Meeting record February 19-20, 2014 - Chemicals Management Plan Science Committee
Chemicals Management Plan (CMP) Science Committee (SC)
The SC was requested to prepare a response to the following Charge Question:
How can Environment Canada (EC) and Health Canada (HC) better capture and communicate uncertainty to decision makers, risk managers and external stakeholders in the context of their regulatory risk assessment reports?
The SC offers five suggestions for consideration by the Departments:
- The SC suggests development of standard Guidance for assessors describing methods for characterizing uncertainty and communicating results of uncertainty analyses. (Currently, we understand assessors in both departments use internal documents to maximize consistency within and between assessments);
- The SC suggests that future assessments under the CMP should have a concise summary. This concise summary should succinctly capture and communicate key uncertainties, as well as identify information which, if available, would reduce uncertainty;
- The SC suggests that a standardized summary table be developed to include in every risk assessment under the CMP;
- The SC suggests that best practices be followed when applying assumptions and reporting impacts of these assumptions on risk assessment to improve communication of uncertainty and to facilitate the distinction between scientific approaches to addressing uncertainty in exposures and hazards and policy decisions;
- The SC suggests the use of relevant case studies to support development of standardized guidance and language for preparing statements of uncertainty; these case studies should use the standard format referred to above to summarize key uncertainties in chemical risk assessments under the CMP.
# 1: Develop and make available to stakeholders a standard guidance for characterizing and communicating uncertainty in chemical risk assessment
The overall aim of this Guidance is to increase the quality, consistency and transparency of communicating uncertainty in risk assessments to facilitate informed decisions and risk management.
The availability of updated and formal guidance documentation for assessors, with an added focus on the subject of uncertainty, will enable improved communication between risk assessors, decision makers, risk managers and other stakeholders, so that all groups involved in decision making better understand the uncertainties inherent in the method and its application. EC/HC should strive to update existing internal Guidance documents and make them available to stakeholders.
The intent is to create a formal and consistent Guidance that helps explain where assumptions are incorporated into the assessments and how best to document such assumptions. For the "hazard" component of an assessment, uncertainties to be addressed include both data-driven considerations (for example, confidence bounds for Species Sensitivity Distribution); justification for uncertainty factors applied for data poor substances (and the basis for these uncertainty factors); decisions made when extrapolations are necessary (for example, physical-chemical properties, low dose toxicity), and policy aspects (for example, threshold vs. non-threshold aspects, relevance to persistence-bioaccumulation-toxic [PBT] policies). For "exposure" considerations, uncertainties to be addressed could include the use of specific confidence bounds in exposure conclusions and the uncertainty associated with predictive models, as well as the limitations associated with monitoring data. When the standard practices outlined in the Guidance are not followed, an explanation should be provided. The guidance documents should address how uncertainty and variability are to be handled - both separately and together.
The Guidance should provide a standardized language for use in risk assessments, including metrics or examples to calibrate levels of overall and key uncertainties, thus providing clear communication of uncertainties to external stakeholders.
The SC also sees value in working towards harmonization of this Guidance with other agencies.
# 2: Provide a concise summary of key uncertainties and ways to reduce them
The goal of this concise summary is to provide insight into the confidence associated with conclusions. It should summarize what we know with respect to critical exposures and hazards and their risk. It is important that key uncertainties and their impact on the decision be clearly conveyed. Information which would reduce key uncertainties and increase confidence in the decision should also be identified.
# 3: Develop and provide a standard format for communicating sources of uncertainty
The Appendix contains several examples of a concise format that could be used to communicate sources of uncertainty in the assessment. The standard summary table should encompass both human health and environmental considerations, and include both exposure and hazard endpoints. Examples of table formats were developed to combine these elements in a single presentation.
The committee had diverse views of how best to capture and express the various sources of uncertainty, and differing approaches are reflected in the examples contained in the Appendix. Considerations which were discussed included:
- Whether the standard format should include only those exposures/endpoints which contributed most to the final conclusion, or attempt to report all the data elements considered throughout the risk assessment. The latter approach would include uncertainties associated with elements of an information inventory, combining aspects of uncertainty communication with a data gap analysis. The more limited approach would report only uncertainties associated with those key elements which were the focus of the targeted risk assessment. Text similar to that contained in current risk assessments would continue to report uncertainty for all the information considered in the assessment;
- Whether the table would communicate most effectively by using symbolic expressions (for example, +, ++, +++) or summary words such as "low", "medium" or "high" to convey uncertainty in each component of the risk assessment. Consideration should also be given to indicating whether the uncertainty biases the assessment outcome, and in which direction (for example, conservative or not); and
- Whether the table should affirmatively report "confidence" in the endpoint evaluation or "uncertainty".
The examples in the Appendix include a data-poor example (Option 1) and a data-rich example (Option 2), using both symbolic and "word" descriptors to describe confidence for a large number of data elements. An example of a summary limited to only those factors considered as key elements of the risk assessment is presented as Option 3.
# 4: Apply best practices to track assumptions and capture uncertainty for communication
To facilitate and enable communication of uncertainty it is important to capture all uncertainty within the assessment. When precaution is routinely applied at all stages, by making conservative assumptions, the possibility of doing an objective uncertainty analysis may be lost. We accept that conservative assumptions will be made, but confidence from stakeholders can be lost when several conservative assumptions become convoluted. When multiplying conservative assumptions, we increase the challenge of communicating what is known and unknown.
Certain best practices would better enable communication of key uncertainties, and identification of where additional information could reduce uncertainty, or influence the decision.
- Where possible, use all available information that meets reasonable quality criteria for a parameter, rather than choosing only values that are considered conservative;
- Quantitative and qualitative uncertainty analysis should be used to capture and convey the breadth and consequences of uncertainty in hazard, exposure and risk assessment;
- Where conservative assumptions are made, these should be described and reported in a transparent manner throughout. The uncertainty (magnitude and direction) associated with the assumptions should be estimated and captured.
It is advised that major uncertainties continue to be summarized but that best available tools (for example, sensitivity analysis, and value of information) be used to (semi)quantify the magnitude and direction of each, and thus determine the importance of each in influencing the final risk decision.
# 5: Use case studies to develop guidance and tools for capturing and communicating uncertainty
To find an optimal approach, and to develop and interrogate the utility of a Guidance document, the concise summary, and standardized format, the SC suggests that case studies be examined, using existing CMP assessments covering a range of chemicals, with consideration of both data rich and data poor chemicals. These case studies can be used to aid development of standardized language and calibrate the descriptors used to describe confidence/uncertainty to assure consistency between assessors/assessments.
Appendix
Examples of tables summarizing uncertainties in hazard and exposure analyses
Option 1: Summary of Uncertainty for Assessment with word descriptors: Compound X (a lanthanide element) (data poor chemical)
| Compartment | Measured | Modeled |
|---|---|---|
| Water |  |
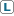 |
| Sediment |  |
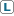 |
| Soil |  |
 |
| Air | 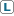 |
 |
Note: The predominance of "H"s reflects the data-poor status of the lanthanides and the fact they are very difficult to study properly (for example, given their low solubility at circumneutral pH) . |
||
| Source | Measured | Modeled |
|---|---|---|
| Direct |  |
 |
| Water |  |
 |
| Air (indoor) |  |
 |
| Air (outdoor) |  |
 |
| Food |  |
 |
| Dust/Soil |  |
 |
Note: The predominance of "H"s reflects the data-poor status of the lanthanides and the fact they are very difficult to study properly (for example, given their low solubility at circumneutral pH) |
||
| Endpoint | Measured | Modeled |
|---|---|---|
| Aquatic SSD |  |
 |
| Aquatic Chron. |  |
 |
| Aquatic Acute |  |
 |
| Terrest. Plant |  |
 |
| Terrest. Invert. |  |
 |
| Bioaccumulation |  |
 |
| Persistence | (irrelevant) | (irrelevant) |
Note: The predominance of "H"s reflects the data-poor status of the lanthanides and the fact they are very difficult to study properly (for example, given their low solubility at circumneutral pH) |
||
| Endpoint | Human | Animal | Analog | In vitro | In silico |
|---|---|---|---|---|---|
| Cancer |  |
 |
 |
 |
 |
| Genetox |  |
 |
 |
 |
 |
| Reprotox |  |
 |
 |
 |
 |
| Teratol |  |
 |
 |
 |
 |
| Neurotox |  |
 |
 |
 |
 |
| Organ |  |
 |
 |
 |
 |
| Acute |  |
 |
 |
 |
 |
Note: The predominance of "H"s reflects the data-poor status of the lanthanides and the fact they are very difficult to study properly (for example, given their low solubility at circumneutral pH) |
|||||
Option 2: Summary of Uncertainty for Assessment of a data rich substance with symbolic descriptors: Compound Y (1 of 16 US EPA priority polycyclic aromatic hydrocarbons or PAH)
| Compartment | Measured | Modeled |
|---|---|---|
| Water | ||
| Sediment |  |
|
| Soil |  |
|
| Air |  |
|
|
||
| Source | Measured | Modeled |
|---|---|---|
| Air (indoor) | ||
| Air (outdoor) |  |
 |
| Water | unknown | |
| Food | ||
| Dust/Soil | ||
| Direct | ||
|
||
| Endpoint | Measured | Modeled |
|---|---|---|
| Aquatic SSD |  |
 |
| Aquatic Chron. | ||
| Aquatic Acute |  |
|
| Terrest. Plant |  |
unknown |
| Terrest. Invert. |  |
|
| Bioaccumulation | ||
| Persistence |  |
|
|
||
| Endpoint | Human | Animal | Analog | In vitro | In silico |
|---|---|---|---|---|---|
| Cancer |  |
 |
no information |  |
 |
| Genetox |  |
 |
no information |  |
 |
| Reprotox |  |
no information |  |
 |
|
| Teratol |  |
 |
no information |  |
 |
| Neurotox |  |
no information | no information | no information | |
| Organ | no information |  |
 |
 |
unknown |
| Acute | not applicable | not applicable |  |
 |
not applicable |
|
|||||
Option 3: Simplified summary of Key Endpoint Uncertainty for Assessment of: Compound Y (1 of 16 US EPA priority polycyclic aromatic hydrocarbons or PAH)
| Compartment | Level of Uncertainty |
|---|---|
| Water | |
| Sediment |  |
|
|
| Source | Level of Uncertainty |
|---|---|
| Air (indoor) | |
| Air (outdoor) |  |
| Food | |
| Direct |  |
|
|
| Endpoint | Level of Uncertainty |
|---|---|
| Aquatic SSD |  |
| Bioaccumulation | |
| Persistence |  |
|
|
| Endpoint | Level of Uncertainty |
|---|---|
| Cancer |  |
| Genetox |  |
| Reprotox | |
| Teratol |  |
|
|
Respectfully submitted, Barbara Hales and Geoff Granville/on behalf of the Chemicals Management Plan Science Committee, 12 March 2014.
View the February 2014 Meeting Record.
Page details
- Date modified: