Speckled dace (Rhinichthys osculus): COSEWIC assessment and status report 2016

Endangered
2016
Table of contents
- Table of contents
- Assessment summary
- Executive summary
- Technical summary
- Preface
- Wildlife species description and significance
- Distribution
- Habitat
- Biology
- Population sizes and trends
- Threats and limiting factors
- Protection, status and ranks
- Acknowledgements and authorities contacted
- Information sources
- Biographical summary of report writers
- Collections examined
List of figures
- Figure 1. Speckled Dace (Rhinichthys osculus; photograph by P. Mylechreest, courtesy of Dr. J. D. McPhail).
- Figure 2. The global distribution of Speckled Dace (with permission of Dr. J. D.
McPhail). - Figure 3. Distribution of Speckled Dace in Canada (based on survey data from BC Conservation Data Centre 2013 and Batty 2010).
- Figure 4. Mean monthly discharge at three Water Survey of Canada stations in the Kettle River system, 2000-2014. The Granby River station (08NN002; gross drainage area 2060 km2) is located at Grand Forks, and has data from 2000-2012. The West Kettle River station (08NN003; gross drainage area 1890 km2) is located at Westbridge, and has data from 2000-2014. The Kettle River station (08NN026; gross drainage area 2140 km2) is located near Westbridge, and has data from 2000-2013.
- Figure 5. Surface and groundwater fluctuations in the Kettle River watershed. Surface water data (blue crosses) are from the Kettle River and groundwater data (red open circles) are from the Grand Forks aquifer (from Brown et al. 2012).
- Figure 6. Mean monthly discharge during low flow periods (2000-2014) in the Kettle River system, with 10% and 20% mean annual discharge rates (MADs) indicated for each river. A 10% MAD is considered the minimum required for fish conservation, while 20% MAD may be the minimum needed to maintain adequate riffle depth and velocity (Government of BC undated; Tennant 1976; Ptolemy and Lewis 2002; Annear et al. 2004). All three rivers fall below the 10% level during low flow periods. Data were collected from the same three Water Survey of Canada stations as in Figure 4.
List of tables
- Table 1. Summary of search effort to establish the Canadian range of Speckled Dace.
- Table 2. Habitat preferences of Speckled Dace in Canada.
- Table 3. Proportional use of different habitat types by Speckled Dace (from Andrusak and Andrusak 2011).
- Table 4. Habitat characteristics used to identify proposed critical habitat for Speckled Dace (from Brown et al. 2012).
List of appendices
- Appendix A. Threats Assessment Calculator
Document information
COSEWIC
Committee on the Status
of Endangered Wildlife
in Canada

COSEPAC
Comité sur la situation
des espèces en péril
au Canada
COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2016. COSEWIC assessment and status report on the Speckled Dace Rhinichthys osculusin Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 51 pp. (Species at Risk Public Registry website).
Previous report(s):
COSEWIC 2006. COSEWIC assessment and update status report on the speckled dace Rhinichthys osculusin Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 27 pp. (Species at Risk Public Registry website).
COSEWIC 2002. COSEWIC assessment and update status report on the speckled dace Rhinichthys osculusin Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 36 pp. (Species at Risk Public Registry website).
Peden, A. 2002. COSEWIC assessment and update status report on the speckled dace Rhinichthys osculus in Canada, in COSEWIC assessment and update status report on the speckled dace Rhinichthys osculus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-36 pp.
Peden, A.E. 1980. COSEWIC status report on the speckled dace Rhinichthys osculus in Canada. Committee on the Status of Endangered Wildlife in Canada. 1-13 pp.
Production note:
COSEWIC would like to acknowledge Andrea Smith (Hutchinson Environmental Sciences Ltd.) for writing the status report on the Speckled Dace, Rhinichthys osculus, in Canada, prepared under contract with Environment and Climate Change Canada. This report was overseen and edited by John Post, Co-chair of the COSEWIC Freshwater Fishes Specialist Subcommittee.
For additional copies contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment and Climate Change Canada
Ottawa, ON
K1A 0H3
Tel.: 819-938-4125
Fax: 819-938-3984
E-mail: COSEWIC E-mail
Website: COSEWIC
Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Naseux moucheté (Rhinichthys osculus) au Canada.
Cover illustration/photo:
Speckled Dace (Rhinichthys osculus); photograph by P. Mylechreest, courtesy of Dr. J. D. McPhail.
COSEWIC assessment summary
Assessment summary – November 2016
- Common name
- Speckled Dace
- Scientific name
- Rhinichthys osculus
- Status
- Endangered
- Reason for designation
- This species reaches its northern limit in south central British Columbia where it is restricted to the Kettle River watershed. While the species has shown some resilience to the effects of drought, it is nevertheless threatened by a combination of low flows due to water extractions and climate change and to forestry and agricultural effluents.
- Occurrence
- British Columbia
- Status history
- Designated Special Concern in April 1980. Status re-examined and designated Endangered in November 2002, April 2006, and in November 2016.
COSEWIC executive summary
Speckled Dace
Rhinichthys osculus
Wildlife species description and significance
The Speckled Dace (Rhinichthys osculus) is a small minnow (51-94 mm in total length) with a robust elongate body. It is grey to brownish grey in colour with dark flecks. Most Speckled Dace in Canada are isolated above a 30.5 m high barrier at Cascade Falls, Columbia River drainage, British Columbia. Speckled Dace above the barrier can be differentiated from US populations by their absence of barbels and higher scale counts.
The Speckled Dace exhibits a high degree of morphological, ecological and genetic variation across its range. Many subspecies and distinct populations are recognized in the US, and several of these isolated populations are listed at risk under the US Endangered Species Act.
The Speckled Dace is one of the most abundant and widely distributed freshwater fish in the western US. In Canada, however, it reaches the northern limit of its range and exists as a peripheral and disjunct population.
Distribution
The Speckled Dace is restricted to western North America. It is found as far south as northern Mexico and as far north as south-central British Columbia. In Canada, it is confined to the Kettle River system (Kettle, West Kettle and Granby Rivers), where it occurs along a 275 km length of river.
Habitat
In Canada, Speckled Dace tends to inhabit shallow slow-moving waters, as well as riffles and runs, with coarse gravel, cobble or boulder substrates. Immature fish prefer the river margin, while adults typically inhabit deeper channel habitat. The Kettle River system is subject to extreme low flows both during the winter and late summer months. Peak flows occur from April through June following snowmelt. Summer surface water temperatures in the river system typically exceed 24°C and winter water temperatures fall below 0°C.
Biology
Little information exists on the biology of Speckled Dace. It is believed to spawn in mid-July in Canada. Males begin breeding at 2+ years and females a year later. Depending on their size, mature females can produce between 400 and 2000 eggs. Newly hatched fry emerge in August and September. The lifespan of Speckled Dace in Canada appears to be over seven years, compared with a maximum of three to four years documented in the US.
Population size and trends
The most recent population estimate from 2010 indicated that approximately 940,000 mature individuals exist in Canada. No long-term studies have been conducted to determine population trends.
Threats and limiting factors
The entire Canadian range of the Speckled Dace is found within a single drainage system characterized by low flows. Most of the Canadian population is isolated above a natural barrier from all other populations. The main threats to Speckled Dace are a reduction in habitat size and quality as a result of water extraction and sedimentation from forestry activity. Climate change may exacerbate low flow conditions during periods of peak water demand. Several non-native fish (e.g., Smallmouth Bass, Micropterus dolomieu; Northern Pike, Esox lucius; Walleye, Sander vitreus) could pose competitive or predatory threats if they were to spread into the Kettle River system above Cascade Falls.
Protection, status, and ranks
Speckled Dace was originally designated Special Concern by COSEWIC in 1980. Following re-examination of its status by COSEWIC in 2002 and in 2006, it was designated Endangered by COSEWIC and listed as Endangered under the federal Species at Risk Act in 2009. No recovery strategy or action plan has yet been approved for the species under federal legislation. Speckled Dace is recognized as a protected species under BC fishery regulations. It is considered a species of least concern by the IUCN Red List and its global rank is G5 (secure). Its national ranking in the US is N5 (secure) and N2 (imperilled) in Canada. Speckled Dace is ranked as S2 (imperilled) on the BC Red List. Several subspecies are listed as at risk under the US Endangered Species Act.
Technical summary
- Scientific name:
- Rhinichthys osculus
- English name:
- Speckled Dace
- French name:
- Naseux moucheté
- Range of occurrence in Canada (province/territory/ocean):
- British Columbia
Demographic information
| Summary items | Information |
|---|---|
| Generation time (usually average age of parents in the population; indicate if another method of estimating generation time indicated in the IUCN guidelines(2011) is being used) | 3 to 4 yrs |
| Is there an [observed, inferred, or projected] continuing decline in number of mature individuals? | Yes |
| Estimated percent of continuing decline in total number of mature individuals within [5 years or 2 generations] | Unknown |
| [Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over the last [10 years, or 3 generations]. | Unknown |
| [Projected or suspected] percent [reduction or increase] in total number of mature individuals over the next [10 years, or 3 generations]. | Unknown |
| [Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over any [10 years, or 3 generations] period, over a time period including both the past and the future. | Unknown |
| Are the causes of the decline a.clearly reversible and b.understood and c. ceased? | a. No b. Yes c. No |
| Are there extreme fluctuations in number of mature individuals? | Unknown |
Extent and occupancy information
| Summary items | information |
|---|---|
| Estimated extent of occurrence | 2809 km2 |
| Index of area of occupancy (IAO) | 160 km2 (discrete) 528 km2 (continuous) |
| Is the population “severely fragmented” i.e., is >50% of its total area of occupancy in habitat patches that are (a) smaller than would be required to support a viable population, and (b) separated from other habitat patches by a distance larger than the species can be expected to disperse? | a. No b. No |
| Number of locations (use plausible range to reflect uncertainty if appropriate) (Note: See Definitions and abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
1-4 based on the combined threats of water withdrawal and climate change impacts on summer and autumn flows |
| Is there an [observed, inferred, or projected] decline in extent of occurrence? | Projected decline as a result of reductions in habitat size and quality from the increasing frequency and severity of summer drought conditions, combined with water withdrawal demands |
| Is there an [observed, inferred, or projected] decline in index of area of occupancy? | Projected decline as a result of reductions in habitat size and quality from the increasing frequency and severity of summer drought conditions, combined with water withdrawal demands |
| Is there an [observed, inferred, or projected] decline in number of subpopulations? | No subpopulations have been identified in Canada. |
| Is there an [observed, inferred, or projected] decline in number of “locations”? (Note: See Definitions and abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
No |
| Is there an [observed, inferred, or projected] decline in [area, extent and/or quality] of habitat? | Projected decline as a result of reductions in habitat size and quality from the increasing frequency and severity of summer drought conditions, combined with water withdrawal demands |
| Are there extreme fluctuations in number of subpopulations? | No |
| Are there extreme fluctuations in number of “locations”? (Note: See Definitions and abbreviations on COSEWIC website and IUCN (Feb 2014) for more information on this term.) |
No |
| Are there extreme fluctuations in extent of occurrence? | No |
| Are there extreme fluctuations in index of area of occupancy? | No |
Number of mature individuals (in each subpopulation)
| Subpopulations (give plausible ranges) | N Mature Individuals |
|---|---|
| - | 940,000 |
| Total | 940,000 (90% CI: 412,000-1,955,000) |
Quantitative analysis
| Summary items | Information |
|---|---|
| Is the probability of extinction in the wild at least [20% within 20 years or 5 generations, or 10% within 100 years]? | Unknown |
Threats (direct, from highest impact to least, as per IUCN Threats Calculator)
Was a threats calculator completed for this species? Yes, on 2015/12/16.
- Natural system modifications
- Climate change & severe weather
- Pollution
- Invasive & other problematic species & genes
The assigned overall threat impact was High to Medium
Rescue effect (immigration from outside Canada)
| Summary items | Information |
|---|---|
| Status of outside population(s) most likely to provide immigrants to Canada. | Closest US populations are Global Rank G5 (secure), US National Rank N5 (secure); 4 subspecies listed at risk under US Endangered Species Act, 11 subspecies listed at risk by NatureServe in US, 1 subspecies presumed extinct and 1 possibly extinct in US |
| Is immigration known or possible? | Immigration not possible from outside populations located below Cascade Falls |
| Would immigrants be adapted to survive in Canada? | Yes |
| Is there sufficient habitat for immigrants in Canada? | Yes |
| Are conditions deteriorating in Canada? See Table 3 (Guidelines for modifying status assessment based on rescue effect). |
Inferred deterioration as a result of reductions in habitat size from low flows combined with water withdrawals |
| Are conditions for the source population deteriorating? See Table 3 (Guidelines for modifying status assessment based on rescue effect). |
Unknown |
| Is the Canadian population considered to be a sink? See Table 3 (Guidelines for modifying status assessment based on rescue effect). |
The main portion of the Canadian population is not a sink because immigration from other populations is unlikely. The small portion of the Canadian population found below the Cascade Falls, however, may be a sink because it is believed to persist only through immigration from upstream sources. |
| Is rescue from outside populations likely? | No, due to 30.5 m high barrier to upstream movement at Cascade Falls |
Data-sensitive species
| Summary items | Information |
|---|---|
| Is this a data sensitive species? | No |
Status history
| Summary items | Information |
|---|---|
| COSEWIC: | Endangered |
| Year Assessed: | 2016 |
COSEWIC Status History: Designated Special Concern in April 1980. Status re-examined and designated Endangered in November 2002, April 2006, and November 2016.
Status and reasons for designation:
| Summary items | Information |
|---|---|
| Status: | Endangered |
| Alpha-numeric code: | B1ab(iii)+2ab(iii) |
| Reasons for designation: | This species reaches its northern limit in south central British Columbia where it is restricted to the Kettle River watershed. While the species has shown some resilience to the effects of drought, it is nevertheless threatened by a combination of low flows due to water extractions and climate change and to forestry and agricultural effluents. |
Applicability of criteria:
| Summary items | Information |
|---|---|
| Criterion A (Decline in total number of mature individuals): |
Not applicable. Population trends unknown. |
| Criterion B (Small distribution range and decline or fluctuation): |
Meets Endangered, B1ab(iii)+2ab(iii), since the EOO, IAO and number of locations are all below thresholds (2,890 km², 160-528 km² and 1-4 respectively) and since there is a projected continuing decline in habitat quality. |
| Criterion C (Small and declining number of mature individuals): |
Not applicable. |
| Criterion D (Very small or restricted population): |
Not applicable. |
| Criterion E (Quantitative analysis): |
Not applicable. |
Preface
Additional information has been collected on Speckled Dace (Rhinichthys osculus) distribution, habitat use and abundance in Canada since the last COSEWIC assessment in 2006. Surveys throughout the Kettle River system in 2008 have expanded the estimated range of the species upstream by 16 km (or 6%) to encompass a 275 km length of river, compared with the 2006 assessment of a 259 km length of river (COSEWIC 2006; Batty 2010). Additional characterization of habitat use in Canada has also occurred since the last assessment (Batty 2010; Andrusak and Andrusak 2011). This research indicates that Speckled Dace has a preference for shallow run or riffle habitat, and supports previous studies demonstrating age-specific differences in habitat use. The 2006 assessment did not provide an estimate of the population size of Speckled Dace in Canada due to a lack of quantitative sampling. Batty’s (2010) extensive surveys throughout the Kettle River system have led to a population estimate of 940,000 mature individuals, which is an order of magnitude higher than previous published estimates. Batty (2010) also collected an individual determined to be more than seven years old, increasing the estimated longevity of the species.
COSEWIC history
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
COSEWIC mandate
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC membership
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
Definitions (2016)
- Wildlife species
- A species, subspecies, variety, or geographically or genetically distinct population of animal, plant or other organism, other than a bacterium or virus, that is wild by nature and is either native to Canada or has extended its range into Canada without human intervention and has been present in Canada for at least 50 years.
- Extinct (X)
- A wildlife species that no longer exists.
- Extirpated (XT)
- A wildlife species no longer existing in the wild in Canada, but occurring elsewhere.
- Endangered (E)
- A wildlife species facing imminent extirpation or extinction.
- Threatened (T)
- A wildlife species likely to become endangered if limiting factors are not reversed.
-
Special concern (SC)
(Note: Formerly described as “Vulnerable” from 1990 to 1999, or “Rare” prior to 1990.) - A wildlife species that may become a threatened or an endangered species because of a combination of biological characteristics and identified threats.
-
Not at risk (NAR)
(Note: Formerly described as “Not In Any Category”, or “No Designation Required.”) - A wildlife species that has been evaluated and found to be not at risk of extinction given the current circumstances.
-
Data deficient (DD)
(Note: Formerly described as “Indeterminate” from 1994 to 1999 or “ISIBD” [insufficient scientific information on which to base a designation] prior to 1994. Definition of the [DD] category revised in 2006.) - A category that applies when the available information is insufficient (a) to resolve a species’ eligibility for assessment or (b) to permit an assessment of the species’ risk of extinction.
The Canadian Wildlife Service, Environment and Climate Change Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Wildlife species description and significance
Name and classification
Phylum: Chordata
Class: Actinopterygii (ray-finned fishes)
Order: Cypriniformes
Family: Cyprinidae
Genus: Rhinichthys
Species: Rhinichthys osculus(Girard 1856)
Common name
English: Speckled Dace (Nelson et al. 2004), also known as Dusky Dace, Pacific Dace, Spring Dace and Western Dace (Nico and Fuller 2015)
French: Naseux moucheté (Scott and Crossman 1973)
The Speckled Dace (Rhinichthys osculus) has an uncertain taxonomy. Some researchers consider it a species complex because of the high degree of variation exhibited among populations, but taxonomic relationships are not well understood (McPhail 2007; Minckley and Marsh 2009; Hoekzema and Sidlauskas 2014). Speckled Dace was originally classified as 12 species under the genus (now subgenus) Apocope (Jordan et al. 1930 in Oakey et al. 2004). Its classification was subsequently revised to a single wide-ranging species (Miller and Miller 1948). Currently, between 15 and 19 subspecies are recognized across its range, as well as several unnamed forms (Minckley and Marsh 2009; USFWS 2014; ITIS 2015; NatureServe 2015). Wiesenfeld (2014) recommended that an additional subspecies be designated in the US. Hoekzema and Sidlauskas (2014) suggested that multiple cryptic species may exist in the Speckled Dace complex. Additionally, there has been debate over whether two closely related species, the Leopard Dace (R. falcatus) and the Umatilla Dace (R. umatilla), represent forms of the Speckled Dace (Oakey et al. 2004; DFO 2008, but see Haas 2001). No subspecies have been named in the Canadian population of the species.
Morphological description
The Speckled Dace is a small minnow, approximately 50-90 mm in total length, although females are occasionally longer (McPhail 2003; DFO 2013a). It has a robust elongate body with a bluntly triangular head and a slight hump behind the head (Scott and Crossman 1973). The Speckled Dace is grey to brownish grey in colour with small dark flecks, generally above the midline. Its underside is yellowish to creamy white (Scott and Crossman 1973).
The Speckled Dace has a long blunt snout, which overhangs the slightly downturned mouth (Figure 1; Scott and Crossman 1973; Williams et al. 2014). The caudal peduncle is moderately narrow and the fork of the caudal fin is shallow with a depth about 6% of the total length (McPhail 2003). Pelvic and pectoral fins are relatively small and rounded, with eight to nine and 13-14 rays respectively (Scott and Crossman 1973). The Speckled Dace has eight to nine dorsal rays, six to seven anal rays and 59-69 scales along the lateral line (McPhail 2003). A faint band extends down the side of the fish, from under the dorsal fin to the caudal peduncle, which ends in a diffuse spot on the caudal fin base. Both the spot and band are more obvious in younger fish (Scott and Crossman 1973). Unlike males, females and juveniles lack irregular dark spots on their back and sides (McPhail 2003). Males resemble Umatilla Dace in their markings, but are less streamlined and ventrally flattened (Haas 2001).

Long description for Figure 1
Photo of a Speckled Dace (lateral view). This small minnow (51 to 94 millimetres total length) has a robust elongate body and is grey to brownish grey in colour with dark flecks.
In Canada, Speckled Dace can be differentiated from Leopard Dace and Umatilla Dace by its terminal mouth, rounded fins, fewer vertebrae, lack of maxillary barbels and pelvic fins without stays. Among these three species, Speckled Dace is the least streamlined and most robust in body form and the darkest in colouration; the eyes are smaller; its fins and fin bases are smaller and rounder in shape; its tail is smaller in size and degree of fork, and caudal peduncle thicker, and the pelvic fin stays are generally weaker or absent (Haas 2001).
Speckling can be greatly reduced or not obvious in the Canadian population of the species (Haas 2001). Speckled Dace in British Columbia (BC) differ from the nearest US populations 80 km to the south by their lack of barbels and higher scale counts around the caudal peduncle (Peden 2002).
Population spatial structure and variability
The Speckled Dace exhibits extensive morphological, ecological and genetic variation across its range. A complex series of morphological forms occurs in many isolated drainages that lie along the US coast, from the Olympic Peninsula to California (McPhail 2003).
The Cascade Falls, situated on the Kettle River five kilometres upstream from the Canadian-US border, represents a natural physical barrier to the upstream movement of Speckled Dace. The majority of the Canadian population is located above the falls, and is thus considered geographically isolated and disjunct from all other Speckled Dace populations (Andrusak and Andrusak 2011; Brown et al. 2012; DFO 2013a). Some individuals are likely swept over the falls during high spring flows, but this downstream population does not appear to be self-sustaining (Peden and Hughes 1984).
Limited genetic analysis suggests that the Canadian population may differ genetically from other populations. Using the mitochondrial cytochrome-b marker (306 base pairs), Haas (2001) found that Speckled Dace from the Granby and Kettle rivers above the falls shared identical sequence with a fish from the Olympic Peninsula in Washington State, but had one to three base pair differences from seven additional populations sampled from other parts of Washington and Oregon. However, samples sizes for the study were low (only three individuals were sampled from BC, one from the Olympic Peninsula, and three each from the rest of Washington and Oregon) and based on a single molecular marker. In addition, no adjacent US populations were sampled.
Extensive morphometric comparisons between Canadian and US populations have not been conducted. Peden (2002) described meristic differences between Speckled Dace in Canada and other populations in the Columbia River drainage (10 populations, including two extinct, in Washington, Idaho and Oregon). Canadian Speckled Dace had higher scale counts around the caudal peduncle than 80% of sampled US populations, while 20% of US populations lacked barbels, like the Kettle River fish. The small sample size involved in the comparison precluded statistical comparison.
The evolutionary history of the Speckled Dace has been shaped dramatically by climatic and geological events (Ardren et al. 2010). Patterns of glaciation, tectonism and climate warming have led to repeated cycles of range fragmentation and reconnection for the species during the Miocene, Pliocene and Pleistocene (Oakey et al. 2004; Pfrender et al. 2004; Ardren et al. 2010; Rusky 2014). This has resulted in high levels of genetic subdivision among populations, often structured by drainage basin or sub-basin. Speckled Dace has maintained substantial genetic variation under these dynamic environmental conditions because it is an ecological generalist, exhibits phenotypic plasticity and has a high reproductive potential (Ardren et al. 2010).
Oakey et al. (2004) documented genetic differentiation in US populations by mapping 112 mitochondrial DNA restriction sites. One hundred and four individuals were sampled representing 59 populations from across the US range. Two major clades were identified, corresponding with the Colorado and Snake river systems. The Colorado River clade further divided into several discrete sub-clades based on sub-basin, likely reflecting population isolation due to regional aridity and tectonic activity (Oakey et al. 2004). There was considerable restriction site variation among populations, with most populations composed of one or more unique haplotypes (Oakey et al. 2004).
Pfrender et al. (2004) examined genetic structure in Speckled Dace across five major drainage systems in Oregon. A 670 base-pair segment of the mitochondrial cytochrome-b region was analyzed in 90 individuals representing 13 localities. High levels of genetic diversity were found across all populations (π = 0.0434; 43 unique haplotypes). In addition, populations in major river basins showed strong genetic differentiation (Nst = 0.823, p =0.027), forming reciprocally monophyletic clades. The amount of sequence divergence among basins ranged from 5.92% (SE = 0.09) to 14.61% (SE = 0.24), which corresponded with species-level differences recorded in other cyprinids (Pfrender et al. 2004). Based on a molecular clock divergence rate of 0.76% per million years for the cyprinid cytochrome b gene, Pfrender et al. (2004) calculated that populations diverged from each other between 3.89 (SE = 0.12) and 9.61 (SE = 0.32) million years ago. Further research, with additional molecular markers and increased sample size, is needed to clarify whether populations in different drainage basins warrant species-level designation (Pfrender et al. 2004).
Gene flow in Speckled Dace may also be restricted by ecological factors. The species was introduced to the Eel River system (northern California) in the mid-1980s. While the population rapidly increased, its distribution did not expand despite the absence of physical barriers (Kinziger et al. 2011). The presence of multiple predators (Prickly Sculpin, Cottus asper; Coastrange Sculpin, C. aleuticus; Sacramento Pikeminnow, Ptychocheilus grandis) appears to limit the introduced range of the fish in this drainage system (Harvey et al. 2004). Such biotic resistance to dispersal could lead to population structure among populations.
Mitochondrial and ribosomal DNA studies based on sequences from the cytochrome-b region (306 base pairs), internal transcribed spacer (250 base pairs), and the ribosomal region (80 base pairs) support the distinction of Speckled Dace, Leopard Dace and Umatilla Dace (Haas 2001). Umatilla Dace is believed to have originated via hybridization between Speckled Dace and Leopard Dace, likely evolving through multiple historical hybridizations following the last glacial period (Haas 2001). Contemporary hybridization between Speckled Dace and Leopard Dace is not believed to occur (McPhail 2007). In Canada, Speckled Dace and Umatilla Dace co-exist in a small section of the Kettle River below Cascade Falls. No evidence of hybrids between the two species has been documented in this area (Peden and Hughes 1988).
Speckled Dace has also been reported to hybridize with Least Chub (Iotichthys phlegethontis), Longnose Dace (R. cataractae), Redside Shiner (Richardsonius balteatus) and Relict Dace (Relictus solitarius) in parts of its US range (Smith 1973; Miller and Behnke 1985; Wiesenfeld 2014). Of these, Longnose Dace and Redside Shiner co-occur with Speckled Dace in Canada.
Designatable units
Currently, Speckled Dace in Canada is not classified below the species level (i.e., no subspecies, varieties or designatable units have been identified). The entire Canadian population occupies a single biogeographic zone and there are no physical barriers to movement upstream of Cascade Falls. No studies have been conducted to test whether fish above and below the falls are genetically and/or morphologically different. Consequently, the Canadian population is considered a single designatable unit, as per COSEWIC guidelines.
Special significance
Speckled Dace is one of the most abundant and widely distributed freshwater fish in the western US, occurring in a diversity of habitats (from small springs and streams to large rivers and deep lakes) and thermal conditions (e.g., summer temperatures of 14° – 33° C; John 1964; Moyle 1976; Peden and Hughes 1981; Oakey et al. 2004; Pfrender et al. 2004; Smith and Dowling 2008; Hoekzema and Sidlauskas 2014).
Speckled Dace reaches the northern limit of its geographic range in south-central BC, where it occurs in a single watershed, the Kettle River system. Despite the species’ ubiquity across the rest of its range, most Speckled Dace in Canada are peripheral and disjunct, isolated from other populations downstream by a 30.5 m natural barrier at Cascade Falls (Peden and Hughes 1984; Haas 2001; McPhail 2003). The small portion of the Canadian population that occurs below the falls may also be isolated from US populations downstream through competitive exclusion by Umatilla Dace (Peden and Hughes 1984). Peden (2002) reported that the morphology of Speckled Dace in Canada is distinct from populations in the US downstream of Cascade Falls.
The Speckled Dace is also of scientific interest as one of the purported parental species (along with Leopard Dace) in the origin of a third species by hybridization, the Umatilla Dace (Haas 2001). Umatilla Dace in Canada are also restricted to a small geographic area in south-central BC (Haas 2001).
Speckled Dace is believed to be an important forage fish linking aquatic and terrestrial food chains, as it serves as prey for piscivorous fish and birds (Scott and Crossman 1973; Brown et al. 2012). It is used as a baitfish in parts of its US range (Scott and Crossman 1973).
The US Fish and Wildlife Service (USFWS) currently recognizes 19 subspecies of Speckled Dace in the US. Of these, six have scientific subspecies nomenclature while thirteen have common names only. Four of the USFWS subspecies are listed as at risk federally in the US: Ash Meadows Speckled Dace (R. osculus nevadensis; endangered), Clover Valley Speckled Dace (R. osculus oligoporus; endangered), Independence Valley Speckled Dace (R. osculus lethoporus; endangered) and Foskett Speckled Dace (R. osculus ssp.; threatened) (USFWS 2014). NatureServe lists an additional 11 subspecies as at risk in the US (NatureServe 2015).
No Aboriginal Traditional Knowledge is available for this species.
Distribution
Global range
Speckled Dace is restricted to western North America (Figure 2). It is found in Pacific drainages from the Columbia River to the Colorado River system, south into northern Mexico (Sonora) and in coastal drainages between the Olympic Peninsula, Washington and southern California (Scott and Crossman 1973; Page and Burr 2011).
Long description for Figure 2
Map of the global distribution of the Speckled Dace, which is restricted to western North America. The species is found in Pacific drainages from the Columbia River to the Colorado River system, south into northern Mexico (Sonora), and in coastal drainages between the Olympic Peninsula, Washington, and southern California.
Canadian range
Speckled Dace reaches its northern limit in Canada, occurring only in the Kettle River and its two main tributaries, the West Kettle River and the Granby River, in south-central British Columbia (Figure 3; Peden and Hughes 1981, 1984; Peden 2002). The Kettle River system is part of the Columbia River drainage and falls entirely within the Pacific biogeographic zone. The river and its tributaries flow south from their headwaters in the Monashee Mountains. The Kettle merges with the West Kettle near Westbridge and continues south, crossing into Washington State below Midway. It then flows north back across the border near Grand Forks, where it joins with the Granby River, flowing east and then south again into the US (Figure 3; Peden and Hughes 1981).
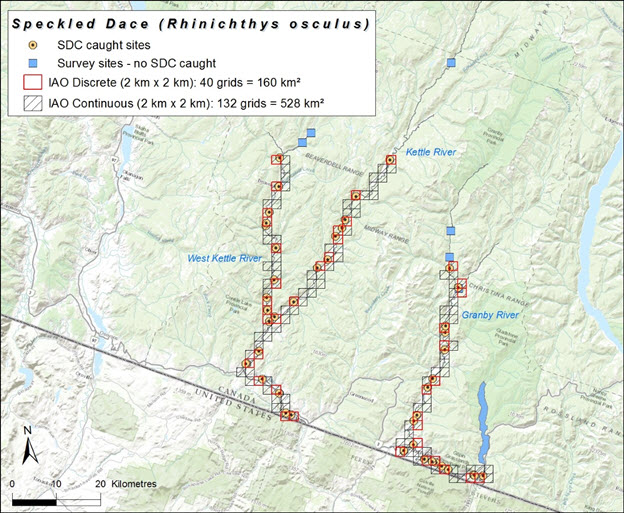
Long description for Figure 3
Map of the distribution of the Speckled Dace in Canada, where it occurs in the Kettle River and its two main tributaries, the West Kettle River and the Granby River, in south-central British Columbia. The map shows survey sites where Speckled Dace were caught and not caught, as well as grid cells indicating discrete and continuous index of area of occupancy.
Most of the Speckled Dace’s Canadian range is isolated from that of other Speckled Dace populations and other Rhinichthys species (Haas 2001; McPhail 2003) by a 30.5 m barrier at Cascade Falls, although the species also has been found in the five kilometre Canadian section of the Kettle River downstream of the falls (Peden and Hughes 1981, 1984, 1988). This downstream population is not believed to be self-sustaining but persists due to individuals being washed over the falls periodically (e.g., during spring snowmelt and flooding; Peden and Hughes 1984; Bradford 2006). Surveys further downstream into Washington State suggest that Speckled Dace is completely replaced by Umatilla Dace within eight kilometres of the border (Peden and Hughes 1984).
An earlier COSEWIC assessment estimated that Speckled Dace had a range of approximately 259 km of river length in Canada (COSEWIC 2006). More recently, Batty (2010) conducted surveys throughout the Kettle-Granby system, expanding the estimated range by 16 km (or 6% greater than the previous COSEWIC estimate). In particular, Batty (2010) recorded Speckled Dace in 118 km of the mid- and upper Kettle Rivers, 43 km of the West Kettle River, 59 km of the lower Kettle River and 55 km of the Granby River. Speckled Dace were found farther upstream (i.e., northward) than previously documented in both the Kettle and Granby rivers.
Andrusak et al. (2012) surveyed portions of the Kettle and Granby Rivers to characterize availability of suitable habitat for Speckled Dace. Over an approximately 33 km stretch of the Kettle River, they estimated that 748 ha of suitable habitat exists upstream of Midway, BC and 316 ha of suitable habitat exists downstream of Grand Forks, BC. Andrusak et al. (2012) estimated that 407 ha of suitable habitat exists in the approximately eight kilometre long section of the Granby River that was sampled.
Andrusak and Andrusak (2011) surveyed four sites along the lower 10 km of the Inonoaklin Creek (located approximately 75 km to the northeast of Carmi near Fauquier BC) but did not find any Speckled Dace. In British Columbia, the species has only been documented in the mainstem of the Kettle River and its two largest tributaries (Granby and West Kettle; MacConnachie pers. comm. 2015).
Extent of occurrence and area of occupancy
The extent of occurrence, based on the minimum convex polygon around all records within Canada’s extent of jurisdiction, is calculated as 2809 km2. The actual biological area of occupancy is estimated by multiplying the total stream length along which Speckled Dace have been recorded (275 km) by the mean wetted width of the Kettle River system (calculated as 30.5 m based on sub-drainage areas and average water yields; COSEWIC 2006), yielding a total area of 8.4 km2 occupied by all known populations. Three approaches were considered to estimate the index of area of occupancy (the surface area of grid cells that intersect the area occupied by the species). First, the continuous stretch of river between all observation records was determined, using a 2 km x 2 km grid, yielding an index of 528 km2 (continuous IAO, Figure 3). Second, the sum of the area of known occupation was overlain by a 2X2 km grid yielding a discrete estimate of IAO of 160 km2 (Figure 3). And third, if appropriately defined in the field, critical habitat can be assumed to represent IAO. Brown et al. (2012) purported to define critical habitat for the Canadian population of Speckled Dace, but we disagree with its use as a surrogate for IAO for COSEWIC assessment is inappropriate (see Habitat section for the rationale). Because it is likely that there is habitat within the Kettle River system that is unfavourable to the species, and also that the species occurs at sites not sampled within its range, the most appropriate estimate of IAO is likely between the discrete and continuous estimates.
Search effort
Prior to 2010, no widespread census of Speckled Dace had been conducted in Canada. Until that time, the known distribution of the species was based on fieldwork conducted during museum collections, Rainbow Trout (Oncorhynchus mykiss) population assessment studies, and an environmental impact assessment for a proposed dam at Cascade Falls (DFO 2008).
Peden and Hughes (1981) sampled an approximately 112 km stretch of the Kettle River (between Carmi and Cascade) and a 27 km stretch of the Granby River (north of Grand Forks), using minnow seines and electrofishing in September and October 1977. Sampling sites were based on river sections accessible by road. Speckled Dace were found at 15 out of 24 (62.5%) sample sites. The majority of individuals captured were young-of-the-year, and most were female (Peden and Hughes 1981; Table 1). The species was not observed during electrofishing surveys conducted for Rainbow Trout in August 2005 along four tributaries of the Kettle (Rendell, Rock, Boundary and McCarthy Creeks, within two kilometres of the confluence with the Kettle; BC Ministry of Environment unpubl. data).
| Survey Method | Area Covered | Number of Sites | Number of Sites with Positive Data | Year | Source |
|---|---|---|---|---|---|
| Minnow seine, electrofishing | ~112 km of Kettle River and ~27 km of Granby River | 24 | 15 | 1977 (September and October) | Peden and Hughes 1981 |
| Single-pass electrofishing | 275 km of Kettle River system | 39 | 29 | 2008 (July and August) | Batty 2010 |
| Electrofishing, snorkel surveys | 1 km of Kettle River, 1 km of West Kettle River | 2 | 2 | 2010 (July and October) | Andrusak and Andrusak 2011 |
| Electrofishing | 10 km of Inonoaklin Creek | 4 | 0 | 2010 (August) | Andrusak and Andrusak 2011 |
Batty (2010) sampled the Kettle River system in July and August 2008, using single-pass electrofishing. Batty (2010) sampled 28 sites quantitatively, which were widely distributed throughout four reaches of the river system: mid- and upper Kettle, West Kettle, lower Kettle and Granby River, at locations accessible by car. Exploratory sampling was conducted at a further 11 sites located in the headwaters of the watershed. At each quantitative sampling site, a 30 m length of the river was surveyed through stratified sampling. Continuous sampling was conducted along the shoreline (since preliminary fieldwork indicated Speckled Dace were more abundant here), while discrete sampling occurred in the river channel (i.e., in 1.5 m x 2.0 m quadrats every two metres across the channel at 0, 15 and 30 m from the downstream end of the site; Batty 2010). Speckled Dace were captured at 29 of the 39 sites (74%; Table 1). Batty (2010) calculated the capture efficiency of the electrofishing method with a mark-recapture study at one site. Seven trials were performed; a total of 203 fish were released and 16 were recaptured. Mean capture efficiency was low and highly variable, ranging from 0 to 0.214, with a mean of 0.079 (SD = 0.08).
To calculate the total range of Speckled Dace throughout the Kettle River system, Batty (2010) assumed a continuous distribution between the furthest downstream and furthest upstream capture locations. The resulting 275 km range calculated may be an underestimate, given that areas of river above the upstream limit of sampling were not surveyed (approximately 18 km for the West Kettle, 26 km for the upper Kettle and three kilometres for the Granby; Brown et al. 2012).
In a study to identify Speckled Dace habitat use and preference, Andrusak and Andrusak (2011) sampled a one kilometre section of both the Kettle and West Kettle Rivers, using electrofishing and snorkel surveys. Surveys were conducted in July and October 2010. For electrofishing, sampling occurred during the day at randomly sampled points six to seven metres apart which encompassed a range of available habitat types. For snorkelling, sampling occurred both during the day and at night (beginning one hour after dusk and ending approximately four hours later) and involved swimming upstream for the one kilometre length of river to record observations of the species (Table 1). A total of 347 Speckled Dace were captured or observed through the two survey methods (223 fish through electrofishing and 124 through snorkeling). Based on fish length, Andrusak and Andrusak (2011) calculated that the majority of the captured fish were juveniles (228 individuals or 66%). More fish were captured on the Kettle River (186 individuals) than the West Kettle River (161 individuals) overall, and no mature fish were found in the West Kettle River during the fall sampling period (Andrusak and Andrusak 2011).
Andrusak and Andrusak (2011) also investigated whether Speckled Dace were present in Inonoaklin Creek, outside the Kettle river watershed, using electrofishing in August 2010. Four sites, roughly 50 m in size, were sampled in the lower 10 km of the waterway and no Speckled Dace were found (Table 1).
There are several limitations to estimation methods used to document Speckled Dace distribution. First, backpack electrofishing is restricted to shallower waters, where smaller individuals tend to occur (and the probability of capturing fish via electrofishing decreases with increasing depth and velocity; Batty 2005; Korman et al. 2010). Conversely, snorkelling, which can be conducted in deeper waters, tends to sample larger individuals (Korman et al. 2010). Using only one of these methods could result in false negative data (i.e., observation of absence from sites when in fact the species may be present). Second, electrofishing was conducted only during the day, potentially missing distributional information if fish habitat use differs nocturnally. In addition, electrofishing was conducted at open rather than closed (e.g., with block nets) sites, which could lower catch rates (Benejam et al. 2011). Furthermore, confining sampling to a single season (e.g., summer or fall) could bias information, as Speckled Dace appear to undergo an ontogenetic shift in habitat use by season (Andrusak and Andrusak 2011).
Habitat
Habitat requirements
Available information on habitat is based on seasonal observations (spring through fall), primarily during daylight hours.
Speckled Dace is known to use a wide variety of habitats in the US part of its range, including small to medium rivers, permanent and intermittent streams, desert springs, and occasionally small and large lakes (Scott and Crossman 1973; NatureServe 2013).
In Canada, habitat use is influenced both by life stage and time of year (McPhail 2007; DFO 2013a; Tables 2 and 3). In general, immature fish are found in shallower slower moving waters than adults, over coarse gravel, small stones or cobble, with low to moderate embeddedness (percentage of surface covered in fine sediment) (McPhail 2007; Andrusak and Andrusak 2011; DFO 2013a; Table 2). Such habitat typically occurs along the river margin, in both pool and run features. Adult fish commonly inhabit deeper water with faster currents, in run, riffle or pool habitat away from the river edge, over boulder or cobble substrate with low embeddedness (Andrusak and Andrusak 2011; DFO 2013a; Tables 2 and 3). The low embeddedness (or high interstitial spaces) of the substrate is believed to be important in providing shelter and concealment from predators for both immature and adult fish (Andrusak and Andrusak 2011). It may also increase food availability by improving habitat quality for macroinvertebrates (Propst and Gido 2004).
| Life Stage | Season | Flow | Depth | Temperature | Substrate | Location | Notes | Source |
|---|---|---|---|---|---|---|---|---|
| Adult | Fall | 0-0.3 m/s 60% mean depth velocity (median 0.15 m/s); 0-0.15 m/sec bottom velocity (median ~0.03 m/s) | 0.05-0.65 m (median 0.3 m) | - | - | Kettle and Granby Rivers | Field sampling limited to maximum depth accessible in chest waders | Haas 2001 |
| Adult | - | 0.3-0.6 m/s 60% mean depth velocity (median 0.45 m/s); 0.25-0.6 m/s bottom velocity (median 0.4 m/s) | - | - | - | Lab (fish taken from Kettle River) | - | Haas 2001 |
| Adult | Late July-October | <0.25 m/s surface velocity; 0.02 m/s bottom velocity | 0.1-0.65 m | - | - | - | - | McPhail 2007 |
| Adult and juvenile | July-August | 0-1.1 m/s | 0.01-1.55 m | 12.7-22.6°C (mean 17.8°C) | Gravel to boulder | Kettle, West Kettle and Granby Rivers | - | Batty 2010 |
| Juvenile | July | <0.24 m/s (0.01 m/s preferred) | 0.05-0.64 m (0.07 m preferred) | - | Small gravel or cobble, mainly in run and riffle habitat along river margin | Kettle and West Kettle Rivers | Field sampling limited to 2 sites | Andrusak and Andrusak 2011 |
| Adult | July | 0-0.67 m/s (0.06 m/s preferred) | 0.2-0.5 m (0.45 m preferred) | - | Boulder and cobble, in run or riffle habitat | Kettle and West Kettle Rivers | Field sampling limited to 2 sites | Andrusak and Andrusak 2011 |
| Season | Habitat | Immature | Adult |
|---|---|---|---|
| Summer (July) | Pool | 9.4% | 0% |
| Summer (July) | Riffle | 35.9% | 20.0% |
| Summer (July) | Run | 54.7% | 80.0% |
| Fall (October) | Pool | 1.3% | 0% |
| Fall (October) | Riffle | 2.6% | 27.3% |
| Fall (October) | Run | 96.1% | 72.7% |
Batty (2010) found that Speckled Dace showed an overall preference for shoreline habitat compared with channel habitat in the Kettle River system, although results were not corrected for size or life stage (Table 2). Within the river channel, the probability of encountering Speckled Dace decreased with increasing depth and current velocity, but increased with substrate size. Sampling constraints, however, may have biased results, as the probability of capturing fish via electrofishing decreases with increasing depth and velocity (Price and Peterson 2010). Batty (2010) concluded that Speckled Dace prefer shallow slow flowing habitat, but recommended that further work be conducted to characterize habitat preferences over a wider range of river conditions, and at night.
Andrusak and Andrusak (2011) found that run and riffle habitat predominated, while pool habitat was less common, along the one kilometre sections of the Kettle and West Kettle Rivers that were surveyed. Correcting for habitat availability, they found that immature fish preferred the margins of pool habitat, compared with mature fish which preferred run habitat (Tables 2 and 3). In a broader scale study, covering 33.1 km of the Kettle River and 8.25 km of the Granby River, Andrusak et al. (2012) determined that more than 50% of available habitat was composed of run habitat, >40% of riffle and less than 7% of pool habitat.
In early spring (i.e., March), Speckled Dace have been recorded in deeper waters in the Kettle River system (more than one metre depth), behind structures such as large rocks, logs and bridge abutments (McPhail 2007). Immature fish have been found in seasonally flooded vegetation during the spring freshet (McPhail 2007). No specific information is available for spawning habitat, although it is believed to occur over clean gravel and cobble, where eggs are deposited in the interstitia (Bradford 2006; Brown et al. 2012; DFO 2013a). During the summer-fall period, young-of-the-year concentrate along the river edge in shallow, still or slow moving water, over clean cobble substrates (Peden and Hughes 1981, 1984; Peden 1994). Immature fish shift to deeper low velocity waters in the fall (Andrusak and Andrusak 2011). Adult microhabitat use may differ between males and females, because males are often missing from samples taken along river margins, suggesting they may prefer deeper or faster moving waters (McPhail 2007).
Boulders and large woody debris may be important habitat features for mature Speckled Dace (particularly during the winter), because these structures can create deep run and pool habitat (Andrusak and Andrusak 2011). Large woody debris is probably relatively rare in the Kettle River system. A short fire interval (<150 years in the upper and <25 years in the lower watershed) and historical riparian logging in the watershed means that currently there are few large trees that fall into the river naturally (Brown et al. 2012). Large woody debris has been added to the system in recent years, however, as part of habitat restoration efforts for Rainbow Trout (Andrusak and Andrusak 2011). These structures create areas of slow-moving waters and deep pools along river margins which are used by Speckled Dace (Rosenfeld and White pers. comm. 2016).
Andrusak et al. (2012) used information on habitat use, combined with flow monitoring data for the Kettle River system to derive estimates of available habitat for Speckled Dace under different discharge rates. They found that immature fish (<55 mm) had a narrow range of usable habitat in relation to discharge in all three rivers (<8 m width of river). Optimal usable widths occurred for immature dace at discharges below 10 m3/s, or 20% of long-term mean annual discharge rates (MAD). Mature dace (>55 mm) had a much wider and more variable range of usable habitat in relation to discharge (<15 m width in the West Kettle but up to 20 m width in the Kettle and Granby rivers). Optimal usable widths for adult fish occurred at discharge rates of 5-10 m3/s, or 20-30% of MAD. Available habitat declined significantly below 10% MAD, especially in the West Kettle River, where low flows may represent a limiting factor for immature Speckled Dace (Andrusak et al. 2012; Epp and Andrusak 2012).
Depth and water velocity preferences in the wild likely vary between systems depending on habitat availability, water temperature, food supply, fish size, abundance and presence of other fish species (Baltz et al. 1982; Moyle and Baltz 1985). For example, in Deer Creek, California, Speckled Dace habitat use is influenced by water temperature and the presence of its competitor, the Riffle Sculpin (Cottus gulosus). Speckled Dace dominate riffle habitat in lower sections of the stream, where summer temperatures reach 32° C. However, higher up the stream, where summer water temperatures are lower (29° C), Riffle Sculpin competitively exclude Speckled Dace from riffle habitat (Baltz et al. 1982).
Speckled Dace densities increased when elevated spring discharge levels were restored in the regulated San Juan River, Colorado (from mean daily discharge of 82.5 m3/s post-impoundment to 98.1 m3/s during the study period; Propst and Gido 2004). Fall densities were significantly positively associated with spring discharge in river channels regardless of habitat (i.e., riffles, runs, pools) or substrate (i.e., cobble, gravel, sand, silt). High spring flows likely improve habitat quality and foraging opportunities for Speckled Dace by flushing fine sediment from cobble and gravel substrates, reducing embeddedness. Fall densities declined, however, with extended periods of low summer discharge (< 14 m3/s; Propst and Gido 2004).
Proposed critical habitat for Speckled Dace was calculated across its Canadian range using key habitat requirements identified from the literature (Table 4) and a minimum population target of 7000 adults in each tributary (Brown et al. 2012; DFO 2013a). Suitable habitat appears abundant in the Kettle River system because the species utilizes the full width of the river (i.e., river margin and channel) and tolerates a relatively wide range of discharge, depth, substrate and temperature conditions (Brown et al. 2012). Determination of critical habitat is based on the length of river necessary to provide enough habitat to maintain a minimum viable population in each of the locations where it currently resides, based on an abundance estimate of 3 fish/m (which is derived from Batty’s (2010) estimate corrected for capture efficiency; Brown et al. 2012). For each of the three rivers where Speckled Dace has been documented, a 2.4 km stretch of habitat was identified, starting at the uppermost site where the species was located by Batty (2010) and extending downstream. Although presented as a measure of critical habitat in this DFO report (Brown et al. 2012), this appears to be incorrect for two reasons. First, the minimum spatial extent necessary to maintain a viable population, while a useful analysis, does not in any way represent critical habitat for long-term persistence. And secondly, identifying the uppermost river section conforming to this minimum spatial extent is arbitrary and does not represent critical habitat. Therefore, despite purporting to identify critical habitat, Brown et al. (2012) does not provide a useful analysis of the particular habitat necessary to successfully complete the Speckled Dace life cycle and ensure population viability throughout its range in Canada. It is therefore not useful in our assessment of IAO for a COSEWIC assessment.
| Life stage | Function | Feature | Attribute |
|---|---|---|---|
| Immature | Rearing | Pool, run, margin | Small gravel/cobble; flow <0.24 m/s and depth < 0.4 m; low to moderate embeddedness |
| Mature | Rearing | Run and riffle | Boulder/cobble; flow 0.18-0.45 m/s; depth 0.2-0.5 m (although may be found at > 1m depths); low embeddedness |
| - | Spawning | Run and riffle | Large clean cobble |
Annual flow patterns in the Kettle River system are similar to those in most interior streams, with high flows in spring following snowmelt (May to July) and low flows from late summer through to spring (August through March; Figure 4). The spring freshet, representing 78% of the annual discharge, tends to peak in late May or early June, with flows exceeding 200 m3/s (DFO 2008, 2013). By late summer, base flows have declined to 5 m3/s or less for all three rivers. Heavy rain in fall periodically leads to temporary increases in stream flows (Andrusak and Andrusak 2011). The upper Kettle River (encompassing the Kettle and West Kettle rivers upstream of Midway) has a MAD of over 40 m3/s, while the West Kettle River has a MAD of over 14 m3/s. The Granby River has a MAD of more than 30 m3/s (Andrusak et al. 2012).
The headwaters of the Kettle River system are located at higher elevations than downstream sites and consequently are generally much cooler in summer. Peden and Hughes (1981) reported a 4° C difference in average summer temperature between upstream (14° C at 884 m elevation) and downstream (18° C at 457 m elevation) sites. Summer surface water temperatures typically rise above 20°C in the Kettle River system, reaching 24°C in the lower Kettle River and over 26° C in the West Kettle River (Dessouki 2009; Andrusak and Andrusak 2011). Although these temperatures exceed BC aquatic life water guidelines, cyprinids in general, and Speckled Dace in particular, seem able to tolerate such elevated thermal conditions (John 1964; Andrusak and Andrusak 2011; BC Ministry of Environment 2016). British Columbia now has a seasonal closure on angling in the system due to excessive water temperatures.
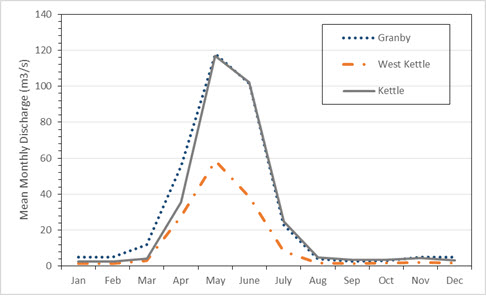
Long description for Figure 4
Chart illustrating mean monthly discharge at three Water Survey of Canada stations in the Kettle River system, from 2000 to 2014. The stations are the Granby River station, the West Kettle River station, and the Kettle River station.
Long-term water quality monitoring (1990-2007) at two sites on the Kettle River (Midway and Carson, both close to the border with Washington State) indicates that water quality is generally good for the waterway (Dessouki 2009). Water chemistry measurements exhibit high seasonal variability correlated with changes in stream flow. Dissolved fluoride levels (naturally occurring) often exceeded BC aquatic life guidelines during low flows, although fish populations in the river may be adapted to these occurrences (Maciak et al. 2007). Several total metal concentrations (e.g., aluminum, cadmium, chromium, iron) also exceeded guidelines seasonally, but these increases are strongly correlated with turbidity (which peaked during high flow periods). Consequently, metals are likely bound to particulate matter and unavailable to biota (Dessouki 2009). Seasonal declines in alkalinity linked to low flow suggest that the river may be moderately sensitive to acid inputs, which are often amplified during spring freshet. Several water quality parameters had significant increasing trends over the sampling period at one or both sites: total hardness (possibly linked to declining flows), turbidity and dissolved fluoride (Dessouki 2009). From 1979-2006, the median pH at the Midway and Carson sites ranged from 7.9-8.0, while median turbidity was 0.5-0.6 NTU, median total hardness was 57.7-69.2 mg/L and median total alkalinity in the range of 58.1-74.2 mg/L (Summit Environmental Consultants Inc. 2012).
Habitat trends
Trends in the availability of suitable habitat can be inferred from patterns of human activity in the watershed. The first Europeans to settle the area were farmers, but around the turn of the 20th century an industrial boom occurred that included the construction of railroads, mines, a smelter and a power plant. Much of the watershed’s lower elevations have been logged and converted to ranchland (Andrusak and Andrusak 2011). Today, the economy of the region is focused on logging, ranching and tourism. Mining activity has declined considerably since the 1900s, but could re-emerge if metal prices rise in the future (DFO 2013a). The human population in the Kettle River watershed has fallen slightly in recent years, while forestry production has dropped by over 40% in the last decade (DFO 2013a).
The main factors affecting trends in Speckled Dace habitat are water withdrawals, climate change and forestry (DFO 2013).
Significant water withdrawals for agricultural purposes, particularly for irrigation, have an impact on habitat availability during the summer months when flows are already naturally low. During low flow periods, licensed surface water withdrawals account for less than five to up to 30% of the average annual flow of the system (Summit Environmental Consultants Inc. 2012). Agriculture accounts for 80-90% of authorized consumptive use of surface water annually in the Kettle River system (Brown et al. 2012; DFO 2013a). Low flows have worsened in the last 75 years, partly due to the increase in water allocations, to a point where the system is now considered ‘regulated’ (i.e., as opposed to ‘natural’, due to the amount of water being withdrawn from the system; Summit Environmental Consultants Inc. 2012). Total water withdrawal (based on the area of land under irrigation), however, has declined since 1981 (Brown et al. 2012). Only approximately 50% of all licensed allocations are currently being used, suggesting adequate flow could further decline if demand for water use increases (Brown et al. 2012). The BC Ministry of Environment has identified the Kettle River as a priority system for management because of the critical low flow that results from intense agricultural demands, combined with the projected impacts of climate change (Andrusak and Andrusak 2011).
In the BC portion of the watershed above Cascade Falls, the rate of growth in the area of land under irrigation licences increased gradually from about two to over 65 hectares/year between 1929 and 1962; between 1963 and 1981 the area under irrigation grew at an average rate of 236 hectares/year, then declined at an average rate of 26 hectares/year as water users in the basin switched from diverting surface water to using groundwater (Aqua Factor Consulting Inc. 2004). There appears to be a strong linkage between the aquifers and the flow in the mainstem rivers, and the switch to groundwater sources may not resolve the chronic low flow problems in the system (Brown et al. 2012). While changes to groundwater levels generally track trends in surface water levels, the relationship between the two is not well understood. Currently, the reduction in flow attributed to groundwater use is believed to be less than if the same volume was pumped directly from the river (Summit Environmental Consultants Inc. 2012). The extent of groundwater extraction, however, cannot be determined because a licence to use groundwater was not required at the time that Brown et al. 2012 was written.
Habitat has been directly and indirectly affected by the forestry industry. Logging leads to loss of woody debris in the riparian zone, which may be important for Speckled Dace habitat use. In addition, logging causes erosion along river banks, increasing siltation and the embeddedness of rocky substrates in rivers, widening the river and lowering water levels. Since 1994, the impact of forestry on riparian areas of fish-bearing streams has declined significantly with the introduction of protective provisions under provincial forestry legislation (Summit Environmental Inc. 2012). Riparian buffers, however, are not required along first and second order streams. Although Speckled Dace are not known to occur in these smallest reaches of the Kettle River system, these streams comprise 80% of the total stream length in the watershed and forestry activity along their banks could influence downstream aquatic conditions (Coleshill and Watt 2015).
Construction of forestry roads is particularly damaging to fish habitat. Sediment from forestry roads constructed along the Granby River smothered cobble and boulder substrate, resulting in the almost complete disappearance of Speckled Dace in affected areas (Brown et al. 2012). A recent riparian threat assessment for the Kettle River watershed identified 15,000 km of resource roads in the watershed, with 221 km within unstable or potentially unstable terrain, and 5107 km within stream riparian areas (Coleshill and Watt 2015). Forestry activity in the watershed is currently concentrated in the headwaters of the Kettle River system, in Douglas Fir and Montane Spruce biogeoclimatic zones (Brown et al. 2012). The southern portion of the watershed, where most Speckled Dace occur, is characterized by the Ponderosa Pine and Bunchgrass zones, which are not suitable for forestry. Logging is unlikely to occur within the proposed critical habitat sections of the Granby River further upstream (Brown et al. 2012).
Climate change is expected to result in increased temperature (British Columbia 2002) and longer and drier summer and autumns resulting in reduced stream flows and contraction of river margins and riffle habitat during these lowest periods of the hydrograph (Leith and Whitfield 1998; Whitfield et al. 2001, Brown et al. 2012). For example, the Lower Kettle River had the lowest water levels in history in 2015. Water extraction for irrigation is also highest during the driest period of lowest flows.
A proposal to develop a run-of-riverFootnote1 28 megawatt hydroelectric generation project at Cascade Falls, downstream of Grand Forks, was approved by the province in 2006. The power project would consist of a weir and intake at the top of the falls, and would be built on the historic site of an abandoned power station, which was constructed in 1899 and operated until 1919.
The footprint of the weir will result in the loss of 537.5 m2 of potential Speckled Dace habitat, representing less than 2% of its total available habitat in the Kettle River system (PDI 2005; Bradford 2006). The weir, however, is located in an area considered to be marginal habitat because Speckled Dace here are already susceptible to being swept over the falls (Hamilton and Associates Ltd. 2005).
Overall, the proposed hydro project is expected to have minimal impact on Speckled Dace populations and habitat for several reasons: (i) important areas including possible spawning habitat and areas where the highest densities of Speckled Dace have been found are located upstream of the backwater area; (ii) the headpond will remain flowing at all times; and (iii) the weir can be deflated during high flows to prevent accumulation of fine sediment on the stream bed within the headpond (Hamilton and Associates 2005; Bradford 2006). Mitigation measures proposed for the power project include establishing a monitoring program to assess changes to Speckled Dace habitat, abundance and entrainment, and habitat restoration initiatives (PDI 2005; DFO 2008).
Low flows and high temperatures during the summer-fall periods have characterized the Kettle River system in recent years (Andrusak et al. 2012). A 2003 drought resulted in the lowest flows on record for the river, but five years later the Speckled Dace population appeared robust, suggesting the species is capable of repopulating quickly following severe drought (Batty 2010). While Speckled Dace seems adapted to drought conditions, there may be limits to what the species can tolerate (Andrusak et al. 2012; Brown et al. 2012). For example, the combination of increased droughts due to climate change and increased water extraction could reduce habitat availability for the species in future.
Instream flow standards have been established for fish populations in BC, although these are largely based on salmonid requirements (Hatfield et al. 2002). A 20% MAD has been recognized as the minimum necessary for optimal riffle conditions, fish incubation, summer-fall rearing of juveniles and fish overwintering, while 10% MAD is sufficient for short-term biological maintenance (Government of BC undated; Ptolemy and Lewis 2002). Flow rates under 10% MAD, however, commonly occur in the Kettle River system during low flow periods (DFO 2008; Andrusak et al. 2012). Riffle habitat in much of the Kettle River is reduced to < 10 cm depths in late summer, and Speckled Dace have been reported stranded in isolated pools during extreme low flow periods (DFO 2008; Andrusak and Andrusak 2011).
Habitat restoration projects have occurred and are planned in the Kettle River system which may benefit Speckled Dace habitat. A stretch of the West Kettle River 16 km south of Beaverdell was a demonstration site profiling various fish habitat rehabilitation techniques under the provincial Watershed Restoration Program in the late 1990s and early 2000s. Approximately 3 km of riverbank was revegetated with Cottonwood, Willow, Dogwood and native grasses, while approximately 7.5 ha of the river was restored for Rainbow Trout habitat (Cleary and Underhill 2001). Restoration work included the addition of submerged habitat reefs and large woody debris-boulder complexes to provide cover and increase habitat complexity, as well as bank stabilization debris groins to reduce erosion (Zaldokas 1999; Underhill 2000; Cleary and Underhill 2001). Pool/run habitat was estimated to have increased by 15% as a result of restoration efforts (Cleary and Underhill 2001).
Andrusak and Andrusak (2011) described a three-year project along part of the Kettle River (seven kilometres upstream from Midway, BC) to increase overwinter habitat for Rainbow Trout through the addition of large woody debris. More recently, the Regional District of Kootenay Boundary received funding from Environment Canada and Trout Unlimited Canada to undertake habitat restoration activities for Speckled Dace along two-kilometre stretches of the Kettle, West Kettle and Granby rivers. The work will entail stabilizing river banks (to stop erosion and the influx of silt) and building up gravel bars (to create deeper pools and increase stream flow) through the planting of native species, such as Cottonwood, Willow and Dogwood (Dalziel 2015).
Biology
There is little information on the basic biology of Speckled Dace in the wild in Canada. The only published sources of information on its biology in Canada are Peden and Hughes (1981, 1984), Peden (1994) and McPhail (2007). McPhail (undated) produced a summary on Speckled Dace [PDF, 284 Kb] that is available on the UBC website.
Life cycle, demographic parameters and reproduction
Increasing photoperiod and increasing water temperature both induce spawning in Speckled Dace (John 1963; Kaya 1991). Under laboratory conditions, Speckled Dace spawned from April to July when maintained at 21 to 29ºC under a natural photoperiod, indicating that spawning can be protracted (Kaya 1991). Individuals kept at 15ºC in a photoperiod of 14 h light and 10 h dark from June through the summer spawned within one to two days once the water temperature was increased to 18 or 24ºC in late August-early September (Kaya 1991). A bimodal reproductive cycle has been described for Speckled Dace in Arizona, with discrete peaks in spawning occurring in early spring and late summer under normal precipitation (John 1963). During prolonged periods of drought, however, populations did not reproduce and mortality was high (John 1963).
Speckled Dace spawns over clean gravel in shallow water in the US part of its range (McPhail 2007). Nest preparation by males has been documented in Arizona, but no evidence of nests was found in New Mexico, where the species has been observed forming spawning clusters of more than 25 fish (John 1963; Mueller 1984). Based on ovarian maturity in female Speckled Dace spawning probably starts in mid-July (Peden and Hughes 1981). Data collected on fish in spawning condition during sampling are consistent with this timeline (PDI 2005). Females considered to be in spawning condition contained relatively few large eggs (usually <500) around 1.5 mm in diameter (Peden and Hughes 1981). The number of large eggs in fall-caught females ranged from about 450 to 2,000, suggesting a single ovarian cycle per year. Newly fertilized eggs are about 1.8 mm in diameter, adhesive and denser than water; in aquaria eggs were deposited at the base of available stones, on filters and in corners (Kaya 1991). A ‘spawning ball’ of bright red Speckled Dace was observed in the West Kettle River in mid-July in water temperatures ranging from 18-24°C. No dug nest was apparent in the gravel substrate, suggesting that the species might practice broadcast spawning in Canada (White and Andrusak pers. comm.)
Egg development is rapid following fertilization, as hatching occurs in four to five days at 24ºC and six to seven days at 18ºC. Newly hatched larvae are about six mm long and become free-swimming about a week later (depending on temperature). They emerge from the substrate at approximately eight mm and begin to actively feed (McPhail 2003).
Newly emerged fry appear in the Kettle River system in early August at a size of around nine mm; by late October they are about 20-30 mm in fork length (McPhail 2003). At least three size classes or age groups are believed to exist, based on length-frequency analysis and otolith examination (PDI 2005; Batty 2010). Most males in the Kettle River mature at the end of their second summer (at age 1+) and spawn for the first time the next summer (age 2+). Females typically become sexually mature one year later than the males. Speckled Dace do not mature until they are around 40 to 50 mm in length (Peden and Hughes 1984). While there are no detailed data on age structure, field sampling indicates that the adult population is comprised mostly of fish <60 mm in fork length (those in their second or third summer); females, which occasionally reach fork lengths over 90 mm were previously thought to be in their fourth summer (age 3+) (Peden and Hughes 1981, 1984; Peden 1994; McPhail 2003). However, based on otolith readings, Batty (2010) estimated the age of one fish over 90 mm collected to be 7+ years. This longevity estimate is considerably higher than in the US, where the species is believed to live a maximum of three to four years in most streams (Batty 2010).
Female Speckled Dace tend to be caught more frequently than males (e.g., Peden and Hughes 1981, 1984; McPhail 2007), suggesting that there may be sexual differences in microhabitat use (e.g., with males occupying deeper or faster waters). Alternatively, mortality rates may be higher in male individuals, although this has not been studied.
Physiology and adaptability
Little information exists on the physiological requirements of Speckled Dace. In a comparison of flow and depth preferences among three dace species (Speckled, Leopard and Umatilla), Haas (2001) documented that Speckled Dace had the lowest flow tolerance (median of 0.4 m/sec at river bottom) and shallowest water depth requirements (median depth ~ 30 cm). Speckled Dace exhibit morphological plasticity to flow conditions, with streamlined forms (having large curved fins) occurring in swift water and robust forms (having small rounded fins) occurring in slow moving water (Smith and Dowling 2008). Speckled Dace have been observed pressing their pectoral fins against the bottom during high flows, which may enable them to inhabit higher velocity areas and/or avoid being washed away during flooding (Ward et al. 2003). Speckled Dace was the least tolerant of low dissolved oxygen levels in a comparison of four freshwater fish species in Arizona (2.0 mg/L; Lowe et al. 1967).
Adaptability to changes in habitat has not been investigated in Speckled Dace in Canada. Generalizations from case studies in the US may be misleading due to the extent of adaptive diversity observed among populations in different drainages (Peden 2002; McPhail 2003). Because Speckled Dace are warm-water adapted they may benefit from the warmer temperatures associated with climate change (Brown et al. 2012). Whether they can also adapt to the associated decrease in summer flows and the consequent degradation of habitat and reduction in food supply from riffles is unknown (although such conditions presumably occur in the southern part of the Speckled Dace range). Peden (2002) speculated that the current presence of large Speckled Dace in the area above the old dam near Cascade Falls may demonstrate the ability of the species to respond to habitat improvement or the restoration of natural flows following weir/dam removal. Batty’s (2010) population estimates suggest that Speckled Dace were numerous in the Kettle River system (over 900,000 individuals) five years after the 2003 drought, which produced the lowest flows on record. Batty (2010) concluded that the species is tolerant of, or at least resilient to, drought conditions.
Dispersal and migration
There are no reports of Speckled Dace migrations in the literature, although Minckley (1973) referred to the ability of Speckled Dace to recolonize isolated refuges in Arizona rivers following devastating floods. It is likely that some larval fish drift downstream from their natal sites (DFO 2013a). Young-of-the-year disperse from shallow, low velocity habitat to deeper, faster water as they grow (Peden and Hughes 1981, 1984). Most Speckled Dace in Canada are reproductively isolated from other populations downstream of Cascade Falls. It is likely that some individuals occasionally spill over the waterfalls, but these fish would be unable to return to the population upstream (Peden and Hughes 1988). Movement across the US border between the Canadian and American sections of the Kettle River above Cascade Falls is possible.
Interspecific interactions
Speckled Dace co-occur with Longnose Dace, Chiselmouth (Acrocheilus alutaceus), Pikeminnow (Ptychocheilus oregonensis), Redside Shiner, Longnose Sucker (Catostomus catostomus), Bridgelip Sucker (Catostomus columbianus), Largescale Sucker (Catostomus macrocheilus), Rainbow Trout, introduced Brook Trout (Salvelinus fontinalis), Mountain Whitefish (Prosopium williamsoni), Columbia Sculpin (Cottus hubbsi) and Slimy Sculpin (Cottus cognatus) in the Kettle River system above the falls (Peden and Hughes 1981). Speckled and Umatilla Dace coexist for a short section below Cascade Falls, but Umatilla Dace appear to completely replace Speckled Dace eight kilometres below the US border likely through competitive exclusion (Peden and Hughes 1988).
Speckled Dace are omnivorous, feeding mainly on aquatic insects and filamentous algae (McPhail 2007). Information on predators is generally lacking, although Rainbow Trout have been documented feeding on the species (Turek et al. 2015).
Species interactions have not been studied in the Kettle River system. Baltz et al. (1982) found that competitive interactions between Speckled Dace and Riffle Sculpin for preferred microhabitat in a California stream were influenced by water temperature. Speckled Dace dominated riffles in warmer downstream waters, while Riffle Sculpin dominated in cooler upstream riffles (where Speckled Dace was limited to riffle edges; Baltz et al. 1982). The restricted distribution of introduced Speckled Dace in the Eel River system, California, may be caused by predation by the introduced Sacramento Pikeminnow and competition and predation by native sculpins (Cottus spp.; Harvey et al. 2004; Kinziger et al. 2011). In the Colorado River, Arizona, predators of Speckled Dace include Rainbow Trout, Brown Trout (Salmo trutta) and Channel Catfish (Ictalurus punctatus), all introduced species (Marsh and Douglas 1997).
The Asian Tapeworm (Bothriocephalus acheilognathi) infects Speckled Dace in the Colorado River system of Utah, Nevada and Arizona (Brouder and Hoffnagle 1997). From 1990-1994, Clarkson et al. (1997) found an average of 8% of individuals were infected in the Little Colorado River within the Grand Canyon. Mortality rates due to the parasite are unknown, but it can cause emaciation, anemia, reduced growth and reproductive capacity and depressed swimming ability (Clarkson et al. 1997). Afflicted fish are also more susceptible to secondary bacterial infections (Clarkson et al. 1997). Cyprinids are the tapeworm’s most common host (Clarkson et al. 1997).
Population sizes and trends
Sampling effort and methods
The most recent and comprehensive population estimate for Speckled Dace in Canada was conducted by Batty (2010). Twenty-eight sites throughout the Kettle River watershed were sampled on July 14-21, 2008 and August 5-8, 2008 using single-pass electrofishing. At each site, a 30 m length of river was surveyed with a stratified design, covering both shoreline and channel habitat. Batty (2010) also calculated the capture efficiency of this sampling method by conducting a mark-recapture study at one site from August 25-27, 2008. Seven trials were carried out at the site and 26-30 Speckled Dace were captured per trial. Individuals were then marked by taking a clip of one pectoral fin, and kept overnight in a container. The following day, fish were released into a 15 m long by three m wide enclosure along the shoreline, and left to acclimatize for three hours. Electrofishing was then conducted in the enclosed area and the number of recaptured Speckled Dace was recorded (Batty 2010).
Previous population estimates for Speckled Dace in Canada have relied on limited data derived from museum collections and environmental impact assessment studies of the Cascade Falls hydroelectric project. Bradford (2006) provided an estimate using survey data from the 1990s taken along a 10 km stretch of the Kettle River upstream of the falls, extrapolated over the 284 km of river length known to be used by the species (including the ~45 km stretch of the river in the US).
Abundance
Batty (2010) estimated that the number of mature individuals in Canada was 940,000 (90% confidence interval: 412,000 – 1,955,000), which is at least 40 times greater than previous estimates. Within each of four reaches of the Kettle River system (i.e., mid- and upper Kettle River, West Kettle River, lower Kettle River and Granby River), Batty (2010) estimated that the population of mature Speckled Dace was at least 110,000. The estimated linear abundance of mature Speckled Dace was 0.22 individuals/m in the Kettle River system, which is higher than densities recorded for populations in the core of the species’ range (Batty 2010). Mature individuals were estimated to comprise 32% of the population (Batty 2010).
The population estimates calculated by Batty (2010) assume that samples were collected in a random and unbiased manner and that sampled sites were representative of the overall available habitat, both in physical and biological features. Batty (2010) assumed that all fish > 56 mm in length were mature.
The mean capture efficiency of the study was low and highly variable, ranging from 0-0.214, with a mean of 0.079 (SD = 0.08). Capture efficiency tends to be reduced in shallow streams (Price and Peterson 2010). Speckled Dace are also bottom-dwelling fish that hide in the substrate, which could further influence capture rates (Batty 2010). Batty (2010) indicated that the abundance estimates were inversely proportional to capture efficiency, with minor changes in the latter resulting in major changes to the former. Consequently, he recommended that future research estimate capture efficiency with more precision, or utilize a sampling method with higher capture efficiency (Batty 2010).
Previous estimates of the Canadian population of Speckled Dace ranged from 3,000-10,000 (Cannings and Ptolemy 1998) to 11,550-23,100 (Bradford 2006). The Bradford (2006) estimate was based on limited sampling along river margins only.
Fluctuations and trends
There have been no long-term studies of Speckled Dace or detailed studies of their habitat that could provide information on trends in abundance. Surveys from 1978 to 1980 indicated populations were stable over that short period (Peden and Hughes 1981). Peden and Hughes (1984) speculated that there may be fluctuations in survival of young-of-the-year fish as a consequence of variability in spring flooding, but also noted that Speckled Dace evolved within the natural flood regime of the river and may have developed adaptations to cope with natural patterns of disturbance. In other systems annual estimates of Specked Dace abundance can fluctuate considerably (e.g., Pearsons et al. 1992).
Rescue effect
Most Speckled Dace in Canada are isolated above a 30.5 m high barrier at Cascade Falls. The Kettle River above Cascade Falls does loop down into the US and Speckled Dace in this section are likely able to move across the border into Canada. This section is only about 45 km long. These fish, however, would likely be affected by the same event affecting Speckled Dace in Canada, if the event were to occur upstream of the US section of the river. Rescue of Canadian populations from downstream populations in the US below the falls is not possible given the physical barrier of the Cascade Falls (DFO 2013a). Furthermore, the small portion of the population occurring downstream of the falls appears to be isolated from downstream US populations through competitive exclusion by Umatilla Dace (Peden and Hughes 1984). This population may represent a sink, as it apparently persists because individuals are swept over the falls (Peden and Hughes 1984; Bradford 2006). Within Canada, larval fish from upstream sites could potentially repopulate lower stretches of river affected by a catastrophic event if they drift downstream (DFO 2013a).
Threats and limiting factors
Threats
The main threats to Speckled Dace in Canada are water withdrawal exacerbated by climate change-related reduction in summer and autumn flows and inputs of fine sediment from forestry operations (Brown et al. 2012; DFO 2013a – see Appendix A for the Threats Assessment Calculator). These threats, however, do not apply equally across the species’ Canadian range, with water demands being greater in the lower reaches of the watershed, logging activity concentrated in the upper reaches of the watershed, and climate change effects likely a basin-wide threat.
The Kettle River system by nature is considered flow-sensitive (i.e., it typically experiences extreme low flows, <10% MAD, in late summer), which is exacerbated by increasing human demands for water-taking. Agricultural activity accounts for approximately 80% of total annual water usage, with the bulk of it occurring during seasonal low flow periods in late summer (DFO 2013b). Agriculture occurs primarily in the lower half of the watershed, affecting approximately half of the Speckled Dace population. While only a portion of licensed surface water withdrawals are currently in use, adequate flow for Speckled Dace could be threatened if consumption rises due to increasing municipal, residential, commercial, irrigation and industrial needs (Brown et al. 2012). Recent assessments of water supply and demand in the Kettle River watershed predicted that future demands could increase by 75 to 116% by 2050 due to agricultural expansion and climate change (van der Gulik et al. 2013; Watt and KRWMP 2014a).
A further concern is the growing practice of groundwater extraction, which until recently was unregulated by the BC government. However, the province’s Water Sustainability Act, which came into force in early 2016, enables groundwater licensing and the protection of environmental flows. The two largest cities in the watershed, Midway and Grand Forks, currently draw their water supply from local aquifers, despite holding licences for surface water diversion from the Kettle River. The City of Greenwood also relies on groundwater extraction, as do an increasing number of ranches in the area (DFO 2013a). There appears to be a close connection between aquifers, groundwater recharge and surface flow in the Kettle River system, with fluctuations in surface water levels matched by fluctuations in groundwater levels (Figure 5; Brown et al. 2012; Watt and KRWMP 2014a). The aquifers under highest use in the region are tightly linked with surface waters, suggesting that increased groundwater extraction could worsen low flow conditions (Watt and KRWMP 2014a). Water withdrawal from both surface and groundwater sources may pose a particular problem for Speckled Dace in the lower portion of the Kettle River watershed, where water demands are greater and rivers are wider, shallower, less sheltered and have more porous substrates than further upstream (Brown et al. 2012).
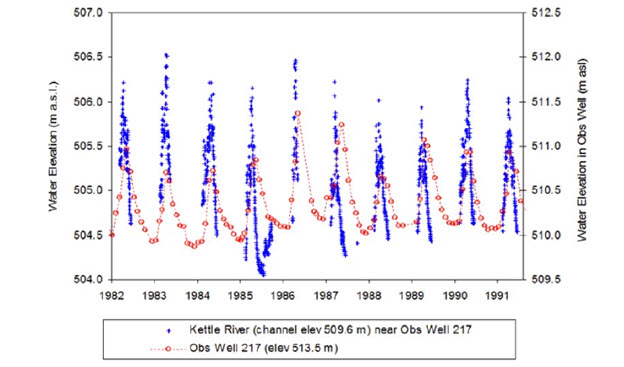
Long description for Figure 5
Chart illustrating surface and groundwater fluctuations in the Kettle River watershed from 1982 to 1992. Data are plotted for both surface water data and groundwater data.
The Tennant Method for instream flow assessment describes instream flows of 30% MAD as the generic threshold at which depth and velocity in riffles are adequate to maintain good habitat for fish and aquatic insects (Tennant 1976). Flows of 10% MAD provide poor or minimum habitat for fish and wildlife (short-term survival only in most cases), while habitat is considered severely degraded at flows below 10% MAD (Tennant 1976; Annear et al. 2004). Summer flows in the Kettle River, however, regularly fall below 10% MAD, and extreme low flows and high water temperatures have been common in the system over the last decade (Figure 6; Andrusak et al. 2012; Epp and Andrusak 2012).
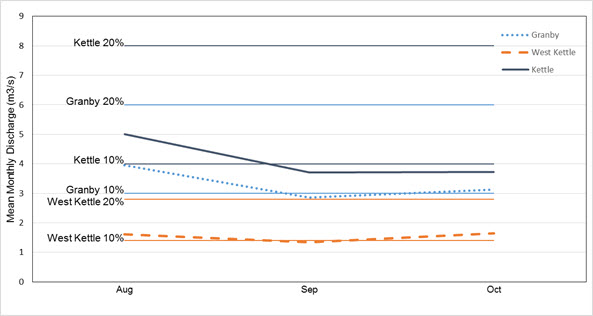
Long description for Figure 6
Chart plotting mean monthly discharge during low flow periods (2000 to 2014) for three rivers in the Kettle River system, with 10 percent and 20 percent mean annual discharge rates indicated for each river.
Speckled Dace is considered a drought-tolerant species, but increasing water demands combined with climate change could exacerbate low flow conditions to the point that Speckled Dace habitat availability is affected (Batty 2005; Andrusak et al. 2012; Brown et al. 2012).
Climate change is expected to increase the severity, duration and frequency of drought conditions, intensifying summer low flow conditions (Brown et al. 2012). The flows measured in the Fraser River at Hope indicate that the date by which one-third and one-half of the annual cumulative flow occurs has advanced by 11 and nine days respectively each century, consistent with an earlier snowmelt (Aqua Factor Consulting Inc. 2004). The earlier onset of the spring freshet is believed to contribute to lower summer flows, a pattern observed in south-central BC streams (Aqua Factor Consulting Inc. 2004; Epp and Andrusak 2012).
Reduction in flow may adversely impact Speckled Dace through habitat loss and degradation, as well as changes to food supply (Brown et al. 2012). Low flow conditions can reduce riffle habitat, which is used by adult fish for spawning and rearing (Andrusak and Andrusak 2011; Brown et al. 2012). Low flows also limit habitat for immature fish, which rely on shallow waters along river margins; this is believed to be a particular problem in the West Kettle River during the summer (Andrusak et al. 2012). In addition, low flow conditions can result in degraded water quality and reduced dissolved oxygen levels (Wetzel 2001). Speckled Dace were absent from waters immediately downstream of sewage discharge at Grand Forks (at the junction of the Kettle and Granby rivers), but this may have been due to lack of suitable habitat rather than a response to pollution (Peden and Hughes 1981). The species was found to be least tolerant of low dissolved oxygen levels in an experimental study comparing responses of four freshwater fish species in Arizona (Lowe et al. 1967). Nonetheless, as a species adapted to drought and high temperatures that is at the northern limits of its range, Speckled Dace may be less vulnerable to low flow conditions than other fish in the Kettle River system (Brown et al. 2012). Batty (2010) for example, found that Speckled Dace were relatively abundant in the watershed five years after a severe drought which produced the lowest flows on record.
Increasing water temperatures associated with climate change are not expected to have a negative impact on Speckled Dace, because it is a warm water species more tolerant of high water temperatures than other fish species in the Kettle River system. Recent fish kills associated with daily temperature maxima exceeding 26°C, for example, have affected salmonids and whitefish, but not Speckled Dace (Andrusak et al. 2012; Epp and Andrusak 2012). As a species adapted to warmer water, Speckled Dace may also gain a competitive advantage over other fish species from rising temperatures (Brown et al. 2012). Projected increases in winter water temperatures would also benefit Speckled Dace by decreasing the risk and severity of winter ice, while increasing the growing season of juveniles (Brown et al. 2012).
Forestry was a major activity in the Kettle River watershed in the past, but has declined by 40% since the mid-2000s (DFO 2013a; Watt and KRWMP 2014b). Today, timber harvesting is concentrated in the northern portion of the Kettle River watershed, affecting the headwaters of the Kettle River and its tributaries. Most of the Speckled Dace’s Canadian range, which falls within the more arid southern portion of the watershed, is not directly affected by logging (Brown et al. 2012).
Forestry operations have led to increased siltation and embeddedness in parts of the Kettle River system. Construction of logging roads is considered the most damaging aspect of forestry activity for Speckled Dace, because it can result in the deposition of sand that smothers their preferred cobble and boulder habitat (Brown et al. 2012). Low embeddedness of rocky substrate is believed important for both immature and adult fish, because it provides shelter, a place to hide from predators and potentially improved access to food (Propst and Gido 2004; Andrusak and Andrusak 2011).
Additional threats include invasive alien species, resource extraction, and hydro development (Brown et al. 2012).
Several non-native fish species could pose a predatory threat to Speckled Dace if they were to spread into the Kettle River system above Cascade Falls. For example, Smallmouth Bass (Micropterus dolomieu) feeds primarily on minnow species and occupies similar habitat to Speckled Dace. It was introduced to Christina Lake in the early 1900s and now occurs below Cascade Falls (Brown et al. 2012). Northern Pike (Esox lucius), a voracious predator, was illegally introduced to Washington State and is expanding its distribution northwards via the Columbia River and its tributaries. Northern Pike is now found in the lower Kettle River just south of the US border, where it is reported to feed on Speckled Dace (Christina Lake Stewardship Society 2015). The abundance of native minnows in the Pend Oreille River, Washington has declined since the Northern Pike’s introduction (Washington Dept. of Fish and Wildlife 2016). The predator could threaten Canadian populations of Speckled Dace if it is illegally introduced above Cascade Falls. Walleye (Sander vitreus) populations have been increasing in the Columbia River since the 1980s and may threaten Speckled Dace below Cascade Falls through competition and predation (Brown et al. 2012). All three of these non-native species are part of the BC recreational fishery, and thus could be spread above the Cascade Falls through sport fishing.
Didymo (Didymosphenia geminata), a species of diatom algae believed native to British Columbia, can form nuisance blooms in rivers and streams that could threaten Speckled Dace populations. It appears to be spread among river systems primarily by anglers wearing felt-soled waders, which can harbour viable algal cells for up to a month (Bothwell et al. 2009). The alga occurs in clear shallow warm waters, where it attaches to rocks, vegetation and other substrates to form thick mats that can cover large areas of stream bottom (BC Ministry of Environment undated). Didymo can negatively affect fish by decreasing availability of benthic invertebrate food and irritating and clogging gills. The algal mats also restrict water flow, reducing oxygen to fish eggs and fry, and deplete oxygen levels during decomposition (Lui et al. 2008; BC Ministry of Environment undated). Didymo infestations have been reported in the Kettle River, but no research has been conducted on Didymo’s effects on Speckled Dace in Canada (Government of BC 2016).
Limited mining currently occurs in the watershed, but could increase if metal prices rise. Mining activity could potentially adversely affect Speckled Dace by introducing chemical pollutants and sediments to the Kettle River system, altering water quality and increasing embeddedness (DFO 2013a). Mining exploration and operations in the watershed are concentrated at present along the southern Granby and Kettle rivers near the US border, a relatively small portion of the Speckled Dace’s Canadian range (Katay 2016). Three active quarries exist in the Grand Forks area, which produce gabbro (Winner Quarry), basalt (Friday Quarry) and slag/silica (Grand Forks Slag). Three exploration projects for precious metals (primarily gold and silver) are currently underway in the Greenwood area: Gold Drop, May Mac and Greenwood Gold (Katay 2016).
A 28 megawatt run-of-river hydroelectric generation project was approved by the BC government for the Cascade Falls in 2006. A 350 m section of river directly upstream of the dam would become a pond, and possible downstream effects could include reduced flow and changed the movement of sand and gravel (Brown et al. 2012). Some Speckled Dace will likely be lost or injured due to entrainment at the intake for the turbine. Juvenile fish could be especially vulnerable, because they are smaller and weaker swimmers than adults. The intake’s location in deeper water (5.2 m), however, may minimize capture of juveniles (Hamilton and Associates Ltd. 2005). Two studies (one for the power company: Hamilton and Associates Ltd. 2005, and one for the federal government: Bradford 2006) concluded that impacts of the project on Speckled Dace would be minimal.
Speckled Dace are numerous throughout the Kettle River system and it is unlikely that a single catastrophic event could drastically reduce or eliminate the Canadian population (Brown et al. 2012; DFO 2013a). Furthermore, although water demands are elevated in the lower part of its Canadian range due to agricultural and domestic water extraction, the species is still widely distributed throughout the three upper reaches of the Kettle River system above the major threat of water withdrawal (Brown et al. 2012).
Limiting factors
The entire range of Speckled Dace in Canada is found within a single drainage system characterized by low flows (Andrusak et al. 2012). Harvey (2007) suggested that the availability of riffle or fast flowing habitat may be a limiting factor for adult Speckled Dace. More recent studies, however, have indicated that Speckled Dace has a preference for slower shallow waters, but also uses deeper pool habitats (Batty 2010; Andrusak and Andrusak 2011). Andrusak et al. (2012) concluded that because of the abundance of slow shallow habitat in the Kettle River system, habitat on its own is likely not a limiting factor for the species. However, existing low flows in the West Kettle River appear to drastically reduce the usable width and available habitat for immature individuals, which rely on river margins. Consequently, in the West Kettle it appears that low flows limit habitat availability for the immature life stage (Andrusak et al. 2012).
Speckled Dace may be inherently more vulnerable than other fish species to disturbance because of its small size. Minimum viable population (MVP) estimates increased exponentially with the probability of catastrophe in small-bodied freshwater fish at risk in Canada, including Speckled Dace (Vélez-Espino and Koops 2012). Small freshwater fish species also appear to be more sensitive to habitat loss than larger species, particularly in the pre-adult life stages (van der Lee and Koops 2015).
Number of locations
The most likely and imminent threat is water withdrawal coupled with climate change-induced reductions in summer and autumn flows. The threat is greatest in the southern part of the Kettle River system, where agricultural and domestic demands are concentrated (Brown et al. 2012; Summit Environmental Consultants Inc. 2012). In addition, river sections at lower elevations tend to be wider with lower gradients, which further predisposes them to low flow conditions. While current low flow patterns in the lower part of the drainage system may not be a problem for the drought-tolerant Speckled Dace, the combination of climate change and increased water extraction poses a substantial threat in the future. The Upper Kettle River and the Granby River are less affected by water withdrawal (Summit Environmental Consultants Inc. 2012). In the upper part of the watershed, meanwhile, the West Kettle River experiences extreme low flows in late summer, which may reduce habitat availability for immature stages. If water withdrawal alone was the primary threat then four locations should be recognized for Canadian Speckled Dace: Lower Kettle River, Granby River, Upper Kettle River, and West Kettle River. However coupled with climate change which is assessed as a basin-wide threat, it can be argued that there is a single location. Therefore, the best inference of number of locations is 1 to 4.
Protection, status and ranks
Legal protection and status
Speckled Dace was originally designated Special Concern by COSEWIC in 1980. Following a re-examination of its status in 2002 the species was designated as Endangered by COSEWIC. In 2006, the species was again designated as Endangered based on an updated status report, which concluded that the species had a small area of occupancy (7.47 km2) and continuing decline observed or projected in the extent and quality of available habitat as a result of increases in water extraction and drought conditions (COSEWIC 2006). Speckled Dace was listed as Endangered under the federal Species at Risk Act (SARA) in 2009. As a result, Speckled Dace receives protection under the federal Species at Risk Act, which prohibits the killing, harm, harassment, capture, taking, possession, collection, or trade of listed species, and prohibits the damage or destruction of their residence. Proposed critical habitat has been identified for Speckled Dace, but not within a recovery strategy or action plan; thus it currently provides no protection for the species (Brown et al. 2012). Fisheries and Oceans Canada is currently working with the Province of British Columbia to develop a recovery strategy for the species.
Speckled Dace is recognized as a protected species in the BC Sport Fishing Regulations (under the federal Fisheries Act), meaning that it cannot be fished for, caught or retained.
There are several forms of Speckled Dace that are listed under the US Endangered Species Act, although the species overall is not considered at risk. Foskett Speckled Dace (R. osculus ssp.; from Oregon) is listed as Threatened. Independence Valley Speckled Dace (R. osculus lethoporus; from Nevada), Ash Meadows Speckled Dace (R. osculus nevadensis, from Nevada) and Clover Valley Speckled Dace (R. osculus oligoporus, from Nevada) are listed as Endangered.
Non-legal status and ranks
NatureServe lists 11 additional subspecies of Speckled Dace at risk in the US: Big Smoky Valley Speckled Dace (R. o. lariversi, critically imperilled, Nevada), Moapa Speckled Dace (R. o. moapae, critically imperilled, Nevada), Amargosa Canyon Speckled Dace (R. o. ssp., critically imperilled, California), Meadow Valley Speckled Dace (R. o. ssp., imperilled, Nevada), Long Valley Speckled Dace (R. o. ssp., critically imperilled, California), Owens Speckled Dace, (R. o. ssp., critically imperilled, California), Monitor Valley Speckled Dace (R. o. ssp., critically imperilled, Nevada), Oasis Valley Speckled Dace (R. o. ssp., critically imperilled, Nevada), White River Speckled Dace (R. o.ssp., imperilled, Nevada), Santa Ana Speckled Dace (R. o. ssp., critically imperilled, California) and Pahranagat Speckled Dace (R. o. velifer, critically imperilled, Nevada). Grass Valley Speckled Dace (R. o. reliquus, Nevada) is presumed extinct, and Diamond Valley Speckled Dace (R. o. ssp., Nevada) is possibly extinct (NatureServe 2015).
Speckled Dace is considered a species of least concern by the IUCN Red List because of its large extent of occurrence, large number of subpopulations, large population size and lack of major threats (NatureServe 2013). The species’ global rank is G5 (secure) because it has a large range across most of the western US and is very abundant in many areas (NatureServe 2015). Its national ranking is N5 (secure) in the US and N2 (imperilled) in Canada, the latter ranking because the species is considered at high risk of extirpation due to a very restricted range, few populations, steep declines or other factors (NatureServe 2015). The BC Conservation Data Centre ranks Speckled Dace on its Red List as S2 (imperilled; BC Conservation Data Centre 2013).
Habitat protection and ownership
Under the Species at Risk Act, it is illegal to destroy critical habitat that has been identified within a recovery strategy or action plan for a listed species. While proposed critical habitat has been described for Speckled Dace (Brown et al. 2012), it has not been delineated within a recovery strategy or action plan. As a result habitat for Speckled Dace is not specifically protected.
Speckled Dace habitat may receive general protection under the federal Fisheries Act, which prohibits serious harm to fish species in or supporting commercial, recreational or Aboriginal fisheries (including death of fish or any permanent alteration or destruction of fish habitat). However, there is no fishery for Speckled Dace, and it is not clear that the species would fall under provisions for species that support fisheries (DFO 2013b). Furthermore, habitat protection provisions are discretionary under the Fisheries Act, compared with more rigorous protection under the Species at Risk Act (Taylor and Pinkus 2013). Provisions under various provincial statutes designed to protect the environment, water quality and fish may also provide habitat protection. None of this legislation, however, specifically protects Speckled Dace habitat. In addition to these broad protection measures, Speckled Dace habitat receives consideration by both the provincial and federal government (e.g., under environmental assessment procedures) because it is listed by the British Columbia Conservation Data Centre and COSEWIC as a species at risk.
The mainstem of the West Kettle River has been designated as a Heritage River under British Columbia’s Heritage Rivers System in recognition of its outstanding natural, cultural and recreational values. The main objective of the Heritage Rivers program is to raise awareness and promote good stewardship of British Columbia’s rivers. The designation identifies rivers considered important components of the province’s geographical diversity, but it does not provide any legally binding protection.
The Kettle River was included on British Columbia’s list of most endangered rivers in 2012, primarily for excessive water withdrawal, but also because of degradation of riparian zones and potential pollution from proposed uranium mining in the Beaverdell area (Angelo 2012).
The Kettle River Watershed Management Plan, a collaborative initiative developed by local and provincial governments, as well as stakeholders from multiple sectors and organizations, was released in 2014. The purpose of the Plan is to ensure a healthy, resilient and sustainable watershed through healthy aquatic ecosystems, safe and secure water supplies and a reliable water system (Regional District of Kootenay Boundary 2014). The Plan will include restoration and conservation planning, as well as public education on the importance of stewardship of the watershed. While not legally enforceable, the Plan is supported by a wide range of parties, and action is underway to secure funding for its implementation.
The waters inhabited by Speckled Dace in Canada are owned by the Crown; however, the private use of surface water is licensed.
Acknowledgements and authorities contacted
The author is grateful to Greg Wilson, Sean MacConnachie and Adam Batty for providing background information that assisted in the preparation of this report. Jenny Wu calculated extent of occurrence and index of area of occupancy for the species and developed associated mapping. Brent Parsons provided comments on an earlier draft. Juanita Ptolemy prepared the 2006 update status report on Speckled Dace in Canada. Funding was provided by the Canadian Wildlife Service, Environment Canada.
List of authorities contacted
Dr. Rhonda Millikin, A/Head Population Assessment, Pacific Wildlife Research Centre, Canadian Wildlife Service, Environment Canada, Delta, British Columbia. Did not respond.
Shelagh Bucknell, Administrative Services Assistant, Pacific Wildlife Research Centre, Canadian Wildlife Service, Environment Canada, Delta, British Columbia. Did not respond.
Dr. Simon Nadeau, Senior Advisor, Fish Population Science, Fisheries and Oceans Canada, Ottawa, Ontario. Did not respond.
Christie Whelan, Science Advisor, Fish Population Science, Fisheries and Oceans Canada, Ottawa, Ontario. Responded June 2, 2015.
Sean MacConnachie, Species at Risk Biologist, Fisheries and Oceans Canada, Vancouver, British Columbia. Responded June 4, 2015.
Dr. Patrick Nantel, Conservation Biologist, Species at Risk Program, Ecological Integrity Branch, Parks Canada, Gatineau, Quebec. Responded June 8, 2015.
Dr. Briar Howes, Ecological Restoration, Parks Canada, Gatineau, Quebec. Did not respond (on maternity leave until March 2016).
Dr. Robert Anderson, Research Scientist, Zoology, Canadian Museum of Nature, Ottawa, Ontario. Did not respond.
Gregory Wilson, Aquatic Species at Risk Specialist, Ecosystem Protection & Sustainability Branch, Ministry of Environment, Victoria, British Columbia. Responded June 2, 2015.
Katrina Stipec, British Columbia Conservation Data Centre, Ministry of Environment, Victoria, British Columbia. Responded September 17, 2015.
Dr. Rick Page, Biologist, Page and Associates Environmental Solutions, Victoria, British Columbia. Did not respond.
Dr. Danna Leaman, Conservation Biologist, Ottawa, Ontario. Responded June 2, 2015.
Dr. Lynda Corkum, Professor, Department of Biological Sciences, University of Windsor, Windsor, Ontario. Responded June 2, 2015.
Adam Batty, Project Manager, Fishery Monitoring, Archipelago Marine Research, Victoria, British Columbia. Responded June 13, 2015.
Graham Watt, Coordinator, Kettle River Watershed Management Plan, Regional District of Kootenay Boundary, Grand Forks, British Columbia. Did not respond.
Sonia Schnobb, Administrative Assistant, COSEWIC Secretariat, Canadian Wildlife Service, Environment Canada, Gatineau, Quebec. Responded June 2, 2015.
Jenny Wu, Science Officer, COSEWIC Secretariat, Canadian Wildlife Service, Environment Canada, Gatineau, Quebec. Responded June 4, 2015.
Neil Jones, Aboriginal Traditional Knowledge Coordinator, COSEWIC Secretariat, Canadian Wildlife Service, Environment Canada, Gatineau, Quebec. Responded June 2, 2015.
Dr. Donald McPhail, Professor Emeritus, University of British Columbia, Vancouver, BC. Responded April 13, 2016.
Information sources
Andrusak, G., and H. Andrusak. 2011. Identification of habitat use and preferences of Speckled Dace within the Kettle River watershed. Prepared for the Department of Fisheries and Oceans Canada and the BC Ministry of the Environment. Penticton, BC. 75 pp.
Andrusak, G., H. Andrusak, and G. Pavan. 2012. Assessment of habitat for Speckled Dace within the Kettle River watershed- 2012. Prepared for the Department of Fisheries and Oceans Canada and the Ministry of Forests, Lands and Natural Resource Operations. Penticton, BC. 47 pp.
Annear, T., I. Chisholm, H. Beecher, A. Locke, P. Aarrestad, C. Coomer, C. Estes, J. Hunt, R. Jacobson, G. Jöbsis, J. Kauffman, J. Marshall, K. Mayes, G. Smith, R. Wentworth, and C. Stainaker. 2004. Instream Flows for Riverine Stewardship, Revised Edition. Instream Flow Council, Cheyenne, Wyoming. 267 pp.
Angelo, M. 2012. BC Most Endangered Rivers List. Outdoor Recreation Council of British Columbia. Vancouver, BC. 22 pp.
Aqua Factor Consulting Inc. 2004. Potential effects of the Cascade Heritage Power Project on the allocation of water in the Kettle River basin. Report prepared for the BC Environmental Assessment Office. Victoria, BC. 96 pp.
Ardren, W. R., J. Baumsteiger, and C. S. Allen. 2010. Genetic analysis and uncertain taxonomic status of threatened Foskett Spring Speckled Dace. Conservation Genetics 11:1299-1315.
Baltz, D. M., P. B. Moyle, and N. J. Knight. 1982. Competitive interactions between benthic stream fishes, Riffle Sculpin, , and Speckled Dace, Rhinichthys osculus. Canadian Journal of Fisheries and Aquatic Sciences 39:1502-1511.
Batty, A. 2010. Examination of Speckled Dace abundance, biology, and habitat in the Canadian range. M. Sc. Thesis, Simon Fraser University, Burnaby, BC. 87 pp.
Benejam, L., C. Alcaraz, J. Benito, N. Caiola, F. Casals, A. Maceda-Veiga, A. de Sostoa and E. García-Berthou. 2012. Fish catchability and comparison of four electrofishing crews in Mediterranean streams. Fisheries Research 123-124:9-15.
Bothwell, M. L., D. R. Lynch, H. Wright, and J. Deniseger. 2009. On the boots of fishermen: the history of Didymo blooms on Vancouver Island, British Columbia. Fisheries 34(8):382-388.
Bradford, M. 2006. Impact of the proposed hydroelectric development at Cascade Falls on the conservation status of Speckled Dace (Rhinichthys osculus) in the Kettle River, British Columbia. Report for the Department of Fisheries and Oceans Canada. 25 pp.
BC CDC (British Columbia Conservation Data Centre). 2013. Conservation Data Centre Mapping Service. Web site: http://webmaps.gov.bc.ca/imfx/imf.jsp?site=imapbc&savessn=Ministry%20of%20Environment /Conservation_Data_Centre.ssn [accessed October 2015].
BC Ministry of Environment. undated. Water Quality. Didymosphenia geminata in British Columbia streams. Environmental Protection Division. Web site: http://www.env.gov.bc.ca/wat/wq/didy_bcstrms.html[accessed April 2016].
BC Ministry of Environment. 2016. British Columbia approved water quality guidelines. Summary report. Water Protection & Sustainability Branch. March 2016. 1-36 pp.
British Columbia. 2002. Indicators of climate change for British Columbia. Water, Air and Climate Branch, Ministry of Water, Land and Air Pollution. Victoria BC.
Brouder, M. J., and T. L. Hoffnagle. 1997. Distribution and prevalence of the Asian Fish Tapeworm, Bothriocephalus acheilognathi, in the Colorado River and tributaries, Grand Canyon, Arizona, including two new host records. Journal of Helminthological Society of Washington 64(2):219-226.
Brown, T., B. Harvey, and M. J. Bradford. 2012. Information in support of the identification of critical habitat for Speckled Dace (Rhinichthys osculus). DFO Canadian Science Advisory Secretariat Research Document 2012/065, iv + 29 pp.
Cannings, S. G., and J. Ptolemy. 1998. Rare Freshwater Fish of British Columbia. BC Ministry of the Environment, Victoria, BC. 214 pp.
Christina Lake Stewardship Society. 2015. The Northern Pike are on their way! Web site: http://blog.lakesteward.ca/the-northern-pike-are-on-their-way/[accessed March 2016].
Clarkson, R. W., A. T. Robinson, and T. L. Hoffnagle. 1997. Asian Tapeworm (Bothriocephalus acheilognathi) in native fishes from the Little Colorado River, Grand Canyon, Arizona. Great Basin Naturalist 57(1):66-69.
Cleary, J. D., and D. Underhill (eds.). 2001. Annual Compendium of Aquatic Rehabilitation Projects for the Watershed Restoration Program 2000-2001. Watershed Restoration Project Report No. 19. Watershed Restoration Program, Ministry of Environment, Lands and Parks, Vancouver, BC.
Coleshill, J., and G. Watt. 2015. Kettle River Riparian Threat Assessment (Final Draft). Granby Wilderness Society, Grand Forks, BC. 97 pp.
COSEWIC. 2006. COSEWIC assessment and update status report on the Speckled Dace Rhinichthys osculus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa, ON. vi + 27 pp.
Dalziel, S. 2015. Small minnow needs big help. Boundary Creek Times, February 22, 2015. Greenwood, BC.
DFO (Department of Fisheries and Oceans Canada). 2008. Recovery Potential for the Speckled Dace (Rhinichthys osculus). DFO Canadian Science Advisory Secretariat Science Advisory Report 2008/030.
DFO. 2013a. Recommendations for Speckled Dace (Kettle River) critical habitat. DFO Canadian Science Advisory Secretariat Science Advisory Report 2013/20.
DFO. 2013b. Science advice to support development of a fisheries protection policy for Canada. DFO Canadian Science Advisory Secretariat Science Advisory Report 2012/063.
Dessouki, T. C. E. 2009. Canada-British Columbia Water Quality Monitoring Agreement- Water quality assessment of the Kettle River at Midway and Carson, British Columbia (1990-2007). Report prepared for the BC Ministry of Environment and Environment Canada. 58 pp.
Epp, P., and G. Andrusak. 2012. Results of the 2011 West Kettle River, Kettle River and Granby River flow, temperature, useable fish habitat & snorkel enumeration survey for Kettle River fish protection planning. Prepared by Trout Creek Hydrology & Soils and Redfish Consulting Ltd. for the Ministry of Natural Resource Operations, Penticton, BC. 73 pp.
Girard, C. 1856. Researches upon the cyprinoid fishes inhabiting the fresh waters of the United States of America, west of the Mississippi Valley, from specimens in the museum of the Smithsonian Institution. Proceedings of the Academy of Natural Sciences of Philadelphia 8:165-214.
Government of British Columbia. undated. British Columbia’s Water Act modernization. Technical background report. 92 pp. Web site: http://www.livingwatersmart.ca/water-act/docs/wam_tbr.pdf[accessed September 2015].
Government of British Columbia. 2016. Water Quality. Didymosphenia geminate in British Columbia Streams. Ministry of Environment, Environmental Protection Division. Web site: http://www.env.gov.bc.ca/wat/wq/didy_bcstrms.html[accessed September 2016].
Haas, G. R. 2001. The evolution through natural hybridizations of the Umatilla Dace (Pisces: Rhinichthys Umatilla), and their associated ecology and systematics. Ph. D. dissertation, University of British Columbia, Vancouver, BC. 218 pp.
Hamilton, S., and Associates Ltd. 2005. Appendix C. Status of Kettle River Speckled Dace (Rhinichthys osculus). Cascade Heritage Power Project. Response to provincial and federal agency comments on their 2003 additional information. Saanich, BC. 5 pp.
Harvey, B. C., J. L. White, and R. J. Nakamoto. 2004. An emergent multiple predator effect may enhance biotic resistance in a stream fish assemblage. Ecology 85(1):127-133.
Harvey, B., 2007. Scientific information used in the recovery potential assessment for the Speckled Dace. DFO Canadian Science Advisory Secretariat Research Document 2007/074.
Hoekzema, K., and B. L. Sidlauskas. 2014. Molecular phylogenetics and microsatellite analysis reveal cryptic species of Speckled Dace (Cyprinidae: Rhinicthys osculus) in Oregon’s Great Basin. Molecular Phylogenetics and Evolution 77:238-250.
ITIS (Integrated Taxonomic Information System). 2015. ITIS Search Results: Speckled Dace. Web site: http://www.itis.gov [accessed August 2015].
John, K. R. 1963. The effect of torrential rains on the reproductive cycle of Rhinichthys osculus in the Chiricahua Mountains, Arizona. Copeia 1963(2):286-291.
John, K. R. 1964. Survival of fish in intermittent streams of the Chiricahua Mountains, Arizona. Ecology 45(1):112-119.
Jordan, D. S., B. W. Evermann, and H. W. Clark. 1930. Checklist of the Fishes and Fishlike Vertebrates of North and Middle America North of the Northern Boundary of Venezuela and Colombia. Report of the US Commission of Fish and Fisheries 1928: 1-670.
Katay, F. 2016. Exploration and mining in the Kootenay-Boundary Region, British Columbia. Pp. 57-87, in Provincial Overview of Exploration and Mining in British Columbia, 2015. British Columbia Geological Survey, Information Circular 2016-1.
Kaya, C. M. 1991. Laboratory spawning and rearing of Speckled Dace. Progressive Fish-Culturist 53:259-260.
Kinziger, A. P., R. J. Nakamoto, E. C. Anderson, and B. C. Harvey. 2011. Small founding number and low genetic diversity in an introduced species exhibiting limited invasion success (Speckled Dace, Rhinichthys osculus). Ecology and Evolution (1):73-84.
Korman, J., A. S. Decker, B. Mossop, and J. Hagen. 2010. Comparison of electrofishing and snorkeling mark-recapture estimation of detection probability and abundance of juvenile Steelhead in a medium-sized river. North American Journal of Fisheries Management 30:1280-1302.
Leith, M. M. and P. H. Whitfield. 1998. Evidence of climate change effects on the hydrology of streams in south-central British Columbia. Canadian Water Resources Journal 23:219-230.
Lowe, C. H., D. S. Hinds, and E. A. Halpern. 1967. Experimental catastrophic selection and tolerances to low oxygen concentration in native Arizona freshwater fishes. Ecology 48:1013-1017.
Lui, K., M. Butler, M. Allen, J. da Silva, and B. Brownson. 2008. Field Guide to Aquatic Invasive Species- Identification, Collection and Reporting of Aquatic Invasive Species in Ontario Waters. Queen’s Printer for Ontario, Ontario, Canada.
MacConnachie, S., pers. comm. 2015. Phone conversation with S. MacConnachie. June 2015. Species at Risk Biologist, DFO Pacific Region, Vancouver, BC.
Maciak, R., T. Ford, and J. Schroeder. 2007. Kettle River watershed analysis: Midway, British Columbia to stream headwaters. Web site: http://www.boundaryalliance.org/kettleriverstudy.pdf[accessed September 2015].
Marsh, P. C., and M. E. Douglas. 1997. Predation by introduced fishes on endangered humpback chub and other native species in the Little Colorado River, Arizona. Transactions of the American Fisheries Society 126:343-346.
McPhail, J. D. undated. Feature fish Speckled Dace Rhinichthys osculus. Web site: http://www.zoology.ubc.ca/~etaylor/nfrg/dace.pdf[accessed July 2015].
McPhail, J. D. 2003. Report on the taxonomy, life history, and habitat use of the four species of Dace (Rhinichthys) inhabiting the Canadian portion of the Columbia drainage system. Report prepared for BC Hydro, Castlegar, BC. 24 pp.
McPhail, J. D. 2007. The Freshwater Fishes of British Columbia. University of Alberta Press, Edmonton, AB. xv + 620 pp.
Miller, D. L., and R. J. Behnke. 1985. Two new intergeneric Cyprinid hybrids from the Bonneville Basin, Utah. Copeia 1985(2):509-515.
Miller, R. R., and R. G. Miller. 1948. The contribution of the Columbia River system to the fish fauna of Nevada: Five species unrecorded from the state. Copeia 1448(3):174-187.
Minckley, W. L. 1973. Fishes of Arizona. Sims Printing, Phoenix, Arizona. 293 pp.
Minckley, W. L., and P. C. Marsh. 2009. Inland fishes of the Greater Southwest: Chronicle of a Vanishing Biota. University of Arizona Press, Tucson, Arizona. xxxiv + 426 pp.
Moyle, P. B. 1976. Inland Fishes of California. University of California Press, Berkeley, California. 504 pp.
Moyle, P. B., and D. M. Baltz. 1985. Microhabitat use by an assemblage of California stream fishes: developing criteria for instream flow determinations. Transactions of the American Fisheries Society 114:695-704.
Mueller, G. A. 1984. Spawning by Rhinichthys osculus (Cyprinidae), in the San Francisco River, New Mexico. Southwest Naturalist 29:354-356.
NatureServe. 2013. Rhinichthys osculus. The IUCN Red List of Threatened Species 2013, e.T62205A18231790. Web site: http://dx.doi.org/10.2305/IUCN.UK.2013-1.RLTS.T62205A18231790.en[accessed March 2016].
NatureServe. 2015. NatureServe Explorer: an online encyclopedia of life. Version 7.1. NatureServe, Arlington, Virginia. Web site: http://explorer.natureserve.org[Accessed September 2015].
Nelson, J. S., E. J. Crossman, H. Espinosa, L. T. Finley, C. R. Gilbert, R. N. Lea, and J. D. Williams. 2004. Common and scientific names of fishes from the United States, Canada and Mexico, 6th Edition. American Fisheries Society, Bethesda, Maryland. 386 pp.
Nico, L., and P. Fuller. 2015. Rhinichthys osculus. USGS Nonindigenous Aquatic Species Database, Gainesville, Florida. Web site: http://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=640[accessed July 2015].
Oakey, D. D., M. E. Douglas, and M. R. Douglas. 2004. Small fish in a large landscape: diversification of Rhinichthys osculus (Cyprinidae) in western North America. Copeia 2004(2):207-221.
Page, L. M., and B. M. Burr. 2011. Peterson Field Guide to Freshwater Fishes. 2nd edition. Houghton Mifflin Harcourt, New York, New York. 663 pp.
Pearsons, T. N., H. W. Li, and G. A. Lamberti. 1992. Influence of habitat complexity on resistance to flooding and resilience of stream fish assemblages. Transactions of the American Fisheries Society 121:427-436.
Peden, A. E. 1994. Updated status report on Canadian populations of Speckled Dace, Rhinichthys osculus. Manuscript submitted to COSEWIC, Subcommittee on Fish and Marine Mammals. 26 pp.
Peden, A. E. 2002. COSEWIC assessment and update status report on the Speckled Dace Rhinichthys osculus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa, ON. 1-36 pp.
Peden, A. E., and G. W. Hughes. 1981. Life history notes relevant to the Canadian status of the Speckled Dace (Rhinichthys osculus). Syesis 14:21-31.
Peden, A. E., and G. W. Hughes. 1984. Status of the Speckled Dace, Rhinichthys osculus, in Canada. Canadian Field-Naturalist 98(1):98-103.
Peden, A. E., and G. W. Hughes. 1988. Sympatry in four species of Rhinichthys (Pisces), including the first documented occurrences of R. umatilla in the Canadian drainages of the Columbia River. Canadian Journal of Zoology 66:1846-1856.
Pfrender, M. E., J. Hicks, and M. Lynch. 2004. Biogeographic patterns and current distribution of molecular-genetic variation among populations of Speckled Dace, Rhinichthys osculus (Girard). Molecular Phylogenetics and Evolution 30:490-502.
PDI (Powerhouse Developments Inc.). 2003. Cascade Heritage Power Project – Additional information, Volume 2, 2.2. Project design and consultation specifications. Response to project specification # 2. Project description. 17 pp. Web page: https://a100.gov.bc.ca/appsdata/epic/documents/p55/d15946/1069266890930_7f2bd4f9497d48f8a27ed476db1e09d2.pdf[accessed September 2015].
PDI. 2005. Cascade Heritage Power Project - Response to provincial and federal agency comments on their 2003 additional information. Web site: https://a100.gov.bc.ca/appsdata/epic/html/deploy/epic_document_55_20409.html[accessed September 2015].
Price, A. L., and J. T. Peterson. 2010. Estimation and modeling of electrofishing capture efficiency for fishes in wadeable warmwater streams. North American Journal of Fisheries Management 30(2):481-498.
Propst, D. L., and K. B. Gido. 2004. Responses of native and non-native fishes to natural flow regime mimicry in the San Juan River. Transactions of the American Fisheries Society 133:922-931.
Ptolemy, R., and A. Lewis. 2002. Rationale for multiple British Columbia instream flow standards to maintain ecosystem function and biodiversity. Draft report prepared for the BC Ministry of Water, Land and Air Protection and the BC Ministry of Sustainable Resource Management.
Regional District of Kootenay Boundary. 2014. Kettle River Watershed Management Plan, version 1.0, November 2014. Regional District of Kootenay Boundary, Kettle River Watershed Steering Committee, Trail, BC.
Rusky, J. A. 2014. Morphological stasis and genetic divergence without reproductive isolation in the Rhinichthys cateractae species complex: insights from a zone of secondary contact in the lower Fraser Valley, British Columbia. M. Sc. Thesis, University of British Columbia, Vancouver, BC. 123 pp.
Scott, W. B., and E. J. Crossman. 1973. Freshwater Fishes of Canada. Bulletin 184, Fisheries Research Board of Canada, Environment Canada, Ottawa, ON. 966 pp.
Smith, G. R., 1973. Analysis of several hybrid Cyprinid fishes from western North America. Copeia 1973 (3):395-410.
Smith, G. R., and T. E. Dowling. 2008. Correlating hydrographic events and divergence times of Speckled Dace (Rhinichthys: Teleostei: Cyprinidae) in the Colorado River drainage. Geological Society of America Special Paper 439: 301-317. Doi: 10.1130/2008.2439(13).
Summit Environmental Consultants Inc. 2012. Kettle River watershed management plan: phase 1 technical assessment. Report prepared for the Regional District of Kootenay Boundary. Vernon, BC. 206 pp.
Taylor, E. B., and S. Pinkus. 2013. The effects of lead agency, nongovernmental organizations, and recovery team membership on the identification of critical habitat for species ar risk: insights from the Canadian experience. Environmental Reviews 21:93-102.
Tennant, D. L. 1976. Instream flow regimens for fish, wildlife, recreation, and related environmental resources. Pp. 359-373, in J. F. Orsborn and C. H. Allman (eds.). Instream Flow Needs, Volume II. Proceedings of the Symposium and Specialty Conference on Instream Flow Needs, Boise, Idaho. American Fisheries Society, Bethesda, Maryland.
Turek, K. C., M. A. Pegg, and K. L. Pope. 2015. Experimental evaluation of Rainbow Trout Oncorhynchus mykiss predation on Longnose Dace Rhinichthys cataractae. Ecology of Freshwater Fish 24:600-607.
Underhill, D. (ed.). 2000. Annual Compendium of Aquatic Rehabilitation Projects for the Watershed Restoration Program 1999-2000. Watershed Restoration Project Report No. 18. Watershed Restoration Program, Ministry of Environment, Lands and Parks, Vancouver, BC.
USFWS (US Fish and Wildlife Service). 2014. Environmental Conservation Online System. Web site: http://ecos.fws.gov/tess_public/pub/SpeciesReport.do[accessed September 2015].
Van der Gulik, T., D. Neilson, R. Fretwell, A. Peterson, and S. Tam. 2013. Agriculture water demand model: Report for the Kettle Watershed. BC Ministry of Agriculture, Victoria, BC.
van der Lee, A. S., and M. A. Koops. 2015. Are small fishes more sensitive to habitat loss? A generic size-based model. Canadian Journal of Fisheries and Aquatic Sciences 72:1-11.
Vélez-Espino, L. A., and M. A. Koops. 2012. Capacity for increase, compensatory reserves, and catastrophes as determinants of minimum viable population in freshwater fishes. Ecological Modelling 247:319-326.
Washington Department of Fish and Wildlife. 2016. Aquatic Invasive Species. Esox lucius(Northern Pike). Web site: http://wdfw.wa.gov/ais/esox_lucius/[accessed March 2016].
Watt, G., and the KRWMP Stakeholder Advisory Group. 2014a. Sustaining the flow: managing water supply and demand to support ecosystem health and community needs. Discussion Paper Three for the Kettle River Watershed Management Plan. Grand Forks, BC. Regional District of Kootenay Boundary. Web site: http://kettleriver.ca/what-we-are-planning/discussion-paper-3.
Watt, G. and the KRWMP Stakeholder Advisory Group. 2014b. Water quality and source water protection issues and strategies in the Kettle River watershed. Discussion Paper Four for the Kettle River Watershed Management Plan. Grand Forks, BC. Regional District of Kootenay Boundary. Web site: http://kettleriver.ca/watershed-management-plan/discussion-paper-4/.
Ward, D. L., A. A. Schultz, and P. G. Morton. 2003. Differences in swimming ability and behavior in response to high water velocities among native and non-native fishes. Environmental Biology of Fishes 68:87-92.
Wetzel, R. G. 2001. Limnology. Lake and River Ecosystems, Third Edition. Academic Press, San Diego, California.1006 pp.
Whitfield, P. H., C. J. Reynolds and A. J. Cannon. 2002. Modelling streamflow in present and future climates: examples from the Georgian Basin British Columbia. Canadian Water Resources Journal 27:427-456.
Wiesenfeld. J. C. 2014. Riverscape genetics identifies a cryptic lineage of Speckled Dace (Rhinichthys osculus) in the Klamath-Trinity Basin. M.Sc. Thesis, Humboldt State University, Arcata, California. 87 pp.
Williams, J. E., G. R. Giannico, and B. Withrow-Robinson. 2014. Field Guide to Common fish of the Willamette Valley Floodplain. Oregon State University Extension Service EM 909.
Zaldokas, D. O. (ed.). 1999. Annual Compendium of Aquatic Rehabilitation Projects for the Watershed Restoration Program 1998-1999. Watershed Restoration Project Report No. 13., Watershed Restoration Program, Ministry of Environment, Lands and Parks, Vancouver, BC, 382 pp.
Biographical summary of report writers
Dr. Andrea Smith is a senior scientist with Hutchinson Environmental Sciences Ltd., based in Bracebridge Ontario. She obtained her M.Sc. in conservation biology and her Ph.D. in evolutionary ecology, both at Queen’s University. Andrea has worked on a variety of research projects relating to species at risk, invasive species, and environmental impact assessment. She has prepared three previous COSEWIC status reports (two on marine fish species and one on an arctic shorebird), and has also developed a prioritized list of crustacean species potentially at risk in Canada for COSEWIC. Andrea’s research interests include documenting the interactive effects of multiple stressors on biodiversity, and applying conservation science to develop meaningful policy.
Collections examined
No collections were examined for this report.
Appendix A. threats assessment calculator.
Threats assessment worksheet
- Species or ecosystem scientific name:
- Speckled Dace Rhinichthys osculus
- Element ID
- -
- Elcode
- -
- Date:
- 16/12/2015
- Assessor(s):
- Dwayne Lepitzki (moderator), John Post (co-chair), Andrea Smith (writer), Todd Hatfield (SSC Member), Dave Fraser (COSEWIC member BC), Ruben Bowles (COSEWIC member CWS), Greg Andrusak (BC expert), Michael Bradford (BC expert), Angele Cyr (Secretariat).
- References:
- draft COSEWIC report;
| Threat impact | Threat impact (descriptions) | Level 1 Threat impact counts: high range |
Level 1 Threat impact counts: low range |
|---|---|---|---|
| A | Very high | 0 | 0 |
| B | High | 0 | 0 |
| C | Medium | 4 | 0 |
| D | Low | 0 | 4 |
| - | Calculated overall threat impact: | High | Medium |
- Assigned overall threat impact:
- BC = High - Medium
- Impact adjustment reasons:
- -
- Overall threat comments
- generation time (according to draft 2-3 years but
| # | Threat | Impact (calculated) |
Scope (next 10 Yrs) |
Severity (10 Yrs or 3 Gen.) |
Timing | Comments |
|---|---|---|---|---|---|---|
| 1 | Residential and commercial development | - | - | - | - | Generation time ~3-4 years so 3 generations = 9-12yrs. |
| 1.1 | Housing and urban areas | - | - | - | - | not applicable. |
| 1.2 | Commercial and industrial areas | - | - | - | - | docks or marinas planned right on the river? Not applicable. |
| 1.3 | Tourism and recreation areas | - | - | - | - | not applicable. |
| 2 | Agriculture and aquaculture | Negligible | Restricted (11-30%) | Negligible (<1%) | High (Continuing) | - |
| 2.1 | Annual and perennial non-timber crops | - | - | - | - | haying? Expansion of agricultural areas? Yes for expansion in crop timing. Most of area has been taken up already but the intensity will increase over the next 10 years. Water use is the issue which is accounted for under threat 9.3. Some riparian areas affected. nurseries in main stem of Kettle River but increase over the next 10 years is unknown. |
| 2.2 | Wood and pulp plantations | - | - | - | - | not applicable. |
| 2.3 | Livestock farming and ranching | Negligible | Restricted (11-30%) | Negligible (<1%) | High (Continuing) | cattle trampling is applicable. |
| 2.4 | Marine and freshwater aquaculture | - | - | - | - | not applcable |
| 3 | Energy production and mining | Negligible | Negligible (<1%) | Unknown | Low (Possibly in the long term, >10 yrs) | - |
| 3.1 | Oil and gas drilling | - | - | - | - | not applicable |
| 3.2 | Mining and quarrying | Negligible | Negligible (<1%) | Unknown | Low (Possibly in the long term, >10 yrs) | Limited mining, dependent on metals market; current moratorium on uranium mining Uranium mine unlikely in the next 10 years. Granby mining is historical. One uranium mine at Beaver Dell. Unknown whether the mine development is planned directly over aquatic habitat. |
| 3.3 | Renewable energy | - | - | - | - | not applicable. |
| 4 | Transportation and service corridors | Negligible | Negligible (<1%) | Unknown | Moderate (Possibly in the short term, < 10 yrs) | - |
| 4.1 | Roads and railroads | Negligible | Negligible (<1%) | Unknown | Moderate (Possibly in the short term, < 10 yrs) | new roads planned for development. Unknown whether spawning habitat or inshore riparian zones are for this species so unknown what impact on the population road development or bridge abutment will have. Scope is limited. Also currently unknown what the current plans and timing are for this area so this should be researched to better quantify this threat (moderate may be changed to high if this is clarified). |
| 4.2 | Utility and service lines | - | - | - | - | applicable in the past. Not future. |
| 4.3 | Shipping lanes | - | - | - | - | not applicable. |
| 4.4 | Flight paths | - | - | - | - | not applicable |
| 5 | Biological resource use | Negligible | Negligible (<1%) | Slight (1-10%) | High (Continuing) | - |
| 5.1 | Hunting and collecting terrestrial animals | - | - | - | - | not applicable |
| 5.2 | Gathering terrestrial plants | - | - | - | - | not applicable |
| 5.3 | Logging and wood harvesting | Negligible | Negligible (<1%) | Slight (1-10%) | High (Continuing) | logging currently occurs in headwaters of Kettle River system; southern portion of watershed, where most Speckled Dace occur, is not suitable for forestry. Logging may be beneficial. Siltation accounted for under pollution 9.3. Riparian habitat logging would be considered (West Kettle and Kettle). Land tenure is unknown. not a lot of crown buffering for regulations on clear cutting because most of land is privately owned. High threat impact over the past 20 yrs but negligible over the next 10 years. Removal of branches over the riparian is detrimental. |
| 5.4 | Fishing and harvesting aquatic resources | - | - | - | - | bait fish not permitted under sport fishing regulations in BC. By catch? Dace is small so unlikely. Not applicable. |
| 6 | Human intrusions and disturbance | Negligible | Small (1-10%) | Negligible (<1%) | - | - |
| 6.1 | Recreational activities | Negligible | Small (1-10%) | Negligible (<1%) | High (Continuing) | recreation is a threat. Boating. |
| 6.2 | War, civil unrest and military exercises | - | - | - | - | not applicable. |
| 6.3 | Work and other activities | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | research? Some bycatch in fisheries research. Very small portion that would be affected by non-targeted research resulting in mortality. Some shocking in these streams. Electrofishing was not authorized for one research proposal for critical habitat identification. |
| 7 | Natural system modifications | CD - Medium - Low | Large (31-70%) | Moderate - Slight (1-30%) | High (Continuing) | - |
| 7.1 | Fire and fire suppression | D - Low | Restricted - Small (1-30%) | Slight (1-10%) | High (Continuing) | clearing of riparian areas to suppress fires as well as fires burning riparian areas and water withdrawal for suppression of natural fires. |
| 7.2 | Dams and water management/use | CD - Medium - Low | Large (31-70%) | Moderate - Slight (1-30%) | High (Continuing) | High rates of water withdrawal occur in southern part of river system during natural low flow period; proposed hydroelectric project expected to have minimal impact. Most of the water use in this area is for agriculture. Upper Kettle and Large Kettle water withdrawal is less of a problem. Proposal for ski resort to increase water extraction for condo development and snow making. Big White is subject to water withdrawal but is negligible and not accounted for in this threat category. Combination of water withdrawal for agriculture and other uses. Distribution of this effect is small in the Kettle, Granby, upper reaches. More important impact in the lower reaches. Run of the river project will also have a small impact though its not a consumptive use of the water. direct mortality to individuals caught in the turbine. |
| 7.3 | Other ecosystem modifications | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | removal of twigs and planting native trees and restoration. Restoration will cause stabilization of the river banks as well as stopping cattle from accessing the streams. Siltation from roads is accounted for under threat 9 (pollution). |
| 8 | Invasive and other problematic species and genes | Medium - Low | Pervasive (71-100%) | Moderate - Slight (1-30%) | High - Moderate | - |
| 8.1 | Invasive non-native/alien species | Medium - Low | Pervasive (71-100%) | Moderate - Slight (1-30%) | High - Moderate | Brook Trout non-predatory (competitive exclusion) throughout Speckled Dace range (lower range accounted). Bass, Pike, Walleye (predatory) below the falls (Cascade Falls) may be accidentally introduced above the falls (higher range accounted). |
| 8.2 | Problematic native species | - | - | - | - | Rainbow Trout habitat restoration planned (beneficial for Dace). Not a threat. Threat of Didyimo needs to be researched and perhaps rescore this threat. |
| 8.3 | Introduced genetic material | - | - | - | - | not applicable |
| 9 | Pollution | Medium - Low | Large (31-70%) | Moderate - Slight (1-30%) | High (Continuing) | - |
| 9.1 | Household sewage and urban waste water | Unknown | Large (31-70%) | Unknown | High (Continuing) | septic tanks? oil residue? absence of fish below the Grand Forks sewage treatment plant but may be artifact of development. Unknown. May be beneficial in terms of increased nutrients. |
| 9.2 | Industrial and military effluents | Negligible | Negligible (<1%) | Unknown | High (Continuing) | low abundance of fish below pulp mill at Midway may have discharge affecting water quality. Unknown. |
| 9.3 | Agricultural and forestry effluents | CD - Medium - Low | Large (31-70%) | Moderate - Slight (1-30%) | High (Continuing) | soil erosion, siltation from cows, sedimentation from logging (forestry) and from road use. nutrient loading from agriculture. |
| 9.4 | Garbage and solid waste | - | - | - | - | not applicable |
| 9.5 | Air-borne pollutants | - | - | - | - | not applicable |
| 9.6 | Excess energy | - | - | - | - | not applicable |
| 10 | Geological events | - | - | - | - | - |
| 10.1 | Volcanoes | - | - | - | - | not applicable |
| 10.2 | Earthquakes/ tsunamis | - | - | - | - | not applicable |
| 10.3 | Avalanches/landslides | - | - | - | - | no landslides in this area in BC. Not applicable. |
| 11 | Climate change and severe weather | CD - Medium - Low | Pervasive (71-100%) | Moderate - Slight (1-30%) | High (Continuing) | - |
| 11.1 | Habitat shifting and alteration | - | - | - | - | not applicable |
| 11.2 | Droughts | - | - | - | - | Increasing severity, duration and frequency of droughts exacerbates low flow conditions, reducing habitat and changing food supply. warmer temperatures may be beneficial in some cases (longer season) and detrimental in others (changes to food supply). reduction in water (quantity). West Kettle had the lowest water levels in history this year and it is likely to persist. Significant loss of habitat. |
| 11.3 | Temperature extremes | - | - | - | - | not applicable |
| 11.4 | Storms and flooding | - | - | - | - | not applicable |
Classification of Threats adopted from IUCN-CMP, Salafsky et al. (2008).