Recovery Strategy for the Common Nighthawk (Chordeiles minor) in Canada - 2016
Common Nighthawk

Photo: © Zoe Crysler and Danielle Fife
2016
- Preface
- Acknowledgments
- Executive Summary
- Recovery Feasibility Summary
- 1. COSEWIC Species Assessment Information
- 2. Species Status Information
- 3. Species Information
- 4. Threats
- 5. Population and Distribution Objectives
- 6. Broad Strategies and General Approaches to Meet Objectives
- 7. Critical Habitat
- 8. Measuring Progress
- 9. Statement on Action Plans
- 10. References
- Appendix A: Effects on the Environment and Other Species
- Appendix B: Additional research for known and suspected threats to the Common Nighthawk, its prey, and their habitats
Recovery Strategy for the Common Nighthawk (Chordeiles minor) in Canada - 2016
Environment Canada. 2016. Recovery Strategy for the Common Nighthawk (Chordeiles minor) in Canada. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. vii + 49 pp.
For copies of the recovery strategy, or for additional information on species at risk, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry.
Cover illustration: © Zoe Crysler and Danielle Fife
Également disponible en français sous le titre « Programme de rétablissement de l'Engoulevent d'Amérique (Chordeiles minor) au Canada [Proposition]»
Content (excluding the illustrations) may be used without permission, with appropriate credit to the source.
The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of recovery strategies for listed Extirpated, Endangered, and Threatened species and are required to report on progress within five years after the publication of the final document on the SAR Public Registry.
The Minister of the Environment and Minister responsible for the Parks Canada Agency is the competent minister under SARA for the Common Nighthawk and have prepared this strategy, as per section 37 of SARA. To the extent possible, it has been prepared in cooperation with the Provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec (Ministère des Forêts, de la Faune et des Parcs), New Brunswick, Prince Edward Island, Nova Scotia, Newfoundland and Labrador, as well as the territories of Yukon and Northwest Territories and others as per section 39(1) of SARA.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy and will not be achieved by Environment Canada and the Parks Canada Agency, or any other jurisdiction alone. All Canadians are invited to join in supporting and implementing this strategy for the benefit of Common Nighthawk and Canadian society as a whole.
This recovery strategy will be followed by one or more action plans that will provide information on recovery measures to be taken by Environment Canada and the Parks Canada Agency and other jurisdictions and/or organizations involved in the conservation of the species. Implementation of this strategy is subject to appropriations, priorities, and budgetary constraints of the participating jurisdictions and organizations.
The recovery strategy sets the strategic direction to arrest or reverse the decline of the species, including identification of critical habitat to the extent possible. It provides all Canadians with information to help take action on species conservation. When the recovery strategy identifies critical habitat, there may be future regulatory implications, depending on where the critical habitat is identified. SARA requires that critical habitat identified within federal protected areas be described in the Canada Gazette, after which prohibitions against its destruction will apply. For critical habitat located on federal lands outside of federal protected areas, the Minister of the Environment must either make a statement on existing legal protection or make an order so that the prohibition against destruction of critical habitat applies. For critical habitat located on non-federal lands, if the Minister of the Environment forms the opinion that any portion of critical habitat is not protected by provisions in or measures under SARA or other Acts of Parliament, and not effectively protected by the laws of the province or territory, SARA requires that the Minister recommend that the Governor in Council make an order to extend the prohibition against destruction of critical habitat to that portion. The discretion to protect critical habitat on non-federal lands that is not otherwise protected rests with the Governor in Council.
This recovery strategy was prepared by Julie McKnight, Krista Baker (Environment Canada, Canadian Wildlife Service (EC-CWS) - Atlantic Region) and Andrew Horn. Early drafts were reviewed by members of the National landbird recovery planning team [Andrew Boyne, Peter Thomas, Becky Whittam (EC-CWS – Atlantic Region), Vincent Carignan, Gilles Falardeau, Mireille Poulin (EC-CWS – Quebec Region), François Fournier and Junior Tremblay (EC – Science & Technology – Quebec region), Kevin Hannah, Rich Russell, Kathy St. Laurent, Ken Tuininga, Russ Weeber (EC-CWS – Ontario Region), Connie Downes, Manon Dubé, Carolyn Seburn, (EC-CWS – National Capital Region), Mark Bidwell, Donna Bigelow, Lisa Mahon, Lisa Pirie, Samantha Song, Steve VanWilgenburg, Karl Zimmer (EC-CWS - Prairie & Northern Region), Saleem Dar, Wendy Easton, Megan Harrison, Craig Machtans, Nancy Mahony, Wendy Nixon, Pam Sinclair (EC-CWS - Pacific & Yukon Region)]. Other contributors provided detailed comments on this recovery strategy: Adam Smith (EC-CWS-National Capital region), Stephen Davis and Samuel Haché (EC-CWS-Prairie & Northern Region). Acknowledgement and thanks are also given to all other parties that provided advice and input used to help inform the development of this recovery strategy including various Aboriginal Organizations and individuals, provincial and territorial governments, other federal departments (e.g., Department of National Defence), landowners, citizens, and stakeholders.
Environment Canada would like to acknowledge the contribution of the thousands of volunteers who generously donate their time and expertise to bird monitoring programs throughout North America, as well as the many professional biologists and technicians working for various government agencies and non-government organizations in Canada and the United States who helped to establish, design, run, and analyze the Breeding Bird Survey and Breeding Bird Atlas results.
Common Nighthawk is a medium-sized mottled grey-brown bird usually seen or heard overhead at dusk and dawn, with long pointed white-barred wings and unique bounding flight. The species is listed as Threatened on Schedule 1 of the federal Species at Risk Act (SARA), because of significant long- and short-term declines across the portion of its range covered by bird population monitoring programs. The species is known to breed in every province and territory except Nunavut. Ten percent of the global population of Common Nighthawks is estimated to breed in Canada (Rich et al. 2004).
Common Nighthawk nests on the ground in open land or forest clearings, and on gravel roofs in cities. Foraging nighthawks require open areas with flying insects and this need is met in a wide range of habitats. Almost any site with shade, camouflage from predators, and an unobstructed flight path for access from the air can be used for roosting. There is virtually no information on habitat needs during migration and wintering habitat is not well known except that a variety of open areas are used for foraging much like at other times of year.
Many threats to Common Nighthawk have been postulated, but data are still lacking to directly link a single threat to observed population declines. The threats to the species are found within the following categories: natural system modifications (e.g., reduced insect prey and fire suppression), habitat loss and degradation, climate change and severe weather, accidental mortality, pollution, and problematic native and invasive non-native species.
The recovery of the Common Nighthawk in Canada is considered feasible; however, there are several unknown factors associated with its potential for recovery. Despite these unknowns and in keeping with the precautionary principle, this recovery strategy has been prepared as per section 41(1) of SARA.
The short-term population objective for the Common Nighthawk in Canada is to halt the national decline by 2025 (i.e., 10 years after this recovery strategy is posted on the Species at Risk Public Registry), while ensuring the population does not decrease more than 10% over this time. The long-term (after 2025) population objective is to ensure a positive 10-year population trend for the Common Nighthawk in Canada. The distribution objective for Common Nighthawk is to maintain the current extent of occurrence (i.e., the area that encompasses the geographic distribution of all known populations) in Canada.
Broad strategies to be taken to address the threats to the survival and recovery of Common Nighthawk are presented in section 6.2: Strategic Direction for Recovery.
At present, the available information is not adequate to identify the habitat necessary for the survival or recovery of the Common Nighthawk in Canada. A schedule of studies is included to obtain the information needed for the identification of critical habitat.
One or more action plans for Common Nighthawk will be posted on the Species at Risk Public Registry within the five years following the posting of this recovery strategy.
Based on the following four criteria outlined by the Government of Canada (2009), there are unknowns regarding the feasibility of recovery for Common Nighthawk. In keeping with the precautionary principle, a recovery strategy has been prepared as per section 41(1) of SARA, as would be done when recovery is determined to be feasible. This recovery strategy addresses the unknowns surrounding the feasibility of recovery.
The species was listed as Threatened in Canada under Schedule 1 of SARA (c. 29) in 2010. Under provincial endangered species legislation, Common Nighthawk is listed as Special Concern in Ontario (ESA 2007) and Threatened in Manitoba (C.C.C.S.M. c. E111 1990), New Brunswick (S.N.B. 2012, c. 6), Nova Scotia (ESA 2002), and Newfoundland and Labrador (SNL2001 CHAPTER E-10.1 [Amended: 2004 cL-3.1 s27; 2004 c36 s11]). In Quebec, the species is listed on the "Liste des espèces susceptibles d'être désignées menacées ou vulnérables" (list of wildlife species likely to be designated threatened or vulnerable). This list is produced according to the "Loi sur les espèces menacées ou vulnérables" (RLRQ, c. E-12.01) (Act respecting threatened or vulnerable species) (CQLR, c. E-12.01). The species is not currently listed in Yukon, the Northwest Territories, British Columbia, Alberta, Saskatchewan, or Prince Edward Island. Although the species is not listed in the USA, it is considered an imperilled (S2) or critically imperilled (S1) breeder in Connecticut, Rhode Island, New Hampshire, Vermont, Delaware, and Massachusetts. Table 1 provides conservation status ranks for Common Nighthawk.
| Global (G) Rank |
National (N) Rank | Sub-national (S) RankNotebof Table 1 |
|---|---|---|
| G5 (secure) |
Canada: N4B (apparently secure) United States: N5B (secure) |
Yukon (S2B) Northwest Territories (S2B) British Columbia (S4B) Alberta (S4) Saskatchewan (S4S5B, S4/S5M) Manitoba (S3B) Ontario (S4B) Quebec (S3) New Brunswick (S3B) Prince Edward Island (S1B) Nova Scotia (S3B) Newfoundland (NR) and Labrador (S2B) |
Common Nighthawk is a medium-sized, slender bird with very long, pointed wings, most commonly heard overhead near dawn or dusk. Its long pointed wings, erratically bounding flight, white bar between the bend and tip of the wing, and nasal peent call are distinctive. Territorial males produce a distinct booming sound caused by air rushing through feathers. This sound is characteristic to Common Nighthawks and is an important indicator of breeding activity. When resting on the ground or a tree branch, it lies flat against the substrate and is well camouflaged by its mottled brown, grey, and black plumage. Males have a white tail band as well as a white throat patch, which is buffy in females and mottled brown in juveniles. Common Nighthawk is most easily distinguished from similar birds, such as Eastern Whip-poor-will (Antrostomus vociferous), by the white bar across its wings and its call (Brigham et al. 2011).
The breeding range of Common Nighthawk includes most of North and Central America (Figure 1). Approximately 37% of the species' breeding range is found in Canada (Rich et al. 2004). It is known to breed in every province and territory except Nunavut, and in every U.S. state except Alaska and Hawaii. It winters in the northeastern half of South America (Figure 1), where its distribution is poorly known, although it might be especially frequent in southern Brazil and eastern Ecuador and Peru (COSEWIC 2007).

Long description for Figure 1
Figure 1 illustrates the distribution of the species in North, Central and South America according to range type. Their breeding range spreads from northern Canada to southern Mexico and parts of Central America. Their migrating range is mainly in Mexico, the Caribbean Islands, and Central America. Their wintering range is limited to South America; from Colombia to north-eastern Argentina.
Ten percent of the global population of Common Nighthawks is estimated to breed in Canada (Rich et al. 2004) and the species' population in Canada, based on Breeding Bird Survey results, was estimated in COSEWIC (2007) as 400,000 adults and underwent an 80% decline between 1968 and 2005 (average: -4.2% per year; Downes et al. 2005). More recently, the Partners in Flight Population Estimates database was updated and now provides the most comprehensive information on North American landbirds. The Canadian population of Common Nighthawks is now estimated to be 900,000 adults (Partners in Flight Science Committee 2013). This does not represent an actual increase in the population but rather is the result of newer analytical techniques and a refined detection distance used to estimate density.
A new approach to produce population trend estimates from Breeding Bird Survey data provides more precise trend estimates and the results show an annual population change of -3.58% (95% credible intervals (CI): -5.33 to -2.1) from 1973 to 2012, and -2.265% (CI: -5.2 to 1.5) from 2002-2012 (Environment Canada 2014a). This annual change indicates that the population declined by almost 76% between 1973 and 2012 and by approximately 20% between 2002 and 2012 (Smith pers. comm. 2014).
Map: © Environment Canada 2014
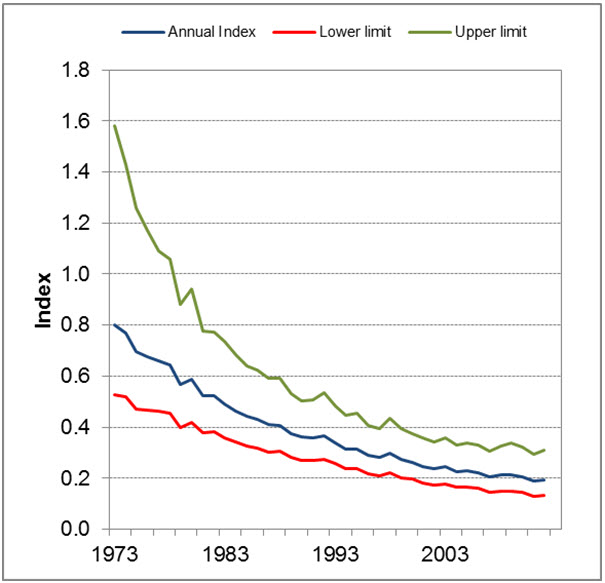
Long description for Figure 2
Figure 2 shows three lines on a graph with years on the horizontal axis and an index from 0 to 1.8 in bounds of 0.2 on the vertical axis. The annual index begins at 0.8 in 1973, slowly descends to 0.2 by 2006. The lower limit of the trend starts at 0.55 in 1973 a lowers progressively to 0.15 by 2006. The third line, the upper limit, begins at 1.6, drops suddenly to 1.0 by 1979 and continues to fall steadily down to 0.3 by 2006.
There are fundamental problems with interpreting Breeding Bird Survey and other point-count survey data for Common Nighthawk. It should be emphasized that Common Nighthawks are not well represented by standard avian point counts because of their crepuscular behavior (i.e., most active at dawn and dusk). Also, the Breeding Bird Survey data does not sample the species' entire range at random, having lower coverage in the boreal forest, urban areas, and remote rocky areas (Status of Birds in Canada 2011, Haché et al. 2014). The majority of Breeding Bird Survey routes tend to be located in southern and disturbed areas of Canada which may bias population estimates of species with northern distributions such as Common Nighthawk (Machtans et al. 2014). Breeding Bird Survey data also tends to overestimate Common Nighthawk densities due to road-side bias (Haché et al. 2014). For these reasons, there is uncertainty in estimating population size for this species.
Regional surveys suggesting declines are reviewed in COSEWIC (2007). For a list of current and past surveys that monitor Common Nighthawk populations refer to section 6.1: Actions Already Completed or Currently Underway.
Common Nighthawks require open ground or clearings for nesting. The species breeds in a wide range of open habitats including sandy areas (e.g., dunes, eskers, and beaches), open forests (e.g., mixedwood and coniferous stands, burns, and clearcuts), grasslands (e.g., short-grass prairies, pastures, and grassy plains), sagebrush, wetlands (e.g., bogs, marshes, lakeshores, and riverbanks), gravelly or rocky areas (e.g., outcrops, barrens, gravel roads, gravel rooftops, railway beds, mines, quarries, and bare mountain tops and ridges), and some cultivated or landscaped areas (e.g., parks, military bases, airports, blueberry fields, orchards, cultivated fields) (Hunt 2005, Campbell et al. 2006, COSEWIC 2007). There is a wide range in published territory sizes, from less than one hectare to 28 hectares (Brigham et al. 2011). However, home ranges can include isolated foraging and roosting areas that may be greater than six kilometers apart (Fisher et al. 2004, Ng 2009). These home ranges can extend well beyond the defended area and range in size from 3.7 - 259 ha (mean = 86 ha; Ng 2009).
Nests have been observed in close proximity to each other (25 – 75 m apart) which suggests that more than one pair may nest in small patches of suitable nesting habitat (Sutherland 1963). There is evidence that some individuals return to the same general area to nest each year (Campbell et al. 2006, Brigham et al. 2011). The female lays the eggs directly on the substrate, sometimes in a depression or scrape. A wide range of substrates are used (Campbell et al. 2006), and primary microsite characteristics include more open ground cover with low or no vegetation, adequate camouflage from predators, and nearby shade (Ng 2009, Lohnes 2010, Brigham et al. 2011, Allen and Peters 2012). Nestlings often change locations daily, moving greater distances (up to 48 m) as they age, perhaps mainly to seek shade and avoid disturbance from predators (Allen and Peters 2012, Kramer and Chalfoun 2012).
Nighthawks forage in open areas with flying insects during crepuscular periods, although they sometimes forage during the day. This need is met in a wide range of habitats, although open water and artificial lighting are particularly favoured and can attract foraging flocks of a few to hundreds of individuals (Campbell et al. 2006 COSEWIC 2007, Ng 2009). The main prey of Common Nighthawk are beetles (Coleoptera), caddisflies (Trichoptera) and moths (Lepidoptera) (Tyler 1940, Cink 2002), some of which are considered agricultural pests.
Suitable roost sites are most likely important for individual survival (Fisher et al. 2004). Almost any site can be used for roosting, including tree limbs, the ground, fenceposts, or rooftops that have shade from overheating, camouflage from predators, and unobstructed flight paths (Fisher et al. 2004, Campbell et al. 2006, Ng 2009).
Open areas are used during migration but detailed habitat needs during this stage are poorly known. Nighthawks can be seen in flocks of a few and up to thousands of individuals during fall migration, but undertake individual spring migrations (COSEWIC 2007, Brigham et al. 2011), suggesting that specific areas or habitat characteristics are optimal for flight efficiency and/or for foraging during migration. In particular, larger flocks are often associated with particular rivers or coastlines (Brigham et al. 2011). There is virtually no information on habitat needs during the winter except that a variety of open areas are used for foraging much like at other times of year (Brigham et al. 2011).
Limiting factors influence a species' survival and reproduction, and play a major role in the recovery of a species. Common Nighthawk is a long-distance Neotropical migrant that arrives late on the breeding grounds and departs earlier than many other landbirds. This constrains the species to one brood per year and clutch size is small (2 eggs). As with all aerial insectivorous birds, the Common Nighthawk specializes in aerial insects which increases its vulnerability to inclement weather. These life history characteristics contribute to this species' intrinsic sensitivity to changes in their environment.
| Threat Category | Threat | Level of ConcernNotecof Table 2 | Extent | Occurrence | Frequency | SeverityNotedofdTable 2 | Causal CertaintyNote eof Table 2 |
|---|---|---|---|---|---|---|---|
| Changes in Ecological Dynamics or Natural Processes | Reduced availability of insect prey (Loss of insect-producing habitats, insect-breeding temporal mismatch, habitat acidification, pesticides, light pollution, increased extreme weather events) |
High | Widespread | Current | Continuous | Moderate | Medium |
| Changes in Ecological Dynamics or Natural Processes | Fire suppression | Medium | Widespread | Current | Recurrent | Moderate | Medium |
| Habitat Loss or Degradation | Loss of breeding habitat: habitat succession | Medium | Widespread (SE Canada) |
Current | Continuous | Moderate | Medium |
| Habitat Loss or Degradation | Loss of breeding habitat: change in roof construction and materials | Low | Localized (urban areas) |
Current | One-time | Low | Medium |
| Habitat Loss or Degradation | Loss of breeding habitat: residential and commercial development | Unknown | Widespread | Current | Continuous | Unknown | Low |
| Habitat Loss or Degradation | Loss of breeding habitat: agriculture | Unknown | Widespread (S Canada) |
Current | Continuous | Unknown | Low |
| Habitat Loss or Degradation | Loss of breeding habitat: logging and wood harvesting | Unknown | Widespread | Current | Continuous | Unknown | Low |
| Habitat Loss or Degradation | Loss of non-breeding habitat | Unknown | Widespread | Current | Continuous | Unknown | Low |
| Climate and Natural Disasters | Temperature extremes and storms | Medium | Widespread | Current | Seasonal | Moderate | Medium |
| Climate and Natural Disasters | Habitat shifting and alteration | Unknown | Widespread | Current | Continuous | Unknown | Low |
| Accidental Mortality | Collisions with vehicles, planes, and human structures | Medium | Localized | Current | Recurrent | Moderate | Medium |
| Pollution | Pesticides (direct effects) | Unknown | Localized | Current | Seasonal | Unknown | Low |
| Pollution | Mercury | Unknown | Widespread (E Canada) |
Current | Continuous | Unknown | Low |
| Pollution | Acid precipitation | Unknown | Widespread (E Canada) |
Current | Continuous | Unknown | Low |
| Exotic, Invasive, or Introduced Species/Genome | Problematic native and invasive non-native species | Unknown | Widespread | Current | Continuous | Unknown | Low |
Many threats to Common Nighthawk have been identified, but none have been directly linked to population declines of the species. At this time, it is unknown whether these population declines are driven by one particular threat or by cumulative effects of numerous threats. They are listed as above in the threat assessment table and are described in more detail below.
Reduced availability of insect prey (loss of insect-producing habitats, insect-breeding temporal mismatch, habitat acidification, pesticides, light pollution, increased extreme weather events)
Populations of aerial insectivores are showing dramatic declines, particularly in northeastern North America (Nebel et al. 2010). The trait common to all species in this diverse group is insectivory, which has led multiple researchers to implicate a reduction in available insect prey in breeding, migratory, and/or wintering areas as a probable contributing factor in the declining population trends (Nebel et al. 2010, Paquette et al. 2014).
Insect populations are exhibiting significant declines worldwide. A recent review of global faunal population trends, noted that 33% of all insects with available IUCN-documented population trends were declining and many also exhibited range retractions (Dirzo et al. 2014). These declines are considered a global pattern, but are more severe in heavily disturbed locations, such as the tropics (Dirzo et al. 2014). The possible causes for reduced availability of insect prey are identified and described below.
Birds often exhibit a strong synchronization between their reproductive timing (i.e., hatching) and peak food abundance, but climate change has caused the timing of peaks in some insects to advance (Both et al. 2010). Warming is less severe in Common Nighthawk's wintering areas than in their breeding areas and they may experience migration cues at dates that are too late for them to arrive at breeding sites at the optimal time (Jones and Cresswell 2010). As a result, climate change is creating a temporal mismatch between reproduction and maximal prey abundance (i.e., insects) for species that are not adapting to the changing climate at the same rate as their prey (Strode 2003). Both et al. (2006) found that an aerial insectivore in the Netherlands, the Pied Flycatcher (Ficedula hypoleuca), had declined 90% between 1987 and 2003 in areas where the prey peaked too early in the breeding season to provide adequate food for nestlings. Great Tits (Parus major) have exhibited a mismatch between optimal timing of nestlings and peak caterpillar biomass as a result of recent warming (Visser et al. 2006). The weight of chicks and the number of chicks that fledged were both affected by their timing in relation to this peak (Visser et al. 2006). An insect-breeding temporal mismatch has also been linked to the population declines of migrant birds across Europe (Møller et al. 2008, Saino et al. 2011), and is believed to be contributing to the declines of other avian species heavily reliant on invertebrates, such as Rusty Blackbird (Euphagus carolinus) (McClure et al. 2012).
Populations of migratory birds that exhibit long-distance migrations and breed in seasonal habitats (such as forests) are more vulnerable to climate change because the temporal mismatch is both more likely and more severe (Both et al. 2006, 2010). Although no species-specific data are currently available, Common Nighthawk is an insectivore that migrates long distances so a climate-induced mismatch between breeding and prey availability is plausible. Areas where the species breeds and forages in seasonal habitats (e.g., open forests) may be more susceptible to this threat (Both et al. 2010).
Many insects are limited to specific habitats for some part of their life cycle and any process that diminishes these habitats may harm them. Over 90% of insect groups considered threatened are impacted by habitat loss or degradation (Price et al. 2011). A number of human activities alter or destroy natural habitats necessary for particular insect life stages, including wetland drainage and peat extraction, intensive agriculture, wetland destruction, industrial activities, and urban development (U.S. Bureau of Land Management 1978, Price et al. 2011, Benton et al. 2002, Brooks et al. 2012). Foster (1991) noted the drainage of wetlands and peat extraction as a significant threat facing insect populations. Benton et al. (2002) found arthropod numbers were lower when farming was more intense, that insect abundance was significantly related to agricultural practices, and that bird density was significantly related to insect abundance in the previous year. Differences in Diptera abundances change between landscapes as the breeding season of Tree Swallows (Tachycineta bicolor) progresses, setting up the potential for an 'ecological trap' in intensively landscaped areas (Paquette et al. 2013). Paquette et al. (2014) found that agriculture intensification did not influence adult body mass, but had a negative effect on the number of chicks fledged.
The effects of habitat loss for insects are not restricted to Common Nighthawk's breeding range, but could also be affecting their migrating and wintering range.
Since the 1980s, there has been a substantial decline in the rate of acid deposition, but acidifying compounds (e.g., sulphur dioxide and nitrogen oxide) are still being released into the environment (Shannon 1999, Environment Canada 2014b). Acidification of surface water can reduce the abundance and diversity of flying insects that are aquatic for part of their life cycle (Graveland 1998). Although some of Common Nighthawk's prey (e.g., some beetles and large moths) do not have an aquatic phase, abundance of caddisflies and populations of alternative prey may be affected by habitat acidification. Reduced reproductive success of Tree Swallows nesting near acidified wetlands in Ontario was linked to changes in available calcium-rich prey for nestlings (Blancher and McNicol 1991) and acidification of forests was implicated in the decline of Wood Thrush (Hylocichla mustelina) (Hames et al. 2002). Nevertheless, a study in central Ontario showed no difference in forest songbird productivity between acidified and non-acidified sites (Mahony et al. 1997). At present, there is no evidence to support a range-wide effect of reduced insect prey as a result of habitat acidification, but it may have implications for Common Nighthawks in areas with local, severe acid deposition and in eastern North America where soil buffering is relatively poor due to low pH.
Aerial insectivores breeding in North America and exhibiting population declines have wintering ranges that consist, or partially consist, of countries with high expenditures on insecticides; insecticide expenditures in wintering ranges was the best, significant predictor of the index of species abundance (Nocera et al. 2014). Nevertheless, the direct mechanisms for the population declines (e.g., reduced insect availability, lethal exposure) are unknown (Nocera et al. 2014).
Most organochlorine pesticides (chemicals in the same family as dichlorodiphenyltricholoroethane – DDT) have been banned in North America for decades, but there is some indication that Neotropical migrant insectivores are still being exposed to organochlorine pesticides throughout their ranges (Sager 1997, Klemens et al. 2000). These chemicals can have long-lasting effects on insect communities and thus the birds that rely on them. Chimney Swift (Chaetura pelagica) dietary records confirm a marked decrease in beetles (Coleoptera) and an increase in true bugs (Hemiptera) temporally correlated with a steep rise in DDT and its metabolites. Nocera et al. (2012) argued that DDT caused declines in beetles and dramatic (possibly permanent) shifts in insect communities, resulting in a nutrient-poor diet and ultimately a declining Chimney Swift population.
The harmful effects of chemical insecticides have led to the increased use of biological insecticides. Currently, insecticides used for forestry operations in Canada are mainly biological (Bacillus thuringiensis var. kurstaki - Btk) and target larval Lepidoptera such as Jack Pine Budworm (Choristoneura pinus) and Spruce Budworm (C. fumiferana). The average area sprayed per year with Btk across Canada's forests between 1988 and 2000 was 273,440 ha (range: 73,209-855,535 ha) (NFD 2014). In 2012, Btk was sprayed in the forests of four Canadian provinces: Quebec (98,044 ha), Manitoba (828 ha), Saskatchewan (15,639 ha), and British Columbia (116,012 ha) (NFD 2014). On average, Quebec sprays the most forest area with Btk per year (1988-2012) (NFD 2014). Although many microbial insecticides are considered non-toxic to birds, their indirect effects caused by changes in available prey items remains inconclusive. A 12,803 ha area of Vancouver Island, British Columbia, exhibited no difference in species richness or relative abundance of songbirds one year after being sprayed with Btk to control for Gypsy Moth (Lymantria dispar) (Sopuck et al. 2002). Holmes (1998) found that the nestling survival and growth of Tennessee Warblers (Vermivora peregrina) were unaffected by sites treated with Btk in Ontario, and although nests in sprayed sites had smaller clutches, smaller broods, and lower hatch rates, the differences were not significant. Other studies have found significant indirect impacts of microbial pesticides to birds. Spruce Grouse (Dendragapus canadensis) chicks had significantly slower growth rates in an area treated with Btk in Ontario compared to chicks raised in study sites not treated with Btk (Norton et al. 2001). Norton et al. (2001) attributed this to the reduction in available Lepidoptera larvae as a result of spraying. In France, House Martins (Delichon urbicum) at sites treated with Bacillus thuringiensis var. israelensis (Bti) exhibited a change in diet from Nematocera (which are Bti-sensitive), spiders, and dragonflies (Nematocera predators) to flying ants (Poulin et al. 2010). This dietary change resulted in lower clutch size and fledgling survival (Poulin et al. 2010). Bti may be used for mosquito and black fly control programs throughout Common Nighthawk's breeding, migratory, and wintering range.
Neonicotinoid insecticides were introduced in the 1990s and although their rates of use are poorly known across Common Nighthawk's range, nearly 11 million hectares of cropland across the Canadian Prairies were estimated to be treated with neonicotinoids (Main et al. 2014). Neonicotinoids are generally used on agricultural lands, but have been detected in wetlands (Main et al. 2014) and waterways in Canada (Environment Canada 2011, Xing et al. 2013). Mineau and Palmer (2013) suggested that the effects of neonicotinoids to birds may not be limited to the farm scale, but likely expand to the watershed or regional scale. Neonicotinoids are adversely affecting insect populations and in 2013 the European Food Safety Authority declared that they posed 'unacceptable' risk to insects (Goulson 2014). In the Netherlands, neonicotinoid concentrations in surface waters were correlated with the declines in farmland insectivorous birds (Hallmann et al. 2014). Hallmann et al. (2014) suggested these declines were likely caused by a reduction of insect prey as a result of insecticide use. The indirect effects of these insecticides have also been noted in Skylark (Alauda arvensis), Yellowhammer (Emberiza citronella), Whinchat (Saxicola rubertra), Reed Bunting (Emberiza schoeniclus), and Corn Bunting (Miliaria calandra) (Boatman et al. 2004, Gibbons et al. 2014).
Many insects that are prey species for Common Nighthawk (beetles, caddisflies, and moths) are known to be drawn to artificial lights (Bruce-White and Shardlow 2011). Insects depend on natural light cycles to complete several stages of their life cycle. There is evidence that artificial light is affecting the breeding and survival of caddisflies (Trichoptera) and other aquatic invertebrates. Artificial light interferes with the cues that such species rely on for adult emergence and disorients adults and large mortality events have been recorded around light sources close to rivers (Bruce-White and Shardlow 2011). Artificial lighting is also likely to have adverse impacts on feeding, reproduction, and movements of insects. This can result in fragmentation and/or a decline in the populations of certain species and changes to the community of insects available to aerial insectivores (Bruce-White and Shardlow 2011, Davies et al. 2012).
Nighthawks and other aerial insectivores exploit patches of flying insects concentrated by frontal systems (Russell 1999, Taylor 2009). For example, Russell and Wilson (1997) counted 85 Common Nighthawks at a convergence zone in Florida during a single 10-minute point count. These frontal systems may be disrupted by increasing storm severity resulting from climate change. On the other hand, storm intensification may increase available habitat for Common Nighthawk through increased forest fires and windthrow.
More frequent and extended droughts as a result of climate change are expected to result in local and possibly regional declines of some insect species, particularly those reliant on aquatic environments (Haile 2000, Boulton and Lake 2008). But, droughts may also lead to dramatic increases (outbreaks) in other insect species (Haile 2000).
Fire in a natural system may provide a mosaic of shifting breeding habitat for Common Nighthawks. North American forests evolved under the influence of fire and the boreal forest, in particular, is strongly connected to the fire regime (FAO 2006). For much of the 20th century, suppression of wildfire to protect forest resources and rural communities was the management norm. In fact, the effectiveness of fire suppression programs in Canada is such that 97 percent of all forest fires are contained before they reach 200 hectares in size (Stocks et al. 2003). Even in the large boreal forest, Cumming (2005) concluded that fire suppression by initial attack has significantly impacted the area burned over recent decades and suggests this will persist into the foreseeable future. Decades of fire suppression have resulted in longer fire intervals with reduced open areas that are used by breeding Common Nighthawks. Availability of habitat in mesic prairie systems is also reduced because of natural succession to shrubland in the absence of natural fire regimes (McCracken 2005). Climate change modelling predicts an increase in fire frequency and intensity in Canada's boreal region (Natural Resources Canada 2013) which would lead to an increase in open habitat for Common Nighthawks. However, the ultimate benefit to Common Nighthawks is unknown especially because any increase in fire frequency may be met with increased fire suppression efforts by forest managers.
Prescribed burns are a forest management tool that can create optimal Common Nighthawk habitat. However, in Canada, prescribed fire as a management technique is relatively uncommon on a landscape scale (Taylor 1998) and is used mainly on Parks Canada and First Nations lands (Weber and Taylor 1992). Because post-fire habitats generally remain suitable for a relatively short period of time, repeated burns on a single parcel of land (Kotliar 2007b), a shifting mosaic of prescribed burns, and/or no-suppression policies are required to ensure long-term availability of suitable habitat in areas where post-burn habitats are important.
Declines of several bird species that use open habitats, including Common Nighthawk, have been attributed in part to succession to forests of lands that were cleared after European settlement (Parody et al. 2001) and the encroachment of woody vegetation due to the abandonment of non-productive farmland. Substantial reforestation has been implicated in declines of native grassland habitat and agricultural land for grassland birds (Askins 1993) and may be the case regionally in southeastern Canada. Furthermore, reforestation in Ontario and Quebec is contributing to the encroachment of woody vegetation in grassland habitat (Bollinger 1995).
The European Nightjar (Caprimulgus europaeus), a close relative with similar nesting habits, population increases in planted forests are directly related to the availability of forest clearings (Langston et al. 2007). Farmland abandonment creates early- and mid-successional forests that can, at first, provide suitable habitat for the species, but succession eventually leads to older forest stages, which are not preferred (Bushman and Therres 1988). In southeastern Canada, succession of abandoned farmland has been the trend in marginal areas (i.e., areas not capable of sustained production of cultivated field crops) (Desponts 1996, Cadman et al. 2007) and forest succession in these areas may have caused some degree of nesting/foraging habitat loss for Common Nighthawk (Mills 1987, Smith 1996).
Natural succession may also pose a problem in native prairies where fire suppression is used as a management technique (McCracken 2005).
In urban environments, Common Nighthawks nest almost exclusively on roofs covered with pea gravel with a source of shade (Marzilli 1989). The change in roofing to tar, rubber, and other materials is thought to be a main cause of the species' decline in urban environments (Brigham et al. 2011). Such surfaces are unsuitable for nesting because rubber gets hotter than gravel in direct sun, does not provide camouflage for birds, and smooth surfaces may allow eggs to roll (Marzialli 1989, Brigham et al. 2011). Locally, declines have also been associated with changes from pea gravel to larger gravel (Wedgwood 1992), walled to unwalled, and drained to water-retaining roofs (Sandilands 2010). Acceptance of green roof technology is growing in North America (Dalglish 2012) but it is too early to tell whether, or how, an increase in green roofs will impact urban-nesting populations of Common Nighthawks.
Residential and commercial development and urban areas in general, have encroached upon a large amount of Canada's land over the last few decades (Cocklin et al. 1983) and leads to permanent habitat loss. This loss of habitat may be associated with the decline of bird species that use open habitats (Valiela and Martinetto 2007). Urban development is a major contributing factor in Canada's diminishing supply of dependable agricultural land and forested lands. Urban development is considered the leading cause of deforestation in the United States, and a major contributing factor in Canada (17%), especially in southern Ontario and Quebec (Radeloff et al. 2005, Robinson et al. 2005 Sun et al. 2007, Masek et al. 2011).
The largest increases in urban and rural landscapes from 2000 to 2011 occurred in Ontario and Quebec (Statistics Canada 2013) and Ontario has the highest concentration of urban land in Canada. More than 10% of Ontario's prime productive land was permanently removed by urban growth between 1971 and 2001, representing a nearly 80% increase in the amount of urban land in Ontario (Hofman et al. 2005).
Native grassland ecosystems have experienced losses greater than any other major biome in North America (Federal, Provincial and Territorial Governments of Canada 2010). To date, it is estimated that approximately 70 percent of Prairie grasslands, and greater than 99% and 97% of Tallgrass prairie and Tallgrass/savannah prairies, respectively, have been lost in Canada. Most of these losses occurred due to conversion of cropland prior to the 1930s (Gauthier et al. 2003, Riley et al. 2007) and while the rate of loss has slowed, grasslands are still being lost today (Federal, Provincial and Territorial Governments of Canada 2010).
Market pressure and increased mechanization available to farmers leads to the elimination of edge and natural features and the conversion of many pastures and hayfields to cereal and row crops, which are less suitable for ground-nesting species (Jobin et al. 1996, Corace et al. 2009) because they provide little or no nesting cover and because the frequency of disturbance during the breeding season is too high.
Agriculture is the largest source of deforestation in Canada (mainly in southern central Canada) (Masek et al. 2011). Forest clearing for agricultural expansion is particularly important in the boreal hardwood transition zone where 73% of the forest cover has been cleared, including a 25% loss from 1966 to 1994 (Hobson et al. 2002).
Although the species is known to use human altered landscapes, the impacts of agriculture and, specifically agriculture intensification, have not been directly assessed for Common Nighthawk.
Rates of harvest in Canada are highest in Quebec, British Columbia, and Ontario. Harvest rates were relatively stable in Canada from the 1980s to 2008 despite a steady increase in the area of harvest in Quebec over this time (Masek et al. 2011). Since 2008, the rates of harvest in Canada are lower than in the early 2000s (NFD 2014). Logging, in general, can have a short term negative impact on nesting birds by disrupting breeding activities (e.g., excessive disturbance, direct destruction of nests, eggs, and young) (Hobson et al. 2013) but some appropriately-timed practices may be beneficial for Common Nighthawks (e.g., through creation of open habitat that can be used for nesting). Although the species is known to use human altered landscapes, the impact of logging and wood harvesting has not been directly assessed for this species.
Migration is not 'well studied' for Common Nighthawk and there is virtually no information on habitat needs during migration or winter except that a variety of open areas are used for foraging (Brigham et al. 2011). It is not possible to assess the loss of nonbreeding habitat with so little information, but given similar declines in aerial insectivores which also migrate long distances, reduced habitat quality on either the wintering or migratory stopover areas may play a role in observed declines (Nebel et al. 2010).
Tropical storms can kill aerial insectivores migrating in the autumn in large numbers; a single hurricane (Hurricane Wilma 2005) had a measurable effect on the population of another aerial insectivore, Chimney Swift (Dionne et al. 2008). Climatic fluctuations in spring reduce survival and reproductive success, especially because nighthawks have a migration schedule that appears to rely on warm weather for flying insects (Brigham et al. 2011) (see section 4.2: Reduced availability of insects). A long period of cold wet weather in June 1903 decimated the nighthawk population throughout Massachusetts (Griscom 1949) and record high precipitation in 1990 resulted in reduced foraging and apparent starvation of nighthawks in British Columbia (Firman et al. 1993). The deleterious effects of cold wet weather during the breeding season are well known for other aerial insectivores (e.g., Brown and Brown 2000) and such weather extremes are expected to occur more frequently due to climate change (Huber and Gulledge 2011).
Migratory bird species which travel long distances are dependent on multiple, spatially disparate, habitats during their annual cycle (breeding, migration, and wintering) and this makes them particularly sensitive to the impacts of climate change because any change along the way could negatively impact the population (Robinson et al. 2008, Newson et al. 2009). There is little information to directly link climate change to the population decline of this species but Cumming et al. (2003) suggested a large potential for avian distributional shifts in response to climate change.
Common Nighthawks, especially males, often rest along gravel roads at night, where they are vulnerable to vehicle collisions (Poulin et al. 1998, Brigham et al. 2011). Red-necked Nightjars (Caprimulgus ruficollis) in Spain were attracted to paved roads (during migration and during cool temperatures while breeding) where the temperatures were significantly warmer than nearby gravel roads and bare ground (Camacho 2013). This behavior caused a significant increase in the number of road casualties (Camacho 2013). Road kills can also be particularly frequent where roads cross areas with concentrations of foraging nighthawks (Stevenson and Anderson 1994). Bishop and Brogan (2013) found Caprimulgiformes represented 1.9% of the birds reported in North American studies of bird mortalities resulting from collisions with vehicles, whereas Loss et al. (2014a) found Common Nighthawks represented 0. 01% of the total bird-vehicle mortalities from studies compiled within the range of the Common Nighthawk in the United States. Although there are exceptions, in general, mortality rates due to vehicle collisions often increase with increasing traffic speed, road corridor width, and road elevation (i.e., when above surrounding land) (Baudvin 1997,Case 1978, Loss et al. 2014a). Nests and broods can also be destroyed by vehicle traffic in managed forests (Bender and Brigham 1995). Vehicle collisions are expected to become a growing source of mortality for Common Nighthawk, as development and associated road infrastructure expand into new parts of their range.
Common Nighthawks are also vulnerable to collisions with airplanes. Common Nighthawks concentrating for roosting and foraging at McConnell Air Force Base (Kansas, USA) accounted for 82% of bird strikes at the Air Base until corrective actions were taken (Cumming et al. 2003). Management of migrating nighthawks on the Air Base is an ongoing issue because traditional hazing techniques are ineffective and birds quickly return to roosting spots which presents an even greater hazard to aircraft.
Collisions with buildings, telephone and power lines, communication towers, wind turbines, and other vertical human structures can also result in localized mortality for many bird species, particularly during migration.
Approximately 25 million birds (of many species) are killed each year in Canada from collisions with windows (Machtans et al. 2013) and between 365 and 988 million are killed each year in the United States (Loss et al. 2014b). Common Nighthawk is at 2.6 times lesser risk than the average species to mortality due to building collisions across all building types and has average risk of collisions with high-rise buildings when compared to other species (Loss et al. 2014b).
It is estimated that 2.5-25.6 million birds (of many species) are killed each year by transmission lines in Canada (Rioux et al. 2013) and between 12 and 64 million birds are killed each year by power lines in the United States (8-57 million of these by collisions, 0.9-11.6 million by electrocution) (Loss et al. 2014c). Common Nighthawks, especially adult males during courtship, are known to collide with telephone and power lines (Erikson 2005). The impact of these collisions has not been quantified for Common Nighthawk but it is presumed to be limited, though such collisions may increase as development expands.
An estimated 6.8 million birds (of many species) are killed by collisions with communication towers each year in the United States and Canada (Longcore et al. 2012). Mortality is most frequent for Neotropical migrants and nocturnal migrants attracted to tower lights (Longcore et al. 2013), but the estimated annual mortality due to collisions with communication towers is less than 1% of Common Nighthawk's total population (Longcore et al. 2013).
Approximately 23,300 birds of many species are killed each year from collisions with wind turbines (Zimmerling et al. 2014). Almost 50% of the deaths from collisions with wind turbines are predicted to occur in Ontario (Zimmerling et al. 2014).
Mineau and Whiteside (2013) suggest that pesticides be strongly considered in efforts to identify the causes of bird population declines in North America. They were unable to separate between the direct (i.e., toxic) and indirect (e.g., habitat or food chain) effects of pesticides and they concluded that both are likely occurring (Mineau and Whiteside 2013). Although largely undocumented for this species, pesticide use on both breeding and wintering grounds has been implicated in direct mortality and habitat loss of many avian species (e.g., Chamberlain et al. 2000, Boatman et al. 2004, Mineau 2005).
Most organochlorine pesticides (chemicals in the same family as dichlorodiphenyltrichloroethane: DDT) have been banned for decades in North America. Little is known about the extent to which Common Nighthawks and other Neotropical migrant passerines were exposed to organochlorine pesticides throughout their lifetime (Gard et al. 1993 Klemens et al. 2000) but there is some indication that Neotropical migrant insectivores are still being exposed to organochlorine pesticides in North America (Sager 1997, Klemens et al. 2000) either legally through exceptions in the restriction laws, or illegally, and they may still be in use in Central and South America (Klemens et al. 2000, Lebbin et al. 2010, Nebel et al. 2010). Endosulfan, which is primarily used on a wide variety of food crops is an exception to the ban of organochlorine pesticides but will be phased out of use in the U.S. by 2016 because it was deemed to pose an 'unacceptable' risk to farmworkers and wildlife (birds, in general, are fairly sensitive to endosulfan poisoning) (U.S. Environmental Protection Agency 2010). Several other counties have followed suit acting to ban the chemical through the Stockholm Convention on Persistent Organic Pollutants (an international environmental treaty signed in 2001) (Secretariat of the Stockholm Convention 2011).
Organophosphate and carbamate compounds have been used increasingly since the majority of organochlorine pesticides (e.g., DDT and dieldrin) were restricted in North America in the 1970s and banned in the 1980s (Commission for Environmental Cooperation of North America 2003). Birds and other vertebrate species are susceptible to these chemicals if they ingest or otherwise absorb enough organophosphate or carbamate pesticides; however, birds appear to be more sensitive than other vertebrates (Freedman 1995, Friend and Franson 1999-2001). Indeed, mass mortalities of other bird species feeding on insects poisoned by organophosphates have been documented on Common Nighthawk's wintering grounds (Goldstein et al. 1999).
Direct impacts of a relatively new class of pesticides, neonicotinoids, are unknown for insectivorous species such as Common Nighthawk, but studies show that seed-eating birds may be exposed to lethal doses of this pesticide (Mineau and Palmer 2013, Goulson 2014) while eating just a few treated seeds. Hallmann et al. 2014 recently published a study that correlated neonicotinoid concentrations in surface waters to declines in insectivorous birds in the Netherlands. Hallmann et al. 2014 suggest the declines are in relation to a reduction of insect prey (see section 4.2: Reduced availability of insects) but they could not rule out direct pathways in which the neonicotinoids may have had an effect on the birds.
Mercury is a naturally occurring element that is enriched in the environment by human activities. Long-range atmospheric transport and deposition is the dominant source of mercury to many aquatic habitats over much of the landscape (Fitzgerald et al. 1998, USGS 2000) but inputs of waterborne mercury have occurred (and potentially are still occurring) in North America and especially the Northeast (Evers et al. 2005). Bio-available mercury is also mobilized within watersheds by forestry activities, hydroelectric reservoir creation, and various industrial-related activities (Porvari et al. 2003, Vuori et al. 2003, and Wiener et al. 2003). Large amounts of mercury accumulated over thousands of years in peatlands, and currently underlain by permafrost, also has the potential to release mercury to the environment (Rydberg et al. 2010) in some parts of their range. Mercury concentrations in aquatic food webs are usually correlated with low-pH levels, and as a result mercury concentrations increase from west to east across Canada in freshwater food webs (Depew et al. 2013).
Mercury exposure can decrease reproductive success, alter immune responsiveness, and cause behavioural and physiological effects in birds (Scheuhammer et al. 2007, Hawley et al. 2009). Research by Rimmer et al. 2010 and Keller et al. 2014 suggests that mercury is biomagnifying in terrestrial songbirds that eat invertebrates and although not currently documented for this species, this requires investigation because Common Nighthawk may be exposed in some parts of its range to elevated methylmercury (MeHg; toxic form of mercury) due to its consumption of predatory insects from acidic wetlands where mercury is easily converted to methylmercury (Evers et al. 2005, Greenberg and Matsuoka 2010, Evers et al. 2011, Edmonds et al. 2012). A recent large-scale study of mercury in an insectivorous bird, Rusty Blackbird, emphasized the potential threat of mercury, especially to the population in northeastern North America (Edmonds et al. 2010). The feathers of Rusty Blackbirds breeding in the Acadian forest ecoregion of New England and the Maritimes (Maine, New Hampshire, Vermont, New Brunswick, and Nova Scotia) had mercury concentrations that were orders of magnitude higher than concentrations observed in the winter regions in the southern U.S. and breeding sites in Alaska (Edmonds et al. 2010).
Acid precipitation has been identified as a contributing factor in the decline of spruce-fir forests throughout the Eastern United States (U.S. Environmental Protection Agency 2014) and this is presumably occurring in Canada as well. Acidification may modify habitat leading to altered soil invertebrate assemblages (see section 4.2: Reduced availability of insects), loss of favoured nesting, roosting, and/or foraging sites (Hames et al. 2002), increased vigilance, and increase predation risk (Brotons et al. 1998).
Acidification of forests also contributes to the leaching of calcium from soils, a phenomenon that is particularly marked in northeastern North America (Driscoll et al. 2001) where soil buffering is relatively poor due to low pH and nitrogen saturation is occurring (i.e., nitrates can remove additional calcium from the soil) (U.S. Environmental Protection Agency 2014). Passerines must obtain calcium from their food during the egg-laying period (Hames et al. 2002) and calcium-deficiency during this time may lead to birds laying egg shells that are thin, weak, and more porous which can lead to breeding failure. Acidification has been implicated in the decline of Wood Thrush (Hylocichla mustelina) (Hames et al. 2002) as well as other passerine birds from northern Europe that nest in acidified parts of their range (Graveland and Drent 1997, Mand et al. 2000).
An increase in predators, such as cats (Felis catus), corvids (Corvus spp.), raccoons (Procyon lotor), and skunks (Mephitis mephitis and Spilogale gracilis) has been proposed as a threat to Common Nighthawks (COSEWIC 2007). Indeed, nest predation rates were highest for ground nesting bird species breeding in fragmented forest tracts near urban areas in a study by Wilcove (1985). Cats, being the number one source of human-related avian mortality in Canada (Calvert et al. 2013), could pose a threat to Common Nighthawks. An estimated two to seven percent of birds in Southern Canada are killed by cats annually (Blancher 2013). Predominately a roof-nester in urban centers, Common Nighthawks may be more susceptible to cat predation in rural areas and possibly during migration.
The spread of gulls nesting on roofs has been suggested as displacing nighthawks from nesting sites in Montreal (Ring-billed Gull, Larus delawarensis; COSEWIC 2007) and in cities of British Columbia (Glaucous-winged Gull, L. glaucescens; Campbell et al. 2006). This highlights the potential for nest predation by gulls in urban centers.
It is not deemed feasible to halt population declines immediately due the number of potential threats to the species, their nature, and ultimately the uncertainty around the cause of the decline.
The short-term population objective for the Common Nighthawk in Canada is to halt the national decline by 2025 (i.e., 10 years after this recovery strategy is posted on the Species at Risk Public Registry), while ensuring the population does not decrease more than 10% over this time. The long-term (i.e., after 2025) population objective is to ensure a positive 10-year population trend for the Common Nighthawk in Canada.
The distribution objective for Common Nighthawk is to maintain the current extent of occurrence (i.e., the area that encompasses the geographic distribution of all known populations) in Canada.
The population objectives address the species' long-term decline, which was the reason for its designation as Threatened (COSEWIC 2007). Short-comings with the Breeding Bird Survey dataset for this species (see section 3.2: Population and Distribution) are acknowledged and this strategy includes approaches to improve monitoring for Common Nighthawk. As new information becomes available, population and distribution objectives might be revised, as appropriate for species recovery.
The 10-year time frame was deemed appropriate to assess population change in Common Nighthawk. This time frame was selected because halting the decline of a species is challenging, and cannot be done in just a few years, and because COSEWIC species assessments occur every 10 years. Their criteria for assessment include reviewing population change within 10-year windows.
These objectives will be reviewed during the development of the report required five years after this strategy is posted on the Species at Risk Public Registry to assess the implementation of this strategy and the progress towards meeting its objectives (s. 46 SARA).
The following list of actions is not exhaustive, but is meant to illustrate the main areas where work is already underway and to give context to the broad strategies to recovery outlined in section 6.2. Actions completed or underway include the following:
- Common Nighthawk is considered and mitigative measures are established for land-use development projects and during environmental assessments across Canada.
- Environment Canada has completed some initial work in Yukon to determine appropriate seasonal and diurnal timing of surveys for Common Nighthawk.
- WildResearch's BC Nightjar Survey uses citizen-science road-side surveys to study Common Nighthawks in British Columbia. Volunteers conduct crepuscular passive point counts for nighthawks in priority regions across the province. Survey results are used to determine current population trends and identify landscapes that are important for conservation of nighthawk populations in British Columbia.
- The Government of Alberta published inventory guidelines for sensitive species including Common Nighthawk (Government of Alberta 2013).
- The Government of Saskatchewan published a Common Nighthawk Survey Protocol (Saskatchewan Ministry of Environment 2014).
- Opportunistic sightings of Common Nighthawks were collected during surveys for Common Poorwill (Phalaenoptilus nuttallii) by the Saskatchewan Wetland Conservation Corporation (now the Saskatchewan Water Security Agency).
- The Ontario Whip-poor-will project, conducted by Bird Studies Canada (BSC) (2010-2014), records incidental Common Nighthawk observations particularly in more northern routes (Central and Eastern Ontario).
- BSC piloted an urban Common Nighthawk count in 2013 and this volunteer survey was conducted again in 2014.
- Common Nighthawk data are collected as part of Ontario SwiftWatch (peak numbers and date of observations).
- Most Department of National Defense installations are conducting surveys or monitoring for the species.
- A volunteer survey of 25 permanent routes in agricultural landscapes has been implemented in Quebec.
- A project was launched in 2014 by Environment Canada in Quebec to assess the possibility of monitoring Common Nighthawk in the boreal forest using Song Meters programmed to record during periods of nighthawk activity and placed near stops on Breeding Bird Survey routes the night before the surveys are conducted.
- Common Nighthawk sightings are opportunistically collected as part of the Maritimes SwiftWatch.
- Incidental sightings of Common Nighthawk are collected by the Wildlife Division of the Newfoundland and Labrador Department of Environment and Conservation.
- Forestry and silviculture practices and initiatives in areas across the country attempt to preserve habitat features thought to be important for Common Nighthawk and/or identify occupied habitat.
In Canada, there has been little conservation work specifically targeting Common Nighthawk. However, several conservation-oriented research, planning, and education projects have been implemented in Canada and the U.S. that either include the species in the framework of activities or specifically target the species as a focus of efforts. These include the following groups and/or projects:
Uncertainties around the cause of the species' decline make it challenging to devise a strategic direction for its recovery. Monitoring and research is deemed to be the highest priority strategy, without which an understanding of recovery for the species cannot be reached. Research and management approaches may be amended when more information becomes available. The species occurs across a huge range and displays regional and ecotype differences across that range. It will be necessary to address such differences in all aspects of recovery for the species.
| Threat or Limitation | Broad Strategy to Recovery | PriorityNotef of Table 3 | General Description of Research and Management Approaches |
|---|---|---|---|
| Knowledge gaps to recovery | Monitoring and research | High |
|
Habitat Loss or Degradation. Changes in Ecological Dynamics or Natural Processes and Pollution Exotic, Invasive or Introduced Species/Genome |
Habitat and species conservation and management | High |
|
Habitat Loss or Degradation. Changes in Ecological Dynamics or Natural Processes and Pollution Exotic, Invasive or Introduced Species/Genome |
Habitat and species conservation and management | Medium |
|
| All threats and knowledge gaps | Education and awareness, stewardship, and partnerships | High |
|
| All threats and knowledge gaps | Education and awareness, stewardship, and partnerships | Medium |
|
| All | Law and policy | Medium |
|
Recovery of Common Nighthawk will require commitment, collaboration, and cooperation among federal, provincial and territorial jurisdictions, wildlife management boards, Aboriginal people, local communities, landowners, industry, and other interested parties. Due to Common Nighthawk's widespread range across the country, it will be important to monitor habitat conditions, population trend, and the distribution of the species so that the effectiveness of the recovery efforts can be evaluated, and adjusted as necessary.
Common Nighthawk is locally abundant but widespread, so an important first step in its recovery is to develop standardized protocols and survey designs for the collection and analysis of population data. There is a need to establish a monitoring program specific to this species to monitor the population trend using a consistent, comprehensive, and reliable survey method. Corresponding habitat models will need to be built to better understand where the species would be expected to breed on the landscape, and to assist with efforts to identify critical habitat.
The mechanisms driving population change in the species and its prey are far from clear (McCracken 2008) and there is simply too little information about the species to know whether any of its basic demographic parameters have changed over time. Even less information is known about the species outside the breeding season. There is only a vague understanding of where Common Nighthawks spend their winters in South America and little to no information on their migration routes and stopover sites. Gathering information on the species during the non-breeding period, what habitats it requires, and what threats it faces away from the breeding grounds will be a challenging, but necessary, endeavor.
Aerial ecology and the aerial biomass which Common Nighthawks utilize are poorly understood. Large-scale programs to monitor population levels of aerial insects are needed to understand how their population dynamics and trends affect aerial insectivore populations and research is needed to identify factors affecting prey species.
Because the population objective for this species includes halting the species' decline and ultimately increasing the population, potentially suitable but currently unoccupied habitat should be identified, as should any areas that are especially important passage or stopover sites during migration.
Conservation, management, and/or restoration of nesting habitat may be required in areas where important habitats have been lost or degraded, for example in grassland and agricultural areas. In some specific instances, it may be necessary to create nesting habitat where anthropogenic factors (e.g., residential and commercial development, agriculture, logging and wood harvesting) have led to a significant reduction of suitable habitat. Trends in aerial insect population dynamics must be better understood to know whether maintaining, enhancing, and/or restoring insect-producing habitats will be of benefit to the species.
Integrated Pest Management is not a new concept in Canada and some regional programs and initiatives are already underway. Such programs may help reduce some of the threats faced by the species and its prey.
Ground-nesting species like Common Nighthawk are especially vulnerable to predation because they are susceptible to a greater range of predators. Development of long-term solutions to address the problem of elevated levels of predator populations will be required in some areas where Common Nighthawks nest in high densities. Attention to predators in environmental assessment reviews will help curb the proliferation of predators, most notably those related to agricultural projects, food and fish processing plants, and mink farms. Such reviews should recommend measures that will result in less favourable conditions for predators.
To be effective, conservation and stewardship actions should be implemented throughout the species' range, including migratory stopovers and wintering grounds and this will require international collaboration for the approaches focused on maintaining habitat and reducing the threat of pollution. Cooperative relations should be fostered with landowners, the forest industry, farmers, industry, and pet owners to name a few, to implement beneficial management practices for the species and its habitat. The quantity of habitat available to Common Nighthawk and the degree of habitat protection on public lands is unknown but the species also nests on private land and this creates an opportunity for public involvement in habitat conservation and other conservation initiatives. Preserving and enhancing habitat for Common Nighthawk populations will require education and stewardship on a broad scale.
Ground-nesting birds like Common Nighthawk may be especially negatively impacted in urban areas by free-roaming cats and feral pet species. Education and stewardship will be required to reduce this threat.
The best management of breeding habitat will fail to recover the species unless wintering habitat and migratory stopovers are maintained. Thus, collaboration with international jurisdictions and non-governmental organizations to identify, preserve, restore, and enhance winter habitat is an equally important component of this strategy. Such collaboration should have a synergistic effect on several other species at risk, whose winter ranges overlap with Common Nighthawk.
There are multiple legislative and voluntary means available to protect Common Nighthawks and their habitat in Canada.
General prohibitions under the Migratory Birds Convention Act (1994) and its regulations protect Common Nighthawk nests and eggs anywhere they are found in Canada, regardless of land ownership. Nevertheless, nests and eggs can be inadvertently harmed or disturbed as a result of many activities. During the breeding period, potential destructive or disruptive activities should be avoided at locations where Common Nighthawks are likely to be encountered or known to occur (Environment Canada 2014c). This mitigation can also be accomplished through various avenues including planning policies and regulations, environmental assessments, etc.
Beneficial management practices for this species must be integrated with those for other species to maintain heterogeneous landscapes that are a dynamic mosaic of habitat conditions which will benefit several species. Beneficial management practices for governments, industry, and even individuals can play an important role for the ongoing efforts across the range of the species and will be needed to promote recovery of Common Nighthawk and conservation on a large scale across the continent and into South America.
Many species will benefit from reductions in air pollutants. Partnerships should be strengthened with government departments to encourage compliance with the Canadian Environmental Protection Act and to continue implementing the Canada-Wide Acid Rain Strategy for Post-2000, and various relevant provincial acts and regulations including the Energy Strategies and Climate Change Action Plans.
The same pesticides that may affect aerial insectivores are almost certainly affecting human health and the aerial insectivore guild may benefit from campaigns and policies aimed at reducing human reliance on pesticides. Indeed, an ecosystem approach to crop production and protection that combines different management strategies and practices is favoured by the Food and Agricultural Organizations of the United Nations to grow healthy crops and minimize the use of pesticides (Integrated Pest Management Program (Plant Production and Protection Division – UN 2013)).
Voluntary private sector standards and codes (e.g., third-party sustainable forest management certification and international rating systems that recognize excellence for green building) may help reduce some of the threats faced by the species and its prey.
Section 41(1)(c) of SARA requires that the recovery strategy include an identification of the species' critical habitat, to the extent possible, as well as examples of activities that are likely to result in its destruction.
The current knowledge of the species, its wide breadth of nesting habitats, and the dynamic nature of landscapes that are used for nesting, roosting, and foraging impart a high degree of uncertainty in the identification of habitat necessary for the survival or recovery of the Common Nighthawk in Canada. Although some habitat suitability modelling has been done (Haché et al.2014), model inconsistencies persist and, at present, it is unknown whether breeding habitat is limiting in Canada. The available information is not adequate to enable the identification of critical habitat for the following reasons:
- There is a lack of understanding and data to indicate the appropriate biophysical attributes required by the species and their configuration at a landscape scale.
- Habitat requirements may vary across the range of the species. Management units (i.e., geographic units within which critical habitat would be managed) need to be identified in such a way to best reflect variation in habitat use and land planning processes.
- There is a lack of data related to presence, site usage where detected (e.g., foraging, roosting, defending a territory, nesting, transiting), and abundance in large portions of the species' range and the northern limit of the species' range is unknown. Without this information any model used to predict critical habitat with current data may have a limited ability to do so.
- For Common Nighthawk, it is unknown whether certain habitats with specific biophysical attributes may be functionally more important than others. For example, specific habitats may have greater densities of individuals or pairs and/or result in higher reproductive success.
- The relationships between anthropogenic disturbance and habitat quality are poorly known. A better understanding of these relationships is needed to ensure sufficient suitable habitat is currently available for Common Nighthawk and to identify at what scale and intensity activities would be likely to destroy critical habitat.
Locating nests is difficult and determining general nesting locations is problematic using typical point-count survey methodology. Common Nighthawks defend a large area and their foraging habitats can be separated from nest sites by many kilometers, so it is not possible to determine how an individual is using the habitat where it is detected (e.g., foraging, defending a territory, transiting). Furthermore, traditional point-count survey methodology in the morning is not appropriate for this crepuscular species (Government of Alberta 2013; Saskatchewan Ministry of Environment 2014).
A schedule of studies has been developed to provide the information necessary to identify the critical habitat that will be sufficient to meet the population and distribution objectives. The identification of critical habitat will be included either in a revised recovery strategy or an action plan.
To inform the Schedule of Studies, a recent project was undertaken by the Boreal Avian Modelling group to help inform habitat use by Common Nighthawk (Haché et al. 2014). Haché et al. (2014) assessed habitat use for Common Nighthawk across Canada based on avian point counts and available land classification metrics (i.e., land cover and topography), and environmental data (i.e., disturbance and climate). The small number of observations available for the modelling exercise may have resulted in inconsistencies reported among habitat models for Common Nighthawk and likely prevented findings of important habitat relationships (i.e., landscape-scale biophysical attributes) (Haché et al. 2014). The dataset available for the modelling exercise was the most comprehensive dataset for the species available to date in Canada.
The following Schedule of Studies is required to complete the identification of critical habitat.
| Description of Activity | Rationale | Timeline |
|---|---|---|
| Determine the appropriate management units based on habitat requirements across the species' range. | Habitat requirements may vary across the range of the species. Management units need to be identified in such a way to best reflect this variation in habitat use. | 2016 |
| Increase monitoring using newly developed protocols for the species. | Common Nighthawks are not well captured by standard point counts resulting in fewer observations than required to identify important habitat relationships and define biophysical attributes at a landscape scale. The northern limit of the species' breeding range is uncertain at present and is required to identify all habitat that is necessary for its survival or recovery. Monitoring using newly developed protocols is necessary to validate and improve recent habitat models (i.e., Haché et al. 2014). | 2016-2022 |
| Determine the appropriate configuration of landscape-scale biophysical attributes. | In order to identify critical habitat at a landscape scale, it is necessary to understand the biophysical attributes required by the species at this scale and to determine how these should be configured to meet the species' needs. | 2017-2020 |
| Determine habitat quality across and within management units. | Information on abundance, productivity, and other measures of habitat quality may lead to the identification of areas that contribute disproportionately to the survival or recovery of the species. | 2018-2022 |
| Determine the scale and intensity at which suitable habitat would likely be destroyed by anthropogenic activities. | A better understanding of the relationship between anthropogenic disturbance and habitat quality is needed to ensure sufficient suitable habitat is currently available for Common Nighthawk and to identify at what scale and intensity activities would be likely to destroy critical habitat. | 2018-2022 |
| Determine how much suitable habitat is required to meet the population and distribution objectives. | It is uncertain whether habitat is limiting in Canada for Common Nighthawk. An assessment of whether there is sufficient habitat in Canada to meet the population and distribution objectives is required. | 2022 |
| Develop and validate habitat models to determine where biophysical attributes are present in required quantity, quality, and configuration within each management unit to meet population and distribution objectives. | Results from studies listed above will allow models to be built to identify the location, quantity, and quality of habitat that should be identified as critical habitat for Common Nighthawk. | 2023 |
The performance indicators presented below provide a way to define and measure progress toward achieving the population and distribution objectives.
- In the short term (i.e., by 2025), declining population trends have been halted or reversed to a point where Canadian populations of Common Nighthawk have declined no more than 10% during this time.
- In the long term (after 2025), a positive 10-year trend is achieved (i.e., the population is increasing).
- The breeding extent of occurrence for Common Nighthawk is maintained throughout Canada.
One or more action plans for Common Nighthawk will be posted on the Species at Risk Public Registry within the five years following the posting of this recovery strategy.
Allen, M.C. and K.A. Peters. 2012. Nest survival, phenology, and nest-site characteristics of Common Nighthawks in a New Jersey Pine Barrens grassland. The Wilson Journal of Ornithology 124:113-118.
Askins, R.A. 1993. Population trends in grassland, shrubland, and forest birds in Eastern North America. Current Ornithology 11:1-34.
Baudvin, H. 1997. Barn Owl (Tyto alba) and Long-eared Owl (Asio otus) mortality along motorways in Bourgogne-Champagne: report and suggestions. Pages 58-61 In Duncan, J.R., D.H. Johnson, T.H. Nicholls (eds). Biology and Conservation of Owls of the Northern Hemisphere: 2nd International Symposium. U.S. Department of Agriculture, St. Paul, MN.
Bender, D.J., and R.M. Brigham.1995. Preliminary inventory manual for sampling goatsuckers (Caprimulgidae) in British Columbia. Resource Inventory Committee. Ministry of Environment, Wildlife Branch.
Benton, T.G., D.M. Bryant, L. Cole, and H.Q.P. Crick. 2002. Linking agricultural practice to insect and bird populations: a historical study over three decades. Journal of Applied Ecology 39: 673-687.
BirdLife International and NatureServe (2014) Bird species distribution maps of the world. BirdLife International, Cambridge, UK and NatureServe, Arlington, USA.
Bishop, C.A., and J.M. Brogan. 2013. Estimates of avian mortality attributed to vehicle collisions in Canada. Avian Conservation and Ecology 8(2): 2. Available: http://dx.doi.org/10.5751/ACE-00604-080202 [accessed: August 2014]
Blancher, Peter. Estimated Number of Birds Killed by House Cats (Felis catus) in Canada / Estimation du nombre d'oiseaux tués par les chats domestiques (Felis catus) au Canada. 2013. Avian Conservation and Ecology 8.2: 3.
Blancher, P.J., and D.K. McNicol. 1991. Tree Swallow diet in relation to wetland acidity. Can. J. Zool. 69: 2629–2637.
Boatman, N.D., N.W. Brickle, J.D. Hart, T.P. Milsom, A.J. Morris, A.W.A. Murray, K.A. Murray, and P.A. Robertsone. 2004. Evidence for the indirect effects of pesticides on farmland birds. Ibis 146: 131-143.
Bollinger, E.K. 1995. Successional changes and habitat selection in hayfield bird communities. The Auk 112:720-732.
Both, C., C.A.M Van Turnhout, R.G. Bijlsma, H. Siepel, A.J. Van Strien, and R.P.B. Foppen. 2010. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proceedings of The Royal Society B 277: 1259-1266.
Both, C.,S. Bouwhuis, C.M. Lessells, and M.E. Visser. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441: 81-83.
Boulton, A.J., and P.S. Lake. 2008. Effects of drought on stream insects and its ecological consequences. Pages 81-102 In Lancaster, J., and R.A. Briers. Aquatic Insects: Challenges to Populations. CABI, Cambridge MA.
Brigham, R. M., J. Ng, R. G. Poulin and S. D. Grindal. 2011. Common Nighthawk (Chordeilesminor), The Birds of North America Online (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology. Available: http://bna.birds.cornell.edu/bna/species/213doi:10.2173/bna.213 [accessed: September 2013]
Brooks, D.R., J.E. Bater, S.J. Clark, D.T. Monteith, C. Andrews, S.J. Corbett, D.A. Beaumont, and J.W. Chapman. 2012. Large carabid beetle declines in a United Kingdom monitoring network increases evidence for a widespread loss in insect biodiversity. Journal of Applied Ecology 49(5): 1009-1019.
Brotons,L., M. Magrans, L. Ferrús, and J. Nadal. 1998. Direct and indirect effects of pollution on the foraging behaviour of forest passerines during the breeding season. Can. J. Zool., 76, 556–565.
Brown, C.R. and M.B. Brown. 2000. Weather-mediated natural selection on arrival time in cliff swallows (Petrochelidon pyrrhonota). Behavioral Ecology and Sociobiology 47:339–345.
Bruce-White, C., and M. Shardlow. 2011. A Review of the Impact of Artificial Light on Invertebrates. Buglife – The Invertebrate Conservation Trust. 32 p.
Bushman, E.S. and G.D. Therres. 1988. Habitat management guidelines for forest interior breeding birds of coastal Maryland. Maryland Department of Natural Resources, Wildlife Tech. Publ. 88–1. 50p.
Cadman, M. D., D.A. Sutherland, G.G. Beck, D. Lepage, and A. R. Couturier. 2007. Atlas of the breeding birds of Ontario, 2001–2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto, Ontario, Canada.
Calvert, Anna M., et al. "A synthesis of human-related avian mortality in Canada." Avian Conservation and Ecology 8.2 (2013): 11.
Camacho, C. 2013. Behavioral thermoregulation in man-made habitats: surface choice and mortality risk in Red-necked Nightjars. Bird Study 60(1): 124-130.
Campbell, R.W., M.K. McNicholl, R.M. Brigham, and J. Ng. 2006. Wildlife data centre featured species: Common Nighthawk. Wildlife Afield 3:32-71.
Case, R.M. 1978. Interstate highway road-killed animals: a data source for biologists. Wildlife Society Bulletin 6: 8-13.
Chamberlain, D.E., R.J. Fuller, R.G.H. Bunce, J.C. Duckworth, and M.J. Shrubb. 2000. Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. J. Appl. Ecol. 37: 71–788.
Cink, C.L. 2002. Eastern Whip-poor-will (Caprimulgus vociferus). The birds of North America. Number 620.
Cocklin, C., E. Gray, and B. Smit. 1983. "Future Urban Growth and Agricultural Land in Ontario", Applied Geography, 3: 91-104.
Commission for Environmental Cooperation of North America 2003 (PDF; 374 KB). [accessed: August 2014]
Corace III R.G., D.J. Flaspohler, and L.M. Shartell. 2009. Geographical patterns in openland cover and hayfield mowing in the Upper Great Lakes region: implications for grassland bird conservation. Landscape Ecology 24:309-323.
COSEWIC. 2007. COSEWIC assessment and status report on the Common Nighthawk Chordeiles minorin Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa. Available: http://www.sararegistry.gc.ca/status/status_e.cfm [accessed: September 2013]
Cumming, S.G. 2005. Effective fire suppression in boreal forests. Canadian Journal of Forest Research 35, 772–786. doi:10.1139/X04-174.
Cumming, J.L., P.A. Pipas, J.C. Luchsinger, J.E. Davis, M.J. Pipas, and J.B. Bourassa. 2003. Managing Common Nighthawks at McConnel Air Force Base, Kansas, to reduce aircraft strikes. In K.A. Fagerstone, and C.W. Witmer (eds.), Proceedings of the 10th Wildlife damage management Conference.
Dalglish, B. 2012. Up on the roof, green takes root. The Globe and Mail. [accessed: August 2014]
Davies, T.W., J. Bennie, and K.J. Gaston (2012). Street lighting changes the composition of invertebrate communities. Biology Letters, 8(5), 764-767. Chicago.
Depew, D.C., Burgess, N.M., and L.M. Campbell. 2013. Modelling mercury concentrations in prey fish: Derivation of a national-scale common indicator of dietary mercury exposure for piscivorous fish and wildlife. Environmental Pollution, 176: 234-243.
Desponts, M. 1996. Biogeography of Québec. Pp. 18-70 In: J. Gauthier and Y. Aubrey, (eds.) Breeding Birds of Québec. Association Québecoise des Groupes d'Ornithologues, Province of Quebec Society for the Protection of Birds, and Canadian Wildlife Service of Environment Canada, Montreal, Quebec.
Dionne, M., C. Maurice, J. Gauthier, and F. Shaffer. 2008. Impact of Hurricane Wilma on migrating birds: the case of the Chimney Swift. The Wilson Journal of Ornithology 120:784-792.
Dirzo, R., H. S. Young, M. Galetti, G. Ceballos, N.J.B. Isaac, and B. Collen. 2014. "Defaunation in the Anthropocene." Science 345(6195): 401-406.
Downes, C.M., B.T. Collins, and M. Damus. 2005. Canadian Bird Trends Web site Version 2.1. Migratory Birds Conservation Division, Canadian Wildlife Service, Gatineau, Quebec.
Driscoll, C.T., G.B. Lawrence, A.J. Bulger, T.J. Butler, C.S. Cronan, C. Eagar, K.F. Lambert, G.E. Likens, J.L. Stoddard, and K.C. Weathers. 2001. Acidic deposition in the northeastern United States: sources and inputs, ecosystem effects, and management strategies. BioScience 51: 180–198.
Dwyer, C.P., J.L. Belant, and R.A. Dolbeer. Distribution and Abundance of Roof-Nesting Gulls in the Great Lakes Region of the United States. 1996 USDA National Wildlife Research Center - Staff Publications. Paper 130. [accessed: January 2014]
eBird. 2014. eBird: An online database of bird distribution and abundance. eBird , Ithaca, New York. [accessed: August 2014]
Edmonds, S.T., N.J. O'Driscoll, N.K. Hillier, J.L. Atwood, and D.C. Evers. 2012. Factors regulating the bioavailability of methylmercury to breeding Rusty Blackbirds in northeastern wetlands. Environmental Pollution 171:148-154.
Edmonds, S.T., D.C.Evers, D.A. Cristol, C. Mettke-Hofmann, L.L. Powell, et. al. 2010. Geographic and seasonal variation in mercury exposure of the declining Rusty Blackbird. The Condor 112(4):789–799.
Environment Canada. 2011. Presence and levels of priorty pesticides in selected Canadian aquatic ecosystems. Water Science and Technology Directorate. Ottawa, Ontario. 102 pp.
Environment Canada. 2013. Species at Risk Act implementation guidance for recovery practitioners. Environment Canada - Canadian Wildlife Service, Ottawa, ON.
Environment Canada, 2014a. North American Breeding Bird Survey - Canadian Trends Website, Data-version 2012. Environment Canada, Gatineau, Quebec, K1A 0H3.
Environment Canada. 2014b. National Pollutant Release Inventory. [accessed: August 2014]
Environment Canada. 2014c. Avoidance guidelines related to incidental take of migratory birds in Canada. Environment Canada, Ottawa, On. [accessed: October 29 2014]
Erikson, L. 2005. Species profile: The Uncommon Common Nighthawk. [accessed: January 2014]
Evers, D.C., K.A. Williams, M.W. Meyer, A.M. Scheuhammer, N. Schoch, A.T. Gilbert, L. Siegel, R.J. Taylor, R. Poppenga, C.R. Perkins. 2011. Spatial gradients of methylmercury for breeding common loons in the Laurentian Great Lakes region. Ecotoxicology 20:1609-1625.
Evers DC, N.M. Burgess, L. Champoux , B. Hoskins, A. Major, W.M. Goodale, R.J. Taylor, R. Poppenga, and T. Daigle. 2005. Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology 14 : 193-221.
FAO. 2006. Global Forest Resources Assessment 2005 – Report on fires in the North American Region. Fire Management Working Paper 15. [accessed: August 2014]
Federal, Provincial and Territorial Governments of Canada. 2010. Canadian Biodiversity: Ecosystem Status and Trends 2010 [online]. Canadian Councils of Resource Ministers. Ottawa, ON.
Firman, M.C., R.M. Brigham, R.M.R. Barclay. 1993. Do free-ranging Common Nighthawks enter torpor? Condor 95, 157–162.
Fisher, R.J., Q.E. Fletcher, C.K.R. Willis, and R.M. Brigham. 2004. Roost selection and roosting behaviour of male Common Nighthawks. American Midland Naturalist 151:79-87.
Fitzgerald WF, Engstrom DR, Mason RP, Nater EA. 1998. The case for atmospheric mercury contamination in remote areas. Environ Science Technol 32:1–7.
Foster, G.N. 1991. Conserving insects of aquatic and wetland habitats, with special reference to beetles. Pages: 238-262 In Collins, N.M., and J.A. Thomas. The Conservation of Insects and their Habitats. 15th Symposium of the Royal Entomological Society of London. 14-15 September 1989. Academic Press Limited, London.
Freedman, B. 1995. Environmental Ecology. The Impacts of Pollution and Other Stresses on Ecosystem Structure and Function. Second Edition. Academic Press. San Diego, CA. 606 pp.
Friend, M. & J.C. Franson (eds) Field Manual of Wildlife Diseases: General Field Procedures and Disease of Birds. U.S. Geological Survey, Biological Resources Division Information and Technology Report 1999-2001: 175-179.
Gard, N.W., Hooper, M.J., Bennett, R.S., 1993. Effects of pesticides and contaminants on Neotropical migrants. In: Finch, D.M., Stangel, P.W. (Eds.), Status and Management of Neotropical Migratory Birds. U.S. Fish Wildl. Serv. Gen. Tech. Report RM-229 pp. 310±314.
Gauthier, D.A., Lafon, A., Toombs, T.P., Hoth, J. and Wiken, E. 2003. Grasslands: toward a North American conservation strategy. Canadian Plains Research Center, University of Regina, Regina, SK, and Commission for Environmental Cooperation, Montreal, QC. 99 p.
Gibbons, D., C. Morrissey, P. Mineau. 2014. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrates wildlife. Environmental Science Pollution Research, doi: 10.1007/s11356-014-3180-5.
Goldstein, M.I., T.E.J. Lacher, B. Woodbridge, M.J. Bechard, S.B. Canavelli, M.E. Saccagnini, G.P. Cobb, E.J. Scollon, R. Tribolet, and M.J. Hooper. 1999. Monocrotophos-induced mass mortality of Swainson's Hawks in Argentina, 1995– 96. Ecotoxicology 8:201-214.
Goulson. 2014. Pesticides linked to bird declines. Nature 511: 295-296.
Government of Alberta. 2013. Sensitive Species Inventory Guidelines (PDF; 1.11 MB). [accessed: August 2014]
Government of Canada. 2009. Species at Risk ActPolicies, Overarching Framework [Draft]. Species at Risk Act Policy and Guideline Series. Environment Canada. Ottawa. 38 pp.
Graveland, J. 1998. Effects of acid rain on bird populations. Environmental Review 6:41-54.
Graveland, J., and R.H. Drent. 1997. Calcium availability limits breeding success of passerines on poor soils. Journal of Animal Ecology 66: 279–288.
Greenberg, R. and S.M. Matsuoka. 2010. Special section: Rangewide ecology of the declining Rusty Blackbird, Rusty Blackbird: Mysteries of a species in decline. Condor 112(4): 770-777.
Griscom, L. 1949. The Birds of Concord: A Study of Population Trends. Harvard University Press.
Haché, S., P. Solymos, T. Fontaine, E. Bayne., S. Cumming, F. Schmiegelow, and D. Stralberg. 2014. Critical habitat of Olive-sided Flycatcher, Canada Warbler, and Common Nighthawk in Canada (Project K4B20-13-0367) [DRAFT]. Boreal Avian Modelling Project.
Haile. F.J. 2000. Drought stress, insects, and yield loss. Pages 117-134. In Peterson, R.K.D., and L.G. Higley (eds). Biotic Stress and Yield Loss. CRC Press, Florida.
Hallmann, C.A., R.P.B. Foppen, C.A.M. van Turnhout, H. de Kroon, and E. Jongejans. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature. 2014. advance online publication, doi:10.1038/nature13531.
Hames, R. S., K.V. Rosenberg, J.D. Lowe, S.E. Barker, and A.A. Dhondt. 2002. Adverse effects of acid rain on the distribution of the Wood Thrush Hylocichla mustelina in North America. Proc. Nat. Acad. Sci. 99: 11235–11240. [accessed: August 2014]
Hawley, D. M., K.K. Hallinger,and D.A. Cristol. 2009. Compromised immune competence in free-living tree swallows exposed to mercury. Ecotoxicology, 2009 Mar 26.
Hobson, K.A., A.G. Wilson, S,L. Van Wilgenburg, and E.M. Bayne. 2013. An estimation of nest loss in Canada due to industrial forestry operations. Avian Conservation and Ecology 8(2):5.
Hobson, K. A., Bayne, E. M. and S. L. van Wilgenburg. 2002. Large-scale conversion of forest to agriculture in the boreal plains of Saskatchewan. Conservation Biology 16(6):1530-1541.
Holmes, S.B. 1998. Reproduction and nest behaviour of Tennessee warblers Vermivora peregrina in forests treated with Lepidoptera-specific insecticides. Journal of Applied Ecology 35: 185-194.
Huber, D.G. and J. Gulledge. 2011. "Extreme Weather and Climate Change: Understanding the Link and Managing the Risk". Science and Impacts Program. Center for Climate and Energy Solutions: Arlington, VA. e [accessed: August 2014]
Hunt, P.D. 2005. Species profile: Common Nighthawk Chordeiles minor. Pages A403-A409 in New Hampshire Wildlife Action Plan. New Hampshire Fish and Game Department, Concord, NH. [accessed: September 2013]
Jobin, B., J.L. Desgranges, and C. Boutin. 1996. Population trends in selected species of farmland birds in relation to recent developments in agriculture in the St. Lawrence Valley. Agriculture, Ecosystems and Environment 57:103-116.
Jones, T. and W. Cresswell. 2010. The phenology mismatch hypothesis: are declines of migrant birds linked to uneven global climate change? Journal of Animal Ecology 79(1): 98-108.
Keller, R.H., L. Xie, D.B. Buchwalter, K.E. Franzreb, and T.R. Simons. 2014. Mercury bioaccumulation in Southern Appalachian birds, assessed through feather concentrations. Ecotoxicology 23: 304–316.
Klemens, J. A., R.G. Harper, J.A. Frick, A.P. Capparella, H.B. Richardson, and M.J. Coffey. 2000. Patterns of organochlorine pesticide contamination in neotropical migrant passerines in relation to diet and winter habitat. Chemosphere 41:1107-1113.
Kotliar, N. 2007b. Olive-sided Flycatcher (Contopus cooperi): A Technical Conservation Assessment Rocky Mountain Region, USDA Forest Service.
Kramer, G.R. and A.D. Chalfoun. 2012. Growth rate and relocation movements of Common Nighthawks (Chordeiles minor) nestlings in relation to age. The Wilson Journal of Ornithology 124: 793-797.
Langston, R.H.W., S.R. Wotton, G.J. Conway, L.J. Wright, J.W. Mallord, F.A. Currie, A.L. Drewitt, P.V. Grice, D.G. Hoccom, and N. Symes. 2007. Nightjar Caprimulguseuropaeus and Woodlark Lullula arborea: recovering species in Britain? Ibis 149:250-260.
Lebbin, D., M. Parr, G. Fenwick. 2010. The American Bird Conservancy Guide to Bird Conservation. Chicago, IL: The University of Chicago Press.
Lohnes, P. 2010. Nest site selection and nest thermal properties of Common Nighthawks on the tallgrass prairie of Kansas. PhD dissertation, Cornell University, Ithaca, NY.
Longcore, T., C. Rich, P. Mineau, B. MacDonald, D.G. Bert, L.M. Sullivan, E. Mutrie, S.A. Gauthreaux Jr, M.L. Avery, R.L. Crawford, A.M. Manville II, E.R. Travis, D. Drake. 2013. Avian mortality at communication towers in the United States and Canada: which species, how many, and where? Biological Conservation 158: 410-419.
Longcore, T., C. Rich, P. Mineau, B. MacDonald, D.G. Bert, L.M. Sullivan, E. Mutrie, S.A. Gauthreaux Jr, M.L. Avery, R.L. Crawford, A.M. Manville II, E.R. Travis, D. Drake. 2012. An estimate of avian mortality at communication towers in the United States and Canada. PLoS One 7(4): e34025. doi:10.1371/journal.pone.0034025.
Loss, S.R., T. Will, and P.P. Marra. 2014a. Estimation of bird-vehicle collision mortality on U.S. roads. The Journal of Wildlife Management 78(5): 763-771.
Loss, S.R., T. Will, S.S. Loss, and P.P. Marra. 2014b. Bird-building collisions in the United States: Estimates of annual mortality and species vulnerability. The Condor 116(1): 8-23.
Loss, S.R., T. Will, and P.P. Marra. 2014c. Refining estimates of bird collision and electrocution mortality at power lines in the United States. PLoS One 9(7): e101565. Doi:10.1371/journal.pone.0101565.
Machtans, C. S., K. J. Kardynal, and P. A. Smith. 2014. How well do regional or national Breeding Bird Survey data predict songbird population trends at an intact boreal site? Avian Conservation and Ecology 9(1): 5. [accessed: August 2014]
Machtans, C. S., C.H.R. Wedeles, and E.M. Bayne. 2013. A first estimate for Canada of the number of birds killed by colliding with building windows. Avian Conservation and Ecology 8(2): 6. [accessed: August 2014]
Mahony, N., E. Nol, and T. Hutchinson. 1997. Food-chain chemistry, reproductive success, and foraging behaviour of songibrds in acidified maple forests of central Ontario. Canadian Journal of Zoology 75: 509-517.
Main, A.R., J.V. Headley, K.M. Peru, N.L. Michel, A.J. Cessna, and C.A. Morrissey. 2014. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada's Prairie Pothole Region. PLOS One 9(3): 12 pp.
Mand, R., V. Tilgar, and A. Leivits. 2000. Calcium, snails, and birds: a case study. Web Ecology 1: 63–69.
Marzilli, V. 1989. Up on the roof. Maine Fish and Wildlife 31:25-29.
Masek, J. G., W. B. Bohen, D. Leckie, M. A. Wulder, R. Vargas, B. de Jong, S. Healey, B. Law, R. Birdsey, R. A. Houghton, D. Mildrexler, S. Goward, and W. B. Smith. 2011. Recent rates of forest harvest and conservation in North America. Journal of Geophysical Research 116, G00K03, doi:10.1029/2010JG001471.
McClure, C.J., B.W. Rolek, K. McDonald, and G.E. Hill. 2012. Climate change and the decline of a once common bird. Ecology and Evolution 2(2): 370-378.
McCracken, J.D. 2005. Where the Bobolinks roam: the plight of North America's grassland birds. Biodiversity 6:20-29.
McCracken, J.D. 2008. Are Aerial insectivores being bugged out? (PDF ; 120 KB) [accessed: December 2013]
Mills, A.M. 1987. Whip-poor-will, pp. 224-225 in Cadman, M.D., P.F.J. Eagles, and F.M. Helleiner, eds. Atlas of the Breeding Birds of Ontario. University of Waterloo Press, Waterloo, Ontario. 617 pp.
Mineau, P. 2005. Direct losses of birds to pesticides – beginnings of a quantification. USDA Forest Service Gen. Tech. Rep. PSW-GTR-191. Pp. 1065-1070.
Mineau, P. and C. Palmer. 2013. The Impact of the Nation's Most Widely Used Insecticides on Birds: Neonicotinoid Insecticides and Birds. American Bird Conservancy p5.
Mineau P. and M. Whiteside. 2013. Pesticide Acute Toxicity Is a Better Correlate of U.S. Grassland Bird Declines than Agricultural Intensification. PLoS ONE 8(2): e57457. doi:10.1371/journal.pone.0057457.
Møller, A.P., D. Rubolini, and E. Lehikoinen. 2008. Populations of migratory bird species that did not show a phenological response to climate change are declining. PNAS 105(42): 16195-16200.
Natural Resources Canada. 2013. Climate change and fire. [accessed: January 2014]
NatureServe. 2013. NatureServe Explorer: An online encyclopedia of life. Version 5.0. NatureServe, Arlington, Virginia. Available: http://www.natureserve.org/explorer [accessed: September 2013]
Nebel, S., A.M. Mills, J.D. McCracken and P.D. Taylor. 2010. Declines of aerial insectivores in North America follow a geographic gradient. Avian Conservation and Ecology 5:1. [accessed: September 2013]
Newson, S.E., S. Mendes, H.Q.P. Crick, N.K. Dulvy and others. 2009. Indicators of the impact of climate change on migratory species. Endang Species Res 7:101–113.
NFD. 2014. National Forestry Database. [accessed: 20 August]
Ng, J. W. 2009. Habitat use and home range characteristics of Common Nighthawks (Chordeiles minor) in mixed-grass prairie. Master's thesis. University of Regina.
Nocera, J.J., J.M. Blais, D.V. Beresford, L.K. Finity, C. Grooms, L.E., Kimpe, K. Kyser, N. Michelutti, M.W. Reudink, and J.P. Smol. 2012. Historical pesticide applications coincided with an altered diet of aerially foraging insectivorous chimney swifts. Proceedings of the Royal Society B. [accessed: January 2014]
Nocera, J.J., M.W. Reudink, and A.J. Campomizzi. Population trends of aerial insectivores breeding in North America can be linked to trade in insecticides on wintering grounds in Central and South America (Abstract). Presented at the Annual meeting of the American Ornithologists' Union (132nd Stated Meeting), the Cooper Ornithological Society (84th Stated Meeting), and the Society of Canadian Ornithologists, 23-28 September, 2014, Estes Park, Colorado.
North American Bird Conservation Initiative Canada. 2012. The State of Canada's Birds, 2012. Environment Canada, Ottawa, Canada. 36 pp.
Norton, M.L., J.F., L.I. Bendell-Young, and C.W. LeBlanc. 2001. Secondary effects of the pesticide Bacillus thuringiensis kurstaki on chicks of Spruce Grouse (Dendragapus canadensis). Archives of Environmental Contamination and Toxicology 41: 369-373.
Parody, J.M., F.J. Cuthbert, and E.H. Decker. 2001. The effect of 50 years of landscape change on species richness and community composition. Global Ecology and Biogeography 10:305-313.
Paquette, S.R., F. Pelletier, D. Garant, and M. Bélisle. 2014. Severe recent decrease of adult body mass in a declining insectivorous bird population. Proceedings of the Royal Society B 281: 9 pp.
Paquette, S.R., D. Garant, F. Pelletier, and M. Bélisle. 2013. Seasonal patterns in tree swallow prey (Diptera) abundance are affected by agricultural intensification. Ecological Applications 23(1): 122-133.
Partners in Flight Science Committee 2013. Population Estimates Database, version 2013. [accessed: October 2013]
Plant Production and Protection Division – UN. 2013. Pest and Pesticide Management. [accessed: January 2014]
Porvari, P., M. Verta, J. Munthe, and M. Haapanen. 2003. Forestry practices increase mercury and methyl mercury output from boreal forest catchments. Environmental Science and Technology 37:2389-2393.
Poulin, B., G. Lefebvre, and L. Paz. 2010. Red flag for green spray: adverse trophic effects of Bti on breeding birds. Journal of Applied Ecology 47: 884-889.
Poulin, R.G., L.D. Todd, R.M. Brigham. 1998. Male Common Nighthawk use of gravel roads at night. The Prairie Naturalist 30(2): 85-90.
Price, P.W., R.F. Denno, M.D. Eubanks, D.L. Finke, I. Kaplan. 2011. Insect Ecology: Behavior, Populations and Communities. Cambridge University Press, New York. 812 pp.
Radeloff, V.C., R.B. Hammer, and S.I. Stewart. 2005. Rural and sub-urban sprawl in the U.S. Midwest from 1940 to 2000 and its relation to forest fragmentation. Conservation Biology 19:793-805.
Rich, T.D., C.J. Beardmore, H. Berlanga, P.J. Blancher, M.S.W. Bradstreet, G.S. Butcher, D.W. Demarest, E.H. Dunn, W.C. Hunter, E.E. Iñigo-Elias, J.A. Kennedy, A.M. Martell, A.O. Panjabi, D.N. Pashley, K.V. Rosenberg, C.M. Rustay, J.S. Wendt, and T.C. Will. 2004. Partners in Flight North American Landbird Conservation Plan. Cornell Lab of Ornithology. Ithaca, NY.
Riley, J.L., Green, S.E. and Brodribb, K.E. 2007. A conservation blueprint for Canada's prairies and parklands. Nature Conservancy of Canada. Toronto, ON. 226 p. and DVD-ROM.
Rimmer, C.C., E.K. Miller, K.P. McFarland, and R.J. Taylor. 2010. Mercury bioaccumulation and trophic transfer in the terrestrial food web of a montane forest. Ecotoxicology 19: 697–709.
Rioux, S., J.-P. L. Savard, and A. A. Gerick. 2013. Avian mortalities due to transmission line collisions: a review of current estimates and field methods with an emphasis on applications to the Canadian electric network. Avian Conservation and Ecology 8(2): 7. [accessed: August 2014]
Robinson, L., J.P. Newell, and J.M. Marzluff. 2005. Twenty-five years of sprawl in the Seattle region: Grown management responses and implications for conservation. Landscape and Urban Planning 71:51-72.
Robinson, R.A., H.Q.P Crick, J.A. Learmonth, I.M.D. Maclean and others. 2009. Travelling through a warming world: climate change and migratory species. Endang Species Res 7: 87–99.
Russell, R.W. 1999. Precipitation scrubbing of aerial plankton: inferences from bird behavior. Oecologia 118:381-387.
Russell, R.W., and J.W. Wilson. 1997. Radar-observed "fine lines" in the optically clear boundary layer: reflectivity contributions from aerial plankton and its predators. Boundary-Layer Meteorology 82: 235-262.
Rydberg, J., J. Klaminder,P. Rosén, and R. Bindler. 2010. Climate driven release of carbon and mercury from permafrost mires increases mercury loading to sub-arctic lakes. Sci. Total Environ. 408, 4778–4783.
Sager, T.A. 1997. Organochlorine Pesticide Contamination in New World Passerines (1997). Honors Projects. Paper 10. [accessed: August 2014]
Saino, N., R. Ambrosini, D. Rubolini, J. von Hardenberg, A. Provenzale, K. Hüppop, O. Hüppop, A. Lehikoinen, E. Lehikoinen, K. Rainio, M.Romano, and L. Sokolov. 2011. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proceedings of the Royal Society B 278: 835-842.
Sandilands, A. 2010. Birds of Ontario: Habitat Requirements, Limiting Factors, and Status. Nonpasserines: Shorebirds through Woodpeckers. UBC Press, Vancouver.
Saskatchewan Ministry of Environment. 2014. Common Nighthawk Survey Protocol. Fish and Wildlife Branch Technical Report (PDF; 364 KB) No. 2014-15.0.3211 Albert Street, Regina, Saskatchewan, 7 pp. [accessed: August 2014]
Sauer, J. R., J. E. Hines, J. E. Fallon, K. L. Pardieck, D. J. Ziolkowski, Jr., and W. A. Link. 2014. The North American Breeding Bird Survey, Results and Analysis 1966 - 2012. Version 02.19.2014 USGS Patuxent Wildlife Research Center, Laurel, MD
Scheuhammer, A.M., M.W. Meyer, M.B. Sandheinrich and M.W. Murray. 2007. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio 36: 12-18.
Secretariat of the Stockholm Convention. 2011. United Nations targets widely-used pesticide endosulfan for phase out. [accessed: August 2014]
Shannon, J.D. 1999. Regional trends in wet deposition of sulfate in the United States and SO2 emissions from 1980 through 1995. Atmospheric Environment 33(5): 807-816.
Smith, A.R. 1996. Atlas of Saskatchewan birds. Canadian Wildlife Service, Natural History Society, Regina, 456 pp.
Sopuck, L., K. Ovaska, and B. Whittington. 2002. Responses of songbirds to aerial spraying of the microbial insecticide Bacillus thuringiensis var. kurstaki (Foray 48B®) on Vancouver Island, British Columbia, Canada.
Statistics Canada. 2013. Measuring ecosystem goods and services in Canada Human Activity and the Environment: Annual Statistics 2013. Statistics Canada Catalogue no. 16-201-x2013000. p. 1 -122. [accessed: April 2014]
Status of Birds in Canada. 2011. Status of Landbirds, Shorebirds, Waterbirds (excluding Waterfowl). [accessed: January 2014]
Stevenson, H.M. and B.H. Anderson. 1994. The Birdlife of Florida. University Presses of Florida, Gainesville.
Stocks, B.J., J.A. Mason, J.B. Todd, E.M. Bosch, B.M. Wotton, B.D. Amiro, M.D. Flannigan, K.G. Hisch, K.A. Logan, D.L. Martell, and W.R. Skinner. 2003. Large forest fires in Canada, 1959–1997. Journal of Geophysical Research 108, 8149. doi:10.1029/2001JD000484.
Strode, P. 2003. Implications of climate change for North American wood warblers (Parulidae). Global Change Biology 9: 1137-1144.
Sun, H., W. Forsythe, and N. Waters. 2007. Modeling urban land use change and urban sprawl: Calgary, Alberta, Canada. Networks and Spatial Economics 7:353-376.
Sutherland, C. A. 1963. Notes on the behavior of Common Nighthawks in Floria. Living Bird 2:31-39.
Taylor, S. W. 1998. Prescribed fire in Canada - a time of transition.
Taylor, P. 2009. Late-summer feeding and migration behaviour and numerical trends of Common Nighthawks, Chordeiles minor, near Pinawa, Manitoba, 1976–2009. Canadian Field-Naturalist 123:338–345.
Tyler, W. M. 1940. Chimney Swift. Pages 271-293 in A. C. Bent, ed. Life histories of North American cuckoos, goatsuckers, hummingbirds and their allies. U.S. Natl. Mus. Bull. 176.
U.S. Environmental Protection Agency. 2014. Environmental Effects of Acid Rain. [accessed: August 2014]
U.S. Environmental Protection Agency. 2010. Endosulfan Phase-out. [accessed: August 2014]
U.S. Bureau of Land Management. 1978. Grass Creek: Oil and Gas Leasing Environmental Assessment Record. Bureau of Land Management, Worland District, Wyoming. 138 pp.
USGS. 2000. Mercury in the Environment. [accessed: August 2014]
Valiela, Ivan and Paula Martinetto. Changes in Bird Abundance in Eastern North America: Urban Sprawl and Global Footprint? BioScience, April 2007, Vol. 5, No. 4, 360-370.
Visser, M.E., L.J.M. Holleman, and P. Gienapp. 2006. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Global Change Ecology 147: 164-172.
Vuori, K.-M., O. Siren, and H. Luotonen. 2003: Metal contamination of streams in relation to catchment silvicultural practices: a comparative study in Finnish and Russian headwaters. Boreal Env. Res. 8: 61–70.
Weber, M. G. and S. W. Taylor. 1992. The use of prescribed fire in the management of Canada's forested lands. Forest Chronicles 68: 324-334.
Wedgwood, J. 1992. Common nighthawks in Saskatoon. Blue Jay 50:211-217.
Wiener, J.G., Krabbenhoft, D.P. Heinz, G.H., and Scheuhammer, A.M. 2003. Ecotoxicology of mercury, Chapter 16 in D.J. Hoffman, B.A. Rattner, G.A. Burton, Jr., and J. Cairns, Jr. (Eds.), Handbook of Ecotoxicology, 2nd edition. CRC Press, Boca Raton, Florida, pp. 409-463.
Wilcove, D. S. (1985). Nest predation in forest tracts and the decline of migratory songbirds. Ecology, 66(4),1211-1214.
Xing, Z., L. Chow, H. Rees, F. Meng, S. Li, B. Ernst, G. Benoy, T. Zha, and L.M. Hewitt. 2013. Influences of sampling methodologies on pesticide-residue detection in stream water. Archives of Environmental Contamination and Toxicology 64(2): 208-218.
Zimmerling, J.R., A.C. Pomeroy, M.V. d'Entremont, and C.M. Francis. 2013. Canadian estimate of bird mortality due to collisions and direct habitat loss associated with wind turbine developments. Avian Conservation and Ecology 8(2): 10.
A strategic environmental assessment (SEA) is conducted on all SARA recovery planning documents, in accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals. The purpose of a SEA is to incorporate environmental considerations into the development of public policies, plans, and program proposals to support environmentally sound decision-making and to evaluate whether the outcomes of a recovery planning document could affect any component of the environment or any of the Federal Sustainable Development Strategy's (FSDS) goals and targets.
Recovery planning is intended to benefit species at risk and biodiversity in general. However, it is recognized that strategies may also inadvertently lead to environmental effects beyond the intended benefits. The planning process based on national guidelines directly incorporates consideration of all environmental effects, with a particular focus on possible impacts upon non-target species or habitats. The results of the SEA are incorporated directly into the strategy itself but are also summarized below in this statement.
All species that depend on aerial insects for prey such as bats, swallows, and flycatchers and specifically, bird species at risk including: Chimney Swift, Eastern Whip-poor-will (Antrostomus vociferus), Olive-sided Flycatcher (Contopus cooperi), Acadian Flycatcher (Empidonax virescens) may benefit from the recommended approaches for Common Nighthawk.
Nonetheless, some species, including other species at risk, may prefer different habitat conditions than Common Nighthawk which needs open areas for nesting. Examples of such species include, but are not limited to Canada Warbler, Bicknell's Thrush, and Rusty Blackbird. Recovery actions for the species must be integrated with beneficial management practices for other species, especially where such practices may conflict.
The possibility that the present recovery strategy inadvertently generates negative effects on the environment and on other species was considered. Some gull species may be negatively affected by predator management where they nest on roofs. Such predator management is already underway in many urban and industrial areas in Canada. It was concluded that this strategy will not result in any significant adverse effects.
The following list is not exhaustive, but illustrates some of the research required to understand the threats to the species, its prey, and their habitats.
- Determine potential links between insect availability and breeding productivity;
- Determine if a temporal mismatch between reproduction and maximal prey abundance is occurring;
- Determine the effects of habitat loss (particularly in wintering areas) on the species' prey availability;
- Determine the relative importance of nonbreeding versus breeding habitat supply in population declines;
- Once non-breeding habitats are identified, identify threats to the species, its prey, and their habitats;
- Determine availability of suitable roof-nesting sites;
- Determine the cause(s) of nest loss for roof-nesting Common Nighthawks;
- Determine the impacts of climate change on the species and its habitat;
- Monitor frequency of collisions and determine site characteristics contributing to high collision rates;
- Determine Common Nighthawks' exposure to pollution (pesticides, mercury) and identify impacts;
- Determine if acidification of the species' environment is negatively affecting Common Nighthawk and its habitat (e.g., through loss of favoured nesting, roosting, and/or foraging sites, increased vigilance, and increase predation risk, calcium deficiency during egg laying and chick rearing phases);
- Determine if gull species are displacing Common Nighthawks from nesting sites in urban areas;
- Determine human-related predation risk in urban areas (e.g., by cats and other species with increased populations due to human habitation).