Wild species 2010: chapter 6
Macrolichens
Lichens - Lichens are fungi that have established a symbiotic relationship with an alga or cyanobacterium or both.

Quick facts
- There are about 2000 species of lichens in Canada, of which 861 can be considered as macrolichens. There are about 15 000 species of macrolichens worldwide.
- When excluding species ranked as Extinct, Extirpated, Undetermined, Not Assessed, Exotic or Accidental, the majority (72%) of macrolichens in Canada have Canada General Status Ranks (Canada ranks) of Secure, while 16% have Canada ranks of May Be At Risk and 11% have Canada ranks of Sensitive. Five macrolichen species (1%) have a Canada rank of At Risk following a detailed assessment by COSEWIC.
- One macrolichen species is recorded as Extirpated from Canada.
- Lichens can grow almost anywhere in the world in environments ranging from extreme heat to extreme cold.
- Moisture is essential for the growth of lichens. Lichens act like a sponge and absorb water over their entire surface. In the same way that they absorb water, they also dry out rapidly and become dormant.
Background
Lichens are fungi that have established a symbiotic relationship with an alga or cyanobacterium or both. In this type of relationship, the fungus and alga live together and both generally benefit from the association. The fungus provides structural support to the alga by surrounding the algal cells with hyphae or strands of fungal tissue. It also provides a relatively stable microhabitat for the algal cells to exist. The algae in turn provide carbohydrates and sugars produced in photosynthesis (the green pigment, chlorophyll, contained in the algal cells utilizes sunlight to produce carbohydrates and sugars from carbon dioxide and water). The fungus and alga have evolved together in such a way that an equilibrium exists, whereby the fungus does not utilize all of the carbohydrates and sugars made by the alga, while the alga derives structural integrity from the fungus. Most lichens obtain their shape from the fungal component (often called the mycobiont) with the algal component (the photobiont) restricted to a narrow band of cells located near the upper surface of the lichen.
The process of a fungus and alga joining to form a lichen is called lichenization. When a lichen is separated in the laboratory into its fungal and algal components and these are cultured separately, they grow perfectly well. Each forms its characteristic fungal or algal form. When these same components are mixed together under the right conditions, the new association takes the form of the parent lichen. The fungus appears to contain all the genetic information it needs to create the characteristic form of the lichen, but requires the alga or cyanobacterium to ‘turn on’ the fungal genes that control this morphogenesis or change in time controlled by an external or internal event.
There are about 15 000 species of macrolichens in the world, but this number is continually growing as more species are described. The names given to these lichens apply to the fungal symbionts and hence all lichens belong to the Kingdom Fungi. In almost all lichens, the fungus is an ascomycete or cup fungus with spores formed in sacs or asci. In a small number of species, the fungus is a basidiomycete or mushroom where the spores are formed on club-shaped basidia.
The lichen body or thallus is made up of three main layers – cortex, algal layer and medulla. The cortex is specialized tissue composed of compact fungal cells that forms a protective covering for the lichen. The algal layer, composed of algal cells intertwined with strands of fungal hyphae, is usually located beneath the outer or upper cortex. The medulla or inner layer is composed only of fungal hyphae.
Lichens can grow almost anywhere in the world. Some lichens are able to colonize the very hot or cold harsh environments of deserts and others can survive in the cold, snowfree alpine habitats where most other plants are not able to exist. In these extreme environments, crustose lichens that are tightly attached to their wood or rock substrate, may only grow a fraction of a millimetre a year and reach several hundred years in age, whereas foliose lichens growing in temperate or tropical areas may grow one centimetre or more per year.
Moisture is essential for the growth of lichens. Lichens act like a sponge and absorb water over their entire surface. In the same way that they absorb water, they also dry out rapidly and become dormant. When in this dormant stage, the physiological process of photosynthesis stops while respiration is greatly slowed. Studies have shown that lichens that have been dormant for several years are capable of physiological function when again moistened.
Status of knowledge
Historically, early botanists in Canada were the first to collect and identify lichens. However, their main focus was to collect and name vascular plants. Today, we still have many of these early collections in herbaria throughout the country. Both amateurs and professionals use these herbaria to aid in identifying and naming lichen species. Modern methods in genetics and DNA sequencing are re-grouping lichen species along evolutionary lines into new genera and species. Lichenology has worldwide organizations, journals and web sites that keep lichenologists in touch with one another and up-to-date regarding research topics and results. Lichens produce secondary chemical compounds that are useful in their identification. Spot tests, for example, using household bleach that turns the lichen medulla red, will identify lecanoric acid in speckled shield lichens (Punctelia). These chemical compounds also protect the lichen from herbivory by animals, insects and other fungi. Botanical and medicinal research uses lichens and their secondary substances for tests in cures for cancer and other medical conditions.
With the onset of the industrial revolution in Europe, it was observed that lichens were disappearing from these industrial areas that were later called “lichen deserts”. Nowadays lichens are widely recognized as sensitive indicators of air pollution, particularly sulphur dioxide. A reason for this sensitivity is that the biology of the lichen is unique in that it has no vascular system for conducting water or nutrients as seen in vascular plants. Thus, lichens have no means to retain water, such as a leaf cuticle or stomate. Consequently, lichen water status varies passively with the environment, although the lichen can absorb moisture and nutrients from fog, dew and highly humid air. From an air pollution perspective, there are several reasons why these attributes are important. The lichen is a long-lived perennial organism that is exposed to air pollutants all year. If injury to either the alga (photobiont) or fungus occurs from these pollutants, the symbiotic association of the lichen may disintegrate. The lack of stomata and cuticle in lichens results in aerosols being absorbed over the entire surface area. Sulphur dioxide (SO2) damages cell-membrane integrity, which affects a lichen’s ability to photosynthesize at low atmospheric SO2 concentrations in sensitive species such as in beard lichens (Usnea), whereas tolerant lichens such as Monk’s Hood Lichen (Hypogymnia physodes) will survive continuous to high exposure. Air pollution studies using lichens as indicators in the 1960s documented lichen desert effects in Montreal, an urban industrial area and Sudbury and Wawa, Ontario, both with iron ore mines that emitted sulphur dioxide into the atmosphere. Nowadays, with strict governmental emissions controls, more subtle effects of air quality exposure are measured in the lichen tissues. Lichen community indicators for nitrogen-containing pollutants such as ammonia can be detected in the macrolichen community structure by using the presence/absence of certain lichen species that tell researchers what areas are being affected.
Lichens have several other wide and varied uses today such as a food source for caribou that eat ground-dwelling reindeer (Cladonia) lichens in winter. Some squirrels make nests from witch’s hair (Bryoria) lichens. Icelandmoss Lichen (Cetraria islandica) can be ground into flour and used to thicken soups and stews. Model railroads often use reindeer (Cladonia) lichens as trees or shrubs on the train platform.
Richness and diversity in Canada
Relative to most other groups covered in this report, the species richness of macrolichens is high across the country (figure 6 and table 6), peaking in British Columbia (573 macrolichens). The flora of British Columbia is particularly diverse within a Canadian context, as 99 species of macrolichens that occur there are found nowhere else in Canada. Macrolichens in British Columbia have been intensively collected relative to other provinces and territories except southern Ontario; this may be reflected in the high diversity of BC macrolichens. Other factors affecting macrolichen richness and diversity are large areas of provinces and territories that are under-collected and when collection sites are mapped show no collections at all. These areas can be targeted for future inventory work.
Species spotlight - Wolf Lichen
Wolf Lichen (Letharia vulpina) is a brightly coloured chartreuse or yellow-green fruticose or shrubby lichen. The tufted thallus or lichen body is characterized by having granular soredia or asexual reproductive structures on angular, pitted branches. This light-loving lichen is widespread and common, growing on the twigs, branches and trunks of conifers, especially Lodgepole Pine (Pinus contorta). Often, Wolf Lichen will form a prominent display partially covering standing dead trees and snags. In winter, skiers will notice this lichen as its vivid chartreuse colour forms a sharp contrast with the snow, forest and bright blue sky.
The common Wolf Lichen is widespread in the mountains throughout southern British Columbia and Alberta, the Pacific Northwest and California, whereas the Brown-eyed Wolf Lichen (Letharia columbiana), a less common species, tends to have a more restricted distribution in high elevation subalpine forests. Wolf Lichen is the sorediate half of the lichen species pair with Brown-eyed Wolf Lichen that has large brown, fringed fruiting bodies or apothecia and lacks soredia. Of species pairs, the sorediate species, e.g. Wolf Lichen, tends to have a broader geographic distribution throughout its range as its options for dispersal are much greater than the apotheciate species where the fungal spore must find the “right” alga before a lichen can be formed. DNA evidence has disclosed six species of wolf lichens (Letharia) in North America that includes the two species above. Some of the species are cryptic and cannot be distinguished morphologically from one another.
The common and scientific name “Wolf Lichen” comes from the bright yellow vulpinic acid, an example of a secondary substance present in most lichens that gives the distinctive yellow colour to this lichen. Wolf Lichen was used traditionally to poison foxes and wolves in northern Europe. Vulpinic acid is not only poisonous to all meat eaters but also to insects and molluscs, but surprisingly is not harmful to rabbits and mice.
Species spotlight - Boreal Felt Lichen
Boreal Felt Lichen (Erioderma pedicellatum) is an epiphytic foliose lichen found in the temperate and boreal northern hemisphere. It is a leafy lichen, light grey when dry to greyish green when wet. The lichen has a covering of fine white hairs on the upper surface and a mat of dense white hairs on the under surface. Mature thalli will have small round reddish fruiting bodies on the upper surface. This lichen is part of a group of lichens known as cyanolichens because the photosynthesizing partner is a cyanobacterium. In the case of Boreal Felt Lichen, the cyanobacterium is in the genus Scytonema.
Boreal Felt Lichen is a globally endangered species known from only a few places in the world. Populations are threatened by air pollution and commercial forestry, and continue to decline. Recent finds in Alaska may be the most promising news for the future of the species. Boreal Felt Lichen is one of the most sensitive species to human disturbances and thus acts as an early warning of ecosystem impacts.
The world population of Boreal Felt Lichen has been listed as critically endangered by the International Union for the Conservation of Nature (IUCN). In Canada, the Atlantic population, which includes Nova Scotia and New Brunswick, was listed as Endangered by COSEWIC (Committee on the Status of Endangered Wildlife in Canada) in 2002. The boreal population on the Island of Newfoundland was listed as Special Concern by COSEWIC in 2002.
Newfoundland hosts the largest population of Boreal Felt Lichen in the world with numbers, according to forestry personnel, probably in the tens of thousands. Unfortunately, recent population modeling indicates the population is declining. The exact causes are unknown, but researchers suspect that acid rain may be a contributing factor. They also suggest that introduced Moose (Alces americanus), with an expanding high density population, have browsed young Balsam Fir (Abies balsamea), the main substrate of Boreal Felt Lichen, to the extent that (regeneration of) habitat is limited. Further, old-growth Balsam Fir, the ideal habitat for Boreal Felt Lichen, is the target of commercial forestry operations.
The known population of Boreal Felt Lichen in Nova Scotia is 180 individuals and although new sites are being found by researchers, old sites are disappearing. One third of lichens monitored since 2005 are dead or dying. At least two locations have been lost due to adjacent forestry operations, although there may be others. Other thalli have been lost due to grazing, possibly by introduced gastropods. Like other cyanolichens, Boreal Felt Lichen is extremely sensitive to air pollution and in North America is predicted to decline in the next 12 years. Large areas of Nova Scotia continue to receive levels of acid deposition in excess of critical loads.
Erioderma pedicellatum is believed to be extirpated from New Brunswick. Despite recent searches by local lichenologists, it hasn’t been found in the province since the early 20th century. Acid rain and fog and air pollution have likely degraded the habitat to the point where it cannot survive there.
In August, 2007, several thalli of Boreal Felt Lichen were collected in Denali National Park and Preserve and later in Denali State Park in Alaska. This was the first collection in western North America and marks a significant range extension. The significance of this find is yet to be understood. The possibility of a larger population in western North America increases the hope for the survival of this species.
Boreal Felt Lichen is sensitive to anthropogenic impacts. The species provides an early warning of human perturbations on the environment. The fate of the global population of Boreal Felt Lichen is uncertain. With only two locations in Europe, survival there is uncertain. Air pollution and commercial forestry continue to be threats in eastern Canada. Only increased effort to reduce threats can ensure the survival of this species.
Results of general status assessment
Just over half of Canada’s 861 species of macrolichens have Canada ranks of Secure (53%, 468 species; figure 6 and table 6), while 12% have Canada ranks of May Be At Risk (100 species), 8% have Canada ranks of Sensitive (68 species) and 1% have Canada ranks of At Risk (five species). Less than 1% of macrolichens have Canada ranks of Extirpated (one species) and none have Canada ranks of Extinct. Less than 1% of macrolichen species have a Canada rank of Exotic (one species). Finally, 25% of Canada’s macrolichens have Canada ranks of Undetermined (211 species) and 1% have Canada ranks of Not Assessed (seven species).
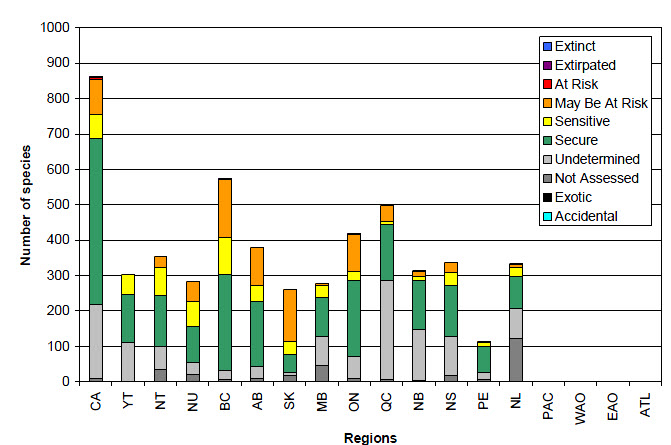
Long description for Figure 6
Figure 6 shows results of the general status assessments for macrolichen species in Canada in the Wild Species 2010 report. The bar graph shows the number of macrolichen species ranked as Extinct, Extirpated, At Risk, May Be At Risk, Sensitive, Secure, Undetermined, Not assessed, Exotic, and Accidental in Canada, each province and territory and the 4 oceanic regions. Of the 861 species occurring in Canada, 1 was ranked as Extirpated, 5 as At Risk, 100 as May Be At Risk, 68 as Sensitive, 468 as Secure, 211 as Undetermined, 7 as Not Assessed, and 1 as Exotic. Of the 304 species occurring in the Yukon, 58 were ranked as Sensitive, 136 as Secure, and 110 as Undetermined. Of the 354 species occurring in the Northwest Territories, 31 were ranked as May Be at Risk, 78 as Sensitive, 145 as Secure, 66 as Undetermined, and 34 as Not Assessed. Of the 283 species occurring in Nunavut, 57 were ranked as May Be at Risk, 70 as Sensitive, 102 as Secure, 34 as Undetermined, and 20 as Not Assessed. Of the 573 species occurring in British Columbia, 1 was ranked as Extirpated, 2 as At Risk, 162 as May Be At Risk, 104 as Sensitive, 273 as Secure, 24 as Undetermined, 6 as Not Assessed, and 1 as Exotic. Of the 380 species occurring in Alberta, 109 were ranked as May Be at Risk, 43 as Sensitive, 186 as Secure, 33 as Undetermined, and 9 as Not Assessed. Of the 260 species occurring in Saskatchewan, 146 were ranked as May Be at Risk, 37 as Sensitive, 52 as Secure, 7 as Undetermined, and 18 as Not Assessed. Of the 278 species occurring in Manitoba, 1 was ranked as At Risk, 5 as May Be at Risk, 33 as Sensitive, 111 as Secure, 82 as Undetermined, and 46 as Not Assessed. Of the 418 species occurring in Ontario, 2 were ranked as Extirpated, 1 as At Risk, 102 as May Be At Risk, 26 as Sensitive, 216 as Secure, 61 as Undetermined, and 10 as Not Assessed. Of the 498 species occurring in Quebec, 45 were ranked as May Be at Risk, 9 as Sensitive, 157 as Secure, 281 as Undetermined, and 6 as Not Assessed. Of the 314 species occurring in New Brunswick, 1 was ranked as At Risk, 16 as May Be at Risk, 11 as Sensitive, 138 as Secure, 145 as Undetermined, and 3 as Not Assessed. Of the 337 species occurring in Nova Scotia, 1 was ranked as At Risk, 27 as May Be at Risk, 37 as Sensitive, 145 as Secure, 110 as Undetermined, and 17 as Not Assessed. Of the 114 species occurring in Prince Edward Island, 4 were ranked as May Be at Risk, 10 as Sensitive, 73 as Secure, 21 as Undetermined, and 6 as Not Assessed. Of the 333 species occurring in Newfoundland and Labrador, 1 was ranked as At Risk, 8 as May Be at Risk, 26 as Sensitive, 92 as Secure, 83 as Undetermined, 122 as Not Assessed, and 1 as Exotic. There were no species listed as occurring in the oceanic regions.
| Canada rank | Number and percentage of species in each rank category |
|---|---|
| 0.2 Extinct | 0 (0%) |
| 0.1 Extirpated | 1 (0%) |
| 1 At Risk | 5 (1%) |
| 2 May Be At Risk | 100 (12%) |
| 3 Sensitive | 68 (8%) |
| 4 Secure | 468 (53%) |
| 5 Undetermined | 211 (25%) |
| 6 Not Assessed | 7 (1%) |
| 7 Exotic | 1 (0%) |
| 8 Accidental | 0 (0%) |
| Total | 861 (100%) |
Threats to Canadian macrolichens
The major threats to Canadian macrolichens are loss of habitat and poor air quality. Loss of habitat can be caused by many different events such as forestry practices, especially clear-cutting and old-growth forest removal; both practices are especially detrimental to lichen biodiversity. Lichens that lack asexual propagules have limited dispersal abilities and may not recolonize disturbed habitats. Growth rates of macrolichens are slow, so once the lichen is removed from its environment, it may take years to return, if ever. Strong wind storms such as along coastal regions, can destroy lichen habitat and the lichens themselves. Flooding will kill lichens if water levels remain high for extended periods of time. Mining operations and clear-cut logging alter habitat so that the microenvironments of lichens will no longer support a rich diversity of lichens.
Air quality in industrial areas is compromised by emissions such as sulphur dioxide, nitrogen oxides and a variety of other noxious compounds. Most lichens are sensitive to these pollutants and will eventually die. Acid rain is harmful to lichens and has caused habitat degradation in Atlantic Canada. Lichens in heavily populated areas in southern Canada are most at risk as their habitat is altered by urban development and air quality.
Conclusion
This general status assessment of 861 of Canada’s macrolichens is an important achievement, involving input from lichenologists, professional biologists and government departments across the country using the most current information to assess the distribution and general status of the macrolichens of Canada. This first compilation of macrolichens is a starting point for awareness of lichens and impetus to continue to build the lichen lists in all provinces and territories in Canada. The macrolichen list will form a platform for further lichen inventories, especially in locations where knowledge is lacking, that will lead to new lichens discovered and new lichens described as well as expanding the provincial and territorial lists to include crustose or microlichens.
Further information
Brodo, I. M., Sharnoff, D. S. and Sharnoff, S. 2001 Lichens of North America. Yale University Press, New Haven & London: 795 pp.
Esslinger, T. L. 2009. A cumulative checklist for the lichen-forming, lichenicolous and allied fungi of the continental United States and Canada. North Dakota State University: (Accessed March 8, 2010).
Goward, T., McCune, B. and Meidinger, D. 1994. The lichens of British Columbia, Illustrated keys. Part 1 - Foliose and squamulose species. Special report series 8, research program, British Columbia Ministry of Forests, Victoria: 181 pp.
Goward, T. 1999. The lichens of British Columbia, Illustrated keys. Part 2 - Fruticose species. British Columbia Ministry of Forests, Victoria: 319 pp.
Hinds, J. W. and Hinds, P. L. 2007. The macrolichens of New England. Memoirs of the New York Botanical Garden, volume 96. The New York Botanical Garden Press: 584 pp.
McCune, B. and Geiser, L. 2009. Macrolichens of the Pacific Northwest. Oregon State University Press, Corvallis: 464 pp.
Nash, T. H. III. (editor). 2008. Lichen biology, second edition. Cambridge University Press, Cambridge: 486 pp.
Purvis, W. 2000. Lichens. Smithsonain Institution Press, Washington in association with the Natural History Museum, London.
Richardson, D. H. S. 1992. Pollution monitoring with lichens. Naturalists’ Handbooks 19, Richmond Publishing Co. Ltd., Slough, England: 76 pp.
References
Cameron, R. P., Neily, T., Clayden, S. R. and Maass, W. S. G. 2009. COSEWIC Draft Status Report on Vole Ears - Erioderma mollissimum. Committee on the Status of Endanagered Wildlife in Canada. Ottawa.
Environment Canada. 2004. Canadian acid deposition science assessment: summary of key results. Environment Canada, Ottawa.
Goudie, I. R., Scheidegger, C., Hanel, C., Munier, A. and Conway, E. 2010. Population model for the globally rare boreal felt lichen (Erioderma pedicellatum) in Newfoundland. Endangered Species Research, in prep.
Holien, H. 2006. Trøderlav (Erioderma pedicellatum) [PDF, 595 KB]. Artsdatabankens Faktaark nr. 3. (Accessed March 8, 2010).
Keeping, B. and Hanel, C. 2006. A 5 year (2006-2011) management plan for the Boreal Felt Lichen (Erioderma pedicellatum) in Newfoundland and Labrador [PDF, 946.5 KB]. Wildlife Division, Department of Environment and Conservation, CornerBrook. (Accessed March 8, 2010).
Nash, T. H. III. 2008. Lichen sensitivity to air pollution. In Lichen Biology, second edition (T. H. III. Nash, editor). Cambridge University Press, Cambridge: 299-314.
Nelson, P., Walton, J. and Roland, C. 2009. Erioderma pedicellatum (Hue) Jørg, P. M. - New to the United States and Western North America, Discovered in Denali National Park and Preserve and Denali State Park, Alaska. Evansia 25: 19-23.
New Brunswick Department of Natural Resources. 2007. Recovery strategy for the Boreal Felt Lichen (Erioderma pedicellatum) in New Brunswick. Natural
Resources, Fredericton. Recovery Strategy for the Boreal Felt Lichen (Erioderma pedicellatum), Atlantic Population,
in Canada [PDF, 1.46 MB] (Accessed March 8, 2010).
Tehler, A. and Wedin, M. 2008. Systematics of lichenized fungi. In Lichen biology, second edition (T. H. III. Nash, editor). Cambridge University Press, Cambridge: 336-352.
Vitt, D. H., Marsh, J. E. and Bovey, R. B. 1988. Mosses, lichens & ferns of Northwest North America. Lone Pine Publishing, Edmonton, Alberta, and University of Washington Press, Seattle: 296 pp.