Guidelines for Canadian drinking water quality boron: Health considerations and health-based value
On this page
- Kinetics
- Health effects
- Mode of action
- Selected key study
- Derivation of the health-based value (HBV)
Kinetics
Absorption
Gastrointestinal absorption of boron compounds is similar in humans and experimental animals (rats, rabbit), and ranged from 64% to 98% (Jansen et al., 1984; Schou et al., 1984; Vanderpool et al., 1994; Hunt et al., 1997; Dourson et al., 1998). Inorganic borates readily hydrolyze to boric acid in the gut (IOM, 2001; Pahl et al., 2001) and uptake is almost exclusively (> 98%) as undissociated boric acid, which is likely absorbed by passive, non-mediated diffusion (IOM, 2001; Pahl et al., 2001). Dermal exposure studies demonstrate that boron absorption through intact skin ranges from 0.5% to 10% (ECCC and Health Canada, 2016), but can be absorbed through damaged skin especially when dissolved in an aqueous vehicle (Draize and Kelley, 1959; Friis-Hansen et al., 1982; Stuttgen et al., 1982; Murray, 1998; See et al., 2010). Boron can also be absorbed across pulmonary tissues following inhalation exposure in humans and rats (Culver et al., 1994; Wilding et al., 1959).
Distribution
Distribution of boron is similar in humans and experimental animals (rats, rabbits), with boron being evenly distributed via passive diffusion throughout body fluids and soft tissues (liver, kidney, muscle, colon, brain, testis, epididymis, seminal vesicles, prostate and adrenals), reaching a steady state within 3 to 4 days (Ku et al., 1991; Treinen and Chapin, 1991; Moseman, 1994; Murray, 1998; Bakirdere et al., 2010). In both animals and humans, boron does not accumulate above plasma levels in soft tissues, including the testes, but does accumulate in bone (2 to 3 times higher than in soft tissues) (Forbes et al., 1954; Forbes and Mitchell, 1957; Ku et al., 1991; Culver et al., 1994; Moseman, 1994; Chapin et al., 1998; Murray, 1998). Average blood boron concentrations of 0.034 μg/ml from Alberta biomonitoring studies have been found to adequately represent levels in the Canadian population and are also representative of exposures in children and adults (Alberta Health and Wellness, 2008; Government of Alberta, 2010; ECCC and Health Canada, 2016). Maximum blood boron values (0.195 μg/ml) are found in Germany and are considered to represent the upper bound concentration in Canadians (Heitland and Köster, 2006; ECCC and Health Canada, 2016). Accumulation in bone is dose-dependent but reversible once exposure is stopped (Moseman, 1994; Chapin et al., 1997). Boron can cross the placenta in humans and has been measured in placental blood and umbilical cord blood (Grella et al., 1976; Huel et al., 2004; Caglar et al., 2014). Furthermore, maternal blood and serum boron levels have been found to be significantly correlated to umbilical blood boron concentrations in humans (Caglar et al., 2012, 2014).
Blood-boron levels resulting from a given boron intake level differ between species, and humans appear to have higher blood-boron levels for a given intake level compared to animals (dogs, rats) (Culver et al., 1994).
Metabolism
There is no evidence that boron compounds are metabolized. Indeed, boric acid is presumed not to be metabolized in the body as a large amount of energy (523 kJ/Mol) would be required to break the boron-oxygen bond (Murray, 1998).
Excretion
The overall extent of boric acid elimination is similar between humans and rodents. In humans, approximately 90% of orally administered boron given as boric acid is excreted unchanged in the urine (Kent and McCance, 1941; Jansen et al., 1984; Schou et al., 1984; Hunt et al., 1997; Naghii and Samman, 1997; Murray, 1998; Samman et al., 1998; Sutherland, 1998). In rats, 95% and 4% of the administered dose was recovered from urine and feces, respectively, within 24 hours of exposure (Vanderpool et al., 1994).
Since boric acid is not metabolized, renal clearance is expected to govern its rate of excretion, and rats have been shown to have a faster clearance rate compared to humans. The glomerular filtration rate (GFR) in rats (163 ml/hour/kg or 2.72 ml/min./kg) is approximately 4 times higher than in humans (41 ml/hour/kg or 0.68 ml/min./kg) when compared on a body weight basis (Dourson et al., 1998; Murray, 1998; Hasegawa et al., 2013). Differences in GFR likely explain the differences in blood boron levels between rats and humans.
Renal clearance rates also increase during pregnancy in both humans and rats (Dourson et al., 1998), although the increases observed are not necessarily statistically significant in individual studies (Pahl et al., 2001; Vaziri et al., 2001). When pooling results across several studies, Dourson et al., (1998) found that mean blood boron clearance was 2.4 times higher in pregnant rats (397 ml/kg/hour) than non-pregnant rats (163 ml/kg/hour). Overall, renal clearance increases in pregnancy by 50% in humans and 21% in rats (Cheung and Lafayette, 2013; Hasegawa et al., 2013).
Physiologically based pharmacokinetic modelling
No models applicable to the current risk assessment were identified.
Health effects
The database for the oral toxicity of boron is well characterized (for example, carcinogenicity, reproduction, development, effects on bone, kidney, liver, nervous system) in both animals and humans (see ATSDR [2010] and U.S. EPA [2008] for detailed reviews) and clearly identifies reproduction and development as the most sensitive targets for boron toxicity in animals (U.S. EPA, 2008; WHO, 2009; ATSDR, 2010; EFSA, 2013). The most recent comprehensive review on boron is by the European Food Safety Authority (EFSA) and covers the literature up to 2012. The present assessment considers the previous data, as well as material published after this period from 2012 to 2018. Health Canada has also previously reviewed the toxicity of boric acid, its salts and its precursors under the Canadian Environmental Protection Act (ECCC and Health Canada, 2016), the Pest Control Products Act (Health Canada, 2012, 2016), and the Natural Health Products Regulations (Health Canada, 2007).
Beneficial effects
A number of studies indicate that boron may be beneficial to human health, but essentiality has not been demonstrated (EFSA, 2004). Boron has been used to treat inflammation, arthritis and menstrual pain and kidney stones (Scorei et al., 2011; Naghii et al., 2011; Naghii, 2013, 2014) and may be protective against certain cancers, bone loss and liver damage (Cui et al., 2004; Barranco et al., 2007; Mahabir et al., 2008; ATSDR, 2010; Hakki et al., 2013; Balabanli and Balaban, 2015; Toker et al., 2016). Some studies also suggest a beneficial role of boron on male reproduction (Korkmaz et al., 2011; Cortés et al., 2017). Beneficial effects on sperm parameters have been seen in men consuming drinking water containing 3.0 mg/L to 7.0 mg/L levels of boron, but negative effects were observed at higher and lower doses, suggestive of a U-shaped dose response curve (Cortés et al., 2017).
Acute toxicity
The literature contains numerous reports of poisoning following acute ingestion or exposure of broken skin to boric acid or its salts. The acute lethal oral dose of boric acid ranges from 15 g to 280 g (3 g to 49 g B) in adults; 1 g to 3 g (0.2 g to 0.5 g B) in newborns; 5 g to 6 g (0.9 g to 1 g B) in infants; and 15 g to 20 g (3 g to 4 g B) in children (Ishii et al., 1993; Corradi et al., 2010; Rani and Meena, 2013). Symptoms of acute exposure vary and include dermal effects such as erythema and desquamation of the skin, nausea, diarrhea, abdominal pain, headaches, shivering, seizures, lethargy, altered mental state, coma and kidney effects (Culver and Hubbard, 1996). While information from poisoning cases is useful in identifying toxic effects and symptoms, it is of limited use in establishing dose-response relationships (Culver and Hubbard, 1996).
Carcinogenicity and genotoxicity
Boron and its compounds have not been classified by the International Agency for Research on Cancer (IARC) or the National Toxicology Program (NTP) with regards to carcinogenicity. The United States Environmental Protection Agency (U.S. EPA) has determined that the available data for boron and its compounds are inadequate for an assessment of human carcinogenic potential (U.S. EPA, 2008). No epidemiological studies were available in the literature linking boron intake to the development of cancer in humans. In vitro and animal studies found no evidence of genotoxicity (Haworth et al., 1983; Benson et al., 1984; NTP, 1987; Arslan et al., 2008; U.S. EPA, 2008) or carcinogenicity (in mice fed boric acid up to 550 mg /kg bw per day (136 mg B/kg bw per day) for 2 years) (NTP, 1987; Dieter, 1994).
Developmental and reproductive effects
As reproduction and development are the most sensitive targets for boron toxicity (U.S. EPA, 2008; WHO, 2009; ATSDR, 2010; EFSA, 2013), the description of the health effects of boron focuses on these effects. Developmental and reproductive effects of boron exposure are described below, with a focus on studies which were considered as candidates for the key study for risk assessment. Studies considered relevant for assessing developmental and reproductive toxicity of boron are summarized in Table 2. These include oral exposure studies in experimental animals that evaluated effects of prenatal boron exposure and repeated dose studies that evaluated reproductive effects.
Developmental effects in humans
Epidemiological evidence for developmental effects is sparse and inconclusive, although some reproductive studies in humans have observed effects such as an increased frequency of spontaneous abortion, and delayed pregnancy (see below). One cohort study demonstrated a possible relationship between boron exposure and birth length and weight. This study followed 180 mothers who were exposed to varying amounts of boron through their drinking water and found that infant birth weight and length were decreased in infants born to mothers with serum boron concentrations > 80 μg/L, although serum boron levels were only weakly correlated with boron concentration in drinking water (Igra et al., 2016). Conversely, a cross-sectional study of 30 pregnant women in Turkey failed to show a relationship between birth weight and either maternal blood or umbilical blood boron levels (Caglar et al., 2014). A more recent cohort study also failed to demonstrate an effect on birth outcomes (that is, spontaneous abortion, miscarriage, infant and neonatal death, preterm birth, congenital abnormalities, sex ratio and birth weight) in infants born to mothers with blood boron concentrations > 0.15 μg/L, although boron levels in drinking water were found to be significantly correlated with blood boron levels (Duydu et al., 2018a). The Calgar et al. (2014) and Duydu et al. (2018a) developmental epidemiology studies have notable deficiencies including small sample size and failure to account for the potential confounding effects of co-exposure to other drinking water contaminants. Additionally, the Calgar et al. (2014) study excluded infants with congenital abnormalities.
Developmental effects in experimental animals
In experimental animals, developmental effects (for example, decreased fetal body weight, skeletal and cardiovascular malformations) were reported at non-maternally toxic doses (Table 2). The lowest observed adverse effect level (LOAEL) identified in the literature was 13.3 mg B/kg bw per day and the lowest no adverse effect level (NOAEL) was 9.6 mg B/kg bw per day, both associated with decreased body weight and skeletal malformations in rats from mothers exposed to boron in their diet (Price et al., 1996). The effects observed in rats are also supported by studies in mice and rabbits (Heindel et al., 1992, 1994). The Price et al. (1996) study is described below as it was considered as a candidate for the key study for risk assessment.
In the Price et al. (1996) study, the developmental toxicity of boric acid was evaluated in rats in two phases: phase I evaluated effects of prenatal exposure, while phase II included a post-natal follow-up portion to evaluate potential reversibility of effects. In phase I, boric acid was given in the diet at 0%, 0.025%, 0.050%, 0.075%, 0.100% or 0.200% (0, 3.3, 6.3, 9.6, 13.3 or 25 mg B/kg bw per day) to time-mated Sprague-Dawley rats (60/dose) from gestational days (GD) 0 to 20. In phase II, the rats received 0, 3.2, 6.3, 9.8, 12.9 or 25.3 mg B/kg bw per day from GD 0 to 20 and were followed until post-natal day (PND) 21. In both phases of the study, no treatment-related effects were observed in maternal animals. However, developmental effects (both pre- and post-natal) were observed, indicating sensitivity of offspring to boron exposure.
In phase I (prenatal study), fetal body weights were significantly reduced in the 13.3 and 25 mg B/kg bw per day groups at GD20. At GD20, there was a dose-related increase in the incidence of skeletal malformations (short rib XIII) in fetus in the same dose groups. A dose-dependent increase in the incidence of wavy ribs was also observed at GD20. Based on the decrease in fetal body weight and increased incidence of skeletal malformations, a developmental NOAEL of 9.6 mg B/kg bw per day can be established.
In phase II (post-natal study), exposure to boric acid was stopped at birth and dams were allowed to rear the offspring until PND21. Reductions in offspring body weight were not observed at PND21, nor was an increase in the incidence of wavy ribs. An increase in the incidence of skeletal malformation (short rib XIII) was seen only in pups in the highest dose group (25.3 mg B/kg bw per day). This study suggests that effects may be reversible following cessation of exposure to boric acid. A developmental NOAEL of 12.9 mg B/kg bw per day can be identified from this phase of the study.
Reproductive effects in humans
Although identified as a reproductive toxicant in the animal literature, evidence of reproductive effects in humans is not as conclusive. Recent reviews by Bonde (2013) and Pizent et al. (2012) found no epidemiological evidence that boric acid impaired male fertility as measured by sperm concentration, motility, morphology or DNA integrity even at high occupational exposure levels. Nevertheless, some studies in boron workers have reported a range of reproductive health outcomes including spontaneous abortions, delayed pregnancy and altered male:female (M:F) sex ratios.
Notable human reproductive studies are summarized below, but the study limitations prevent their use in a quantitative risk assessment. Limitations in the epidemiological studies include absence of a clear point of departure (POD) needed for dose-response analysis, lack of individual exposure data, small sample sizes, poor disease ascertainment and failure to control for confounders. Nevertheless, the results of these studies can be used qualitatively to support the choice of the key endpoint used for quantitative assessment in animals.
In a series of studies conducted on Turkish men occupationally exposed to boron (4.46 to 106.8 mg B/day; primarily via exposure to boron-contaminated drinking water), no effects were seen on sperm parameters (sperm morphology, sperm motility, sperm concentration) or reproductive hormones (follicle stimulating hormone [FSH], luteinizing hormone [LH], total testosterone and prostate specific antigen [PSA]) when compared to control individuals (exposed to 4.68 mg B/day), although boron was shown to accumulate in semen in a dose-dependent manner (Duydu et al., 2011; Basaran et al., 2012; Duydu et al., 2012, 2015, 2016, 2018b). Earlier Turkish studies also show that fertility rates were not affected by boron exposure (0.04 mg/L to 29 mg/L in drinking water), and while effects were observed on M:F sex ratios, they were not statistically significant (Sayli et al., 1998a, 1998b). A follow-up survey of some study participants also showed no differences in the frequency of infant deaths, stillbirths, spontaneous abortion or congenital malformations (Tüccar et al., 1998). In contrast, a similar survey showed a higher prevalence of spontaneous abortion and delayed pregnancy in the wives of boron workers compared to those of control workers (Liu et al., 2005). An observational study of male boron workers in California also found altered M:F sex ratios, but these failed to reach significance (Whorton et al., 1994a, 1994b). The toxicological significance of altered sex ratios is unclear but may be indicative of an adverse effect on fertility.
Several epidemiological studies have also been conducted on occupationally exposed men in China (for example, Chang et al., 2006; Robbins et al., 2008, 2010; reviewed by Scialli et al., 2010). In these studies, exposure to boron (up to 51.1 mg B/day) did not significantly affect sperm parameters, fertility or sperm DNA integrity measures (for example, aneuploidy, DNA strand breakage and apoptosis). Although effects on sperm X:Y ratios and sex ratio of offspring were observed, they were not statistically significant. The Chang et al. (2006) study also showed a delay in pregnancy (defined as the inability to conceive a child within 1 year of desiring a child) in the wives of boron workers compared to controls (although this effect was not statistically significant). However, no effect was observed on the number of multiple births or spontaneous abortions.
Blood boron concentrations measured in the men followed in the Turkish studies were much lower than those anticipated to elicit reproductive and developmental effects in experimental animals. Blood boron levels in the most highly exposed workers were 1 100 ng B/g (Duydu et al., 2018b), while the blood level associated with reproductive toxicity in animals is calculated as 2 020 ng/g (corresponding to a NOAEL of 17.5 mg B/kg bw per day) (Bolt et al., 2012; Duydu et al., 2012, 2016).
Reproductive effects in experimental animals
In experimental animals, the male reproductive tract is a consistent target for boron toxicity as indicated by testicular, sperm and fertility effects observed in dogs and rodents at concentrations ranging from 23.7 to 94.2 mg B/kg bw per day (Table 2). The lowest NOAEL identified in the literature was 3.9 mg B/kg bw per day, observed for decreased testis:bw ratio, testicular atrophy and degeneration of spermatogenic epithelium in dogs after 90 days (Weir and Fisher, 1972). The lowest LOAEL of 23.7 mg/kg bw per day was observed for decreased testes weight and impaired spermatogenesis in rats after 70 days (Seal and Weath, 1980).
In the Weir and Fisher (1972) study, the effects of 90-day and 2-year exposure to boric acid and borax were evaluated in both rats and dogs. This study is described below as it is considered the most appropriate reproductive study for consideration as the key study for risk assessment.
In the 90-day rat study, Sprague-Dawley rats (10/sex/dose/substance) were administered borax or boric acid in the diet at concentrations of 52.5, 175, 525, 1 750 or 5 250 ppm (calculated as 0, 2.6, 8.8, 26.3, 87.5 and 262.5 mg B/kg bw per day in U.S. EPA, 2008) for 90 days (Weir and Fisher, 1972). The highest dose caused 100% mortality. Complete atrophy of testes was seen in male rats in the 87.5 mg B/kg bw per day dose group, and partial atrophy was reported in 4 males in 26.3 mg B/kg bw per day dose group. Effects observed at these 2 doses included rapid respiration, inflamed eyes, swollen paws and desquamated (peeling) skin on paws and tail. No clinical signs of toxicity were seen below the dose of 26.3 mg B/kg bw per day. Based on systemic toxicity, the NOAEL of 8.8 mg B/kg bw per day can be identified from this 90-day study.
In the 2-year rat study, Sprague-Dawley rats (35/sex/dose/substance in treated groups, 70 unexposed controls/sex) were exposed to 0, 117, 350, or 1 170 ppm of boron as borax or boric acid daily in the diet for 2 years (Weir and Fisher, 1972). Boron equivalent doses were estimated as 0, 5.9, 17.5 or 58.5 mg B/kg bw per day for both sexes (U.S. EPA, 2008). Five rats/sex/dose were sacrificed at 6 and 12 months and all surviving animals were sacrificed after 2 years. No treatment-related effects were observed in rats receiving 5.9 or 17.5 mg B/kg bw per day. Signs of toxicity observed in rats in the 58.5 mg B/kg bw per day dose group included swelling and desquamation of the paws, scaly tails, inflammation of the eyelids and bloody discharge from the eyes. Moreover, in the high dose group, the scrotum appeared shrunken in male rats, and both males and females showed decreased food consumption and suppressed growth. A significant (p < 0.05) decrease (80% to 84%) was reported in testes weight and the testes:body weight ratio as early as 6 months in the 58.5 mg B/kg bw per day group, and remained significantly below that of controls at 12 and 24 months. Brain and thyroid:body weight ratios were significantly (p < 0.05) increased in the 58.5 mg B/kg bw per day animals but no microscopic changes were observed in these organs. Severe testicular atrophy was observed in all high-dose males at 6, 12 and 24 months. The seminiferous epithelium was atrophied and the tubular size in the testes also decreased in male rats. Based on systemic and testicular effects, a NOAEL of 17.5 mg B/kg bw per day can be identified from the 2-year rat study.
Weir and Fisher (1972) also evaluated the effects of repeated dose boric acid and borax exposure in young dogs. It should be noted, however, that in their assessment, Health Canada's Pest Management Regulatory Agency (PMRA) identified several discrepancies in the published dog studies compared to the original study data (also coordinated or supervised by Weir) (Health Canada, 2012, 2016). Where possible, and as was previously done in the PMRA assessment, this assessment relies on the original study data. (Note: The 90-day studies correspond to studies no. 1237735 and 1249382, the 2-year studies correspond to studies no.1249414 and 1249387, and the 38-week study corresponds to studies no. 1249410 and 1249383 (as cited in Health Canada, 2012).
In the 90-day study, young beagle dogs (5/sex/dose/substance) were given borax or boric acid in diet for 90 days at concentration of 17.5, 175 and 1 750 ppm (doses calculated as 0, 0.33, 3.9 and 30.4 in males and 0, 0.24, 2.5 and 21.8 mg B/kg bw per day in females in U.S. EPA, 2008). No clinical signs of toxicity were observed, and all dogs appeared normal for 90 days (except 1 high-dose male which died on day 68 from complications of diarrhea and severe congestion of the kidneys and intestinal mucosa). A decrease in testis:body weight ratio was seen in 2 mid-dose (3.9 mg B/kg bw per day) dogs and testicular atrophy was noted in all males in the highest dose group (30.4 mg B/kg bw per day). Exposure to 30.4 mg B/kg bw per day also caused breakdown of red blood cells and effects on the thyroid gland in both sexes. Based on reproductive toxicity in males and systemic toxicity in males and females, NOAELs of 3.9 and 2.5 mg B/kg bw per day can be identified for males and females, respectively. In their assessment of boron, the PMRA combined the results of the 90-day boric acid and borax studies to calculate a benchmark dose lower 95% confidence limit (BMDL) of 2.90 mg/kg bw per day, based on testicular effects (Health Canada, 2012, 2016).
In the 2-year study, young beagle dogs (4/sex/dose) were exposed to borax or boric acid in the diet at doses of 0, 58, 117 and 350 ppm boron (doses calculated as 0, 1.4, 2.9 and 8.8 mg B/kg bw per day by U.S. EPA, 2008) for 104 weeks. A 52-week interim sacrifice and a 13-week recovery period were allowed for some dogs after cessation of exposure. One control male dog was sacrificed at week 52, 2 dogs were sacrificed after 104 weeks and 1 dog was sacrificed after 104 weeks of treatment followed by a subsequent 13 weeks of the recovery period. Testicular atrophy was observed in 1 control dog sacrificed after the 104-week exposure + 13-week recovery period and in 1 dog in the high-dose group sacrificed after 104 weeks of exposure. A NOAEL of 8.8 mg B/kg bw per day can be derived from this study.
An additional test group of dogs (n = 4/sex/dose/substance) were given borax or boric acid in the diet at 0 and 1 170 ppm (doses calculated as 0 and 29.2 mg B/kg bw per day by U.S. EPA, 2008) for 38 weeks. Interim sacrifice of 2 dogs at 26 weeks revealed testicular atrophy and spermatogenic arrest. After 38 weeks of exposure, 1 dog showed a decrease in spermatogenesis and the other had testicular atrophy. Following a 25-day recovery period, testicular degeneration was not as severe as those found in the control dogs. Based on observed testicular atrophy and spermatogenic arrest observed in this 38-week study, a LOAEL of 29.2 mg B/kg bw per day was identified.
Mode of action
Although numerous studies have attempted to elucidate the mode of action of boron toxicity, no single mechanism has been agreed upon in the literature. The mechanism implicated in boron's reproductive effects is proposed to be related to a delay in spermiation followed by testicular atrophy at higher doses. Studies conducted in rats suggest that boron affects the sertoli cell by impairing energy production which eventually results in delayed spermiation and disruption of spermatogenesis (Fail et al., 1998). The mechanism implicated in boron's developmental effects may be related to inhibition of mitosis by boric acid (Fail et al., 1998) and/or inhibition of histone deacetylase (Di Renzo et al., 2007). It has also been proposed that boron binds to and is a reversible inhibitor of cyclic adenosine diphosphate ribose, which can lead to a decrease in intracellular calcium release that is necessary for many processes including insulin release, bone formation and brain function (Nielsen, 2014).
The literature to date provides no indication of a difference between the mode of action in animals as compared to humans. A full analysis of the mode of action of boron toxicity was not conducted as it is not critical to the selection of a POD or to the derivation of a HBV for boron.
| Species, sex (number) | Exposure | POD (mg B/kg bw per day) | Critical effect(s) | Key strengths and/or weakness | Ref. | |
|---|---|---|---|---|---|---|
| Duration | Compound; dosesTable 2 Footnote a (mg B/kg bw per day) | |||||
| Developmental studies | ||||||
Mice, CD-1, F (29/group) |
GD 0-17 |
Boric acid (diet); 0, 43.4, 79, 175 |
|
Maternal effects : increased kidney weight, increased incidence of renal tubular dilation Fetal effects: decreased body weight, increased frequency of fetal resorptions and fetal malformations (most commonly short rib XIII) |
Well-conducted study; limited evaluation of maternal toxicity |
Heindel et al., 1992, 1994 |
Rats, Sprague-Dawley, F (29/group) |
(i) GD 0-20 (ii) GD 6-15 |
Boric acid (diet); (i) 0, 13.6, 28.5, 57.7 and (ii) 0, 94.2 |
|
Maternal effects: increased liver and kidney weights Fetal effects: decreased body weight, increased fetal resorptions and malformations (most commonly observed as enlargement of brain ventricles and short rib XIII) |
Well-conducted study; limited evaluation of maternal toxicity |
|
Rabbits, New Zealand, F (20-23/ group) |
GD 6-19 |
Boric acid (gavage); 0, 10.9, 21.9, 43.7 |
|
Maternal effects: decreased body weight, increased kidney weight Fetal effects: increased frequency of fetal resorptions, increased frequency of litters with no live fetuses, fetal malformations (primarily cardiovascular) |
Well-conducted study; limited evaluation of maternal toxicity |
Heindel et al., 1994 |
Rats, Sprague Dawley, F (60/group) |
(i) GD 0-20 (ii)GD 0-20, follow-up until PND21 |
Boric acid (diet); (i) 0, 3.3, 6.3, 9.6, 13.3 and (ii) 0, 3.2, 6.3, 9.8, 12.9, 25.3 |
|
Maternal effects: None Fetal effects: Decreased body weight (only in rats exposed on GD 0-20), skeletal malformations (primarily short rib XIII) |
Well-conducted study; limited evaluation of maternal toxicity |
Price et al., 1996 |
| Repeated dose studies with reproductive endpoints | ||||||
Dogs, beagle M&F (5/group) |
90 days |
Boric acid, borax (diet); M: 0, 0.33; 3.9, 30.4; F: 0, 0.24, 2.5 and 21.8 |
|
Reproductive effects: Decreased testis:bw ratio, testicular atrophy, degeneration of spermatogenic epithelium Systemic effects: Decreased thyroid weight, histopathological changes in thyroid gland (for example, presence of solid epithelial nests, minute follicles), increased brain:bw ratio, elevated breakdown of red blood cells |
Use of young dogs; the same control animals were used in the boric acid and borax studies |
Weir and Fisher, 1972 |
Dogs, beagle M&F (4/group) |
(i) 2 years (ii) 38 weeks |
Boric acid, borax (diet): (i) 0, 1.4, 2.9 and 8.8 (ii) 0, 29.2 |
|
Reproductive effects : 2-year study: histopathological changes in thyroid. 38-wk study: testicular atrophy and degeneration (reversible after 25-wk recovery period in 1 dog), decreased spermatogenesis Systemic effects: None |
Use of young dogs; the same control animals were used in the boric acid and borax studies |
Weir and Fisher, 1972 |
Rat, Sprague Dawley M&F (10/group) |
90 days |
Boric acid, borax (diet): 0, 2.6, 8.8, 26.3, 87.5, 262.5 |
NOAEL = 8.8 |
Reproductive effects: Testicular atrophy Systemic effects: Mortality, rapid respiration, inflamed eyes, swollen paws, desquamated skin on paws and tail |
Well-conducted study |
Weir and Fisher, 1972 |
Rat, Sprague Dawley M&F (35/group) |
2 years |
Boric acid, borax (diet): 0, 5.9, 17.5, 58.5 |
NOAEL = 17.5 |
Reproductive effects: Increased testes weight, testicular atrophy, atrophy of seminiferous epithelium Systemic effects: Swelling and desquamation of paws, scaly tails, inflamed eyes, bloody discharge from eyes, decreased food consumption, suppressed growth rates, reduction in body weight gain, increased brain and thyroid:body weight ratios |
Well-conducted study |
|
Mice, B6C3F1, M&F (10/group) |
90 days |
Boric acid (diet); M: 0, 34, 70, 141, 281, 563; F: 0, 47, 97, 194, 388, 776 |
LOAEL = 34 |
Reproductive effects: testicular degeneration, atrophy of seminiferous tubules Systemic effects: mortality, extramedullary hematopoiesis |
High doses |
Dieter, 1994 |
Mice, B6C3F1, M&F (10/group) |
2 years |
Boric acid (diet); 0, 48, 96 |
LOAEL = 48 |
Reproductive effects: testicular atrophy interstitial, cell hyperplasia Systemic effects: mortality (males only), reduced body weight gain, increased splenic lymphoid depletion |
Only two doses tested |
|
Rats, Evans, M (15/group) |
70 days |
Borax (drinking water); 0, 23.7, 44.7 |
LOAEL = 23.7 |
Reproductive effects: Decreased testes weight, impaired spermatogenesis Systemic effects: Decreased body weight |
Drinking water exposure |
Seal and Weeth, 1980 |
bw – body weight; F – females; GD – gestational day; LOAEL – lowest observed adverse effect; M – males; NOAEL – no observed adverse effect; PND – post-natal day; POD – point of departure
|
||||||
Selected key study
Two key studies were considered in the risk assessment for boron: a reproductive study (testicular effects) in dogs and rats by Weir and Fisher (1972) and a developmental study (reduced fetal body weights and skeletal malformations) in rats by Price et al. (1996).
The male reproductive tract is a target of boron toxicity in animals as indicated by testicular, sperm and fertility effects observed in dogs and rodents, with dogs being the most sensitive species. Of all the longer-term animal studies in the literature showing male reproductive effects, the lowest POD observed was from the 90-day dog study by Weir and Fisher (1972). Based on the combined results of the 90-day borax and boric acid studies, a BMDL1SD (lower 95% confidence limit on the benchmark dose associated with a change of 1 standard deviation from the controls) of 2.90 mg/kg bw per day was previously derived by PMRA (Health Canada, 2012, 2016) using the model that provided the most conservative POD (that is, the Hill model) in Benchmark Dose Software (U.S. EPA version 2.12). Selection of the Weir and Fisher (1972) 90-day dog study is consistent with other Canadian assessments of boron, conducted under the Canadian Environmental Protection Act (ECCC and Health Canada, 2016) and the Pest Control Products Act (Health Canada, 2012, 2016). While this study did have some limitations (for example, the same control animals were used in the boric acid and borax studies, and there may be difference in responses between young and adult dogs), it does still provide strong evidence for male reproductive effects. Moreover, while the epidemiological studies on boron are insufficient for deriving a POD for risk assessment (see Developmental and reproductive effects, above), they are also considered insufficient to confirm the absence of effects in humans (ECCC and Health Canada, 2016). Effects observed in boron workers qualitatively support the selection of animal reproductive toxicity studies for risk assessment.
The Price et al. (1996) developmental rat study was also considered as a candidate for selection of the key study. This study provided a developmental NOAEL of 9.6 mg/kg bw per day, based on decreased fetal body weight, an effect that was observed in the absence of maternal toxicity, indicating the potential for sensitivity of offspring to boron exposure. Benchmark dose (BMD) modelling of the fetal body weight data from this study carried out using the Benchmark Dose Software (U.S. EPA version 2.7) yields a BMDL05 (lower 95% confidence limit on the benchmark dose associated with a response rate that differs from the control response rate by 5%) of 10.6 mg B/kg bw per day. This is consistent with the BMD05 of 10.3 mg B/kg bw per day established by Allen et al. (1996) using the same dataset.
While this rat study was generally well conducted, the exposure was limited to a 20-day in utero exposure. The study also included only limited evaluations of fetal and maternal toxicity, and the reduced fetal body weights observed appeared to be reversible following birth (at PND21).
Selection of the Weir and Fisher (1972) 90-day dog study, which has a lower POD, is considered more conservative, and is still considered to be adequately protective of developmental effects that may occur.
Derivation of the health-based value (HBV)
Reproductive and developmental effects are the most sensitive effects, and most frequently and consistently observed across a variety of animal species following boron exposure. The critical effect considered most appropriate for deriving a POD is the reduction in testicular weight observed in a repeated dose study in dogs, which are considered to be the most sensitive species (Weir and Fisher, 1972).
To derive a HBV for boron, the BMDL1SD of 2.90 mg/kg bw per day was employed (Health Canada, 2012, 2016; ECCC and Health Canada, 2016). This BMDL is based on testicular effects and was the lowest BMDL calculated using four models for continuous data. BMD modelling was used over the NOAEL/LOAEL approach because it offers better dose-response characterization by including all experimental data to determine PODs independently of pre-established dose levels.
A total uncertainty factor of 300 was considered appropriate for assessment of boron in drinking water. This is comprised of uncertainty factors of 10 for interspecies variability, 10 for intraspecies variability and 3 for database uncertainties.
A default 10-fold interspecies uncertainty factor was employed because little to no data on the toxicokinetics of boron in dogs exists which would allow refinement of this uncertainty factor.
A default 10-fold intraspecies uncertainty factor was employed to account for variability within the human population (for example, difference in clearance during pregnancy, which may not necessarily protect the fetus from developmental effects of boron exposure, and individual difference in boron toxicokinetics).
The 3-fold database uncertainty factor was selected to account for the quality of the database, and the fact that histological changes in the testes likely occur at lower dose levels than those associated with decreases in testicular weight (Fail et al., 1998; Ku et al., 1993; Health Canada, 2012, 2016).
Using the BMDL of 2.90 mg B/kg bw per day, the tolerable daily intake (TDI) for boron is calculated as follows:
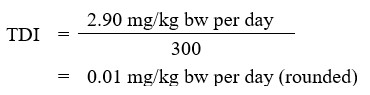
Alt text
The tolerable daily intake (TDI) of boron is 0.01 mg/kg body weight per day. This is calculated by dividing the BMDL of 2.90 mg/kg body weight per day by the uncertainty factor of 300.
The tolerable daily intake (TDI) of boron is 0.01 mg/kg body weight per day. This is calculated by dividing the BMDL of 2.90 mg/kg body weight per day by the uncertainty factor of 300.
where:
- 2.90 mg/kg bw per day is the BMDL, calculated based on decreased testicular weight observed in the dog study of Weir and Fisher (1972) (Health Canada, 2012, 2016)
- 300 is the total uncertainty factor, which accounts for interspecies variation (×10), intraspecies variation (×10), and database deficiencies (×3)
Using this TDI, the HBV for boron in drinking water is calculated as follows:

Alt text
The health-based value (HBV) for boron in drinking water is 0.1 mg/L. This is calculated by multiplying the TDI for boron of 0.01 mg/kg body weight per day by the average adult body weight of 74 kg, then by the drinking water allocation factor of 0.2. The result is divided by 1.53 L/day, which is the drinking water intake rate for an adult.
The health-based value (HBV) for boron in drinking water is 0.1 mg/L. This is calculated by multiplying the TDI for boron of 0.01 mg/kg body weight per day by the average adult body weight of 74 kg, then by the drinking water allocation factor of 0.2. The result is divided by 1.53 L/day, which is the drinking water intake rate for an adult.
where:
- 0.01 mg/kg bw per day is the TDI derived above
- 74 kg is the average body weight for an adult (Health Canada, 2021)
- 0.2 is the allocation factor for drinking water. Based on Canadian drinking water intake estimates of 3% to 16% (see Exposure in Exposure considerations), the floor value of 20% for drinking water is appropriate (Krishnan and Carrier, 2013)
- 1.53 L/day is the drinking water intake rate for an adult (Health Canada, 2021). A multi-route exposure assessment (Krishnan and Carrier, 2008) found that, based on phys-chem properties, dermal and inhalation exposures through showering or bathing represent negligible routes of exposure to boron through drinking water
An alternative approach was considered using the data from Price et al. (1996) for reduced fetal body weight in rats. Using the BMDL05 of 10.6 mg/kg bw per day, and an appropriate uncertainty factor of 60 (6 for intraspecies differences, 10 for interspecies differences), would yield a TDI of 0.18 mg/kg bw per day. Employing the above assumptions for body weight, drinking water intake and default allocation factor would then yield an alternative value of 1.7 mg/L.