Cannabis voluntary recall guide
Download the alternative format
(PDF format, 453 KB, 31 pages)
Organization: Health Canada
Date published: 2019-08-27
Table of Contents
- 1.0 Purpose
- 2.0 Scope
- 3.0 Definitions
- 4.0 What is a recall?
- 5.0 Roles and responsibilities
- 6.0 Establishing and using documented procedures
- 7.0 Keeping sale, distribution and export records
- 8.0 Recall process
- 9.0 Contact us
- 10.0 Feedback—Help us improve
- Appendix A: Checklist for recalls
- Appendix B: Voluntary recall process flow chart
- Appendix C: Reports to Health Canada
1.0 Purpose
This guide provides information on the requirements of the Cannabis Act and Cannabis Regulations related to voluntary recalls of cannabis and cannabis products. It helps licence holders understand their role in a voluntary recall and promotes their compliance with recall requirements.
Federal licence holders
- Cultivation (nurseries, standard and micro)
- Processing (standard and micro)
- Sales for Medical Purposes
- Research
Federal permit holders
- Import (medical or scientific purposes)
- Export (medical or scientific purposes)
In addition, provincial and territorial distributors and retailers that are authorized under subsection 69(1) of the Cannabis Act are subject to some but not all recall requirements under the Cannabis Regulations.
The Cannabis Regulations do not apply to a holder of a licence under the Industrial Hemp Regulations.
Sections 46 and 235 (i.e., requiring a system of control for recalls) of the Cannabis Regulations do not apply to a holder of a licence for analytical testing or to a holder of a cannabis drug licence (exempted by section 3 of the Cannabis Regulations). For cannabis drug licences, recall requirements under the Food and Drugs Act and its Regulations apply.
This guide provides information on the requirements for licence holders to:
- Establish and maintain a system of control for the voluntary recall of cannabis or cannabis products, including conducting a recall simulation
- Keep sale, distribution and export records for cannabis or cannabis products
- Report voluntary recalls of cannabis or cannabis products to Health Canada
- Conduct a voluntary recall of cannabis or cannabis products
2.0 Scope
This guide applies to the recall requirements that are set out in the following sections of the Cannabis Regulations:
- Section 5.3: Prohibitions on selling voluntarily recalled cannabis products
- Sections 224, 225, and 226: Records on cannabis inventories
- Section 227: Record on cannabis sold, distributed or exported
- Sections 46 and 235: System of control for recalls and recall simulation
- Section 247: Recall reporting process
These requirements apply to licence holders engaged in cannabis activities that are subject to the Cannabis Regulations, with the exception of analytical testing and cannabis drug licence holders. Although the recall requirements do apply to licence holders for research, the nature and extent of the recall simulation will depend on the scope of the activities being conducted by the research licence holders (i.e., if they are authorized to sell or distribute).
Under section 5.3 of the Cannabis Regulations, provincial and territorial distributors and retailers that are authorized under subsection 69(1) of the Cannabis Act are prohibited from selling a cannabis product that they know is the subject of a voluntary recall related to the quality of the cannabis product or to non-compliance with Parts 5 or 6 of the Cannabis Regulations.
Health Canada uses a voluntary and collaborative approach to work with licence holders and other parties to ensure effective recalls.
However, this does not preclude Health Canada from taking other actions consistent with the Compliance and enforcement policy for the Cannabis Act. For example, in certain circumstances, a ministerial order requiring a recall or other measures could be used under section 75 or section 76 of the Cannabis Act.
3.0 Definitions
3.1 Definitions
The Cannabis Act and the Cannabis Regulations contain definitions of terms, some of which are included here for ease of use. The source is indicated in brackets. In addition to terms defined in the Act and Regulations, other terms are also included below for the purpose of interpreting this document.
- Brand element
- Includes a brand name, trademark, trade name, distinguishing logo, graphic arrangement, design or slogan that is reasonably associated with a brand of cannabis. (Cannabis Act)
- Cannabis
- A cannabis plant and anything referred to in Schedule 1 to the Act. Does not include anything in Schedule 2 to the Act. (Cannabis Act)
- Cannabis product
- Cannabis of only one of the classes that are set out in Schedule 4 to the Act – or a cannabis accessory if that accessory contains such cannabis – after it has been packaged and labelled for sale to a consumer at the retail level, but does not include cannabis that is intended for an animal, a cannabis accessory that contains cannabis that is intended for an animal, or a drug containing cannabis. (Cannabis Regulations)
- Client
- In respect of a holder of a licence for sale for medical purposes, an individual who is registered with that holder of the licence under subsection 282(1). (Cannabis Regulations)
- Consumer
- An individual who has obtained a cannabis product to be used for non-commercial purposes. This definition includes a client.
- Distribute
- Include administering, giving, transferring, transporting, sending, delivering, providing or otherwise making available in any manner, whether directly or indirectly, and offering to distribute. (Cannabis Act)
- Effectiveness check
- A review of the recall process that includes a survey of those affected by a cannabis recall (e.g., supply chain customers) to verify they have received the recall notification and are aware of their responsibilities with respect to the recall. The effectiveness check may include verification of the actions taken.
- Licence holder
- The holder of a licence issued under the Cannabis Act, other than a holder of a licence that is subject to the Industrial Hemp Regulations.
- Produce
- In respect to cannabis, means to obtain it by any method or process, including by manufacturing; synthesis; altering its chemical or physical properties by any means; or cultivating, propagating or harvesting it or any living thing from which it may be extracted or otherwise obtained. (Cannabis Act)
- Recalling licence holder
- A licence holder who commenced and is responsible for conducting a recall.
- Recall strategy
- A planned course of action taken by a recalling licence holder to conduct a recall.
- Risk type
- The numerical designation assigned to a recall; that corresponds to the relative degree of risk presented by the cannabis or cannabis product being recalled. There are three recall types (i.e., Type I, II or III) based on the degree of risk for health and safety.
- Sell
- Include offer for sale, expose for sale and have in possession for sale. (Cannabis Act)
- Supply chain customer
- Anyone who received, purchased or used cannabis or cannabis products that the recalling licence holder can identify and contact directly or indirectly, including federal licence holders, persons authorized to sell cannabis under a provincial or territorial Act, and clients.
3.2 Icons
This icon is used in this guide to highlight information of interest.
Important: Key or cautionary information.
4.0 What is a recall?
A recall, in respect of cannabis or a cannabis product that has been sold, distributed or exported, includes any action taken by a licence holder to correct or remove the cannabis or cannabis product from sale and distribution, and to notify all affected supply chain customers and the public of a problem or potential problem with the cannabis or cannabis product. Reasons for initiating a voluntary recall can include, but are not limited to, a licence holder becoming aware that the cannabis or cannabis product presents or may present a health or safety risk or that it may not meet the requirements of the Act and its Regulations.
During a recall, typical actions to be taken by a recalling licence holder with regard to the affected cannabis or cannabis product include:
- Informing Health Canada
- Ceasing the production, distribution and sale of the cannabis or cannabis product
- Removing the cannabis or cannabis product from the supply chain
- Correcting or destroying the cannabis or cannabis product, if applicable
- Contacting affected supply chain customers to notify them to stop further distribution and sale of the cannabis or cannabis product
- Contacting affected consumers to advise against use of the cannabis or cannabis product, if applicable
- Providing instructions to supply chain customers and consumers on what to do with the cannabis or cannabis products remaining in possession
- Assessing the effectiveness of the recall
- Taking corrective measures to prevent the problem underlying the recall from recurring
In addition, Health Canada posts a recall notice on its Recalls and Safety Alerts website.
A recall is intended to minimize the risk associated with a problem or potential problem with the cannabis or cannabis product by removing it from the supply chain and providing important health and safety information to the public.
5.0 Roles and responsibilities
While the requirements related to voluntary recalls apply to all licence holders in the supply chain, the most readily identifiable licence holder is encouraged to take responsibility for initiating a recall. Typically, the most readily identifiable licence holder–known as the recalling licence holder once the recall is initiated–is would be the licence holder who owns the product brand or whose information is listed on the label.
Under the Cannabis Regulations, the recalling licence holders haves specific responsibilities related to recalls, including conducting recall simulations, reporting and notification obligations. Nevertheless, a recall is a collaborative process among all licence holders and other parties in the supply chain.
The recalling licence holder should communicate the decision to commence a recall to all supply chain customers.
The effectiveness of the collaboration between the recalling licence holder and its supply chain customers depends, in part, on the extent to which each supply chain customer:
- Understands its own roles and responsibilities
- Defines and documents its expectations with other parties in the supply chain
- Communicates and shares recall information
- Maintains effective documentation with respect to distribution, inventory and sale
Health Canada documents and monitors recalls, provides guidance to licence holders and verifies compliance (e.g., request for information or inspection) with the recall requirements of the Cannabis Regulations. Health Canada is committed to making data and information available to Canadians, including posting recall notices on the Recall and Safety Alert website.
Under section 5.3 of the Cannabis Regulations, provincial and territorial distributors and retailers that are authorized under subsection 69(1) of the Cannabis Act are prohibited from selling a cannabis product that they know is the subject of a voluntary recall in Canada if the recall was initiated for one of the following reasons:
- There is concern about the quality of the cannabis product.
- Good production practice requirements (Part 5 of the Cannabis Regulations) have not been met.
- Cannabis product requirements (Part 6 of the Cannabis Regulations) have not been met.
6.0 Establishing and using documented procedures
Under section 46(1) of the Cannabis Regulations, licence holders, other than a licence holder for analytical testing or a cannabis drug licence holder, must establish and maintain a system of control that permits the rapid and complete recall of every lot or batch of cannabis that has been sold or distributed. This means that well-documented processes must be in place and that documents must be retained, as outlined under section 235 of the Cannabis Regulations.
Licence holders must establish documented processes with regard to maintaining sale and distribution records and carrying out recalls, including recall reporting (section 247). Each step of the recall process may be documented as a single procedure or as a number of procedures, depending on the structure of the quality system.
Licence holders must be able to demonstrate that they follow their established procedures during a recall. In addition, licence holders who use multiple procedures must be able to show that all parts of the distribution record and recall processes are reflected in the overall system of control.
The written recall procedures should:
- Define key activities
- Assign responsibilities
- Provide a detailed description of steps taken from the beginning to the end of the process
- Address the information required by the Cannabis Regulations, including keeping records (sections 226 and 227) and recall reporting (section 247)
When a recall is commenced or a recall notification is received, the licence holders involved should:
- Follow their written procedures
- Ensure that each employee responsible for any step in the procedure:
- Has access to the procedure
- Understands their responsibilities
- Is appropriately trained and qualified
- Receives support from management to ensure they follow the procedure
- Keep records as required, as outlined in section 7.0 of this guide
Activities described in recall procedures should have a time frame, where appropriate. Time frames should be based on the level of risk: the higher the risk, the faster the action needs to take place. In particular, timing needs to be specified for:
- Notifying Health Canada
- Notifying affected supply chain customers
- Following up with anyone who does not respond to the recall notification
To ensure an effective recall, everyone involved in the process, including supply chain customers, must understand their roles and responsibilities and how to complete their parts of the recall including the management of records.
A checklist of recall steps and a sample recall process flow chart can be found in Appendices A and B, respectively.
6.1 Recall Simulation
Licence holders, other than a licence holder for analytical testing or a cannabis drug licence holder, must conduct a recall simulation (or mock recall) at least every 12 months in order to ensure a recall plan is effective (subsection 46(2) of the Cannabis Regulations). Licence holders must conduct the simulation based on their established recall procedures.Existing licence holders as of October 17, 2019, must complete a recall simulation within 12 months of October 17, 2019. Any licence holders who receive their licence after October 17, 2019, must complete a recall simulation within 12 months of the date the licence was received.
After the recall simulation has been completed, licence holders must document the details of how the recall was conducted and the results, and retain this document for at least two years after the day on which the recall simulation was completed. Licence holders are not required to notify Health Canada of the recall simulation or provide the recall simulation document at the time of the simulation. However, Health Canada may request it at any time or verify compliance during an inspection.
For example, a licence holder might decide to conduct a recall simulation within its own supply chain for a lot of cannabis or cannabis product that the licence holder knows has reached the consumer market. The simulation could include identifying the problem as well as all of the supply chain customers who are affected, to ensure the licence holder’s contacts are up to date.
To document the details of the recall simulation, including how it was conducted and the results, the licence holder could include:
- A description of the scenario
- The date and time of the simulation
- The effectiveness of the simulation, such as
- Whether employees followed the recall procedure
- Whether all affected supply chain customers were identified quickly
- Whether the amount of recalled product could be reconciled with the amount produced, distributed and in inventory
- Any problems encountered during the simulation and when and how they will be corrected (e.g., updates to training and procedures)
Section 8.5.2 of this guide provides information on evaluation a recall’s effectiveness that could also be used to evaluate a recall simulation.
As noted in Section 2.0 of this document, although the recall requirements do apply to licence holders for research, the nature and extent of the simulation will depend on the scope of the activities being conducted by the research licence holders (i.e., if they are authorized to sell or distribute).
7.0 Keeping sale, distribution and export records
The process that licence holders follow to create and keep sale, distribution and export records varies depending on sales as well as accounting and shipping procedures. Keeping records may involve a number of procedures and personnel. Records that identify the current location of affected cannabis and cannabis products during a recall should be readily available.
To conduct a timely and effective recall, licence holders must create and maintain records for all cannabis sold, distributed, and in inventory. It is expected that information listed in the records will be sufficient to allow licence holders to trace and account for all of the affected cannabis and cannabis products being recalled. In addition, processing licence holders must keep records of ingredients that are obtained or produced to make a cannabis extract, cannabis topical, or edible cannabis. These records must be retained for two years.
- Sections 224 to 227 of the Cannabis Regulations provide more information about the requirement related to inventory and distribution records.
- Section 224: Inventory
- Section 225: Inventory cannabis exract, cannabis topical or edible cannabis
- Section 226: Cannabis obtained from another person
- Section 226.1: Things to be used as ingredients
- Section 227: Sale, distribution and export of cannabis
Licence holders should ensure that their records are stored in a way that protects the integrity of the records and allows them to be easily retrieved.
The time needed to access records should be identified as part of the recall process. To permit rapid and complete recalls, licence holders should be able to retrieve the relevant records within one business day.
If the record keeping system is changed, licence holders must ensure access to records generated prior to the change is maintained.
8.0 Recall process
The recall process has six steps, divided into two main parts: initiating a recall (sections 8.1 to 8.3 of this guide) and commencing a recall (sections 8.4 to 8.6 of this guide).
Initiating a recall
- Identify the need to initiate a recall
- Develop a recall strategy and determine scope of the recall
- Inform Health Canada of the recall
Commencing a recall
- Notify supply chain customers and begin the recall
- Follow up with Health Canada and supply chain customers
- Review and close the recall
Details on each step are outlined below, in order, but some may occur simultaneously.
The recalling licence holder is involved in all six steps. Other licence holders and supply chain customers participating in the recall are involved in steps 4, 5 and 6.
To manage or participate in a recall, licence holders are expected to follow their established recall procedures, as outlined in section 6 of this guide.
8.1 Identify the need to initiate a recall
A licence holder should consider initiating a recall if they become aware that cannabis or a cannabis product:
- Presents a risk to health or safety
- Does not or may not meet the requirements of the Act or Regulations
There are many ways a licence holder might become aware of a problem or potential problem with cannabis or a cannabis product. These include a complaint and subsequent investigation, inspections, internal quality control testing, pesticide testing, or internal audits. Product issues may be related to non-compliance and /or substandard quality, such as:
- Improper packaging and labelling
- Lack of adherence to good production practices
- Issues arising from improper storage, shipping or handling
Quality systems should include ways to identify product issues.
It is the responsibility of the licence holder to perform a risk evaluation (section 8.2.1 of this guide) and determine the actions required to correct the problem. Once the need to initiate a recall has been identified, the recalling licence holder should review its current inventory to identify and quarantine any affected cannabis and cannabis product still under its control. Quarantine measures can include physical and/or electronic methods to prevent affected cannabis or cannabis products from being sold or distributed.
8.2 Develop a recall strategy and determine scope of the recall
Once the licence holder has determined that a recall is necessary, the scope of the recall (i.e., determining which cannabis and cannabis products need to be recalled) should be defined and a strategy to commence the recall should be developed. The recalling licence holder should follow its established recall procedures to develop the recall strategy and quickly identify supply chain customers who may be affected.
The recall strategy should include:
- A risk evaluation
- The scope of the recall within the supply chain
- Timelines
- A communications plan
- The content of the recall notifications that will be sent to supply chain customers and, if applicable, to consumers
- The initiation date, the date(s) progress reports will be submitted to Health Canada and the anticipated closure date of the recall
8.2.1 Risk evaluation
The licence holder's recall procedures should provide clear instructions on how to evaluate the risk associated with the affected cannabis or cannabis product and how this information will be provided to Health Canada. The extent and type of recall action depends on the risk associated with the affected cannabis or cannabis product or the potential problems that underlie the recall.
When evaluating the risk, the recalling licence holder should take into account:
- The nature and degree of the problem or potential problem
- The nature of the population at risk
- The size of the population at risk
- The extent of supply chain customer awareness of the problem
- Whether adverse health consequences have occurred from using the affected cannabis or cannabis product
The recalling licence holder must provide Health Canada with a risk evaluation within 72 hours of notifying Health Canada that the recall has been initiated, as per subsection 247(3) of the Cannabis Regulations.
Health Canada assigns risk types to recalls based on the information provided in the notification received from the licence holder. The risk type is used to establish the timeline to commence the recall.
Table 1 describes the three risk type classifications for recalls and provides illustrative examples.
| Risk type | Description | Example |
|---|---|---|
Type I |
There is a reasonable probability that the use of or exposure to the affected cannabis or cannabis product will cause serious adverse health consequences or death |
One lot of edible cannabis with E. coli. |
Type II |
The use of or exposure to the affected cannabis or cannabis product may cause temporary adverse health consequences or the probability of serious adverse health consequences is remote |
Cannabis extract was bottled and mistakenly labelled with a lower concentration of total THC than what was in the product
|
Type III |
The use of, or exposure to, the affected cannabis or cannabis product is not likely to cause any adverse health consequences |
One run of a product label was printed without the mandatory health warning messages. |
These examples are illustrations, as each recall scenario is unique. Health Canada assesses the risk based on the risk type descriptions above.
8.2.2 Scope of recall within the supply chain
The recall strategy should define the scope of the recall, which is the extent to which the affected cannabis or cannabis product has been distributed in the supply chain and the number of supply chain customers who are involved. The recalling licence holder should base the scope of the recall on the amount of affected cannabis or cannabis products, where and how it was or is being used, and the risk it poses to the public.
8.2.3 Timelines
The recall strategy must define timelines for key activities. Table 2 outlines suggested maximum timelines for making the first contact with supply chain customers, based on the risk type assigned by Health Canada.
| Recall type | Timeline for action |
|---|---|
| Type I | Initial contact should be made as soon as possible, and at most within one business day of commencing the recall |
| Type II | Initial contact should be made as soon as possible, and at most within four business days of commencing the recall |
| Type III | Initial contact should be made as soon as possible, and at most within seven business days of commencing the recall |
The recalling licence holder must develop a detailed plan that shows estimated timelines for each action, based on:
- The complexity of recall actions
- The number of supply chain customers and geographic distribution
- The risk associated with the affected cannabis or cannabis product
8.2.4 Recall communications plan and content
The recall strategy must include a communications plan that defines the method and content for all communications associated with the recall. Recall notifications to supply chain customers should be brief and to the point and should not contain irrelevant information, promotional material or any element that may detract from the message and the risk.
To prevent further sale and distribution of the affected cannabis or cannabis product, it is important to instruct supply chain customers to immediately stop the sale and distribution of the cannabis and cannabis product and quarantine any stock. The recall notification should include detailed instructions to notify other supply chain customers who may be selling or distributing the affected cannabis or cannabis product.
In general, recall notifications to supply chain customers should include the following, in English and French:
- The date the recall notification is being sent
- The name of the affected cannabis or cannabis product that is subject to the recall
- A description of the affected cannabis or cannabis product being recalled, including catalogue number, lot number(s), serial number(s) or other descriptive information to enable the immediate and accurate identification of the cannabis
- The reason for recall and any risk associated with the use of the affected cannabis or cannabis product
- Instructions to immediately cease further sale, distribution or use of any of the remaining affected cannabis or cannabis product
- Instructions regarding disposal of the affected cannabis or cannabis product, with specific steps for destruction or return
- A request for a prompt response to confirm receipt of the notification as well as understanding of the actions required
The recalling licence holder is responsible for sending out recall notifications to its supply chain customers. To encourage a quick response, recall notifications might include:
- Pre-addressed post cards
- A toll-free number for telephone replies
- A form to complete and return by fax or email
- A link in an email that the recipient can click to acknowledge receipt of the recall notification
The recalling licence holder should clearly mark recall notifications with Cannabis Recall in a bold and easily identifiable manner.
If supply chain customers are carrying the recall forward, they may also develop their own communications to be included with those from the recalling licence holder. If they do, the recalling licence holder’s original notification, with the risk information and directions, should not be changed.
8.3 Inform Health Canada of the recall
Section 247 of the Cannabis Regulations outlines the requirements for to reporting a voluntary recall of cannabis or a cannabis product to Health Canada. The recalling licence holder must report the recall to Health Canada by e-mailing at hc.compliance-cannabis-conformite.sc@canada.ca
Health Canada must be informed prior to commencing the recall (i.e., before notifying supply chain customers) The report to Health Canada must specify whether the affected cannabis or cannabis product was sold or distributed in Canada or exported from Canada.
Based on the information received, Health Canada will publish a recall notice on the Recalls and Safety Alerts website that identifies the issue, the risk type and any actions that consumers should take.
The recalling licence holder sends Health Canada three types of reports during a recall:
- An initial report must be sent containing information that must be provided prior to commencing a recall (subsections 247(1) and 247(2) of the Cannabis Regulations)
- A risk evaluation must be sent within 72 hours of providing the initial report (subsection 247(3) of the Cannabis Regulations)
- Progress reports (subparagraphs 247(1)(j)(ii) and (2)(j)(ii) of the Cannabis Regulations)
- A final report must be sent (subsections 247(4) and (5) of the Cannabis Regulations)
In addition to other information about the affected cannabis and cannabis product, the anticipated completion date of the recall must be provided in the initial report to Health Canada. A rationale should be provided if the completion is expected to take longer than two weeks.
Appendix C provides a checklist of the information that must be included in reports.
8.4 Commerce the recall and notify supply chain customers
Commencing the recall begins when the recalling licence holder notifies its affected supply chain customers of the recall. It involves the recalling licence holder and includes any supply chain customers who have further sold, distributed or exported the affected cannabis or cannabis product.
8.4.1 Identifying affected supply chain customers
The recalling licence holder's recall procedures should describe how to generate a list of affected supply chain customers and include a method to locate consumer contact information.
Sale, distribution and export records will help determine how many supply chain customers will need to be contacted.
8.4.2 Method of notification
The recall procedures should describe how the recall will be communicated to affected supply chain customers. Both primary and secondary methods of communication should be identified.
Every effort should be made to ensure the most appropriate person in the supply chain is contacted and a record of that contact should be kept.
Some of the ways that recall communications can be accomplished include:
- Telephone calls
- Fax
- Special delivery letters that have tracking and receipt confirmation (i.e., registered mail, courier)
If telephone calls are used, it is recommended that a written follow-up be done to ensure all details are shared and to establish a clear record of the communication.
Depending on the scope of the recall, additional distribution methods could be considered for recall notices, as outlined in table 3.
| Method | Examples |
|---|---|
Websites |
|
Social media platforms |
|
Media/marketing outlets |
|
Direct notice |
|
Posters |
|
8.4.3 Tracking responses to recall notifications
Recall procedures should describe how the recalling licence holder will record and track responses to recall notifications. Records showing that appropriate efforts have been made to contact all supply chain customers should be maintained. These records could include:
- Dates of attempted contact
- Name and title of person contacted
- Means of contact (e.g., phone, email, mail)
- A record of the discussion once contact was successful
- Whether recall instructions were understood and carried out
- Completed response forms
- Related correspondence
Documentation and confirmation of contact could include a fax-back form, email response or telephone log. The details of all contact made with a supply chain customer should be documented and appropriate follow-up with supply chain customers who do not respond should be completed.
8.4.4 Completing and tracking recall actions
The recalling licence holder must also complete and track other required actions related to the affected cannabis or cannabis product, such as receipt of returned affected product. Once acknowledgement that the initial notification of the recall is received, the recalling licence holder should complete other actions related to the recall, as applicable, including:
- Having the affected cannabis or cannabis product returned for destruction
- Having the supply chain customer destroy the affected cannabis or cannabis product
- Providing new labelling
- Correcting the affected cannabis or cannabis product
The recalling licence holder should track each action that is completed. Because some recalls involve multiple actions, the licence holder may choose to use spreadsheets or databases to track completion of recall actions.
8.4.5 Controlling the affected cannabis or cannabis product
Recall procedures must identify how the affected cannabis or cannabis product is to be handled until it is corrected or destroyed. Any returned cannabis or cannabis product must be controlled to prevent it from being sold, distributed, exported or used in error.
8.5 Follow up with Health Canada and supply chain customers
The recalling licence holder is required to complete a number of steps to follow up with Health Canada and supply chain customers. These can include:
- Submitting progress reports to Health Canada
- Evaluating the recall's effectiveness
- Checking that recall actions have been completed
- Product correction, if applicable
- Product disposal (destruction or return), if applicable
8.5.1 Submitting progress reports to Health Canada
The recalling licence holder should submit progress reports to Health Canada at agreed-upon intervals in the initial report. They should contain the following:
- The number of supply chain customers notified of the recall and date and method of notification
- The quantity of affected cannabis and cannabis product in possession of each supply chain customer
- The number of respondents supply chain customers
- The number of non-respondents supply chain customers
- The number of subsequent attempts to contact non-respondent supply chain customers
- The quantity of affected cannabis and cannabis product returned and/or destroyed
- The estimated time frame for completion of the recall if revised from the original date
8.5.2 Evaluating the recall's effectiveness
The recall strategy should specify how the effectiveness of the recall will be evaluated. This is known as an effectiveness check. In most cases, effectiveness can be monitored from the initial notification through to the supply chain customers' responses.
Responses may involve written acknowledgement that the supply chain customers received, read and understood the recall. The recalling licence holder may also request supply chain customers to provide information about the status of the affected cannabis or cannabis products. The recalling licence holder should evaluate the effectiveness of each recall action.
Table 4 sets out some best practices to use when determining recall effectiveness.
| What to review | Action |
|---|---|
Overall process |
Confirm with certainty that the recall procedure and process have been implemented |
Supply chain customer feedback |
Verify the following:
If few supply chain customers respond to confirm receipt of the information, more effort may be required to reach them to confirm that action has been taken. |
Consumer feedback, if applicable |
Determine the following:
If few consumers have responded, more work may be required to understand the reason and additional effort may be necessary to ensure the recall has been properly communicated. |
8.5.3 Following up with non-respondent supply chain customers
If supply chain customers do not respond to the first notification, the recalling licence holder must follow up with them. Non-responders are supply chain customers from whom the recalling licence holder does not receive confirmation of receipt of the recall.
Health Canada expects recalling licence holders to follow up with non-responders. Table 5 outlines guidelines for follow-up efforts.
| Recall risk type | Follow-up efforts |
|---|---|
| Type I | There should be no non-responders. If there are non-responders, justification should be provided and records should be maintained. |
| Type II | Three follow-up efforts have been made using different contact methods as appropriate. Records should be maintained. |
| Type III | Two follow-up efforts have been made using different contact methods as appropriate. Records should be maintained. |
8.5.4 Checking completion of recall actions
The recalling licence holder should review tracking mechanisms for its corrective actions, which could include contacting supply chain customers or retrieving and/or destroying affected cannabis or cannabis products, to ensure all of the affected cannabis and cannabis products have been addressed. Recalling licence holders are responsible for ensuring that all recall actions are complete.
Health Canada recognizes that recall actions depend on the consent and cooperation of supply chain customers. If the supply chain customer does not permit or conduct the actions required by the recall despite repeated attempts to communicate their importance, the recalling licence holder should include this information in its recall records and notify Health Canada.
The recalling licence holder should review all information collected during this step to determine if additional action must be taken to address the problem or potential problem that underlies the recall. This may include revising or adding to its recall strategy.
8.5.5 Product correction
If the affected cannabis or cannabis product will be corrected, the corrections should be done as outlined in the recall strategy and standard operating procedures. The recalling licence holder should keep appropriate records that clearly show how the affected cannabis or cannabis product has been corrected, such as putting a corrected label on a product if the initial label was incorrect or contained missing information.
8.5.6 Product disposal
When affected cannabis or a cannabis product has been returned and will not be corrected, the recalling licence holder should dispose of it as outlined in its recall strategy and standard operating procedures. The recalling licence holder should keep appropriate records to show that disposal of the affected cannabis or cannabis product has been completed.
8.6 Review and close the recall
The steps required to review and close recalls are:
- Completing a final review of all recall actions
- Submitting a final report to Health Canada
- Closing the recall
- Completing and maintaining final documentation
8.6.1 Completing a final review
The recall procedure requires a final review to determine if the recall is ready to be closed. A recall may only be closed once it has been completed, meaning that all notifications and follow-up actions have been completed and the problem or potential problem has been addressed. A qualified person or group must do a final review to ensure the recall file contains the necessary documentation related to all recall actions that were taken.
The recalling licence holder must review the following information, as applicable, before determining that a recall is complete and ready to be closed:
- The number of units affected
- The number of units returned, if applicable
- The number of units destroyed, if applicable
- The number of units corrected, if applicable
- The number of units that could not be located, if applicable
- The final completion date for the recall
- Assurance that all supply chain customers received the recall information
- A detailed plan to prevent the problem from recurring, including any steps that will be taken to improve quality control, if applicable
The final review can also provide valuable information about the recalling licence holder's recall strategy and procedures, and this may be used to refine the strategy for future recalls.
8.6.2 Submitting a final report to Health Canada
The recalling licence holder must submit a final report to Health Canada within 30 days after the recall has been completed, as per subsection 247(4) of the Cannabis Regulations.
8.6.3 Closing the recall
The recalling licence holder must document the completion of the recall. The person who determines that the recall is complete and can be closed should be familiar with all aspects of the recall process.
Once Health Canada receives the final recall report, Health Canada sends a response to the recalling licence holder to confirm that the recall is closed.
8.6.4 Completing and maintaining final documentation
The recalling licence holder must keep copies of the initial report, risk assessment, progress reports and final report for at least two years as per subsection 247(6) of the Cannabis Regulations. Health Canada may request this information at any time or during inspection.
9.0 Contact us
Voluntary recalls for cannabis or cannabis products should be reported to Health Canada by contacting hc.compliance-cannabis-conformite.sc@canada.ca.
If you have specific questions about cannabis or cannabis product voluntary recalls, email Health Canada at cannabis@canada.ca.
If you have general questions about the Cannabis Act and its Regulations, email cannabis@canada.ca.
Alternatively, you can reach the Controlled Substances and Cannabis Branch at 1-866-337-7705.
10.0 Feedback—Help us improve
Health Canada is committed to providing all stakeholders with timely, accurate and reliable information. This includes providing applicants and licence holders with the information they need to comply with the Cannabis Act and its Regulations.
We would appreciate receiving your feedback on whether this guide was useful, and we welcome your suggestions for improvement. Email your feedback to us at cannabis@canada.ca and indicate in the subject line Feedback on the Cannabis Voluntary Recall Guide.
Appendix A: Checklist for recalls
This checklist summarizes the steps recalling licence holders should follow during a recall. It can be used in conjunction with the recall procedures outlined in section 8 of this guide.
Step 1: Identify the need for a recall
- The licence holder has identified an issue with the cannabis or cannabis product. The licence holder should assess the need for a recall by reviewing issues such as:
- Does the cannabis or cannabis product pose a risk to health and safety?
- Does it meet the requirements of the Cannabis Act and Cannabis Regulations?
- Does it have quality deficiencies?
- Is action required to mitigate the risk?
- If the product has been sold, distributed or exported and the recalling licence holder determines that it should be recalled, proceed to step 2.
Step 2: Develop a recall strategy and determine scope of the recall
- Define the recall scope from sale, distribution and export records
- How many products are affected?
- How many affected supply chain customers are affected?
- What risk type has been assigned?
- What action(s) are required?
- Develop a recall notification
- Develop a notification that provides a description of the affected cannabis or cannabis product, the reason for recall, the risk associated with use, instructions on the actions to take with regard to remaining product, and a request for acknowledgement
- Include a clear stop sale request, as applicable in the notification to supply chain customers
Step 3: Inform Health Canada of the recall
- Contact Health Canada by e-mail at hc.compliance-cannabis-conformite.sc@canada.ca and provide:
- The name of the recalling licence holder, the licence number, and a description of the affected cannabis or cannabis product that is being recalled, including the brand name
- The number of each affected lot or batch of affected cannabis or cannabis products
- If known, the number of any lot or batch that was used to make the cannabis or cannabis product
- If applicable, information on who produced, imported, packaged or labelled the affected cannabis or cannabis product
- If the recall is related to a cannabis extract, cannabis topical or edible cannabis that is a cannabis product or that is contained in a cannabis accessory that is a cannabis product, the list of ingredients that appears on the label of the cannabis product
- If the recall is related to a cannabis accessory that is a cannabis product, the name and address of each person that produced or imported the affected cannabis accessory or any part of it
- The reasons for initiating a recall
- The quantity of affected cannabis or cannabis product that was produced or imported by the licence holder
- The quantity of affected cannabis or cannabis product that was sold or distributed by the licence holder
- The quantity of affected cannabis or cannabis product that remains in possession of the recalling licence holder
- The number of supply chain customers to whom of the affected cannabis or cannabis product was sold or distributed
- The period of time during which the affected cannabis or cannabis product product was sold or distributed
- The recall strategy, including the intended date to commence the recall, how and when Health Canada will be notified of the progress of the recall and the proposed date for completion of the recall
- A description of any other action that is being taken or is intended to be taken with respect to the recall
- An evaluation of the risk associated with the problem or possible problem
- Contact information for a representative of the recalling licence holder
- The communications plan
Step 4: Notify supply chain customers and perform other recall actions
- Identify and communicate with supply chain customers
- Monitor responses and acknowledgements
- Perform other actions, as required, such as quarantine or collection or destruction of the affected cannabis or cannabis product
Step 5: Follow up with Health Canada and supply chain customers
- Perform an effectiveness check
- Follow up with non-respondent supply chain customers
- Ensure completion of required actions (e.g., return affected cannabis or cannabis product)
- Take additional action if required
- Submit progress reports to Health Canada as per agreed-upon timelines that include:
- The number of respondent supply chain customers
- The number of non-respondent supply chain customers
- The quantity of affected cannabis or cannabis product returned or destroyed
- The estimated time frame for completion if revised from original
Step 6: Review and close the recall
- Review recall records and documentation and ensure they are properly retained
- Dispose of affected cannabis or cannabis products as per procedures
- Submit final report to Health Canada within 30 days of recall completion or agreed-upon timeframe with Health Canada that outlines the following
- The results of the recall, including:
- The number of units affected
- The number of units returned, if applicable
- The number of units destroyed, if applicable
- The number of units that could not be located, if applicable
- The date of completion for the recall
- Assurance that all supply chain customers have received the recall information
- The measures taken to prevent the problem from recurring and steps taken to resolve the problem
- The results of the recall, including:
Appendix B: Voluntary recall process flow chart
Responsibilities and relationships of recalling licence holders, supply chain customers and Health Canada
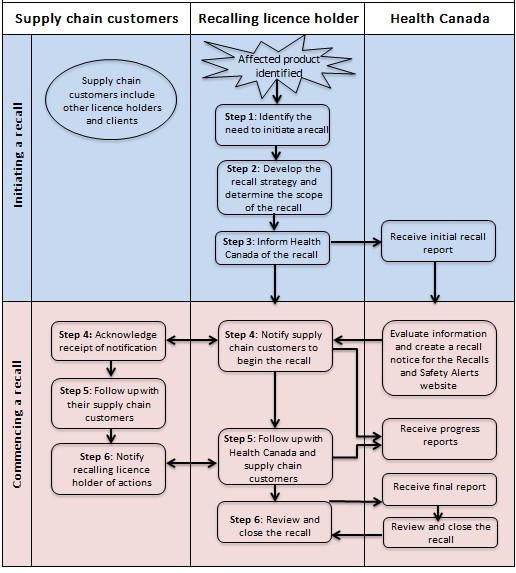
Text description
Appendix B is a Process flow chart of the cannabis recall process. It outlines the responsibilities and relationships between recalling licensees, supply chain customers and Health Canada.
The supply chain could include other licence holders and their clients.
Launching the recall: Once the affected product is identified by the licence holder initiating the recall, the first step is to determine the need for a recall. The next step is to define a recall strategy and determine its scope, and the third step is to inform Health Canada of the recall. The Department also requires the initial recall report.
Start of Recall: Health Canada evaluates the information received and creates a recall and safety alert. It is posted on the Recall & Safety Alerts web site.
In the 4th step the licence holder sends a notice to all the supply chain customers that may have received the affected product, to start the recall. Customers confirm receipt of the notification.
For the fifth step, a follow-up is made to all the affected supply chain customers by the licence holder initiating the recall. The licence holder is required to send these progress reports to Health Canada.
In the final step, the customers in the supply chain must notify the licence holder of the actions taken. The license holder reviews and ends the recall. This is done in conjunction with a final report to Health Canada. The Department also reviews the recall and concludes it.
Appendix C: Reports to Health Canada
Initial report to Health Canada
Subsections 247(1) and (2) of the Cannabis Regulations outline the following information that must be submitted in the initial report to Health Canada, prior to commencing a recall.
- The full name and address of the recalling licence holder, as indicated on the licence
- A description of the affected cannabis or cannabis product, including:
- Brand name
- Identifier (catalogue number, product code, bar code, if applicable)
- The number of each lot or batch of the affected cannabis or cannabis product
- The full name and address of the licence holder(s) who imported, produced, packaged, or labelled the cannabis or cannabis product, if applicable
- If the affected cannabis or cannabis product was obtained from another licence holder, the source should be identified.
- The reasons for commencing the recall
- A description of the problem or potential problem with the affected cannabis or cannabis product. This should be as simple as possible, as this information will be used to create a recall notice for the Recalls and Safety Alerts website.
- The quantity of affected cannabis or cannabis product that the recalling licence holder produced or imported into Canada
- When the recall is for cannabis or cannabis product that has been sold or distributed in Canada, indicate:
- The quantity produced or imported by the licence holder
- The quantity sold or distributed in Canada by the licence holder
- The quantity that remains in possession of the licence holder, if applicable
- The number of supply chain customers to whom the licence holder sold or distributed the affected cannabis or cannabis product, and number of consumers, if applicable
- The period during which the recalling licence holder sold or distributed the affected cannabis or cannabis product in Canada, including the first date of sale or distribution and the last date of sale
- When the recall is for cannabis or cannabis product that has been exported from Canada:
- The quantity that was produced or imported into Canada by the recalling licence holder, if applicable
- The quantity that was sold or distributed by the recalling licence holder in foreign countries
- The quantity that remains in the possession of the recalling licence holder, if applicable
- The number of persons to whom the recalling licence holder sold or distributed the or cannabis product cannabis in foreign countries
- The period during which the recalling licence holder sold or distributed the cannabis or cannabis product in foreign countries
- The recall strategy, indicating the timelines and manner that the recall will be carried out:
- The expected date of commencement of the recall
- The intended start date to contact supply chain customers
- The recall communications plan, including
- Content of the recall notification
- Primary and secondary methods of contacting supply chain customers
- How and when progress reports will be provided to Health Canada
- Proposed date for completion of the recall
- Copies of all intended communications about the recall in both official languages
- Letters, notices, telephone script(s), emails to supply chain customers
- Acknowledgement forms
- Public notices or press releases
- Description of any other measures the recalling licence holder is taking or intends to take related to the recall, including a description of how the licence holder plans to prevent the problem or potential problem from recurring again. This should include:
- A root cause analysis, if one has been completed
- If the recalling licence holder does not yet have a detailed plan, the report should indicate what the licence holder plans to do to understand and resolve the problem
- Contact information for a representative of the recalling licence holder
- An evaluation of the risk associated with the problem or possible problem, submitted within 72 hours of submitting the initial report
Progress reports to Health Canada
After providing the initial report to Health Canada, the recalling licence holder should provide progress reports at agreed-upon intervals, containing the following information:
- The number of supply chain customers notified of the recall
- The number of respondent supply chain customers
- The number of non-respondent supply chain customers
- The number of products returned
- The estimated time frame for completion, if revised
Final report to Health Canada
After completion of the recall, the recalling licence holder must provide a final report that contains the following information, as applicable:
- Results of the recall, including:
- The quantity of affected cannabis or cannabis product recovered
- The quantity of affected cannabis or cannabis product used and not recovered
- The quantity of affected cannabis or cannabis product destroyed, if applicable
- The number of non-respondent supply chain customers
- Measures taken to prevent a recurrence of the problem
- Completion date
The final report must be provided to Health Canada within 30 days after the completion of the recall, or within an agreed-upon extended timeline, as per subsection 247(4) and (5) of the Cannabis Regulations.