DEET in people living in Canada
Information on human biomonitoring of DEET in Canada with results from the Canadian Health Measures Survey.
On this page
Background
What is DEET?
DEET (CAS RN 134-62-3), or N,N-diethyl-m-toluamide, is one of the most common and effective active ingredients in personal insect repellents. It is used to repel biting insects such as mosquitoes, blackflies, and ticks. Health Canada recommends using insect repellents containing DEET to protect against mosquito and tick bites, which could lead to vector-borne diseases including West Nile virus and Lyme disease.
Where is DEET found?
Personal insect repellents containing DEET are available in the form of sprays, lotions, foams and moist towelettes.
How are people exposed to DEET?
People are exposed to DEET mainly through skin contact from intentional application of insect repellents that contain DEET.
How is DEET measured in people?
After DEET is applied to the skin, a small amount can be absorbed through the skin. Once absorbed, it is rapidly broken down in the body to form metabolites or excreted unchanged in the urine. The main metabolites are 3-diethylcarbamoyl benzoic acid (DCBA) and N,N-diethyl-3-hydroxymethylbenzamide (DHMB). Measurement of DEET and its metabolites in urine reflects relatively recent exposure. It is important to note that the presence of a substance in the body does not necessarily mean a health effect will occur.
What are the potential health impacts of DEET?
Personal insect repellents containing DEET that are registered in Canada are safe when used according to label directions, which includes using the right concentration, depending on a person's age. Adverse health effects reported by users to Health Canada were typically not serious and resolved quickly without medical treatment. Commonly reported effects include minor skin or eye irritation shortly after exposure, as well as the occurrence of mild respiratory or general reactions (for example, cough, headache or nausea) soon after the product was applied.
What is the Government of Canada doing to minimize potential health impacts of human exposures to DEET?
All pesticides, including DEET, must be approved before they can be sold or used in Canada. Science-based risk assessments are conducted by Health Canada's Pest Management Regulatory Agency (PMRA) under the Pest Control Products Act to ensure that pesticide products meet current health and environmental requirements. Pesticides are registered for use in Canada under specific conditions, as indicated on each product label, and are re-evaluated every 15 years to ensure their use continues to meet the requirements for protecting human health and the environment. DEET is currently under re-evaluation by the PMRA.
Biomonitoring data contribute to the understanding of pesticide exposures in people living in Canada and allow Health Canada to evaluate the effectiveness of any regulatory actions taken. The Government of Canada continues to monitor and evaluate new information as it becomes available. Health Canada will also continue to monitor scientific literature and international regulatory activities and will take appropriate action if human or environmental risks of concern are identified.
For guidance on using personal insect repellents safely, including choosing the right concentration of DEET depending on age, visit Health Canada's personal insect repellents web page.
Data sources
| Initiative | Target population |
|---|---|
| Canadian Health Measures Survey (CHMS) | General Canadian population living in the 10 provinces |
| U.S. National Health and Nutrition Examination Survey (NHANES) | General U.S. population |
This fact sheet presents data from the CHMS. These data are compared with data from the U.S. NHANES for the DEET metabolite DCBA. DEET and its other metabolite, DHMB, were detected in too few people in the Canadian and U.S. populations to be included in the comparison.
| Collection period | Age range (years) | Matrix | Biomarker |
|---|---|---|---|
| CHMS | |||
| 2014–2015 | 3 to 79 | Urine | DCBA |
| 2018–2019 | 3 to 79 | Urine | DCBA |
| U.S. NHANES | |||
| 2007–2008 | 6+ | Urine | DCBA |
| 2009–2010 | 6+ | Urine | DCBA |
| 2011–2012 | 6+ | Urine | DCBA |
| 2013–2014 | 6+ | Urine | DCBA |
| 2015–2016 | 3+ | Urine | DCBA |
Results
Canadian population
Figure 1. DCBA concentrations in the Canadian population aged 3 to 79.
This figure shows the geometric mean concentrations (that is, average levels) of the DEET metabolite DCBA in urine (µg/L) in the Canadian population from the CHMS (2014–2015 and 2018–2019).
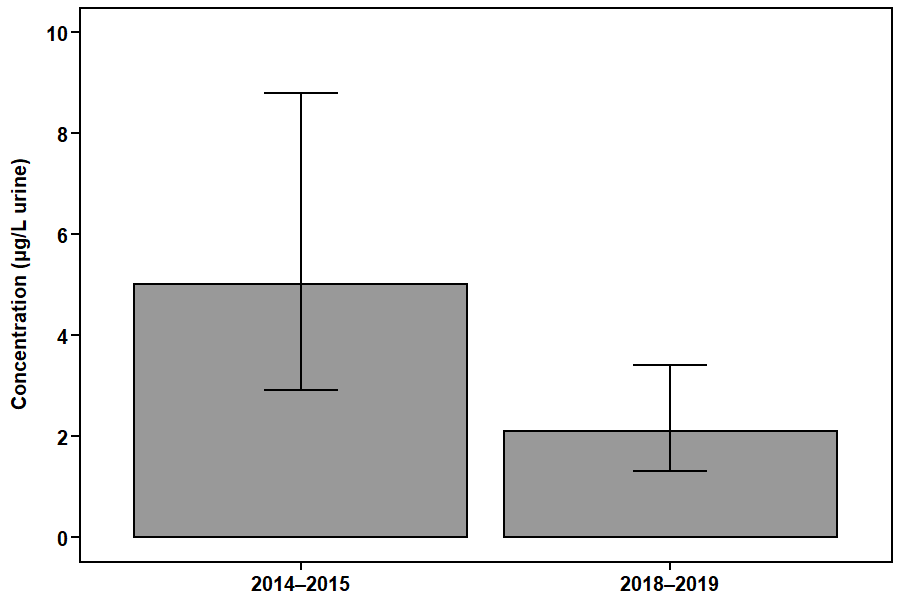
Figure 1 - Text description
| Collection period | Geometric mean |
|---|---|
| 2014–2015 | 5.0 |
| 2018–2019 | 2.1 |
There was a statistically significant decrease (P = 0.011) in DCBA concentrations between the two collection periods in the Canadian population aged 3 to 79. Concentrations decreased by 57% between 2014–2015 and 2018–2019. The average levels of the DEET metabolite DCBA for the Canadian population were below the biomonitoring screening level. The screening level is the level above which further analysis by Health Canada would be triggered. A health assessment by Health Canada has also confirmed there are no risks of concern to human health when DEET-containing products are used properly according to product label instructions.
Canadian population, by age group
Figure 2. DCBA concentrations in the Canadian population, by age group.
This figure shows the geometric mean concentrations (that is, average levels) of the DEET metabolite DCBA in urine (µg/L) in the Canadian population by age group from the CHMS (2014–2015 and 2018–2019).

Figure 2 - Text description
| Collection period | Age group (years) | Geometric mean |
|---|---|---|
| 2014–2015 | 3 to 5 | 5.2 |
| 2014–2015 | 6 to 11 | 6.4 |
| 2014–2015 | 12 to 19 | 9.1 |
| 2014–2015 | 20 to 39 | 6.0 |
| 2014–2015 | 40 to 59 | 4.4 |
| 2014–2015 | 60 to 79 | 3.3 |
| 2018–2019 | 3 to 5 | 2.2 |
| 2018–2019 | 6 to 11 | 3.5 |
| 2018–2019 | 12 to 19 | 3.3 |
| 2018–2019 | 20 to 39 | 2.0 |
| 2018–2019 | 40 to 59 | 2.2 |
| 2018–2019 | 60 to 79 | 1.7 |
Concentrations of DCBA were similar across age groups in the Canadian population. The average levels of the DEET metabolite DCBA for all age groups were below the biomonitoring screening level. The screening level is the level above which further analysis by Health Canada would be triggered. A health assessment by Health Canada has also confirmed there are no risks of concern to human health when DEET-containing products are used properly according to product label instructions.
Canadian population, by sex
Figure 3. DCBA concentrations in the Canadian population aged 3 to 79, by sex.
This figure shows the geometric mean concentrations (that is, average levels) of the DEET metabolite DCBA in urine (µg/L) in the Canadian population by sex from the CHMS (2014–2015 and 2018–2019).
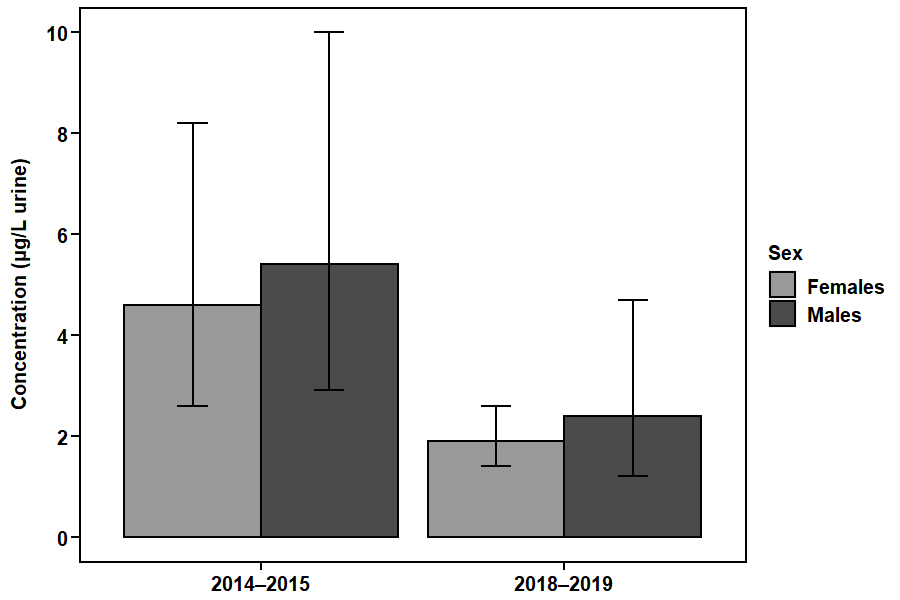
Figure 3 - Text description
| Collection period | Sex | Geometric mean |
|---|---|---|
| 2014–2015 | Females | 4.6 |
| 2014–2015 | Males | 5.4 |
| 2018–2019 | Females | 1.9 |
| 2018–2019 | Males | 2.4 |
Concentrations of DCBA were similar between females and males in the Canadian population. The average levels of DCBA for females and males were below the biomonitoring screening level. The screening level is the level above which further analysis would be triggered. A health assessment by Health Canada has also confirmed there are no risks of concern to human health when DEET-containing products are used properly according to product label instructions.
Comparison of the Canadian and U.S. populations
Figure 4. DCBA concentrations in the Canadian and U.S. populations.
This figure shows the geometric mean concentrations (that is, average levels) of DCBA in urine (µg/L) in the Canadian population from the CHMS (2014–2015 and 2018–2019) and in the U.S. population from the NHANES (2007–2016). Note that there are slight differences between the surveys in sampling (such as the age ranges of participants) and analysis (such as the limits of detection).
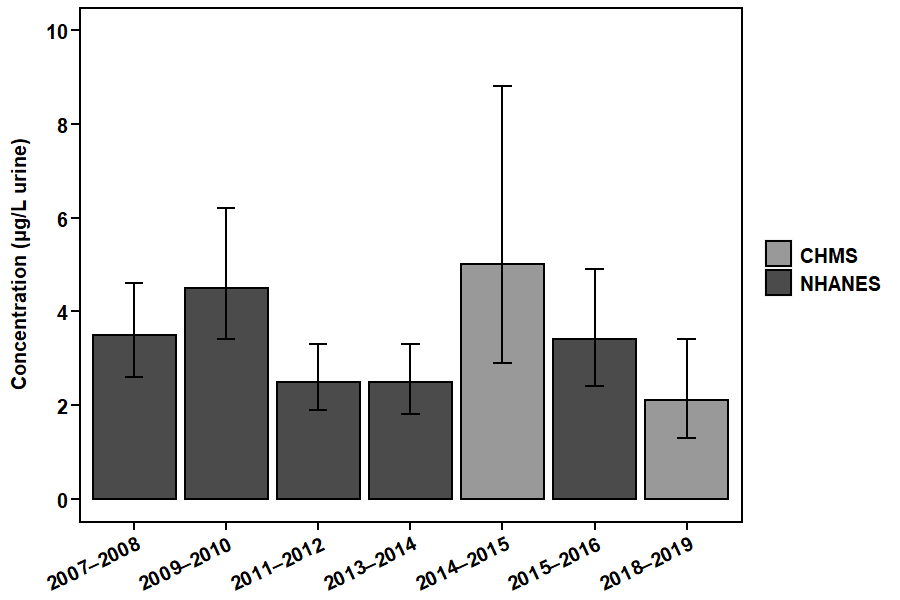
Figure 4 - Text description
| Biomonitoring initiative | Collection period | Geometric mean |
|---|---|---|
| CHMS | 2014–2015 | 5.0 |
| CHMS | 2018–2019 | 2.1 |
| NHANES | 2007–2008 | 3.5 |
| NHANES | 2009–2010 | 4.5 |
| NHANES | 2011–2012 | 2.5 |
| NHANES | 2013–2014 | 2.5 |
| NHANES | 2015–2016 | 3.4 |
Concentrations of DCBA were similar between the Canadian and U.S. populations.
Suggested citation
Health Canada. 2023. DEET in people living in Canada. Ottawa, ON. Available: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/environmental-contaminants/human-biomonitoring-resources/deet-in-people.html
Additional information
Centers for Disease Control and Prevention. 2022. National Report on Human Exposure to Environmental Chemicals. Atlanta, GA, USA.
Hays, SM, Kirman, CR. 2023. Biomonitoring Equivalents for N,N-Diethyl-meta-toluamide (DEET). Regulatory Toxicology and Pharmacology.
Health Canada. 2021. Personal Insect repellents. Ottawa, ON, Canada.
Health Canada. 2023. Re-evaluation Note REV2023-01, Pest Management Regulatory Agency Re-evaluation and Special Review Work Plan 2023-2028. Ottawa, ON, Canada.
Health Canada. 2023. Canadian Biomonitoring Dashboard. Ottawa, ON, Canada.