Page 4: Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – 2-Methyl-4-chlorophenoxyacetic Acid (MCPA)
9.0 Health effects
9.1 Effects in humans
The lowest published lethal oral dose for MCPA in humans is 814 mg/kg bw (RTECS, 2005). Symptoms of acute exposure to large doses of MCPA have been reported as a result of poisoning from accidental ingestion and accidental exposure during manufacturing or application in the field. The symptoms include fatigue, weakness, anoxia, nausea, vomiting, diarrhoea, lowering of the blood pressure, body temperature disturbance, progressive hypotension, ataxia, neuromuscular irritability and convulsion (Popham and Davis, 1964; Johnson and Koumides, 1965; Jones et al., 1967; Palva et al., 1975; Bovey, 1980b; Timonen and Palva, 1980; U.S. EPA, 1984).
Various epidemiological studies have been conducted with phenoxy herbicides in Canada, the United States, Australia, New Zealand and several European countries. Most epidemiological studies conducted to date have dealt with multiple exposures to various chlorophenoxy herbicides, including principally 2,4-D and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), and to other pesticides, raw materials, intermediates and processing chemicals. It has been reported that some phenoxy herbicides, such as 2,4,5-T, were contaminated by dioxins and furans during production, but MCPA was not shown to be contaminated with dioxin (Wiklund et al., 1988; Eriksson et al., 1990; Mannetje et al., 2005). Controversy exists as to whether the presence of dioxin increased the risk of certain cancers. Cases of soft tissue sarcoma (STS), non-Hodgkin's lymphoma (NHL) and Hodgkin's disease have been associated with phenoxy herbicides, including those contaminated with dioxin; however, results were not consistent (Mannetje et al., 2005).
Some of these epidemiological studies have included MCPA as part of the repertoire of herbicides examined (Hardell and Sandström, 1979; Eriksson et al., 1981; Hardell et al., 1981; Vineis et al., 1986, 1991; Wiklund et al., 1987, 1988, 1989; Eriksson et al., 1990; Saracci et al., 1991; Bueno de Mesquito et al., 1993; Becher et al., 1996; Lynge, 1998); however, very few studies report outcomes specific to MCPA (and related compounds), and it is therefore difficult to isolate exposure to MCPA alone. These studies, as reported below, are limited, since the sample sizes were small, co-exposure to other pesticides likely occurred, and there was no detailed information on dose used and amount of time spent applying pesticides. More definitive studies with accurate assessment of MCPA-specific exposure are needed.
A population-based case-control study in northern and central Sweden investigated the potential association between exposure to a group of several classes of pesticides including MCPA and the risk of NHL. Male cases (404) with NHL were selected during 1987-1990, along with twice as many controls. The authors found that among the pesticides, herbicide use was marginally associated with NHL, with an odds ratio (OR) of 1.6 and a 95% confidence interval (CI) of 1.0-2.5; however, the sample size was small (61 cases and 81 controls). Further analysis, looking at subjects exposed to specific classes of herbicides, showed that the phenoxyacetic acids group predominated, with an OR of 1.5 (CI = 0.9-2.4), which was not significant. However, among the phenoxyacetic acid group, MCPA showed an OR of 2.7 (CI = 1.0-6.9). Because of the small number of exposed cases and controls for MCPA (12 cases and 11 controls), not much reliance can be placed on the results (Hardell and Eriksson, 1999).
Two nested case-control studies looking at STS and NHL were conducted within an international cohort of workers exposed to phenoxy herbicides, chlorophenols and dioxin, as well as other pesticides in the workplace (Kogevinas et al., 1995). Results showed an excess risk of STS for exposure to any phenoxy herbicide (OR = 10.3; CI = 1.2-90.6; 10 exposed cases of STS were observed vs. 30 controls) and to each of the three major classes of phenoxy herbicides, including the class of MCPA (including MCPA, MCPP [(±)-2-(4-chloro-2-methylphenoxy)propanoic acid or mecoprop] and MCPB; OR = 11.3; CI = 1.3-98; 10 exposed cases vs. 29 controls). The association for NHL was weaker than those observed for STS; no excess risk was strongly associated with any of the exposures. According to the authors, it is complicated to evaluate the independent effect of each herbicide or contaminant on cancer risk, since workers are rarely exposed to a single herbicide. The authors found no specific association between risk and those herbicides contaminated with dioxin.
A cohort study was conducted in Denmark on the health effects related to potential herbicide exposure in 4461 workers (3390 males and 1071 females) working between 1947 and 1993 in two phenoxy herbicide production facilities. MCPA, 2-(2,4-dichlorophenoxy)propanoic acid (2,4-DP or dichlorprop) and MCPP were the main herbicides produced in these facilities, but other compounds, such as dyes and pigments, were also manufactured (Lynge, 1993, 1998). A non-significant twofold risk of STS was observed in persons potentially exposed to phenoxy herbicides compared with the Danish population; four cases were observed compared with 1.68 expected cases (standardized incidence ratio = 2.38; CI = 0.7-6.1). No excess of NHL was observed among workers potentially exposed to phenoxy herbicides compared with the Danish population. The overall cancer risk was found to be similar for both the Danish population and workers. However, the contribution of MCPA could not be determined due to exposure to multiple herbicides. The authors suggested that their findings (although based on small numbers) continue to suggest a possible association between exposure to MCPA and exposure to other related phenoxy herbicides, such as MCPP and 2,4-DP, and the risk of STS. Previous Swedish case-control studies with phenoxy herbicides by Eriksson et al. (1981) and Hardell et al. (1981) suggested a possible association (i.e., an increased risk) between STS and exposure to phenoxy herbicides as well as chlorophenols and their contaminants.
In another cohort study (1947-1975), mortality and cancer incidences were examined in 5754 workers from a British factory that manufactured, formulated and sprayed principally MCPA, but also other agricultural chemicals during that same period (Coggon et al., 1986). The overall mortality as well as mortality from cancer were less than expected from national rates; however, when the rural correction factor was applied, the deficit of deaths from cancer became a slight excess, but was not statistically significant. Only one death from STS was observed in this study, compared with the expected number of 0.9 in the complete cohort and 0.6 in workers potentially exposed to phenoxy acids. Mortalities from Hodgkin's disease and NHL were less than expected from the national rates. The authors concluded that "if there is a hazard of STS due to MCPA, then the risk is less than suggested by earlier studies of 2,4,5-T and 2,4,5-trichlorophenol, and it is small in absolute terms."
Because of the small size of most of the cohorts, not much reliance could be placed on the results, whether positive or negative.
Although several epidemiological studies were conducted on the reproductive and developmental outcomes of pesticide exposures, only a few included MCPA and are reported below.
A study was conducted to analyse the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population (Arbuckle et al., 1999). A questionnaire was used to obtain information from 2110 women on 3936 pregnancies, including 395 spontaneous abortions. Preconception (4-month period from 3 months before conception to the calendar month of conception) and post-conception (4-month period from calendar month of conception to the end of the first trimester) exposure windows were examined separately, in addition to differentiating between early (< 12 weeks) and late (12-19 weeks) spontaneous abortions for these same windows of exposure. Results showed a weak association between the risk of early spontaneous abortion and preconception exposure to any phenoxyacetic acid herbicides (adjusted OR = 1.1; CI = 0.6-1.9). However, when restricted to early abortions, the adjusted OR increased to 2.5 (CI = 1.0-6.4); in contrast, the adjusted OR for late abortions decreased to 0.4 (CI = 0.2-1.0). When the analysis accounted for the use of protective equipment or clothing in male applicators exposed to a phenoxy herbicide, the OR (for all spontaneous abortions or for early abortions subgroup) was less than 1 in males reporting the use of such equipment/clothing. In contrast, it was considerably higher in the early spontaneous abortion subgroup when males failed to use the protective equipment/clothing while being exposed. When the analysis was restricted to exposure to MCPA, the results showed no elevated risk of spontaneous abortion from preconception exposure for all gestational ages; the adjusted OR was 0.8 (CI = 0.4-1.7). In contrast, the adjusted OR for MCPA for early abortions increased to 2.3 (CI = 0.8-6.5), whereas the adjusted OR for late abortions decreased to 0.4 (CI = 0.1-1.2). Further analysis suggested that if preconception exposure to MCPA occurred for 1 month or more, the adjusted OR increased to 5.4 (CI = 1.7-17.3) for early abortions; the OR was less than 1 for late abortions for MCPA, as well as for all the other phenoxy herbicides analysed.
Limitations of the study (Arbuckle et al., 1999) include small sample size (e.g., the unexposed group and the MCPA subgroup), use of memory recall (quality of information limited by the memory of the respondents and the length of recall for pesticide usage generally greater than 10 years) and the lack of detailed information on dose used and amount of time spent applying pesticides. According to the authors, the findings of the study suggest that there may be a preconceptual effect with MCPA and phenoxy herbicides in adverse pregnancy outcomes, particularly early spontaneous abortions (which may suggest a male-mediated effect due to the higher risk seen as a result of not using protective equipment or clothing). However, given the limitations of the study, additional studies are needed to confirm these findings.
In order to evaluate the possibility that offspring of agricultural pesticide applicators might have increased risks of birth anomalies, Garry et al. (1996) compared the births (n = 4935) of state-licensed, private pesticide applicators (n = 34 772) in Minnesota between 1989 and 1992 with those of the state's birth registry (containing 210 723 live births within the same period). The birth defect rate for all birth anomalies was significantly increased in children born to private applicators. The pattern of excess frequency of birth anomalies was analysed by region, pesticide use, season and male/female sex ratio. Analyses of the various regions showed western Minnesota, a major growing region for wheat, sugar beet and potato, as having the highest rate of birth anomalies per 1000 live births. Data for MCPA and 2,4-D were pooled because of their similarities of chemical structure, spectrum of biocidal activity and crop use, although 2,4-D was used more than MCPA. In the case of 2,4-D and MCPA, the frequency of anomalies in high-use areas was not significantly increased (OR = 1.86; CI = 0.69-2.05) for combined births with central nervous system, circulatory/respiratory, urogenital and muscular anomalies; however, a significant increase was reported when all anomalies were combined (OR = 1.51; CI = 1.40-1.62). No individual data were available for MCPA in this cohort study (Garry et al., 1996).
Very few epidemiological studies have been conducted with regards to reproductive and developmental outcomes with MCPA, and those that have been conducted are limited by small sample sizes. Because workers may be exposed to multiple phenoxy herbicides and other compounds, as well as possibly to contaminants such as dioxin, it is difficult to isolate exposure to MCPA alone.
Another key limitation of the studies above was that there was only potential for exposure to have occurred, and none of the studies had any direct measurements of exposure for the individuals in the study.
9.2 Effects on laboratory animals and in vitro test systems
9.2.1 Acute toxicity
Details on the available acute oral toxicity studies are provided in Table 3.
| Forms of MCPA | Oral LD50 (mg/kg bw)Table 3 footnote 1 | ||
|---|---|---|---|
| Rats | Mice | Guinea pigs | |
a References are as follows: 1. Ben-Dyke et al. (1979); 2. Rowe and Hymas (1954); 3. U.S. EPA (2003); 4. U.S. EPA (1990); 5. RTECS (2005); 6. Weed Science Society of America (1989); 7. Gurd et al. (1965). |
|||
| MCPA acid | 700-1383Table 1 footnote 2,Table 1 footnote 3,Table 3 footnote 4Table 3 footnote 5 | 439-800Table 1 footnote 6,Table 1 footnote 7 | 700Table 1 footnote 6 |
| MCPA-EHE | 2235Table 1 footnote 4 | ||
| MCPA-DMAS | 1200-1867Table 1 footnote 3,Table 1 footnote 4 | 550Table 1 footnote 8 | |
| MCPA sodium salt | 3500Table 1 footnote 4 | 560Table 1 footnote 8 | |
9.2.2 Short-term exposure studies
Short-term studies were conducted on the effects of MCPA, MCPA-DMAS and MCPA-EHE in mice, rats and dogs. Adverse health effects were noted in the liver and kidney. In general, dogs appear to be more sensitive than rats to the adverse effects of MCPA after repeated exposure. According to the U.S. EPA (2003, 2004e), this increased sensitivity appears to be a consequence of reduced capacity in the elimination of MCPA in dogs relative to rats.
In a 28-day oral range-finding toxicity study, MCPA (94.8%) was administered to mice (4-6 per sex per dose) in the diet at concentrations of 0, 100, 300, 900 or 2700 ppm (equivalent to doses of 0, 19.1-22.0, 56.3-67.7, 173.4-184.8 and 453.7-820.1 mg/kg bw per day for males and 0, 20.7-26.2, 69.2-73.9, 193.4-223.9 and 442.3-956.3 mg/kg bw per day for females). Observed effects at the highest dose in both sexes included motor disturbances, significant cumulative body weight loss, hepatotoxicity (increased serum alanine aminotransferase [ALT] and alkaline phosphatase [ALP] activities, increased liver weight and related histopathological findings), spleen effects (decreased mean spleen weights, gross observation of involution, increased neutrophil counts and decreased lymphocyte counts) and decreased kidney weights. High-dose males had decreased mean testicular weight with histopathological findings of testicular atrophy, whereas females had atrophy of the ovaries and uterine glands. At necropsy, high-dose animals were shown to be cachectic (state of general ill health characterized by malnutrition, weakness and emaciation). Liver effects (cloudy swelling) were observed in one mid-dose female. In female mice, the lowest-observed-adverse-effect level (LOAEL) is 900 ppm (193.4-223.9 mg/kg bw per day) based on cloudy swelling in the liver, and the no-observed-adverse-effect level (NOAEL) is 300 ppm (69.2-73.9 mg/kg bw per day). In male mice, the LOAEL is 2700 mg/kg (453.7-820.1 mg/kg bw per day), and the NOAEL is 900 ppm (173.4-184.8 mg/kg bw per day) (Kirsch, 1985a). The highest dose used in this study was within range of the LD50 for mice, which may explain the significant effects at this dose.
Effects on the kidney were also noted in 90-day studies in rats. Technical-grade MCPA (94.8%) was administered to rats (15 per sex per dose) at dietary concentrations of 0, 50, 150 or 450 ppm (equivalent to doses of 0, 3.6, 10.9 and 32.6 mg/kg bw per day for males and 0, 4.0, 12.1 and 35.8 mg/kg bw per day for females). At 450 ppm, an increase in creatinine values in the plasma of females was observed. At the same level, decreases in cholesterol and calcium values in the males were observed. The authors mentioned that these effects are difficult to evaluate, as they occur only in males. Also, an increase in absolute and relative kidney weights in males was observed. At 150 ppm, increased absolute kidney weights (108% of controls) were noted (p < 0.05). No changes were observed at the lowest level (50 ppm). The authors concluded that the NOAEL lies between 50 and 150 ppm and is based on renal impairment related to calcium and kidney weight changes, but no histopathological alterations were detected that correlated with this increase in kidney weight (Kirsch, 1985b). In this same study, PMRA (2007) established a NOAEL of 3.6 mg/kg bw per day and a LOAEL of 10.9 mg/kg bw per day based on kidney effects (increased absolute and relative weights, urinary bilirubin, crystals and pH).
In a combined subchronic toxicity and neurotoxicity study, MCPA (94.2%) was administered to groups of 15 male and 15 female Wistar rats for 3 months at dietary concentrations of 0, 50, 500 or 2500 ppm (equivalent to doses of 0, 3, 34 or 177 mg/kg bw per day for males and 0, 4, 42 or 188 mg/kg bw per day for females) (Mellert et al., 1994). At the highest dose, a significant decrease in body weight was observed in both sexes beginning on day 7 of the administration period and continuing until the end of the study. At the highest dose, in both sexes, a significant decrease in haematological parameters (red blood cells, haemoglobin and haematocrit), a significant increase in liver enzymes (ALT, ALP and aspartate aminotransferase [AST]) and alteration of hepatocytes, characterized by cytoplasmic eosinophilia and granular cytoplasm in the liver, were observed. In addition, a higher incidence/grading of foam cell accumulations in the lung and myeloid atrophy of the haematopoietic marrow were seen in both sexes. In high-dose males, a decrease in testes weights, testicular atrophy and atrophy of the seminal vesicles and prostate, aspermia or oligospermia in the epididymides were observed. For the neurotoxicity evaluation, functional observational battery and motor activity assessments were performed on 10 animals per sex per group prior to the treatment and on treatment days 22, 50 and 85. Effects observed at 2500 ppm were a decreased value of hindlimb grip strength in females on day 85, decreased foot splay test values (p < 0.02) in males on day 22 and reduced values (p < 0.02) of forelimb grip strength in males on day 50. No significant treatment-related changes were seen at the two lowest doses. The U.S. EPA set the NOAEL at 500 ppm (34 and 42 mg/kg bw per day for males and females, respectively) and the LOAEL at 2500 ppm (177 and 188 mg/kg bw per day for males and females, respectively), based on decreased body weight and body weight gains, liver pathology, changes in clinical chemistry and haematological parameters, testicular atrophy and changes in motor activity.
U.S. EPA (2004e) considered MCPA "a neurotoxicant based on clinical signs of neurotoxicity"
based on the study by Mellert et al. (1994) and an acute neurotoxicity study conducted with rats (U.S. EPA, 2003). Additional studies (acute and subchronic oral studies) conducted with MCPA-DMAS and MCPA-EHE and rats also showed adverse neurotoxic effects, with MCPA-EHE being the most toxic of the three forms of MCPA (U.S. EPA, 2004e). Neurotoxic effects seen in these studies included impaired coordination and gait, reduced motor activity, ataxia (muscle incoordination), decrease in arousal and reduced hind grip strength.
MCPA was administered in the diet to Charles River rats (10 per sex per dose) at dose levels of 0, 4, 8 or 16 mg/kg bw per day for 90 days. No treatment-related effects were observed other than a significant increase in both relative and absolute kidney weights for males at the high dose, with moderate increases at the other two treatment levels in males. There were no treatment-related histopathological alterations in either sex at any dose level tested. The no-observed-effect level (NOEL) for increased kidney weights in rats was 8 mg/kg bw per day (Holsing and Kundzin, 1970).
In two separate 90-day studies, technical MCPA (94.8%) was given in the diet to beagle dogs (four per sex per dose) at dose levels of 0, 3, 12 or 48 mg/kg bw per day and at dose levels of 0, 0.3, 1 or 12 mg/kg bw per day, respectively. Severe toxicity and mortality were seen at the highest dose (48 mg/kg bw per day): one female died during week 5, and the remaining three females and three out of four males were sacrificed moribund during weeks 7-8. A decrease in kidney and liver function, characterized by an increase in blood urea, ALT and creatinine levels, was noted at 3 mg/kg bw per day and above. A NOAEL of 1 mg/kg bw per day was reported in this study, and a LOAEL of 3 mg/kg bw per day based on impaired renal function, without histopathological change, was also reported. This lack of histopathological change probably indicates that the effects are reversible (Reuzel and Hendriksen, 1980).
In a 1-year study, male and female beagle dogs (six per sex per dose) were administered technical-grade MCPA (94.8%) in the diet at concentrations of 0, 6, 30 or 150 ppm (equivalent to doses of 0, 0.22, 1.02 or 5.32 mg/kg bw per day in males and 0, 0.21, 1.02 or 5.12 mg/kg bw per day in females) (Hellwig, 1986). No treatment-related clinical signs or deaths were seen during the study. In the high-dose males, mean cumulative body weight gain was 77% of controls for the 1- to 364-day interval. At the high dose (both sexes), statistically significant increases were observed in serum creatinine concentration (at 13, 26 and 52 weeks) and serum ALT and AST activities at most sampling intervals. Gross necropsy showed dark brown discoloration of the kidney in four of six mid-dose females, in four of six high-dose males and in all high-dose females. Histopathological changes were observed in the kidney and included a dose-related increase in severity in kidney pigment deposition in the proximal tubular epithelium in all females and three of the six males at the highest dose and in four females at the middle dose. No treatment-related effects were seen at the low dose (Hellwig, 1986). Based on this study, the U.S. EPA (2003) established a NOAEL of 6 ppm (0.22 and 0.21 mg/kg bw per day for males and females, respectively) and a LOAEL of 30 ppm (1.02 mg/kg bw per day in both sexes) based on nephrotoxicity (evident as gross necropsy and hispathological changes).
Two subchronic studies were also conducted with the other forms of MCPA. MCPA-DMAS (99.9% active ingredient) was administered in beagle dogs at dietary concentrations of 0, 20, 80 or 360 ppm (equivalent to doses of 0, 0.6, 2.4 and 10.9 mg/kg bw per day in the males and to 0, 0.7, 2.9 and 12.8 mg/kg bw per day in the females) for a period of 110-118 days (Hellwig et al., 1995a). At the highest dose, both sexes had increases in creatinine, urea and ALT levels, whereas males had increases in partial thromboplastin times. At 80 ppm, increases in creatinine and urea levels were observed in both sexes, whereas increases in ALT levels and partial thromboplastin times were observed in females. Increases in ALT levels correlated with a subacute to chronic interstitial inflammation of the liver. No significant changes were observed at the lowest doses in either sex. The U.S. EPA (2003) set the LOAEL at 80 ppm (2.4 mg/kg bw per day in males and 2.9 mg/kg per day in females) based on histopathology changes (increases in a subacute to chronic interstitial inflammation of the liver), haematology and clinical chemistry and the NOAEL at 20 ppm (0.6 mg/kg bw per day in males and 0.7 mg/kg bw per day in females). No statistical details were provided in the review.
Beagle dogs were administered MCPA-EHE (93.5% active ingredient) at dietary concentrations of 0, 20, 80 or 360 ppm (equivalent in the males to 0, 0.6, 2.5 and 11.1 mg/kg bw per day and in the females to 0, 0.7, 2.8 and 12.7 mg/kg bw per day) for a period of 110-118 days. The administered doses are equivalent to 0, 0.4, 1.6 and 7.1 mg/kg bw per day in males and 0, 0.4, 1.8 and 8.1 mg/kg bw per day in females as MCPA acid. At 360 ppm, both sexes had increases in clinical chemistry parameters (creatinine, urea and ALT levels), partial thromboplastin times, relative and absolute thyroid weights and relative ovary weights (females). Increases in ALT levels correlated with a subacute to chronic interstitial inflammation of the liver. At 80 ppm, both sexes had increases in creatinine and urea levels, whereas females had increases in ALT levels. No treatment-related changes were observed at the lowest dose in either sex. The U.S. EPA (2003) set the LOAEL for systemic toxicity in beagle dogs at 80 ppm (1.6 mg/kg bw per day as MCPA acid in males and 1.8 mg/kg bw per day in females) based on changes in clinical chemistry parameters (increases in creatinine, and urea and ALT levels). The NOAEL is 20 ppm (0.4 mg/kg bw per day as MCPA acid in both sexes) (Hellwig et al., 1995b). No statistical details were provided in the review.
9.2.3 Long-term exposure (chronic toxicity) and carcinogenicity
Long-term exposure and/or carcinogenicity studies with MCPA were conducted in rats and mice. No evidence of carcinogenicity was seen in either species; however, both rats and mice showed evidence of adverse effects in the liver and kidney similar to those observed in the subchronic studies, and at comparable dose levels. No long-term study in dogs was located.
Wistar rats (50 per sex per dose) received MCPA (94.8%) in their diet for 2 years at 0, 20, 80 or 320 ppm (corresponding to doses of 0, 1.1, 4.4 or 17.6 mg/kg bw per day in males and 0, 1.4, 5.7 or 23 mg/kg bw per day in females) (Kirsch, 1988; Bellet et al., 1999). Two satellite groups (n = 10-15 per sex per dose per group) were also studied. No treatment-related effects were seen at 20 ppm. Changes in clinical chemistry in the absence of histopathology were seen at 80 ppm in both sexes. Effects at the high dose included a minimal decrease in body weight in males (day 14 and beyond; p < 0.05) and a small but sporadic increase in body weight in females (p < 0.05). Clinical chemistry parameters showed a significant decrease in triglycerides in males at weeks 78 and 104 and an elevation of ALT levels in females at weeks 52, 78 and 104. At the high dose, gross and microscopic pathological changes in male rats indicated a chronic progressive nephropathy, as seen by an increase in the retraction and glandular surface of the kidney. At the doses tested, there was no treatment-related increase in tumour incidence when compared with controls. The authors reported the systemic NOEL at 20 ppm (1.1 mg/kg bw per day in males and 1.4 mg/kg bw per day in females). However, the U.S. EPA (2003) in their review set the NOAEL for systemic toxicity at 80 ppm (4.4 mg/kg bw per day in males and 5.7 mg/kg bw per day in females) and the LOAEL at 320 ppm (17.6 mg/kg bw per day in males and 23 mg/kg bw per day in females) based on hepatotoxicity (increased ALT levels) in females and kidney effects in males. However, PMRA (2005b, 2006) found that this study did not test high enough doses to reach a maximum tolerated dose (MTD) and, as a result, was not adequate to fully assess the overall oncogenic potential of MCPA.
B6C3F1 mice (50 per sex per dose) received MCPA (94.6%) for 2 years in the diet at 0, 20, 100 or 500 ppm, corresponding to doses of 0, 3.2, 15.7 or 79.5 mg/kg bw per day in males and 0, 3.9, 19.5 or 97.2 mg/kg bw per day in females (Kuhborth et al., 1988; Bellet et al., 1999). One satellite group (n = 10 per sex per dose) was used for haematological examination. Body weight changes were observed in both sexes during the course of the study; however, the final body weights in males and females were not statistically different from those of the control group. The kidney was the only organ with treatment-related lesions. Kidney weights were statistically significantly higher in high-dose females. The kidney lesions included increased incidences of intratubular calcification and tubular hyaline-proteinaceous casts in high-dose males and females. In the mid- and high-dose females, there was a non-dose-related increase in the incidence of slight renal hyperplasia (7/50 and 4/50, respectively) compared with controls and the low dose, at which none was observed. In addition, there was an increased incidence of renal tubular epithelial focal hyperplasia in high-dose males (not specified if statistically significant). The authors reported the systemic NOEL at 100 ppm (15.7 mg/kg bw per day in males and 19.5 mg/kg bw per day in females) (Kuhborth et al., 1988; Bellet et al., 1999). However, the U.S. EPA (2003) set the systemic NOAEL in males at 100 ppm (15.7 mg/kg bw per day) and the LOAEL in males at 500 ppm (79.5 mg/kg bw per day) based on histopathological changes in the kidneys. In female mice, U.S. EPA (2003) set the systemic NOAEL at 20 ppm (3.9 mg/kg bw per day) and the LOAEL at 100 ppm (19.5 mg/kg bw per day), based on renal hyperplasia, which was considered toxicologically significant at that dose.
9.2.4 Mutagenicity/genotoxicity
Based on the weight of evidence, MCPA acid and its other forms are not considered to be of genotoxic concern in vivo. As outlined below, MCPA was not mutagenic in the majority of bacterial and mammalian cell gene mutation assays reported and did not induce DNA damage in the SOS chromotest. In addition, no in vivo evidence was found to suggest clastogenicity in bone marrow, and sister chromatid exchange tests gave negative or weakly positive results.
With the exception of one positive and two weakly positive results (reviewed in Elliott, 2005), MCPA acid was negative in in vitro bacterial assays using Salmonella typhimurium (several strains) (Räsänen et al., 1977; Nishimura et al., 1982; Moriya et al., 1983; Kappas, 1988; Jones et al., 1993a), and both MCPA-DMAS and MCPA-EHE were also negative (Jones et al., 1992, 1993b; Elliott, 2005).
Negative results with MCPA acid, MCPA-DMAS and MCPA-EHE were observed in mammalian cell mutation assays (Chinese hamster ovary HPRT with and without S9) (Adams et al., 1993a,b,c; Elliott, 2005).
One positive result was obtained with MCPA acid in a mitotic recombination assay with Aspergillus nidulans (Kappas, 1988). A weakly positive result was found in the yeast mutagenicity test in Saccharomyces cerevisiae with MCPA acid (Zetterberg, 1978, 1979).
Negative results with MCPA acid were observed in an SOS chromotest (Escherichia coli PQ37 with and without S9) (Mersch-Sundermann et al., 1989).
Chromosomal aberrations in vitro were detected in human peripheral lymphocytes (with and without S9) at high cytotoxic concentrations with MCPA acid and MCPA-DMAS, but not with MCPA-EHE (Akhurst et al., 1993a,b,c; Elliott, 2005).
In vivo chromosomal aberration assays with MCPA acid were negative in the Chinese hamster for doses up to 1200 mg/kg bw administered by oral gavage (Gelbke and Engelhardt, 1985a,d), whereas sister chromatid exchange assays were either negative or weakly positive at the same dose level and for the same route of exposure (Linnainmaa, 1984; Gelbke and Engelhardt, 1985b,c; Mustonen et al., 1989). Negative results were observed in in vivo bone marrow micronucleus assays with MCPA acid, MCPA-DMAS and MCPA-EHE in which mice were given a 384 mg/kg bw dose by oral gavage (Proudlock et al., 1993a,b,c).
This overall lack of genotoxicity following MCPA exposure is consistent with the lack of carcinogenicity in animals (Elliott, 2005).
9.2.5 Reproductive and developmental toxicity
Developmental effects in the presence of maternal toxicity were seen with rats when dosed with MCPA, MCPA-DMAS and MCPA-EHE. No developmental effects in the presence of maternal toxicity were seen in the rabbit. No reproductive effects were seen in a two-generation study involving rats.
MCPA (94.22%) was administered daily by gavage to pregnant female Wistar rats on days 6-15 of gestation at dose levels of 0, 15, 60 or 120 mg/kg bw per day (Hellwig and Hildebrand, 1993a). The LOAEL for maternal toxicity was 120 mg/kg bw per day, based on treatment-related decreases in body weight, body weight gain and food consumption during treatment and for the remainder of the gestation period. The LOAEL for development toxicity was 120 mg/kg bw per day, based on decreased placental and fetal body weights and on an increase in the number of fetuses with skeletal retardation. The NOAEL for maternal and developmental toxicity was 60 mg/kg bw per day.
MCPA (94.22%) was administered once daily by gavage to Himalayan rabbits on days 7-19 of gestation at dose levels of 0, 15, 30 or 60 mg/kg bw per day (Hellwig and Hildebrand, 1993b). At 60 mg/kg bw per day, treatment-related decreases were noted in maternal body weight, body weight gain and food consumption. The LOAEL for maternal toxicity was 60 mg/kg bw per day based on significant decreases in body weight, body weight gain and food consumption during the treatment period. The NOAEL for maternal toxicity was 30 mg/kg bw per day. The NOAEL for developmental toxicity was equal to or greater than the highest dose tested, 60 mg/kg bw per day. No developmental LOAEL was determined.
MCPA-DMAS (78.2%) was administered to 17-25 pregnant CD rats per group by gavage in 0.5% methylcellulose at doses of 0, 18.5, 62 or 185 mg/kg bw per day (equivalent to doses of 0, 15, 50 and 150 mg MCPA free acid/kg bw per day) on gestation days 6-19, inclusive (Cappon, 1999b). Maternal toxicity, consisting of clinical signs and mortality of one rat, was observed in high-dose females. When adjusted to account for gravid uterine weights, the body weight in high-dose females was comparable to the controls. Gravid uterine weights were significantly reduced in the high-dose group compared with the controls. Complete litter resorption occurred in five high-dose dams; this post-implantation loss was significantly higher than in controls (41.8% compared with 3.8% for the controls). The mean fetal body weight in the high-dose group was significantly less than the controls (2.1 g compared with 3.5 g for the controls). An increase in the incidence of major external and/or skeletal malformations was seen in the fetuses of the 150 mg/kg bw per day groups (11/17 litters) compared with controls (3/25 litters). In addition, there was an increase in the incidence of skeletal variations at the high dose compared with controls. The LOAEL for maternal toxicity was 150 mg MCPA acid/kg bw per day based on mortality and clinical signs, with a NOAEL of 50 mg/kg bw per day as MCPA acid. U.S. EPA (2003) set the LOAEL for developmental toxicity as 150 mg/kg bw per day as MCPA acid based on increased resorptions, decreased fetal body weight and external and skeletal malformations/variations. The NOAEL for developmental toxicity was set at 50 mg/kg bw per day as MCPA acid (U.S. EPA, 2003).
MCPA-EHE (99.9%) was administered to 25 pregnant CD rats per group by gavage in 0.5% methylcellulose at doses of 0, 23.5, 62.7 or 188 mg/kg bw per day (equivalent to 0, 15, 40 and 120 mg/kg bw per day as MCPA acid) on gestation days 6-19, inclusive (Cappon, 1999a). Food consumption was significantly reduced in high-dose females during treatment (83-91% of control group values). As a result, mean net body weight and mean net body weight gain (when adjusted to account for gravid uterine weights) were statistically decreased by 5% and 28%, respectively, compared with controls. Complete litter resorption was seen in two high-dose dams, and there were corresponding slight (but not significant) increases in post-implantation loss, total resorptions and early resorption. At the highest dose, mean fetal weight was significantly decreased compared with controls (2.5 vs. 3.7 g for controls). Significant increases in the incidence of several skeletal variations, including unossified sternebrae and bent ribs, were observed at this dose. The incidence of an ossified cervical centrum number 1 was statistically decreased at 62.7 and 188 mg/kg bw per day (13.7, 12.2, 4.6 and 0.6% per litter for the control, low-, mid- and high-dose groups, respectively). U.S. EPA (2003) set the LOAEL for developmental toxicity at 188 mg/kg bw per day as MCPA-EHE (120 mg/kg bw per day for MCPA acid) based on total litter resorptions, decreased fetal weight and altered growth, and the NOAEL for developmental toxicity was 62.7 mg/kg bw per day as MCPA-EHE (40 mg/kg bw per day for MCPA acid). The LOAEL for maternal toxicity was 188 mg/kg bw per day as MCPA-EHE (120 mg/kg bw per day as MCPA acid) based on reduced body weight gains and reduced food consumption (U.S. EPA, 2003). The NOAEL for maternal toxicity was 62.7 mg/kg bw per day as MCPA-EHE (40 mg/kg bw per day as MCPA acid).
No reproductive effects were observed when male and female albino rats (n = 25 per sex per dose per generation) were exposed to MCPA (94.8%) in the diet at concentrations of 0, 50, 150 or 450 ppm (corresponding to doses of 0, 2.5, 7.5 or 22.5 mg/kg bw per day) for two generations (MacKenzie, 1986; Bellet et al., 2001). There were no treatment-related effects on mean live litter sizes, sex ratios at birth or pup survival of either litter of the treated groups of either generation. At the high dietary level (450 ppm), statistically significant differences were noted in body weight gain for both male pups (F1a) and female pups (F1a and F1b) and in body weight and body weight gains for both male and female pups from F2a and F2b. No significant effects on the reproductive function for either generation (both sexes) were observed (MacKenzie, 1986; Bellet et al., 2001).
In this study, the NOEL for reproductive function in rats administered MCPA was determined to be 450 ppm (approximately 22.5 mg/kg bw per day). The NOEL for general systemic toxicity based on body weight in adult animals in the F1b generation and for effects on the offspring of the F1b generation (based on reduced pup weight and pup weight gains) was 150 ppm (approximately 7.5 mg/kg bw per day). Based on the results of the study, MCPA is not a reproductive toxicant for rats, which was confirmed by the U.S. EPA (2003). However, PMRA (2006) determined that the MTD had not been achieved in this study. According to PMRA (2006), MCPA showed a potential for increased sensitivity of the young in the absence of maternal toxicity due to decreases seen in body weight and body weight gain of the pups from both generations during lactation. As a result of the evaluation of this two-generation study, the MCPA Task Force Three submitted two additional one-generation confidential studies to PMRA (2007), where they administered either the acid or MCPA-EHE using higher doses than in the above two-generation study. No adverse effects were seen on pup body weight until the time of weaning, indicating that there was no increased sensitivity of the young relative to maternal animals.
The U.S. EPA (2004e), in its Hazard Identification Assessment Review Committee report, reported that because of its concerns with neurotoxicity in the acute and subchronic studies with the various forms of MCPA, the currently available data supported the need to perform a developmental neurotoxicity study.
10.0 Classification and assessment
Based on inadequate data from epidemiological studies and the lack of adequate animal studies, Health Canada classifies MCPA as Group VIA (unclassifiable with respect to carcinogenicity in humans), as defined in Health Canada (1994). IARC (1983) evaluated MCPA and concluded that "no adequate data were available to evaluate the carcinogenicity of MCPA to experimental animals. The data on humans were also considered to be inadequate. The available data are insufficient to evaluate the carcinogenicity to humans of MCPA alone."
IARC (1986) also evaluated the family of chlorophenoxy herbicides and concluded that "there is limited evidence that occupational exposures to chlorophenoxy herbicides are carcinogenic to humans."
The U.S. EPA (2003, 2004c,e) has classified MCPA as "not likely to be carcinogenic to humans,"
based on the lack of evidence of carcinogenicity in mice and rats.
Although a limited number of epidemiological studies have been conducted on the effects of MCPA and related chlorophenoxy compounds, the evidence for carcinogenicity and reproductive effects remains inconclusive. Available studies have dealt with multiple exposures to mixtures of chlorophenoxy herbicides, other pesticides as well as other organic compounds. The results were difficult to interpret, and the studies were considered limited for several reasons, such as the lack of consideration of confounding factors and small sample size.
There was no evidence of carcinogenicity in the long-term mouse or rat studies using MCPA acid. However, PMRA (2005b, 2006, 2007) indicated that the long-term rat study did not reach the maximum tolerated dose and that it was not considered adequate for assessing the overall potential for carcinogenicity. Tests for genotoxicity and mutagenicity were largely negative.
Subchronic studies have shown that dogs are more sensitive than rats or mice to the effects of MCPA and related compounds, with effects seen at levels at least 10 times lower in dogs than in rats or mice based on chronic and subchronic studies. Allometric scaling of data from rats, dogs and humans indicates that the renal clearance of MCPA in dogs is approximately 10 times slower than in humans (Timchalk, 2004). The unique sensitivity of dogs to MCPA-mediated effects observed in the literature, therefore, may be attributed to the reduced renal clearance of organic acids (e.g., MCPA) leading to higher concentrations in blood compared to humans and other species. This evidence suggests that the dog is not an appropriate indicator species for MCPA-mediated toxicity in humans (Timchalk, 2004). Based on the results of the allometric scaling across species, reported by Timchalk (2004), and the available animal database for MCPA, the PMRA (2007) deemed the rat as the most appropriate model for the human health risk assessment for agricultural and other non-turf uses of MCPA. No long-term studies on dogs were located in the literature.
PMRA (2006, 2007, 2008) has derived a chronic (lifetime) dietary reference dose, or acceptable daily intake (ADI), for MCPA of 0.012 mg/kg bw per day using the NOAEL of 3.6 mg/kg bw per day from the 90-day study in rats by Kirsch (1985b). The NOAEL was based on kidney effects (increased absolute and relative weights, urinary bilirubin, crystals and pH).
A TDI is established as follows, using the NOAEL and uncertainty factors identified by PMRA in their ADI calculation:

Figure 1 - Text Description
The tolerable daily intake for MCPA is 0.012 milligrams per kilogram body weight per day. This value is calculated by dividing 3.6 milligrams per kilogram body weight per day by 300.where:
- 3.6 mg/kg bw per day is the NOAEL in the Kirsch (1985b) 90-day study in rats,
- 300 is the uncertainty factor: × 10 for interspecies variability; × 10 for intraspecies variability; × 3 for database deficiencies - inadequate carcinogenicity assessment, since the MTD was not achieved in the two-year rat study (Bellet, 1999).
Although the NOAEL is obtained from a sub-chronic study, a × 10 uncertainty factor to account for database deficiencies (use of a sub-chronic study instead of a chronic study) is not warranted, since the kidney effects noted after 90 days of dosing were not evident after 2 years of exposure to higher levels of MCPA, suggesting that the rat may be able to adapt to these effects. However, the 2-year rat study by Bellet (1999) did not achieve the MTD, and the effects at 90 days were considered to be indicative of an initial toxic insult to the kidney, because the kidney is the target organ in all species tested. Since the NOAEL for kidney effects in the 90-day rat study is the lowest NOAEL reported in the animal toxicity database for MCPA (excluding the dog), the TDI for MCPA is also protective of all other adverse effects reported in the sub-chronic and chronic rat studies, including systemic, kidney, liver, testicular, reproductive/developmental and nervous system effects.
Using the TDI of 0.012 mg/kg bw per day, the Maximum Acceptable Concentration (MAC) for MCPA in drinking water is derived as follows:
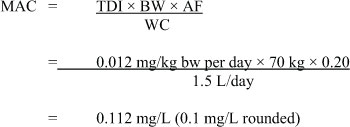
Figure 2 - Text Description
The maximum acceptable concentration for MCPA is 0.1 milligrams per litre (rounded). This value is calculated by taking the tolerable daily intake (previously calculated) of 0.012 milligrams per kilogram body weight per day and multiplying it by 70 kilograms and by 0.2. This value is then divided by 1.5 litres per day to achieve the maximum acceptable concentration.where:
- TDI = the tolerable daily intake derived above;
- BW = body weight; the mean adult body weight estimated for a Canadian is 70 kg;
- WC = water consumption; 1.5 L/day is the daily volume of tap water consumed by an adult;
- AF = allocation factor; the proportion of the total daily intake of MCPA estimated to originate from exposure via drinking water, compared to other sources (food, soil, air and consumer products). In the absence of comprehensive or appropriate exposure data for all relevant environmental media, a default allocation factor of 20% is used.
10.1 International Considerations
The U.S. EPA has not established a maximum contaminant level (MCL) for MCPA in drinking water. It did establish a life-time health advisory in 1988, to serve as informal technical guidance to assist federal, state and local officials responsible for protecting public health when emergency spills or contamination situations occur. They are not to be construed as legally enforced standards (U.S. EPA, 1987; 2006).
The WHO (1996) established a drinking water guideline for MCPA of 0.002 mg/L based on the one-year feeding study in dogs indicating a NOAEL of 0.15 mg/kg/day for renal and liver toxicity published by Hellwig (1986). The derivation of the guideline used an uncertainty factor of 300 to account for interspecies (× 10) and intraspecies (× 10) variation, and the inadequacy of the database (× 3), as well as an allocation of 10% of the TDI for drinking water exposure (WHO, 1996, 2003).
11.0 Rationale
MCPA is a commonly used herbicide in Canada. It is registered for use in Canada for agricultural sites, for fine turf, lawns and sod, in forestry and at industrial sites. MCPA is used everywhere in Canada, particularly in the Prairies, and is among the top 10 pesticides sold in Canada. Although MCPA is used widely in Canada, exposure data do not indicate significant levels in drinking water.
Health Canada classifies MCPA as unclassifiable with respect to carcinogenicity in humans, based on inadequate data from epidemiological studies and the lack of adequate animal studies. This is consistent with the classifications established by IARC and the U.S. EPA. The Maximum Acceptable Concentration for MCPA in drinking water was established based on kidney effects in the rat.
A MAC of 0.1 mg/L (100 µg/L) is established for MCPA in drinking water. This MAC is achievable by available treatment technology, and measurable by available analytical methods. As part of its ongoing guideline review process, Health Canada will continue to monitor new research in this area and recommend any change to the guideline that it deems necessary.