Chloramines in Drinking Water - Guideline Technical Document for Public Consultation
Consultation period ends January 25, 2019
Table of Contents
- Purpose of consultation
- Part I. Overview and Application
- 1.0 Proposed guideline
- 2.0 Executive summary
- 3.0 Application of the guideline
- Part II. Science and Technical Considerations
- 4.0 Identity, use and sources in the environment
- 5.0 Exposure
- 6.0 Analytical methods
- 7.0 Treatment technology and distribution system considerations
- 8.0 Kinetics and metabolism
- 9.0 Health effects
- 10.0 Classification and assessment
- 11.0 Rationale
- 12.0 References
- Appendix A: List of acronyms

Download the entire report
(PDF format, 600 KB, 63 pages)
Organization: Health Canada
Type: Consultation
Date published: 2018-11-23
Purpose of consultation
The available information on chloramines has been assessed with the intent of updating the current drinking water guideline and guideline technical document. The draft guideline technical document proposes that it is no longer considered necessary to establish a guideline for chloramines in drinking water. However, based on the use of chloramines in the disinfection of drinking water, a guideline technical document is still considered necessary.
The document is being made available for a 60-day public consultation period. The purpose of this consultation is to solicit comments on the proposed approach, as well as to determine the availability of additional exposure data. Comments are appreciated, with accompanying rationale, where required. Comments can be sent to Health Canada via email at HC.water-eau.SC@canada.ca. If this is not feasible, comments may be sent by mail to the Water and Air Quality Bureau, Health Canada, 269 Laurier Avenue West, A.L. 4903D, Ottawa, Ontario K1A 0K9. All comments must be received before January 25, 2019.
The existing guideline on chloramines, last updated in 1995, established a maximum acceptable concentration (MAC) of 3.0 mg/L (3,000 μg/L) based on reduction in body weight gain. This new document provides updated scientific data and information related to the health effects of chloramines, and proposes that it is no longer considered necessary to establish a guideline for chloramines in drinking water, based on recent studies that show a very low toxicity for monochloramine in drinking water. These studies also show that the health effect observed in earlier studies was due to a decreased water consumption related to taste aversion to monochloramine in drinking water.
Comments received as part of this consultation will be shared with members of the Federal-Provincial-Territorial Committee on Drinking Water (CDW), along with the name and affiliation of their author. Authors who do not want their name and affiliation to be shared with CDW should provide a statement to this effect along with their comments.
It should be noted that this guideline technical document on chloramines in drinking water will be revised following evaluation of comments received. This document should be considered as a draft for comment only.
Part I. Overview and Application
1.0 Proposed guideline
It is not considered necessary to establish a guideline for chloramines in drinking water, based on the low toxicity of monochloramine at concentrations found in drinking water. Any measures taken to limit the concentration of chloramines or their by-products in drinking water supplies must not compromise the effectiveness of disinfection.
2.0 Executive summary
The term "chloramines" refers to both inorganic and organic chloramines. This document focuses on inorganic chloramines, which consist of monochloramine, dichloramine and trichloramine. Unless specified otherwise, the term "chloramines" will refer to inorganic chloramines throughout the document.
Chloramines are found in drinking water mainly as a result of treatment, either intentionally as a disinfectant in the distribution system, or unintentionally as a by-product of the chlorination of drinking water in the presence of natural ammonia. As monochloramine is more stable and provides longer-lasting disinfection, it is commonly used in the distribution system, whereas chlorine is more effective at disinfecting water in the treatment plant. Chloramines have also been used in the distribution system to help reduce formation of common disinfection by-products such as trihalomethanes and haloacetic acids. However, chloramines also react with natural organic matter to form other disinfection by-products.
All drinking water supplies should be disinfected, unless specifically exempted by the responsible authority. Disinfection is an essential component of public drinking water treatment; the health risks associated with disinfection by-products are much less than the risks from consuming water that has not been adequately disinfected. Most Canadian drinking water supplies maintain a chloramine residual below 4 mg/L in the distribution system.
This guideline technical document focuses on the health effects related to exposure to chloramines in drinking water supplies, also taking in consideration taste and odour concerns. It does not review the benefits or the processes of chloramination; nor does it assess the health risks related to exposure to by-products formed as a result of the chloramination process. The Federal-Provincial-Territorial Committee on Drinking Water has determined that an aesthetic objective is not necessary, since levels commonly found in drinking water are within an acceptable range for taste and odour, and since protection of consumers from microbial health risks is paramount.
During its Fall 2017 meeting, the Federal-Provincial-Territorial Committee on Drinking Water reviewed the guideline technical document for chloramines and gave its endorsement for this document to undergo public consultation.
2.1 Health effects
The International Agency for Research on Cancer and the United States Environmental Protection Agency (U.S. EPA) have classified monochloramine as "not classifiable as to its carcinogenicity to humans" based on inadequate evidence in animals and in humans. The information on dichloramine and trichloramine is insufficient to establish any link with unwanted health effects in animals or in humans. These forms are also less frequently detected in drinking water. Studies have found minimal effects in humans and animals following ingestion of monochloramine in drinking water, with the most significant effect being decreased body weight gain in animals. However, this effect is due to reduced water consumption caused by taste aversion.
2.2 Exposure
Human exposure to chloramines primarily results from their presence in treated drinking water; monochloramine is usually the predominant chloramine. Intake of monochloramine and dichloramine from drinking water is not expected through either skin contact or inhalation. Intake of trichloramine from drinking water might be expected from inhalation; however, it is relatively unstable in water and is only formed in specific conditions (at very high chlorine to ammonia ratios or under low pH), which are unlikely to occur in treated drinking water. Consequently, exposure to chloramines via inhalation and skin contact during showering or bathing is expected to be negligible.
2.3 Analysis and treatment
Although there are no U.S. EPA-approved methods for the direct measurement of chloramines, there are several such methods for the measurement of total and free chlorine. The results from these methods can be used to calculate the levels of combined chlorine (or chloramines). However, it is also important to determine the levels of organic chloramines to avoid overestimating the disinfectant residual.
For municipal plants, a change in disinfectant (such as changing the disinfectant residual to chloramine) can impact water quality. When considering conversion to chloramine, utilities should assess the impacts on their water quality and system materials, including the potential for corrosion, nitrification and the formation of disinfection by-products.
Chloramines may be found in drinking water at the treatment plant, in the distribution system and in premise plumbing. For consumers that find the taste of chloramines objectionable, there are residential drinking water treatment devices that can decrease concentrations of chloramines in drinking water. However, removal of the disinfectant residual is not recommended.
2.4 International considerations
Drinking water quality guidelines, standards and/or guidance established by foreign governments or international agencies may vary due to the science available at the time of assessment, as well as the utilization of different policies and approaches, such as the choice of key study, and the use of different consumption rates, body weights and allocation factors.
Several organizations have set guidelines or regulations for chloramines in drinking water, all based on the same study which found no health effects at the highest dose administered.
The U.S. EPA has established a maximum residual disinfectant level of 4 mg/L for chloramine, recognizing the benefits of adding a disinfectant to water on a continuous basis and of maintaining a residual to control for pathogens in the distribution system. The World Health Organization and Australia National Health and Medical Research Council both established a drinking water guideline of 3 mg/L.
3.0 Application of the guideline
Note: Specific guidance related to the implementation of drinking water guidelines should be obtained from the appropriate drinking water authority in the affected jurisdiction.
Chloramines are formed when chlorine and ammonia are combined in water and comprise three chemical species: monochloramine (NH2Cl), dichloramine (NHCl2) and trichloramine (NCl3). The relative amounts formed are dependent on numerous factors, including pH, chlorine:ammonia ratio (Cl2:NH3), temperature and contact time. When chloramines are used as a disinfectant in drinking water systems, the desired species is monochloramine. When treatment processes are optimized for monochloramine stability (Cl2:NH3 weight ratio of 4.5:1–5:1, pH >8.0), almost all of the chloramines are present as monochloramine. Since chloramines can also be formed when ammonia is present in source water, utilities should characterize their source water to assess the presence of and variability of ammonia levels. When utilities are considering conversion from chlorine to chloramine, they should assess the impacts on their water quality and system materials, including the potential for corrosion, nitrification and the formation of disinfection by-products.
Maintenance of adequate disinfectant residual will minimize bacterial regrowth in the distribution system and provide a measurable level of chloramine; therefore, a drop in monochloramine level, suggesting unexpected changes in water quality, can be more quickly detected. Specific requirements for chloramine residual concentrations are set by the regulatory authority and may vary among jurisdictions. Monochloramine, used as a secondary disinfectant, should be applied so as to maintain a stable residual concentration throughout the distribution system. The appropriate amount of disinfectant needed to maintain water quality in the distribution system will depend on (among other factors), the characteristics of the distribution system, the species of bacteria, the presence of biofilms, the temperature, the pH and the amount of biodegradable material present in the treated water. Water utilities should be aware that a minimum target chloramine residual of "detectable" will not be sufficient to effectively limit bacterial growth in the distribution system. Regular monitoring of distribution system water quality (e.g., disinfectant residual, microbial indicators, turbidity, pH) and having operations and maintenance programs in place (water mains cleaning, cross-connection control, replacements and repairs) are important for ensuring that drinking water is transported to the consumer with minimum loss of quality.
Depending on the water system, chloramine residual concentrations of >1.0 mg/L may be required to maintain lower heterotrophic bacterial counts, to reduce coliform occurrences and to control biofilm development. Some utilities may require monochloramine concentrations much higher than this to address their specific distribution system water quality. Nitrification in the distribution system is also a potential problem for municipal systems that chloraminate. The concerns for utilities from nitrification are the depletion of the disinfectant residual, increased bacterial growth and biofilm development in the distribution system, as well as decreased pH, which can result in corrosion issues. When used as part of a program for nitrification prevention and control, suggested best operational practices for a chloramine residual are 2 mg/L leaving the treatment plant and preferably greater than 1.5 mg/L at all monitoring points in the distribution system. Information on strategies for controlling nitrification can also be found in the guideline technical document for ammonia.
Most Canadian drinking water supplies maintain a chloramine residual range below 4 mg/L in the distribution system. At these concentrations, taste and odour related to chloramines are generally within the range of acceptability for most consumers. Individual sensitivities in the population are widely variable, but generally, taste and odour complaints have resulted at levels of 3–3.7 mg/L monochloramine. Taste and odour concerns should be taken into account during the selection of operational and management strategies for the water treatment and distribution systems, although they do not make the water unsafe to consume. The protection of public health by maintaining the microbiological safety of the drinking water supply during distribution is the primary concern when using monochloramine for secondary disinfection.
Taste and odour issues can be indicators that operational changes may be required to address causal issues (i.e., water age, loss of monochloramine stability, formation of dichloramine, etc.). Utilities should establish operational targets for a disinfectant residual concentration appropriate for their system: one that allows them to meet their water quality objectives (i.e., microbial protection, minimal formation of disinfection by-products, nitrification prevention, biological stability and corrosion control).
Dialysis treatment providers at all levels (e.g., large facilities/hospitals, small community facilities, mobile units, providers for independent/home dialysis) should be notified that water is chloraminated.
3.1 Monitoring
Utilities using chloramine for secondary disinfection should, at minimum, monitor total chlorine residual daily in water leaving the treatment plant and throughout the distribution system. Disinfectant residual sampling should be conducted at the point of entry (baseline) and throughout the distribution system. This ensures that the target chloramine level is being applied at all times and provides a comparison for residual levels observed throughout the distribution system. Sample locations should be chosen to represent all areas of the distribution system. Key points for sampling also include entry point to distribution system (baseline), storage facilities, upstream and downstream of booster stations, in areas of low flow or high water age, in areas of various system pressures, in mixed zones (blended water) and in areas with various sizes and types of pipe material. Some utilities should also consider increasing the frequency of sampling during warmer months. Targeting more remote locations with fewer samples versus taking more samples at fewer locations can be a useful strategy for providing a more representative assessment of residual achieved and for detecting problem areas. Dedicated sampling taps are an ideal approach for residual sampling, and customer taps can be used as an alternative. In the absence of suitable tap access, hydrants can be used for residual sampling, although taking a meaningful hydrant sample can be challenging. Additional samples can be added for investigative purposes. Having operators well trained in the use of field testing methods for free and total chlorine will also be important for ensuring the accuracy of measurements.
Operational parameters (including finished water pH, free chlorine, free ammonia, temperature, total organic carbon and alkalinity) should be monitored at the treatment plant when using chloramines. It is also recommended that water leaving the treatment plant and throughout the distribution system be tested at least weekly for nitrite and ammonia. Nitrite should also be analyzed weekly at storage facilities and in areas of low flow and high water age to monitor for nitrification. Utilities should also monitor weekly for free ammonia at locations such as reservoir outlets and areas with long water detention times (e.g., dead ends). Changes in the trends of nitrification parameters in the distribution system (i.e., total chlorine residual, nitrite and nitrate) should trigger more frequent monitoring of free ammonia. Utilities that undertake comprehensive preventive measures and have baseline data indicating that nitrification does not occur in the system may conduct less frequent monitoring of free ammonia and nitrite. Heterotrophic plate count (HPC) monitoring on a monthly basis, at minimum, is also useful as a tool to assess system water quality.
More information on monitoring for nitrite, ammonia and HPCs can be found in the guideline technical documents on nitrate and nitrite and on ammonia and in the guidance document on the use of HPCs in Canadian drinking water supplies.
Part II. Science and Technical Considerations
4.0 Identity, use and sources in the environment
Chloramines are oxidizing compounds containing one or more chlorine atoms attached to a nitrogen atom. In the literature, the term "chloramine" refers to both inorganic and organic chloramines. Health effects of organic chloramines are beyond the scope of the present document and will not be discussed. Throughout the document, the term "chloramines" will refer only to inorganic chloramines, unless otherwise specified.
Inorganic chloramines consist of three chemically related compounds: monochloramine, dichloramine and trichloramine. Only mono- and dichloramine are really soluble in water. The volatility varies depending on the compound, with trichloramine being the most volatile. Their physical properties are provided in the table below:
| Parameter | Inorganic chloramine compounds | ||
|---|---|---|---|
| Monochloramine | Dichloramine | Trichloramine | |
| Synonym | ChloramideFootnote d | ChlorimideFootnote d | Nitrogen trichlorideFootnote b |
| CAS No. | 10599-90-3 | 3400-09-7 | 10025-85-1 |
| Molecular formula | NH2Cl | NHCl2 | NCl3 |
| Molecular weightFootnote a | 51.48 | 85.92 | 120.37 |
| Water solubilityFootnote a | Soluble | Soluble | Limited to hydrophobic |
| Boiling point | 486°C (predicted)Footnote e | 494°C (predicted)Footnote e | NA |
| pKFootnote a | 14 ± 2 | 7 ± 3 | NA |
| Henry's Law constant—Kaw (estimated, at 25°C)Footnote c | 0.00271 | 0.00703 | 1 |
| Vapour pressure (at 25°C) | 1.55 × 10 –7 PaFootnote c | 8.84 × 10 –8 PaFootnote c | 19.99 kPaFootnote b |
Chloramines have been used for almost 90 years as disinfectants to treat drinking water. Although chloramines are less efficient than free chlorine in killing or inactivating pathogens, they generate less trihalomethanes (THMs) and haloacetic acids (HAAs). They are also more stable than free chlorine, thus providing longer disinfection contact time within the drinking water distribution system. Because of these properties, chloramines are mainly used as secondary disinfectants to maintain a disinfectant residual in the distribution system and are generally not used as primary disinfectants (White, 1992).
Of the three chloramines, monochloramine is the preferred species for use in disinfecting drinking water because of its biocidal properties and relative stability, and because it rarely causes taste and odour problems when compared with dichloramine and trichloramine (Kirmeyer et al., 2004).
As a secondary usage, monochloramine has been used for the organic synthesis of amines and substituted hydrazines used as pharmaceutical intermediates, while dichloramine has been used for the preparation of diazirine, a labelling reagent (Graham, 1965; Kirk-Othmer, 2004; El-Dakdouki, 2014). Trichloramine gas was previously used in the food industry for bleaching flour (agene method), but the practice was discontinued in 1950 in the United States and the United Kingdom due to adverse health effects seen in animals (Shaw and Bains, 1998). Other uses have included paper manufacturing and fungicide treatment for fruit (Kirk-Othmer, 2004).
Chloramines do not occur naturally (IARC, 2004). They may be intentionally produced or generated as by-products of drinking water chlorination, including in groundwater systems that undergo chlorination in the presence of natural ammonia, as well as in chlorinated wastewater effluents (WHO, 2004; Hach, 2017).
For disinfection purposes, chloramines are formed through a process called chloramination (U.S. EPA, 1999). Chloramination involves the addition of ammonia (NH3) to free aqueous chlorine (hypochlorous acid, HOCl). This mixture can lead to the formation of inorganic compounds, such as monochloramine, dichloramine, and trichloramine (NHMRC, 2011).
NH3 + HOCl –> NH2Cl (monochloramine) + H2O
NH2Cl + HOCl –> NHCl2 (dichloramine) + H2O
NHCl2 + HOCl –> NCl3 (trichloramine) + H2O
Chloramine speciation mainly depends on the chlorine to ammonia ratio (Cl2:NH3) and the pH, but also depends on the temperature and the contact time (U.S. EPA, 1999). Cl2:NH3 ratios of ≤5:1 by weight (equivalent to ratios of ≤1:1 by mole) are optimum for monochloramine formation. The Cl2:NH3 ratio by weight is defined as the amount of chlorine added in proportion to the amount of ammonia added (in milligrams); all the Cl2:NH3 ratios presented in the following document are reported by weight. Ratios between 5:1 and 7.6:1 favour dichloramine production, whereas trichloramine is produced at higher ratios. Neutral to alkaline conditions (pH 6.5–9.0) are optimum for monochloramine formation (monochloramine formation occurs most rapidly at a pH of 8.3), whereas acidic conditions are optimum for the formation of dichloramine (pH 4.0–6.0) and trichloramine (pH <4.4) (Kirmeyer et al., 2004).
Under typical drinking water treatment conditions (pH 6.5–8.5) and with a Cl2:NH3 ratio of <5:1 (a ratio of 4:1 is typically accepted as optimal for chloramination), both monochloramine and dichloramine are formed with a much higher proportion of monochloramine (U.S. EPA, 1999). For example, when water is chlorinated with a Cl2:NH3 ratio of 5:1 at 25°C and pH 7.0, the proportions of monochloramine and dichloramine are 88% and 12%, respectively (U.S. EPA, 1994a). For its part, trichloramine can be formed in drinking water at pH 7.0 and 8.0, but only if the Cl2:NH3 ratio is increased to 15:1 (Kirmeyer et al., 2004). Thus, under usual water treatment conditions, monochloramine is the principal chloramine encountered in drinking water. Elevated levels of dichloramine and trichloramine in drinking water may occur, but would be due to variations in the quality of the raw water (e.g., pH changes) or accidental changes in the Cl2:NH3 ratio (Nakai et al., 2000; Valentine, 2007).
Chloramines (mono-, di- and trichloramines) can be found in media other than drinking water. In swimming pools, for example, they are disinfection by-products (DBPs) incidentally formed from the decomposition, via chlorination, of organic-nitrogen precursors, such as urea, creatinine and amino acids, originating from human excretions (e.g., sweat, feces, skin squama, urine) (Li and Blatchley, 2007; Blatchley and Cheng, 2010; Lian et al., 2014).
Chloramines are also formed when wastewater effluents or cooling waters are treated with chlorine (U.S. EPA, 1994a). In the food industry, they may result from the reaction between hypochlorite and nitrogen compounds coming from the proteins released by vegetables or animals (Massin et al., 2007). At home, chloramine fumes (a combination of monochloramine and dichloramine forming a noxious gas) can be produced when bleach and ammonia are accidentally mixed for cleaning purposes (Gapany-Gapanavicius et al., 1982).
4.1 Environmental fate
Because chloramines are found mainly in the aqueous phase, their fate in the environment will be ruled by processes relevant to this media.
The (auto) decomposition of monochloramine in water is affected by many factors (Wilczak et al., 2003b); its rate will increase as the temperature and inorganic carbon increase, the Cl2:NH3 ratio increases and the initial chloramine concentration, as well as the pH decrease. Vikesland et al. (2001), using decay experiments, reported that at pH 7.5, monochloramine has a half-life of over 300 h at 4°C, whereas it decreases to 75 h at 35°C. Autodecomposition of aqueous monochloramine to dichloramine will occur by one of two pathways: hydrolysis and acid-catalyzed disproportionation, both of which are described in Wilczak et al. (2003b).
When the pH is neutral, trichloramine in water will slowly decompose by autocatalysis to form ammonia and HOCl (U.S. EPA, 1994a). Trichloramine has limited solubility. Since it is extremely volatile, it will volatilize into air (U.S. EPA, 1994b; Environment Canada and Health Canada, 2001). By contrast, according the physico-chemical properties listed in Table 1, mono- and dichloramine are very soluble in water and not very volatile.
4.1.1 Impact of chloramines on aquatic life
Chloramines enter the Canadian aquatic environment primarily through municipal wastewater release (73%) and drinking water release (14%), along with other minor sources (Pasternak et al., 2003). Release of chloraminated water (total chlorine = 2.53 mg/L and 2.75 mg/L) as a result of a drinking water main break reportedly caused two large fish kills in the Lower Fraser River watershed (Nikl and Nikl, 1992). To mitigate the impact of chlorine or chloramines, aquarium owners must ensure the use of proper aeration or chlorine/chloramine quenching (Roberts and Palmeiro, 2008).
4.2 Terminology
This section provides definitions for some relevant terms used in this document, as adapted from the American Water Works Association (AWWA, 1999; Symons et al., 2000):
- Total chlorine: all chemical species containing chlorine in an oxidized state; usually the sum of free and combined chlorine concentrations present in water;
- Free chlorine: the amount of chlorine present in water as dissolved gas (Cl2), hypochlorous acid (HOCl), and/or hypochlorite ion (OCl-) that is not combined with ammonia or other compounds in water;
- Combined chlorine: the sum of the species resulting from the reaction of free chlorine with ammonia (NH3), including inorganic chloramines: monochloramine (NH2Cl), dichloramine (NHCl2), and trichloramine (nitrogen trichloride, NCl3);
- Chlorine residual: the concentration of chlorine species present in water after the oxidant demand has been satisfied;
- Primary disinfection: the application of a disinfectant at the drinking water treatment plant, with a primary objective to achieve the necessary microbial inactivation; and
- Secondary disinfection: the subsequent application of a disinfectant, either at the exit of the treatment plant or in the distribution system, with the objective of ensuring that a disinfectant residual is present throughout the distribution system.
4.3 Chemistry in aqueous media
The objectives of chloramination are to maximize monochloramine formation, to minimize free ammonia, and to prevent excess dichloramine formation and breakpoint chlorination. Chloramine formation is governed by the reactions of ammonia (oxidized) and chlorine (reduced); its speciation is principally determined by the pH and the Cl2:NH3 weight ratio. The reaction rate of monochloramine formation depends on the pH, the temperature and the Cl2:NH3 weight ratio. Ideally, a weight ratio of 4.5:1–5:1 will help minimize free ammonia and reduce the risk of nitrification (AWWA, 2006b).
The breakpoint chlorination curve can be used to illustrate the ideal weight ratio where monochloramine production can be maximized. For a utility wishing to produce monochloramine, the breakpoint ratio should be determined experimentally for each water supply (Hill and Arweiler, 2006). Figure 1 below shows an idealized breakpoint curve that occurs between pH 6.5 and 8.5 (Griffin and Chamberlin, 1941; Black and Veatch Corporation, 2010). Initially, monochloramine is formed, and once the Cl2:NH3 weight ratio is greater than 5:1, monochloramine formation decreases, because no free ammonia is available to react with the free chlorine being added. The reaction of free chlorine with monochloramine leads to the formation of dichloramine. When high enough Cl2:NH3 weight ratios are achieved, breakpoint chlorination will occur. The breakpoint curve is characterized by the “hump and dip” shape (Figure 1). Dichloramine undergoes a series of decomposition and oxidation reactions to form nitrogen-containing products, including nitrogen, nitrate, nitrous oxide gas and nitric oxide (AWWA, 2006b). Trichloramine, or nitrogen trichloride, is an intermediate during the complete decomposition of chloramines. Its formation depends on the pH and the Cl2:NH3 weight ratio and may appear after the breakpoint (Kirmeyer et al., 2004; Hill and Arweiler, 2006; Randtke, 2010).
After breakpoint, free chlorine is the predominant chlorine residual, not monochloramine. However, the reaction rate of breakpoint chlorination is determined by the formation of monochloramine and the formation and decay rates of dichloramine and trichloramine, reactions that are highly dependent on pH. The theoretical Cl2:NH3 weight ratio for breakpoint chlorination is 7.6:1; however, the actual Cl2:NH3 ratio varies from 8:1 to 10:1, depending on the pH, the temperature and the presence of reducing agents. The presence of iron, manganese, sulphide and organic compounds creates a chlorine demand; i.e., they compete with the free chlorine added, potentially limiting the chlorine available to react with ammonia (Kirmeyer et al., 2004; AWWA, 2006b; Muylwyk, 2009). It is therefore important that each utility generates a site-specific breakpoint curve, experimentally. Additionally, periodic switching to chlorine (breakpoint chlorination) for seasonal nitrification control has also been observed to increase DBPs such as trihalomethanes (chloroform) (Vikesland et al., 2006; Rosenfeldt et al., 2009). However, Rosenfeldt et al. (2009) also observed that flushing was an effective mitigation strategy.
Automated systems can be used to monitor and maintain weight ratio. Hutcherson (2007) reported that the application of programmable logic control and human-to-machine interface resulted in an intuitive and effective dosing control strategy for Newport Beach, California. The 5:1 ideal weight ratio was maintained and monitored 24 h/day. At the time of the report, the system had been in use for 2 years of successful operation.
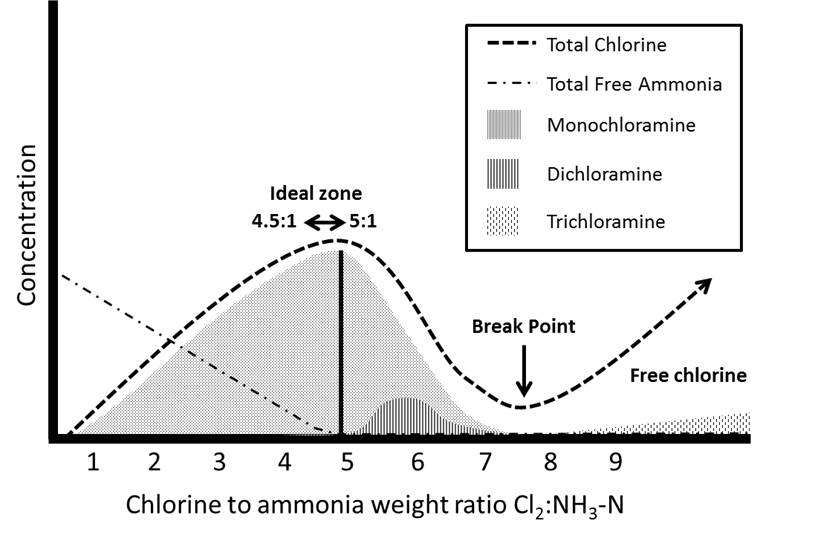
Figure 1 - Text Description
A graph that shows the idealized breakpoint chlorination curve for achieving maximum monochloramine formation while also minimizing free ammonia. The verticle axis shows concentration with no units and the horizontal axis shows a scale of chlorine to ammonia weight ratios (Cl2:NH3-N) ranging from 1 to 9. The graph shows that at a weight ratio ranging from 4.5:1 to 5:1 (the ideal zone), monochloramine formation and total chlorine are at a maximum while free ammonia reaches a concentration of zero. As the weight ratio increases, dichloramine formation begins increasing after a ratio of about 5.2:1, reaches a maximum at about 5.8:1 and drops to zero at about 7.2:1, where monochoramine formation also reaches zero. At a ratio of about 7.7:1, break point chlorination is depicted with the total chlorine curve reaching its minimum concentration. Beyond break point chlorination and beyond a ratio of 9:1, both total chlorine and trichloramine formation steadily increase.
4.4 Application to drinking water treatment
This document focuses on the health effects related to exposure to chloramine in drinking water supplies, whether formed intentionally as a secondary disinfectant, or unintentionally as a by-product of chlorination. It does not review the benefits or the processes of chloramination, nor does it assess the health risks related to exposure to by-products formed as a result of the chloramination process.
4.4.1 Chloramines in water treatment
The mechanisms by which monochloramine inactivates microbiological organisms are not fully understood (Jacangelo et al., 1991; Coburn et al., 2016). It is suggested that free chlorine and chloramines react with different functional groups in the cell membrane (LeChevallier and Au, 2004). The proposed mode of action of monochloramine is the inhibition of such protein-mediated processes as bacterial transport of substrates, respiration and substrate dehydrogenation (Jacangelo et al., 1991; Coburn et al., 2016). Experiments with bacteria indicated monochloramine was most reactive with sulphur-containing amino acids (LeChevallier and Au, 2004; Rose et al., 2007). Monochloramine did not severely damage the cell membrane or react strongly with nucleic acids. Monochloramine is a more selective reactant than free chlorine and seems to act in more subtle ways at drinking water concentrations (Jacangelo et al., 1991). Inactivation with monochloramine appears to require reactions at multiple sensitive sites (Jacangelo et al., 1991). More research is needed for a better understanding of the process behind chloramine disinfection.
4.4.2 Primary disinfection
Primary disinfection is the application of a disinfectant in the drinking water treatment plant, with the primary objective of achieving the necessary microbial inactivation.
Monochloramine is much less reactive than free chlorine, has lower disinfecting power and is generally not used as a primary disinfectant because it requires extremely high CT valuesFootnote 1 to achieve the same level of inactivation as free chlorine (Jacangelo et al., 1991, 2002; Taylor et al., 2000; Gagnon et al., 2004; LeChevallier and Au, 2004; Rose et al., 2007; Cromeans et al., 2010).
4.4.3 Secondary disinfection
Secondary disinfection may be applied to the treated water as it leaves the treatment plant or at rechlorination points throughout the distribution system, to introduce and maintain a disinfectant residual in the drinking water distribution system.
The main function of the residual is to protect against microbial regrowth (LeChevallier and Au, 2004). The disinfectant residual can also serve as a sentinel for water quality changes. A drop in residual concentration can provide an indication of treatment process malfunction, inadequate treatment or a break in the integrity of the distribution system (LeChevallier, 1998; Haas, 1999; Health Canada, 2012a, 2012b).
Monochloramine is slower to react and, in treated drinking water, can provide a more stable and longer-lasting disinfectant residual in the distribution system (Jacangelo et al., 1991; U.S. EPA, 1999; LeChevallier and Au, 2004; Cromeans et al., 2010). However, combined chlorine residuals are less functional as sentinels of potential post-treatment contamination events than free chlorine residuals. Declines in combined chlorine measurements may not always be large enough or rapid enough to alert utilities that a contamination problem has occurred within the distribution system (Snead et al., 1980; Wahman and Pressman, 2015). Also, a drop in residual may be due to nitrification and not post-treatment contamination (Wahman and Pressman, 2015). Disinfectant residual monitoring should be conducted alongside other parameters as part of broader programs for microbiological quality and nitrification.
Organic chloramines provide little to no disinfection (Feng, 1966; Donnermair and Blatchley, 2003). In bench-scale experiments, Lee and Westerhoff (2009) demonstrated that organic chloramines comprise a greater proportion of the chlorine residual in systems where chloramines are formed by adding ammonia after a 10-min contact time with free chlorine than in those where preformed monochloramine is used. Organic chloramines formed more rapidly in the presence of free chlorine than in the presence of monochloramine. However, as it is common practice to provide short chlorine contact time followed by ammonia addition, the risk of generating organic chloramines is an important consideration.
4.4.4 Formation of chloraminated disinfection by-products
Chloramines are often used to meet DBP compliance based on HAAs and THMs; however, chloramines also react with natural organic matter (NOM) to form other DBPs such as iodinated disinfection by-products (I-DBPs) and nitrosamines (Richardson and Ternes, 2005; Charrois and Hrudey, 2007; Hua and Reckhow, 2007; Richardson et al., 2008; Nawrocki and Andrzejewski, 2011). Hydrazine can also form as a result of abiotic reactions of ammonia and monochloramine (Najm et al., 2006).
I-DBPs are more readily formed in chloraminated systems. Monochloramine oxidizes iodide to hypoiodous acid quickly, but the reaction with NOM is slow, allowing sufficient time for the formation of I-DBPs (Singer and Reckhow, 2011). Chlorine and ozone can also oxidize iodide to hypoiodous acid; however, the iodide is further oxidized to iodate, forming only trace to minimal amounts of I-DBPs (Hua and Reckhow, 2007). In bench-scale formation experiments using simulated raw water, Pan et al. (2016) found that chloraminated water formed more polar I-DBPs than water treated with either chlorine dioxide or chlorine. The authors also noted that as pH increased (from 6 to 9), the formation of polar I-DBPs decreased. Water quality factors such as pH and ratios of dissolved organic carbon, iodide and bromide have been demonstrated to play an important role in determining the species and abundance of iodated trihalomethanes formed under drinking water conditions (Jones et al., 2012).
N-Nitrosodimethylamine (NDMA) is a nitrogen-containing DBP that may be formed during the treatment of drinking water, particularly during chloramination and, to a lesser extent, chlorination (Richardson and Ternes, 2005; Charrois and Hrudey, 2007; Nawrocki and Andrzejewski, 2011). The key to controlling the formation of NDMA lies in limiting its precursors, including dichloramine; thus optimization and control of free ammonia are important elements in preventing NDMA formation (Health Canada, 2011). Additionally, Krasner et al. (2015) demonstrated that several pre-oxidation technologies were effective in destroying the watershed-derived NDMA precursors (ozone > chlorine > medium pressure UV > low pressure UV > permanganate). Uzen et al. (2016) observed that site-specific factors such as upstream reservoirs, wastewater discharge, and mixing conditions can affect NDMA formation potential and should be characterized for each individual site. More detailed descriptions of precursors and treatment options can be found in that report (Krasner et al., 2015; Uzen et al., 2016). Cationic polymers containing diallyldimethylammonium chloride, used in water treatment, can also act as a source of NDMA precursors (Wilczak et al., 2003a).
Under certain conditions, hydrazine can form through a reaction between ammonia and monochloramine. Najm et al. (2006) found that at low concentrations of free ammonia-nitrogen (<0.5 mg/L) and at pH <9, less than 5 ng/L of hydrazine was formed, but that increasing either ammonia concentrations or pH also increased hydrazine formation. Davis and Li (2008) obtained 13 samples from six chloraminated drinking water utilities and found hydrazine above the detection limit of 0.5 ng/L in 7 of the samples (0.53–2.5 ng/L). Hydrazine production was found to be greatest where treatment processes had high pH (i.e., lime softening). Several treatment practices were identified to minimize hydrazine production, including delaying chloramination until the pH was adjusted (i.e., recarbonation step) and managing the Cl2:NH3 ratio to minimize the free ammonia concentration (Najm et al., 2011).
4.4.5 Taste and odour considerations
Chloramines have been described as having a flavour profile of chlorine-like, musty and/or chalky, ammonia, slat, soapy-slimy feel, sour, bitter, dry mouth. Trichloramine has also been described as having a geranium-like odour (White, 1972). Consumer concerns regarding chloramines in drinking water are often related to taste and odour issues, although the taste and odour of chloramines are generally less noticeable and less offensive to consumers than those of free chlorine. The principal chloramine species, monochloramine, normally does not contribute significantly to the objectionable taste and odour of drinking water when present at concentrations of less than 5 mg/L (Kirmeyer et al., 2004). Di- and tri- chloramines are more likely to cause complaints, especially if they comprise more than 20% of the chloramine concentration in the drinking water (Mallevialle and Suffett, 1987).
Several studies conducted with panels or volunteers to determine the taste and odour thresholds of chloramines in water showed that taste and odour were highly subjective. Krasner and Barrett (1984) used linear regression of data compiled from a trained panel of moderate- to highly-sensitive individuals to derive a taste threshold of 0.48 mg/L and an odour threshold of 0.65 mg/L for monochloramine. Only the most sensitive panelists could detect monochloramine in the range of 0.5–1.5 mg/L (Krasner and Barrett, 1984). By contrast, a taste threshold of 3.7 mg/L was determined using untrained volunteers from the public (Mackey et al., 2004). Similarly, White (1992) found that concentrations of 3 mg/L and even 5 mg/L of monochloramine in drinking water were unlikely to cause taste and odour complaints. Lubbers and Bianchine (1984) found a wide variability in individual perception of chloramine taste. Although a dose of 24 mg/L was slightly (6/10) to very (2/10) unpleasant to most volunteer test subjects (n = 10), one subject could not detect a taste and another did not find it objectionable.
By contrast, the presence of dichloramine and trichloramine was detected at much lower concentrations. Krasner and Barrett (1984) determined the taste and odour thresholds for dichloramine to be 0.13 mg/L and 0.15 mg/L, respectively. Taste and odour problems were not expected when dichloramine was below 0.8 mg/L (White, 1992). However, Krasner and Barrett (1984) felt that 0.5 mg/l was a better cutoff since objectionable taste and odour were noted at 0.9–1.3 mg/L, and at a lesser level at 0.7 mg/L (Krasner and Barrett, 1984). A similar odour threshold concentration was seen for trichloramine at 0.02 mg/L (White, 1972).
Taste and odour complaints should be monitored and tracked. Utilities can address taste and odour issues through a variety of operational strategies to address water age, disinfection demand, hydraulic issues (such as dead ends and low-flow areas), bacteria growth and dosing issues (Kirmeyer et al., 2004).
Optimizing treatment for monochloramine production reduces the potential to form dichloramine and trichloramine, resulting in water with the least flavour. Reactions of chloramines with organic compounds in water can form by-products that also cause tastes and odours.
Operational strategies to reduce tastes and odours include treating the water to remove taste and odour precursors, flushing the distribution system and reducing the detention time and water age in the distribution system. When using monochloramine for secondary disinfection the primary concern is the protection of public health by maintaining the microbiological safety of the drinking water supply during distribution.
Available studies (see Section 9.0) and surveys have not indicated evidence of adverse health effects associated with exposure to monochloramine at concentrations used in drinking water disinfection. Although levels commonly found in drinking water are within an acceptable range for taste and odour, individual sensitivities regarding the acceptability of water supplies can be varied. In addition, where elevated chloramine concentrations are required in order to maintain an effective disinfectant residual throughout the distribution system, the median taste thresholds may be exceeded. Therefore it is important that utilities contemplating a conversion to monochloramine remain aware of the potential for taste and odour concerns during the selection of operational and management strategies.
Communication with consumers is a key part of assessing and promoting the acceptability of drinking water supplies with the public. Consumer feedback on drinking water quality is an important source of data for utilities to assess the quality of drinking water distributed to residences and businesses. Evaluation of consumer acceptability and knowledge of consumer complaints are also recognized as important components of water quality verification under a Water Safety Plan approach to drinking water delivery. Guidance material to help water utilities develop programs for communication and consumer feedback is available elsewhere (Whelton et al., 2007 ).
5.0 Exposure
No environmental data were found for inorganic chloramines in sediments, soils and ambient air (Environment Canada and Health Canada, 2001). As a result, drinking water is considered the primary source of exposure for this assessment.
5.1 Water
Chloramines are usually measured as combined chlorine residuals, corresponding to the difference between total chlorine residual and free chlorine residual. This method has limitations, because the combined chlorine value does not determine the individual concentrations of mono- and dichloramines present in drinking water, and because the free chlorine measurement is not always accurate in the presence of high levels of chloramines. Individual chloramines can be differentiated using multi-stage procedures, but interferences such as organic chloramines may in some cases result in misleading measurements (e.g., overestimation of monochloramine concentrations) (Lee et al., 2007; Ward, 2013) (see Section 6 for additional information).
Limited provincial data are reported in Table 2. Generally, levels of chloramines or combined chlorine are below 3 mg/L; only a few values exceeding 3 mg/L were reported.
The City of Ottawa (2017) uses chloramines for secondary disinfection, and results from 2016 show an average monochloramine residual of 1.62 mg/L (range 1.21–2.12 mg/L) for the Britannia Water Purification Plant (WPP) and 1.56 mg/L (range 1.21–2.03 mg/L) for the Lemieux Island WPP. The remaining chloramines measured (i.e., total of di- and trichloramines) only make up a small portion of the chloramine residual for each of the two treatment plants (averages of 0.06 mg/L and 0.12 mg/L, respectively).
| Province(Year) | # of sites | Range: min–max (No. non-detects/total no. of samples) | ||||||
|---|---|---|---|---|---|---|---|---|
| Chlorine | Chloramines | |||||||
| Total | Free | Combined | Chloramines | Mono- | Di- | Tri- | ||
| NSFootnote e (2008–2015) |
39 | — | — | — | <0.00002–1.75 mg/L (1/81) |
<0.1–0.1 mg/L (0/9) |
<0.1 (0/9) | <0.1–0.4 mg/L (0/9) |
| QCFootnote f (2013–2015) |
3Footnote a | — | — | — | 0.07–1.80 mg/L (0/7) |
— | — | — |
| QCFootnote f,Footnote j (2013–2015) |
5Footnote b | 0.01–4.6 mg/L (0/3432) |
0–4.2 mg/L (544/3432) |
— | 0–3.24 mg/L (9/3432) |
— | — | — |
| QCFootnote f (2013–2015) |
11Footnote c | 0–7.05 mg/L (8/2924) |
0–5.5 mg/L (239/2924) |
— | 0–2.47 mg/L (8/2924) |
— | — | — |
| ONFootnote g (2012–2017) |
108 | — | — | 0–2.64 mg/L (2/1500) |
— | — | — | |
| SKFootnote d,Footnote h,Footnote j (2006–2015) |
18 | 0.62–3.24 mg/L (0/25) |
0.01–1.18 mg/L (0/24) |
— | 0.01–3.9 mg/L (0/28) |
1.28–3.11 mg/L (0/24) |
— | — |
| BCFootnote i (2015) |
37 | 0.01–1.86 ppm (10/1923) |
0–0.97 ppm (10/1918) |
— | — | 0.6–1.51 mg/L (0/51) |
0–0.7 µg/L (4/51) |
— |
| BCFootnote i (2016) |
37 | 0.03–1.89 ppm (0/1931) |
0–0.93 ppm (11/1929) |
— | — | 0–1.3 mg/L (1/51) |
0–0.61 µg/L (2/50) |
— |
5.2 Air
Chloramines may be encountered in the ambient air of food industry facilities that typically use large quantities of disinfecting products. For example, total concentrations of chloramines (mainly trichloramine) have been reported in the ambient air of green salad processing plants (e.g., 0.4–16 mg/m3; Hery et al., 1998) and of turkey processing plants (e.g., 0.6–1 mg/m3 average concentrations; Kiefer et al., 2000).
5.3 Swimming pools and hot tubs
Chloramines are present in indoor swimming pools as DBPs of chlorination. Due to its high volatility and low solubility, trichloramine is the predominant species present in the swimming pool atmosphere. Numerous papers report trichloramine concentrations in the atmosphere of indoor swimming pools (including water parks), with mean concentrations ranging from approximately 114 µg/m3 to 670 µg/m3 (Carbonnelle et al., 2002; Thickett et al., 2002; Jacobs et al., 2007; Dang et al., 2010; Parrat et al., 2012). The levels of airborne trichloramine are influenced by such factors as number of swimmers, organic compounds (mainly urine and sweat) introduced into the water by swimmers, air ventilation, as well as water temperature, circulation and movement (splashing, waves, etc.) (Carbonnelle et al., 2002; Jacobs et al., 2007; Parrat et al., 2012).
5.4 Multiroute exposure through drinking water
Mono- and dichloramine do not possess the physico-chemical characteristics to consider their contribution via the dermal or inhalation route, since both are water soluble but not volatile (see Table 1). Conversely, trichloramine is very volatile and not soluble in water. In addition, trichloramine is relatively unstable in water and is only formed beyond breakpoint (see Figure 1) or under low pH, conditions that are unlikely to occur in treated drinking water. Therefore under normal usage conditions, the ratio of trichloramine to total chloramines is very low and would not contribute significantly to the dermal or inhalation routes of exposure. Consequently, exposure to chloramines via inhalation and dermal routes during showering or bathing is expected to be negligible.
6.0 Analytical methods
There are no U.S. EPA approved methods for the direct measurement of chloramines. There are, however, several U.S. EPA approved methods for the measurement of total and free chlorine (Table 3). Free chlorine is the sum of chlorinated species that do not contain ammonia or organic nitrogen (i.e., Cl2, HOCl, OCl-, and Cl3-), whereas combined chlorine is the sum of chlorine species that are combined with ammonia (NH2Cl, NHCl2, and NCl3) (Black and Veatch Corporation, 2010). Since total chlorine is often used as an assumed proxy for combined chlorine (chloramines), it is important that free chlorine be measured to validate the assumption that none is present. Although there is no method for directly measuring chloramines, equation 1 below shows how chloramines can be determined by subtracting free chlorine from total chlorine:
Combined chlorine (chloramines) = Total chlorine – Free chlorine (1)
When using the DPD colorimetric test, it is important to ensure that field staff are well trained to do both free and total chlorine measurements. This ensures that false positive results are not inadvertently reported (Spon, 2008). Users should consult with the manufacturer regarding method interferences, interfering substances and any associated corrective steps that may be necessary. Although they are not approved by the U.S. EPA for direct measurement of chloramine species, several methods (SM 4500 D, 4500 F, and 4500 G) have additional steps (beyond total, free and combined chlorine) that can be used to distinguish between the various chloramine species. Both dichloramine and trichloramine are relatively unstable, and their formation reactions do not proceed to completion under typical drinking water conditions (Black and Veatch Corporation, 2010). Specific instructions for mitigating effects of interfering agents (including interference from other chlorine species), optimal analytical performance (including the use of reagent blanks), and reaction times for accurate sample readings are available in the method documents.
| Method(Reference) | Methodology | Residual measured (MDL) | Comments |
|---|---|---|---|
| ASTM D1253 (ASTM, 1986, 2003, 2008, 2014) |
Amperometric titration | Total, free, combined (NA) | Reaction is slower at pH >8 and requires buffering to pH 7. A maximum concentration of 10 mg/L is recommended. Interferences include cupric, cuprous and silver ions; trichloramine; some N-chloro compounds; chlorine dioxide; dichloramine; ozone; peroxide; iodine; bromine; ferrate; Caro's acid. |
| SM 4500-Cl D (APHA et al., 1995, 1998, 2000, 2005, 2012) |
Amperometric titration | Total, free, combined (NA) | Dilutions are recommended for concentrations above 2 mg/L. Interferences are trichloramine, chlorine dioxide, free halogens, iodide, organic chloramines, copper, silver. Monochloramine can interfere with free chlorine, and dichloramine can interfere with monochloramine. Method can also be used to characterize species (monochloramine and dichloramine). |
SM 4500-Cl G (colorimetric) SM 4500-Cl F
(ferrous) |
N,N-diethyl-p-phenylenediamine (DPD) | Total, free, combined
Total, free, combined |
Interferences include oxidized manganese, copper, chromate, iodide, organic chloramines. Method can also be used to characterize species (monochloramine, dichloramine, and trichloramine) in a laboratory setting. |
| Hach 10260 Rev 1.0 (HACH, 2013) |
DPD Chemkey | Total (0.04 mg/L), free (0.04 mg/L), combined (NA) | Interferences include acidity >150 mg/L CaCO3, alkalinity >250 mg/L as CaCO3 highly buffered or extreme pH samples, bromine, chlorine dioxide, iodine, ozone, organic chloramines, peroxides, oxidized manganese, oxidized chromium. |
Organic chloramines are formed when dissolved organic nitrogen reacts with either free chlorine or inorganic chloramines (Lee and Westerhoff, 2009) and are known interfering agents for both the amperometric and the DPD methods (APHA et al., 2012). Wahman and Pressman (2015) highlighted that organic chloramines can provide false positives. Lee and Westerhoff (2009) estimated that utilities are likely to overestimate chloramine residuals by approximately 10% as a result of interference from organic chloramines. Similarly, in Gagnon et al. (2008), a pipe loop distribution system study identified that organic chloramines comprised approximately 10–20% of the total chlorine residual in a chloraminated system. The contribution of organic chloramines to the total chlorine residual can be calculated by subtracting the monochloramine concentration from the total chlorine residual. Although not approved by the U.S. EPA, the Hach 10241 U.S. EPA Indophenol method (Hach, 2017) can be used to measure monochloramine using a DPD method. This method could be used to help inform day-to-day operations by providing an approximation of organic chloramines.
6.1 Recommended method
Key points for sampling also include the entry point to the distribution system (baseline), storage facilities, upstream and downstream locations of booster stations, areas of low flow or high water age, areas of various system pressures, mixed zones (blended water), and areas with various sizes and types of pipe material. Dedicated sampling taps are an ideal approach for residual sampling (AWWA, 2013), and customer taps can be used as an alternative. In the absence of suitable tap access hydrants can be used for residual sampling. However, taking a meaningful hydrant sample can be challenging. The U.S. EPA (2016a) has developed a hydrant sampling best practice manual that can be used to estimate the flush time required for a representative sample. Alexander (2017) recommended targeting remote locations of a distribution system, suggested that it is preferable to target more locations with fewer samples at those locations vs. more samples at fewer locations, and recommended taking additional investigative samples. An investigation of disinfectant residuals in Flint, Michigan, revealed that the previous number and location of sampling sites (10 sites) were insufficient. These few locations demonstrated adequate disinfectant (chlorine) residual; however, expanding the number and location of sample sites to more representative locations (an additional 24 sites) revealed that the disinfectant residual was problematic (Pressman, 2017). Although chloramine residuals are consumed less readily in the distribution system, it is still important to have an adequate and representative set of sampling sites. Resources outlining approaches for determining the number and location of sampling points for monochloramine disinfectant residual monitoring in the distribution system are available elsewhere (Louisiana Department of Health and Hospitals, 2016).
Alexander (2017) highlighted the importance of proper field testing techniques. Sample vials can become scratched (during transportation in a truck) or dirty, leading to inaccurate readings. Additionally, plastic vials are prone to the formation of fine bubbles, which can be resolved with slow inversion of the sample. It is important that operators be aware of the challenges of disinfectant residual sampling.
Sampling programs should be reviewed annually and the review should examine historical data, water use patterns/changes, as well as any changes in water treatment or distribution system operation (AWWA, 2013).
7.0 Treatment technology and distribution system considerations
As chloramines are added to drinking water to maintain a residual concentration in the distribution system, or are formed as a by-product of the chlorination of drinking water in the presence of natural ammonia, they are expected to be found in drinking water at the treatment plant, as well as in the distribution and plumbing systems.
7.1 Municipal scale
The literature indicates that monochloramine CT requirements are one to many orders of magnitude greater than those required for free chlorine for achieving similar levels of inactivation of heterotrophic bacteria, E. coli, nitrifying bacteria, enteric viruses and Giardia (LeChevallier and Au, 2004; Wojcicka et al., 2007; Cromeans et al., 2010; Health Canada, 2012b, 2017, 2018a). Reported CT values also demonstrate that similarly to free chlorine, monochloramine is not effective for inactivation of Cryptosporidium (LeChevallier and Au, 2004; Health Canada, 2017).
Given the operational benefits of secondary disinfection, operators should strive to maintain a stable disinfectant residual throughout the system. Regularly monitoring distribution system water quality (e.g., disinfectant residual, microbial indicators, turbidity, pH) and having operational and maintenance programs in place (water mains cleaning, cross-connection control, replacements and repairs) are important for ensuring that drinking water is transported to the consumer with minimum loss of quality (Kirmeyer et al., 2001, 2014).
7.1.1 Disinfectant residual and microbial control
To control bacterial regrowth and biofilm formation, a strong, stable disinfectant residual is needed that is capable of reaching the ends of the distribution system. The amount of disinfectant needed to maintain water quality depends on the characteristics of the distribution system, the species of bacteria, the type of disinfectant used, the temperature, the pH and the amount of biodegradable organic material present (Kirmeyer et al., 2004). When applying monochloramine as a disinfectant, utilities should be aware of their target disinfectant residual value, and adjustments should be made to address monochloramine demand and decay. Manuals have been developed to guide water utilities in establishing goals and disinfection objectives. These manuals recommend that utilities include setting system-specific disinfectant residual targets based on their water quality objectives and system characteristics, and that they ensure chloramine concentrations leaving the treatment plant are sufficient to achieve their established target residual (Kirmeyer et al., 2004; AWWA, 2006a). Full-scale and laboratory-scale studies have been conducted to examine the effectiveness of monochloramine residual concentrations in controlling biofilm growth in drinking water distribution systems. Camper et al. (2003) observed that a monochloramine residual of 0.2 mg/L did not completely control biofilm growth on coupons of epoxy, ductile iron, polyvinyl chloride (PVC) or cement. Similarly, Pintar and Slawson (2003) noted that maintaining a low monochloramine residual of 0.2–0.6 mg/L was not sufficient to inhibit the establishment of biofilms containing nitrifying bacteria and heterotrophic bacteria on PVC coupons. Wahman and Pressman (2015) have noted that when using inorganic chloramines (monochloramine), a “detectable” total chlorine residual is not sufficient to effectively limit bacterial growth in the distribution system. In full-scale investigations conducted by Norton and LeChevallier (1997) and Volk and LeChevallier (2000), water samples from drinking water systems maintaining monochloramine residual concentrations of >1.0 mg/L maintained lower heterotrophic bacterial counts and substantially fewer coliform occurrences than systems with lower monochloramine levels. In laboratory experiments a monochloramine residual of >1.0 mg/L reduced viable counts by greater than 1.5 log for biofilms grown on PVC, galvanized iron, copper and polycarbonate (LeChevallier et al., 1990; Gagnon et al., 2004; Murphy et al., 2008). When biofilms were grown on iron pipe, a monochloramine residual of >2.0 mg/L was needed to reduce bacterial numbers by greater than 2 log (LeChevallier et al., 1990; Ollos et al., 1998; Gagnon et al., 2004). Monochloramine residual concentrations of >2 mg/L may similarly be required to control the development of nitrifying biofilms.
Monochloramine has been perceived to penetrate biofilms better because of its low reactivity with polysaccharides—the primary component of the biofilm matrix (Vu et al., 2009; Xue et al., 2014). In disinfection transport experiments using chlorine/monochloramine-sensitive microelectrodes, monochloramine was shown to penetrate biofilm faster and further than free chlorine but not to result in complete inactivation (Lee et al., 2011, Pressman et al., 2012). Work by Xue et al. (2013, 2014) suggests that monochloramine disinfection efficacy and persistence may be affected by the combination of polysaccharide extracellular polymeric substances (EPSs) obstructing monochloramine reactive sites on bacterial cells, and protein EPS components reacting with monochloramine. Free chlorine, on the other hand, was more effective near the biofilm surface, where it penetrated but had little biofilm penetration (Lee et al., 2011). The authors noted that these findings highlight the challenges for chloraminating drinking water utilities that employ free chlorine application periods for nitrification control and also help explain why nitrification is so hard to stop once it has started.
An understanding of system-specific factors that may make it difficult to achieve target residuals is important for maximizing the effectiveness of strategies for secondary disinfection.
7.1.2 Considerations when changing disinfection practices to chloramination
A change in disinfectant (oxidant), including changing secondary disinfection to chloramine, can impact water quality. It is always recommended that site-specific water quality/corrosion studies be conducted to capture the complex interactions of water quality, distribution system materials and treatment chemicals used in each individual water system. Some considerations when applying chloramine treatment include nitrification, biofilm, residual loss, the formation of DBPs, the degradation of elastomer materials and the impacts the distribution system/premise plumbing materials may have on corrosion.
7.1.3 Presence of ammonia in source water
Ammonia may be present in source water and strategies to treat it will depend on many factors, including the characteristics of the raw water supply, the source and concentration of ammonia (including variation), the operational conditions of the specific treatment method and the utility's treatment goal. In some cases, utilities will form chloramines as a strategy to remove naturally occurring ammonia in the raw water supply, while others may use breakpoint chlorination. In both cases, frequent monitoring of relevant parameters (ammonia; combined, total and free chlorine) is needed to ensure that objectives are achieved at all times. More details on ammonia removal technologies and strategies, including biological treatment, can be found in Health Canada (2013a).
7.2 Distribution system considerations
The use of chloramines as a secondary disinfectant can have impacts on the distribution system water quality, including corrosion, nitrification, microbial regrowth and the growth of opportunistic pathogens.
7.2.1 Residual loss
Chloramines auto-decompose through a series of reactions (Jafvert and Valentine, 1992). However, decomposition of chloramines can also be impacted by interactions with pipe materials and NOM, resulting in loss of residual.
Vikesland and Valentine (2000) demonstrated a direct reaction between Fe(II) and the monochloramine molecule, ultimately forming ammonia. Iron oxides play an autocatalytic role in the oxidation of Fe(II) by monochloramine (Vikesland and Valentine, 2002). In bench-scale experiments, Westbrook and DiGiano (2009) compared the rates of chloramine loss at two pipe surfaces (cement-lined ductile iron and tuberculated, unlined cast iron pipe). Chloramine decay occurred faster (from 3.8 mg/L to <1 mg/L in 2 h) for the unlined cast iron pipe compared with the cement-lined ductile iron pipe (from 3.7 mg/L to 1.5 mg/L in 2 h).
Duirk et al. (2005) described two reaction pathways between chloramines and NOM: one fast reaction and a slow reaction where chloramines were hypothesized to hydrolyze to HOCl. The slow reaction was reported to account for the majority of the chloramine loss in this mechanism. Work by Zhou et al. (2013) reported that the mixing ratio of chloramines impacted their decay rate in the presence of NOM: a higher decay rate was observed for chloramines mixed in a ratio of 3:1 than for those mixed at 4:1. Wilczak et al. (2003b) suggested that the nature and characteristics of NOM are important factors in determining how reactive chloramine is with NOM. Additionally, the authors observed that bacterial cell fragments shed from full-scale biologically active granular activated carbon (GAC) filters exerted a significant chloramine demand.
Carbonate has been observed to accelerate monochloramine decay by acting as a general acid catalyst (Vikesland et al., 2001). This study showed that nitrite exerted a significant long-term monochloramine demand and demonstrated that although bromide affects chloramine loss, at low concentrations (<0.1 mg/L), bromide did not have a significant role. Wahman and Speitel (2012) explored the important role of HOCl in nitrite oxidation and found that it peaked in the pH range of 7.5–8.5. A 5:1 Cl2:NH3 ratio was also associated with increased nitrite oxidation (compared with a 3:1 Cl2:NH3 ratio). Increased oxidation of nitrite by HOCl was also associated with lower nitrite concentration (0.5 mg N/L compared with 2 mg N/L) and decreased monochloramine concentrations (1 mg Cl2/L compared with 4 mg Cl2/L).
Factors such as mixing ratios, pH, and temperature have also been shown to play an important role in chloramine decay rates. In experiments by Zhou et al. (2013), chloramine decay rate was to shown to increase over time (72 h). At pH >7, the decay rate was higher for chloramines mixed at a ratio of 3:1 than for those mixed at 4:1. However, at pH <7, that ratio did not play an obvious role. Chloramine loss was greater at low pH. Generally, chloramine loss increased with increasing temperature, and the decay rate was higher for chloramines mixed at a ratio of 3:1 than at 4:1. Research conducted by the Philadelphia Water Department similarly showed monochloramine decay was slow at lower water temperatures (38% decay at 7°C) and increased at higher temperatures (84% decay at 30°C) (Kirmeyer et al. 2004). Web-based chloramine formation and decay models have been developed by Wahman (2015), based on the unified models described by Jafvert and Valentine (1992) and Vikesland et al. (2001), as well as NOM reactions described by Duirk et al. (2005). A user-input model is also publicly available (U.S. EPA, 2016b).
7.2.2 Rechlorination and temporary breakpoint chlorination
As part of a comprehensive distribution management plan, both rechlorination and temporary breakpoint chlorination could be considered as potential tools. Maintaining sufficient disinfectant residual is important, and recombining the (released) free ammonia in the distribution system by booster chlorination can be used in order to maintain the ratio near 5:1 throughout the system (Wilczak, 2006). Free ammonia residual needs to be measured before chemical addition. If sufficient free ammonia is still present, only chlorine needs to be added.
A temporary disinfectant switch to chlorine as a method for controlling nitrification is common practice (Vikesland et al., 2006). Depending on whether nitrification is localized or widespread, the practice may range from a short-term targeted breakpoint of an isolated area of the distribution system to a longer system-wide chlorine burnout (Hill and Arweiller, 2006; Skadsen and Cohen, 2006). Periodic free chlorination has not been demonstrated to be effective for long-term nitrification control (Vikesland et al., 2006). In a full-scale study, the process of switching from chloramines to chlorine and back to chloramines created a breakpoint chlorination reaction, which resulted in a low chlorine residual “transitional front” in the distribution system. Although the HPC temporarily decreased, THMs also increased upon switching back to chloramines. While conducting breakpoint chlorination experiments in distribution systems, Rosenfeldt et al. (2009) observed that incorporating systematic flushing was important to achieving chlorine residual and decreasing chloroform production.
7.2.3 Opportunistic pathogens
The microbiological ecology of distribution systems and piped plumbing supplies can be influenced by factors such as the disinfection strategy, the operational and water quality parameters, the system materials and the age of the system (Baron et al., 2014; Ji et al., 2015). Research has shown the existence of differences in the relative abundance of certain bacterial groups in bulk water and biofilms in the distribution system when using monochloramine compared with free chlorine when providing a disinfectant residual (Norton et al., 2004; Williams et al., 2005; Hwang et al., 2012; Chiao et al., 2014; Mi et al., 2015).
Distribution system and premise plumbing biofilms can serve as reservoirs for opportunistic premise plumbing pathogens (OPPPs) such as Legionella pneumophila, non-tuberculous mycobacteria (e.g., M. avium, M. intracellulare), Pseudomonas aeruginosa and Acinetobacter baumanii (Fricker, 2003; Falkinham, 2015). In developed countries, Legionella and non-tuberculous mycobacteria are becoming increasingly recognized as important causes of waterborne disease for vulnerable sections of the population (Wang et al., 2012, Beer et al., 2015; Falkinham, 2015).
It has been proposed that the use of chloramines in the distribution system may contribute to environmental conditions that are less favourable for Legionella and more favourable for non-tuberculous mycobacteria (Norton et al., 2004; Williams et al., 2005; Flannery et al., 2006; Moore et al., 2006; Gomez-Alverez et al., 2012, 2016; Revetta et al., 2013; Baron et al., 2014; Chiao et al., 2014; Gomez-Smith et al., 2015; Mancini et al., 2015). The free-living amoeba Naegleria fowleri has also received an increased profile as a drinking water pathogen of emerging concern in the southern United States as a result of recent infections linked to municipal drinking water supplies in Arizona and Louisiana (Bartrand et al., 2014). N. fowleri causes primary amoebic meningoencephalitis (PAM), an extremely rare, but commonly fatal disease that has been predominantly associated with water inhalation during recreational water activities at warm freshwater locations (Yoder et al., 2012; Bartrand et al., 2014). Two cases in Louisiana represent the first time disinfected tap water has been implicated in N. fowleri infection (Yoder et al., 2012). Recommendations for maintaining a minimum monochloramine residual of 0.5 mg/L throughout the distribution system and monitoring for nitrification have been included within strategies developed for the control of N. fowleri at impacted water utilities that use chloramines (Robinson and Christy, 1984; NHMRC, 2011; Louisiana Department of Health and Hospitals, 2016).
A well-maintained distribution system is an important component in minimizing microbiological growth in distributed water in premise plumbing systems. Utilities also need to be aware that changes in the microbiological diversity of drinking water systems can occur with changes to disinfection strategies. An understanding of the effects different disinfectants may have on drinking water system ecology is necessary to maximize the effectiveness of disinfection strategies and minimize such unintended consequences as the potential enrichment of specific microbial groups (Williams et al., 2005; Baron et al., 2014).
7.2.4 Nitrification
Nitrification, the microbiological process whereby ammonia is sequentially oxidized to nitrite and nitrate by ammonia-oxidizing bacteria and nitrite-oxidizing bacteria, respectively, is a significant concern for utilities using chloramines for secondary disinfection. Free ammonia can be present as a result of treatment (e.g., improper dosing or incomplete reactions) or release during chloramine demand and decay (Cunliffe, 1991; U.S. EPA, 2002).
Water quality problems caused by nitrification include the formation of nitrite and nitrate, loss of disinfectant residual, bacterial regrowth and biofilm formation, DBP formation, and decreases in pH and alkalinity that can lead to corrosion issues, including the release of lead and copper (U.S. EPA, 2002; Cohen and Friedman, 2006; Zhang et al., 2009, 2010). Growth of nitrifying bacteria leads to a loss in disinfectant residual and increased biofilm production, which further escalates chlorine demand, ammonia release and bacterial regrowth (Kirmeyer et al., 1995; Pintar and Slawson, 2003; Scott et al., 2015). Cometabolism of monochloramine by ammonia-oxidizing bacteria and reactions between chloramines and nitrite are also significant mechanisms for the loss of chloramine residual (Health Canada, 2013b; Wang et al., 2014, Wahman et al., 2016).
Factors favouring nitrification include warm water temperatures, long detention times, and the presence of high levels of organic matter in the distribution system (Kirmeyer et al., 2004; AWWA, 2006a). Nitrification episodes have typically been reported during the summer months when water temperatures range from 20°C to 25°C (Pintar et al., 2000). Utilities should consider increasing the frequency of sampling during warmer months for this reason (AWWA, 2013). Full-scale distribution systems may also be impacted by nitrifying bacteria during typical North American winter conditions, in addition to the summer and fall seasons (Pintar et al., 2000).
In general, to prevent nitrification it is recommended that utilities maintain a minimum monochloramine concentration of 2 mg/L leaving the treatment plant and preferably greater than >1.5 mg/L at all monitoring points in the distribution system (U.S. EPA, 1999; Norton and LeChevallier, 1997; Skadsen and Cohen, 2006). Once it has started, nitrification cannot be easily stopped, even by applying high chloramine doses. Nitrifying bacteria have been detected in drinking water distribution systems in the presence of chloramine residuals exceeding 5 mg/L (Cunliffe, 1991; Baribeau, 2006). Nitrifying biofilms may harbour viable bacteria even after complete penetration with monochloramine at elevated concentrations (Wolfe et al., 1990; Pressman et al., 2012). Utilities should be aware of the chemistry of their water and system materials before considering conversion from chloramines to free chlorine and vice versa (Hill and Arweiler, 2006; Wilczak, 2006). When establishing monitoring programs for nitrification in the distribution system, the most useful parameters are total chlorine, free ammonia, nitrite, temperature and pH (Smith, 2006). HPCs or adenosine triphosphate measurements as indicators of biological growth or biostability are also considered valuable (Smith, 2006; LeChevallier et al., 2015; Health Canada, 2018b).
Detailed information on the causes of nitrification and its monitoring, prevention and mitigation are available elsewhere (AWWA, 2006a). It is important to note that even the stringent control of excess free ammonia and the maintenance of a proper Cl2:NH3 ratio may not always be effective in preventing nitrification. This is due to the fact that chloramine in the distribution system will start to decay based on water quality conditions and water age, releasing free ammonia into the water (Cohen and Friedman, 2006).
7.2.5 Lead and copper release
Several studies have compared the impact of chlorine and chloramines on lead release. In a study where three utilities had converted from chlorine to chloramines, Dyksen et al. (2007) found that one utility had decreased lead levels, a second utility experienced an increase in lead release that was below the U.S. EPA Lead and Copper Rule (LCR) action level, while the third utility experienced increases that exceeded the LCR action level. As part of an all-encompassing strategy to reduce lead exposure and provide effective corrosion control, the San Francisco Public Utilities Commission increased the pH of its low alkalinity, soft water supply (pH 8.6–9.4). As a result, San Francisco did not experience lead and copper release; nor did it impact the utility’s ability to meet the LCR action levels following conversion to chloramines from chlorine (Wilczak et al., 2010).
Conversely, conversion from chlorine to chloramines as a secondary disinfectant was implicated as a cause of increased lead release in the Washington, DC, water crisis in 2004 (Renner, 2004; Dyksen et al., 2007). High oxidation–reduction potential (ORP) conditions favour the formation and accumulation of lead dioxide (PbO2) scales (Schock and Gardels, 1983; Schock et al., 1996). Since chlorine is a powerful oxidant, switching from chlorine to chloramines reduced the oxidizing potential of the distributed water and destabilized the PbO2 scale, resulting in increased lead release (Schock and Giani, 2004; Lytle and Schock, 2005). A number of studies confirmed that stable PbO2 deposits could be readily formed and subsequently destabilized and reduced to (soluble) Pb(II) in a short period (weeks to months) under realistic conditions of distribution system pH, ORP and alkalinity (Edwards and Dudi, 2004; Lytle and Schock, 2005; Switzer et al., 2006; Valentine and Lin, 2009). Valentine and Lin (2009) suggested that for chloraminating systems, this type of lead release could be managed through having higher pH (>8), having carbonate levels >50mg/L, minimizing the monochloramine dose and using a lower chlorine:ammonia (Cl:N) molar ratio of 0.7:1. However, the authors noted that this lower ratio could enhance nitrification when adequate residual is not maintained. They also noted that NOM was a more significant agent for reducing lead oxide than monochloramine. In pipe loop experiments (water with <5 mg/L of CaCO3 and a pH of 7.3, zinc orthophosphate of 0.8 mg/L as PO4), lead release was significantly higher (approximately one order of magnitude) after a 30-min and 24-h stagnation period for the chloraminated (1 mg/L and 5 mg/L residual) pipe loops compared with chlorinated (1 mg/L) pipe loops (Woszczynski et al., 2013). Similarly, in simulated partial lead service line replacement experiments, sodium silicate-treated water at pH 8 with a monochloramine residual of 3 mg/L was found to increase lead release compared with the chlorinated water (1 mg/L) (Zhou et al., 2015).
Edwards and Dudi (2004) found that high initial concentrations of lead were leached from in-line devices such as meters and shut-off valves, and they decreased exponentially over the 58-day test period. The authors found that more lead was leached from brass treated with chloramines than that treated with chlorine, although this was only significant at the 85% confidence level, which the authors attributed to the small sample size. In Lin et al. (1997), in the absence of corrosion inhibitors, chloramines were found to suppress copper release and increase lead release from brass (3% lead content) compared with chlorine. The authors also examined lead and copper release from brass in the presence of zinc orthophosphate, sodium phosphate blend, long-chain phosphate blend, and a commercial sodium phosphate; they found that lead and copper release were elevated in the chloraminated systems compared with the chlorinated systems.
A study assessed the leaching potential for several components tested under NSF International (NSF)/American National Standards Institute (ANSI) Standard 61 protocols with both chlorinated and chloraminated water. This study also included results from a benchmarking study undertaken to assess 28 waters (chlorinated and chloraminated), collected from utilities across the United States, against the NSF/ANSI Standard 61 exposures waters (pH 5 and pH 10 water [Section 8] and pH 8 water [Section 9]) with respect to the leaching of lead, copper, and zinc from brass samples (rods). Results indicated that neither chlorine nor chloramines were a dominant factor in the metal release of the brass rods (Sandvig et al., 2012). Laboratory experiments were conducted to quantify the levels of lead leached from seven commercially available low-lead brass alloys containing lead at 0.25% or less. The tests were conducted with two different waters, including an NSF /ANSI Standard 61 section 9 test water and a low-pH, low-alkalinity, chloraminated water expected to be aggressive with respect to lead leaching. The concentrations of lead leached from all low-lead alloys were below 1 µg/L under both leaching conditions for the 4-week duration of the experiment (Triantafyllidou and Edwards, 2010).
Douglas et al. (2004) determined that nitrification within the distribution system was the cause of a reduction in pH that resulted in high lead levels in an area of the city with lead service lines. Concentrations of lead at the tap (10–15 µg/L for flowing samples) were seen in the distribution system where a reduction in pH from 8.5 to a range of 7.8–8.2 was observed.
Limited information has been reported in the literature about the effect of other disinfectants on copper. In a 2007 utility survey, 2 of 11 participating utilities reported copper data before and after conversion from chlorine to chloramines (Dyksen et al., 2007). The two utilities both reported decreases in copper release post-chloramination. A bench-scale study by Rahman et al. (2007) examined the effects of three disinfectants (chlorine, chlorine dioxide, and chloramines) under two pH (7.2 and 8.5) and alkalinity (10 or 100 mg CaCO3/L) conditions. The authors found that there was no significant difference in dissolved copper release for the disinfectant-free control compared with any of the disinfectants used in the study. Generally, the study found that the application of disinfectant lowered the copper release, with the lowest total copper release being found under the pH 8.5, 10 mg CaCO3/L and 1.0 mg Cl2/L conditions.
As part of a study assessing the leaching potential for brass components tested under NSF/ANSI Standard 61 protocols using chlorinated and chloraminated water, utilities throughout the United States were surveyed on copper and lead release issues. The survey responses indicated few problems with copper and lead releases when chloramines were used for secondary disinfection. However, the authors cautioned that their observations did not specifically examine switching from chlorine to chloramines (Sandvig et al., 2012).
Boyd et al. (2008) studied the effects of changing disinfectants from free chlorine to chloramines and vice versa on leaching rates of metals and concentrations of metals from lead, brass, and copper components in the distribution system. Local tap water was used and water quality parameters (disinfectant residual, pH, alkalinity, and orthophosphate concentration) were monitored and maintained. The authors conducted pipe loop testing using new copper tubing and bronze piping (as a surrogate for standard brass) with copper–bronze, lead–bronze, and lead–copper galvanic couplings. Copper concentrations in unpassivated copper and bronze pipe loops were sensitive to free chlorine and chloramines, but the effects were transient and not related to a specific disinfectant.
For a more detailed discussion on corrosion control, see Health Canada (2009).
7.2.6 Iron
There is limited information on the impacts of chloramines on iron. However, in a utility survey by Dyksen et al. (2007), three utilities that had converted from chlorine to chloramines responded with data on “red water.” Generally, discoloured water complaints decreased following the switch to chloramines; however, two utilities observed an initial increase in complaints for several months before they decreased to below complaint levels observed during chlorine treatment. The implications of discoloured water events should not be ignored, as trace inorganics such as lead (Camara et al. 2013; Masters and Edwards, 2015; Trueman and Gagnon, 2016) as well as arsenic, chromium, uranium, and vanadium (Friedman et al., 2010) have been associated with iron scale release. For a more detailed discussion on corrosion control, see Health Canada (2009).
7.2.7 Elastomeric materials
The effect of chloramines on gasket material is a common concern. In a study by Seidel et al. (2005), 16% of utilities reported that they experienced gasket deterioration with the use of chloramines. Rockaway et al. (2007a) conducted a series of accelerated degradation experiments at 23°C, 45°C, and 75°C and at 1 mg/L, 30 mg/L, and 60 mg/L of chlorine and chloramines for six different elastomer materials: natural rubber, neoprene rubber, ethylene propylene diene monomer-peroxide-cured (EPDM-P), ethylene propylene diene monomer-sulphur-cured (EPDM-S), styrene butadiene rubber (SBR), and nitrile rubber. Generally, elastomers were more susceptible to degradation in the presence of chloramines compared with chlorine. Elastomers exposed to chloramine exhibited greater loss of hardness and tensile strength and increased welling. The authors evaluated the overall performance of the materials and their sensitivity to degradation and rated the EPDM-P and EPDM-S as the least sensitive, the natural rubber, SBR, and nitrile rubber as sensitive, and the neoprene rubber as acutely sensitive. Nagisetty et al. (2014) determined that degrading elastomers can release organic materials that are the result of leaching of additives from the elastomer itself or by-products of the reaction of chloramines and the elastomer. Using accelerated degradation processes at 40 mg/L and 30 mg/L chloramines, they found that a total of 18 different organic compounds were released from natural rubber, SBR, and EPDM-S after 3 and 30 days. Bonds (2004) compared the degradation of fluorocarbons (FKM), SBR, EPDM, nitriles, and neoprenes as mechanical joints and push-on joints exposed to a chloramine solution (110 mg/L) over 364 days. The author reported that with the exception of FKM, all the materials showed significant signs of deterioration when exposed in the sheet (large surface area) configuration, but that the same extent of degradation was not observed when the surface area was small (as in the fitted gasket formation). Rockaway et al. (2007a, 2007b) stressed that the selection of elastomer material is an engineering decision, as it required assessment of the suitability of each material for the intended environment as well as other considerations for the consequences of critical failure. The possibility of release of other compounds and degradation by-products should also be considered.
7.3 Residential scale
Generally, it is not necessary to use drinking water treatment devices with municipally treated water. The use of residential-scale treatment devices on municipally treated water is based primarily on individual choice. Although private residential drinking water treatment devices may be an option for reducing concentrations of chloramines in drinking water if the consumer finds the taste objectionable, removal of the disinfectant is not recommended. Treatment devices for removal of chloramines for aesthetic concerns are covered under NSF/ANSI Standard 42, which establishes minimum requirements for materials, design and construction, and performance of drinking water treatment systems that are designed to reduce specific aesthetic-related (taste and odour) contaminants (NSF/ANSI, 2017). Systems certified under NSF/ANSI Standard 42 must be able to reduce an influent concentration of 3.0 ± 0.3 mg/L monochloramine to ≤0.5 mg/L monochloramine (NSF/ANSI, 2017).
Health Canada does not recommend specific brands of drinking water treatment devices, but it strongly recommends that consumers use devices that have been certified by an accredited certification body as meeting the appropriate NSF/ANSI drinking water treatment unit standards. These standards have been designed to safeguard drinking water by helping to ensure the material safety and performance of products that come into contact with drinking water. Certification organizations provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada (SCC). In Canada, the following organizations have been accredited by the SCC to certify drinking water devices and materials as meeting NSF/ANSI standards (SCC, 2016):
- CSA Group (www.csagroup.org)
- NSF International (www.nsf.org)
- Water Quality Association (www.wqa.org)
- UL LLC (www.ul.com)
- Bureau de normalisation du Québec (www.bnq.qc.ca)
- International Association of Plumbing and Mechanical Officials (www.iapmo.org)
- Truesdail Laboratories Inc. (www.truesdail.com)
An up-to-date list of accredited certification organizations can be obtained from the SCC (www.scc.ca).
8.0 Kinetics and metabolism
No information relating to dermal or inhalation exposure to either mono-, di- or trichloramine was located in the literature. The only available kinetics studies were oral and in vitro studies that used monochloramine. As an oxidant, monochloramine is unlikely to undergo absorption, distribution and excretion intact (National Research Council, 1987). Rather, it will react with organic and inorganic substrates in saliva and stomach fluid to form organic chloramines and iodinated compounds (National Research Council, 1987; Scully and White, 1991). Metabolism studies demonstrated that the chloride ion and not monochloramine is absorbed into the bloodstream, distributed into the tissues and excreted in the urine (Abdel-Rahman et al., 1983).
8.1 Absorption
Based on in vitro time persistence studies and estimated chemical reactions in saliva and gastric juice, monochloramine is unlikely to be absorbed intact following ingestion (Scully and White, 1991; Kotiaho et al., 1992). By adding water containing monochloramine at levels found in drinking water (typically 1–3 ppm) to samples of human saliva, Kotiaho et al. (1992) showed that the reaction of monochloramine with saliva was concentration dependent. At a low concentration of 0.7 ppm, monochloramine remained in human saliva for up to 5 min, while at a concentration of 1.8 ppm it persisted for up to 50 min. At higher concentrations (3.6–15 ppm) monochloramine did not completely react with saliva even after 2 h (Kotiaho et al., 1992). These measured decay times of monochloramine in saliva and the short residence time in the mouth suggest that the majority of monochloramine in treated drinking water likely reaches the stomach unchanged.
However, in stomach fluid, depletion of monochloramine was so rapid (within 30 sec for concentrations of 0.7–15 ppm) that intact monochloramine is unlikely to enter systemic circulation (Kotiaho et al., 1992). A metabolism study in male Sprague–Dawley rats (n = 4) given 36Cl radiolabelled monochloramine (1.1 mg/rat) orally showed 36Cl- appeared in the bloodstream and that chlorite and chlorate were not seen as metabolites. Chloride reached a peak plasma level of 10.3 µg/L 8 h post-dosing (Abdel-Rahman et al., 1983). The absorption rate constant was 0.278 h-1 and the absorption half-life was 2.5 h for the labelled chloride.
8.2 Distribution
Abdel-Rahman et al. (1983) examined the distribution of the 36Cl portion of radiolabelled monochloramine given orally to male rats (1.1 mg/rat) and found plasma contained the highest amount (3.15 µg36Cl/g of tissue), followed by whole blood (2.66 µg/g), skin (2.13 µg/g), testes (2.09 µg/g) packed cells (1.90 µg/g), bone marrow (1.82 µg/g), kidney (1.62 µg/g), lung (1.58 µg/g), stomach (1.53 µg/g), thyroid (1.36 µg/g), thymus (1.36 µg/g), duodenum (1.20 µg/g), spleen (1.11 µg/g), carcass (0.77 µg/g), liver (0.74 µg/g), ileum (0.59 µg/g), and fat (0.18 µg/g).
8.3 Metabolism
Ingested monochloramine is quickly broken down in the stomach (Kotiaho et al., 1992). The reaction products formed are influenced by the concentration of monochloramine, the pH and composition of stomach fluid, and diet (Scully and White, 1991). The pH of the stomach ranges from 1 to 8 (Scully and White, 1991). At a pH near 1, the protonated form of monochloramine is more prevalent and more likely to transfer its chlorine to amino acids present in the gastric juice, thereby producing N-chlorinated amino acids (Scully and White, 1991). Monochloramine may also generate iodinated organic compounds in gastric juice and saliva in the presence of nutrients such as tyrosine, 4-aminobenzoic acid, arachidonic acid, and folic acid (Bercz and Bawa, 1986). Formation of dichloramine, trichloramine or molecular chlorine did not occur in gastric juice in vitro (Kotiaho et al., 1992). The presence of food would likely increase reaction rates of organics with monochloramine, resulting in its having a shorter half-life in saliva or stomach fluid (Scully and White, 1991). A metabolism study by Abdel-Rahman et al. (1983) that focused on chloride, chlorite, and chlorate as metabolites found only chloride present in the bloodstream of rats following oral dosing with 36Cl radiolabelled monochloramine (1.1 mg/rat).
The results obtained by Kotiaho et al. (1992), Abdel-Rahman et al. (1983) and Scully and White (1991) suggest that any observed toxicity associated with the ingestion of monochloramine is likely due to the formation of reaction products in the stomach rather than absorption of intact inorganic chloramines.
8.4 Excretion
Abdel-Rahman et al. (1983) showed that monochloramine is partly excreted in the urine as chloride. Excretion data for male Sprague–Dawley rats (n = 4) given 36Cl radiolabelled monochloramine (1.1 mg/rat) showed only 0.40% and 0.08% of the administered dose was excreted in the urine and feces, respectively, in the first 24 h post-treatment. After 120 h, the values were 25.15% in urine and 1.98% in feces. No 36Cl was detected in expired air at any time point (Abdel-Rahman et al., 1983).
9.0 Health effects
The following discussion on health effects will focus primarily on studies conducted via the oral route, since it is the most relevant route of exposure for chloramines. Most available studies are for monochloramine, the predominant species in drinking water. Dichloramine and trichloramine are infrequently detected in drinking water; only one oral study of each was available from the literature. Minimal to no effects were seen in both humans and animals following ingestion of monochloramine, with the most significant effect (decreased body weight gain) attributed to a reduction in water intake caused by decreased palatability from high levels of monochloramine.
9.1 Acute toxicity
Despite more than 90 years' history of using monochloramine as a drinking water disinfectant (U.S. EPA, 2009; Vermont Department of Health, 2012), little data exist concerning its health effects via drinking water. Only a few clinical and case–control studies and surveys are available from the literature. Results from these studies did not show any adverse health effects, although the limitations of the studies (e.g., weak exposure assessment, chloramination used as control, sample bias) do not allow for definitive conclusions to be drawn.
By contrast, numerous studies have been published regarding the toxicity of chloramines via inhalation. These studies are related to accidental intoxication from chloramine gas (monochloramine and dichloramine) due to improper handling of cleaning agents (Reisz and Gammon, 1986; Tanen et al., 1999; Cohle et al., 2001) or occupational/recreational exposure to trichloramine from its use in food industry facilities or from swimming pools, scenarios that are not relevant to drinking water exposure. Although several papers have reported negative health effects (decreased haptoglobin, decreased hemoglobin, formation of Heinz bodies, hemolytic anemia, methemoglobinemia) in hemodialysis patients when tap water containing chloramines was used for dialysis (Eaton et al., 1973; Kjellstrand et al., 1974; Klein, 1986; Fluck et al., 1999; Weinstein et al., 2000; Junglee et al., 2010), these changes were not observed in healthy volunteers deliberately ingesting chloraminated drinking water (Lubbers et al., 1982, 1984).
9.1.1 Beneficial effects
Only two short-term studies were located regarding the human health effects following ingestion of drinking water containing monochloramine. These did not show any adverse effects at doses of up to 24 mg/L.
In phase I of a double-blind acute rising-dose tolerance study, no effects in liver, thyroid function, kidney function, hematology or overall health were observed in male volunteers consuming distilled water containing up to 24 mg/L of monochloramine (Lubbers et al., 1982). Males (10/group, aged 21–35) with normal methaemoglobin levels, thyroid function and glutathione levels drank two 500 ml portions of either organic-free demineralized deionized water alone or water containing monochloramine, with a 4-h interval between portions. Treatment days occurred every 3 days for a total of 5 treatment days over a period of 13 days. Doses of monochloramine were increased over the course of the treatments, beginning with 0.01 mg/L on Treatment Day 1 and increasing to 24 mg/L by the end of treatment. A battery of tests, including blood and urine biochemistry (e.g., cholesterol, triglycerides, triiodothyronine [T3]), hematology (blood cell counts, hemoglobin, haptoglobin and methemoglobin levels), cell morphology, and a physical examination found no difference between pre- and post-treatment values (Lubbers et al., 1982).
In a randomized, controlled parallel trial study by Wones et al. (1993), thyroid and lipid effects were generally absent, except for a slight increase in apolipoprotein B levels at 15 ppm/day of monochloramine. Three groups of healthy men (16/group) were given distilled water and were fed a diet high in fat and cholesterol for the first 4 weeks of the study to establish a baseline. For the 4-week treatment period, all subjects continued on the study diet and were given 1.5 L/day of water containing monochloramine at 0 ppm (control), 2 ppm or 15 ppm. Blood levels were taken at the end of each 4-week period to assess lipid (total cholesterol, high-density lipoprotein [HDL], low-density lipoprotein [LDL], triglycerides, apolipoprotein A1, A2 and B) and thyroid (thyroid-stimulating hormone [TSH], T3, thyroxine [T4]) metabolism. No changes were observed in thyroid function in either treatment group. No change in lipid function was seen except for a slight, statistically significant increase in apolipoprotein B (a component of LDL cholesterol) observed at the 15 ppm (15 mg/L) dosage. The significance of increased apolipoprotein B independent of changes in LDL cholesterol is unknown. Study limitations included relatively brief baseline and treatment periods, and previous consumption of chlorinated drinking water by most subjects.
9.1.2 Subchronic and chronic toxicity and carcinogenicity
Except for a slight increase (although within normal range) in T3 seen at the 5 mg/L/day dosage (Lubbers et al., 1984), the available long-term studies did not find any association between chloramination and health effects, including dermal symptoms, upper respiratory effects and bladder cancer (Zierler et al., 1986, 1988; McGeehin et al., 1993; Weintraub et al., 2006; CDC, 2008; Vermont Department of Health, 2012). Most long-term chloramine studies compared the effects of different disinfection treatment methods (e.g., chlorination vs. chloramination) on specific health outcomes (e.g., bladder cancer) or used chloramination exposure as the control group. Other shortcomings of these studies included lack of exposure characterization, previous or concurrent exposure to chlorine, and recall bias, making the results unsuitable for risk assessment (Zierler et al., 1986, 1988; McGeehin et al., 1993; Weintraub et al., 2006; CDC, 2008; Vermont Department of Health, 2012).
In a continuation of an earlier study (described in Section 9.1.1), two groups of healthy male volunteers (n = 10/group; aged 21–35) were given unlimited organic-free demineralized deionized water to drink and asked to refrain from drinking tap water. One group also consumed 500 ml of water containing 5 ppm of monochloramine daily for 12 weeks followed by an 8-week recovery period (Lubbers et al., 1984). The same battery of tests as used in the previous study (Lubbers et al., 1982) was conducted. The only statistically significant effect was an increase in T3 uptake, which changed over time when compared with the control group; however, this change remained within the normal T3 range and was clinically irrelevant.
No direct link between reported health effects and chloramination could be established in two surveys conducted in the United States following the switch from chlorine to chloramination as a secondary disinfectant by local public utilities (Weintraub et al., 2006; CDC, 2008).
After the local water utility switched from chlorine to chloramine as its secondary disinfectant, the San Fransico Health Department received a small number of complaints of dermatitis (Weintraub et al., 2006). These complaints prompted a questionnaire-based study. Despite widespread publicity, only 17 respondents completed the questionnaire from a total population of 2.4 million in the service area. Of these, 11 had histories of allergies while 8 had histories of skin issues (e.g., hives, shingles, eczema). Of the 14 that sought medical treatment, no diagnoses were recalled by respondents. Since the complaints were heterogeneous (no consistency between individual complaints) and respondents had underlying or pre-existing conditions that plausibly explained the reported symptoms, the effects were likely unrelated to chloramination, according to the authors. Study shortcomings included use of a convenience sample, small sample size, no dermatological exam, and no validation of the questionnaire. Media coverage linking health complaints to chloramination may have influenced symptom reporting (Weintraub et al., 2006).
Similarly, Vermont's Champlain Water District and the Public Health Service of the Centers for Disease Control and Prevention (CDC) conducted a joint investigation into health complaints (n = 74) such as upper respiratory tract symptoms, watery eyes/nose, scratchy throat, gastrointestinal ailments, and skin rashes/itching following the water district's change to chloramine as a secondary disinfectant (CDC, 2008). The switch to monochloramine was widely publicized and a mass media campaign against the use of chloramination was undertaken by People Concerned About Chloramine (PCAC) prior to the switch. Both home visits and telephone interviews (46/50 subjects met the inclusion criteria) were conducted with persons expressing health concerns. Twenty-five (54%) respondents had at least one pre-existing health condition (allergies/asthma, skin issues, diabetes, autoimmune disorders, cardiac history, respiratory, arthritis, cancer). Of the 32 who sought medical help, 8 received a diagnosis (five cases of psoriasis/eczema and one each of fluid in lungs, writer's rash and surface eye irritation). Only 23 of the 34 participants who changed their water use habits to treat their symptoms showed improvement. Chloramine concentrations of 1.8–2.3 mg/L were reported at the distribution entry point. Consumer exposure was not measured but was expected to be lower than in the distribution system. Symptoms may have been biased by self-reporting, the media campaign and the presence of PCAC members at home visits. No clear link could be drawn between treatment and symptoms (CDC, 2008).
A follow-up survey of 173 health care providers, including family practice and primary care physicians and specialists, was conducted by the Department of Health in Vermont to assess the prevalence of health problems related to monochloramine use. Of the 81 surveys returned, no patients were reported to have an illness directly related to monochloramine use, while only two patients were considered to have an underlying disease that was exacerbated by chloraminated water use. Eleven providers reported they were unsure if patient complaints were related to the water, while 59 providers reported the water did not cause patient complaints. It was concluded that some people may be sensitive to monochloramine in the water (Vermont Department of Health, 2012).
No association was seen between chloraminated drinking water and bladder cancer when compared with chlorinated drinking water in three studies (Zierler et al., 1986, 1988; McGeehin et al., 1993).
McGeehin et al. (1993) investigated the possible association between bladder cancer and drinking water disinfection methods using a population-based, case–control study in Colorado. Cases (327 subjects with bladder cancer) and controls (261 subjects with other cancers, except colorectal and lung) were identified through the Colorado Central Cancer Registry and were limited to living white subjects, aged 21 to 84. Residential and water source histories and information on potential confounders were obtained through telephone interviews. Residences were linked to water utilities information (e.g., water source, disinfection type) and used to create exposure profiles. Logistic regression was used to adjust for cigarette smoking, consumption of tap water and coffee, and medical history. The mean person-year level of exposure to chloraminated surface water was lower (p = 0.02) for cases (mean of 14.8 years) than for controls (mean of 18.8 years). The risk of bladder cancer decreased with increasing duration of exposure to chloraminated surface water (p < 0.01) with a risk estimate of 0.7 (95% confidence interval [CI] 0.4–1.1) for subjects who consumed chloraminated water for 21–40 years, while those who consumed chloraminated water for more than 40 years had an odds ratio (OR) of 0.6 (95% CI 0.4–1.0). The decreased risk seen likely corresponded to the decrease in years of exposure to chlorinated water and not to any protective effect of chloramination, since there is no biological evidence that suggests chloramination inhibits neoplastic transformation of bladder epithelium. Some potential shortcomings of the study included disinfection methods changes made by some water utilities over time, the assumption that water parameters and individual consumption patterns were constant over time, gaps in historical data on water treatment and individual exposure profiles, and the potential for recall bias.
Using Massachusetts Department of Public Health death records, Zierler et al. (1986) examined mortality patterns between state residents (≥45 years of age) consuming either chlorine-treated or chloramine-treated drinking water. Subjects were assigned to either group based on residence at time of death. No correlation was seen between cancer mortality and consumption of chloraminated water, but the chloramine group did have a slight excess of deaths from pneumonia and influenza (standardized mortality ratio [SMR] of 118; CI 116–120 for chloraminated water vs. SMR of 98; CI 95–100 for chlorinated water). Bladder cancer deaths were moderately associated with chlorine-treated (SMR of 105; CI 99–111) but not chloramine-treated (SMR of 91; CI 88–98) drinking water. The results should be considered preliminary and qualitative, since the study had a number of shortcomings (e.g., exposure history based on residence at time of death, error in assigning disease status, failure to assess previous residence histories, length of exposure and water quality) and may have been influenced by unidentified or uncontrolled confounding factors. The authors also suggested that deaths from pneumonia may have been a secondary cause of death, with cancer or some other underlying illness likely being the primary cause.
In a follow-up case–control study that considered exposure duration, Zierler et al. (1988) again found no correlation between bladder cancer mortality and consumption of chloraminated surface water. The study looked at data on 614 individuals who died of primary bladder cancer and had been exposed to surface water treated by chlorination or chloramination for either a lifetime or 50% of residence. The control group (1,074 individuals) consisted of five disease groups: cardiovascular disease, cerebrovascular disease, lung cancer, chronic obstructive pulmonary disease, and lymphatic cancer. Potential confounders (e.g., age, sex, pack-years of smoking, occupation, socioeconomic status) were provided through proxy interviews and were controlled by a multiple logistic regression model. The frequency of bladder cancer mortality increased among lifetime residents of communities receiving chlorinated drinking water compared with residents of communities receiving chloraminated drinking water. However, the U.S. EPA (1994b) pointed out that it is impossible to find control subjects who have not been exposed to chlorine or chloramines, and indicated that the relationship between chloraminated water consumption and bladder cancer incidence cannot be defined based on the results of these studies.
9.1.3 Developmental and reproductive toxicity
In the only developmental study available, chloramination was less likely to cause adverse pregnancy outcomes than chlorination. Aschengrau et al. (1993) conducted a case–control study comparing adverse pregnancy outcomes (congenital anomalies, stillbirths, neonatal deaths) with a variety of community drinking water quality parameters (source, treatment, routine chemistry). Residential address at the time of pregnancy outcome or during the first trimester was matched to routine water quality analyses conducted by the Massachusetts Department of Environmental Protection and used to characterize exposure. After adjusting for potential confounding factors (e.g., maternal race, age, previous birth with congenital anomaly, medical insurance coverage type, alcohol consumption, water source), chlorinated surface water for drinking was associated with an increase in both stillbirths (adjusted OR = 2.6; 95% CI 0.9–7.5) and major malformations (adjusted OR = 1.5; 95% CI 0.7–2.1) when compared with chloraminated drinking water. A number of study shortcomings (such as the lack of a non-treated control group, no consideration of other maternal health issues, the rough estimates of individual exposure, no water consumption data, no measurement of chloramine levels) make the results difficult to interpret (Aschengrau et al., 1993).
9.2 Effects on experimental animals
The main effects seen in studies with monochloramine were decreased body weight gain, decreased organ weights and alterations of blood parameters. However, paired water studies indicate that these effects are likely caused by decreased water consumption and not the toxicity of monochloramine (Daniel et al., 1990, 1991; Poon et al., 1997).
No dermal studies were located in the literature for any of the chloramines. The majority of available oral studies in animals were for monochloramine; only one subchronic oral study was available for both di- and trichloramine. No inhalation studies were found for either mono- or dichloramine. Two acute inhalation studies were available for trichloramine (Barbee et al., 1983; Gagnaire et al., 1994).
9.2.1 Acute toxicity
Acute oral exposure to monochloramine caused stomach irritation at high doses (Kato et al., 1997; Nishiwaki et al., 1997; Umeda et al., 1999) while its effects on blood parameters (e.g., glutathione [GSH]) were transient (Abdel-Rahman et al., 1984). Inhalation of high doses of trichloramine resulted in severe respiratory irritation (Barbee et al., 1983; Gagnaire et al., 1994).
9.2.1.1 Oral
No significant effects on blood parameters were observed in a limited number of acute oral studies using monochloramine, although stomach effects were seen in high-dose studies.
Monochloramine given orally to male rats (5/dose) at high concentrations (20 mM, 60 mM, and/or 120 mM, corresponding to approximately 1,000 mg/L, 3,100 mg/L, and 6,200 mg/L) was shown to irritate the gastric mucosa and impair the ulcerogenic/repair process in the stomach, with doses of ≥60 mM (corresponding to 11.25 mg/kg bw, calculated) inducing severe mucosal lesions (Kato et al., 1997; Nishiwaki et al., 1997; Umeda et al., 1999).
In another study examining several blood parameters, male Sprague–Dawley rats (4/dose) were gavaged with a single dose of monochloramine in deionized water (0, 10 mg/L, 20 mg/L or 40 mg/L) (Abdel-Rahman et al., 1984). Osmotic fragility was unaffected, whereas increases in blood GSH, an antioxidant, were noted at 20 mg/L and 40 mg/L 15 min after dosing; they continued for up to 60 min but returned to normal after 2 h.
9.2.1.2 Inhalation
Barbee et al. (1983) reported an inhalation LC50 (1 h) of 112 ppm (550 mg/m3) in rats exposed to trichloramine. Pulmonary edema and clear fluid in the lung and trachea were seen in the animals that died following exposure. No mortality was seen at the lowest concentration of 58 ppm. At the highest concentration of 157 ppm, rats displayed effects such as eye irritation, excessive lacrimation, salivation, gasping and inactivity, as well as convulsions and nasal discharge.
In another inhalation study to evaluate upper airway irritation from airborne chemicals, male OF1 mice (n ~ 8/concentration) were exposed oronasally to 0.19–5.0 ppm trichloramine for 60 min (Gagnaire et al., 1994). The RD50, an exposure concentration producing a 50% respiratory rate decrease and an indication or respiratory irritation, was calculated at 2.5 ppm. Once exposure was discontinued, recovery was fairly rapid except for the highest dose, where mice had a slower recovery.
9.2.2 Short-term and sub-chronic exposure
9.2.2.1 Monochloramine: decreased body weights and blood effects
Daniel et al. (1990) exposed Crl:CD BR Sprague–Dawley rats (10/sex/dose) to 0, 25, 50, 100 and 200 mg/L monochloramine in drinking water (equivalent to 0, 1.8, 3.4, 5.8, and 9.0 mg/kg bw per day for males and 0, 2.6, 4.3, 7.7, and 12.1 mg/kg bw per day for females) for 90 days. A significant dose-related reduction in daily water consumption was seen in both sexes at all doses. In addition, a significant reduction in body weight gain (males at ≥50 mg/L, and females at 200 mg/L) and in final body weight (both sexes at the highest dose) were noted. Changes in some hematological parameters and in absolute and relative organ weights were also noted in the absence of histopathological effects. Clinical chemistry results showed a dose-dependent decrease in the level of calcium at all doses in treated males, whereas an increase was seen in females at the highest dose only. The hematological and clinical chemistry changes were either not dose-dependent, not biologically significant or within the normal range for rats of that age and strain. A no-observed-adverse-effect level (NOAEL) of 100 mg/L (equivalent to 7.7 mg/kg bw per day for females and 5.8 mg/kg bw per day for males) was proposed in the study based on final body weight. The authors suggested conducting a matched watering and feeding study (where controls are given reduced water to match the treated group) to help distinguish between systemic toxic effects and effects on weight gain due to taste aversion.
A decrease in body weight gain (≥50 mg/L in males and ≥100 mg/L in females), final body weight (at ≥100 mg/L in both sexes), water consumption (≥12.5 mg/L for females; ≥100 mg/L for males) and food consumption (females only at 100 ppm and above) were also observed in B6C3F1 mice exposed to monochloramine at 0, 12.5, 25, 50, 100 and 200 mg/L (equivalent to 0, 2.5, 5, 8.6, 11.1, and 15.6 mg/kg bw per day for males and 0, 2.8, 5.3, 9.2, 12.9, and 15.8 mg/kg bw per day for females) in their drinking water for 90 days (Daniel et al., 1991). Changes in relative and/or absolute organ weights at ≥100 ppm were seen in the absence of compound-related gross or microscopic lesions. Dose-dependent increases in white blood cells was seen in female mice at 25 mg/L and above; similarly, increases were seen in males but were not dose-dependent. In addition, significant decreases in the percentage of neutrophils were seen in both male and female mice at the top two doses, and an increase in the number of lymphocytes was seen in males only at the top two doses. Other hematological and clinical chemistry changes were also noted at 12.5 mg/L and above but were inconsistent and not dose-dependent. The authors report a NOAEL of 50 mg/L (8.6 mg/kg bw per day for males and 9.2 mg/kg bw per day for females) based on decreased organ weights, decreased weight gain and decreased consumption of food and water. They indicated that the observed effects could be a consequence of the decreased water consumption associated with taste aversion (Daniel et al., 1991).
The impact of taste aversion and reduced water intake on body and organ weights was assessed by Poon et al. (1997) using male Sprague–Dawley rats (10/dose) exposed to 0 or 200 ppm of monochloramine (equivalent to 0 or 21.6 mg/kg bw per day, as calculated by the study authors) for 13 weeks. A paired-water control (with restricted water intake) and a control group (given bicarbonate-buffered water ad libitum) were used. Over the course of the study, the monochloramine group consistently drank less water (p < 0.05) than the control given water ad libitum. Both the monochloramine group and the paired-water control group showed similar reduced body weight gain, final body weights and food consumption. Changes in organ weights and minor changes in biochemistry, hematology, immunological and histopathology were also seen in the treated group as well as in the paired-water controls. The authors concluded that these changes were related to the reduced water and food consumption seen in these two groups and not a result of monochloramine toxicity.
Body weight loss (50 ppm and above) and decreased water consumption (25 ppm and above) were observed in male A/J mice (12/dose) administered monochloramine in drinking water (0, 2.5, 25, 50, 100 or 200 ppm) for 30 days (Moore et al., 1980). No evidence of hemolysis or other blood effects was reported.
By contrast, adult African green monkeys (five males and seven females) exposed to monochloramine in drinking water up to 100 mg/L per day (10 mg/kg bw per day) using a rising dose protocolFootnote 2 did not show adverse effects on body weight or on thyroid function (serum thyroxine [T4] concentrations), hematology, hematological oxidative functions or serum chemistry (Bercz et al., 1982). Three other chemicals (chlorine dioxide, sodium chlorite and sodium chlorate) were also tested on the same monkeys with a 9-week rest period before a new chemical was introduced. No separate control group was used; however, the monkeys served as their own control.
Similarly, no signs of overt toxicity, decreased body weight gain or hematological changes were observed in rats exposed to monochloramine in drinking water (up to 100 mg/L) for 45 days in a study sponsored by the U.S. EPA's Health Effects Research Laboratory (Bull, 1980). However, a decrease in the amount of methemoglobin in blood was noted at the highest dose, contrary to the authors' expectations of an increase, based on observations in patients dialyzed with chloraminated tap water (Eaton et al., 1973; Kjellstrand et al., 1974; Klein, 1986; Fluck et al., 1999; Junglee et al., 2010).
Body weights were significantly decreased starting at 3 months and above at the highest dose in male Sprague–Dawley rats (4/dose) exposed to monochloramine in drinking water at 0, 1, 10 or 100 mg/L for up to 12 months (Abdel-Rahman et al., 1984). Blood parameters were also investigated; however, the observed changes (increased blood osmotic fragility and decreased blood GSH) were not consistent among the treatment groups and were not dose- or time-dependent. Other parameters (decreased red blood cell count and hematocrit) were seen at mid-time points but not at 10 months. Hemoglobin and mean corpuscular hemoglobin concentrations were reduced at 100 mg/L after 10 months; this, according to the authors, was an indication of erythrocyte damage. No data on water consumption was provided in this study; however, the authors noted significant decreases in body weights at 100 mg/L, which may have had effects on blood parameters, as seen in previous studies cited above.
9.2.2.2 Monochloramine: immunotoxic effects
Immunotoxic effects of monochloramine were investigated by Exon et al. (1987). Male Sprague–Dawley rats (12/dose) were exposed to monochloramine concentrations in drinking water (0, 9, 19 or 38 ppm) from weaning (3 weeks old) for 9 weeks. A reduction in relative and absolute spleen weight at the highest dose and an increased production of prostaglandin E2 by adherent resident peritoneal cells were observed at the two highest doses. Other effects observed were a decrease (inverse dose-dependent) in serum antibody synthesis at the two lowest doses (9 and 19 ppm). A review by the U.S. EPA (1994a) indicated that the biological importance of these immunotoxic effects from monochloramine was not clear, as there was no correlation among the various immunological endpoints studied. They also noted deficiencies in some of the methodologies used (e.g., ELISA test to analyze antibodies). No other remarkable findings were noted with respect to body weights, or in the battery of other immunoassays used (Exon et al., 1987).
No immunotoxic effectsFootnote 3 related to monochloramine exposure were observed when female B6C3F1 mice (8/dose) were exposed to monochloramine in drinking water at 0, 2, 10, 20, 100 and 200 ppm (0, 0.4, 2, 4, 20 and 40 mg/kg bw per day) for 28 days (NTP, 2000; Guo et al., 2011).
In a 13-week drinking water study, a small, statistically significant increase in Concanavalin A-mediated lymphocyte transformation activity (a measure of cell agglutination) was observed in male Sprague–Dawley rats (10/group) given 200 ppm (21.6 mg/kg bw per day) monochloramine in drinking water compared with paired-water controls (Poon et al., 1997). A second control group was given bicarbonate-buffered water ad libitum throughout the study. Both the monochloramine group and the paired-water control group showed similarly reduced body weight gain, which the authors concluded was related to the reduced water and food consumption seen in these two groups. The authors also concluded that monochloramine was not likely to be a significant immunotoxicant, given that there were no effects observed on the relative thymus weight and no treatment-related histopathological changes in the thymus and spleen.
9.2.2.3 Dichloramine and trichloramine
Only one subchronic study assessed the oral toxicity of di-and trichloramine in rats. Biochemical and mild to minimal histological effects were seen at doses of 0.2 ppm and above in females and males in the absence of clinical signs or weight gain changes.
Sprague–Dawley rats (10/sex/dose) were exposed to dichloramine (0, 0.2, 2, 20 and 200 ppm; calculated as 0.019, 0.19, 1.9 and 18 mg/kg bw per day for males and 0.025, 0.26, 2.5 and 24 mg/kg bw per day for females) or trichloramine (0, 0.2, 2, 10 and 90 ppm; calculated as 0.020, 0.23, 1.1, and 9.6 mg/kg bw per day for males and 0.028, 0.29, 1.3 and 13 mg/kg bw per day for females) in drinking water for 13 weeks (Nakai et al., 2000). Dichloramine produced minimal histological effects (epithelial hyperplasia) in the gastric cardia at ≥2 ppm (≥0.19 mg/kg bw per day) in males, and at ≥20 ppm (≥2.5 mg/kg bw per day) in females. Minimal to mild effects were seen in the kidney and thyroid without any clear dose–response link and in the absence of changes in relative organ weight or clinical manifestations. Other relatively mild effects, considered adaptive, were also seen in the liver. Trichloramine exposure produced minimal to mild histological effects in the thyroid at 2 ppm and above in both males (≥0.23 mg/kg bw per day) and females (≥0.29 mg/kg bw per day). Minimal to mild changes (considered adaptive by the authors) in the liver of males were noted at ≥0.2 ppm (≥0.02 mg/kg bw per day), whereas at 90 ppm (13 mg/kg bw per day), females showed increases in hepatic enzyme activity. At the highest dose, minimal histopathological changes in the kidney were also seen in males and increased relative kidney weights were observed in both males and females. Although histological changes in the kidney were noted in females at all doses, no dose–response relationship in incidence or severity was observed.
It must be noted that water consumption was decreased in both studies and that the minimal effects reported did not have clinical manifestations over the dose range examined.
9.2.3 Long-term exposure and carcinogenicity
The National Toxicological Program (NTP, 1992; Dunnick and Melnick, 1993), exposed F344/N rats and B6C3F1 mice (n = 70/sex/species/group) to monochloramine in drinking waterFootnote 4 (0, 50, 100 and 200 ppm) for 2 years. The calculated weighted mean equivalent dosesFootnote 5 were 0, 2.6, 4.8 and 8.7 mg/kg bw per day in male rats; 0, 2.7, 5.2, and 9.5 mg/kg bw per day in female rats; 0, 7.4, 14.0, and 23.8 mg/kg bw per day in male mice; and 0, 7.6, 14.2 and 24.2 mg/kg bw per day in female mice. Interim sacrifices (10/sex/species/dose) were conducted at weeks 14 and 66. Both rats and mice showed a dose-related decrease in water consumption throughout most of the study that was attributed to palatability.
In treated F344/N rats, feed consumption was similar to that of the controls. However, mean body weights in the high-dose rat group (both sexes) were decreased (5–10%) compared with the low- and mid-dosed rats. Changes in absolute and relative liver, brain and/or kidney weight were also observed but were considered by the authors to be related to the lower body weights and not biologically significant. In female F344/N rats, there was a marginal statistically significant increase in the incidence of mononuclear cell leukemia (MNCL) at the top two doses, but this increase was not dose-dependent, since the responses at these two doses were very similar: 8/50 (16%), 11/50 (22%), 15/50 (30%), and 16/50 (32%) for control, low-, mid- and high-dose groups, respectively. In addition, this marginal increase was within the range (although in the upper range) reported for historical controls (16–33%) and no reduced latency of MNCL in treated female rats was noted. According to NTP (1992), the evidence was weak in support of an association between MNCL in female rats and the consumption of chloraminated water. No increase in MNCL was observed in male rats. No other clinical findings or hematological effects or effects on survival or gross microscopic lesions were attributable to the consumption of chloraminated water. A NOAEL was set at 8.7 mg/kg bw per day (200 ppm) based on the absence of biologically significant adverse effects in male rats exposed at the highest concentration tested. Although lower body weights were observed at this level of exposure, they were likely caused by the unpalatability of the drinking water.
In mice (both sexes), there were also dose-related decreases in mean body weights during most of the study. Feed consumption was similar in treated male mice compared with controls and only slightly lower in high-dose females compared with controls. Changes in absolute and relative organ weight (kidney, liver and brain) were also observed in the high-dose mice and were also considered to be related to the lower body weights. No other clinical findings, effects on survival rates or gross microscopic lesions were attributable to the consumption of chloraminated water.
At the time of the study, the NTP (1992) concluded that under conditions of the bioassay, there was equivocal evidence of carcinogenicity in female F344/N rats based on MNCL but no evidence of carcinogenicity in either male F344/N rats or in B6C3F1 mice of either sex. Beginning in 2006, NTP discontinued the use of F344 rats in carcinogenicity studies (King-Herbert and Thayer, 2006; Maronpot et al., 2016) because of the high and variable background incidence of MNCL in F344 rats, the species- and strain-specificity of MNCL and its questionable significance to humans, especially when rates fall within normal historical control values (King-Herbert and Thayer, 2006; Maronpot et al., 2016).
In a rat liver foci assay, chloramine failed to induce an increase in γ-glutamyl-transpeptidase foci (GGT foci; an indicator of carcinogenicity) in male Sprague–Dawley rats given 14.75 mg/kg of chloramine in drinking water (Herren-Freund and Pereira, 1986). Based on this study, monochloramine did not show any potential for tumour initiation. Negative results were also seen using concentrated drinking water samples disinfected with monochloramine in another liver foci assay in rats (Miller et al., 1986).
9.2.4 Carcinogenicity
The literature on the genotoxicity of chloramines is limited to four in vitro studies and two in vivo studies; these studies investigated the genetic toxicology of monochloramine only. Monochloramine was not genotoxic in in vivo studies or in a modified Ames test but was weakly mutagenic in Bacillus substilis assay.
9.2.4.1 In vitro findings
In in vitro studies, monochloramine was shown to be weakly mutagenic in Bacillus subtilis (Shih and Lederberg, 1976a) but not mutagenic in Salmonella typhimurium TA100 in a modified Ames test without metabolic activation (Thomas et al., 1987).
Monochloramine induced single-strand breaks in Bacillus subtilis (Shih and Lederberg, 1976b), double-strand plasmid DNA breakage (Shibata et al., 1999), DNA fragmentation and DNA double-strand breaks, as well as chromatin condensation in rabbit gastric mucosal cells and human gastric cancer cells (Suzuki et al., 1997, 1998).
9.2.4.2 In vivo findings
Monochloramine did not induce chromosomal damage in vivo in either bone marrow or sperm of orally dosed mice.
No increases in micronucleated polychromatic erythrocytes or in chromosomal aberrations were seen in the bone marrow of Swiss CD-1 mice (5/sex/dose) gavaged with 1 ml of 0, 50 mg/L, 100 mg/L, or 200 mg/L of monochloramine per day for 5 days and then sacrificed (Meier et al., 1985). In the same study, single, gavage doses of monochloramine (0, 50 mg/L, 100 mg/L, or 200 mg/L) in Swiss CD-1 mice (4/sex/dose) also did not cause any chromosomal aberrations.
Abdel-Rahman et al. (1984) investigated DNA synthesis in various organs (via incorporation of [3H] thymidine into the nuclei), by administering methyl thymidine intraperitoneally to rats treated with 0, 1 mg/L, 10 mg/L, and 100 mg/L monochloramine for 3 months. Increased DNA synthesis (as indicated by increased [3H] thymidine uptake into the testes cell nuclei) was noted at 100 mg/L after the 3-month treatment, whereas increased uptake into the kidney and spleen cell nuclei were noted at the two lower doses (1 mg/L and 10 mg/L) only. Monochloramine did not increase the number of sperm-head abnormalities in male B6C3F1 mice (10/group) gavaged with 1 ml of 0, 50 mg/L, 100 mg/L, or 200 mg/L of monochloramine per day for 5 days and then sacrificed (Meier et al., 1985).
9.2.5 Reproductive and developmental toxicity
No development or reproductive effects were seen in two limited oral rat studies using monochloramine in drinking water. No studies were located for di- and trichloramine.
Adult male (12/dose) and female Long–Evans rats (24/dose) were gavaged with monochloramine in deionized water at doses of 0, 2.5, 5, and 10 mg/kg bw per day for 66 days (males) and 76 days (females) before and during mating. Dosing continued throughout gestation and up to day 21 of lactation for females (Carlton et al., 1986). No changes in body weight gain, hematology, fertility, reproductive histopathology or sperm parameters (including motility, morphology and count) were seen in adults. Lactation, litter viability, litter size, mean pup weight and day of eye opening were not affected up to postnatal day (PND) 21. Several female pups were retained until PNDs 28–40 for observation and showed no effect on average days of vaginal patency. Statistical analysis of the data is not provided in the study.
No significant external or soft tissue anomalies or resorption were seen in fetuses, when female Sprague–Dawley rats (6/dose) were exposed to monochloramine in drinking water (0, 1 mg/L, 10 mg/L, or 100 mg/L) for 2½ months prior to mating and up to gestation day 20 (Abdel-Rahman et al., 1982). Information on statistical analyses, maternal weight, drinking water intake, and monochloramine intake were not provided in the study. The study also used a small number of animals per dose and reported fetal effects based on individual pups rather than by litter or maternal unit.
10.0 Classification and assessment
The International Agency for Research on Cancer (IARC, 2004) has classified monochloramine as “not classifiable as to its carcinogenicity to humans” (Group 3) based on inadequate evidence in humans and in experimental animals. Similarly, the U.S. EPA (1994a) classified monochloramine as not classifiable to human carcinogenicity (Group D), based on inadequate evidence of cancer in both humans and animals. The NTP (1992) study concluded that there was equivocal (marginal) evidence of carcinogenic activity in female rats based on an increase in the incidence of MNCL and that there was no evidence of carcinogenic activity of chloraminated water in male rats, male mice, or female mice. Although an increased incidence of MNCL in female rats was observed, there was no evidence of a dose–response relationship and no evidence of a temporal relationship between increasing dose and incidence of tumours. In addition, it was noted that MNCL has a high spontaneous rate of occurrence in female F344 rats and that the levels reported in the NTP (1992) study were within the historical control range (U.S. EPA, 1994a). The use of F344 rats by NTP has since been discontinued in carcinogenicity studies because of the high and variable background incidence of MNCL in F344 rats, the species- and strain-specificity of MNCL and its questionable significance to humans, especially when rates fall within normal historical control values (King-Herbert and Thayer, 2006; Maronpot et al., 2016).
The databases for di- and trichloramine were weak, with only two subchronic oral or drinking water studies available. Although additional studies are available for trichloramine via inhalation, these pertain to occupational and swimming pool exposures, which occurred at different conditions than those found in drinking water systems. It is unlikely that trichloramine would be produced during regular residential use of chloraminated drinking water (e.g., during showering or bathing).
The database for monochloramine is better characterized and consists of studies conducted orally, with several studies dosing in drinking water. In drinking water, monochloramine is the predominant chloramine present while dichloramine and trichloramine are infrequently detected.
A decrease in animal body weight gain was observed in a number of subchronic and chronic studies conducted using monochloramine with rats and mice. These effects occurred at doses of 50–200 ppm (2.6–9.5 mg/kg bw per day for rats; 7.4–24.2 mg/kg bw per day for mice). Several authors have suggested that this response is probably linked to decreased water consumption by rodents (Daniel et al., 1990, 1991; NTP, 1992) most likely due to aversion to the taste of high levels of chloramine in drinking water and thus not considered biologically significant. This opinion is also shared by the U.S. EPA (1994b) and the WHO (2011). Poon et al. (1997) demonstrated this effect by adding a paired-water control to their study and also found changes in body weights, organ weights, and minor changes in biochemistry, hematology, immunological and histopathology in the treated group as well as in the paired-water controls. The authors concluded that these changes were related to the reduced water and food consumption seen and not as a result of monochloramine toxicity.
Some possible immunological effects have been reported, although the biological significance of these effects is unclear. No treatment-related developmental or reproductive effects have been observed in rats exposed to monochloramine in drinking water in limited studies. It should also be noted that monochloramine rapidly breaks down in the stomach (see Section 9.2.3), forming other potentially toxic compounds (e.g., DBPs) depending on the stomach contents and pH.
In humans, results from available studies and surveys did not indicate any adverse health effects associated with exposure to monochloramine in drinking water. Because there were limitations with these studies (e.g., weak exposure assessment, chloramination used as control, sample bias), no definitive conclusions could be drawn. There have been no published reports of dermal or ocular irritation in humans following bathing or showering with chloraminated tap water. In addition, no information is available on any potential systemic toxicity that can be caused by exposure to chloramine via the dermal route.
A toxic endpoint from exposure to monochloramine (or di- or trichloramine) has not been identified due to the lack of toxicity observed in rodent or human studies. Hence, it was not deemed necessary to establish a Health Based Value for chloramine in drinking water.
10.1 Aesthetic considerations
Chloramines are in general less noticeable and less offensive than free chlorine. Monochloramine normally does not contribute significantly to objectionable taste and odour issues when present at concentrations typically found in drinking water; however, the presence of dichloramine and trichloramines is more likely to cause customer taste and odour complaints. These complaints can be an important source of information for utilities, and operational strategies to reduce tastes and odours include treating water to remove taste and odour precursors, flushing the distribution system and reducing detention time and water age in the distribution system. The main objective of monochloramine is to provide secondary disinfection, protecting public health by maintaining the microbiological safety of the drinking water supply during distribution. Therefore, where elevated chloramine concentrations are required in order to maintain an effective disinfectant residual throughout the distribution system, the taste thresholds may be exceeded.
10.2 International considerations
Several organizations have set guidelines or regulations pertaining to the concentration of chloramines in drinking water, all based on the same chronic 2-year study (NTP, 1992), which indicated a NOAEL of 200 ppm, the highest dose of the study. None of the agencies applied an additional uncertainty factor for possible carcinogenicity, as the carcinogenic effects reported in the study were equivocal and the tumour incidences were within the range observed in historical controls.
The U.S. EPA (1998) has established a maximum residual disinfectant level (MRDL) and a maximum residual disinfectant level goal (MRDLG) for chloramine, both set at 4.0 mg/L (4,000 μg/L) and measured as combined total chlorine (as Cl2). The U.S. EPA (1998) considers MRDLs to be enforceable standards, analogous to maximum contaminant levels (MCLs), which recognize the benefits of adding a disinfectant to water on a continuous basis and of maintaining a residual to control for pathogens in the distribution system. The MRDL and MRDLG are the same because there are no limitations imposed by the analytical methods or treatment technology.
The World Health Organization (2004, 2011) established a drinking water guideline of 3 mg/L (or 3,000 μg/L) based on the same NTP (1992) study. WHO (2011) noted that there was insufficient data to establish a guideline for the other two forms of inorganic chloramines, di-and trichloramine. The Australian drinking water guideline for chloramine is also set at 3.0 mg/L (NHMRC, 2011), based on the same NTP (1992) study.
11.0 Rationale
The protection of public health by maintaining the microbiological safety of the drinking water supply during distribution is the primary concern when using monochloramine for secondary disinfection. Health risks from chloramines or from any of its DBPs are much lower than the risks from consuming water that has not been adequately disinfected. Minimal to no effects were seen in both humans and animals following ingestion of monochloramine, with the most significant effect being decreased body weight gain in animal studies. However, this effect is attributed to a reduction in water consumption caused by taste aversion to drinking water with high levels of monochloramine. The information on dichloramine and trichloramine is insufficient to establish any link with unwanted health effects in animals or in humans. These forms are also not frequently detected in drinking water.
Health-based values have been established for chloramines in other countries or by international organizations. The WHO and Australia have established guideline values for chloramines of 3 mg/L in drinking water, whereas the U.S. EPA has set a maximum residual disinfectant level goal of 4 mg/L for chloramines. These health-based values have all been derived from the same NTP (1992) study, using the highest dose administered. These standards or guidelines are considered to be conservative, since there were no documented health effects in that study or any other available studies. A true NOAEL was not identified in the NTP study due to aesthetic considerations limiting the highest doses tested in rodents. Based on the lack of toxicity observed in rodent studies, the Federal-Provincial-Territorial Committee on Drinking Water has deemed that there is no need to establish a guideline for chloramines in drinking water. The Committee has also determined that an aesthetic objective is not necessary, since levels commonly found in drinking water are within an acceptable range for taste and odour, and since protection of consumers from microbial health risks is paramount.
Where chloramines are used as a drinking water disinfectant, it is recommended that their concentration be determined on a system-specific basis to ensure effectiveness of disinfection and maintenance of an appropriate residual while minimizing by-product formation and aesthetic concerns.
12.0 References
-
Abdel-Rahman, M.S., Berardi, M.R. and Bull, R.J. (1982). Effect of chlorine and monochloramine in drinking water on the developing rat fetus. J. Appl. Toxicol., 2(3): 156–159.
-
Abdel-Rahman, M.S., Waldron, D.M. and Bull, R.J. (1983). A comparative kinetics study of monochloramine and hypochlorous acid in rats. J. Appl. Toxicol., 3(4): 175–179.
-
Abdel-Rahman, M.S., Suh, H.D. and Bull, R.J. (1984). Toxicity of monochloramine in rat: An alternative drinking water disinfectant. J. Toxicol. Environ. Health, 13: 825–834.
-
Alexander, M. (2017). Development of a disinfectant residual monitoring plan. U.S. EPA Small Systems Webinar Series. Jan 31, 2017.
-
APHA/AWWA/WEF (1995). Standard methods for the examination of water and wastewater. 19th edition. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC.
-
APHA/AWWA/WEF (1998). Standard methods for the examination of water and wastewater. 20th edition. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC.
-
APHA/AWWA/ WEF (2000). Standard methods for the examination of water and wastewater (online version). American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC.
-
APHA/AWWA/WEF (2005). Standard methods for the examination of water and wastewater. 21st edition. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC.
-
APHA/AWWA/WEF (2012). Standard methods for the examination of water and wastewater. 22nd edition. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC.
-
Aschengrau, A., Zierler, S. and Cohen, A. (1993). Quality of community drinking water and the occurrence of late adverse pregnancy outcomes. Arch. Environ. Health, 48(2): 105–113.
-
ASTM (1986). ASTM D1253-86 Standard test method for residual chlorine in water. ASTM International, West Conshohocken, Pennsylvania.
-
ASTM (2003). ASTM D1253-03 Standard test method for residual chlorine in water. ASTM International, West Conshohocken, Pennsylvania.
-
ASTM (2008). ASTM D1253-08 Standard test method for residual chlorine in water. ASTM International, West Conshohocken, Pennsylvania.
-
ASTM (2014). ASTM D1253-14 Standard test method for residual chlorine in water. ASTM International, West Conshohocken, Pennsylvania.
-
AWWA (1999). Water quality & treatment: A handbook of community water supplies. 5th edition. Published for the American Water Works Association by McGraw-Hill, New York, New York.
-
AWWA (2006a). Fundamentals and control of nitrification in chloraminated drinking water systems. Manual of water supply practices – M56. 1st edition. American Water Works Association, Denver, Colorado.
-
AWWA (2006b). Water chlorination/chloramination practices and principles. Manual of water supply practices – M20. 2nd edition. American Water Works Association, Denver, Colorado.
-
AWWA (2013). Fundamentals and control of nitrification in chloraminated drinking water systems. Manual of water supply practices – M56. 2nd edition. American Water Works Association, Denver, Colorado.
-
Barbee, S.J. Thackara, J.W. and Rinehart, W.E. (1983). Acute inhalation toxicology of nitrogen trichloride. Am. Ind. Hyg. Assoc. J., 44(2): 145–146.
-
Baribeau, H. (2006). Chapter 6: Growth and inactivation of nitrifying bacteria. In: Fundamentals and control of nitrification in chloraminated drinking water distribution systems. Manual of water supply practices – M56. 1st edition. American Water Works Association, Denver, Colorado. pp. 99–127.
-
Baron, J.L., Vikram, A., Duda, S., Stout, J.E. and Bibby, K. (2014). Shift in the microbial ecology of a hospital hot water system following the introduction of an on-site monochloramine disinfection system. PLoS One, 9(7): e102679.
-
Bartrand, T.A., Causey, J.J. and Clancy, J.L. (2014). Naegleria fowleri: An emerging drinking water pathogen. J. Am. Water Works Assoc., 106(10): E418–E432.
-
Beer, K.D., Gargano, J.W., Roberts, V.A., Hill, V.R., Garrison, L.E., Kutty, P.K., Hilborn, E.D., Wade, T.J., Fullerton, K.E. and Yoder, J.S. (2015). Surveillance for waterborne disease outbreaks associated with drinking water — United States, 2011–2012. Morb. Mortal. Wkly Rep., 64(31): 842–848.
-
Bercz, J.P., Jones, L., Garner, L., Murray, D., Ludwig, A. and Boston, J. (1982). Subchronic toxicity of chlorine dioxide and related compounds in drinking water in the nonhuman primate. Environ. Health Perspect., 46: 47–55.
Bercz, J.P. and Bawa, R. (1986). Iodination of nutrients in the presence of chlorine based disinfectants used in drinking water treatment. Toxicol. Lett., 34: 141–147. -
Black and Veatch Corporation (2010). White's handbook of chlorination and alternative disinfectants. 5th edition. John Wiley and Sons, Hoboken, New Jersey.
-
Blatchley, E.R. and Cheng, M. (2010). Reaction mechanism for chlorination of urea. Environ. Sci. Technol., 44(22): 8529–8534.
-
Bonds, R.W. (2004). Effect of chloramines on ductile-iron pipe gaskets of various elastomer compounds. J. Am. Water Works Assoc., 96(4): 153–160.
-
Boyd, G.R., Dewis, K.M., Korshin, G.V., Reiber, S.H., Schock, M.R., Sandvig, A.M. and Giani, R. (2008). Effects of changing disinfectants on lead and copper release. J. Am. Water Works Assoc., 100(11): 75–87.
-
British Columbia Ministry of Health (2017). Personal communication from D. Fishwick, Drinking Water Manager.
-
Bull, R.J. (1980). Health effects of alternate disinfectants and their reaction products. J. Am. Water Works Assoc., 72(5): 299–303.
-
Camara, E., Montreuil, K.R., Knowles, A.K. and Gagnon, G.A. (2013). Role of the water main in lead service line replacement: A utility case study. J. Am. Water Works Assoc., 105(8): E423–E431.
-
Camper, A.K., Brastrup, K., Sandvig, A., Clement, J., Spencer, C. and Capuzzi, A.J. (2003). Effect of distribution system materials on bacterial regrowth. J. Am. Water Works Assoc., 7(95): 107–121.
-
Carbonnelle, S., Francaux, M., Doyle, I., Dumont, X., de Burbure, C., Morel, G., et al. (2002). Changes in serum pneumoproteins caused by short-term exposures to nitrogen trichloride in indoor chlorinated swimming pools. Biomarkers, 7(6): 464–478.
-
Carlton, B.D., Barlett, P., Basaran, A., Colling, K., Osis, I. and Smith, M.K. (1986). Reproductive effects of alternative disinfectants. Environ. Health Perspect., 69: 237–241.
-
CDC (Centers for Disease Control) (2008). Epi-aid trip report: An assessment of health concerns in a community exposed to chloramine treated tap water in Vermont, 2006–2007. Health Studies Branch, Centers for Disease Control and Prevention, Epi-2007-054. Available at: www.mawc.org/sites/default/files/department_of_health.pdf
-
Charrois, J.W.A. and Hrudey, S.E. (2007). Breakpoint chlorination and free-chlorine contact time: Implications for drinking water N-nitrosodimethylamine concentrations. Water Res., 41(3): 674–682.
-
Chiao, T.H., Clancy, T.M., Pinto, A., Xi, C. and Raskin, L. (2014). Differential resistance of drinking water bacterial populations to monochloramine disinfection. Environ. Sci. Technol., 48(7): 4038–4047.
-
City of Ottawa (2017). Personal communication with I. Douglas and P. Wilson, Drinking Water Services.
-
Coburn, K.M., Wang, Q., Rediske, D., Viola, R.E., Hanson, B.L., Xue, Z. and Seo, Y. (2016). Effects of extracellular polymeric substance composition on bacteria disinfection by monochloramine: Application of MALDI-TOF/TOF-MS and multivariate analysis. Environ. Sci. Technol., 50(17): 9197–9205.
-
Cohen, Y.K. and Friedman, M. (2006). Chapter 1: Introduction to nitrification in drinking water and its impact on regulatory compliance. In: Fundamentals and control of nitrification in chloraminated drinking water distribution systems. Manual of water supply practices – M56. 1st edition. American Water Works Association, Denver, Colorado. pp. 43–67.
-
Cohle, S.D., Thompson, W., Eisenga, B.H. and Cottingham, S.L. (2001). Unexpected death due to chloramine toxicity in a woman with a brain tumor. Forensic Sci. Int., 124: 137–139.
-
Cromeans, T.L., Kahler, A.M. and Hill, V.R. (2010). Inactivation of adenoviruses, enteroviruses, and murine norovirus in water by free chlorine and monochloramine. Appl. Environ. Microbiol., 76(4): 1028–1033.
-
Cunliffe, D.A. (1991). Bacterial nitrification in chloraminated water supplies. Appl. Environ. Microbiol., 57(11): 3399–3402.
-
Dang, B., Chen, L., Mueller, C., Dunn, K.H., Almaguer, D., Roberts, J.L., et al. (2010). Ocular and respiratory symptoms among lifeguards at a hotel indoor waterpark resort. J. Occup. Environ. Med., 52(2): 207–213.
-
Daniel, F.B., Condie, L.W., Robinson, M., Stober, J.A., York, R.G., Olson, G.R., et al. (1990). Comparative subchronic toxicity studies of three disinfectants. J. Am. Water Works Assoc., 82(10): 61–69.
-
Daniel, F.B., Ringhand, H.P., Robinson, M., Stober, J.A. and Olson, G.R. (1991). Comparative subchronic toxicity of chlorine and monochloramine in the B6C3F1 mouse. J. Am. Water Works Assoc., 83(11): 68–75.
-
Davis II, W.E. and Li, Y. (2008). Analysis of hydrazine in drinking water by isotope dilution gas chromatography/tandem mass spectrometry with derivatization and liquid–liquid extraction. Anal. Chem., 80(14): 5449–5453.
-
Donnermair, M.M. and Blatchley, E.R. (2003). Disinfection efficacy of organic chloramines. Water Res., 37(7): 1557–1570.
-
Douglas, I., Guthmann, J., Muylwyk, Q. and Snoeyink, V. (2004). Corrosion control in the City of Ottawa—Comparison of alternatives and case study for lead reduction in drinking water. In: W. Robertson and T. Brooks (eds.), 11th Canadian National Drinking Water Conference and 2nd Policy Forum, April 3–6, Calgary, Alberta. Canadian Water and Wastewater Association, Ottawa, Ontario.
-
Duirk, S.E., Gombert, B., Croué, J. and Valentine, R.L. (2005). Modeling monochloramine loss in the presence of natural organic matter. Water Res., 39(14): 3418–3431.
-
Dunnick, J.K. and Melnick, R.L. (1993). Assessment of the carcinogenic potential of chlorinated water: Experimental studies of chlorine, chloramine, and trihalomethanes. J. Natl Cancer Inst., 85: 817–822.
-
Dyksen, J.E., Spencer, C., Hoehn, R., Clement, J., Brandt-Edwards, J., Friedman, M.J., Hanson, A., Spellecacy, R., McGuire, M.J., Singer, P.C. and Camper, A. (2007). Long-term effects of disinfection changes on water quality. AWWA Research Foundation and American Water Works Association, Denver, Colorado.
-
Eaton, J.W., Kolpin, C.F., Swofford, H.A., Kjellstrand, C.M. and Jacob, H.S. (1973). Chlorinated urban water: A cause of dialysis-induced hemolytic anemia. Science, 181: 463–464.
-
Edwards, M. and Dudi, A. (2004). Role of chlorine and chloramine in corrosion of lead-bearing plumbing materials. J. Am. Water Works Assoc., 96(10): 69–81.
-
El-Dakdouki, M. H., El-Boubbou, K., Xia, J., Kavunja, H. and Huang, X. (2014). Methods for magnetic nanoparticle synthesis and functionalization. In: Narain, R. (ed.). Chemistry of bioconjugates: Synthesis, characterization, and biomedical applications. John Wiley & Sons, Inc., Hoboken, NJ, USA. doi: 10.1002/9781118775882.ch10. Available at: http://onlinelibrary.wiley.com/doi/10.1002/9781118775882.ch10/summary
Environment Canada and Health Canada (2001). Priority substances list assessment report. Inorganic chloramines. Canadian Environmental Protection Act, 1999. Available at: www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/environmental-contaminants/canadian-environmental-protection-act-1999-priority-substances-list-assessment-report-inorganic-chloramines.html -
Exon, J.H., Koller, L.D., O’Reilly, C.A. and Berck, J.P. (1987). Immunotoxicologic evaluation of chlorine-based drinking water disinfectants, sodium hypochlorite and monochloramine. Toxicology, 44(3): 257–269.
-
Falkinham, J.O. (2015). Common features of opportunistic premise plumbing pathogens. Int. J. Environ. Res. Public Health, 12(5): 4533–4545.
-
Feng, T.H. (1966). Behavior of organic chloramines in disinfection. J. Water Pollut. Control Fed. 38(4): 614–628.
-
Flannery, B., Gelling, L.B., Vugia, D.J., Weintraub, J.M., Salerno, J.J., Conroy, M.J., Stevens, V.A., Rose, C.E., Moore, M.R., Fields, B.S. and Besser, R.E. (2006). Reducing Legionella colonization of water systems with monochloramine. Emerg. Infect. Dis., 12(4): 588–596.
-
Fluck, S., McKane, W., Cairns, T., Fairchild, V., Lawrence, A., Lee, J., et al. (1999). Chloramine-induced haemolysis presenting as erythropoietin resistance. Nephrol. Dial. Transplant., 14(7): 1687–1691.
-
Fricker, C.R. (2003). The presence of bacteria in water after regrowth. In: Heterotrophic plate counts and drinking-water safety. Bartram, J., Cotruvo, J., Exner, M., Fricker, C. and Glasmacher, A. (eds.). IWA Publishing, London, United Kingdom. pp. 137–145.
-
Friedman, M.J., Hill, A.S., Reiber, S.H., Valentine, R.L. and Korshin, G.V. (2010). Assessment of inorganics accumulation in drinking water system scales and sediments. Water Research Foundation, Denver, Colorado.
Gagnaire, F., Azim, S., Bonnet, P., Hecht, G. and Hery, M. (1994). Comparison of the sensory irritation response in mice to chlorine and nitrogen trichloride. J. Appl. Toxicol., 14(6): 405–409. -
Gagnon, G.A., O'Leary, K.C., Volk, C.J., Chauret, C., Stover, L. and Andrews, R.C. (2004). Comparative analysis of chlorine dioxide, free chlorine and chloramines on bacterial water quality in model distribution systems. J. Environ. Eng., 130(11): 1269–1279.
-
Gagnon, G.A., Baribeau, H., Rutledge, S.O., Dumancic, R., Oehmen, A., Chauret, C. and Andrews, S. (2008). Disinfectant efficacy in distribution systems: A pilot-scale assessment. J. Water Supply Res. T., 57(7): 507–518.
Gapany-Gapanavicius, M., Molho, M. and Tirosh, M. (1982). Chloramine-induced pneumonitis from mixing household cleaning agents. Br. Med. J. (Clin. Res. Ed.), 285(6348): 1086. -
Gomez-Alvarez, V., Revetta, R.P. and Domingo, J.W.S. (2012). Metagenomic analyses of drinking water receiving different disinfection treatments. Appl. Environ. Microbiol., 78(17): 6095–6102.
-
Gomez-Alvarez, V., Pfaller, S., Pressman, J.G., Wahman, D.G. and Revetta, R.P. (2016). Resilience of microbial communities in a simulated drinking water distribution system subjected to disturbances: Role of conditionally rare taxa and potential implications for antibiotic-resistant bacteria. Environ. Sci. Water Res. Technol., 2(4): 645–657.
-
Gomez-Smith, C.K., Lapara, T.M. and Hozalski, R.M. (2015). Sulfate reducing bacteria and mycobacteria dominate the biofilm communities in a chloraminated drinking water distribution system. Environ. Sci. Technol., 49(14): 8432–8440.
-
Graham, W. H. (1965). Reactions of dichloramine. I. A convenient method of preparation of diazirine. J. Org. Chem. 30: 2108.
-
Griffin, A.E. and Chamberlin, N.S. (1941). Relation of ammonia-nitrogen to break-point chlorination. Am. J. Public Health Nations Health, 31(8): 803–808.
-
Guo, T.L., Germolec, D.R., Collins, B.J., Luebke, R.W., Auttachoat, W., Smith, M.J., et al. (2011). Immunotoxicological profile of chloramine in female B6C3F1 mice when administered in the drinking water for 28 days. J. Immunotoxicol., 8(4): 381–388.
-
Haas, C.N. (1999). Benefits of using a disinfectant residual. J. Am. Water Works Assoc., 91(1): 65–69.
-
Hach (2013). USEPA DPD Method 10260. 4th Edition. Hach Company, Loveland, Colorado.
-
Hach (2017). USEPA Indophenol Method. 10th Edition. Hach Company, Loveland, Colorado.
-
Harp, D.L., Wiese, P. and Franklin, S., inventors; Hach Company, assignee. Controlling chlorination of wastewater and chloramination of drinking water. United States patent US6315950 B1. November 13, 2001.
-
Health Canada (2009). Guidance on controlling corrosion in drinking water distribution systems. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at: www.canada.ca/en/health-canada/services/publications/healthy-living/guidance-controlling-corrosion-drinking-water-distribution-systems.html
-
Health Canada (2011). Guidelines for Canadian drinking water quality: Guideline technical document — N-Nitrosodimethylamine. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No H128-1/11-662E).
-
Health Canada (2012a). Guidelines for Canadian drinking water quality: Guideline technical document — Total coliforms. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No H144-8/2013EPDF).
-
Health Canada (2012b). Guidelines for Canadian drinking water quality: Guideline technical document — Escherichia coli. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No H144-7/2013EPDF).
-
Health Canada (2013a). Guidelines for Canadian drinking water quality: Guideline technical document — Ammonia. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No H144-13/5-2013E-PDF).
-
Health Canada (2013b). Guidelines for Canadian drinking water quality: Guideline technical document — Nitrate and nitrite. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No H144-13/2-2013EPDF).
-
Health Canada (2017). Guidelines for Canadian drinking water quality: Guideline technical document — Enteric protozoa: Giardia and cryptosporidium. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario.
-
Health Canada (2018a). Guidelines for Canadian drinking water quality: Guideline technical document — Enteric viruses. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario.
-
Health Canada (2018b). Guidelines for Canadian drinking water quality: Guideline technical document — Natural organic matter. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario.
-
Herren-Freund, S.L. and Pereira, M.A. (1986). Carcinogenicity of by-products of disinfection in mouse and rat liver. Environ. Health Perspect., 69: 59–65.
-
Hery, M., Gerber, J.M., Hecht, G., Subra, I., Possoz, C., Aubert, S., et al. (1998). Exposure to chloramines in a green salad processing plant. Ann. Occup. Hyg., 42(7): 437–451.
-
Hill, C.P. and Arweiler, S. (2006). Chapter 9: Assessment and operational responses to nitrification episodes. In: Fundamentals and control of nitrification in chloraminated drinking water distribution systems. Manual of water supply practices – M56. 1st edition. American Water Works Association. Denver, Colorado. pp. 189–220.
-
Hua, G. and Reckhow, D.A. (2007). Comparison of disinfection byproduct formation from chlorine and alternative disinfectants. Water Res., 41(8): 1667–1678.
-
Hutcherson, D. (2007). Maintaining a continuous target ratio of chlorine and ammonia. J. Am. Water Works Assoc., 99(2): 44–46.
-
Hwang, C., Ling, F., Andersen, G.L., LeChevallier, M.W. and Liu, W.T. (2012). Microbial community dynamics of an urban drinking water distribution system subjected to phases of chloramination and chlorination treatments. Appl. Environ. Microbiol., 78(22): 7856–7865.
-
IARC (2004). Some drinking-water disinfectants and contaminants, including arsenic. Chloramine. International Agency for Research on Cancer . IARC Monogr. Eval. Carcinog. Risks Hum., 84: 295–316. Available at: http://monographs.iarc.fr/ENG/Monographs/vol84/mono84.pdf
-
Jacangelo, J.G., Olivieri, V.P. and Kawata, K. (1991). Investigating the mechanism of inactivation of Escherichia coli B by monochloramine. J. Am. Water Works Assoc., 83(5): 80–87.
-
Jacangelo, J.G., Patania, N.L., Trussell, R.R., Haas, C.N. and Gerba, C. (2002). Inactivation of waterborne emerging pathogens by selected disinfectants. AWWA Research Foundation and American Water Works Association, Denver, Colorado.
-
Jacobs, J.H., Spaan, S., van Rooy, G.B.G.J., Meliefste, C., Zaat, V.A.C., Rooyackers, J.M., et al. (2007). Exposure to trichloramine and respiratory symptoms in indoor swimming pool workers. Eur. Respir. J., 29(4): 690–698.
Jafvert, C.T. and Valentine, R.L. (1992). Reaction scheme for the chlorination of ammoniacal water. Environ. Sci. Technol., 26(3): 577–786. -
Ji, P., Parks, J., Edwards, M.A. and Pruden, A. (2015). Impact of water chemistry, pipe material and stagnation on the building plumbing microbiome. PLoS One, 10(10): e0141087.
-
Jones, D.B., Saglam, A., Song, H. and Karanfil, T. (2012). The impact of bromide/iodide concentration and ratio on iodinated trihalomethane formation and speciation. Water Res., 46(1): 11–20.
-
Junglee, N.A., Rahman, S.U., Wild, M., Wilms, A., Hirst, S., Jibani, M. and Seale, J.R.C. (2010). When pure is not so pure: Chloramine-related hemolytic anemia in home hemodialysis patients. Hemodial. Int., 14: 327–332.
-
Kato, S., Nishiwaki, H., Konaka, A. and Takeuchi, K. (1997). Mucosal ulcerogenic action of monochloramine in rat stomachs (effects of polaprezinc and sucralfate). Dig. Dis. Sci., 42(10): 2156–2163.
-
Kiefer, M., Sanderson, W.T., Lenhart, S.W. and Weber, A. (2000). NIOSH Health hazard evaluation of Wampler Foods, Inc.: Evaluation of eye, nose, and throat irritation in the First Processing Department. National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Washington, D.C.
-
King-Herbert, A. and Thayer, K (2006). NTP Workshop: Animal models for the NTP rodent cancer bioassay: Stocks and strains—should we switch? Toxicol. Pathol. 34(6): 802–805.
-
Kirk-Othmer (ed.) (2004). N-halamines. In: Kirk-Othmer encyclopedia of chemical technology. Vol. 13. 5th edition. John Wiley & Sons, Inc. Online version.
-
Kirmeyer, G.J., Odell, L.H., Jacangelo, J., Wilczak, A. and Wolfe, R.L. (1995). Nitrification occurrence and control in chloraminated water systems. AWWA Research Foundation and American Water Works Association, Denver, Colorado.
-
Kirmeyer, G.J., Friedman, M., Martel, K., Howie, D., LeChevallier, M., Abbaszadegan, M., Karim, M., Funk, J. and Harbour, J. (2001). Pathogen intrusion into the distribution system. AWWA Research Foundation and American Water Works Association, Denver, Colorado.
-
Kirmeyer, G.J., Martel, K., Thompson, G., Radder, L., Klement, W., LeChevallier, M., Baribeau, H. and Flores, A. (2004). Optimizing chloramine treatment. 2nd edition. AWWA Research Foundation and American Water Works Association, Denver, Colorado.
-
Kirmeyer, G.J., Thomure, T.M., Rahman, R., Marie, J.L., LeChevallier, M.W., Yang, J., Hughes, D.M. and Schneider, O. (2014). Effective microbial control strategies for main breaks and depressurization. Water Research Foundation, Denver, Colorado.
-
Kjellstrand, C.M., Eaton, J.W., Yawata, Y., Swofford, H.S., Kolpin, C.F., Buselmeier, T.J., von Hartitzsch, B. and Jacob, H.S. (1974). Hemolysis in dialyzed patients caused by chloramines. Nephron, 13: 427–433.
Klein, E. (1986). Effects of disinfectants in renal dialysis patients. Environ. Health Perspect., 69: 45–47. -
Kotiaho, T., Wood, J.M., Wick Jr., P.L., Dejarme, L.E., Ranasinghe, A. and Cooks, R.G. (1992). Time persistence of monochloramine in human saliva and stomach fluid. Environ. Sci. Technol., 26(2): 302–306.
-
Krasner, S.W. and Barret, S.E. (1984). Aroma and flavor characteristics of free chlorine and chloramines. In: Proc. AWWA water quality technology conference. AWWA, 1984, Denver, Colorado. pp. 381–398.
-
Krasner, S.W., Shirkhani, R., Westerhoff, P., Hanigan, D., Mitch, W.A., McCurry, D.L., Chen, C., Skadsen, J. and von Gunten, U. (2015). Controlling the formation of nitrosamines during water treatment. Water Research Foundation and U.S. Environmental Protection Agency, Denver, Colorado.
-
LeChevallier, M.W., Lowry, C.D., and Lee, R.G. (1990). Disinfecting biofilms in a model distribution system. J. Am. Water Works Assoc., 82(7): 87–99.
-
LeChevallier, M.W. (1998). Benefits of employing a disinfectant residual in distribution systems. Water Supp., 16(3–4): 61–73.
-
LeChevallier, M.W. and Au, K.K. (2004). Water treatment and pathogen control: Process efficiency in achieving safe drinking water. IWA Publishing, London, UK, on behalf of the World Health Organization, Geneva.
-
LeChevallier, M.W., Schneider, O.D., Weinrich, L.A., Jjemba, P.K., Evans, P.J., Hooper, J.L. and Chappell, R.W. (2015). Guidance manual for control of biostability in drinking water. Water Research Foundation, Denver, Colorado.
-
Lee, W. and Westerhoff, P. (2009). Formation of organic chloramines during water disinfection – chlorination versus chloramination. Water Res., 43(8): 2233–2239.
-
Lee, W., Westerhoff, P., Yang, X. and Shang, C. (2007). Comparison of colorimetric and membrane introduction mass spectrometry techniques for chloramine analysis. Water Res., 41(14): 3097–3102.
-
Lee, W.H., Wahman, D.G., Bishop, P.L. and Pressman, J.G. (2011). Free chlorine and monochloramine application to nitrifying biofilm: Comparison of biofilm penetration, activity, and viability. Environ. Sci. Technol., 45(4): 1412–1419.
-
Li, J. and Blatchley, E.R. (2007). Volatile disinfection byproduct formation resulting from chlorination of organic-nitrogen precursors in swimming pools. Environ. Sci. Technol., 41(19): 6732–6739.
-
Lian, L., E, Y., Li, J. and Blatchley, E.R. (2014). Volatile disinfection byproducts resulting from chlorination of uric acid: Implications for swimming pools. Environ. Sci. Technol, 48(6): 3210–3217.
-
Lin, N.H., Torrents, A., Davis, A.P., Zeinali, M. and Taylor, F.A. (1997). Lead corrosion control from lead, copper-lead solder, and brass coupons in drinking water employing free and combined chlorine. J. Environ. Sci. Health. A, 32(4): 865–884.
-
Louisiana Department of Health and Hospitals (2016). Rule: Sanitary code/Water supplies minimum disinfectant residual levels in public water systems. Louisiana Register, 42(03). March. Available from: www.dhh.la.gov
-
Lubbers, J.R. and Bianchine, J.R. (1984). Effects of the acute rising dose administration of chlorine dioxide, chlorate and chlorite to normal healthy adult male volunteers. J. Environ. Pathol. Toxicol. Oncol., 5: 215–228.
-
Lubbers, J.R., Chauan, S. and Bianchine, J.R. (1982). Controlled clinical evaluations of chlorine dioxide, chlorite and chlorate in man. Environ. Health Perspect., 46: 57–62.
-
Lubbers, J.R., Chauhan, S., Miller, J.K. and Bianchine, J.R. (1984). The effects of chronic administration of chlorine dioxide, chlorite and chlorate to normal healthy adult male volunteers. J. Environ. Pathol. Toxicol. Oncol., 5: 229–238.
-
Lytle, D.A. and Schock, M.R. (2005). Formation of Pb(IV) oxides in chlorinated water. J. Am. Water Works Assoc., 97(11): 102–114.
-
Mackey, E.D., Baribeau, H., Crozes, G.F., Suffet, I.H. and Piriou, P. (2004). Public thresholds for chlorinous flavors in U.S. tap water. Water Sci. Technol., 49(9): 335–340.
-
Mallevialle, J. and Suffet, J.H. (1987). Identification and treatment of tastes and odors in drinking water. American Water Works Association Research Foundation, Denver, Colorado [cited in Health Canada, 1996].
-
Mancini, B., Scurti, M., Dormi, A., Grottola, A., Zanotti, A. and Cristino, S. (2015). Effect of monochloramine treatment on colonization of a hospital water distribution system by Legionella spp.: A 1 year experience study. Environ. Sci. Technol., 49(7): 4551–4558.
-
Maronpot, R.R., Nyska, A., Foreman, J.E., and Ramot, Y. (2016). The legacy of the F344 rat as a cancer bioassay model (a retrospective summary of three common F344 rat neoplasms). Crit. Rev. Toxicol., 46(8): 641–675.
-
Massin, N., Hecht, G., Ambroise, D., Héry, M., Toamain, J.P., Hubert, G., et al. (2007). Respiratory symptoms and bronchial responsiveness among cleaning and disinfecting workers in the food industry. Occup. Environ. Med., 64(2): 75–81.
-
Masters, S. and Edwards, M. (2015). Increased lead in water associated with iron corrosion. Environ. Eng. Sci., 32(5): 361–369.
-
McGeehin, M.A., Reif, J.S., Becher, J.C. and Mangione, E.J. (1993). Case-control study of bladder cancer and water disinfection methods in Colorado. Am. J. Epidemiol., 138(7): 492–501.
-
Meier, J.R., Bull, R.J., Stober, J.A. and Cimino, M.C. (1985). Evaluation of chemicals used for drinking water disinfection for production of chromosomal damage and sperm-head abnormalities in mice. Environ. Mutagen., 7(2): 201–211.
-
Mi, Z., Dai, Y., Xie, S., Chen, C. and Zhang, X. (2015). Impact of disinfection on drinking water biofilm bacterial community. J. Environ. Sci. (China), 37: 200–205.
-
Miller, R.G., Kopfler, F.C., Condie, L.W., Pereira, M.A., Meier, J.R., Ringhand, H.P., et al. (1986). Results of toxicological testing of Jefferson Parish pilot plant samples. Environ. Health Perspect., 69: 129–139.
-
Ministère du Développement durable, de l’Environnement et des Parcs du Québec. (2017). Personal communication from C. Robert, Direction de l’eau potable et des eaux souterraines.
-
Moore, G.S., Calabrese, E.J., and McGee, M. (1980). Health effects of monochloramines in drinking water. J. Environ. Sci. Health A., 15(3): 239–258.
-
Moore, M.R., Pryor, M., Fields, B., Lucas, C., Phelan, M. and Besser, R.E. (2006). Introduction of monochloramine into a municipal water system: Impact on colonization of buildings by Legionella spp. Appl. Environ. Microbiol., 72(1): 378–383.
-
Murphy, H.M., Payne, S.J. and Gagnon, G.A. (2008). Sequential UV- and chlorine-based disinfection to mitigate Escherichia coli in drinking water biofilms. Water Res., 42(8–9): 2083–2092.
-
Muylwyk, Q. (2009). Chloramine 101: Introduction to planning, design, implementation, and operation with chloramine. A little bit of chloramines chemistry. American Water Works Association Annual Conference and Exposition, June 14–18, 2009, San Diego, California.
-
Nagisetty, R.M., Rockaway, T.D. and Willing, G.A. (2014). Drinking water quality concerns from chloramine-induced degradation of elastomeric compounds. J. Am. Water Works Assoc., 106(9): E402–E407.
-
Najm, I., Brown, N.P., Guo, Y.C., Hwang, C.J. and Barrett, S.E. (2006). Formation of hydrazine as a chloramine by-product. AWWA Research Foundation and American Water Works Association, Denver, Colorado.
-
Najm, I., Brown, N.P. and Gramith, K. (2011). Quantifying hydrazine in chloraminated water. Water Research Foundation and American Water Works Association, Denver, Colorado.
-
Nakai, J.S., Poon, R., Lecavalier, P., Chu, I., Yagminas, A. and Valli, V.E. (2000). Effects of subchronic exposure of rats to dichloramine and trichloramine in drinking water. Regul. Toxicol. Phar., 31(2): 200–209.
-
National Research Council (U.S.) (1987). Drinking water and health. Volume 7. Commission on Life Sciences, Subcommittee on Disinfectants and Disinfectant By-Products. Available at: www.nap.edu/catalog/1008/drinking-water-and-health-volume-7-disinfectants-and-disinfectant-by
-
Nawrocki, J. and Andrzejewski, P. (2011). Nitrosamines and water. J. Hazard. Mater., 189(1): 1–18.
-
NHMRC (2011). National water quality management strategy. Australian Drinking Water Guidelines 6. 2011. Version 3.3. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra. Updated November 2016.
-
Nikl, L. and Nikl, D. (1992). Environmental impacts associated with monochloramine-disinfected municipal potable water. Robertson, W., Tobin, R. and Kjartanson, K. (eds.). Proceedings of the Fifth National Conference on Drinking Water, Winnipeg, Manitoba. American Water Works Association, Denver, Colorado. p. 291.
-
Nishiwaki, H., Kato, S. and Takeuchi, K. (1997). Irritant action of monochloramine in rat stomachs: effects of zinc l-camosine (polaprezinc). Gen. Pharmacol. Vasc. S., 29(5): 713–718.
-
Norton, C.D. and LeChevallier, M.W. (1997). Chloramination: Its effect on distribution system water quality. J. Am. Water Works Assoc., 89(7): 66–77.
-
Norton, C.D., LeChevallier, M.W. and Falkinham, J.O., III. (2004). Survival of Mycobacterium avium in a model distribution system. Water Res., 38(6): 1457–1466.
-
Nova Scotia Environment (2017). Personal communication from A. Polegato, Drinking Water Management Unit, Science Division.
-
NSF/ANSI (2017). NSF International/American National Standards Institute standard 42: Aesthetic effects. NSF International, Ann Arbor, Michigan.
-
NTP (1992). NTP technical report on the toxicology and carcinogenesis studies of chlorinated water (CAS Nos. 7782-50-5 and 7681-52-9) and chloraminated water (CAS No. 10599-90-3) (deionized and charcoal-filtered) in F344/N rats and B6C3F1 mice (drinking water studies). NTP TR 392. p. 482. Available at: http://ntp.niehs. nih.gov/results/pubs/longterm/reports/longterm/tr300399/abstracts/tr392/index.html
-
NTP (2000). IMM20007: Immunotoxicity of chloramine (CASRN 10599-90-3) in female B6C3F1 mice. National Toxicology Program, National Institute of Environmental Health Sciences, Washington, D.C. Abstract.
Ollos, P.J., Slawson, R.M. and Huck, P.M. (1998). Bench scale investigations of bacterial regrowth in drinking water distribution systems. Wat. Sci. Technol., 38(8–9): 275–282. -
Ontario Ministry of the Environment and Climate Change (2017). Personal communication from S. Deshpande, Water Standards Section, Standards Development Branch.
-
OSHA (2007). Chemical sampling information: Nitrogen trichloride. Occupational Safety and Health Administration, U.S. Department of Labor. Available at: www.osha.gov/dts/chemicalsampling/data/CH_257450.html
Pan, Y., Li, W., An, H., Cui, H. and Wang, Y. (2016). Formation and occurrence of new polar iodinated disinfection byproducts in drinking water. Chemosphere, 144: 2312–2320. -
Parrat, J., Donzé, G., Iseli, C., Perret, D., Tomicic, C. and Schenk, O. (2012). Assessment of occupational and public exposure to trichloramine in Swiss indoor swimming pools: A proposal for an occupational exposure limit. Ann. Occup. Hyg., 56(3): 264–277.
-
Pasternak, J.P., Moore, D.R.J. and Teed, R.S. (2003). An ecological risk assessment of inorganic chloramines in surface water. Hum. Ecol. Risk Assess., 9(2): 453–482.
-
Pintar, K.D.M. and Slawson, R.M. (2003). Effect of temperature and disinfection strategies on ammonia-oxidizing bacteria in a bench-scale drinking water distribution system. Water Res., 37(8): 1805–1817.
-
Pintar, K.D.M., Slawson, R.M. and Huck, P.M. (2000). Investigation of conditions and control strategies influencing nitrification in a chloraminated bench-scale distribution system. In: Proceedings of the AWWA Water Quality Technology Conference, American Water Works Association, Denver, Colorado.
-
Poon, R., Lecavalier, P., Tryphonas, H., Bondy, G., Chen, M., Chu, I., et al. (1997). Effects of subchronic exposure of monochloramine in drinking water on male rats. Regul. Toxicol. Phar., 25(2): 166–175.
-
Pressman, J.G. (2017). Disinfectant residual – Representative monitoring and minimum residual implications. U.S. EPA Small Systems Webinar Series. Jan 31, 2017.
-
Pressman, J.G., Lee, W.H., Bishop, P.L. and Wahman, D.G. (2012). Effect of free ammonia concentration on monochloramine penetration within a nitrifying biofilm and its effect on activity, viability, and recovery. Water Res., 46(3): 882–894.
-
PubMed (2005a). Open chemistry database: Monochloramine. Available at: www.ncbi.nlm.nih.gov/pccompound/
-
PubMed (2005b). Open chemistry database: Dichloramine. Modified in 2017. Available at: www.ncbi.nlm.nih.gov/pccompound/
-
Rahman, S., McDonald, B.C. and Gagnon, G.A. (2007). Impact of secondary disinfectants on copper corrosion under stagnation conditions. J. Environ. Eng., 133(2): 180–185.
-
Randtke, S. (2010). Chapter 2: Chemistry of aqueous chlorine. In: White’s handbook of chlorination and alternative disinfectants. 5th edition. John Wiley and Sons, Hoboken, New Jersey.
-
Reisz, G.R. and Gammon, R.S. (1986). Toxic pneumonitis from mixing household cleaners. Chest, 89(1): 49–52.
-
Renner, R. (2004). Leading to lead. Sci. Am., 291(1): 22–24.
-
Revetta, R.P., Gomez-Alvarez, V., Gerke, T.L., Curioso, C., Santo Domingo, J.W. and Ashbolt, N.J. (2013). Establishment and early succession of bacterial communities in monochloramine-treated drinking water biofilms. FEMS Microbiol. Ecol., 86(3): 404–414.
-
Richardson, S.D. and Ternes, T.A. (2005). Water analysis: Emerging contaminants and current issues. Anal. Chem., 77(12): 3807–3838.
-
Richardson, S.D., Fasano, F., Ellington, J.J., Crumley, F.G., Buettner, K.M., Evans, J.J., Blount, B.C., Silva, L.K., Waite, T.J., Luther, G.W., Mckague, A.B., Miltner, R.J., Wagner, E.D. and Plewa, M.J. (2008). Occurrence and mammalian cell toxicity of iodinated disinfection byproducts in drinking water. Environ. Sci. Technol., 42(22): 8330–8338.
-
Roberts, H. and Palmeiro, B.S. (2008). Toxicology of aquarium fish. Vet. Clin.North Am. Exot. Anim. Pract., 11(2): 359–374.
-
Robinson, B.S. and Christy, P.E. (1984). Disinfection of water for control of amoebae. Water, 11: 21–24.
-
Rockaway, T.D., Willing, G.A. and Nagisetty, R.M. (2007a). Life predictions of elastomers in drinking water distribution systems. J. Am. Water Works Assoc., 99(12): 99–110.
-
Rockaway, T.D., Willing, G.A., Schreck, R.M. and Davis, K.A. (2007b). Performance of elastomeric components in contact with potable water. AWWA Research Foundation and U.S. Environmental Protection Agency, Denver, Colorado.
-
Rose, L.J., Rice, E.W., Hodges, L., Peterson, A. and Arduino, M.J. (2007). Monochloramine inactivation of bacterial select agents. Appl. Environ. Microbiol., 73(10): 3437–3439.
-
Rosenfeldt, E.J., Baeza, C., Knappe, D.R.U. (2009). Effect of free chlorine application on microbial quality of drinking water in chloraminated distribution systems. J. Am. Water Works Assoc., 101(10): 60–70.
-
Sandvig, A., Martel, K., Beggs, K., Murray, A.J., Greiner, P., Kneen, K., McLellan, C., Bennet, D. and Terrell, J. (2012). Is NSF 61 relevant for chloraminating utilities? Water Research Foundation and U.S. Environmental Protection Agency, Denver, Colorado.
-
Saskatchewan Water Security Agency (2017). Personal communication from S. Ferris, Environmental and Municipal Management Services Division.
-
SCC (2016). Directory of accredited product, process and service certification bodies. Standards Council of Canada, Ottawa, Ontario. Available at: www.scc.ca
-
Schock, M.R. and Gardels, M.C. (1983). Plumbosolvency reduction by high pH and low carbonate–solubility relationships. J. Am. Water Works Assoc., 75(2): 87–91.
-
Schock, M.R. and Giani, R. (2004). Oxidant/disinfectant chemistry and impacts on lead corrosion. In: Proceedings of the 2004 AWWA Water Quality Technology Conference, San Antonio, Texas. American Water Works Association, Denver, Colorado.
-
Schock, M.R., Wagner, I. and Oliphant, R.J. (1996). Corrosion and solubility of lead in drinking water. In: Internal corrosion of water distribution systems. 2nd edition. American Water Works Association Research Foundation and DVGW Technologiezentrum Wasser, Denver, Colorado. pp. 131–230.
-
Scott, D.B., Van Dyke, M.I., Anderson, W.B. and Huck, P.M. (2015). Influence of water quality on nitrifier regrowth in two full-scale drinking water distribution systems. Can. J. Microbiol., 61(12): 965–976.
-
Scully Jr., F.E. and White, W.N. (1991). Reactions of chlorine, monochloramine in the GI tract. Environ. Sci. Technol., 25(5): 820–828.
-
Seidel, C.J., McGuire, M.J., Summers, R.S. and Via, S. (2005). Have utilities switched to chloramines? J. Am. Water Works Assoc., 97(10): 87–97.
-
Shaw, C.A. and Bains, S.J. (1998). Did consumption of flour bleached by the agene process contribute to the incidence of neurological disease? Med. Hypotheses, 51: 477–481.
-
Shibata, H., Sakamoto, Y., Oka, M. and Kono, Y. (1999). Natural antioxidant, chlorogenic acid, protects against DNA breakage caused by monochloramine. Biosci. Biotechnol. Biochem., 63: 1295–1297.
-
Shih, K.L. and Lederberg, J. (1976a). Chloramine mutagenesis in Bacillus subtilis. Science, 192(4244): 1141–1143.
-
Shih, K.L. and Lederberg, J. (1976b). Effects of chloramine on Bacillus subtilis deoxyribonucleic acid. J. Bacteriol., 125(3): 934–935.
-
Singer, P.C. and Reckhow, D.A. (2011). Chapter 7: Chemical oxidation. In: Water quality and treatment: A handbook on drinking water. Edzwald, J.K. (ed.). 6th edition. American Water Works Association. McGraw-Hill, New York; Toronto. pp. 7.1–7.52.
-
Skadsen, J. and Cohen, Y.K. (2006). Chapter 8: Operational and treatment practices to prevent nitrification. In: Fundamentals and control of nitrification in chloraminated drinking water distribution systems. Manual of water supply practices – M56. 1st edition. American Water Works Association, Denver, Colorado. pp. 151–187.
-
Smith, C.D. (2006). Chapter 7: Monitoring for nitrification prevention and control. In: Fundamentals and control of nitrification in chloraminated drinking water distribution systems. Manual of water supply practices – M56. 1st edition. American Water Works Association, Denver, Colorado. pp. 129–149.
-
Snead, M.C., Oliveri, V.P., Kruse, C.W. and Kawata, K. (1980). Benefits of maintaining a chlorine residual in water supply systems. U.S. Environmental Protection Agency, EPA 600/2-80-010, Washington, DC.
-
Spon, R. (2008). Do you really have a free chlorine residual? Opflow, 34(6): 24–27.
-
Suzuki, H., Mori, M., Suzuki, M., Sakurai, K., Miura, S. and Ishii, H. (1997). Extensive DNA damage induced by monochloramine in gastric cells. Cancer Lett., 115(2): 243–248.
-
Suzuki, H., Seto, K, Mori, M., Suzuki, M., Miura, S. and Ishii, H. (1998). Monochloramine induced DNA fragmentation in gastric cell line. Am. J. Physiol. Gastrointest. Liver Physiol., 275: G712–716.
-
Switzer, J.A., Rajasekharan, V.V., Boonsalee, S., Kulp, E.A. and Bohannan, E.M.W. (2006). Evidence that monochloramine disinfectant could lead to elevated Pb levels in drinking water. Environ. Sci. Technol., 40(10): 3384–3387.
-
Symons, J.M., Bradley, L.C., and Cleveland, T.C. (eds.) (2000). The drinking water dictionary. American Water Works Association, Denver, Colorado [cited in Health Canada, 2009].
-
Tanen, D.A., Graeme, K.A. and Raschke, R. (1999). Severe lung injury after exposure to chloramine gas from household cleaners. N. Engl. J. Med., 341(11): 848–849.
-
Taylor, R.H., Falkinham, J.O., III., Norton, C.D. and LeChevallier, M.W. (2000). Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol., 66(4): 1702–1705.
-
Thickett, K.M., McCoach, J.S., Gerber, J.M., Sadhra, S. and Burge, P.S. (2002). Occupational asthma caused by chloramines in indoor swimming-pool air. Euro. Respir. J., 19(5): 827–832.
-
Thomas, E.L., Jefferson, M.M., Bennett, J.J. and Learn, D.B. (1987). Mutagenic activity of chloramines. Mutat. Res., 188(1): 35–43.
-
Triantafyllidou, S. and Edwards, M. (2010). Contribution of galvanic corrosion to lead in water after partial lead service line replacements. Water Research Foundation, Denver, Colorado (Water Research Foundation Project No. 4088b).
-
Trueman, B.F. and Gagnon, G.A. (2016). Understanding the role of particulate iron in lead release to drinking water. Environ. Sci. Technol., 50(17): 9053–9060.
-
U.S. EPA (1994a). Drinking water criteria document for chloramine. U.S. Environmental Protection Agency, Washington, DC, ECAO-CIN-D002.
-
U.S. EPA (1994b). Integrated risk information system (IRIS): Monochloramine; CASRN 10599-90-3. National Center for Environmental Assessment. Available at: https://cfpub.epa.gov/ncea/iris2/chemicalLanding. cfm?substance_nmbr=644
-
U.S. EPA (1998) National primary drinking water regulations: Disinfectants and disinfection byproducts; final rule, Stage 1 D/DBPR 63 FR 69360-69476, Dec 16, 1998. 63 Federal Register 241 (16 December 1998), pp. 69390–69412. Available at: http://water.epa.gov/lawsregs/rulesregs/sdwa/mdbp/upload/2001_10_23_mdbp_stage1dbprfactsheet.pdf
-
U.S. EPA (1999). Alternative disinfectants and oxidants guidance manual. EPA 815-R-99-014, Office of Water, U.S. Environmental Protection Agency, Washington, DC.
-
U.S. EPA (2002). Nitrification. Distribution system issue paper. Office of Water, Office of Ground Water and Drinking Water, U.S. Environmental Protection Agency, Washington, DC.
-
U.S. EPA (2009). EPA chloramines Q & A’s. Basic information about chloramines. EPA 815-B-09-001, Office of Water 4607-M. U.S. Environmental Protection Agency, Washington, DC. Available at: http://nepis.epa.gov/Exe/ZyPDF.cgi/P10051XS.PDF?Dockey=P10051XS.PDF
-
U.S. EPA (2012). EPI Suite Version 4.11 Estimation Programs Interface Suite™ for Microsoft® Windows, U.S. Environmental Protection Agency, Washington, DC. Latest version available at: www.epa.gov/tsca-screening-tools/download-epi-suitetm-estimation-program-interface-v411
-
U.S. EPA (2016a). Hydrant sampler procedure. Office of Water (MS-140), U.S. Environmental Protection Agency, Washington, DC. Available at: www.epa.gov/sites/production/files/2016-05/documents/hydrant-sampler-procedure.pdf
-
U.S. EPA (2016b). Batch (plug flow) reactor simulation of drinking water chloramine formation and decay (Version 0.52, Updated 02/16/2016). Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC. Available at: https://usepaord.shinyapps.io/Unified-Combo/
-
U.S. EPA (2017). Chemical dashboard. Chloramine. 10599-90-3. DTXSID8023842. U.S. Environmental Protection Agency, Washington, DC. Available at: https://comptox.epa.gov/dashboard/dsstoxdb/results?utf8=% E2%9C%93&search=chloramine
-
Umeda, M., Fujita, A., Nishiwaki, H. and Takeuchi, K. (1999). Monochloramine and gastric lesions. Effect of lafutidine, a novel histamine H2-receptor antagonist, on monochloramine-induced gastric lesions in rats: Role of capsaicin-sensitive sensory neurons. J. Gastroenterol. Hepatol., 14(9): 859–865.
-
Uzen, H., Kim, D., Beita-Sandi, W., Ersan, M.S., Karanfil, T. and Petry, C. (2016). Seasonal changes of NDMA formation potential and its removal during water treatment. Water Research Foundation, Denver, Colorado.
-
Valentine, R.L. (2007). Opinion as to probable chloramine speciation in San Francisco public utilities drinking water distribution system. Available at: https://sfwater.org/modules/showdocument.aspx?documentid=4107
Valentine, R.L. and Lin, Y. (2009). The role of free chlorine, chloramines, and NOM on the release of lead into drinking water. Water Research Foundation, Denver, CO. -
Vermont Department of Health (2012). Public health review of monochloramine. October 19, 2012. Available at: www.healthvermont.gov/sites/default/files/documents/2016/12/Env_DW_public_health_review_of_monochloramine.pdf
-
Vikesland, P.J. and Valentine, R.L. (2000). Reaction pathways involved in the reduction of monochloramine by ferrous iron. Environ. Sci. Technol., 34(1): 83–90.
-
Vikesland, P.J. and Valentine, R.L. (2002). Iron oxide surface-catalyzed oxidation of ferrous iron by monochloramine: Implications of oxide type and carbonate on reactivity. Environ. Sci. Technol., 36(3): 512–519.
-
Vikesland, P.J., Ozekin, K. and Valentine, R.L. (2001). Monochloramine decay in model and distribution system waters. Wat. Res., 35(7): 1766–1776.
-
Vikesland, P.J., Love, N.G., Chandran, K., Fiss, E.M., Rebodos, R., Zaklikowski, A.E., DiGiano, F.A. and Ferguson, B. (2006). Seasonal chlorination practices and impacts to chloraminating utilities. AWWA Research Foundation and U.S. Environmental Protection Agency, Denver, Colorado.
-
Volk, C.J. and LeChevallier, M.W. (2000). Assessing biodegradable organic matter. J. Am. Water Works Assoc., 92 (5): 64–76.
-
Vu, B., Chen, M., Crawford, R.J. and Ivanova, E.P. (2009). Bacterial extracellular polysaccharides involved in biofilm formation. Molecules, 14(7): 2535–2554.
-
Wahman, D.G. (2015). Chloramine chemistry – web based application. In: Proceedings of the 2015 AWWA Water Quality Technology Conference, Salt Lake City, Utah. American Water Works Association, Denver, Colorado.
-
Wahman, D.G. and Speitel, G.E. (2012). Relative importance of nitrite oxidation by hypochlorous acid under chloramination conditions. Environ. Sci. Technol., 46: 6056–6064.
-
Wahman, D.G. and Pressman, J.G. (2015). Distribution system residuals-is "detectable" still acceptable for chloramines? J. Am. Water Works Assoc., 107(8): 53–63.
-
Wahman, D.G., Maestre, J.R. and Speitel, G.E. (2016). Monochloramine cometabolism by nitrifying biofilm relevant to drinking water. J. Am. Water Works Assoc., 108(7): E362–E373.
-
Wang, H., Edwards, M., Falkinham, J.O. and Pruden, A. (2012). Molecular survey of the occurrence of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa, and amoeba hosts in two chloraminated drinking water distribution systems. Appl. Environ. Microbiol., 78(17): 6285–6294.
-
Wang, H., Proctor, C.R., Edwards, M.A., Pryor, M., Domingo, J.W.S., Ryu, H., Camper, A.K., Olson, A. and Pruden, A. (2014). Microbial community response to chlorine conversion in a chloraminated drinking water distribution system. Environ. Sci. Technol., 48(18): 10624–10633.
-
Ward, A. (2013). Chlorine residual measurement in chloraminated systems. 76th Annual Victorian Water Industry Operations Conference and Exhibition. Bendigo Exhibition Centre, 3–5 September, 2013. Available at: www.wioa.org.au/conference_papers/2013_vic/documents/Aaron_Ward.pdf
-
Weinstein, T., Chagnac, A., Korzets, A., Boaz, M., Ori, Y., Herman, M., Malachi, T. and Gafter, U. (2000). Haemolysis in haemodialysis patients: evidence for impaired defence mechanisms against oxidative stress. Nephrol. Dial. Transplant., 15(6): 883–887.
-
Weintraub, J.M., Berger, M. and Bhatia, R. (2006). Heterogeneous dermatitis complaints after change in drinking water treatment: A case report. Environ. Health, 5: 18–20.
-
Whelton, A.J., Dietrich, A.M., Gallagher, D.L. and Roberson, J.A. (2007). Using customer feedback for improved water quality and infrastructure monitoring. Am. Water Works Assoc., 99(11): 62–76.
-
Westbrook, A. and DiGiano, F.A. (2009). Rate of chloramine decay at pipe surfaces. J. Am. Water Works Assoc., 101(7): 59–70.
-
White, G.C., (1992). The handbook of chlorination and alternative disinfectants. 3rd edition. Van Nostrand Reinhold, New York, New York [cited in Krasner et al., 1985].
-
WHO (2004). Monochloramine in drinking-water. Background document for development of WHO guidelines for drinking-water quality. World Health Organization, Geneva, Switzerland (WHO/SDE/WSH/03.04/67). Available at: www.who.int/water_sanitation_health/water-quality/guidelines/chemicals/chloramine-background.pdf
-
WHO (2011). Guidelines for drinking-water quality, 4th edition. World Health Organization, Geneva, Switzerland. ISBN 978 924 154815 1. Available at: http://apps.who.int/iris/bitstream/10665/44584/1/9789241548151_eng.pdf
-
Wilczak, A. (2006). Chapter 4: Overview of causes and control of nitrification in water distribution systems. In: Fundamentals and control of nitrification in chloraminated drinking water distribution systems. Manual of water supply practices – M56. 1st edition. American Water Works Association, Denver, Colorado. pp. 43–67.
-
Wilczak, A., Assadi-Rad, A., Lai, H.H., Hoover, L.L., Smith, J.F., Berger, R., Rodigari, F., Beland, J.W., Lazzelle, L.J., Kincannon, E.G., Baker, H. and Heaney, C.T. (2003a). Formation of NDMA in chloraminated water coagulated with DADMAC cationic polymer. J. Am. Water Works Assoc., 95(9): 94–106.
-
Wilczak, A., Hoover, L.L. and Lai, H.H. (2003b). Effects of treatment changes on chloramine demand and decay. J. Am. Water Works Assoc., 95(7): 94–106.
-
Wilczak, A., Hokanson, D.R., Trussell, R.R., Boozarpour, M. and DeGraca, A.F. (2010). Water conditioning for LCR compliance and control of metals release in San Francisco's water system. J. Am. Water Works Assoc., 102(3): 52–64.
-
Williams, M.M., Santo Domingo, J.W. and Meckes, M.C. (2005). Population diversity in model potable water biofilms receiving chlorine or chloramine residual. Biofouling, 21(5–6): 279–288.
-
Wojcicka, L., Hofmann, R., Baxter, C., Andrews, R.C., Auvray, I., Lière, J., Miller, T., Chauret, C. and Baribeau, H. (2007). Inactivation of environmental and reference strains of heterotrophic bacteria and Escherichia coli O157:H7 by free chlorine and monochloramine. J. Water Supply Res. T., 56(2): 137–150.
-
Wolfe, R.L., Lieu, N.I., Izaguirre, G. and Means, E.G. (1990). Ammonia-oxidizing bacteria in a chloraminated distribution system: Seasonal occurrence, distribution, and disinfection resistance. Appl. Environ. Microbiol., 56(2): 451–462.
-
Wones, R.G., Deck, C.C., Stadler, B., Roark, S., Hogg, E. and Frohman, L.A. (1993). Effects of drinking water monochloramine on lipid and thyroid metabolism in healthy men. Environ. Health Perspect., 99: 369–374.
-
Woszczynski, M., Bergese, J. and Gagnon, G.A. (2013). Comparison of chlorine and chloramines on lead release from copper pipe rigs. J. Environ. Eng., 139(8): 1099–1107.
-
Xue, Z., Hessler, C.M., Panmanee, W., Hassett, D.J. and Seo, Y. (2013). Pseudomonas aeruginosa inactivation mechanism is affected by capsular extracellular polymeric substances reactivity with chlorine and monochloramine. FEMS Microbiol. Ecol., 83(1): 101–111.
-
Xue, Z., Lee, W.H., Coburn, K.M. and Seo, Y. (2014). Selective reactivity of monochloramine with extracellular matrix components affects the disinfection of biofilm and detached clusters. Environ. Sci. Technol., 48(7): 3832–3839.
-
Yoder, J.S., Straif-Bourgeois, S., Roy, S.L., Moore, T.A., Visvesvara, G.S., Ratard, R.C., Hill, V.R., Wilson, J.D., Linscott, A.J., Crager, R., Kozak, N.A., Sriram, R., Narayanan, J., Mull, B., Kahler, A.M., Schneeberger, C., Da Silva, A.J., Poudel, M., Baumgarten, K.L., Xiao, L. and Beach, M.J. (2012). Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin. Infect. Dis., 55(9): E79–85.
-
Zhang, Y., Griffin, A., Rahman, M., Camper, A., Baribeau, H. and Edwards, M. (2009). Lead contamination of potable water due to nitrification. Environ. Sci. Technol., 43(6): 1890–1895.
-
Zhang, Y., Edwards, M., Pinto, A., Love, N., Camper, A., Rahman, C. and Baribeau, H. (2010). Effect of nitrification on corrosion in the distribution system. Water Research Foundation and U.S. Environmental Protection Agency, Denver, Colorado.
-
Zhou, L., Zhang, Y. and Zeng, G. (2013). Monochloramine decay for two chlorine to ammonia ratios in bulk water. Water Environ. Res., 85(11): 2194–2200.
-
Zhou, E., Payne, S.J.O., Hofmann, R. and Andrews, R.C. (2015). Factors affecting lead release in sodium silicate-treated partial lead service line replacements. J. Environ. Sci. Health A, 50(9): 922–930.
-
Zierler, S., Danley, R.A. and Feingold, L. (1986). Type of disinfectant in drinking water and patterns of mortality in Massachusetts. Environ. Health Perspect., 69: 275–279.
-
Zierler, S., Feingold, L., Danley, R.A. and Craun, G. (1988). Bladder cancer in Massachusetts related to chlorinated and chloraminated drinking water: A case-control study. Arch. Environ. Health, 43(2): 195–200.
Appendix A: List of acronyms
- ANSI
- American National Standards Institute
- APHA
- American Public Health Association
- bw
- body weight
- CDC
- Centers for Disease Control and Prevention (United States)
- CI
- confidence interval
- Cl2:NH3
- chlorine:ammonia weight ratio
- CT
- concentration × time
- DBPs
- disinfection by-products
- DPD
- N,N-diethyl-p-phenylenediamine
- EPA
- Environmental Protection Agency (U.S.)
- GSH
- glutathione
- HAAs
- haloacetic acids
- HOCl
- hypochlorous acid
- HPC
- heterotrophic plate count
- IARC
- International Agency for Research on Cancer
- I-DBPs
- iodinated disinfection by-products
- MNCL
- mononuclear cell leukemia
- MRDL
- maximum residual disinfectant level (United States)
- MRDLG
- maximum residual disinfectant level goal (United States)
- NA
- not available
- NDMA
- N-nitrosodimethylamine
- NOAEL
- no-observed-adverse-effect-level
- NOM
- natural organic matter
- NSF
- NSF International
- NTP
- National Toxicology Program (United States)
- OPPPs
- opportunistic premise plumbing pathogens
- OR
- odds ratio
- T3
- triiodothyronine
- T4
- thyroxine
- THMs
- trihalomethanes
- WHO
- World Health Organization