Scientific opinion on the impact of somatic cell nuclear transfer (SCNT) cloning of cattle and swine on food and feed safety, animal health and the environment
Draft: For internal consultation
Contributors:
Agriculture and Agri-Food Canada
Canadian Food Inspection Agency
Environment and Climate Change Canada
Health Canada
November 21, 2023
Disclaimer
This document has been drafted to develop a scientific opinion concerning the impact of somatic cell nuclear transfer (SCNT) technologies, primarily in cattle and swine, with respect to food and feed safety, animal health, and its impact on the environment, including indirect human health effects. It is not a full assessment of potential impacts of the technology. This scientific opinion will serve as the basis for updating existing and/or developing new science-based policies related to cattle and swine SCNT clones, their progeny, their products, and by-products in Canada. All existing policies on these animals and their products will continue to apply until such a time as each federal Department or Agency responsible for individual policies has updated them.
Contents
- Summary
- Section 1. Introduction
- Section 2. Review of data, potential impact of SCNT cloning in Canada, and international opinions and decisions
- Section 3. Lifecycle of clones and progeny
- Section 4. Summary of findings for cattle and swine somatic cell nuclear transfer (SCNT) clones and their progeny
- Section 5. Glossary and List of Acronyms
- Section 6. References
Summary
This scientific opinion was developed by expert scientists drawn from federal departments/agencies to review the common understanding of the current knowledge, including any gaps and other associated issues related to the impacts of somatic cell nuclear transfer (SCNT) on food and feed safety, animal health, and the environment (including indirect human health). For the purposes of this document, the focus is primarily cattle (Bos taurus) and swine (Sus scrofa domestica) produced by SCNT technologies, their progeny and their derived products. However, considerations are given to data derived from a variety of agricultural and other domestic species.
Currently in Canada, SCNT animal clones, their progeny and derived products are subject to the same rigorous health and safety regulations that apply to conventional animals and their derived products administered by the Canadian Food Inspection Agency (CFIA) and Health Canada (HC). These include the Health of Animals Act and Regulations, the Food and Drugs Act and Regulations, the Safe Food for Canadians Act and Regulations , and the Feeds Act and Regulations. Moreover, SCNT animal clones and their progeny are also considered as "new" living organisms under Part 6 of the Canadian Environmental Protection Act, 1999 (CEPA).
SCNT is considered a reproductive cloning technique. Research on SCNT has spanned 50 years with the first successful cloned live animals being developed in the last 20 years. Species that have been the subject of this research includes mouse, cattle, goat, pig, rabbit, cat, mule, horse, rat, dog, deer, wolf, camel, coyote and macaque. Despite this lengthy period of research, SCNT remains an inefficient process in most mammalian species.
Although animal clones have been successfully derived from various somatic cells in several mammalian species, there are problems associated with SCNT animal cloning, including lower rates of reproductive success, altered birth weights and higher organ failure rates. The origins and mechanisms underlying these problems are not completely understood. However, they are believed to be the result of factors such as the asynchronous stages of development between the embryo and the uterus, and/or incorrect reprogramming of the genetic material and other chromosomal abnormalities in the embryo. These problems are not unique to SCNT and have been observed in natural breeding and other assisted reproductive techniques (ARTs), although in lower frequencies. Of note, healthy cattle and swine clones have been produced using SCNT technologies, indicating that correct epigenetic reprogramming of the embryo clone is possible.
Cellular and reproductive manipulations associated with SCNT also have the potential to impact the health, welfare, and survival of all animals involved in the cloning process. Parameters relevant to animal health include: pre-natal and post-natal survival rates for animal clones, birth weights, growth rates, freedom from anatomic defects or metabolic abnormalities, transmission of infectious diseases, fertility and longevity. The reported problems tend to be most evident during the prenatal and neonatal period, and often gradually diminish with maturity, so that some individual animals appear to exhibit no detectable anatomic or physiologic abnormalities by the time they reach sexual maturity. Based on empirical findings, there are no known significant differences between the progeny from SCNT animal clones versus other sexually reproduced animals, in terms of their health status.
There are eight natural control points during embryonic and foetal development, which effectively act as filters to prevent the continuance of faulty genomes or epigenetic reprogramming errors. These natural control points in sexual reproduction decrease the likelihood that any anomalies encountered in SCNT animal clones are passed on to their progeny.
When considering their use as food or feed, available data indicates that there are no biologically significant differences in the composition of foods derived from healthy SCNT cattle and swine clones versus food from healthy animals produced through natural breeding and other ARTs. Most animal clones will be used primarily as breeding stock and their disposal into the food and feed chain will occur later in the life of the animal, long past the normal slaughter age. Based on empirical findings reviewed, there are no known significant differences between the progeny from SCNT animal clones versus other sexually reproduced animals, in terms of the composition of their derived edible products.
Considering the expected low likelihood of human exposure to SCNT clones in Canada and the fact that there is no difference in hazard potential compared to conventionally bred cattle and swine, there is no evidence to indicate the potential for an indirect risk to human health from the import or manufacture of SCNT cattle and swine clones in Canada. Moreover, although there is limited information on the impact of SCNT-derived livestock clones on the environment, it is expected that they will exhibit the same variation in traits that exist in their original natural counterparts and thus likely pose no additional impact on the environment.
Based on the available scientific information, the apparent impact of SCNT-derived cattle and swine clones in Canada is not expected to be different from conventionally bred animals with respect to food and feed safety, animal health, impact on the environment – including impacts on biodiversity - and indirect human health.
Section 1: Introduction
1.1 Purpose of document
The purpose of this document is to assess available information regarding primarily cattle and swine produced by somatic cell nuclear transfer (SCNT) technologies, and including their progeny and their derived products with respect to potential impacts on food, feed, the environment and indirect human health. This will assist in developing a common understanding of the current knowledge, including the gaps and other associated issues related to the potential impacts of SCNT derived animals. In addition, this opinion will serve as the scientific basis on which the Government of Canada will review policies as they relate to the regulation of SCNT animals, their progeny and their derived products. This document has been developed by expert scientists drawn from federal departments/agencies in Canada and does not purport to express any opinion on the benefits and/or disadvantages of SCNT cloning other than where necessary for informing policy options. Furthermore, this document may serve as an information resource for interested stakeholders in Canada as part of a public consultation regarding the potential impacts, policies, and regulation of such animals on food and feed safety, animal health, and the environment.
1.2 Scope of scientific opinion
SCNT animal cloning is a set of relatively new and developing technologies and includes a wide range of species, sources of cells, cell culture media, manipulation and cell fusion techniques. Therefore, it is necessary to define the scope of this scientific opinion – both for what it will include as well as what it will exclude. The scientific opinion will be limited to SCNT animal clones of two livestock species for which there is sufficient data for proper analysis: cattle (Bos taurus) and swine (Sus scrofa domestica), their progeny and products; in the context of current agricultural breeding practices. The scientific opinion will also specifically exclude:
- Interspecies cloning, where any of the involved animals are of a different species, including "closely-related" species
- The development of transgenic animals which may have been produced using SCNTFootnote 1
- Archaeological cloning of extinct species
- Other assisted reproductive technologies (ARTs) such as artificial insemination, in vitro fertilization, and embryo transfer, as well as embryo splitting and embryonic cell nuclear transfer cloning.
Although this scientific opinion is limited to cattle and swine, information from the cloning of other species has been considered for the purposes of clarifying and understanding the molecular and physiological basis on which SCNT animal clone development takes place, and for identifying potential hazards in need of consideration. All available information will be considered in developing this scientific opinion on SCNT cloning of cattle and swine and its impact on food and feed safety, animal health and the environment.
1.3 Background
Assisted reproductive technologies (ARTs), which aim to complement or transcend natural livestock breeding (McEvoy et al., 2006), have a long history of safe use. They are an integral part of domestic livestock breeding programs and date back to at least the early 1900s with the introduction of artificial insemination (Foote, 2002). The Canadian livestock sector has been involved for many years in the commercial use of ARTs, such as artificial insemination, embryo transfer and in vitro fertilization. These ARTs have been valuable for extending the effective reproductive potential of breeding animals, increasing flexibility for managing breeding populations, and enhancing the access to and distribution of superior genetic lineages. Yet, other techniques, such as embryo splitting, embryonic cell nuclear transfer (ECNT), and SCNT, which may collectively be referred to as "cloning", allow the production of near-identical genetic copies of animals.
Cloning is the process of creating an identical copy of the original. There are three types of cloning techniques: gene, reproductive, and therapeutic. All aim to produce genetically nearly identical copies of a biological entity that could be a gene, a cell, a tissue or an entire organism.
Reproductive cloning does not involve putting new genetic material into the genome so it is not a genetic modification. However, perpetuation of genetically modified lines often involves cloning.
SCNT is considered a reproductive cloning technique. It has been attempted over the last 50 years with first successful cloned live animals being developed in the last 20 years (Campbell et al., 1996). SCNT animal cloning involves the transfer and fusion of a donor animal's somatic cell with an enucleated oocyte (Figure 1). Donor somatic cells are those derived from tissues of animals that have differentiated to perform specialized functions, such as skin cells or mammary cells. Once their nucleus is fused with the oocyte using an electric shock, the reconstructed embryo is placed in the uterus of a foster mother where it is reprogrammed to return to a state similar to a conventional embryo. It will then develop into the various tissue types required in the growing embryo and subsequent foetus (for reviews of SCNT animal cloning technologies, see AFSSA, 2005; US FDA, 2008; EFSA, 2008; EFSA 2012; Verma et al. 2015, Wilmut et al., 2015).
The advantage of SCNT cloning over natural breeding and other ARTs is that it allows near-identical genetic copies of valuable animals with known and desirable traits to be reproduced. The SCNT cloning procedures, which are currently used in domestic animals, are adaptations of a technique developed by Ian Wilmut and colleagues at the Roslin Institute in Scotland, as described in papers published in Nature in the mid-1990s (Campbell et al., 1996; Wilmut et al., 1997). Using cells from the mammary gland of an adult sheep, Wilmut's group successfully used SCNT cloning in 1996 to produce the first clone of an adult mammal - the sheep named "Dolly".
In the years following the original report, the SCNT cloning technique has been used successfully to produce animal clones in various livestock and other species (for review see Wells, 2006, Wilmut et al., 2015). There have also been a few reports of successful cloning of endangered wildlife species, using somatic cells from the endangered species and enucleated oocytes and surrogate dams of a closely related domestic species (Kim et al., 2007; Wani et al., 2017).
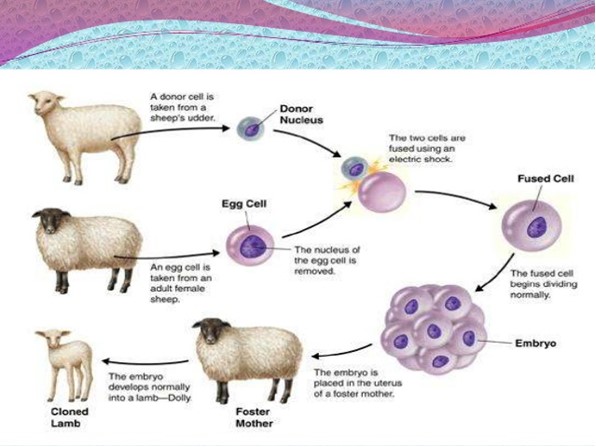
Figure 1 - Text description
The cloning process in a sheep. The image begins with two sheep. At the same time, a donor cell, containing the donor nucleus is taken from one of the sheep's udder. An egg cell is taken from another adult female sheep and the nucleus of the egg cell is removed. The two cells are then fused using an electric shock, becoming a fused cell. The fused cell begins dividing normally turning into an embryo. The embryo is then placed in the uterus of a foster mother sheep. The embryo develops normally into a cloned lamb – Dolly.
1.4 Applications of SCNT Cloning
1.4.1 SCNT Technologies
Dolly the sheep, born in 1996 at the Roslin Institute in Scotland, was the first live mammal SCNT clone produced using cells from the mammary gland of an adult sheep (Campbell et al., 1996; Wilmut et al., 1997). Prior to the birth of Dolly, it was unknown whether the nucleus of a terminally differentiated somatic cell could be reprogrammed to allow development into a viable embryo. Dolly's birth demonstrated that cloning higher organisms from a single adult somatic cell was feasible. Dolly was euthanized at the age of six due to progressive lung disease and advanced arthritis; her progeny have been studied and had health profiles similar to other sheep (Sinclair et al., 2016). Starbuck II, a bull, was cloned in 2000 by researchers from the University of Montreal and L'Alliance Boviteq and was the first reported SCNT livestock clone in Canada.
The technology is progressing at different rates in different species as there has been greater success in producing healthy clones in some species than in others. However, there have been numerous reports of SCNT cloning in mouse, cattle, goat, pig, rabbit, cat, mule, horse, rat, dog, deer, wolf, camel, coyote and macaque (Wakayama et al., 1998; Kato et al., 1998; Baguisi et al., 1999; Betthauser et al., 2000; Chesné et al., 2002; Shin et al., 2002; Woods et al., 2003; Galli et al., 2003; Zhou et al,. 2003; Lee et al., 2005; Berg et al. 2007; Kim et al., 2007;Wani et al., 2010; Hwang et al., 2013; Wani et al., 2017; Liu et al., 2018).
Cloning through SCNT is currently an inefficient process in most mammalian species. The rates of healthy progeny produced with this technique vary considerably, with an average less than 10% of reconstructed embryos resulting in live animals (Cibelli et al., 2002; Paranace et al., 2007; US FDA, 2008; EFSA 2012). There are multiple steps in the SCNT process that have been reported to be responsible for the relatively low success rates observed for the cloning process versus success rates for natural breeding or other ARTs. However, with the fast-paced progress in DNA editing technology, there is a recent shift of perspective on SCNT cloning. This is due to a better understanding and control of the SCNT tool by achieving more effective reprogramming strategies based on knowledge gained from genome-wide and single-gene editing technologies. This can serve better biomedical research and improve the application of SCNT cloning (Loi et al., 2017).
1.4.2 Current and Potential SCNT Applications
SCNT cloning has several potential research and commercial applications (McLaren and Southee, 1997; Smith et al., 2000b; Alberio and Campbell, 2003; Niemann and Lucas-Hahn, 2012). Based on a review of the literature, SCNT cloning is used in four sectors: research, livestock breeding, pets and prize winning animals, and biodiversity conservation.
Research
SCNT cloning in mice, cattle and other species has proven to be useful to study developmental genetics. Production of genetically uniform animals could also be valuable for other research applications (e.g., to compare various treatments).
In the Canadian research and development (R&D) sector, academic research on SCNT livestock in Canada (pig, cow) is being conducted mostly at veterinary colleges. These include the University of Guelph (cattle, pig, sheep, bison, telomere length reprogramming), University of Montreal at the Faculté de médecine vétérinaire (St. Hyacinthe) (cattle, horse, rat), and McGill University (pig, bovine). Apart from the livestock sector, researchers at University of Toronto use SCNT cloned mice for research purposes to improve the cloning technique and study developmental and genetic anomalies and gene expression. Researchers at the University of Guelph focus their research projects on improving the SCNT technique and interspecies cloning of endangered species.
Further, most SCNT cloning is expected to be valuable for use in conjunction with genetic modification technologies. For example, SCNT could be used: 1) to produce several copies of transgenic animals that were bioengineered to have novel traits, such as the production of biopharmaceuticals, other biochemicals, or enhanced nutritional profiles; or 2) as animal models bioengineered to study human disease. In addition, SCNT is an irreplaceable tool to understand nuclear reprogramming and totipotency (Loi et al, 2016).
Livestock sector
SCNT cloning could accelerate the distribution of genetics from animals with superior or rare traits (e.g., product quality attributes, immune tolerance to endemic disease), or be an adjunct to genetic modification (rDNA, gene editing) for dissemination of genetics in the population. Genetic modification is excluded from the scope of this scientific opinion.
In the livestock sector, SCNT cloning is primarily expected to be of value for breeding of elite genetics. At the outset, SCNT cloning offered the advantage of both expanding access to the superior genetics of animals at the top of the classical breeding pyramid (Figure 2), and acting as a form of insurance policy against the untimely loss of an elite and highly proven breeding animal. The livestock SCNT clones are not produced for end-market food and feed use; they are rather used to produce eggs or semen for breeding purposes. In 2000, the Holstein bull Starbuck II was the first mammalian clone born in Canada. He was a clone of the well-known Holstein bull, Hanoverhill Starbuck. Hanoverhill Starbuck sired some 200,000 Holstein daughters distributed in 45 countries (communiqué from CIAQ, Saint-Hyacinthe, Québec), and produced 209 proven sons and 406 grandsons which further extended his influence and market value. At the time of his death in 1998, Starbuck had an estimated total market value of upwards of 25 million dollars to the industry, with continued interest in his genetics from export markets.
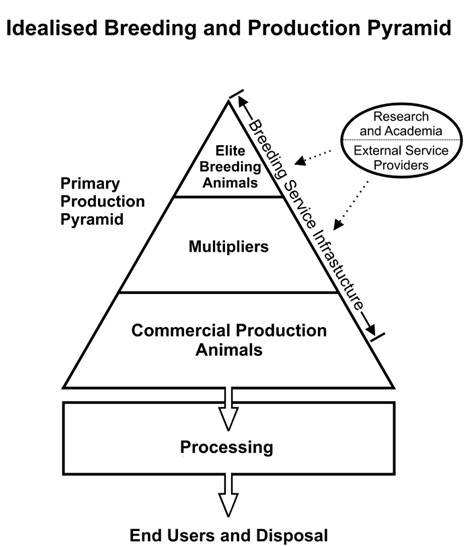
Figure 2 - Text description
The idealised breeding and production pyramid. An image of a Primary Production Pyramid with four layers. At the top of the pyramid is Elite Breeding Animals. The second section is Multipliers. The third section, Commercial Production Animals. An arrow pointing down from the third section, Commercial Production animals goes to the 4 section, indicated as Processing. The fourth, Processing section also has an arrow pointing down to the final step, End Users and Disposal. A bidirectional arrow, indicating Breeding Service Infrastructure, runs on the right side of the pyramid, from the top, Elite Breeding animals section to the third section, Commercial Production Animals. Pointing to the Breeding Service Infrastructure is Research and Academia and External Service Providers in a circle.
Figure 2: A breeding pyramid is typically used to show how genetic improvement achieved in a relatively small number of elite breeding populations (plants or animals) flows through to successive generations. Natural breeding and assisted reproductive technologies (ARTs) both contribute to this flow. Animal cloning can potentially be used for animals of superior market value, either at the elite breeding or multiplier stage. Cloning technologies services may be supplied by existing industry breeding service suppliers or be contracted by external service providers. Imports and exports may occur at any point along the pyramid. Assurance requirements may also be demanded by buyers at any point along the pyramid.
However, the implementation of genomics technologies for use by the Canadian dairy sector in 2009 greatly enhanced the accuracy of genetic evaluations of younger animals without having to wait for several years in order to have data on the productivity of their daughters. As a result, the value of top proven bulls dropped rapidly and consequently the value of cloning to the Canadian dairy industry also dropped. Starbuck II was euthanized in 2010 and the Canadian livestock genetic dissemination industry has since discontinued much of its research and development of cloning technology. Meanwhile, SCNT cloning may still hold value for the livestock breeding sector as a means of introducing genetics from a gene edited or recombinant DNA animals into an adapted population, more rapidly than would be feasible through traditional breeding.
Although healthy animals from any point in the breeding pyramid could potentially enter the food or feed chain, the top of the pyramid is where cloning is most likely to be applied. They typically represent only a very small proportion of all animals used, and many of the mature elite breeding animals may never enter the food chain but may be used only for feed.
Several companies involved in developing ARTs commercialize the products of SCNT livestock clones and their progeny worldwide and in Canada. These companies are mostly located in the US, Argentina, Australia, China and Korea. Examples are: ViaGen, Transova Genetics, Clone International, Boya Life, and Sooam.
Pets and prize winning animals
Other commercial applications have also been developed to meet specific consumer desires and market demands worldwide (e.g., cloning pets, cloning prize-winning animals). In the USA and Canada, at least five companies offering cloning of horses, deer, cat and dogs (CBC, 2017), were found in the public domain. Examples are: ViaGen Pets, Moore Equine, and Timber Creek.
Biodiversity Conservation
Since the advent of procedures for cloning animals, conservation biologists have tried using SCNT technology to preserve endangered species or to expand and restore, animal populations threatened with extinction (Loi et al., 2001; Saragusty et al., 2016). Examples where SCNT has been used for endangered species are: Pyrenean Ibex (Folch. et al., 2009), bison, Gaur, Bateng, Mouflon, African wild cat (Mastromonaco and King, 2007), Bactrian Camel (Wani et al., 2017). Although many basic scientific questions have been answered and more than 30 wild species (mammals, fishes, birds, reptiles and amphibians) have been investigated, very few successes have been reported due mostly to issues related to nuclear reprogramming, abnormal gene expression, and epigenetic deregulation (Mastromonaco et al., 2014; Loi et al 2013). It is still difficult to adapt the technology to wildlife species; however, the rapid gain in the understanding of the molecular clues underlying nuclear reprogramming using editing at the gene and whole genome levels, will help accelerate successful cloning for wildlife conservation purposes.
1.5 Canadian Regulations and Policies on SCNT Animal Cloning
Before any SCNT animal clones, their progeny or their derived products are imported, manufactured or released into Canada, the developer must notify the responsible Canadian federal government authorities, which may trigger one or more Acts and Regulations. This often involves coordinated regulatory oversight among the various involved departments and agencies.
In Canada, SCNT animal clones, their progeny and derived products are subject to the same rigorous health and safety regulations that apply to conventional animals and their derived products administered by the Canadian Food Inspection Agency (CFIA) and Health Canada (HC). These include the Health of Animals Act and Regulations, the Food and Drugs Act and Regulations, the Safe Food for Canadians Act and Regulations, and the Feeds Act and Regulations. In addition, edible products from SCNT animal clones and their progeny are considered as "novel foods" and "novel feeds"; therefore, assessment for food safety and standards regulations, and feed safety, are required under the Food and Drugs Regulations and the Feeds Regulations, administered by HC and the CFIA, respectively. SCNT animal clones and their progeny are also considered as "new" living organisms under Part 6 of the Canadian Environmental Protection Act, 1999 (CEPA). Therefore, they must meet the notification requirements under the New Substances Notification Regulations (Organisms) [NSNR (O)], jointly administered by Environment and Climate Change Canada (ECCC) and Health Canada (HC).
1.5.1 Food and Drug Act and Regulations
In July 2003, as an interim policy, Health Canada stated that foods produced from livestock animals developed using SCNT cloning and from the progeny of such animals, will be considered to fall under the definition of "novel food." Novel foods are subject to the regulations in Division 28, Part B, of the Food and Drug Regulations (Health Canada, 2003). Therefore, developers producing food from animal clones through SCNT must not introduce the products or by-products of any animal clones or their progeny into the human food supply in Canada, unless they have been subject to the pre-market safety assessment required for novel foods. However, because there was insufficient data at that time to guide the pre-market safety assessment of these products, developers who wished to use SCNT technologies for producing livestock, were requested to withhold novel food notifications until requirements were determined and guidance made available. This interim policy is posted on the Health Canada's website and still applies until Health Canada advises otherwise.
1.5.2 Feeds Act and Regulations
The CFIA also considers feed ingredients derived from SCNT animal clones and their progeny to be "novel feeds" and subject to the Feeds Regulations; therefore, assessment is required before any products and by-products derived from these animals are released in the feed chain. This assessment considers the safety of the feed to livestock, to humans via worker/bystander exposure and consumption of animal products, and to the environment. More information regarding the use of products and by-products derived from SCNT-animal clones into feeds can be found at the CFIA's web site Novel feeds from biotechnology-derived animals.
1.5.3 CEPA and NSNR (O)
SCNT animal clones and their progeny are considered "new" living organisms under Part 6 of the Canadian Environmental Protection Act, 1999 and, therefore, are subject to notification requirements under the New Substances Notification Regulations (Organisms) before import and manufacture. More information regarding the requirements for notification of new living organisms is posted on Environment and Climate Change Canada's New substances website.
The Domestic Substances List (DSL) is an inventory of approximately 23 000 substances (including living organisms) manufactured in, imported into or used in Canada on a commercial scale. The DSL is the sole standard against which a substance is judged to be "new" to Canada. Substances not on this list are considered "new" and must be notified prior to importation or manufacture in order that they can be assessed to determine if they are, or could become, toxic to the environment or human health.
A few companies and academics have approached ECCC and HC to request advice on meeting their regulatory obligations for release in the environment of cloned livestock (bull semen from Starbuck II in 2005, and for cloned horses in 2007). However, to date, there has been one formal risk assessment conducted for the import of semen from the progeny of an SCNT Holstein bovine for breeding purposes in Canadian dairy farms (NSN-19594). The assessment concluded this SCNT breed of cattle does not pose any environmental or human health concerns over and above that of existing cattle. In November 2023, "Cattle descended from a cloned Bos taurus (Breed: Holstein) founder" was added to the DSL. As a result, live cattle clones descending from a cloned Bos taurus founder of the breed Holstein, and their germplasms are no longer considered "new" and therefore not subject to notification under the NSNR(O).
1.5.4 Exemptions of R&D activities for higher organisms
The regulatory system in Canada applies to commercial activities involving higher organisms such as their proposed use for food, feed, drug production, or any other use. However, higher organisms used only in research and development are exempt from regulatory oversight given that industries and individuals are obligated to conduct such activities in strict containment dictated by specific criteria under the various Acts and Regulations in Canada. At the international level, there is oversight of R&D activities in Australia, New Zealand, United Kingdom, USA and Brazil through notification or licencing systems or as a requirement to receive research funding. (An annex is available for reference).
At the international level, before products derived from such new technologies can be introduced on the market, potential risks to human health, food and feed safety, animal health and the environment must be addressed. The US Food and Drug Administration, European Food Safety Authority, Japan Food Safety Commission and Food Standards Australia New Zealand have all concluded that food products from cloned animals and their offspring are as safe as food products from conventionally bred animals (https://www.foodstandards.gov.au/consumer/foodtech/clone).
Section 2: Review of molecular data, potential impact of SCNT cloning in Canada, and international opinions and decisions
2.1 Epigenetics and Genetics
2.1.1 Potential Mechanisms of Variability in SCNT Clones
Although animal clones have been successfully derived from various somatic cells in several mammalian species, there are health problems associated with SCNT animal cloning, including lower rates of reproductive success, altered birth weights and higher organ failure rates. The origins and mechanisms underlying these problems are not completely understood, however, they are believed to be the result of factors such as the asynchronous stages of development between the embryo and the uterus, and/or incorrect reprogramming of the genetic material and other chromosomal abnormalities in the embryo. These problems are not unique to SCNT and have also been observed in natural breeding and other ARTs, albeit at lower frequencies (Walker et al., 1996; Ortegon et al., 2007).
The observed problems might arise from transfer of the somatic nucleus, reprogramming of the nucleus, embryo culture conditions, embryo transfer methods, or a combination of some or all of the above (Wilson et al., 1995; Kruip and den Daas, 1997; Van Wagtendonk-de Leeuw et al., 1998). There is variation among studies in the incidence of health and welfare problems of animals produced through SCNT technologies (Cibelli et al., 2002; Paranace et al. 2007; US FDA, 2008). Varying levels of expertise with the techniques could be a contributing factor to this range of effects. Nuclear transfer represents only one step in a process that involves harvesting an egg, removing its nuclear material, synchronization of somatic cell cycles, nuclear transfer, fusion and activation, in vitro culture, and embryo transfer. Abnormalities can arise due to any of these manipulations, and may not be exclusive to SCNT animal cloning. For example, in vitro manipulations of embryos or gametes may compromise the ability of the resultant embryo to develop normally. Studies in mice have shown that in vitro manipulations of the embryos can result in long-term changes, including retarded growth (Reik et al., 1993). The in vitro culture of embryos is also associated with large offspring syndrome, a set of symptoms, of which the most predominant is an increased birth weight as compared with animals resulting from natural reproduction (Young et al., 1998). In cattle, developmental and perinatal problems may be caused by the in vitro culture technology as well as the nuclear transfer (Kruip et al., 1997; Van Wagtendonk-de Leeuw et al., 1998). Most of the abnormalities observed in animal clones, whether produced through SCNT or other ARTs, are also seen with natural breeding, but at much lower rates (Cibelli et al., 2002; Paranace et al., 2007; US FDA, 2008).
2.1.2 Chromatin Remodelling
Chromatin is a complex made up of DNA and basic proteins in the nucleus. Several proteins (e.g., histones) associated with the DNA are known to play key roles in governing how chromatin is assembled. This assembly can influence the expression of genes by changing their transcription rates. Chromatin remodelling appears to be an important part of the overall reprogramming of cells (Wade and Kikyo, 2002). As part of the oocyte reprogramming process, an exchange of somatic proteins for oocyte proteins must occur followed by remodelling of donor nucleus and reactivation of pluripotency genes. Unlike the stochastic reprogramming induced by transcription factors, these events are considered to be deterministic and should be studied more closely. In a study by Wen et al., 2014, it was found that histone variant h4.3 is an essential maternal factor for oocyte reprogramming and is required in the reactivation of many pluripotency genes and the development of the embryo following SCNT. The characterization of such elements can shed light towards understanding the plethora of modifications that occur during reprogramming.
In the normal sexual reproduction process, nuclear reprogramming takes place during gamete (i.e., sperm and egg) formation and during early embryonic development. The cloning process could interfere with chromatin formation and normal reprogramming through several ways, including the bypassing of meiosis. There is evidence that incomplete or altered chromatin remodelling may be a primary cause of health problems in mammalian clones (Rideout et al., 2001; Chavette-Palmer et al., 2012; Niemann, 2016).
The epigenetic states of donor cells can significantly affect the development of SCNT animals (Zhai Y et al., 2018). Embryos derived from porcine bone marrow-derived mesenchymal stem cells had more active epigenetic markers and fewer repressive epigenetic markers than fetal fibroblast donor cells. As such, donor cells that have a more open chromatin state are generally more "conductive" to nuclear reprogramming as concluded by the authors. Furthermore, neural stem cells were found to be more efficient than terminally differentiated neuronal cells when used as donors for nuclear transfer (Blelloch et al., 2006).
A number of studies also point out specific epigenetic constraints seen in SCNT embryos, such as abnormal histone modification and aberrant DNA methylation patterns. For example, signals for key epigenetic markers such as h4K4me2 (transcriptionally active chromatin marker) and h4K9 (inactive chromatin marker) have been observed at a lower level in cloned embryos compared to their in vivo counterparts (Shao et al., 2008 and Wang et al., 2007).
A potential solution for this problem is to treat the zygotes (post nuclear transfer) (in vitro) with histone deacetylase inhibitors such as trichostatin A (TSA). TSA showed promising improvements in cloning efficiency for bovine, pig, mouse and rabbit (Enright et al., 2003; Kishigami et al., 2006; Shi et al., 2008 and Das et al., 2010) by causing hyper acetylation, thereby enhancing the acetylation state of the cloned embryos to levels close to those of fertilized embryos. TSA is able to improve nuclear reprograming by unfolding chromatin, making it more accessible to different transcriptional factors leading to gene transcription (Mason et al., 2012). Nevertheless, further studies in chromatin-modifying agents (CMAs) concluded that treatment of nuclear donor cells or cloned zygotes with CMAs such as TSA has no positive effect on pre and post-implantation development in cloned cattle which questions the effectiveness of these methods (Sangalli et al., 2012).
In a separate study by Russo et al., it was found that DNA methylation and chromatin remodelling induced by homologous DNA repair may be a source of permanent variation of gene expression in somatic cells. As such it was concluded that both stochastic and deterministic factors control the stable DNA methylation profile. Such variability in chromatin remodelling and methylation can further elucidate the complexity of successful cell reprogramming (Russo et al., 2016).
Looking deeper into the chromatin remodelling process, it was found that polyADP-ribosylation which is catalyzed by poly(ADP-ribose) polymerase 1 (parp1) is a major post-translational modification that facilitates DNA repair and chromatin remodelling. Research showed that in the absence of Parp1, DNA breaks are slowly repaired and may result in delayed entry into the S phase. A decrease in histone h4 monomethylation at lysine 4 and h4 trimethylation at lysine 27 was also observed in the Parp 1 -/- donor embryos. As such, it was concluded that Parp1 plays an important role in the plastic remodelling of chromatin structure following nuclear transfer by supporting DNA repair and specific histone modifications (Osada et al., 2016).
Kim et al., 2018 found that silencing of growth arrest-specific gene 6 (Gas6) in oocytes impaired cytoplasmic maturation resulting in failure of sperm chromatin de-condensation (SCD) and pronuclear (PN) formation after fertilization. Disruption of the Gas6 expression led to the direct inhibition of heparin sulfate (HS) biosynthesis through a reduction in several HS enzymes. Considering these results, the authors proposed that the addition of HS to sperm and/or oocyte maturation would improve the efficiency of somatic cell nuclear transfer reprogramming.
To fully understand the principles and mechanisms of chromatic remodelling as a result of SCNT further research is needed to identify key factors responsible in the process and how they may be affected by the surrounding, dynamic environment.
2.1.3 Epigenetic Modification of Telomeres
Telomere length regulation and maintenance have been shown to contribute to normal cellular aging and diseases, such as cancer (for reviews see Blasco, 2007; Hornsby, 2007). During normal cellular aging, telomere length shortens – a phenomenon that has been associated with cell senescence. In the sexual reproductive process, the telomere length is restored during embryo development. In SCNT animal clones, the nucleus comes from the donor organism, and as such, has undergone multiple rounds of chromosome replication during cell division and has gone through the normal aging process.
In Dolly, telomere length was found to be shortened when compared to an age-matched conventional sheep. Dolly's telomere length was comparable to the telomere length of the sheep that was used to develop her, i.e., her telomere length corresponded to that of a six year older sheep (Sheils et al., 1999). Several researchers have postulated that telomere length may have contributed to the premature deaths of some SCNT animal clones animals (reviewed by Kühholzer-Cabot and Brem, 2002). Telomere-dysfunction diseases such as telomere dyskeratosis do not seem to be prevalent among cloned animals (Burgstaller and Brem, 2017).
Most studies on the progeny of SCNT animal clones indicate that telomere length is normal in both germline and somatic cells (Miyashita et al., 2002; Miyashita et al., 2003; Alexander et al., 2007; Ortegon et al., 2007). However, one report of the progeny of goat clones indicates that abnormal telomere length may be heritable, although those progeny appeared healthy for their chronological age at the time of tissue extraction (Betts et al., 2005). Many questions remain to be answered regarding the relationship between telomere length and the lifespan of SCNT animal clones and for conventionally bred animals (Miyashita et al., 2003; Burgstaller and Brem, 2017).
More recently, studies of telomere length in domestic SCNT animal clones versus those measured in their age-matched counterparts have reported that telomere lengths may be shorter, similar in length or sometimes longer (reviewed in Alexander et al. (2007) and Burgstaller and Brem (2017)). Niemann (2016) reports that most studies report that telomere length in cloned cattle, pigs, goat, and mice are comparable to their age-matched naturally bred animals. Burgstaller and Breum (2017) indicate for sheep, telomere length is usually shortened. Telomere length elongation (restoration) happens during the transition from morula to blastocyst stage of embryogenesis through telomerase activity (Dang-Nguyen et al., 2012, Schaetzlein et al., 2004; Wang et al., 2012, Miyashita et. al., 2011; Niemann, 2016). It is unclear why this does not happen perfectly. Burgstaller (2017) indicates that species differences, donor cell origin and of course the NT protocol itself may be responsible for improper telomere length restoration. Niemann (2016) also indicates that telomere length is associated with donor cell type. The sex of the donor cell was also shown to have significant effects on the telomere length of cloned goats (telomere length of female goats were shorter than those of donor cells; those of males were longer (Liu et al., 2016). Burgstaller (2017) also indicates that the degree of telomere lengthening was found to be associated with nuclear reprogramming (Huang et al., 2011). Currently, the application of trichostatin A, improves the success rate of cloning but also to favourably influence the telomere length (Kong et al., 2014). Telomere length can also be restored in the embryo during the SCNT cloning process by adding histone deacetylase inhibitor (Wakayama et al., 2013).
2.1.4 Genomic Imprinting and DNA Methylation
Genomic imprinting is an epigenetic parent-specific modification by which genes or chromosomes are expressed or repressed in progeny. This process determines the parent-dependent expression of certain genes during gametogenesis and embryonic development. Egg and sperm contribute equally to the DNA of the zygote but the genomic imprinting determines which parental genes will be expressed.
During embryogenesis, the two parental genomes (from the egg and the sperm) respond to the environment of the egg and proceed through development (Rideout et al., 2001). In SCNT animal clones, chromosomes are inherited from one donor source, bypassing the normal processes of sexual reproduction. Developmental problems associated with one-donor chromosome inheritance are well documented and are known to be caused by changes in genomic imprinting (Shi et al., 2003). To successfully mimic the normal embryogenesis process, the reconstructed embryo clone must be reprogrammed to express genes required for early development (Jaenisch, 1997). Genome-wide imbalance or disruption of imprinted gene expression results in post-implantation lethality. The most common phenotypes observed in SCNT animal clones are foetal growth abnormalities, such as altered placental and birth weights, which may be due to aberrant expression of imprinted genes. The observed altered growth patterns in foetus clones and placentas may result from the cumulative dysregulation of several imprinted genes (Rideout et al., 2001). In cattle low efficiency of cloning may result from removal of imprinting marks during the reprogramming of the somatic cell nucleus during early development (Smith et al., 2012).
DNA methylation refers to the addition of methyl groups to certain nucleotides in genomic DNA and is the main contributor to genomic imprinting. The methylation state of the genomic DNA differs between different somatic cell types, as well as between somatic cells and germline cells. One effect of DNA methylation is on gene expression – methylated DNA is not easily transcribed – which can result in gene repression. Methylation pattern is by far the best studied mechanism among the potential epigenetic modifications likely involved in nuclear reprogramming (Jafarpour et al., 2011; Peat and Reik, 2012; Matoba et al., 2014).
An inappropriate methylation state could lead to developmental abnormalities in animals produced through SCNT (Niemann, 2016; Zhang et al., 2016; Zhai et al., 2018). There have been a variety of mechanisms employed to combat aberrant epigenetic expression in SCNT but a frequent method that appears is the overexpression of DNA methyltransferase inhibitors to prevent hypermethylation (Huang et al., 2016; No et al., 2018; Zhang et al., 2018). The methylation status of SCNT bovine and swine in relation to blastocyst maturation and development has been well-studied with a clear link between hypermethylation and developmental deficiencies (Oh et al., 2012; Zhao et al., 2014; Zhang et al., 2018).
There are reports of abnormal methylation in SCNT mice clones compared with animals produced using in vitro fertilization (Cibelli et al., 2002). However, the ability of some SCNT animal clones to develop and reproduce normally implies that functional methylation can be restored (Lanza et al., 2001; and reviewed in AFSSA, 2005; EFSA, 2008; EFSA, 2012; US FDA, 2008). Based on the available data, there is no evidence that defects in DNA methylation are passed on from the animal clone to its progeny, likely due to the natural control points in sexual reproduction (Yamanaka et al., 2011; Couldrey et al., 2011).
2.1.5 Genetics
2.1.5.1 Mitochondrial Inheritance
In embryos resulting from sexual reproduction, the mitochondria are inherited from the maternal egg cell. Paternal mitochondria (from the sperm cell) also penetrate the egg upon fertilization but they are quickly destroyed during the first few divisions of the fertilized egg (Evans et al., 1999). For sexually reproduced embryos, therefore, mitochondrial inheritance is maternal. In embryos that are derived from nuclear transfer, the mitochondria could be derived from the enucleated recipient egg (homoplasmy) or from both the egg and the donor somatic cell (heteroplasmy) (Smith et al., 2000a; Burgstaller et al., 2007).
There are several unknowns regarding the outcomes of SCNT cloning in regards to mitochondrial inheritance. Much of the evidence is confounded with effects from epigenetic factors (reviewed by Hiendleder et al., 2005; Hiendleder, 2007). Some studies suggest that mitochondrial source could theoretically affect production traits (via novel recombination due to heteroplasmy) as well as the development and functions of various organs (Smith et al., 2000a), that somatic cell mitochondria may be detrimental to development (Takeda et al., 2005; Sansinena et al., 2011; Srirattana and St. John, 2017) or that potential incompatibility between the somatic cell nucleus and the mitochondrial DNA of the recipient oocyte may represent another hurdle to successful SCNT cloning (Hwang et al., 2013; Burgstaller and Brem 2017).
The reporting of mitochondrial heteroplasmy in SCNT animal clones has been inconsistent. Some researchers have suggested that incompatibility of mitochondrial DNA haplotypes between donor cells and host oocytes causes early embryo development arrest (Yan et al., 2010; Hua et al., 2011). Other studies demonstrate that mitochondrial heteroplasmy can be successfully overcome by using mitochondria depleted donor cells so that the mitochondrial DNA is inherited from the recipient oocyte only (Lee et al., 2010; Srirattana and St. John, 2017). The progress in DNA editing technologies may have led to mitochondrial heteroplasmy no longer being a barrier in SCNT technology (Hua et al., 2011;Srirattana and St. John 2017); However, in either cases, there have not been reports of mitochondrial heteroplasmy linked to adverse effects on animal health and meat and milk composition (EFSA, 2008; US FDA, 2008).
2.1.5.2 Other Genetic Effects
Other potential genetic effects of SCNT technologies were examined by the European Food Safety Authority (EFSA, 2008), including the extent to which SCNT: 1) induces silent mutations in the nuclear DNA of clones that could be transmitted to later generations; and 2) induces adverse modifications of the oocyte cytoplasm. EFSA concluded that there was insufficient information on these other genetic effects to draw any conclusions (EFSA, 2008).
2.1.6 Summary for Epigenetics and Genetics
Factors such as the asynchronous stages of development between the embryo and the uterus, and/or incorrect reprogramming of the genetic material and other chromosomal abnormalities in the embryo are the most likely source of problems encountered in using SCNT technologies to clone animals.
These problems are not unique to SCNT and have been observed in natural breeding and other ARTs, although in lower frequencies.
Healthy cattle and swine clones have been produced using SCNT technologies, indicating that correct epigenetic reprogramming of the embryo clone is possible.
2.2 Animal Health Considerations
2.2.1 IntroductionFootnote 2
The cellular and reproductive manipulations associated with SCNT have the potential to impact the health, welfare, and survival of all animals involved in the cloning process. The surrogate dam, the animal clone, the sexually reproduced progeny of the animal clone, and any animals re-cloned from the animal clone, may all be affected. Parameters relevant to animal health include: pre-natal and post-natal survival rates for animal clones, birth weights, growth rates, freedom from anatomic defects or metabolic abnormalities, transmission of infectious diseases, fertility and longevity.
Much of the animal health data for SCNT animal cloning was derived from studies in cattle, swine, sheep, and goats. Unless otherwise indicated, the information in this section applies to the application of SCNT cloning technologies in cattle and swine. Many of the abnormalities discussed below do not seem to be as severe or common in swine or goats as they are in cattle and sheep.
The reported problems tend to be most evident during the prenatal and neonatal period, and often gradually diminish with maturity, so that some individual animals appear to exhibit no detectable anatomic or physiologic abnormalities by the time they reach sexual maturity.
In 2008, the OIE (now the WOAH) agreed to adopt the following Articles as a starting point for identifying, characterising and providing a basis for discussion on the animal health risks associated with SCNT cloning technologies, including their implication for environmental safety and food and feed safety. This chapter of the Terrestrial Animal Health Code is entitled: Somatic Cell Nuclear Transfer in Production Livestock and Horses (OIE, 2008 and 2018).
In addition, the International Embryo Transfer Society (IETS) has released a guidance document for practitioners of SCNT technologies regarding animal health entitled: Health Assessment and Care for Animals Involved in the Cloning Process (IETS, 2008).
2.2.2 Pregnancy
This section includes animal health considerations during pregnancy, including maternal and foetal effects during the prenatal period from embryo implantation to parturition. The health and welfare of some of the surrogate dams of clones can be adversely affected due to complications in pregnancy from abnormal placentation (e.g., abortions, placental deformities, large foetuses, difficult birth) (EFSA, 2012). Additionally, the transfer of the cloned embryos requires a surgical approach (incision in the midline to expose the oviduct in the abdomen in order to deposit the embryos). This can result in complications in healing, post-operative pain, etc. could result for the surrogate dam. (References that outline the procedure can be provided.)
However, neither of these phenomena appears to affect the offspring of clones, born through conventional breeding techniques (EFSA, 2008).
Similar to other ARTs, embryonic loss early in pregnancy represents a primary impediment to SCNT cloning efficiency. Studies suggest that early embryonic death (prior to gestation day 30 in cattle) accounts for the loss of about half of the transferred SCNT embryos, which appears to be similar to early embryonic death rates for other ARTs (Heyman et al., 2002; Lee et al., 2004; Panarace et al., 2007). Early embryonic death is not expected to be of significant health concern to the surrogate dam in SCNT, since the embryonic tissues are generally reabsorbed and the female typically resumes estrus cycling, with minimal effects on overall health or fertility.
In contrast to other ARTs, SCNT is associated with a significantly higher rate of mid-term to late-term abortions in cattle. In this species, foetal losses from gestation day 30 to term has been reported to be as high as 70-80% of established pregnancies (Hasler et al., 1987; Hill et al., 2000; Heyman et al., 2002; Edwards et al., 2003; Panarace et al., 2007). In comparison, other ARTs, such as in vitro fertilization and embryo transfer, have been associated with mid- term to late-term pregnancy losses of approximately 5% or less (Hasler et al., 1987; Hasler et al., 1995; Heyman et al., 2002). Although foetal organ abnormalities are sometimes observed, the primary cause of mid-term to late-term abortions appears to be related to aberrant placental development (Fletcher et al., 2007; Lee et al., 2004). Placental malformations affect the supply of vital oxygen and nutrients to the foetus, and impair foetal waste removal. A possible correlation has been noted between placental abnormalities/pregnancy failure and the type of donor cell selected for nuclear transfer (Wells et al., 2003; Urakawa et al., 2004; Ideta et al., 2005). Aside from the obvious implications for foetal health, late-term abortions can also pose a significant health risk to the surrogate dam, as failure to fully expel the foetus, placenta, and uterine fluids may lead to uterine infections which may impair future fertility, and could lead to illness and death if untreated. There are animal welfare considerations for SCNT cloning, since there are health consequences for animals involved in this process, including the recipient of the aborted embryo.
Another relatively common complication of SCNT pregnancy in cattle is hydrops, a condition involving the accumulation of oedema fluids within the foetus (hydrops foetalis/foetal oedema) and/or its associated membranes (hydroallantois). Published reports indicate that the incidence of hydrops has consistently been higher in embryo clones than in embryos derived from natural breeding or other ARTs. In cattle, the observed rates have been in the range of 13- 61% for embryos clones versus 0.1-0.5 % for embryos derived from natural breeding or other ARTs (Hasler et al., 1995; Pace et al., 2002; Panarace et al., 2007). If severe, hydrops can represent a significant health concern to both the SCNT foetus and the surrogate dam. Hydrops foetalis is often characterized by ascites, pleural effusion, and/or pericardial effusion, and can be fatal to the foetus, while hydrallantois or placental oedema can result in illness or death of the surrogate dam as well as the foetus.
2.2.3 Perinatal Period
At birth, calves and lambs derived from embryos manipulated in vitro, especially SCNT cloning, tend to display a higher rate of large offspring syndrome (Farin et al., 2004, McEvoy et al., 2000), which is associated with birthing difficulties often requiring caesarean section. In addition to their large size at birth, some affected animals may exhibit a delayed ability to stand after birth and weak suckle reflex (Young et al., 1998). The most common clinical abnormalities observed in neonatal SCNT clones are respiratory distress, circulatory problems, metabolic problems, thermoregulatory deficiencies, enlarged umbilical cord, umbilical hernia, and contracted flexor tendons (Cibelli et al., 2002; Pace et al., 2002; Chavatte-Palmer et al., 2004; Panarace et al., 2007). These abnormalities are observed much more frequently in SCNT animal cloning than in natural breeding, artificial insemination or embryo transfer. For SCNT animal clones, the proportion of live births surviving the first few days after birth varies considerably between studies, but is typically 50% or greater (Solter, 2000; Chavatte-Palmer et al., 2004; Panarace et al., 2007). In comparison, in vitro fertilization studies in cattle generally report perinatal survival rates over 75%, while embryo transfer and normal conception each have survival rates of greater than 90% (Kruip and den Daas, 1997; US FDA, 2008). As such, there are animal welfare considerations for the quality of life of the offspring. According to the Agence Française de Sécurité Sanitaire des Aliments, it is possible that these problems will be mitigated by more careful control of environmental and animal care conditions (AFSSA, 2005).
The other ARTs differ from SCNT in that they are based on the physical transfer of intact oocytes, spermatozoa, or embryos, without the additional interventions at the sub-cellular level that are employed in SCNT animal cloning. Examples are exposure to biochemical reagents to synchronize the cell cycle; micro-manipulation of the donor cell and host oocyte; oocyte enucleation to form the ooplast; introduction of the donor cell with its nucleus into the ooplast; and oocyte reconstruction through the fusion of membranes and activation of the reconstructed oocyte to initiate mitosis.
Taken together, the available data suggest that survival rates for embryos derived from SCNT cloning are lower than those observed with natural breeding or other less invasive ARTs (artificial insemination, embryo transfer, or in vitro fertilization), with a correspondingly increased vulnerability during in utero development and the immediate postnatal period. It appears that rates of early embryonic death and incidence of abnormality correlate with the degree of manipulation involved in a particular ART (US FDA, 2008).
2.2.4 Growth, Maturation, and Aging
Relatively few studies have examined the health of livestock animal clones beyond the perinatal stage, through juvenile development, up to early reproductive life. Some studies in cattle have reported post-natal survival ratios of 38/44 (to 6 months; Chavatte-Palmer et al., 2004), 24/26 (to 1-4 years; Lanza et al., 2001), 82/90 (post-weaning to 2 years; Pace et al., 2002) and 20/21 (starting after 4 months up to 3 years; Heyman et al., 2007). Incidence of death loss due to diseases in SCNT cattle during the first 30 days after birth was higher compared to conventionally bred cattle; however SCNT cattle surviving more than 200 days after birth displayed similar disease-induced mortality (Watanabe and Nagai, 2009). The health status, growth, meat and milk production and reproductive performances were found similar SCNT cattle clones that survived to adulthood compared to conventionally bred cattle (Watanabe and Nagai, 2008). Panarace et al. (2007) reported that cloned cows and bulls exhibit normal fertility.
Polejaeva et al., (2013) report similar reproductive performance of cloned cows, in terms of ability to produce transferrable-quality embryos, compare to cows produced through conventional breeding practices.
Based on the limited number of animals studied to date, it appears that most physiologic abnormalities and death loss are observed during the perinatal period. In addition, cloned offspring that survive beyond the neonatal period are healthy with physiological parameters (e.g., growth rate, blood chemistry, fertility, etc.) within their acceptable ranges (Ortegon et al., 2007; US FDA 2008; Watanabe and Nagai, 2009).
Considerably less information is known about the long-term effects on health and fertility of SCNT animal clones, mainly because cloning in livestock animals is a relatively new technology, so few clones have reached the advanced stages of their normal life expectancy. Dolly, the first SCNT mammal clone, was euthanized at six years of age (approximately half the expected lifespan for its breed of sheep) after developing a respiratory disease. A post-mortem examination confirmed that the respiratory disease was associated with a Maedi-Visna virus infection, a retroviral-induced pulmonary neoplasm and pneumonia of sheep (ovine pulmonary adenocarcinoma, Jaagsiekte). A report from the Roslin Research Institute concluded that there was no reason to believe that the cloning procedure used on Dolly made her more vulnerable to ovine pulmonary adenocarcinoma than conventionally bred sheep, since the disease also affected the conventionally bred animals housed in the same barn (Rind et al., 2004). The first study to assess the long-term effect of SCNT in large mammals showed SCNT had no detrimental long-term effects in 13 cloned sheep aged between 7 and 9 years, including 4 clones derived from the mammary gland cell line that gave rise to Dolly (Sinclair et al., 2016).
All of the clinical abnormalities associated with SCNT animal cloning have been observed in natural breeding and other ART procedures, although at a higher frequency in the ARTs and even higher in SCNT technologies. Supported by the general trend of increasing efficiency in SCNT technologies that is being achieved and documented in the literature, as the cloning methods are further refined and animal health hazards are better identified, improvements are being made in the survival rates and overall health of animal clones. This improvement is similar to what has been observed with the development of other ARTs.
2.2.5 Progeny of Clones
Empirical data indicate that the progeny of animal clones do not seem to exhibit the animal health problems which have been observed in SCNT clones themselves. For example, in Wells et al., 2004), the offspring of cloned cattle exhibited normal survival rates. The progeny of Starbuck II (a Holstein bull clone which exhibited normal health parameters), were found to have normal phenotypic characteristics, behaviour, growth, hematologic and reproductive parameters, and overall health, compared to age-matched controls (Ortegon et al., 2007). A survey of 202 progeny of cloned cattle produced in Japan reported similar lifespans compared to conventionally bred cattle, as observed from Developmental node 2 (perinatal period), 3 (juvenile development), and 4 (reproductive period) during the first year of life after birth (Watanabe and Nagai, 2009). The health status, growth, reproduction, and the quality of milk and meat production were found to be similar in offspring of SCNT cattle compared to conventionally bred cattle (Wells et al., 2004; Watanabe and Nagai, 2008). Similar findings have been reported in studies on pig clones (Mir et al., 2005; Walker et al., 2007), sheep clones (Wells et al., 1998), and goat clones (Gauthier et al., 2001; Reggio et al., 2001). The production of normal progeny has been attributed to the fact that: 1) the subtle genetic imprinting errors that may exist in the animal clone are reset during the epigenetic reprogramming events of gametogenesis; and 2) only those animal clones without gross genetic defects survive and are reproductively fit.
2.2.6 Re-cloning or Serial Cloning
Kubota et al. (2004) were the first group to successfully re-clone a livestock animal when they produced two second-generation clones from a three-month-old bull clone. One bull calf died of anaemia shortly after birth. The other calf was reported to be alive and well at four years of age. This second generation bull was fertile and reportedly sired six healthy progeny by artificial insemination. There was no evidence of telomere shortening in these animals.
The serial cloning of pigs through three generations has been described (Cho et al., 2007; Kurome et al., 2008). In Cho et al., 2007, the authors reported that they produced five first-generation piglet clones, one of which had a dimorphic facial appearance with severe hypertelorism and a broad prominent nasal bridge. The piglet clone with the abnormal phenotype was used to produce second and then third generation piglet clones. The second and third generation serial clones did not express the abnormal phenotype. The authors concluded that the first generation clone's phenotypic abnormality was likely due to epigenetic dysregulation, which was not observed in the subsequent serial cloning. Kurome et al, (2008) report that the telomere lengths of cloned pigs from the first to third generations are normal.
Kim et al. (2017) report successful recloning of Snuppy, the first cloned dog. One reclone died of acute diarrhea shortly after birth, however when the report was written the three other recloned puppies were 9-month old and healthy. The serial cloning of mice has been achieved up to the 25th generation and the cloned mice exhibit normal health and life expectancy (Wakayama et al., 2013).
Taken together, although there is very little data available on re-cloning of domestic livestock, these preliminary studies indicate that serial cloning is technically possible. The re-cloned animals do not seem to exhibit any increased health problems. Further studies would be required to more conclusively evaluate the potential health effects associated with re-cloning.
From an environmental and animal health point of view, genetic diversity is essential for maintaining the genetic well-being of a breeding population. While it has been posited that SCNT cloning could be used to create a highly uniform population of animals for commercial production purposes, it will remain essential that the germinal breeding populations continue to retain sufficient genetic diversity. Therefore, while ARTs such as SCNT enhance the ability to increase prevalence of unique germ lines in a population, prudent breeding also requires the maintenance of sufficient genetic diversity for population genetic health and to enable ongoing genetic improvement.
2.2.7 Infectious Disease and Animal Cloning
There are three main potential routes for the introduction of pathogens during the SCNT procedure. These include contaminated donor somatic cells, oocytes and the system used to reconstruct embryos. Similar to IVF, another important source of contamination can be the laboratory environment and personnel who handle cell cultures (Bielanski 2014). Cells for SCNT are usually obtained either from the existing established cell lines or from live animals with desirable phenotypes. When somatic cells are harvested, health status of donors should be taken into consideration, since microorganisms can be present in blood and many tissues in acutely and persistently infected animals (reported in Bielanski, 2014). Similar to IVF, oocytes for SCNT recovered from slaughtered animals with unknown health status may be a potential source of infectious agents for cloned embryos (Bielanski 2014). In addition, the nuclear transfer procedure produces small openings on the zona pellucida, which serves as the embryo's protective outer covering, raising the concern that infectious agents could be introduced into the reconstructed embryo.
There are international guidelines for reducing the risk of disease and infection for embryo transfer technologies. These guidelines are published by the World Organization for Animal Health (OIE; http://oie.int), and were developed in cooperation with the International Embryo Transfer Society (IETS; http://www.iets.org). These guidelines indicate that the main factors to avoid disease transmission are to ensure that the donor of the nucleus, the recipient oocyte, and the surrogate dam are healthy (Terrestrial Animal Health Code 2023, Articles 4.11.5 & 4.11.6).
The risks of transmission of the bovine viral diarrhoea virus (BVDV), porcine reproductive and respiratory syndrome virus (PRRSV) and equine infectious anaemia virus (EIAV) via in vitro embryo production by SCNT have been extensively assessed. The data indicate that risks are reduced to negligible when appropriate precautions in line with the OIE and IETS guidelines are adopted in the SCNT embryo production and transfer (EFSA, 2012). The risk of transmission of other infectious agents by SCNT remains to be investigated (Bielanski 2014).
There is a theoretical risk of reactivation of endogenous retroviruses (ERV) (dormant viruses) via introduction of a foreign nucleus into an enucleated oocyte. However, to date there have not been any reports of such outcomes occurring during the cloning process. Experimentation with bovine clones revealed that ERV sequences were not transcribed and no RNA was detected in the blood of clones, donor animals or controls (reported in Bielanski 2014).
As described in 2.2.6, SCNT cloning adds to the range of ARTs that enable enhanced usage and thus prevalence of highly desired germ lines in a population. Nevertheless, it remains essential for ongoing genetic health of breeding populations and enabling genetic improvement, to preserve sufficient genetic diversity.
2.2.8 Implications for Animal Welfare
Animal welfare means "how an animal is coping with the conditions in which it lives" (Terrestrial Animal Health Code 2023). Stressors constantly pose challenges to animals' abilities to cope, whether they are living in the wild, raised domestically with limited intervention, or are intensively raised. Stressors can be physical, physiological, or psychological and can affect the development and functioning of animals at all stages of their lifecycle. The manipulations involved in ARTs have been observed to create additional stresses.
In Canada, oversight of the use of animals for research and testing to ensure good welfare practices is the responsibility of the Canadian Council on Animal Care (CCAC). The CCAC assesses and certifies institutional animal care programs. It is mandatory for all Canadian institutions conducting research on animals and receiving funding from the three main federal research granting organisations (CIHR, NSERC, SSHRC) to maintain a CCAC Certificate of GAP – Good Animal Practice. Similar requirements have been established by some provincial funders and charitable organisations. Private organisations also may choose to be CCAC certified. Given the potential animal welfare complications that can arise with SCNT cloning, it would be prudent for those organisations conducting SCNT research and testing to become CCAC certified.
Published reports indicate that while SCNT cloning does not cause new risks compared to other ARTs, there is evidence of a higher incidence of health and welfare issues. Particularly during the pre-natal and peri-natal periods (Cibelli et al., 2002; Panarace et al., 2007), SCNT cloning tends to increase the chance of problems including physiological abnormalities. However, SCNT cloning does not pose any new risks compared to other ARTs (IETS, 2008).
The ability of an animal to cope with natural stressors is critical for maintaining homeostasis of key system functions, including the endocrine and immune systems. The peri-natal period is a particularly critical time for managing additional animal health and welfare implications such as posed by ARTs. From an animal welfare point of view, a few factors can be considered—these include the health consequences for the animals involved in this process including the recipient of the embryos if midterm or late term abortion rate increase and the quality of life for the offspring's (large offspring syndrome, genetic abnormalities, overall health). To minimize and address potential implications for the health and welfare of the surrogate dam and neonates, the International Embryo Transfer Society (IETS) developed a consensus recommendation Health Assessment and Care for Animals Involved in the Cloning Process (IETS, 2008).
To address any potential animal welfare problems that could arise in relation to clones, it would be prudent for practitioners employing SCNT technology to be aware of the IETS recommendations. As well, for organisations in the livestock industry that may consider developing animal clones or purchasing such animals, industry awareness is recommended. In particular, it would be prudent for the national Codes of Practice for dairy cattle and other species (National Farm Animal Care Council) reference to the IETS recommendations as appropriate.
2.2.9 Summary for Animal Health
The technologies for producing SCNT animal clones are still in development and success rates remain low, including higher incidences of animal health and welfare problems than observed for naturally bred animals or for animals produced through other ARTs.
Perinatal mortality and morbidity rates tend to be higher in clones and surrogate dams than when animals are conceived naturally or even by other ARTs (Panarace et al., 2007). Higher rates of large offspring syndrome have been noted in cattle clones, and higher placental abnormalities observed in surrogate dams, than with naturally bred animals or other ARTs. Although the incidences of animal health and welfare problems are higher, no new issues or illnesses have been identified compared to other ARTs.
In most cases, embryonic or foetal mortality will naturally eliminate animals with defective genetics, independent from the reproductive method used to create them.
Published studies shows that most surviving animals produced by SCNT cloning are healthy and develop normally (Sinclair et al., 2016; Burgstaller and Brem, 2017; Kim et al., 2017). There is now widespread empirical evidence which shows that healthy cattle and swine clones have been produced using SCNT technologies.
Based on empirical findings, there are no known significant differences between the progeny from SCNT animal clones versus other sexually reproduced animals, in terms of their health status.
2.3 Food and Feed Safety Considerations
2.3.1 Food Safety Considerations
2.3.1.1 Introduction
The purpose of this chapter is to assess the safety of foods derived from animal clones and their progeny based on the information currently available. Food safety assessments of novel foods are typically based on assessing compositional, toxicological, nutritional, and allergenicity factors, sometimes in comparison with a conventional counterpartFootnote 3. One of the main sources of information which was used for this scientific opinion was the data presented in the United States of America Food and Drug Administration's (US FDA, 2008) risk assessment of animal cloning which covered the impact of SCNT animal cloning on animal health and food safety. In addition to the peer-reviewed literature referenced by the US FDA (2008), the document also contains data generated by industry and academia spanning from 2003 to 2007.
To identify food consumption risks associated with the use of SCNT, the US FDA developed a two-pronged approach, which included a Critical Biological Systems Approach (CBSA) and compositional analysis approach. The CBSA evaluates animal health data using the premise that if significant differences exist between SCNT clones and conventionally bred animals then these differences would be reflected in the health status of the animal clones. Regarding the compositional analysis approach, the US FDA suggested in their risk assessment that if food products from healthy animal clones and their progeny meet the local, state, and federal regulatory requirements set forth for those products, and are not materially different from products from conventionally bred animals, then they would pose no more food consumption risk(s) than corresponding products derived from conventionally bred animals.
The comparative approach suggested by the US FDA is in line with the approach used in Canada to assess foods and feeds derived from other new technologies, i.e., the food/feed is compared to its conventional counterpart to determine if there are any changes in the safety or nutritional quality of the food/feed. Data on the impact of SCNT technologies on the health of the animal clones and their progeny is also informative in the food safety context, and further adds to the weight of evidence. This will be further discussed in this document.
2.3.1.2 Compositional/Nutritional Data
The food safety assessment was focused mainly on Chapter VI: Food Consumption Risks of the US FDA risk assessment (US FDA, 2008). The US FDA has reported on several studies of composition of milk, beef and pork from clones, including one large study of five boar clones compared with 15 related control barrows, and 264 clone progeny derived from these clones compared with related control animals (US FDA, 2008). There is little to no data available on composition of meat and milk from sheep and goat clones, in addition to little or no data on older livestock animals.
The US FDA suggested, for the purpose of the risk assessment, that key nutrients or compositional parameters to be considered in the characterisation of food from clones should be nutrients which make a major or moderate contribution to the total daily diet of milk or meat consumers, and/or which are the results of complex biochemical pathways in the animal (e.g., saturated fats, vitamins). The examination of this data provides indirect support that the clones are not materially different from conventionally bred animals. The Canadian reviewers concur with the scientific basis for this comparative approach.
Based on the US Center for Disease Control's National Health and Nutrition Examination Survey of 2000-2001, the major and moderate nutrients suggested by the US FDA were as follows:
- For meat: Vitamin B12, Vitamin B6, niacin, riboflavin, zinc, phosphorus, iron, and selenium.
- For milk: Vitamins B12, B1, B2, B6, pantothenic acid, calcium, phosphorus, selenium, zinc, potassium, and magnesium.
Essential amino acids, fatty acids and proteins were also identified as key components in the group of analytes selected for a comparison. The US FDA also suggested that additional nutrients might be included as a useful tool to further demonstrate the similarity of the products from animal clones versus conventionally bred animals (US FDA, 2008). The Canadian reviewers concur with this approach; to begin with a broad list of components, then to extend the list of "key" components as emerging compositional data becomes available.
In its final risk assessment, the US FDA considered recently published compositional information for milk obtained from two peer-reviewed papers published in 2007 (Heyman et al., 2007 and Laible et al., 2007). These two studies further support the general conclusion reached in the US FDA's initial draft risk assessment, i.e., that there is no evidence to suggest that milk from clones differs in any nutritionally important way in composition from milk from conventionally bred animals, or presents any different food safety risk. Three additional studies (Yang et al., 2007; Shibata et al., 2006; Heyman et al., 2007) on carcass quality and meat composition for cattle and swine were also considered in the final risk assessment. These studies provided further evidence that meat from cattle and swine clones and the progeny of swine clones does not differ from meat obtained from conventionally bred animals.
In one of the milk composition studies (Heyman et al., 2007), fatty acid composition differed between clones and comparators. Specifically, stearic acid was lower, and C18:0 and C18:2-c9-t-11 were higher, in milk from clones. This difference was also identified in muscle biopsy samples from these clones. The US FDA assessment of the data noted that this difference may reflect a difference in lipid metabolism in this group of clones (i.e., higher delta-9 desaturase activity in clones), but also that in the other, more comprehensive study of nine clones versus five control cows (Laible et al., 2007), fatty acid composition was not found to be different in milk from clones versus the control animals. In the Laible et al. (2007) study, all of the suggested key nutrients identified in the US FDA draft assessment were analysed, and the authors concluded that milk from the nine clones was broadly similar to their breed-matched comparators, and generally within reference ranges for normal bovine milk. No biologically important or safety-relevant differences were noted when composition was compared to standard databases or contemporary comparator controls. Three other studies from New Zealand on composition of milk from clones did not show altered fatty acid composition. The US FDA assessment further suggested that additional studies with more genotypes would be needed to further explore the differences observed by Heyman et al. (2007). It is important to note that the composition of milk and meat obtained from conventionally-bred animals vary rather widely and can be influenced by external factors such as feed composition, disease status of the animal, and environmental conditions.
In the three additional studies of carcass and meat composition and quality, Yang et al. (2007) showed no differences in meat composition between 11 cattle clones and their comparators. They also reported that the variability in composition in meat from clones was not different from that reported for conventionally-bred comparators. Shibata et al. (2006) compared progeny of female swine clones with comparator progeny, and showed some differences in weight and length of certain cuts of pork, but no differences in back fat thickness, loin area, water content, drip loss or fat content. The authors noted that meat quality characteristics of the particular breed of swine were retained in the progeny of the clones. Heyman et al. (2007) showed a difference in fatty acid profile in muscle biopsy samples of clones versus conventionally bred animals, suggesting a difference in lipid metabolism. However, in a study by ViaGen (Walker et al., 2007), specifically planned to compare the levels of key nutrients in meat from swine clones and their progeny with comparators, the authors found "no biologically relevant differences" in food composition values in muscle tissue. Further, in a study 242 progeny of clones vs. 162 progeny of comparator boars, only 0.2% of the 14,036 data points collected for the progeny of clones were different from the controls, and only 2 values were greater that 10% different from the controls. This study clearly showed that pork from the progeny of clones is not different from that of conventionally bred animals (US FDA, 2008).
From the review of the available information on composition, there is no evidence to suggest that meat and milk products from clones differ in composition from those from conventionally bred animals, or present an altered food safety risk (US FDA, 2008). The possibility of altered lipid metabolism in some clone genotypes is noted (US FDA, 2008), but more data would be needed to further investigate this possibility.
The rationale for the conclusion that edible products from the progeny of clones pose no additional food consumption risks relative to the products of conventionally bred animals is well demonstrated (US FDA, 2008), and further supported by compositional analysis of foods from these animals. The direct and indirect evidence for the assumption that the progeny of clones are essentially indistinguishable from the progeny of conventionally bred animals is very well supported. This conclusion was also reached by EFSA (2008) in their review of the available data.
Watanabe and Nagai (2008) further support these conclusions by conducting a review of 65 reports generated in Japan from 2000 onward. Data was generated on 171 cattle clones and 32 progeny, and covered and food composition (meat and milk) and animal health issues. The data on food composition available in this review also indicated no significant difference between these animals, comparators and reference data.
On the basis of the data reviewed, it is possible to conclude that there are no biologically important differences in composition of foods from clones versus conventionally bred animals during their normal age for slaughter. Animal clones will most likely be used primarily as breeding stock, which are typically bred well past slaughter age. Given the lack of empirical data, it is not possible to arrive at any significant conclusions on the composition of foods derived from cattle and swine clones of advanced age.
2.3.1.3 Toxicity and Allergenicity
Another consideration is whether the use of SCNT gives rise to compounds of unknown toxicity or to new allergenic substances in foods derived from animal clones or their progeny. Food animals, unlike food crops, do not typically synthesize toxicants or anti-nutrients. It is therefore unlikely that any alteration in the epigenetic reprogramming of animal clones induced by the SCNT process would result in production of endogenous toxins or anti-nutrients. In addition, while changes to the metabolic pathways of organisms could result in over-expression of bio-active compounds, which could be harmful to humans, significant changes to the metabolic pathways of animal clones would also likely result in detectable changes to the health status of the animals. Consequently, the US FDA risk assessment focused on the health comparability (both physiological and compositional) of the animal clones or their progeny with their conventionally bred counterparts as the foundation for a safety assessment (US FDA, 2008). With respect to allergenicity, there is no reason to suspect that SCNT cloning will induce the synthesis of new proteins in apparently healthy and normal animals (US FDA, 2008), however, should the allergenic risk theoretically increase in food products from animal clones, two (2) hypotheses may be pursued to explain such an increase (US FDA, 2008): 1) an increase in the relative amount of an individual allergenic protein in milk or meat that might typically be present (e.g., casein) and/or 2) an altered processing of animal proteins in vivo that changes their antigenic presentation. It should be considered, however, that people who are allergic or sensitive to certain foods of animal origin should continue avoiding these foods, whether they were derived from an animal clone or not. There is therefore no data to support an increased allergic risk from foods derived from clones or progeny, versus those derived from conventionally-bred animals.
2.3.1.4 Microbiological safety
In addition to the implications of SCNT in relation to infectious diseases in the animal clone (in section 2.2.7), consideration was also given to the food as carrier of food borne pathogens. However, regarding the impact of the technologies on the microbiological safety of foods derived from SCNT animal clones and their progeny, there is no data that has been collected to date on the impact of SCNT on the microflora of these animals.
Meats of healthy animals, apart from exposed surfaces, are essentially free of microflora. Contamination by food borne pathogens is most often attributed to slaughter practices which transfer microflora from the hide, from the GI tract or from the slaughterhouse environment to the meat surfaces. Milk should also be quasi-sterile in healthy animals at secretion, but it is usually contaminated as it leaves the udder. In microbiological terms, contamination of foods of animal origin is inevitable, but the impact of the contamination can always be mitigated. The purpose of any food safety system is to mitigate and reduce these risks as close to zero as possible. Whether an animal is cloned or not it will be subject to the same slaughter and processing procedures which are designed to mitigate such contamination.
2.3.1.5 Chemical Residues
Consideration was given to the food as carrier of chemical residues, whether from feeding practices, environmental conditions or use of veterinary treatments. However, there is no data that has been collected to date on the impact of SCNT on the levels of chemical residues in the meat and milk of animal clones or of their progeny.
For the animal clones, the assessment conducted by EFSA (2008) noted that animal clones "...generally need more intensive care, especially in the early life stages of growth and development, the levels of veterinary medicinal products treatment are likely to be higher than those of their natural comparators, but no reliable data are available on comparative levels of veterinary drug residue levels...". It is important to note that levels of agricultural chemical and veterinary drugs residues must comply with applicable Canadian regulations, whether the milk and meats are from clones or not. These levels are set in a manner that is protective of human health and animal health.
2.3.2 Feed Safety Considerations
The purpose of this section is to address the impact of SCNT on livestock feed safety. Food safety considerations considered in Section 2.3.1 would also apply for feed safety, including the general assumption that the health of an animal can be an important indicator for feed safety. If an animal is unfit to enter the human food supply, it could represent safety concerns for livestock feed. Feed safety concerns may arise from the incorporation of the rendered products derived from animal clone tissues into livestock feeds, and the feeding of animal products, such as milk whey protein, derived from animal clones to livestock. Incorporation of unsafe products into livestock feed can have adverse effects on animal health and may potentially affect human health through its impact on food safety.
Given the low proportion of SCNT clones that develop into healthy adult animals at the present time, SCNT clone developers may wish to use rendering as a means of disposal of animal clones which are unhealthy or possess gross deformities making them unfit for breeding or food production purposes. However, Canadian Feed RegulationsFootnote 4 prohibit the use in livestock feed of proteins derived from food animals or fish that were not raised and/or slaughtered for human consumption as food. In many cases, SCNT animal clones of food-producing species would be considered research animals, and thus prohibited from being rendered into livestock feed. Animal clones that are unhealthy or possess gross deformities are prohibited from being rendered, under the Canadian regulations.
2.3.3 Linkage of Animal Health with Food/Feed Safety
In assessing novel foods and feeds using a comparative approach, it is important to consider a broad range of issues. For example, animal health is inextricably linked to the safety of foods of animal origin. Therefore, animal health is an indirect indicator adding to the weight of evidence for safety of food and feed products from SCNT animal clones and their progeny, based on the assumption that a healthy animal will yield safe food and feed.
In the data reviewed on composition of edible products, the assumption is that the products analyzed were derived from healthy animal clones and/or from healthy progeny from animal clones. In some articles, such as Heyman et al. (2007), the health status of the animals was clearly indicated, but in most reports on food composition, the health status was not directly stated or necessarily assessed. It should be noted, however, that all of the animals in these studies were raised using intensive management practices, going beyond the typical practices used in feedlots.
Although healthy animal clones can be produced using the SCNT process, there is a higher incidence of health problems associated with SCNT cloning technologies than with natural breeding and other ARTs. This higher incidence of health problems does not increase the confidence that foods derived from animal clones are as safe as their natural bred counterparts or as those produced through other ARTs. However, under the current regulations, any animals with signs of health problems or deformities would not be authorized for slaughter for human food and may not be authorized for rendering for livestock feed.
On the other hand, based on the empirical data reviewed, the progeny of animal clones do not display a higher incidence of health problems than naturally bred animals or those produced through other ARTs. This fact adds to the confidence that foods derived from the progeny of animal clones are as safe as foods derived from naturally bred animals and those produced through other ARTs. All progeny of clones would be also subject to the current Canadian regulations for slaughter and rendering.
2.3.4 Summary for Food/Feed Safety
Available data indicates that there are no biologically significant differences in the composition of foods derived from healthy SCNT cattle and swine clones versus food from healthy animals produced through natural breeding and other ARTs.
Under current Canadian regulations, animals must pass health inspections prior to entering the food/feed chain. These conditions would apply equally to animals produced through conventional breeding, as well as through SCNT technologies.
Most animal clones will be used primarily as breeding stock and their disposal into the food and feed chain will occur later in the life of the animal, long past the normal slaughter age. There is a lack of empirical data on animal health and food and feed safety for livestock clones of advanced age, although, unhealthy animals would be excluded from the food chain and may be excluded from the feed chain.
Based on empirical findings reviewed, there are no known significant differences between the progeny from SCNT animal clones versus other sexually reproduced animals, in terms of the composition of their derived edible products.
2.4 Impacts on the Canadian Environment
2.4.1 Environmental Impacts
The purpose of this section is to review and identify any potential environmental and indirect human health concerns regarding the release of live SCNT cattle and swine clones under CEPA 1999 and the NSNR(O). Published information on the potential risks associated with SCNT food animal cloning, including the US FDA's risk assessment (US FDA, 2008), the EFSA scientific opinion (EFSA, 2008), and available scientific literature have focused on animal health and food safety concerns. In general, data on the environmental impacts of SCNT animal clones is lacking. EFSA (2008) concluded that, based on available knowledge, it is not expected that SCNT clones or their progeny would pose any new or additional environmental risks compared to naturally bred animals or those produced through other ARTs. There is also no information available to suggest that such risk may exist (EFSA, 2012). No new elements have emerged that would change the previous EFSA Opinion on the possible environmental impact of cloning: i.e., cloning of farm animals in itself poses no particular threats for genetic diversity or biodiversity. There are no indications from the limited data available, that would suggest new or additional environmental risks from farmed animal clones when compared to conventionally bred farmed animals. There is also no new information available to suggest that such risk may exist.
In the event of an unmanaged release of cattle or swine SCNT clones into the environment in Canada, the potential impacts of possible interactions with wild relatives (bison, wild boars) and with other organisms, including humans, is expected to be the same as for their conventionally bred counterparts. There is no information available to suggest that there might be differences in behavioural, reproductive, or health characteristics due to cloning which may have ecological implications when SCNT animal clones are released into the wider environment.
Savage et al. (2003) reported no changes in behavioural characteristics with aging between cattle clones and their age-matched controls: the cattle clones were similar to their counterparts of comparable chronological age for essentially all the assessed behaviour traits.
To date, there is no evidence to suggest that, when compared to their conventionally produced counterparts, SCNT animal clones and their sexually produced progeny would have any greater impact on the environment due to any changes induced by the use of SCNT technologies. It is expected that SCNT cloned livestock animals will exhibit the same range of trait variation as that seen for naturally existing species.
2.4.2 Considerations for other species (other than livestock)
2.4.2.1 Pets and prize winning animals
The impact of cloned pets and prize winning animals into the Canadian environment is expected to be low given that they are expected to mainly be kept in private care, thus greatly reducing the likelihood of release into the environment. In addition, cloned pets would likely originate from species that have well-established populations in Canada and thus no new species would be introduced into the environment.
2.4.2.2 Wildlife species
It is still difficult to adapt the technology to wildlife species; rapid gain in the understanding of the molecular clues underlying nuclear reprogramming using gene editing at the gene and whole genome levels, will help accelerate successful cloning for wildlife conservation. There is currently no available information on the impact of SCNT restored wildlife species on the environment.
2.4.3 Indirect Human Health Impacts
Based on experiences and assessments from other jurisdictions (Niemann and Lucas-Hahn, 2012; Taylor-Robinson et al., 2014; Hur, 2017), the most common sources of human exposure to cloned animals are anticipated to be from agronomical applications mainly involving cattle, pigs and sheep. Most human exposure to live cattle and swine in Canada would be expected to occur through direct contact from farm visits, agricultural fairs, and petting zoos (Conrad et al., 2017). There is generally a lack of data on numbers and trends in the extent of livestock animal cloning in Canada, although there are likely some animals that are being generated for research purposes (Bordignon et al., 2013). However, with a success rate of less than 10% of reconstructed embryos resulting in live animals (Cibelli et al., 2002; Paranace et al., 2007; US FDA, 2008), the likelihood of human exposure to SCNT livestock animal clones is considered to be very low. For non-agricultural purposes, there are the beginnings of a trend towards importing into Canada SCNT cloned animals as pets from the U.S. or other countries for personal use (See section 1.4.2; CBC, 2017). Should this trend continue in the future, it will likely increase the potential for human exposure to SCNT pet clones in Canada.
Since this opinion covers only non-genetically modified SCNT clones, there are no new proteins to be introduced. Because farm animals such as poultry and ruminants are reservoirs of zoonotic pathogens (Conrad et al., 2017), one common human health hazard associated with handling of animal clones is in relation to transmission of diseases from animals to humans. Considering that there are no differences between cloned and non-cloned animals with regard to the composition of meat and milk (US FDA, 2008; EFSA, 2012; Hur, 2017) and in blood chemistry (Kim et al., 2013), it is not expected that the potential for allergenicity and toxicity in SCNT clones will be significantly different from conventionally produced animals. According to Chavatte-Palmer et al. (2009), an extensive survey in cloned heifers of leukocyte subsets and the humoral and T-cell immune responses to exogenous antigens revealed a normal representation of leukocyte subsets with no modifications to functional immunity. This extensive analysis suggests that cloned cattle have normal immune function and hence the risk of transmitting diseases or suffering from other health issues would be no different from their non-cloned counterparts. In the absence of a clear indication of potential hazards associated with SCNT cattle and swine clones, they are unlikely to possess more indirect hazards to human health compared to conventionally bred counterparts. The same can likely be concluded for SCNT clones produced for non-agricultural purposes (eg., as pets), although this is based on very limited experiences with these animals in the U.S. and Canada.
2.4.4 Summary for Environmental and Indirect Human Health Impacts
Based on the available scientific information, there is no indication that healthy cattle and swine clones would interact with the Canadian environment any differently than healthy cattle or swine produced through conventional breeding.
In addition, it is not expected that the progeny of SCNT cattle and swine clones would interact with the Canadian environment any differently than other sexually produced animals.
Considering the expected low likelihood of human exposure to SCNT clones in Canada and the fact that there is no difference in hazard potential compared to conventionally bred cattle and swine, there is no evidence to indicate the potential for an indirect risk to human health from the import or manufacture of SCNT cattle and swine clones in Canada. This was supported by a formal risk assessment conducted in 2020 (NSN-19594) that concluded SCNT Holstein cattle and their progeny do not pose an increased risk for zoonotic potential, allergenicity, or other adverse human health effects compared to conventionally bred cattle.
Although there is limited information on of livestock SCNT clones impact on environment, it is expected that they exhibit the same variation in traits that exist in their original natural counterparts and thus likely pose no additional impact on the environment.
In the case of SCNT clones produced for non-agricultural purposed (eg., as pets), many of the same considerations apply and the same conclusions can be drawn as those for SCNT livestock animals, although this is currently based on very limited experiences in the U.S. and Canada with animals for these purposes.
Section 3: Lifecycle of clones and progeny
3.1 Lifecycle analysis
In addition to the empirical evidence reviewed in the previous sections, consideration was also given to the theoretical basis of the development cycle of clones and progeny. The mechanisms responsible for the apparent higher incidence of health problems in SCNT animal clones have not been precisely determined. These problems might arise from the nuclear transfer process itself, nuclear reprogramming, embryo culture conditions, embryo transfer methods, or a combination of some or all of the above. SCNT cloning has been observed to affect prenatal and perinatal viability. On the other hand, cell and foetal death losses are indicators that there are natural control points during the embryonic and foetal development stages, which effectively act as filters to prevent the continuance of faulty genomes or epigenetic reprogramming errors. Eight such primary natural control points are characterized in Figure 3.
These control points exist, whether reproduction is through natural breeding, SCNT or other ARTs. In the event that any major problems were to arise at any one of these natural control points, it would be unlikely that the foetus would survive to term.
The first three natural control points (Figure 3) are carried out in vitro when performing SCNT technologies. In order to increase experimental success rates and, therefore, the health status of the resultant animal clones, it is essential that high standards for laboratory and experimental techniques are followed. The principles of Good Laboratory Practices (GLP), as a means of standardisation, quality control and quality assurance, have been used in other areas so as to provide a basis to promote consistency and reliability of results. The Organisation for Economic Co-operation and Development Principles of GLP outlines the basis of GLP and various national regulations require that scientific data be gathered under these principles.
Good Laboratory Practice (GLP) embodies a set of principles that provides a framework within which laboratory studies are planned, performed, monitored, recorded, reported and archived. These studies are undertaken to generate data by which the hazards and risks to users, consumers and third parties, including the environment, can be assessed for pharmaceuticals, agrochemicals, cosmetics, food and feed additives and contaminants, novel foods and biocides. GLP helps assure regulatory authorities that the data submitted are a true reflection of the results obtained during the study and can therefore be relied upon when making risk/safety assessments.
The first and second control points encompass donor cell and oocyte sourcing. Factors influencing the first control point include: donor health status and age; tissue source; differentiation stage of the cell; and the tissue culture environment. Factors influencing the second control point include: oocyte donor health status; physical trauma; and breaching of the zona pellucida.
The third control point encompasses the act of fusion of the donor cell and oocyte to form the reconstructed embryo and includes the early stages of embryonic development. Failures at this stage could be due to: manipulation of the donor cell and oocyte; previous damage to the oocyte by enucleation; and epigenetic defects in the early embryo (at control points 3 and 4). At the nuclear level, the resulting epigenetic defects may manifest in the form of altered chromatin remodelling or aberrant methylation patterns and imprinting (reviewed in Niemann et al., 2008). If any of these factors lead to severe enough defects, cell death will occur.
If the embryo survives to the embryo transfer stage (natural control point 4), the next challenge is implantation of the embryo in the uterus of the surrogate dam. If the embryo fails to implant properly, resorption of the embryo will occur.
At natural control point 5, the implanted embryo survives and reaches a stage where there is formation and migration of Primordial Germ Cells (PGCs) to the genital ridge in the early embryo. At this point, the genome of these PGCs undergoes further reprogramming. Before this event, the epigenetic marks of these cells would be similar to those of other epiblast cells from which PGCs are derived, which includes any epigenetic alterations incurred by the SCNT process. Many of these epigenetic marks are erased about the time PGCs arrive at the genital ridges. There are some parts of the genome which are relatively resistant to the demethylation process; therefore, it is impossible to rule out some epigenetic markings being retained and passed down to the progeny. However, since most epigenetic markings, including defects, are erased, this process effectively acts as a first biological filter of epigenetic defects for the progeny of clones.
Figure 3 - Text description
Natural control points in the development cycle of SCNT clones and progeny, with non-exhaustive examples of factors influencing success at each step.
- Step 1 box. Donor cell sources (harvest, storage, preparation):
- Organ or tissue source
- Age of donor animal
- Differentiation stage of the cell
- Mitotic stage of the cell
- Tissue culture environment
- Step 2 box. Oocyte sources (harvest, enucleation):
- Origin of oocyte
- Trauma due to enucleation, including staining, removal/damage of key structural and functional proteins through cytoplast removal
- Breaching of zona pellucida
- Damage caused by handling (e.g., cumulus cell removal)
Step 1 and step 2 boxes have arrows pointing to:
- Step 3 box. Fusion of donor cell and oocyte (nuclear transfer) and early embryo division to blastocyst:
- Impact of fusion (electrical or chemical)
- Incomplete or inappropriate epigenetic reprogramming in the early embryo
- Embryo culture and environmental conditions
- Mitotic anomalies due to structural damage to oocyte architecture (e.g., leading to aneuploidy)
Step 3 box has an arrow pointing to a box, to the right, indicating, Embryonic mortality, and another arrow pointing down to:
- Step 4 box. Embryo survives, transferred in surrogate:
- Implantation problems
Step 4 box has an arrow pointing to a box, to the right, indicating, Resorption, and another arrow pointing down to:
- Step 5 box. Implanted embryo survives and reaches stage where there is formation of Primordial Germ Cells (PGCs) in the early embryo:
- Germline reprogramming re-sets and re-establishes many imprinting marks (FILTER 1)
Step 5 box has an arrow pointing to a box, to the right, indicating, Abortion, and another arrow pointing down to:
- Step 6 box. Fetal development to term and parturition
Step 6 box has arrows pointing to 2 boxes, to the right, indicating, Perinatal death and Morbidity, Morbidity is pointing towards Perinatal death and another box, Premature death. Step 6 box is also pointing down to:
- Step 7 box. Birth of animal clone, and reaches reproductive maturity:
- Meiosis and differentiation of germ cells into mature gametes
- Meiosis introduces genetic variety in gametes through homologous recombination
A bidirectional arrow indicates a connection between the Step 7 box and the Morbidity.
Step 7 box has arrows pointing to 2 boxes, to the right, indicating, Premature death and Healthy animal clone.
The Perinatal death and Premature death boxes both point to another box, Disposal.
The Healthy animal clone box points to another box, Intended use in Breeding. The Intended use in breeding box also points up the Disposal box.
Step 7 box is also pointing down to:
- Step 8 box. Sexual reproduction leading to progeny:
- One gamete from a clone and one gamete from a conventionally-bred animal OR
- One gamete from a clone and one gamete from another clone:
- Zygotic reprogramming occurs (FILTER 2)
Step 8 box has a arrow pointing to a box, Healthy animal. The Healthy animal box has an arrow pointing to another box, with Direct: Human food, Indirect: Livestock feed.
The Intended use in breeding box also points to the box with Direct: Human food, Indirect: Livestock feed.
Additional information:
- Events in Steps 3 and 4 may occur at the nuclear level. Their severity may influence the outcome of the embryo surviving or not
- Altered chromatin remodelling
- Aberrant methylation patterns
- Aberrant imprinting
- Steps 5-8 are important natural control points where potential mutations and epigenetic effects may be repaired or where broad genetic change can be achieved through meiosis and sexual reproduction.
Figure 3: Natural control points in the development cycle of SCNT clones and progeny, with non-exhaustive examples of factors influencing success at each step. There is possibility of failure at each step, in which case the animal clone will never be born and will not enter the food and feed chain or the environment. Steps 5-8 are important natural control points where potential mutations and epigenetic effects may be repaired or where broad genetic change can be achieved through meiosis and sexual reproduction.
As foetal development continues (control point 6), and once the animal is born (control point 7) any of the defects caused by the SCNT process could still lead to perinatal death or premature death. Studies have shown (US FDA, 2008) that once a clone passes the critical perinatal stage, its chance of survival increases significantly. Then, as it reaches reproductive maturity, meiosis, which is usually arrested in early stages of PGC formation, proceeds and the cells continue to differentiate into gametes. During meiosis I, chromosomal crossover occurs so that homologous chromosomes randomly exchange segments of genetic information over regions of homology. One important purpose of meiosis is to produce genetic variety in gametes, and ultimately, the progeny. The final control point (8) and major biological filter, is sexual reproduction leading to progeny, wherein, one gamete from a clone and one gamete from a conventionally bred animal or from another clone are combined to create a zygote which will eventually become the progeny. Normal zygotic reprogramming also occurs, further erasing and/or changing epigenetic markings (and defects). Thus, epigenetic defects leading to the possibility of biological variants in animal clones are not only reduced leading up to gametogenesis, but also again at time of formation of the zygote which will give rise to progeny. Empirical evidence (Shimozawa et al,. 2002; Tamashiro et al., 2003) demonstrating that phenotypic anomalies observed in mouse SCNT clones were not transmitted to their progeny, illustrates the effectiveness of the multiple biological filters or natural control points. Others have observed that neither progeny of animal clones nor foods derived from them differ phenotypically from conventionally bred animals (swine: Mir et al. 2005, Shibata et al., 2006, Yamaguchi et al., 2007 and cattle: Kasai et al., 2007, Ortegon et al., 2007). Even if the transmission of epigenetic changes to progeny cannot be completely ruled out, based on the literature available to date, there have been no reports that indicate that the resulting phenotypes would impact the health of the progeny or the safety of foods and feeds derived from them.
3.2 Summary for Lifecycle analysis
There are eight natural control points during embryonic and foetal development stages, which effectively act as filters to prevent the continuance of faulty genomes or epigenetic reprogramming errors.
These control points exist, whether reproduction is through natural breeding, SCNT or other ARTs. In the event that any major problems were to arise at any one of these natural control points, it would be unlikely that the foetus would survive to term.
The natural control points in sexual reproduction decrease the likelihood that any anomalies encountered in SCNT animal clones are passed on to their progeny. Therefore, conventionally bred progeny of clones do not represent the same risk profile as clones, due to the following:
- progeny of clones pass through additional natural control points or biological filters, resulting in reprogramming of the genome;
- during the life cycle of clones leading up to gametogenesis, several additional control points contribute to reduce the occurrence of improper reprogramming; and
- empirical evidence shows that progeny of clones do not exhibit health problems at a higher rate than animals produced through conventional breeding.
Section 4: Summary of findings for cattle and swine somatic cell nuclear transfer (SCNT) clones and their progeny
4.1 Cattle and swine SCNT clones
There is empirical evidence which shows that healthy cattle and swine clones have been produced using SCNT technologies.
The technologies for producing SCNT animal clones are still in development and success rates/efficiency remain low; there is an observed higher incidences of animal health and welfare problems are observed in SCNT animal clones compared to naturally bred animals or animals produced through other ARTs.
The incidences of animal health and welfare problems are higher, partially due to: 1) the technical challenges often results in relatively lower survival rates; and 2) some animals that appear healthy at birth may still have latent health issues and may therefore lead to a higher prevalence of health problems. Apart from these considerations, no new ecological, food or latent feed safety, or indirect human health issues have been identified.
Available data indicates that there are no biologically significant differences in the composition of foods derived from healthy SCNT cattle and swine clones versus food from healthy animals produced through natural breeding and other ARTs.
Under current Canadian regulations, animals must pass health inspections prior to entering the food/feed chain. These conditions would apply equally to animals produced through conventional breeding, as well as through SCNT technologies.
Most animal clones will be used primarily as breeding stock and their disposal into the food and feed chain will occur later in the life of the animal, long past the normal slaughter age. There is little empirical data on animal health and food and feed safety for livestock clones of advanced age, although, unhealthy animals would be excluded from the food/feed chain.
Based on the available scientific information, there is no indication that healthy cattle and swine clones would interact with the Canadian environment any differently than healthy cattle or swine produced through conventional breeding. The same conclusions can reasonably be drawn for SCNT clones used for non-agricultural purposes (e.g., pets), although in both cases, this is based on very limited data and information.
4.2 Progeny of cattle and swine SCNT clones
The natural control points in sexual reproduction decrease the likelihood that any anomalies encountered in SCNT animal clones are passed on to their progeny.
In addition, based on the empirical findings reviewed, there are no known significant differences between the progeny from SCNT animal clones versus other sexually reproduced animals, in terms of their health status or the composition of their derived edible products.
Based on the available scientific information, it is not expected that the progeny of SCNT cattle and swine clones would interact with the Canadian environment any differently than other sexually produced animals.
4.3 Conclusion
Healthy cloned animals, their progeny and derived products are no different from other sexually reproduced animals and no new characteristics have been observed in these animals in Canada. Generally, the primary usage of SCNT cloning is to enhance the propagation of unique, high value animals. Consequently, based on the available scientific information, the apparent impact of SCNT technologies in healthy cattle and swine populations in Canada is not expected to be different from those used in conventionally bred animals with respect to food and feed safety, animal health, and impact on the environment – including impacts on biodiversity - and indirect human health effects.
SCNT cattle and swine clones themselves and their derived-products exhibit the same variation in traits that exist naturally in the livestock species even though there are higher incidences of birth defects/malformations, and pregnancy related complications in the clones themselves compared to their conventionally-bred counterparts. These latter issues do not appear to be present in the progeny of SCNT cattle and swine clones.
Despite the fact that there is little empirical data on animal health and food and feed safety for livestock clones of advanced age, nor on species other than cattle and swine, any new data based on these factors are not expected to raise any new concerns for SCNT cattle and swine clones.
As indicated in the disclaimer text at the beginning of this document, the conclusion of this scientific opinion may serve as the basis for updating existing and/or developing new science-based policies related to cattle and swine SCNT clones, their progeny, their products, and by-products in Canada. All existing policies on these animals and their products will continue to apply until such a time as each federal Department or Agency responsible for these individual policies has updated them.
Section 5: Glossary and list of acronyms
5.1 Glossary
- Assisted reproductive technologies (ARTs)
- A group of therapies that employ manipulation of the egg and/or sperm and/or early conceptus in order to establish a pregnancy.
- Chromatin
- The complex of DNA, RNA and proteins that makes up the chromosomes.
- Chromosome
- Any strands composed of chromatin found in the nucleus of cells, which carry the coded information for heredity.
- Clone
- Commonly defined as a copy of a gene, cell or organism.
- Cloning
- A general term used for the process of copying a biological entity (a gene or cell or organism).
- Conventional breeding
- includes natural breeding and other ARTs, but excludes SCNT cloning.
- Differentiation
- Process in the development of a multicellular organism by which cells become specialized for particular functions.
- DNA Methylation
- See Methylation.
- Enucleated egg (or enucleated oocyte)
- An egg cell from which the nucleus has been removed.
- Epigenetic effect
- A term referring to the non-genetic causes of a phenotype; the best studied epigenetic modification is DNA methylation.
- Gamete
- A reproductive cell (i.e., sperm or egg).
- Gametogenesis
- The formation of gametes.
- Genetic effect
- A term referring to the effect of (heritable) genes on phenotype.
- Genome (of an animal)
- the totality of the hereditary information encoded in the DNA (nuclear and mitochondrial), including both the genes and the non-coding sequences.
- Genomic Imprinting
- Gene expression resulting from the parent-specific expression or repression of genes or chromosomes in offspring.
- Genotype
- the genetic constitution of a cell, an organism, or an individual (i.e., the specific allele makeup of the individual) usually with reference to a specific character under consideration.
- Germline cells
- Precursor cells that will give rise to gametes.
- Germplasm
- The genetic material that forms the physical basis of heredity.
- Hazard
- Capability of causing harm.
- In vitro
- Describing a process occurring outside of a living organism.
- Methylation
- A biochemical process involving the addition of chemical tags called methyl groups to DNA. Methylation can be a signal for a gene or a section of a chromosome to turn off gene expression and become inactive or "silent".
- Mitochondria (singular, mitochondrion)
- A cellular structure containing DNA, which provides energy to the cell.
- Mitosis
- The process where a single cell divides resulting in generally two identical cells, each containing the same number of chromosomes and genetic content as that of the original cell.
- Meiosis
- The process of germ cell formation in sexually reproducing organisms by which two consecutive nuclear divisions (meiosis I and meiosis II) occur without the chromosomal replication in between, leading to the production of four haploid gametes, each containing one of every pair of homologous chromosomes (that is, with the maternal and paternal chromosomes being distributed randomly between the cells).
- Natural breeding
- excludes all intervention through ARTs and/or cloning technologies.
- Nuclear Reprogramming
- Restoring the totipotency of a cloned cell.
- Nuclear Transfer
- The incorporation of the nucleus of a cell from the donor animal into an enucleated egg.
- Nucleus (plural, nuclei)
- A dense membrane-bound cellular structure containing DNA organized in chromosomes.
- Oocyte
- The egg cell.
- Perinatal
- The time just before, during and immediately after birth.
- Phenotype
- The observable traits of an organism. The phenotype results from the combination of genetic, epigenetic and environmental factors.
- Senescence
- A physiological aging process that limits the lifespan of cells and prevents unlimited cell proliferation.
- Somatic cell
- Any cell of a multicellular organism other than a germline cell.
- Somatic cell nuclear transfer (SCNT)
- the transfer of the nucleus of a somatic cell into an enucleated egg.
- Telomeres
- Repetitive sequences at the ends of chromosomes, which protect the ends of the chromosome from damage, facilitate the replication of linear chromosomes, and prevent the chromosomes from fusing into rings or binding to other DNA in the cell nucleus.
- Totipotent
- Having the potential of forming all the types of cells of the organism.
- Transcription
- The process of synthesizing RNA molecules from DNA.
- Transgenic
- An organism which has been altered by the introduction of recombinant DNA in order to stably integrate a foreign gene (a transgene) in its genome.
- Zygote
- The fertilized egg.
5.2 List of acronyms
- AFSSA
- Agence Française de Sécurité Sanitaire des Aliments
- ART
- Assisted Reproductive Technology
- CBSA
- Critical Biological Systems Approach
- CEPA
- Canadian Environmental Protection Act
- CFIA
- Canadian Food Inspection Agency
- ECCC
- Environment and Climate Change Canada
- EFSA
- European Food Safety Authority
- ERV
- Endogenous Retrovirus
- GLP
- Good Laboratory Practice
- HC
- Health Canada
- IETS
- International Embryo Transfer Society
- NSNR(O)
- New Substances Notification Regulations (Organisms)
- OIE
- World Organisation for Animal Health
- PGC
- Primordial Germ Cell
- SCNT
- Somatic Cell Nuclear Transfer
- US FDA
- United States Food and Drug Administration
Section 6: References
- AFSSA. Risks and benefits related to livestock cloning applications. Agence Française de Sécurité Sanitaire des Aliments. 2005; 1-54.
- Alberio R, Campbell KH. Epigenetics and nuclear transfer. Lancet. 2003; 361:1239-1240.
- Alexander B, Coppola G, Perrault SD, Peura TT, Betts DH, King WA. Telomere length status of somatic cell sheep clones and their offspring. Mol Reprod Dev. 2007;74(12):1525-1537.
- Baguisi A, Behboodi E, Melican DT, Pollock JS, Destrempes MM, Cammuso C, Williams JL, Nims SD, Porter CA, Midura P, Palacios MJ, Ayres SL, Denniston RS, Hayes ML, Ziomek CA, Meade HM, Godke RA, Gavin WG, Overström EW, Echelard Y. Production of goats by somatic cell nuclear transfer. Nat. Biotechnol. 1999; 17:456-461.
- Blelloch R, Wang Z, Meissner A, Pollard S, Smith A, Jaenisch R. Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells. 2006 Sep;24(9):2007-13. Epub 2006 May 18. PubMed PMID: 16709876; PubMed Central PMCID:PMC3000431.
- Berg DK, Li C, Asher G, Wells DN, Oback B. Red deer cloned from antler stem cells and their differentiated progeny. Biology of Reproduction 2007; 77(3): 384–394. https://doi.org/10.1095/biolreprod.106.058172
- Betthauser J, Forsberg E, Augenstein M, Childs L, Eilertsen K, Enos J, Forsythe T, Golueke P, Jurgella G, Koppang R, Lesmeister T, Mallon K, Mell G, Misica P, Pace M, Pfister-Genskow M, Strelchenko N, Voelker G, Watt S, Thompson S, Bishop M. Production of cloned pigs from in vitro systems. Nat. Biotechnol. 2000; 18:1055-1059.
- Betts DH, Perrault SD, Petrik J, Lin L, Favetta LA, Keefer CL, King WA. Telomere length analysis in goat clones and their offspring. Mol. Reprod. Dev. 2005; 72:461-470.
- Bielanski A. (2014) Biosafety in Embryos and Semen Cryopreservation, Storage, Management and Transport. In: Holt W., Brown J., Comizzoli P. (eds) Reproductive Sciences in Animal Conservation. Advances in Experimental Medicine and Biology, vol 753. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0820-2_17
- Blasco, MA. The epigenetic regulation of mammalian telomeres. Nat. Rev. Gen. 2007; 8:299-309.
- Bordignon V, El-Beirouthi N, Gasperin BG, Albornoz MS, Martinez-Diaz MA, Schneider C, Agellon, LB. (2013). Production of cloned pigs with targeted attenuation of gene expression. Plos one, 8(5), e64613.
- Burgstaller JP, Brem G. Aging of Cloned Animals: A Mini-Review Gerontology 2017;63:417–425. doi: 10.1159/000452444
- Burgstaller JP, Schinogl P, Dinnyes A, Müller M, Steinborn R. Mitochondrial DNA heteroplasmy in ovine fetuses and sheep cloned by somatic cell nuclear transfer. BMC Dev Biol. 2007; 21: 7-141.
- Campbell KHS, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996; 380:64-66.
- CBC News Website (2017). Newmarket man clones beloved dog Woofie in a Canadian first that cost him $90K. URL: https://www.cbc.ca/news/canada/toronto/newmarket-man-clones-beloved-dog-woofie-in-a-canadian-first-that-cost-him-90k-1.4105694 (accessed 19th September 2018.
- Centner TJ, Petetin L. Divergent approaches regulating beta agonists and cloning of animals for food: USA and European Union. Society and Animals. 2020;28(5).
- Chavatte-Palmer, Pascale, M, Heyman Y, Richard C, Urien C, Renard JP, Schwartz-Cornil I. The immune status of bovine somatic clones. Cloning Stem Cells. 2009 Jun;11(2):309-18. doi: 10.1089/clo.2008.0080. PubMed PMID: 19508113.
- Chavatte-Palmer P, Remy D, Cordonnier N, Richard C, Issenman H, Laigre P, Heyman Y, Mialot JP. Health status of cloned cattle at different ages. Cloning Stem Cells. 2004; 6:94-100.
- Chesné P, Adenot PG, Viglietta C, Baratte M, Boulanger L, Renard JP. Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat. Biotechnol. 2002; 20:366-69.
- Cho SK, Kim JH, Park JY, Choi YJ, Bang JI, Hwang KC, Cho EJ, Sohn SH, Uhm SJ, Koo DB, Lee KK, Kim T, Kim JH. Serial cloning of pigs by somatic cell nuclear transfer: Restoration of phenotypic normality during serial cloning. Dev. Dynam. 2007; 236:3369-3382.
- Cibelli JB, Campbell KH, Seidel GE, West MD, Lanza RP. The health profile of cloned animals. Nat. Biotechnol. 2002; 20:13-14.
- Conrad CC, Stanford K, Narvaez-Bravo C, Callaway T, McAllister T. (2017). Farm fairs and petting zoos: A review of animal contact as a source of zoonotic enteric disease. Foodborne pathogens and disease, 14(2), 59-73.
- Couldrey C, Wells DN, Lee RS. DNA methylation patterns are appropriately established in the sperm of bulls generated by somatic cell nuclear transfer. Cell Reprogram. 2011 Apr;13(2):171-7. doi: 10.1089/cell.2010.0065. PubMed PMID: 21473693
- Curtasu MV, Knudsen KEB, Callesen H, Purup S, Stagsted J, Hedemann MS. Obesity development in a miniature Yucatan pig model: A multi-compartmental metabolomics study on cloned and normal pigs fed restricted or ad libitum high-energy diets. Journal of Proteome Research. 2019;18(1).
- Dang-Nguyen TQ, Haraguchi S, Akagi S, Somfai T, Kaneda M, Watanabe S, Kikuchi K, Tajima A, Nagai T. Telomere elongation during morula-to-blastocyst transition in cloned porcine embryos. Cell Reprogram. 2012 Dec;14(6):514-9. doi: 10.1089/cell.2012.0045. PubMed PMID: 23194454.
- Das ZC, Gupta MK, Uhm SJ, Lee HT. Increasing histone acetylation of cloned embryos, but not donor cells, by sodium butyrate improves their in vitro development in pigs. Cell Reprogram. 2010 Feb;12(1):95-104. doi: 10.1089/cell.2009.0068. PubMed PMID: 20132017.
- Edwards JL, Schrick FN, McCracken MD, van Amstel SR, Hopkins FM, Welborn MG, Davies CJ. Cloning adult farm animals: a review of the possibilities and problems associated with somatic cell nuclear transfer. Am. J. Reprod. Immunol. 2003; 50:113-123.
- Enright BP, Kubota C, Yang X, Tian XC. Epigenetic characteristics and development of embryos cloned from donor cells treated by trichostatin A or 5-aza-2'-deoxycytidine. Biol Reprod. 2003 Sep;69(3):896-901. Epub 2003 May 14. PubMed PMID: 12748129.
- EFSA. Draft Scientific Opinion on Food Safety, Animal Health and Welfare and Environmental Impact of Animals [cattle and pigs] derived from Cloning by Somatic Cell Nucleus Transfer (SCNT) and their Offspring and Products Obtained from those Animals. European Food Safety Authority. 2008; 1-47.
- EFSA (European Food safety Authority) Update on the state of play of Animal Health and Welfare and Environmental Impact of Animals derived from SCNT Cloning and their Offspring, and Food Safety of Products Obtained from those Animals. EFSA Journal 2012; 10(7): 2794-2835.
- Evans MJ, Gurer C, Loike JD, Wilmut I, Schnieke AE, Schon EA. Mitochondrial DNA genotypes in nuclear transfer-derived cloned sheep. Nat. Genet. 1999; 23:90-93.
- Farin CE, Farin PW, Piedrahita JA. Development of fetuses from in vitro-produced and cloned bovine embryos. J. Anim. Sci. 2004; 82 E-Suppl: E53-E62.
- Fletcher CJ, Roberts CT, Hartwich KM, Walker SK, McMillen IC. Somatic cell nuclear transfer in the sheep induces placental defects that likely precede fetal demise. Reproduction. 2007; 133:243-255.
- Folch J, Cocero MJ, Chesné P, Alabart JL, Domínguez V, Cognié Y, Roche A, Fernández-Arias A, Martí JI, Sánchez P, Echegoyen E, Beckers JF, Bonastre AS, Vignon X. First birth of an animal from an extinct subspecies (Capra pyrenaica pyrenaica) by cloning. Theriogenology. 2009 Apr 1;71(6):1026-34. doi: 10.1016/j.theriogenology.2008.11.005. Epub 2009 Jan 23. PubMed PMID: 19167744.
- Foote RH. The history of artificial insemination: Selected notes and notables. J. Anim. Sci. 2002; 80:1-10
- Galli C, Lagutina I, Crotti G, Colleoni S, Turini P, Ponderato N, Duchi1 R, Lazzariet G. Pregnancy: a cloned foal born to its dam twin. Nature. 2003; 424:635.
- Gao K, Luo Z, Han S, et al. Analysis of meat color, meat tenderness and fatty acid composition of meat in second filial hybrid offspring of MSTN mutant pigs. Meat Sci. 2022;193
- Gauthier M, Pierson J, Drolet M, Bhatia B, Baldassarre H, Keefer CL. Sexual maturation and fertility of male Nigerian Dwarf goat (Capra hircus) clones produced by somatic cell nuclear transfer. Cloning Stem Cells. 2001; 3:151-155.
- Gu T, Shi J, Luo L, et al. Study on hematological and biochemical characters of cloned duroc pigs and their progeny. Animals. 2019;9(11)
- Hasler JF, Henderson WB, Hurtgen PJ, Jin ZQ, McCauley AD, Mower SA, Neely B, Shuey LS, Stokes JE, Trimmer SA. Production, freezing and transfer of bovine IVF embryos and subsequent calving results. Theriogenology. 1995; 43:141-152.
- Hasler JF, McCauley AD, Lathrop WF, Foote RH. Effect of donor-embryo-recipient interactions on pregnancy rate in a large-scale bovine embryo transfer program. Theriogenology. 1987; 27:139-168
- Health Canada. 2003. Food Directorate Interim Policy on Foods from Cloned Animals. FD-FSNP-075, Version Number: 2.0. Food Directorate, Health Products and Food Branch
- Health Canada, Ottawa.
- Heyman Y, Chapatti-Palmer P, Berthold V, Fromentin G, Hachette JF, Martiana L, Regard JP. Assessing the quality of products from cloned cattle: An integrative approach. Theriogenology. 2007; 67:134-141.
- Heyman Y, Chavatte-Palmer P, LeBourhis D, Camous S, Mignon X, Regard JP. Frequency and occurrence of late-gestation losses from cattle cloned embryos. Biol. Reprod. 2002; 66:6-13.
- Hiendleder S. Mitochondrial DNA inheritance after SCNT. Adv. Exp. Med. Biol. 2007; 591:103-116.
- Hiendleder S, Zakhartchenko V, Wolf E. Mitochondria and the success of somatic cell nuclear transfer cloning: from nuclear-mitochondrial interactions to mitochondrial complementation and mitochondrial DNA recombination. Reprod. Fert. Dev. 2005; 17:69-83.
- Hill JR, Bernhardt RC, Jones K, Long CR, Looney CR, Shin T, Spencer TE, Thompson JA, Winger QA, Westhusin ME. Evidence for placental abnormality as the major cause of mortality in first-trimester somatic cell cloned bovine fetuses. Biol. Reprod. 2000; 63:1787-1794.
- Hornsby PJ. Telomerase and the Aging Process. Exp. Gerontol. 2007; 42:575-581.
- Hua S, Zhang H, Su JM, Zhang T, Quan FS, Liu J, Wang YS, Zhang Y. Effects of the removal of cytoplasm on the development of early cloned bovine embryos. Anim Reprod Sci. 2011 Jun;126(1-2):37-44. doi: 10.1016/j.anireprosci.2011.05.002. Epub 2011 May 14. PubMed PMID: 21632190.
- Huang J, Wang F, Okuka M, Liu N, Ji G, Ye X, Zuo B, Li M, Liang P, Ge WW, Tsibris JC, Keefe DL, Liu L. Association of telomere length with authentic pluripotency of ES/iPS cells. Cell Res. 2011 May;21(5):779-92. doi: 10.1038/cr.2011.16. Epub 2011 Feb 1. PubMed PMID: 21283131; PubMed Central PMCID: PMC3203670.
- Huang J, Zhang H, Yao J, Qin G, Wang F, Wang X, Luo A, Zheng Q, Cao C, Zhao J. BIX-01294 increases pig cloning efficiency by improving epigenetic reprogramming of somatic cell nuclei. Reproduction. 2016 Jan;151(1):39-49. doi: 10.1530/REP-15-0460. PubMed PMID: 26604326.
- Hur, Sun Jin. "A study on current risk assessments and guidelines on the use of food animal products derived from cloned animals." Food and Chemical Toxicology 108 (2017): 85-92.
- Hwang I, Jeong YW, Kim JJ, Lee HJ, Kang M, Park KB, Park JH, Kim YW, Kim WT, Shin T, Hyun SH, Jeung EB, Hwang WS. (2013) Successful cloning of coyotes through interspecies somatic cell nuclear transfer using domestic dog oocytes. Reproduction, Fertility and Development 2012; 25: 1142-1148. doi.org/10.1071/RD12256
- Ideta A, Urakawa M, Aoyagi Y, Saek K. Early morphological nuclear events and developmental capacity of embryos reconstructed with fetal fibroblasts at the M or G1 phase after intra cytoplasmic nuclear injection in cattle. J. Reprod. Dev. 2005; 51:187-194.
- IETS. Health Assessment and Care for Animals Involved in the Cloning Process. International Embryo Transfer Society. 2008; 1- 35. http://www.iets.org/pdf/HASAC-HealthAssessmentCare.pdf Accessed February 9, 2009.
- Jaenisch R. DNA methylation and imprinting: why bother? Trends Genet.1997; 13:323-29.
- Jafarpour F, Hosseini SM, Hajian M, Forouzanfar M, Ostadhosseini S, Abedi P, Gholami S, Ghaedi K, Gourabi H, Shahverdi AH, Vosough AD, Nasr-Esfahani MH. Somatic cell-induced hyperacetylation, but not hypomethylation, positively and reversibly affects the efficiency of in vitro cloned blastocyst production in cattle. Cell Reprogram. 2011 Dec;13(6):483-93. doi: 10.1089/cell.2011.0005. Epub 2011 Sep 15. PubMed PMID: 21919704; PubMed Central PMCID: PMC3229819.
- Kasai K, Sano F, Miyashita N, Watanabe S, Nagai T. Comparison of the growth performances of offspring produced by a pair of cloned cattle and their nuclear donor animals. J Reprod Dev. 2007 Feb;53(1):135-42. Epub 2006 Sep 29. PubMed PMID: 17008756.
- Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science. 1998; 282:2095-98.
- Kim KH, Kim EY, Lee SY, Ko JJ, Lee KA. Oocyte Cytoplasmic Gas6 and Heparan Sulfate (HS) are Required to Establish the Open Chromatin State in Nuclei During Remodeling and Reprogramming. Cell Physiol Biochem. 2018;45(1):37-53. doi: 10.1159/000486221. Epub 2017 Dec 22. PubMed PMID: 29316553.
- Kim MJ, Oh HJ, Kim GA, Setyawan EMN, Choi YB, Lee SH, Petersen-Jones SM, Ko CJ, Lee BC. Birth of clones of the world's first cloned dog. Sci Rep. 2017; 7(1):15235. doi: 10.1038/s41598-017-15328-2.
- Kim, EY, Song, DH, Park, MJ, Park, HY, Lee, SE, Choi, HY, Moon, JJ, Kim, YH, Mun, SH, Oh, CE and Ko, MS. (2013). Post-death cloning of endangered Jeju black cattle (Korean native cattle): fertility and serum chemistry in a cloned bull and cow and their offspring. Journal of Reproduction and Development, 59(6), 536-543.
- Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, Hwang WS, Hossein MS, Kim JJ, Shin NS, Kang SK, Lee BC. Endangered wolves cloned from adult somatic cells. Cloning Stem Cells.2007; 9(1):130-137. DOI: 10.1089/clo.2006.0034
- Kishigami S, Mizutani E, Ohta H, Hikichi T, Thuan NV, Wakayama S, Bui HT, Wakayama T. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem Biophys Res Commun. 2006 Feb 3;340(1):183-9. Epub 2005 Dec 9. PubMed PMID: 16356478.
- Kong Q, Ji G, Xie B, Li J, Mao J, Wang J, Liu S, Liu L, Liu Z. Telomere elongation facilitated by trichostatin a in cloned embryos and pigs by somatic cell nuclear transfer. Stem Cell Rev. 2014 Jun;10(3):399-407. doi: 10.1007/s12015-014-9499-y. PubMed PMID: 24510582.
- Kruip T, den Daas JH. in vitro produced and cloned embryos: Effects on pregnancy, parturition and offspring. Theriogenology. 1997; 47:43-52.
- Kubota C, Tian XC, Yang X. Serial bull cloning by somatic cell nuclear transfer. Nat. Biotechnol. 2004; 22:693–694.
- Kurome M, Hisatomi H, Matsumoto S, Tomii R, Ueno S, Hiruma K, Saito H, Nakamura K, Okumura K, Matsumoto M, Kaji Y, Endo F, Nagashima H. Production efficiency and telomere length of the cloned pigs following serial somatic cell nuclear transfer. J Reprod Dev. 2008; 54(4):254-258.
- Kühholzer-Cabot B, Brem G. Aging of Animals Produced by Somatic Cell Nuclear Transfer. Exp. Gerontol. 2002; 37:1317–1323.
- Laible G, Brophy B, Knighton D, Wells DN. Compositional analysis of dairy products derived from clones and cloned transgenic cattle. Theriogenology. 2007; 1:166-177.
- Lanza RP, Cibelli JB, Faber D, Sweeney RW, Henderson B, Nevada W, West MD, Wheatstone PJ. Cloned cattle can be healthy and normal. Science. 2001; 294:1893-1894.
- Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, Hossein MS, Kim JJ, Kang SK, Schatten G, Hwang WS. Dogs cloned from adult somatic cells. Nature 2005; 436:641.
- Lee JH, Peters A, Fisher P, Bowles EJ, St John JC, Campbell KH. Generation of mtDNA homoplasmic cloned lambs. Cell Reprogram. 2010 Jun;12(3):347-55. doi: 10.1089/cell.2009.0096. PubMed PMID: 20698774.
- Lee RS, Peterson PJ, Denistone PJ, Ravelich S, Ledgard AM, Li N, Oliver JE, Miller AL, Tucker FC, Bremer B, Wells DN. Cloned cattle fetuses with the same nuclear genetics are more variable than contemporary half-siblings resulting from artificial insemination and exhibit fetal and placental growth deregulation even in the first trimester. Biol. Reprod. 2004; 70:1-11.
- Liang X, Lan J, Xu M, et al. Impact of KIT editing on coat pigmentation and fresh meat color in Yorkshire pigs. CRISPR Journal. 2022;5(6)
- Liu HJ, Peng H, Hu CC, Li XY, Zhang JL, Zheng Z, Zhang WC. Effects of donor cells' sex on nuclear transfer efficiency and telomere lengths of cloned goats. Reprod Domest Anim. 2016 Oct;51(5):789-94. doi: 10.1111/rda.12752. Epub 2016 Aug PubMed PMID: 27558653.
- Liu Z, Cai Y, Wang Y1, Nie Y, Zhang C, Xu Y, Zhang X, Lu Y, Wang Z, Poo M, Sun Q. Cloning of Macaque Monkeys by Somatic Cell Nuclear Transfer Cell. 2018; 172:881-887. doi: 10.1016/j.cell.2018.01.020.
- Loi P, Iuso D, Toschi P, Palazzese L, Czernik M. Alternative strategies for nuclear reprogramming in Somatic Cell Nuclear transfer (SCNT). Anim. Reprod. 2017; 14(2): 377-382.
- Loi P, Iuso D, Czernik M, Ogura A. A New, Dynamic Era for Somatic Cell Nuclear Transfer? Trends Biotechnol. 2016 Oct;34(10):791-797. doi: 10.1016/j.tibtech.2016.03.008. Epub 2016 Apr 22. Review. PubMed PMID: 27118511.
- Loi P, Ptak G, Barboni B, Fulka J Jr, Cappai P, Clinton M. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat Biotechnol. 2001 Oct;19(10):962-4. PubMed PMID: 11581663.
- Mason K, Liu Z, Aguirre-Lavin T, Beaujean N. Chromatin and epigenetic modifications during early mammalian development (2012). Animal Reproduction Science. Vol 134(1-2): pp 45-55.
- Mastromonaco GF, González-Grajales LA, Filice M, Comizzoli P. Somatic cells, stem cells, and induced pluripotent stem cells: how do they now contribute to conservation? Adv Exp Med Biol. 2014;753:385 427. doi: 10.1007/978-1-4939-0820-2_16. Review. PubMed PMID: 25091918.
- Mastromonaco GF, King WA. Cloning in companion animal, non-domestic and endangered species: can the technology become a practical reality? Reprod Fertil Dev. 2007;19(6):748-61. Review. PubMed PMID: 17714629.
- Matoba S, Liu Y, Lu F, Iwabuchi KA, Shen L, Inoue A, Zhang Y. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell. 2014 Nov 6;159(4):884-95. doi: 10.1016/j.cell.2014.09.055. Epub 2014 Oct 30. PubMed PMID: 25417163; PubMed Central PMCID: PMC4243038.
- McEvoy TG, Sinclair KD, Young LE, Wilmot I, Robinson JJ. Large offspring syndrome and other consequences of ruminant embryo culture in vitro: relevance to blastocyst culture in human ART. Hum. Fertile. 2000; 3:238-246.
- McEvoy TG, Alink FM, Moreira VC, Watt RG, Powell KA. Embryo technologies and animal health – consequences for the animal following ovum pick-up, in vitro embryo production and somatic cell nuclear transfer. Theriogenology. 2006; 65:926-942.
- McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev. Biol. 1997; 187:107-113.
- Mir B, Zaunbrecher G, Archer GS, Friend TH, Piedrahita JA. Progeny of somatic cell nuclear transfer (SCNT) pig clones are phenotypical similar to non-cloned pigs. Cloning Stem Cells. 2005; 7:119-125.
- Miyashita N, Kubo Y, Yonai M, Kaneyama K, Saito N, Sawai K, Minamihashi A, Suzuki T, Kojima T, Nagai T. Cloned cows with short telomeres deliver healthy offspring with normal-length telomeres. J Reprod Dev. 2011 Oct;57(5):636-42. Epub 2011 Aug 5. PubMed PMID: 21817835.
- Miyashita N, Shiga K, Fujita T, Umeki H, Sato W, Suzuki T, Nagai T. Normal telomere lengths of spermatozoa in somatic cell-cloned bulls. Theriogenology. 2003; 59:1557–1565.
- Miyashita N, Shiga K, Yonai M, Kaneyama K, Kobayashi S, Kojima T, Goto Y, Kishi M, Aso H, Suzuki T, Sakaguchi M, Nagai T. Remarkable differences in telomere lengths among cloned cattle derived from different cell types. Biol. Reprod. 2002; 66:1649–1655.
- Monzani PS, Adona PR, Long SA, Wheeler MB. Cows as Bioreactors for the Production of Nutritionally and Biomedically Significant Proteins. Advances in Experimental Medicine and Biology. 2022;1354
- Niemann H, Tian XC, King WA, Lee RSF. Epigenetic reprogramming in embryonic and foetal development upon somatic cell nuclear transfer cloning. Reproduction. 2008; 135:151-163.
- Niemann H and Lucas-Hahn A. Somatic Cell Nuclear Transfer Cloning: Practical Applications and Current Legislation. Reprod Dom Anim. 2012; 47 (Suppl. 5): 2-10. doi: 10.1111/j.1439-0531.2012.02121.x
- Niemann H. Epigenetic reprogramming in mammalian species after SCNT-based cloning. Theriogenology. 2016; 86(1): 80-90. doi: 10.1016/j.theriogenology.2016.04.021.
- No JG, Hur TY, Zhao M, Lee S, Choi MK, Nam YS, Yeom DH, Im GS, Kim DH. Scriptaid improves the reprogramming of donor cells and enhances canine-porcine interspecies embryo development. Reprod Biol. 2018 Mar;18(1):18-26. doi: 10.1016/j.repbio.2017.11.001. Epub 2017 Nov 20. PubMed PMID: 29162325.
- Oh HJ, Lee TH, Lee JH, Lee BC. Trichostatin a improves preimplantation development of bovine cloned embryos and alters expression of epigenetic and pluripotency genes in cloned blastocysts. J Vet Med Sci. 2012 Nov;74(11):1409-15. Epub 2012 Jun 18. PubMed PMID: 22785124.
- OIE. Somatic Cell Nuclear Transfer in Production Livestock and Horses. World Organisation for Animal Health, 2008; Chapter 4.12. http://www.oie.int/eng/normes/MCODE/en_chapitre_1.4.12.htm Accessed February 9, 2009.
- OIE. 2018. Terrestrial Animal Health Code. Somatic Cell Nuclear Transfer in Production Livestock and Horses. World Organisation for Animal Health, 2018. http://wahis2-devt.oie.int/fileadmin/Home/eng/Health_standards/tahc/2010/en_chapitre_somatic_cell.htm), Articles 4.11.5 & 4.11.6). Accessed August 30, 2018.
- Ortegon H, Betts DH, Lin L, Capella G, Paroled SD, Blond in P, King WA. Genomic Stability and physiological assessments of live offspring sired by a bull clone, Starbuck II. Theriogenology. 2007; 67:116-126.
- Osada T, Nozaki T, Masutani M. 2016. Parp1 Deficiency Confers Defects in Chromatin Surveillance and Remodeling During Reprogramming by Nuclear Transfer. Curr Protein Pept Sci. Vol 17(7):693-704.
- Pace MM, Eganstown ML, Betthauser JM, Children LA, Eilertsen KH, Egos JM, Forster EJ, Gaelic PJ, Glaber DF, Kemper JH, Capping RW, Lange G, Lesmeister TL, Maldon KS, Mel GD, Mazowiecki PM, Pfister-Genskow M, Strelchenko NS, Volcker GR, Watt SR, Bishop MD. Ontogeny of cloned cattle to lactation. Biol. Reprod. 2002; 67:334-339.
- Panarace, M, Aegir, JI, Garrote M, Garage G, Segovia A, Can L, Gutierrez J, Moorfowl M, RigaliF, Puggiest M, Young S, Lagioia J, Garni C, Forte Pontes JE, Erenow Julio JH, Mower S, Medina M. How healthy are clones and their progeny: 5 years of field experience. Theriogenology. 2007; 67:142-151.
- Peat JR, Reik W. Incomplete methylation reprogramming in SCNT embryos. Nat Genet. 2012 Sep;44(9):965-6. doi: 10.1038/ng.2393. PubMed PMID: 22932499.
- Perota A, Lagutina I, Duchi R, et al. Generation of cattle knockout for galactose-a1,3-galactose and N-glycolylneuraminic acid antigens. Xenotransplantation. 2019;26(5)
- Polejaeva IA, Broek DM, Walker SC, Zhou W, Walton M, Benninghoff AD, Faber DC. 2013. Longitudinal Study of Reproductive Performance of Female Cattle Produced by Somatic Cell Nuclear Transfer. PLoS ONE 8(12): e84283. doi:10.1371/journal.pone.0084283
- Reggio BC, James AN, Green HL, Gavin WG, Behboodi E, Accelerate Y, Gotcha RA. Cloned transgenic offspring resulting from somatic cell nuclear transfer in the goat: oocytes derived from both follicle-stimulating hormone-stimulated and nonstimulated abattoir-derived ovaries. Biol. Reprod. 2001; 65:1528-1533.
- Reik W, Romer I, Barton SC, Surani MA, Howlett SK, Klose J. Adult phenotype in the mouse can be affected by epigenetic events in the early embryo. Development. 1993; 119:933-942.
- Rideout III W, Eggan K, Jaenisch R. Nuclear cloning and epigenetic reprogramming of the genome. Science. 2001; 293:1093-1098.
- Rind S, Cui W, King T, Ritchie, WA, Wylie, D, Wilmut I. Dolly: A final report (Poster presented at the International Embryo Transfer Society meeting in Portland, Oregon, January 2004) http://www.roslin.ac.uk/downloads/dolly_final_report_pstr.pdf Accessed February 19, 2008.
- Russo G, Landi R, Pezone A, Morano A, Zuchegna C, Romano A, Muller M, Gottesman ME, Porcellini A and Avvedimento EV. 2016. DNA damage and repair modify DNA methylation and chromatin domain of the targeted locus: mechanism of allele methylation polymorphism. Sci Rep. Vol 6: 33222.
- Sangalli JR, De Bem TH, Perecin F, Chiaratti MR, Oliveira Lde J, de Araujo RR, Valim Pimentel JR, Smith LC and Meirelles FV. 2012. Treatment of nuclear-donor cells or cloned zygotes with chromatin-modifying agents increases histone acetylation but does not improve full term development of cloned cattle. Cell Reprogram. Vol 14(3): 235-47
- Sansinena MJ, Lynn J, Bondioli KR, Denniston RS, Godke RA. Ooplasm transfer and interspecies somatic cell nuclear transfer: heteroplasmy, pattern of mitochondrial migration and effect on embryo development. Zygote. 2011 May;19(2):147-56. doi: 10.1017/S0967199410000419. Epub 2010 Aug 25. PubMed PMID: 20735895
- Saragusty J, Diecke S, Hildebrandt T, et al. Rewinding the process of mammalian extinction. Zoo Biology [serial online]. 2016;35(4):280-292. Available from: CAB Abstracts, Ipswich, MA. Accessed August 23, 2018.
- Savage A, Maulla J, Tian XC, Taneja M, Katz L, Darre M, Yang X. Behavioral observations of adolescent Holstein heifers cloned from adult somatic cells. Theriogenology. 2003; 60:1097-1110.
- Schaetzlein S, Lucas-Hahn A, Lemme E, Kues WA, Dorsch M, Manns MP, Niemann H, Rudolph KL. Telomere length is reset during early mammalian embryogenesis. Proc Natl Acad Sci U S A. 2004 May 25;101(21):8034-8. Epub 2004 May 17. PubMed PMID: 15148368; PubMed Central PMCID: PMC419552.
- Selokar NL, Saini M, Kumar D, Sharma RK, Yadav PS. 10 Years after the birth of India's first cloned farm animal, where is buffalo cloning heading. Indian Journal of Biotechnology. 2019;18
- Shao GB, Ding HM, Gong AH, Xiao DS. Inheritance of histone h4 methylation in reprogramming of somatic nuclei following nuclear transfer. J Reprod Dev. 2008 Jun;54(3):233-8. Epub 2008 Apr 14. PubMed PMID: 18408353.
- Sinclair KD, Corr SA, Gutierrez CG, Fisher PA, Lee JH, Rathbone AJ, Choi I, Campbell KH, Gardner DS. Healthy ageing of cloned sheep. Nat Commun. 2016 Jul 26;7:12359. doi: 10.1038/ncomms12359. PubMed PMID: 27459299; PubMed Central PMCID: PMC4963533.
- Shiels PG, Kind AJ, Campbell KH, Waddington D, Wilmut I, Colman A, Schnieke AE. Analysis of telomere lengths in cloned sheep. Nature. 1999 May 27;399(6734):316-7. PubMed PMID: 10360570.
- Shi LH, Ai JS, Ouyang YC, Huang JC, Lei ZL, Wang Q, Yin S, Han ZM, Sun QY, Chen DY. Trichostatin A and nuclear reprogramming of cloned rabbit embryos. J Anim Sci. 2008 May;86(5):1106-13. doi: 10.2527/jas.2007-0718. Epub 2008 Feb 1. PubMed PMID: 18245503.
- Shi W, Zakhartchenko V, Wolf E. Epigenetic reprogramming in mammalian nuclear transfer. Differentiation. 2003; 71:91-113.
- Shimozawa, Nobuhiro, Yukiko Ono, Shingo Kimoto, Kyoji Hioki, Yoshihiko Araki, Yoichi Shinkai, Tomohiro Kono, and Mamoru Ito. "Abnormalities in cloned mice are not transmitted to the progeny." Genesis 34, no. 3 (2002): 203-207.
- Shin T, Kraemer D, Pryor J, Liu L, Rugila J, Howe L, Buck S, Murphy K, Lyons L, Westhusin M. A cat cloned by nuclear transplantation. Nature. 2002; 415:859.
- Shibata M, Otake M, Tsuchiya S, Chikyu M, Horiuchi A, and Kawarasaki T. Reproductive and growth performance in Jin Hua pigs cloned from somatic cell nuclei and the meat quality of their offspring. J. Reprod. Dev. 2006; 52:583-590
- Smith LC, Suzuki J Jr, Goff AK, Filion F, Therrien J, Murphy BD, Kohan-Ghadr HR, Lefebvre R, Brisville AC, Buczinski S, Fecteau G, Perecin F, Meirelles FV. Developmental and epigenetic anomalies in cloned cattle. Reprod Domest Anim. 2012;47 Suppl 4:107-114. doi: 10.1111/j.1439-0531.2012.02063.x
- Smith LC, Bordignon V, Garcia JM, Meirelles FV. Mitochondrial genotype segregation and effects during mammalian development: Applications to biotechnology. Theriogenology. 2000a; 53(1):35-46.
- Smith L, Bordignon V, Babkine M, Fecteau G, Keefer C. Benefits and Problems with Cloning Animals. Can. Vet. J. 2000b; 41:919-924.
- Solter D. Mammalian cloning: advances and limitations. Nat. Rev. Genet. 2000; 1:199-207.
- Sperber JL, Lust DG, Veneklasen GO, Hawkins DE, McEvers TJ, Lawrence TE. Live and carcass production traits for progeny of purebred sires in comparison with the clone of a USDA prime yield grade one carcass. Translational animal science. 2023;7(1)
- Srirattana K, St John JC. Manipulating the mitochondrial genome to enhance cattle embryo development. G3 (Bethesda). 2017; 7(7):2065-2080. doi: 10.1534/g3.117.042655.
- Takeda K, Tasai M, Iwamoto M, Onishi A, Tagami T, Nirasawa K, Hanada H, Pinkert CA. Microinjection of cytoplasm or mitochondria derived from somatic cells affects parthenogenetic development of murine oocytes. Biol Reprod. 2005; 72(6):1397-1404.
- Tamashiro, Kellie LK, Teruhiko Wakayama, Yukiko Yamazaki, Hidenori Akutsu, Stephen C. Woods, Sylvia Kondo, Ryuzo Yanagimachi, and Randall R. Sakai. "Phenotype of cloned mice: development, behavior, and physiology." Experimental Biology and Medicine 228, no. 10 (2003): 1193-1200.
- Taylor-Robinson, Andrew W., Simon Walton, David L. Swain, Kerry B. Walsh, and Gábor Vajta. "The potential for modification in cloning and vitrification technology to enhance genetic progress in beef cattle in Northern Australia." Animal reproduction science 148, no. 3-4 (2014): 91-96.
- Urakawa M, Ideta A, Saada T, Aoyagi Y. Examination of a modified cell cycle synchronization method and bovine nuclear transfer using synchronized early G1 phase fibroblast cells. Theriogenology. 2004; 62:714-728.
- US FDA. Animal Cloning: A Risk Assessment. Center for Veterinary Medicine, US Food and Drug Administration. 2008; 1-968.
- van der Berg JP, Kleter GA, Kok EJ. Regulation and safety considerations for somatic cell nuclear transfer-cloned farm animals and their offspring used for food production. Theriogenology. 2019;135.
- Van Wagtendonk-de Leeuw AM; Aerts BJG, den Daas JHG. Abnormal offspring following in vitro production of bovine preimplantation embryos: A field study. Theriogenology. 1998; 49:883-894.
- Verma G, Arora JS, Sethi RS, Mukhopadhyay CS, Verma R. Handmade cloning: recent advances,potential and pitfalls. Journal of Animal Science and Biotechnology. 2015; 6:43.
- Wade PA, Kikyo N. Chromatin remodeling in nuclear cloning. Eur. J. Biochem. 2002; 269:2284-2287.
- Wakayama T, Perry ACF, Zucotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998; 394:369-374.
- Wakayama S, Kohda T, Obokata H, Tokoro M, Li C, Terashita Y, Mizutani E, Nguyen VT, Kishigami S, Ishino F, Wakayama T. Successful serial recloning in the mouse over multiple generations. Cell Stem Cell. 2013 Mar 7;12(3):293-7. doi: 10.1016/j.stem.2013.01.005. PubMed PMID: 23472871.
- Walker SC, Christenson RK, Ruiz RP, Reeves DE, Pratt SL, Arenivas F, Williams NE, Bruner BL, and Polejaeva IA. Comparison of meat composition from offspring of cloned and conventionally produced boars. Theriogenology. 2007; 67: 178-184
- Walker SK, Hartwich KM, Seamark RF. The production of unusually large offsprings following embryonic manipulation: concepts and challenges. Theriogenology. 1996; 45:111-120.
- Wang F, Kou Z, Zhang Y, Gao S. Dynamic reprogramming of histone acetylation and methylation in the first cell cycle of cloned mouse embryos. Biol Reprod. 2007 Dec;77(6):1007-16. Epub 2007 Sep 5. PubMed PMID: 17823087.
- Wang F, Yin Y, Ye X, Liu K, Zhu H, Wang L, Chiourea M, Okuka M, Ji G, Dan J, Zuo B, Li M, Zhang Q, Liu N, Chen L, Pan X, Gagos S, Keefe DL, Liu L. Molecular insights into the heterogeneity of telomere reprogramming in induced pluripotent stem cells. Cell Res. 2012 Apr;22(4):757-68. doi: 10.1038/cr.2011.201. Epub 2011 Dec 20. PubMed PMID: 22184006; PubMed Central PMCID: PMC3317559.
- Wang H, Li G, Zhong C, et al. Generation of multi-transgenic pigs using PiggyBac transposons co-expressing pectinase, xylanase, cellulase, beta-1.3-1.4-glucanase and phytase. Frontiers in Genetics. 2020;11
- Wani NA, Wernery U, Hassan FAH, Wernery R, Skidmore JA. Production of the First Cloned Camel by Somatic Cell Nuclear Transfer. Biology of Reproduction 2010; 82(2):373–379. https://doi.org/10.1095/biolreprod.109.081083
- Wani NA, Vettical BS, Hong SB. First cloned Bactrian camel (Camelus bactrianus) calf produced by interspecies somatic cell nuclear transfer: A step towards preserving the critically endangered wild Bactrian camels. PLoS ONE. 2017;12(5):e0177800. doi:10.1371/journal.pone.0177800.
- Watanabe S, Nagai T. Death losses due to stillbirth, neonatal death and diseases in cloned cattle derived from somatic cell nuclear transfer and their progeny: a result of nationwide survey in Japanas Animal Science Journal. 2009; 80:233–238. doi: 10.1111/j.1740-0929.2009.00640.x
- Watanabe S, Nagai T. Health Status and Productive Performance of Somatic Cell Cloned Cattle and Their Offspring Produced in Japan. J. Reprod. Dev. 2008; 54:6-17
- Wells DN. Cloning Livestock. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources. 2006; 39:1-21.
- Wells DN, Forsyth JT, McMillen V, Back B. The health of somatic cell cloned cattle and their offspring. Cloning Stem Cells. 2004; 6:101-110.
- Wells DN, Mazowiecki PM, Day AM, Peterson PJ, Tervit HR. Cloning sheep from cultured embryonic cells. Reprod. Fertile. Dev. 1998; 10:615-626.
- Wells DN, Laible G, Tucker FC, Miller AL, Oliver JE, Xian T, Forsyth JT, Berg MC, Cockrem K, L'Huillier PJ, Tervit HR, Back B. Coordination between donor cell type and cell cycle stage improves nuclear cloning efficiency in cattle. Theriogenology. 2003; 59:45-59.
- Wilmut I, Bai Y, Taylor J. Somatic cell nuclear transfer: origins, the present position and future opportunities. Phil. Trans. R. Soc. B 2015; 370: 20140366. http://dx.doi.org/10.1098/rstb.2014.0366
- Wilmut I, Schnieke EA, McWhir J, Kind AJ, Campbell KHS. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997; 385:810-813.
- Wilson JM, Williams JD, Bondioli KR, Looney CR, Westhuin ME, McCalla DF. Comparison of birth weight and growth characteristics of bovine calves produced by nuclear transfer (cloning), embryo transfer and natural mating. Anim. Reprod. Sci. 1995; 38:73-84.
- Woods GL, White KL, Vanderwall DK, Li GP, Aston KI, Bunch TD, Meerdo LN, Pate BJ. A mule cloned from fetal cells by nuclear transfer. Science. 2003; 301:1063.
- Wu G, Bazer FW. Application of new biotechnologies for improvements in swine nutrition and pork production. Journal of Animal Science and Biotechnology. 2019;10(1)
- XiangXing Z, QunMei Z, YanYan W, et al. CRISPR/Cas9-mediated MSTN disruption accelerates the growth of Chinese bama pigs. Reproduction in Domestic Animals. 2020;55(10).
- Yamaguchi M, Yoshihiko I, Seiya T. "Fourteen-week feeding test of meat and milk derived from cloned cattle in the rat." Theriogenology 67, no. 1 (2007): 152-165.
- Yamanaka K, Kaneda M, Inaba Y, Saito K, Kubota K, Sakatani M, Sugimura S, Imai K, Watanabe S, Takahashi M. DNA methylation analysis on satellite I region in blastocysts obtained from somatic cell cloned cattle. Anim Sci J. 2011 Aug;82(4):523-30. doi: 10.1111/j.1740-0929.2011.00881.x. Epub 2011 Apr 20. PubMed PMID: 21794009.
- Yan ZH, Zhou YY, Fu J, Jiao F, Zhao LW, Guan PF, Huang SZ, Zeng YT, Zeng F. Donor-host mitochondrial compatibility improves efficiency of bovine somatic cell nuclear transfer. BMC Dev Biol. 2010 Mar 19;10:31. doi: 10.1186/1471-213X-10-31. PubMed PMID: 20302653; PubMed Central PMCID: PMC2858029.
- Yang X, Tian XC, Kubota C, Page R, Xu J, Cibelli J, and Seidel Jr G. Risk assessment of meat and milk from cloned animals. Nat. Biotechnol. 2007; 25:77-83.
- Young LE, Sinclair KD, Wilmut I. Large offspring syndrome in cattle and sheep. Rev. Reprod. 1998; 3:155-163.
- Zhai Y, Wei L, Zhiren Z, Yunqing C, Zhengzhu W, Sheng Z, Ziyi L. Epigenetic states of donor cells significantly affect the development of somatic cell nuclear transfer (SCNT) embryos in pigs. Mol reprod Dev. 2018; Jan 85(1):26-37. doi: 10.1002/mrd.22935. Epub 2017 Dec 18. PubMed PMID: 29205617.
- Zhang S, Chen X, Wang F, An X, Tang B, Zhang X, Sun L, Li Z. Aberrant DNA methylation reprogramming in bovine SCNT preimplantation embryos. Sci Rep. 2016 Jul 26;6:30345. doi: 10.1038/srep30345. PubMed PMID: 27456302; PubMed Central PMCID: PMC4960566.
- Zhang Z, Zhai Y, Ma X, Zhang S, An X, Yu H, Li Z. Down-Regulation of h4K4me3 by MM-102 Facilitates Epigenetic Reprogramming of Porcine Somatic Cell Nuclear Transfer Embryos. Cell Physiol Biochem. 2018;45(4):1529-1540. doi:10.1159/000487579. Epub 2018 Feb 16. PubMed PMID: 29466785.
- Zhao ShuJun, Hu JiaQi, Zhang MingYue, et al. DNA methylation and imprinting status of Meg8 in somatic cell nuclear transfer bovines. Acta Veterinaria et Zootechnica Sinica. 2014;45(9):1417-1423.
- Zhou Q, Renard JP, Le Friec G, Brochard V, Beaujean N, Cherifi Y, Fraichard A, Cozzi J. Generation of fertile cloned rats by regulating oocyte activation. Science 2003; 302(5648): 1179. DOI: 10.1126/science.1088313
Footnotes:
- Footnote 1
-
Transgenic animals are animals which have been genetically altered by the introduction of recombinant DNA in order to stably integrate a foreign gene (a transgene) in their genome.
- Footnote 2
-
Livestock animals are deemed to be healthy according to the provisions of the Health of Animals Act and Regulations and the Meat Inspection Act and Regulations, including, but not limited to, the mandatory ante-mortem and post-mortem inspections. The Canadian Health of Animals Act and Regulations and the Meat Inspection Act and Regulations may be found on the Canadian Department of Justice website: http://laws.justice.gc.ca/en/index.html.
- Footnote 3
-
The concept of substantial equivalence is a key step in the safety assessment process for many novel foods and novel feeds. It is not a safety assessment in itself as it does not characterise the hazard, but it is used as a starting point to structure the safety assessment of a new food or feed relative to its conventional counterpart. This concept is used to identify similarities and differences between the new food or feed and its conventional counterpart. It aids in the identification of potential safety and nutritional issues and is currently considered the most appropriate strategy for safety assessments conducted on novel foods and novel feeds. The use of this concept in a safety assessment does not imply absolute safety of the new product; rather, it focuses on assessing the safety of any identified differences between the new product and its conventional counterpart so that the safety of the new product can be considered relative to its counterpart. The concept of substantial equivalence is endorsed by international groups such as the Organization for Economic Cooperation and Development, the Food and Agriculture Organization of the United Nation, the World Health Organization, and the Codex Alimentarius Commission. This concept will be applied in this scientific opinion on the safety of food and feeds derived from SCNT livestock clones and their progeny.
- Footnote 4
-
The Canadian Feeds Act and Regulations may be found on the Canadian Department of Justice website: http://laws.justice.gc.ca/en/index.html.