COSEWIC Assessment and Status Report on the Phantom Orchid Cephalanthera austiniae in Canada - 2014

Photo: © Bruce Bennett.
Long description for Banner
The flowering stem is almost totally white. White sheaths clasp a smooth leafless stock topped by a loose raceme composed of up to 20 white flowers
- Document Information
- COSEWIC Assessment Summary
- COSEWIC Executive Summary
- Technical Summary
- COSEWIC History
- Preface
- Wildlife Species Description and Significance
- Distribution
- Habitat
- Biology
- Population Sizes and Trends
- Threats and Limiting Factors
- 1. Residential and Commercial Development (1.1)
- 2. Recreational Activities (6.1)
- 3. Invasive Non-native Plants (8.1)
- 4. Logging and Wood Harvesting (5.3)
- 5. Problematic Native Species Grazing (8.2)
- 6. Livestock Farming and Ranching (2.3)
- 7. Gathering Terrestrial Plants (5.2)
- 8. Transportation and Service Corridors (4.1)
- 9. Mining and Quarrying (3.2)
- 10. Storms and Flooding (11.4)
- Limiting Factors
- Small Isolated Subpopulations
- Threats to Partner Species
- Number of Locations
- Protection, Status and Ranks
- Acknowledgements and Authorities Contacted
- Information Sources
- Biographical Summary of Report Writers
- Collections Examined
- Figure 1. Phantom Orchid.
- Figure 2. Close-up photograph of Phantom Orchid showing yellow gland at the base of the lower lip. Photo credit: C. Maslovat.
- Figure 3. Phantom Orchid distribution in North America.
- Figure 4. Distribution of Phantom Orchid in British Columbia with reference to the subpopulation numbers. (Table 4)
- Figure 5. Typical habitat of Phantom Orchid with minimal understory vegetation.
- Table 1. Phantom Orchid subpopulations known to occur in Canada. Table Footnoted Table Footnotee
- Table 2. Number of flowering stems over time 1982-2013 Table Footnotef, Table Footnoteg, Table Footnoteh
- Table 3. Number of Phantom Orchid flowering stems in Katherine Tye Ecological Reserve from 1964-1978 (data from Long 1979 in COSEWIC 2000). All observations were made by the landowner at the time (Katherine Tye) who made consistent counts in the same location using the same methodology. Table listing numbers of Phantom Orchid flowering stems recorded in Katherine Tye Ecological Reserve between 1964 and 1978.
- Table 4. Number of sites and primary threats.
COSEWIC
Committee on the Status
of Endangered Wildlife
in Canada

COSEPAC
Comité sur la situation
des espèces en péril
au Cananda
COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2014. COSEWIC assessment and status report on the Phantom Orchid Rhynchospora macrostachya in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 45 pp.
COSEWIC. 2000. COSEWIC assessment and update status report on the phantom orchid Cephalanthera austiniae in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 21 pp.
Klinkenberg, B., and R. Klinkenberg. 2000. Update COSEWIC status report on the phantom orchid Cephalanthera austiniae in Canada, in COSEWIC assessment and update status report on the phantom orchid Cephalanthera austiniae in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-21 pp.
Klinkenberg, B., and R. Klinkenberg. 1992. COSEWIC status report on the phantom orchid Cephalanthera austiniae in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 28 pp.
COSEWIC would like to acknowledge Carrina Maslovat for writing the status report on the Phantom Orchid (Cephalanthera austiniae) in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Bruce Bennett, Co-chair of the COSEWIC Vascular Plants Specialist Subcommittee.
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment Canada
Ottawa, ON
K1A 0H3
Tel.: 819-938-4125
Fax: 819-938-3984
E-mail: COSEWIC/COSEPAC
Website: COSEWIC
Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Céphalanthère d'Austin (Cephalanthera austiniae) au Canada.
Phantom Orchid -- Photo credit: Bruce Bennett.


The Phantom Orchid (Cephalanthera austiniae) is a myco-heterotrophic epiparasite that lacks chlorophyll and derives its food from a three-way partnership with an underground fungus and a tree species. The white flowering stem stands up to 55 cm tall. White sheaths up to 10 cm long clasp a smooth leafless stock topped by up to 20 white flowers. The noticeably vanilla-scented, aromatic flowers have a yellow throat. Fibrous roots branch from a slender rhizome.
The Phantom Orchid is the only North American representative of the genus Cephalanthera. It is found only in the Pacific Northwest, in California, Oregon, Washington, Idaho, and British Columbia (BC). In BC, it occurs only in the extreme southwest, with subpopulations reported from southeast Vancouver Island, Saltspring Island, and the lower Fraser Valley.
In BC, the Phantom Orchid is found in relatively undisturbed old growth, mature and occasionally older second growth forests. It is typically found in coniferous or mixed forests and it requires an intact below-ground (ectomycorrhizal) fungal network. In BC, the Phantom Orchid usually grows in sites with sparse ground cover and thick leaf litter although it is also occasionally found in areas with a high cover of forbs and shrubs. In BC, the Phantom Orchid is found at elevations ranging from 0-550 m, on a range of slopes (0-92%) and the majority of sites are south to southwest-facing. Some sites in BC occur on soils with elevated pH including bedrock with carbonate materials, shell middens, and limestone quarry tailings. Litter from Bigleaf Maple or other trees may play a role in making the soil pH more alkaline than in other sites.
Phantom Orchid does not flower every year and although the flowers indicate the presence of the orchid, they do not reflect the full extent of the below-ground plants. Plants may have periods of dormancy and it is unclear what factors trigger the production of flowering stems. Flowering is staggered over the growing season from early May to mid-July with unconfirmed reports of flowering stems emerging as late as September. The pollinators of the Phantom Orchid in BC are not known. The Phantom Orchid can self-pollinate and other Cephalanthera species are known to have substantial levels of inbreeding, suggesting that they also self-pollinate. Like other orchids, Cephalanthera species produce large numbers of very tiny seeds that are dispersed by wind, generally with short dispersal distances (i.e. less than 6 m). In BC, very few of the flowering stems produce capsules or mature seed.
The Phantom Orchid receives its food via a parasitic connection to mycorrhizal fungi, which are in turn associated with the roots of a tree species. The health of both the tree species and the mycorrhizal fungus is critical to the survival of the orchid. Molecular studies of populations in the United States found the Phantom Orchid was exclusively associated with a fungus of the family Thelephoraceae.
The previous status report (COSEWIC 2000) documented nine subpopulations. Since that time, three sites within two different subpopulations have been extirpated and one subpopulation is presumed extirpated. At two other subpopulations, plants have not been seen since 2000 and 2006. Because these subpopulations have not been consistently surveyed and Phantom Orchids may be dormant at these sites, the subpopulations are presumed extant, but they may also be extirpated. Since the previous status report, nine new Phantom Orchid subpopulations have been found and new sites have also been found within previously known subpopulations. There are currently 20 known Phantom Orchid subpopulations in Canada, with 76 extant sites. In 2013, the number of flowering stems in each subpopulation ranged from 0 (dormant plants) to 76.
Trends in the total number of flowering stems are difficult to determine due to irregular monitoring, periods of dormancy, and annual weather variation, which may influence flowering. Based on 2013 subpopulation estimates during which all but 4 sites were re-measured, the total population included approximately 344 flowering stems. The number of flowering stems represents a slight overestimation of the number of mature individuals because flowering stems that are close to each other may be part of the same individual (this is impossible to determine without excavation, which would kill the plants). However, the total count may also be an underestimation because dormant individuals were not included.
The 2013 population estimate is greater than that reported previously (i.e. 49 flowering stems in the 2000 COSEWIC status report) owing to increased search effort compared to the previous report rather than increasing numbers at previously known sites. The population is severely fragmented because the majority of individuals are found in small and relatively isolated subpopulations, most with low estimated viability.
The primary threat to Phantom Orchid is habitat destruction from the rapid increase of new housing development. The majority of Phantom Orchid sites occur on private property (12 of the 20 subpopulations have some or all sites on private land). Phantom Orchid occurs on private property owned by 22 different landowners and several of the landowners intend to subdivide. Homeowner activities including maintenance and construction of both buildings and gardens, inadvertent mowing and trampling can threaten the Phantom Orchid. The Phantom Orchid is also threatened by forest harvest activities, which can destroy habitat directly and/or by altering hydrology/light conditions, removing host trees, destroying the fungal partner, creating edge effects, and increasing fragmentation. Recreational activities including hiking and dirt-biking can also damage plants and habitat. Other threats include competition from invasive plants, plant collection, overgrazing by deer, impacts associated with small isolated populations, and threats to partner species.
The Phantom Orchid is protected under Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora and is listed as Threatened under Canada's Species at Risk Act (SARA) on Schedule 1. A draft provincial recovery strategy for the Phantom Orchid has been prepared.
In BC, the Phantom Orchid has a provincial status of Imperilled (S2) and is on the BC Conservation Data Centre Red List. In Canada, the Phantom Orchid has a National NatureServe Status of Imperilled (N2). Globally it is ranked Apparently Secure (G4).
Although 12 of the 20 of Phantom Orchid subpopulations occur either solely or partially on private land, ten of the subpopulations are afforded some protection from development by their locality either entirely or partially within provincial parks, regional parks, provincial Crown land, municipal Crown land, BC Parks Ecological Reserve and federally owned Department of National Defence land. One subpopulation on provincial Crown land is currently protected from logging within a Wildlife Habitat Area.
Generation time
- 5-6+ years Content Footnote1
Is there an [observed, inferred, or projected] continuing decline in number of mature individuals?
- Yes
Estimated percent of continuing decline in total number of mature individuals within [5 years or 2 generations] (52-60 years)
- Unknown
Observed percent reduction in total number of mature individuals over the last [10 years, or 3 generations] (78-90 years)
- Unknown
Suspected percent reduction in total number of mature individuals over the next 100 years.
- Unknown
Observedpercent reduction in total number of mature individuals over any [10 years, or 3 generations] period, over a time period including both the past and the future.
- Unknown
Are the causes of the decline clearly reversible and understood and ceased?
- No
Are there extreme fluctuations in number of mature individuals?
- No
Estimated extent of occurrence
- 2018 km2
Index of area of occupancy (IAO, 2 x 2 km² grid values)
- 96 km2
Is the population severely fragmented?
- Yes
Number of locations (see Table 4.)
- 36
Is there a projected continuing decline in extent of occurrence?
Overall EO has increased because new subpopulations have been found since the last report. It is believed these are new detections through increased search effort. The extirpation of some sites, in particular Mount Shannon, would decrease the size of the convex polygon from historical levels if all sites had been known.
- Yes
Is there a projected continuing decline in index of area of occupancy?
The documented IAO has increased due to the discovery of new sites through increased search effort, but actual IAO is inferred to have declined because some sites are known to have been extirpated in the last 3 generations.
- Yes
Is there an inferred continuing decline in number of subpopulations?
- Yes
Is there an inferred continuing decline in number of locations ?
- Yes
Is there an observed/inferred continuing decline in quality of habitat?
- Yes
Are there extreme fluctuations in number of subpopulations?
- No
Are there extreme fluctuations in number of locations ?
- No
Are there extreme fluctuations in extent of occurrence?
- No
Are there extreme fluctuations in index of area of occupancy?
- No
| The number of flowering stems is used as an index of the number of mature individuals, but it represents an underestimate because of the ability of individuals to remain dormant for one or more years. | Observed number flowering stems (2013) used as an index of population size |
|---|---|
| Gowlland Todd | 21 |
| Colwood | 4 |
| Horth Hill (this site has not been surveyed consistently so the plants may still be extant but dormant) | 0 |
| Saltspring Island, Musgrave Landing | 1 |
| Saltspring Island, Mount Tuam | 43 |
| Vedder Mountain, S. foot of | 76 |
| Lindell Beach | 4 |
| Sumas Mountain | 2 |
| McKee Peak | 5 |
| Cultus Lake | 16 |
| Katherine Tye | 14 |
| Mt. Tom | 4 |
| Ryder Lake, 1.5 km SW of | 18 |
| Mt. Tom, Southside Road | 26 |
| Bench Road | 24 |
| Chilliwack, DND | 53 |
| Kent | 30 |
| Sumas River/Vedder Canal | 1 |
| Promontory, Chilliwack | 2 |
| Mission, Westminster Abbey (this site has not been surveyed consistently so the plants may still be extant) | 0 |
| Total | N = 344 |
Probability of extinction in the wild.
- No Quantitative Analysis
- In order of importance: Housing development, logging operations, habitat alteration/homeowner activities, recreational activities, invasive plants, plant collecting, overgrazing, small isolated subpopulations, and threats to partner species.
- Status of outside population(s)?
Apparently Secure in Washington, Vulnerable (S3) in Idaho.
- G4 Apparently Secure
Is immigration known or possible?
- Not known
Would immigrants be adapted to survive in Canada?
- Yes
Is there sufficient habitat for immigrants in Canada?
Habitat in Canada is declining in extent and quality.
- Unknown
Is rescue from outside populations likely?
- No
- COSEWIC: Designated Special Concern in April 1992. Status re-examined and designated Threatened in May 2000. Status re-examined and designated Endangered in November 2014.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
Note: The Canadian Wildlife Service, Environment Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Since the previous status report and COSEWIC assessment, nine new Phantom Orchid subpopulations have been recorded and new sites have also been found within previously known subpopulations. The discovery of these new sites is thought to represent increased search effort rather than an increase in the distribution of the species. The total number of flowering stems is estimated at 344, an increase from the 49 flowering stems documented in the previous status report. The increased number of flowering stems is a direct result of the increased search effort rather than increasing numbers at previously known sites. The discovery of new sites has resulted in a slight increase in the extent of occurrence and area of occupancy. Although the index of area of occupancy (IAO) has increased due to the discovery of new sites, overall the IAO is inferred to have declined because some sites are known to be extirpated. The population remains severely fragmented.
Although the total number of known subpopulations is greater, there have been declining trends. Since the previous status report, two sites within two different subpopulations have been extirpated and one subpopulation is presumed extirpated. At two other subpopulations, plants have not been seen for many years and these subpopulations, although presumed extant, may be extirpated as well. Trends at the remaining sites are difficult to determine.
Scientific name: Cephalanthera austiniae (A. Gray) Heller
Synonyms:
Eburophyton austiniae (A. Gray) Heller
Serapias austiniae(A. Gray) A.A. Eaton
Epipactis austiniae(A. Gray) Wettstein
Cephalanthera oregana Reichenbach
Chloraea austiniae A. Gray
Common name: Phantom Orchid
French common name: Céphalanthère d'Austin
Family: Orchidaceae; Orchid Family
The Phantom Orchid is a myco-heterotrophic Content Footnote2 epiparasite Content Footnote3 that derives its food from a three-way partnership with a fungus and one of several possible tree species (COSEWIC 2000; Phantom Orchid Recovery Team 2008).
Most of the Phantom Orchid occurs underground as a branched creeping rhizome with only the intermittent flowering stems visible above ground (Figure 1). The flowering stem is almost totally white, and stands up to 55 cm tall. White sheaths 2-6(10) cm long clasp a smooth leafless stock topped by a loose raceme composed of up to 20 white flowers. The noticeably vanilla-smelling flowers have a yellow gland at the base (Figure 2). Plants occur in colonies. In some years, the flowering stalks can vary from tall and robust, to short and weak-looking within a single colony (COSEWIC 2000; Douglas et al. 2001).
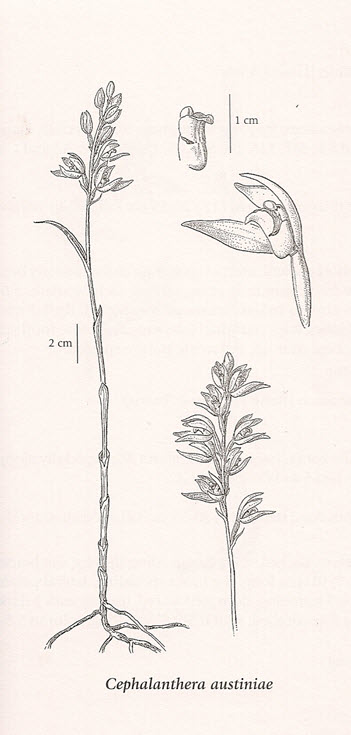
Illustration by Jeanne R. Janish, used with permission by the © University of Washington Press.
Long description for Figure 1
Illustration of the Phantom Orchid showing details of the flowering stem, which may be up to 55 centimetres tall. White sheaths between 2 and 6 centimetres long clasp a smooth leafless stock topped by a loose raceme composed of up to 20 white flowers.

Photo: © C. Maslovat.
Long description for Figure 2
Close-up photo of the white Phantom Orchid flower showing the yellow gland at the base of the lower lip.
In Canada, subpopulations of the Phantom Orchid are geographically isolated from each other with subpopulations on Vancouver Island, Saltspring Island and the Fraser Valley. The Canadian population is at the northern limit of the Phantom Orchid's geographic range (Figure 3).
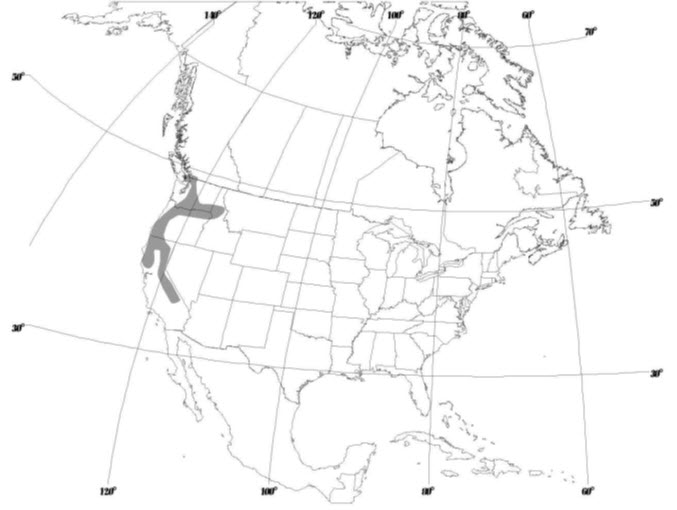
--.
Long description for Figure 3
Map showing the distribution of the Phantom Orchid in the Pacific Northwest of North America, where it is found in four U.S. states (California, Oregon, Washington, and Idaho) and in southwestern British Columbia.
The Phantom Orchid is considered one designatable unit in Canada. Currently, there is no evidence to support dividing the Canadian population into more than one Designatable Unit: 1) genetic distinctiveness of Canadian populations has not been assessed 2) this species is only known from a relatively small region within the Pacific Ecological Area.
- it is the only representative of the genus Cephalanthera in North America and is the only non-photosynthetic member of this genus,
- it occurs in British Columbia (BC) at the northern limit of its geographic range and therefore may be more genetically and ecologically divergent than central populations,
- it may have extended periods of dormancy of unknown length and the mechanisms of its dormancy are poorly understood,
- it is a myco-heterotrophic species deriving its nourishment from fungi that are in turn associated with shrub or tree species. It has scientific importance as the first orchid confirmed through molecular studies to have an association with a specific ectomycorrhizal Content Footnote4 fungus (Taylor and Bruns 1997; Taylor pers. comm. 2003 in Phantom Orchid Recovery Team 2008).
The Phantom Orchid is endemic to the Pacific Northwest and is found in four US states (California, Oregon, Washington, and Idaho). In Canada, it is found only in southwestern BC where it occurs at the northern limit of its range (Figure 3; COSEWIC 2000). It is possible there has been an historical shift northwards as information from the southern part of its range is sparse both historically and presently (Lazar pers. comm. 2014).
In BC, the Phantom Orchid is found in both the Coastal Douglas-fir and the Coastal Western Hemlock biogeoclimatic zones. Occurrences are restricted to the lower Fraser Valley between Mission and Chilliwack, Saltspring Island, and Greater Victoria, including the Saanich Peninsula, on Vancouver Island. The Canadian population represents less than 1% of the global distribution.
The closest documented subpopulations in the United States are found 2 km south of the border near Abbotsford, BC. There are also subpopulations in the San Juan Islands (Orcas Island and Lummi Island) approximately 30-40 km south of the border (Arnett pers. comm. 2013).
The most recent Phantom Orchid status report (COSEWIC 2000) reported nine extant subpopulations, one extirpated subpopulation and two historical (pre-1950) subpopulations known from herbarium records. In the absence of information on demographic or genetic exchange of this species, this report follows the BC Conservation Data Centre and defines subpopulations (referred to by the CDC as an element occurrence) based on a separation of more than 1 km (NatureServe 2004). This definition lumped two of the records from the 2000 report into one subpopulation (Gowlland Todd and Hill Property) and split one record (Mt. Tom Content Footnote5) into four distinct subpopulations (Katherine Tye Ecological Reserve; Mt Thom; Ryder Lake; and Mt. Tom, Southside Road; Table 1).
Since the 2000 report, nine new subpopulations have been recorded (Horth Hill, North Saanich; Saltspring Island, Mount Tuam; Sumas Mountain, Ryder Trail; McKee Peak; Bench Road; Kent; Chilliwack DND; Sumas River/Vedder Canal; and Promontory, Chilliwack) and an additional historical record has been noted for Brentwood Bay Content Footnote6. Numerous newly discovered sites within these subpopulations have also been documented. These new sites are thought to represent increased search effort rather than an increase in the distribution of the species. Since 2001, two sites have been extirpated at two different subpopulations (Mt. Thom, Chilliwack and Gowlland Todd) and one subpopulation (Mt. Shannon) is presumed extirpated because no flowering individuals have been observed since 1993. Four of the subpopulations were not visited in preparation of this status report but are presumed extant (Figure 4).
| Subpop. Number (CDC number) | CDC Subpop. Name | Status of Subpop. | Total Number of Sites within Subpop. | Ownership (Number of Sites shown in brackets) | Total Number of Foral Stems (2013 or latest year recorded) | Most Recent Surveyor | Date Last Surveyed | First Obs. Date |
|---|---|---|---|---|---|---|---|---|
| 1 (1) | Gowlland Todd Provincial Park | Extant (2 private sites extirpated: Quarry Lake and Hill property) |
6 Table Footnoted | Provincial Park (4) Private (2) [2 different landowners] |
21 | Maslovat | 2013 | 1984 |
| 2 (15) | Colwood | Extant | 1 | Private (1) | 4 | Maslovat | 2013 | 2000 |
| 3 (16) | Horth Hill, North Saanich Table Footnotee | Unknown | 1 | Regional Park (1) | 0 | Keogh | 2013 | 2000 |
| 4 (7) | Saltspring Island, Musgrave Landing | Extant | 3 | Provincial Crown (3) | 1 | Annschild | 2013 | 1985 |
| 5 (23) | Saltspring Island, Mount Tuam, 2.4km W of Table Footnotee | Extant | 2 | Private (2) [1 landowner] |
43 | Maslovat | 2013 | 2013 |
| 6 (14) | Vedder Mountain, S foot of | Extant | 10 Table Footnoted | Provincial Crown (6), Unknown (2) Private (2) [2 different landowners] |
76 | Maslovat Ferguson Knopp |
2013 | 1982 |
| 7 (10) | Lindell Beach, W of Cultus Lake | Extant | 2 Table Footnoted | Private (2) [1 landowner] |
4 | Ferguson | 2013 | 1982 |
| 8 (18) | Sumas Mountain, Ryder trail Table Footnotee | Extant | 2 | Provincial Crown (2) | 2 | Maslovat | 2013 | 2003 |
| 9 (12) | McKee Peak, SW slope of Table Footnotee | Extant | 5 | Municipal Crown (1) Private (4) [2 different landowners] |
5 | Maslovat 1 not surveyed |
2013 | 2003 |
| 10 (2) | Cultus Lake Provincial Park, Teapot Hill | Extant | 6 Table Footnoted | Provincial Park (6) | 16 | Maslovat | 2013 | 1987 |
| 11 (3) | Katherine Tye Ecological Reserve, Chilliwack | Extant | 9 Table Footnoted | Ecological Reserve (5) Private (4) [4 different landowners] |
14 | Maslovat Knopp Catling 2 not surveyed |
2013 | 1964 |
| 12 (4) | Mt. Tom, Chilliwack (Mt. Thom 2) |
Extant (1 private site extirpated) |
3 Table Footnoted | Regional Park (2) Private (1) |
4 | Maslovat Catling DeBoer |
2013 | 1966 |
| 13 (19) | Ryder Lake, 1.5 km NW of (Mt. Thom 4) | Extant | 2 | Private (2) [1 landowner] |
18 | Maslovat | 2013 | 2000 |
| 14 (5) | Mt. Tom, Southside Road (Mt. Thom 3, 5, and 6) | Extant | 14 Table Footnoted | Private (14) [6 different landowners] | 26 | Ferguson 2 Not surveyed | 2013 | 1988 |
| 15 | Bench Road Table Footnotee | Extant | 1 | Private (1) | 24 | Catling | 2013 | 2013 |
| 16 (17) | Chilliwack, DND, training site Table Footnotee | Extant | 5 | Federal (5) | 53 | Nernberg | 2013 | 2003 |
| 17 (20) | Kent Table Footnotee | Extant | Unknown | Unknown | 30 | Lomer | 2012 | 2007 |
| 18 (21) | Sumas River/ Vedder Canal, 1.4 km SW of (East Pumptown Quarry) Table Footnotee | Assumed Extant – in a reserve area protected from the quarry | 1 | Private (1) | 1 | Welsted | 2008 | 2008 |
| 19 (22) | Promontory, Chilliwack (Mt. Thom 7) | Assumed Extant | 1 | Private (1) | 2 | Slater | 2008 | 2007 |
| 20 (9) | Mission, Westminster Abbey | Assumed Extant | 1 | Private (1) | 0 | Barsanti | 2006 | 1984 |
| (13) | Mount Shannon, Chilliwack | Presumed Extirpated | - | Private | 0 | Barsanti | 2006 | 1993 |
| - | North Saanich | Extirpated | - | - | - | Roemer | - | 1968 |
| - | Agassiz | Historical (herbarium specimen) | - | Unknown | - | Ross | - | 1926 |
| - | Chilliwack | Historical (herbarium specimen) |
- | Unknown | - | Morkill | - | 1943 |
| - | Brentwood Bay | Historical | - | Unknown | - | - | - | - |

--.
Long description for Figure 4
Map showing occurrences (extant, presumed extant, extirpated, historical) of the Phantom Orchid in British Columbia, where the species is found in both the Coastal Douglas-fir and the Coastal Western Hemlock biogeoclimatic zones. Occurrences are restricted to the lower Fraser Valley between Mission and Chilliwack, Saltspring Island, and Greater Victoria, including the Saanich Peninsula, on Vancouver Island.
The EO of Phantom Orchid is estimated as 2018 km2 based on a minimum convex polygon around extant and unknown status sites. Overall EO has increased because new subpopulations have been found since the last report. The extirpation of some sites, in particular Mount Shannon, would decrease the size of the convex polygon from historical levels if all sites had been known. The index of area of occupancy (IAO) based on a 2 km x 2 km grid including all presumed extant sites is 96 km2.
There has been substantial search effort for Phantom Orchids over the last 10 years. During three years of Phantom Orchid monitoring, the BC Conservation Corps (BCCC) conducted a targeted search in potential sites including Cole Hill, Crown land on Vedder, Elk, and Sumas mountains, Cultus Lake Provincial Park, and private properties in Promontory and Ryder Lake communities (Barsanti and Iredale 2005; Barsanti et al. 2006; Kerr 2007). In 2007 alone, BCCC crews searched 22 ha of land in the Fraser Valley (Kerr 2007). In 2007, 2008, and 2009 the Department of National Defence (DND) searched for the Phantom Orchid in the DND property immediately south of Gowlland Todd (Miskelly pers. comm. 2013) and a portion of another DND property next to the Colwood site was searched in 2013 (Maslovat pers. obs. 2013). In 2013, 11 GPS track logs documented the survey of 18,327 linear metres through suitable habitat in the Fraser Valley (Ferguson 2013).
Other efforts to identify new sites included the development of a public outreach brochure "Have you seen the phantom?" (Fraser Valley Conservancy 2009) to help identify sites on private lands. Predictive mapping based on known Phantom Orchid locations, relevant environmental factors and analysis with habitat prediction models was used to identify potential sites for the Phantom Orchid in the Fraser Valley (Klinkenberg 2009). Some of the predicted habitat localities were surveyed in 2013 by Ferguson (2013) but there has been no funding for targeted searches of the majority of potential habitat (Welstead pers. comm. 2014).
To date, the search effort described above has resulted in the discovery of nine new subpopulations. However, the potential habitat for the Phantom Orchid covers a very large area and it is unlikely that all subpopulations of the species have been found. Search efforts are complicated by the staggered emergence time of the floral stems and potential dormancy in some years. In larger subpopulations, floral stems are generally found every year. In smaller subpopulations, dormancy of even a few stems would result in lack of detection.
In BC, the Phantom Orchid is found in relatively undisturbed old growth, mature and occasionally older (50-60 years) second growth forests. An essential habitat factor is the presence of an intact, below ground ectomycorrihizal (ECM) fungal network and associated host trees. Molecular studies of populations in the United States found the Phantom Orchid was exclusively associated with a fungus of the family Thelephoraceae (Taylor and Bruns 1997). It is typically found in conifer or mixed woodland/conifer forests with Bigleaf Maple (Acer macrophyllum), Douglas-fir (Pseudotsuga menziesii), Paper Birch (Betula papyrifera),Western Hemlock (Tsuga heterophylla) and/or Western Redcedar (Thuja plicata) (COSEWIC 2000; Dunster 2008a). At present, it is not known which tree species are associated with the Phantom Orchid via the fungal network. Both Douglas-fir (Dunster 2008b) and Paper Birch (COSEWIC 2000) have been proposed as possible ECM host trees although neither is present at all sites.
The Phantom Orchid is shade-tolerant and total tree canopy cover ranges from 30-100% (Dunster 2008a). At most sites, the Phantom Orchid usually grows under conifers, with sparse ground cover, thick leaf litter and a well-developed Ah horizon Content Footnote7 (Figure 5) (Dunster 2008a). The Phantom Orchid is occasionally found in areas with a high cover of forbs and shrubs, possibly as relic populations in areas where habitat has changed with increased ingrowth. Phantom Orchid sites have also included horse or cow paddocks, grazed pastures, along deer trails, close to recreational trails, along fence lines and within a deer bed (COSEWIC 2000; Barsanti et al. 2006; Klinkenberg pers. obs. in Phantom Orchid Recovery Team 2008). It is uncertain if these represent relic sites with limited viability.
It has been speculated that light disturbance from grazing or trampling may help to reduce competition for space or soil nutrients, benefiting the Phantom Orchid and/or its fungal host. In one subpopulation with a well-developed understory, the number of flowering stems was higher when grazing occurred and the number of flowering stems declined dramatically after grazers were removed (COSEWIC 2000). However, it is not known whether the previous grazing facilitated the later increase in ground cover by changing soil conditions and altering the vegetation.

Photo: © C. Maslovat.
Long description for Figure 5
Photo illustrating typical Phantom Orchid habitat, with sparse ground cover and thick leaf litter.
The slope of known Phantom Orchid sites ranges from 0-92%. Of the 20 known subpopulations, twelve have at least some sites located on mid-slope benches with deeper soil, often within 500 m of ridges. Sites on Vancouver Island are at low elevations (30-50 m) and both sites on Saltspring occur next to the shoreline (0-20 m). Fraser Valley sites range in elevation from 100-560 m (Dunster 2008a; Phantom Orchid Recovery Strategy 2008). Most Phantom Orchid sites are south to southwest-facing although aspect can range from north, northeast, east, and southeast. Plants emerge earlier at sites with more southerly aspects (Dunster 2008a).
The Phantom Orchid grows in sites that are well-drained, with moist to mesic loamy soil. Predictive mapping, which analyzed relevant environmental factors (e.g. climate, geology, soil and forest cover) associated with known Phantom Orchid localities and used habitat prediction models and GIS, determined there may be a significant correlation between Lonzo Creek soils and Phantom Orchid localities in the Fraser Valley (Klinkenberg 2009). Other soil types that Phantom Orchid uses throughout its range, as determined from soil maps in BC include: Poignant (PT), Cannell (CE) and Columbia (CL) soils. These soils are sandy loams developed in gravelly glaciofluvial deposits, often in association with Abbotsford (AD) silty loam soils and Ryder (RD) silt to sandy loams (Dunster 2008a). On Saltspring Island, the soil type is Musgrave (MG), which is a gravelly sandy loam to gravelly loamy sand colluvium and glacial drift material that is less than 100 cm deep over metamorphosed sedimentary bedrock (Dunster 2008a). In both Phantom Orchid sites on Saltspring Island, the soil is covered with shell midden and organic material (Maslovat pers. obs. 2013). At Gowlland Todd Provincial Park on Vancouver Island, soil type is Ragbark (RJ), rapidly drained gravelly sandy loams have formed on colluvium. At Horth Hill, soil types are Saanichton (SA), well-drained soils on marine sediments such as clay loams, Tagner (TT) silty clay loams, or Dashwood Creek (DD) gravelly loamy sands (Dunster 2008a).
Some species in the genus Cephalanthera found in Europe require calcareous soils, growing on chalk or limestone but others are found on acidic soils (Phantom Orchid Recovery Team 2008). In the Fraser Valley, some but not all Phantom Orchid sites are found on bedrock with some carbonate materials (Klinkenberg 2009). Some but not all sites on Vancouver Island and Saltspring Island appear to have basic soils occurring on limestone quarry tailings, old shell middens, and a former heavily limed compost pile (COSEWIC 2000). Bigleaf Maple, Paper Birch,and to a lesser extent Western Redcedar, can raise the pH of acidic soils and provide calcium for the Phantom Orchid, the fungi and the host tree (Dunster 2008b; Turk et al. 2008). Researchers have identified nitrogen as an important environmental factor for the regulation of mycorrhiza and different ectomycorrhizal species have different responses to increased nitrogen levels (e.g. Avis and Charvat 2005; Parrent and Vilgalys 2007; Kjoller et al. 2012). The impact of nitrogen on the thelephorid fungi associated with Phantom Orchid and the potential impact on flowering are not known (Taylor pers. comm. 2014).
Habitat for the Phantom Orchid is being rapidly lost and remaining habitat is becoming increasingly fragmented. Development in the Fraser Valley is rapid as new subdivisions are built and the owners of two known Phantom Orchid subpopulations may be subdivided and developed in the near future. This trend will likely increase in the future as the human population in the Fraser Valley continues to expand. From 2012-2040, the population of Chilliwack (the municipality with the greatest number of Phantom Orchid sites) is projected to increase by 4.6% per year with a total population increase of 48,000 to a total of 133,000. The total number of housing starts from 2012-2040 is projected to be 25,675, with only 1,703 of these as demolition units (City of Chilliwack 2013). This projected growth will necessarily develop a substantial amount of land. Development in the Abbotsford area will likely proceed at a similar rate.
There are also significant development pressures in the Capital Regional District. Colwood, the municipality with a single subpopulation, is one of the fastest growing regions in British Columbia and the city is poised for significant growth. School district projections expect the student population to expand by 55% between 2012-2022 (City of Colwood 2014). Projected population growth on the Saanich Peninsula is more moderate with an anticipated increase of 17% in Central Saanich from 15,160 in 1996 to 17,700 in 2026, and an increase of 21%in North Saanich from 10,770 to 13,000 over the same period (Capital Regional District 2001). Population growth for the Gulf Islands is projected to rise from 13,870 in 1996 to 19,900 in 2026 (Capital Regional District 2001).
Information on the biology of the Phantom Orchid comes primarily from fieldwork on the species in BC. This is augmented by published information on the species in the United States and related species in other parts of the world.
The Phantom Orchid flowers sporadically throughout its lifetime and although the flowers indicate the presence of the orchid, they do not capture the full extent of below-ground plants (Phantom Orchid Recovery Team 2008), and necessarily underestimate the total number of mature individuals within subpopulations. Prolonged dormancy periods occur in Phantom Orchid subpopulations in California and dormancy does occur in other species of Cephalanthera (Coleman 1995 in Phantom Orchid Recovery Team 2008; Winnal 1999 in Phantom Orchid Recovery Team 2008; Shefferson et al. 2005). Extended dormancy has not been confirmed in BC subpopulations although the number of flowering stems can fluctuate significantly between years (COSEWIC 2000; Barsanti et al. 2006; Phantom Orchid Recovery Team 2008), suggesting that dormancy is also a feature of the Canadian population. In 2013, 15 of 16 Canadian subpopulations surveyed had flowering plants although plants were not found at all of the sites within these subpopulations.
It is not known what factors trigger the production of flowering stems in this species (COSEWIC 2000; BC CDC 2013b). Taylor (pers. comm. 1999 in COSEWIC 2000) believes that flowering stems fluctuate with annual weather variation between years.
The Phantom Orchid produces new flowering stems throughout the growing season (Barsanti et al. 2006). Newly emerged flowering stems have been observed from early May (earliest reported date May 7th) to mid-July (COSEWIC 2000; Barsanti et al. 2006; Osterhold pers. comm. 2013). A flowering herbarium specimen from Agassiz is dated August 1926 and there is an unconfirmed report of flowering stems in September on Teapot Hill (MacDougall pers. comm. 2000 in COSEWIC 2000). Flowering stems appear to last approximately three weeks (COSEWIC 2000; BC CDC 2013b).
The pollinators of the Phantom Orchid in BC are not known (Phantom Orchid Recovery Team 2008). The Phantom Orchid can self-pollinate (Van der Cingel 2001 in Phantom Orchid Recovery Team 2008; Kipping 1971 in Argue 2012) and other Cephalanthera species have substantial levels of inbreeding (e.g. Chung et al. 2004). Pollination of the Phantom Orchid by small, solitary sweat bees (Lasioglossum pillilabre and Lasioglossum nigrescens, Family Halictidae) has been observed in California (Kipping 1971 in Argue 2012), but neither of these species of halictid bee are known to occur in BC (Heron pers. comm. 2014).
It is unknown if the vanilla-like fragrance of the flowers attracts pollinators or if this is a vestigial character (Jersáková et al. 2006 in Phantom Orchid Recovery Team 2008). It is unknown whether insect pollinators receive rewards for visiting flowers or if the Phantom Orchid produces "pseudopollen" in order to mimic the food plants of the pollinating insects (van der Cingel 2001 in Phantom Orchid Recovery Team 2008; Argue 2012). Orchids in the genus Cephalanthera produce fewer pollen grains in a pollinium than other orchid species resulting in much smaller chance of pollination if pollen is robbed (Johnson and Edwards 2000 in Dunster 2008b).
Capsule production and seed dispersal occurs from August through November and the number of capsules produced per floral stem ranges from 1 to 16 (Barsanti and Iredale 2005; Barsanti et al. 2006). Capsules and seed pods are produced infrequently in BC (COSEWIC 2000; BC CDC 2013b). For example, in 2006 a total of 15 subpopulations were surveyed repeatedly over the season and it was found that only 8 of 163 flowering stems produced seed capsules and only 4 of these were confirmed to have dehisced (Barsanti et al. 2006).
Poor seed production may be due to a lack of pollinators associated with occurrences at the northern limits of their range (Klinkenberg 2013). Hand-pollinated plants have been reported to produce capsules and seeds but the vigour and viability of the seeds produced is unknown (Herperger pers. comm. 2004 in Phantom Orchid Recovery Team 2008; BC CDC 2013b).
The longevity of Phantom Orchids is unknown. The related species, Narrow-leaved Helleborine (Cephalanthera longifolia) can live for 22 years (Latr et al. 2008) and some estimates suggest as long as 37 years (Shefferson et al. 2005). Reddoch (pers. comm. 2014) found that Hooker's Orchid (Platanthera hookeri) plants can live as long as 25 years and likely longer, that Case's Ladies'-tresses (Spiranthes casei) plants can live for a decade or so, and that Downy Rattlesnake-plantain (Goodyera pubescens) plants can live for much longer than four decades (Reddoch and Reddoch 2014). However, Catling (pers. comm. 2013) estimates a generation time of 5-6 years based on his knowledge and experience of other terrestrial orchids. This estimate is based on the requirement for the Phantom Orchid to colonize and reproduce in a relatively short period of time as well as the time required to grow to maturity from a tiny seed.
The Phantom Orchid is a myco-heterotrophic epiparasite and receives its food via a parasitic connection to mycorrhizal fungi that is associated with tree species. The health of both the tree species and the mycorrhizal fungi is critical to the survival of the orchid (Taylor pers. comm. cited in Klinkenberg 2013). While studying Helleborine (Epipactis helleborine) Light and MacConaill (2006, 2011) postulated that ecological effect of large trees may be critical to the persistence of mycorrhizal associates of both the trees and the orchids and hence contribute to the extended hypogeal survival of the orchid.
Most of the plant occurs below the ground and the infrequent flowering stems are the only visible part of the plant. The full extent of the underground portion of the plant is not known although it has been described as "roots fleshy, slender, [and] scattered along slender rhizome" (Sheviak and Catling 2002). The full extent of the belowground fungal distribution associated with Phantom Orchid is unknown. Some genets of ectomycorrhizal fungi can extend over 40 metres in association with the root tips of the host plant (Taylor pers. comm. 2014).
Orchids produce large numbers of very tiny seeds that are dispersed by wind (Taylor et al. 2002; Chung et al. 2004). Dispersal experiments with Bamboo-leaved Silver Orchid (Cephalanthera longibracteata) resulted in short distance dispersal with the majority of seeds falling less than 6 m from the maternal plant, a dispersal distance that was consistent with the genetic structure of the subpopulations studied (Chung et al. 2004). However, even if the majority of seeds fall near the parent plant, it does not preclude long-distance seed dispersal.
The small seeds have limited energetic reserves; therefore seedlings must form a mycorrhizal association with fungi for food immediately after the seeds germinate (Taylor et al. 2002; Leake 2004; Bidartondo and Read 2008). In other species of myco-heterotrophic plants, seeds will only germinate when fungi are in the immediate vicinity (Leake 2004).
Molecular studies of fungal associations from 26 individual Phantom Orchids at 11 sites in California, Oregon, and Washington found that the orchids were exclusively associated with fungi identified as a species in the ectomycorrhizal family Thelephoraceae --the black thelephorids (Taylor and Bruns 1997; Taylor pers. comm. 2003 in Phantom Orchid Recovery Team 2008). The thelephorids are found only in intact, mature or maturing forests (Taylor and Bruns 1997; Taylor pers. comm. 2014). The association with this specific fungus has not been confirmed in BC.
It is unknown which tree species is associated with the Phantom Orchid via the fungi in BC, but different authors have suggested Paper Birch (COSEWIC 2000), Bigleaf Maple, Western Redcedar or Douglas-fir (Dunster 2008b) may be essential habitat components. Shrub species could also be an important habitat component although these understory species also vary from site to site and many sites lack shrubs entirely and have very little understory vegetation.
It has been suggested that light grazing by livestock, which creates bare ground for germination and reduces competition for space and soil nutrients, may benefit the Phantom Orchid and/or the fungal host. It has also been suggested that the fungal partner may also respond positively to the increased nutrients associated with livestock grazing (COSEWIC 2000). Alternatively, grazing may negatively impact Phantom Orchid by increasing soil compaction, changing soil nutrient levels and spreading non-native invasive plants. Most ectomycorrhizal fungal species decrease in abundance with increased soil fertility (Taylor pers. comm. 2014). Grazing can also prevent Phantom Orchid from flowering and setting seed: one group of four flowering stems next to a deer trail at one Saltspring Island subpopulation were grazed (COSEWIC 2000). Teeth marks from an herbivore were observed on a broken stem at the Cultus Lake subpopulation. Two sites, which were logged and grazed by goats, no longer support Phantom Orchids (Ferguson 2013).
Aphids have been observed on Phantom Orchid plants in some of the Fraser Valley subpopulations. In 2013, aphids destroyed between 25-40% of the flowers at three sites and are probably substantially reducing the reproductive capability of the plants (Catling pers. comm. 2013).
Field verification of eleven subpopulations was made by the report writer between May 15 and June 15, 2013; when the majority of sites would have had flowering stems. Some sites were visited multiple times and the floral stems recounted. At each site, suitable habitat was surveyed next to known sites and a habitat description including aspect, slope, associated species, elevation, etc. was recorded. A number of other surveyors (Annschild, Catling, Keogh, and Ferguson; Table 1) supplemented these observations at other subpopulations. A new subpopulation and many new sites within known subpopulations were identified (Catling pers. comm. 2013; Ferguson 2013; Maslovat pers. obs. 2013). The most recent BC Conservation Data Centre records were included for the four subpopulations and five sites within surveyed subpopulations that were not revisited in 2013 (BC CDC 2013a) (Table 1).
Population size is based on the total number of flowering stems counted at each site. If more than one flowering stem appeared to come from the same base, this was counted as a clump and assumed to be part of the same mature individual. However, 98% of flowering stems were farther than 15 cm apart and were therefore considered to be separate individuals: surveyors other than the report writer did not make this distinction but counted each flowering stem.
As noted in other reports (e.g. COSEWIC 2000; Barsanti et al. 2006) the staggered emergence of flowering stems means that a single survey date in a given year may not give an accurate count of the total number of mature individuals in a given year. The numbers of flowering stems at each locality changes over time as early emerging stems fade and new stems emerge. Each visit provides only a snapshot in time of the subpopulation and cannot be considered a thorough estimate of the total number of flowering stems (Barsanti et al. 2006; Maslovat pers. obs. 2013). Furthermore, dormant plants do not produce flowering stems leading to a further underestimation of population size.
There are currently 20 known Phantom Orchid subpopulations in Canada (Table 1), with a total of 76 sites. A site is defined as a distinct group of plants that is likely to have genetic or demographic exchange with nearby groups of plants and therefore cannot be a distinct subpopulation. Each subpopulation can have either just one site or multiple sites, often with different land tenures.
The number of flowering stems counted in each subpopulation in 2013 ranges from 0 to 76 with half the subpopulations having fewer than 10 mature individuals (Table 1). Unless the number of mature individuals is greatly underestimated by the number of flowering stems, it is unlikely that most sites contain enough individuals to maintain viable subpopulations. The disappearance of one subpopulation (Mt. Shannon) and three sites at two different subpopulations (Mt. Thom, Chilliwack, and Gowlland Todd), all with small numbers of observed flowering stems, suggests some of these small subpopulations are not viable. The subpopulations are likely to be genetically isolated from other habitat patches, assuming that average seed dispersal distances are much smaller (<6 m) than the distance between subpopulations (>1 km). Therefore, the total population is considered severely fragmented (IUCN 2013). The majority of the population is found in small and relatively isolated subpopulations, and the viability of the subpopulations and the ability to recolonized new sites is reduced or eliminated by the increasing human fragmentation of the habitat, which is complicated by the need to support the orchid, its fungal associate and host tree (See Rescue Effect).
The 2013 population estimate is greater than that previously reported (i.e. 49 flowering stems in the 2000 COSEWIC status report) owing to an increased search effort. A total of 311 flowering stems were counted in 16 subpopulations during 2013 surveys. Four subpopulations which were not counted in 2013 are presumed extant: one had 30 flowering stems in 2012 (Kent); one had 1 flowering stem in 2008 (Sumas River/Vedder Canal); one had 2 flowering stems in 2008 (Promontory, Chilliwack); and one had 0 stems in 2006 (Mission, Westminster Abbey). Assuming there has been no decline in numbers at the four sites not revisited in 2013, the total number of flowering stems is estimated as 344 (Table 1). Plants have not been observed at two of the sites (Horth Hill and Mission Hill) since 2005 and 2000 respectively. These sites have not been consistently surveyed and therefore may have dormant plants and are presumed extant.
The number of mature individuals is likely slightly less than this because flowering stems that emerge close to each other may be part of the same individual (although this is impossible to determine without excavation, which would likely kill the plants). However, the presence of dormant individuals would result in a larger number of mature individuals than those counted.
Interpreting trends in subpopulation sizes based on the number of flowering stems counted from year to year is complicated. Different observers used different counting techniques (Table 2); data from 2006 and 2007 were compiled from multiple surveys to each site to catch the full range of flowering stems (Barsanti et al. 2006; Kerr 2007). Due to the staggered emergence of flowering stems, these surveys are associated with higher totals than single surveys conducted in other years; a total of 361 flowering stems was counted with multiple site visits in 2007 compared to 205 at the same sites in 2013 with a single survey.
| Site | 82 | 84 | 85 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 00 | 01 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 11 | 12 | 13Table Footnotef |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gowlland Todd (including quarry site) | - | - | - | 0 | - | - | - | - | - | 35 | 17 | 10 | 12 | - | 11 | - | 16 | 12 | - | - | 7 | 7 | 25 | 20 | - | 4 | 14 | 7 | 21 (10) |
| Hill Property (part of 1.) | - | 4 | - | - | - | - | 0 | 0 | - | - | - | - | - | - | - | - | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - |
| 2. Colwood | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 8 | - | - | - | 0 | 1 | - | - | - | - | - | 4 (4) |
| 3. Horth Hill | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4 | 4 | - | 3 | 4 | 0 | 0 | - | - | - | - | 0 |
| Site | 82 | 84 | 85 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 00 | 01 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 11 | 12 | 13 Table Footnotef |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4. Musgrave Landing | - | - | 1 | - | - | 3 | 8 | - | - | - | - | - | - | - | - | - | 0 | 19 | - | - | 1 | - | - | - | - | - | - | - | 1 (1) |
| 5. Saltspring Island, Mount Tuam | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 43 |
| Site | 82 | 84 | 85 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 00 | 01 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 11 | 12 | 13 Table Footnotef |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6. Vedder, S. foot of Table Footnoteg | 6? | - | - | - | 11? | - | - | - | - | - | - | - | 5? | - | - | - | - | - | - | - | 2 | 6 | 27 | 127 | 4 | - | - | - | 76 |
| 7. Lindell Beach | 6 | - | - | - | 11 | - | - | - | - | - | - | - | 5 | - | - | - | - | - | - | - | - | - | - | 40 | - | - | - | - | 4 (0?) |
| 8. Sumas Mtn | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 6 | 5 | - | - | - | - | 2 |
| 9. McKee Peak | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4 | - | 0 | - | 31 | - | 5 | 2 | - | 5 |
| 10. Cultus Lake | - | - | - | - | 25 | 29 | 3 | 12 | 15 | Table Footnoteh | - | - | - | - | - | - | 7 | - | - | - | - | - | 34 | 52 | - | - | 8 | - | 16 (14?) |
| 11. Katherine Tye | - | - | - | - | - | 15 | 3 | 9 | 10 | 14 | - | - | 21 | 2 | 5 | - | 1 | 5 | - | - | - | 3 | 11 | 10 | - | - | - | - | 14 (4) |
| 12. Mt. Tom, Chilliwack | - | - | - | - | - | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | 0 | 0 | 3 | - | - | - | - | 4 (0) |
| 13. Ryder Lake | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3 | - | - | - | 6 | 27 | 21 | - | - | - | - | 18 (18) |
| 14. Mt. Tom, Southside Rd. | - | - | - | - | - | 7 | 7 | 5 | Table Footnoteh | Table Footnoteh | - | 3 | 5 | Table Footnoteh | - | Table Footnoteh | 9 | 6 | 1 | - | 12 | 6 | 23 | 49 | 11 | - | - | - | 26 (?) |
| 15. Bench Rd. | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 24 |
| 16. Chilliwack DND, training | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 28 | 6 | 4 | 13 | 74 | - | - | - | - | 53 |
| 17. Kent | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 30 | - |
| 18. Sumas River/Vedder Canal | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | - | - |
| 19.Promontory, Chilliwack | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 2 | - | - | - | - |
| 20. Mission, Westminster | - | 15 | - | - | - | - | 0 | 0 | - | - | - | - | - | - | - | - | 0 | 1 | - | - | - | 0 | 0 | - | - | - | - | - | - |
| 21. Mt. Shannon | - | - | - | - | - | - | - | - | - | - | 3 | - | - | - | - | - | - | 0 | 0 | - | - | 0 | 0 | - | - | - | - | - | - |
| All Sites | 82 | 84 | 85 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 00 | 01 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 11 | 12 | 13Table Footnotef |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 6 | 19 | 1 | 0 | 36 | 55 | 21 | 26 | 25 | 49 | 20 | 13 | 43 | 2 | 16 | Table Footnoteh | 33 | 58 | 5 | 32 | 31 | 37 | 167 | 434 | 18 | 9 | 24 | 37 | 311 (51) |
Furthermore, comparing annual counts is complicated by the presence of multiple sites within each subpopulation, not all of which are counted by a given surveyor. Previous status reports do not list the number of sites, further complicating comparisons. Comparing annual counts is further complicated because the flowering stems emerge in different areas several metres apart from year to year, suggesting they belong to different genetic individuals (Erlandson pers. comm. 2013; Welstead pers. comm. 2014), making them difficult to find especially in areas with dense undergrowth. Annual weather variation can also influence the number of flowering stems (COSEWIC 2000). Possible long periods of dormancy complicate the picture because plants may be present even if flowering does not occur; fluctuations in the number of flowering stems counted may represent fluctuations in flowering events rather than fluctuation in the number of plants present because flowering each year is unlikely to be essential for survival.
Some degree of natural fluctuation may be the norm for this species (Taylor pers. comm. 1999 in COSEWIC 2000). At the site most consistently studied by a single observer with a single count per season (Katherine Tye Ecological Reserve), the number of flowering stems observed fluctuated between 0 and over 100+ flowering stems from 1964 to 1978 (Table 3). There is currently no evidence of extreme fluctuations (greater than one order of magnitude in population size) in Phantom Orchid subpopulations.
| Date | Number of Flowering Stems |
|---|---|
| 1964 | 8 |
| 1965 | 35 |
| 1966 | 75 |
| 1967 | 100+ |
| 1968 | 50 |
| 1969 | 85 |
| 1970 | 60 |
| 1971 | 15 |
| 1972 | 7 |
| 1973 | 0 |
| 1974 | 9 |
| 1975 | 12 |
| 1976 | 22 |
| 1977 | 18 |
| 1978 | 11 |
Although the current total of 344 flowering stems is much increased from the 49 flowering stems documented in the previous status report (COSEWIC 2000), this represents an increase in search effort rather than increasing numbers at previously known sites.
Since the previous status report, one subpopulation (Mt. Shannon) is presumed extirpated with no plants observed since 1993, and severely degraded habitat at this site. There are now extirpated sites within two of the subpopulations listed in the previous status report (Quarry Lake and Hill property within Gowlland Todd and 25 A within Mt. Thom, Chilliwack). No plants have been observed at Horth Hill since 2005 or at Mission, Westminster Abbey since 2000 and these subpopulations may be extirpated. Using sites that correspond with the previous status report (Table 2), the total number of flowering stems has decreased from 64 in 1999/2000 to 51 in 2013. However, further years of monitoring are required to know whether this is a reflection of seasonal weather variation, differences in survey techniques or a declining trend.
Rescue for the Phantom Orchid is expected to be unlikely. Although there are sites known just south of the border (see Canadian Range), the seeds are small and may rarely be dispersed long distances by wind; studies on related species have shown that short dispersal distances are predominant. Suitable habitat, which must include the associated fungus and associated host tree, is isolated, occurring on islands separated by large distances (e.g. Vancouver Island and Saltspring Island) or by large expanses of altered habitat destroyed by urban development (Lower Mainland).
A threat calculator for Phantom Orchid is included in Appendix A. The numbers after each threat correspond to their designation in the threat calculator and correspond to those in Salafsky et al. 2008).
Residential development continues to be the highest threat to the Phantom Orchid. More details on the development pressures can be found in the Habitat Trends section. In the Chilliwack and Abbotsford area, new large-scale subdivisions, which drastically alter the topography and substrate of a site, are being constructed at a rapid rate in potential Phantom Orchid habitat. There has been no comprehensive inventory to detect new subpopulations in many of these areas (Phantom Orchid Recovery Team 2008; Welstead pers. comm. 2014). Private lands with known Phantom Orchid sites in the Fraser Valley continue to be subdivided and developed (Ferguson 2013; Kenny pers. comm. 2013). At one Phantom Orchid subpopulation an application was made in 2011 for zoning to be changed to allow development of 128 residences (Fraser Valley Regional District 2008). On Saltspring Island, one subpopulation has been reduced in size as surrounding development constricted the subpopulation to a small reserve established for Phantom Orchid protection (Annschild pers. comm. 2013). Development on Vancouver Island may be responsible for the extirpation of at least one known site (private site in Gowlland Todd subpopulation).
A number of remaining Phantom Orchid sites occur in suburban areas, in some cases quite close to residences and gardens. It is unlikely that the Phantom Orchid established in these sites after construction and their presence suggests the plants survived construction because there was minimal disturbance to the substrate, below-ground fungus, host trees and Phantom Orchid rhizomes. In sites where the Phantom Orchid is found close to homes, it may be threatened by maintenance and construction activities including tree removal, piling of debris (e.g. brush, leaves, lawn clippings), garden maintenance (e.g. mowing, applications of herbicide or fertilizer), and construction activities associated with gardens, outbuildings, residences or septic fields. Trampling is also a concern in privately owned suburban sites: although some landowners place cages around plants to protect them, trampling may still damage roots and below-ground fungi.
Recreational activities are considered a medium threat. Phantom Orchid habitat can be damaged by trail-building for hiking, mountain biking and dirt-biking, which disturbs and/or compacts the soil. Dirt bikes and/or mountain bikes frequently use trails next to at least three subpopulations (Barsanti and Iredale 2005; BC CDC 2013a; Maslovat pers. obs. 2013). Trampling of stems by people or dogs has been observed in provincial parks next to recreational trails (Hirner pers. comm. 2013; Maslovat pers. obs. 2013). Breakage of the floral stems compromises the reproductive capacity of the plants.
Trail maintenance activities including drainage ditching, which can alter hydrology, and removing trees, which may inadvertently remove host trees and sources of leaf litter, can also harm Phantom Orchids (Ceska pers. comm. 2005, 2008 in Phantom Orchid Recovery Strategy 2008).
Invasive terrestrial plants are considered a medium to low threat. Non-native invasive plants may compete with the Phantom Orchid, with the host plants, and/or alter plant community composition. Non-native forbs next to Phantom Orchids include minor amounts of Herb-Robert (Geranium robertianum), Common Bedstraw (Galium aparine), Wild Chervil (Anthriscus sylvestris), and Creeping Buttercup (Ranunculus repens). Invasive ornamental plants including Dead-nettle (Lamium sp.) and Common Periwinkle (Vinca major) are also found close to Phantom Orchids in some sites (Ferguson 2013; Maslovat pers. obs. 2013). The full impact of these invasive species on Phantom Orchid is not known although flowering stems appear to less robust in sites with a denser cover of non-native invasive species (Maslovat pers. obs. 2013).
Woody invasive species including European Filbert (Corylus avellana), English Holly (Ilex aquifolium), Himalayan Blackberry (Rubus armeniacus), and English Ivy (Hedera helix) are also found at a number of Phantom Orchid sites (Barsanti and Iredale 2005; Ferguson 2013; Knopp pers. comm. 2013; Maslovat pers. obs. 2013). Although the full impact of the presence of these woody invasive species is not known, they may compete with either the Phantom Orchid or the host plants as well as altering the habitat structure.
Logging and wood harvesting is considered to be a low threat. Clearcut and selective logging operations threaten the Phantom Orchid both directly and indirectly. This threat is greatest in the Fraser Valley but it is also a potential threat on the privately owned subpopulation on Saltspring Island.
The direct impacts of logging operations include the alteration of site conditions such as changes to hydrology either from tree removal up slope or next to subpopulations, removal or disturbance of host trees, destruction of the fungal partner(s) either directly or by changing hydrology or light conditions, and direct destruction of habitat (Dunster 2008b). Even selective tree cutting can irreparably harm the host tree or disturb the ECM fungal network (Dunster 2008b).
The indirect impacts created by adjacent logging activities can include creating edge effects (Chen et al. 1992 in Phantom Orchid Recovery Team 2008) that may alter growing conditions for the partner tree, fungus or competing understory vegetation (Phantom Orchid Recovery Team 2008). Logging operations also contribute to increased habitat fragmentation, loss of connectivity among sites and subpopulations, loss of pollinators, influx of invasive species as competitors, increased herbivory, and post-logging treatments that include herbicide spray, and seeding of non-native species (Phantom Orchid Recovery Team 2008).
The proliferation of deer is considered a low threat. Floral stems are grazed by Columbian Black-tailed Deer (Odocoileus hemionus columbianus) (COSEWIC 2000; Knopp pers. comm. 2013; Maslovat pers. obs. 2013), which limits the reproductive capacity of the plants. Deer populations are increasing in some areas because of forest clearing and the associated creation of edge habitats as well as a reduction in the number of predators next to populated areas. However, deer may play a role in the ecology of this species because Phantom Orchids have been observed in deer beds and along deer trails (Phantom Orchid Recovery Team 2013). Grazing also damages flower heads before seeds develop and trampling can damage the flowering stems (COSEWIC 2000; Barsanti et al. 2006; Maslovat pers. obs. 2013).
Livestock are considered to be a low threat. The interaction between the abundance of Phantom Orchids and grazing by livestock (horses, cattle and goats) is unclear. At most (but not all) sites, Phantom Orchids are found in areas with very little undergrowth and the plants may be sensitive to competition from other species. Removing livestock grazing at Katherine Tye Ecological Reserve and private sites in the same subpopulation appears to have resulted in a dramatic reduction in the number of plants and a large increase in the amount of competing understory vegetation (Barsanti and Iredale 2005; Barsanti et al. 2006, Hayens pers. comm. in Ferguson 2013). However, at two of the private sites, Phantom Orchids are no longer found in an area that was logged and then grazed by goats (Ferguson 2013).
The remaining threats are considered to have negligible effects on the Canadian population but individually have been noted due to their effects to subpopulations
There was evidence of digging in the exact locality where Phantom Orchids occurred and it is presumed that orchid growers were attempting to transplant wild plants even though without host fungi and trees these efforts will be unsuccessful (Phantom Orchid Recovery Team 2008). Digging of other species has also been observed in the proximity (presumably for the nursery trade because of the large numbers of plants dug) and Phantom Orchids have been trampled (COSEWIC 2000). There is also the potential at some private sites for purposeful alteration of land (i.e., removal of plants) so as not to prevent future land subdivision.
At two private residences, Phantom Orchids growing along the roadside have been mowed by municipal maintenance workers (Hayens pers. comm. 2013 in Ferguson 2013; Osterhold pers. comm. 2013).
One site (Sumas River/Vedder Canal) is an active rock quarry site. In order to extract the rock, the site is first clear cut prior to blasting. Although a setback was created to protect the Phantom Orchid, this protection relies on voluntary cooperation from the mining company. Recent logging and road construction (in 2013) appear to be close to the Phantom Orchid site although there have not been recent surveys to determine if the subpopulation has been impacted (Welstead pers. comm. 2014).
There are mine tenures throughout the Chilliwack area, and in the future other mining operations may impact Phantom Orchid sites. Construction of independent power production facilities may also alter habitat in the future (Welstead pers. comm. 2014).
At three of the subpopulations, windthrow above natural background levels has been observed (BC CDC 2013a). Windthrow can harm host trees or the fungal host although the full impact of windthrow on Phantom Orchid is unknown.
The entire population of the Phantom Orchid in Canada is extremely small, totalling only 344 floral stems (see Abundance, Table 1). The population is separated into isolated subpopulations occurring in three distinct geographic areas (2 islands and the Fraser Valley). Small, isolated subpopulations are particularly vulnerable to stochastic catastrophic events and often suffer from low genetic diversity. Although Phantom Orchids may self-pollinate, isolated small subpopulations can suffer from a lack of pollinators. The Phantom Orchid may be vulnerable to changing climatic conditions.
The Phantom Orchid depends entirely on its fungal and tree partners for survival. If conditions are not suitable for the fungal partner (cool, moist shaded sites) or if the fungus is impacted by disturbance or the introduction of pathogens, the health of the orchids will be affected (Taylor pers. comm. 2005 in Phantom Orchid Recovery Team 2008). Activities that impact the tree partner (species as yet unknown), will affect the health and vigour of the Phantom Orchid (Phantom Orchid Recovery Team 2008).
Many of the 20 Phantom Orchid subpopulations have multiple sites, often occurring on property owned by different adjacent landowners. The 20 Phantom Orchid subpopulations occur at sites with varying land tenure, and the overall distribution implicates multiple landowners. Accordingly, the threat of property development is site-specific (Table 4). There are 36 Phantom Orchid locations (Table 4), defined by COSEWIC as geographically distinct areas in which a single threatening event can rapidly affect all individuals of the wildlife species present (COSEWIC 2013).
| Subpopulation Name and Number | Number of Sites and Ownership (36 locations) | Major Threat |
|---|---|---|
| 1. Gowlland Todd Provincial Park | 4 Provincial Park | Recreational activities |
| 1. Gowlland Todd Provincial Park | 2 Private (Hill Property and Quarry Lake) | Extirpated |
| 2. Colwood | 1 Private | Residential and commercial development: homeowner activities |
| 3. Horth Hill, North Saanich | 1 Regional Park | Recreational activities |
| 4. Saltspring Island, Musgrave Landing | 3 Provincial Crown | Residential and commercial development: small isolated population |
| 5. Saltspring Island, Mount Tuam, 2.4 km W of | 2 Private | Residential and commercial activities: homeowner activities Potential future logging and wood harvesting |
| 6. Vedder Mountain, S foot of | 6 Provincial Crown (several parcels) | Logging and wood harvesting |
| 6. Vedder Mountain, S foot of | 1 Private Owner AA | Unknown |
| 6. Vedder Mountain, S foot of | 1 Private Owner AB | Unknown |
| 6. Vedder Mountain, S foot of | 2 Unknown Ownership | Unknown |
| 7. Lindell Beach, W of Cultus Lake | 2 Private | Residential and commercial development |
| 8. Sumas Mountain, Ryder trail | 2 Provincial Crown | Recreational activities |
| 9. McKee Peak, SW Slope of | 1 Municipal Crown | Invasive plants |
| 9. McKee Peak, SW Slope of | 3 Private Owner BA | Residential and commercial development |
| 9. McKee Peak, SW Slope of | 1 Private Owner BB | Residential and commercial development |
| 10. Cultus Lake Provincial Park, Teapot Hill | 6 Provincial Park | Recreational activities |
| 11. Katherine Tye Ecological Reserve, Chilliwack | 5 Ecological Reserve | Invasive non-native plants |
| 11. Katherine Tye Ecological Reserve, Chilliwack | 1 Private Owner CA | Invasive non-native plants |
| 11. Katherine Tye Ecological Reserve, Chilliwack | 1 Private Owner CB | Invasive non-native plants/logging and wood harvesting |
| 11. Katherine Tye Ecological Reserve, Chilliwack | 1 Private Owner CC | Residential and commercial development |
| 11. Katherine Tye Ecological Reserve, Chilliwack | 1 Private Owner CD | Residential and commercial development |
| 12. Mt. Tom, Chilliwack (Mt. Thom 2) | 2 Regional Park | Recreational activities/Residential and commercial development: small isolated subpopulation |
| 12. Mt. Tom, Chilliwack (Mt. Thom 2) | 1 Private | Extirpated |
| 13. Ryder Lake, 1.5 km NW of (Mt. Thom 4) | 2 Private | Transportation and service corridors |
| 14. Mt. Tom, Southside Road (Mt. Thom 3, 5, and 6) | 1 Private Owner DA | Residential and commercial development: homeowner activities |
| 14. Mt. Tom, Southside Road (Mt. Thom 3, 5, and 6) | 1 Private Owner DC | Unknown |
| 14. Mt. Tom, Southside Road (Mt. Thom 3, 5, and 6) | 1 Private Owner DD | Unknown |
| 14. Mt. Tom, Southside Road (Mt. Thom 3, 5, and 6) | 4 Private Owner DE | Invasive non-native plants |
| 14. Mt. Tom, Southside Road (Mt. Thom 3, 5, and 6) | 4 Private Owner DF | Residential and commercial development: homeowner activities |
| 14. Mt. Tom, Southside Road (Mt. Thom 3, 5, and 6) | 3 Private Owner DG | Livestock farming and ranching |
| 15. Bench Road | 1 Private | Unknown |
| 16. Chilliwack, DND Training site | 5 Federal | Recreational activities |
| 17. Kent | (Unknown) Unknown Ownership | Unknown |
| 18. Sumas River/ Vedder Canal 1.4km SW of (East Pumptown Quarry) | 1 Private | Mining and quarrying/Logging and wood harvesting |
| 19. Promontory, Chilliwack (Mt. Thom 7) | 1 Private | Residential and commercial development |
| 20. Mission, Westminster Abbey | 1 Private | Gathering terrestrial plants/Residential and commercial development: homeowner activities |
Many of the threats are localized and restricted to a specific parcel of land. Most of the publicly owned land (provincial parks, regional parks, federal land and one of the provincial Crown sites) is characterized by threats from recreational activities including trampling from hikers and dogs as well as bikes. Although different parcels of public land face the same threat, they are geographically distinct and are therefore considered separate locations.
There are at least two private landowners who intend to subdivide their property in the near future, bringing the associated risk of housing development. Threats to other locations include logging operations for view clearing, overgrazing from livestock, plant collecting, and habitat alteration from homeowner activities depending on the intensions and priorities of the landowners. All of these threats are largely restricted to within the property boundaries although edge effects from logging may impact adjacent lots. The threat from invasive plants has a slightly wider extent as propagules of invasive species will readily cross property lines. Primary threats for seven of the sites in four subpopulations are unknown.
The most significant threat to the publicly owned Katherine Tye Ecological Reserve and adjacent private property is the spread of invasive plants associated with a cessation of grazing. This threat has a slightly broader extent because grazing was practised over several lots and the spread of invasive species propagules is not restricted by property lines. The second location on provincial Crown land is in a Wildlife Habitat area suffering from the edge effects of logging, and these edge effects may also affect other sites in the vicinity. The third provincial Crown land is a small reserve with the Phantom Orchids threatened as a small, isolated subpopulation. The municipal Crown location is under a hydro right-of-way and although protected from development, the Phantom Orchid habitat is threatened by invasive plants.
The Phantom Orchid is protected under Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES 2013).
The Phantom Orchid is listed under the Canadian Species at Risk Act (SARA) on Schedule 1 as Threatened (May 2000). Critical habitat for this species has not yet been identified in a federal recovery strategy.
A draft provincial recovery strategy for the Phantom Orchid has been prepared (Phantom Orchid Recovery Team 2008). Key actions that have been implemented from the Recovery Strategy include:
- Draft Best Management Practices for the Phantom Orchid have been developed (Klinkenberg and Klinkenberg 2006).
- Ecological monitoring and landowner contact at some Phantom Orchid subpopulations was undertaken from 2005-2008 (Barsanti and Iredale 2005; Barsanti et al. 2006; Kerr 2007; Slater 2008 in Phantom Orchid Recovery Team 2008). This has included an ongoing effort to obtain more accurate subpopulation counts within a single flowering season and monitoring of capsule and seed production.
- Permanent plots were established at many subpopulations in order to monitor change over time. Mark-recapture was initiated in order to obtain accurate counts of flowering stems within a single season; however, the permanent plots were difficult to monitor because the flowering stems emerged in different spots.
- Predictive mapping for Fraser Valley subpopulations has been completed (Klinkenberg 2009).
- A public information brochure has been prepared and distributed (Fraser Valley Conservancy 2009).
- Draft survival critical habitat with a 200 metre buffer has been mapped for some known Phantom Orchid sites.
The Phantom Orchid is not protected under the Forest and Range Practices Act or as Identified Wildlife under the provincial Wildlife Act although protection has been recommended (Phantom Orchid Recovery Team 2008; Province of British Columbia 2013).
In BC, the Phantom Orchid has a Provincial Status of S2 (Imperilled) and is on the BC Conservation Data Centre Red List with a Conservation Framework Priority of 2 (BC CDC 2013b). In Canada, it has a National Status of N2 (Imperilled) (NatureServe 2012). The Phantom Orchid has a current General Status Rank in Canada and BC of 1 (At Risk) (National General Status Working Group 2012).
The Phantom Orchid has a Global Status of G4 (Apparently Secure) and is not ranked in California, Oregon, and Washington. It is ranked as S3 (Vulnerable) in Idaho (NatureServe 2012). Washington state no longer tracks this species, having decided that it is widespread and abundant enough in Washington to be considered secure (Arnett 2013).
The Phantom Orchid has not been assessed for the IUCN Red List (IUCN 2013).
The current level of habitat protection for the Phantom Orchid is insufficient for ensuring the long-term survival of the species. The distribution of Phantom Orchid implicates multiple landowners including 22 private landowners, and the threat of local development is site-specific for private lands.
The ownership of the non-private subpopulations are as follows: provincial parks (2), regional parks (2), provincial Crown (3), municipal Crown (1), Nature Trust of BC managed by BC Parks Ecological Reserve (1) and federally owned Department of National Defence land (1).
Some of the Phantom Orchid sites on Crown land in one subpopulation on Vedder Mountain are currently protected from logging within a Wildlife Habitat Area (WHA) (BC CDC 2013a). However, the WHA was created to protect Mountain Beaver (Aplondontia rufa), which is no longer listed as Identified Wildlife under the Identified Wildlife Management Strategy. Phantom Orchid is not currently listed as Identified Wildlife so there is no legislation that would prevent revoking the WHA (Welstead pers comm. 2014).
The subpopulation of Phantom Orchid on federally owned Department of National Defence land is protected under the Species at Risk Act general prohibitions (Government of Canada 2012).
The report writer would like to thank Environment Canada for commissioning and funding this report.
The writer also thanks the following individuals for provision of data, and for their field assistance and support during the preparation of this report:
Jenifer Penny and Katrina Stipec of the BC Conservation Data Centre for providing data and support for the project. The substantial efforts of Malissa Smith and Kym Welstead for providing background material and providing advice on fieldwork were greatly appreciated. Many thanks to Brian and Rose Klinkenberg for their comprehensive work on the two previous Phantom Orchid status reports, preparation of the draft recovery strategy, and other pertinent documents for this species. Bruce Bennett provided important advice for how to count individuals and define subpopulations in addition to careful reviews of early drafts of this report. Special thanks to Jenny Wu for her spectacular patience and expertise in the preparation of area calculations and distribution maps.
The author wishes to offer many thanks to the many people who volunteered their time to help survey for Phantom Orchids including Malissa Smith, Greg Ferguson, Joy Newham, Tony MacLeod, Laura Matthias, Joanna Hirner, Joe Benning, Kathy Wilkinson, Stan Olsen, Jason Osterhold, and Denis Knopp.
Special thanks from the author are extended to Greg Ferguson for his extensive additional surveys for this species and detailed survey report. The report writer would like to thank Robin Annschild, Paul Catling, Joanna Hirner, Ruth Keogh, Kevin deBoer, Dean Nernberg and Glenna Erlandson for sharing details of Phantom Orchid localities. Thanks also from the author to Brian Brett for searching his property for the orchids.
Annschild, Robin. Biologist, Saltspring Island Conservancy. Saltspring Island, BC.
Bennett, Bruce. Co-chair Vascular Plant Specialist Subcommittee, COSEWIC, Whitehorse, YT.
Brett, Brian. Landowner, Saltspring Island, BC.
Catling, Paul. Research Scientist and Curator, Biodiversity, National Program on Environmental Health, Agriculture and Agri-Food Canada. Ottawa, ON.
Doubt, Jennifer. Chief Collection Manager- Botany, Canadian Museum of Nature. Ottawa, ON.
Erlandson, Glenna. Natural Resource Technician. 1 Area Support Group, Department of National Defence. Chilliwack, BC.
Fraser, Dave. Unit Head, Scientific Authority Assessment, Ecosystem Branch, Ministry of Environment, Victoria BC.
Jones, Neil. Scientific Project Officer and Aboriginal Traditional Knowledge (ATK) Coordinator, COSEWIC, Gatineau, QC.
Kirkby, Jan. Landscape Ecologist. Environment Canada-Canadian Wildlife Service, Pacific and Yukon Region, Delta, BC.
Millikin, Rhonda. Head, Population Assessment. Environment Canada-Canadian Wildlife Service, Pacific and Yukon Region, BC.
Miskelly, James. Conservation Biologist, Department of National Defence. Victoria, BC.
Nantel, Patrick. Conservation Biologist, Species at Risk Program, Parks Canada. Gatineau, QC.
Nernberg, Dean. Species at Risk Officer, Director General of Environment, Directorate of Environmental Stewardship, National Defence Headquarters, Ottawa, ON.
Sadler, Kella. Senior Species at Risk Biologist, Species at Risk Recovery Unit. Environment Canada-Canadian Wildlife Service, Pacific and Yukon Region, Delta, BC.
Schnobb, Sonia. Administrative Assistant, COSEWIC Secretariat, Canadian Wildlife Service, Environment Canada. Gatineau, QC.
Stipec, Katrina. BC Conservation Data Centre, Ministry of Environment, Victoria, BC.
Welstead, Kym. Chair of Phantom Orchid Recovery Team, Species at Risk Biologist, Ministry of Forests, Lands and Natural Resource Operations, Surrey, BC.
Wu, Jenny. Scientific Project Officer, COSEWIC Secretariat, Canadian Wildlife Service, Environment Canada, Gatineau, QC.
Arnett, J., pers. comm. 2013. Email correspondence to B. Bennett. October 2013. Rare Plant Botanist, Washington Natural Heritage Program. Olympia, Washington.
Annschild, R., pers. comm. 2013. Email correspondence to C. Maslovat. June 2013. Biologist, Saltspring Island Conservancy. Saltspring Island, British Columbia.
Argue, C.L. 2012. The Pollination Biology of North American Orchids: Volume 2 North of Florida and Mexico. Springer, New York, Dordrecht, Heidelberg, London. ix + 202 pp.
Avis, P., and E. Charvat. 2005. The response of ectomycorrhizal fungal inoculum to long-term increases in nitrogen supply. Mycologia 97(2): 329-337.
Barsanti, J., and F. Iredale. 2005. Inventory and Monitoring of the Phantom Orchid (Cephalanthera austiniae (A. Gray) Heller) in Canada. Unpublished BC Conservation Corps report by the submitted to the BC Ministry of Environment. 31pp.
Barsanti, J., S. Pinkus, J. Neilson, and N. Sands. 2006. Population and Site Assessment of the Phantom Orchid (Cephalanthera austiniae (A. Gray) Heller) in Canada. Unpublished BC Conservation Corps report by the submitted to the BC Ministry of Environment, BC Conservation Foundation. 63 pp.
BC CDC (BC Conservation Data Centre). 2013a. Element occurrence records. BC CDC, Victoria, BC.
BC CDC (BC Conservation Data Centre) 2013b. Species Summary: Cephalanthera austiniae. BC. Ministry of Environment. [accessed March 2013].
Bidartondo M.I., and D.J. Read. 2008. Fungal specificity bottlenecks during orchid germination and development. Molecular Ecology 17:3707-3716.
Capital Regional District. 2001. Demographics: Population Forecast, 2026 Capital Region. PDF file [accessed April 2014].
Catling, P.M., pers. comm. 2013. Email correspondence to C. Maslovat. October 2013. Research Scientist and Curator, Biodiversity, National Program on Environmental Health, Agriculture and Agri-food Canada. Ottawa, Ontario.
Chung, M.Y., J.D. Nason, and M.G. Chung. 2004. Spatial genetic structure in populations of the terrestrial Cephalanthera longibracteata (Orchidaceae). American Journal of Botany 91 (1):52-57.
CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora). 2013. CITES Species Databas. [accessed October 2013].
City of Chilliwack. 2013. Population and Household Projections. Unpublished data provided by the City of Chilliwack.
City of Colwood. 2014. Colwood is Open for Business. [accessed April 2014].
COSEWIC. 2000. COSEWIC assessment and update status report on the Phantom Orchid Cephalanthera austiniae in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa.vii + 21 pp.
COSEWIC. 2013. Status Reports: Definitions and Abbreviations. [accessed September 2014].
Douglas, G., D. Meidinger, and J. Pojar. 2001. Illustrated Flora of British Columbia: Volume 7 Monocotyledons (Orchidaceae through Zosteraceae). BC Ministry of Sustainable Resource Management and Ministry of Forests. Victoria BC. 379 pp.
Dunster, K. 2008a. Preliminary Partial Survival Habitat Identification for Phantom Orchid (Cephalanthera austiniae). Unpublished draft report prepared by the Phantom Orchid Recovery Team. November 15, 2008. vii + 12 pp.
Dunster, K. 2008b. Cephalanthera austiniae: Survival Habitat Literature Review. Unpublished report prepared by the Phantom Orchid Recovery Team. 27pp.
Erlandson, G., pers. comm. 2013. Email correspondence to C. Maslovat. May 2013. Natural Resource Technician. 1 Area Support Group, Department of National Defence. Chilliwack, British Columbia.
Ferguson, G. 2013. Inventory, Monitoring and Habitat Assessment of Phantom Orchid (Cephalanthera austiniae) in Southwestern British Columbia. Draft report submitted to the British Columbia Ministry of Environment and Environment Canada. iv + 29pp.
Fraser Valley Conservancy. 2009. Have You Seen The Phantom? Public outreach brochure. Fraser Valley Conservancy. Abbotsford BC.
Fraser Valley Regional District. 2008. Development Approvals. [accessed October 2013].
Government of Canada. 2012. Species at Risk Public Registry. [accessed September 2014].
Heron, J. pers. comm. 2014. Email correspondence to B. Bennett. 18 November 2014. BC Ministry of Environment Ecosystem Protection & Sustainability Branch, Vancouver, BC.
Hirner, J., pers. comm. 2013. Email correspondence to C. Maslovat. July 2013.Conservation Specialist, BC Parks. Vancouver, British Columbia.
IUCN (International Union for Conservation of Nature and Natural Resources). 2013. The IUCN Red List of Threatened Species. [accessed March 2013].
Kenny, A., pers. comm. 2013. In person conversation with C. Maslovat. May 2013. Private landowner. Abbotsford, British Columbia.
Kerr, T. 2007. Inventory, Monitoring and Habitat Assessment of Phantom Orchid (Cephalanthera austiniae (A. Gray) Heller) in Canada. Unpublished BC Conservation Corps report submitted to the BC Ministry of Environment. 50 pp.
Kjoller, R. L. Nilsoon, K. Hansen, I. Kappel Schmidt, L. Vesterdal, and P. Gundersen. 2012. Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New phytologist. PDF file [accessed April 2014].
Klinkenberg, R., and B. Klinkenberg. 2006. Best Management Practices Guidelines for Phantom Orchid in Urban and Rural Areas. Draft unpublished report prepared for the Phantom Orchid Recovery Team. Vancouver, BC. 3 pp.
Klinkenberg, B. 2009. Predictive Mapping of Potential Locations for Phantom Orchid (Cephalanthera austiniae) in the Lower Fraser Valley of British Columbia. Unpublished report prepared for the BC Ministry of Environment and the National Phantom Orchid Recovery Team. Vancouver, BC. v + 26 pp.
Klinkenberg, B. (editor) 2013. Electronic Atlas of the Plants of British Columbia Lab for Advanced Spatial Analysis, Department of Geography, University of British Columbia, Vancouver. [accessed March 2013].
Knopp, D., pers. comm. 2013. In person conversation with C. Maslovat. May 2013. Biologist. Chilliwack, British Columbia.
Latr, A., M. Curikova, M. Balaz, and J. Jurcak. 2008. Mycorrhizas of Cephalanthera longifolia and Dactylorhiza majalis, two terrestrial orchids. Ann. Bot. Fennici 45:281-289.
Lazar, K., pers. comm. 2013. Email correspondence to B. Bennett. October 2013. California Natural Diversity Database, Environmental Scientist-Lead Botanist. Sacramento, California.
Leake J.R. 2004. Myco-heterotroph/epiparasitic plant interactions with ectomycorrhizal and arbuscular mycorrhizal fungi. Current Opinion in Plant Biology 7:422-428.
Light, H.S., and M. MacConaill. 2006. Appearance and disappearance of a weedy orchid, Epipactis helleborine. Folia Geobotanica 41:77-93.
Light, M.H.S., and M. MacConaill. The role of common orchids in appreciating the complexity of biodiversity conservation. Lakesteriana 11(3):293-300.
Long, R. 1979. Eburophyton austiniae (A. Gray). Heller, Phantom Orchid. Davidsonia 10(1):30-33.
Miskelly, J., pers. comm. 2013. Email correspondence to C. Maslovat. May 2013. Conservation Biologist, Department of National Defence. Victoria, British Columbia.
National General Status Working Group. 2012. Phantom Orchid. Wild Species The General Status of Species in Canada. [accessed March 2013].
NatureServe. 2004. A Habitat-Based Strategy for Delimiting Plant Element Occurrences: Guidance from the 2004 Working Group. Web site: PDF file [accessed November 2013].
NatureServe. 2012. Cephalanthera austiniae: Phantom Orchid. NatureServe Explorer: An Online Encyclopedia of Life. [accessed March 2013].
Osterhold, J., pers. comm. 2013. In person conversation with C. Maslovat. May 2013. Landowner, Chilliwack, British Columbia.
Parrent, J., and R. Vilgalys. 2007. Biomass and compositional responses of ectomycorrhizal fungi hyphae to elevated CO2 and nitrogen fertilization. New phytologist 176 (1):164-174.
Phantom Orchid Recovery Team. 2008. Draft Recovery Strategy for the Phantom Orchid (Cephalanthera austiniae) in British Columbia. Prepared for the BC Ministry of Environment, Victoria, BC. 20 pp.
Province of British Columbia. 2013. Accounts and Measures for Managing Identified Wildlife [accessed October 2013].
Reddoch, J.M., pers. comm. 2014. Email correspondence with B. Bennett, May 14. Orchid researcher, Gatineau Quebec.
Reddoch, J.M., and A.H. Reddoch. 2014. Orchid longevities in Gatineau Park: final summary. Trail & Landscape 48(2):62-67.
Salafsky, N., D. Salzer, A.J. Stattersfield, C. Hilton-Taylor, R. Neugarten, S. H. Butchart, B. Collen, N. Cox, L.L. Master, S. O'Connor and D. Wilkie. 2008. A Standard Lexicon for Biodiversity Conservation: Unified Classifications of Threats and Actions. Conservation Biology 22 (4): 897-911.
Shefferson, R.P, T. Kull, and K. Tali. 2005. Adult whole-plant dormancy induced by stress in long-lived orchids. Ecology 86(11):3099-3104.
Sheviak, C.J., and P.M. Catling. 2002. Flora of North America Volume 26. p. 494-583. [accessed April 2013].
Taylor, D.L., and T.D. Bruns, 1997. Independent, specialized invasions of ectomycorrhizal mutualism by two nonphotosynthetic orchids. Proceedings of the National Academy of Science 94:4510-4515.
Taylor D.L., T.D. Bruns, J.R. Leake, and D.J. Read. 2002 PDF file. Mycorrhizal specificity and function in myco-heterotrophic plants. Pp 375-414. in: I.R. Sanders and M. van der Heijden, (eds.) The Ecology of Mycorrhizas. Ecological Studies Volume 157. Springer-Verlag, Berlin.
Turk, T.D., M.G. Schmidt, and N.J. Roberts. 2008. The influence of bigleaf maple on forest floor and mineral soil properties in a coniferous forest in coastal British Columbia. Forest Ecology and Management 255:1874-1882. PDF file [accessed April 2014].
Welstead, K., pers. comm. 2014. Telephone and email conversation with C. Maslovat. April 2014. Chair, Phantom Orchid Recovery Team, Vancouver, British Columbia.
Carrina Maslovat currently works as a consultant in plant communities at risk, primarily Garry Oak ecosystems. She has conducted plant inventories in a range of regional, municipal, federal and provincial parks, finding new populations of species at risk and monitoring rare plant populations' abundance and vitality over time. She has developed management plans and Best Management Practices to minimize the impact to plant species at risk. She is the author of the Prairie Lupine status report update and several recovery planning documents including acting as the lead author of the Recovery Strategy for Multi-Species at Risk in Maritime Meadows Associated with Garry Oak Ecosystems in Canada.
Canadian Museum of Nature (no specimens)
University of British Columbia (5 specimens)
| Threat Impact | Threat Impact (descriptions) | Level 1 Threat Impact Counts: high range |
Level 1 Threat Impact Counts: low range |
|---|---|---|---|
| A | Very High | 0 | 0 |
| B | High | 1 | 1 |
| C | Medium | 2 | 1 |
| D | Low | 2 | 3 |
| - | Calculated Overall Threat Impact: | Very High | High |
| # | Threat | Impact (calculated) Criterion |
Impact (calculated) |
Scope (next 10 Yrs) |
Severity (10 Yrs or 3 Gen.) |
Timing | Comments |
|---|---|---|---|---|---|---|---|
| 1 | Residential & commercial development | B | High | Large (31-70%) | Extreme (71-100%) | High (Continuing) | - |
| 1.1 | Housing & urban areas | B | High | Large (31-70%) | Extreme (71-100%) | High (Continuing) | Note this is done site by site basis in Table 4. |
| 1.2 | Commercial & industrial areas | - | - | - | - | - | - |
| 1.3 | Tourism & recreation areas | - | - | - | - | - | - |
| 2 | Agriculture & aquaculture | D | Low | Small (1-10%) | Serious (31-70%) | High (Continuing) | - |
| 2.1 | Annual & perennial non-timber crops | - | - | - | - | - | - |
| 2.2 | Wood & pulp plantations | - | - | - | - | - | - |
| 2.3 | Livestock farming & ranching | D | Low | Small (1-10%) | Serious (31-70%) | High (Continuing) | Vegt site has two sites on property, one intact, the other has had land cleared and goats grazing. Goats will graze down more intensely than other species (ie horse, cattle) |
| 2.4 | Marine & freshwater aquaculture | - | - | - | - | - | - |
| 3 | Energy production & mining | - | Negligible | Negligible (<1%) | Extreme (71-100%) | High (Continuing) | - |
| 3.1 | Oil & gas drilling | - | - | - | - | - | - |
| 3.2 | Mining & quarrying | - | Negligible | Negligible (<1%) | Extreme (71-100%) | High (Continuing) | There may have been plans to quarry at Pumptown or Sumas River/Vedder Site. Secretariat to follow up with Kym Welstead. |
| 3.3 | Renewable energy | - | - | - | - | - | - |
| 4 | Transportation & service corridors | - | Negligible | Negligible (<1%) | Unknown | High (Continuing) | - |
| 4.1 | Roads & railroads | - | Negligible | Negligible (<1%) | Unknown | High (Continuing) | Some roadside mowing Rider Lake Area |
| 4.2 | Utility & service lines | - | - | - | - | - | - |
| 4.3 | Shipping lanes | - | - | - | - | - | - |
| 4.4 | Flight paths | - | - | - | - | - | - |
| 5 | Biological resource use | D | Low | Small (1-10%) | Extreme (71-100%) | High (Continuing) | - |
| 5.1 | Hunting & collecting terrestrial animals | - | - | - | - | - | - |
| 5.2 | Gathering terrestrial plants | - | Negligible | Negligible (<1%) | Extreme (71-100%) | High (Continuing) | Speculated that some sites dug up (evidence of digging) and likely flower picking as a very showy and fragrant plant. Potential at some private sites for purposeful alteration of land (ie removal of plants) so as not to prevent future land subdividing. |
| 5.3 | Logging & wood harvesting | D | Low | Small (1-10%) | Extreme (71-100%) | High (Continuing) | Secretariat to follow up with Kym Welstead regarding logging on non-Crown land sites. Crown land sites protected under WHAs. some illegal harvesting of cedars but likely not a threat in next 10 years. Some sites private woodlots. Private landowner on one Saltspring I. site has indicated interest in logging or selling parcel - this site has the highest occurrence of plants and the highest potential for this particular threat. |
| 5.4 | Fishing & harvesting aquatic resources | - | - | - | - | - | - |
| 6 | Human intrusions & disturbance | C | Medium | Restricted (11-30%) | Serious (31-70%) | High (Continuing) | - |
| 6.1 | Recreational activities | C | Medium | Restricted (11-30%) | Serious (31-70%) | High (Continuing) | Issue in most of public sites. Mostly trampling, especially on sides of trail where soil could get impacted. Jenifer Penney to investigate potential historic records of threat on Gowlland Todd referring to collecting plants. |
| 6.2 | War, civil unrest & military exercises | - | Unknown | Unknown | Unknown | High (Continuing) | Impacts uncertain, author unable to visit that site. Follow-up by DND needed - does DND restrict activity around this plant? If so, these estimates should be revised. |
| 6.3 | Work & other activities | - | - | - | - | - | - |
| 7 | Natural system modifications | - | - | - | - | - | - |
| 7.1 | Fire & fire suppression | - | - | - | - | - | - |
| 7.2 | Dams & water management/use | - | - | - | - | - | - |
| 7.3 | Other ecosystem modifications | - | - | - | - | - | Roadside mowing was captured elsewhere so not added here. |
| 8 | Invasive & other problematic species & genes | CD | Medium - Low | Restricted (11-30%) | Serious - Moderate (11-70%) | High (Continuing) | - |
| 8.1 | Invasive non-native/alien species | CD | Medium - Low | Restricted (11-30%) | Moderate - Slight (1-30%) | High (Continuing) | Some species threats to plant, some to host trees but impacts unknown. Potential for at least 4 sites total where invasives and weedy plants could be an issue where they outcompete. Impact of introduced earthworms, gastropods, and rabbits are unknown. |
| 8.2 | Problematic native species | D | Low | Small (1-10%) | Serious - Moderate (11-70%) | High (Continuing) | Mountain Beaver, Banana Slug, deer, and succession plants considered. |
| 8.3 | Introduced genetic material | - | - | - | - | - | - |
| 9 | Pollution | - | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | - |
| 9.1 | Household sewage & urban waste water | - | - | - | - | - | - |
| 9.2 | Industrial & military effluents | - | - | - | - | - | - |
| 9.3 | Agricultural & forestry effluents | - | - | - | - | - | - |
| 9.4 | Garbage & solid waste | - | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | Some garbage dumping on Mount Shannon site but is an extirpated site. |
| 9.5 | Air-borne pollutants | - | - | - | - | - | - |
| 9.6 | Excess energy | - | - | - | - | - | - |
| 10 | Geological events | - | - | - | - | - | - |
| 10.1 | Volcanoes | - | - | - | - | - | - |
| 10.2 | Earthquakes/ tsunamis | - | - | - | - | - | - |
| 10.3 | Avalanches/landslides | - | - | - | - | - | Some sites on steep slopes and ridges, there is potential but very low given age of trees on sites and surrounding physical environment does not appear to be an influence. However, any sort of development or activity that causes instability a potential threat on these slopes. |
| 11 | Climate change & severe weather | - | Unknown | Unknown | Unknown | Moderate (Possibly in the short term, < 10 yrs) | - |
| 11.1 | Habitat shifting & alteration | - | - | - | - | - | This can be an issue in the longer term but not likely in the next 10 years. |
| 11.2 | Droughts | - | - | - | - | - | - |
| 11.3 | Temperature extremes | - | - | - | - | - | - |
| 11.4 | Storms & flooding | - | Unknown | Unknown | Unknown | Moderate (Possibly in the short term, < 10 yrs) | Windthrow above natural background levels observed at a few sites. |
Classification of Threats adopted from IUCN-CMP, Salafsky et al. (2008).