Recovery Strategy for White Sturgeon (Acipenser transmontanus) in Canada [Proposed]-2013
- Introduction
- Description of Needs of the Species / Threats / Habitat Trends / Knowledge Gaps
- Recovery / Critical Habitat
- Upper Fraser River Population
- Nechako River Population
- Upper Columbia River Population
- Kootenay River Population / Activities Likely to Result in the Destruction of Critical Habitat / Schedule of Studies to Identify Critical Habitat
- Basin Overview for each Recovery Population / Implementation
- Activities Permitted by the Recovery Strategy / References Cited
- Appendix A
- Appendix B
- Appendices C – E
- Preface
- Responsible jurisdictions
- Contributors / Authors
- Acknowledgements
- Strategic environmental assessment statement
- Residence
- Executive summary
- 1. Species information
- 2. Description of the Species
- 3. Description of Needs of the Species
- 4. Threats
- 5. Habitat Trends
- 6. Knowledge Gaps
- 7. Recovery
- 8. Critical Habitat
- 8.1 Information and Methods used to Identify Critical Habitat
- 8.2 Identification of Critical Habitat
- 8.3 Upper Fraser River Population
- 8.4 Nechako River Population
- 8.5 Upper Columbia River Population
- 8.6 Kootenay River Population
- 8.7 Activities Likely to Result in the Destruction of Critical Habitat
- 8.8 Schedule of Studies to Identify Critical Habitat
- 9. Basin Overview for Each Recovery Population
- 10. Implementation
- 11. Activities Permitted by the Recovery Strategy
- 12. References Cited
- Appendix A Studies to Address Identified Knowledge Gaps for White Sturgeon
- Appendix B Recovery of Middle and Lower Fraser River White Sturgeon Populations and Important Habitats
- Appendix C Acronyms used in the Recovery Strategy
- Appendix D Glossary of Terms
- Appendix E Record of Consultation and Cooperation
- Figure 1. The white sturgeon, Acipenser transmontanus.
- Figure 2. Map of the Fraser River basin depicting the approximate ranges for each of the four white sturgeon populations in the Fraser River watershed. The species is principally found in the mainstem habitats of the Fraser and Nechako rivers, although they also make extensive use of tributaries and large lakes (such as in the Harrison or Stuart watersheds). Anecdotal records indicate that sturgeon were present in several watersheds beyond the described boundaries.
- Figure 3. Map of the Columbia and Kootenay basins depicting the approximate ranges for two of the white sturgeon populations in British Columbia. Records indicate that sturgeon historically occurred beyond the described boundaries, but at low abundance. Small remnant populations occur upstream of Duncan Dam and in Slocan Lake (see text for details). White sturgeon are present in the Columbia to its confluence with the Pacific Ocean, but this document addresses only sturgeon upstream of the Canada-U.S. border.
- Figure 4. Reference map for locations of Upper Fraser River white sturgeon critical habitats.
- Figure 5. Map of critical habitat for Upper Fraser River white sturgeon: Penny.
- Figure 6. Map of critical habitat for Upper Fraser River white sturgeon: Longworth Grand Canyon.
- Figure 7. Map of critical habitat for Upper Fraser River white sturgeon: Bowron River confluence with the Fraser River.
- Figure 8. Map of critical habitat for Upper Fraser River white sturgeon: McGregor River confluence with the Fraser River to Limestone Creek confluence.
- Figure 9. Map of critical habitat for Upper Fraser River white sturgeon: Giscome at Tay Creek.
- Figure 10. Map of critical habitat for Upper Fraser River white sturgeon: Willow River confluence with the Fraser River.
- Figure 11. Map of critical habitat for Upper Fraser River white sturgeon: Salmon River confluence with the Fraser River.
- Figure 12. Map of critical habitat for Upper Fraser River white sturgeon: Nechako River confluence with the Fraser River.
- Figure 13. Map of critical habitat for Upper Fraser River white sturgeon: Red Rock.
- Figure 14. Reference map for locations of Nechako River white sturgeon critical habitats.
- Figure 15. Map of critical habitat for Nechako River white sturgeon: Vanderhoof braided section of the Nechako River.
- Figure 16. Map of critical habitat for Nechako white sturgeon: Sinkut River confluence with the Nechako River.
- Figure 17. Map of critical habitat for Nechako River white sturgeon: Leduc Creek confluence with the Nechako River.
- Figure 18. Map of critical habitat for Nechako River white sturgeon: Finmoore.
- Figure 19. Map of critical habitat for Nechako River white sturgeon: Keilor’s Point.
- Figure 20. Map of critical habitat for Nechako River white sturgeon: Culvert Hole.
- Figure 21. Map of critical habitat for Nechako River white sturgeon: Powerline.
- Figure 22. Map of critical habitat for Nechako River white sturgeon: Sturgeon Point.
- Figure 23. Map of critical habitat for Nechako River white sturgeon: Pinchi Bay on Stuart Lake.
- Figure 24. Map of critical habitat for Nechako River white sturgeon: Tachie River confluence with Stuart Lake.
- Figure 25. Map of critical habitat for Nechako River white sturgeon: Lower Half of Stuart Lake.
- Figure 26. Map of critical habitat for Nechako River white sturgeon: Middle River confluence with Trembleur Lake.
- Figure 27. Map of critical habitat for Nechako River white sturgeon: Fraser Lake and Nautley River confluence with the Nechako River.
- Figure 28. Reference map for locations of Upper Columbia River white sturgeon critical habitats.
- Figure 29. Map of critical habitat for Upper Columbia River white sturgeon: Columbia River adjacent to Revelstoke Golf Course, Big Eddy and Salmon Rocks.
- Figure 30. Map of critical habitat for Upper Columbia River white sturgeon: Beaton Reach.
- Figure 31. Map of critical habitat for Upper Columbia River white sturgeon: Narrow Burton Reach.
- Figure 32. Map of critical habitat for Upper Columbia River white sturgeon: Robson Reach.
- Figure 33. Map of critical habitat for Upper Columbia River white sturgeon: Kootenay Eddy, Bridge Hole and Brilliant Tailrace.
- Figure 34. Map of critical habitat for Upper Columbia River white sturgeon: Fort Shepherd Eddy.
- Figure 35. Map of critical habitat for Upper Columbia River white sturgeon: Waneta Eddy and Pend d’Oreille confluence with the Columbia River.
- Figure 36. Reference map for locations of Kootenay River white sturgeon critical habitats.
- Figure 37. Map of critical habitat for Kootenay River white sturgeon: Kootenay River Delta and Lower Kootenay River.
- Figure 38. Map of critical habitat for Kootenay River white sturgeon: Duncan Delta on Kootenay Lake.
- Figure 39. Map of critical habitat for Kootenay River white sturgeon: Crawford Creek Delta on Kootenay Lake.
- Table 1. Abundance estimates for wild white sturgeon in Canada. Estimates are for fish > 40 cm, unless noted otherwise in footnotes.
- Table 2. Abundance estimates for mature (greater than 160 cm) white sturgeon in Canada. Estimates for the Nechako River, Kootenay River, and Columbia River populations have been updated to 2012 using the best available estimates of annual survival rates. Lower, middle and upper Fraser River populations are thought to be relatively stable, so the most recent census data are provided for these populations.
- Table 3. Definition for levels of relative risk to the viability for the white sturgeon population and its recovery.
- Table 4. Summary of historic and ongoing threats to white sturgeon and their habitats. Impacts are listed by type, and the affected populations are indicated. To some extent these threats include natural limitations such as habitat or productivity.
- Table 5. Recommended activities and actions to meet population and distribution objectives for SARA-listed populations.
- Table 6. Summary of information base for white sturgeon critical habitats in the Upper Fraser River. A blank cell means that the life stage does not consistently use the habitat.
- Table 7. Summary of the biophysical functions, features, attributes and locations of critical habitat for Upper Fraser white sturgeon.
- Table 8. Geographic Coordinates of Critical Habitat Areas for Upper Fraser River white sturgeon.
- Table 9. Summary of information base for white sturgeon critical habitats in the Nechako system.
- Table 10. Summary of the biophysical functions, features, attributes and locations of critical habitat for Nechako white sturgeon.
- Table 11. Geographic Coordinates of Critical Habitat Areas for Nechako River white sturgeon.
- Table 12. Summary of information base for white sturgeon critical habitats in the ALR area of the Columbia River.
- Table 13. Summary of information base for white sturgeon critical habitats in the transboundary area of the Columbia River.
- Table 14. Summary of the biophysical functions, features, attributes and locations of critical habitat for Upper Columbia River white sturgeon in Arrow Lakes Reservoir.
- Table 15. Summary of the biophysical functions, features, attributes and locations of critical habitat for Upper Columbia River white sturgeon in the Columbia Transboundary Reach.
- Table 16. Geographic Coordinates of Critical Habitat Areas for Upper Columbia River white sturgeon.
- Table 17. Summary of information base for white sturgeon critical habitats in the Kootenay River. A blank cell means that the life stage does not consistently use the habitat.
- Table 18. Summary of the biophysical functions, features, attributes and locations of critical habitat for Kootenay River white sturgeon.
- Table 19. Geographic Coordinates of Critical Habitat Areas for Kootenay River white sturgeon.
- Table 20. Activities Likely to Destroy Critical Habitat – Upper Fraser River Population.
- Table 21. Activities Likely to Destroy Critical Habitat – Nechako River Population.
- Table 22. Activities Likely to Destroy Critical Habitat – Upper Columbia River Population.
- Table 23. Activities Likely to Destroy Critical Habitat – Kootenay River Population.
- Table 24. Schedule of Studies to Identify Critical Habitats.
- Table 25. Fish welfare risk level, mitigative actions, and associated references for risk reduction during conservation fish culture activities at the Freshwater Fisheries Society of B.C.

Recovery Strategy for White Sturgeon (Acipenser transmontanus)
in Canada [Proposed]
2013
About the Species at Risk Act Recovery Strategy Series
What is the Species at Risk Act (SARA)?
SARA is the Act developed by the federal government as a key contribution to the common national effort to protect and conserve species at risk in Canada. SARA came into force in 2003 and one of its purposes is “to provide for the recovery of wildlife species that are extirpated, endangered or threatened as a result of human activity.”
What is recovery?
In the context of species at risk conservation, recovery is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed and threats are removed or reduced to improve the likelihood of the species’ persistence in the wild. A species will be considered recovered when its long-term persistence in the wild has been secured.
What is a recovery strategy?
A recovery strategy is a planning document that identifies what needs to be done to arrest or reverse the decline of a species. It sets goals and objectives and identifies the main areas of activities to be undertaken. Detailed planning is done at the action plan stage.
Recovery strategy development is a commitment of all provinces and territories and of three federal agencies -- Environment Canada, Parks Canada Agency, and Fisheries and Oceans Canada -- under the Accord for the Protection of Species at Risk. Sections 37–46 of SARA outline both the required content and the process for developing recovery strategies published in this series.
Depending on the status of the species and when it was assessed, a recovery strategy has to be developed within one to two years after the species is added to the List of Wildlife Species at Risk. Three to four years is allowed for those species that were automatically listed when SARA came into force.
What’s next?
In most cases, one or more action plans will be developed to define and guide implementation of the recovery strategy. Nevertheless, directions set in the recovery strategy are sufficient to begin involving communities, land and water users, and conservationists in recovery implementation.
The series
This series presents the recovery strategies prepared or adopted by the federal government under SARA. New documents will be added regularly as species get listed and as strategies are updated.
To learn more
To learn more about the Species at Risk Act and recovery initiatives, please consult the SARA Public Registry.
Recommended citation:
Fisheries and Oceans Canada. 2013. Recovery strategy for White Sturgeon (Acipenser 79 transmontanus) in Canada [Proposed]. In Species at Risk Act Recovery Strategy Series. 80 Ottawa: Fisheries and Oceans Canada. 245 pp.
Additional copies:
You can download additional copies from the SARA Public Registry.
Cover illustration: Juvenile white sturgeon. Photo by David Gluns.
Également disponible en français sous le titre
« Programme de rétablissement de l'esturgeon blanc (Acipenser transmontanus) au Canada »
©Her Majesty the Queen in Right of Canada, represented by the Minister of Fisheries and Oceans, 2012. All rights reserved.
ISBN To come
Catalogue no. To come
Content (excluding the illustrations) may be used without permission, with appropriate credit to the source.
The white sturgeon is a freshwater fish, with six populations in Canada (Lower Fraser River, Mid Fraser River, Nechako River, Upper Fraser River, Upper Columbia River, and Kootenay River). All populations are managed by the British Columbia (B.C.) Ministry of Environment (MOE), while four SARA-listed populations are under the responsibility of the federal government. The Species at Risk Act (SARA, Section 37) requires the competent minister to prepare recovery strategies for listed Extirpated, Endangered and Threatened species. Four populations of white sturgeon (Nechako River, Upper Fraser River, Upper Columbia River, and Kootenay River) were listed as Endangered under SARA in August 2006. The development of this recovery strategy was led by Fisheries and Oceans Canada – Pacific Region and the B.C. MOE in cooperation and consultation with many individuals, organizations and government agencies, as indicated below. The strategy meets SARA requirements in terms of content and process (Sections 39-41).
Success in the recovery of these listed populations depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy, and will not be achieved by Fisheries and Oceans Canada (DFO) or any other party alone. This strategy provides advice to jurisdictions and organizations that may be involved or wish to become involved in the recovery of the species. In the spirit of the National Accord for the Protection of Species at Risk, the Minister of Fisheries and Oceans invites all responsible jurisdictions and Canadians to join DFO in supporting and implementing this strategy for the benefit of the white sturgeon and Canadian society as a whole. DFO will support implementation of this strategy to the extent possible, given available resources and its overall responsibility for species at risk conservation.
The goals, objectives and recovery approaches identified in the strategy are based on the best existing knowledge at the time the strategy was developed, and are subject to modifications resulting from new information. Studies related to white sturgeon populations are ongoing, and understanding of threats and recovery approaches is evolving. DFO will continue to be guided by new information and it is recognized that adaptations to recovery approaches and scales may be required in future. The Minister of Fisheries and Oceans will report on progress within five years.
This strategy will be complemented by one or more action plans that will provide details on specific recovery measures to be taken to support conservation of the species. The Minister of Fisheries and Oceans will take steps to ensure that, to the extent possible, Canadians interested in or affected by these measures will be consulted.
Much of this report was originally written prior to 2010, and revised in 2012 to accommodate new information. At the time of revision, the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was undertaking its review of an update status report with an aim of providing a status decision in late fall 2012. In November 2012, COSEWIC reassessed white sturgeon, and divided the species into four Designatable Units (DUs). These DUs differ in structure somewhat from the six Nationally Significant Populations previously identified in the 2003 assessment; however, this recovery strategy was prepared to provide publicly available direction for recovery of the species at a national scale and in response to the 2006 SARA listing of the Upper Columbia, Nechako, Upper Fraser, and Kootenay River populations, with the understanding that adaptations to recovery approaches and scales may be required in the future.
The responsible jurisdiction for white sturgeon under the Species at Risk Act is DFO. White sturgeon occur in British Columbia and its respective governments also contributed to the production of this recovery strategy.
Contributors/authors to/of this recovery strategy provided valuable technical and scientific advice towards the development of this product. Their involvement is greatly appreciated and should not be interpreted to signify the support by their employer for all the contents of this recovery strategy.
Note: The following are past or present members of the National Recovery Team for White Sturgeon (formerly the National Technical Coordinating Committee). Italics denote former team members.
Steve McAdam (B.C. Ministry of Environment, Chair of National Recovery Team)
Courtney Druce (Fisheries and Oceans Canada, co-chair of the National Recovery Team)
Gary Birch (B.C. Hydro – former Chair of the Upper Columbia River Technical Working Group)
Mike Bradford (Fisheries and Oceans Canada)
Tola Coopper (Fisheries and Oceans Canada, former co-chair of the National Recovery Team)
Bill Green (Canadian Columbia River Intertribal Fisheries Commission)
Todd Hatfield (Ecofish Research)
Ken Malloway (Sto:lo Tribal Council)
Troy Nelson (Fraser River Sturgeon Conservation Society)
Matt Neufeld (Ministry of Forests, Lands and Natural Resource Operations – Canadian Representative on the Kootenay Recovery Team)
Louise Porto (AMEC Earth & Environmental)
Jim Powell (Freshwater Fisheries Society of British Columbia – Canadian Chair of the Upper Columbia River Technical Working Group)
Mike Ramsay (B.C. Ministry of Forests, Lands and Natural Resource Operations – Lower and Mid Fraser Technical Working Group)
Dan Sneep (Fisheries and Oceans Canada)
Colin Spence (B.C. Ministry of Environment)
Erin Stoddard (B.C. Ministry of Forests, Lands and Natural Resource Operations – Chair of the Lower and Mid Fraser Technical Working Group)
Brian Toth (Lheidli T'enneh First Nation and the Carrier Sekani Tribal Council)
Cory Williamson (B.C. Ministry of Forests, Lands and Natural Resource Operations – Chair of the Nechako and Upper Fraser Technical Working Group)
Lee Williston (B.C. Ministry of Forests, Lands and Natural Resource Operations – Lower and Mid Fraser Technical Working Group)
Chris Wood (Fisheries and Oceans Canada)
Development of this recovery strategy was partially funded by DFO and the Living Rivers Trust Fund of British Columbia. Grateful acknowledgement is also made for the time and effort put in by many individuals involved in this province-wide process.
A strategic environmental assessment (SEA) is conducted on all SARA recovery planning documents, in accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals. The purpose of a SEA is to incorporate environmental considerations into the development of public policies, plans, and program proposals to support environmentally-sound decision making.
Recovery planning is intended to benefit species at risk and biodiversity in general. However, it is recognized that strategies may also inadvertently lead to environmental effects beyond the intended benefits. The recovery planning process based on national guidelines directly incorporates consideration of all environmental effects, with a particular focus on possible impacts on non-target species or habitats. The results of the SEA are incorporated directly in the strategy itself, but are also summarized below.
While this recovery strategy will clearly benefit the environment by promoting the recovery of white sturgeon, potential effects of the recovery strategy on other species was considered. For example, the strategy calls for protection of various habitats highly used by white sturgeon, which may require restoration. However, habitat restoration for white sturgeon may impact residing species that are suited to the existing conditions. An increase in white sturgeon populations due to conservation aquaculture may lead to additional encounters or by-catch in recreational fisheries, which in turn may require increased management attention. Further information on potential interactions with other species can be found in Section 10.1 (Potential Impacts on Other Species). Restoration activities required for the protection of white sturgeon will be based on a risk assessment of effects on other species. Taking these approaches into account, it was concluded that the benefits of this recovery strategy far outweigh any adverse effects that may result.
SARA defines residence as: “a dwelling-place, such as a den, nest or other similar area or place, that is occupied or habitually occupied by one or more individuals during all or part of their life cycles, including breeding, rearing, staging, wintering, feeding or
hibernating” [SARA S2(1)].
The existence of a Residence indicates that a species at risk has invested in a specific structure that it relies upon to carry out certain life-cycle processes. Any damage or destruction of this structure would have an impact on the fitness of individual(s) of the species. With the current understanding of residence, it is not applicable for white sturgeon at this time and “residence” has not been proposed for this species.
Residence descriptions are posted when available on the SARA Public Registry.
Within Canada, white sturgeon occur only in British Columbia and are divided into six populations, based on geography, demographics and genetics: the lower, middle and upper Fraser River; Nechako River; Upper Columbia River; and, Kootenay River. All populations were assessed as Endangered by COSEWIC in 2003; the latter four are legally listed under SARA. This document is a SARA-compliant recovery strategy for the four SARA-listed populations, and also provides recovery and management recommendations for the lower and middle Fraser River populations.
White sturgeon, Acipenser transmontanus, is the largest, longest-lived freshwater fish species in North America. The species’ most distinguishing features include a mainly cartilaginous skeleton, long scaleless body covered with rows of large bony plates (called scutes) on the back and sides, shark-like (heterocercal) tail, and four barbels between the mouth and an elongated snout. Fish of over 6 m in length and over 100 years of age have been reported in the Fraser River. To complete their full life cycle, white sturgeon require sufficient suitable habitat, an abundant food base, and appropriate water conditions. These needs are discussed in the recovery strategy.
The intrinsic biologic factors most limiting to white sturgeon population growth are very low early life stage survival and delayed maturation. Females and males may spawn for the first time at 26 and 11 years of age respectively, but often it is later. Estimated survival is very low during the first year (0.000396%), but is substantially higher in subsequent years (between 91% and 97% or greater for ages equal to or greater than 1). The combined effect of low early life stage survival and the compounding effects of survival rates from age one onward means that a small proportion of fish actually reach the old ages frequently cited. If juvenile survival rates drop slightly, the effect over multiple years can be substantial. Furthermore, delayed maturity means that even if juvenile recruitment (i.e., survival past the egg and larval stages) starts to improve immediately, recruitment to the spawning population may be delayed for two decades or more.
Long-term trend data on fluctuations in population size or density are generally lacking for all white sturgeon populations because most studies are relatively recent. Various lines of evidence can be used to infer that population abundance has declined in many parts of the Canadian range, particularly in the Nechako, Columbia and Kootenay rivers. In the lower Fraser River, the decline is primarily related to historic harvest and habitat loss, and juveniles continue to recruit to the population. In the Columbia, Kootenay and Nechako rivers the cause of decline is primarily ongoing recruitment failure (i.e., absence of sufficient juvenile abundance capable of sustaining a population) associated with changes in habitat, flows, and the ecological community. In the middle and upper Fraser River, abundance is likely food and habitat limited and white sturgeon are thought to be at or near historic levels. In the Columbia, Kootenay and Nechako rivers, abundances of wild spawned fish are low and consist mainly of mature, adult white sturgeon with few naturally produced juveniles. Current abundance estimates for each population are presented in the recovery strategy along with summaries of present hatchery supplementation programs.
The primary human activities that threaten white sturgeon in the wild are direct habitat alienation and loss where sturgeon can longer access previously used habitats, river regulation, harvest of prey/food, introduction of invasive non-native fish species, direct and indirect harvest, release of pollutants, and floodplain development. Primary threats to white sturgeon, both current and historic, are discussed in this recovery strategy. Activities and threats that are likely to destroy critical habitat are also provided. However, addressing conservation needs for sturgeon will require more than simply limiting or prohibiting these human activities. For example, at this time it is not possible or feasible to remove large dams or flood control dikes to reclaim lost habitats. Additionally, the populations occur over large spatial scales, creating distinct challenges for recovery. It is necessary to understand the underlying mechanisms that control sturgeon abundance and distribution across populations, and use this information to develop acceptable strategies for protecting and recovering sturgeon populations.
There are four main knowledge gaps that need to be addressed: causes of recruitment failure in basins that are regulated by dams; design and implementation details of conservation fish culture; clarification of existing threats; and, basic biology information needs. Knowledge gaps for the species as a whole are discussed separately from the Schedule of Studies to identify or further refine critical habitat areas.
The recovery goal for white sturgeon is to ensure that all populations are sustainable throughout their natural range, are self-sustaining through natural reproduction, and that opportunities for beneficial use are increased or restored, if and when feasible. To achieve this goal, a series of population and distribution objectives and broad strategies to address threats have been identified, including specific recovery measures, research, and ongoing monitoring. Objectives and strategies are presented separately for SARA-listed and non-SARA-listed populations. The National Recovery Team (NRT) has prioritized actions to meet population and distribution objectives, and these are presented in the recovery strategy, along with their status as of 2012.
Critical habitat for each life stage are discussed and identified, to the extent possible, based on the best available information. Maps of the critical habitat areas, along with geographic coordinates, and applicable river kilometers are provided. Activities Likely to Result in Destruction of Critical Habitat are also presented, along with a Schedule of Studies to identify or further refine critical habitat areas.
Under SARA, critical habitat must be legally protected from destruction within 180 days of being identified in a recovery strategy or action plan. For white sturgeon, it is anticipated that protection will be accomplished through a SARA Ministerial Order made under subsections 58(4) and (5), which will prohibit the destruction of the identified critical habitat.
Recovery-related actions have been underway in most areas for several years, and are communicated regularly through basin-level teams. Basin-level plans are available in separate documents for each population of white sturgeon. An overview summary for each basin and actions completed or initiated at both the national and the basin level for each population is provided.
Together with partners, DFO will prepare one or more Action Plans for white sturgeon within five years of the final posting of this Recovery Strategy.
SARA allows for certain activities to be exempt from its general prohibitions provided that they are permitted by a recovery strategy, an action plan or a management plan and are also authorized under a federal Act of Parliament. These activities must also not jeopardise species survival or recovery. Activities currently permitted by this recovery strategy are focused on conservation aquaculture for white sturgeon recovery. However as long as specific conditions outlined in this document are met, exemptions may be considered in the future for First Nations Food, Social, Ceremonial (FSC) by-catch and First Nations direct harvest. Any activity, or the cumulative activities, that may be permitted by this recovery strategy will not jeopardize survival or recovery of white sturgeon and regular monitoring and assessment will be required to ensure that no jeopardy is maintained.
Certain terms and acronyms used in this recovery strategy have been defined for the reader, for ease of understanding. These definitions can be found in Appendices C and D.
Common Name: white sturgeon
Scientific Name: Acipenser transmontanus
COSEWIC Assessment Summary: November 2003
COSEWIC Status: Endangered
COSEWIC Reason for Designation: A long-lived species with a 30-40 year generation time and late maturity, that has suffered over a 50% decline in the total population trend in the last three generations1. Three of six populations are in imminent threat of extirpation. Extant populations are subject to threats of habitat degradation and loss due to dams, impoundments, channelization, dyking and pollution. Illegal fishing (poaching) and incidental catches are also limiting. In addition, a developing commercial aquaculture industry may also impose additional genetic, health and ecological risks to wild populations.
Canadian Occurrence: British Columbia
COSEWIC Status History: Designated Special Concern in April 1990. Status re-examined and changed to Endangered in November 2003. Last assessment based on an update status report.
SARA Status: Endangered – Schedule 1 (2006)
Note: In November 2012, COSEWIC reassessed white sturgeon, and divided the species into four Designatable Units (DUs). These DUs differ somewhat in genetic structure and distribution from the six Nationally Significant Populations previously identified in the 2003 assessment; however, this recovery strategy was prepared to provide publicly available direction for recovery of the species at a national scale and in response to the 2006 SARA listing of the Upper Columbia, Nechako, Upper Fraser, and Kootenay River populations, with the understanding that adaptations to recovery approaches and scales may be required in the future.
White sturgeon occur only in British Columbia within Canada, and have been divided into six “nationally significant populations” (NSPs), based on geography, demographics and genetics: the lower, middle and upper Fraser River, Nechako River, Columbia River, and Kootenay River. All populations were assigned an Endangered status by COSEWIC in 2003; the latter four were legally listed under SARA in 2006. This document is a SARA-compliant recovery strategy for the four SARA-listed populations, but it also provides recovery and management recommendations for the lower and middle Fraser River populations (i.e., non-SARA-listed). Sections 2 and 3 (Description of the Species, and Description of Needs of the Species) and Section 10 (Implementation) of this document discuss all populations, whereas recovery and management recommendations for SARA-listed and non-SARA-listed populations are discussed separately in other sections.
White sturgeon, Acipenser transmontanus, is the largest, longest-lived freshwater fish species in North America (Scott and Crossman 1973). Fish of over 6 m in length and over 100 years of age have been reported in the Fraser River (Scott and Crossman 1973). The species’ most distinguishing features include a mainly cartilaginous skeleton, long scaleless body covered with rows of large bony plates (called scutes) on the back and sides, shark-like (heterocercal) tail, and four barbels between the mouth and an elongated snout (Figure 1 ). It has a protrusible mouth with which it creates suction to capture and pick up food. Body colouration ranges from black to olive or light grey on the dorsal surface and upper edge of scutes, but is consistently white on the ventral surface (Scott and Crossman 1973).
Figure 1. The white sturgeon, Acipenser transmontanus. (Drawing by Paul Vecsei provided courtesy of Golder Associates Ltd.)
Figure 1. Lateral view of the right side of an adult white sturgeon black and white drawing (Paul Vecsei provided courtesy of Golder Associates Ltd.). The fish has a long scale-less body with three lengthwise lines of bony plates running along the back and sides of the body (called scutes and denticles respectively). Four barbels are situated between the protrusible mouth and elongated snout that make up a flattened head. This fish has a shark-like (heterocercal) tail with a caudal fin extending from the bottom half. Moving forward from the tail, this fish has an anal fin (on the bottom), dorsal fin (on the top), pelvic fin (on the bottom), and pectoral fins (on each side) just before reaching the head.

White sturgeon are slow-growing with a delayed onset of sexual maturity. Growth rates and maturity vary significantly throughout the white sturgeon’s range. Growth rates tend to be highest where waters are warmer, growing seasons are longer, and food is abundant.
Males tend to mature at a younger age and smaller size than females. Females and males may spawn for the first time at 26 and 11 years respectively, but often it is later (Semakula and Larkin 1968). White sturgeon may also spawn multiple times throughout their life. For example, limited data for white sturgeon in the lower Fraser River suggest that spawning intervals for females may vary from 4 to 11 years, with the interval increasing with age (Semakula and Larkin 1968, Scott and Crossman 1973). Hatchery programs for white sturgeon in the Nechako and Kootenay rivers have identified spawning intervals as short as 3 to 4 years (Steve McAdam, B.C. Ministry of Environment, personal communication). Estimated survival is very low during the first year (estimated at 0.000396% in Gross et al. 2002) and is higher for hatchery-released juveniles over 1.5 years of age (e.g., 29% in Golder Associates Ltd. 2007, Beamesderfer and Justice 2008). Survival can also be very low in some year classes due to density dependant juvenile survival (Justice et al. 2009). Survival rates are substantially higher in the juvenile and adult life stages, with estimates ranging between 91% and 97% (Gross et al. 2002, Walters et al. 2005, Irvine et al. 2007) . The combined effect of low early survival and the compounding effects of subsequent mortality over many years means that relatively few individuals actually reach the old ages often cited for this species.
For consistency, the following major life stages will be used throughout this document. This terminology has been reviewed by the NRT, but a variety of other terms may be used in the literature. The divisions into the following life stages are useful for facilitating discussion of the life stage-specific biotic processes, habitats, including the biological function, features and attributes of critical habitat, and population and distribution objectives discussed in this document.
Spawning -- The spawning life stage refers to the primary period of active reproduction for mature individuals. Typically this is shortly after the peak of the spring freshet, but the actual timing varies considerably among locations. Where logical to do so, the spawning period may include staging near spawning areas immediately prior to spawning events.
Incubation -- The incubation life stage refers to the period from fertilization to hatch. Hatch occurs 5 to 10 days after fertilization depending on water temperature, with temperatures in excess of 20˚ C leading to abnormal development (Wang et al. 1985). Since incubation habitats are the same or contiguous with spawning habitats, the discussion of spawning and incubation is usually combined in this document.
Yolk sac larvae (0 – 12 days post-hatch) -- During the yolk sac larvae period individuals tend to remain hidden (typically in interstitial spaces within river bed substrates) until the yolk sac is exhausted, but at the beginning of this period drift may occur until yolk sac larvae find appropriate hiding locations. The life stage ends at the onset of exogenous feeding. First feeding varies from 8–16 days post-hatch, depending on water temperature (Doroshov et al. 1983; Buddington and Christofferson 1985; Gawlicka et al. 1995). The period to 12 days post-hatch is used to capture the typical developmental range.
Feeding Larvae (12 – 40 days post-hatch) -- At the onset of the larval period individuals emerge from hiding habitats, show nocturnal drift, and initiate exogenous feeding. First feeding varies from 8–16 days post-hatch, depending on water temperature (Doroshov et al. 1983; Buddington and Christofferson 1985; Gawlicka et al. 1995). A larva’s first feeding occurs after about 200 accumulated temperature units (Boucher 2012). Nocturnal drift likely decreases predation and drift is presumed to allow feeding larvae to move to low velocity feeding habitats (e.g. side channels or floodplain). The division between the feeding larvae and early juvenile life stage is termed metamorphosis and occurs when fish take on the features of the adult form. Various ages have been suggested for metamorphosis and an age of 40 days is used here as an appropriate timeframe (Buddington and Christofferson, 1985; Deng et al. 2002).
Early Juvenile (40 days to 2 years) -- While early juvenile white sturgeon are morphologically very similar to later life stages after metamorphosis, habitat use and diets may be substantially different than later life stages, primarily due to differences in body size. The 40 days to 2 years stage is one in which young fish become less susceptible to predation and by one year old fish are often observed holding in habitats that are similar to adult habitat types. The division between this life stage and the next has been set somewhat arbitrarily at two years of age. In general, once white sturgeon are one year old they tend to occupy habitat that is similar to that preferred by adults.
Late Juvenile and Adult (greater than 2 years) -- Individuals greater than two years old differ in size and sexual maturity from late juveniles, but habitat for these stages is similar, so these fish can be grouped together during the rearing and overwintering phases. Food resources likely shift during this stage with an increasing trend toward piscivory in older fish. This life stage may include activities such as staging, overwintering, migration and rearing.
White sturgeon release large numbers of eggs and sperm over bottom substrates in the water column of turbulent river habitats. Spawning occurs in the late spring and early summer, typically following the highest water levels of freshet. During this time, water temperatures rise, with fast water velocities over coarse substrates (Parsley et al. 1993, RL&L Environmental Services Ltd. 1994a, Parsley and Kappenman 2000, Paragamian et al. 2002, Parsley et al. 2002, Perrin et al. 2003, Sykes et al. 2007) , though there are deviations from this general pattern. Recent studies indicate that velocity and depth preferences supersede substrate preference during spawning (Paragamian et al. 2009, McDonald et al. 2010, Sykes 2010) , though substrate condition appears to have a critical effect on egg and very early life stage survival (Paragamian et al. 2009, McAdam 2011, Boucher 2012, McAdam 2012) . In the Fraser River, the only unregulated river examined, spawning has been documented primarily in large side channel habitats from mid-June through July (RL&L Environmental Services Ltd. 2000a, Perrin et al. 2003, Paradis et al. 2011) . In the mainstem Columbia and Snake rivers, spawning has occurred largely in the tailwater areas of large dams (e.g., Parsley et al. 1993, Parsley and Kappenman 2000, Lepla et al. 2001, Terraquatic Resource Management 2011) or at the confluences of large tributaries possibly indicating that sturgeon select higher velocity spawning areas. In the Kootenay River, white sturgeon spawn in the mainstem far downstream of Libby Dam (Idaho, USA) whereas in the Nechako River, fish also spawn far downstream of the Kenney Dam (in and upstream of a braided section of the river) near Vanderhoof, B.C.
The number of eggs that white sturgeon females can produce (i.e., fecundity) is directly proportional to body size and has been reported by Scott and Crossman (1973) to range from 0.7 to 4.0 million eggs. For example, a 239 cm female contained approximately 0.7 million eggs (Scott and Crossman 1973). Eggs are approximately 3.5 mm in diameter, adhesive, and demersal (Deng et al. 2002).
During incubation (the life stage that begins at fertilization and ends at hatch), white sturgeon embryos gain nutrition endogenously. Hatch timing depends on water temperature and has been observed after approximately 5 to 10 days at water temperatures ranging from 11° to 21.5˚C (Wang et al. 1985). Abnormal development has been observed when early life stages are reared at temperatures in excess of 20˚C (Wang et al. 1985). For several days after hatch, yolk sac larvae continue to receive all nutrition endogenously from a yolk sac (van der Leeuw et al. 2006). After the yolk sac is exhausted, exogenous feeding begins and the larvae are referred to as feeding larvae. Metamorphosis is complete, including the development of adult osteological features, approximately 40 to 60 days after hatching, depending on ambient water temperature; the young fish are identical in most ways to the adult form (Wang et al. 1985, Kynard et al. 2007).
Under culture conditions the highest daily mortality rate of young sturgeon is associated with the onset of exogenous feeding (Gisbert and Williot 2002). First feeding in white sturgeon varies from 8 to 16 days post-hatch, depending on water temperature (Doroshov et al. 1983, Buddington and Christofferson 1985, Gawlicka et al. 1995). During early development under cultured conditions, a mixture of food organisms produces faster growth than diets of single species (Gisbert and Williot 2002). Studies of larval feeding under natural conditions are limited (e.g., Muir et al. 2000), but feeding observations in the wild suggest that larvae eat a variety of foods and are likely more limited by the gape size of their mouth than by food type (Steve McAdam, B.C Ministry of Environment, personal communication).
Movement and dispersal during early development are poorly understood, especially considering the importance of this life stage to recruitment. After hatching, yolk sac larvae tend to hide in interstitial spaces while endogenous yolk reserves are used (McAdam 2011, McAdam 2012). Drifting may occur during this phase until larvae reach suitable hiding locations (Howell and McLellan 2006, McAdam 2011, McAdam 2012). Emergence from hiding occurs at the initiation of exogenous feeding and leads to nocturnal drift, which is believed to allow larvae to move to preferred feeding locations (McAdam 2012). Larval drift can result in very long range dispersal: larvae were collected more than 180 km downstream of the spawning area at Bonneville Dam on the Columbia River (McCabe and Tracy 1994). This pattern of hiding and drift is consistent with a variety of field and laboratory studies for white sturgeon and other sturgeon species (Kempinger 1988, Parsley et al. 2002, Kynard et al. 2007, McAdam 2011, McAdam 2012); however, the importance of these phases to recruitment emphasizes the need to clearly identify factors affecting stage-specific survival rates.
Adult and juvenile white sturgeon are adapted to feeding in dark, benthic habitats, where prey are often located through direct contact, facilitated by highly sensitive taste receptors on barbels near the mouth (Brannon et al. 1985). Juvenile white sturgeon are primarily benthic feeders and prey include a variety of aquatic insects, isopods, mysids, clams, snails, small fish, and fish eggs (Scott and Crossman 1973, McCabe et al. 1993), but diets also vary throughout the year and among locations. In the upper Columbia River, Mysis relicta, a non-native pelagic crustacean, is the most common prey item for hatchery-released juveniles that are between one and two years of age, followed by Trichopteran larvae (Golder Associates Ltd. 2006a). Adult white sturgeon feed predominantly on fish, particularly migratory salmonids where available, although crayfish and chironomids are also consumed (Scott and Crossman 1973). In the lower Fraser population, white sturgeon have access to a broader range of food sources than in other areas of B.C., including marine and estuarine fish and invertebrates, anadromous fish, as well as seasonally abundant eulachon (Thaleichthys pacificus) and Pacific salmon runs.
Adult and late juvenile movement and migration is linked to feeding, overwintering, and spawning activities. Movement patterns appear primarily related to food type and availability, and habitat type and availability; the presence of dams and river regulation may alter natural movement patterns. For example, in the unimpounded Fraser River most individuals seem to remain on feeding grounds and exhibit relatively localized movements during the summer (RL&L Environmental Services Ltd. 2000a). Migration behaviour is observed during the fall or winter (if overwintering habitat is not immediately available), followed by a period of relatively low activity during the winter, with the timing and length of inactivity variable among populations (RL&L Environmental Services Ltd. 2000b, Nelson et al. 2004). Spring spawning migrations are more extensive compared to feeding and overwintering movements (RL&L Environmental Services Ltd. 2000a). Telemetry studies in the Columbia River indicate that while white sturgeon remain in preferred high use areas throughout the year, some individuals also move between these areas for spawning and/or feeding (e.g., Golder Associates Ltd. 2006b). Long distance migrations (greater than 1,000 km) have been observed in a few individuals that have access to the ocean (e.g., Welch et al. 2006). In the lower Fraser River, extensive migratory movements have been recorded (greater than 100 km); these movements are likely a consequence of the greater variety of prey available and their timing in river, estuary and marine waters.
Populations of self-sustaining white sturgeon occur in three major drainages on the Pacific coast of North America: the Fraser, Columbia and Sacramento river systems. They are found in the mainstem of these rivers, as well as several of the larger tributaries. White sturgeon can exhibit facultative anadromy and have been observed in several coastal inlets and estuaries, typically near creek and river mouths. Some migration occurs via the ocean between the three major drainages and to other coastal watersheds (Pacific States Marine Fisheries Commission 1992). While these movements are apparently rare, the extent of marine migration and exchange is poorly understood. Studies are currently being undertaken to better understand estuary and marine use in the lower Fraser population.
Six white sturgeon NSPs exist in Canada (all in B.C.) and are referred to as the Lower Fraser River, Middle Fraser River, Upper Fraser River, Nechako River, Upper Columbia River, and Kootenay River populations (Figure 2 and Figure 3) (Smith et al. 2002, COSEWIC 2003). In this document populations are often discussed separately to accommodate differences in biology, threats, and recovery measures. Populations are discussed in geographic order from west to east--no significance is implied by this order.
The lower, middle and upper Fraser River populations are the only ones not directly fragmented by dams, as no dams are present on the Fraser River mainstem. Within the Fraser River, white sturgeon have been observed in the mainstem from the marine estuary upstream past the Morkill River, northwest of McBride, a distance of approximately 1,100 km (Yarmish and Toth 2002). They are also found in a number of large tributaries including the Nechako and Stuart systems (a total of 400 km in length), the Harrison and Pitt rivers, and the confluences or lower reaches of numerous large and small tributaries, such as the Bowron, McGregor and Torpy rivers (Ptolemy and Vennesland 2003). Present distribution in the Fraser River is believed to be the same or similar to historic distribution with the exception of Seton, Thompson, and Nechako rivers, where white sturgeon may now be more restricted than they were historically due to dams. White sturgeon have also been confirmed in several large lakes including Seton, Pitt and Harrison lakes, and reported in several others, including Williams and Kamloops lakes.
Movement within the Fraser River mainstem is generally unrestricted, with the possible exception of seasonal rapids within the Fraser Canyon, such as at Hells Gate. This confined canyon section, which is about 210 km from the mouth is an upstream movement barrier to many fish species. Salmon, trout, char, and white sturgeon have nevertheless been recorded moving upstream and downstream. There have been two documented downstream movements and one upstream movement of white sturgeon through Hells Gate (Fraser River Sturgeon Conservation Society 2012).
Although movements between the lower, middle, and upper Fraser River and Nechako River populations are possible and have occasionally been documented (Lheidli T’enneh Band 2001, Golder Associates Ltd. 2003a, Fraser River Sturgeon Conservation Society 2012), genetic differentiation in mitochondrial DNA suggests that Fraser River white sturgeon exist as reproductively isolated populations in these sections of the watershed (Smith et al. 2002). Tagging and genetic data also strongly suggest that the Nechako River population does not interbreed or mix significantly with the upper Fraser mainstem population (Smith et al. 2002). Schreier et al., (2012), using microsatellites, found evidence supporting genetic differentiation between Fraser white sturgeon populations above and below Hell’s Gate, and evidence of population substructure in the Fraser River above Hell’s Gate and the Nechako River. Additional research on population substructure and spawning groups in the Fraser River above Hell’s Gate may help guide conservation efforts for these populations (Schreier et al., 2012).
The lower Fraser River population is thought to be relatively productive, as they have access to marine derived nutrients (e.g. salmon) and estuarine habitats not available to other Canadian populations. Juveniles and adults of the lower Fraser River population disperse widely, especially when food sources are abundant, such as during smelt and eulachon spawning, and during sockeye, Chinook pink and chum salmon migration and spawning. During these periods sturgeon move extensively between holding, prey spawning and carcass depositional areas.
In the Nechako system, white sturgeon can be found from the confluence with the Fraser River, upstream to Cheslatta Falls, and throughout most of the Stuart River, which is a major tributary, and several large lakes such as Fraser, Takla, Stuart and Trembleur lakes. Some Nechako River white sturgeon have been observed to move into the Fraser River confluence for feeding and overwintering, but these movements are limited (Lheidli T’enneh First Nation 2008, Sykes 2008). Seasonal migrations to the Stuart and Fraser Lake systems coincide with the migrations of salmon, suggesting that both lake systems are important feeding and rearing areas for this population (Liebe et al. 2004). The Kenney Dam has altered the natural flow regime and instream habitats, which may have affected movement patterns of white sturgeon (Nechako White Sturgeon Recovery Initiative 2004).
Figure 2. Map of the Fraser River basin depicting the approximate ranges for each of the four white sturgeon populations in the Fraser River watershed. The species is principally found in the mainstem habitats of the Fraser and Nechako rivers, although they also make extensive use of tributaries and large lakes (such as in the Harrison or Stuart watersheds). Anecdotal records indicate that sturgeon were present in several watersheds beyond the described boundaries (see text for details).
Figure 2. Map of the Fraser River basin depicting the approximate ranges for each of the four white sturgeon populations in the Fraser River watershed. One tributary of the Nechako population (highlighted in pale pink) begins about 75km northwest of Fort St. John, flowing south-eastward. A second and third tributary begin near Fraser Lake and the Kenney Dam respectively, joining together between Fraser Lake and Vanderhoof. All three tributaries of the population join together and flow ~50km eastward ending in Prince George. The Upper Fraser population highlighted in pale yellow extends north-westward from just past McBride near the Alberta border to Prince George. This population reaches a “mixing point” with the Mid Fraser population between Quesnel and Prince George. The Mid Fraser population extends southward from this mixing point to about halfway between Hope and Lytton in the Fraser Canyon. In orange the Lower Fraser population’s range is highlighted flowing southward from the Fraser Canyon, westward through the Fraser Valley and ending in Delta. A scale and legend are provided along with an inset map showing locations are primarily in the southwestern corner and interior of British Columbia. The map is oriented in a “north is up” direction.
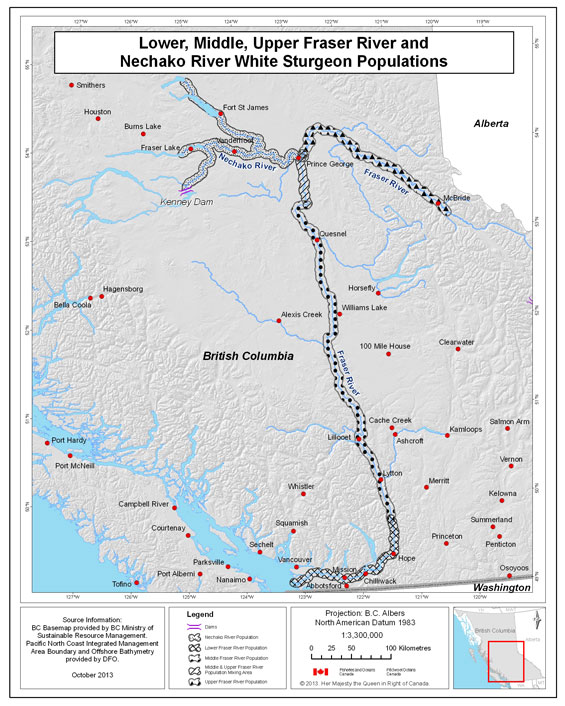
Figure 3. Map of the Columbia and Kootenay basins depicting the approximate ranges for two of the white sturgeon populations in British Columbia. Records indicate that sturgeon historically occurred beyond the described boundaries, but at low abundance. Small remnant populations occur upstream of Duncan Dam and in Slocan Lake (see text for details). White sturgeon are present in the Columbia to its confluence with the Pacific Ocean, but this document addresses only sturgeon upstream of the Canada-U.S. border.
Figure 3. Map of the Columbia and Kootenay basins depicting the approximate ranges for two white sturgeon populations in British Columbia. The Upper Columbia River population (highlighted in orange) begins in Revelstoke (where it is restricted by the Revelstoke Dam), extending southward past the Hugh L. Keenleyside Dam, Castlegar, and Trail until it reaches the Grand Coulee Dam in Northeastern Washington State. The Kootenay River population (highlighted in pink) begins near Meadow Creek and extends southward to Balfour, forming a confluence with this tributary, also part of the Kootenay population, flowing northward from the Libby Dam. The population extends southwestward from the confluence, ending in Nelson. A scale and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.

White sturgeon historically had access from the ocean to Columbia Lake in the upper Columbia River and to Shoshone Falls in the upper Snake River. Populations in the upper reaches of the basin were most likely resident and benefited from the seasonal availability of anadromous salmon. White sturgeon inhabited the upper Columbia mainstem, lower Spokane River, lower Pend d’Oreille River, and lower Kootenay River to Bonnington Falls, and likely also used portions of smaller tributaries including the Sanpoil, Kettle, Slocan, and Salmo rivers (Hildebrand and Birch 1996, Prince 2001). Distribution was probably patchy with fish concentrated in areas of favourable habitat. Concentrations of white sturgeon were reported during the early 1900s in the Columbia River mainstem downstream from Castlegar, the lower Kootenay River downstream of Bonnington Falls, Arrow Lakes, Big Eddy near Revelstoke, and at the present site of Mica Dam (Prince 2001).
The current distribution of white sturgeon in the upper Columbia River extends from Revelstoke Dam (REV) to Grand Coulee Dam in Washington, and the lower Kootenay River from its confluence with the Columbia River to Brilliant Dam (Figure 3). Studies on this population focus on three geographic areas: i) Arrow Lakes Reservoir (ALR), upstream of Hugh L. Keenleyside Dam (HLK); ii) the transboundary reach, which extends downstream from HLK to Roosevelt Reservoir (FDR) (Upper Columbia White Sturgeon Recovery Initiative 2012); and iii) Roosevelt Reach (i.e., FDR). This recovery strategy addresses only white sturgeon and habitats upstream of the Canada-U.S. border.
The ALR component of the Columbia River white sturgeon population has access to approximately 230 km of riverine and lacustrine habitat from REV downstream to HLK. Abundance of the ALR component is substantially lower than that of the other two population components. Radio-tagged sturgeon have been observed to overwinter at Beaton Flats and several move during spring and summer upstream to Revelstoke or into Beaton Arm near the confluence with the Incomappleux River (see Section 8: Critical Habitat). Most assessment effort has concentrated on the upper ALR and a total of 32 unique fish have been captured there (Golder Associates Ltd. 2006c). More recently, considerable effort was expended on assessments in the narrows section of ALR (see Section 8: Critical Habitat), which resulted in an additional 10 unique captures (Prince 2002, 2003, 2004).
White sturgeon in the transboundary population have access to habitats from HLK to Grand Coulee Dam (Figure 3). Habitat in Canada for this population includes approximately 56 km of riverine habitat located between HLK and the Canada-U.S. border, and the small section of river in the lower Kootenay River below Brilliant Dam. Studies have been conducted on the transboundary population component since 1990 (Upper Columbia White Sturgeon Recovery Initiative 2002). Movements of white sturgeon between Canada and the U.S. have been observed (Golder Associates Ltd. 2006b), but white sturgeon within the transboundary reach tend to remain within fairly localized areas and some fish may make larger movements related to spawning activity (Golder Associates Ltd. 2006b, Nelson and McAdam 2012). Habitats within this reach include large, deep, eddy areas that are preferred areas for both white sturgeon and their prey items (Golder Associates Ltd. 2006b). Concentrations of overwintering adult white sturgeon have been observed mostly between HLK eddy and Norns Creek (7 km downstream of HLK), as well as Fort Shepherd eddy, Waneta eddy, and, to a lesser extent, in the lower portion of the Kootenay River below Brilliant Dam (i.e., Kootenay eddy, near Brilliant Bridge, Brilliant Dam plunge pool; see Section 8: Critical Habitat).
Research since 2005 has substantially increased our knowledge of sturgeon distribution and density in the approximately 40 km section of river between the Canada-U.S. border and FDR (Howell and McLellan 2007a, b, Howell and McLellan 2009, 2011). Most adult sturgeon occur in the river-reservoir transition zone and upstream to the border, with much lower densities in the FDR main pool (Howell and McLellan 2007a, b). Spawning has been identified at two locations near Northport and China Bend (Washington). Although these fish are also undergoing recruitment failure, occasional low levels of recruitment have been detected (e.g., 1997). Although fish in Lake Roosevelt do not mix freely across the border, both demographic patterns (McAdam 2012) and movements during the spawning season (Howell and McLellan 2007a, b) suggest some cross border exchange, particularly with the Waneta spawning site.
Remnant population components may also exist upstream of the ALR component (i.e., between REV and Mica Dam, and in Kinbasket Reservoir) but investigations have not captured white sturgeon despite considerable effort (RL&L Environmental Services Ltd. 1996a, 2000b, Prince 2009). Given the large size of these reservoirs, the failure to catch white sturgeon does not prove their absence, but suggests that population abundance is at most very low (RL&L Environmental Services Ltd. 2000b).
There are several hypotheses regarding the historic distribution and population structure of upper Columbia River white sturgeon. For example, recent research suggests that white sturgeon in the Canadian portion of the Columbia River historically consisted of three or more reproductively isolated populations (Nelson and McAdam 2012). Investigations of historic genetic population structure are ongoing. However, for the purpose of recovery planning white sturgeon in the Columbia River from REV to the Canada-U.S. border are considered to be a single population or designatable unit. This recovery strategy does not consider the FDR population component that resides in the U.S. and it does not consider remnant, demographically isolated population components (e.g., Revelstoke, Kinbasket, and Slocan systems)2.
The Kootenay3 River population of white sturgeon extends from Kootenai Falls, Montana, located 50 river-kilometres below Libby Dam (Idaho), downstream through Kootenay Lake to Corra Linn Dam on the lower West Arm of Kootenay Lake, British Columbia.
Kootenai Falls likely represented an impassable natural barrier to upstream migration of white sturgeon, although there are a handful of anecdotal accounts describing the presence of white sturgeon upstream of Kootenai Falls in Montana and British Columbia (Jason Flory, U.S. Fish and Wildlife Service, personal communication). During the mid-1970s, after construction of Libby Dam, Montana Fish, Wildlife & Parks introduced five adult white sturgeon into Koocanusa Reservoir. One of these was captured at Wardner Bridge in 1980, but the fate of the other sturgeon is unknown, and some or all of these fish may have been captured by anglers.
A natural barrier at Bonnington Falls downstream of Kootenay Lake has isolated the Kootenay River white sturgeon from other white sturgeon populations in the Columbia River basin since the end of the Pleistocene, approximately 10,000 years ago (Northcote 1973). Spawning habitat is located in the U.S., whereas much of the adult and juvenile rearing habitat is located in Kootenay Lake and the Canadian portion of Kootenay River. White sturgeon occur in very small numbers in Duncan Reservoir and Slocan Lake (RL&L Environmental Services Ltd. 1998a, b).
The Slocan River is a tributary of the Kootenay River and several white sturgeon have been captured in Slocan Lake (RL&L Environmental Services Ltd. 1996b). Two white sturgeon captured in Slocan Lake were aged using fin ray samples and assessed to be younger than Brilliant Dam (RL&L Environmental Services Ltd. 1996b). It is thought that these fish may have en entrained from upstream on the Kootenay River and subsequently moved into Slocan Lake (RL&L Environmental Services Ltd. 1996b, 1997).
Tracking studies in Kootenay Lake located sturgeon released upstream of the Canada-US border (Neufeld and Spence 2004a, Neufeld and Rust 2009). A 2005 study tracked the dispersal of tagged juvenile white sturgeon from release sites in the US (n=4) and Canada (n=1) upstream of Kootenay Lake (Neufeld and Rust 2009). All fish released in the shallow, higher gradient reach above Bonners Ferry, Idaho moved downstream to the lower gradient reach below Bonners Ferry within 2 months of release (Neufeld and Rust 2009). Meanwhile, juveniles released within this lower gradient section showed movement both upstream and downstream, with 9% (n=3) of tagged fish moving from river release sites to Kootenay Lake (Neufeld and Rust 2009). High gradient locations are characterized by typical gradients of 0.6 m/km-1 and velocities greater than 0.8 m/sec-1, whereas low gradient reaches were characterized by typical gradients of 0.02 m/km-1 and velocities less than 0.4 m/sec-1 (Neufeld and Rust 2009).
Long-term trend data are generally lacking for all white sturgeon populations because most studies are relatively recent (though see Walters et al. 2005, Whitlock and McAllister 2012). Various lines of evidence can be used to demonstrate that population abundance has declined in many parts of the Canadian range, particularly in the Nechako, Columbia and Kootenay rivers, where the timing of the decline is associated with the installation of dams and subsequent river regulation. In the lower Fraser River, the decline is primarily related to historic harvest and habitat loss. In the middle and upper Fraser River, abundance is likely food and habitat limited and white sturgeon are thought to be at or near historic levels. Recruitment failure4 is ongoing in the Nechako, Columbia and Kootenay, which has resulted in highly skewed age structures, but regular recruitment continues to occur in the upper, mid and lower Fraser and age structure appears normal (Ptolemy and Vennesland 2003).
The total estimated population abundance for Fraser, Nechako, Columbia and Kootenay white sturgeon populations are presented in Table 1. The estimates are for wild fish (i.e., not hatchery produced fish) greater than 40 cm total length. Three population estimates are provided for the Columbia River population, corresponding to census studies that divide this population into the transboundary, ALR, and FDR population components. The estimated abundance for the Kootenay River population includes fish captured in both Canada and the U.S., since these components cannot be separated.
Updated abundance estimates of mature white sturgeon are provided in Table 2 for the year 2012. The estimates provide information on the reproductive potential of each population and are based on estimates of fish greater than160 cm total length and the latest estimates of survival.
Table 1. Abundance estimates for wild white sturgeon in Canada. Estimates are for fish > 40 cm, unless noted otherwise in footnotes. There are five columns read left to right: Population or Population Component, Abundance Estimate, 95% CI, Year of Estimate, and Reference. Directly below the column headings are eleven rows. Row 6 has three sub-categories under the “Population or Population Component – Columbia” category as follows: ALR, Transboundary, and FDR. The contents of the rows are as follows reading the table read left to right:
Row 1: Lower Fraser (est. 1), 44,713, 42,634 – 46,792, 2011, Nelson et al. 2012. A footnote on 44,713 states the following: The 2011 estimate is for fish > 40 cm and < 279 cm. The estimate does not include individuals larger and smaller due to the small number of tagged individuals and low capture rates in these size groups (Nelson et al. 2013). This population estimate, generated through a mark-recapture study, is the estimate that is used for lower Fraser population management and recovery planning as it is current and likely reflects population trends. Additional population estimates have been completed using mark recapture and other data by Walters et al. (2005) and Whitlock and McAllister (2012). These modeling exercises and their associated estimates and management recommendations are also considered in the management of the lower Fraser population and development of recovery actions. A footnote on 42,634 – 46,792 states the following: Values for the lower Fraser describe the 95% highest density rather than a parametric confidence interval (see Nelson et al. 2004). Row 2: Lower Fraser (est. 2), 97,658, 73,582 – 121,734, 2004, Whitlock and McAllister 2012. A footnote on 73,582 – 121-74 states the following: Mean plus minus standard deviation. Row 3: Middle Fraser, 3,745, 3,064 – 4,813, 2000, RL&L 2000a. Row 4: Upper Fraser, 815 677 – 953, 2002, Yarmish and Toth 2002. Row 5: Nechako, 571, 4 421 – 890, 1999, RL&L 2000a. A footnote on 571 states the following: Estimates are for fish > 50 cm fork length. Row 6: Columbia, empty cell, empty cell, empty cell, empty cell. A footnote on “Columbia” states the following: These 3 segments of the Columbia River population are tabulated separately and correspond to separate census studies for each population component. Row 7: ALR, 52, 37-92, 2003, Golder 2006b. A footnote on Golder 2006b states the following: These values are for the year 2003. Row 8: Transboundary 1,157 414 – 1,900 2003 Irvine 2007. A footnote on Irvine 2007 states the following: These values are for the year 2003 and combine separate estimates for two river sections from HLK to the U.S. border (see text for further information). Row 9: FDR 2,037 1,093 – 3,223 2007 Howell and McLellan 2007a, b. Row 10: Kootenay 1,000 8 800 – 14,000 2007 Beamesderfer et al. 2009. A footnote on 1,000 states the following: This is a revised draft estimate based on analysis of 1977-2008 data for naturally-produced Kootenai sturgeon in the population in 2007 (Beamesderfer et al. 2009). This estimate is for wild (i.e., naturally produced) white sturgeon -- there are many juvenile hatchery releases that are now >40 cm. This population estimate is also based on a combined Canadian and U.S. estimate since components cannot be separated. Row 11: total in Canada, 54,090, 49,140 – 72,663, empty cell, empty cell. A footnote on “total in Canada” states the following: The total uses the estimate for the most recent year in the lower Fraser River.
1 The 2011 estimate is for fish > 40 cm and < 279 cm. The estimate does not include individuals larger and smaller due to the small number of tagged individuals and low capture rates in these size groups (Nelson et al. 2013). This population estimate, generated through a mark-recapture study, is the estimate that is used for lower Fraser population management and recovery planning as it is current and likely reflects population trends. Additional population estimates have been completed using mark-recapture and other data by Walters et al. (2005) and Whitlock and McAllister (2012). These modeling exercises and their associated estimates and management recommendations are also considered in the management of the lower Fraser population and development of recovery actions.
2 Values for the lower Fraser describe the 95% highest density rather than a parametric confidence interval (see Nelson et al. 2004).
3 Mean ± standard deviation.
4 Estimates are for fish > 50 cm fork length.
5 These 3 segments of the Columbia River population are tabulated separately and correspond to separate census studies for each population component.
6 These values are for the year 2003.
7 These values are for the year 2003 and combine separate estimates for two river sections from HLK to the U.S. border (see text for further information).
8 This is a revised draft estimate based on analysis of 1977-2008 data for naturally-produced Kootenai sturgeon in the population in 2007 (Beamesderfer et al. 2009). This estimate is for wild (i.e., naturally produced) white sturgeon -- there are many juvenile hatchery releases that are now >40 cm. This population estimate is also based on a combined Canadian and U.S. estimate since components cannot be separated.
9 The total uses the estimate for the most recent year in the lower Fraser River.
Table 2. Abundance estimates for mature (greater than 160 cm) white sturgeon in Canada. Estimates for the Nechako River, Kootenay River, and Columbia River populations have been updated to 2012 using the best available estimates of annual survival rates. Lower, middle and upper Fraser River populations are thought to be relatively stable, so the most recent census data are provided for these populations.
There are four columns read left to right: Population, Population Reference for uncorrected abundance estimate, Survival estimate, and Number of mature fish in 2012. Directly below column headings are ten rows. Row 6 has three sub-categories under the “Population – Columbia” category as follows: ALR, Transboundary, and FDR. The contents are as follows reading from left to right:
Row 1: Lower Fraser, Nelson et al. 2012, 0.96, 8,460. Row 2: Mid Fraser, RL&L 2000a, 0.96, 749. Row 3: Upper Fraser, Yarmish and Toth, 2002, 0.96, 185. Row 4: Nechako, RL&L 2000a, 0.94, 243. A footnote on 243 states the following: Population estimated in 1999 and assumes 95% of uncorrected estimate are mature with 94% survival rate per year projected to 2012. There are no adult survival estimates specific to the Nechako River population; this assumed survival value is taken from Whitlock (2007) and Irvine et al. (2007). Row 5: Columbia, empty cell, empty cell, empty cell, empty cell. A footnote on “Columbia” states the following: These 3 segments of the Columbia River population are tabulated separately and correspond to census studies that divide the population. Further information on these population components are provided in Section 2.4 (Distribution). Row 6: ALR, Golder 2006a, 0.97, 40. A footnote on 40 states the following: Population estimated in 2003 and is projected to 2012 based on a 97% survival rate (Irvine et al. 2007) per year. Row 7: Transboundary, Irvine, 2007, 0.97, 790. A footnote on 790 states the following: The uncorrected abundance estimate is for the year 2003. The 2012 estimate assumes 90% are mature with a mean annual survival rate of 97% (Irvine et al. 2007) per year projected to 2012. Abundance estimates are for wild (i.e., naturally produced) fish. Row 8: FDR, Golder 2005b, 0.97, 1,749. A footnote on 1749 states the following: Population estimated in 2006 and assumes 70% of the uncorrected abundance estimate are mature (Howell and McLellan 2007b) with 97% survival rate (Irvine et al. 2007) per year projected to 2012. Row 9: Kootenay, Beamesderfer 2009, 0.96, 815. A footnote on 815 states the following: This is a revised draft estimate based on analysis of 1977-2008 data for naturally-produced Kootenai sturgeon in the population in 2007 (Beamesderfer et al. 2009). Current analyses estimates a 96% survival rate per year (Beamesderfer et al. 2009; Matt Neufeld, B.C. Ministry of Forests, Lands and Natural Resource Operations, personal communication) projected to 2012. This population estimate is based on a combined Canadian and U.S. estimate since components cannot be separated out. Row 10: total in Canada, empty cell, empty cell, 12,215.
Table 2. Abundance estimates for mature (greater than 160 cm) white sturgeon in Canada. Estimates for the Nechako River, Kootenay River, and Columbia River populations have been updated to 2012 using the best available estimates of annual survival rates. Lower, middle and upper Fraser River populations are thought to be relatively stable, so the most recent census data are provided for these populations.
There are four columns read left to right: Population, Population Reference for uncorrected abundance estimate, Survival estimate, and Number of mature fish in 2012. Directly below column headings are ten rows. Row 6 has three sub-categories under the “Population – Columbia” category as follows: ALR, Transboundary, and FDR. The contents are as follows reading from left to right:
Row 1: Lower Fraser, Nelson et al. 2012, 0.96, 8,460. Row 2: Mid Fraser, RL&L 2000a, 0.96, 749. Row 3: Upper Fraser, Yarmish and Toth, 2002, 0.96, 185. Row 4: Nechako, RL&L 2000a, 0.94, 243. A footnote on 243 states the following: Population estimated in 1999 and assumes 95% of uncorrected estimate are mature with 94% survival rate per year projected to 2012. There are no adult survival estimates specific to the Nechako River population; this assumed survival value is taken from Whitlock (2007) and Irvine et al. (2007). Row 5: Columbia, empty cell, empty cell, empty cell, empty cell. A footnote on “Columbia” states the following: These 3 segments of the Columbia River population are tabulated separately and correspond to census studies that divide the population. Further information on these population components are provided in Section 2.4 (Distribution). Row 6: ALR, Golder 2006a, 0.97, 40. A footnote on 40 states the following: Population estimated in 2003 and is projected to 2012 based on a 97% survival rate (Irvine et al. 2007) per year. Row 7: Transboundary, Irvine, 2007, 0.97, 790. A footnote on 790 states the following: The uncorrected abundance estimate is for the year 2003. The 2012 estimate assumes 90% are mature with a mean annual survival rate of 97% (Irvine et al. 2007) per year projected to 2012. Abundance estimates are for wild (i.e., naturally produced) fish. Row 8: FDR, Golder 2005b, 0.97, 1,749. A footnote on 1749 states the following: Population estimated in 2006 and assumes 70% of the uncorrected abundance estimate are mature (Howell and McLellan 2007b) with 97% survival rate (Irvine et al. 2007) per year projected to 2012. Row 9: Kootenay, Beamesderfer 2009, 0.96, 815. A footnote on 815 states the following: This is a revised draft estimate based on analysis of 1977-2008 data for naturally-produced Kootenai sturgeon in the population in 2007 (Beamesderfer et al. 2009). Current analyses estimates a 96% survival rate per year (Beamesderfer et al. 2009; Matt Neufeld, B.C. Ministry of Forests, Lands and Natural Resource Operations, personal communication) projected to 2012. This population estimate is based on a combined Canadian and U.S. estimate since components cannot be separated out. Row 10: total in Canada, empty cell, empty cell, 12,215.
1 Population estimated in 1999 and assumes 95% of uncorrected estimate are mature with 94% survival rate per year projected to 2012. There are no adult survival estimates specific to the Nechako River population; this assumed survival value is taken from Whitlock (2007) and Irvine et al. (2007).
2 These 3 segments of the Columbia River population are tabulated separately and correspond to census studies that divide the population. Further information on these population components are provided in Section 2.4 (Distribution).
3 Population estimated in 2003 and is projected to 2012 based on a 97% survival rate (Irvine et al. 2007) per year.
4 The uncorrected abundance estimate is for the year 2003. The 2012 estimate assumes 90% are mature with a mean annual survival rate of 97% (Irvine et al. 2007) per year projected to 2012. Abundance estimates are for wild (i.e., naturally produced) fish.
5 Population estimated in 2006 and assumes 70% of the uncorrected abundance estimate are mature (Howell and McLellan 2007b) with 97% survival rate (Irvine et al. 2007) per year projected to 2012.
6 This is a revised draft estimate based on analysis of 1977-2008 data for naturally-produced Kootenai sturgeon in the population in 2007 (Beamesderfer et al. 2009). Current analyses estimates a 96% survival rate per year (Beamesderfer et al. 2009; Matt Neufeld, B.C. Ministry of Forests, Lands and Natural Resource Operations, personal communication) projected to 2012. This population estimate is based on a combined Canadian and U.S. estimate since components cannot be separated out.
Based on a size criterion of 160 cm minimum fork length5, a total of at least 9,394 adults were estimated to be present throughout the Fraser River: 8,460, 749 and 185 adults in the lower, middle and upper Fraser River, respectively (Ptolemy and Vennesland 2003, Nelson et al. 2008; Table 2). The estimates indicate that approximately 90% of white sturgeon in the Fraser River occur downstream of Hell’s Gate.
No trend data are available for white sturgeon populations in the upper and mid–Fraser River, but abundance is believed to be naturally low in this region and within the historic range (Ptolemy and Vennesland 2003, Lheidli T’enneh First Nation 2009). This conclusion is based on repeated sampling, evidence for consistent recruitment (age structure is typical of a self-sustaining population), and the general absence, both now and historically, of direct threats to sturgeon or their habitats. However, abundance of prey such as anadromous salmon is significantly less than historic levels.
White sturgeon abundance in the lower Fraser River was significantly reduced by unsustainable harvesting through the commercial fishery for sturgeon in the late 1800s and early 1900s (Echols 1995, Walters et al. 2005). Low abundance continued after this time due to unregulated by-catch retention in the First Nations, and commercial salmon net fisheries (Waters et al. 2005). Catch management was also limited in the recreational kill fishery until the early 1990s (Walters et al. 2005). Habitat loss and prey reductions have been implicated in keeping abundance below historic levels; the lower Fraser has had significant losses of estuary, floodplain and side channel habitats (Rosenau and Angelo 2005), and salmon, smelt and eulachon spawning returns are lower than historic levels (Fisheries and Oceans Canada 2010a). Abundance monitoring in the lower Fraser River has been underway since 1985, and has been more intensive since 1995, utilizing techniques such as radio telemetry, statistical analysis of commercial and recreational catches, mark-recapture, and life history studies (Lane 1991, Swiatkiewicz 1992, RL&L Environmental Services Ltd. 2000a, Nelson et al. 2004, Nelson et al. 2006, 2007, Nelson et al. 2008, 2009, Nelson et al. 2010, Nelson et al. 2011, Nelson et al. 2012). Recent population estimates by Whitlock and McAllister (2012) are somewhat higher than prior estimates (Walters et al. 2005, Nelson et al. 2011); however, this difference was attributed to the more explicit incorporation of fish movement by Whitlock and McAllister (2012). Recent reports (Nelson et al. 2012) draw attention to declines in total population since 2003, but the current population is within 10% of the 2001 estimate and has overlapping confidence limits, and abundance of mature individuals has increased markedly since 2004. Additionally, lower abundance of smaller size classes may reflect a recruitment pulse that apparently occurred in 1996-1997 (Whitlock 2007). Considering the longevity of the species and the potential for long term recruitment patterns, current research indicates a relatively normal age structure and variable recruitment (Nelson et al. 2009, Nelson et al. 2012).
Population monitoring on the Nechako River began in 1982 and became more intensive in 1995 (Dixon 1986, RL&L Environmental Services Ltd. 2000a) using radio telemetry, recreational catch statistics, mark-recapture estimates and life history studies. Age distribution is highly skewed to older individuals and indicates little or no recruitment to the population since 1967 (McAdam et al. 2005). Population models developed by Korman and Walters (2001) estimated that approximately 150 mature females remain in the Nechako River and that this number would decline to 25 by 2025. Immediate recovery of juvenile recruitment would not improve spawner abundance for at least 25 years due to late maturation of this species. Total abundance was estimated to be 571 in 1999 (Table 1). Assuming a mortality rate of 0.06, the expected population in 2012 is 243 (Table 2). Collecting additional census data has been a lower priority than efforts to address recruitment failure (Cory Williamson, B.C. Ministry of Forests, Lands and Natural Resource Operations, personal communication).
A hatchery program focusing on conservation objectives operated as a pilot program for three years, and released a total of 15,000 juveniles between 2006 and 2008 (Cory Williamson, B.C. Ministry of Forest, Lands, and Natural Resource Operations, personal communication). The Nechako White Sturgeon Recovery Initiative has completed detailed design for a hatchery and research centre in Vanderhoof. An agreement to begin construction of the facility was announced in early 2013, and construction is currently underway. The aquaculture program will be used to produce the founder population and eggs/larvae for habitat restoration research.
In the Columbia River, population monitoring has been underway since 1990 (Hildebrand et al. 1999, Upper Columbia White Sturgeon Recovery Initiative 2002). Separate estimates are provided for components of the population above and below HLK based on census studies (Table 1). From HLK to the Canada-U.S. border (transboundary reach) the combined population was estimated to be 1,157 (95% CI: 414 - 1900; Irvine et al. 2007), with an additional 2037 (95% CI 1093 – 3223) fish estimated downstream of the Canada-U.S. border in FDR (Howell and McLellan 2007a). The population estimate for the transboundary reach was further divided into two strata: A) HLK to 20 km downstream; and, B) from km 20 to the U.S. border (Irvine et al. 2007). For Strata A, the 2004 population was estimated to be 590 (95% CI 254-925), whereas Strata B was estimated at 566 (95% CI 158-974) (Robyn Irvine, Poisson Consulting Ltd., personal communication).
For the ALR population component, the number of mature wild white sturgeon in 2012 is estimated at approximately 40 fish (Table 2). Anecdotal evidence indicates that white sturgeon have been observed upstream of REV, but efforts to confirm this empirically have been unsuccessful (RL&L Environmental Services Ltd. 1996a, Hildebrand et al. 1999, Prince 2009, Nelson and McAdam 2012).
The Columbia River population is considered at significant risk of extinction in the wild given strong evidence that, for several decades, natural recruitment has been too low to sustain itself. Yearly spawning has been documented for the transboundary population component, since 1993 (Upper Columbia White Sturgeon Recovery Initiative 2002, Golder Associates Ltd. 2004). Spawning has also been documented for the ALR population component, but it has been intermittent (Golder Associates Ltd. 2006c). Analyses of age structure data are still being developed, but preliminary information suggests that recruitment began to decline in 1969, and has been very low since 1985 (RL&L Environmental Services Ltd. 1994a; Steve McAdam, personal communication, Hildebrand et al. 1999).
Spawning has been confirmed at four locations: i) the Pend d’Oreille River at its confluence with the Columbia River, which is referred to as the Waneta spawning site; ii) a small riverine section below REV along the bank of the golf course; iii) immediately downstream of the Arrow Lakes Generating Station at HLK (Terraquatic Resource Management 2011) and, iv) two sites below the U.S. border at Northport, Washington Section 8: Critical Habitat). An additional spawning site has been identified based on the capture of larvae at Kinnaird (near the Kootenay-Columbia River confluence), but further study is required to confirm the precise spawning location (Golder Associates Ltd. 2008). It is unclear whether current spawning sites in the Columbia were used prior to construction of the dams, and the extent to which the dams artificially separated individuals from the same population. Preliminary evidence indicates that white sturgeon within the ALR and some fish downstream of HLK are genetically similar (Nelson and McAdam 2012), and evaluation of fin ray chemistry indicates that most fish residing immediately downstream of Keenleyside Dam may have reared upstream of that location (Clarke et al. 2011).
Current data indicate that abundance of wild white sturgeon in the Columbia River will decline by an additional 50% over the next 25 years (Golder Associates Ltd. 2005a). In response, a hatchery program has been initiated as a conservation measure. Hatchery releases started in 2002, with fish that were hatched in 2001. As of January 1, 2012, approximately 164,585 hatchery juveniles have been released into the Upper Columbia recovery area: 38,368 into the Roosevelt Reach, 93,524 into the Keenleyside Reach, and 36,693 into ALR Reach (James Crossman, B.C. Hydro, personal communication). In addition, 1,454,010 larvae have been released into the ALR Reach (James Crossman, B.C. Hydro, personal communication).
Studies were initiated in the ALR to determine distribution and habitat use by released hatchery fish. Early indications suggest the areas with highest sustained presence of juveniles were between Wells and Crawford creeks (Golder Associates Ltd. 2009). Hatchery juveniles have been observed concentrated in the Robson reach between HLK and the Norns Creek fan, and in a series of smaller eddies downstream to Waneta Eddy near the Canada-US border; these areas are likely used for feeding and overwintering (Golder Associates Ltd. 2006b). Hatchery-produced juveniles are regularly observed, along with a few wild juveniles, especially around the mouths of the Kettle and Colville rivers and at Marcus Flats (see Section 8: Critical Habitat). Concentrations of juveniles may be influenced by the river/reservoir interface in the ALR (Golder Associates Ltd. 2009). It is assumed that wild fish would behave similarly to hatchery fish. Survival through the first 6 months for fish age 1 to 1.5 was estimated at 29% (approximate 95% CI = 11%-54%) for juveniles released into the Columbia River downstream of HLK in 2001 (Golder Associates Ltd. 2007 as reported by Beamesderfer and Justice 2008). There is currently no evidence of density-dependence associated with the releases into the Upper Columbia River (James Crossman, B.C. Hydro, personal communication).
Population surveys in the Kootenay River began in 1977 and became more intensive after 1990, with radio telemetry, recreational catch monitoring, mark-recapture estimates and life history studies being conducted (Duke et al. 1999, Paragamian et al. 2005). Existing abundance estimates are for wild adult fish throughout the transboundary reach from Libby Dam to Bonnington Falls (Table 1 and Table 2). Analysis of age structure indicates recruitment began to decline in the mid-1960s (Partridge 1983 cited in Duke et al. 1999, Paragamian et al. 2005), and has been negligible since 1974, the year Libby Dam became operational. Total abundance was estimated in 2000 to be 760 individuals. Beamesderfer et al. (2009) conducted revised analyses based on 1977-2008 data and estimated approximately 1,000 naturally-produced Kootenai sturgeon in the population for 2007 (Table 1 and 2). The same analysis indicated that approximately 3,000 fish were present in 1989 within both Canada and the U.S. At an estimated mortality rate of 4% per year the remaining wild population will fall below 50 fish in 2080 (Beamesderfer et al. 2009).
The U.S. Fish and Wildlife Service listed the Kootenai River white sturgeon population as an endangered species on September 6, 1994 under the authority of the Endangered Species Act of 1973 (U.S. Fish and Wildlife Service 1999). Conservation aquaculture operations by the Kootenai Tribe of Idaho (KTOI) were initiated in 1990 with funding provided by the Bonneville Power Administration. Joint biological investigations began in 1994 with the cooperation of the Idaho Department of Fish and Game, the B.C. Ministry of Environment, and KTOI (Neufeld 2006). A total of 200,274 Kootenai white sturgeon were released from 1992 through 2011 (Kootenay Tribe of Idaho 2012). Significant releases began in 1997 after the hatchery was identified as a critical component of the Recovery Plan; hatchery releases prior to 1997 were largely experimental. Annual releases have ranged from about 3,000 to 37,000 fish per year from 2003 to 2009 (average 21,000) (Kootenay Tribe of Idaho 2012). The hatchery and release program is ongoing (Matt Neufeld, B.C. Ministry of Forests, Lands and Natural Resource Operations, personal communication). Current production is approximately 15,000 per year from the combined U.S. and Canadian facilities.
Subsequently, 2,938 Passive Integrated Transponder (PIT)-tagged fish were recaptured as part of a long term monitoring program (Justice et al. 2009). Annual survival rates of marked groups ranged from 0.01 to 0.84 (mean = 0.45) during the first year, from 0.48 to 1.0 (mean = 0.84) in the second year, and averaged 1.0 during all subsequent years (Justice et al. 2009). Results to date provide strong evidence of density- and size-dependent mortality in hatchery-reared juvenile white sturgeon in the Kootenai River, and have led to recommendations for management actions that prioritize the release of fewer, larger-sized fish (Justice et al. 2009).
White sturgeon have been important to the spiritual, aesthetic and economic history of the people of British Columbia. Sturgeon have traditional cultural values to many First Nations in B.C. and these values continue to be maintained. The predominant values of white sturgeon to non-aboriginal communities have shifted from those of a natural resource commodity to those in which white sturgeon are appreciated as a species, and as a component of a healthy ecosystem. Consequently it is expected that the general public would have high non-market (existence and bequest) values for this iconic species. Other values for white sturgeon include recreational and guided angling, Aboriginal fishing, scientific inquiry, aquaculture, and ecosystem education. These values are discussed in greater detail for Fraser populations in the Fraser River White Sturgeon Conservation Plan (Fraser River White Sturgeon Working Group 2005). In addition, a grade school level education program implemented by the Fraser River Sturgeon Conservation Society and HSBC Bank of Canada has been ongoing throughout the province since 2005. Many stakeholders have been working to support white sturgeon recovery since before the species was designated as endangered. For example, the Upper Columbia White Sturgeon Recovery Initiative ( UCWSRI) has conducted a communication and public awareness program since 2000, in conjunction with the B.C. Hydro Fish and Wildlife Compensation Program – Columbia Region. One component of this program provides primary school teaching aids, and has involved primary and high school students in annual hatchery release programs since 2003. Efforts by the Nechako Community Working Group were also underway for the Nechako River population prior to its listing.
1 This 50% decline is based on the total population trend as a whole for all affected populations. Based on the COSEWIC technical document (COSEWIC 2003 and Ptolemy and Vennesland 2003) the following % declines are listed as reasons for COSEWIC designation for each population: for the lower Fraser (SP1) this decline is ≥ a 50% decline (over the last 100 years), whereas for the Middle Fraser (SP2) and Upper Fraser (SP3) populations this decline trend is unknown; the Nechako River (SP4) population decline is rated as significant but undetermined % since 1960’s and is projected to be at 83% in the next 25 years; this is similar for the upper Columbia (SP5) and Kootenay River (SP6) white sturgeon populations.
2 Although Duncan Reservoir, Slocan Lake and Revelstoke Reservoir are within the historic range of white sturgeon, recovery of populations in these relatively small waterbodies has been deemed infeasible. There is insufficient data on Kinbasket Reservoir to recommend whether or not the establishment of a self-sustaining population in that area is feasible or recommended.
3 Portions of the Kootenay River that occur within the U.S. are referred to as the “Kootenai.” The American spelling is used to refer to American portions of the river, and to the “Recovery Plan for the Kootenai River Population of the White Sturgeon (Acipenser transmontanus).” The plan was developed by the U.S. Fish and Wildlife Service, with input from Canadian agencies, and refers to white sturgeon recovery in both Canadian and American portions of the river.
4 Recruitment refers to juveniles of a particular age entering the population. Recruitment failure, as used here, refers to insufficient juvenile abundance to support a self-sustaining population. Low levels of recruitment are observed in all populations, but at levels that are insufficient to sustain the population.
5 This size criterion is somewhat arbitrary, but is assumed to be a reasonable indicator of mature adults. Males may be mature at 100 cm FL; females may not mature until 170 cm FL. It is difficult to accurately sex live white sturgeon, so a fork length of 160 cm was used to estimate combined (male and female) adult populations for each stock.
To complete their long lived life cycle, white sturgeon require suitable habitats, an abundant food base, and appropriate flows and water conditions. These needs are discussed separately in the following sections.
White sturgeon inhabit large rivers where they are often associated with slow, deep mainstem channels interspersed with zones of swift and turbulent water; extensive floodplains with sloughs and side channels; and, a snowmelt-driven hydrograph with prolonged spring floods (Coutant 2004). Most habitat use studies are recent and have been conducted on regulated rivers, particularly on the upper Columbia and Kootenay rivers. The few studies completed on the Fraser River, which is the only unregulated system in the species’ range, indicate that habitat use there may be quite different. Care must therefore be exercised when extrapolating observations and conclusions regarding sturgeon habitat and related behaviours from regulated systems, where in some cases white sturgeon are not presently successful in utilizing habitat for their life functions.
White sturgeon naturally spawn during the spring freshet. There has been a considerable amount of work done to characterize white sturgeon spawning habitats, but much of the information has come from regulated rivers (e.g., Parsley and Beckman 1994; Parsley et al. 1993; Paragamian et al. 2001; Golder Associates Ltd. 2005c). These studies indicate strict requirements for deep, swift water and coarse substrates. Mean water column velocities typically range from 0.5 to 2.5 m/sec-1 (Parsely et al. 1993).
Spawning has occurred in the Kootenai River in an area characterized by large mobile sand deposits, but this area is believed to have extremely poor egg survival, since collected eggs were coated in sand (Duke et al. 1999; Kock et al. 2006) and only one wild sturgeon larvae has ever been collected (hatched on an egg collection mat; Vaughn L. Paragamian, Idaho Dept. of Fish and Game personal communication) despite significant collection effort. Spawning was observed in 2004 in the Nechako River over substrates dominated by gravel and fines, but these conditions appear to be one of the causes of ongoing recruitment failure experienced by this population (McAdam et al. 2005).
Evidence from the unregulated lower Fraser River, where there is successful recruitment, indicates that white sturgeon use large side channels for spawning (Perrin et al. 2003) as well as more turbulent areas downstream of the Fraser canyon (RL&L 2000a). Physical characteristics of the side channels included gravel, cobble and sand substrates, and mostly laminar flows with near-bed velocities averaging 1.7 m/sec-1 (Perrin et al. 2003). Boulder and cobble substrates predominate at the mainstem study site (Perrin et al. 2003). All sites were within a portion of the lower Fraser that is unconfined and largely unaffected by floodplain development (Appendix B). Coutant (2004) noted that successful spawning is most often associated with turbulent or turbid river sections areas upstream of floodplains.
There appears to be a natural mechanism by which spawning is initiated when temperature exceeds a rough threshold. While other factors may have a secondary influence on spawn timing (e.g. flow and photoperiod – see Liebe et al. 2004) temperature appears to have a dominant effect. Threshold temperatures of 14ºC and 13ºC have been reported for the Columbia River at Waneta and the Nechako River respectively. Spawning in the Kootenai River occurs at a lower temperature (8.5-12ºC; Paragamian et al. 2001) as does spawning at the Revelstoke spawning site (10-11ºC; Golder Associates Ltd. 2009). In these two latter cases it is challenging to identify such thresholds due to historic anthropogenic changes. For example, it is possible that the fish have a biological threshold that is not reached by the current thermal regime (e.g. Revelstoke spawning site) or are uniquely adapted to a cooler thermal regime (suggested for the Kootenai population). Maximum temperatures may also be a concern, particularly at the Waneta spawning site where spawning has occurred above the 20ºC level where Wang et al. (1985) indicates that abnormal development may occur.
Incubation success is thought to be at its greatest when discharges are high and steady ( UCWSRI 2002). High velocities in egg deposition areas may exclude some predators and provide high turbidity, which may limit predator efficiency (Gadomski and Parsley 2005a). Substrate condition may also influence larval survival (Gessner et al. 2005; McAdam 2011). Recent research has indicated a preference of early yolk sac larvae for gravel sizes between 12 and 22 mm (Bennett et al. 2007; McAdam 2011).
Hiding in interstitial habitats is the predominant behaviour during this phase, and substrate quality affects survival both directly and indirectly (McAdam 2011, 2012; Boucher 2012; Crossman and Hildebrand 2012). Hiding immediately after hatching in the vicinity of the spawning location, and continuously until the initiation of exogenous feeding, should lead to the greatest survival under good habitat conditions. When substrate conditions are not optimal (e.g. embedded with fine substrates), drift may occur prior to hiding, or yolk sac larvae may be displaced. However, such movements are not considered optimal strategies for survival (McAdam 2011) due to the possibility for increased mortality. Similar to spawning and incubation phases, preferred substrates vary in grain size, but provide interstitial spaces suitable for yolk sac larvae hiding (for example, the interstitial spaces created by ¾” to 1 ½” gravel; McAdam 2011). Under natural conditions preferred substrate conditions will likely include a mixture of particle sizes ranging from gravel to large cobble.
Optimal temperatures for this phase are between 14ºC and 18ºC (Wang et al. 1985). Similar to eggs, lower temperatures delay development (Tiley 2006; Boucher 2012) and higher temperatures lead to increased deformities and mortality (Wang et al. 1985; Boucher 2012). Due to the interstitial location of this life phase, optimal water velocities (e.g. near bed velocities) would be primarily determined by their ability to maintain interstitial water quality (likely site dependant) and exclude some fish predation (e.g. velocity greater than 0.8 m/sec-1 has been suggested at the Waneta spawning site). This life phase likely prefers very low to no velocity within interstitial habitats (McAdam unpubl.).
White sturgeon early life history phases are particularly sensitive to contaminant effects and an LC50 (the concentration required to kill 50% of tested individuals) for copper (Cu) was identified at 22 ųg/mL (Vardy et al. 2013). Due to their residence within interstitial habitats, the ambient levels of contaminants may not be accurately reflected by measurements within the water column.
Habitat use by feeding larvae is quite uncertain, in part due to the rarity of this life stage in populations undergoing recruitment failure. Nocturnal drift at the beginning of this period allows dispersal to subsequent feeding habitats, however, characterisation of this process is limited by small larval sizes and sampling challenges in larger rivers. Laboratory studies suggest that feeding larvae forage over the open bottom and use less cover with increased age (Brannon et al. 1985). Important habitat attributes may include the presence of fluvial habitat downstream of spawning sites, and low velocity feeding habitats such as side channels or floodplains. The presence of white sturgeon in some areas where low velocity lateral habitats are limited (e.g. the middle Fraser River) also suggests the possibility that both feeding larvae and later stages can utilize mainstem habitat in some cases (e.g. the river bottom or margins).
Water temperature preferences of feeding larvae were characterized by Wang et al. (1985) and vary from 14ºC to 18ºC. Similar to yolk sac larvae evaluations of contaminant effects suggest white sturgeon are sensitive relative to other species (Bennett and Farrell 1998; Vardy et al. 2013; Little et al. 2012). Some contaminants have the potential for sub-lethal effects such as growth limitations (e.g. Didecyldimethylammonium chloride (DDAC); Teh et al. 2003); however, there are no confirmed occurrences of ambient contaminant levels exceeding tolerance limits. Between 19 to 30 dph toxicology studies regarding the effects of Cu, cadmium (Cd) and zinc (Zn) exposure show increased mortality (based on LC50s), at concentrations of 4.1-9.9 ųg/L, 21.4 ųg/L and 340 ųg/L, respectively (Vardy et al. 2011; Little et al. 2012). Little et al. (2012) also identified sub-lethal effects at concentrations less than 4.1 ųg/L. Notably Vardy et al. (2013) show that feeding larvae were more sensitive to Cu (LC50= 10ųg/mL at 15 dph) than yolk sac larvae. Vardy et al. (2011) suggest that ambient water quality criteria should provide sufficient protection for the metals they tested, however Little et al. (2012) suggest that some water quality thresholds may not be sufficiently conservative.
The presence of fin deformities from hatchery progeny in the Columbia River (and not other hatcheries) suggests a possible non-lethal effect of contaminants; however, this has not been confirmed.
Juvenile (less than 2 years) habitat for white sturgeon varies considerably with stage of development. In general, information is limited about natural juvenile habitat use for white sturgeon populations in B.C., with most information coming from laboratory studies or studies in other river systems. Parsley et al. (1993) defined physical habitat for juvenile white sturgeon in the lower Columbia River as 2 to 58 m depth, 0.1 to 1.2 m/sec-1 mean column velocity, and near-substrate velocity of 0.1 to 0.8 m/sec-1. While the study was conducted downstream of McNary Dam, and the upper end of this depth range rarely exists in natural rivers, their observations nevertheless suggest that juvenile white sturgeon may be found at a range of depths, but that they prefer slow to moderate water velocities.
Observations and traditional ecological knowledge regarding a number of locations within the Canadian range (e.g., Bennett et al. 2005; Failing et al. 2003) shows that juveniles are often associated with the lower reaches or confluences of tributaries, large backwaters, side channels and sloughs. Sampling of side channel and slough habitat on the Kootenay has, however, shown little use of such habitats in comparison to the mainstem (Neufeld and Spence 2002). Extensive use of deep, low velocity mainstem habitats also occurs (RL&L Environmental Services 2000a; Golder Associates Ltd. 2003b; Neufeld and Spence 2004b), especially as fish grow larger. Substrates at collection sites have varied from finer particles through to boulder and hard clay (Parsley et al. 1993; Young and Scarnecchia 2005). Feeding juveniles showed a slight preference for sand substrates, but occupied other substrates if food was present (Brannon et al. 1985). In the Kootenay system, and perhaps in other systems, there is use of lake habitat by juveniles.
Late juvenile (over 2 years) and mature adult habitat use is variable, depending on time of year and function, such as spawning, feeding, overwintering, and movements to and from these key habitats (RL&L Environmental Services 2000a; Neufeld 2005). In general, white sturgeon adults are found in deep areas, adjacent to heavy flows, defined by deposits of sand and fine gravels with backwater and eddy flow characteristics (RL&L Environmental Services 1994a, 2000a). Adults in the upper Fraser River may be widely dispersed including use of tributaries, and may require long migrations to reach feeding and spawning habitats (Yarmish and Toth 2002). Most studies of adult habitat use have focussed on the physical features of spawning habitat. Considerably less attention has been given to other adult habitat requirements including overwintering, feeding, holding habitats, or migration habitats. Large lakes and rivers, where available, are extensively used at all times of the year (e.g., RL&L Environmental Services 1999b; Golder Associates Ltd. 2006c).
Summer
During this period, which is typically July to September, the movements of white sturgeon in most populations are volitional and tend to be more localized than in the spring to early summer or fall. In the Columbia (RL&L Environmental Services 1994a; Brannon and Setter 1992) and Kootenay rivers (Apperson and Anders 1991), white sturgeon were reported to use shallower depths during the spring to summer period and exhibited frequent, short distance forays between shallow and deep-water areas to feed.
High-use areas in the upper Columbia River are generally depositional areas where food items settle out. In Kootenay Lake, adults undertake an annual migration from the south end of the lake to the outlet of the Duncan River at the north end, where large numbers of spawning kokanee provide an excellent food source (RL&L Environmental Services 1999b). In the upper Fraser, the pattern of habitat use in summer is linked to sturgeon spawning, but potentially also feeding on spawning cyprinids and the end of the period is clearly linked to the upstream migration of spawning salmon, especially sockeye.
Overwintering
Reduced activity is generally observed during winter months (e.g., RL&L Environmental Services Ltd. 2000a; Nelson et al. 2004). Individuals in all populations tend to utilize deeper, lower-velocity areas during this period. Large lakes and rivers are extensively used, where available (e.g., RL&L Environmental Services 1999b; Golder Associates Ltd. 2006c).
Migration Movements
Migration is defined in RL&L Environmental Services (2000a) as sustained, unidirectional movements, either upstream or downstream but not both, likely for feeding, spawning or overwintering. Migration patterns are being studied in many populations, but are not well understood for any of the B.C. populations. However, white sturgeon movements are volitional and may be attributed to spawning and feeding. For example, migrations have been observed during the spring/summer period and have been associated with staging, and spawning, as well as feeding activities in response to spring invertebrate hatches and spawning of other fish species. Fall movements may be associated with feeding opportunities (e.g., kokanee spawning near creek confluences). White sturgeon do not generally reproduce annually, especially females, so male and female spawning migration patterns may vary between years. The proximity between overwintering, spawning, and feeding areas is a key determinant of the extent of white sturgeon movements; however, further analysis on sturgeon movement is required.
Connectivity among habitats is necessary, since fish must be able to move freely between feeding, holding and spawning areas to complete their life cycle. At present, connectivity is maintained throughout much of the species’ range, with the exception of some habitat for the transboundary component of the Columbia River population. Connectivity is acknowledged as key for conservation planning of this species.
For the ALR component in the Columbia River, connectivity is required for the segment upstream from the Highway 1 Bridge to Big Eddy and from Big Eddy to the spawning site at the Columbia River adjacent to Revelstoke Golf Course. Connectivity is identified specifically for those locations because flow releases from REV Dam are required to ensure their connectivity. Since the addition of unit 5 turbine the minimum flow at REV Dam has gone from 8.5cm to 142cm increasing total wetted riverbed area by 37%; monitoring is ongoing to determine improvements in habitat connectivity as well as hydraulic properties within incubation and early rearing habitats (Jamie Crossman, B.C. Hydro, personal communication). For the transboundary component in the Columbia River, connectivity is also an issue. Based on preliminary genetic data, it is believed that HLK currently divides a formerly contiguous population. The limited ability to alter present connectivity levels at HLK is acknowledged; however, connectivity was likely critical prior to dam construction. At a minimum the present levels of connectivity within the transboundary reach should be maintained.
An adequate prey source is an important habitat feature for all white sturgeon populations. White sturgeon feeding behaviour is specialized for dark, benthic habitats where prey are often located through direct contact, using highly sensitive taste receptors on barbels near the mouth (Brannon et al. 1985). Juvenile white sturgeon are primarily benthic feeders, feeding on a range of invertebrate and fish species. Diet varies throughout the year and with location depending on availability. Juveniles reportedly eat a variety of aquatic insects, isopods, mysids, clams, snails, small fishes, and fish eggs (Scott and Crossman 1973; McCabe et al. 1993). In the Upper Columbia River, Mysis relicta, a non-native pelagic crustacean, is the most common prey item of 1 – 2 year old juveniles (Golder Associates Ltd. 2006a). Adults feed predominantly on fish, particularly migratory salmonids where available, although crayfish and chironomids are also consumed (Scott and Crossman 1973; Partridge 1980).
The Columbia, Kootenay, Nechako and Fraser are large rivers, and are the receiving waters for a wide variety of point and non-point source pollutant discharges over a broad geographic range. These include point source discharges from pulp mills and smelters, industrial plants, treated and untreated municipal and private sewage, and various other industrial, agricultural and urban discharges, as well as non-point sources of pollution from agriculture, forestry, and urban areas.
Aquatic species are at risk when water conditions degrade beyond specific thresholds for oxygen, temperature, pH, or pollutants. The current provincial water quality guidelines provide general direction for the protection of aquatic life and may be sufficient for describing overall requirements for white sturgeon until further studies are completed. Additional research is needed to determine if certain aspects of these water quality guidelines require lower tolerance limits for different white sturgeon life stages. Specific concerns include treated and untreated municipal and private sewage, and various other industrial and urban discharges, and non-point sources of pollution from agriculture, forestry, and urban areas. Benthic sediment contamination (e.g., metals, organochlorine compounds) in and downstream of urban and industrialized areas may also provide a pathway for uptake and accumulation of harmful contaminants by white sturgeon (Fairchild et al. 2012).
Sturgeon are susceptible in particular to chemicals that can result in bioaccumulation. Current water quality guidelines should provide adequate protection; however, significant point or non-point discharges could result in impacts to habitat and/or individuals. Toxicology studies regarding the effects of Cu, Cd and Zn exposure have shown increased mortality at concentrations of 1.5 ųg/L, 5.5 ųg/L and 112 ųg/L (Vardy et al. 2011) and U.S. studies have shown behavioural effects at lower concentrations (Little et al. 2012).
One water quality variable for which there is a better understanding is the interaction between temperature and spawn timing. There appears to be a natural mechanism by which spawning is initiated when temperature exceeds a rough threshold. While other factors may have a secondary influence on spawn timing (e.g., flow and photoperiod – see Liebe et al. 2004) temperature appears to have a dominant effect. Threshold temperatures of 14ºC and 13ºC have been reported for the Columbia River at Waneta and the Nechako River respectively. Spawning in the Kootenai River occurs at a lower temperature (8.5-12ºC; Paragamian et al. 2001) as does spawning at the Revelstoke site (10-11ºC; Golder Associates Ltd. 2009). In these two latter cases it is challenging to identify such thresholds due to historic anthropogenic changes. For example, it is possible that the fish have a biological threshold that is not reached by the current thermal regime (e.g., Revelstoke spawning site) or are uniquely adapted to a cooler thermal regime (suggested for the Kootenai population). Maximum temperatures may also be a concern, particularly at the Waneta spawning site where spawning has occurred at temperatures above 20ºC, a level that Wang et al. (1985) observed can lead to abnormal development. Unfortunately, temperature requirements, and limitations, for other life stages are not as well understood at this time.
For all rivers where white sturgeon are undergoing recruitment failure it is important to note that thermal regime alterations are not considered to be the primary cause of recruitment failure (e.g. Revelstoke – Gregory and Long 2008, McAdam 2012). The Nechako River is perhaps the best example, since thermal regime would have been affected by flow regulation for 15 years prior to the initiation of recruitment failure (1967). Ongoing monitoring under the current thermal regime indicates that both past and existing thermal regimes were/are not a primary cause of recruitment failure. However, they may have secondary effects including a delay in the timing of spawning and duration of embryo development. In addition, with warmer winters and cooler spring summer temperatures fish are metabolically somewhat more active in the winter and have less growth potential in the summer (Jamie Crossman, B.C. Hydro, personal communication).
White sturgeon are both predator and prey. During early life stages, white sturgeon are preyed on by other fishes and wildlife and thereby form part of the food base of those species. As they increase in size, and with the help of their sharp scutes, sturgeon avoid predation by all species except humans and large marine mammals, such as pinnipeds (Stansell et al. 2010). The extent to which white sturgeon play a role in limiting abundance of prey species is not known. White sturgeon are known more as an opportunistic feeder than a voracious predator, but Scott and Crossman (1973) indicated that even one year old sturgeon take live fish as prey, and white sturgeon are known to be more piscivorous than any other North American sturgeon species. Abundance of fish populations that are highly-aggregated in space and/or time, such as spawning anadromous species, or spawning congregations of cyprinids, for example, would be heavily preyed on and could be affected by white sturgeon.
The intrinsic biological factors most limiting to white sturgeon population growth are very low early survival and delayed maturation. Gross et al. (2002) used a form of population modeling called “elasticity analysis” to assess the sensitivity of sturgeon population growth to changes in age and life-stage specific survival and fecundity. The analysis indicated that population growth is most sensitive to changes in early survival rates. Changes in survival of older fish and changes in fecundity had considerably less effect on population growth. The authors stress that since white sturgeon survival during the first year is extremely low, the overall opportunity for affecting population growth is strongest in that age class because of its exceptional potential for increased survival rates. Older fish already have fairly high survival rates, so there is less potential to effect higher population growth through increases in survival of these age classes.
However, even small changes in early juvenile survival, when compounded over many years, can have substantial effects on the number of fish reaching maturity.
In the Columbia, Kootenay and Nechako rivers the cause of decline is primarily ongoing recruitment failure associated with changes in habitat, flows, and the ecological community. In each of the three rivers, regular spawning occurs, but viable offspring do not recruit to the juvenile stage in sufficient numbers to sustain the population. In addition, spawning habitat is limited and may be degraded such that it is impacting egg or larval survival and unknowns remain about the requirements of early juvenile habitat. These are key factors for addressing recruitment failure. This pattern has been well documented and there have been numerous general studies, but detailed research into the specific causes of recruitment failure is relatively recent. The research is complicated by the possibility of multiple causal factors that may differ among populations.
There are clearly many natural and anthropogenic factors influencing abundance and distribution of all life stages of white sturgeon (see Section 3: Description of Needs of the Species and Section 4: Threats). Recruitment failure is strongest in dam-affected rivers, yet reasonable recruitment rates occur in the lower Columbia River, which is also affected by multiple mainstem dams, so it is more complicated than simply the presence of dams. Gregory and Long (2008) examined recruitment failure hypotheses for the upper Columbia River population, defined key hypotheses, and identified potential programs for assessment and recovery, but there was surprisingly little agreement among experts on the primary drivers of recruitment failure in that system. More recently, greater agreement seems to be emerging, based on new research in the laboratory and in the field, across multiple basins.
The general pattern of recruitment failure points to an interaction of geomorphology, substrate conditions, flow and fish behaviour: adults spawn successfully (i.e., viable gametes are produced and eggs are fertilized), but spawning occurs in areas in which substrates are inappropriate for egg incubation and survival and development of yolk sac larvae. In the Kootenay River, adults spawn in a restricted area where high levels of sand substrate lead to essentially zero survival (e.g., Paragamian et al. 2001, Paragamian et al. 2002, Paragamian et al. 2005). For the Nechako River population, substrate conditions at the spawning site diminish both egg and yolk sac larvae survival (McAdam et al. 2005, McAdam 2011, McAdam 2012). Additional layers of complexity seem to be part of the recruitment failure pattern, including high levels of fidelity (see Forsythe et al. 2012 for related work on lake sturgeon) for sites that have suitable water velocities and depths across a range of flows (Paragamian et al. 2009, McDonald et al. 2010), little or no direct preference for substrate type and condition, and larval behaviour, growth and survival that are highly dependent on substrate conditions (McAdam 2011, Boucher 2012, McAdam 2012). Further limitations likely exist, such as water temperature (Boucher 2012), predation (Gadomski and Parsley 2005a, b) and other factors (Gregory and Long 2008), which may interact with the primary drivers of recruitment failure. Understanding the causal factors related to recruitment failure is further complicated by biological characteristics of white sturgeon including very low early life stage survival and late maturation; efforts to resolve recruitment failure through habitat restoration must take these considerations into account.
Adequate assessment of threats to white sturgeon in Canada not only requires identification of those threats, but also understanding the relative importance of different threats. It is also necessary to assess the supporting evidence for each threat and the populations to which they apply. Threats differ among the white sturgeon populations and are analysed and discussed in detail elsewhere: threats to lower, middle, and upper Fraser River populations are discussed in Hatfield et al. (2004); threats to Nechako River white sturgeon are presented in Nechako White Sturgeon Recovery Initiative (2004); threats to the Kootenay River population are found in U.S. Fish and Wildlife Service (1999); and, threats to Columbia River white sturgeon can be found in UCWSRI 2002), Gregory and Long (2008) and McAdam (McAdam 2012).
The following discussion includes primary threats that historically may have caused a population decline (e.g., demonstrated by recruitment failure) and may have ongoing effects as well as threats that may presently cause declines or limit recovery. The risk assigned to each threat for white sturgeon include ratings of negligible, low, moderate, high, and unknown, as defined in Table 3.
Table 3. Definition for levels of relative risk to the viability for the white sturgeon population and its recovery.
Negligible: Threat has no detectable effect on the population or does not occur at this time.
Low: Threat produces measurable habitat, behavioural and/or physiological effects but does not affect population viability and recovery potential.
Moderate: Threat reduces habitat suitability, produces chronic behavioural effects and/or promotes physiological changes, which reduce population viability and recovery potential.
High: Threat results in habitat destruction and/or lethal effects that produce a severe, continuous and near-term effect on population viability and recovery potential.
Unknown: Available information is insufficient to gauge the degree to which the threat may affect the population viability and recovery potential.
Threats to each white sturgeon population are summarized in Table 4 and discussed briefly in the following sections. Threats are plausible mechanisms, caused by human activities, which influence abundance, distribution, and health of white sturgeon. Much of this information is based on expert opinion as knowledge of many of these threats is limited. Different expert groups have assessed threats for each population as part of watershed-based conservation planning processes, and Technical Working Group (TWG) members for each population were involved in these threat assessments. Detailed hypotheses and population level impacts will be addressed as further information on the specific nature of impacts is developed.
The threats are grouped as abiotic or biotic, and ordered within each of these groups by threat severity. Severities were assigned based on related laboratory or field evidence, correlations between historic ecosystem changes and observed changes in population structure, or the strength of underlying components of the logic pathway for each hypothesis (Table 4). Not all threats are equally valid for each population, and there are differing degrees of support for each stressor and its ability to influence the abundance, distribution, and health of each population of white sturgeon. Also, there may not be one single threat that caused population declines, but several threats may have cumulatively affected white sturgeon.
Threat rankings, presented in Table 4, are not directly comparable across populations. Table 4 also introduces the term “dam-affected system” to describe the state of the river basin. Dam-affected system is a generic way of describing the Columbia, Kootenay, and Nechako river systems, which are regulated by dams. Other anthropogenic factors are also present in these watersheds, particularly the Columbia and Kootenay rivers. River regulation is not necessarily the sole cause of recruitment failure; however, recruitment failure consistently occurs in systems with significant flow regulation.
Overall, the primary human activities that threaten white sturgeon in the wild are direct habitat loss, river regulation, harvest of prey/food, introduction of invasive non-native fish species, direct and indirect harvest, and release of pollutants (Table 4). However, addressing conservation needs for sturgeon will require more than simply limiting or prohibiting these activities. For example, at this time it is not feasible to remove large dams or flood control dikes to reclaim lost habitats. It may be necessary to understand the underlying mechanisms that control sturgeon abundance and distribution and use this information to develop acceptable strategies for protecting and restoring sturgeon populations. Further information about each threat presented in Table 4 is provided below. The National Recovery Team has also prioritized research and management activities needed to meet population and distribution objectives across all watersheds and these priorities are presented in Section 7.5 (Research and Management Activities Needed to Meet Population and Distribution Objectives).
Table 4. Table 4 describes historic and ongoing threats to white sturgeon and their habitats. Definitions for the levels of relative risk are provided in Table 3 and include the following categories: negligible, low, moderate (mod), high, and unknown. These are not comparable across populations. The table has two main columns: Potential Threat to Species and Habitat, and Level of Relative Risk. Under the “Potential Threat to Species and habitat” header are two sub-categories: Stressor and Activity. Under the “Level of Relative Risk” header are six sub-categories as follows: Lower Fraser, Mid Fraser, Upper Fraser, Nechako, Columbia and Kootenay; these last three sub-categories are indicated as Dam Affected Systems. Below the column headings there are seven abiotic and five biotic threats, each with its own dedicated row. As the table is read from left to right each threat is described and ranked as low, moderate or high impact for each population respectively.
| Potential Threat to Species or Habitat | Level of Relative Risk | ||||||
|---|---|---|---|---|---|---|---|
| Dam Affected System | |||||||
| Stressor | Activity | Lower Fraser | Mid Fraser | Upper Fraser | Nechako | Columbia | Kootenay |
| Abiotic | |||||||
| Loss of habitat quality and quantity1 | Habitat changes are associated with flow regulation (e.g. affecting geomorphology, depth, velocity, substrate), as well as gravel and sand extraction; upland, foreshore, floodplain and estuary use and development, including bank protection, dyking and infilling, and other in-channel works. | high | mod | mod | high | high | high |
| Habitat fragmentation | Habitat fragmentation occurs where there are impassable dams and/or dykes (e.g., Kootenay River) and through inadequate flows or water level changes. | low | low | low | low | high | low-mod |
| Altered hydrograph components | Altered hydrograph components may be related to flow regulation, flow diversion, and anthropogenic activities causing climate change. | low | low | low | high | high | high |
| Pollution | Pollutant sources include industrial inputs (pulp mill effluents, various wastewater, and smelting effluents), municipal and domestic sanitary and storm sewage, non-point source urban runoff, point source agricultural discharges and chemical over-sprays, and non-point source agricultural runoff. | mod | mod | low | low | mod | low |
| Fishing and industrial effects (direct and indirect) | Fishing effects are related to poaching (illegal retention), recreational catch-and-release fishery, scientific inquiry and monitoring, aboriginal and commercial net fisheries, and by-catch in the aboriginal and recreational fisheries. Industrial effects include interactions with industrial facilities or operations, including equipment at hydro-electric facilities (turbines, draft tubes, locks), | high | mod | low | mod | mod | low |
| Reduced turbidity | Reduced turbidity may be related to flow regulation and stream channelization, which can influence water clarity | low | low | low | low | mod | low |
| Altered thermal regime | Thermal regimes are affected by flow regulation and anthropogenic activities causing climate change. | low | low | low | low | mod | mod |
| Biotic | |||||||
| Effects of small population size | Anthropogenic factors causing recruitment failure. | low | mod | high | high | low | high |
| Hatchery and aquaculture effects on health and population | These effects may occur from conservation aquaculture and commercial aquaculture. | low | mod | mod | mod | low | low |
| Reduced or altered food supply (including fishing of white sturgeon prey base) | Food supply is affected by commercial, Aboriginal, and recreational fishing, upland, foreshore, floodplain and estuary development, dams (fragmentation and hydrograph changes) and anthropogenic activities causing climate change. | mod | high | high | high | mod | mod |
| Change in ecological community (predation/competition) | Ecological community composition can be affected by flow regulation, species introductions and movements, fishing effects, habitat alteration, and anthropogenic activities causing climate change. | mod | low | low | low | high | low-mod |
| Disease | Disease rates can be affected by aquaculture, thermal regime changes (e.g., anthropogenic activities causing climate change, river regulation), introduction of pathogens, and introduction of pollutant stressors. | low | low | low | low | low | low |
Description -- The large rivers occupied by white sturgeon have a variety of interlinked habitats, including main channel, tributary confluence, foreshore, seasonally-inundated, and tidal and estuarine areas. Both habitat quality and quantity have declined throughout the species’ range, particularly in the regulated systems and the lower Fraser. Changes to white sturgeon habitat or to the habitats of prey species are believed to be directly related to impacts on recruitment and overall carrying capacity.
Potential influences -- Habitat changes are associated with flow regulation (e.g., geomorphology, depth, velocity, substrate), as well as gravel and sand extraction, upland, foreshore, floodplain and estuary use and development, including bank protection, dyking and infilling, and other in-channel works.
Assessment and level of confidence -- This is a very broad category and it is difficult to tease out the effects of quality from quantity. White sturgeon abundance and distribution is believed to be related to the availability of suitable habitat. Habitat use by white sturgeon is best known in the regulated watersheds, and least known in the upper and middle Fraser River. Changes in habitat associated with flow regulation are clearly implicated in the recruitment failure of the Nechako, Columbia, and Kootenay river populations. However, less is known about how other physical habitat modifications might have affected, or might now constrain the abundance and distribution of white sturgeon.
Sturgeon habitat can be directly impacted by river regulation in two important ways: i) changes in abundance and distribution of hydraulically suitable habitat; and, ii) geomorphic changes to instream habitats. If the regulated regime is sufficiently different from the natural flow regime then there is a potential for reduced function or loss of key spawning, incubation and rearing areas. Predation impacts may also be exacerbated due to increased water clarity below dams. In regulated rivers, fine sediments may build up both in the river channel and within reservoirs, and fine sediments have been shown to directly reduce embryo survival (Kock et al. 2006). The effects of fine sediment accumulation at spawning sites has now been identified as a likely mechanism for all three dam-affected populations (McAdam et al. 2005, Paragamian et al. 2009, McAdam 2012). However, this mechanism is yet to be proven by successfully restoring recruitment.
Upland, foreshore, floodplain and estuary use and development, floodplain and estuary removal through dyking, and instream modifications have led to habitat changes in most of the systems and are implicated in overall reductions in carrying capacity. Many of the foreshore and instream impacts continue to occur, but most large scale floodplain and estuary changes occurred several decades ago.
In both the upper and middle Fraser River the primary limiting factor appears to be habitat carrying capacity. River gradient is higher and the confined canyon means habitat is spatially limited. Prey species populations like salmon have also been reduced from historic levels and overall biological productivity is lower.
Description -- Dam and reservoir construction has had a large influence on the distribution of aquatic habitat within the natural range of white sturgeon (Figure 2 and Figure 3 ). Formerly continuous habitats were split into smaller portions by impassable dams. Usable habitat has become permanently alienated, and dams have subdivided continuous populations. Habitat fragmentation is most pronounced in the Columbia and Kootenay rivers, although other systems are also affected.
Potential influences -- Habitat fragmentation occurs where there are impassable dams and/or dykes (e.g., Kootenay River) and through inadequate flows or water level changes.
Assessment and level of confidence -- In the upper Columbia River, white sturgeon are fragmented by impassable dams at Mica, REV and HLK. Habitat above Mica (now Kinbasket Reservoir) is no longer accessible, but was used historically (Prince 2001). Anecdotal and other evidence indicates white sturgeon may still occur upstream of REV and Mica (Prince 2001). HLK divides white sturgeon in the ALR from the transboundary population component; although the lock at the dam is used at least on a limited basis for passage, it is unknown whether it is directly used by white sturgeon to access upstream areas. However, white sturgeon have been observed in draft tubes at HLK dam. In the Kootenay system, Duncan Dam has alienated habitat in the Duncan River and a small number of white sturgeon are known to reside in Duncan Reservoir, but there is no viable spawning habitat in that area. Multiple dams on the Kootenay River downstream of Kootenay Lake are impassable, but most have been integrated with natural water falls located near Bonnington (i.e., South Slocan, upper Bonnington and lower Bonnington dams) and the level of fragmentation has not increased with the addition of these facilities. However, Brilliant and Corra Linn Dams in the lower Kootenay River were not constructed on natural water falls. In the Kootenai River upstream of Kootenay Lake, Libby Dam is located upstream of Kootenai Falls (Idaho), the suspected historic upstream limit of white sturgeon.
In the Fraser watershed, Seton Dam precludes use of Seton Lake, which was used historically by white sturgeon. In contrast, the Kenney Dam on the Nechako River does not fragment historic white sturgeon habitat. In the lower Fraser River a significant amount of floodplain and estuary habitat that was available historically is now isolated from the mainstem by dykes and is currently unavailable for use by sturgeon.
Description -- The life history of white sturgeon is closely linked to river hydrology. White sturgeon are endangered in the Columbia, Kootenay, and Nechako rivers where the flows are highly regulated; additionally the Nechako River is subject to substantial out of basin diversion. The precise mechanisms responsible for population decline and recruitment failure are still unproven, but river regulation is heavily implicated. This hypothesis is not meant to address geomorphic-related impacts, which are discussed under Loss of Habitat Quality and Quantity.
Potential influences -- Altered hydrograph components may be related to flow regulation, flow diversion, and anthropogenic activities causing climate change.
Assessment and level of confidence -- Sturgeon are adapted to the natural hydrograph, having persisted successfully in large rivers for millennia. This impact hypothesis is built around the idea that sturgeon require a natural hydrograph, with seasonal fluctuations in river flow, particularly a pronounced spring freshet with bed mobilizing flows. The importance of winter flows has been considered by the Nechako River White Sturgeon Recovery Initiative (NWSRI) and the Kootenay River White Sturgeon Recovery Team (KRWSRT), but has received less attention elsewhere. In some cases, there is attention paid to flow-related microhabitat conditions in the regulated systems such as immediately below REV, but in general more attention has been paid to effects of flow regulation on substrate conditions, turbidity, and geomorphic influences on habitat.It is important to note that hydrographs may also affect lake and reservoir levels, with potentially significant impacts on white sturgeon. For example, Kootenay Lake water elevations in combination with Libby Dam operations influence sediment transport and water depth in spawning habitat on the Kootenai River (upstream in Idaho). Ongoing work in Washington State (Lake Roosevelt) is assessing the relationship between decreased flows and access to feeding habitats (by drifting larvae).
This impact hypothesis is not believed to be relevant at present to white sturgeon in the lower, middle, and upper Fraser River, due to the absence of mainstem dams, though the increasing demands for hydroelectric power and anthropogenic activities causing climate change may affect the hydrograph in the future and thereby affect localized areas.
Description -- The large rivers in which white sturgeon reside are the receiving waters for a wide variety of point and non-point source pollutant discharges, which are introduced over a very broad geographic scale, especially in the more urbanized or industrialized areas such as the densely populated Lower Mainland. This concern is important because white sturgeon are more sensitive to contaminants than other species (Bennett and Farrell 1998, Vardy et al. 2011). Some point and non-point discharges, like antisapstain chemicals (Bennett and Farrell 1998), industrial effluents (Bruno 2004) and metals (Vardy et al. 2011, Little et al. 2012, Vardy et al. 2013) are known to be acutely toxic to juvenile white sturgeon. Waterborne toxins and pollutants in sediments may both present risks (Vardy et al. 2011, Fairchild et al. 2012). This threat refers to inputs of both liquid and solid waste discharges to rivers.
Potential influences -- Pollutant sources include industrial inputs (pulp mill effluents, various wastewater, and smelting effluents), municipal and domestic sanitary and storm sewage, non-point source urban runoff, point source agricultural discharges and chemical over-sprays, and non-point source agricultural runoff.
Assessment and level of confidence -- Laboratory studies assessing pollution impacts to white sturgeon have not been confirmed or disproven by field studies to date (Bruno 2004). Additionally, despite the sensitivity of white sturgeon, current water quality criteria apparently provide sufficient protection against metals like Cu, Pb and Zn (Vardy et al. 2011, Vardy et al. 2013). Point source discharges include pulp mill effluent, municipal and private sewage, and other industrial and agricultural effluents. Non-point sources include industrial, urban and agricultural runoff. Because white sturgeon are long lived and large in size they are susceptible to not only the direct effects of pollutants, but also to bioaccumulation of toxins in tissue and organs. Sturgeon in the Fraser River system that consume marine-derived foods such as salmon and eulachon may be less susceptible to local pollutants that could bioaccumulate through prey items, but may be more susceptible to global marine-derived pollutants. White sturgeon that depend solely on locally-derived food sources such as benthic invertebrates and resident fish may be more susceptible to bioaccumulation of “local” pollutants. Contaminant loads likely differ within and among populations depending in part on proximity to pollutant sources.
Description -- Drift by white sturgeon larvae may expose them to predation, which may decrease when water is turbid (e.g., during freshet). Regulated systems tend to have reduced turbidity due to sediment settlement in reservoirs and the diminished erosive potential of lower peak flows. Since predators of sturgeon larvae are primarily visual hunters, increased water clarity in regulated systems may allow for a higher predation rate on early juveniles (i.e., less than 1 year old).
Potential influences -- Reduced turbidity may be related to flow regulation and stream channelization, which can influence water clarity.
Assessment and level of confidence -- Laboratory tests indicate that predation rates decreased when turbidities increased above 60 NTU, but showed no statistically discernible effects at lower turbidity levels (Gadomski and Parsley 2005a). Natural turbidity levels associated with successful sturgeon spawning and recruitment were 6 to 92 NTU in the lower Fraser with an average during the spawning period of 42 NTU (Perrin et al. 2000). In the Columbia River below HLK, turbidities have been observed to range from 1 to 3 NTU (Upper Columbia White Sturgeon Recovery Initiative 2002), whereas limited pre-regulation data are only slightly higher (Steve McAdam, B.C. Ministry of Environment, personal communication).
This hypothesis has received the most attention in the Columbia River, and has some limited support from the Nechako and Kootenay Recovery Teams. The impact hypothesis is not considered to be currently relevant in any of the Fraser River populations.
Description -- White sturgeon (adults and late juveniles) are caught both intentionally and incidentally by fisheries that use a range of capture methods and gear, with effects varying by timing and gear type. White sturgeon also occasionally interact with industrial sites, including hydro-electric facilities, which can cause negative effects such as harm or mortality.
Potential influences -- Fishing effects are related to poaching (illegal retention), recreational catch-and-release fishery, scientific inquiry and monitoring, aboriginal and commercial net fisheries, and by-catch in the aboriginal and recreational fisheries. Industrial effects are related to interactions with hydro-electric facilities (e.g. turbines and draft tubes) and other industrial sites.
Assessment and level of confidence -- Late juveniles and adult white sturgeon have relatively high catchability, highlighting the potential for this impact mechanism. Multiple recaptures from many years of set line fishing for research purposes indicate this technique appears to cause little mortality, if set-lines are well-monitored. Information for size classes less than 140 cm also suggests that mortality due to catch-and-release fishing appears to be low (Robichaud et al. 2006). The same gear comparison study indicated that set salmon gillnet by-catch has a more notable mortality effect, than by-catch from drift gill nets or from catch-and-release angling. Effects on reproductive fitness from catch-and-release fisheries are unknown. The DFO Recovery Potential Assessment for White Sturgeon (Wood et al. 2007) indicated that the estimated level of by-catch in 2006 would not prevent recovery if natural recruitment were restored to historic levels . However, as juvenile populations increase from conservation aquaculture efforts, particularly in the Columbia, Kootenay and Nechako rivers, by-catch of these additional juveniles from angling or other capture methods is becoming more common.
Recent information indicates that incidental capture of juvenile white sturgeon might occasionally cause injury or mortality. Anecdotal information from provincial Conservation Officers and regional biologists suggest that juvenile (and potentially adult) by-catch in the Columbia River walleye (Stizostedion vitreum) and rainbow trout (Oncorhynchus mykiss) fishery (transboundary population component) is noteworthy, though it is unquantified at present. Recent autopsies of juvenile white sturgeon from the Columbia River suggest angling has caused mortality, but again, the frequency of deaths from angling is uncertain, and the impact on population viability is uncertain. Efforts to quantify mortality from recreational by-catch of juvenile white sturgeon will be necessary to determine the level of threat this poses to the Columbia River population. Further study of this issue as a whole is required to determine fishing effects on juveniles.
Directed harvest of white sturgeon is prohibited at present, not only for the SARA-listed populations, but the non-SARA-listed populations as well. The release of white sturgeon by-catch in recreational and commercial fisheries is mandatory and strictly regulated. The same is true for First Nations salmon gillnet fisheries, though in recent years First Nations have been known to retain white sturgeon caught as by-catch if they are found dead in their gillnets. Efforts to reduce or eliminate white sturgeon encounters in these FSC fisheries are underway. In the lower Fraser there is anecdotal information that illegal retention of sturgeon is increasing but the level of this impact is not well known. Studies of delayed mortality associated with net interception and angling in the lower Fraser River suggest that sturgeon captured in both set and drifted gill nets may be subjected to considerable levels of mortality (Robichaud et al. 2006). Mitigation of fishing impacts is presently being pursued on the Nechako River, as evidence indicates continuing pressure from set lines, gillnets, and rod and reel gear.
Ongoing industrial activities on the river are known to occasionally result in impacts to sturgeon and work is ongoing to mitigate those impacts. Several white sturgeon mortalities have been noted on the Kootenay and Columbia River that are thought to be associated with dams, likely through attempted upstream or downstream passage (Wood et al., 2007). More research is needed to understand the specific causal mechanism in some cases (Wood et al., 2007), but interaction with turbines, draft tubes, and locks may lead to negative effects. Several mortalities in and around Columbia River and Kootenay River dams and other industrial sites have been reported to DFO in the past several years, and mitigation efforts for industrial activities continue.
Description -- White sturgeon metabolic rates are directly related to water temperature, so changes to temperature may affect multiple aspects of sturgeon biology. Temperature has also been implicated in terminating pre-spawning behaviour at certain locations (e.g., Paragamian and Kruse 2001). Increased winter temperatures along with higher flows may impact winter survival, although this has not been investigated yet.
Potential influences -- Thermal regimes are affected by flow regulation and anthropogenic activities causing climate change.
Assessment and level of confidence -- Increased winter temperature can increase energy demands during periods of low food abundance. Water temperature has been identified as an important spawning cue (Golder Associates Ltd. 2005a, Triton 2009), and altered temperatures during the spawning period have been associated with shifts in the timing of spawning (Tiley 2006). High temperatures have occasionally exceeded egg viability thresholds (e.g., at Waneta spawning site), and high temperatures were implicated as a potential cause in lower Fraser River adult white sturgeon die-offs in the mid-1990s (McAdam 1995). However, McAdam (2001) suggested that summer temperature changes below REV may not have been substantial, whereas in the fall, water temperatures below REV may actually be warmer than historic due to thermal retention. Further research on the effects of changes to the thermal regime on larval and juvenile stages is currently underway.
Water temperatures in the Nechako, Columbia and Kootenay rivers have been affected by river regulation. Thermal inertia in reservoirs and other large bodies of water can act to slow downstream river warming in the spring, alter peak summer temperatures (either up or down), and delay or reduce winter cooling (Hamblin and McAdam 2003). Scientific data also clearly indicate that the climate is changing and animal and plant distributions are responding to these changes (Parmesan and Yohe 2003). River temperatures have changed in response to global climate change and this trend is expected to continue (Morrison et al. 2002). Impacts from climate change may exacerbate other impacts to white sturgeon and their prey, including either competition or predation from invasive, non-native fish species. The direct and indirect response of white sturgeon populations to climate change is of concern. However, these changes are beyond the scope of this strategy.
For all rivers where white sturgeon are undergoing recruitment failure it is important to note that thermal regime alterations are not considered the primary cause of recruitment failure. The Nechako River is perhaps the best example, since thermal regime would have been affected by flow regulation for 15 years prior to the initiation of recruitment failure (1967). Ongoing monitoring under the current thermal regime also indicates that both past and existing thermal regimes were/are not a primary cause of recruitment failure. However, an altered thermal regime may nevertheless limit recovery of the species, with the best example being the effects of temperature at the Revelstoke spawning site (Tiley 2006).
Description -- Small populations face substantial risks to their long-term viability, even when habitat and food resources are not limiting. For example, small populations are more susceptible to random demographic and environmental variability, and mortality can begin to increase as the population declines below a specific abundance threshold (Allee effect), due to changes in predation or mating success. Genetic effects also become significant, leading to further reductions in survival through inbreeding depression and a loss of genetic variance that compromises adaptability to future conditions.
Potential influences -- Small population size may occur from historic influences (e.g., food supply, habitat availability, water quality, disease, etc.), or anthropogenic factors causing recruitment failure.
Assessment and level of confidence -- The general effects of small population size are well-known and well-supported in the scientific literature through empirical and theoretical studies. The extent to which specific risks apply to white sturgeon has not been well-studied, but several of the populations are below general guidelines for long-term viability, and some are below guidelines for short- to medium-term viability. The ratings in Table 4 are based on knowledge of current population size, and do not include consideration of past and potential current isolation between components of these populations.
Description -- Hatchery effects are well-known for salmon and other species with captive breeding programs. There are specific risks to naturally-reproducing white sturgeon from conservation and commercial aquaculture programs, including population effects, genetic effects, and disease transfer. There is also concern regarding imprinting behaviour and whether hatchery-reared fish will be able to find or select suitable spawning locations at maturity.
Potential influences -- These effects may occur from conservation aquaculture and commercial aquaculture.
Assessment and level of confidence -- Specific risks to white sturgeon include displacement of wild fish by those of hatchery origin, loss of genetic integrity of the wild population, accidental release of a large number of closely-related individuals, and wild population reduction from wild broodstock capture. Many of these effects are well-known from the scientific literature. The populations at greatest risk are those with proposed or ongoing supplementation programs, since migration among populations is likely very low. However, downstream or adjacent (e.g., Upper Fraser) populations could also be put at risk. The proposed or ongoing conservation fish culture programs have been designed around breeding plans (Kootenai Tribe of Idaho 2004, Nechako White Sturgeon Recovery Initiative 2005), so the risk of unintended effects is deemed to be low to moderate (Table 4), and is considered a net-benefit when compared to the unacceptably high risk of population extirpation in the absence of these programs. Current risks from commercial aquaculture are low, due to the low intensity of upland contained commercial operations and the absence of these operations from marine waters or the floodplain of sturgeon-bearing rivers. These risks could increase if sturgeon aquaculture intensifies or if rearing methods change, though commercial aquaculture is not permitted for the SARA-listed populations. Impacts related to this hypothesis have not been observed, and observation of such effects would likely increase the assessed threat ranking.
Description -- The elimination, reduction or alteration of white sturgeon prey base may have important effects on white sturgeon abundance and distribution.
Potential influences -- Food supply is affected by commercial, Aboriginal, and recreational fishing, upland, foreshore, floodplain and estuary development, dams (fragmentation and hydrograph changes) and anthropogenic activities causing climate change.
Assessment and level of confidence -- Anadromous species including smelt, eulachon and salmon, are an important part of the food base for Fraser and Nechako white sturgeon, and anadromous salmon were formerly an important part of the prey base for upper Columbia white sturgeon. In Pacific Canadian waters, salmon are harvested in commercial, Aboriginal, and recreational fisheries, smelt are harvested in limited commercial and recreational fisheries, eulachon are harvested in Aboriginal fisheries. White sturgeon are acknowledged as predators of these species, but currently there are no explicit management actions in Canadian smelt, salmon or eulachon fisheries related to these species’ roles as a critical food source for white sturgeon. The construction of Grand Coulee Dam (Washington, U.S.) eliminated anadromous salmon runs to the upper Columbia River. Changing nutrient regimes may also cause diet shifts or impact prey species. For example, the introduction of Mysis relicta has provided an additional food source for juvenile white sturgeon in the Columbia River. Land use and development changes in some systems continue to have a large impact on foreshore, floodplain and estuarine habitats that provided substantial food inputs. Identification of critical habitat for each population in areas where sturgeon are known to feed may help in protecting these habitats not only for sturgeon, but for the prey species critical to their survival (see Section 8: Critical Habitat). The extent to which changes in food supply have affected or continue to influence white sturgeon abundance has not been quantified, but diminished food production has likely had some impact. The extent to which each of the white sturgeon populations are currently food limited is not known.
Description -- Increased predator and/or competitor abundance may be an important threat to white sturgeon, especially in the Columbia, Kootenay and lower Fraser rivers. This could include shifts in native fauna and/or introduction of non-native species.
Potential influences -- Ecological community composition can be affected by flow regulation, species introductions and movements, fishing effects, habitat alteration, and anthropogenic activities causing climate change.
Assessment and level of confidence -- The ecological community has changed dramatically in most basins due to habitat alterations (e.g., hydroelectric dams, channelization) and non-native species introductions. Introduced species may be able to take advantage of habitat alterations and out-compete or prey on the native fish community. Non-native species may increase predation on white sturgeon eggs and larvae or out-compete early juveniles for food. Non-native species introductions include walleye and Mysis relicta into the Columbia River and increased native or non-native cyprinid and centrarchid species in the Nechako and Fraser river systems.
After deliberate introduction into the Columbia River system in the U.S., non-native walleye have dispersed into the upper Columbia River and some of its tributaries. Predation of early juveniles and eggs by walleye has been a suggested cause of recruitment failure of white sturgeon in the Columbia River. Other community changes in the Columbia River include the loss of anadromous fish populations, increases in the rainbow trout population, and introductions of warm-water species such as perch, pumpkinseed, crappie, bass, etc. Anecdotal information indicates that an increase in cyprinid species has been observed in the Nechako River and may be related to white sturgeon decline. Non-native fishes such as largemouth bass, black crappie and carp have spread and increased significantly over the last decade in the lower and mid-Fraser River through range extension and continued unauthorized introductions (Tovey et al. 2008; Erin Stoddard, B.C. Ministry of Forests, Lands and Natural Resource Operations, personal communication). The cyprinid population in the lower Fraser is also significant and appears to be changing.
The plausibility of this hypothesis was initially rated as high for the Nechako River (Korman and Walters 2001), but support for it has not been maintained by the Nechako Recovery Team, in part because the hypothesis does not explain the rapidity of white sturgeon decline. Predators such as walleye are a substantial concern in the lower Columbia River, but effects are mostly unstudied. If potential impacts are proven in any area, impact rankings would likely increase. If valid, this impact may be one of several causes of declines in early juvenile survival.
Description -- Several parasites and diseases of white sturgeon are known to be present in British Columbia, and additional pathogens may be introduced. For example, the white sturgeon papova-like virus was identified from one wild, subyearling Columbia River white sturgeon (Canadian Columbia River Inter-Tribal Fisheries Commission 2005). No obvious external signs were noted, but microscopic lesions were detected in the gill, liver, spleen and kidney (Canadian Columbia River Inter-Tribal Fisheries Commission 2005). Also, five parasites have been documented in the Columbia River including three trematodes (Nitzschia quadritestes, Tubulovesicula lindbergi, Cestrahelmins rivularis), a cestode (Amphilina bipunctata), and a nematode (Cystoopsis acipenseri) (Canadian Columbia River Inter-Tribal Fisheries Commission 2005). There was a recent occurrence at the Kootenay Trout Hatchery of the cnidarian parasite Polypodium hydriforme (Ron Ek, Kootenay Trout Hatchery, personal communication), which is known to infect the ovaries and eggs of several species of sturgeon and similar fishes (Raikova 2002). Diseased juvenile sturgeon are regularly encountered in the lower Fraser, but the causes of disease have not been diagnosed (Erin Stoddard, B.C. Ministry of Forests, Lands and Natural Resource Operations, personal communication).
Potential influences -- Disease rates can be affected by aquaculture, thermal regime changes (e.g., anthropogenic activities causing climate change, river regulation), introduction of pathogens, and introduction of pollutant stressors.
Assessment and level of confidence -- The persistence of white sturgeon demonstrates their ability to co-exist with naturally-occurring diseases and parasites. However, risks of disease outbreak would increase under stressful conditions (e.g., increased temperature, high pollutant concentration). Risks associated with introduced diseases and their vectors or mechanisms of spread are unknown. The risks of disease are generally not well-defined at this time. The movement of large numbers of fish between watersheds (e.g., release from hatcheries) is also a potential threat, but is amenable to control. Disease transfer may also occur from hatchery and aquaculture facilities. Introduced species may also be a vector for diseases, but the risks associated with this are unknown.
White sturgeon habitat has declined in both quality and quantity throughout the species’ range in Canada. The primary influences on habitat are river regulation, discharge of pollutants, and foreshore, floodplain and estuarine development. The extent and magnitude of these influences vary among geographic locations. River regulation from hydroelectric developments has had a large influence on habitat in the Columbia, Kootenay and Nechako systems. The effects of hydroelectric dams and developments may include creation of migration barriers, changes to water quality (turbidity, nutrient status, Total Gas Pressure (TGP), etc.), streamflow patterns, water temperature, and physically suitable habitat. These factors can also alter the ecological communities that reside in these systems. Floodplain isolation and development has altered habitats in the lower Fraser and Kootenay rivers, and to a lesser extent in other systems. Estuary development has altered estuarine habitats that are used by lower Fraser River white sturgeon. Pollutant discharges and spills occur on all systems but are most notable in the highly populated areas of the lower Fraser River (where there is considerable urban, agricultural and industrial use and development) and on the upper Columbia and Kootenay rivers (with a long history of mining, smelting and pulp mill operations). Below is a discussion of general habitat trends, focusing on alterations that may impact white sturgeon for each geographic region.
Overall, numerous anthropogenic changes have occurred in this system, particularly in the lower Fraser River with its large urban population, but few linkages exist to demonstrate direct impacts from these changes to white sturgeon distribution, abundance or biology. In the middle and upper Fraser River there have been relatively few changes in physical habitat, although some impacts to water quality have occurred from industrial and municipal effluent discharges (e.g., pulp mills).
White sturgeon populations in the Fraser River are the only populations still experiencing relatively natural hydrographs. The Fraser River mainstem does not have any hydroelectric developments, thus these populations are not considered to be directly affected by dams. However, dams exist on tributary systems (e.g., Nechako, Bridge, Seton, Stave, Coquitlam, and Allouette rivers). While flow regulation is believed to have had only a minor influence on overall flows in the Fraser River (Hatfield and Long 2004), localized habitat changes may occur in the vicinity of some facilities (Erin Stoddard, B.C. Ministry of Forests, Lands and Natural Resource Operations, personal communication). Tributary impoundments can affect downstream food/prey availability, and since they are all salmon spawning streams they have the potential to affect salmon abundance. White sturgeon rely heavily on returning spawning salmon for food.
Historically, significant flooding occurred annually in the braided channels at the Fraser-Nechako confluence near Prince George. These flooded reaches were likely used by Nechako and upper Fraser River white sturgeon. Extensive seasonal flooding also occurred throughout the lower Fraser River, where vast braided reaches and enormous areas of connected swamp and marsh occurred (North and Teversham 1984, Perry 1984, Boyle et al. 1997). However,, most of the floodplain and estuary areas in the lower Fraser River were dyked and drained in the 1940s for agriculture and urban development (e.g., Sumas Lake), which affected white sturgeon habitat areas. There are currently over 600 km of dykes that isolate braided channels and floodplain areas in the lower Fraser River (B.C. Ministry of Water, Land and Air Protection 2002). Hardened bank protection, dredging, gravel mining, land reclamation, and channelization activities occurred historically and continues in the lower Fraser River (Lane and Rosenau 1995, RL&L Environmental Services Ltd. 2000a, Rosenau and Angelo 2000, Rosenau and Angelo 2005). In contrast, the limited floodplain areas in the middle Fraser River are primarily intact. Dyking and floodplain drainage has not been widespread in these reaches upstream of the town of Hope due to the natural scarcity of floodplain areas, and the river’s higher gradient and confined channels associated with the steeper topography.
With often steep, naturally confined channels and less abundant spawning salmon, habitat and prey/food availability in the upper and middle Fraser River is likely a limiting factor for sturgeon population size. Habitat availability in the lower Fraser River is less abundant compared to historic levels, due to human activities and development, though detailed information on habitat use by age-class and life history stage is limited. The extent of estuarine or marine habitat use, or habitat used for spawning remains unclear. However, it is likely that sturgeon productivity is increasingly limited by the availability of prey/food. For example, spawning eulachon and salmon returns have been significantly lower in recent years, and demands for human use of returning salmon have been increasing (e.g., Fisheries and Oceans Canada 2010a, b).
The Fraser River acts as the receiving waters for many point and non-point source pollutant discharges, especially since the Fraser River Basin supports most of the province’s economy and human population. Pollutants are introduced into the Fraser River over a broad geographic scale, though the majority are downstream of the Fraser-Nechako River confluence, and the highest levels of discharge occur in the Lower Mainland downstream of Mission (Fraser River White Sturgeon Working Group 2005).
The Nechako River is affected by anthropogenic impacts and currently has the smallest SARA-listed white sturgeon population. The main impact in the watershed was the construction of the Kenney Dam in 1952, which created the Nechako Reservoir and diverted water into the Kemano River; downstream habitat is influenced by this dam. Subsequent water management in the Nechako River system has significantly altered its hydrology by decreasing overall annual flow and by altering its seasonality. However, the thermal regime of the river is not substantially different than that observed pre-dam because summer water temperatures are actively managed primarily for migrating sockeye salmon; without this active management the thermal regime would likely show substantial impacts (Nechako White Sturgeon Recovery Initiative 2004).
Post-impoundment, the Nechako River’s channel has become more simplified. Side channels near Vanderhoof decreased in the 1960s due to the combined effect of flow regulation and a large upstream avulsion at Cheslatta Falls (Rood and Neill 1987, Hay and Company Consultants Inc. 2000). Fine sediments were deposited over the white sturgeon spawning reach near Vanderhoof and marked the initiation of recruitment failure (McAdam et al. 2005). Hydrodynamic modeling (Northwest Hydraulic Consultants 2006, 2008) has confirmed that hydraulic characteristics of the site have changed following regulation, and indicate that flow restoration may not be sufficient to restore the site to its previous state.
Water quality characteristics (e.g., temperature, turbidity, nutrients, pollutants) in the Nechako River are influenced primarily by river regulation from Nechako Reservoir, local weather effects and discharge of municipal effluent at Vanderhoof, but their effects on white sturgeon are unknown. Pulp mills, located at the mouth of the Nechako River, discharge into the Fraser River and are assumed to have had minimal influence on Nechako River white sturgeon.
The Columbia River is affected by several anthropogenic impacts, but the main influence on aquatic habitat has been the construction of large mainstem dams for flood control and electricity generation. Impacts from hydroelectric developments on upper Columbia River white sturgeon likely first occurred in 1941 with the completion of Grand Coulee Dam on the Columbia River downstream of the Canada-U.S. border (Figure 3). The construction of Grand Coulee Dam resulted in the loss of anadromous salmon returns to the upper Columbia River, which were likely a main source of prey for white sturgeon. Completion of the third powerhouse at Grand Coulee Dam in 1974 subsequently altered the operations of FDR. Massive drawdowns of Lake Roosevelt occurred in 1969 and 1974 related to the powerhouse construction activities, in both cases exposing Kettle Falls. In 1968, the construction of HLK isolated white sturgeon in the former Arrow Lakes system and created the ALR (Figure 3). Analysis of mitochondrial DNA indicates that white sturgeon within the ALR and fish located immediately downstream of HLK Dam are genetically similar, which suggests that they historically spawned in the same location (McAdam 2012). It is currently unclear whether this group (ALR and HLK fish combined) historically spawned upstream of HLK, but fin ray chemistry indicates that juveniles may have reared upstream (Clarke et al. 2011). The ecosystem above ALR was further fragmented by the construction of Mica Dam in 1973, which flooded over 250 km of the Columbia River mainstem, and replaced riverine habitat with a reservoir environment (Upper Columbia White Sturgeon Recovery Initiative 2002). The construction of REV in 1984 effectively eliminated the 130 km section of flowing river between Mica Dam and ALR and replaced it with reservoir habitat (Upper Columbia White Sturgeon Recovery Initiative 2002).
On the upper Columbia River, habitat diversity and contiguity was lost as a direct result of impoundment, and habitat suitability was affected by flow regulation (Upper Columbia White Sturgeon Recovery Initiative 2002). Regulation of flows on the Columbia River have resulted in a more uniform river channel, decreased the overall annual flow, and altered flow seasonality (Upper Columbia White Sturgeon Recovery Initiative 2002). These changes have reduced aquatic habitat diversity, altered flow conditions at known and potential spawning and nursery areas, and may have altered substrates in rearing habitats necessary for survival. Complex habitats were lost that may have provided important seasonal forage areas and refuges from high discharges, such as side channels and low-lying marshlands.
In addition to physical changes, dams on the Columbia River have caused a number of changes in water quality, including temperature, turbidity, total gas pressure and nutrient status. Upstream of REV, water temperatures are cooler in summer and warmer in fall and winter, in comparison to the pre-impoundment period (McAdam 2001, Tiley 2005). Downstream of HLK, average fall and winter temperatures are similar, but temperatures from May through September are approximately 2° to 3°C warmer than occurred historically (Hamblin and McAdam 2003). On the Pend d’Oreille River, a regulated tributary to the Columbia River located just upstream of the border, water temperatures may be warmer than they were historically; relative to the Columbia River they rise faster during the spawning season and become much warmer (e.g., 24˚C in 1998). However, pre-impoundment data are lacking for comparison. A wider range of water temperatures and more complex thermal environment occurs in FDR relative to historic conditions in the river.
Water quality has been influenced by a variety of industrial and municipal activities, but has improved in recent years through better waste discharge and management practices. Residual effects are still a concern, since historic and current industrial activity and residential development along the river have contributed metals and a myriad of organic compounds to water and sediments.
Turbidity in the Columbia River at Birchbank, downstream of the Kootenay River confluence, is generally below 3 NTU year round (Ministry of Environment data – EMS database; Golder Associates Ltd. 2006d). Anecdotal evidence indicates that turbidity was historically higher due to runoff from glacial systems; though historical data are limited (see Van Winkle 1914, McAdam 2012), they do not indicate that turbidity during the freshet period has declined substantially. Settlement within the historical Arrow Lakes may explain the presence of naturally reduced turbidity downstream in the Columbia River. Turbidity data on Pend d’Oreille River is limited, but indicates higher turbidity events during some peak freshets (e.g., 13 NTU in 1997) even after dams were installed.
Natural nutrient inputs into the upper Columbia River system have been reduced by the combined effects of the elimination of anadromous salmon runs and reservoir construction. Prior to the construction of Grand Coulee Dam, anadromous fish runs were likely an important food source for white sturgeon and a significant source of nutrients (e.g., nitrogen, phosphorus, and trace elements) for aquatic food webs. Reservoirs act as nutrient sinks and reduce downstream transport from the upper basin. These changes have likely reduced the carrying capacity of the system for many fish species, including white sturgeon. The ongoing ALR fertilization program is designed to offset some of this impact by improving overall pelagic production, with the specific aim to improve kokanee abundance (Schindler et al. 2006). The entrainment of Mysis relicta through HLK Dam has become an important downstream food source for juvenile sturgeon (Golder Associates Ltd. 2007).
Substantial changes have also occurred within the aquatic ecological community through the introduction of exotic species, which have flourished because of anthropogenic changes in the upper Columbia River. The pre-development fish community included large numbers of anadromous fishes including spring and summer Chinook salmon (Oncorhynchus tshawytscha), sockeye salmon (Oncorhynchus nerka), steelhead (Oncorhynchus mykiss), and possibly Pacific lamprey (Lampetra tridentata). The resident fish community included bull trout (Salvelinus confluentus) and burbot (Lota lota). The primary changes have been the elimination of anadromous species and an increase in introduced predatory species such as walleye.
There is a long history of development within the Kootenay River watershed that includes hydroelectric development, as well as channel stabilization and modification for flood control. Channel alteration and other factors apparently affected white sturgeon recruitment prior to the completion of Libby Dam in 1972; however, Libby Dam operations appear to have been the primary factor that led to complete recruitment failure (Duke et al. 1999).
Several dams occur in the Kootenay watershed, including five facilities on the lower Kootenay River (Corra Linn Dam, Upper and Lower Bonnington Dams, South Slocan Dam, and Brilliant Dam), Libby Dam on the Kootenai River, and Duncan Dam on the Duncan River, a tributary to Kootenay Lake (Figure 3). The construction and operation of these dams led to habitat alterations similar to those discussed for the Columbia River population. Upper and Lower Bonnington and South Slocan dams did not fragment the white sturgeon population because they were built on natural waterfalls that have existed for approximately 10,000 years (Northcote 1973).
Changes to aquatic habitat with the construction of Libby Dam in 1972 have been notable because of the significant flow alterations and their effect on spawning habitats (Duke et al. 1999). For example, average spring peak flows have been reduced by more than 50 percent, and winter flows have increased by 300 percent compared to pre-dam values (Paragamian et al. 2005). The naturally high spring flows, thought to be required by white sturgeon for reproduction, now rarely occur. Lower freshet flows at Libby Dam and lower spring maximum elevation of Kootenay Lake are thought to have contributed to white sturgeon decline by affecting depths at their spawning location and causing white sturgeon to spawn in locations with sub-optimal conditions (U.S. Fish and Wildlife Service 2006; Matt Neufeld, B.C. Ministry of Forests, Lands and Natural Resource Operations, personal communication). Habitat changes from construction and operation of Duncan Dam (e.g., nutrient retention, lack of access to prey habitats) have also been implicated in contributing to the decline of Kootenay River white sturgeon (Duke et al. 1999).
River regulation has altered water temperatures in the Kootenai River so that they are now typically warmer (by approximately 3˚C) during the winter and colder (by approximately 1 to 2˚C) during the summer compared to pre-Libby Dam conditions (Partridge 1983, Duke et al. 1999). Spring temperature conditions are also currently cooler compared to pre-dam conditions due to thermal stratification in the reservoir and constraints around elevation control for withdrawals (Brian Marotz, Montana Fish, Wildlife & Parks, personal communication).
Alterations to floodplain habitats in the Kootenay system are a contributing factor to white sturgeon decline (Duke et al. 1999). For example, side-channel and slough habitats were eliminated from Bonners Ferry (Idaho) to Creston (B.C.) due to dyking and bank stabilization in the early 1950s for flood protection (Figure 3; Constable 1957, Duke et al. 1999). These habitat changes resulted in reduced aquatic habitat diversity, altered access or flow conditions at potential spawning and nursery areas, and altered substrates in incubation and rearing habitats (Partridge 1983, Apperson and Anders 1991, Duke et al. 1999).
Kootenay Lake is an important habitat area for Kootenay River white sturgeon. Based on a review of limnological studies of Kootenay Lake, Daley et al. (1981) concluded that biological productivity decreased markedly since construction of Libby Dam. Reduced productivity is thought to have decreased prey abundance and food availability for some life stages of sturgeon downstream of Libby Dam, and possible reduction in the carrying capacity of Kootenai River and Kootenay Lake. The ongoing Kootenay Lake Nutrient Restoration Program has been successful in partially offsetting nutrient declines and increasing biological productivity in the lake (Binsted and Ashley 2006, Sebastian et al. 2010). Monitoring confirms that the density and abundance of kokanee is higher than before fertilization started (Sebastian et al. 2010).
Excessive nutrients were once a problem in the upper Kootenai River, prior to the construction and operation of Libby Dam. Waste water control and effluent recycling measures were initiated in the late 1960s and significant improvements in Kootenai River water quality were noted by 1977 (Duke et al. 1999). Fertilizer processing, sewage, lead-zinc mine, and vermiculite discharges have been eliminated, but many of these pollutants and contaminants persist, and are primarily bound in sediments (Duke et al. 1999). Ultimately, the effects of these pollutants on sturgeon reproduction and survival are unknown but are likely minimal, especially in the Canadian section.
There are a number of key knowledge gaps that apply to almost all white sturgeon populations, whereas others are specific only to certain populations. General knowledge gaps are discussed in this section and are separated from knowledge gaps specific to the task of identifying critical habitat (see Section 8: Critical Habitat). Studies to address the knowledge gaps identified here are provided in Appendix A. In some cases it will not be necessary to address data gaps separately for each population because there are opportunities to fill gaps in one area and to transpose that learning across the species’ range. Whereas impacts across populations are more likely to be similar in nature, it is possible that impact mechanisms may differ. Activities needed to meet population and distribution objectives for SARA-listed populations, including those addressing population-specific and general knowledge gaps, are provided in Section 7.5 (Research and Management Activities Needed to Meet Population and Distribution Objectives).
The four most significant data gaps across the entire species’ range are: i) causes of recruitment failure in dam-affected systems; ii) design and implementation details of conservation fish culture; iii) clarification of existing threats; and, iv) basic biological information requirements. These are discussed further below.
Chronic recruitment failure is the primary cause of population declines in dam-affected systems. In all the three rivers showing recruitment failure (i.e., Columbia, Kootenay and Nechako) regular spawning occurs, but viable offspring do not recruit to the juvenile stage in sufficient numbers to sustain the population. This pattern has been well described, but detailed research into the causes of recruitment failure is relatively recent. Such research is complicated by the possibility of multiple causal factors and likely variation in the strength of particular factors among populations.
Recent reviews completed for the Columbia River population examined a variety of recruitment failure hypotheses, defined key hypotheses, and identified potential programs for assessment and recovery (Gregory and Long 2008, McAdam 2012). Key hypotheses for the Columbia River population included: effects of hydrograph alterations on predation of yolk sac larvae and feeding larvae; effects of diminished substrate suitability on early life stage habitat availability; effects of changes in the fish community on early life stage predation rates; and, effects of altered substrate on decreasing prey availability for early life stages and early juveniles. Nearly all hypotheses apply to the first year of life, and principally the early life history stages of egg, yolk sac larvae and feeding larvae. Although evaluations based on expert elicitation indicated similar levels of support for multiple hypotheses (Gregory and Long 2008) a recent weight of evidence evaluation suggests substrate change may be the proximate cause of recruitment failure. Similar findings have also been achieved for the Nechako (McAdam et al. 2005) and Kootenay (Paragamian et al. 2009, McDonald et al. 2010). Research on the Kootenay River population has gathered a broad variety of evidence over multiple years of studies (e.g., Paragamian et al. 2001, Paragamian et al. 2002, Paragamian et al. 2005), the culmination of which suggests that adults are spawning in areas where high levels of sand substrate in the river lead to essentially zero survival to the yolk sac larvae stage. For the Nechako River population, the presence of a clear association between historic habitat changes and recruitment patterns (McAdam et al. 2005), in conjunction with subsequent laboratory and field studies, provides further evidence that substrate alterations have led to habitat changes that diminish both egg and yolk sac larvae survival (McAdam 2012). While both the Kootenay and Nechako River findings emphasize survival at either the egg or yolk sac larvae stage, evidence from spawning sites in Washington State indicates that survival during the initiation of exogenous feeding may also be limiting (Howell and McLellan 2011).
Studies on all three dam-affected populations represent important advances in understanding recruitment failure. However, significant information gaps will remain until experimental recruitment restoration can be successfully demonstrated. To that end, laboratory and in situ (i.e. in-river) studies are required to identify factors within these rivers currently limiting early life history survival and particularly how habitats might be effectively altered to restore recruitment. It is particularly important to emphasize the importance of in situ studies for these challenging early life stages, since it is critically important that the results can be demonstrated under natural conditions. A useful approach may be the sequential increase in the scale of field studies in order to build an understanding sufficient to confidently examine recruitment restoration methods at the whole-river scale. Recent experimental substrate restoration studies in the Nechako (McAdam 2012) and Columbia (Crossman and Hildebrand 2012) represent very important steps in the direction toward larger scale restoration.
The following knowledge gaps are particularly important.
- Improved understanding of survival rates at the egg, yolk sac larvae and feeding larvae stages.
- Manipulative experiments, and particularly in situ experiments, to understand factors affecting survival during early life history.
- Understanding of habitat changes that were likely associated with both historic recruitment failure and contemporary recruitment pulses.
- Additional experimental evaluation of habitat restoration measures (and their maintenance) at a scale sufficient to create detectable recruitment. This could be done with out-planted eggs/yolk sac larvae/feeding larvae, but ultimately must address habitat restoration in conjunction with wild spawning fish.
- Investigation of feasible means by which identified habitat restoration measures can be implemented and maintained in a manner that leads to sustained recruitment in conjunction with wild spawning.
Population supplementation is proposed as a temporary, but long term (potentially 40+ years) measure to prevent extirpation of impacted white sturgeon populations. The general techniques required to spawn and rear white sturgeon in a hatchery environment are well-understood and conservation aquaculture measures have been successfully used in the Nechako, Columbia and Kootenay rivers. However, there are still some knowledge gaps that should be investigated to maximize the effectiveness of this recovery technique, including:
- Investigate the role of environmental imprinting in the hatchery as it may relate to later life stages.
- Determine optimal size at release to maximize survival rates.
- Investigate and adapt CFC practices to include captive broodstock strategies should they become required.
- Update breeding plans as new genetic information becomes available.
- Investigate improvements in hatchery rearing practices.
- Update hatchery practices as technology and methods become available.
- Develop and implement best management practices for white sturgeon CFC.
Many threats to white sturgeon are known and have been described in some detail (see Section 4: Threats), although in most cases it remains difficult to quantify risks or the efficacy of different mitigation measures. Clarifying threats would allow resources to be more effectively distributed to the most useful mitigation measures. Specific needs include:
- An assessment of direct and indirect effects from various targeted and non-targeted incidental recreational, commercial and Aboriginal fisheries. This work should build on results from previous studies (e.g., Robichaud et al. 2006), which indicated significant potential effects from direct and indirect catch. This could also include determination of the potential threat of the white sturgeon recreational kill fishery in northern Washington State marine waters.
- Assessment of whether ecological shifts have occurred to such an extent as to inhibit recovery efforts (e.g., multiple stable ecological states, introduced and invasive species).
- Effect of pollutants on wild sturgeon:
- Identify the major pollutants that affect each white sturgeon life stage;
- Develop an understanding of the lethal and sublethal effects of these pollutants on each white sturgeon life stage; and,
- Develop an understanding of bioaccumulation in sturgeon tissues and effects of pollution on sturgeon populations.
- Flow issues have been identified as a significant threat in the three dam-affected populations. However, it is unclear how factors such as flow, river stage, and temperature influence these populations. The dam-affected systems are regulated under Canadian and international regulations and agreements and there is no current plan to remove the dams, so it will be necessary to understand how to manage flows to address recruitment failure, habitat quantity and quality, predation and other threats to white sturgeon.
- An assessment and/or monitoring program for angling effects on juvenile white sturgeon and their survival is required for areas supplemented by conservation fish culture. This is currently most needed for the transboundary reach of the Columbia River.
There are numerous knowledge gaps in our understanding of the biology of white sturgeon. Such information is of key importance in helping to monitor populations, model population dynamics, and inform mitigation and recovery techniques. These gaps include:
1. Demography:
- Population abundance and trends;
- Population structure (size, age, sex ratios, genetic differences within and among populations);
- Genetically effective population sizes; and,
- Age-specific mortality rates and mortality sources.
2. Life history ecology:
- Ecology of early life stages (particularly habitat use, survival rates, growth rates and recruitment);
- Frequency of spawning;
- Fidelity and frequency of spawning habitat use, and,
- Influences on timing and location of spawning, such as spawning cues and habitat requirements.
3. Spawning Migration:
- Spawning triggers in populations not affected by dams;
- Gene flow between and within populations.
4. Feeding:
- Food sources by life stage and population, an understanding of historic trends in these prey species, and a functional relationship between sturgeon indicators (e.g., survival, growth, reproduction, etc.) and food supply.
5. Habitat:
- Abundance and distribution of available habitats;
- Quality of available habitat;
- Historic habitat availability (this information provides context for discussions regarding population targets and what can be achieved in terms of white sturgeon abundance);
- Limiting habitats of major life stages (this relates to critical habitat definitions); and,
- Carrying capacity of currently available habitats, and assessment of whether abundance targets can be met with this (i.e., is habitat restoration required?).
6. Monitoring Methods:
- Development of good field indicators of sex, age, sexual maturity, and population identity;
- Development of additional nuclear DNA markers to allow identification of parentage for hatchery-produced sturgeon (for Kootenay and Nechako populations only); and
- Development of additional techniques for monitoring early life stages (e.g., stage specific survival, larval quality indicators).
White sturgeon in Canada, comprised of six Nationally Significant Populations, were assessed as endangered by COSEWIC in 2003. In 2006 the Governor in Council listed four populations (Nechako River, Upper Fraser River, Upper Columbia River, and Kootenay River) under Schedule 1 of SARA, and declined to list two populations (Lower Fraser River and Mid Fraser River). This document is a SARA-compliant recovery strategy for the four SARA-listed populations, and also provides information to support recovery of the lower and middle Fraser River populations (Appendix B).
This recovery strategy uses a modified single species approach (rather than an ecosystem approach) because it addresses a single taxonomic unit with multiple distinct populations. Given the unique life history of white sturgeon and its use of large river habitats there may be limited opportunities for sharing resources with recovery efforts aimed at other species. There are no apparent opportunities to combine such efforts across the whole range of white sturgeon, although general studies and recovery information from one area should inform efforts in other areas. There may to be opportunities to combine efforts over smaller geographic ranges, and such opportunities should be sought wherever possible.
The recovery goal for white sturgeon is to ensure that each of the populations are sustainable throughout their natural range, are self-sustaining through natural reproduction, and to increase or restore opportunities for beneficial use, if and when feasible.
To achieve this goal, a series of population and distribution objectives and general activities have been identified, including specific recovery measures, research, and ongoing monitoring. The objectives and timelines will be revisited as new information is collected and possible changes to priorities will be evaluated. Objectives and activities are presented below for SARA-listed populations and in Appendix B for non-SARA-listed populations.
As part of the SARA process, the competent minister must determine the biological and technical feasibility of recovery for each species at risk. To help standardize these determinations, four directives are posed and answered here for the SARA-listed populations of white sturgeon (Draft Policy on the Feasibility of Recovery, Species at Risk Act Policy. January 2005). It is important to note that there may be substantial challenges in meeting these directives and these challenges are further discussed below, where applicable.
- Individuals of the wildlife species that are capable of reproduction are available now or in the foreseeable future to sustain the population or improve its abundance.
The upper Fraser population is currently self-sustaining at or near its historic abundance. However, the natural population size is small (185 mature fish), and consequently, its long-term viability remains a concern.
- Sufficient suitable habitat is available to support the species or could be made available through habitat management or restoration.
The extent of suitable habitat for the upper Fraser population is at or near historic levels.
- The primary threats to the species or its habitat (including threats outside Canada) can be avoided or mitigated.
The Upper Fraser population exists entirely within Canada and is not currently threatened by human activities.
- Recovery techniques exist to achieve the recovery goal or could quickly be developed.
Recovery of the upper Fraser population does not require special techniques, but rather, effective management of future threats to maintain abundance and habitat. Long-term viability might be enhanced by increasing the genetically effective population size by artificially promoting an appropriate level of immigration (gene flow) from other, more diverse populations.
Recovery feasibility for the dam-affected populations in the Nechako, Columbia, and Kootenay rivers depend primarily on whether prolonged failures in natural recruitment can be reversed. Accordingly, even though some differences exist between these populations they are considered together, due to their many similarities and for brevity. Directives 2 through 4 pose significant challenges, since they depend on outcomes of ongoing research programs.
- Individuals of the wildlife species that are capable of reproduction are available now or in the foreseeable future to sustain the population or improve its abundance.
Each population still comprises enough mature individuals to become self-supporting given favourable conditions for natural recruitment. In the meantime, conservation aquaculture programs have been initiated in each population to ensure that adequate numbers will be available for the foreseeable future.
- Sufficient suitable habitat is available to support the species or could be made available through habitat management or restoration.
Present habitat is not adequate to support sufficient survival through early life history stages (egg, yolk sac larvae, and larvae) for the Kootenay, Columbia, and Nechako river populations. It is possible that conditions can be made more suitable through habitat management or restoration efforts. For the Kootenay River, spawning habitat is in the U.S. and Canadian recovery efforts are limited for restoring this habitat. Therefore, restoration of Kootenay River spawning habitats requires coordinated efforts between Canada and the U.S.
- The primary threats to the species or its habitat (including threats outside Canada) can be avoided or mitigated.
Based on current hypotheses and evidence, mitigating threats may be feasible. However, proposed mechanisms leading to recruitment failure are still being studied and will dictate the mitigation measures required for reducing threats. For example, water management for power production and flood control is considered a primary threat to the Kootenay, Columbia, and Nechako River populations. On the Columbia River the Columbia River Treaty regulates overall water management for both the U.S. and Canada, whereas on the Nechako River flows are controlled by provincial and federal agreements within Canada. On the Kootenay River, water is also managed under the International Joint Commission between Canada and the U.S. Managing these and other threats will require working cooperatively with stakeholders in both Canada and the U.S. The feasibility of mitigation options is dependent upon clarifying the primary mechanisms of recruitment failure to ensure that options are focused, efficient, and as effective as possible.
- Recovery techniques exist to achieve the recovery goal or could quickly be developed.
Conservation aquaculture has been initiated for the Kootenay and Columbia River populations, and is currently being initiated for the Nechako River population, to help avoid extirpation until natural recruitment can be restored. Research is continuing to identify the factors limiting natural recruitment, and to develop appropriate methods for restoring it. Examination of habitat restoration measures is underway in the Nechako, Columbia and Kootenay rivers; however, success of these efforts has not yet been demonstrated.
Currently, white sturgeon are seriously imperilled through much of their natural range. Given their very long generation time and the enormity of some of the threats, it is likely that white sturgeon will remain at risk for some time. Recovery actions for all populations will be aimed at maintaining and enhancing current habitat conditions, monitoring populations and specific threats, and undertaking necessary research tasks. With the support of governments, First Nations, industry, user groups and the general public, recovery is deemed to be technically and biologically feasible. With the transboundary nature of Kootenay River and Columbia River populations, cooperation between Canada and the U.S. is critically important.
The population and distribution objectives below have been reviewed in the DFO Recovery Potential Assessment for White Sturgeon (Wood et al. 2007), and were modified slightly relative to earlier drafts of the recovery strategy. The only population objective that is directly applicable for the Upper Fraser population is objective one, since the population is thought to be at or near historic levels. All population and distribution objectives are applicable for the other three SARA-listed populations.
- Prevent extirpation of white sturgeon in each of the four identified populations by preventing net loss of reproductive potential.
- Initiate, within 5 years, pilot studies towards restoration of natural recruitment for each population that is affected by dams. Within 10 years, identify methods for each population that, if and when implemented, have a high likelihood of restoring recruitment to a level sufficient to achieve the other recovery measures listed herein.
- Reach or exceed all of the following population and distribution targets for survival or recovery within 50 years:
- 1,000 mature individuals in an approximately 1:1 sex ratio at maturity;
- distribution over the natural range, with the exception of Duncan Reservoir and Slocan Lake, and the Columbia River upstream of REV6; and,
- ongoing natural recruitment sufficient to meet all other targets.
- Reach or exceed population and distribution targets for beneficial use within specified timeframes. As success is achieved in meeting the biological recovery targets, the beneficial use targets and timelines will be established and adjusted. Such targets may vary among populations.
This section provides a more detailed discussion of population recovery targets in general and the rationale for the white sturgeon targets proposed here. The rationale and feasibility of these targets have been reviewed in detail in the DFO Recovery Potential Assessment for White Sturgeon (Wood et al. 2007).
The ultimate performance measure of any conservation program is long-term viability of a species. The recovery strategy follows McElhany et al. (2000) in defining a viable population as one “that has a negligible risk of extinction due to threats from demographic variation (random or directional), local environmental variation, and genetic diversity changes (random or directional) over a 100-year time frame.” Viability over longer timeframes is a laudable conservation goal, but one that is more logically part of general species management than recovery efforts per se, as long as recovery goals and management do not conflict. The role of recovery efforts is to attain sufficient viability beyond which general species management can take over.
In assessing viability under the above definition, McElhany et al. (2000) suggest that it is necessary to consider abundance, population growth rate, population structure, and population diversity, and note that the values of these four parameters would be lower or less functional in an endangered population than in a viable population.
7.4.2.1 Abundance
Abundance is a key component of population viability, because all else being equal, small populations are at greater risk of extinction than large ones. Several processes affect population dynamics differently in small populations (McElhany et al. 2000). These processes include density-dependence, environmental variation, genetic processes, demographic stochasticity, ecological feedback and catastrophes. McElhany et al. (2000)7 suggest that, to be viable, a population’s abundance must be large enough to satisfy the following criteria:
- a high probability of surviving historic and expected future environmental variation;
- resilience to environmental and anthropogenic perturbation; and,
- maintenance of genetic diversity over the long-term.
In setting abundance targets for conservation one must consider external threats to the population, and inherent causes of population vulnerability. External threats tend to affect mean vital rates (differences in vital rates across different years or seasons) and carrying capacity. For example, mean fecundity or survival may be reduced by pollution, carrying capacity may be low due to habitat destruction, or harvest may affect the abundance of mature individuals. These factors are clearly important and must be addressed to meet conservation targets of threatened and endangered species. Various threats to white sturgeon are discussed in Section 4 (Threats)and mitigation strategies are presented in Section 7.5 (Research and Management Activities Needed to Meet Population and Distribution Objectives). However, factors affecting temporal variability in vital rates must also be considered when setting population targets for long-term viability. Both natural and threatened populations face temporal variability in vital rates, but such variability is generally a greater concern at low abundance. Some years (and individuals, habitats, etc.) tend to be better than others and this can have a substantial influence on population trajectories and overall population recovery probabilities.
There are multiple causes of temporal variability in vital rates, but they fall into three categories: demographic, environmental and genetic stochasticity. Demographic stochasticity is temporal variation in population growth driven by chance variation in the fates of individuals within years (Morris and Doak 2002). The effects of demographic stochasticity are strongly dependent on population size, and become a concern as abundance declines below several hundred spawners.
Environmental stochasticity can be defined as among-year variation in vital rates caused by changes in environmental factors. Population viability analyses (PVAs) tend to focus on effects of environmental stochasticity on survival and reproduction rates, and population-level processes, such as density-dependence, because these are the main sources of temporal variability in population vulnerability over short and medium timeframes. Special cases of environmental stochasticity include long-term trends in environmental factors (e.g., climate change), and bonanzas and catastrophes (i.e., especially good or especially bad years that are outside the normal range of variation). Population targets to account for environmental stochasticity are invariably greater than and therefore supersede those for demographic stochasticity, meaning the latter can be essentially ignored if a target properly accommodates environmental stochasticity.
Random genetic changes can accumulate in small populations and further increase population vulnerability. Inbreeding increases via genetic drift at a rate that is inversely proportional to 2Ne, where Ne is the number of breeders in an idealized population of constant size with no immigration, no natural selection, discrete non-overlapping generations and Poisson distributed variance in among-individual reproductive rates. To assess the magnitude of genetic effects in non-ideal populations, census population size is converted to the corresponding genetically effective population size (Ne) -- the size of an idealized population that would respond genetically in the same way. To constrain random genetic changes within acceptable levels, an early rule of thumb was the “50:500” rule, which stated that Ne > 50 individuals will eliminate short-term risks from inbreeding, and Ne > 500 individuals will maintain heterozygosity over the long-term. More recent evidence indicates that these thresholds may have to be considerably higher (Ne of at least 1,000) to ensure evolutionary potential over the long-term (Lynch and Lande 1998, Allendorf and Ryman 2002). Converting Ne to N can be done directly, or can be based on published Ne to N ratios, which average around 0.1 for a wide range of wildlife species (Frankham 1995). A population target based on Ne = 1,000 should therefore be around 10,000 reproductively mature individuals.
Since there is scarce data on population-specific vital rates of white sturgeon it is useful to consider some “rules of thumb” that have been developed in the literature. In a review of population variability in relation to population persistence, Thomas (1990) concluded that a population of “1,000 is adequate for species of normal variability, and 10,000 should permit medium- to long-term persistence of most of the most variable birds and mammals.” In a more formal review of PVA results, Reed et al. (2003) found that minimum viable populations (MVPs) for vertebrates tend to be on the order of 1,000 to 10,000 breeding pairs in single closed populations. They suggest a population target of 7,000 adults is appropriate for long-term persistence. There are often good reasons to extend recovery targets beyond the MVP. For example, to account for restricted geographic distribution or to accommodate additional safety factors to offset threats.
In setting population targets for white sturgeon effects of environmental stochasticity were focused on; it is believed that this is an adequate interim target for rebuilding over at least the next 10 years. There is currently insufficient information to conduct a full PVA for any of the white sturgeon populations in Canada. As new information becomes available these targets may be updated. The targets are considered sufficient for ongoing recovery planning of white sturgeon over the next 10 years.
An interim abundance target of 1,000 mature individuals (25 years of age or older) is proposed for each SARA-listed population of white sturgeon, with the exception of the Upper Fraser population. Based on the available scientific literature, this target should be sufficient to buffer variability due to demographic and environmental stochasticity (but not catastrophes), and to maintain genetic diversity over the next 100 years. The abundance target of 1,000 mature fish is considered interim because it may be less than needed to maintain genetic variation indefinitely. However, given the extraordinary generation time of white sturgeon (~ 40 years), the target should be adequate for recovery planning over the foreseeable future. The small Upper Fraser population is believed to be maintaining itself at historic levels of just under 200 mature fish, and is likely constrained by natural limitations on the extent of suitable habitat. Accordingly, the abundance goal for this population is to maintain the current abundance of mature fish, meaning only the first population objective applies. Genetic concerns about the long-term viability of such a small population could be addressed in other ways, for example, by creating gene flow with another large population through artificial transfer.
7.4.2.2 Population Growth Rate
Population growth rate is a measure of how well a population is performing, and clearly links to population abundance. McElhany et al. (2000) suggest that a salmonid population must meet the following population growth criteria to be viable:
- natural productivity (i.e., in the absence of hatchery supplementation) should be sufficient to maintain abundance above the viability threshold,
- the population should not exhibit sustained declines in abundance,
- the population should not exhibit trends or shifts in traits that portend declines in population growth rate, and
- population status evaluations should take into account uncertainty in estimates of population growth rate and productivity-related parameters.
The following growth rate targets are proposed for all SARA-listed populations of white sturgeon:
- ongoing natural recruitment, supplemented by conservation aquaculture where needed; and,
- increasing trend in abundance for all populations that are below the abundance target.
The long-term objective is to have all populations self-supporting, but conservation aquaculture is required in some instances as an interim measure. Minimum recruitment targets, to meet the abundance target of 1000 mature individuals, are specified by a simple population model that assumes 20% survival in the first year of release (currently 1 to 2 years of age) and 92% survival for subsequent ages (Golder Associates Ltd. 2005a). Under these assumptions a minimum of approximately 4,000 individuals are required to recruit to the 1 year old age class each year. At this rate the abundance target of 1000 mature fish would be reached after 40 years. Increased recruitment or hatchery releases would allow a population to reach the abundance target sooner, but it should be remembered that a lower limit of 25 years is imposed by the late maturation of this species.
7.4.2.3 Population Structure
White sturgeon in Canada occur as six distinct populations (see Section 2.4: Distribution). The populations are geographically separate and genetically distinct; the genetic separation indicating they have had little or no effective mixing for a considerable period of time (Smith et al. 2002). Differences among the populations indicate that it is reasonable to consider each population distinct and manage for viability in each. This rationale was supported by the 2003 COSEWIC status report, which acknowledged the six populations, and the 2006 SARA listing decision, which considered each population separately. Recent evidence suggests that the designated Columbia River population might comprise two or more reproductively isolated populations (Nelson and McAdam 2004), but current understanding is too uncertain and too limited to attempt to delineate finer populations in this recovery planning document. The 2012 COSEWIC reassessment of white sturgeon delineated the species into four Desginatable Units, following recent guidelines (COSEWIC 2011), though current species management and recovery efforts remain focused at the population level.
The following targets for population structure are proposed for all SARA-listed populations of white sturgeon:
- natural sex ratio (currently defined as 1:1), and
- natural age structure.
Age structure can be defined using the same model noted in the previous paragraph. Under this scenario, the stable age distribution is highly skewed, with the majority of individuals in immature age classes, and approximately 1000 individuals 25 years and older.
McElhany et al. (2000) note that long-term viability of a species hinges in part on its ability to respond to changes in the environment, which in turn is dependent on maintenance of sufficient phenotypic and genotypic diversity. Population diversity can be maintained by ensuring a sufficient spatial and temporal array of habitat types, that dispersal among them is not altered, and general processes that give rise to diversity are maintained. Based in large part on the need to maintain population diversity, the distribution objective focuses over most of the natural range of white sturgeon in Canada.
7.4.3.1 Distribution Objective
The target proposed for species diversity is distribution across the species’ natural range. Duncan Reservoir, Slocan Lake, and Revelstoke Reservoir are excluded from this distribution because recovery of populations in these relatively small waterbodies has been deemed infeasible, and the Kinbasket Reservoir is excluded because there is insufficient data to recommend whether or not the establishment of a self-sustaining population in that area is feasible or recommended. At this time there is insufficient information on this topic to propose meaningful targets, beyond this distribution target.
Table 5. Recommended activities and actions to meet population and distribution objectives for SARA-listed populations. The table has four columns read left to right: Priority, Strategy, Activities, and Performance Measure. A footnote on “Priority” states the following: Priority has been assigned based on professional judgement of the National Recovery Team into one of three groups, from highest to lowest: necessary, primary, secondary. A footnote on “Performance Measure” states the following: As white sturgeon is a slow-growing, late-maturing, and long-lived species, performance measures were developed that could be measured repeatedly throughout the recovery process. Therefore, performance measures plot the progress toward meeting the stated objectives. The performance measures are presented here as questions, the answers to which can be plotted in time to monitor progress.
Directly below the column headings are seven rows. The contents are as follows read from left to right:
Row 1: Necessary, Meet or exceed recovery population targets within specified timeframe,
1. Set up conservation aquaculture where needed, 2. Monitor population trends, 3. Establish parameters for beneficial use, Have targets been achieved? Row 2: Necessary, Protect critical habitats, 1. Identify habitat requirements for all life stages, 2. Define critical habitat (including related ecological processes), 3. Identify critical habitats for designation and protection, 4. Protect, maintain and enhance critical habitat for white sturgeon, 5. Ensure habitat diversity, connectivity & productivity, 6. Work cooperatively to develop plans to protect habitat, Has critical habitat been identified? Row 3: Necessary, Restore natural recruitment, 1. Determine accuracy of recruitment index time series, 2. Identify temporal correlations between significant recruitment shifts (peaks or drops) and environmental changes, 3. Examine potential mechanism of recruitment effect, 4. Under take meso-scale field trials, 5. Undertake larger scale field trials, 6. Design and implement longer term restoration program, 7. Determine habitat requirements for dam-affected population enhancement or recovery, 8. Initiate, within 5 years, pilot studies towards restoration of natural recruitment for each population that is affected by dams, 9. Within 10 years, identify methods for each population that, if and when implemented, can restore recruitment to a level sufficient to achieve the other recovery measures listed herein, Have experimental trials been initiated to restore natural recruitment? Have experimental results shown that natural recruitment can be restored to necessary levels? Has recruitment been restored? Row 4: Necessary, Clarify and mitigate threats, 1. Clarify the following threats and their relative risks: a. fishing; b. pollution; c. predation; 2. Clarify threats to: a. food supply; and, b. habitat (including effects of flow regulation) 3. Undertake specific actions to address risks: a. protect, maintain and enhance critical habitat; b. address illegal harvest; c. minimize bycatch and mitigate impacts from fisheries through regulation and best practices; d. limit and address pollutant discharges and contaminant loading, especially adjacent to important or critical habitats; e. protect, maintain and enhance water quality; f. mitigate entrainment of white sturgeon; g. manage risks from conservation hatchery introductions; and, h. better understand, maintain and enhance food availability for all life stages of each population. 4. Monitor threat indicators and population trends. 5. Work cooperatively to develop plans to mitigate threats to white sturgeon, Have the most serious threats been defined? Have these threats been sufficiently mitigated? Row 5: Primary, Address information gaps that inhibit conservation of white sturgeon, Address basic biological data gaps (see Section 7: Knowledge Gaps and Appendix A: Studies to Address Knowledge Gaps for list of priorities), Have key information gaps been filled? Row 6: Primary, Increase stakeholder and general public awareness of white sturgeon and its conservation needs, 1. Maintain and where possible increase awareness and stewardship of white sturgeon throughout its natural range 2. Engage in effective public education of the species and its conservation needs 3. Support learning and communication across all working groups 4. Ensure participation from community and technical experts, Has general awareness of sturgeon conservation been increased? Row 7: Secondary, Maintain and where necessary restore ecosystem functions relevant to white sturgeon, 1. Incorporate the needs of healthy white sturgeon populations into the management of white sturgeon prey species, especially salmon and resident sportfish. 2. Accommodate other species’ needs during recovery of white sturgeon 3. Closely manage non-native predatory fish species 4. Dialogue with regulatory agencies that have influence or jurisdiction over white sturgeon prey species, Is the ecosystem “healthy” for white sturgeon?
| Priority8 | Strategy | Activities | Performance Measure 9 |
|---|---|---|---|
| Necessary | Meet or exceed recovery population targets within specified timeframe. |
|
Have targets been achieved? |
| Necessary | Protect critical habitats |
|
Has critical habitat been identified? |
| Necessary | Restore natural recruitment |
|
Have experimental trials been initiated to restore natural recruitment? Have experimental results shown that natural recruitment can be restored to necessary levels? Has recruitment been restored in dam-affected populations? |
| Necessary | Clarify and mitigate threats |
|
Have the most serious threats been defined? Have these threats been sufficiently mitigated? |
| Primary | Address information gaps that inhibit conservation of white sturgeon. | Address basic biological data gaps (see Section 6: Knowledge Gaps and Appendix A: Studies to Address Knowledge Gaps for list of priorities). | Have key information gaps been filled? |
| Primary | Increase stakeholder and general public awareness of white sturgeon and its conservation needs. |
|
Has general awareness of sturgeon conservation been increased? |
| Secondary | Maintain and where necessary restore ecosystem functions relevant to white sturgeon |
|
Is the ecosystem “healthy” for white sturgeon? |
Critical habitat is identified for the four SARA-listed white sturgeon populations to the extent possible, using the best available information. The critical habitat identified describes the geospatial areas that contain the biophysical functions, features, and attributes necessary for survival or recovery of the species. Activities likely to destroy these habitats, data gaps and data sources are also presented. Maps of critical habitat areas are provided for each watershed, with each map indicating critical habitat boundaries. Corresponding geographic coordinates and river kilometers (where applicable) are also identified for critical habitat areas. The Schedule of Studies outlines the research required to identify additional critical habitats (where applicable), acquire more information about the critical habitat identified or refine the description of existing critical habitats in order to support its protection.
The critical habitat identified in this recovery strategy, when combined with functioning recruitment in each population, is thought to be sufficient to achieve the species’ population and distribution objectives. Although there remains some uncertainty regarding factors such as the precise timing and functionality of some specific critical habitats proposed here, the greater uncertainty is the cause of persistent recruitment failure and identification of a feasible means of restoration. This focus is reflected in the knowledge gaps identified in the recovery strategy, which provides a guide to future studies with a strong focus on recruitment failure diagnosis and restoration. As a result, studies identified here emphasize those which are designed to understand habitat functionality and recruitment restoration within critical habitats. While studies of the species biology and movement may provide further information on definition of particular critical habitats, such studies should not supersede investigations of recruitment failure and its restoration because doing so may not be in the best interest of the species.
Critical habitat is defined in SARA as “…the habitat that is necessary for the survival or recovery of a listed wildlife species and that is identified as the species’ critical habitat in a recovery strategy or in an action plan for the species.” [s. 2(1)]
SARA defines habitat for aquatic species at risk as “… spawning grounds and nursery, rearing, food supply, migration and any other areas on which aquatic species depend directly or indirectly in order to carry out their life processes, or areas where aquatic species formerly occurred and have the potential to be reintroduced.” [s. 2(1)]
A reasonable approach to determine critical habitat for a species at risk is to consider the amount and type of habitat required for the species to meet and maintain its recovery target, an approach supported by existing guidance including recent DFO guidance documents (Fisheries and Oceans Canada 2012). As all SARA-listed white sturgeon populations are at a very low abundance and hence a quantitative relationship between habitat and population size would not be possible, areas of present high use are identified as critical habitat at current population levels. As populations expand toward target recovery levels additional critical habitats may need to be added to those identified here.
The priority for recovery efforts should focus on resolving recruitment failure in each of the dam-affected populations. The ongoing research, particularly to understand the causes of recruitment failure, will benefit critical habitat maintenance and restoration needs which in turn will support critical habitat protection. Identification of additional critical habitats, or further clarification of critical habitat functions, features or attributes is a task that will be addressed with future studies as recovery efforts proceed.
To the extent possible, critical habitat identification was based on habitat associations developed from detailed empirical work. Where detailed studies were lacking or inconclusive, expert opinion and a precautionary approach was used, as described in SARA and DFO guidance documents (e.g., Fisheries and Oceans Canada 2012). The precautionary approach recognizes that, where there is a risk of serious or irreversible harm, cost-effective measures to prevent the reduction or loss of a species should not be postponed for a lack of full scientific certainty. This approach allowed critical habitat to be identified for less-well studied populations such as the Upper Fraser River and for early life stages in areas currently afflicted by recruitment failure.
Information related to the degree of habitat use (high, medium, low) and the overall level of certainty (confirmed use, suspected, unknown) were both categorized, based on abundance and frequency of use. In assessing habitat use the relative size of the population or group of fish using the area was considered. Since different expert groups have assessed habitat use for each population using their own understanding of these terms, caution is warranted when making comparisons between watersheds.
The following guidance was used to identify critical habitats for white sturgeon:
- DFO Operational Guidelines for the Identification of Critical Habitat for Aquatic Species at Risk (Fisheries and Oceans Canada 2012) outline the process to identify the geographic location of critical habitat and the biophysical features of that area, and their attributes, that support functions necessary for the species at risk to carry out the life processes necessary for its survival or recovery.
- The geographic or biophysical features of critical habitat can include: riparian vegetation; areas not presently occupied, or degraded, but required for recovery; the availability of food supply, water depth or flow, physical structures or substrate (i.e., for cover, spawning, rearing and forage activities); etc.
- The DFO Science document, Documenting Habitat Use of Species at Risk and Quantifying Habitat Quality (Fisheries and Oceans Canada 2007), suggests a series of guiding principles to consider when trying to determine critical habitat for a species at risk.
- Provide functional descriptions of the features or attributes that a species’ aquatic habitat must have to allow successful completion of all life history stages.
- Provide information on the spatial extent of the areas that are likely to have the necessary features or attributes.
- Provide advice on how much habitat of various qualities / properties exists at present.
- Provide advice on the degree to which supply of suitable habitat meets the demands of the species both at present, and when the species reaches biologically based recovery targets for abundance, range, and number of populations.
- Provide advice on the extent to which various threats can alter the quality and/or quantity of habitat that is available.
- Provide advice on feasibility of restoring habitat to higher values, if supply may not meet demand by the time recovery targets would be reached.
The guidance outlined above was considered in order to identify, to the extent possible, based on the best available information, the habitat necessary for survival or recovery of white sturgeon, the features of that habitat, the life stages it supports and any threats to that habitat.
On June 23, 2009 a meeting was held by DFO’s internal scientific advisory body - the Pacific Science Advisory Review Committee (PSARC). In attendance were BC MOE staff and invited external white sturgeon experts, First Nations, Environmental Non-Government Organizations (ENGOs), and industry stakeholders that could provide relevant technical advice on critical habitat designations. As the responsible agency, DFO assessed the information and the opinions it received during the PSARC meeting and made a precautionary identification of white sturgeon critical habitats; further information is discussed in the Canadian Science Advisory Secretariat Research Document titled Scientific Information in Support of Identifying Critical Habitat for SARA listed White Sturgeon Populations in Canada: Nechako, Columbia, Kootenay and Upper Fraser (2009) (Hatfield et al. 2012). The scientific information from the research document was used to identify critical habitat for white sturgeon in this recovery strategy10, as outlined in DFO’s operational guidance.
In the following sections, critical habitat for each population of white sturgeon is described, to the extent possible, including the geographic locations and biophysical functions, features and attributes of the critical habitat identified.
Critical habitat identification includes describing the biophysical functions, features, and attributes necessary to support survival or recovery of the species. A function is a characteristic of critical habitat that corresponds to a biological need or life process requirement of the species such as spawning, rearing, feeding and migration. Every function is the result of a single or multiple features, which are physical components or conditions of the critical habitat such as riffles, pools, riparian habitat, etc. Features describe how the habitat provides the critical function to meet the species needs and are always associated with a function. Attributes provide information about a feature that explain how the feature supports the function necessary for the species life process, for example the attributes of a riffle feature that supports the function of rearing may include substrate size, velocity, water chemistry, prey species and temperature.
General information on life stages and biophysical functions, features and attributes for white sturgeon is provided in Section 2.3 (Life Stages) and Section 3 (Description of Needs of the Species) and more specific information for each population follows in tables 7 (Upper Fraser), 10 (Nechako), 14 (Upper Columbia – ALR) , 15 (Upper Columbia - Transboundary, and 17 (Kootenay).
These tables summarize the best available information of the functions, features and attributes for each life-stage of the white sturgeon (refer to Sections 2.3 and 3 for full references). Note that in tables 7, 10, 14, 15, and 17, not all attributes may be known or identified for given features that are identified as critical habitat and in some cases existing habitat attributes are not ideal for the current function sturgeon use it for. The areas described in tables 7, 10, 14, 15,and 17 are considered critical habitat for the species, even though some of the associated attributes might be outside of the range indicated in the tables. Ongoing studies related to the recruitment failure hypothesis will provide further clarity to this section.
Maps of the identified critical habitat areas are provided for each specific population (sections 8.3-8.6). The locations of the critical habitat’s functions, features and attributes have been identified using the critical habitat parcel approach (CHPA). The CHPA is defined as when:
Critical habitat is the exact area within the identified boundaries and it is understood that this area supports the functions and features necessary for the species’ survival or recovery.
The identified critical habitat areas contain the biophysical functions, features, and attributes necessary to achieve the species’ recovery goal and population and distribution objectives (Section 7), and to support survival or recovery of the species. For geographic coordinate points situated at the wetted boundary, this boundary is meant to represent the annual high water mark (Hatfield et al., 2012).
6 Although Duncan Reservoir, Slocan Lake and Revelstoke Reservoir are within the historic range of white sturgeon, recovery of populations in these relatively small waterbodies has been deemed infeasible. There is insufficient data on Kinbasket Reservoir to recommend whether or not the establishment of a self-sustaining population in that area is feasible or recommended.
7 McElhany et al. (2000) were concerned explicitly with Pacific salmon (Oncorhynchus spp.) populations. Their discussion provides much general guidance, but some of their criteria and definitions have been adjusted for this recovery strategy to remain relevant to the biology of white sturgeon.
8 Priority has been assigned based on professional judgement of the National Recovery Team into one of three groups, from highest to lowest: necessary, primary, secondary.
9 As white sturgeon is a slow-growing, late-maturing, and long-lived species, performance measures were developed that could be measured repeatedly throughout the recovery process. Therefore, performance measures plot the progress toward meeting the stated objectives. The performance measures are presented here as questions, the answers to which can be plotted in time to monitor progress.
10 One critical habitat area boundary in the Columbia River (Robson Reach) was amended, through consensus of the National Recovery Team, in February 2013.
Knowledge of habitat use for the Upper Fraser white sturgeon population is more limited than for other populations. A variety of studies have been completed showing habitat associations based on capture rates, but no spawning sites have been confirmed at this time. Recent, but limited, telemetry data exists for mature sturgeon (Cory Williamson, B.C. Ministry of Environment, personal communication). High use habitats have been identified for juvenile rearing and feeding, adult holding and feeding, and adult overwintering life stages. This information is summarized in Table 6.
Two of the locations identified (Red Rock and Cottonwood Canyon) are downstream of the Nechako-Fraser confluence: therefore are in an area of overlap between the Upper Fraser population and the mid-Fraser population. These areas are identified as critical habitat based solely on their influence on the Upper Fraser population.
Table 6. Summary of information base for white sturgeon critical habitats in the Upper Fraser River. An empty cell means that the life stage does not consistently use the habitat. The table has two main columns from left to right: Location (see Figure 14 for basin overview), and Confirmed (Checkmark), Suspected (S), or Possible (question mark) Use by Life Stage and Degree of Use (H=High, M=Moderate, L=Low). The latter column has seven sub-columns from left to right: Spawn, Yolk sac larvae/feeding larvae, Early juvenile, Late Juvenile and Adult, Overwintering, Staging, and Overall assessment. Directly below column headings are 9 rows, read from left to right.
Row 1: Penny, question mark, question mark, blank cell, checkmark H, checkmark H, blank cell, Critical. Row 2: Longworth Grand Canyon, (S)M, question mark, blank cell, checkmark H, checkmark H, question mark, Critical. Row 3: Bowron River Confluence, (S) H, question mark, (checkmark) question mark, (checkmark) H, (S) H, question mark, Critical. Row 4: McGregor River to Limestone Creek, question mark, question mark, empty cell, (checkmark) H, (checkmark) H, question mark, Critical. Row 5: Giscome at Tay Creek, question mark, question mark, empty cell, (checkmark) H, (S) H, empty cell, Critical. Row 6: Willow River Confluence, (S) M, question mark, empty cell, (checkmark) H, (checkmark) H, empty cell, Critical. Row 7: Salmon River Confluence, question mark, question mark, empty cell, (checkmark) H, (checkmark) H, empty cell, Critical. Row 8: Nechako River Confluence, (S) M, question mark, empty cell, (checkmark) H, (S) H, empty cell, Critical. Row 9: Red Rock, (S) H, question mark, empty cell, (checkmark) H, (S) H, empty cell, Critical.
Confirmed (√), Suspected (S), or Possible (?) Use by Life Stage and Degree of Use (H=High, M=Moderate, L=Low)
Table 7 summarizes the critical habitat function(s), features and attributes, to the extent possible, for the Upper Fraser population of white sturgeon.
Table 7. Table 7 provides a summary of the biophysical features, functions, attributes and locations of critical habitat for Upper Fraser River white sturgeon. The first column describes the geographic locations of the critical habitat, which encompass areas within the Upper Fraser River where white sturgeon reside. The second column indicates the life stage that uses each respective critical habitat area. The third column indicates the function that the particular life stage undertakes in each area. The fourth column describes the critical habitat feature that provides the function, and the fifth column details the attributes that the critical habitat feature must have in order to provide the biological function needed to support Upper Fraser River white sturgeon survival or recovery. The final column contains notes.
1 Depositional Area - typically lower velocity areas where fish can rest and prey species may congregate; often in close proximity to confluences with other water bodies providing further access to food sources
The following locations of the critical habitat’s functions, features and attributes have been identified using the critical habitat parcel approach.
Figure 4. Reference map for locations of Upper Fraser River white sturgeon critical habitats.
Figure 4. This is a map of the Upper Fraser River system showing an overview of critical habitat locations. Critical habitat includes aquatic habitat features and attributes that Upper Fraser River white sturgeon use to carry out life functions. Nine locations within the vicinity of Prince George are labelled on a map of British Columbia as follows: Red Rock, Nechako River Confluence, Salmon River Confluence, Willow River Confluence, Giscome Tay Creek, McGregor River to Limestone Creek, Bowron River Confluence, Grand Canyon Longworth and Penny. A scale and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.
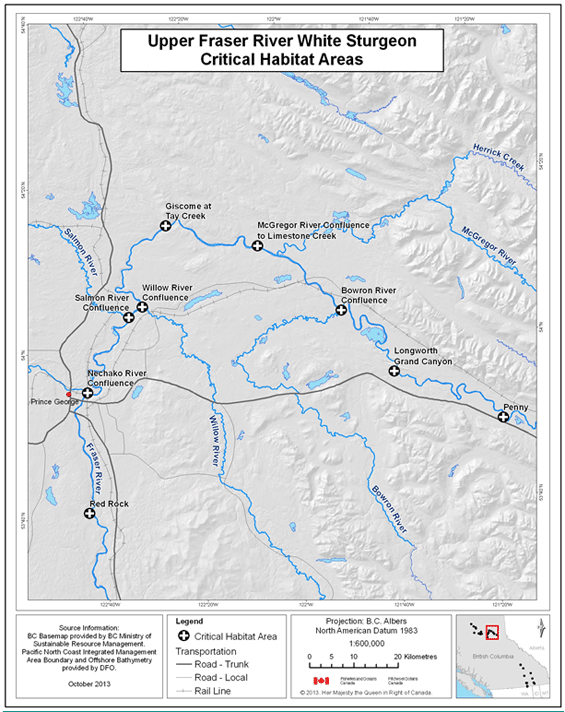
Figure 5. Map of critical habitat for Upper Fraser River white sturgeon: Penny.
Figure 5. Figure 5 is a map of a section of the Upper Fraser River, British Columbia, showing the critical habitat location of Penny. The map depicts a polygon that has been identified as critical habitat for Upper Fraser River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Fraser River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 8. The critical habitat polygon in the Penny map is also labeled with codes that correspond to codes used to identify the polygon in Table 8. A scale of 1:15,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 6. Map of critical habitat for Upper Fraser River white sturgeon: Longworth Grand Canyon.
Figure 6. Figure 6 is a map of a section of the Upper Fraser River, British Columbia, showing the critical habitat location of Longworth Grand Canyon. The map depicts a polygon that has been identified as critical habitat for Upper Fraser River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Fraser River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 8. The critical habitat polygon in the Longworth Grand Canyon map is also labeled with codes that correspond to codes used to identify the polygon in Table 8. A scale of 1:36,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.
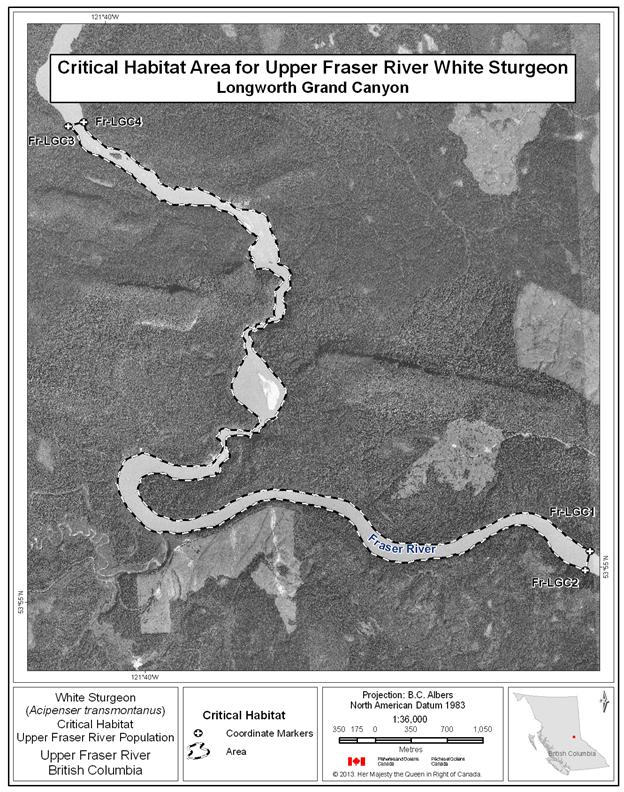
Figure 7. Map of critical habitat for Upper Fraser River white sturgeon: Bowron River confluence with the Fraser River.
Figure 7. Figure 7 is a map of a section of the Upper Fraser River, British Columbia, showing the critical habitat location Bowron River confluence with the Fraser River. The map depicts a polygon that has been identified as critical habitat for Upper Fraser River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Fraser River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 8. The critical habitat polygon in the Bowron River confluence with the Fraser River map is also labeled with codes that correspond to codes used to identify the polygon in Table 8. A scale of 1:18,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.
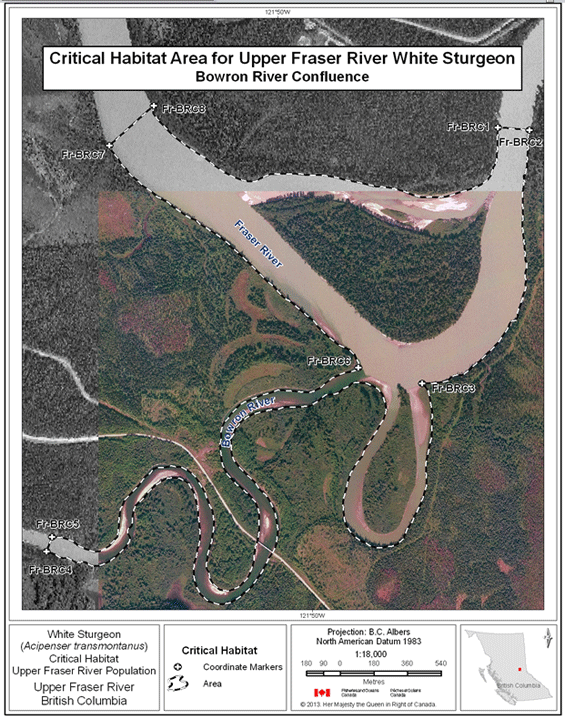
Figure 8. Map of critical habitat for Upper Fraser River white sturgeon: McGregor River confluence with the Fraser River to Limestone Creek confluence.
Figure 8. Figure 8 is a map of a section of the Upper Fraser River, British Columbia, showing the critical habitat location McGregor River confluence with the Fraser River to Limestone Creek confluence. The map depicts a polygon that has been identified as critical habitat for Upper Fraser River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Fraser River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 8. The critical habitat polygon in the McGregor River confluence with the Fraser River to Limestone Creek confluence map is also labeled with codes that correspond to codes used to identify the polygon in Table 8. A scale of 1:107,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 9. Map of critical habitat for Upper Fraser River white sturgeon: Giscome at Tay Creek.
Figure 9. Figure 9 is a map of a section of the Upper Fraser River, British Columbia, showing the critical habitat location Giscome at Tay Creek. The map depicts a polygon that has been identified as critical habitat for Upper Fraser River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Fraser River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 8. The critical habitat polygon in the Giscome at Tay Creek map is also labeled with codes that correspond to codes used to identify the polygon in Table 8. A scale of 1:16,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 10. Map of critical habitat for Upper Fraser River white sturgeon: Willow River confluence with the Fraser River.
Figure 10. Figure 10 is a map of a section of the Upper Fraser River, British Columbia, showing the critical habitat location Willow River confluence with the Fraser River. The map depicts a polygon that has been identified as critical habitat for Upper Fraser River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Fraser River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 8. The critical habitat polygon in the Willow River confluence with the Fraser River map is also labeled with codes that correspond to codes used to identify the polygon in Table 8. A scale of 1:30,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 11. Map of critical habitat for Upper Fraser River white sturgeon: Salmon River confluence with the Fraser River.
Figure 11. Figure 11 is a map of a section of the Upper Fraser River, British Columbia, showing the critical habitat location Salmon River confluence with the Fraser River. The map depicts a polygon that has been identified as critical habitat for Upper Fraser River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Fraser River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 8. The critical habitat polygon in the Salmon River confluence with the Fraser River map is also labeled with codes that correspond to codes used to identify the polygon in Table 8. A scale of 1:15,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.
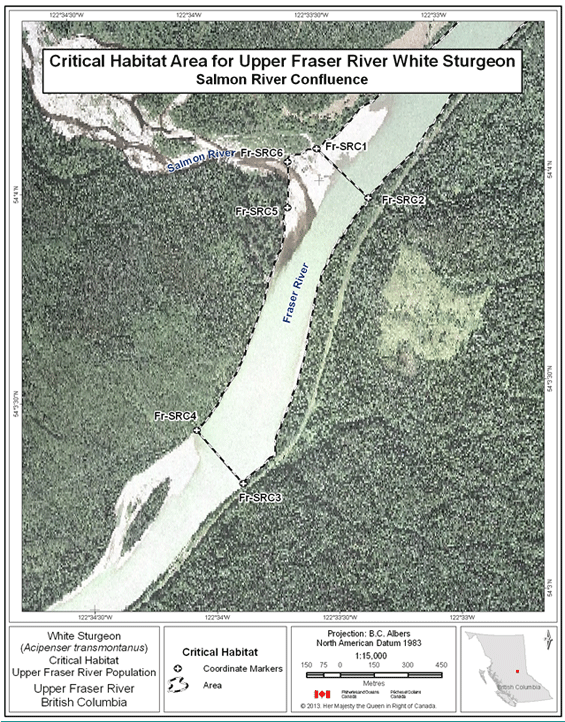
Figure 12. Map of critical habitat for Upper Fraser River white sturgeon: Nechako River confluence with the Fraser River.
Figure 12. Figure 12 is a map of a section of the Upper Fraser River, British Columbia, showing the critical habitat location Nechako River confluence with the Fraser River. The map depicts a polygon that has been identified as critical habitat for Upper Fraser River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Fraser River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 8. The critical habitat polygon in the Nechako River confluence with the Fraser River map is also labeled with codes that correspond to codes used to identify the polygon in Table 8. A scale of 1:24,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 13. Map of critical habitat for Upper Fraser River white sturgeon: Red Rock.
Figure 13. Figure 13 is a map of a section of the Upper Fraser River, British Columbia, showing the critical habitat location Red Rock. The map depicts a polygon that has been identified as critical habitat for Upper Fraser River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Fraser River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 8. The critical habitat polygon in the Red Rock map is also labeled with codes that correspond to codes used to identify the polygon in Table 8. A scale of 1:70,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Table 8. Geographic Coordinates of Critical Habitat Areas for Upper Fraser River white sturgeon. A footnote on the word “coordinates” in the previous sentence states the following: Coordinate points were digitized using various orthophotos provided by Fisheries and Oceans Canada. The resolution of the various orthophotos varied significantly - ranging from 0.2 m cell size to 24 m cell size. This should be taken into consideration when evaluating the accuracy of the coordinates associated with these points. For geographic coordinate points situated at the wetted boundary, this boundary is meant to represent the annual high water mark (Hatfield et al., 2012). Note: For the Fraser River, relative locations are measured as “river kilometers”, which increase from the river mouth (Rkm 0) upstream to the farthest extent possible.
The table has eight columns read left to right: Critical Habitat Name, Coordinate Marker, Waterbody, River Kilometer, Latitude (DD), Longitude (DD), Latitude (DMS), Longitude (DMS). DD refers to Decimal Degrees and DMS refers to Degrees, Minutes, Seconds. Directly below the column headings there are 46 rows. Six rows correspond to the Fraser – Bowron River Confluence area, four to the Fraser – Giscombe at Tay Creek area, four to Fraser-Longworth Grand Canyon area, four to the Fraser McGregor River Confluence to Limestone Creek area, six to the Fraser - Nechako River Confluence area, four to the Fraser – Penny area, four to the Fraser – Red Rock area, four to the Fraser – Salmon River Confluence area, and six to the Fraser – Willow River Confluence area.
| Critical Habitat Name | Coordinate Marker | Waterbody | River Kilometer | Latitude (DD) | Longitude (DD) | Latitude (DMS) | Longitude (DMS) |
|---|---|---|---|---|---|---|---|
| Fraser - Bowron River Confluence | Fr-BRC1 | Fraser River | 922.7 | 54.070 | -121.816 | 54° 4' 11" N | 121° 48' 57" W |
| Fraser - Bowron River Confluence | Fr-BRC2 | Fraser River | 922.7 | 54.069 | -121.813 | 54° 4' 10" N | 121° 48' 48" W |
| Fraser - Bowron River Confluence | Fr-BRC3 | Bowron River | 0.1 | 54.058 | -121.823 | 54° 3' 28" N | 121° 49' 24" W |
| Fraser - Bowron River Confluence | Fr- BRC4 | Bowron River | 3.8 | 54.051 | -121.855 | 54° 3' 3" N | 121° 51' 17" W |
| Fraser - Bowron River Confluence | Fr- BRC5 | Bowron River | 3.8 | 54.051 | -121.854 | 54° 3' 5" N | 121° 51' 15" W |
| Fraser - Bowron River Confluence | Fr-BRC6 | Bowron River | 0.1 | 54.059 | -121.828 | 54° 3' 31" N | 121° 49' 42" W |
| Fraser - Bowron River Confluence | Fr- BRC7 | Fraser River | 919.1 | 54.070 | -121.848 | 54° 4' 12" N | 121° 50' 52" W |
| Fraser - Bowron River Confluence | Fr- BRC8 | Fraser River | 919.1 | 54.072 | -121.844 | 54° 4' 18" N | 121° 50' 38" W |
| Fraser - Giscome at Tay Creek | Fr-GTC1 | Fraser River | 868.7 | 54.248 | -122.412 | 54° 14' 53" N | 122° 24' 42" W |
| Fraser - Giscome at Tay Creek | Fr-GTC2 | Fraser River | 868.7 | 54.246 | -122.410 | 54° 14' 45" N | 122° 24' 36" W |
| Fraser - Giscome at Tay Creek | Fr-GTC3 | Fraser River | 866.7 | 54.238 | -122.436 | 54° 14' 15" N | 122° 26' 10" W |
| Fraser - Giscome at Tay Creek | Fr-GTC4 | Fraser River | 866.7 | 54.240 | -122.438 | 54° 14' 24" N | 122° 26' 17" W |
| Fraser - Longworth Grand Canyon | Fr-LGC1 | Fraser River | 963.7 | 53.918 | -121.600 | 53° 55' 5" N | 121° 35' 58" W |
| Fraser - Longworth Grand Canyon | Fr-LGC2 | Fraser River | 963.7 | 53.916 | -121.600 | 53° 54' 59" N | 121° 36' 2" W |
| Fraser - Longworth Grand Canyon | Fr-LGC3 | Fraser River | 952.3 | 53.958 | -121.673 | 53° 57' 29" N | 121° 40' 23" W |
| Fraser - Longworth Grand Canyon | Fr-LGC4 | Fraser River | 952.3 | 53.958 | -121.671 | 53° 57' 30" N | 121° 40' 15" W |
| Fraser - McGregor River Confluence to Limestone Creek | Fr-MRLC1 | Fraser River | 902.2 | 54.173 | -122.000 | 54° 10' 22" N | 122° 0' 1" W |
| Fraser - McGregor River Confluence to Limestone Creek | Fr-MRLC2 | Fraser River | 902.2 | 54.169 | -122.002 | 54° 10' 9" N | 122° 0' 7" W |
| Fraser - McGregor River Confluence to Limestone Creek | Fr-MRLC3 | Fraser River | 883.7 | 54.202 | -122.229 | 54° 12' 7" N | 122° 13' 45" W |
| Fraser - McGregor River Confluence to Limestone Creek | Fr-MRLC4 | Fraser River | 883.7 | 54.206 | -122.229 | 54° 12' 23" N | 122° 13' 43" W |
| Fraser - McGregor River Confluence to Limestone Creek | Fr-MRLC5 | McGregor River | 0.3 | 54.180 | -122.035 | 54° 10' 47" N | 122° 2' 7" W |
| Fraser - McGregor River Confluence to Limestone Creek | Fr-MRLC6 | McGregor River | 0.3 | 54.179 | -122.033 | 54° 10' 45" N | 122° 1' 59" W |
| Fraser - Nechako River Confluence | Fr-NRC1 | Fraser River | 799.0 | 53.918 | -122.698 | 53° 55' 3" N | 122° 41' 53" W |
| Fraser - Nechako River Confluence | Fr-NRC2 | Fraser River | 799.0 | 53.915 | -122.701 | 53° 54' 56" N | 122° 42' 3" W |
| Fraser - Nechako River Confluence | Fr-NRC3 | Fraser River | 797.0 | 53.909 | -122.723 | 53° 54' 32" N | 122° 43' 23" W |
| Fraser - Nechako River Confluence | Fr-NRC4 | Fraser River | 797.0 | 53.911 | -122.726 | 53° 54' 40" N | 122° 43' 35" W |
| Fraser - Nechako River Confluence | Fr-NRC5 | Nechako River | 0.3 | 53.916 | -122.720 | 53° 54' 59" N | 122° 43' 12" W |
| Fraser - Nechako River Confluence | Fr- NRC6 | Nechako River | 2.9 | 53.927 | -122.748 | 53° 55' 36" N | 122° 44' 54" W |
| Fraser - Nechako River Confluence | Fr- NRC7 | Nechako River | 2.9 | 53.928 | -122.749 | 53° 55' 41" N | 122° 44' 55" W |
| Fraser - Nechako River Confluence | Fr-NRC8 | Nechako River | 0.3 | 53.919 | -122.710 | 53° 55' 8" N | 122° 42' 35" W |
| Fraser - Penny | Fr-PNY1 | Fraser River | 998.6 | 53.827 | -121.290 | 53° 49' 37" N | 121° 17' 25" W |
| Fraser - Penny | Fr-PNY2 | Fraser River | 998.6 | 53.827 | -121.294 | 53° 49' 36" N | 121° 17' 37" W |
| Fraser - Penny | Fr-PNY3 | Fraser River | 995.5 | 53.839 | -121.320 | 53° 50' 20" N | 121° 19' 12" W |
| Fraser - Penny | Fr-PNY4 | Fraser River | 995.5 | 53.840 | -121.317 | 53° 50' 23" N | 121° 19' 1" W |
| Fraser - Red Rock | Fr-RR1 | Fraser River | 759.1 | 53.622 | -122.679 | 53° 37' 19" N | 122° 40' 43" W |
| Fraser - Red Rock | Fr-RR2 | Fraser River | 759.1 | 53.622 | -122.682 | 53° 37' 20" N | 122° 40' 55" W |
| Fraser - Red Rock | Fr-RR3 | Fraser River | 774.2 | 53.726 | -122.710 | 53° 43' 34" N | 122° 42' 36" W |
| Fraser - Red Rock | Fr-RR4 | Fraser River | 774.2 | 53.726 | -122.708 | 53° 43' 34" N | 122° 42' 27" W |
| Fraser - Salmon River Confluence | Fr-SRC1 | Fraser River | 832.2 | 54.068 | -122.558 | 54° 4' 5" N | 122° 33' 30" W |
| Fraser - Salmon River Confluence | Fr-SRC2 | Fraser River | 832.2 | 54.066 | -122.555 | 54° 3' 57" N | 122° 33' 18" W |
| Fraser - Salmon River Confluence | Fr-SRC3 | Fraser River | 830.8 | 54.055 | -122.564 | 54° 3' 17" N | 122° 33' 52" W |
| Fraser - Salmon River Confluence | Fr-SRC4 | Fraser River | 830.8 | 54.057 | -122.567 | 54° 3' 25" N | 122° 34' 3" W |
| Fraser - Salmon River Confluence | Fr-SRC5 | Salmon River | 54.066 | -122.561 | 54° 3' 56" N | 122° 33' 38" W | |
| Fraser - Salmon River Confluence | Fr-SRC6 | Salmon River | 54.067 | -122.560 | 54° 4' 3" N | 122° 33' 38" W | |
| Fraser - Willow River Confluence | Fr-WRC1 | Fraser River | 837.5 | 54.097 | -122.519 | 54° 5' 48" N | 122° 31' 7" W |
| Fraser - Willow River Confluence | Fr-WRC2 | Fraser River | 837.5 | 54.099 | -122.514 | 54° 5' 57" N | 122° 30' 52" W |
| Fraser - Willow River Confluence | Fr-WRC3 | Willow River | 0.2 | 54.087 | -122.508 | 54° 5' 13" N | 122° 30' 28" W |
| Fraser - Willow River Confluence | Fr- WRC4 | Willow River | 2.3 | 54.080 | -122.492 | 54° 4' 47" N | 122° 29' 32" W |
| Fraser - Willow River Confluence | Fr- WRC5 | Willow River | 2.3 | 54.080 | -122.493 | 54° 4' 47" N | 122° 29' 34" W |
| Fraser - Willow River Confluence | Fr-WRC6 | Willow River | 0.2 | 54.086 | -122.508 | 54° 5' 10" N | 122° 30' 30" W |
| Fraser - Willow River Confluence | Fr- WRC7 | Fraser River | 832.2 | 54.066 | -122.555 | 54° 3' 57" N | 122° 33' 18" W |
| Fraser - Willow River Confluence | Fr- WRC8 | Fraser River | 832.2 | 54.068 | -122.558 | 54° 4' 5" N | 122° 33' 30" W |
11 Coordinate points were digitized using various orthophotos provided by Fisheries and Oceans Canada. The resolution of the various orthophotos varied significantly - ranging from 0.2 m cell size to 24 m cell size. This should be taken into consideration when evaluating the accuracy of the coordinates associated with these points. For geographic coordinate points situated at the wetted boundary, this boundary is meant to represent the annual high water mark (Hatfield et al., 2012).
fix(original) Note: For the Fraser River, relative locations are measured as “river kilometers”, which increase from the river mouth (Rkm 0) upstream to the farthest extent possible.
In the Nechako system, white sturgeon occur from the confluence with the Fraser River, upstream to Cheslatta Falls including Fraser Lake, and through much of the Stuart River watershed, a major tributary. This population is referred to as Nechako River white sturgeon. Data indicate limited movement of Nechako white sturgeon into the Fraser; however, feeding at the Fraser River confluence has been observed. Current distribution in the Nechako may be limited by population declines and the alteration of flows (and related effects) below Kenney Dam (NWSRI 2005).
The information base for the Nechako white sturgeon population is substantial, based on many years of intensive study. High use habitats have been identified for all life stages, and this information is summarized in Table 9Error! Reference source not found.. As additional information is collected through recruitment failure diagnosis, it will become possible to refine existing information on critical habitat.
Table 9. Summary of information base for white sturgeon critical habitats in the Nechako system. An empty cell means that the life stage does not consistently use the habitat. The table has two main columns from left to right: Location (see Figure 14 for basin overview) and Confirmed (Checkmark), Suspected (S), or Possible (question mark) Use by Life Stage and Degree of Use (H=High, M=Moderate, L=Low). The latter column has seven sub-columns from left to right: Spawn, Yolk sac larvae/feeding larvae, Early juvenile, Late Juvenile and Adult, Overwintering, Staging, and Overall assessment. Directly below column headings are 14 rows, read from left to right.
Row 1: Vanderhoof braided section, checkmark (H), checkmark (H), empty cell, empty cell, empty cell, checkmark (H), Critical. Row 2: Sinkut River Confluence, empty cell, empty cell, checkmark (H), checkmark (H), checkmark (H), checkmark (H), Critical. Row 3: Leduc Creek Confluence, empty cell, empty cell, checkmark (H), checkmark (H), checkmark (H), checkmark (H), Critical. Row 4: Finmoore, empty cell, empty cell, checkmark (H), checkmark (H), empty cell, empty cell, Critical. Row 5: Keilor's Point, empty cell, empty cell, checkmark (H), checkmark (H), checkmark (M) checkmark (M), Critical. Row 6: Culvert Hole, empty cell, empty cell, question mark (H), empty cell, checkmark (M), empty cell, Critical. Row 7: Powerline, empty cell, empty cell, question mark (H), empty cell, checkmark (M), empty cell, Critical. Row 8: Sturgeon Point, empty cell, empty cell, empty cell, checkmark (M), (S) M, empty cell, Critical. Row 9: Pinchi Bay on Stuart Lake, empty cell, empty cell, empty cell, checkmark (H), (checkmark) M, empty cell, Critical. Row 10: Tachie River Confluence with Stuart Lake, empty cell, empty cell, empty cell, checkmark (H), (checkmark) M, empty cell, Critical. Row 11: Lower Half of Stuart Lake, empty cell, empty cell, empty cell, checkmark (M), (S) M, empty cell, Critical. Row 12: Middle River, empty cell, empty cell, empty cell, checkmark (M), (S) M, empty cell, Critical. Row 13: Confluence with Trembleur Lake, empty cell, empty cell, empty cell, empty cell, empty cell, empty cell, empty cell. Row 14: Fraser Lake, empty cell, empty cell, empty cell, checkmark (M), (S) M, empty cell, Critical.
Confirmed (√), Suspected (S), or Possible (?) Use by Life Stage and Degree of Use (H=High, M=Moderate, L=Low)
Table 10 summarizes the critical habitat function(s), features and attributes, to the extent possible, for the Nechako population of white sturgeon.
Table 10. This table provides a summary of the biophysical features, functions, attributes and locations of critical habitat for Nechako white sturgeon. The first column describes the geographic locations of the critical habitat, which encompass areas within the Nechako River where white sturgeon reside. The second column indicates the life stage that uses each respective critical habitat area. The third column indicates the function that the particular life stage undertakes in each area. The fourth column describes the critical habitat feature that provides the function, and the fifth column details the attributes that the critical habitat feature must have in order to provide the biological function needed to support Nechako River white sturgeon survival or recovery. The final column contains notes.
The following locations of the critical habitat’s functions, features and attributes have been identified using the critical habitat parcel approach.
Figure 14. Reference map for locations of Nechako River white sturgeon critical habitats.
Figure 14. This is a map of the Nechako River system showing an overview of critical habitat locations. Critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. Thirteen locations centered roughly around Fort St. James are labelled on a map of British Columbia as follows: Mid River Confluence, Tachie River Confluence, Pinchi Bay, Lower Half Stuart Lake, Fraser Lake, Vanderhoof Braided Section, Culvert Hole, Leduc Creek Confluence, Powerline Crossing, Keilor’s Point, Sinkut River, Nechako Finmoore, and Sturgeon Point. A scale and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.
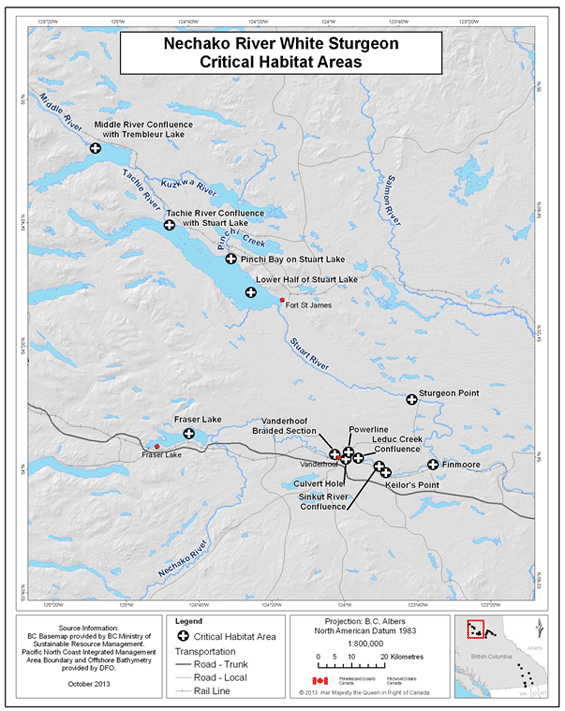
Figure 15. Map of critical habitat for Nechako River white sturgeon: Vanderhoof braided section of the Nechako River.
Figure 15. Figure 15 is a map of a section of the Nechako River, British Columbia, showing the critical habitat location Vanderhoof braided section of the Nechako River. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Vanderhoof braided section of the Nechako River map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:39,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.
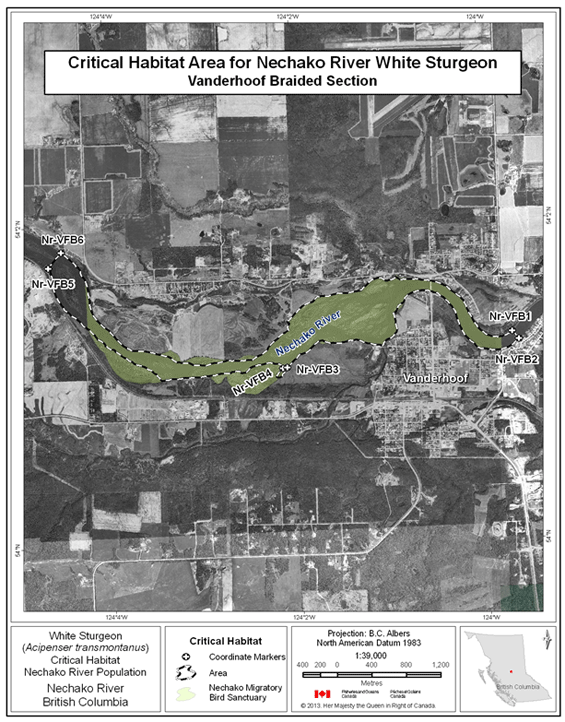
Figure 16. Map of critical habitat for Nechako white sturgeon: Sinkut River confluence with the Nechako River.
Figure 16. Figure 16 is a map of a section of the Nechako River, British Columbia, showing the critical habitat location Sinkut River confluence with the Nechako River. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Sinkut River confluence with the Nechako River map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:12,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 17. Map of critical habitat for Nechako River white sturgeon: Leduc Creek confluence with the Nechako River.
Figure 17. Figure 17 is a map of a section of the Nechako River, British Columbia, showing the critical habitat location Leduc Creek confluence with the Nechako River. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Leduc Creek confluence with the Nechako River map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:10,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 18. Map of critical habitat for Nechako River white sturgeon: Finmoore.
Figure 18. Figure 18 is a map of a section of the Nechako River, British Columbia, showing the critical habitat location Finmoore. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Finmoore map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:50,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 19. Map of critical habitat for Nechako River white sturgeon: Keilor’s Point.
Figure 19. Figure 19 is a map of a section of the Nechako River, British Columbia, showing the critical habitat location Keilor’s Point. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Keilor’s Point map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:10,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.
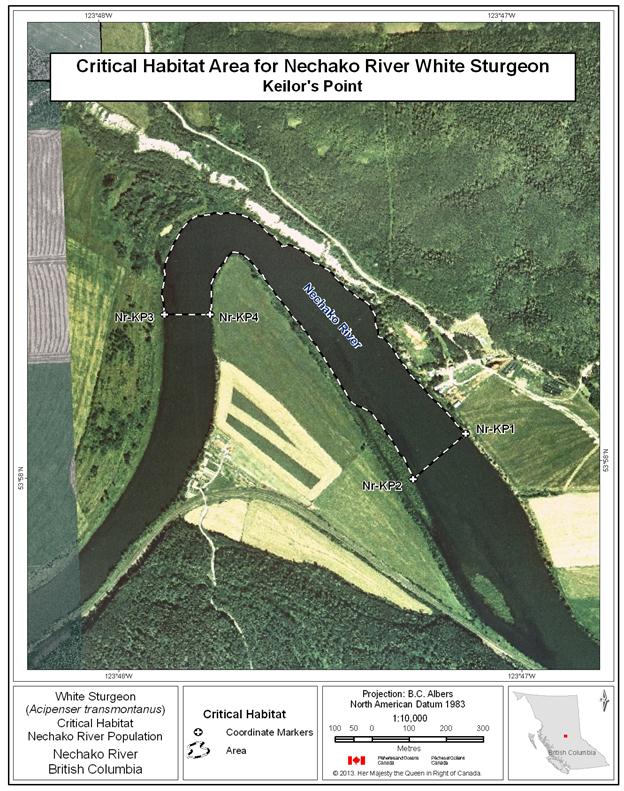
Figure 20. Map of critical habitat for Nechako River white sturgeon: Culvert Hole.
Figure 20. Figure 20 is a map of a section of the Nechako River, British Columbia, showing the critical habitat location Culvert Hole. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Culvert Hole map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:4,500 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 21. Map of critical habitat for Nechako River white sturgeon: Powerline.
Figure 21. Figure 21 is a map of a section of the Nechako River, British Columbia, showing the critical habitat location Powerline. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Powerline map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:7,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 22. Map of critical habitat for Nechako River white sturgeon: Sturgeon Point.
Figure 22. Figure 22 is a map of a section of the Stuart River, British Columbia, showing the critical habitat location Sturgeon Point. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Sturgeon Point map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:11,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 23. Map of critical habitat for Nechako River white sturgeon: Pinchi Bay on Stuart Lake.
Figure 23. Figure 23 is a map of a section of Stuart Lake, British Columbia, showing the critical habitat location Pinchi Bay on Stuart Lake. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Pinchi Bay on Stuart Lake map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:25,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.

Figure 24. Map of critical habitat for Nechako River white sturgeon: Tachie River confluence with Stuart Lake.
Figure 24. Figure 24 is a map of a section of Stuart Lake, British Columbia, showing the critical habitat location Tachie River confluence with Stuart Lake. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Tachie River confluence with Stuart Lake map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:22,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.
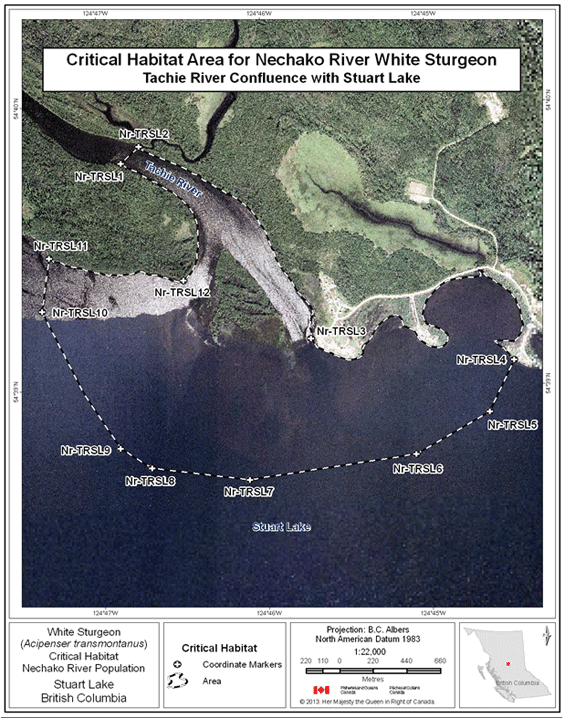
Figure 25. Map of critical habitat for Nechako River white sturgeon: Lower Half of Stuart Lake.
Figure 25. Figure 25 is a map of a section of Stuart Lake, British Columbia, showing the critical habitat location Lower Half of Stuart Lake. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Lower Half of Stuart Lake map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:110,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.
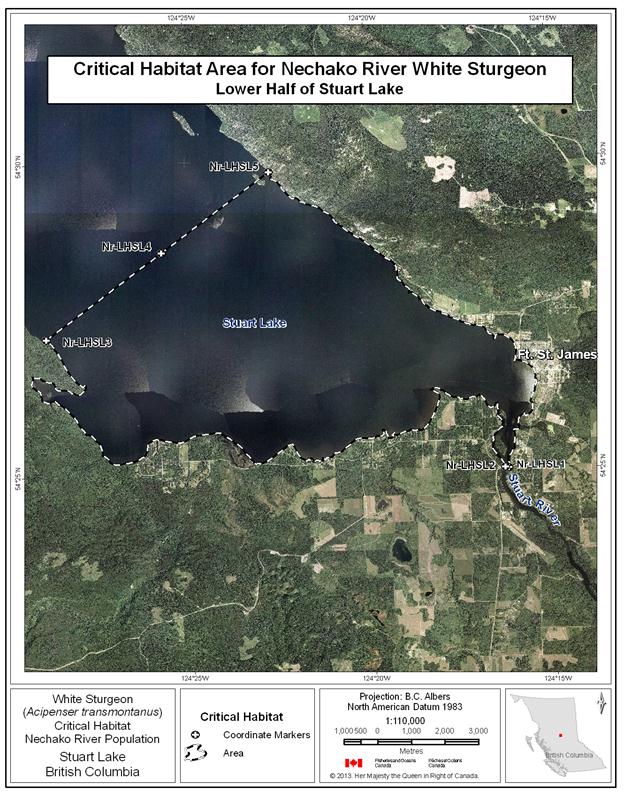
Figure 26. Map of critical habitat for Nechako River white sturgeon: Middle River confluence with Trembleur Lake.
Figure 26. Figure 26 is a map of a section of Trembleur Lake, British Columbia, showing the critical habitat location Middle River confluence with Trembleur Lake. The map depicts a polygon that has been identified as critical habitat for Nechako River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 11. The critical habitat polygon in the Middle River confluence with Trembleur Lake map is also labeled with codes that correspond to codes used to identify the polygon in Table 11. A scale of 1:21,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.
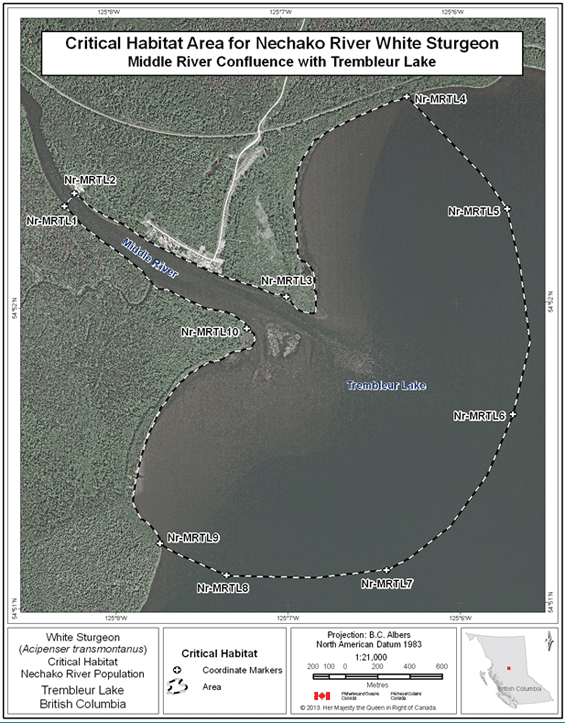
Figure 27. Map of critical habitat for Nechako River white sturgeon: Fraser Lake and Nautley River confluence with the Nechako River.
Figure 27. Figure 27 is a map of Fraser Lake, British Columbia, showing the critical habitat locations of Fraser Lake and the Nautley River confluence with the Nechako River. The map depicts two polygons that have been identified as critical habitat for Nechako River white sturgeon. In the identified polygons, critical habitat includes aquatic habitat features and attributes that Nechako River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygons’ boundaries are listed in Table 11. The critical habitat polygons in Fraser Lake and the Nautley River confluence with the Nechako River map are also labeled with codes that correspond to codes used to identify the polygons in Table 11. A scale of 1:149,000 and legend are provided along with an inset map showing locations are primarily in the Omineca zone of British Columbia. The map is oriented in a “north is up” direction.
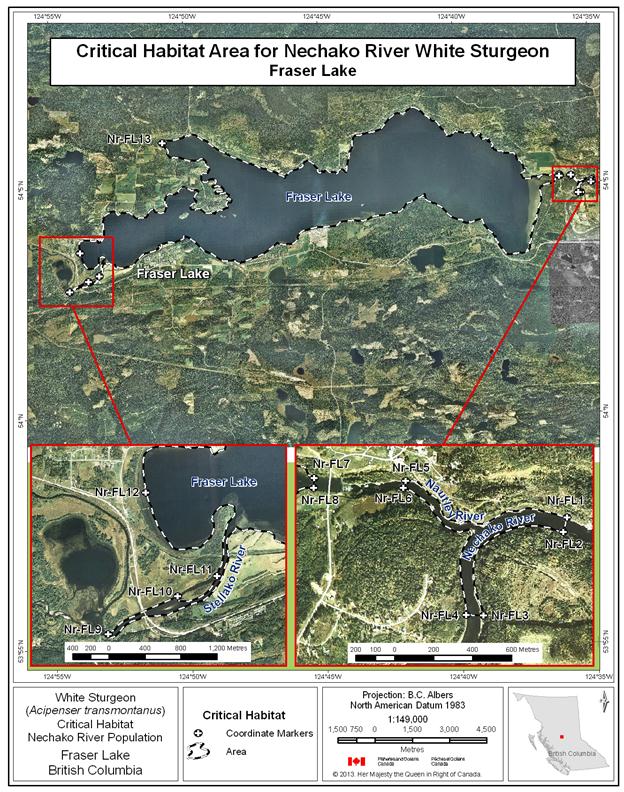
Table 11. Geographic Coordinates of Critical Habitat Areas for Nechako River white sturgeon. A footnote on the word “coordinates” in the previous sentence states the following: Coordinate points were digitized using various orthophotos provided by Fisheries and Oceans Canada. The resolution of the various orthophotos varied significantly - ranging from 0.2 m cell size to 24 m cell size. This should be taken into consideration when evaluating the accuracy of the coordinates associated with these points. For geographic coordinate points situated at the wetted boundary, this boundary is meant to represent the annual high water mark (Hatfield et al., 2012). Note: For the Nechako River, relative locations are measured as “river kilometers”, which increase from the river mouth (Rkm 0) upstream to the farthest extent possible.
The table has eight columns read left to right: Critical Habitat Name, Coordinate Marker, Waterbody, River Kilometer, Latitude (DD), Longitude (DD), Latitude (DMS), Longitude (DMS). DD refers to Decimal Degrees and DMS refers to Degrees, Minutes, Seconds. Directly below the column headings there are 80 rows. Four rows correspond to the Nechako – Culvert Hole area, four to the Nechako – Finmoore area, 13 to the Nechako – Fraser Lake area, four to the Nechako – Keilor’s Point area, four to the Nechako – Leduc Creek Confluence area, five to the Nechako – Lower Half of Stuart Lake area, eight to the Nechako – Middle River Confluence with Trembleur area, six to the Nechako – Pinchi Bay on Stuart Lake area, four to the Nechako – Sturgeon Point area, eight to the Nechako – Tachie River Confluence with Stuart Lake area, and four to the Nechako – Vanderhoof Braided Section area.
| Critical Habitat Name | Coordinate Marker | Waterbody | River Kilometer | Latitude (DD) | Longitude (DD) | Latitude (DMS) | Longitude (DMS) |
|---|---|---|---|---|---|---|---|
| Nechako - Culvert Hole | Nr-CH1 | Nechako River | 132.2 | 54.011 | -123.975 | 54° 0' 40" N | 123° 58' 30" W |
| Nechako - Culvert Hole | Nr-CH2 | Nechako River | 132.2 | 54.010 | -123.975 | 54° 0' 38" N | 123° 58' 28" W |
| Nechako - Culvert Hole | Nr-CH3 | Nechako River | 132.8 | 54.011 | -123.984 | 54° 0' 41" N | 123° 59' 3" W |
| Nechako - Culvert Hole | Nr-CH4 | Nechako River | 132.8 | 54.012 | -123.982 | 54° 0' 43" N | 123° 58' 56" W |
| Nechako - Finmoore | Nr-FMR1 | Nechako River | 90.3 | 53.993 | -123.525 | 53° 59' 36" N | 123° 31' 30" W |
| Nechako - Finmoore | Nr-FMR2 | Nechako River | 90.3 | 53.993 | -123.524 | 53° 59' 33" N | 123° 31' 26" W |
| Nechako - Finmoore | Nr-FMR3 | Nechako River | 98.4 | 53.983 | -123.634 | 53° 58' 59" N | 123° 38' 2" W |
| Nechako - Finmoore | Nr-FMR4 | Nechako River | 98.4 | 53.984 | -123.636 | 53° 59' 3" N | 123° 38' 10" W |
| Nechako - Finmoore | Nr-FMR5 | Stuart River | 0.2 | 53.990 | -123.542 | 53° 59' 24" N | 123° 32' 33" W |
| Nechako - Finmoore | Nr-FMR6 | Stuart River | 0.2 | 53.989 | -123.539 | 53° 59' 21" N | 123° 32' 22" W |
| Nechako - Fraser Lake | Nr-FL1 | Nechako River | 191.3 | 54.084 | -124.582 | 54° 5' 1" N | 124° 34' 56" W |
| Nechako - Fraser Lake | Nr-FL2 | Nechako River | 191.3 | 54.083 | -124.583 | 54° 4' 59" N | 124° 34' 57" W |
| Nechako - Fraser Lake | Nr-FL3 | Nechako River | 192.3 | 54.079 | -124.589 | 54° 4' 45" N | 124° 35' 20" W |
| Nechako - Fraser Lake | Nr-FL4 | Nechako River | 192.3 | 54.079 | -124.590 | 54° 4' 45" N | 124° 35' 25" W |
| Nechako - Fraser Lake | Nr-FL5 | Nautley River | 0.5 | 54.085 | -124.595 | 54° 5' 7" N | 124° 35' 42" W |
| Nechako - Fraser Lake | Nr-FL6 | Nautley River | 0.5 | 54.086 | -124.595 | 54° 5' 8" N | 124° 35' 42" W |
| Nechako - Fraser Lake | Nr-FL7 | Fraser Lake | 1.0 | 54.086 | -124.602 | 54° 5' 9" N | 124° 36' 8" W |
| Nechako - Fraser Lake | Nr-FL8 | Fraser Lake | 1.0 | 54.085 | -124.602 | 54° 5' 7" N | 124° 36' 8" W |
| Nechako - Fraser Lake | Nr-FL9 | Stellako River | 54.046 | -124.906 | 54° 2' 46" N | 124° 54' 20" W | |
| Nechako - Fraser Lake | Nr-FL10 | Stellako River | 54.050 | -124.894 | 54° 2' 59" N | 124° 53' 37" W | |
| Nechako - Fraser Lake | Nr-FL11 | Stellako River | 54.052 | -124.887 | 54° 3' 6" N | 124° 53' 13" W | |
| Nechako - Fraser Lake | Nr-FL12 | Fraser Lake | 54.060 | -124.899 | 54° 3' 37" N | 124° 53' 56" W | |
| Nechako - Fraser Lake | Nr-FL13 | Fraser Lake | 54.100 | -124.847 | 54° 5' 59" N | 124° 50' 50" W | |
| Nechako - Keilor's Point | Nr-KP1 | Nechako River | 109.6 | 53.967 | -123.785 | 53° 58' 3" N | 123° 47' 7" W |
| Nechako - Keilor's Point | Nr-KP2 | Nechako River | 109.6 | 53.966 | -123.788 | 53° 57' 59" N | 123° 47' 15" W |
| Nechako - Keilor's Point | Nr-KP3 | Nechako River | 110.8 | 53.971 | -123.798 | 53° 58' 14" N | 123° 47' 52" W |
| Nechako - Keilor's Point | Nr-KP4 | Nechako River | 110.8 | 53.970 | -123.796 | 53° 58' 14" N | 123° 47' 45" W |
| Nechako - Leduc Creek Confluence | Nr-LCC1 | Nechako River | 124.8 | 54.007 | -123.918 | 54° 0' 23" N | 123° 55' 4" W |
| Nechako - Leduc Creek Confluence | Nr-LCC2 | Nechako River | 124.8 | 54.006 | -123.919 | 54° 0' 21" N | 123° 55' 7" W |
| Nechako - Leduc Creek Confluence | Nr-LCC3 | Nechako River | 126.2 | 54.012 | -123.927 | 54° 0' 42" N | 123° 55' 36" W |
| Nechako - Leduc Creek Confluence | Nr-LCC4 | Nechako River | 126.2 | 54.013 | -123.928 | 54° 0' 46" N | 123° 55' 39" W |
| Nechako - Lower Half of Stuart Lake | Nr-LHSL1 | Stuart River | 109.3 | 54.417 | -124.271 | 54° 25' 1" N | 124° 16' 14" W |
| Nechako - Lower Half of Stuart Lake | Nr-LHSL2 | Stuart River | 109.3 | 54.417 | -124.272 | 54° 25' 1" N | 124° 16' 20" W |
| Nechako - Lower Half of Stuart Lake | Nr-LHSL3 | Stuart Lake | 13.2 | 54.453 | -124.482 | 54° 27' 12" N | 124° 28' 55" W |
| Nechako - Lower Half of Stuart Lake | Nr-LHSL4 | Stuart Lake | 13.2 | 54.476 | -124.428 | 54° 28' 34" N | 124° 25' 41" W |
| Nechako - Lower Half of Stuart Lake | Nr-LHSL5 | Stuart Lake | 13.2 | 54.497 | -124.378 | 54° 29' 49" N | 124° 22' 41" W |
| Nechako - Middle River Confluence with Trembleur Lake | Nr-MRTL1 | Middle River | 1.4 | 54.872 | -125.138 | 54° 52' 20" N | 125° 8' 16" W |
| Nechako - Middle River Confluence with Trembleur Lake | Nr-MRTL2 | Middle River | 1.4 | 54.873 | -125.137 | 54° 52' 23" N | 125° 8' 13" W |
| Nechako - Middle River Confluence with Trembleur Lake | Nr-MRTL3 | Trembleur Lake | 13.5 | 54.867 | -125.116 | 54° 52' 2" N | 125° 6' 59" W |
| Nechako - Middle River Confluence with Trembleur Lake | Nr- MRTL4 | Trembleur Lake | 54.878 | -125.105 | 54° 52' 41" N | 125° 6' 16" W | |
| Nechako - Middle River Confluence with Trembleur Lake | Nr- MRTL5 | Trembleur Lake | 54.872 | -125.095 | 54° 52' 19" N | 125° 5' 42" W | |
| Nechako - Middle River Confluence with Trembleur Lake | Nr- MRTL6 | Trembleur Lake | 11.2 | 54.860 | -125.095 | 54° 51' 38" N | 125° 5' 41" W |
| Nechako - Middle River Confluence with Trembleur Lake | Nr- MRTL7 | Trembleur Lake | 54.852 | -125.107 | 54° 51' 7" N | 125° 6' 26" W | |
| Nechako - Middle River Confluence with Trembleur Lake | Nr- MRTL8 | Trembleur Lake | 54.852 | -125.123 | 54° 51' 6" N | 125° 7' 21" W | |
| Nechako - Middle River Confluence with Trembleur Lake | Nr- MRTL9 | Trembleur Lake | 54.854 | -125.129 | 54° 51' 13" N | 125° 7' 44" W | |
| Nechako - Middle River Confluence with Trembleur Lake | Nr-MRTL10 | Trembleur Lake | 0.0 | 54.865 | -125.120 | 54° 51' 55" N | 125° 7' 13" W |
| Nechako - Pinchi Bay on Stuart Lake | Nr-PBSL1 | Pinchi Creek | 54.567 | -124.484 | 54° 34' 0" N | 124° 29' 3" W | |
| Nechako - Pinchi Bay on Stuart Lake | Nr-PBSL2 | Stuart Lake | 54.562 | -124.486 | 54° 33' 44" N | 124° 29' 8" W | |
| Nechako - Pinchi Bay on Stuart Lake | Nr-PBSL3 | Stuart Lake | 54.562 | -124.487 | 54° 33' 43" N | 124° 29' 12" W | |
| Nechako - Pinchi Bay on Stuart Lake | Nr-PBSL4 | Stuart Lake | 54.563 | -124.489 | 54° 33' 46" N | 124° 29' 19" W | |
| Nechako - Pinchi Bay on Stuart Lake | Nr- PBSL5 | Stuart Lake | 54.558 | -124.474 | 54° 33' 30" N | 124° 28' 25" W | |
| Nechako - Pinchi Bay on Stuart Lake | Nr- PBSL6 | Stuart Lake | 54.556 | -124.488 | 54° 33' 21" N | 124° 29' 18" W | |
| Nechako - Pinchi Bay on Stuart Lake | Nr-PBSL7 | Stuart Lake | 54.557 | -124.498 | 54° 33' 24" N | 124° 29' 53" W | |
| Nechako - Pinchi Bay on Stuart Lake | Nr- PBSL8 | Stuart Lake | 54.559 | -124.502 | 54° 33' 32" N | 124° 30' 9" W | |
| Nechako - Pinchi Bay on Stuart Lake | Nr- PBSL9 | Stuart Lake | 54.564 | -124.507 | 54° 33' 52" N | 124° 30' 24" W | |
| Nechako - Pinchi Bay on Stuart Lake | Nr- PBSL10 | Stuart Lake | 54.568 | -124.506 | 54° 34' 5" N | 124° 30' 21" W | |
| Nechako - Pinchi Bay on Stuart Lake | Nr-PBSL11 | Stuart Lake | 54.564 | -124.490 | 54° 33' 50" N | 124° 29' 23" W | |
| Nechako - Powerline | Nr-PL1 | Nechako River | 129.5 | 54.027 | -123.964 | 54° 1' 37" N | 123° 57' 49" W |
| Nechako - Powerline | Nr-PL2 | Nechako River | 129.5 | 54.026 | -123.964 | 54° 1' 34" N | 123° 57' 51" W |
| Nechako - Powerline | Nr-PL3 | Nechako River | 130.4 | 54.024 | -123.972 | 54° 1' 27" N | 123° 58' 20" W |
| Nechako - Powerline | Nr-PL4 | Nechako River | 130.4 | 54.025 | -123.970 | 54° 1' 28" N | 123° 58' 13" W |
| Nechako - Sinkut River Confluence | Nr-SRC1 | Nechako River | 115.2 | 53.982 | -123.841 | 53° 58' 55" N | 123° 50' 28" W |
| Nechako - Sinkut River Confluence | Nr-SRC2 | Nechako River | 115.2 | 53.981 | -123.843 | 53° 58' 52" N | 123° 50' 34" W |
| Nechako - Sinkut River Confluence | Nr-SRC3 | Nechako River | 118.3 | 53.992 | -123.832 | 53° 59' 32" N | 123° 49' 55" W |
| Nechako - Sinkut River Confluence | Nr-SRC4 | Nechako River | 118.3 | 53.994 | -123.831 | 53° 59' 40" N | 123° 49' 52" W |
| Nechako - Sturgeon Point | Nr-SP1 | Stuart River | 48.4 | 54.164 | -123.651 | 54° 9' 52" N | 123° 39' 4" W |
| Nechako - Sturgeon Point | Nr-SP2 | Stuart River | 48.4 | 54.164 | -123.651 | 54° 9' 49" N | 123° 39' 4" W |
| Nechako - Sturgeon Point | Nr-SP3 | Stuart River | 51.4 | 54.177 | -123.653 | 54° 10' 39" N | 123° 39' 12" W |
| Nechako - Sturgeon Point | Nr-SP4 | Stuart River | 51.4 | 54.178 | -123.653 | 54° 10' 42" N | 123° 39' 12" W |
| Nechako - Tachie River Confluence with Stuart Lake | Nr-TRSL1 | Tachie River | 0.9 | 54.663 | -124.781 | 54° 39' 48" N | 124° 46' 52" W |
| Nechako - Tachie River Confluence with Stuart Lake | Nr-TRSL2 | Tachie River | 0.9 | 54.664 | -124.779 | 54° 39' 51" N | 124° 46' 45" W |
| Nechako - Tachie River Confluence with Stuart Lake | Nr-TRSL3 | Stuart Lake | 45.7 | 54.653 | -124.762 | 54° 39' 10" N | 124° 45' 43" W |
| Nechako - Tachie River Confluence with Stuart Lake | Nr- TRSL4 | Stuart Lake | 54.651 | -124.741 | 54° 39' 5" N | 124° 44' 29" W | |
| Nechako - Tachie River Confluence with Stuart Lake | Nr-TRSL5 | Stuart Lake | 54.648 | -124.744 | 54° 38' 54" N | 124° 44' 38" W | |
| Nechako - Tachie River Confluence with Stuart Lake | Nr- TRSL6 | Stuart Lake | 54.646 | -124.751 | 54° 38' 45" N | 124° 45' 5" W | |
| Nechako - Tachie River Confluence with Stuart Lake | Nr- TRSL7 | Stuart Lake | 44.1 | 54.645 | -124.768 | 54° 38' 41" N | 124° 46' 6" W |
| Nechako - Tachie River Confluence with Stuart Lake | Nr- TRSL8 | Stuart Lake | 54.645 | -124.778 | 54° 38' 43" N | 124° 46' 42" W | |
| Nechako - Tachie River Confluence with Stuart Lake | Nr-TRSL9 | Stuart Lake | 54.647 | -124.782 | 54° 38' 48" N | 124° 46' 54" W | |
| Nechako - Tachie River Confluence with Stuart Lake | Nr- TRSL10 | Stuart Lake | 54.655 | -124.789 | 54° 39' 17" N | 124° 47' 21" W | |
| Nechako - Tachie River Confluence with Stuart Lake | Nr- TRSL11 | Stuart Lake | 54.658 | -124.788 | 54° 39' 28" N | 124° 47' 18" W | |
| Nechako - Tachie River Confluence with Stuart Lake | Nr-TRSL12 | Stuart Lake | 45.7 | 54.656 | -124.775 | 54° 39' 23" N | 124° 46' 29" W |
| Nechako - Vanderhoof Braided Section | Nr-VFB1 | Nechako River | 135.1 | 54.021 | -123.995 | 54° 1' 17" N | 123° 59' 42" W |
| Nechako - Vanderhoof Braided Section | Nr-VFB2 | Nechako River | 135.1 | 54.021 | -123.994 | 54° 1' 14" N | 123° 59' 37" W |
| Nechako - Vanderhoof Braided Section | Nr-VFB3 | Stoney Creek | 54.018 | -124.035 | 54° 1' 6" N | 124° 2' 6" W | |
| Nechako - Vanderhoof Braided Section | Nr-VFB4 | Stoney Creek | 54.018 | -124.036 | 54° 1' 6" N | 124° 2' 9" W | |
| Nechako - Vanderhoof Braided Section | Nr- VFB5 | Nechako River | 141.9 | 54.029 | -124.077 | 54° 1' 45" N | 124° 4' 38" W |
| Nechako - Vanderhoof Braided Section | Nr- VFB6 | Nechako River | 141.9 | 54.031 | -124.075 | 54° 1' 51" N | 124° 4' 29" W |
12 Coordinate points were digitized using various orthophotos provided by Fisheries and Oceans Canada. The resolution of the various orthophotos varied significantly - ranging from 0.2 m cell size to 24 m cell size. This should be taken into consideration when evaluating the accuracy of the coordinates associated with these points. For geographic coordinate points situated at the wetted boundary, this boundary is meant to represent the annual high water mark (Hatfield et al., 2012).
Note: For the Nechako River, relative locations are measured as “river kilometers”, which increase from the river mouth (Rkm 0) upstream to the farthest extent possible.
The current distribution of the Columbia River population resides in the upper Columbia River from Revelstoke (REV) Dam to Grand Coulee Dam (Washington), as well as in the lower Kootenay River from its confluence with the Columbia River to Brilliant Dam (Figure 3). Studies conducted on Columbia River white sturgeon are divided among the following population components: i) Transboundary Reach, 56 km of riverine habitat located between HLK Dam and the Canada-U.S. border, including the small section of river in the lower Kootenay River below Brilliant Dam; ii) Arrow Lakes Reservoir (ALR), 230 km of riverine and lacustrine habitat located from REV to HLK Dams; and, iii) Roosevelt Reach (FDR), from the U.S. border downstream. The transboundary nature of this population requires that recovery efforts be coordinated across multiple jurisdictions. Since SARA is Canadian legislation, only critical habitat identification in Canada is addressed.
Remnant population components may also exist upstream of the ALR component (i.e. between REV and Mica Dams and in the Kinbasket Reservoir) but investigations have not captured white sturgeon at this time. Given the large size of these reservoirs, the failure to catch a white sturgeon does not necessarily preclude their existence, but would suggest that population densities are very low (RL&L Environmental Services Ltd. 2000b).
The following sections discuss the ALR component (Table 12) and the transboundary component (Table 13) separately.
Table 12. Summary of information base for white sturgeon critical habitats in the ALR area of the Columbia River. An empty cell means that the life stage does not consistently use the habitat. The table has two main columns from left to right: Location (see Figure 28 for basin overview) and Confirmed (Checkmark), Suspected (S), or Possible (question mark) Use by Life Stage and Degree of Use (H=High, M=Moderate, L=Low). The latter column has seven sub-columns from left to right: Spawn, Yolk sac larvae/feeding larvae, Early juvenile, Late Juvenile and Adult, Overwintering, Staging, and Overall assessment. Directly below column headings are five rows, read from left to right.
Row 1: Columbia River adjacent to Revelstoke Golf Course, checkmark (M), S (L), empty cell, checkmark (L), empty cell, empty cell, Critical. Row 2: Big Eddy, empty cell, question mark, empty cell, checkmark (L), empty cell, checkmark (M), Critical. Row 3: Salmon Rocks, empty cell, question mark, empty cell, checkmark (L), empty cell, checkmark (M), Critical. Row 4: Beaton Reach, empty cell, empty cell, checkmark (M), checkmark (H), checkmark (H), empty cell, Critical. Row 5: Narrow Burton Reach, empty cell, empty cell, S (L), checkmark (M), empty cell, empty cell, Critical.
Confirmed (√), Suspected (S), or Possible (?) Use by Life Stage and Degree of Use (H=High, M=Moderate, L=Low)
Table 13. Summary of information base for white sturgeon critical habitats in the transboundary area of the Columbia River. An empty cell means that the life stage does not consistently use the habitat. The table has two main columns from left to right: Location (see Figure 28 for basin overview) and Confirmed (checkmark), Suspected (S), or Possible (question mark) Use by Life Stage and Degree of Use (H=High, M=Moderate, L=Low). The latter column has seven sub-columns from left to right: Spawn, Yolk sac larvae/feeding larvae, Early juvenile, Late Juvenile and Adult, Overwintering, Staging, and Overall Assessment. Directly below column headings are seven rows, read from left to right.
Row 1: Robson Reach, checkmark (H), question mark (H), checkmark (H), checkmark (H), checkmark (H), checkmark (M), Critical. Row 2: Kootenay Eddy, empty cell, empty cell, checkmark (M), checkmark (H), checkmark (M), checkmark (L-M), Critical. Row 3: Fort Shepherd Eddy, empty cell, empty cell, checkmark (H), checkmark (H), checkmark (H), checkmark (H), Critical. Row 4: Waneta Eddy, empty cell, empty cell, checkmark (H), checkmark (H), checkmark (H), checkmark (H), Critical. Row 5: Pend d’Oreille – Columbia Confluence, checkmark (H), checkmark (H), checkmark (L), checkmark (M), empty cell, checkmark (H), Critical. Row 6: Bridge Hole, empty cell, empty cell, S (L), checkmark (M), checkmark (M), empty cell, Critical. Row 7: Brilliant Tailrace, empty cell, empty cell, S (L), checkmark (M), checkmark (M), empty cell, Critical.
Confirmed (√),Suspected (S), or Possible (?) Use by Life Stage and Degree of Use (H=High, M=Moderate, L=Low)
Tables 14 and 15 summarize the critical habitat function(s), features and attributes, to the extent possible, for the Upper Columbia River population of white sturgeon.
Table 14. This table provides a summary of the biophysical features, functions, attributes and locations of critical habitat for Upper Columbia River white sturgeon in Arrow Lakes Reservoir. The first column describes the geographic locations of the critical habitat, which encompass areas within the Upper Columbia River system where white sturgeon reside. The second column indicates the life stage that uses each respective critical habitat area. The third column indicates the function that the particular life stage undertakes in each area. The fourth column describes the critical habitat feature that provides the function, and the fifth column details the attributes that the critical habitat feature must have in order to provide the biological function needed to support Upper Columbia River white sturgeon survival or recovery. The final column contains notes.
1 Depositional area– typically lower velocity areas where fish can rest and prey species may congregate; often in close proximity to confluences with other water bodies providing further access to food sources
Table 15. This table provides a summary of the biophysical features, functions, attributes and locations of critical habitat for Upper Columbia River white sturgeon in the Columbia Transboundary Reach. The first column describes the geographic locations of the critical habitat, which encompass areas within the Upper Columbia River system where white sturgeon reside. The second column indicates the life stage that uses each respective critical habitat area. The third column indicates the function that the particular life stage undertakes in each area. The fourth column describes the critical habitat feature that provides the function, and the fifth column details the attributes that the critical habitat feature must have in order to provide the biological function needed to support Upper Columbia River white sturgeon survival or recovery. The final column contains notes.
1 Depositional area– typically lower velocity areas where fish can rest and prey species may congregate; often in close proximity to confluences with other water bodies providing further access to food sources
The following locations of the critical habitat’s functions, features and attributes have been identified using the critical habitat parcel approach. Critical habitat downstream of existing hydroelectric facilities does not include the physical structure of the dam, although it may include anthropogenic features such as rip rap downstream of the facilities.
Figure 28. Reference map for locations of Upper Columbia River white sturgeon critical habitats.
Figure 28. This is a map of the Upper Columbia River showing an overview of critical habitat locations. Critical habitat includes aquatic habitat features and attributes that Upper Columbia River white sturgeon use to carry out life functions. Nine locations in the vicinity of Revelstoke, Castlegar and Trail are labelled on a map of British Columbia as follows: Columbia River adjacent to Revelstoke Golf Course, Salmon Rocks/Big Eddy, Beaton Reach, Narrow Burton Reach, Robson Reach, Bridge Hole, Brilliant Tailrace, Kootenay Eddy, Ft. Shepherd Eddy, Waneta Eddy/Pend d’Oreille River Confluence. A scale and legend are provided along with an inset map showing locations are primarily in the south-eastern corner of British Columbia. The map is oriented in a “north is up” direction.

Figure 29. Map of critical habitat for Upper Columbia River white sturgeon: Columbia River adjacent to Revelstoke Golf Course, Big Eddy and Salmon Rocks.
Figure 29. Figure 29 is a map of a section of Arrow Lakes Reservoir, British Columbia, showing the critical habitat locations of Big Eddy, Salmon Rocks, and the Columbia River adjacent to Revelstoke Golf Course. The map depicts three polygons that have been identified as critical habitat for Upper Columbia River white sturgeon. In the identified polygons, critical habitat includes aquatic habitat features and attributes that Upper Columbia River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygons’ boundaries are listed in Table 16. The critical habitat polygons in the Big Eddy, Salmon Rocks, and the Columbia River adjacent to Revelstoke Golf Course map are also labeled with codes that correspond to codes used to identify the polygons in Table 16. A scale of 1:29,000 and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.
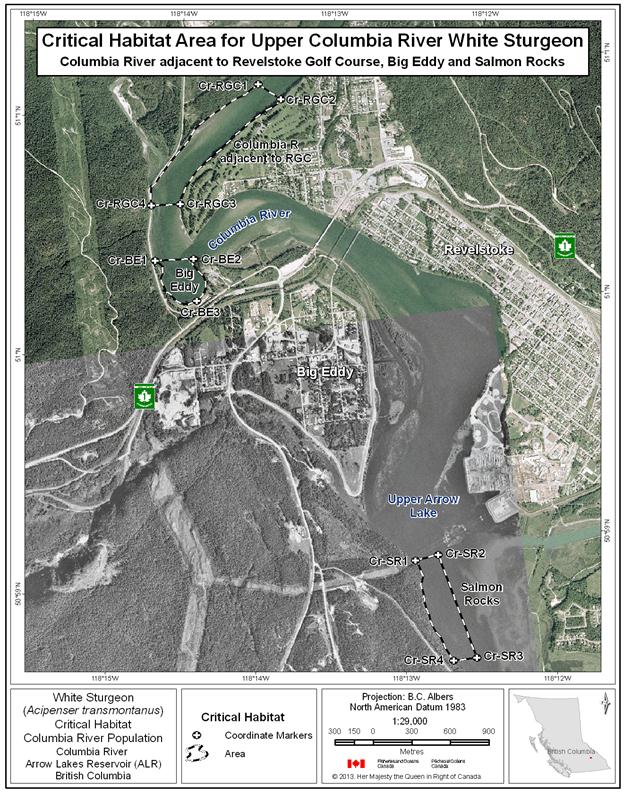
Figure 30. Map of critical habitat for Upper Columbia River white sturgeon: Beaton Reach.
Figure 30. Figure 30 is a map of a section of Arrow Lakes Reservoir, British Columbia, showing the critical habitat location Beaton Reach. The map depicts a polygon that has been identified as critical habitat for Upper Columbia River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Columbia River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 16. The critical habitat polygon in the Beaton Reach map is also labeled with codes that correspond to codes used to identify the polygon in Table 16. A scale of 1:73,000 and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.

Figure 31. Map of critical habitat for Upper Columbia River white sturgeon: Narrow Burton Reach.
Figure 31. Figure 31 is a map of a section of Arrow Lakes Reservoir, British Columbia, showing the critical habitat location Narrow Burton Reach. The map depicts a polygon that has been identified as critical habitat for Upper Columbia River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Columbia River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 16. The critical habitat polygon in the Narrow Burton Reach map is also labeled with codes that correspond to codes used to identify the polygon in Table 16. A scale of 1:73,000 and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.
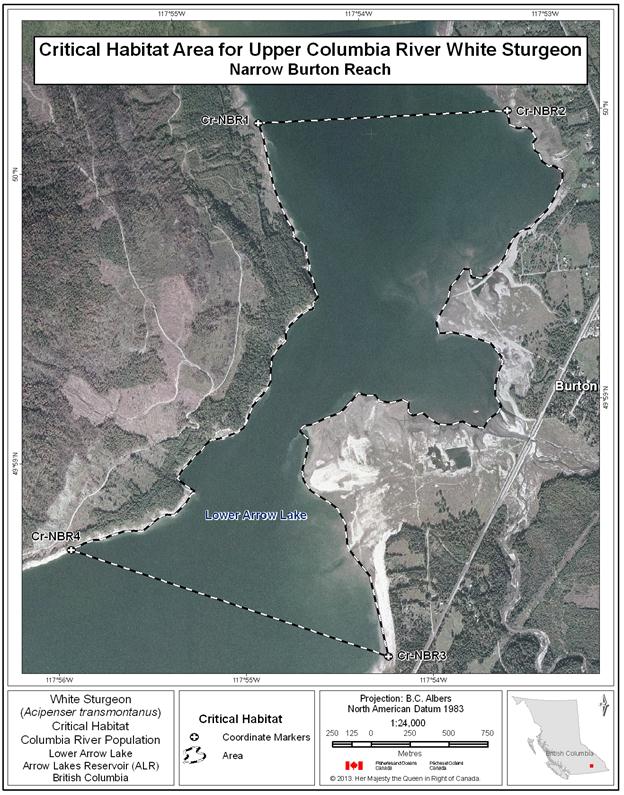
Figure 32. Map of critical habitat for Upper Columbia River white sturgeon: Robson Reach.
Figure 32. Figure 32 is a map of a section of the Columbia River, British Columbia, showing the critical habitat location Robson Reach. The map depicts a polygon that has been identified as critical habitat for Upper Columbia River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Columbia River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 16. The critical habitat polygon in the Robson Reach map is also labeled with codes that correspond to codes used to identify the polygon in Table 16. A scale of 1:55,000 and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.
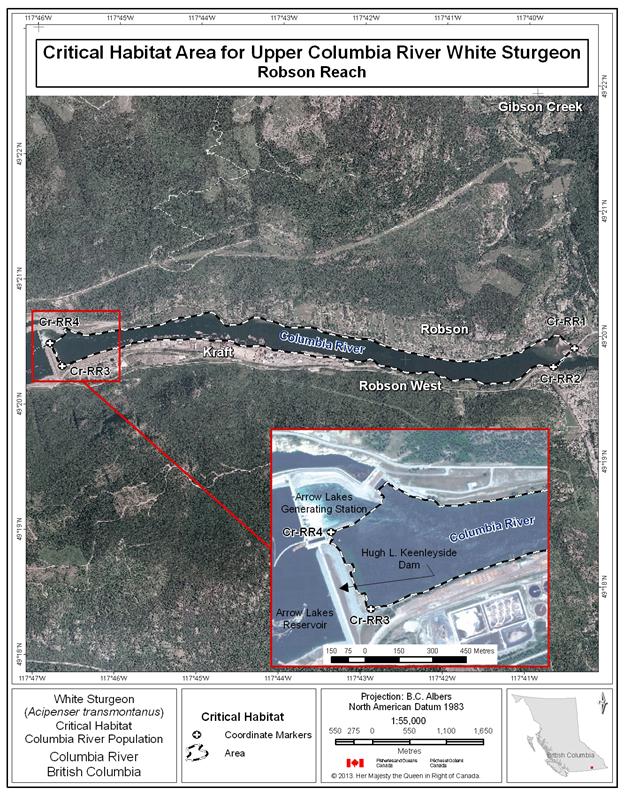
Figure 33. Map of critical habitat for Upper Columbia River white sturgeon: Kootenay Eddy, Bridge Hole and Brilliant Tailrace.
Figure 33. Figure 33 is a map of a section of the Lower Kootenay River, British Columbia, showing the critical habitat locations of Kootenay Eddy, Bridge Hole and Brilliant Tailrace. The map depicts three polygons that have been identified as critical habitat for Upper Columbia River white sturgeon. In the identified polygons, critical habitat includes aquatic habitat features and attributes that Upper Columbia River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygons’ boundaries are listed in Table 16. The critical habitat polygons in the Kootenay Eddy, Bridge Hole and Brilliant Tailrace map are also labeled with codes that correspond to codes used to identify the polygons in Table 16. A scale of 1:16,000 and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.
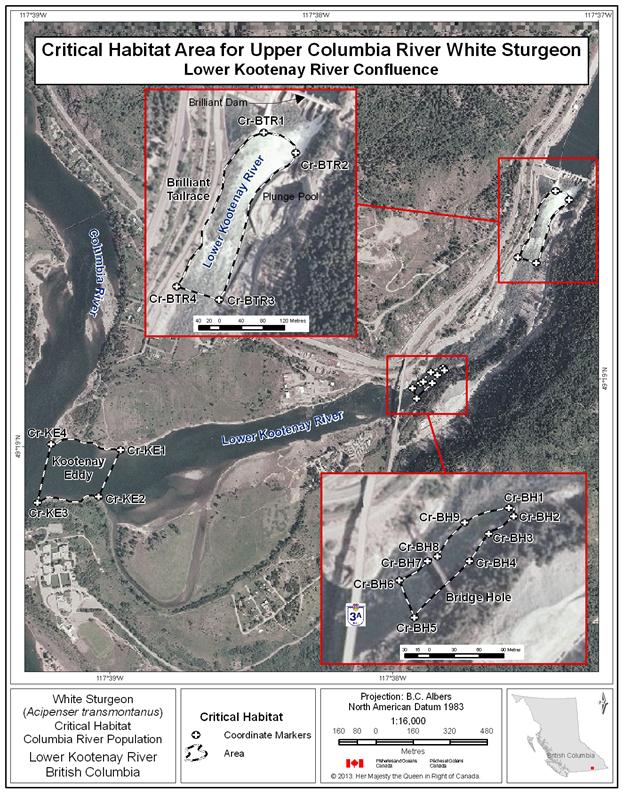
Figure 34. Map of critical habitat for Upper Columbia River white sturgeon: Fort Shepherd Eddy.
Figure 34. Figure 34 is a map of a section of the Columbia River, British Columbia, showing the critical habitat location Fort Shepherd Eddy. The map depicts a polygon that has been identified as critical habitat for Upper Columbia River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Upper Columbia River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 16. The critical habitat polygon in the Fort Shepherd Eddy map is also labeled with codes that correspond to codes used to identify the polygon in Table 16. A scale of 1:7,000 and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.

Figure 35. Map of critical habitat for Upper Columbia River white sturgeon: Waneta Eddy and Pend d’Oreille confluence with the Columbia River.
Figure 35. Figure 35 is a map of a section of the Columbia River, British Columbia, showing the critical habitat locations Waneta Eddy and the Pend d’Oreille confluence with the Columbia River. The map depicts two polygons that have been identified as critical habitat for Upper Columbia River white sturgeon. In the identified polygons, critical habitat includes aquatic habitat features and attributes that Upper Columbia River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygons’ boundaries are listed in Table 16. The critical habitat polygons in the Waneta Eddy and Pend d’Oreille confluence with the Columbia River map are also labeled with codes that correspond to codes used to identify the polygons in Table 16. A scale of 1:9,000 and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.

Table 16. Geospatial Coordinates of Critical Habitat Areas for Upper Columbia River white sturgeon. A footnote on the word “coordinates” in the previous sentence states the following: Coordinate points were digitized using various orthophotos provided by Fisheries and Oceans Canada. The resolution of the various orthophotos varied significantly - ranging from 0.2 m cell size to 24 m cell size. This should be taken into consideration when evaluating the accuracy of the coordinates associated with these points. For geographic coordinate points situated at the wetted boundary, this boundary is meant to represent the annual high water mark (Hatfield et al., 2012). Note: For the Canadian portion of the Columbia River, river kilometres start at Hugh L. Keenleyside Dam (HLK) Dam in Castlegar and increase moving downstream to the Canada/U.S. border (HLK = 0 km, Canada/U.S. border ~ 57.0 km). River kilometres also increase on the upstream side of HLK Dam, starting at 0 km at the dam and increasing to the headwaters of the Columbia River.
The table has eight columns read left to right: Critical Habitat Name, Coordinate Marker, Waterbody, River Kilometer, Latitude (DD), Longitude (DD), Latitude (DMS), Longitude (DMS). DD refers to Decimal Degrees and DMS refers to Degrees, Minutes, Seconds. Directly below the column headings there are 63 rows. Nine rows correspond to the Columbia – Bridge Hole area, four to the Columbia – Brilliant Tailrace area, four to the Fort Shepherd Eddy area, five to the Columbia – Pend d’Oreille Confluence area, four to the Columbia – Robson Reach area, four to the Columbia – Waneta Eddy area, six to the Columbia (ALR) – Beaton Reach area, three to the Columbia (ALR) – Big Eddy area, four to the Columbia (ALR) – Columbia River adjacent to Revelstoke Golf Course area, four to the Columbia (ALR) – Narrow Burton Reach, and four to the Columbia (ALR) – Salmon Rocks area.
| Critical Habitat Name | Coordinate Marker | Waterbody | River Kilometer | Latitude (DD) | Longitude (DD) | Latitude (DMS) | Longitude (DMS) |
|---|---|---|---|---|---|---|---|
| Columbia - Bridge Hole | Cr-BH1 | Lower Kootenay River | 49.318 | -117.628 | 49° 19' 4" N | 117° 37' 42" W | |
| Columbia - Bridge Hole | Cr-BH2 | Lower Kootenay River | 49.318 | -117.628 | 49° 19' 4" N | 117° 37' 41" W | |
| Columbia - Bridge Hole | Cr-BH3 | Lower Kootenay River | 49.318 | -117.629 | 49° 19' 3" N | 117° 37' 43" W | |
| Columbia - Bridge Hole | Cr-BH4 | Lower Kootenay River | 49.317 | -117.629 | 49° 19' 2" N | 117° 37' 44" W | |
| Columbia - Bridge Hole | Cr-BH5 | Lower Kootenay River | 49.317 | -117.630 | 49° 19' 0" N | 117° 37' 48" W | |
| Columbia - Bridge Hole | Cr-BH6 | Lower Kootenay River | 49.317 | -117.630 | 49° 19' 2" N | 117° 37' 49" W | |
| Columbia - Bridge Hole | Cr-BH7 | Lower Kootenay River | 49.317 | -117.630 | 49° 19' 2" N | 117° 37' 47" W | |
| Columbia - Bridge Hole | Cr-BH8 | Lower Kootenay River | 49.317 | -117.630 | 49° 19' 2" N | 117° 37' 46" W | |
| Columbia - Bridge Hole | Cr-BH9 | Lower Kootenay River | 49.318 | -117.629 | 49° 19' 4" N | 117° 37' 44" W | |
| Columbia - Brilliant Tailrace | Cr-BTR1 | Lower Kootenay River | 49.324 | -117.620 | 49° 19' 27" N | 117° 37' 13" W | |
| Columbia - Brilliant Tailrace | Cr-BTR2 | Lower Kootenay River | 49.324 | -117.620 | 49° 19' 25" N | 117° 37' 11" W | |
| Columbia - Brilliant Tailrace | Cr-BTR3 | Lower Kootenay River | 49.321 | -117.622 | 49° 19' 17" N | 117° 37' 19" W | |
| Columbia - Brilliant Tailrace | Cr-BTR4 | Lower Kootenay River | 49.322 | -117.623 | 49° 19' 18" N | 117° 37' 23" W | |
| Columbia - Fort Shepherd Eddy | Cr-FSE1 | Columbia River | 49.028 | -117.603 | 49° 1' 40" N | 117° 36' 11" W | |
| Columbia - Fort Shepherd Eddy | Cr-FSE2 | Columbia River | 49.027 | -117.606 | 49° 1' 35" N | 117° 36' 22" W | |
| Columbia - Fort Shepherd Eddy | Cr-FSE3 | Columbia River | 49.032 | -117.615 | 49° 1' 55" N | 117° 36' 53" W | |
| Columbia - Fort Shepherd Eddy | Cr-FSE4 | Columbia River | 49.033 | -117.613 | 49° 2' 0" N | 117° 36' 45" W | |
| Columbia - Kootenay Eddy | Cr-KE1 | Lower Kootenay River | 49.316 | -117.648 | 49° 18' 58" N | 117° 38' 52" W | |
| Columbia - Kootenay Eddy | Cr-KE2 | Lower Kootenay River | 49.314 | -117.649 | 49° 18' 52" N | 117° 38' 58" W | |
| Columbia - Kootenay Eddy | Cr-KE3 | Lower Kootenay River | 49.314 | -117.653 | 49° 18' 52" N | 117° 39' 11" W | |
| Columbia - Kootenay Eddy | Cr-KE4 | Lower Kootenay River | 49.317 | -117.652 | 49° 19' 0" N | 117° 39' 7" W | |
| Columbia - Pend d'Oreille Confluence | Cr-POC1 | Pend d'Oreille River | 49.005 | -117.619 | 49° 0' 17" N | 117° 37' 7" W | |
| Columbia - Pend d'Oreille Confluence | Cr-POC2 | Pend d'Oreille River | 49.003 | -117.619 | 49° 0' 12" N | 117° 37' 7" W | |
| Columbia - Pend d'Oreille Confluence | Cr-POC3 | Columbia River | 49.001 | -117.630 | 49° 0' 3" N | 117° 37' 46" W | |
| Columbia - Pend d'Oreille Confluence | Cr-POC4 | Columbia River | 49.001 | -117.633 | 49° 0' 3" N | 117° 38' 0" W | |
| Columbia - Pend d'Oreille Confluence | Cr-POC5 | Columbia River | 49.002 | -117.631 | 49° 0' 7" N | 117° 37' 52" W | |
| Columbia - Pend d'Oreille Confluence | Cr- POC6 | Columbia River | 49.003 | -117.630 | 49° 0' 11" N | 117° 37' 46" W | |
| Columbia - Pend d'Oreille Confluence | Cr-POC7 | Columbia River | 49.004 | -117.627 | 49° 0' 13" N | 117° 37' 36" W | |
| Columbia - Robson Reach | Cr-RR1 | Columbia River | 49.332 | -117.665 | 49° 19' 56" N | 117° 39' 55" W | |
| Columbia - Robson Reach | Cr-RR2 | Columbia River | 49.330 | -117.670 | 49° 19' 48" N | 117° 40' 12" W | |
| Columbia - Robson Reach | Cr-RR3 | Columbia River | 49.338 | -117.770 | 49° 20' 16" N | 117° 46' 12" W | |
| Columbia - Robson Reach | Cr-RR4 | Columbia River | 49.341 | -117.772 | 49° 20' 28" N | 117° 46' 19" W | |
| Columbia - Waneta Eddy | Cr-WE1 | Columbia River | 49.006 | -117.622 | 49° 0' 23" N | 117° 37' 19" W | |
| Columbia - Waneta Eddy | Cr-WE2 | Columbia River | 49.007 | -117.620 | 49° 0' 24" N | 117° 37' 13" W | |
| Columbia - Waneta Eddy | Cr-WE3 | Columbia River | 49.007 | -117.618 | 49° 0' 26" N | 117° 37' 6" W | |
| Columbia - Waneta Eddy | Cr- WE4 | Columbia River | 49.008 | -117.616 | 49° 0' 28" N | 117° 36' 58" W | |
| Columbia - Waneta Eddy | Cr- WE5 | Columbia River | 49.005 | -117.619 | 49° 0' 17" N | 117° 37' 7" W | |
| Columbia - Waneta Eddy | Cr- WE6 | Columbia River | 49.004 | -117.625 | 49° 0' 15" N | 117° 37' 28" W | |
| Columbia (ALR) - Beaton Reach | Cr-BR1 | Upper Arrow Lake | 188.0 | 50.698 | -117.984 | 50° 41' 53" N | 117° 59' 1" W |
| Columbia (ALR) - Beaton Reach | Cr-BR2 | Upper Arrow Lake | 188.0 | 50.706 | -117.948 | 50° 42' 23" N | 117° 56' 51" W |
| Columbia (ALR) - Beaton Reach | Cr-BR3 | Upper Arrow Lake | 6.5 | 50.703 | -117.848 | 50° 42' 11" N | 117° 50' 53" W |
| Columbia (ALR) - Beaton Reach | Cr-BR4 | Upper Arrow Lake | 6.5 | 50.691 | -117.838 | 50° 41' 29" N | 117° 50' 17" W |
| Columbia (ALR) - Beaton Reach | Cr-BR5 | Upper Arrow Lake | 180.0 | 50.652 | -117.876 | 50° 39' 7" N | 117° 52' 33" W |
| Columbia (ALR) - Beaton Reach | Cr-BR6 | Upper Arrow Lake | 180.0 | 50.634 | -117.921 | 50° 38' 3" N | 117° 55' 14" W |
| Columbia (ALR) - Big Eddy | Cr-BE1 | Columbia River | 51.006 | -118.239 | 51° 0' 20" N | 118° 14' 22" W | |
| Columbia (ALR) - Big Eddy | Cr-BE2 | Columbia River | 51.005 | -118.235 | 51° 0' 19" N | 118° 14' 7" W | |
| Columbia (ALR) - Big Eddy | Cr-BE3 | Columbia River | 51.002 | -118.235 | 51° 0' 9" N | 118° 14' 7" W | |
| Columbia (ALR) - Columbia River adjacent to Revelstoke Golf Course | Cr-RGC1 | Columbia River | 232.8 | 51.017 | -118.226 | 51° 1' 1" N | 118° 13' 33" W |
| Columbia (ALR) - Columbia River adjacent to Revelstoke Golf Course | Cr-RGC2 | Columbia River | 232.8 | 51.016 | -118.224 | 51° 0' 57" N | 118° 13' 25" W |
| Columbia (ALR) - Columbia River adjacent to Revelstoke Golf Course | Cr-RGC3 | Columbia River | 231.6 | 51.009 | -118.236 | 51° 0' 33" N | 118° 14' 9" W |
| Columbia (ALR) - Columbia River adjacent to Revelstoke Golf Course | Cr-RGC4 | Columbia River | 231.6 | 51.009 | -118.239 | 51° 0' 34" N | 118° 14' 21" W |
| Columbia (ALR) - Narrow Burton Reach | Cr-NBR1 | Columbia River | 99.0 | 50.001 | -117.910 | 50° 0' 5" N | 117° 54' 36" W |
| Columbia (ALR) - Narrow Burton Reach | Cr-NBR2 | Columbia River | 99.0 | 50.000 | -117.888 | 50° 0' 2" N | 117° 53' 15" W |
| Columbia (ALR) - Narrow Burton Reach | Cr-NBR3 | Lower Arrow Lake | 95.5 | 49.970 | -117.904 | 49° 58' 11" N | 117° 54' 14" W |
| Columbia (ALR) - Narrow Burton Reach | Cr-NBR4 | Lower Arrow Lake | 95.5 | 49.978 | -117.931 | 49° 58' 41" N | 117° 55' 52" W |
| Columbia (ALR) - Salmon Rocks | Cr-SR1 | Upper Arrow Lake | 226.8 | 50.983 | -118.214 | 50° 58' 58" N | 118° 12' 52" W |
| Columbia (ALR) - Salmon Rocks | Cr-SR2 | Upper Arrow Lake | 226.8 | 50.983 | -118.212 | 50° 58' 58" N | 118° 12' 42" W |
| Columbia (ALR) - Salmon Rocks | Cr-SR3 | Upper Arrow Lake | 226.1 | 50.975 | -118.209 | 50° 58' 32" N | 118° 12' 32" W |
| Columbia (ALR) - Salmon Rocks | Cr-SR4 | Upper Arrow Lake | 226.1 | 50.975 | -118.211 | 50° 58' 31" N | 118° 12' 41" W |
13 Coordinate points were digitized using various orthophotos provided by Fisheries and Oceans Canada. The resolution of the various orthophotos varied significantly - ranging from 0.2 m cell size to 24 m cell size. This should be taken into consideration when evaluating the accuracy of the coordinates associated with these points. For geographic coordinate points situated at the wetted boundary, this boundary is meant to represent the annual high water mark (Hatfield et al., 2012).
Note: For the Canadian portion of the Columbia River, river kilometres start at Hugh L. Keenleyside Dam (HLK) Dam in Castlegar and increase moving downstream to the Canada/U.S. border (HLK = 0 km, Canada/U.S. border ~ 57.0 km). River kilometres also increase on the upstream side of HLK Dam, starting at 0 km at the dam and increasing to the headwaters of the Columbia River.
The Kootenay River population of white sturgeon extends from Kootenai Falls, Montana, located 50 river-kilometres downstream of Libby Dam (Idaho, U.S.), through Kootenay Lake to Corra Linn Dam on the lower West Arm of Kootenay Lake, British Columbia. Spawning habitat is located in the U.S., whereas much of the adult and juvenile rearing habitat is located in the Canadian portion of Kootenay River plus Kootenay Lake (e.g. Kootenay delta and tributary creek mouths). Off channel wetland habitat is likely valuable for early life stages, and historically was in greater abundance than at present. The transboundary nature of the Kootenay River population requires that recovery efforts be coordinated across multiple jurisdictions. Recovery of this population will require critical habitats to be designated and managed in both countries in a coordinated manner.
The information base for the Kootenay River white sturgeon population is substantial, based on many years of intensive study, although relatively more information is available for habitats in the U.S. than in Canada. High use habitats within the Canadian portion of its range have been identified for all life stages, and this information is summarized in Table 17. As additional information is collected, it will become possible to refine critical habitat designations.
Table 17. Summary of information base for white sturgeon critical habitats in the Kootenay River. A blank cell means that the life stage does not consistently use the habitat. The table has two main columns from left to right: Location (see Figure 36 for basin overview) and Confirmed (Checkmark), Suspected (S), or Possible (question mark) Use by Life Stage and Degree of Use (H=High, M=Moderate, L=Low, U=Unknown). The latter column has seven sub-columns from left to right: Spawn, Yolk sac larvae/feeding larvae, Early juvenile, Late Juvenile and Adult, Overwintering, Staging, and Overall assessment. Directly below column headings are four rows, read from left to right.
Row 1: Lower Kootenay River, empty cell, S (U), checkmark (M), checkmark (M), checkmark (M), checkmark (M), Critical. Row 2: Kootenay River Delta, empty cell, question mark (U), checkmark (H), checkmark (H), checkmark (H), checkmark (M), Critical. Row 3: Duncan Delta on Kootenay Lake, empty cell, empty cell, S (L), checkmark (H), checkmark (M), checkmark (L), Critical. Row 4: Crawford Creek Delta on Kootenay Lake, empty cell, empty cell, checkmark (L), checkmark (M), checkmark (M), checkmark (L), Critical.
Confirmed (√), Suspected (S), or Possible (?) Use by Life Stage and Relative Density (H=High, M=Moderate, L=Low, U=Unknown)
Table 18 summarizes the critical habitat function(s), features and attributes, to the extent possible, for the Kootenay River population of white sturgeon.
Table 18. This table provides a summary of the biophysical features, functions, attributes and locations of critical habitat for Kootenay River white sturgeon. The first column describes the geographic locations of the critical habitat, which encompass areas within the Kootenay River system where white sturgeon reside. The second column indicates the life stage that uses each respective critical habitat area. The third column indicates the function that the particular life stage undertakes in each area. The fourth column describes the critical habitat feature that provides the function, and the fifth column details the attributes that the critical habitat feature must have in order to provide the biological function needed to support Kootenay River white sturgeon survival or recovery. The final column contains notes.
*Depositional area– typically lower velocity areas where fish can rest and prey species may congregate; often in close proximity to confluences with other water bodies providing further access to food sources
The following locations of the critical habitat’s functions, features and attributes have been identified using the critical habitat parcel approach.
Figure 36. Figure 36 is a map of Kootenay Lake and portions of the Kootenay River showing an overview of critical habitat locations. Critical habitat includes aquatic habitat features and attributes that Kootenay River white sturgeon use to carry out life functions. Four locations between Meadow Creek and Creston are labelled on the map as follows: Duncan Delta, Crawford Creek Delta, Kootenay River Delta, and Kootenay River. The coordinates denoting various points of the critical habitat boundaries are listed in Table 19. A scale and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.

Figure 37. Figure 37 is a map of a section of the Kootenay River, British Columbia, showing the critical habitat locations Kootenay River Delta and Lower Kootenay River. The map depicts two polygons that have been identified as critical habitat for Kootenay River white sturgeon. In the identified polygons, critical habitat includes aquatic habitat features and attributes that Kootenay River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygons’ boundaries are listed in Table 19. The critical habitat polygons in the Kootenay River Delta and Lower Kootenay River map are also labeled with codes that correspond to codes used to identify the polygons in Table 19. A scale of 1:75,000 and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.

Figure 38. Figure 38 is a map of a section of Kootenay Lake, British Columbia, showing the critical habitat location Duncan Delta on Kootenay Lake. The map depicts a polygon that has been identified as critical habitat for Kootenay River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Kootenay River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 19. The critical habitat polygon in the Duncan Delta on Kootenay Lake map is also labeled with codes that correspond to codes used to identify the polygon in Table 19. A scale of 1:18,000 and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.

Figure 39. Figure 39 is a map of a section of Kootenay Lake, British Columbia, showing the critical habitat location Crawford Creek Delta on Kootenay Lake. The map depicts a polygon that has been identified as critical habitat for Kootenay River white sturgeon. In the identified polygon, critical habitat includes aquatic habitat features and attributes that Kootenay River white sturgeon use to carry out life functions. The coordinates denoting various points of the polygon’s boundary are listed in Table 19. The critical habitat polygon in the Crawford Creek Delta on Kootenay Lake map is also labeled with codes that correspond to codes used to identify the polygon in Table 19. A scale of 1:30,000 and legend are provided along with an inset map showing locations are primarily in the Kootenay region of British Columbia. The map is oriented in a “north is up” direction.

Table 19. Geographic Coordinates of Critical Habitat Areas for Kootenay River white sturgeon. A footnote on the word “coordinates” in the previous sentence states the following: Coordinate points were digitized using various orthophotos provided by Fisheries and Oceans Canada. The resolution of the various orthophotos varied significantly - ranging from 0.2 m cell size to 24 m cell size. This should be taken into consideration when evaluating the accuracy of the coordinates associated with these points. For geographic coordinate points situated at the wetted boundary, this boundary is meant to represent the annual high water mark (Hatfield et al., 2012), except where otherwise noted for the Kootenay River population where the areal extent is 533 m elevation at the extreme south end of Kootenay Lake to a depth of 100 m (the transition from depositional delta to regular lake bottom). This area includes the Kootenay River downstream from the CP train bridge near rkm 122. Note: For the Kootenay River, relative locations are measured as “river kilometers”, which increase from the river mouth (Rkm 0) upstream to the farthest extent possible.
The table has eight columns read left to right: Critical Habitat Name, Coordinate Marker, Waterbody, River Kilometer, Latitude (DD), Longitude (DD), Latitude (DMS), Longitude (DMS). DD refers to Decimal Degrees and DMS refers to Degrees, Minutes, Seconds. Directly below the column headings there are 12 rows. Four rows correspond to the Kootenay – Crawford Creek Delta on Kootenay Lake area, four to the Kootenay – Duncan Delta on Kootenay Lake area, and four to the Kootenay – Kootenay River Delta and Lower Kootenay River area.
| Critical Habitat Name | Coordinate Marker | Waterbody | River Kilometer | Latitude (DD) | Longitude (DD) | Latitude (DMS) | Longitude (DMS) |
|---|---|---|---|---|---|---|---|
| Kootenay - Crawford Creek Delta on Kootenay Lake | Kr-CCD1 | Kootenay Lake | 49.623 | -116.791 | 49° 37' 24" N | 116° 47' 27" W | |
| Kootenay - Crawford Creek Delta on Kootenay Lake | Kr-CCD2 | Kootenay Lake | 49.625 | -116.806 | 49° 37' 30" N | 116° 48' 22" W | |
| Kootenay - Crawford Creek Delta on Kootenay Lake | Kr-CCD3 | Kootenay Lake | 49.663 | -116.828 | 49° 39' 46" N | 116° 49' 39" W | |
| Kootenay - Crawford Creek Delta on Kootenay Lake | Kr-CCD4 | Kootenay Lake | 49.666 | -116.811 | 49° 39' 57" N | 116° 48' 40" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL1 | Kootenay Lake | 50.166 | -116.923 | 50° 9' 58" N | 116° 55' 22" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL2 | Kootenay Lake | 50.166 | -116.928 | 50° 9' 57" N | 116° 55' 40" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL3 | Kootenay Lake | 50.167 | -116.929 | 50° 10' 2" N | 116° 55' 46" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL4 | Kootenay Lake | 50.166 | -116.931 | 50° 9' 58" N | 116° 55' 51" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL5 | Kootenay Lake | 50.167 | -116.935 | 50° 9' 59" N | 116° 56' 7" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL6 | Kootenay Lake | 50.166 | -116.940 | 50° 9' 58" N | 116° 56' 23" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL7 | Kootenay Lake | 50.166 | -116.944 | 50° 9' 57" N | 116° 56' 38" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL8 | Kootenay Lake | 50.165 | -116.945 | 50° 9' 53" N | 116° 56' 43" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL9 | Kootenay Lake | 50.162 | -116.945 | 50° 9' 44" N | 116° 56' 44" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL10 | Kootenay Lake | 50.160 | -116.952 | 50° 9' 37" N | 116° 57' 6" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr- DDKL11 | Kootenay Lake | 50.159 | -116.958 | 50° 9' 33" N | 116° 57' 28" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr- DDKL12 | Kootenay Lake | 50.167 | -116.957 | 50° 10' 2" N | 116° 57' 27" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL13 | Kootenay Lake | 50.171 | -116.947 | 50° 10' 14" N | 116° 56' 49" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL14 | Kootenay Lake | 50.172 | -116.940 | 50° 10' 17" N | 116° 56' 24" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL15 | Kootenay Lake | 50.174 | -116.936 | 50° 10' 25" N | 116° 56' 8" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL16 | Kootenay Lake | 50.174 | -116.934 | 50° 10' 25" N | 116° 56' 3" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL17 | Kootenay Lake | 50.172 | -116.934 | 50° 10' 21" N | 116° 56' 3" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL18 | Kootenay Lake | 50.173 | -116.928 | 50° 10' 24" N | 116° 55' 42" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr-DDKL19 | Kootenay Lake | 50.174 | -116.929 | 50° 10' 27" N | 116° 55' 45" W | |
| Kootenay - Duncan Delta on Kootenay Lake | Kr- DDKL20 | Kootenay Lake | 50.175 | -116.926 | 50° 10' 29" N | 116° 55' 34" W | |
| Kootenay - Kootenay River Delta and Lower Kootenay River | Kr-KRD1 | Lower Kootenay River | 122.0 | 49.187 | -116.630 | 49° 11' 11" N | 116° 37' 48" W |
| Kootenay - Kootenay River Delta and Lower Kootenay River | Kr-KRD2 | Lower Kootenay River | 122.0 | 49.185 | -116.629 | 49° 11' 6" N | 116° 37' 45" W |
| Kootenay - Kootenay River Delta and Lower Kootenay River | Kr-KRD3 | Kootenay Lake | 132.5 | 49.258 | -116.703 | 49° 15' 29" N | 116° 42' 12" W |
| Kootenay - Kootenay River Delta and Lower Kootenay River | Kr-KRD4 | Kootenay Lake | 0.0 | 49.264 | -116.700 | 49° 15' 50" N | 116° 42' 0" W |
| Kootenay - Kootenay River Delta and Lower Kootenay River | Kr-KRD5 | Kootenay Lake | 0.0 | 49.271 | -116.696 | 49° 16' 16" N | 116° 41' 46" W |
| Kootenay - Kootenay River Delta and Lower Kootenay River | Kr-KRD6 | Kootenay Lake | 0.0 | 49.276 | -116.692 | 49° 16' 35" N | 116° 41' 31" W |
| Kootenay - Kootenay River Delta and Lower Kootenay River | Kr-KRD7 | Kootenay Lake | 0.0 | 49.279 | -116.686 | 49° 16' 44" N | 116° 41' 10" W |
| Kootenay - Kootenay River Delta and Lower Kootenay River | Kr-KRD8 | Kootenay Lake | 0.0 | 49.279 | -116.681 | 49° 16' 46" N | 116° 40' 52" W |
| Kootenay - Kootenay River Delta and Lower Kootenay River | Kr-KRD9 | Kootenay Lake | 0.0 | 49.281 | -116.677 | 49° 16' 52" N | 116° 40' 38" W |
| Kootenay - Kootenay River Delta and Lower Kootenay River | Kr-KRD10 | Kootenay Lake | 0.0 | 49.289 | -116.675 | 49° 17' 21" N | 116° 40' 31" W |
| Kootenay - Kootenay River Delta and Lower Kootenay River | Kr- KRD11 | Kootenay Lake | 132.5 | 49.301 | -116.664 | 49° 18' 2" N | 116° 39' 50" W |
Under SARA, critical habitat must be legally protected from destruction within 180 days of being identified in a recovery strategy or action plan. For white sturgeon, it is anticipated that protection will be accomplished through a SARA Ministerial Order made under subsections 58(4) and (5), which will engage the prohibition under s.58(1) of SARA against the destruction of critical habitat. It is important to keep in mind that critical habitat can be destroyed from activities both within and outside of its geographic extent.
The activities described in this section are neither exhaustive nor exclusive and have been guided by the threats in section 4. The absence of a specific human activity does not preclude the department’s ability to regulate it pursuant to SARA. Furthermore, the inclusion of an activity does not necessarily result in its prohibition. The prohibition against the destruction of critical habitat is engaged if a critical habitat protection order is made. Also, activities that impact critical habitat but do not result in its destruction are not prohibited. Since habitat use is often temporal in nature, every activity is assessed on a case-by-case basis and site-specific mitigation measures are applied where they are reliable and available. In every case, where information is available, thresholds and limits are associated with attributes to better inform management and regulatory decision-making. However, in many cases the knowledge of a species and its critical habitat may be lacking. In particular, information associated with a species’ or habitat’s thresholds of tolerance to disturbance from human activities is lacking and must be acquired.
For each population of white sturgeon, known critical habitat threats have been assessed to provide examples of activities likely to result in the destruction of critical habitat and are summarized below.
Activities that could destroy critical habitat for the Upper Fraser population include instream activities such as gravel or sand dredging, linear developments, riparian alterations or developments to instream or adjacent habitats, and upstream land and water uses. There is concern around the cumulative impact of sedimentation, habitat fragmentation and works that may cumulatively impact food supply. There are also unknowns about juvenile habitat use that make these impacts potentially significant. Changes to the habitat as a result of pine beetle deforestation will need to be carefully monitored for impacts to sturgeon life functions, though complete mitigation in this case may not be possible.
Table 20. Activities likely to destroy critical habitat for the Upper Fraser River population. The table has five columns read from left to right as follows: Threat, Activity, Effect – Pathway, Function Affected, and Thresholds / Range / Qualitative Characteristics of the CH feature and Attribute beyond which the activity could negatively impact the function such that destruction of CH is likely; this last column has two sub-headings: Feature Affected, and Attribute Affected. The Threat column lists the nature of the threat to critical habitat. The Activity column lists individual activities that may result in a threat. The Effect – Pathway column details the mechanisms through which a particular activity results in a threat to critical habitat. The Function Affected column lists which functions of critical habitat are threatened by a particular activity. The remaining two sub-headings (Feature Affected and Attribute Affected) describe the thresholds, ranges, and qualitative characteristics of features and attributes of critical habitat that are threatened by a particular activity.
*Depositional area– typically lower velocity areas where fish can rest and prey species may congregate; often in close proximity to confluences with other water bodies providing further access to food sources
There are several activities that could destroy critical habitat in the Nechako system. River regulation is believed to have had a significant influence on habitat quality at the spawning site, in particular by removing peak flows from the system. This has led to less frequent flooding of gravel bars, increased vegetation on bars and islands, and generally less movement of stream substrates (with concomitant decrease in substrate suitability for white sturgeon). Other activities such as gravel or sand dredging, linear developments, riparian, foreshore, floodplain alterations or developments, upstream land and water uses, and point and non-point source effluent discharges are possible concerns for all critical habitats in the watershed depending on details of the activities.
There is concern around the cumulative impact of sedimentation, habitat fragmentation and works that may cumulatively impact food supply. There are also unknowns about juvenile habitat use that make these impacts potentially significant. Changes to the habitat as a result of pine beetle deforestation will need to be carefully monitored for impacts to surgeon life functions, though complete mitigation in this case may not be possible.
Federal and Provincial agencies continue to work with hydroelectric facilities to mitigate impacts to sturgeon and balance residual impacts where possible. Continued co-operation and monitoring of impacts from river regulation are required.
Table 21. Activities likely to destroy critical habitat for the Nechako River population. The table has five columns read from left to right as follows: Threat, Activity, Effect – Pathway, Function Affected, and Thresholds / Range / Qualitative Characteristics of the CH feature and Attribute beyond which the activity could negatively impact the function such that destruction of CH is likely; this last column has two sub-headings: Feature Affected, and Attribute Affected. The Threat column lists the nature of the threat to critical habitat. The Activity column lists individual activities that may result in a threat. The Effect – Pathway column details the mechanisms through which a particular activity results in a threat to critical habitat. The Function Affected column lists which functions of critical habitat are threatened by a particular activity. The remaining two sub-headings (Feature Affected and Attribute Affected) describe the thresholds, ranges, and qualitative characteristics of features and attributes of critical habitat that are threatened by a particular activity.
Activities that could destroy critical habitat for juvenile and adult life stages include: several aspects of river regulation (impoundment operations and load-following (i.e. adjusting power output to meet daily demand fluctuations)); gravel or sand dredging; linear developments; riparian, foreshore, floodplain alterations or developments; upstream land and water uses; and point and non-point source effluent discharges. The exact concerns vary depending on details of the activities and their location within the Columbia drainage.
There is some concern around the cumulative impact of sedimentation, habitat fragmentation and works on ecosystem pathways and trophic interactions. There are also unknowns about juvenile habitat use that make these impacts potentially significant.
Federal and Provincial agencies continue to work with hydroelectric facilities to mitigate impacts to sturgeon and balance residual impacts where possible. Continued co-operation and monitoring of impacts from river regulation are required.
ALR component -- The spawning site for the ALR component is downstream from REV Dam, which is currently operated as a load-following facility. Operations of REV Dam are believed to influence the viability of incubation habitat by dewatering eggs and embryos. Hypolimnetic releases (i.e., from deep, cold water) from REV Dam are thought to have contributed to altered water temperatures and are believed to influence timing of spawning and duration of embryo development. Elevation of ALR is believed to alter channel depth and velocity parameters below REV Dam due to a backwatering effect, which may influence suitability of spawning and incubation habitats.
Operations at REV Dam prior to unit 5 coming online in December 20, 2010 may have limited connectivity among habitats from ALR to the spawning site adjacent to the Revelstoke golf course during minimum flow periods. Since the addition of unit 5 the minimum flow at REV Dam has gone from 8.5cm to 142cm increasing total wetted riverbed area by 37%; monitoring is ongoing to determine improvements in habitat connectivity as well as hydraulic properties within incubation and early rearing habitats (Jamie Crossman, B.C. Hydro, personal communication)
Transboundary component -- The Waneta spawning area is influenced by load-following and water storage associated with a series of dams on the Pend d’Oreille River, primarily within facilities in the U.S., but including Seven Mile and Waneta Dams within Canada. There are restrictions on load-following currently in place during the spawning season to address this threat. Spawning occurs primarily beyond the Pend d’Oreille River channel, into the confluence with the Columbia River mainstem and downstream a short distance. Thus, upstream storage dams on the Columbia and Kootenay rivers may influence spawning and incubation habitats in the Waneta area, although the consequences have been limited. Backwater influence from Lake Roosevelt in the U.S. does not reach upstream to these habitats, but may influence downstream rearing habitat. Contaminant impacts are of concern primarily in the U.S. portion of the river (this was rated low in Canada).
*Depositional area– typically lower velocity areas where fish can rest and prey species may congregate; often in close proximity to confluences with other water bodies providing further access to food sources
Activities that could destroy critical habitat for the Kootenay River population include flow regulation, instream activities such as gravel or sand dredging, linear developments, alterations or developments to instream and adjacent habitats, and upstream land and water uses.
There is concern around the cumulative impact of sedimentation, habitat fragmentation and works that may cumulatively impact food supply. There are also unknowns about juvenile habitat use that make these impacts potentially significant.
Federal and Provincial agencies continue to work with hydroelectric facilities to mitigate impacts to sturgeon and balance residual impacts where possible. Continued co-operation and monitoring of impacts from river regulation are required.
Table 23. Activities likely to destroy critical habitat for the Kootenay River population. The table has five columns read from left to right as follows: Threat, Activity, Effect – Pathway, Function Affected, and Thresholds / Range / Qualitative Characteristics of the CH feature and Attribute beyond which the activity could negatively impact the function such that destruction of CH is likely; this last column has two sub-headings: Feature Affected, and Attribute Affected. The Threat column lists the nature of the threat to critical habitat. The Activity column lists individual activities that may result in a threat. The Effect – Pathway column details the mechanisms through which a particular activity results in a threat to critical habitat. The Function Affected column lists which functions of critical habitat are threatened by a particular activity. The remaining two sub-headings (Feature Affected and Attribute Affected) describe the thresholds, ranges, and qualitative characteristics of features and attributes of critical habitat that are threatened by a particular activity.
*Depositional area– typically lower velocity areas where fish can rest and prey species may congregate; often in close proximity to confluences with other water bodies providing further access to food sources
Further research is required to identify and/or refine additional critical habitat necessary to support the species’ population and distribution objectives and protect critical habitat from destruction. This additional work includes the following studies and is directly related to the Recruitment Failure Hypothesis.
Critical habitats defined in this document, when combined with functioning recruitment in each population, should be sufficient for survival and provide a solid basis for population recovery. While it is possible that additional habitat may be required for recovery of the species, it is not known at this time. The principle limitation to recovery is the functionality of currently identified habitats rather than their spatial extent. This focus is reflected in the knowledge gaps identified in the recovery strategy, which provides a guide to future studies with a strong focus on recruitment failure diagnosis and restoration. As a result, studies identified here emphasize understanding habitat functionality and recruitment restoration within critical habitats. While studies of the species biology and movement may provide further information on definition of particular critical habitats, such studies should not supersede investigations of recruitment failure and its restoration because doing so may not be in the best interest of the species.
Table 24. Schedule of Studies to identify critical habitats. The table has four column headings read from left to right: Population(s), Description of Study, Rationale, and Timeline. A footnote on the heading “Timeline” states the following: The timelines denoted here represent an estimation of how long each study would take in years. Since some studies are dependent on others, or on specific hydraulic or biological conditions being present, the timelines provided do not specific start and end dates. Flexibility to undertake studies opportunistically as conditions allow should be maintained.
Directly below the column headings are five rows, read from left to right.
Row 1: Upper Columbia, Nechako, Upper Fraser populations, Confirm locations of spawning sites. Not all spawning locations are currently known or confirmed, 3 years. Row 2: All SARA listed populations*, Initiate lab and/or in situ studies to investigate habitat use by eggs (e.g. survival), yolk sac larvae (e.g. survival), and feeding larvae (e.g. cover, and food availability). Eggs/yolk sac larvae - interstitial habitats are beneficial, micro habitat use requires further study Feeding larvae - habitat use by feeding larvae is highly uncertain *Only yolk sac and feeding larvae studies would be undertaken for Kootenay River white sturgeon, because spawning occurs outside Canada, 2-4 years, contingent on having enhanced Substrate placed and having appropriate river conditions. Row 3: Upper Columbia, Nechako populations, Undertake pre-requisite studies in support of spawning habitat restoration. Investigate hydraulic conditions required to sustain preferred incubation substrates, To better understand substrate preferences that determine habitat selection, 4-5 years. Row 4: Upper Columbia, Nechako populations, Investigate biological determinants of spawning micro habitat selection. This would include evaluation of physical conditions (e.g. hydraulics), social/chemical cues (e.g. presence of other fish, pheromones), as well as investigation of manipulating habitat attributes to attract/deter spawning at specific locations, To better understand substrate preferences that determine habitat selection, 4-5 years, contingent upon spawning cycles. Row 5: Upper Columbia, Initiate experimental spawning habitat restoration (one location minimum) in the Transboundary reach, Suggested for the Transboundary reach of the Upper Columbia because experiments already initiated for the Nechako Population, and for the Revelstoke reach of the Upper Columbia. Kootenay white sturgeon spawning habitat is not in Canada, 4-5 years.
| Population(s) | Description of Study | Rationale | Timeline15 |
|---|---|---|---|
| Upper Columbia, Nechako, Upper Fraser populations | Confirm locations of spawning sites. | Not all spawning locations are currently known or confirmed | 3 years |
| All SARA-listed populations* | Initiate lab and/or in situ studies to investigate habitat use by eggs (e.g. survival), yolk sac larvae (e.g. survival), and feeding larvae (e.g. cover, and food availability). | Eggs/yolk sac larvae - interstitial habitats are beneficial, micro habitat use requires further study Feeding larvae - habitat use by feeding larvae is highly uncertain *Only yolk sac and feeding larvae studies would be undertaken for Kootenay River white sturgeon, because spawning occurs outside Canada |
2-4 years, contingent on having enhanced substrate placed and having appropriate river conditions |
| Upper Columbia, Nechako populations | Undertake pre-requisite studies in support of spawning habitat restoration. Investigate hydraulic conditions required to sustain preferred incubation substrates. | To better understand substrate preferences that determine habitat selection | 4-5 years |
| Upper Columbia, Nechako populations | Investigate biological determinants of spawning micro habitat selection. This would include evaluation of physical conditions (e.g. hydraulics), social/chemical cues (e.g. presence of other fish, pheromones), as well as investigation of manipulating habitat attributes to attract/deter spawning at specific locations. | To better understand substrate preferences that determine habitat selection | 4-5 years, contingent upon spawning cycles |
| Upper Columbia | Initiate experimental spawning habitat restoration (one location minimum) in the Transboundary reach. | Suggested for the Transboundary reach of the Upper Columbia because experiments already initiated for the Nechako Population, and for the Revelstoke reach of the Upper Columbia. Kootenay white sturgeon spawning habitat is not in Canada. | 4-5 years |
14 Coordinate points were digitized using various orthophotos provided by Fisheries and Oceans Canada. The resolution of the various orthophotos varied significantly - ranging from 0.2 m cell size to 24 m cell size. This should be taken into consideration when evaluating the accuracy of the coordinates associated with these points. For geographic coordinate points situated at the wetted boundary, this boundary is meant to represent the annual high water mark (Hatfield et al., 2012), except where otherwise noted for the Kootenay River population where the areal extent is 533 m elevation at the extreme south end of Kootenay Lake to a depth of 100 m (the transition from depositional delta to regular lake bottom). This area includes the Kootenay River downstream from the CP train bridge near rkm 122.
Note: For the Kootenay River, relative locations are measured as “river kilometers”, which increase from the river mouth (Rkm 0) upstream to the farthest extent possible.
15 The timelines denoted here represent an estimation of how long each study would take in years. Since some studies are dependent on others, or on specific hydraulic or biological conditions being present, the timelines provided do not specific start and end dates. Flexibility to undertake studies opportunistically as conditions allow should be maintained.
An overview of the general structure and directives for the Fraser, Nechako, Columbia and Kootenay River basin teams is provided below.
In 2003, the B.C. Ministry of Environment together with First Nations, federal government, commercial fishers, and conservation stakeholders, initiated and supported a working group to address the recovery of Fraser River basin white sturgeon populations (excluding the Nechako River stock groups). The group reviewed information availability and needs, and conducted assessments of impacts and threats to the populations. The primary role of the group was the development of a conservation plan for the upper, middle, and lower Fraser River white sturgeon populations, which was completed in December 2005 (Fraser River White Sturgeon Working Group 2005). In close association with that earlier process, and with many of the same participants, an informal recovery planning process was initiated for the lower and middle Fraser River populations in 2005. (At that time consideration of the Upper Fraser population was shifted to the NWSRI Technical Working Group, due to the similar geography and SARA classification of both populations.) Technical and community working groups were formed to provide an informative and cooperative means to develop recovery action plans, and undertake and support recovery initiatives for the lower and middle Fraser River white sturgeon populations.
The Lower and Mid Fraser White Sturgeon TWG is a group of sturgeon experts and is made up of members from First Nations, B.C. Ministry of Environment sturgeon biologists, DFO fishery biologists, educational institutions, angling guides and the Fraser River Sturgeon Conservation Society (FRSCS). The role of the TWG is to represent sturgeon. The TWG interprets the available information and science to develop recovery action plans and initiatives for lower and middle Fraser white sturgeon, and also assists in developing protection and management objectives for the populations.
The Lower and Mid Fraser White Sturgeon Community Working Group (CWG) is a group of community stakeholders that have an interest in lower and middle Fraser sturgeon management and recovery. This group is currently made up of representatives from local First Nations, B.C. Ministry of Environment sturgeon biologists, DFO fishery biologists, educational institutions, the FRSCS, independent angling guides, the Fraser Valley Angling Guide Association, independent anglers, and a representative from the DFO Sportfish Advisory Committee. The role of this group is to represent the interests of the various stakeholders in the recovery of the lower and middle Fraser sturgeon populations. CWG members have assisted the TWG in the development of recovery plans and initiatives. The role of the CWG has also been to review any recovery plans or initiatives that were developed by the TWG and provide their concerns, recommendations and/or support.
The TWG and CWG use the Fraser River White Sturgeon Conservation Plan (Fraser River White Sturgeon Working Group 2005) as their higher level guiding document for recovery planning, and in 2007, developed a living recovery planning worksheet to further guide recovery activities. Over the last decade, the support for recovery initiatives has come from First Nations, provincial and federal governments, substantial in-kind contributions from the angling community and from private benefactors. The majority of studies associated with recovery have been undertaken or lead by the FRSCS in cooperation with provincial and federal biologists and stakeholders. The B.C. Ministry of Environment implemented a sturgeon conservation surcharge for sturgeon angling in non-tidal waters in September of 2008 to better record and manage recreational angling activities and to help raise funds to support recovery initiatives for these populations.
Additional information on Fraser River white sturgeon, recovery initiatives and the FRSCS can be found on the B.C. Ministry of Environment website, and on the Fraser River Sturgeon Conservation Society website. The TWG and CWG chair for the Fraser population is Erin Stoddard (B.C. Ministry of Forests, Lands and Natural Resource Operations).
A recovery planning process was initiated for Nechako River (and Upper Fraser) white sturgeon by the province of B.C. in 2000 (Nechako White Sturgeon Recovery Initiative 2009). The Nechako River White Sturgeon Recovery Initiative (NWSRI) was formed and is “responsible for identifying the reasons why white sturgeon are no longer successfully spawning and surviving in the Nechako River watershed, and for the design and implementation of habitat protection, restoration and management options” (Nechako White Sturgeon Recovery Initiative 2009). The NWSRI is composed of two committees: the TWG and the CWG.
The Nechako TWG (formerly called the Recovery Team) was formed in 2000, and consists of technical experts including federal and provincial biologists, First Nations and industry experts (Nechako White Sturgeon Recovery Initiative 2009). The TWG develops hypotheses for the decline of the Nechako River white sturgeon population and plans for recovery to a self-sustaining population based on the best-available science, local and traditional knowledge (Nechako White Sturgeon Recovery Initiative 2009). The Nechako TWG has undertaken a series of investigations into the causes of recruitment failure, leading up to current in-river habitat experiments.
The Nechako CWG (formerly called the Action Planning Group) was assembled in 2001. The CWG was created to provide input in the NWSRI from various stakeholders and act as a public advocate for Nechako white sturgeon recovery (Nechako White Sturgeon Recovery Initiative 2009). It is comprised of representatives from First Nations, non-government environmental organizations, industry, local and regional governments and affected public. “The CWG provides an opportunity for key groups essential to the success of a recovery plan to become involved in the process. The group focuses on increasing the public’s awareness and knowledge about the recovery process, as well as the ecological problems facing the Nechako River white sturgeon. It is also concerned with building and maintaining community support for the recovery plan.” (Nechako White Sturgeon Recovery Initiative 2009)
In 2004, a basin-specific Recovery Plan was developed to “ensure technical soundness and meaningful participation of the public” in recovery efforts (Nechako White Sturgeon Recovery Initiative 2004). In 2007, a Strategic Plan for the Nechako River white sturgeon recovery facility and interpretive centre was also developed in order to secure resources for capital and long-term funding for constructing and operating a conservation aquaculture facility in the Saik’uz Territory in the District of Vanderhoof (Nechako White Sturgeon Recovery Initiative 2009). In early 2013, an agreement was reached to begin construction on this facility, and construction is currently underway.
In addition to the Recovery Plan, the Nechako River White Sturgeon Habitat Management Plan was developed in 2008 to provide habitat requirements and rehabilitation/enhancement programs that will actively work to support the conservation of Nechako River white sturgeon (Nechako White Sturgeon Recovery Initiative 2009).
Further information on the NWSRI and supporting documents can be found on their website. The TWG chair for the Nechako River population is Cory Williamson (B.C. Ministry of Forests, Lands and Natural Resource Operations), and the CWG chair is Brian Frenkel (Avison Management).
The Upper Columbia River White Sturgeon Recovery Initiative ( UCWSRI) was formed in 2000 to aid the recovery of the Columbia River white sturgeon population. The initiative began with a 2-year agreement signed between the provincial and federal governments and B.C. Hydro formalizing a common commitment to address the endangered status of upper Columbia River white sturgeon ( UCWSRI 2009). Similar to the other basins, two core teams are involved in recovery planning and include the TWG (formerly the Recovery Team), and the CWG (formerly the Action Planning Group).
The Upper Columbia White Sturgeon TWG is made up of technical experts responsible for the development and implementation of the Recovery Plan, and is comprised of biologists, researchers, and other sturgeon experts from provincial, federal and state governments, B.C. Hydro, Teck Metals, Columbia Power Corporation, Bonneville Power Administration, Spokane Tribe of Indians, Colville Confederated Tribes and other groups (Upper Columbia White Sturgeon Recovery Initiative 2009a). In November 2002, the TWG completed a Recovery Plan and technical appendices that identifies goals and recovery measures for rebuilding the white sturgeon population (Upper Columbia White Sturgeon Recovery Initiative 2002), which has now been updated after ten years (Upper Columbia White Sturgeon Recovery Initiative 2012). The Recovery plan summarizes white sturgeon biology, factors leading to the decline of white sturgeon in the upper Columbia River, as well as conservation measures and recommendations for recovery (Upper Columbia White Sturgeon Recovery Initiative 2002). The TWG also undertook a Recruitment Failure Hypothesis Review (RFHR) and evaluation of historic recruitment patterns to develop and evaluate a set of hypotheses to guide future work on recruitment failure of white sturgeon populations in the upper Columbia River (Gregory and Long 2008, McAdam 2012).
The Upper Columbia White Sturgeon CWG is made up of public stakeholders including federal, provincial and local governments, First Nations, public and industry from the U.S. and Canada that are “responsible for developing a common vision and public support for sturgeon recovery, providing information and feedback on recovery operations, and informing the public and seeking funding for recovery projects” (Upper Columbia White Sturgeon Recovery Initiative 2009a).
Further information on the UCWSRI and supporting documents can be found on their website. The TWG chair for the Columbia River population is Jim Powell (Freshwater Fisheries Society of B.C., Victoria) and the CWG chair is Gerry Nellestijn (Salmo Watershed Streamkeepers Society, Salmo, B.C.).
The Kootenai River population of white sturgeon was listed as Endangered under the U.S. federal Endangered Species Act in 1994 (Duke et al. 1999). The Kootenai River White Sturgeon Recovery Team is made up of members from U.S. Fish and Wildlife Service (Idaho and Washington), Kootenai Tribe of Idaho, U.S. Army Corps of Engineers, Montana Fish, Wildlife and Parks, Bonneville Power Administration, B.C. Ministry of Forests, Lands and Natural Resource Operations and the Idaho Department of Fish and Game. Further information on the Kootenai River white sturgeon recovery process and supporting documents can be found on the U.S. Fish and Wildlife Service website. The Canadian representative for the Kootenai River White Sturgeon Recovery Team is Matthew Neufeld (B.C. Ministry of Forests, Lands and Natural Resource Operations – Nelson).
Enhancement of presently degraded ecosystem conditions is believed to be required for effective recovery of white sturgeon throughout much of its range. Habitat enhancement for the benefit of white sturgeon may have positive influences on abundance and distribution of some other species. However, recovery efforts aimed at white sturgeon may have negative effects on other native fish or wildlife species, but this impact is considered unlikely to have a population level effect and, as such, risks are considered reasonable when compared to the risks of losing an endangered species. There are four specific activities that deserve mention here.
First, if alterations to flows in dam-affected systems are recommended for white sturgeon recovery or to further refine data gaps, these flow changes may affect other fish species in the river. The impacts of recovery actions on other species in the affected river systems may need to be further evaluated and consideration will have to be given to potential negative impacts prior to implementation.
Second, some concerns have been raised regarding the use of aquaculture to supplement natural recruitment in three of the populations of white sturgeon in British Columbia. Concerns include genetic effects on target and to adjacent populations, the introduction of diseases into wild populations, competition with target angling species (i.e., their prey), and by-catch of white sturgeon which may influence the angling experience. The peer-reviewed breeding plans (Kincaid 1993 , Pollard 2002 ) aim to maximize genetic diversity, and have been assessed to be low risk (Williamson et al. 2003 ) . The implementation plans include monitoring programs and stop criteria to avoid long-term genetic impacts to the population or connected populations of white sturgeon. The strict implementation of these plans and management of conservation aquaculture hatcheries will ensure that disease introductions and genetic effects are low risk, whereas without aquaculture programs the risk of extirpation of white sturgeon would be high. Further evaluation of aquaculture inputs on prey species and by-catch is likely required.
Third, a long-term strategy is needed for potentially introducing white sturgeon to Kinbasket Reservoir to establish a population upstream of Mica Dam on the Columbia River. Planning for this activity is still preliminary. As part of the planning process, Westslope Fisheries and Canadian Columbia River Intertribal Fisheries Commission (CCRIFC) (2005 ) conducted an ecological risk assessment of the proposed introduction and are in the midst of conducting a multiyear sampling and habitat assessment program (2009). CCRIFC (2005 ) has also conducted a pathogen risk assessment. However, both assessments were qualitative as there are few relevant data available to develop a quantitative assessment. Nevertheless, the assessment was thorough in identifying a range of potential effects, but acknowledged considerable uncertainty in its predictive ability. Studies concluded that there are few substantial risks associated with the introduction and that the greatest risks observed were to burbot, kokanee, mountain whitefish and prickly sculpin because of predation by white sturgeon. The ecological risks need to be further assessed relative to the benefits of white sturgeon introduction. Further review of these issues is expected in conjunction with activities under the Columbia River Water Use Plan (Columbia River Water Use Plan Consultative Committee 2005 ) . The Kootenai Recovery Team is considering a similar transplant of white sturgeon to Lake Koocanusa, which extends into Canada, but a risk assessment has not been completed.
Fourth, the management of Aboriginal, commercial, and recreational fisheries does not consider the needs of white sturgeon. Returning salmon in the Fraser and Nechako rivers are important food sources for white sturgeon; in the lower Fraser eulachon and smelt are also important foods. There is evidence that white sturgeon located in the upper Columbia River at Waneta Eddy are food limited (Van Poorten and McAdam 2010 ) . The extent to which most of the white sturgeon populations are food-limited is not known, but it is possible that management of prey species to meet sturgeon needs will be required for recovery.
Recovery-related actions have been underway in most areas for several years, and are communicated regularly through basin-level teams. The following provides a short summary of actions completed or initiated at both the national and the basin level.
- The National Recovery Team for White Sturgeon, with national and basin level subcommittees, was established.
- Terms of reference for the National Recovery Team and basin level subcommittees were developed.
- A COSEWIC status report was completed in 2003 (Ptolemy and Vennesland 2003 ) and is available through the SAR Public Registry website. A subsequent COSEWIC reassessment was also completed; the status report will become available on the SARA Public Registry website in Fall 2013.
- The SARA listing process has been completed following the 2003 COSEWIC assessment. A SARA listing process following the 2012 COSEWIC reassessment will commence in 2013-2014.
- A Recovery Potential Assessment for White Sturgeon (Wood et al. 2007) was developed to inform species recovery
- Critical habitat has been identified in this recovery strategy.
Lower and Mid-Fraser River
- A Conservation Plan for Fraser River White Sturgeon was developed with input from DFO, B.C. Ministry of Environment, Sto:lo First Nation, B.C. Aboriginal Fisheries Commission, Fraser Basin Council, and the Fraser River Sturgeon Conservation Society. The plan summarizes existing information, assesses threats, and recommends mitigation and management procedures.
- A Community Working Group and a Technical Working Group for the Middle and Lower Fraser have been established. Members that represented the upper Fraser River area joined a separate Nechako and Upper Fraser River group.
- Some fisheries management actions have been implemented to prevent, limit or mitigate by-catch of Fraser River white sturgeon in the commercial salmon gillnet fisheries (e.g. all white sturgeon by-catch must, by regulation, be released).
- Public awareness and education about Fraser River white sturgeon has been ongoing through a number of efforts by agencies and non-governmental organizations. Efforts include the publication of a white sturgeon brochure in the Wildlife in British Columbia at Risk series, ongoing communications, fund raising and study efforts through the Fraser River Sturgeon Conservation Society, development and implementation of Fraser River white sturgeon education programs for delivery to schools and communities, education and awareness programs, research partnerships, and community outreach initiatives aimed at First Nations fishermen and communities. See the Fraser River Sturgeon Conservation Society website for more details.
- A wide variety of scientific investigations have been completed or are ongoing, including:
- monitoring and assessment of white sturgeon abundance through mark-recapture studies in the lower Fraser River and portions of the mid–Fraser River;
- genetics research to identify populations;
- preliminary studies on the effects of various gear capture types on the short term survival of white sturgeon;
- radio and acoustic telemetry tagging studies to determine white sturgeon habitat use and movement;
- studies on habitat use by juvenile white sturgeon
- ecological research (e.g., habitat use, movements and migration, diets, growth and feeding, behaviour);
- habitat research (spawning habitat research, effects of dredging in the lower Fraser River mainstem, juvenile habitat use); and,
- numerous scientific documents and publications have been written about Fraser River white sturgeon (see Appendix C of Fraser River White Sturgeon Working Group 2005, and Fraser River Sturgeon Conservation Society, 2013) .
Nechako River and Upper Fraser River
- A Recovery Plan for Nechako River White Sturgeon was completed in 2004, and a Conservation Plan for Upper Fraser River White Sturgeon was completed in 2005. The plans summarize existing information, assess threats, and recommend mitigation and management procedures. The planning processes included diverse participants, including First Nations, B.C. Ministry of Environment, Fisheries & Oceans Canada, and industry.
- Planning groups assembled to provide input to the earlier plans have formed into a Community Working Group and Technical Working Group for the Nechako and Upper Fraser rivers, and operate as components of the National Recovery Team for White Sturgeon.
- Fisheries management actions, and stewardship efforts have been undertaken to prevent direct or by-catch harvest of Nechako and Upper Fraser River white sturgeon in local fisheries.
- Public awareness and education about Nechako and Upper Fraser River white sturgeon has been ongoing through a number of efforts by agencies and non-governmental organizations, and efforts of the CWG.
- Conservation aquaculture plans have been completed for the Nechako River, including breeding plans to minimize genetic consequences of captive breeding and release. Artificial propagation of Nechako River white sturgeon began in 2006 with a pilot project that lasted until 2010. Demonstration of the feasibility of the conservation fish culture program is complete; efforts to implement a long-term conservation aquaculture program are underway.
- A wide variety of scientific investigations have been completed or are ongoing, including:
- population status investigations for the upper Fraser, led by the Lheidli T’enneh First Nation;
- population assessment and monitoring of white sturgeon abundance;
- ecological research (e.g., habitat use, spawning, movements and migration, diets, growth and feeding, behaviour);
- habitat research (spawning site habitat work including substrate studies, juvenile habitat studies);
- recruitment restoration studies (e.g., field studies of larval habitat use, growth and survival);
- risk assessment of white sturgeon introductions; and,
- collection of local and traditional knowledge.
Kootenay River
- Kootenai white sturgeon was listed as endangered under the U.S. Endangered Species Act in 1994. A U.S. Recovery Team was formed in 1996, with Canadian participation.
- A Recovery Plan for Kootenai River White Sturgeon was completed in 1999 by the U.S. Fish and Wildlife Service, with input from Canadian agencies. The plan summarizes existing information, assesses threats, and recommends mitigation and management procedures. The recovery planning process for Kootenai River white sturgeon continues to be led by U.S. agencies with active participation from Canadian agencies.
- A Kootenai River White Sturgeon Implementation Plan and Schedule was completed in 2005. Preparation of this Implementation Plan was administered by the Kootenai Tribe of Idaho through a contract from the Bonneville Power Administration as part of the Northwest Power and Conservation Council’s Fish and Wildlife Program, with assistance from the Kootenai River White Sturgeon Recovery Team. This 5 year Plan and Schedule delineated research, monitoring and evaluation actions believed necessary to protect, rehabilitate, and maintain Kootenai River white sturgeon in conjunction with activities highlighted in the population’s Recovery Plan (USFWS 1999). Information in this Plan and Schedule is intended to complement current Recovery Plan activities and provide valuable information in its update (Kootenai Tribe of Idaho 2005 ) .
- A conservation aquaculture program was established in 1990 and has released more than 50,000 juveniles as a stop gap measure to prevent extinction.
- A conservation aquaculture breeding plan was completed in 1993 and recently updated to reduce the risk of genetic effects from hatchery releases. Broodstock are captured wild Kootenay white sturgeon, which are then released alive after gamete extraction.
- Fisheries management actions were implemented to prevent harvest of Kootenay River white sturgeon.
- Population assessments in 1989 confirmed almost total recruitment failure since 1974.
- A wide variety of scientific investigations have been completed or are ongoing, including:
- monitoring of white sturgeon abundance in Kootenay River and Kootenay Lake;
- annual indexing programs for wild and hatchery origin juveniles and larvae;
- ecological research (e.g., habitat use, spawning, movements and migration, diets, growth and feeding, behaviour);
- habitat research (spawning site habitat work including substrate studies, juvenile habitat studies);
- experimental creation and remediation of white sturgeon spawning habitat; and,
- implementation of operational changes at Libby Dam (e.g., improved temperature control, tiered sturgeon flow volumes, Variable Flow (VARQ) flood control).
Columbia River
- The Upper Columbia River White Sturgeon Technical Working Group was formed with representation from B.C. Hydro, B.C. Ministry of Environment, First Nations (Canada), Bonneville Power Administration, Fisheries and Oceans Canada, Teck Metals Ltd., U.S. Fish and Wildlife Service, U.S. Tribal agencies, U.S. Geological Survey, and Washington Department of Fish and Wildlife. The Working Group completed a Recovery Plan in 2002, and revised it in 2013. The plan summarizes existing information, assesses threats, and recommends mitigation and management procedures.
- A Community Working Group was formed in 2001 to provide outreach support and provide local and socio-economic input on recovery actions proposed by the Technical Working Group.
- The Community Working Group and Technical Working Group now operate as components of the National Recovery Team for White Sturgeon.
- Fisheries management actions have been implemented to prevent harvest of upper Columbia River white sturgeon in local fisheries.
- Public awareness and education about upper Columbia River white sturgeon has been ongoing through a number of efforts by agencies and NGOs. Efforts include brochures, annual reports, development of school curriculum materials, school participation in hatchery sturgeon releases, and participation at numerous conferences and local events.
- Conservation aquaculture plans have been completed including breeding plans to minimize genetic consequences of captive breeding and release. Artificial propagation is ongoing, with the intent of meeting the breeding plan objectives every year.
- A wide variety of scientific and adaptive management investigations have been completed or are ongoing, including:
- population assessment and monitoring of white sturgeon abundance;
- life history research (e.g., habitat use, spawning, movements and migration, diets, growth and feeding, behaviour);
- habitat research (spawning site and juvenile habitat quantification studies, juvenile habitat studies) including location, flow, temperature, substrate and other parameters);
- feasibility investigation of alternative mitigation measures, and subsequent pilot tests and related monitoring;
- risk assessment of white sturgeon introductions; and,
- collection of local and traditional knowledge.
Within five years of posting the final Recovery Strategy on the SARA Public Registry, one or more basin-specific Action Plans for White Sturgeon will be developed by DFO, in collaboration with the National Recovery Team and basin partners.
Section 83(4) of SARA allows for certain activities to be exempt from the general prohibitions of the Act provided the activities are permitted by a recovery strategy, an action plan or a management plan and the persons carrying out the activities are authorized under a federal Act of Parliament to engage in the activities. Any activity, or the cumulative activities, permitted by this recovery strategy must not jeopardize survival or recovery of white sturgeon and regular monitoring and assessment will be required to ensure no jeopardy. The activities permitted in this recovery strategy will be reconsidered if survival and/or recovery are negatively affected at any time, changes to the activities are anticipated that may affect survival and/or recovery, or the conditions for exemption can no longer be met.
Although there is risk associated with routine fish culture procedures, activities, and/or processes (as discussed below in section 11.1.1), conservation aquaculture is necessary to ensure the persistence of white sturgeon into the future. Therefore, conservation aquaculture activities that follow the relevant annual TWG-endorsed broodstock and/or stocking plans are permitted by this recovery strategy, in accordance with section 83(4) of SARA, and are exempt from the SARA prohibitions for impacts to white sturgeon, provided the activities also have a valid authorization under an Act of Parliament.).
The conservation fish culture (“CFC”) component of white sturgeon recovery activities in British Columbia are currently undertaken by the Freshwater Fisheries Society of British Columbia (FFSBC). The following identifies activities undertaken during their hatchery operations for white sturgeon conservation aquaculture and details the mitigation procedures that minimize risk to white sturgeon welfare.
The premise of CFC activities undertaken by the FFSBC is that they are essential to the survival of the species and may involve activities that pose a risk to all life stages at some time while in captivity. The key to this premise is that risks are calculated and minimized based on an adaptive management approach where new research and technologies are used in concert with best fish culture practices to ensure the optimal outcomes from these efforts. TWG members and the FFSBC have established methods of conduct and fish culture protocols that minimize risks to fish health. The FFSBC has a provincially-approved Fish Health Management Plan (FHMP) that provides guidance on fish culture practices through Standard Operating Procedures and is designed to minimize fish health risks and optimize fish welfare. In addition, the FFSBC has fish health resources including a fully-equipped fish health laboratory and trained fish health professionals that aid in identifying culture/collection practices that may pose a risk to various life stages. Further, fish handling and culture guidelines have been set or agreed to by the TWGs to ensure fish welfare standards are maintained. Finally, only trained and experienced staff are involved in the capture, culture and release of white sturgeon, with the exception of the release of cultured juveniles by the public at stewardship events. FFSBC oversees these events.
In the course of CFC, it is necessary to euthanize young fish during routine culling and the removal of malformed individuals. This is necessary for several reasons. First, some culling occurs prior to release in order to equalize genetic family contributions (i.e., excess or surplus family contributions are not released). This is undertaken to prevent ‘genetic swamping’ in released fish and to try to achieve equal genetic representation of a wide population base. Culling is done under the direction of relevant government agencies, with technical guidance also provided by the TWG. Secondly, malformed fish occur under all conditions including natural ones where they presumably do not survive in the wild. Third, another instance of euthanizing fish is following experimentation. Fish involved in experiments that are reviewed and approved by relevant government agencies such as toxicological, ecological or physiological studies are not released to the wild (unless directed otherwise by the agencies) and are euthanized as their condition may be compromised compared to cohorts raised under normal CFC conditions. These experiments may be carried out by private sector contractors who subscribe established protocols and who obtain required approvals, including section 73 permits under SARA. Fourth, fish that may be undergoing a fish health event may be culled for biosecurity reasons to prevent a facility-wide outbreak and to identify pathogens or diseases within white sturgeon progeny. In all cases, records that identify the cause of the mortality and the number of fish affected are maintained rigorously in accordance with FFSBC’s provincially-approved FHMP and the UCWSRI Suggested Protocol for Sampling White Sturgeon Mortality (Upper Columbia White Sturgeon Recovery Initiative 2009b). The actions taken to euthanize fish are strictly controlled and are for the benefit of the population as a whole.
All white sturgeon produced by CFC methods and released to the Columbia or Nechako rivers are marked (scute removal) and PIT-tagged. Both procedures are moderately invasive. External marking is done by the surgical removal of scutes according to an established code published in the UCWSRI Recovery Plan. A PIT tag is inserted into the dorsal musculature of the fish using a trocar following Canadian Council on Animal Care (CCAC) guidelines and procedures described in Parker et al.(1990) and Morton et al.(2003). In both procedures, fish health risks and trauma are minimized by using standard techniques and are performed by trained and experienced staff.
Larval fish are from time to time released to support experimental studies of recruitment failure. Although these fish are not marked for immediate identification, they have traceability through genetic ‘marking’ of their progenitors who were sampled for DNA during spawning events.
Transport of white sturgeon is potentially conducted at four life stages: adult, egg, larvae and juveniles. In all cases, transport is conducted under conditions that are compliant with the CCAC Guidelines on the care and use of fish in research, teaching and testing. In addition, the FFSBC and TWG activities adhere to the Upper Columbia River Sturgeon Capture, Transport and Handling Manual (UC T&H), May 2006 (Golder Associates Ltd. 2006e). Conditions of transport are controlled to minimize stress and negative effects.
Spawning of white sturgeon may necessitate the surgical removal of eggs from ripe female fish. The conditions of the surgical procedures follow those detailed in the Hatchery Manual for the White Sturgeon (WSHM) (Conte et al. 1988) and are regarded as the standard method. Likewise for male sturgeon, fish are handled and spawned using non-invasive techniques and using methods that minimize duress (Conte et al. 1988). Table 25 summarizes the actions that involve risk, the mitigative actions that govern that activity, and associated reference.
Table 25. Fish welfare risk level, mitigative actions, and associated references for risk reduction during conservation fish culture activities at the Freshwater Fisheries Society of B.C. The table has four columns read left to right: Activity, Risk, Mitigation, and Reference. The column heading “Mitigation” has a footnote that states the following: Water quality parameters are detailed in the Canadian Committee on Animal Care, Guidelines on: The care and use of fish in research, teaching and testing. The column heading “Reference” has a footnote that states the following: UC T&H: (Golder Associates Ltd. 2006e), CCAC: (Canadian Council on Animal Care 2003), WSHM: (Conte et al. 1988), FHMP: (B.C. Ministry of Agriculture and Lands 2009).
Directly below the column headings are nine rows. The third row has five sub-categories of “Activity” under the name Husbandry as follows: Spawning, Incubation, First feeding, Culling, Marking / tagging, and Experimentation. The table reads as follows from left to right.
Row 1: Transport (all), Minimal, Water Quality & Time, UC T&H/CCAC. Row 2: Release, Minimal, Water Quality & Time, UC T&H. Row 3: Husbandry, empty cell, empty cell, empty cell. Row 4: (Begin sub-categories) Spawning, Minimal/Moderate, Experience & Technique, WSHM. Row 5: Incubation, Minimal, Standard Procedures, WSHM. Row 6: First feeding, Minimal, Optimal Feed/Feeding, WSHM. Row 7: Culling, Minimal, Experience & Technique, FHMP. Row 8: (last sub-category) Marking / tagging, Minimal/Moderate, Experience & Technique, FHMP. Row 9: Experimentation, Minimal/Moderate, TWG Review, empty cell.
1 Water quality parameters are detailed in the Canadian Committee on Animal Care, Guidelines on: The care and use of fish in research, teaching and testing
2 UC T&H: (Golder Associates Ltd. 2006e)
CCAC: (Canadian Council on Animal Care 2003)
WSHM: (Conte et al. 1988)
FHMP: (B.C. Ministry of Agriculture and Lands 2009)
Activities undertaken by the TWGs, the FFSBC and its contractors are structured and monitored to minimize risk and harm to white sturgeon adults, eggs, larvae and juveniles.
The FFSBC, or any other organization conducting white sturgeon CFC, is required to have a joint federal-provincial Introductions and Transfers Committee (ITC) permit for each of the sturgeon stocks that they work with. An ITC permit is held for white sturgeon broodstock transport, holding and spawning, and an additional ITC permit is held for larval releases, and for juvenile rearing, and releases (Ron Ek, Kootenay Trout Hatchery, personal communication).
DFO is of the view that conservation fish culture activities for white sturgeon as described above benefit the species and are required to enhance its chances of survival in the wild.
In accordance with subsection 83(4) of SARA, this recovery strategy authorizes conservation fish culture activities related to transfer of individuals to a hatchery facility, operation of the facility, and releasing the offspring into fish habitat. This exemption is subject to the following conditions:
- a) transfer of live individuals to a fish rearing facility is carried out under the authority of a licence issued under section 56 of the Fishery (General) Regulations, SOR/93-53;
- b) operation of the aquaculture component of the conservation fish culture activities are carried out under the authority of a licence issued under section 3 of the Pacific Aquaculture Regulations, SOR/2010-270; and
- c) release of live individuals into fish habitat is carried out under the authority of a licence issued under section 56 of the Fishery (General) Regulations, SOR/93-53.
Broodstock capture activities must still be permitted in accordance with section 73 of SARA, including all pre-conditions under section 73(3).
Section 73 of SARA allows for the permitting of activities that would otherwise contravene the prohibitions of SARA, provided certain conditions are met. As it is not possible to permit the direct harvest of a species at risk under section 73 of SARA, the only mechanism to authorize the direct harvest of white sturgeon is though a section 83(4) exemption. First Nations may be interested in seeking such an exemption to harvest white sturgeon for Food, Social, and Ceremonial (FSC) purposes. Due to increased threats to population recovery, directed FSC fisheries for white sturgeon are not supported by the recovery strategy at this time. At a time when the population can support cumulative impacts of incidental harm and directed harvest, DFO will consider amending this recovery strategy to permit First Nations FSC harvest of white sturgeon under section 83(4). The following conditions will have to be satisfied:
- First Nations and DFO, in consultation with B.C. MOE, enter into a SARA section 11 conservation agreement that ensures or confirms:
- a net gain to sturgeon population and distribution objectives;
- First Nations involvement in the development and implementation of recovery strategy goals and population and distribution objectives; and
- monitoring to ensure the conditions of the agreement are being met (e.g., monitoring reports are submitted to DFO and the regional B.C. MOE sturgeon biologist immediately after each fishery).
- A valid authorization under an Act of Parliament is obtained, along with appropriate conditions, to harvest white sturgeon for FSC purposes.
First Nations Food, Social, and Ceremonial (FSC) fisheries for salmon can result in by-catch of white sturgeon. The Recovery Potential Assessment for White Sturgeon (Wood et al. 2007) indicates that so long as hatchery releases and habitat restoration to fully restore historic rates of natural recruitment are in place, some allowable harm may be permissible for white sturgeon populations. As a result, DFO may consider permitting by-catch through an amendment to this recovery strategy. As an alternative, permitting provisions of SARA section 73 could apply to the FSC salmon fishery. DFO will seek to engage First Nations regarding these various approaches.
Apperson, K. A. and P. J. Anders. 1991. Kootenai River white sturgeon investigations and experimental culture (PDF). Annual progress report for FY90 by Idaho Department of Fish and Game. Report DOE/BPA 93497-2. Portland, OR: U. S. Department of Energy, Bonneville Power Administration.
B.C. Ministry of Agriculture and Lands. 2009. Fish Health Management Plan Template and Manual of Fish Health Practices .
B.C. Ministry of Water, Land and Air Protection. 2002. Flood Hazard Information: Lower Fraser River Floodplain. April 2002. Pamphlet.
Beamesderfer, R. and C. Justice. 2008. Sturgeon hatchery release targets: report to the Upper Columbia White Sturgeon Recovery Initiative Technical Working Group [PDF 851 KB]. Report prepared with the assistance of Cramer Fish Sciences. July 2008, 36 p.
Bennett, W. R., G. Edmondson, K. Williamson and J. Gelley. 2007. An investigation of the substrate preference of white sturgeon (Acipenser transmontanus) eleutheroembryos. Journal of Applied Ichthyology 23: 539–542.
Brannon, E., S. Brewer, A. Setter, M. Miller, F. Utter, and W. Hershberger. 1985. Columbia River white sturgeon (Acipenser transmontanus) early life history and genetics study (PDF 5,2 MB). Final report August 1, 1985 to December 1985 (Project Number 83-316), Report DOE/BP-18952-1. Portland, OR: U. S. Department of Energy, Bonneville Power Administration.
Canadian Council on Animal Care. 2003. CCAC guidelines on: the care and use of fish in research, teaching and testing . 87pp. Ottawa ON: CCAC.
COSEWIC. 2003. COSEWIC assessment and update status report on the white sturgeon Acipenser transmontanus in Canada (PDF). Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 51 pp.
COSEWIC. 2011. COSEWIC guidelines on for recognizing designatable units. Committee on the Status of Endangered Wildlife in Canada. Ottawa.
Fish and Wildlife Compensation Program. 2012. Columbia Basin Plan (PDF). Draft, June 2012. Available online at:
Fisheries and Oceans Canada. 2010a. Eulachon in the Pacific Region. Accessed on 11 March 2010.
Fisheries and Oceans Canada. 2010b. Salmon in the Pacific Region. Accessed on 11 March 2010.
Fisheries and Oceans Canada. 2012. Operational Guidelines for the Identification of Critical Habitat for Aquatic Species at Risk Species at Risk Act (SARA). Draft October 2012. 51 pp.
Gessner, J., S. Wurtz, and C.M. Kamerichs. 2005. Substrate related behavioural response in early life stages of American Atlantic sturgeon A. oxyrinchus oxyrinchus. Presented at the 5th International Symposium on Sturgeon. May 9-13, 2005. Ramsar, Iran.
Golder Associates Ltd. 2005b. Upper Columbia River White Sturgeon Stock Monitoring and Data Management Program: Annual Report No. 2, 1 April 2004 – 31 March 2005. Report prepared for B.C. Ministry of Water, Land and Air Protection, Nelson, B.C., Golder Associates Ltd. Report No. 031480078A2F: 22 pp. + 2 app.
Golder Associates Ltd. 2005c. White sturgeon spawning in relation to the White Sturgeon Flow Augmentation Program. Report prepared for Teck Cominco Metals Ltd., Trail Operations, Trail, B.C.
Golder Associates Ltd. 2006b. Upper Columbia White Sturgeon Stock Monitoring and Data Management Program: Synthesis Report, 1 November 2003 - 31 March 2006. Report prepared for British Columbia Ministry of Water, Land and Air Protection, Nelson, B.C. Golder Report No. 05-1480-025F: 52 p. + 2 app.
Government of Canada. 2009. Species at Risk Act Policies: Overarching Policy Framework [Draft]. Pp ii+ 38pp. In Species at Risk Act Policies and Guidelines Series, Environment Canada. Accessed June 2010.
Hatfield, T., S. McAdam, and T. Nelson. 2004. Impacts to abundance and distribution of Fraser River white sturgeon. A summary of existing information and presentation of impact hypotheses. Report prepared for the Fraser River White Sturgeon Working Group.
Korman, J. and C. Walters. 2001. Nechako River white sturgeon recovery planning: summary of stock assessment and Oct. 2-3 2000 Workshop. Prepared for Ministry of Water, Land and Air Protection, Victoria, B.C. 26 pp.
Lane, E. D. 1991. Status of the white sturgeon in Canada. The Canadian Field Naturalist 105:161-168.
National Recovery Working Group. 2004. Recovery Handbook (ROMAN). October 2004. Working Draft.
Nechako White Sturgeon Recovery Initiative. 2009. Nechako White Sturgeon Recovery Initiative (NWSRI) . Accessed 16 June 2009.
Nelson, T. C., W. J. Gazey, K. K. English, and M. L. Rosenau. 2004. Status of white sturgeon in the lower Fraser River. Report on the findings of the lower Fraser River white sturgeon monitoring and assessment program, 1999-2004. Prepared by LGL Limited for the Fraser River Sturgeon Conservation Society, Vancouver, B.C.
Nelson, T. C., W. J. Gazey, K. K. English, and M. L. Rosenau. 2013. Status of White Sturgeon in the Lower Fraser River, British Columbia. Fisheries, 38:5, 197-209.
Neufeld, M. D. and C. R. Spence. 2002. Kootenay River White Sturgeon Studies, Juvenile Sampling, 2001. Report prepared for Ministry of Environment, Lands and Parks, Nelson B.C.
Neufeld, M. D. and C. R. Spence. 2004b. White Sturgeon and Burbot Recovery Progress 2003-04. Report prepared for Ministry of Environment, Lands and Parks, Nelson B.C.
Neufeld, M. D. 2005. White Sturgeon and Burbot Recovery Progress in British Columbia 2004-05. Report prepared for Ministry of Environment, Lands and Parks, Nelson B.C.
Paradis, E., G. Sykes, R. Liebe, A. English, and S. Johnston. 2011. Fraser River sturgeon sampling and monitoring program 2011 [PDF 50 MB]. Prepared for Emergency Management B.C., Victoria B.C. (draft) December 2011.
Partridge, F. 1980. Federal aid to fish and wildlife restoration, Job Performance Report Project F-73-R-2, Subproject IV: River and stream investigations study, VI: Kootenai River fisheries investigations. Idaho Department of Fish and Game.
RL&L Environmental Services Ltd. 1994a. Status of white sturgeon in the Columbia River, B.C. Prepared for B.C. Hydro, Environmental Affairs. Vancouver B.C. 377F: 101 p.
RL&L Environmental Services Ltd. 1994b. Waneta expansion feasibility study: 1990-1993 Fisheries Investigations. Report prepared for B. C. Hydro, Environmental Resources. R.L. & L. Report No. 380D. 51 p. + 5 app.
RL&L Environmental Services Ltd. 1997. The status of white sturgeon in Slocan Lake, B.C., 1996 study results. Report prepared for B.C. Ministry of Environment, Lands and Parks. R. L. & L. Report No. SL-515F: 12 p. + 2 app.
RL&L Environmental Services Ltd. 1999a. Fraser River White Sturgeon Monitoring Program. Region 2 (Lower Mainland) – 1997 Data Report. Prepared for B.C. Fisheries and Fraser River Sturgeon Conservation Society. 671F: 22 p.
RL&L Environmental Services Ltd. 1999b. Movements of white sturgeon in Kootenay Lake, 1994-1997. Report prepared for B.C. Ministry of Environment, Lands and Parks, Nelson, B.C. R.L. & L. Report No. 613F: 22 p. + 4 app.
RL&L Environmental Services Ltd. 2000a. Fraser River White Sturgeon Monitoring Program - Comprehensive Report (1995 to 1999). Final Report Prepared for B.C. Fisheries. RL&L Report No. 815F: 92 p + app.
RL&L Environmental Services Ltd. 2000b. A summary of white sturgeon investigations in isolated water bodies within the Columbia River Basin in B.C. 1995 to 1999. Report to B.C. Ministry of Environment, Lands and Parks.
Rosenfeld, J.S. and T. Hatfield. 2006. Information needs for assessing critical habitat of freshwater fish. Canadian Journal of Fisheries and Aquatic Sciences. 63: 683–698.
Schreier, A.D., Mahardja, B., and B. May. 2012. Hierarchical patters of population sturcture in the endangered Fraser Riverw white sturgeon (Acipenser transmontanus) and implications for conservation. Canadian Journal of Fisheries and Aquatic Sciences, 69: 1968-1980.
Teh, S. J., Wong, C., Furtula, V. and Teh, F.-C. 2003, Lethal and sublethal toxicity of didecyldimethylammonium chloride in early life stages of white sturgeon, Acipenser transmontanus. Environmental Toxicology and Chemistry, 22: 2152–2158.
Terraquatic Resource Management. 2011. Arrow Lakes Generating Station White Sturgeon Spawn Monitoring Program. Report prepared for Columbia Power Corporation, Castlegar B.C. 19 p.
Upper Columbia White Sturgeon Recovery Initiative. 2009a. Upper Columbia White Sturgeon Recovery Initiative ( UCWSRI). Accessed 16 June 2009.
Upper Columbia White Sturgeon Recovery Initiative. 2009b. Recovery Plan and Technical Appendices (WS Capture, Transport and Handling Manual, May 2006 and Suggested Protocol for Sampling White Sturgeon Mortality) . Accessed16 June 2009.
Upper Columbia White Sturgeon Recovery Initiative. 2012. Upper Columbia River white sturgeon recovery and management plan – 2012 revision . Accessed November 2012.
Young, W.T., and D.L. Scarnecchia. 2005. Habitat use of juvenile white sturgeon in the Kootenai River, Idaho and British Columbia. Hydrobiologia 537:265-271.
Note: This table was developed as part of the National Recovery Strategy for the six populations of white sturgeon. Scores were provided in this table to give some idea of priority across the different populations (Steve McAdam, B.C. Ministry of Environment, personal communication). The Columbia Basin Team used the original scoring method to update priorities from the 2006 document. Basins sharing the same score are presented based on geography from west to east. In 2012, this table was updated to include the status of each study, as an indication of progress towards addressing knowledge gaps. Some changes to the study descriptions and rationales have also been made for increased clarity. Though studies are prioritized, studies may also be completed opportunistically by partners, as resources allow.
Table A-1. Prioritized studies to address identified knowledge gaps for white sturgeon. There are six columns read left to right: Life Stage, Study, Rationale for study, Score, and Status. There are 98 rows, each representing the characteristics of a single study that addresses knowledge gaps for white sturgeon. Of these studies, 33 were directed at Columbia populations (five fully, and seven partially completed), 19 were directed at the Nechako population (seven partially completed), five were directed at the Kootenay population (one partially completed), 11 were directed at the Mid-Fraser population (one partially completed), 12 were directed at the Lower Fraser
| Population | Life Stage | Study | Rationale for study | Score | Status |
|---|---|---|---|---|---|
| Columbia - downstream of HLK Dam | Incubation - Juvenile Rearing | Investigate substrate preferences of yolk sac larvae to assist in understanding which habitats and life stages are limiting, including a literature review for evidence that substrate condition can be responsible for limited recruitment among sturgeon species. | Releases of hatchery juveniles have resulted in reasonable survivals, and wild eggs are also known to be viable. Combined with links between substrate change at spawning sites and recruitment failure, this strongly suggests substrate mediated effects on early life stage survival. Understanding the nature of substrate conditions affecting early life stage habitats of sturgeons should assist in characterizing survival bottlenecks. Early life stage habitat preferences will need to be documented to determine which habitats are limiting and how they can be restored. | 24 | Completed. Key results are presented in: Crossman, J. A. and L. R. Hildebrand. 2011. Evaluation of spawning substrate enhancement for white sturgeon in a regulated river: Effects on larval retention and dispersal. Revelstoke Unit 5 Project, B.C. Hydro, Castlegar, BC. McAdam, S. 2011. Effects of substrate condition on habitat use and survival by white sturgeon (Acipenser transmontanus) larvae and potential implications for recruitment. Canadian Journal of Fisheries and Aquatic Sciences 68:812-822. McAdam, S. O. 2012. Diagnosing white sturgeon (Acipenser transmontanus) recruitment failure and the importance of substrate condition to yolksac larvae survival. Ph.D. Thesis. University of British Columbia, Vancouver, B.C. |
| Columbia - transboundary | Early life history | Investigate behaviour of yolk sac larvae and larvae to determine factors affecting habitat selection, drift triggers and mortality. This would include experimental releases of yolk sac larvae from the conservation aquaculture program. | There is continued uncertainty regarding white sturgeon larval behaviour and factors that affect them (e.g., drift vs. hiding behaviours). Early life stage behaviours, habitat preferences and sources of mortality will need to be documented to characterize recruitment failure mechanism(s). | 24 | Completed. Key results are presented in: Crossman, J. A. and L. R. Hildebrand. 2011. Evaluation of spawning substrate enhancement for white sturgeon in a regulated river: Effects on larval retention and dispersal. Revelstoke Unit 5 Project, B.C. Hydro, Castlegar, B.C. McAdam, S. 2011. Effects of substrate condition on habitat use and survival by white sturgeon (Acipenser transmontanus) larvae and potential implications for recruitment. Canadian Journal of Fisheries and Aquatic Sciences 68:812-822. McAdam, S. O. 2012. Diagnosing white sturgeon (Acipenser transmontanus) recruitment failure and the importance of substrate condition to yolksac larvae survival. Ph.D. Thesis. University of British Columbia, Vancouver, B.C. |
| Nechako | Early life history | Investigation of habitat preference and utilization during early life history. | Due to the links between substrate change ad recruitment failure, substrate preferences during early life history will be an important determinant of mitigative measures. | 23 | Partially Completed. Results from lab studies and field studies in the Nechako and Columbia rivers provide useful results. Key results are presented in: Crossman, J. A. and L. R. Hildebrand. 2011. Evaluation of spawning substrate enhancement for white sturgeon in a regulated river: Effects on larval retention and dispersal. Revelstoke Unit 5 Project, B.C. Hydro, Castlegar, B.C. McAdam, S. 2011. Effects of substrate condition on habitat use and survival by white sturgeon (Acipenser transmontanus) larvae and potential implications for recruitment. Canadian Journal of Fisheries and Aquatic Sciences 68:812-822. McAdam, S. O. 2012. Diagnosing white sturgeon (Acipenser transmontanus) recruitment failure and the importance of substrate condition to yolksac larvae survival. Ph.D. Thesis. University of British Columbia, Vancouver, B.C. |
| Columbia - downstream of HLK Dam | Early life history | Investigate food preferences and availability of food sources for larvae during initial stages of exogenous feeding. | Although early life stage survival is the likely recruitment bottleneck, the impact mechanisms are uncertain. Early life stage food preferences and availability need to be documented to determine whether they are limiting. | 23 | Ongoing. Studies are examining food consumption of wild larvae, and food availability. Future studies also plan to address this question by evaluating recruitment success following experimental larval releases. |
| Kootenay | Larval | Lab, modelling and in situ study of larval drift and behaviour. | Understanding substrate preference as well as spatial and temporal extent of larval drift will help define critical habitat for this life stage below the Idaho spawning reach in Canada. | 23 | Partially Completed. Results from lab studies and field studies in the Columbia River provide useful results. Key results are presented in: Paragamian, V. L., R. McDonald, G. J. Nelson, and G. Barton. 2009. Kootenai River velocities, depth, and white sturgeon spawning site selection – a mystery unraveled? Journal of Applied Ichthyology 5:640-646. Crossman, J. A. and L. R. Hildebrand. 2011. Evaluation of spawning substrate enhancement for white sturgeon in a regulated river: Effects on larval retention and dispersal. Revelstoke Unit 5 Project, B.C. Hydro, Castlegar, B.C. McAdam, S. 2011. Effects of substrate condition on habitat use and survival by white sturgeon (Acipenser transmontanus) larvae and potential implications for recruitment. Canadian Journal of Fisheries and Aquatic Sciences 68:812-822. McAdam, S. O. 2012. Diagnosing white sturgeon (Acipenser transmontanus) recruitment failure and the importance of substrate condition to yolksac larvae survival. Ph.D. Thesis. University of British Columbia, Vancouver, B.C. Kynard, B., E. Parker, and B. Kynard. 2010. Ontogenetic behavior of Kootenai River White Sturgeon, Acipenser transmontanus, with a note on body color: A laboratory study. Environmental Biology of Fishes 88:65-77. |
| Nechako | Early life history | Drift duration and timing. | Spatial and temporal extent of larval drift will define habitat- recruitment bottleneck as well as the spatial extent and location of habitat restoration needs. | 22 | Partially Completed. Key results are presented in: McAdam, S. 2011. Effects of substrate condition on habitat use and survival by white sturgeon (Acipenser transmontanus) larvae and potential implications for recruitment. Canadian Journal of Fisheries and Aquatic Sciences 68:812-822. McAdam, S. O. 2012. Diagnosing white sturgeon (Acipenser transmontanus) recruitment failure and the importance of substrate condition to yolksac larvae survival. Ph.D. Thesis. University of British Columbia, Vancouver, B.C. |
| Columbia - downstream of HLK Dam | Spawning - Juvenile Rearing | Once the life stage and habitat conditions contributing to recruitment failure have been identified, suitable habitat remediation options will be reviewed and assessed for feasibility as appropriate below HLK Dam. | Based on Recruitment Failure Hypothesis Review (RFHR) hypotheses, this work would identify the likely causes of recruitment failure and research required to assess the feasibility of mitigative options and define pilot experiments. | 22 | Partially Completed. Results from lab studies and field studies in the Columbia River provide useful results. Key results are presented in: Crossman, J. A. and L. R. Hildebrand. 2011. Evaluation of spawning substrate enhancement for white sturgeon in a regulated river: Effects on larval retention and dispersal. Revelstoke Unit 5 Project, B.C. Hydro, Castlegar, B.C. McAdam, S. O. 2012. Diagnosing white sturgeon (Acipenser transmontanus) recruitment failure and the importance of substrate condition to yolksac larvae survival. Ph.D. Thesis. University of British Columbia, Vancouver, B.C. |
| Columbia - upstream of HLK Dam | Early life history- Juvenile Rearing | Assess effects of low temperatures on larval and juvenile development, growth and survival. | Cooler spawning, incubation and early life stage thermal regimes may limit the potential for establishing sustainable sturgeon stocks in upper Columbia reaches. | 22 | Partially Completed. Results from lab studies are useful: Parsley, M.J., E. Kofoot, T. Blubaugh, 2011. Mid-Columbia sturgeon incubation and rearing study (Year 2) (PDF) . Administrative Report to British Columbia Hydro and Power Authority, 2010-2011. Castlegar, B.C. 29 p. Boucher, M. 2012. The effect of substrate rearing on the growth, development, and survival of larval white sturgeon (Acipenser transmontanus) during early ontogeny. M.Sc. Thesis. University of Northern British Columbia, Prince George, B.C. |
| Columbia - upstream of HLK Dam | Spawning - Incubation | Assess effects of low temperatures on spawning activity and success. | Cooler spawning, incubation and early life stage thermal regimes may limit the potential for establishing sustainable sturgeon stocks in upper Columbia reaches. | 22 | Complete. Water Licence Requirement monitoring study CLBMON-27 assessed influence of temperature on habitats downstream of REV. Results are available in: B.C. Hydro. 2012. Columbia River White Sturgeon Management Plan Monitoring Program and Physical Works Annual Report: 2012. Prepared by B.C. Hydro, Castlegar B.C. |
| Columbia - upstream of HLK Dam | Early life history | Investigation of habitat requirements of yolk sac larvae and larvae to assist in understanding which habitats and life stages are limiting. | Early life stage habitat preferences will need to be documented to determine which habitats are limiting and how they can be restored. | 22 | Partially Completed. Results from lab studies and field studies in the Columbia River provide useful results. Key results are presented in: Crossman, J. A. and L. R. Hildebrand. 2011. Evaluation of spawning substrate enhancement for white sturgeon in a regulated river: Effects on larval retention and dispersal. Revelstoke Unit 5 Project, B.C. Hydro, Castlegar, B.C. McAdam, S. O. 2012. Diagnosing white sturgeon (Acipenser transmontanus) recruitment failure and the importance of substrate condition to yolksac larvae survival. Ph.D. Thesis. University of British Columbia, Vancouver, B.C. |
| Columbia - downstream of Hugh L. Keenleyside (HLK) Dam | Incubation - Juvenile Rearing | Plan and implement a baseline juvenile monitoring program to track trends in recruitment and survival in response to opportunistic high flow events and other environmental variables. | Dams have resulted in hydrograph changes including reductions to freshet flows. Limited natural recruitment still occurs in the Columbia downstream from HLK, and may be related to higher than average flow conditions or possibly lower FDR elevations during the post-hatch period. | 21 | Ongoing. B.C. Hydro monitors juvenile abundance and distribution as part of its Water Licence Requirements programs. |
| Columbia - downstream of HLK Dam | Early life history | Develop and apply sampling procedures to understand factors affecting habitat and food suitability and availability for 15-40 day old larvae. | Releases of hatchery juveniles have resulted in reasonable survivals, and wild eggs are also known to be viable. This strongly suggests an early life stage bottleneck as the mechanism of recruitment failure. Early life stage habitat preferences will need to be documented to determine which habitats are limiting and how they can be restored. | 21 | Not completed. Initial work on food availability in the Robson Reach will begin in 2013. |
| Columbia - upstream of HLK Dam | Spawning | Spawn monitoring and flow modelling associated with proposed spawning operations tests used to assess hydrograph effects on spawning habitat and activity. | Spawning habitats below REV Dam are impacted by diel load shaping, as well as backwatering of the Arrow Lakes Reservoir. Depth, velocity, and substrate conditions associated with operations should be determined. Flow options from REV Dam should be tested and sturgeon spawning activity and success monitored to determine if these impacts can be mitigated. | 21 | Ongoing. Water Licence Requirement monitoring study CLBMON-20 is detailed hydraulic modeling of habitats downstream of REV. Results are available in: B.C. Hydro. 2012. Columbia River White Sturgeon Management Plan Monitoring Program and Physical Works Annual Report: 2012. Prepared by B.C. Hydro, Castlegar B.C. |
| Columbia - upstream of HLK Dam | Spawning - Juvenile Rearing | Assess engineering options to provide warmer temperatures below REV Dam, which may result in improved spawning success and better egg, larval and juvenile development and survival. | If feasible, remove or reduce the effects of upstream dams on thermal regime in the mid-Columbia River. | 21 | Partially Complete. Water Licence Requirement monitoring study CLBWORKS-28 assessed feasibility of various physical works associated with White Sturgeon in the Columbia River. Results are available in: B.C. Hydro. 2012. Columbia River White Sturgeon Management Plan Monitoring Program and Physical Works Annual Report: 2012. Prepared by B.C. Hydro, Castlegar B.C. Further temperature monitoring work will begin in 2013. |
| Lower Fraser | Early life history | Conduct spawning and early juvenile rearing field assessments to determine important larval to early juvenile transition rearing habitats. | Some Fraser mainstem and side channel spawning sites identified and timing confirmed through works conducted in 1999. Additional works needed to confirm timing and full extent of habitat use including potential sub-populations. Habitats undergo significant annual physical changes. Potential to have greatest opportunity to affect recruitment in this population. | 20 | Partially completed. Some assessments were conducted in 2010 and 2011 see: Paradis, E., Sykes, G., Liebe, R. English, A. and Johnston, S. 2011 (draft). Fraser River sturgeon sampling and monitoring program 2011. Prepared for Emergency Management B.C., Victoria B.C. December 2011[PDF 50 MB]. |
| Lower Fraser | Juvenile Rearing | Conduct dedicated field assessments to determine or re-confirm extent of important juvenile rearing habitats. Compare results of assessments with previous assessments if available. | Very limited information available for 25 day to 1 year age class. Previous comprehensive assessment conducted between 1985 and 1993, and recent less comprehensive study conducted in 2007 and 2008 confirmed age 1+ use in some habitats. Habitat use confirmed similar to late juvenile and adult. | 20 | Not completed. |
| Nechako | Spawning | Identify spawning window (temporal). | Hydrograph and thermograph in regulated system variable (cues for spawning), therefore need to better understand. | 20 | Ongoing. Spawn monitoring was conducted in most years since 2004. See: Triton Environmental Consultants. 2009. Nechako white sturgeon monitoring 2009. Unpubl. report for B.C. Ministry of Environment, Prince George, B.C. 73 pp. |
| Nechako | Juvenile Rearing | Review environmental/ biological variables in context of recruitment pulses. | Very little information exists on this life stage. | 20 | Not completed. |
| Columbia - downstream of HLK Dam | Early life history | Investigate the habitats of yolk sac larvae and larvae to determine where and how survival bottlenecks for these life stages occur. | Modify as in similar rows above. Substrate condition in the vicinity of spawning sites is strongly implicated as the mechanism of recruitment failure. The location of early life stage habitats need to be documented to determine the nature of these habitats and how they can be restored. | 20 | Partially Completed. Key results are available for other locations and are presented in: Crossman, J. A. and L. R. Hildebrand. 2011. Evaluation of spawning substrate enhancement for white sturgeon in a regulated river: Effects on larval retention and dispersal. Revelstoke Unit 5 Project, B.C. Hydro, Castlegar, B.C. McAdam, S. 2011. Effects of substrate condition on habitat use and survival by white sturgeon (Acipenser transmontanus) larvae and potential implications for recruitment. Canadian Journal of Fisheries and Aquatic Sciences 68:812-822. McAdam, S. O. 2012. Diagnosing white sturgeon (Acipenser transmontanus) recruitment failure and the importance of substrate condition to yolksac larvae survival. Ph.D. Thesis. University of British Columbia, Vancouver, B.C. |
| Columbia - downstream of HLK Dam | Early life history - Juvenile Rearing | Determine the relationship between habitat availability, use and growth and survival. | This work is required to assess appropriate levels of stocking for ongoing conservation aquaculture. As well, the work can be applied to earlier life stages to assess the exact life stage timing of recruitment failure. | 20 | Several Water Licence Requirement monitoring studies are assessing juvenile survival and habitat use. Results are available in: B.C. Hydro. 2012. Columbia River White Sturgeon Management Plan Monitoring Program and Physical Works Annual Report: 2012. Prepared by B.C. Hydro, Castlegar B.C. |
| Columbia - downstream of HLK Dam | Incubation - Juvenile Rearing | Complete literature review to assess role of predation in recruitment failure. | Predation is a component in a number of hypotheses' impact pathways and may therefore be linked to recruitment reduction or failure. Changes in freshet volume, turbidity levels, substrate condition, and other habitat parameters should be explored to understand effects on the relationship between young sturgeon and their predators. | 20 | Not completed. |
| Columbia - upstream of HLK Dam | Early life history - Juvenile Rearing | Early life stage habitat flow modelling associated with proposed spawning operations tests to assess changing availability of habitat under a range of river discharge and reservoir operation regimes. | Associated with spawn monitoring and flow modelling below REV Dam, this study would assess the effect of proposed spawning flows on availability of early life stage habitat. | 20 | Ongoing. Water Licence Requirement monitoring study CLBMON-20 is detailed hydraulic modeling of habitats downstream of REV. Results are available in: B.C. Hydro. 2012. Columbia River White Sturgeon Management Plan Monitoring Program and Physical Works Annual Report: 2012. Prepared by B.C. Hydro, Castlegar B.C. |
| Kootenay | Spawning | Impact of lake levels on spawner access upstream of Bonners Ferry, Idaho. | Implications of US studies suggest lack of lake backflooding has affected spawning site selection in US. | 20 | Not completed. |
| Upper Fraser | Incubation | Spatial and Temporal. | No information exists on this life stage. | 19 | Not completed. |
| Upper Fraser | Incubation | Habitat/ Habitat Quality. | No information exists on this life stage. | 19 | Not completed. |
| Upper Fraser | Early life history | Spatial and Temporal. | No information exists on this life stage. | 19 | Not completed. |
| Upper Fraser | Early life history | Food requirements. | No information exists on this life stage. | 19 | Not completed. |
| Nechako | Incubation | Egg deposition and drift. | Need for refinement of spatial extent of incubation habitat. | 19 | Partially completed. Hydraulic modeling of the Vanderhoof spawning area was completed by Northwest Hydraulic Consultants for current and pre-dam conditions. Northwest Hydraulic Consultants. 2008. Nechako River at Vanderhoof: hydrodynamic model upgrade. Unpubl. report for B.C. Ministry of Environment, Victoria, B.C. 39 pp. |
| Columbia - all locations | Incubation - Juvenile Rearing | Examine whether Upper Columbia substrate conditions within key areas may have limited recruitment. | Understanding the location and nature of substrate conditions throughout the area of early life stage habitats should assist in characterizing the survival bottleneck. Pre-dam substrate modelling could describe conditions in which recruitment successfully occurred. | 19 | Partially Completed. Results from lab studies and field studies in the Columbia River provide useful results. Key results are presented in: Crossman, J. A. and L. R. Hildebrand. 2011. Evaluation of spawning substrate enhancement for white sturgeon in a regulated river: Effects on larval retention and dispersal. Revelstoke Unit 5 Project, B.C. Hydro, Castlegar, B.C. McAdam, S. 2011. Effects of substrate condition on habitat use and survival by white sturgeon (Acipenser transmontanus) larvae and potential implications for recruitment. Canadian Journal of Fisheries and Aquatic Sciences 68:812-822. McAdam, S. O. 2012. Diagnosing white sturgeon (Acipenser transmontanus) recruitment failure and the importance of substrate condition to yolksac larvae survival. Ph.D. Thesis. University of British Columbia, Vancouver, B.C. Golder Associates Ltd. 2006. White sturgeon spawning at Waneta, 2005 investigations. Unpubl. report #05-1480-030F for Teck Cominco Metals, Trail, B.C. and B.C. Hydro, Castlegar, B.C. 40 pp. + 1 app. |
| Columbia - downstream of HLK Dam | Early life history - Juvenile Rearing | Determine the differences in habitat conditions in the reach between Northport and Kettle Falls during spawning and early rearing periods, and explore the relationship between habitat and hydro-operations in years with and without recruitment. | There have been years during the period of virtual recruitment failure when natural recruitment has been detected. The intent of this study is to examine the potential differences in habitat conditions under various snow pack levels and resulting hydro-operations that may facilitate increases in natural recruitment. | 19 | Not completed. Note: this study would occur in the US. |
| Columbia - downstream of HLK Dam | Spawning - incubation | Assess effects of high temperatures on spawning and incubation success. | Spawning at Waneta is affected by high water temperatures during the latter part of the spawning period. These are high enough to affect embryo survival and development, and while unlikely to be a causal agent for recruitment failure, may be an exacerbating issue. | 19 | Not completed. Some relevant information contained in: Parsley, M.J., E. Kofoot, T. Blubaugh, 2011. Mid-Columbia sturgeon incubation and rearing study (Year 2). Administrative Report to British Columbia Hydro and Power Authority, 2010-2011. Castlegar, B.C. 29 p. |
| Kootenay | Adult Migration | Impacts of low flow, temperature (high or low) on migration. | Key information is lacking regarding cues for fish to move into spawning reach. | 19 | Not completed. |
| Nechako | Spawning | Identify additional spawning sites (spatial). | Fish could spawn at more the one spawning site or spawning sites may shift based on river conditions (temperature /flow). | 18 | Partially completed. Information is collected as opportunities allow. Some information exists of apparent spawning aggregations and behaviour. |
| Nechako | Incubation | Temperature limits of incubation. | Nechako thermograph is variable with periodically high temperature, based on Wang (1985) may be link to recruitment failure. | 18 | Not completed. Some relevant information contained in: Boucher, M. 2012. The effect of substrate rearing on the growth, development, and survival of larval white sturgeon (Acipenser transmontanus) during early ontogeny. M.Sc. Thesis. University of Northern British Columbia, Prince George, B.C. |
| Nechako | 25d to 1y | Food supply. | Limited information exists for this life stage. | 18 | Not completed. Questions related to possible food limitations during early life stages may be addressed in part through studies of recruitment following hatchery releases. |
| Nechako | Staging | Monitor pre-spawning movements. | Little information exists- suspected that overlap occurs with overwintering. | 18 | Partially completed. Information is collected as opportunities allow. Some information exists of apparent spawning aggregations and behaviour. |
| Nechako | Staging | Monitor habitat use at putative staging locations. | Little information exists for this life stage. | 18 | Partially completed. Information is collected as opportunities allow. Some information exists of apparent spawning aggregations and behaviour. |
| Columbia - all locations | All life stages | Align stock structure analysis with information on known ecosystem changes to develop impact timelines for each recruitment failure hypothesis. | Hypotheses refer to those defined as significant by the RFHR. Historic recruitment patterns among various components of the population are key to understanding impacts to recruitment (pre and post failure and other river comparisons). | 18 | Completed. See chapter 4 of: McAdam, S. O. 2012. Diagnosing white sturgeon (Acipenser transmontanus) recruitment failure and the importance of substrate condition to yolksac larvae survival. Ph.D. Thesis. University of British Columbia, Vancouver, B.C. |
| Columbia - all locations | All life stages | Synthesis of studies relating to historic population function, with workshop to assist in completing interpretation and developing report. | This key step in the interpretation of historic data and recruitment trends was identified by the TWG during the RFHR and will guide future research and recovery efforts. | 18 | Not completed. |
| Columbia - downstream of HLK Dam | Early life history | Investigate the influence of predation on eggs under various Pend d'Oreille River flow regimes (specific to Waneta Expansion power development impact assessment). | The Waneta spawning area is affected by dam operations on the Pend d’Oreille system, which are operated as load shaping facilities. The Waneta Expansion Project, if developed, may add additional load shaping to the system. Load shaped flows pre-date recruitment failure; however, some impacts to embryo survivals may occur from increased predation enhanced by shaped flows. The extent of this impact should be investigated, particularly if additional impacts are forecast from new developments. | 18 | Partially completed. Results presented in: Golder Associates Ltd. 2009b. Environment and Public Safety Issues: Technical Due Diligence Review: Waneta Generating Station. Report 09-1480-0037, prepared for B.C. Hydro Engineering, Aboriginal Relations and Generation, 6911 Southpoint Dr. Burnaby, B.C. |
| Columbia - upstream of HLK Dam | Spawning | Assess impacts of REV Dam load shaping on connectivity to spawning area for adults attempting to access from Big Eddy. | Big Eddy is located in Revelstoke a short distance downstream from the spawning site at the Golf Course. This site is a staging location for sturgeon using the upstream spawning area. Depending on the flows from REV Dam, a habitat connectivity issue could exist between the eddy and spawning area. Minimum flows will be instituted in 2010 and may resolve this concern, but monitoring of adult movements should be conducted. | 18 | Not completed. |
| Columbia - upstream of HLK Dam | Early life history - Juvenile Rearing | Investigate habitat and feeding requirements and related availability between REV Dam and the Arrow Lakes reservoir. | Juvenile rearing habitat in this area may be of limited availability due to unsuitable habitat parameters and food resources. These limitations may be historical in nature or cause by current operating regimes, but should be investigated to understand their effect on growth, survival and recruitment. | 18 | Ongoing. Several Water Licence Requirement monitoring studies are assessing juvenile survival and habitat use. Results are available in: B.C. Hydro. 2012. Columbia River White Sturgeon Management Plan Monitoring Program and Physical Works Annual Report: 2012. Prepared by B.C. Hydro, Castlegar B.C. |
| Lower Fraser | Incubation | Conduct dedicated spawning habitat field assessments to determine or re-confirm important incubation habitats. | As for spawning sites. Some Fraser mainstem and side channel spawning sites identified and timing confirmed through works conducted in 1999. Additional works needed to confirm timing and full extent of habitat use including potential sub-populations. Habitats undergo significant annual physical changes. No recent work has been conducted. | 17 | Not completed. |
| Upper Fraser | Spawning | Spatial and Temporal. | No information-required for other populations. | 17 | Not completed. |
| Upper Fraser | Spawning | Environmental Cues. | No information-required for other populations. | 17 | Not completed. |
| Upper Fraser | Juvenile Rearing | Spatial and Temporal. | No information exists on this life stage. | 17 | Not completed. |
| Nechako | Spawning | Refine spatial extent of known site. | Spatial extent of current site based on limited data (3 years). | 17 | Partially completed. Information is collected as opportunities allow. |
| Nechako | Spawning | Genetic/ bony structure studies | Genetics has identified population structure in Columbia, finray microchemical/ genetics could help identify or determine likelihood of other spawning groups and/or sites in the Nechako River. | 17 | Not completed. |
| Nechako | Adult Holding & Feeding | Food requirements. | Very little information exists for this stage in Nechako River. | 17 | Not completed. |
| Columbia - all locations | All life stages | Finalize stock structure analysis including completion of fin ray microchemistry, genetics, aging re-evaluation and movement studies to determine possible historic breeding groups. | Historic recruitment patterns among various components of the population are key to understanding impacts to recruitment (pre and post failure and other river comparisons). | 17 | Completed. Results presented in: Nelson, R.J., and D.S.O. McAdam. 2012. Historical population structure of White Sturgeon in the Upper Columbia River detected with combined analysis of capture, telemetry and genetics. Journal of Applied Ichthyology 28: 1–7 Clarke, A., S. McAdam and K. Telmer. 2011. Upper Columbia white sturgeon final analysis and data report: microchemical analysis of fin-rays to evaluate historical habitat use. Unpubl. report for Canadian Columbia Intertribal Fisheries Commission, Cranbrook, B.C. 70 pp. Drauch-Schrier, A., B. Mahardja and B. May. 2010. Investigation of white sturgeon population structure in the transboundary reach of the Columbia River using polysomic microsatellite markers. Report for the Spokane Tribe of Indians. 20 pp. (draft). McAdam, S. O. 2012. Diagnosing white sturgeon (Acipenser transmontanus) recruitment failure and the importance of substrate condition to yolksac larvae survival. Ph.D. Thesis. University of British Columbia, Vancouver, B.C. |
| Columbia - All locations | Juvenile | Investigation of food availability in relation to growth & fecundity. | Dams have caused significant changes to the availability of food in this area (especially anadromous salmon and other sources reliant on salmon). This could result in lower growth, survival or reproductive frequency. | 17 | Ongoing. Juvenile growth studies are ongoing. Some relevant information presented in: Van Poorten, B. T. and S. O. McAdam. 2010. Estimating differences in growth and metabolism in two spatially segregated groups of Columbia River white sturgeon using a field based bioenergetics model. Open Fish Sci. J. 3: 132-141. |
| Columbia - upstream of HLK Dam | Juvenile Rearing | Release of hatchery fish, various ages, with follow-up sampling and telemetry to assess: i) approximate juvenile habitat requirements and suitability; and, ii) approximate impacts of operational variations on juvenile location, growth and survival. | Survival rates for early life stages need to be assessed to determine if natural recruitment is possible in this area. This research involves follow-up assessments of a series of hatchery release groups, or differing age, from yearlings to larvae. Survivals of each release group should be able to provide an indication of limitations to natural growth and survival. | 17 | Several Water Licence Requirement monitoring studies are assessing juvenile survival and habitat use. Results are available in: B.C. Hydro. 2012. Columbia River White Sturgeon Management Plan Monitoring Program and Physical Works Annual Report: 2012. Prepared by B.C. Hydro, Castlegar B.C. |
| Lower Fraser | Spawning | Conduct dedicated spawning habitat field assessments to determine or re-confirm specific spawning habitats and the spawning window (spatial). | Some Fraser mainstem and side channel spawning sites identified and timing confirmed through works conducted in 1999. Additional works needed to confirm timing and full extent of habitat use including potential sub-populations. Habitats undergo significant annual physical changes. No recent work has been conducted. | 16 | Not completed. |
| Upper Fraser | 25d to 1year | Food requirements. | No information exists on this life stage. | 16 | Not completed. |
| Columbia - downstream of HLK Dam | Juveniles - Adults | Assess energetic effects of the rivers thermal regime; winter temperature has increased, which may have energetic consequences for white sturgeon over winter energy use. | Reservoir thermal inertia, exacerbated by climate change, is thought to have led to changes in the temperature regime downstream of HLK. Early and late winter temperatures could be warm enough to result in higher metabolic demand during the period when food resources are limited. This could result in lower growth, survival or ultimately reproductive frequency. | 16 | Not completed. |
| Kootenay | Juvenile (1-2 years) | Release of YOY hatchery fish to assess habitat use. | Very little is known about critical habitat for juvenile white sturgeon in Kootenay River/Lake. | 16 | Ongoing. Hatchery releases are ongoing and some individuals are released with PIT tags. Studies of recruitment and habitat use are ongoing. |
| Upper Fraser | 1-2 years | Habitat. | No information exists on this life stage. | 15 | Not completed. |
| Nechako | 1-2 years | Habitat. | No information exists on this life stage. | 15 | Not completed. |
| Nechako | Rearing 2+ | Spatial Extent of habitat (Upstream Vanderhoof is priority). | Limited information exists for this stage in Nechako River. | 15 | Not completed. Annual juvenile monitoring studies continue to monitoring habitat use. |
| Nechako | Rearing 2+ | Food and habitat requirements. | No information exists for this stage in Nechako River. | 15 | Not completed. |
| Nechako | Adult Holding & Feeding | Key feeding habitats. | Little information exists for this stage in Nechako River. | 15 | Not completed. |
| Columbia - all locations | Adults | Undertake assessment of abundance to better understand survival and population trajectory, including, where feasible, assessment of sub-population status. | This Columbia population (from REV Dam) downstream into Lake Roosevelt in the US) has not been assessed since the early 1990s. A new assessment of stock status will help guide the timing of recovery actions and inform projections of the outcome of hatchery stocking. | 15 | Ongoing. New estimates of adult abundance are underway (James Crossman, B.C. Hydro, personal communication). |
| Columbia - downstream of HLK Dam | Adults | Assess factors influencing the use of Kootenay Eddy, and the consequences of providing flows to protect depths. | Kootenay Eddy is used fairly extensively by adult sturgeon but does not appear to be as consistently used as other eddies in this area. Sturgeon may abandon use of the area when the depth decreases below 18 m. | 15 | Not completed. |
| Columbia - all except Kootenay | Adults | Assess the impact and recovery implications from disruption of movement and related habitat fragmentation caused by HLK, Brilliant and Waneta dams. | Sturgeon from the Columbia River historically accessed the lower Pend d'Oreille, Kootenay and Slocan, and upper Columbia rivers (as far as Kinbasket Lake). Some of these areas were probably important for seasonal forage and some may have provided additional spawning and early life stage habitats. Altered access to downstream habitats may also have been an issue for those fish residing between dams. The extent of disruption in the system, and implications for recovery needs to be assessed. | 15 | Not completed. |
| Kootenay | Rearing 2+ | Kokanee impacts on growth, survival and reproduction. | Reductions in kokanee populations may be limiting adult spawner frequency and increasing the age at maturity. | 15 | Not completed. |
| Mid Fraser | Spawning | Conduct field assessments to confirm the spawning window (temporal). | Limited information exists on spawning habitat use and the influence of environmental cues within a natural river. | 14 | Partially completed. Ongoing radiotracking studies are providing information about spatial and temporal patterns of habitat use. |
| Mid Fraser | Early life history | Conduct field assessments to determine important larval rearing habitats. | Greatest opportunity to affect recruitment, however, little apparent impact to recruitment. | 14 | Not completed. |
| Mid Fraser | Adult Holding & Feeding | Conduct field assessments to determine important habitats, and food requirements and preferences. Compare results of assessments with previous limited assessments. | Limited information, but access to salmon available. | 14 | Not completed. |
| Columbia - downstream of HLK Dam | Adults | Assess effects of hatchery supplemented fish on food availability, habitat use, fecundity and growth. | This work is required to assess appropriate levels of stocking for ongoing conservation aquaculture. Stocking targets should be based on a combination of long-term adult population targets, and some measure of habitat capacity based on physical assessment, growth and survival. | 14 | Ongoing. Hatchery releases are being monitored through WLR programs. There is no indication of density dependence at this time (James Crossman, B.C. Hydro, personal communication). |
| Nechako | Overwintering | Habitat Use. | Not known whether habitat is limiting or is use limited due to low population size. | 14 | Not completed. |
| Nechako | Overwintering | Attributes of habitat. | One specific site seems to be preferred- not known why or if other sites exist. | 14 | Not completed. |
| Lower Fraser | Spawning | Conduct field assessments to confirm the spawning window (temporal). | Limited information exists on spawning habitat use and the influence of environmental cues within a natural river. | 13 | Not completed. |
| Mid Fraser | Spawning | Conduct field assessments to confirm specific spawning habitats and the spawning window (spatial). | No sites identified; medium due to link to habitat issues. | 13 | Not completed. Ongoing radiotracking work has identified possible spawning sites, but spawning has not been confirmed. |
| Mid Fraser | Juvenile Rearing | Conduct field assessments to determine important juvenile rearing habitats. Compare results of assessments with previous assessments. | Very limited information available; could infer from other studies. | 13 | Not completed. |
| Columbia - All locations | Juvenile Rearing | Assess kokanee impacts on growth, survival and reproduction. | Kokanee are believed to provide an important annual food source during and immediately following the spawning period. The nutrients provided by these fish may ultimately affect sturgeon reproductive cycles and population productivity. A better understanding of the importance of kokanee and their role in the recovery process is required. This also relates to the issue of habitat fragmentation, where dams prevented access to kokanee resources for sturgeon in the recovery area. | 13 | Not completed. |
| Columbia - All locations | Adults | Determine the extent of kokanee distribution in each tributary, and assess potential benefits to sturgeon. | Kokanee are believed to provide an important annual food source during and immediately following the spawning period. The nutrients provided by these fish ultimately affect sturgeon reproductive cycles and population productivity. A better understanding of the importance of kokanee and their role in the recovery process is required. This also relates to the issue of habitat fragmentation, where dams prevented access to kokanee resources for sturgeon in the recovery area. | 13 | Not completed. |
| Lower Fraser | 25d to 1 year | Conduct dedicated field assessments to determine important early juvenile rearing habitats for this life history stage. | Very limited information available for this life history stage. Habitat type use has not been confirmed. | 12 | Partially completed. See: Glova et al. 2009 [PDF 1.4 MB]. |
| Lower Fraser | Adult Holding & Feeding | Conduct field assessments to determine important habitats, and food requirements and preferences. Compare results of assessments with previous limited assessments. | Limited information, however, access to marine derived food is reasonably abundant. | 12 | Not completed. |
| Mid Fraser | 25d to 1year | Conduct field assessments to determine important juvenile rearing habitats. Compare results of assessments with previous assessments. | Limited information, but variable likely not limiting past 6 months of age. | 12 | Not completed. |
| Mid Fraser | Rearing 2+ | Conduct field assessments to determine important juvenile rearing habitats. Compare results of assessments with previous assessments. | Limited information, possible use of edge habitats creates potential anthropogenic impact | 12 | Not completed. |
| Mid Fraser | Adult Holding & Feeding | Conduct field assessments to determine important habitats, and food requirements and preferences. Compare results of assessments with previous limited assessments. | Adult locations poorly understood, and would provide inference about juvenile habitat use. | 12 | Not completed. An ongoing radiotracking study is providing information about spatial and temporal patterns of habitat use. |
| Upper Fraser | Rearing 2+ | Spatial and Temporal. | No information exists for this stage in Upper Fraser River. | 12 | Not completed. |
| Upper Fraser | Rearing 2+ | Food requirements. | No information exists for this stage in Upper Fraser River. | 12 | Not completed. |
| Upper Fraser | Rearing 2+ | Habitat. | No information exists for this stage in Upper Fraser River. | 12 | Not completed. |
| Upper Fraser | Adult Holding & Feeding | Spatial and Temporal. | No information exists on this life stage. | 12 | Not completed. |
| Upper Fraser | Adult Holding & Feeding | Habitat. | No information exists on this life stage. | 12 | Not completed. |
| Upper Fraser | Overwintering | Habitat Use. | No information exists on this life stage. | 12 | Not completed. |
| Upper Fraser | Overwintering | Attributes of habitat. | No information exists on this life stage. | 12 | Not completed. |
| Upper Fraser | Staging | Monitor pre-spawning movements. | No information exists on this life stage. | 12 | Not completed. |
| Upper Fraser | Staging | Monitor habitat use at putative staging locations. | No information exists on this life stage. | 12 | Not completed. |
| Lower Fraser | 1-2 years | Conduct dedicated field assessments to determine or confirm important early juvenile rearing habitats for this life history stage. Compare results of assessments with previous assessments to determine change or effects. | Limited information available. Previous comprehensive assessment conducted between 1985 and 1993, and recent less comprehensive study conducted in 2007 and 2008. Habitat use confirmed similar to late juvenile and adult. | 11 | Not completed. |
| Lower Fraser | Overwintering | Conduct field assessments to determine important habitats, and food requirements and preferences. Compare results of assessments with previous limited assessments. | Adult locations reasonably well understood, not felt to limit population. | 11 | Not completed. |
| Lower Fraser | Staging | Monitor habitat use at putative staging locations. | Limited information, however, habitat and access not felt to be limiting. | 11 | Not completed. |
| Mid Fraser | Incubation | Conduct field assessments to determine important larval rearing habitats. | Can be derived from spawning location. | 11 | Not completed. |
| Mid Fraser | 1-2 years | Conduct field assessments to determine important juvenile rearing habitats. Compare results of assessments with previous assessments. | Limited information available, most important for 21 days to 1 year. | 11 | Not completed. |
| Mid Fraser | Staging | Conduct field assessments to determine important habitats, and food requirements and preferences. Compare results of assessments with previous limited assessments. | Limited information, however, habitat and access not felt to be limiting. | 11 | Not completed. |
| Lower Fraser | Spawning | Conduct field assessments to confirm sub-population spawning habitats. (e.g. Pitt or Harrison) (spatial & temporal). Conduct analysis of collected tissue samples from sub-populations to confirm genetic variability. | Genetics has identified population structure in Columbia, and evidence in the Lower Fraser from genetics and movement, will influence monitoring and population estimates. | 10 | Not completed. Ongoing studies of fin ray chemistry may provide useful insight. |
| Lower Fraser | Rearing 2+ | Conduct field assessments to determine important juvenile rearing habitats. Compare results of assessments with previous assessments. | Limited information, possible use of edge habitats creates potential anthropogenic impact. | 10 | Not completed. |
Note: The Lower and Middle Fraser white sturgeon populations were assessed by COSEWIC as Endangered in 2003, but were declined for SARA listing in 2006. As such, SARA does not apply to these populations, and they are not subject to the automatic prohibitions or requirement to identify critical habitat. Relevant recovery information for these populations is included as an appendix to the Recovery Strategy to provide a comprehensive picture of white sturgeon conservation in British Columbia.
The following recovery objectives apply to the middle and lower Fraser River populations of white sturgeon:
- Prevent extirpation of white sturgeon in each of the identified populations by ensuring no net loss of reproductive potential.
- Reach or exceed the following population and distribution targets for conservation within 50 years:
- 10,000 mature individuals in the lower Fraser River;
- current mature individuals in the middle Fraser River;
- approximately 1:1 sex ratio at maturity;
- distribution over the natural range;
- natural age structure;
- natural recruitment in the lower Fraser River sufficient to meet goal (a);
- maintain current levels of natural recruitment in middle Fraser River;
- Determine the current levels of beneficial use through the FSC and commercial salmon gillnet and beach seine fisheries, and in the catch and release recreational fishery. Maintain current levels of beneficial use provided they do not threaten population recovery as indicated above.
- Reach or exceed population and distribution targets for expanded beneficial use within specified timeframes. As success is achieved in meeting the biological recovery targets, the beneficial use targets and timelines will be established and adjusted. Such targets may vary among populations.
The recovery strategy proposes the following targets for long-term population viability as defined in the Recovery Population Targets section of the Recovery Strategy. As new information becomes available these targets may be updated. The targets are considered sufficient for ongoing recovery planning of white sturgeon over the next 10 years.
An interim abundance target of 10,000 mature individuals is proposed for the lower Fraser River population and a target of current abundance is proposed for the mid-Fraser River. Mature is defined as 1.6 metres fork length or 18-20 years of age. These abundance targets are higher than for the four listed populations, although for the lower Fraser River in particular the habitat carrying capacity is much greater than in other areas. Based on the available scientific literature these targets are believed sufficient to meet abundance criteria to offset threats from demographic, environmental and genetic changes over the next 100 years. Because genetic variation in these populations is poorly understood it is assumed that this will be adequate given the long generation time (~ 40 years) and the planning time frame considered here. However, these abundance targets provide little protection against environmental catastrophes or significant increases in beneficial use. It should be noted also, that the current population estimate for the mid-Fraser River is weak, and needs to be improved to support the abundance target.
Natural populations fluctuate in abundance, which can make trends difficult to detect over the short term. The target is continued growth in all populations over the long-term when populations are below the target abundance level. The following growth rate targets are proposed:
- continued natural recruitment level in the middle Fraser River;
- natural recruitment in the lower Fraser River that allows population targets to be achieved;
- maintenance of at least the present abundance for all age classes in the middle Fraser River populations; and,
- increasing abundance for all age classes in the lower Fraser River population in order to meet population targets.
The following targets for population structure are proposed:
- distribution across the populations’ natural range;
- natural sex ratio (currently defined as 1:1); and,
- natural age structure.
The stable age distribution should be highly skewed, with the majority of individuals in immature age classes.
To understand historic and current sub-populations and their associated habitats, to protect important habitats and where possible restore habitats required for population maintenance or recovery.
Table B-1. Recommended strategies to meet management objectives for non-SARA-listed populations. The table has four columns read from left to right: Priority, Strategy, Actions, Performance Measure. Directly below column headings are seven rows read from left to right as follows.
Row 1: Necessary, Meet or exceed population targets within specified timeframe, 1. Monitor population trends. 2. Establish parameters for beneficial use. Have targets been achieved? Row 2: Necessary, Protect important habitats, 1. Identify habitat requirements for major life stages. 2. Define important habitats (including related ecological processes). 3. Protect, maintain and enhance important white sturgeon habitats. 4. Ensure habitat diversity, connectivity & productivity. 5. Ensure coordination between regulatory agencies with jurisdiction over white sturgeon habitat. Has important habitat been defined? Have important habitats been protected? Row 3: Necessary, Clarify, manage and mitigate threats, 1. Clarify the following threats and their relative risks: a. Directed and non-directed fishing, b. pollution, c. food supply, d. habitat. 2. Undertake specific actions to manage risks: a. protect, maintain and enhance important habitats, b. manage illegal harvest, c. mitigate impacts from fisheries through regulation and best practices, d. limit and manage pollutant discharges and contaminant loading, especially adjacent to important habitats, e. protect, maintain and enhance water quality, f. manage and mitigate the growth of commercial sturgeon aquaculture, g. better understand, maintain and enhance food availability for all life stages of each population. 3. Monitor threat indicators and population trends. 4. Clarify regulatory roles and responsibilities of agencies with jurisdiction over threats to white sturgeon and their habitats; Have the threats been clarified? Have the regulatory roles and responsibilities been clarified? Have the threats been sufficiently managed or mitigated? Row 4: Primary, Address information gaps that inhibit conservation of white sturgeon. 1. Address study needs to define important habitats (see Appendix A for the Studies to Address Knowledge Gaps). 2. Address basic biological data gaps (see Section 7: Knowledge Gaps in the Recovery Strategy for discussion of data gaps), Have information gaps been filled? Row 5: Primary Increase stakeholder and general public awareness of white sturgeon and its conservation needs. 1. Increase awareness and stewardship of white sturgeon throughout its natural range. 2. Engage in effective public education of the species and its conservation needs. 3. Support learning and communication across all working groups. 4. Ensure participation from community and technical experts. Has general awareness of sturgeon conservation been achieved? Row 6: Secondary, Maintain and where necessary restore ecosystem functions relevant to white sturgeon, 1. Incorporate the needs of healthy white sturgeon populations into the management of white sturgeon prey species including salmon, eulachon, and resident fish. 2. Accommodate other species’ needs during recovery of white sturgeon. 3. Manage non-native predatory fish species to reduce impacts to white sturgeon. 4. Coordinate with regulatory agencies that have influence or jurisdiction over white sturgeon prey species. Is the ecosystem “healthy” for white sturgeon? Row 7: Secondary, Implement the recovery strategy, 1. Protect the species with existing legislation. 2. Secure funding for implementation of recovery actions. 3. Update and revise the strategy at least every 5 years. Has the recovery strategy been updated as needed?
| Priority | Strategy | Actions | Performance Measure |
|---|---|---|---|
| Necessary | Meet or exceed population targets within specified timeframe. |
|
Have targets been achieved? |
| Necessary | Protect important habitats |
|
Has important habitat been defined? Have important habitats been protected? |
| Necessary | Clarify, manage and mitigate threats |
|
Have the threats been clarified? Have the regulatory roles and responsibilities been clarified? Have the threats been sufficiently managed or mitigated? |
| Primary | Address information gaps that inhibit conservation of white sturgeon. |
|
Have information gaps been filled? |
| Primary | Increase stakeholder and general public awareness of white sturgeon and its conservation needs. |
|
Has general awareness of sturgeon conservation been achieved? |
| Secondary | Maintain and where necessary restore ecosystem functions relevant to white sturgeon |
|
Is the ecosystem “healthy” for white sturgeon? |
| Secondary | Implement the recovery strategy |
|
Has the recovery strategy been updated as needed? |
With the inclusion of the non-SARA listed middle and lower Fraser River white sturgeon populations to the recovery strategy it was decided by the Recovery Team that important habitats for these populations should be identified and included in the strategy. To undertake this evaluation, a summary of current knowledge about the freshwater habitat that is important to the survival or recovery of each population is required.
The purpose of this section is to review existing information relevant to the determination of important habitat for white sturgeon in each of the two non-SARA listed populations. This section introduces the concept of important habitat as it relates to the non-SARA listed populations and summarizes existing information about the location, extent, current status, and potential threats to freshwater habitat that is important to survival and recovery of white sturgeon in the non-SARA listed populations in Canada. This section provides sources of information, reliability of the information, and provides a brief discussion of data gaps. The document also includes maps to indicate the geographic areas containing important habitat features. Much of the information on white sturgeon in the section comes from main body of the National White Sturgeon Recovery Strategy.
Though the important habitat identified for the non-SARA listed populations of white sturgeon in B.C. has no legal meaning or bearing under SARA it can be used to guide decisions by various parties including regulatory agencies. The document will provide an initial direction to users on potential impacts to specific habitat types, additional habitat or sturgeon use assessment needs, and potential mitigation activities needed to avoid impacts to habitats, to population recovery potential or to individual sturgeon. Fisheries and Oceans Canada (DFO) may use the section to inform decisions on habitat management within the area.
Identification of habitats that are required to complete necessary life stages is vital for the management of species in decline, and is one of the most challenging aspects of their management. Despite its complexity, the core issue is the same for all species: to determine the role of habitat in population limitation, and to answer the question, “How much habitat, and of what type, is required to maintain or restore viable populations of the species?”
Important habitat for non-SARA listed individuals can be defined as habitat that is required to complete essential life stages and that may play a key role in the maintenance, survival or recovery of the species. Important habitat designation includes geographic areas as well as habitat features such as water quality, large woody debris associations, spawning habitat qualities etc. It is distinct from ‘critical habitat’, which is legally identified for SARA-listed populations only
To complement this definition, Rosenfeld and Hatfield (2006) suggest several practical working definitions that provide general guidance and screening criteria for evaluating potentially important habitats.
- Habitat that is disproportionately important. The litmus test is whether loss of a particular habitat unit will result in significant population level effects for a population at the abundance level of the recovery target. This emphasizes prioritization of habitat protection based on the population consequences of habitat loss or gain, with the understanding that if a particular habitat unit can be lost without population level effects, it is unlikely to be critical.
- The minimum subset of habitats required for a species or population to persist. This emphasizes that the default objective may not be to protect the entire range of a species, and that for some species different configurations or subsets of habitat of varying quality may ensure species persistence.
- Habitats that are necessary to maintain ecosystem integrity and function. This emphasizes that discrete habitat patches must function “properly,” and processes that influence habitat quality must be maintained (e.g., river flooding, riparian buffer, etc.).
A reasonable approach to determine important habitat for a species in decline is to consider the amount and type of habitat required for the species to persist and recover. At present it is not possible to complete a quantitative relationship between habitat and population size due to a lack of data. The lower and mid-Fraser River populations represent somewhat different conditions than the SARA listed populations. In the mid-Fraser River the population is near its historic level, and relative to the Upper Fraser River the higher population offers greater resilience. The lower Fraser River on the other hand has the highest population in the province and the largest available habitat area, including the marine environment. As such, the default assumption that present habitat use is the best indicator of a minimum set of habitats required into the future may not be as appropriate in these two cases. Despite this, and because of the lack of data to pursue alternate approaches, this same assumption was used to define important habitats for the lower and mid-Fraser River populations. To the extent feasible, habitat delineations were based on habitat associations developed from detailed empirical work. Current data limitations suggest that additional important habitats may be identified with further information.
Some of the important habitats proposed here encompass large areas of the Fraser River. In part this reflects the variety of habitats used by this species, and its extensive movements. However, it is also important to note that the proposed definition of large areas also results from the significant uncertainty in our understanding of habitat use. The large spatial area of the lower and mid-Fraser River, and the comparative complexity of the river channel make precise delineation of habitat use more challenging than for other populations. As such, important habitats proposed here represent a broad delineation of habitats in many cases. In many cases further assessment will be required in future in order to provide more definitive and accurate delineation of habitats, and habitat features that will be required for species recovery. Further information might define only a subset of habitats within these larger areas.
It should be noted that this document was not peer reviewed and important habitat has no legal meaning or bearing under SARA.
Providing information on important habitat was a separate and discrete task during development of the Recovery Strategy. The National Recovery Team for White Sturgeon has representation from a number of basin-specific technical and community working groups. Members of the National Recovery Team were tasked with leading the basin-specific groups through a process to collate and evaluate relevant existing information on critical habitat for the SARA-listed populations, and important habitat for the non-listed populations. Lee Williston (Ministry of Environment) led the process for the middle Fraser River; and Erin Stoddard (Ministry of Environment) led the process for the lower Fraser River. They were supported in this role by other National and basin-specific members. The basin-specific groups were tasked with determining candidate habitats and providing a recommendation for important habitats. They were asked to document sources of information, reliability of the information, and descriptions of data gaps. The results of these basin-specific discussions are provided in the following sections. All background information in this document was assembled during development of basin-specific recovery or conservation plans.
In addition to deciding which habitats are important the basin groups were required to provide help in defining the boundaries of these habitats. There is inevitably some subjectivity associated with this task, but it was based on consideration of the available scientific information. The elevational boundary of important habitats was limited to the annual high water mark. All boundaries were demarcated on maps.
The early juvenile stage is defined as occurring from hatch to 2 years, and may be broken into two stages as follows:
0 - 21 days -- This is the period from hatch to successful initiation of exogenous feeding. It includes phases of hiding in interstitial spaces, drift and swim-up, and the initiation of exogenous feeding. Little is known about this phase in the wild, but it is known to be an important phase for recruitment as larvae are very vulnerable to predation. These habitats would be closely associated with spawning and incubation habitats. Current research in the Nechako River indicates that newly hatched sturgeon undergo a hiding phase which lasts until the onset of feeding, so drift for long distances downstream from these habitats is likely limited (Steve McAdam, B.C. Ministry of Environment, personal communication).
21 days to 2 years -- Habitat needs within this 2 year period may be considerably different than in the life stage immediately preceding it. Information about this age group is limited, however, especially from age 1 year onward they appear to use similar habitats as immature and adult sturgeon. Recent information indicates that this phase may not use lower estuary or marine habitats, as no juveniles less than 2 years old have been captured downstream of km 10.5 (Glova et al. 2008, 2009). However, limited sampling in these habitats cannot definitively exclude such use, especially since these areas are used extensively by late juvenile and mature fish. In general terms Glova et al. (2008, 2009) confirmed that 1+ year old sturgeon appear concentrated in some areas more than others, and that they use similar habitats to adults. However, no 0+ year old (in their first year) sturgeon have been captured, so the assumed similarity in habitat use for fish less than 1 year old requires further evaluation.
The late juvenile and adult stages include fish which are greater than 2 years old, with maturity occurring at approximately 20 years of age. The lifespan of white sturgeon is likely greater than 80 years so this is a much extended period. Further research into the life history of the natural middle and lower Fraser River populations including the marine and estuary use in the lower Fraser River may result in this period being further categorized.
2 years to greater than 80 years -- Although the diets of younger fish may be considerably different from those of large adult fish, habitat use during this stage is likely similar. However, it will depend on the target prey or food, its availability, and the river and/or tidal flow levels and patterns. In general, individuals in this class occupy similar habitats for the purposes of feeding, holding and likely for overwintering. Habitat preference differences likely occur between age classes in this group due to competition, significant differences in energetic requirements and expenditures, and associated caloric requirements. Habitat use during this stage can be separated into five general types as follows:
Overwintering -- Overwintering habitats are typically deeper lower flow areas, which are downstream of salmon spawning tributaries or mainstem areas that would act as carcass depositional areas. Congregations of sturgeon in these areas are known to be significant, but the size, extent or activity of these congregations has not been delineated due to difficulties with studying these parameters in a large, deep, turbid river. These congregations have been shown to be very large (tens of thousands) in similar lower Columbia River populations (Michael J. Parsley, United States Geological Survey, personal communication). Sturgeon typically inhabit these areas throughout the year. However, congregations appear to be larger during the overwintering period, which starts around the decrease of fall spawning salmon near the middle of December, and ends around the start of spring spawning fish species near the middle of March. Sturgeon still actively feed during the overwintering period, even when water temperatures are colder in January and February, though their activity levels appear reduced.
Spring Migration & Feeding -- The spring migration starts in association with the beginning of spring spawning prey fish species activity. During this period, sturgeon likely concentrate on all congregations of spring spawners for food, including trout, catastomids and cyprinids. In the lower Fraser River, sturgeon concentrate strongly on spring spawning smelt and eulachon, with their initial migration off the overwintering areas closely associated with the start of the upstream migration of spawning eulachon.
Staging & Spawning -- It is unclear to what degree pre-spawning adults participate in the spring migration for feeding or whether they are primarily migrating to pre-spawn staging areas. However, in the lower Fraser River there is anecdotal and traditional aboriginal knowledge that supports large fish migrating downstream to feed on eulachon near the Fraser River mouth and then migrating upstream out of the area prior to or soon after the start of the spring freshet. Staging occurs in deep lower flow areas adjacent to spawning habitats. Sturgeon move onto spawning areas on the falling hydrograph after the peak snow melt freshet. The timing of spawning is likely somewhat variable, has not been recorded in detail, and is temperature and flow dependent. However, it appears to occur between the middle of June and the beginning of August.
Early Summer Feeding -- This includes the period from the end of spring spawner feeding to the start of upstream salmon migration. Sturgeon use is typically associated with spring spawner habitats or carcass depositional areas during this period. The first spawning salmon species to enter the river are early Chinook which starts in May to June, but there appears to be only limited changes in sturgeon migration associated with the upstream movement of this salmon species. The start of the early sockeye salmon spawning migration at the beginning of July appears to trigger the end of this period when sturgeon start migrating to sockeye spawning rivers or associated carcass depositional areas.
Late Summer & Fall Migration and Feeding -- The start of the early sockeye salmon spawning migration at the beginning of July is associated with the beginning of this period. In the lower Fraser River, this period lasts well into December as numerous salmon species and stocks are migrating into the rivers to spawn. In the middle Fraser River, this period is likely less extended due to the reduced number of salmon species and stocks, or other fall spawning fish for sturgeon to target. During this period, sturgeon movement can be extensive while they feed on different salmon spawning populations, especially in the lower Fraser River. The habitat use at the end of this period is closely associated with the overwintering habitats, which are often depositional areas for the last fall spawning salmon population.
In the middle Fraser River, white sturgeon occur from the confluence of the Nechako River at Prince George, downstream to Hell’s Gate canyon, 210 km from the mouth, and at the confluence and lower reaches of major salmon spawning tributaries. Migration data is limited; however, movement between holding, spawning and feeding areas has been observed and recorded. Current abundance and distribution in the middle Fraser River is likely limited by habitat and prey availability, which is limited due to higher gradient areas, and by the abundance of migrating sockeye salmon as prey. The middle Fraser River also likely has significantly lower overall and seasonal productivity than the lower Fraser River due to significant climate differences and fewer anadromous fish species and stocks. The section upstream of km 674 to the confluence of the Nechako River is believed to have sparse numbers of sturgeon due to even more limited habitat availability, though there are some habitats dispersed throughout this section that are similar to those preferred by sturgeon. Limited research was conducted through this entire section of the Fraser River through the provincial study in the early 1990s. However, only limited studies have been conducted through km 410 to km 674 since then. Some information has been available and is provided here for the section from km 210 to km 410 from the recreational catch and release fishery. This fishery has been established here for some time, and has been recently expanding.
Only two mainstem spawning areas have been identified by radiotracking in the middle Fraser River. One spawning area is at and near the confluence of the Cottonwood River. However, the precise spawning location changes from year to year depending on substrate and flow conditions. The second spawning area is located approximately 10 kilometres downstream of Hawks Creek. It is suspected through anecdotal information that there are spawning sites downstream of the Hawks Creek site between km 210 and km 500. However, very few sturgeon studies have been undertaken through this reach of the middle Fraser River. It is likely that additional spawning and incubation sites will be identified in the future. Recent studies indicate that in natural habitats, white sturgeon eggs do not drift far, so incubation habitats are assumed to be coincident with spawning habitats.
Important Spawning & Incubation Habitat -- The Cottonwood River and Hawks Creek areas are the only identified spawning areas for middle Fraser River white sturgeon. This habitat would be deemed important on an annual basis during June and July, based on known timing of spawning and incubation. This period is sufficient to encompass known annual variability in the onset of spawning. The degree of certainty in the delineation of important spawning habitat for the Cottonwood River area is rated as high, based on repeated radiotracking of fish to this area during the spawning season. The degree of certainty in the delineation of important spawning habitat for Hawks Creek area is also rated as high, based on tracking of mature radio tagged sturgeon.
These areas are depicted in Figures B-1 and B-2 and are described as:
- Cottonwood River (km 669-674); and,
- Hawks Creek (km 539-542).
The area km 325-332 may also be a potential important spawning and incubation site through repeated use of the area by large sturgeon during the spawning period as identified by recreational angler captures in the area. The potential spawning area is depicted in Figure B-6 and described as:
- Near the Seton River confluence (km 325-332).
Data Gaps -- There are minor data gaps for determining the geographic boundaries of this important habitat. However, there are significant gaps in determining similar habitats, particularly downstream of the Williams Creek confluence. There are also significant uncertainties with respect to the qualities of habitat that are required to maintain this habitat unit function for incubation and early life stages.
0 - 21 days -- See Figures B-1, B-2 and B-6. Important habitat for this life stage is assumed to include areas within and immediately adjacent to spawning and incubation areas and a short distance downstream as follows:
- Cottonwood River (km 669-674);
- Hawks Creek (km 539-542); and,
- Near the Seton River confluence (km 325-332).
21 days to 2 years -- Habitat needs within this 2 year period may be considerably different than in the life stage immediately preceding it. It is likely that this stage uses habitat types similar to those preferred by immature and adult sturgeon.
Important Early Juvenile Habitats -- The important habitats for the 0 - 21 days stage are the Cottonwood River confluence and Hawks Creek areas, the same habitat areas noted as important for spawning and incubation. This habitat would be deemed important on an annual basis during June, July and August, based on suspected timing of spawning and incubation. This period is sufficient to encompass known annual variability in onset of spawning, hatch and initial rearing. Important habitat would extend downstream beyond the boundaries of spawning and incubation habitat, but the downstream limit cannot be described at this time. The degree of certainty in these important habitat determinations is rated as high, based on repeated observations of spawning at the Cottonwood River confluence site and presence of mature radio tagged sturgeon at the Hawks Creek site throughout the time of known spawning, although there is insufficient certainty to define the downstream limit of this habitat beyond that already defined for spawning and incubation. The km 330-332 site has been offered as a potential spawning, incubation and early rearing site through repeated use of the area by large sturgeon during the spawning period as identified by recreational angler captures in the area. The degree of certainty for this site is moderate.
Based on the assumed similarity in habitat use by fish aged 21 days to 2 years, the following areas are identified as important:
- Cottonwood River (km 669-674);
- Quesnel River (km 643-645);
- Hawks Creek (km 539-552);
- Chilcotin River (km 475-482);
- Word Creek (km 468-470);
- Grinder and Lone Cabin Creek (km 428-432);
- French Bar Creek (km 408-411);
- km 405-407;
- km 395-401;
- km 368;
- km 350;
- km 320-344;
- km 317-320;
- km 310-315;
- km 282;
- km 273-274; and
- km 250-255.
These areas are reflected in Figures B-1 through B-9. This habitat would be deemed important throughout the year, based on the assumption of continuous occupation. The degree of certainty in this important habitat determination is rated as low to moderate, given that they are based on assumed habitat use patterns.
Data Gaps -- There are moderate data gaps for determining the geographic boundaries of this important habitat; river kilometres discussed here are approximate. These areas defined as important habitat are fairly broad, and are based on existing information of white sturgeon habitat use. Additional studies may increase the confidence in these boundaries and may permit greater precision in defining the geographic areas of interest.
The late juvenile and adult stage is defined as occurring from 2 years onward. Though feeding requirements and preferences are expected to be quite different, it is expected that in general, all individuals in this class occupy the same habitats for the purposes of feeding and overwintering.
Important Late Juvenile and Adult Overwintering Habitat -- Overwintering habitat of white sturgeon is characterized as habitat where fish can maintain their position with minimal energy use. These areas are typically also carcass depositional areas for salmon. The deepest holes in the river are known to be used for overwintering. The specific locations noted as important overwintering areas are indicated in Figures B-1 through B-5 and include:
- Quesnel River (km 643-645);
- Hawks Creek (km 539-552);
- Chimney Creek (km 520-525);
- Chilcotin River (km 475-482);
- Word Creek (km 468-470);
- Grinder and Lone Cabin Creek (km 428-432); and,
- French Bar Creek (km 408-411).
No overwintering areas have been identified between Hells Gate (km 210) and French Bar Creek (km 408-411) due to current data limitations.
These habitats would be deemed important on an annual basis from November to May, based on known overwintering periods. The degree of certainty in this important habitat determination is rated as moderate to high, based on radio telemetry and repeated observations of high use areas for overwintering. Other areas within km 411-470 may also be important habitats for feeding and staging, but additional work is required to support these recommendations.
Important Adult Staging Habitat -- Middle Fraser River white sturgeon are known to “stage” in specific areas in April, May, June & July prior to spawning. Recent work observed mature fish staging in several areas from Cottonwood River (km 669-674) (Mike Ramsay, B.C. Ministry of Environment, personal communication). There are deep holes in this river section used for staging and fish were observed to move frequently throughout adjacent areas. Tracking of radio tagged sturgeon has also identified sites from Hawks Creek (km 539-552) where mature fish were staging. The confirmed important staging areas are indicated in Figures B-1 and B-2, and include:
- Cottonwood River (km 669-674); and,
- Hawks Creek (km 539-552).
In many cases important habitat features for staging are redundant to adult holding and overwintering important habitats identified, as staging fish commonly occupy adult habitats that are used for other purposes prior to migrating to spawning areas. Additional important staging areas could include the following as indicated in Figures B-1, B-3 and B-6:
- Quesnel River (km 643-645);
- Chilcotin River (km 475-482); and,
- Near the Seton River confluence (km 325-332).
Important Late Juvenile and Adult Feeding Habitat -- There doesn’t appear to be a distinct spring migration and feeding stage as in the lower Fraser River. However, it is known that sturgeon in this area do feed on cyprinids, so it is likely that migrations do occur to cyprinid spawning areas to feed.Feeding habitats are generally similar to overwintering habitat. Numerous high use areas have been identified for this life stage in the middle Fraser River. Based on known habitat use, several areas are proposed as important habitats for feeding and include (see also Figures B-1 through B-9):
- Cottonwood River (km 669-674);
- Quesnel River (km 643-645);
- Hawks Creek (km 539-552);
- Chimney Creek (km 520-525);
- Riske Creek (km 485-495);
- Chilcotin River (km 475-482);
- Word Creek (km 468-470);
- Grinder and Lone Cabin Creek (km 428-432);
- French Bar Creek (km 408-411);
- km 405-407;
- km 395-401;
- km 368;
- km 350;
- km 320-344;
- km 317-320;
- km 310-315;
- km 282;
- km 273-274; and
- km 250-255.
These habitats would be deemed important year-round. The degree of certainty in this important habitat determination is rated as moderate to high, based on radio telemetry and repeated observations of white sturgeon at these sites.
Data Gaps -- There are moderate data gaps for determining the geographic boundaries of this important habitat component; river kilometres discussed here are approximate. These areas defined as important habitat are fairly broad, and are based on existing information of white sturgeon habitat use. Additional studies may increase the confidence in these boundaries and may permit greater precision in defining the geographic areas of interest.
In the lower Fraser River, white sturgeon occur from the Hell’s Gate canyon at km 210, downstream to the estuary and marine confluence near Vancouver, within major salmon spawning tributaries, Pitt and Harrison Lakes, and in the upper Pitt and Lillooet rivers, as well as in the Georgia Strait and other near and offshore marine waters along the Pacific Coast. Individuals from the middle Fraser River population have also been recorded migrating downstream into the lower Fraser River. In-river migration is moderately well known, and movements between holding, spawning and feeding areas can be extensive (greater than 100 km). The extent of migration to estuary and marine waters is poorly understood, but is currently being studied. However, individual sturgeon that were tagged in the lower Columbia River were captured nearly 100 km upstream in the lower Fraser River indicating that extensive estuarine and marine use and migration is probable. This section of the river has several different reaches, including the restricted Fraser Canyon from km 210 to 160, the gravel reach from km 160 to approximately km 90, the lower gradient tidal influenced km 83 to approximately km 10, and the estuary from approximately km 10 to 5 out from the mouth. In addition, lower Fraser River sturgeon have access to the marine waters of Georgia Strait and the Pacific, to the large tidal Pitt Lake, to the very large Harrison Lake and to numerous larger salmon producing tributaries.
Current abundance and distribution in the lower Fraser River is likely limited by habitat availability, which has been historically affected by significant floodplain and estuary development and disturbance. It is also likely limited by the availability of prey/food, and as such the abundance of migrating eulachon, salmon and other fishes, by impacts from First Nations and commercial salmon gillnet and beach seine fisheries, and by impacts from the recreational fisheries. The lower Fraser River population is also subjected to significant industrial developments, discharges and in-river activities, especially in the section downstream of km 94. There are also numerous municipal and agricultural point and non-point source discharges in this section. This area of the province houses more than approximately 60% of the human population of British Columbia and has numerous port facilities within and immediately adjacent to the lower river.
The lower Fraser River white sturgeon population was also assessed during the provincial study in the early 1990s. Since 2000, an extensive ongoing volunteer based mark-recapture program has been conducted throughout this section of the river through the Fraser River Sturgeon Conservation Society (FRSCS). Numerous other sturgeon studies have been recently conducted by the FRSCS and others on the lower river sturgeon population including recent juvenile sturgeon habitat use studies (Glova et al. 2008, 2009).
Spawning areas have been confirmed for mainstem and side channel locations within the gravel reach (river km 94-160) and within the Fraser Canyon from Hope upstream of Yale (km 160- 187). However, the precise spawning locations may change from year to year depending on channel and substrate configurations, and flow conditions. It is likely that the Harrison and Pitt Lake and River systems also have spawning and incubation areas that have yet to be identified. Additional Fraser River mainstem spawning and incubation sites may also be identified in the future. Recent research data indicates that white sturgeon eggs do not drift far, so incubation and early juvenile habitats are assumed to coincide with spawning habitats.
Important Spawning and Incubation Habitats -- The higher gradient side channels and large tributary river fans from the confluence of the Sumas/Vedder River upstream to the Coquihalla River and within the Fraser Canyon upstream to Saddle Rock are confirmed spawning and incubation areas for lower Fraser River white sturgeon. This habitat would be deemed “important” on an annual basis during June, July and August, based on known timing of spawning and incubation. This period is sufficient to encompass known annual variability in the onset of spawning. The degree of certainty in this important habitat determination is rated as moderate; despite observations of spawning at these sites long term site specific studies are lacking. The delineation of broad areas for important spawning habitats for the lower Fraser River population is due to the combination of a lack of long term studies, the potential for habitat conditions to change from year to year, and the likelihood that various areas may be required for recovery. Additionally, these areas may not be adequate for recovery of any sub-populations that may be delineated in the future. The mainstem Fraser areas are indicated in Figures B-10 and B-11 and described as:
- Fraser River canyon (km 160-187); and,
- Fraser River side channels and main channel (km 94-160).
Data Gaps -- There are data gaps for determining the detailed specific geographic boundaries of these important habitats due to ongoing changes in channel and substrate configurations, variable flows and the high cost of conducting research on a large turbid river. As such, it is difficult to determine and maintain precise habitat boundaries for multiple years over such a large area, or to confirm similar habitats. There are also significant uncertainties with respect to the quality of habitat that is required to maintain this functionality for spawning, incubation and early life stages for the recovery of this population.
0 - 21 days -- Important habitat for this life stage is assumed to include areas within and immediately adjacent to spawning and incubation areas and a short distance downstream as indicated in Figures B-10 and B-11 and described by the following:
- Fraser River canyon (km 160-187); and,
- Fraser River side channels and main channel (km 94-160).
21 days to 2 years -- Habitat needs within this 2 year period may be considerably different than in the life stage immediately preceding it. It is likely that from age 1 onward, this stage uses habitat types similar to those preferred by immature and adult sturgeon, however habitat use prior to age 1 is uncertain. Recent initial studies on habitat use indicate that fish from 1-2 years old use similar habitat to older juveniles and adults. While concentrations in specific habitats may occur during particular times of the year no distinct concentrations have been identified.
Important Early Juvenile Habitats -- The important habitat for the 0 - 21 days stage is the same habitat area noted as important for spawning and incubation. This habitat would be deemed important on an annual basis during June, July and August, based on suspected timing of spawning and incubation. This period is sufficient to encompass expected annual variability in onset of spawning. Important habitat would extend downstream beyond the boundaries of spawning and incubation habitat, but the downstream limit cannot be described at this time. The degree of certainty in this important habitat determination is rated as high, based on repeated observations of spawning at this site, although there is insufficient certainty to define the downstream limit of this habitat beyond that already defined for spawning and incubation.
The important habitat for the 0 to 21 days stage is identified in Figures B-10 and B-11 and includes the following areas, which are also identified as spawning areas:
- Fraser River Canyon (km 160-187); and,
- Fraser River side channels and main channel (km 94-160).
The important habitat for the 21 days to 2 years stage includes the following areas, which are also identified as high use areas for multiple life stages:
- Peg Leg Bar (km 112-115);
- Grassy Bar and Island 22 (km 103-109);
- Vedder River Confluence (km 88-99);
- Hatzic Bend (km 80-89);
- Matsqui Island Channels (km 74-77);
- Annacis Island (km 19-25); and,
- Canoe Pass/Deas Island (km 9-12).
- km 200-205;
- km 198-199; and,
- km 185-187.
The following more general areas are also likely used as they are high use for late juvenile and adults during the salmon spawning period:
- Fraser Canyon (km 160-185); and,
- Fraser mainstem & wetted side channels (km 89-160).
These areas are depicted in Figures B-11 through B-13. This habitat would be deemed important year-round, based on knowledge of continuous occupation of these sites. The degree of certainty in this important habitat determination is rated as high, based on repeated observations and captures at these sites.
Data Gaps -- There are moderate data gaps for determining the geographic boundaries of this important habitat; river kilometres discussed here are approximate. These areas defined as important habitat are fairly broad, and are based on existing information of white sturgeon habitat use. Additional studies may increase the confidence in these boundaries and may permit greater precision in defining the more specific areas of interest within the larger areas currently identified.
The late juvenile and adult stage is defined as occurring from 2 years onward. Diets of younger fish may be considerably different than those of large adult fish, but in general, all individuals in this class occupy the same habitats for the purposes of feeding, and presumably for overwintering.
Important Late Juvenile and Adult OverwinteringHabitats -- In the lower Fraser River, overwintering habitats are typically associated with fall feeding habitats. They are often carcass deposition areas downstream of salmon spawning areas and are slower deep areas that require minimal energy expenditure to inhabit. The deepest holes in the river are known to be used for overwintering. Confirmed locations noted as important overwintering areas are indicated in Figure B-12 and include:
- Peg Leg Bar (km 112-115);
- Grassy Bar and Island 22 (km 103-109);
- Hatzic Bend (km 80-89); and
- Matsqui Island Channels (km 74-77).
These areas support very high densities of sturgeon from the middle of December through to the middle of March, but are typically used extensively by sturgeon year-round. The degree of certainty in this important habitat determination is rated as moderate, based on repeated observations of high use areas for overwintering. Other important overwintering areas likely exist throughout the lower Fraser River and its larger tributaries, but have not been confirmed and additional work would be required to support any recommendations as important habitat.
Important Spring Migration & Feeding Habitats -- In the lower Fraser River, the onset of sturgeon spring migrations to feeding habitats is strongly associated with spring spawning anadromous or resident fish or their migrations, and with their spawning and downstream carcass deposition areas. Important fish prey species during this period include eulachon and smelt, and likely trout, cyprinids and catastomids. It appears that late juvenile and non-spawning adult sturgeon stay associated with these habitats through the freshet to the onset of upstream salmon migration in July and August. Important spring spawning eulachon feeding areas are indicated in Figure B-13 and are described as follows:
- Pitt River Confluence (km P1-P4);
- Barnston Island (km 41-48);
- Douglas Island (km 37-40); and,
- Annacis Island (km 20-24).
These areas would be considered important for spring and summer feeding during the period from approximately April 1st – August 1st. Additional upstream eulachon spawning and sturgeon feeding areas have also been identified, but less frequently, and traditional ecological knowledge has confirmed that eulachon regularly migrated over 100 km upstream. Additional spring feeding areas likely exist throughout the lower Fraser River, but have not yet been identified or confirmed. These would include any spring spawning habitats including the confluences of large and smaller tributaries.
Important Adult Staging Habitats -- There are numerous deep holes in this river section used for staging and fish are known to move frequently throughout the entire section. Fish were observed to move from these locations to the spawning site at or prior to the spawning period (RL & L 2000a). Important habitat features for staging are likely somewhat redundant with confirmed important adult feeding and overwintering habitats, as staging fish commonly occupy these areas just prior to spawning. However, there also appears to be participation of large mature fish in the spring migration and feeding.Based on known habitat use, several areas have been confirmed as important habitats for adult staging. These areas are indicated in Figure B-12 and include:
- Peg Leg Bar (km 112-115); and,
- Grassy Bar and Island 22 (km 103-109).
Areas within the section km 115 – 135 are considered important habitats for staging; however, additional work is required to confirm this area. Other potential Fraser River side channels and main channel sites likely exist in deep holes in the following areas as indicated in Figures B-10 and B-11:
- Fraser River canyon (km 160-187); and,
- Fraser mainstem and wetted side channels (km 94-160).
These habitats would be deemed important during pre-spawn staging. This would likely include the period from approximately May 15th to September 1st. The degree of certainty in this important habitat determination is rated as high for confirmed sites and moderate for potential sites, based on repeated captures of white sturgeon at these sites and past studies.
Important Fall/Summer Migration & Late Summer & Fall Feeding Habitats -- In the lower Fraser River, the onset of sturgeon migrations to late summer and fall feeding habitats is strongly associated with spawning anadromous salmon migrations, and with their spawning and downstream carcass deposition areas. Some of the lower Fraser River salmon spawning and carcass depositional areas that are important for sturgeon feeding are indicated in Figures B-10, B-11 and B-12 and described as follows:
- Fraser Canyon (km 160- 185);
- Fraser mainstem and wetted side channels (km 89-160);
- Peg Leg Bar (km 112-115);
- Grassy Bar and Island 22 (km 103-109);
- Vedder River Confluence (km 88-99);
- Hatzic Bend (km 80-89);
- Lower Stave River and Stave River Confluence (km 62-70); and,
- Lower Harrison River (km H1-H19)
- km 200-205;
- km 198-199; and,
- km 185-187.
Numerous other sites exist throughout this section of the river depending on the timing and extent of the spawning salmon species. For example, feeding becomes more widespread and voracious annually during chum salmon migration and spawning, and biannually on odd years during pink salmon migration and spawning. Sturgeon feed across channels at all depths during these periods. Other heavily used feeding sites include the confluences and lower reaches of all large and small salmon spawning tributaries. The timing of this activity varies with the timing and extent of the salmon migrations. It typically occurs from approximately July 15th to December 15th. However, salmon runs in the lower Fraser River continue through the winter into the early spring.
Data Gaps -- There are moderate data gaps for determining the geographic boundaries of this important habitat component; river kilometres discussed here are approximate. These areas defined as important habitat are fairly broad, and are based on existing information of white sturgeon habitat use. Further studies should increase the confidence in these boundaries and greater precision in defining more specific geographic locations.
Activities that may impact important habitat for mid and lower Fraser River white sturgeon include river regulation, gravel or sand dredging, bank protection, dyke construction and bank armouring, linear developments, riparian, foreshore, floodplain alterations or developments, upstream land and water uses, and point and non-point source pollution discharges. The exact concerns would vary depending on details of the activities, location and applied mitigation measures. General threats to white sturgeon and their habitats are discussed in the Recovery Strategy.
The following graphics are at a smaller scale for practical presentation of strategic information. Users or regulatory agencies should consult regional B.C. Ministry of Environment sturgeon biologists and/or the lower and middle Fraser River white sturgeon working groups to confirm accurate locations of important areas.
Figure B-1. Important habitat for middle Fraser River white sturgeon. A section of the middle Fraser River is depicted flowing in a southeastward direction. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. An upstream section of river is circled in red in the vicinity of the Cottonwood confluence (roughly 674.5 to 670.5); this is one of only two designated spawning habitats for this population and is identified as important habitat for spawning, incubation and early juvenile (0 – 21 days) life stages. A meander is present south of the upper red polygon. Downstream, another stretch of river (roughly 642.5 to 646) is outlined in red in the vicinity of the Quesnel River confluence, important for all other life history stages from early juvenile to overwintering. The map is oriented in a “north is up” direction.

Figure B-2. Important habitat for middle Fraser River white sturgeon. A section of the middle Fraser River is depicted flowing in a southward direction through Williams Lake. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. The lower portion of an upstream red outlined polygon is located between Hawks Creek and Williams Creek. This is one of only two designated spawning habitats for this population and is identified as important habitat for spawning, incubation and early juvenile (0-21 days) life stages. The remainder of this polygon and a downstream red outlined polygon are important for all other life history stages from early juvenile to overwintering. The upstream polygon runs from roughly 538 to 552 and the downstream polygon runs roughly from 519 to 525. The map is oriented in a “north is up” direction.

Figure B-3. Important habitat for middle Fraser River white sturgeon. A section of the middle Fraser River is depicted flowing in a southward direction roughly between Dog Creek Dome and Junction Sheep Range Provincial Park. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. A meander is present at the northern end of this river section. Three red outlined polygons indicate important habitats for life history stages, including early juvenile and overwintering. No spawning habitat has been confirmed in this section of the river. The upstream polygon runs roughly from 484 to 495, the middle polygon from 473 to 482, and the downstream polygon from 467 to 470. The map is oriented in a “north is up” direction.

Figure B-4. Important habitat for middle Fraser River white sturgeon. A section of the middle Fraser River is depicted flowing in a southeastward direction through the Churn Creek Protected Area. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. Four red outlined polygons indicate important habitats for all life history stages, including early juvenile and overwintering. No spawning habitat has been confirmed in this section of the river. The map is oriented in a “north is up” direction.
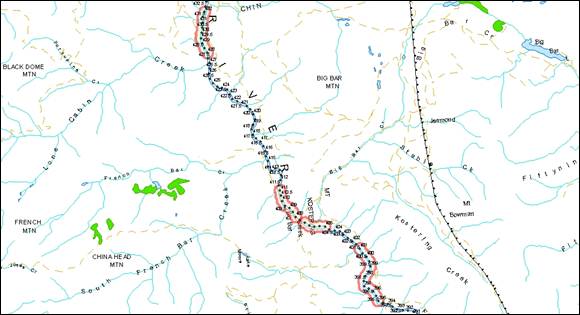
Figure B-5. Important habitat for middle Fraser River white sturgeon. A section of the middle Fraser River is depicted flowing in a southward direction roughly between Lillooet and Edge Hills Provincial Park. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. A meander is present at the southern end of this river section. Two red outlined polygons indicate important habitats for all life history stages including early juvenile and overwintering. No spawning habitat has been confirmed in this section of the river. The upstream polygon runs roughly from 357 to 358.5 and the downstream polygon from 349.5 to 350.5. The map is oriented in a “north is up” direction.

Figure B-6. Important habitat for middle Fraser River white sturgeon. A section of the middle Fraser River is depicted flowing in a southeastward direction through Lillooet. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. A meander is present at the northern end of this river section. Two red outlined polygons indicate important habitats for all life history stages including early juvenile and overwintering. Spawning habitat is suspected, but has not been confirmed in the lower section of the upper polygon. The upstream polygon runs roughly from 367 to 368.5 and the downstream polygon from 349.5 to 350.5. The map is oriented in a “north is up” direction.
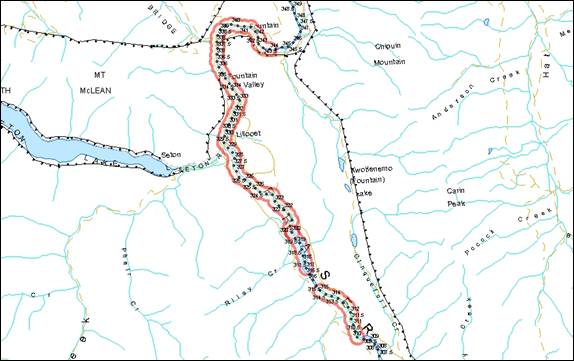
Figure B-7. Important habitat for middle Fraser River white sturgeon. A section of the middle Fraser River is depicted flowing in a southward direction through Lytton. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. Three red outlined polygons indicate important habitats for all life history stages including early juvenile and overwintering. No spawning habitat has been confirmed in this section of the river. The map is oriented in a “north is up” direction.
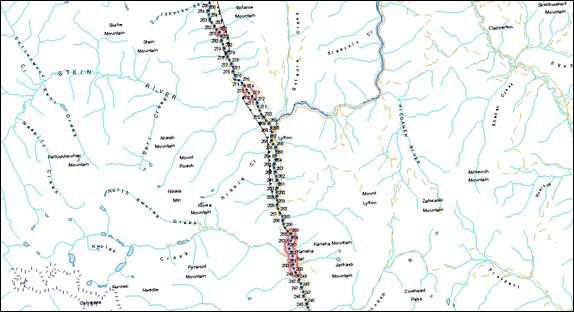
Figure B-8. Important habitat for middle Fraser River white sturgeon. A section of the middle Fraser River is depicted flowing in a southward direction roughly through Spuzzum and Yale. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. A meander is present in the middle (near Saddle Rock) and southern end (near Yale) of this river section. Three red outlined polygons indicate important habitats for all life history stages including early juvenile and overwintering. No spawning habitat has been confirmed in this section of the river. The upstream polygon runs roughly from 199 to 205, the middle polygon from 197 to 199 and the downstream polygon from 184 to 187. The map is oriented in a “north is up” direction.

Figure B-9. Important habitats for lower Fraser River white sturgeon. A section of the lower Fraser River is depicted flowing in a southward direction through Yale. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. A meander is present in the northern and southern ends of this river section. A red outlined polygon indicates areas known of sturgeon spawning habitat, running roughly from 159 - 187. These habitats are also used extensively for summer to late fall feeding habitats. The purple shaded areas are additional confirmed important juvenile and adult sturgeon holding and feeding areas. The map is oriented in a “north is up” direction.

Figure B-10. Important habitats for lower Fraser River white sturgeon. A section of the lower Fraser River is depicted flowing in a southwestward direction through Hope, Harrison Hot Springs, Agassiz, and Chilliwack. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. A red outlined polygon indicates areas known to contain sturgeon spawning habitats, with the exception of the Harrison River. The polygon runs from roughly 93 to 156 and H1 to H18. These habitats are also used extensively for summer to late fall feeding habitats. The purple shaded areas are additional confirmed important juvenile and adult sturgeon holding and feeding areas. The map is oriented in a “north is up” direction.

Figure B-11. Important habitats for lower Fraser River white sturgeon. A section of the lower Fraser River is depicted flowing in a westward direction through Agassiz, Chilliwack, Abbotsford and Mission. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. Five red outlined polygons indicate areas known to be used extensively for overwintering habitats, and for spring and summer to late fall feeding habitats. Moving upstream to downstream, the first polygon runs roughly from 116-111, the second from 102 – 109.5, the third from 89.5 – 99, the fourth from 79 – 89.5, and the fifth from 73.5 – 77. The purple shaded areas are additional confirmed important juvenile and adult sturgeon holding and feeding areas. The map is oriented in a “north is up” direction.

Figure B-12. Important habitats for lower Fraser River white sturgeon. A section of the lower Fraser River is depicted flowing in a westward direction through Maple Ridge, Pitt Meadows, Port-Coquitlam, Coquitlam, New Westminster, Surrey, Richmond, and Delta. Points along the river labeled with numbers represent sequentially numbered kilometers of river, increasing in an upstream direction. Four red outlined polygons indicate areas known to be used extensively for spring and summer to late fall feeding habitats. Moving from upstream to downstream, the first polygon runs roughly from 36 to 48, the second polygon from P1 to P4.5, the third polygon from 17.5 to 25.5, and the fourth polygon from 7.5 to 11.5. The purple shaded areas are additional confirmed important juvenile and adult sturgeon holding and feeding areas. The map is oriented in a “north is up” direction.
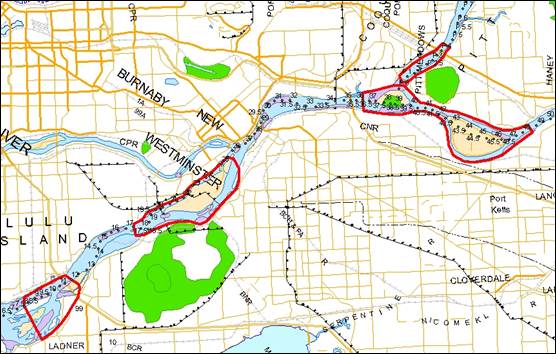
Glova, G., T. Nelson, K. English, and T. Mochizuki. 2009. A further report on juvenile white sturgeon habitat use in the lower Fraser River, 2008-2009. Prepared by LGL Limited for the Fraser River Sturgeon Conservation Society, Vancouver, B.C.
Glova, G., T. Nelson, K. English, and T. Mochizuki. 2008. A preliminary report on juvenile white sturgeon habitat use in the lower Fraser River, 2007-2008. Prepared by LGL Limited for the Fraser River Sturgeon Conservation Society, Vancouver, B.C.
16 Note: information in the Recovery of Lower and Mid Fraser Populations appendix is current to 2009.
Table D-1. Glossary (with two columns: “Term” and “Definition”) for the following 68 terms (read from top to bottom): Abiotic; Accumulated Temperature Unit (ATU); Action Plan; ALR Arrow Lakes Reservoir; Aquatic Species; B.C. MOE; Beneficial Use; Biological Function, Feature and Attribute; Biotic; Biotic Diversity; Bycatch; Competent Minister; Conservation Fish Culture; COSEWIC; Critical Habitat; Dam-Affected System; DFO; Ecological; Ecosystem; Effective Population Size (Ne); Endangered species; Environmental stochasticity; Exemption; Existing Facilities; Extinct species; Extirpated species; FDR; Fecundity; Federal Land; Fish; Food, Social, Ceremonial (FSC); Habitat; Habitat for Aquatic Species; Habitat Variable or Feature; HLK Dam; Hydraulic Condition; Hydrograph; Important habitat Non-SARA Listed Populations; Individual; Invasive / Non-Native Species; Life Stage (Spawning, Incubation, Yolk sac, larvae, Larvae, Early Juvenile, Late Juvenile and Adult); List; Migration; National Recovery Team for white sturgeon; Overwintering; Physical Habitat; Population Viability Analyses; Precautionary Approach; Prey; Prohibitions; Recovery; Recovery Strategy; Recruitment; Recruitment Failure; Regulated River; Regulation; Residence; REV Dam; SARA; SARA Public Registry; Self-Sustaining; Spawning; Substrates; Species at Risk; Status Report; Survival; Threats; Threatened species; Turbidity.
| Term | Definition |
|---|---|
| Abiotic | Not associated with or derived from living organisms. Non-living chemical and physical factors in the environment. Abiotic factors in an environment include such items as sunlight, temperature, wind patterns, and precipitation. |
| Accumulated Temperature Unit (ATU) | A unit of measurement used to describe the cumulative effect of temperature over time. |
| Action Plan | Means an action plan included in the Species at Risk Act (SARA) public registry and includes any amendment to it included in the public registry. A document that establishes the activities that may be undertaken to meet the objectives outlined in the recovery strategy for a wildlife species. |
| ALR | Arrow Lakes Reservoir. |
| Aquatic Species | A wildlife species that is a fish as defined in section 2 of the Fisheries Act, or a marine plant as defined in section 47 of the Act. |
| Beneficial Use | Beneficial use in the context of this recovery strategy means use of white sturgeon, if and when feasible, in Aboriginal Food, Social, and Ceremonial fisheries, and recreational fisheries, including those with and without retention (harvest). |
| B.C. MOE | British Columbia Ministry of Environment. |
| Biological Function, Feature and Attribute | A biological function is a characteristic of critical habitat that corresponds to a biological need or life process requirement of the species such as spawning, rearing, feeding and migration. Every function is the result of a single or multiple features which are physical components or conditions of the critical habitat such as riffles, pools, riparian habitat etc. Features describe how the habitat provides the critical function to meet the species needs and are always associated with a function. Attributes provide information about a features that tell us how the feature supports the function necessary for the species life process, for example the attributes of a riffle feature that supports the function of rearing may include substrate size, velocity, water chemistry, prey species and temperature. |
| Biotic | Associated with or derived from living organisms. The biotic factors in an environment include the organisms themselves as well as such items as predation, competition for food resources, and symbiotic relationships. |
| Biotic Diversity | 1) the number of different native speciesand individuals in a habitat or geographical area; 2) the variety of different habitatswithin an area; 3) the variety of interactions that occurbetween different species in a habitat; and, 4) the range of genetic variationamong individuals within a species. |
| Bycatch | The incidental catch of a species of fish in a fishery when intending to catch another species of fish. |
| Competent Minister | a) the Minister of Canadian Heritage with respect to individuals in or on federal lands that are administered by that Minister and that are national parks, national historic sites or other protected heritage areas as those expressions are defined in subsection 2(1) of the Parks Canada Agency Act; b) the Minister of Fisheries and Oceans with respect to aquatic species, other than individuals mentioned in paragraph (a); and c) the Minister of the Environment with respect to all other individuals. |
| Conservation Fish Culture | The artificial production of fish in a hatchery to support recovery in the wild. |
| COSEWIC | Committee on the Status of Endangered Wildlife in Canada (www.cosewic.gc.ca); a committee of experts that assesses and designates which wild species are in some danger of disappearing from Canada. |
| Critical Habitat | The habitat necessary for the survival or recovery of a listed wildlife species and that is identified as the species’ critical habitat in the recovery strategy or in an action plan for the species. |
| Dam-Affected System | A generic way of describing the Columbia, Kootenay, and Nechako River systems that are regulated by dams. Other anthropogenic factors are also present in these watersheds in particular the Columbia and Kootenay rivers. This term is used to describe the state of the river basin. River regulation is not necessarily the sole cause of recruitment failure but it occurs in systems where significant flow regulation activities occur. |
| DFO | Fisheries and Oceans Canada. |
| Ecological | Of, or having to do with, the environments of living things or with the pattern of relations between living things and their environments; of or relating to the interdependence of organisms. |
| Ecosystem | An ecological community considered together with the nonliving factors of its environment considered as a unit |
| Effective Population Size (Ne) | A term commonly used in the study of population genetics; the effective population size is the size of an ideal population which acts the same as the real population in question, where ideal is defined as: (1) no selection; (2) random mating; and, (3) random chance of each offspring having a particular parent. |
| Endangered species | Facing imminent extirpation or extinction. |
| Environmental stochasticity | Environmental stochasticity refers to variability that result from environmental conditions such as weather. For example, environmental stochasticity leads to underlying variation in populations due to environmentally induced variation in birth and mortality rates. . |
| Exemption | SARA section 83(4) Subsections 32(1) and (2), section 33 and subsections 36(1), 58(1), 60(1) and 61(1) do not apply to a person who is engaging in activities that are permitted by a recovery strategy, an action plan or a management plan and who is also authorized under an Act of Parliament to engage in that activity, including a regulation made under section 53, 59 or 71. |
| Existing Facilities | A structure or ongoing operation that may be impacting species at risk or their habitats that was in place prior to SARA coming into effect or prior to a species being listed. |
| Extinct species | A wildlife species that no longer exists. |
| Extirpated species | Under SARA this is defined as a wildlife species that no longer exists in the wild in Canada, but exists elsewhere in the wild. This differs from the biological definition which refers to the loss of distinct subcomponent of a species, irrespective of political boundaries. |
| FDR | Lake Roosevelt, impounded by Grand Coulee Dam. |
| Fecundity | The potential reproductive capacity of an organism or population, measured by the number of gametes (eggs). |
| Federal Land | Includes any land owned by the federal government, the internal waters and territorial sea of Canada, and reserves and other land set apart for the use and benefit of a band under the Indian Act. |
| Fish | From the Fisheries Act: (a) parts of fish, (b) shellfish, crustaceans, marine animals and any parts of shellfish, crustaceans or marine animals, and (c) the eggs, sperm, spawn larvae, spat and juvenile stages of fish, shellfish, crustaceans and marine animals. |
| Food, Social, Ceremonial (FSC) | An Aboriginal group has a right to fish for food, social and ceremonial purposes; it takes priority, after conservation, over other uses of the resource. |
| Habitat | The type of environment in which an organism or group of organisms normally lives or occurs; a habitat is composed of and influenced by both abiotic and biotic factors. |
| Habitat for Aquatic Species | Spawning grounds and nursery, rearing, food supply, migration and any other areas on which aquatic species depend directly or indirectly in order to carry out their life processes, or areas where aquatic species formerly occurred and have the potential to be reintroduced |
| Habitat Variable or Feature | A specific biotic or abiotic measure of the habitat in question. |
| HLK Dam | Hugh L. Keenleyside Dam. |
| Hydraulic Condition | The interaction of flow and substrate to create a specific habitat feature. |
| Hydrograph | A record of the stage and/or discharge of a river as a function of time. |
| Important habitat; Non-SARA Listed Populations | Habitat that is required to complete essential life stages and may play a key role in survival or recovery of the species. |
| Individual | Includes an individual of a wildlife species, whether living or dead, at any developmental stage and includes larvae, embryos, eggs, sperm, seeds, pollen, spores and asexual propagules. |
| Invasive / Non-Native Species | Species that do not naturally occur in a specific area (e.g. plants or animals) that adversely affect the habitats they invade economically, environmentally or ecologically. |
| Life Stage (Spawning, Incubation, Yolk sac larvae, Larvae, Early Juvenile, Late Juvenile and Adult) | Physical maturation. See recovery strategy text for definitions of specific white sturgeon life stages. |
| List | The List of Wildlife Species at Risk set out in Schedule 1 of SARA. |
| Migration | To move from one place to another for feeding, breeding, etc. |
| National Recovery Team for White Sturgeon | A group of experts that come together to facilitate recovery planning for white sturgeon. |
| Overwintering | To pass through or wait out the winter season, or to pass through that period of the year when “winter” conditions (cold or sub-zero temperatures, ice, snow, limited food supplies) make normal activity or even survival difficult. |
| Physical Habitat | A physical measure of habitat; in streams this often includes depth, velocity, substrate and cover. |
| Population Viability Analyses | A species-specific method of risk assessment frequently used in conservation biology. It is traditionally defined as the process that determines the probability that a population will go extinct within a given number of years. |
| Precautionary Approach | Recognizing that the reduction or loss of the species should not be postponed for lack of full scientific certainty. |
| Prey | An animal taken by a predator as food. |
| Prohibitions | A law forbidding an action. |
| Recovery | The process by which the decline of an endangered, threatened or extirpated species is stopped or reversed, and threats reduced to improve the likelihood of the species’ persistence in the wild. |
| Recovery Goal | A recovery goal sets the strategic course for recovery planning by defining what ‘recovery’ means for each species. |
| Recovery Strategy | A document that outlines the long-term goals and short-term objectives for recovering a species at risk, based on the best available scientific information. |
| Recruitment | Recruitment refers to juveniles of a particular age entering the population. When an individual survives one life stage they are said to recruit to the next stage; recruitment therefore also refers to the process by which individuals survive to the next life stage. |
| Recruitment Failure | Recruitment failure, as used here, refers to the absence of juvenile abundance capable of supporting a sustainable population. Detection of low levels of recruitment is observed in all populations but at levels that are not sufficient to sustain the population. Recruitment failure in many of the SARA-listed white sturgeon populations has been severe and ongoing over a period of at least 25 years. |
| Regulated River | A river or creek in which flow is determined primarily by a major dam. |
| Regulation | A regulation is a rule that indicates how a statute is to be enforced. |
| Residence | The specific dwelling place, such as a den, nest or other similar area or a place that is occupied or habitually occupied by one or more individuals during all or part of their life cycles, including breeding, rearing, staging, wintering, feeding, or hibernating. |
| REV Dam | Revelstoke Dam. |
| SARA | Species at Risk Act. |
| SARA Public Registry | The SARA Public Registry web site has been designed to help interested parties better understand Canada's approach to protecting and recovering species at risk, learn about species at risk and what's being done to help them, and get involved in decision making and recovery activities. The Public Registry fulfills the requirement under SARA for the federal Minister of the Environment to establish a public registry for the purpose of facilitating access to SARA-related documents. |
| Self-Sustaining | Maintaining or able to maintain oneself or itself by independent effort. |
| Spawning Substrates | Surfaces on which fish prefer to deposit their eggs. |
| Species at Risk | An extirpated, endangered, threatened species or a species of special concern. |
| Status Report | A report containing a summary of the best available information on the status of a wildlife species, including scientific knowledge, community knowledge and aboriginal traditional knowledge. |
| Survival | The continuation of life or existence. |
| Threats | Plausible mechanisms, caused by human activities, which influence abundance, distribution, and health of white sturgeon. |
| Threatened species | A wildlife species that is likely to become endangered if nothing is done to reverse the factors leading to its extirpation or extinction. |
| Turbidity | The cloudiness of a fluid caused by suspended particles that are generally invisible to the naked eye, similar to smoke in air. |
The white sturgeon is a freshwater fish and is under the responsibility of the federal government and management by the B.C. Ministry of Environment. Four populations of white sturgeon (Nechako River, Upper Fraser River, Kootenay River, and Upper Columbia River) are listed as Endangered under Schedule 1 of SARA. In 2005, DFO and the B.C. Ministry of Environment established a National Recovery Team for White Sturgeon in Canada. Its membership comprises technical experts from DFO, the Province of B.C., First Nations, and the chairs of basin-specific Technical Working Groups, and the Team was tasked with preparing the draft recovery strategy. See the Contributors/Authors section of this document for a list of National Recovery Team Members.
This draft recovery strategy was posted to the DFO Pacific Region Consultation website for a regional consultation from September 16 to October 16, 2009. Online consultations solicited feedback on the draft recovery strategy through discussion guides and feedback forms. In addition to online consultations, community dialogue sessions were held in Castlegar and Prince George in September 2009 where presentations were given on the draft recovery strategy, followed by question and answer periods. Twenty-three people attended the meeting in Castlegar, and six people attended the meeting in Prince George. Notification of the consultations and the community meetings included mail-outs to First Nations, industry stakeholders, ENGOs, government agencies, and other interested parties, notices in local community newspapers, and information on the Pacific Region Consultation website. The draft recovery strategy, and discussion guides and feedback forms were available at the meetings. DFO also received meeting requests and held additional meetings with industry stakeholders, ENGO stakeholders, and First Nations. Feedback was received from industry and ENGO stakeholders, as well as the Province of B.C. No First Nations groups provided written comments, but verbal support of the draft recovery strategy was provided to DFO during the community meetings. All feedback received during this consultation period has been incorporated into the proposed recovery strategy as appropriate.
*IMPORTANT NOTICE AND DISCLAIMER: DFO does not assume any responsibility for the quality of information, products or services listed in the Web sites provided above. Users should also be aware that information from external sources is available only in the language in which it was provided.