ARCHIVED - Polychlorinated Dibenzodioxins and Polychlorinated Dibenzofurans - PSL1
Environment Canada
Health Canada
1990
ISBN: 0-662-17644-8
Cat. no.: En40-215/1E
Canadian Environmental Protection Act
Table of Contents
- Summary
- Introduction
- Identity and Properties
- Toxicity Equivalency Factors
- Sources of Environmental Contamination
- Environmental Fate and Levels
- Kinetics and Metabolism
- Toxicology
- Effects on the Ecosystem
- Effects on Humans
- Currents Canadian Objectives, Guidelines and Regulations
- Environmental Risk Assessment
- Human Health Risk Assessment
- References
List of Tables
- Table 1 Dioxins and the numbering system used to name them
- Table 2 Furans and the numbering system used to name them
- Table 3 Log octanol-water partition coefficients for selected dioxins
- Table 4 International Toxicity Equivalency Factors
- Table 5 Canadian sales, in tonnes, for dioxin-containing chemicals
- Table 6 Dioxin and furan levels in Canada
- Table 7 Estimates of Canadian intake of dioxins and furans
List of Figures
A summary of this report, together with recommendations, has been published in the Canada Gazette Part I. This report is published as required by Section 13 of the Canadian Environmental Protection Act (CEPA).
This report has been prepared for the Ministers of Environment and of National Health and Welfare by the following staff members:
M.J. Boddington (Environment Canada)
A.P. Gilman (Health and Welfare Canada)
R.C. Newhook (Health and Welfare Canada)
B.M. Braune (Environment Canada)
D.J. Hay (Environment Canada)
V. Shantora (Environment Canada)
Any inquires concerning this publication or requests for copies should be directed to either of the following officials:
Summary
Polychlorinated dibenzodioxins (dioxins) and polychlorinated dibenzofurans (furans) are highly persistent compounds with a strong affinity for sediments and a high potential for accumulating in biological tissues. They have been found in all compartments of the ecosystem, including: air, water, soil, sediments, animals and foods. All animals and humans in Canada are exposed to these substances.
Dioxins and furans enter the environment as complex mixtures from four major sources: commercial chemicals (eg. pentachlorophenol); incineration; pulp and paper mills that use chlorine bleaching; and both accidental fires and spills involving polychlorinated biphenyls (PCBs, which contain principally furan contaminants).
Both the number of chlorine atoms and their positions on the molecule determine the properties of dioxins and furans. It is primarily those dioxins and furans with chlorines in the 2, 3, 7 and 8 positions that are retained by animals and humans, and which concentrate selectively in body fat and fatty organs such as the liver. Although dioxins and furans can be metabolized and excreted, this is a relatively slow process in humans, with half-lives of several years reported for some dioxins and furans.
The compound 2,3,7,8-tetrachlorodibenzodioxin (and to a lesser extent, the other dioxins and furans substituted in the 2, 3, 7 and 8 positions) is extremely toxic to mammals, with a wide variation in sensitivity among species. In animals, death results from exposure to amounts ranging from less than one microgram to a few milligrams per kilogram of body weight. The bird and fish species tested are more sensitive than most mammals to short-term exposures to dioxins. Longer-term exposure of test mammals to lesser amounts can affect reproduction, cause birth defects, damage the liver and suppress the immune system. Exposure to 2,3,7,8- tetrachlorodibenzodioxin at certain doses causes cancer in rodents.
The ecosystem has clearly been affected in several well documented cases. Deaths of animals were noted after accidental exposures at Seveso, Italy and in Missouri, U.S.A. Adverse effects on reproduction of fish-eating birds have been linked to high ambient exposures in the Great Lakes and on Canada's West Coast. Malformed embryos and reproductive failure have been observed in fish-eating birds in the Great Lakes.
Studies of human populations indicate that exposure to several milligrams of mixtures of dioxins and furans can lead to a variety of effects on skin, eyes, and sensory and behavioural processes. Exposure of women to several milligrams of furans in contaminated rice oil in Japan and Taiwan may have been responsible for reproductive anomalies and infant mortality. To date, there has been no adequate demonstration that human populations exposed to dioxins and furans have suffered excess cancer.
Lifetime daily doses of 1 nanogram of 2,3,7,8-tetrachlorodibenzodioxin per kilogram of body weight have no effect on the incidence of cancer in rodents and did not affect fertility, litter size, fetal resorption and organ function in rats exposed through three generations. This dose is considered to be the no-observed-adverse-effect-level for 2,3,7,8-tetrachlorodibenzodioxin.
A recent Federal-Ontario multimedia study has estimated the average total exposure of Canadian adults and children to dioxins and furans from all pathways. These estimates are based on the average intakes and representative concentrations of these substances in the media to which Canadians are exposed. The average daily Canadian intake of dioxins and furans over a lifetime is estimated to be between 2.0 and 4.2 picograms [see page ix] of 2,3,7,8-tetrachlorodibenzodioxin toxic equivalents per kilogram of body weight per day. Virtually all of this intake comes from food.
Based on the no-observed-adverse-effect-level of 1 nanogram per kilogram of body weight per day and a 100-fold uncertainty factor, it is concluded that human intakes should be below 10 picograms toxic equivalents per kilogram of body weight per day averaged over a lifetime. This value may also be applicable to populations of wild mammals. While the estimated average Canadian exposure to dioxins and furans is below this 10 picogram intake level, persons eating highly contaminated fish in quantities well in excess of the general population norm may approach or exceed the guideline for intake. Wildlife that consumes highly contaminated fish may also be taking in a dose of dioxins and furans that exceeds their tolerable daily intake. Elevated concentrations of these substances in some shellfish near some pulp and paper mills that use chlorine bleaching have necessitated closure of the affected fisheries in order to protect human health. Estimates of dioxin and furan intakes by infants through breast milk are also relatively high. Although medical opinion is that the known benefits of breast feeding outweigh any potential risks, further contamination of human breast milk may lead to significant and unacceptable exposures to dioxins and furans.
Some dioxins and furans are very persistent, and continued release of these chemicals into the environment could unnecessarily prolong exposures, with a resultant increase in the risk to the environment and to human health. Those chemicals which are sources of dioxins and furans are already subject to stringent controls. However, dioxins and furans continue to be released from other sources such as incinerators which employ old technology and from pulp mills that use chlorine bleaching. Large quantities of PCBs (which are a potential source of furans) are currently in storage awaiting the establishment of suitable destruction facilities, which are starting to become available in Canada.
The most significant dioxin sources are the wood preservative pentachloro-phenol, municipal incinerators, and pulp and paper mills using chlorine for the bleaching process. Polychlorinated biphenyls (PCBs) are the most significant potential source of furans.
Based on these considerations the Ministers of the Environment and of National Health and Welfare have concluded that polychlorinated dibenzodioxins and polychlorinated dibenzofurans may enter the environment in quantities which have immediate and long-term harmful effects on the environment, and which constitute a danger in Canada to human health. These substances are therefore considered "toxic" as defined under Sections 11(a) and 11(c) of the Canadian Environmental Protection Act.
1 picogram = 1 x 10-12 grams
1 nanogram = 1 x 10-9 grams
1 microgram = 1 x 10-6 grams
1 milligram = 1 x 10-3 grams
Introduction
The new Canadian Environmental Protection Act (CEPA) requires the Ministers of the Environment and of National Health and Welfare to prepare and publish a Priority Substances List that identifies substances, including chemicals, groups of chemicals, effluents and wastes which may be harmful to the environment or constitute a danger to human health. The Act requires the federal Ministers of the Environment and of National Health and Welfare to assess these substances and determine whether they are "toxic" as defined in Section 11 of the Act which states:
"A substance is toxic if it is entering, or may enter, the environment in a quantity or concentration, or under conditions:
- having, or that may have, an immediate or long-term harmful effect on the environment;
- constituting, or that may constitute, a danger to the environment on which human life depends; or
- constituting, or that may constitute, a danger in Canada to human life or health."
While research and information collection can be undertaken, the primary objective is to determine whether these substances are "toxic" according to the definition under the Act, in which case they are to be placed on Schedule I of the Act, which allows for the making of regulations to control any aspect of their life cycle, from the research and development stage through manufacture, use, storage, transport and ultimate disposal.
This report assesses the "toxicity" of the first substances to be evaluated: polychlorinated dibenzodioxins (dioxins) and polychlorinated dibenzofurans (furans), two related families of chemicals.1
Dioxins and furans have been reviewed extensively by a variety of experts and organizations.2 For the purpose of this assessment it is not necessary to perform a detailed review of the subject matter. Instead, this report is designed to be a general summary of the key findings of these previous reviews, and uses this information as the basis for the assessment of "toxicity".
1 For simplicity, this report will use the terms "dioxins" and "furans" when referring to polychlorinated dibenzodioxins and polychlorinated dibenzofurans. However, when identifying a specific member of either of these families, such as 2,3,7,8-tetrachlorodibenzodioxin, the full name will be used.
2 Reviews have been conducted by: Moore, 1973; Blair, 1973; IARC, 1977 Cattabeni et al., 1978; Esposito et al., 1980; Hutzinger et al., 1981; NRCC, 1981a, b, 1984; OME, 1985; Kimbrough, 1980; Kimbrough et al., 1984; FRG, 1985; U.S.EPA, 1985, 1988; WHO, 1987, 1989 (in press). Extensive compilations of papers produced at symposia include: Tucker et al., 1983; Boddington et al., 1985; Hutzinger et al., 1983, 1986; McNelis et al., 1989.
Identity and Properties
Dioxins and furans are two large groups of chlorinated organic chemicals with properties that indicate a strong affinity for sediments and a high potential for accumulating in fish, birds, animals and humans.
Dioxins and furans are groups of chemicals whose structural formulae are shown in Figure 1. A dioxin or furan molecule can have as few as one, or as many as eight chlorine atoms attached to the molecule at any of eight locations.
Figure 1 Dioxin, Furan

Dioxin |
Furan |
It is both the number of chlorine atoms, and their position in the molecule, that determine the physical and chemical properties, as well as the toxic potency, of a given dioxin or furan. The most hazardous dioxin has chlorine atoms attached at positions 2, 3, 7 and 8, and is therefore referred to as "2,3,7,8-tetrachlorodibenzodioxin" (Figure 2).
Figure 2
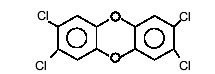 |
| 2,3,7,8-tetrachlorodibenzodioxin |
|---|
In total, there are 210 dioxins and furans: 75 from the dioxin group (Table 1), and 135 from the furan group (Table 2). The most studied of these is 2,3,7,8-tetrachlorodibenzodioxin, but information regarding selected characteristics of some of the others is also available.3
MONOCHLORO-(2)1 |
PENTACHLORO-(14) |
||
1- |
2- |
1,2,3,4,6- |
1,2,3,4,7- |
1,2,3,6,7- |
1,2,3,6,8- |
||
1,2,3,6,9- |
1,2,3,7,8- |
||
DICHLORO-(10) |
1,2,3,7,9- |
1,2,3,8,9- |
|
1,2- 1,3- 1,4- |
1,2,4,6,7- |
1,2,4,6,8- |
|
1,6- 1,7- 1,8- |
1,2,4,6,9- |
1,2,4,7,8- |
|
1,9- 2,3- 2,7- |
1,2,4,7,9- |
1,2,4,8,9- |
|
2,8- |
|
|
|
TRICHLORO-(14) |
HEXACHLORO-(10) |
||
1,2,3- |
1,2,4- |
1,2,3,4,6,7- |
|
1,2,6- |
1,2,7- |
1,2,3,4,6,8- |
|
1,2,8- |
1,2,9- |
1,2,3,4,6,9- |
|
1,3,6- |
1,3,7- |
1,2,3,4,7,8- |
|
1,3,8- |
1,3,9- |
1,2,3,6,7,8- |
|
1,4,6- |
1,4,7- |
1,2,3,6,7,9- |
|
1,7,8- |
2,3,7- |
1,2,3,6,8,9- |
|
TETRACHLORO-(22) |
1,2,3,7,8,9- |
||
1,2,3,4- |
1,2,3,6- |
1,2,4,6,7,9- |
|
1,2,3,7- |
1,2,3,8- |
1,2,4,6,8,9- |
|
1,2,3,9- |
1,2,4,6- |
HEPTACHLORO-(2) |
|
1,2,4,7- |
1,2,4,8- |
1,2,3,4,6,7,8- |
|
1,2,4,9- |
1,2,6,8- |
1,2,3,4,6,7,9- |
|
1,2,6,7- |
1,2,7,8- |
OCTACHLORO-(1) |
|
1,2,6,9- |
1,2,8,9- |
1,2,3,4,6,7,8,9- |
|
1,2,7,9- |
1,3,6,9- |
||
1,3,6,8- |
1,3,7,9- |
||
1,3,7,8- |
1,4,7,8- |
||
1,4,6,9- |
2,3,7,8- |
||
1 Number of Isomers in that group
MONOCHLORO-(4)1 |
HEXACHLORO-(16) |
||||
1- |
2- |
3- |
4- |
1,2,3,4,6,7- |
1,2,3,4,6,8- |
1,2,3,4,6,9- |
1,2,3,4,7,8- |
||||
1,2,3,4,7,9- |
1,2,3,4,8,9- |
||||
DICHLORO-(16) |
1,2,3,6,7,8- |
1,2,3,6,7,9- |
|||
1,2- |
1,3- |
1,4- |
1,6- |
1,2,3,6,8,9- |
1,2,3,7,8,9- |
1,7- |
1,8- |
1,9- |
2,3- |
1,2,4,6,7,8- |
1,2,4,6,7,9- |
2,4- |
2,6- |
2,7- |
2,8- |
1,2,4,6,8,9- |
1,3,4,6,7,8- |
3,4- |
3,6- |
3,7- |
4,6- |
1,3,4,6,7,9- |
2,3,4,6,7,8- |
TRICHLORO-(28) |
HEPTACHLORO-(4) |
||||
1,2,3- |
1,2,4- |
1,2,6- |
1,2,7- |
1,2,3,4,6,7,8- |
|
1,2,8- |
1,2,9- |
1,3,4- |
1,3,6- |
1,2,3,4,6,7,9- |
|
1,3,7- |
1,3,8- |
1,3,9- |
1,4,6- |
1,2,3,4,6,8,9- |
|
1,4,7- |
1,4,8- |
1,4,9- |
1,6,7- |
1,2,3,4,7,8,9- |
|
1,6,8- |
1,7,8- |
2,3,4- |
2,3,6- |
OCTACHLORO-(1) |
|
2,3,7- |
2,3,8- |
2,4,6- |
2,4,7- |
1,2,3,4,6,7,8,9- |
|
2,4,8- |
2,6,8- |
3,4,6- |
3,4,7- |
||
TETRACHLORO-(38) |
|||||
1,2,3,4- |
1,2,3,6- |
1,2,3,7- |
|
||
1,2,3,8- |
1,2,3,9- |
1,2,4,6- |
|||
1,2,4,7- |
1,2,4,8- |
1,2,4,9- |
|||
1,2,6,7- |
1,2,6,8- |
1,2,6,9- |
|||
1,2,7,8- |
1,2,7,9- |
1,2,8,9- |
|||
1,3,4,6- |
1,3,4,7- |
1,3,4,8- |
|||
1,3,4,9- |
1,3,6,7- |
1,3,6,8- |
|||
1,3,6,9- |
1,3,7,8- |
1,3,7,9- |
|||
1,4,6,7- |
1,4,6,8- |
1,4,6,9- |
|||
1,4,7,8- |
1,6,7,8- |
2,3,4,6- |
|||
2,3,4,7- |
2,3,4,8- |
2,3,6,7- |
|||
2,3,7,8- |
2,3,8,9- |
2,4,6,7- |
|||
2,4,6,8- |
3,4,6,7- |
|
|||
PENTACHLORO-(28) |
|||||
1,2,3,4,6- |
1,2,3,4,7- |
1,2,3,4,8- |
1,2,3,4,9- |
||
1,2,3,6,7- |
1,2,3,6,8- |
1,2,3,6,9- |
1,2,3,7,8- |
||
1,2,3,7,9- |
1,2,3,8,9- |
1,2,4,6,7- |
1,2,4,6,8- |
||
1,2,6,7,8- |
1,2,6,7,9- |
1,3,4,6,7- |
1,3,4,6,8- |
||
1,2,4,6,9- |
1,2,4,7,8- |
1,3,4,7,9- |
1,2,4,8,9- |
||
1,3,4,6,9- |
1,3,4,7,8- |
1,3,4,7,9- |
1,3,6,7,8- |
||
1,4,6,7,8- |
2,3,4,6,7- |
2,3,4,6,8- |
2,3,4,7,8- |
||
1 Number of Isomers in that group.
The fate of contaminants in the environment depends on both the properties of the chemical and the properties of the ecosystem itself.4 One can make predictions about how dioxins and furans partition among water, soil, sediments, plants and animals by examining such properties as the contaminants' molecular weights, vapour pressures, and solubilities in water. One measure that is particularly relevant for predicting the environmental fate of dioxins and furans is the octanol-water partition coefficient. A high octanol-water value means that a chemical is more attracted to animal or human fat tissues; compounds with a high octanol-water partition coefficient have a low water solubility and tend to accumulate in soils, aquatic sediments and animals. The octanol-water partition coefficient of dioxins and furans increases as the number of chlorine atoms in the molecule increases (see Table 3). Therefore, the higher the chlorine content of the dioxins and furans, the greater their potential to accumulate in sediments, soils or living organisms. However, after the log octanol-water coefficient reaches a value of about 6, increased molecular size and decreased solubility usually result in decreased bioconcentration. Substances with high octanol-water coefficients also tend to be singly bound to sediments and therefore generally unavailable to animals.5
1-chlorodibenzodioxin |
4.75 |
2-chlorodibenzodioxin |
5.00 |
2,3-dich1orodibenzodioxin |
5.60 |
2,7-dich1orodibenzodioxin |
5.75 |
2,8-dich1orodibenzodioxin |
5.60 |
1,2,4-trich1orodibenzodioxin |
6.35 |
1,2,3,4-tetrach1orodibenzodioxin |
6.60 |
1,2,3,7-tetrachlorodibenzodioxin |
6.90 |
1,3,6,8-tetrach1orodibenzodioxin |
7.10 |
1,3,7,8-tetrach1orodibenzodioxin |
6.80 |
1,2,3,4,7-pentachlorodibenzodioxin |
7.40 |
1,2,3,4,7,8-hexachlorodibenzodioxin |
7.80 |
1 ,2,3,4,6,7,8-heptachlorodibenzodioxin |
8.00 |
octachlorodibenzodioxin |
8.20 |
3 Recent estimates of the physical and chemical characteristics of 2,3,7,8-tetrachlorodibenzodioxin are given in U.S.EPA, 1988. Information on selected properties of some other dioxins and furans is also available (Shiu et al., 1988; U.S.EPA, 1988).
Toxicity Equivalency Factors
The toxicity of dioxin and furan mixtures in the environment can be assessed by using an internationally accepted system of comparison known as "toxicity equivalency factors." The scientific and regulatory communities of eight countries6 have agreed upon a standard set of International Toxicity Equivalency Factors that compare the toxicity of the 17 most toxic dioxins and furans found in complex mixtures to that of 2,3,7,8-tetrachlorodibenzodioxin. This system has been broadly corroborated in laboratory studies.
Dioxins and furans usually enter and are present in the environment as complex mixtures. While it is possible to separate and study each type of dioxin and furan individually, it would be time-consuming and very expensive (if not impossible) to test each of the 75 dioxins and 135 furans as well as all conceivable combinations.
Fortunately, individual testing can be avoided. Many dioxin and furan compounds have been tested for their toxic effects on living animals and in cell cultures, as well as for their ability to induce one or more of a number of liver enzymes. Researchers have developed a method for expressing the toxicity of different dioxins and furans on a common basis; International Toxicity Equivalency Factors recognize and compare the similarities and differences between the toxic action of the dioxins and furans.7
International Toxicity Equivalency Factors are assigned to individual dioxins and furans on the basis of how toxic they are in comparison with the toxicity of 2,3,7,8-tetrachlorodibenzodioxin, the most potent dioxin. This contaminant has been assigned the value of 1.0.8 By comparison, animal and cell tests show that 2,3,7,8-tetrachlorodibenzofuran is approximately one-tenth as toxic as 2,3,7,8-tetrachlorodibenzodioxin. Consequently, its toxic equivalent value is 0.1. International Toxicity Equivalency Factors have been developed for those dioxins and furans that contribute most to the toxicity of a complex mixture, which are those that have chlorines in at least the 2, 3, 7 and 8 positions. The toxicity is decreased from that of 2,3,7,8-tetrachlorodibenzodioxin in a predictable manner for those dioxins or furans which have either fewer or more chlorines than this compound, and/or which have chlorines in positions 1, 4, 6 or 9.
Dioxin/Furan |
Equivalency Factor |
|---|---|
2,3,7,8-tetrachlorodibenzodioxin |
1 |
1,2,3,7,8-pentachlorodibenzodioxin |
0.5 |
1,2,3,4,7,8-hexachlorodibenzodioxin |
0.1 |
1,2,3,7,8,9-hexachlorodibenzodioxin |
|
1,2,3,6,7,8-hexachlorodibenzodioxin |
|
1,2,4,6,7,8-heptachlorodibenzodioxin octachlorodibenzodioxin |
0.01 |
2,3,7,8-tetrachlorodibenzofuran |
0.001 |
2,3,4,7,8-pentachlorodibenzofuran |
0.5 |
1,2,3,7,8-pentachlorodibenzofuran |
0.05 |
1,2,3,4,7,8-hexachlorodibenzofuran |
0.1 |
1,2,3,7,8,9-hexachlorodibenzofuran |
|
1,2,3,6,7,8-hexachlorodibenzofuran |
|
2,3,4,6,7,8-hexachlorodibenzofuran |
|
1,2,3,4,6,7,8-heptachlorodibenzofuran |
0.01 |
1,2,3,4,7,8,9-heptachlorodibenzofuran |
|
octachlorodibenzofuran |
0.001 |
Of the 210 dioxins and furans, 17 contribute most to the toxicity of a complex mixture and are of the greatest concern. This does not mean that the remaining 193 dioxins and furans are not toxic, but merely that they contribute comparatively little to the toxicity of a complex mixture.
6 Canada, Denmark, the Federal Republic of Germany, Italy, the Netherlands, Norway, the United Kingdom and the United States.
Sources of Environmental Contamination
The most significant dioxin sources are the wood preservative pentachlorophenol, municipal incinerators, and pulp and paper mills using chlorine for the bleaching process. Polychlorinated biphenyls (PCBs) are the most significant potential source of furans.
Pentachlorophenol as a wood protection agent is being phased out of use and replaced by alternatives. Substantial quantities of dioxins and furans continue to be emitted from municipal incinerators employing old technology and from pulp mills using chlorine for the bleaching process. The technology to limit these releases is available. PCBs are subject to strict storage, handling, and inventory controls, and await the establishment of suitable facilities for their destruction.
Dioxins and furans may enter the environment from four broad categories of sources: chemical products, combustion, natural and industrial.
Most of these sources produce complex mixtures containing both dioxins and furans. For instance, the herbicide 2,4-D contains a mixture of dichloro-, trichloro- and tetrachloro-dioxins, while the wood preservative pentachloro-phenol contains hexachloro-, heptachloro-, and octachloro-dioxins and furans. Incinerators produce a wide range of tetrachloro-, pentachloro-, hexachloro-, heptachloro-, and octachloro-dioxins and furans. The dioxin and furan contaminants most often associated with pulp and paper mills are predominantly 2,3,7,8-tetrachlorodibenzofuran and 2,3,7,8- tetrachlorodibenzodioxin. Complex mixtures of furans are found in PCBs.
Chemical sources
A wide variety of commercial chemicals contain dioxins or furans as impurities. None of these is currently manufactured in Canada, and the use of chemicals containing these contaminants has decreased considerably over the past ten years (see Table 5), a trend that is expected to continue.
Pentachlorophenol (include tetrachlorophenol) is still widely used in Canada to preserve and protect wood. Although in 1981 this compound was one of the largest potential sources of dioxins to the environment, the dioxin content and the amounts used have since been reduced.
Consequently, today its potential as a source is only one tenth of that noted earlier by the National Research Council of Canada (NRCC),9 and this is likely to decrease further as pentachlorophenol is replaced by alternative wood protection chemicals or processes in the near future.10
The second largest chemical source for dioxins is the herbicide 2,4-D. The dioxins contained in 2,4-D are not substituted in 2, 3, 7 and 8 positions, and are considered to be relatively benign. Their concentration in 2,4-D is now strictly controlled. The chemical known as 2,4,5-T, and mixtures such as Agent Orange, both of which contained 2,3,7,8-tetrachlorodibenzodioxin, are no longer registered or used in Canada. The same is true for the closely related 2,4,5-trichlorophenol. Hexachlorophene, which is believed to contain 2,3,7,8-tetrachlorodibenzodioxin, is still used for a number of registered health care products.
|
19801 |
1987 |
|---|---|---|
Pentachlorophenol |
2300 |
1340 |
Sodium pentachlorophenate and tetrachlorophenate |
1200 |
402 |
2,4,5-T |
50 |
0 |
2,4-D |
3800 |
4546 |
Hexachlorophene |
7 |
7 |
1approximate values for me period from 1978 to 1981 (See NRCC, 1981a)
The most significant potential source of furans is PCBs. The total amount of furans contained in PCBs in storage or in use in Canada is estimated to be 75 kilograms.11 While the use and storage of PCBs is now strictly controlled, the potential for releases through accidental spills, or fires in equipment containing PCBs, remains. Existing stocks of PCBs await the establishment of suitable destruction facilities, which are now starting to become available.
Combustion sources
Dioxins and furans are released by numerous combustion sources. In Canada, municipal incinerators are the major concern. Environment Canada's National Incinerator Testing and Evaluation Program (NITEP) has demonstrated that emissions from even the oldest of incinerators can be controlled to acceptable levels, provided correct management practices and modern control technologies are applied. Newer incinerators can be controlled even more successfully. Environment Canada's current estimates for total dioxin and furan emissions from all Canadian incinerators range widely, depending on the assumptions used, from between 20 grams per year if all incinerators were operating with Best Available Practicable Technology to 6000 grams per year if all were old and poorly managed incinerators.
A variety of other combustion sources have been reported to give rise to dioxins and furans but their contribution to total exposures is uncertain. Burning wood in stoves and fireplaces and barbecuing produce dioxins and furans in small amounts. Cigarette smoke also contains a variety of dioxins.12
Natural sources
There may be two natural sources of dioxins and furans: forest fires and volcanoes. There are no data specific to these sources, although sediments that are 300 to 1000 years old have low concentrations of the less toxic dioxins and furans.13
Industrial sources
Several industries release dioxins and furans in their effluents. Of significance to Canada are the pulp and paper mills that use chlorine in the bleaching process. Effluents and products from these mills contain concentrations of dioxins and furans at the parts-per-trillion level. Environment Canada estimates of the total amount of dioxins and furans released, based on the number of mills, the estimated annual bleached-pulp production, and the estimated effluent discharges, are approximately 100 to 150 grams per year of 2,3,7,8-tetrachlorodibenzodioxin and 2000 to 3000 grams per year of 2,3,7,8-tetrachlorodibenzofuran. Dioxin and furan production from pulp and paper mills can be reduced by changing the processing system and reducing the demand for bleached pulp.
Considering only the total quantity of dioxins and furans emitted from a source does not necessarily reflect the true toxic potential. The use of International Toxicity Equivalency Factors enables us to compare the toxic potential of the different sources of these chemicals. For example, the toxic potential of both pentachlorophenol and incinerator emissions would be reduced significantly by applying these factors while the toxic potential of pulp mill effluents would hardly change at all. PCBs would remain the most significant potential source of furans.
Environmental Fate and Levels
Dioxins and furans occur throughout the Canadian environment and are found in all compartments of the ecosystem, including air, water and soil. These contaminants are highly persistent. They are transported to remote parts of the country by the prevailing winds and are deposited in sediments and soils. They accumulate in animals and have been found in most species of wildlife surveyed. They also appear in the natural and human food chains. All Canadians have detectable concentrations in their body fat. In this respect, Canadians are no different from individuals living in other industrialized countries.
Transport and persistence
Dioxins and furans do not move readily through soils and sediments because they generally attach to the particles. Soils and sediments represent the most significant "sink" for dioxins and furans. Once dioxins, particularly 2,3,7,8-tetrachlorodibenzodioxin, enter the soil and sediments, they are very slow to degrade. 2,3,7,8-Tetrachlorodibenzodioxin has a degradation half-life of ten years or longer. Dioxins and furans also accumulate in biological tissue where they also have long half-lives. Recent studies of aerial transport14 reveal that prevailing winds can play a significant role in environmental contamination. Contamination of wildlife in the Far North appears to be primarily the result of aerial transport.
Levels
Since the 1981 report of the NRCC,15 further research has confirmed that dioxins occur everywhere in the environment. A summary of many recent data for air, water, soil, foods and consumer products was prepared by the Federal-Ontario Ad Hoc Working Group on Multimedia Standards.16 The summary's findings are condensed in Table 6.
1ND: not detectable
2fw: fresh weight
Dioxin and furan levels in North American air, as summarized in Table 6, ranged from 0.4 to 36.7 picograms per cubic metre. In drinking water, one sample Out of 800 contained 46 picograms per litre of octachlorodibenzo-dioxin. Soil values varied from a low of 50 picograms per gram to a high of 14,100 picograms total dioxins and furans per gram of soil in highly contaminated areas.
The high water-octanol partition coefficients of dioxins and furans indicate a potential for considerable accumulation in biological tissues. The compounds which are substituted in the 2, 3, 7 and 8 positions are not only accumulated but are also metabolized slowly and therefore persist in the body tissues for considerable lengths of time. More than 100 species of invertebrates, fish, reptiles, amphibians, birds and mammals across Canada have been demonstrated to contain detectable levels of dioxins and furans.17
Environment Canada surveys revealed that levels, of 2,3,7,8-tetrachloro-dibenzodioxin ranged up to 37 nanograms per kilogram in mammals; up to 1996 nanograms per kilogram in birds; up to 474 nanograms per kilogram in reptiles; and up to 35 nanograms per kilogram in amphibians.
The department of Fisheries and Oceans coordinated a National Dioxin and Furan Fish Sampling Program to assess the level of contamination in fish and shellfish from the vicinity of 47 Canadian pulp mills employing the chlorine bleaching process. Levels of 2,3,7,8-tetrachlorodibenzodioxin reported in this survey ranged up to 662 nanograms per kilogram in Dungeness crab hepatopancreas; up to 31 nanograms per kilogram in shellfish tissue; and up to 137 nanograms per kilogram in fish.18 Mean values of 2,3,7,8-tetrachlorodibenzodioxin for whole fish lake trout samples from Lake Ontario averaged more than 35 nanograms per kilogram from 1977 through 1988.19 Much of the Lake Ontario contamination is related to upstream losses from chlorophenol manufacturing waste dumps.
A long-term study of 2,3,7,8-tetrachlorodibenzodioxin residue levels in herring gull eggs from Lake Ontario showed that the highest level of 1996 nanograms of 2,3,7,8-tetrachlorodibenzodioxin per kilogram was found in eggs collected in the early 1970s and the levels declined steadily to 50 nanograms per kilogram by 1980.20 During the 1980s, levels have been fairly constant and similar to background levels found in wildlife across the country.
Levels of dioxins and furans in the eggs of great blue herons from British Columbia exceed those currently found in herring gull eggs from the Great Lakes. This indicates the extent of the contamination of certain near shore zones in the southern Strait of Georgia off Canada's West Coast.21 Studies have linked the 2,3,7,8-tetrachlorodibenzodioxin in the herons to emissions from pulp mills using chlorine in the bleaching process, whereas the pentachloro-dioxins and hexachloro-dioxins were related to a chlorophenol source, possibly the use of chlorophenol-contaminated wood chips in the pulping process. Dioxin and furan contamination in some species of shellfish in the vicinity of certain pulp mills resulted in the closure of certain fisheries in November of 1988 and again in 1989.
Dioxins and furans, particularly 2,3,7,8-tetrachlorodibenzodioxin and octa-chlorodibenzodioxin, have been found in the tissues of polar bears, ringed seals and beluga whales from areas in the Canadian Arctic that are far from sources of these compounds. This indicates distribution by long-range transport mechanisms such as the prevailing winds.22
Dioxins and furans move along the natural food chain and are found in the food consumed by humans. Canadian foods that have been tested include fish, beef, pork, poultry, eggs, milk, fruits, vegetables and wheat-based products (Table 6). Detectable levels of octachlorodibenzodioxin have been reported in all of these food types; hexachloro- and heptachloro-dioxins and furans, and octachlorodibenzofuran have been reported in some fatty foods. Maximum total dibenzodioxin concentrations in whole fish samples from the Great Lakes have been reported at greater than 200 nanograms per kilogram. 23
A recent report indicates that smoking represents another potential source of exposure to dioxins. The total concentration of dioxins in cigarette smoke was determined to be approximately 5 micrograms per cubic metre (or 1.8 nanograms toxic equivalents per cubic metre).24
Human fat tissues sampled from several industrialized countries, including Canada, contain primarily dioxins and furans substituted in the 2, 3, 7 and 8 positions.25 The average background concentrations of tetrachloro-dioxins range from approximately 3 to 10 nanograms per kilogram; pentachloro-dioxins from 10 to 15 nanograms per kilogram; hexachloro-dioxins from 20 to 100 nanograms per kilogram; heptachloro-dioxins from 100 to 250 nanograms per kilogram; and octachlorodibenzodioxin from 250 to 1000 nanograms per kilogram. In general, the concentrations for furans are considerably lower than those for dioxins.
Persons exposed to substances containing dioxins and furans through occupational or accidental exposures have elevated body fat burdens of the 2,3,7,8-substituted compounds to which they were exposed. Some victims of a 1968 mass poisoning in Japan, the "Yusho" incident, had concentrations of up to more than two orders of magnitude higher than background levels in the general population (see Effects on Humans).
The types and concentrations of dioxin and furan compounds in human milk fat are similar to those in fat tissue. This indicates that the proximate source of these contaminants for breast milk is the fat stores of the mother.25 The average milk fat concentration in people from industrialized countries is about 2 nanograms per kilogram of 2,3,7,8-tetrachlorodibenzodioxin. Other dioxins are present at higher concentrations and in most instances more than one half is octachlorodibenzodioxin. Furans are generally found at lower concentrations. Levels in milk fat decline significantly as a function of the number of offspring borne if the mother breast feeds her children. The World Health Organization26 reports that the average background concentrations of dioxins and furans in human milk fat in industrialized countries range from 5 to 53 nanograms toxic equivalents per kilogram of milk fat.
Recent improvements in analytical sensitivity enable the detection of dioxins and furans in all samples of blood from members of the general population at the parts-per-quadrillion level.27 The blood of people exposed both occupationally and through transformer fires shows higher levels of contamination. In some instances these levels reach tens or even hundreds of parts-per-trillion of the dioxins or furans to which they have been exposed. Initial blood concentrations of total furans in the Taiwanese accidentally exposed to PCBs in the "Yu-Cheng" incident (see Effects on Humans) were similarly elevated.
17 Norstrom et al., 1982, 1986; Norstrom and Simon 1983; Stalling et al., 1983, 1985, 1986; ICTC 1986; Braune and Norstrom 1989; Fox et al., 1988; Kubiak et al., 1989; Elliott et al., 1988, 1989; Ryan et al., 1986.
Kinetics and Metabolism
When mixtures of dioxins and furans enter living tissue, those compounds with chlorine in the 2, 3, 7 and 8 positions are the ones most predominantly absorbed and retained. They are most highly concentrated in body fat and in fatty organs such as the liver. While they can be metabolized and excreted, this is a relatively slow process in humans. Half-lives are several years. Their excretion in breast milk can significantly affect the body burden of both the mother (as a source of elimination) and the breast-fed infant (as a source of exposure).
In experimental studies, the absorption of dioxins and furans varies, depending on the medium in which they are administered, the specific compound involved and whether exposure took place through the mouth, skin or lungs.28 Examples include: rats exposed orally to 2,3,7,8-tetrachlorodibenzodioxin absorb 70 to 80 percent of a dose delivered in oil; 50 to 60 percent of a dose mixed in food; 10 to 20 percent of a dose in soil or fly ash; and virtually none of a dose absorbed onto activated carbon. The higher chlorinated dioxins and furans that have been tested seem to be less well absorbed than 2,3,7,8-tetrachlorodibenzodioxin.
In general, absorption is greater when dioxins and furans are ingested than when applied to the skin. Absorption by the lung is probably high but inadequately tested. Human absorption is probably similar to that demonstrated in animal studies. A recent study29 estimated that 87 percent of a 1.14 nanogram per kilogram dose of 2,3,7,8-tetracblorodibenzodioxin ingested by a volunteer was absorbed.
When dioxins or furans are absorbed into the body, they are distributed to a variety of tissues. The liver and fat tissues are the major storage sites in all animal species, including humans.30 Small amounts may also be found in skin, muscle and other organs. Dioxins and furans tend to be found in concentrations in different tissues within the body in proportion to the fat content of the tissue. Distribution of the specific dioxins and furans is dependent on the route of exposure, the amount of exposure and the length of time since exposure began.
The metabolism of dioxins and furans has been studied in laboratory animals but not in humans. While similar paths of metabolism exist in most animals studied, including fish, the rates at which metabolism occurs vary among species.31 Rats metabolize 2,3,7 ,8-tetrachlorodibenzodioxin relatively readily, mice and monkeys, less readily, and metabolism by guinea pigs is quite limited. The furans that have been studied appear to be metabolized by similar pathways to the dioxins. Octachloro-dioxin and -furan are not readily broken down in the body.
The rate at which dioxins or furans are eliminated from the body varies from species to species.31 However, the route of elimination is predominantly the same across species, i.e. the faeces. For most test animals 50 to 80 percent of an administered dose of 2,3,7,8-tetrachlorodibenzodioxin is excreted in the faeces. A lesser amount is excreted in urine. A single dose of octachloro-dibenzodioxin tends to be excreted virtually unchanged, reflecting its poor absorption into the body following ingestion and its resistance to metabolic degradation. Breast milk can be a significant route of elimination, because its high fat content allows the dioxins and furans to dissolve in the fat and leave the body with the milk. Such elimination can significantly lower the body burden of nursing mothers, but the dioxins and furans will be absorbed by their infants.
Whole-body half-lives have been estimated for 2,3,7,8-tetrachlorodibenzo-dioxin in several test species.31 These estimates are similar for rats (range: 17.4 to 31 days) and mice (9.6 to 24.4 days), similar or longer for guinea pigs (22 to 93.7 days), and somewhat shorter for hamsters (12.0 to 15.0 days). Monkeys appear to excrete 2,3,7,8-tetrachlorodibenzodioxin much more slowly, with a half-life of approximately one year.32 Furan compounds are eliminated more rapidly than the corresponding dioxins, i.e. those with the same substitution pattern.
In humans, the half-life of 2,3,7,8-tetrachlorodibenzodioxin has been estimated to be 5.8 years33 and between 5 and 8 years.33 The half-life for 2,3,7,8-tetrachlorodibenzofuran in humans is somewhat shorter (1 to 2 years).
Toxicology
The compound 2,3,7,8-tetrachlorodibenzodioxin (and to a lesser extent, the other dioxins and furans which are substituted in the 2, 3, 7 and 8 positions) are extremely toxic to mammals, with a wide variation in sensitivity among species. Bird and fish species tested seem to be more sensitive than most mammals to acute exposures to dioxins. In laboratory animals, death results from a single exposure to amounts ranging from less than one microgram to a few milligrams per kilogram of body weight. Longer-term exposure to lesser amounts can activate enzymes or lead to tissue damage. It can also affect reproduction, cause developmental deformities in the fetus and cause cancer. However, 2,3,7,8-tetrachlorodibenzodioxin does not appear to damage the genetic material or chromosomes.
The no-observed-adverse-effect-level for 2,3,7,8- tetrachlorodibenzodioxin for chronic exposure in rats (cancer and reproduction) is approximately 1 nanogram per kilogram of body weight per day.
Dioxins and furans that are not substituted in the 2, 3, 7 or 8 positions are far less toxic and are less capable of inducing enzymes.
Short-term exposure in mammals
Mammals vary widely in their sensitivity to single-dose exposures of dioxins and furans. For 2,3,7,8-tetrachlorodibenzodioxin -- the most acutely toxic of all dioxins and furans -- the lethal oral dose resulting in death for 50 percent of the exposed animals ranges from 0.6 micrograms per kilogram of body weight for guinea pigs to 5051 micrograms per kilogram of body weight for hamsters.35 Recent data on mink show them to be a sensitive species with a median lethal dose of 4.2 micrograms per kilogram.36 Guinea pigs are more sensitive than rats or monkeys, which are in turn more sensitive than rabbits or mice. The common effects on all mammals exposed to 2,3,7,8-tetra-chlorodibenzodioxin are loss of body weight and shrinkage of the thymus gland, followed by death approximately three weeks after exposure. Other effects commonly observed in one or more test mammals include: skin changes, such as eruptions, thickening or discolouration; hair loss; liver damage; and changes in the white cells of the blood and bone marrow.
While other dioxins and furans are less acutely toxic than 2,3,7,8-tetrachloro-dibenzodioxin, they produce similar symptoms. Their toxicity varies widely and depends on the number and position of the chlorine atoms on the molecule. The most toxic dioxins and furans are those with four, five or six chlorines, particularly when substitution is in the 2, 3, 7 and 8 positions.37
Long-term exposure in mammals
Test animals subjected to longer-term oral exposures of 2,3,7,8-tetrachlorodibenzodioxin exhibit many of the symptoms produced by acute exposures.37 Exposed animals display a "wasting syndrome," which includes reduced food consumption and decreased body weight, often followed by death. Several species develop acne-like lesions. Some lose facial hair, and some suffer fluid build-up under the skin or in the body cavity.
Animals exposed to lethal or sublethal doses of 2,3,7,8-tetrachlorodibenzo-dioxin may also exhibit a marked loss of fat; shrinkage of the thymus, spleen and other lymphoid tissues; changes in numbers of blood cells; elevation of several serum enzymes indicative of tissue damage; changes in the liver; and thickening of the gastrointestinal tract, the gall bladder or the bile duct.
Exposure studies of other dioxins and furans substituted in the 2, 3, 7 and 8 positions have shown similar effects. However, higher doses were needed to elicit the same response as a given dose of 2,3,7,8-tetrachlorodibenzodioxin.
Other effects of concern that result from long-term exposure to dioxins and furans must also be considered in the assessment of health risk. For example, most dioxins and furans substituted in the 2, 3, 7 and 8 positions affect the mammalian immune system. 37 Sustained exposures in mammals have led to suppression of both cell-mediated and humoral-mediated immune responses. This is significant because immune suppression compromises the body's ability to fight infection.
Animal studies indicate that long-term exposure to 2,3,7,8-tetrachlorodi-benzodioxin can result in the formation of tumours and carcinomas.37 38 Mice exposed orally for a lifetime to 2,3,7,8-tetrachlorodibenzodioxin developed cancer of the liver and thyroid. Rats exposed the same way developed cancer of the liver, lung, tongue, hard palate and nose. Lifetime doses in rats of 0.1 microgram per kilogram of body weight per day resulted in tumours predominantly in the liver, but no increases in liver tumours or nodules were observed in rats or mice at 0.001 microgram per kilogram of body weight per day. Both repeated skin applications and injections of 2,3,7,8- tetrachlorodibenzodioxin have resulted in an increase in malignant tumours in different animal species.
Over the lifetime of rats, oral exposures, to a mixture of hexachloro-dioxins led to an increase in liver cancer.39 However, exposure to 2,7-dichloro-dibenzodioxin did not.
Most studies suggest that 2,3,7,8-tetrachlorodibenzodioxin acts only as a promoter of cancer and not as a complete carcinogen. In addition, the presence of tumours both at, and remote from, the treatment site indicates 2,3,7,8-tetrachlorodibenzodioxin can act both locally and throughout the body.
Despite its ability to promote or cause cancer, 2,3,7,8-tetrachlorodibenzo-dioxin does not appear to affect the genetic material of cells (i.e., it is not genotoxic).39 This is supported by negative findings in a variety of studies on well established mutagenicity test systems. Also, 2,3,7,8- tetrachlorodibenzodioxin does not appear to cause changes in chromosome structure. Together, these data suggest that 2,3,7,8-tetrachlorodibenzodioxin and possibly other dioxins and furans which are substituted in the 2, 3, 7 and 8 positions induce cancer by a non-genetic mechanism, which has implications for the method of estimating risk to humans.
Dioxins and furans can also affect reproduction and the development of offspring depending on the dose, the specific compound involved, the route of administration and the animal species. The reproductive ability of rats exposed to 2,3,7,8-tetrachlorodibenzodioxin was clearly affected in a three-generation study.40 Effects on fertility, litter size, fetal resorption and organ function occurred at 0.1 and 0.01 micrograms per kilogram of body weight per day, but not at 0.001 micrograms per kilogram of body weight per day. The reproductive functions of monkeys are quite sensitive to the effects of 2,3,7,8-tetrachlorodibenzodioxin. Spontaneous abortions occurred at 1 microgram per kilogram of body weight per day, but not at 0.2 micrograms per kilogram of body weight per day.41 Several studies have reported degeneration of the testes and functional impairment in the males of several exposed mammalian species.
Oral administration of 2,3,7,8-tetrachlorodibenzodioxin to pregnant mice has resulted in the development of malformations of the palate and kidney in the fetus.42 Other species are less sensitive in this regard than mice. In general, dioxins and furans substituted in the 2, 3, 7 and 8 positions have some potential to produce deformities in mice, while other chlorinated dioxins and furans do not.
Another factor to consider while assessing health risks is that most dioxins and furans substituted in the 2, 3, 7 and 8 positions are potent inducers of enzymes,42 including the liver enzymes that play a role in metabolizing foreign substances. The induction of enzymes is not necessarily dangerous, but indicates that the cells have recognized the presence of a foreign substance and have heightened their response readiness. The toxic dioxins and furans are potent inducers of enzymes, whereas the less toxic dioxins and furans are less able to induce this effect.
Fish
Yellow perch, carp, bullhead, rainbow trout, largemouth bass and bluegill have an 80-day lethal dose for 2,3,7,8-tetrachlorodibenzodioxin ranging from 2 to 23 micrograms per kilogram of body weight (centring around 10 micrograms per kilogram of body weight43). At the highest treatment concentration, the time to death varied from 7 days in the perch to 44 days in the carp. The remaining species had a time to death in the range of 16 to 22 days, which is similar to that of mammals. The only common lethal effect between species was the degeneration of the fin tissue. Some species exhibited effects such as weight loss and hyperpigmentation.
Recent data on rainbow trout show effects upon growth, survival and behaviour at water concentrations of less than 38 picograms of 2,3,7,8-tetrachlorodibenzodioxin per litre.44 The "no-effect concentration" could not be determined. The mortality curve was time- and concentration-dependent; that is, the longer the time, the lower the concentration that was lethal.
Growth inhibition was dose-dependent, as were behaviourial responses including lethargic swimming, feeding inhibition and lack of response to external stimuli. These effects on fish are in addition to the earlier observations of fluid build-up (edema).45
Birds
A recent review has examined the toxicity of 2,3,7,8-tetrachlorodibenzodioxin to birds.46 Lethal dose values, computed 37 days after one oral dose of 2,3,7,8-tetrachlorodibenzodioxin, vary from 15 micrograms per kilogram of body weight in the northern bobwhite, to more than 810 micrograms per kilogram of body weight for the ringed turtle-dove. Mallards were intermediate in sensitivity with an acute oral lethal dose value of more than 108 micrograms per kilogram of body weight.47 Signs of toxicity began seven days after treatment. For all three species, death was delayed, occurring 13 to 37 days after exposure.
Autopsies of ringed turtle-doves that survived treatment showed livers enlarged to twice the normal size. Bobwhites showed severe emaciation, high accumulations of uric acid salts in connective tissues, and fluid accumulations in the lung, heart and abdominal cavities.47
Domestic chickens have an estimated oral lethal dose range of 25 to 50 micrograms of 2,3,7, 8-tetrachlorodibenzodioxin per kilogram of body weight.47 Chickens fed lower doses (which were often lethal) showed signs of extensive fluid buildup under the skin and in the body cavity. This is referred to as chick edema disease.48 Autopsies of poultry that died following a chlorophenol reactor explosion in Seveso, Italy, in 1976, showed signs characteristic of chick edema disease.49 Large amounts of dioxins substituted in the 2, 3, 7 and 8 positions were released at the time of the accident.
Effects on the Ecosystem
Several incidents have focused the public's attention on the dioxin and furan issue. Deaths occurred in exposed animals at Seveso, Italy and in Missouri, USA. Adverse effects on reproduction and malformations in offspring of fish-eating birds have been noted in the Great Lakes and on the West Coast of Canada. It is difficult to determine the full extent to which dioxin and furan contamination affects the environment because of the parallel presence of a large number of other chlorinated organic compounds.
Over the years a number of incidents involving dioxins have focused public attention on these chemicals and on the subject of environmental contamination in general. One of the earliest incidents involved the death of chicken flocks in the United States during the mid-1950s. The causative agent (identified only twelve years later) was hexachloro-dioxin from pentachlorophenol. It had been accidentally added to feed stock from a tallow by-product of (he leather industry. Since that event, there have been many additional incidents, but few have successfully documented specific effects of dioxins and furans alone as their effects are difficult to separate from those of other chemicals in the environment.
Two well-documented incidents took place in Seveso, Italy and Missouri, USA.
Seveso, a small Italian town near Milan, was contaminated by several chemicals, including 2,3,7,8-tetrachlorodibenzodioxin, when a chlorophenol reactor exploded in 1976. While this was only one among many such explosions in the United States and Europe, it differed from the others because the resultant widespread environmental contamination was well documented. Many wildlife deaths, particularly of rabbits, were noted.
The Missouri incident, which occurred in the early 1970s, involved the death of a number of horses at an arena after it had been sprayed with waste oils for dust control. Birds also died and some children became ill. The cause was eventually attributed to 2,3,7,8-tetrachlorodibenzodioxin. The source was waste from the manufacture of 2,4,5-trichlorophenol that had been mixed with PCBs and waste oils. The arena was only one of many areas that was sprayed for dust control. After 10 years, the true dimensions of the Missouri dust spraying incident became clear with the discovery of widespread contamination in the town of Times Beach.50
Great Lakes
During the 1970s, reports of chick edema disease in herring gulls, together with egg failure and birth deformities, were ascribed to dioxin contamination. The incidence of birth anomalies was 100- to 200-fold above the background level for the period 1971 to 1975.51 At that time, levels of 2,3,7,8-tetrachlorodibenzodioxin ranged from 489 to 1996 nanograms per kilogram in Lake Ontario herring gull eggs.
Reproduction rates and the incidence of anomalies returned to normal after 1976.52 By this time levels of 2,3,7,8-tetrachlorodibenzodioxin dropped below 500 nanograms per kilogram in eggs. A cause-effect relationship between 2,3,7,8-tetrachlorodibenzodioxin and reproductive failure could not be established because many other contaminants were also present at very high levels in the eggs and adults.53
West Coast
In 1982, unexpectedly high concentrations of dioxins and furans were found in the eggs of great blue herons in the Eraser River estuary.54 Dioxin levels were also significantly elevated at a colony near a pulp mill at Crofton, British Columbia, that used chlorine in the bleaching process.53 In 1987, heron productivity was normal at three other colonies, while the colony near Crofton failed to produce any young. Mean levels of 2,3,7,8-tetrachlorodibenzodioxin in eggs from the Crofton colony increased threefold from 66 nanograms per kilogram in 1986 to 210 nanograms per kilogram in 1987.
As a result of these studies, as well as recent discoveries of dioxins and furans in pulp mill effluents from plants using chlorine in the bleaching process, a national sampling program was undertaken by the federal government.
Following the first set of results, issued in November 1988, the prawn, shrimp and crab fisheries were closed in the immediate vicinity of the Woodfibre and Port Mellon pulp mills. The crab fishery near the Prince Rupert pulp mill in British Columbia was also closed. Additional commercial shellfish closures took place at seven coastal areas in B.C. in 1989. Furthermore, health advisories were issued by Health and Welfare Canada for some recreational and native shellfish fisheries at nine British Columbia coastal sites as well as for fish species at four British Columbia inland locations and at one Quebec inland location.
Effects on Humans
Human populations appear to be less sensitive to the effects of dioxins and furans than most animal species which have been tested. Exposures in the workplace, or from industrial accidents or the Yusho and Yu-Cheng incidents, have resulted in changes to the skin (including chloracne) that can persist for many years. Such extensive exposures have also produced neurological and psychological effects (including sexual dysfunction), elevated blood levels of some enzymes, and adverse effects on the fetus in some instances. The evidence of a link between cancer incidence or mortality and exposure of human populations to dioxins and furans is equivocal. It is difficult to assess the effects that dioxins and furans may have on human health because of concomitant exposures to other chemicals and imprecise information on exposures.
Dioxins and furans occur only in mixtures as by-products of industrial emissions, combustion, or chemical production. Consequently, it is often difficult to identify clearly how they affect humans, because the influence of co-occurring exposures to chemicals other than dioxins and furans always confounds our ability to establish cause-effect and dose-response relationships.
Dioxins
Numerous industrial accidents around the world, although not in Canada, involving out-of-control reaction vessels used to manufacture chlorinated phenols or phenoxy herbicides have exposed workers to short-term, high-level doses of the dioxins that occur as contaminants of these substances. Although there is documentation describing the effects on more than 1300 exposed workers from 1910 to 1976, the rate, route and length of exposure differs in each report, and therefore the time-to-onset and severity of the signs and symptoms also differ. Nevertheless, exposures have frequently been associated with such effects as: acne-like skin lesions ("chloracne") and dermatitis; fluctuations in serum levels of liver enzymes; pulmonary deficiency; sensory changes such as numbness, nausea, headaches, loss of hearing, sleep disturbances, tiredness; sexual dysfunction; depression; and loss of appetite.55
Populations exposed to dioxin-contaminated materials through non-occupational sources have experienced similar effects. For example, a small number of adults and children exposed to dioxin-contaminated soils in Missouri, USA, developed skin rashes, chloracne, headaches and lethargy.55 The soil contained approximately 6000 micrograms of trichlorophenol, 33 micrograms of 2,3,7,8-tetrachlorodibenzodioxin and 1500 micrograms of PCBs in each gram of soil.
The best-studied exposure of a general population to dioxins occurred following the trichlorophenol reactor explosion in Seveso, Italy in 1976. Some 190 people developed chloracne. No other sustained or clear effects have been attributed to 2,3,7,8-tetrachlorodibenzodioxin exposure in eight years of study.55
Some studies of populations in areas of Vietnam sprayed with dioxin-contaminated herbicides have concluded that these herbicides have had effects on human reproduction.56 In contrast, studies of Vietnam veterans in the United States who fought in sprayed areas or worked with the defoliants have not revealed any significant related adverse health effects.57
On two occasions scientists were exposed to pure mixtures of 2,3,7,8-tetrachlorodibenzodioxin in the laboratory.58 They reported chloracne, insomnia, depression, inability to concentrate, decreased libido and, in some cases, effects on personality that emerged two years after exposure.
Epidemiological studies of exposed workers have not effects beyond prolonged chloracne. Some studies mortality59 but others have not.60
The linkage between cancer and exposure of workers to dioxin-containing chemicals such as herbicides or chlorophenols remains equivocal. Workers in Canada who used defoliants containing dioxin have claimed that cancer rates are higher than expected. In contrast, an excess incidence of cancer has not been observed in U.S. army technicians who used the same defoliants in the 1970s. The International Agency for Research on Cancer (IARC)61 has concluded that while there is limited evidence of carcinogenicity in humans exposed to chlorophenoxy herbicides and chlorophenols (substances that contain dioxins and furans), the supporting animal data are inadequate. Nevertheless, IARC considers these chemical groups to be possibly carcinogenic to humans. For 2,3,7,8-tetrachlorodibenzodioxin itself, IARC indicates that there is inadequate human data but sufficient animal data to conclude that this compound is possibly carcinogenic to humans.
Furans
There have been three notable accidental human population exposures to high concentrations of furans. All have been confounded by co-exposure to other chemicals.
In 1981, an electrical transformer, filled with transformer fluid containing 65 percent PCBs and 35 percent tri- and tetra-chlorobenzenes, exploded and burned in a Binghamton, New York office tower. The building was contaminated with soot that contained large amounts of furans and lesser amounts of dioxins.62
The approximately 500 exposed individuals were not systematically monitored. However, of 50 that were clinically assessed, some had chloracne, some skin rashes, some minor serum enzyme changes and some effects on behaviour symptoms. No estimate of individual exposures was made in this study.
In 1968, a mass poisoning incident occurred in Japan due to the ingestion of rice oil contaminated with PCBs, furans and polychlorinated quaterphenyls.63 The most common signs and symptoms of the resultant Yusho disease included: acne-like lesions of the skin; thickened rough texture of the skin on hands and feet; blackening of nails; dark discolouration of gums and skin; eye secretions; swelling of the eyelid and increased redness of the eyelids; sweating of the palms; and sensory changes, including weakness, itching, hearing and sight deficiencies.64 While most of these effects subsided over a ten-year period, others were still evident ten years after exposure. Among the most persistent symptoms were:: nail pigmentation and deformation; skin cysts that replaced acne-like lesions; swelling of the eyelid and eye discharges; some sensory complaints; and impairment of lung function.
In the fat tissues of the Yusho patients, furan concentrations ranged from 6 to 13 nanograms per gram of fat, and furan concentrations in the liver from 3 to 25 nanograms per gram of fat. Although approximately 40 furan compounds were identified in the contaminated rice oil, many of these were not detected in the tissues of Yusho patients. The compounds that were retained included 2,3,6,8-tetrachlorodibenzofuran; 2,3,7,8-tetrachlorodibenzofuran; 1,2,4,7,8-pentachlorodibenzofuran; 2,3,4,7,8-pentachlorodibenzofuran; and 1,2,3,4,7,8-hexachlorodibenzofuran.65
A similar mass food poisoning occurred in central Taiwan in 1979, again involving rice oils contaminated with PCBs, furans and polychlorinated quaterphenyls. Yu-Cheng disease resembled Yusho disease. The exposure occurred over approximately three to nine months and the onset of visible signs appeared over an average of three to four months. The furan compounds consumed and the total intakes of furans were similar to those in the Yusho poisoning.64 Rough estimates of intakes of PCB, furans and poly-chlorinated quaterphenyls for Yu-Cheng patients are 973, 3.8 and 586 milligrams per adult, respectively66 and for Yusho patients 633, 3.3 and 596 milligrams per adult, respectively.67
Yu-Cheng children who were exposed while in the womb often died soon after birth, and had reduced size at birth, abnormal gums, skin, nails, teeth and lungs. Delays and deficits in mental development were also observed in surviving children.68 Similar effects have been reported for Yusho children exposed in the same manner.
On the basis of animal studies with contaminated and uncontaminated PCBs, as well as correlation of the clinical signs and symptoms of disease with exposure concentrations, there is reasonably good evidence that Yusho and Yu-Cheng disease were caused primarily by furan contaminants in the rice oi1.69
Current Canadian Objectives, Guidelines and Regulations
Medium |
Agency1 |
Regulation/Objective/Guideline |
|---|---|---|
Fish: |
NHW (Food Tolerance) |
20 pans-per-trillion 2,3,7,8-tetrachlorodibenzodioxin (edible portion) |
Other Food: |
NHW (Food Tolerance) |
No detectable dioxin |
Drinking Water: |
OME (Interim Drinking Water Objective) |
15 parts-per-quadrillion 2,3,7,8-tetrachlorodibenzodioxin toxic equivalents |
Ambient Water: |
IJC (Water Quality Objective) |
10 parts-per-quadrillion 2,3,7,8-tetrachlorodibenzodioxin |
Ambient Air: |
OME (Provisional Air Quality Guideline) |
30 picograms (total dioxins and 1/50 total furans per m3 as an annual average) |
Other: |
IJC (Water Quality Objective) |
10 parts-per-trillion 2,3,7,8-tetrachlorodibenzodioxin (sediment or tissue of aquatic organisms) |
1 NHW (National Health and Welfare) OME (Ontario Ministry of Environment) IJC (International Joint Commission)
Environmental Risk Assessment
The dioxins and furans substituted in the 2, 3, 7 and 8 positions are highly toxic substances. They are pervasive in the environment and bioaccumulate readily in the food chain. When wildlife consumes fish from the Great Lakes contaminated with dioxins and furans their intake could exceed the no-observed-adverse-effect- level. A growing body of evidence indicates that dioxins and furans or related compounds are already adversely affecting some wildlife populations.
As a result of these considerations, the Minister of the Environment has concluded that polychlorinated dibenzodioxins and polychlorinated dibenzofurans, occurring in complex mixtures of environmental contaminants, have immediate and long-term harmful effects on the environment.
They are considered toxic as defined under Section 11(a) of the Canadian Environmental Protection Act.
Dioxins and furans are highly persistent compounds that bioaccumulate in the food chain. From the Atlantic to the Pacific and throughout the Far North, dioxins and furans have been found in all compartments of the ecosystem, including air, water, soil, sediments, and foods. All animals and humans in Canada have been, and continue to be exposed to these substances.
The Canadian environment is subjected to four major sources of dioxins and furans: commercial chemicals containing dioxins, incineration activities, releases from pulp and paper mills that use chlorine for the bleaching process, and both accidental fires and spills involving PCBs (which contain principally furan contaminants). In addition, transborder contamination from the United States and long-range aerial transport from other areas contribute to the environmental load. While the chemical sources are, for the most part, subject to stringent regulations, dioxins and furans continue to be released in large quantities from incinerators and pulp and paper mills that use chlorine in the bleaching process. The use and storage of PCBs is now strictly controlled, but there remains the potential for releases through accidental spills and uncontrolled fires.
A review of the scientific literature shows that dioxins and furans tested in the laboratory are highly toxic to a wide variety of mammals (including humans), birds and fish. These compounds are both extremely toxic in acute exposure and some dioxins and furans which have been tested are capable of causing birth defects and cancer, and adversely affecting reproduction and the immune system, following repeated exposures. Several act together with other substances to cause cancer. Most sources release these substances as complex mixtures that are highly toxic to a variety of organisms.
The calculated70 total body intake of 2,3,7,8-tetrachlorodibenzodioxin in daily food that produces a semi-chronic no-effect level varies for mammals, birds and fish. In mammals, it is estimated to be 550 to 5200 nanograms per kilogram of body weight; in birds, 2100 nanograms per kilogram of body weight; and in fish, 760 nanograms per kilogram of body weight. The threshold dose at which there is no effect in chronic studies of carcinogenicity and reproduction in rodents is approximately 1 nanogram per kilogram body weight per day.
A diet of the more highly contaminated fish from the Great Lakes could conceivably reach a cumulative dose in toxic equivalents exceeding these no-effect levels for sensitive wildlife species such as mink. Reproductive failure and anomalies in fish-eating birds on the Great Lakes and on the West Coast of Canada are ongoing concerns. They correlate strongly with dioxin and furan levels in eggs and adult tissues. Studies of fish-eating, colonially-nesting birds attribute low reproductive success to two major factors: embryotoxic chemicals in eggs, and aberrant behaviour caused by pollutants, resulting in poor nest incubation and care of nestlings. Congenital malformations have been reported in Great Lakes terns, gulls and double-crested cormorants. Such effects on reproduction and development are characteristic of exposure to dioxins and some of the other organochlorine contaminants that co-occur with dioxins and furans.
If dioxins and furans continue to be released into the environment, exposures to the more persistent and toxic ones will unnecessarily be prolonged. A growing body of evidence indicates that dioxins and furans in mixtures with other contaminants adversely affect some wildlife populations in Canada.
As a result of these considerations, the Minister of Environment has concluded that polychlorinated dibenzodioxins and polychlorinated dibenzofurans, occurring in complex mixtures of environmental contaminants, have immediate and long-term harmful effects on the environment. They are considered "toxic" as defined under Section 11(a) of the Canadian Environmental Protection Act.
Human Health Risk Assessment
The dioxins and furans substituted in the 2, 3, 7 and 8 positions are highly toxic substances. They are pervasive in the environment and bioaccumulate readily in the food chain. Some Canadian fisheries have been closed as a result of dioxin and furan contamination. Current estimates of general population exposures indicate that certain high-risk sub-groups may have exposures that approach or exceed the guideline for tolerable daily intake of dioxins and furans. Since some toxic dioxins and furans are very persistent, exposure will be unnecessarily prolonged if these substances continue to be released, with a resultant increase in the risk to the health of high-exposure sub-populations. Human exposure should be kept to a minimum, and efforts to control sources of dioxins and furans should continue to be vigorous.
As a result of these considerations, the Minister of National Health and Welfare has concluded that polychlorinated dibenzodioxins and polychlorinated dibenzofurans may enter the environment in quantities which constitute a danger to human health. They are considered "toxic" as defined under Section 11(c) of the Canadian Environmental Protection Act.
Animal studies confirm that most mammalian species respond in the same qualitative way to dioxins and furans. However, dose-effect relationships vary markedly between species for the various dioxin and furan compounds. The dioxins and furans substituted in the 2, 3, 7 and 8 positions produce several effects in animals that are significant for consideration of human health. Among these are their effects on the reproductive system of non-human primates and rats, their tendency to cause hyperplasia in rodents, their carcinogenic effects on livers in rats and their effects on the immune system in several species.
Studies of human populations indicate that short-term exposure to several milligrams of mixtures of dioxins and furans can lead to a variety of effects on skin, eyes, and sensory and behavioural processes. Most effects have been reported to be reversible, although a return to normal may take several years. High-level exposure of women to furans in contaminated rice-oil may have been responsible for reproductive anomalies and infant mortalities. There is no adequate demonstration that populations exposed to dioxins and Furans have suffered excess cancer. However, the evidence is conflicting and data are confounded by exposure to other chemicals, incomplete health records, inadequate case identification and small sample size.
A recent Federal-Ontario study has estimated the average total exposure of Canadian adults and children to dioxin and furan compounds from all possible pathways.71 These estimates are based on average Canadian intakes of air, water, soil and food with representative concentrations of dioxins and furans. Between 94 and 96 percent of the intake by non-smoking adults is estimated to be from food, with the remainder equally split between air and all other routes (Table 7).
Substrate/Medium |
Estimated Intake (picograms toxic equivalents per kilogram of body weight per day) |
|||
|---|---|---|---|---|
Adult1 |
Child2 |
Infant3 |
Neonate4 |
|
Food: |
0.495 to 2.06 |
1.185to 4.786 |
2.65to 10.76 |
165 |
Air: |
0.04 |
0.07 |
0.1 |
0.04 |
Soil: |
0.01 |
0.027 to 0.03 |
0.34 to 0.038 |
- |
Water: |
<0.01 to 0.05 |
<0.01 to 0.07 |
<0.002 to 0.11 |
- |
Consumer7 Products: |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
Total Estimated Intake |
0.56 to 2.1 |
1.3 to 5.0 |
3.1 to 11.0 |
165 |
Exposure Period: |
53 years |
14 years |
2.5 years |
0.5 years |
- Adult differ, slightly from quoted reference due to small difference, in calculations and an assumed body weight of 70 kilograms.
- Child: Weighs 33 kilograms; Breathes 15m3 air: Intake 1 litre water, 0.02 gm soil, 113% of adult food (NH&W, 977).
- Infant: Weighs 13 kilograms; Breathes l0 m 3 air: Intake 0.6 litre water, 0.1 gm soil, 100% of adult food (NH&W, 1977).
- Neonate: Weighs 5 kilograms; Breathes l m3 air: Intake 750 millilitres, 3% fat breast milk containing 36.5 picograms toxic equivalents per gram of fat Ryan et al., 1985).
- Lower end of all ranges assumes a reported Not Detectable = 0.
- Upper end of all ranges assumes a reported Not Detectable = Limit of Detection.
- Does not include intake from cigarette smoking.
The average daily intake of dioxins and furans over a lifetime can be calculated from Table 7 by adding total intakes during the exposure periods of neonates, infants, children, and adults, and then dividing the total by 70 years. This estimate of 2.0 to 4.2 picograms toxic equivalents per kilogram of body weight per day is based on assumptions that are likely to overestimate exposure in the interest of protecting human health.
Pharmacokinetic modelling of Canadian general population exposure to dioxins and furans has corroborated the above exposure assessment with an estimate of 1.86 picograms toxic equivalents per kilogram body weight per day. The model used the average concentration of dioxins and furans in Canadian fat tissue72 to estimate body burden, a half-life of 7.1 years, and a first-order kinetic model.73
Data from a recent report74 suggest tobacco smoking as another pathway of general population exposure to dioxins. The potential exposure of a person who smokes 20 cigarettes a day is 0.5 picograms toxic equivalents per kilogram of body weight per day (this assumes that a 70 kilogram person inhales 1 litre of smoke per cigarette75 containing dioxins at the concentration of 1.8 nanograms toxic equivalents per cubic metre,72 and that uptake is 100%). This estimate is preliminary in that it does not consider sidestream smoke, the potential presence of furans, nor the differences between the experimental protocol and actual smoking. This pathway has not been included in the above estimates of general population exposure because of these uncertainties. Nonetheless, this calculation indicates that smoking may contribute significantly to dioxin and furan exposure.
Based on a traditional approach which uses the no-observed-adverse-effect-levels from animal tests and standard uncertainty factors, it is estimated that human intakes should be below 10 picograms per kilogram of body weight per day of toxic equivalents averaged over a lifetime. This estimate assumes that 2,3,7,8-substituted dioxins and furans are non-genotoxic carcinogens for which a threshold dose exists at approximately 1 nanogram toxic equivalents per kilogram of body weight per day. The no-observed-adverse-effect-level for reproduction in rodents is also approximately 1 nanogram of 2,3,7,8-tetrachlorodibenzodioxin per kilogram of body weight per day. Humans appear to be less sensitive to dioxins and furans than most laboratory species (especially rats and monkeys) and the effect threshold values in rodents were based on lifetime exposures. An uncertainty factor of 100 was applied to the no-observed-adverse-effect-level to obtain the 10 picogram toxic equivalents per kilogram of body weight per day value, to take account of variations in response between individuals and to account for the seriousness of the potential effects. In a recent review, Rozman76 reached the same conclusion, deriving Acceptable Daily Intake estimates for 2,3,7,8- tetrachlorodibenzodioxin of 10 picograms per kilogram of body weight per day for tumour promotion; 14 picograms per kilogram of body weight per day for porphyria; and 21 picograms per kilogram of body weight per day for dermal effects.
Intake via breast milk during the first half year of life is estimated to be relatively high (Table 7). This estimate was based on levels of dioxins and furans in Canadian adipose tissue. Subsequent analyses of human milk reduce this estimate by approximately one half, to 70 picograms toxic equivalents per kilogram body weight per day.77 Although estimated intakes for neonates would still exceed the permissible daily intake, breast feeding only occurs for a short part of the life span (i.e., less than 4%) and lower exposures throughout the remainder of the life span reduce lifetime exposure to below this guideline. The concentrations of dioxins and furans in target organs are not expected to be dramatically increased as a consequence of breast feeding because of the rapid increase in the amount of the infant's fatty tissue. The known benefits of breast feeding currently outweigh any potential risks that may be associated with dioxins and furans in breast milk.77
The foregoing exposure estimates indicate that average Canadian lifetime intakes are currently below 10 picograms toxic equivalents per kilogram of body weight per day. However, these substances bioaccumulate in the food chain, and concern for the health of human populations living in some areas where shellfish are highly contaminated has already necessitated closure of the affected fisheries near some pulp and paper mills that use chlorine bleaching. Exposures are estimated to approach or exceed 10 picograms toxic equivalents per kilogram of body weight per day for persons consuming highly contaminated fish in quantities well in excess of the general population norm. Furthermore, there is considerable uncertainty associated with the estimates of exposure, as they do not consider other sources that may contribute significantly to exposure, such as cigarette smoking. Finally, some toxic dioxins and furans are very persistent, and their continued release into the environment will unnecessarily prolong exposure, with a resultant increase in the risk to the health of high-exposure sub-populations.
While most chemical sources are already subject to stringent regulations, these dioxins and furans continue to be released from incinerators employing old technology and pulp mills employing chlorine in the bleaching process. The use, handling and storage of PCBs is controlled. Existing stocks of PCBs await the establishment of suitable destruction facilities, which are starting to become available.
As a result of these considerations, the Minister of National Health and Welfare has concluded that polychlorinated dibenzodioxins and polychlorinated dibenzofurans may enter the environment in quantities which constitute a danger to human health. They are considered "toxic" as defined under Section 11(c) of the Canadian Environmental Protection Act.
References
Arthur, M.F. and Frea, J.I., 1988. Microbial activity in soils contaminated with 2,3,7,8-TCDD. Environ. Toxicol. Chem. 7: 5-13.
Astle, J.W., Gobas, F.A.P.C., Shiu, Wan-Ying and Mackay, D., 1987. Lake sediments as historic records of atmospheric contamination by organic chemicals. Adv. Chem. Ser. 216: 57-77.
ATSDR (Agency for Toxic Substances and Disease Registry), 1987. Toxicological profile for 2,3,7,8-tetrachlorodibenzo-p-dioxin U.S. Public Health Service. 122 pp.
Baumann, P.C. and D.M. Whittle, 1988. The status of selected organics in the Laurentian Great Lakes: an overview of DDT, PCP's, dioxins, furans, and aromatic hydrocarbons. Aquatic Toxicology. 11, 241-257.
Birmingham, B., Gilman, . A., Grant, D., Salminen, J., Boddington, M., Thorpe, B., Wile I.,Toft, P. and Armstrong, V., 1989. PCDD/PCDF multimedia exposure analysis for the Canadian population: detailed exposure analysis for the Chemosphere 19 (1-6): 637-642.
Blair, E.H. (ed)., 1973. Chlorodioxins - origin and fate. Adv. Chem. Ser. 120: 144 pp.
Boddington, M.J., Barrette, P., Grant, D., Norstrom, R.J., Ryan, J.J. and Whitby, L. (eds), 1985. Chlorinated dioxins and related compounds 1984. Chemosphere 14: 571-989.
Braune, B.M. and Norstrom, R.J., 1989. Dynamics of organochlorine compounds in herring gulls: III, Tissue distribution and bioaccumulation in Lake Ontario gulls. Environ. Toxicol. Chem. 8: 957-968.
Cattabeni, F., Cavallaro, A., and Galli, G. (eds), 1978. Dioxin: Toxicological and chemical aspects. SP Medical and Scientific Books: New York. 222 pp.
Chen, P.H., Wong, C.-K., Rappe, C. and Nygren, M., 1985. Polychlorinated biphenyls, dibenzofurans and quaterphenyls in toxic rice-bran oil in the blood and tissues of patients with PCB poisoning (Yu-Cheng) in Taiwan. Environ. Health Persp. 59: 59-65.
Connell, D.W. and Hawker, D.W., 1988. Use of polynomial expressions to describe the bioconcentration of hydrophobic chemicals by fish. Ecotoxicol. Environ. Safety 16: 242-257.
Cook, R.R., Townsend, J.C., Ott, M.G. and Silverstein, L.G., 1980. Mortality experience of employees exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). J. Occup. Med. 22: 530-532.
Cook, R.R., Bond, G.G., Olson, R.A. and Ott, M.G., 1987. Update of the mortality experience of workers exposed to chlorinated dioxins. Chemosphere 16: 2111-2116.
Czuczuwa, J.M. and Hites, R.A., 1986. Sources and fate of PCDD and PCDF. Chemosphere 15: 1417-1420. Eisler, R., 1986. Dioxin hazards for fish, wildlife and invertebrates. U.S. Fish and Wildlife Service. Biological Report 85. 36 pp.
Elliott, J.E., Butler, R.W., Norstrom, R.J. and Whitehead, P.E., 1988. Levels of polychlorinated dibenzodioxins and polychlorinated bidenzofurans in eggs of great blue herons (Ardea herodias) in British Columbia, 1983-87: possible impacts on reproductive success. Progress Notes: Canadian Wildlife Service. 176: 7 pp.
Elliott, J.E., Butler, R.W., Norstrom, R.J. and Whitehead, P.E., 1989. Environmental contaminants and reproductive success of great blue herons Ardea herodias in British Columbia, 1986-87. Environ. Pollu. 59: 91-114.
Esposito, M.P., Tiernan, T.O., and Dryden, F.E., 1980. Dioxins. U.S. Environmental Protection Agency. EPA-600-80-197. 351 pp.
Fanelli, R., Bertoni, M.P., Castelli, M.G., Chiabrando, C., Martelli, G.P., Noseda, A., Garattini, S.,
Binagh, C., Marazza, V. and Pezza, F., 1980. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxic effects and tissue levels in cows milk from the contaminated area of Seveso, Italy. Arch. Environ. Contam. Toxicol. 9: 569-577.
Fox, G.A., Kennedy, S.W., Norstrom, R.J. and Wigfield, D.C., 1988. Porphyria in herring gulls: a biochemical response to chemical contamination of Great Lakes food chains. Environ. Toxicol. Chem. 7: 831-839.
FRG (Federal Republic of Germany), 1985. Sachstand Dioxine - Stand November 1984. Umweltdunesamt und Bundesgesundeitsamt. 385 pp.
Gilbertson, M., 1983. Etiology of chick edema disease in herring gulls in the lower Great Lakes. Chemosphere 12: 357-370.
Government of Canada, 1988-89. National Dioxin and Furan Sampling Program. Results released publicly in May and November, 1988 and in May, June and November, 1989.
Government of Canada, 1989. Discussion document on anti-sapstain chemicals., October 1989. 118 pp.
Hayabuchi, H., Yoshimura, T. and Kuratsune, M. 1979. Consumption of toxic rice oil by 'Yusho' patients and its relation to the clinical response and latent period. Fd. Cosmet. Toxicol. 17: 455-461.
Helder, T., 1980. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on early life stages of the pike (Esox Lucius L.). Sci. Total Environ. 14: 255-264.
Helder, T., 1981. The effects of 2,3,7,8,-tetrachlorodibenzo-p-dioxin (TCDD) on early life stages of rainbow trout ( Salmo gairdneri, Richardson). Toxicology 19: 101-112.
Hochstein, J.R., Aulerich, R.J., and Bursian, S.J., 1988. Acute toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin to mink. Arch. Environ. Contam. Toxicol. 17: 33-37.
Holmstedt, B., 1980. Prolegomena to Seveso. Arch. Toxicol. 44: 211-230.
Honchar, P.A. and Halperin, W.E., 1981. 2,4,5-T, trichlorophenol, and soft tissue sarcoma. Lancet 1: 268-269.
Hudson, R.H., Tucker, R.K., and Haegele, M.A., 1984. Handbook of toxicity of pesticides to wildlife. U.S. Fish Wildlife Service. Resource Publ. 153: 99 pp.
Hutzinger, O., Frei, R.W., Merian, E. and Pocchiari, F. (eds), 1981. Impact of chlorinated dioxins and related compounds on the environment. Pergamon Series on Environ. Sci. 5: 658 pp.
Hutzinger, O., Frei, R.W., Merian, E., and Regiani, G. (eds), 1983. Chlorinated dioxins and related compounds, 1982. Chemosphere 12: 425-790.
Hutzinger, O., Crummett, W., Karasek, F.W., Merian, E., Regiani, G., Reissinger, M. and Safe, S. (eds), 1986. Chlorinated dioxins and related compounds 1985. Chemosphere 15: 1079-2132.
IARC (International Agency for Research on Cancer), 1977. Chlorinated dibenzodioxins. In: IARC Monographs on the evaluation of the carcinogenic risk of chemicals to man. 15: 41-102.
IARC, 1976. Overall evaluations of carcinogenicity: an updating of IARC monographs Volumes 1 to 42. In: IARC Monographs on the evaluation of carcinogenic risks to humans. Supplement 7: 350-351.
IJC (International Joint Commission), 1983. 1983 Report on Great Lakes Water Quality. Appendix: Great Lakes Surveillance. Great Lakes Water Quality Board. 129 pp.
IJC, 1989. The 1987 Report on Great Lakes Water Quality, Appendix B, Great Lakes Surveillance, Volume 1. 70 pp.
ICTC (Interdepartmental Committee for Toxic Chemicals: Canada), 1986. Dioxins in Canada: The Federal Approach. First Progress Report. Environment Canada. 13 pp.
Jansson, B., Alsberg, T., Bergman, A., Renberg, L., and Reutergardh, L., 1987. Persistent organic compounds in the marine environment. National Swedish Environmental Protection Boards, Report #3395, 8 pp.
Jensen, A.A., 1987. Polychlorobiphenyls (PCBs), polychlorodibenzo-p- dioxins (PCDDs) and polychlorodibenzofurans (PCDFs) in human milk, blood and adipose tissue. Sci. Total Environ. 64: 259-293.
Kenaga, E.E. and Norris, L.A., 1983. Environmental toxicity of TCDD. In. R.E. Tucker, A.L. Young, A.P. Gray (eds). Human and environmental risks of chlorinated dioxins and related compounds. Environ. Sci. Res. 26: 277-299.
Kimbrough, R.D. (ed.), 1980. Halogenated biphenyls, terphenyls, napthalenes, dibenzodioxins Health 4: 406 pp. and related products. Top. Environ.
Kimbrough, R.D. 1984. The epidemiology and toxicology of TCDD. Bull, Environ. Contam. Toxicol. 33: 636-647.
Kimbrough, R.D., Falk, H., Stehr, P., Fries, G., 1984. Health implication of 2,3,7,8-tetrachlorodibenzodioxin contamination of residential soil. J. Toxicol. Environ. Health 14: 47-94.
Kleeman, J.M., Olson, J.R., Peterson, R.E., 1988. Species Differences in 2,3,7,8-tetrachlorodibenzo-p-idoxin Toxicity and Biotransformation in Fish. Fund. and Appl. Technol. 10: 206-213.
Kubiak, J.J., Harris, H.J., Smith, L.M., Schwartz, T.R., Stalling D.L., Trick, J.A., Sileo, L., Doherty, D.E. and Erdman, T.C., 1989. Microcontaminants and reproductive impairment of the Forster's tern on Green Bay, Lake Michigan - 1983. Arch. Environ. Contam. Toxicol. 18: 706-727.
Kuratsune, M., Yoshimura, T., Matsuzaka, J. and Yamaguchi, A., 1972. Epidemiological study on Yusho, a poisoning caused by ingestion of rice oil contaminated with a commercial brand of polychlorinated biphenyls. Environ. Health Persp. 1: 119-128.
Masuda, Y., Kuroki, H., Haraguchi, K. and Nagayama, J., 1985. PCB and PCDF congeners in the
blood and tissues of Yusho and Yu-Cheng patients. Environ. Health Persp. 59: 53-58.
Masuda, Y. and Yoshimura, H., 1984. Polychlorinated biphenyls and dibenzofurans in patients with Yusho and their toxicological significance: a review. Amer. J. Indust. Med. 5: 31-44.
McNulty, W.P., Nielsen-Smith, K.A., Lay, J.O., Jr., Lippstreu, D.L., Kangas, N.L., Lyon, P.A., and Gross, M.L., 1982. Persistence of TCDD in monkey adipose tissue. Fd. Cosmet. Toxicol. 20: 985-987.
McNelis, D.N., Nauman, C.H., Fenstermaker, L.K., Safe, S., Clement, R.E., Campana, J., Kahn, P., Fingerhut, J., desRosiers, P., Barnes, D.J., Hileman, F. and Keith, L.H. (eds), 1989. Chlorinated dioxins and related compounds 1987. Chemosphere 18: 1-1339.
Mehrle, P.M. Buckler, D.R., Little, E.E., Smith, L.M., Petty, J.D., Peterman, P.H., Stalling, D.L., Degraeve, G.M., Coyle, J.J. and Adams, W.J., 1988. Toxicity and bioconcentration of 2,3,7,8- tetrachlorodibenzodioxin and 2,3,7,8-tetrachlorodibenzofuran in rainbow trout. Environ. Toxicol. Chem. 7: 47-62.
Mineau, P., Fox, G.A., Norstrom, R.J., Weseloh, D.V., Hallette, D.J. and Ellenton, J.A., 1984. Using the herring gull to monitor levels and effects of organochlorine contamination in the Canadian Great Lakes. In. J.O. Nriagu and J.J. Simmons (eds). Toxic Contaminants in the Great Lakes. J. Wiley and Sons Inc.: Toronto. Chp. 19: 437-452.
Moore, J.A., (ed), 1973. Perspective on chlorinated dibenzodioxins and dibenzofurans. Environ. Health Persp. 5: 313 pp.
Murray, F.J., Smith, F.A., Nitschke, K.D., Humiston, C.G., Kociba, R.J., and Schwetz, B.Z., 1979. Three-generation reproduction study of rats given 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the diet. Toxicol. Appl. Pharmacol. 50: 241-252.
Muto, H. and Takizawa, Y., 1989. Dioxins in cigarette smoke. Arch. Environ. Health 44: 171-174.
NATO (North Atlantic Treaty Organization), 1988a. International Toxicity Equivalency Factor (I-TEF) method of risk assessment for complex mixtures of dioxins and related compounds. Pilot study on international information exchange on dioxins and related compounds. Committee on the Challenges of Modern Society. #186: 26 pp.
NATO, 1988b. Scientific basis for the development of the International Toxicity Equivalency Factor (I-TEF) method of risk assessment for complex mixtures of dioxins and related compounds. Pilot study on international information exchange on dioxins and related compounds. Committee on the Challenges of Modern Society. #188: 56 pp.
NH&W (National Health and Welfare), 1977. Nutrition Canada Survey, food consumption patterns report 1970-1972. Bureau of Nutritional Services, Health Protection Branch, 248 pp.
Norstrom, R.J., Clark, T.P. and Weseloh, D.V., 1985. Great Lakes monitoring using herring gulls, In. T.C. Hutchinson and S.M. Evans (eds). Hazardous contaminants in Ontario: Human and Environmental effects. Institute for Environmental Studies, University of Toronto, pp. 86-98.
Norstrom, R.J. and Simon, M., 1983. Preliminary appraisal of tetra- to octachlorodibenzodioxin contamination in eggs of various species of wildlife in Canada. In. J. Miyamoto et al. (ed). IUPAC Pesticide Chemistry, Human Welfare and Environment. Pergamon Press: New York. 165-170.
Norstrom, R.J., Hallett, D.J., Simon, M., Mulvihill, M.J., 1982. Analysis of Great Lakes herring gull eggs for tetrachlorodibenzo-p-dioxins. In. Hutzinger et al., (eds) Chlorinated Dioxins and Related compounds: Impact on the Environment. Pergamon Press: New York. 173-181.
Norstrom, R.J., Clark, T.P., Jeffrey, D.A., Won, H.T. and Gilman, A.P., 1986. Dynamics of organochlorine compounds in herring gulls (Larus argentatus): I. Distribution and clearance of [14C] DDE in free-living herring gulls (Larus argentatus). Environ. Toxicol. Chem. 5: 41-48.
Norstrom, R.J., Simon, M. and Muir, D.C.G., (submitted). Chlorinated dioxins and furans in marine mammals from the Canadian Arctic. Arch. Environ. Contam. Toxicol.
NRCC, (National Research Council of Canada), 1981a. Polychlorinated dibenzo-p-dioxins: Criteria for their effects on man and his environment. Associate Committee on Scientific Criteria for Environmental Quality. NRCC #18574: 251 pp.
NRCC, 1981b. Polychlorinated dibenzo-p-dioxins: Limitation to the current analytical techniques. Associate Committee on Scientific Criteria for Environmental Quality. NRCC #18576: 184 pp.
NRCC, 1984. Polychlorinated dibenzofurans: Criteria for their effects on humans and the environment. Associate Committee on Scientific Criteria for Environmental Quality. NRCC #22846: 243 pp.
Oliver, R.M., 1975. Toxic effects of 2,3,7,8-tetrachlorodibenzo-1,4-dioxin in laboratory workers. Br. J. Ind. Med. 32: 49-53.
OME (Ontario Ministry of the Environment), 1985. Scientific Criteria Document for Standard Development No. 4-84: Polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs).
Ott, M.G., Holder, B.B., and Olson, R.D., 1980. A mortality analysis of employees engaged in the manufacture of 2,4,5- trichlorophenoxyacetic acid. J. Occup. Med. 22: 47-50.
Peakall, D.B. and Fox, G.A., 1987. Toxicological investigations of pollutant-related effects in Great Lakes gulls. Environ. Health Persp. 71: 187-193.
Poiger, H. and Schlatter, C., 1986. Pharmacokinetics of 2,3,7,8-TCDD in man. Chemosphere 15: 1489-1494.
Rickert, W.S., Collishaw, NE.., Bray, D.F. and Robinson, J.C., 1986. Estimates of maximum or average cigarette tar, nicotine, and carbon monoxide yields can be obtained from yields under standard conditions. Prevent. Med. 15: 82-91.
Roberts, R.J. and Boddington, M.J., 1983. The theory of accumulation and its relationship to the choice of monitoring matrices for dioxins. In. Tucker, R.E. et al. (eds). Human and environmental risks of chlorinated dioxins and related compounds. Environ. Sci. Res. 26. 301-321.
Rogan, W.J., Gladen, B.C., Hung, H.-L., Koong, S-L., Shih, L-Y., Taylor, J.S., Wu, Y-C., Yang, D., Ragan, N.B., and Hsu, C-C., 1988. Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science 241: 334-336.
Rozman, K., 1989. A critical view of the mechanism(s) of toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin: implications for human safety assessment. Dermatosen 37(3): 82-92.
Ryan, J.J. and Norstrom, R., (In press). Occurrence in humans and major exposure routes. In. Environmental Carcinogens: Methods of Analysis and Exposure Measurement, Vol. 11, Polychlorinated dibenzo-p-dioxins, Dibenzofurans and Biphenyls, Chapter 6.
Ryan, J.J., Lizotte, R. and Lau, B.P.-Y., 1985. Chlorinated dibenzo-p-dioxins and chlorinated dibenzofurans in Canadian human adipose tissue. Chemosphere 14: 697-706.
Ryan, J.J., Lau, B.P.-Y., Hardy, J.A., Stone, W.B., O'Keefe, P. and Gierty, J.F., 1986. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related dioxins and furans in snapping turtle (Chelydra serpentina) tissues from the upper St. Lawrence River. Chemosphere 15: 537-548.
Schecter, A. and Ryan, J.J., 1989. Blood and adipose tissue levels of PCDDs/PCDFs over three
years in a patient after exposure to polychlorinated dioxins and dibenzofurans. Chemosphere 18: 635-642.
Schecter, A. and Tiernan, T., 1985. Occupational exposure to polychlorinated dioxins, polychlorinated furans, polychlorinated biphenyls, and biphenylenes after an electrical panel and transformer accident in an office building in Binghamton, N.Y. Environ. Health Persp. 60: 305-313.
Sheffield, A., 1985. Polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) sources and releases. Environment Canada. EPS 5/HA/2. 47 pp.
Shiu, W.Y., Doucette, W., Gobas, A.P.C., Andren, A. and Mackay, D., 1988. Physical-Chemical Properties of Chlorinated Dibenzo-p-dioxins. Environ. Sci. Technol. 22: 651-658.
Stalling, D.L., Smith, L.M., Petty, J.D., Hogin, J.W., Johnson, J.L., Rappe, C. and Buser, H.P., 1983. Residues of polychlorinated dibenzo-p-dioxins and dibenzofurans in Laurentian Great Lakes fish. In. R.F. Tucker et al., (eds). Human and environmental risks of chlorinated dioxins and related compounds. Plenum Press: New York. 221-240.
Stalling, D.L., Norstrom, R.J., Smith, L.M., and Simon, M., 1985. Patterns of Great Lakes fish and birds, and their characteristics by principal component analysis. Chemosphere 14: 627-643.
Stalling DL.L., Peterman, P.H., Smith, L.M., Norstrom, R.J. and Simon, M., 1986. Use of pattern recognition in the evaluation of PCDD and PCDF residue data from GC/MS analyses. Chemosphere 15: 1435-1443.
Thiess, A.M., Frentzel-Beyme, R., and Link, R., 1982. Mortality study of persons exposed to dioxin in a trichlorophenol-process accident that occurred in the BASF AG on November 17,1 953. Am. J. Ind. Med. 3: 179-189.
Tucker, R.E., Young, A.L. and Gray, A.P. ( eds), 1983. Human and environmental risks of chlorinated dioxins and related compounds. Env. Sci. Res. 26. Plenum Press: New York. 823 pp.
U.S.EPA (United States Environmental Protection Agency), 1985. Health Assessment Document for Polychlorinated dibenzo-p-dioxins. Office for Health and Environmental Assessment. EPA/600/8-884/014F. PB #86-122546.
U.S.EPA, 1988. Estimating exposure to 2,3,7,8-TCDD. Office of Health and Environmental Assessment. EPA/600/6-68/005A. PB #88-231196.
WHO (World Health Organization), 1987. PCDD and PCDF emissions from incinerators for municipal sewage sludge and solid waste -evaluation of human exposure. Environmental Health #17, Regional Office for Europe, Copenhagen, Denmark. 56 pp.
WHO, 1988. Assessment of health risks in infants associated with exposure to PCBs, PCDDs and PCDFs in breast milk. Report on a WHO Working Group: Abano Terme/Padua, 16-19 February, 1987. WHO Regional Office for Europe: Copenhagen.
WHO, 1989 (In press). Polychlorinated dibenzo-para-dioxins and dibenzofurans. Environmental Health Criteria #88. International Programme on Chemical Safety. Geneva, Switzerland.
Zack, J. and Gaffey, W., 1983. A mortality study of workers employed at the Monsanto Company plant in Nitro, West Virginia. In. Tucker, R.E., et al. (eds). Human and environmental risks of chlorinated dioxins and related compounds. Plenum Press: New York. 575-591.
Zack, J.A. and Suskind, R.R., 1980. The mortality experience of workers exposed to tetrachlorodibenzodioxin in a trichlorophenol process accident. J. Occup. Med. 22: 11-14.
Page details
- Date modified: