Pink Coreopsis (Coreopsis rosea): COSEWIC assessment and status report
Endangered 2012
Photo of the Pink Coreopsis, Coreopsis rosea

Description for cover photo
Photo of the Pink Coreopsis, Coreopsis rosea, providing a detailed view of the flower arrangement. Flowers are aggregated at the top of the stem in a daisy-like head composed of two flower types: yellow tubular florets forming the central disc and petal-like (ligulate) ray florets forming the outer margin of the head.
Table of contents
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Assessment Summary
- COSEWIC Executive Summary
- Technical Summary
- Preface
- Wildlife Species Description and Significance
- Distribution
- Habitat
- Biology
- Population Sizes and Trends
- Threats and Limiting Factors
- Protection, Status, and Ranks
- Acknowledgements and Authorities Contacted
- Information Sources
- Biographical Summary of Report Writers
- Collections Examined
List of figures
List of tables
- Table 1. Number of individuals, primary threats and waterfront protection and ownership for all extant Pink Coreopsis populations in Canada. Populations are mapped in Figure 3.
- Table 2. Total phosphorus and trophic status* of Pink Coreopsis lakes or lakes upstream from Pink Coreopsis lakes from 2002 (Eaton and Boates 2003) and 2011 (Submair 2011).
List of appendices
Document Information
COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC . 2012. COSEWIC assessment and status report on the Pink Coreopsisin Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 42 pp.
Previous report(s):
COSEWIC . 2000. COSEWIC assessment and update status report on the pink coreopsis Coreopsis rosea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 11 pp.
Newell, R.E. 1999. Update COSEWIC status report on the pink coreopsis Coreopsis rosea in Canada, In COSEWIC assessment and update status report on the pink coreopsis Coreopsis rosea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-11 pp.
Keddy, C., and P. Keddy. 1984. COSEWIC status report on the pink coreopsis Coreopsis rosea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 26 pp.
Production note:
COSEWIC would like to acknowledge David Mazerolle and Sean Blaney for writing the status report on the Pink Coreopsis, Coreopsis rosea, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Bruce Bennett, Co-chair of the COSEWIC Vascular Plants Specialist Subcommittee.
For additional copies contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment Canada
Ottawa, ON
K1A 0H3
Tel.: 819-953-3215
Fax: 819-994-3684
COSEWIC E-mail
COSEWIC Website
Également disponible en français sous le titre Évaluation et rapport de situation du COSEPAC sur le coréopsis rose (Coreopsis rosea) au Canada.
Cover illustration/photo:
Pink Coreopsis -- Photo by David Mazerolle, Atlantic Canada Conservation Data Centre.
©Her Majesty the Queen in Right of Canada, 2013.
Catalogue No. CW69-14/136-2013E-PDF
ISBN 978-1-100-22222-6
COSEWIC Assessment Summary
Assessment Summary – November 2012
Common name
Pink Coreopsis
Scientific name
Coreopsis rosea
Status
Endangered
Reason for designation
This showy perennial lake and river shore plant has a restricted global range with a disjunct distribution limited to southernmost Nova Scotia. There is a concern regarding potential widespread and rapid habitat degradation due to recent increases in levels of phosphorus in lakes, tied to a rapidly growing mink farming industry. Though the population size is now known to be larger than previously documented due to greatly increased survey effort, the species is also at risk due to the continuing impacts associated with shoreline development, and historical hydro development.
Occurrence
Nova Scotia
Status history
Designated Endangered in April 1984. Status re-examined and confirmed Endangered in April 1999, May 2000, and November 2012.
COSEWIC Executive Summary
Wildlife Species Description and Significance
Pink Coreopsis is a slender, perennial, herbaceous member of the Aster family, arising from a rhizomatous root system. It is 20-60 cm tall, with simple or branched stems bearing opposite linear leaves. Flowers are aggregated into daisy-like heads containing both yellow, tubular disk florets and pink to white, petal-like ray florets.
Pink Coreopsis is globally rare and co-occurs in extreme southwest Nova Scotia with a large suite of other disjunct species of the Atlantic Coastal Plain. Its attractive flowers encourage the public and landowners to appreciate and to become good stewards of co-occurring but less-showy rare species and their habitats. Canadian populations are 400+ km from the nearest occurrences in Massachusetts and exhibit significant genetic differentiation. The species is widely available as an ornamental, with various cultivars developed.
Distribution
Pink Coreopsis is restricted to three disjunct regions: 1) coastal areas of Massachusetts, Rhode Island, New York, Pennsylvania (now extirpated), New Jersey, Delaware and Maryland; 2) South Carolina and Georgia; 3) southern Yarmouth County in southwestern Nova Scotia on the shores of eight lakes in three river systems (Annis, Carleton and Tusket rivers), all of which flow into the Tusket River estuary. Roughly 10% of its global range is in Canada.
Habitat
In Canada, Pink Coreopsis grows exclusively on lakeshores and occurs primarily on the shores of larger, lower-watershed lakes which experience significant water-level fluctuations. It occurs on broad, open, gently sloping shores of sand, gravel, fine cobble and shallow peat, on exposed substrates above the water line and as an emergent in shallow water. As is typical of disjunct Atlantic Coastal Plain Flora in Canada, Pink Coreopsis is restricted to areas where nutrient availability is low and disturbances such as flooding, wave action and ice scour inhibit more competitive, higher biomass species.
Biology
Pink Coreopsis is an herbaceous perennial that reproduces vegetatively by clonal shoots formed at rhizome tips and by rooting from the nodes of fragmented rhizomes or stems. Flowering requires at least two growing seasons following germination. In Canada, Pink Coreopsis flowers from mid-July to late September. The species is insect-pollinated and largely self-incompatible. Seeds mature in late summer and early fall, with each fertilized flower in the head bearing a single, dry, one-seeded fruit (cypsela). Dispersal is not well understood, but seeds and stem or rhizome fragments could be carried some distance by water. Seedling recruitment has been observed in Canada, but its frequency is unknown. Available data suggest a limited soil seed bank. Lifespan of individual rhizome segments (“mature individuals”) is unknown, but genetic individuals could be long-lived. Pink Coreopsis is a stress-tolerant species well adapted to withstand nutrient-poor conditions and prolonged flooding. It exhibits a low competitive ability and is easily excluded by faster-growing, high-biomass species.
Population Sizes and Trends
The number of stems in Canada is estimated at 276,600 to 328,000 in four populations on eight lakes. The number of genetic individuals is much lower. Most of the Canadian population is on three lakes: Sloans Lake (>35%), Wilsons Lake (>31%) and Bennetts Lake (>18%). The Canadian population is believed to have remained relatively stable within the past 10 to15 years, with minor declines (<2.2%) due to shoreline development.
Threats and Limiting Factors
Eutrophication is the most significant and widespread threat, with potential to affect Pink Coreopsis by increasing competition from more common species. Mink farm waste is causing eutrophication of the Carleton River system where major cyanobacterial blooms have occurred since at least 2007 in a formerly oligotrophic system, and Pink Coreopsis habitat on Raynards Lake may be affected. Total phosphorus has increased 608% to 819% from 2002 to 2011 on the Tusket River lakes (59-65% of the Canadian population), with causes unknown. A major mink farm was built in 2010 800 m upslope from Sloans Lake (>35% of Canadian population), and the industry is expanding throughout the region occupied by Pink Coreopsis. Eutrophication-induced habitat effects do not yet appear to be affecting a significant portion of the population, but could result in significant future population declines.
Shoreline alteration associated with waterfront development and recreational activity (including off-road vehicle use) is causing ongoing, localized declines throughout the Canadian range. Total declines associated with shoreline development are estimated to have been less than 2.2% and additional declines are not expected to exceed that amount in the next 10 to15 years.
Water-level regulation by hydroelectric development is presumed to have caused significant historical declines. It may still be impacting the Raynards Lake population and is likely limiting recolonization of other dammed lakes on the lower Tusket River, but additional dams are unlikely.
Protection, Status, and Ranks
COSEWIC reassessed the Pink Coreopsis as Endangered in November 2012. Pink Coreopsis is listed as Endangered under the federal Species at Risk Act and the Nova Scotia Endangered Species Act and is therefore protected on all lands on which it occurs in Canada. It has a NatureServe global rank of Vulnerable (G3), and in Canada and Nova Scotia it is ranked as Critically Imperilled (N1 and S1). In the United States, Pink Coreopsis has a NatureServe national rank of Vulnerable (N3), and is Critically Imperilled to Vulnerable (S1S3) in all eight states in which it presently occurs. It is extirpated (SX) in Pennsylvania. It is legally protected in New York and Maryland. It has a General Status rank of At Risk in Canada and Nova Scotia.
Technical Summary
Coreopsis rosea
Pink Coreopsis
Coréopsis rose
Range of occurrence in Canada: Nova Scotia
Demographic Information
Generation time (average age of parents in the population)
Minimum time from seed to sexual reproduction is two years. Clonal patches likely long-lived. Longevity of rhizome segments (COSEWIC individuals) unclear, potentially several or many years.
estimated ~ 5 years
Is there an [observed, inferred, or projected] continuing decline in number of mature individuals?
Small ongoing decline inferred from impacts of shoreline development. Eutrophication could lead to significant future population declines. Significant historical declines due to damming.
Yes
Estimated percent of continuing decline in total number of mature individuals within [5 years or 2 generations].
Small ongoing declines (<<2.2%) due to shoreline development and off-road vehicle use. Future declines from nutrient enrichment potentially more severe, but likely acting over longer than two generations.
Unknown
[Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over the last [10 years or 3 generations].
Small local declines (<<2.2%) inferred from impacts of shoreline development and off-road vehicle use.
Likely <<2.2%
[Projected or suspected] percent [reduction or increase] in total number of mature individuals over the next [10 years or 3 generations].
Significant eutrophication in 4 occupied lakes, with potential to impact remainder given proximity of existing mink farms and expansion of the industry. Effects could be significant over time, but magnitude and timing of effects is unclear.
Unknown
[Observed, estimated, inferred, or suspected] percent reduction in total number of mature individuals over any (10 year or 3 generations) period, over a time period including both the past and the future.
See above.
Unknown
Are the causes of the decline clearly reversible and understood and ceased?
Eutrophication effects largely reversible but not ceased. Causes are understood in Carleton River but not in Tusket River.
No
Are there extreme fluctuations in number of mature individuals?
No
Extent and Occupancy Information
Estimated extent of occurrence
133 km²
Index of area of occupancy (IAO) (2 x 2 km grid)
72 km²
Is the total population severely fragmented? Most individuals are in large, apparently stable and presumably viable occurrences.
No
Number of “locations*” Locations defined by threat of eutrophication.
4
Is there an [observed, inferred, or projected] continuing decline in extent of occurrence? Low numbers in Agard, Pleasant & Gillfillan lakes occurrences increase susceptibility to localized disturbances and stochastic events. Extirpation at Gillfillan Lake would greatly reduce the extent of occurrence.
No
Is there an [observed, inferred, or projected] continuing decline in index of area of occupancy? Small ongoing declines are not expected to reduce number of 2 x 2 km grid squares occupied, but as noted above, Agard, Pleasant & Gillfillan lakes could be susceptible to unexpected loss, which would reduce IAO.
No
Is there an [observed, inferred, or projected] continuing decline in number of populations?
No
Is there an [observed, inferred, or projected] continuing decline in number of locations?
No
Is there an [observed, inferred, or projected] continuing decline in quality of habitat? Minor declines observed due to shoreline disturbance and possibly eutrophication. Future declines due to eutrophication anticipated.
Yes
Are there extreme fluctuations in number of populations?
No
Are there extreme fluctuations in number of locations*?
No
Are there extreme fluctuations in extent of occurrence?
No
Are there extreme fluctuations in index of area of occupancy?
No
* See definition of location.
| Population | Sub-population | N Mature Individuals See Life Cycle and Reproduction |
|---|---|---|
| 1 Annis River | 1 – Agard Lake | 187 |
| 1 Annis River | 2 – Salmon Lake | ~6,700 |
| 1 Annis River | 3 – Pleasant Lake | 29 |
| 2 Carleton River | 1 – Sloans Lake | ~114,000 |
| 2 Carleton River | 2 – Raynards Lake | 2,569 to 4,000 |
| 3. Tusket River (Bennetts/ Wilsons) |
1 – Bennetts Lake | 50,000 to 100,000 |
| 3. Tusket River (Bennetts/ Wilsons) |
2 – Wilsons Lake | ~103,000 |
| 4. Tusket River (Gillfillan) | 1 – Gillfillan Lake | 114 |
| Total | 276,600 to 328,000 |
Quantitative Analysis
Probability of extinction in the wild is at least [20% within 20 years or 5 generations, or 10% within 100 years] : N/A
Threats (actual or imminent, to populations or habitats)
Eutrophication (nutrient enrichment) is the most significant threat and is expected to increase into the future. It may already be affecting Raynards Lake plants, and has recently been documented on Wilsons and Bennetts lakes (~59-65% of Canadian population), and inferred on Gillfillan Lake. New mink farm development on Sloans Lake (>35% of Canadian population) increases its likelihood there.
Shoreline disturbance associated with waterfront development and recreational activity (including off-road vehicle use) is a localized, ongoing threat at all occupied lakes but population impacts have been small and are expected to be small over last/next three generations.
Water-level regulation for hydroelectric power generation is presumed to have caused significant historical declines. Raynards Lake plants are subject to water-level regulation with unknown consequences but new dam construction is unlikely.
Rescue Effect (immigration from outside Canada)
Status of outside population(s)?
Vulnerable in USA (N3). S3 in Massachusetts (nearest to Canada). Vulnerable to Critically Imperilled in all states where extant and extirpated in Pennsylvania. Extirpated or possibly extirpated in 30% of counties where it has been documented.
Is immigration known or possible? Canadian population is 400+ km across open ocean from nearest occurrences in Massachusetts, making immigration very unlikely.
Very unlikely
Would immigrants be adapted to survive in Canada? Canadian population is found in a climate zone similar to that of northern Massachusetts.
Possibly
Is there sufficient habitat for immigrants in Canada?
Yes
Is rescue from outside populations likely?
No
Status History
Designated Endangered in April 1984. Status re-examined and confirmed Endangered in April 1999, May 2000, and November 2012.
Status and Reasons for Designation
Status: Endangered
Alpha-numeric code: B1ab(iii,v)+2ab(iii,v)
Reasons for designation: This showy perennial lake and river shore plant has a restricted global range with a disjunct distribution limited to southernmost Nova Scotia. There is a concern regarding potential widespread and rapid habitat degradation due to recent increases in levels of phosphorus in lakes, tied to a rapidly growing mink farming industry. Though the population size is now known to be larger than previously documented due to greatly increased survey effort, the species is also at risk due to the continuing impacts associated with shoreline development, and historical hydro development.
Applicability of Criteria
- Criterion A (Decline in Total Number of Mature Individuals):
- Not met: Declines below thresholds.
- Criterion B (Small Distribution Range and Decline or Fluctuation):
- Meets Endangered as extent of occurrence (EO) < 5,000 km² (133 km²) and IAO < 500 km² (72 km²), it is known to exist at 4 locations and there is a continuing decline in the extent and quality of habitat and an inferred decline in the number of individuals.
- Criterion C (Small and Declining Number of Mature Individuals):
- Not met: number of mature individuals exceeds thresholds.
- Criterion D (Very Small or Restricted Total Population):
- Meets Threatened D2 with 4 locations, and the effects of recent nutrient enrichment could cause declines in a short period.
- Criterion E (Quantitative Analysis):
- Not done.
Preface
Since the previous status report (COSEWIC 2000), eutrophication has changed from a theoretical threat to the most significant actual threat. Effluent from upstream mink farming operations is strongly implicated as the cause of eutrophication of the Carleton River system (Brylinsky 2012), including the Raynards Lake population where eutrophication may be impacting Pink Coreopsis habitat and individuals. Water testing in 2011 shows eutrophication to be a widespread threat, documenting 608% to 819% increases in total phosphorus over 2002 levels throughout the main branch of the Tusket River (Mersey Tobeatic Research Institute (MTRI) 2011), which supports roughly 59% to 65% of the Canadian population. One mink farm near Kemptville is upstream of the affected lakes. It is a potential, but unconfirmed, source of increased phosphorus levels in the Tusket River lakes (Pearl, Third, Gillfillan, Lac de l’Ecole, Wilson, Bennetts).
The threats posed by shoreline development and off-road vehicle use have been more clearly quantified since the previous status report, and although both are still in effect, they are now believed to have had only relatively small impacts on populations and are not anticipated to cause large population reductions in the near future.
Extensive fieldwork has produced detailed distribution and abundance data for all lakes on which the species occurs except for Bennetts Lake. The species has been discovered on two new lakes in the Carleton River system (Sloans and Raynards lakes). The Sloans Lake occurrence supports over 35% of the known Canadian population, and documentation of widespread occurrence in limited numbers on Raynards Lake demonstrates that Pink Coreopsis can persist in lakes with dam-controlled water levels. New localities and more detailed population data have increased the estimated population in Canada from a few thousand to 277,000 to 328,000 stems. The extensive fieldwork in southwestern Nova Scotia has also documented the species’ absence from a large number of lakes in the vicinity of known population sites and within the potential range of the species, confirming that its distribution in Canada is extremely limited.
The small population on Gillfillan Lake has been entirely protected within a new provincial nature reserve. New nature reserves owned by the Nature Conservancy of Canada and the Nova Scotia Nature Trust have protected a portion of the large population at Bennetts Lake and a very small portion of the large population at Wilsons Lake. These have increased the proportion of the Canadian population in protected areas from about 7% to about 10 to 15%. These areas protect the upland portion of the species’ habitat, but have very limited effect on protecting the waters from eutrophication.
COSEWIC History
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
COSEWIC Mandate
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC ) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC Membership
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
Definitions (2012)
- Wildlife Species
- A species, subspecies, variety, or geographically or genetically distinct population of animal, plant or other organism, other than a bacterium or virus, that is wild by nature and is either native to Canada or has extended its range into Canada without human intervention and has been present in Canada for at least 50 years.
- Extinct (X)
- A wildlife species that no longer exists.
- Extirpated (XT)
- A wildlife species no longer existing in the wild in Canada, but occurring elsewhere.
- Endangered (E)
- A wildlife species facing imminent extirpation or extinction.
- Threatened (T)
- A wildlife species likely to become endangered if limiting factors are not reversed.
- Special Concern (SC)*
- A wildlife species that may become a threatened or an endangered species because of a combination of biological characteristics and identified threats.
- Not at Risk (NAR)**
- A wildlife species that has been evaluated and found to be not at risk of extinction given the current circumstances.
- Data Deficient (DD)***
- A category that applies when the available information is insufficient (a) to resolve a species’ eligibility for assessment or (b) to permit an assessment of the species’ risk of extinction.
* Formerly described as “Vulnerable” from 1990 to 1999, or “Rare” prior to 1990.
** Formerly described as “Not In Any Category”, or “No Designation Required.”
***Formerly described as “Indeterminate” from 1994 to 1999 or “ISIBD” (insufficient scientific information on which to base a designation) prior to 1994. Definition of the (DD) category revised in 2006.
The Canadian Wildlife Service, Environment Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Wildlife Species Description and Significance
Name and Classification
Scientific Name: Coreopsis rosea Nutt.
Synonyms: Calliopsis rosea Spreng. 1826
Coreopsis rosea f. leucantha Fern.
Type Specimen: Lectotype: T. Nuttall s.n. at BM
English vernacular name: Pink Coreopsis, Rose Coreopsis, Pink Tickseed
French vernacular name: Coréopsis rose
Family: Asteraceae, aster family
Major plant group: Angiosperms, Eudicotyledons
Pink Coreopsis (Coreopsis rosea) was first described by Thomas Nuttall (1818). Sprengel (1826) placed the species in the genus Calliopsis, but all references from Gray (1884) onward appear to have treated the species as Coreopsis rosea. Fernald (1919) described the white-rayed forma leucanthafrom Massachusetts. The genus Coreopsis has approximately 35 New World species (Strother 2006), including several globally rare endemics (Sorrie and Weakley 2001). Life forms vary from herbaceous annuals to long-lived shrubs (Strother 2006), with highest diversity in eastern North America, Mexico and the Andes (Tadesse et al. 1995). Pink Coreopsis is within section Eublepharis, which includes four eastern North American perennial herbaceous species (Strother 2006) and appears to be monophyletic (Kim et al.1999). All other species in the section have native ranges entirely restricted to the southern Atlantic Coastal Plain and the Gulf of Mexico (Biota of North America Program (BONAP) 2010).
Pink Coreopsis is a diploid species with a chromosome number of n=13 (Smith 1983; Crawford et al. 1991; Strother 2006). This is the most common base chromosome number in the genus and is believed to have originated from an ancestral base number of n=14 and shifted through descending aneuploidy (Smith 1975).
Morphological Description
The following is derived from detailed descriptions in Fernald (1950), Gleason and Cronquist (1991) and Strother (2006). Figure 1 illustrates the species in its natural habitat, with a detailed view of flower arrangement.
Figure 1. Pink Coreopsis (Coreopsis rosea) flowering in an emergent shoreline meadow at Wilsons Lake, Yarmouth County, Nova Scotia (photo by David Mazerolle, Atlantic Canada Conservation Data Centre).

Description of Figure 1
Photo of the Pink Coreopsis, providing a detailed view of the flower arrangement. Flowers are aggregated at the top of the stem in a daisy-like head composed of two flower types: yellow tubular florets forming the central disc and petal-like (ligulate) ray florets forming the outer margin of the head.
Pink Coreopsis is a slender perennial herb arising from a rhizomatous root system. The erect stems, 20-60 cm tall, are generally minimally branched and have opposite, sessile, narrowly linear leaves, often also having axillary fascicles of reduced leaves. Flowers are aggregated at the top of the stem in a daisy-like head composed of two flower types: 1) yellow tubular florets forming the 5-10 mm wide central disc; and 2) petal-like (ligulate) ray florets forming the outer margin of the head. These are 9-15 mm long, three-toothed at their tips and deep pink, fading toward white with age.
Flowers produce flat, 1.3-1.8 mm long, narrowly oblong, dry fruit (cypselae). Within its Canadian range and habitat, Pink Coreopsis is fairly distinctive. Superficial similarity of non-flowering plants to vegetative Carolina Grass-leaved Goldenrod (Euthamia caroliniana), which is generally abundant with Pink Coreopsis, makes vegetative individuals easy to miss in the field. Pink Coreopsis is readily distinguished upon closer examination by its opposite (vs. alternate) leaves. Plymouth Gentian (Sabatia kennedyana), which also co-occurs with Pink Coreopsis and has superficially similar stems, has wider lance-shaped stem leaves, and generally has well developed basal rosettes, which are lacking in Pink Coreopsis.
Population Spatial Structure and Variability
Most of the Canadian population is concentrated along the shores of Sloans, Wilsons, and Bennetts lakes. These three lakes respectively contain at least 35%, 31% and 18% of the total Canadian population (Table 1).
| Population | Lake | Number of locations | Primary current or potential threats | Number of individuals1 | % of shoreline and % of individuals protected |
Number and ownership of properties supporting Pink Coreopsis |
|---|---|---|---|---|---|---|
| 1. Annis River | 1.1 Agard | 1 | - nutrient enrichment - shoreline development |
187 | 32% (0%) | 2 private |
| 1. Annis River | 1.2 Salmon | 1 | - nutrient enrichment - shoreline development |
6,700 (accurate est.) | 6% (<1%) | 23 private, 1 Crown |
| 1. Annis River | 1.3 Pleasant | 1 | - nutrient enrichment - shoreline development |
29 | 0% | 1 private |
| 2. Carleton River | 2.1 Sloans | 1 | - nutrient enrichment - shoreline development |
114,000 (accurate est.) |
0% | 14 private |
| 2. Carleton River | 2.2 Raynards* | 1 | - nutrient enrichment - water-level regulation - shoreline development |
2,569 – 4,000 (accurate est.)* | 0% | >11* private |
| 2. Carleton River | 2.3 Lake Vaughan | 0 | - nutrient enrichment - water-level regulation (both likely limiting recolonization) |
- Believed extirpated by flooding from hydroelectric dam. Not recorded since 1920. | ||
| 3. Tusket River (Bennetts/ Wilsons) |
3.1 Bennetts | 1 | - nutrient enrichment - shoreline development |
50,000 to 100,000 (very rough est.) |
33% (likely <25%) | 37 private2, 2 NGO protected |
| 3. Tusket River (Bennetts/ Wilsons) |
3.2 Wilsons | 1 | - nutrient enrichment - off-road vehicle use - shoreline development |
103,000 (accurate est.) |
17% (20%) | 43 private, 1 NGO protected, 1 prov. nature reserve |
| 3. Tusket River (Bennetts/ Wilsons) |
3.3 Gavels Lake | 1 | - nutrient enrichment - water-level regulation (both likely limiting recolonization) |
- Believed extirpated by flooding from hydroelectric dam. Not recorded since 1920. | ||
| 4. Tusket River (Gillfillan) | Gillfillan | 1 | - nutrient enrichment | 114 | 30% (100%) | 1 provincial nature reserve |
| Total | 4 extantpopulations on 8 lakes | 4 | 277,000 – 328,000 | 13% (10-15%) | >131 private, 3 NGO protected, 2 prov. naturereserve |
1 Population estimates from MTRI (2010-2011) and Blaney and Mazerolle (2011), except Bennetts Lake where population was estimated using Wilsons Lake densities (see Abundance).
2 Additional surveys would likely increase number of private properties with Pink Coreopsis. There are 52 lakeshore private properties on Bennetts Lake.
* Detailed surveys have only been conducted over approximately 65% of the lake’s total shoreline; upper population estimate extrapolates observed density in surveyed area across unsurveyed shore.
A landowner on Agard Lake has stated that his father transplanted a sod from Wilsons Lake (where Pink Coreopsis is widespread and locally abundant) to Agard Lake in 1990 in order to introduce Plymouth Gentian there (Hill pers. comm. 2011; COSEWIC in prep.). The small occurrence of Pink Coreopsis on Agard Lake (discovered in 1997, 5 km upstream from the Salmon Lake occurrence) is in the immediate area of the same landowner’s property, so the Agard Lake population may also have been introduced, intentionally or unintentionally, with the Plymouth Gentian. Whatever the origins of Agard Lake plants, they are considered a wild occurrence because they are from a native source and have persisted and spread for 20+ years, and because COSEWIC follows the International Union for Conservation of Nature (IUCN) recommendation that self-sustaining populations resulting from translocations be included in wildlife species assessments regardless of the intent or means of the original introduction (Standards and Petitions Working Group 2006).
Patterns of genetic diversity in South Carolina and Massachusetts Pink Coreopsis were investigated by Cosner and Crawford (1994). They found Pink Coreopsis had relatively high levels of allozyme diversity compared to other Coreopsis species. They also found very high population differentiation, with more than one third of genetic diversity occurring between rather than within populations. Genetic distance between populations strongly correlated with spatial distance, and southern populations were more genetically diverse than northern ones, possibly suggesting that they were ancestral to northern populations that had been through a genetic bottleneck. They also suggested that clonal reproduction does not appear to play a major role in genetic structuring of populations in Pink Coreopsis, citing Mueller (1974) and Smith (1976).
Wood (2006) found significant isolation and genetic differentiation between populations in Nova Scotia and Massachusetts, with Canadian diversity lower than in Massachusetts and South Carolina (compared with Cosner and Crawford 1994), but she found no relationship between genetic and geographic distance within Canadian populations.
Designatable Units
Pink Coreopsis is restricted to a very small portion of the COSEWIC Atlantic Ecological Area in southwestern Nova Scotia. Canadian populations are thus considered a single designatable unit.
Special Significance
Pink Coreopsis is one of three native Coreopsis species in Canada, and is a globally vulnerable species restricted to three small, disjunct regions along the Atlantic coast. It is rare in all jurisdictions in which it occurs and is considered extirpated or possibly extirpated in roughly 30% of counties where it has been documented (NatureServe 2011), which further indicates the global significance of Canadian populations.
The species is extremely rare in Canada, where it co-occurs with a large suite of other disjunct southern species of the Atlantic Coastal Plain, including the Threatened Plymouth Gentian and Water Pennywort (Hydrocotyle umbellata). The attractive flowers of Pink Coreopsis encourage the public to value habitats occupied by Atlantic Coastal Plain flora and provide cottagers with an easily appreciated reason for good stewardship of what they might otherwise consider weeds of their beaches. Canadian populations are significantly isolated from the species’ main range, situated roughly 400 km from the nearest populations in Massachusetts and are significantly genetically differentiated from populations in the United States (Wood 2006). This could indicate that Canadian populations have a disproportionate significance to the species as a whole (Lesica and Allendorf 1995; Garcia-Ramos and Kirkpatrick 1997; Eckert et al. 2008).
Pink Coreopsis is a popular garden ornamental, with many named varieties and hybrid crosses readily available for sale throughout North America.
No evidence of local Aboriginal traditional knowledge for this species was found during the preparation of this report (Hurlburt pers. comm. 2011).
Distribution
Global Range
Pink Coreopsis is a globally rare species of very strong Atlantic Coastal Plain affinity. It is limited to three small, disjunct regions (Figure 2). The largest of these extends from eastern Massachusetts through Rhode Island, New York, New Jersey and Delaware, south to eastern Maryland, with all occurrences within 100 km of the Atlantic coast. Massachusetts holds the largest concentration of populations, supporting over half of all extant occurrences (Gravuer 2009). The species is extirpated in Pennsylvania (NatureServe 2011).
Figure 2. Global distribution of Pink Coreopsis (Coreopsis rosea) based on county-level distribution (NatureServe 2011; USDA 2011; Patrick pers. comm.2011)
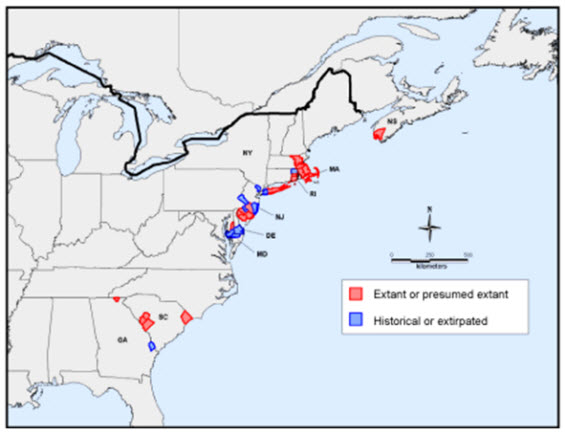
Description of Figure 2
Map of the global range of the Pink Coreopsis in eastern North America based on county-level distribution. The species occurs in three small, disjunct regions. The largest of these extends from eastern Massachusetts through Rhode Island, New York, New Jersey and Delaware, south to eastern Maryland, with all occurrences within 100 kilometres of the Atlantic coast. The southernmost region of occurrence is in South Carolina and Georgia, about 600 kilometres south of the nearest extant population in Maryland, where scattered occurrences are on the southern Atlantic Coastal Plain and in the Appalachian Mountains. Canadian populations are restricted to a small area around the lower Tusket River in extreme southwestern Nova Scotia and are isolated from other occurrences by roughly 400 kilometres.
The southernmost region of occurrence is in South Carolina and Georgia, about 600 km south of the nearest extant population in Maryland, where scattered occurrences are on the southern Atlantic Coastal Plain and in the Appalachian Mountains. Strother (2006) suggested South Carolina occurrences may have resulted from human-mediated dispersal, but Pink Coreopsis is now known to occur in several of the state’s counties and the South Carolina Natural Heritage Program considers the species native (Gravuer 2009). Until very recently, the species was known in Georgia from one historical occurrence documented by Thomas Nuttall in 1815 (Patrick pers. comm. 2011) but in 2010, a second occurrence was discovered in Georgia at Chatuge Lake in the Appalachian Mountains near the North Carolina border (Patrick pers. comm. 2011).
Canadian populations are restricted to a small area around the lower Tusket River in extreme southwestern Nova Scotia and are isolated from other occurrences by roughly 400 km. Approximately 10% of its global range is within Canada.
Canadian Range
In Canada, Pink Coreopsis is restricted to a 20 km by 15 kmarea in southwestern Nova Scotia in the Tusket River Ecodistrict of the Nova Scotia Uplands Ecoregion (Webb and Marshall 1999). Canadian occurrences are limited to eight lakes on the Tusket, Carleton and Annis river systems in south-central Yarmouth County (Figure 3), all of which flow into the Tusket River estuary.
Figure 3. Canadian distribution of Pink Coreopsis (Coreopsis rosea)
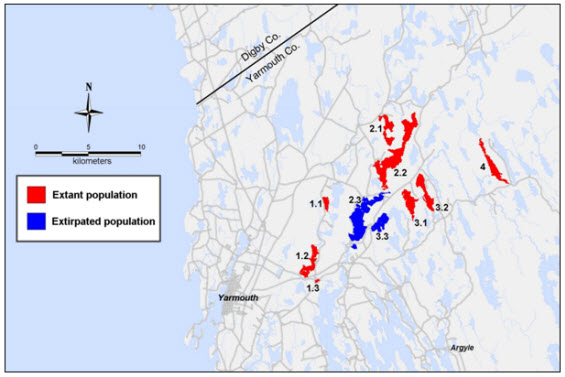
Description of Figure 3
Map of the Canadian distribution of the Pink Coreopsis. The species occurs in an area measuring 20 by 15 kilometres in the Tusket River Ecodistrict of the Nova Scotia Uplands Ecoregion. Canadian occurrences are limited to eight lakes on the Tusket, Carleton and Annis river systems in south-central Yarmouth County, all of which flow into the Tusket River estuary. Numbers used to identify populations and lakes correspond to those in Table 1, as follows: 1 – Annis River system: 1.1-Agard Lake, 1.2-Salmon Lake, 1.3-Pleasant Lake; 2 – Carleton River system: 2.1-Sloans Lake, 2.2-Raynards Lake; 3 and 4 – Tusket River system: 3.1-Bennetts Lake, 3.2-Wilsons Lake, 4-Gillfillan Lake; Extirpated occurrences: 2.3-Lake Vaughan and Tusket Falls; 3.3-Gavels Lake.
Extent of Occurrence and Area of Occupancy
Following COSEWIC guidelines (COSEWIC 2009), the extent of occurrence (EO) for extant populations in Canada is 133 km². Index of area of occupancy (IAO) derived using a 2 x 2 km grid aligned with 10 x 10 km UTM grid squares is 72 km² when limited to extant sites. The species has apparently been lost on Lake Vaughan and Gavels Lake because of flooding associated with hydroelectric development around 1929. This significantly reduced IAO but did not affect EO. IAO and EO are believed stable within the past three generations (15 years).
Delimitation of Populations
COSEWIC separates populations if there is typically less than one successful genetic exchange per generation (COSEWIC 2009). Because rates of genetic exchange are unknown for Pink Coreopsis, populations are defined in this report using NatureServe (2004), under which occurrences meeting one of the following conditions are grouped into a single population: 1) occurrences separated by less than 1 km, 2) occurrences separated by 1 to 3 kmwith no break in suitable habitat between them exceeding 1 km, 3) occurrences separated by 3 to 10 km but connected by linear water flow with no break in suitable habitat between them exceeding 3 km. Four extant populations are thus known to occur in Canada: 1) Agard, Salmon and Pleasant lakes (Annis River), 2) Sloans and Raynards lakes (Carleton River), 3) Wilsons and Bennetts lakes (Tusket River) and 4) Gillfillan Lake (Tusket River). The historical occurrences at Lake Vaughan and Gavels Lake are believed extirpated by hydroelectric dam flooding, although recent documentation of plants on the dam-controlled Raynards Lake suggests the possibility of some persistence.
Search Effort
The presence of Atlantic Coastal Plain flora in southern Nova Scotia has been well known since Merritt Fernald’s expeditions (Fernald 1921, 1922), which included the discovery of at least five Pink Coreopsis occurrences. Extensive floristic work focused on coastal plain flora in southern Nova Scotia has since been undertaken, starting in the 1950s to the 1970s by Chalmers Smith and students, and by Albert Roland, and John and David Erskine (as documented in Roland and Smith 1969). Paul and Cathy Keddy, Irene Wisheu, Nicholas Hill and their collaborators conducted detailed studies on the ecology, distribution and local diversity of Nova Scotian coastal plain flora with a focus on conservation implications (Keddy 1984, 1985, 1989; Keddy and Wisheu 1989; Wisheu and Keddy 1991; Hill and Keddy 1992; Wisheu and Keddy 1989; 1994; Wisheu et al. 1994; Holt et al. 1995; Morris et al. 2002). This work included visits to all major lakes through which the lower Tusket River flows and many other lakes nearby (Appendix 1). More recently, extensive floristic and conservation work has been conducted by Atlantic Canada Conservation Data Centre (AC CDC), Nova Scotia Department of Natural Resources, Nova Scotia Nature Trust and Mersey Tobeatic Research Institute (MTRI) (e.g. Eaton and Boates 2003; Blaney 2002, 2004, 2005a, 2005b; Blaney and Mazerolle 2009, 2010; MTRI 2010; Blaney and Mazerolle unpubl. 2011). Fieldwork in 2011-2012 included visits to 21 additional lakes in the vicinity of known Pink Coreopsis occurrences (Appendix 1). The species has not been found on at least 60 lakes in or near the Tusket watershed surveyed in the last 30 years (Appendix 1) as well as dozens more lakes further afield within the region of Atlantic Coastal Plain flora occurrence in Queens and Lunenburg counties. Despite the extensive search effort, only three new occurrences (Agard Lake in 1997, Sloans Lake in 2002 and Raynards Lake in 2005) have been found since the original status report (Keddy and Keddy 1984).
Since 1920, there have been several hundred field days spent on lakeshores in the potential range of Pink Coreopsis by botanists capable of identifying the species. Search effort is certainly sufficient to conclude that the species is very infrequent within the coastal plain zone of Nova Scotia. Pink Coreopsis records in Nova Scotia are primarily from lakes with large watersheds above them, and there are no such lakes around the species’ lower Tusket River area of occurrence that remain unsurveyed. Additional populations could occur on unsurveyed lakes but the species’ pattern of occurrence and the number of lakes on which the species has been confirmed absent suggests few additional populations are likely. The documentation of extensive occurrence of Pink Coreopsis at Raynards Lake demonstrates that it can persist on reservoir shorelines. Reservoirs have generally not been as intensively surveyed by botanists as natural lakes, so the shorelines of the lower Tusket reservoir lakes (Kings Lake, Gavels Lake, Lake Vaughan and the unsurveyed portions of Raynards Lake), which are immediately downstream from large Pink Coreopsis populations and within the historical range, could support additional plants, which would be included within populations and localities already documented.
Habitat
Habitat Requirements
Range wide, Pink Coreopsis occurs on lake, pond and vernal pool shores and on river or stream margins (Gravuer 2009). In Canada, it grows exclusively in lakeshore habitats and occurs primarily on larger, lower-watershed lakes having a large upstream catchment area (Keddy 1985; Lusk 2006). These lakes tend to have wider shorelines because of larger annual water-level fluctuations and their greater wave and ice action helps maintain infertile conditions by removing fine particles and nutrients from the soil (Keddy 1983, 1984, 1985; Hill and Keddy 1992; Hill et al. 1998; Morris et al. 2002). This limits competition from shrubs and other high biomass shoreline species (Keddy and Wisheu 1989; Sweeney and Ogilvie 1993; Morris et al. 2002).
Pink Coreopsis populations within a lake are often concentrated on the windward (eastern or northeastern) shore. Plants are typically on broad, gently sloping shores of sand, gravel, fine cobble and shallow peat (Keddy 1985; Keddy and Wisheu 1989; Blaney and Mazerolle pers. obs. 2009-2011), in areas with low standing crop and little or no litter (Keddy and Wisheu 1989; Craine and Orians 2004; Blaney and Mazerolle pers. obs.2009-2011). In these habitats, Pink Coreopsis occupies a zone below the shrub line where flooding is frequent and it occurs both on exposed substrates and as an emergent in shallow water (to a depth of about 15 cm at low water periods, Blaney and Mazerolle pers. obs. 2011). It is most often found in association with Carolina Grass-leaved Goldenrod, Twig-rush (Cladium mariscoides), Swamp Loosestrife (Lysimachia terrestris), Carolina Yellow-eyed Grass (Xyris difformis), Canada Rush (Juncus canadensis), Golden Hedge-hyssop (Gratiola aurea), Seven-angled Pipewort (Eriocaulon aquaticum), Redtop Panic Grass (Panicum rigidulum var. pubescens) and Plymouth Gentian.
In Canada, climate may play a key role in limiting the species’ occurrence. Oceanic moderation in the coastal zone of extreme southwest Nova Scotia creates some of the warmest Canadian winters outside of southern British Columbia (United States Department of Agriculture (USDA) 1990; Agriculture and Agri-Food Canada 2000), with more than 180 frost-free days immediately along the coast, and a moderating effect extending inland to a lesser degree into the range of Pink Coreopsis (Energy, Mines and Resources Canada 1981). Winter flooding over rhizomes may also be important in insulating the species against freezing as in Plymouth Gentian (Hazel 2004).
Habitat Trends
a) Historical habitat loss
Pink Coreopsis occurrences on at least two lakes are believed to have been extirpated by construction of hydroelectric and headpond dams at Lake Vaughan, Raynards Lake and Gavels Lake starting in 1929. These dams flooded the original shores, transforming Lake Vaughan, Raynards, Gavels and Kings lakes into artificially regulated reservoirs. Fernald (1921, 1922) and AC CDC (2011) document historical records of Pink Coreopsis at Lake Vaughan and Gavels Lake (but not Raynards Lake, where it occurs at present and presumably occurred historically). It likely also occurred at Kings Lake given its presence immediately upstream and downstream, and modelling that predicts hydrologically suitable habitat (Hill et al. 1998). Precise maps of the area from prior to 1929 are not available, but depth data (Nova Scotia Power 2009) suggests there were more than four lakes (likely all occupied by Pink Coreopsis) connected by narrow river segments prior to damming. Current habitat at Raynards Lake is also likely much reduced from pre-dam levels. The proportion of original habitat in the original Canadian range of Pink Coreopsis that was lost or altered because of the dams may have been over 50%, given that shoreline on the reservoir lakes is presently about 63 km and total shoreline distance on the lakes (Bennetts, Wilsons, Salmon and Sloans) currently occupied by large Pink Coreopsis populations is only 50 km.
b) Recent and future habitat change
Eutrophication is a major new issue affecting Pink Coreopsis habitat to varying degrees in all extant occurrences (see detailed discussion in Threats – Eutrophication). Impacts on habitat are primarily through the potential to increase competition from more common shoreline plants, but potentially also through covering of seedlings by cyanobacterial mats. As noted below, eutrophication-induced habitat changes are not believed to have yet had significant impacts on Pink Coreopsis, but could become problematic in future.
As noted under Threats, other factors aside from eutrophication are not believed to have greatly impacted Pink Coreopsis habitats or populations in the past 10 to 15 years (estimated to equal three generations) and are not expected to have large impacts over the next three generations, although shoreline development and off-highway vehicle (OHV) use are having continuing localized effects on habitat.
Biology
Life Cycle and Reproduction
Pink Coreopsis is an herbaceous, obligate perennial, requiring at least two full growing seasons after germination before it can set seed (Shipley and Parent 1991). In Nova Scotia, plants begin growing actively in early April, while still submerged by high springtime water levels (Lusk and Reekie 2007). Throughout the growing season, Pink Coreopsis readily reproduces vegetatively by forming clonal shoots at the apex of rhizomes (Gleason 1952; Keddy 1985). The species is also known to sprout from nodes of fallen stems (Wisheu and Keddy 1991) and can even root from broken stem fragments (Lusk 2006).
In its Canadian range, Pink Coreopsis flowers from mid-July to late September. Average time from appearance of flower bud to development of seeds is approximately 20 days in cultivation, with flower heads receptive to pollination over a four-day period (Siqueira 2003). The species is known to be largely self-incompatible and therefore depends on cross-pollination for sexual reproduction to take place (Smith 1975, 1983; Siqueira 2003; Loehrlein and Siqueira 2005). Preliminary work cited in Wood (2006) suggests that Nova Scotia Pink Coreopsis has weak pollen tube growth for both self-and cross-pollinations, suggesting that its self-incompatibility system may be breaking down. Evolution of self-compatibility is a common trend among members of the Asteraceae coping with low levels of genetic diversity (Reinartz and Les 1994). Potential pollinating insects are discussed in Interspecific Interactions.
Ovaries mature in late summer and early fall, with each fertilized flower in the head producing a single, dry, one-seeded fruit (cypsela). Shipley and Parent (1991) noted that cold-stratified Nova Scotia seeds kept at an alternating temperature of 20/30°C and a 15-hour photoperiod began to germinate after six days and achieved a maximum germination of 50% after 30 days. Hazel (2004) found only 2% germination of Wilsons Lake seeds under similar treatment in a small trial (50 seeds). Seedling recruitment has been noted in the field (Keddy and Keddy 1984), but has never been quantified. Soil seed banking was limited (11 seeds/m²) within a dense population at Wilsons Lake (Wisheu and Keddy 1991). Seed longevity in Pink Coreopsis has not been investigated. In Sand Coreopsis (Coreopsis lanceolata), a more widespread species of dry sandy habitats, seed longevity ranges from 2 years in smaller seeds to 13 years in larger ones, with 99% of all seeds surviving less than a decade (Banovetz and Scheiner 1994).
Because individual shoots (or ramets) are relatively discrete, are capable of asexual reproduction, and can survive in the field to produce new shoots after fragmentation (Lusk 2006), both flowering and vegetative shoots are considered mature individuals for the purposes of determining population and generation time (COSEWIC in prep.). Shoots are annual, although new shoots generated from buds at the base of a previous year’s shoot are considered the same COSEWIC individual to be consistent with the treatment of non-rhizomatous perennial plants (COSEWIC 2010). Field observation (Blaney and Mazerolle pers. obs. 2011) suggests that vegetative reproduction in an individual shoot is limited or non-existent in its first year of growth, but shoot longevity and patterns of rhizome spread and yearly re-growth from shoot bases are not otherwise known. Generation time (average age of individuals capable of vegetative and/or sexual reproduction, COSEWIC 2010) is thus unclear but is estimated at five years for this report. The lifespan of individual shoots is unknown, but the genet (individual plant) could likely live many years.
Physiology and Adaptability
Pink Coreopsis, like many of the lakeshore species of Nova Scotia’s Atlantic Coastal Plain flora, is a stress-tolerant species adapted to survive in infertile and periodically flooded habitats (Wisheu and Keddy 1989; Sweeney and Ogilvie 1993; Wisheu et al. 1994). This stress-tolerance allows it to escape competition and to thrive in habitats that are inhospitable to many other species (Keddy and Wisheu 1989; Wisheu and Keddy 1989, 1994). Pink Coreopsis is capable of growth in both aquatic and terrestrial conditions, although growth and survival are much higher in individuals that are not completely submerged for extended periods (Lusk 2006; Lusk and Reekie 2007). Plants are believed to need at least some time above water and have been shown to fare much better when exposed for 100 days or more, particularly when most of this period coincides with July and August (Lusk and Reekie 2007).
Dispersal and Migration
Pink Coreopsis readily disperses vegetatively through rhizome growth, enabling the species to spread over short distances into adjacent suitable habitat. New shoots can also sprout from the nodes of fallen stems, allowing the species to spread by distances equalling the height of plants (Wisheu and Keddy 1991). Broken stem segments can sometimes root as well (Lusk 2006). Disturbance by ice, animals or off-road vehicles may break rhizome segments, or stem segments (Lusk 2006), or dislodge small sections of turf that could be further transported by water and ice, as has been observed in Plymouth Gentian (COSEWIC in prep.).
Although Pink Coreopsis shows no adaptation for wind dispersal, small seed size (1.3-1.8 mm long) and weight (0.11 mg average, Shipley and Parent 1991) suggest that strong winds could carry seeds over moderate distances, especially over ice in winter. Because all Canadian populations are situated on lakeshores, long-distance seed dispersal across water bodies and downstream along river systems may be possible, although the buoyancy and floatation period of Pink Coreopsis are not known.
No information is readily available on the role of animal dispersal in Coreopsis. Although Pink Coreopsis does not exhibit adaptations for zoochory (animal-mediated dispersal) such as fleshy fruits or fruits/seeds bearing stiff hairs, seeds could be transported over longer distances if lodged in animal fur or feathers. Fallen seeds could also be carried in mud clinging to animals, people or to OHVs passing through population sites.
Interspecific Interactions
Pink Coreopsis is a stress-tolerant plant with low competitive ability. In an experiment investigating the competitive response of a number of rare species of the Atlantic Coastal Plain Flora, the mean biomass of Pink Coreopsis individuals grown alone was more than 4.6 times that of plants grown within a sward of several other species (Keddy et al. 1998). Any habitat alterations that increase nutrient availability and/or suppress natural water level fluctuations can lead to encroachment by more competitive species and could result in habitat loss and local extirpations (Keddy 1989; Wisheu and Keddy 1994).
In species of the Aster family, pollination is carried out by a wide variety of insects, including bees, wasps, flies, moths, butterflies and beetles (Jones 1978; Semple et al. 1996; Robson 2010). Pink Coreopsis flowers in the United States are visited by hover flies (family Syrphidae), lance flies (family Lonchaeidae), ebony bugs (family Thyreocoridae), butterflies of the family Pieridae, bumblebees and honey bees (family Apidae) and tachinid flies (family Tachinidae) (Siqueira 2003). It is not clear which of these are effective pollinators. Both bees and flower flies were observed on flowers during recent surveys at Wilsons Lake (Blaney and Mazerolle, pers. obs.2011).
Pink Coreopsis is somewhat toxic to certain invertebrates, such as the European Corn Borer Moth (Ostrinia nubilalis), a highly polyphagous introduced species (McCanny et al. 1990). The larvae of several moth species are known to feed on the foliage of other Coreopsis species, but information specific to Pink Coreopsis is limited to possible herbivory by plant bugs (family Miridae) in the United States (Siqueira 2003). No significant insect damage has been noted in Canada. Egg-laying in stems of Pink Coreopsis has been observed, although identification of the species responsible was not attempted (Lusk 2006, pers. comm. 2011).
Browsing by White-tailed Deer (Odocoileus virginianus), Muskrat (Ondatra zibethicus) or Beaver (Castor canadensis), mostly involving the consumption of flower heads and the upper part of plants, has been detected at Gillfillan Lake and Wilsons Lake and may be fairly common in Nova Scotia. At Gillfillan Lake, the heads of most plants in the small population were consumed in 2011 (Blaney and Mazerolle, pers. obs. 2011). Given the small population (114 stems) at the site, this level of browsing might impact population viability if present annually. Browsing was common enough at Wilsons Lake in 1995 and 2011 (Hill pers. comm. 2011; Blaney and Mazerolle pers. obs. 2011) to suggest that Pink Coreopsis may be a favoured lakeshore species for deer, but impacts of browsing did not appear to be significantly affecting populations on the lake.
Population Sizes and Trends
Sampling Effort and Methods
Populations were estimated by lake (Appendix 2). Fieldwork covered Wilsons Lake and Gillfillan Lake populations completely. An MTRI project had completed comprehensive counts on Sloans, Agard, Salmon, and Pleasant lakes in 2010 and partial counts on Raynards Lake, so those lakes were not revisited. Four lakes in close proximity to known occurrences where Pink Coreopsis was not previously known were also comprehensively surveyed for this status report (Canoe, Lac a Pic, Springhaven Duck and Long lakes). The highest potential habitat on English Clearwater Lake was also surveyed.
No attempt was made to quantify error or uncertainty in any of the lake-specific population estimates in this report. The counts on Gillfillan, Agard, and Pleasant lakes should accurately represent population numbers because the small numbers of stems were counted individually and populations are believed to have been comprehensively documented. The population estimates for Salmon, Sloans and Wilsons lakes involved comprehensive coverage of populations and are largely derived by extrapolation of population values per metre of shoreline extrapolated over GPS-measured distances. These estimates should represent actual numbers fairly accurately (with actual values very likely within plus or minus 30% of the given values). Individual stems were counted over the 65% of Raynards Lake walked in 2011 by MTRI staff, and error relative to actual numbers in this area should be small. Uncertainty over total numbers on Raynards Lake is because of the portion of shoreline not covered. Bennetts Lake was not visited for this report. The population estimate is a guess based on observed densities and total shoreline distance at Bennetts Lake being similar to Wilsons Lake. The high degree of uncertainty of that population estimate is reflected in the broad population range given (50,000 to 100,000).
Abundance
The total Canadian population of Pink Coreopsis is approximately 276,600 to 328,000 stems in four populations on three river systems (Table 1; Appendix 2). As noted in Life Cycle and Reproduction, this likely over-estimates COSEWIC “mature individuals” to some degree, but is the only available metric for population.
The Annis River population includes occurrences on three small to medium-sized lakes and contains less than 3% of the total Canadian population. With an estimated 6,700 stems widely distributed along its shores, Salmon Lake holds 97% of all stems found in this watershed. Agard Lake, 5 km upstream, and Pleasant Lake, just downstream, each hold single small occurrences (187 and 29 stems respectively).
The Carleton River population is estimated at 116,600 to 118,000 stems on Sloans Lake and the Raynards Lake reservoir (MTRI 2010; Belliveau pers. comm. 2011). Pink Coreopsis is particularly abundant and widespread along the shores of Sloans Lake, which supports an estimated 114,000 stems, representing at least 35% of the total Canadian population. Suitable habitat is much less common on the dam-regulated Raynards Lake, where occurrences are smaller and scattered and are estimated at 2,600 to 4,000 stems.
The Tusket River system supports two populations. In the lower part of the watershed Wilsons and Bennetts lakes (separated by 600 m of river and thus a single population), contain extensive high-quality Atlantic Coastal Plain shoreline habitat and represent the largest Canadian population. Pink Coreopsis is common and widespread at both lakes, with highest abundances found along north, northeastern and northwestern shores. Comprehensive shoreline surveys in 2011 (Blaney et al. 2011) counted approximately 103,000 stems at Wilsons Lake. Although extensively surveyed, Bennetts Lake does not have comparable, comprehensive distribution and abundance data but numbers are probably similar to those at Wilsons Lake and are estimated for this report at 50,000 to 100,000+ stems (see Appendix 2). The Wilsons and Bennetts lakes occurrences collectively represent about 59% to 65% of the Canadian population. The second Tusket River population is 16 km upstream at Gillfillan Lake, where Pink Coreopsis is limited to a single occurrence of 114 stems over 20 m of shore (Blaney et al. 2011).
Fluctuations and Trends
There is no evidence of significant natural population fluctuations in Canada. The massively increased population sizes in this report vs. previous status reports (Keddy and Keddy 1984; COSEWIC 2000) are almost certainly due to more systematic and comprehensive surveys. Surveys repeated at particular sites by various botanists over many years (i.e. Nick Hill 1988 to 2011, Sean Blaney 2002 to 2011, Ruth Newell 1980 to 2010, Pamela Mills 1997 to 2008) have not produced any anecdotal reports of major declines or increases. Detectability at a site is likely to fluctuate with water level, but Pink Coreopsis is very tolerant of substantial water level fluctuation, frequently flowering in 15+ cm of water (Lusk 2006; Lusk and Reekie 2007), so any actual population changes related to water level are likely much less than one order of magnitude.
The only site for which past population information is comparable to recent information is Salmon Lake, where 5,000 plants were estimated in 1997 (COSEWIC 2000) and 6,700 stems were counted in 2010, with the difference between the values probably within the margin of error of the 1997 count, meaning that numbers do not indicate significant change.
There is insufficient information to assess population effects of eutrophication, although they do not appear to have been large to this point (Blaney pers. obs. 2002-2011). Existing data on shoreline development, water level management, and OHV damage suggest only limited recent population effects (see Habitat Trends and Threats). Aside from small numbers lost with localized human disturbance, populations seem to have been relatively stable within the past 10 to 15 years, and the population trend for the next 10 to 15 years will be largely determined by the extent to which eutrophication impacts Pink Coreopsis.
Severe Fragmentation
The Canadian Pink Coreopsis population is not considered severely fragmented (COSEWIC 2009) because most individuals occur within large populations that are presumed to have good viability. Two small and isolated occurrences do, however, indicate some degree of fragmentation. The uppermost occurrences on the Annis and Tusket rivers (on Agard and Gillfillan lakes, with 147 and 114 individuals respectively) are significantly isolated from others downstream and would be unlikely to be re-colonized in the event of local extirpation. Hydroelectric development has contributed to the fragmentation of the Canadian population, as loss of habitat between Raynards and Bennetts lakes has greatly decreased the potential for genetic exchange between occurrences on the Tusket and Carleton rivers.
Rescue Effect
The Canadian Pink Coreopsis population is isolated from the next nearest occurrence in Massachusetts by 400+ km, with most of that distance being across open ocean. Potential for rescue is therefore extremely limited.
Threats and Limiting Factors
Eutrophication
Since the last status report (COSEWIC 2000), eutrophication has changed from a theoretical threat to Pink Coreopsis (Wisheu et al. 1991; Eaton and Boates 2003; Environment Canada and Parks Canada Agency 2010; Brylinsky 2011a) to the most significant actual threat. Eutrophication of previously oligotrophic wetlands can have a detrimental impact on Atlantic Coastal Plain shoreline plant communities (Ehrenfeld 1983; Moore et al. 1989; Zaremba and Lamont 1993), causing establishment and increased abundance of competitive, high-biomass species, which can reduce species richness (Ehrenfeld 1983; Wilson and Keddy 1988) and cause eventual loss of rare species (Morgan and Philipp 1986; Moore et al. 1989). Pink Coreopsis has limited tolerance of competition for light and nutrients from larger and faster growing plants (Wisheu and Keddy 1989), so major increases in nutrient availability could have a significant impact on populations.
Eutrophication impacts on the Carleton River system, which includes the Raynards Lake population, have become a major public issue with local landowners since 2007 when annual cyanobacterial blooms were first noted (Brylinsky 2011a, b). Algal blooms occur with the addition of nitrogen and phosphorus to the river system. The decay of the algae depletes oxygen, kills fish and bottom-dwelling animals, and thereby creates “dead zones” in the body of water (Carpenter 2008). Effluent from mink farming is identified as the primary cause of increased nutrient levels (Brylinsky 2012). “Most of the phosphorus was present in the dissolved inorganic form which is not typically found in high concentrations in aquatic ecosystems because of its rapid assimilation by aquatic plants. This suggests that the major source of phosphorus is most likely to be a result of mink farm operations that utilize superphosphate, a substance used to increase the shelf of mink feed and to reduce the occurrence of kidney stones in mink livestock” (Brylinsky 2011a). Lake Fanning, just upstream from Raynards Lake, was a very nutrient poor (ultra-oligotrophic) lake in 1986 (Brylinsky 2011a, b) and 2002 (Eaton and Boates 2003), but total phosphorus has increased 1000% since that time and phosphorus and chlorophyll a levels are now in the eutrophic range (MTRI2011). The density of competing native vegetation, especially Golden Hedge-hyssop, are evident over much of the Raynards Lake shoreline (Belliveau pers. comm. 2011) and appears to be limiting the vigour of the Threatened Plymouth Gentian on Lake Fanning. The invasive exotic Reed Canary Grass (Phalaris arundinacea), a species almost never seen in nutrient-poor lakes, has also expanded (COSEWIC in prep.) and could become an issue for Pink Coreopsis habitat on Raynards Lake, as it is likely present in the surrounding area already (Blaney pers. obs. 2011).
At Raynards Lake itself, total phosphorus in the upper mesotrophic range was recorded in 2009 and 2010 (Brylinsky 2011b), but it is unclear if habitat has changed with eutrophication in the past 10 years, because almost all known plants on the lake were first discovered in 2011. Competing plants at Raynards Lake have much higher standing crops than other Pink Coreopsis lakes, a feature that would be consistent with a response to eutrophication.
Eutrophication is also occurring in the Tusket River system, which supports an estimated 59% to 65% of the Canadian population of Pink Coreopsis at Wilsons and Bennetts lakes, and at Gillfillan Lake. Recent nutrient level testing (MTRI 2011) has shown 608% to 819% increases in total phosphorus since 2002 at the Wilsons and Bennetts lakes population (Table 2). Gillfillan Lake, is probably similarly affected since phosphorus levels are elevated both upstream (at Pearl Lake) and downstream. The cause of the eutrophication is unknown, but one mink farm is present upstream near Pearl Lake. No habitat or population impacts on Pink Coreopsis have yet been noted and future testing will be required to determine if 2011 results were an anomaly, but total phosphorus on Pearl and Bennetts lakes was in the same range as on Lake Fanning, so declines in habitat quality and population similar to those suspected on Lake Fanning seem likely if nutrient levels remain high.
| Lake | Watershed | Total Phosphorus 2002 (µg/L) |
Total Phosphorus 2011 (µg/L) |
% Change | Trophic Status 2002* | Trophic Status 2011* |
|---|---|---|---|---|---|---|
| Bennetts Lake | Tusket R | 9.67 | 77.00 | 797% | Oligotrophic | Eutrophic |
| Wilsons Lake | Tusket R | 8.33 | 50.67 | 608% | Oligotrophic | Mesotrophic |
| Pearl Lake (upstream from Gillfillan Lake) | Tusket R | 11.67 | 95.50 | 819% | Mesotrophic | Eutrophic |
| Lake Fanning (upstream from Raynards Lake) | Carleton R | 10.33 | 103.33 | 1000% | Mesotrophic | Eutrophic |
| Raynards Lake | Carleton R | --- | 64.00 | --- | --- | Eutrophic |
| Sloans Lake | unnamed branch, Carleton R | 3.00 | 1.00 | -333% | Oligotrophic | Oligotrophic |
| Salmon Lake | Annis R | 13.00 | 17.33 | 133% | Mesotrophic | Mesotrophic |
* Trophic status based on Carlson’s Trophic Status Index (Carlson 1977).
Mink farming is one of the fastest growing agricultural sectors in Nova Scotia (Nova Scotia Department of Agriculture 2009). Statistics Canada (2007) reported that Nova Scotia was the largest producer of mink fur in the country, with an 89% increase in provincial mink production from 2001 to 2006. Approximately 75% of Nova Scotia mink farms are in Yarmouth and Digby counties, including 40 mink farms within the Carleton River watershed alone (David Suzuki Foundation 2011). Provincial regulation of mink farm waste discharge has been very limited up to 2011, when specific regulations were developed (Nova Scotia Department of Agriculture 2011). These regulations are still in public consultation and are unlikely to be in effect for several years, and it is not clear if the new regulations or available enforcement will be sufficient to limit the impacts of new farms as they are built. Thus eutrophication from mink farming is a potential future threat to all other populations. The threat is most immediate at Sloans Lake, where construction of a large new mink farm 800 m upslope from the lake was completed in 2010 (Brylinsky 2011b). Sloans Lake supports a large majority of Pink Coreopsis plants in the Carleton River watershed and over 35% of the total Canadian population. Water quality surveys at Sloans Lake in 2002, 2009, 2010 and 2011 showed no eutrophication (Nova Scotia Environment 2010; Brylinsky 2011b; MTRI 2011) but the lake has a very low flushing rate of 0.7 times/year (Brylinsky 2011b), making it especially susceptible to future nutrient accumulation. Once phosphorus has entered the system, the recovery of eutrophic lakes following a reduction in the external phosphorus loading may be slow as the phosphorus is stored in the lake sediments (Marsden 1989; White et al. 2002).
Water quality monitoring at Salmon Lake has shown stable nutrient levels since 2002 (MTRI 2011). This result likely applies to Pleasant Lake immediately downstream and is also suggestive of an absence of major nutrient inputs to Agard Lake upstream. The Pink Coreopsis population at Salmon Lake has been stable since 1997 (see Fluctuations and Trends), but a facility for processing fish into mink food is present on the shore of Salmon Lake. It could be contributing nutrients to the lake and thereby limiting Pink Coreopsis to some extent, because the lake is in the mesotrophic rather than oligotrophic range (MTRI 2011). Any increases in effluent from the fish plant could affect the Salmon Lake - Pleasant Lake population, which represents 97% of plants known in the Annis River system, and expansion of mink farming in the region could change the apparently stable nutrient levels of the watershed within a short period.
Shoreline Development
Shoreline development, primarily for cottages, is considered a significant threat to rare Atlantic Coastal Plain plant populations in Canada (Wisheu and Keddy 1989; Eaton and Boates 2002; Environment Canada and Parks Canada Agency 2010). It is having small, but widespread and ongoing impacts on Pink Coreopsis populations, because the gently sloping lakeshores where Pink Coreopsis is most abundant tend to be considered prime sites for cottage or rural residential development.
Impacts of shoreline development on Pink Coreopsis populations appear relatively minor to this point. A large portion of the Canadian population is relatively sheltered from direct disturbance from cottage construction because of its occurrence well below the mean high-water mark. Disturbances in these areas are largely limited to vegetation removal and substrate alteration to enhance swimming or for trail, boat launch, dock, or patio construction. Shoreline development thus often impacts but rarely eliminates the Pink Coreopsis from a property. Cottagers typically use a limited portion of their shorefront intensively, reducing or eliminating the species in that area, while the remaining shorefront is used less intensively in ways that may allow persistence of most or all plants that occurred prior to development and that frequently leave relatively undisturbed portions of shoreline between adjacent cottages. However, impacts can continue after cottage construction as shorelines are further landscaped and maintained for swimming and as new structures are added. Development also leads to a higher incidence of trampling and OHV passage along lakeshores.
Aerial photography from 1973 and from 2010 shows the total number of buildings within 50 metres of the Pink Coreopsis lakes has increased from approximately 75 to approximately 160 (Mazerolle unpubl. 2011). Eaton and Boates (2002) calculated that on five of eight Pink Coreopsis lakes (Sloans, Agard, Bennetts, Wilsons and Gillfillan lakes), shoreline development between 1945 and 2000 had eliminated 2.2% of the natural vegetation cover within 100 mof the shoreline. This would translate to much less than 2.2% population loss because the species generally persists at some level after shoreline alteration, and because most of that habitat alteration would be in upland habitat not occupied by Pink Coreopsis. Population losses to development within the last three generations would be even less, because the majority of existing development is more than 10 to 15 years old.
Shoreline development impacts are ongoing with intensification of existing development and with new development. A particularly significant potential development is at the north end of Wilsons Lake, where a new access road to the shore was built about 2008 for a large property where 41,000 stems were estimated over the property’s one km of shoreline in 2011 (Blaney and Mazerolle pers. obs. 2009-2011). Only about 12 to 16% of the Canadian population is in areas with protected shorefront (non-governmental and provincial Nature Reserves and Crown land), so there is significant potential for additional population loss from future development. However, if development continues at the pace of the past 10 to 15 years, total population impacts in the next three generations are unlikely exceed the < 2.2% impacts thus far.
Off-highway Vehicle Traffic (OHV)
Off-highway vehicle traffic is considered a threat to several coastal plain flora species in Nova Scotia (Wisheu and Keddy 1991; Environment Canada and Parks Canada Agency 2010). Coastal plain species’ slow growth rates increase their vulnerability to disturbance (Sharp and Keddy 1985; Keddy and Wisheu 1989) and even infrequent vehicle traffic could allow more common species (especially rushes, Juncusspp.) which are especially abundant in the seed bank, to colonize areas once occupied by rare species (Keddy and Wisheu 1989).
Presently, off-highway traffic is a significant threat to Pink Coreopsis only at Wilsons Lake, where a well-used trail runs along the lake’s northeastern and eastern shore over one to two kilometres, causing damage or loss of plants and degradation of habitat. Less than five percent of plants on the lake, representing less than three percent of the Canadian population are believed to be affected by this trail (Blaney and Mazerolle pers. obs. 2011). In past years, OHV use has also been noted as a problem along the northwest shore of Wilsons Lake (COSEWIC 2000) and locally on Gillfillan Lake (Wisheu and Keddy 1994; Sutton 2008), but only very limited signs of vehicle traffic were observed in these areas during surveys in 2011 and habitats seem to have recovered from disturbance. Off-highway vehicle impacts on other population sites were minimal to non-existent in 2011 (Blaney and Mazerolle pers. obs.2011).
Anthropogenic Manipulation of Water Levels
Water-level fluctuations play an essential role in maintaining species’ richness and habitat zonation patterns on shorelines (Dynesius and Nilsson 1994), and the actions of flooding, erosion and ice scouring are crucial in maintaining shoreline Atlantic Coastal Plain flora habitat in Canada (Wisheu and Keddy 1989; Hill et al. 1998). Artificial regulation of water levels typically brings about severe changes in shoreline vegetation communities (Hill et al. 1998; Nilsson and Berggren 2000) and is widely recognized as having potential to reduce or eliminate Pink Coreopsis populations (Wisheu and Keddy 1994; Lusk and Reekie 2007; Environment Canada and Parks Canada Agency 2010).
Hydroelectric development on the Tusket River system starting about 1929 is believed to have resulted in the extirpation of Pink Coreopsis from Gavels Lake and Lake Vaughan and likely caused population losses at other lakes that may have totalled more than 50% of the original population (see Habitat Trends). Additional dams on Pink Coreopsis lakes are unlikely because of legal protection for Species at Risk, but existing dams are likely limiting re-colonization of dammed lakes because of unsuitable water level fluctuations. Prolonged summer flooding and winter drought may both be significant. Winter flooding is known to be important in insulating the co-occurring Plymouth Gentian from freezing temperatures (Hazel 2004), but prolonged summer flooding greatly reduces Pink Coreopsis’ above ground to below ground biomass ratios, making plants much more susceptible to storm disturbance (Lusk 2006) and reducing survival. One of the mechanisms explaining extirpations or smaller populations in many reservoirs may be demonstrated in Raynards Lake, which experienced a drop of three metres in fall of 2004 (Lusk and Reekie 2007). Reservoirs can support Pink Coreopsis, as shown by the extant occurrences on Raynards Lake. The species is not common at this lake, however, and field observations suggest that several stands are in suboptimal habitat and are subjected to higher levels of competition from other species (Belliveau pers. comm. 2011). Additionally, because summer flow out of dammed lakes is likely reduced compared to natural rates, damming likely intensifies impacts of eutrophication.
Invasive Species
Competition from invasive species has thus far been only a theoretical threat to Pink Coreopsis (Eaton and Boates 2003). Occupied lakeshores are generally quite resistant to the establishment of invasive alien plants (Blaney et al. 2002; Eaton and Boates 2003; Blaney and Mazerolle pers. obs.2010, 2011). Artificial water level management and nutrient enrichment can, however, make habitats much more susceptible to invasion by exotic species (Wisheu and Keddy 1994; Hill et al. 1998; Environment Canada and Parks Canada Agency 2010). The significant eutrophication of the Carleton and Tusket River systems, coupled with new development bringing new invasive species, could increase the threat invasive plants pose to Pink Coreopsis populations. Reed Canary-grass (Phalaris arundinacea), a well-documented invasive of shorelines (Lavergne and Molovsky 2004; IPANE 2011) known from Lake Fanning (COSEWIC in press) and probably also present just downstream at Raynards Lake, poses the most immediate threat. European Common Reed (Phragmites australisssp. australis), although not known from the immediate vicinity of Pink Coreopsis occurrences, is known from southern Nova Scotia (Saltonstall 2002) and could become a threat in future, as could other invasives of shorelines.
Number of Locations
There are four Pink Coreopsis locations in Canada (as defined by the scale of the most immediate threat at each site, COSEWIC 2009; Table 1). Eutrophication, which can lead to competitive exclusion of coastal plain flora by more common native and exotic species, is clearly the most significant threat to Pink Coreopsis on all lakes. Four different sources and states of eutrophication serve to define the following four locations for Pink Coreopsis: 1) Raynards Lake has experienced major eutrophication and cyanobacterial blooms because of mink waste since at least 2007 (Brylinsky 2011a, 2011b, 2012). 2) The hydrologically connected Bennetts, Wilsons and Gillfillan Lakes on the Tusket River have recently had major increases in phosphorus levels (MTRI 2011), possibly from a single mink farm upstream near Kemptville. No impacts on lake ecology or on Pink Coreopsis have yet been observed. 3) Sloans Lake has had no eutrophication thus far, but a very large mink farm was completed 800 m upslope in 2010. 4) Salmon Lake (and presumably Pleasant Lake immediately downstream) has had stable moderate nutrient measurements possibly influenced by a fish processing facility on its shore. This facility could quickly cause significant eutrophication if its waste management were to change. The small Agard Lake occurrence occurs on two adjacent private properties and represents a location if eutrophication is considered the greatest threat. Agard Lake has had no water quality monitoring, but Salmon Lake data (MTRI 2011) further downstream suggests that no major upstream changes affecting the Annis River system have occurred since 2002. Given the expansion of mink farming in Yarmouth County, eutrophication could still be a greater threat at Agard Lake than shoreline alteration. If locations on Agard Lake are defined by the threat of eutrophication there would be a single location combined with the downstream occurrences on Salmon and Pleasant lakes.
Protection, Status, and Ranks
Legal Protection and Status
In Canada, Pink Coreopsis was assessed as Endangered by COSEWIC in 1984, a designation which was reviewed three times and upheld in November 2012. It is currently a Schedule 1 species listed as Endangered under the federal Species at Risk Act (Government of Canada 2011) and is provincially Endangered and legally protected under the Nova Scotia Endangered Species Act (Nova Scotia Department of Natural Resources 2011). The provincial act prohibits the disturbance or destruction of plants or their habitat on all lands. In the United States, Pink Coreopsis is protected from harvest and disturbance through its designation as Endangered in Maryland under the Nongame and Endangered Species Conservation Act (Maryland Natural Heritage Program 2010), and as Rare in New York under the New York State Environmental Conservation Law (New York NHP 2010). It has no legal protection in any other state in which it occurs.
Non-Legal Status and Ranks
Pink Coreopsis has a NatureServe global status rank of Vulnerable (G3, last reviewed in 2009) and national status ranks of Critically Imperilled (N1) in Canada and Vulnerable (N3) in the United States (NatureServe 2011). In Nova Scotia, the species has a NatureServe status rank of Critically Imperilled (S1) and a “Red” rank under the NS Department of Natural Resources ranking system. It has been ranked At Risk in Canada and Nova Scotia under the General Status of Wild Species process (CESCC 2011).
In the United Sates, Pink Coreopsis is rare in all states in which it occurs, with the subnational rank of S1 in Delaware and Maryland, S2 in New Jersey, Rhode Island and South Carolina, S3 in Massachusetts and New York, and SX (extirpated) in Pennsylvania (NatureServe 2011). The species’ subnational status rank in Georgia was very recently changed from SH to S1 by the Georgia Natural Heritage Program because of a recently discovered population (Patrick pers. comm. 2011). Pink Coreopsis is a Species of Concern in Rhode Island (Enser 2007), is on the Watch List in Georgia (Georgia DNR 2011) and is listed by the New England Plant Conservation Program as a Division 1 species (a globally rare species, rare in New England, Brumback et al. 1996).
Habitat Protection and Ownership
All or almost all Canadian Pink Coreopsis occurs below the mean lakeshore high water mark and is therefore on Crown land. However, 65% of plants occur on shorelines fronted by private land. Waterfront landowners generally perceive lakeshores as private property and lakeshores are often disturbed by installation of docks and landscaping of shores and swimming areas. Thus the ownership of upland and shorelines adjacent to Pink Coreopsis occurrence is more relevant to threats than is actual ownership of the occupied habitat. The analyses below therefore describe ownership of occurrences based on the land ownership immediately up from the shore.
Lakes supporting known extant populations of Pink Coreopsis in Canada have a total waterfront of approximately 103 km, divided into 354 private and nine Crown-owned parcels of land. Pink Coreopsis is found on at least 133 of these properties. Five of these properties are within provincial or non-government nature reserves and one is on Crown land with no special designation, where no development is likely to be permitted. Protected and/or Crown-owned lands represent 13% of the shoreline of occupied lakes and are estimated to contain 10% to 15% of the Canadian population. A large portion of Canadian occurrences are on highly subdivided and developed privately owned waterfront. Table 1 includes information on property subdivision and ownership for all occupied lakes.
Pink Coreopsis occurs within a provincial nature reserve at the Tusket River Ecological Site, protected since 1987 under Nova Scotia’s Special Places Act. This reserve includes 700 m of shoreline on the northwest shore of Wilsons Lake, representing about 6.5% of the lake’s shoreline and including about 20% of Pink Coreopsis individuals on the lake. The parcel of land supporting the single, small occurrence (114 stems) of Pink Coreopsis on Gillfillan Lake was added to this reserve in 2006.
Large nature reserves containing some Pink Coreopsis were also created on Wilsons and Bennetts lakes in 2010. The Nature Conservancy of Canada (NCC) now owns 118 ha on the southeast side of Bennetts Lake, which includes 22% of the lake’s total shoreline, and the Nova Scotia Nature Trust (NSNT) owns the adjacent 186 ha that includes part of the eastern shore of Bennetts Lake (9% of total shoreline), most of the southwest shore of Wilsons Lake (10.5% of total shoreline), and Absaloms Run, the 900 m stretch of the Tusket River between the lakes. The NCC land on Wilsons Lake supports very limited numbers of Pink Coreopsis, with only about 50 plants counted in 2011 (Blaney and Mazerolle pers. obs. 2011). Numbers within NCC and NSNT reserves on Bennetts Lake are not thoroughly counted, but include at least several thousand plants (Newell pers. comm. 2011). The protection offered by nature reserves mayhave very limited effect on protecting the waters from the primary threat of eutrophication.
The single provincial Crown land occurrence that is not within a nature reserve is on Salmon Lake, where 24 stems were recorded within the property and an additional 165 stems were recorded on the property border. These represent no more than about 3% of plants on Salmon Lake.
Pink Coreopsis habitat receives indirect protection from provincial laws and policies regulating shoreline development and pertaining to the protection of water quality, watercourses, wetlands and riparian buffers. These include the Nova Scotia Wetlands Conservation Policy, Activities Designation Regulations and Environmental Assessment Regulations, all under the Environment Act, the Forest Act - Wildlife Habitat and Watercourses Protection Regulations, and the Off Highway Vehicle Act. Though projects involving lakeshore or wetland alterations are required to go through a permitting process, not all private landowners make efforts to acquire necessary permits and enforcement is strictly complaint-based.
Acknowledgements and Authorities Contacted
Contract botanists Nicholas M. Hill and Alain Belliveau assisted with fieldwork and also provided survey data including stem counts and notes on habitats, disturbances and threats. The Mersey Tobeatic Research Institute provided recent Pink Coreopsis survey data and the results of water quality monitoring in the Tusket River Watershed. David S. MacKinnon of the Nova Scotia Department of Environment contributed valuable information on protected areas in southwestern Nova Scotia. Jennifer Lusk related useful observations on the ecology of Pink Coreopsis and assisted in identifying recent pertinent research. Ruth Newell provided information on recent survey coverage at Bennetts Lake. Tom Patrick of the Georgia Department of Natural Resources provided information on the status of Pink Coreopsis in the state of Georgia. Sherman Boates, Manager, Biodiversity Wildlife Division, Department of Natural Resources, Government of Nova Scotia provided additional information on eutrophication.
Information sources
Agriculture and Agrifood Canada. 2000. Plant Hardiness Zones of Canada. Website: http://sis.agr.gc.ca/cansis/nsdb/climate/hardiness/intro.html. [accessed November 2010].
Atlantic Canada Conservation Data Centre (AC CDC). 2011. Digital database of rare species locations for Nova Scotia. Atlantic Canada Conservation Data Centre, Sackville, NB.
Banovetz, S.J., and S.M. Scheiner. 1994. The effects of seed mass on the seed ecology of Coreopsis lanceolata. American Midland Naturalist 131(1):65-74.
Belliveau, A., pers. comm. 2011. Email correspondence with David Mazerolle regarding field observations made during surveys of Raynards Lake. November 2011. Botanist, Mersey Tobeatic Research Institute, Kempt, NS.
Blaney, C.S. 2002. 2001 Rare plant surveys on Bowater Mersey Woodlands land. Report to Bowater Mersey, Inc. Liverpool, NS. Atlantic Canada Conservation Data Centre, Sackville, NB. 31 pp.
Blaney, C.S. 2004. A vascular plant inventory and Pseudocyphellaria lichen survey on Bowater property at Bog Lakes, Lunenburg County, Nova Scotia. Atlantic Canada Conservation Data Centre, Sackville, NB. 15 pp.
Blaney, C.S. 2005a. 2004 Vascular Plant Surveys in Yarmouth and Shelburne Counties, Nova Scotia. Report to NS Department of Natural Resources (DNR). Atlantic Canada Conservation Data Centre, Sackville, NB. 28 pp.
Blaney, C.S. 2005b. 2005 Vascular Plant Surveys in Yarmouth and Shelburne Counties, Nova Scotia. Report to NS DNR. Atlantic Canada Conservation Data Centre, Sackville, NB. 38 pp.
Blaney, C.S., and D.M. Mazerolle. 2009. Rare Plant Inventory of Lakes in the Ponhook-Molega Lakes region, Nova Scotia. Report to the Endangered Species Recovery Fund and Nova Scotia Species at Risk Conservation Fund. Atlantic Canada Conservation Data Centre, Sackville, NB. 27 pp.
Blaney, C.S., and D.M. Mazerolle (pers. obs.). 2009-2011. Field observations from Atlantic Canada Conservation Data Centre rare species surveys in southwestern Nova Scotia. [unpublished data; rare species location data stored in Atlantic Canada Conservation Data Centre Database, Sackville, NB].
Blaney, C.S., and D.M. Mazerolle. 2011. Unpublished personal observations of Sean Blaney and David Mazerolle made during fieldwork for COSEWIC Coreopsis rosea update status report. Botanists, Atlantic Canada Conservation Data Centre, Sackville, NB.
Blaney, C.S., C.D. Spicer, T.M. Popma, and C. Hanel. 2002. Field observations from Atlantic Canada Conservation Data Centre rare species surveys in southwestern Nova Scotia. (unpublished data; rare species location data stored in Atlantic Canada Conservation Data Centre Database, Sackville, NB).
BONAP (Biota of North America Program). 2010. North American Plant Atlas - Coreopsis. [http://www.bonap.org/BONAPmaps2010/Coreopsis.html, accessed November 2011].
Brumback, W.E., L.J. Mehrhoff, R.W. Enser, S.C. Gawler, R.G. Popp, P. Somers, D. Sperduto, W.D. Countryman, and C.B. Hellquist. 1996. Flora Conservanda: New England. The New England Plant Conservation Program (NEPCoP) list of plants in need of conservation. Rhodora 98:233-361.
Brylinsky, M. 2011a. An assessment of the sources and magnitudes of nutrient inputs responsible for degradation of water quality in seven lakes located within the Carleton River watershed area of Digby and Yarmouth counties, Nova Scotia. Prepared for the Nova Scotia Department of Environment. 25 pp.
Brylinsky, M. 2011b. Water quality survey of ten lakes located in the Carleton River watershed area of Digby and Yarmouth counties, Nova Scotia. Prepared for the Nova Scotia Department of Environment. 63 pp. + app.
Brylinsky, M. 2012. Results of the 2011 Water Quality Survey of Ten Lakes Located in the Carleton River Watershed Area of Digby and Yarmouth Counties, Nova Scotia. Prepared for Nova Scotia Environment. Acadia Center for Estuarine Research, Acadia University, Wolfville, NS. 37 pp.
Canadian Endangered Species Conservation Council (CESCC). 2011. Wild Species 2010: The General Status of Species in Canada. National General Status Working Group: 302 pp.
Carlson, R.E. 1977. A trophic state index for lakes. Limnology and Oceanography. 22(2):361-369.
Carpenter, S.R. 2008. Phosphorus control is critical to mitigating eutrophication. PNRS. 105(32):11039-11040. Website: [accessed Dec. 2012].
COSEWIC. 2000. COSEWIC assessment and update status report on the Pink Coreopsis Coreopsis rosea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 11 pp.
COSEWIC. 2009. Instructions for the Preparation of COSEWIC Status Reports. Online document: [accessed November 2011].
COSEWIC. (In prep.). COSEWIC assessment and update status report on the Plymouth Gentian Sabatia kennedyana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa.
Cosner, M.E., and D.J. Crawford. 1994. Comparisons of isozyme diversity in three rare species of Coreopsis (Asteraceae). Systematic Botany 19(3):350-358.
Craine, S.I., and C.M. Orians. 2004. Pitch Pine (Pinus rigida Mill.) invasion of Cape Cod pond shores alters abiotic environment and inhibits indigenous herbaceous species. Biological Conservation 116:181-189.
Crawford, D.J., J.D. Palmer, and M. Kobayashi. 1991. Chloroplast DNA restriction site variation, phylogenetic relationships, and character evolution among sections of North American Coreopsis (Asteraceae). Systematic Botany 16:211-224.
David Suzuki Foundation. 2011. The impacts of the mink industry on freshwater lakes in Nova Scotia: an overview of concerns. Technical Brief prepared by the Tri-County Watershed Protection Association, Yarmouth, NS. 8 pp.
Dynesius, M., and C. Nilsson. 1994. Fragmentation and flow regulation of river systems in the northern third of the world. Science 266:753-762.
Eaton, S.T., and J.S. Boates. 2002. Assessing Threats to Coastal Plain Flora in the Tusket River Watershed. Centre for Wildlife and Conservation Biology, Acadia University; Wildlife Division, Nova Scotia Department of Natural Resources. The Federal Habitat Stewardship Program for Species at Risk. 105 pp.
Eaton, S.T., and J.S. Boates. 2003. Securing the science foundation for responsible stewardship and recovery of Atlantic Coastal Plain Flora (ACPF) species at risk. NS Department of Natural Resources, Kentville, NS.
Eckert, C.G., K.E. Samis, and S.C. Lougheed. 2008. Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Molecular Ecology 17:1170-1188.
Ehrenfeld, J.G. 1983. The effects of changes in land use on swamps of the New Jersey Pine Barrens. Biological Conservation 25:353-375.
Energy, Mines and Resources Canada. 1981. Atlas of Canada, Fifth Edition. Canada – Frost-Free Period. Online document: http://atlas.nrcan.gc.ca/site/english/maps/archives/5thedition/environment/climate/mcr4037/#download [accessed January 8, 2012].
Enser, R.W. 2007. Rare Native Plants of Rhode Island (PDF 601 Kb). Rhode Island Natural History Survey, Providence, RI. 17 pp. [accessed November 2011].
Environment Canada and Parks Canada Agency. 2010. Recovery Strategy and Management Plan for Multiple Species of Atlantic Coastal Plain Flora in Canada. Species at Risk Act Recovery Strategy Series. Environment Canada and Parks Canada Agency. Ottawa. 96 pp. + app.
Fernald, M.L. 1919. Coreopsis rosea Nutt. forma leucantha n.f. Rhodora 21:171.
Fernald. M.L. 1921. The Gray Herbarium expedition to Nova Scotia 1920. Rhodora 23:89-111, 130-152, 153-171, 184-195, 233-245, 257-78, 284-300.
Fernald, M.L. 1922. Notes on the flora of western Nova Scotia. Rhodora 24:157-164, 165–181, 201-208.
Fernald, M.L. 1950. Gray’s Manual of Botany. A handbook of the flowering plants of the central and northeastern United States and adjacent Canada. 8th Edition. American Book Company. New York. 1632 pp.
García-Ramos, G.. and M. Kirkpatrick. 1997. Genetic models of rapid evolutionary divergence in peripheral populations. Evolution 51: 21-28.
Georgia Department of Natural Resources (DNR). 2011. Watched Plant Species of Georgia. Website: [http://georgiawildlife.com/sites/default/files/uploads/%20wildlife/nongame/text/html/et_lists/etwpl.html, accessed December 10, 2011].
Gleason, H.A. 1952. The New Britton and Brown Illustrated Flora of the Northeastern United States and Adjacent Canada. New York Botanical Garden, New York.
Gleason, H.A., and A. Cronquist. 1991. Manual of the Vascular Plants of Northeastern United States and Adjacent Canada, Second Edition. New York Botanical Garden, New York.
Government of Canada. 2011. Species at Risk Public Registry. Website: Species Profile Goldencrest [accessed November 2011].
Gray, A. 1884. Compositae. In Synoptical Flora of North America. Vol. 1, pt. 2. Ivison, Blakeman, Taylor and Co., New York.
Gravuer, K. 2009. NatureServe Explorer comprehensive report for Coreopsis rosea. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. [accessed: November 2011].
Hazel, S.N. 2004. Hydrological alterations and rare species of the Atlantic Coastal Plain Flora in Nova Scotia. Masters thesis, Acadia University, Wolfville, NS.
Hill, N., pers. comm. 2011. Email correspondence with David Mazerolle regarding mammal herbivory on Pink Coreopsis in Canadian populations, general threats to the species and signs of nutrient enrichment at various sites. November 2011. Botanist and researcher, Fern Hill Institute for Plant Conservation, South Berwick, NS.
Hill, N.M., and P.A. Keddy. 1992. Prediction of rarities from habitat variables: coastal plain plants on Nova Scotian lakeshores. Ecology 73:1852-1859.
Hill, N.M., P.A. Keddy, and I.C. Wisheu. 1998. A hydrological model for predicting the effects of dams on the shoreline vegetation of lakes and reservoirs. Environmental Management 22(5):723-736.
Holt, T.D., I. Blum, and N.M. Hill. 1995. A watershed analysis of the lakeshore plant community. Canadian Journal of Botany 73: 598–607.
Hurlburt, D., pers. comm. 2011. Email communication with Sean Blaney regarding aboriginal traditional knowledge of Pink Coreopsis (Coreopsis rosea). December 2011. COSEWIC Aboriginal Traditional Knowledge Specialist Committee representative to the COSEWIC Vascular Plants Species Specialist Committee, and Wilsons Lake cottager, Yarmouth, NS.
Invasive Plant Atlas of New England (IPANE). 2011. Reed Canary Grass (Phalaris arundinacea) Website: http://nbii-nin.ciesin.columbia.edu/ipane/icat/browse.do?specieId=84 [accessed November 30, 2011].
Jones, A.G. 1978. Observations on reproduction and phenology in some perennial asters. American Midland Naturalist 99:184-197.
Keddy, C.J., and P.A. Keddy. 1984. COSEWIC assessment and status report on the Pink Coreopsis Coreopsis rosea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 21 pp.
Keddy, P.A. 1983. Shoreline vegetation in Axe Lake, Ontario: effects of exposure on zonation patterns. Ecology 64:331-344.
Keddy, P.A. 1984. Quantifying a within-lake gradient of wave energy in Gillfillan Lake Nova Scotia. Canadian Journal of Botany 62:301-309.
Keddy, P.A. 1985. Lakeshores in the Tusket River Valley, Nova Scotia: distribution and status of some rare species, including Coreopsis rosea Nutt. and Sabatia kennedyana Fern. Rhodora 87:309-320.
Keddy, P.A. 1989. Effect of competition from shrubs on herbaceous wetland plants: a 4 year field experiment. Canadian Journal of Botany 67:708-716.
Keddy, P.A, L.H. Fraser, and I.C. Wisheu. 1998. A comparative approach to examine competitive response of 48 wetland plant species. Journal of Vegetation Science 9:777-786.
Keddy, P.A., and I.C. Wisheu. 1989. Ecology, biogeography, and conservation of coastal plain plants: some general principles from the study of Nova Scotian wetlands. Rhodora 91:72–94.
Kim, S.-C., D.J. Crawford, M. Tadesse, M. Berbee, F.R. Ganders, M. Pirseyedi, and E.J. Esselman. 1999. ITS sequences and phylogenetic relationships in Bidens and Coreopsis (Asteraceae). Systematic Botany 24:480-493.
Lavergne, S., and J. Molovsky. 2004. Reed canary grass (Phalaris arundinacea) as a biological model in the study of plant invasions. Critical Reviews in Plant Sciences 23:415-429.
Lesica, P., and F.W. Allendorf. 1995. When Are Peripheral Populations Valuable for Conservation? Conservation Biology 9(4):753-760.
Loehrlein, M., and S. Siqueira. 2005. Self-incompatibility in Pink Tickseed, Coreopsis rosea Nutt. HortScience 40:1001-1002.
Lusk, J., pers. comm. 2011. Telephone conversation and email correspondence with David Mazerolle regarding research on Pink Coreopsis, the ecology of the species, personal field observations and the discovery of the species at Raynards Lake. November 2011. Researcher and naturalist, Wolfville, NS.
Lusk, J. M. 2006. Environmental limitations of two rare Atlantic Coastal Plain Flora species and the impact of hydrological alterations. Masters thesis. Acadia University, Wolfville, NS.
Lusk, J.M., and E.G. Reekie. 2007. The effect of growing season length and water level fluctuations on growth and survival of two rare and at risk Atlantic Coastal Plain flora species, Coreopsis rosea and Hydrocotyle umbellata. Canadian Journal of Botany 85:119-131.
Marsden, M.W. 1989. Lake restoration by reducing external phosphorus loading: the influence of sediment phosphorus release. Freshwater Biology 21(2):139-162.
McCanny, S.J., P.A. Keddy, T.J. Arnason, C.L. Gaudet, D.R.J. Moore, and B. Shipley. 1990. Fertility and food quality of wetland plants: a test of the resource availability hypothesis. Oikos 59(3):373-381.
Maryland Natural Heritage Program. 2010. Rare, Threatened and Endangered Plants of Maryland. April 2010 edition. Maryland Department of Natural Resources, Wildlife and Heritage Service, Annapolis, Maryland. Online Document: http://www.dnr.state.md.us/wildlife/Plants_Wildlife/rte/pdfs/rte _Plant_List.pdf [accessed December 10, 2011].
Mazerolle, D.M. 2011. Unpublished analysis of shoreline development based on maps and aerial photography from 1973 and 2010. Botanist, Atlantic Canada Conservation Data Centre, Sackville, NB.
Mazerolle, D.M., and C.S. Blaney. 2011. Unpublished observations made during examination of 2010 aerial photography. Botanists, Atlantic Canada Conservation Data Centre, Sackville, NB.
Mersey Tobeatic Research Institute (MTRI). 2010-2011. Data from rare coastal plain species surveys in southwestern Nova Scotia [unpublished data].
Mersey Tobeatic Research Institute (MTRI). 2011. Results of water quality monitoring carried out in 2002, 2010 and 2011 in Tusket River region [unpublished data].
Moore, D.R.J., P.A. Keddy, C. Gaudet, and I.C. Wisheu. 1989. Conservation of wetlands: do infertile wetlands deserve a higher priority? Biological Conservation 47:203-217.
Morgan, M.D., and K.R. Philipp. 1986. The effect of agricultural and residential development on aquatic macrophytes in the New Jersey Pine Barrens. Biological Conservation 35:143-158.
Morris, P.A., N.M. Hill, E.G. Reekie, and H.L. Hewlin. 2002. Lakeshore diversity and rarity relationships along interacting disturbance gradients: catchment area, wave action and depth. Biological Conservation 106(1):79-90.
Mueller, A.M. 1974. An evolutionary study of Coreopsis section Palmatae (Compositae). Ph.D. Dissertation University of North Carolina, Chapel Hill.
National General Status Working Group. 2010. Wild Species 2010: The General Status of Species in Canada. General Status Search Tool. [accessed November 2011].
NatureServe. 2004. Habitat-based Plant Element Occurrence Delimitation Guidelines. [accessed November 2011].
NatureServe. 2011. NatureServe Explorer database. Web site: [accessed November 2011].
New York Natural Heritage Program (New York NHP). 2010. New York Rare Plant Status Lists June 2010 (S.M. Young, ed.). New York Natural Heritage Program, Albany, NY. Online Document: http://www.dec.ny.gov/docs/fish_marine_ pdf/2010rareplantstatus.pdf [accessed December 10, 2011].
Newell, R., pers. comm. 2011. Email correspondence with David Mazerolle on the abundance of Pink Coreopsis on Bennetts Lake. November 2011. Herbarium Curator, Acadia University, Wolfville, NS.
Nilsson, C., and K. Berggren. 2000. Alterations of riparian ecosystems caused by river regulation. BioScience 50(9):783-792.
Nova Scotia Department of Agriculture. 2011. Draft Fur Industry Regulations. Online document: http://gov.ns.ca/agri/mink/pdf/FurIndustryRegulations.pdf [accessed December 20, 2011].
Nova Scotia Environment. 2010. A water quality survey of ten lakes in the Carleton River watershed area, Yarmouth and Digby counties, Nova Scotia. Prepared by the Water and Wastewater Branch, (D. Taylor, project lead). 16 pp. + app.
Nova Scotia Power. 2009. Bathymetry map of Raynards Lake. Unpublished digital map. Nova Scotia Power, Halifax.
Nuttall, T. 1818. The Genera of North American Plants, and Catalogue of the Species, to the Year 1817. Philadelphia. 2 vols. 2:179.
Patrick, T., pers. comm. 2011. Email correspondence with David Mazerolle regarding the status of Pink Coreopsis in Georgia and the possible discovery of a previously unknown population. November 2011. Botanist, Georgia Department of Natural Resources, Wildlife Resource Conservation Center, Social Circle, GA.
Reinartz, J.A., and D.H. Les. 1994. Bottleneck-induced dissolution of self-incompatibility and breeding system consequences in Aster furcatus(Asteraceae). American Journal of Botany 81:446-455.
Robson, D.B. 2010. A comparison of flower-visiting insects to rare Symphyotrichum sericeumand common Solidago nemoralis(Asteraceae). Botany 88:241-249.
Roland, A.E., and E.C. Smith. 1969. The Flora of Nova Scotia. Nova Scotia Museum, Halifax. 743 pp.
Saltonstall, K. 2002. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proceedings of the National Academy of Sciences USA 99:2445–2449.
Semple, J.C., S.B. Heard, and C. Xiang. 1996. The asters of Ontario (Compositae: Astereae): Diplactis Raf., Oclemena Greene, Doellingeria Nees, and Aster L. (including Canadanthus Nesom, Symphyotrichum Nees, and Virgulus Raf.). University of Waterloo Biology Series 38:1-94.
Sharp, M.J., and P.A. Keddy. 1985. Biomass accumulation by Rhexia virginica and Triadenum fraseri along two lakeshore gradients: a field experiment. Canadian Journal of Botany 63:1806-1810.
Shipley, B., and M. Parent. 1991. Germination responses of 64 wetland species in relation to seed size, minimum time to reproduction and seedling relative growth rate. Functional Ecology 5(1):111-118.
Siqueira, S.S. 2003. Breeding system in Coreopsis rosea Nutt. (Asteraceae). Masters thesis, Western Illinois University, Macomb, IL.
Smith, E.B. 1975. The chromosome numbers of North American Coreopsis with phyletic interpretations. Botanical Gazette (Crawfordsville) 136:78-86.
Smith, E.B.1976. A biosystematic survey of Coreopsis in eastern United States and Canada. Sida 6:123-215.
Smith, E.B. 1983. Phyletic trends in sections Eublepharis and Calliopsis of the genus Coreopsis (Compositae). American Journal of Botany70:549-554.
Sorrie, B.A., and A.S. Weakley. 2001. Coastal plain vascular plant endemics: phytogeographic patterns. Castanea 66(1-2):50-82.
Sprengel, C.P.J. 1826. Systema vegetabilium 3: 611. Sumtibus Librariae Dieterichianae, Gottingen, Germany.
Standards and Petitions Working Group. 2006. Guidelines for using the IUCN Red List Categories and Criteria. Version 6.2. Prepared by the Standards and Petitions Working Group of the IUCN Species Survival Commission (SSC) Biodiversity Assessments Sub-Committee in December 2006.
Statistics Canada. 2007. 2006 Census of Agriculture. Ottawa. [accessed December 2010].
Strother, J.L. 2006. Coreopsis Linnaeus. In Flora of North America Editorial Committee (Eds.) Flora of North America North of Mexico, Vol. 21: Asteraceae. New York and Oxford. [accessed November 2011].
Sutton, J. 2008. Effects of latitude and habitat disturbance on morphology, fruit and seed set, genetic variation, spatial genetic structure and gene flow in a rare Atlantic Coastal Plain Flower Sabatia kennedyana Fern. M.Sc. Thesis, Acadia University, Wolfville, NS. 115 pp.
Sweeney, S., and R. Ogilvie. 1993. The conservation of coastal plain flora in Nova Scotia. Maine Naturalist 1(3):131-144.
Tadesse, M., D.J. Crawford, and E.B. Smith. 1995. Comparative capitular morphology and anatomy of Coreopsis L. and Bidens L., including a review of generic boundaries. Brittonia 47:61-91.
USDA (United States Department of Agriculture). 1990. The USDA Plant Hardiness Zone Map. USDA Miscellaneous Publication No. 1475.
USDA (United States Department of Agriculture). 2011. USDA Plants Database profile for Coreopsis rosea Nutt. Pink Tickseed. [accessed November 2011].
Webb, K.T., and I.B. Marshall. 1999. Ecoregions and ecodistricts of Nova Scotia. Crops and Livestock Research Centre, Research Branch, Agriculture and Agri-Food Canada, Truro, Nova Scotia; Indicators and Assessment Office, Environmental Quality Branch, Environment Canada, Hull, Quebec. 39 pp.and 1 map.
White, D.J., J.C. Makarewicz, and T.W. Lewis. 2002. The significance of phosphorus released from the sediment under anoxic conditions in Sodus Bay, N.Y. Environmental Sciences Program, Department of Biological Sciences SUNY Brockport Brockport, New York, 33 pp.
Wilson, S.D., and P.A. Keddy. 1988. Species richness, survivorship, and biomass accumulation along an environmental gradient. Oikos 53:375-380.
Wisheu, I.C., C.J. Keddy, P.A. Keddy, and N.M. Hill. 1994. Disjunct Atlantic coastal plain species in Nova Scotia: distribution, habitat and conservation priorities. Biological Conservation 68:217–224.
Wisheu, I.E., and P.A. Keddy. 1989. The conservation and management of a threatened coastal plain plant community in eastern North America (Nova Scotia, Canada). Biological Conservation 48:229-238.
Wisheu, I.C., and P.A. Keddy. 1991. Seed banks of a rare wetland plant community: distribution patterns and effects of human-induced disturbance. Journal of Vegetation Science 2:81-88.
Wisheu, I.C., and P.A. Keddy. 1994. The low competitive ability of Canada’s Atlantic Coastal Plain shoreline flora: implications for conservation. Biological Conservation 68:247-252.
Wisheu, I.C., P.A. Keddy, D.R.J. Moore,S.J. McCanny, and C.L. Gaudet. 1991. Effects of eutrophication on wetland vegetation. In Wetlands of the Great Lakes: Protection and Restoration Policies; Status of the Science (eds J. Kusler & R. Smardon), pp. 112-121. Symposium proceedings, Niagara, New York, 1990.
Wood, S.C. 2006. Genetic structure of endangered populations of Coreopsis rosea in Nova Scotia. Honours Thesis, Acadia University, Wolfville , NS.
Zaremba, R.E., and E.E. Lamont. 1993. The status of the Coastal Plain Pondshore community in New York. Bulletin of the Torrey Botanical Club 120:180-187.
Biographical summary of report writers
David Mazerolle completed an undergraduate degree with a major in biology and a minor in geography, followed by a master’s degree in environmental studies, both at the Université de Moncton. For his M.Sc. he studied the geography of exotic vegetation in Kouchibouguac National Park and created a strategy for the management of the park’s exotic invasive flora. David has worked as a botanist at the AC CDC since 2006. Prior to this he was coordinator for rare plant survey and monitoring projects at the Bouctouche Dune Irving Eco-Centre from 2003 to 2006, where his work focused on the rare coastal plants of New Brunswick’s Northumberland Coast. He has over ten years’ experience working on various research, survey and monitoring projects and has authored and coauthored numerous status reports and technical reports pertaining to rare plants in Atlantic Canada.
Sean Blaney is the Botanist and Assistant Director of the AC CDC, where he is responsible for maintaining status ranks and a rare plant occurrence database for plants in each of the three Maritime provinces. Since beginning with the AC CDC in 1999, he has discovered dozens of new provincial records for vascular plants and documented several thousand rare plant occurrences during extensive fieldwork across the Maritimes. Sean is a member of the COSEWIC Vascular Plant Species Specialist Committee, the Nova Scotia Atlantic Coastal Plain Flora Recovery Team, and has authored or co-authored numerous COSEWIC and provincial status reports. Prior to employment with AC CDC, Sean received a B.Sc. in Biology (Botany Minor) from the University of Guelph and an M.Sc. in Plant Ecology from the University of Toronto, and worked on a number of biological inventory projects in Ontario as well as spending eight summers as a naturalist in Algonquin Park, where he co-authored the second edition of the park's plant checklist.
Collections examined
All known specimens from Nova Scotia herbaria were already documented in the AC CDC database (AC CDC 2010) prior to the preparation of this report, so no further examination of herbarium specimens was undertaken. Historical specimen collections had been made by expert botanists familiar with Atlantic Coastal Plain flora, so it was not considered necessary to verify identifications.
Appendix 1. Lakes in or near the Tusket River watershed visited by botanists in the past 30 years, where Pink Coreopsis was not found
| Site | Date | Observers | Survey Type |
|---|---|---|---|
| Back Lake | 1988 | NH | Six plots visited around the lakeshore |
| Beaver Lake | 2002 | SB | Casual botanizing of much of shore from canoe |
| Beaverhouse Lake | 1988 | NH | Six plots visited around the lakeshore |
| Biggars Lake | 1988 | NH | Six plots visited around the lakeshore |
| Cedarwood Lake (includes Bazalgette & Mud Lakes) |
2011 | SB, DM | Comprehensive shoreline walk/paddle |
| Churchills Lake | 1983 | PK | Extensive perimeter search by canoe |
| Clearwater Lake (Digby Co.) |
2002 | SB | Casual botanizing of much of shore from canoe |
| Clearwater Lake (Yarmouth Co.) | 1983 | PK | Extensive perimeter search by canoe |
| Cranberry Lake | 2010 | SB | Spot check at one end of lake, limited suitable habitat visible |
| Duck Lake | 2012 | SB, DM | Comprehensive shoreline coverage by canoe |
| East Corning Lake | 2010 | NH | Spot check at one end, no suitable habitat seen |
| Eel Lake (Digby Co.) | 2002 | SB | Casual botanizing of much of shore from canoe |
| Ellenwood Lake | 2002 | SB, CS | Comprehensive shoreline walk |
| English Clearwater Lake | 2011 | NH | East side (~3km) of lake covered on foot |
| Fanning Lake | 2011 | SB, DM | Comprehensive shoreline walk |
| French Clearwater Lake | 2011 | DM | Comprehensive shoreline walk |
| Gavels Lake | 1988 | NH | Six plots visited around the lakeshore |
| Georges Lake | 2004 | SB | Full shoreline coverage by canoe |
| Germain Lake | 2002 | SB | Casual botanizing of much of shore from canoe |
| Halfway Lake | 1988 | NH | Six plots visited around the lakeshore |
| Hamilton Pond | 1988 | NH | Six plots visited around the lakeshore |
| Harris Lake | 2012 | SB, DM | Comprehensive shoreline coverage by canoe |
| Hibbards Lake | 2012 | SB, DM | Comprehensive shoreline coverage by canoe |
| Hog Lake | 2012 | SB, DM | Comprehensive shoreline coverage by canoe |
| Hoopers Lake | 1988 | NH | Six plots visited around the lakeshore |
| Janes Lake | 1988 | NH | Six plots visited around the lakeshore |
| Kegeshook Lake | 2003 | PM | Comprehensive shoreline walk |
| Kempt Back Lake | 2012 | SB, DM | Extensive perimeter search by canoe |
| Kempt Snare Lake | 2012 | SB, DM | Comprehensive shoreline coverage by canoe |
| Lac a Pic | 2011 | SB, DM, NH | Comprehensive shoreline coverage, primarily by canoe |
| Lac de l’Ecole | 2002 | SB, CS | Comprehensive shoreline walk |
| Lake Vaughn | 1988 | NH | Six plots visited around the lakeshore |
| Langford Lake | 2012 | SB, DM | Comprehensive shoreline coverage by canoe |
| Little Tusket Lake | 2012 | SB, DM | Comprehensive shoreline coverage, by foot and canoe |
| Long Lake (Southwest of Wilsons Lake) | 2011 | SB, DM | Comprehensive shoreline coverage, primarily by canoe |
| Louis Lake | 1988 | NH | Six plots visited around the lakeshore |
| Lower Crawleys Lake | 1999 | SB | Spot check at one end, limited suitable habitat |
| Marcel Lake | 2012 | SB, DM | Comprehensive shoreline coverage by canoe |
| Mill Lake | 2011 | DM, AB | Comprehensive shoreline walk |
| Mingo Beck Lake | 2012 | SB, DM | Comprehensive shoreline coverage by canoe |
| Mink Lake | 1988 | NH | Six plots visited around the lakeshore |
| Mushpauk Lake | 2011 | SB, DM | Partial shoreline coverage (~7km) on foot |
| Oakleaf Lake | 2002 | SB | Casual botanizing of much of shore from canoe |
| Ogden Lake | 1988 | NH | Six plots visited around the lakeshore |
| Parr Lake | 1988 | NH | Six plots visited around the lakeshore |
| Pearl Lake | 2002 | SB, CS | Comprehensive shoreline walk |
| Petes Lake | 2011 | SB, NH | Comprehensive shoreline walk |
| Pothiers Millpond | 2012 | SB, DM | Comprehensive shoreline coverage by canoe |
| Randals Lake | 2011 | SB, DM | Comprehensive shoreline walk |
| Rounding Lake | 1988 | NH | Six plots visited around the lakeshore |
| Rushy Lake | 1988 | NH | Six plots visited around the lakeshore |
| Salmon River Lake | 2002 | SB | Casual botanizing of much of shore from canoe |
| Solomon Lake | 1988 | NH | Six plots visited around the lakeshore |
| Somes Lake | 1988 | NH | Six plots visited around the lakeshore |
| South Wallace Lake | 1988 | NH | Six plots visited around the lakeshore |
| Springhaven Duck Lake | 2011 | SB, DM | Comprehensive shoreline coverage, primarily by canoe |
| Sunday Lake | 1983 | PK | Extensive perimeter search by canoe |
| Third Lake | 2003 | SB, CS | Comprehensive shoreline walk |
| Travis Lake | 1983 | DMc | Extensive perimeter search by canoe by Keddys ~1983 |
| Wentworth Lake | 2011 | DM | Partial shoreline coverage (~6km) on foot |
Appendix 2. Methods used to derive population estimates of Pink Coreopsis on each lake on which extant populations are known
| Population | Lake | Methods for Population Estimate |
|---|---|---|
| 1. Annis River | 1. Agard | Nicholas Hill walked part of the shoreline in 2012, covering all of the area in which the plant was found during a comprehensive shoreline survey by Pamela Mills in 1997. The plant is not suspected to occur elsewhere in the lake, but further surveys to confirm this would be valuable. |
| 1. Annis River | 2. Salmon | Comprehensive shoreline walk in 2010 (Nicholas Hill). Plants counted individually or estimated visually site by site. |
| 1. Annis River | 3. Pleasant | Comprehensive shoreline walk in 2010 (Nicholas Hill). Plants counted individually or estimated visually site by site. |
| 2. Carleton River | 1. Sloans | Comprehensive shoreline walk in 2010 (Nicholas Hill). Plants counted individually or estimated visually site by site. |
| 2. Carleton River | 2. Raynards* | About 65% of lakeshore walked by Alain Belliveau in 2011, with area covered not selected for habitat. 2,600 stems recorded by individual counts/visual estimates. If density is similar in unsurveyed portion (and there is no obvious reason to think otherwise) about 4,000 stems would be present. |
| 3. Tusket River (Bennetts/Wilsons) | 1. Bennetts | No recent comprehensive shoreline survey. Areas of occurrence were mostly delimited in a comprehensive shoreline walking survey in 2002 (Sean Blaney, Cindy Spicer, Pamela Mills), but counts were not attempted. Sean Blaney and Nicholas Hill believe densities are similar to Wilson’s Lake based on personal observation. Lake sizes are similar (12.1 km shoreline on Wilsons Lake, 13.0 km of shoreline on Bennetts Lake). Population on Bennetts Lake is thus probably similar to that on Wilsons Lake (est. 103,000). For this report population is estimated at 50,000 to 100,000+. |
| 3. Tusket River (Bennetts/Wilsons) | 2. Wilsons | Comprehensive shoreline walking survey in 2011 (Sean Blaney, David Mazerolle). Stems counted individually, visually estimated site by site, or in extensive areas of occurrence, estimated by marking start and end points with Global Positioning System (GPS) to get shoreline length and multiplying by an estimate of number of stems per metre derived for each individual locality. |
| 4. Tusket River (Gillfillan) | 1. Gillfillan | Comprehensive shoreline walking survey in 2002 (Sean Blaney, Claudia Hanel, Theo Popma, Pamela Mills) did not find the plant (presumably overlooking the very small population). Comprehensive shoreline walking survey over 12.1 km of the lake’s 18.3 km 2011 only found the plant in the single locality known at least since 1999 (and possibly since Fernald in 1921). Stems were counted individually. Substantial additional field survey on the lake has occurred in the last 30 years with no additional population found. |