Recovery Strategy for Olive-sided Flycatcher (Contopus cooperi) in Canada - 2015 - Proposed
- Preface
- Acknowledgments
- Executive Summary
- Recovery Feasibility Summary
- 1. COSEWIC, Species Assessment Information
- 2. Species Status Information
- 3. Species Information
- 4. Threats
- 5. Population and Distribution Objectives
- 6. Broad Strategies and General Approaches to Meet Objectives
- 7.Critical Habitat
- 8. Measuring Progress
- 9. Statement on Action Plans
- 10. References
Environment Canada. 2015. Recovery Strategy for Olive-sided Flycatcher (Contopus cooperi) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. vi + 52 pp.
For copies of the recovery strategy, or for additional information on species at risk, including COSEWIC Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the SAR Public Registry.
Cover illustration: © Dan Busby (provided with permission)
Également disponible en français sous le titre
« Programme de rétablissement du Moucherolle à côtés olive (Contopus cooperi) au Canada [Proposition] »
© Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment and the Parks Canada Agency, 2015. All rights reserved.
ISBN
Catalogue no.
Content (excluding the illustrations) may be used without permission, with appropriate credit to the source.
The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of recovery strategies for listed Extirpated, Endangered, and Threatened species and are required to report on progress five years after the publication of the final document on the SAR Public Registry.
The Minister of the Environment and the Minister responsible for the Parks Canada Agency are the competent ministers for the recovery of Olive-sided Flycatcher and have prepared this strategy, as per section 37 of SARA. It has been prepared in cooperation, to the extent possible, with the Provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec (Ministère des Forêts, de la Faune et des Parcs), New Brunswick, Prince Edward Island, Nova Scotia, Newfoundland and Labrador, as well as the territories of Yukon, Northwest Territories, and Nunavut, Gwich’in Renewable Resources Board, and others as per section 39(1) of SARA.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy and will not be achieved by Environment Canada and the Parks Canada Agency, or any other jurisdiction alone. All Canadians are invited to join in supporting and implementing this strategy for the benefit of Olive-sided Flycatcher and Canadian society as a whole.
This recovery strategy will be followed by one or more action plans that will provide information on recovery measures to be taken by Environment Canada and the Parks Canada Agency, and other jurisdictions and/or organizations involved in the conservation of the species. Implementation of this strategy is subject to appropriations, priorities, and budgetary constraints of the participating jurisdictions and organizations.
This recovery strategy was prepared by Krista Baker, Julie McKnight, David Andrews, and Peter Thomas (EC-CWS – Atlantic region). An early draft of the document was prepared by Dan Busby. An initial draft was reviewed by the EC Landbird Technical Committee and subsequent reviews were undertaken by Manon Dubé (EC CWS National Capital region), Andrew Boyne, Samara Eaton, and Becky Whitham (EC-CWS – Atlantic region), Vincent Carignan, Bruno Drolet, Renée Langevin, and Gilles Falardeau (EC-CWS – Quebec region), François Fournier and Junior Tremblay (EC-Science & Technology – Quebec region), Connie Downes (EC-NCR – NWRC), Kathy St. Laurent, Rich Russell, Kevin Hannah, Russ Weeber, Madeline Austen, Lesley Dunn, Krista Holmes, and Elizabeth Rezek (EC-CWS – Ontario region), Lisa Mahon, Mark Bidwell, Steven Van Wilgenburg, Nancy Mahony, Samuel Haché, and Donna Bigelow (EC-CWS – Prairie & Northern region), Pam Sinclair, Megan Harrison, Wendy Easton, and Craig Machtans (EC-CWS – Pacific & Yukon region), and Darroch Whitaker (Parks Canada).
Acknowledgement and thanks are given to all other parties that provided advice and input used to help inform the development of this recovery strategy, including various Aboriginal Organizations and individuals, provincial governments, other federal departments (e.g., Department of National Defence), landowners, citizens, and stakeholders.
Other contributors provided comments on the recovery strategy: Adam Smith (EC-NWRC) and Francesco Lai (CCIW, Burlington).
Olive-sided Flycatcher (Contopus cooperi) is a medium-sized songbird of Canada's forests. The species breeds in open coniferous or mixedwood forests, often located near water or wetlands with the presence of tall snags. It was designated as Threatened by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) in 2007 and has been listed according to the same status under Schedule 1 of the Species at Risk Act (SARA) since 2010.
An estimated 900,000 individuals (53% of the global population) breed in Canada. The species has a relatively wide, yet sparse, distribution across Canada's coniferous and coniferous-dominated forests from Newfoundland and Labrador to the Yukon Territory. Population trends follow widespread and unabated declines; Breeding Bird Surveys estimated an annual rate of population decline equal to 3.4%.
The causes of the population decreases are not well understood, although several possible factors have been suggested and some studies have provided empirical evidence for these factors. Probable significant threats include reduced availability of insect prey, fire suppression, deforestation and land conversion in nonbreeding habitat, forest harvesting and silviculture, energy and mining exploration and extraction, and residential and commercial development. It is currently unknown whether the availability of breeding habitat is a limiting factor in Canada. The significance of each threat varies across Olive-sided Flycatcher's geographical range.
There are several unknown factors associated with the feasibility of recovering Olive-sided Flycatcher. Despite these unknowns, and in keeping with the precautionary principle, a recovery strategy has been prepared as per section 41(1) of SARA.
This recovery strategy identifies both short-term and long-term objectives for Olive-sided Flycatcher. The short-term population objective for Olive-sided Flycatcher in Canada is to halt the national decline by 2025 (i.e., 10 years after this recovery strategy is posted on the Species at Risk Public Registry), while ensuring the population does not decrease more than 10% over this time. The long-term (after 2025) population objective is to ensure a positive 10-year population trend for Olive-sided Flycatcher in Canada. The distribution objective for Olive-sided Flycatcher is to maintain the current extent of occurrence (the area that encompasses the geographic distribution of all known populations) in Canada. Broad strategies and approaches to achieve these objectives are outlined in this recovery strategy.
At present, the available information is not adequate to identify the habitat necessary for the survival or recovery of Olive-sided Flycatcher in Canada. A Schedule of Studies is provided to obtain the information needed for the identification of critical habitat.
An action plan for Olive-sided Flycatcher will be posted on the Species at Risk Public Registry within 5 years following the publication of this recovery strategy.
In considering the criteria established by Government of Canada (2009), unknowns remain as to the recovery feasibility of Olive-sided Flycatcher. Nevertheless, in keeping with the precautionary principle, this recovery strategy has been prepared as per section 41(1) of SARA as would be done when recovery is determined to be feasible. This recovery strategy addresses the unknowns surrounding the feasibility of recovery.
- Individuals of the wildlife species that are capable of reproduction are available now or in the foreseeable future to sustain the population or improve its abundance.
Yes. The species is still common throughout its range and breeding individuals are currently distributed throughout the Canadian range, as well as in the United States. The Canadian population is estimated to be 900,000 individuals (Partners in Flight Science Committee 2013). It is believed there are currently adequate numbers of individuals available to sustain the species in Canada or increase its abundance with the implementation of proper conservation actions. - Sufficient suitable habitat is available to support the species or could be made available through habitat management or restoration.
Unknown. Sufficient suitable breeding habitat is likely available to support the species, and habitats could be enhanced (e.g., by prescribed burning). Habitat suitability is still insufficiently understood to adequately identify unoccupied habitats that could be used to enhance population size.
Large tracts of forest have been lost throughout Olive-sided Flycatcher's migratory range. It is unknown whether sufficient habitat is available on their migratory route.
Altman and Sallabanks (2012) hypothesized that the loss or alteration of wintering habitat is possibly the most limiting factor for this species. Olive-sided Flycatcher can tolerate a degree of habitat disturbance on the wintering grounds (e.g., forest edges), but in general, wintering habitat in South America is declining. It is therefore unknown whether sufficient suitable habitat remains for this species in wintering areas. - The primary threats to the species or its habitat (including outside of Canada) can be avoided or mitigated.
Unknown. It is expected that the primary threats on the breeding grounds in Canada can be mitigated or avoided through targeted conservation actions driven by focused research and stewardship efforts. These threats include fire suppression and possibly forest harvesting.
A significant threat to the species may be the degradation and loss of wintering habitat. However, it is uncertain whether there is a direct-causal relationship between population declines and wintering habitat availability. If a cause–effect relationship was established, it remains unclear what mechanisms could be employed to protect or restore wintering habitat. Nevertheless, there are numerous programs/organizations (e.g., Southern Wings and Important Bird Areas) currently in operation and aimed at the conservation of wintering habitat for Neotropical migrants.
Populations of aerial insects, including bees, are declining and there may be a climate-induced prey–breeding temporal mismatch decreasing prey availability. Population levels of aerial insects are not monitored on a large-scale and very little is known about their population dynamics. Without this information, it is impossible to conclude whether there is sufficient prey available for Olive-sided Flycatcher and other aerial insectivores to recover. - Recovery techniques exist to achieve the population and distribution objectives or can be expected to be developed within a reasonable timeframe.
Unknown. It is expected that one of the main recovery techniques will be to maintain existing breeding and nonbreeding habitats. Habitat management and stewardship could be effective for this species. Availability of suitable habitat on the breeding grounds may not be limiting. Research is required to identify critical habitat and inform land-use practices and habitat management that will benefit the species in breeding and nonbreeding areas. Mitigating the threat of reduced insect availability will be a continuing challenge.
A significant challenge will be to conduct the necessary research on the importance of wintering habitat loss and work toward protecting existing suitable habitats. Existing programs and organizations can be used (whenever possible) to help ensure these measures are conducted within a reasonable timeframe.
Assessment Summary
Canada hosts approximately 53% of the global breeding population of Olive-sided Flycatcher (Contopus cooperi) (Partners in Flight Science Committee 2013). The species was listed as Threatened under Schedule 1 of the Species at Risk Act (SARA; S.C. 2002, c. 29) in 2010. Under provincial endangered species legislation, Olive-sided Flycatcher is listed as Special Concern in Ontario (S.O. 2007, CHAPTER 6) and as Threatened in Newfoundland and Labrador (SNL2001 CHAPTER E-10.1 [Amended: 2004 cL-3.1 s27; 2004 c36 s11]), New Brunswick (S.N.B. 2012, c. 6), and Nova Scotia (Endangered Species Act 1998, c. 11, s. 1. [amended 2010, c. 2, s. 99]). In Quebec, the species is listed on the Liste des espèces susceptibles d'être désignées menacées ou vulnérables (list of wildlife species likely to be designated threatened or vulnerable. This list is produced according to the Loi sur les espèces menacées ou vulnérables (RLRQ, c E-12.01) (Act respecting threatened or vulnerable species) (CQLR, c E-12.01). The species is not listed under provincial/territorial endangered species legislation in British Columbia, Yukon, the Northwest Territories, Nunavut, Alberta, Saskatchewan, Manitoba, or Prince Edward Island.
The International Union for Conservation of Nature (IUCN) ranks the species as Near Threatened because of the decreasing population (IUCN 2013). Partners in Flight ranks the species as a Temperate Breeder of High Tri-National Concern (Partners in Flight Science Committee 2012). NatureServe (2013) ranks the species globally as G4 -- Apparently Secure. Other NatureServe rankings include those in Table 1.
| Species | Global (G) Rank Noteadu tableau 1 | National (N) Rank Notebdu tableau 1 | Sub-national (S) Rank Notecdu tableau 1 |
|---|---|---|---|
| Olive-sided Flycatcher | G4 | Canada: N4B (September 2011) United States: N4B (March 2001) |
Alberta (S3) British Columbia (S3S4B) Labrador (S2S3) Manitoba (S3S4B) New Brunswick (S3S4B) Newfoundland Island (S3S4B) Northwest Territories (SUB) Nova Scotia (S3B) Ontario (S4B) Prince Edward Island (S3B) Quebec (S3B) Saskatchewan (S4B,S4M) Yukon (S2S3B) |
Olive-sided Flycatcher is a medium-sized songbird (body weight of approximately 34 g for males and 31 g for females) that reaches a total length of 18–20 cm (Altman and Sallabanks 2012). Its plumage is a deep brownish olive-grey above, with a whitish throat and breast and contrasting streaked olive-grey flanks. It has a relatively short tail, robust beak, and often shows an erected crest on the head. The species is known to perch in open areas and sing a loud and distinctive three-note whistle: quick, THREE BEERS (COSEWIC 2008, Altman and Sallabanks 2012). Olive-sided Flycatcher is an aerial sallying specialist and will often forage for insects near or above the canopy level of the surrounding forest where light intensity is at its maximum (Altman and Sallabanks 2012).
According to the COSEWIC status report, Olive-sided Flycatcher's Canadian population was estimated as 450,000 (COSEWIC 2008), but the latest Canadian population estimate presented in the Partners in Flight Population Estimates Database is 900,000 individuals (Partners in Flight Science Committee 2013). The data quality rating for the Partners in Flight estimate is considered "Beige", which is second only to a rating of "Green". In this case, "Beige" corresponds to a Breeding Bird Survey (BBS) range coverage equal to 1/3 or more of routes (Blancher et al. 2007). The change in the population estimate does not represent an actual increase in the population. It is the result of newer analytical techniques, largely of a refined detection distance used to estimate density. Because of uncertainty in precisely estimating absolute population numbers, the trend of the population is the key metric used to judge population health in this recovery strategy.
Figure 1. Breeding, migrating, and wintering distribution of Olive-sided Flycatcher (adapted from BirdLife International and NatureServe (2013), using data from Haché et al. (2014) and eBird (2014)).
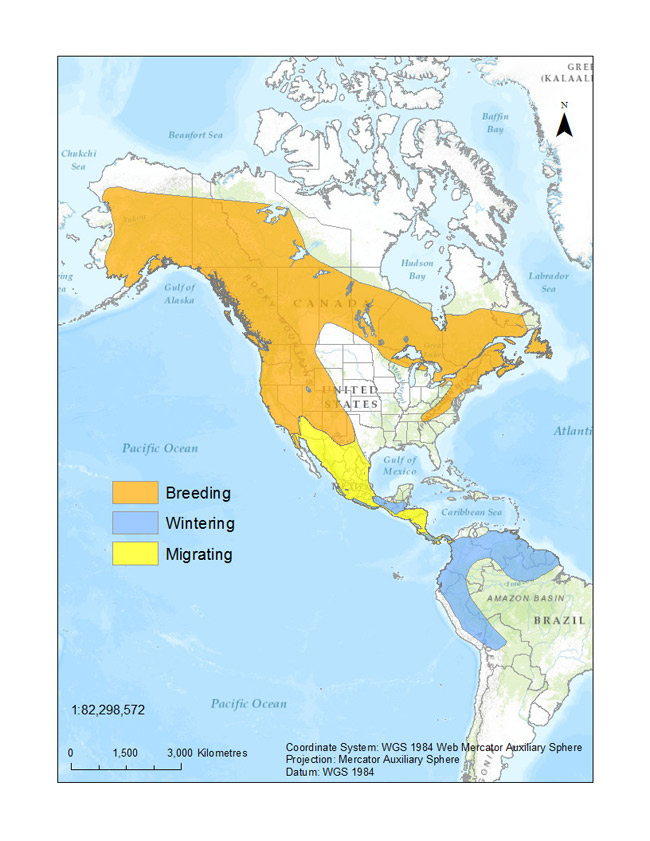
Map: © Environment Canada
Long description for Figure 1
Figure 1 illustrates the distribution of the species in North, Central and South America according to range type. Their breeding range spreads from the southern Northwest Territories in Canada to the north-eastern coast of the United States. Their migrating range is mainly in the eastern United States; from North Dakota to Texas and to the eastern coast. It then makes its way down the eastern coast of Mexico all the way to Costa Rica. Their wintering range begins in Costa Rica and descends into northern South America; from Venezuela to Peru.
In Canada, Olive-sided Flycatcher breeds primarily in boreal, sub-boreal, interior, and coastal forest regions of the country (Figure 1). Although widely distributed across Canada's boreal region and south through the southwestern United States, the highest densities of breeding individuals are reported in the mountainous parts of western Canada and the United States (Figure 2). Based on BBS results (which are largely constrained to the southern portion of the breeding range), 75% percent of the global population breeds in Alaska, British Columbia, the Northwest Territories, Yukon, California, Ontario, Oregon, and Manitoba. Figure 2 underrepresents the distribution of the species because occurrences outside the extent of established road networks, particularly in northern Canada (grey area in Figure 2), are not well captured by the BBS (Cumming et al. 2010).
The BBS analyses indicate that the abundance of this species has decreased significantly, at least in more southern areas of their range where data are available for assessment. Results from the BBS suggest an annual rate of decline in population size of 3.4% (95% confidence interval: –4.4 to –2.35) in southern Canada between 1973 and 2012 (Environment Canada 2014d). This annual rate of decline corresponds to an approximately 80% decline in population size from 1973 to 2009 (Environment Canada 2011c). Similar decreases have also been demonstrated in the United States (Sauer et al. 2011, Altman and Sallabanks 2012). However, there are fundamental problems with interpreting BBS data that relate to coverage and bias (Machtans et al. 2014). BBS data do not sample the species' entire range at random, having lower coverage in locations such as the boreal forest (Haché et al. 2014). The majority of BBS routes tend to be located in southern and disturbed areas of Canada, which may bias population estimates of species with northern distributions, such as Olive-sided Flycatcher (Machtans et al. 2014). BBS data also tend to overestimate Olive-sided Flycatcher densities because of a positive road-side bias (Haché et al.2014). For these reasons, there is uncertainty in estimating population sizes and range-wide trends for this species.
Sub-regional analyses indicate that within the area covered by the BBS, the declines are of similar magnitude across the breeding range of the species, from east to west and north to south. Decreases appear to be greatest in the Northwest Territories, Saskatchewan, Manitoba, Quebec, and New Brunswick (Environment Canada 2014d).
Olive-sided Flycatcher primarily winters in northern South America (Figure 1), but there is limited information available concerning habitat use and key wintering locations. They are most often found in Panama and the Andes Mountains, from northwest Venezuela through Colombia, Ecuador, and eastern Peru to western Bolivia (Altman and Sallabanks 2012).
Figure 2. Relative abundance of Olive-sided Flycatcher recorded on Breeding Bird Survey routes (1993–2012) (map produced by Peter Blancher, Science and Technology Branch, Environment Canada, based on data from the North American Breeding Bird Survey - BBS). Measurement unit = individuals / BBS route / year within each degree block.
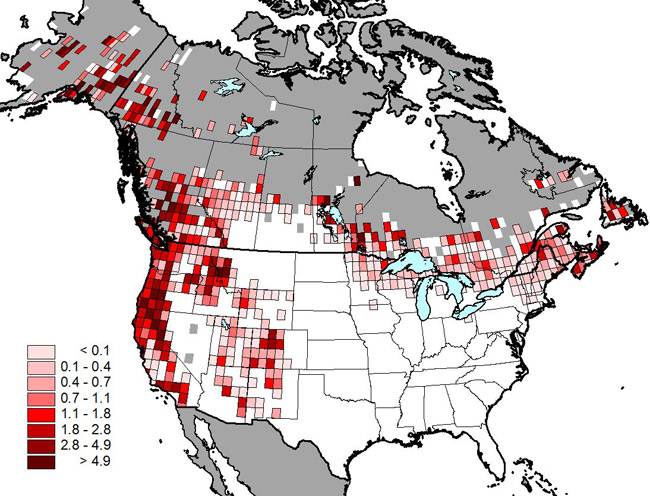
Map: © Environment Canada
Long description for Figure 2
Figure 2 shows that the species is more abundant on the western coast of the United States and Canada, with approximate two times more degree blocks containing two individuals per BBS route per year than in central and eastern North America.
Olive-sided Flycatcher has specific habitat requirements for nesting, brood-rearing, feeding, wintering, and migration. Current understanding of the ecological needs of Olive-sided Flycatcher may be biased because the selection of study sites and associated findings are influenced by site accessibility.
Olive-sided Flycatcher has been widely observed in open coniferous or mixed-coniferous forests, often located near water or wetlands (Cheskey 2007, Altman and Sallabanks 2012) with the presence of tall snags or trees from which the species sallies for prey (flies out to catch prey and returns to perch) and advertises its territory (Brandy 2001, Altman and Sallabanks 2012).
Data gathered from points across Canada indicate that mature conifer stands within patchy landscapes influenced by natural disturbance (e.g., recent burns) support the highest densities of Olive-sided Flycatcher (Haché et al. 2014). Wet areas have a positive effect on the density of Olive-sided Flycatcher at a landscape scale, but a negative effect at a local scale (Haché et al. 2014).
Olive-sided Flycatcher prefers post-burn areas that create open habitats for the species to forage (Hutto 1995, Kotliar et al. 2002, Altman and Sallabanks 2012). In fact, the species may have evolved as a post-fire-dependent species (Hutto and Young 1999). Harvested forests also create these open spaces and are regularly used by Olive-sided Flycatcher (but see section 4.2 Description of Threats -- Breeding Habitat: Forest Harvesting and Silviculture).
The following is a summary of habitat features that are most often associated with high densities and reproductive success in breeding areas:
- Primarily montane and northern coniferous or mixedwood forest (Altman and Sallabanks 2012).
- Open to semi-open areas within forested regions, mostly in early seral Note2de bas de page (including clearcuts) or mature to late-seral forest (Altman and Sallabanks 2012).
- Presence of tall snags and/or residual live trees for nests, singing, and foraging perches (Wright 1997, Altman and Sallabanks 2012).
- Near water or wetlands supporting a high abundance of aerial insects (Altman and Sallabanks 2012).
- Areas where fire, especially intense burns, has created clearings (Robertson and Hutto 2007).
Nests are generally placed toward the tip of coniferous branches (although other tree types have been used), are constructed of twigs, rootlets, and arboreal lichens, and may be lined with grasses and pine needles (Altman and Sallabanks 2012). After fledging, young often remain close to the nest (and each other) for several days and may remain as a family unit until fall migration (Altman and Sallabanks 2012).
Olive-sided Flycatchers have relatively large territories. Documented territory sizes vary depending on landscape features (Wright 1997, Altman and Sallabanks 2012), but are usually between 10 and 20 ha (Altman and Sallabanks 2012). Territories have been noted as large as 45 ha in California (Bock and Lynch 1970).
Olive-sided Flycatcher most often feed on Hymenoptera (bees, wasps, flying ants, etc.), but also prey on a variety of other insects including flies (Diptera), moths (Lepidoptera), grasshoppers (Orthoptera), beetles (Coleoptera), and dragonflies (Odonata) (Altman and Sallabanks 2012).
There is limited information regarding Olive-sided Flycatcher habitat use during the nonbreeding season, but the species is thought to use a wide diversity of habitat types along migration pathways (Kotliar 2007a). In general, it uses substantially more riparian and non-coniferous habitats during migration than while breeding. Habitats during migration include pine–oak, evergreen and semi-deciduous forests and edges (Mexico and northern Central America), highlands (Honduras), pine and oak forests and edges (Guatemala), and second-growth scrubby woodland (Costa Rica) (Altman and Sallabanks 2012).
While wintering, the required habitat elements are thought to be similar to the needs on the breeding grounds -- mature forest with openings containing snags or live trees from which individuals perch and forage for aerial insects (COSEWIC 2008), as well as forest edges associated with wetlands, burns, blowdowns, or clearcuts where remnant trees and snags remain. Although the species is generally found at elevations ranging between 1,000 and 2,000 m, it has been recorded in habitats ranging from 400 to 3,400 m (Altman and Sallabanks 2012).
Olive-sided Flycatcher has life history traits that can reduce its potential for population growth and recovery. Despite its relatively lengthy nesting period (egg-laying, incubation, and nestling period are 36–46 days), its overall time on the breeding grounds is among the shortest of all passerines. Its clutch size is relatively small (3 eggs); it is not known to produce more than one brood per season (common amongst boreal songbirds); it specializes in aerial insects, which increases its vulnerability to inclement weather (Altman and Sallabanks 2012); growth rates of young are slow thereby increasing the nesting period length of the species (Robertson and Hutto 2007); and it has the longest migration distance of any North American flycatcher species (Murphy 1989). Further, the lengthy nesting period increases its likelihood of nest predation (Kotliar 2007a). The result of these limitations is that members of the genus Contopus have the lowest reproductive rate of all passerines in North America (Murphy 1989, Altman and Sallabanks 2012). These factors limit the species' ability to adapt to threats and possibly recover from such threats once they are alleviated.
| Threat Category | Threat | Level of Concern Notea.1du tableau 2 | Extent | Occurrence | Frequency | Severity Noteb.1du tableau 2 | Causal Certainty Notec.1du tableau 2 |
|---|---|---|---|---|---|---|---|
| Changes in Ecological Dynamics or Natural Processes | Reduced availability of insect prey (ultimate causes: loss of insect-producing habitats, prey–breeding temporal mismatch due to climate change, habitat acidification, and pesticides) | High | Widespread | Current | Continuous | Moderate | Medium |
| Changes in Ecological Dynamics or Natural Processes | Fire suppression | High | Widespread | Current | Recurrent | Moderate | Medium |
| Habitat Loss or Degradation | Nonbreeding habitat: deforestation and land conversion | High | Widespread | Current | Continuous | Moderate | Medium |
| Habitat Loss or Degradation | Breeding habitat: forest harvesting and silviculture | Medium | Widespread | Current | Continuous | Moderate | Medium |
| Habitat Loss or Degradation | Energy and mining (exploration and extraction) | Medium | Widespread | Current | Continuous | Moderate | Low |
| Habitat Loss or Degradation | Breeding habitat: residential and commercial development | Unknown | Widespread | Current | Continuous | Unknown | Low |
| Climate and Natural Disasters | Habitat shifting and alteration | Unknown | Widespread | Current | Continuous | Unknown | Low |
| Climate and Natural Disasters | Temperature extremes and storms | Unknown | Widespread | Current | Seasonal | Unknown | Low |
| Pollution | Pesticides (direct effects) | Unknown | Localized | Current | Seasonal | Unknown | Low |
| Pollution | Mercury | Unknown | Widespread (E. Canada) | Current | Continuous | Unknown | Low |
| Pollution | Acid precipitation | Unknown | Widespread (E. Canada) | Current | Continuous | Unknown | Low |
| Accidental Mortality | Collisions with anthropogenic structures and vehicles | Low | Localized | Current | Recurrent | Low | Low |
| Exotic, Invasive, or Introduced Species/Genome | Problematic native and non-native species | Unknown | Widespread | Current | Continuous | Unknown | Low |
Threats are listed here in the order in which they are presented in Table 2. The Boreal Songbird Initiative estimated that 17% of Olive-sided Flycatcher habitat in the boreal forest of Canada has been disturbed by anthropogenic activities (Boreal Songbird Initiative 2012), but the extent to which those disturbances directly affect the species' abundance, survival, and productivity has not been quantified.
Most information pertaining to threats is a result of studies on the breeding grounds. Nonetheless, threats in nonbreeding areas may be particularly important for this species (Altman and Sallabanks 2012).
The populations of aerial insectivores are showing dramatic declines, particularly in northeast North America (Nebel et al. 2010). The trait common to all species in this diverse group is insectivory, which has led multiple researchers to implicate a reduction in available insect prey in breeding, migratory, and/or wintering areas as a probable contributing factor in declining population trends (Nebel et al. 2010, Paquette et al. 2014).
Insect populations are exhibiting significant declines worldwide. A recent review of global faunal population trends noted that 33% of all insects with available IUCN-documented population trends were declining and many also exhibited range retractions (Dirzo et al. 2014). These declines are considered a global pattern, but are more severe in heavily disturbed locations, such as the tropics (Dirzo et al. 2014). Specifically, bees, a significant component of Olive-sided Flycatcher's diet, have exhibited dramatic population declines and range retractions (Cameron et al. 2011). The possible causes for reduced availability of insect prey are identified and described below.
Many insects are limited to specific habitat for some part of their life cycle, and any process that diminishes these habitats may harm them. Over 90% of insect groups considered threatened are impacted by habitat loss or degradation (Price et al. 2011). A number of human activities alter or destroy natural habitats necessary for particular insect life stages, including wetland drainage and peat extraction, intensive agriculture, wetland destruction, industrial activities, and urban development (U.S. Bureau of Land Management 1978, Benton et al. 2002, Price et al. 2011, Brooks et al. 2012). For example, the drilling, construction, and development associated with oil and gas extraction can lead to the loss of insect habitat and result in reductions in insect populations and changes in species compositions (U.S. Bureau of Land Management 1978). Foster (1991) noted the drainage of wetlands and peat extraction as a significant threat facing insect populations.
The effects of habitat loss for insects are not restricted to Olive-sided Flycatcher's breeding range; they could also be affecting their migrating and wintering range. In general, insect responses to changes in land use in the tropical Andes are context dependent, but some research has shown that species richness, diversity, and abundance decline in response to land-use disturbances (Larsen et al. 2011).
Birds often exhibit a strong synchronization between their reproductive timing (i.e., hatching) and peak food abundance, but climate change has caused the timing of peaks in some insects to advance (Both et al. 2009). Because warming is less severe in Olive-sided Flycatcher’s wintering areas than in their breeding grounds, they may experience migration cues at dates that are too late for them to arrive at breeding grounds at the optimal time (Jones and Cresswell 2010). As a result, climate change is creating a temporal mismatch between reproduction and maximal prey abundance for species that are not adapting to the changing climate at the same rate as their prey (Strode 2003). Both et al. (2006) found that an aerial insectivore in the Netherlands, Pied Flycatcher (Ficedula hypoleuca), had declined by 90% between 1987 and 2003 in areas where the prey peaked too early in the breeding season to provide adequate food for nestlings. Great Tits (Parus major) have exhibited a mismatch between optimal timing of nestlings and peak caterpillar biomass as a result of recent warming (Visser et al. 2006). Both the weight and the number of chicks that fledged were affected by their timing in relation to this peak (Visser et al. 2006). A prey–breeding temporal mismatch has also been linked to the population declines of migrant birds across Europe (Møller et al. 2008, Saino et al. 2011) and is believed to be contributing to the declines of other avian species heavily reliant on invertebrates, such as Rusty Blackbird (Euphagus carolinus) (McClure et al. 2012).
Populations of migratory birds that exhibit long-distance migrations and breed in seasonal habitats are more vulnerable to climate change because the temporal mismatch is more probable and more severe (Both et al. 2006, Both et al. 2009). Although no species-specific data are currently available, Olive-sided Flycatcher is an insectivore, migrates long distances, and breeds/forages in seasonal habitats, so a climate-induced mismatch between breeding and prey availability is probable
Since the 1980s, there has been a substantial decline in the rate of acid deposition, but acidifying compounds (e.g., sulphur dioxide and nitrogen oxide) are still being released into the environment (Shannon 1999, Environment Canada 2014c). Acidification of surface water can reduce the abundance and diversity of flying insects that are aquatic for part of their life cycle (Graveland 1998). Although much of Olive-sided Flycatcher's prey (e.g., bees and wasps) do not have an aquatic phase, other reported prey items such as dragonflies and flies (Altman and Sallabanks 2012) that have aquatic phases may be affected by habitat acidification. Reduced reproductive success of Tree Swallows (Tachycineta bicolor) nesting near acidified wetlands in Ontario was linked to changes in available calcium-rich prey for nestlings (Blancher and McNicol 1991) and acidification of forests was implicated in the decline of the Wood Thrush (Hylocichla mustelina) (Hames et al. 2002). Nevertheless, a study in central Ontario showed no difference in forest songbird productivity between acidified and non-acidified sites (Mahony et al. 1997). Habitat acidification has implications for birds in areas of local, severe acid deposition and eastern North America where soil buffering is relatively poor due to low pH. For example, acid rain has been suggested as a threat in the southern Appalachian Mountains (LeGrand and Hall 1989). Nevertheless, at present, there is no evidence to support a range-wide effect of reduced insect prey as a result of habitat acidification.
Aerial insectivores breeding in North America and exhibiting population declines have wintering ranges that consist, or partially consist, of countries with high expenditures on insecticides; insecticide expenditures in wintering ranges was the best significant predictor of the index of abundance of these species (Nocera et al. 2014). Nevertheless, the direct mechanisms for the population declines (e.g., reduced insect availability and lethal exposure) are unknown (Nocera et al. 2014).
Most organochlorine pesticides (chemicals in the same family as dichlorodiphenyltrichloroethane -- DDT) have been banned in North America for decades, but there is indication that Neotropical migrant insectivores are still being exposed to organochlorine pesticides throughout their ranges (Sager 1997, Klemens et al. 2000). These chemicals can have long-lasting effects on insect communities and thus the birds that rely on them. Dietary records of Chimney Swifts (Chaetura pelagica) confirmed a marked decrease in beetlesand an increase in true bugs (Hemiptera) that was temporally correlated with a steep rise in DDT and its metabolites. Nocera et al. (2012) argued that DDT caused declines in Coleoptera and dramatic (possibly permanent) shifts in the insect communities, resulting in a nutrient-poor diet and ultimately a declining Chimney Swift population.
The harmful effects of chemical insecticides have led to the increased use of biological insecticides. Currently, insecticides used for forestry operations in Canada are mainly biological (Bacillus thuringiensis var. kurstaki (Btk)) and target larval Lepidoptera such as Jack Pine Budworm (Choristoneura pinus) and Spruce Budworm (Choristoneura fumiferana). The average area sprayed per year with Btk across Canada's forests between 1988 and 2000 was 273,440 ha (range: 73,209–855,535 ha) (NFD 2014). In 2012, Btk was sprayed in the forests of four Canadian provinces: Quebec (98,044 ha), Manitoba (828 ha), Saskatchewan (15,639 ha), and British Columbia (116,012 ha) (NFD 2014). On average, Quebec sprays the most forest area with Btk per year (1988–2012) (NFD 2014). Although many microbial insecticides are considered non-toxic to birds, their indirect effects caused by changes in available prey items remain inconclusive. A 12,803 ha area of Vancouver Island, British Columbia, exhibited no difference in species richness or relative abundance of songbirds 1 year after being sprayed with Btk to control for Gypsy Moth (Lymantria dispar) (Sopuck et al. 2002). Holmes (1998) found that the nestling survival and growth of Tennessee Warblers (Vermivora peregrina) were unaffected by sites treated with Btk in Ontario, and although nests in sprayed sites had smaller clutches, smaller broods, and lower hatch rates, the differences were not significant. Other studies have found significant indirect impacts of microbial pesticides to birds. Spruce Grouse (Dendragapus canadensis) chicks had significantly slower growth rates in an area treated with Btk in Ontario compared with chicks raised in study sites not treated with Btk (Norton et al. 2001). Norton et al. (2001) attributed this to the reduction in available Lepidoptera larvae as a result of spraying. In France, House Martins (Delichon urbicum) at sites treated with Bacillus thuringiensis var. israelensis (Bti) exhibited a change in diet from Nematocera (which are Bti-sensitive), spiders (Araneae), and dragonflies (Nematocera predators) to flying ants (Poulin et al. 2010). This dietary change resulted in lower clutch size and fledgling survival (Poulin et al. 2010). Bti may be used for mosquito and black fly control programs throughout Olive-sided Flycatcher's breeding, migratory, and wintering range.
Neonicotinoid insecticides were introduced in the 1990s, and although their rates of use are poorly known across Olive-sided Flycatcher's range, nearly 11 million ha of cropland across the Canadian Prairies were estimated to be treated with neonicotinoids (Main et al. 2014). Neonicotinoids are generally used on agricultural lands, but have been detected in wetlands (Main et al. 2014) and waterways in Canada (Environment Canada 2011b, Xing et al. 2013). Bees, a major food source of Olive-sided Flycatcher, are exhibiting substantial population declines, thought to be in part due to neonicotinoid use (Gill et al. 2012, Whitehorn et al. 2012). Olive-sided Flycatcher's habitat generally does not include cropland, so the impacts of neonicotinoids may be small, even given the insecticides mobility and persistence in the environment (Hladik et al. 2014). Nevertheless, Mineau and Palmer (2013) suggested that the effects of neonicotinoids to birds may not be limited to the farm scale, but likely expand to the watershed or regional scale; therefore, neonicotinoids could be impacting insect and bird species found outside of the arable lands and have been included here as a contributing factor to the threat. Neonicotinoids are adversely affecting insect populations and in 2013 the European Food Safety Authority declared that they posed "unacceptable" risk to insects (Goulson 2014). In the Netherlands, neonicotinoid concentrations in surface waters were correlated with the declines in farmland insectivorous birds (Hallmann et al. 2014). Hallmann et al. (2014) suggested these declines were likely caused by a reduction of insect prey as a result of insecticide use. The indirect effects of these insecticides have also been noted in Skylark (Alauda arvensis), Yellowhammer (Emberiza citronella), Whinchat (Saxicola rubetra), Reed Bunting (Emberiza schoeniclus), and Corn Bunting (Miliaria calandra) (Boatman et al. 2004, Gibbons et al. 2014).
Wildfires create a spatio-temporal variation in Olive-sided Flycatcher habitat across the landscape (Kotliar 2007b). For much of the 20th century, suppression of wildfire to protect forest resources and rural communities was the management norm. The effectiveness of fire suppression programs in Canada is such that 97% of all forest fires are contained before they reach 200 ha in size (Stocks et al. 2003). Even in the boreal forest, Cumming (2005) concluded that fire suppression by initial attack of fires significantly reduced the area burned over recent decades and suggested this will persist into the foreseeable future. Decades of fire suppression have resulted in longer fire intervals with reduced available burned habitat for Olive-sided Flycatcher.
Prescribed burns are a forest management tool that can create optimal Olive-sided Flycatcher habitat. However, in Canada, prescribed fire as a management technique is relatively uncommon on a landscape scale (Taylor 1998) and is used mainly on Parks Canada and First Nations lands (Weber and Taylor 1992).
Because post-fire habitats generally remain suitable as Olive-sided Flycatcher habitat for a relatively short period of time, repeated burns on a single parcel of land (Kotliar 2007b), a shifting mosaic of prescribed burns, and/or no-suppression policies are required to ensure long-term availability of suitable habitat in areas where post-burn habitats are important.
Deforestation on the wintering grounds has been identified as a threat to Olive-sided Flycatcher and other avian species that winter on and along the northern slopes of the Andes Mountains of South America (Environment Canada 2011a, Altman and Sallabanks 2012, BirdLife International 2014). This conclusion is generally based on the intense levels of deforestation in these areas, rather than a direct causal relationship between the Olive-sided Flycatcher population and this threat.
In 1991, it was determined that the forested area in the northern Andes (Peru, Ecuador, and Colombia) had declined by approximately 90% from its historical levels (Henderson et al. 1991), and by 1998, an estimated 180,600 km2 (69%) of the Andean forests in Colombia were cleared for agriculture (Etter et al. 2006). Although there may have been some local gains over the past decade (Sánchez-Cuervo et al. 2012), large tracts of forested area within Olive-sided Flycatcher's wintering range (particularly in Colombia) are continuing to exhibit overall trends of forest loss (Portillo-Quintero et al. 2012, Hansen et al. 2013). The ultimate causes of deforestation vary locally, but have been identified as human encroachment, increased pasture area, conversion of shade coffee to sun-tolerant coffee, timber harvest, plantations of native fruits (naranjilla), agricultural activities, and monocultures (Davis et al. 1997, Portillo-Quintero et al. 2012, BirdLife International 2014).
Hansen et al. (2013) also noted substantial forest loss between 2000 and 2012 throughout large portions of Olive-sided Flycatcher's migratory range, particularly in Central America.
Logging and wood harvesting is the third largest cause of deforestation in Canada, after agriculture and residential and commercial development (Masek et al. 2011). Harvest rates in Canada are highest in Quebec, British Columbia, and Ontario and have been relatively stable in Canada since the 1980s (Masek et al. 2011). Between 2000 and 2012, approximately 11,041,217 ha of forest were harvested throughout Canada (NFD 2014). Forestry practices vary across Olive-sided Flycatcher's range.
Forest harvesting and silviculture, in general, can have a short-term negative impact on nesting birds by disrupting breeding activities (Hobson et al. 2013). The nests and/or eggs can be inadvertently harmed or disturbed as a result of clearing trees and other vegetation (e.g., pre-commercial thinning) (Environment Canada 2014a). Nesting failure could also result from disruptive activities experienced by a nesting bird (Environment Canada 2014a). Hobson et al. (2013) estimated that between 616,000 and 2.09 million nests (of many species) are lost annually as a result of industrial forest harvesting.
Many studies have shown that numbers of Olive-sided Flycatcher respond positively to some types of forest harvesting, particularly when snags and residual trees remain for perching and nesting (Altman and Sallabanks 2012). For example, Olive-sided Flycatcher moved into areas within 3 years of shelterwood and green tree retention harvesting (but not patch cuts and clearcuts) being applied to an old-growth forest in British Columbia (Beese and Bryant 1999). Chambers et al. (1999) regularly found Olive-sided Flycatcher in two-story (i.e., green tree retention) stands and modified clearcuts, but the species was rarely observed in small-patch and unharvested stands. Olive-sided Flycatcher also increased after silviculture thinning in Oregon (Hagen et al. 1997). It is unknown whether there is a threshold (e.g., % area of landscape) beyond which the species may begin to exhibit a negative numerical response to forest harvesting.
Although some forest harvesting techniques attempt to mimic natural disturbances and Olive-sided Flycatcher is often attracted to post-harvest areas, forest harvesting results in features that are unlike post-fire habitats (e.g., green trees and coarse woody debris often remain). Robertson and Hutto (2007) found that nest success was twice as high in a burned plot, compared with a selectively harvested plot. Selectively harvested forests could be functioning as ecological traps for Olive-sided Flycatchers, where the habitat superficially appears to be optimal, thus attracting the birds, but does not offer the lower nest predator abundance that a post-burn habitat would offer (Robertson and Hutto 2007). Nest predators (Red Squirrels – Tamiasciurus hudsonicus, Common Raven – Corvus corax, and Gray Jays – Perisoreus canadensis) were twice as abundant in the harvested plot, compared with the burned plot. Currently, only one study (Robertson and Hutto 2007) has provided evidence of this ecological trap for Olive-sided Flycatcher, and contrary to these findings, Olive-sided Flycatcher in California had higher reproductive success in logged habitats (Meehan et al. 2003).
An increase in nest predation may be linked to the habitat structure that exists following anthropogenic activities. It is likely that the structural elements that remain after anthropogenic disturbance (e.g., density of snags, size of remaining standing trees, and extent of open areas) determine the quality of the habitat and the abundance of predators and brood parasites, rather than specifically how the habitat was disturbed (e.g., fire burn versus cutting) (COSEWIC 2008).
Forest management activities also involve other practices that may adversely affect features considered important for Olive-sided Flycatcher habitat. These activities can include (depending on location) large-scale, single-species, even-aged plantings that lack diversity and structure and result in undesirable habitat, short stand rotations that reduce the abundance of old forest habitats with appropriate gap dynamics and high snag densities, herbicide use and insecticide use that reduce insects and vegetation, alteration of water drainage patterns, lack of retention buffers along water edges, and salvage logging. The practice of post-fire salvage logging reduces snag and remnant stand availability and, therefore, lowers habitat suitability. Olive-sided Flycatcher (in fact, most insectivorous birds) were less numerous in salvaged versus unsalvaged burned forest in Saskatchewan (Morissette et al. 2002).
Exploration to find energy (e.g., oil, gas, and hydroelectricity) and mineral resources, exploitation of these resources (e.g., flooding of large areas to create reservoirs and mine residues), and the creation of corridors for transportation (e.g., pipelines, transmission lines, and roads) have caused substantial habitat loss, degradation, and fragmentation in some portions of the Olive-sided Flycatcher's range (Drummond and Loveland 2010, Masek et al. 2011, Birch and Kaye 2012). Activities associated with these industries can also lead to the unintentional destruction of nests, eggs, nestlings, and/or adults (Van Wilgenburg et al. 2013).
Van Wilgenburg et al. (2013) estimated that approximately 48,400 ha of areas are disturbed annually from the construction of wells, pipelines, and seismic lines in the Boreal Ecozone of the Western Canadian Sedimentary Basin. This coincides with approximately 7,301 nests (of many species) being lost annually in this area (Van Wilgenburg et al. 2013). The construction of wells, pipelines, and seismic lines is especially prevalent in northern Alberta and northeastern British Columbia (Schneider et al. 2003, Calvert et al. 2013, Van Wilgenburg et al. 2013). Although Olive-sided Flycatcher tends to associate with fragmented landscapes, Haché et al. (2014) found that the density of this species was negatively affected by linear disturbances on the landscape, and therefore, the species may be negatively affected by such construction. Pipeline and associated road construction are also proceeding locally in the species' wintering range in the northern Andes (Davis et al. 1997).
Mining activities occur across the Canadian range of Olive-sided Flycatcher and include a large variety of targets (e.g., gold, diamonds, zinc, lead, and copper) (Stothart 2011). The total area under mineral lease in Canada is 2.1 million ha (Cheng and Lee 2014); provinces/territories with the most boreal forest zone dedicated to mineral leases include Alberta (3,206 km2), Ontario (1,686 km2), Manitoba (1,463 km2), and the Northwest Territories (1,431 km2) (Cheng and Lee 2014).
In northern parts of the species' range, especially the boreal forest, Olive-sided Flycatchers are often associated with water, such as lakes, rivers, bogs, ponds, muskegs, and wooded shorelines (Altman and Sallabanks 2012). This likely results from higher insect abundance in these areas (Altman and Sallabanks 2012). As a result, it seems reasonable to expect that activities that permanently remove forest habitat and alter hydrological regimes would have an effect on Olive-sided Flycatcher where the activities and the species overlap.
Residential and commercial development leads to permanent habitat loss and is considered the leading cause of deforestation in the United States and a contributing factor to deforestation in Canada, especially in southern Ontario and Quebec and southwestern British Columbia (Radeloff et al. 2005, Sun et al. 2007, Latendresse 2008, Masek et al. 2011). Urban development results in habitat loss for Olive-sided Flycatcher, but the effects of this development on its population size are unknown. Manley et al. (2006) found that the abundance of Olive-sided Flycatcher in remnant forest stands near the Lake Tahoe Basin decreased with increasing amounts of development activity in the area. As a bird of the northern boreal forests and mountainous regions of the west, urbanization has likely been only a minor factor in loss of habitat for the species, although it may have been more significant in southern regions of Olive-sided Flycatcher habitat and near isolated urban areas in western Canada.
Migratory bird species that travel long distances are dependent on multiple, spatially disparate habitats during their annual cycle (breeding, migration, and wintering). This makes them particularly sensitive to the impacts of climate change because any change along the route could negatively impact the population (Newson et al. 2009, Robinson et al. 2009). There is little information to directly link climate change to the population decline of Olive-sided Flycatcher, but Cumming et al. (2014) suggested a large potential for avian distributional shifts in response to climate change.
Tropical storms can kill large numbers of aerial insectivores migrating in the autumn; for example, a single hurricane (Hurricane Wilma 2005) had a measurable effect on the population of another aerial insectivore, the Chimney Swift (Dionne et al. 2008). The deleterious effects of cold, wet weather during the breeding season are well known for other aerial insectivores (e.g., Brown and Brown 2000) and such weather extremes are expected to occur more frequently because of climate change (Huber and Gulledge 2011).
Although it is plausible that these weather extremes negatively affect Olive-sided Flycatcher populations, it is also possible that these extremes could have positive effects. Fire activity is strongly influenced by weather (Flannigan et al. 2009), and the extent, intensity, and frequency of forest fires are projected to increase because of warmer springs and summers and decreases in water availability (Flannigan et al. 2009, North American Bird Conservation Initiative US Committee 2010, de Groot et al. 2013, Girardin et al. 2013). This could create post-burn habitat that is suitable for Olive-sided Flycatcher.
Mineau and Whiteside (2013) suggested that pesticides be strongly considered in efforts to identify the causes of bird population declines in North America, especially for those species that breed, winter, or migrate through agricultural areas. They were unable to separate between the direct (i.e., toxicity through ingestion of products such as coated seeds, inhalation, absorption through the skin, or by eating contaminated prey) and indirect (e.g., habitat or disruption of the food chain) effects of pesticides, and they concluded that both are likely occurring (Mineau and Whiteside 2013). Although largely undocumented for this species, pesticide use on both breeding and wintering grounds has been implicated in direct mortality and habitat loss of avian species (e.g., Chamberlain et al. 2000, Boatman et al. 2004, Mineau 2005).
Most organochlorine pesticides (chemicals in the same family as DDT) have been banned for decades in North America. Little is known about the extent to which Olive-sided Flycatcher and other Neotropical migrant passerines were exposed to organochlorine pesticides throughout their lifetime (Gard et al. 1993, Klemens et al. 2000), but there is some indication that Neotropical migrant insectivores are still being exposed to organochlorine pesticides in North America (Sager 1997, Klemens et al. 2000). This may be legally through loopholes in the restriction laws or illegally. These pesticides may still be in use in Central and South America (Klemens et al. 2000, Lebbin et al. 2010, Nebel et al. 2010) for nuisance mosquito control and agricultural or other applications. Endosulfan (which is primarily used on a wide variety of food crops) is an exception to the ban of organochlorine pesticides, but will be phased out of use in the United States by 2016 because it was deemed to pose an unacceptable risk to farmworkers and wildlife (U.S. Environmental Protection Agency 2010). Birds, in general, are fairly sensitive to endosulfan poisoning (U.S. Environmental Protection Agency 2010). Several other countries have followed suit acting to ban the chemical through the Stockholm Convention on Persistent Organic Pollutants: an international environmental treaty signed in 2001 (Secretariat of the Stockholm Convention 2011).
Organophosphorus/organophosphate and carbamate compounds have been used increasingly since the majority of organochlorine pesticides were restricted in North America in the 1970s and banned in the 1980s (Commission for Environmental Cooperation of North America 2003). Birds and other vertebrate species are susceptible if they ingest or otherwise absorb enough organophosphate or carbamate pesticides, and birds appear to be more sensitive than other vertebrates (Freedman 1995, Friend and Franson 1999).
The direct impacts of a relatively new class of pesticides, neonicotinoids, are unknown for insectivorous species such as Olive-sided Flycatcher (Mineau and Palmer 2013, Goulson 2014). Hallmann et al. (2014) correlated neonicotinoid concentrations in surface waters to declines in insectivorous birds in the Netherlands. They suggested the declines are in relation to a reduction of insect prey, but they could not rule out direct pathways in which the neonicotinoids may have had an effect on the birds.
The exposure of Olive-sided Flycatcher to neonicotinoid pesticides is unknown but, given its habitat preferences, is probably low on its breeding grounds even given the pesticide's mobility and persistence in the environment (Hladik et al. 2014).
Mercury is a naturally occurring element that is enriched in the environment by human activities. Long-range atmospheric transport and deposition is the dominant source of mercury to many aquatic habitats over much of the landscape (Fitzgerald et al. 1998, U.S. Geological Survey 2000). Bio-available mercury is also mobilized within watersheds by forestry activities, hydroelectric reservoir creation, and various industrial-related activities (Porvari et al. 2003, Vuori et al. 2003, Wiener et al. 2003). Large amounts of mercury accumulated over thousands of years in peatlands, and currently underlain by permafrost, also have the potential to release mercury to the environment (Rydberg et al. 2010) in some parts of the Olive-sided Flycatcher's range. Mercury concentrations in aquatic food webs are usually correlated with low pH levels, and as a result, mercury concentrations increase from west to east across Canada in freshwater food webs (Depew et al. 2013).
Mercury exposure can decrease reproductive success, alter immune responsiveness, and cause behavioural and physiological effects in birds (Scheuhammer et al. 2007, Hawley et al. 2009). Research by Keller et al. (2014) and Rimmer et al. (2010) suggested that mercury is biomagnifying in terrestrial songbirds that eat invertebrates. Olive-sided Flycatcher may be exposed in some parts of its range to elevated methylmercury (MeHg; toxic form of mercury) because it consumes predatory insects from acidic wetlands where mercury is easily converted to methylmercury (Greenberg and Matsuoka 2010, Evers et al. 2011, Edmonds et al. 2012). A recent large-scale study of mercury in an insectivorous bird, the Rusty Blackbird, emphasized the potential threat of mercury, especially to the population in northeastern North America (Edmonds et al. 2010). The feathers of Rusty Blackbirds breeding in the Acadian forest ecoregion of New England and the Maritimes (Maine, New Hampshire, Vermont, New Brunswick, and Nova Scotia) had mercury concentrations that were orders of magnitude higher than concentrations observed in the wintering sites in the southern United States and breeding sites in Alaska (Edmonds et al. 2010).
Acid precipitation has been identified as a contributing factor in the decline of spruce–fir forests throughout eastern United States (U.S. Environmental Protection Agency 2014), and this is presumably occurring in Canada as well. Acidification may modify habitat leading to altered soil invertebrate assemblages (see section Reduced Availability of Insect Prey), loss of favoured nesting and/or foraging sites (Hames et al. 2002), increased vigilance and incubation, and increased predation risk (Brotons et al. 1998). Acidification of forests also contributes to the leaching of calcium from soils, a phenomenon that is particularly marked in the northeastern part of the continent (Driscoll et al. 2001), where soil buffering is relatively poor due to low pH and nitrogen saturation (i.e., nitrates can remove additional calcium from the soil) (U.S. Environmental Protection Agency 2014). Passerines must obtain calcium from their food during the egg-laying period (Hames et al. 2002), and calcium deficiency during this time may lead to birds laying eggs with shells that are thin, weak, and more porous that can lead to breeding failure. Although there is no direct evidence for Olive-sided Flycatcher, acidification of its breeding habitat could negatively affect the species. Acidification has been implicated in the decline of Wood Thrush (Hames et al. 2002), as well as other passerine birds from northern Europe (Graveland and Drent 1997, Mänd et al. 2000).
Collisions with buildings, telephone and power lines, communication towers, wind turbines, and other vertical human structures can result in localized mortality for many bird species, particularly during migration.
Approximately 25 million birds (of many species) are killed annually in Canada from collisions with windows (Machtans et al. 2013) and between 365 and 988 million birds are killed each year in the United States (Loss et al. 2014a). Olive-sided Flycatchers had approximately average risk of mortality due to collisions across all building types when compared with all other species with available data (1.1 times at greater risk than the average species) (Loss et al. 2014a), but were at 3.2 times at greater risk of collisions with high-rise buildings compared with the average species' collision risk (Loss et al. 2014a). It is unclear whether the number of fatalities at windows is sufficient to affect population levels.
It is estimated that 2.5–25.6 million birds (of many species) are killed each year by transmission lines in Canada (Rioux et al. 2013) and between 12 and 64 million birds are killed each year by power lines in the United States (8–57 million of these by collisions and 0.9–11.6 million by electrocution) (Loss et al. 2014c). The impact of these collisions has not been quantified for Olive-sided Flycatcher.
An estimated 6.8 million birds (of many species) are killed by collisions with communication towers each year in the United States and Canada (Longcore et al. 2012). Mortality is most frequent for Neotropical migrants, but the ratio of collision mortality to estimated population size for Olive-sided Flycatcher was among the lowest of all species recorded (Longcore et al. 2013).
Approximately 23,300 birds of many species are killed each year from collisions with wind turbines (Zimmerling et al.2013). Almost 50% of the deaths from collisions with wind turbines are predicted to occur in Ontario (Zimmerling et al.2013). The impact of these types of collisions has not been quantified for Olive-sided Flycatcher.
Bishop and Brogan (2013) estimated that approximately 3,462 birds (of many species) were killed per 100 km of 1- and 2-lane paved roads outside of major urban centers in Canada during each breeding season, and Loss et al. (2014b) estimated that between 89 and 340 million birds die each year in the United States from vehicle collisions. Although there are exceptions, in general, mortality rates due to vehicle collisions often increase with increasing traffic speed, road corridor width, and road elevation (above surrounding land) (Case 1978, Baudvin 1997, Loss et al. 2014b). Passeriformes make up 40% of all avian vehicle-collision casualities in North America, but Olive-sided Flycatcher was not recorded in any of the 28 studies that surveyed roads in North America reviewed by Bishop and Brogan (2013).
Domestic and feral cats are the largest source of human-related mortality of birds in Canada (Calvert et al. 2013). An estimated 2%–7% of all birds in southern Canada are killed by cats annually (Blancher 2013). Although cats are less of a concern in more northern areas, Olive-sided Flycatcher would be vulnerable to cats in southern and rural parts of its breeding range, as well as along migration routes.
Gray Jays, Steller's Jays (Cyanocitta stelleri), Common Ravens, Northern Flying Squirrels (Glaucomys sabrinus), Red Squirrels, and Douglas Squirrels (Tamiascriurus douglasii) are suspected predators of Olive-sided Flycatcher eggs and nestlings (Altman and Sallabanks 2012). Peregrine Falcon (Falco peregrinus) are suspected predators of adult Olive-sided Flycatchers (Altman and Sallabanks 2012).
Owing to the large and possibly irreversible changes to Olive-sided Flycatcher habitat over its breeding, migratory, and wintering range, the restoration of this population to some former larger level is likely unachievable. However, because there are currently adequate numbers of individuals to ensure continuing reproductive output sufficient to maintain breeding populations, it is reasonable to set objectives to halt the population decline and subsequently increase the population over a period of time.
- The short-term population objective for Olive-sided Flycatcher in Canada is to halt the national decline by 2025 (i.e., 10 years after this recovery strategy is posted on the Species at Risk Public Registry), while ensuring the population does not decrease more than 10% over this time.
- The long-term (after 2025) population objective is to ensure a positive 10-year population trend for Olive-sided Flycatcher in Canada.
- The distribution objective for Olive-sided Flycatcher is to maintain the current extent of occurrence (the area that encompasses the geographic distribution of all known populations) in Canada.
The population objectives address the species' long-term decline, which was the reason for its designation as Threatened (COSEWIC 2008). Short comings with the BBS dataset (see section 3.2 Population and Distribution) for this species are acknowledged and this strategy includes approaches to improve monitoring for Olive-sided Flycatcher. As new information becomes available, population and distribution objectives might be revised, as appropriate to species recovery.
The 10-year time frame was deemed appropriate to assess population change in the Olive-sided Flycatcher. This time frame was selected because halting the decline of a species is challenging, and cannot be done in just a few years, and because COSEWIC species assessments occur every 10 years. Their criteria for assessment include reviewing population change within 10-year windows.
These objectives will be reviewed during the development of the report required 5 years after this strategy is posted to assess the implementation of the strategy and the progress towards meeting its objectives (s. 46 SARA).
Numerous activities have been initiated since the latest COSEWIC assessment (COSEWIC 2008). The following list is not exhaustive, but is meant to illustrate the main areas where work is already underway, to give context to the broad strategies to recovery outlined in section 6.2. Actions completed or underway include the following:
- The American Bird Conservancy and other partner groups have initiated a research project to determine migratory connections between Olive-sided Flycatcher breeding and wintering populations and to determine the role of prey resources as a limiting factor on the breeding grounds. Subsequent work will target locations and partnerships for conservation action based on the results of the initial efforts (Hagelin et al. 2013).
- A multi-species effects assessment (which includes Olive-sided Flycatcher), as part of the Joint Oil Sands Monitoring project.
- Relative habitat suitability mapping has been completed by the Boreal Avian Modelling Project (Haché et al.2014).
- Observational studies of habitat use to identify the characteristics that distinguish suitable habitat and create species distribution models for the Maritimes (A. Westwood, pers. comm.).
- Investigation into the impacts of climate change, habitat degradation, migratory behaviour and connectivity, and productivity of Olive-sided Flycatchers breeding in Alaska (T. Stehelin, pers. comm.).
- Completion and publication of Bird Conservation Region plans for Canada that identify conservation objectives and actions for priority bird species (including Olive-sided Flycatcher) (Environment Canada 2014b).
- Research regarding the broad-scale predictors of aerial insectivore declines across North America (e.g., Morrissey et al. (2014)).
- The Newfoundland Landbird Recovery Team is currently recovery planning for Olive-sided Flycatcher.
- Forestry and silviculture practices and initiatives in areas across the country that attempt to preserve habitat features thought to be important for Olive-sided Flycatcher and/or identify occupied habitat.
- During environmental assessments and land-use development projects across Canada, Olive-sided Flycatcher is considered and mitigative measures are established.
In Canada, there has been little conservation work specifically targeting Olive-sided Flycatcher. However, several conservation-oriented research, planning, and education projects have been implemented in Canada and the United States that either include Olive-sided Flycatcher in the framework of activities or specifically target the species as a focus of efforts. These include the following groups and/or projects:
| Threat or Limitation | Broad Strategy to Recovery | Priority | General Description of Research and Management Approaches |
|---|---|---|---|
| All threats | Habitat and species conservation and management | High |
|
| All threats | Habitat and species conservation and management | Medium |
|
| All threats | Habitat and species conservation and management | Low |
|
| Knowledge gaps to recovery | Monitoring and research | High |
|
| Knowledge gaps to recovery | Monitoring and research | High |
|
| Knowledge gaps to recovery | Monitoring and research | High |
|
| Knowledge gaps to recovery | Monitoring and research | High |
|
| Knowledge gaps to recovery | Monitoring and research | High |
|
| Knowledge gaps to recovery | Monitoring and research | High |
|
| Knowledge gaps to recovery | Monitoring and research | Medium |
|
| Knowledge gaps to recovery | Monitoring and research | Low |
|
| All threats | Law and policy | High |
|
| All threats | Law and policy | Medium |
|
| All threats | Law and policy | Medium |
|
| All threats | Law and policy | Low |
|
| All threats | Education and awareness, stewardship, and partnerships | High |
|
| All threats | Education and awareness, stewardship, and partnerships | High |
|
| All threats | Education and awareness, stewardship, and partnerships | High |
|
| All threats | Education and awareness, stewardship, and partnerships | High |
|
| All threats | Education and awareness, stewardship,ewardship, and partnerships | Medium |
|
| All threats | Education and awareness, stewardship, and partnerships | Medium |
|
| All threats | Education and awareness, stewardship, and partnerships | Medium |
|
Recovery of Olive-sided Flycatcher will require the commitment, collaboration, and cooperation among international, federal, provincial and territorial jurisdictions, wildlife management boards, aboriginal people, local communities, landowners, industry, and other interested parties. Owing to Olive-sided Flycatcher's widespread range, it will be important to monitor habitat conditions, population trend, and the distribution of the species so that the effectiveness of the recovery efforts can be evaluated and adjusted as necessary.
Aerial insectivores are in decline across Canada (e.g., McCracken 2008, Nebel et al. 2010, North American Bird Conservation Initiative 2012). Undertaking monitoring and research and moving forward with other broad strategies identified in this recovery strategy will undoubtedly benefit a wide range of aerial insectivorous species.
It is currently unknown whether breeding habitat is limiting in Canada. Nevertheless, adequate breeding habitat must be managed and protected to ensure species survival, particularly in areas where large portions of habitat are at risk of being lost or degraded. As well, trends in prey population dynamics must be better understood to know whether maintaining, enhancing, and/or restoring insect-producing habitats will significantly benefit Olive-sided Flycatcher populations.
Identifying key factors limiting populations of Olive-sided Flycatcher and resolving how to determine the relative importance of nonbreeding versus breeding habitat supply is an important activity for the recovery of this species. Knowing where to most effectively place economic support and research and monitoring is important for habitat and species conservation.
The best management of breeding habitat will fail to recover the species unless migration and wintering habitat is also maintained. Thus, collaboration with international jurisdictions and non-governmental organizations to preserve, restore, and enhance winter and migration habitat is an equally important component of this strategy. Such collaboration should have an additive effect on other species at risk, whose winter and migration ranges overlap with Olive-sided Flycatcher (see Appendix A).
It is unclear to what extent threats in Canada are affecting population decline of Olive-sided Flycatcher or whether the significant drivers of population decline are occurring elsewhere during another part of the species' annual cycle. A comprehensive approach to research and monitoring (which includes all stages of the annual life cycle and the entire range of occupancy) will be required to more completely understand the status of the species, as well as its threats and limiting factors in Canada and beyond. Currently, adequate monitoring of the species is primarily limited to areas near human-populations. It is currently unknown whether the trends observed from road-based surveys (i.e., BBS) in the southern portion of its range reflect the actual trend in the national breeding population. Additional survey and monitoring effort is required in more northern extents of their range and in medium- to high-elevation habitats, where they are currently not adequately monitored by BBS.
Since the population objective for this species includes halting the decline and ultimately increasing the population over time, identifying potentially suitable habitat that is currently unoccupied is necessary. Furthermore, identifying migratory routes, stopover sites, and migratory connectivity is also important. Determining key demographic parameters through Olive-sided Flycatcher's annual cycle (e.g., survival and reproductive success in different habitat types) will provide insight into the most suitable habitat characteristics, as well as activities/locations of concern for the species, population size, etc. Associated with these efforts, there is a need to build and validate corresponding habitat models at national and regional scales to better understand where on the landscape the species would be expected to breed and assist with efforts to protect habitat. There are fewer monitoring programs established on the wintering grounds, but these are essential and need to be developed and implemented to provide better information on habitat use and local habitat and population trends.
Research is needed to better understand the effects of the threats on the species. Examples of these research needs are listed in Appendix B.
While necessary monitoring and research occurs, the current state of available science can provide a base of knowledge to protect known habitats and mitigate threats for the species.
There are multiple legal means available to protect Olive-sided Flycatchers and their habitat in Canada. It is important that these tools are fully realized and utilized for the protection of the species. Four Canadian provinces (Ontario, Quebec, New Brunswick, and Newfoundland and Labrador) presently have this species listed on their respective species-at-risk legislation, and fully realizing the potential of the protection offered by these designations is important.
General prohibitions under the Migratory Birds Convention Act (1994) and its regulations protect Olive-sided Flycatcher nests and eggs anywhere they are found in Canada, regardless of land ownership. Nevertheless, nests and eggs can be inadvertently harmed or disturbed as a result of many activities, including but not limited to clearing trees. During the breeding period, potential destructive or disruptive activities should be avoided at locations where Olive-sided Flycatcher is likely to be encountered or known to occur (Environment Canada 2014a). This mitigation can also be accomplished through various avenues including planning policies and regulations, environmental assessments, etc.
Beneficial management practices (BMPs) and associated policies for Olive-sided Flycatcher, its prey, and their habitat must be developed and implemented based on the best available science. These can include BMPs and policies related to a variety of known and suspected threats, including forest harvesting and silviculture (e.g., salvage logging), fire suppression (and prescribed burning), energy and mining exploration and extraction, residential and commercial development, and problematic species. Beneficial management practices for this species must be integrated with those for other species to maintain heterogeneous landscapes that are a dynamic mosaic of habitat conditions that will benefit several species. Whenever possible a multi-species approach to recovery should be considered. Beneficial management practices for governments, industry, and even individuals can play an important role for the ongoing efforts across the range of the species and will be needed to promote recovery of the Olive-sided Flycatcher and conservation on a large scale across the continent and into South America.
Beyond Canada's borders, international collaboration on research and stewardship programs will be important for Olive-sided Flycatcher recovery and protection considering the potential threats to habitat in nonbreeding areas. An example of such an approach has already been established for the Canada Warbler (Cardellina canadensis); the Canada Warbler Conservation Initiative will be tackling these types of initiatives on an international scale in the years to come. The expansion of this effort to include other species, such as Olive-sided Flycatcher, or the establishment of a comparable effort for Olive-sided Flycatcher or aerial insectivores may be warranted.
Cooperative relations should be fostered with various levels of governments, landowners, foresters, industry, and pet owners (to name a few). Stewardship initiatives need to be pursued in strategic locations throughout Olive-sided Flycatcher's range, especially in areas of increased likelihood of development in the near future. Within these areas of potential development, the need for BMPs and appropriate policies will be increasingly important. Regulations, policies, and BMPs that provide protection for the species should be promoted to encourage compliance. Some parts of the northern range are experiencing increased exploration, and the threats in this area will only increase with time.
Preserving and enhancing Olive-sided Flycatcher breeding habitat will require promotion of conservation and stewardship on a broad scale. The key actions that can be promoted include the forest harvesting and silviculture practices that provide breeding habitat and reduce the risk of nest and egg disruption and/or destruction and prescribed burning to increase suitable habitat supply. In addition, because broad-scale monitoring and surveying is complicated by the large range of the species and the relative inaccessibility of portions of its range (e.g., northern range), developing targeted surveys, innovated survey approaches (e.g., acoustic monitoring), and promoting volunteer participation and collaborations are critical. Volunteers whose efforts should be promoted include local bird clubs who have local knowledge of areas with high breeding densities and citizen scientists participating in bird atlases and BBS programs. As well, citizen-based data collection (e.g., eBird) should continue to be encouraged to aid in ongoing research and monitoring.
Section 41(1)(c) of SARA requires that the recovery strategy include an identification of the species' critical habitat to the extent possible.
An examination of the geographic range of the species, its habitat specificity, its population size, and threats indicates that critical habitat should be identified at a landscape scale Note3de bas de page. Although habitat suitability is generally understood (see section 3.3) and some habitat suitability modeling has been done (Haché et al. 2014), currently, it is unknown whether habitat is limiting in Canada. The available information is not adequate to enable the identification of critical habitat at the landscape scale for the following reasons:
- There is a lack of understanding and data to indicate the suitable configuration of important landscape biophysical attributes.
- Habitat requirements may vary across the range of the species. Management units (i.e., geographic units within which critical habitat would be managed) need to be identified in such a way to best reflect variation in habitat use.
- There is a lack of data related to Olive-sided Flycatcher presence and abundance in large portions of its range. Without this information any model used to predict critical habitat with current data may have a limited ability to do so in these areas.
- For Olive-sided Flycatcher, it is unknown whether certain habitats with specific biophysical attributes may be functionally more important than others. For example, specific habitats may have greater densities of individuals or pairs and/or result in higher reproductive success. There are few data regarding the relative importance of suitable habitat types for Olive-sided Flycatcher population numbers and indices of habitat quality.
- The relationships between anthropogenic disturbance and habitat quality are poorly known. A better understanding of these relationships is needed to ensure sufficient suitable habitat is available for Olive-sided Flycatcher and to identify at what scale and intensity activities would be likely to destroy the critical habitat.
A Schedule of Studies (Table 4) has been developed to provide the information necessary to identify the critical habitat that will be sufficient to meet the population and distribution objectives. The identification of critical habitat will be included in a revised recovery strategy or an action plan.
To inform the Schedule of Studies (see Table 4) a recent project was undertaken by the Boreal Avian Modelling group to help identify patterns of habitat use for Olive-sided Flycatcher (Haché et al. 2014). Haché et al. (2014) assessed habitat attributes of Olive-sided Flycatcher across Canada based on avian point counts, available land classification metrics (i.e., land cover, disturbance, and topography), and environmental data (i.e., climate). While the results have added to our knowledge of habitat use by Olive-sided Flycatcher in Canada, the work cannot be used to identify habitat that is critical for the survival or recovery of the species because of the lack of adequate information (outlined in section 7.1).
The following Schedule of Studies is required to identify critical habitat.
| Description of Activity | Rationale | Timeline |
|---|---|---|
| Determine the appropriate management units based on habitat requirements across the species range. | Habitat requirements may vary across the range of the species. Management units need to be identified to best reflect this variation in habitat use. | 2016 |
| Increase monitoring at strategic locations. | Information on abundance, productivity, and other measures of habitat quality is poor in many regions of the country. Increased monitoring in pre-determined locations is necessary to validate and improve recent habitat models (i.e., Haché et al. 2014). | 2016–2020 |
| Determine the appropriate configuration of landscape biophysical attributes | To identify critical habitat at a landscape scale, it is necessary to understand the biophysical attributes required by the species at this scale and determine how they should be configured to meet the species' needs. | 2016–2019 |
| Determine habitat quality across and within management units. | Information on abundance, productivity, and other measures of habitat quality may lead to the identification of areas that contribute disproportionately to the survival or recovery of Olive-sided Flycatcher. | 2016–2020 |
| Determine the scale and intensity at which suitable habitat would likely be destroyed by anthropogenic activities. | A better understanding of the relationship between anthropogenic disturbance and habitat quality is needed to ensure sufficient suitable habitat is available for the Olive-sided Flycatcher and to identify at what scale and intensity activities would be likely to destroy critical habitat. | 2016–2020 |
| Determine how much suitable habitat is required to support the population and distribution objectives for Olive-sided Flycatcher. | It is uncertain whether habitat is limiting in Canada for Olive-sided Flycatcher. An assessment of whether there is sufficient habitat in Canada to meet the population and distribution objectives is required. | 2020 |
| Develop and validate habitat models to determine where biophysical attributes are present in required quantity, quality, and configuration within each management unit to meet population and distribution objectives. | Results from studies listed above will allow models to be built to identify the location, quantity, and quality of habitat that should be identified as critical habitat for Olive-sided Flycatcher. | 2021 |
The performance indicators presented below provide a way to define and measure progress toward achieving the population and distribution objectives.
- In the short term (10 years; before 2025), declining population trends have been halted or reversed to a point where Canadian populations of Olive-sided Flycatcher have declined no more than 10% during this time.
- In the long term (after 2025), a positive 10-year trend is achieved (i.e., the population is increasing).
- The breeding extent of occurrence for Olive-sided Flycatcher is maintained throughout Canada.
One or more action plans for Olive-sided Flycatcher will be developed and posted on the Species at Risk Public Registry within the 5 years following the publication of the recovery strategy in the Species at Risk Public Registry.
- Altman, B. and R. Sallabanks. 2012. Olive-sided Flycatcher (Contopus cooperi). Cornell Lab of Ornithology, Ithaca, NY. [accessed: 17 September 2014].
- Baudvin, H. 1997. Barn Owl (Tyto alba) and Long-eared Owl (Asio otus) mortality along motorways in Bourgogne-Champagne: report and suggestions. Pages 58-61 In Biology and Conservation of Owls of the Northern Hemisphere: 2nd International Symposium. U.S. Dept. of Agriculture, Forest Service, North Central Forest Experiment Station. St. Paul, MN.
- Beese, W. J. and A. A. Bryant. 1999. Effect of alternative silvicultural systems on vegetation and bird communities in coastal montane forests of British Columbia, Canada. Forest Ecology and Management 115(2): 231-242.
- Benton, T. G., D. M. Bryant, L. Cole, and H. Q. Crick. 2002. Linking agricultural practice to insect and bird populations: a historical study over three decades. Journal of Applied Ecology 39(4): 673-687.
- Birch, R. and D. Kaye. 2012. Global mining finance: 2012. Toronto, ON.
- BirdLife International. 2014. Canada Warbler (Cardellina canadensis) wintering distribution and ecology: South America. BirdLife International, Quito, Ecuador.
- BirdLife International and NatureServe. 2013. Bird species distribution maps of the world. BirdLife International and NatureServe, Cambridge, UK and Arlington, VA. Available: Upon request. [accessed: August 2014].
- Bishop, C. A. and J. M. Brogan. 2013. Estimates of avian mortality attributed to vehicle collisions in Canada. Avian Conservation and Ecology 8(2): 2.
- Blancher, P. 2013. Estimated number of birds killed by house cats (Felis catus) in Canada. Avian Conservation and Ecology 8(2): 3.
- Blancher, P. J. and D. K. McNicol. 1991. Tree swallow diet in relation to wetland acidity. Canadian Journal of Zoology 69(10): 2629-2637.
- Blancher, P. J., K. V. Rosenberg, A. O. Panjabi, B. Altman, J. Bart, C. J. Beardmore, G. S. Butcher, D. Demarest, R. Dettmers, E. H. Dunn, W. Easton, W. C. Hunter, E. E. Inigo-Elias, D. N. Pashley, C. J. Ralph, C. Rich, C. M. Rustay, J. M. Ruth, and T. Will. 2007. Guide to the Partners in Flight Population Estimates Database. Version: North American Landbird Conservation Plan 2004. Partners in Flight Technical Series No. 5.
- Boatman, N. D., N. W. Brickle, J. D. Hart, T. P. Milsom, A. J. Morris, A. W. Murray, K. A. Murray, and P. A. Robertson. 2004. Evidence for the indirect effects of pesticides on farmland birds. Ibis 146(s2): 131-143.
- Bock, C. E. and J. F. Lynch. 1970. Breeding bird populations of burned and unburned conifer forest in the Sierra Nevada. The Condor 72: 182-189.
- Boreal bird declines and human disturbances fact sheet.Available:[accessed: 20 August 2014].
- Both, C., S. Bouwhuis, C. Lessells, and M. E. Visser. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441(7089): 81-83.
- Both, C., C. A. Van Turnhout, R. G. Bijlsma, H. Siepel, A. J. Van Strien, and R. P. Foppen. 2009. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proceedings of the Royal Society B: Biological Sciences 277: 1259-1266.
- Brandy, P. M. 2001. A hierarchical analysis of Olive-sided Flycatcher habitat use in a managed landscape. M.Sc. Thesis. Humboldt State University.
- Brooks, D. R., J. E. Bater, S. J. Clark, D. T. Monteith, C. Andrews, S. J. Corbett, D. A. Beaumont, and J. W. Chapman. 2012. Large carabid beetle declines in a United Kingdom monitoring network increases evidence for a widespread loss in insect biodiversity. Journal of Applied Ecology 49(5): 1009-1019.
- Brotons, L., M. Magrans, L. Ferrús, and J. Nadal. 1998. Direct and indirect effects of pollution on the foraging behaviour of forest passerines during the breeding season. Canadian Journal of Zoology 76(3): 556-565.
- Brown, C. R. and M. B. Brown. 2000. Weather-mediated natural selection on arrival time in Cliff Swallows (Petrochelidon pyrrhonota). Behavioral Ecology and Sociobiology 47(5): 339-345.
- Calvert, A. M., C. A. Bishop, R. D. Elliot, E. A. Krebs, T. M. Kydd, C. S. Machtans, and G. J. Robertson. 2013. A synthesis of human-related avian mortality in Canada. Avian Conservation and Ecology 8(2): 11.
- Cameron, S. A., J. D. Lozier, J. P. Strange, J. B. Koch, N. Cordes, L. F. Solter, and T. L. Griswold. 2011. Patterns of widespread decline in North American bumble bees. Proceedings of the National Academy of Sciences 108(2): 662-667.
- Case, R. M. 1978. Interstate highway road-killed animals: a data source for biologists. Wildlife Society Bulletin 6(1): 8-13.
- Chamberlain, D. E., R. J. Fuller, R. G. H. Bunce, J. C. Duckworth, and M. Shrubb. 2000. Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. Journal of Applied Ecology 37(5): 771-788.
- Chambers, C. L., W. C. McComb, and J. C. Tappeiner. 1999. Breeding bird responses to three silvicultural treatments in the Oregon Coast Range. Ecological Applications 9(1): 171-185.
- Cheng, R. and P. Lee. 2014. Canada's industrial concessions: a spatial analysis. Global Forest Watch Canada.
- Cheskey, E. 2007. Olive-sided Flycatcher. Pages 338-339 In M. D. Cadman, D. A. Sutherland, G. G. Beck, D. Lepage, and A. R. Couturier (eds.). Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature.
- Commission for Environmental Cooperation of North America. 2003. DDT no longer used in North America. Commission for Environmental Cooperation of North America, Montreal, QC.
- COSEWIC. 2008. COSEWIC assessment and status report on the Olive-sided Flycatcher Contopus cooperi in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa, ON.
- Cumming, S. 2005. Effective fire suppression in boreal forests. Canadian Journal of Forest Research 35(4): 772-786.
- Cumming, S. G., K. L. Lefevre, E. Bayne, T. Fontaine, F. K. Schmiegelow, and S. J. Song. 2010. Toward conservation of Canada's boreal forest avifauna: design and application of ecological models at continental extents Avian Conservation and Ecology 5(2): 8.
- Cumming, S. G., D. Stralberg, K. L. Lefevre, P. Sólymos, E. M. Bayne, S. Fang, T. Fontaine, D. Mazerolle, F. K. A. Schmiegelow, and S. J. Song. 2014. Climate and vegetation hierarchically structure patterns of songbird distribution in the Canadian boreal region. Ecography 37(2): 137-151.
- Davis, S., V. Heywood, O. Herrera-Macbryde, J. Villa-Lobos, and A. Hamilton. 1997. Centres of plant diversity: a guide and strategy for their conservation. Volume 3. IUCN Publications Unit, Cambridge, UK. Available: [accessed: August 2014].
- de Groot, W. J., M. D. Flannigan, and A. S. Cantin. 2013. Climate change impacts on future boreal fire regimes. Forest Ecology and Management 294: 35-44.
- Depew, D. C., N. M. Burgess, and L. M. Campbell. 2013. Modelling mercury concentrations in prey fish: derivation of a national-scale common indicator of dietary mercury exposure for piscivorous fish and wildlife. Environmental Pollution 176: 234-243.
- Dionne, M., C. Maurice, J. Gauthier, and F. Shaffer. 2008. Impact of Hurricane Wilma on migrating birds: the case of the Chimney Swift. The Wilson Journal of Ornithology 120(4): 784-792.
- Dirzo, R., H. S. Young, M. Galetti, G. Ceballos, N. J. Isaac, and B. Collen. 2014. Defaunation in the Anthropocene. Science 345(6195): 401-406.
- Driscoll, C. T., G. B. Lawrence, A. J. Bulger, T. J. Butler, C. S. Cronan, C. Eagar, K. F. Lambert, G. E. Likens, J. L. Stoddard, and K. C. Weathers. 2001. Acidic deposition in the northeastern United States: sources and inputs, ecosystem effects, and management strategies. BioScience 51(3): 180-198.
- Drummond, M. A. and T. R. Loveland. 2010. Land-use pressure and a transition to forest-cover loss in the eastern United States. BioScience 60(4): 286-298.
- eBird. 2014. eBird an online database of bird distribution and abundance. Version 2. Audubon and Cornell Lab of Ornithology, Ithaca, NY. Available: [accessed: 10 September 2014].
- Edmonds, S. T., D. C. Evers, D. A. Cristol, C. Mettke-Hofmann, L. L. Powell, A. J. McGann, J. W. Armiger, O. P. Lane, D. F. Tessler, and P. Newell. 2010. Geographic and seasonal variation in mercury exposure of the declining Rusty Blackbird. The Condor 112(4): 789-799.
- Edmonds, S. T., N. J. O'Driscoll, N. K. Hillier, J. L. Atwood, and D. C. Evers. 2012. Factors regulating the bioavailability of methylmercury to breeding Rusty Blackbirds in northeastern wetlands. Environmental Pollution 171: 148-154.
- Environment Canada. 2011a. Management plan for the Cerulean Warbler (Dendroica cerulea) in Canada. Environment Canada, Ottawa, ON.
- Environment Canada. 2011b. Presence and levels of priority pesticides in selected Canadian aquatic ecosystems. Environment Canada, Water Science and Technology Directorate, Gatineau, QC.
- Environment Canada. 2011c. Status of birds in Canada. Environment Canada, Gatineau, QC.
- Environment Canada. 2013. Species at Risk Act implementation guidance for recovery practitioners. Environment Canada - Canadian Wildlife Service, Ottawa, ON.
- Environment Canada. 2014a. Avoidance guidelines related to incidental take of migratory birds in Canada. Environment Canada, Gatineau, QC.
- Environment Canada 2014b. Bird Conservation Regions and Conservation Strategies. Environment Canada, Ottawa, ON. Available: [accessed: 19 September 2014].
- Environment Canada 2014c. National Pollutant Release Inventory. Environment Canada, Gatineau, QC. Available:[accessed: 4 September 2014].
- Environment Canada 2014d. North American Breeding Bird Survey - Canadian Trends Website. Environment Canada, Gatineau, QC. Available: [accessed: 15 August 2014].
- Etter, A., C. McAlpine, K. Wilson, S. Phinn, and H. Possingham. 2006. Regional patterns of agricultural land use and deforestation in Colombia. Agriculture, Ecosystems & Environment 114(2): 369-386.
- Evers, D. C., K. A. Williams, M. W. Meyer, A. M. Scheuhammer, N. Schoch, A. T. Gilbert, L. Siegel, R. J. Taylor, R. Poppenga, and C. R. Perkins. 2011. Spatial gradients of methylmercury for breeding common loons in the Laurentian Great Lakes region. Ecotoxicology 20(7): 1609-1625.
- Fitzgerald, W. F., D. R. Engstrom, R. P. Mason, and E. A. Nater. 1998. The case for atmospheric mercury contamination in remote areas. Environmental Science &Technology 32(1): 1-7.
- Flannigan, M., B. Stocks, M. Turetsky, and M. Wotton. 2009. Impacts of climate change on fire activity and fire management in the circumboreal forest. Global Change Biology 15(3): 549-560.
- Foster, G. N. 1991. Conserving insects of aquatic and wetland habitats, with special reference to beetles. Pages 237-262 In The Conservation of Insects and their Habitats. 15th Symposium of the Royal Entomological Society of London. Academic Press. London, UK.
- Freedman, B. 1995. Environmental ecology: the ecological effects of pollution, disturbance, and other stresses. Academic Press. San Diego, CA. 606 pp.
- Friend, M. and J. C. Franson. 1999. Field manual of wildlife diseases: general field procedures and diseases of birds. US Geological Survey, Biological Resources Division Information and Technology Report 1999-2001, DTIC Document.
- Gard, N. W., M. J. Hooper, and R. S. Bennett. 1993. Effects of pesticides and contaminants on neotropical migrants. Pages 310-314 In D. M. Finch and P. W. Strange (eds.). Status and Management of Neotropical Migratory Birds. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. Fort Collins, CO.
- Gibbons, D., C. Morrissey, and P. Mineau. 2014. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environmental Science and Pollution Research: 1-16.
- Gill, R. J., O. Ramos-Rodriguez, and N. E. Raine. 2012. Combined pesticide exposure severely affects individual-and colony-level traits in bees. Nature 491(7422): 105-108.
- Girardin, M. P., A. A. Ali, C. Carcaillet, O. Blarquez, C. Hély, A. Terrier, A. Genries, and Y. Bergeron. 2013. Vegetation limits the impact of a warm climate on boreal wildfires. New Phytologist 199(4): 1001-1011.
- Goulson, D. 2014. Pesticides linked to bird declines. Nature 511: 295-296.
- Government of Canada. 2009. Species at Risk Act Policies, Overarching Framework [Draft]. Environment Canada, Ottawa, ON.
- Graveland, J. 1998. Effects of acid rain on bird populations. Environmental Reviews 6(1): 41-54.
- Graveland, J. and R. Drent. 1997. Calcium availability limits breeding success of passerines on poor soils. Journal of Animal Ecology 66(2): 279-288.
- Greenberg, R. and S. M. Matsuoka. 2010. Special section: rangewide ecology of the declining Rusty Blackbird - Rusty Blackbird: mysteries of a species in decline. The Condor 112(4): 770-777.
- Haché, S., P. Solymos, T. Fontaine, E. Bayne, S. Cumming, F. Schmiegelow, and D. Stralberg. 2014. Habitat of Olive-sided Flycatcher, Canada Warbler, and Common Nighthawk in Canada. Boreal Avian Modelling Project, Edmonton, AB.
- Hagelin, J., A. Brinkman, J. Johnson, S. M. Matsuoka, L. DeCicco, and N. Hajdukovich. 2013. Preliminary report: Olive-sided Flycatcher migration and breeding biology. Pages 17-18 In J. Hagelin (ed.). Summary of Landbird Projects for Boreal Partners in Flight. Partners in Flight. Fairbanks, AK.
- Hagen, J. M., P. S. McKinley, A. L. Meehan, and S. L. Grove. 1997. Diversity and abundance of landbirds in a northeastern industrial forest. Journal of Wildlife Management 61: 718-735.
- Hallmann, C. A., R. P. Foppen, C. A. van Turnhout, H. de Kroon, and E. Jongejans. 2014. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511: 341-343.
- Hames, R. S., K. V. Rosenberg, J. D. Lowe, S. E. Barker, and A. A. Dhondt. 2002. Adverse effects of acid rain on the distribution of the Wood Thrush Hylocichla mustelina in North America. Proceedings of the National Academy of Sciences 99(17): 11235-11240.
- Hansen, M., P. Potapov, R. Moore, M. Hancher, S. Turubanova, A. Tyukavina, D. Thau, S. Stehman, S. Goetz, and T. Loveland. 2013. High-resolution global maps of 21st-century forest cover change. Science 342(6160): 850-853 [Data available: http://www.earthenginepartners.appspot.com/science-2013-global-forest]
- Hawley, D. M., K. K. Hallinger, and D. A. Cristol. 2009. Compromised immune competence in free-living Tree Swallows exposed to mercury. Ecotoxicology 18(5): 499-503.
- Henderson, A., S. P. Churchill, and J. L. Luteyn. 1991. Neotropical plant diversity. Nature 351(6321): 21-22.
- Hladik, M. L., D. W. Kolpin, and K. M. Kuivila. 2014. Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA. Environmental Pollution 193: 189-196.
- Hobson, K. A., A. G. Wilson, S. L. Van Wilgenburg, and E. M. Bayne. 2013. An estimate of nest loss in Canada due to industrial forestry operations. Avian Conservation and Ecology 8(2): 5.
- Holmes, S. B. 1998. Reproduction and nest behaviour of Tennessee Warblers Vermivora peregrina in forests treated with Lepidoptera-specific insecticides. Journal of Applied Ecology 35(2): 185-194.
- Huber, D. G. and J. Gulledge. 2011. Extreme weather and climate change: understanding the link, managing the risk. Center for Climate and Energy Solutions, Arlington, VA.
- Hutto, R. L. 1995. Composition of bird communities following stand-replacement fires in northern Rocky Mountain (USA) conifer forests. Conservation Biology 9(5): 1041-1058.
- Hutto, R. L. and J. S. Young. 1999. Habitat relationships of landbirds in the Northern Region. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Ogden, UT.
- IUCN. 2013. The IUCN The IUCN Red List of Threatened Species. Version 2013.2. IUCN. Available: [accessed: 4 September 2014].
- Jones, T. and W. Cresswell. 2010. The phenology mismatch hypothesis: are declines of migrant birds linked to uneven global climate change? Journal of Animal Ecology 79(1): 98-108.
- Keller, R. H., L. Xie, D. B. Buchwalter, K. E. Franzreb, and T. R. Simons. 2014. Mercury bioaccumulation in Southern Appalachian birds, assessed through feather concentrations. Ecotoxicology 23(2): 304-316.
- Klemens, J., R. Harper, J. Frick, A. Capparella, H. Richardson, and M. Coffey. 2000. Patterns of organochlorine pesticide contamination in neotropical migrant passerines in relation to diet and winter habitat. Chemosphere 41(7): 1107-1113.
- Kotliar, N. 2007a. Olive-sided Flycatcher (Contopus cooperi): a technical conservation assessment USDA Forest Service, Rocky Mountain Region Available: [accessed: August 2014].
- Kotliar, N. 2007b. Olive-sided Flycatcher, (PDF Format) (Contopus cooperi): A Technical Conservation Assessment Rocky Mountain Region, USDA Forest Service.
- Kotliar, N. B., S. J. Hejl, R. L. Hutto, V. A. Saab, C. P. Melcher, and M. E. McFadzen. 2002. Effects of fire and post-fire salvage logging on avian communities in conifer-dominated forests of the western United States. Studies in Avian Biology 25: 49-64.
- Larsen, T. H., F. Escobar, and I. Armbrecht. 2011. Insects of the tropical Andes: diversity patterns, processes and global change. Pages 228-244 In S. K. Herzog, R. Martinez, P. M. Jorgensen, and H. Tiessen (eds.). Climate Change and Biodiversity in the Tropical Andes. Inter-American Institute of Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE), São José dos Campos and Paris.
- Latendresse, C. 2008. Synthèse de connaissances sur l'habitat de reproduction de l'Engoulevent d'Amérique et de l'Engoulement bois-pourri au Québec. Service Canadien de la Faune, Montreal, QC.
- Lebbin, D. J., M. J. Parr, and G. H. Fenwick. 2010. The American Bird Conservancy guide to bird conservation. University of Chicago Press. Chicago, IL. 456 pp.
- LeGrand, H. E. and S. P. Hall. 1989. Element stewardship abstract – Contopus borealis, The Nature Conservancy, Arlington, VA. Cited In J. M. C Peterson and C. Fichtel. 1992. Olive-sided Flycatcher Contopus borealis. Pages 353-367 In K. J. Schneider and D. M. Pence (eds.) Migratory non-game birds of management concern in the northeast. U.S. Fish and Wildlife Service. Newton Corner, MA.
- Longcore, T., C. Rich, P. Mineau, B. MacDonald, D. G. Bert, L. M. Sullivan, E. Mutrie, S. A. Gauthreaux Jr, M. L. Avery, and R. L. Crawford. 2012. An estimate of avian mortality at communication towers in the United States and Canada. PLoS One 7(4): e34025.
- Longcore, T., C. Rich, P. Mineau, B. MacDonald, D. G. Bert, L. M. Sullivan, E. Mutrie, S. A. Gauthreaux Jr, M. L. Avery, and R. L. Crawford. 2013. Avian mortality at communication towers in the United States and Canada: which species, how many, and where? Biological Conservation 158: 410-419.
- Loss, S. R., T. Will, S. S. Loss, and P. P. Marra. 2014a. Bird-building collisions in the United States: estimates of annual mortality and species vulnerability. The Condor 116(1): 8-23.
- Loss, S. R., T. Will, and P. P. Marra. 2014b. Estimation of bird-vehicle collision mortality on US roads. The Journal of Wildlife Management 78(5): 763-771.
- Loss, S. R., T. Will, and P. P. Marra. 2014c. Refining estimates of bird collision and electrocution mortality at power lines in the United States. PLoS One 9(7): e101565.
- Machtans, C. S., K. J. Kardynal, and P. A. Smith. 2014. How well do regional or national Breeding Bird Survey data predict songbird population trends at an intact boreal site? Avian Conservation and Ecology 9(1): 5.
- Machtans, C. S., C. H. Wedeles, and E. M. Bayne. 2013. A first estimate for Canada of the number of birds killed by colliding with building windows. Avian Conservation and Ecology 8(2): 6.
- Mahony, N., E. Nol, and T. Hutchinson. 1997. Food-chain chemistry, reproductive success, and foraging behaviour of songbirds in acidified maple forests of central Ontario. Canadian Journal of Zoology 75(4): 509-517.
- Main, A. R., J. V. Headley, K. M. Peru, N. L. Michel, A. J. Cessna, and C. A. Morrissey. 2014. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada's prairie pothole region. PLoS One 9(3): e92821.
- Mänd, R., V. Tilgar, and A. Leivits. 2000. Calcium, snails, and birds: a case study. Web Ecology 1: 63-69.
- Manley, P., D. Murphy, L. Campbell, K. Heckmann, S. Merideth, S. Parks, M. Sanford, and M. Schlesinger. 2006. Biotic diversity interfaces with urbanization in the Lake Tahoe Basin. California Agriculture 60(2): 59-64.
- Masek, J. G., W. B. Cohen, D. Leckie, M. A. Wulder, R. Vargas, B. de Jong, S. Healey, B. Law, R. Birdsey, and R. Houghton. 2011. Recent rates of forest harvest and conversion in North America. Journal of Geophysical Research: Biogeosciences (2005–2012) 116(G4).
- McClure, C. J., B. W. Rolek, K. McDonald, and G. E. Hill. 2012. Climate change and the decline of a once common bird. Ecology and Evolution 2(2): 370-378.
- McCracken, J. D. 2008. Are aerial insectivores being bugged out? , Bird Studies Canada, Port Rowan, ON.
- Meehan, T. D., T. L. George, and A. Powell. 2003. Short-term effects of moderate-to high-severity wildfire on a disturbance-dependent flycatcher in northwest California. The Auk 120(4): 1102-1113.
- Mineau, P. 2005. Direct losses of birds to pesticides - beginnings of a quantification. Pages 1065-1070 In Bird Conservation Implementation and Integration in the Americas: Proceedings of the Third International Partners in Flight Conference 2002. USDA Forest Service, GTR-PSW-191. Albany, CA.
- Mineau, P. and C. Palmer. 2013. The impact of the nation's most widely used insecticides on birds: neonicotinoid insecticides and birds. American Bird Conservancy, Washington, DC.
- Mineau, P. and M. Whiteside. 2013. Pesticide acute toxicity is a better correlate of US grassland bird declines than agricultural intensification. PLoS One 8(2): e57457.
- Møller, A. P., D. Rubolini, and E. Lehikoinen. 2008. Populations of migratory bird species that did not show a phenological response to climate change are declining. Proceedings of the National Academy of Sciences 105(42): 16195-16200.
- Morissette, J., T. Cobb, R. Brigham, and P. James. 2002. The response of boreal forest songbird communities to fire and post-fire harvesting. Canadian Journal of Forest Research 32(12): 2169-2183.
- Morrissey, C., B. Clark, and K. Hobson. 2014. Exploring intercontinental patterns and potential drivers of aerial insectivorous bird declines. University of Saskatchewan, Saskatoon, SK. Available: [accessed: 19 September 2014].
- Murphy, M. T. 1989. Life history variability in North American breeding tyrant flycatchers: phylogeny, size or ecology? Oikos 54: 3-14.
- NatureServe 2013. NatureServe Explorer: an online encyclopedia of life. Version 7.1. NatureServe, Arlington, VA. Available [accessed:]
- Nebel, S., A. Mills, J. D. McCracken, and P. D. Taylor. 2010. Declines of aerial insectivores in North America follow a geographic gradient. Avian Conservation and Ecology 5(2): 1.
- Newson, S., S. Mendes, H. Crick, N. Dulvy, J. Houghton, G. Hays, A. Hutson, C. MacLeod, G. Pierce, and R. Robinson. 2009. Indicators of the impact of climate change on migratory species. Endangered Species Research 7(2): 101-113.
- NFD. 2014. National Forestry Database. Natural Resources Canada and Canadian Forest Service, Ottawa, ON. Available: [accessed: 20 August 2014].
- Nocera, J. J., J. M. Blais, D. V. Beresford, L. K. Finity, C. Grooms, L. E. Kimpe, K. Kyser, N. Michelutti, M. W. Reudink, and J. P. Smol. 2012. Historical pesticide applications coincided with an altered diet of aerially foraging insectivorous Chimney Swifts. Proceedings of the Royal Society B: Biological Sciences 279(1740): 3114-3120.
- Nocera, J. J., M. W. Reudink, and A. J. Campomizzi. 2014. Population trends of aerial insectivores breeding in North America can be linked to trade. American Ornithologists' Union, Cooper Ornithological Society, and Society of Canadian Ornithologists 2014 Joint Meeting. AOU, COS, SCO, Estes Park, CO.
- North American Bird Conservation Initiative. 2012. The state of Canada's birds, 2012. Environment Canada, Ottawa, ON.
- North American Bird Conservation Initiative US Committee. 2010. The state of the birds 2010 - report on climate change, United States of America. US Department of the Interior, Washington, D.C.
- Norton, M., J. Bendell, L. Bendell-Young, and C. LeBlanc. 2001. Secondary effects of the pesticide Bacillus thuringiensis kurstaki on chicks of Spruce Grouse (Dendragapus canadensis). Archives of environmental contamination and toxicology 41(3): 369-373.
- Paquette, S. R., F. Pelletier, D. Garant, and M. Bélisle. 2014. Severe recent decrease of adult body mass in a declining insectivorous bird population. Proceedings of the Royal Society B: Biological Sciences 281(1786): 1-9.
- Partners in Flight Science Committee. 2012. Species Assessment Database Version 2012. Laurel, MD. [accessed: 16 September 2014].
- Partners in Flight Science Committee. 2013. Population Estimates Database Version 2013. Laurel, MD. [accessed: 16 September 2014].
- Portillo-Quintero, C., A. Sanchez, C. Valbuena, Y. Gonzalez, and J. Larreal. 2012. Forest cover and deforestation patterns in the Northern Andes (Lake Maracaibo Basin): a synoptic assessment using MODIS and Landsat imagery. Applied Geography 35(1): 152-163. Porvari, P., M. Verta, J. Munthe, and M. Haapanen. 2003. Forestry practices increase mercury and methyl mercury output from boreal forest catchments. Environmental Science & Technology 37(11): 2389-2393.
- Poulin, B., G. Lefebvre, and L. Paz. 2010. Red flag for green spray: adverse trophic effects of Bti on breeding birds. Journal of Applied Ecology 47(4): 884-889.
- Price, P. W., R. F. Denno, M. D. Eubanks, D. L. Finke, and I. Kaplan. 2011. Insect ecology: behavior, populations and communities. Cambridge University Press. New York, NY. 812 pp.
- Radeloff, V. C., R. B. Hammer, and S. I. Stewart. 2005. Rural and suburban sprawl in the US Midwest from 1940 to 2000 and its relation to forest fragmentation. Conservation Biology 19(3): 793-805.
- Rimmer, C. C., E. K. Miller, K. P. McFarland, R. J. Taylor, and S. D. Faccio. 2010. Mercury bioaccumulation and trophic transfer in the terrestrial food web of a montane forest. Ecotoxicology 19(4): 697-709.
- Rioux, S., J.-P. L. Savard, and A. A. Gerick. 2013. Avian mortalities due to transmission line collisions: a review of current estimates and field methods with an emphasis on applications to the Canadian electric network. Avian Conservation and Ecology 8(2): 7.
- Robertson, B. A. and R. L. Hutto. 2007. Is selectively harvested forest an ecological trap for Olive-sided Flycatchers? The Condor 109(1): 109-121.
- Robinson, A., H. Q. Crick, J. A. Learmonth, I. M. Maclean, C. D. Thomas, F. Bairlein, M. C. Forchhammer, C. M. Francis, J. A. Gill, B. J. Godley, J. Harwood, G. C. Hays, B. Huntley, A. M. Hutson, G. J. Pierce, M. M. Rehfisch, D. W. Sims, M. B. Santos, T. H. Sparks, D. A. Stroud, and M. E. Visser. 2009. Travelling through a warming world: climate change and migratory species. Endangered Species Research 7: 87-89.
- Rydberg, J., J. Klaminder, P. Rosén, and R. Bindler. 2010. Climate driven release of carbon and mercury from permafrost mires increases mercury loading to sub-arctic lakes. Science of the total environment 408(20): 4778-4783.
- Sager, T. A. 1997. Organochlorine pesticide contamination in New World passerines. Honors Project, Paper 10. Illinois Wesleyan University.
- Saino, N., R. Ambrosini, D. Rubolini, J. von Hardenberg, A. Provenzale, K. Hüppop, O. Hüppop, A. Lehikoinen, E. Lehikoinen, and K. Rainio. 2011. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proceedings of the Royal Society B: Biological Sciences 278(1707): 835-842.
- Sánchez-Cuervo, A. M., T. M. Aide, M. L. Clark, and A. Etter. 2012. Land cover change in Colombia: surprising forest recovery trends between 2001 and 2010. PLoS One 7(8): e43943.
- Sauer, J. R., J. E. Hines, J. E. Fallon, K. L. Pardieck, D. J. Ziolkowski, and W. A. Link. 2011. The North American Breeding Bird Survey, results and analysis 1966 - 2009. Version 3.23.2011. USGS Patuxent Wildlife Research Center, Laurel, MD.
- Scheuhammer, A. M., M. W. Meyer, M. B. Sandheinrich, and M. W. Murray. 2007. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. AMBIO: A Journal of the Human Environment 36(1): 12-19.
- Schneider, R. R., J. B. Stelfox, S. Boutin, and S. Wasel. 2003. Managing the cumulative impacts of land-uses in the Western Canadian Sedimentary Basin: a modeling approach. Conservation Ecology 7(1): 8.
- Secretariat of the Stockholm Convention. 2011. United Nations targets widely-used pesticide endosulfan for phase out. [accessed: August 2014].
- Shannon, J. D. 1999. Regional trends in wet deposition of sulfate in the United States and SO2 emissions from 1980 through 1995. Atmospheric Environment 33(5): 807-816.
- Sopuck, L., K. Ovaska, and B. Whittington. 2002. Responses of songbirds to aerial spraying of the microbial insecticide Bacillus thuringiensis var. kurstaki (Foray 48B®) on Vancouver Island, British Columbia, Canada. Environmental Toxicology and Chemistry 21(8): 1664-1672.
- Stocks, B., J. Mason, J. Todd, E. Bosch, B. Wotton, B. Amiro, M. Flannigan, K. Hirsch, K. Logan, and D. Martell. 2003. Large forest fires in Canada, 1959–1997. Journal of Geophysical Research 108: 8149.
- Stothart, P. 2011. F&F 2011: Fact$ and Figure$ of the Canadian Mining Industry. The Mining Association of Canada.
- Strode, P. K. 2003. Implications of climate change for North American wood warblers (Parulidae). Global Change Biology 9(8): 1137-1144.
- Sun, H., W. Forsythe, and N. Waters. 2007. Modeling urban land use change and urban sprawl: Calgary, Alberta, Canada. Networks and Spatial Economics 7: 353-376.
- Taylor, S. W. 1998. Prescribed fire in Canada - a time of transition.
- U.S. Bureau of Land Management. 1978. Grass Creek: oil and gas leasing environmental assessment record. U.S. Bureau of Land Management, Worland District, WY.
- U.S. Environmental Protection Agency. 2010. Endosulfan phase-out. U.S. Environmental Protection Agency. [accessed: August 2014].
- U.S. Environmental Protection Agency. 2014. Environmental effects of acid rain. [accessed: August 2014]
- U.S. Geological Survey. 2000. Mercury in the environment, fact sheet 146-00. [accessed: August 2014].
- Van Wilgenburg, S. L., K. A. Hobson, E. M. Bayne, and N. Koper. 2013. Estimated avian nest loss associated with oil and gas exploration and extraction in the Western Canadian Sedimentary Basin. Avian Conservation and Ecology 8(2): 9.
- Visser, M. E., L. J. Holleman, and P. Gienapp. 2006. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147(1): 164-172.
- Vuori, K.-M., O. Siren, and H. Luotonen. 2003. Metal contamination of streams in relation to catchment silvicultural practices: a comparative study in Finnish and Russian headwaters. Boreal environment research 8(1): 61-70.
- Weber, M. G. and S. W. Taylor. 1992. The use of prescribed fire in the management of Canada's forested lands. Forest Chronicles 68: 324-334.
- Whitehorn, P. R., S. O'Connor, F. L. Wackers, and D. Goulson. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336(6079): 351-352.
- Wiener, J. G., D. P. Krabbenhoft, G. H. Heinz, and A. M. Scheuhammer. 2003. Ecotoxicology of mercury. Pages 409-463 In D. J. Hoffman, B. A. Rattner, G. A. Burton, Jr., and J. Cairns, Jr. (eds.). Handbook of Ecotoxicology, 2nd Edition. CRC Press. Boca Raton, FL.
- Wright, J. M. 1997. Preliminary study of Olive-sided Flycatchers, July 1994-April 1997. Endangered Species Conservation Fund Federal Aid Studies Alaska Department of Fish and Game, Division of Wildlife Conservation, Juneau, AK.
- Xing, Z., L. Chow, H. Rees, F. Meng, S. Li, B. Ernst, G. Benoy, T. Zha, and L. M. Hewitt. 2013. Influences of sampling methodologies on pesticide-residue detection in stream water. Archives of environmental contamination and toxicology 64(2): 208-218.
- Zimmerling, J. R., A. C. Pomeroy, M. V. d'Entremont, and C. M. Francis. 2013. Canadian estimate of bird mortality due to collisions and direct habitat loss associated with wind turbine developments. Avian Conservation and Ecology 8(2): 10.
T. Stehelin. 2014. Ph.D. Candidate, School of Science, Yukon College, Whitehorse, YK.
A. Westwood. 2014. Ph.D. Candidate, Department of Biology, Dalhousie University, Halifax, NS.
A strategic environmental assessment (SEA) is conducted on all SARA recovery planning documents, in accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals. The purpose of a SEA is to incorporate environmental considerations into the development of public policies, plans, and program proposals to support environmentally sound decision-making and to evaluate whether the outcomes of a recovery planning document could affect any component of the environment or any of the Federal Sustainable Development Strategy's (FSDS) goals and targets.
Recovery planning is intended to benefit species at risk and biodiversity in general. However, it is recognized that strategies may also inadvertently lead to environmental effects beyond the intended benefits. The planning process based on national guidelines directly incorporates consideration of all environmental effects, with a particular focus on possible impacts upon non-target species or habitats. The results of the SEA are incorporated directly into the strategy itself, but are also summarized below in this statement.
All species that depend on aerial insects for prey such as bats, dragonflies, wasps, swallows, nighthawks, and flycatchers and specifically bird species at risk including the Chimney Swift, Eastern Whip-poor-will (Antrostomus vociferus), Common Nighthawk (Chordeiles minor), and Acadian Flycatcher (Empidonax virescens) may benefit from the recommended approaches for Olive-sided Flycatcher in their breeding, wintering, and/or migration areas that aim to increase the availability of insects. Other species found along the migratory route of Olive-sided Flycatcher (e.g., Alder Flycatcher (Empidonax alnorum), Barn Swallow (Hirundo rustica), and Bank Swallow (Riparia riparia)) may also benefit from recovery approaches in these areas. Actions that enhance wintering habitat may have additive effects favouring several other landbirds that share Olive-sided Flycatcher’s wintering habitat or have overlapping ranges (e.g., Canada Warbler).
Nonetheless, some species, including other species at risk, may prefer different forest conditions. For example, two landbirds require different forest breeding habitat than Canada Warbler: Eastern Wood-pewee, assessed as special concern by COSEWIC, breeds in intermediate and mature forest stands with little underbrush (COSEWIC 2012) and the Threatened Eastern Whip-poor-will breeds in semi-open forests with little understory vegetation (COSEWIC 2009). Recovery actions for the species must be integrated with best practices for other songbird and wildlife species.
The possibility that the present recovery strategy inadvertently generates negative effects on the environment and on other species was considered. The majority of recommended actions are non-intrusive in nature, including surveys and outreach. It is unlikely that the present recovery strategy will produce significant negative effects.
The following list is not exhaustive, but illustrates some of the research required to understand the threats to the species, its prey, and their habitats.
- Determine whether the ecological trap of forest harvesting is a widespread phenomenon.
- Determine the role of wildfire versus forest harvest and other disturbance sources (e.g., insect outbreaks, windthrow, and blowdown) in the creation and maintenance of habitat.
- Determine the amount (and characteristics) of forest harvesting and silviculture that can be completed while maintaining enough suitable habitat at a landscape scale for Olive-sided Flycatcher populations.
- Determine the relative importance of nonbreeding versus breeding habitat supply in population declines.
- Investigate the rate and impact of various types of habitat loss throughout breeding and nonbreeding areas.
- Determine the effects of alterations in hydrological regimes on Olive-sided Flycatchers.
- Determine potential links between insect availability and breeding productivity.
- Determine whether there is a temporal mismatch between reproduction and maximal prey abundance.
- Determine the effects of habitat loss on Olive-sided Flycatcher’s prey availability.
- Determine the exposure of pesticides, mercury, and acidification throughout Olive sided Flycatcher’s range and their potential effects on prey availability.
- Monitor frequency of collisions and determine site characteristics contributing to high collision rates.
- Determine human-related predation risk in urban and rural areas (e.g., by cats and other species with increased populations due to human habitation).
- Determine the potential impacts of climate change on the species and its habitat.
- Determine Olive-sided Flycatcher’s exposure to pollution (pesticides and mercury) throughout its range and identify impacts.
- Determine whether acidification of the species’ environment is negatively affecting Olive-sided Flycatcher and its habitat (e.g., through loss of favoured nesting and/or foraging sites, increased vigilance, increased predation risk, and calcium deficiency during egg-laying and chick-rearing phases).