Committee report – July 18-19 2018
Chemicals Management Plan Science Committee
Advancing consideration of endocrine-disrupting chemicals under the Canadian Environmental Protection Act, 1999
The introductory portion of the report was provided by the departments in pre-meeting material.
On this page
- Background
- Context and meeting objective
- Current approach in Canada for addressing endocrine disrupting chemicals
- Overview of Science Committee deliberation process
- Charge Question 1
- Charge Question 2
- Charge Question 3
- Summary
- Acknowledgements
- Annex 1
- Appendix A
- Appendix B
Background
The Chemicals Management Plan (CMP) is a Government of Canada initiative launched in 2006 which set clear priorities for assessing and managing chemical substances used in Canada, including the new and existing substances programs of the Canadian Environmental Protection Act, 1999 (CEPA 1999). The CMP Science Committee (SC) was established by Health Canada (HC) and Environment and Climate Change Canada (ECCC) (herein referred to as the departments) in 2013 to contribute expertise pertaining to scientific considerations in the delivery of the CMP.
At the meeting held in July 2018, the departments sought input from the SC on scientific considerations related to how the Government of Canada could evolve the current approach for the identification and assessment of endocrine-disrupting chemicals (EDCs).
This SC report includes excerpts from pre-meeting materials provided by the departments to outline the context and meeting objective as well as provide background information on the current approach in Canada for addressing EDCs and includes the Annex and Appendix material. These excerpts are identified within the text.
Context and meeting objectives
(as provided by the departments in pre-meeting material)
In 2017, the House of Commons Standing Committee on Environment and Sustainable Development released a report with recommendations on strengthening CEPA 1999 as part of its 5 year review cycle (section 343), including recommendations pertaining to addressing EDCs (Parliament of Canada, 2017). Moreover, EDC identification and assessment is an area where some stakeholders have provided the departments with recommendations for actions moving forward (CELA, 2017).
An EDC is an exogenous chemical that interacts or interferes with the function of the endocrine system. This may include the control of growth and maturation; reproduction and development; behaviour and reaction to stimuli; the production, use, and storage of energy; and balance and maintenance of water and electrolytes in the body. As defined by the World Health Organization (WHO) and adopted by the Organisation for Economic Co-Operation and Development (OECD), "an endocrine disruptor is an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations, "while" a potential endocrine disrupter is an exogenous substance or mixture that possesses properties that might be expected to lead to endocrine disruption in an intact organism, or its progeny, or (sub)populations" (OECD, 2018d). As such, exposure to an EDC may change the production, transport, metabolism, receptor activation, or downstream action of a hormone, resulting in disrupted messages received by a target tissue. Exposure to EDCs during critically susceptible periods of development (for example, development/differentiation of the brain, reproductive tract, or reproductive organs) can result in adverse effects (that is, long-term and possibly multigenerational changes in function).
The goal of this meeting was to focus on the scientific considerations needed to guide the advancement of a potential program of work on EDCs in Canada that builds on international best practices and benefits from new and emerging methodologies and data. Towards this objective, the SC was asked to answer charge questions identified by the departments. Given that the departments carry out various activities related to chemicals management (that is, information gathering, priority setting, risk assessment and risk management, research, monitoring, and surveillance), the SC was requested to use a "fit-for-purpose" lens, identifying uncertainties as relevant to the context of the decision.
Current approach in Canada for addressing endocrine disrupting chemicals
(as provided by the departments in pre-meeting material)
CEPA 1999 defines a hormone-disrupting substance as a substance having the ability to disrupt the synthesis, secretion, transport, binding, action, or elimination of natural hormones in an organism, or its progeny, that are responsible for the maintenance of homeostasis, reproduction, development, or behaviour of the organism (Canada, 1999). Although CEPA 1999 does not explicitly require the identification of chemicals as EDCs, the risk-based approach taken by the departments to assess new and existing substances involves the review of scientific literature, including human epidemiologic studies and available effects information from acute and repeated-dose tests, which includes EDCs for reproduction and development endpoints. Based on structural information and available hazard data, the EDC potential of a substance is considered in the assessment where possible. Assessments under the CMP, including those for EDCs and potential EDCs, consider expected and potential sources, pathways, and routes of exposure in order to characterize risk. When data sets are limited, the evaluation of an EDC potential can also consider alternative data sources such as in silico models, in vitro assays, read-across, and information from other regulatory jurisdictions.
Additional to the information sources described in the preceding paragraph, the New Substances Notification Regulations (Chemicals and Polymers) (NSNR) (Canada, 2005) require that physical-chemical, hazard, and exposure information be submitted prior to the import or manufacture of a new substance, following stepwise quantity triggers (see Appendix A for a tabular summary of the data requirements). At quantities >10,000 kg/year for Schedules 5 and 6, the required toxicity information includes repeated-dose mammalian toxicity testing (Appendix A; Table A-1) which, while not specifically designed for the detection of endocrine-disrupting potential, can be used, to a certain extent, to identify potential endocrine-related adverse effects. The ecotoxicity tests (Appendix A; Table A-2) are not designed for informing endocrine effects due to being short-term (acute) tests. The NSNR also requires that a notifier submit all other relevant data in their possession relevant to the assessment of a hazard, which could include submission of the specific endocrine-related tests if available.
In addition to the strategies noted above, the departments can solicit information directly from stakeholders. The New Substances (NS) Program can follow up with notifiers directly to solicit more information, either on a voluntary basis or through implementation of risk-management measures. For example, the NS Program may require additional testing data through measures available in CEPA 1999, such as Significant New Activity (SNAc) notices (section 81), Ministerial requests for additional information [paragraph 84(1)c], and written notices [paragraph 71(1)c]. Similarly, for existing substances, information can be requested under section 71 data-gathering activities or through other voluntary submissions (Canada, 2017b). Furthermore, under section 70 the onus is on industry to provide information in their possession that reasonably supports the conclusion that a substance is toxic or capable of becoming toxic, as defined under section 64 of CEPA 1999. When available, information on endocrine-disruption potential can be provided by industry under these requirements.
Further, the departments conduct and monitor research related to chemicals (including EDCs), and this research informs the identification of new priorities for risk assessment or if there is a need to take additional action. The approach for the identification of chemicals and polymers as risk-assessment priorities under Part 5 of CEPA 1999 is outlined in Canada (2017c). An overview of the departments' activities up until 2012 pertaining to EDCs can be found in the federal government's response to the Ecojustice and Canadian Environmental Law Association Petition 340, Federal Research on Hormone Disrupting Substances as required under the Canadian Environmental Protection Act, 1999 (Canada, 2012). Also, Appendix B lists more recent and active intramural research activities, which includes biomonitoring and exposures to various chemicals, including potential EDCs (Table B-1), effects due to exposures to potential EDCs (Table B-2), and research activities and expertise relevant to EDC (Table B-3). HC and ECCC scientists continue to contribute to international initiatives and programs, including those of the OECD, for the development, standardization, and validation of internationally recognized toxicity test methods (in vitro and in vivo) to assess substances for endocrine disruption and toxicity endpoints associated with these mechanisms.
For an international context, Annex 1 provides an overview of select international approaches, frameworks, and guidance for consideration with regard to the identification and evaluation of EDCs.
Overview of Science Committee deliberation process
The CMP SC was asked to deliberate on scientific approaches to identify and assess EDCs in the context of the CMP. As previously noted, this request was borne out of recommendations of the House of Commons Standing Committee on Environment and Sustainable Development and positions of some stakeholders regarding EDCs. In this context, the SC initially discussed the overall charge from the departments in plenary, and then broke into 3 small groups to consider the 3 individual Charge Questions. Each of the 3 groups discussed all the Charge Questions and held brief plenary sessions to encourage debate. Finally, a longer plenary was held to share final thoughts and suggestions. The report was subsequently drafted and completed after the meeting.
At the outset, the SC discussed whether EDCs present a unique challenge for risk assessment or if EDCs can be readily handled within the existing framework and methods of the Canadian CMP. Most, but not all, members opined that EDCs do not present a unique challenge from a science perspective in that the issues encountered in evaluating EDCs are also present with chemicals that affect non-endocrine biological processes. Several examples were provided as illustration, including the challenge in distinguishing the dose response at low chemical exposures and the potential transgenerational effects, which were identified as concerns for EDCs but are also ongoing challenges with non-endocrine endpoints. All members were in agreement that EDCs have been instrumental in driving efforts to better understand a number of issues applicable across the field of toxicology, and that these issues have often been more explicitly evaluated for EDCs than for chemicals with other modes of action. Examples presented here included the dose-response relationship at "low" doses (defined as being in the range of environmental exposures); complexities of receptor interactions; and the challenge of identifying a meaningful point of departure (POD) from "early" or "upstream" effects that are not generally considered "apical" endpoints (that is, indicative of a disease state). Thus, most but not all members considered that scientific approaches to evaluating hazards and risks presented by EDCs are broadly consistent with the methods and approaches currently in use and those under development, including approaches to address the potential of transgenerational effects. However, some members considered that the latter concern constituted a distinct and potentially unique challenge. Some members of the SC also noted that questions about EDCs have and are causing societal concerns and that they present immediate challenges to the regulatory process charged with managing EDCs.
Many of the responses to the Charge Questions do not necessarily pertain only to EDCs but rather speak to evolving risk assessment methods that could be used in the future under the CMP for all substances. This is another example of EDCs driving the science of health and environmental risk assessment.
Charge Question 1
How could the considerations introduced above (and any additional identified by the SC) influence current priority-setting and assessment practices as the departments work to advance and expand approaches for EDCs and potential EDCs?
Key areas to examine could include:
- the dose response and threshold for adversity
- the role of data related to less well studied endocrine-disruption modalities [that is, non-estrogen receptor, -androgen receptor, -thyroid hormone signalling, and -steroidogenesis (non-EATS)]
- the applicability of human health-focused data to inform ecological assessments and vice versa
1.1 General comments on key considerations
Based on input provided by the departments plus further discussion, the SC developed a list of topics which pertain to the issue of priority setting and assessment practices for EDCs and potential EDCs, as follows:
- Dose response, including non-monotonic dose response (NMDR) and low-dose toxicology*
- Endocrine-disruption modalities, including not only estrogen receptor, androgen receptor, thyroid hormone signalling, and steroidogenesis (EATS), but also non-EATS modalities
- Windows of susceptibility
- Multigenerational effects, including epigenetics
- Cross-species extrapolation, including humans, mammalian models, and ecological species (vertebrates and invertebrates)
- Environmentally relevant exposures
- Population variability, susceptibility, and vulnerability (beyond windows of susceptibility noted above)*
- Cumulative risk due to mixtures, co-exposures, and non-chemical stressors*
- Complexities of interpreting results of alternative test systems
Comments on these 9 topics are woven into the SC's responses to Charge Question 1. Furthermore, the SC had some additional general comments on the 3 topics marked by an asterisk (*), which are discussed in the following sub-sections.
1.1.1 Non-monotonic dose-response curves
For risk assessment, the POD is typically derived from the critical effect defined as the first adverse apical effect that occurs at the lowest exposure level on the toxicological dose-response curve in the most sensitive species. In developing exposure guidelines based on a risk assessment, a level of exposure protective for that critical effect is used, and in doing so, exposed populations should be protected against all other apical effects of concern because such effects would require higher doses to manifest. However, one of the key concerns from stakeholders is that the possibility of NMDR curves for EDCs may imply that current risk assessment practices are inadequately protective. While the SC was not charged with providing an opinion on this specific question, the following points may be relevant for addressing this concern:
- There are multiple processes by which NMDR curves may be manifest; for example:
- an "apparent" NMDR due to different receptors being "activated" in different dose ranges. One classic example is essential nutrients in which at low intakes, the dose-response curve is downward sloping due to the reduction of the deficiency effects; and at high intakes, the dose-response curve is upward sloping due to the increased effects of toxicity. In such cases, the downstream adverse effects of deficiency and toxicity are qualitatively different.
- an "integrated" NMDR due to an adverse effect being modulated by multiple pathways with different directionality. For instance, estrogen-mediated proliferation can be modulated upwards or downwards, depending on the molecular interaction or receptor target. Therefore, if a substance has multiple targets with different potencies, then the response may initially be in one direction and then shift in the opposite direction as the dose increases. For this reason, more integrated assays (for example, medium-throughput, discussed below) may be better able to discern such cases versus assays that focus on only initiating targets (for example, receptor-ligand binding).
- The NMDR issue is potentially applicable across all types of toxicants, not just EDCs (for example, high-dose toxicity confounding the dose response for effects first observed at lower doses). For example, there is the well-known inverted "U-shaped" curve on behavioural activity for amphetamine. For EDCs that specifically affect hormone levels (for example, altered synthesis, transport or degradation), distinct effects may occur at low versus high levels.
- More detailed discussions of NMDR issues can be found in the United States (U.S.) Environmental Protection Agency (EPA) report, "State of the Science Evaluation: Nonmonotonic Dose Responses As They Apply to Estrogen, Androgen, and Thyroid Pathways and EPA Testing and Assessment Procedures" (EPA, 2013); the U.S. National Academies of Sciences (NAS) report, "Review of the Environmental Protection Agency's State-of-the-Science Evaluation of Nonmonotonic Dose-Response Relationships As They Apply to Endocrine Disruptors" (NASEM, 2014); and the U.S. NAS report, "Application of Systematic Review Methods in an Overall Strategy for Evaluating Low-Dose Toxicity from Endocrine Active Chemicals" (NASEM, 2017).
Further commentary on NMDRs in the context of EDCs is provided in section 1.7.
1.1.2 Population variability and susceptibility (beyond windows of susceptibility)
A critical component of chemical management is characterizing variability in how organisms respond to stressors (Zeise et al., 2013). Although there has been much progress in characterizing exposure variability (NASEM, 2012), characterizing human variability in hazard and dose response remains a challenge, particularly because most testing is done in genetically homogeneous test systems that do not mimic the variation within (or between) species. This challenge also exists in ecological risk assessments where only a few models are presently tested and used to represent thousands of potentially susceptible species. Chiu et al. (2018) and Harrill and McAllister (2017) provide overviews of mammalian models, as well as some of the computational tools available, to help characterize variability in hazard and dose response. In many cases, a shift away from point estimates to probabilistic analyses will enable a richer characterization of individual risk, population incidence, and statistical confidence.
The above comment applies to all substances being considered for risk assessment and, if required, subsequent risk management. However, a key issue for EDCs is that there can be substantial baseline variability, both in hormonal "set points" as well as in the functional reserve for homeostatic control. For instance, at any given point in time, a fraction of the population may already be "outside the expected range" (for example, due to impacts from multiple stressors) and may be at higher risk of being impacted when exposures occur. Another example is the developing organism, which may have much less compensatory ability compared to an adult. Thus, environmental exposures of early life stages to EDCs may have the effect of pushing more individuals across a threshold of adversity compared to adults (for example, Woodruff et al., 2008). The NAS (2017) low-dose report noted that current toxicity-testing methods used to identify hazard may substantially underestimate exposures at which adverse effects occur due to variability, susceptibility, exposure to multiple stressors, and so forth, which typify "natural" human populations. Safety or uncertainty factors have been used in risk assessments to account for uncertainties. The SC questioned whether additional safety factors/uncertainty factors were needed to fully account for the range of factors discussed. The NAS expert panel report also recommended a strategy to "facilitate more regular consideration of the adequacy of toxicity testing" (2017, pp. 29-34), but noted that questions regarding the amount of evidence needed to evaluate potential changes to existing test methods (for example, establishing the sensitivity, specificity, and reproducibility of new endpoints) could be substantial and concluded that "these questions might be more appropriately addressed through policy decisions."
The following additional issues and questions were raised:
- How are vulnerability and susceptibility best defined, since risk assessments should be protective of individuals, populations, and species?
- Can computational toxicology approaches (in silico) be used to identify sentinels that are defined as more sensitive/susceptible individuals or populations?
- Can biological variability be defined based on the genomic background of a population that can then be compared with primary cells or tissues (in vitro assay) to define the relevant biologic space and to identify sentinels based on genomic background? (An example is the possibility of using lung tissue to predict gut response.)
- How can phenotypic variability be considered? The case was presented for the need to consider "current background" levels of exposure, particularly in areas where ecological receptors may have acclimated to pollutant levels. When testing for low-dose "EDC effects," the response to a toxicant or toxicant mixture could vary, depending on whether ecological test subjects (or a population) have been acclimated to a substance or not.
- In an effort to consider environmentally relevant exposures, test conditions could be improved from the current practice. Here, examples include coming closer to biologically plausible doses/concentrations that better model human and ecological environmental exposures, using more relevant test species, and considering across specific life stages.
- It was suggested that induced pluripotent stem cell (iPSC) models provide a means of evaluating genetic susceptibility, and that case studies demonstrating the relationship across and within in vitro and in vivo models could increase confidence in predicting safe levels of exposure for sensitive populations.
1.1.3 Cumulative risk due to mixtures, co-exposures, and non-chemical stressors
Cumulative risk presents a similar set of issues as those discussed above for population variability. The U.S. EPA report, "Guidance on Cumulative Risk Assessment of Pesticide Chemicals that Have a Common Mechanism of Toxicity" (2002) discussed the use of relative potency when evaluating cumulative exposure to substances acting via a common mechanism of action, which may have applicability for certain classes of EDCs. Chemical mixtures have traditionally posed challenges for risk assessment due to the difficulty in characterizing mixed exposures and designing studies that can adequately address exposure scenarios, potential outcomes, and the various possible dose-response curves. Accounting for possible complex dose-response curves and defining the nature of chemical interaction (for example, additive versus synergistic versus antagonistic) necessitates the use of complex study designs and for in vivo studies that require very large numbers of animals. In vitro studies hold some potential for characterizing mixture toxicity (for example, Abdo et al. 2015b; Neal et al. 2015). However, analytical challenges remain in characterizing mixtures and additional challenges will be encountered in translating in vitro test conditions to in vivo exposures [in vitro to in vivo extrapolation (IVIVE)]. While high-throughput IVIVE approaches have demonstrated utility in translating in vitro bioactivity to in vivo points of departure, particularly for human health risk assessment, these efforts have largely focused on individual substances.
Recently, Zeise and colleagues (2013) included co-exposures, mixtures, and non-chemical stressors as components of variability. The interconnectedness of the pathways of most biological systems, including endocrine, means that there are many potential areas of interaction across multiple chemicals and other stressors. The potential areas of interaction with physical, genetic, and social risk factors are not unique to mixtures or to individual EDCs, but nevertheless are particularly important here. Addressing non-chemical stressors in both in vitro and in vivo laboratory test systems remains a challenge.
As noted below, in vitro studies are currently limited in their ability to capture multiple interconnected pathways, though the development of organotypic models promises to improve these capabilities. With respect to non-chemical stressors or other factors affecting endocrine homeostasis, it is well known that the endocrine system responds to a variety of non-chemical stimuli, including generalized stress and anxiety, and seasonality. Examples include effects of stress on thyroid hormone levels in humans and seasonal changes (for example, temperature, day lengths) on reproductive signalling in many animals (for example, Helmreich and Tylee, 2011). However, further research is needed to incorporate these factors into risk assessment. Recognizing that the development of disease is a multifactorial process, this knowledge could be applied to improve EDC risk assessment as research advances to elucidate the relative contributions of physical, chemical, social, and genetic determinants of disease. In the interim, approaches that rely on the use of uncertainty factors to account for uncertainties in risk assessments, including exposures to mixtures, should be applied based on appropriate scientific justification, and more research is warranted to evaluate the sufficiency of these factors.
1.2 The science committee's scope and approach to Charge Question 1
The SC discussed the context necessitated by the definitions of "EDC" and "potential EDC." The SC noted that the definition of EDC requires establishing both that a substance "causes adverse health effects" and that it "alters function(s) of the endocrine system." The SC concluded that from the point of view of "current priority-setting and assessment practices," establishing that a substance "causes adverse health effects" is sufficient in and of itself for justifying prioritization and early assessment. Specifically, the SC felt that having established an adverse health effect, it was not necessary to establish that the mechanism is through alterations of the endocrine system. As a consequence, the SC focused more on the issue of identifying potential EDCs, particularly for "data-poor" substances that increasingly constitute most of the effort within the CMP.
Taking into consideration the current approach in Canada for addressing EDCs under the CMP and incorporating potential enhancements discussed by the SC, Figure 1 illustrates the major steps that could be taken to better address EDCs from priority-setting through assessment. This includes, in a tiered approach, the integration of high-throughput screening (HTS) assays, evaluation of exposure and toxicokinetics (TK), use of bioactivity-to-exposure ratios (BERs), and then, finally, in vivo testing. Note the phrase "Action Required" means the assignment of either a higher priority for an assessment or the actual conduct of one, depending on the specific outcome.
Following the order depicted in Figure 1 can guide prioritization, subsequent assessment, and potential protection, particularly if the risk assessor can access or generate the different elements depicted. Another enhancement raised by some members of the SC, consistent with the recommendations of Thomas and colleagues (2013), would be to integrate exposure at each decision node when bioactivity is detected. Including exposure would permit the use of BERs to inform the need for further testing. In cases of significant uncertainty in the "non-in vivo" boxes, the absence of a robust in vivo dataset may lead to the need for animal testing. This option is critically important in identifying and regulating potential EDCs, because it is currently where adversity can be best confirmed. Having noted this, the approach outlined is pragmatic in terms of identifying possible EDCs and confirming concerns. In summary, the absence of endocrine-disrupting properties can often be strongly indicated by current and rapidly maturing non-in vivo approaches, but in some instances should be better confirmed by in vivo testing. Thus, "Action Required" in Figure 1 indicates that more in-depth consideration be given to the results to inform specific decision contexts (for example, priority setting, hazard evaluation, or risk assessment).
Figure 1. Conceptual outlineFootnote 1
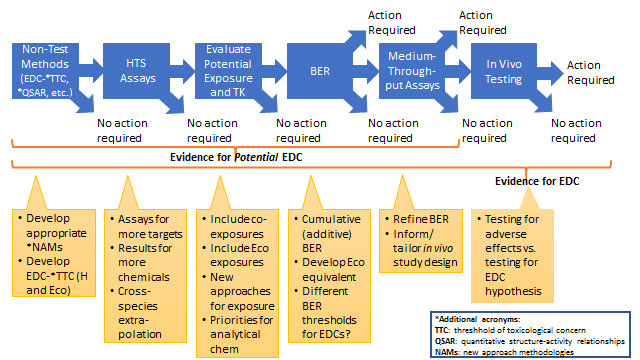
Figure 1 Text Description
The diagram shows a conceptual strategy that illustrates the major steps that could be taken to better address EDCs under the CMP from priority-setting through assessment. The diagram is a series of steps that moves from left to right, in a tiered manner, showing the integration of:
- non-test methods [for example, the thresholds of toxicological (TTC) and the quantitative structure-activity relationship (QSAR)],
- high-throughput screening (HTS) assays,
- evaluation of exposure and toxicokinetics (TK),
- use of bioactivity-to-exposure ratios (BERs),
- medium throughput assays, and
- in vivo testing
At each step there are arrows illustrating decisions for "action required", "no action required" or to proceed to the next step. In this context, "action required" means the assignment of either a higher priority for an assessment or the actual conduct of one, depending on the specific outcome. The diagram also illustrates the information from each of the steps that can provide support that a substance is a potential EDC (that is, QSARs, HT screening assays, and medium through-put assays) or an EDC (that is, previous steps with the addition of in vivo animal testing). Finally, the diagram includes suggestions or questions pertaining to each step that require further exploration or development which are expanded upon in detail in the main body text.
- Footnote 1
-
This is a conceptual outline, including potential enhancements to current practices as they may apply to EDCs and potential EDCs (see text for details and explanations of acronyms.). "Non-Test Methods" refers to in silico approaches. "H" refers to human and "Eco" refers to ecological receptors.
Therefore, in response to Charge Question 1, the SC's deliberations and recommendations focused on how to enhance these practices for the evaluation of potential EDCs. The SC noted that the overall conceptual workflow illustrated in Figure 1 can enhance the current approaches as described in the excerpt section prepared by the departments through the incorporation of additional steps as new approach methodologies (NAMs) continue to mature. NAMs have recently been defined as "any technology, methodology, approach, or combination thereof that can be used to provide information on chemical hazard and risk assessment that avoids the use of intact animals" (EPA, 2018). However, the term has also been considered to be synonymous with alternative test methods and strategies to reduce, refine, or replace vertebrate animals. Within the context of the CMP, a broader definition, which includes but is not limited to non-animal tests, is being applied. Consistent with this approach, NAMs are being used in the international risk assessment and research community to broadly describe approaches that make use of in silico methods and in chemico and/or in vitro assays for the purposes of chemical hazard characterization and risk assessment. The SC's suggestions are illustrated in Figure 1, and are discussed in more detail below. The SC's recommended enhancements are organized first with respect to the steps in the overall conceptual level, and then within each step the considerations identified above with respect to potential EDCs and EDCs are addressed as appropriate. Finally, the SC commented on several cross-cutting issues. Details of approaches and methods discussed here are expanded upon under Charge Question 2.
1.3 "Non-Test" Methods
The Non-Test (or in silico) Methods box in Figure 1 represents the use of predictive modelling methods that do not involve the collection of additional empirical data using in vitro or in vivo methods. However, these non-test methods rely heavily on previously collected empirical data for model development and evaluation, and they are likely to be continually improved as new data are made available. The relationship between test and non-test methods should be iterative, where new data are used to improve model predictions, and model evaluation can help guide targeted testing strategies. Caution is needed to ensure that non-test methods are used within their domain of applicability.
Considerable progress has occurred in the development and use of in silico modelling of the potential hazards and exposure for chemicals. Commonly used methods for predicting relative bioactivity include quantitative structure-activity relationship (QSAR) models, read-across (Patlewicz et al., 2017), and thresholds of toxicological concern (TTCs), which rely on chemical structure to predict potential hazard and/or relative potency. The TTC (WHO/EFSA, 2016b; Patlewicz et al., 2018) and QSAR-based "conditional toxicity values" (CTVs) (Wignall et al., 2018) have been used for rapid prioritization or screening for human health, including by the departments within CMP (Canada, 2016). The TTC is a defined exposure value for chemicals, below which no appreciable risk is expected based on a de minimus value for toxicity (Belanger et al., 2015; Hartung, 2017; Hennes, 2012; Kroes et al., 2004). The development of similar approaches, specifically in the context of EDCs (such as an EDC-TTC and EDC-CTV), in addition to an expansion to address ecological health, could provide substantial enhancements to the ability of current practices to identify potential EDCs. More details are provided under Charge Question 2.
Models for rapid exposure estimation and biokinetics models have also been evolving into useful tools for the early prioritization of substances, from in silico exposure models (Biryol et al., 2017; Wambaugh et al., 2014) to high-throughput TK models (Pearce et al., 2017) and high-throughput IVIVE models (Bell et al., 2018; Wambaugh et al., 2018).
Currently available in silico models for hazard and exposure have merits and limitations (Cohen Hubal et al., 2018). Regardless, there are opportunities to apply in silico methods prior to in vitro high-throughput hazard or exposure screening. For example, the U.S. EPA's Endocrine Disruption Screening Program (EDSP) considered in silico exposure modelling (ExpoCast) for hundreds of substances, which was then combined with bioactivity data (ToxCast) to produce a high-throughput risk-based prioritization and screening for human health (U.S. EPA, 2014; Wambaugh et al. 2014). More recently, QSAR models have been used to predict estrogenic and androgenic activity for thousands of substances (Mansouri et al., 2016; Trisciuzzi et al., 2017).
Exposure model results present opportunities for improving inputs to priority-setting and assessments (Becker et al., 2015). Using environmental levels of exposure, a similar BER approach has been used to prioritize EDC chemicals and specific sites for ecological receptors (Blackwell et al., 2017).
1.4 Improving the utility of high-throughput screening assays
The second box in Figure 1 represents high-throughput assays for exposure, kinetics, and bioactivity that can be conducted in a time-cost efficient manner relative to in vivo assays. While currently available high-throughput hazard and exposure screening methods have merits and limitations (Cohen Hubal et al., 2018; Coussens et al., 2018; Thorne, Auld, and Inglese, 2010) they can be useful in EDC risk assessments. Examples of recent methods/models include the ToxCast/Tox21 bioactivity assays (Attene-Ramos et al., 2013; Kavlock et al., 2012) and in vitro measurement of pharmacokinetic (PK) parameters (Nicolas et al., 2018).
The SC noted that considerable progress has been made in the last decade in the HTS of chemicals in the U.S. EPA's ToxCast and Tox21 programs (Dix, 2010); however, data are still limited. The SC also noted that data are not yet available across the entire chemical space and biological space, and filling such gaps inevitably involves substantial "investment" in relevant in vivo studies to provide additional read-across/extrapolation ability. Such an investment comes with significant associated impacts in terms of animal usage and financial cost. To expand testing across the chemical space (for example, to cover the totality of chemicals and substances under consideration), testing needs to include substances of high volatility, substances with low solubility in water and/or in dimethyl sulfoxide, and environmental degradation products (Richard et al., 2016). In addition, due to the lack of metabolic competence of most in vitro assays, more testing needs to be conducted on environmentally relevant metabolites, which may have increased or decreased endocrine disrupting (ED) bioactivity compared to the parent substance. For example, certain prototypical EDCs are inactive as the parent substance, but become active after in vivo metabolism. Similarly, some prototypical substances that can interact with hormone receptors in vitro are inactive in vivo because they are rapidly metabolized to structures that can no longer bind to the receptor. Recent work has begun to address this issue by retrofitting in vitro assays with metabolic competence (for example, DeGroot et al., 2018; Yu et al., 2018). There is also a concern for the lack of coverage of potential EDCs not included in the current EATS testing efforts (that is, non-EATS; see below).
Some key challenges and issues that are cross-cutting with other levels of testing are as follows:
- EATS and non-EATS. EDC-related targets that have greater coverage include estrogen, androgen, and steroidogenesis pathways (Browne et al., 2015; Haggard et al., 2018; Kleinstreuer et al., 2018). In addition, there has been progress on a number of high-throughput assays for molecular-initiating events (MIEs) that may predict disruptions in thyroid homeostasis. These include thyroperoxidase (Paul et al., 2014), deiodinases (Hornung et al., 2018), the sodium-iodide symporter (Wang et al., 2018), thyroid receptor transactivation (K Paul-Friedman, personal communication, January 2, 2019), and the thyroid-stimulating hormone receptor and thyrotropin-releasing hormone receptor (U.S. EPA's Toxicity Forecaster [ToxCast]/U.S. Department of Health and Human Services' Toxicology in the 21st Century [Tox 21]). Technologies exist for many other thyroid MIEs, but presently some MIEs either lack in vitro assays or the assays have not been used to screen large numbers of environmental chemicals [for example, cellular transporters (Dong and Wade, 2017); serum-binding proteins (Marchesini et al., 2008)]. The identification and testing of non-EATS pathways remain a significant challenge. High-throughput transcriptomics, in conjunction with fast-improving bioinformatics tools, present a promising path forward for rapid hazard identification and prioritization based on bioactivity for EDCs, in particular non-EATS, as well as non-EDCs due to the broad biological coverage and decreasing cost of high throughput sequencing methods. This is expanded on under Charge Question 2.
- Population variability is being addressed in some emerging high-throughput approaches. These include the use of populations of up to 1,000 human lymphoblasts derived from diverse donors to simulate a broad "population" (Abdo et al., 2015a,b; Chiu, Wright, and Rusyn, 2017), and more recently iPSC-derived cardiomyocytes (Grimm et al., 2018). None of these approaches are specific to ED-related effects, but the potential exists in the future for using iPSC-based technologies. An additional possibility raised by the SC was the identification and development of "sentinels" that represent more sensitive/susceptible individuals, as noted above.
- Cross-species extrapolation, particularly from human to ecological health and vice versa, is currently possible in principle but has not been examined in any great detail, particularly with respect to quantitative bioactivity measures. Such extrapolations are likely to have large uncertainties, which should be quantifiable through appropriate analyses (Hecker, 2018). On the other hand, it is generally accepted that allometric scaling is appropriate for in vivo oral doses across mammalian species, with residual uncertainties that have been characterized quantitatively (for example, Bokkers and Slob, 2007). Less work has been done for in vitro test concentrations, but similar approaches could be attempted in this space across a broader range of species. Also, there are few examples of in vitro approaches that have successfully been used to predict apical responses across species based on receptor activation, such as the aryl hydrocarbon receptor (Doering et al., 2018). Another potential starting point is the U.S. EPA's Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS) tool (LaLone et al., 2016). SeqAPASS evaluates the similarities of amino acid sequences and protein structure to estimate the degree of homology for genes across species (LaLone et al., 2013). Some limitations of this approach are that (1) uncertainty is characterized only qualitatively, and (2) it requires both molecular targets and genome sequence information as inputs. Nonetheless, it represents a promising approach that can be used, with recognized uncertainties, when no other cross-species models or empirical data are available.
- Cumulative risk, mixtures, and co-exposures. In vitro assays could show promise in assessing the hazards of chemical mixtures. These have involved "designed" mixtures (that is, deliberately created for testing purposes) (Meek et al., 2011), mixtures derived from environmental samples (Neale et al., 2015), and commercial product mixtures (Grimm et al., 2016). The incorporation of non-chemical stressors into a cumulative risk assessment, however, has not been well investigated.
- Windows of susceptibility, multigenerational effects, and epigenetics are several challenging areas for which the SC knew of little or no high-throughput approaches in development that could inform these issues for EDCs.
1.5 Expanding the evaluation of exposure and toxic kinetics
The SC noted that there have been several recent efforts to improve exposure assessment, of which the CMP could take advantage in the context of EDCs. For existing substances, expanded biomonitoring and environmental monitoring provide a plethora of potential new data sources to estimate exposures and apply exposure models. New measurement methods are emerging to characterize human exposure, such as silicone passive samplers (for example, wrist bands) (Hammel et al. 2016, Okeme et al. 2018), although at this point they are used for qualitative and not for quantitative exposure assessment. Passive air samplers have also been developed for birds (Sorais et al., 2017). In the future, these efforts could be applied to estimate the exposure to mixtures of targeted substances to estimate co-exposures. Additionally, there is growing interest in the use of non-target chemical analyses to screen environmental samples, which can also contribute to the understanding of co-exposures and mixtures (for example, Moschet et al., 2018; Rager et al., 2016). Furthermore, reverse toxicokinetics based on IVIVE have become a "routine" part of the chemical assessment workflow for individual chemicals, and have been limited by analytical chemistry methods. New exposure models based on limited empirical or predicted kinetic parameters have been used for extrapolation of in vitro bioactivity data to daily human exposure dose, with uncertainty bounds, for thousands of chemicals (Wambaugh et al., 2018) and more specifically for EDCs [estrogen receptor (Casey et al., 2018); glucocorticoid receptor (Hartman et al., 2018)]. Further, computational approaches have proven useful for evaluating population susceptibility due to pharmacokinetic variability (Ring et al., 2017). Notably, approaches that use physiologically based pharmacokinetic modelling or related dosimetry methods to interpret human biomonitoring data in a risk context, both for EDC and non-EDC substances, have been used in the Canadian Health Measures Survey (Haines et al., 2017; St-Amand et al., 2014) and in the U.S. National Health and Nutrition Examination Survey (NHANES) initiative (Aylward et al., 2013).
For further improvements in this area, the SC has several suggestions for possible approaches:
- Continue to support fate and exposure modelling. Near-field models would be of particular interest for EDCs due to their general higher spatial and temporal resolution, which may better inform exposures during specific windows of susceptibility. Of high importance is estimating and predicting fetal or early life-stage exposure.
- Explore the ability of QSAR and other high-throughput methods to predict the biological activity of metabolites, degradation productions, and their associated pathways.
- Combine exposure data with QSAR and/or high-throughput bioactivity data to help prioritize future analytical chemistry method developments in terms of compound selection and analytical sensitivity.
- Work towards a better understanding of exposure variability, both for individual substances and for co-exposures and mixtures.
- Continue to support the development of predictive high-throughput kinetics and exposure models.
Additional emphasis on TK is also needed in the context of medium-throughput and in vivo studies, as discussed below.
1.6 Extending the use of bioactivity-exposure ratios
The fourth box in Figure 1 represents the use of the BER estimates for human risk assessment from in vitro testing. This approach is being increasingly used as an important screening and prioritization metric for data-poor chemicals. In this arena, BERs are calculated as the difference between a predicted oral-equivalent dose from in vitro bioactivity data and the predicted exposure estimates (Sipes et al., 2017; Wetmore et al., 2015). As noted, although Figure 1 depicts the use of the BER following high-throughput screening, the BER approach can be used at each node where bioactivity is estimated or measured, including non-test methods such as the TTC and medium-throughput assays. The SC reviewed several potential enhancements in the use of BERs for EDCs:
- The SC discussed the possibility of requiring a larger BER for a "no-action" decision for EDC-related targets compared to other types of bioactivity due to special concerns related to the 9 key considerations listed above (see bulleted points 1.1 General Comments on Key Considerations). Some members supported this idea, while others considered that a case-by-case approach was preferable. Ultimately, the SC recognized that this is a risk management policy rather than a scientific decision. However, it could be informed by retrospective analyses comparing bioactivity PODs with in vivo PODs for EDCs.
- The SC supported the development of an ecological risk equivalent to the BER that included the consideration of TK in ecological receptors, and would be based on the according relevant exposure route (for example, dietary vs. immersion/contact). Such an approach could build from Blackwell et al. (2017).
- The SC discussed supporting research on developing a cumulative-risk version of a BER to address co-exposures to multiple potential EDCs. The SC noted that such a cumulative risk "research project" could consider both substances with varying potencies that act via the same mode of action, and substances that act by different modes of action but converge at the same adverse outcome or an earlier key event (KE).
- The SC supported the refinement of the BER via testing in more integrated systems, such as those that are emerging as "medium-throughput" or whole-organism assays, and which are discussed next.
1.7 Use of medium-throughput assays prior to in vivo testing
A number of emerging "medium-throughput" assays show substantial promise in providing both qualitative as well as quantitative information related to EDCs (for example, Hartman et al., 2018; Miller et al., 2016). Specifically, the aim of these assays would be to provide a more integrated system to model ED rather than to only address individual molecular targets, which may not provide an accurate characterization of the overall dose response due to the presence of multiple interconnected pathways (Miller et al., 2017; Yoon et al., 2016). Potential approaches include the following:
- The use of non-traditional animal models for human health and ecological assessment (for example, zebrafish and Daphnia). These assays are discussed further under Charge Question 2.
- The identification and development of appropriate sentinels to address both population and sensitivity (for example, phenotypic) variabilities. One idea is to first map the "space" of biological variability across individuals and species; for instance, via gene-expression mapping using cell lines and primary cells/tissues from different sources. From this "space," one could test the variability in sensitivity across the range of variation and identify "extremes" in the distribution that could serve as sentinels.
- Better modelling of dosimetry for IVIVE, which will be an important aspect for all new models. Given the concern for developmental and reproductive toxicity of EDCs, traditional plasma concentration-based IVIVE may reflect maternal dosimetry but may not necessarily translate into tissue (or embryo) dosimetry. Explicit consideration of bioactivity in both maternal and embryo/fetal exposures should be explored.
As they mature, medium-throughput assays are first likely to provide more confidence in identifying potential EDCs. Thus, the results of these assays may be useful for refining and informing more targeted in vivo testing. In the future (likely in combination with computational models), such medium-throughput assays may be able to confidently predict adverse effects and thereby provide evidence to identify EDCs in the absence of in vivo tests.
1.8 Refining in vivo testing
Due to the complex interconnectedness of endocrine-related pathways and toxicity, the SC does not envision that a full-scale replacement of in vivo testing will be feasible for the foreseeable future. Therefore, in vivo testing will still be needed. However, the SC envisions that in vitro and in silico approaches will not only be useful for prioritization and screening, but also provide key data by which in vivo testing could be refined and prioritized in order to better address ED-related effects and to reduce the use of animals when possible. While the use of in vitro assays in prioritization and screening is being implemented now (for example, Browne et al., 2015), ultimately, the SC anticipates that the combination of in silico methods, high throughput testing, and medium-throughput testing will provide sufficient information to generate specific hypotheses to focus in vivo testing for adverse health effects (human or ecological). The design of the in vivo studies should then be adapted to the hypothesized adverse health effects, such as the following:
- Design features for in vivo tests may become better tailored based on the adverse health effects suggested by bioactivity and medium-throughput assays in terms of:
- windows of susceptibility
- the use of appropriate positive and negative controls
- The use of more dose groups with fewer replicates per dose, in combination with benchmark-dose modelling, may become a more efficient in vivo study design than traditional (guideline) designs, particularly if the aim is to better characterize the dose-response relationship (especially at low environmentally relevant dose ranges for EDCs). Moreover, dose selection can be substantially informed by:
- exposure estimates
- target levels of internal dose based on bioactivity and medium-throughput assays results, in combination with reverse toxicokinetics;
- greater detail of the biotransformation of the test substance (if known), whether with respect to bioactivation or detoxification.
The "fewer replicates" suggestion, however, has its limitations. Reducing the number of litters may not prove to be justified from a research perspective because in developmental and reproductive toxicity studies, the litter is the experimental unit (Festing, 2006). Moreover, from a statistical power viewpoint, Elswick, Welsch, and Janszen (2000) noted that highly variable endpoints may not be amenable to study designs with lower numbers of test animals because of normal biological intra-litter variability.
Additionally, the SC noted the need for retrospective case studies to better understand the "value of information" of in vivo testing. Points that are particularly germane to EDCs could include the following:
- The value of adding a two-generation study (or an extended one-generation study) in terms of level of protectiveness of the POD compared to in vitro or shorter-term (for example, acute, short-term, or repeat-dose) in vivo studies.
- The use of precursor effects (for example, hormone changes) that are traditionally not considered "adverse" at the individual level to protect against downstream adverse events, which could be important to discern multigenerational and population-level effects.
- The comparison of the applied equivalent doses derived from lower-tiered NAMs to PODs from higher-tiered in vivo multigenerational effect studies to determine if NAMs are protective of health.
Charge Question 2
A) Identify assays and methods, including NAMs, which could:
- supplement the current information requirements under the NSNR
- inform assessments carried out for existing substances
- be of utility to address data needs for screening and evaluation of the ED potential of a substance.
B) How should data on perturbations/modulations in the absence of apical adverse effects be considered for:
- priority setting
- regulatory decision-making
2.1 General Themes
In identifying assays and methods that could be used to evaluate new and existing substances, the SC discussed short-, medium-, and longer-term needs for the CMP. The SC did not comment on who would generate data, for example, government, industry, or another source. Immediate needs were discussed relative to currently available approaches and methods that could be readily used or applied. Data gaps, research, and methods development were identified for the medium- and longer-term needs.
Fit-for-purpose considerations and acceptable degree of uncertainty underpinned most of the discussions of this Charge Question. The SC stressed that a clear statement of the regulatory context/need and necessary level of confidence should guide the selection of appropriate methods and data requests.
Throughout the discussions, the SC recognized that because estrogens and androgens act via receptor-mediated biological pathways, the shift towards more mechanistic/mode of action-based toxicology has allowed for EDCs to lead the way in transitioning from traditional approach methodologies (TAMs) to NAMs. This is best illustrated with the use of the mode of action (MOA) framework (Boobis et al., 2006; 2008) and the adverse outcome pathway (AOP) framework (Ankley et al., 2010). These frameworks have led to increased understanding of the sequence of KEs in the pathways involved that result in observed apical effects. Further, the frameworks have guided the development of assays for molecular initiating and other KEs upstream from apical endpoints for EATS and non-EATS pathways.
Both in vivo TAMs and NAMs can be used to address the needs for screening and testing of EDCs. It is important to stress that many TAMs do capture EDC (EATS and many non-EATS) endpoints (Manibusan and Touart, 2016). In addition, adding satellite groups for specific use for interim evaluations during the course of an investigation of hormone levels or other endocrine pathway endpoints to TAMs can provide additional information to further inform EDC chemical MOAs without extensive additional tests. As with any method, an understanding and description of the applicability domain is critical. Priority for inclusion should be given to methods that can capture several pathways or endpoints for NAMs and TAMs.
Data availability, access, and communication are critical to ensure the efficient use of limited resources. As methods are developed and data are generated using TAMs and NAMs, efforts must be made to make this information available in an accessible format across the government and internationally. Here, examples include the U.S. EPA's ToxRef, ToxCast, and Tox21 databases; the U.S. EPA's CompTox Dashboard; and the European Chemicals Agency's open databases. Continued enhancement of data and decision outcomes will foster the development of additional NAMs, including QSARs and other non-testing approaches, and facilitate the expansion of the chemical space tested using new methods.
Many of the NAMs and TAMs currently focused on identifying potential EDCs pertain to the EATS pathways. As noted previously, additional work is needed to prioritize method development for the non-EATS pathways. The SC was unable to recommend the implementation of specific non-EATS assays and methods due to the limited availability of well-validated assays; a further discussion on this point is given in section 2.3.2.
2.2 Short-Term Recommendations
2.2.1 Exposure
Understanding exposure is critical to apply a risk-based approach to evaluate EDCs. As noted under Charge Question 1, the needs related to exposure involve better estimates of external (environmental) exposure concentrations that result from the production and release of the chemicals, as well as a better understanding of concentrations within the test system (as opposed to nominal concentrations, which are not necessarily reflective of actual concentrations).
Many of the in vitro and in vivo studies developed to date do not appropriately address TK, and therefore IVIVE extrapolation across species (rodents to humans), and from laboratory to real-world exposures is uncertain (see also Charge Question 1). Reducing the uncertainties in TK estimates and IVIVE will be necessary to remove the reliance on in vivo studies to derive a POD for use in assessments. Understanding dose in the test system is needed to utilize in vitro methods for regulatory decision-making.
For the NS Program, detailed toxicity testing for EDC endpoints would only be requested when a potential risk for ED has been identified through an initial risk assessment, if not previously received. The risk assessment is based on information provided in a notification package, which is submitted by an importer or manufacturer prior to exceeding an annual import or manufacture volume threshold (100-10,000 kg/yr) for a substance in "neat" form or within a finished formulated product (not including manufactured items). It is noted that low-volume chemicals that would be of concern due to high biological activity and potency may fall below the notification thresholds (for example, minor components of personal care products). In addition, the volume thresholds are not always by themselves reliable indicators of human exposure. The regulations do not specifically require a test for ED, but ED will be investigated if there is some indication of ED activity identified; notably, if there is no measure of ED specifically in the notification requirements. As a minimum, if there is uncertainty, a flag will be noted for future assessments.
If a potential ED issue is identified, then control measures may be developed. As a control measure, a confirmatory endocrine test may be requested in a published SNAc notice when the substance is used for a new activity defined by the NS Program. Alternatively, a substance may be prohibited from import or manufacture until a confirmatory endocrine test is provided. Conditions on the import or manufacture of a substance, and prohibition, may also be imposed when toxicity is confirmed. The SC commented that multiple lines of evidence should be considered for required toxicity testing. In particular, some SC members suggested that exposure estimates should be obtained through monitoring or modelling (such as exposure models or QSARs), or a combination of approaches. The long-term approach to triggering testing requirements should evolve from annual volumes to more holistic and relevant estimates of exposure and hence potential risk of effects specific to the use of EDCs. The SC understands that this evolution is underway but requires more effort.
2.2.2 Targeted data generation
As noted in section 1.4, many of the methods developed to evaluate EDC endpoints have been tested, evaluated, and validated for a variety of chemicals in high-throughput programs (for example, ToxCast, Tox21; Dix, 2010). The EDC bioactivity models have performed well for estrogen and androgen pathways, typically producing balanced accuracy results of 0.8 to close to 1.0 relative to the U.S. EPA EDSP's Tier 1 assays (Browne et al., 2015; Cox et al., 2014; Kleinstreuer et al., 2017; Rotroff et al., 2013) Initially, prediction models for steroidogenesis had low accuracy (on the order of 0.5), but newer assays have improved the prediction modelling, yielding balanced accuracies that ranged from 0.75 to 0.9 relative to the OECD-validated low-throughput H295R assay (Haggard et al., 2018).
In prioritizing additional testing needs to further expand the applicability domain, the focus should be given to specific data gaps, as discussed in section 1.4. Importantly, any additional data that are generated should be made available to help refine and improve in silico methods as discussed in section 1.3.
2.2.3 Utilize the AOP framework to organize EDC assays and information, and identify gaps
AOPs provide an organizational framework for the methods that may be used or developed to help evaluate EDCs. Using this framework to organize information can help to provide context on data and method development needs. Browne and colleagues (2017) provide an example of how EDC test methods can be aligned using the AOP framework-examples of relevant pathways and available test methods at the various levels of biological organization are presented (for example, MIE à organism or population adverse outcome for both human health and ecological receptors). The SC noted that while there are a number of endocrine AOPs under development, very few of these AOPs have been endorsed by the OECD to date (OECD, 2018). Clearly, more work is required and should be done to expand this list.
This general framework can also be used to compare information on known chemicals (either positive or negative in EDC tests) with available information on a new chemical for which only limited information may be available. This may facilitate a "read-across" type of strategy and identify where specific, targeted testing would be of highest value (Figure 2).
Figure 2. Alignment of available EDC methodologies with the AOP framework
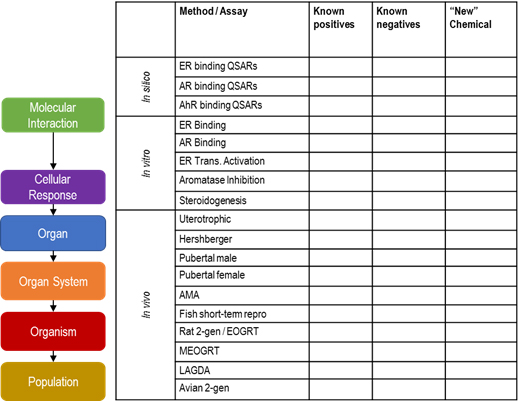
Figure 2 Text Description
The diagram shows the alignment of the levels of biological organization that make up an adverse outcome pathway (AOP) to a table containing a selection of various tools and assays that are available for testing EDCs. Receptor binding QSARs and in vitro assays are aligned with the molecular initiating event of the AOP while other in vitro assays that measure events such as transcriptional activation are aligned with the cellular response level within the AOP. In vivo tests, such as the uterotrophic assay, are aligned with the organ response while tests such as the generation reproduction study are aligned with the organism response level. Finally, the diagram shows that the assay table includes columns where the results for known positives, known negatives and "new" chemicals can be entered. Limited information on the new chemical when the table is complete can be used to identify gaps and testing needs or information on the known chemicals can be used for read-across.
The table may be used to facilitate a "read-across" type of strategy when evaluating a new chemical for which little information is available. For each assay, chemicals that are known positives or negatives can be listed, and the limited information on the new chemical shown in the last column can be used to identify gaps and testing needs. (Adapted from Browne et al., 2017; and Borgert et al., 2011.)
2.2.4 Exploration of an "EDC-TTC"
As mentioned under Charge Question 1, a TTC approach could be used to prioritize chemicals for evaluation of potential ED risk. For human health applications, the TTC is based on the lower 5% of the distribution of rodent "no observable adverse effect level" for chemicals with similar structural features (Kroes et al., 2004). The TTC is derived by applying the standardly used 100-fold uncertainty factor (10× inter- and 10× intra-species), noting uncertainties in these factors (see previous comments about the application of "uncertainty"; for example, in sections 1.1.2 and 1.1.3). For the ecological context, it has been proposed, but not yet tested, that the values be derived based on the lower 5% distribution of the "predicted no-effect concentration" (Belanger et al., 2015). The TTC can then be compared to an estimate of the likely exposure to a chemical to develop a screening-level assessment for a given route of exposure. A recent state-of-the-science review by the European Food Safety Authority/WHO (WHO/EFSA, 2016b) indicated that the TTC represents a fit-for-purpose approach that has broad applicability as a risk assessment tool. The TTC can be used as a health guidance value when no in vivo data are available, and to calculate a chemical-specific toxicity reference dose or "tolerable daily intake."
Because 1 of the stakeholder concerns regarding EDCs is the potential for exquisite differential sensitivity (that is, the potential for effects at lower doses than non-EDC modes of action), the SC proposed exploring the development of a TTC that is specifically applicable to EDCs, under CMP activities. This development could, perhaps, start with those MOAs that are data rich (for example, estrogen and androgen pathways); in other words, an "EDC-TTC" for both human and ecological assessments. If feasible, this could be used as a health protective screening value for chemicals where EDC toxicity data are lacking, and used for prioritization purposes. Foundational work to this effect has been presented by Kroes and colleagues (2000) for a TTC relevant for estrogenic disruption, by Borgert, Matthews, and Baker (2018) for a human-relevant potency relative to 17β-estradiol, and by Gross and colleagues (2010) for a TTC for endocrine-active substances in the aquatic environment. Data are currently available to begin the development of TTCs for androgen, thyroid, and steroidogenic chemicals.
2.2.5 Biotransformation
Efforts continue to incorporate the consideration of biotransformation when evaluating chemicals (including but not limited to EDCs), as most in vitro test systems are not metabolically competent. Examples include incorporating microspheres containing human hepatic liver homogenates into ToxCast assays (U.S. EPA, 2016), and transfecting the messenger ribonucleic acid (RNA) of human liver cytochrome P450 enzymes into existing HTS cell-based assays (DeGroot et al., 2018.)
As mentioned in section 1.4, a critical consideration is the potential for the parent chemical to be activated to an EDC-active form in the environment, either via biotic or abiotic transformation.
2.2.6 Risk-based framework to assess for EATS pathways
The U.S. EPA's EDSP and the OECD's report, "Conceptual Framework for Testing and Assessment of Endocrine Disruptors" (OECD, 2012) provide comprehensive listings of assays available to evaluate mainly EATS-related EDCs. The tiered manner by which these methods should be used provides a resource-appropriate approach for EDC evaluation. The SC supported the Tier 1 (U.S. EPA)/Levels 1 and 2 (OECD) methods, as reviewed and discussed by Manibusan and Touart (2017), as an appropriate list of available methods to evaluate EATS pathways. However, a more specific weight-of-evidence decision framework should be developed to evaluate the results of these lower-tiered test methods, integrating various streams of information to determine targeted necessary next steps (for example, whether additional data are needed; whether the level of uncertainty resulting from lower-tiered tests is acceptable for decision-making). The SC recognized that asking for a full suite of in vitro and in vivo methods is not feasible, and that efforts should be made to develop a streamlined yet protective approach that uses non-testing approaches, in vitro methods, and consideration of exposure before requiring additional higher-tiered tests.
The U.S. EPA's weight-of-evidence approach to evaluate results of Tier 1 screening (EPA, 2011) and the OECD Guidance Document 150 (OECD, 2018b) may be good starting points, with additional considerations for QSAR and other in silico methods at the initial steps. Exposure information may also be considered at this stage.
The SC did not discuss the details of specific methods or assays that should be routinely requested, nor did they provide a critical evaluation of the individual methods. However, the SC recommended that a critical evaluation of each method be performed to ensure that useful and fit-for-purpose information is obtained.
Considerable scientific advancement has been made in the development of QSAR/in silico models since the development of the tiered testing framework in the EDSP and the tiered OECD Conceptual Framework in the 1990s to early 2000s. Accordingly, as indicated in Figure 1, the use of these types of NAMs is supported by the SC. This approach integrates currently available reliable, predictive, and appropriate QSAR/in silico models (that is, those that meet the OECD QSAR Principles; OECD, 2004) to identify chemicals that are likely to interact with the estrogen receptors (ERs), androgen receptors (ARs), and aryl hydrocarbon receptors (AhRs). These available QSARs have demonstrated an approximate 90% predictivity for the in vitro receptor binding assays (ER, AR, and AhR) and, therefore, running further in vitro screening assays would not add significant new information [OECD QSAR Toolbox v4.2 (OECD, 2017); LMC Oasis TIMES model; ACD Labs Endocrine System Disruption RBA Model] in terms of defining potential bioactivity/MOA. Thyroid QSAR models, limited to thyroid receptor (TR) and thyroperoxidase, are available (DEPA, 2018) but they are not as robust nor well developed as the ER, AR, or AhR QSARs. More information related to these in silico approaches can be found in the following references: Mekenyan et al. (2000), Mekenyan and Serafimova (2009), Petkov et al. (2010), Schmieder et al. (2003), Schmieder et al. (2009), and Serafimova et al. (2007). Additional work is underway to further develop and refine QSAR and consensus modelling approaches [for example, Collaborative Modeling Project for Androgen Receptor Activity (CoMPARA; Mansouri et al., 2017) and Collaborative Estrogen Receptor Activity Prediction Project (CERAPP; Mansouri et al., 2016)]. It is anticipated that this first QSAR step will be expanded to allow for the evaluation of additional pathways in this manner.
If the initial QSAR models indicate that the chemical is positive for ER, AR, or AhR binding (or that additional pathways are indicated by reliable, predictive, and appropriate QSAR models), then more toxicity information could be generated. These follow-up investigations should include targeted assays with the aim of obtaining a dose-response relationship to derive a POD, then use IVIVE to obtain an applied-dose equivalent and integrate this with exposure modelling results to derive a BER. This should be conducted consistent with the Integrated Approaches to Testing and Assessment Framework (OECD, 2016) to ensure that any new data generated for exposure or hazard is performed in a very targeted and focused manner, led by the initial QSAR evaluations. The SC believed that the current QSARs for ER, AR, and AhR are significantly reliable to negate the need for in vitro receptor-binding assays, with the exception of certain chemicals that are outside of the applicability domain of the models, such as chemical substances of unknown or variable composition and polymers. More specific assays and data are needed for thyroid, steroidogenesis, and non-EATS pathways.
If the initial QSAR models do not indicate that the chemical is likely to bind to the ER, AR, or AhR, then targeted Level 1/Tier 1-type assays (for example, in vitro tests) for other potential EATS and non-EATS pathways should be considered. For such ER, AR, or AhR QSAR negatives (depending on other specific factors such as chemical structure, structural alerts, etc.), additional approaches may be considered to address potential concerns for bioactivities in other endocrine pathways or by other MOAs. This could include read-across, using EPA's GenRA, or other suitable methods; in vitro whole transcriptomics; or targeted testing in Level 1/Tier 1-type assays (for example, in vitro tests). As stated above, increased data generation, coupled with analysis and accessibility, will hopefully continue to spark the development, application, and evaluation of additional QSAR and computational approaches, broadening this initial step beyond ERs, ARs, and AhRs, thereby reducing the need to run Level 1/Tier 1-type assays.
As discussed for the workflow presented under Charge Question 1 (Figure 1), focused, higher-tiered data needs should be determined based on an initial evaluation of the acceptable BER and acceptable degree of uncertainty as it pertains to the overall purpose of the assessment (that is, fit for purpose). This risk-based evaluation will then drive targeted data needs. For example, an Integrated Approach to Testing and Assessment could begin at Tier 1 with use of a QSAR model, read-across, or TTC coupled to high-throughput exposure assessment (for example, ExpoCast) to identify substances that have a sufficiently high BER, to allow them to be set aside as "No Action" (Figure 1). Then, substances that require additional hazard evaluation could be screened using targeted high-throughput mechanistic assays (for example, ToxCast assay battery) and then, using a suitable POD, IVIVE, and exposure modelling, refined BERs could be derived. Those substances still warranting closer evaluation could be subjected to a fit-for-purpose cell-based assay (for example, the estrogenicity cell-based assay; Miller et al., 2017) or medium-throughput assay (see section 1.7 and section 2.3) and then again, using a suitable POD and perhaps a refined exposure model, more refined BERs could be derived. If a substance still requires more intense testing, a suitably tailored in vivo animal test could be designed and carried out, as discussed under Charge Question 1.
2.2.7 Case studies
Case studies are needed to evaluate the ability of lower-tiered (QSAR-in silico; Level 1/2; Tier 1) approaches to appropriately identify EDCs (see section 1.8 and section 3). It would be useful to explore the extent to which PODs of lower-tiered results align with PODs of higher-tiered studies to determine if lower-tiered results would be protective, even if they cannot fully predict higher-tiered adverse effects. These case studies would address both parts of the Charge Question in that they would help to evaluate real-world application of these lower-tiered methods (that is, NAMs) to appropriately predict potential apical adverse effects (that is, TAMs) and also help to illustrate the most feasible and useful methods and assays. The SC extensively scoped this suggestion. Case studies should involve data-rich examples where higher-tiered data are available (for example, legacy chemicals, pesticides) and where a retrospective analysis could be performed. Perchlorate was discussed as a potential candidate for thyroid disruption, but additional scoping is needed to identify possible case study substances. Support for conducting case studies is also expressed under the response to Charge Question 3.
The importance of appropriately capturing the diversity of circumstances under which organisms are exposed (for example, heat stress, habitat stress, life stage, cumulative risk, multiple generations, reproductive cycling) was raised in sections 1.1.2 and 1.1.3. The SC commented that investigating these issues using case studies could help to advance knowledge here because the SC had few specific examples of methods that have been used or that are under development.
2.3 Medium - and longer-term recommendations
2.3.1 Embryo assays
Zebrafish embryo assays may represent a promising area to explore in the near term. As noted in section 1.7, embryo-based approaches provide more complex, integrated assays than the current cell-based in vitro methods. Embryo assays would allow for a more "systems biology" type of approach to the evaluation of EDC (and other)-mediated effects. The outcomes of these assays could be applied to human and ecological questions by using toxicogenomics/transcriptomics knowledge. Further, these assays would allow the evaluation of apical endpoints, which is a clear limitation of the existing in silico/in vitro methods that often preclude their application beyond screening and prioritization. The SC noted that it is important to standardize medium- and longer-term assays (such as the zebrafish assay) so that reliable and comparable dose-response information can be obtained from the test(s).
Zebrafish have been studied and explored as a model for both human and ecological health (Bambino and Chu, 2017; Garcia, Noyes, and Tanguay, 2016). Assays using zebrafish embryos to predict developmental toxicity have been adapted in many cases as a "medium-throughput" approach in, for example, ToxCast and the U.S. National Toxicology Program testing. The recent adoption of the fish embryo test by the OECD (see: TG 236; OECD, 2013) has also led to an increase in laboratory capacity to work with this model, likely facilitating easier use and uptake of these methods.
Although well studied, application of the zebrafish embryo model specifically for the identification of EDC chemicals will require additional coordination to develop a road map for the existing methods and approaches. The SC commented that the CMP should promote the development of the zebrafish assay by convening a group of experts that are studying zebrafish in Canada (and elsewhere) to develop this strategic plan.
Regulatory guidelines currently do not exist for the recommended use of zebrafish to detect thyroid disruption. However, some relatively new zebrafish assays are available that hold promise (for example, Jarque et al., 2018; Raldúa, Thienpont, and Babin, 2012), as well as a Xenopus tadpole assay (Mughal, Demeneix, and Fini, 2018). These assays, as well as the frog embryo teratogenesis assay-Xenopus (FETAX) test, should be further explored to address this gap as a parallel to the zebrafish developmental toxicity assay.
In addition to whole-animal models, 3D organotypic models and organ-on-a-chip models provide a promising path forward for more comprehensive in vitro evaluation of EDCs. 3D models, such as the breast cancer spheroid model, allow for the evaluation of tissue-level responses, including morphology and functionality (that is, in vitro pathology; Vantangoli et al., 2015, 2016). Organ-on-a-chip models have been developed for the female reproductive tract; EVATAR™ is a miniaturized 3D representation of the female reproductive tract and liver on a handheld, interconnected platform (Xiao et al., 2017). These assays are progressing towards in vitro replacement of in vivo toxicology models; however, these types of systems are still in development and their implementation in regulatory assessments is hindered by the need for validation in the context of chemical dose response.
2.3.2 Non-EATS pathways
Relatively few test guidelines for mechanistic methods exist to evaluate non-EATS endocrine pathways, although several promising approaches were summarized in the departments' pre-meeting materials, and were further discussed at the meeting (see, in particular, section 1.6. and section 2.2.6.). An example of a promising approach is the evaluation of adipogenesis using alternative models. While no in vivo standard exists for the potential for environmental chemicals to affect adipocyte maturation, several alternative methods have been developed, including mammalian in vitro and zebrafish models (Foley et al., 2017; Hartman et al., 2018; Lyssimachou et al., 2015; Sargis et al., 2010).
To further prioritize needs and target method development, the SC proposes that HC and ECCC convene an expert group to discuss high-priority non-EATS endocrine pathways/targets, driven by public health and ecological concerns. Examples of these non-EATS pathway outcomes include developmental neurotoxicity, obesity, behavioural issues, and diabetes. This expert group should be tasked with identifying the AOPs of highest priority and those KEs in each of the above pathways that should be the highest priority for method development, and evaluating the confidence in the causal linkages of the key event relationships in each pathway in accordance with OECD guidance (OECD, 2018c).
The SC also noted that data will need to be generated on a large scale and in a fit-for-purpose and focused manner once targeted non-EATS methods are developed and validated. Such data generation can be helped by various mechanisms, including research project grant programs and increasing government flexibility for collaboration and data sharing (see Charge Question 3). This data-generation effort is critical to increase the domain of applicability of the methods and to improve/feed into future QSARs and other predictive models.
2.3.3 Evaluation of population-level research
The SC discussed the need to better understand POD considerations around issues such as susceptible populations and multigenerational effects. Here, empirical data could be used to address public concerns regarding the putative connection between chemical exposure and disease states. The SC noted the importance of population-level research but had few specific comments, although the SC explored this concern in Charge Question 3.
Charge Question 3
Where would future government efforts be best placed in the areas of research, risk assessment, and risk management for EDCs?
The SC discussed specific areas for future efforts under Charge Questions 1 and 2. The following response offers "high-level" observations and commentary to address this question.
- The departments should conduct a formal "SWOT" type of review (strengths, weaknesses, opportunities, and threats) of EDC-related issues (summarized as the numerous "considerations" and research directions in the departments' pre-meeting materials) for which they intend to lead, influence, or follow/monitor at the international level. This should be broken up, as per the Charge Question, according to the areas of research, risk assessment, risk management, and risk communication. The SWOT analysis should be performed retrospectively and prospectively over two time frames: the previous 5-10 years and future 5+ years. The outcome of this exercise would help inform the science-based priorities raised elsewhere in this report, and it could also be used to communicate regularly with the public and stakeholders and to confirm that the government is indeed playing an important international leadership role with respect to EDC assessment methods and management.
- Canada should continue to provide international leadership in terms of conducting and coordinating relevant and strategic EDC case studies. The benefit of conducting case studies was raised under Charge Questions 1 and 2. Here, the SC suggested that involvement and leadership in efforts such as "Accelerating the Pace of Chemical Risk Assessment" (APCRA) (Kavlock et al., 2018) are particularly noteworthy. Case studies can also be designed to build internal capacity and networking (for example, issue internal challenges, as elaborated on below). Case studies are invaluable because they provide a formal and systematic process of reviewing and learning from previous efforts and outcomes. Case study types should be driven to address the needs (1) for regulatory purposes (for example, NAMs vs. the "gold standard" of animal studies; how best to address population-wide variability); (2) for scientific research (for example, identified clusters of "EDC-associated genes" that represent key signatures to predict some apical endocrine outcome; methods for considering multiple stressors); (3) for society and stakeholders (for example, new approaches, which are indeed faster, more ethical, and cheaper); and (4) as confirmation that previous risk assessment and risk management decisions made under the CMP have proved to be sufficiently protective.
- While some members of the SC were confident with proceeding with actualizing the toxicity testing in the 21st-century paradigm encapsulated in Figure 1 and discussed in previous sections of this report, other SC members encouraged asking whether a more holistic, public health, and innovative approach to assess EDCs could be designed that gets closer to evaluating population-wide (including vulnerable populations) cumulative exposures and multigenerational effects under "real-world" exposure to multiple stressors. Some within the public have expressed concerns with respect to EDCs, including the assertion that "status quo" approaches using the CEPA 1999 framework may not be sufficient. This concern extends beyond potential EDCs to include broader considerations of physical, chemical, and social determinants of human and ecological health. In the post-2020 time frame, the SC suggests a future evaluation of the entire approach and a review of "lessons" learned from EDC (and other) experiences. This "big and bold" thinking is especially needed to address the challenges faced with EDCs (and other mechanisms of toxicity of concern) from multidisciplinary dimensions, including uncertainties, exposure to chemical mixtures, vulnerable populations, and risk communication. Taking a big and bold approach would enable the evolution from a chemical-by-chemical approach (which has been the focus up to now) towards more disease-based scenarios that are more indicative of real-world exposures. As scientific understanding of the multifactorial nature and pathogenesis of diseases advances, such advances can be used to better understand the totality of genetic, physical, nutritional, chemical, and social determinants of diseases across all life stages to identify risk factors, including exposures to natural and synthetic chemicals. This knowledge can be used to identify how, and the extent to which, these determinants of disease, individually and in combination, act to impact health. On the ecological side, we may learn lessons from environmental effects monitoring programs that target effluents and complex mixtures, and integrate such studies with non-chemical stressors to consider physical, biological, and other co-stressors.
- In a new era of big data, these data must become more open and accessible nationally and internationally. Translating data into user-driven knowledge is a key consideration. Making data open and accessible needs to carefully consider database design and the user experience, and this needs to be done up front. Also, data sharing is motivated by the Government of Canada's Open Data Initiative (Canada, 2017). It is conceivable that some concerns over data unavailability due to being confidential business information may be overcome with digital cryptography (blockchain), which would be a Canadian innovation.
- Strategically targeted funding and other resources (for example, staffing positions) are needed to prioritize and advance the knowledge of potential EDC issues, particularly related to NAMs, case studies, consideration of unfamiliar chemicals/classes, and risk assessment and risk management. Targeted funding should be driven by a formal process, which would include all engaged stakeholders. The Toxic Substances Research Initiative (TSRI) supported by the Canadian government in the 1990s was a successful program that could be reinstated as one that leverages inter-sectoral as well as interdisciplinary strengths; and/or the departments could review the U.S. EPA STAR model and other existing challenge programs as possible models. Due consideration should be given to funding such a program at a level that would make this initiative impactful and worthwhile.
- The SC encourages formalizing the centralization and coordination of an already strong nucleus of EDC activities in the departments to ensure that key subgroups (for example, researchers and risk assessors; NAM developers and those using standard tests; ecologists and biomedical scientists) continue to actively collaborate. EDCs transcend CEPA 1999/CMP, and so stakeholders with interests regarding other Parliamentary Acts that cover EDCs (such as the Canada Consumer Product Safety Act, Pest Control Products Act or Fisheries Act) need to be involved.
The SC discussed many other suggestions for "future opportunities" but is hesitant to present a large shopping list. The outcome of the SWOT type of analysis, if carefully scoped, will identify major areas for future focus.
4. Summary
- Many aspects associated with potential EDCs comprise "cutting-edge" science. In addition, the public has a keen interest in ensuring that human and ecological health will not be compromised by exposure to EDCs. During the meeting, it became clear that some members of the SC considered that the identification, assessment, and management of EDCs can be adequately conducted according to current and evolving science-based processes. Other members of the SC considered that EDCs could be unique, and therefore concluded that additional policy-based responses may be appropriate. The SC was not requested to address these differing perspectives.
- The SC responded to all 3 Charge Questions. Highlights comprise the following:
- For Charge Questions 1 and 2, the SC offers a workflow consisting of a tiered testing and evaluation framework, starting with non-test methods (for example, in silico and models), and including high-throughput in vitro testing, medium-throughput assays (which include greater complexity), and ending with in vivo testing if warranted. This workflow aligns with the risk characterizations for non-EDC pathways, but the discussions focused on EDC-specific pathways.
- The greatest certainty lies with characterizing EATS pathways through high- and medium-throughput assays (estrogen, androgen, thyroid, and steroidogenesis). However, non-EATS pathways (such as developmental neurotoxicity and obesity) require additional development.
- Issues that arose but were not, or only partially, resolved included: (1) adequately capturing population variability (including vulnerable populations and life stages), (2) performing cross-species extrapolation, and (3) assessing cumulative risk from exposures to chemical mixtures and non-chemical stressors. In addition, the SC noted the need to better consider the full range of chemicals, metabolites, and degradation products of parent compounds.
- The SC discussed using the AOP framework to organize future work (as a paradigm to translate between MIEs and KEs), which can inform assessment endpoints for high-throughput or medium-throughput assays, and apical endpoints.
- In discussing the TTC approach for human risk assessment, the SC suggested developing an EDC-TTC for both human and ecological health.
- Improvements to exposure assessment should be considered (such as greater reliance on biomonitoring and environmental effects monitoring).
- In response to Charge Question 3, the SC made 6 major recommendations, including conducting a SWOT analysis, conducting case studies to glean "lessons learned," convening expert panels to advance EDC-related risk assessment activities, improved data sharing, and stepping towards "big and bold" thinking to address the challenges with respect to EDCs from a multidisciplinary perspective.
Acknowledgements
We thank ad hoc members of the SC for their valuable contributions: Prof. Markus Hecker (University of Saskatchewan), Dr. Rebecca Clewell (ToxStrategies, Inc.) and Dr. Kevin Crofton (R3Fellows, LLC).
References
Abdo N., Xia M., Brown C. C., et al. 2015a. "Population-based in vitro hazard and concentration-response assessment of chemicals: the 1000 genomes high-throughput screening study." Environ Health Perspect. 123(5):458-66. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4421772/pdf/ehp.1408775.pdf.
Abdo N., Wetmore B. A., Chappell G. A., et al. 2015b. "In vitro screening for population variability in toxicity of pesticide-containing mixtures." Environ Int. 85:147-55. Available at: http://www.rusynlab.org/publications/2015/Abdo%20Wetmore%20et%20al%202015.pdf.
Ankley G. T., Bennett R. S., Erickson R. J., et al. 2010. "Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment." Environ Toxicol Chem. 29(3):730-41. Available at: https://setac.onlinelibrary.wiley.com/doi/epdf/10.1002/etc.34.
Attene-Ramos M. S., Miller N., Huang R., et al. 2013. "The Tox21 robotic platform for the assessment of environmental chemicals--from vision to reality." Drug Discov Today. 18(15-16):716-23. Available at: https://www.sciencedirect.com/science/article/pii/S135964461300161X?via%3Dihub.
Aylward L. L., Kirman C. R., Schoeny R., et al. 2013. "Evaluation of biomonitoring data from the CDC National Exposure Report in a risk assessment context: Perspectives across chemicals." Environ Health Perspect. 121(3):287-94. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3621178/
Bambino K., Chu J. 2017. "Zebrafish in toxicology and environmental health." Curr Top Dev Biol. 124:331-367. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0070215316301867.
Becker R. A., Friedman K. P., Simon T. W., et al. 2015. An exposure:activity profiling method for prioritizing potential EDCs interpreting high-throughput screening data for further testing estrogenic activity-proof of concept. Regul Toxicol Pharmacol. 71(3), 398−408). Available at: https://ac.els-cdn.com/S0273230015000100/1-s2.0-S0273230015000100-main.pdf?_tid=d29f4ef0-3158-400f-a013-a58e65a92f82&acdnat=1542219796_2d22ebb408bff722e73e6a7dbb0746db.
Belanger E., Sanderson H., Embry M. R., et al. 2015. "It is time to develop ecological thresholds of toxicological concern to assist environmental hazard assessment." Environ Toxicol Chem. 34(12):2864-9. Available at: https://setac.onlinelibrary.wiley.com/doi/epdf/10.1002/etc.3132.
Bell S. M., Chang X., Wambaugh J. F., et al. 2018. "In vitro to in vivo extrapolation for high throughput prioritization and decision making." Toxicol In Vitro. Mar;47:213-27. doi: 10.1016/j.tiv.2017.11.016. Available at: https://www.sciencedirect.com/science/article/pii/S0887233317303661?via%3Dihub.
Biryol D., Nicolas C. I., Wambaugh J., et al. 2017. "High-throughput dietary exposure predictions for chemical migrants from food contact substances for use in chemical prioritization."Environ Int. Nov;108:185-94. doi: 10.1016/j.envint.2017.08.004. Available at: https://www.sciencedirect.com/science/article/pii/S0160412016304767?via%3Dihub.
Blackwell B. R., Ankley G. T., Corsi S. R., et al. 2017. "An 'EAR' on environmental surveillance and monitoring: A case study on the use of exposure-activity ratios (EARs) to prioritize sites, chemicals, and bioactivities of concern in Great Lakes waters." Environ Sci Technol. 51(15):8713-8724. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6132252/pdf/nihms-1504076.pdf.
Bokkers B. G., Slob W. 2007. "Deriving a data-based interspecies assessment factor using the NOAEL and the benchmark dose approach." Crit Rev Toxicol. 37(5):355-73. Available at: https://www.tandfonline.com/doi/pdf/10.1080/10408440701249224?needAccess=true.
Boobis A. R., Cohen S. M., Dellarco V., et al. 2006. "IPCS framework for analyzing the relevance of a cancer mode of action for humans." Crit Rev Toxicol. 36(10):781-92. Available at: https://www.tandfonline.com/doi/pdf/10.1080/10408440600977677?needAccess=true.
Boobis A. R., Doe J. E., Heinrich-Hirsch B., et al. 2008. "IPCS framework for analyzing the relevance of a noncancer mode of action for humans." Crit Rev Toxicol. 38(2):87-96. Available at: https://www.tandfonline.com/doi/pdf/10.1080/10408440701749421?needAccess=true.
Borgert C. J., Mihaich E. M., Ortego L. S., et al. 2011. "Hypothesis-driven weight of evidence framework for evaluating data within the US EPA's Endocrine Disruptor Screening Program." Regul Toxicol Pharmacol. Nov;61(2):185-91. https://www.sciencedirect.com/science/article/pii/S0273230011001528?via%3Dihub.
Borgert C. J., Matthews J. C., Baker S. P. 2018. "Human-relevant potency threshold (HRPT) for ERα agonism." Arch Toxicol. 92(5): 1685-1702. Available at: https://link.springer.com/article/10.1007/s00204-018-2186-z
Browne P., Judson R. S., Casey W. M., et al. 2015. "Screening chemicals for estrogen receptor bioactivity using a computational model." Environ Sci Technol. 49(14):8804-14. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26066997.
Browne P., Noyes P. D. Casey W. M., et al. 2017. "Application of adverse outcome pathways to U.S. EPA's Endocrine Disruptor Screening Progam." Environ Health Perspect. 125(9):1-11. Available at: https://ehp.niehs.nih.gov/doi/full/10.1289/EHP1304?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed.
[Canada] Government of Canada. 2005. Guidelines for the Notification and Testing of New Substances: Chemicals and Polymers. Pursuant to Section 69 of the Canadian Environmental Protection Act, 1999. Available at: http://publications.gc.ca/collections/Collection/En84-25-2005E.pdf
[Canada] Government of Canada. 2012. "Response to Petition 340: Federal research on hormone disrupting substances as required under the Canadian Environmental Protection Act, 1999." Available at: http://www.oag-bvg.gc.ca/internet/English/pet_340_e_37607.html
[Canada] Government of Canada. 2016. "Science Approach Document: Threshold of Toxicological Concern (TTC)-based Approach for Certain Substance." Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=326E3E17-1
[Canada] Government of Canada. 2017. "Open Data 101." Available at: https://open.canada.ca/en/open-data-principles.
[Canada] Government of Canada. 2017b. "Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA 1999)." Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=A10191AD-1#s7
[Canada] Government of Canada. 2017c. "Information Gathering." Available at: https://www.canada.ca/en/health-canada/services/chemical-substances/canada-approach-chemicals/information-gathering.html
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Casey W. M., Chang X., Allen D. G., et al. 2018. "Evaluation and optimization of pharmacokinetic models for in vitro to in vivo extrapolation of estrogenic activity for environmental chemicals." Environ Health Perspect. 126(9):97001. Available at: https://ehp.niehs.nih.gov/doi/pdf/10.1289/EHP1655.
[CELA] Canadian Environmental Law Association. 2017. "Scientific Justification to Address Endocrine Disrupting Chemicals (EDCs): A Roadmap for Action." Available at: http://www.cela.ca/sites/cela.ca/files/EDCs-CEPA-Review-Subm.pdf
Chiu W. A., Wright F. A., Rusyn I. 2017. "A tiered, Bayesian approach to estimating of population variability for regulatory decision-making." ALTEX. 34(3):377-388. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5468494/pdf/nihms849877.pdf.
Cohen Hubal E. A, Wetmore B. A, Wambaugh J. F., et al. 2018. "Advancing internal exposure and physiologically-based toxicokinetic modeling for 21st-centruy risk assessments." J Expo Sci Environ Epidemiol. doi: 10.1038/s41370-018-0046-9. Epub 2018 Aug 16. Available at: https://www.nature.com/articles/s41370-018-0046-9.
Coussens N. P., Sittampalam G. S., Guha R., et al. 2018. "Assay guidance manual: Quantitative biology and pharmacology in preclinical drug discovery." Clin Transl Sci. 11(5):461-70. Available at: https://ascpt.onlinelibrary.wiley.com/doi/epdf/10.1111/cts.12570.
Cox L. A., Popken D., Marty M. S., et al. 2014. "Developing scientific confidence in HTS-derived prediction models: Lessons learned from an endocrine study." Regul Toxicol Pharmacol. 69(3): 443-50. Available at: https://www.sciencedirect.com/science/article/pii/S0273230014000920?via%3Dihub.
DeGroot D., Thomas R., Simmons, S. 2016. "Metabolism Retrofit Strategies for ToxCast Assays (BOSC)." Presented at BOSC CSS Meeting, RTP, NC, November 16-18, 2016. Available at: https://doi.org/10.23645/epacomptox.5179093.
DeGroot D. E., Swank A., Thomas R. S., et al. 2018. "mRNA transfection retrofits cell-based assays with xenobiotic metabolism." J Pharmacol Toxicol Methods. 92:77-94. Available at: https://www.sciencedirect.com/science/article/pii/S1056871918305665?via%3Dihub.
[DEPA] Danish Environmental Protection Agency. 2018. Danish (Q)SAR database. Available at: http://qsar.food.dtu.dk/.
Dix, D. 2010. "ToxCast and Tox21: High Throughput Screening for Hazard & Risk of Environmental Chemicals." U.S. Environmental Protection Agency, Office of Research and Development, National Center for Computational Toxicology. Available at: https://www.toxicology.org/groups/rc/nlsot/docs/Dix.pdf.
Doering J. A., Lee S., Kristiansen K., et al. 2018. "In silico site-directed mutagenesis informs species-specific predictions of chemical susceptibility derived from the Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS) tool." Toxicol Sci. 166(1):131-145. Available at: https://academic.oup.com/toxsci/advance-article/doi/10.1093/toxsci/kfy186/5060571.
Dong H., Wade M. G. 2017. "Application of a nonradioactive assay for high throughput screening for inhibition of thyroid hormone uptake via the transmembrane transporter MCT8." Toxicol In Vitro. 40:234-42. Available at: https://reader.elsevier.com/reader/sd/pii/S0887233317300140?token=9300F4460F3EFF657A74169F7AC09AEF43A3FD6720E38D60CE808364C908C11A1BD1E1E4AF27C65A29E75FDD94DC8059.
Elswick B. A., Welsch F., Janszen D. B. 2000. "Effect of different sampling designs on outcome of endocrine disruptor studies." Reprod Toxicol. 14(4):359-67. Available at: https://ac.els-cdn.com/S0890623800000927/1-s2.0-S0890623800000927-main.pdf?_tid=b5920b3c-4373-494c-ac45-c5710039cce5&acdnat=1542314701_7e2774e70d0ee90566e76f615b25bae5.
[EPA] U.S. Environmental Protection Agency. 2002. "Guidance on Cumulative Risk Assessment of Pesticide Chemicals that Have a Common Mechanism of Toxicity." Office of Pesticide Programs, Office of Prevention, Pesticides, and Toxic Substances. Washington, DC. Available at: https://www.epa.gov/sites/production/files/2015-07/documents/guidance_on_common_mechanism.pdf.
[EPA] U.S. Environmental Protection Agency. 2011. "Endocrine Disruptor Screening Program: Weight-of-Evidence- Evaluating Results of EDSP Tier 1 Screening to Identify the Need for Tier 2 Testing." Available at: https://www.regulations.gov/document?D=EPA-HQ-OPPT-2010-0877-0021.
[EPA] U.S. Environmental Protection Agency. 2014. "FIFRA SAP Meeting on Endocrine Activity and Exposure-based Prioritization and Screening." Available at: https://www.regulations.gov/docket?D=EPA-HQ-OPP-2014-0614.
Festing M. F. 2006. "Design and statistical methods in studies using animal models of development." ILAR J. 47(1):5-14. Available at: https://academic.oup.com/ilarjournal/article/47/1/5/662269.
Foley, B., Doheny, D. L., Black, M. B., et al. 2017. "Editor's highlight: Screening ToxCast prioritized chemicals for PPARG function in a human adipose-derived stem cell model of Adipogenesis." Toxicol Sci. 155(1):85-100. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5216650/.
Garcia G. R., Noyes P. D., Tanguay R. L. 2016. "Advancements in zebrafish applications for 21st century toxicology." Pharmacol Ther. 161:11-21. Available at: https://ac.els-cdn.com/S0163725816300171/1-s2.0-S0163725816300171-main.pdf?_tid=d23b2f07-3c79-4c27-9cbd-2bff236cdb37&acdnat=1539791117_2bcf2c529842fcdde842c3a37fba33f6.
Grimm F. A., Iwata Y., Sirenko O., et al. 2016. "A chemical-biological similarity-based grouping of complex substances as a prototype approach for evaluating chemical alternatives." Green Chem. 18(16):4407-19. Available at: https://www.concawe.eu/wp-content/uploads/2017/03/grimm-et-al-2016-final.pdf.
Grimm F. A., Blanchette A., House J. S., et al. 2018. "A human population-based organotypic in vitro model for cardiotoxicity screening." ALTEX. 35(4):441-52. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6231908/pdf/nihms-994287.pdf.
Gross M., Daginnus K., Deviller G., et al. 2010. "Thresholds of toxicological concern for endocrine active substances in the aquatic environment." Integr Environ Assess Manag. 6(1): 2-11. Available at: https://setac.onlinelibrary.wiley.com/doi/full/10.1897/IEAM_2008-092.1.
Haggard D. E., Karmaus A. L., Martin M. T., et al. 2018. "High-throughput H295R steroidogenesis assay: Utility as an alternative and a statistical approach to characterize effects on steroidogenesis." Toxicol Sci. 162(2):509-34. Available at: https://academic.oup.com/toxsci/article/162/2/509/4690670.
Haines D. A., Saravanabhavan G., Werry K., et al. 2017. "An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007-2019." Int J Hyg Environ Health. 220(2 Pt A):13-28. Available at: https://reader.elsevier.com/reader/sd/pii/S1438463916300888?token=49DD0D1B7C856C1ED44EBF837FD411FA2EF1261B82268A6E8158C45EA2F9D39070644D3AAE876B05455BB84D8BA518FD.
Hammel S. C., Hoffman K., Webster T. F., et al. 2016. "Measuring personal exposure to organophosphate flame retardants using silicone wristbands and hand wipes." Environ Sci Technol. 50(8):4483-91. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4872512/pdf/nihms-786160.pdf.
Hartman J. K., Beames T., Parks B., et al. 2018. "An in vitro approach for prioritization and evaluation of chemical effects on glucocorticoid receptor mediated adipogenesis." Toxicol Appl Pharmacol. 355:112-26. Available at: https://reader.elsevier.com/reader/sd/pii/S0041008X18302217?token=CAE4EEF03E80F494A5797E181414040773301848866A448A7517293A81A71720072DD90F270E124742CC3C0BC309E387.
Hartung T. 2017. "Thresholds of toxicological concern-setting a threshold for testing below which there is little concern." ALTEX. 34(3):331-51. Available at: https://www.ncbi.nlm.nih.gov/pubmed/28735337.
Hecker M. 2018. "Non-Model Species in Ecological Risk Assessment." In: A Systems Biology Approach to Advancing Adverse Outcome Pathways for Risk Assessment. Murphy C. and Reyero N., Eds., pp 107-132. Springer: New York. Available at: https://www.springer.com/gp/book/9783319660820.
Helmreich D. L. and Tylee D. 2011. "Thyroid hormone regulation by stress and behavioral differences in adult male rats" Horm Behav. 60(3): 284-91. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3148770/pdf/nihms-304715.pdf.
Hennes E. C. 2012. "An overview of values for the threshold of toxicological concern." Toxicol Lett. 211:296-303. Available at: https://ac.els-cdn.com/S0378427412008971/1-s2.0-S0378427412008971-main.pdf?_tid=b13ebc75-0c03-4e90-893f-f4a23be7f2b8&acdnat=1542215357_59ac5a8bd8dfb417a8d363a4de7cf656.
Hornung M. W., Korte J. J., Olker J. H., et al. 2018. "Screening the ToxCast Phase 1 chemical library for inhibition of deiodinase type 1 activity." Toxicol Sci. 162(2):570-81. Available at: https://academic.oup.com/toxsci/article/162/2/570/4706013.
Jarque S., Fetter E., Veneman W. J., et al. 2018. "An automated screening method for detecting compounds with goitrogenic activity using transgenic zebrafish embryos." PLOS One. 13(8). Available at: https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0203087&type=printable.
Kavlock R., Chandler K., Houck K., et al. 2012. "Update on EPA's ToxCast program: Providing high throughput decision support tools for chemical risk management." Chem Res Toxicol. 25(7):1287-302. Available at: https://pubs.acs.org/doi/10.1021/tx3000939.
Kavlock R. J., Bahadori T., Barton-Maclaren T. S., et al. 2018. "Accelerating the pace of chemical risk assessment." Chem Res Toxicol. 31(5):287-90. Available at: https://pubs.acs.org/doi/10.1021/acs.chemrestox.7b00339.
Kleinstreuer N. C., Ceger P., Watt E. D., et al. 2017. "Development and validation of a computational model for androgen receptor activity." Chem Res Toxicol. 30(4):946-64. Available at: https://pubs.acs.org/doi/10.1021/acs.chemrestox.6b00347.
Kleinstreuer N. C., Browne P., Chang X., et al. 2018. "Evaluation of androgen assay results using a curated Hershberger database." Repord Toxicol. 81:272-80. Available at: https://www.sciencedirect.com/science/article/pii/S0890623818302235?via%3Dihub.
Kroes R., Galli C., Munro I., et al. 2000. "Threshold of toxicological concern for chemical substances present in the diet: A practical tool for assessing the need for toxicity testing." Food Chem Toxicol. 38(2-3): 255-312. Available at: https://www.sciencedirect.com/science/article/pii/S0278691599001209?via%3Dihub.
Kroes R., Renwick A. G., Cheeseman M., et al. 2004. "Structure-based thresholds of toxicological concern (TTC): Guidance for application to substances present at low levels in the diet." Food Chem Toxicol. 42(1):65-83. Available at: https://www.sciencedirect.com/science/article/pii/S0278691503002412?via%3Dihub.
LaLone C. A., Villeneuve D. L., Burgoon L. D., et al. 2013. "Molecular target sequence similarity as a basis for species extrapolation to assess the ecological risk of chemicals with known modes of action." Aquat Toxicol. 144-145:141- 54. Available at: https://www.sciencedirect.com/science/article/pii/S0166445X13002312?via%3Dihub.
LaLone C.A., Villneuve D.L., Lyons D., et al. 2016. "Editor's Highlight: Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS): A Web-Based Tool for Addressing the Challenges of Cross-Species Extrapolation of Chemical Toxicity." Toxicological Sciences. 153(2):228-245. Available at: http://ct4xw3qj9z.search.serialssolutions.com/?rft_id=info%3Adoi%2F10.1093%2Ftoxsci%2Fkfw119.
Lyssimachou, A., Santos, J. G., Andre, A., et al. 2015. "The mammalian 'Obesogen' tributyltin targets hepatic triglyceride accumulation and the transcriptional regulation of lipid metabolism in the liver and brain of zebrafish." PLOS ONE. 10(12):e0143911. Available at: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0143911.
Manibusan M. K., Touart L. W. 2016. A comprehensive review of regulatory test methods for endocrine adverse health effects." Crit Rev Toxicol. 47:440-88. Available at: https://www.tandfonline.com/doi/full/10.1080/10408444.2016.1272095.
Manibusan M. K., Touart L. W. 2017. "A comprehensive review of regulatory test methods for endocrine adverse health effects." Crit Rev Toxicol. 47(6):433-81. Available at: https://www.tandfonline.com/doi/pdf/10.1080/10408444.2016.1272095?needAccess=true.
Mansouri K., Abdelaziz A., Rybacka A., et al. 2016. "CERAPP: Collaborative Estrogen Receptor Activity Prediction Project." Environ Health Perspect. 124(7):1023-33. Available at: https://ehp.niehs.nih.gov/cms/attachment/525c8061-1f72-467b-9278-8dd4954b47e0/ehp.1510267.pdf.
Mansouri K., Kleinstreuer N., Watt E., et al. 2017. "CoMPARA: Collaborative Modeling Project for Androgen Receptor Activity." The United States Environmental Protection Agency's National Center for Computational Toxicology. Poster presented at the Annual Society of Toxicology meeting, March 12-16, 2017. Available at: https://doi.org/10.23645/epacomptox.5176876.
Marchesini G. R., Meimaridou A., Haasnoot W., et al. 2008 "Biosensor discovery of thyroxine transport disrupting chemicals." Toxicol Appl Pharmacol. 232(1):150-60. Available at: https://ac.els-cdn.com/S0041008X0800269X/1-s2.0-S0041008X0800269X-main.pdf?_tid=94e2b6cf-8829-4a2a-8894-5fc725eb3c2c&acdnat=1539792267_02c32add501b4864c865e4faffc7de49
Meek M. E., Boobis A. R., Crofton K. M., et al. 2011. "Risk assessment of combined exposure to multiple chemicals: A WHO/IPCS framework." Regul Toxicol Pharmacol. 60(2):Suppl. S1-S14. Available at: https://www.sciencedirect.com/science/article/pii/S0273230011000638?via%3Dihub.
Mekenyan O. G., Kamenska V., Schmieder P. K., et al. 2000. "A computationally based identification algorithm for estrogen receptor ligands: Part 2. Evaluation of hERalpha binding affinity model." Toxicol Sci. 58(2):270-81.
Mekenyan O., Serafimova R. 2009. "Mechanism-based modeling of estrogen receptor binding affinity: A COREPA implementation." In: Endocrine Disruption Modeling, J. Devillers, ed., pp. 259-293. Boca Raton: CRC Press.
Miller M. M., Alyea R. A., LeSommer C., et al. 2016. "Editor's highlight: Development of an in vitro assay measuring uterine specific estrogenic responses for use in chemical safety assessment." Toxicolo Sci. 154(1):162-73. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5091368/pdf/kfw152.pdf.
Miller M. M., McMullen P. D., Andersen M. E., et al. 2017. "Multiple receptors shape the estrogen response pathway and are critical considerations for the future of in vitro-based risk assessment efforts." Crit Rev Toxicol. 47(7):564-80. Available at: https://www.tandfonline.com/doi/pdf/10.1080/10408444.2017.1289150?needAccess=true.
Moschet C., Anumol T., Lew B. M., et al. 2018. "Household dust as a repository of chemical accumulation: New insights from a comprehensive high-resolution mass spectrometric study." Environ Sci Technol. 52(5):2878-87. Available at: https://pubs.acs.org/doi/10.1021/acs.est.7b05767.
Mughal B. B., Demeneix B. A., Fini J. B. 2018. "Evaluating thyroid disrupting chemicals in vivo using Xenopus laevis." Methods Mol Bio. 1801:183-92. Available at: https://link.springer.com/protocol/10.1007%2F978-1-4939-7902-8_15.
[NASEM] National Academies of Sciences, Engineering, and Medicine. 2012. Exposure Science in the 21st Century: A Vision and a Strategy. Washington, DC: The National Academies Press. doi: http:s//doi.org/10.17226/13507. Available at: https://www.nap.edu/catalog/13507/exposure-science-in-the-21st-century-a-vision-and-a.
[NASEM] National Academies of Sciences, Engineering, and Medicine. 2014. Review of the Environmental Protection Agency's State-of-the-Science Evaluation of Nonmonotonic Dose-Response Relationships as they Apply to Endocrine Disruptors. Washington, DC: The National Academies Press. doi: https://doi.org/10.17226/18608. Available at: https://www.nap.edu/catalog/18608/review-of-the-environmental-protection-agencys-state-of-the-science-evaluation-of-nonmonotonic-dose-response-relationships-as-they-apply-to-endocrine-disruptors.
[NASEM] National Academies of Sciences, Engineering, and Medicine. 2017. Application of Systematic Review Methods in an Overall Strategy for Evaluating Low-Dose Toxicity from Endocrine Active Chemicals. Washington, DC: The National Academies Press. doi: https://doi.org/10.17226/24758. Available at: https://www.nap.edu/catalog/24758/application-of-systematic-review-methods-in-an-overall-strategy-for-evaluating-low-dose-toxicity-from-endocrine-active-chemicals.
Neale P. A., Ait-Aissa S., Brack W., et al. 2015. "Linking in vitro effects and detected organic micropollutants in surface water using mixture-toxicity modeling." Environ Sci Technol. 49(24):14614-24. Available at: https://pubs.acs.org/doi/pdf/10.1021/acs.est.5b04083.
Nicolas X., Djebli N., Rauch C., et al. 2018. "Population pharmacokinetic/pharmacodynamic analysis of alirocumab in healthy volunteers or hypercholesterolemic subjects using an indirect response model to predict low-density lipoprotein cholesterol lowering: Support for a biologics license application submission: Part II." Clin Pharmacokinet. Epub 2018 May 3, 1-16. doi: https://doi.org/10.1007/s40262-018-0670-5. Available at: https://link.springer.com/article/10.1007%2Fs40262-018-0670-5.
[OECD] Organisation for Economic Co-operation and Development. 2004. "OECD Principles for the Validation, for Regulatory Purposes, of (Quantitative) Structure-Activity Relationship Models." Available at: http://www.oecd.org/chemicalsafety/risk-assessment/37849783.pdf.
[OECD] Organisation for Economic Co-operation and Development. 2012. "OECD Conceptual Framework for Testing and Assessment of Endocrine Disrupters (as revised in 2012)." Available at: https://www.oecd.org/env/ehs/testing/OECD%20Conceptual%20Framework%20for%20Testing%20and%20Assessment%20of%20Endocrine%20Disrupters%20for%20the%20public%20website.pdf.
[OECD] Organisation for Economic Co-operation and Development. (2013). "Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals, Section 2." OECD, Paris 2013. Available at: https://doi.org/10.1787/9789264203709-en.
[OECD] Organisation for Economic Co-operation and Development. 2016. "OECD Series on Testing and Assessment No. 260: Guidance Document on the Use of Adverse Outcome Pathways in Developing Integrated Approaches to Testing and Assessment (IATA)." OECD, Paris, 2016. Available at: https://one.oecd.org/document/ENV/JM/MONO(2016)67/en/pdf.
[OECD] Organisation for Economic Co-operation and Development. 2017. "The OECD QSAR Toolbox." Available at: http://www.oecd.org/chemicalsafety/oecd-qsar-toolbox.htm.
[OECD] Organisation for Economic Co-operation and Development. 2018. "AOPs with OECD Status." Available at: https://aopwiki.org/oecd_page.
[OECD] Organisation for Economic Co-operation and Development. 2018b. "Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption." Available at: http://www.oecd.org/publications/guidance-document-on-standardised-test-guidelines-for-evaluating-chemicals-for-endocrine-disruption-2nd-edition-9789264304741-en.htm.
[OECD] Organisation for Economic Co-operation and Development. 2018c. "OECD Series on Adverse Outcome Pathways." Available at: https://www.oecd-ilibrary.org/environment/users-handbook-supplement-to-the-guidance-document-for-developing-and-assessing-adverse-outcome-pathways_5jlv1m9d1g32-en.
[OECD] Organisation for Economic Co-Operation and Development. 2018d. "OECD Work on Endocrine Disrupting Chemicals." Available at: http://www.oecd.org/chemicalsafety/testing/OECD%20Work%20on%20Endocrine%20Disrupting%20Chemicals.pdf.
Okeme J. O., Yang C., Abdollahi A., et al. 2018. "Passive air sampling of flame retardants and plasticizers in Canadian homes using PDMS, XAD-coated PDMS and PUF samplers." Environ Pollut. 239:109-17. Available at: https://reader.elsevier.com/reader/sd/pii/S0269749118300149?token=08F16079E3291F153221D4A5871A8DC18CFBAFC4F9FDBB6FA70C4B034BFB0895E8ADE68817837326B85FB8A31B0DD329.
Parliament of Canada (2017). Standing Committee on Environment and Sustainable Development. Healthy Environment, Healthy Canadians, Healthy Economy: Strengthening the Canadian Environmental Protection Act, 1999. 42ndParl., 1st sess. Available at: http://www.ourcommons.ca/Content/Committee/421/ENVI/Reports/RP9037962/envirp08/envirp08-e.pdf
Patlewicz G., Helman G., Pradeep P., et al. 2017. "Navigating through the minefield of read-across tools: A review of in silico tools for grouping." Comput Toxicol. 3:1-18. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6134855/pdf/nihms-1504045.pdf.
Patlewicz G., Wambaugh J. F., Felter S. P., et al. 2018. "Utilizing threshold of toxicological concern (TTC) with high throughput exposure predictions (HTE) as a risk-based prioritization approach for thousands of chemicals." Comput Toxicol. 7:58-67. Available at: https://www.sciencedirect.com/science/article/pii/S2468111318300689
Paul K. B., Hedge J. M., Rotroff D. M., et al. 2014. "Development of a thyroperoxidase inhibition assay for high-throughput screening." Chem Res Toxicol. 27(3):387-99. Available at: https://pubs.acs.org/doi/10.1021/tx400310w.
Pearce R. G., Setzer R. W., Davis J. L., et al. 2017. "Evaluation and calibration of high-throughput predictions of chemical distribution to tissues." J Pharmacokinet Pharmacodyn. 44(6):549-565. Available at: https://link.springer.com/content/pdf/10.1007%2Fs10928-017-9548-7.pdf.
Petkov P. I., Rowlands J. C. Budinsky R., et al. 2010. "Mechanism-based common reactivity pattern (COREPA) modelling of aryl hydrocarbon receptor binding affinity." SAR QSAR Environ Res., 21(1): 187-214. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3036575/.
Rager J. E., Strynar M. J., Liang S., et al. 2016. "Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring." Environ Int. 88:269-280. Available at: https://ac.els-cdn.com/S0160412015301112/1-s2.0-S0160412015301112-main.pdf?_tid=8cd6a384-a330-4ddb-8f74-f395c18646f4&acdnat=1542305505_7ae87765bf02f7492f2c630916f96069.
Raldúa D., Thienpont B., Babin P. J. 2012. "Zebrafish eleutheroembryos as an alternative system for screening chemicals disrupting the mammalian thyroid gland morphogenesis and function." Reprod Toxicol. 33(2):188-97. Available at: https://ac.els-cdn.com/S0890623811003595/1-s2.0-S0890623811003595-main.pdf?_tid=d091c37b-516f-492c-bcc4-93da419ba84e&acdnat=1539792564_cda8ae1c3cefe709c18778dc6ffd60b9.
Richard A. M., Judson R. S., Houck K. A., et al. 2016. "ToxCast chemical landscape: Paving the road to 21st century toxicology." Chem Res Toxicol. 29(8):1225-51. Available at: https://pubs.acs.org/doi/10.1021/acs.chemrestox.6b00135.
Thomas R. S., Philbert M. A., Auerbach S. S., et al. 2013. "Incorporating new technologies into toxicity testing and risk assessment: Moving from 21st century vision to a data-driven framework." Toxicol Sci. 136(1): 4-18. Available at: https://academic.oup.com/toxsci/article/136/1/4/1653723.
Thorne N., Auld D. S., Inglese J. 2010. "Apparent activity in high-throughput screening: Origins of compound-dependent assay interference." Curr Opin Chem Biol. 14(3):315-24. Available at: https://ac.els-cdn.com/S1367593110000463/1-s2.0-S1367593110000463-main.pdf?_tid=fe990c4c-d49b-44b4-81ae-f34a035085be&acdnat=1539792882_350877138a7450addcc67073678bdb61.
Trisciuzzi D., Alberga D., Mansouri K., et al. 2017. "Predictive structure-based toxicology approaches to assess the androgenic potential of chemicals." J Chem Inf Model. 57(11):2874-2884. Available at: https://pubs.acs.org/doi/pdf/10.1021/acs.jcim.7b00420.
Vantangoli M. M., Madnick S. J., Huse S. M., et al. 2015. "MCF-7 human breast cancer cells from differentiated microtissues in scaffold-free hydrogels." PLOS One, 10(8): e0135426. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4534042/.
Vantangoli M. M., Wilson S., Madnick S. J., et al. 2016. "Morphologic effects of estrogen stimulation on 3D MCF-7 microtissues." Toxicol Lett. 248:1-8. Available at: https://www.sciencedirect.com/science/article/pii/S0378427416300303?via%3Dihub.
Wambaugh J. F., Wang A., Dionisio K. L., et al. 2014. "High throughput heuristics for prioritizing human exposure to environmental chemicals." Environ Sci Technol., 48(21):12760-7. Available at: https://pubs.acs.org/doi/pdf/10.1021/es503583j.
Wambaugh J. F., Hughes M. F., Ring C. L., et al. 2018. "Evaluating in vitro-in vivo extrapolation of toxicokinetics." Toxicol Sci. 163(1):152-169. Available at: https://academic.oup.com/toxsci/article/163/1/152/4827578.
Wang J., Hallinger D. R., Murr A. S., et al. 2018. "High-throughput screening and quantitative chemical ranking for sodium-iodide symporter inhibitors in ToxCast Phase I chemical library." Environ Sci Technol. 52(9):5417-5426. Available at: https://pubs.acs.org/doi/10.1021/acs.est.7b06145.
Wetmore B. A., Wambaugh J. F., Allen B., et al. 2015. "Incorporating high-throughput exposure predictions with dosimetry-adjusted in vitro bioactivity to inform chemical toxicity testing." Toxicol Sci. 148(1):121-36. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4620046/pdf/kfv171.pdf.
[WHO/EFSA] World Health Organisation and European Food Safety Authority. 2016a. "EFSA/WHO report makes recommendations on Threshold of Toxicological Concern approach." Available at: https://www.efsa.europa.eu/en/press/news/160310a.
[WHO/EFSA] World Health Organisation and European Food Safety Authority. 2016b. "Review of the Threshold of Toxicological Concern (TTC) approach and development of new TTC decision tree." Available at: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2016.EN-1006.
Wignall J. A., Muratov E., Sedykh A., et al. 2018. "Conditional toxicity value (CTV) predictor: An in silico approach for generating quantitative risk estimates for chemicals." Environ Health Perspect. 126(5). Available at: https://www.ncbi.nlm.nih.gov/pubmed/29847084.
Xiao S., Coppeta J. R., Rogers H. B., et al. 2017. "A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle." Nat Commun. 8:14584. Available at: https://www.nature.com/articles/ncomms14584.
Yoon, M., Adeleye, Y., Clewell, R., et al. 2016. "Moving beyond prioritization toward true in vitro safety assessment." Appl In Vitro Toxicol. 2(2):67-73. Available at: https://www.liebertpub.com/doi/pdf/10.1089/aivt.2016.29005.rtl.
Yu K. N., Kang S. Y., Hong S., et al. 2018. "High-throughput metabolism-induced toxicity assays demonstrated on a 384-pillar plate." Arch Toxicol. 92(8):2501-2516. Available at: https://link.springer.com/content/pdf/10.1007%2Fs00204-018-2249-1.pdf.
Zeise L., Bois F. Y., Chiu W. A., et al. 2013. "Addressing human variability in next-generation human health risk assessments of environmental chemicals." Environ Health Perspect. 121(1):23-31. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3553440/pdf/ehp.1205687.pdfAnnex 1: Summary of select international approaches, frameworks, and guidance (as provided by the departments in pre-meeting material)
Provided below is a brief summary of some approaches for addressing EDCs and potential EDCs in the U.S., Europe, and Japan. The U.S. EPA EDSP tests chemicals in a tiered fashion to identify endocrine activity and establish dose-response relationships that are used in regulatory programs. Under certain European legislation, evidence that a chemical is an EDC may be used to inform hazard-based restrictions (Parrott et al., 2016; Solecki et al., 2017). Japan's program continues to expand the scope of work relate to EDCs and has established an assessment framework for integration into existing risk assessment practices. The organizations discussed in this section are particularly influential in the development of test protocols, frameworks, and guidance for considering EDCs, and generally coordinate their efforts, including input from the Government of Canada.
U.S.
The U.S. EPA has established the EDSP where chemicals regulated under the Federal Food, Drug, and Cosmetic Act (FFDCA), the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), and the Safe Drinking Water Act (SDWA) may be screened for their endocrine-disrupting potential using a tiered testing approach. The EDSP chemical universe includes pesticides (active and inert ingredients) as well as other industrial or commercial chemicals that may be found in sources of drinking water. The Tier 1 test battery includes a suite of in vitro and in vivo screening assays to identify the potential to interact with the estrogen, androgen, or thyroid hormonal systems. Where testing in Tier 1 shows endocrine-disrupting potential, testing under Tier 2 (which includes test protocols for fish and amphibians) may be proposed, which further identifies adverse endocrine-related effects caused by the substance and establishes a quantitative relationship between the dose and that adverse effect. Testing guidelines have been developed for both Tier 1 and Tier 2 assays. To date, the U.S. EPA has issued mandatory test orders for 52 pesticides in Tier 1 tests, with follow-up Tier 2 testing being proposed for a subset of these chemicals that showed endocrine-related activity (EPA, 2017).
More recent screening activity has been to develop and add in vitro and in silico screening methods for molecular targets related to estrogen, androgen, and thyroid toxicity. This initiative, referred to as EDSP in the 21st Century (or EDSP 21), aims to increase the amount of relevant data for a broader array of chemicals across the mandates of all three acts to set priorities for further testing, and replaces some of the animal tests in the EDSP Tier 1. Data from this high-throughput initiative is shared through a public online database to ensure transparency and support the global assessment of EDCs (https://comptox.epa.gov/dashboard/chemical_lists/toxcast_e1k).
Europe
The European Chemicals Agency (ECHA) has considered endocrine-disrupting potential while implementing the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulations for chemical substances. ED properties can be used as evidence to support that a chemical has an equivalent level of concern to substances that are classified as carcinogenic, mutagenic, or toxic for reproduction (CMR), or (very) persistent, (very) bioaccumulative, or toxic (PBT/vPvB), and as a result are considered as substances of very high concern (SVHC). The SVHC designation is based on intrinsic hazard properties, and these substances may ultimately be placed on the authorization list whereby use is prohibited unless authorization is granted by the ECHA.Priority to determine if a chemical is to be placed on the authorization list is given to substances with wide dispersive use or high volumes (or PBT or vPvB properties). This has recently been applied for bisphenol A, which is considered an SVHC and is currently on the candidate list for authorization based on its endocrine-disrupting properties in addition to its effects on reproduction (ECHA, 2018).
The ECHA and the European Food Safety Authority (EFSA) recently published a draft guidance document for the implementation of the hazard-based criteria to identify endocrine disruptors, which was compiled with support from the European Commission's Joint Research Centre (JRC) (EFSA, 2018). The guidance document was drafted for the purposes of regulating plant protection products and the use of biocidal products. The guidance provides a stepwise process whereby the endocrine-disruptor potential of a substance is determined from an evaluation of its relevant endocrine hazard properties. This includes steps on how to gather, evaluate, and consider all relevant information for the assessment, as well as how to conduct a mode-of-action analysis and apply a weight-of-evidence approach for the determination. The guidance is limited to the EATS modalities.
Japan
In 1998, Japan's Ministry of Environment Protection (MEP) first established the Strategic Program on Environmental Endocrine Disruptors (SPEED), which is focused on researching and testing substances with significant exposure to humans and wildlife. Under this program, 67 substances were prioritized for further investigation based on suspicion of endocrine disruption (there is no list of confirmed endocrine disruptors in Japan). The MEP continued to expand the program of work to address further regulatory issues with Extended Tasks on Endocrine Disruption (EXTEND) 2005 and EXTEND 2010, which aimed to accelerate the establishment and implementation of assessment methodologies towards the goal of assessing the environmental risk of endocrine-disrupting effects of chemical substances and to take management measures, if necessary. EXTEND 2010 was based on several pillars, including: (1) observation of wildlife, (2) a survey on environmental concentrations and measurement of exposure levels, (3) the promotion of fundamental studies, (4) a hazard assessment, (5) a risk assessment, (6) risk management, and (7) the promotion of information sharing and risk communication. Japan has now updated the Strategic Plan in the finalized EXTEND 2016. According to Manibusan and Touart (2017), this fourth program emphasizes hazard and risk assessment in support of regulatory risk management decisions. The assessment framework will be integrated into existing regulatory assessment practices, including setting environmental water quality standards, setting a tiered risk assessment for industrial chemicals under the Chemical Substances Control Law, and setting standards for registration decisions under the Agricultural Chemicals Regulation Law.
Appendix A: New Substances Notification Regulations data requirements (as provided by the departments in pre-meeting material)
| Data requirement | Schedules | Example of acceptable test method(s) |
|---|---|---|
| Acute mammalian toxicity | 5, 6, 10, 11 | OECD Test Guidelines (TGs) 402, 403, 420, 423, 425, 436 |
| Skin irritation | 6, 11 | OECD TG 404, 430, 431, 439 |
| Skin sensitization | 6, 11 | OECD TGs 406, 429, 442 (A-D) |
| Repeated-dose toxicityFootnote 1 | 6, 11, and high release [subsection 7(2), 7(3), 11(2), and 11(3) of the Regulations] | OECD TGs 407, 408, 409, 410, 412, 422 |
| Mutagenicity | 5, 6, 11, and high release [subsection 7(3), 11(2), and 11(3) of the Regulations] | OECD TGs 471, 473, 474, 475, 476,Footnote 2 487,Footnote 2 488, 489 |
|
||
| Data requirement | Schedules | Test method |
|---|---|---|
| Acute fish toxicity | 5, 6, 10, 11 | OECD TG 203; ECCC method EPS1/RM/9 and EPS1/RM/13 |
| Acute Daphnia toxicity | 5, 6, 10, 11 | OECD TG 202; ECCC method EPS1/RM/11 |
| Algae toxicity | 5, 6, 10, 11 | OECD TG 201; ECCC method EPS1/RM/25 |
| Ready biodegradability | 5, 11 | OECD TG 301 |
Appendix B: Government of Canada research activities related to endocrine disrupting chemicals (as provided by the departments in pre-meeting material)
| Project | Description | Leads |
|---|---|---|
| Canadian Health Measures Survey | Biomonitoring of environmental chemicals in urine, blood, hair, and indoor environment (air and tap water) samples were collected, along with direct health measures and self-report questionnaire data in a representative population of Canadians. | HC Statistics Canada |
| Maternal-Infant Research on Environmental Chemicals (MIREC) research platform | Cross-Canada prospective pregnancy and birth cohort study examining early-life exposures (prenatal, early childhood) to diverse chemicals, and tracking maternal and child health outcomes into the teen years. | HC |
| Canadian House Dust Study | Dust samples from >1,000 homes in major urban areas across Canada were analyzed for metals/chemicals, including suspected EDCs. | HC |
| Total Diet Survey | Chemicals [persistent organic pollutants (POPs), pesticides, and metals] were analyzed in a composite of foods representing an approximation of a diet. Representative food items purchased within a given city each year. | HC |
| Great Lakes Bird Egg Monitoring Survey | Aquatic bird eggs (for example, herring gulls) were collected from key nesting colonies throughout and analyzed for POPs, metals, and substances of concern. Gulls eggs collected annually from the 1970s. | ECCC |
| Targeted monitoring of environmental samples | Diverse activities analyzing chemicals, including potential EDCs (in air, surface water, wastewaters, sediments, biota, wildlife tissues, etc.). Includes method development for novel chemicals of concern. Some sampling is repeated to assess historic trends. | ECCC |
| Targeted monitoring of human-relevant samples | Similar to monitoring of environmental samples but that analyzes air, dust materials from built environments, foods, drinking water, occupational settings, and so forth that are relevant for estimating human exposure. | HC |
| Project | Description | Leads |
|---|---|---|
| Assessment of fish health in rivers receiving effluents | Fish health assessment (growth, development, tumours, gonad size) is being studied in areas where pulp mill effluent or municipal effluents are being discharged. Similar environmental effects monitoring techniques are being used in areas of oil sands development. | ECCC |
| Fish life cycle studies of pharmaceuticals of concern to HC-CMP | Metformin (diabetes drug) fish life cycle study, assessing growth, development, reproduction, vitellogenin, intersex. | ECCC-HC |
| Assessing mussel health and bioaccumulation of pharmaceuticals downstream of municipal wastewaters | Assessment of mussel health and tissue accumulation of pharmaceuticals and personal care products downstream of large municipal wastewater discharges on the Grand River, Ontario. | ECCC-HC |
| Assessing EDC effects of pharmaceuticals using 'omics endpoints in cell lines and cells | Assessment of changes in gene expression and metabolites related to endocrine pathways in cells and cell lines from aquatic organisms. | ECCC-HC |
| Toxicity of flame-retardant chemicals | Assessing effects of flame retardant exposure on reproduction, growth, development, metabolism, and hormone physiology in cell cultures and various animal models. | ECCC-HC |
| Development of standardized tests for endocrine-disrupting properties | Development and validation of novel methods to test chemicals for activity on key molecular or cellular events involved in hormone production, action, or metabolism (thyroid, adipogenesis, etc.). Includes contribution to the development of adverse outcome pathways. | ECCC-HC |
| Research area | Lead | Principle investigatorFootnote 1 |
|---|---|---|
| Reproductive developmental toxicology-mammalian | HC/ECCC | M. Wade I. Curran A. M. Gannon J. E. Elliott |
| Reproductive developmental toxicology-avian | ECCC | K. J. Fernie D. Crump J. L. Shutt J. E. Elliott |
| Reproductive developmental toxicology-amphibian | ECCC | B. D. Pauli |
| Reproductive developmental toxicology-fish | ECCC | J. L. Parrott M. McMaster J. Sherry |
| Thyroid hormone toxicity | HC/ECCC | M. G. Wade D. Crump R. J. Letcher K. J. Fernie |
| Nuclear receptors and steroid hormones | HC/ECCC | E. Atlas J. Sherry |
| Pulp mill/wastewater effects | ECCC | M. McMaster L. M. Hewitt |
| Metabolic effects | HC | E. Atlas T. E. Arbuckle |
| Endocrine and developmental toxicity of air pollution | HC/ECCC | E. M. Thomson J. R. Brook |
| Epidemiology of fetal and developmental exposures in humans | HC | T. E. Arbuckle |
|
||