Guidelines for Canadian Drinking Water Quality: Operational Parameters
Download in PDF format
(1,938 KB, 139 pages)
Organization: Health Canada
Date published: March 2025
Cat.: H144-137/2025E-PDF
ISBN: 978-0-660-74972-3
Pub.: 240720
Table of contents
- Guideline
- Executive summary
- Exposure
- Health effects
- Aesthetic considerations
- Analytical and treatment considerations
- Application of the guidelines
- 1.0 Exposure consideration
- 2.0 Health Considerations
- 3.0 Derivation of the health-based values (HBVs)
- 4.0 Analytical considerations
- 5.0 Treatment considerations
- 6.0 Management strategies
- 7.0 International considerations
- 8.0 Rationale for aesthetic objectives
- 9.0 References
- Appendix A: Abbreviations
- Appendix B: Provincial data tables
- Appendix C: Health Canada Drinking Water Survey Data
- Appendix D: Summary of total hardness removal for residential scale technologies
- Appendix E: Intake of sodium as a result of water softener use, by hardness level
Guideline Technical Document
Guideline
Aesthetic objectives (AOs) are established for the following parameters:
- chloride ≤ 250 mg/L
- sulphate ≤ 500 mg/L
- total dissolved solids (TDS) ≤ 500 mg/L
- sulphide (as hydrogen sulphide) ≤ 0.05 mg/L in drinking water
Executive summary
This guideline technical document was prepared in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water (CDW). It consolidates and updates all relevant information for the seven parameters: calcium, magnesium, hardness, chloride, sulphate, total dissolved solids (TDS) and hydrogen sulphide.
Exposure
Calcium, magnesium, hardness, chloride, sulphate, TDS and hydrogen sulphide occur naturally and are found in most Canadian waters. They are most prevalent in groundwater aquifers.
Health effects
Calcium, magnesium, chloride and sulphate are essential for human health.
Studies in humans have found that intake of calcium supplements may increase the risk of kidney stone formation. Excess calcium intake and hypercalcemia from foods and water alone are unlikely. A health based value (HBV) of 300 mg/L is established for calcium based on an elevated risk of kidney stone formation.
Studies in humans have found that increased intake of chloride, as sodium chloride, may elevate blood pressure. An HBV of 470 mg/L is established for chloride based on an increased risk of elevated blood pressure.
An HBV represents a concentration of a chemical in drinking water that can be consumed over a lifetime without significant health risk. The HBV is developed using available epidemiological and/or animal toxicological information and may serve as the basis for establishing a maximum acceptable concentration (MAC) if required.
Since levels of calcium and chloride in Canadian water are typically below their HBVs, Health Canada and the CDW have determined that there is no need to establish MACs for these substances in drinking water. However, in the unlikely event that these substances are present in drinking water at high levels, the HBVs are provided to help jurisdictions and the public understand the potential health effects of these substances.
Currently, there is insufficient evidence to support the need for HBVs or MACs for magnesium, hardness, sulphate, TDS or hydrogen sulphide.
Aesthetic considerations
Calcium, magnesium, hardness, chloride, sulphate, TDS and hydrogen sulphide are considered to have operational significance for drinking water utilities and residential water consumers.
Increased chloride levels can result in an objectionable water taste when it is in the presence of sodium, calcium, potassium and magnesium. Sulphate also has a taste threshold, with most consumers accepting only moderate concentrations from a taste perspective. Hydrogen sulphide is predominantly an issue due to its offensive rotten egg odour and its low odour threshold. High levels of TDS can lead to excessive scaling in water pipes, heaters, boilers and home appliances. Concerns regarding the presence of these substances in drinking water are often related to consumer complaints.
The AOs for chloride (≤ 250 mg/L), sulphate (≤ 500 mg/L), TDS (≤ 500 mg/L) and hydrogen sulphide (≤ 0.05 mg/L) are intended to minimize the occurrence of complaints based on unacceptable taste, odour or excessive scaling, and to improve consumer confidence in drinking water quality. The AOs are primarily based on taste and odour acceptance, which varies based on source water, local conditions, habituation, pH and water temperature.
Analytical and treatment considerations
The development of a drinking water guideline takes into consideration the ability to measure the contaminant and to remove it from drinking water supplies. Several analytical methods are available for measuring all of the operational parameters well below their respective AO values.
At the municipal level, treatment technologies that are available to decrease the levels of calcium, magnesium, hardness, chloride, sulphate, TDS and hydrogen sulphide in drinking water include softening, membrane filtration, ion exchange (IX) and aeration. Most well-operated and optimized treatment plants can achieve concentrations in the treated water below the AO established for each parameter. Prior to full-scale implementation, bench- and/or pilot-scale studies should be conducted using source water to ensure sufficient removal and to optimize performance.
In cases where removal of these substances is desired at a small-system or household level, for example, a private well, a residential drinking water treatment unit may be an option. While several treatment technologies can be effective for reducing these substances, water softeners are the best available technology for the overall reduction of hardness, calcium and magnesium. When using a residential drinking water treatment unit, it is important to take samples of water entering and leaving the treatment unit and send them to an accredited laboratory for analysis, to ensure that adequate removal of the substances of concern is achieved. Routine operation and maintenance of treatment units, including replacement of filter components, should be conducted according to manufacturer specifications.
Individuals on sodium-restricted diets or needing to limit their exposure to sodium should be aware that residential water softening systems will increase the concentration of sodium in the treated water. In this case, it is recommended that a portion of the water most frequently consumed (from the kitchen tap) bypass the softener altogether to avoid excessive sodium intake. Generally, children under 8 years of age should not drink water containing sodium from a water softener as they may exceed the recommended upper limit of 1.5–1.9 g of sodium/day. However, due to calcium being a predominant cation in water hardness, individuals should be mindful that in rare instances where the drinking water source exhibits very high hardness (> 750 mg CaCO3/L), it could result in calcium exposure exceeding the HBV (300 mg calcium/L). In this instance, an alternate drinking water source should be sought.
Application of the guidelines
Specific guidance related to implementing drinking water guidelines should be obtained from the appropriate drinking water authority.
This document is intended to update, consolidate and replace the current guideline technical documents for seven parameters: calcium, magnesium, hardness, chloride, sulphate, TDS and hydrogen sulphide. For the purpose of this guideline document, these seven parameters are defined as operational parameters.
All water utilities should implement a risk management approach such as the source-to-tap or water safety plan approach to ensure water safety. These approaches require a system assessment to characterize the source water, describe the treatment barriers that prevent or reduce contamination, identify the conditions that can result in contamination and implement control measures. Operational monitoring is then established and operational and management protocols such as standard operating procedures, corrective actions and incident responses are instituted. Other protocols are also implemented to validate the water safety plan, such as recordkeeping and consumer satisfaction. Operator training is also required to ensure the effectiveness of the water safety plan at all times.
Considering that the levels of these operational parameters can vary significantly in source water, within treatment plants and in distribution systems, monitoring programs should be system-specific to enable utilities to have a good understanding of their water quality from source to tap. Monitoring programs should be designed based on risk factors that contribute to the likelihood that calcium and chloride may be elevated within the drinking water system. These factors may include source water chemistry and use of road salt, among others. The locations, frequency and types of samples that should be collected will differ from one system to the next, depending on the desired objective and site-specific considerations. Suggested monitoring details are provided in this document.
1.0 Exposure consideration
Calcium, magnesium, hardness, chloride, sulphate, total dissolved solids (TDS) and hydrogen sulphide are naturally co-occurring in most Canadian waters, but their presence is most prevalent in groundwater aquifers. These parameters are considered to have operational and aesthetic significance, particularly for groundwater systems and private well owners, and addressing them will help ensure good quality, palatable drinking water. For the purposes of this document, operational parameters are defined as calcium, magnesium, hardness, chloride, sulphate, TDS and hydrogen sulphide. Other operational parameters of importance (for example, pH, alkalinity, and total organic carbon) are addressed elsewhere (Health Canada, 2024a).
Water in nature comes into contact with minerals, salts, metals and vegetation, which can then dissolve into the water. The measure of all these dissolved combined substances in water is known as TDS. TDS comprises mostly ions such as calcium, magnesium, sodium, bicarbonates, chloride and sulphate. Hydrogen sulphide is produced from the breakdown of organic matter in the absence of oxygen but may also be reduced directly from sulphate in the presence of sulphate-reducing bacteria. It is widely present in sediments and water, as well as in biological wastes.
Since some of these parameters are typically measured, handled and monitored together, they are discussed together in the document as follows:
- Calcium and magnesium: these are the primary contributing cations for water hardness.
- Chloride and sulphate: these are primarily related to aesthetic concerns but there are also operational considerations related to corrosion.
- TDS: these are a main determinant in the taste of water and people's acceptance. High TDS are also of operational concern due to the formation of scale deposits.
- Hydrogen sulphide: this substance has an offensive rotten egg odour that is often the primary reason for its removal during the water treatment process.
1.1 Identity, uses and sources in the environment
The parameters discussed in this guideline document are major cations and anions that are naturally occurring in Canadian waters and are most significant in groundwater aquifers.
1.1.1 Calcium, magnesium and hardness
Water hardness is defined as the sum of all multivalent cations in a solution. The principal hardness -causing ions are calcium and magnesium. Although hardness is caused by calcium, magnesium and a variety of other metals, the simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Strontium, iron, barium and manganese ions also contribute to the overall hardness but are generally present in lower concentrations. From a consumer perspective, hard water may be better observed as a reduced ability of water to react with soap. Hard water requires a considerable amount of soap to produce a lather, and it also causes scaling of hot water pipes, boilers and other household appliances (Davis, 2010; Crittenden et al., 2012).
Groundwater is generally harder than surface water, richer in carbonic acid or dissolved carbon dioxide, and usually has a high solvating power. Longer residence times within calcium-rich formations (for example, calcite, gypsum and dolomite) can lead to hardness levels as high as several thousand milligrams per litre. Residence times and solubility can vary seasonally in some aquifers.
Ferromagnesian mineral igneous rocks and magnesium carbonates in sedimentary rocks are generally considered to be the principal sources of magnesium in natural waters. The principal natural sources of hardness in water are sedimentary rocks, seepage and runoff from soils.
| Property | Calcium | Magnesium |
|---|---|---|
| Chemical Abstracts Service Registry Number (CAS RN) | 7440-70-2 | 7439-95-4 |
| Molecular formula | Ca | Mg |
| Molecular Weight (g/mol) | 40.078 | 24.3050 |
| Melting point | 842°C, 1115 K | 648.8°C, 921.8K |
| Boiling Point | 1484°C, 1757 K | 1090°C, 1363K |
| Density at room temp (g•cm-3) | 1.55 | 1.738 |
Calcium is the fifth most abundant natural element and is the primary source of hardness. Some of the common forms of calcium are calcium carbonate (CaCO3), gypsum (CaSO4·2H2O), anhydrite (CaSO4) and fluorite (CaF2) (Yaroshevsky, 2006; Crittenden et al., 2012). Surface water generally contains lower concentrations of calcium than groundwater. Notably, some areas of the country have observed decreasing calcium concentrations in surface water sources. There have been reductions in calcium in several boreal lakes with already low levels of calcium when comparing concentrations observed in the 1980s versus the 2000s (Jeziorski et al., 2008). This is thought to be due to reduced calcium contributions to water bodies from soil as acidic precipitation has been greatly reduced over this time period. The increased prevalence of invasive zebra mussels is also thought to cause decreased calcium concentrations in some surface water sources (Chapra et al., 2012).
Magnesium is the eighth most abundant natural element and is commonly found in such minerals as magnesite, dolomite, olivine, serpentine, talc and asbestos. It is present in all natural waters and is a major contributor to water hardness. Water from areas rich in magnesium-containing rocks may contain magnesium in the range of 10 to 50 mg/L. The sulphates and chlorides of magnesium are very soluble, and water in contact with such deposits may contain several hundred milligrams of magnesium per litre. Industrial effluents may contain similarly high levels of magnesium. Calcium and magnesium may also be introduced to a water supply intentionally as part of water treatment. Where hardness is extremely low (such as soft water) in a water system, the addition of calcium or magnesium to the water may be needed to decrease corrosion effects downstream. Sources of hardness may include a limestone or pellet contactor, or direct injection of a solution or slurry consisting of calcium or magnesium hydroxides.
Due to the relationship between calcium, magnesium and hardness, practitioners often convert the concentrations of calcium and magnesium into their equivalents as CaCO3. This is the conventional unit of measure for hardness. The calcium concentration is multiplied by 2.5 (based on the molar ratio) to convert it to a unit of CaCO3 mg/L. Similarly, the magnesium concentration is multiplied by 4.1 (based on the molar ratio) to provide a result in mg/L as CaCO3.
The degree of hardness of drinking water may be classified in terms of its concentration expressed in mg/L as CaCO3 as soft, medium hard (or moderately hard), hard, and very hard. Different ranges to characterize these classifications are encountered in the literature (Table 2).
| Extremely soft (mg/L) | Soft (mg/L) | Medium hard or Moderately hard (mg/L) | Hard (mg/L) | Very hard (mg/L) | Reference |
|---|---|---|---|---|---|
| 0–50 | 50–100 | 100–150 | 150–300 | > 300 | Davis (2010) |
| N/A | 0–75 | 75–150 | 150–300 | > 300 | AWWA and ASCE (2005) |
| N/A | 0 to < 50 | 50 to < 100 | 100 to < 150 | > 150 | Crittenden et al. (2012) |
| N/A | 0 to < 60 | 60 to < 120 | 120 < 180 | > 180 | As cited in Crittenden et al. (2012) |
| N/A | 0–60 | 61–120 | 121–180 | > 180 | USGS (2018) |
| N/A | N/A | 60–120 | 120–180 | > 180 | Droste (2019) |
| N/A – not applicable. | |||||
Public acceptance of hardness varies considerably according to the local conditions, individual tolerance, pH and temperature of the water; consumers may get used to higher levels of hardness in their water. Water supplies with a hardness greater than 200 mg/L are considered poor but have been tolerated by consumers; those in excess of 500 mg/L are unacceptable for many domestic purposes and may require softening. The palatability of the water also depends on the ionic makeup of the water being consumed.
Water softening by sodium ion exchange (IX) may introduce undesirably high levels of sodium into drinking water. Generally, children under 8 years of age should not drink water containing sodium that may exceed the recommended upper limit of 1.5–1.9 g of sodium/day (IOM, 2005). Similarly, softening by potassium IX may introduce undesirable levels of potassium which may adversely impact certain segments of the population (Health Canada, 2008). In both cases, a proportion of the water most frequently consumed (such as from the kitchen tap) should bypass the softener altogether to avoid excessive sodium or potassium intake.
The aesthetic concerns for water hardness come from the tendency of hardness ions to precipitate out of solution (primarily as hydroxide and carbonate salts) and form scale on the inside of hot water–bearing pipes and water heating appliances. This is generally related to pH and temperature. These two characteristics change the solubility of calcium and magnesium and may result in oversaturation of a solution, resulting in precipitation of scale. The precipitated hardness may lead to particles or turbidity that are visible to the naked eye. Depending on the primary chemical element responsible for contributing to hardness, consumers may also note discoloration of the water. The precipitation of calcium and magnesium scales are generally white in colour.
Although hardness is caused by multivalent cations, it is often discussed in terms of carbonate and non-carbonate hardness. Carbonate hardness refers to the amount of carbonates (CO32-) and bicarbonates (HCO3-) that can be precipitated out of solution with heating. This type of hardness is responsible for the scale that may be deposited in hot water pipes and kettles. Non-carbonate hardness is caused by the association of the hardness causing cations with sulphates (SO4-), chlorides (Cl-) and nitrates (NO3-), as well as the salts of calcium and magnesium such as calcium sulphate (CaSO4), calcium chloride (CaCl2), magnesium chloride (MgCl2) and magnesium sulphate (MgSO4) (AWWA, 2016). Non-carbonate hardness is more readily kept in solution but still participates in the inhibition of soaping functions (Crittenden et al., 2012).
Drinking water systems can determine the amount of CaCO3 that will precipitate calcium using the calcium carbonate precipitation potential (CCPP) to predict the potential for scaling (Schock and Lytle, 2011; Tang et al., 2021). It can be calculated by a variety of computer programs or spreadsheet-based applications (RTW, 2008; AWWA, 2017; APHA et al., 2018).
In areas with hard water, household pipes can become clogged with scale (Coleman, 1976). Hard waters can also cause incrustations on kitchen utensils and increase soap consumption. Hard water is thus both a nuisance and an economic burden to the consumer.
Hard water is generally less corrosive than soft water (Schock, 1999). It has been suggested that a hardness level of 80 to 100 mg/L as CaCO3 provides an acceptable balance between corrosion and incrustation (Bean, 1968). Other taste thresholds for minor hardness constituents are discussed in the drinking water guidelines for manganese and iron (Health Canada, 2019, 2024b).
Alkalinity serves to control the buffer intensity of most water systems through bicarbonate and carbonate ions. Alkalinity and hardness are related through common ions formed in aquatic systems. Since the carbonate fraction of hardness (expressed as CaCO3 equivalents) is chemically equivalent to the bicarbonates of alkalinity present in water, it is also expressed in milligrams of CaCO3 per litre (mg/L as CaCO3). As the alkalinity of most Canadian surface waters is due to the presence of carbonates and bicarbonates, their alkalinity is similar to their hardness (Thomas, 1953). Additional information on alkalinity can be found in the pH guideline technical document (Health Canada, 2015).
Hardness can also be measured as grain per gallon, where a grain per gallon of hardness is equivalent to 17.1 mg CaCO3/L of hardness (Appendix E).
1.1.2 Chloride and sulphate
Chloride is widely distributed in nature, generally as sodium (NaCl) and potassium (KCl) salts; it constitutes approximately 0.05% of the lithosphere. By far the greatest amount of chloride found in the environment is in the oceans. Underground salt deposits have been found in most Canadian provinces. Bedded deposits occur in southwestern Ontario, Saskatchewan and Alberta; dome deposits are found in Nova Scotia, New Brunswick, Ontario, Manitoba, Saskatchewan and Alberta.
Chapra et al. (2012) describe the long-term trends of major ions in the Great Lakes system. They note that minimum chloride concentrations in Lake Ontario and Lake Erie were reached in approximately 1995 and 1985, respectively, with a slow rise in subsequent years. The Geological Survey of Canada (2014) noted that chloride levels have been found to increase in urban municipal wells and that the shallow groundwater near highways in Toronto has been found to have chloride levels as high as 14 000 mg/L due to the use of road salt during winter. The upper Great Lakes have all shown steady increases in chloride concentrations since the 1960s. The trend towards higher chloride levels has been noted across North America (Kaushal et al., 2018, 2021).
Sulphate occurs naturally in numerous minerals, including barite (BaSO4), epsomite (MgSO4 ·7H2O) and gypsum (CaSO4·2H2O). Sulphates are discharged into the aquatic environment in wastes from industries that use sulphates and sulphuric acid, such as mining and smelting operations, kraft pulp and paper mills, textile mills and tanneries. Aluminum sulphate (alum) is used as a coagulant in the treatment of drinking water, and copper sulphate has been used for the control of blue-green algae/cyanobacteria in both raw water and public water supplies in the United States. Sulphate concentrations are slowly decreasing in Lake Erie and Lake Ontario as a result of reduced impacts from acid rain caused by industrial activities. In the other Great Lakes – Lakes Michigan, Huron and Superior – concentrations of sulphate have remained stable over the past 50 years (Chapra et al., 2012).
Atmospheric sulphur dioxide (SO2), formed by the combustion of fossil fuels and by the metallurgical roasting process, may also contribute to the sulphate content of surface waters. Sulphates or sulphuric acid products are also used in the manufacture of numerous chemicals, dyes, glass, paper, soaps, textiles, fungicides, insecticides, astringents and emetics. They are also used in the mining, pulping, metal and plating industries, in sewage treatment and in leather processing.
| Property | Chloride | Interpretation | Sulphate | Interpretation |
|---|---|---|---|---|
| Chemical Abstracts Service Registry Number (CAS RN) | 16887-00-6 | Not applicable | 14808-79-8 | Not applicable |
| Molecular formula | Cl- | Not applicable | SO4-2 | Not applicable |
| Molecular Weight (g/mol) | 35.45 | Not applicable | 96.064 | Not applicable |
| Melting point | 101°C | Not applicable | Not available | Not applicable |
| Boiling Point | Not available | Not applicable | Not available | Not applicable |
| Density at room temp | Not available | Not applicable | Not available | Not applicable |
| Solubility | 6.3 mg/mL at 25°C | High solubility | Not available | Not applicable |
Studies have shown that both chloride and sulphate have an impact on corrosion in the distribution system, especially with metallic pipe and components. The chloride-to-sulphate mass ratio (CSMR) is used as an indicator of galvanic corrosion potential, particularly for lead. Dudi and Edwards (2004) conclusively demonstrated that a chloride-to-sulphate mass ratio greater than 0.58 increased lead leaching from brass due to galvanic connections. The type of coagulant used can have an impact on the distributed water CSMR (Edwards et al., 2007; Renner, 2006). Further information on corrosion control is available (Health Canada, 2025). The Larson Index (the ratio of the sum of chloride and sulphate to bicarbonate) is also important, with a higher ratio indicating water that is more corrosive to iron (Larson and Skold, 1958). Sulphate has also been identified as a nutrient that has a role in microbial growth, either in serving as a fuel for microorganisms or by consuming disinfectant residuals in the distribution system. For further information, refer to the Guidance on Monitoring the Biological Stability of Drinking Water in Distribution Systems (Health Canada, 2022a).
Chlorides and sulphates can play a role in water hardness, where they may contribute to the stability of non-carbonate hardness. Non-carbonate hardness involves salts of calcium chloride, calcium sulphate, magnesium chloride or magnesium sulphate, which present as hardness when titrated with ethylene diaminetetra acetic acid (EDTA) but are not considered scale forming. They will not precipitate when heated but still cause reduced lathering of soap. Other minor contributions of non-carbonate hardness include chloride or sulphate salts of barium or strontium.
Higher concentrations of chloride are most often present in drinking water derived from groundwater sources. The presence of chloride in drinking water sources can be attributed to the dissolution of salt deposits, salting of highways to control ice and snow, effluents from chemical industries, oil well operations, sewage, irrigation drainage, refuse leachates, volcanic emanations, sea spray and seawater intrusion in coastal areas. Each of these sources may result in local contamination of surface water and groundwater. Chloride ions are highly mobile and are eventually transported into closed basins or to the oceans.
Sodium chloride is widely used in the production of industrial chemicals such as caustic soda (sodium hydroxide), chlorine, soda ash (sodium carbonate), sodium chlorite, sodium bicarbonate and sodium hypochlorite. Sodium chloride and, to a lesser extent, calcium chloride (CaCl2) are used for snow and ice control in Canada. Annual usage is estimated to be about 5 million tonnes of salt during winter months (Environment and Climate Change Canada, 2018). Much of this road-applied salt directly enters local surface water bodies during the spring melt (Pieper et al., 2018). However, some salt has been shown to accumulate in soils and subsurface formations. This leads to delayed release to the surrounding aquatic environment in subsequent seasons and causes elevated sodium and chloride levels throughout the year (Robinson et al., 2017). Elevated chloride levels increase the corrosivity of water to well and plumbing components and household appliances, and can mobilize heavy metals, as well as radionuclides, from natural geologic deposits and soils (Pieper et al., 2018; Lazur et al., 2020). Road salt contamination in drinking water is generally limited to wells near paved roads and areas with heavy applications and is affected by the topography of the area (Geological Survey of Canada, 2014). Annual usage of salt during winter months has increased from 3.6 million tonnes in 1984 (Prud'homme, 1985) to an estimated 5 million tonnes as of 2018 (Environment and Climate Change Canada, 2001, 2018).
Sulphate salts of sodium, potassium and magnesium are all soluble in water, whereas calcium and barium sulphates and the heavy metal sulphates are not. Sulphates occur naturally in numerous minerals, including barite (BaSO4), epsomite (MgSO4·7H2O) and gypsum (CaSO4·2H2O). The reversible interconversion of sulphate and sulphide in the natural environment is known as the "sulphur cycle." Sulphates are discharged into the aquatic environment in wastes from industries that use sulphates and sulphuric acid, such as mining and smelting operations, kraft pulp and paper mills, textile mills and tanneries.
The discharge of sodium chloride-based resin regeneration solutions may contribute chloride to the environment, as well as other contaminants that were removed from the water (such as barium or arsenic). Resources for homeowners using IX resins include a factsheet published with the goal of minimizing regeneration salt quantities (Missouri Department of Natural Resources, 2024).
1.1.3 Total dissolved solids (TDS)
TDS are a measure of all dissolved substances that are found in a water sample, including all ionic, molecular and colloidal matter. The primary ions contributing to TDS include calcium, magnesium, sodium, chloride and sulphate.
TDS in water supplies originate from natural sources, sewage, urban and agricultural runoff, and industrial wastewater (Droste, 1997). The concentration of TDS is influenced by the solubility of the soil and rock and the contact time, which can vary seasonally in some aquifers. In Canada, salts used for road de-icing can contribute significantly to the TDS loading of water supplies (Chapra et al., 2012).
Water containing less than 1 000 mg/L of TDS is considered freshwater while water with TDS levels between 1 000 mg/L and 10 000 mg/L is considered brackish water (Crittenden et al., 2012). Concentrations of TDS in water vary owing to different mineral solubility in geological regions. The concentration of TDS in water in contact with granite, siliceous sand, well-leached soil or other relatively insoluble materials may be below 30 mg/L.
TDS is usually associated with high concentrations of ions that increase the conductivity of water and may affect the formation of a protective film. When hardness is the main contributor to TDS, the water may be corrosive toward copper. When sulphate and chloride are the main anionic contributors to TDS, the water may be corrosive to iron-based materials (Schock, 1999). High TDS may also lead to scale deposits in distribution systems and home appliances (Van der Aa, 2003).
1.1.4 Hydrogen sulphide
Hydrogen sulphide (H2S) is a naturally occurring gas produced from the breakdown of organic matter in the absence of oxygen and may also be formed by the direct reduction of sulphate by sulphate-reducing bacteria. It is widely present in sediments and water, as well as in biological wastes.
It has been estimated that natural sources account for 60% to 90% of the hydrogen sulphide in the atmosphere globally (U.S. EPA, 1993a; Watts, 2000). Hydrogen sulphide is produced naturally through non-specific and anaerobic bacterial reduction of sulphates and sulphur-containing organic compounds, such as proteins and amino acids (Hill, 1973). It is found naturally in crude petroleum, natural gas, volcanic gases and hot springs, and is released primarily as a gas. Hydrogen sulphide is found naturally in a variety of environmental media, including anaerobic aquatic sediments and groundwater, owing primarily to the bacterial reduction of other forms of sulphur.
| Property | Hydrogen sulphide | Interpretation |
|---|---|---|
| Chemical Abstracts Service Registry Number (CAS RN) | 7783-06-4 | Not applicable |
| Molecular formula | H2S | Not applicable |
| Molecular weight (g/mol) | 35.45 | Not applicable |
| Melting point (°C) | -85.49 | Not applicable |
| Boiling point (°C) | -60.33 | Not applicable |
| Density at room temp | 1.5392 g/L at 0°C at 760 mm Hg; | Not applicable |
| Solubility | 3980 mg/L at 20°C | High solubility |
Hydrogen sulphide can be released as a result of agricultural activities or industrial processes. These include releases as a by-product from petroleum sector activities since natural gas and gases associated with crude oil contain hydrogen sulphide at levels varying from trace amounts to 70%–80% by volume (Pouliquen et al., 1989; Environment Canada, 2004a). Hydrogen sulphide can be generated during hydraulic fracturing (Kahrilas et al., 2015; Marriott et al., 2016). Other anthropogenic sources include liquid manure storage (Blunden and Aneja, 2008; Kim et al., 2008), kraft pulp and paper mills (Teschke et al., 1999; IPCS, 2003; ATSDR, 2006; Janssen et al., 2009), landfills (IPCS, 2003; ATSDR, 2006; Kim, 2006), decomposition of organic waste from wastewater treatment (Muezzinoglu, 2003) and other industrial processes such as metal refining (OMOE, 2007; NPRI, 2023). Releases to the environment are primarily in the form of emissions to ambient air, although sulphides (including hydrogen sulphide) may also be released to water under specific environmental conditions.
Hydrogen sulphide can accelerate corrosion by reacting with metal ions but this may not be evident for months. Hydrogen sulphide can react with iron, steel copper and galvanized piping to form black water, even when oxygen is absent (Schock and Lytle, 2011). Studies have shown that hydrogen sulphide plays a role in the degradation of concrete and asbestos-cement pipe in some water (LeRoy et al., 1996; Vollertsen et al., 2008; Correa et al., 2010; Radlinksi and Wolf, 2016).
1.2 Exposure
The concentrations of these parameters in raw groundwater are typically higher than in raw surface water. The concentrations of raw groundwater are presented in Tables 5 and 6 and the full tables for each parameter in different water types are presented in Appendix B.
In general, higher concentration of calcium, magnesium, hardness, chloride, sulphate and TDS were found in raw groundwater when compared with raw surface water. However, it should be noted that, in Saskatchewan, the raw surface water generally had a higher level than the raw groundwater of calcium, magnesium, hardness, sulphate and TDS. Private wells showed trends similar to those of raw water from public utilities. Fluctuations between treated water and distributed water were observed for several parameters.
The median values for calcium and chloride were generally below the health-based values (HBVs) determined for these substances of 300 mg/L and of 470 mg/L respectively (see section 3.0). However, in most provinces (British Columbia, Manitoba, Newfoundland, Nova Scotia, Ontario, Prince Edward Island, Quebec and Saskatchewan), the maximum value recorded for calcium exceeded the HBV. For chloride, the maximum value observed in each province exceeded the HBV. Limited monitoring data were provided for hydrogen sulphide.
| Provinces | Calcium (mg/L) |
Magnesium (mg/L) |
Hardness (mg/L as CaCO3) |
Chloride (mg/L) |
Sulphate (mg/L) |
TDS (mg/L) |
Sulphide (mg/L) |
|---|---|---|---|---|---|---|---|
| British Columbia | 59.1 | 13.2 | 215 | 6.3 | 29.7 | N/A | N/A |
| Manitoba | 73.7 | 40 | 368 | 18.4 | 60.4 | 521 | N/A |
| New Brunswick | 27.4 | 3.9 | 92 | 35.6 | 15 | 131 | 0.05 |
| Newfoundland | 27.5 | 6 | 96 | 25.5 | 8 | 181 | N/A |
| Nova Scotia | 33.8 | 5.8 | 120 | 30 | 13 | 202 | 0.05 |
| Ontario | 85.7 | 25.3 | 320 | 70.3 | 34 | 448 | 0.03 |
| Prince Edward Island | 36.6 | 12.5 | 159 | 18.5 | 7.8 | 208 | N/A |
| Saskatchewan | 128 | 50.5 | 532 | 13.2 | 320 | 1210 | N/A |
| N/A – not applicable; TDS – total dissolved solids. | |||||||
| Provinces | Calcium (mg/L) |
Magnesium (mg/L) |
Hardness (mg/L as CaCO3) |
Chloride (mg/L) |
Sulphate (mg/L) |
TDS (mg/L) |
Sulphide (mg/L) |
|---|---|---|---|---|---|---|---|
| British Columbia | 59.1 | 12.9 | 234.5 | 6.4 | 32.6 | N/A | N/A |
| Nova Scotia | 27 | 4 | 98 | 21 | 11 | 180 | N/A |
| Prince Edward Island | 32.5 | 13.1 | 136.4 | 14.8 | 6.4 | 214 | N/A |
| Quebec | 44.3 | 10.9 | 166 | 91.2 | 40.7 | 684 | 0.075 |
| N/A – not applicable; TDS – total dissolved solids. | |||||||
Health Canada has completed several targeted drinking water surveys that included measurements of these operational parameters (Appendix C; Health Canada, 2022b).
- Data from the 2009–2010 National Drinking Water Survey conducted by Health Canada can be found in Appendix C.1.
- In 2007, a survey targeting water plants using water sources with elevated bromide was conducted. In this survey, data on calcium, magnesium, hardness, chloride, sulphate and TDS were also collected and the results can be found in Appendix C.2.
- In 2012–2013, a targeted national survey of water treatment plants with source water containing high sodium and naturally present ammonium/chloramines was conducted. In this survey, data on calcium, magnesium, hardness, chloride, sulphate and TDS were also collected and the results can be found in Appendix C.3.
Hydrogeological mapping on the concentration of calcium, magnesium, hardness, chloride, sulphate and TDS in surface and groundwater are available (Department of Fisheries and the Environment, 1978a, 1978b, 1978c). A detailed overview of Canada's groundwater is available (Geological Survey of Canada, 2014).
2.0 Health Considerations
2.1 Calcium, magnesium and hardness
2.1.1 Essentiality
Magnesium and calcium, the two predominant cations that make up water hardness, are essential minerals and beneficial to human health in numerous ways (IOM, 1997, 2011; Silva et al., 2019). Other essential minerals that contribute to water hardness include copper, iron, manganese and zinc (Silva et al., 2019; Water Resources, 2019). Aluminum, barium, cadmium and lead are also part of hardness but are non-essential elements (Exley, 2013; Chellan and Sadler, 2015; Water Resources, 2019). Strontium is likely a non-essential trace element (Bain et al., 2009; Zhao et al., 2015).
Magnesium is a cofactor for more than 300 enzymatic reactions and plays an essential role in electrolytic homeostasis, for the synthesis of carbohydrates, lipids, nucleic acids and proteins, as well as for specific actions in various organs such as the neuromuscular or cardiovascular systems (Wacker and Parisi, 1968; Cowan, 2002; Romani, 2013; EFSA, 2015a).
Calcium plays an important role in the formation and resorption of bone, as well as in mediating vascular contraction and vasodilation, muscle function, nerve transmission, intracellular signalling, blood clotting and hormonal secretion (Campbell, 1990; Brown, 1991; Peacock, 2010; IOM, 2011; EFSA, 2015b).
If the dietary intake of calcium is insufficient to meet physiological requirements, calcium is resorbed from the skeleton to maintain blood concentrations within the range required for normal cellular and tissue functions. This may lead to rickets, osteomalacia, osteoporosis and increased risk of fractures (EFSA, 2015b). Inadequate intake of calcium has also been associated with increased risks of kidney stones, colorectal cancer, hypertension and stroke, coronary artery disease, insulin resistance and obesity (WHO, 2011).
Magnesium and calcium deficiency may be detrimental to human health, while increasing intake generally results in health benefits. Magnesium deficiency has been reported to be linked to an increased risk of cardiovascular disease, hypertension, diabetes, osteoporosis, cancers, and renal and gastrointestinal dysfunctions (Tucker et al., 1999; Anastassopoulou and Theophanides, 2002; Catling et al., 2008; Rude et al., 2009; Dong et al., 2011; Rodríguez-Morán et al., 2011; Kass et al., 2012; Del Gobbo et al., 2013; EFSA, 2015a; Zhang et al., 2016; Rapant et al., 2019). Hypocalcemia and hypokalemia may also occur, which can lead to neurological or cardiac symptoms when it is associated with marked hypomagnesemia (< 0.5 mmol/L) (EFSA, 2015a). Loss of appetite, fatigue, muscle spasm and weakness may be signs of magnesium deficiency (Bowman and Russell, 2006).
2.1.2 Beneficial effects
It has been suggested that consuming hard water is protective against osteoporosis, decreased cognitive function in the elderly, decreased birth weight, various cancers and diabetes mellitus (Burton and Comhill, 1977; Yang et al., 1997, 1998, 1999, 2000a; Rosborg and Kozisek, 2020). Higher magnesium and/or calcium intake has been reported to offer a protective effect against cardiovascular disease, stroke, pre-eclampsia in pregnant women, high blood pressure and metabolic syndrome (Melles and Kiss, 1992; Catling et al., 2008; Nie et al., 2013; Poursafa et al., 2014; Chen et al., 2015; Khan et al., 2015; Moore-Schiltz et al., 2015; Anderson et al., 2016; Hofmeyr et al., 2018; Cormick et al., 2022).
Increasing magnesium and calcium intake has also been suggested as protective against various cancers, including colorectal, prostate, breast, ovarian and liver (Yang et al., 2000a, 2000b; Kesse et al., 2006; Chen et al., 2010; Keum et al., 2014; Aune et al., 2015; Bonovas et al., 2016; Hidayat et al., 2016; Song et al., 2017; Wesselink et al., 2020; Zhong et al., 2020; Shah et al., 2021). Increasing calcium intake has a positive effect on bone health, increasing bone mineral density, reducing circulating parathyroid hormone levels and bone turnover markers, and reducing the risk of fractures (Guillemant et al., 2000; Meunier et al., 2005; Silk et al., 2015; Tai et al., 2015; Weaver et al., 2016; Liu et al., 2020).
2.1.3 Adverse effects
Hardness, magnesium and calcium have low potential for toxicity to humans through drinking water. Adverse effects associated with excess intake of magnesium, calcium and/or hardness at elevated levels are seldom reported (WHO, 2009, 2011; Cotruvo et al., 2017). No adverse effects have been associated with the ingestion of magnesium from food sources, while supplementation of magnesium in excess of the daily recommended allowance may lead to adverse symptoms such as osmotic diarrhea (IOM, 1997; WHO, 2009, 2011). Water with very high magnesium levels together with high sulphate (> 400 mg/L combined) may cause transient diarrhea (Rosborg and Kozisek, 2020). Water with very high levels of magnesium, together with high level of TDS, may increase the risk of renal and other types of stones and arthritis problems (Kozisek, 2020). Symptoms of excess magnesium may include change in mental status, diarrhea, loss of appetite, muscle weakness, difficulty breathing, low blood pressure and irregular heartbeat. However, adverse effects associated with magnesium intake are most likely due to excess magnesium from supplements and do not generally happen to people with normal kidney function (WHO 2009, 2011; Rosborg and Kozisek, 2020). Similarly, excess calcium intake from foods alone is difficult or impossible to achieve, and hypercalcemia is unlikely to occur with high intake of calcium from the diet alone due to a tightly regulated intestinal absorption mechanism, where excess calcium is excreted by the kidneys (WHO, 2009, 2011; IOM, 2011). Excess calcium intake and hypercalcemia may be caused by high-dose calcium supplements, especially when accompanied by vitamin D supplements, as these can increase calcium absorption (Aloia et al., 2014; EFSA, 2015b). Intake of calcium supplements above the Tolerable Upper Intake Level (UL) (1 000 to 3 000 mg/day dependent on the life stage) increases the risk of hypercalcemia, hypercalciuria, vascular and soft tissue calcification, kidney stones, prostate cancer, constipation and interactions with iron and zinc (IOM, 2011). Clinical symptoms of persistent hypercalcemia are fatigue, muscular weakness, anorexia, nausea, vomiting, constipation, tachycardic arrhythmia, vascular and soft tissue calcification, failure to thrive and weight loss (EFSA, 2015b). Hypercalcemia can cause renal insufficiency and vascular and soft tissue calcification, including calcinosis, leading to nephrocalcinosis and kidney stones (IOM, 2011). Dermal exposure to water with high hardness may exacerbate atopic dermatitis (McNally et al., 1998; Miyake et al., 2004; Perkin et al., 2016).
2.1.4 Genotoxicity and Carcinogenicity
The mutagenicity of magnesium and calcium was reported to be negligible either with or without S9 mix by Fujii et al. (2016), who completed the Ames test using 0.031 to 0.25 mol/L mg(II), 0.031 to 0.25 mol/L Ca(II) with Salmonella typhimurium TA100 as the bacterial strain. Sanders et al. (2015) used a comet assay to assess magnesium sulphate genotoxicity on pheochromocytoma (PC-12) cells developed from the rat adrenal medulla. A concentration-dependent increase of DNA damage was evident, with a damage percentage of 8.1% at the 5.01 µg/mL treatment. At 50.01 µg/mL, the percentage of DNA damage was 10.8%.
Ribeiro et al. (2004) investigated the genotoxic potential of calcium hydroxide by the comet assay using mouse lymphoma cells and human fibroblasts cells. The results showed that calcium hydroxide at 20 µg/mL to 80 µg/mL did not promote DNA damage in mammalian cells.
Magnesium appears to play a protective role at the early stages of carcinogenesis but contributes to the proliferation of existing tumours at the later stages (Anastassopoulou and Theophanides, 2002). This is because magnesium is required for cellular proliferation. In neoplastic cells, intracellular magnesium is increased (due to a decrease in binding affinity) and protein and DNA synthesis is promoted (Leidi et al., 2011). Parsons et al. (1974) reported that maintaining plasma-magnesium levels below 0.8 mg/100 mL in patients with existing tumours generally resulted in regression of the tumours.
In a study comprising 142 520 European adult men, a high intake of calcium from dairy products (but not from other foods) was positively associated with prostate cancer risk (Allen et al., 2008). This association with dairy calcium intake may be due to its high correlation with other aspects of dairy food, particularly protein (Allen et al., 2008).
2.2 Chloride and sulphate
2.2.1 Essentiality
Chloride and sulphate are essential for human health. Chloride contributes to gastric hydrochloric acid production, electrical activity in general (for example, muscular and myocardial activities), the maintenance of blood pressure and renal function, and the volume and electrolyte balance of body fluids (Kataoka, 2021). Chloride also plays a central role in oxygen transport, gas exchange and regulation of renin produced by the juxtaglomerular apparatus (McCallum et al., 2015; Kataoka, 2021). Dietary chloride deficiency is rare. Low intakes of chloride have been described in two breast-fed infants whose mothers' milk was deficient in chloride, in infants given chloride-deficient formula milks, and among children and adult patients provided with chloride-deficient liquid nutritional products (Asnes et al., 1982; Hill and Bowie, 1983; Rodriguez-Soriano et al., 1983; Kaleita, 1986; Miyahara et al., 2009). In infants, hypochloremia features include growth failure, lethargy, irritability, anorexia, gastrointestinal symptoms, weakness, hypokalemic metabolic alkalosis and hematuria (Gross et al., 1980).
Inorganic sulphate is required for the synthesis of 3'-phosphoadenosine-5'-phosphosulphate (PAPS). PAPS, also known as "active sulphate," is required for the biosynthesis of many essential sulphur-containing compounds in the body, including chondroitin sulphate, cerebroside sulphate, dermatan sulphate, heparin sulphate, tyrosine-o-sulphate, taurolithocholate sulphate (bile salt) and estrone 3-sulphate. There are hundreds of sulphur-containing compounds in the human body and the body synthesizes all of them, with the exception of the vitamins thiamin and biotin (IOM, 2005). Sulphate requirements are met when intakes meet recommended levels of sulphur amino acids since the major source of inorganic sulphate for humans is due to body protein turnover of the sulphur amino acids methionine and cysteine. Thus, a deficiency of sulphate is not found in humans consuming normal protein intakes with adequate sulphur amino acids (IOM, 2005). However, sulphate deficiency may decrease blood coagulation and blood vessel stability, and low intake from drinking water may contribute to constipation (Rosborg and Kozisek, 2020).
2.2.2 Beneficial effects
Observational studies showed an inverse association (protective effect) between serum chloride and all-cause mortality in hypertensive patients. A serum chloride concentration lower than 100 milliequivalents per litre (mEq/L) was associated with a higher risk of mortality (all-cause, cardiovascular and non-cardiovascular). A 1.5% reduction in all-cause mortality was observed for every 1 mEq/L increase in serum chloride (McCallum et al., 2013). However, the serum chloride concentration cannot be used as a marker for chloride intake, and no studies are available which investigate the association between chloride intake or urinary excretion and cardiovascular disease–related health outcomes (EFSA, 2019).
Sulphate in drinking water decreases the health risks correlated with consumption of heavy metals by acting as an antagonist (Watts, 1997).
2.2.3 Adverse effects
The major adverse effect of increased intake of chloride, as sodium chloride, is elevated blood pressure, which can lead to cardiovascular and renal disease (Luft et al., 1979; MacGregor et al., 1989; Johnson et al., 2001; Sacks et al., 2001; IOM, 2005; EFSA, 2019). Elevation of blood pressure has been shown to rely on the concomitant presence of both sodium and chloride. In normotensive and hypertensive subjects, sodium chloride caused a greater elevation of mean blood pressure than sodium combined with other anions (Kurtz et al., 1987; Shore et al., 1988; Kotchen and Kotchen, 1997; McCallum et al., 2015). On average, blood pressure rises progressively with increased sodium chloride intake (IOM, 2005). In normotensive individuals, significant increases in blood pressure were observed when receiving approximately 7 500–13 900 mg/day sodium chloride (Mascioli et al., 1991; Ganry et al., 1993). Individuals with hypertension, diabetes and chronic kidney disease, as well as older-age individuals and African Americans, tend to be more sensitive to the blood pressure–raising effects of sodium chloride (Tuck et al., 1990; Weinberger, 1993; Morimoto et al., 1997; Morris et al., 1999; Johnson et al., 2001; Vollmer et al., 2001; du Cailar et al., 2002; IOM, 2005). Genetic factors also influence the blood pressure response to sodium chloride (Hunt et al., 1999; Lifton et al., 2002; IOM, 2005). Although rare, acute toxicity may be caused by ingestion of 500–1 000 mg sodium chloride/kg bw (Expert Group on Vitamins and Minerals, 2003). Symptoms include vomiting, ulceration of the gastrointestinal tract, muscle weakness and renal damage, leading to dehydration, metabolic acidosis and severe peripheral and central neural effects. High sodium chloride intakes increase calcium excretion and may increase the risk of kidney stone formation (Castenmiller et al., 1985; McParland et al., 1989; Zarkadas et al., 1989; Sakhaee et al., 1993; Evans et al., 1997; Lietz et al., 1997; Expert Group on Vitamins and Minerals, 2003; Lin et al., 2003; IOM, 2005). However, there is no substantial evidence to suggest a relationship between excess sodium chloride intake and reduced bone mineral density effects (Expert Group on Vitamins and Minerals, 2003). Both sodium and chloride contribute to the worsening of exercise-induced asthma symptoms that are seen after consuming a normal or high sodium chloride diet (Mickleborough et al., 2001). Individuals on sodium-restricted diets or needing to limit their exposure to sodium should be aware that residential softening systems will increase the sodium concentration in the treated water. Appendix E contains information on the intake of sodium as a result of water softener use, by hardness level.
Ingestion of sulphate has been associated with osmotic diarrhea and ulcerative colitis. Osmotic diarrhea is usually short term but may be more severe in infants (Chien et al., 1968; Backer, 2000; IOM, 2005). The extent and nature of the laxative effect are dependent on the specific sulphate salt. Laxative effects are commonly experienced by people consuming drinking water containing sulphate in concentrations > 500 mg/L (Chien et al., 1968; Esteban et al., 1997; Heizer et al., 1997; U.S. EPA, 1999b, 2003a). Laxative effects may occur at lower concentrations when both magnesium and sulphate are present (> 400 mg/L combined) (Rosborg and Kozisek, 2020). Dehydration may also occur if fluid replacement is not maintained (Arnaud, 2003). Humans appear to develop a tolerance to water containing high sulphate concentrations (Schofield and Hsieh, 1983). Although the acclimation concentration and rate have not been determined, it generally occurs in adults within one to two weeks (U.S. EPA, 1999a, 2003a).
2.2.4 Genotoxicity and carcinogenicity
Epidemiological data have indicated that there is a positive association between excess sodium chloride intake and risk of gastric cancer (Expert Group on Vitamins and Minerals, 2003; Wang et al., 2009; D'Elia et al., 2014).
Potassium sulphate was not mutagenic at 0.83 mg/plate, 1.66 mg/plate, 3.33 mg/plate and 5.00 mg/plate on TA98 (with and without S9) and TA100 (with S9) strains of Salmonella typhimurium. However, potassium sulphate showed a weak mutagenic effect on the TA100 strain in the absence of S9 but not in a dose-dependent manner (Kayraldiz et al., 2006). Kasprzak et al. (1983) reported that nickel(II) sulphate was not toxic or carcinogenic two years after intramuscular injections of 20 µL doses of 0.2 M nickel(II) sulphate (4.4 µmol/rat) or sodium sulphate (used as a control) every other day for four weeks (rats were injected with 15 x 20 µL doses of 0.2 M nickel(II) sulphate or sodium sulphate). After reviewing toxicity data on sulphate food additives, the United States Environmental Protection Agency (U.S. EPA) Select Committee concluded that there was no evidence that sulphuric acid or ammonium, calcium, potassium and sodium sulphates presented a hazard to public health when they are used at current levels or levels that might reasonably be expected in the future (U.S. EPA, 2003a).
The International Agency for Research on Cancer (IARC) and the U.S. EPA have not reviewed the carcinogenicity of calcium or sulphate.
2.3 Total dissolved solids (TDS)
Recent data on health effects associated with ingestion of TDS in drinking water are scarce. Recent studies appear to focus on health effects correlated with hardness rather than TDS.
2.3.1 Essentiality
Many ions that make up TDS, such as magnesium, calcium, sodium, chloride and potassium, are essential minerals and consuming adequate levels of these ions is beneficial to human health in numerous ways. Regular consumption of distilled or demineralized water (that is, low TDS) for a few weeks or months can lead to deficiencies in calcium, magnesium and/or sodium, leading to extreme fatigue, malaise, nausea, headache, brittleness of nails and hair, pre-eclampsia, twitch, leg and abdominal cramps, metabolic acidosis, higher diuresis and cardiovascular disorders (Kozisek, 2005, 2020).
2.3.2 Beneficial effects
An older study showed a significant negative (protective) correlation between regions supplied with water with high TDS and mortality from cardiovascular diseases in adult men 45 to 64 years old (Schroeder, 1960). However, new data have shown this correlation is likely due to high magnesium or calcium content rather than high TDS (Catling et al., 2008; Del Gobbo et al., 2013; Khan et al., 2015). Other studies reported inverse relationships between TDS concentrations in drinking water and the incidence of cancer and arteriosclerotic heart disease (Schroeder, 1966; Burton and Comhill, 1977). Epidemiological data among Russian populations suggest that high-mineral drinking water may reduce the risk of hypertension, coronary heart disease, ulcers, chronic gastritis, goitre, pregnancy complications, cholecystitis, nephritis, slower physical development in children and complications in newborns and infants (Lutaĭ, 1992; Mudryi, 1999).
2.3.3 Adverse effects
High levels of TDS in water are generally not harmful to humans. However, while TDS is made up of numerous essential minerals that are beneficial to human health, many other potentially harmful ions may also be present. Although TDS may include the presence of salts that can cause adverse health effects (for example, arsenic, boron, cadmium, chromium, fluoride and nitrate), these are unlikely to make up a substantial fraction of TDS in drinking water sources.
High levels (> 1 000 mg/L) of TDS may cause some individuals to experience a laxative or constipation effect, and increase the risk of renal stones, arthritis problems, and eye and skin irritation (Kahlown et al., 2006; Hussain et al., 2014; Meride and Ayenew, 2016; Kozisek, 2020). Studies in Russia suggest that regular and long-term intake of extremely mineral-rich water (TDS > 1 000–2 000 mg/L) increases the risk of developing excretory system diseases (such as kidneys and urinary tract), gastrointestinal tract diseases, diseases affecting female reproductive functions, developmental problems in children, arthritis and calculi (Shtannikov and Obyedkova, 1984; Shtannikov et al., 1986; Lagutina et al., 1990; Muzalevskaya et al., 1993; Rylova, 2005). In Sri Lanka, serum creatinine levels (a clinical sign and symptom of chronic kidney disease of unknown etiology) were significantly and positively correlated with TDS content in the drinking water (range: 136.3–3 750 mg/L; mean: 687 mg/L) (Gobalarajah et al., 2020).
2.3.4 Genotoxicity and carcinogenicity
Since TDS is made up of numerous salts, it is not possible to conduct a meaningful evaluation of the genotoxicity or carcinogenicity of TDS. Instead, the genotoxic and carcinogenic potential correlated with specific salts must be assessed separately. The heavy metals arsenic, beryllium, cadmium and chromium(VI) are classified as carcinogens in humans by the IARC (IARC, 2018; Rahman et al., 2021), and may be present in TDS. Nitrate may also be present in TDS, and is classified as "probably carcinogenic to humans" (Group 2A) (IARC, 2018). The IARC has also reviewed the potential carcinogenicity of numerous sodium-, potassium- and sulphate-containing molecules (IARC, 2018). However, it is unlikely that these salts will make up a substantial fraction of TDS in drinking water sources.
2.4 Hydrogen sulphide
2.4.1 Biological Role
Hydrogen sulphide is not an essential element and is endogenously biosynthesized mainly by cystathionine β-lyase and the tandem enzymes cysteine aminotransferase and 3-mercaptopyruvate sulphurtransferase (Kashfi and Olson, 2013). A portion of endogenous hydrogen sulphide is also derived via non-enzymatic chemical reduction of reactive sulphur species (such as persulphides, thiosulphate and polysulphides) in the presence of reducing equivalents such as nicotinamide adenine dinucleotide phosphate (NADPH) and nicotinamide adenine dinucleotide (NADH) (Cao et al., 2019).
2.4.2 Adverse effects
No epidemiological data are available on the oral toxicity of hydrogen sulphide (WHO, 2003; ATSDR, 2016). However, alkali sulphides irritate mucous membranes and can cause nausea, vomiting and epigastric pain following ingestion (WHO, 2003). The oral dose of sodium sulphide that is fatal to humans has been estimated at 10–14 g (WHO, 1981).
When inhaled, hydrogen sulphide is acutely toxic to humans (Gosselin, 1984). Irritation of the eyes and respiratory tract can be observed at 15–30 mg/m3, and concentrations of 700–1 400 mg/m3 can cause unconsciousness and respiratory paralysis resulting in death (WHO, 1987). Hydrogen sulphide exposure levels that result in semi-consciousness or temporary unconsciousness (for example, 15–30 minutes) can cause persistent neurophysical, neurobehavioural, neurocognitive, respiratory and ophthalmologic deficits (Hagley and South, 1983; Tvedt et al., 1991a; Kilburn, 1993; Snyder et al., 1995; U.S. EPA 2003b). Prolonged unconsciousness can lead to respiratory failure, hypoxia and death (Milby, 1962; Wasch et al., 1989; Khan et al., 1990; Tvedt et al., 1991b; U.S. EPA, 2003b). Overexposure to hydrogen sulphide may lead to a variety of central nervous system transitory symptoms such as dizziness, nausea, headache and more long-acting effects such as abrupt physical collapse or "knockdown," all of which have been attributed to direct effects of hydrogen sulphide on the brain (Milby and Baselt, 1999a). Levels associated with "knockdown" and pulmonary edema have been estimated to be in the range of 500 to 1 000 ppm (695 to 1 390 mg/m3) and 250 to 500 ppm (348 to 695 mg/m3), respectively (Milby and Baselt, 1999a, 1999b; Reiffenstein et al., 1992).
2.4.3 Genotoxicity and carcinogenicity
Attene-Ramos et al. (2010) measured the genotoxicity of hydrogen sulphide using the comet assay in human intestinal epithelial cells (FHs 74 Int). Hydrogen sulphide was genotoxic in concentrations from 250 µM to 2 000 µM. Changes in gene expression were analyzed after exposure to a single genotoxic, but not cytotoxic, concentration of hydrogen sulphide (500 µM). Significant changes in gene expression were predominately observed after the four-hour exposure period as compared to the 30-minute exposure. Cultured human lung fibroblasts were treated with the hydrogen sulphide donor, sodium hydrosulphide (10–75 µM; 12–48 hours). Sodium hydrosulphide caused a concentration-dependent increase in micronuclei formation (indicating DNA damage) and cell cycle arrest (G1 phase) (Baskar et al., 2007).
Based on limited data, hydrogen sulphide has not been shown to cause cancer in humans (ATSDR, 2016). The U.S. EPA has determined that data for hydrogen sulphide are inadequate for carcinogenic assessment (U.S. EPA, 2003b). The IARC has not reviewed the carcinogenicity of hydrogen sulphide.
3.0 Derivation of the health-based values (HBVs)
An HBV represents a concentration of a chemical in drinking water that can be consumed over a lifetime without significant health risk. The HBV is developed using available epidemiological and/or animal toxicological information and may serve as the basis for establishing a maximum acceptable concentration (MAC) if required. A MAC is established for a chemical in drinking water if it meets all of the following criteria:
- exposure to the contaminant could lead to adverse health effects;
- the contaminant is frequently detected or could be expected to be found in a large number of drinking water supplies throughout Canada; and
- the contaminant is detected, or could be expected to be detected, at a level that is of possible health significance.
If a chemical in drinking water does not meet all these criteria, Health Canada in collaboration with the CDW may choose not to establish a MAC but instead provide health guidance for jurisdictions to use when interpreting monitoring data. This may include developing an HBV, such as the ones derived for calcium and for chloride.
3.1 Magnesium, calcium and hardness
3.1.1 Magnesium
The toxicological data on magnesium are insufficient to serve as the basis for developing an HBV due to lack of available data on excess magnesium level toxicity. The Institute of Medicine (IOM) derived a UL for magnesium of 2 500 mg/day for children older than 8 years, adolescents and adults (IOM, 1997). An HBV cannot be derived using the reported UL by IOM (1997) because the UL does not apply to magnesium naturally found in drinking water or in food. Magnesium, when ingested as a naturally occurring substance in drinking water or foods, has not been demonstrated to exert any adverse effects (IOM, 1997). However, adverse effects of excess magnesium intake have been observed with intakes from non-food sources such as various magnesium salts used for pharmacologic purposes, including osmotic laxatives. Ingestion of adequate levels of magnesium has a protective effect on human health while deficiencies can result in toxicologically adverse effects. Thus, no HBV is established for magnesium.
3.1.2 Calcium
The IOM (2011) derived ULs for calcium based on calcium excretion for younger age groups and kidney stone formation for older age groups. The established UL of 2 000 mg/day for adults older than 50 years old was selected as the most appropriate UL to derive an HBV for calcium, as it is the lowest UL for individuals 1+ years old. An HBV for calcium can be calculated as follows:
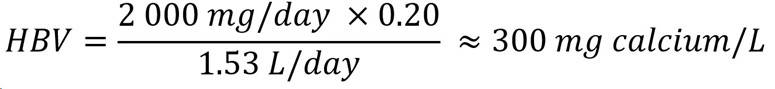
Figure 1 : Descriptive text
The health-based value for calcium is 300 mg/L. This is calculated by multiplying the tolerable upper intake level of 2 000 mg/day by the allocation factor of 0.20. The product is then divided by 1.53 L/day which is the daily volume of water consumed by an adult.
Where:
- 2 000 mg/day is the UL established for adults older than 50 years old and is the most conservative UL for individuals 1+ years old (IOM, 2011); there are no data indicating that infants are more sensitive to excess calcium compared to adults.
- 20 is the allocation factor for drinking water; it is used as a "floor value," since drinking water is not a major source of exposure to calcium, and there is evidence of the widespread presence of calcium in one of the other media (such as food) (Krishnan and Carrier, 2013).
- 53 L/day is the daily volume of water consumed by an adult (Health Canada, 2021).
The HBV is protective against health effects from exposure to calcium in drinking water over a lifetime Since levels of calcium in Canadian water are typically below the HBV, it was determined that there is no need to establish a MAC for this substance in drinking water. However, in the unlikely event that calcium is present in drinking water at high levels, the HBV is provided to help jurisdictions and the public understand the potential health effects of this substance. The responsible authority may decide to implement corrective actions using water treatment or other strategies to reduce exposure.
3.1.3 Hardness
Hardness is most often measured as the sum of magnesium and calcium present, expressed as equivalent CaCO3, which is the traditional unit of measurement for hardness (see section 1.1.1 Calcium, magnesium, hardness). Thus, an HBV for water hardness can be derived if both magnesium and calcium have HBVs. However, it is not possible to calculate a relevant HBV for magnesium since the UL for magnesium only applies to magnesium from non-food sources such as supplements (see section 3.1.1 Magnesium). Detrimental health effects caused by excess magnesium and/or calcium (i.e., the two principal ions that make up water hardness) are generally caused by consumption of supplements rather than food and drinking water. Thus, an HBV for hardness is not warranted.
3.2 Chloride and sulphate
3.2.1 Chloride
To protect against the risk of elevated blood pressure associated with sodium chloride intake, the IOM (2005) derived ULs for both sodium and chloride. An UL of 3 600 mg/day for individuals 13+ years old was established for chloride. An HBV can be derived using the reported UL by the IOM (2005) as follows:
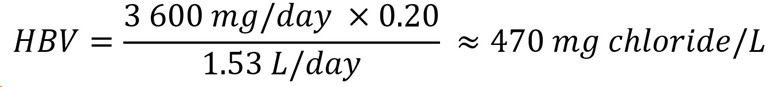
Figure 2 : Descriptive text
The health-based value for chloride is 470 mg/L. This is calculated by multiplying the tolerable upper intake level of 3 600 mg/day by the allocation factor of 0.20. The product is then divided by 1.53 L/day which is the daily volume of water consumed by an adult.
Where:
- 3 600 mg/day is the UL for chloride for individuals 13+ years old (IOM, 2005).
- 20 is the allocation factor for drinking water; it is used as a "floor value," since drinking water is not a major source of exposure to chloride, and there is evidence of the widespread presence of chloride in one of the other media (such as food) (Krishnan and Carrier, 2013).
- 53 L/day is the daily volume of water consumed by an adult (Health Canada, 2021).
The HBV is protective against health effects from exposure to chloride in drinking water over a lifetime Since levels of chloride in Canadian water are typically below the HBV, Health Canada and the CDW have determined that there is no need to establish a MAC for this substance in drinking water. However, in the unlikely event that chloride is present in drinking water at high levels, the HBV is provided to help jurisdictions and the public understand the potential health effects of this substance. The responsible authority may decide to implement corrective actions using water treatment or other strategies to reduce exposure.
For information on sodium in drinking water, please refer to the Guidelines for Canadian Drinking Water for Sodium (Health Canada, 1992).
3.2.2 Sulphate
Epidemiological data are insufficient to use as the basis for developing an HBV for sulphate. Although several studies have examined the effects of exposure of humans to sulphate in drinking water, none can be used to derive a dose-response characterization. Peterson (1951), Moore (1952) and Cass (1953) published long-term toxicological data showing a correlation between sulphate consumption and laxative effects. These data are insufficient because they were based on recall with little scientific weight (based on a YES/NO survey) and there were varying levels of magnesium and TDS in the water samples (U.S. EPA, 2003a). The majority of short-term toxicological studies did not find a significant association between sulphate consumption and diarrhea (Esteban et al., 1997; Heizer et al., 1997; U.S. EPA, 1999b).
Adverse effects correlated with ingestion of sulphate were noted in two animal studies. However, neither of these studies are suitable for deriving an HBV. Narotsky et al. (2012) noted dose-related frequency of diarrhea in rats consuming sodium sulphate in drinking water. It was not stated if the frequency of diarrhea was statistically significant between dosage groups, thus the requirements for benchmark dose modelling are not met. In addition, neither a no observed adverse effect level (NOAEL) nor lowest observed adverse effect level (LOAEL) can be calculated, since sodium sulphate dosages were given in g/L in drinking water, and body weight and water consumption changed throughout the experiment. Therefore, accurate dosages could not be obtained when converting the provided g/L sodium sulphate dosages to mg/kg bw per day for the HBV calculation. Gomez et al. (1995) noted diarrhea in piglets with ingestion of dietary sulphate ≥ 1 600 mg/L. This study is also not ideal to derive an HBV for reasons similar to Narotsky et al. (2012). The IOM has considered this study and concluded it was not suitable to derive an UL (IOM, 2005).
An HBV for sulphate is therefore not proposed. However, multiple international agencies have stated that catharsis/laxative effects and gastrointestinal irritation can occur when drinking water with sulphate levels ≥ 500 mg/L is ingested (NHMRC, NRMMC, 2011; WHO, 2017).
3.3 Total dissolved solids (TDS)
Since TDS is made up of numerous salts, it is not possible to derive a meaningful HBV for this parameter. The health effects correlated with specific salts that make up TDS must be assessed separately. Many salts that make up TDS that may cause adverse health effects (for example, boron, fluoride, nitrate, arsenic and chromium) already have separate established HBVs and are not expected to make up a substantial fraction of TDS in drinking water sources. Thus, an HBV for TDS is not warranted.
3.4 Hydrogen sulphide
The toxicological data on hydrogen sulphide are insufficient to use as the basis for developing an HBV because all the available studies, except one, are based on inhalation/air exposure of hydrogen sulphide and not oral exposure (Beauchamp et al., 1984; Arnold et al., 1985; Jäppinen and Tola, 1990; Haahtela et al., 1992; Kilburn and Warshaw, 1995; Richardson, 1995; Vanhoorne et al., 1995; Bates et al., 1997, 1998; Hessel et al., 1997; Legator et al., 2001; WHO, 2003; Lewis and Copley, 2015; ATSDR, 2016). Only one oral animal study was found in the literature (Wetterau et al., 1964). There was a 23% decrease in body weight gain at 6.7 mg/kg bw per day in pigs exposed for 104 days and diarrheic digestive disturbances in pigs exposed to 15 mg/kg bw per day for a few days. Interpretation of this study is limited because very few details are reported (for example, no information on methods used, strain used, number of animals studied or statistics) (ATSDR, 2016). Thus, an HBV cannot be derived using animal data. The IOM and Health Canada have not derived recommended daily intakes for sulphide. Thus, an HBV cannot be derived using a recommended daily intake such as the UL. An HBV is therefore not established for hydrogen sulphide.
4.0 Analytical considerations
Standardized methods, commercial online analyzers and portable test kits (Table 7) are available for the analysis of calcium, magnesium, hardness, chloride, sulphate, TDS and hydrogen sulphide in source and drinking water. Method detection limits (MDLs) are dependent on the sample matrix, instrumentation and selected operating conditions, and will vary between individual laboratories. These methods are subject to a variety of interferences which are outlined in the respective references or instructions.
To accurately measure the concentration of operational parameters using online analyzers and test kits, utilities should develop a quality assurance and quality control program such as those outlined in Standard Method (SM) 3020 (APHA et al., 2018). Periodic verification of results using an accredited laboratory is recommended. Drinking water utilities should check with the responsible drinking water authority to determine whether results from analyzers are acceptable for compliance reporting.
Drinking water utilities should discuss sampling requirements with the accredited laboratory conducting the analysis to ensure that quality control procedures are met and that method reporting limits are low enough to ensure accurate monitoring.
| Parameter | Standardized Method | Online Method | Test KitFootnote * |
|---|---|---|---|
| Calcium | Yes | Yes | Yes |
| Magnesium | Yes | Yes | Yes |
| Hardness | Yes | Yes | Yes |
| Sulphate | Yes | Yes | Yes |
| Chloride | Yes | Yes | Yes |
| TDS | Yes | Yes | Yes |
| Hydrogen sulphide | Yes | Yes | Yes |
|
|||
4.1 Standardized methods
4.1.1 Calcium, magnesium, hardness
Standardized methods using atomic absorption spectroscopy and titration can be used for measuring the concentration of calcium and magnesium (Tables 8 and 9). SM 2340B (APHA et al., 2018) is the preferred method for calculating total hardness as the sum of the results from the individual analysis of calcium and magnesium.
| Method (Reference) | Calcium | Magnesium | Interferences/Comments |
|---|---|---|---|
| EPA 200.5 Rev. 4.2 (U.S. EPA, 2003c) |
Yes | Yes |
|
| EPA 200.7 Rev. 4.4 (U.S. EPA, 1994) |
Yes | Yes |
|
| EPA NERL 215.1 (U.S. EPA, 1978a) |
Yes | N/A |
|
| EPA NERL 242.1 (U.S. EPA, 1978b) |
N/A | Yes |
|
| SM 3120B (APHA et al., 2018) |
Yes | Yes |
|
| SM 3111B (APHA et al., 2018) |
Yes | Yes |
|
| SM 3111D (APHA et al., 2018) |
Yes | N/A |
|
| USGS-NWQL: I-7152 (USGS, 1985a) |
Yes | N/A |
|
| USGS-NWQL: I-4447 (USGS, 1985b) |
N/A | Yes |
|
| N/A – Not applicable; TDS –Total dissolved solids. | |||
| Method (Reference) | Calcium | Hardness | Interferences/Comments |
|---|---|---|---|
| EPA NERL 130.2 (U.S. EPA, 1971a) |
N/A | Yes |
|
| EPA NERL 215.2 (U.S. EPA, 1978c) |
Yes | N/A |
|
| SM 3500-Ca B (APHA et al., 2018) |
Yes | N/A |
|
| SM 2340 Hardness C (APHA et al., 2018) |
N/A | Yes |
|
| USGS-NWQL: I-3338 (USGS, 1985c) |
N/A | Yes |
|
| EDTA – ethylene diaminetetra acetic acid; N/A – Not applicable. | |||
4.1.2 Chloride and sulphate
The standardized method for measuring chloride and sulphate uses ion chromatography (Table 10). Turbidimetric, gravimetric and potentiometric standardized methods are also available (Tables 11–13). SM 4500-Cl- and SM 4500-SO42- can be used to aid in the selection of method for the determination of chloride and sulphate respectively.
| Method (Reference) | Chloride | Sulphate | Interferences/Comments |
|---|---|---|---|
| U.S. EPA 300.1, Rev. 1.0 (U.S. EPA, 1999c) |
Yes | Yes |
|
| SM 4110 B (APHA et al., 2017) |
Yes | Yes |
|
| SM 4110 C (APHA et al., 2017) |
Yes | Yes |
|
| EPA-NERL: 375.4 (U.S. EPA, 1978d) |
N/A | Yes |
|
| N/A – Not applicable | |||
| Method (Reference) | Chloride | Sulphate | Interferences/Comments |
|---|---|---|---|
| SM 4500- SO42- E (APHA et al., 2017) |
N/A | Yes |
|
| SM 4500-Cl- B (APHA et al., 2017) |
Yes | N/A |
|
| SM 4500-Cl- C (APHA et al., 2017) |
Yes | N/A |
|
| N/A – Not applicable. | |||
| Method (Reference) | Sulphate | Interferences/Comments |
|---|---|---|
| SM 4500-SO42- C (APHA et al., 2017) |
Yes |
|
| SM 4500- SO42- D (APHA et al., 2017) |
Yes |
|
| Method (Reference) | Chloride | Interferences/Comments |
|---|---|---|
| SM 4500-Cl- D (APHA et al., 2017) |
Yes |
|
4.1.3 Total Dissolved Solids (TDS)
Standardized methods are available for measuring TDS. SM 2540 B-D (APHA et al., 2020) is suitable for TDS concentrations ranging from 2.5 to 200 mg/L. The major ionic constituents of TDS (sodium, calcium, magnesium, chlorides, sulphates, etc.) can also be measured individually and summed to produce an estimate of overall TDS using SM 2510 A 3020 (APHA et al., 2017).
Alternatively, SM 2510 (APHA et al., 2017) and Hach Method 8160 (Hach Co., 2021) considers conductivity as a surrogate measure of TDS that may be used for rapid quantification and process monitoring capabilities. This method uses a conductivity probe and should only be used if a calibration curve has previously established the correction factor for the specific water type.
4.1.4 Hydrogen sulphide
There are four categories of sulphide in water (total sulphides, dissolved sulphides, acid-volatile sulphide or un-ionized hydrogen sulphide) that can be measured (Table 14 Figure 4500-S2-:1) found in Standard Methods (APHA et al., 2017). This table can be used to aid in the selection of methods for the determination of sulphide under various conditions. SM 4500-S2- H provides guidance calculating un-ionized hydrogen sulphide (APHA et al., 2017).
| Method (References) | Methodology | Interferences/Comments |
|---|---|---|
| SM 4500-S2- D (APHA et al., 2017) |
Colorimetric |
|
| SM 4500-S2- E (APHA et al., 2017) |
Gas Dialysis with Methylene Blue |
|
| SM 4500-S2- I (APHA et al., 2017) |
Distillation with Methylene Blue |
|
| SM 4500-S2- F (APHA et al., 2017) |
Iodometric |
|
| SM 4500-S2- G (APHA et al., 2017) |
Ion-selective electrode |
|
| USGS-NWQL: I-3840 (USGS, 1985d) |
Iodometric titration |
|
4.1.4.1 Sample preservation and preparation for hydrogen sulphide
Ballinger and Lloyd (1981) noted that gentle shaking of the sample for 10 seconds resulted in the loss of 15% of hydrogen sulphide from the solution. Therefore, it is important that samples are collected with minimum agitation and aeration to minimize loss of hydrogen sulphide from solution, and to ensure the analytical results reflect the hydrogen sulphide concentration at the time and point of sample collection.
Sample preservation considerations for the analysis of hydrogen sulphide in drinking water can be found in the references listed in Table 14. SM 4500-S2- C provides guidance on sample pretreatment to remove interfering substances (such as reducing agents) for the methylene blue and iodometric methods (APHA et al., 2017). Zinc acetate is commonly used for preserving the water sample at the time of collection (Goodwin et al., 1998; APHA et al., 2017). It is important to ensure that sample preservation follows the sampling handling procedure stated in the selected method.
4.2 Online analyzers and portable test kits
Commercial online analyzers and/or portable test kits are available for quantifying operational parameters. Online analyzers can be used to obtain a rapid or continuous (online units only) indication of changes in concentrations of the parameter of interest. Generally, portable test kits are used for routine monitoring of treatment facilities and distribution systems where rapid results are favoured over analytical accuracy.
4.2.1 Calcium, magnesium and hardness
Commercial online and portable analyzers based on conductivity, electrochemical and optical sensing principles are available for quantifying hardness. For monitoring of softening processes, the use of continuous online methods may be appropriate. Semi-batch colorimetric titrations with EDTA can provide process control signals for optimized control of softening processes. Kruse (2018) noted that conductivity methods to measure hardness have a limitation as there are minerals that do not contribute to hardness but contribute to conductivity.
Portable (field) test kits (Table 15) for the analysis of calcium, magnesium and total hardness are also available and suitable for routine monitoring of treatment facilities and distribution systems where rapid results are favoured over analytical accuracy. Calcium, magnesium and hardness are all expected to remain stable throughout a water distribution network. However, field testing may be required for remote locations and to verify the stability of the concentrations throughout the distribution system. Several manufacturers provide colorimetric test kits that can be read with either a visual colour comparator or a portable spectrophotometer.
| Method (Reference) | Calcium | Magnesium | Total Hardness | Interferences/Comments |
|---|---|---|---|---|
| Hach 8204- Titration with EDTA (Hach, 2019) |
Yes | N/A | N/A |
|
| Hach 8030- Calmagite colorimetric (Hach, 2015a) |
Yes | Yes | N/A |
|
| Hach 8213- Titration with EDTA (Hach, 2015b) |
N/A | N/A | Yes |
|
| EDTA – ethylene diaminetetra acetic acid; N/A – Not applicable. | ||||
4.2.2 Chloride and sulphate
Online and portable analyzers are available for quantifying sulphate. Some of these analyzers are based on the colorimetric method (Table 16) titration systems using solid state lead/sulphate-ion-selective electrode. A variety of test kits are available commercially for the detection of chloride and sulphate in drinking water based on mercuric nitrate and turbidimetric methods, respectively.
| Method (Reference) | Sulphate | Interferences/Comments |
|---|---|---|
| EPA 375.2, Rev. 2.0 (U.S. EPA, 1993b) |
Yes |
|
| EPA-NERL: 375.1 (U.S. EPA, 1971b) |
Yes |
|
| SM 4500-SO42- F (APHA et al., 2017) |
Yes |
|
4.2.3 Total dissolved solids (TDS)
Commercial online and portable probes based on conductivity are also available for providing quick measurements of TDS in water. Depending on the manufacturer and/or calibration, these portable probes can typically measure TDS levels up to 1 g/L and some can measure as high as 500 g/L.
4.2.4 Hydrogen sulphide
A variety of test kits are available commercially for the detection of hydrogen sulphide in drinking water. Test strips are based on a colorimetric method where the paper will develop a colour when exposed to hydrogen sulphide. This method typically provides a reading within a minute. However, the tests are not as precise as laboratory methods.
Test kits are available and are more precise than test strips. However, some of the test kits require a portable colorimeter or spectrophotometer to determine the hydrogen sulphide concentration. Real-time analyzers are also available to obtain rapid measurement of hydrogen sulphide concentration in drinking water. Pandey et al. (2012) completed a review of the sensor-based method commonly used for monitoring hydrogen sulphide, including comparing the general response time, limit of detection, common operating range and limitations of the different technologies. As with the laboratory methods, it is important that samples that will be tested with kits or strips are collected with minimum agitation and aeration to minimize loss of hydrogen sulphide from solution to ensure the analytical results reflect the hydrogen sulphide concentration at the time and point of sample collection.
5.0 Treatment considerations
5.1 Municipal-scale treatment
Treatment technologies are available for the reduction of all seven operational parameters at the municipal level. Treatment plants should balance aesthetic (visual, taste and odour) and health-based considerations and operational concerns (scaling in distribution and plumbing systems and household appliances, fouling of ultraviolet units, corrosion control) with proper removal and disinfection of microorganisms. Measures taken to reduce the parameters in this guideline (calcium, magnesium, hardness, chlorides, sulphates, TDS and hydrogen sulphide) should not compromise the disinfection process. It is also possible that treatment strategies that aim to reduce one parameter could potentially lead to unintended downstream effects.
The selection of an appropriate treatment technology for a specific water supply will depend on many factors, including the characteristics of the raw water supply, the concentration of the parameter and the operational conditions of the specific treatment method. For example, ionic strength of the source water may impact the efficacy of coagulants, and jar testing is necessary when using a coagulation process. These factors should be taken into consideration to ensure that the treatment technology selected is capable of reducing the parameters of interest in the drinking water. Since these parameters are considered to have both operational and aesthetic significance for drinking water, it is important to ensure that the consumers find the treated water acceptable for drinking or they may obtain water from unsafe alternative sources.
As these naturally occurring parameters exist in the environment together, the treatment technologies for these parameters are often grouped together in the scientific literature. As such, the reduction of calcium, magnesium, hardness, chloride, sulphate and TDS are discussed in a grouped approach in this guideline document. Due to the offensive odour of hydrogen sulphide, this parameter is often removed to the lowest possible concentration.
When introducing a new source water and implementing or modifying treatment processes, bench- or pilot-scale testing is necessary to ensure the water can be successfully treated and an optimal process design is established. The impact on the distribution system must also be considered.
5.1.1 Calcium, magnesium and hardness
Total hardness is generally not an aesthetic concern unless it is related to the taste threshold for calcium or magnesium. Treatment technologies available to reduce hardness level include traditional softening, IX and membrane filtration treatment.
Treatment plants and private consumers may choose not to treat these aesthetic concerns on the basis that they do not affect the safety of water. Elevated hardness in distribution systems can lead to scale formation or incrustation on pipe walls resulting in frictional pressure losses throughout the distribution system. Hardness-related pipe fouling can be mitigated by reducing hardness levels or by adjusting the pH to increase the solubility of the precipitating ions. Scale formation is particularly noticeable within premise plumbing or on plumbing fixtures as a white powdery or scaly deposit. Deposits within hot water tanks and other heating vessels can impede heat transfer resulting in lower energy efficiencies and premature failure (Hofman et al., 2006). Davis (2010) noted that magnesium levels in excess of approximate 40 mg/L as CaCO3 can form scales in hot water heaters.
In cases where water providers seek to reduce the impact of calcium and magnesium, they often aim to reduce hardness rather than designing for complete removal as soft water can lead to corrosion in drinking water distribution systems and in household plumbing. More information on corrosion control can be found in Health Canada's Guidance on Sampling and Mitigation Measures for Controlling Lead Corrosion (Health Canada, 2025). Hardness can be reduced through chemical precipitation (softening), IX or membrane filtration processes. The selection of removal technologies will depend on the type of hardness (carbonate or non-carbonate) that needs to be reduced.
While calcium and magnesium are often the most prevalent cations in solution, it is important to note the presence of sodium and potassium as they also contribute to the ionic strength of a solution. More information on potassium can be found in the Health Canada drinking water guideline document (Health Canada, 2008).
5.1.1.1 Lime softening
Softening refers to the removal of the multivalent ions that cause hardness. At the municipal scale, this is often accomplished through the chemical precipitation of hardness ions by the addition of hydrated lime (calcium hydroxide Ca(OH)2), soda ash (sodium carbonate Na2CO3) and/or caustic soda (sodium hydroxide NaOH). The lime softening process achieves removal of Ca and Mg, principally calcium and magnesium bicarbonate (Ca(HCO3)2 and Mg(HCO3)2), by raising the pH to precipitate the soluble Ca and Mg salts. Quicklime (calcium oxide CaO) is often used and is rehydrated by the addition of water to create a slurry of hydrated lime which is then used for softening (Davis, 2010). The extent of hardness reduction depends on the type of precipitation agent applied, the dosage, the temperature, the pH of a water system and the contact time achieved in the process. Chemical stoichiometry, solution of simultaneous equilibria equations, softening diagrams and appropriate testing (for example jar tests, pilot test) can be used for determining the appropriate precipitation agent and dosage (Benefield and Morgan, 1999; Davis, 2010; Crittenden et al., 2012). As softening raises the pH level of the water to above 10 and sometimes to as high as 11.5, the process can inactivate some microorganisms as many cannot survive above a pH of 10.5 (Logsdon et al., 1994; Benefield and Morgan, 1999, 2016).
For source water with high carbonate hardness and low magnesium hardness, single-stage lime softening is the simplest method. Hydrated lime is added to raise the pH of the water to approximately 9.5–10 to precipitate calcium-related hardness (Crittenden et al., 2012). The amount of CaCO3 and Mg(OH)2 precipitation increases with pH up to an equilibrium of 10.3 and 10.8, respectively. However, this pH may vary slightly due to the interactions of calcium and magnesium with other solutes in the water (Benefield and Morgan, 1999). In raising the pH, the bicarbonate ions are converted to non-soluble carbonate ions (Droste, 2019; Benefield and Morgan, 1999). When the source water contains high concentrations of calcium and magnesium, excess lime is used to raise the pH above 11.0 in order to precipitate the magnesium carbonate and magnesium hydroxide along with the calcium (Benefield and Morgan, 1999; Lawler and Kweon, 2003; Crittenden et al., 2012).
When there are significant levels of magnesium and/or non-carbonate hardness present in the source water, soda ash can be added in a subsequent stage to achieve the desired hardness reduction at a pH greater than 10.5 (Cadena et al., 1974; Crittenden et al., 2012). Caustic soda may also be used to precipitate calcium hardness when there is insufficient carbonate hardness to react with lime (Crittenden et al., 2012). This reaction produces less sludge but is generally more costly compared with the lime softening methods due to the wide availability of lime (Mercer et al., 2005; Davis, 2010).
Generally, the lowest hardness level that can be achieved with the lime softening for calcium is 30 mg/L as CaCO3 and for magnesium, 10 mg/L as CaCO3. This limitation is due to the solubility of CaCO3 and Mg(OH)2, the physical constraint of mixing and contact, and the lack of sufficient time for the reaction to go to completion (Davis, 2010). The theoretical limit for the reduction of hardness in water through the lime-soda ash softening processes is 13.5 mg/L as CaCO3 (Cadena et al., 1974).
If present, natural organic matter (NOM) may also precipitate and slow the precipitation of hardness (Mercer et al., 2005). Carbon dioxide (CO2) and carbonic acid need to be neutralized prior to raising the pH as they will compete to react with the lime before softening can occur (Benefield and Morgan, 1999; AWWA, 2016).
Where the pH has been increased above the CaCO3 saturation point, it is often necessary to recarbonate the water through the addition of CO2. This will stop the precipitation reaction, preventing CaCO3 deposition in the downstream of the treatment process and in the distribution system (Davis, 2010). The addition of CO2 results in the conversion of carbonate into bicarbonate alkalinity and lowers the bulk water pH to a more neutral range (Hill, 1924). Careful control of CO2 is important as it can react with excess lime to form CaCO3 (AWWA, 2016) and increase the hardness level. Post-chlorination (with chlorine gas) may be sufficient to reduce the pH to saturation pH without the need to recarbonate the water (Crittenden et al., 2012).
Contact times for lime-softening are highly dependent on temperature, with longer reaction times required under cold water conditions. Contact times may not be sufficient to achieve a chemical equilibrium state in the chemical mixing plants (Hoover and Langelier, 1938), but rather reach the point at which reactions slow down considerably. Downstream filters will need to be monitored for excess precipitation of hardness solids if the reactions are incomplete in the lime-softening stage of the treatment plant. The detailed effects of each of these conditions (for example contact time, pH, etc.) are discussed in several literature resources (Droste et al., 1997; Crittenden et al., 2012). The chemical equations and process flow diagrams of the softening processes described above are available in Droste et al., 1997; Benefield and Morgan, 1999; Davis, 2010; Crittenden et al., 2012; and AWWA, 2016.
In a study of integrated water treatment of softening and ultrafiltration, it was noted that the characteristics (for example, size, structure, surface charge) of the precipitates (for example, CaCO3, Mg(OH)2) formed during the softening process can influence hardness removal (Lawler and Kweon, 2003).
Randtke et al. (1982) found that, when lime is used for softening, it produces a low degree of supersaturation that results in the formation of few nuclei, which grow slowly in well-structured, dense calcite crystals, leading to high removal of Ca2+. When NaOH is used to initiate the process, it produces a large degree of supersaturation that leads to the rapid precipitation of many nuclei and small particles with a relatively high solubility, resulting in a higher residual calcium concentration. Soda-induced precipitates resulted in a lower amount of calcite. These properties affect the removal of hardness ions as they affect the settling velocity and chemical affinity for individual compounds (Lawler and Kweon, 2003). Manganese and iron are examples of other contaminants that can be removed by lime softening (Health Canada, 2019, 2024b). The softening process can reduce other parameters in the raw water concurrently. A list of inorganic parameters and their observed removal rate is available (Benefield and Morgan, 1999; Davis, 2010).
Lime softening lowers TDS through the removal of calcium and magnesium bicarbonates as CaCO3 and Mg(OH)2 solids, respectively. It is important to note that it will also remove alkalinity and may require additional treatment to address corrosion issues (Clifford, 1999).
5.1.1.2 Blending
Blending or split treatment is a more advanced form of precipitative softening, where the treatment train includes a secondary flow that bypasses the lime-softening basins and is recombined with the completely softened water downstream from the lime-softening basins (Davis, 2010; Crittenden et al., 2012). It is intended as a simple and economical process control that requires far less operator intervention than a traditional lime-softening plant (Cherry, 1955; Rossum, 1955; Black, 1966). The split stream design allows for reduced operational costs by only treating a portion of the water for hardness removal followed by blending with other water within the treatment plant (Cherry, 1955; Shuey, 1966; Davis, 2010). If the raw water contains high levels of other contaminants at levels that exceed applicable guidelines, particular attention should be paid to the water stream bypassing the lime-softening step to ensure it is also treated appropriately.
This process allows for greater removal of magnesium hardness as the basins can achieve oversaturation and precipitation of both calcium and magnesium in a single step (Zipf et al., 1981). Split treatment with excess lime can reduce magnesium to its solubility limit of 10 mg/L as CaCO3 (Benefield and Morgan, 1999). Soda ash may be added to the recombined stream to reduce non-carbonate hardness (Zipf et al., 1981). The recombined stream may need to be recarbonated through the addition of CO2 gas to achieve stable pH and water quality characteristics. Zipf et al. (1981) noted that recarbonation may not be needed for the lime-soda ash process and or if there is an adequate return of sludge with the recombined water. The authors also developed a methodology for understanding the equilibria relationships to determine the desired effluent concentrations and operating variables that may be useful for plant operators. Attention must be given to the water quality of a new source prior to making any changes (such as switching, blending and interconnecting) to an existing supply. For example, if the new water source is more corrosive, it may cause leaching of lead or copper in the distribution system. The water quality of the recombined stream should be analyzed to ensure it meets the desired levels.
5.1.1.3 Enhanced precipitative softening
Enhanced precipitative softening aims to reduce the levels of hardness as well as dissolved organic carbon (DOC), ultimately reducing the formation of disinfection by-products (DBPs) (U.S. EPA, 1999b; Carlson et al., 2000). Enhanced precipitative softening is typically used for source water with high concentrations of NOM as the precipitated Mg(OH)2 solids could effectively remove the NOM (Lawler and Kweon, 2003). The high lime dosage required for magnesium removal may lead to voluminous sludge and operational problems (Lawler and Kweon, 2003). Rantke et al. (1982) noted that although the DOC reduction had been observed in softening processes, it was unclear what the mechanisms were for this process. They also highlighted the differing effectiveness between softening processes using lime versus soda ash. The presence of multivalent cations (for example, Ca2+, Mg2+) may also cause charge neutralization of negatively charged NOM compounds. However, that is not thought to be the main mechanism of NOM removal in this precipitative softening process. Leentvaar and Rebhun (1982) suggested that much of the total organic carbon removal associated with CaCO3 precipitation was through sweep flocculation, while the removal of magnesium hydroxide was achieved through charge neutralization. Guidance is available on the implementation of enhanced coagulation and precipitative softening (U.S. EPA, 1999b).
Sludge resulting from the addition of lime and/or soda ash can be a major operational burden for treatment plants that apply chemical precipitation processes (AWWA, 1981). Water utilities should remove these solids on a regular basis, to avoid entrainment of fine particles in the finished water. It is also possible to reduce the quantity of sludge by partially substituting caustic soda in place of either lime or soda ash.
5.1.1.4 Filtration
While much of the precipitated solids will settle out in the treatment basins, all softening processes may require a filtration step to remove fine particulate matter. Rapid granular filters are traditionally included in lime-softening plants. However, they may be subject to fouling of the filter media if there is excess particulate carry-over from the lime-softening process. Similarly, membrane elements may also be fouled. In a study of integrated water treatment of softening and ultrafiltration by Lawler and Kweon (2003), it was noted that the degree of pretreatment (for example, extent of softening) will influence the degree of fouling and efficacy of the membrane filtration treatment. Due to the kinetics of the softening process, precipitation can continue during settling on the surface or in the pores of the membrane unless the precipitation is chemically stopped. Precipitation occurring on the surface or in the pores will cause the flux to decline. CCPP can be used to control precipitation on the filters. Targets for CCPP after recarbonation are normally less than 5 mg/L entering the filter.
5.1.1.5 IX Softening
IX is a physicochemical process in which there is an exchange of ions in the raw water with ions within the solid phase of a resin (AWWA and ASCE, 2005; Crittenden et al., 2012). As raw water ions displace ions on the resin, the capacity of the resin is gradually exhausted, resulting in contaminant breakthrough. Once the resin has reached its capacity, the resin must be regenerated to reverse the process. Exchange resins exhibit a degree of selectivity for various ions, depending on ion type and concentration in solution, and the type of resin selected (Davis, 2005; Crittenden et al., 2012). The process is governed by stoichiometric ratios and electroneutrality where the total surface charge of resin beads must be maintained at all times (Crittenden et al., 2012). An exchange resin is initially saturated with monovalent cations (usually sodium) (AWWA and ASCE, 2005).
Strong acid cation (SAC) exchange resins typically use a strong acid sulphonate group that allows for full dissociation of salts in a water stream (Davis, 2010; Crittenden et al., 2012). Full dissociation of salts allows for the reduction of both carbonate and non-carbonate hardness. This technology is appropriate for a wide pH range and can be regenerated using either a strong acid rinse solution or a sodium salt brine soak (AWWA and ASCE, 2005; Crittenden et al., 2012). Treatment plants using SAC resins in the sodium form should be aware that this process may introduce undesirable quantities of sodium in the treated water.
In a bench-scale study conducted in Texas using IX softening (exchange of sodium for Ca and Mg), the system was able to reduce the hardness level from 388 mg/L down to 7 mg/L, the calcium level from 88 mg/L to 1 mg/L and the magnesium level from 41 mg/L to 1 mg/L (AWWA, 2003). The softened water was then blended with other streams to produce a blended product as finished water. The researchers estimated approximately 2.6 tons of salt per million gallons of blended product was needed for the softening process. The sodium level increased from 110 mg/L in untreated water to 286 mg/L in the softened water.
IX may present challenges related to managing the potential environmental impact of spent regeneration solutions, which are usually NaCl-based. Measures to limit the impact of discharging these spent solutions into receiving environments may be needed. Mitigation measures to limit potential impacts should be considered, and are reviewed in Liu et al. (2021).
5.1.1.6 Membrane filtration
Membrane filtration technologies for hardness reduction in drinking water include reverse osmosis (RO) and nanofiltration (NF) (U.S. EPA, 1999; Odell, 2010). RO membranes can lower TDS and monovalent ions, while NF membranes are mainly used for the reduction of hardness (Ca2+, Mg2+) (Bergman et al,. 1995). RO treatment systems typically require prefiltration for particle removal and often include other pretreatment steps, such as the addition of anti-scaling agents and dechlorination. The membrane permeate has a low pH and is very corrosive due to the acid pretreatment preventing scaling (Davis, 2010). Lime softening followed by filtration and pH adjustment is an effective pretreatment to improve the performance of the RO membrane for enhanced reduction of mineral salt scaling from water sources. If the raw water contains high levels of other contaminants at levels that exceed applicable guidelines, particular attention should be paid to the water stream bypassing the membranes to ensure it is also treated appropriately.
Pretreatment is required to preserve membrane life because the presence of chlorine residuals, particulates, NOM and scale-forming ions (Ca2+, Ba2+, iron and silica) in the feed water can adversely affect the performance of RO membrane technologies. Site-specific testing is recommended to determine the design criteria, potential fouling and pretreatment needs when treatment plants consider RO membranes. As membrane filtration technologies such as NF and RO can remove a high concentration of bivalent cations (Ca2+ and Mg2+), the permeate is sometimes blended with the feed water to achieve acceptable levels of hardness.
Post-treatment for RO permeate (finished water) typically includes pH adjustment, addition of corrosion inhibitors and disinfection. RO concentrate disposal must also be considered in the design and operation of RO membrane plants. Inorganic scale formation from the precipitation of salts within the membrane module leads to permeate flux decline and shortening of the membrane life. Additional information on CaCO3 scale control can be found in AWWA and ASCE (2005).
In a pilot-scale study conducted in Texas using RO membranes (pressure at 130 psi, recovery at 81%), hardness was lowered from 388 mg/L down to 3 mg/L, calcium was reduced from 88 mg/L to < 1 mg/L and magnesium was lowered from 41 mg/L to < 1 mg/L (AWWA, 2003). The RO permeate (68%) was then blended with untreated groundwater (32%) to produce a blended water with hardness at 149 mg CaCO3 per litre, calcium at 34 mg Ca per litre, and magnesium at 16 mg per litre. The RO membrane filtration was also able to lower the TDS level from 702 mg/L down to 14 mg/L in the permeate. The permeate pH was considerably lower than the feed water as acid was added to the feed water to increase recovery. Remineralization with lime, CO2 and sodium bicarbonate is generally needed to reduce the corrosivity of water. Since low mineral content water can leave a bitter, stale, or unpalatable taste, remineralization may improve the aesthetic quality of the finished water (Vingerhoeds et al., 2016; Biyoune et al., 2017; Lesimple et al., 2020).
NF membrane filtration can achieve upwards of 98% rejection of magnesium sulphate.
NF membranes (molecular weight cut-off of 500 Daltons) have a much tighter pore size than other types of membranes such as microfiltration and ultrafiltration membranes, and reject many larger ions (Conlon and McClellan, 1989). In a pilot study of two commercial membranes using a mix of distilled water with groundwater, the NF membranes achieved a rejection rate between 79.5% and 98.4% for calcium, 83.3% and 99% for magnesium, and 37.8% and 83.4% for hardness (Nasr et al., 2013).
Mulford et al. (1999) completed an NF membrane filtration study at full and pilot scale in Florida with different treatment train set-ups. In this study, the full-scale plant was able to reduce hardness from 290–320 mg/L down to 28–46 mg/L, achieving > 84% reduction in hardness. In a full-scale study at two drinking water treatment plants in Florida, the NF membrane filtration was able to reduce hardness levels from around 250 mg/L down to the range of 10 to 20 mg/L; this water was blended with that from the lime softening process to produce finished water that met quality requirements (Bartels and Wilf, 2007). The authors found that the NF membrane greatly reduced total organic carbon in the finished water compared with lime softening. Tang et al. (2019) evaluated NF membranes for a full-scale water treatment plant in the Netherlands and found that NF membrane filtration was able to reduce hardness levels from 200 mg/L to 130 mg/L, noting that target hardness is achieved by blending non-softened bypass water with softened permeate water.
In a pilot-scale study conducted in Germany, Gorenflo et al. (2002) found that water with a high concentration of sulphate and potentially the complexation of Ca2+ with humic acids resulted in the NF membrane rejecting Ca2+ and Mg2+ at rates that were higher than the manufacturer's data. The rejection rate for calcium was > 74% and magnesium was > 86%, whereas the concentration of calcium was 114.7 mg/L, magnesium was 12.3 mg/L and sulphate was 99 mg/L in the raw water.
Several bench-scale studies have shown that NF membrane filtration is capable of reducing hardness. Van de Bruggen et al. (2001) studied four types of NF membranes and, depending on the membrane, the hardness level was reduced from 280 mg/L down to between 14 mg/L and 140 mg/L. The authors noted the NF membranes with higher rejection rates were due to pore size and charge effects and interactions of Ca2+ and Mg2+ with membranes that had a positive charge surface. Ghizellaoui et al. (2005) studied the impact of pressure on NF membranes for a very hard water (600 ppm) and found that higher pressure (4–16 bar) resulted in higher retention rates of calcium and bicarbonates (50% and 40%, respectively), while lower pressure (1 and 2 bar) resulted in lower retention rates (34% and 30%, respectively).
5.1.2 Chloride and sulphate
Chloride and sulphate ions are difficult to remove in most water treatment processes. High salt content source waters (such as brackish or marine-influenced aquifers) may require treatment to reduce customer complaints and to achieve a chemically stable water that will not be corrosive for distribution systems and internal building plumbing.
Reductions in chloride and sulphate concentrations can be achieved through anion exchange or RO. Both technologies also have waste streams (concentrated brine) that require further handling to mitigate environmental concerns (U.S. EPA, 1999b).
5.1.2.1 Membrane filtration
RO is capable of > 90% rejection of chlorides and > 69% of sulphates, depending on the membrane unit implemented (Biesheuvel et al., 2019). The specific ionic composition, pH and temperature of the influent stream can have an impact on the overall efficacy of a particular membrane unit. In some cases, a membrane will preferentially remove greater concentrations of the sulphate form of specific salts (sodium sulphate > sodium chloride) due to size differences between the ions of concern. Particular attention should be paid to the concentration of each parameter in the bypass water to ensure that the finished water concentration does not exceed the applicable guidelines.
NF units generally have poor rejection of small monovalent anions such as chlorides (as low as 7%) and are often better suited for the removal of larger multivalent anions such as sulphates (removal > 90%) as shown in a pilot study by Nasr et al. (2013).
A pilot-scale study of an ultrafiltration treatment plant in the Netherlands found that the sulphate concentration was reduced from 140 mg/L to 0.1 mg/L (Duranceau, 2001).
In an NF bench-scale study, Schaep et al. (1998) observed the influence of temperature on the rejection rate of chloride. The rejection was approximately 10% lower at 30 oC (approximately 60% as read from graph) compared with 10 oC (approximately 70%). This may be due to the temperature influencing the viscosity of the water, which impacts the flux. The same was not observed for calcium, magnesium or sulphate in the same study.
RO technology is used primarily to remove inorganic contaminants, and some treatment plants may choose to blend the treated water with raw water to achieve a non-corrosive product water. In many cases, the water produced by RO treatment units is considered corrosive towards distribution systems and household plumbing. Additional treatment may be required to remineralize water prior to distribution. In all cases, system operators must ensure that all microbial treatment requirements are being met prior to delivering water to consumers.
Inorganic scale formation (for example, silica, barium sulphate and CaCO3) remains a serious impediment to achieving high RO recovery and leads to permeate flux decline and shorter membrane life. Lime softening followed by filtration and pH adjustment is an effective pretreatment to improve the performance of RO for enhanced reduction of mineral salt scaling from water sources. Additional information on sulphate scale control can be found in AWWA and ASCE (2005).
5.1.2.2 Anion exchange
Although anion exchange technology is capable of reducing sulphate, most systems will increase the concentration of chlorides in the discharge. Thus, anion exchange is rarely implemented in municipal drinking water systems for this purpose. Some systems may practice anion exchange for the reduction of nitrate concentrations which will lead to increased chloride levels. Therefore, chloride levels should be monitored when using anion exchange to evaluate if they meet the HBV. Alternative regenerants for IX that do not contain chloride are also available but may increase the cost of operation.
5.1.2.3 Treatment chemicals
Many inorganic coagulants are chloride- or sulphate-based salts such as aluminum sulphate, ferric sulphate, polyaluminum sulphate, polyaluminum chloride or high-polyaluminum chloride sulphate coagulants (Wu et al., 2020). These coagulants all contribute soluble chlorides and sulphates and may contribute to a large portion of the total of the treated water for conventional coagulation and filtration plants. Direct filtration plants will see a more moderate impact from the chlorides and sulphates during associated coagulation. Disinfection with chlorine gas yields free chloride ions when the aqueous chlorine dissociates to form hydrochloric and hypochlorous acids, with the hydrochloric acid further dissociating into H+ and Cl-.
IX units will also lead to the addition of chlorides as they typically use sodium chloride or potassium chloride as regeneration brines for the IX. While the sodium and/or potassium ions are active in the regeneration exchange reactions, the chloride ions are simply discharged to the waste stream when regeneration is complete. This additional loading of chlorides on the environment is a concern both in local discharge to weeping fields or discharge to the sanitary sewers. Storage or concentration of this brine may be required in order to limit the downstream impacts on the environment. In all applications (including cleaning chemicals), it is important to ensure that all treatment chemical used are certified according to NSF/ANSI/CAN Standards 60: Drinking Water Treatment Chemicals – Health Effects (NSF/ANSI/CAN, 2024).
5.1.3 Total dissolved solids (TDS)
RO and NF membranes are the most commonly used and well established techniques for reducing TDS (AWWA and ASCE, 2005; Pushpalatha et al., 2022). Switching between water sources with significantly different TDS on a seasonal basis may be detectable for some consumers and cause undue concerns regarding water quality.
5.1.3.1 Membrane filtration
RO membranes can be used to reduce TDS and monovalent ions (Bergman et al., 1995; Pushpalatha et al., 2022). RO treatment systems typically require prefiltration for particle removal and often include other pretreatment steps, such as the addition of anti-scaling agents and dechlorination. Pre-treatment for hardness reduction may be required to prevent scaling on the membrane elements. Lime softening followed by filtration and pH adjustment is an effective pretreatment to improve the performance of RO for enhanced reduction of mineral salt scaling from water sources.
A review article on TDS removal in various water types and by a variety of technologies identified a removal efficacy of 90% for TDS by RO at a municipal groundwater system. The article stated an influent concentration of 1 100 ppm (mg/L) was reduced to a treated water concentration of 10-60 ppm (mg/L). Removal efficacy was dependent on factors such as water pH and temperature, membrane type and operating pressure (Pushpalatha et al., 2022). A U.S. EPA full-scale demonstration program evaluated various treatment technologies at small and semi-public systems, including RO. The systems were monitored over several years. Although the program was primarily focused on arsenic removal, the removal efficacy for other contaminants such as TDS was also evaluated. The long-term performance of one point-of-entry (POE) RO system and nine point-of-use (POU) RO units were evaluated at 2 sites for 8 or 12 months. The influent TDS concentrations ranged from 246-698 mg/L and an average concentration of 255 mg/L was achieved in the treated water, resulting in an average removal of 97% (Chen et al., 2020).
NF membranes were capable of reducing some dissolved ions by size exclusion in full-scale treatment plants in Florida, achieving a 33%–75% reduction of TDS (Bergman, 1995). Saitua et al. (2011) found 53% removal of TDS through a pilot-scale membrane filtration using a polyamide membrane treating water with an influent TDS concentration of 1 290 mg/L. TDS removal efficiency of 97.4% was realized by NF, where TDS concentration was reduced from 500 ppm to 13 ppm (Pushpalatha et al., 2022). In general, larger multivalent ions such as those that cause hardness will be removed through NF membrane filtration. However, monovalent ions will still pass through.
5.1.3.2 IX
TDS are often not significantly impacted by IX systems as this technology is designed to exchange hardness ions for non-hardness ions (Crittenden et al., 2012). Davis (2010) noted that strong base anion exchange is capable of reducing TDS when the concentration is below 500 mg/L and the sulphate concentration is < 50 mg/L. The removal of arsenic and TDS from groundwater was examined using alumina media by IX technology. The results in this article showed that the alumina media IX system achieved 90% TDS removal. However, they concluded that this system is less effective for higher TDS (> 500 ppm) or sulfate (> 150 ppm) concentrations. The formation of fouling at electrodes is one of the major disadvantages of this technology. However, for small-scale water treatment plants, it may be a viable solution due to the ease of operations and lower maintenance costs than other technologies (Pushpalatha et al., 2022).
5.1.4 Hydrogen sulphide
Hydrogen sulphide is predominantly an issue due to its offensive odour and low odour threshold. Treatment technologies able to remove hydrogen sulphide to ≤ 0.05 mg/L include oxidation, aeration and adsorption (Levine et al., 2004a; Crittenden et al., 2012; Lemley et al., 1999; Duranceau et al., 2010; Odell, 2010).
5.1.4.1 Oxidation
Oxidants for hydrogen sulphide control include chlorine, hydrogen peroxide (H2O2), potassium permanganate (KMnO4) and ozone (O3) (Thompson et al., 1995; Levine et al., 2004a; Crittenden et al., 2012). A summarized comparison of different oxidation and the dosage requirements is found in Table 17. A list of their advantages and disadvantages can be found in Duranceau et al. (2010).
| Oxidant | Oxidation reaction | Dose. (mg/mg H2S) |
|---|---|---|
| Chlorine | H2S + Cl2 → S0 +2HCl | 2.08 |
| H2S + 4H2O + 4Cl2 → H2SO4 + 8HCl | 8.33 | |
| Ferrate | 4H2S + 3HFeO4- + 7H+ → 3Fe+2 + S2O3-2 + 2S0 +9H2O | 2.66 |
| 16H2S + 20HFeO4- + 10H2O → 20Fe(OH)3 + 3H2S2 + SO3-2 + 3S2O3-2 + 3SO4-2 + 6OH- | 4.44 | |
| Hydrogen peroxide | H2S + H2O2 → S0 + 2H2O | 1.03 |
| HS- + 4H2O2 → SO4-2 + 4H2O + H+ (pH>8) | 4.11 | |
| Ozone | S-2 + 4O3 + 4H2O → SO4-2 + 4O2 | 5.64 |
| Potassium permanganate | 3H2S + 2KMnO4→3S0 + 2MnO2 + 2KOH + 2H2O | 3.09 |
| 3S-2 + 8KMnO4 + 4H2O→8MnO2 + 3SO4-2 + 8KOH | 12.39 |
Chemical requirements for complete sulphide oxidation depend on the solution pH and temperature. Generally, the oxidant dose increases with increasing pH (Cadena and Peters, 1988). Typically, a higher oxidant dose will form sulphate rather than elemental sulphur, resulting in increased turbidity (Cadena and Peters, 1988; Thompson et al., 1995; Levine et al., 2004a).
Crittenden et al. (2012) noted the potential formation of polysulphides when using oxidation to remove hydrogen sulphide. The formation of polysulphides occurs when the hydrogen sulphide concentration is > 1 mg/L and pH < 9. Conversion to sulphate requires an oxidant dose in excess of the stoichiometric requirements and a pH > 8. Polysulphides are difficult to remove, have unique taste and odour, and can complex with metals in distribution systems leading to the formation of black water.
Oxidation of hydrogen sulphide with hydrogen peroxide requires long contact times and large doses of oxidants. This subsequently results in the formation of not only sulphate but also colloidal sulphur, which can increase turbidity in the water (Duranceau et al., 2010; Thompson et al.,1995).
Levine et al. (2004a) conducted a pilot study using hydrogen peroxide coupled with filtration to oxidize groundwater from a wellfield in Florida. The pilot study showed that hydrogen peroxide resulted in > 60% removal at 6.5 minutes of contact time. The tandem use of oxidation catalyzed by ferric sulphate to coagulate the colloidal sulphur showed an increase in hydrogen sulphide removal to > 80% with a shorter contact time (< 6 minutes). The authors concluded that the use of hydrogen peroxide catalyzed by ferric sulphate coupled with filtration was capable of reducing hydrogen sulphide (removal data not provided) and produced low turbidity water.
Levine et al. (2004b) further conducted pilot-scale studies to compare inline hydrogen peroxide oxidation coupled with a two-stage upflow filtration system. Studies were conducted with and without the addition of a low dosage of ferric sulphate as catalyzer for the removal of hydrogen sulphide. The addition of ferric sulphate increased the removal of hydrogen sulphide from 20% to 40% (contact time of approximately 2 to 6 minutes) to over 80% (contact time of 2 minutes) and produced water with turbidity below 0.1 nephelometric turbidity unit (NTU), while lowering hydrogen sulphide and minimizing chlorine demand.
Continuous chlorination is a common method for oxidizing hydrogen sulphide using a dose of 2.0 mg/L for every 1.0 mg/L of hydrogen sulphide (Odell, 2010). In a field study by Lyn and Taylor (1992), chlorine oxidation resulted in the formation of colloidal sulphur at pH > 3.8 and that increasing pH resulted in increased turbidity. The authors also found that aerobic conditions at low pH favoured sulphate formation whereas anaerobic conditions at high pH favoured elemental sulphur formation. The use of chlorine for sulphide oxidation can result in the formation of DBPs such as trihalomethanes and haloacetic acids (Levine et al., 2004a, 2006; Thompson et al., 2010; Stefen et al., 2018).
Ortenberg et al. (2000) found that oxidizing hydrogen sulphide with chlorine was able to completely remove hydrogen sulphide but an objectionable taste was still present. They postulated that the oxidation of hydrogen sulphide with chlorine produced elemental sulphur and polysulphides (H2Sn), which in turn hydrolyzed back into hydrogen sulphide. Stefen et al. (2018) noted that a higher contact time is needed to increase the efficacy of chlorine. However, it can also result in the formation of trihalomethanes.
The use of chlorine dioxide to oxidize hydrogen sulphide resulted in the DBPs chlorite and chlorate, presumably due to the presence of inorganic constituents in the reduced form (Ortenberg et al., 2000). The removal of hydrogen sulphide using ozone was capable of producing sulphate and did not result in any trihalomethanes (Stefen et al., 2018).
Duranceau et al. (2010) noted that using oxygen can result in incomplete oxidation, creating colloidal sulphur and polysulphides. The authors also found that using ferrate as an oxidant reduced sulphide to below the MDL (< 0.3 mg/L). However, the turbidity level increased significantly after contact with this oxidant.
Hydrogen sulphide can be removed from water with potassium permanganate pretreated with sulphuric acid to reduce pH followed by a degasser (Willey et al., 1964). The kinetics for potassium permanganate are rapid with chemical equilibrium occurring 5 minutes after the addition of the oxidant (Cadena and Peters, 1988). The reaction produces floc particles of MnO2 and elemental sulphur, which can be removed by filtration (Duranceau et al., 2010; Edwards et al., 2011). Careful control of the potassium permanganate dose is necessary to prevent the generation of pink water due to excess manganese (Levine et al., 2004a). In addition, the manganese dioxide formed by the reaction can produce excess turbidity in the distribution system (Levine et al., 2004a).
Pilot-scale studies by Duranceau et al. (2010) used hypochlorite as an oxidant for Florida groundwater. The oxidant was used before a proprietary filtration process or a manganese green sand filter. The process was continuously regenerated with bleach to remove hydrogen sulphide from the groundwater. Preliminary results showed that the combination of these technologies was able to reduce sulphides from between 1.4 and 2.6 mg/L to below 1.0 mg/L (DL). Another pilot study conducted by Duranceau and Trupiano (2011) using Florida groundwater evaluated two different oxidized media filtration processes: NaOCl oxidation preceding a proprietary filtration, and NaOCl preceding MnO2 filtration. Both processes were able to reduce sulphide to below DL (< 0.1 mg/L) and produce finished water with turbidity levels < 1.0 NTU.
5.1.4.2 Aeration
Aeration brings water and air in close contact in order to remove dissolved gases (such as CO2) and oxidize dissolved metals such as iron, hydrogen sulphide and volatile organic compounds. Aeration is a common method for treating sulphides when the concentration is below 2.0 mg/L and when present in the gas phase (Lemley et al., 1999; Duranceau et al., 2010; Odell, 2010). Pre-oxidation is not recommended with aeration of hydrogen sulphide as it may produce sulphide, bisulphide or solid sulphur, which are not air-strippable and would subsequently need to be filtered from the treated water (Odell, 2010).
The pH of water plays a significant role in the form of hydrogen sulphide present. It is difficult to remove hydrogen sulphide in water with a pH > 7 since most of the hydrogen sulphide is present in the forms of HS- and H+ ions. Decreasing the pH of the water entering an aeration system can improve hydrogen sulphide removal efficacy and lower turbidity levels but may result in the formation of some DBPs and increase the copper corrosion rate (Thompson et al., 1995; Edwards et al., 2011). The removal of sulphides at a pH of 6 is roughly 80% and at a pH of 7 is only 70%. (Lemley et al., 1999; Odell, 2010). As water is aerated, CO2 is released, increasing the pH as hydrogen sulphide is converted to HS- thereby reducing the overall efficacy of the stripping process (Levine et al., 2004b; Munter and Vilu, 2008; Crittenden et al., 2012).
Cascade or tray aeration and volatilization in ground storage facilities are only partially effective for sulphide removal which is dependent on pH and atmospheric conditions (for example, more sulphides are removed on windy, warm days) (Duranceau et al., 2010). Montgomery (1985) reported using a cascade tray aerator on the top of a groundwater storage tank and found a removal of only 20% of hydrogen sulphide at ambient pH and temperature. The remaining sulphide was oxidized by chlorine to elemental sulphur in the storage tank. The author noted that the process produced high levels of turbidity and an offensive rotten egg odour.
Packed towers have higher stripping efficacies but CO2 is released faster than hydrogen sulphide. As a result, the pH changes as the water flows down the packing, impacting the removal efficacy (Duranceau et al., 2010). The use of carbonic acid for pH adjustment prior to packed tower aeration can be an effective pretreatment and can also aid in corrosion control (Duranceau et al., 2010).
Aeration technology is widely applied in Estonia through the contact of a thin descending film of water with air on the surface of wood, ceramic or plastic packing. Munter et al. (1999) noted that the sulphide concentration was reduced to 0.3 mg/L from concentrations ranging from an average value of 0.003–0.5 mg/L. A full-scale study on the western coast of Estonia involving groundwater treated by aeration followed by filtration with manganese greensand was able to reduce hydrogen sulphide from a range of 0.64 to 3.4 mg/L down to 0.04 to 0.89 mg/L.
A full-scale trickling filter was able to remove hydrogen sulphide from well water in Greece (Terkerlekopoulou et al., 2010). Water from the two wells was stored in an elevated water tower at 15 m above ground. The water cascaded into the homogenization tank and water aeration occurred through the filter, leading to an elimination of hydrogen sulphide (raw water concentration between 1 and 1.3 mg/L) at the filter outlet (effluent data were not provided).
5.1.4.3 IX
Hydrogen sulphide can be removed using anion-exchange resins since a significant amount of hydrogen sulphide present in water is in ionized form. The effectiveness of the system depends on the resin selected and the concentration of competing anions (sulphate, total organic carbon, and alkalinity) as well as the pH of the water. Typically, chloride resin is used for hydrogen sulphide removal (Lemley et al., 1999; Odell, 2010). The resin can also foul because of the growth of sulphur-related bacteria, which can affect removal efficacy (Duranceau et al., 2010).
Packed-bed anion exchange was capable of removing an influent concentration of 0.82–3.22 mg/L sulphide during a pilot study in Florida (Levine et al., 2006). The removal and effluent data were not provided. The authors also examined the biological enhancements of the anion exchange as the low concentration of dissolved oxygen (< 2 mg/L) promoted the growth of sulphur oxidizing bacteria at the upper surface of the resin, improving the removal of hydrogen sulphide by converting it to either elemental sulphur or sulphate. The by-products of the biological sulphur oxidation, sulphate and elemental sulphur, were removed through the anion exchange. The authors noted the benefit that coupling biological sulphur oxidation with anion exchange increased the exchange capacity, resulting in about two- to three-fold longer operating cycles, thus decreasing the frequency of regenerating the column.
In a pilot plant study by Vidović et al. (2010) using IX followed by an adsorption column, it was shown that up to 60% of hydrogen sulphide in an acid medium (pH 6.6–7.2) can be removed by an IX column and that there is no removal in alkaline medium. The hydrogen sulphide that was not fully removed by the IX was subsequently removed by adsorption.
5.1.4.4 Adsorptive media
Granular activated carbon (GAC) can generally remove hydrogen sulphide to concentrations below 0.3 mg/L (Lemley, 1999; Odell, 2010). Catalytic carbon, a type of activated carbon with a modified surface, has the ability to promote or catalyze chemical reactions. Catalytic carbon can adsorb sulphides onto the carbon surface and, in the presence of dissolved oxygen, it oxidizes the sulphides into elemental sulphur (S8) and sulphate (Megonnell and Spotts, 1994). A minimum dissolved oxygen of 4.0 mg/L is necessary for complete oxidation of hydrogen sulphide into elemental sulphur, which can then be filtered (Saunders and Urbans, 1995; Lemley et al., 1999; Odell, 2010).
A series of pilot-scale studies were performed by Ikehata et al. (2015) in the City of Huntington Beach, California, using coconut shell GAC media (certified to NSF/ANSI/CAN 61) to remove hydrogen sulphide. Using five GAC filters in series, the hydrogen sulphide concentration was reduced from 0.02 and 0.7 mg/L to below MDL of 0.01 mg/L.
Manganese greensand filtration can be used to treat water containing less than 5.0 mg/L of hydrogen sulphide. The manganese dioxide coating on the filter catalyzes hydrogen sulphide gas to solid sulphur which is then filtered (Odell, 2010). Willey et al. (1964) conducted a full-scale study at a water treatment plant in central Indiana. The plant had been operating for 2 years and the study showed that hydrogen sulphide can be removed from water using a regeneration process consisting of a continuous feed of potassium permanganate to the influent of a manganese greensand filter. The authors also concluded that a dilute sulphuric acid feed and aeration eliminated a considerable portion of the hydrogen sulphide (from 11.3 ppm to below MDL) mechanically to reduce the demand for potassium permanganate. Saunders and Lee (1996) noted that, when potassium permanganate is used with manganese greensand, the greensand media may become fouled by iron and other contaminants, allowing sulphur to break through if the process runs out of potassium permanganate. Thus, it is important to monitor the manganese greensand filter to ensure it is working optimally.
5.1.4.5 Microbiological filtration
Bacteria can oxidize sulphide into sulphur under oxygen-limited conditions. When dissolved oxygen is < 0.1 mg/L, the dominant product is elemental sulphur while, at high levels of dissolved oxygen, the dominant product is sulphate (Janssen et al., 1998). Microbiological filtration can chemically reduce hydrogen sulphide but the organisms can slough off surfaces and cause turbidity downstream of storage facilities (Duranceau et al., 2010).
5.2 Residential-scale treatment
Several treatment technologies can effectively reduce these substances at a residential scale, for example, a small system or household whose drinking water supply is from a private well.
Before a treatment unit is installed, the water should be tested to determine the general water chemistry and the concentration of the parameters of interest found in the source water. Periodic testing by an accredited laboratory should be conducted on both the water entering the treatment unit and the treated water to verify that the treatment unit is effective. Units can lose removal capacity through use and time and need to be maintained and/or replaced. Consumers should verify the expected longevity of the components in the treatment unit according to the manufacturer's recommendations and service it when required. Systems classified as residential scale may have a rated capacity to treat volumes greater than that needed for a single residence, so they may also be used in small systems.
Certified residential treatment units are available for the reduction of hardness, sulphate, chloride, TDS and hydrogen sulphide.
5.2.1 Calcium, magnesium, hardness
A number of certified residential treatment devices are currently available for the removal of calcium, magnesium or other hardness-contributing elements from drinking water. These devices rely on cation exchange, RO and distillation systems.
An IX (water softening) system certified to NSF/ANSI Standard 44 (Residential Cation Exchange Water Softeners) can reduce hardness in drinking water. To be certified for hardness reduction under Standard 44, the device must be capable of reducing hardness to below 1.0 gpg (17.1 mg/L) from an influent hardness of 20 gpg (342 mg/L) (NSF/ANSI, 2024). Higher concentrations of hardness mean that the IX unit will consume greater amounts of salt for regeneration to achieve an acceptable level of hardness.
Homeowners with private wells using IX softeners in sodium form should be aware that the treatment unit may introduce undesirable quantities of sodium into the treated water. It is recommended that a separate supply be used for potable water consumption and culinary purposes. Similarly, softening by potassium IX may introduce undesirable levels of potassium which may adversely impact certain segments of the population (Health Canada, 2008). In both cases, when a water softener is used, it is recommended that a portion of the water most frequently consumed (such as from the kitchen tap) bypass the softener altogether to avoid excessive sodium or potassium intake. Appendix E contains information on the intake of sodium as a result of water softener use, by hardness level.
An RO system should be able to remove hardness but there are no certified systems available. Additionally, the use of an RO system for removal of high concentrations of hardness would foul the membrane more quickly and require more frequent maintenance as well as shorten the service life of the RO membrane. Consumers may need to pretreat the influent water to reduce fouling and extend the service life of the RO membrane. RO systems are generally not practical for residential-scale POE systems as larger quantities of influent water are needed to obtain the required volume of treated water.
The distillation process should also be able to remove hardness but there are no certified systems available.
Water that has been treated using RO or distillation may be corrosive to internal plumbing components. Therefore, these units should be installed only at the POU. As large quantities of influent water are needed to obtain the required volume of treated water, RO systems are generally not practical for POE installation. A consumer may need to pretreat the influent water to reduce fouling and extend the service life of the RO membrane.
A detailed report prepared by Brodeur and Barbeau (2015) using the data from Barbeau et al. (2011) studied the effectiveness of treatment technologies for the removal of manganese in groundwater. This report also included results for hardness removal for 96 systems using various technologies at an average influent concentration of 136 356 µg/L (136.36 mg/L) and treated water concentration of 49 549 µg/L (49.55 mg/L). The authors found that the IX and RO systems were capable of reducing hardness with median removals of 99% and 72%, respectively. Results from the individual technologies are summarized in Appendix D (Table D.1).
5.2.2 Chloride, sulphate
The technologies available for the reduction of chlorides and sulphates at a residential scale are limited. Both chlorides and sulphates tend to remain in solution and are not involved in many chemical reactions. Reductions in the concentrations of chlorides and sulphates may be achieved through anion exchange or RO. A number of certified residential treatment devices are currently available for the removal of chloride and sulphate from drinking water. These devices rely on POU/POE filtration systems.
Distillation systems for sulphate and chloride removal must be capable of reducing an average influent concentration of 800 mg/L to a maximum concentration of 250 mg/L (NSF/ANSI, 2023a).
Homeowners may opt to seek out water sources and aquifers with lower concentrations of these parameters if they find their existing levels are too high.
5.2.3 Total dissolved solids (TDS)
A number of certified residential treatment devices are currently available for the removal of TDS from drinking water. These devices rely on POU/POE filtration systems, RO and distillation systems.
Reduction requirements for TDS are included under NSF/ANSI Standard 42 (Drinking Water Treatment Units – Aesthetic Effects). For a device to be certified for TDS removal under Standard 42, it must be capable of reducing an average influent concentration of 1 500 mg/L to a maximum treated water concentration of 500 mg/L (NSF/ANSI, 2023b).
Reduction requirements for TDS are included under NSF/ANSI Standard 58 (Reverse Osmosis Drinking Water Treatment Systems). For a device to be certified for TDS removal under Standard 58, it must be capable of reducing an average influent concentration of 750 mg/L by at least 75% (down to 187 mg/L) (NSF/ANSI, 2023c).
A distillation system certified to NSF/ANSI Standard 62 (Drinking Water Distillation Systems) includes reduction of TDS using sodium chloride as a surrogate. To be certified for TDS removal under Standard 62, a device must be capable of reducing an average influent concentration of 1 000 mg/L with a minimum reduction of 97% (30 mg/L) (NSF/ANSI, 2023a).
Water that has been treated using RO or distillation may be corrosive to internal plumbing components. Therefore, these units should be installed only at the POU. As large quantities of influent water are needed to obtain the required volume of treated water, RO systems are generally not practical for POE installation. A consumer may need to pretreat the influent water to reduce fouling and extend the service life of the RO membrane.
5.2.4 Hydrogen sulphide
Certified residential treatment devices are currently available for the removal of hydrogen sulphide from drinking water. These devices rely on POU/POE filtration systems. Reduction requirements for hydrogen sulphide are included under NSF/ANSI Standard 42 (Drinking Water Treatment Units –Aesthetic Effects). For a device to be certified for hydrogen sulphide removal under Standard 42, it must be capable of reducing an average influent concentration of 1.0 mg/L to a maximum permissible product water concentration of 0.05 mg/L (NSF/ANSI, 2023b).
6.0 Management strategies
All water utilities should implement a risk management approach such as the source-to-tap or water safety plan approach to ensure water safety. These approaches require a system assessment to characterize the source water, describe the treatment barriers that prevent or reduce contamination, identify the conditions that can result in contamination and implement control measures. Operational monitoring is then established and operational and management protocols (for example, standard operating procedures, corrective actions and incident responses) are instituted. Other protocols are also implemented (for example, recordkeeping, consumer satisfaction) to validate the water safety plan (AWWA, 2024). Operator training is also required to ensure the effectiveness of the water safety plan at all times (Smeets et al., 2009).
Management strategies to reduce concentrations of operational parameters in drinking water may be achieved by:
- Adopting centralized treatment for parameter(s) of concern while considering downstream effects on distribution systems.
- Considering blending source waters with lower concentrations of these parameters.
- Reviewing source water characteristics and the concentration of parameter(s) of concern, and possibly adopting a new source with lower concentrations.
- Addressing any anthropogenic sources of the parameters of concern.
- Providing public information on the appropriateness of POE or POU systems for individual consumers.
Many useful references on water safety plans have been published (for example, Breach 2012, Jackson et al. 2023, WHO 2017, 2012). The World Health Organization (WHO) and International Water Association (IWA) have also maintained an online portal to provide access to reference materials in support of water safety planning https://wsportal.org.
6.1 Control strategies
In water sources with higher than acceptable concentrations of operational parameters, one or more treatment options (see section 5.0) may be implemented. Other control strategies may include controlled blending prior to system entry point, interconnecting with and/or purchasing water from another water system, or use of alternative water supplies. Attention must be given to the water quality of a new source prior to making any changes (such as switching, blending and interconnecting) to an existing supply. For example, if the new water source is more corrosive, it may cause leaching of lead or copper in the distribution system. The water quality of the recombined stream should be analyzed to ensure it meets the desired concentrations.
6.1.1 Blending
A common practice in water softening is bypass blending, which involves diverting a portion of the influent flow around the treatment vessel and blending the diverted water with the treated water. Blending of finished water with raw water may stabilize finished water and decrease the cost of treatment by reducing the volume of water treated. This can result in less frequent regeneration and a savings in chemical and brine disposal costs (U.S. EPA, 1999b). However, the concentration of each parameter in the bypass water needs to be considered to ensure that the finished water concentration does not exceed the treatment objectives (for example, guidelines).
Utilities implementing control options that involve a new, blended or interconnected source of water for addressing the hardness concentration should assess the water quality of new sources and blended water to ensure that it does not interfere with the existing treatment processes, impact the distribution system and/or cause other water quality issues.
Other options typically considered for calcium and magnesium reduction include sequestration or chelation. Both of these processes act to stabilize the divalent ions and prevent precipitation under normal conditions. Typically, a sodium or potassium polyphosphate is used to isolate and stabilize the hardness-causing ions in solution. This can be applied to calcium and magnesium hardness as well as minor hardness contributors such as iron and manganese. In general, sequestration is considered a temporary control measure because its effect is time limited (Kohl and Medlar, 2006).
The chelation effects can degrade over time, leading to precipitation in downstream pipes and fixtures. Sequestration is generally not recommended as a strategy since it can also have a negative impact on other metals (for example, lead). The use of polyphosphates is discussed in greater detail in Health Canada's Guidance on Sampling and Mitigation Measures for Controlling Lead Corrosion (Health Canada, 2025).
6.2 Monitoring
Routine sampling at the plant intake is recommended for all surface water supplies in order to characterize the seasonal variability of these parameters. Depending on the watershed, snow melt may dilute the concentration of most parameters unless the snow melt is affected by road salt. However, it is also possible that it will allow for migration of groundwater into the primary surface water system, which may actually increase the concentrations for a short period during the spring melt. Groundwater supplies generally have a more stable concentration of these parameters but would benefit from regular monitoring on a quarterly basis to ensure that the aquifer has not changed drastically.
6.2.1 Source water characterization
Characterization of the water quality must be carried out to ensure that changes in water quality resulting from control or treatment options are assessed and that potential impacts to the distribution system are determined. Any change in water quality should not result in compliance issues. Pilot testing of the selected treatment method or control option for hardness is also an important step to assess unintended consequences such as water quality changes.
6.2.2 Treatment
When treatment is in place for hardness reduction (including control options), it is recommended that monitoring be conducted quarterly, at minimum, to confirm that the AO is not exceeded. Samples should be collected after treatment prior to distribution (typically at the entry point to the distribution system). Paired samples of source and treated water should be taken to confirm the efficacy of the treatment or control option.
RO and IX are often operated with a bypass that blends a portion of the influent (incoming) flow with the treated water to obtain the desired water quality. It is important to monitor blended treated water to determine final hardness concentrations when this control option is used.
6.2.3 Operational
Treatment plants using lime softening for hardness should conduct operational monitoring of calcium and magnesium concentrations, and pH.
Treatment plants using IX water softening for lowering hardness in their source water should monitor for hardness breakthrough in each IX vessel to identify the timing for resin regeneration and achieve desired hardness control. An operational consideration when using SAC resins in hydrogen form includes the potential rapid chromatographic peaking of contaminants. In IX, some components of a mixture will have more affinity for the sorbent than others. The more strongly sorbed component will displace a less strongly sorbed component out of the sorption bed first, causing a "peak" of concentration of the less strongly sorbed component at the time water exits the column (Clifford, 1999). Since barium and calcium are the cations most preferred by these IX resins, chromatographic peaking may be observed for ions such as sodium and magnesium in the treated water. The hydrogen form of SAC and weak-acid cation exchange resins must be followed by a CO2 stripping process and a pH or alkalinity adjustment step to reduce the corrosivity of the treated water.
Treatment plants using cation exchange resins in sodium form should be aware that this process may introduce undesirable quantities of sodium into the treated water.
RO and IX are often operated with a bypass that blends a portion of the influent (incoming) flow with the treated water to obtain the desired water quality. It is important to monitor blended treated water to determine final hardness concentrations when this control option is used.
6.2.4 Blending
It is important to monitor blended treated water to determine final hardness concentrations. The disinfectant type (chlorine or chloramine) should be the same to avoid water quality and disinfection issues. Corrosion issues should be considered when blending different water qualities. An increase in pH during the lime softening process may be detrimental to the efficacy of primary disinfection treatment units. There is also potential for increased removal of pathogens through the sedimentation phase of precipitative hardness removal processes (Cornwell et al., 2003).
6.2.5 Residential
Homeowners with private wells as well as operators of small systems are encouraged to have their water tested for hardness to ensure that the concentration in their water supply is below the AO. Homeowners with private wells using residential treatment devices should conduct routine testing on both the water entering the treatment device and the treated water to verify that the treatment device is effective. Homeowners using IX softeners should be aware that the treatment unit may introduce undesirable quantities of sodium into the treated water.
7.0 International considerations
The U.S. EPA has not established a standard for hardness concentration in drinking water. Calcium and magnesium are addressed only indirectly as components of hardness and are considered aesthetic parameters (USGS, 2018, 2023). The U.S. EPA and the WHO categorize overall hardness (Table 18) similarly as soft, moderate, hard and very hard (McGowan, 2000; USGS, 2023). In the European Union, the values for hardness vary by country, with some countries recommending minimum calcium and magnesium levels to avoid corrosion and taste issues (EU, 2020).
| Organisation | Soft (mg/L) |
Moderate (mg/L) |
Hard (mg/L) |
Very hard (mg/L) |
|---|---|---|---|---|
| WHO (WHO, 2009) | < 60 | 61–120 | 121–180 | > 180 |
| U.S. EPA (USGS, 2023) | < 60 | 61–120 | 121–180 | > 180 |
| Australia (NHMRC, NRMMC, 2011) | < 60 Soft but corrosive | 60–200 Good | 200–500 Increasing scale | > 500 Severe scale |
| U.S. EPA – United States Environmental Protection Agency; WHO – World Health Organization. | ||||
Chlorides and sulphates have aesthetic limits of approximately 250 mg/L in multiple jurisdictions, including the United States, Australia and the European Union (Table 19). The WHO and Australia also note a potential laxative effect for sulphate at 500 mg/L.
TDS as a bulk measure of dissolved solids has been placed on the U.S. EPA secondary contaminants list with some concerns around taste and aesthetics. The WHO and Australia established an aesthetic limit of 0.05 mg/L for hydrogen sulphide.
| Organisation | Chloride (mg/L) |
Sulphate (mg/L) |
TDS (mg/L) |
H2S (mg/L) |
|---|---|---|---|---|
| WHO (WHO, 2009) | 250 |
|
500–1 000 | 0.05 (odour) |
| U.S. EPA (U.S. EPA, 2020) | 250 | 250 | 500 | N/A |
| Australia (NHMRC, NRMMC (2011) | 250 |
|
|
0.05 |
| European Union (EU, 2020) | 250 | 250 | N/A | N/A |
| N/A – Not Applicable; TDS – total dissolved solids; U.S. EPA – United States Environmental Protection Agency; WHO – World Health Organisation. | ||||
8.0 Rationale for aesthetic objectives
The taste threshold of calcium has been reported to be between 100 mg/L and 300 mg/L (Burlingame et al., 2007). Magnesium may also contribute undesirable tastes (for example, bitterness) to drinking water with a taste threshold between 100 mg/L and 500 mg/L (Burlingame et al., 2007).
Increased chloride levels can result in an objectionable taste to drinking water when it is in the presence of sodium, calcium, potassium and magnesium (Burlingame et al., 2007). The taste threshold for chloride is estimated to be 200–300 mg/L (Dietrich and Burlingame, 2015). However, it is thought to act in concert with the concentration of sodium ions, with chloride only slightly modifying the taste perception.
Sulphate has been reported to have a taste threshold of 250 mg/L, with sodium sulphate having a threshold of 250 mg/L and calcium sulphate a threshold of 1 000 mg/L (Lin et al., 2019). Sulphate in moderate concentrations is more amenable to most consumers from a taste perspective. Sulphate can compliment other TDS content to provide a balanced taste profile that is acceptable up to moderate levels of TDS.
TDS is a main determinant in the taste of water and people's acceptance include prior exposure and to what they are accustomed (Lin et al., 2019). For example, in France, where consumers are accustomed to mineral water, there is acceptance for water with 300–350 mg/L, while consumers in California prefer water free from mineral taste with approximate 80 mg/L of TDS (Lin et al., 2019). Van der Aa (2003) noted that consumers generally accept water with a TDS level lower than 1 000 mg/L and that water with a low TDS level may taste flat. Generally, consumers were able to identify a difference in taste when the TDS concentration changed by about 150 mg/L (Devesa and Dietrich, 2018).
Sulphide (as hydrogen sulphide) is known for its rotten egg odour. It has a low olfactory threshold, from less than 0.01 ppm to 0.3 ppm. There is uncertainty associated with determination of a specific odour threshold, as it varies with individual sensitivity (WHO, 2000; Greenberg et al., 2013). The median odour detection threshold for hydrogen sulphide reported by Amoore and Hautala (1983), based on a compilation of 25 published reports of odour threshold, is 0.008 ppm.
Based primarily on these taste and odour considerations (which vary based on source water, local conditions, habituation, pH and the temperature of the water), AOs are established for:
- chloride at ≤ 250 mg/L
- sulphate at ≤ 500 mg/L
- TDS ≤ 500 mg/L
- sulphide (as hydrogen sulphide) at ≤ 0.05 mg/L
The AOs are intended to minimize the occurrence of complaints based on unacceptable taste, odour or excessive scaling, and to improve consumer confidence in drinking water quality. The concentrations of these operational parameters can be readily measured by available analytical methods, and effective treatment technology is available at the municipal and residential scales.
9.0 References
- Allen, N.E., Key, T.J., Appleby, P.N., Travis, R.C., Roddam, A.W., Tjønneland, A., Johnsen, N.F., Overvad, K., Linseisen, J., Rohrmann, S., Boeing, H., Pischon, T., Bueno-De-Mesquita, H.B., Kiemeney, L., Tagliabue, G., Palli, D., Vineis, P., Tumino, R., Trichopoulou, A., Kassapa, C., Trichopoulos, D., Ardanaz, E., Larrãaga, N., Tormo, M.J., González, C.A., Quirós, J.R., Sánchez, M.J., Bingham, S., Khaw, K.T., Manjer, J., Berglund, G., Stattin, P., Hallmans, G., Slimani, N., Ferrari, P., Rinaldi, S. and Riboli, E. (2008). Animal foods, protein, calcium and prostate cancer risk: The European prospective investigation into cancer and nutrition. Br. J. Cancer, 98(9): 1574–1581.
- Aloia, J.F., Dhaliwal, R., Shieh, A., Mikhail, M., Fazzari, M., Ragolia, L. and Abrams, S.A. (2014). Vitamin D supplementation increases calcium absorption without a threshold effect. Am. J. Clin. Nutr., 99(3): 624–631.
- Amoore, J.E. and Hautala, E. (1983). Odor as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3(6): 272–290.
- Anastassopoulou, J. and Theophanides, T. (2002). Magnesium-DNA interactions and the possible relation of magnesium to carcinogenesis. Irradiation and free radicals. Crit. Rev. Oncol. Hematol., 42(1): 79–91.
- Anderson, J.J., Kruszka, B., Delaney, J.A., He, K., Burke, G.L., Alonso, A., Bild, D.E., Budoff, M. and Michos, E.D. (2016). Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J. Am. Heart Assoc., 5(10): e003815.
- APHA, AWWA, and WEF (2017). Standard methods for the examination of water and wastewater. 23rd Ed. (Online version). Standard methods for the examination of water and wastewater. American Public Health Association. Available at: https://www.standardmethods.org/
- Arnaud, M. (2003). Mild dehydration: A risk factor of constipation? Eur. J. Clin. Nutr., 57(2): S88–S95.
- Arnold, I.M., Dufresne, R.M., Alleyne, B.C. and Stuart, P.J. (1985). Health implication of occupational exposures to hydrogen sulfide. J. Occup. Med., 27(5): 373–376.
- Asnes, R.S., Wisotsky, D.H., Migel, P.F., Seigle, R.L. and Levy, J. (1982). The dietary chloride deficiency syndrome occurring in a breast-fed infant. J. Pediatr., 100(6): 923–924.
- ATSDR (2006). Toxicological profile for hydrogen sulfide. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia.
- ATSDR (2016). Toxicological profile for hydrogen sulphide and carbonyl sulphide. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia. Available at: https://www.atsdr.cdc.gov/ToxProfiles/tp114.pdf
- Attene-Ramos, M.S., Nava, G.M., Muellner, M.G., Wagner, E.D., Plewa, M.J. and Gaskins, H.R. (2010). DNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHs 74 int cells. Environ. Mol. Mutagen., 51(4): 304–314.
- Aune, D., Navarro Rosenblatt, D.A., Chan, D.S., Vieira, A.R., Vieira, R., Greenwood, D.C., Vatten, L.J. and Norat, T. (2015). Dairy products, calcium, and prostate cancer risk: A systematic review and meta-analysis of cohort studies. Am. J. Clin. Nutr., 101(1): 87–117.
- AWWA (1981). Lime softening sludge treatment and disposal. Committee Report. J. Am. Water Works Assoc., 73(11): 600–608.
- AWWA and ASCE (2005). Water treatment plant design. Fourth edition. American Water Works Association, and American Society of Civil Engineers. Denver, Colorado.
- AWWA (2016). Water system operations (WSO) Treatment, grade III&IV. American Water Works Association (AWWA). Denver, Colorado.
- AWWA (2024). Managing drinking water aesthetics with water safety planning. J. Am. Water Works Assoc., 116(3): 6–17.
- Backer, L.C. (2000). Assessing the acute gastrointestinal effects of ingesting naturally occurring, high levels of sulfate in drinking water. Crit. Rev. Clin. Lab. Sci., 37(4): 389–400.
- Bain, S.D., Jerome, C., Shen, V., Dupin-Roger, I. and Ammann, P. (2009). Strontium ranelate improves bone strength in ovariectomized rat by positively influencing bone resistance determinants. Osteoporosis Int., 20(8): 1417–1428.
- Ballinger, D. and Lloyd, A. (1981). A method for the determination of sulphides in water, sewage, and effluents. Water Pollut. Control, 80(5): 648–654.
- Barbeau, B., Carrière, A. and Bouchard, M. (2011). Spatial and temporal variations in manganese concentrations in drinking water. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng., 46(6): 608–616.
- Bartels, C., Lai, K. and Wilf, M. (2007). New generation of low fouling nanofiltration membranes. Proceedings of the 2007 EDS Conference, 2007, Halkidiki, Greece. Hydranautics, Oceanside, California.
- Baskar, R., Li, L. and Moore, P.K. (2007). Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. Faseb j., 21(1): 247–255.
- Bates, M.N., Garrett, N., Graham, B. and Read, D. (1997). Air pollution and mortality in the Rotorua geothermal area. Aust. New Zealand J. Public Health, 21(6): 581–586.
- Bates, M.N., Garrett, N., Graham, B. and Read, D. (1998). Cancer incidence, morbidity and geothermal air pollution in Rotorua, New Zealand. Int. J. Epidemiol., 27(1): 10–14.
- Baylis, J.R. (1926). Factors other than dissolved oxygen influencing the corrosion of iron pipes. Ind. Eng. Chem., 18(4): 370–380.
- Bean, E.L. (1968). Quality goals for potable water. J. Am. Water Works Assoc., 60: 1317
- Beauchamp, R.O., Jr, Bus, J.S., Popp, J.A., Boreiko, C.J. and Andjelkovich, D.A. (1984). A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol., 13(1): 25–97.
- Benefield, L.D. and Morgan, J.M. (1999). Chemical Precipitation Chapter 10 in: Water quality and treatment: a handbook of community water supplies, 5th edition. Letterman, R.D. (ed). American Water Works Association. Denver, Colorado.
- Bergman, R.A. (1995). Membrane softening versus lime softening in Florida: A cost comparison update. Desalination, 102: 11–24.
- Biesheuvel, P., Zhang, L., Gasquet, P., Blankert, B., Elimelech, M. and Van Der Meer, W. (2019). Ion selectivity in brackish water desalination by reverse osmosis: Theory, measurements, and implications. Environ. Sci. Technol. Lett., 7(1): 42–47.
- Biyoune, M.G., Atbir, A., Bari, H., Hassnaoui, L., Mongach, E., Khadir, A., Boukbir, L., Bellajrou, R. and Elhadek, M. (2017). Remineralization of permeate water by calcite bed in the Daoura's plant (south of Morocco). Eur. Phys. J. Spec. Top., 226: 931–941.
- Black, A. (1966). Split‐treatment water softening at Dayton. J. Am. Water Works Assoc., 58(1): 97–106.
- Blunden J., and Aneja V.P. (2008). Characterizing ammonia and hydrogen sulfide emissions from a swine waste treatment lagoon in North Carolina. Atmos. Environ., 42(14): 3277–3290.
- Bonovas, S., Fiorino, G., Lytras, T., Malesci, A. and Danese, S. (2016). Calcium supplementation for the prevention of colorectal adenomas: A systematic review and meta-analysis of randomized controlled trials. World J. Gastroenterol., 22(18): 4594–4603.
- Bowman, B.A. and Russell, R.M. (2006). Nutrition. 9th. Nutrition. ILSI Press, Washington D.C.
- British Columbia Ministry of Health (2021). Personal communication with D. Fishwick.
- Brodeur, M.E. and Barbeau, B. (2015). Performance of point-of-use and point-of-entry technologies for the removal of manganese in drinking water: Summary of the EDU-MANGO epidemiological study. Report prepared for and available on request from Health Canada.
- Brown, E.M. (1991). Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol. Rev., 71(2): 371–411.
- Burlingame, G.A., Dietrich, A.M. and Whelton, A.J. (2007). Understanding the basics of tap water taste. J. Am. Water Works Assoc., 99(5): 100–111.
- Burton, A.C. and Comhill, J.F. (1977). Correlation of cancer death rates with altitude and with the quality of water supply of the 100 largest cities in the United States. J. Toxicol. Environ. Health, 3(3): 465–478.
- Cadena, F.C., Midkiff, W.S., and O'Connor, G.A. (1974). Calcium carbonate ion-pair as a limit to hardness removal. J. Am. Water Works Assoc., 66(9): 524–526.
- Cadena, F. and Peters, R.W. (1988). Evaluation of chemical oxidizers for hydrogen sulfide control. J. Water Pollut. Control Fed., 60(7): 1259–1263.
- Campbell, A.K. (1990). Calcium as an intracellular regulator. Proc. Nutr. Soc., 49(1): 51–56.
- Cao, X., Ding, L., Xie, Z.Z., Yang, Y., Whiteman, M., Moore, P.K. and Bian, J.S. (2019). A review of hydrogen sulfide synthesis, metabolism, and measurement: Is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxid. Redox Signal., 31(1): 1–38.
- Carlson, K., Via, S., Bellamy, B. and Carlson, M. (2000). Secondary effects of enhanced coagulation and softening. J. Am. Water Works Assoc., 92(6): 63–75.
- Cass, J. (1953). Report on the physiological effects of some common inorganic salts in water on man and domestic animals. Cincinnati, Ohio: The Ohio River Valley Water Sanitation Commission.
- Castenmiller, J.J., Mensink, R.P., van der Heijden, L., Kouwenhoven, T., Hautvast, J.G., de Leeuw, P.W. and Schaafsma, G. (1985). The effect of dietary sodium on urinary calcium and potassium excretion in normotensive men with different calcium intakes. Am. J. Clin. Nutr., 41(1): 52–60.
- Catling, L.A., Abubakar, I., Lake, I.R., Swift, L. and Hunter, P.R. (2008). A systematic review of analytical observational studies investigating the association between cardiovascular disease and drinking water hardness. J. Water Health, 6(4): 433–442.
- Chapra, S.C., Dove, A. and Warren, G.J. (2012). Long-term trends of great lakes major ion chemistry. J. Great Lakes Res., 38(3): 550–560.
- Chellan, P. and Sadler, P.J. (2015). The elements of life and medicines. Philos. Trans. A. Math. Phys. Eng. Sci., 373(2037): 20140182.
- Chen, P., Hu, P., Xie, D., Qin, Y., Wang, F. and Wang, H. (2010). Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res. Treat., 121(2): 469–477.
- Chen, Y., Strasser, S., Cao, Y., Wang, K.S. and Zheng, S. (2015). Calcium intake and hypertension among obese adults in United States: Associations and implications explored. J. Hum. Hypertens., 29(9): 541–547.
- Cherry, A.K. (1955). Split-treatment method of water softening. J. Am. Water Works Assoc., 47(4): 393–397.
- Chien, L., Robertson, H. and Gerrard, J.W. (1968). Infantile gastroenteritis due to water with high sulfate content. Can. Med. Assoc. J., 99(3): 102–104.
- Clifford, D.A. (1999). Ion exchange and inorganic adsorption. Chapter 9 in: Water quality and treatment: a handbook of community water supplies, 5th edition. Letterman, R.D. (ed). American Water Works Association. Denver, Colorado.
- Coleman, R.L. (1976). Potential public health aspects of trace elements and drinking water quality. Ann. Okla. Acad. Sci., 5: 57.
- Conlon, W.J., and McClellan, S.A. (1989). Membrane softening: A treatment process comes of age. J. Am. Water Works Assoc., 81(11): 47–51.
- Cormick, G., Ciapponi, A., Cafferata, M.L., Cormick, M.S. and Belizán, J.M. (2022). Calcium supplementation for prevention of primary hypertension. Cochrane Database Syst. Rev., 1(1): CD010037.
- Cornwell, D.A., MacPhee, M.J., Brown, R.A. and Via, S.H. (2003). Demonstrating Cryptosporidium removal using spore monitoring at lime-softening plants. J. Am. Water Works Assoc., 95(5): 124–133.
- Correa, J., Hahn, D. and Ellison, D. (2010). A variety of construction methods and pipeline materials help create a new sewer system. Proceedings of the Pipelines 2010 Conference, 2010, Anonymous, pp. 13–22.
- Cotruvo, J.A., Costello, R. and Weglicki, W.B. (2017). Magnesium, hard water, and health. J. Am. Water Works Assoc., 109(11): 62–68.
- Cowan, J.A. (2002). Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals, 15(3): 225–235.
- Crittenden, J.C., Trussell, R.R., Hand, D.W., Howe, K.J. and Tchobanoglous, G. (2012). MW's water treatment: Principles and design. Third edition. John Wiley & Sons, Hoboken, New Jersey.
- Davis, M.L. (2010). Water and wastewater engineering: Design principles and practice. McGraw-Hill, New York, New York.
- Del Gobbo, L.C., Imamura, F., Wu, J.H.Y., de Oliveira Otto, Marcia C, Chiuve, S.E. and Mozaffarian, D. (2013). Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr., 98(1): 160–173.
- D'Elia, L., Galletti, F. and Strazzullo, P. (2014). Dietary salt intake and risk of gastric cancer. Cancer Treat. Res., 159: 83–95.
- Department of Fisheries and the Environment (1978a). Plate 28. Water quality of surface water. Hydrological Atlas of Canada. Available at: https://ftp.maps.canada.ca/pub/nrcan_rncan/raster/atlas/eng/hydro_1978/water_quality/28_Water_Quality_Surface_Waters_1978_150.jpg
- Department of Fisheries and the Environment (1978b). Plate 30. Surficial hydrogeology. Hydrological Atlas of Canada. Available at: https://ftp.maps.canada.ca/pub/nrcan_rncan/raster/atlas/eng/hydro_1978/hydrogeology/30_Surficial_Hydrogeology_1978_150.jpg
- Department of Fisheries and the Environment (1978c). Plate 31. Bedrock hydrogeology. Hydrological Atlas of Canada. Available at: https://ftp.maps.canada.ca/pub/nrcan_rncan/raster/atlas/eng/hydro_1978/hydrogeology/31_Bedrock_Hydrogeology_1978_150.jpg
- Devesa, R. and Dietrich, A.M. (2018). Guidance for optimizing drinking water taste by adjusting mineralization as measured by total dissolved solids (TDS). Desalination, 439: 147–154.
- Dietrich, A.M. and Burlingame, G.A. (2015). Critical review and rethinking of USEPA secondary standards for maintaining organoleptic quality of drinking water. Environ. Sci. Technol., 49(2): 708–720.
- Dong, J., Xun, P., He, K. and Qin, L. (2011). Magnesium intake and risk of type 2 diabetes: Meta-analysis of prospective cohort studies. Diabetes Care, 34(9): 2116–2122.
- Droste, R.L. (1997). Theory and practice of water and wastewater treatment. First Edition. Wiley. Hoboken, New Jersey.
- Droste, R.L. (2019). Theory and practice of water and wastewater treatment. Second Edition. Wiley. Hoboken, New Jersey.
- du Cailar, G., Ribstein, J. and Mimran, A. (2002). Dietary sodium and target organ damage in essential hypertension. Am. J. Hypertens., 15(3): 222–229.
- Dudi, A. and Edwards, M. (2004). Galvanic corrosion of lead bearing plumbing devices. In: Reconsidering lead corrosion in drinking water: Product testing, direct chloramine attack and galvanic corrosion. Faculty of the Virginia Polytechnic Institute and State University, Blacksburg, Virginia. pp. 69–105 (A. Dudi Master's Thesis).
- Duranceau, S.J. (2001). Membrane practices for water treatment. First edition. American Water Works Association, Denver, Colorado.
- Duranceau, S.J., Trupiano, V.M., Lowenstine, M., Whidden, S. and Hopp, J. (2010). You cannot hide from hydrogen sulfide: But there are alternatives for treatment. Proceedings of the American Water Works Association Annual Conference and Exposition, 2010, American Water Works Association, Denver, Colorado.
- Duranceau, S.J. and Trupiano, V.M. (2011). Evaluation of oxidized media filtration for removing sulfides from groundwater. Desalination Water Treat., 28(1–3): 366–377.
- Edwards, M. and Triantafyllidou, S. (2007). Chloride-to-sulfate mass ratio and lead leaching to water. J. Am. Water Works Assoc., 99(7): 96–109.
- Edwards, S., Alharthi, R. and Ghaly, A.E. (2011). Removal of hydrogen sulphide from water. Am. J. Environ. Sci., 7(4): 295–305.
- EFSA (2015a). Scientific opinion on dietary reference values for magnesium. EFSA panel on dietetic products, nutrition and allergies (NDA). 13(7): 4186. Available at: https://www.efsa.europa.eu/en/efsajournal/pub/4186
- EFSA (2015b). Scientific opinion on dietary reference values for calcium. EFSA panel on dietetic products, nutrition and allergies (NDA). 13(5): 4101. Available at: https://www.efsa.europa.eu/en/efsajournal/pub/4101
- EFSA (2019). Dietary reference values for chloride. 27(9): 5779. Available at: https://www.efsa.europa.eu/en/efsajournal/pub/5779
- Environment Canada and Health Canada (2001). Priority substances list assessment report for road salts (Archived). Canadian Environmental Protection Act, 1999. ISBN: 0-662-31018-7. Available at: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/environmental-contaminants/canadian-environmental-protection-act-1999-priority-substances-list-assessment-report-road-salts.html
- Environment and Climate Change Canada (2018). Road salts: frequently asked questions. Available at: https://www.canada.ca/en/environment-climate-change/services/pollutants/road-salts/frequently-asked-questions.html
- Esteban, E., Rubin, C.H., McGeehin, M.A., Flanders, W.D., Baker, M.J. and Sinks, T.H. (1997). Evaluation of infant diarrhea associated with elevated levels of sulfate in drinking water: A case-control investigation in South Dakota. Int. J. Occup. Environ. Health, 3(3): 171–176.
- EU (2020). Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast) (Text with EEA relevance). Off. J. EC. L435/1 Available at: https://eur-lex.europa.eu/eli/dir/2020/2184/oj#d1e32-54-1
- Evans, C.E., Chughtai, A.Y., Blumsohn, A., Giles, M. and Eastell, R. (1997). The effect of dietary sodium on calcium metabolism in premenopausal and postmenopausal women. Eur. J. Clin. Nutr., 51(6): 394–399.
- Exley, C. (2013). Aluminum in biological systems. In: Encyclopedia of metalloproteins. Kretsinger, R.H., Uversky, V.N. and Permyakov, E.A. (eds.). Springer New York, pp. 33–34.
- Expert Group on Vitamins and Minerals (2003). Safe upper levels for vitamins and minerals. Available at: https://cot.food.gov.uk/sites/default/files/vitmin2003.pdf
- Fang, W., Shi, L., and Wang, R. (2014). Mixed polyamide-based composite nanofiltration hollow fiber membranes with improved low-pressure water softening capability. J. Memb. Sci., 468: 52–61.
- Fujii, N., Yano, S. and Takeshita, K. (2016). Selective enhancing effect of metal ions on mutagenicity. Genes Environ., 38(1): 21.
- Ganry, O., Boudet, J., Wargon, C., Hornych, A. and Meyer, P. (1993). Effect of sodium bicarbonate and sodium chloride on arterial blood pressure, plasma renin activity and urinary prostaglandins in healthy volunteers. J. Hypertens. Suppl., 11(5): S202–3.
- Geological Survey of Canada (2014). Canada's groundwater resources. Fitzhenry & Whiteside. Markham, Ontario. Available at: https://publications.gc.ca/site/eng/9.854795/publication.html
- Ghizellaoui, S., Chibani, A. and Ghizellaoui, S. (2005). Use of nanofiltration for partial softening of very hard water. Desalination, 179: 315–322.
- Gobalarajah, K., Subramaniam, P., Jayawardena, U.A., Rasiah, G., Rajendra, S. and Prabagar, J. (2020). Impact of water quality on chronic kidney disease of unknown etiology (CKDu) in Thunukkai Division in Mullaitivu District, Sri Lanka. BMC Nephrol., 21(1).
- Gomez, G.G., Sandler, R.S. and Seal Jr, E. (1995). High levels of inorganic sulfate cause diarrhea in neonatal piglets. J. Nutr., 125(9): 2325–2332.
- Goodwin, L.R., Francom, D., Urso, A. and Dieken, F.P. (1988). Determination of trace sulfides in turbid waters by gas dialysis/ion chromatography. Analytical chemistry. Anal. Chem., 60(3): 216–219.
- Gorenflo, A., Velázquez-Padrón, D. and Frimmel, F.H. (2002). Nanofiltration of a German groundwater of high hardness and NOM content: Performance and costs. Desalination, 151: 253–265.
- Gosselin, R. (1984). Hydrogen sulfide. In: Clinical toxicology of commercial products. Hydrogen sulfide. 5th ed. Williams & Wilkins, Baltimore, Maryland, pp. 198–202.
- Greenberg, M.I., Curtis, J.A. and Vearrier D. (2013). The perception of odor is not a surrogate marker for chemical exposure: a review of factors influencing human odor perception. Clin. Toxicol. (Phila.), 51(2): 70–76.
- Gross, S.J., David, R.J., Bauman, L. and Tomarelli, R.M. (1980). Nutritional composition of milk produced by mothers delivering preterm. J. Pediatr., 96(4): 641–644.
- Guillemant, J., Le, H.T., Accarie, C., du Montcel, S.T., Delabroise, A.M., Arnaud, M.J. and Guillemant, S. (2000). Mineral water as a source of dietary calcium: Acute effects on parathyroid function and bone resorption in young men. Am. J. Clin. Nutr., 71(4): 999–1002.
- Haahtela, T., Marttila, O., Vilkka, V., Jäppinen, P. and Jaakkola, J.J. (1992). The South Karelia air pollution study: Acute health effects of malodorous sulfur air pollutants released by a pulp mill. Am. J. Public Health, 82(4): 603–605.
- Hach Co. (2015a). Hardness, calcium and magnesium: Calmagite colorimetric method 8030. 10th ed. Hach Company, Available at: https://www.hach.com/WAH#H
- Hach Co. (2015b). Hardness, total: Titration method with EDTA method 8213. 8th ed. Hach Company, Available at: https://www.hach.com/WAH#H
- Hach Co. (2019). Hardness, calcium: Titration method with EDTA method 8204. 9th ed. Hach Company, Available at: https://www.hach.com/WAH#H
- Hach Co. (2021). Conductivity: USEPA direct measurement method 8160. 10th ed. Hach Company, Available at: https://www.hach.com/WAH#H
- Hagley, S.R. and South, D.L. (1983). Fatal inhalation of liquid manure gas. Med. J. Aust., 2(9): 459–460.
- Health Canada (2008). Guidance on potassium from water softeners. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidance-potassium-water-softeners.html
- Health Canada (2019). Guidelines for Canadian drinking water quality: Guideline technical document – manganese. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-manganese.html
- Health Canada (2021). Canadian exposure factors used in human health risk assessments. Fact sheet. Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/chemical-substances/fact-sheets/canadian-exposure-factors-human-health-risk-assessments.html
- Health Canada (2022a). Guidance on monitoring the biological stability of drinking water in distribution systems. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidance-monitoring-biological-stability-drinking-water-distribution-systems.html
- Health Canada (2022b). Personal communication from Anca-Maria Tugulea, Environmental and Health Sciences Research Bureau.
- Health Canada (2024a). Guidelines for Canadian drinking water quality – summary table. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/water-quality/guidelines-canadian-drinking-water-quality-summary-table.html
- Health Canada (2024b). Guidelines for Canadian drinking water quality: Guideline technical document – iron: Guideline Technical Document for Public Consultation. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-iron.html
- Health Canada (2025). Guidance on sampling and mitigation measures for controlling lead corrosion. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidance-controlling-corrosion-drinking-water-distribution-systems.html
- Heizer, W.D., Sandler, R.S., Seal Jr., E., Murray, S.C., Busby, M.G., Schliebe, B.G. and Pusek, S.N. (1997). Intestinal effects of sulfate in drinking water on normal human subjects. Dig. Dis. Sci., 42(5): 1055–1061.
- Hessel, P.A., Herbert, F.A., Melenka, L.S., Yoshida, K. and Nakaza, M. (1997). Lung health in relation to hydrogen sulfide exposure in oil and gas workers in Alberta, Canada. Am. J. Ind. Med., 31(5): 554–557.
- Hidayat, K., Chen, G.C., Zhang, R., Du, X., Zou, S.Y., Shi, B.M. and Qin, L.Q. (2016). Calcium intake and breast cancer risk: Meta-analysis of prospective cohort studies. Br. J. Nutr., 116(1): 158–166.
- Hill, F.B. (1973). Atmospheric sulfur and its links to the biota. Brookhaven Symp. Biol., 1(30): 159.
- Hill, N.S. Jr. (1924). Recarbonization of softened water. Proceedings of the Chemical and Bacteriological Section, Detroit Convention, May 25, 1923.
- Hill, I.D. and Bowie, M.D. (1983). Chloride deficiency syndrome due to chloride-deficient breast milk. Arch. Dis. Child., 58(3): 224–226.
- Hofman, J., Kramer, O., van der Hoek, J.P., Nederlof, M. and Groenendijk, M. (2006). Twenty years of experience with central softening in Netherlands: Water quality, environmental benefits, and costs. In: Proceedings of the International Symposium on Health Aspects of Calcium and Magnesium in Drinking Water, Washington D.C., pp. 24–26.
- Hofmeyr, G.J., Lawrie, T.A., Atallah, Á. and Torloni, M.R. (2018). Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev., 10(10): CD001059.
- Hoover, C.P., and Langelier, W.F. (1938). Practical application of the Langelier Method. J. Am. Water Works Assoc., 30(11), 1802–1807.
- Hunt, S.C., Geleijnse, J.M., Wu, L.L., Witteman, J.C., Williams, R.R. and Grobbee, D.E. (1999). Enhanced blood pressure response to mild sodium reduction in subjects with the 235T variant of the angiotensinogen gene. Am. J. Hypertens., 12(5): 460–466.
- Hussain, I., Shakeel, M., Faisal, M., Soomro, Z.A., Hussain, M. and Hussain, T. (2014). Distribution of total dissolved solids in drinking water by means of Bayesian kriging and Gaussian spatial predictive process. Water Quality, Exposure and Health, 6(4): 177–185.
- IARC (2018). Agents classified by the IARC monographs, volumes 1–123. Available at: https://monographs.iarc.who.int/wp-content/uploads/2018/09/ClassificationsAlphaOrder.pdf
- Ikehata., K., Komor, A.T., Li, Y., Qu, X., Kleinheiz, J., Lee, D.S., Trussell, C.B. and Hokanson, D.R. (2015). Removal of hydrogen sulfide from groundwater by granular activated carbon. 2015 CA-NV AWWA Annual Fall Conference, October 26–29, 2015, Las Vegas, Nevada.
- IOM (1997). Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride (1997). The National Academies Press, Washington D.C. Available at: https://doi.org/10.17226/5776
- IOM (2005). Dietary reference intakes for water, potassium, sodium, chloride, and sulfate (2005). The National Academies Press, Washington D.C. Available at: https://doi.org/10.17226/10925
- IOM (2011). Dietary reference intakes for calcium and vitamin D. The National Academies Press, Washington D.C. Available at: https://nap.nationalacademies.org/read/13050/chapter/1
- IPCS (2003). Hydrogen sulfide: human health aspects. Concise international chemical assessment Document 53. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, the World Health Organization and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals, World Health Organization, Geneva, Switzerland.
- Janssen, A.J.H., Meijer, S., Bontsema, J. and Lettinga, G. (1998). Application of the redox potential for controlling a sulfide oxidizing bioreactor. Biotechnol. Bioeng., 60(2): 147–155.
- Janssen, A.J.H., Lens, P.N., Stams, A.J., Plugge, C.M., Sorokin, D.Y., Muyzer, G., Dijkman, H., Van Zessen, E., Luimes, P. and Buisman, C.J. (2009). Application of bacteria involved in the biological sulfur cycle for paper mill effluent purification. Sci. Total Environ. 407(4): 1333–1343.
- Jäppinen, P. and Tola, S. (1990). Cardiovascular mortality among pulp mill workers. Br. J. Ind. Med., 47(4): 259–262.
- Jeziorski, A., Yan, N.D., Paterson, A.M., DeSellas, A.M., Turner, M.A., Jeffries, D.S., Keller, B., Weeber, R.C., McNicol, D.K., Palmer, M.E., McIver, K., Arseneau, K., Ginn, B.K., Cumming, B.F. and Smol, J.P. (2008). The widespread threat of calcium decline in fresh waters. Science. 322(5906), 1374–1377.
- Jiang, L., He, P., Chen, J., Liu, Y., Liu, D., Qin, G. and Tan, N. (2016). Magnesium levels in drinking water and coronary heart disease mortality risk: A meta-analysis. Nutrients, 8(1).
- Johnson, A.G., Nguyen, T.V. and Davis, D. (2001). Blood pressure is linked to salt intake and modulated by the angiotensinogen gene in normotensive and hypertensive elderly subjects. J. Hypertens., 19(6): 1053–1060.
- Kahlown, M.A., Ashraf, M., Hussain, M., Salam, H.A. and Bhatti, A.Z. (2006). Impact assessment of sewerage and industrial effluents on water resources, soil, crops and human health in Faisalabad. Pakistan Council of Research in Water Resources, Islamabad, Pakistan. Available at: https://pcrwr.gov.pk/wp-content/uploads/2020/Water-Management-Reports/Impact%20Assessment%20of%20Sewerage%20and%20Industrial%20Effluents%20on%20Water.pdf
- Kahrilas, G.A., Blotevogel, J., Stewart, S.P. and Borch, T. (2015). Biocides in hydraulic fracturing fluids: A critical review of their usage, mobility degradation, and toxicity. Environ. Sci. Technol., 49(1): 16–32.
- Kaleita, T.A. (1986). Neurologic/behavioral syndrome associated with ingestion of chloride-deficient infant formula. Pediatrics, 78(4): 714–715.
- Karagas, M.R., Wang, A., Dorman, D.C., Hall, A.L., Pi, J., Sergi, C.M., Symanski, E., Ward, E.M., Arrandale, V.H., Azuma, K., Brambila, E., Calaf, G.M., Fritz, J.M., Fukushima, S., Gaitens, J.M., Grimsrud, T.K., Guo, L., Lynge, E., Marinho-Reis, A.P., McDiarmid, M.A., Middleton, D.R.S., Ong, T.P., Polya, D.A., Quintanilla-Vega, B., Roberts, G.K., Santonen, T., Sauni, R., Silva, M.J., Wild, P., Zhang, C.W., Zhang, Q., Grosse, Y., Benbrahim-Tallaa, L., de Conti, A., DeBono, N.L., El Ghissassi, F., Madia, F., Reisfeld, B., Stayner, L.T., Suonio, E., Viegas, S., Wedekind, R., Ahmadi, S., Mattock, H., Gwinn, W.M. and Schubauer-Berigan, M.K. (2022). Carcinogenicity of cobalt, antimony compounds, and weapons-grade tungsten alloy. Lancet Oncol., 23(5): 577–578.
- Kashfi, K. and Olson, K.R. (2013). Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem. Pharmacol., 85(5): 689–703.
- Kasprzak, K.S., Gabryel, P. and Jarczewska, K. (1983). Carcinogenicity of nickel(II)hydroxides and nickel(II)sulfate in Wistar rats and its relation to the in vitro dissolution rates. Carcinogenesis, 4(3): 275–279.
- Kass, L., Weekes, J. and Carpenter, L. (2012). Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr., 66(4): 411–418.
- Kataoka, H. (2021). Chloride in heart failure syndrome: Its pathophysiologic role and therapeutic implication. Cardiol. Ther., 10(2): 407–428.
- Kaushal, S.S., Likens, G.E., Pace, M.L., Utz, R.M., Haq, S., Gorman, J. and Grese, M. (2018). Freshwater salinization syndrome on a continental scale. Proc. Natl. Acad. Sci. U.S.A., 115(4), E574–E583.
- Kaushal, S.S., Likens, G.E., Pace, M.L., Reimer, J.E., Maas, C.M., Galella, J.G., Woglo, S.A. (2021).
- Freshwater salinization syndrome: from emerging global problem to managing risks. Biogeochemistry, 154: 255–292.
- Kayraldiz, A., Kaya, F.F., Canimoǧlu, S. and Rencüzoǧullari, E. (2006). Mutagenicity of five food additives in Ames/Salmonella/microsome test. Ann. Microbiol., 56(2): 129–133.
- Kesse, E., Bertrais, S., Astorg, P., Jaouen, A., Arnault, N., Galan, P. and Hercberg, S. (2006). Dairy products, calcium and phosphorus intake, and the risk of prostate cancer: Results of the French prospective SU.VI.MAX (supplémentation en vitamines et minéraux antioxydants) study. Br. J. Nutr., 95(3): 539–545.
- Keum, N., Aune, D., Greenwood, D.C., Ju, W. and Giovannucci, E.L. (2014). Calcium intake and colorectal cancer risk: Dose-response meta-analysis of prospective observational studies. Int. J. Cancer, 135(8): 1940–1948.
- Khan, A.A., Schuler, M.M., Prior, M.G., Yong, S., Coppock, R.W., Florence, L.Z. and Lillie, L.E. (1990). Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol. Appl. Pharmacol., 103(3): 482–490.
- Khan, B., Nowson, C.A., Daly, R.M., English, D.R., Hodge, A.M., Giles, G.G. and Ebeling, P.R. (2015). Higher dietary calcium intakes are associated with reduced risks of fractures, cardiovascular events, and mortality: A prospective cohort study of older men and women. J. Bone Miner. Res., 30(10): 1758–1766.
- Kilburn, K.H. (1993). Case report: Profound neurobehavioral deficits in an oil field worker overcome by hydrogen sulfide. Am. J. Med. Sci., 306(5): 301–305.
- Kilburn, K.H. and Warshaw, R.H. (1995). Hydrogen sulfide and reduced-sulfur gases adversely affect neurophysiological functions. Toxicol. Ind. Health, 11(2): 185–197.
- Kim, K.H. (2006) Emissions of reduced sulphur compounds (RSC) as a landfill gas (LFG): a comparative study of young and old landfill facilities. Atmos Environ. 40:6567–6578.
- Kim, K.Y., Ko, H.J., Kim, H.T., Kim, Y.S., Young, M.R., Lee, C.M. and Kim, C.N. (2008). Quantification of ammonia and hydrogen sulphide emitted from pig buildings in Korea. J. Environ. Manage., 88(2): 195–202.
- Kohl, P.M. and Medlar, S.J. (2006). Occurrence of manganese in drinking water and manganese control. American Water Works Association, Denver, Colorado.
- Kotchen, T.A. and Kotchen, J.M. (1997). Dietary sodium and blood pressure: Interactions with other nutrients. Am. J. Clin. Nutr., 65(2 Suppl): 708S–711S.
- Kozisek, F. (2020). Regulations for calcium, magnesium or hardness in drinking water in the European Union member states. Regul. Toxicol. Pharmacol., 112: 104589.
- Krishnan, K. and Carrier, R. (2013). The use of exposure source allocation factor in the risk assessment of drinking-water contaminants. J. Toxicol. Environ. Health B Crit. Rev., 16(1): 39–51.
- Kruse, P. (2018). Review on water quality sensors. J. Phys. D: Appl. Phys. 51:203002
- Kurtz, T.W., Al-Bander, H.A. and Morris, R.C.J. (1987). "Salt-sensitive" essential hypertension in men. Is the sodium ion alone important? N. Engl. J. Med., 317(17): 1043–1048.
- Lagutina L.E., Shtannikov, E.V. and Makarova, O.A. (1990). Health status and some indicators of function of excretory system in children in conditions of long term consumption of water with unsuitable mineral composition (in Russian). Pediatria, (9): 40-44.
- Larson, T.E. and Skold, R.V. (1958). Current research on corrosion and tuberculation of cast iron. J. Am. Water Works Assoc., 50(11): 1429–1432.
- Lawler, D.S. and Kweon, J.H. (2003). Integrated water treatment: Softening and ultrafiltration. AWWA Research Foundation, Denver, Colorado.
- Lazur, A., VanDerwerker, T. and Koepenick, K. (2020). Review of implications of road salt use on groundwater quality—corrosivity and mobilization of heavy metals and radionuclides. Water, Air, & Soil Pollution, 231(9). DOI: 10.1007/s11270-020-04843-0.
- Leentvaar, J., and Rebhun, M. (1982). Effect of magnesium and calcium precipitation on coagulation-flocculation with lime. Water Res., 16(5): 655–662.
- Legator, M.S., Singleton, C.R., Morris, D.L. and Philips, D.L. (2001). Health effects from chronic low-level exposure to hydrogen sulfide. Arch. Environ. Health, 56(2): 123–131.
- Leidi, M., Wolf, M. and Maier, J.A.M. (2011). Magnesium and cancer: More questions than answers. In: Magnesium in the central nervous system [internet]. Vink, R. and Nechifor, M. (eds.). Adelaide, Australia: University of Adelaide Press, Available at: https://www.ncbi.nlm.nih.gov/books/NBK507261/
- Lemley, A.T., Schwartz, J.J. and Wagenet, L.P. (1999). Hydrogen sulphide in household drinking water. Cornell University, Fact Sheet 7.
- LeRoy, P., Schock, M.R., Wagner, I. and Holtschulte, H. (1996). Cement-based materials. In: Internal Corrosion of Water Distribution Systems. 2nd ed. American Water Works Association Research Foundation and DVGW Technologiezentrum Wasser, Denver, Colorado. pp. 313–388.
- Lesimple, A., Ahmed, F.E., and Hilal, N. (2020). Remineralization of desalinated water: Methods and environmental impact. Desalination, 496: 114692.
- Levine, A.D., Raymer, B.J. and Jahn, J. (2004a). Hydrogen sulfide and turbidity control using catalysed oxidation coupled with filtration for groundwater treatment. J. Water Supply Res. Technol., 53(5): 325–337.
- Levine, A.D., Raymer, B.J. and Jahn, J. (2004b). Evaluation of biological hydrogen sulfide oxidation coupled with two-stage upflow filtration for groundwater treatment. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng., 39(5): 1263–1279.
- Levine, A.D., Romero-Cotrino, C., Minnis, R.J., Perone, J. and Amitzoglou, P. (2006). Biologically enhanced anion exchange for removal of hydrogen sulfide from groundwater. WQTC conference. American Water Works Association, Denver, Colorado.
- Lewis, R.J. and Copley, G.B. (2015). Chronic low-level hydrogen sulfide exposure and potential effects on human health: A review of the epidemiological evidence. Crit. Rev. Toxicol., 45(2): 93–123.
- Lietz, G., Avenell, A. and Robins, S.P. (1997). Short-term effects of dietary sodium intake on bone metabolism in postmenopausal women measured using urinary deoxypyridinoline excretion. Br. J. Nutr., 78(1): 73–82.
- Lifton, R.P., Wilson, F.H., Choate, K.A. and Geller, D.S. (2002). Salt and blood pressure: New insight from human genetic studies. Cold Spring Harb. Symp. Quant. Biol., 67: 445–450.
- Lin, P., Ginty, F., Appel, L.J., Aickin, M., Bohannon, A., Garnero, P., Barclay, D. and Svetkey, L.P. (2003). The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J. Nutr., 133(10): 3130655–3136.
- Lin, T.F., Watson, S., Dietrich, A.M., and Suffet, I. H. (2019). Taste and odour in source and drinking water: causes, controls, and consequences. IWA Publishing, London, United Kingdom. DOI: 10.2166/9781780406664.
- Liu, C., Kuang, X., Li, K., Guo, X., Deng, Q. and Li, D. (2020). Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Food Funct., 11(12): 10817–10827.
- Liu, Z., Haddad, M., Sauvé, S., and Barbeau, B. (2021). Alleviating the burden of ion exchange in brine water treatment: From operational strategies to brine management. Water Res., (205): 117728.
- Logsdon, G. S., Frey, M.M., Stefanich, M.D., Johnson, S.L., Feely, D.E., Rose, J.B. and Sobsey, M. (1994). The removal and disinfection efficiency of lime softening processes for Giardia and Viruses. Awwa Research Foundation and American Water Works Association, Denver, Colorado (Project #90648).
- Logue, J.N., Ramaswamy, K. and Hersh, J.H. (2001). Investigation of illness associated with exposure to hydrogen sulfide among Pennsylvania school students. J. Environ. Health, 63(6): 9–13.
- Luft, F.C., Rankin, L.I., Bloch, R., Weyman, A.E., Willis, L.R., Murray, R.H., Grim, C.E. and Weinberger, M.H. (1979). Cardiovascular and humoral responses to extremes of sodium intake in normal black and white men. Circulation, 60(3): 697–706.
- Lutaĭ, G.F. (1992). Chemical composition of the drinking water and the health of the population (in Russian). Gig. Sanit., 1(1): 13–15.
- Lyn, T.L and Taylor, J.S. (1992). Assessing sulfur turbidity formation following chlorination of hydrogen sulfide in groundwater. J. Am. Water Works Assoc., 84(9): 103–112.
- MacGregor, G.A., Markandu, N.D., Sagnella, G.A., Singer, D.R. and Cappuccio, F.P. (1989). Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet, 2(8674): 1244–1247.
- Manitoba Sustainable Development (2021). Personal communication with K. Philip, Water Quality Management Section.
- Marriott, R.A., Payman, P., Marrugo-Hernandez, J.J. and Raval, S. (2016). Hydrogen sulfide formation in oil and gas. Can. J. Chem., 94(4): 406–413.
- Mascioli, S., Grimm, R., Jr, Launer, C., Svendsen, K., Flack, J., Gonzalez, N., Elmer, P. and Neaton, J. (1991). Sodium chloride raises blood pressure in normotensive subjects. The study of sodium and blood pressure. Hypertension, 17(1 Suppl): I21–I26.
- McCallum, L., Jeemon, P., Hastie, C.E., Patel, R.K., Williamson, C., Redzuan, A.M., Dawson, J., Sloan, W., Muir, S., Morrison, D., McInnes, G.T., Freel, E.M., Walters, M., Dominiczak, A.F., Sattar, N. and Padmanabhan, S. (2013). Serum chloride is an independent predictor of mortality in hypertensive patients. Hypertension, 62(5): 836–843.
- McCallum, L., Lip, S. and Padmanabhan, S. (2015). The hidden hand of chloride in hypertension. Pflugers Arch., 467(3): 595–603.
- McGowan, W. (2000). Water processing: Residential, commercial, light-industrial. 3rd ed. Water Quality Association.
- McNally, N.J., Williams, H.C., Phillips, D.R., Smallman-Raynor, M., Lewis, S., Venn, A. and Britton, J. (1998). Atopic eczema and domestic water hardness. Lancet, 352(9127): 527–531.
- McParland, B.E., Goulding, A. and Campbell, A.J. (1989). Dietary salt affects biochemical markers of resorption and formation of bone in elderly women. BMJ, 299(6703): 834–835.
- Megonnell, N.E. and Spotts S.D. (1994). Can "sulfur water" be cured with carbon? It may help oxidize one odor-causing gas. Water technol., 19(3): 128–32.
- Melles, Z. and Kiss, S.A. (1992). Influence of the magnesium content of drinking water and of magnesium therapy on the occurrence of preeclampsia. Magnes. Res., 5(4): 277–279.
- Mercer, K.L., Lin, Y.-P. and Singer, P.C. (2005). Enhancing calcium carbonate precipitation by heterogeneous nucleation during chemical softening. J. Am. Water Works Assoc., 97(12) : 116–125.
- Meride, Y. and Ayenew, B. (2016). Drinking water quality assessment and its effects on residents health in Wondo Genet campus, Ethiopia. Environmental Systems Research, 5(1): 1–7.
- Meunier, P.J., Jenvrin, C., Munoz, F., de la Gueronnière, V., Garnero, P. and Menz, M. (2005). Consumption of a high calcium mineral water lowers biochemical indices of bone remodeling in postmenopausal women with low calcium intake. Osteoporos. Int., 16(10): 1203–1209.
- Mickleborough, T.D., Gotshall, R.W., Kluka, E.M., Miller, C.W. and Cordain, L. (2001). Dietary chloride as a possible determinant of the severity of exercise-induced asthma. Eur. J. Appl. Physiol., 85(5): 450–456.
- Milby, T.H. (1962). Hydrogen sulfide intoxication. Review of the literature and report of unusual accident resulting in two cases of nonfatal poisoning. J. Occup. Med., 4: 431–437.
- Milby, T.H. and Baselt, R.C. (1999a). Hydrogen sulfide poisoning: Clarification of some controversial issues. Am. J. Ind. Med., 35(2): 192–195.
- Milby, T.H. and Baselt, R.C. (1999b). Health hazards of hydrogen sulfide: Current status and future directions. Environ. Epidemiol. Toxicol., 1: 262–269.
- Ministère de l'Environnement et de la Lutte contre les changements climatiques (2021). Personal communication with P. Cantin.
- Missouri Department of Natural Resources (2024). Chloride factsheet. Available at: https://dnr.mo.gov/water/hows-water/pollutants-sources/chloride
- Miyahara, J., Aramaki, S. and Yokochi, K. (2009). Dietary chloride deficiency due to new liquid nutritional products. Pediatr. Int., 51(2): 197–200.
- Miyake, Y., Yokoyama, T., Yura, A., Iki, M. and Shimizu, T. (2004). Ecological association of water hardness with prevalence of childhood atopic dermatitis in a Japanese urban area. Environ. Res., 94(1): 33–37.
- Montgomery, J.M. (1985). Water treatment: Principles and design. John Wiley & Sons, Hoboken, New Jersey.
- Moore, E. (1952). Physiological effects of the consumption of saline drinking water. A progress report to the 16th meeting of the subcommittee on sanitary engineering and environment. Appendix B. Anonymous Washington D.C.: National Academy of Sciences. pp. 221–227.
- Moore-Schiltz, L., Albert, J.M., Singer, M.E., Swain, J. and Nock, N.L. (2015). Dietary intake of calcium and magnesium and the metabolic syndrome in the national health and nutrition examination (NHANES) 2001–2010 data. Br. J. Nutr., 114(6): 924–935.
- Morimoto, A., Uzu, T., Fujii, T., Nishimura, M., Kuroda, S., Nakamura, S., Inenaga, T. and Kimura, G. (1997). Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet, 350(9093): 1734–1737.
- Morris, R.C.J., Sebastian, A., Forman, A., Tanaka, M. and Schmidlin, O. (1999). Normotensive salt sensitivity: Effects of race and dietary potassium. Hypertension, 33(1): 18–23.
- Mudryi, I.V. (1999). Effects of the mineral composition of drinking water on the population´s health (review) (in Russian). Gig. Sanit., 1: 13–15.
- Muezzinoglu A. (2003). A study of volatile organic sulfur emissions causing urban odors. Chemosphere, 51(4): 245–252.
- Mulford, L.A., Taylor, J.S., Nickerson, D.M., and Chen, S.-S. (1999). NF performance at full and pilot scale. J. Am. Water Works Assoc., 91(6): 64–76.
- Munter, R., Kallas, J., Trapido, M., Veressinina, Y. and Veil, H. (1999). Estonian ground water: Quality problems and technology for improvement. Kem.-Kemi, 26(7): 552–556.
- Munter, R. and Vilu, H. (2008). Drinking water production from well water with high sulfur and sulfur bacteria content. J. Environ. Eng., 134(5): 376–381.
- Muzalevskaya, L.S., Lobkovskiy A.G. and Kukarina, N.I. (1993). Prevalence of cholelithiasis and urolithiasis, osteoporosis and salt arthropathy in relation to water hardness (in Russian). Gig. Sanit., 58(12): 17–20.
- Narotsky, M.G., Pressman, J.G., Miltner, R.J., Speth, T.F., Teuschler, L.K., Rice, G.E., Richardson, S.D., Best, D.S., McDonald, A., Hunter III, E.S. and Simmons, J.E. (2012). Developmental toxicity evaluations of whole mixtures of disinfection by-products using concentrated drinking water in rats: Gestational and lactational effects of sulfate and sodium. Birth Defects Res. Part B Dev. Reprod. Toxicol., 95(3): 202–212.
- Nasr, A.B., Charcosset, C., Amar, R.B. and Walha, K. (2013). Defluoridation of water by nanofiltration. J. Fluor. Chem., 150: 92–97.
- National Drinking Water Clearinghouse (1998). Iron and manganese removal. Tech Brief 9 – A National Drinking Water Clearinghouse Fact Sheet. Available at: https://actat.wvu.edu/files/d/a0d141dd-f559-412b-91df-bd1d14c52545/iron-mn-removal.pdf
- New Brunswick Department of Health (2021). Personal communication with K. Gould.
- Newfoundland and Labrador Municipal Affairs and Environment (2021). Personal communication with H. Khan.
- NHMRC, NRMMC (2011). Australian Drinking Water Guidelines Paper 6 National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia. Available at: https://www.nhmrc.gov.au/about-us/publications/australian-drinking-water-guidelines
- Nie, Z., Wang, Z., Zhou, B., Tang, Z. and Wang, S. (2013). Magnesium intake and incidence of stroke: Meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis., 23(3): 169–176.
- Nova Scotia Environment (2021). Personal communication with A. Polegato.
- NPRI [database on the Internet]. (2022). 2013–2017. H2S data for various reporting years. National Pollutant Release Inventory Gatineau, Quebec: Environment Canada. Available at: https://pollution-waste.canada.ca/national-release-inventory/
- NSF/ANSI (2023a). NSF International/American National Standards Institute Standard 62: Drinking water distillation systems. NSF International, Ann Arbor, Michigan.
- NSF/ANSI (2023b). NSF International/American National Standards Institute Standard 42: Drinking water treatment units-Aesthetic effects. NSF International, Ann Arbor, Michigan.
- NSF/ANSI (2023c). NSF International/American National Standards Institute Standard 58: Reverse osmosis
- drinking water treatment systems. NSF International, Ann Arbor, Michigan.
- NSF/ANSI (2024). NSF International/American National Standards Institute Standard 44: Residential cation exchange water softeners. NSF International, Ann Arbor, Michigan.
- NSF/ANSI/CAN (2024). NSF International/American National Standards/National Standard of Canada Standard 60: Drinking water treatment chemicals – Health effects. NSF International, Ann Arbor, Michigan.
- Odell, L.H. (2010). Treatment technologies for groundwater. American Water Works Association, Denver, Colorado.
- OMOE (2007). Ontario air standard for total reduced sulphur. Standards Development Branch, Ontario Ministry of the Environment.
- Ontario Ministry of the Environment, Conservation and Parks (2021). Personal communication with S. Deshpande.
- Ortenberg, E., Groisman, L. and Rav-Acha, C. (2000). Taste and odour removal from an urban groundwater establishment- A case study. Water Sci. Technol., 42(1–2): 123–128.
- Pandey, S.K., Kim, K.-H. and Tang, K.-T. (2012). A review of sensor-based methods for monitoring hydrogen sulfide. Trends Analyt. Chem., 32: 87–99.
- Parsons, F.M., Edwards, G.F., Anderson, C.K., Ahmad, S., Clark, P.B., Hetherington, C. and Young, G.A. (1974). Regression of malignant tumours in magnesium and potassium depletion induced by diet and haemodialysis. Lancet, 1(7851): 243–244.
- Peacock, M. (2010). Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol., 5 Suppl 1: S23–30.
- PEI Department of Communities, Land and Environment (2021). Personal communication with G. Somers.
- Perkin, M.R., Craven, J., Logan, K., Strachan, D., Marrs, T., Radulovic, S., Campbell, L.E., MacCallum, S.F., McLean, W.H., Lack, G., Flohr, C. and Enquiring About Tolerance Study Team (2016). Association between domestic water hardness, chlorine, and atopic dermatitis risk in early life: A population-based cross-sectional study. J. Allergy Clin. Immunol., 138(2): 509–516.
- Peterson, N. (1951). Sulfates in drinking water. Official Bulletin: North Dakota Water and Sewage Works Conference (ed.). pp. 6–11.
- Pieper, K.J., Tang, M., Jones, C.N., Weiss, S., Greene, A., Mohsin, H., Parks, J. and Edwards, M.A. (2018). Impact of road salt on drinking water quality and infrastructure corrosion in private wells. Environ. Sci. Technol., 52(24) : 14078–14087.
- Pouliquen, F., Blanc, C. and Arretz, E. (1989). Hydrogen sulfide. In: Elvers, B., Hawkins, S., Ravenscroft, M. and Schultz, G., eds. Ullmann's Encyclopedia of Industrial Chemistry. Deerfield Beach, Florida. VCH Publishers. A13:467–485.
- Poursafa, P., Kelishadi, R., Amin, M.M., Hashemi, M. and Amin, M. (2014). First report on the association of drinking water hardness and endothelial function in children and adolescents. Arch. Med. Sci., 10(4): 746–751.
- Prud'Homme, M. (1985). Salt. In : Canadian minerals yearbook, 1985: review and outlook, report on minerals nº 34. Energy, Mines and Resources Canada. Ottawa, Ontario.
- Pushpalatha, N., Sreeja, V., Karthik, R. and Saravanan, G. (2022). Total dissolved solids and their removal techniques. Int. J. Environ. Sustain. Prot., 2(2): 13-20 DOI: 10.35745/ijesp2022v02.02.0002
- Radlinski, M. and Wolf, J. (2016). Condition assessment and service life analysis of an asbestos-cement sewer pipe. Proceedings of the Pipelines 2016 Conference, 2016, Kansas City, Missouri. American Society of Civil Engineers (ASCE), pp. 321–333.
- Rahman, M.A., Islam, M.R., Kumar, S. and Al-Reza, S.M. (2021). Drinking water quality, exposure and health risk assessment for the school-going children at school time in the southwest coastal of Bangladesh. J. Water Sanit. Hyg. Develop., 11(4): 612–628.
- Randtke, S.J., Thiel, C.E., Liao, M.Y. and Yamaya, C.N. (1982). Removing soluble organic contaminants by lime-softening. J. Am. Water Works Assoc., 74(4): 192–202.
- Rapant, S., Cvečková, V., Fajčíková, K., Hajdúk, I., Hiller, E. and Stehlíková, B. (2019). Hard water, more elastic arteries: A case study from Krupina District, Slovakia. Int. J. Environ. Res. Public. Health., 16(9): 1521.
- Reiffenstein, R.J., Hulbert, W.C. and Roth, S.H. (1992). Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol., 32: 109–134.
- Renner, R. (2006). Lead in water linked to coagulant. Environ. Sci. Technol., 40(17): 5164–5165.
- Ribeiro, D.A., Marques, M.E.A. and Salvadori, D.M.F. (2004). Lack of genotoxicity of formocresol, paramonochlorophenol, and calcium hydroxide on mammalian cells by comet assay. J. Endod., 30(8): 593–596.
- Richardson, D.B. (1995). Respiratory effects of chronic hydrogen sulfide exposure. Am. J. Ind. Med., 28(1): 99–108.
- Robinson, H.K., Hasenmueller, E.A. and Chambers, L.G. (2017). Soil as a reservoir for road salt retention leading to its gradual release to groundwater. Appl. Geochem., 83: 72–85.
- Rodríguez-Morán, M., Simental Mendía, L.E., Zambrano Galván, G. and Guerrero-Romero, F. (2011). The role of magnesium in type 2 diabetes: A brief based-clinical review. Magnes. Res., 24(4): 156–162.
- Rodriguez-Soriano, J., Vallo, A., Castillo, G., Oliveros, R., Cea, J.M. and Balzategui, M.J. (1983). Biochemical features of dietary chloride deficiency syndrome: A comparative study of 30 cases. J. Pediatr., 103(2): 209–214.
- Romani, A.M.P. (2013). Magnesium in health and disease. In: Interrelations between essential metal ions and human diseases. Sigel, A., Sigel, H. and Sigel, R.K.O., eds. Springer, Germany, pp. 49–79.
- Rosborg, I. and Kozisek, F. (2020). Drinking water minerals and mineral balance: Importance, health significance, safety precautions. 2nd ed. Springer, Cham, Switzerland.
- Rossum, J. (1955). Split-treatment softening of water. Ind. Eng. Chem., 47(11): 2313–2317.
- RTW, Inc. (2008). Tetra Tech (RTW) model for water chemistry, process and corrosion control. Rothberg, Tamburini and Winsor, Inc. (Tetra Tech RTW). AWWA, Denver, Colorado.
- Rude, R.K., Singer, F.R. and Gruber, H.E. (2009). Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr., 28(2): 131–141.
- Rylova, N.V. (2005). Influence of mineral composition of drinking water on health status of children (in Russian). Gig. Sanit., 70(1): 45–46.
- Sacks, F.M., Svetkey, L.P., Vollmer, W.M., Appel, L.J., Bray, G.A., Harsha, D., Obarzanek, E., Conlin, P.R., Miller, E.R.3rd, Simons-Morton, D.G., Karanja, N., Lin, P.H. and DASH-Sodium Collaborative Research Group (2001). Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-sodium collaborative research group. N. Engl. J. Med., 344(1): 3–10.
- Saitua, H., Gil, R. and Padilla, A.P. (2011). Experimental investigation on arsenic removal with a nanofiltration pilot plant from naturally contaminated groundwater. Desalination, 274: 1–6.
- Sakhaee, K., Harvey, J.A., Padalino, P.K., Whitson, P. and Pak, C.Y. (1993). The potential role of salt abuse on the risk for kidney stone formation. J. Urol., 150(2 Pt 1): 310–312.
- Sanders, T., Liu, Y.M. and Tchounwou, P.B. (2015). Cytotoxic, genotoxic, and neurotoxic effects of Mg, Pb, and Fe on pheochromocytoma (PC-12) cells. Environ. Toxicol., 30(12): 1445–1458.
- Saskatchewan Water Security Agency (2021). Personal communication with S. Hase.
- Saunders, S. and Urbans, M.J. (1995). Count on catalytic carbons for hydrogen sulfide. They can help you handle its offensive odors. Water technol. 18(6): 41–44.
- Saunders., S. and Lee, M. (1996). The bottom line on sulfide removal. Catalytic carbon can provide cost-effective removal. Water technol. 19(7): 24–26.
- Schock, M.R (1999). Internal corrosion and deposition control. Chapter 17 in: Water quality and treatment: a handbook of community water supplies, 5th edition. Letterman, R.D. (ed). American Water Works Association. Denver, Colorado.
- Schock, M.R. and Lytle, D.A. (2011). Chapter 20: Internal corrosion and deposition control. In: Edzwald, J.K., ed., Water quality and treatment: A handbook on drinking water. Sixth edition. McGraw-Hill, New York, New York.
- Schofield, R. and Hsieh, D. (1983). Criteria and recommendations for standards for sulfate in military field supplies. Lawrence Livermore National Laboratory, Contract no. UCRL-53481-4, Livermore, California.
- Schroeder, H.A. (1960). Relation between mortality from cardiovascular disease and treated water supplies: Variations in states and 163 largest municipalities of the United States. J. Am. Med Assoc., 172(17): 1902–1908.
- Schroeder, H.A. (1966). Municipal drinking water and cardiovascular death rates. J. Am. Med. Assoc., 195(2): 81–85.
- Shah, S.C., Zhu, X., Dai, Q., Peek, R.M. and Shrubsole, M.J. (2021). Magnesium intake is associated with a reduced risk of incident liver cancer, based on an analysis of the NIH-American Association of Retired Persons (NIH-AARP) diet and health study prospective cohort. J. Clin. Nutr., 113(3): 630–638.
- Shore, A.C., Markandu, N.D. and MacGregor, G.A. (1988). A randomized crossover study to compare the blood pressure response to sodium loading with and without chloride in patients with essential hypertension. J. Hypertens., 6(8): 613–617.
- Shtannikov, E.V. and Obyedkova, G.Y. (1984). Effect of level of drinking water mineralization on female reproductive functions (in Russian). Gig. Sanit., 49(9): 20–23.
- Shtannikov E.V., Lagutina, L.E. and Maneshina O.A. (1986). Effect of drinking water of high total dissolved solids on urinary tract in children (in Russian). Gig. Sanit., 51(10): 90–92.
- Shuey, B.S. (1966). Economics of split‐treatment water softening. J. Am. Water Works Assoc., 58(1): 107–112.
- Silk, L.N., Greene, D.A. and Baker, M.K. (2015). The effect of calcium or calcium and vitamin D supplementation on bone mineral density in healthy males: A systematic review and meta-analysis. Int. J. Sport Nutr. Exerc. Metab., 25(5): 510–524.
- Silva, C.S., Moutinho, C., Ferreira da Vinha, A. and Matos, C. (2019). Trace minerals in human health: Iron, zinc, copper, manganese and fluorine. International Journal of Science and Research Methodology, 13(3): 57–80.
- Smeets, P.W.M.H., Medema, G.J. and van Dijk, J.C. (2009). The Dutch secret: how to provide safe drinking water without chlorine in the Netherlands. Drink. Water Eng. Sci., 2: 1–14.
- Snyder, J.W., Safir, E.F., Summerville, G.P. and Middleberg, R.A. (1995). Occupational fatality and persistent neurological sequelae after mass exposure to hydrogen sulfide. Am. J. Emerg. Med., 13(2): 199–203.
- Song, X., Li, Z., Ji, X. and Zhang, D. (2017). Calcium intake and the risk of ovarian cancer: A meta-analysis. Nutrients, 9(7): 679.
- Stefan, D.S., Meghea, I. and Stefan, D.M. (2018). Comparative study on the removal of hydrogen sulfide and ammonia from natural water using oxygen, air and chlorine. Proceedings of the 18th International Multidisciplinary Scientific GeoConference SGEM, July 2018, 18: pp. 227–234.
- Stets., E.G., Lee, C.J., Lytle, D.A. and Schock, M.R. (2018) Increasing chloride in rivers of the conterminous U.S. and linkages to potential corrosivity and lead action level exceedances in drinking water. Sci. Total Environ.,. 613(2018): 1498–1509.
- Tai, V., Leung, W., Grey, A., Reid, I.R. and Bolland, M.J. (2015). Calcium intake and bone mineral density: Systematic review and meta-analysis. BMJ, 351: h4183.
- Tang, C., Merks, C.W.A.M. and Albrechtsen, H.-J. (2019). Water softeners add comfort and consume water – comparison of selected centralised and decentralised softening technologies. Water Supply, 19(7): 2088–2097.
- Tang, C., Godskesen, B., Aktor, H., van Rijn, M., Kristensen, J.B., Rosshaug, P.S., Albrechtsen, H.-J. and Rygaard, M. (2021). Procedure for calculating the calcium carbonate precipitation potential (CCPP) in drinking water supply: Importance of temperature, ionic species and open/closed system. Water, 13(42): 42.
- Tekerlekopoulou, A.G., Papazafiris, P.G.D. and Vayenas, D.V. (2010). A full-scale trickling filter for the simultaneous removal of ammonium, iron and manganese from potable water. J. Chem. Technol. Biotechnol., 85(7): 1023–1026.
- Teschke, K., Ahrens, W., Andersen, A., Boffetta, P., Fincham, S., Finkelstein, M., Henneberger, P., Kauppinen, T., Kogevinas, M., Korhonen, K., Liss, G., Liukkonnen, T., Osvoll, P., Savela, A., Szadkowska-Stanczyk, I., Westberg, H. and Widerkiewicz, K. (1999). Occupational exposure to chemical and biological agents in the nonproduction departments of pulp, paper, and paper product mills: An international study. Am. Ind. Hyg. Assoc. J., 60(1): 73–83.
- Thomas, J.F.J. (1953). Industrial water resources of Canada: Scope, procedure, and interpretation of survey studies. Dept. of Mines and Technical Surveys, Ottawa, Ontario.
- Thompson, M.A., Kelkar, U.G. and Vickers, J.C. (1995). The treatment of groundwater containing hydrogen sulfide using microfiltration. Desalination, 102(1–3): 287–291.
- Tuck, M., Corry, D. and Trujillo, A. (1990). Salt-sensitive blood pressure and exaggerated vascular reactivity in the hypertension of diabetes mellitus. Am. J. Med., 88(3): 210–216.
- Tucker, K.L., Hannan, M.T., Chen, H., Cupples, L.A., Wilson, P.W. and Kiel, D.P. (1999). Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am. J. Clin. Nutr., 69(4): 727–736.
- Tvedt, B., Skyberg, K., Aaserud, O., Hobbesland, A. and Mathiesen, T. (1991a). Brain damage caused by hydrogen sulfide: A follow-up study of six patients. Am. J. Ind. Med., 20(1): 91–101.
- Tvedt, B., Edland, A., Skyberg, K. and Forberg, O. (1991b). Delayed neuropsychiatric sequelae after acute hydrogen sulfide poisoning: Affection of motor function, memory, vision and hearing. Acta Neurol. Scand., 84(4): 348–351.
- U.S. EPA (1971a). EPA-NERL: 130.2: Hardness, total (mg/L as CaCO3) (titrimetric, ethylenediamine tetraacetate). Environmental Monitoring Systems Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
- U.S. EPA (1971b). EPA-NERL: 375.1: Sulfate (colorimetric, automated, chloranilate). Environmental Monitoring Systems Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
- U.S. EPA (1978a). EPA-NERL: 215.1: Calcium (atomic absorption, direct aspiration). Environmental Monitoring Systems Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
- U.S. EPA (1978b). EPA-NERL: 242.1: Magnesium (atomic absorption, direct aspiration). Environmental Monitoring Systems Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
- U.S. EPA (1978c). EPA-NERL: 215.2: Calcium (titrimetric, ethylenediamine tetraacetate). Environmental Monitoring Systems Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
- U.S. EPA (1978d). EPA-NERL: 375.4: Sulfate (turbidimetric). Environmental Monitoring Systems Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
- U.S. EPA (1988). National Secondary Drinking Water Regulations. CFR 40 Part 143, 53 FR 37412, Sept. 26, 1988. United States Environmental Protection Agency, Washington D.C.
- U.S. EPA (1993a). Health assessment document for hydrogen sulfide. Office of Research and Development, United States Environmental Protection Agency, Washington D.C. (Document ID: EPA/600/8-86/026F).
- U.S. EPA (1993b). EPA-NERL: 375.2: Determination of sulfate by automated colorimetry. Revision 2.0. Environmental Monitoring Systems Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
- U.S. EPA (1994). EPA-NERL: 200.7: Determination of metals and trace elements in water and wastes by inductively coupled plasma-atomic emission spectrometry. Revision 4.4. Environmental Monitoring Systems Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
- U.S. EPA (1999a). Health effects from exposure to sulfate in drinking water workshop. Office of Water, EPA 815-R-99-002, Washington D.C. Available at: https://nepis.epa.gov/Exe/ZyPDF.cgi/200021JF.PDF?Dockey=200021JF.PDF
- U.S. EPA (1999b). Health effects from exposure to high levels of sulfate in drinking water study. Office of Water, EPA 815-R-99-001, Available at: https://nepis.epa.gov/Exe/ZyPDF.cgi/200021IN.PDF?Dockey=200021IN.PDF
- U.S. EPA (1999c). METHOD 300.1 revision 1.0: Determination of inorganic anions in drinking water by ion chromatography. National Exposure Research Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio. Available at: https://www.epa.gov/sites/default/files/2015-06/documents/epa-300.1.pdf
- U.S. EPA (1999d). Enhanced coagulation and enhanced precipitative softening guidance manual. Office of Water, United States Environmental Protection Agency (Document ID: EPA-815-R-99-012).
- U.S. EPA (2003a). Drinking water advisory: Consumer acceptability advice and health effects analysis on sulfate. Washington D.C. Available at: https://www.epa.gov/sites/default/files/2014-09/documents/support_cc1_sulfate_healtheffects.pdf
- U.S. EPA (2003b). Toxicological review of hydrogen sulfide (CAS no. 7783-06-4) in support of summary information on the integrated risk information system (IRIS). Washington D.C. Available at: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0061tr.pdf
- U.S. EPA (2003c). Method 200.5 revision 4.2: Determination of trace elements in drinking water by axially viewed inductively coupled plasma-atomic emission spectrometry. Environmental Monitoring Systems Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio. Available at: https://www.epa.gov/sites/default/files/2015-08/documents/method_200-5_rev_4-2_2003.pdf
- USGS (1985a). Method: I-7152: Calcium, suspended-recoverable, water, FLAA. United States Geological Survey, National Water Quality Laboratory.
- USGS (1985b). Method: I-1447: Magnesium, dissolved, FLAA. United States Geological Survey, National Water Quality Laboratory.
- USGS (1985c). Method: I-1338: Hardness in water by colorimetric titration. United States Geological Survey, National Water Quality Laboratory.
- USGS (1985d). Method L: I-3840: Sulfide, titrimetric, iodometric. United States Geological Survey, National Water Quality Laboratory.
- USGS (2018). Hardness of water. USGS Water Science School. United States Geological Survey. Washington DC. Available at: https://www.usgs.gov/special-topics/water-science-school/science/hardness-water
- USGS (2023). Frequently asked questions about water. United States Geological Survey. Washington DC. Available at: https://www.usgs.gov/faqs/do-you-have-information-about-water-hardness-united-states
- Van Der Aa, M. (2003). Classification of mineral water types and comparison with drinking water standards. Environ. Geol., 44(5): 554–563.
- Van der Bruggen, B., Everaert, K., Wilms, D. and Vandecasteele, C. (2001). Application of nanofiltration for removal of pesticides, nitrate and hardness from ground water: Rejection properties and economic evaluation. J. Memb. Sci., 193: 239–248.
- Vanhoorne, M., de Rouck, A. and de Bacquer, D. (1995). Epidemiological study of eye irritation by hydrogen sulphide and/or carbon disulphide exposure in viscose rayon workers. Ann. Occup. Hyg., 39(3): 307–315.
- Vidović, M.M., Jovićević, J.N., Krstić, J.D., Tomić, I.D. and Rogan, S.S. (2010). The influence of pH on removal of H2S and natural organic matter by anion resin. Desalination Water Treat., 21(1–3): 255–263.
- Vingerhoeds, M.H., Nijenhuis-de Vries, M.A., Ruepert, N., van der Laan, H., Bredie, W.L.P. and Kremer, S. (2016). Sensory quality of drinking water produced by reverse osmosis membrane filtration followed by remineralisation. Water Res., 94: 42–51.
- Vollertsen, J., Nielsen, A.H., Jensen, H.S., Wium-Andersen, T. and Hvitved-Jacobsen, T. (2008). Corrosion of concrete sewers-the kinetics of hydrogen sulfide oxidation. Sci. Total Environ. 394(1): 162–170.
- Vollmer, W.M., Sacks, F.M., Ard, J., Appel, L.J., Bray, G.A., Simons-Morton, D.G., Conlin, P.R., Svetkey, L.P., Erlinger, T.P., Moore, T.J., Karanja, N. and DASH-Sodium Trial Collaborative Research Group (2001). Effects of diet and sodium intake on blood pressure: Subgroup analysis of the DASH-sodium trial. Ann. Intern. Med., 135(12): 1019–1028.
- Wacker, W.E.C. and Parisi, A.F. (1968). Magnesium metabolism. N. Engl. J. Med., 278(13): 712–717.
- Wang, X., Terry, P. and Yan, H. (2009). Review of salt consumption and stomach cancer risk: Epidemiological and biological evidence. World J. Gastroenterol., 15(18): 2204–2213.
- Wasch, H.H., Estrin, W.J., Yip, P., Bowler, R. and Cone, J.E. (1989). Prolongation of the P-300 latency associated with hydrogen sulfide exposure. Arch. Neurol., 46(8): 902–904.
- Watts, D.L. (1997). Trace elements and other essential nutrients: Clinical application of tissue mineral analysis. 2nd writer's B-l-o-c-k edition.
- Watts, S.F. (2000). The mass budgets of carbonyl sulfide, dimethyl sulfide, carbon disulfide and hydrogen sulfide. Atmos. Environ. 34(5): 761–779.
- Weaver, C.M., Alexander, D.D., Boushey, C.J., Dawson-Hughes, B., Lappe, J.M., LeBoff, M.S., Liu, S., Looker, A.C., Wallace, T.C. and Wang, D.D. (2016). Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the national osteoporosis foundation. Osteoporos. Int., 27(1): 367–376.
- Weinberger, M.H. (1993). Racial differences in renal sodium excretion: Relationship to hypertension. Am. J. Kidney Dis., 21(4 Suppl 1): 41–45.
- Wesselink, E., Kok, D.E., Bours, M.J.L., De Wilt, J.H.W., Van Baar, H., Van Zutphen, M., Geijsen, A.M.J.R., Keulen, E.T.P., Hansson, B.M.E., Van Den Ouweland, J., Witkamp, R.F., Weijenberg, M.P., Kampman, E. and Van Duijnhoven, F.J.B. (2020). Vitamin D, magnesium, calcium, and their interaction in relation to colorectal cancer recurrence and all-cause mortality. Am. J. Clin. Nutr., 111(5): 1007–1017.
- Wetterau, H., Oekert, W. and Knape, U.G. (1964). Tests for the application of dried green fodder with higher hydrogen sulfide content (experiments with poultry and fattened pigs). Fetterung. 5: 383–393 (In German).
- WHO (1981). Hydrogen sulfide. (Environmental Health Criteria, No. 19), Geneva, Switzerland.
- WHO (1987). Air quality guidelines for Europe. Copenhagen, Denmark: WHO Regional Office for Europe, Available at: https://apps.who.int/iris/handle/10665/107364
- WHO (2000). Chapter 6.6: Hydrogen sulfide. In: Air quality guidelines: Part II. Evaluation of human health risks. Regional Office for Europe, World Health Organization, Copenhagen, Denmark.
- WHO (2003). Hydrogen sulfide in drinking-water. Background document for development of WHO guidelines for drinking-water quality. WHO/SDE/WSH/03.04/07, Geneva, Switzerland. Available at: https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/hydrogensulfide-bd.pdf?sfvrsn=2f03ad98_4
- WHO (2009). Calcium and magnesium in drinking water: Public health significance. Geneva, Switzerland. Available at: https://apps.who.int/iris/bitstream/handle/10665/43836/9789241563550_eng.pdf
- WHO (2011). Hardness in drinking-water. Background document for development of WHO guidelines for drinking-water quality. WHO/HSE/WSH/10.01/10, Geneva, Switzerland. Available at: https://apps.who.int/iris/handle/10665/70168
- WHO (2017). Guidelines for drinking-water quality, 4th edition, incorporating the 1st addendum. Available at: https://www.who.int/publications/i/item/9789241549950
- Willey, B.F., Jennings, H. and Muroski, F. (1964). Removal of hydrogen sulfide with potassium permanganate. J. Am. Water Works Assoc., 56(4): 475–479.
- Wu, Z., Zhang, X., Pang, J., Li, J., Li, J. and Zhang, P. (2020). High-poly-aluminum chloride sulfate coagulants and their coagulation performances for removal of humic acid. RSC Adv., 10(12): 7155–7162.
- Yang, C.Y., Chiu, H.F., Chiu, J.F., Tsai, S.S. and Cheng, M.F. (1997). Calcium and magnesium in drinking water and risk of death from colon cancer. Jpn. J. Cancer Res., 88(10): 928–933.
- Yang, C.Y., Cheng, M.F., Tsai, S.S. and Hsieh, Y.L. (1998). Calcium, magnesium, and nitrate in drinking water and gastric cancer mortality. Jpn. J. Cancer Res., 89(2): 124–130.
- Yang, C.Y., Tsai, S.S., Lai, T.C., Hung, C.F. and Chiu, H.F. (1999). Rectal cancer mortality and total hardness levels in Taiwan's drinking water. Environ. Res., 80(4): 311–316.
- Yang, C.Y., Chiu, H.F., Cheng, B.H., Hsu, T.Y., Cheng, M.F. and Wu, T.N. (2000a). Calcium and magnesium in drinking water and the risk of death from breast cancer. J. Toxicol. Environ. Health Part A, 60(4): 231–241.
- Yang, C.Y., Chiu, H.F., Tsai, S.S., Cheng, M.F., Lin, M.C. and Sung, F.C. (2000b). Calcium and magnesium in drinking water and risk of death from prostate cancer. J. Toxicol. Environ. Health A, 60(1): 17–26.
- Yaroshevsky, A.A. (2006). Abundances of chemical elements in the earth's crust. Geochem. Int., 44(1): 48–55.
- Zarkadas, M., Gougeon-Reyburn, R., Marliss, E.B., Block, E. and Alton-Mackey, M. (1989). Sodium chloride supplementation and urinary calcium excretion in postmenopausal women. Am. J. Clin. Nutr., 50(5): 1088–1094.
- Zhang, X., Li, Y., Del Gobbo, L.C., Rosanoff, A., Wang, J., Zhang, W. and Song, Y. (2016). Effects of magnesium supplementation on blood pressure: A meta-analysis of randomized double-blind placebo-controlled trials. Hypertension, 68(2): 324–333.
- Zhao, S., Wang, X., Li, N., Chen, Y., Su, Y. and Zhang, J. (2015). Effects of strontium ranelate on bone formation in the mid-palatal suture after rapid maxillary expansion. Drug Des. Dev. Ther., 9: 2725–2734.
- Zhong, G.C., Peng, Y., Wang, K., Wan, L., Wu, Y.Q.L., Hao, F.B., Hu, J.J. and Gu, H.T. (2020). Magnesium intake and primary liver cancer incidence and mortality in the prostate, lung, colorectal and ovarian cancer screening trial. International journal of cancer. Int. J. Cancer, 147(6): 1577–1586.
- Zipf, K.A. Jr. and Luthy, R.G. (1981). Chemical equilibria in split-treatment softening of water. J. Am. Water Works Assoc., 73(6): 304–311.
Appendix A: Abbreviations
- ANSI
- American National Standards Institute
- AWWA
- American Water Works Association
- AO
- aesthetic objective
- CCPP
- calcium carbonate precipitation potential
- DOC
- dissolved organic carbon
- EDTA
- ethylene diaminetetra acetic acid
- GAC
- granular activated carbon
- HBV
- health-based value
- IARC
- International Agency for Research on Cancer
- IOM
- Institute of Medicine
- IX
- ion exchange
- MAC
- maximum acceptable concentrations
- MDL
- method detection limit
- NF
- nanofiltration
- NSF
- NSF International
- NOM
- natural organic matter
- NTU
- nephelometric turbidity unit
- POE
- point of entry
- POU
- point of use
- RDL
- reporting detection limit
- RO
- reverse osmosis
- SAC
- strong acid cation
- TDS
- total dissolved solids
- UL
- tolerable upper intake level
- U.S. EPA
- United States Environmental Protection Agency
- USGS
- United States Geological Survey
- WHO
- World Health Organization
Appendix B: Provincial data tables
Data provided by the provinces were from a variety of water supplies in Canada, including surface water and groundwater, as well as treated and distributed water where monitoring occurred (British Columbia Ministry of Health, 2021; Ontario Ministry of the Environment, Conservation and Parks, 2021; Manitoba Sustainable Development, 2021; Ministère de l'Environnement et de la Lutte contre les changements climatiques, 2021; Nova Scotia Environment, 2021; Saskatchewan Water Security Agency, 2021; PEI Department of Communities, Land and Environment, 2021; New Brunswick Department of Health, 2021; Newfoundland and Labrador Municipal Affairs and Environment, 2021).
| Jurisdiction (DL mg/L) | Municipal/ Non-municipal | Water type | No. detects/ samples | Concentration (mg/L) | ||
|---|---|---|---|---|---|---|
| Median | 90th percentile | Max | ||||
| British Columbia 2016–2021 | Municipal | Ground-raw | 117/117 | 59.1 | 95.8 | 531 |
| Ground-distributed | 5/5 | 67.2 | NC | 90.3 | ||
| Ground &/or surface – raw | 54/54 | 39.9 | 100.8 | 141 | ||
| Ground &/or surface – treated | 13/13 | 17 | 84.4 | 90.3 | ||
| Ground &/or surface – distributed | 358/366 | 38.7 | 85.7 | 195 | ||
| Surface – raw | 10/10 | 14.4 | 75.2 | 107 | ||
| Surface – distributed | 5/5 | 27.9 | NC | 38.2 | ||
| Non-municipal | Ground – raw | 127/127 | 59.1 | 97.3 | 531 | |
| Ground – treated | 3/3 | 72.5 | NC | 90.3 | ||
| Ground – distributed | 1/1 | NC | NC | 69.8 | ||
| Ground – unspecified | 212/215 | 45.6 | 85.6 | 195 | ||
| Ground &/or surface –raw | 33/33 | 41.3 | 91.7 | 107 | ||
| Ground &/or surface – treated | 6/6 | 7.6 | NC | 87.4 | ||
| Ground &/or surface – unspecified | 205/211 | 39.1 | 91.1 | 193 | ||
| Surface – raw | 24/24 | 13.9 | 59.2 | 96 | ||
| Surface – treated | 10/10 | 14.9 | 33.2 | 33.6 | ||
| Surface – distributed | 2/2 | NC | NC | 35.8 | ||
| Surface – unspecified | 46/46 | 27 | 57.4 | 124 | ||
| Manitoba 2009–2020 | Municipal and non-municipal | Ground & GUDI – raw | 581/581 | 73.7 | 150 | 546 |
| Ground & GUDI – undisinfected | 323/323 | 65.7 | 118 | 383 | ||
| Ground & GUDI – treated | 571/572 | 57.2 | 115 | 347 | ||
| Ground & GUDI – distributed | 282/282 | 62.8 | 133 | 360 | ||
| Ground &/or surface – raw | 55/55 | 30.9 | 94.2 | 125 | ||
| Ground &/or surface – undisinfected | 22/22 | 45.4 | 112.3 | 126 | ||
| Ground &/or surface – treated | 55/55 | 23.1 | 53.7 | 120 | ||
| Ground &/or surface – distributed | 20/20 | 28.6 | 62.2 | 161 | ||
| Surface – raw | 306/306 | 23.5 | 88.6 | 219 | ||
| Surface –treated | 306/306 | 19 | 58.2 | 227 | ||
| Surface – distributed | 194/194 | 19.7 | 63.5 | 232 | ||
| New Brunswick (0.05–0.2) 2016–2019 | Municipal and Semi-public | Ground-raw | 977/977 | 27.4 | 66 | 188 |
| Ground – treated | 338/338 | 24.7 | 67.4 | 162 | ||
| Ground – distributed | 554/554 | 33.2 | 56 | 132 | ||
| Ground – unspecified | 161/161 | 46.5 | 112 | 191 | ||
| Ground & surface – raw | 288/288 | 5.5 | 7.7 | 42.3 | ||
| Ground &surface – treated | 5/5 | 6.9 | NC | 9.2 | ||
| Ground & surface – distributed | 552/552 | 6.1 | 64.8 | 89.5 | ||
| Surface – raw | 97/97 | 4.4 | 32.1 | 78.1 | ||
| Surface – treated | 1/1 | NC | NC | 2.6 | ||
| Surface – distributed | 259/259 | 7.9 | 28.1 | 55.1 | ||
| Newfoundland and Labrador (0.1–1) 2015–2020 | Municipal | Ground – raw | 252/254 | 27.5 | 55 | 250 |
| Ground – distributed | 1 317/1 357 | 28 | 64 | 266 | ||
| Surface – raw | 657/735 | 2 | 19 | 88 | ||
| Surface – distributed | 3 409/3 823 | 3 | 19 | 95 | ||
| Nova Scotia 2016–2020 | Municipal/semi-public | Ground – raw | 186/186 | 33.8 | 85.1 | 438 |
| Ground – treated | 314/319 | 29 | 66.1 | 330 | ||
| Ground – distributed | 4/4 | 37.9 | NC | 63.1 | ||
| Surface – raw | 79/79 | 2.2 | 7.1 | 29 | ||
| Surface – treated | 400/401 | 5.8 | 20.2 | 56 | ||
| Surface – distributed | 24/24 | 6.8 | 12.4 | 16.8 | ||
| Non-municipal | Ground – raw | 752/752 | 27 | 71.9 | 609 | |
| Ontario 2018–2021 | Municipal | Ground – raw | 2 157/2 159 | 85.7 | 130 | 453 |
| Ground – treated | 1 147/1 148 | 84.5 | 125 | 253 | ||
| Ground – distributed | 821/821 | 80.6 | 106 | 139 | ||
| Ground & surface – raw | 218/218 | 66.5 | 99.2 | 177 | ||
| Ground & surface – treated | 41/41 | 81.4 | 92.5 | 97.9 | ||
| Ground & surface – distributed | 699/699 | 36.3 | 42.4 | 150 | ||
| Surface – raw | 894/894 | 32.4 | 37.8 | 216 | ||
| Surface – treated | 1 124/1 124 | 33.7 | 37.6 | 117 | ||
| Surface – distributed | 1 611/1 611 | 8 | 32.4 | 114 | ||
| PEI (0.02 mg/L) 2016–2021 | Municipal | Ground – raw | 852/857 | 36.6 | 91.4 | 188.2 |
| Non – municipal | Ground – raw | 14 515/15 141 | 32.5 | 62 | 2 783 | |
| Quebec 2010–2014 | Non – municipal | Ground – raw | 1 915/1 945 | 44.3 | 87 | 1 900 |
| Saskatchewan 2015–2020 | Municipal | Ground – raw | 270/270 | 128 | 205.1 | 358 |
| Ground & surface – treated | 117/136 | 48 | 134 | 369 | ||
| Ground & surface – distributed | 3 084/3 183 | 62 | 179.8 | 593 | ||
| Surface – raw | 136/137 | 75 | 103 | 172 | ||
| DL – detection limit; GUDI – groundwater under the direct influence of surface water; NC – not calculated due to insufficient sample size; Unspecified –unspecified as to whether raw, treated or distributed. | ||||||
| Jurisdiction (DL mg/L) | Municipal/ Non-municipal | Water type | No. detects/ samples | Concentration (mg/L) | ||
|---|---|---|---|---|---|---|
| Median | 90th percentile | Max | ||||
| British Columbia (2016–2021) | Municipal | Ground – raw | 107/107 | 6.3 | 36.9 | 793 |
| Ground – distributed | 5/5 | 4.1 | NC | 68.1 | ||
| Surface – raw | 9/9 | 0.7 | 80.7 | 210 | ||
| Surface – distributed | 5/5 | 2.2 | 6.5 | 6.8 | ||
| Non-municipal | Ground – raw | 116/117 | 6.4 | 50 | 793 | |
| Ground – treated | 3/3 | 9.4 | NC | 43.4 | ||
| Ground – distributed | 1/1 | NC | NC | 1.3 | ||
| Ground – unspecified | 189/191 | 4.8 | 51.2 | 293 | ||
| Surface – raw | 23/23 | 0.5 | 5.6 | 210 | ||
| Surface – treated | 10/10 | 0.8 | 1.8 | 3.2 | ||
| Surface – distributed | 2/2 | NC | NC | 27.2 | ||
| Surface – unspecified | 41/42 | 1.6 | 21.4 | 57.3 | ||
| Manitoba (2009–2020) | Municipal and non-municipal | Ground & GUDI – raw | 571/571 | 18.4 | 172 | 1 280 |
| Ground & GUDI – undisinfected | 305/305 | 20.5 | 262.2 | 1 390 | ||
| Ground & GUDI – treated | 541/541 | 19.8 | 139 | 879 | ||
| Ground & GUDI – distributed | 4/4 | 49.8 | NC | 85.4 | ||
| Surface – raw | 305/305 | 4.7 | 50.4 | 1 830 | ||
| Surface – treated | 306/306 | 14 | 70.9 | 352 | ||
| Surface – distributed | 6/6 | 18 | NC | 39.3 | ||
| New Brunswick (0.01–0.2) 2016–2019 | Municipal | Ground – raw | 1 328/1 367 | 35.6 | 78.6 | 280 |
| Ground – treated | 338/338 | 37.3 | 149.6 | 540 | ||
| Ground – distributed | 554/554 | 27.4 | 83.2 | 407 | ||
| Ground – unspecified | 156/156 | 39.8 | 225.5 | 503 | ||
| Ground & surface – raw | 288/288 | 5.8 | 9.9 | 33.1 | ||
| Ground & surface – treated | 5/5 | 11.4 | NC | 14.3 | ||
| Ground & surface – distributed | 552/552 | 10.7 | 68.2 | 78 | ||
| Surface – raw | 97/97 | 3.8 | 10.9 | 134 | ||
| Surface – treated | 1/1 | NC | NC | 5 | ||
| Surface – distributed | 259/259 | 5.8 | 15.4 | 106 | ||
| Newfoundland and Labrador (0.1–1) 2015–2020 | Municipal | Ground – raw | 253/254 | 25.5 | 73 | 405 |
| Ground – distributed | 1 354/1 357 | 29 | 83 | 610 | ||
| Surface – raw | 727/735 | 8 | 20 | 570 | ||
| Surface – distributed | 3 722/3 823 | 12 | 25 | 610 | ||
| Nova Scotia 2016–2020 | Municipal/ non--municipal | Ground – raw | 201/201 | 30 | 142 | 960 |
| Ground – treated | 285/285 | 36 | 140 | 1 600 | ||
| Ground – distributed | 4/4 | 11.5 | NC | 67 | ||
| Surface – raw | 91/91 | 8 | 21 | 51 | ||
| Surface – treated | 397/404 | 15 | 31.7 | 88 | ||
| Surface – distributed | 24/24 | 6 | 13 | 14 | ||
| Non-municipal | Ground – raw | 743/743 | 21 | 170 | 2 200 | |
| Ontario 2018–2021 | Municipal | Ground – raw | 2 790/2 801 | 70.3 | 270 | 1 715 |
| Ground – treated | 1 272/1 272 | 53 | 177 | 561.6 | ||
| Ground – distributed | 1 155/1 155 | 55.7 | 173.6 | 330 | ||
| Ground & surface – raw | 168/168 | 70.2 | 124.5 | 331 | ||
| Ground & surface – treated | 223/223 | 13.8 | 74.5 | 116 | ||
| Ground & surface – distributed | 815/815 | 28.9 | 37.8 | 100 | ||
| Surface – raw | 894/895 | 24.5 | 34 | 190 | ||
| Surface – treated | 941/941 | 27 | 39.3 | 170 | ||
| Surface – distributed | 1 804/1 806 | 6.1 | 87.8 | 160 | ||
| PEI (0.26) 2016–2021 | Municipal | Ground – raw | 856/856 | 18.5 | 117.5 | 2 140 |
| Non-municipal | Ground – raw | 14 590/14 615 | 14.8 | 59.8 | 15 000 | |
| Quebec 2010–2014 | Non-municipal | Ground – raw | 1 999/2 065 | 91.2 | 130 | 11 000 |
| Saskatchewan 2015–2020 | Municipal | Ground – raw | 266/267 | 13.2 | 151.5 | 616.5 |
| Ground & surface – treated | 135/136 | 17.1 | 60.8 | 394.7 | ||
| Ground & surface – distributed | 3 032/3 049 | 21.6 | 87 | 1 120 | ||
| Surface – raw | 159/159 | 18.6 | 52 | 1 065.6 | ||
| DL – detection limit; GUDI – groundwater under the direct influence of surface water; NC – not calculated due to insufficient sample size; Unspecified –unspecified as to whether raw, treated or distributed. | ||||||
| Jurisdiction (DL mg/L as CaCO3) | Municipal/ Non municipal | Water type | No. detects/ samples | Concentration (mg/L as CaCO3) | ||
|---|---|---|---|---|---|---|
| Median | 90th percentile | Max | ||||
| British Columbia (2016–2021) | Municipal | Ground – raw | 111/111 | 215 | 454 | 1 650 |
| Ground – distributed | 5/5 | 236 | NC | 389 | ||
| Surface – raw | 10/10 | 44 | 399.3 | 1 140 | ||
| Surface – distributed | 5/5 | 95.1 | NC | 162 | ||
| Non-municipal | Ground – raw | 120/120 | 234.5 | 469.1 | 1 650 | |
| Ground – treated | 3/3 | 286 | NC | 367 | ||
| Ground – distributed | 1/1 | NC | NC | 200 | ||
| Ground – unspecified | 199/203 | 183 | 452.4 | 1 150 | ||
| Surface – raw | 24/24 | 41.8 | 207.8 | 1 140 | ||
| Surface – treated | 10/10 | 46.3 | 101.1 | 102 | ||
| Surface – distributed | 2/2 | NC | NC | 319 | ||
| Surface – Unspecified | 46/46 | 86.4 | 282.5 | 606 | ||
| Manitoba (2009–2020) | Municipal and non-municipal | Ground & GUDI – raw | 571/571 | 368 | 677 | 1 570 |
| Ground & GUDI – undisinfected | 305/305 | 337 | 649 | 1 650 | ||
| Ground & GUDI – treated | 538/541 | 278 | 546 | 1 360 | ||
| Ground & GUDI – distributed | 4/4 | 111 | NC | 170 | ||
| Surface – raw | 305/305 | 91.7 | 416.2 | 1 130 | ||
| Surface – treated | 306/306 | 76.7 | 245.5 | 861 | ||
| Surface – distributed | 6/6 | 143.4 | NC | 310 | ||
| New Brunswick (0.2-0.7) 2016–2019 | Municipal | Ground – raw | 1 176/1 176 | 91.8 | 196 | 543 |
| Ground – treated | 338/338 | 78.5 | 207 | 476 | ||
| Ground – distributed | 551/551 | 102 | 174 | 392 | ||
| Ground – Unspecified | 161/161 | 125 | 296 | 499 | ||
| Ground & surface – raw | 61/61 | 23 | 111 | 126 | ||
| Ground & surface – treated | 5/5 | 20 | NC | 25 | ||
| Ground & surface – distributed | 306/306 | 23 | 216 | 289 | ||
| Surface – raw | 97/97 | 14 | 88.4 | 224 | ||
| Surface – treated | 1/1 | 8.5 | 8.5 | 8.5 | ||
| Surface – distributed | 259/259 | 22.8 | 81 | 158 | ||
| Newfoundland and Labrador (1) 2015–2020 | Municipal | Ground – raw | 251/253 | 96 | 190 | 700 |
| Ground – distributed | 1 316/1 357 | 95 | 218.4 | 747 | ||
| Surface – raw | 666/735 | 7 | 83 | 273 | ||
| Surface – distributed | 3 419/3 823 | 10 | 70.8 | 291 | ||
| Nova Scotia 2016–2020 | Municipal/ non-municipal | Ground – raw | 182/183 | 120 | 257.2 | 1 250 |
| Ground – treated | 252/263 | 84.2 | 208 | 850 | ||
| Ground – distributed | 4/4 | 104 | NC | 210 | ||
| Surface – raw | 76/76 | 8.6 | 23.2 | 82 | ||
| Surface – treated | 382/383 | 17.8 | 52 | 190 | ||
| Surface – distributed | 23/23 | 18.7 | 36.4 | 47 | ||
| Non-municipal | Ground – raw | 692/692 | 98 | 260 | 1 740 | |
| Ontario 2018–2021 | Municipal | Ground – raw | 2 142/2 143 | 320 | 458 | 1 410 |
| Ground – treated | 1 240/1 240 | 318 | 460 | 924 | ||
| Ground – distributed | 1 119/1 119 | 236 | 383 | 548 | ||
| Ground & surface – raw | 167/167 | 278 | 362.4 | 577 | ||
| Ground & surface – treated | 49/49 | 305 | 407.2 | 434 | ||
| Ground & surface – distributed | 702/702 | 126 | 158 | 476 | ||
| Surface – raw | 1 148/1 148 | 115 | 131 | 668 | ||
| Surface – treated | 1 442/1 442 | 118 | 131 | 476 | ||
| Surface – distributed | 945/945 | 26 | 123 | 415 | ||
| PEI 2016–2021 | Municipal | Ground – raw | 857/857 | 159 | 247.8 | 1 164 |
| Non-municipal | Ground – raw | 14 665/14 723 | 136.4 | 202.3 | 11 090 | |
| Quebec 2010–2014 | Non-municipal | Ground – raw | 604/604 | 166 | 337.7 | 3902 |
| Saskatchewan 2015–2020 | Municipal | Ground – raw | 260/260 | 531.5 | 909.7 | 1 528 |
| Ground & surface – treated | 136/136 | 194 | 640.5 | 1 475 | ||
| Ground & surface – distributed | 2 977/2 996 | 298.5 | 848.5 | 4 980 | ||
| Surface – raw | 134/134 | 407 | 596.7 | 1 023 | ||
| DL – detection limit; GUDI – groundwater under the direct influence of surface water; NC – not calculated due to insufficient sample size; Unspecified –unspecified as to whether raw, treated or distributed. | ||||||
| Jurisdiction (DL mg/L) | Municipal/ Non-municipal | Water type | No. detects/ samples | Concentration (mg/L) | ||
|---|---|---|---|---|---|---|
| Median | 90th percentile | Max | ||||
| British Columbia (2016–2021) | Municipal | Ground – raw | 121/121 | 13.2 | 72.5 | 177 |
| Ground – distributed | 5/5 | 16.6 | NC | 49.1 | ||
| Surface – raw | 9/9 | 2 | 10.5 | 12.4 | ||
| Surface – distributed | 5/5 | 10.8 | NC | 16.2 | ||
| Non-municipal | Ground – raw | 131/131 | 12.9 | 72.5 | 177 | |
| Ground – treated | 3/3 | 14.7 | NC | 45.1 | ||
| Ground – distributed | 1/1 | NC | 6.2 | 6.2 | ||
| Ground – unspecified | 211/214 | 12.5 | 52.3 | 176 | ||
| Surface – raw | 23/23 | 2 | 15.7 | 25 | ||
| Surface – treated | 10/10 | 2.7 | 4.5 | 5.8 | ||
| Surface – distributed | 2/2 | 60 | NC | 65 | ||
| Surface – unspecified | 45/45 | 5.8 | 28.8 | 93.6 | ||
| Manitoba (2009–2020) | Municipal and non-municipal | Ground &GUDI – raw | 581/581 | 40 | 83.8 | 153 |
| Ground &GUDI – undisinfected | 325/325 | 37.2 | 93.1 | 214 | ||
| Ground &GUDI – treated | 575/576 | 28.8 | 69.7 | 145 | ||
| Ground &GUDI – distributed | 282/285 | 29.4 | 72.2 | 127 | ||
| Surface – raw | 306/306 | 7.1 | 50.3 | 147 | ||
| Surface – treated | 307/307 | 5.9 | 23.8 | 90.3 | ||
| Surface – distributed | 195/196 | 6.8 | 25.6 | 89 | ||
| New Brunswick (0.05–1) 2016–2019 | Municipal | Ground – raw | 973/973 | 3.9 | 11.3 | 38 |
| Ground – treated | 338/338 | 2.6 | 9.4 | 20.1 | ||
| Ground – distributed | 554/554 | 3.6 | 10.1 | 16.3 | ||
| Ground – unspecified | 161/161 | 4.1 | 9.6 | 22.5 | ||
| Ground & surface – raw | 61/61 | 1.1 | 6.2 | 6.8 | ||
| Ground & surface – treated | 5/5 | 0.7 | NC | 0.8 | ||
| Ground & surface – distributed | 306/306 | 0.9 | 11.3 | 15.8 | ||
| Surface – raw | 97/97 | 0.8 | 2.1 | 7 | ||
| Surface – treated | 1/1 | NC | NC | 0.5 | ||
| Surface – distributed | 259/259 | 0.9 | 2.6 | 5 | ||
| Newfoundland and Labrador (1) 2015–2020 | Municipal | Ground – raw | 236/254 | 6 | 14 | 27 |
| Ground – distributed | 1 287/1 357 | 5 | 15 | 41 | ||
| Surface – raw | 445/735 | 0.5 | 7 | 37 | ||
| Surface – distributed | 2 284/3 823 | 0.6 | 5 | 39 | ||
| Nova Scotia 2016–2020 | Municipal/Semi-public | Ground – raw | 182/186 | 5.8 | 14 | 57.7 |
| Ground – treated | 263/286 | 4 | 14 | 110 | ||
| Ground – distributed | 4/4 | 2.5 | NC | 11.8 | ||
| Surface – raw | 77/77 | 0.8 | 1.3 | 545 | ||
| Surface – treated | 390/392 | 0.9 | 3.4 | 580 | ||
| Surface – distributed | 24/24 | 0.4 | 1.2 | 1.4 | ||
| Non Municipal | Ground – raw | 750/751 | 4 | 16 | 75 | |
| Ontario 2018–2021 | Municipal | Ground – raw | 1 984/1 986 | 25.3 | 37.3 | 84.6 |
| Ground – treated | 986/987 | 25.5 | 39.5 | 71.7 | ||
| Ground – distributed | 665/665 | 27 | 35.9 | 46.3 | ||
| Ground & surface – raw | 214/214 | 19.7 | 26.2 | 34.2 | ||
| Ground & surface – treated | 41/41 | 23.7 | 25.7 | 32.4 | ||
| Ground & surface – distributed | 553/553 | 8.9 | 13.9 | 36.9 | ||
| Surface – raw | 856/864 | 8.3 | 9.2 | 31.2 | ||
| Surface – treated | 1 065/1 075 | 8.6 | 9.3 | 29.9 | ||
| Surface – distributed | 1 535/1 563 | 2 | 8.1 | 28.6 | ||
| PEI (0.01) 2016–2021 | Municipal | Ground – raw | 852/857 | 12.5 | 20.1 | 168.6 |
| Non-municipal | Ground – raw | 14 424/15 141 | 13.1 | 20 | 1 006 | |
| Quebec 2010–2014 | Non-municipal | Ground – raw | 1 895/1 996 | 10.9 | 25 | 327.9 |
| Saskatchewan 2015–2020 | Municipal | Ground – raw | 266/270 | 50.5 | 94 | 168 |
| Ground & surface – treated | 116/136 | 19 | 71.5 | 152 | ||
| Ground & surface – distributed | 2 941/3 155 | 30 | 97 | 924 | ||
| Surface – raw | 136/137 | 52 | 91.4 | 166 | ||
| DL – detection limit; GUDI – groundwater under the direct influence of surface water; NC – not calculated due to insufficient sample size; Unspecified –unspecified as to whether raw, treated or distributed. | ||||||
| Jurisdiction (DL mg/L) | Municipal/ Non-municipal | Water type | No. detects/ samples | Concentration (mg/L) | ||
|---|---|---|---|---|---|---|
| Median | 90th percentile | Max | ||||
| British Columbia 2016–2021 | Municipal | Ground – raw | 105/106 | 29.7 | 128.5 | 1 490 |
| Ground – distributed | 5/5 | 38.1 | NC | 137 | ||
| Surface – raw | 9/9 | 7.1 | 57.2 | 147 | ||
| Surface – distributed | 5/5 | 6 | NC | 47.7 | ||
| Non-municipal | Ground – raw | 115/116 | 32.6 | 140.5 | 1 490 | |
| Ground – treated | 2/3 | 16.5 | NC | 116 | ||
| Ground – distributed | 1/1 | NC | NC | 32.3 | ||
| Ground – unspecified | 185/187 | 27 | 134.6 | 582 | ||
| Surface – raw | 23/23 | 7.1 | 33.3 | 147 | ||
| Surface – treated | 9/9 | 7.3 | 14.7 | 14.8 | ||
| Surface – distributed | 2/2 | 11 | NC | 12.3 | ||
| Surface – unspecified | 40/41 | 10.9 | 47.2 | 273 | ||
| Manitoba 2009–2020 | Municipal and non-municipal | Ground & GUDI – raw | 555/571 | 60.4 | 389 | 1 310 |
| Ground & GUDI – undisinfected | 290/305 | 51.6 | 358.8 | 2 260 | ||
| Ground & GUDI – treated | 522/541 | 49.7 | 295 | 1 220 | ||
| Ground & GUDI – distributed | 4/4 | 34.1 | NC | 101 | ||
| Surface – raw | 303/305 | 4.4 | 205 | 497 | ||
| Surface – treated | 273/306 | 6.2 | 142 | 468 | ||
| Surface – distributed | 5/6 | 55.8 | NC | 277 | ||
| New Brunswick (0.05–2) 2016–2009 | Municipal | Ground – raw | 1 327/1 366 | 15 | 47 | 83 |
| Ground – treated | 338/338 | 10 | 34 | 440 | ||
| Ground – distributed | 554/554 | 12 | 30 | 73 | ||
| Ground &/or surface – unspecified | 156/156 | 13 | 23 | 104 | ||
| Ground & surface – raw | 54/54 | 5.5 | 8.7 | 18 | ||
| Ground & surface – treated | 5/5 | 2 | NC | 3 | ||
| Ground & surface – distributed | 298/298 | 4 | 37 | 51 | ||
| Surface – raw | 87/87 | 2 | 3.6 | 23 | ||
| Surface – treated | 1/1 | NC | NC | 2 | ||
| Surface – distributed | 251/251 | 12 | 31 | 47 | ||
| Newfoundland and Labrador (1–2) 2015–2020 | Municipal | Ground – raw | 244/254 | 8 | 24 | 550 |
| Ground – distributed | 1 299/1 357 | 9 | 25 | 598 | ||
| Surface – raw | 368/735 | 1 | 3 | 120 | ||
| Surface – distributed | 2 224/3 823 | 1 | 4 | 120 | ||
| Nova Scotia 2016–2020 | Municipal/ non-municipal | Ground – raw | 191/202 | 13 | 66.4 | 1 100 |
| Ground – treated | 264/279 | 13 | 100 | 620 | ||
| Ground – distributed | 4/4 | 30 | NC | 34 | ||
| Surface – raw | 65/91 | 3 | 14 | 31 | ||
| Surface – treated | 361/406 | 10 | 31 | 71 | ||
| Surface – distributed | 24/24 | 16.5 | 26 | 29 | ||
| Non-municipal | Ground – raw | 723/742 | 11 | 94.9 | 1 600 | |
| Ontario 2018–2021 | Municipal | Ground – raw | 1 825/1 865 | 39.7 | 110 | 1 000 |
| Ground – treated | 1 003/1 059 | 34 | 78.3 | 827 | ||
| Ground – distributed | 980/989 | 27.6 | 62.5 | 190 | ||
| Ground & surface – raw | 159/159 | 31.2 | 44.8 | 69.4 | ||
| Ground & surface – treated | 37/37 | 44.9 | 51.2 | 53 | ||
| Ground & surface – distributed | 793/793 | 25 | 26.7 | 66 | ||
| Surface – raw | 745/745 | 23.8 | 26 | 91 | ||
| Surface – treated | 921/922 | 26 | 34.3 | 92 | ||
| Surface – distributed | 852/852 | 26.7 | 34.5 | 45 | ||
| PEI (0.03) 2016–2021 | Municipal | Ground – raw | 857/857 | 7.8 | 15.6 | 287.1 |
| Non-municipal | Ground – raw | 14 946/15 121 | 6.4 | 14.8 | 2 126 | |
| Quebec 2010–2014 | Non-municipal | Ground – raw | 1 842/1 999 | 40.7 | 52 | 3 624 |
| Saskatchewan 2015–2020 | Municipal | Ground – raw | 263/263 | 320 | 867.5 | 1 710 |
| Ground & surface – treated | 136/136 | 90.4 | 764 | 1 381.6 | ||
| Ground & surface – distributed | 2 981/3 014 | 148.2 | 805.5 | 8 560 | ||
| Surface – raw | 137/137 | 350 | 808.2 | 1 936.2 | ||
| DL – detection limit; GUDI – groundwater under the direct influence of surface water; NC – not calculated due to insufficient sample size; Unspecified –unspecified as to whether raw, treated or distributed. | ||||||
| Jurisdiction (DL mg/L) | Municipal/ Non-municipal | Water type | No. detects/ samples | Concentration (mg/L) | ||
|---|---|---|---|---|---|---|
| Median | 90th percentile | Max | ||||
| Manitoba | Municipal and non-municipal | Ground & GUDI – raw | 571/571 | 521 | 1 320 | 3 120 |
| Ground & GUDI – undisinfected | 305/305 | 521 | 1 206 | 4 890 | ||
| Ground & GUDI – treated | 541/541 | 456 | 1 030 | 3 030 | ||
| Ground & GUDI – distributed | 4/4 | 300 | NC | 486 | ||
| Ground &/or surface – raw | 56/56 | 219 | 848.5 | 981 | ||
| Ground &/or surface – undisinfected | 21/21 | 620 | 1 290 | 1 650 | ||
| Ground &/or surface – treated | 56/56 | 194 | 656.5 | 1 030 | ||
| Ground &/or surface – distributed | 2/2 | 114 | NC | 163 | ||
| Surface – raw | 305/305 | 120 | 640.2 | 3 160 | ||
| Surface – treated | 305/306 | 136.5 | 472 | 1 410 | ||
| Surface – distributed | 6/6 | 224 | NC | 593 | ||
| New Brunswick (1–5) | Municipal | Ground – raw | 103/103 | 131 | 179 | 429 |
| Ground – distributed | 50/50 | 123 | 205.2 | 493 | ||
| Ground & surface – raw | 5/5 | 22 | NC | 25 | ||
| Ground & surface – treated | 5/5 | 49 | NC | 58 | ||
| Surface – raw | 8/8 | 61 | NC | 101 | ||
| Surface – distributed | 12/12 | 52 | 98.5 | 101 | ||
| Newfoundland and Labrador (1–5) 2015–2020 | Municipal | Ground – raw | 253/253 | 181 | 309.2 | 1 030 |
| Ground – distributed | 1 356/1 357 | 190 | 367 | 1 550 | ||
| Surface – raw | 735/735 | 30 | 120 | 1 000 | ||
| Surface – distributed | 3 772/3 823 | 43 | 128.9 | 1 100 | ||
| Nova Scotia 2016–2020 | Municipal/ non-municipal | Ground – raw | 194/194 | 201.5 | 477.6 | 2 380 |
| Ground – treated | 303/304 | 248 | 432.8 | 2 900 | ||
| Ground – distributed | 4/4 | 80 | NC | 345 | ||
| Surface – raw | 90/92 | 24.5 | 65.8 | 119 | ||
| Surface – treated | 407/409 | 67 | 115 | 396 | ||
| Surface – distributed | 24/24 | 42.5 | 80.2 | 95 | ||
| Non-municipal | Ground – raw | 737/737 | 180 | 598 | 4 200 | |
| Ontario 2018–2021 | Municipal | Ground – raw | 711/711 | 448 | 870 | 1 880 |
| Ground – treated | 540/540 | 478 | 810.1 | 1 530 | ||
| Ground – distributed | 336/337 | 442 | 667.8 | 952 | ||
| Ground & surface – raw | 124/124 | 374 | 514.3 | 797 | ||
| Ground & surface – treated | 70/70 | 202 | 444.5 | 507 | ||
| Ground & surface – distributed | 605/605 | 187 | 212 | 728 | ||
| Surface – raw | 429/435 | 83 | 187 | 860 | ||
| Surface – treated | 579/581 | 134 | 210 | 634 | ||
| Surface – distributed | 820/831 | 100 | 160 | 1 120 | ||
| PEI 2016–2021 | Municipal | Ground – raw | 1/1 | 208 | NC | 208 |
| Non-municipal | Ground – raw | 5/5 | 214 | NC | 513 | |
| Quebec 2010–2014 | Non-municipal | Ground – raw | 443/444 | 684.3 | 1 125.7 | 13 837.4 |
| Saskatchewan 2015–2020 | Municipal | Ground – raw | 210/210 | 1 210.5 | 2 149.4 | 3 320 |
| Ground & surface – treated | 80/80 | 810 | 2 201.3 | 3 043 | ||
| Ground & surface – distributed | 2 364/2 364 | 745.5 | 2 067.8 | 13 395 | ||
| Surface – raw | 109/109 | 903 | 1 606.8 | 3 426 | ||
| DL – detection limit; GUDI – groundwater under the direct influence of surface water; NC – not calculated due to insufficient sample size; TDS – total dissolved solids; Unspecified – unspecified as to whether raw, treated or distributed. | ||||||
| Jurisdiction (DL mg/L) | Municipal/ Non-municipal | Water type | No. detects/ samples | Concentration (mg/L) | ||
|---|---|---|---|---|---|---|
| Median | 90th percentile | Max | ||||
| New Brunswick (0.05) 2016–2019 | Municipal | Ground – raw | 3/3 | 0.05 | NC | 0.05 |
| Ground – distributed | 1/1 | NC | NC | 0.05 | ||
| Nova Scotia 2016–2020 | Municipal+ Semi-public | Ground – raw | 1/23 | 0.05 | 0.05 | 0.1 |
| Ground – treated | 0/4 | < DL | NC | < DL | ||
| Surface – raw | 0/21 | < DL | NC | < DL | ||
| Surface – treated | 25/25 | 0.01 | 0.05 | 0.05 | ||
| Surface – distributed | 1/1 | NC | NC | 0.005 | ||
| Ontario 2018–2021 | Municipal | Ground – raw | 218/590 | < DL | 0.0261 | 2.6 |
| Ground – treated | 1/64 | 0.01 | NC | < DL | ||
| Ground & surface – distributed | 0/6 | < DL | NC | < DL | ||
| Surface – raw | 1/63 | 0.005 | NC | 0.01 | ||
| Surface – treated | 0/12 | < DL | < DL | < DL | ||
| Surface – distributed | 0/1 | NC | < DL | < DL | ||
| Quebec 2010–2014 | Non-municipal | Ground – raw | 86/948 | < DL | < DL | 8.7 |
| DL – detection limit; NC – not calculated due to insufficient sample size; Unspecified – unspecified as to whether raw, treated or distributed. | ||||||
Appendix C: Health Canada Drinking Water Survey Data
C.1 National Drinking Water Survey 2009–2010
| Water type | Summer (mg/L) | Winter (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 18/18 | 33.5 | 42.1 | 87.5 | 140 | 17/17 | 32.0 | 42.8 | 93.4 | 130 |
| Well – treated | 17/17 | 33.0 | 42.7 | 89.4 | 140 | 16/16 | 31.0 | 41.8 | 92.0 | 130 |
| Well –distribution | 54/54 | 33.5 | 39.3 | 77.2 | 150 | 27/27 | 29.0 | 28.9 | 48.4 | 50 |
| Lake – raw | 21/21 | 13.0 | 17.0 | 37.0 | 41 | 20/20 | 17.5 | 20.2 | 44.7 | 55 |
| Lake – treated | 21/21 | 11.0 | 16.9 | 38.0 | 42 | 20/20 | 12.5 | 20.3 | 44.0 | 53 |
| Lake –distribution | 57/57 | 10.0 | 17.6 | 39.0 | 47 | 31/31 | 11.0 | 18.9 | 42.0 | 54 |
| River – raw | 26/26 | 33.0 | 34.6 | 61.5 | 81 | 22/22 | 45.5 | 42.8 | 77.0 | 100 |
| River –treated | 26/26 | 33.5 | 34.9 | 60.5 | 81 | 22/22 | 42.0 | 39.9 | 73.8 | 100 |
| River –distribution | 77/77 | 33.0 | 36.3 | 66.8 | 120 | 36/36 | 44.0 | 44.1 | 63.0 | 100 |
| Reporting detection limit (RDL) = 0.2 mg/L. | ||||||||||
| Water type | Summer (mg/L) | Winter (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 18/18 | 9.9 | 14.0 | 32.2 | 68 | 17/17 | 14.0 | 14.6 | 25.2 | 67 |
| Well – treated | 17/17 | 9.1 | 13.6 | 24.6 | 69 | 16/16 | 12.0 | 14.6 | 27.0 | 67 |
| Well –distribution | 54/54 | 8.8 | 14.0 | 28.0 | 76 | 27/27 | 6.7 | 9.0 | 18.4 | 22 |
| Lake – raw | 21/21 | 2.2 | 5.0 | 11.0 | 28 | 20/20 | 2.2 | 5.9 | 11.9 | 33 |
| Lake – treated | 21/21 | 2.0 | 4.7 | 11.0 | 27 | 20/20 | 2.3 | 5.6 | 11.0 | 33 |
| Lake –distribution | 57/57 | 1.7 | 4.5 | 11.4 | 28 | 31/31 | 1.3 | 6.0 | 10.0 | 34 |
| River – raw | 26/26 | 5.9 | 11.0 | 22.0 | 54 | 22/22 | 8.0 | 12.7 | 26.5 | 43 |
| River – treated | 26/26 | 5.5 | 8.3 | 18.0 | 22 | 22/22 | 8.2 | 10.0 | 19.7 | 28 |
| River –distribution | 77/77 | 5.5 | 8.5 | 18.4 | 36 | 36/36 | 13.0 | 12.7 | 19.5 | 28 |
| Reporting detection limit (RDL) = 0.05 mg/L. | ||||||||||
| Water type | Summer (mg/L as CaCO3) | Winter (mg/L as CaCO3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 18/18 | 130.0 | 161.9 | 291.0 | 620 | 16/16 | 140.0 | 165.7 | 335.0 | 590 |
| Well – treated | 17/17 | 130.0 | 162.0 | 302.0 | 630 | 15/15 | 150.0 | 163.9 | 330.0 | 590 |
| Well –distribution | 54/54 | 130.0 | 156.5 | 305.0 | 690 | 27/27 | 99.0 | 108.8 | 180.0 | 200 |
| Lake – raw | 21/21 | 38.0 | 64.1 | 140.0 | 220 | 20/20 | 60.0 | 74.2 | 141.0 | 270 |
| Lake – treated | 21/21 | 39.0 | 62.0 | 130.0 | 210 | 20/20 | 42.5 | 74.2 | 150.0 | 270 |
| Lake –distribution | 57/57 | 38.0 | 62.7 | 132.0 | 220 | 25/25 | 40.0 | 83.9 | 216.0 | 270 |
| River – raw | 26/26 | 115.0 | 131.9 | 255.0 | 420 | 21/21 | 160.0 | 164.5 | 340.0 | 370 |
| River – treated | 26/26 | 120.0 | 121.8 | 225.0 | 280 | 21/21 | 150.0 | 145.9 | 240.0 | 370 |
| River –distribution | 77/77 | 120.0 | 125.9 | 248.0 | 440 | 30/30 | 150.0 | 161.8 | 250.0 | 370 |
| Reporting detection limit (RDL) = 1 mg/L. | ||||||||||
| Water type | Summer (mg/L) | Winter (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detects*/samples | Median | Mean | 90th | Max | Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 18/18 | 13.0 | 29.2 | 87.6 | 120 | 16/16 | 18.0 | 39.1 | 88.5 | 240 |
| Well – treated | 17/17 | 16.0 | 33.1 | 108.0 | 120 | 15/15 | 13.0 | 41.5 | 106.0 | 240 |
| Well – distribution | 54/54 | 15.0 | 28.4 | 99.7 | 150 | 27/27 | 8.0 | 25.1 | 89.2 | 110 |
| Lake – raw | 17/21 | 9.0 | 25.8 | 34.2 | 260 | 16/20 | 9.0 | 34.4 | 54.0 | 330 |
| Lake – treated | 21/21 | 12.0 | 26.8 | 36.0 | 280 | 20/20 | 11.5 | 33.3 | 45.0 | 350 |
| Lake – distribution | 57/57 | 13.0 | 28.6 | 36.4 | 290 | 24/25 | 10.0 | 61.1 | 266.6 | 360 |
| River – raw | 24/26 | 11.5 | 18.6 | 47.5 | 80 | 20/21 | 6.0 | 19.7 | 47.9 | 110 |
| River – treated | 26/26 | 13.0 | 21.1 | 51.0 | 97 | 20/21 | 10.0 | 25.5 | 58.1 | 140 |
| River – distribution | 77/77 | 12.0 | 22.1 | 47.4 | 220 | 28/30 | 9.0 | 31.9 | 93.1 | 140 |
| Reporting detection limit (RDL) = 1 mg/L. | ||||||||||
| Water type | Summer (mg/L) | Winter (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detects*/samples | Median | Mean | 90th | Max | Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 17/18 | 16.0 | 84.5 | 204.0 | 630 | 15/16 | 16.0 | 92.8 | 276.4 | 640 |
| Well – treated | 17/17 | 37.0 | 85.9 | 205.0 | 620 | 15/15 | 21.0 | 95.3 | 268.0 | 650 |
| Well – distribution | 54/54 | 16.0 | 77.2 | 250.8 | 650 | 27/27 | 37.0 | 35.7 | 62.4 | 65 |
| Lake – raw | 16/21 | 9.0 | 14.0 | 36.5 | 49 | 16/20 | 10.0 | 16.4 | 35.0 | 75 |
| Lake – treated | 18/21 | 11.5 | 16.4 | 37.0 | 50 | 17/20 | 17.0 | 21.2 | 46.6 | 73 |
| Lake – distribution | 49/57 | 12.0 | 17.9 | 39.0 | 53 | 25/25 | 23.0 | 27.4 | 60.6 | 75 |
| River – raw | 22/26 | 21.5 | 41.6 | 85.1 | 260 | 18/21 | 34.5 | 53.5 | 118.3 | 260 |
| River – treated | 25/26 | 39.0 | 64.8 | 126.0 | 280 | 20/21 | 46.0 | 65.8 | 114.9 | 290 |
| River – distribution | 72/77 | 46.0 | 68.3 | 120.0 | 280 | 30/30 | 52.0 | 83.0 | 163.0 | 280 |
| Reporting detection limit (RDL) = 1 mg/L. | ||||||||||
| Water type | Summer (mg/L) | Winter (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 18/18 | 185.5 | 372.5 | 801.6 | 1710 | 16/16 | 211.5 | 412.6 | 914.0 | 1710 |
| Well – treated | 17/17 | 195.0 | 387.7 | 877.0 | 1710 | 15/15 | 216.0 | 426.7 | 962.0 | 1690 |
| Well – distribution | 54/54 | 190.5 | 356.8 | 868.9 | 1720 | 27/27 | 219.0 | 301.5 | 660.2 | 833 |
| Lake – raw | 21/21 | 61.0 | 101.5 | 159.0 | 673 | 20/20 | 73.0 | 125.7 | 193.9 | 874 |
| Lake – treated | 21/21 | 60.0 | 110.5 | 161.0 | 683 | 20/20 | 74.5 | 135.9 | 200.3 | 880 |
| Lake – distribution | 57/57 | 64.0 | 114.8 | 164.4 | 706 | 25/25 | 87.0 | 198.2 | 620.0 | 894 |
| River – raw | 26/26 | 149.0 | 183.2 | 360.0 | 710 | 21/21 | 186.0 | 226.0 | 495.0 | 650 |
| River – treated | 26/26 | 161.0 | 201.7 | 352.0 | 542 | 21/21 | 225.0 | 230.5 | 375.0 | 609 |
| River – distribution | 77/77 | 163.0 | 209.2 | 381.2 | 768 | 30/30 | 222.0 | 279.3 | 533.6 | 612 |
TDS – total dissolved solids. Reporting detection limit (RDL) = 1 mg/L. |
||||||||||
C.2 Targeted Drinking Water Survey 2007
| Water type | Calcium (mg/L), RDL = 0.2 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 12/12 | 33.5 | 45.9 | 148.2 | 180 |
| Well – treated | 12/12 | 33.5 | 46.9 | 148.2 | 190 |
| Lake – raw | 5/5 | 1.9 | 18.9 | NC | 47 |
| Lake – treated | 5/5 | 13 | 15.6 | NC | 31 |
| River – raw | 2/2 | 89.5 | 89.5 | NC | 110 |
| River – treated | 2/2 | 92 | 92.0 | NC | 110 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit. | |||||
| Water Type | Magnesium (mg/L), RDL = 0.05 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 12/12 | 15 | 23.7 | 66.8 | 79 |
| Well – treated | 12/12 | 14 | 21.7 | 64.8 | 80 |
| Lake – raw | 5/5 | 0.94 | 7.5 | NC | 18 |
| Lake – treated | 5/5 | 0.94 | 7.5 | NC | 18 |
| River – raw | 2/2 | 25.5 | 25.5 | NC | 28 |
| River – treated | 2/2 | 26 | 26.0 | NC | 28 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit. | |||||
| Water Type | CaCO3 (mg/L), RDL = 1 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 16/16 | 155 | 206.06 | 490 | 770 |
| Well – treated | 16/16 | 145 | 197.6 | 475 | 800 |
| Lake – raw | 8/8 | 7.5 | 51.9 | NC | 190 |
| Lake – treated | 8/8 | 23 | 53.25 | NC | 150 |
| River – raw | 2/2 | 330 | 330 | NC | 390 |
| River – treated | 2/2 | 340 | 340 | NC | 390 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit. | |||||
| Water type | Dissolved Chloride (mg/L), RDL = 1 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 16/16 | 72 | 70.3 | 140 | 190 |
| Well – treated | 16/16 | 85 | 80.4 | 170 | 210 |
| Lake – raw | 8/8 | 8.5 | 7.4 | NC | 10 |
| Lake – treated | 8/8 | 10 | 10.3 | NC | 15 |
| River – raw | 2/2 | 125 | 125.0 | NC | 150 |
| River – treated | 2/2 | 155 | 155.0 | NC | 190 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit. | |||||
| Water type | Dissolved SO4 (mg/L), RDL = 1 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 16/16 | 9.5 | 90.5 | 306.5 | 484 |
| Well – treated | 16/16 | 48 | 111.1 | 307 | 456 |
| Lake – raw | 8/8 | 2.5 | 19.0 | NC | 73 |
| Lake – treated | 8/8 | 15 | 29.9 | NC | 89 |
| River – raw | 2/2 | 82.5 | 82.5 | NC | 90 |
| River – treated | 2/2 | 90.5 | 90.5 | NC | 96 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit. | |||||
| Water type | TDS (mg/L), RDL = 1 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 16/16 | 662.5 | 715.5 | 1245 | 1550 |
| Well – treated | 16/16 | 748.5 | 725.8 | 1235 | 1540 |
| Lake – raw | 8/8 | 22 | 80.6 | NC | 279 |
| Lake – treated | 8/8 | 50.5 | 92.3 | NC | 245 |
| River – raw | 2/2 | 558.5 | 558.5 | NC | 644 |
| River – treated | 2/2 | 597.5 | 597.5 | NC | 695 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit; TDS – total dissolved solids. | |||||
C.3 Targeted Drinking Water Survey 2012–2013
| Water type | Calcium (mg/L), RDL = 0.2 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 19/19 | 13.0 | 31.6 | 92.2 | 140.0 |
| Well – treated | 19/19 | 6.8 | 24.4 | 93.0 | 99.0 |
| Well – distribution | 14/14 | 12.0 | 30.2 | 89.8 | 96.0 |
| Lake – raw | 1/1 | 52 | 52 | 52 | 52 |
| Lake – treated | 1/1 | 50 | NC | NC | 50 |
| Lake – distribution | 1/1 | 52 | NC | NC | 52 |
| River – raw | 6/6 | 85.0 | 84.3 | NC | 91.0 |
| River – treated | 6/6 | 80 | 80.5 | NC | 90 |
| River – distribution | 6/6 | 78 | 76.8 | NC | 91 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit. | |||||
| Water type | Magnesium (mg/L), RDL = 0.05 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 19/19 | 2.5 | 9.6 | 17.4 | 73 |
| Well – treated | 19/19 | 2 | 5.7 | 16.4 | 24 |
| Well – distribution | 14/14 | 3.3 | 7.4 | 18.9 | 23 |
| Lake – raw | 1/1 | 8.6 | NC | NC | 8.6 |
| Lake – treated | 1/1 | 8.5 | NC | NC | 8.5 |
| Lake – distribution | 1/1 | 9 | NC | NC | 9 |
| River – raw | 6/6 | 20.5 | 19.5 | NC | 24 |
| River – treated | 6/6 | 20.5 | 19.7 | NC | 24 |
| River – distribution | 6/6 | 19.5 | 19.3 | NC | 24 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit. | |||||
| Water Type | CaCO3 (mg/L), RDL = 1 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 19/19 | 42 | 118.4 | 306 | 650 |
| Well – treated | 19/19 | 22 | 84.5 | 304 | 340 |
| Well – distribution | 14/14 | 41.5 | 105.5 | 305 | 330 |
| Lake – raw | 1/1 | 160 | 160 | NC | 160 |
| Lake – treated | 1/1 | 160 | NC | NC | 160 |
| Lake – distribution | 1/1 | 170 | NC | NC | 170 |
| River – raw | 6/6 | 290 | 290 | NC | 300 |
| River – treated | 6/6 | 290 | 283.3 | NC | 300 |
| River – distribution | 6/6 | 290 | 273.3 | NC | 300 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit. | |||||
| Water type | Dissolved chloride (mg/L), RDL = 1 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 19/19 | 66 | 70.3 | 152 | 170 |
| Well – treated | 19/19 | 76 | 82.1 | 162 | 180 |
| Well – distribution | 14/14 | 77 | 87.8 | 155 | 170 |
| Lake – raw | 1/1 | 55 | NC | NC | 55 |
| Lake – treated | 1/1 | 56 | NC | NC | 56 |
| Lake – distribution | 1/1 | 59 | NC | NC | 59 |
| River – raw | 6/6 | 86.5 | 67.7 | NC | 100 |
| River – treated | 6/6 | 94.5 | 78.0 | NC | 120 |
| River – distribution | 6/6 | 93.5 | 73.8 | NC | 110 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit. | |||||
| Water type | Dissolved sulphate (mg/L), RDL = 1 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 16/19 | 275 | 399.5 | 910 | 920 |
| Well – treated | 17/19 | 360 | 400.6 | 900 | 920 |
| Well – distribution | 13/14 | 370 | 405.8 | 868 | 920 |
| Lake – raw | 1/1 | 21 | NC | NC | 21 |
| Lake – treated | 1/1 | 20 | NC | NC | 20 |
| Lake – distribution | 1/1 | 21 | NC | NC | 21 |
| River – raw | 6/6 | 56 | 57.5 | NC | 71 |
| River – treated | 6/6 | 55.5 | 58 | NC | 73 |
| River – distribution | 6/6 | 58 | 57 | NC | 72 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit. | |||||
| Water type | TDS (mg/L), RDL = 1 mg/L | ||||
|---|---|---|---|---|---|
| Detects/samples | Median | Mean | 90th | Max | |
| Well – raw | 19/19 | 1 090 | 1 132.5 | 1 852 | 2 060 |
| Well – treated | 19/19 | 1 080 | 1 212.6 | 1 868 | 2 050 |
| Well – distribution | 14/14 | 1 285 | 1 243.7 | 1 858 | 1 910 |
| Lake – raw | 1/1 | 260 | NC | NC | 260 |
| Lake – treated | 1/1 | 250 | NC | NC | 250 |
| Lake – distribution | 1/1 | 260 | NC | NC | 260 |
| River – raw | 6/6 | 446 | 409.5 | NC | 451 |
| River – treated | 6/6 | 449 | 411.5 | NC | 470 |
| River – distribution | 6/6 | 424 | 394.5 | NC | 470 |
| NC – not calculated due to insufficient sample size; RDL – reporting detection limit. | |||||
Appendix D: Summary of total hardness removal for residential scale technologies
| Water treatment units | Average | Median | Min | Max | Percentage ofsamples above | Percentile | |||
|---|---|---|---|---|---|---|---|---|---|
| 120 mg/L | 180 mg/L | 75th | 95th | ||||||
| Total (n=96) | Influent (µg/L) | 136 356 | 112 581 | 156 | 419 661 | 44.8% | 24.0% | 175 631 | 369 694 |
| Effluent (µg/L) | 49 549 | 3 632 | 44 | 376 034 | 17.7% | 7.3% | 72 369 | 204 207 | |
| % reduction | 67% | 95% | -111% | 99.97% | N/A | N/A | 99.58% | 100% | |
| Ion exchange (n=54) | Influent (µg/L) | 155 740 | 115 706 | 469 | 419 661 | 48.1% | 27.8% | 237 422.8 | 385 947 |
| Effluent (µg/L) | 29 507 | 1 886 | 83 | 326 359 | 7.4% | 3.7% | 39 467.25 | 179 062 | |
| % reduction | 81% | 99% | -111% | 99.9% | N/A | N/A | 99.8% | 100% | |
| Activated carbon (n=18) | Influent (µg/L) | 115 749 | 103 011 | 156 | 279 420 | 44.4% | 22.2% | 156 814 | N/A |
| Effluent (µg/L) | 108 350 | 100 743 | 83 | 292 259 | 50% | 16.7% | 153 558 | N/A | |
| % reduction | 19% | 8% | -8% | 90.6% | N/A | N/A | 31% | N/A | |
| Reverse osmosis (n=9) | Influent (µg/L) | 30 865 | 2 803 | 269 | 138 639 | 22.2% | 0% | 65 002 | N/A |
| Effluent (µg/L) | 880 | 287 | 44 | 5 144 | 0% | 0% | 760 | N/A | |
| % reduction | 66% | 72% | 21.4% | 99.97% | N/A | N/A | 95% | N/A | |
| Green sand (n=3) | Influent (µg/L) | 107 478 | 73 769 | 67 743 | 180 921 | 33% | 33% | N/A | N/A |
| Effluent (µg/L) | 113 275 | 73 694 | 71 146 | 194 985 | 33% | 33% | N/A | N/A | |
| % reduction | -4% | -5% | -7.8% | 0.1% | N/A | N/A | N/A | N/A | |
| Ceramic microfilter (n=1) | Influent (µg/L) | 104 048 | N/A | N/A | N/A | 0% | 0% | N/A | N/A |
| Effluent (µg/L) | 101 113 | N/A | N/A | N/A | 0% | 0% | N/A | N/A | |
| % reduction | 2.82% | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Sediment filter (n=1) | Influent (µg/L) | 173 939 | N/A | N/A | N/A | 100% | 0% | N/A | N/A |
| Effluent (µg/L) | 173 947 | N/A | N/A | N/A | 100% | 0% | N/A | N/A | |
| % reduction | 0% | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Combinations (n=10) | Influent (µg/L) | 171 856 | 131 206 | 40 475 | 369 333 | 50% | 30% | 325 567 | N/A |
| Effluent (µg/L) | 59 027 | 2 569 | 119 | 376 034 | 20% | 10% | 62 554 | N/A | |
| % reduction | 87% | 99% | -1.8% | 99.9% | N/A | N/A | 100% | N/A | |
| Note: Effluent = treated water. N/A – not applicable; POE – point of entry; POU – point of use. | |||||||||
Appendix E: Intake of sodium as a result of water softener use, by hardness level
Depending on the type of softener used, sodium may be added to the water during the water softening process. The contribution of sodium to water from a water softener will vary depending on the level of hardness of the water. A higher concentration of hardness will result in higher levels of sodium being added to the water. The table below shows that a water softener using sodium chloride can add significantly to the intake of the sodium, compared with the amount that adults in Canada typically consume in drinking water.
| Drinking water hardness (CaCO3 mg/L) | Hardness (Grains per gallon)Footnote a | Sodium added (mg/L) |
|---|---|---|
| 100 (acceptable)Footnote b | 5.8 | 46 |
| 200 (poor) | 11.7 | 92 |
| 500 (unacceptable) | 29.2 | 230 |
|
||
When a water softener is used, it is recommended that a portion of the water most frequently consumed (such as from the kitchen tap) bypass the softener altogether. As a general rule, children under 8 years of age should not drink water containing sodium from a water softener as they may exceed the recommended upper limit (1.5–1.9 g/day) (IOM, 2005). Persons on a sodium restricted diet should consult their physician.
