COSEWIC Assessment and Status Report on the Tall Beakrush Rhynchospora macrostachya in Canada - 2014

Photograph by Sean Blaney, AC CDC.
Long description for Banner
The inflorescences consist of long-peduncled, dense clusters of elongated brown spikelets that are predominantly in terminal clusters.
- Document Information
- COSEWIC Assessment Summary
- COSEWIC Executive Summary
- Technical Summary
- COSEWIC History
- Wildlife Species Description and Significance
- Distribution
- Habitat
- Biology
- Population Sizes and Trends
- Threats and Limiting Factors
- Protection, Status and Ranks
- Acknowledgements and Authorities Contacted
- Information Sources
- Biographical Summary of Report Writers
- Collections Examined
- Figure 1. Tall Beakrush (Rhynchospora macrostachya) inflorescence at Carrigan Lake, Queens County, Nova Scotia.
- Figure 2. Tall Beakrush in lakeshore peatland on Carrigan Lake at the mouth of the brook draining Murphy Lake. Several large individuals of the invasive shrub Glossy Buckthorn (Frangula alnus) are visible in the background (the greyer-green mid-sized shrubs at the margin of the taller Red Maples (Acer rubrum)).
- Figure 3. Tall Beakrush at Keddy Cove, Molega Lake with cottage beach development visible in background across the bay. Shoreline development was also present immediately southeast (right in this picture) from the shoreline area occupied by Tall Beakrush.
- Figure 4. Native range (counties with light green shading in the United States, and light green dots in Canada) of Tall Beakrush (Rhynchospora macrostachya). The . In the United States a whole county is shaded light green if at least one record is known. The Mississippi record may be in error (see Global Range).”
- Figure 5. Distribution of Tall Beakrush (Rhynchospora macrostachya; red dots) in Nova Scotia at 1 – Carrigan Lake, 2 – Keddy Cove, Molega Lake. Inset map indicates location of the larger map within Nova Scotia.
- Appendix 1. Named lLakes within 10 km of known Tall Beakrush occurrences, with extent and date(s) of shoreline plant surveys since 2007.
- Appendix 2. Species associated with Tall Beakrush in order of frequency, based on AC CDC (2013) records of associated species at 30 occurrences of Tall Beakrush at Carrigan and Molega Lakes.
COSEWIC
Committee on the Status
of Endangered Wildlife
in Canada

COSEPAC
Comité sur la situation
des espèces en péril
au Canada
COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2014. COSEWIC assessment and status report on the Tall Beakrush Rhynchospora macrostachya in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 49 pp.
COSEWIC would like to acknowledge Sean Blaney (Atlantic Conservation Data Centre) for writing the status report on the Tall Beakrush, Rhynchospora macrostachya, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Bruce Bennett, Co-chair of the COSEWIC Vascular Plants Specialist Subcommittee.
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment Canada
Ottawa, ON
K1A 0H3
Tel.: 819-938-4125
Fax: 819-938-3984
E-mail: COSEWIC email
Website: COSEWIC
Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Rhynchospore à gros épillets (Rhynchospora macrostachya) au Canada.
Tall Beakrush -- Photo: C. Smith.


Tall Beakrush is a perennial, herbaceous sedge. Flowering stems, arising from a dense clump of basal leaves, reach 150 – 170 cm in the United States and about 100 cm in Canada. Flowers are enclosed within brown scales, with each having male and female parts and six elongate, barbed bristles. Fertilized flowers develop into a hard, flattened achene 5 to 6 mm long, topped by a greatly elongated tubercle.
Tall Beakrush is one of many species of the Atlantic Coastal Plain that are disjunct and nationally rare in southern Nova Scotia, and that have received fairly widespread attention and appreciation in the region through ongoing outreach programs. The Canadian population is isolated from others by 468 km and is the northernmost worldwide, suggesting potential significance to the species' range-wide genetic diversity. The seed-like achenes of Tall Beakrush can also be an important food for wild ducks in the southern United States.
Tall Beakrush is predominantly a species of the Atlantic and Gulf Coastal Plains between southern Maine, northeastern Florida, and Louisiana, but it also occurs in southeast Michigan and adjacent Indiana, eastern Oklahoma and adjacent areas of Kansas, Missouri and Arkansas, and along the Tennessee-Alabama border. Isolated records are reported for Kentucky, and northern New York. Reports from Illinois, Mississippi and Vermont are erroneous. Canadian occurrence is restricted to two lakes 23 km apart in southern Nova Scotia. Canada supports less than 1% of the global population.
Tall Beakrush is an obligate wetland plant occurring in Canada on shallow acidic open lakeshores that are fully exposed (or nearly so) during summer low water levels. Substrates are mostly gravelly, often with a thin layer of peaty organic soil on top, but some plants are on deeper peat or on shallow organic soil within cracks in exposed bedrock. In the southern United States, Tall Beakrush also occupies freshwater and slightly saline tidal marshes, swamp forests, and marshes and sloughs within tallgrass prairies, and it can occur in disturbed habitats such as ditches, all-terrain vehicle tracks, pipeline rights-of-way, rice fields and impoundments.
In Nova Scotia, Tall Beakrush flowers from July to September. Pollination is presumed to be largely or entirely by wind, as is the case with most sedges. It is believed to be self-compatible. Seed-like achenes are dispersed from the parent plant in the fall and their long bristles may facilitate dispersal via floatation or on animals. Internal and external dispersal by waterfowl over longer distances is also likely. In a closely related species, germination occurs best in drier periods than are ideal for growth. Reproduction before age one occurs in the United States but probably requires at least two or three years in Nova Scotia, based on observation of mid-sized, non-flowering rosettes. The species is non-rhizomatous but vegetative reproduction occurs over very short distances via production of new rosettes to the side of existing ones. Demographics of vegetative reproduction are unknown, as are longevity of genetic individuals and ramets, and generation time.
A 2013 comprehensive count of the Canadian population found 688 individuals, 648 (95%) of which were in a 1.3 km x 0.7 km area on Carrigan Lake and 36 (5%) of which were in a 30 m stretch of shoreline on Keddy Cove on Molega Lake. Survey effort is sufficient to suggest that it is unlikely that large numbers of additional individuals would be found on these lakes, or that many additional undiscovered subpopulations are present in Canada. Trends are unknown but habitat near current subpopulations suggests stability or small population declines in the past three generations and potentially significant historical subpopulation losses from damming.
Lakeshore development has not yet affected Carrigan Lake plants but 38% of the Canadian population there is adjacent to private land potentially subject to shoreline development, and an additional 39% is on land owned by Nova Scotia Power that might one day be sold. All of the Molega Lake subpopulation (5% of the Canadian population) is in a small, undeveloped zone within shoreline otherwise occupied by cottages, and is under significant threat of further development. All plants at Carrigan Lake (95% of the Canadian population) occur within shoreline for which Nova Scotia Power has flooding rights associated with hydroelectric power generation. Nova Scotia Power believes that anthropogenic flooding has never occurred on Carrigan Lake, and suggests it is unlikely for the foreseeable future. The invasive exotic shrub Glossy Buckthorn is already present immediately around some occurrences at Carrigan Lake and occurs within 950 m of the Molega Lake subpopulation, but is believed unlikely to impact most occupied lakeshore habitat. Competitive exclusion by more aggressive plants responding to eutrophication from mink farm waste or from the cumulative effects of hundreds of additional cottages on Molega Lake is a potential future threat.
Tall Beakrush has no legal protected status in Canada and no occurrences are within protected areas, but it has legal protection in Maine, Connecticut and Tennessee. Tall Beakrush is Critically Imperilled (N1) in Canada and in Nova Scotia (S1) and is ranked as May Be At Risk in Nova Scotia and Canada under the General Status process. It is globally secure (G4) and nationally secure in the United States (N4), but Critically Imperilled (S1) in Kentucky, Maine, Missouri and Rhode Island, borderline Critically Imperilled (S1S2) in Connecticut and Tennessee, Imperilled (S2) in Arkansas, Indiana and Kansas, Vulnerable (S3 or S3?) in New York, Virginia and North Carolina and marginally Vulnerable (S3S4) in Michigan. Tall Beakrush is Apparently Secure (S4) in Delaware, and is unranked (SNR) in Alabama, Florida, Louisiana, Maryland, Massachusetts, New Jersey, Oklahoma, South Carolina, Texas, and the District of Columbia, and Unrankable (SU) in Georgia. It may be Imperilled in Florida and marginally vulnerable in Alabama, Georgia and Massachusetts.
Generation time
Reproduction by seed and by vegetative production of new rosettes. Potential for independence of vegetatively produced rosettes (and their countability as COSEWIC individuals) unknown. See “Life Cycle and Reproduction”
- Estimated 3 to 5 years
Is there an [observed, inferred, or projected] continuing decline in number of mature individuals?
Small Molega Lake subpopulation may have declined before discovery due to cottage development in past 20 years.
- Unknown
Estimated percent of continuing decline in total number of mature individuals within [5 years or 2 generations] (52-60 years)
Decline or loss of Molega Lake subpopulation (5% of Canadian total) could occur due to development impacts. 39% to 78% of Canadian population at Carrigan Lake is adjacent to private land potentially subject to development, or on Nova Scotia Power land that might eventually be sold for development.
- Unknown
[Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over the last [10 years, or 3 generations] (78-90 years)
Large declines unlikely because Carrigan Lake subpopulation (95% of Canadian total) has seen no significant habitat changes.
- Unknown
Projected percent reduction in total number of mature individuals over the next 100 years.
Decline or loss of Molega Lake subpopulation (5% of Canadian total) could occur with future development impacts. Up to 78% of Canadian population at Carrigan Lake also on land potentially subject to future development.
- Unknown
[Observed, estimated, inferred or suspected] percent [reduction or increase] in total number of mature individuals over any [10 years, or 3 generations] period, over a time period including both the past and the future.(As above)
- Unknown
Are the causes of the decline clearly reversible and understood and ceased?
Declines not clearly demonstrated, but any historical declines from damming, and historical or recent declines from development have limited reversibility.
- Understood, partly ceased, limited reversibility
Are there extreme fluctuations in number of mature individuals?
Close relative shows rapid increases from seed bank during low water periods, but this is not known in Tall Beakrush.
- None known
Estimated extent of occurrence
Actual value of 11.46 km2 reverts to the larger IAO value below.
- 12 km2
Index of area of occupancy (IAO, 2 x 2 km² grid values)
From 2 x 2 km grid aligned with 10 x 10 km UTM grid squares.
- 12 km2
Is the population severely fragmented?
See "Population Spatial Structure and Variability"
- No
Number of locations Table Footnotea
Two landowners at Molega Lake. One location at Carrigan Lake if water level management is primary threat.
- 3
Is there a projected continuing decline in extent of occurrence?
Possible decline with cottage construction at Molega Lake.
- Unknown
Is there a projected continuing decline in index of area of occupancy?
Possible decline with cottage construction at Molega Lake, but less likely than above given that a theoretical lost occurrence would need to be in a separate 4 km2 block.
- Unknown
Is there an [observed, inferred, or projected] continuing decline in number of subpopulations?
- Unknown
Is there an [observed, inferred, or projected] continuing decline in number of locations Table Footnotea ?
- Unknown
Is there an [observed, inferred, or projected] continuing decline in quality of habitat?
Cottage development is ongoing at Molega Lake, reducing area, extent and quality of potential habitat near known occurrence, and is likely to affect the occurrence in future.
- Yes
Are there extreme fluctuations in number of subpopulations?
- No
Are there extreme fluctuations in number of locations Table Footnotea ?
- No
Are there extreme fluctuations in extent of occurrence?
- No
Are there extreme fluctuations in index of area of occupancy?
- No
Population:
- Subpop. 1 – Carrigan Lake (N Mature Individuals): 648
- Subpop. 2 – Keddy Cove, Molega Lake (N Mature Individuals): 36
Total
- 684
Probability of extinction in the wild is at least [20% within 20 years or 5 generations, or 10% within 100 years].
- N/A
- Shoreline Development (1.1) Removal of individuals and habitat loss from shoreline development (actual threat at both sites, likely most imminent at Molega Lake)
- Dams & Water Management (7.2) Habitat alteration from artificial water level management (non-imminent but high magnitude threat at Carrigan Lake)
- Invasive Species (8.1) Shading from invasion of exotic Glossy Buckthorn (actual and imminent threat of low magnitude at Carrigan Lake)
- Household (9.1) and Agricultural Effluents (9.3) Increased competition from other plants responding to eutrophication from mink farm waste or cottage septic systems (potential threat of high magnitude at Molega Lake, but not imminent)
- Status of outside population(s)?
Globally Apparently Secure (G4) and Nationally Apparently Secure (N4) in the U.S.: Critically Imperilled (S1) in Kentucky, Maine, Missouri and Rhode Island. Critically Imperilled to Imperilled (S1S2) in Connecticut and Tennessee. Imperilled (S2) in Arkansas, Indiana and Kansas. Vulnerable (S3 or S3?) in New York, Virginia and North Carolina and Vulnerable to Apparently Secure (S3S4) in Michigan. Apparently Secure (S4) in Delaware. Unranked (SNR) and probably secure in Maryland, New Jersey, South Carolina and Texas. Unranked but possibly Imperilled in Florida. Unranked and uncommon in Alabama and Massachusetts. Unrankable (SU) and uncommon in Georgia.
Is immigration known or possible?
- Not known and unlikely
Would immigrants be adapted to survive in Canada?
Southern Maine and Massachusetts populations occur in a similar climate zone
- Probably
Is there sufficient habitat for immigrants in Canada?
Extensive apparently suitable but unoccupied lakeshore in southern NS
- Yes
Is rescue from outside populations likely?
Tall Beakrush is disjunct from nearest populations in Maine (where very rare – S1) by 468 km across open ocean. Disjunct from areas where more common by 500+ km across open ocean.
- No
- COSEWIC: Designated Endangered in November 2014.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
Note: The Canadian Wildlife Service, Environment Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Scientific name: Rhynchospora macrostachya Torr. ex A.Gray
Annals of the Lyceum of Natural History of New York 3: 206. 1835
Synonyms:
Ceratoschoenus macrostachys Torr.
Ceratoschoenus macrostachys (Torr. ex A. Gray) A. Gray in Torr.
Rhynchospora corniculata var. macrostachya (Torr. ex A. Gray) Britton
Rhynchospora macrostachya Torr. ex A. Gray var. colpophila Fernald & Gale
English vernacular names:
Tall Beakrush
Tall Beaked-rush
Tall Horned Beak Sedge
French vernacular name: Rhynchospore à gros épillets
Genus: Rhynchospora
Family: Cyperaceae
Order: Poales
Class: Commelinid clade (APG 2003)
Major plant group: Angiosperms, Monocots
Tall Beakrush was first described by Torrey in Gray (1835) as Rhynchospora macrostachya. It was subsequently called Ceratoschoenus macrostachys by Gray in Torrey (1836), but the species has generally been treated under Rhynchospora since about 1860 (IPNI 2005). The species concept for Rhynchospora macrostachya has varied in the past. The Narrow-fruit Beakrush (Rhynchospora inundata) was originally described as a variety of Tall Beakrush under the name Ceratoschoenus macrostachys var. inundatus by Oakes (1841) before being segregated at the species level by Fernald (1918). Britton (1892) treated Tall Beakrush as variety macrostachya of the Short-bristle Beakrush(Rhynchospora corniculata). Chapman treated some or all of the Broad-fruit Beakrush (Rhynchospora careyana) as a variety of Tall Beakrush under the names Ceratoschoenus macrostachyus [sic] var. patulus (Chapman 1860) and Rhynchospora macrostachya var. patula (Chapman 1897). All these species are today considered quite distinct from Tall Beakrush (Kral 2002) and none of them occur in Canada. Tall Beakrush is thus completely distinct from all Canadian Rhynchospora species. A variety specific to freshwater tidal estuarine marshes (Rhynchospora macrostachya var. colpophila) was described from Maryland and Virginia by Fernald (1940) but has not been recognized in more recent works (Gleason and Cronquist 1991; Kral 2002; Weakley 2012).
Tall Beakrush (Figure 1, Figure 2 and Figure 3) is a perennial, clump-forming, herbaceous species. Culms (flowering stems) are erect and reported as 80 to 150 cm and occasionally up to 170 cm in the United States (U.S.) (Kral 2002), but flowering sometimes occurs on plants as small as about 20 cm in the Canadian population, where maximum height is about 1 m and average culm height is probably less than 60 cm (Blaney and Mazerolle pers. obs. 2009-2013). Flowering on smaller stems also occurs in the U.S., based on a culm height range of 15 to 110 cm given in Fernald (1950). The narrowly elongate and attenuate leaves occur in a dense cluster around the base of the plant and sparsely up the culm and are 3 to 12 mm (rarely 15 mm) wide. The inflorescence is composed of long-peduncled, dense clusters of elongated brown spikelets that are predominantly in terminal clusters but can also be in axillary clusters as much as 1 m below the top of the plant (Fernald 1950). The flowers are enclosed within brown scales, with each flower having male and female parts and six elongate, barbed bristles interpreted as reduced sepals and petals. Fertilized flowers develop into a single hard, flattened achene 5 to 6 mm long, which is topped by a remarkably long tubercle (the hardened remains of the style base and neck) up to 21 mm. The tubercle and spikelets of Tall Beakrush are likely the largest of all 250+ species in the genus Rhynchospora (Kral 2002). Tall Beakrush is reported as non-rhizomatous (Kral 2002) but in Nova Scotia can spread up to a few centimetres via production of offset rosettes of basal leaves (Blaney and Mazerolle pers. obs. 2009-2013). Tall Beakrush has a chromosome number of 2n = 18 as do the other three species in the Rhynchospora corniculata species complex (Moore 1997).

Photograph by Sean Blaney, AC CDC.
Long description for Figure 1
Photo showing Tall Beakrush inflorescences at Carrigan Lake, Nova Scotia. The inflorescences are composed of long-peduncled, dense clusters of elongated brown spikelets that are predominantly in terminal clusters.

Photograph by Sean Blaney, AC CDC.
Long description for Figure 2
Photo of the Tall Beakrush in lakeshore peatland at Carrigan Lake in Nova Scotia, at the mouth of the brook draining Murphy Lake. Red Maples, Acer rubrum, and several large individuals of the invasive shrub Glossy Buckthorn, Frangula alnus, are visible in the background.

Photograph by Sean Blaney, AC CDC.
Long description for Figure 3
Photo of Tall Beakrush plants at Keddy Cove, Molega Lake, Nova Scotia, with cottage beach development in background across the bay.
Canadian occurrences of Tall Beakrush are presumed to be completely genetically isolated from those in the U.S. They are 468 km disjunct from the next nearest documented U.S. occurrence at York in southernmost Maine (the only extant Maine occurrence, St. Hilaire pers. comm. 2013), and 500+ km from coastal Massachusetts, where the species is considered secure (NatureServe 2013).
Tall Beakrush is known in Canada from two lakes (Carrigan and Molega, see Defining Subpopulations) in southern Nova Scotia, with known occurrences separated by 23 km. Genetic exchange via pollen movement between the two sites may be possible, because beakrush species, with few exceptions, are believed to be predominantly or entirely wind-pollinated (Moore 1997; Costa and Machado 2012). On the decadal or century scale, genetic exchange between Carrigan and Molega Lakes via new colonization might be possible because there is considerable unoccupied but presumed suitable lakeshore habitat between the two sites, and although they are on different drainages (Carrigan Lake drains into the Mersey River via the Lake Rossignol reservoir and Molega Lake drains into the Medway River via the Wildcat River and Ponhook Lake), Carrigan Lake is separated from the Medway drainage at Apple Tree Lake by only 650 m and drainage flows from there almost directly toward the Molega Lake occurrence. Genetic exchange via wind pollination and seed dispersal is presumed possible between all plants within subpopulations. Plants occur over only 30 m at Molega Lake and over 1.3 km x 0.7 km at Carrigan Lake with maximum distance between plants of 540 m.
No work on genetic structure of American populations of Tall Beakrush has been completed, and the relatively recent discovery of Tall Beakrush in Canada (Blaney and Mazerolle 2009) means that genetic diversity of the species within Canada and in comparison with American populations has never been investigated.
Although occupied habitat at Carrigan Lake (supporting 95% of the Canadian population) is quite small, probably amounting to less than 1000 m2 (Blaney and Mazerolle pers. obs. 2009-2013), it is believed sufficiently large to support a viable subpopulation and therefore Tall Beakrush is not considered severely fragmented in Canada (COSEWIC 2010).
In Canada, Tall Beakrush is restricted to a small portion of the COSEWIC Atlantic Ecological Area in southwestern Nova Scotia, thus Canadian subpopulations should be considered a single designatable unit (DU).
Tall Beakrush is one of a large suite of southern species of the Atlantic Coastal Plain disjunct in southern Nova Scotia, many of which are rare in Canada (Environment Canada and Parks Canada Agency 2010). Ongoing stewardship and outreach programs have resulted in these rare species being known and appreciated by many cottagers, residents and visitors in southern Nova Scotia. Tall Beakrush is considered one of the characteristic species of Atlantic Coastal Plain Northern Pondshores from Massachusetts to Delaware Bay and it can sometimes achieve local co-dominance in such communities (i.e., Coastal Plain Muck Pondshore, Westervelt et al. 2006).
Canadian subpopulations of Tall Beakrush are isolated from other occurrences by 468 km and are at the northeastern range limit for the species. Canadian subpopulations could thus have a disproportionate significance for the species' rangewide genetic diversity (Lesica and Allendorf 1995; Garcia-Ramos and Kirkpatrick 1997; Eckert et al. 2008).
The achenes of Tall Beakrush and its close relatives are an important food source for ducks in the southern U.S. (Martin and Uhler 1939), but a wide variety of Internet searches produced no other species-specific information on human use.
Tall Beakrush is sufficiently large and striking that it could be used as an ornamental species in water gardens, as is the case with White Star-Sedge (Rhynchospora colorata) (e.g., Growing Wild Nursery 2013; Watergarden Paradise 2013). Internet sources do not suggest, however, that there is significant current horticultural use of Tall Beakrush.
No evidence of Canadian Aboriginal traditional knowledge of this species was found during the preparation of this report (Hurlburt pers. comm. 2013).
Tall Beakrush is predominantly a species of the Atlantic and Gulf Coastal Plains between southern Maine and eastern Texas, but it also occurs in several disjunct areas within the eastern U.S. (Figure 4). County level distribution data from Kartesz (2011) suggests Tall Beakrush to be relatively frequent near the coast from Massachusetts to South Carolina and along the Gulf Coastal Plain in Louisiana and east Texas, but uncommon to rare from Georgia to Louisiana. Additional concentrations of occupied counties occur at the southeast end of Lake Michigan in Michigan and Indiana (an area supporting a high diversity of disjunct Atlantic Coastal Plain flora, Reznicek 1994), in eastern Oklahoma and adjacent areas of Kansas, Missouri and Arkansas, and along the Tennessee-Alabama border (Kartesz 2011). Isolated records are reported for Kentucky and northern New York (Kartesz 2011; Weldy et al. 2013). Reports from Mississippi and Vermont appear to be in error Content Footnote1. There is also an Illinois report based on misidentification (Bowles et al. 1991). Canadian occurrence is restricted to two sites 23 km apart in Queens and Lunenburg counties, southern Nova Scotia. Canada supports less than 1% of the global population.
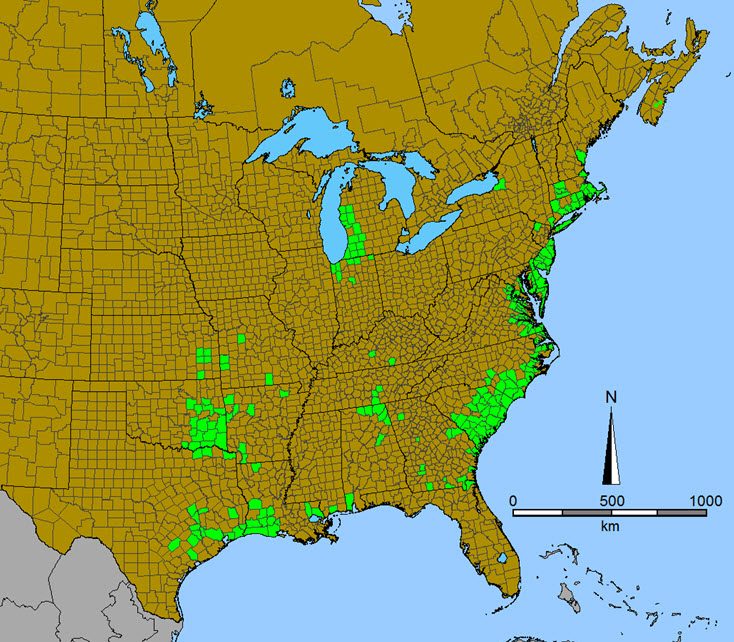
Map is modified from Kartesz (2013)
Long description for Figure 4
Map of the distribution of the Tall Beakrush in the United States and Canada. In the United States, the species occurs predominantly in the Atlantic and Gulf Coastal Plains between southern Maine and eastern Texas but also in several disjunct areas within the eastern U.S. The Canadian distribution is restricted to two sites in Queens and Lunenburg counties, southern Nova Scotia.
In Canada, Tall Beakrush is restricted to the COSEWIC Atlantic National Ecological Area in southwest Nova Scotia (Figure 5). It is known only from Carrigan Lake in the Mersey River watershed and 23 km east-northeast at Keddy Cove on Molega Lake in the Medway River watershed.
Despite the species' discovery in Nova Scotia (Blaney and Mazerolle 2009) after the vast majority of native species had been documented for the province (Roland and Smith 1969), the phytogeographic patterns and the context of the occurrences strongly suggest that Tall Beakrush should be considered a naturally established, native species in Nova Scotia. Tall Beakrush distribution in the U.S. and its disjunction into southern Nova Scotia from southern Maine fits a pattern shared by many other Atlantic Coastal Plain flora that are clearly native to Nova Scotia (Roland and Smith 1969; Wisheu and Keddy 1994; Clayden et al. 2009). Additionally, both Canadian occurrences are in habitats not significantly altered by humans and supporting a high diversity of other native and disjunct Atlantic Coastal Plain species, including the very rare and highly disjunct Redroot (Lachnanthes caroliniana) at Molega Lake and Poison Sumac (Toxicodendron vernix) in the vicinity of Carrigan Lake. The absence of earlier records of Tall Beakrush does not suggest recent introduction because specimen records (AC CDC 2013) do not indicate any botanical fieldwork prior to 2007 at Keddy Cove on Molega Lake, or prior to 2009 at Carrigan Lake.
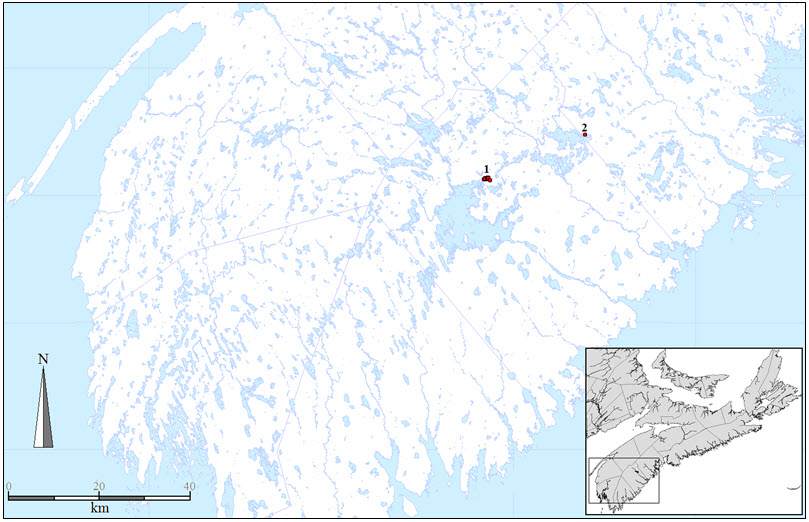
Long description for Figure 5
Map of the Canadian distribution of the Tall Beakrush at Carrigan and Molega Lakes, in the COSEWIC Atlantic National Ecological Area in southwestern Nova Scotia.
Actual extent of occurrence (EO) in Canada is 11.46 km2 but under COSEWIC guidelines (COSEWIC 2010), if EO is less than index of area of occupancy (IAO), the EO is considered to be the IAO value. IAO for Tall Beakrush in Canada, derived using a 2 x 2 km grid aligned with 10 km x 10 km UTM grid squares, is 12 km2. EO is, therefore, also 12 km2.
Although Tall Beakrush was not discovered in Nova Scotia until 2009 (Blaney and Mazerolle 2009), it is clearly a native species. The absence of reports prior to 2009 does not suggest recent introduction because collection records (AC CDC 2013) give no indication that any botanists ever visited the currently occupied areas before that time. The presence of Atlantic Coastal Plain flora in southern Nova Scotia has been well known since Merritt Fernald's expeditions (Fernald 1921, 1922), and search effort for coastal plain species has been extensive. Coastal plain floristic work in southern Nova Scotia has continued from the 1950s to the present. Academic work on the ecology, distribution and local diversity of Nova Scotian coastal plain flora with a focus on conservation implications has been ongoing since the 1980s (see references in COSEWIC 2012a). The AC CDC (2013) database of vascular plant records and COSEWIC (2012a) document site visits to 322 lakes within the potential range of Tall Beakrush Content Footnote2, of which 220 were visited up to 2000. Fieldwork since 2000, predominantly by Atlantic Canada Conservation Data Centre (AC CDC), Nova Scotia Department of Natural Resources and Mersey Tobeatic Research Institute (MTRI) (see references in COSEWIC 2012a) has been more intensive, with 179 lakes visited, including 102 lakes not visited prior to 2000. Most of the 102 newly visited lakes have had comprehensive coverage of their shorelines for rare plants. These figures, especially those from before 2000, are conservative estimates of the number of lakes visited by botanists because of incomplete databasing of existing specimens and lakes visited where no data were collected. They would, however, include the majority of southern Nova Scotia lakes visited by botanists.
Search effort for rare lakeshore plants has been much more intense in the vicinity of known Tall Beakrush occurrences than in most other parts of southern Nova Scotia. Excluding the Lake Rossignol reservoir, there are 60 named lakes within 10 km of the known occurrences of Tall Beakrush (Appendix 1). Of these, 36 have been comprehensively (33 lakes) or extensively (three lakes) surveyed for rare plants since 2007 (Appendix 1; AC CDC 2013). Six additional lakes have been visited by botanists since 2008 but not extensively surveyed. Lakes selected for survey in recent years were the most promising for rare plant occurrence based on: larger size and high water level fluctuation (associated with lower position in their watersheds; Hill and Keddy 1992; Hill et al. 1998); and on the presence of pre-existing rare plant records. As a result, the 17 largest of these
lakes had already been comprehensively or extensively surveyed for rare plants prior to fieldwork for this status report. The unsurveyed lakes within 10 km of Tall Beakrush occurrences are all small (maximum 1.14 km2, only Black Lake and McLean Lake are over 1 km2, and most are less than 0.3 km2) and high in their watersheds, suggesting limited water level fluctuation (Hill and Keddy 1992; Hill et al. 1998) and lower potential for rare Atlantic Coastal Plain shoreline flora.
The documentation of Tall Beakrush on only two of the 322 southern Nova Scotia lakes with some botanical survey effort strongly indicates that the very limited known range is not a result of inadequate survey effort. Although Molega Lake is a well-known hotspot for rare Atlantic Coastal Plain flora, with provincially uncommon characteristics that promote coastal plain flora diversity (Hill and Keddy 1992; Hill et al. 1998; Blaney and Mazerolle 2009; Environment Canada and Parks Canada Agency 2010; AC CDC 2013), the habitats occupied by Tall Beakrush at Molega Lake and especially at Carrigan Lake do not appear especially unusual for southern Nova Scotia lakes (Blaney pers. obs. 1999-2012). Similar habitats should be present to some degree on a fair proportion of the roughly 1,128 lakes and ponds (out of 1,450 total, Natural Resources Canada 2003) within the potential range of Tall Beakrush for which no record of a botanist visit is available. Thus although Tall Beakrush is clearly very rare in southern Nova Scotia, there is a reasonable possibility that small numbers of additional occurrences may eventually be found.
Tall Beakrush is considered an obligate wetland plant in Nova Scotia (Blaney 2011) and in all U.S. regions in which it occurs (Lichvar 2013). Kral (2002) summarizes rangewide habitat preferences as “acidic sunny wetlands, mostly pond shores, seeps, bogs, marshlands; 0–400m”.
Nova Scotian habitat information is entirely based on Blaney and Mazerolle (pers. obs. 2009-2013). Tall Beakrush occurs in Nova Scotia along untreed lakeshores within a zone that experiences shallow flooding through much of the year but is fully exposed or nearly so during low water levels in mid- or late summer. In 2013, under slightly higher than seasonal average water levels, some flowering plants occurred in water up to about 25 cm deep in early September and most were in at least a few cm of water. Substrates are mostly gravelly, often with a thin layer of peaty organic soil on top, but some plants are on deeper peat or on shallow organic or peat soil within cracks in exposed bedrock. Associated species are mostly herbaceous, sometimes with low to moderate cover of the shrubs Sweet Gale (Myrica gale), Leatherleaf (Chamaedaphne calyculata) and Meadowsweet (Spiraea alba var. latifolia) (Blaney and Mazerolle pers. obs. 2009-2013). The most frequently associated species, in order of frequency, are Virginia Marsh St. John’s Wort (Triadenum virginicum s.st.), Eaton’s Witchgrass (Dichanthelium spretum), Three-Way Sedge (Dulichium arundinaceum), Bog Aster (Oclemena nemoralis), Sweet Gale, Twig Rush (Cladium mariscoides), Pickerel Weed (Pontederia cordata), Large Cranberry (Vaccinium macrocarpon), Royal Fern (Osmunda regalis var. spectabilis), and Swamp Loosestrife (Lysimachia terrestris) (AC CDC 2013; see detailed analysis of associate species in Appendix 2). The lakeshore habitats occupied by Tall Beakrush in Nova Scotia support a high diversity of restricted and rare plants with affinity to the coastal plains of the eastern and southern U.S. These low biomass, high diversity lakeshore communities are maintained by acidic, nutrient-poor conditions and disturbance from fluctuating water levels, ice scour and wave action (Keddy 1985; Keddy and Wisheu 1989; Hill and Keddy 1992; Wisheu and Keddy 1994; Hill et al. 1998).
Occupied habitats are relatively similar in the northern part of its American range. Tall Beakrush is noted as being characteristic of Coastal Plain Muck Pondshore and Northern Peatland Sedge Pondshore community types within Atlantic Coastal Plain Northern Pondshore systems that are found near the coast between Massachusetts and northern Virginia (Westervelt et al. 2006). These share many characteristics and species with southwestern Nova Scotia lakeshores. In Michigan, its habitat is described as “…areas with a fluctuating water table such as coastal plain marshes, sandy lake edges, dune swales, seepages, sandy marshes, sandy and peaty edges of wetlands, and intermittent wetlands” (Michigan Natural Features Inventory 2013). Tall Beakrush also occurs in some habitats that differ from those in which it occurs in Nova Scotia. It is noted as frequent in Atlantic White-Cedar (Chamaecyparis thyoides) – Seaside Alder (Alnus maritima) Swamp in Delaware and Maryland (Westervelt et al. 2006). In Kansas, Tall Beakrush has been recorded in and near freshwater marshes and sloughs in unglaciated tallgrass prairies where the substrate is shale, limestone, or sandstone (Magrath and Johnson 1971; Freeman pers. comm. 2013). These occurrences and others in prairie areas of the southcentral U.S. are likely in significantly more alkaline habitats than those on the Atlantic Coastal Plain (University of Wisconsin – Madison 2002; Freeman pers. comm. 2013).
Further south, Tall Beakrush also occupies open coastal plain pondshores, as well as freshwater and slightly saline tidal marshes, swamps and interdune ponds in Virginia (Virginia Botanical Associates 2013), with the variety colpophila (no longer recognized; Kral 2002) having been described as restricted to fresh tidal marshes of the Chesapeake Bay and Albemarle Sound regions of Maryland and Virginia (Fernald 1918; Fernald 1950). Tall Beakrush is also noted from wet depressions and seasonal ponds within pine savannahs and flatwoods, and from Pond Cypress (Taxodium ascendens) swamps and hardwood swamps in South Carolina, Alabama and Louisiana (University of South Carolina 2013; Kral et al. 2013; Louisiana State University 2013). Herbarium records suggest that in the south it is more likely, as compared to the northern U.S., to occur in disturbed habitats such as ditches, all-terrain vehicle tracks, pipeline rights-of-way, rice fields and impoundments (Louisiana State University 2013; University of South Carolina 2013).
It is quite likely that the 1929 damming of the Mersey River, which created the Lake Rossignol reservoir, destroyed occupied Tall Beakrush habitat, because it flooded eleven named lakes with 100+ km of shoreline immediately downstream from Carrigan Lake (Belliveau and Gray 2011). Tall Beakrush habitat at Carrigan Lake has, however, likely been stable for at least the past decade, and probably longer. Despite Nova Scotia Power ownership or flowage rights along the entire shoreline, water levels on Carrigan Lake appear not to have been manipulated by Nova Scotia Power and there is currently no plan to manage them in the future (Peck pers. comm. 2013; see Threats – Artificial Regulation of Water Levels). There is no shoreline development on the lake at present. Camping activity is evident at the end of a vehicle track leading to the lakeshore from an abandoned farm at Carrigan Hill just north of the lake, and at least one other fire pit was found near Tall Beakrush, but neither appears to impact the species, and obvious human impacts on the lakeshore are otherwise absent (Blaney and Mazerolle pers. obs. 2009-2013).
At Keddy Cove on Molega Lake, the Tall Beakrush habitat that is currently occupied has likely been stable over the past 20+ years, but most nearby cottages appear younger than 20 years old (Blaney et al. pers. obs. 2013) so nearby sites could have been lost with development in that period. At Molega Lake, Tall Beakrush occurs on or near the shoreline margins of one property with a roughly 30 year old cottage (five of 35 individuals) and a larger private property with no current development (30 of 35 individuals). The roughly 30 m x 3 m area currently occupied by Tall Beakrush shows no obvious evidence of human impact. However, past impacts are visible within 20 m of these plants on the above cottage property, where the soil excavated for construction of the cottage foundation was dumped into the lake (Fielding-Croft pers. comm. 2013), creating a roughly 10 m long by up to 5 m wide berm and covering whatever plants might have been originally present.
Potential for further shoreline development impacts at Molega Lake is high as most of the Tall Beakrush occurs on a property without a cottage in an otherwise developed area, and access is good via Keddy Cove Road 100 m away. The cottage property with Tall Beakrush could change hands within the next decade, after which additional shoreline alterations associated with property improvement would be likely (Blaney et al. pers. obs. 2013; Fielding-Croft pers. comm. 2013). Various other shoreline impacts are present at the 15 other cottages on Keddy Cove as well as the roughly 300 cottages elsewhere on Molega Lake (Blaney and Mazerolle pers. obs. 2009-2013), but at which there are not also currently any Tall Beakrush plants. Any undiscovered occurrences on Molega Lake would also have a high potential for future impacts from shoreline development, because the shoreline is almost entirely in private ownership and new cottages are built every year (Blaney pers. obs. 2007-2013).
Biology of Tall Beakrush in Canada is incompletely understood because of limited field observation. Fruiting is reported for the U.S. from late July to October (Fernald 1950). In Canada, flowering likely begins in July under favourable conditions, based on the earliest available observation of mature inflorescences (August 18 at Molega Lake; AC CDC 2013). The latest observation of mature inflorescences (with achenes of unknown maturity) is September 10 (AC CDC 2013) but fruiting likely occurs from August into October), based on observations on September 6, 2013 of some plants in deeper water at Carrigan Lake having inflorescences that were not yet fully elongated, releasing pollen or exposing receptive stigmas.
Several Rhynchospora species have independently evolved characteristics for insect pollination (Leppik 1955; Costa and Machado 2012), but Tall Beakrush lacks such characters and pollination is probably largely or exclusively by wind as with most sedges (Leppik 1955; Reznicek 1990; Friedman and Barrett 2012), including closely related beakrush species (Moore 1997, in Craine 2003). Moore (1997, in Craine 2003) noted that within each fascicle (cluster of florets), Tall Beakrush florets develop synchronously and that “the species appears to be self-compatible, unlike its close relative Narrow-fruit Beakrush”. It is thus less likely to hybridize with related species.
Narrow-fruit Beakrush is reported to produce only 100 to 200 achenes per plant in Massachusetts (Craine 2003), but Tall Beakrush has larger flowering heads composed of more spikelets (Weakley 2012) and a large plant might produce several times that amount (Blaney and Mazerolle pers. obs. 2009-2013). Achenes are probably shed from the parent plant through the autumn and some likely remain on the plant until the stems, which are not especially sturdy, fall over in late fall or early winter providing a means for short-distance dispersal. Seed banking is clearly an important aspect of life history given the following: a) Tall Beakrush’s use of some habitats that are only occasionally exposed above water; b) Reznicek’s (pers. comm. 2013) observations of rapid response to seasonal water draw-down in Michigan; and c) the documented importance of seed banking in the closely related Narrow-fruit Beakrush (Gerritsen and Greening 1989; who found up to 1,200 viable seeds per m2 in deep marsh habitat not dominated by Narrow-fruit Beakrush in high water periods). Gerritsen and Greening (1989) and Conti and Gunter (1984) found that Narrow-fruit Beakrush from deep marsh seed banks required oxygenated conditions for germination and found a germination response from light and heat as would occur upon exposure with lowering water levels. Narrow-fruit Beakrush germinated poorly in 3 – 4 cm of water compared to a moist but not inundated treatment, but grew best in a treatment in which it was inundated a few weeks after germination, indicating the importance of fluctuating water levels, a result that likely applies to Tall Beakrush as well. Tall Beakrush seeds might be very long-lived in soil seed banks. Gunther et al. (1984) found that seeds of Narrow-fruit Beakrush germinated from the oldest peat layers of any species they could identify, and their study found germination of unidentified sedge seeds that may have been Narrow-fruit Beakrush from 400+ year old peat layers (as cited in Clark 2003).
Tall Beakrush is non-rhizomatous, in contrast to closely related species that can form large, dense patches (Kral 2002). Vegetative reproduction in Tall Beakrush occurs over short distances by the production of new rosettes to the side of the parent rosette. Fernald (1950) notes that plants are “bulbous-thickened at base, with erect autumnal shoots usually crowded about the fruiting culm”. The bulbous-thickened base functions as a nutrient storage organ, and the autumnal shoots and associated new rosettes overwinter and flower the next spring (Reznicek pers. comm. 2013). Narrow-fruit Beakrush grown in a greenhouse in Georgia required less than three months from germination to reach flowering. Reznicek (pers. comm. 2013) reports occasional field observations and specimens of Tall Beakrush from Michigan and Indiana indicative of fruiting within the same growing season as germination. He describes the species as a facultative annual or short-lived perennial. Growth to flowering in one year might be possible under ideal conditions in Nova Scotia, but has not yet been observed (Blaney and Mazerolle pers. obs. 2009-2013). Most individuals likely take at least two years to reach maturity, as in Michigan, where herbarium specimens having older rosette bases alongside fresh ones indicate longevity of genetic individuals of at least three years and likely longer (Reznicek pers. comm. 2013). Individual rosettes may not last into the season after flowering as the leaves and stems are soft and spongy, and likely decay rapidly (Reznicek pers. comm. 2013). Without knowing the demographics of vegetatively produced rosettes, especially the extent to which vegetatively produced rosettes could function independently of their parent rosette, generation time is hard to determine. It is roughly and conservatively estimated at three to five years based on information above and observations at Carrigan Lake of some smaller rosettes, likely more than one year old, that were infertile (Blaney et al. pers. obs. 2013).
There is little published information on physiology of Tall Beakrush, but certain features can be inferred. With Nova Scotia at the species' extreme northern geographic range limit, cold temperatures could limit its occurrence. The range of Tall Beakrush extends across U.S. Plant Hardiness Zones 5b to 9b, corresponding to minimum temperatures of about -26oC to -4oC (USDA 2012). Its status as an obligate wetland plant throughout its range (Blaney 2011; Lichvar 2013) and its occurrence on the wetter end of the seasonally exposed lakeshore zone in areas that may not be fully exposed every year (Blaney et al. pers. obs. 2013) suggest that it has a high tolerance for flooding and a low tolerance for drought. As outlined in Habitat Requirements, occurrence in various swamp forest habitats in the southern U.S. suggest that it has some tolerance for shading, although it appears to be known primarily or exclusively from full sun habitats north of Delaware. Tall Beakrush has some tolerance for slight salinity and for tidal fluctuations based on habitats documented in Virginia (Virginia Botanical Associates 2013) and the past recognition of the freshwater tidal estuarine specialist variety colpophila. The occurrence of Tall Beakrush in prairie marshes in the Great Plains (i.e.,Magrath and Johnson 1971; Freeman pers. comm. 2013) suggests that although it is typically a species of acidic soils (Gray 1950; Kral 2002), it can also occur in alkaline sites. As noted in Habitat Requirements, Tall Beakrush is able to utilize certain anthropogenic habitats in the southern part of its range, but association with disturbance appears limited in the northern part of its range.
Small-scale dispersal of Tall Beakrush is accomplished through the growth of new rosettes a few cm to the side of existing ones, and through the falling over of the fruiting stems at the end of the growing season. Stems can reach about 1 m in Canada and up to 1.7 m in the U.S. The achenes (seeds) of Tall Beakrush are relatively large and heavy for a sedge and do not appear well adapted for long-distance dispersal by wind or water. The six long, barbed bristles attaching at the base of the achene likely aid in dispersal. For the closely related species Narrow-fruit Beakrush, the bristles spread outward with wetting, which increases floating time (Moore 1997). Tall Beakrush may be similar. The bristles likely also contribute to animal-mediated dispersal by catching on fur or feathers (Moore 1997). Waterfowl provide a means of longer-distance dispersal. Ducks are known to eat the seeds of Tall Beakrush and closely related large beakrush species (Martin and Uhler 1939; Center for Aquatic and Invasive Plants 2002) and a certain portion may pass through the duck's gut unharmed (Mueller and van der Valk 2002 and references therein). Waterfowl can also move aquatic plant seeds within mud attached to their feet (Stiles 2000).
The traditional view on colonization of Atlantic Coastal Plain plant species into present-day Nova Scotia (Roland and Smith 1969) is that these plants reached Nova Scotia after having colonized (or having persisted throughout the period of glaciations on) land exposed by lower sea levels between present-day southern Nova Scotia and Massachusetts. This suggests a slow migration to Nova Scotia via shorter-distance dispersal events over thousands of years. A recent evaluation (Clayden et al. 2009) suggests this scenario may be unlikely for southern species like Tall Beakrush because offshore land is now known to have had high boreal or arctic climate, and to have been more limited in time and space than previously believed. Thus very long distance dispersal (on the scale of 450+ km between occupied areas of southern Nova Scotia and New England) may be possible for Tall Beakrush over geological time.
Limited observation has documented no significant insect or mammalian herbivory of Tall Beakrush within the Canadian population (Blaney and Mazerolle pers. obs. 2009-2013). In the U.S., the closely related Short-bristle Beakrush (which does not occur in Canada) is known as a larval food plant for the buprestid (metallic wood-boring) beetle Taphrocerus gracilis (MacRae 2004; Nelson et al. 2008, as cited in Webster and DeMerchant 2012), the larvae of which are leaf, root and stem miners. This beetle occurs in Nova Scotia (Webster and DeMerchant 2012) and could likely feed on Tall Beakrush given that MacRae (2004) found larval feeding by T. gracilis on no species other than Short-bristle Beakrush in Missouri. The fact that the beetle has been detected in Nova Scotia would suggest, however, that it is unlikely to be restricted to feeding on the very rare Tall Beakrush there. In Europe, White Beakrush (Rhynchospora alba), which is common in lakeshore peatlands within the Canadian range of Tall Beakrush, is known as a food plant for the Common Ringlet butterfly (Coenonympha tullia; seven references given in Biological Records Center 2013), which also occurs in Nova Scotia and might also feed on Tall Beakrush.
Ducks and other waterfowl feed on seeds of Tall Beakrush and closely related species in the southern U.S. (Martin and Uhler 1939; Center for Aquatic and Invasive Plants 2002) and are likely important for seed dispersal. The potential value of Tall Beakrush seeds to waterfowl is indicated by the fact that beakrush seeds (species unknown) constituted 12 to 32% of stomach contents in Fulvous Whistling Ducks (Dendrocygna bicolor) in Louisiana (Hohman et al. 1992).
Competition from high biomass and potentially dominant species like Canada Bluejoint (Calamagrostis canadensis), Royal Fern, Sweet Gale and other shrubs may be an important limiting factor in the distribution of Tall Beakrush in its Canadian range. Rare Atlantic Coastal Plain shoreline flora are typically stress-tolerant species with low competitive ability (Keddy 1985; Keddy and Wisheu 1989; Hill and Keddy 1992; Wisheu and Keddy 1994; Hill et al. 1998). Exclusion effects from neighbouring plants (by herbicide treatment) on the growth of two close relatives of Tall Beakrush (Short-bristle Beakrush and Narrow-fruit Beakrush) were examined in a brackish marsh in Louisiana by Geho et al. (2007). They found significant increases in biomass as a result of exclusion of neighbours. They also found that exclusion of mammalian herbivores (primarily Nutria, Myocastor coypus) did not significantly affect biomass.
The mycorrhizal status of Tall Beakrush is undocumented, but Dighton et al. 2013, documented the rare Atlantic Coastal Plain endemic Knieskern's Beakrush (Rhynchospora knieskernii) as facultatively mycorrhizal in fluctuating wetlands in New Jersey sand barrens, and several other species of beakrush have been documented as mycorrhizal (Lovera and Cuenca 1996; Silva et al. 2001) or facultatively mycorrhizal (Silva et al. 2001).
Fisher (1953, as cited in Clark 2003) listed Tall Beakrush and five other northeastern beakrush species as prone to smut infection of inflorescences. The fungus involved is unknown, but smut fungi in the genera Cintractia and Trichocintractia infect tropical Rhynchospora, inhibiting floral and inflorescence development respectively (Piepenbring 1995, in Clark 2003), and the Australian smut fungus Leucocintractia scleriae is known as Rhynchospora Smut because of its association with beakrushes (PADIL 2013). No smut infection has been observed on Canadian Tall Beakrush (Blaney and Mazerolle pers. obs. 2009-2013).
Fieldwork conducted in 2013 for this report concentrated on known Tall Beakrush sites and on the highest potential unsurveyed lakes within 10 km of known Tall Beakrush occurrences. As described below, extensive recent field surveys conducted prior to 2013 significantly shaped sampling strategy for 2013 fieldwork.
Tall Beakrush was first discovered on Carrigan Lake in 2009, during comprehensive shoreline surveys that combined walking and canoeing (Blaney and Mazerolle 2009; AC CDC 2013). Survey effort in parts of the central portion of the lake was less intense than it would have been were the surveyors focused exclusively on Tall Beakrush (Blaney and Mazerolle pers. obs. 2009-2013). The east and west ends of Carrigan Lake were considered to have been surveyed intensively enough in 2009, and/or to have been lacking in high potential habitat, such that the lack of Tall Beakrush records in those areas was thought to represent true absence. Three person-days of survey effort in 2013 were thus focused on the central portion of the lake. The extent to which additional sites were found at Carrigan Lake in 2013 was slightly surprising and suggests that some undocumented sites may also be present in the east or west ends of the lake, although undiscovered large occurrences on Carrigan Lake are still considered very unlikely (Blaney and Mazerolle pers. obs. 2009-2013).
Molega Lake is a large lake with more than 110 km of lakeshore, including islands. Extensive fieldwork since 2007 focused on mapping rare plant occurrences has covered 94 km (85%) of that shoreline (AC CDC 2013; Toms pers. comm. 2013). Molega Lake fieldwork for this report in 2013 was limited to two person days, visiting the known site and the surrounding area at the head of Keddy Cove on foot, and covering high potential peaty shoreline meadows at Cranberry Island, Softwood Island and the Black Rattle Lake channel within 3 km of the known occurrence on foot and by canoe.
As described in Search Effort, the highest potential lakes for rare Atlantic Coastal Plain flora within 10 km of Tall Beakrush occurrences had already been surveyed between 2007 and 2012. Fieldwork in 2013 for this status report combined on-foot surveys (especially in the highest potential habitats) with canoe-based survey to comprehensively cover the shorelines of an additional seven lakes (Payzant, Murphy, McBride, Bradley, Loon, and Cannon lakes, plus a lake-like but unnamed segment of the Wildcat River). An additional lake (McGuire Lake) was visited briefly. All but Loon Lake had seen no previous botanical survey effort. These sites were selected based on potential habitat visible on aerial photographs and occurrence within a 10 km radius of known sites. Loon Lake on the Mersey River and the Wildcat River segment were prioritized because their major water level fluctuations associated with connection to major river channels were anticipated to promote the occurrence of rare Atlantic Coastal Plain shoreline flora (Hill and Keddy 1992; Hill et al. 1998).
COSEWIC separates subpopulations if there is typically less than one successful genetic exchange per generation. Although pollen movement via wind might be possible between the Carrigan Lake and Molega Lake occurrences, the 23 km distance and relatively small subpopulation sizes at both sites probably make this unlikely and the two lakes are assumed to represent separate subpopulations. At Molega Lake all known plants are within 30 m of shoreline and are clearly a single subpopulation. At Carrigan Lake there are no gaps in occurrence larger than 540 m (AC CDC 2013), which is presumed to be within the potential dispersal distance of pollen and seeds along a lakeshore, meaning that plants at Carrigan Lake are here treated as a single subpopulation.
Comprehensive subpopulation counts in 2013 recorded 684 mature individuals, with 648 at Carrigan Lake and 36 at Molega Lake. As a perennial that produces new rosettes to the side of existing ones, individuals can be hard to determine in cases where plants are growing in loose patches. For the above counts, individuals were defined as rosettes separated from adjacent rosettes such that leaves were not extensively overlapping and some substrate was visible between them, under the assumption that such rosettes were either independent from others or would have sufficient roots to support themselves if separated from attached rosettes by ice scour or other disturbance. The relationship between the above counts and the number of genetic individuals is unclear.
As noted under Search Effort and Methods, although Carrigan and Molega lakes are very well and fairly well surveyed respectively, some additional undetected individuals could be present at both subpopulations. Survey effort is sufficient to suggest that the actual total population is possibly, but not likely, over 1,000 individuals and is unlikely to be over 2,500 individuals (Blaney and Mazerolle pers. obs. 2009-2013; AC CDC 2013).
Population counts have been undertaken only twice at each site since 2009 and thus provide limited evidence on population fluctuation and trends in Canada. At Molega Lake the two counts were nearly identical (32 in 2010, 35 in 2013), and the difference could easily reflect slight differences in interpretation of “individuals” or in detectability because of extent of flowering and water levels. At Carrigan Lake, the 2013 count (653 mature individuals) was considerably higher than that in 2009 (346+) and plants were found over a wider area, but the extent to which this represents actual fluctuation is unclear. The 2009 survey was a general shoreline rare plant inventory with no expectation of finding Tall Beakrush and most of the lake was covered before it was realized that Tall Beakrush was present, whereas the 2013 survey was specifically focused on finding Tall Beakrush.
As noted in Habitat Trends, flooding caused by construction of the Lake Rossignol dam in 1929 is quite likely to have caused significant historical population loss at the eleven flooded lakes downstream from Carrigan Lake. Limited understanding of the timing and effects of water level fluctuation at Carrigan Lake means that inferring more recent population trends there is not possible. Small, local subpopulation declines caused by cottage development on Molega Lake over the past 40 years may have occurred, but are unlikely to have been extensive. Although about 300 cottages are present on the lake, the shoreline is still intact enough and has been surveyed well enough (i.e. 322 GPS locations of the COSEWIC Special Concern Redroot spread extensively around the lake; AC CDC 2013) that one would expect Tall Beakrush to have been detected elsewhere if it had been at least somewhat widespread on the lake historically.
Zaremba and Lamont (1993) noted that Tall Beakrush was not among the species visible only in low water years in coastal plain ponds on Long Island, New York, meaning that extreme fluctuations are likely not typical for the species. The species’ occurrence in up to 25 cm of water at the depth limits of occurrence for shoreline species (Blaney and Mazerolle pers. obs. 2009-2013) does suggest, however, that multi-year periods of especially high or low water levels could cause a population fluctuation. Observations in Nova Scotia (Blaney and Mazerolle pers. obs. 2009-2013) also suggest there could be a difference in detectability associated with water level if high water levels inhibited flowering. The rosettes of Tall Beakrush are quite similar to small rosettes of the common Wool-grass Bulrush (Scirpus cyperinus) and are thus easily overlooked if the striking flowering stem is not present. The possible effects of changes in detectability should be kept in mind when assessing future population changes.
| Sub-pop.# | Name | Subpopulation (2013) | Previous Count (year) |
|---|---|---|---|
| 1 | Carrigan Lake | 653 | 346+ (2009) |
| 2 | Keddy Cove, Molega Lake | 35 | 32 (2010) |
| Total | - | 688 | - |
Although long distance dispersal was likely significant in establishing Tall Beakrush in Nova Scotia (see Dispersal and Migration), rescue is likely limited for the species because the Nova Scotia population is separated from the nearest documented American occurrence in York County, Maine, by at least 468 km and from northern coastal Massachusetts, where the species is more widespread, by 490 km. Minimum distances to the nearest potential habitat in southernmost Nova Scotia (about 100 km southwest of Carrigan Lake) are about 380 km from southern Maine and 400 km from northern Massachusetts. In all cases the majority of the distance is across the open ocean of the Gulf of Maine. Rescue from subpopulations in Maine is further limited because of the species' rarity there (a single extant occurrence with 13 plants documented, St. Hilaire pers. comm. 2013). Establishment in Nova Scotia from United States populations is thus likely to be extremely rare.
The primary threat to Carrigan Lake is the impact of long-term water level management. The primary threat to Molega Lake is shoreline development. Additional threats identified include invasive alien plant species, particularly Glossy Buckthorn, Frangula alnus, and the potential of changes in the lake water nutrient levels due to eutrophication by agriculture and household effluents.
The impacts of long-term water level management on Tall Beakrush at Carrigan Lake could be severe. Artificial regulation of water levels through dams can directly eliminate coastal plain shoreline species through flooding and can alter community composition because reduced disturbance allows competitive species to displace rarer, less competitive ones (Keddy 1989; Hill and Keddy 1992; Wisheu and Keddy 1994; Nilsson and Jansson 1995; Hill et al. 1998; Merritt and Cooper 2000). In addition, drainage of reservoirs in winter can expose climate-sensitive Atlantic Coastal Plain species in Nova Scotia to fatal cold temperatures (Hazel 2004; Lusk and Reekie 2007), and artificially high water levels in summer and autumn can reduce or prevent flowering and seed production (Johannson and Nilsson 2002). The elevation band occupied by Tall Beakrush at Carrigan Lake is very narrow (probably under 1 m), and 506 of 653 individuals at Carrigan Lake (78% of the subpopulation) are in areas that may be especially susceptible to water level increases (narrow shoreline zones adjacent to steep-sided drumlins that may not allow for migration toward shore, and a long, narrow point that might be almost entirely flooded with a small rise in water level).
The Carrigan Lake shoreline adjacent to areas occupied by Tall Beakrush is either owned by Nova Scotia Power or is Crown land over which Nova Scotia Power has flowage rights associated with the Lake Rossignol reservoir. Nova Scotia Power is a private corporation that generates 95% of the province’s electricity and is regulated under the provincially appointed Nova Scotia Utility and Review Board (Nova Scotia Power 2013). Flowage rights are defined under the provincial Land Registration Act of 2001, Chapter 6, Section 1, Item 73 (1), which states that a “utility interest” (in this case the right to maintain water levels for power generation such that the land in question is flooded), “shall be enforced with priority over all other interests according to law” (Nova Scotia Office of the Legislative Counsel 2001). Nova Scotia Power does not currently regulate Carrigan Lake’s water level and could not do so within the ten-year period ending in 2020 covered by their licence to operate the Lake Rossignol dam. The upper limit of Nova Scotia Power flowage rights at Carrigan Lake corresponds to the 279 foot elevation (85.04 m) contour, and the maximum operating level of the Lake Rossignol dam is 274 feet (83.51 m), with no foreseen modifications to water levels beyond that range in the future (all figures from Peck pers. comm. 2013). The elevation of Carrigan Lake shorelines occupied by Tall Beakrush is not known exactly but would be just above the Lake Rossignol maximum (Blaney and Mazerolle pers. obs. 2009-2013). Thus the only way for Nova Scotia Power to exercise its flowage rights would be to build a dam at the outlet of Carrigan Lake. There are several large lakes flowing into Lake Rossignol that are regulated by Nova Scotia Power to provide water for hydroelectric power generation, so there is precedence for similar water level management. However, the infrastructure requirements (a dam and more than 1 km of new road crossing private and Crown land) and regulatory issues associated with damming Carrigan Lake mean that if it was economically viable, it would be unlikely to occur within the next ten years. Nova Scotia Power’s shoreline ownership and flowage rights on Carrigan Lake probably date from the construction of the dam that created Lake Rossignol in 1929. At that time, Nova Scotia Power was a Crown corporation and all Carrigan Lake’s shoreline may have been Crown land. With little limitation on Crown to Crown transfer of land and flowage rights in that era, Nova Scotia Power acquired land and rights in areas that had only remote possibilities of ever being used (Peck pers. comm. 2013).
Historical water level regulation has likely affected Tall Beakrush subpopulations in Nova Scotia. Upon completion in 1929, the Indian Falls dam on the Mersey River flooded eleven lakes downstream from Carrigan Lake and created Lake Rossignol, Nova Scotia’s largest reservoir, which extends to within 75 m of the outlet of Carrigan Lake. Suitable shoreline habitat for Tall Beakrush was undoubtedly lost to the flooding and it is quite likely that subpopulations were lost. Molega Lake has also been regulated by a small dam at its outlet that may have reduced a once-larger subpopulation. The dam was constructed in 1880 to assist river driving of logs and to regulate flow for a mill downstream at Charleston and it held 1.7 m of water. It was inconsistently maintained up until about 1965 but has not been maintained since. The remains of the dam still hold water about 25 cm above the level downstream at Hog Lake (all Molega Lake dam information from D. Freeman pers. comm. 2013).
Shoreline development is considered a significant threat to Atlantic Coastal Plain flora communities on lakeshores (Wisheu and Keddy 1994; Eaton and Boates 2003; Environment Canada and Parks Canada Agency 2010). It is a threat at both Canadian subpopulations, but is an especially acute threat at Molega Lake. The 110 km of Molega Lake’s shoreline is heavily subdivided into about 770 properties with lakeshore frontage, excluding islands. Of these, 760 are privately owned. Only about 300 properties currently have cottages (estimated from 690 buildings registered on Ponhook, Molega and adjacent lakes, COSEWIC 2009), but new cottages are built every year (Blaney pers. obs. 2007-2013) and the extensive property subdivision clearly shows the potential for hundreds of additional cottages in the future. Most of the larger undivided private land shoreline parcels are also owned by developers. The subpopulation of Tall Beakrush at Molega Lake occurs along the boundary of two private properties in Keddy Cove, extending into Crown-owned waters where plants might also be affected by the shoreline alterations of adjacent landowners (see Habitat Protection and Ownership). The southern property with five of 35 individuals has an existing cottage, the development of which appears to have had little impact on currently occupied habitat. As noted in Habitat Trends, adjacent shoreline habitat on this property 20 m away from Tall Beakrush plants was impacted by dumping of excavated soil during cottage construction about 30 years ago. Impacts on Tall Beakrush could increase at this property at any time if a decision was made to “tidy up” shoreline vegetation. This might be particularly likely if the elderly owner, who visits infrequently (Fielding-Croft pers. comm. 2013), were to sell to a new owner interested in “upgrading” the property. The adjacent property with 30 of the 35 Tall Beakrush individuals on Molega Lake currently has no cottages but is at high risk of future development given that about 15 of 28 properties on Keddy Cove already have cottages and there is good road access via Keddy Cove Road 100 m away. The undeveloped property has about 400 m of shoreline frontage and could thus house four or five cottages if subdivided. Tall Beakrush could likely co-exist with limited cottage development on this property as long as impacts were kept away from occupied habitat. Potential cumulative impacts of cottage development on Molega Lake are discussed under Eutrophication, below.
There are currently no cottages on Carrigan Lake but there is a high likelihood of future development. The lake is accessible from a provincial highway at the village of Caledonia by 11 km of fairly good road that is drivable to within about 170 m of Tall Beakrush plants, and an all-terrain vehicle trail extends from the end of the road to the shore about 135 m east of Tall Beakrush plants. Electrical power servicing cottages on nearby Murphy Lake extends to within about 750 m of Tall Beakrush plants, making larger-scale cottage development more likely. There is a single private property relevant to Tall Beakrush on Carrigan Lake. This property extends to the edge of a shoreline zone between 4 m and 20 m wide that is owned by Nova Scotia Power. There are 267 Tall Beakrush individuals within 15 m of the margin of this private property, representing 41% of the Carrigan Lake subpopulation and 39% of the Canadian population. These plants could be affected by cottage development on the adjacent private property because cottage owners would be likely to develop access trails to the lakeshore across the narrow zone of Nova Scotia Power ownership and otherwise alter the lakeshore as if they were the landowners. Sandall (pers. comm. 2013) reports “…in a lot of cases where Nova Scotia Power owns flowage around reservoirs, land owners don’t seem to recognize that fact and build their docks without our knowledge or permission.”
If Nova Scotia Power were to determine that they have no interest in using Carrigan Lake as a reservoir they might eventually sell their shoreline land at the lake. In that case future lakeshore development impacts could also extend to the narrow point owned by Nova Scotia Power extending 230 m out into Carrigan Lake from the above private property. This land supports an additional 266 Tall Beakrush individuals (39% of the Canadian population).
The exotic shrub Glossy Buckthorn isone of the most problematic invasive plant species in Canada and the northeast U.S. (Catling and Porebski 1994; Frappier et al. 2003a; Catling and Mitrow 2012; IPANE 2012). Glossy Buckthorn is scattered in an old field at an abandoned farm 150 m from the majority of Tall Beakrush plants in Canada (Blaney et al. pers. obs. 2013). It is also well established and rapidly spreading in the open Red Maple (Acer rubrum) and alder (Alnus incana ssp. rugosa and Alnus serrulata) swamp along the brook flowing from Murphy Lake into Carrigan Lake (Blaney et al. pers. obs. 2013), extending to within about 5 m of 12 Tall Beakrush plants on a peat mat at the brook mouth (Figure 2). It is these individuals on deeper peat that are believed most threatened by Glossy Buckthorn. Peaty wetlands have a well-documented susceptibility to Glossy Buckthorn invasion in Wisconsin (Reinartz and Kline 1998, where it was noted as having “over-run the 1,000 ha Cedarburg Bog in 20 years”), Illinois (Taft and Solecki 1990), Michigan (Fiedler and Landis 2013), Ontario (Catling and Mitrow 2012), and Nova Scotia (Hill and Blaney 2009). As noted under Habitat Requirements, in the northern part of its range Tall Beakrush is restricted to open habitats and the development of a canopy of Glossy Buckthorn would likely have a significant negative impact on Tall Beakrush individuals.
Several other Tall Beakrush occurrences on rocky and gravelly lakeshore at Carrigan Lake occur within a few metres of Glossy Buckthorn individuals (Blaney et al. pers. obs. 2013). However, based on observations elsewhere in southern Nova Scotia (Blaney pers. obs. 1999-2013), Glossy Buckthorn does not appear capable of establishing dense populations on open, gravelly, seasonally flooded lakeshore, likely because of ice impacts in winter. At Molega Lake, no Glossy Buckthorn were observed in the vicinity of Tall Beakrush. Although buckthorn is sparsely established around Molega Lake, is known within 950 m of the Tall Beakrush subpopulation, and is likely spreading rapidly from larger populations nearby (Blaney pers. obs. 2007-2013), the threat to Tall Beakrush from Glossy Buckthorn is believed to be similarly low because of unsuitable shoreline habitat. Glossy Buckthorn is thus considered only a medium impact threat to Tall Beakrush in Canada at present.
In recent years, eutrophication has changed from a theoretical threat to Atlantic Coastal Plain flora to one of the most significant actual threats in Nova Scotia (COSEWIC 2012a; 2012b; 2013). This is based primarily on documentation of its effects elsewhere (Ehrenfeld 1983; Moore et al. 1989; Zaremba and Lamont 1993; Environment Canada and Parks Canada Agency 2010), and on increased recent mink farm development. Tall Beakrush was among the suite of species considered at risk from eutrophication in coastal plain ponds in Long Island, New York, where significant losses of rare species on some ponds are presumed to have been a result of eutrophication effects (Zaremba and Lamont 1993).
Mink farming is the most significant source of inland nutrient pollution within the Atlantic Coastal Plain flora region of Nova Scotia (Brylinsky 2011, 2012), and has had significant effects on plant communities and on the status of rare Atlantic Coastal Plain flora in southern Nova Scotia (COSEWIC 2012a, b, 2013). It is an especially large source of phosphorus pollution because mink feed is treated with superphosphate to increase shelf life and reduce the occurrence of kidney stones in mink (Brylinsky 2011). Mink farm waste, including manure, carcasses and surplus food, tends to leak from inadequate storage facilities at some farms, entering local watercourses and changing them from nutrient-poor (oligotrophic) to nutrient rich (eutrophic) systems (Brylinsky 2011, 2012). Once phosphorus has entered a lake, the recovery from eutrophic conditions following a reduction in the external phosphorus loading may be slow as the phosphorus is stored in the lake sediments (Marsden 1989; White et al. 2002). Mink farming has undergone rapid expansion in Nova Scotia over the past decade and is the province's largest agricultural export with 1.4 million pelts produced annually by 152 farms (Flemming pers. comm. 2011). There are currently no mink farms within the watersheds flowing into Carrigan and Molega lakes, and new regulations on fur farm waste treatment (Government of Nova Scotia 2013) should require any new farms pose a reduced threat to natural systems. Eutrophication from mink farming does, however, warrant mention as a potential future threat because ongoing expansion of the industry means that any road-accessible areas with limited human population could see mink farm development, and there are many such areas upstream from Molega Lake. The total area with mink farm development potential upstream from Carrigan Lake is more limited because the lake is higher in its watershed.
Eutrophication from the cumulative effects of hundreds of additional cottage septic systems on Molega Lake is also a possible future threat. As noted in Threats – Shoreline Development, there are roughly 300 cottages on Molega Lake, about 500 subdivided but undeveloped cottage lots and potential for several hundred additional lots on larger undeveloped private land shoreline. To this point, the shoreline flora of the lake remains dominated by species of acidic, nutrient poor conditions and shows no obvious evidence of eutrophication effects (Blaney pers. obs. 2007-2013). One would expect, however, that there could be some tipping point in the future at which nutrient inputs from septic systems begin to change shoreline plant communities in favour of more common and competitive species. The Region of Queens Municipality municipal planning strategy recognizes that lakes have a “limited carrying capacity to support new development” (Region of Queens Municipality 2009) and has regulations and recommendations related to shoreline alteration, but unlike neighbouring Municipality of the County of Kings (2013) does not attempt to protect water quality by identifying an upper limit on the number of cottages that can be built around lakes.
COSEWIC “locations” are defined at the scale of the most significant threat to each population (COSEWIC 2010). The threats differ between the two Canadian subpopulations of Tall Beakrush, meaning that each represents at least one location. Number of locations in Canada is between two and five, depending on interpretation of threats as outlined below.
At Carrigan Lake, with 95% of the Canadian population, Nova Scotia Power has flowage rights to the entire shore, and this could also make water level management for hydroelectricity production the most significant threat. This threat has very high magnitude (potential to eliminate 95% of the Canadian population), despite what is believed to be low likelihood and immediacy (see Threats – Artificial Regulation of Water Levels). Tall Beakrush plants at Carrigan Lake are within a very narrow elevation range along the lakeshore and would be affected relatively uniformly by water level changes, making the subpopulation a single location.
Shoreline development has the potential to impact a reasonable proportion of the Canadian population and is relatively likely to occur to some degree within ten years (see Threats – Shoreline Development). This would make two or three locations at Carrigan Lake.
The occurrence at Molega Lake is within a portion of the lake having extensive shoreline cottage development, and expansion of cottage-related impacts is the most significant and imminent threat there (see Threats – Shoreline Development). Tall Beakrush at Molega Lake is on Crown land lake margin a few metres beyond the lakeshore margin of two private properties with separate owners. The Molega Lake subpopulation is probably best considered two locations because of the differing ownership and development states of the properties (one currently undeveloped, one with an existing cottage). An argument could be made to consider it a single location because its very limited extent (about 30 m x 3 m) means that most or all individuals could be affected by a single landowner management action (such as clearing shoreline vegetation for a boat launch or swimming beach) that extended across the property boundary. All individuals are within 16 m of the boundary, which is not well marked.
Tall Beakrush does not currently have any legal protected status in Canada. It receives protection in Maine through the state's Natural Resource Protection Act and under Maine's Site Law, which governs approval for development (Cameron pers. comm. 2013). Tall Beakrush is also protected as a Threatened species in Connecticut under the Connecticut Endangered Species Act (Connecticut DEEP 2013), and is listed as Special Concern in Tennessee under the state's Rare Plant Protection and Conservation Act of 1985 (Crabtree 2012). No other states have legal protection for Tall Beakrush.
Tall Beakrush is Critically Imperilled (N1, last assessed in 2012) in Canada and in Nova Scotia (S1) (NatureServe 2013) and is ranked as May Be At Risk in Nova Scotia and Canada under the General Status process (Canadian Endangered Species Conservation Council 2011). NatureServe ranks (from NatureServe [2013], unless otherwise noted) suggest Tall Beakrush is globally secure (G4; last assessed in 1985) and nationally secure in the U.S. (N4), but is Critically Imperilled (S1) in Kentucky, Maine, Missouri (Missouri Natural Heritage Program 2014) and Rhode Island, Critically Imperilled to Imperilled (S1S2) in Connecticut and Tennessee, Imperilled (S2) in Arkansas (Arkansas Natural Heritage Commission 2013), Indiana and Kansas, Vulnerable (S3 or S3?) in New York, Virginia, and North Carolina and Vulnerable to Apparently Secure (S3S4) in Michigan. Tall Beakrush is Apparently Secure (S4) in Delaware, and is unranked (SNR) in Alabama, Florida, Louisiana, Maryland, Massachusetts, New Jersey, Oklahoma, South Carolina, Texas, and the District of Columbia, and is Unrankable (SU) in Georgia. Tall Beakrush is also unranked (SNR) in Vermont and Mississippi but records for both states appear to be erroneous (see Global Range). Tall Beakrush may warrant ranking as a rare species in Florida (Jenkins pers. comm. 2013), given that it is mapped for only one county (Kartesz 2011; Hansen pers. comm. 2013; Wunderlin and Hansen 2013). Additionally, relatively limited occurrence in Alabama, Georgia and Massachusetts (Kartesz 2011) suggests that Tall Beakrush may be marginally rare in those jurisdictions.
Tall Beakrush has the following non-legal state status ranks: Endangered in Kentucky (Kentucky State Nature Preserves Commission 2012), State Threatened in Rhode Island (Enser 2007), “Inventory Element” in Arkansas (Arkansas Natural Heritage Commission 2013), and State Rare in Indiana (Indiana Natural Heritage Data Centre 2013).
Most or all Tall Beakrush occurrences are within the seasonally flooded zone along the shore that is under Crown ownership (LIANS 2008). Lakeshore plants such as Tall Beakrush receive indirect protection through provincial laws and policies regulating shoreline development and pertaining to the protection of water quality, watercourses, wetlands and riparian buffers. The Nova Scotia Wetlands Conservation Policy, Activities Designation Regulations and Environmental Assessment Regulations, all under the Environment Act, the Forest Act - Wildlife Habitat and Watercourses Protection Regulations, and the Off Highway Vehicle Act all may apply. In practice, however, these regulations are not always known to landowners or may be ignored, especially in relation to activities like removing rocks and removing or mowing vegetation for the creation and maintenance of beaches and boat launches on seasonally exposed Crown-owned shoreline in front of private properties.
At Molega Lake, land ownership adjacent to Tall Beakrush occurrences is with two private landowners. Five of 35 individuals are adjacent to a property with an existing cottage and the remaining 30 are on the adjacent property that is currently undeveloped. All plants are within 16 m of the boundary between these two properties. The boundary is not well marked (Blaney et al. pers. obs. 2013).
At Carrigan Lake, the lakeshore adjacent to the areas occupied by Tall Beakrush is either owned outright by Nova Scotia Power (506 of 653 individuals), or is Crown land over which Nova Scotia Power has flowage rights (147 of 653 individuals). Flowage rights are defined under the provincial Land Registration Act of 2001, Chapter 6, Section 1, Item 73 (1), which states that a “utility interest” (in this case the right to maintain water levels for power generation such that the land in question is flooded), “shall be enforced with priority over all other interests according to law” (Nova Scotia Office of the Legislative Counsel 2001). Nova Scotia Power is a private corporation that generates 95% of the province’s electricity and is regulated under the provincially appointed Nova Scotia Utility and Review Board (Nova Scotia Power 2013). Nova Scotia Power’s landholdings on the shore of Carrigan Lake are as narrow as 4 m, and 263 of 653 individuals on the lake are adjacent to very narrow Nova Scotia Power ownership, behind which land is privately owned and potentially subject to development. An additional three individuals on the south shore of the lake are near to the boundary of the provincial Lake Rossignol Wilderness area, which is separated from the lakeshore by a 4 m wide zone of Nova Scotia Power ownership and a 26 m wide zone of undesignated Crown land over which Nova Scotia Power has flowage rights.
David Mazerolle of the Atlantic Canada Conservation Data Centre (who originally discovered the species in Canada), Alain Belliveau of Mersey Tobeatic Research Institute and Shalan Joudry of Bear River First Nation assisted with fieldwork. Nicholas Hill of Fern Hill Institute for Plant Conservation provided directions and site descriptions that allowed the relocation of the Molega Lake occurrence. Tony Reznicek, University of Michigan and Alan Weakley of University of North Carolina provided information on Tall Beakrush ecology in their areas. Brad Toms of Mersey Tobeatic Research Institute provided GIS files documenting search effort on Molega Lake. David Freeman, landowner and local historian at Molega Lake, Alain Belliveau and Shalan Joudry provided information on the Molega Lake dam. Catherine Fielding-Croft, cottage owner at Keddy Cove on Molega Lake, provided information on the cottage history and landowner at that occurrence. Jeremy Peck and Jim Sandall of Nova Scotia Power provided information on water levels and land ownership on Carrigan Lake and Lake Rossignol. Julia Flemming of Nova Scotia Department of Agriculture provided information on mink farming. The following botanists provided information on local status and/or habitat of Tall Beakrush in their regions: Don Cameron and Lisa St. Hilaire (Maine), Bob Popp (Vermont Natural Heritage Inventory), Craig Freeman (Kansas Natural Heritage Inventory), Bruce Hansen (University of South Florida), Amy Jenkins (Florida Natural Areas Inventory), and Lucille McCook (University of Mississippi).
AC CDC (Atlantic Canada Conservation Data Centre). 2013. Digital database of rare species locations for Nova Scotia. Atlantic Canada Conservation Data Centre, Sackville, NB.
APG (Angiosperm Phylogeny Group).2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnaean Society 141:399-436.
Arkansas Natural Heritage Commission. 2013. Rare Species Search – Rhynchospora macrostachya. [accessed November 7, 2013].
Biological Records Center. 2013. Database of Insects and their Foodplants. [accessed November 15, 2013].
Blaney, C.S. 2011. Nova Scotia Wetland Plant Indicator List. Nova Scotia Department of Environment. [accessed February, 2013].
Blaney, C.S. pers. obs. 1999-2013. Personal observations on Atlantic Coastal Plain flora and associated communities in southern Nova Scotia. Botanist and Assistant Director, Atlantic Canada Conservation Data Centre, Sackville NB.
Blaney, C.S. pers. obs. 2007-2013. Personal observations on Atlantic Coastal Plain flora and associated communities in the vicinity of Ponhook and Molega Lakes in Queens and Lunenburg Co., Nova Scotia. Botanist and Assistant Director, Atlantic Canada Conservation Data Centre, Sackville NB.
Blaney, C.S., and D.M. Mazerolle. 2009. Rare plant inventory of lakes in the Ponhook – Molega Lakes region. Report to the Endangered Species Recovery Fund and Nova Scotia Species at Risk Conservation Fund. Atlantic Canada Conservation Data Centre, Sackville NB. 23 pp.
Blaney, C.S., and D.M. Mazerolle pers. obs. 2009-2013. Personal observations on Tall Beakrush (Rhynchospora macrostachya) at Carrigan Lake, 2009 and 2013. Botanist and Assistant Director, Atlantic Canada Conservation Data Centre, Sackville NB.
Blaney, C.S., D.M. Mazerolle, and A. Belliveau pers. obs. 2013. Personal observations on Tall Beakrush (Rhynchospora macrostachya) at Carrigan Lake and Molega Lake, Nova Scotia, August 6, September 6 & 9, 2013. Botanist and Assistant Director, Atlantic Canada Conservation Data Centre, Sackville NB.
Bowles, M.L., J.B. Taft, E.F. Ulaszek, M.K. Solecki, D.M. Ketzner, L.R. Phillipe, A. Dennis, P.J. Burton, K.R. Robertson. 1991. Rarely seen endangered plants, rediscoveries and species new to Illinois. Erigenia 11:27-30.
Britton, N.L. 1892. A list of the species of the genera Scirpus and Rhynchospora occurring in North. America. Trans. New York Acad. Sci. 11:74-93.
Brylinsky, M. 2011. An assessment of the sources and magnitudes of nutrient inputs responsible for degradation of water quality in seven lakes located within the Carleton River watershed area of Digby and Yarmouth counties, Nova Scotia. Prepared for the Nova Scotia Department of Environment. 25 pp.
Brylinsky, M. 2012. Results of the 2011 water quality survey of ten lakes located in the Carleton River watershed area of Digby and Yarmouth Counties, Nova Scotia. Prepared for Nova Scotia Environment. Acadia Center for Estuarine Research, Acadia University, Wolfville NS. 37 pp.
Cameron, D. pers. comm. 2013. November 13, 2013. Email to Sean Blaney regarding legal protection for Tall Beakrush in Maine. Botanist, Maine Natural Areas Program, Augusta, ME.
Canadian Endangered Species Conservation Council. 2011. Wild Species 2010: The General Status of Species in Canada. National General Status Working Group. 302 pp.
Catling, P.M., and G. Mitrow. 2012. Major invasive alien plants of natural habitats in Canada: 4. Glossy Buckthorn. Canadian Botanical Association Bulletin 45:70-77.
Catling, P.M., and Z.S. Porebski. 1994. The history of invasion and current status of Glossy Buckthorn, Rhamnus frangula, in southern Ontario. Can. Field-Nat. 108:305-310.
Center for Aquatic and Invasive Plants. 2002. Rhynchospora inundata, inundated beakrush. University of Florida, Gainesville, FL. Website: (www.plants.ifas.ufl.edu/node/368) [accessed November 27, 2013].
Chapman, A.W. 1860. Ceratoschoenus macrostachyus (Torrey ex. A. Gray) A. Gray var. patulus Chapman. Flora of the Southern United States, ed. 1. 529.
Chapman, A.W. 1897. Rhynchospora macrostachya Torr. ex A. Gray var. patula Chapm. Flora of the Southern United States ed. 3. 556.
Clark, F.H. 2003. Rhynchospora nitens (Vahl) A. Gray – Short-beaked bald-sedge Conservation and Research Plan for New England. New England Plant Conservation Program, New England Wildflower Society, Framingham MA. Online document: PDF file [accessed November 15, 2013]
Clayden, S.R., M.C. Munro, C.S. Blaney, and S.P. Vander Kloet. 2009. Vascular flora of the Atlantic Maritime Ecozone: some new perspectives. Ch. 10, pp. 197-214 in D.F. McAlpine, and I.M. Smith (eds.). Assessment of Species Diversity in the Atlantic Maritime Ecozone. NRC Research Press, Ottawa, ON. 785 pp.
Connecticut Department of Energy and Environmental Protection (DEEP). 2013. Endangered, Threatened & Special Concern Plants. [accessed November 6, 2013]
Conti, R.S., and P.P. Gunther. 1984. Relations of phenology and seed germination to the distribution of dominant plants in Okefenokee Swamp. pp. 144-167 in A. D. Cohen, D. J. Casagrande, M. J. Andrejko, and G. R. Best (Eds.). The Okefenokee Swamp: Its Natural History, Geology, and Geochemistry. Wetland Surveys, Los Alamos, New Mexico, USA.
COSEWIC. 2009. COSEWIC assessment and update status report on Redroot Lachnanthes caroliniana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa.
COSEWIC. 2010. COSEWIC’s Assessment Process and Criteria. Online document: PDF File [accessed November 15, 2013].
COSEWIC. 2012a. COSEWIC assessment and update status report on Plymouth Gentian Sabatia kennedyana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa.
COSEWIC. 2012b. COSEWIC assessment and update status report on Pink Coreopsis Coreopsis rosea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa.
COSEWIC. 2013. COSEWIC assessment and update status report on Water Pennywort Hydrocotyle umbellata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa.
Costa. A.C.G., and I.C. Machado. 2012. Flowering dynamics and pollination system of the sedge Rhynchospora ciliata (Vahl) Kükenth (Cyperaceae): does ambophily enhance its reproductive success? Plant Biology 14: 881-887. DOI: 10.1111/j.1438-8677.2012.00574.x
Crabtree, T. 2012. Tennessee Heritage Program Rare Plant List. Division of Natural Areas, Tennessee Department of Environment and Conservation, Nashville TN. 48 pp. Website: PDF file [accessed March 10, 2013].
Craine, S.I. 2003. Rhynchospora inundata (Oakes) Fern. – Inundated Beak-rush Conservation and Research Plan for New England. New England Plant Conservation Program, New England Wildflower Society, Framingham MA. Online document: PDF file [accessed November 15, 2013].
Dighton, J., T. Gordon, R. Meija, and M. Sobel. 2013. Mycorrhizal Status of Knieskern’s Beaked Sedge (Rhynchospora knieskernii) in the New Jersey Pine Barrens. Bartonia 66:24-27.
Eaton, S.T., and J.S. Boates. 2003. Securing the science foundation for responsible stewardship and recovery of ACPF species at risk. NS Department of Natural Resources, Kentville, NS.
Eckert, C.G., K.E. Samis, and S.C. Lougheed. 2008. Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Molecular Ecology 17:1170–1188.
Ehrenfeld, J.G. 1983. The effects of changes in land use on swamps of the New Jersey Pine Barrens. Biological Conservation 25:353-375.
Enser, R.W. 2007. Rare Native Plants of Rhode Island. Rhode Island Natural Heritage Program, Rhode Island Department of Environmental Management. Providence, RI. 17 pp. Website: PDF file [accessed November 2013].
Environment Canada and Parks Canada Agency. 2010. Recovery Strategy and Management Plan for Multiple Species of Atlantic Coastal Plain Flora in Canada. Species at Risk Act Recovery Strategy Series. Environment Canada and Parks Canada Agency. Ottawa. 96 pp. + appendices.
Fernald, M.L. 1918. Some allies of Rhynchospora macrostachya. Rhodora 20: 138-140.
Fernald, M.L. 1921. The Gray Herbarium expedition to Nova Scotia 1920. Rhodora 23:89-111, 130-152, 153-171, 184-195, 233-245, 257-78, 284-300.
Fernald, M.L. 1922. Notes on the flora of western Nova Scotia. Rhodora 24:157-164, 165–181, 201-208.
Fernald, M.L. 1940. Rhynchospora macrostachya Torr. ex A.Gray var. colpophila Fernald and Gale, p. 421 in A century of additions to the flora of Virginia.Rhodora 42:419-521.
Fernald, M.L. 1950. Gray’s Manual of Botany. A handbook of the flowering plants of the central and northeastern United States and adjacent Canada. 8th Edition. American Book Company. New York. 1632 pp.
Fiedler, A.K., and D.A. Landis. 2012. Biotic and abiotic conditions in Michigan Prairie Fen invaded by Glossy Buckthorn (Frangula alnus). Natural Areas Journal 32:41-53.
Fielding-Croft, C. pers. comm. 2013. August 3, 2013. In-person discussion with Sean Blaney about ownership and land use at cottage property supporting Tall Beakrush on Keddy Cove, Molega Lake, Nova Scotia. Owner of adjacent cottage property. Keddy Cove NS.
Fisher, G. W. 1953. Manual of North American Smut Fungi. Ronald Press, New York, New York, USA. 343 pp.
Flemming, J., pers. comm. 2011. December 7, 2011. Telephone conversation with Sean Blaney regarding mink farming in southern Nova Scotia. Permitting officer, Laboratory Services Section, Nova Scotia Department of Agriculture, Truro NS.
Frappier, B., R.T. Eckert, and T.D. Lee. 2003a. Potential impacts of the invasive exotic shrub Rhamnus frangula L. (glossy buckthorn) on forests of southern New Hampshire. Northeast. Nat. 10:277-296.
Frappier, B., T.D. Lee, K.F. Olson, and R.T. Eckert. 2003b. Small-scale invasion pattern, spread rate, and lag-phase behavior of Rhamnus frangula L. For. Ecol. Manag. 186:1-6.
Freeman, C.C. pers. comm. 2013. November 21, 2013. Email to Sean Blaney about habitat of Tall Beakrush in Kansas, especially in relation to soil pH. Botanist, Kansas Natural Heritage Inventory, University of Kansas. Lawrence, KS.
Freeman, D. pers. comm. 2013. November 26, 2013. Telephone conversation with Sean Blaney about the history and water level impacts of the Molega Lake Dam. Cottage owner at dam site and local historian. Molega Lake, NS.
Friedman, J., and S.C.H. Barrett. 2012. The consequences of monoecy and protogyny for mating in wind-pollinated Carex. New Phytologist 181:489–497. doi: 10.1111/j.1469-8137.2008.02664.x
García-Ramos, G., and M. Kirkpatrick. 1997. Genetic models of rapid evolutionary divergence in peripheral populations. Evolution 51:21-28.
Geho, E.M., D. Campbell, and P. A. Keddy. 2007. Quantifying ecological filters: the relative impact of herbivory, neighbours, and sediment on an oligohaline marsh. Oikos 116:1006-1016.
Gerritsen, J., and H.S. Greening. 1989. Marsh seed banks of the Okefenokee Swamp: Effects of hydrologic regime and nutrients. Ecology 70:750-763.
Gleason, H. A., and A. Cronquist. 1991. Manual of the Vascular Plants of Northeastern United States and Adjacent Canada. 2nd ed. New York Botanical Garden, NY. 910 p.
Government of Canada. 2011. Species at Risk Public Registry [accessed November 2011].
Government of Nova Scotia. 2013. Fur Industry Regulations made under Section 36 of the Fur Industry Act S.N.S. 2010, c. 4 O.I.C. 2013-2 (January 11, 2013), N.S. Reg. 4/2013 [accessed November 14, 2013].
Gray, A. 1835. A monograph of the North American species of Rhynchospora. Annals of the Lyceum of Natural History, New York. 3: 25-220.
Growing Wild Nursery. 2013. Coastal Plain Native Plants - White Bracted Sedge Rhynchospora colorata. Website: (http://www.growingwildnursery.net/plants/rhynchospora-colorata.html) [accessed November 5, 2013].
Gunther, P.P., D.J. Casagrande, and R.R. Cherney. 1984. The viability and fate of seeds as a function of depth in the peats of Okefenokee Swamp. Pages 168-179 in A.D. Cohen, D.J. Casagrande, M.J. Andrejko, and G.R. Best (Editors). The Okefenokee Swamp: Its Natural History, Geology, and Geochemistry. Wetland Surveys, Los Alamos, New Mexico, USA.
Hansen, B.F. pers. comm. 2013. November 8, 2013. Email to Sean Blaney confirming that Tall Beakrush has been validly reported for Florida. Curator of the Herbarium, Institute for Systematic Botany, University of South Florida. Tampa, FL.
Hazel, S.N. 2004. Hydrological alterations and rare species of the Atlantic Coastal Plain Flora in Nova Scotia. M.Sc. Thesis, Acadia University, Wolfville, NS.
Hill, N. M., and C. S. Blaney. 2009. Exotic and invasive vascular plants of the Atlantic Maritime Ecozone. Pages 1-18 in Assessment of species diversity in the Atlantic Maritime Ecozone. Edited by D. F. McAlpine and I. M. Smith. NRC Research Press, Ottawa.
Hill, N., and P.A. Keddy. 1992. Predicting numbers of rarities from habitat variables: coastal plain plants of Nova Scotian lakeshores. Ecology 73:1852-1859.
Hill, N.M., P.A. Keddy, and I.C. Wisheu. 1998. A hydrological model for predicting the effects of dams on the shoreline vegetation of lakes and reservoirs. Environmental Management 22:723-736.
Hohman, W.L., T.M. Stark, and J.L. Moore. 1992. Food availability and feeding preferences of breeding Fulvous Whistling-Ducks in Louisiana rice field. The Wilson Bulletin 108: 137-150.
Hurlburt, D., pers. comm. 2013. Email communication with Sean Blaney regarding aboriginal traditional knowledge of Tall Beakrush (Rhynchospora macrostachya). November 28, 2013. COSEWIC Aboriginal Traditional Knowledge Specialist Committee representative to the COSEWIC Vascular Plants Species Specialist Committee, Yarmouth, NS.
Indiana Natural Heritage Data Centre. 2013. Endangered, Threatened, Rare and Extirpated Plants of Indiana. Online document: PDF file [accessed November 8, 2013].
IPANE (Invasive Plant Atlas of New England IPANE). 2012.[accessed December 2012].
IPNI (International Plant Names Index). 2005. Rhynchospora macrostachya. Website: (http://www.ipni.org/ipni/advPlantNameSearch.do?find_family=&find_genus=Rhynchospora&find_species=macrostachya) [accessed November 5, 2013].
Jenkins, A. pers. comm. 2013. November 12, 2013. Email to Sean Blaney regarding status of Tall Beakrush in Florida. Senior Botanist, Florida Natural Areas Inventory. Tallahassee, FL.
Johansson, M.E., and C. Nilsson. 2002. Responses of riparian plants to flooding in free-flowing and regulated boreal rivers: an experimental study. Journal of Applied Ecology. 39: 971-986. DOI: 10.1046/j.1365-2664.2002.00770.x
Kartesz, J.T. 2011. North American Plant Atlas. [maps generated from Kartesz, J.T. 2010. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). Chapel Hill, NC. [accessed November 2013].
Kartesz, J.T. pers. comm. 2013. December 4, 2013. Email to Sean Blaney with digital image of North American range of Rhynchospora macrostachya. Director, Biota of North America Project. Chapel Hill, NC.
Kavaljian, L.G. 1952. The floral morphology of Clethra alnifolia with some notes on C. acuminata and C. arborea. Bot. Gaz. 113:392-413.
Keddy, P.A. 1985. Wave disturbance on lakeshores and the within-lake distribution of Ontario’s Atlantic coastal plain flora. Can. J. Bot. 63:656-660.
Keddy, P.A. 1989. Effect of competition from shrubs on herbaceous wetland plants: a 4-year field experiment. Canadian Journal of Botany 67:708–716.
Keddy, P.A., and I.C. Wisheu. 1989. Ecology, biogeography and conservation of Coastal Plain plants: Some general principles from the study of Nova Scotian wetlands.
Kentucky State Nature Preserves Commission. 2012. Rare and Extirpated Biota and Natural Communities of Kentucky. Online Document: PDF file [accessed November 8, 2013].
Kral, R. 2002. Rhynchospora. Pages 200-239 in Flora of North America Editorial Committee, editors. Flora of North America north of Mexico. Volume 23. Oxford University Press, New York, New York.
Kral, R., A.R. Diamond Jr, S.L. Ginzbarg, C.J. Hansen, R.R. Haynes, B.R. Keener, M.G. Lelong, D.D. Spaulding, and M. Woods. 2013 Alabama Plant Atlas: Rhynchospora macrostachya. Alabama Plant Atlas [accessed November 15, 2013].
Kral, R., A.R. Diamond Jr, S.L. Ginzbarg, C.J. Hansen, R.R. Haynes, B.R. Keener, M.G. Lelong, D.D. Spaulding, and M. Woods. 2013. Rhynchospora macrostachya in Alabama Plant Atlas. University of West Alabama, Livingston, Alabama. Alabama Plant Atlas [accessed November 14, 2013].
Lee, T.D., and J.H. Thompson. 2012. Effects of logging history on invasion of eastern white pine forests by exotic glossy buckthorn (Frangula alnus P. Mill.). For. Ecol. Manag. 265:201-210.
Leppik, E.E. 1955. Dichronema ciliata, a noteworthy entomophilous plant among Cyperaceae. American Journal of Botany 42:455–458.
Lesica, P., and F.W. Allendorf. 1995. When are peripheral populations valuable for conservation? Conservation Biology 9:753-760.
LIANS (Lawyers’ Insurance Association of Nova Scotia). 2008. The Ebb and Flow of Water Law in Nova Scotia. Online document: (file:///E:\www.lians.ca\documents\EbbAndFlowOfWaterLaw-CorsanoRisk%20(00012380).pdf) [accessed September 12, 2013].
Lichvar, R.W. 2013. The National Wetland Plant List: 2013 wetland ratings. PDF file Phytoneuron 49:1-241
Louisiana State University. 2013. Online Herbarium. [accessed November 10, 2013].
Lovera, M., and G. Cuenca. 1996. Arbuscular mycorrhizal infection in Cyperaceae and Gramineae from natural, disturbed and restored savannas in La Gran Sabana, Venezuela. Mycorrhiza 6:111–113.
Lusk J.M., and E.G. Reekie. 2007. The effect of growing season length and water level fluctuations on growth and survival of two rare and at risk Atlantic coastal plain flora species, Coreopsis rosea and Hydrocotyle umbellata. Canadian Journal of Botany 85:119-131.
MacRae, T.R. 2004. Notes on host associations of Taphrocerus gracilis (Say) (Coleoptera: Buprestidae) and its life history in Missour. The Coleopterists Bulletin 58: 388-390. Website: [accessed November 2013].
Magrath, L.K., and K.L. Johnson. 1971. The genus Rhynchospora (Cyperaceae) in Kansas. Southwest Naturalist 15:389.
Marsden, M.W. 1989. Lake restoration by reducing external phosphorus loading: the influence of sediment phosphorus release. Freshwater Biology 21:139-162.
Martin, A.C., and F.M. Uhler. 1939. Food of game ducks in the United States and Canada. United States Department of Agriculture Technical Bulletin No. 634. Washington D.C.
McCook, L. pers. comm. 2013. November 11, 2013. Email to Sean Blaney regarding the occurrence of Tall Beakrush in Mississippi, confirming that the report was likely based on a misidentified specimen. Herbarium curator, University of Mississippi. Oxford MS.
Merritt, D.M., and D.J. Cooper. 2000. Riparian vegetation and channel change in response to river regulation: A comparative study of regulated and unregulated streams in the Green River Basin, USA. Regulated Rivers: Research and Management 16:543–564.
Michigan Natural Features Inventory. 2013. Rare Species Explorer – Rhynchospora macrostacya (Tall Beakrush). [accessed November 9, 2013].
Missouri Natural Heritage Program. 2014. Missouri species and communities of conservation concern checklist. Missouri Department of Conservation. Jefferson City, Missouri. pp. 52. Available online at: PDF file.
Moore, G. 1997. A taxonomic investigation of Rhynchospora Section Longirostres Kunth. Ph.D. Dissertation, Vanderbilt University, Nashville, Tennessee, USA. 298 pp.
Moore, D.R.J., P.A. Keddy, C.L. Gaudet, and I. Wisheu. 1989. Conservation of Wetlands: Do Infertile Wetlands Deserve a Higher Priority? Biological Conservation 47:203-217.
Mueller, M.H. and A.G. van der Valk. 2002. The potential role of ducks in wetland seed dispersal. Wetlands 22:170-178.
Municipality of the County of Kings. 2013. Transforming Theory into Practice: Lakeshore Planning in Kings County, Nova Scotia. Online document: PDF file [accessed November 26, 2013].
Natural Resources Canada. 2003. Canadian Geographical Names. Geomatics Canada, Earth Sciences Sector, Centre for Topographic Information, Geographical Names Section. Ottawa ON. Geobase [Downloaded 2007; sorted for “lake” and “pond” and by latitude and longitude to produce list of relevant waterbodies].
NatureServe. 2013. NatureServe Explorer – Rhynchospora macrostachya. [accessed November 17, 2013].
Nelson G.H., G.C. Walters Jr, R.D. Haines, C.L. Bellamy. 2008. A catalogue and bibliography of the Buprestoidea of America north of Mexico. Coleopterists Society Special Publication 4, Sacramento, California, USA, 274 pp.
Nilsson, C., and R. Jansson. 1995. Floristic differences between riparian corridors of regulated and free-flowing boreal rivers. Regulated Rivers: Research and Management 11: 55-66.
Nova Scotia DNR (Department of Natural Resources). 2013. Species at Risk List Regulations made under Sections 10 and 12 of the Endangered Species Act. [accessed March 2013].
Nova Scotia Office of the Legislative Counsel. 2001. Land Registration Act. Chapter 6 of the Acts of 2001. [accessed November 20, 2013].
Nova Scotia Power. 2013. How We Operate – Regulations. [accessed November 20, 2013].
Oakes, W. 1841. Ceratoschoenus macrostachys var. inundatus Oakes in Mag. Hort. Bot. 7:185.
PADIL (Pests and Diseases Image Library). 2013. Smut Fungi of Australia. [accessed November 15, 2013].
Peck, J. pers. comm. 2013. November 19, 2013. Telephone conversation with Sean Blaney about water levels on Carrigan Lake and Nova Scotia Power’s flowage rights at the lake. Hydrologist, Nova Scotia Power, Halifax NS.
Piepenbring, M. 1995. Trichoncintractia, a new genus for Cintractia utriculicola (Ustailaginales). Canadian Journal of Botany 73:1089-1096.
Popp, B. pers. comm. 2013. November 11, 2013. Email to Sean Blaney regarding Vermont record of Tall Beakrush, confirming that it was based on a misidentified specimen. Botanist, Vermont Natural Heritage Inventory, Barre VT.
Region of Queens Municipality. 2009. Municipal Planning Strategy. Region of Queens Municipality, Liverpool NS. [accessed November 25, 2013].
Reinartz, J.A., and J. Kline. 1998. Glossy buckthorn (Rhamnus frangula), a threat to the vegetation of the Cedarburg Bog. Field Station Bulletin, University of Wisconsin – Milwaukee 21:20-35.
Reznicek, A.A. 1990. Evolution in sedges (Carex, Cyperaceae). Canadian Journal of Botany 68:1409–1432.
Reznicek, A.A. 1994. The disjunct Coastal Plain flora in the Great Lakes region. Biological Conservation 68:203-215.
Reznicek, A.A. 2013. pers. comm. November 27, 2013. Email to Sean Blaney regarding longevity, age to first reproduction and ecology of Tall Beakrush. Herbarium Curator, University of Michigan. Ann Arbor, MI.
Reznicek, A.A., E.G. Voss, and B.S. Walters. 2011. Michigan Flora Online. February 2011. University of Michigan. [accessed December 4, 2012].
Roland, A.E., and E.C. Smith. 1969. The Flora of Nova Scotia. Nova Scotia Museum, Halifax. 743 pp.
Sandall, J. pers. comm. 2013. November 27, 2013. Email to Sean Blaney regarding development on shoreline owned by or with flowage right to Nova Scotia Power. Survey Technologist, Nova Scotia Power. Halifax NS.
Silva, G.A.D., B.A.D. Santos, M.V. Alves, L.C. Maia. 2001. Arbuscular mycorrhiza in species of Commelinidae (Liliopsida) in the state of Pernambuco (Brazil). Acta Bot. Bras. 15:155–165.
St. Hilaire, L. pers. comm. 2013. November 14, 2013. Email to Sean Blaney regarding occurrences of Tall Beakrush in Maine. Data Manager, Maine Natural Areas Program. Augusta, ME.
Stiles, E.W. 2000. Animals as seed dispersers in Seeds: The Ecology of Regeneration in Plant Communities (ed. M. Fenner), pp. 111-124. CAB International, London UK. 415 pp.
Taft, J.B., and M.K. Solecki. 1990. Vascular flora of the wetland and prairie communities of Gavin Bog and Prairie Nature Preserve, Lake County, Illinois. Rhodora. 92(871):142-165.
Tiner, R.W., Jr. 1987. A field guide to coastal wetland plants of the northeastern United States. University of Massachusetts Press, Amherst, MA. 285 pp.
Toms, B. pers. comm. 2013. November 22, 2013. Email to Sean Blaney with GIS data files outlining shoreline areas on Molega Lake walked in Atlantic Coastal Plain flora lakeshore survey effort. Wildlife Biologist, Mersey Tobeatic Research Institute, Kempt NS.
Torrey, J. 1836.Monograph of the North American Cyperaceae. Annals of the Lyceum Natural History, New York 3: 239-450.
USDA (United States Department of Agriculture). 2012. Plant Hardiness Zone Map. [accessed March 17, 2013].
University of South Carolina. 2013. Flora Caroliniana: Rhynchospora macrostachya. [accessed November 17, 2013].
University of Wisconsin – Madison, Center for Sustainability and the Global Environment. 2002. Soil pH – North America. Website: (http://www.sage.wisc.edu/atlas/maps/soilph/atl_soilph_nam.jpg) [accessed November 12, 2013].
Virginia Botanical Associates. 2013. Digital Atlas of the Virginia Flora: Rhynchospora macrostachya.
Virginia Botanical Associates, Blacksburg VA. [accessed November 9, 2013].
Watergarden Paradise. 2013. Medium Height Marginal Aquatic Plants. [accessed November 5, 2013].
Weakley, A.S. 2012. Flora of the Southern and Mid-Atlantic States. University of North Carolina Herbarium, Chapel Hill NC. 1225 pp. Website: PDF file [accessed November 27, 2013].
Weakley, A.S. 2013. pers. comm. November 27, 2013. Email to Sean Blaney regarding ecology and life history of Tall Beakrush. Herbarium Curator, University of North Carolina. Chapel Hill, NC.
Webster, R.P., and I. DeMerchant. 2012. New Coleoptera records from New Brunswick, Canada: Buprestidae. Zookeys 179:55-65. doi:
Weldy, T., D. Werier, and A. Nelson. 2013. New York Flora Atlas. [S.M. Landry, and K.N. Campbell, original application development], Florida Center for Community Design and Research, University of South Florida, New York Flora Association. Albany, New York. [accessed November 20, 2013].
Westervelt, K., E. Largay, R. Coxe, W. McAvoy, S. Perles, G. Podniesinski, L. Sneddon, and K. Walz. 2006. A Guide to the Natural Communities of the Delaware Estuary: Version I. NatureServe, Arlington, Virginia. PDF file
White, D.J., J.C. Makarewicz, and T.W. Lewis. 2002. The significance of phosphorus released from the sediment under anoxic conditions in Sodus Bay, N.Y. Environmental Sciences Program, Department of Biological Sciences SUNY Brockport Brockport, New York, 33 pp.
Wisheu, I.C., and P.A. Keddy. 1994. The low competitive ability of Canada’s Atlantic Coastal Plain shoreline flora: implications for conservation. Biological Conservation 68: 247–252.
Wunderlin, R.P., and B.F. Hansen. 2008. Atlas of Florida Vascular Plants – Rhynchospora macrostachya. S. M. Landry and K. N. Campbell (application development), Florida Center for Community Design and Research. Institute for Systematic Botany, University of South Florida, Tampa. [accessed November 15, 2013].
Zaremba, R.E., and E.E. Lamont. 1993. The status of the Coastal Plain Pondshore community in New York. Bulletin of the Torrey Botanical Club 120:180-187.
Sean Blaney is the Botanist and Assistant Director of the Atlantic Canada Conservation Data Centre (AC CDC), where he maintains status ranks and a rare plant occurrence database for each of the three Maritime provinces. Since beginning with the AC CDC in 1999, he has documented dozens of new provincial records for vascular plants and thousands of rare plant localities during extensive fieldwork across the Maritimes. Sean is a member of the COSEWIC Vascular Plant Species Specialist Subcommittee, the Nova Scotia Atlantic Coastal Plain Flora Recovery Team, and has authored or co-authored numerous COSEWIC and provincial status reports. Prior to employment with AC CDC, Sean received a B.Sc. in Biology (Botany Minor) from the University of Guelph and an M.Sc. in Plant Ecology from the University of Toronto, and worked on biological inventory projects in Ontario as well as spending eight summers as a naturalist in Algonquin Park, where he co-authored the second edition of the park's plant checklist.
No specimens were examined during preparation of the report. The only Canadian specimens were collected upon the initial discovery of the Carrigan Lake subpopulation in 2008 by AC CDC, and the Keddy Cove, Molega Lake subpopulation by Nick Hill in 2009.
| Lake Name | Extent of Shoreline Coverage | Within 10 km of: |
|---|---|---|
| Black Rattle | Comprehensive (2007, 2008, 2013) | Molega |
| Beartrap [& Cameron] | Comprehensive (2007, 2012) | Carrigan |
| Cameron [& Beartrap] | Comprehensive (2007, 2012) | Carrigan |
| Second Christopher | Comprehensive (2007, 2012) | Carrigan |
| Beavertail | Comprehensive (2008) | Molega |
| Elizabeth | Comprehensive (2008) | Molega |
| Laurel [& Third Christopher] | Comprehensive (2008) | Carrigan |
| Long | Comprehensive (2008) | Molega |
| Russell | Comprehensive (2008) | Carrigan |
| Third Christopher [& Laurel] | Comprehensive (2008) | Carrigan |
| Whynott | Comprehensive (2008) | Molega |
| Bull Moose | Comprehensive (2009) | Carrigan |
| Little Rocky | Comprehensive (2009) | Carrigan |
| Carrigan | Comprehensive (2009, 2013) | Carrigan |
| Annis | Comprehensive (2010) | Molega |
| Apple Tree | Comprehensive (2010) | Carrigan |
| Big Rocky | Comprehensive (2010) | Carrigan |
| First Christopher | Comprehensive (2010) | Carrigan |
| Fourth Christopher | Comprehensive (2010) | Carrigan |
| Little Ponhook | Comprehensive (2010) | Molega |
| Moccassin | Comprehensive (2010) | Carrigan |
| Telfer | Comprehensive (2010) | Carrigan |
| Hog | Comprehensive (2011) | Molega |
| Moosehorn | Comprehensive (2012) | Carrigan |
| Bradley | Comprehensive (2013) | Carrigan |
| Cannon | Comprehensive (2013) | Carrigan |
| Loon | Comprehensive (2013) | Carrigan |
| McBride | Comprehensive (2013) | Carrigan |
| Murphy | Comprehensive (2013) | Carrigan |
| Payzant | Comprehensive (2013) | Carrigan |
| Beaverdam | Near comprehensive (2007) | Molega |
| Seven Mile | Near comprehensive (2008) | Molega |
| Fox | Near comprehensive (2011) | Molega |
| Ponhook | ~75% coverage (2007-2012) | Molega |
| Shingle | ~75% coverage (2008-2012) | Molega |
| Molega | 85% coverage (2007-2013) | Molega |
| Turtle | Casual shore coverage (~2009) | Carrigan |
| McLean | Casual shore coverage (2012) | Carrigan |
| Cow Moose [West] | spot visit (2009) | Carrigan |
| Gludogan | spot visit (2009) | Carrigan |
| McGuire | spot visit (2013) | Carrigan |
| Barney | never visited | Carrigan |
| Black | never visited | Carrigan |
| Cow Moose [East] | never visited | Molega |
| Faulkner | never visited | Molega |
| Fire | never visited | Molega |
| Fisher | never visited | Molega |
| Harley | never visited | Molega |
| Horseshoe | never visited | Molega |
| Huey | never visited | Molega |
| Irwin | never visited | Carrigan |
| Little Moose | never visited | Molega |
| Menchan | never visited | Carrigan |
| Oickle | never visited | Molega |
| Pollock | never visited | Carrigan |
| Prescott | never visited | Molega |
| Rhyno | never visited | Molega |
| Slauenwhite | never visited | Molega |
| Weagle | never visited | Molega |
| Woolenhaupt | never visited | Molega |
| Common Name | Species | # Sites (n=30) |
|---|---|---|
| Virginia Marsh St. John's Wort | Triadenum virginicum Table Footnotee | 19 |
| Eaton's Witchgrass | Dichanthelium spretum Table Footnotee | 17 |
| Three-Way Sedge | Dulichium arundinaceum | 15 |
| Bog Aster | Oclemena nemoralis | 13 |
| Sweet Gale | Myrica gale | 10 |
| Twig Rush | Cladium mariscoides | 10 |
| Pickerel Weed | Pontederia cordata | 10 |
| Large Cranberry | Vaccinium macrocarpon | 9 |
| Royal Fern | Osmunda regalis var. spectabilis | 8 |
| Swamp Loosestrife | Lysimachia terrestris | 8 |
| Blue-Joint Reedgrass | Calamagrostis canadensis | 6 |
| Carolina Grass-Leaved Goldenrod | Euthamia caroliniana Table Footnotee | 5 |
| Canada Rush | Juncus canadensis | 5 |
| Lance-Leaf Violet | Viola lanceolata | 5 |
| Leatherleaf | Chamaedaphne calyculata | 4 |
| Spoon-Leaved Sundew | Drosera intermedia | 4 |
| Glossy Buckthorn | Frangula alnus | 3 |
| Brown Beakrush | Rhynchospora fusca | 3 |
| New Belgium American-Aster | Symphyotrichum novi-belgii | 3 |
| Broadleaf Arrowhead | Sagittaria latifolia | 2 |
| Brook-Side Alder | Alnus serrulata Table Footnotee | 2 |
| Roundleaf Sundew | Drosera rotundifolia | 2 |
| Brown-Fruited Rush | Juncus pelocarpus | 2 |
| American Water-Lily | Nymphaea odorata | 2 |
| Rough Bentgrass | Agrostis scabra | 2 |
| Northern Bugleweed | Lycopus uniflorus | 2 |
| Little Prickly Sedge | Carex echinata | 2 |
| Long Sedge | Carex folliculata | 1 |
| Creeping Spike-Rush | Eleocharis palustris | 1 |
| Marsh Fern | Thelypteris palustris var. pubescens | 1 |
| Northern Meadow-Sweet | Spiraea alba var. latifolia | 1 |
| Robbins Spikerush | Eleocharis robbinsii | 1 |
| Perennial Bentgrass | Agrostis perennans | 1 |
| Hemlock Water-Parsnip | Sium suave | 1 |
| Virginia Chainfern | Woodwardia virginica Table Footnotee | 1 |
| Seven-Angled Pipewort | Eriocaulon aquaticum | 1 |
| Carolina Yellow-Eyed-Grass | Xyris difformis Table Footnotee | 1 |
| Broad-Leaf Cattail | Typha latifolia | 1 |
| Common Buttonbush | Cephalanthus occidentalis | 1 |