Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Canada - 2017 [Proposed]
Small-mouthed Salamander

- Part 1 - Federal Addition to the Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario, prepared by Environment and Climate Change Canada
- Table of contents
- Preface
- Acknowledgements
- Additions and modifications to the adopted document
- 1 Recovery feasibility summary
- 2 COSEWIC i species assessment information
- 3 Species status information
- 4 Species information
- 5 Threats
- 6 Population and distribution objectives
- 7 Broad strategies and general approaches to meet objectives
- 8 Critical habitat
- 9 Measuring progress
- 10 Statement on action plans
- 11 Effects on the environment and other species
- References
- Figure 1. Grid squares that contain critical habitat for the Small-mouthed Salamander in Canada.
- Table 1. Detailed biophysical attributes of critical habitat for specific life cycle activities of the Small-mouthed Salamander in Ontario.
- Table 2. Grid squares that contain critical habitat for the Small-mouthed Salamander in Canada.
- Table 3. Activities Likely to Result in the Destruction of Critical Habitat.
- Appendix A. Subnational conservation ranks (S-Ranks) of Small-mouthed Salamander (Ambystoma texanum) in Canada and the United-States
- Part 2 - Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario, prepared by Stewart E. Hamill for the Ontario Ministry of Natural Resources and Forestry
- Table 1. Protection and recovery objectives
- Table 2. Approaches to recovery of the Small-mouthed Salamander in Ontario
- 1. Protect and maintain the quality and quantity of habitat on Pelee Island where the Small-mouthed Salamander occurs.
- 2. Implement a monitoring program for salamander populations, habitats and threats on Pelee Island including surveys of suitable habitat.
- 3. Promote and carry out research on Small-mouthed Salamander genetics, populations and threats.
- 4. Investigate existing, former and potential Small-mouthed Salamander habitats on Pelee Island to determine if restoration, re-introduction or population interventions would be appropriate.
- 5. Implement education, stewardship and communication programs for private landowners, residents and visitors on Pelee Island.
Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Canada [Proposed] - 2017

Environment and Climate Change Canada. 2017. Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment and Climate Change Canada, Ottawa. 2 parts, 27 pp. + vi + 18 pp.
For copies of the recovery strategy, or for additional information on species at risk, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry.
Cover illustration: © Jim Bogart
Également disponible en français sous le titre
« Programme de rétablissement de la salamandre à nez court (Ambystoma texanum) au Canada [Proposition] »
Content (excluding the illustrations) may be used without permission, with appropriate credit to the source.
Under the Accord for the Protection of Species at Risk (1996), the federal, provincial, and territorial governments agreed to work together on legislation, programs, and policies to protect wildlife species at risk throughout Canada.
In the spirit of cooperation of the Accord, the Government of Ontario has given permission to the Government of Canada to adopt the Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario (Part 2) under Section 44 of the Species at Risk Act (SARA). Environment and Climate Change Canada has included a federal addition (Part 1) which completes the SARA requirements for this federal recovery strategy.
The federal recovery strategy for the Small-mouthed Salamander in Canada consists of two parts:
Part 1 - Federal Addition to the Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario, prepared by Environment Canada.
Part 2 - Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario, prepared by Stewart E. Hamill for the Ontario Ministry of Natural Resources and Forestry
The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of recovery strategies for listed Extirpated, Endangered, and Threatened species and are required to report on progress within five years after the publication of the final document on the SAR Public Registry.
The Minister of Environment and Climate Change is the competent minister under SARA for the Small-mouthed Salamander and has prepared the federal component of this recovery strategy (Part 1), as per section 37 of SARA. To the extent possible, it has been prepared in cooperation with the Province of Ontario, as per section 39(1) of SARA. SARA section 44 allows the Minister to adopt all or part of an existing plan for the species if it meets the requirements under SARA for content (sub-sections 41(1) or (2)). The Ontario Ministry of Natural Resources and Forestry led the development of the attached recovery strategy for the species (Part 2) in cooperation with Environment and Climate Change Canada.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy and will not be achieved by Environment and Climate Change Canada, or any other jurisdiction alone. All Canadians are invited to join in supporting and implementing this strategy for the benefit of the Small-mouthed Salamander and Canadian society as a whole.
This recovery strategy will be followed by one or more action plans that will provide information on recovery measures to be taken by Environment and Climate Change Canada and other jurisdictions and/or organizations involved in the conservation of the species. Implementation of this strategy is subject to appropriations, priorities, and budgetary constraints of the participating jurisdictions and organizations.
The recovery strategy sets the strategic direction to arrest or reverse the decline of the species, including identification of critical habitat to the extent possible. It provides all Canadians with information to help take action on species conservation. When critical habitat is identified, either in a recovery strategy or an action plan, SARA requires that critical habitat then be protected.
In the case of critical habitat identified for terrestrial species including migratory birds SARA requires that critical habitat identified in a federally protected area Footnote1 be described in the Canada Gazette within 90 days after the recovery strategy or action plan that identified the critical habitat is included in the public registry. A prohibition against destruction of critical habitat under ss. 58(1) will apply 90 days after the description of the critical habitat is published in the Canada Gazette.
For critical habitat located on other federal lands, the competent minister must either make a statement on existing legal protection or make an order so that the prohibition against destruction of critical habitat applies.
If the critical habitat for a migratory bird is not within a federal protected area and is not on federal land, within the exclusive economic zone or on the continental shelf of Canada, the prohibition against destruction can only apply to those portions of the critical habitat that are habitat to which the Migratory Birds Convention Act , 1994 applies as per SARA ss. 58(5.1) and ss. 58(5.2).
For any part of critical habitat located on non-federal lands, if the competent minister forms the opinion that any portion of critical habitat is not protected by provisions in or measures under SARA or other Acts of Parliament, or the laws of the province or territory, SARA requires that the Minister recommend that the Governor in Council make an order to prohibit destruction of critical habitat. The discretion to protect critical habitat on non-federal lands that is not otherwise protected rests with the Governor in Council.
This recovery strategy was prepared by John Brett of Environment and Climate Change Canada, Canadian Wildlife Service - Ontario Region, with contributions from Jennie L. Pearce of Pearce & Associates Ecological Research and David A. Kirk of Aquila Conservation and Environment Consulting. This recovery strategy benefited from input, review, and suggestions from the following individuals and organizations: Allison Foran, Krista Holmes, Angela Darwin, Marie-Claude Archambault, and Judith Girard (Environment and Climate Change Canada, Canadian Wildlife Service - Ontario Region) and Jay Fitzsimmons, Joe Crowley, Glenn Desy, and Vivian Brownell (Ontario Ministry of Natural Resources and Forestry). The Ontario Natural Heritage Information Centre (NHIC), Jim Bogart, Thomas Hossie, and Dennis Murray provided species occurrence information.
Acknowledgement and thanks is given to all other parties that provided advice and input used to help inform the development of this recovery strategy including various Aboriginal organizations and individuals, individual citizens, and stakeholders who provided input and/or participated in consultation meetings.
The following sections have been included to address specific requirements of the federal Species at Risk Act (SARA) that are not addressed in the Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario (Part 2 of this document, referred to henceforth as "the provincial recovery strategy") and/or to provide updated or additional information.
Environment and Climate Change Canada is adopting the provincial recovery strategy, including section 2, Recovery, with the exception of approaches 1.1, 2.1, 3.1, 3.2, and 3.3. Approaches 1.1, 2.1, 3.1, and 3.3 have been modified for the purposes of this recovery strategy. Environment and Climate Change Canada has established its own population and distribution objective that is consistent with the provincial recovery goal.
Under SARA, there are specific requirements and processes set out regarding the protection of critical habitat. Therefore, statements in the provincial recovery strategy referring to protection of the species' habitat may not directly correspond to federal requirements. Recovery measures dealing with the protection of habitat are adopted; however, whether these measures will result in protection of critical habitat under SARA will be assessed following publication of the final federal recovery strategy.
Based on the following four criteria that Environment and Climate Change Canada uses to establish recovery feasibility, there are unknowns regarding the feasibility of recovery of the Small-mouthed Salamander. In keeping with the precautionary principle, this recovery strategy has been prepared as per section 41(1) of SARA, as would be done when recovery is determined to be technically and biologically feasible. This recovery strategy addresses the unknowns surrounding the feasibility of recovery.
Due to its very limited distribution and the low probability of natural range expansion, the Small-mouthed Salamander will likely continue to be considered rare in Canada despite applying available recovery techniques and maintaining existing populations. In addition, based on the fact that the species inhabits a small island in Canada, it will likely always be vulnerable to human-caused stressors and natural, chance events (MacArthur and Wilson 1963).
i COSEWIC (Committee on the Status of Endangered Wildlife in Canada)
Globally, the Small-mouthed Salamander is ranked Secure (G5) (NatureServe 2015). At the national scale, it is ranked as Critically Imperiled (N1) in Canada and Secure (N5) in the United States. At the sub-national level, it is ranked as Critically Imperiled (S1) in Ontario, and Critically Imperiled to Secure across its range in the United States (Appendix A).
The Small-mouthed Salamander is listed as Endangered Footnote3 under the Ontario Endangered Species Act, 2007 (ESA), and is currently listed as Endangered Footnote4 on Schedule 1 of the federal SARA.
4.1 Species population and distribution
The Small-mouthed Salamander occurs in North America, ranging from Ontario, Pennsylvania, and Michigan in the north, to Texas, Louisiana, Mississippi and Alabama in the south.
In Canada, the species only occurs on Pelee Island, at the southwestern end of Lake Erie in Ontario. Five breeding locations were identified in the 1991 COSEWIC Status Report (Bogart and Licht 1991), but two of those sites have since been lost (Bogart and Licht 2004); the 2014 COSEWIC species assessment and the Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario identifies three extant Footnote5 sites on Pelee Island (Hamill 2015). Since the publication of the last COSEWIC assessment and the provincial recovery strategy, Small-mouthed Salamanders have been documented at three additional locations on Pelee Island, bringing the total number of extant sites to six (Hossie and Murray 2016).
At the northern limit of its global range, the Canadian population of the Small-mouthed Salamander constitutes less than one percent of the species' global distribution (Bogart and Licht 2004).
Estimates of population size for the Small-mouthed Salamander in Canada are difficult to obtain because of the presence of unisexual polyploid salamanders Footnote6, which are morphologically Footnote7 similar to the Small-mouthed Salamander, and comprise approximately 78% of the Ambystoma salamander population sampled on Pelee Island (Bogart and Licht 2004).
As described in the provincial recovery strategy (Part 2, section 1.6), habitat alteration, loss and fragmentation, invasive and introduced species, climate change, pollution, predation, road mortality and competition and hybridization are considered threats to the Small-mouthed Salamander in Canada.
In addition to those threats identified in Part 2, additional threats may potentially affect this species.
The Small-mouthed Salamander in Canada could be particularly vulnerable in the face of pathogen introduction due to its small population size and geographic isolation. Pathogens like chytrid fungi Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans are introduced through regional and international trade of animals, and have caused important declines in amphibians and other ectotherms around the world (Duffus et al. 2015, Yap et al. 2015). Mortalities caused by Ranaviruses and B. dendrobatidis have been documented in various amphibian species of Canada. (Hughey et al. 2014; Duffus et al. 2015;). B. salamandrivorans, a pathogen specific to salamanders, has not yet been recorded in North America, but introduction is considered likely given considerable salamander imports from Asia, where the disease originates (Yap et al. 2015).
Trematode parasites have been observed in Small-mouthed Salamanders in the United States (McAllister et al. 2008, 2010). However, the impact of this threat on the viability of the Canadian population is unknown (COSEWIC 2014).
Environmental contaminants (e.g., pesticides, de-icing salt) and potential habitation
alteration and predation caused by Wild Turkeys may be additional threats to this species.
The provincial recovery strategy contains the following recovery goal for the recovery of the Small-mouthed Salamander in Ontario:
- The recovery goal is to ensure that threats to populations and habitat are sufficiently managed to allow for long-term persistence and expansion of the Small-mouthed Salamander population within its Ontario range on Pelee Island.
Under SARA, a population and distribution objective for the species must be established. Consistent with the goal provided in the provincial recovery strategy, Environment and Climate Change Canada's population and distribution objective for the Small-mouthed Salamander in Canada is to:
- Ensure the persistence of Small-mouthed Salamander at extant sites on Pelee Island and, where biologically and technically feasible, promote population expansion within its range on the island.
Estimates of population size for the Small-mouthed Salamander are difficult to obtain because of the presence of unisexual polyploid salamanders, which are morphologically similar to the Small-mouthed Salamander, and comprise approximately 78% of the sampled Ambystoma salamander population on Pelee Island (Bogart and Licht 2004). Adults are also difficult to observe or capture except at breeding ponds, where they may be present for only a few days. As a result, there is not enough information about population size and trends of the Small-mouthed Salamander in Canada to set a quantitative objective based on population abundance. Therefore, the population and distribution objective is based on ensuring persistence of the local populations at extant sites rather than targeting a specific population abundance.
Maintaining local populations of the Small-mouthed Salamander at extant sites on Pelee Island will require reducing and mitigating threats to this species, especially those related to a loss of suitable habitat. Suitable habitat for the Small-mouthed Salamander is limited within its Canadian range on Pelee Island (Hamill 2015). Conserving suitable habitat and promoting connectivity between important habitats is essential for the persistence of Small-mouthed Salamander population on Pelee Island. Provided other threats to Small-mouthed Salamander individuals (e.g., habitat loss, alteration and fragmentation) are managed and mitigated, populations would be expected to persist over long time frames where sufficient suitable habitat exists. Given the limited range and small population size of this species in Canada, a single catastrophic natural or human-induced event could threaten the survival of the entire Canadian population. The natural expansion of the existing population to different areas of Pelee Island may be encouraged through the stewardship and management of potential recovery habitat (not currently occupied by the salamander). Therefore, this recovery strategy also provides direction for communication, outreach, and habitat management activities. Engaging landowners, residents and visitors in habitat management and conservation will continue to be an important part of recovery for the Small-mouthed Salamander and its habitat in Canada. Implementing those broad strategies adopted from the provincial Recovery Strategy for the Small-mouthed Salamander in Ontario (Part 2) will aid in the understanding and recovery of this species.
Environment and Climate Change Canada is adopting the approaches identified in section 2.3 of the Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario (Part 2) as the broad strategies and general approaches to meet the population and distribution objective, with the exception of approaches 1.1, 2.1, 3.1, 3.2, and 3.3. Approaches 1.1, 2.1, 3.1, and 3.3 have been modified, for the purposes of this recovery strategy, to read as follows:
1.1 Encourage the Ontario Ministry of Natural Resources and Forestry to develop a habitat regulation or habitat description to define the area protected as habitat for Small-mouthed Salamander in Ontario.
2.1 Carry out regular inventory, monitoring, surveying and sampling activities for populations, reproduction, water levels, habitat quality, new locations, and known threats.
3.1 Study the impact of Wild Turkeys on salamanders in general, and on Small-mouthed Salamander in particular.
3.3 Investigate the impact of climate change, pathogens, contaminants, and trematode parasites on Small-mouthed Salamander populations.
8.1 Identification of the species' critical habitat
Section 41 (1)c of SARA requires that recovery strategies include an identification of the species' critical habitat, to the extent possible, as well as examples of activities that are likely to result in its destruction. Under SARA, critical habitat is "the habitat that is necessary for the survival or recovery of a listed wildlife species and that is identified as the species' critical habitat in the recovery strategy or in an action plan for the species".
Identification of critical habitat is not a component of provincial recovery strategies under the Province of Ontario's ESA. Under the ESA, when a species becomes listed as endangered or threatened on the Species at Risk in Ontario List, it automatically receives general habitat protection. The Small-mouthed Salamander currently receives general habitat protection under the ESA; however, a description of the general habitat has not yet been developed. In some cases, a habitat regulation may be developed that replaces the general habitat protection. A habitat regulation is a legal instrument that prescribes an area that will be protected Footnote8 as the habitat of the species by the Province of Ontario. A habitat regulation has not been developed for Small-mouthed Salamander under the ESA; however, the provincial recovery strategy (Part 2) contains a recommendation on the area for consideration in developing a habitat regulation. This federal recovery strategy identifies critical habitat for the Small-mouthed Salamander in Canada to the extent possible, based on this recommendation and on the best available information as of March 2016.
Critical habitat is identified for the six extant sites of Small-mouthed Salamander in Ontario, all occurring on Pelee Island (See Figure 1.; see also Table 2). More precise boundaries may be mapped, and additional critical habitat may be added in the future if new or additional information supports the inclusion of areas beyond those currently identified (e.g., species dispersal into adjacent areas, or Small-mouthed Salamander presence is confirmed at other locations).
The identification of the Small-mouthed Salamander critical habitat is based on two criteria: habitat occupancy and habitat suitability, which are discussed in detail below.
8.1.1 Habitat occupancy
The habitat occupancy criterion refers to areas where there is a reasonable degree of certainty of current use by the species. Occupancy is based on occurrence reports from the Natural Heritage Information Centre (NHIC, also known as the Ontario Conservation Data Centre), documented reports from targeted Small-mouthed Salamander surveys in 2015, and Environment and Climate Change Canada's Canadian Wildlife Service. These records must provide enough detail to be associated to a specific location (e.g., an individual's home range) to be considered adequate to satisfy the habitat occupancy criterion.
Habitat is considered occupied when:
- One or more Small-mouthed Salamander individuals (includes all life stages) have been observed, and
- The location has not been classified as extirpated by the NHIC.
In Canada, the population of Small-mouthed Salamanders includes only a small number of individuals (Bogart and Licht 2004, COSEWIC 2014). Therefore, a precautionary approach is used where a single observation (confirmed and unconfirmed individuals identified as Small-mouthed Salamander) may be indicative of a local population or important habitat features. While unisexual female polyploid Ambystoma salamanders will not be protected under the ESA or SARA, unisexuals can resemble the Small-mouthed Salamander which causes issues with species identification (Hamill 2015). Due to difficulties in providing genetic identification, it is possible that some observations of Small-mouthed Salamanders may actually be unisexual individuals. Therefore, taking the precautionary approach, observations of Small-mouthed Salamander individuals that are not genetically confirmed will also indicate habitat occupancy. The presence of unisexuals indicates that a pure breeding Small-mouthed Salamander or a pure Blue-spotted Salamander is present to act as a sperm donor (Hedges et al. 1992). Furthermore, unisexuals use the same habitats as pure Small-mouthed Salamanders and, therefore, a unisexual associated with a specific habitat (e.g., breeding pond) provides a good indication that pure Small-mouthed Salamanders may also use that habitat.
As two of the known breeding sites in Canada have been lost (Hamill 2015), only observations from extant occurrence reports (i.e., not extirpated) were considered in the application of the habitat occupancy criterion. If new observations become available, they will be considered for the identification of additional critical habitat.
8.1.2 Habitat suitability
Habitat suitability refers to areas possessing a specific set of biophysical attributes that support individuals of the species to carry out essential life cycle activities (e.g., breeding, foraging, movement, growth, and hibernation). Suitable habitat for the Small-mouthed Salamander can therefore be described as a mosaic of habitats including core breeding areas and dispersal corridors between core breeding areas. Core breeding areas are comprised of breeding ponds as well as the terrestrial areas within 300 m of breeding ponds that provide suitable conditions for foraging, growth, and hibernation. The 300 m distance is based on data from telemetry studies of a similar species within Ontario, the Jefferson Salamander (Ambystoma jeffersonianum), and is the habitat area expected to support 90% of the adult population for each breeding site (Hamill 2015). Dispersal corridors include habitat that connect core breeding areas and provide suitable conditions for seasonal migrations and other movements. Within the area of suitable habitat, the biophysical attributes required by the Small-mouthed Salamander will vary over space and time with the dynamic nature of ecosystems. In addition, particular biophysical attributes will be of greater importance to salamanders at different points in time (e.g., during different life processes, seasons or at various times of the year).
The biophysical attributes of suitable habitat include the characteristics described in Table 1.
| Life Stage and/or Need | Biophysical Attributes | Reference |
|---|---|---|
| Breeding (i.e., mating and egg-laying) |
|
|
| All life processes (i.e., foraging, growth, breeding, hibernation and movement) |
|
|
| Hibernation |
|
|
| Movement (including seasonal migrations) |
|
|
a As conditions may vary year-to-year, breeding ponds that are temporary in nature may only contain ponded water for a sufficient duration in some years.
b Habitat containing a moderate amount of moisture.
At this time, breeding ponds that provide the basis for delineating critical habitat are represented using available wetland habitat mapping. Because small, temporal or ephemeral features are not well captured through existing land classification mapping, especially where field verification has not taken place, caution should be taken when biophysical attributes of suitable habitat are represented by available thematic data.
Critical habitat includes all of the biophysical attributes described in Table 1 above that extend radially 300 m from the edge of the breeding pond.
To support adult migration and juvenile dispersal between breeding wetlands, the present recovery strategy also includes connecting corridors as part of the critical habitat. These correspond to the areas of contiguous suitable habitat connecting two breeding wetlands that meet the habitat occupancy criteria and that are separated by a maximum distance of 1 km, which is based on the maximum migratory distance of adults of similar species (Hamill 2015). Should new information about the movements of the Small-mouthed Salamander become known, the criteria above may be refined.
8.1.3 Application of criteria to identify critical habitat for small-mouthed salamander
Critical habitat for the Small-mouthed Salamander is identified as the extent of suitable habitat (section 7.1.2), where the habitat occupancy criterion is met (section 7.1.1). In applying the critical habitat criteria above to the best available data, critical habitat is identified for the six extant populations of the Small-mouthed Salamander in Canada (Figure 1, See also Table 2). The critical habitat identified is considered a full identification of critical habitat and is sufficient to meet the population and distribution objective for the Small-mouthed Salamander.
Newly created artificial habitat (i.e. created breeding ponds) will not be included in the identification of critical habitat until there is evidence of use. In addition, the following features are not considered suitable (do not meet the biophysical attributes described above) and are not part of critical habitat: existing houses, buildings, structures, quarries, and other pre-existing industrial land uses, and major roads.
Critical habitat identified for the Small-mouthed Salamander is presented using a 10 x 10 km UTM grid. The UTM grid squares presented in Figure 1 are part of a standardized grid system that indicates the general geographic areas containing critical habitat, which can be used for land use planning and/or environmental assessment purposes. In addition to providing these benefits, the 10 x 10 km Standardized UTM grid respects data-sharing agreements with the province of Ontario. Critical habitat within each grid square occurs where the description of occupancy (section 7.1.1) and habitat suitability (section 7.1.2) is met. More detailed information on critical habitat to support protection of the species and its habitat may be requested on a need-to-know basis by contacting Environment and Climate Change Canada - Canadian Wildlife Service at ec.planificationduretablissement-recoveryplanning.ec@canada.ca.
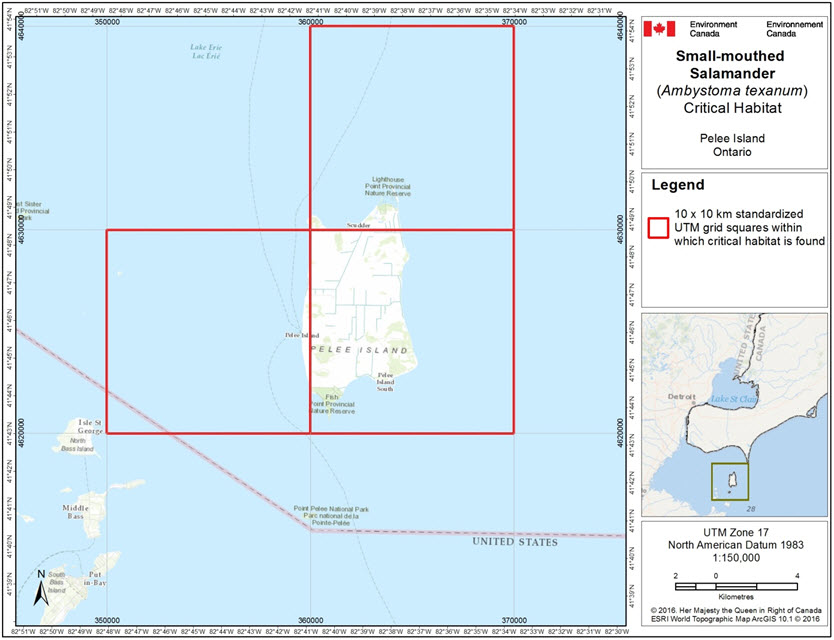
Long description for Figure 1
Figure 1 shows the three grid squares which cover critical habitat for this species. One square covers most of Pelee Island, one square covers the northern portion of the island, and the final square covers its western coast.
| 10 x 10 km Standardized UTM grid square ID c | UTM Grid Square Coordinates d Easting |
UTM Grid Square Coordinates d Northing |
Land tenure e |
|---|---|---|---|
| 17TLG52 | 350000 | 4620000 | Non-federal Land |
| 17TLG62 | 360000 | 4620000 | Non-federal Land |
| 17TLG63 | 360000 | 4630000 | Non-federal Land |
c Based on the standard UTM Military Grid Reference System, where the first 2 digits and letter represent the UTM Zone, the following 2 letters indicate the 100 x 100 km standardized UTM grid followed by 2 digits to represent the 10 x 10 km standardized UTM grid containing all or a portion of the critical habitat unit. This unique alphanumeric code is based on the methodology produced from the Breeding Bird Atlases of Canada (for more information on breeding bird atlases).
d The listed coordinates are a cartographic representation of where critical habitat can be found, presented as the southwest corner of the 10 x 10 km standardized UTM grid square containing all or a portion of the critical habitat unit. The coordinates may not fall within critical habitat and are provided as a general location only.
e Land tenure is provided as an approximation of the types of land ownership that exist at the critical habitat units and should be used for guidance purposes only. Accurate land tenure will require cross referencing critical habitat boundaries with surveyed land parcel information.
8.2 Activities likely to result in destruction of critical habitat
Understanding what constitutes destruction of critical habitat is necessary for the protection and management of critical habitat. Destruction is determined on a case by case basis. Destruction would result if part of the critical habitat was degraded, either permanently or temporarily, such that it would not serve its function when needed by the species. Destruction may result from a single activity or multiple activities at one point in time or from the cumulative effects of one or more activities over time, and can occur at a variety of scales and in both aquatic and terrestrial habitats. It may occur from an activity taking place either within or outside of the critical habitat boundary and it may occur in any season of the year. Within the critical habitat boundary, activities may affect core breeding areas which include breeding ponds and the areas within 300 m of occupied breeding ponds that provide suitable conditions for foraging, dispersal, migration or hibernation (i.e., the areas described in Table 1). Activities may also affect dispersal corridors that connect core areas (i.e., areas that allow movement, described in Table 1). Within dispersal corridors it is most important to maintain habitat permeability (movement through connective habitat to access adjacent core areas) and, as a result, certain activities that are likely to cause destruction in core areas may not cause destruction in corridors, so long as sufficient habitat permeability is maintained. It should be noted that not all activities that occur in or near critical habitat are likely to cause its destruction.
Activities described in Table 3 are examples of those likely to cause destruction of critical habitat for the species; however, destructive activities are not necessarily limited to those listed.
| Description of activity | Description of effect in relation to function loss | Where activity may cause destruction of critical habitat Within critical habitat boundary Core breeding areas |
Where activity may cause destruction of critical habitat Within critical habitat boundary Dispersal corridor |
Where activity may cause destruction of critical habitat Outside critical habitat boundary |
Details of effect |
|---|---|---|---|---|---|
| Development activities (e.g. building construction) or other activities that alter site cover and/or hydrology (e.g., drainage for agriculture, tree harvesting, site clearing and grading, stormwater management, surface paving, etc.) | Tree harvesting, site clearing, and drainage (e.g., for agriculture) and activities that result in the net removal, disturbance or destruction of cover objects (e.g., rocks, logs or debris) may result in the direct loss of suitable terrestrial microhabitat characteristics which the species relies on for foraging, for maintaining hydration, for protective cover, and for overwintering. Site clearing and grading may alter the topography and the hydrology (drainage patterns, water table, groundwater flow) of the site. Stormwater management and increases in impervious surfaces (e.g. paving) may also alter site hydrology. These topographic and hydrologic changes may destroy or degrade breeding and/or foraging habitat by modifying or disrupting water flow, water balance, wetland hydroperiods f, which in turn could modify or disrupt soil moisture or wetland composition and function. Development may remove canopy, alter watercourses for snowmelt and runoff, and/or draw down the water table and therefore may result in the reduction of ephemeral ponds or premature drying of ponds, and thereby destroy, damage or fragment habitat. | X | X | X | Activities within critical habitat core breeding areas that alter cover or the hydrologic regime are highly likely to result in direct destruction at any time of the year because they would degrade habitat required by all life stages for survival. If grading or other activities that alter water flows occur outside of critical habitat or in corridors, it could result in the indirect destruction of core breeding critical habitat by altering water regimes within critical habitat thereby reducing or eliminating breeding habitat. Large-scale developments within or adjacent to critical habitat may cause destruction of critical habitat at any time of year. If it occurs within critical habitat core breeding areas, it is highly likely to cause destruction; if it occurs in corridors or adjacent to critical habitat, effects would most likely be cumulative and whether or not they result in destruction would likely depend on the extent and location of the development. |
| Complete or partial drainage or filling of wetlands and water management | Complete or partial draining (or other significant hydrological changes) or filling of wetlands (e.g., breeding ponds) at any time of the year may cause permanent loss of habitat or degradation of habitat (e.g., reduced soil moisture) required for all life stages (e.g., breeding, foraging, hibernation and movement). | X | X | X | If these activities were to occur outside the bounds of critical habitat, it could result in destruction of critical habitat if the wetland characteristics that contribute to critical habitat suitability are not maintained (e.g., sufficient soil moisture for hibernation). A single event could cause critical habitat destruction. |
| Activities that introduce exotic and/or invasive species (e.g., planting non-native species, moving fill) | Introduction of exotic and/or invasive species may lead to the reduction of wetland habitat. For example, dense stands of non-native Common Reed (Phragmites australis) can overgrow breeding sites, preventing the species from carrying out this life process. Such stands can also fill in wetland habitat and prevent salamanders from being able to forage easily for food. | X | X | - | Activities which introduce exotic and/or invasive species which occur within the bounds of critical habitat could lead to habitat destruction. A single event within critical habitat could lead to habitat destruction because once seeds are introduced it can lead to rapid expansion of invasive species. |
| Erecting barriers (e.g. silt fences or drainage ditches) | Temporary and permanent structures including silt fences erected during construction, or drainage ditches, create physical barriers within the habitat that may hinder or prevent migration of salamanders, thereby preventing movement and restricting access to habitats required to carry out life processes (e.g., breeding, growth, and hibernation) or to migrate among sites. | X | X | - | This activity must occur within the bounds of critical habitat to cause its destruction, and the likelihood of it causing destruction would depend in large part on the configuration of the barrier and the time of year that it is in place. If this activity occurs within critical habitat core breeding areas, it could cause destruction if it prevents access to areas required by the Small-mouthed Salamander at one or more life stages. If this activity were to occur in early spring it could prevent or impair breeding, or prevent or impair adults from returning to foraging habitat. If this activity were to occur in late summer/early fall, this activity could prevent or impede dispersal by juveniles from breeding habitat to foraging and overwintering habitat. If this activity occurs within critical habitat corridors, it may cause destruction if it eliminates the function of the corridor. |
| Building or upgrading roads | If it occurs within critical habitat, this activity could result in the loss or degradation of suitable habitat for all life stages through removal of vegetative cover. The construction or upgrading of roads can also lead to fragmentation of critical habitat by forming physical barriers that impede dispersal (e.g., steep roadside slopes, large roads with concrete lane dividers), thereby preventing Small-mouthed Salamander from accessing habitats required to carry out life processes or to migrate among sites, and by increasing mortality (e.g., greater risk of desiccation, vehicle collision and predation). If this activity occurs within or near critical habitat, chemicals and pollutants from roads (e.g., salt, metals, products of combustion) can enter breeding ponds and alter pond water chemistry, reducing habitat suitability and altering the availability of suitable aquatic prey items. | X | X | X | The effects of this activity can be both direct (e.g., loss of cover, creation of a barrier that fragments habitat) and indirect or cumulative (e.g., pollution). If the effects of the activity are permanent (e.g., paving natural habitat), the activity is likely to cause destruction of habitat if undertaken at any time of the year. However, if activities do not have lasting effects (e.g., upgrading of a road that does not result in further reducing habitat permeability or increasing pollution), it may only cause destruction if conducted when the species is undertaking terrestrial movements. This activity is highly likely to cause destruction if it occurs within critical habitat core breeding areas, because it would reduce access to areas required by the Small-mouthed Salamander at one or more life stages. If this activity occurs within critical habitat corridors, it is also highly likely to cause destruction by eliminating the function of the corridor. If this activity occurs outside of critical habitat, it may result in the indirect destruction of critical habitat through the introduction of chemical pollution (e.g., salt) into wetlands within critical habitat or changes to the hydrology |
| Intensification of agricultural practices near breeding wetlands | Increased runoff of pesticides and fertilizers into adjacent habitats can reduce water quality and lead to reduced prey availability. | - | X | X | If this activity occurs within critical habitat dispersal areas, it could cause destruction if it prevents access to areas required by the Small-mouthed Salamander at one or more life stages. If this activity occurs outside of critical habitat, it may result in the indirect destruction of critical habitat through the introduction of chemical pollution (e.g., pesticides and fertilizers) into wetlands within critical habitat. |
| Addition of carnivorous fish to breeding ponds | Addition of carnivorous fish to ponds would destroy breeding habitat because fish prey upon all life stages of salamanders and would therefore reduce the survival and reproductive success of Small-mouthed Salamander individuals. | X | - | - | If this activity occurs within critical habitat core breeding areas, at any time of the year, it is highly likely to result in its destruction because it would directly eliminate breeding habitat. |
f The duration of time in which water is present in a wetland.
The performance indicators presented below provide a way to define and measure progress toward achieving the population and distribution objective. Every five years, success of recovery strategy implementation will be measured against the following performance indicators:
- The number of sites occupied by Small-mouthed Salamander in Canada has been maintained, and if feasible, increased.
One or more action plans will be completed for Small-mouthed Salamander by December 31, 2022.
A strategic environmental assessment (SEA) is conducted on all SARA recovery planning documents, in accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals. The purpose of a SEA is to incorporate environmental considerations into the development of public policies, plans, and program proposals to support environmentally sound decision-making and to evaluate whether the outcomes of a recovery planning document could affect any component of the environment or any of the Federal Sustainable Development Strategy's (FSDS) goals and targets.
Recovery planning is intended to benefit species at risk and biodiversity in general. However, it is recognized that strategies may also inadvertently lead to environmental effects beyond the intended benefits. The planning process based on national guidelines directly incorporates consideration of all environmental effects, with a particular focus on possible impacts upon non-target species or habitats. The results of the SEA are incorporated directly into the strategy itself, but are also summarized below in this statement.
Recovery efforts that are focused on the Small-mouthed Salamander will likely benefit species inhabiting extant breeding ponds and the surrounding habitat, including many Carolinian plant species, Blue-spotted Salamander (Ambystoma laterale), Eastern Foxsnake (Pantherophis gloydi) and Blue Racer (Coluber constrictor foxii). It is also expected to benefit the populations of unisexual polyploid salamanders on Pelee Island. No species of conservation concern are expected to be detrimentally affected.
Bogart, J.P. and L.E. Licht. 1991. COSEWIC status report on the smallmouthed salamander Ambystoma texanum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 21 pp.
Bogart, J.P., and L.E. Licht. 2004. Update COSEWIC status report on the small-mouthed salamander Ambystoma texanum in Canada in COSEWIC assessment and update status report on the small-mouthed salamander Ambystoma texanum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-20 pp.
Brodman, R. and H.D. Krouse. 2007. How Blue-spotted and Small-mouthed Salamander larvae coexist with their unisexual counterparts. Herpetologica 63 (2):135-143.
COSEWIC. 2014. COSEWIC status appraisal summary on the Small-mouthed Salamander Ambystoma texanum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x pp. (Species at Risk Public Registry).
Duffus, A.L.J., T.B. Waltzek, A.C. Stöhr, M.C. Allender, M. Gotesman, R.J. Whittington, P. Hick, M.K. Hines, and R.E. Marschang. 2015. Distribution and host range of ranaviruses. Pp. 9-57. In M.J. Gray and V.G. Chinchar (eds.). Ranaviruses: Lethal pathogens of ectothermic vertebrates. Springer International Publishing.
Hamill, S.E. 2015. Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. vi + 18 pp.
Hedges, S.B., J.P. Bogart, and L.M. Maxson. 1992. Ancestry of unisexual salamanders. Nature 356:708-710.
Hossie, T.J. & D. Murray. 2016. Assessing the population size, genetic structure, critical habitat, and predation threats in Small-mouthed Salamanders. Prepared for the Ontario Ministry of Natural Resources and Forestry, Ontario. 39 pp.
MacArthur, R.H. and E.O. Wilson. 1963. An equilibrium theory of insular zoogeography. 1051 Evolution 17:373-387.
McAllister, C.T., C.R. Bursey, and S.E. Trauth. 2008. New host and geographic distribution records for some endoparasites (Myxosporea, Trematoda, Cestoidea, Nematoda) of amphibians and reptiles from Arkansas and Texas, USA. Comparative Parasitology 75:241-254.
McAllister, C.T., C.R. Bursey, J.A. Crawford, A.R. Kuhns, C. Shaffer, and S.E. Trauth. 2010. Metacercariae of Clinostomum (Trematoda: Digenea) from three species of Ambystoma (Caudata: Ambystomatidae) from Arkansas and Illinois, USA. Comparative Parasitology 77:25-30.
NatureServe. 2015. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. Available. [Accessed: May 19, 2015 ].
Spight, T. 1967. The water economy of salamanders: exchange of water with the soil. Biol. Bull. 132: 126-132.
Trauth, S. E. 2005. In Ambystoma texanum (Matthes, 1855) Small-mouthed Salamander. Lannoo, M., editor. Amphibian Declines: The Conservation Status of United States Species. 634-636. Berkeley, California, University of California Press.
Yap, T.A., M.S. Koo, R.F. Ambrose, D.B. Wake, and V.T. Vredenburg. 2015. Averting a North American biodiversity crisis. Science 349(6247):481-482.
| S-rank | State/Province |
|---|---|
| S1 (Critically Imperiled) | Ontario, Michigan, Nebraska, West Virginia |
| S3 (Vulnerable) | Alabama, Iowa |
| S4 (Apparently Secure) | Indiana |
| S5 (Secure) | Arkansas, Illinois, Kansas, Louisiana, Mississippi, Missouri, Oklahoma, Tennessee, Texas |
| SNR (Unranked) | Ohio |
Rank Definitions (NatureServe 2015)
S1: Critically Imperiled - At very high risk of extirpation in the jurisdiction (i.e., N - nation, or S -state/province) due to very restricted range, very few populations or occurrences, very steep declines, severe threats, or other factors.
S3: Vulnerable - At moderate risk of extirpation in the jurisdiction due to a fairly restricted range, relatively few populations or occurrences, recent and widespread declines, threats or other factors.
S4: Apparently Secure - At a fairly low risk of extirpation in the jurisdiction due to an extensive range and/or many populations or occurrences but with possible cause for some concern as a result of local recent declines, threats or other factors.
S5: Secure - At very low risk of extinction or elimination due to a very extensive range, abundant populations or occurrences, and little to no concern from declines or threats.
SNR/NNR: Unranked - National or subnational conservation status
Recovery Strategy for Small-mouthed Salamander (Ambystoma texanum) in Ontario

Prepared by the Stewart Hamill for the Ontario Ministry of Natural Resources and Forestry
British Columbia Ministry of Environment
2015
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada.
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. There was a transition period of five years (until June 30, 2013) to develop recovery strategies for those species listed as endangered or threatened in the schedules of the ESA. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources and Forestry Species at Risk.
Hamill, Stewart E. 2015. Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. vi + 18 pp.
© Scott Gillingwater
Additional copies can be downloaded from the B.C. Ministry of Environment Recovery Planning webpage
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Stewart E. Hamill - Wildlife Biologist, Merrickville
My contacts at the Ontario Ministry of Natural Resources and Forestry (OMNRF) office in Peterborough, Megan McAndrew and Amelia Argue, Species at Risk Biologists, assisted with guidance and information for this project. I would also like to thank those who reviewed and commented on the drafts. The individuals listed below provided details on the species, the locations where it occurs and the habitat, and on threats.
Karine Bériault
Species at Risk Biologist, OMNRF
Vineland, ON
James Bogart
Professor Emeritus, University of Guelph
Guelph, ON
Joe Crowley
Herpetology Species at Risk Specialist, OMNRF
Peterborough, ON
Ron Gould
Zone Ecologist, OMNRF
Aylmer, ON
David Green
Director, Redpath Museum, McGill University
Montreal, QC
Michael Oldham
Botanist/Herpetologist
Natural Heritage Information Centre, OMNRF
Peterborough, ON
John Urquhart
Conservation Science Manager, Ontario Nature
Toronto, ON
Allen Woodliffe
District Ecologist (retired), OMNRF
Chatham, ON
The recovery strategy for the Small-mouthed Salamander was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Ontario Ministry of Natural Resources and Forestry
Environment Canada - Canadian Wildlife Service, Ontario
The Small-mouthed Salamander is a medium to large salamander classified as endangered on the Species at Risk in Ontario List. It spends most of its adult life in underground burrows or under leaf litter, or living under cover objects such as logs and rocks. In early spring the adults make an annual nocturnal overland migration to breeding wetlands for mating and egg-laying. The larvae remain in water until emergence as adults in mid-summer.
Habitat requirements include an integrated complex with:
- a shallow fish-free wetland which can retain water until mid-summer;
- surrounding habitat which provides cover for migration and adult life;
- shaded soft moist soils for burrowing; and
- habitat connections which permit dispersal and longer migrations of up to one km.
The Small-mouthed Salamander reaches the northern limit of its range in Michigan, Ohio and on Pelee Island in Ontario. On Pelee Island it is known from only three wetlands, two of which are protected. The third site is on private land. Due to the relative isolation of the island, monitoring and surveillance programs are not frequent, no comprehensive population census data are available and threat assessment is limited. Nevertheless, the abilities of the species to withstand temporary droughts and avoid predators mean that this salamander could continue to thrive in Ontario if its habitat is maintained.
Threats to the Small-mouthed Salamander in Ontario include:
- habitat alteration, loss, and fragmentation;
- invasive and introduced species, such as the European Common Reed (Phragmites australis ssp. australis), the Emerald Ash Borer (Agrilus planipennis) and fish introduced to breeding ponds;
- climate change, which could bring warmer, drier conditions with prolonged drought;
- pollution, which is a particular threat to salamanders due to their sensitivity;
- predation, particularly by the recently-introduced Wild Turkey (Meleagris gallopavo) which could be an effective salamander predator;
- mortality on roads from vehicles; and
- competition and hybridization, although the species appears to be capable of managing this threat.
The recovery goal is to ensure that threats to populations and habitat are sufficiently managed to allow for long-term persistence and expansion of the Small-mouthed Salamander population within its Ontario range on Pelee Island. The strategy describes protection and recovery objectives for this species in Ontario, including to:
- protect and maintain the quality and quantity of habitat on Pelee Island where the Small-mouthed Salamander occurs;
- implement a monitoring program for salamander populations, habitats, and threats on Pelee Island including surveys of suitable habitat;
- promote and carry out research on Small-mouthed Salamander genetics, populations and threats;
- investigate existing, former, and potential Small-mouthed Salamander habitats on Pelee Island to determine if restoration, re-introduction or population interventions would be appropriate; and
- implement education, stewardship and communication programs for private landowners, residents and visitors on Pelee Island.
This recovery strategy also recommends that a habitat regulation be developed which includes:
- all wetland habitats where the Small-mouthed Salamander is known to breed;
- any new locations found or any locations where the salamander is re-introduced;
- all suitable terrestrial areas and features that extend radially 300 m from the edge of any breeding wetland; and
- corridors that provide contiguous connections between breeding locations extending up to a maximum of one kilometre.
The glossary provides definitions for technical terms, including the abbreviations above.
Species Description
The Small-mouthed Salamander (Ambystoma texanum) is a typical member of the Mole Salamander family (Ambystomatidae), being a medium to large salamander (maximum length 18 cm) with prominent costal grooves, robust limbs and body and a broad head (Harding 1997). The head in this species, however, is noticeably smaller than that of other mole salamanders and the snout is short and blunt. The back is black or very dark brown and the belly is black with a few light spots. The flanks and the long tail are covered with light grey-blue flecking (Petranka 1998). The adult Small-mouthed Salamander resembles the sympatric Blue-spotted Salamander (Ambystoma laterale) adult although the latter species usually has blue spots rather than grey flecks on the body. The adult Small-mouthed Salamander also has a proportionally smaller head (MacCulloch 2002).
Larvae are much smaller than adults and have external gills and a large fin on the tail. Identification of larvae is difficult because adult colouring develops only when juveniles leave the water as adults (MacCulloch 2002).
Species Biology
The common family name "mole" salamander refers to the habit of usually staying underground or beneath cover objects except when breeding. In early spring (late March or early April) the adults migrate overland at night to shallow fish-free bodies of water for mating and egg-laying. Fertilization is internal. The female lays 200 to 300 eggs individually or in small groups, on dead leaves and twigs on the bottom of the breeding pond. The eggs hatch after 9 or 10 days to aquatic gilled larvae that transform three months later, by mid-summer (June or July), to terrestrial adults. Adult salamanders reach breeding age two years after metamorphosis (Government of Canada 2012, Harding 1997). After breeding activities, the adults return to their underground or under cover haunts where they also spend the winter in hibernation.
The only location where the Small-mouthed Salamander is found in Canada is Pelee Island, in Ontario. This habitat is shared with another Ambystoma species, the Blue-spotted Salamander, and with a group of unisexual female polyploid Ambystoma salamanders (King et al. 1997) which are more common than the pure Small-mouthed Salamander (Bogart and Licht 2004). These polyploids have a unique mode of reproduction involving heterosexual mating and asexual development of the egg, but with incorporation of the genome from the sperm which increases the chromosome complement to diploid (double), triploid (triple), or even tetraploid (quadruple) (Bogart and Licht 2004). Molecular studies (Bogart et al. 1985, Bogart and Licht 1986, Bogart et al. 1987, Bi and Bogart 2010) suggest that the original unisexual polyploids on Pelee Island were not produced through crosses involving the two species there (A. laterale and A. texanum). Apparently, they were isolated on the island at the same time as the two pure species and now exchange nuclear genomes with those two species. Small-mouthed Salamander DNA can make up anywhere from a minority to a majority of the unisexual polyploid genome, and unisexual salamanders can thus resemble the Small-mouthed Salamander. The polyploids on Pelee Island are all females and each must mate with a male Small-mouthed or Blue-spotted Salamander for reproductive success (Hedges et al. 1992). These Pelee Island polyploid hybrids are more correctly called 'unisexuals' as they are not true hybrids.
Unisexual salamander larvae are larger than Blue-spotted and Small-mouthed Salamander larvae (Wilbur 1972). These larger unisexual larvae have been observed to attack and bite the smaller larvae of both of those species in artificial ponds (Brodman and Krouse 2007). The result was reduced survival and growth of Small-mouthed Salamander larvae in the presence of unisexual larvae. However this effect was less than the effect of competition with other Small-mouthed Salamander larvae. When raised with unisexuals, Small-mouthed Salamander larvae spent more time concealed in vegetation and were able to minimize the effects of competition and predation (Brodman and Krouse 2007).
Adult Small-mouthed Salamanders feed on insects, slugs and earthworms, while larvae eat a variety of small aquatic invertebrates. The larvae are eaten by crayfish, predaceous aquatic insects, birds and snakes, while adults are consumed by snakes and other vertebrate predators (Harding 1997).
Small-mouthed Salamander is primarily a central southern United States species, ranging from Texas, Louisiana, Mississippi and western Alabama north to extreme southeastern Michigan, northern Ohio (including islands in Lake Erie) and Pelee Island in Ontario (Bogart and Licht 2004).
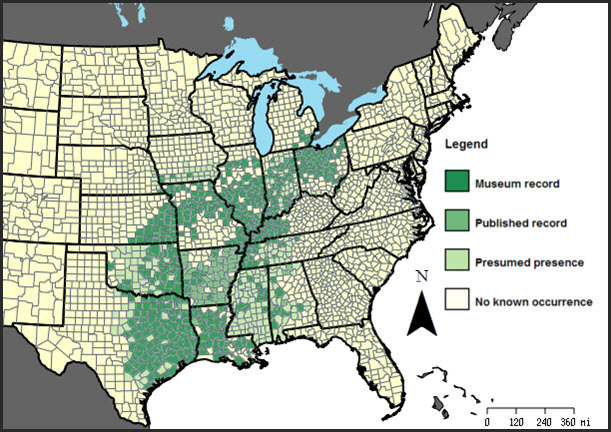
Long description for Figure 1
Figure 1 the historical range of the species on a map of central and eastern United States. The main extent of its museum record, published record and presumed presence covers a large portion of the Mississippi basin: from eastern Texas to southern Iowa and from central Alabama to northern Ohio. Approximately 75% of this coverage is museum record, 10% is published record and 15% is presumed presence.
The abundance and status of the species range from widespread, common and secure in southern portions of the range, to rare and endangered at its northern limit in Michigan and Ontario (Harding 1997). The only location for the species in Canada and Ontario is Pelee Island in Lake Erie.
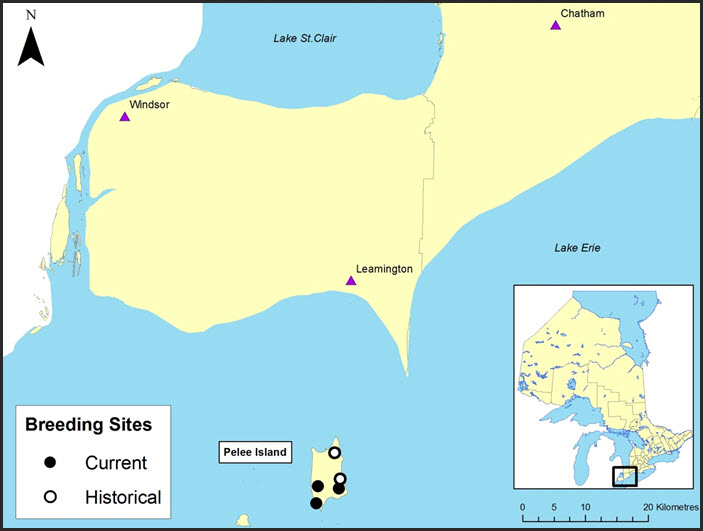
Long description for Figure 2
Figure 2 shows the southern-most tip of Ontario, where historical and current breeding sites for the species are found. All five of these sites are found on Pelee Island. Two of these are historical sites, one is on the north-eastern tip of the island, and the other is in the center of the eastern coast. The three current sites are on the southern portion of the island; two on the western coast and one on the eastern coast.
In 1991 the Small-mouthed Salamander occupied five breeding sites on Pelee Island (Bogart and Licht 1991). By the year 2000 two of these had been eliminated by development activities and the permanent loss of water (Bogart and Licht 2004). Three breeding locations with surrounding woodland habitat remain:
- a flooded woodlot within the provincial Fish Point Nature Reserve;
- a flooded woodlot on a nature reserve jointly owned by Ontario Nature, Essex Region Conservation Authority and the Nature Conservancy of Canada; and
- a pond on private land.
It is impossible to determine the density of "pure" populations of Small-mouthed Salamander or to assess trends due to the difficulties associated with identification in the field. Collections by J. Bogart and L. Licht on Pelee Island in 2000 indicate that unisexuals made up 78 percent of the population, but there are currently no population estimates (Government of Canada 2012).
In Ontario, the Small-mouthed Salamander is found in several types of moist habitats, including tall-grass prairies (Ecological Land Classification (ELC) unit TP), dense hardwood forests (ELC unit FOD) and agricultural lands (ELC unit CU) if such areas provide suitable breeding ponds (Government of Canada 2012). These habitats must also have soils soft enough to enable adults to find burrows, such as those created by crayfish.
Shallow fish-free bodies of water are needed by the Small-mouthed Salamander for breeding activities including mating and egg-laying. Suitable water bodies are usually less than a metre in depth and contain woody debris, submerged grasses and reeds, and emergent vegetation. These water bodies must retain water throughout the larval stage which normally lasts from March through July (Bogart and Licht 2004). At times other than the spring breeding season the adults generally remain hidden underground in soft moist (shaded) soils or beneath rotting logs, rocks or leaf litter (Harding 1997). Like other mole salamanders, adult Small-mouthed Salamanders retreat below the frost line into deep rock fissures and rodent burrows (Jefferson Salamander Recovery Team 2009) during the winter.
Radio-tracking studies and literature searches have documented that the migratory distance of adults of various pond-breeding salamander species can range from hundreds of metres up to one km from the breeding pond into surrounding habitat (Semlitsch 1998, Faccio 2003, Bériault 2005). Based on those studies (none of which included the Small-mouthed Salamander) the adult Small-mouthed Salamander is presumed to travel only this limited distance (up to one km, but usually not more than 300 m) from the breeding site. This is calculated as the habitat area utilized by 90 percent of the adult population for each breeding location based on the movements of tracked individuals. The combination of water connected to suitable terrestrial habitat is therefore essential. Adult Small-mouthed Salamanders do cross roads (Bogart and Licht 2004) and the road itself would therefore probably not be a habitat barrier although heavy traffic could be.
These habitat needs are similar to those of other mole salamanders (Spotted Salamander (Ambystoma maculatum), Blue-spotted Salamander, Jefferson Salamander, and Eastern Tiger Salamander (Ambystoma tigrinum)). The Blue-spotted Salamander is more likely to be found above ground during the warmer months and the Eastern Tiger Salamander is less dependent on forested habitats than most other Ambystoma (Harding 1997, Jefferson Salamander Recovery Team 2009).
Within its current range, the Small-mouthed Salamander is limited by the availability of shallow water bodies which hold water until mid-summer, the absence of fish (which eat all life stages of salamanders) in those water bodies and the presence of soft moist shaded soils with burrows. Given the current fragmentation of wooded and wetland habitat on Pelee Island and the salamander's dispersal abilities, in Ontario the species is restricted to a few sites on the island. However it may occur in more locations there than where it has been found to date (R. Gould, pers. comm. 2013).
Climate change bringing warmer temperatures and drier conditions with less snow and less water in vernal pools could further shrink the number of suitable locations and the area of suitable habitat on Pelee Island.
Threats to the Small-mouthed Salamander in Ontario are listed below.
Habitat Alteration, Loss and Fragmentation
Because of the very limited distribution of the Small-mouthed Salamander in Ontario, any change in habitat (pollution, drainage, permanent drier conditions or development activities) could have serious impacts on the Ontario population. Two of the five breeding sites on Pelee Island have already been lost. One of these was drained, cleared and filled for development (A. Woodliffe, pers. comm. 2013), which follows the general history of land use on the island: clearing and draining for agriculture and development (J. Crowley, pers. comm. 2013). Two of the remaining three breeding sites are on protected lands but the third is privately-owned. However, drainage of both private and public lands on the island is an ongoing concern for island residents as most of the island is below the average lake level. Continued drainage without sufficient consideration for natural processes could threaten the scattered wetland areas (A. Woodliffe, pers. comm. 2013).
Removal of tree cover and rotting logs is a particular threat. Trees keep the ground moist by providing shade and maintain water levels in breeding areas by retarding evaporation. Rotting logs provide habitat for adult salamanders and their invertebrate prey. Such removals not only eliminate habitat but also fragment remaining natural areas, making it more difficult for adult salamanders to travel. Loss of tree canopy has been shown to stop egg deposition in a mole salamander species (Felix et al. 2010) and to cause adult Ambystoma salamanders to move out of the area (Semlitsch et al. 2008).
Invasive and Introduced Species
The ongoing encroachment of European Common Reed (Phragmites australis ssp. australis) into wetlands and riparian areas on Pelee Island could degrade wetland habitat and reduce the availability of suitable egg placement sites (R. Gould, pers. comm. 2013). Although the specific impacts on salamanders are unknown, an analysis by Greenberg and Green (2013) has shown that population decline in Fowler's Toad (Anaxyrus fowleri) populations is associated with the spread of the European Common Reed. Uncontrolled growth of dense stands of Common Reed stems can effectively eliminate shallow, sparsely vegetated, aquatic areas which are needed by both Fowler's Toad and Small-mouthed Salamander.
The loss of shade due to the death of ash trees caused by the Emerald Ash Borer (Agrilus planipennis), an invasive, non-native species, may change wetland conditions, making them less suitable for salamanders (A. Woodliffe, pers. comm. 2013).
An incidental human threat is the introduction of carnivorous fish, which are predators on all life stages of salamanders, to breeding ponds, which could eliminate salamander populations.
Climate Change
If the Small-mouthed Salamander were unable to breed for a few years due to temporary low water levels, it is unlikely that significant population declines would result. However, if climatic changes (such as higher temperatures, less snow, lowered water table, less water in vernal pools) eliminate a breeding site, the population in that area would disappear. Bogart and Licht (2004) noted in their 2000 visit to Pelee Island that water levels had lowered at one of the breeding wetlands and that this could be related to a lower water level in Lake Erie. This could, however, have positive effects by reducing the possibilities of flooding and the invasion of fish from Lake Erie (J. Bogart, pers. comm. 2013).
Pollution
Salamanders have been shown to be particularly sensitive to various pollutants, which can kill outright but also induce sublethal affects in embryos, larvae and adults. Given the agricultural character of Pelee Island, agricultural pesticides are a particular threat as they can reduce survival and metamorphosis of Ambystoma larvae by killing zooplankton thereby reducing food resources (Metts et al. 2005). De-icing salt runoff from Pelee Island roads is another pollutant threat as experimental concentrations have been shown to cause mass loss in Ambystoma egg clutches (Karraker and Gibbs 2011). In addition to pollution and sometimes working synergistically with pollutants, increased ultraviolet radiation caused by reduction of ozone in the stratosphere is another widespread threat to amphibians in general leading to population declines (Blaustein et al 2003).
Predation
The Small-mouthed Salamander has evolved several strategies to avoid predation: adults live under cover or underground most of the year; above-ground movements during the breeding season are nocturnal; and larvae are aquatic and have hiding and avoidance strategies. These characteristics help to protect them from predators, including humans. However, a new potential predator with unknown impacts is now present on Pelee Island: the Wild Turkey (Meleagris gallopavo), introduced in 2002 (R. Gould, pers. comm. 2013). Wild Turkeys scratch to find food and in so doing, can disturb cover habitat for adult salamanders while eating any salamanders uncovered.
Road Mortality
Small-mouthed Salamanders travel overland every spring during the breeding season and every summer after transformation. On Pelee Island, as elsewhere, this means that they sometimes cross roads. Due to the low traffic levels on the island, it is thought that this is not currently a large threat to the species (R. Gould, pers. comm. 2013). However, the potential remains for mass mortality caused by traffic and subsequent population decline. Gibbs and Shriver (2005) showed that even a small annual road mortality risk can lead to local population extirpation of a mole salamander species. Beebee (2013) concluded from a literature review that the long-term effects of road mortality on populations of amphibians are often severe.
Competition and Hybridization
The threat of competition from unisexuals and the Blue-spotted Salamander was discussed by Bogart and Licht (2004) and examined by Brodman and Krouse (2007). It was concluded that competition is probably not a concern to the continued existence of the Small-mouthed Salamander. Although there is insufficient evidence to rule definitively on this, it is known that unisexuals require a pure male of either species to reproduce. Other details considered as part of this analysis include the following:
- the Small-mouthed Salamander is larger than the Blue-spotted Salamander;
- the Small-mouthed Salamander can produce more eggs than the Blue-spotted Salamander;
- the Small-mouthed Salamander can occupy a wider diversity of habitats than the Blue-spotted Salamander; and
- Small-mouthed Salamander larvae have strategies to avoid the larger unisexual larvae.
The isolated location of the Small-mouthed Salamander on Pelee Island has protected the species from human disturbance but has also limited visitation by naturalists and biologists. There are no monitoring programs on the island for water levels, habitat or salamander numbers, and no frequent enforcement or surveillance presence in the nature reserves. Without regular observations, identification and assessment of threats has been limited. In particular there has been little or no work done on the impact of introducing Wild Turkey to the island (R. Gould, pers. comm. 2013), the effects of European Common Reed on salamander habitat, the amount of road mortality on Pelee Island, or the potential future impacts of climate change on Pelee Island wetlands and salamander habitat.
Little scientific work has been done recently on the Small-mouthed Salamander in general and none in Canada. The density of "pure" populations of Small-mouthed Salamander is unknown and it has not been possible to assess trends in hybridization and polyploidy due to the difficulties associated with identification in the field (Bogart and Licht 2004). There are no size estimates for the Small-mouthed Salamander population. This information would be needed before any population restoration or re-introduction could be considered (J. Bogart, pers. comm. 2013).
Various organizations have undertaken the acquisition and protection of land on Pelee Island in order to maintain species and habitat; Ontario Parks, Essex Region Conservation Authority, Ontario Nature and the Nature Conservancy of Canada are included. Each provides some level of infrequent visitation and surveillance. Ontario Nature has a local steward group that checks on the Stone Road site annually (J. Urquhart, pers. comm. 2013). No active management or regular monitoring programs are carried out on these nature reserves (Fish Point, Stone Road).
The Province of Ontario provides species and habitat protection for the Small-mouthed Salamander under the Endangered Species Act, 2007 . Activities that may impact the species or its habitat are subject to provisions of the act and applicable regulations.
The recovery goal is to ensure that threats to populations and habitat are sufficiently managed to allow for long-term persistence and expansion of the Small-mouthed Salamander population within its Ontario range on Pelee Island.
| No. | Protection or Recovery Objective |
|---|---|
| 1 | Protect and maintain the quality and quantity of habitat on Pelee Island where the Small-mouthed Salamander occurs. |
| 2 | Implement a monitoring program for salamander populations, habitats and threats on Pelee Island including surveys of suitable habitat. |
| 3 | Promote and carry out research on Small-mouthed Salamander genetics, populations and threats. |
| 4 | Investigate existing, former and potential Small-mouthed Salamander habitats on Pelee Island to determine if restoration, re-introduction or population interventions would be appropriate. |
| 5 | Implement education, stewardship and communication programs for private landowners, residents and visitors on Pelee Island. |
Table 2. Approaches to recovery of the Small-mouthed Salamander in Ontario
| Relative Priority |
Relative Timeframe |
Recovery Theme |
Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Ongoing | Protection | 1.1 Develop a habitat regulation or habitat description to define the area protected as habitat for Small-mouthed Salamander in Ontario. |
|
| Necessary | Ongoing | Protection, Management | 1.2 Engage landowners, residents and visitors to develop habitat management and protection programs, which might include:
|
|
| Relative Priority |
Relative Timeframe |
Recovery Theme |
Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Necessary | Ongoing | Inventory, Monitoring and Assessment | 2.1 Carry out regular inventory, monitoring, surveying and sampling activities for populations, reproduction, water levels, habitat quality, new locations and threats including road mortality, climate change, and European Common Reed invasion. |
|
| Beneficial | Short-term | Inventory, Monitoring and Assessment | 2.2 Survey suitable habitats to find unknown populations and locations which could be used in population interventions. |
|
| Relative Priority |
Relative Timeframe |
Recovery Theme |
Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Beneficial | Short-term | Research | 3.1 Study the impact of Wild Turkey predation on salamanders in general, and on Small-mouthed Salamander in particular. |
|
| Beneficial | Long-term | Research | 3.2 Promote research on Small-mouthed Salamander genetics, hybridization, polyploidy and population structure. |
|
| Beneficial | Long-term | Research | 3.3 Investigate the impact of climate change on Small-mouthed Salamander populations. |
|
| Relative Priority |
Relative Timeframe |
Recovery Theme |
Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Beneficial | Long-term | Management | 4.1 Carry out investigations on existing, former and potential Small-mouthed Salamander habitats on Pelee Island in order to gather information on current conditions, human activities and land uses which would be needed to develop and implement programs for restoration or re-introduction, if appropriate. |
|
| Relative Priority |
Relative Timeframe |
Recovery Theme |
Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Beneficial | Long-term | Education and Outreach, Communications, Stewardship | 5.1 Develop and implement programs for residents, landowners and visitors to deal with land management, road mortality and disturbance to salamanders and habitat. |
|
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources and Forestry on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister when developing the habitat regulation for this species.
Breeding Habitat
All permanent and seasonal wetland habitats where the Small-mouthed Salamander is known to breed should be included in a habitat regulation. This would include vernal pools, ponds, flooded quarries, swamps and marshes, which at present totals only three locations. Any new locations found or any locations where the salamander is re-introduced should be added to the area regulated.
Terrestrial Habitat
The terrestrial component of Small-mouthed Salamander habitat consists of woodlands, upland forests, swamps, successional areas, meadows, old fields, agricultural fields and other vegetated areas that provide conditions required for foraging, seasonal migration, growth and hibernation. Terrestrial habitat includes all of the areas and features described above that extend radially 300 m from the edge of any breeding wetland. The 300 m distance is based on the findings of telemetry studies of other similar species (K. Bériault, pers. comm. 2013, as reported in Semlitsch 1998, Faccio 2003, Bériault 2005) and is calculated as the habitat area utilized by 90 percent of the adult population for each breeding location based on the movements of individuals tracked for three months. Terrestrial habitat that meets these requirements should be included within the habitat regulation, similar to that recommended for the Jefferson Salamander (Jefferson Salamander Recovery Team 2009).
Connecting corridors that provide continuous extensions of the terrestrial habitats listed above, between the edge of one wetland breeding location and another, can extend up to one kilometre, the maximum presumed travel distance of an adult Small-mouthed Salamander (based on studies of other similar salamander species, such as Bériault 2005). These contiguous corridors should also be included within the habitat regulation. Non-vegetated open areas such as cultivated fields should also be included if part of a dispersal corridor, as these areas are not known to be a barrier to Small-mouthed Salamander dispersal.
Beebee, T.J.C. 2013. Effects of road mortality and mitigation measures on amphibian populations. Conservation Biology 27(4):657-668.
Bériault, Karine, pers. comm. 2013. Telephone conversation with S. Hamill. April
2013. Species at Risk Biologist, OMNR, Vineland, ON.
Bériault, K.R.D. 2005. Critical Habitat of Jefferson Salamanders in Ontario: An Examination through Radiotelemetry and Ecological Surveys. M.Sc. Thesis, University of Guelph, 69 pp.
Bi, K. and J.P. Bogart. 2010. Time and time again: unisexual salamanders (genus Ambystoma) are the oldest unisexual vertebrates. BMC Evolutionary Biology 10:238.
Blaustein, A.R., J.M. Romansic, J.M. Kiesecker, and A.C. Hatch. 2003. Ultraviolet radiation, toxic chemicals, and amphibian declines. Diversity and Distributions 9: 123-140.
Bogart, James, pers. comm. 2013. Telephone conversation with S. Hamill. April 2013. Professor Emeritus, University of Guelph, Guelph, ON.
Bogart, J.P. and L.E. Licht. 1986. Reproduction and the origin of polyploids in hybrid salamanders of the genus Ambystoma. Canadian Journal of Genetics and Cytology 28: 605-617.
Bogart, J.P. and L.E. Licht. 1991. Status Report on the Small-mouthed Salamander Ambystoma texanum in Canada. Report for the Committee on the Status of Endangered Wildlife in Canada (COSEWIC). 19 pp.
Bogart, J.P. and L.E. Licht. 2004. Update COSEWIC Status Report on the Small-mouthed Salamander Ambystoma texanum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa.
Bogart, J.P., L.E. Licht, M.J. Oldham, and S.J. Darbyshire. 1985. Electrophoretic identification of Ambystoma laterale and Ambystoma texanum as well as their diploid and triploid interspecific hybrids (Amphibia: Caudata) on Pelee Island, Ontario. Canadian Journal of Zoology 63: 340-347.
Bogart, J.P., L.A. Lowcock, C.W. Zeyl, and B.K. Mable. 1987. Genome constitution and reproductive biology of the Ambystoma hybrid salamanders on Kelleys Island in Lake Erie. Canadian Journal of Zoology 65: 2188-2201.
Brodman, R. and H.D. Krouse. 2007. How Blue-spotted and Small-mouthed Salamander larvae coexist with their unisexual counterparts. Herpetologica 63 (2):135-143.
Crowley, Joe, pers. comm. 2013. E-mail correspondence with S. Hamill. August 2013 Herpetology Species at Risk Specialist, OMNR, Peterborough, ON.
Faccio, S.D. 2003. Post breeding emigration and habitat use by Jefferson and Spotted salamanders in Vermont. Journal of Herpetology 37:479-489.
Felix, Z.I., Y. Wang, and C.J. Schweitzer. 2010. Effects of experimental canopy manipulation on amphibian egg deposition. Journal of Wildlife Management 74(3):496-503.
Gibbs, J.P. and W.G. Shriver. 2005. Can road mortality limit populations of pool breeding amphibians? Wetlands Ecology and Management 13:281-289.
Gould, Ron, pers. comm. 2013. Telephone conversation and e-mail correspondence with S. Hamill. April and August 2013. Zone Ecologist, OMNR, Aylmer, ON.
Government of Canada. 2012. Species Profile (Small-mouthed Salamander). Species at Risk Public Registry. [accessed April 2013].
Greenberg, D.A. and D.M. Green. 2013. Effects of an invasive plant on population dynamics in toads. Conservation Biology 27(5):1049-1057.
Harding, J.H. 1997. Amphibians and Reptiles of the Great Lakes Region. University of Michigan Press, Ann Arbor, 378 pp.
Hedges, S.B., J.P. Bogart, and L.M. Maxson. 1992. Ancestry of unisexual salamanders. Nature 356:708-710.
Jefferson Salamander Recovery Team. 2009. Draft Recovery Strategy for the Jefferson Salamander (Ambystoma jeffersonianum) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 27pp.
Karraker, N.E. and J.P. Gibbs. 2011. Road deicing salt irreversibly disrupts osmoregulation of salamander egg clutches. Environmental Pollution 159:833-835.
King, R.B., M.J. Oldham, and W.F. Weller. 1997. Historic and current amphibian and reptile distributions in the island region of western Lake Erie. American Midland Naturalist 138:153-173.
MacCulloch, R.D. 2002. The ROM Field Guide to Amphibians and Reptiles of Ontario. Royal Ontario Museum, Toronto, ON.
Metts, B.S., W.A. Hopkins, and J.P. Nestor. 2005. Interaction of an insecticide with larval density in pond-breeding salamanders (Ambystoma). Freshwater Biology 50:685-696.
Petranka, J. 1998. Salamanders of the United States and Canada. Smithsonian Institution Press, Washington and London, 587 pp.
Semlitsch, R.D. 1998. Biological delineation of terrestrial buffer zones for pond-breeding salamanders. Conservation Biology 12(5):1113-1119.
Semlitsch, R.D., C.A. Conner, D.J. Hocking, T.A.G. Rittenhouse, and E.B. Harper. 2008. Effects of timber harvesting on pond-breeding amphibian persistence: testing the evacuation hypothesis. Ecological Applications 18(2):283-289.
Urquhart, John, pers. comm. 2013. Telephone conversation with S. Hamill. April 2013. Conservation Science Manager, Ontario Nature, Toronto, ON.
USGS National Amphibian Atlas. 2012. Small-Mouthed Salamander (Ambystoma texanum). Version 2.2 USGS Patuxent Wildlife Research Center, Laurel, Maryland. .
Wilbur, H.M. 1972. Competition, predation, and the structure of the Ambystoma – Rana sylvatica community. Ecology 53:3-21.
Woodliffe, P.A. 2013. E-mail correspondence with S. Hamill. August 2013. District Ecologist (retired), OMNR.