some assumptions in his calculations, Tener (1963:22) asserted that “…there is little doubt, however, that the total caribou population is in the thousands, far more than hitherto believed.”
Miller (1987, 1988) surveyed Prince Patrick, Eglinton and Emerald islands in 1986 (181 ± SE 59 caribou) and Melville Island (943 ± SE 126) and Byam Martin Island (98 ± SE 37) in 1987; the combined estimates for the two years total 1,222 (Appendix 1). In 1997, Gunn and Dragon (2002) found 907 adult and yearling caribou on three islands: Melville Island (787 ± SE 97), Prince Patrick Island (84 ± SE 34), and Mackenzie King Island (36 ± SE 22), with no live caribou on Eglinton, Byam Martin, Emerald, or Brock islands. Borden Island was not surveyed. In summer 1997, dead caribou made up 43% of the 1+ year old caribou surveyed in summer 1997 on the WQEI, although mortality rates varied by island (30% for Melville Island, 84% for Bathurst, 22% for Lougheed, 40% for the Prime Minister Group; Gunn and Dragon 2002).
Lougheed, Ringnes and Devon islands: Tener’s (1963) 1961 estimate was 566 caribou on the Amund Ringnes and Ellef Ringnes islands (13% calves), 269 on King Christian and Cornwall islands (30% calves), and 1,325 caribou on Lougheed Island (22.1% calves). Ground surveys by Stefansson (1921) estimated 300 caribou, which was also Macpherson’s (1961) extrapolation from a geologist who counted 56 caribou from a high hill where he could observe about a quarter of the island. Resolute Bay hunters reported that Lougheed Island had “plenty of healthy caribou” in the early 1970s (Tony Manik in Taylor 2005). After the 1973–1974 crash, no caribou were documented (although surveys were infrequent) until Gunn and Dragon (2002) estimated 101 ± SE 73 adults and yearlings living on the island in 1997. Like the other island groups in WQEI, Lougheed was affected by the mid-1990s die-offs, with about 22% of the population represented by dead caribou in 1997 (Gunn and Dragon 2002). The most recent estimate was 372 ± CI 234 adults plus “short yearlings” on Lougheed Island and the four smaller islands extending south of it (collectively the Findlay Group) in 2007 (Jenkins et al. 2011). Caribou were only seen on Lougheed Island.
On western Devon Island, Jenkins et al. (2011) counted 35 caribou (no calves), mostly off transect in 2002, and gave a rough estimate of 40 caribou. In a more extensive survey (7,985 km) of all non-glaciated areas of Devon Island and small proximal islands in 2008, they found just 17 Peary Caribou.
At 239,413 km2, the EQEI occupy a larger area than WQEI and are made up of only two large remote islands: Ellesmere and Axel Heiberg. There have only been a few surveys since Tener (1963), with the most recent published accounts in 2005-2007 (Jenkins et al., 2011) and 2015 for southern Ellesmere Island (Anderson and Kingsley 2015). Available information suggests that numbers have increased since the 1990s, but it is important to note that recent surveys have covered more areas than in the past ( Table 3 ).
In 1961, Tener (1963), acknowledging uncertainty based on low coverage and other factors, gave “provisional” estimates of 300 (14% calves) on Axel Heiberg Island (which he characterized as an “intuitive guess”) and 200 on Ellesmere Island (11% calves), the latter based on very low coverage, particularly in the north. Miller et al. (2005b), recalculated the 1961 estimates from Tener’s original maps and field records, almost doubling the total number of Peary Caribou. Hendrigan (in MacPherson 1963) estimated 150 caribou on Axel Heiberg in 1960, more than half in the north from Cape Stallworthy to Nansen Sound, which is also where Tener recorded animals in 1961. Since that time, a few partial surveys were completed. For example, Riewe (1973) estimated 35 caribou around Skaare and Wolf fiords on southeast Axel Heiberg in 1973, Zoltai et al. (1981) saw no caribou in their study area on the east slopes of Axel Heiberg in 1980, while Gauthier (1996) reported a minimum count of 25 caribou in June 1995 on Axel Heiberg (Skaare Fiord to Mokka Fiord and west to Li Fiord). The island was not completely surveyed until 2007, with an estimate of 2,291 caribou of ≥1 year, mostly along the eastern slopes (Jenkins et al. 2011). However, reconnaissance flights in summer 2014 along eastern and southeastern Axel Heiberg only reported sightings of three bulls and a cow-calf pair at Skaare and Wolf fiords (M. Anderson, pers. comm. 2015). This island is too remote for hunters to access, with the most frequent access being researchers at Expedition Fiord who report seeing caribou occasionally in the limited ground they cover (M. Anderson, pers. comm. 2015).
Since Tener’s survey on Ellesmere, several surveys have covered parts of the island, particularly in the south. Riewe (1973) estimated 150 caribou in 1973 on southern Ellesmere. Case and Ellsworth (1991) estimated 89 ± 31 (SE) caribou on southern Ellesmere Island. Gauthier (1996) counted 38 caribou on southern Ellesmere in June 1995. Southern Ellesmere was surveyed in 2005, along with Graham Island, with an estimate of 219 adults (109-442 95% CI). A survey was flown in March 2015 in the same area, with an estimate of 183 ± 128 (SE) indicating stability at a low density on southern Ellesmere Island (Anderson and Kingsley 2015). Central and northern Ellesmere were last flown in 2006, with an estimate of 802 adults (531-1207 95% CI) (Jenkins et al. 2011).
IQ emphasized the continued presence but general scarcity of caribou on southern Ellesmere Island until the early 2000s when they began to increase; Grise Fiord residents also reported fluctuations in numbers and more particularly in distribution, on southern Ellesmere Island (Taylor 2005). Peary Caribou have also been reported on Axel Heiberg Island by residents of Grise Fiord and Resolute when they (rarely) visit the island, and by the pilots and researchers working there in the spring and summer. The evidence could also suggest that caribou are re-colonizing areas that have been unoccupied for 15-25 years (Campbell 2006).
In light of the inconsistent surveys (different islands in different years, which may not accurately reflect subpopulations), large data gaps, and variable survey techniques and coverage, overall trends for Peary Caribou and each of its four subpopulations must be considered approximations and interpretations should be made with caution.
COSEWIC (2004) provided a rough total estimate of 50,000 Peary Caribou in the 1960s-70s when the first counts were made; in 1987, roughly three generations ago, the population was ca. 22,000 mature individuals (including some calves, especially from WQEI). Peary Caribou were at their overall lowest in 1996 at ca. 5,400 mature individuals ( Table 3 ). The population estimate for the last COSEWIC assessment was 7,000 (COSEWIC 2004), while the current estimate is 13,700. In spite an increasing overall two-generation population trend of ca. 150%, the three-generation decline is just over 35% ( Table 3 ).
WQEI experienced profound declines in the mid-1990s, related to icing events, whereas declines of both the Banks-Victoria and Prince of Wales-Somerset-Boothia subpopulations commenced almost a decade earlier and took place more gradually and for reasons that are less understood. One subpopulation (POW-Somerset-Boothia), which comprised almost half (10,000 mature individuals) of the estimated Peary Caribou population in 1987, has shown no signs of recovery. Banks-Victoria numbers have been increasing in the past decade, but not on Victoria Island. The WQEI subpopulation has increased overall since the mid-1990s, but with some fluctuations. EQEI numbers appear to be increasing as well, although baseline numbers are highly uncertain (Table 3).
Peary Caribou does not meet the IUCN definition of “extreme fluctuations” (IUCN 2014) because the magnitude of the population changes has been less than 10-fold, they are not synchronous for the four subpopulations, and are more reflective of population reductions (followed by some recovery) in response to threatening processes, rather than naturally recurring patterns of increases and decreases. However, ATK does indicate a tendency for population numbers to fluctuate over time over the past century (Ekaluktutiak HTA 2013; Resolute Bay HTO 2013; Sachs Harbour HTC 2013; Spence Bay HTO 2013), and many island surveys indicate considerable variability around the mean (Appendix 1).
The only potential source for rescue of Peary Caribou from outside Canada would have been from northwestern Greenland at one time, but there is little evidence of a present-day extant population (see Global Range).
Direct threats facing Peary Caribou assessed in this report were organized and evaluated based on the IUCN-CMP (World Conservation Union-Conservation Measures Partnership) unified threats classification system (Master et al. 2009). Threats are defined as the proximate activities or processes that directly and negatively affect the Peary Caribou population. Results on the impact, scope, severity, and timing of threats are presented in tabular form in Appendix 2. The overall calculated and assigned threat impact is Very High-Medium for Peary Caribou. This wide range rank of threat impacts is due to the combined effect of the high number of mostly low-impact threats, and the considerable uncertainty, unpredictability, and potential overlap and interaction of individual threats.
Narrative descriptions of the threats are provided below in the general order of highest to lowest overall impact threats.
The highest-impact threat to Peary Caribou arises from the myriad effects of a changing climate. Climate change has already affected the Arctic, and is occurring at higher rates than in other global ecosystems (ENR 2011; IPPC 2013; Stern and Gaden 2015). Measurable signs of a warmer Arctic and observed and predicted ecological consequences are commonly reported (Hinzman et al. 2005; Lim et al. 2008; Post et al., 2013). Inuit of the Kitikmeot region reported for the mainland a variety of changes, including longer summers, unusual freeze-thaw cycles in the spring, earlier spring break-up and open sea-ice, later fall freeze-up, thinner ice (both lakes and sea-ice), lower water levels, and less snowfall (Golder Associates Ltd. 2003). For the Arctic islands, community representatives reported effects similar to those in the Kitikmeot region, plus icebergs having disappeared north of King William Island, the extent of multi-year ice reduced, harder and rougher snowpack, and altered prevailing wind direction and causing altered orientation of snowdrifts (Golder Associates Ltd. 2003 and sources therein).
For Peary Caribou, changes in three Arctic climate (abiotic) variables – temperature, precipitation and severe weather events – account for most population-level effects of climate change (reviewed in Johnson et al. in prep.). This leads to both negative and positive changes in forage accessibility and decreased extent and thickness of sea ice. The primary population-level impacts range from shifts in migration and movement patterns to periodic mortality events, including population crashes. Climate change may also have a positive effect through extension of the growing season and increases in forage biomass. The accessibility of caribou to hunters will also be influenced by ice conditions and snow cover.
Habitat Shifting and Alteration (#11.1)
Annual average temperatures have increased across the Canadian Arctic from 1950 to 2007, with implications for the timing and amount of plant growth and diversity (Zhang et al. 2011). Arctic surface air temperatures since 2005 have been higher than for any five-year period since first measured in the 1880s, and evidence from lake sediments, tree rings, and ice cores suggest that recent summer temperatures have been higher than at any time in the past 2,000 years (AMAP 2012). Other documented changes include higher inflows of warm water entering the Arctic Ocean from the Pacific, declines in the extent and duration of snow cover, with the Arctic land area covered by snow in early summer reduced by 18% since 1966, and Arctic sea-ice decline at a rate that has been faster during the past ten years than averaged over the previous 20 years. Sea-ice thickness is also decreasing and sea-ice cover is increasingly dominated by younger, thinner ice (AMAP 2012).
Future temperatures in the Arctic are difficult to model because of uncertainties regarding extent of snow cover and retreat of sea ice, which are already accelerating much faster than previously predicted (see below). Nevertheless, experts agree that by 2100, mean projections for Arctic winter air temperatures under various CO2 concentration scenarios will be an increase of 2–9 °C above the 1986–2005 average; the highest projections range up to about 15 °C above the 1986–2005 average (IPCC 2013b). By 2035, Christensen et al. (2013) predicted mean annual surface temperature in the Arctic to rise by 1.5°C, with mean winter (December to February) temperature expected to increase more than mean summer (June-August) temperature (+1.7°C winter vs. 1°C summer). Mean projections for sea surface temperatures will be an increase from 4 to 14 °C under reasonably foreseeable CO2 concentration scenarios, with estimates for the highest CO2 concentration scenario at about 23 °C above the 1986–2005 average (IPPC 2013).
From 1951 to 2008, mean annual precipitation increased by 0.63-5.83 mm/yr/decade across the Arctic (IPCC 2013). Records from NWT climate stations indicate an increase in snowfall by 20-40% in the Arctic tundra (GNWT 2014). Mean annual precipitation is projected to further increase by 6% in 2035, more in winter than summer (Christensen et al. 2013).
This threat category is made up of three principal components: terrestrial habitat changes, sea ice loss, and sea level rise. Collectively, these are expected to affect most if not all of Peary Caribou range, with overall impact ranging from moderate to serious, depending on many competing factors.
Terrestrial Habitat Changes:
Temperature increases (and other climate changes such as increased CO2) have increased plant biomass. Ahern (2010) used analysis of the satellite-sensed normalized-difference vegetation index (NDVI) to show that plant growth has increased in southern and western parts of the range of Peary Caribou over the past 30 years. In short, “the Arctic is getting greener and primary productivity is increasing” (Eamer et al. 2013). These changes include plants leafing out and blooming earlier, which correlates with the general warming over the same time period (Oberbauer et al. 2013). With greening due primarily to increased shrub biomass (especially evergreen shrubs), however, the extent to which it will improve habitat or forage, and be of sufficient nutritional content for Peary Caribou is unknown. A spatially explicit modelling effort by Tews et al. (2007a) concluded that under scenarios where the frequency of extreme weather events did not change during this century, a projected 50% increase in biomass might alleviate the severity of population die-offs during disturbance years. However, when forage inaccessibility in poor winters increased by more than 30% over the same time period, as might be expected if the frequency and severity of disturbance events increases (as has been predicted to be a result of climate change; Larsen et al. 2014), models suggested net negative effects for Peary Caribou population dynamics.
Several authors have suggested that a phenological mismatch could threaten Peary Caribou if climate change were to alter the current synchrony between calving and lactation on one hand, and plant greening and blooming on the other (Festa-Bianchet et al. 2011; Gunn 1995, 1998; Gunn and Skogland 1997; Oberbauer et al. 2013; Parks Canada 2010; Tews et al. 2007b). This may have already occurred in other Arctic caribou ranges: in West Greenland, advancement of the plant-growing season during a period of temperature increase led to increased calf mortality, and a fourfold drop in calf recruitment over about a ten-year period (Kerby and Post 2013; Post and Forchhammer 2008).
Sea Ice Loss:
Sea ice decline is occurring at a faster pace than predicted by earlier modelling efforts (Overland and Wang 2013). In 2012, seasonal ice shrank to its lowest extent ever, continuing a trend that accelerated after 2000. The 2012 extent was about half that of the average summertime extent from 1979 to 2000, while the maximum winter extent was the fifth lowest in the past 35 years (Vinas 2013). September sea ice extent could shrink another 43%–94% by 2100; “a nearly ice-free Arctic Ocean in September before mid-century is likely” for the highest CO2 emission scenario (IPCC 2013). The extent of Arctic perennial and multi-year sea ice decreased between 1979 and 2012 and the thickness of average winter sea ice within the Arctic Basin decreased by between 1.3 and 2.3 m between 1980 and 2008 (IPCC 2013). Relevant to Peary Caribou sea ice crossings (Figure 2), declines of total sea-ice concentration that occurred from 2001-2010 were 50% for the M’Clintock Channel and 38% for the Eastern Arctic Channel (Stern and Gaden 2015). A general trend is for freeze-up to be occurring later and thawing events to happen more frequently during winter today than in the past (Ekaluktituiak HTA 2013).
The extent to which loss of sea ice could interrupt the inter-island migrations and other movements in parts of the range of Peary Caribou with population-level impacts is unknown. Hunters reported drowning events in the 1950s of Peary Caribou crossing between islands, and some suspected such events to be responsible for local declines (William Kagyut in Elias 1993; Kassam 2009). The nature of the impact to Peary Caribou populations would relate to the timing of the sea-ice freeze up in fall and break up in summer. This can affect migration patterns and the ability of individuals to move from island to island safely on time. Higher mortality rates can result from drownings that occur when animals fall through the ice as they seek to reach more suitable winter foraging areas. Because multi-island range rotation is known to enable recovery and growth of forage plants on summer ranges (Miller et al. 2005b; Resolute Bay HTO 2013), if Peary Caribou are forced to remain on any one island, there may be consequences to forage quality and nutritional state of stranded animals.
Sea Level Rise:
Sea level has risen about 0.19 m in the last 110 years (IPCC 2013). In the next 90 years, sea level is likely to rise further between 0.26 to 0.82 m (IPCC 2013). Such an increase could inundate large areas of Prince of Wales Island, Prince Patrick Island and islands in the Prime Minister and Ringnes groups (Pelletier and Medioli 2014) where isostatic rebound does not counter sea level rise.
Storms and Flooding (11.4)
Several high-mortality incidences following severe weather events have been recorded over the past four decades. Peary Caribou die-offs in the WQEI were linked to unusually warm weather in early winter, which caused the upper few centimetres of snow to melt and then subsequently freeze solid, preventing access to forage (COSEWIC 2004 and others). This resulted in 46% (1973-74) and 30% (1996-97) mortality in one winter, and >90% when there were three successive years of severe weather. An event such as this tends to occur as an ice crust on top of the snow, or the melted snow, percolates through the snowpack and refreezes at depth or on contact with the ground. In support of this, IQ reported up to 5 cm of ice in some years (Jenkins et al. 2010a;b; Taylor 2005). Similar ATK observations on Banks Island were reported: “in the fall, we get freeze-up on the whole island. Then, before the snow is really deep, we get our mild weather and rain. Then it’s cold enough for the rain to freeze on top the snow and that’s when the caribou try to leave the island, even go out into the ocean…. they were eating mostly ice” (Frank Carpenter quoted in Nagy 1999:163).
How much of a threat climate change may be to Peary Caribou will depend on the frequency and severity of icing (rain-on-snow and melt-freeze) events. Although severe weather events are predicted to increase in frequency and severity, there is considerable uncertainty with respect to location and timing of such events, and the consequent effects on population dynamics within the next three generations. There have been many reports that the frequency of rain-on-snow icing events have increased within Peary Caribou range (Festa-Bianchet et al. 2011; Gunn 1998; Gunn and Skogland 1997; Harding 2004; Miller and Gunn 2003a; Sharma et al. 2009; Tews et al. 2007b, 2012; Vors and Boyce 2009), and are predicted to continue increasing into the future (Hansen et al. 2011; IPCC 2013). Erratic weather is linked to the prevalence of freezing rain, and indications are that stochastic weather events are becoming more common on Banks Island due to climate change (Riedlinger 2001).
Miller and Barry (2009) argued that major population declines in Peary Caribou have followed severe winter weather due to forage inaccessibility bringing about starvation, and Arctic community members also consider this to be a major threat to Peary Caribou (Resolute Bay HTO 2013; Sachs Harbour HTC 2013; Spence Bay HTO 2013). The negative effects of severe weather events such as icing on populations appear to be predominantly through increased mortality from reduced forage in winter (“locked pastures”; Hansen et al. 2011) or reduced production of calves (Miller et al., 1977; Miller, 1991a; Gunn and Dragon, 2002; Miller and Gunn, 2003; Tews et al., 2007b). Contrastingly, Tyler (2010) argued that the effect of above-zero temperatures when snow is on the ground depends on snow depth: while warm weather may cause melting and a hard crust in deep snow, in shallow snow it could improve forage availability by melting the snow and baring the foliage.
The potential role of disease in Peary Caribou population dynamics is not well understood. ATK on Prince of Wales-Somerset indicated that increased observations of disease were accompanied by population declines in the 1980s (ATK in Taylor 2005). The literature on disease in Peary Caribou is sparse, thus potential issues are extrapolated from what is known in other caribou ecotypes and Muskoxen.
Known pathogens of potential concern that have impacts on reproductive success or survival in caribou include Brucella suis biovar 4, Erysipelothrix rhusiopathiae, Cervid herpes virus, parapox virus, Neospora, Besnoitia, and gastrointestinal parasites. Of these, the most important threats may be Brucella and Erysipelothrix.
Brucella suis biovar 4 is a bacterium that can cause arthritis, bursitis, and infertility. It has been associated with substantial population decline of the Southampton caribou since 2000 (Campbell, 2013). Brucella has not previously been reported in Peary Caribou and a serological survey on Banks Island in 1993-94 did not detect antibodies to this disease (e.g., serum samples were negative for brucellosis--Larter et al. 1996). However, clinical cases weredetected in Muskoxen on Victoria Island near Minto Inlet and Ekalluk River between 1996-1998 (B. Elkin pers. comm. 2015), and more recently (2014) in a sport-hunted Muskox near Cambridge Bay (M. Tomaselli pers. comm. 2015). The bacteria is well known in mainland Barren-ground Caribou with fluctuating prevalence (Leighton 2011; Curry 2012), and was reported as an emerging disease issue in the 1980s by hunters near Taloyoak, Kugaaruk, and Gjoa Haven, Nunavut, but presumably from Barren-ground Caribou (Gunn et al., 1991). There is no reason to think that this bacterium will not, if it has not already, invade Peary Caribou populations. The population-level impacts will depend on transmission dynamics; low densities of Peary Caribou may limit spread.
Erysipelothrix rhusiopathiae is a bacterium recently identified as a significant cause of widespread mortality in Muskoxen on Banks and Victoria islands, and likely at least in part responsible for the observed declines approaching 70% on Banks Island since 2010 (Kutz et al., 2013). This is a generalist and opportunistic pathogen, and is often found infecting domestic animals that are considered ‘stressed’. In Muskoxen and caribou it can cause sudden death, and in Muskoxen this is of all age classes. Several Barren-ground Caribou herds have tested positive for exposure to this bacterium (S. Kutz pers. comm. 2015) and it was considered the cause of death for Mountain Caribou in British Columbia (Forde 2015). While there remain many uncertainties about the origin and ecology of this bacterium in the Arctic, early data suggest that it should be considered a pathogen of interest for all arctic ungulates, including Peary Caribou (Forde 2015).
In general, under current climate warming scenarios, range expansion of several other pathogens is anticipated, and has already occurred for at least one parasite, the lungworm, Varestrongylus eleguneniensis (Kutz et al., 2013). In 2010 this parasite, which affects both caribou and Muskoxen, was detected for the first time on Victoria Island. It was probably introduced by the migrations of the Dolphin and Union caribou, and sporadic movement of Muskoxen to the island from the mainland. The recently permissive climatic conditions appear to have allowed this parasite to now be maintained, and expand its geographic range as far north as Surrey River area (P. Kafle pers. comm. 2015). The parasite requires slug or snail intermediate hosts, so its distribution may be limited by the abundance of these hosts. However, a related lungworm of Muskoxen has also expanded its range onto the island and occurs near Ulukhaktok; thus further range expansion of the lungworm into Peary Caribou range is anticipated. Although V. eleguneniensis is not considered to be particularly pathogenic, this recent range expansion highlights that climate change is already driving changes in distribution and abundance of pathogens of caribou.
Climate warming may also act by increasing susceptibility of caribou to infectious disease and insect harassment. Inuit have confirmed that hot weather can cause caribou to lose body condition and they have noted an increase in deaths from heat-related and insect-induced exhaustion that they attributed to climate change (ATK in Dumond 2007; Thorpe et al. 2001).
Summer weather influences the activity of warble flies. There has been an increase in suitable weather and a longer fly season from 1957–2009 on Barren-ground Caribou ranges (Gunn et al. 2011 and references therein). Warble flies are considerably less common on the High Arctic islands (e.g., 97% to 100% of Beverly herd caribou had warbles, but only 14% of Peary Caribou; Thomas and Kiliaan 1990), but the adult fly as the infective stage could be prolonged with warmer summers their prevalence could increase with continued global warming.
On the other hand, warmer temperatures may not favour all parasites, i.e., gastro-intestinal worms (Hoar et al. 2012). A warmer climate will not only affect the existing parasites and diseases but also increase the likelihood of invasive species (Kutz 2007; Davidson et al. 2011).
The projected decline of sea ice extent increases the possibility of year-round shipping routes within the Canadian Arctic Archipelago, particularly the opening of the Northwest Passage (NWP). It is assumed that increasingly lighter ice conditions will allow the navigation season to lengthen and shipping traffic to increase. In 1990-2011 shipping increased by 75%, reaching a maximum of 19 transits in 2010 (NORDREG in ENR 2011, updated to 2012 by SARC 2012), with some large icebreakers taking the northern route between Melville and Banks islands (McClure Strait: 6 times from 1993-2011; SARC 2012). Passages of cruise ships have already increased more than threefold between 1993 and 2007 (Judson, 2010, cited in Gunn et al. 2011). Shipping traffic experienced a 75% increase in Canadian Arctic waters from 1990 to 2012, while extent of sea ice declined, and is expected to increase further. Increased icebreaker-supported shipping would exacerbate the climate-induced effect of thinner ice and more lengthy ice-free periods (Gunn et al. 2011; Poole et al. 2010).
Shipping as a potential threat is a consideration for Peary Caribou due to seasonal migrations between islands (Paulatuk HTC 2013; Resolute Bay HTO 2013). In addition to potential population consequences of changes to ice thickness (discussed above), opening of shipping channels during winter would curtail certain island crossings altogether. The severity of impact to the overall population will depend on which island crossings are affected, how consistently across years, and the sizes of the populations. Shipping channels (Figure 6) could open between Prince of Wales and Somerset islands (Prince of Wales-Somerset and QEI-Prince of Wales crossings) and Bathurst-Cornwallis, but are less likely to affect Ellesmere, Axel Heiberg, or the Ringnes group, all of which are largely in pack ice (Figure 3) and not on any trade route.
Mine and energy exploration and development (discussed below; Figure 6) could also precipitate increases in shipping traffic in the region. Overall, shipping traffic is expected to increase in the Canadian Arctic Archipelago in the near future.
Modern Inuit and the cultures that preceded them, including the Thule from whom Inuit are descended, and the unrelated Dorset and pre-Dorset cultures have been hunting caribou in the region for at least 4,000 years (Fitzhugh 1976; Friesen 2013; Howse 2008; Manseau et al. 2005; Meldgaard 1960). Large-scale hunting and purchase of caribou meat by European explorers, and their introduction of firearms to Inuit (e.g., by Peary in the 1890s; Roby et al. 1984), caused or accelerated some declines, for example on Ellesmere Island (Petersen et al. 2010).
Much of Peary Caribou range is too inaccessible from settlements for resident hunters to reach by snow machine. There are no settled communities in the Melville-Prince Patrick group, Prime Minister Group, Ringnes group, Axel Heiberg Island or northern Ellesmere Island (with the exception of the Alert military base). Mould Bay (Prince Patrick Island) and Isachsen (Ellef Ringnes Island) weather stations are currently uninhabited. Therefore, modern-day Peary Caribou hunting takes place in areas accessible from settlements in and adjacent to the population’s range.
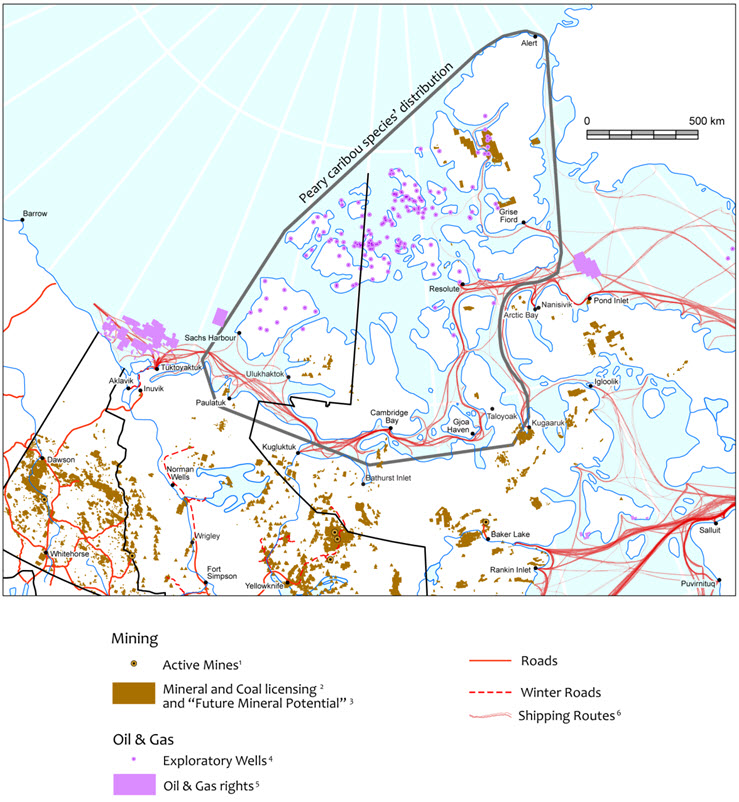
1Active Mines : Canada Northern… = 1Mines actives : Agence canadienne de développement économique du Nord, (http://www.cannor.gc.ca/DAM/DAM-CANNOR-CANNOR/staging/texte-text/nat_res_dev_project_map_1380647429535.eng.pdf) (consulté le 15 décembre 2015)
2Mineral and Coal Licensing: Geomatics Yukon, Government of Yukon. Aboriginal Affairs and… = 2Permis d’exploitation de charbon2 et de minéraux : Géomatique Yukon, Gouvernement du Yukon (http://mapservices.gov.yk.ca/GeoYukon/SL/) (consulté le 15 décembre 2015). Affaires autochtones et Développement du Nord Canada (http://nwt-tno.inac-ainc.gc.ca/ism-sid/minftp_f.asp) (consulté le 15 décembre 2015)
3Future Mineral Potential: Energy, Mines and Resources, Government of Yukon. Northwest Territories Geological Survey…NunavutGeoscience.ca = 3Potentiel minier futur : Gouvernement du Yukon, ministère de l’Énergie, des Mines et des Ressources du Yukon (http://www.emr.gov.yk.ca/mining/pdf/yukon_exploration_projects_december_2012_13x17.pdf (consulté le 15 décembre 2015). Commission géologique des Territoires du Nord-Ouest, base de données NORMIN (http://ntgodata.nwtgeoscience.ca/) (consulté le 15 décembre 2015). NunavutGeoscience.ca, Mineral Exploration Overview Magazine 2012 (http://www.nunavutgeoscience.ca/eo/index_f.html) (consulté le 15 décembre 2015)
4 OIl & Gas Exploratory Wells: Natural Resources Canada, BASIN database = 4Puits d’exploration : Ressources naturelles Canada, base de données BASIN (http://basin.gdr.nrcan.gc.ca/index_f.php) (consulté le 15 décembre 2015)
5Oil & Gas rights : Aboriginal Affairs and… = 5Droits pétroliers et gaziers : Affaires autochtones et du Nord Canada, Fichiers numériques des droits pétroliers et gaziers (http://www.aadnc-aandc.gc.ca/fra/1100100036298/1100100036301) (consulté le 15 décembre 2015)
6Shipping Routes : Generalized from Canadian Arctic… = 6Voies de navigation : Généralisation à partir de Routes de navigation dans l’Arctique canadien en 2010, Projets spéciaux et navigation dans l’Arctique, 2011, Transports Canada
Long description for Figure 6
Map illustrating the location of resource development in the Canadian Arctic. For mining, the map shows the locations of active mines, mineral and coal licensing, and future mineral potential. For oil and gas, it shows the locations of exploratory wells and oil and gas rights. Additional information includes roads, winter roads, and shipping routes.
Beneficiaries of the Nunavut Land Claim Agreement (NLCA), i.e. Inuit, are not restricted through legislation from hunting caribou, unless a conservation issue arises that results in establishing a total allowable harvest (TAH); absent a TAH, there is no reporting requirement. Specifically, Section 5 of the NLCA states: “Where a total allowable harvest for a stock or population of wildlife has not been established by the NWMB…an Inuk shall have the right to harvest that stock or population in the Nunavut Settlement Area up to the full level of his or her economic, social, and cultural needs, subject to the terms of this Article.” The parallel situation also pertains to the Inuvialuit Final Agreement.
An absence of hunting limits and mandatory reporting means that hunting records are not kept consistently, which prevents quantitative analysis or enumeration of trends. In addition, even when hunting levels are monitored, effort is unrecorded, adding to the difficulty of determining when hunting reaches unsustainable levels. Other evidence does suggest, however, that current offtake rates are low where hunting occurs within Peary Caribou range. A compilation of voluntary reporting of Peary Caribou hunt in Nunavut during the last decade showed about 10-36 animals per year hunted by residents from Resolute Bay (mostly on Bathurst Island), and another 10-60 hunted by residents of Grise Fiord on Ellesmere and Devon islands (Government of Nunavut 2011). Annual harvests during the last decade for the Northwest Territories were reported as 12 or fewer on Banks Island, and 0 from both WQEI and Minto Inlet (Gissing and Fleck 2011).
There is a history of voluntarily curtailing of hunting of Peary Caribou by Inuit and Inuvialuit hunters, through their local associations, when caribou populations were known to be at low levels (Ferguson 1987; Ferguson et al. 2001; Larter and Nagy 1995, 2000a; Miller and Gunn 1978; Taylor 2005). For example, from 1974 to 1989, the Resolute Bay Hunters and Trappers Association (HTA) prohibited Peary Caribou hunting on Bathurst Island. In 1982, upon noticing Bathurst Island caribou moving to Cornwallis Island, the ban was extended to include that island as well. From 1989 to 1996, as the population increased, the HTA allowed limited hunt in consultation with government biologists. After the 1995-1997 die-off, however, the hunt was halted again. Similarly, Inuit hunters from Grise Fiord instituted a 10-year moratorium on caribou hunting on most of southern Ellesmere Island from 1986 to 1996 while caribou numbers were low. There are currently no harvest limits imposed on NLCA beneficiaries hunting Peary Caribou in Nunavut.
Hunting may have been a factor in the declining trend of Peary Caribou on northwestern Victoria Island (Gunn et al. 1998). In response to the decline, the Olokhaktomiut Hunters and Trappers Committee initiated a zero-harvest by-law that is now enforced by GNWT legislation (Gunn 2005). Approximately 300-450 caribou (mostly females) were hunted annually on Banks Island in the 1970s and 1980s, skewing the subpopulation towards males and younger animals (Larter and Nagy 2000a). Despite action by Sachs Harbor to institute a voluntary quota in 1990 for Banks Island, the caribou subpopulation continued to decline. The voluntary quota is still in place (GNWT 2011 cited by SARC 2012); surveys since 1998 have shown an increasing trend (see Fluctuations and Trends). SARC (2012) reports a harvest rate on Banks Island of 1-3% since the mid-2000s. Miller et al. al. (2007 a and c) rationalized from estimated harvest rates and abundance how hunting on the Boothia Peninsula may have contributed to the 98% decline (1980-1995) of the Prince of Wales-Somerset subpopulation (see Fluctuations and Trends).
In summary, there is a history of cooperation between local community associations and biologists to implement community-based management in recognition of potential population-level impacts of hunting of Peary Caribou under certain conditions. Accordingly, current hunting rates of Inuit and Inuvialuit communities situated within Peary Caribou range appear to be low relative to before the 1990s. However, inconsistently collected hunting statistics, insufficiently-frequent population surveys and limited demographic sampling to quantify recruitment, age-specific mortality and fecundity collectively provide substantial uncertainty in population trends, hunting levels, and their interaction. The continued success of community harvest management as a dynamic component of Peary Caribou conservation will rely on both adequate monitoring and the ability to account for shifting trends, which include the steep declines of Baffin Island caribou and several mainland Barren-ground herds as well as Banks and Victoria Island Muskoxen (Kutz et al., 2015), the increasing demand for caribou from rapidly growing human populations, and a rising interest in country food and potential commercial harvest implications.
Possible multi-prey (especially Muskoxen) and Wolf interactions were noted earlier (see Interspecific Interactions). Although the impact of Wolf predation on Peary Caribou population dynamics is unknown, many authors consider it likely to be a major threat to recovery when population sizes are low (Nagy et al. 1996; Gunn et al. 2000b; SARC 2012). How such interactions might change with a warming and greening environment adds a new dimension to the question, which is why they are considered a threat (albeit) low in this status report, rather than a limiting factor.
Industrial activities are currently restricted, with market prices being an important determinant of the extent and intensity of activity at any given time. Mineral exploration, particularly for coal on Ellesmere Island, is currently occurring within Peary Caribou range (CWS 2013; 2014; 2015), but there is little current seismic activity or oil and gas development occurring in the range at large (Figure 6).
The most active period for oil and gas exploration in Peary Caribou range was in the 1960s and 1970s, when it was widespread on Banks, Melville and Prince Patrick islands (Usher 1971; Miller et al. 1977a). Polaris mine − located on Little Cornwallis Island from 1980-2002 − was the one mine (Zn-Pb) that has been operational in Peary Caribou range. A surge in oil-related exploration and other factors in the 1960s led to the initial discovery and exploration of the deposit; logistic support through the mine’s operation offered opportunities for continued exploration until the closure of the mine in September 2002 (Dewing et al. 2006). Mineral exploration took place in the Shaler Mountains of northwest Victoria Island in the 1990s, but this has not led to any development (SARC 2012). The known potential for oil and gas as well as minerals exists throughout Peary Caribou range, and exploratory wells have been drilled all over the WQEI and Banks Island (Figure 6). High-grade thermal coal deposits, with the potential for metallurgical coal, at or near the surface on Axel Heiberg and central Ellesmere islands have previously been proposed for development by West Star Resources, and more recently by Canada Coal, the company which owns the licences on the Fosheim Peninsula, although they withdrew their application from the Nunavut Impact Review Board pending more consultation in 2013. Boundaries for the recently gazetted Qausuittuq National Park on northern Bathurst Island reflect the recommendations of the Senior Mineral Energy & Resource Assessment Committee, which rated high potential for lead zinc mineralization on the northeast coast of Bathurst Island, and petroleum potential on southwest Cameron Island, and therefore excluded this island from within the park boundaries in spite of its known importance for caribou (Resolute Bay HTO 2013; Poole et al. 2015).
ATK concerns about strong negative influence of industrial activities on Peary Caribou include:
- direct, negative effects on animal health from smoke and dust from seismic explosions and fuel or rust leaking from oil drums (Taylor 2005; Ivig HTA 2013; Resolute Bay HTO 2013; Sachs Harbour HTC 2013);
- avoidance behaviour due to sensory disturbance (Taylor 2005; CWS 2013; Ivig HTA 2013; Resolute Bay HTO 2013) or barriers to movement (Urquhart 1973; Slaney and Co., Ltd. 1975), seismic drill rigs and camps (Riewe 1973; Urquhart 1973; Slaney and Co., Ltd. 1975; Sachs Harbour HTC 2013); and
- habitat loss, especially in critical areas for calving and higher-density areas (SARC 2012; Resolute Bay HTO 2013; Sachs Harbour HTC 2013).
Inuit in Resolute Bay and Grise Fiord suggested that disturbance by oil and gas exploration activities and prospecting for coal and base metals inhibited Peary Caribou from moving into areas necessary for their survival during years of high snow accumulation (Jenkins et al. 2010a, b; Taylor 2005).
Habitat loss from cumulative impacts of individual projects and associated infrastructure is the chief cause of concern for Peary Caribou; impacts have been well documented for caribou in general (Vistnes et al. 2008; Festa-Bianchet et al. 2011). The scale of development currently being contemplated by industry and the Government of Canada – new ports, mines, roads and expanding human populations (Government of Canada 2013) – may be a threat to Peary Caribou if not managed as to location and timing (e.g., migration routes, calving and rutting areas) of construction. Peary Caribou avoid industrial activities including roads and off-road vehicle traffic, although some individuals may approach a single vehicle out of curiosity (Slaney and Co., Ltd. 1974, 1975; Nellemann and Cameron 1998), they also avoid helicopters (Gunn 1984; Gunn and Miller 1980). Although these effects are localized, they may involve increased energy expenditure during nutritionally challenging periods and displacement from preferred habitats. The cumulative stressors may also lead to increased susceptibility to infectious diseases.
There are signs that human intrusions from work (non-tourist) activities and year-round military exercises are increasing in some parts of Peary Caribou range, with increases in traffic from snowmobiles, helicopters, and airplanes (including unscheduled flights). If such human activities interrupt caribou foraging or lead to avoidance behaviour affecting movements, this may increase caribou energetic costs (Weladji and Forbes 2002). Grise Fiord and Resolute Bay Inuit have also documented concerns about potential negative impacts of netting, collaring, and other research activities on Peary Caribou (Iviq HTA 2013, Resolute Bay HTO 2013). No Peary Caribou captures have been undertaken in Nunavut since 2003 due to community concerns.
Global climate systems bring certain volatile organic compounds from southern to northern regions, where they condense, precipitate, and accumulate (e.g., Prowse et al. 2009). Mainland and Baffin Island Barren-ground Caribou have trace amounts of organic contaminants such as HCB (hexachlorobenzene) and PCB (polychlorinated biphenyl) that are probably transported atmospherically from other continents such as Asia (Elkin and Bethke 1995). In the 1990s, contaminant levels were measured in Peary Caribou on Banks Island, and it was found that these caribou had the lowest levels reported in the study of 15 Canadian caribou subpopulations and are similar to background levels found in humans (MacDonald et al. 1996; Larter and Nagy 2000b). Inuit and Inuvialuit communities have voiced concerns about contaminant levels, e.g., on Bathurst Island (Resolute Bay HTO 2013).
Peary Caribou on Banks had lower levels of kidney heavy metals than mainland Barren-ground Caribou, which Larter and Nagy (2000b) attributed to low levels of lichen in their diet. Those metals are naturally occurring elements with no known local anthropogenic sources.
The highest threat to Peary Caribou is from climate change-induced habitat changes (e.g., severe weather events and sea ice loss), but the timing and geographic location of threatening events that might take place as a result makes it impossible to estimate the number of discrete locations, as defined by IUCN (2014).
Peary Caribou are co-managed in Nunavut according to the Nunavut Land Claims Agreement and in NWT according to the Inuvialuit Final Agreement. These agreements confer primary wildlife management authority on the respective management boards: the Nunavut Wildlife Management Board and the Wildlife Management Advisory Council (NWT).
COSEWIC most recently assessed this species as Threatened in 2015. Peary Caribou are currently listed under Schedule 1 as Endangered under the federal Species at Risk Act (2011); Canada Gazette Part II, Vol. 145, No. 4, 2011-02-16). Under the Species at Risk Act (NWT), Peary Caribou are listed as threatened in NWT. Provisions for Species at Risk designation under the Nunavut Wildlife Act have not yet been enacted.
The NatureServe global status rank of Peary Caribou is G5T1 (2012), signifying this as a critically imperiled subspecies of an otherwise widespread and common species. Its national status is N1; it is S1 in NWT and SNR (unranked) in Nunavut (NatureServe 2014).
All land except owned privately, by Inuit Organizations or by municipalities, is Crown Land in right of the respective territories. Figure 7 shows the national parks and other federally protected areas. National parks in the range of Peary Caribou are Quttinirpaaq National Park (Ellesmere Island), Qausuittuq National Park ( Bathurst Island), and Aulavik National Park (Banks Island).
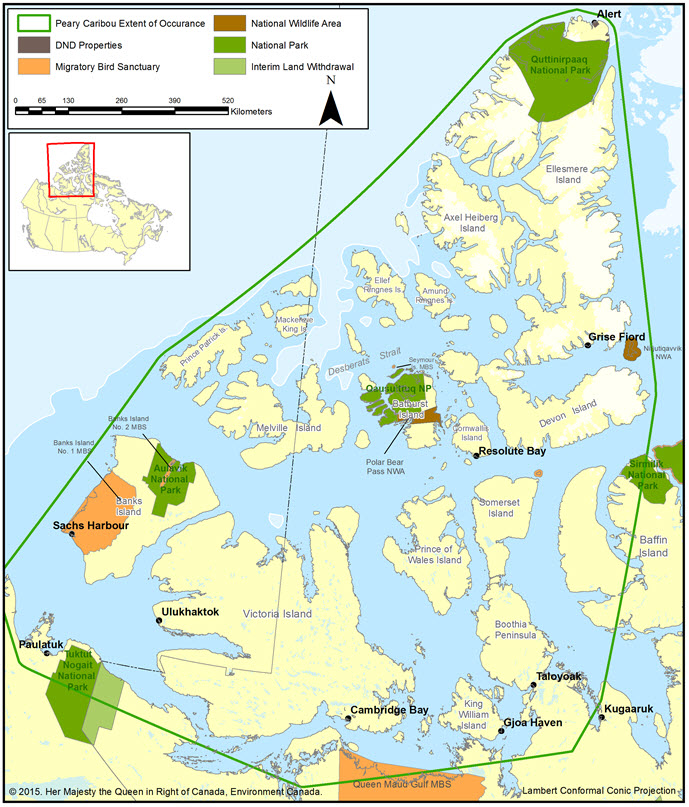
Long description for Figure 7
Map illustrating the locations of national parks and other protected areas, such as Wildlife Management Areas and Migratory Bird Sanctuaries, within the Peary Caribou extent of occurrence.
Members of the COSEWIC Terrestrial Mammal Subcommittee provided careful reviews, advice, and analyses throughout the process of putting this status report together. Special thanks are extended from the author to Agnes Richards, Cheryl Johnson, and Erin Neaves from Environment Canada for freely providing information related to the technical aspects of Peary Caribou recovery, especially the population numbers. Special thanks from the author to Dawn Andrews, Jeff Bowman, and Andrew Murray for making the figures. The author wishes to thank the following individuals for furnishing information and analyses, and for providing thoughtful and thorough reviews at various stages:
| Jurisdiction | Name of contact |
|---|---|
| Canadian Wildlife Service | Siu-ling Han, Lisa Pririe, Bruce MacDonald Vanessa Charlwood, Ruben Boles, Syd Cannings, Donna Bigelow, Dawn Andrews, Cheryl A. Johnson, Agnes Richards, Erin Neave, Pauline Quesnelle |
| Parks Canada | Patrick Nantel, Micheline Manseau, Andrew Maher |
| NWT | Suzanne Carrière, Nic Larter, Joanna Wilson, Claire Singer, Kendra McGreish, Michelle Henderson, Tracy Davison |
| Nunavut | Morgan Anderson, Lynda Orman, Lisa-Marie Leclerc |
| Conservation Data Centre(s) or Natural Heritage Information Centre(s) | Robert Anderson, Jennifer Doubt |
| Wildlife Management Board(s): (WMAC; Nunavut Wildlife Management Board) | Rebecca Jeppesen, Sarah Spencer, Peter Kydd, Deborah Simmons, Walter Bezha, Chris Hopkins, Larry Carpenter |
| COSEWIC Secretariat | Neil Jones, Jenny Wu |
| Other relevant contacts | John Nagy, Nic Larter, Jack Brink, Anne Gunn, Georg Tews, Kim Poole, Keri McFarlane, Deb Jenkins, Nicolas Lecomte |
| COSEWIC Terrestrial Mammals Subcommittee | Jeff Bowman, Nicolas Lecomte, Susan Kutz, Christopher Kyle, Chris Johnson, Martin-Hughes St.-Laurent, Graham Forbes, Hugh Broders, Scott Gilbert, Stephen Petersen, Ian Thompson |
Ahern, F. 2010. NDVI trends 1985 to 2006. Canadian Council of Resource Ministers, Ottawa, Ontario.
title="Link to a resource that is not part of a Government of Canada Web site. For more information, read the Important Terms and Conditions in the footer of this page."
Allen, J. A. 1902. A new caribou from Ellesmereland. Bulletin of the American Museum of Natural History 16:409 412.
Allen, J. A. 1908. The Peary caribou (Rangifer pearyi Allen). Bulletin of the American Museum of Natural History 24:487-504.
AMAP (Arctic Monitoring and Assessment Programme), 2012. Arctic Climate Issues 2011: Changes in Arctic Snow, Water, Ice and Permafrost. SWIPA 2011 Overview Report. [Accessed September 2015].
Anderson, M. 2014. Distribution and abundance of Peary caribou (Rangifer tarandus pearyii) and muskoxen (Ovibos moschatus) on the Bathurst Island Group, May 2013. Nunavut Department of Environment, Wildlife Research Section, NWRT PROJECT 2-13-18, Igloolik, NU. 39 pp.
Anderson, M. and M. C. S. Kingsley. 2015. Distribution and abundance of Peary caribou (Rangifer tarandus pearyii) and muskoxen (Ovibos moschatus) on southern Ellesmere Island, March 2015. Nunavut Department of Environment, Wildlife Research Section, Status Report, Igloolik, NU. 46 pp.
Anderson, R. M. 1946. Catalogue of Canadian recent mammals. Ottawa, National Museum of Canada Bulletin No. 102, Biological Series 31. 238 pp.
Anonymous. 2008. Olokhaktomiut Community Conservation Plan (OCCP). 2008. Prepared by the Community of Holman, Wildlife Management Advisory Council (NWT) and Joint Secretariat.
Bandringa, R. 2010. Inuvialuit Nautchiangit- Relationships between people and plants. Inuvialuit Cultural Resource Centre, 320 pp.
Banfield, A. W. F. 1961. A revision of the reindeer and caribou, genus Rangifer. National Museum of Canada Bulletin No. 177, Biological Series 66. 137 pp.
Berger, T. 1976. Transcripts of the proceedings at the community hearings of the Mackenzie Valley Pipeline Inquiry before the Honourable Mr. Justice Berger, Commissioner. March 4, 1976. Volume 42. Sachs Harbour, N.W.T.
Bernier, J. E. 1910. Report on the Dominion of Canada government expedition to the Arctic islands and Hudson Strait on board C. G. S. Arctic. Ottawa, Ontario, Government Printing Bureau.
Buckland, A. T., K. P. Burnham, J. L. Laake, D. L. Borchers, and L. Thomas. 2001. Introduction to distance sampling: estimating abundance of biological populations. Oxford University Press, New York.
Callaghan, T., L. O. Björn, E. S. Chapin III, Y. Chernov, T. R. Christensen, B. Huntley, R. Ims, M. Johanson, D. Jolly, S. Jonasson, N. Matveyeva, N. Panikov, W. Oechel and G. Shaver. 2005. Chapter 7 Arctic tundra and polar desert ecosystems. Pp Chapter 7 1-172 in Arctic Climate Impact Assessment (ACIA) Scientific Report, Cambridge University Press.
Campbell, M. W. 2013. Population estimate of a declining population of island bound barren-ground caribou (Rangifer tarandus groenlandicus), Southampton Island NU. Research Update to the Department of Environment. Interim Report Department of Environment, Kivalliq Region, Arviat, Nunavut.
Campbell, M. W. 2006. Estimating Peary caribou (Rangifer tarandus pearyi) and muskox (Ovibos moschatus) numbers, composition and distributions on Ellesmere Island, Nunavut. Government of Nunavut, Department of Environment, Status Report No. 19, Iqaluit, Nunavut. 12 pp.
Curry, P.S. 2012. Blood on filter paper for monitoring caribou health: efficacy, community-based collection, and disease ecology in circumpolar herds. Ph.D. Thesis, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada. 308pp.
CWS (Canadian Wildlife Service). 2012. Summary of Discussions at the 2012 Meeting of the Peary Caribou Recovery Strategy Development Group - October 16-18, 2012. Canadian Wildlife Service unpublished report. Yellowknife, NT.
CWS. 2013. Summary of Discussions at the 2013 Meeting of the Peary Caribou Recovery Strategy Development Group - October 22-24, 2013. Canadian Wildlife Service unpublished report. Yellowknife, NT.
CWS. 2015. Summary of Discussions at the 2015 Meeting of the Peary Caribou Recovery Strategy Development Group - February 17-19, 2015. Canadian Wildlife Service unpublished report. Yellowknife, NT.
Case, R. and T. Ellsworth. 1991. Distribution and abundance of muskoxen and Peary caribou on southern Ellesmere Island, NWT, July 1989. Northwest Territories Department of Renewable Resources Manuscript Report No. 41, Yellowknife, NWT.
Christensen, J.H., K. Krishna Kumar, E. Aldrian, S. I. An, I. F.A. Cavalcanti, M. de Castro, W. Dong, P. Goswami, A. Hall, J. K. Kanyanga, A. Kitoh, J. Kossin, N. C. Lau, J. Renwick, D. B. Stephenson, S. P. Xie, S.P., and T. Zhou. 2013. Climate Phenomena and their Relevance for Future Regional Climate Change. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex. V., and P.M. Midgley (eds.). Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
Collings, P., and R. Condon. 1996. Blood on the ice: status, self-Esteem, and ritual injury among Inuit hockey players. Human Organization 55: 253-262.
Condon, R. 1996. The Northern Copper Inuit: a history. Toronto, Ontariio, University of Toronto Press,.
COSEWIC. 2004. COSEWIC assessment and update status report on the Peary caribou Rangifer tarandus pearyi and the barren-ground caribou Rangifer tarandus groenlandicus (Dolphin and Union population) in Canada (www.sararegistry.gc.ca/status/status_e.cfm). Committee on the Status of Endangered Wildlife in Canada (COSEWIC), Ottawa. x + 91 pp.
COSEWIC. 2011. Designatable units for caribou (Rangifer tarandus) in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa, Ontario. 88 pp.
Davison, T., J. Adamczewski, and J. Williams. 2014. Peary caribou and muskox survey on Banks Island, 2014 Summary. Amended unpublished report. Department of Environment and Natural Resources, GNWT, Inuvik Region.
Davison, T., J. Pongracz and J. Williams. 2013. Population survey of Peary caribou (Rangifer tarandus pearyi) and muskoxen (Ovibos moschatus) on Banks Island, Northwest Territories, July 2010. Rangifer 33 Special Issue 21:135-140.
Davison, T. and J. Williams. 2012. Caribou and muskoxen survey on Melville and Prince Patrick Islands, 2012 summary. Department of Environment and Natural Resources, GNWT Inuvik Region, Inuvik, NWT.
Davison, T. and J. Williams. 2013. Peary caribou (Rangifer tarandus pearyi) and muskoxen (Ovibos moschatus) on northwest Victoria Island, Northwest Territories. Rangifer 33 Special Issue 21: 129-134.
Davison, T. and J. Williams. 2015. Caribou and muskox survey on Northwest Victoria Island, April/May 2015. Unpublished preliminary report. Department of Environment and Natural Resources, GNWT, Inuvik Region.
Dewing, K., R. J. Sharp, and T. Muraro. 2006. Exploration history and mineral potential of the central Arctic Zn-Pb district, Nunavut. Arctic 59:415-427.
Dumond, M. 2006. Muskoxen abundance and distribution, and caribou distribution and calving areas on Boothia Peninsula, Nunavut. Nunavut Department of Environment Field Work Summary. Kuglutuk, NU.
Dumond, M. (ed) 2007. Western Kitikmeot caribou workshop. Iqaluit, Nunavut. Government of Nunavut, Department of Environment, Final Wildlife Report No. 19. 47 pp.
Eamer, J., G. Henry, A. Gunn and L. Harding. 2013. Arctic Ecozone+ status and trends assessment-draft September 2013. Environment Canada, Ottawa, Ontario.
Ecological Stratification Working Group. 1996. A national ecological framework for Canada. Ottawa, Ontario, Agriculture and Agri-Food Canada, Research Branch, Centre for Land and Biological Resources Research; and Ottawa: Environment Canada, State of the Environment Directorate, Ecozone Analysis Branch. 119 pp.
Edlund, S.A., and Alt, B.T. 1989. Regional congruence of vegetation and summer climate patterns in the Queen Elizabeth Islands, Northwest Territories, Canada. Arctic 42:3–23.
Eger, J. L., T. P. Birt, A. Gunn and A. J. Baker. 2009. Genetic diversity and history of Peary caribou (Rangifer tarandus) in North America. K. McFarlane, A. Gunn, and C. Strobeck, (eds) Proceedings from the caribou genetics and relationships workshop, March 8-9, 2003, Edmonton, Alberta Wildlife and Economic Development, Government of the Northwest Territories, Yellowknife, Pp 73.
Ekaluktutiak HTA. (2013). Summary of HTA and Public Peary Caribou Federal Recovery Strategy Development Community Technical Meetings - February 26, 2013. Canadian Wildlife Service unpublished report. Cambridge Bay, NU.
Elias, A. 1993. Appendix A Survey of elders’ traditional knowledge of caribou in the Homan area in A. Gunn, The decline of caribou on Northwest Victoria Island: a review. Government of the Northwest Territories Draft File Report, Yellowknife, Northwest Territories. 39-44 pp.
Elkin, B. T. and R. W. Bethke. 1995. Environmental contaminants in caribou in the Northwest Territories, Canada. Science of the total environment 160:307-321.
ENR (Environment and Natural Resources). 2011. State of the Environment Report. Department of Environment and Natural Resources, Yellowknife, NT.
Environment Canada. 2015. Recovery Strategy for Peary Caribou (Rangifer tarandus pearyi) in Canada [In Preparation].
Ferguson, M. A. D. 1987. Status of Peary caribou and muskox populations on Bathurst Island, N.W.T., August 981. Arctic 40:131-137.
Ferguson, M. A. D., A. Idlout and J. Akeeagok. 2001. Conservation of the endangered Peary Caribou by Inuit in Canada’s High Arctic. Presented at 9th North American Caribou Workshop, Kuujjuaq, Quebec, 23-27 April 2001.
Festa-Bianchet, M., J. C. Ray, S. Boutin, S. D. Côté and A. Gunn. 2011. Conservation of caribou (Rangifer tarandus) in Canada: an uncertain future. Canadian Journal of Zoology 89:419-434.
Fischer, C. A. and E. A. Duncan. 1976. Ecological studies of caribou and muskoxen in the Arctic Archipelago and northern Keewatin. Renewable Resources Consulting Services Ltd., Edmonton, Alberta. 194 pp.
Fitzhugh, W. 1976. Environmental factors in the evolution of Dorset culture: A marginal proposal for Hudson Bay. Memoirs of the Society for American Archaeology:139-149.
Flerov, K. K. 1952. Mammals: musk deeer and deer. Fauna of the USSR, v. 1. Moscow, Russia, Academy of Science USSR.
Forde, T. L. 2015. Heightened resolution in wildlife health monitoring and outbreak investigations through the use of molecular and genomic tools. PhD Thesis. Veterinary Medical Sciences, Faculty of Veterinary Medicine, University of Calgary. 210pp.
Friesen, T. M. 2013. The impact of weapon technology on caribou drive system variability in the prehistoric Canadian Arctic. Quaternary International 297:13-23.
Gauthier, L. 1996. Observations of wildlife on Ellesmere and Axel Heiberg islands between June 12-21, 1995. Northwest Territories Department of Renewable Resources Manuscript Report No. 86, Pond Inlet, NT.
Geist, V. 1998. Deer of the world: their evolution, behavior, and ecology. Mechanicsburg, Pennsylvania, Stackpole Books.
Gissing, D. and S. Fleck. 2011. Petition To list the Peary caribou and Dolphin and Union caribou as endangered or threatened. Letter from Government of Northwest Territories and Government of Nunavut to U.S. Fish and Wildlife Service, Arlington, Va, June 3, 2011.
Gjoa Haven HTA. (2013). Summary of HTA Peary Caribou Federal Recovery Strategy Development Community Technical Meeting - February 28, 2013. Canadian Wildlife Service unpublished report. Gjoa Haven, NU.
GNWT (Government of the Northwest Territories). 2014. State of the Environment Report. Department of Environment and Natural Resources, Government of the Northwest Territories. [Accessed September 2015].
Golder Associates Ltd. 2003. Report on Inuit qaujimajatuqangit literature, GAP analysis and workshop results related to the Doris North project, Hope Bay belt, Nunavut. Submitted to Miramar Hope Bay Limited, North Vancouver, B.C., November 13, 2003, Victoria, British Columbia.
Gould, W. A., M. Raynolds and D. A. Walker. 2003. Vegetation, plant biomass, and net primary productivity patterns in the Canadian Arctic. Journal of Geophysical Research 108:8167.
Government of Canada 2015. CanVec - Hydrography data for Canada. (Accessed September, 2015).
Government of Nunavut. 2011. Muskoxen and Peary caribou harvest database summary report December, 2011 version 2.0. Wildlife Research Section, Baffin Region, Department of Environment, Pond Inlet, Nunavut.
Groves, D. J. and E. J. Mallek. 2011. Migratory bird surveys in the Canadian Arctic 2009. U.S. Fish and Wildlife Service. Available from: PDF. Accessed Sept. 27, 2015.
Gunn, A. 1984. A review of research on the effects of human activities on barren-ground caribou of the Beverly and Kaminuriak herds, Northwest Territories. N.W.T. Wildlife Service File Report No. 43, Yellowknife, Northwest Territories. 66 pp.
Gunn, A. 1995. Responses of Arctic ungulates to climate change. Pp 90-106 in D. L. Peterson, and D. R. Johnson (eds), Human ecology and climate change: people and resources in the far north. Washington D.C., Taylor and Francis.
Gunn, A. 1998. Weather, climate and Peary caribou and arctic-island caribou, Pp 1-19 in Conservation Breeding Specialist Group (SSC/IUCN), (ed), Population and habitat viability assessment workshop for the Peary caribou (Rangifer tarandus pearyi)-Briefing BookApple Valley, Minnesota.
Gunn, A. 2005. The decline of caribou on northwest Victoria Island: 1980-93. Department of Resources and Economic Development File report No. 133, Yellowknife, Northwest Territories. 64 pp.
Gunn, A. and J. Ashevak. 1990. Distribution, abundance and history of caribou and muskoxen north and south of the Boothia Isthmus, NWT May-June 1985. Department of Renewable Resources File Report 90, Coppermine, NWT.
Gunn, A. and R. Decker. 1984. Numbers and distribution of Peary caribou and muskoxen in July 1980 on Prince of Wales, Russell and Somerset Islands, Northwest Territories. Department of Renewable Resources File Report No. 38, Yellowknife, Northwest Territories. 56 pp.
Gunn, A. and J. Dragon. 1998. Status of caribou and muskox populations within the Prince of Wales Island-Somerset Island-Boothia Peninsula complex, NWT, July-August 1995. Department of Resources, Wildlife & Economic Development, Government of the Northwest Territories File Report No. 122, Yellowknife, NWT. 45 pp.
Gunn, A. and J. Dragon. 2002. Peary caribou and muskox abundance and distribution on the western Queen Elizabeth Islands, Northwest Territories and Nunavut June-July 1997. Department of Resources, Wildlife and Economic Development. File Report No. 130, Yellowknife, Northwest Territories. 93 pp.
Gunn, A. and B. Fournier. 2000. Caribou herd delimitation and seasonal movements on Victoria Island 1987-1989. Department of Resources, Wildlife and Economic Development File Report No. 125, Yellowknife, Northwest Territories. 104 pp.
Gunn, A. and F. L. Miller. 1980. Responses of Peary caribou cow-calf pairs to helicopter harassment in the Canadian High Arctic. Pp 497-507 in E. Reimers, E. Gaare, and S. Skjenneberg (eds), 2nd International Reindeer/Caribou Symposium. Røros, Norway, Direktoratet for vilt og ferskvannsfisk, Trondheim.
Gunn, A.and K. Poole. 2014. Summary report on survey design and methods to estimate trends in Peary caribou abundance between 1961 and 2013. Contract prepared for Environment Canada, Science and Technology, Landscape Science and Technology Division, Ottawa, Ontario, Canada.
Gunn, A. and T. Skogland. 1997. Responses of caribou and reindeer to global warming. Pp 191 in W. C. Oechl (ed) Global Change and Arctic Terrestial Ecosystems. New York, Springer-Verlag.
Gunn, A., F. L. Miller and D. C. Thomas. 1981. The current status and future of Peary caribou (Rangifer tarandus pearyi) on the Arctic Islands of Canada. Biological Conservation 19:283-296.
Gunn, A., C. Shank and B. McLean. 1991. The history, status and management of muskoxen on Banks Island. Arctic 44:188-195.
Gunn, A., U. S. Seal and P. S. Miller (eds), 1998. Population and habitat viability assessment workshop for the Peary caribou (Rangifer tarandus pearyi). Yellowknife, Northwest Territories. Conservation Breeding Specialist Group (SSC/IUCN), Apple Valley, Minnesota.
Gunn, A., B. Fournier and R. Morrison. 2000a. Seasonal movements and distribution of satellite-collared caribou cows on the Boothia and Simpson Peninsula areas, Northwest Territories, 1991-93. Department of Resources, Wildlife and Economic Development. Manuscript Report No. 126, Yellowknife, Northwest Territories. 77 pp.
Gunn, A., F. L. Miller and J. Nishi. 2000b. Status of endangered and threatened caribou on Canada’s Arctic islands. Rangifer Special Issue 12:39-50.
Gunn, A., F. L. Miller, S. J. Barry and A. Buchan. 2006. A near-total decline in caribou on Prince of Wales, Somerset, and Russell islands, Canadian Arctic. Arctic 59:1-13.
Gunn, A., D. Russell and J. Eamer. 2011. Northern caribou population trends in Canada. Canadian Biodiversity: Ecosystem Status and Trends 2010. Ottawa, Ontario, Canadian Councils of Resource Ministers, Technical Thematic Report No. 10 71 pp.
Hansen, B.B., R. Aanes, I. Herfindal,J. Kohler, B.E. Sæther. 2011. Climate, icing, and wild arctic reindeer: past relationships and future prospects. Ecology 92:1917-1923.
Harding, L. E. 2004. The future of Peary caribou (Rangifer tarandus pearyi) in a changing climate. T. Hooper, (ed) Species at Risk 2004: Pathways to Recovery Conference Proceedings, Victoria, British Columbia, March 2-6, 2004, Ministry of Environment. Available on CD.
Heard, D. C. 1984. Historical and Present Status of Wolves in the Northwest Territories. Department of Renewable Resources. Government of the Northwest Territories. Information Series Report No. 4, Yellowknie, N.W.T. 21 pp.
Hinzman, L.D. et al. 2005. Evidence and implications of recent climate change in northern Alaska and other arctic regions. Climatic Change 72:251-298.
Holand, Ø., K. R. Askim, K. H. Røed, R. B. Weladji, H. Gjøstein and M. Nieminen. 2007. No evidence of inbreeding in a polygynous ungulate: the reindeer (Rangifer tarandus). Biology Letters 3:36-39.
Howse, L. 2008. Late Dorset caribou hunters: zooarchaeology of the Bell site, Victoria Island. Arctic anthropology 45:22-40.
Hughes, J., S. D. Albon, R. J. Irvine and S. Woodin. 2009. Is there a cost of parasites to caribou? Parasitology 136:253-265
Irvine, R. J., Stien, A., Dallas, J.F., Halvorsen, O. & Langvatn, R. 2001. Contrasting regulation of fecundity in two abomasal nematodes of Svalbard reindeer (Rangifer tarandus platyrhynchus). Parasitology 122, 673-681.
IPCC (Intergovernmental Panel on Climate Change). 2013. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker, T.F., D. Qin, G.K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. 1535 pp.
IUCN Standards and Petitions Subcommittee. 2014. Guidelines for Using the IUCN Red List Categories and Criteria. Version 11. Prepared by the Standards and Petitions Subcommittee. Available from PDF. Accessed 27 September 2015.
Iviq HTA. (2013). Summary of HTA and Public Peary Caribou Federal Recovery Strategy Development Community Technical Meetings - February 20 and 21, 2013. Canadian Wildlife Service unpublished report. Grise Fiord, NU.
Jackimchuk, R. D. and D. R. Carruthers. 1980. Caribou and muskoxen on Victoria Island, N.W.T. R.D. Jackimchuk Management Associates Ltd. for Polar Gas Project, Sidney, British Columbia. 93 pp.
Jenkins, D. 2006. Estimating Peary caribou (Rangifer tarandus pearyi) and muskox (Ovibos moschatus) numbers, composition and distributions on Ellesmere Island, Nunavut. Government of Nunavut, Department of Environment, Status Report No. 18, Iqaluit, Nunavut. 8 pp.
Jenkins, D. and N. Lecomte. 2012. All about ice: Peary caribou movements in the Bathurst Islands Complex: Highlights report. Department of Environment, Government of Nunavut.
Jenkins, D., P. Curry, A. Millar, D. Karadag and S. Akeeagok. 2010a. Peary caribou workshop: sharing what we know about caribou. Grise Fiord, summary report. Department of Environment, Government of Nunavut, Iqaluit, Nunavut.
Jenkins, D., P. Curry, A. Millar, D. Karadag and S. Akeeagok. 2010b. Peary caribou workshop: sharing what we know about caribou. Resolute Bay, summary report. Department of Environment, Government of Nunavut, Iqaluit, Nunavut.
Jenkins, D. A., M. Campbell, G. Hope and J. Goor. 2011. Recent trends in abundance of Peary Caribou (Rangifer tarandus pearyi) and Muskoxen (Ovibos moschatus) in the Canadian Arctic Archipelago, Nunavut. Wildlife Research Section, Department of Environment Wildlife Report, No.1, Version 2, Pond Inlet, Nunavut. 184 pp.
Johnson, C.A., E. Neave, A. Richards, S.N. Banks, and P. Quesnelle. In prep. Knowledge assessment to inform the identification of critical habitat for Peary caribou, Rangifer tarandus pearyi, in the Canadian Arctic. Environment Canada, Science and Technology, Ottawa, Ontario, Canada.
Kassam, K.-A. S. 2009. Biocultural diversity and indigenous ways of knowing: human ecology in the Arctic. Calgary, Alberta, University of Calgary Press,.
Kerby, J. T. and E. Post. 2013. Advancing plant phenology and reduced herbivore production in a terrestrial system associated with sea ice decline. Nature Communications. doi: 10.1038/ncomms3514.
Kevan, P. G. 1974. Peary caribou and muskoxen on Banks Island. Arctic 27:256-264.
Klütsch, C. F., M. Micheline and P. J. Wilson. 2012. Phylogeographical analysis of mtDNA data indicates postglacial expansion from multiple glacial refugia in woodland caribou (Rangifer tarandus caribou). PloS one 7:e52661.
Kutz S. J., J. Ducrocq, G. G. Verocai, B. M. Hoar, D. D. Colwell, K. Beckmen, L. Polley, B. T. Elkin, and E. P. Hoberg. 2012. Parasites of ungulates of arctic North America and Greenland: A view of contemporary diversity, ecology, and impact in a world under change. Advances in Parasitology 79:99-252.
Kutz S. J., S. Checkley,G. Verocai, M. Dumond, E. Hoberg, R. Peacock, J. P. Wu, K. Orsel, K. Seegers, A. L. Warren, and A. Abrams. 2013. Invasion, establishment, and range expansion of two parasitic nematodes in the Canadian Arctic. Global Change Biology 19:3254-3262.
Kutz, S. J., E. P. Hobert, P. K. Molnar, A. Dobson and G. G. Verocai. 2014. A walk on the tundra: host-parasite interactions in an extreme environment. International Journal for Parasitology: Parasites and Wildlife 3:198-208.
Kutz S. J., T. Bollinger, M. Branigan, S. Checkley, T.Davison, M. Dumond, B. Elkin, T. Forde, W. Hutchins, A. Niptanatiak, and K. Orsel. 2015. Erysipelothrix rhusiopathiae associated with recent widespread muskox mortalities in the Canadian Arctic. Canadian Veterinary Journal 56:560-563.
Larsen, J.N., O. A. Anisimov, A. Constable, A. B. Hollowed,N. Maynard, P. Prestrud, T D. Prowse, and J.M.R. Stone. 2014. Polar Regions. In: Climate Change 2014: Impacts, Adaptation and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Barros, V.R., Field, C.B., Dokken, D.J., Mastrandrea, M.D., Mach, K.J., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., Girma, B., Kissel, E.S., Levy, A.N., MacCracken, S., Mastrandrea, P.R., White, L.L. (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. p 1567-1612.
Larter, N.C., and J.A. Nagy. 1996. Caribou collection, Banks Island November 1993-February 1994. Department of Renewable Resources, Government of the Northwest Territories, Yellowknife, NT. Manuscript Report No. 89. 54 pp.
Larter, N. C. and J. A. Nagy. 1997. Peary caribou, muskoxen and Banks Island forage: assessing seasonal diet similarities. Rangifer Special Issue 17:9-16.
Larter, N. C. and J. A. Nagy. 2000a. Calf production and overwinter survival estimates for Peary caribou, Rangifer tarandus pearyi, on Banks Island, Northwest Territories. Canadian Field-Naturalist 114:661-670.
Larter, N. C. and J. A. Nagy. 2000b. A comparison of heavy metal levels in the kidneys of High Arctic and mainland caribou populations in the Northwest Territories of Canada. Science of the total environment 246:109-119.
Larter, N. C. and J. A. Nagy. 2001a. Seasonal and annual variability in the quality of important forage plants on Banks Island, Canadian High Arctic. Applied Vegetation Science 4:115-128.
Larter, N. C. and J. A. Nagy. 2001b. Variation between snow conditions at Peary caribou and muskox feeding sites and elsewhere in foraging habitats on Banks Island in the Canadian High Arctic. Arctic, Antarctic, and Alpine Research 33:123-130.
Larter, N.C. and J.A. Nagy. 2003. Upland barren habitat of southern Banks Island excluded from grazing by large herbivores for five years: effects on aboveground standing crop. Manuscript Report No. 148, Inuvik, NWT. 14 pp.
Larter, N. C. and J. A. Nagy. 2004. Seasonal changes in the composition of the diets of Peary caribou and muskoxen on Banks Island. Polar Research 23:131-140.
Lee, A.M., E. M. Bjørkvoll, B. B. Hansen, S. D. Albon,A. Stien, B. E. Sæther, V. Steinar; V. Veiberg, L. E. Loe, and V. Grøtan. 2015. An integrated population model for a long-lived ungulate: more efficient data use with Bayesian methods. Oikos. 124: 806–816.
Leighton, F. A. 2011. Wildlife pathogens and diseases in Canada. Canadian Biodiversity: Ecosystem Status and Trends 2010. Technical Thematic Report No. 7. Ottawa, Ontario, Canadian Councils of Resource Ministers.
Lenart, E. A., R. T. Bowyer, J. V. Hoef and R. W. Ruess. 2002. Climate change and caribou: effects of summer weather on forage. Canadian Journal of Zoology 80: 664-678.
Lim, D. S. S., J. P. Smol, and M. S. V. Douglas. 2008. Recent environmental changes on Banks Island (N.W.T., Canadian Arctic) quantified using fossil diatom assemblages. Journal of Paleolimnology 40: 385-398.
M’Dougall, G. F. 1857. The eventful voyage H. M. Discovery ship Resolute to the Arctic regions in search of Sir John Franklin and the missing crews of H, M. Discovery ships Erebus and Terror, 1852, 1853, 1854. London, UK, Longman, Brown, Green, Longmans, and Roberts.
MacDonald C.R, L.L. Ewing, B.T. Elkin, and A.M. Wiewel. 1996. Regional variation in radionuclide concentrations and radiation doses in caribou (Rangifer tarandus) in the Canadian Arctic; 1992-94. Science in the Total Environment 182:53-73.
MacPherson, A. H. 1959. A preliminary survey of arctic wildlife resources. Canadian Wildlife Service, unpublished report, Ottawa, Ontario.
MacPherson, A. H. 1960. Notes on abundance and distribution of mammals on Banks Island and Victoria Island, N.W.T. in the summer of 1959. Canadian Wildlife Service unpublished report, Ottawa, Ontario.
MacPherson, A. H. 1961. On the abundance and distribution of certain mammals in the Western Arctic Islands in 1958-9. The Arctic Circular XIV:1-17.
Manning, T. H. 1960. The relationship of the Peary and barren ground caribou. Arctic Institute of North America Technical Paper No. 4Manning, T. H. and A. H.
MacPherson. 1958. The mammals of Banks Island. Arctic Institute of North America Technical Paper No. 2:1-74.
Manning, T. H. and A. H. MacPherson. 1961. A biological investigation of Prince of Wales Island, N.W.T. Transactions Royal Canadian Institute 33:116-239.
Manseau, M., L. Dick, N. Lyons, C. St.-Pierre and J. Wood. 2004. Ecological history of Peary caribou and muskox on northern Ellesmere Island, ca. 4300 to present. Research Links 12:1-8.
Manseau, M., L. Dick and N. Lyons. 2005. People, caribou and muskoxen on northern Ellesmere Island: historical interactions and population ecology ca. 4300 BP to present. Winnipeg, Manitoba, Parks Canada, Western Canada Service Centre. 67 pp.
Master, L., D. Faber-Langendoen, R. Bittman, G.A. Hammerson, B. Heidel, J. Nichols, L. Ramsay, and A. Tomaino. 2009. NatureServe conservation status assessments: factors for assessing extinction risk. NatureServe, Arlington, VA. PDF [Accessed September 2015].
Maxwell, J. B. 1981. Climatic regions of the Canadian Arctic Islands. Arctic 34(3): 225-240.
McFarlane, K., A. Gunn and C. Strobeck (eds), 2009. Proceedings from the Caribou Genetics and Relationships Workshop, March 8-9, 2003. Edmonton, Alberta. Department of Natural Resources and Environment, Government of the Northwest Territories Manuscript Report No. 183.
McFarlane, K., F. L. Mille, S. J. Barry and G. A. Wilson. 2014. An enigmatic group of arctic island caribou and the potential implications for conservation of biodiversity. Rangifer 34:73-94.
Meland, M. 2014. Partial migration as a response to ground icing events in a high arctic ungulate. MSc thesis, Norwegian University of Life Sciences.
Meldgaard, J. 1960. Origin and evolution of Eskimo cultures in the Eastern Arctic, February 1960. Canadian Geographical Journal 60:64-75.
Meldgaard, M. 1986. The Greenland caribou - zoogeography, taxonomy, and population dynamics. Meddelelser om Groenland Bioscience 20:88.
Miller, F. L. 1987a. Peary caribou and muskoxen on Prince Patrick Island, Eglinton Island, and Emerald Isle, Northwest Territories, July 1986. Technical Report Sereries No. 29, Canadian Wildlife Service, Western and Northern Region Edmonton, Alberta. 65 pp.
Miller, F. L. 1987b. Peary caribou and muskoxen on Bathurst, Alexander, Marc, Massey, Vanier, Cameron, Helena, Lougheed, and Edmund Wlaker Islands, Northwest Territories, July 1985. Canadian Wildlife Service, Prairie and Northern Region Technical Report Series No. 20, Edmonton, AB.
Miller, F. L. 1988. Peary caribou and muskoxen on Melville and Byam Martin islands, Northwest Territories, July 1987. Technical Report Series No. 37, Canadian Wildlife Service, Prairie & Northern Region, Edmonton, Alberta. 58 pp.
Miller, F. L. 1989. Reevaluation of the status of Peary caribou and muskox populations within the Bathurst Island complex, Northwest Territories, July 1988. Canadian Wildlife Service, Prairie and Northern Range Technical Report No. 78, Edmonton, AB.
Miller, F. L. 1990. Inter-island movements of Peary caribou: a review and appraisement of their ecological importance. Pp 608-632 in C. R. Harington (ed) Canada’s missing dimension - science and history in the Canadian Arctic Islands. Ottawa, Ontario, Canadian Museum of Nature No. 2.
Miller, F. L. 1991. Updated status report on the Peary caribou, Rangifer tarandus pearyi, in Canada. Committee on Status of Endangered Wildlife in Canada (COSEWIC), Ottawa, Ontario. 116 pp.
Miller, F. L. 1992. Peary caribou calving and postcalving periods, Bathurst Island complex, Northwest Territories, 1990. Canadian Wildlife Services, Prairie and Northern Region Technical Report Series No. 151, Edmonton, AB.
Miller, F. L. 1993. Peary caribou calving and postcalving periods, Bathurst Island complex, Northwest Territories, 1991. Canadian Wildlife Service, Prairie and Northern Region Technical Report Series No. 166, Edmonton, AB.
Miller, F. L. 1994. Peary caribou calving and postcalving periods, Bathurst Island complex, Northwest Territories, Canada, 1992. Canadian Wildlife Service Technical Report Series No. 186, Edmonton, Alberta. 99 pp.
Miller, F. L. 1995a. Inter-island water crossings by Peary caribou, south-central Queen Elizabeth Islands. Arctic 48:8-12.
Miller, F. L. 1995b. Peary caribou studies, Bathurst Island complex, Northwest Territories, July-August 1993. Canadian Wildlife Service Technical Report Series No. 35, Edmonton, Alberta. 76 pp.
Miller, F. L. 1997a. Late winter absence of caribou on Prince of Wales, Russell and Somerset islands, Northwest Territories, April-May 1996. Canadian Wildlife Serivce Technical Report Series No. 291, Edmonton, Alberta. 34 pp.
Miller, F. L. 1997b. Peary caribou conservation studies, Bathurst Island complex, Northwest Territories, April-August 1994 and June-July 1995. Canadian Wildlife Service Technical Report Series No. 295,155 pp.
Miller, F. L. 1998. Status of Peary caribou and muskox populations within the Bathurst Island complex, south-central Queen Elizabeth Islands, Northwest Territories, July 1996. Canadian Wildlife Service Technical Report Series No. 317, Ottawa, Ontario. 147 pp.
Miller, F. L. 2002. Multi-island seasonal home range use by two Peary caribou, Canadian High Arctic, 1993-94. Arctic 55:133-142.
Miller, F. L. and S. J. Barry. 2009. Long-term control of Peary caribou numbers by unpredictable, exceptionally severe snow or ice conditions in a non-equilibrium grazing system. Arctic 62:175-189.
Miller, F. L. and A. Gunn. 1978. Inter-island movements of Peary caribou south of Viscount Melville Sound, Northwest Territories. Canadian Field-Naturalist 92:327-331.
Miller, F. L. and A. Gunn. 2003a. Catastrophic die-off of Peary caribou on the Western Queen Elizabeth Islands, Canadian High Arctic. Arctic 56:381-390.
Miller, F. L. and A. Gunn. 2003b. Status, population fluctuations and ecological relationships of Peary caribou on the Queen Elizabeth Islands: Implications for their survival. Rangifer Special Issue 14:213-226.
Miller, F. L. and H. P. L. Kiliaan. 1981. Inter-island movements of Peary caribou in the Prince of Wales Island-Somerset Island-Boothia Peninsula complex, Northwest Territories, June 1980. Canadian Wildlife Service Progress Notes 120:1-7
Miller, F. L., R. H. Russell and A. Gunn. 1975. The recent decline of Peary caribou on Western Queen Elizabeth Islands of Artic Canada. Polarforschung 45:17-21.
Miller, F. L., R. H. Russell and A. Gunn. 1977a. Interisland movements of Peary caribou (Rangifer tarandus pearyi) on western Queen Elizabeth Islands, Arctic Canada. Canadian Journal of Zoology 55:1029-1037.
Miller, F. L., R. H. Russell and A. Gunn. 1977b. Distributions, movements and numbers of Peary caribou and muskoxen on western Queen Elizabeth Islands, Northwest Territories, 1972-74. Canadian Wildlife Service Report Series No. 40, Edmonton, Alberta. 55 pp.
Miller, F. L., E. J. Edmonds and A. Gunn. 1982. Foraging behaviour of Peary caribou in response to springtime snow and ice conditions. Canadian Wildlife Service Occasional Papers No. 48, 41 pp.
Miller, F. L., S. J. Barry and W. A. Calvert. 2005a. Sea-ice crossings by caribou in the south-central Canadian Arctic Archipelago and their ecological importance. Rangifer Special Issue 16:77-88.
Miller, F. L., S. J. Barry and W. A. Calvert. 2005b. Conservation of Peary caribou based on a recalculation of the 1961 aerial survey on the Queen Elizabeth Islands, Arctic Canada. Rangifer Special Issue 16:65-75.
Miller, F. L., S. J. Barry and W. A. Calvert. 2007a. Near-total loss of caribou on south-central Canadian Arctic Islands and the role of seasonal migration in their demise. Arctic 60:23-36.
Miller, F. L., S. J. Barry, W. A. Calvert and K. A. Zittlau. 2007b. Rethinking the basic conservation unit and associated protocol for augmentation of an” endangered” caribou population: An opinion. Rangifer 27:13-24.
Miller, F. L., S. J. Barry and W. A. Calvert. 2007c. The role of seasonal migration in the near-total loss of caribou on south-central Canadian Arctic Islands. Rangifer Special Issue 17:243-245.
Nagy, J. A., N. C. Larter and V. P. Fraser. 1996. Population demography of Peary caribou and muskox on Banks Island, N.W.T. 1982-1992. Rangifer Special Issue 9:213-222.
Nagy, J. A., N. Larter and W. H. Wright. 2006. Population estimates for Peary caribou and muskox on Banks Island, NT, July 2001. Department of Environment and Natural Resources Government of the Northwest Territories Manuscript Report No. 199, Inuvik, Northwest Territories.
Nagy, J. A., N. Larter and W. H. Wright. 2009a. Population estimates for Peary caribou (Minto Inlet herd), Dolphin and Union caribou, and muskox on Northest Victoria Island, NT, July 2001. Department of Environment and Natural Resources Government of the Northwest Territories, Manuscript Report No. 202, Inuvik, Northwest Territories.
Nagy, J. A., A. Gunn and W. H. Wright. 2009b. Population estimates for Peary caribou (Minto Inlet herd), Dolphin and Union caribou, and muskox on Northwest Victoria Island, NT, July 2005 Department of Environment and Natural Resources Manuscript Report No. 203, Inuvik, Northwest Territories.
Nagy, J. A., N. Larter and W. H. Wright. 2009c. Population estimates for Peary caribou (Minto Inlet herd), Dolphin and Untion caribou, and muskox on Northwest Victoria Island NT, July 1998 Department of Environment and Natural Resources Manuscript Report No. 201 Inuvik, Northwest Territories.
Nagy, J. A., P. B. Latour, W. H. Wright and N. Territories. 2009d. Population estimates for Peary caribou and muskox on Banks Island, NT, July 1982: a retrospective analysis. Yellowknife, Northwest Territories, Department of Environment and Natural Resources, Government of the Northwest Territories Manuscript No. 197.
Nagy, J. A., A. Gunn and W. H. Wright. 2009e. Population estimates for Peary caribou and muskox on Banks Island, NT, July 2005 Department of Environment and Natural Resources Manuscript Report No. 200 Yellowknife, Northwest Territories.
Nagy, J.A., A. Gunn and W.H. Wright. 2009f. Population estimates for Peary caribou and muskox on Banks Island, NT, August 1992. Environment and Natural Resources, Government of the Northwest Territories Manuscript Report 198. Yellowknife.
Nagy, J. A., N. Larter and W. H. Wright. 2013a. Population estimates for Peary caribou and muskox on Banks Island, NWT, July 1998 Environment and Natural Resources Manuscript Report No. 224 Inuvik, Northwest Territories.
Nagy, J.A., N. Larter and W.H. Wright. 2013b. Population estimates for Peary caribou and muskox on Banks Island, NWT, July 1994. Environment and Natural Resources, Government of the Northwest Territories Manuscript Report 223. Yellowknife.
Nagy, M. 1999. Aulavik oral history project on Banks Island, NWT: final report. Presented to Parks Canada - Western District, for the Inuvialuit Social Development Program, Inuvik, N.T.
NatureServe. 2014. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. (Accessed: September 2015).
Nellemann, C. and R. D. Cameron. 1998. Cumulative impacts of an evolving oil-field complex on the distribution of calving caribou. Canadian Journal of Zoology 76:1425-1430.
Nishi, J. and L. Buckland. 2000. An aerial survey of caribou on Victoria Island, 15-17 June 1994. Northwest Territories Department of Resources, Wildlife and Economic Development File Report No. 128, Yellowknife, Northwest Territories. 88 pp.
Oberbauer, S. F., S. C. Elmendorf, T. G. Troxler, R. D. Hollister, A. V. Rocha, M. S. Bret-Harte, M. A. Dawes, A. M. Fosaa, G. H. Henry, T. T. Høve, F. C. Jarrad, I. S. Jónsdóttir, K. Klanderud, J. A. Klein, U. Molau, C. Rixen, N. M. Schmidt, G. R. Shaver, R. T. Slider, O. Totland, C. H. Wahren and J. M. Welker. 2013. Phenological responses of tundra plants to background climate variation tested using the International Tundra Experiment (ITEX). Philosophical Transactions of the Royal Society B 368:1624.
Olohaktomiut HTC. (2013). Summary of HTC and Public Peary Caribou Federal Recovery Strategy Development Community Technical Meetings - March 4, 2013. Canadian Wildlife Service unpublished report. Ulukhaktok, NT.
Olson, D. M., E. Dinerstein, E. D. Wikramanayake, N. D. Burgess, G. V. Powell, E. C. Underwood, J. A. D’amico, I. Itoua, H. E. Strand and J. C. Morrison. 2001. Terrestrial Ecoregions of the World: A New Map of Life on Earth A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. Bioscience 51:933-938.
Overland, J. E., and M. Wang. 2013. When will the summer Arctic be nearly sea ice free? Geophysical Research Letters, 40: 2097–2101, doi:10.1002/grl.50316.
Paulatuk HTC. (2013). Summary of HTC and Public Peary Caribou Federal Recovery Strategy Development Community Technical Meeting - March 6, 2013. Canadian Wildlife Service unpublished report. Paulatuk, NT.
Parker, G. R. 1978. The diets of muskoxen and Peary caribou on some islands in the Canadian High Arctic. Canadian Wildlife Service Occasional Papers No. 35, Edmonton, Alberta. 21 pp.
Parker, G. R., and R. K. Ross. 1976. Summer habitat use by muskoxen (Ovibos moschatus) and Peary caribou (Rangifer tarandus pearyi) in the Canadian High Arctic. Polarforschung 46:12-25.
Parks Canada. 2010. State of the Park Report 2010: Aulavik National Park of Canada. Parks Canada, Hull, Quebec. 50 pp.
Parry, W. E. 1821. Journal of a voyage for the discovery of a Northwest passage from the Atlantic to the Pacific; performed in the years 1819-20, in His Majesty’s ships Hecla and Griper, under the Orders of William Edward Parry, with an appendix containing the scientific and other observations, Second Edition. London, UK, John Murray.
Pearce, T., H. Wright, R. Notaina, A. Kudlak, B. Smit, J. Ford, and C. Furgal. 2011. Transmission of environmental knowledge and land skills among Inuit men in Ulukhaktok, Northwest Territories, Canada. Human Ecology 39: 271-288.
Pelletier, B. R. and B. E. Medioli (eds.). 2014. Environmental Atlas of the Beaufort Coastlands. Geological Survey of Canada, Open File 7619. doi:10.4095/294601. 271 pp.
Petersen, S. D., M. Manseau and P. J. Wilson. 2010. Bottlenecks, isolation, and life at the northern range limit: Peary caribou on Ellesmere Island, Canada. Journal of Mammalogy 91:698-711.
Poole, K. G., A. Gunn, B. R. Patterson and M. Dumond. 2010. Sea ice and migration of the Dolphin and Union caribou herd in the Canadian Arctic: an uncertain future. Arctic 63:414-428.
Poole, K. G., A. Gunn, J. Wierzchowsk and M. Anderson. 2015. Peary caribou distribution within the Bathurst Island Complex relative to the boundary proposed for Qausuittuq National Park, Nunavut. Rangifer. Rangifer 35 (Spec. Iss. No. 23): 81-98.
Post, E. and M. C. Forchhammer. 2008. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philosophical Transactions of the Royal Society of London B 363:2369–2375.
Post, E., U. S. Bhatt, C. M. Bitz, J. F. Brodie, T. L. Fulton, M. Hebblewhite, J. Kerby, S. J. Kutz,I. Stirling, I., and D.A. Walker. 2013. Ecological consequences of sea-ice decline. Science 341:519-524.
Prowse, T. D., C. Furgal, F. J. Wrona and J. D. Reist. 2009. Implications of climate change for northern Canada: freshwater, marine, and terrestrial ecosystems. AMBIO: A Journal of the Human Environment 38:282-289.
Reimers, E. 2012. Svalbard reindeer population size and trends in four sub-areas of Edgeøya. Polar Research 31: 11089.
Riewe, R. R. 1973. Final report on a survey of ungulate poplutions on the Bjorne Peninsula, Ellesmere Island. Determination of numbers and distribution and assessment of the effects of seismic activities on the behaviour of these populations. Univeristy of Manitoba, Winnipeg, for Department of Indian and Northern Affairs Ottawa, ON.
Rennert, K. J., G. Roe, J. Putkonen and C. Bitz. 2009. Soil thermal and ecological impacts of rain on snow events in the Circumpolar Arctic. Journal of Climate 22:2302-2315.
Resolute Bay HTO. (2013). Summary of HTO and Public Peary Caribou Federal Recovery Strategy Development Community Technical Meetings - February 19, 2013. Canadian Wildlife Service unpublished report. Resolute Bay, NU.
Riedlinger, D. 2001. Community-based assessments of change: Contributions of Inuvialuit knowledge to understanding climate change in the Canadian Arctic. University of Manitoba, Winnipeg, Manitoba. 139 pp.
Roby, D. D., H. Thing and K. L. Brink. 1984. History, status and taxonomic identity of caribou (Rangifer tarandus) in Northwest Greenland. Arctic 37:23-30.
Russell, R. H., E. J. Edmonds and J. Roland. 1978. Caribou and muskoxen habitat studies. Canadian Wildlife Service Environmental Social Program, Northern Pipelines, ESCOM Report No. A1-26, Edmonton, Alberta. 40 pp.
Russell, D.E., P.H. Whitfield, J. Cai, A. Gunn, R.G. White and K. Poole. 2013. CARMA’s MERRA-based caribou climate database. Rangifer, 33, Special Issue No. 21:145-152.
Sachs Harbour HTC. (2013). Summary of HTC, Elder and Public Peary Caribou Federal Recovery Strategy Development Community Technical Meetings - March 5, 2013. Canadian Wildlife Service unpublished report. Sachs Harbour, NT.
SARC (Species at Risk Committee). 2012. Species status report for Peary Caribou (Rangifer tarandus pearyi) in the Northwest Territories. Species at Risk Committee, Yellowknife, NT.. 137 pp.
SARC. 2013. Species status report for Dolphin and Union caribou (Rangifer tarandus groenlandicus x pearyi) in the Northwest Territories. Northwest Territories, Yellowknife, NT. 106 pp.
Serrouya, R., D. Paetkau, B. McLellan, S. Boutin, M. Campbell and D. Jenkins. 2012. Population size and major valleys explain microsatellite variation better than taxonomic units for caribou in western Canada. Molecular Ecology, 21:2588-2601.
Shank, C. C., P. F. Wilkinson and D. F. Penner. 1978. Diet of Peary caribou, Banks Island, N.W.T. Arctic 31:125-132.
Sharma, S., S. Couturier and S. D. Côté. 2009. Impacts of climate change on the seasonal distribution of migratory caribou. Global Change Biology 15:2549-2562.
Slaney, F. F. and Co. Ltd. 1974. Peary caribou and muskoxen and Panarctic’s seismic operations on Bathurst Island, N.W.T., 1974. Panarctic Oils Ltd., Calgary, Alberta. 156 pp.
Slaney, F. F. and Co. Ltd. 1975. Peary caribou and muskoxen and Panarctic’s seismic operations on Bathurst Island, N.W.T., 1975. Supplemental Report. Panarctic Oils Ltd., Calgary, Alberta. 11 pp
Spence Bay HTO. 2013. Summary of HTO Peary Caribou Federal Recovery Strategy Development Community Technical Meeting - February 27, 2013. Canadian Wildlife Service unpublished report. Taloyoak, NU.
Steele, J. F. 2013. The devil’s in the diversity: Divergent parasite faunas and their impact on body condition in two Greenland caribou populations. Masters of Science. Veterinary Medical Sciences, Department of Ecosystem and Public Health, Faculty of Veterinary Medicine, University of Calgary, Calgary, Alberta, Canada.
Steele J., K. Orsel, C. Cuyler, E. P. Hoberg, N. M. Schmidt, and S. J. Kutz. 2013. Divergent parasite faunas in adjacent populations of West Greenland caribou: Natural and anthropogenic influences on diversity.International Journal for Parasitology: Parasites and Wildlife 2:197-202.
Stefansson, V. 1921. The Friendly Arctic. New York, Macmillan and Co.
Stern, G. A. and A. Gaden. 2015. From Science to Policy in the Western and Central Canadian Arctic: An Integrated Regional Impact Study (IRIS) of Climate Change and Modernization. ArcticNet, Quebec City, 432 pp.
Svoboda, J. 1977. Ecology and primary production of raised beach communities. Pp 185-216 in L. C. Bliss (ed) Truelove Lowland, Devon Island, Canada: a High Arctic Ecosystem. Edmonton, Alberta, University of Alberta Press.
Taylor, A. D. M. 2005. Inuit Qaujimajatuqangit about population changes and ecology of Peary caribou and muskoxen on the High Arctic islands of Nunavut. MA Thesis, Queen’s University, Kingston, Ontario.
Tener, J. S. 1963. Queen Elizabeth Islands game survey, 1961. Canadian Wildlife Service Occasional Papers No. 4, Edmonton, Alberta. 50 pp.
Tews, J., M. A. D. Ferguson and L. Fahrig. 2007a. Modeling density dependence and climatic disturbances in caribou: a case study from the Bathurst Island complex, Canadian High Arctic. Journal of Zoology 272:209-217.
Tews, J., M. A. D. Ferguson and L. Fahrig. 2007b. Potential net effects of climate change on High Arctic Peary caribou: lessons from a spatially explicit simulation model. Ecological Modelling 207:85-98.
Thomas, D. C. 1982. The relationship between fertility and fat reserves of Peary caribou. Canadian Journal of Zoology 60:597-602.
Thomas, D. C. and J. Edmonds. 1983. Rumen contents and habitat selection of Peary caribou in winter, Canadian Arctic Archipelago. Arctic and Alpine Research 15:97-105.
Thomas, D. C. and P. Everson. 1982. Geographic variation in caribou on the Canadian Arctic Islands. Canadian Journal of Zoology 60:2442-2454.
Thomas, D. D. and H. P. L. Kiliaan. 1990. Warble infestations in some Canadian caribou and their significance. Rangifer 10:409-417.
Thomas, D. C. and P. Kroeger. 1980. In vitro digestibilities of plants in rumen fluids of Peary caribou. Arctic 33:757-767.
Thomas, D. C., R. H. Russell, E. Broughton and P. L. Madore. 1976. Investigations of Peary caribou populations on some Canadian Arctic Islands, March 1975. Canadian Wildlife Service Progress Notes No. 64, Ottawa, Ontario. 13 pp.
Thomas, D. C., E. J. Edmonds and H. J. Armbruster. 1999. Range types and their relative use by Peary caribou and muskoxen on Melville Island, NWT. Canadian Wildlife Service Technical Report Series No. 343, Edmonton, Alberta. 146 pp.
Thompson, D. C. and C. A. Fischer. 1980. Numbers and distribution of caribou on the Boothia Peninsula, Northwest Territories. Canadian Field-Naturalist 94:171-174.
Thorpe, N., N. Hakongak, S. Eyegetok and Kitikmeot Elders. 2001. Thunder on the tundra: Inuit Qaujimajatuqangit of the Bathurst Caribou - Tuktu and Nogak Project. Generation Printing, Vancouver, British Columbia. 208 pp.
Tyler, N. J. C. 2010. Climate, snow, ice, crashes, and declines in populations of reindeer and caribou (Rangifer tarandus L.). Ecological Monographs 80:197-219.
Ulukhaktok, Wildlife Management Advisory Council (NWT), and Joint Secretariat. 2008. Olokhaktomiut Community Conservation Plan. Version 18 December 2009.
Urquhart, D. R. 1973. Oil exploration and Banks Island wildlife: a guideline for the preservation of caribou, muskox, and arctic fox populations on Banks Island, N.W.T. Northwest Territories Game Management Division, Yellowknife, Northwest Territories. 105 pp.
Usher, P. J. 1971. The Bankslanders: economy and ecology of a frontier trapping community. Volume 2 - Economy and ecology. Northern Science Research Group, Department of Indian Affairs and Northern Development, Ottawa, Ontario. 169 pp.
Van der Wal, R., N. Madan, S. Van Lieshout, C. Dormann, R. Langvatn, and S. D. Albon. 2000. Trading forage quality for quantity? Plant phenology and patch choice by Svalbard reindeer. Oecologia 123:108–115.
Van der Wal, R., Brooker, R., Cooper, E. and Langvatn, R. 2001. Differential effects of reindeer on high Arctic lichens. Journal of Vegetation Science, 12, 705-710.
Van der Wal, R. 2006. Do herbivores cause habitat degradation or vegetation state transition? Evidence from the tundra. Oikos, 114, 177-186.
Vincent, D. and A. Gunn. 1981. Population increase of muskoxen on Banks Island and implications for competition with Peary caribou. Arctic 34:175-179.
Vistnes, I., C. Nellemann, P. Jordhoy and O. Stoen. 2008. Summer distribution of wild reindeer in relation to human activity and insect stress. Polar Biology 31:1307-1317.
Vors, L. S. and M. S. Boyce. 2009. Global declines of caribou and reindeer. Global Change Biology 15:2626-2633.
Weladji, R.B. and B.C. Forbes. 2002. Disturbance effects of human activities on Rangifer tarandus habitat: implications for life history and population dynamics. Polar Geography 26:171-186.
Wilkinson, P. F., C. C. Shank and D. F. Penner. 1976. Muskox-caribou summer range relations on Banks Island, N.W.T. Journal of Wildlife Management 40:151-162.
Yannic, G. et al. 2013. Genetic diversity in caribou linked to past and future climate change. Nature Climate Change. DOI: 10.1038/NCLIMATE2074.
Youngman, P. M. 1975. Mammals of the Yukon Territory. National Museum of Canada, Publications in Zoology No. 10. 192 pp.
Ytrehus, B., Davidson, R., and Isaksen, K. 2015. Single Causative Factor for Severe Pneumonia Epizootics in Muskoxen? EcoHealth: 1-3. doi:10.1007/s10393-015-1033-4.
Zhang, X., R. Brown, L. Vincent, W. Skinner, Y. Feng and E. Mekis. 2011. Canadian climate trends, 1950-2007. Canadian Biodiversity: Ecosystem Status and Trends 2010, Technical Thematic Report No. 5. Ottawa, Ontario, Canadian Councils of Resource Ministers.
Zittlau, K., J. Nagy, A. Gunn and C. Strobeck. 2009. Do subspecific divisions make good conservation units? Pp 135-145 in K. McFarlane, A. Gunn, and C. Strobeck (eds), Proceedings from the Caribou Genetics and Relationships Workshop, March 8-9, 2003. Edmonton, Alberta, Department of Natural Resources and Environment, Government of the Northwest Territories Manuscript Report No. 183.
Zoltai, S.C., P.N. Boothroyd, and G.W. Scotter. 1981. A natural resource survey of eastern Axel Heiberg Island, Northwest Territories. Parks Canada.
Dr. Lee E. Harding has a BSc in wildlife management and a PhD in wildlife toxicology. He is the principal of SciWrite Environmental Sciences Ltd. and was formerly a senior biologist and science program manager with Environment Canada from 1976 until he took early retirement in 1997. From 1977 to 1980 he managed the Impact Assessment division of the Environmental Protection Service district office in Yellowknife, NWT. During 1972-1976, as an environmental consultant assessing the impact of industrial developments in the Arctic, he studied Barren-ground Caribou and reindeer in the Mackenzie Delta, mountain caribou in British Columbia and Yukon and Peary Caribou on Bathurst, Melville and Little Cornwallis Islands. He first called attention to the possible endangered status of British Columbia’s mountain caribou in a magazine article in 1975. He was the author of the 2004 COSEWIC re-assessment of Peary Caribou.
Collections were not examined for this reassessment.
| Year | Month | Survey Estimate | Error type | Age Class | Area sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1970 | June | 5300 | - | inc. calves | 38804 | 1.8301 | 9699 | Kevan 1974 |
| 1971 | June | 10327 | - | inc. calves | 74333 | 0.9554 | 9866 | Urquhart 1973 |
| 1972 | Sept. | 12098 | - | inc. calves | 74333 | 0.9554 | 11558 | Urquhart 1973 |
| 1982 | July | 9036 ± 2927 | 95% CI | Non-Calf | 70582 | 1.0061 | 9091 | Nagy et al. 2009d |
| 1985 | July | 4931 ± 914 | SE | Non-Calf | 70266 | 1.0064 | 4983 | Nagy et al. 1996 |
| 1987 | June | 4251 ± 663 | SE | Non-Calf | 70266 | 1.0064 | 4296 | Nagy et al. 1996 |
| 1989 | June | 2641 ± 344 | SE | Non-Calf | 70266 | 1.0164 | 2669 | Nagy et al. 1996 |
| 1991 | June - July | 897 ± 151 | SE | Non-Calf | 70266 | 1.0164 | 907 | Nagy et al. 1996 |
| 1992 | August | 1018 ± 270 | 95% CI | Non-Calf | 70583 | 1.0061 | 1024 | Nagy et al. 2009f |
| 1994 | July | 742 ± 132 | 95% CI | Non-Calf | 70583 | 1.0061 | 747 | Nagy et al. 2013b |
| 1998 | July | 451 ± 123 | 95% CI | Non-Calf | 70583 | 1.0061 | 454 | Nagy et al. 2013a |
| 2001 | July | 1142 ± 324 | 95% CI | Non-Calf | 70583 | 1.0061 | 1149 | Nagy et al. 2006 |
| 2005 | July - Aug. | 929 ± 289 | 95% CI | Non-Calf | 70585 | 1.0061 | 935 | Nagy et al. 2009e |
| 2010 | July | 1097 ± 343 | 95% CI | Non-Calf | 70579 | 1.0061 | 1104 | Davison et al. 2013 |
| 2014 | July | 2234 ± 830 | 95% CI | Non-Calf | 70580 | 1.0061 | 2248 | Davison et al. 2014 |
| Year | Month | Survey Estimate | Error type | Age Class | Area sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1980 | August | 4512 ± 988 | SE | inc. calves | 33520 | 1.0668 | 4,814 | Jakimchuk and Carruthers 1980 |
| 1987 | June | 2600 | - | non-calf | 32710 | 1.0932 | 2800 | Gunn 2005; Gunn and Fournier, 2000 |
| 1993 | June | 159 | - | inc calves | 22363 | 1.5990 | 250 | Gunn 2005 |
| 1994 | June | 39 ± 28 | SE | inc calves | 26992 | 1.3248 | 52 | Nishi and Buckland 2000 |
| 1998 | July | 95 ± 60 | 95% CI | non-calf | 24880 | 1.4373 | 137 | Nagy et al. 2009c |
| 2001 | July | 204 ± 103 | 95% CI | non-calf | 20364 | 1.7560 | 358 | Nagy et al. 2009a |
| 2005 | July | 66 ± 61 | 95% CI | non-calf | 20364 | 1.7560 | 116 | Nagy et al. 2009b |
| 2010 | Jul-Aug. | 150 ± 104 | 95% CI | non-calf | 20364 | 1.7560 | 263 | Davison and Williams 2013 |
| 2015 | Apr.-May | 2 | - | Min. num. (non-calf) | 20364 | 1.7560 | 4 | Davison and Williams 2015 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1974 | August | 561 | - | includes calves | 33000 | 0.9723 | 545 | Fischer and Duncan 1976 |
| 1975 | June | 1739 | - | includes calves | 32811 | 0.9779 | 1701 | Fischer and Duncan 1976 |
| 1976 | March | 1120 | - | includes calves | 32941 | 0.9740 | 1091 | Thompson and Fischer 1980 |
| 1985 | June | 4831±543 | SE | adults and 1year olds | 32715 | 0.9808 | 4738 | Gunn and Ashevak 1990, Gunn and Dragon 1998 |
| 1995 | July and August | 3329 | - | adults and 1year olds | 32715 | 0.9808 | 3265 | Gunn and Dragon 1998 |
| 2006 | June | 1 | - | minimum count | 32715 | 0.9808 | 1 | Dumond 2006 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1974 | July | 5437 | - | includes calves | 33770 | 1.0000 | 5437 | Fischer and Duncan 1976 |
| 1975 | June | 3768 | - | includes calves | 33643 | 1.0038 | 3768 | Fischer and Duncan 1976 |
| 1980 | July | 3952±932 | 95% CI | adults and 1year olds | 31686 | 1.0658 | 3952 | Gunn and Decker 1984 |
| 1995 | July | 5 | - | minimum count | 32946 | 1.0251 | 5 | Gunn and Dragon 1998 |
| 1996 | April and May | 0 | - | - | 33340 | 1.0129 | 0 | Miller 1997a |
| 2004 | April | 0 | - | - | 33274 | 1.0150 | 0 | Jenkins et al. 2011 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1974 | June | 245 | - | includes calves | 24786 | 0.9892 | 242 | Fischer and Duncan 1976 |
| 1975 | June | 903 | - | includes calves | 24786 | 0.9892 | 893 | Fischer and Duncan 1976 |
| 1980 | July | 561±300 | 95% CI | adults and 1year olds | 23818 | 1.0294 | 577 | Gunn and Decker 1984 |
| 1995 | July and August | 2 | - | minimum count | 8544 | 2.8695 | 115 | Gunn and Dragon 1998 |
| 1996 | April and May | 2 | - | minimum count | 23818 | 1.0294 | 49 | Miller 1997a |
| 2004 | April | 0 | - | - | 25549 | 0.9596 | 0 | Jenkins et al. 2011 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1975 | June | 159 | - | includes calves | 940 | 1.0251 | 163 | Fischer and Duncan 1976 |
| 1980 | July | 584±90 | 95% CI | adults and 1year olds | 930 | 1.0362 | 605 | Gunn and Decker 1984 |
| 1995 | July | 0 | - | - | 975 | 0.9883 | 0 | Gunn and Dragon 1998 |
| 1996 | April and May | 0 | - | - | 940 | 1.0251 | 2 | Miller 1997a |
| 2004 | April | 0 | - | - | 937 | 1.0284 | 0 | Jenkins et al. 2011 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | August | 300 | - | includes calves | 30232 | 1.0053 | 302 | Tener 1963 |
| 1973 | July | 35 | - | includes calves | 1010 | 30.0867 | 1053 | Riewe 1973 |
| 1995 | June | 25 | - | minimum count (includes calves) | 8101 | 3.7515 | 94 | Gauthier 1996 |
| 2007 | Apr.-May | 2291 (1636-3208) | 95% CI | 10 month olds and adults | 30877 | 0.9842 | 2255 | Jenkins et al. 2011 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1973 | July | 450 | - | includes calves | 19788 | 5.6389 | 2538 | Riewe 1973 |
| 1973 | July | 450 | - | includes calves | 19788 | 5.6389 | 2538 | Riewe 1973 |
| 1989 | July | 89±31 | SE | includes calves | 25050 | 4.4543 | 396 | Case and Ellesworth 1991 |
| 2005 | May | 219 (109-442) | 95% CI | adults and 1year olds | 22243 | 5.0164 | 1099 | Jenkins et al. 2011 |
| 2015 | March | 183 ± 128 | SE | adults and 10-month | 22243 | 5.0164 | 918 | Anderson and Kingsley 2015 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1995 | June | 38 | - | minimum count (includes calves) | 28383 | 3.9313 | 149 | Gauthier 1996 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 2006 | Apr,-May | 802 (531-1207) | 95% CI | adults and 1year olds | 96567 | 1.1555 | 927 | Jenkins et al. 2011 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | August | 12,799 | - | Includes Calves | 41334 | 1.0349 | 13246 | Tener 1963 |
| 1972 | August | 2,551 ± 724 | SE | Includes Calves | 42220 | 1.0132 | 2585 | Miller et al. 1977b; SARC 2012: Jenkins et al. 2011 |
| 1973 | July and August | 3,425 ± 618 | SE | Includes Calves | 42220 | 1.0132 | 3470 | Miller et al. 1977b; SARC 2012: Jenkins et al. 2011 |
| 1974 | July and August | 1679 | - | Includes Calves | 42220 | 1.0132 | 1701 | Miller et al. 1977b; SARC 2012: Jenkins et al. 2011 |
| 1987 | July | 943 ±126 | SE | Includes Calves | 42220 | 1.0132 | 955 | Miller 1988 |
| 1997 | July | 787 ± 97 | SE | No calves seen | 42220 | 1.0132 | 797 | Gunn and Dragon 2002 |
| 2012 | July-August | 2,728 ± 647 | 95% CI | 1+ yr old | 42583 | 1.0045 | 2740 | Davison and Williams 2012 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | July | 2,254 | - | Includes Calves | 15750 | 1.0360 | 2335 | Tener 1963 |
| 1973 | July-August | 807 ± 259 | SE | Includes Calves | 15830 | 1.0307 | 832 | Miller et al. 1977b; SARC 2012: Jenkins et al. 2011 |
| 1974 | July-August | 621 ± 177 | SE | Includes Calves | 15830 | 1.0307 | 640 | Miller et al. 1977b; SARC 2012: Jenkins et al. 2011 |
| 1986 | July | 151 | - | Includes Calves | 15830 | 1.0307 | 156 | Miller 1987 |
| 1997 | June | 84 ± 34 | SE | 1+ yr old | 15830 | 1.0307 | 87 | Gunn and Dragon 2002 |
| 2012 | July-August | 2,708 ± 855 | 95% CI | 1+ yr old | 16090 | 1.0141 | 2746 | Davison and Williams 2012 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | July | 204 | - | Includes Calves | 1427 | 1.0917 | 223 | Tener 1963 |
| 1972 | August | 83 ± 59 | SE | Includes Calves | 1550 | 1.0051 | 83 | Miller et al. 1977b |
| 1973 | August | 12 ± 9 | SE | Includes Calves | 1550 | 1.0051 | 12 | Miller et al. 1977b |
| 1974 | July | 18 ± 10 | SE | Includes Calves | 1550 | 1.0051 | 18 | Miller et al. 1977b |
| 1986 | July | 79 | - | Includes Calves | 1550 | 1.0051 | 79 | Miller 1987 |
| 1997 | July | 0 | SE | - | 1550 | 1.0051 | 0 | Gunn and Dragon 2002 |
| 2012 | July-August | 181 ± 134 | 95% CI | 1+ yr old | 1573 | 0.9902 | 181 | Davison and Williams 2012 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | July | 161 | - | Includes Calves | 650 | 0.8556 | 138 | Tener 1963 |
| 1973 | July | 39 | - | Includes Calves | 550 | 1.0113 | 39 | Miller et al.1977b |
| 1974 | July | 20 | - | Includes Calves | 550 | 1.0113 | 20 | Miller et al. 1977b |
| 1986 | July | 14 (0-49) | 95% CI | Includes Calves | 550 | 1.0113 | 14 | Miller 1987 |
| 1997 | July | 0 | - | - | 550 | 1.0113 | 0 | Gunn and Dragon 2002 |
| 2012 | July-August | 46±78 | 95% CI | 1+ yr old | 570 | 0.9756 | 45 | Davison and Williams 2012 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1972 | August | 86 ± 65 | SE | Includes Calves | 1160 | 1.0189 | 88 | Miller et al. 1977b; Jenkins et al. 2011 |
| 1973 | July | 43 ± 36 | SE | Includes Calves | 1160 | 1.0189 | 44 | Milleret al. 1977b; Jenkinset al. 2011 |
| 1974 | August | 6±4 | SE | Includes Calves | 1160 | 1.0189 | 6 | Miller et al. 1977b; Jenkins et al.2011 |
| 1987 | July | 98 ± 37 | SE | Includes Calves | 1160 | 1.0189 | 100 | Miller 1988; Jenkinset al. 2011 |
| 1997 | July | 0 | - | - | 1160 | 1.0189 | 0 | Gunn and Dragon 2002; Jenkins et al.2011 |
| 2012 | July-August | 119 ± 73 | 95% CI | non-calves | 1158 | 1.0207 | 121 | Davison and Williams 2012 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | August | 2,192 | - | All | - | - | 2192 | Tener 1963 |
| 1973 | April | 3 | - | Minimum count | - | - | 3 | Miller et al. 1977b |
| 1974 | April | 60 | - | All | - | - | 60 | Miller et al. 1977b |
| 1997 | July | 36 ± 22 | SE | 1+ yr old | - | - | 36 | Gunn and Dragon 2002 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | August | 1,630 | - | All | - | - | 1630 | Tener 1963 |
| 1973 | April | 16 | - | All | - | - | 16 | Miller et al. 1977b |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | August | 190 | - | All | - | - | 190 | Tener 1963 |
| 1973 | April | 24 | - | All | - | - | 24 | Miller et al. 1977b |
| 1997 | July | 0 | - | - | - | - | 0 | Gunn and Dragon 2002 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | June | 150 | - | includes calves | 37550 | 1.0323 | 155 | Tener 1963 |
| 2002 | May | 35 | - | min. count (includes calves) | 12316 | 3.1475 | 110 | Jenkins et al.2011 |
| 2008 | April-May | 17 | - | min. count (includes calves) | 39731 | 0.9757 | 17 | Jenkins et al. 2011 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | August | 1325 | - | includes calves | 808 | 1.6458 | 2181 | Tener 1963 |
| 1973 | April | 66 | - | includes calves | 1300 | 1.0230 | 68 | Miller et al. 1977b |
| 1974 | April | 0 | - | - | 1300 | 1.0230 | 0 | Miller et al. 1977b |
| 1985 | July | 0 | - | - | 1300 | 1.0230 | 0 | Miller 1987b |
| 1997 | July | 101±73 | SE | 1+year | 1300 | 1.0230 | 103 | Gunn and Dragon 2002 |
| 2007 | April | 372 (205-672) | 95% CI | 1+year | 1319 | 1.0083 | 375 | Jenkins et al. 2011 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | June and July | 3509 | - | Includes calves | - | - | 3509 | Tener 1963; adjusted by Miller et al. 2005 |
| 1973 | March-April | 990 | - | Includes calves | 19266 | 1.0350 | 1025 | Miller et al. 1977b |
| 1974 | August | 269 | - | Includes calves | 19266 | 1.0350 | 278 | Miller et al. 1977b |
| 1985 | July | 724 (460-987) | 95% CI | Includes calves | 19266 | 1.0350 | 749 | Miller 1987b |
| 1988 | July | 1034±146 | SE | Includes calves | 19266 | 1.0350 | 1070 | Miller 1989 |
| 1990 | July | 871 | - | min. count (includes calves) | 19266 | 1.0350 | 901 | Miller 1992 |
| 1991 | June and July | 949 | - | min. count (includes calves) | 19266 | 1.0350 | 982 | Miller 1993 |
| 1992 | July | 1644 | - | min. count (includes calves) | 19266 | 1.0350 | 1701 | Miller 1994 |
| 1993 | August | 2387 | - | min. count (includes calves) | 19266 | 1.0350 | 2470 | Miller 1995b |
| 1994 | July | 3100 | - | Includes calves | 27550 | 0.7238 | 2244 | Miller 1997b; Miller 1998 |
| 1995 | July | 2200 | - | min. count (includes calves) | 27550 | 0.7238 | 1592 | Miller 1997b; Miller 1998 |
| 1996 | July | 552±108 | SE | Includes calves | 27550 | 0.7238 | 400 | Miller 1998 |
| 1997 | June and July | 78 | - | 1+ year old | 19266 | 1.0350 | 81 | Gunn and Dragon 2002 |
| 2001 | May | 187 (104-330) | 95% CI | 1+ year old | 19644 | 1.0150 | 190 | Jenkins et al. 2011 |
| 2013 | May | 1482±387 | 95% CI | Includes calves | 20200 | 0.9871 | 1463 | Anderson 2014 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | June | 43 | - | Includes calves | 6915 | 1.0338 | 44 | Tener 1963 |
| 1988 | July | 51 (0-107) | 95% CI | Includes calves | 7000 | 1.0213 | 52 | Miller 1989 |
| 2002 | May | 1 | - | Minimum count | 3411 | 2.0958 | 2 | Jenkins et al. 2011 |
| 2013 | May | 2 | - | min. count (includes calves) | 3411 | 2.0958 | 4 | Anderson 2014 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1961 | June | 0 | - | - | 412 | 1.0249 | 0 | Tener 1963 |
| 1973 | Mach and August | 9 | - | Includes calves | 410 | 1.0294 | 9 | Miller et al. 1977b |
| 1974 | March | 12 | - | Includes calves | 410 | 1.0294 | 12 | Miller et al. 1977b |
| 1988 | July | 0 | - | - | 410 | 1.0294 | 0 | Miller 1989 |
| 2002 | May | 0 | - | - | 381 | 1.1077 | 0 | Jenkins et al. 2011 |
| 2013 | May | 1 | - | minimum total count | 381 | 1.1077 | 1 | Anderson 2014 |
| Year | Month | Survey Estimate | Error type | Age Class | Area Sampled (km2) | Scaling factor | Area-corrected population est. | References |
|---|---|---|---|---|---|---|---|---|
| 1973 | April | 0 | - | Includes calves | 220 | 1.5043 | 0 | Miller et al. 1977b |
| 1974 | March | 3 | - | Includes calves | 220 | 1.5043 | 5 | Miller et al.1977b |
| 1985 | July | 0 | - | - | 220 | 1.5043 | 0 | Miller 1987 |
| 1988 | July | 17 (0-42) | 95% CI | Includes calves | 220 | 1.5043 | 26 | Miller 1989 |
| 1990 | July | 34 | - | min. count (includes calves) | 220 | 1.5043 | 51 | Miller 1992 |
| 1991 | June | 22 | - | min. count (includes calves) | 220 | 1.5043 | 33 | Miller 1993 |
| 1992 | June | 46 | - | min. count (includes calves) | 220 | 1.5043 | 69 | Miller 1994 |
| 1995 | June | 49 | - | min. count (includes calves) | 220 | 1.5043 | 74 | Miller 1997b |
| 1997 | July | 0 | - | - | 220 | 1.5043 | 0 | Gunn and Dragon 2002 |
| 2001 | May | 2 | - | min. count (includes calves) | - | - | - | Jenkins et al 2011 |
| 2013 | May | 2 | - | min. count (includes calves) | - | - | - | Anderson 2014 |
| Threat Impact | Threat Impact (descriptions) | Level 1 Threat Impact Counts: high range |
Level 1 Threat Impact Counts: low range |
|---|---|---|---|
| A | Very High | 0 | 0 |
| B | High | 1 | 0 |
| C | Medium | 2 | 1 |
| D | Low | 3 | 5 |
| - | Calculated Overall Threat Impact: | Very High | High |
| Threat number | Threat | Impact (calculated) |
Impact (calculated) Definition |
Scope (next 10 Yrs) |
Severity (10 Yrs or 3 Gen.) |
Timing | Comments |
|---|---|---|---|---|---|---|---|
| 1 | Residential & commercial development | - | Negligible | Negligible (<1%) |
Extreme (71-100%) |
High (Continuing) |
- |
| 1.1 | Housing & urban areas | - | Negligible | Negligible (<1%) |
Extreme (71-100%) |
High (Continuing) |
Scope includes portion of species range that is alienated by human settlements plus a buffer zone for animals displaced by disturbance. |
| 3 | Energy production & mining | D | Low | Restricted - Small (1-30%) |
Slight (1-10%) |
High (Continuing) |
- |
| 3.1 | Oil & gas drilling | D | Low | Restricted - Small (1-30%) |
Slight (1-10%) |
Moderate (Possibly in the short term, < 10 yrs) |
No seismic activity or O&G development at present but an expectation was expressed by participants that this is very likely to increase within the next 10 years. There is some experience of impacts to caribou populations from seismic drilling activities (particularly blasting) in the 1970s, although difficult to tease apart from other sources of decline. Impacts will be higher if high intensity activities occur where most of the population is at that time. |
| 3.2 | Mining & quarrying | D | Low | Small (1-10%) |
Slight (1-10%) |
High (Continuing) |
There is mineral exploration underway, e.g., coal on Foshein Peninsula on Ellesmere Island and on Axel Heiberg Island, staking for coal on Banks Island but these activities ceased when markets fell. A number of old sites on Prince Patrick Island and Victoria Island require clean-up. |
| 4 | Transportation & service corridors | CD | Medium - Low | Restricted - Small (1-30%) |
Serious - Moderate (11-70%) |
High (Continuing) |
- |
| 4.1 | Roads & railroads | D | Low | Small (1-10%) |
Slight (1-10%) |
Moderate (Possibly in the short term, < 10 yrs) |
- |
| 4.2 | Utility & service lines | - | Negligible | Negligible (<1%) |
Negligible (<1%) |
Unknown | - |
| 4.3 | Shipping lanes | CD | Medium - Low | Restricted - Small (1-30%) |
Serious - Moderate (11-70%) |
High (Continuing) |
There is a large range of uncertainty associated with this threat, particularly looking out to the next 10 years. The severity to the overall population will depend on which island crossings are affected and how big are the populations. Shipping channels could open in Prince of Wales complex (PoW-Somerset and Queen Elizabeth-PoW crossings), Bathurst – Cornwallis; less likely Banks-Victoria, Ellesmere complex. For Peary Caribou, island crossings between islands are exceptionally important. In next 10 years develop projects that require shipping could have high impact on available crossings for caribou, as well as cruise ships. Ships & ice breakers come earlier and earlier every year and stay and keep breaking the ice to make it safer for the cruise ships continue to break ice until season is over. Kitikmeot region opening of NW Passage increase transport minerals south. |
| 4.4 | Flight paths | - | Negligible | Negligible (<1%) |
Slight (1-10%) |
Moderate - Low | Regularly scheduled commercial flights |
| 5 | Biological resource use | D | Low | Small (1-10%) | Slight (1-10%) | High (Continuing) |
- |
| 5.1 | Hunting & collecting terrestrial animals | D | Low | Small (1-10%) |
Slight (1-10%) |
High (Continuing) | There are many other threats and circumstances that can interact with this one when it comes to determining severity: climate, management response, and quality of survey information. In terms of scope, a large portion of range not accessible. Severity: there are quotas in place where they are hunted, and not all caribou that encounter a hunter will be killed. If management is doing its job, there should be no decline. Increasing the severity to slight takes into account other factors that may lead to a decline, including unreported mortality and inaccurate knowledge of population status. |
| 6 | Human intrusions & disturbance | D | Low | Restricted (11-30%) | Slight (1-10%) | High (Continuing) |
- |
| 6.1 | Recreational activities | - | Negligible | Negligible (<1%) |
Negligible (<1%) |
High (Continuing) |
- |
| 6.2 | War, civil unrest & military exercises | D | Low | Restricted (11-30%) |
Slight (1-10%) |
High (Continuing) |
Year-round military exercises are increasing in Peary Caribou range; mostly ships and land exercises. Military personnel are travelling long distances, from island to island. We can expect this to increase in the future. |
| 6.3 | Work & other activities | D | Low | Restricted (11-30%) |
Slight (1-10%) |
High (Continuing) |
This relates to activities on land for work: i.e., snowmobiles, helicopters, airplanes. Includes unscheduled flights. More research (e.g., climate change) is taking place and traffic is increasing as a result. |
| 8 | Invasive & other problematic species & genes | CD | Medium - Low | Pervasive (71-100%) | Moderate - Slight (1-30%) | High (Continuing) |
- |
| 8.1 | Invasive non-native/alien species | CD | Medium - Low | Large - Restricted (11-70%) |
Moderate - Slight (1-30%) |
High (Continuing) |
Pathogens include native & non-native species in this category. In terms of the scope, there is much uncertainty as to how much of the population will be affected by pathogens within the next 10 years; probably not over 50% given current evidence and accounting for uncertainty. Need to consider the interaction of a changing climate on pathogen-host relationships that is already being documented. Could have more cycles of parasites with increased temperatures. |
| 8.2 | Problematic native species | D | Low | Pervasive (71-100%) |
Slight (1-10%) |
High (Continuing) |
Muskoxen, wolves, wolverines, and grizzly bears considered in this category, not disease. Scope must be pervasive because all Peary Caribou encounter one or more of these species. The direct impact is uncertain but likely to be low. There is, however, evidence for an inverse relationship between caribou and muskox in some areas, although this is variable throughout the distribution of Peary Caribou. The mechanism for this is unknown, but could be aversion. In some areas, elders say that muskox need to be controlled to keep Peary Caribou populations healthy. |
| 8.3 | Introduced genetic material | - | Unknown | Small (1-10%) |
Unknown | High (Continuing) |
The future depends on climate change and the extent to which Barren-ground and Peary or D&U and Peary meet and hybridize. The only place where there is a real possibility of mixing is on NW Victoria, affecting 10% of the overall population. Results from genetic analyses are showing a lot of Peary Caribou gene flow southward and not a corresponding northward flow of Barren-ground genes; As such, the impact would expect to be felt by D&U and Barren-ground. However, the impact (severity) on Peary Caribou is fundamentally unknown. |
| 9 | Pollution | - | Unknown | Pervasive (71-100%) |
Unknown | High (Continuing) |
- |
| 9.5 | Air-borne pollutants | - | Unknown | Pervasive (71-100%) |
Unknown | High (Continuing) |
There are few sources of contaminants in NU or NWT, but they can be sink holes for southern air-borne pollution. Because of wind currents scope is everywhere. Although lichen does tend to collect air-borne pollution, it is a small part of Peary Caribou diet. It would be more of a concern if arctic willow sucked up pollutants. Studies have shown that Banks Island caribou have lower pollution load than mainland. There is a growing concern around pollinated bromiles (used in fire retardants), which may act like DDT and are showing up in wildlife in NWT; Unknown effects. Air currents bring pollutants from India/China to arctic; volatile contents condense; precipitate out in arctic where they land on snow or ice and go into aquatic systems; lighter fractions that are more volatile are showing up in arctic ecosystems. |
| 11 | Climate change & severe weather | BC | High - Medium | Pervasive (71-100%) |
Serious - Moderate (11-70%) |
High (Continuing) |
- |
| 11 | Habitat shifting & alteration | BC | High - Medium | Pervasive (71-100%) |
Serious - Moderate (11-70%) |
High (Continuing) |
This category includes sea ice loss; sea level rise; habitat changes as result of climate change and severe weather. Negative effects may be countered in some places by positive aspects like vegetation growth and biomass. But because much of this is shrubs, unclear how much Peary Caribou will actually benefit from this enhanced vegetation growth. If changes occur gradually, then there may be more opportunities for adaptation. This category does not include icing events (see 11.4) |
| 11 | Storms & flooding | CD | Medium - Low | Restricted - Small (1-30%) |
Serious - Moderate (11-70%) |
Moderate (Possibly in the short term, < 10 yrs) |
Peary Caribou can move to avoid smaller icing events, but frequent small events or one larger event (which may happen once every 1-2 generations) can have a high impact, as has been the case on at least two major occasions since monitoring of Peary Caribou began in the 60s. Although there is an expectation that the frequency of these events will increase in the next 3 generations due to climate change, there is considerable uncertainty regarding the impact to the overall Peary Caribou that can be expected. |
Classification of Threats adopted from IUCN-CMP, Salafsky et al. (2008).