Combined government discussion paper and science committee report on informed substitution

Download the entire report
(PDF format, 1.20 MB, 67 pages)
Published: 2018-08-28
Health Canada and Environment and Climate Change Canada
Chemicals Mavnagement Plan (CMP) Science Committee (SC)
January 2018
Combined government discussion paper and science committee report on informed substitution
On this page
- Executive summary
- Meeting objectives and scope
- Context and background
- Part I: Opportunities to support information substitution under the CMP
- Part II: Comparative chemical hazard evaluation tools
- Part III: CMP data
- CMP SC concluding comments
- References
- Appendix 1: Key reading material and links to comparative hazard evaluation tools
- Appendix 2: Research initiatives with CMP funding
- Appendix 3: Data sources that may be used to identify potential alternatives during the risk management phase
- Appendix 4: Data requirements for Schedule 6 New Substance Notification
Executive summary
The Chemicals Management Plan (CMP) is a Government of Canada initiative that, launched in 2006, that set clear priorities for assessing and managing chemical substances used in Canada, including in the context of the new and existing substances programs of the Canadian Environmental Protection Act, 1999 (CEPA 1999). The CMP Science Committee (SC) was established by Health Canada (HC) and Environment and Climate Change Canada (ECCC) (herein referred to as the departments) in 2013 to contribute expertise pertaining to scientific considerations in the delivery of the CMP.
At the meeting held in January 2018, the SC was asked to provide input on opportunities for the departments to advance informed substitutionFootnote 1 as part of core Government of Canada chemicals management activities and means by which the departments could play a greater role in supporting industry and other stakeholders in the transition to safer chemicals or non-chemical alternatives. The departments are exploring ways to advance the responsible replacement of chemicals of concern, and from a program design perspective, are looking to consider how applying an informed substitution lens could support chemicals management.
The SC is comprised of 9 core members. Three ad hoc members were invited to provide specific expertise on this topic; Dr. Joel Tickner (Associate Professor, Department of Public Health, University of Massachusetts Lowell), Dr. David Widawsky [Director of the Chemistry, Economics and Sustainable Strategies Division, United States (U.S.) Environmental Protection Agency (EPA)] and Dr. Meredith Williams (Deputy Director, California Department of Toxic Substances Control).
This report is a combination of the objectives paper that was prepared by HC and ECCC in advance of the CMP SC meeting, and SC input submitted, as a report, after the meeting. SC input has been identified in this discussion paper as text boxes at the end of Parts I, II and III.
The CMP SC considered 3 charge questions on considering opportunities to support informed substitution (IS) under the CMP, exploring comparative chemical hazard evaluation tools, and building on CMP work and information to date. The committee briefly considered the main difference between IS and alternatives assessment (AA), however the committee did not distinguish between the two in its deliberations and thus most comments and responses were general to both. In their deliberations, the SC noted that adopting an explicit IS approach in Canada will have new and unique challenges, as has been the case with adoption of IS frameworks by other jurisdictions. The SC believes the comments and suggestions in this report would, if addressed, accelerate formal activities to support the adoption in Canada. The SC also noted that developing an approach to support IS in Canada can be guided by considerable efforts undertaken within other jurisdictions.
Opportunities to support IS under the CMP
The SC was asked, within the context of the CMP, to provide input regarding opportunities to support IS through the departments' current chemicals management activities (for example, priority setting, information gathering, risk assessment and management of new and existing substances and research/monitoring).
The SC concluded that there are a range of opportunities that could be explored to advance IS in Canada under the current chemicals management framework. Some of the numerous suggestions made included:
- consider developing a Potentially Acceptable Substitute List (PASL) based on the data/work done under CMP and new substance notifications to incentivize companies to further develop and submit data on safer substitutes; the SC cautioned that PASL candidates should have a robust data set and a high level of certainty to avoid regrettable substitution
- the preparation and publication of problem formulations for an assessment, where appropriate, to provide opportunities for input of data that would inform AA, and clearly state the context(s) of use and targeted outcomes (for example, avoiding regrettable substitution versus ensuring a measurable benefit in terms of the risk profile)
- collating data from existing screening and assessment activities to support future AA
- the risk management phase could routinely include consideration of alternatives assessment and/or additional information requirements, including consideration of chemical and product functionality
- when practicable and appropriate, include assessment of potential alternatives on a functional basis, characterizing both the chemical function of a substance and the exposure profile (for example, conditions of use)
It was noted that roles and responsibilities for conducting the AA would need to be developed. Additional suggestions outside of the traditional CMP activities included the development of AA guidance and issuing substitution challenges (for example, green chemistry initiatives).
Comparative chemical hazard evaluation tools
The SC was asked to consider comparative chemical hazard screening tools that are available to industry and, from a scientific perspective, identify the strengths and weaknesses of these tools, key endpoints that are necessary for basic hazard characterization of a substitute, and how new approach methodologies (NAM) could enhance these tools/toolboxes.
Some SC members highlighted the need to consider comparative exposure-oriented activities in parallel with the more traditional hazard-related options for AA/IS initiatives, and both considerations were included during its deliberations. The SC noted there are many methods and approaches available to conduct comparative hazard and exposure screening; it also cautioned against reinventing the wheel. Broad consensus was reached that a panel approach of key endpoints tailored to a functional use and exposure profile are preferred over an approach that aggregates information into a single overall score.
For ecological risk assessment, the SC recognized that advancements in the applicability and domain of NAMs, moving beyond aquatic ecological receptors, and increased information on inherent exposure potential will improve comparative screening. While this charge question was focussed on comparative hazard evaluation tools, the SC noted that overall risk (that is, the inclusion of exposure considerations) is also a key consideration for a broader perspective to identify safer alternatives and support IS.
CMP data
The SC was asked to consider the amount of information on substances that has been collected, generated and analyzed throughout the CMP, and provide suggestions on how to use these data to support industry and other stakeholders in evaluating and selecting safer chemicals.
The SC agreed that making data collected, generated and analyzed throughout the CMP available to industry and other jurisdictions would support IS. It was recognized that these data could be used to contribute to existing tools, inform the development of new tools, and evaluate existing models to support IS. The SC observed it is important to understand end-user needs, and stakeholders should be consulted to ensure the structure and format of these data are useable to support IS. Similarly, coordination internationally would facilitate standardization efforts and data sharing across jurisdictions.
The SC agreed that implementing IS will be complex and global activities in this area should be leveraged. The SC strongly encourages the departments to extend their significant international engagement on chemicals management and identify opportunities for data sharing, tool development; and to formalize an informed substitution paradigm. While a more universal AA approach is under development, a case-by-case approach may be necessary to support IS, particularly in order to avoid decisions that could lead to regrettable substitutions.
Meeting objectives and scope
The objective of the January 2018 SC meeting was twofold. First, input was sought on means by which the departments could play a greater role in supporting industry and other stakeholders in the transition to safer chemicals or non-chemical alternatives, considering data collected or generated and subsequently analyzed by the program to date, as well as existing comparative chemical hazard evaluation tools. Second, input was sought on opportunities to advance IS as part of core Government of Canada chemicals management activities (that is, information gathering, priority-setting, risk assessment, risk management, research and monitoring).
The CMP SC was requested to consider the 3 charge questions in the discussion paper (pp. 12, 19, and 33) in the context of the new and existing substances program of CEPA 1999. Key reading material and links to tools that further informed the discussion of the charge questions are provided in Appendix 1.
The departments are exploring ways to advance the responsible replacement of chemicals of concern. From a program design perspective, the departments are looking to consider how applying an IS lens could support chemicals management.
There are many definitions of the substitution principle in the literature, which have been summarized in a recent report from the European Commission (EC, 2017, Appendix 1). For the purposes of the SC deliberations, IS was defined using the definition from Hansson et al., 2011:
"Informed substitution is the considered transition from a chemical of particular concern to safer chemicals or non-chemical alternatives."
IS may be encouraged and facilitated by a number of different policy means, including mandatory restrictions of certain substances in certain applications, the development of tools for risk management and for the assessment of potential alternatives, and the provision of support for research, development, and innovation (EC, 2017). Furthermore, IS involves a broad array of science and policy disciplines, including social sciences and commerce/economics considerations (for example, the consideration of technical and economic feasibility of alternatives). The focus of this meeting is on specific biological and chemical science considerations related to IS.
Context and backgroundFootnote 2
In December 2006, through the introduction of the CMP, the Government of Canada committed to addressing 4,300 substances that were identified as priorities for action following the categorization of the Domestic Substances List (DSL). To date, the departments have assessed approximately 3,300 substances, implemented approximately 80 risk management actions for existing substances (with additional actions currently in development), and are on track to meet their commitment to address legacy chemicals by 2020. Additionally, there is ongoing pre-market assessment of substances pursuant to the " New Substances Notification Regulations (Chemicals and Polymers)" (NSNR) of CEPA 1999, with approximately 17,400 notifications received since 1994, when the NSNR came into force. Approximately 160 risk management measures for new substances have been implemented to manage potential risks to Canadians and the environment. Additional CMP accomplishments have included the implementation of robust monitoring programs and the strengthening of research programs to address key data needs and to identify emerging risk issues and advance methodology development for risk assessment.
As part of recent consultations on a potential post-2020 program of work in chemicals management, stakeholders have suggested a focus on advancing IS. Additionally, several recommendations in the 2017 review of CEPA 1999 by the Standing Committee on Environment and Sustainable Development (ENVI, 2017) pertain to IS:
- The standing committee recommends that CEPA 1999 be amended to add a mandatory duty to assess alternatives as part of all screening assessments of existing substances.
- The standing committee also recommends that CEPA 1999 be amended to add a mandatory substitution test to the regulation of substances under Part 5, to ensure that decisions about how to regulate toxic substances are based in part on information about substitutes, with a goal of replacing toxic substances with safer alternatives.
- The standing committee recommends that CEPA 1999 be amended to ensure that alternative assessments include the following aspects:
- consideration of the opportunities, costs and feasibility of adopting and implementing safer alternatives
- clear recommendations for the elimination, or limited use of, a toxic substance
- efforts to ensure transparency across the supply chain regarding key information and the process to be used in the development of alternatives assessments
- a review of data on a consistent basis to ensure up-to-date and accurate information.
- The standing committee recommends that CEPA 1999 be amended to mandate that the government prepare national safer alternatives action plans for substances for which reports on safer alternatives have been prepared.
Input from the CMP SC will inform the Government of Canada's actions in response to these recommendations.
Many international regulatory and non-regulatory policies and programs have provisions for IS and include alternatives assessmentFootnote 3 frameworks that can inform potential future activity in Canada. The University of Massachusetts Lowell (UMass Lowell, 2017), the Organisation for Economic Co-operation and Development (OECD, 2013) and Jacobs et al. (2016) have reviewed these initiatives. Additionally, a report by the Committee on the Design and Evaluation of Safer Chemical Substitutions, sponsored by the U.S. EPA, presents the SC's consideration of existing frameworks as well as a proposed framework, recommendations for implementation, and future research needs (NRC, 2014).
The departments are interested in leveraging the information generated, collected, and analyzed to date as part of the CMP, including its potential utility in IS. Specifically, this SC topic focused on scientific considerations pertaining to supporting industry and other stakeholders in proactively evaluating and selecting safer chemicals. To support deliberations on informed substitution in the Canadian context, key areas of the CMP are described below. While the CMP brings together various federal chemicals programs under a single strategy (for example, product classes such as foods, health products, pesticides, and consumer products), the focus of this paper is primarily on CEPA 1999-related activities. Additionally, the extent to which IS currently informs specific activities, as applicable, is noted.
New substance notifications
Under CEPA 1999, the departments use a preventative approach to managing the risks new substances may pose to human health or to the Canadian environment. A substance is considered to be new to Canada if it is not listed on the DSL. Prior to new a substance being permitted into commerce in Canada, the substance must undergo an ecological and human health risk assessment. This process begins with a pre-import or pre-manufacture notification of the substance. Any person intending to import or manufacture a new substance in Canada is subject to the NSNR, and is required to submit a package containing all information prescribed in the regulation. These regulations apply to chemicals and polymers (including nanomaterials), biochemicals, biopolymers, and biotechnology (living organisms). The assessment process carried out by the departments, which must be completed within a time limit specified by the NSNR, results in either:
- a determination that the substance is not suspected of being "toxic" or capable of becoming "toxic"Footnote 4
- a suspicion that the substance is "toxic" or capable of becoming "toxic"
- a suspicion that a significant new activity (SNAc) may result in the substance becoming "toxic"
When the risk assessment identifies a risk, CEPA 1999 provides the authority to impose conditions to mitigate the risk. The implementation of risk management must also be undertaken within specified time periods.
The NSNR have ensured that evaluation of substitute chemicals new to Canada takes place prior to manufacture or import. Government officials administering the NSNR are in a unique position to discern trends and potential new chemistries of concern. Companies are not required to indicate whether a notified substance is a replacement for anything currently on the market; however, the departments are considering modifying the notification form to allow companies the opportunity to voluntarily provide this information. Emerging issues identified can be flagged for further consideration through an internal nomination process for the Identification of Risk Assessment Priorities (IRAP).
Priority-setting
In 2006, Canada completed the prioritization of 23,000 chemical substances in use in Canada between January 1, 1984, and December 31, 1986, and identified 4,300 chemicals as priorities, a process known as categorization, for action by 2020. Most of the risk assessment activity under the CMP is currently focused on priorities established as an outcome of this process. Potential substitution was not considered in this prioritization process.
The categorization process was 1 of 7 feeders that help to identify candidates for risk assessment. Other feeders include, for example, industry information and emerging science and monitoring. These other feeders have been formalized within the identification of risk assessment priorities throughout the CMP as new information becomes available. A systematic approach for the IRAP was developed in 2014 to identify substances where there is a potential for risk (that is, evidence of hazard and Canadian exposure) (Environment Canada and Health Canada, 2014). Two cycles of prioritization have taken place to date, identifying 38 substances for risk assessment and 378 substances for information gathering. Knowledge of chemicals recognized as substitutes for substances of concern have informed this exercise. For example, some potential bisphenol A (BPA) alternatives have been flagged as priorities for further information gathering through feeders, including the internal nomination process, international activities, and emerging science. Substances identified as priorities for risk assessment are listed in the published results documents (ECCC and HC, 2015 and 2016).
Information gathering from industry
A variety of information-gathering methods are used to inform prioritization, risk assessment, and risk-management initiatives. Information from industry on quantities manufactured, imported, and used, as well as types of uses, releases, and, in some cases, scientific data, have been collected since the 1990s. Section 71 of CEPA 1999, for example, authorizes mandatory information gathering, and is it regularly used to collect information on the commercial status of specific substances. Since the launch of the CMP in 2006, more than 30 section 71 surveys have been published on over 6,000 substances.
An IS lens has been applied to information gathering on occasion. Specifically, in 2009, a section 71 notice entitled "Notice with respect to alternative substances to phosphorus compounds in household laundry detergents, household dishwashing compounds and household cleaners" was issued, and did not include a list of substances. This was published following the draft regulations to control the phosphorus concentration in certain household cleaning applications. In this way, alternative substances to phosphorous compounds were identified by manufacturers and importers so that the Government could determine hazards associated with the alternative substances based on likely levels of exposure to the environment (Canada, 2009).
Industry is also required to report on pollutant releases (to air, water, and land), disposals, and transfers to recycling through the legislated National Pollutant Release Inventory (NPRI).
Research, monitoring, and surveillance
Scientists in the departments also conduct monitoring and surveillance and research to inform prioritization, risk assessment, and risk management initiatives. HC's monitoring and surveillance initiatives under the CMP include national biomonitoring initiatives, targeted population biomonitoring initiatives, biomonitoring supportive research, and targeted environmental monitoring. Examples of such projects include the Canadian Health Measures Survey (CHMS) biomonitoring program, the Maternal-Infant Research on Environmental Chemicals (MIREC) program, the Northern Contaminants Program (NCP), and the Canadian House Dust Study. Data from some of these initiatives are used to meet international commitments (for example, Stockholm Convention on Persistent Organic Pollutants, Minamata Convention on Mercury).
ECCC's monitoring and surveillance work builds upon a comprehensive series of environmental monitoring programs in place, often for decades, to monitor substances in air, water, and organisms (such as fish and birds). These programs were integrated and augmented under the CMP to provide a fully national program, capable of meeting the Government's monitoring commitments (such as the Great Lakes Water Quality Agreement and the Stockholm Convention on Persistent Organic Pollutants), as well as being responsive to newer emerging chemistries of concern. This includes a network of integrated, continuous environmental monitoring and surveillance. A national monitoring program has also been established under the CMP to measure what chemicals are found in wastewater, where these chemicals are entering wastewater and in what quantities, and to assess the ability of treatments systems to remove these chemicals from wastewater.
The departments' research programs under the CMP focus on enhancing our understanding of the environmental and health effects, as well as exposures related to environmental contaminants. This research involves the generation and dissemination of science-based information necessary to understand the risks chemicals may pose to both human health and the environment. It involves identifying the hazardous properties of a chemical, its fate in the environment, and how people and wildlife may be exposed and affected. While a single compilation of all research is not available, a list of recently funded ECCC and HC research provided in Appendix 2 is illustrative of the types of information that are generated.
Existing substances - risk assessment
As outlined in the CMP Risk Assessment Toolbox, several types of assessment are used to address substances or groups of substances, to ensure a fit-for-purpose approach so that efforts are focused on substances of higher concern. Types of assessment include the development and application of tools that identify the relative risk of substances (for example, the ecological risk characterization of organic substances) and substances of low concern (for example, the threshold of toxicological concern approach) (ECCC, 2016; HC, 2016a).
IS was a consideration during the development of the Substance Groupings Initiative in the second phase of the CMP. For some substances, groupings were identified based, in part, on structural or functional similarities to provide industry with assessments that could inform their substitution decisions (for example, flame retardants and substituted diphenylamines). As the scope of these assessments was typically limited to substances that were identified through categorization,Footnote 5 these assessments were not considered to be inclusive of all potential alternatives for a particular function. There have been instances within the CMP where there were shifts in the market from "toxic" substances to substances of similar hazard. For example, in the case of some flame retardants, the use of polybrominated diphenyl ethers (PBDEs) were replaced in some cases with tris(2-chloroethyl) phosphate (TCEP) and other organophosphate flame retardants. Under the CMP, each of these substances were assessed and managed at different points in time (see Figure 1), whereas in the U.S., an approach was taken to work with industry and nongovernment organizations to identify viable alternatives to penta-BDE in polyurethane foam, and conduct a hazard-based alternatives assessment on 19 substances, which included both TCEP and 2-propanol, 1-chloro-, phosphate (3:1) (TCPP) (U.S. EPA, 2015). The original U.S. alternatives assessment was conducted from 2003 to 2005 and subsequently updated from 2013 to 2015.
Figure 1. CMP risk assessment and management of certain flame retardants.
(decaBDE, Ecological State of the Science Report on Decabromodiphenyl Ether; risk management, reference method; TDCPP, tris(1,3-dichloroisopropyl)phosphate)
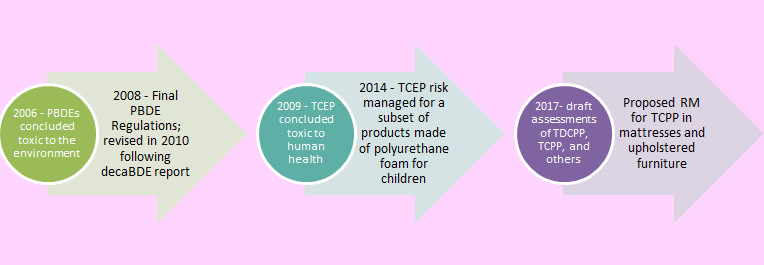
Text Description
Figure 1 illustrates key milestones in the assessment and management of certain flame retardants under the CMP since 2006.
In 2006, PBDE's were concluded toxic to the environment. In 2008, the Final PBDE Regulations were put in place, and then revised in 2010 following the publication of the decaBDE State of the Science Report.
In 2009, the Final risk assessment for TCEP was published and concluded that it was toxic to human health. In 2014, risk management was implemented for TCEP for a subset of products made of polyurethane foam for children.
The draft assessments for TDCPP, TCPP and others were published in 2017, and risk management has been proposed for TCPP in mattresses and upholstered furniture.
Existing substances - risk management
When developing risk management approaches, Government officials consider costs and availability of alternatives when determining the appropriate actions to address risk. Different strategies, tools, and data sources (see Appendix 3) are used to obtain information on existing chemical alternatives to inform the risk management actions that are selected to mitigate the risks; however, a formal, systematic evaluation of the hazard of alternatives is not carried out by the departments in the risk management phase.
If known alternatives exist for the function of the substance of concern and there are products on the market that do not contain the substance, then it may be possible for industry to reformulate the entire product or reduce the concentration of the substance in the product to levels that would not cause harm. However, departmental officials may not have the expertise to know if the alternative is suitable for its specific use (for example, relative hazard). For example, in the case of BPA, under the Canada Consumer Product Safety Act, polycarbonate baby bottles were prohibited because alternatives were available on the market that did not release BPA. An AA, including establishment of the any available hazard profiles of alternatives, was not undertaken.
If no alternatives exist for the substance of concern and the substance is critical for the function of the product, an option is to require industry to lower the concentration of the substance to the minimum level required for its function and further reduce the risks by using other means such as labelling and/or an educational campaign [for example, a campaign on how to create a well-ventilated area when using products containing butanone oxime (methyl ethyl ketoxime; MEKO)] (HC, 2014). These decisions would also depend on the severity of the risks that would be incurred in that the risk management actions would need to be proportional to the risks.
In some cases, consideration of alternatives has been incorporated into pollution prevention plans under CEPA 1999. For example, the nonylphenol and its ethoxylates pollution prevention planning notice required that persons subject to the notice consider choosing alternatives and identified alternatives that would not be suitable substitutes (Canada, 2004a). Other pollution prevention planning notices have suggested the consideration of alternatives, including siloxane D4 in industrial effluents, BPA in industrial effluent, and inorganic chloramines and chlorinated wastewater effluents (Canada, 2012a and 2004b).
Part I: Opportunities to support informed substitution under the CMP
Informed substitution (IS) has not been the driver for work under the CMP to date; however, as shown in the Context and background sections, efforts have been made in some instances to support IS (for example, through the formation of substance groupings). Best practices adopted in other jurisdictions to advance IS [as outlined by UMass Lowell (2017)] and lessons learned from the CMP to date informed deliberations on the following charge question:
I. Considering Opportunities for Supporting IS in the CMP
Charge question 1: Within the context of the CMP, can the CMP SC provide input regarding opportunities to support IS through the departments' current chemicals management activities (that is, priority setting, information gathering, risk assessment, and management of new and existing substances and research/monitoring)?
Currently, bisphenols provide an example of where IS considerations are at play in the decision-making around prioritization, information gathering, and potential risk assessment activities, as summarized in Figure 2 and described further below.
Figure 2. CMP activities related to BPA and certain bisphenols
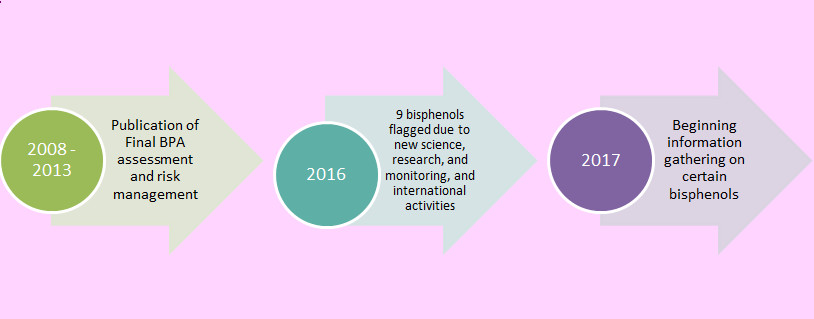
Text Description
Figure 2 describes the CMP activities related to BPA and certain bisphenols.
In 2008, the Final assessment concluded that BPA is "toxic" to human health and the environment. A number of risk management measures were implemented between 2009 and 2013.
In 2016, 9 bisphenols were flagged during the IRAP process due to new science, research and monitoring and international activities.
In 2017, information gathering on certain bisphenols is planned to begin, which will inform the need for future assessment.
BPA was assessed under the first phase of the CMP, where the final screening assessment concluded that BPA is "toxic" to human health and the risk management measures were implemented between 2009 and 2013 to reduce exposure to BPA to protect infants and the environment. The departments recognized that there were key knowledge gaps and have invested in research and monitoring for BPA.
The BPA assessment did not, however, consider potential substitutes, although structurally similar substances such as bisphenol S (BPS) and bisphenol F (BPF) were on the DSL and the Non-domestic Substances List (NDSL), respectively. The IRAP process has subsequently identified a number of bisphenols (9 substances, including BPS, BPF, and their isomers or derivatives) through a variety of feeders, including internal nomination, new science, and international activities. All of these substances have the bisphenol backbone and/or have the methane group substituted (see Table 1), have potentially similar human health toxicological and exposure profiles as BPA (the route of exposure is ingestion), and have potential for similar applications as BPA for which there was an initial concern for human health in 2008.
The commercial status of 8 of these substances in Canada, however, has not been quantified. BPS was included in the 2012 Inventory Update (Canada, 2012b) and there were no reports of import or manufacturing of this substance above the reporting threshold of 100 kg. Information gathering is planned to inform future actions on these 9 bisphenols. This includes obtaining information on BPA use patterns (including the identification and use of alternatives), obtaining information on its commercial status, and identifying who should be engaged for more targeted information-gathering requests, as needed.
There are other substances that are potential BPA substitutes, including substances that are not structurally similar to BPA, substances that may have different toxicological or exposure profiles as BPA, or substances that are not currently used in similar applications as BPA.
In terms of scientific data on BPA substitutes, the departments' activities would be informed by the work and NAM data generated from the Canadian Institutes of Health Research team grant on Endocrine Disrupting Chemicals: Towards Responsible Replacements.
| Chemical identifier | Chemical structure | DSL status |
|---|---|---|
| BPA Bisphenol A CAS RN 80-05-7 |
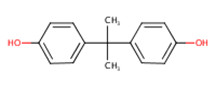 |
DSL - CMP Final assessment with Toxic Conclusion |
| BPS Bisphenol S CAS RN 80-09-1 |
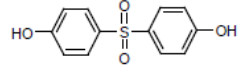 |
DSL |
| 2,4-BPS CAS RN 5397-34-2 |
 |
NDSL |
| TGSH CAS RN 41481-66-7 |
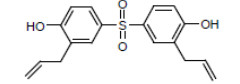 |
NDSL |
| BPS-MPE CAS RN 63134-33-8 |
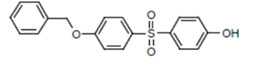 |
NDSL |
| BPF Bisphenol F CAS RN 620-92-8 |
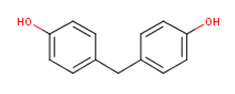 |
NDSL |
| BPF (mixed isomers) CAS RN 1333-16-0 |
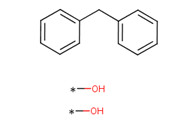 |
Not on DSL or NDSL |
| BPF Bisphenol F CAS RN 87139-40-0 |
 |
Not on DSL or NDSL |
| BADGE Bisphenol A diglycidyl ether CAS RN 1675-54-3 |
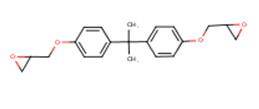 |
DSL |
| BP-AP Bisphenol AP CAS RN 1571-75-1 |
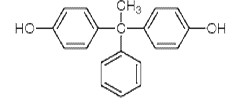 |
Not on DSL, or NDSL |
CMP SC response to charge question 1
Within the context of the CMP, can the CMP SC provide input regarding opportunities to support IS through the departments' current chemicals management activities (that is, priority setting, information gathering, risk assessment and management of new and existing substances and research/monitoring)?
The SC addressed this question by mapping out a risk assessment and management framework that integrated opportunities for AA and activities to support IS to reduce risk. This framework was developed in light of, but not constrained by, the current CMP process (that is, this use of IS is within a risk-based statute).
Based on the current CMP framework, the SC considered substances from the 2 lenses of existing and new substances. For both work-flows, the following were general considerations:
- The SC looked for opportunities to encourage and facilitate stakeholders, such as industry, to conduct AA as per charge question 2.
- The SC considered the assembly of a "Potentially Acceptable Substitute List" (PASL) as has been done by the U.S. EPA.Footnote 6 The U.S. EPA has developed criteria for inclusion to the list. As discussed below, developing a PASL could be informed by mining data already collected for substance assessment, an issue also related to charge question 3.
- The SC suggested that opportunities should be identified for masking and generalizing information that otherwise would be unavailable to stakeholders due to confidential business information considerations.
- For existing and new substances that will see use in Canada, opportunities should be sought to demonstrate and promote the co-benefits of IS and pollution prevention.
- When practicable and appropriate, include an assessment of potential alternatives on a functional basis, characterizing both the chemical function of a substance (for example, solvent, surfactant) and the exposure profile (for example, conditions of use). Anticipate regrettable substitutions based on information gathered on new chemical function-use-hazard information.
- The departments might prioritize important substances and/or functions for consideration of AA or activities to support IS.
- Opportunities were also sought to promote cross-jurisdictional collaboration and to use approaches consistent with those used by other jurisdictions such as the U.S. EPA and the European Union, including work with organizations such as the OECD.
- Although beyond the purview of the SC and beyond the role for government, it was acknowledged that performance, commercial, and economic considerations are of critical importance to the concept and application of IS.
1.1. Existing substances
Government officials advised that the CMP is on target to have assessed the approximately 4,300 substances identified in the categorization phase of the CMP by 2020. Currently, about 900 are yet to be assessed. The assessment process of these 900 substances has already involved the collection of data. In addition, two-thirds of those reviews are underway and no further requests under section 71 of CEPA 1999 will be made.
The SC considered 2 situations for existing substances. The first pertains to the substances already considered under categorization and that have undergone assessment. The second is for substances that could undergo assessment in the future.
For the substances that have already undergone categorization and assessment, or that are undergoing assessment, the SC discussed whether the data collected for these substances could be parsed and used to populate a PASL. In considering a PASL, the SC recognized that a specific group of endpoints and criteria for endpoints could be valuable information for determining inclusion in a PASL. In addition, functional and structural information should be used to develop a PASL.
The SC was concerned about the level of uncertainty with the data that would be used to develop a PASL. Specifically, the SC was keenly aware that "absence of evidence" is not "evidence of absence" for the purpose of populating a PASL. Nevertheless, maximizing the (public) availability and encouraging the use of the available information in AA was seen as a possible positive contribution to IS, at least in the context of avoiding regrettable substitution (see also charge question 3).
For substances that could undergo assessment in the future, the SC considered opportunities to conduct AA within the current CMP workflow, as follows:
- As a best practice, a problem formulation document should be developed and disseminated, clearly describing the substances undergoing risk assessment, which would allow for stakeholders to have input to, or conduct an AA.Footnote 7 Before gathering the specific parameters used to estimate risk, a problem formulation document would provide interested parties information on the conditions of use that will be assessed for the chemical substance(s), along with the types of toxicological endpoints that will be assessed. This information would convey the chemical landscape within which one might seek alternatives for which initial scoping work could occur, rather than waiting to start AA until a final, refined risk assessment is complete.
- Under CEPA 1999, the first step in risk assessment is the collection of information on each substance or substance group. A range of information is considered in an assessment, including: chemical properties, releases to and concentrations in the environment, environmental fate and behaviour, hazards, and nature of exposure. As a best practice, the SC noted an opportunity at this stage of the workflow to consider and facilitate the collection of information that would inform AA and activities to support IS. A problem formulation document clearly describing the substances under assessment could be disseminated that would allow for stakeholders to have input into AA and IS considerations, or to begin conducting an AA where the stakeholders deem this type of analysis to be useful.
- For future assessments, data can be collected under section 71 whereby the collected data could be used to consider AA and IS. To facilitate AA, the substances could be grouped according to functionality. As discussed under charge question 3, efforts should be made to share these data with stakeholders and other jurisdictions.
- Currently under CEPA 1999, if a substance is determined to be toxic or capable of becoming toxic, then risk management measures are considered to prevent or control the identified risks. Follow-up activities may be undertaken for those substances recognized for their potential effects of concern. At this stage in the workflow, the SC sees an opportunity where appropriate (based on results of the risk assessment), for the government to conduct a thorough AA that might support subsequent informed, safer substitutions and enhance the efficacy of final risk management measures. Roles and responsibilities for conducting the AA would need to be developed. This AA would use data collected for substances at the previous stages and additional data gathered under section 71.
- As a general note, the SC suggested including an additional focus in the CMP workflow on potential and differential exposure scenarios for the individual members of the substance grouping and potential alternatives.
Finally, for existing substances, the SC noted that there may be a need to use the data from completed CMP assessments, groupings, and classes to inform IS activities, such as a PASL or AA.
For substances that will undergo AA, the need was noted for ongoing tracking and surveillance to ensure a reduced risk with regard to the substituted substance and to evaluate the impacts of identified alternatives. This activity can feed into future program assessments.
1.2. New substances
Currently, the New Substances Notification (NSN) and assessment program results in either prohibition; restriction, depending on quantities involved; restrictions for specified uses, with the requirement to further notify for significant new activities (uses and/or quantities); and unrestricted entry of substances. The SC noted that one early potential enhancement to existing NSN documentation could include information leading to the recognition and designation of the substance as a candidate for activities that would support IS or inclusion in a PASL. Information provided by the submitter to earn such recognition could be voluntary, but in order to earn recognition, data elements would need to be included, such as characterizing the functional use, identifying the health/environmental benefits compared to incumbent substances for those uses, and identifying the potential incumbents or substances to be replaced.
For approved new substances with identified potential health or environmental risks, the goal of an enhanced IS focus would be met by making an additional data request to the submitter of the NSN to provide information relevant to possible IS activities. It is recognized that it may not always be possible for the submitter to respond with specific information and that additional follow-up activity must be considered on a case-by-case basis. Information requirements can include hazard- and exposure-related components. The SC additionally observed that current control measures activities under the NSN program, particularly the SNAc mechanism, might be informed, or even enhanced, by adding an IS dimension to the program.
1.3. General characteristics of managing a program compatible with an IS paradigm
The SC considered other general components that could form part of an IS-assessment and management program and that shares the following concepts/activities for future consideration:
- Prepare clear guidance documents to inform and increase awareness on how to conduct AA and consider IS, to identify potential AA/IS actions and opportunities (focusing particularly on problem formulation, context, and stopping points), and to create and communicate the appropriate tools.
- Pay particular attention to problem formulation with regard to specifying the "decision criteria" of a particular AA/IS activity (for example, if the outcome is meant to avoid regrettable substitutions versus ensure a measurable benefit in terms of the hazard/risk profile). Different "decision criteria" can imply different metrics for comparison and different tolerances for uncertainty.
- Consider comparative exposure-oriented activities in parallel with the more traditional hazard-related options for AA. The SC noted that most work on AA focuses on hazard-related properties and, while not minimizing the hazard aspect, confirmed that examining exposure information (including the intrinsic properties that affect exposure potential) will also provide opportunities for successful IS by identifying potential exposure trade-offs relevant to inherent properties of a substance or its use.
- Conduct case studies to identify the benefits of AA versus the disadvantages of not performing one, and to identify and recognize success stories. Once a formal program that supports IS is underway and knowledge is gained, retrospective case studies would be useful to glean "lessons learned," including the use of traditional versus NAMs data information. Further discussion of retrospective analysis can be found under charge question 3.
- Identify specific multi-sector (for example, industry, academic, nongovernmental organization) stakeholders for which to issue substitution challenges using knowledge gained to date and, potentially, via a multi-stakeholder process. A specific example would be to engage with green chemistry initiatives.
- Link regulatory priorities to research and development priorities, both within government and with external groups, in order to inform future activities to support IS.
- Consider establishing programs that incentivize companies or organizations to develop, curate, and submit data on safer substitutes (recognition, safer chemical lists, labelling programs, etc.).Footnote 8 Generating such data can be expensive, particularly where there are multiple potential candidate substitute substances. Where companies can take advantage of market opportunities through recognition or labelling, they have incentives to generate and submit chemical profile data and embrace the substitution challenges mentioned above.
- Consider establishing an integrating function/unit to support a portfolio approach to identifying alternatives. Earlier, this report mentioned chemical data collection on substitutes through section 71 as well as voluntary data submission through incentive programs, signalling data on substitutes through the NSN program (including the recognition and building out of chemical profiles for promising new chemicals), and surveillance of the green chemistry research landscape for new innovations and approaches to safer chemicals/chemistry. It would not necessarily involve high costs, but there could be substantial benefits from an integrating function for these disparate information streams.
Part II: Comparative chemical hazard evaluation tools
A number of tools have been developed over the years to assist industry and other stakeholders and, to a lesser extent, government, in evaluating the hazards of alternatives and identifying safer substitutes in a consistent and transparent way. The OECD Substitution and Alternatives Assessment Toolbox (SAAT) glossary defines a "tool" as a means (computer-based or not, automatic or manual) of converting data into an outcome useful to alternatives assessment. They vary widely in complexity and the level of expertise needed to apply them. Some tools simply draw from existing lists of substances of concern to identify known chemicals of concern, while others are designed to compare chemical alternatives over a range of endpoints and involve a review of the toxicological literature. Tools have also been developed to assist with other aspects of alternatives assessment, such as cost and availability, exposure/risk, technical feasibility, and product performance; however, these are outside the scope of these deliberations. Comparative chemical hazard evaluation tools were the focus of the discussion below to address the following charge questions:
II. Exploring Comparative Chemical Hazard Evaluation Tools
Charge question 2a: Based on the comparative chemical hazard screening tools that are available to industry, from a scientific perspective, what are the strengths and weaknesses of these tools?
For consideration:
- Are the available screening tools effectively identifying critical toxicities?
- Are they effectively identifying key data gaps?
- Are there endpoints that are not adequately screened?
Charge question 2b: Are there key endpoints that are necessary for basic hazard characterization of a substitute?
Charge question 2c: How could New Approach Methodologies (NAM)Footnote 9 enhance these tools/toolboxes (see CMP SC Report, 2016)? How can emerging data contribute to filling gaps, decreasing uncertainty about the hazard, and increasing the reliability and relevance of existing screening tools?
There are many similarities between a hazard assessment of potential alternatives and a hazard assessment in a traditional risk assessment. They are similar in that the types of endpoints and the data sources considered are largely the same. In the ecological assessment, however, the effects on different species have not to-date been routinely differentiated in the comparative assessments (for example, invertebrates versus vertebrates versus plants). The National Research Council (NRC) notes that it is not necessary to be precise in comparisons across species for the purposes of informing substitution, as the goal is to choose a chemical that has substantially less potential hazard, and the variability in the measured endpoints across various species tests precludes precise comparisons (NRC, 2014).
Comparative chemical hazard evaluation tools generally consider a wide range of hazard endpoints, integrate the information and present it in a way that identifies safer alternatives. Some tools include a decision framework to guide the user in the identification of the safer alternative (for example, the GreenScreen benchmarks), whereas other tools array the data and leave the decision-making about alternatives to the user. The online OECD Substitution and Alternatives Assessment Tools Selector of the SAAT was designed to assist users in identifying the tool that is best suited for their goal based on filterable criteria. It includes 14 tools, many of which are described below, and presents descriptions of data input, data output, and limitations. These tools have been compared in a number of recent studies, including UMass Lowell (2017) and Panko et al. (2017). Descriptions of these tools are summarized in Table 2. A more comprehensive summary of many of the tools is available in Annex 3, Table 2, of the UMass Lowell report (2017) and in the OECD SAAT Tool Selector. Weblinks to the comparative chemical hazard evaluation tools from the OECD SAAT Tool Selector are also provided in Appendix 1.
| Tool | Developer | Description |
|---|---|---|
| GreenScreen for Safer ChemicalsTable 2 Footnote A, Table 2 Footnote B, Table 2 Footnote C | U.S.-based nongovernment organization Clean Production Action | A manual method where the user collects data and employs expert judgement to classify and assign a level of confidence to the hazard level of 18 hazard endpoints for human and environmental health, according to GreenScreen criteria. Each chemical is then classified in one of four benchmark categories defining the safety of the chemical. |
| Quick Chemical Assessment Tool (QCAT)Table 2 Footnote B, Table 2 Footnote C | Washington State Department of Ecology | Evaluates 9 hazard endpoints and rates substances based on an aggregated score. The first step is an automated and based on QCAT's authoritative database and data sources; the second step is a manual method where users must collect and evaluate hazard endpoint data and interpret results. |
| Pollution Prevention Options Assessment System (P2OASys) Table 2 Footnote B, Table 2 Footnote C | Massachusetts Toxics Use Reduction Institute | Users input both quantitative and qualitative data on hazard and then compare current processes to alternatives based on 11 endpoints. The user can set data certainty and weighting factors for each endpoint. The tool automatically calculates an aggregated score for each alternative, and the user can also manually compare alternatives based on individual endpoint categories. |
| U.S. EPA DfE Alternatives Assessment Criteria for Hazard EvaluationTable 2 Footnote B | Design for the Environment (DfE) Program at the U.S. EPA | Uses existing primary data and predictive modelling to determine hazards for each alternative, and provides a results table where each endpoint is ranked (high, moderate, or low) with colour codes. Criteria are generally based on the Globally Harmonized System (GHS) criteria (UN, 2015). Results are not aggregated. |
| Column ModelTable 2 Footnote B, Table 2 Footnote C | German Institute for Occupational Safety and Health | A manual tool that allows for comparison of chemicals or materials based on 6 hazard endpoints. Endpoints are compared individually and collectively, and the user makes the final evaluation. Users must consult external data sources (primarily safety data sheets) and compare against the tool's internal standards database. |
| Chemical Hazard Data Commons-PilotTable 2 Footnote B | U.S.-based nongovernment organization Healthy Building Network | Hazard endpoints are consistent with those considered in the GreenScreen tool. For each endpoint, the criteria define "High," "Moderate," and "Low" concern, and are color-coded according to the ranking. Results are not aggregated. Information comes from authoritative hazard lists and GreenScreen assessments. |
| GreenSuiteTable 2 Footnote A | Chemical Compliance Systems | A set of modules that includes a proprietary database of approximately 28,000 chemicals and their associated hazard and physical-chemical properties. Considers both lists and toxicological data for more than 44 endpoints (not all are hazard endpoints; also includes life cycle endpoints). Uses a web-based software to design a screening model that is tied to a database of scientific information to perform the hazard analysis based on scientific data. Includes normalization process, where raw data for each endpoint is assigned a value from 0 to 100, so that the score for each chemical in a product can be added, allowing a comparison across products. |
| SciVera Lens Chemical Safety AssessmentTable 2 Footnote A, Table 2 Footnote C | SciVera, LLC | A manual tool for toxicology professionals to assess hazard of chemical ingredients in a product with risk assessment option. Considers both lists and data for 22 endpoints; with carcinogenic, mutagenic, or toxic for reproduction (CMR) designated as the core endpoints. Endpoints with the highest hazard drive the overall chemical score. |
While the outputs of the tools listed in Table 2 are informed by the scientific literature, GreenScreen and DfE also use authoritative lists to classify hazards. Classifications from authoritative lists from agencies such as the U.S. National Toxicology Program, the International Agency for Research on Cancer (IARC), and hazard classifications by countries using the GHS are used to classify carcinogenicity in the 2 tools. The European Union Categories for substances classified as CMR is used to classify mutagenicity/genotoxicity in the DfE framework (NRC, 2014). The use of authoritative lists is recommended in the NRC framework, as it maximizes the use of existing evaluations of scientific information and helps ensure that alternatives assessments are efficient and based on consistent science (NRC, 2014).
Panko et al. (2017) found that when the results of 5 different tools were compared for 7 substances, the outcomes varied when the same substance was run through each tool. This was due to the differences in endpoints considered, endpoint weighting, and differences that result from the use of expert judgement.
There have been several other initiatives in recent years to review and compare these tools, and alternatives assessment tools in general (for example, OECD, 2013; NRC, 2014) and, in some cases, identify gaps and opportunities for further work. In 2013, the OECD published its meta-review of the current landscape of alternatives assessment practice. Included in this meta-review is a discussion of the tools available to support alternatives assessment (including comparative chemical hazard evaluation tools) (OECD, 2013). In 2014, the NRC published the "Framework to Guide the Selection of Chemical Alternatives" developed by a multi-stakeholder committee for governments. A series of recommendations were made after comparing existing alternatives assessment frameworks or tools, including GreenScreen and DfE (NRC, 2014). While the scope of this review was much broader than comparative hazard tools, recommendations on the ecological and hazard evaluation are relevant to this discussion and included in the sections below.
There is an opportunity to leverage these aforementioned resources, and build on them to advance informed substitution. Here, we focus on 3 key scientific areas or factors likely to present challenges, but also opportunities to advance the science in the use of comparative chemical hazard screening tools. These areas include the consideration of health and ecological hazard endpoints, the handling of absence of empirical data/low-quality data, and the use of NAM.
Hazard endpoints
Although several of the tools are generally similar, there are some notable differences in the specific endpoints evaluated and criteria applied for determining the degree of hazard. Table 3 highlights the endpoints considered in 5 comparative chemical hazard screening tools. Many of these are GHS health hazards, with the exception of endocrine activity, which is not included in GHS. Hazard endpoints and criteria used in comparative chemical hazard evaluation tools are generally available online in guidance documents (CPA, 2017a; Stone, 2016; U.S. EPA, 2011a).
| Human health effects | Aquatic toxicity | Environmental fate | Total number of hazard endpoints | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute toxicity | Carcinogenicity | Mutagenicity / genotoxicity | Reproductive | Developmental | Neurotoxicity | Repeated dose | Skin sensitization | Respiratory sensitization | Eye irritation | Skin/dermal irritation | Endocrine activity | Systemic toxicity and organ effects | Chronic toxicity | Acute | Chronic | Persistence | Bioaccumulation | ||
| GreenScreen | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 16 | ||
| QCAT | X | X | X | X | X | X | X | X | X | 9 | |||||||||
| P2OASys | X | X | X | X | X | 5 | |||||||||||||
| DfETable 3 Footnote 1 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 15 | ||
| GreenSuiteTable 3 Footnote 2 | X | X | X | X | X | X | X | X | X | X | X | X | 12 | ||||||
| Total | 5 | 4 | 4 | 4 | 3 | 3 | 1 | 3 | 2 | 3 | 3 | 3 | 2 | 1 | 5 | 3 | 5 | 4 | |
Many of these criteria follow GHS, which can be used when traditional data is available. An overview of the GHS classification scheme in hazard classification and how it is used in the GreenScreen and DfE tools are provided in Appendix D of the NRC framework (NRC, 2014). The NRC framework recommends using GHS-tied criteria with a few refinements, including using health hazard assessment guidance to classify substances for endpoints where GHS criteria require expert judgement. Certain endpoints such as reproductive toxicity require expert judgement to apply GHS criteria; therefore, the NRC recommends that guidance should be followed to ensure consistency and transparency (NRC, 2014).
A limitation of the comparative ecotoxicity assessment of many frameworks highlighted by the NRC is the sole focus on aquatic toxicity. The NRC recommends that in their framework, the analyst should focus on gathering ecotoxicological data for the ecosystem of concern (that is, aquatic, sedimentary, or terrestrial). Aquatic toxicity is generally the focus, as these test data are most likely to be available for comparison and robust models have been developed. However, the DfE framework includes placeholders in the Additional Endpoints section of the criteria should the data and criteria become available (for example, impacts on wildlife growth; survival; development and reproductive toxicity) (U.S. EPA, 2011b).
Endocrine activity is not included as a health hazard in the GHS, although it is included as an effect in several comparative chemical hazard evaluation tools and alternatives assessment frameworks. The evaluation criteria vary. The DfE framework, for example, evaluates endocrine activity of chemicals in the narrative of the report but does not use that information to characterize hazard in the comparative table, citing that the data for evaluation of endocrine activity for most chemicals is limited and robust tools are not available for modelling endocrine activity, making it difficult to compare results (U.S. EPA, 2011a and 2011b). The framework recognizes that as the science advances, the criteria could be updated to include a level of hazard for an endocrine activity and/or endocrine-related effects (U.S. EPA 2011b). Indeed, significant advances have been made in this area since the publication of the DfE framework in 2011, including work under the U.S. EPA Endocrine Disruption Screening Program. GreenScreen evaluates chemicals for endocrine activity and assigns hazard values based on adverse endocrine-related health effects (NRC, 2014). The classification of endocrine activity in GreenScreen (that is, moderate hazard classification) is modified if there is a high (or very high) plausibility that the endocrine activity is related to carcinogenicity, reproductive toxicity, developmental toxicity, or systemic toxicity. For example, if the initial endocrine activity classification is "moderate," it will be modified to "high" if there is a high likelihood that carcinogenicity is due to the endocrine mode of action (CPA, 2017b).
Some frameworks identify priority endpoints that will have a greater weight in the overall assessment. For example, GreenScreen gives the highest weighting for the endpoints of PBT (persistent, bioaccumulative, and toxic), vPvB (very persistent and very bioaccumulating), and CMRs (carcinogenic, mutagenic/genotoxic or toxic for reproduction) and leads to a Benchmark 1 designation (avoid). In SciVera Lens, CMR drives the overall assessment. GreenSuite, however, allows the user to choose the default weighting or adjust them in advance of the analysis (Panko et al., 2017). DfE simply presents a comprehensive picture of the hazards, and refers the user to other tools such as GreenScreen to weigh the hazard endpoints and evaluate trade-offs (U.S. EPA, 2011a). NRC recommends an output that would not include an integrated score across human health endpoints, but simply a tabular array showing the health points considered/not considered, hazard level, certainty, and gaps. The NRC did not recommend integrating information across health endpoint domains for 3 primary reasons: (1) there is no established consensus on which effects are of greater concern, (2) doing so unnecessarily carries forward the impact of benchmarking cut-offs, and (3) it is important to carry forward the certainty and the level of the hazard into the integration of other data in the decision-making step of an alternatives assessment.
Data quality and gaps
Comparative chemical hazard screening tools require the practitioner to conduct a review of toxicological literature to identify potential human health and environmental effects. As is the case in a traditional hazard assessment, data availability and quality can vary, and it is important to take this into account in the hazard characterization and uncertainty analysis. If measured data are lacking or are of low quality, the practitioner may need to read across from empirical data on analogs or estimated data from predictive modelling to characterize potential hazards.
Data quality (however, not specifically data uncertainty) is addressed in several frameworks and tools. For example, the DfE framework refers to the U.S. EPA HPV Challenge Program and the OECD HPV Programme data adequacy guidelines (U.S. EPA, 2011a). They also use "data hierarchies" that indicate the types of data that are preferred in the hazard assessment process (NRC, 2014).
The GreenScreen method addresses data quality by having the practitioner assign a high or low level of confidence for each hazard level assigned to an endpoint, which is clearly communicated in the hazard summary table. If the hazard level is determined using equivocal results, measured data for a weak analog, and/or modelled data, it is assigned "low" confidence. If studies are truly inadequate for characterizing some aspect of a chemical, GreenScreen identifies it as a data gap in the hazard summary table (CPA, 2017b). Certain endpoints in GreenScreen are informed by authoritative lists, and some of these lists are given a "high" confidence level (for example, IARC classifications for carcinogenicity), while other screening lists have lower confidence [for example, DSL categorization data, due to reliance on Quantitative Structure-Activity Relationships (QSAR) predictions] (NRC, 2014).
Capturing and communicating uncertainty has been a priority in CMP risk assessments and was the focus of a previous CMP SC meeting (CMP SC, 2014). Similarly, the NRC report (2014) highlights the importance of indicating the certainty of the data in an alternatives assessment framework. They recommend that the tabulated health and ecological hazard endpoints indicate the certainty of the data that is, high, medium, or low) and suggest a colour code to identify the level of certainty for each endpoint.
DfE, GreenScreen, GreenSuite, and SciVera Lens all consider a data gap as having a negative effect on the overall score (Panko et al., 2017). GreenScreen, for example, defines a minimum data set and describes the permissible data gaps for each hazard effect. The failure to meet minimum data requirements is negative and benchmark specific; for example, no data gaps are permitted in the best benchmark that identifies a substance as "Preferred - Safer Chemical" (NRC, 2014).
The NRC strongly supports the use of in vitro screening and in silico data to fill data gaps when the necessary information is not available in the traditional epidemiological and animal testing data (NRC, 2014 and 2017). This is also recognized by the European Chemicals Agency (ECHA), as demonstrated by investments in the OECD QSAR Toolbox, to help fill data gaps in toxicity/ecotoxicity to facilitate substitution away from chemicals of concern (ECHA, 2017). Challenges and opportunities in this area are discussed in the next section.
When empirical data are not available or deemed inadequate, the DfE framework requires that a hazard concern level be assigned based on structure-activity relationship (SAR) considerations and professional judgement so that all effects are covered and the hazard profile is complete. GreenScreen requires that in the absence of data, expert judgement and estimated data from an analog and SAR analysis is used. If information is still deemed insufficient for classification, the endpoint is assigned a "data gap" or "no data" designation.
Given the paucity of data for soil, sediment, and terrestrial toxicity compared to aquatic toxicity, there is an opportunity for high throughput in vitro studies coupled with adverse outcome pathways (AOPs) as more are developed, appropriately predictive for species other than humans, to be used as a substitute to experimental data or provide a basis for extrapolating aquatic toxicity data to other species (NRC, 2014). The coupling of in silico, macro-molecular interactions and cellular responses with relevant AOPs (as available) is also a future opportunity for predicting adverse outcomes.
New approach methodologies
Jacobs et al. (2016) evaluated several alternatives assessment frameworks and noted that very few frameworks were identified that offered methods for addressing incomplete hazard data for the hazard assessment element. Ticker et al. (2015) point out that comprehensive and useable chemical hazard data are required to advance informed substitution, including the use of nontraditional tools and data streams, such as in silico modelling and in vitro high-throughput screening to fill data gaps.
When empirical data are not available for some hazard endpoints within the DfE framework, QSARs are used to inform hazard classification (Jacobs et al., 2016). GreenScreen recommends using modelled data to fill in for missing measured data (CPA, 2017b). Among the suite of models recommended are those that use QSAR methods that apply statistical tools correlating biological activity of chemicals with descriptors representative of molecular structure and properties, such as EPISUITE, ECOSAR, ONCOLOGIC, and other models such as the OECD QSAR Toolbox (CPA, 2017b).
Many computational tools used in toxicity assessments rely either on chemical or biological data. Specifically, QSARs attempt to predict toxicity from a chemical structure only, while biologically based bioinformatics do not inherently take advantage of the chemical features based on structure (NRC, 2014). As advances continue to be made in NAM, the increased integration of both QSAR-based and biologically based information and modelling will enhance the accuracy of predictions, to provide insights previously uncovered by either informatics discipline alone (NRC, 2014, 2015, and 2017).
Another critical reason supporting the move towards integrative chemical-biological modelling is to take advantage of the ever-growing number of novel high-throughput data streams, which effectively capture chemical-biological interactions. High-throughput in vitro data could be used as primary evidence for an endpoint of concern (for example, in vitro mutagenicity is included in GHS as a primary data type) to fill data gaps for an endpoint of concern (for example, several ToxCast in vitro assays may identify the potential for reproductive and developmental toxicants based on endocrine activity), or to screen out possible unintended consequences of data-poor chemicals (for example, looking at mode-of-action information, evidence of nonselective chemical activity at low concentrations, or effects associated with particularly susceptible subpopulations) (NRC, 2014). While the use of novel in vitro assays and statistical methods is promising, there are continued efforts to better define and demonstrate their predictive power and classification accuracy. As such, at the time that the NRC (2014) published the report, it was concluded that novel methodologies and approaches with limited or uncertain predictivity should generally only be used in alternatives assessments to fill data gaps or screen for indicators of the potential for toxicity, with the exception of certain mutagenicity and endocrine/reproductive toxicity assays that are more developed (NCR, 2014).
Since the publication of the NRC 2014 report on alternatives assessment, there have been important advancements that demonstrate further application for NAMs in various risk assessment activities, including supporting read across, prioritization, and screening, as well as activities for improving mechanistic understanding. Furthermore, significant progress is being made to adapt new methodologies and models to chemical assessments as new data and case studies are generated (NRC, 2017); these developments would also be relevant to alternatives assessment approaches. Notably, advancements in the last decade that demonstrate the applications for high-throughput technologies have reduced the limitations that were characterized in the NRC 2014 report (NRC, 2017). Toxicological in vitro models are probing more biologically relevant interactions in more complex biological organizations, such as 3D-model tissues. The NRC 2017 report provides several examples of the progress in the use of high-throughput technology and integrated methods for green chemistry.
Further, large-scale efforts to generate emerging data streams (such as the Tox21 and ToxCast programs) are key examples of new data sources that can inform gap analysis when traditional data from human and experimental animal studies are lacking. A recent analysis by HC scientists aggregated various international chemical inventory lists to identify a chemical space of common interest between regulatory jurisdictions, while simultaneously conducting a data availability analysis that incorporated traditional risk assessment data on hazard and exposure, as well as an analysis of available NAM data. Over 50 data sources were included in the analysis; the steps followed for data collection, filtering, and analysis are shown in Figure 3 (data quality was not evaluated in the context of this exercise). The results of the analysis (based on the data available to August 2016) indicate that there are over 900 substances with both traditional and NAM data that could be used to investigate the application and confidence of NAMs to support informed substitution. In this context, new methods in in vitro toxicology could have significant utility by providing information on which molecular characteristics are associated with greater or less toxicity and by helping to identify chemicals that do not affect known biological pathways of toxicity; an application relevant for the screening of new chemistries is being considered as potential replacements for those with greater toxicity.
Figure 3. Workflow for the identification of data sources available for substances in commerce internationally.

Text Description
Figure 3 provides the workflow for the identification of data sources available for substances in commerce internationally. The first step includes aggregating substance inventories from regulatory bodies (Australia, Canada, Europe, Japan, Korea and the U.S.). The second step includes the incorporation of data, such as DSSTox structures, exposure information, phys-chem property data, chemical category information, risk assessments and toxicity data. The third step involves filtering substances into groups based on data and data availability.
The NRC points out that there is a need for scientists and regulatory agencies to determine which high-throughput toxicology assays, end points, and model systems are most informative in assessing hazard types used in chemical alternatives assessment (NRC, 2014). This is consistent with the overall vision and strategy driving the global paradigm shift in testing and assessment to address current challenges in chemical risk assessments. The NRC also notes that there is a need to further invest into more expertise and resources to help advance these high-throughput and predictive tools and demonstrate pragmatic approaches for integrating emerging results, as they also show promise for new chemical screening (NRC, 2017).
As more data become available and advances in assay technology reduce experimental variability, integrative approaches are expected to play a greater role in alternatives assessment (NRC, 2014). The NRC notes that ensuring that high-throughput data can be searched using appropriate data mining tools will be equally important to enable practitioners and stakeholders to access the novel data for comparative analyses and tools development (NRC, 2014 and 2017).
CMP SC response to charge question 2
Charge question 2a: b ased on the comparative chemical hazard screening tools that are available to industry, from a scientific perspective, what are the strengths and weaknesses of these tools? Further questions for consideration are provided below.
Are the available screening tools effectively identifying critical toxicities?
The SC noted that the introductory part of this charge question could have been worded as"...comparative chemical hazard and exposure screening tools...," which would encourage a broader perspective for addressing IS. The SC observed that many methods and approaches are already available and cautioned against "reinventing the wheel" for AA (see jurisdictional scans by, for example, Jacobs et al., 2016; and UMass Lowell, 2017). These methods and approaches include those for comparative chemical hazard and exposure assessment and, increasingly, comparative risk assessments (that is, a combination of intrinsic hazard and exposure potential). These tools are relatively effective for identifying relatively higher-risk situations, and should be deployed in a fit-for-purpose manner (depending on specific scenarios of what is being assessed) for the purpose and stage of the workflow.
Broad consensus was reached that indexing approaches should be avoided (that is, approaches that aggregate information into a single overall score). Such methods hide from the assessor potentially important specific data on hazards or exposure trade-offs. Instead, the SC preferred methods that take a panel approach [such as the U.S. EPA's Design for the Environment (DfE) method] for pools of key endpoints tailored to a functional use and exposure profile.
Are they effectively identifying key data gaps?
Data gaps and poor or variable data quality are critical challenges associated with AA/IS activities, particularly for newer, less studied chemicals, as is the case for risk assessment in general. In addition to concerns about data gaps, the SC stressed that data quality can vary tremendously and needs to be properly evaluated (that is, questions concerning uncertainty, measurement error and bias, and potential replicability should be asked). The SC recognized the importance of data gaps and poor quality data, particularly when assessing substances for CMR-related and PBT-related endpoints. However, the SC cautioned against raising the bar higher that is, demanding a more complete database) for the assessment of potential alternatives than exists for current risk assessments, because this would dis-incentivize substitution. Identification of, and language regarding, data gaps should include a discussion of associated uncertainties, which may serve as points of caution; however, this will not be easily managed in practice.
The SC identified a number of specific areas where data gaps can be particularly problematic and opportunities to fill those gaps. These comprise the following (in no specific order) for ecological risk assessment:
- Efforts should continue to move beyond traditional approaches that rely on aquatic receptors, given that a substance's fate can ultimately be in non-aquatic ecosystems or expose non-aquatic ecological receptors.
- Chemical partitioning, fate, and transport properties could be used to help pinpoint ecological receptors of concern and thus target subsequent activities (for example, potential trade-offs).
- Standard lab animal models and test guidelines exist for major taxa (for example, invertebrates, fish, frogs, birds). Many researchers in Canada (especially ECCC) are developing alternative (that is, non-animal) models, such as tests on embryos and cell-based tools, in an effort to move beyond testing of protected life stages for these taxa, and also to increase testing efficiencies (that is, test more chemicals using less resources).
- Work continues to develop tools for species native to Canada, as these are most appropriate in characterizing ecosystem risk. For example, ECCC scientists are developing and testing both exposure and hazard tools for relevant native species (such as herring gulls, cormorants, and polar bears) to replace less-relevant but otherwise standard tests.
- Data from existing monitoring and surveillance programs should be used to understand ecosystems and species of concern (for example, herring gulls, polar bears, top trophic-level salmonids). The data from these programs can be used to identify "new" substances of concern and to track temporal trends of existing substances, particularly after applying risk mitigation actions. This activity currently applies to current substances of concern but can readily be extended to substitutes (the future will deem this "regrettable substitution" if substitutes are subsequently found to be accumulating and/or impacting an ecosystem's health).
- Continue to expand current efforts to develop species sensitivity distributions for use in screening assessments.
- Consider using "Eco TTC"Footnote 10 as a new approach methodology; this could be considered to be an "Eco NAM" (further discussed below).
- The aforementioned strategies will result in big data, with the consequential opportunity to develop harmonized, centralized, and intuitive data tools and access portals that amalgamate exposure and hazard data for the purposes of ecological risk assessment to support IS.
The following points were raised that are pertinent to considerations of human and ecosystem risks:
- It is widely recognized that AA of components of mixtures are much more difficult than for single substances. Nevertheless, mechanisms and models need to be developed to deal with this challenge.
- Transformation and/or metabolic breakdown products that could be PBT should be identified.
Few comments or suggestions were made regarding inorganic substances with respect to activities to support IS. This is a potentially large gap in the knowledge base.
Are there endpoints that are not adequately screened?
Although consensus was not reached on the balance between seeking information on hazard versus risk, the SC recognized the need for more information on comparative actual and inherent (that is, persistent- and bioaccumulative-based) exposure potential, particularly as it pertains to products and articles. The following data would be needed to facilitate this analysis:
- physical/chemical property data
- emission/release information
- fate and transport information (currently addressed through persistence and bioavailability, but could be augmented; for example, identifying environmental compartment[s] of concern)
- for human health concerns, opportunities and rates of substance exposure via contact pathways should be identified (such as dermal and hand-to-mouth transfer). The SC noted that emission/release and contact rates may be use-/context-specific as related to performance, efficacy, and so forth
- bioavailability approximated using in vitro and QSAR methods in the absence of in vivo data
- toxicokinetics estimated using in vitro and in silico methods in absence of in vivo data
The additional points were also made with respect to information-gathering tools:
- Build on the current use of PBT characteristics (that is, as a "bright line" for early indication of whether or not a substance is potentially of concern and/or a priority), facilitate the adaptation and repurposing of fate and exposure models (already in use by ECCC and HC) to allow comparative exposure and risk-based estimates as an input to AA activities.
- Identify chemical spaces and domains for which estimation tools are appropriate.
The following points were raised specifically in terms of addressing hazard:
- Recognize the wide variation in maximum tolerated dose rates that can be incorporated in in vivo animal studies.
- Ensure that a clear distinction remains between evidence for causality versus estimates of potency.
- Equally, keep clear the distinction between "absence of evidence" and "evidence of absence" of any specific effect of concern.
- Acknowledge that many traditional potency indicators [no observed adverse effect level (NOAEL); lowest observed adverse effect level (LOAEL)] are ill-suited for comparative evaluations; more statistically based measures (such as benchmark doses or probabilistic estimates) have more utility for such evaluations
Charge question 2b: a re there key endpoints that are necessary for basic hazard characterization of a substitute?
Because any hazard endpoint may be of significant concern from an IS perspective, a "case-by-case" approach to AA/IS is required. It is nevertheless reasonable to suggest that a reasonable "minimum set of information needs" for specific endpoints and properties will routinely comprise the following:
- PBT considerations with the associated physical/chemical properties
- quantities/release rates (and the associated ability to identify "pseudo-persistence" in the environment)
- in general, indicators of CMR hazards
- ecotoxicity data for substances with potential broad environmental distribution
Charge question 2c: how could New Approach Methodologies (NAMs) enhance these tools/toolboxes (see CMP SC report, 2016)? How can emerging data contribute to filling gaps, decreasing uncertainty about the hazard and increasing the reliability and relevance of existing screening tools?
The SC recognizes the potential for NAMs to significantly advance the assessment of chemical substances and potentially facilitate the introduction of a meaningful AA/IS framework. The November 2016 report of the SC regarding NAMs is instructive here (CMP SC, 2016).
In the context of current workflow, the SC offered the following comments:
- The use of NAMs can be promoted by data assembled throughout the CMP assessment process, as discussed under charge question 1.
- NAMs have the potential to be used to estimate the relevant and relative toxicity values for identified potential alternatives.
- NAMs are being developed which may allow for rapid exposure modeling in the context of AA/IS.
- Currently available tools/NAMs, such as ToxCast/Tox21, TTC, httk (high-throughput toxicokinetics; Ring et al., 2017), QSAR/QSUR,Footnote 11 and ToxPiFootnote 12, could be employed by stakeholders such as industry and government (during the various stages of AA/IS analysis discussed under charge question 1) to characterize and refine chemical profiles, as well as fill in data gaps.
Considerations for encouraging/enabling future enhanced use of NAMs:
- Support research and development; for example, to expand NAMs applicability and domains that span a broader range of chemical space (for example, more volatile and/or less water soluble, and/or ionizable substances) as well as biological space such that a variety of toxicological pathways and systems are covered.
- Some NAMs are co-addressing bioactivity and exposure considerations (for example, the bioactivity-to-exposure ratio (BER) model discussed in CMP SC 2016). Encouragement should continue for developing this and other NAMs with the ability to, for example, more explicitly investigate doses (internal versus exposure), toxicokinetic considerations, and more sophisticated hazard and exposure investigation.
- Develop NAMs for assessing the integration of fate, transport, and transformation characteristics; and for alternative (non-animal) testing for vertebrates.
- Document case studies (as mentioned previously), which will be powerful in documenting the potential of coupling of NAMs into AA.
Part III: CMP Data
The data collected, generated, and analyzed under the CMP to assess, and manage as appropriate, thousands of substances is considerable and may have utility in advancing informed substitution. A 2013 OECD meta-review of the current landscape of alternatives assessment practice identified access to robust technical data sources, including toxicity data, as a key need for alternatives assessment audiences (OECD, 2013). Other jurisdictions have made efforts to share data on substances; the meta-review cites the U.S. EPA ChemView portal and the OECD's eChemPortal as 2 data sources that aggregate data on chemicals. The eChemPortal provides direct access to data from different jurisdictions, including the non-confidential information from registration dossiers submitted to ECHA. The OECD review indicates that there are still significant gaps in data availability that impede the ability to understand and compare hazards associated with chemicals. The ECHA has recently proposed to further develop the integration, interpretation, and public access to data as part of its proposed strategy to promote the substitution of hazardous chemicals (ECHA, 2017).
Over the past decade, the CMP has collected, generated, and analyzed a wide range of data that could be helpful in supporting informed substitution, including data on the commercial status of substances in Canada (for example, quantities, uses), empirical scientific data (for example, physical-chemical properties, toxicological information, monitoring data), and predicted values (for example., physical-chemical properties, predicted estimates of exposure). Some of these data are confidential. There are provisions within CEPA 1999 whereby those submitting information can require that the information be treated as confidential in order to protect Canadian commercial interests, and the departments have an obligation to protect this information and store it in secure, internal systems. The departments are currently proposing an approach to achieve an appropriate balance between transparency and industry's right to protect confidential information.
To inform deliberations on the following charge question, CMP data,Footnote 13 including data capture formats and confidentiality considerations as applicable, are elaborated upon below.
III. Building on CMP Work and Information to Date
Charge question 3: Given the amount of information on substances that has been collected, generated and analyzed throughout the CMP (for example, hazard data, use/quantity information, monitoring), what suggestions does the CMP SC have on how to use the data to support industry and other stakeholders in evaluating and selecting safer chemicals?
New substances notifications
The NSNR set out the information requirements for substances new to Canada. This information is tailored to the use and quantity of the chemical or polymer being manufactured or imported, and are listed in the "Schedules" of the NSNR. A number of factors must be considered when identifying the information to be submitted. These factors include:
- whether the new substance meets the definition of a chemical or a polymer
- whether the new substance falls within any of the prescribed special categories (for example, research and development, contained site-limited intermediate, or contained export only)
- whether the new substance is listed on the Non-domestic Substances List (NDSL)Footnote 14
- the annual quantities of the new substance that will be manufactured in or imported into Canada
- if the new substance is a polymer, whether it meets the definition of a Reduced Regulatory Requirement Polymer
- if the new substance is a polymer, whether it is manufactured solely from monomers and reactants that are listed on the DSL or the NDSL
- whether the new substance will be released to the aquatic environment in significant quantities and/or if the public may be significantly exposed to the substance in a product
All notifications require submission of information regarding the identity of the substance, its anticipated uses and quantity, and, depending on the factors above, there may also be the requirement for data from tests for certain physical-chemical properties, biodegradation, ecotoxicity (for example, fish, daphnia, or algae toxicity), and toxicity studies (for example, acute, repeated-dose, skin irritation, skin sensitization, and genotoxicity). Information on uses is generally limited, with descriptions such as "used in cosmetics" or "pigment" provided. All notifications require a summary of all other information and test data in the notifiers' possession, or to which they ought to have access, that are relevant to identifying hazards to the environment and human health and the degree of environmental and public exposure to the substance. Specific data requirements for the highest level of notification for a chemical not listed on the NDSL are listed in Appendix 4.
Information may be provided through an electronic submission form, by email, or by using a hard-copy form. As noted in the preceding section, information in the submission can be claimed confidential and is stored in secure internal databases. The departments currently use 2 ChemFinder databases, 1 for chemicals and 1 for polymers. The software has been customized with interfaces to facilitate viewing of the data and conduct searches on data values, company, and chemical structure. A screen shot showing the blank data fields and an example substance structure (not a new substance) is provided in Figure 4. The database contains information on approximately 15,000 substances notified under the NSNR since 1994. Approximately 500 new substances notification submissions have been received for the highest quantities.
Figure 4. Illustrative view of ChemFinder interface for New Substances Notification information.

Text Description
Figure 4 provides a screen shot of blank data fields, including an example substance structure of the ChemFinder interface used to capture information provided in a New Substances Notification. It includes fields for substance identifiers, physical chemical properties, mammalian toxicity data and ecotoxicity data.
Detailed information on the mammalian toxicity studies provided in the notification package is entered into the International Uniform Chemical Information Database (IUCLID), an application developed by ECHA and the OECD that is widely used by European government and industry. Under the REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) European Union regulation, information submitted to ECHA must be in IUCLID format. It is widely considered to be the gold standard for capturing and sharing chemicals data throughout the international community.
The NSNR specify that test data are to be generated using OECD Test Guidelines that are current at the time the data is generated, and for certain tests the laboratory practices must comply with Good Laboratory Practices. However, the New Substances Program does accept data generated using other test protocols, computational models, and read-across, where the use of this information is supported by sufficient scientific justification (Environment Canada, 2005).
In addition to the information provided by the notifier, officials search for relevant information in the published literature, as well as internal databases, to consider as part of the assessment.
Risk assessments on new substances are not available publicly; however, risk assessment summaries are published, on a biannual basis, for new chemicals and polymers for which the risk assessment has been completed, a restriction has been imposed, and the restriction was published in the Canada Gazette.
Priority-setting
The categorization process involved the collection of data on the 23,000 substances on the DSL, including modelled and experimental data on physical-chemical properties, persistence, bioaccumulation, and ecological and human health toxicity. Each substance also has a "categorization decision," indicating if it met the criteria for persistence, bioaccumulation, and ecological and human health toxicity (that is, yes or no). The final decisions are available on the Government of Canada website using a search engine that is searchable by chemical name or Chemical Abstracts Service Registry Number (CAS RN) and an exportable Microsoft Excel spreadsheet file. These results do not show the supporting data that were used to reach the categorization decision and only provide a snapshot in time (2006); results do not reflect the outcome of any subsequent prioritization or assessment activities. Categorization results are also available in the OECD eChemPortal, including supporting data for the ecological decisions (that is, type of data, and the values and references that formed the basis for the decisions on inherent toxicity, persistence, and bioaccumulation), as illustrated in Figure 5. Supporting data are also kept internally as Excel spreadsheets.
Figure 5. Screenshot of ecological data supporting a categorization decision for CAS RN 78-63-7 in eChemPortal
Text Description
Figure 5 provides a screen shot of the ecological data used for Categorization decisions for CAS RN 78-63-7 in eChemPortal. It depicts model predictions on the persistence, bioaccumulation and inherent toxicity to aquatic organisms (experimental data was not available).
Substances that have been prioritized for assessment following the more recent IRAP process are identified in the online IRAP documents; however, detailed data that were available for all substances considered is only available in an internal Microsoft Access database.
Information gathering from industry
Data collected from industry may include quantities of substances in commerce, information on uses, and, to a limited extent, toxicological data. Some of it is claimed as confidential business information; therefore, all data are stored in Excel spreadsheets on secure internal systems. A summary of non-confidential information received for many notices is available online and available for export in Excel spreadsheet format. A portion of one of these reports is shown in Figure 6.
Figure 6. Screenshot of information available in the summary of non-confidential information received from the second phase of the DSL Inventory Update (Canada, 2012b).

Text Description
Figure 6 provides a screen shot of information available in the summary of non-confidential information received from the second phase of the Domestic Substances List Inventory Update, including the Substance Use Function Codes and their descriptions that were reported for five substances.
Pollutant releases (to air, water, and land), disposals, and transfers to recycling reported to the NPRI are stored in an internal database. Most of the data reported are non-confidential, and that data are publicly accessible and searchable by facility, substance, location, industry type, or release/disposal/transfer category. There are also a number of predefined queries available, including total on-site releases by substance name or industrial sector and highest on-site substance releases by quantity or province. Data are available for download in Microsoft Access, comma-separated values (CSV), and Excel formats. Release data are available from 1994 to 2016 (2016 NPRI data are preliminary). For the year 2015, 7,284 facilities reported to the NPRI on 343 listed substances. Figure 7 illustrates a subset of the search results for sulphur dioxide releases for 2016.
Figure 7. Screenshot of results of a search of the NPRI for sulphur dioxide releases in 2016.

Text Description
Figure 7 provides a screen shot of the first nine results of a search of the NPRI search engine for sulphur dioxide releases in 2016. The results return data on the facility name, city, province and on-site releases to air, water, land, and total on-site releases.
The Government of Canada has an Open Data Portal that makes structured data freely accessible in a machine-readable format so that it can be built on without restrictions. CMP data have not been added widely to the portal to date; however, all public NPRI datasets are available in the Open Data Portal. These include a downloadable database with the full dataset from 1993, geographic distribution of NPRI-reporting facilities and releases as map layers in KMZ file format (Keyhole Markup Language) for use in Google Earth™ and other virtual globe software, and most commonly used data in tabulated format (.xlsx and.csv).
Research, monitoring, and surveillance
The ways in which the departments' research and monitoring data are made available varies across projects. Data are provided internally to evaluators via internal reports for use in risk assessments. Results may also be published in quality, peer-reviewed journals. For example, the results of the Canadian House Dust Study are published in journals such as Environmental Science and Technology (Rasmussen et al., 2011). Similarly, the cohort profile of the MIREC study is published in the journal Paediatric and Perinatal Epidemiology (Arbuckle et al., 2013), and the results or the MIREC study on phthalate and BPA exposure among pregnant women in Canada are published in Environment International (Arbuckle et al., 2014). Data from NCP-funded projects are available online through the Polar Data Catalogue, a searchable database of metadata and data generated by Arctic and Antarctic researchers from a range of disciplines, from natural sciences and policy to health and social sciences. Annual synopsis research reports from the NCP are also published by Indigenous and Northern Affairs Canada.
Some environmental monitoring and surveillance data are available in the Open Data Portal, including the National Air Pollution Surveillance (NAPS) Network, Great Lakes Basin (GLB) Monitoring and Surveillance, and Ambient Gasses and Particles data.
Human biomonitoring data for each environmental chemical measured in the CHMS are available in the Open Data Portal. Between 2007 and 2015, the biomonitoring component measured 176 chemicals in individual blood and urine samples collected during the first 4 cycles. Data are presented for each chemical by biological matrix, cycle, sex, and age group and are available in downloadable.csv files. A screen shot is shown in Figure 8.
Figure 8. Screenshot of CHMS data in the Open Data Portal.
Text Description
Figure 8 provides a screen shot of the first sixteen results of the CHMS data in excel accessed through the Open Data Portal. Data are presented in a table with information including chemical group, chemical, matrix, units, group, age, cycle, percentage of results that fall below the limit of detection, arithmetic mean, geometric mean, the 10th, 25th, 50th, 75th, 90th and 95th percentiles.
Results of Statistics Canada-led projects, such as CHMS, are also housed in Research Data Centres, which are situated in secure university settings across Canada. Due to confidentiality, the centres are accessible only to researchers with approved projects who have been sworn in under the Statistics Act as "deemed employees."
Online "supplementary information" offered by many journals is another way research data are made available, as it allows authors to post data related to their manuscripts using a variety of formats. This is a useful alternative for projects where Research Ethics Board, legal and privacy restrictions on projects prevent it from being able to be shared in the Open Data Portal (for example, when the Canadian House Dust Study was designed, participants were assured that the information would be published in scientific journals in aggregated form only). For example, the Canadian House Dust Study published population-based distributions using this option, including the recent Indoor Air article (Rasmussen et al., 2017), which provided complete aggregated datasets on rare earth elements in indoor environments.
Existing substances risk assessment
During the risk assessment process, scientific data and information on commercial activity are collected from a variety of sources and mechanisms. For example, the HC search strategy involves a stepwise approach to checking search engines, databases, specific data sources, and primary literature to collect human health hazard data, and documents found are recorded in a checklist. Similarly, at ECCC, the search strategy involves over 60 recommended search engines, domestic and international databases and websites, and the data collected are typically compiled in internal spreadsheets. To evaluate the reliability and applicability of data, ECCC has developed Robust Study Summary (RSS) templates in Microsoft Excel for the evaluation of data on ecotoxicity (for aquatic, sediment, and soil-dwelling organisms), degradation, aquatic bioconcentration, field bioaccumulation studies, and physical-chemical properties. Completed RSSs are maintained internally and are often made available through supporting documents. Key data are presented in the published screening assessment reports or in supporting documents.
Certain information gathered from the published literature to support the human health risk assessment is entered into IUCLID. The software is used by HC to capture and store critical toxicity data used in risk assessments. The level of data entered into IUCLID is related to the type of assessment approach being followed (that is, consistent with the Risk Assessment Toolbox where the level of effort is commensurate with the level of anticipated risk; a more complex risk assessment approach will similarly involve more data capture than a less complex approach). Generally, critical studies used in the human health risk characterization, or those that support the weight-of-evidence for the critical study, are entered into IUCLID. The data entered into IUCLID can be exported in a tabular format, as illustrated in Figure 9.
Figure 9. Screenshot of an IUCLID report generated for studies on repeated-dose toxicity after oral administration.

Text Description
Figure 9 provides a screen shot of the IUCLID report generated for studies on repeated dose toxicity after oral administration. A table shows the methods, results and reference for two different studies.
IUCLID also includes functionality for physical/chemical properties and, to a limited extent, exposure/use data. However, these functionalities are not used at this time, due to challenges with harmonizing Canadian use information (for example, use codes reported in section 71 surveys), as well as input/output of modelled exposure scenarios and physical/chemical properties with the OECD harmonized templates used in IUCLID. ECCC has used the IUCLID software to enter data from studies that were used in some groupings assessed as part of the second phase of the CMP (for example, boron, phthalates, organic flame retardants, methylenediphenyl diisocyanates, and diamines).
Toxicological data stored in IUCLID can be used by the OECD QSAR Toolbox. Through IUCLID, data can be structured in a way that it can be used to address data gaps for related substances in a systematic and transparent way.
The IUCLID software does not currently have the chemical structure searching functionality like the ChemFinder program used for new substances, although this has been identified as a high priority for future development. Health Canada scientists have built a prototype for a chemical structure searching tool using robust, free, open-source software. The prototype uses chemical structure information to search the IUCLID database and other internal systems to return substance endpoints (for example, toxicological data), substance decisions (for example, risk assessment conclusions), and related documentation (for example, risk assessment files). A screenshot of the search results of this prototype is shown in Figure 10.
Figure 10. Screenshot of search results from an HC prototype to search IUCLID and other databases by chemical structure.
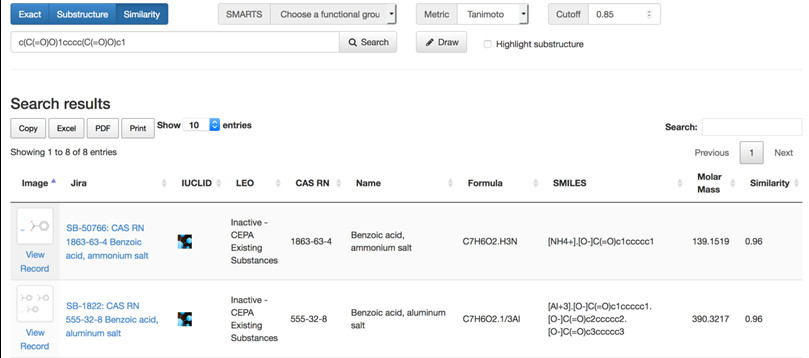
Text Description
Figure 10 provides a screen shot of the search results from a HC prototype to search IUCLID and other databases by chemical structure. A SMILES string was used to search and two of eight results are shown, including the image of the returned structures, links to internal databases, CAS number, substance name, formula, SMILES, molar mass and percent similarity.
Science approaches
As noted in the CMP Context section, a number of approaches have been developed to screen large numbers of substances. Science Approach Documents (SciADs) provide descriptions of these approaches, and the results are available in.html and.pdf format online. Four SciADs have been published to date:
- Biomonitoring-based approach 1 for beryllium, vanadium, trichlorooxo, and vanadium oxide (3 substances) (HC, 2016b)
- Biomonitoring-based approach 2 for barium-containing substances, molybdenum-containing substances, silver-containing substances, thallium-containing substances, and inorganic tin-containing substances (17 substances) (HC, 2016c)
- Threshold of toxicological concern (TTC)-based approach for certain substances (237 candidate substances, 89 substances of low human health concern) (HC, 2016a)
- Ecological risk classification (ERC) of organic substances (640 candidate substances, 542 substances of low ecological concern) (ECCC, 2016)
The TTC approach, for example, is based on the principle of establishing human exposure threshold values for chemicals, below which there is a low probability of risk. In the TTC approach, a threshold value is assigned to a chemical based on structural features, then comparing this threshold value to an estimate of human exposure. Substances that have exposure below the assigned TTC value may be considered to be of low concern for human health. Results of this approach are published online and available in.html or.pdf format. It includes tables of data by CAS RN on information such as the assigned TTC values and associated data/predictions for substances with exposures lower than their associated TTC values (see Figure 11), total volumes in commerce and highest total environmental intake estimates, and direct-exposure scenarios and estimates (HC, 2016a).
Figure 11. Screen shot of TTC values and associated data from the SciAD, Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances.
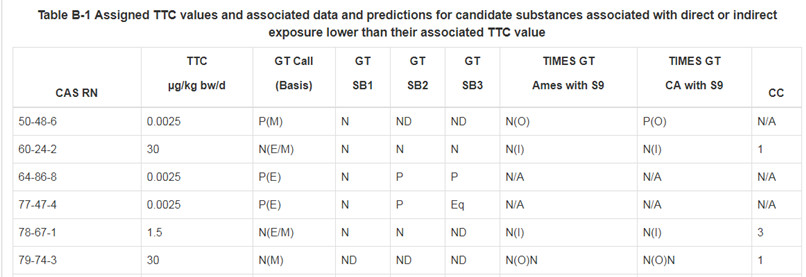
Text Description
Figure 11 provides a screen shot of TTC values and associated data for six substances from the Science Approach Document, TTC-based Approach for certain substances.
The ERC approach uses empirical and modelled data to classify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing ecological harm. Hazard and exposure profiles were developed for individual substances based on multiple metrics. The ERC describes the hazard or potency of a substance using key parameters, including mode of action, chemical reactivity, internal toxicity thresholds, bioavailability, and chemical activity and bioactivity. The possible exposure of organisms in the aquatic and terrestrial environments is characterized based on factors that include potential emission rates, overall persistence, and long-range transport potential in air. Results of the approach were published online in the SciADs (ECCC, 2016). The data used to create substance-specific hazard and exposure profiles and assign risk classifications in the ERC are available in Excel spreadsheets upon request. Figure 12 shows part of the hazard data contained in the spreadsheet. Of note, certain substances classified by ERC as having low potential for risk on the basis of current use patterns have been flagged as being possible substitutes in the future for structurally similar substances having a higher potential for risk. Examples of these are found in screening assessments of these substances of low concern that are available online (ECCC and HC, 2017a and 2017b).
Figure 12. Screenshot of hazard data used in the ERC approach (which is available upon request).
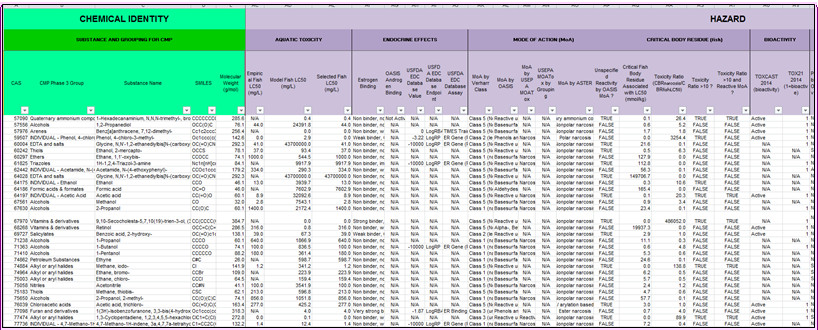
Text Description
Figure 12 provides a screen shot of the excel table of hazard data used in the ERC approach that is available upon request. Details included relate to chemical identity, aquatic toxicity, endocrine effect, mode of action, critical body residue and bioactivity.
Existing substances risk management
The information collected to inform risk management is compiled in the internal risk management search document and published in the risk management scope and approach documents.
The risk management search document (Figure 13) is an internal document created to provide information on all of the current risk management actions or strategies that are in place for a particular substance, both in Canada and internationally. This document informs risk management of substances of concern. During the preparation of this document, potential chemical alternatives to the substances(s) being evaluated may be identified from public information sources (see Appendix 3).
Figure 13. Screenshot of one tab of the risk management search document template
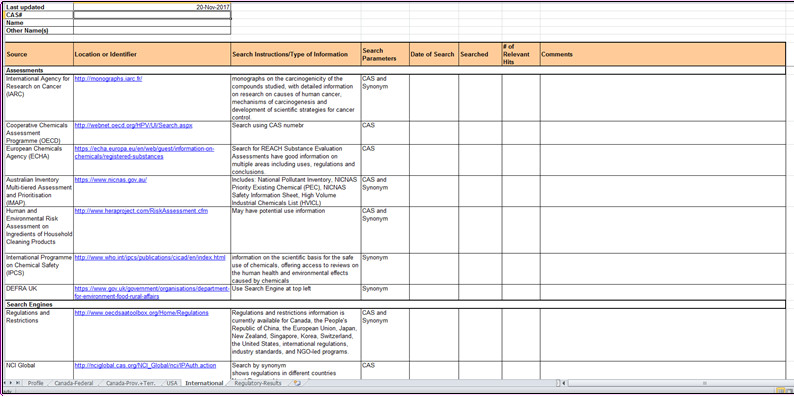
Text Description
Figure 13 provides a screen shot of one tab of the risk management search document template listing 9 sources, their weblinks, search instructions and blank fields for the risk manager to complete.
The published scope and approach documents contain a section on chemical alternatives and alternate technologies that briefly summarizes information on alternatives and are used to communicate this information to stakeholders. These documents may also indicate a need for additional information on alternatives in order to encourage stakeholders to provide this information to the departments.
CMP SC response to charge question 3
Charge question 3: given the amount of information on substances that has been collected, generated and analyzed throughout the CMP (for example, hazard data, use/quantity information, monitoring), what suggestions does the CMP SC have on how to use the data to support industry and other stakeholders in evaluating and selecting safer chemicals?
3.1 As discussed under charge question 1, the SC noted several opportunities in the CMP work flow for which data could be generated, collated, and made available to assist with supporting IS and AA within the CMP and to enable stakeholders such as industry to conduct AA. The SC offered the following considerations in response to charge question 3.
3.1.1. Make the information generated under the CMP, plus the tools developed, more accessible to stakeholders and other jurisdictions. This necessitates making the data available in a usable format. The following points were made:
- Engage with stakeholders, including industry sector groups, to identify end-users needs regarding how CMP-generated data could be used to help with IS. This information will inform how best to structure and format the data, while noting the need for reporting uniformity. These consultations can also help to identify additional information, tools, and policies needed to help with IS. Examples raised by the SC are included under the responses to charge question 2.
- Continue to work on improved coordination at the national and international levels. One specific example suggested by the SC would be to move to an "IUCLID-based" mechanism for information capture. This would facilitate standardization of basic use and function information, and would continue to support and contribute to ongoing standardization efforts within the OECD and other international groups.
- Make uncertainty information available when possible within the database/data capture format (refer back to the previous CMP SC position on communicating uncertainty; CMP SC, 2016).
3.1.2. Identify and promote specific tools that could be developed to facilitate IS. Examples are as follows:
- Data visualization tools (for example, ToxPi, Comp Tox dashboard) already exist and thus should be integrated with CMP-generated data.
- Tools that allow for groupings/categorization relevant to AA and IS (including an analysis of or focus on functional use criteria) may prove to be a breakthrough "technology."
3.1.3. Initiate and conduct retrospective analyses with a focus on IS to evaluate the following:
- Were opportunities missed in managing chemical risk where a discussion on IS would have helped?
- Should the regulator frame the policy intent at the time of the previous decision and re-frame it with an IS lens in terms of what the regulated community could do? In practice, such an approach might best be developed via a de novo approach, possibly by engaging the regulated community in a trial exercise.
- How can the chemical space be better defined? Can one draw insights from grouping substances based on common functional use or on another basis for substitution, leading one to re-frame some of these assessments/decisions with an IS lens? Can the nomenclature of these groupings be standardized (for example, through the OECD)?
- Can we learn about IS in terms of outcomes and conclusions from previous assessments under the CMP?
- Assess specific examples where questions of regrettable substitution have been raised; for example, organic flame retardants, azo dyes, phthalates (which included a cumulative risk assessment), bisphenols such as BPA and BPS (note that the experience associated with identifying potential alternatives to BPA has a strong educational/external communication component), and siloxanes.
- As noted under charge question 1, for the proposed creation of a possible PASL, determine how to best commence a review of substances of lower risk; this activity should include the +/- 19000 substances that did not meet the categorization criteria in addition to those identified for assessment and found not to be "toxic."
- Potential learnings from review of substances with "not CEPA-toxic" conclusions that were relatively close to a toxic determination.
- Assess whether more granular "use" information helps identify how best to move forward. If so, are there learnings on how to better use this information in future prioritization steps? Is this an additional step to help with prioritization?
- Identify and assess arguments based on "value of information" in an AA context.
3.1.4. Identify opportunities for use of existing information to evaluate models (for example, QSUR; Phillips et al., 2017) even when input data are classified as confidential business information such that it cannot be publicly released. This activity would assist in the evaluation, refinement, and further definition of the domain of acceptability of particular models; results could be offered to the outside community to help identify data gaps and to allow for their use; and it could help to spark the generation and release of data. The U.S. Food and Drug Administration has successfully used a similar approach (Matthews, 2007).
3.1.5. To spark innovation, research and data generation, look to the larger stakeholder community. Use the analysis of existing databases to help focus efforts and identify research needs.
CMP SC concluding comments
The SC's efforts were focused on responding to the charge questions and the SC believes the responses described herein comprise a reasonable summary of its consensus perspective on this exciting and emerging topic.
The SC noted that the subject of AA and IS cannot be easily summarized into a "one-size-fits-all" approach. A case-by-case approach may be necessary, particularly in order to avoid decisions that are subsequently found to belong in the "regrettable substitution" group. However, the SC believes the comments and suggestions in this report would, if addressed, allow for the acceleration of formal AA and activities to support IS within the CMP. The SC also noted that developing an approach for IS within the Government of Canada's chemicals management program can be guided by considerable efforts undertaken within other jurisdictions.
The subject of whether to focus more on "hazard" over "exposure" considerations was raised often during the committee's deliberations. The SC agreed that more emphasis has been placed to date on the hazard component, and opinion was split on whether this is appropriate. Some of the members discussed the question of whether/when exposure is relatively constant (for example, for "drop-in" replacement chemicals) or whether exposure varies with the substances (or chemical manufacturing processes) under consideration; therefore, "exposure" may be of varying relevance in a given AA (hence, the recurring theme of a case-by-case approach). The answer (to whether exposure is of equal importance to hazard) in the legislated risk-based process underpinning CEPA 1999, if there is one, can only be elucidated by continued review and retrospective analyses (such as those promoted in 3.1.3.).
The SC agreed that the subject of IS is complicated and requires some degree of global collaboration. The SC strongly encourage the departments to continue with their international engagement on chemicals management and to work with other jurisdictions to identify opportunities for data sharing, create consistent databases, and generally work towards formalizing a generic IS paradigm.
The SC wishes to recognize the 3 ad hoc members, Joel Tickner (University of Massachusetts Lowell), David Widawsky (U.S. EPA) and Meredith Williams (Department of Toxics Substances Control, California) for their significant contributions to this report.
Respectfully submitted on behalf of the CMP Science Committee,
Miriam Diamond & Geoff Granville, Co-chairs
References
Arbuckle T. E., Fraser W. D., Fisher M., Davis K., Liang C. L., Lupien N., Bastien S., Velez M. P., von Dadelszen P., Hemmings D. G., Wang J., Helewa M., Taback S., Sermer M., Foster W., Ross G., Fredette P., Smith G., Walker M., Shear R., Dodds L., Ettinger A. S., Weber J. P., D'Amour M., Legrand M., Kumarathasan P., Vincent R., Luo Z. C., Platt R. W., Mitchell G., Hidiroglou N., Cockell K., Villeneuve M., Rawn D. F., Dabeka R., Cao X. L., Becalski A., Ratnayake N., Bondy G., Jin X., Wang Z., Tittlemier S., Julien P., Avard D., Weiler H., Leblanc A., Muckle G., Boivin M., Dionne G., Ayotte P., Lanphear B., Séguin J. R., Saint-Amour D., Dewailly E., Monnier P., Koren G., Ouellet E. 2013. "Cohort profile: The maternal-infant research on environmental chemicals research platform." Paediatr and Perinat Epidemiol, 27(4):415-425. Available at: https://www.ncbi.nlm.nih.gov/pubmed/23772943.
Arbuckle T. E., Davis K., Marro L., Fisher M., Legrand M., LeBlanc A., Gaudreau E., Foster W. G., Choeurng V., Fraser W. D. 2014. "Phthalate and bisphenol A exposure among pregnant women in Canada-results from the MIREC study." Environ Int, 68: 55-65. Available at: https://www.ncbi.nlm.nih.gov/pubmed/24709781.
Auer, C. 2016. U.S. experience in applying 'Informed Substitution' as a component in risk reduction and alternatives analyses. Transcript of an oral presentation given at the Chemicals, Health, and the Environment Conference, Ottawa, Ontario, Canada, October 2006.
Canada, Department of the Environment. 2004a. "Canadian Environmental Protection Act, 1999: Notice requiring the preparation and implementation of pollution prevention plans in respect of effluents from textile mills that use wet processing (TMEs) and nonylphenol (NP) and its ethoxylates (NPEs)." Canada Gazette, Part I, Vol. 138, No. 49, p. 3522 - 3563. Available at: http://publications.gc.ca/collections/Collection/SP2-1-138-49.pdf.
Canada, Department of the Environment. 2004b. "Canadian Environmental Protection Act, 1999: Notice requiring the preparation and implementation of pollution prevention plans for inorganic chloramines and chlorinated wastewater effluents." Canada Gazette, Part I, Vol. 138, No. 49, p. 3497-3522. Available at: http://publications.gc.ca/gazette/archives/p1/2004/2004-12-04/pdf/g1-13849.pdf.
Canada, Department of the Environment. 2009. "Canadian Environmental Protection Act, 1999: Notice with respect to alternative substances to phosphorus compounds in household laundry detergents, household dish-washing compounds and household cleaners." Canada Gazette, Part I, Vol. 143, No. 17, p. 1216-1222. Available at: http://www.gazette.gc.ca/rp-pr/p1/2009/2009-04-25/pdf/g1-14317.pdf.
Canada, Department of the Environment. 2012a. "Canadian Environmental Protection Act, 1999: Notice requiring the preparation and implementation of pollution prevention plans in respect of cyclotetrasiloxane, octamethyl- (siloxane D4) in industrial effluents." Canada Gazette, Part I, Vol. 146, No. 22. Available at: http://www.gazette.gc.ca/rp-pr/p1/2012/2012-06-02/html/sup2-eng.html.
Canada, Department of the Environment. 2012b. "Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List." Canada Gazette, Part I, Vol. 146, No. 48. Available at: http://www.gazette.gc.ca/rp-pr/p1/2012/2012-12-01/html/sup-eng.html.
[CMP SC] Chemicals Management Plan Science Committee. 2014. "Committee report - February 19-20, 2014." Available at: https://www.canada.ca/en/health-canada/services/chemical-substances/chemicals-management-plan/science-committee/meeting-records-reports/meeting-record-february-19-20-2014.html.
[CMP SC Chemicals Management Plan Science Committee. 2016. "Committee report - November 16-17, 2016." Available at: https://www.canada.ca/en/health-canada/services/chemical-substances/chemicals-management-plan/science-committee/meeting-records-reports/committee-report-november-16-17-2016.html.
[CPA] Clean Production Action. 2017a. "GreenScreen Version 1.3 (2e) hazard criteria." Available at: https://www.greenscreenchemicals.org/static/ee_images/uploads/resources/GreenScreen_Hazard_Criteria_v1.3(2e)_2-20-2017_final.pdf.
[CPA] Clean Production Action. 2017b. "GreenScreen for safer chemicals hazard assessment guidance Version 1.3 (2e)." Available at: https://www.greenscreenchemicals.org/static/ee_images/uploads/resources/GreenScreen_Guidance_v1.3(2e)_Feb_2017_final.pdf.
[EC] European Commission. 2017. "Study for the strategy for a non-toxic environment of the 7th EAP: Sub-study A: Substitution, including grouping of chemicals & measures to support substitution." Available at: http://ec.europa.eu/environment/chemicals/non-toxic/pdf/Sub-study%20a%20substitution%20grouping%20NTE%20final.pdf.
[ECCC] Environment and Climate Change Canada. 2016. "Science approach document: Ecological risk classification of organic substances." Available at: https://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=A96E2E98-1.
[ECCC and HC] Environment and Climate Change Canada and Health Canada. 2015. "Identification of risk assessment priorities: Results of the 2015 review." Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=9E41BB6B-1.
[ECCC and HC] Environment and Climate Change Canada and Health Canada. 2016. "Identification of Risk Assessment Priorities (IRAP): Results of the 2016 review." Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=ADF217F9-1.
[ECCC and HC] Environment and Climate Change Canada and Health Canada. 2017a. "Rapid screening of substances with limited general population exposure." Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=1D06D668-1.
[ECCC and HC] Environment and Climate Change Canada, Health Canada. 2017b. "Draft screening assessment: Substances identified as being of low concern based on the Ecological Risk Classification of organic substances and the Threshold of Toxicological Concern (TTC)-based approach for certain substances." Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=8A598C49-1.
[ECHA] European Chemicals Agency. 2017. "Strategy to promote the substitution of hazardous chemicals-a thought starter." Available at: https://echa.europa.eu/documents/10162/13630/substitution_strategy_thought_starter_en.pdf/d1158bef-a5aa-9dd4-fb70-9a1fdb82cc93.
[ENVI 2017] Standing Committee on Environment and Sustainable Development. 2017. "Healthy Environment, Health Canadians, Health Economy: Strengthening the Canadian Environmental Protection Act, 1999." Available at: https://www.ourcommons.ca/DocumentViewer/en/42-1/ENVI/report-8/
Environment Canada. 2005. "Guideline for the notification and testing of new substances-chemicals and polymers: Pursuant to Section 69 of the Canadian Environmental Protection Act, 1999." Available at: http://publications.gc.ca/site/eng/280464/publication.html.
Environment Canada and Health Canada. 2008. "Screening assessment for the challenge: Phenol, 4,4'-(1-methylethylidene)bis- (bisphenol A)." Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=3C756383-1.
Environment Canada and Health Canada. 2014. "Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999." Available at: http://www.ec.gc.ca/ese-ees/A10191AD-973D-4059-AFC4-4DEEC36C4DB1/Assessment%20Triggers%20Approach_EN.pdf.
Hansson S. O., Molander L., Rudén C. 2011. "The substitution principle." Regul Toxicol Pharmacol, 59(3):454-60. doi: 10.1016/j.yrtph.2011.01.011. Epub 2011 Feb 2.
[HC] Health Canada. 2014. "Code of practice for 2-butanone, oxime (butanone oxime) associated with the interior application of consumer alkyd paint and coating products." Available at: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewh-semt/alt_formats/pdf/pubs/contaminants/butanone-oxime/butanone-oxime-eng.pdf.
[HC] Health Canada. 2016a. "Science approach document: Threshold of Toxicological Concern (TTC)-based approach for certain substances." Available at: https://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=326E3E17-1.
[HC] Health Canada. 2016b. "Science approach document: Biomonitoring-based approach 1 for beryllium vanadium, trichlorooxo vanadium oxide." Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=B0FA951B-1.
[HC] Health Canada. 2016c. "Science approach document: Biomonitoring-based approach 2 for barium-containing substances, molybdenum-containing substances, silver-containing substances, thallium-containing substances, inorganic tin-containing substances." Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=D335D89F-1.
Jacobs M. M., Malloy T. F., Tickner J. A., Edwards S. 2016. "Alternatives assessment frameworks: Research needs for the informed substitution of hazardous chemicals." Env Health Persp, 124(3): 265-280. Available at: https://ehp.niehs.nih.gov/1409581/.
Matthews, E. J. 2007. Quantitative structure activity tools used by the U.S. FDA. OEHHA-COEH Workshop, October 1-2, 2007. Available at: https://oehha.ca.gov/media/downloads/risk-assessment/presentation/matthewsoct207.pdf.
[NRC] National Research Council. 2014. A Framework to Guide Selection of Chemical Alternatives. Washington, D.C.: The National Academies Press. Available at: https://www.nap.edu/catalog/18872/a-framework-to-guide-selection-of-chemical-alternatives.
[NRC] National Research Council. 2015. Application of Modern Toxicology Approaches for Predicting Acute Toxicity for Chemical Defense. Washington, D.C.: The National Academies Press. Available at: https://doi.org/10.17226/21775.
[NRC] National Research Council. 2017. Using 21st Century Science to Improve Risk-Related Evaluations. Washington, D.C.: The National Academies Press. Available at: https://doi.org/10.17226/24635.
[OECD] Organisation for Economic Co-operation and Development. 2013. "Current landscape of alternatives assessment practice: A meta-review." Series on Risk Management. No. 26. Available at: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO%282013%2924&docLanguage=En.
Panko J. M., Hitchcock K., Fung M., Spencer P. J., Kingsbury T., Mason A. M. 2017. "A comparative evaluation of five hazard screening tools." Integr Environ Assess Manag, 13(1): 139-154. Available at: http://onlinelibrary.wiley.com/doi/10.1002/ieam.1757/abstract;jsessionid=7EAE078C181069523920392CDC9C398D.f03t02.
Phillips, K. A., Wambaugh, J. F., Grulke, C. M., Dionisio, K. L., Isaacs, K. K. 2017. High-throughput screening of chemicals as functional substitutes using structure-based classification models. Green Chem., 19(4): 1063-1074.
Rasmussen P. E., Beauchemin S., Chénier M., Levesque C., MacLean L. C. W., Marro L., Jones-Otazo H., Petrovic S., McDonald L. T., Gardner H. D. 2011. "Canadian house dust study: Lead bioaccessibility and speciation." Environ Sci and Technol, 45: 4959-4965. Available at: http://pubs.acs.org/doi/pdf/10.1021/es104056m.
Rasmussen P. E., Levesque C., Chénier M., Gardner H. D. 2017. "Supporting information for: Rare earth elements and selected actinoids in the Canadian house dust study." Int J Environ Health, 27(5): 965-976. Available at: http://onlinelibrary.wiley.com/doi/10.1111/ina.12379/abstract;jsessionid=9797C1C3DB7B3B704E98B6ECB2075FCC.f03t04
Ring, C. L., Pearce, R. G., Setzer, R. W., Wambaugh, J. F. 2017. Identifying populations sensitive to environmental chemicals by simulating toxicokinetic variability. Environ Internat, 106: 105-118. doi: 10.1016/j.envint.2017.06.004.
Stone A. 2016. "Quick Chemical Assessment Tool Version 2.0." Washington State Department of Ecology. Available at: https://fortress.wa.gov/ecy/publications/documents/1404033.pdf.
Tickner J. A., Schifano J. N., Blake A., Rudisill C., Mulvill M. J. 2015. "Advancing safer alternatives through functional substitution." Environ Sci Technol, 49: 742-749. Available at: http://pubs.acs.org/doi/full/10.1021/es503328m.
[UMass Lowell] University of Massachusetts Lowell, Lowell Center of Sustainable Production. 2017. "Examining opportunities to support the transition to safer chemicals in Canada: International landscape on chemical substitution and alternatives assessment." Report prepared under contract with Environment and Climate Change Canada.
[UN] United Nations. 2015. "Globally harmonized system of classification and labelling of chemicals (GHS)." Sixth revised edition. Available at: http://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev06/English/ST-SG-AC10-30-Rev6e.pdf.
U.S. EPA. 2011a. "Design for the Environment program alternatives assessment criteria for hazard evaluation." Version 2.0. Available at: https://www.epa.gov/sites/production/files/2014-01/documents/aa_criteria_v2.pdf.
U.S. EPA. 2011b. "Response to comments on the DfE alternatives assessment criteria for hazard evaluation." Available at: https://www.epa.gov/sites/production/files/2014-01/documents/aa_criteria_comments_response.pdf.
U.S. EPA. 2015. "Flame retardants used in flexible polyurethane foam: An alternatives assessment update." Available at: https://www.epa.gov/sites/production/files/2015-08/documents/ffr_final.pdf.
Appendix 1: Key reading material and links to comparative hazard evaluation tools (note: this list is updated to add new resources/databases)
Jacobs M. M., Malloy T. F., Tickner J. A., Edwards S. 2016. "Alternatives assessment frameworks: Research needs for the informed substitution of hazardous chemicals." Env Health Persp, 124(3): 265-280. Available at: https://ehp.niehs.nih.gov/1409581/.
[NRC] National Research Council. 2014. A Framework to Guide Selection of Chemical Alternatives. Sections 7 and 8: pp. 81-121. Washington, D.C.: The National Academies Press. Available at: https://www.nap.edu/catalog/18872/a-framework-to-guide-selection-of-chemical-alternatives.
[OECD] Organisation for Economic Co-operation and Development. 2013. "Current landscape of alternatives assessment practice: A meta-review." Series on Risk Management. No. 26. Available at: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO%282013%2924&docLanguage=En.
Panko J. M., Hitchcock K., Fung M., Spencer P. J., Kingsbury T., Mason A. M. 2017. "A comparative evaluation of five hazard screening tools." Integr Environ Assess Manag, 13(1): 139-154. Available at: http://onlinelibrary.wiley.com/doi/10.1002/ieam.1757/abstract;jsessionid=7EAE078C181069523920392CDC9C398D.f03t02.
Tickner J. A., Schifano J. N., Blake A., Rudisill C., Mulvill M. J. 2015. "Advancing safer alternatives through functional substitution." Environ Sci Technol, 49: 742-749. Available at: http://pubs.acs.org/doi/full/10.1021/es503328m.
[UMass Lowell] University of Massachusetts Lowell, Lowell Center of Sustainable Production. 2017. "Examining opportunities to support the transition to safer chemicals in Canada: International landscape on chemical substitution and alternatives assessment." Report prepared under contract with Environment and Climate Change Canada.
| Tool | Developer | Weblink |
|---|---|---|
| GreenScreen® for Safer Chemicals | U.S.-based nongovernment organization Clean Production Action | http://www.greenscreenchemicals.org/ |
| Quick Chemical Assessment Tool (QCAT) | Washington State Department of Ecology | http://www.ecy.wa.gov/programs/hwtr/chemalternatives/QCAT.html |
| P2OASys (Pollution Prevention Options Assessment System) | Massachusetts Toxics Use Reduction Institute | https://www.turi.org/Our_Work/Research/Alternatives_Assessment/Tools_and_Methods/P2OASys_Tool_to_Compare_Materials |
| Column Model | German Institute for Occupational Safety and Health | http://www.dguv.de/ifa/Praxishilfen/GHS-Spaltenmodell-zur-Substitutionspr%C3%BCfung/index-2.jsp |
| SciVera Lens Chemical Safety Assessment | SciVera, LLC | http://www.scivera.com/index.php |
Appendix 2: Research initiatives with CMP funding
2017-2021 CMP-funded HC research projects Footnote 15
- National indoor air survey
- Determination of additional volatile organic compounds in blood
- Development of pathogenicity test methods for assessing the hazard of microorganisms used in biotechnology
- Multimedia exposure to replacement chemicals of emerging concern and selected CMP3 chemicals
- Assessment of the carcinogenic potential of CMP chemicals through the application and investigation of the Syrian hamster embryo cell transformation assay (SHE-CTA)
- Developing in vitro screening methods for metabolic disruptors in adipocytes
- An integrated testing strategy to assess somatic and germ cell mutations using the OECD's transgenic rodent test guideline TG 488 and the MutaMouse model
- Assessment of the performance and predictiveness of an optimized in vitro developmental neurotoxicity assay using proven developmental neurotoxicants and negative controls
- Development of a high-throughput chamber test method for the determination of semi-volatile organic compounds from consumer products
- Development and validation of rapid methods to assess endocrine toxicity
- In vitro to in vivo extrapolation (IVIVE) toxicokinetics of CMP chemicals
- The impact of dissolution behaviour of metal oxide nanomaterials on toxicological response
- Characterization of residential exposures to CMP metals and organics
- GeneTox21-an integrated, high-throughput (HT) platform for in vitro genetic toxicity assessment of new and existing chemicals
- Refining and deploying a quantitative framework for the analysis and regulatory interpretation of genetic toxicity dose-response data
- Development of methodology for home dust microbiome analysis towards Canadian exposure assessments of biotechnology microbes
- Implementation of whole-genome sequence data towards hazard characterization and identification of biotechnology microbes in mixtures
- Relative toxic potency of silica and titanium dioxide nanoparticle variants
- Development of non-targeted and screening analysis approaches for identifying emerging metabolites and chemicals in human fluids as exposure biomarkers using high-resolution mass spectrometry
- Designing cost-effective drinking water surveys in the 21st century: Optimizing target analytes, site selection, sampling, and analytical methods
- Animal studies to support the interpretation of biomonitoring data for the organophosphate flame retardant TBOEP (ethanol, 2-butoxy-, phosphate (3:1), CAS no. 78-51-3)
- In vitro pharmacokinetics for high-throughput data interpretation (Part 2)
- Derivation of biomonitoring equivalents for organics and inorganics for interpreting biomonitoring data to support chemical risk assessment (Part 1)
- Derivation of biomonitoring equivalents for organics and inorganics for interpreting biomonitoring data to support chemical risk assessment (Part 2)
- Retrospective analysis of GC/MS data from CHMS Cycle 3 indoor air samples
- Residential exposures (dust and airborne) to CMP3 inorganics
- Canadian biomonitoring data, reference ranges and association with health outcomes of selected CMP-3 priority metals and trace elements
- Animal studies to support the interpretation of biomonitoring data rare earth metals in the CMP Cycle 3
- Development of request for proposals for dermal absorption testing of existing and new priority chemicals under the CMP (Part 1)
- Development of request for proposals for dermal absorption testing of existing and new priority chemicals under the CMP (Part 2)
- Direct comparison of the sub-acute toxicities of bisphenol A, F, and S, using a standard OECD exposure protocol
- Uptake rates of silicone based personal sampling devices - proof of principle
- Biostatistics data analysis for Existing Substance Risk Assessment Bureau (ESRAB)
- Maternal-infant research on environmental chemicals (MIREC) and associated follow-up studies
- Application of ex vivo precision lung slice technique to determine silica nanoparticle-induced cellular responses: role of size, surface charge, and modification
- Development of rapid testing methodology models for assessing the pathogenic potential of emerging products of biotechnology
- Assessment of toxicity determinants of non-porous silica nanoparticles with varied size and surface functionality
- Assessing the potential utility of Tox21 high-throughput screening assays for assessment of chemicals on the revised In Commerce List (ICL)
2017-2018 CMP-funded ECCC research projects
- The environmental fate, distribution, and effects of naphthalene sulfonic acids (NSAs): developing analytical methods, investigating toxicity, and evaluating bioaccumulation
- First steps towards characterization of halogenated alkene flame retardants
- Environmental transformation processes and bioaccumulation, fate, and effects of CMP3 priority organic flame retardants in wildlife and fish within an adverse outcome pathway (AOP) framework
- Evaluation of estrogenic and thyroid-disrupting activities of targeted CMP3 priority substances: benzotriazole, thiocarbamate, hindered phenols, and a brominated organophosphate flame retardant
- Study of rare earth elements (REEs) and platinum group elements (PGEs) on aquatic and terrestrial biota (crops, native plants, and invertebrates): implications for ecological risk assessment at contaminated sites
- Using a multi-tiered screening approach and the adverse outcome pathway (AOP) framework to determine the effects of new and existing priority CMP3 substances, primarily organic flame retardants, on key neuroendocrine pathways
- Environmental fate and disposition of CMP3 priority polar organic substances
- Exposure, uptake, and adverse effects on birds exposed to new and Existing CMP3-priority organic flame retardants: identifying in vivo changes within an avian adverse outcome pathway
- Chronic toxicity and modes of action of benzotriazoles/benzothiazoles and flame retardants in aquatic organisms
- Atmospheric fate studies on CMP priority chemicals
- Aquatic ecotoxicology of lanthanides
- Lead for air; lead for water and wastewater; lead for sediment and biosolids; lead for mass balance component-source, environmental fate, and toxicity of synthetic musks in Canada
- Chronic toxicity of thiocarbamate and benzothiazole compounds to survival, growth, and reproduction of freshwater invertebrates
- Effect of six rare earth element lanthanides in boreal forest soil on invertebrates and soil microbial community
- Survival, developmental toxicity, and tumour inducing potency of a model benzotriazole/benzothiazole (for example, 2-mercaptobenzothiazole) in fish as a step towards an adverse outcome pathway (AOP) for this class of compounds
- Aquatic nanotoxicology
- Understanding the atmospheric fate and toxicity of engineered nanoparticles through transformation studies
- Fate, transformation and bioaccumulation of silver nanoparticles (nAg) and metal oxide nanoparticles (nCeO2, nCuO, nZnO) in the aquatic environment
- Fate and effects of nanotechnology in bacterial cultures and complex communities
- Environmental fate, effects and bioaccumulation of priority nanomaterials in soil
Appendix 3: Data sources that may be used to identify potential alternatives during the risk management phase
The OECD Substitution and Alternatives Assessment Toolbox is used to search through a compilation of resources relevant to chemical substitution and alternatives assessments. Resources that are available include:
- The Alternatives Assessment Tool Selector, which is a filterable inventory of chemical hazard assessment tools and data resources to help identify tools most relevant to the chemical substitution and alternatives. However, not all databases are free to use, and some need more technical expertise than others.
- The regulations and restrictions section provides access to regulations and restrictions throughout OECD member countries.
- The OECD case studies and other resources section include descriptions of alternative assessments that have been conducted by manufacturers, academic institutions, nongovernment organizations, or government bodies, and may offer relevant information.
The U.S. EPA offers a Safer Chemical Ingredient List that is used to obtain information on chemical alternatives grouped by their functional-use class, and includes many chemicals evaluated through the Safer Choice Program.
Another resource is the Institute for Research and Technical Assistance (IRTA), a non-profit organization, which has expertise in finding alternatives in a variety of applications.
Another non-profit organization is the Environmental Working Group (EWG) that can offer information on substances in different sectors.
For cosmetics, both the Good Scent Company Information System and the European Commission cosmetic ingredient database (CosIng) websites can be used to search for chemicals based on their uses, such as ultraviolet absorbers or preservatives.
Appendix 4: Data requirements for Schedule 6 New Substance Notification
Information respecting other chemicals and biochemicals not on the NDSL (10,000 kg):
1. The following identification information with respect to the chemical:
- the chemical name
- the trade name(s) of the chemical and the synonyms of its chemical name
- the CAS RN of the chemical
- its molecular formula
- its structural formula
- its gram molecular weight
- the degree of purity in its technical-grade composition
- known impurities present and their concentration by weight
- any additives, stabilizers, and solvents present when the chemical is tested, and their concentration by weight
A material safety data sheet with respect to the chemical, if available:
2. The following exposure information regarding the chemical:
- the anticipated annual quantity to be manufactured, if applicable
- the anticipated annual quantity to be imported, if applicable
- the anticipated uses within Canada
- its anticipated concentration in products and, if known, in end-use products
- a description of the expected modes for its transportation and storage
- a description of the size and type of container used for its transportation and storage
- an identification of the components of the environment into which it is anticipated to be released
- its anticipated releases into municipal wastewater systems
- a description of the methods recommended for its destruction or disposal
- whether it is anticipated to be used in products intended for use by or for children
- the anticipated degree of direct human exposure to the chemical, including concentration, duration, frequency, and circumstances of exposure and factors that may limit direct human exposure
- the three sites in Canada (if known) where the greatest quantity of the chemical, manufactured or imported by the person, is anticipated to be used or processed, and the estimated quantity by site
- its historical and other likely uses
- any factors that may limit environmental exposure
3. The following physical and chemical data 1 with respect to the chemical:
- its melting point or the temperature at which the chemical decomposes
- its boiling point or the temperature at which the chemical decomposes
- its density
- its vapour pressure if it has a standard boiling point of 0°C or greater
- its water solubility
- its octanol-water partition coefficient for chemicals having a water solubility of less than or equal to 5 g/L
- one of an infrared, ultraviolet, mass, or nuclear-magnetic resonance spectrum suitable for characterization of the chemical
- the adsorption-desorption screening test data for chemicals having a water solubility of greater than or equal to 200 μg/L
- its hydrolysis rate as a function of pH and, if known, an identification of the products of the hydrolysis for chemicals having a water solubility of greater than or equal to 200 μg/L
4. Ready biodegradation test data and, if known, identification of the products of biodegradation.
5. Data from acute fish, daphnia, and algae toxicity tests.
6. Data from two acute mammalian toxicity tests selected on the basis of the most significant routes of potential human exposure to the chemical; namely, oral, dermal, or inhalation.
7. Information sufficient to assess skin irritation.
8. Data from a skin sensitization test
9. Data from one repeated-dose mammalian toxicity test, of at least 28 days duration, selected on the basis of the most significant route of potential human exposure to the chemical; namely, oral, dermal or inhalation.
10. Mutagenicity data obtained from the following:
- one in vitro test, with and without metabolic activation, for gene mutations
- one in vitro test, with and without metabolic activation, for chromosomal aberrations in mammalian cells
- one in vivo mammalian test for chromosomal aberrations or gene mutations or another indicator of mutagenicity that, together with data substantiating that the tissue investigated was exposed to the chemical or its metabolites, permits an assessment of in vivo mutagenicity
11. A summary of all other information and test data with respect to the chemical that is in the possession of the manufacturer or importer or to which they ought to have access, and that are relevant to identifying hazards to the environment and human health and the degree of environmental and public exposure to the chemical.
Footnotes
- Footnote 1
-
For the purposes of this meeting, informed substitution is defined as the considered transition from a chemical of particular concern to safer chemicals or non-chemical alternatives (Hansson et al. 2011).
- Footnote 2
-
The following information was provided to the SC in advance of the meeting, and SC input has been identified in text boxes at the end of Parts I, II and III.
- Footnote 3
-
An alternatives assessment may be defined as a process for identifying and comparing potential chemical and non-chemical alternatives that could replace chemicals of concern on the basis of their hazards, performance, and economic viability (UMass Lowell, 2017); in this way, it is a means by which to support informed substitution.
- Footnote 4
-
According to the definition in Section 64 of CEPA 1999.
- Footnote 5
-
In some cases, substances that were not identified through Categorization were included in the scope of the work (for example, substances added to the DSL through new substances notifications).
- Footnote 6
-
The U.S. EPA has developed a Safer Chemicals Ingredients List (SCIL), which is based on evaluating a chemical substance against a set of human health and ecological hazard criteria. Chemical substances that meet the criteria thresholds for all endpoints are eligible to be listed on the SCIL. Hazard data underlying the SCIL are most commonly generated by companies seeking recognition and use of the EPA's Safer Choice label. Information on both the SCIL and the Safer Choice program can be found, respectively, at: www.epa.gov/saferchoice/safer-ingredients and at www.epa.gov/saferchoice.
- Footnote 7
-
An example of a risk assessment scoping document for the U.S. EPA's work on cyclic aliphatic bromides can be found at: www.epa.gov/sites/production/files/2017-06/documents/hbcd_scope_06-22-17_0.pdf and an example of a risk assessment problem formulation document is at: www.epa.gov/sites/production/files/2015-09/documents/hbcd_problem_formulation.pdf.
- Footnote 8
-
see the US EPA's Safer Choice and Safer Chemical Ingredients List programs: www.epa.gov/saferchoice.
- Footnote 9
-
The international risk assessment community has not narrowly defined NAM, but the broad context may include in silico approaches, and in chemico and in vitro assays.
- Footnote 10
-
TTC: threshold of toxicological concern for environmental assessment. HC has published an approach for TTC within the human health assessment paradigm; see http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=326E3E17-1.
- Footnote 11
-
Quantitative structure activity relationships/quantitative structure-use relationships. See Phillips, K.A., J.F. Wambaugh, C.M. Grulke, K.L. Dionisio, K.K. Isaacs. 2017. High-throughput screening of chemicals as functional substitutes using structure-based classification models. Green Chemistry doi: 10.1039/c6gc02744j.
- Footnote 12
-
See, for example, Gangwal, S. and D. Rief. 2010. Toxicological Priority Index (ToxPi) as a platform for incorporation of exposure data for chemical prioritization. U.S. EPA, https://www.epa.gov/sites/production/files/2014-08/documents/toxpi_framework_exposuredata_gangwal_22april2010_.pdf.
- Footnote 13
-
The focus is on CEPA-related data. Substance data are also generated/collected by programs under the Food and Drugs Act, Pest Control Products Act, and Canada Consumer Product Safety Act.
- Footnote 14
-
The NDSL includes substances that are on the U.S. EPA's Toxic Substances Control Act Chemicals Substances Inventory for 1985 that are not already on the Canadian DSL. Substances that are not on the DSL but are listed on the NDSL are subject to lesser information requirements.
- Footnote 15
-
These are only projects led by scientists in HC's Environmental Health Sciences Research Bureau; there may be other relevant CMP research projects in other HC Branches (such as Health Products and Foods Branch).