The identification of risk assessment priorities
Fact sheet series: Topics in risk assessment of substances under the Canadian Environmental Protection Act, 1999 (CEPA)
On this page
Overview
The Government of Canada assesses and manages risks to human health and the environment posed by chemical substances that can be found in the environment, food and food products, consumer products, cosmetics, drugs, drinking water and industrial releases.
Since 2006, priorities for assessment of existing substances under CEPA have largely been based on the results of the categorization of the Domestic Substances List (DSL). However, as our knowledge of chemicals continues to evolve, it is important to consider new information to identify substances that may have the potential to cause harm to the environment or human health. Once identified, these substances may then be recommended as priorities for assessment and proposed for addition to the Plan of Priorities.
In 2014, the Government of Canada published an approach outlining the systematic collection, consolidation and analysis of information, undertaken in order to determine appropriate action, including assessment, for substances with new information. This approach has been applied periodically since then to identify emerging priorities for assessment. Building on experience gained from these activities, key drivers for the selection of substances as priorities for assessment were identified, including:
- substances that are hazardous to human health or the environment, including carcinogens, mutagens, reproductive toxicants as well as endocrine disrupting substances
- substances that are impacting populations or environments that may be at increased risk due to either greater exposure or greater susceptibility
- substances with the potential to contribute to cumulative risks
- very hazardous substances that are capable of long-range transport (VH-LRT)
- substances with known hazardous properties that are used in products available to consumers; and
- potential substitutes for substances with known toxicity
This fact sheet describes the steps and considerations used as a basis for the identification of priorities for assessment under CEPA.
Approach for prioritization
There are 3 steps involved in the identification of risk assessment priorities (IRAP):
- Acquisition of information relevant to the potential health and ecological risks of substances
- Evaluation of the information available for each substance and identification of appropriate
- Action for each substance
The acquisition of new information occurs on an ongoing basis, while the other 2 steps are generally performed at regular intervals. The steps in the IRAP approach are outlined in Figure 1.
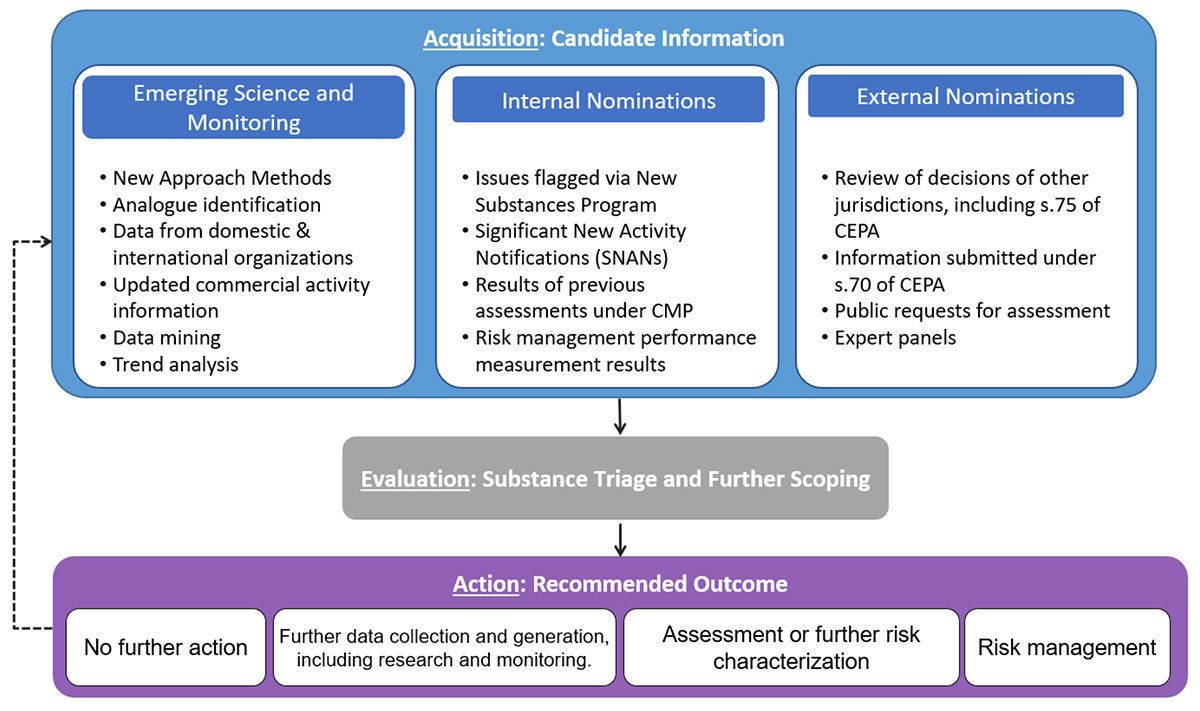
Figure 1: Text description
This figure depicts the overall IRAP process. Starting at the top, is Step 1, acquisition, where sources of candidate information are collected to help inform the prioritization approach. While both internal and external nominations are sources of candidate information, the focus in this graph is on the emerging science and monitoring information sources, which represent the place where HAWPr fits into this overall process. The emerging science and monitoring information includes elements such as new approach methods, analogue identification, data from domestic and international organizations, updated commercial activity information, data mining, as well as trend analysis. After collection of data and information in the first step, the process moves to Step 2, evaluation where substance triage and further scoping takes place. Finally, the last step is action, where a decision on a recommended outcome of the prioritization process is made. Recommended outcomes can include further data collection and generatio
Acquisition refers to the ongoing collection and compilation of data on substances for further consideration in the evaluation phase. Multiple information sources and candidate identification mechanisms are used in the IRAP approach and these are summarized in Figure 1. They include, for example, emerging science and monitoring including data mining, analysis of trends, and incorporation of the results of new approach methods (NAMs). External nominations may be identified through international hazard classifications, decisions made in other jurisdictions, toxicity data submitted under section 70 of CEPA, or public requests for assessment. Internal nominations may also be made from within the Government of Canada that take into consideration information from the New Substances program and other relevant Government of Canada program areas. The IRAP approach is not prescriptive in the data sources used, and additional data sources are considered in each review as new science, methods, and information become available. Examples of sources for hazard or exposure used by Environment and Climate Change Canada (ECCC) and Health Canada (HC) for the identification of assessment priorities can be found on the information gathering web page.
Evaluation refers to the periodic review of the data and subsequent analysis that is performed by scientists at ECCC and HC. A series of factors are considered and weighed, and judgments are made on the relative importance of different indicators (for example, reliability of the information, number of information sources, potential concern for hazard and exposure). Evaluation can be a complex process because substances may have different types of information available and prior activities on a substance must also be taken into account (for example, outcomes of previous assessments). Various prioritization tools have been developed to assist with the review and evaluation of data. These approaches, such as ECCC’s Ecological Risk Classification (ERC) and the HC Automated Workflow for Prioritization (HAWPr), are decision support tools that integrate emerging tools and datasets with traditionally available data to evolve the process for identification of priorities for further work. This offers the opportunity to modernize and expand on the data considerations, including improvements to previous methods for how data and information is gathered, how it is reviewed and considered, as well as how it is weighed and compared. This ensures that substances are not only prioritized based on the availability of traditional toxicity data, which is often limited for the diverse types of chemicals on the DSL, but that information provided by non-animal methods such as predictive models and read-across approaches are used to capture data poor chemicals that merit further consideration. This step also includes further scoping of the identified priority, which helps ensure the appropriateness of the future work. For example, further scoping would be used to consider the feasibility and suitability of using a group or class approach, or the potential for cumulative effects from exposure to multiple substances.
Action refers to recommended next steps for a given substance based on the evaluation of the information. Decisions are guided by a set of principles and considerations as outlined in the IRAP approach. A substance may be recommended for assessment and proposed to be added to the Plan of Priorities. Or it could be recommended for further information gathering or data generation where additional information would be beneficial to determining the appropriate next step. HC or ECCC program areas or stakeholders may be engaged to collect or generate additional information (including research, monitoring, and/or surveillance). Additional actions may be considered when warranted, including risk management or no further action.
Generally, for a substance to be identified as a priority for assessment, the approach would identify information suggesting a potential risk – that is, the presence of both a hazard and a significant potential for exposure in Canada. If only hazard information is identified (or if only international exposure data are available), the recommended action would typically be to confirm, through further information gathering, whether there are activities with this substance in Canada that could lead to exposure of the substance to humans or to the environment. If results of this information gathering indicate that there is a significant potential for exposure, then the substance may be recommended for assessment and proposed to be added to the Plan of Priorities.