Page 11: Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Carbon Tetrachloride
Part II. Science and Technical Considerations - Continued
Carbon tetrachloride has been classified in Group IIIC (Health Canada, 1994), possibly carcinogenic to humans, based on inadequate evidence of carcinogenicity in humans, but sufficient evidence in experimental animals. Although Health Canada's previous guideline was based on carcinogenesis, the carcinogenicity of carbon tetrachloride now appears to be secondary to its hepatotoxic effects in animal studies, suggesting that a threshold may exist. This is consistent with the classifications established by the International Agency for Research on Cancer of Group 2B (IARC, 1999), possibly carcinogenic to humans, based on inadequate evidence in humans, but sufficient evidence in animals, and by the U.S. EPA of Group B2 (IRIS, 1991), probably carcinogenic to humans, based on inadequate evidence of carcinogenicity in humans, but sufficient evidence in experimental animals. The U.S. Department of Health and Human Services has determined that carbon tetrachloride may reasonably be anticipated to be a carcinogen (ATSDR, 2005).
Epidemiological studies suggesting an association between carbon tetrachloride exposure and cancer were confounded by many factors, including exposure to other substances in drinking water or in industrial settings, lack of adequate control groups, and lack of statistical significance of effects. These studies are therefore inadequate to infer a causal relationship between carbon tetrachloride and cancer in humans (IPCS, 1999).
Studies in animals have shown an increased incidence of hepatic tumours in rats, mice, and hamsters following oral or inhalation exposure to carbon tetrachloride. IPCS (1999) has indicated that many genotoxicity assays have been conducted with carbon tetrachloride. On the basis of available data, carbon tetrachloride can be considered as a non-genotoxic compound. Carbon tetrachloride induces hepatomas and hepatocellular carcinomas in mice and rats, however the doses inducing hepatic tumours are higher than those inducing cell toxicity. Despite evidence of the carcinogenicity of carbon tetrachloride in animals, there are numerous deficiencies identified in the cancer studies (e.g., inappropriate routes of exposure, poor dose response, excessive mortality, inadequate sample size). Consequently, the TDI approach is considered appropriate to calculate the guideline for carbon tetrachloride in drinking water. In addition, the carcinogenicity of carbon tetrachloride appears to be secondary to its hepatotoxic effects, suggesting that a threshold may exist. There is evidence that the mechanism of carbon tetrachloride carcinogenicity involves both genotoxic and non-genotoxic processes. The ability of reactive carbon tetrachloride metabolites and lipid peroxidation products to bind to DNA indicates potential for genotoxicity. However, results of most in vivo studies in animals suggest that genotoxic effects may be secondary to cytotoxicity. Carbon tetrachloride may also cause cancer by non-genotoxic mechanisms involving regenerative hyperplasia.
As there are no adequate long-term studies on carbon tetrachloride, the subchronic rat study by Bruckner et al. (1986) was chosen as the most appropriate study for risk assessment. The subchronic rodent studies conducted by Condie et al. (1986), Hayes et al. (1986), and Allis et al. (1990) support the choice of the Bruckner et al. (1986) study, as similar hepatotoxic effects were observed in these studies at similar doses. It should be noted that carbon tetrachloride was administered to rats as a single oral bolus in corn oil in Bruckner et al. (1986), which does not represent the typical exposure scenario in humans. Sanzgiri et al. (1995) demonstrated that carbon tetrachloride was significantly more hepatotoxic when administered as a single oral bolus than when it was administered by gastric infusion over a period of 2 hours. Furthermore, Bruckner et al. (1986) dosed the rats with carbon tetrachloride soon after the beginning of their light/inactive period, which has since been shown to result in increased susceptibility to carbon tetrachloride hepatotoxicity. This effect is likely related to restricted food intake during the inactive period, as fasting is known to increase CYP2E1 activity and potentiate carbon tetrachloride hepatotoxicity (Bruckner et al., 2002). Given the experimental evidence suggesting that the dosing regimen and timing used in the Bruckner et al. (1986) study may have influenced the observed toxicity, the guideline derived from this study is based on a conservative approach.
The TDI for carbon tetrachloride is calculated as follows:
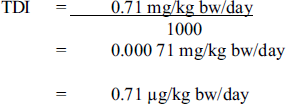
Figure 3 - Text Equivalent
The tolerable daily intake for carbon tetrachloride is 0.00071 milligrams per kilogram body weight per day. This value is calculated by dividing 0.71 milligrams per kilogram body weight per day by 1000.
where:
- 0.71 mg/kg bw/day is the adjusted NOAEL; a NOAEL of 1 mg/kg bw/day determined from Bruckner et al. (1986) was multiplied by 5/7 to correct for a dosing schedule of 5 days per week; and
- 1000 is the uncertainty factor (×10 for interspecies variability; ×10 for intraspecies variability; ×10 for major database deficiencies, including lack of adequate chronic studies and evidence regarding carcinogenic mode of action in animals).
Using this TDI, the maximum acceptable concentration (MAC) for carbon tetrachloride in drinking water is derived as follows:
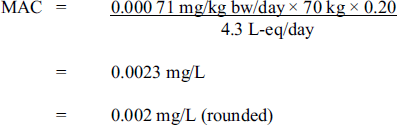
Figure 4 - Text Equivalent
The maximum acceptable concentration for carbon tetrachloride is 0.002 milligrams per litre (rounded). This value is calculated by taking the tolerable daily intake (previously calculated) 0.00071 milligrams per kilogram body weight per day and multiplying it by 70 kilograms and then multiplying by 0.2. This value is then divided by 4.3 litre-equivalents to achieve the maximum acceptable concentration.
where:
- 0.000 71 mg/kg bw/day is the TDI derived above;
- 70 kg is the average body weight of an adult;
- 0.20 is the default allocation factor for drinking water in the absence of adequate exposure data from all exposure media; and
- 4.3 L-eq/day is the daily volume of water consumed by an adult, which accounts for the following multi-route exposure: 1.0 L-eq/day from dermal absorption, and 1.8 L-eq/day from inhalation.
A quantitative cancer risk assessment was conducted by Health Canada (2006b) to estimate the unit risks associated with hepatocellular carcinomas and adenomas in rats and mice from two studies (Weisburger, 1977; Nagano et al., 1998). Concentrations of carbon tetrachloride in drinking water yielding risks of 1 × 10−5 and 1 × 10−6 range from 1.0 to 30 µg/L and 0.1 to 3.0 µg/L, respectively. Health Canada's confidence in these estimated unit risks is low due to the quality of the studies and the lack of fit of the Nagano et al. (1998) data to the statistical model applied. This further supports the choice of the TDI approach as the most appropriate approach for developing a MAC for carbon tetrachloride in drinking water.
The current U.S. EPA maximum contaminant level (MCL) for carbon tetrachloride is 5 µg/L (U.S. EPA, 1998b) based on the carcinogenic potential of carbon tetrachloride. The U.S. EPA approach calculated the geometric mean of the upper limit unit risk estimates (3.7 × 10−6) from data from four animal studies, and selected this mean as the unit risk corresponding to drinking water containing 1 µg/L. A human water consumption of 2 L/d and a human body weight of 70 kg were used to derive a slope factor of 1.3 × 10−1 (mg/kg bw/d)−1 from the above unit risk (U.S. EPA, 1984, 1989).
The WHO established a drinking water guideline of 4.0 µg/L based on a NOAEL of 1 mg/kg from Bruckner et al. (1986) for hepatotoxic effects in rats. The guideline value is based on 10% allocation of the TDI to drinking water and assuming a 60 kg adult drinking 2 litres of water per day. WHO reported this value to be lower than the range of values associated with lifetime upperbound excess cancer risks of 10−4, 10−5 and 10−6 calculated by linear extrapolation (WHO, 2004a).
The California EPA has developed a public health goal (PHG) of 0.1 µg/L (or 0.1 ppb) for carbon tetrachloride in drinking water (OEHHA, 2000). The PHG is based on an increased incidence of hepatocellular carcinomas in mice, an estimated cancer potency of 1.8 × 10−1 (mg/kg bw/day)−1 and a de minimis theoretical excess individual cancer risk level of 10−6. California's current drinking water standard for carbon tetrachloride is 0.5 µg/L. The California Department of Health Services (DHS) adopted this standard, referred to as the state maximum contaminant level (MCL), in 1988.
The Australian drinking water guideline for carbon tetrachloride is 3 μg/L based on a NOAEL of 1.2 mg/kg body weight per day for hepatotoxicity in mice. The guideline value is based on 10% allocation of the TDI to drinking water and assuming a 70 kg adult drinking 2 litres of water per day (NHMRC, 2004).