Red crossbill (percna subspecies, Loxia curvirostra percna): COSEWIC assessment and status report 2016

Threatened
2016
Table of Contents
- Document Information
- Assessment Summary
- Executive Summary
- Technical Summary
- Preface
- Wildlife Species Description and Significance
- Distribution
- Habitat
- Biology
- Population Sizes and Trends
- Threats and Limiting Factors
- Protection, Status and Ranks
- Acknowledgements and Authorities Contacted
- Information Sources
- Biographical Summary of Report Writers
- Collections Examined
List of Figures
- Figure 1. Male Red Crossbill on Red Pine in Howley, western Newfoundland and Labrador. Image courtesy of D.M. Whitaker.
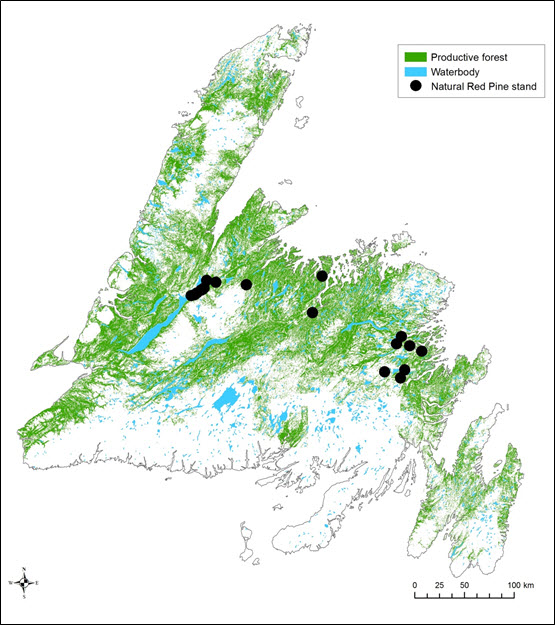
- Figure 2. Global distribution of Red Crossbill percna (solid black), which is endemic to Canada.
- Figure 3. Productive forest in insular Newfoundland and locations of the 20 remaining natural Red Pine stands. Data provided by NL Forestry and Agrifoods Agency.
- Figure 4. Locations of Christmas Bird Counts, Breeding Bird Survey routes, and winter bird surveys conducted in insular Newfoundland and Labrador. Winter bird survey locations where Red Crossbills have been detected in at least one year are shown with enlarged symbols. CBC circles and winter bird survey pentagons are not to scale; lengths of BBS routes are to scale. The scale of the map prevents the display of all winter bird survey pentagons; pentagons mapped thus represent approximate geographical locations where one or more winter bird surveys have been conducted.
- Figure 5. Locations of 10 km by 10 km survey squares surveyed during the Québec Breeding Bird Atlas in the Anticosti Island and Québec North Shore region. Yellow squares: Red Crossbills detected were possible breeders; orange squares: Red Crossbills detected were probable breeders; grey squares: Red Crossbills were not observed; white squares: not surveyed. Yellow dots: Red Crossbills were detected in the second Atlas (2010-2014) but not the first (1984-1989); black dots: Red Crossbills were detected in the first Atlas (1984-1989) but not the second (2010-2014). Data and map obtained from www.atlas-oiseaux.qc.ca (Atlas of the breeding birds of Québec, 2015).
- Figure 6. Estimated average abundance (annual index) of Red Crossbills on Breeding Bird Survey routes in insular Newfoundland and Labrador from 1980 to 2012. Red lines indicate upper and lower 95% credible limits. The vertical axis has been scaled to highlight trends in the annual indices; upper credible limits are not visible due to the imprecision and overall low reliability of the model (after Environment Canada 2014).
List of Tables
- Table 1. Historical taxonomic revisions affecting the nomenclature of Red Crossbill percna.
- Table 2. First and last years in which Christmas Bird Counts (CBC) and Breeding Bird Surveys (BBS) have been conducted at each circle/route in insular Newfoundland and Labrador, and the number of times (n) each count circle/route has been surveyed. Bolded circles/routes are those where Red Crossbill (subspecies unknown) was recorded at least once.
- Table 3. Contemporary systematic songbird surveys conducted during the breeding season in forests of insular Newfoundland and Labrador. Adapted from Whitaker (2010).
List of Appendices
- Appendix 1. Body, wing, tail, and tarsus measurements of Red Crossbill percna subspecies. Values include single measurements, means + SEs, or ranges. Sample sizes in parentheses, when available; f: female, m: male, juv: juvenile.
- Appendix 2. Bill measurements of Red Crossbill percna subspecies. Values include single measurements, means + SEs, or ranges. Sample sizes in ( ), when available; f: female, m: male, juv: juvenile.
- Appendix 3. Threats Assessment for Red Crossbill percna ssp.
Document Information
COSEWIC
Committee on the Status
of Endangered Wildlife
in Canada

COSEPAC
Comité sur la situation
des espèces en péril
au Canada
COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2016. COSEWIC assessment and status report on the Red Crossbill percna subspecies Loxia curvirostra percna in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 62 pp. Species at Risk Public Registry website.
Previous report(s):
COSEWIC 2004. COSEWIC assessment and status report on the Red Crossbill percna subspecies Loxia curvirostra percna in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 46 pp. Species at Risk Public Registry website.
Production note:
COSEWIC would like to acknowledge Tina D. Leonard for writing the status report on Red Crossbill, percna subspecies (Loxia curvirostra percna) in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Marcel Gahbauer, Co-chair of the COSEWIC Birds Specialist Subcommittee.
For additional copies contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment and Climate Change Canada
Ottawa, ON
K1A 0H3
Tel.: 819-938-4125
Fax: 819-938-3984
E-mail: COSEWIC E-mail
Website: COSEWIC
Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Bec-croisé des sapins de la sous-espèce percna ( Loxia curvirostra percna ) au Canada.
Cover illustration/photo:
Red Crossbill percna subspecies -- Image courtesy of D.M. Whitaker.
COSEWIC Assessment Summary
Assessment Summary – May 2016
- Common name
- Red Crossbill percna subspecies
- Scientific name
- Loxia curvirostra percna
- Status
- Threatened
- Reason for designation
- This subspecies is a distinctive taxonomic group endemic to Canada. Previously known to breed only on the island of Newfoundland, it has within the past five years also been documented nesting on Anticosti Island. While the Canadian population is thought to be greater than was understood previously due to the recent discovery of a breeding population component on Anticosti Island, there is no evidence of an increasing trend. On the contrary, this taxon has experienced a substantial long-term decline. Further population decrease is expected based on identified threats, most notably competition and predation from introduced squirrels in Newfoundland, habitat loss due to logging, and a fungal disease affecting Red Pine.
- Occurrence
- Quebec, Newfoundland and Labrador
- Status history
- Designated Endangered in May 2004. Status re-examined and designated Threatened April 2016.
COSEWIC Executive Summary
Red Crossbill percna subspecies
Loxia curvirostra percna
Wildlife Species Description and Significance
Red Crossbill percna is one of 10 recognized forms of Red Crossbill in North America. It is a medium-sized finch and a specialized seed eater having curved and crossed mandibles, muscular hinged jaws, and strong clasping feet for prying open conifer cone scales to access the seeds. Red Crossbill males are dull red, females are greyish-olive, and juveniles are dull grey to brownish and heavily streaked. Compared to other Red Crossbill forms in North America, percna has a relatively stout and deep (tall) bill, larger body size, and darker, duskier plumage.
Each form of Red Crossbill in North America is characterized by minor differences in morphology, genetics, and behaviour. Forms are also referred to as vocal types; each is most readily and reliably identified by spectrographic analysis of their unique flight vocalizations. Recent research suggests that Red Crossbill percna may correspond with Type 8. North American Red Crossbills likely represent a complex of cryptic species. Though weakly differentiated genetically, vocalization may promote reproductive isolation even among groups that are not geographically separated. Red Crossbill percna is significant because it is a distinct taxonomic group restricted to insular Newfoundland and Labrador (hereafter “Newfoundland”) and surrounding islands, and Anticosti Island (QC).
Distribution
Red Crossbills (form/vocal type(s) unknown) were historically considered to occur throughout most of Newfoundland, but with an erratic and localized distribution. Their range apparently has contracted since the first half of the 20th century; the current distribution of Red Crossbill (both percna and other forms) in Newfoundland is not fully understood. Presence of percna/Type 8 in Newfoundland was confirmed during 2005-2011 via audiospectrographic and morphometric analyses on the Avalon Peninsula, and in eastern, central, and western insular Newfoundland. Probable breeding of Type 8 Red Crossbills having morphology within the documented range of values for percna was also documented on Anticosti Island, QC, in summer 2014.
Birds that possibly are percna(i.e., have large bills) have been documented in Nova Scotia, New Brunswick, Québec (on the mainland and Magdalen Islands), and in New England (USA); these sightings may represent areas of irregular irruptions during years of food shortages in core areas of occurrence.
Habitat
All Red Crossbill forms are closely associated with cone-productive forests. Forms vary with respect to bill morphology, with each specialized to feed on particular conifer species. All large-billed crossbills, including percna, are pine forest associates. In Newfoundland, Red and White Pine stands likely represented a significant portion of important habitat for percna in the past; however, these native pines (particularly Red Pine) are currently rare on the Island and do not occur on Anticosti Island. Mature Black Spruce forests, and to a lesser extent Balsam Fir and White Spruce forests, historically and currently provide additional important habitat for percna. Throughout recent history, habitat conversion, forest harvesting, fire, insect damage, and fungal infestations have led to reductions in conifer seed abundance in Newfoundland. Cone consumption by Red Squirrels introduced to Newfoundland in 1963 is implicated as causing significant recent declines in cone availability. Recent projections by the Newfoundland and Labrador (NL) Department of Natural Resources indicate a significant increase in cone production on Newfoundland’s Avalon Peninsula over the next two decades. However, a major Spruce Budworm outbreak is expected to occur in Newfoundland and Anticosti Island in the near future; such an outbreak could have a negative effect on cone availability, but may provide some food in the form of insect larvae and pupae.
Biology
All forms of Red Crossbill are dependent on conifer forests for the food resources they provide in the form of conifer seeds; availability of cones highly influences survival and breeding. Red Crossbills are irruptive and undertake movements across a range of spatial scales in search of sufficient cone crops, though some populations (possibly including percna) tend to exhibit more sedentary behaviour. Irrupting birds tend to be reasonably faithful to core breeding areas, to which some return within a few years of the irruption. Red Crossbills are monogamous, form pair bonds, nest in loose aggregations, and forage in flocks. They have a flexible breeding strategy, can have multiple broods, and nest in colder months if conifer seeds are abundant. Other adaptations to extreme variability in conifer seed crops include sexual maturity at a relatively young age, accelerated succession of broods, and tolerance of repeated cooling and slow development of young when food is relatively scarce.
Population Size and Trends
Red Crossbills were once relatively common in Newfoundland but have been precipitously and continuously declining since the 1950s. Currently they are rare, with infrequent and erratic sightings on both formal and informal surveys. Numbers of percna comprising the recently confirmed population on Anticosti Island (which is probably breeding there) are unknown but are estimated to be in the high hundreds. The Canadian population of Red Crossbill percna is estimated to be in the low thousands (i.e., 1,000-2,500 mature individuals), based on recent bioacoustic analyses and localized systematic surveys targeting Red Crossbills, as well as data from Christmas Bird Counts, Breeding Bird Surveys, the Québec Breeding Bird Atlas, and anecdotal reports from birdwatchers. Much uncertainty is associated with this estimate because of relatively limited sampling (particularly in remote areas), difficulties associated with surveying irruptive birds, and the possibility that percna likely moves very large distances during times of food shortage.
Threats and Limiting Factors
Threats to percna are not clearly understood due to the general lack of information on the taxon in Newfoundland and Anticosti Island. Probable threats (from highest to lowest apparent/predicted impact) include: i) invasive, non-native species and problematic native species (i.e., competition for food resources and nest predation by introduced Red Squirrels in Newfoundland, fungal infestations affecting native and non-native pines in Newfoundland, and insect outbreaks resulting in reduced cone production or tree mortality); ii) biological resource use (i.e., forest harvesting); iii) natural system modifications (i.e., forest fires and forest fire suppression); iv) transportation and service corridors (i.e., roadways); v) mining and quarrying; and vi) agriculture. At times, birds face starvation if cone crops fail across wide geographic areas; additional causes of mortality for percna are vehicle strikes and predation.
Protection, Status, and Ranks
Red Crossbill percna has been listed as Endangered since 2004 under the federal Species at Risk Act and the NL provincial Endangered Species Act. It is also protected under the Migratory Birds Convention Act. It is considered At Risk in the General Status of Wild Species. NatureServe ranks Red Crossbill percna as nationally imperilled (N2) but has not ranked it provincially although it is recommended as S2 in Québec; Red Crossbill in general has been ranked as S2S3 for Newfoundland and S4 for Québec.
Technical Summary
- Scientific Name:
- Loxia curvirostra percna
- English Name:
- Red Crossbill percna subspecies
- French Name:
- Bec-croisé des sapins de la sous-espèce percna
- Range of occurrence in Canada (province/territory/ocean):
- Newfoundland and Labrador (island of Newfoundland only), and Québec (Anticosti Island only)
Demographic Information
| Summary Items | Information |
|---|---|
| Generation time (average age of parents in the population) | Yes, but based on limited data, and therefore with high uncertainty. -16.2% per year (2003-2012) |
| Estimated percent of continuing decline in total number of mature individuals within 5 years or 2 generations | Unknown |
| Inferred percent reduction in total number of mature individuals over the last 10 years. | Conflicting short-term results, with high uncertainty due to small sample sizes: Christmas Bird count data: 0.0% change (2004-2013) Breeding Bird Survey data: -83% change (2003-2012) |
| Suspected percent increase in total number of mature individuals over the next 10 years. | Unknown |
| Suspected percent increase in total number of mature individuals over any [10 years, or 3 generations] period, over a time period including both the past and the future. | Unknown |
| Are the causes of the decline a. clearly reversible and b. understood and c. ceased? | No. Causes of decline are not fully understood; some may be reversible (e.g., if the Newfoundland population of Red Pine was enhanced) while some likely are not (e.g., it is not plausible to eradicate non-native competitive Red Squirrels from Newfoundland); those that are understood have not ceased. |
| Are there extreme fluctuations in number of mature individuals? | Unlikely; when cone crops fail, birds likely move large distances to find sufficient resources but numbers of individuals likely do not fluctuate widely. |
Extent and Occupancy Information
| Summary Items | Information |
|---|---|
| Estimated extent of occurrence | 261,500 km2 |
| Index of area of occupancy (IAO) (Based on a 2-km × 2-km grid). | Unknown, but likely at least 300 km2 |
| Is the population “severely fragmented” i.e., is >50% of its total area of occupancy in habitat patches that are (a) smaller than would be required to support a viable population, and (b) separated from other habitat patches by a distance larger than the species can be expected to disperse? | a. Unlikely b. No |
| Number of locations (Note: See Definitions and Abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
Unknown, but >10 |
| Is there an observed decline in extent of occurrence? | Unknown. While a subpopulation was recently confirmed on Anticosti Island (QC), it is unclear whether or not that subpopulation has been extant for some time and simply went undetected; thus the apparent increase in extent of occurrence since the 2004 assessment may be an artifact of wider survey coverage and an improved understanding of occurrence and distribution. |
| Is there an observed, inferred, or projected decline in index of area of occupancy? | Unknown. Had IAO been estimated in the 2004 assessment using 2x2 km grid squares, it would have been smaller than now because percnahas since been confirmed on Anticosti Island, but this may not represent a true increase. |
| Is there an observed decline in number of subpopulations? | No |
| Is there an observed, inferred, or projected decline in number of “locations”? (Note: See Definitions and Abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
Unknown |
| Is there an observed or projected decline in area, extent and/or quality of habitat? | Unknown. An observed and projected decline of Red Pine in Newfoundland in the next decade or so may be offset by a projected increase in cones of Black Spruce, White Spruce, and Balsam Fir on Newfoundland’s Avalon Peninsula. |
| Are there extreme fluctuations in number of subpopulations? | Not applicable |
Are there extreme fluctuations in number of “locations”? |
Not applicable |
| Are there extreme fluctuations in extent of occurrence? | No |
| Are there extreme fluctuations in index of area of occupancy? | No |
Number of Mature Individuals (in each subpopulation)
| Subpopulations (give plausible ranges) | N Mature Individuals |
|---|---|
| Newfoundland | 500-1,500 |
| Anticosti Island | 500-1,000 |
| Total | 1,000-2,500 |
Quantitative Analysis
| Summary Items | Information |
|---|---|
| Probability of extinction in the wild is at least [20% within 20 years or 5 generations, or 10% within 100 years]. | Unknown (no analysis done) |
Threats (actual or imminent, to populations or habitats, from highest impact to least)
- Invasive, non-native species and problematic native species (competition for food resources and nest predation by introduced Red Squirrels in Newfoundland, fungal infestations affecting native and non-native pines in Newfoundland, and insect outbreaks resulting in reduced cone production or tree mortality)
- Biological resource use (forest harvesting)
- Natural system modifications (forest fires and forest fire suppression)
- Transportation and service corridors (roadways)
- Mining and quarrying; and
- Agriculture
Was a threats calculator completed for this species and if so, by whom? Yes; completed by Tina Leonard, Marcel Gahbauer, Darroch Whitaker, Bruno Drolet, Shelley Pardy, Karen Timm, Jessica Humber, and Mary Sabine
Rescue Effect (immigration from outside Canada)
| Summary Items | Information |
|---|---|
| Status of outside population(s) most likely to provide immigrants to Canada. | Red Crossbill percna is endemic to Canada |
| Is immigration known or possible? | Not applicable |
| Would immigrants be adapted to survive in Canada? | Not applicable |
| Is there sufficient habitat for immigrants in Canada? | Not applicable |
| Are conditions deteriorating in Canada? See Table 3 (Guidelines for modifying status assessment based on rescue effect) |
Not applicable |
Are conditions for the source population deteriorating? |
Not applicable |
Is the Canadian population considered to be a sink? |
Not applicable |
| Is rescue from outside populations likely? | Not applicable |
Data-Sensitive Species
| Summary Items | Information |
|---|---|
| Is this a data sensitive species? | No |
Status History
COSEWIC: Designated Endangered in May 2004. Status re-examined and designated Threatened April 2016.
Status and Reasons for Designation:
- Status:
- Threatened
- Alpha-numeric code:
- C2a(ii)
- Reasons for designation:
- This subspecies is a distinctive taxonomic group endemic to Canada. Previously known to breed only on the island of Newfoundland, it has within the past five years also been documented nesting on Anticosti Island. While the Canadian population is thought to be greater than was understood previously due to the recent discovery of a breeding population component on Anticosti Island, there is no evidence of an increasing trend. On the contrary, this taxon has experienced a substantial long-term decline. Further population decrease is expected based on identified threats, most notably competition and predation from introduced squirrels in Newfoundland, habitat loss due to logging, and a fungal disease affecting Red Pine.
Applicability of Criteria
- Criterion A (Decline in Total Number of Mature Individuals):
- Not applicable. There are no reliable data supporting a recent or future rate of decline exceeding 30%.
- Criterion B (Small Distribution Range and Decline or Fluctuation):
- Not applicable. The population is not severely fragmented, occurs at more than 10 locations, and does not experience extreme fluctuations
- Criterion C (Small and Declining Number of Mature Individuals):
- Meets Threatened C2a(ii). The population of mature individuals is <10,000, continuing decline is inferred from threats, and one subpopulation has 100% of mature individuals. May meet Endangered given that the population estimate is 1,000-2,500, but there is uncertainty regarding these numbers, and no evidence that it is at imminent risk of extinction
- Criterion D (Very Small or Restricted Population):
- Not applicable. Estimates suggest a population estimate >1,000 mature individuals
- Criterion E(Quantitative Analysis):
- The information necessary to conduct a quantitative analysis is not available.
Preface
Red Crossbill percna is endemic to Canada. It was first assessed in 2004 as Endangered by the Committee on the Status of Wildlife in Canada (COSEWIC). It was listed under the Newfoundland and Labrador Endangered Species Act in 2004 and the Canadian Species at Risk Act in 2005. A federal recovery strategy (Environment Canada 2006) and a federal action plan (Environment Canada 2012) have been completed.
New information on Red Crossbill percna is available since the subspecies was first assessed in 2004. Spectrographic analyses of Red Crossbill vocalizations recorded in 2011 in insular Newfoundland and Labrador (hereafter “Newfoundland”) confirmed the presence of a vocal type (i.e., Type 8) which at that time was unknown from elsewhere in North America; this vocal type appears to be synonymous with percna (Young et al. 2012; Hynes and Miller 2014). Morphological measurements (wing chord, bill depth at nares, and bill length from nares to tip) obtained from birds confirmed as Type 8 are near the known morphological means of percna (Young et al. 2012). These studies confirm that percnaremains extant in Newfoundland, contrary to earlier suggestions that an endemic Newfoundland form is probably extinct.
The presence of other vocal types of Red Crossbill in Newfoundland was also confirmed via audiospectrographic analyses. At the time of the 2004 assessment, it was unclear whether all individuals observed in Newfoundland were percna or perhaps other forms also occurred on the Island (however, all 54 Red Crossbills collected from Newfoundland since the early 20th century and housed at the Canadian Nature Museum are uniformly dark and heavy-billed, suggesting none have been mainland subspecies; M. Gosselin pers. comm. 2014). In a study of 83 birds recorded in Newfoundland during 2010 and 2011, 78 (94%) were identified as Type 8; five individuals were identified as Types 2, 4, and 10 (Hynes and Miller 2014). Type 2 occurs throughout continental USA, southern Canada, and northern Mexico; Type 4 is primarily found in the Pacific Northwest and secondarily in the Intermontane West of the USA; and Type 10 occurs throughout North America, primarily on the Pacific Northwest coast of northern California to central Oregon but is also uncommon year-round in the northeastern USA and southeastern Canada (Young 2012).
Our understanding of the geographic range of percna has improved since the original 2004 assessment. During the 2011 and 2014 field seasons of the 2nd Québec Breeding Bird Atlas, numerous Red Crossbills were observed on Anticosti Island (M. Robert pers. comm. 2014). Observations by experienced field personnel in 2011 first suggested that some of these birds were probably percna (based on large bill size). In summer 2014, multiple vocalization recordings were obtained from more than 20 Red Crossbills; some individuals have been confirmed as Type 8. Both adults and hatch-year birds with large bill size (that could correspond to percna) were observed on Anticosti Island in 2014, but there was no evidence to confirm breeding there (M. Robert pers. comm. 2014). Blood samples and bill measurements were also taken from two adult males and one juvenile Red Crossbill; these individuals were collected and are currently housed at the Canadian Museum of Nature. Morphometric data from these three birds fall within the range of known values for percna (M. Gosselin pers. comm. 2014). These findings confirm previous reports of percnaon Anticosti Island (Ouellet 1969; Benkman 1993c). As such they increase the current known extent of occurrence and area of occupancy for percna. Incidentally, Red Crossbill was also detected on Anticosti Island during the original 1984-1989 Québec Breeding Bird Atlas, but subspecies identification was not pursued at that time.
Finally, 10 additional years of Christmas Bird Count data and 12 additional years of Breeding Bird Survey data are available since the original 2004 assessment. Data from winter bird surveys designed to detect Red Crossbills conducted in Newfoundland since 2008 are also available, as are datasets obtained from the NL Boreal Landbird Monitoring Program, the 2nd Québec Breeding Bird Atlas, various other summer bird surveys, and anecdotal reports of birds by birdwatchers in Newfoundland.
Given this new information, the current population of Red Crossbill percna is estimated to be in the low thousands (i.e., 1,000-2,500 mature individuals). This estimate is larger than the original status assessment estimate of 500-1,500 individuals because of recent evidence suggesting that a breeding population probably occurs on Anticosti Island; this newly found population was likely there for some time, such that the change in population size estimates should not be interpreted as an actual increase in real population size. The population estimate for birds in Newfoundland remains at 500-1,500 individuals due to lack of strong empirical evidence of a change in population size, because the apparent increase in the number of Red Crossbills reported by Newfoundland birdwatchers in recent years is offset by a declining BBS trend. It is also unclear whether the increase in reporting is due to an actual population increase, change(s) in distribution, improved education and public awareness, increased use of feeders (by both people and the birds), enhanced communication between observers (e.g., social media), or a combination of all of these factors.
COSEWIC History
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
COSEWIC Mandate
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC Membership
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
Definitions (2015)
- Wildlife Species
- A species, subspecies, variety, or geographically or genetically distinct population of animal, plant or other organism, other than a bacterium or virus, that is wild by nature and is either native to Canada or has extended its range into Canada without human intervention and has been present in Canada for at least 50 years.
- Extinct (X)
- A wildlife species that no longer exists.
- Extirpated (XT)
- A wildlife species no longer existing in the wild in Canada, but occurring elsewhere.
- Endangered (E)
- A wildlife species facing imminent extirpation or extinction.
- Threatened (T)
- A wildlife species likely to become endangered if limiting factors are not reversed.
-
Special Concern (SC)
(Note: Formerly described as “Vulnerable” from 1990 to 1999, or “Rare” prior to 1990.) - A wildlife species that may become a threatened or an endangered species because of a combination of biological characteristics and identified threats.
-
Not at Risk (NAR)
(Note: Formerly described as “Not In Any Category”, or “No Designation Required.”) - A wildlife species that has been evaluated and found to be not at risk of extinction given the current circumstances.
-
Data Deficient (DD)
(Note: Formerly described as “Indeterminate” from 1994 to 1999 or “ISIBD” [insufficient scientific information on which to base a designation] prior to 1994. Definition of the [DD] category revised in 2006.) - A category that applies when the available information is insufficient (a) to resolve a species’ eligibility for assessment or (b) to permit an assessment of the species’ risk of extinction.
The Canadian Wildlife Service, Environment and Climate Change Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Wildlife Species Description and Significance
Name and Classification
- Scientific name:
- Loxia curvirostra percna
- English name:
- Red Crossbill percna; Newfoundland Red Crossbill; Red Crossbill Type 8
- French name:
- Bec-croisé des sapins de la sous-espèce percna
- Classification:
-
Class – Aves
Order – Passeriformes
Family – Fringillidae
Subfamily – Carduelinae
Genus – Loxia
Species – Loxia curvirostra
Subspecies – Loxia curvirostra percna
Vernacular names: Vernacular synonyms for Red Crossbill in Newfoundland include Spruce Mope, Large Spruce Bird, and Spruce Bird (Reeks 1869; Peters and Burleigh 1951; Montevecchi and Wells 1987).
Subspecies nomenclature: Red Crossbills (Loxia curvirostra) in North America historically have been separated into a number of subspecies based on differences in plumage and size and shape of body and bill (Griscom 1937). Variation in bill size between subspecies corresponds with differing preferences for conifer food sources (Lack 1944; Benkman 1993a).
Newfoundland Red Crossbills were first described as a new subspecies (i.e., L. c. percna) in 1912 by Arthur Cleveland Bent. He examined 11 specimens collected from Fox Island River and Flat Bay River in southwestern Newfoundland and noted differences in plumage colouration, bill depth, and wing, tail, and culmen length compared to other North American subspecies known at that time (Bent 1912). In 1934, Red Crossbills from Newfoundland were renamed L. c. pusilla based on similarities between the Newfoundland birds and pusilla birds collected from the European nation of Georgia (Groth 1993a).
By the 1980s, many authors recognized that the use of pusilla was likely in error and reverted to the use of percna in reference to the Newfoundland subspecies (Phillips 1981; Dickerman 1987; Payne 1987). At least one current authority continues to use L. c. pusilla when referring to Newfoundland Red Crossbills (Clements et al. 2014).
Another method of differentiating Red Crossbill forms involves using recordings of crossbill vocalizations, because each form or “type” produces a series of unique, identifiable call notes when in flight. At least 10 Red Crossbill vocal types occur in North America, each having minor differences in morphology (bill size, palate structure, and plumage), genetics, and behaviour (Groth 1993a; Irwin 2010; Young 2012). Individuals of a given vocal type appear to preferentially mate with others of that same call type (i.e., positive assortative mating; Groth 1993b; Young et al. 2012). Some authorities suggest that vocal types therefore represent the early stages of unique species (e.g., Parchman et al. 2006). Based on morphological differences and on two 4-second recordings that differed from all other recorded North American Red Crossbill calls, Groth (1993a) labelled Red Crossbills from Newfoundland as Type 8 Red Crossbill.
Few genetic studies have been conducted on the North American Red Crossbill complex, and these are limited to Red Crossbill types other than percna. In one study, Groth (1993a) analyzed allozyme variation among seven forms of North American Red Crossbills and found genetic distances among forms was low, and that while one form (Type 1) was most genetically divergent of those analyzed, allozyme differences were not statistically significant. However, the markers used by Groth (1993a) inherently have low levels of variability (Parchman et al. 2006). A second study which analyzed 440 amplified fragment length polymorphic markers of eight North American Red Crossbill call types revealed greater genetic structuring based on allele frequency variation than Groth’s (1993a) allozyme study, but still did not find evidence that call types cluster together monophyletically, and found only minor differentiation among the types (Parchman et al. 2006). The authors of that study attributed the lack of monophyly to be the result of recent divergence and ongoing introgression, which are consistent with incipient species groups and recent adaptive radiation with low levels of ongoing gene flow among diverging taxa (Parchman et al. 2006). Genetic analysis of part of the mtDNA control region from study skin samples of Newfoundland Red Crossbills suggests that they differ from European Red Crossbills but not from other North American birds (Hynes and Miller 2014).
Recent bioacoustic research conducted from 2005 to 2011 confirmed the presence of Type 8 from multiple localities in Newfoundland; morphometric measurements from some of the recorded individuals fall within the known range of values for percna (Young et al. 2012; Hynes and Miller 2014). It thus appears that Type 8 and percna are synonymous though further validation using acoustical and morphological analysis is needed in other regions of Eastern Canada (Hynes and Miller 2014). Young et al. (2012) also discovered that their sample of 30 recordings (all were Type 8) did not match spectrographs of the original 4-second recordings of Newfoundland birds used by Groth (1993a) to conclude that Type 8 occurred in Newfoundland (i.e., Groth misidentified them). The original recordings instead most closely resemble Type 4 Red Crossbill, which most regularly occurs in the Pacific Northwest (Young 2012; Young et al. 2012). The presence of other vocal types of Red Crossbill in Newfoundland has recently been confirmed via audiospectrographic analyses. Of 83 birds recorded during 2010 and 2011, 78 (94%) were identified as Type 8; five individuals were identified as Types 2, 4, and 10 (Hynes and Miller 2014). The proportion of Type 8 birds in Newfoundland in other years is unknown. All 54 of the Red Crossbills collected from Newfoundland and housed at the Canadian Nature Museum have morphologies consistent with percna (M. Gosselin pers. comm. 2014).
Historical taxonomic revisions affecting the nomenclature of the Red Crossbill percna are presented in Table 1.
| Name | Year | Range | Source |
|---|---|---|---|
| L. c. percna | 1912 | Terre-Neuve seulement | Bent (1912) |
| L. c. pusilla | 1934 | Terre-Neuve | Van Rossem (Groth, 1988) |
| L. c. pusilla | 1937 | Terre-Neuve, avec nomadisme vers le sud sur la côte est du continent | Griscom (1937) |
| L. c. pusilla | 1966 | Terre-Neuve, avec nomadisme au Canada jusqu’en Ontario, au Québec, au Nouveau-Brunswick et en Nouvelle-Écosse | Godfrey (1966) |
| Classe de taille III | 1981 | Terre-Neuve, mais incluant d’autres « gros » becs-croisés d’Amérique du Nord présents sur le continent | Phillips (Dickerman, 1987) |
| L. c. percna | 1987 | Terre-Neuve, avec nomadisme jusque dans le nord-est des États-Unis et dans des régions canadiennes adjacentes | Dickerman (1987) |
| Type vocal 8 | 1993 | Terre-Neuve; occurrence sur le continent inconnue | Groth (1993a) |
| Type vocal 8 | 2012 | Terre-Neuve | Young et al. (2012) |
| Type vocal 8 | 2014 | Île d’Anticosti (Québec) | M. Robert, SCF-Région de Québec (données inédites) |
It has been suggested that when they are temporarily sympatric during irruptions, each of the Red Crossbill subspecies should be considered “pseudospecies” because there is no evidence that they interbreed even while nesting alongside each other (Knox 1992). Others argue that at least some of the North American Red Crossbills are in fact separate species because they are reproductively isolated and gene flow has not resulted in homogenous morphology (Benkman et al. 2009), they pair assortatively based on vocal type (summarized in Edelaar 2008), and they display the same divergences as Old World Red Crossbills which are recognized as separate species (DeBenedictis 1995). The possibility of full species status for L. curvirostra forms has also been demonstrated in Europe (Robb 2000; Summers et al. 2002, 2007).
In summary, Red Crossbill percna is a distinct form that is morphologically and vocally different from other North American Red Crossbill forms, and is likely at least partially reproductively isolated. The past use of the subspecies name pusillawas most likely erroneous; the more accurate subspecies name for this Red Crossbill taxon is percna, which recent research suggests corresponds to Type 8.
Morphological Description
All Red Crossbills are sparrow-sized finches identifiable by their unique crossed mandibles (i.e., bill tips overlap in the vertical plane) and lack of white wing bars (characteristic of White-winged Crossbills, L. leucoptera, which also occur in North America). Adult males are generally dull red (Figure 1), and adult females are generally brownish-green or greyish-olive. Juveniles are heavily streaked and are pale grey or brown in colour with hints of olive or yellow. Red Crossbills may not undergo regular seasonal moults, and plumages can vary throughout the year (e.g., males can range from deep brick red to reddish yellow or greenish). Body size and bill length and depth (height of bill from base of lower mandible to top of upper mandible where the bill meets the face) vary according to the form/vocal type (Godfrey 1986; Adkisson 1996; Pyle 1997). In an examination of Canadian Red Crossbills, left and right bill crossover occurred with the same regularity (James et al. 1987). A study of European Red Crossbills found no support for a genetic basis of mandible crossing direction (Edelaar et al. 2005).
Compared to other forms of North American Red Crossbills, percna is larger overall, has a more massive bill, and has darker and duskier plumage (Bent 1912; Pyle 1997). Adult male Red Crossbills in Newfoundland are dull red in colour with brighter rumps and blackish wings and tail; adult females are dull olive-grey with yellow rumps and underparts, and greyish wings and tail; and juveniles are variable in plumage, which ranges from olive-green to yellow to reddish (Peters and Burleigh 1951). Morphometric and bill measurements for Red Crossbill percna from various sources are presented in appendices 1 and 2.

Long description for Figure 1
Photo of a male Red Crossbill percna subspecies on a branch, showing side and underparts. The Red Crossbill percna subspecies has the crossed mandibles common to all crossbills. Adult male Red Crossbills in Newfoundland, like the one shown here, have dull red plumage with brighter rumps and blackish wings and tail. Compared with other forms of North American Red Crossbills, the percna subspecies is larger overall, has a more massive bill, and has darker and duskier plumage.
Population Spatial Structure and Variability
There appear to be two subpopulations of percna in Canada: one on Newfoundland and one on Anticosti Island (Figure 2). However, the amount of demographic and genetic exchange between these two groups is unknown. The Newfoundland subpopulation occurs on the main Island of Newfoundland as well as some forested nearshore islands (population estimate of 500-1,500 mature individuals). In all likelihood, a breeding population also occurs on Anticosti Island, QC (population estimate of 500-1,000 mature individuals; M. Robert pers. comm. 2014). Large-billed Red Crossbills that may be percna have been observed elsewhere in Québec, and in Nova Scotia, New Brunswick, and New England; these sightings are likely due to irruptions of birds to the mainland during times of food shortages in their core range of occurrence (Godfrey 1986).
Long description for Figure 2
Map of the global distribution of the Red Crossbill percna subspecies, a Canadian endemic occurring in insular Newfoundland and Labrador and on Anticosti Island.
Designatable Units
North American Red Crossbills likely represent a complex in the early stages of speciation (Parchman et al. 2006). At least 10 forms are recognized for Red Crossbill in North America, eight of which occur in Canada (Young 2012). These forms differ in their morphology, genetics, ecological associations, and behaviour (Groth 1993a; Parchman et al. 2006; Young 2012). Each is also characterized by its unique vocalizations made during flight. At least one of these forms appears to remain reproductively isolated even when it is not geographically separated (Benkman et al. 2009).
Red Crossbill percna is one of the eight recognized forms occurring in Canada. It occurs within a single national ecological area, the Boreal Shield ecozone. Its distribution is currently thought to be restricted to Newfoundland and Anticosti Island.
Special Significance
Red Crossbill percna is significant because it is a distinct taxonomic group (morphologically and behaviourally) having a distribution restricted to the island of Newfoundland and Anticosti Island. There is no Aboriginal Traditional Knowledge available for percna.
Distribution
Global and Canadian Range
Red Crossbill percna is endemic to Canada. Large-billed Red Crossbills are annually observed on the French islands of St. Pierre et Miquelon, off the south coast of Newfoundland, but there is no evidence that they breed there (Tuck and Borotra 1972; R. Etcheberry pers. comm. 2014). The subspecies’ range is restricted and limited to the islands of Newfoundland (and surrounding islands such as Merasheen, Fogo, and Bell Islands) and Anticosti Island (Figure 2). At the time of the original 2004 status assessment, Red Crossbill percna was considered to breed only in Newfoundland (COSEWIC 2004). Evidence to suggest that percna also breeds on Anticosti Island was collected during the Québec Breeding Bird Atlas in 2011 and 2014 – numerous birds with large bill size (that could correspond to percna) were observed feeding juveniles; recordings of Type 8 individuals were also confirmed and body measurements from three birds fall within the known range of values for percna (M. Robert pers. comm. 2014). Red Crossbills had been previously detected on Anticosti Island during the 1984-1989 Québec Breeding Bird Atlas (in much lower numbers than during the 2011-2014 Atlas), but subspecies identification was not attempted at that time. Birdwatchers on Anticosti reported low numbers of Red Crossbills (n = 1-2 every year or so) to Étude des populations d’oiseaux du Québec (ÉPOQ) between 2001 and 2007 (J. Larivée, pers. comm. 2015). However, it is possible that percna has always occurred on Anticosti Island and simply went largely undetected until the most recent Québec Breeding Bird Atlas (M. Robert pers. comm. 2014), aside from a specimen collected there in 1963 (Ouellet 1969; Benkman 1993c). It is unknown whether the Newfoundland and Anticosti Island populations are disjunct or if mixing occurs.
Red Crossbill percna is dependent on cone-productive boreal forest which occurs in the Boreal Ecozone. These birds may wander to New Brunswick, Nova Scotia, and elsewhere in Québec, Ontario, and the northeastern United States in search of food during periods of cone crop failures in insular Newfoundland (Godfrey 1986; Dickerman 1987; COSEWIC 2004). Large-billed Red Crossbills have been observed and collected in eastern Canada and New England (e.g., Griscom 1937; Peters and Burleigh 1951; P. Thomas pers. comm. 2014), though these were not considered to be breeding birds and at least some may not have been percna. Dickerman (1986) discussed nine Red Crossbill specimens from New York State that were identified as pusilla, but while all of the specimens were large-billed, they were not as heavy-billed as specimens from Newfoundland or Anticosti Island. Large-billed specimens collected in Massachusetts have been identified as pusilla (Griscom and Snyder 1955). A photograph of a large-billed female Red Crossbill taken in Goose Bay in June 2003 represents the only confirmed sighting of the species in Labrador (P. Linegar pers. comm. 2014). There are no reliable breeding records of Red Crossbills from Labrador (Todd 1963). Peters and Burleigh (1951) stated that the Newfoundland race of Red Crossbill bred in Nova Scotia but did not substantiate this claim. A female Red Crossbill designated as pusilla was collected from Anticosti Island in 1963 (Ouellet 1969); its morphology is consistent with the known range of values for percna (M. Gosselin pers. comm. 2014).
Extent of Occurrence and Area of Occupancy
Extent of occurrence of Red Crossbill percna is estimated to be 261,500 km2; nearly 55% of which is open water. The index of area of occupancy (IAO) is measured as the surface area of 2-km × 2-km grid cells that intersect the actual area occupied by a taxon at any one time (i.e., the biological area of occupancy). IAO is difficult to estimate for percna because it is itinerant, breeds at any time of year, and regularly makes very large movements (i.e., island-wide or even off-island), therefore the area occupied by the species can vary throughout the year. This propensity for large spatial movements and a flexible breeding strategy are tied to the availability of cone crops, which fluctuate across years. In addition, only a small proportion of potential habitat has been surveyed in Newfoundland. While the IAO is unknown, it is most likely smaller than the threshold value of 2,000 km2 relevant to COSEWIC’s designation criteria. Because the population of percna is relatively small and the birds are somewhat colonial, IAO may be as small as 300 km2. This estimate takes into consideration that at any given time, Red Crossbills are estimated to occupy approximately 40 2x2 km cells in Newfoundland, and the area occupied on Anticosti Island is likely similar or smaller.
The number of locations for percna is unknown and particularly difficult to determine for an irruptive finch such as percna. However, given the extent of occurrence and nature of threats to the species, the number is expected to be >10.
Search Effort
Determining Red Crossbill percna’s range in Canada is complicated given the difficulty associated with identifying the subspecies in the field. On their own, morphometric data can be used to state that an individual is probably percna. Bioacoustic data are required to confirm the identity of birds as Type 8. The combination of these data from individual birds is required to definitively identify individuals as percna; this is lacking for the vast majority of Red Crossbills in Newfoundland (but see Young et al. 2012) and on Anticosti Island.
Red Crossbill percna’s distribution in Canada is largely based on comparisons of specimens collected in Newfoundland to those collected elsewhere in North America; these comparisons have resulted in the conclusion that percna is generally restricted to Newfoundland (e.g., Griscom 1937; Burleigh and Peters 1948; Payne 1987). Bioacoustic data have more recently been used to improve our understanding of the range of Type 8. Based on a sample size of 83 individuals observed and recorded during one study in 2010/2011, the majority of Red Crossbills observed in Newfoundland are assumed to be Type 8 (approximately 94%; Hynes and Miller 2014). However, only a small proportion of all Red Crossbill sightings from Newfoundland have been identified as percna or Type 8 via morphometrics or bioacoustics.
During the 2nd Québec Breeding Bird Atlas (2010-2014), Red Crossbills (subspecies unknown) were detected at a small number of widely dispersed sites in mainland Québec (Atlas of the Breeding Birds of Québec 2015). On Anticosti Island, however, Red Crossbills were regularly and frequently recorded; the majority of those birds were suspected to be percna based on morphology and vocalizations (M. Robert pers. comm. 2014); further research on this is scheduled for summer 2016.
Large-billed Red Crossbills have been irregularly and infrequently observed throughout eastern Canada but are assumed to represent irruptions during periods of low cone crops in core areas of occurrence (i.e., Newfoundland and Anticosti Island). A limited number of surveys have been conducted in Labrador, including general searches during Christmas Bird Counts at two sites (Labrador City-Wabush in western Labrador and Happy Valley-Goose Bay in south-central Labrador) and 9 Breeding Bird Survey routes, and targeted Red Crossbill winter surveys conducted at 42 points in 2012 in southeastern Labrador. Red Crossbills have not been detected on those surveys.
Habitat
Habitat Requirements
All forms of Red Crossbill are cone-obligate seed eaters extremely specialized for foraging in coniferous habitat (Adkisson 1996). Conifer forests are also required for roosting and nesting (although nesting and roosting sites may be distant from foraging sites). All large-billed crossbills are pine associates (e.g., Benkman 2010; Benkman et al. 2001; Summers et al. 2002). Prior to European settlement in Newfoundland, Red Pine (Pinus resinosa) and White Pine (Pinus strobus) stands likely provided key habitat for percna, but currently these conifers are rare and exist in small, isolated fragments scattered across the island, and Black Spruce is also important for Red Crossbill in Newfoundland (Rajora et al. 1998; Parchman and Benkman 2002; COSEWIC 2004; Roberts 2011).
Conifer seed availability is the main factor affecting occurrence, distribution, breeding, and survival of Red Crossbills. A key habitat feature is a mosaic of cone-bearing trees over a large (i.e., island-wide) spatial scale such that sufficient food resources are available in any geographical area or from any species of conifer at any given time. Red Crossbills typically irrupt into other areas (and/or species of conifers) that provide sufficient seed resources when core areas of occurrence become depleted of seed-bearing cones. If they find suitable food resources during these movements, they may settle and nest for one or more seasons. However, irrupting birds tend to be reasonably faithful to core breeding areas and may return within a few years of the irruption (Knox 1992).
In Newfoundland, Red Crossbills are consistently recorded in Red Pine stands (e.g., at West Brook Ecological Reserve in central Newfoundland and Terra Nova National Park in eastern Newfoundland), even though very few stands remain. Because of the rarity of Red Pine in Newfoundland, its status as a species at risk is currently being assessed by the NL Species Status Advisory Committee (S. Pardy pers. comm. 2015). Black Spruce (Picea mariana) forests currently provide conifer seeds on a large scale. Balsam Fir (Abies balsamea), White Spruce (Picea glauca) and Eastern Larch (Larix laricinia) also provide food for percna. Red Crossbills in Newfoundland are known to feed on cones of all conifers, including Red and White Pines, Black and White Spruce, Balsam Fir, Eastern Larch, and non-native Austrian (Pinus nigra), Jack (P. banksiana), Mugho (P. mugo), and Scots Pines (P. sylvestris; Lamberton 1976; Benkman 1989; COSEWIC 2004; NL Landbird Recovery Team pers. comm. 2014). They have also been observed feeding on maple seeds and buds of other deciduous trees, as well as weed seeds, wild fruits, and insects and larvae (Peters and Burleigh 1951).
On Anticosti Island, the majority of forest is characterized by White Spruce, with smaller proportions of Balsam Fir and Black Spruce (Potvin et al. 2003). On Anticosti Island, percna feeds mainly on cones of White Spruce and to a lesser extent Black Spruce (M. Robert pers. comm. 2014). Balsam Fir forests likely do not provide much habitat, as they have been significantly overgrazed by hyper-abundant, introduced White-tailed Deer (Odocoileus virginianus) and are being replaced by forests dominated by White Spruce (Côté et al. 2008). The crop of one percna specimen taken from Anticosti Island in 2014 contained pupae of Spruce Budworm (Choristoneura fumiferana) (M. Robert pers. comm. 2014).
Well-defined habitat associations are difficult to identify given the sporadic nature of percna’s occurrence across the landscape, and its flexible breeding strategy (determined by cone availability). While percna is predominantly found in natural forest habitat, the subspecies is also known to occur in urban areas and frequents bird feeders between February and May on Newfoundland’s Avalon Peninsula, and in eastern, central, and western Newfoundland (Young et al. 2012; NL Landbird Recovery Team pers. comm. 2014; TDL pers. obs.).
Critical habitat for Red Crossbill percna has not been identified. However, in Newfoundland percna is reliably detected each year in a small number of Red Pine stands (i.e., at Terra Nova National Park in eastern Newfoundland, West Brook Ecological Reserve in central Newfoundland, and near the community of Howley in western Newfoundland) and at backyard bird feeders (i.e., at Whitbourne on the Avalon Peninsula and Glovertown/Traytown near Terra Nova National Park in eastern Newfoundland).
Habitat Trends
Approximately 31% of the landbase (35,000 km2) in Newfoundland is characterized by productive forest (i.e., capable of producing, at rotation age and under natural conditions, at least 30 m3 per hectare of merchantable timber; Government of Newfoundland and Labrador 2014a; Figure 3). It is possible that this represents the total amount of habitat available to Red Crossbills in Newfoundland. The majority of this habitat has not been surveyed for Red Crossbill. However, the majority of Red Pine stands (where Red Crossbills are consistently detected and which therefore apparently provide important habitat) have been surveyed only once or a few times each. Availability of seed-bearing cones is a key determinant of Red Crossbill survival, breeding, and spatial movement patterns. Recent projections by the NL Forestry and Agrifoods Agency indicate that cone production in spruces and Balsam Fir is likely to significantly increase across Newfoundland’s Avalon Peninsula over the next two decades because they will be of the most productive age class during that 20-year period (B. English pers. comm. 2014). However, an increase in cone production may be strongly affected by a major Spruce Budworm outbreak which is expected to occur in the near future.
Forests in Newfoundland are highly fragmented, both naturally (due to an abundance of waterbodies and wetlands, and large- and small-scale natural disturbances such as forest fires, insect outbreaks, and wind-driven gap dynamics) and anthropogenically (due to human habitation, forest harvesting, agriculture, and linear features such as roads, trails, and hydroelectric rights of way).
Approximately 72% of Anticosti Island (5,720 km2) is characterized by productive forest (i.e., is capable of regenerating such that canopy recovery is at least 25% in the next 100 years; P. Beaupré pers. comm. 2015).
Long description for Figure 3
Map showing areas of productive forest in insular Newfoundland and Labrador and the locations of the 20 remaining natural Red Pine stands. Productive forest makes up approximately 31 percent of the island’s land base (35,000 square kilometres).
On Anticosti Island, forests are naturally fragmented due to fire, insect outbreaks, wind-driven gap dynamics, and (in the eastern portion of the island) by large peatlands (Côté et al. 2008). Anthropogenic fragmentation is largely characterized by forest harvesting and associated resource roads. The only human settlement is located on the western side of the island and has a year-round population of 230 inhabitants (Potvin et al. 2003).
There has been a net loss in amount and quality of Red Crossbill habitat since the time of European settlement in Newfoundland. Direct loss of habitat has occurred due to the permanent conversion of forests for human habitation and to a lesser extent agriculture, as well as the extensive removal of pines, human-caused forest fires, and fire suppression. Habitat degradation continues to occur due to mechanized forest harvesting, construction of linear features such as roads and hydroelectric rights of way, and insect and fungal outbreaks. These factors are detailed below and in the Threats and Limiting Factors section.
Forest harvesting and anthropogenic habitat fragmentation
The boreal forest landscape that currently exists in Newfoundland is different from that of previous centuries. The cumulative impact of anthropogenic (permanent conversion of forest, forest harvesting, creation of linear features, and human-caused fires) and natural (insect and fungal infestations, fire cycle, and forest regeneration patterns) factors has led to changes in the composition, age structure, and succession patterns of Newfoundland forests.
Populations of both White and Red Pine have undergone major declines since Newfoundland was first settled by Europeans in the 17th century. In Newfoundland, Red Pine is at the northeastern extreme of its range, where it has never been abundant but may have had the same range as White Pine (Mosseler et al. 1992; Roberts and Mallik 1994). White Pine was widely spread across central and western Newfoundland at the time of European arrival (Rajora et al. 1998). Both species of pines were intensively harvested in the 1600-1800s; forest fires, urbanization, disease, and depressed recruitment levels have also contributed to present-day low population numbers (Page et al. 1974; Whitaker et al. 1996; Rajora et al. 1998; Roberts 2011). Red Pine is currently the rarest native conifer in Newfoundland and occurs in only 20 widely dispersed natural stands covering less than 1,500 ha; each stand has fewer than 25,000 mature and semi-mature trees (Roberts 2011). White Pine is found in only a small number of isolated fragments scattered about the Island; many patches contain fewer than 30-50 individuals (Rajora et al. 1998). Since the late 1990s, the NL Forestry and Agrifoods Agency has implemented a policy against cutting Red or White Pine on Crown Land (Government of Newfoundland and Labrador 2014a; B. English pers. comm. 2014), which presumably protects important food resources and habitat for Red Crossbills. Of the 20 remaining natural Red Pine stands in Newfoundland (surveyed in 1985 and then re-surveyed in 2010-2011), numbers of mature trees have been decreasing in 4 stands and increasing in 10 stands. Most important current and ongoing threats to these 20 stands include cottage and road development, insect infestation, cone predation by non-native Red Squirrels (Tamiasciurus hudsonicus), all-terrain vehicle use, and gravel extraction (Roberts 2011). Given these factors, a 10%-20% decline in Red Pine populations over the next decade or so is possible (particularly for central Newfoundland stands), although more study is needed (B.A. Roberts pers. comm. 2015).
Coniferous forest in Newfoundland has been anthropogenically fragmented by mechanized forest harvesting (clearcut harvesting and selective logging) since the second half of the 20thcentury. Harvesting regimes have led to shorter rotation times (i.e., younger trees are harvested) for conifer stands in Newfoundland (Thompson et al. 1999, 2003). This has resulted in relatively fewer and more fragmented mature and old-growth stands, which are the most productive habitat for Red Crossbills elsewhere (e.g., Holimon et al. 1998). Of the 35,000 km2 of productive forest in Newfoundland, 48% is old-growth/late-seral forest (i.e., 81 years of age of older; Government of Newfoundland and Labrador 2014a,b). While well-defined habitat associations are not known for percna, it is likely that late-seral forests are important. The highest incidences of Red Crossbills detected on breeding bird surveys conducted from 1980 to 1985 were in old-growth forests of western Newfoundland. In other parts of the Red Crossbill’s range, managed habitat must contain old-growth or at least mature conifers to be beneficial to crossbills, because they offer the best yield of cones (Newton 1972; Benkman 1993b; Holimon et al. 1998). Some species of conifer in Newfoundland produce seed-bearing cones as young as age ten (e.g., Black Spruce; B. English pers. comm. 2014). These stands may provide food resources although the caloric value to Red Crossbills of seeds produced by young trees is unknown. In a two-year study in Terra Nova National Park, where Red Crossbills regularly occur, Black Spruce trees aged 21-60 years produced larger cones with more filled seed per cone than did Black Spruce trees aged 61-81 or more years (Tulk 2004).
While forest management regimes in the past resulted in large swaths of forest being removed, recent practices have shifted to an ecosystem-based approach where biodiversity and other forest values are considered during the planning process. In addition, continual downturns in the forest industry since the 1990s have led to decreased demand for timber. At the time of the last percna status assessment in 2004, two major pulp and paper companies held timber rights to 59% of the island’s productive timber lands (Government of Newfoundland and Labrador 2003). Since 2008, only one company has held timber rights to approximately 31% of productive forest (Government of Newfoundland and Labrador 2014a; F. Knott pers. comm. 2014). Forest volumes harvested annually since 2008 have been reduced to approximately half of what was harvested during peak years of forestry activity in Newfoundland (B. Adams pers. comm. 2015). In its most recent Sustainable Forest Management Strategy, the Forestry Services Branch (FSB) of the NL Department of Natural Resources has committed to maintaining at least 15% of late-seral forest within commercial forest management areas across Newfoundland (Government of Newfoundland and Labrador 2014a). The FSB and Corner Brook Pulp and Paper Ltd. have also recently begun to incorporate into planning regimes the inherent ecological values of large, intact landscapes (LILs) and species-specific areas aimed at species at risk. For LILs, the use of mechanized equipment, construction of roads, and creation of cuts larger than 0.05 km2 have been deferred over an area covering approximately 40,000 km2 until at least 2024 (Government of Newfoundland and Labrador 2014a). These LILs are mainly characterized by non-productive forest and barren lands, but contain some productive forest which is expected to provide habitat for percna. Forest management criteria vary in species-specific areas, where habitat for Woodland Caribou (Rangifer tarandus caribou) and the federally endangered Newfoundland Marten (Martes americana atrata) represent the largest species-specific areas (Government of Newfoundland and Labrador 2014a), the latter of which likely also provides much suitable habitat for percna.
Linear features also result in fragmentation and loss of boreal forest habitat. In Newfoundland, there are more than 20,000 kilometres of primary and secondary roads (A. Bassan pers. comm. 2015), and approximately 12,000 km of active/passable forestry resource roads (S. Payne pers. comm. 2015). In addition, there are more than 10,000 km of hydroelectric rights of way and associated distribution lines (J. Linfield pers. comm. 2015).
On Anticosti Island, the forest is publicly owned and managed as a timber reserve (reserve forestière; Gouvernement du Québec 2004a). The forest harvesting capacity is limited to 190,000 m3 with the possibility of 1,600 ha/yr of clearcutting and 1,200 ha/yr of silvicultural treatments (Gouvernement du Québec 2009). At the time of the last percna status assessment in 2004, only one timber company held timber rights to 38% of productive forest on Anticosti Island (Gouvernement du Québec 2009), and approximately 20,000 m3 of forest were being harvested annually (Gouvernement du Québec 2004b).
Specific research regarding the effects of habitat fragmentation and changes to landscape connectivity on Red Crossbill has not been conducted. However, the degree to which animals can move about their surroundings on a range of spatial scales has decreased in anthropogenically fragmented boreal forest for a number of taxa, including highly vagile animals such as songbirds (e.g., Bayne and Hobson 2002; Leonard et al. 2008; Whitaker et al. 2008). It is possible that changes to forest connectivity in Newfoundland over the last 400 years have had a negative impact on Red Crossbill movements, productivity, and survivorship. However, direct loss of habitat is likely to be a more important factor in crossbill productivity and survivorship than habitat fragmentation.
Fire
Fire has had a major cumulative influence on forest composition in Newfoundland, particularly in central and eastern parts of the Island. During European settlement in the early 17th century, settlers used fire to clear large tracts of land along the coasts. These uncontrolled fires removed many tens of thousands of hectares of coniferous boreal forest, much of which has never regenerated but exists today as wetland and barrens habitat (Wilton and Evans 1974; Damman 1983). During the 20th century, extensive fires were often started by cinders from locomotive engines (Wilton and Evans 1974; Montevecchi and Tuck 1987). Other human-caused and natural fires since the late 1800s have contributed to the decline of Red Pines across the island, as fires in the boreal forest tend to be hotter and more intense than Red Pines can withstand (Roberts 1985, 2011; Bergeron and Brison 1990). Boreal forest fires promote the regeneration of Black Spruce, but fire suppression activities likely inhibit the regeneration and spread of both Black Spruce and Red Pine (Fowells 1965; Bergeron and Brison 1990). Controlled burns have been undertaken in small areas of forest (including in Terra Nova National Park, where Red Crossbills are regularly detected) in an attempt to simulate natural ecosystem processes.
Insects and fungal diseases
Outbreaks of insects and fungi have and continue to defoliate large tracts of forests in Newfoundland. Tree loss from these two factors is higher than loss from harvesting and an order of magnitude higher than loss from fire. While infestations are not always lethal for the tree, cone production can be significantly reduced (Lavigne 2014). Since the 1960s, Spruce Budworm, Hemlock Looper (Lambdina fiscellaria fiscellaria), and Balsam Fir Sawfly (Neodiprion abietis) have defoliated approximately 57,000 km2, 13,000 km2, and 4,300 km2 of coniferous forest in Newfoundland, respectively (D. Lavigne pers. comm. 2015).
Natural stands of Red Pine in eastern and western Newfoundland have been threatened by Fir Coneworm (Dioryctria abietivorella) since 1989. During the first few years after initial detections, up to 100% of trees in eastern and 25% of trees in western Newfoundland stands were infected. Those infestations reduced cone size by 17% and the number of full seeds per cone by up to 93% in eastern stands; cone size in western stands did not change but the number of seeds per cone declined by 11% (Mosseler et al. 1992). Fir Coneworm was still present in 2000-2001, and most severe infestations continued to be in eastern stands (B.A. Roberts unpubl. data). In 2010, eastern stands were still severely infested (90%-100% incidence rate), central stands had less than 25% incidence rate, and Fir Coneworm was difficult to find in western stands (<5% incidence rate, likely due to the younger age class in western Newfoundland; Roberts 2011). Fir Coneworm is a serious and long-term threat to regeneration of Red Pine (and thus Red Pine populations), and impacts to regeneration will be further magnified if wildfire occurs in infected stands (B.A. Roberts pers. comm. 2015).
It is possible that insect outbreaks could also positively affect percna in Newfoundland and Anticosti Island by providing a rich and alternate source of food in the form of insect larvae and pupae (Peters and Burleigh 1951). One percna specimen sampled from Anticosti Island in 2014 had Spruce Budworm pupae in its crop (M. Robert pers. comm. 2014).
Pines in Newfoundland are also affected by fungal outbreaks. Introductions of the non-native White Pine Blister Rust (Cronartium ribicola) in the mid-1900s devastated the small number of surviving White Pine trees and continues to prevent regeneration (Rajora et al. 1998; Bérubé 2003). Red Pine stands in Newfoundland are currently threatened by the European race of Scleroderris canker (Gremmeniella abietina var. abietina), a serious fungus of hard pines which causes stem dieback and tree mortality in both immature and mature Red Pines. Non-native pines such as Jack, Austrian, and Scots Pine are less susceptible but can harbour the disease (Lavigne 2014). The fungus was first detected in planted Red Pine stands on the Avalon Peninsula in 1979 and a federal quarantine zone for the Peninsula was enacted. In 1988, the disease was detected in two Red Pine plantations north of the quarantine zone; those stands were eradicated in an attempt to control the disease. The quarantine remained effective until 2007 when Scleroderris canker was detected in a planted Red Pine stand 400 km west of the Avalon Peninsula. Surveys conducted in 2011-2012 identified another six planted stands containing the fungus. To date Scleroderris has been found in planted stands only, with some stands having 100% infection rates and up to 48% mortality of trees. Sanitation programs are being carried out by the Department of Natural Resources in an attempt to prevent the disease from spreading to natural and other planted stands (Lavigne 2014). There is a high probability that Red Pine stands infected with Scleroderris could decline by more than 10% in the next 10 years (B.A. Roberts pers. comm. 2015). Possible transmission pathways for spores of Scleroderris include non-native Moose (Alces alces), humans, and Red Squirrel. The fungus Sirococcus Shoot Blight (Sirococcus strobilinus) has also resulted in significant damage to and mortality of Red Pines in at least one planted stand (Lavigne 2013, 2014). In a survey conducted in 2012, the fungus Red Band Needle Blight (Dothistroma septosporum) was detected in 76% of planted and natural stands of Red Pine; this disease causes premature needle loss and results in tree mortality only after severe defoliation over consecutive years (Lavigne 2013). It is possible that the decline in Red Crossbill percna numbers is correlated with significant declines in the populations of mature pines, as has been observed elsewhere (e.g., Dickerman 1987; Erskine 1992).
Biology
Few data on the biology of Red Crossbill percna exist; information presented in the following sections is for Red Crossbills in general and is summarized from Adkisson (1996) and references therein, unless otherwise noted.
Life Cycle, Demographic Parameters and Reproduction
Little information is available on Red Crossbill lifespan. Based on banding data returns, the North American longevity record for the species is six years and one month from a female banded and recovered in South Dakota (Lutmerding and Love 2014). Captive birds may live up to 8 years, where rates of annual mortality are higher for females than for males.
All forms of Red Crossbills are monogamous, form pair bonds, and nest in loose aggregations. Like many gregarious songbird species, Red Crossbills do not appear to defend territories, although observations of agonistic interactions between males in breeding condition have been interpreted as territoriality. Red Crossbills have the most erratic breeding schedule of any North American bird, and throughout their range can be found breeding during any month of the year. Their annual breeding (i.e., gonadal) cycle appears to be regulated by photoperiod, and opportunistic responses to food supply and social factors are superimposed on this cycle. This breeding strategy allows Red Crossbill to adapt to predictable (seasonality) and unpredictable (food availability) environmental factors (Hahn 1995). Breeding can occur over a range of many months in mixed conifer forests having different species of seeds available at different times. Average clutch size is three eggs and females have one to four broods per year. Bills are not crossed at fledging but are usually fully crossed by 30 days old. Young are able to extract and shell seeds at 45 days old.
Evidence of temporal flexibility for reproduction in percna is sparse, as few adequate nest records exist. However, this temporal flexibility is well established for Red Crossbill in general (Adkisson 1996). Based on the NestWatch data collected in Canada (Bird Studies Canada 2013), the nesting period for Red Crossbill can range from early January to mid-September (B. Drolet pers. comm. 2015). Reeks (1869) considered Red Crossbills to be “common early nesters” in western Newfoundland. Rooke (1935) assumed that most crossbills seen in August/September in Newfoundland were nesting. Peters and Burleigh (1951) described Red Crossbills in Newfoundland as “often very early nesters, sometimes nesting in January or February and at other times not until mid-summer.” McCabe and McCabe (1933) presented information on one percna pair breeding in April. Red Crossbills in Newfoundland have been observed nesting or with dependent young in March-September, December, and February (COSEWIC 2004 and references therein; Hynes 2013; anecdotal observations reported to online discussion forums).
There is little information available on hatch rate for Red Crossbill. Fledging rate (i.e., proportion of eggs surviving to fledglings) ranges from 33% to 48%. No data exist on lifetime reproductive success in wild birds.
Red Crossbills apparently can breed in the same year in which they were born. Both males and females in immature plumage and with incomplete ossification of their skulls have been found in breeding condition. Generation time for Red Crossbill is estimated to be between 2.9 years and 3.4 years (Benkman et al. 2005; BirdLife International 2014).
No quantitative data on survivorship in the wild are available for Red Crossbill.
Physiology and Adaptability
While most forms of Red Crossbill are considered to be pine-forest associates, and Red Crossbills in Newfoundland are reliably detected in Red Pine stands, they are also routinely observed foraging on cones of Black and White Spruce, Balsam Fir, Tamarack, ornamental pines, maple seeds and buds of other deciduous trees, as well as weed seeds, wild fruits, and insects and larvae (Peters and Burleigh 1951). Fieldwork conducted as part of the second Québec Breeding Bird Atlas indicate that Red Crossbills on Anticosti Island apparently feed mainly on cones of White Spruce and to a lesser extent on those of Black Spruce (M. Robert pers. comm. 2014). Other adaptations include those related to extreme variability in conifer seed crops, such as sexual maturity at a relatively young age, accelerated succession of broods, multiple broods per year, and tolerance of repeated cooling and slow development of young when food is relatively scarce.
Dispersal and Migration
Red Crossbills, like other cardueline finches, do not undertake regular seasonal long-distance migrations. Instead, they exhibit periodic irruptive movement patterns that reflect the productivity of the cone-bearing trees on which they depend. Movements occur across a range of spatial scales, from local and regional movements to continental ones (Bock and Lepthien 1976). Irruptions usually occur in autumn when cones ripen, but movements may also be undertaken in the spring or early summer. Red Crossbills may be stimulated to move by food shortage or possibly based on social assessments of cone and seed abundance in an area. Overcrowding within the birds’ regular range may also encourage birds to move. There is no evidence of nocturnal movements; Red Crossbills throughout the Appalachian region have been observed undertaking long flights during the day.
Movements of percna are not well understood. Their sporadic patterns of occurrence across Newfoundland are likely the result of their irruptive movement behaviour. There is some speculation that Red Crossbills move off Newfoundland during conifer seed shortages on the Island. Other bird species with subspecific designations are sedentary on insular Newfoundland (Peters and Burleigh 1951). Large-billed island species of crossbills, including the Scottish Crossbill (L. scotica) and the Parrot Crossbill (L. pytyopsittacus) have also been shown to be more sedentary than sympatric L. curvirostra, which has a smaller bill (Marquiss and Rae 2002; Summers et al. 2002). Thus a pattern of limited movements is not a wholly unexpected strategy for percna. Red Crossbills are occasionally seen on the French islands of Saint Pierre and Miquelon that lie off the Burin Peninsula on Newfoundland’s south coast, but there is no evidence that they breed there (R. Etcheberry pers. comm. 2014). Red Squirrels were unsuccessfully introduced to the islands; introductions may have failed due to the small number of conifer trees there, which likely also impedes crossbill breeding.
Large-billed Red Crossbills that may be percna are irregularly and infrequently reported from Nova Scotia, New Brunswick, mainland Québec and the Magdalen Islands, and New England. These sightings likely represent irruptions of birds off Newfoundland when food resources are scarce.
Interspecific Interactions
Potential predators of Red Crossbills throughout their range include the Sharp-shinned Hawk (Accipiter striatus), Cooper’s Hawk (A. cooperii), Merlin (Falco columbarius), Peregrine Falcon (F. peregrinus), and Northern Shrike (Lanius excubitor); all except Cooper’s Hawk are present in Newfoundland and Anticosti Island, and Peregrine Falcons are rare/accidental on Anticosti Island (Lepage 2014). Red Crossbill nests are vulnerable to predation by non-native Red Squirrels (present in Newfoundland but not Anticosti Island), and Gray Jays (Perisoreus canadensis), both of which are the most common nest predators in boreal forests of Newfoundland and Québec (Lewis 1999; Boulet et al. 2000). Other avian nest predators such as American Crow (Corvus brachrhynchos), Blue Jay (Cyanocitta cristata), and Common Grackle (Quiscalus quiscula) may also take eggs or nestlings.
Population Sizes and Trends
Estimates of total population size are difficult to effectively obtain for songbird populations. It can be particularly challenging to determine population trends of finches because of their irruptive and nomadic behaviours (Bock and Lepthien 1976; Koenig and Knops 2001; Wren 2001). Red Crossbill populations fluctuate with cone availability, and they often move in large flocks over continental scales and in areas sparsely populated by birdwatchers (National Audubon Society 2014).
Sampling Effort and Methods
Multiple datasets are available for estimating Red Crossbill abundance in Newfoundland, including Christmas Bird Counts (CBC), Breeding Bird Surveys (BBS), winter bird surveys designed to detect Red Crossbills, other standardized summer bird surveys, and local birdwatching reports. For Anticosti Island, data were obtained during the 2nd Québec Breeding Bird Atlas. Of these, only the Newfoundland CBCs (and, to some extent, BBSs) provide long-term datasets useful for analyzing population trends, but even they have considerable limitations. Sampling effort varies with survey type and across years and locations.
Christmas Bird Counts (CBCs)
The longest and most consistent dataset on Red Crossbills in Newfoundland has been compiled through annual, volunteer-based CBCs. CBCs are general searches conducted by varying numbers of observers on one day within a 3-week period from mid-December to early January in discrete 24-km diameter circles. Numbers of all species of birds encountered in each circle are recorded. CBCs have been conducted in 17 count circles scattered across Newfoundland since 1936; many of these have been active for decades, although not all have been active each year (Figure 4; Table 2). Because only a small number of count circles have been surveyed, and these generally overlap areas of human occupation, the vast majority of potential Red Crossbill habitat (i.e., cone-bearing forest) in Newfoundland has not been surveyed by CBCs. Of 340 CBCs conducted since 1936, Red Crossbills were detected on 71 occasions (13 of 17 count circles during one or more years; Table 2).
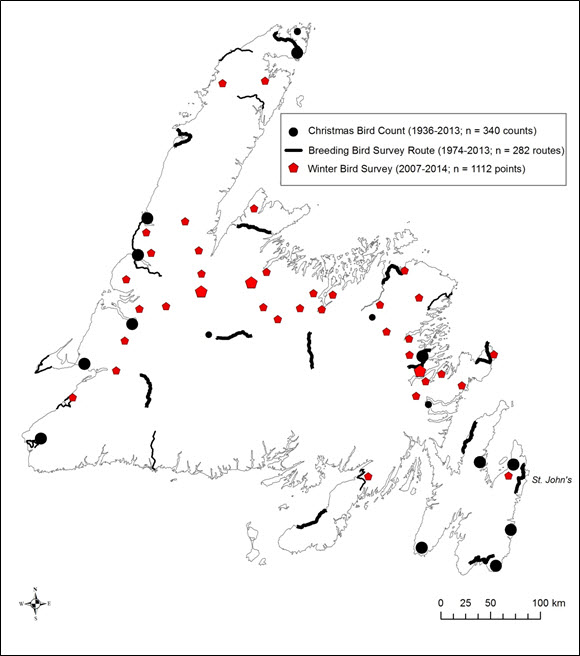
Long description for Figure 4
Map showing locations of Christmas Bird Counts, Breeding Bird Survey routes, and winter bird surveys conducted in insular Newfoundland and Labrador.
| Survey | Route name | First year surveyed | Last year surveyed | n |
|---|---|---|---|---|
| CBC | Bonne Bay | 1971 | 2013 | 39 |
| CBC | Buchans | 2000 | 2010 | 7 |
| CBC | Cape Race | 1977 | 2012 | 33 |
| CBC | Cape St. Mary’s | 1979 | 2010 | 29 |
| CBC | Clarenville | 2001 | 2010 | 8 |
| CBC | Codroy | 1985 | 1986 | 2 |
| CBC | Conception Bay North | 1987 | 1991 | 5 |
| CBC | Corner Brook | 1981 | 2013 | 33 |
| CBC | Ferryland | 1991 | 2013 | 22 |
| CBC | Gander | 2000 | 2012 | 13 |
| CBC | L’Anse aux Meadows | 1976 | 1986 | 11 |
| CBC | Terra Nova | 1968 | 1984 | 17 |
| CBC | Terra Nova north | 1987 | 2011 | 24 |
| CBC | St. Anthony | 1936 | 1986 | 18 |
| CBC | St. John’s | 1966 | 2013 | 48 |
| CBC | St. Paul’s | 1980 | 2013 | 14 |
| CBC | Stephenville | 1991 | 2013 | 17 |
| BBS | 57001 (Trepassey) | 1980 | 2012 | 11 |
| BBS | 57003 (St. John’s) | 1976 | 2013 | 24 |
| BBS | 57004 (Heart’s Delight) | 1976 | 2011 | 13 |
| BBS | 57006 (Grand Bank) | 1982 | 2013 | 14 |
| BBS | 57008 (Burgeo South) | 1983 | 2013 | 6 |
| BBS | 57009 (Sheaves Cove) | 2011 | 2013 | 2 |
| BBS | 57010 (O’Reagans) | 1980 | 2013 | 24 |
| BBS | 57011 (Bonavista) | 1981 | 2011 | 8 |
| BBS | 57012 (Terra Nova) | 1979 | 2010 | 21 |
| BBS | 57013 (NW Gander River) | 1980 | 2013 | 21 |
| BBS | 57014 (Buchans) | 1981 | 2013 | 12 |
| BBS | 57015 (Burgeo Road) | 1981 | 2012 | 15 |
| BBS | 57016 (St. David’s) | 1980 | 2013 | 13 |
| BBS | 57017 (Wareham) | 1980 | 2012 | 9 |
| BBS | 57018 (Gander Bay) | 1981 | 2011 | 17 |
| BBS | 57020 (Burlington) | 1982 | 2011 | 7 |
| BBS | 57021 (St. Paul’s) | 1974 | 2012 | 18 |
| BBS | 57022 (Roddickton) | 1975 | 2013 | 12 |
| BBS | 57023 (Port Saunders) | 1974 | 2012 | 10 |
| BBS | 57024 (St. Anthony) | 1975 | 2013 | 14 |
| BBS | 57105 (Bay L’Argent) | 2007 | 2012 | 3 |
| BBS | 57121 (Woody Point) | 1995 | 2013 | 12 |
| BBS | 57125 (Flower’s Cove) | 1980 | 2012 | 6 |
| Date | Location | Area | Source | Methods | Abundance |
|---|---|---|---|---|---|
| 1974, 1975 | Gros Morne N.P. | 1,805 km2 | Lamberton (1976) | Territory mapping | “single birds and small flocks” in 1975 only |
| 1991, 1992 | Humber River south to Little Grand Lake | NA | Thompson et al. (1999) | point counts (n=175) | not detected |
| 1994, 1995 | Grindstone Pond south to Corner Brook Lake | 60×75 km | Whitaker and Montevecchi (1997, 1999) | 200 m transects (n=52) | not detected |
| 1998-2000 | Greater Gros Morne Ecosystem | 30×40 km | Taylor and Krawchuk (2005) | point counts (n=1,263) | not detected |
| 2003, 2004 | Main River Watershed | 17×11 km | Powell (2005) | point counts (n=120) | not detected |
| 2003-2006 | Main River Watershed | 17×11 km | Whitaker et al. (2008); P. Taylor, I. Warkentin and D. Whitaker (unpubl. data) | Passive mist netting using 25 x 12 m nets on 18 sites | not detected |
| 2006, 2007 | Main River Watershed | 200 km2 | P. Taylor, I. Warkentin and D. Whitaker (unpubl. data) | 1-min point counts followed by 3-min playback of Black-capped Chickadee mobbing calls | not detected |
| 2008, 2009 | Provincial protected areas across Nfld | NA | TDL, NL Dept of Environment and Conservation (unpubl. data) | 5-min point counts followed by 3-min playback of Black-capped Chickadee mobbing calls (n=150 points) | not detected |
| 2010 | Gros Morne National Park | Park lowlands | Rae et al. (2014) | 8-min point counts followed by 8-min playback of Black-capped Chickadee mobbing calls (n = 607) | not detected |
| 2012 | Boreal forest across Nfld | NA | CWS Boreal Landbird Monitoring | 12-min silent point counts | 11 individuals detected at 2 of 9000 locations |
| 2013, 2014 | Provincial protected areas across Nfld | NA | TDL, NL Dept of Environment and Conservation (unpubl. data) | 5-min point counts followed by 3-min playback of Black-capped Chickadee mobbing calls (n=110 points) | 2 individuals detected at West Brook Ecological Reserve in 2014 |
Breeding Bird Surveys (BBSs)
The BBS is a North America-wide, volunteer-based program that surveys breeding bird populations. Skilled observers conduct a single morning survey by driving a roadside route of 39.2 km during the breeding season (early June to early July), stopping for three minutes every 0.8 km (for a total of 50 stops and 150 min), and recording all birds (except dependent young) that are heard or seen within a 0.4-km radius at each of the 50 stops (Environment Canada 2014). The majority of detected birds are identified by song. The BBS has been conducted across 23 survey routes in Newfoundland since 1974, though not all routes have been surveyed annually (Figure 4; Table 2). Because survey routes are located along roadways only and not all routes are active each year, a large proportion of potential Red Crossbill habitat in Newfoundland has not been surveyed by BBSs. Of 292 BBSs conducted since 1974, Red Crossbills were detected on 28 occasions (13 of 23 routes during one or more years; Table 2). There are no BBS routes on Anticosti Island.
Winter Bird Surveys
As a result of recovery actions and research priorities highlighted in the Canada Recovery Strategy for Red Crossbill percna (Environment Canada 2006), winter landbird surveys targeted at detecting Red Crossbills in Newfoundland were initiated in 2007 and have been conducted each winter since then. Surveys are conducted during mid-February to end of March by federal and provincial government agencies (i.e., Parks Canada, Environment Canada, and NL Department of Environment and Conservation). During pilot years (2007-2009), surveys consisted of 4-km line transects of 9 points separated by 500 m each. Since 2010, surveys have been composed of five points arranged in a pentagonal shape; each point is separated by 500 m. Each survey covers an approximately circular area with a diameter of about 800 m. At each of the five points per survey, a standard 5-minute silent point count is conducted, followed first by a 3-minute broadcast playback of Red Crossbill vocalizations and then by a 3-minute playback of Black-capped Chickadee (Poecile atricapillus) predator mobbing calls. Broadcasts of bird calls are used because they increase detection rates; winter birds are otherwise typically silent, reclusive, and difficult to detect. All birds seen or heard during the 11-min survey are recorded. All surveys conducted since 2007 have been located in coniferous stands across Newfoundland; mature forests containing Black Spruce, Balsam Fir, White Spruce, Red Pine, and White Pine were sampled (Figures 3 and 4). A total of 1,112 points were surveyed between 2007 and 2014; a portion of those points were sampled across multiple years. Due to the relatively recent initiation of these surveys, field personnel limitations, and the remoteness of a large proportion of productive forest, the majority of potential Red Crossbill habitat in Newfoundland has not been surveyed by winter bird surveys. Red Crossbills have been detected in three areas only (Terra Nova National Park in eastern Newfoundland, West Brook Ecological Reserve in central Newfoundland, and near the community of Howley in western Newfoundland). The last two of these three areas have natural stands of Red Pine, while Terra Nova National Park does not (although natural and planted stands of Red Pine occur within approximately 10 km of survey locations in the Park).
Other Standardized Summer Bird Surveys
A number of contemporary studies on songbird demographics have occurred during the summer breeding season throughout the conifer forests of Newfoundland (with the majority occurring in the western region). Search effort and sampling methodology vary among the surveys; few Red Crossbills were detected (Table 3).
Incidental Observations
Incidental Red Crossbill sightings are reported by the local birdwatching community in Newfoundland on the online “nf.birds” discussion group, the eBird continental database for bird observations, to various personal online blogs, and to provincial and federal authorities. It is not possible to estimate search effort for these types of observations. Most birdwatchers posting to online forums are located on Newfoundland’s Avalon Peninsula, which contains a small proportion of the island’s conifer forests. The majority of Newfoundland is underrepresented on these online forums but contains a much higher proportion of potential habitat. Red Crossbills are regularly but infrequently reported by birdwatchers in Newfoundland.
Québec Breeding Bird Atlas
The Québec Breeding Bird Atlas is a volunteer-based survey of breeding bird populations throughout the breeding season in 10 km by 10 km survey squares. The objective of the Atlas is to collect breeding evidence for the largest number of species possible; 5-minute silent point counts are optional and are conducted only by observers who can identify birds by song. The Atlas surveys were conducted during the breeding seasons of 2010 to 2014; a total of 5,529 squares were surveyed and 36,056 point counts were conducted over approximately 97,934 hours. Anticosti Island was surveyed during the breeding seasons of 2011 and 2014, where a total of 76 squares were surveyed and 349 point counts were conducted over approximately 769 hours. Red Crossbills were detected in 175 survey squares, 36 (20%) of which were on Anticosti Island, and nearly half (36 of 76; 47%) of the squares surveyed on Anticosti Island had records of Red Crossbill (Atlas of the Breeding Birds of Québec 2015; Figure 5). Individuals confirmed as percna were identified on Anticosti Island only. A large proportion of potential Red Crossbill habitat was surveyed during the Atlas.
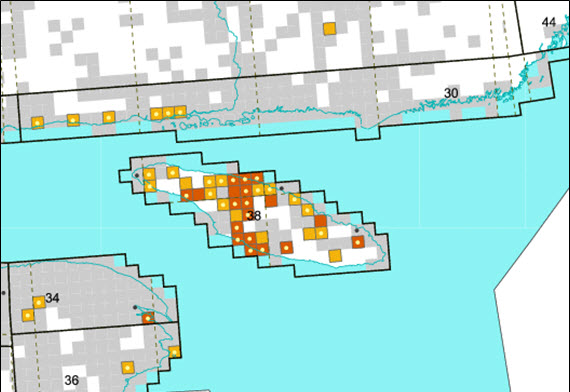
Long description for Figure 5
Map showing the locations of survey blocks surveyed during the Québec Breeding Bird Atlas in the Anticosti Island and Quebec North Shore region. Blocks are 10 by 10 kilometre squares. They are colour-coded to signify whether Red Crossbills were detected and, if so, whether they were possible breeders or probable breeders. Symbols within blocks indicate when Red Crossbills were detected in the second Atlas (2010 to 2014) but not the first (1984 to 1989) or were detected in the first Atlas but not the second.
Abundance
Estimating the number of percna individuals is difficult. An estimate of 500-1,500 individuals was suggested in the original 2004 status assessment based on field experience of the writers, CBCs, BBSs, and other surveys (COSEWIC 2004). Given recent bioacoustic studies and winter bird surveys targeting Red Crossbills in Newfoundland, along with another decade of CBCs, BBSs, and citizen science observations, and the 2nd Québec Breeding Bird Atlas, the NL Landbird Recovery Team has estimated the number of percna birds to be approximately 1,000-2,500 mature individuals (NL Landbird Recovery Team pers. comm. 2014). This estimate is larger than the original 2004 status assessment estimate of 500-1,500 individuals because of recent confirmation that percna occurs on and is probably breeding on Anticosti Island. Much uncertainty is associated with this population estimate because observer effort across the majority of apparently available habitat in Newfoundland is limited and the irruptive nature of the taxon makes accurate surveying difficult. The population estimate for birds in Newfoundland remains at 500-1,500 individuals due to lack of strong empirical evidence despite the apparent increase in the number of Red Crossbill reports by Newfoundland birdwatchers in recent years. It is unclear whether these increased reports are due to an actual population increase, change(s) in distribution, improved education and public awareness, increased use of feeders (by both people and the birds), enhanced communication between observers (e.g., social media), or a combination of all of these factors. The increase in observer reports is offset by a significant long-term decline in Breeding Bird Survey results, which has not abated over the past decade. Therefore, given conflicting evidence and lack of thorough census data, the population estimate for Newfoundland remains unchanged (see the Fluctuation and Trends section).
The conjectures by Parchman and Benkman (2002) that “the formerly abundant Newfoundland Crossbill is probably extinct” and by Benkman (1992) that “although Red Crossbills are still reported from Newfoundland, most of these are probably other, mainland subspecies of Red Crossbill, which apparently move on and off the island like White-wings” are not supported by recent bioacoustic and morphometric data which confirm the presence of percna/Type 8 adults and accompanying juveniles in insular Newfoundland (Young et al. 2012; Hynes and Miller 2014).
Fluctuations and Trends
Although no quantitative information exists, anecdotal evidence suggests that Red Crossbills were historically abundant in Newfoundland. In the mid-19th century, Reeks (1869) described them as “common” early nesters on the west coast of Newfoundland in the northern portion of what is now Gros Morne National Park.
Around the turn of the 20th century, many songbird specimens were collected in western, central, and eastern Newfoundland (Montevecchi and Tuck 1987). Interestingly, among the specimens collected in Newfoundland in the 1890s and early 1900s, Red Crossbills outnumbered White-winged Crossbills by more than a 2:1 ratio (i.e., 21:9; W.A. Montevecchi unpubl. data). If collectors did not favour Red Crossbills over White-winged Crossbills (although they may have, because they tended to target distinctive subspecies) and if Red Crossbills were not more vulnerable to collectors, then it is possible that Red Crossbills could have been more common in Newfoundland than White-winged Crossbills around the turn of the century.
Peters and Burleigh (1951) considered the Red Crossbill in Newfoundland to be fairly common locally in summer, common in the Codroy Valley (in southwestern Newfoundland) in September, and uncommon in winter, with an erratic and local distribution.
Red Crossbills were considered to be “a fairly regular resident” and “similar” in abundance to White-winged Crossbills in western Newfoundland during 1957-1963 by (Deichmann in Benkman 1993c). Erskine (1977) describes Red Crossbills as “common” in spruces in Newfoundland in 1968. In the late 1960s and 1970s, nests and fledglings of Red Crossbills were observed in the St. John’s area and elsewhere in eastern Newfoundland.
The 1975 Newfoundland bird checklist lists both Red and White-winged Crossbills as common (likely to be found daily in appropriate season/habitat) resident breeders (Tuck and Maunder 1975). That same year, however, Red Crossbills were considered to be “uncommon at best” and “occurring in smaller numbers and less often than White-winged Crossbills” in Gros Morne National Park in western Newfoundland (Lamberton 1976). By 1982 and again in 1989, the Newfoundland bird checklists considered both Red and White-winged Crossbills to be uncommon (likely to be found monthly in appropriate season/habitat; may be locally common) resident breeders (Maunder and Montevecchi 1982; Mactavish et al. 1989). The 1999 and 2003 checklists (the latter being the most recent checklist produced) classified White-winged Crossbills as common, irruptive resident breeders, while Red Crossbills were considered to be very uncommon (likely to be found annually in appropriate season/habitat; may be locally uncommon) resident breeders (Mactavish et al. 1999, 2003).
Red Crossbill nests have not been observed in Newfoundland since 1977 (W.A. Montevecchi unpubl. data) but juveniles have been observed in recent years (2009, 2011-2014) on the Avalon Peninsula and in central Newfoundland, and in some cases were accompanied by adults confirmed as Vocal Type 8 birds via audiospectrographic analyses (Hynes 2013).
Christmas Bird Counts (CBCs)
While the irruptive nature of Red Crossbills makes them unlikely to turn up on CBCs with annual regularity, these volunteer-based surveys provide the longest-running dataset on Red Crossbill occurrence in Newfoundland. Analyses of the numbers of Red Crossbills (vocal type and morphological form unknown) detected per party hour over the time period 1967-2013 have been conducted by the National Audubon Society. A Bayesian log-linear hierarchical model was used to control for variation in survey effort among CBC circles (n = 12) and the non-random distribution of circle locations (Link et al. 2006). Results suggest that detections of Red Crossbills on CBCs in Newfoundland declined by 11.2% per year (95% CI: -17.3%, -3.6%) over the interval 1966-2013. This annual trend can be extrapolated into an overall trend which indicates that Red Crossbills detected on CBCs declined by 99.6% over the interval 1966-2013 (C. Soykan pers. comm. 2015). Similar analyses were conducted for the most recent decade of data (2004-2013); model results indicate a point estimate of 0.0% change (95% CI: -5.1%, 9.0%), but with insufficient sample size to estimate trends precisely (C. Soykan pers. comm. 2015).
Results of the CBC data models presented above should be interpreted with caution. The data were collected by varying numbers of observers having a range of skills and detection capabilities in non-randomly located count circles (Dunn and Sauer 1997; Dunn et al. 2005). The CBC data provide a snapshot of early-winter bird occurrences in restricted geographic areas. Because Red Crossbills can exhibit irruptive behaviour, their abundance over an entire winter season may not be accurately sampled by CBC methodology (i.e., Red Crossbills could be counted in a large flock which does not represent their abundance over a larger geographic area, or crossbills could fail to turn up on a census when they are relatively abundant in nearby areas outside the count circle). Additionally, it is possible that some of the Red Crossbill records on Newfoundland CBCs pertain to subspecies other than percna, which can be nomadic in winter. With respect to Newfoundland in particular, only a small number of count circles exist, and relatively few Red Crossbills have been observed in those circles on CBCs. Thus the estimated 99.6% decline over the interval 1966-2013 likely does not accurately reflect the true situation in Newfoundland. However, it might be expected that large foraging flocks of conspicuous, irruptive species like crossbills would be detected more consistently than other rarer and less conspicuous species.
Breeding Bird Surveys (BBSs)
Analyses presented here were conducted by Environment Canada (2014) and are available on the North American BBS Canadian Trends website. Count data were assumed to have a Poisson distribution and hierarchical models were built with year, stratum, and observer-route combinations as predictor variables using Bayesian inference (Link and Sauer 2011). Data collected during the period 1980-2012 were analyzed, and only those routes on which Red Crossbill was recorded were included (n = 13 routes). Model results suggest that detections of Red Crossbills on BBSs in Newfoundland declined by 16.3% per year (95% C.I.: -26.4%, -7.0%) over the interval 1980-2012, with a 99.8% probability that the population has declined since 1980, and a 99.6% probability that the population decreased by more than 50% during 1980-2012 (Figure 6). However, overall reliability for this model was considered to be low (based on geographic coverage, model fit, and precision; Environment Canada 2014). Similar analyses for the last decade (2003-2012; n=12 routes) indicated an estimated annual declining population trend of 16.2% (95% C.I.: -38.0%, 13.3%), with a 92.6% probability that the population has declined since 2003, and 85.7% probability that the population has decreased by more than 50% during 2002-2012. Overall reliability for this model was also considered to be low (Environment Canada 2014). Low overall reliability of these models is partly attributable to the irregular appearance of Red Crossbills on BBS surveys, their irruptive nature, and their tendency to occur in areas that are very sparsely populated by people and may not be accessible to Breeding Bird Surveys (Link and Sauer 1998; National Audubon Society 2014). However, while the reliability of the estimates is low, gross trends suggested by the data indicate that detections of Red Crossbills on the BBS have declined over the past 30 years, including over the past decade. The Newfoundland BBS dataset is also useful as an annual measure of presence/absence: Red Crossbills were reported in eight consecutive years of BBSs (1979-1986) but since then have been reported only four times (Pardieck et al. 2014).
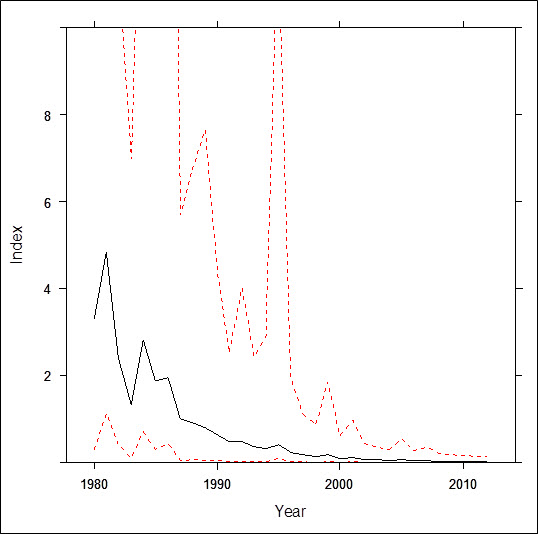
Long description for Figure 6
Chart illustrating trend in estimated average abundance (annual index) of Red Crossbills on Breeding Bird Survey routes in insular Newfoundland and Labrador from 1980 to 2012, with upper and lower 95 percent credible limits.
Trends Summary
In summary, both CBC and BBS data indicate long-term declines of Red Crossbill in Newfoundland, although confidence in results is somewhat limited. Short-term trends have particularly low reliability given the sparseness of Red Crossbill sightings data on CBCs and BBSs, but the BBS data suggest a continuing decline. Conversely, anecdotal reports of Red Crossbills in Newfoundland appear to have increased in recent years, but whether this increase is due to an increase in the Red Crossbill population, change(s) in distribution, improved education and public awareness, increased use of feeders (by both people and the birds), enhanced communication among observers (e.g., social media), or a combination of all of these factors is unknown. The amount of non-pine forest habitat in Newfoundland is not likely to decrease by a significant amount in the short- or long-term (the impending Spruce Budworm outbreak is expected to take more than a decade to move across Newfoundland, and overall tree mortality is expected to be low). Cone production in Black Spruce and Balsam Fir forests on the Avalon Peninsula is expected to increase in the next 20 years. In contrast, it is possible that the amount of pine forest in Newfoundland (particularly Red Pine) will decrease by 10%-20% in the short- and long-term; the effect of such a decrease on the percna subpopulation in Newfoundland is unknown, given that percna feeds on cones of other conifers which are abundant and ubiquitous in Newfoundland. The recent discovery of a subpopulation of percna on Anticosti Island (which is probably breeding there) is highly significant and will require future monitoring to discern its overall effect on percna population trends in Canada.
Rescue Effect
Rescue of the percna population is likely not possible, as the subspecies occurs only on Newfoundland and Anticosti Island. It is unknown whether the recently discovered population on Anticosti Island could rescue the Newfoundland population or vice versa.
Threats and Limiting Factors
A number of threats and limiting factors expected to affect percna have been identified (see also Appendix 3); these include (from highest to lowest apparent/predicted impact): invasive, non-native species and problematic native species; biological resource use; natural system modifications; transportation and service corridors; mining and quarrying; and agriculture.
Invasive, Non-native Species and Problematic Native Species
One hypothesis for the cause of the decline in Red Crossbill percna is related to the presence of non-native Red Squirrel, which was introduced to Newfoundland in 1963 (Payne 1976); Red Squirrels do not occur on Anticosti Island (M. Robert pers. comm. 2014). Red Squirrels are pervasive throughout Newfoundland and the threat they pose to percna is a continuous one of serious to moderate severity. Red Squirrels may be competitively excluding Red Crossbills by outcompeting them for food resources (Benkman 1989, 1993c; Pimm 1990). For example, in western North America, Red Crossbills are relatively uncommon in areas inhabited by Red Squirrels because crossbills are outcompeted for seeds in Lodgepole Pine (Pinus contorta) cones (Benkman et al. 2001). Red Crossbills also prefer to forage on older cones, as resinous bonds and cone scales weather and deteriorate so that seeds are more easily accessed from within the cones (Elliott 1988). In forests dominated by serotinous cones, crossbills rely on cones that have remained in the trees for a number of years (Benkman et al. 2001). Black Spruce and Red Pine seeds are held in semi-serotinous cones into the winter, when food resources in the boreal forest are scarce. All other native conifers in Newfoundland shed their seeds in the fall (B. English pers. comm. 2014). Because of their widespread distribution across Newfoundland, Black Spruce cones likely represent an important resource for percna for a large portion of the year. Red Squirrels are abundant in Black Spruce forests in Newfoundland and in some areas are four times more abundant in Black Spruce compared to Balsam Fir stands (Wren 2001). The impact of squirrels as seed predators in Newfoundland forests is most pronounced during years of relatively low seed availability. In years of relatively high numbers of cones, Red Squirrels have been documented to harvest less than 1% of cones from Black Spruce trees by October, while in small cone crop years Red Squirrels removed up to 96% of Black Spruce cones by October (West 1989). During years of low to medium cone abundance, Black Spruce cone selection by Red Squirrels at Terra Nova National Park throughout the fall/winter/spring harvest resulted in significantly smaller cones remaining on trees in June compared to those on trees in the previous August. These smaller cones had fewer filled seeds (i.e., seeds having tissues essential for germination) and thus may have been of lower nutritional value (Tulk 2004). Because larger Black Spruce cones contained larger and heavier seed in similar forests in New Brunswick (Caron and Powell 1989), it is possible that cone predation by Red Squirrels in Terra Nova (and throughout Newfoundland) may lead to reduced quality of food resources for Red Crossbills.
It has been suggested that Red Squirrel predation pressure on Black Spruce cones in Newfoundland over the past 50 years has led to thicker cone scales, smaller cone size, and lower seed mass to cone mass ratio. Some authors have postulated that these changes to cone morphology may be detrimental to percna, which co-evolved with conifers in Newfoundland in the absence of Red Squirrel (Benkman 1989, 1993c; Parchman and Benkman 2002). However, a recent replicate study on the influence of Red Squirrels on Black Spruce cone morphology in Newfoundland was unable to reproduce the results obtained by Parchman and Benkman (2002) and instead found a lack of evidence to support changing cone morphology in areas with squirrels versus small offshore islands without squirrels (R. Skinner and I. Warkentin unpubl. data). Because percna population declines were first noted at essentially the same time (late 1950-early 1960s) as the introduction of Red Squirrel, a nearly immediate detrimental effect of Red Squirrel on cone morphology and thus on percna survival is highly unlikely (W.A. Montevecchi pers. comm. 2014). There is disagreement concerning whether the introduction of Red Squirrel and potential changes to Black Spruce cone morphology is the mechanism for Red Crossbill percna population decline.
For Red Pine, 10%-90% of cones in all stands surveyed in September and October were removed by Red Squirrels; this is considered to be a major threat to the already-rare Red Pine population in Newfoundland (B.A. Roberts pers. comm. 2015). Similarly, suspected Red Squirrel cone predation in Newfoundland White Pine stands resulted in mean cone loss of 87% of near-mature and mature cones over a three-year period (range 32 to 100%); Red Squirrels therefore may be a significant impediment to natural regeneration of White Pine in Newfoundland (English 1998, 2000).
The extent to which Red Crossbills can escape competition from Red Squirrels by switching to other food resources is unknown, as are the ultimate population level impacts of competition with Red Squirrels. In Scotland, crossbills and squirrels show preferences for different shapes and density of pines; squirrels prefer more dense stands, possibly because they can avoid crossing ground to access other trees, whereas the crossbills more often used the older more open pine stands (Summers and Proctor 1999). These behavioural patterns likely reduce competition between squirrels and crossbills, as competitively inferior crossbills may avoid competitive exclusion during periods of low cone abundance by moving to other sites. It is unknown whether a similar relationship exists between Red Squirrels and percna in Newfoundland. There is no evidence to suggest that other species of birds dependent on conifer seed resources have undergone population declines (Environment Canada 2006).
Red Squirrels are also major predators of bird nests in Balsam Fir forests in Newfoundland (Lewis 1999). While it is expected that Red Squirrels are exerting considerable predation pressure on Red Crossbills, the impacts of Red Squirrel egg and nestling predation on percna (or on other Red Crossbill subspecies in North America) have not been quantified.
Several fungal species are also considered to be threats to percna in Newfoundland because of their impact on food resources. These fungi are a continuous and pervasive threat of serious to moderate severity throughout Newfoundland pine stands. The non-native European strain of Scleroderris canker causes stem dieback and tree mortality in both immature and mature Red Pines. The fungus has only been found in planted stands of Red Pine in Newfoundland, with some stands having 100% infection rates and up to 48% mortality of trees. The Department of Natural Resources has an active management program that aims to prevent the disease from spreading to natural and other planted stands (Lavigne 2014); there is a high probability that Red Pine stands currently infected with Scleroderris could decline by more than 10% in the next 10 years (B.A. Roberts pers. comm. 2015). The effect of a 10% decrease in Red Pine on the percna population in Newfoundland is unknown, given that percna feeds on cones of other conifers which are abundant and ubiquitous in Newfoundland. Other fungal species affecting pines in Newfoundland are Sirococcus Shoot Blight, which has caused significant damage to and mortality of Red Pines in at least one planted stand (Lavigne 2013, 2014), and Red Band Needle Blight, which was detected in 76% of planted and natural stands of Red Pine. Red Band Needle Blight causes tree mortality only after severe defoliation over consecutive years (Lavigne 2013).
Insect outbreaks pose a threat to percna throughout their range due to the potential for a significant reduction in food resources and, to a lesser extent, loss of habitat due to tree mortality (Lavigne 2014; A. Arsenault pers. comm. 2015). During the last Spruce Budworm outbreak in Newfoundland during the 1970s and 1980s, approximately 90% of forests were defoliated. Populations of this insect are cyclical, with outbreaks occurring every 30 to 40 years, and the next major outbreak is expected to occur in Newfoundland and Anticosti Island in the very near future. Early evidence of Spruce Budworm moth immigration into Newfoundland was detected in 2012 and 2013, and a large outbreak is currently underway along the north shore of the St. Lawrence and Gaspé regions of Québec (Natural Resources Canada 2015). An outbreak of Hemlock Looper is also expected to occur in Newfoundland in the near future, but will have less of an effect than Spruce Budworm. Populations of this insect re-emerged on the Northern Peninsula in 2014, and the NL Forestry and Agrifoods Agency predicts that approximately 210 km2 of forest will undergo moderate to severe defoliation in 2015. These outbreaks, however, are considered to be integral to and part of the natural system and not expected to be beyond the scope of natural disturbance in the next 10 years (A. Arsenault pers. comm. 2015; D. Lavigne pers. comm. 2015). The effects on percna of this supposed natural level of disturbance due to insect outbreaks are unknown,
Biological Resource Use
Loss of habitat and habitat fragmentation due to forest harvesting is a threat to percna, albeit to a lesser extent now and into the future than in previous years due to ongoing downturns in the forestry industry (Government of Newfoundland and Labrador 2014a). Notwithstanding these relatively lower levels of forestry activity, clearcut harvesting and selective logging result in temporary loss of habitat (i.e., lasting a few generations until trees produce seed-bearing cones), and if conducted in areas where Red Crossbills are nesting, can cause direct and immediate losses of nests and young. Forest harvesting also leads to fragmented patches of mature and late-seral forest. While some species of passerines in Newfoundland may be resilient to anthropogenic disturbances such as forest harvesting, which mimics natural disturbance regimes (e.g., Leonard et al. 2008; Whitaker et al. 2008), extreme specialists such as Red Crossbills may be more vulnerable to habitat loss because they tend to temporarily concentrate in regions of good cone crops (compared to generalist songbird species that are more evenly distributed in a habitat; Benkman 1993a; Holimon et al. 1998). Fragmented habitat has been shown to negatively impact crossbills in Finland because fragments may have lower cone production than contiguous stands (Helle 1985). A reduction of cone crops due to forest change, whether it is of possible “key conifers” such as pines or Black Spruce, or alternate conifer species like Balsam Fir and White Spruce, might negatively impact Red Crossbill breeding activity and lower recruitment to the population. While cone crops can be cyclical and irruptive finches such as Red Crossbills tend to move in response to those cycles, the age structure of forests in Newfoundland is such that seed-bearing cones from multiple species of conifers are produced at relatively high densities at any given time (B. Hearn pers. comm. 2014). There have not been population declines in other species of birds dependent on conifer seed resources in Newfoundland (Environment Canada 2006). Thus, the relative loss and fragmentation of cone-bearing habitat alone likely does not explain the apparent decline in Newfoundland Red Crossbills. Forest harvesting in the next 10 years is expected to occur over a limited geographical range in Newfoundland and the severity of the threat is uncertain (i.e., slight to serious).
Natural System Modifications
Human-caused forest fires and forest fire suppression both pose a continuous and medium to low impact threat to percna, and over a restricted geographic range. Forest fires lead to direct habitat loss (especially as in some areas the landscape may be slow to return to forest), and contribute to the decline of Red Pine habitat and cone crops because boreal forest fires tend to be hotter and more intense than Red Pines can withstand (Roberts 1985; Bergeron 1990; Roberts 2011). Paradoxically, forest fire suppression can also lead to loss of habitat and food for percna because Black Spruce and Red Pines are fire-dependent species (i.e., their cones open in the presence of fire, allowing seeds to disperse and the trees to reproduce and spread; Fowells 1965; Bergeron 1990). Forests in central Newfoundland are more fire-prone than those in other areas of Newfoundland.
Transportation and Service Corridors
Roadways pose a continuous and medium to low impact threat to percna, and over a large to restricted geographic range. Crossbills may be killed by vehicle strikes when flying over roadways, or when perched on the roadway when ingesting grit and salt on the road surface (e.g., a male Red Crossbill was killed in a vehicle collision in Terra Nova National Park in 1997). A large proportion of birds is expected to be exposed to a roadway in their lifetime, but direct mortality due to vehicle strikes likely affects a small proportion of the total population.
Mining and Quarrying
Mining and quarrying pose a continuous and low impact threat to percna throughout Newfoundland. The industry in Newfoundland is currently declining and this trend is expected to continue in the near future. Active and new projects may cause loss of habitat at the sites. Quarries are typically smaller than 1 km2 and the majority of quarry activity is generally situated along major transportation routes and/or near population centres (i.e., previously disturbed areas). Mine sites tend to be larger in size, but very few, if any, new mining projects are expected in Newfoundland in the next 10 years or the foreseeable long-term future. Mineral exploration activity in Newfoundland continues to decline in response to low commodity prices, lack of investment, and global financial crises (J. Hinchey pers. comm. 2015). The overall impact of mining and quarrying is likely to be low due to the large amount of remaining apparently available habitat.
Agriculture
Agriculture in Newfoundland poses a continuous but low impact threat to percna and over a limited geographic area. Only a small percentage of Newfoundland’s land base is suited to large-scale commercial agricultural activities. Between 2000 and 2010, the area of land in production in Newfoundland increased by 10% due to expansion of existing farms (Government of Newfoundland and Labrador 2010). However, not all of the land being converted for agricultural purposes results in loss of habitat for percna; a portion of the land is bog and peatland which is converted into cranberry farms. No more than 10% of land is expected to be converted for agriculture in the next decade or in the foreseeable future thereafter (R. Carey pers. comm. 2015).
Minor and negligible threats in the next 10 years include residential and commercial development, human intrusions and disturbance, pollution, geological events, and climate change.
The potential for growth of the Red Crossbill population under current conditions is likely compromised by low numbers and consequent reduced population viability (i.e., the Allee effect, whereby a decline in individual fitness occurs at low population size or density; Courchamp et al. 2008; Environment Canada 2012). Population growth potential is also contingent on years of good cone crops within boreal forests where percna is known to occur. Successive years of heavy cone crops cannot guarantee population growth, however, unless breeding pairs are naturally situated to take advantage of conifer seed abundance. There is the potential for population growth to be larger under managed conditions. In Britain, afforestation through the creation of plantations has greatly increased the diversity and abundance of conifer seeds (Marquiss and Rae 1994). This has contributed to an independent breeding population of Common Crossbills in Britain, where the population used to depend on immigration from the European mainland (Newton 1972; Avery and Leslie 1990). To be beneficial to Red Crossbills, habitat management must focus on provision of high cone yields from old-growth or at least mature conifers (Newton 1972; Benkman 1993b; Holimon et al. 1998). Enhanced pine regeneration and production (particularly of Red Pine which may be at risk in Newfoundland) has the potential to increase population growth among Red Crossbills in Newfoundland. Large protected expanses of old growth forest which include pines (e.g., the proposed Little Grand Lake Ecological Reserve) could also be beneficial to Newfoundland crossbills over the long term.
Number of Locations
All threats outlined above, aside from interactions with Red Squirrel, have the potential to impact Red Crossbill percna in both Newfoundland and Anticosti Island. Potential Red Squirrel impacts on Red Crossbill percna are confined to the Newfoundland population only, as Red Squirrels have not yet become established on Anticosti Island; similarly there is no agriculture there. Overall, the number of locations is unknown, but very likely >10.
Protection, Status and Ranks
Legal Protection and Status
Red Crossbill percna is listed as Endangered on Schedule 1 of the Canadian Species at Risk Act; it occurs within Terra Nova National Park and most likely Gros Morne National Park and is protected by the Canada National Parks Act in those localities. It is also listed as Endangered under the Newfoundland and Labrador Endangered Species Act and occurs within West Brook Ecological Reserve where it is protected under the NL Wilderness and Ecological Reserves Act. It is not listed under the QC Act Respecting Threatened or Vulnerable Species; it likely occurs within Parc national d’Anticosti, which is a protected area under the QC Parks Act. Red Crossbills across Canada are protected under the federal Migratory Birds Convention Act.
The Recovery Strategy for the Red Crossbill, percna subspecies (Loxia curvirostra percna) in Canada was completed in 2006 (Environment Canada 2006). Some of the recovery actions identified in the strategy have been completed, including confirmation that percna and other subspecies of Red Crossbill are present in Newfoundland and that percna comprises approximately 95% of individuals detected, survey protocols for long-term monitoring have been developed, and bird surveys targeted at detecting Red Crossbills have been conducted annually since 2007. The Action Plan for the Red Crossbill, percna subspecies (Loxia curvirostra percna), in Canada was produced in 2012 (Environment Canada 2012).
Non-Legal Status and Ranks
NatureServe ranks Red Crossbill percna as nationally imperilled (N2) but has not ranked it provincially, although it is recommended for ranking as imperilled (S2) in Québec (J. Tardif pers. comm. 2016); Red Crossbill in general has been ranked as S2S3 for Newfoundland and S4 for Québec (NatureServe 2015). Red Crossbill percna is considered At Risk in the General Status of Wild Species (CESCC 2011).
Habitat Protection and Ownership
Critical Habitat for Red Crossbill percna has not been identified. Approximately 95% of the landbase in Newfoundland and 99% of productive forest in Newfoundland is provincially and federally owned Crown land. One private pulp and paper company holds long-term timber rights to approximately 31% of productive forest; these rights expire in 2037 (Government of Newfoundland and Labrador 2014a; F. Knott pers. comm. 2014). Approximately 7.7% (9,196 km2) of the landbase of insular Newfoundland is protected (as Wilderness, Wildlife, and Ecological Reserves, and Provincial and National Parks and Historic Sites; Government of Newfoundland and Labrador 2014c), a portion of which is expected to provide habitat for Red Crossbills. Protected areas where Red Crossbills are known to occur include West Brook Ecological Reserve (11 km2; the largest natural Red Pine stand in Newfoundland) and Terra Nova National Park (400 km2). Red Crossbills are also regularly but infrequently recorded at Grant’s Pit in eastern Newfoundland, which is protected from development as a Crown Lands reserve (P. Hearns pers. comm. 2015). Historical and recent anecdotal records exist for Gros Morne National Park (1,805 km2), and potentially important habitat for Red Crossbills is protected in Little Grand Lake Provisional Ecological Reserve (729 km2; which is currently under consideration by the Government of Newfoundland and Labrador for permanent status). Red Crossbill adults and juveniles infrequently but regularly attend bird feeders on private lands in eastern, central, and western Newfoundland.
All of Anticosti Island is considered protected area either as a “white-tailed deer yard” (93% of the island) under the Act respecting the conservation and development of wildlife or within Québec’s Parc national d’Anticosti (572 km2; 7% of the island; Sépaq 2014), the majority of which likely provides habitat for percna. Although protected, timber harvest was allowed outside the national park to a maximum of 190,000 m3 between 2009 and 2013 (Gouvernement du Québec 2009) but no timber activity took place in 2013 (Université Laval 2016).
Acknowledgements and Authorities Contacted
The report writer thanks W.A. Montevecchi and S. Wren for authoring the original status assessment and providing insightful discussions about Red Crossbill in Newfoundland. Much gratitude is extended by the report writer to D.M. Whitaker for statistical advice, general support, and the cover photo, D. Hynes for interpreting sound files and fielding questions, M. Young for analyzing sound files, and M. Robert for his efforts in confirming percna on Anticosti Island and sharing his insights into percna in Québec. W. Clarke, B. English, B. Hearn, D. Lavigne, and B.A. Roberts provided valuable discussions about forestry and forest ecology in Newfoundland. Thanks are extended from the report writer also to C. Soykan who analyzed CBC data for percna in Newfoundland, and to the Regroupement Québec Oiseaux, Environment Canada’s Canadian Wildlife Service, and Bird Studies Canada for supplying data from the Atlas of the breeding birds of Québec. Special recognition is offered to the many hundreds of volunteers and coordinators who conducted Christmas Bird Counts, Breeding Bird Surveys, Québec Breeding Bird Atlas surveys, winter bird surveys, and collected recorded Red Crossbill vocalizations, and to all birdwatchers in Newfoundland who reported their sightings. Without all these volunteers, our understanding of Red Crossbill abundance and distribution would be sorely lacking. The NL Landbird Recovery Team provided helpful advice, and Environment Canada provided funding for this report.
Authorities Contacted
Baker, K. Biologist and Co-chair of the NL Landbirds Recovery Team. Canadian Wildlife Service, Environment Canada. Mount Pearl, NL.
Blaney, S. Executive Director and Senior Scientist. Atlantic Canada Conservation Data Centre. Sackville, NB.
Clarke, J. eBird.org Editor, Newfoundland and Labrador. St. John’s, NL.
Clarke. W.M. Senior Forest Ecologist. Forestry and Agrifoods Agency, NL Department of Natural Resources. St. John’s, NL.
Durochers, A. Assistant Data Manager. Atlantic Canada Conservation Data Centre. Corner Brook, NL.
Feltham, J. Ecologist. Terra Nova National Park, Parks Canada. Glovertown, NL.
Finnis, J. Professor, Department of Geography. Memorial University of Newfoundland. St. John’s, NL.
Hynes, D. Contract Biologist and Sound Engineer. Kentville, NS.
Jones, N. Aboriginal Traditional Knowledge Coordinator, COSEWIC Secretariat. Canadian Wildlife Service, Environment Canada. Gatineau, QC.
Mactavish, B. Wildlife Ecologist. LGL Ltd Environmental Research Associates. St. John’s, NL.
Newfoundland and Labrador Landbird Recovery Team. 2014 Meeting. Corner Brook, NL.
Rodrigues, B. Biologist and Co-chair of the NL Landbirds Recovery Team. Wildlife Division, NL Department of Environment and Conservation. Corner Brook, NL.
Warkentin, I. Professor, Environmental Science. Memorial University of Newfoundland. Corner Brook, NL.
Whitaker, D.M. Species Conservation Specialist, Atlantic Parks. Parks Canada. Rocky Harbour, NL.
Wu, J. Science Officer, COSEWIC Secretariat. Canadian Wildlife Service, Environment Canada. Gatineau, QC.
Young, M.A. Biologist and Sound Engineer. Macaulay Library, Cornell Lab of Ornithology. Ithaca, NY, USA.
Information Sources
Adams, B., pers. comm. 2015. E-mail correspondence to T. Leonard. Director, Centre for Forest Science and Innovation, NL Department of Natural Resources. Gander, NL.
Adkisson, C.S. 1996. Red Crossbill. In A. Poole and F. Gill (eds.). The Birds of North America, No. 256. American Ornithologists' Union, Washington, D.C.
Arsenault, A., pers. comm. 2015. E-mail correspondence to T. Leonard. Forest Ecologist. Atlantic Forestry Centre, Canadian Forest Service, Natural Resources Canada. Corner Brook, NL.
Atlas of the breeding birds of Québec. 2015. Data consulted on and obtained from the website of the Atlas of the breeding birds of Québec. Regroupement Québec Oiseaux, Environment Canada’s Canadian Wildlife Service and Bird Studies Canada. Québec, Québec, Canada. Web site: [accessed December 2015].
Avery, M., and R. Leslie. 1990. Birds and Forestry. Poyser, London, UK. 312 pp.
Bassan, A., pers. comm. 2015. E-mail correspondence to T. Leonard. Road Network Administrator. NL Department of Finance/NL Statistics Agency. St. John’s, NL.
Bayne, E.M., and K.A. Hobson. 2002. Apparent survival of male Ovenbirds in fragmented and forested boreal landscapes. Ecology 83:1307-1316.
Beaupré, P., pers. comm. 2015. E-mail correspondence to T. Leonard. Chargé de Projet des Guides Sylvicoles. Direction de l’Aaménagement et de l’Environnement Forestiers. Ministère des Forêts, de la Faune et des Parcs. Québec, QC.
Benkman, C.W. 1989. On the evolution and ecology of island populations of crossbills. Evolution 43:1324-1330.
Benkman, C.W. 1992. A crossbill's twist of fate. Natural History 101:38-42.
Benkman, C. W. 1993a. Adaptation to single resources and the evolution of crossbill (Loxia) diversity. Ecological Monographs 63:305-325.
Benkman, C.W. 1993b. Logging, conifers, and the conservation of crossbills. Conservation Biology 7:473-479.
Benkman, C.W. 1993c. The evolution, ecology, and decline of the Red Crossbill of Newfoundland. American Birds 47:225-229.
Benkman, C.W. 2010. Diversifying coevolution between crossbills and conifers. Evolution: Education and Outreach 3:47-53.
Benkman, C.W., W.C. Holimon, and J.W. Smith. 2001. The influence of a competitor on the geographic mosaic of coevolution between crossbills and Lodgepole Pine. Evolution 55:282-294.
Benkman, C.W., J.S. Colquitt, W.R. Gould, T. Fetz, P.C. Keenan, and L. Santisteban. 2005. Can selection by an ectoparasite drive a population of red crossbills from its adaptive peak? Evolution 59:2025-2032.
Benkman, C.W., J.W. Smith, P.C. Keenan, T.L. Parchman, and L. Santisteban. 2009. A new species of the Red Crossbill (Fringillidae: Loxia) from Idaho. Condor 111:169-176.
Bent, A.C. 1912. A new subspecies of crossbill from Newfoundland. Smithsonian Miscellaneous Collections 60:1-3.
Bergeron, Y., and J. Brisson. 1990. Fire regime in Red Pine stands at the northern limit of the species’ range. Ecology 71:1352-1364.
Bérubé, J.A. 2003. Incidence of white pine blister rust in Newfoundland. Canadian Plant Disease. Survey 83:144-145.
Bird Studies Canada. 2013. Project NestWatch. Bird Studies Canada / Études d’Oiseaux Canada. Web site: [accessed January 2016].
BirdLife International. 2014. Species factsheet: Loxia curvirostra. Web site: [accessed October 2014].
Bock, C.E. and L.W. Lepthien. 1976. Synchronous eruptions of boreal seed-eating birds. American Naturalist 110:559-571.
Boulet, M., M. Darveau, and L. Bélanger. 2000. A landscape perspective of bird nest predation in a managed boreal black spruce forest. Écoscience 7:281-289.
Burleigh, T.D. and H.S. Peters. 1948. Geographic variation in Newfoundland birds. Proceedings of the Biological Society of Washington 61:111-126.
Canadian Endangered Species Conservation Council (CESCC). 2011. Wild Species 2010: The General Status of Species in Canada.
Carey, R., pers. comm. 2015. E-mail correspondence to T. Leonard. Director, Land Resource Stewardship Division, NL Forestry and Agrifoods Agency. Corner Brook, NL.
Caron, G.E., and G.R. Powell. 1989. Cone size and seed yield in young Picea mariana trees. Canadian Journal of Forest Research 19:351-358.
Clements, J.F., T.S. Schulenberg, M.J. Iliff, D. Roberson, T.A. Fredericks, B.L. Sullivan, and C.L. Wood. 2014. The eBird/Clements checklist of birds of the world: Version 6.9. Web site: [accessed July 2014].
COSEWIC. 2004. COSEWIC assessment and status report on the Red Crossbill percna subspecies Loxia curvirostra percna in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa, ON. vii + 46 pp.
Côté, S.D., C. Dussault, J. Huot, F. Potvin, J.-P. Tremblay, and V. Viera. 2008. High herbivore density and boreal forest ecology: white-tailed deer on Anticosti Island. Pp. 154-161. in A.J. Gaston, T.E. Golumbia, J.L. Martin, and S.T. Sharpe (eds.). Lessons from the Islands: introduced species and what they tell us about how ecosystems work. Proceedings from the Research Group on Introduced Species 2002 Symposium, Queen Charlotte City, Queen Charlotte Islands, British Columbia. Canadian Wildlife Service, Environment Canada, Ottawa, ON.
Courchamp, F., L. Berec, and J. Gascoigne. 2008. Allee Effects in Ecology and Conservation. Oxford University Press, Oxford, UK. x + 256 pp.
Damman, A.W.H. 1983. An ecological subdivision of the island of Newfoundland. Pp. 163-206 in G.R. South (ed.). Biogeography and Ecology of the Island of Newfoundland. Dr W. Junk Publishers, The Hague, Netherlands.
DeBenedictis, P.A. 1995. Red Crossbills, one through eight. Birding 27:494-501.
Dickerman, R.W. 1986. A review of the Red Crossbill in New York State - Part 2. Identification of specimens from New York. Kingbird 36:127-134.
Dickerman, R.W. 1987. The "Old Northeastern" subspecies of Red Crossbill. American Birds 41:189-194.
Drolet, B., pers. comm. 2015. E-mail correspondence to T. Leonard. Senior Landbird Biologist, Canadian Wildlife Service, Environment and Climate Change Canada. Québec, QC.
Dunn, E., C.M. Francis, P.J. Blancher, S.R. Drennan, M.A. Howe, D. Lepage, C.S. Robbins, K.V. Rosenberg, J.R. Sauer, and K.G. Smith. 2005. Enhancing the scientific value of the Christmas Bird Count. Auk 122:338-346.
Dunn, E.H., and J.R. Sauer. 1997. Monitoring Canadian bird populations with winter counts. Pp. 49-55. in E. H.
Dunn, M. D. Cadman, and J. B. Falls (eds.). Monitoring Bird Populations: The Canadian Experience. Canadian Wildlife Service Occasional Paper Number 95. Environment Canada, Ottawa, ON.
Edelaar, P. 2008. Assortative mating also indicates that common crossbill Loxia curvirostra vocal types are species. Journal of Avian Biology 39:9-12.
Edelaar, P., E. Postma, P. Knops , and R. Phillips. 2005. No support for a genetic basis of mandible crossing direction in crossbills (Loxia spp.). Auk 122:1123-1139.
Elliott, P.F. 1988. The influence of population density and body size on the behavioral ecology of the pine squirrel (Tamiasciurus hudsonicus). Natural History Paper no. 1. Mountain Research Center, University of Colorado, Boulder.
English, B. 1998. Impact of squirrel predation on White Pine cone collection efforts in Newfoundland. Newfoundland Forest Service Silviculture Notebook 42:1-4.
English, B. 2000. Squirrel predation of White Pine cones: second year update. Newfoundland Forest Service Silviculture Notebook 51:1-4.
English, B., pers. comm. 2014. E-mail correspondence to T. Leonard. Supervisor of Silviculture and Research. Forestry and Agrifoods Agency, NL Department of Natural Resources. Corner Brook, NL.
Environment Canada. 2006. Recovery Strategy for the Red Crossbill, percna subspecies (Loxia curvirostra percna), in Canada. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa, ON. vii + 29 pp.
Environment Canada. 2012. Action Plan for the Red Crossbill, percna subspecies (Loxia curvirostra percna) in Canada. Species at Risk Act Action Plan Series. Environment Canada, Ottawa, ON. iii + 21 pp.
Environment Canada. 2014. North American Breeding Bird Survey - Canadian Trends Website, Data-version 2012. Environment Canada, Gatineau, QC. Web site: [accessed October 2014].
Erskine, A.J. 1977. Birds in boreal Canada: communities, densities and adaptations. Canadian Wildlife Service Report Series Number 41. Environment Canada, Ottawa, ON.
Erskine, A.J. 1992. Atlas of Breeding Birds of the Maritime Provinces. Nimbus Publishing Ltd. and the Nova Scotia Museum, Halifax, NS. 280 pp.
Etcheberry, R. , pers. comm. 2014. E-mail correspondence to T. Leonard. Local naturalist and Christmas Bird Count Compiler. Miquelon, St. Pierre et Miquelon, France.
Fowells, H.A. 1965. Red pine (Pinus resinosa Ait.). Pp. 432-446 in Silvics of Forest Trees of the United States, Agriculture Handbook No. 271. U.S. Dept. of Agriculture, Washington DC.
Godfrey, W.E. 1966. The Birds of Canada. Bulletin of the National Museum of Canada, No. 203, Biological Series No. 73. National Museum of Canada, Ottawa, ON. 428 pp.
Godfrey, W.E. 1986. The Birds of Canada. Revised Edition. National Museum of Natural Sciences (Canada), Ottawa, ON. 585 pp.
Gosselin, M., pers. comm. 2014. E-mail correspondence to T. Leonard. Collection Manager, Zoology. Canadian Museum of Nature. Ottawa, ON.
Gouvernement du Québec. 2004a. Portrait forestier de la region de la Côte-Nord. Document d’information sur la gestion de la forêt publique prepare par le ministère des Ressources naturelles, de la Faune et des Parcs, direction générale de la Côte-Nord. Québec, QC.
Gouvernement du Québec. 2004b. Portrait forestier de la region de la Gaspésie-Îles-de-la-Madeleine. Document d’information sur la gestion de la forêt publique prepare par le ministère des Ressources naturelles, de la Faune et des Parcs, direction générale de la Gaspésie-Îles-de-la-Madeleine. Québec, QC.
Gouvernement du Québec. 2009. Résultats des calculs des possibilités applicables aux reserves forestières, division du Nord-Est. Bureau du forestier en chef. Québec, QC.
Government of Newfoundland and Labrador. 2003. Provincial Sustainable Forest Management Strategy, 2003. NL Department of Forest Resources and Agrifoods, St. John’s, NL. 77 pp.
Government of Newfoundland and Labrador. 2010. Our Farms, Our Food, Our Future: Agriculture and Agrifoods Strategy. NL Department of Natural Resources. St. John’s, NL. 44 pp.
Government of Newfoundland and Labrador. 2014a. Provincial Sustainable Forest Management Strategy, 2014-2024. Centre for Forest Science and Innovation, NL Department of Natural Resources, Corner Brook, NL. 65 pp.
Government of Newfoundland and Labrador. 2014b. Timber Resource Analysis. NL Department of Natural Resources.
Government of Newfoundland and Labrador. 2014c. Protected Areas in Newfoundland and Labrador. NL Department of Environment and Conservation. Web site: [accessed September 2014].
Griscom, L. 1937. A monographic study of the Red Crossbill. Proceedings of the Boston Society of Natural History 41:77-210.
Griscom, L., and D.E. Snyder. 1955. The Birds of Massachusetts. Peabody Museum, Salem, MA. 295 pp.
Groth, J.G. 1988. Resolution of cryptic species in Appalachian Red Crossbills. Condor 90:745-760.
Groth, J.G. 1993a. Evolutionary differentiation in morphology, vocalizations, and allozymes among nomadic sibling species in the North American Red Crossbill (Loxia curvirostra) complex. University of California Publications in Zoology 127:1-143.
Groth, J.G. 1993b. Call matching and positive assortative mating in Red Crossbills. Auk 110:398-401.
Hahn, T.P. 1995. Integration of photoperiodic and food cues to time changes in reproductive physiology by an opportunistic breeder, the Red Crossbill, Loxia curvirostra(Aves: Carduelinae). Journal of Experimental Zoology 272:213-226.
Hearn, B., pers. comm. 2014. E-mail correspondence to T. Leonard. Science Director. Atlantic Forestry Centre, Canadian Forest Service, Natural Resources Canada. Corner Brook, NL.
Hearns, P., pers. comm. 2015. E-mail correspondence to T. Leonard. Manager of Resource Evaluation and Policy Integration, Land Management Division, NL Department of Municipal and Intergovernmental Affairs. St. John’s, NL.
Helle, P. 1985. Effects of forest fragmentation on bird densities in northern boreal forests. Ornis Fennica 62:35-41.
Hinchey, J., pers. comm. 2015. E-mail correspondence to T. Leonard. Director, Mines - Mineral Lands Division, NL Department of Natural Resources. St. John’s, NL.
Holimon, W.C., C.W. Benkman, and M.F. Willson. 1998. The importance of mature conifers to red crossbills in southeast Alaska. Forest Ecology and Management 102:167-172.
Hynes, D.P. 2013. A bioacoustic analysis of Red Crossbill (Loxia curvirostra) vocalizations from the island of Newfoundland, Canada. M.Sc. thesis, Memorial University, St. John’s, NL. 131 pp.
Hynes, D.P., and E.H. Miller. 2014. Vocal distinctiveness of the Red Crossbill (Loxia curvirostra) on the island of Newfoundland, Canada. Auk 131:421-433.
Irwin, K. 2010. A new and cryptic call type of the Red Crossbill. Western Birds 41:10-25.
James, P.C., T.W. Barry, A.R. Smith, and S.J. Barry. 1987. Bill crossover ratios in Canadian crossbills Loxia spp. Ornis Scandinavica 18:310-312.
Knott, F., pers. comm. 2014. E-mail correspondence to T. Leonard. Environmental Management Representative. Corner Brook Pulp and Paper Ltd. Corner Brook, NL.
Knox, A.G. 1992. Species and pseudospecies: the structure of crossbill populations. Biological Journal of the Linnean Society 47:325-335.
Koenig, W.D., and J.M.H. Knops. 2001. Seed-crop size and eruptions of North America boreal seed-eating birds. Journal of Animal Ecology 70:609-620.
Lack, D. 1944. Correlation between beak and food in the crossbill, Loxia curvirostra Linnaeus. Ibis 86:552-553.
Lamberton, R.D. 1976. Avifaunal Survey of Gros Morne National Park. Parks Canada Contract Report. Report 74-46. Parks Canada, Ottawa, ON. 163 pp.
Larivée, J., pers. comm. 2015. E-mail correspondence to T. Leonard. Database Manager, Étude des populations d'oiseaux du Québec (ÉPOQ). Québec.
Lavigne, D. 2013. Forest insect and disease control and monitoring activities in Newfoundland and Labrador in 2012 and outlook for 2013. NL Department of Natural Resources, Corner Brook, NL. viii + 57 pp.
Lavigne, D. 2014. Forest insect and disease control and monitoring activities in Newfoundland and Labrador in 2013 and outlook for 2014. NL Department of Natural Resources, Corner Brook, NL. ix + 56 pp.
Lavigne, D., pers. comm. 2015. E-mail correspondence to T. Leonard. Supervisor of Insect and Disease Control. Forestry and Agrifoods Agency, NL Department of Natural Resources. Corner Brook, NL.
Leonard T.D., P.D. Taylor, and I.G. Warkentin. 2008. Space use by songbirds in naturally patchy and harvested boreal forests. Condor 110:467-481.
Lepage, D. 2014. Avibase – Bird Checklists of the World: Anticosti. Web site: [accessed October 2014].
Lewis, K.P. 1999. Nest Predation in Riparian Buffer Strips in a Balsam Fir Forest in Western Newfoundland. M.Sc. Thesis, Memorial University of Newfoundland, St. John's, NL. 79 pp.
Linegar, P., pers. comm. 2015. Local naturalist. St. John’s, NL.
Linfield, J., pers. comm. 2015. E-mail correspondence to T. Leonard. Environmental Co-ordinator, Environmental Services. NL Hydro. St. John’s, NL.
Link, W.A., and J.R. Sauer. 1998. Estimating population change from count data: application to the North American Breeding Bird Survey. Ecological Applications 8: 258-268.
Link, W.A. and J.R. Sauer. 2011. Analysis of the North American Breeding Bird Survey using hierarchical models. Auk 128: 87-98.
Link, W. A., J.R. Sauer, and D.K. Niven. 2006. A hierarchical model for regional analysis of population change using Christmas Bird Count data, with application to the American Black Duck. Condor 108:13-24.
Lutmerding, J. A., and A. S. Love. 2014. Longevity Records of North American Birds. Version 2014.1. USGS Patuxent Wildlife Research Center. Bird Banding Laboratory. Web site: [accessed July 2014].
Mactavish, B., J.E. Maunder, and W.A. Montevecchi. 1989. Field checklist of the birds of insular Newfoundland and its continental shelf waters. Osprey 20:139-144.
Mactavish, B., J.E. Maunder, and W.A. Montevecchi. 1999. Field checklist of the birds of insular Newfoundland and its continental shelf waters. Osprey 30:22-27.
Mactavish, B., J.E. Maunder, W.A. Montevecchi, J.L. Wells and D. Fifield. 2003. Checklist of the birds of insular Newfoundland and its continental shelf waters. Natural History Society of Newfoundland and Labrador, St. John’s, NL.
Marquiss, M., and R. Rae. 1994. Seasonal trends in abundance, diet and breeding of Common Crossbills (Loxia curvirostra) in an area of mixed species conifer plantation following the 1990 crossbill 'irruption'. Forestry 67:31-47.
Marquiss, M., and R. Rae. 2002. Ecological differentiation in relation to bill size amongst sympatric, genetically undifferentiated crossbills Loxia spp. Ibis 144:494-508.
Maunder, J.E., and W.A. Montevecchi. 1982. Field checklist of the birds of insular Newfoundland. Osprey 13:59-62.
McCabe, T.T. and E.B. McCabe. 1933. Notes on the anatomy and breeding habits of crossbills. Condor 35:136-147.
Montevecchi, W.A., pers. comm. 2014. E-mail correspondence to T. Leonard. Professor, Biopsychology and Ocean Sciences. Memorial University of Newfoundland. St. John’s, NL.
Montevecchi, W.A., and L.M. Tuck. 1987. Newfoundland Birds: Exploitation, Study, Conservation. Publications of the Nuttall Ornithological Club, No. 21. Nuttall Ornithological Club, Cambridge, MA. 273 pp.
Montevecchi, W.A., and J. Wells. 1987. Vernacular Bird Names of Newfoundland. Pp. 232-245 in W. A.
Montevecchi and L.M. Tuck. Newfoundland Birds: Exploitation, Study, Conservation. Nuttall Ornithological Club, Cambridge, MA.
Mosseler, A., B.A. Roberts, and P. Tricco. 1992. The effect of fir coneworm, Dioryctria abientivorella (Grote) (Lepidoptera: Pyralidae) on seed production in small, isolated populations of Red Pine. Forest Ecology and Management 53:15-27.
National Audubon Society. 2014. The Christmas Bird Count Historical Results [Online]. Web site: [accessed April 2014].
Natural Resources Canada. 2015. Top Forest Insects and Diseases in Canada. Government of Canada. Site Web : [consulté en mai 2015]. [Également disponible en français : Ressources naturelles Canada. 2015. Principaux insectes et maladies des forêts au Canada. Gouvernement du Canada.]
NatureServe 2014. NatureServeExplorer: An online encyclopedia of life [application Web]. Version 7.1. NatureServe, Arlington, VA. Site Web : [consulté en octobre 2014].
Newton, I. 1972. Finches. Harper Collins, London, UK. 227 pp.
Ouellet, H. 1969. Les oiseaux de l'île Anticosti, province de Québec, Canada. National Museums of Canada (Natural Sciences) Publications in Zoology, Number 1. National Museums of Canada, Ottawa, ON. 79 pp.
Page, G., W.C. Wilton, and T. Thomas. 1974. Forestry in Newfoundland. Newfoundland Forest Research Centre, St. John's, NL, 117 pp.
Parchman, T.L., and C.W. Benkman. 2002. Diversifying coevolution between crossbills and Black Spruce on Newfoundland. Evolution 56:1663-1672.
Parchman, T.L., C.W. Benkman, and S.C. Britch. 2006. Patterns of genetic variation in the adaptive radiation of New World crossbills (Aves: Loxia). Molecular Ecology 15:1873-1887.
Pardieck, K.L., D.J. Ziolkowski Jr., M.-A.R. Hudson. 2014. North American Breeding Bird Survey Dataset 1966 - 2013, version 2013.0. U.S. Geological Survey, Patuxent Wildlife Research Center. Web site: [accessed October 2014].
Pardy, S., pers. comm. 2015. E-mail correspondence to T. Leonard. Senior Manager, Biodiversity and Endangered Species Program, Wildlife Division, NL Department of Environment and Conservation. Corner Brook, NL.
Payne, N.F. 1976. Red Squirrel introduction to Newfoundland. Canadian Field Naturalist 90:60-64.
Payne, R.B. 1987. Populations and type specimens of a nomadic bird: comments on the North American crossbills Loxia pusilla Gloger 1834 and Crucirostra minor Brehm 1845. Occasional Papers of the Museum of Zoology University of Michigan 714:1-37.
Payne, S., pers. comm. 2015. Supervisor of Data Acquisition. Forestry and Agrifoods Agency, NL Department of Natural Resources. Corner Brook, NL.
Peters, H.S., and T.D. Burleigh. 1951. The Birds of Newfoundland. Newfoundland Department of Natural Resources, St. John’s, NL. 431 pp.
Phillips, A.R. 1981. The races of the Red Crossbill, Loxia curvirostra in Arizona. Pp 223-230 in G. Monson and A.R.
Phillips (ed.). Annotated Checklist of the Birds of Arizona, Second Edition. University of Arizona Press, AZ.
Pimm, S.L. 1990. The decline of the Newfoundland crossbill. Trends in Ecology and Evolution 5:350-351.
Potvin, F., P. Beaupré, and G. Laprise. 2003. The eradication of balsam fir stands by white-tailed deer on Anticosti Island, Québec: a 150-year process. Écoscience 10:487-495.
Powell, K.G. 2005. Songbird movement, relative abundance and species composition in natural and managed forest landscapes in western Newfoundland. M.Sc. thesis, Acadia University, Wolfville, NS. 84 pp.
Pyle, P. 1997. Identification Guide to North American Birds. Part 1: Columbidae to Ploceidae. Slate Creek Press, Bolinas, CA. 732 pp.
Rae, L.F., D.M. Whitaker, and I.G. Warkentin. 2014. Multiscale impacts of herbivore-induced forest degradation on songbird assemblages. Diversity and Distributions 20:382-395.
Rajora, O.P., L. DeVerno, A. Mosseler, and D.J. Innes. 1998. Genetic diversity and population structure of disjunct Newfoundland and central Ontario populations of Eastern White Pine (Pinus strobus). Canadian Journal of Botany 76:500-508.
Reeks, H. 1869. Notes on the zoology of Newfoundland, Letters 1-4, Ornithology. Zoologist 4:1609-1614, 1689-1695, 1741-1759, 1849-1858.
Robb, M.S. 2000. Introduction to vocalizations of crossbills in north-western Europe. Dutch Birding 22:61-107.
Robert, M. , pers. comm. 2014. E-mail correspondence to T. Leonard. Biologist and Québec Breeding Bird Atlas Coordinator. Canadian Wildlife Service, Environment Canada. Québec, QC.
Roberts, B.A. 1985. Distribution and extent of Pinus resinosa Ait. in Newfoundland. Rhodora 87:341-356.
Roberts, B.A. 2011. The status of Pinus resinosa Ait. in Newfoundland and Labrador. The Centre for Forest Science and Innovation, Department of Natural Resources. Corner Brook, NL. 28 pp.
Roberts, B.A. , pers. comm. 2015. E-mail correspondence to T. Leonard. Forest Ecologist (Ret.). Atlantic Forestry Centre, Natural Resources Canada. Corner Brook, NL.
Roberts, B.A., and A.U. Mallik. 1994. Responses of Pinus resinosa in Newfoundland to wildfire. Journal of Vegetation Science 5:185-196.
Rooke, K.B. 1935. Observations on the birds of Newfoundland during the 1934 expedition of the Public Schools Exploring Society. Ibis 5:856-879.
Salafsky, N., D., Salzer, A.J., Stattersfield, C. Hilton-Taylor, R. Neugarten, S.H.M. Butchart, B. Collen, N. Cox, L.L. Master, S. O’Connor, and D. Wilkie. 2008. A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conservation Biology 22:897-911.
Sépaq. 2014. Parc national d’Anticosti. Web site: [Accessed December 2014].
Soykan, C., pers. comm. 2015. E-mail correspondence to T. Leonard. Quantitative Ecologist. National Audubon Society. San Francisco, CA, USA.
Summers, R.W., and R. Proctor. 1999. Tree and cone selection by crossbills Loxia sp. and red squirrels Sciurus vulgaris at Abernethy forest, Strathspey. Forest Ecology and Management 118:173-182.
Summers, R.W., D.C. Jardine, M. Marquiss, and R. Rae. 2002. The distribution and habitats of crossbills Loxia spp. in Britain, with special reference to the Scottish Crossbill Loxia scotica. Ibis 144:393-410.
Summers, R.W., R.J.G. Dawson, and R.E. Phillips. 2007. Assortative mating and patterns of inheritance indicate that the three crossbill taxa in Scotland are species. Journal of Avian Biology 38:153-162.
Tardif, J., pers. comm. 2016. E-mail correspondence to T. Leonard. Senior Biologist for migratory bird species at risk, Canadian Wildlife Service, Environment Canada. Québec, QC.
Taylor, P.D. and M.A. Krawchuk. 2005. Scale and sensitivity of songbird occurrence to landscape structure in a harvested boreal forest. Avian Conservation and Ecology 1(1): 5. Web site: [accessed November 2014].
Thomas, P. pers. comm. 2014. E-mail correspondence to T. Leonard. Biologist. Canadian Wildlife Service, Environment Canada. Sackville, NB.
Thompson, I.D., H.A. Hogan, and W.A. Montevecchi. 1999. Avian communities of mature Balsam Fir forests in Newfoundland: age-dependence and implications for timber harvesting. Condor 101:311-323.
Thompson, I.D., D.J. Larson, and W.A. Montevecchi. 2003. Characterization of old "wet boreal" forests, with an example from balsam fir forests of western Newfoundland. Environmental Reviews 11:S23-S46.
Todd, W.E.C. 1963. Birds of the Labrador Peninsula and Adjacent Areas. Carnegie Museum and the University of Toronto Press, Pittsburgh, PA. 822 pp.
Tuck, L.M. and M.J. Borotra. 1972. Additions to the Avifauna of St. Pierre and Miquelon. Canadian Field Naturalist 86: 279-284.
Tuck, L.M. and J.E. Maunder. 1975. Field checklist (1975) of the birds of the Island of Newfoundland. Natural History Society of Newfoundland and Labrador, St. John’s, NL.
Tulk, K.A. 2004. Foraging ecology of Red Squirrels in Newfoundland: potential impacts on forest renewal. M.Sc. thesis, University of New Brunswick, Fredericton, NB. 116 pp.
Université Laval. 2016. Chaire de recherche industrielle CRSNG en aménagement des ressources de l’île d’Anticosti. Web site: [accessed January 2016].
West, R.J. 1989. Cone depredations by the Red Squirrel in Black Spruce stands in Newfoundland: implications for commercial cone collection. Canadian Journal of Forest Research 19:1207-1210.
Whitaker, D.M. 2010. The status of Gray-cheeked Thrush (Catharus minimus) in Newfoundland and Labrador, The Species Status Advisory Committee Report Number 24. NL Department of Environment and Conservation. Corner Brook, NL. 40 pp.
Whitaker, D.M., W.A. Montevecchi, and A. Mosseler. 1996. Red Pine, wildlife and Scleroderris canker in Newfoundland. Osprey 27:64-71.
Whitaker, D.M., P.D. Taylor, and I.G. Warkentin 2008. Survival of adult songbirds in boreal forest landscapes fragmented by clearcuts and natural openings. Avian Conservation and Ecology 3(1): 5. Web site: [accessed April 2014].
Wilton, W.C. and C.H. Evans. 1974. Newfoundland Forest Fire History, 1619 - 1960. Forestry Canada Newfoundland and Labrador Information Report. Newfoundland Forest Research Centre, Report N-X-116.
Wren, L.S. 2001. Continental and Regional Distribution and Abundance Patterns of Boreal Cardueline Finches: Influences of Conifer Seed Availability. M.Sc. Thesis, Memorial University of Newfoundland, St. John's, NL. 142 pp.
Young, M.A. 2012. North American Red Crossbill Types: Status and flight call identification. Audubon and Cornell Laboratory of Ornithology. Web Site: [accessed May 2014].
Young, M.A., D.A. Fifield, and W.A. Montevecchi. 2012. New evidence in support of a distinctive Red Crossbill (Loxia curvirostra) type in Newfoundland. North American Birds 66:29-33.
Biographical Summary of Report Writers
Tina D. Leonard holds a B.Sc. (Hons.) in Environmental Biology and an M.Sc. in Biology, where she examined the influence of natural and anthropogenic forest fragmentation on breeding passerines in Newfoundland’s boreal forest. She has been employed as a Protected Areas Ecologist in the NL Department of Environment and Conservation since 2008, and has conducted winter bird surveys designed specifically for the detection of Red Crossbills since that time. She has also collected audio recordings of Red Crossbills in Newfoundland for analysis by the Cornell Laboratory of Ornithology. She has participated in Christmas Bird Counts since 2004 and has conducted Breeding Bird Surveys since 2010. Tina has held a permit to capture and band landbirds in Newfoundland since 2004, and is co-bander-in-charge at a Monitoring Avian Productivity and Survivorship (MAPS) banding station in western Newfoundland. Since 2009, she has been an active member of the NL Landbirds, Shorebirds, and Lichens Recovery Teams, and an alternate member for the Limestone Barrens Species at Risk, Labrador Woodland Caribou, and Newfoundland Marten Recovery Teams. She has published peer-reviewed scientific papers, and is author of the Caspian Tern Status Report for the NL Species Status Advisory Committee.
Collections Examined
No collections were examined for the current report.
The following collections of museum specimens of Red Crossbills taken from Newfoundland were examined for the previous status report (COSEWIC 2004):
Avian Collection, Canadian Museum of Nature, Natural Heritage Building, 1740 Pink Road, Aylmer, Québec, J9H 5E1.
Department of Ornithology. Museum of Comparative Zoology. Harvard University. 25 Oxford Street, Cambridge, MA 02138. (WAM unpublished files)
| Source | Body (mm) | Wing (mm) | Tail (mm) | Tarsus (mm) |
|---|---|---|---|---|
| Griscom (1937) | - | f: 91.0-96.5 | - | - |
| Peters and Burleigh (1951) | 140.0-165.0 | approx. 95.0 | - | - |
| Godfrey (1966) | - | adult m: 88.3-97.7 (mean 91.7) adult f: 87.2-92.3 (mean 89.5) |
53.0-59.4 (mean 55.9) | 16.3-17.8; mean 17.1 |
| Payne (1987) | - | wing arc; m: 92.7 ± 1.7 (43) f: 90.1 ± 2.3 (21) chord; m: 92.0 ± 1.7 (43) |
- | m: 16.5 ± 0.6 (43) f: 16.5 ± 0.8 (22) |
| Pyle (1997) | - | m: 89.0-97.0 (43) f: 85.0-93.0 (21) |
m: 52.0-59.0 (10) m: 49.0-56.0 (10) |
- |
| S. Wren (unpubl. data) | 160.4 ± 5.8 (8) | - | - | - |
| Young et al. (2012) | - | 87.0-91.5 (5) | - | - |
| M. Robert, CWS-Québec Region (unpubl. data) | - | chord; adult m: 86.0-90.0 (2) juv: 90.0 flattened; adult m: 88.0-91.0 (2) juv: 92.0 |
- | - |
| Source | Length (mm) | Depth (mm) | Width (mm) |
|---|---|---|---|
| Griscom (1937) | culmen; m: 17.0-19.0 | m: 10.3-11.5 | - |
| Peters and Burleigh (1951) | 17.8 | - | - |
| Godfrey (1966) | exposed culmen: 17.0-18.6 (mean 17.7) |
- | - |
| Payne (1987) | upper mandible (nares to tip); upper mandible (base of feathers to tip); |
m: 10.5 ± 0.3 (41) f: 10.5 ± 0.4 (21) |
upper mandible at anterior edge of nares; m: 7.2 ± 0.3 (42) f: 7.2 ± 0.3 (21) lower mandible at margins of rami; m: 10.0 ± 0.6 (42) f: 9.7 ± 0.4 (21) |
| Benkman (1989; 1993c) | upper mandible (nares to tip); 14.9 ± 0.18 (10) lower mandible (nares to tip); 11.0 ± 0.16 (10) |
at nares; 10.6 ± 0.1 (10) | upper mandible at anterior edge of nares; 8.1 ± 0.1 (10) |
| Pyle (1997) | nares to tip; 13.5-16.4 | at tip of nares; 9.6 -11.4 | - |
| S. Wren (unpubl. data) | upper mandible; 14.3 ± 1.0 (33) lower mandible; 11.0 ± 0.5 (33) |
upper mandible; 6.4 ± 0.3 (33) lower mandible; 4.5 ± 0.5 (33) |
upper mandible; 6.3 ± 0.6 (33) lower mandible; 8.0 ± 0.7 (33) |
| Young et al. (2012) | 13.9-15.7 (5) | 10.2-10.3 (5) | - |
| M. Robert, CWS-Québec Region (unpubl. data) | upper mandible; m: 14.5-15.7 (2) juv:14.6 lower mandible; m:10.2-11.2 (2) juv:10.3 |
m: 10.1-10.4 (2) juv: 10.2 |
- |
Appendix 3. Threats Assessment for Red Crossbill percna ssp.
Threats Assessment Worksheet
- Species or Ecosystem Scientific Name:
- Red Crossbill percna subspecies
- Element ID
- -
- Elcode
- -
- Date:
- 26/03/2015
- Assessor(s):
- Tina Leonard, Marcel Gahbauer, Darroch Whitaker, Bruno Drolet, Shelley Pardy, Jessica Humber, Mary Sabine, Karen Timm
- References:
- -
| Threat Impact | Threat Impact (descriptions) | Level 1 Threat Impact Counts: high range |
Level 1 Threat Impact Counts: low range |
|---|---|---|---|
| A | Very High | 0 | 0 |
| B | High | 0 | 0 |
| C | Medium | 3 | 2 |
| D | Low | 3 | 4 |
| - | Calculated Overall Threat Impact: | High | High |
- Assigned Overall Threat Impact:
- BC = High - Medium
- Impact Adjustment Reasons:
- Although the calculator suggests High, that assessment seems unrealistically inflated, given that there is considerable overlap among the categories for this species, and the observed rate of decline does not match such a high level of concern. High - Medium seems to more realistically characterize the blend of low-medium threats that were noted, while still acknowledging the potentially more severe impact of invasive species.
- Overall Threat Comments
- Generation time 3.4 years × 3 = 10 years. Possibly some cumulative interactions between invasive species, forestry, and fire suppression.
| # | Threat | Impact (calculated) |
Scope (next 10 Yrs) |
Severity (10 Yrs or 3 Gen.) |
Timing | Comments |
|---|---|---|---|---|---|---|
| 1 | Residential and commercial development | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | - |
| 1.1 | Housing and urban areas | Not a Threat | Negligible (<1%) | Neutral or Potential Benefit | High (Continuing) | Includes habitat conversion due to urbanization. Possibility that development can be beneficial in part through providing a food source (bird feeders) during the harsh winter months. Window strikes were discussed but frequency is rare. |
| 1.2 | Commercial and industrial areas | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | Avalon Peninsula may be at the peak of development with respect to oil and not much more is expected. However, new Galway residential, shopping, and industrial area development project is ongoing. Not much outside of this area in NL. |
| 1.3 | Tourism and recreation areas | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | - |
| 2 | Agriculture and aquaculture | D - Low | Small (1-10%) | Slight (1-10%) | High (Continuing) | - |
| 2.1 | Annual and perennial non-timber crops | D - Low | Small (1-10%) | Slight (1-10%) | High (Continuing) | Habitat loss due to semi-permanent or permanent conversion of forest habitat due to agriculture in Newfoundland is projected to be small in geographic and temporal scope. No net increase in agricultural conversion. No agricultural activities occur on Anticosti Island. |
| 2.2 | Wood and pulp plantations | Not a Threat | Negligible (<1%) | Neutral or Potential Benefit | High (Continuing) | Even though some plantations may be red pine, seed stock may not be local. Red pine of native seed stock would be of greater benefit. Pulp and paper harvesting activity (including pre-commercial thinning) significantly reduced compared to previous decades. |
| 2.3 | Livestock farming and ranching | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | Proposed growth in dairy forecast for NL. Some big expansions of dairy in Deer Lake but overall scale negligible. Mink farms likely of negligible impact. Some abandoned farmland reverting to forest. |
| 3 | Energy production and mining | D - Low | Small (1-10%) | Slight (1-10%) | High (Continuing) | - |
| 3.1 | Oil and gas drilling | Negligible | Negligible (<1%) | Moderate - Slight (1-30%) | Low (Possibly in the long term, >10 yr) | Anticosti - some potential oil development forecast (exploration has begun) and would likely be of impact if fracking used (due to loss of forest) but likely of low scope overall (depending on where the development will be located). There is the potential for nearshore and onshore oil development on the west coast of NL. Habitat loss and increased noise likely but may not influence direct mortality. More information needed. |
| 3.2 | Mining and quarrying | D - Low | Small (1-10%) | Slight (1-10%) | High (Continuing) | Very small scale. Mining industry currently declining and this trend is expected to continue in the next few years. Any operational projects may cause loss of habitat at each of the sites, which are typically smaller than 1 km2; overall impact likely to be low due to remaining available habitat. |
| 3.3 | Renewable energy | Negligible | Negligible (<1%) | Negligible (<1%) | Unknown | No new wind farm proposals known. Small number of wind farms in production currently where species has been found. Possible threat of negligible impact. |
| 4 | Transportation and service corridors | D - Low | Large - Restricted (11-70%) | Slight (1-10%) | High (Continuing) | - |
| 4.1 | Roads and railroads | D - Low | Large - Restricted (11-70%) | Slight (1-10%) | High (Continuing) | Species attracted to grit on roads; effect of ingestion may be negative but unknown. Large proportion of birds is expected be exposed to a roadway in their lifetime, but risk of mortality due to vehicle strikes is expected to be low. |
| 4.2 | Utility and service lines | Negligible | Negligible (<1%) | Moderate - Slight (1-30%) | High (Continuing) | Majority of service and utility lines are hydro lines. Muskrat Falls hydroelectric power corridor project has already begun and will continue in the coming few years. Nesting chronology is not specifically known for this species so difficult to know how construction plans will consider nesting times; however, if work done at same time as nesting would be of negative impact (depending on area of project). |
| 4.4 | Flight paths | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | DND, SAR, and scientific research helicopter flights occur low over trees where birds may be feeding and/or nesting. |
| 5 | Biological resource use | CD - Medium - Low | Restricted (11-30%) | Serious - Slight (1-70%) | High (Continuing) | - |
| 5.3 | Logging and wood harvesting | CD - Medium - Low | Restricted (11-30%) | Serious - Slight (1-70%) | High (Continuing) | Due to potential for loss of nests with logging, and that nesting can occur throughout the year, this would be of likely negative impact. Forestry may also affect proportion of Balsam Fir to Black or White Spruce (both harvested and replanted) and possibly preferred cone source. |
| 6 | Human intrusions and disturbance | Negligible | Small (1-10%) | Negligible (<1%) | High (Continuing) | - |
| 6.1 | Recreational activities | Negligible | Small (1-10%) | Negligible (<1%) | High (Continuing) | - |
| 6.2 | War, civil unrest and military exercises | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | DND and SAR exercises considered in NL and Anticosti. Several military exercises per year in NL and 3-4 live fire ranges, but overall negligible overlap/impact for this species. |
| 6.3 | Work and other activities | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | All scientific research work (small scale overall) requires permits and would not be of high negative impact. |
| 7 | Natural system modifications | C - Medium | Large (31-70%) | Moderate (11-30%) | High (Continuing) | - |
| 7.1 | Fire and fire suppression | CD - Medium - Low | Restricted (11-30%) | Moderate - Slight (1-30%) | High (Continuing) | Forest fire suppression can have negative impact on Red Pine due to bigger, hotter fires when they do occur. Central NL forest type has a more fire-prone ecosystem than other areas. Difficult to determine. |
| 7.2 | Dams and water management/use | Negligible | Negligible (<1%) | Negligible (<1%) | Low (Possibly in the long term, >10 yr) | Not many new projects known but would not be of a large scope. |
| 7.3 | Other ecosystem modifications | C - Medium | Large (31-70%) | Moderate (11-30%) | High (continuing) | Red Squirrels (nest predators and competitors for food cones) present on NL but not Anticosti. |
| 8 | Invasive and other problematic species and genes | C - Medium | Large (31-70%) | Moderate (11-30%) | High (Continuing) | - |
| 8.1 | Invasive non-native/alien species | D - Low | Restricted (11-30%) | Moderate (11-30%) | High (Continuing) | Scleroderris (red pine fungus, European strain) can affect pine survival in NL plantations. Possible human (travel, firewood) and moose transmission of Scleroderris spores. |
| 8.2 | Problematic native species | C - Medium | Large (31-70%) | Moderate (11-30%) | Moderate (Possibly in the short term, < 10 yr) | Insect outbreaks (future spruce budworm outbreak predicted for NL and Anticosti), but these outbreaks are integral to and part of the natural system and not expected to be beyond the scope of natural disturbance in the next 10 years. |
| 9 | Pollution | - | - | - | - | - |
| 10 | Geological events | - | - | - | - | - |
| 11 | Climate change and severe weather | Negligible | Pervasive - Large (31-100%) | Negligible (<1%) | High (Continuing) | Climate change projections for Newfoundland were discussed and are considered to likely not have future impacts on species. Significant shifts in climate due to anthropogenic factors are not expected in the next 10 years. Climate change projections indicate an increase of 2 degrees Celsius in Newfoundland and Labrador during the period 2038-2070; extrapolating back from this trend indicates an approximate increase of 1 degree Celsius by 2025. Natural variability is expected to exert a stronger influence in climate in the next decade due to the North Atlantic Oscillation and Atlantic Multidecadal Oscillation. |
Classification of Threats adopted from IUCN-CMP, Salafsky et al. (2008).
Glossary
- Impact
- The degree to which a species is observed, inferred, or suspected to be directly or indirectly threatened in the area of interest. The impact of each threat is based on Severity and Scope rating and considers only present and future threats. Threat impact reflects a reduction of a species population or decline/degradation of the area of an ecosystem. The median rate of population reduction or area decline for each combination of scope and severity corresponds to the following classes of threat impact: Very High (75% declines), High (40%), Medium (15%), and Low (3%). Unknown: used when impact cannot be determined (e.g., if values for either scope or severity are unknown); Not Calculated: impact not calculated as threat is outside the assessment timeframe (e.g., timing is insignificant/negligible or low as threat is only considered to be in the past); Negligible: when scope or severity is negligible; Not a Threat: when severity is scored as neutral or potential benefit.
- Scope
- Proportion of the species that can reasonably be expected to be affected by the threat within 10 years. Usually measured as a proportion of the species’ population in the area of interest. (Pervasive = 71–100%; Large = 31–70%; Restricted = 11–30%; Small = 1–10%; Negligible < 1%).
- Severity
- Within the scope, the level of damage to the species from the threat that can reasonably be expected to be affected by the threat within a 10-year or three-generation timeframe. Usually measured as the degree of reduction of the species’ population. (Extreme = 71–100%; Serious = 31–70%; Moderate = 11–30%; Slight = 1–10%; Negligible < 1%; Neutral or Potential Benefit > 0%).
- Timing
- High = continuing; Moderate = only in the future (could happen in the short term [< 10 years or 3 generations]) or now suspended (could come back in the short term); Low = only in the future (could happen in the long term) or now suspended (could come back in the long term); Insignificant/Negligible = only in the past and unlikely to return, or no direct effect but limiting.