Sweet pepperbush (Clethra alnifolia): COSEWIC assessment status report 2014

THREATENED
2014
Table of Contents
- Document Information
- COSEWIC Assessment Summary
- COSEWIC Executive Summary
- Technical Summary
- Preface
- Wildlife Species Description and Significance
- Distribution
- Habitat
- Biology
- Population Sizes and Trends
- Threats and Limiting Factors
- Protection, Status, and Ranks
- Acknowledgements and Authorities Contacted
- Information Sources
- Biographical Summary of Report Writer(s)
- Collections Examined
List of Figures
- Figure 1. Sweet Pepperbush (Clethra alnifolia) stand at the outlet of Mudflat Lake. The shrubby lakeshore margin extending to the rear of the picture is dominated by Sweet Pepperbush. Photograph by Megan Crowley, Parks Canada
- Figure 2. Native range (green shading) of Sweet Pepperbush (Clethra alnifolia). The map is modified from Kartesz (2011). In the United States a whole county is shaded if at least one record is known
- Figure 3. Distribution of Sweet Pepperbush (Clethra alnifolia; red dots) in Nova Scotia at 1 – Belliveau Lake, 2 – Pretty Mary Lake, Mudflat Lake and Mill Lake, 3a – Louis Lake and 3b – Canoe Lake. Dark lines are county boundaries. Inset map indicates location of the larger map within Nova Scotia
Document Information

Committee on the Status of Endangered Wildlife in Canada (COSEWIC) status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2014. COSEWIC assessment and status report on the Sweet Pepperbush Clethra alnifolia in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 41 pp. (Species at Risk Public Registry website).
Previous report(s):
COSEWIC. 2001. COSEWIC assessment and update status report on the sweet pepperbush Clethra alnifoliain Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 11 pp.
Newell, R.E. 2001. Update COSEWIC status report on the sweet pepperbush Clethra alnifolia in Canada in COSEWIC assessment and update status report on the sweet pepperbush Clethra alnifolia in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-11 pp.
Taschereau, P.M. 1986. COSEWIC status report on the sweet pepperbush Clethra alnifolia in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 58 pp.
Production note:
COSEWIC would like to acknowledge Sean Blaney of the Atlantic Canada Conservation Data Centre for writing the status report on Sweet Pepperbush, Clethra alnifolia, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Bruce Bennett, Co-chair of the COSEWIC Vascular Plants Specialist Subcommittee.
For additional copies contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment Canada
Ottawa, ON
K1A 0H3
Tel.: 819-953-3215
Fax: 819-994-3684
COSEWIC E-mail
COSEWIC web site
Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur le Clèthre à feuilles d'aulne (Clethra alnifolia) au Canada.
Cover illustration/photo:
Sweet Pepperbush -- Photo provided by author
©Her Majesty the Queen in Right of Canada, 2014.
Catalogue No. CW69-14/157-2014E-PDF
ISBN 978-1-100-24012-1

COSEWIC Assessment Summary
Assessment Summary - November 2014
- Common name
- Sweet Pepperbush
- Scientific name
- Clethra alnifolia
- Status
- Threatened
- Reason for designation
- This disjunct Atlantic Coastal Plain clonal shrub is restricted to the shores of six lakes in a small area of southern Nova Scotia. Newly identified threats from the invasive exotic shrub Glossy Buckthorn and eutrophication have put this species at increased risk of extirpation. Shoreline development also remains a threat.
- Occurrence
- Nova Scotia
- Status history
- Designated Threatened in April 1986. Status re-examined and confirmed in April 1998. Status re-examined and designated Special Concern in May 2001. Status re-examined and designated Threatened in May 2014.
COSEWIC Executive Summary
Sweet Pepperbush
Clethra alnifolia
Wildlife Species Description and Significance
Sweet Pepperbush is a deciduous, woody, wetland shrub 1 to 3 m tall that can grow in a clumped form or with single stems arising from a spreading rhizome (underground stem). The dense, narrowly elongate flower clusters are 4 to 12 cm long and composed of small, white, 5-petalled flowers that are strongly fragrant. Fertilized flowers mature into dry, round capsules with many small seeds, though seed production has been reported as sometimes absent or rare in Canada.
Sweet Pepperbush is one of many nationally rare, disjunct species of the Atlantic Coastal Plain in southern Nova Scotia. Outreach programs have resulted in fairly wide understanding and appreciation of this rare flora. Sweet Pepperbush is particularly appreciated by some landowners because of its showy flowers and strong, pleasant fragrance, characteristics that have made it a widely used ornamental species with many developed cultivars. Canadian populations are isolated from others by 200+ km and are the northernmost worldwide, suggesting potential significance to the species’ range-wide genetic diversity.
Distribution
Sweet Pepperbush is native to the eastern United States and southern Nova Scotia, from Maine to western Texas, primarily along the Atlantic Coastal Plain (excluding southern Florida) and into the Piedmont plateau region of the eastern USA within about 150 km of the coast. In Canada, Sweet Pepperbush is restricted to three subpopulations on six lakes in southern Nova Scotia within a 70 km by 60 km area. It has become marginally established after escaping from cultivation in Belgium, The Netherlands, and England. Canada supports less than 1% of the global population.
Habitat
In Nova Scotia, Sweet Pepperbush is a species of acidic upper lakeshores and lakeshore forest margins, also occurring locally along shrubby and semi-forested stream margins and under Red Maple-dominated swamp forest canopy within about 20 m of shorelines. It has not been observed to flower when under dense forest canopy in Nova Scotia. Similar habitats are occupied throughout its range, but prevalence in shaded and upland areas is more frequent in the United States where occurrence in upper salt marsh margins is also noted.
Biology
Sweet Pepperbush flowers in Nova Scotia from late July to early September. Pollination is primarily or exclusively by insects, especially bees. Sweet Pepperbush exhibits strong, but not complete self-incompatibility. Coupled with theorized low genetic variability, this could cause the limited seed production noted at Belliveau Lake and suspected elsewhere in Nova Scotia, where seedling establishment is rare. The tiny seeds remain in the capsules into late fall or winter and could be moved by water, wind, and vertebrates (via clinging mud). Seeds can germinate immediately after dispersal but germination is enhanced by cold stratification. Seed longevity is unknown. Average time to first flowering from seed in the field is probably more than ten years. Individual stems can live at least 28 years. Most reproduction is by spreading rhizomes which can produce new shoots up to 2.4 m from the parent plant. These allow colonization of wetter areas where seedling establishment is difficult and form a "sprout bank" that can respond rapidly to canopy openings. Time to flowering and to vegetative reproduction for new vegetative shoots is likely at least several years. Generation time could be at least 10 years. Clumps of stems (which continually resprout from the base) and complexes of connected genetic individuals are presumably much longer-lived.
Population Sizes and Trends
The Canadian population is not more than 45,471 individuals based on stem numbers estimated from comprehensive 2011 and 2012 surveys. Stems counts overestimate number of mature individuals because some tightly clumped stems are best classed as single individuals and smaller stems may be unable to reproduce sexually or vegetatively. The degree of this overestimation is unknown. Stem estimates by subpopulation are: 1) Belliveau Lake – 16,070; 2) Pretty Mary, Mudflat, and Mill lakes – 27,700; 3) Louis Lake and Canoe Lake – 1,700 individuals, with only a single individual at Canoe Lake.
Threats and Limiting Factors
Competition from the invasive exotic shrub Glossy Buckthorn is already occurring to a very limited extent and is likely to become more severe at the Pretty Mary-Mudflat-Mill lakes subpopulation, where thousands of mature Glossy Buckthorn plants are present on abandoned farmland adjacent to the lakes. Glossy Buckthorn is perhaps 10 km away from Belliveau Lake and 40 km away from Louis Lake and is likely to reach those sites within one to several decades. The timing and magnitude of its impact is uncertain.
Eutrophication from leaching sewage ponds on an abandoned hog farm at Belliveau Lake is changing habitat on one corner of the lake where Sweet Pepperbush occurs. Impacts on the species are unclear, but could become significant, especially if coupled with Glossy Buckthorn invasion.
Shoreline development has slowly but steadily increased on Belliveau, Pretty Mary, and Mudflat lakes over the past 30 years and will likely continue. It is also a threat on currently undeveloped Mill Lake. Landowners frequently cut and remove some (but generally not all) Sweet Pepperbush for shore access and to enhance views, with overall losses to shoreline development up to the present roughly estimated at less than 4.6%.
A long-standing but poorly maintained dam on Mill Lake may be limiting occurrence there and if it was breached it might make conditions less suitable for existing Sweet Pepperbush and allow rapid influx of Glossy Buckthorn from large nearby populations. Limited genetic variability resulting in limited seed production is speculated to be a major limiting factor in Nova Scotia, which would explain the absence of Sweet Pepperbush over vast areas of suitable habitat.
Protection, Status, and Ranks
About 94% of Sweet Pepperbush habitat in Canada is on private land. All of the Louis Lake – Canoe Lake population and 10% of the Belliveau Lake population are on provincial Crown land that is likely to be included in new nature reserves soon to be finalized.
Sweet Pepperbush is currently listed as Special Concern in Canada by COSEWIC and under Schedule 1 of the Species at Risk Act and Vulnerable under the Nova Scotia Endangered Species Act. It is Endangered in Tennessee under the state’s Rare Plant Protection and Conservation Act of 1985, but has no legal protection elsewhere. Non-legal NatureServe ranks are: Globally secure (G5) and nationally (N5) secure in the United States; Critically Imperilled (N1) in Canada, Nova Scotia (S1), Tennessee (S1), and Imperiled (S2) in Maine. It is considered Sensitive in Canada and Nova Scotia by the National General Status Working Group.
Technical Summary
Clethra alnifolia
Sweet Pepperbush
Clèthre à feuilles d'aulne
- Range of occurrence in Canada (province/territory/ocean):
- Nova Scotia
Demographic Information
-
Generation time (usually average age of parents in the population)
Incompletely understood, but individual stems up to at least 28 years old. See Life Cycle and Reproduction. - Perhaps 10+ years
-
Is there an [observed, inferred, or projected] continuing decline in number of mature individuals?
Small declines inferred from observed and projected losses to shoreline development, otherwise apparent stability. - Yes
-
Estimated percent of continuing decline in total number of mature individuals within 2 generations.
Small declines likely to continue due to development and perhaps invasives and eutrophication. - Unknown
-
Estimated percent reduction in total number of mature individuals over the last 3 generations.
Inferred from observed habitat loss; See Threats – Shoreline Development - Probably under 4.6%
-
[Projected or suspected] percent [reduction or increase] in total number of mature individuals over the next [10 years, or 3 generations].
Small declines from shoreline development; further potential declines from Glossy Buckthorn invasion and eutrophication - Unknown
-
[Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over any [10 years, or 3 generations] period, over a time period including both the past and the future.
As above - Unknown
-
Are the causes of the decline clearly reversible and understood and ceased?
Shoreline development is not readily reversible; Glossy Buckthorn invasion and eutrophication potentially reversible with intensive management action. Causes are understood, but not ceased. - Causes are understood and partially reversible but not ceased
- Are there extreme fluctuations in number of mature individuals?
- No
Extent and Occupancy Information
- Estimated extent of occurrence
- 1,984 km2
-
Index of area of occupancy (IAO) – 2 x 2 km grid
Derived from a 2 x 2 km grid aligned with 10 x 10 km UTM grid squares. - 52 km2
- Is the total population severely fragmented?
- Insert text here
-
Number of locations
Extent and Occupancy Information Footnote1
3 locations if defined by watercourse based on threat of Glossy Buckthorn and eutrophication. If Belliveau Lake locations are defined by threat of shoreline development, total number of Canadian locations is between 5 and 46. See "Number of Locations". - 3
- Is there an [observed, inferred, or projected] continuing decline in extent of occurrence?
- No
- Is there an [observed, inferred, or projected] continuing decline in index of area of occupancy?
- No
- Is there an [observed, inferred, or projected] continuing decline in number of populations?
- No
- Is there an [observed, inferred, or projected] continuing decline in number of locations?
- No
-
Is there an [observed, inferred, or projected] continuing decline in [area, extent and/or quality] of habitat?
Glossy Buckthorn, shoreline development and eutrophication are all contributing to reduced habitat quality. Shoreline development reducing habitat area. - Yes
- Are there extreme fluctuations in number of populations?
- No
- Are there extreme fluctuations in number of locations Extent and Occupancy Information Footnote1?
- No
- Are there extreme fluctuations in extent of occurrence?
- No
- Are there extreme fluctuations in index of area of occupancy?
- No
| Population | N Mature Individuals (Except for Canoe Lake, numbers below are based on stem counts, which are believed to overestimate individuals; see Sampling Effort and Methods and Life Cycle and Reproduction) |
|---|---|
| Subpop. 1 – Belliveau Lake | < 16,070 |
| Subpop. 2 – Pretty Mary, Mudflat and Mill lakes | < 27,700 |
| Subpop. 3, site a – Louis Lake | < 1,700 |
| Subpop. 3, site b – Canoe Lake | 1 individual (4 stems) |
| Total | < 45,471 |
Quantitative Analysis
- Probability of extinction in the wild is at least [20% within 20 years or 5 generations, or 10% within 100 years].
- N/A
Threats (actual or imminent, to populations or habitats)
- Competition from invasive Glossy Buckthorn (actual threat at Pretty Mary, Mudflat and Mill lakes, future threat elsewhere)
- Increased competition caused by eutrophication (actual threat of uncertain magnitude at Belliveau Lake only)
- Habitat loss and cutting of individuals associated with existing and new shoreline development (actual threat at all lakes but Louis and Canoe)
- Habitat change and potential facilitation of Glossy Buckthorn invasion if Mill Lake dam gives way (immediacy of threat hard to assess)
Rescue Effect (immigration from outside Canada)
- Status of outside population(s):
- USA: Secure (S5). Critically Imperiled (S1) in Tennessee. Imperiled (S2) in Maine. Secure (S5) in Alabama, Delaware, New Jersey, New York and Virginia. Not ranked (SNR; generally because it is considered secure) in Connecticut, District of Columbia, Florida, Georgia, Louisiana, Maryland, Massachusetts, Mississippi, New Hampshire, North Carolina, Pennsylvania, Rhode Island, South Carolina, and Texas. Introduced but only marginally established in England, Belgium, The Netherlands and likely elsewhere in Europe.
- Is immigration known or possible?
- Not known and unlikely
-
Would immigrants be adapted to survive in Canada?
Southern Maine populations occur in a similar climate zone - Probably
- Is there sufficient habitat for immigrants in Canada?
- Yes
-
Is rescue from outside populations likely?
Disjunct from nearest Maine populations (where rare – S2) by 200+ km across open ocean. Disjunct from areas where more common by 400+ km across open ocean. - No
Data-Sensitive Species
- Is this a data-sensitive species?
- No
Status History
- COSEWIC:
- Disjunct from nearest Maine populations (where rare – S2) by 200+ km across open ocean. Disjunct from areas where more common by 400+ km across open ocean.
Status and Reasons for Designation
- Status:
- Threatened
- Alpha-numeric code:
- Met criteria for Endangered, B1ab(iii,v)+2ab(iii,v), but designated Threatened, B1ab(iii,v)+2ab(iii,v), due to the long lifespan of the species and the slow-acting main threat of competition from Glossy Buckthorn.
- Reason for Designation:
- This disjunct Atlantic Coastal Plain clonal shrub is restricted to the shores of six lakes in a small area of southern Nova Scotia. Newly identified threats from the invasive exotic shrub Glossy Buckthorn and eutrophication have put this species at increased risk of extirpation. Shoreline development also remains a threat.
Applicability of Criteria
- Criterion A (Decline in Total Number of Mature Individuals):
- Not met. Declines below thresholds.
- Criterion B (Small Distribution Range and Decline or Fluctuation):
- Meets Endangered B1ab(iii,v)+2ab(iii,v) because the EO (1,984 km 2) and IAO (52 km 2) are below thresholds, 3 locations are threatened by invasive plants and eutrophication, and the habitat generally is being degraded by the invading Glossy Buckthorn, shoreline development, and eutrophication. Shoreline development continues to reduce habitat area. There is a decline in the number of mature individuals lost through shoreline development. The population does not undergo extreme fluctuations and is currently not severely fragmented.
- Criterion C (Small and Declining Number of Mature Individuals):
- Not met. Number of mature individuals exceeds thresholds.
- Criterion D (Very Small or Restricted Total Population): :
- Not met. Number of mature individuals exceeds thresholds and a rapid deterioration is not likely to occur in a short time frame (1-2 generations).
- Criterion E (Quantitative Analysis):
- Not done.
Preface
The Canadian population of Sweet Pepperbush appears to have experienced minor declines from shoreline development since its last status assessment in 2001, but new threats have been identified that could cause significant future declines. Competition from the invasive exotic shrub Glossy Buckthorn is already present to a limited extent and has potential to become significant in the near future at Pretty Mary, Mudflat, and Mill lakes. Glossy Buckthorn is also likely to become established at other Sweet Pepperbush sites within three generations with impacts within this timeframe unclear. Nutrient enrichment from the leaching sewage ponds of an inactive hog farm are likely responsible for a dense Broadleaf Cattail mat covering a few hectares (an exceptionally large occurrence for a southern Nova Scotia lake), within an area of dense Sweet Pepperbush occurrence at Belliveau Lake. Nutrient enrichment is a threat to Atlantic Coastal Plain flora generally and probably to Sweet Pepperbush specifically, but no obvious population impacts on the plants at Belliveau Lake are yet evident. A long-standing 1.5 m dam on Mill Lake is another newly recognized issue. It may have reduced local Sweet Pepperbush subpopulations from pre-dam levels and likely limits its current distribution. The largest threat posed by the dam is that it will give way and result in conditions less suitable for existing Sweet Pepperbush while exposing unoccupied habitat that could be rapidly taken over by Glossy Buckthorn. The threat of shoreline development has been more clearly quantified since 2001.
Sweet Pepperbush abundance and distribution have been much more accurately determined since 2001, with comprehensive shoreline surveys delimiting distribution on all known lakes and producing population counts on Belliveau, Mill and Mudflat lakes. Despite survey effort on 172 lakes within its potential range since 2001, no new occurrences have been found and area of occupancy and extent of occurrence are unchanged. The absence of new occurrences despite extensive survey effort has further demonstrated the species’ rarity in Canada.
Protection has improved somewhat since 2001. Crown land occurrences (Louis Lake, Canoe Lake, and 460 m of shore at three Belliveau Lake properties) are likely to be designated provincial nature reserves in the near future, though this will not affect the threats associated with Glossy Buckthorn and eutrophication. Landowner knowledge, appreciation and stewardship of Sweet Pepperbush are also likely improved due to outreach initiatives at Pretty Mary and Mudflat lakes.
Sweet Pepperbush is now more extensively available in the horticultural trade in Nova Scotia, and is known in cultivation throughout most of the province, including one lakeshore record 12 km from the Louis Lake population.

COSEWIC History
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
COSEWIC Mandate
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC Membership
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
Definitions (2014)
Wildlife Species
A species, subspecies, variety, or geographically or genetically distinct population of animal, plant or other organism, other than a bacterium or virus, that is wild by nature and is either native to Canada or has extended its range into Canada without human intervention and has been present in Canada for at least 50 years.
- Extinct (X)
- A wildlife species that no longer exists.
- Extirpated (XT)
- A wildlife species no longer existing in the wild in Canada, but occurring elsewhere.
- Endangered (E)
- A wildlife species facing imminent extirpation or extinction.
- Threatened (T)
- A wildlife species likely to become endangered if limiting factors are not reversed.
- Special Concern (SC)Footnotea
- A wildlife species that may become a threatened or an endangered species because of a combination of biological characteristics and identified threats.
- Not at Risk (NAR)Footnoteb
- A wildlife species that has been evaluated and found to be not at risk of extinction given the current circumstances.
- Data Deficient (DD)Footnotec
- A category that applies when the available information is insufficient (a) to resolve a species’ eligibility for assessment or (b) to permit an assessment of the species’ risk of extinction.
The Canadian Wildlife Service, Environment Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Wildlife Species Description and Significance
Name and Classification
- Scientific Name:
- Magnoliopsida, asterid clade (APG 2003)
- Original Description:
- Linnaeus, Species Plantarum 2 (1753)
- Synonym:
-
Clethra alnifolia Blanco
Clethra angustifolia Raf.
Clethra glauca Hortul. ex Steud.
Clethra nana Raf.
Clethra paniculata Ait.
Clethra pubescens Willd.
Clethra pumila Raf.
Clethra tomentosa Lamk.
Clethra alnifolia L. var. denudata Ait.
Clethra alnifolia L. var. glabella Michx.
Clethra alnifolia L. var. michauxii Zabel
Clethra alnifolia L. var. paniculata (Ait.) Nicholson
Clethra alnifolia L. var. scabra Rehder
Clethra alnifolia L. var. scabra Zabel
Clethra alnifolia L. var. tomentosa Michx.
Clethra alnifolia f. roseaRehder - Class:
- Magnoliopsida, asterid clade (APG 2003)
- Order:
- Ericales
- Family:
- Clethraceae
- Class :
- Magnoliopsida, asterid clade (APG 2003)
- Major Plant Group :
- Angiosperms, Eudicotyledons
- English Vernacular Names:
- Sweet Pepperbush, White Alder, Summer-Sweet
- French Vernacular Name:
- Clèthre à feuilles d’aulne
Clethra alnifolia was first described by Linnaeus in 1753. Eight species and seven varieties described between 1789 and 1841 (above; IPNI 2005) have been treated in synonymy with Clethra alnifolia in major North American floras back at least to Fernald (1950). Sleumer (1967) and many previous authors (summarized in Wilbur and Hespenheide 1967) recognized two varieties: alnifolia (occurring throughout the species’ range and presumably including Nova Scotia plants, which were not known at the time) and tomentosa, with white tomentose leaf undersides, occurring from southern North Carolina to Louisiana. Wilbur and Hespenheide (1967), however, examined all characters used to distinguish var. tomentosa, and found no basis to recognize it at any taxonomic rank. Clethra alnifolia var. tomentosa is still sometimes distinguished (i.e. Reed et al. 2002; Weakley 2012), as is the pink-flowered C. alnifolia f. rosea.
The family Clethraceae has at times been placed within the heath family Ericaceae (i.e. Robinson and Fernald 1908; Benson 1979), but recent analyses of plastid, mitochondrial and nuclear DNA confirm its treatment as a separate family within the ericad clade (APG 1998; Anderberg et al. 2002; Fior et al. 2003).
Morphological Description
Sweet Pepperbush (Clethra alnifolia) is a deciduous woody shrub 1 to 3 m tall (Figure 1). Aerial stems arise from a branching rhizome and can be close together giving a clumped appearance, or spread along the rhizome as far as 2.4 m apart (Laycock 1967). Sweet Pepperbush leaves are alternate, obovate (egg-shaped and widest near the tip), pointed at both ends and have toothed margins. Inflorescences are at the branch tips and are dense upright racemes (narrowly elongate clusters), 4 to 12 cm long, of small, white, radially symmetrical, 5-petalled flowers (Figure 1). The flowers have a strong, pleasant odour described as "deliciously fragrant" (Fernald 1950). Fertilized flowers mature into dry, round capsules with many (up to 30+, Reed et al. 2002) small seeds, though seed production has been reported as locally absent or rare in Canada (Taschereau 1986). The capsules remain on the shrubs into the winter. Sweet Pepperbush has a chromosome number of 2n = 32 (Hagerup 1928; Tanaka and Oginuma 1980; Reed 2005).

Photo: © Environment Canada 2014.
Long description for Figure 1
Photo of a stand of Sweet Pepperbush shrubs at the outlet of Mudflat Lake, Nova Scotia. These woody shrubs are 1 to 3 metres tall and have a clumped appearance. The leafy stems bear narrowly elongate clusters (4 to 12 centimetres long) of small white flowers at their tips.
Population Spatial Structure and Variability
Sweet Pepperbush is known from three areas in southern Nova Scotia, each of which is 50 to 70 km from the others. Seed production was reportedly absent from the large Belliveau Lake subpopulation (Taschereau 1986), but seeds of unknown viability have since been collected from the site. Seed production has not been reported at the Pretty Mary – Mudflat – Mill lakes subpopulation, and no seedlings were observed there in 2012 surveys (Belliveau pers. comm. 2012). Limited numbers of seedlings have been observed at the Louis Lake subpopulation in the two most recent surveys in 2000 and 2012 (Hill et al. 2000; COSEWIC 2001; Hill pers. comm. 2012). The distances between the Canadian subpopulations and the possibility of limited seed production (which would further limit dispersal potential) suggest that the three Canadian subpopulations are genetically isolated from one another.
Sweet Pepperbush is strongly, though not completely, self-incompatible (Reed et al.2002; Reed 2006) and reproduces most frequently by production of vegetative shoots (Jordan 1993; Jordan and Hartman 1995). Genetic diversity of Canadian subpopulations has not been investigated, but if limited seed production is a general condition in Canada it is most likely caused by limited genetic diversity given extensive pollinator activity (COSEWIC 2001; Belliveau pers. comm. 2012; Hill pers. comm. 2012) and climate similar to the northern part of its American range where seed production is not limited (Jordan and Hartman 1995).
All three Canadian subpopulations have large numbers of individuals with gaps between occurrences not exceeding 0.7 km (except for Canoe Lake, discussed below). Movement of pollen by bees (the primary pollinators of the Sweet Pepperbush, see Interspecific Interactions), and potentially other pollinators, over that distance is likely frequent. Osborne et al. (2008) found foraging distances of at least 1.5 km in studies of the Buff-tailed Bumblebee, Bombus terrestris. Walter-Hellwig and Frankl (2000) recorded a similar maximum forage distance of 1.75 km for that species.
The single shrub at Canoe Lake is 1.9 km from the nearest known Sweet Pepperbush on Louis Lake and pollinations over that distance may be limited. Given the species' self in compatibility, the single shrub at the Canoe Lake occurrence is not considered a viable subpopulation on its own.
Sweet Pepperbush subpopulations in Canada are composed of large numbers of "individuals" as defined by COSEWIC (2010), within large areas of suitable habitat, and appear to have good viability at least as clonal subpopulations. The species is thus not considered severely fragmented (COSEWIC 2010).
Designatable Units
In Canada, Sweet Pepperbush is restricted to a small portion of the COSEWIC Atlantic Ecological Area in southwestern Nova Scotia, thus Canadian subpopulations should be considered a single designatable unit.
Special Significance
Sweet Pepperbush is one of a large suite of disjunct southern species of the Atlantic Coastal Plain in southern Nova Scotia, many of which are rare in Canada (Environment Canada and Parks Canada Agency 2010). Ongoing stewardship and outreach programs have resulted in these rare species being known and appreciated by many cottagers, residents and visitors. Sweet Pepperbush is particularly appreciated by some cottagers because of its showy flowers and strong, pleasant fragrance.
These characteristics have resulted in Sweet Pepperbush being a widely used ornamental species in North America and in Europe, where it has been cultivated since 1731 (Taschereau 1986). There are many named cultivars of Sweet Pepperbush available commercially and this species is especially recommended for planting to attract native pollinators and as an alternative to invasive, non-North American shrub species (Cullina 2003; Clemson Extension 2010; Missouri Botanical Garden 2013).
Canadian subpopulations of Sweet Pepperbush are isolated from other occurrences by more than 200 km and are the northernmost occurrences worldwide. Canadian subpopulations could thus have a disproportionate significance to the species’ range-wide genetic diversity (Lesica and Allendorf 1995; Garcia-Ramos and Kirkpatrick 1997; Eckert et al. 2008).
There appears to be little or no evidence of medicinal use of Sweet Pepperbush in the literature, which is somewhat surprising given the medicinal use of other Clethra species (below), Sweet Pepperbush’s abundance in the eastern United States, and its apparent resistance to herbivores (see Interspecific Interactions and Physiology and Adaptability). The Japanese Summersweet (Clethra barbinervis, closely related to Sweet Pepperbush) and the South American Clethra castaneifolia have been investigated chemically because of traditional medicinal uses, with antifungal and antibacterial properties documented (Tanabe et al. 1966; Takani et al. 1977; Takahashi and Takani 1978; Furumai et al. 2003; Bussman et al. 2010, 2011; Murata et al. 2013).
Sweet Pepperbush is well known to beekeepers of the eastern United States as a good honey plant (Hemingson 1986 and references therein).
No evidence of Canadian aboriginal traditional knowledge of this species was found during the preparation of this report (Hurlburt pers. comm. 2013).
Distribution
Global Range
Sweet Pepperbush is native to the eastern United States and southern Nova Scotia (Figure 2). It occurs from southern Maine to western Texas, primarily along the Atlantic Coastal Plain (excluding southern Florida) and into the Piedmont plateau region of the eastern USA within about 150 km of the coast. Occurrence west of eastern Louisiana and in areas further inland is restricted to scattered localities in Texas, Louisiana, Tennessee, western New York and western Pennsylvania (Kartesz 2011). In Canada, Sweet Pepperbush is restricted to three subpopulations on six lakes in southern Nova Scotia within a 70 km by 60 km area.
Sweet Pepperbush is widely planted as an ornamental species but has not been recorded as establishing beyond its native range in North America (Kartesz 2011), although it might be difficult to identify peripheral occurrences as non-native. It is documented as marginally established via persistence of cultivated individuals or localized spread from cultivation in Belgium (Verloove 2006), The Netherlands (van der Meijden 2005 in National Botanic Garden of Belgium 2013) and England (Clement and Foster 1994 in National Botanic Garden of Belgium 2013). Reports of the species as a well-established invasive on Sao Miguel in the Azores Archipelago (Ramos 1995) are in error and actually refer to the Lily of the Valley Tree (Clethra arborea; Silva and Smith 2004).
Canada supports less than 1% of the global population.
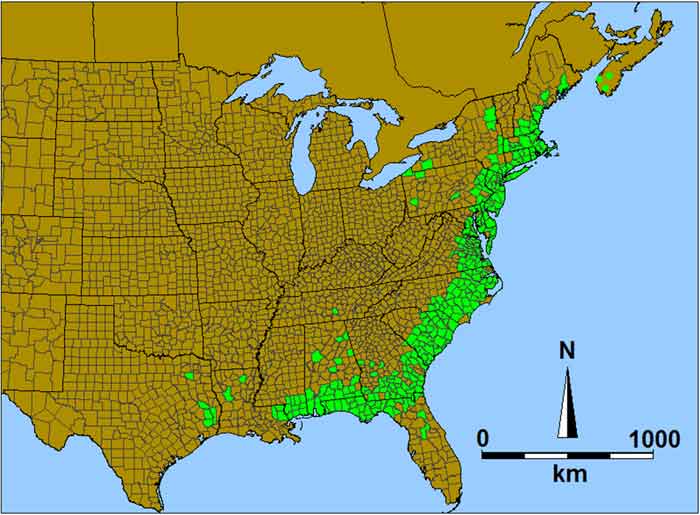
Map: Native range (green shading) of Sweet Pepperbush © Environment Canada 2014.
Long description for Figure 2
Map showing the native range of the Sweet Pepperbush in the eastern United States and southern Nova Scotia. The Sweet Pepperbush occurs from southern Maine to western Texas, primarily along the Atlantic Coastal Plain (excluding southern Florida) and into the Piedmont plateau region of the eastern United States, within about 150 kilometres of the coast. Occurrence west of eastern Louisiana and in areas farther inland is restricted to scattered localities in Texas, Louisiana, Tennessee, western New York, and western Pennsylvania. In Canada, Sweet Pepperbush is restricted to three subpopulations on six lakes in southern Nova Scotia within a 70 by 60 kilometre area.
Canadian Range
In Canada, Sweet Pepperbush is restricted to the COSEWIC Atlantic National Ecological Area in southwest Nova Scotia (Figure 3). It is known only from Louis Lake and nearby Canoe Lake (one shrub only) in southern Yarmouth County, from Belliveau Lake 50 km northwest in Digby County and from three adjacent and hydrologically connected lakes in southern Annapolis County (Pretty Mary, Mudflat, and Mill lakes) 75 km northeast of Louis Lake and 72 km east of Belliveau Lake.
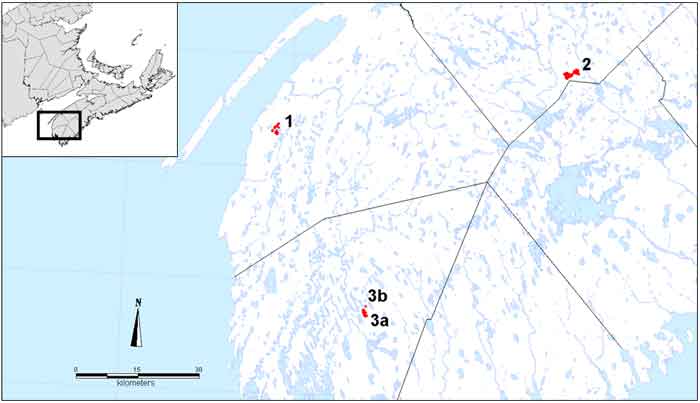
Map: Distribution of Sweet Pepperbush © Environment Canada 2014.
Long description for Figure 3
Map of showing the Canadian distribution of the Sweet Pepperbush in the COSEWIC Atlantic National Ecological Area in southwest Nova Scotia. Sweet Pepperbush is known from three areas, each of which is 50 to 70 kilometres from the others:
- Belliveau Lake;
- Pretty Mary, Mudflat, and Mill lakes; and
- Louis Lake and nearby Canoe Lake.
Sweet Pepperbush is a widely used ornamental species. It seems to have been infrequently planted in Nova Scotia prior to 1990 and is still not frequently seen in cultivation, but it is now widely available at nurseries and major retailers (Blaney pers. obs. 1999-2012). The origins of all cultivated plants are ultimately in the United States, although pepperbush has been in cultivation, and probably subject to horticultural selection, in Europe for several hundred years as well (see Special Significance). Canadian occurrences can be discounted as a potential source for cultivated plants because they were discovered relatively recently (Taschereau 1969) and research for this report uncovered no evidence of attempts to bring Nova Scotia genotypes into commercial cultivation. Herbarium specimens that are clearly from cultivated individuals are known from 1970 and 1973 well outside the natural range on McNabs Island in Halifax Harbour (persistent from historical cultivation but not widely spreading, Taschereau 1986), and from 2012 in a yard on the shore of Marcel Lake in southern Yarmouth County (a single individual, AC CDC 2013), just 12 km from the Louis Lake occurrence. These and other cultivated occurrences (observed as far north as Iona in southern Cape Breton, 380 km northeast of Pretty Mary, Mudflat, and Mill lakes, Blaney pers. obs. 1999-2012) are not considered wild occurrences for the purposes of this report.
Despite the species’ use as an ornamental and its discovery in Nova Scotia (Taschereau 1969) after the vast majority of native species had been documented for the province (Roland and Smith 1969), two lines of evidence strongly indicate that it is a long-established native species in Nova Scotia. First, Sweet Pepperbush occurs quite widely and abundantly at all three subpopulations, with occurrences spread over several kilometres. This suggests long-term residency (likely exceeding several hundred years) because field observation suggests limited seedling establishment in Nova Scotia (see Life Cycle and Reproduction), and because lateral spread by rhizome is unlikely to be much more than 1 m per year (based on maximum rates of growth observed in above-ground stems, Blaney et al. pers. obs. 2013). Second, Sweet Pepperbush distribution in the United States and its disjunction into southern Nova Scotia from southern Maine fits a pattern shared by many other Atlantic Coastal Plain flora (Roland and Smith 1969; Wisheu and Keddy 1994; Clayden et al. 2009) and is thus not suggestive of human introduction. The absence of earlier records of Sweet Pepperbush does not suggest recent introduction because, except for Canoe Lake (visited by Fernald in 1921) where only a single shrub is currently present and the species could have been easily missed, herbarium records indicate that no botanists visited the lakes at which Sweet Pepperbush occurs prior to the discovery of the species.
Extent of Occurrence and Area of Occupancy
Under COSEWIC guidelines (COSEWIC 2010), extent of occurrence (EO) for extant sites in Canada is 1,984 km2. Index of area of occupancy (IAO) for extant sites, derived using a 2 x 2 km grid aligned with 10 km x 10 km UTM grid squares, is 52 km2. These values include the single Canoe Lake individual, which is not a viable subpopulation on its own but is considered part of a larger Louis Lake subpopulation.
Search Effort
Although Sweet Pepperbush was not discovered in Nova Scotia until 1968 (Taschereau 1969), the presence of Atlantic Coastal Plain flora in southern Nova Scotia has been well known since Merritt Fernald’s expeditions (Fernald 1921, 1922) and search effort for coastal plain species has been extensive. Floristic work focused on coastal plain flora in southern Nova Scotia has continued from the 1950s to the present and academic work on the ecology, distribution and local diversity of Nova Scotian coastal plain flora with a focus on conservation implications has been ongoing since the 1980s (see references in COSEWIC 2012a). The AC CDC (2013) database of vascular plant records and COSEWIC (2012a) document site visits to at least 315 lakes within the potential range of Sweet Pepperbush Footnote1.1. Of these, 220 were visited up to 2000 when the last status report (COSEWIC 2001) was prepared. Fieldwork since 2000, predominantly by Atlantic Canada Conservation Data Centre (AC CDC), Nova Scotia Department of Natural Resources and Mersey Tobeatic Research Institute (MTRI) (see references in COSEWIC 2012a) has been more intensive, with 172 lakes visited, including 95 lakes not visited prior to 2000. Most of the 95 newly visited lakes have had comprehensive coverage of their shorelines for rare plants. These figures, especially those from before 2000, are conservative estimates of the number of lakes visited by botanists because of incomplete databasing of existing specimens and lakes visited where no data were collected. They would, however, include a majority of southern Nova Scotia lakes visited by botanists.
Sweet Pepperbush is an exceptionally showy species when in flower, meaning that if it were at all frequent in unsurveyed areas it would likely have been noticed by naturalists, canoe trippers, hunters and other outdoors enthusiasts and brought to the attention of botanists. Hundreds of additional lakes in southern Nova Scotia would thus have had some level of incidental survey for Sweet Pepperbush.
Despite the extensive recent fieldwork, only three new occurrences have been found since Taschereau (1969): Louis Lake in the late 1980s, Canoe Lake (one shrub) in 1995 and Pretty Mary, Mudflat, and Mill lakes in 1998 (COSEWIC 2001; AC CDC 2013). The lack of records of Sweet Pepperbush from 309 of 315 surveyed southern Nova Scotia lakes strongly indicates that the very limited known range is not a result of inadequate survey effort. Habitat occupied by Sweet Pepperbush does not, however, seem at all unusual for southern Nova Scotia (Blaney pers. obs. 1999-2012). The lakes on which Sweet Pepperbush is known include several smaller lakes at or near headwater areas. Similar lakes make up a large proportion of the roughly 1,135 lakes and ponds (out of 1,450 total, Natural Resources Canada 2003) within the potential range of Sweet Pepperbush for which no record of a botanist visit is available. Although Sweet Pepperbush is clearly rare, there is thus a reasonable possibility that small numbers of additional occurrences may eventually be found.
Habitat
Habitat Requirements
In Nova Scotia, Sweet Pepperbush is a species of acidic upper lakeshores and lakeshore forest margins. It also occurs locally along shrubby and semi-forested stream margins and to a limited extent under Red Maple (Acer rubrum)-dominated swamp forest canopy within about 20 m of shorelines (Hill pers. comm. 2012). It has not been observed to flower when under dense forest canopy in Nova Scotia (Hill pers. comm. 2012). Nova Scotian Sweet Pepperbush occurs in gravelly, sandy, peat and muck soils, sometimes within the zone of shoreline boulders pushed up by ice. It is considered an obligate wetland plant in Nova Scotia (Blaney 2011), and can grow in sites with shallow standing water for most of the summer (Belliveau pers. comm. 2012), although it is more often in sites just above the low summer water level. Similar habitats are occupied throughout its range, but there appears to be a greater tendency to extend into shaded and non-wetland conditions further south.
Sweet Pepperbush is a facultative wetland plant throughout its United States" range range (USFWS 1997). Relatively open conditions, swamps, shores, and acidic soils are frequently mentioned themes in habitat descriptions. Examples include: wooded swamps, shores and the borders of shrub swamps, lakes, ponds and streams, rarely in woodlands and ridge tops in New England (Magee and Ahles 1999; Haines 2011); "open bog or swamp habitats in sandy, or peaty, acid, low-nutrient soils" in New York (Gargiullo 2007); "…abundant in hardwood swamp, common in Atlantic White Cedar (Chamaecyparis thyoides) swamp and pine transition communities, and rare throughout the upland" in New Jersey pine barrens (Laycock 1967); "mesic to rather dry, acidic upland forests, wet flatwoods, nonriverine swamps, seepage swamps, and bogs" in Virginia (Virginia Botanical Associates 2013); pine flatwoods, various acidic, peaty or sandy shrub and treed swamps (referred to as "pocosins", "blackwater riverine swamps" and "non-riverine swamp forests") and swamp edges in the southeastern Coastal Plain (Nelson 2006; Weakley 2012); "acidic wetlands and swamps" (Crabtree 2012) in Tennessee; and "edges of acidic wetlands" in Alabama (Kral et al.2013). Tiner (1987) noted Sweet Pepperbush as also occurring in "…irregularly flooded tidal swamps and at the margins of tidal fresh marshes". In the United States, Sweet Pepperbush is frequently noted for salt tolerance (especially relative to salt spray) in horticultural (i.e. Dirr 1990; Ohio State University 2013) and natural situations (Glen 2005), though salt tolerance in soil is limited. Anstey (1999) found significantly reduced growth and heavy mortality in Sweet Pepperbush seedlings watered and/or flooded at 1/7th to 1/11th the salinity of seawater.
Habitat Trends
Sweet Pepperbush habitat at Louis Lake and Canoe Lake is believed to have been stable since the last status report, and likely for a longer period into the past as these sites are on Crown land with no development. Some forest harvesting has taken place within about 100 m of the lakeshores in the past 20 or 30 years (COSEWIC 2001; Google Earth 2013), but does not appear to have directly affected Sweet Pepperbush (Hill pers. comm. 2012). The invasive exotic shrub Glossy Buckthorn (Frangula alnus), presently known about 40 km away from Louis and Canoe lakes, is likely to reach these lakes within decades and may eventually reduce habitat quality, but is not an immediate threat.
Habitat quality is just starting to be impacted by Glossy Buckthorn at Pretty Mary, Mudflat, and Mill lakes, where thousands of mature Glossy Buckthorn in adjacent abandoned farmland provides a large seed source. Impacts on Sweet Pepperbush thus far have likely been minimal, but at least one mature Glossy Buckthorn has been observed growing through a Sweet Pepperbush stand and over-topping it, suggesting potential for future competitive effects. The possible impacts of such shading are indicated by the fact that occurrences in naturally shaded conditions in Nova Scotia do not appear to produce flowers (Hill pers. comm. 2012), and that shading reduces productivity and sexual reproduction in the American range (Jordan and Hartman 1996). Glossy Buckthorn may be within about 10 km of Belliveau Lake already (see Threats – Invasive Species) and is likely to eventually reduce habitat quality there, although it is not believed to be impacting habitat at present.
As discussed in detail under Threats – Shoreline Development, habitat at Belliveau Lake and on Pretty Mary, Mudflat, and Mill lakes has been impacted by shoreline development, primarily within the last 30 years. Shoreline development effects on Sweet Pepperbush habitat are more significant than with many herbaceous Atlantic Coastal Plain species because the species occurs on the upper shore often within the development footprint, and because it is tall enough to block lake views and tends to be removed for that reason. Multiple new single-cottage developments on Belliveau, Pretty Mary, and Mudflat lakes have been observed in recent years (Belliveau pers. comm. 2012; Gray pers. comm. 2012) but total impacts of all development on Sweet Pepperbush habitat are roughly estimated at not more than 4.6% (based on 10 m of loss per cottage within 11 km of occupied shoreline). Good road access and highly divided land ownership mean that small-scale developments are likely to continue. Future development is also likely on Mill Lake, where most of the shoreline is owned by an offshore development company and a group of American lawyers (Gray pers. comm. 2012).
Habitat quality is believed to have declined on Belliveau Lake as a result of eutrophication because of leaching sewage ponds from an abandoned hog farm (see Threats – Eutrophication). The extent to which this threatens Sweet Pepperbush is unclear, but the species’ adaptation to acidic, nutrient-poor soils suggests it may be less competitive than more common species (including the invasive Glossy Buckthorn) in high nutrient conditions. Future mink farm development could cause further eutrophication at Belliveau Lake, but is unlikely to affect other subpopulations (see Threats – Eutrophication).
Biology
Life Cycle and Reproduction
Sweet Pepperbush flowers in Nova Scotia from late July to early September, with peak flowering in mid-August (Belliveau pers. comm. 2012; Hill pers. comm. 2012). An identical flowering period (July 21 to early September) was noted for Storrs, Connecticut by Hemingson (1986). Detailed descriptions of floral morphology and phenology are given in Kavaljian (1952) and Reed (2006). Pollination is primarily or exclusively by insects, which are attracted to the large and highly fragrant inflorescences that produce large quantities of nectar. Bees appear to be the major pollinators, but many other insects are attracted to the flowers (see Interspecific Interactions). The porose anthers suggest buzz pollination (in which larger bees vibrate their bodies while on the flowers to release pollen, Buchmann 1983) is important, as is the case with many species of the heath family Ericaceae and related families (Hemingson 1986). Sweet Pepperbush exhibits strong, but not complete self-incompatibility, perhaps governed by systems in the ovaries or ovules (Hemingson 1986; Reed et al. 2002; Reed 2006. This would be "late acting self-incompatibility" as opposed to self-incompatibility through mechanisms in the stigma or style). Alternatively, there may be early acting inbreeding depression that prevents maturation of seed produced by self-pollination (Reed 2006). Hemingson's (1986) experiments showed major reductions in seed set under various floral bagging treatments, and led her to calculate that 16% to 34% of fruit set could be due to wind. She also found that anther removal coupled with floral bagging produced no seed set, suggesting apomixis does not occur. The species' self-incompatibility may be an important factor in Canada. No seeds were found in plants examined at Belliveau Lake (Taschereau 1986), although seeds of unknown viability have been collected there and recently at Mudflat Lake (Belliveau pers. comm. 2012; Blaney et al. pers. obs. 2013). Seedling establishment seems to be rare in all subpopulations (see Threats - Limited Genetic Diversity and Lack of Seed Production).
Seeds mature through late summer and autumn and remain in the capsules relatively late into the fall. Jordan and Hartman (1995) reported peak of seed dispersal from the capsules in November in New Jersey and noted 6 to 17 seeds per capsule with total seed production per plant ranging from 1,348 to 7,920. Reed et al. (2002) reported a mean of 30 seeds per capsule in hand pollinations between Sweet Pepperbush cultivars. The tiny seeds have no obvious adaptations for dispersal but are known to be moved short distances by wind (Jordan and Hartman 1995). Water, wind and vertebrates moving seeds within mud attached to their bodies could all contribute to longer distance dispersal (see Dispersal and Migration). Seeds do not require stratification and can germinate immediately after dispersal (Dirr and Heuser 1987; Jordan and Hartman1995) but seeds from New Jersey populations germinated more readily under mid-summer conditions in a growth chamber after five months cold (0oC to 2oC), dark stratification than did unstratified seeds (40% to 59% vs. 36%; Jordan and Hartman 1995). Deno (1993 in Jordan and Hartman 1995) also reported 60% germination in controlled conditions (the highest rate of nine shrub species tested). Jordan and Hartman (1995) found that in New Jersey hardwood swamps, seedling establishment was the largest limitation to sexual reproduction, with availability of slightly drier elevated sites, which are good for establishment, being the key factor influencing the ratio of seedling to vegetative regeneration.
Sweet Pepperbush can be propagated by stem cuttings or by sucker division (Wennerberg 2004; Williamson 2010). Softwood stem cuttings are best harvested in early summer. It is not known if reproduction from stem cuttings has been attempted in Canada.
Seed longevity in the field is unknown, and there is no reference to soil seed-banking. Average time to first flowering from seed in the field is probably more than ten years given that flowering is mostly restricted to larger stems and an especially large stem was 28+ years old (Taschereau 1986). In Nova Scotia (Taschereau 1986; COSEWIC 2001) and in studied American populations, most reproduction is by spreading rhizomes which can produce new shoots up to 2.4 m from the parent plant (Laycock 1967). Vegetative shoots can be produced in tight clumps which would typically be counted as a single individual for COSEWIC assessment or as shoots separated from others by as much as 2.4 m (Laycock 1967). Most commonly shoots are produced as sparsely to densely aggregated but distinctly separate stems at densities ranging from a few to 20+ per m2 (Belliveau pers. comm. 2012; Blaney et al. pers. obs. 2013). When greater densities are present, many stems are of very small sizes (Blaney et al. pers. obs. 2013). In New Jersey hardwood swamps, Jordan and Hartman (1995, 1996) found vegetative sprout densities were 20 times those of seedlings, and suggested that establishment via vegetative spread allows occupancy of wetter habitats where seedling establishment is difficult. They also suggested that the development of an extensive network of vegetative shoots created a "sprout bank" that could respond rapidly to canopy openings independent of sexual reproduction. Time to flowering for new shoots is likely to be somewhat faster than from seed because the shoots can draw on parental resources (Huenneke 1987; Huenneke and Marks 1987; Jordan and Hartman 1995). The time required for a new vegetative shoot to produce additional vegetative shoots at a distance sufficient for them to be counted as separate "individuals" is unknown but may be at least several years. Individual shoots can last many years. Taschereau (1986) counted 28 years of annual growth rings on one of the largest stems he could find on Belliveau Lake. If many larger stems are 20 to 30+ years old, generation time (average age of parents) could be at least 10 years. Clumps of stems, which continually resprout from the base, and genetic individuals (complexes of connected stems and clumps) are presumably much longer-lived.
Physiology and Adaptability
Horticultural references suggest Sweet Pepperbush has broad tolerances for: climate (United States Plant Hardiness Zones 4 to 9 [Christman 2011; Dave's Garden 2013], corresponding to minimum temperatures of about -35oC to -4oC, USDA 2012); soil pH (acidic to neutral, Plants for a Future 2013); light levels (from full sun to shade, with a noteworthy characteristic being its ability to produce flowers in the shade, Clemson Extension 2010; Missouri Botanical Garden 2013); and moisture (i.e. "prefers consistently moist to wet soil conditions", Missouri Botanical Garden 2013; "once established in cultivation, it thrives on drier, well drained soils", Christman 2011). The species' broad geographic range from the Gulf Coast of the United States to southern Maine and Nova Scotia confirms a broad climatic tolerance in the wild. Habitat descriptions from the United States show that although it is predominantly a wetland species it does occur in drier upland conditions with some frequency and that it is frequently present in both full sun and deep shade (see Habitat Requirements and Jordan and Hartman 1995, 1996). Anstey (1999) conducted detailed analysis of soil nutrient characteristics of eight sites at four populations in Virginia, finding strongly acidic pH values between 4.0 and 5.2. She also conducted a laboratory study of the interactive effects of flooding and salinity, showing significantly reduced performance of seedlings at 1/11th seawater salinity and complete mortality at 1/7th seawater salinity.
Jordan and Hartman (1996) investigated Sweet Pepperbush's response to varying light levels. They found that Sweet Pepperbush responded to large canopy openings with increased stem density and recruitment, seedling density, growth rate, and flowering, but decreased vertical growth.
Sweet Pepperbush has a high accumulation rate of cobalt, manganese, zinc, and calcium, making it potentially useful in evaluation of those compounds in soils (Beeson et al. 1965). It has also been shown to produce compounds that mimic insect growth regulators and likely serve to reduce insect herbivory (Jacobson et al. 1975).
Dispersal and Migration
Seed production and/or seedling establishment appears to be limited in Canadian subpopulations of Sweet Pepperbush. Taschereau (1986) reported no maturation of seed on Belliveau Lake, though seeds of unknown viability have been collected there recently (Belliveau pers. comm. 2012). Seedlings have only been observed at Louis Lake, where a small number of seedlings were observed in the two most recent surveys in 2000 and 2012 (Hill et al. 2000; COSEWIC 2001; Hill pers. comm. 2012). The presence of the single mature plant at Canoe Lake, one kilometre from Louis Lake, suggests a history of seed production. Seed production has not been investigated at other Nova Scotian sites but likely occurs at least occasionally given that the wide distribution of Sweet Pepperbush around the lakes, including gaps in occurrence of several hundred metres, is unlikely to be explained by vegetative reproduction alone. Limited seed production and a consequent inability to disperse from lake to lake does seem to be a likely explanation for the absence of Sweet Pepperbush from hundreds of apparently suitable lakes in southern Nova Scotia, including many within one or a few kilometres of large subpopulations.
Sweet Pepperbush seeds lack any obvious dispersal mechanisms, but their very small size (0.5 mm x 0.75 mm) suggests they could be moved by wind (Sleumer 1967). Jordan and Hartman (1995) measured seed dispersal within 3 m of parent plants and found maximum dispersal between one and two metres from the parents, with a tendency toward dispersal in the direction of prevailing winds. The fact that seeds are retained in the capsules well into the fall and potentially into the winter means that dispersal by wind across ice could produce longer-distance dispersal within lakes. Dispersal of seeds in mud attached to vertebrates (Porter 1983) would also be possible, and would be a plausible means of long-distance dispersal into Nova Scotia. Information on buoyancy and flotation time of the seeds is not available, but water dispersal may also be important given Sweet Pepperbush’s shoreline habitat.
The traditional view on migration of Sweet Pepperbush and other Atlantic Coastal Plain plant species into present-day Nova Scotia (Roland and Smith 1969) is that these plants reached Nova Scotia after having colonized (or having persisted throughout the period of glaciations on) land between present-day southern Nova Scotia and Massachusetts exposed by lower sea levels. This suggests a slow migration to Nova Scotia via shorter-distance dispersal events over thousands of years. A recent evaluation (Clayden et al. 2009) suggests this scenario may be unlikely for southern species like Sweet Pepperbush because offshore land is now known to have had high boreal or arctic climate, and to have been more limited in time and space than previously believed. Thus very long-distance dispersal (on the scale of 200 to 400+ km between southern Nova Scotia and New England) may be possible for Sweet Pepperbush over geological time.
Interspecific Interactions
Sweet Pepperbush is well known as an attractive plant for pollinating insects, especially bees (Taschereau 1986; COSEWIC 2001; Bhattacharya et al. 2003; Clemson Extension 2010; Missouri Botanical Garden 2013). The pollination biology of Sweet Pepperbush in New England was investigated in detail by Hemingson (1986). She collected insects from Sweet Pepperbush flowers, finding 140 species in the orders Hymenoptera (bees, wasps and ants – 58 species in 13 families), Coleoptera (beetles – 32 species in 12 families), Diptera (flies – 15 species in five families), Hemiptera (true bugs, treated in the broad sense here – 21 species of 9 families) and Lepidoptera (butterflies and moths – 14 species in 6 families). Hemingson (1986) found Black-headed Soldier Beetle (Cantharis nigriceps), Common Eastern Bumble Bee (Bombus impatiens) and Honey Bee (Apis mellifera) were the most abundant flower visitors. Her observations of insect behaviour and insect pollen loads led her to conclude that bumble bees (Bombus spp. sensu lato) were the main pollinators. Jordan and Hartman (1995) reported visits to the flowers from many species of bees, flies and beetles in New Jersey and butterflies are also reported as frequent visitors (Clemson Extension 2010; Missouri Botanical Garden 2013). Bees (species not noted) are abundant visitors to Canadian subpopulations of Sweet Pepperbush (Belliveau pers. comm. 2012; Hill pers. comm. 2012). Bhattacharya et al. (2003) investigated movement of bumble bees and carpenter bees (Bombus impatiens, B. affinis, and Xylocopaspecies) between patches of Sweet Pepperbush in Massachusetts. Only three of 113 bumble bees recaptured in five to six day studies moved away from the patches of Sweet Pepperbush (between 240 m2 and 820 m2 in size, all within a 150 m x 150 m area) at which they were originally captured. The site fidelity of these highly mobile generalist pollinators suggests high nectar and/or pollen rewards within pepperbush patches.
Sweet Pepperbush seems to be relatively resistant to herbivores. Plants in the field in Canada (Belliveau pers. comm. 2012; Hill pers. comm. 2012) and in most images online appear to have relatively limited insect damage. An exception was noted at Mudflat Lake in September 2013, where numerous shoots had been affected by an unknown caterpillar which cut the growing branch tip and used silk to connect the upper three to six leaves into a tube around the stem (Blaney et al. pers. obs. 2013). One moth (Celama clethrae) reported as a specialist herbivore of Sweet Pepperbush (Smith 1909; Brower 1974) is known from Maine and should be looked for around Sweet Pepperbush occurrences in Nova Scotia. It is not currently known to occur in Canada (Troubridge and Lafontaine 2004). No other specialist insect herbivores are apparent in Internet references, and reference to invertebrate feeding of any kind is very limited. The Southern Red Mite (Oligonychus ilicis), a generalist leaf herbivore, is known to feed on Sweet Pepperbush (Denmark et al.2009), as is the introduced Japanese Beetle (Popilia japonica, Fleming 1972, in Hemingson 1986). Jordan and Hartman (1995) reported predation on seed capsules (usually involving removal of the entire capsule), but did not speculate on a cause. Extracts from the stem, leaves and fruits of Sweet Pepperbush have been found to have compounds that mimic insect juvenile hormones (Jacobson et al. 1975) and likely contribute to prevention of insect herbivory. Sweet Pepperbush is noted as being unpalatable to deer (Wood 1988; Jull 2001) and no references to herbivory by other vertebrates (including beavers) were found in preparing this report. Beavers (Castor canadensis) likely cut some stems. One stem was cut by a Beaver from a cultivated lakeshore plant at Marcel Lake, Yarmouth County (Blaney pers. obs. 2012), and Beaver herbivory has also been documented at Mudflat Lake, but has not been observed to have significant effects there or elsewhere in Nova Scotia (Belliveau pers. comm. 2012; Gray pers. comm. 2012; Hill pers. comm. 2012).
Sweet Pepperbush is also somewhat resistant to decomposers. Clethra species are reportedly strongly resistant to Honey Fungus (Armillaria spp.; Huxley et al. 1992 in Plants for a Future 2013) and Sweet Pepperbush leaves are more resistant to decomposition than would be predicted by their high nutrient and low lignin contents (Short 2010).
Sweet Pepperbush is known to develop vesicular arbuscular mycorrhizal associations in the wild (Henry 1934), and in cultivation (Sylvia 1988, 1990). Sylvia (1988, 1990) found that mycorrhizal colonization by inoculated Glomus spp. did not produce significantly increased growth of Sweet Pepperbush in sterilized soils in which most other woody species showed a positive growth response from mycorrhizal inoculation.
Population Sizes and Trends
Sampling Effort and Methods
No fieldwork was undertaken specifically for this report, with the exception of a 1.5 hour visit to Mudflat and Mill Lakes on September 5, 2013 (Blaney et al. pers. obs. 2013) to assess the accuracy of population estimates and the distribution along the stream between the lakes. Distribution of Sweet Pepperbush was comprehensively documented at all lakes on which it is known in 2011 and 2012 through complete walking surveys of shorelines (MTRI unpubl. 2012; AC CDC 2013). At Canoe Lake, the shoreline was covered comprehensively and only a single shrub with four stems was found. For Belliveau Lake, Mudflat Lake, and Mill Lake, stems were counted directly for localized zones of occurrence having small numbers (Belliveau pers. comm. 2012). For larger zones of occurrence, stem density was described as one of "low" (4 stems per metre), "low-medium" (6 stems per metre), "medium" (8 stems per metre), "medium-high" (10 stems per metre) or "high" (12 stems per metre) density. These stem per metre values were derived immediately prior to fieldwork from counts made at Mudflat Lake and subsequently confirmed on Belliveau Lake. Stem numbers were derived by multiplying shoreline distance occupied by the above stem per metre values (Belliveau pers. comm. 2012). Pretty Mary Lake and Louis Lake distribution was comprehensively mapped through shoreline walking surveys and some notes on area occupied were taken, but stems were not counted. Numbers for these lakes are estimated based on assessments by Nick Hill and Alain Belliveau that Pretty Mary Lake numbers are roughly halfway between those on Mudflat and Mill Lake and that Louis Lake numbers are similar to those on Mill Lake (Belliveau pers. comm. 2012; Hill pers. comm. 2012). For all counts above, first-year shoots with no hard woody growth were not included in stem count totals (Belliveau pers. comm. 2012).
Converting stems counts to numbers of COSEWIC "mature individuals" is not possible. Field observation (Blaney et al. pers. obs. 2013) confirms that almost all stems are vegetatively produced from spreading rhizomes (see Biology – Life Cycle and Reproduction). Some are tightly clumped and best considered part of a larger "mature individual" and others are separated by at least 1 m from other stems with root systems along the rhizome suggesting they could survive if severed (Blaney et al. pers. obs. 2013), meaning that they should be considered "individuals" if large enough to reproduce either sexually or vegetatively. Most shoots are not producing flowers in any one year, and the minimum size at which a stem is likely to reproduce via rhizome spread is unknown. Some portion of the smaller stems may be incapable of sexual or vegetative reproduction and therefore not countable as "individuals".
Defining Subpopulations
COSEWIC separates subpopulations if there is typically less than one successful genetic exchange per generation. With distances between them of 50 to 76 km and seed production possibly limited (at least on Belliveau Lake), the occurrences on Belliveau Lake, Pretty Mary – Mudflat – Mill lakes and Louis – Canoe lakes can reasonably be assumed to be three separate subpopulations.
The occurrences on Pretty Mary, Mudflat and Mill lakes are considered a single subpopulation because occurrence is almost continuous with gaps in occupied areas not exceeding 250 m (Blaney et al. pers. obs. 2013), well within the potential pollination distance of larger bees (see Dispersal and Migration) and the potential dispersal distance of small seeds over a frozen lake or in water current. Similarly, Louis Lake and Belliveau Lake are each defined as single populations because occupied habitats are all relatively close to one another with maximum within-lake separation distances under 500 m (Figure 3). The single plant on Canoe Lake is considered part of the Louis Lake subpopulation because of its proximity (1.1 km - within potential pollination distance, and with a gap in suitable habitat of only 420 m) and because it is likely not viable as a subpopulation on its own given significant self-incompatibility (Reed et al.2002; Reed 2006) and the very low subpopulation size, and that risks associated with stochastic events render it non-viable at this time.
Abundance
The only population index assessed during 2011 and 2012 fieldwork was number of stems. As noted in Life Cycle and Reproduction and Search Effort and Methods, total stem counts are a useful index of population size representing a maximum potential population, but stem counts probably greatly overestimate number of individuals because of the inclusion of stems that are insufficiently separate from others to survive on their own or are not mature enough to reproduce by vegetative or sexual means.
Surveys in 2011 and 2012 estimate total stem count in Canada at 45,471 with an estimated 16,070 stems at Belliveau Lake, 1,700 stems at the Louis Lake – Canoe Lake subpopulation and 27,700 stems at the Pretty Mary – Mudflat – Mill lakes subpopulation. Lake-specific estimates for the latter subpopulation are 8,100 stems at Pretty Mary Lake, 17,900 stems at Mudflat Lake and 1,700 stems at Mill Lake (Table 1; see Search Effort and Methodsfor derivations of all estimates).
| Location# | Name | Population | Latest Survey | Land status | Derivation of Stem Count Estimate |
|---|---|---|---|---|---|
| 1 | Belliveau Lake | 16,071 stems | 2012 |
|
Conversion of complete stem count |
| 2 | Pretty Mary Lake – Mudflat Lake – Mill LakeFootnotea.1 | 27,700 stems | 2012 |
|
Estimated stem counts for Mudflat and Mill Lake. Estimate for Pretty Mary Lake based on population size roughly halfway between Mudflat and Mill lakes. |
| 3 | Subpop. 3a - Louis Lake | 1,700 stems | 2012 | Crown | Estimate based on similar population size to Mill Lake |
| 3 | Subpop. 3b - Canoe LakeFootnoteb.1 | 1 individual (4 stems) |
2011 | Crown | Comprehensive count |
| TOTAL | - | 45,471 stems | - | - | - |
The number of genetic individuals is undoubtedly vastly lower than the values above given the predominance of vegetative reproduction in the species generally and in Nova Scotia especially (Taschereau 1986). Jordan and Hartman (1995, 1996) found vegetative sprout densities 20 times that of seedlings.
Fluctuations and Trends
Population counts were not documented in COSEWIC (2001). Qualitative assessments of populations on Belliveau Lake by Alain Belliveau (2009 and 2012, Belliveau pers. comm. 2012), by Nick Hill on Louis Lake (1988 and 2012, Hill pers. comm. 2012) and by Colin Gray on Pretty Mary, Mudflat, and Mill lakes (1998 to present, Gray pers. comm. 2012) suggest that shoreline development is causing a slight decline, likely not exceeding 4.6% (see Threats – Shoreline Development), but that the population is otherwise stable at present. Relative stability excepting development-related losses is further suggested at Belliveau Lake by a comparison of the distribution mapping in Taschereau (1986) with that of Alain Belliveau in 2009-2011 (AC CDC 2013), which shows very similar areas occupied. The threats of Glossy Buckthorn invasion (see Threats – Invasive Species) and increased competition resulting from nutrient enrichment (see Threats – Eutrophication) could have significant effects over several generations into the future, but do not yet appear to have reduced Sweet Pepperbush populations.
No natural fluctuations have been noted and none would be expected over the shorter term for a slow-growing, long-lived, perennial species such as Sweet Pepperbush.
Rescue Effect
Rescue is likely limited for Sweet Pepperbush because Nova Scotia populations are separated from the nearest subpopulations in Hancock County, Maine (Kartesz 2011) by at least 200 km, with most of that distance being open water of the Bay of Fundy. Rescue effect from subpopulations in Maine is further limited because of the species’ rarity there (S2 in Maine, NatureServe 2013). Distance from Nova Scotia occurrences to regions south of Maine where the species is more common are at least 400 km across the Gulf of Maine.
Threats and Limiting Factors
Invasive Species
Competition from the invasive exotic shrub Glossy Buckthorn, one of the most problematic invasive plant species in Canada and the northeast United States (Catling and Porebski 1994; Frappier et al. 2003a; Catling and Mitrow 2012; IPANE 2012) is a newly recognized threat to Sweet Pepperbush. There are thousands of reproducing Glossy Buckthorn on abandoned farmland between Pretty Mary and Mudflat Lakes within about 100 m of both lakeshores (Belliveau pers. comm. 2012), from which seeds would be readily dispersed by birds around all three lakes. At Mudflat Lake at least two large, mature Glossy Buckthorns have grown through dense pepperbush occurrences which they are now overtopping, suggesting strong potential for competitive suppression in the future (Belliveau pers. comm. 2012). There are likely already smaller Glossy Buckthorn individuals within pepperbush occurrences around Pretty Mary, Mudflat, and Mill lakes given the very large numbers nearby. Peaty wetlands similar to those occupied by Sweet Pepperbush in Nova Scotia have a well-documented susceptibility to Glossy Buckthorn invasion in Wisconsin (Reinartz and Kline 1998, where it was noted as having "over-run the 1,000 ha Cedarburg Bog in 20 years"), Illinois (Taft and Solecki 1990), Michigan (Fiedler and Landis 2013), Ontario (Catling and Mitrow 2012), and Nova Scotia (Hill and Blaney 2009). The potential of Glossy Buckthorn to invade swamps and shorelines occupied by Sweet Pepperbush in Nova Scotia is further indicated by occurrence at densities that likely impact native plant performance and diversity in relatively undisturbed Red Maple swamp forest along the Medway River within 2.5 km of Mill Lake (Blaney pers. obs. 2010).
Glossy Buckthorn is not yet known at other Sweet Pepperbush sites in Nova Scotia, but its potential for rapid spread via bird dispersal (Catling and Porebski 1994; Hampe and Bairlein 2000; Frappier et al. 2003a; Frappier et al. 2003b; observations of single shrubs 10 to 20 km from large occurrences – Blaney pers. obs. 1999-2012; AC CDC 2013) and huge source populations in the Digby – Annapolis Royal – Caledonia region and locally around Barrington suggest establishment throughout southern Nova Scotia is likely in the next 20 to 50 years (~1 to 4 generations). Well-established occurrences exist within about 35 km of Belliveau Lake near Digby (S. Blaney pers. obs. 2012), and perhaps within about 10 km around Weymouth given the presence of ideal abandoned farmland habitat. Glossy Buckthorn is also well-established within about 40 km of Louis Lake near Barrington (S. Blaney pers. obs. 2010). Glossy Buckthorn does especially well in cleared, disturbed areas (Reznicek et al. 2011; Lee and Thompson 2012) and its impacts may be especially significant in suppressing recovery of Sweet Pepperbush following disturbance, potentially including water level changes associated with failure of the Mill Lake dam (see Artificial Regulation of Water Levels).
Eutrophication
Since the last status report (COSEWIC 2001), eutrophication has changed from a theoretical threat to Atlantic Coastal Plain flora in Nova Scotia (Ehrenfeld 1983; Moore et al. 1989; Zaremba and Lamont 1993; Environment Canada and Parks Canada Agency 2010) to one of the most significant actual threats to the suite of rare flora as a whole (COSEWIC 2012a, b).
Nutrient enrichment appears to be having effects on occupied Sweet Pepperbush habitat at the south end of Belliveau Lake, where a dense stand of several hectares of Broadleaf Cattail (Typha latifolia, a native species that is rare on nutrient-poor southern Nova Scotia lakes and never seen in stands that large on unaltered lakes, Blaney pers. obs. 1999-2012) is present at the inflow of a stream draining sewage ponds from an inactive hog farm 600 m upslope from the lake (Belliveau pers. comm. 2012; Figure 3). Effects of eutrophication on Sweet Pepperbush are not yet evident at Belliveau Lake and the magnitude of potential future impacts is unclear. Nutrient enrichment is specifically documented as a threat to peatland communities in Massachusetts in which Sweet Pepperbush is a common species (Swain and Cursley 2001). Impacts from increased competition are likely to act much more slowly on a tall woody shrub like Sweet Pepperbush than on low herbaceous shoreline species, though the longer generation time of pepperbush means that a longer period into the future is considered in assessing eutrophication threats. Despite uncertainties regarding its effects on Sweet Pepperbush, this report applies a precautionary principle and treats eutrophication as a threat that could favour more competitive common shoreline species (including the invasive Glossy Buckthorn).
Mink farming is the most significant source of inland nutrient pollution within the Atlantic Coastal Plain flora region of Nova Scotia (Brylinsky 2011, 2012). It is an especially large source of phosphorus pollution because mink feed is treated with superphosphate to increase shelf life and reduce the occurrence of kidney stones in mink (Brylinsky 2011). Once phosphorus has entered a lake, the recovery from eutrophic conditions following a reduction in the external phosphorus loading may be slow as the phosphorus is stored in the lake sediments (Marsden 1989; White et al. 2002). Mink farming has undergone rapid expansion in Nova Scotia over the past decade and is the province’s largest agricultural export with 1.4 million pelts produced annually by 152 farms, about 75% of which are in Yarmouth County and adjacent Digby County (Flemming pers. comm. 2011). Belliveau Lake is within the region of Digby County with the highest concentration of mink farms in Nova Scotia, and although it is a headwater lake there is some area within its drainage basin (especially at the abandoned hog farm) in which future expansion of mink farming would be possible because of good road access and limited population nearby. The Pretty Mary – Mudflat – Mill lakes population is also at a headwater lake and may be somewhat less likely to be affected by mink farm expansion because of limited mink farm activity in the area and a relatively dense cottage population nearby that would object to farm development. Louis and Canoe lakes would be unlikely to be affected by mink farm expansion because of most of their drainage basins are on Crown land, which has not been leased for mink farms in Nova Scotia.
Shoreline Development
Shoreline development is considered a significant threat to Atlantic Coastal Plain flora communities on lakeshores (Wisheu and Keddy 1994; Eaton and Boates 2003; Environment Canada and Parks Canada Agency 2010). As documented in COSEWIC (2001), lakeshore cottage and residential development is an ongoing threat to Sweet Pepperbush at Belliveau, Pretty Mary, Mudflat, and Mill lakes, but not at Louis and Canoe lakes where occurrences are on Crown land. As a shrub of the upper shoreline and forest margin, Sweet Pepperbush is more susceptible to removal and damage by cottage landowners than herbaceous species that occur completely below the high water mark, because some cottagers remove most or all shrubs along their shores.
There are currently about 20 cottages completed or under construction on Pretty Mary Lake, five on Mudflat Lake, and one on Mill Lake. All but two or three of these have been constructed since the late 1970s or 1980s (within a three-generation period in the past), and about eight have been constructed in the past five years (Gray pers. comm. 2012). There are about 26 cottages on Belliveau Lake (Belliveau pers. comm. 2012; Google Earth 2013), with all but four built since 1984 and about 15 built since 2000. Taschereau (1986) reported one cottage and one cabin on Belliveau Lake in 1967 and four cottages on Belliveau Lake in 1984. COSEWIC (2001) reported an additional five cottages. Development is ongoing and in 2012 three lots were observed to have had construction impacts since 2009, including one in which all shoreline shrubs had been removed over a distance of about 15 m (Belliveau pers. comm. 2012). Given the good road access near much of their shores, shoreline development on all these lakes is likely to continue through the future. Belliveau Lake, where property ownership is extremely divided (129 private lots over 15.8 km of lakeshore), and Mill Lake, where a large property is owned by an offshore development company, are especially likely to see significant cottage development in the near future.
Sweet Pepperbush generally persists along cottage shorefronts if landowners do not remove it. A large portion of the population on Pretty Mary Lake occurs within cottage shorefront, and landowners on all private land lakes typically leave some Sweet Pepperbush on their shorefronts while removing vegetation from areas on the scale of a few metres to 20 m (Belliveau pers. comm. 2012; Hill pers. comm. 2012). Total shoreline currently occupied (at various densities) by Sweet Pepperbush is about 11 km, and if each of the 51 cottages on pepperbush lakes removed all pepperbush from 10 m of shoreline (probably an overestimate of actual loss), total loss of occupied habitat would be 4.6%. This is an imprecise calculation of actual population loss but suggests that losses up to the present have been relatively small.
Artificial Regulation of Water Levels
The artificial regulation of water levels through dam construction can directly eliminate coastal plain shoreline species through flooding and can alter community composition as reduced disturbance allows competitive species to displace rarer, less competitive ones (Keddy 1989; Wisheu and Keddy 1994; Nilsson and Jansson 1995; Hill et al. 1998; Merritt and Cooper 2000). Artificial water level regulation was not identified as a threat in the 2001 status report, and it is unlikely that additional dams will be constructed on lakes occupied by Sweet Pepperbush; however, Mill Lake is controlled by a dam that raises its water level about 1.5m. This dam has likely been present for at least 70 years (Gray pers. comm. 2012) and may have reduced pepperbush populations from pre-dam levels, because the species is absent from the most significantly affected area within about 500 m of the dam and is less common on Mill Lake than either Pretty Mary or Mudflat lakes. The largest potential threat related to this dam is that it will give way and result in conditions less suitable for existing pepperbush while exposing unoccupied potential habitat that could be rapidly taken over by Glossy Buckthorn.
Limited Genetic Diversity and Lack of Seed Production
As discussed in Dispersal and Migration, dispersal and/or establishment of Sweet Pepperbush from seed appears limited given the species’ absence from vast areas of apparently suitable habitat around southern Nova Scotia’s many lakes, rivers and streams. Seed production elsewhere is documented as being extensive (Jordan and Hartman 1995). If the limitation is one of seed production as suggested for Belliveau Lake by Taschereau (1986; but note the recent observation of seed production, with viability unknown, by Belliveau pers. comm. 2012), the most likely cause is lack of genetic diversity. Using horticultural cultivars, Reed et al. (2002) recorded no viable seed production with self-pollination, and Reed (2006) found self-pollinations produced less than 10% of the seed produced by cross-pollinations. Except for Canoe Lake, where the single individual could be lost to an unexpected event, eliminating the sub-population, a lack of genetic diversity and inability to produce seed would seem to be a factor limiting range expansion rather than a short-term threat to persistence. Sweet Pepperbush reproduces extensively via rhizome and vegetative shoot growth, and the relatively large and spatially extensive populations at all three Canadian sites are unlikely to disappear quickly.
Number of Locations
C COSEWIC "locations" are defined at the scale of the most significant threat to each population (COSEWIC 2010). Differences in watershed, land ownership and land use mean that threats vary at each of the three subpopulations of Sweet Pepperbush, and these must represent at least three locations. The threat of Glossy Buckthorn invasion, which is relatively uniform across the whole Pretty Mary – Mudflat – Mill Lake occurrence, makes it a single location. The threat of future Glossy Buckthorn invasion and an absence of other threats makes Louis Lake a single occurrence. The single individual at Canoe Lake, which has shown no evidence of vegetative spread beyond the densely clumped stems discovered in 1995, is not considered a viable occurrence and is not counted as a separate location. If nutrient enrichment and/or future Glossy Buckthorn invasion are considered the most significant threat(s) at Belliveau Lake, it is also a single location. If shoreline development is considered the most significant threat at Belliveau Lake, the number of occurrences there could be three (based on Crown, developed private and undeveloped private shorelines) or up to 44 (the number of separate land parcels on the shoreline in which Sweet Pepperbush is known). Use of the higher number would be based on the idea that individual landowners are making independent decisions on land management. Total number of Sweet Pepperbush locations in Canada is thus three, five or a total of not more than 46, depending on interpretation of the most significant threat and the scale at which the threat of shoreline development is considered.
Protection, Status, and Ranks
Legal Protection and Status
Sweet Pepperbush is currently listed as Special Concern in Canada under Schedule 1 of the Species at Risk Act(Government of Canada 2011). It was designated Threatened in April 1986 with status re-examined and confirmed in April 1998 and then it was re-examined and designated Special Concern in May 2001. It is also listed as Vulnerable under the Nova Scotia Endangered Species Act (Nova Scotia DNR 2013).
Sweet Pepperbush is Endangered in Tennessee under the state’s Rare Plant Protection and Conservation Act of 1985 (Crabtree 2012, 2013), but has no legal protection elsewhere in its non-Canadian range.
Non-Legal Status and Ranks
Sweet Pepperbush is Critically Imperilled (N1) in Canada and in Nova Scotia (S1) (NatureServe 2013) and is ranked as Sensitive in Nova Scotia and Canada (Canadian Endangered Species Conservation Council 2011). It is globally secure (G5) and nationally secure in the United States (N5) and is Critically Imperiled (S1) in Tennessee (Crabtree 2012, 2013) and Imperiled (S2) and listed as Special Concern in Maine, but otherwise has no special non-legal designations within its range outside Canada (NatureServe 2013).
Habitat Protection and Ownership
Roughly 94% of Sweet Pepperbush in Nova Scotia is on private land. The entire population on Louis Lake and Canoe Lake (3 or 4% of the Canadian population) and 460 m of shoreline on three different lots on BelliveauLake (with an estimated 1.8 to 2.5% of the Canadian population, assuming even distribution around the lake) are the only occurrences on provincial Crown land. Occurrences on Crown land are not subject to the direct threats associated with property development. The occurrence on Louis Lake is on Crown land designated as a Significant Ecological Site, which confers no specific protections but generally limits forest harvesting (MacKinnon pers. comm. 2012). All Crown land occurrences are likely to be designated as nature reserves in the near future (MacKinnon pers. comm. 2012), providing legal protection from direct human disturbance, but not from the threats associated with invasive Glossy Buckthorn and nutrient enrichment.
Cutting of Sweet Pepperbush, in contravention of the Nova Scotia Species at Risk Act, still occurs at some private land sites (Belliveau pers. comm. 2012; Gray pers. comm. 2012), but stewardship by private landowners has undoubtedly improved somewhat since 2001 due to efforts at landowner contact and education, which have resulted in general familiarity with Sweet Pepperbush by landowners at the Pretty Mary – Mudflat – Mill lake population (Gray pers. comm. 2012).
Sweet Pepperbush occurrences at the water’s edge receive indirect protection afforded by provincial laws and policies regulating shoreline development and pertaining to the protection of water quality, watercourses, wetlands and riparian buffers, although these regulations do not always provide protection in practice. The Nova Scotia Wetlands Conservation Policy, Activities Designation Regulations and Environmental Assessment Regulations, all under the Environment Act, the Forest Act - Wildlife Habitat and Watercourses Protection Regulations, and the Off Highway Vehicle Actall may apply.
Acknowledgements and Authorities Contacted
Alain Belliveau and Colin Gray of Mersey Tobeatic Research Institute and Nicholas Hill of Fern Hill Institute for Plant Conservation provided invaluable insights based on their field observations of Sweet Pepperbush. Brad Toms of Mersey Tobeatic Research Institute provided field data from their comprehensive mapping of Sweet Pepperbush distribution. Megan Crowley of Kejimkujik National Park provided photographs. David MacKinnon of the Nova Scotia Department of Environment, Protected Areas Division provided information on the status of Crown land on which Sweet Pepperbush occurs. Julia Flemming of Nova Scotia Department of Agriculture provided information on mink farming.
Information Sources
AC CDC (Atlantic Canada Conservation Data Centre). 2013. Digital database of rare species locations for Nova Scotia. Atlantic Canada Conservation Data Centre, Sackville, NB.
Anderberg, A.A., C. Rydin, and M. Kallersjo. 2002. Phylogenetic relationships in the order Ericales s.l.: analyses of molecular data from five genes from the plastid and mitochondrial genomes. Amer. J. Bot. 89:677-687.
Anstey, A. 1999. Effects of flooding and salinity on the growth and distribution of Clethra alnifolia L. (sweet pepper bush). M.Sc. thesis, Virginia Commonwealth University, Richmond, VA. 47 pp.
APG (Angiosperm Phylogeny Group). 1998. An ordinal classification for the families of flowering plants. Ann. Missouri Bot. Gard. 85:531-553.
APG (Angiosperm Phylogeny Group).2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnaean Society 141:399-436.
Beeson, K.C., V.A. Lazar, and S.G. Boyce. 1955. Some plant accumulators of micronutrient elements. Ecology 36:155-156.
Belliveau, A., pers. comm. 2012. Telephone and email communication with Sean Blaney regarding populations of and threats to Sweet Pepperbush at Annapolis and Digby County occurrences.December 2012. Ecosystems Researcher, Mersey Tobeatic Research Institute, Kempt NS.
Benson, L. 1979. Plant Classification, 2nd Ed. D.C. Heath & Co., Lexington MA.
Bhattacharya, M., R.B. Primack, and J. Gerwein. 2003. Are roads and railroads barriers to bumblebee movement in a temperate suburban conservation area? Biological Conservation 109:37–45.
Blaney, C.S., pers. obs. 1999-2012. Personal observations on Atlantic Coastal Plain flora in southern Nova Scotia. Botanist and Assistant Director, Atlantic Canada Conservation Data Centre, Sackville NB.
Blaney, C.S. pers. obs. 2010. Personal observations on Glossy Buckthorn (Frangula alnus) on the Medway River near Kempt and near Barrington, Nova Scotia. Botanist and Assistant Director, Atlantic Canada Conservation Data Centre, Sackville NB.
Blaney, C.S. 2011. Nova Scotia Wetland Plant Indicator List Online document: [accessed February, 2013]. Nova Scotia Department of Environment.
Blaney, C.S. pers. obs. 2012. Personal observations on Glossy Buckthorn (Frangula alnus) near Digby and on cultivated Sweet Pepperbush (Clethra alnifolia) on Marcel Lake, Nova Scotia. Botanist and Assistant Director, Atlantic Canada Conservation Data Centre, Sackville NB.
Blaney, C.S., D.M. Mazerolle, and A. Belliveau pers. obs. 2013. Personal observations on Sweet Pepperbush (Clethra alnifolia) at Mudflat Lake and the area between Mudflat and Mill Lake, September 5, 2013. Botanist and Assistant Director, Atlantic Canada Conservation Data Centre, Sackville NB.
Brower, A.E. 1974. A List of the Lepidoptera of Maine – Part I (PDF ; 11,812 Kb), The Macrolepidoptera. University of Maine at Orono, Life Sciences and Agriculture Experimental Station Technical Bulletin No. 66. 136 pp. [accessed March 11, 2013].
Brylinsky, M. 2011. An assessment of the sources and magnitudes of nutrient inputs responsible for degradation of water quality in seven lakes located within the Carleton River watershed area of Digby and Yarmouth counties, Nova Scotia. Prepared for the Nova Scotia Department of Environment. 25 pp.
Brylinsky, M. 2012. Results of the 2011 Water Quality Survey of Ten Lakes Located in the Carleton River Watershed Area of Digby and Yarmouth Counties, Nova Scotia. Prepared for Nova Scotia Environment. Acadia Center for Estuarine Research, Acadia University, Wolfville NS. 37 pp.
Buchmann, S.L. 1983. Buzz pollination in Angiosperms. pp. 73-113 in: Handbook of Experimental Pollination Biology. C.E. Jones and R.J. Little, Eds. Scientific and Academic Editions, New York.
Bussmann, R.W., G. Ashley, D. Sharon, G. Chait, D. Diaz, K. Pourmand, B. Jonat, S. Somogy, G. Guardado, C. Aguirre, R. Chan, K. Meyer, A. Rothrock, and A. Townesmith. 2011. Proving that Traditional Knowledge Works: The antibacterial activity of Northern Peruvian medicinal plants. Ethnobotany Research & Applications 9:67-96.
Bussmann, R.W., G. Malca-García, A. Glenn, D. Sharon, G. Chait, D. Díaz, K. Pourmand, B. Jonat, S. Somogy, G. Guardado, C. Aguirre, R. Chan, K. Meyer, A. Kuhlman, A. Townesmith, J. Effio-Carbajal, F. Frías-Fernandez, and M. Benito. 2010. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. Journal of Ethnopharmacology 132:101–108.
Canadian Endangered Species Conservation Council. 2011. Wild Species 2010: The General Status of Species in Canada. National General Status Working Group. 302 pp.
Catling, P.M., and Z.S. Porebski. 1994. The history of invasion and current status of Glossy Buckthorn, Rhamnus frangula, in southern Ontario. Can. Field-Nat. 108:305-310.
Catling, P.M., and G. Mitrow. 2012. Major invasive alien plants of natural habitats in Canada: 4. Glossy Buckthorn. Canadian Botanical Association Bulletin 45:70-77.
Christman, S. 2011. Floridata – Clethra alnifolia. Website: [accessed March 14, 2013].
Clayden, S.R., M.C. Munro, C.S. Blaney, and S.P. Vander Kloet. 2009. Vascular flora of the Atlantic Maritime Ecozone: some new perspectives. Ch. 10, pp. 197-214 in D.F. McAlpine, and I.M. Smith (eds.). Assessment of Species Diversity in the Atlantic Maritime Ecozone. NRC Research Press, Ottawa, ON. 785 pp.
Clement E.J., and Foster M.C. 1994. Alien plants of the British Isles. BSBI, London. xviii + 590 pp.
Clemson Extension. 2010. Home and Garden Information Centre: Summersweet Clethra. [accessed March 17, 2013].
COSEWIC. 2001. COSEWIC assessment and update status report on the Sweet Pepperbush Clethra alnifolia in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 11 pp.
COSEWIC. 2010. COSEWIC's Assessment Process and Criteria (PDF ; 529 Kb). [accessed February 22, 2013].
COSEWIC. 2012a. COSEWIC assessment and update status report on Plymouth Gentian Sabatia kennedyana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa.
COSEWIC. 2012b. COSEWIC assessment and update status report on Pink Coreopsis Coreopsis rosea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa.
Crabtree, T. 2012. Tennessee Heritage Program Rare Plant List. Division of Natural Areas, Tennessee Department of Environment and Conservation, Nashville TN. 48 pp. Website: Department of Environment & Conservation Sitemap [accessed March 10, 2013].
Crabtree. T., pers. comm. 2013. Email communication with Bruce Bennett regarding legal and non-legal status of Sweet Pepperbush in Tennessee. September 2013. State Botanist, Tennessee Natural Heritage Program, Nashville, TN.
Cullina, W. 2003. Alternatives to invasive or pot entially invasive exotic species (PDF ; 515 Kb). New England Wildflower Society, Framingham MA. Website: [accessed March 20, 2013].
Dave's Garden. 2013. PlantFiles: Summersweet, Sweet Pepper BushClethra alnifolia. [accessed March 13, 2013].
Denmark, H.A., W.C. Welbourn, and T.R. Fasulo. 2009. Southern Red Mite (PDF ; 2,261 Kb), Oligonychus ilicis (McGregor) (Arachnida: Acari: Tetranychidae). EENY-376 (IN680), DPI Entomology Circular 79, Entomology and Nematology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Website: [accessed March 11, 2013].
Deno, N.C. 1993. Seed germination: theory and practice, 2nd ed. (self-published). State College, PA.
Dirr, M.A. 1990. Manual of Woody Landscape Plants. Stipes Publishing Company, Champaign, IL. 1007 pp.
Dirr, M.A., and C.W. Heuser Jr. 1987. The reference manual of woody plant propagation: from seed to tissue culture. Varsity Press, Athens GA. 239 pp.
Eaton, S.T., and J.S. Boates. 2003. Securing the science foundation for responsible stewardship and recovery of ACPF species at risk. NS Department of Natural Resources, Kentville, NS.
Eckert, C.G., K .E. Samis, and S.C. Lougheed. 2008. Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Molecular Ecology 17:1170–1188.
Ehrenfeld, J.G. 1983. The effects of changes in land use on swamps of the New Jersey Pine Barrens. Biological Conservation 25:353-375.
Environment Canada and Parks Canada Agency. 2010. Recovery Strategy and Management Plan for Multiple Species of Atlantic Coastal Plain Flora in Canada. Species at Risk ActRecovery Strategy Series. Environment Canada and Parks Canada Agency. Ottawa. 96 pp. + appendices.
Fernald, M.L. 1921. The Gray Herbarium expedition to Nova Scotia 1920. Rhodora 23:89-111, 130-152, 153-171, 184-195, 233-245, 257-78, 284-300.
Fernald, M.L. 1922. Notes on the flora of western Nova Scotia. Rhodora 24:157-164, 165–181, 201-208.
Fernald, M.L. 1950. Gray’s Manual of Botany. A handbook of the flowering plants of the central and northeastern United States and adjacent Canada. 8th Edition. American Book Company. New York. 1632 pp.
Fiedler, A.K., and D.A. Landis. 2012. Biotic and Abiotic Conditions in Michigan Prairie Fen Invaded by Glossy Buckthorn (Frangula alnus). Natural Areas Journal 32: 41-53.
Fior, S., P.O. Karis, and A.A. Arneberg. 2003. Phylogeny, Taxonomy, and Systematic Position of Clethra (Clethraceae, Ericales) with Notes on Biogeography: Evidence from Plastid and Nuclear DNA Sequences. International Journal of Plant Sciences 16:997-1006.
Fleming, W.E. 1972. Biology of the Japanese beetle. USDA Tech. Bull. No. 1449. Agricultural Research Service, Washington. 129 pp.
Flemming, J., pers. comm. 2011. December 7, 2011. Telephone conversation with Sean Blaney regarding mink farming in southern Nova Scotia. Permitting officer, Laboratory Services Section, Nova Scotia Department of Agriculture, Truro NS.
Frappier, B., R.T. Eckert, and T.D. Lee. 2003a. Potential impacts of the invasive exotic shrub Rhamnus frangula L. (glossy buckthorn) on forests of southern New Hampshire. Northeast. Nat. 10:277-296.
Frappier, B., T.D. Lee, K.F. Olson, and R.T. Eckert. 2003b. Small-scale invasion pattern, spread rate, and lag-phase behavior of Rhamnus frangula L. For. Ecol. Manag. 186:1-6.
Furumai, T., T. Yamakawa, R. Yoshida, and Y. Igarashi. 2003. Clethramycin, a new inhibitor of pollen tube growth with antifungal activity from Streptomyces hygroscopicus TP-A0623. I. Screening, taxonomy, fermentation, isolation and biological properties. J. Antibiot. (Tokyo).56:700-704.
García-Ramos, G., and M. Kirkpatrick. 1997. Genetic models of rapid evolutionary divergence in peripheral populations. Evolution 51:21-28.
Gargiullo, M. 2007. A Guide to Native Plants of the New York City Region. New York City Department of Parks and Recreation. New York. 307 pp.
Glen,C. 2005. Salt Tolerant Plants. North Carolina State University Cooperative Extension, Pender County Center (PDF ; 531 Kb) . Website: [accessed March 13, 2013].
Google Earth. 2013. 2010 aerial photographs of Belliveau Lake, Louis Lake and Canoe Lake, Nova Scotia and 2003 aerial photographs of Pretty Mary, Mudflat and Mill lakes. Online application. [Aerial photographs accessed December 2012].
Government of Canada. 2011. Species at Risk Public Registry. Website: Species Profile: Goldencrest [accessed November 2011].
Gray, C., pers. comm. 2012. Email and phone communication on populations of and threats to Sweet Pepperbush at Pretty Mary, Mudflat and Mill Lakes. December 2012. Chair, Mersey Tobeatic Research Institute and resident of Mudflat Lake, NS.
Hagerup, O. 1928. Morphological and cytological studies of Bicornes. Dansk. Bot. Ark. 6:27.
Haines, A. 2011. New England Wild Flower Society's Flora Novae Angliae: A Manual for the Identification of Native and Naturalised Higher Vascular Plants of New England. Yale University Press, New Haven CT. 992 pp.
Hampe, A., and F. Bairlein. 2000. Modified dispersal-related traits in disjunct populations of bird-dispersed Frangula alnus (Rhamnaceae): A result of its quaternary distribution shifts? Ecography 23:603-613.
Hemingson, J.C. 1986. The pollination biology of Clethra alnifolia L. (Clethraceae). Ph.D. dissertation. University of Connecticut, Storrs, CT. 276 pp.
Henry, L.K. 1934. Mycorrhizae of Wading River region, Long Island. Torrey 34:111-115.
Hill, N., pers. comm. 2012. Email and phone communication on populations of and threats to Sweet Pepperbush in Nova Scotia. December 2012. Research Scientist and Botanical Consultant, Fern Hill Institute for Plant Conservation, Berwick NS.
Hill N.M., Boates J.S., Elderkin M.F. 2000. Low catchment area lakes: new records for rare coastal plain shrubs and Utricularia species in Nova Scotia. Rhodora 102: 518-522.
Hill, N. M., and, C. S. Blaney. 2009. Exotic and invasive vascular plants of the Atlantic Maritime Ecozone. Pages 1-18 in Assessment of species diversity in the Atlantic Maritime Ecozone. Edited by D. F. McAlpine and I. M. Smith. NRC Research Press, Ottawa.
Hill, N.M., P.A. Keddy, and I.C. Wisheu. 1998. A hydrological model for predicting the effects of dams on the shoreline vegetation of lakes and reservoirs. Environmental Management 22:723-736.
Huenneke, L.F. 1987. Demography of a clonal shrub, Alnus incana ssp. rugosa (Betulaceae). The American Midland Naturalist 117:43-55.
Huenneke, L.F., and P.L. Marks. 1987. Stem Dynamics of the Shrub Alnus incana ssp. rugosa: Transition Matrix Models Ecology 68:1234-1242.
Hurlburt, D., pers. comm. 2013. Email communication with Sean Blaney regarding aboriginal traditional knowledge of Sweet Pepperbush (Clethra alnifolia). March 20, 2013. COSEWIC Aboriginal Traditional Knowledge Specialist Committee representative to the COSEWIC Vascular Plants Species Specialist Committee, Yarmouth, NS.
Huxley, A., M. Griffiths, and M. Levy. 1992. The New Royal Horticultural Society Dictionary of Gardening. MacMillan Reference, London.
IPANE (Invasive Plant Atlas of New England IPANE). 2012. [accessed December 2012].
IPNI (International Plant Names Index). 2005. Clethra alnifolia. [accessed March 5, 2013].
Jacobson, M., R.E. Redfern, and G.D. Mills Jr. 1975. Naturally occurring insect growth regulators. II. Screening of insect and plant extracts as insect juvenile hormone mimics. Lloydia 38:455-472.
Jordan, R.A. 1993. The ecology of Clethra alnifolia L. (Clethraceae) in wetland forests of central New Jersey. Ph.D. Dissertation. Rutgers University, New Brunswick, NJ. 232 pp.
Jordan, R.A., and J.M. Hartman. 1995. Safe sites and the regeneration of Clethra alnifolia L. (Clethraceae) in wetland forests of central New Jersey. Am. Midl. Nat. 133:112–123.
Jordan, R.A., and J.M. Hartman. 1996. Effects of canopy opening on recruitment in Clethra alnifolia L. (Clethraceae) populations in central New Jersey wetland forests. Bulletin of the Torrey Botanical Club 123:286-294.
Jull, L.G. 2001. Plants not Favoured by Deer. University of Wisconsin Extension, Madison WI. [accessed March 11, 2013].
Kartesz, J.T. 2011. North American Plant Atlas. maps generated from Kartesz, J.T. 2010. Floristic Synthesis of North America, Version 1.0.The Biota of North America Program (BONAP). Chapel Hill, NC. [accessed February 2013].
Kavaljian, L.G. 1952. The floral morphology of Clethra alnifolia with some notes on C. acuminata and C. arborea. Bot. Gaz. 113:392-413.
Keddy, P.A. 1989. Effect of competition from shrubs on herbaceous wetland plants: a 4-year field experiment. Canadian Journal of Botany67: 708–716.
Kral, R., A.R. Diamond Jr, S.L. Ginzbarg, C.J. Hansen, R.R. Haynes, B.R. Keener, M.G. Lelong, D.D. Spaulding, and M. Woods. 2013. Clethra alnifolia in Alabama Plant Atlas. University of West Alabama, Livingston, Alabama. Alabama Plant Atlas - Clethra alnifolia [accessed March 12, 2013].
Laycock, W.A. 1967. Distribution of Roots and Rhizomes in Different Soil Types in the Pine Barrens of New Jersey (PDF ; 5137 Kb). United States Department of the Interior, Geological Survey Professional Paper 563-C.
Lee, T.D., and J.H. Thompson. 2012. Effects of logging history on invasion of eastern white pine forests by exotic glossy buckthorn (Frangula alnus P. Mill.). For. Ecol. Manag. 265: 201-210.
Lesica, P., and F.W. Allendorf. 1995. When Are Peripheral Populations Valuable for Conservation? Conservation Biology 9:753-760.
MacKinnon, D., pers. comm. 2012. Email conversation with Sean Blaney about the status of Crown land supporting Sweet Pepperbush. November, 2012. Protected Areas Planner, Nova Scotia Department of Environment and Labour, Halifax, NS.
Magee, D.W., and H.E. Ahles. 1999. Flora of the Northeast, A Manual of the Vascular Flora of New England and adjacent New York. University of Massachusetts Press, Amherst MA. 1213 pp.
Marsden, M.W. 1989. Lake restoration by reducing external phosphorus loading: the influence of sediment phosphorus release. Freshwater Biology 21: 139-162.
Merritt, D.M., and D.J. Cooper. 2000. Riparian vegetation and channel change in response to river regulation: A comparative study of regulated and unregulated streams in the Green River Basin, USA. Regulated Rivers: Research and Management 16: 543–564.
Missouri Botanical Garden. 2013. Gardening Help: Clethra alnifolia. [accessed March 17, 2013].
Moore, D.R.J., P.A. Keddy, C.L. Gaudet, and I. Wisheu. 1989. Conservation of Wetlands: Do Infertile Wetlands Deserve a Higher Priority? Biological Conservation 47: 203-217.
MTRI (Mersey Tobeatic Research Institute). 2012. Rare coastal plain species surveys by Alain Belliveau on lakes supporting Sweet Pepperbush. Unpublished data, held at Mersey Tobeatic Research Institute, Caledonia, NS and Atlantic Canada Conservation Data Centre, Sackville NB.
Murata, T., A. Suzuki, N. Mafune, E. Sato, T. Miyase, and F. Yoshizaki. 2013. Triterpene Saponins from Clethra barbinervis and Their Hyaluronidase Inhibitory Activities. Chem. Pharm. Bull. (Tokyo) 61:134-43.
National Botanic Garden of Belgium. 2013. Manual of the Alien Plants of Belgium – Clethra alnifolia. National Botanic Garden of Belgium, Muise, Belgium. [accessed March 10, 2013].
Natural Resources Canada. 2003. Canadian Geographical Names. Geomatics Canada, Earth Sciences Sector, Centre for Topographic Information, Geographical Names Section. Ottawa ON. [Downloaded 2007; sorted for “lake” and “pond” and by latitude and longitude to produce list of relevant waterbodies].
NatureServe. 2013. NatureServe Explorer – Clethra alnifolia. [accessed February 2013].
Nelson, G. 2006. Atlantic Coastal Plain Wildflowers: A Guide to Common Wildflowers of the Coastal Regions of Virginia, North Carolina, South Carolina, Georgia and Northeastern Florida. FalconGuide, The Globe Pequot Press, Guilford, CT. 265 pp.
Nilsson, C., and R. Jansson. 1995. Floristic differences between riparian corridors of regulated and free-flowing boreal rivers. Regulated Rivers: Research and Management11: 55-66.
Nova Scotia DNR (Department of Natural Resources). 2013. Species at Risk List Regulations made under Sections 10 and 12 of the Endangered Species Act. [accessed March 2013].
Ohio State University. 2013. Clethra alnifolia. Ohio State Department of Horticulture and Crop Sciences, College of Food, Agricultural and Environmental Sciences. Website: Horticulture in Virtual Perspective [accessed March 13, 2013].
Osborne, J.L., A.P. Martin, N.L. Carreck, J.L. Swain, M.E. Knight, D. Goulson, R.J. Hale, and R.A. Sanderson. 2008. Bumblebee flight distances in relation to the forage landscape, Journal of Animal Ecology 77:406-415.
Plants for a Future. 2013. Clethra alnifolia L. [accessed March 10, 2013].
Porter, D.M. 1983. Vascular plants of the Galapagos: origins and dispersal. In: R.I. Bowman, M. Berson, and A.E. Leviton, eds. Galapagos organisms. San Francisco, CA, USA: AAAS, 33–96.
Ramos, J. A. 1995. The diet of the Azores bullfinch Pyrrhula murina and the floristic variation within its range. Biological Conservation 71:237–249.
Reed, S.M. 2005. Cytological analysis of a Clethra alnifolia 'Hokie Pink' x C. pringlei hybrid. HortScience 40: 339-342.
Reed, S.M. 2006. Reproductive biology of Clethra alnifolia. HortScience 41: 567-570.
Reed, S.M., Y. Joung, and M. Roh. 2002. Interspecific hybridization in Clethra. HortScience 37: 393-397.
Reinartz, J.A., and J. Kline. 1998. Glossy buckthorn (Rhamnus frangula), a threat to the vegetation of the Cedarburg Bog. Field Station Bulletin, The University of Wisconsin – Milwaukee 21:20-35.
Reznicek, A.A., E. G. Voss, and B. S. Walters. 2011. Michigan Flora Online. February 2011. University of Michigan. [accessed December 4, 2012].
Robinson, B.L., and M.L. Fernald. 1908. Gray's Manual of Botany, 7th Ed. American Book Co., New York.
Roland, A.E., and E.C. Smith. 1969. The Flora of Nova Scotia. Nova Scotia Museum, Halifax. 743 pp.
Short, M. 2010. Contrasting nutrient dynamics of litter decomposition in a deciduous forest and pond ecosystem. Brown University, Providence RI. Website: http://ecosystems.mbl.edu/ses/2010%20projects/Short.pdf [accessed March 11, 2013].
Silva, L., and C.W. Smith. 2004. A characterization of the non-indigenous flora of the Azores Archipelago. Biological Invasions 6:193–204.
Sleumer, H. 1967. Monographia Clethraceum, Parts I & II. Bot. Jahrb. Syst. Pflanzengesch. Pflanzengeorg 87:36-175.
Smith, J.B. 1909. A report on the insects of New Jersey, p. 435 in Annual Report of the New Jersey State Museum. Trenton, NJ.
Swain, P.C., and J.B. Kearsley. 2001. Classification of the Natural Communities of Massachusetts. Version 1.3. Natural Heritage & Endangered Species Program, Division of Fisheries & Wildlife. Westborough, MA. Online document: http://www.mass.gov/dfwele/dfw/nhesp/natural_communities/pdf/acidic_shrub_fen.pdf; http://www.mass.gov/dfwele/dfw/nhesp/natural_communities/pdf/acidic_ graminoid_fen.pdf [accessed December 2012].
Sylvia, D.M. 1988. Growth response of native woody plants to inoculation with Glomus species in phosphate mine soil. Proceedings - Soil and Crop Science Society of Florida. v. 47.
Sylvia, D.M. 1990. Inoculation of native woody plants with vesicular-arbuscular mycorrhizal fungi for phosphate mine land reclamation. Agriculture, Ecosystems & Environment 31:253-261.
Taft, J.B. and, M.K. Solecki. 1990. Vascular flora of the wetland and prairie communities of Gavin Bog and Prairie Nature Preserve, Lake County, Illinois. Rhodora. 92(871): 142-165.
Takahasi, K., and M. Takani. 1978. Studies on the constituents of the Medicinal Plants, Part 21, Constituents of the leaves of Clethra barbinervis and the carbon-13 NMR spectra of 19-alpha hydroxyl URS-12-EN-28-OIC-acid type of triterpinoids. Chemical and Pharmaceutical Bulletin 26:2689-2693.
Takani, M., K. Kubota, M. Nozawa, T. Ushiki, and K. Takahasi. 1977. Studies on the constituents of the Medicinal Plants, Part 18, Constituents of the leaves of Clethra barbinervis. Chemical and Pharmaceutical Bulletin 25:981-985.
Tanabe, Y., T. Oda, and K. Takahashi. 1966. Studies on the constituents of Medicinal Plants: VII. The constituents of the bark and fruit of Clethra barbinervis Sieb. ex Zucc. J. Pharm. Soc. Jap. 86:414-417.
Tanaka, R., and K. Oginuma. 1980. Karyomorphological studies on Clethra barbinervis and two allied species. J. Jpn. Bot. 55:65-72.
Taschereau, P.M. 1969. Clethraceae: A Plant Family New to Canada. Canadian Field-Naturalist 83:166.
Taschereau, P.M. 1986. Status Report on the Sweet Pepperbush Clethra alnifolia in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa, ON. 50 pp.
Tiner, R.W., Jr. 1987. A field guide to coastal wetland plants of the northeastern United States. University of Massachusetts Press, Amherst, MA. 285 pp.
Troubridge, J.T., and J.D. Lafontaine. 2004. The Noctuoidea of Eastern Canada. Agriculture and Agrifood Canada, Canadian Biodiversity Information Facility. [accessed March 11, 2013].
USDA (United States Department of Agriculture). 2012. Plant Hardiness Zone Map. [accessed March 17, 2013].
USFWS (United States Fish and Wildlife Service). 1997. National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary (PDF ; 1370 Kb). USFWS Ecology Section, National Wetlands Inventory. [accessed February 13, 2013].
Van der Meijden, R. 2005. Heukels' Flora van Nederland (23e druk). Wolters-Noordhoff, Groningen. 685 pp.
Verloove, F. 2006. Catalogue of neophytes in Belgium (1800-2005) (PDF ; 1575 Kb). National Botanic Garden of Belgium, Meise, Belgium. 89 pp. [accessed March 8, 2013].
Virginia Botanical Associates. 2013. Digital Atlas of the Virginia Flora. Virginia Botanical Associates, Blacksburg VA. [accessed March 1, 2013].
Walther-Hellwig, K., and R. Frankl. 2000. Foraging distances of Bombus muscorum, Bombus lapidarius, and Bombus terrestris (Hymenoptera, Apidae). J. Insect Behav. 13:239–246.
Weakley, A.S. 2012. Flora of the Southern and Mid-Atlantic States (PDF ; 54993 Kb). University of North Carolina Herbarium, Chapel Hill NC. 1225 pp. [accessed March 12, 2013].
Wennerberg, S. 2004. Coastal Sweet Pepperbush Clethra alnifolia L. Plant Guide(PDF ; 583 Kb). USDA Plants Database. [accessed September 17, 2013].
White, D.J., J.C. Makarewicz, and T.W. Lewis. 2002. The significance of phosphorus released from the sediment under anoxic conditions in Sodus Bay, N.Y. Environmental Sciences Program, Department of Biological Sciences SUNY Brockport Brockport, New York, 33 pp.
Wilbur, R.L., and H.A. Hespenheide. 1967. The genus Clethra (Clethraceae) in the United States. J. Elisha Mitchell Soc. 83: 82-88. .
Williamson, J. 2010. Summersweet Clethra. Home & Garden Information Center. Clemson Cooperative Extension (PDF ; 682 Kb). Clemson University. Clemson, South Carolina. [accessed Spetember 17, 2013].
Wisheu, I.C., and P.A. Keddy. 1994. The low competitive ability of Canada's Atlantic Coastal Plain shoreline flora: implications for conservation. Biological Conservation 68: 247–252.
Wood, G.W. 1988. Effects of prescribed fire on deer forage and nutrients. Wildlife Society Bulletin 16:180-186.
Zaremba, R.E., and E.E. Lamont. 1993. The status of the Coastal Plain Pondshore community in New York. Bulletin of the Torrey Botanical Club120: 180-187.
Biographical Summary of Report Writer(s)
Sean Blaney is the Botanist and Assistant Director of the Atlantic Canada Conservation Data Centre (AC CDC), where he maintains status ranks and a rare plant occurrence database for each of the three Maritime provinces. Since beginning with the AC CDC in 1999, he has documented dozens of new provincial records for vascular plants and thousands of rare plant localities during extensive fieldwork across the Maritimes. Sean is a member of the COSEWIC Vascular Plant Species Specialist Committee, the Nova Scotia Atlantic Coastal Plain Flora Recovery Team, and has authored or co-authored numerous COSEWIC and provincial status reports. Prior to employment with AC CDC, Sean received a B.Sc. in Biology (Botany Minor) from the University of Guelph and an M.Sc. in Plant Ecology from the University of Toronto, and worked biological inventory projects in Ontario as well as spending eight summers as a naturalist in Algonquin Park, where he co-authored the second edition of the park’s plant checklist.
Collections Examined
No specimens were examined during preparation of the report. Specimens from the E.C. Smith Herbarium, Acadia University (ACAD) and the Nova Scotia Museum of Natural History (NSPM) were already documented in the Atlantic Canada Conservation Data Centre database (AC CDC 2011) prior to the preparation of the report.