Drinking water screening value for diazinon
Download the alternative format
(PDF format, 559 KB, 11 pages)
Organization: Health Canada
Date published: January 2022
A drinking water screening value of 0.015 mg/L (15 ug/L) is established for diazinon.
Screening values
Health Canada's screening values identify limits for contaminants in water that could be used as a source of drinking water. A lifetime of exposure to these contaminants up to the screening value, both by drinking the water or by using it for showering or bathing, is not expected to increase health risks for any Canadian, including children.
Screening values are established for contaminants that are not commonly found in Canadian drinking water (either source or treated) and therefore Guidelines for Canadian Drinking Water Quality are not established. Health Canada establishes screening values for contaminants at the request of federal departments, provinces and territories (jurisdictions). These requests are usually made when there is a concern for human health because the presence of a contaminant is suspected or detected in local source water and that contaminant does not have an established limit in drinking water. Since 2020, the technical summaries for screening values are typically published online when Health Canada expects that screening values may be needed by more than one stakeholder or jurisdiction.
Screening values do not replace or supersede existing regulations. However, screening values may help jurisdictions and the public understand the potential health effects of a contaminant.
Screening values are based on a review of scientific research and international regulatory information available at the time of their development. For pesticides, screening values align with the evaluations done by the Pest Management Regulatory Agency (PMRA), the lead authority for the safety of pesticide use in Canada, to ensure consistency.
Health Canada is committed to keeping pace with new science, including the potential health risks from contaminants that are not typically found in drinking water and do not have Guidelines for Canadian Drinking Water Quality. To this end, Health Canada includes contaminants with screening values in its cyclical prioritization of contaminants for full guideline development.
Table of contents
- 1.0 Exposure considerations
- 2.0 Health considerations
- 3.0 Derivation of the screening value
- 4.0 International considerations
- 5.0 References
1.0 Exposure considerations
1.1 Identity and sources
Diazinon (O,O-diethyl O-[6- methyl -2-(1-methylethyl)-4-pyrimidinyl phosphorothiate) is a broad-spectrum organophosphate insecticide that inhibits the enzyme acetylcholinesterase (AChE), disrupting nerve impulse transmission in target organisms. It works through contact, ingestion and vapor action. Diazinon is used on a variety of food, greenhouse and ornamental crops, as well as for seed treatments, on livestock and in forest/woodlot applications (Health Canada, 2005). In 2018, between 1000 and 5000 kg of diazinon was sold in Canada (Health Canada, 2018).
As indicated in Table 1, diazinon is moderately soluble in water, moderately volatile and slightly volatile from moist surfaces or water.
| Property | Value | Interpretation |
|---|---|---|
| CAS# | 333-41-5 | N/A |
| Molecular weight (g/mol) | 304.3 | N/A |
| Water solubility (mg/L) at 20°C | 60 | Moderate solubility |
| Vapour pressure (mmHg) | 1.4 x 10-4 | Moderate volatility |
| Henry’s law constant (atm∙m3 mol-1) | 9.3 × 10-7 | Low to moderate volatility from water or moist soil |
| n-Octanol: water partition coefficient (Log Kow) | 3.3 | Moderate potential to bioaccumulate |
In the aquatic environment under aerobic conditions such as a pond or lake, diazinon is considered non-persistent (50% dissociation in 3 to 15 days). In aerobic soil, diazinon is considered non-persistent to slightly persistent (50% dissipation in 5 to 80 days). Under anaerobic conditions, the biotransformation half-life (50% dissipation in 34 days) indicates that diazinon is slightly persistent. Abiotic transformation is generally not an important factor in the environmental dissipation of diazinon. The hydrolysis of diazinon is not important at environmentally relevant pHs (pH 5–9). Phototransformation of diazinon in water is also not an important dissipation route (84.5 days half-life); however, on the soil surface it may be an important route of transformation with a half-life of 20 hours (Health Canada, 2005). Environmental degradation products of diazinon include diazoxon, a toxic degradate, and 2-isopropyl-6-methyl-4-hydroxypyimidine (IMHP or oxypyrimidine) which is the main soil and water degradate that is less toxic and persistent than the parent compound (EPA 2004).
Diazinon has a low to moderate potential for mobility in a variety of soil types (Koc = 7752–440). Under field conditions, persistence ranges from non-persistent to slightly persistent (50% dissipation in 5 to 17 days) with leaching to 30 cm of soil depth. The major biotransformation product of diazinon, oxypyrimidine, was found to leach to 180 cm in soil, indicating a concern to groundwater (Health Canada, 2005).
1.2 Exposure
Canadians may be exposed to diazinon through food and water, working as a mixer/loader/ applicator, handling treated nursery plants or entering treated sites (Health Canada, 2009).
Seven Canadian provinces provided drinking water monitoring data for diazinon in support of the withdrawal of the Guideline for Canadian Drinking Water Quality in 2021. Monitoring data from Alberta, Manitoba, New Brunswick, Nova Scotia, Ontario, Quebec and Saskatchewan from 2007–2015 indicate that diazinon is rarely detected in Canadian drinking water supplies. Water samples taken from raw, treated and distribution systems showed that only 14 out of 5518 samples (0.3%) had detections of diazinon. The maximum detected level was 0.43 µg/L (Health Canada, 2021a).
Canadian monitoring data indicate that diazinon readily reaches surface water. The maximum surface water concentration detected in Canada was reported as 25 µg of active ingredient/L (Health Canada, 2005).
The Canadian Total Diet Study (TDS) is a food surveillance program that monitors the concentrations of chemical contaminants in foods that are typically consumed by Canadians. Over the period of 1993–1998, diazinon was measured in foods acquired in seven different Canadian cities (Montreal, Halifax, Winnipeg, Vancouver, Ottawa, Toronto and Whitehorse). Levels ranged from 0.25–31.45 ppm for a variety of foods including fruits (cherries, peaches, pears, apples, berries and melons), vegetables (broccoli, cabbage, carrots, celery, corn, cucumbers, lettuce, peas, peppers, potatoes, turnip), wheat products (flour, breads, cereals, cookies, crackers, muffins, oatmeal, pastas), peanuts and peanut butter, seeds, popcorn and chocolate bars (Health Canada, 2013). The maximum residue limits (MRL) established for diazinon in foods range from 0.25 ppm to 0.75 ppm (Health Canada, 2021b).
The National Chemical Residue Monitoring Program and the Food Safety Oversight Program are conducted by the Canadian Food Inspection Agency to verify compliance for chemical residues and contaminants in foods (domestic and imported) with Canadian standards and guidelines. From April 1, 2015 to March 31, 2016, diazinon was detected in various domestic and imported fresh and processed fruits and vegetables with a mean range of 0.0005 to 0.083 ppm (CFIA, 2019).
2.0 Health considerations
2.1 Kinetics
Diazinon is readily absorbed by oral exposure in both animals and humans. In humans, a group of five volunteers that ingested 0.011 mg/kg of diazinon (94% purity) excreted approximately 60% of the dose (as dialkyl phosphate metabolites) in the urine, with 90% of the excretion occurring within 14 hours following exposure (Garfitt et al. 2002). In a woman that ingested a lethal amount of diazinon (10% diazinon formulation, 293 mg/kg estimated dose), diazinon was detected largely in the blood and bile, and to a lesser extent in the adipose tissue, liver, brain and kidney (Poklis et al. 1980).
In animals, rats orally exposed to diazinon showed almost complete absorption with elimination mainly via the urine. Diazinon was not found to accumulate in tissues following single or multiple exposures. No dose-related differences appeared in the metabolism of diazinon (Health Canada, 2005). Data from animals show that in the liver, diazinon is degraded by CYP450-catalyzed oxidation generating phosphooxythiran as an intermediate which is then converted to diazoxon by spontaneous desulfuration. Through hydrolysis catalyzed by hepatic and extrahepatic A- and B-esterases, diazoxon is then converted to 2-isopropyl-4-methyl-6-hydroxypyrimidine (IMHP) and diethylphopshate (DEP) for excretion in the urine. Alternatively, phosphooxythiran can undergo hydrolysis, desulfuration, and deoxygenation to form IMHP, diethylthiophosphate (DETP), and DEP, which are excreted in the urine (Yang et al., 1971; Fabrizi et al., 1999; Poet et al., 2003). Female rats appear to excrete degradation products at a slower rate compared to males (Health Canada, 2005).
2.2 Health effects
All pesticides, including diazinon, are regulated by Health Canada’sPest Management Regulatory Agency (PMRA). PMRA conducts extensive evaluations and cyclical reviews of pesticides, including unpublished and proprietary information, as well as foreign reviews by other regulatory agencies such as the United States Environmental Protection Agency (US EPA). As such, this health assessment is based on the most recent PMRA evaluations (Health Canada, 2005, 2016) and supporting documentation.
Although human studies are available for diazinon, the data are not considered adequate for risk assessment due to data originating from poisoning events or study limitations including co-exposure to other pesticides or inadequate quantification of doses.
Slight to moderate acute toxicity has been reported in rats orally exposed to diazinon. Dermal exposure in rabbits, and inhalation exposure in rats, has resulted in low toxicity. Signs of acute toxicity include: tremors, convulsions, salivation and dyspnea which are consistent with AChE inhibition (Health Canada, 2005).
Subchronic and chronic toxicity studies in mice, rats, rabbits and dogs, show that the
most sensitive endpoint following diazinon exposure is inhibition of AChE activity in the plasma, erythrocytes and brain. AChE inhibition was generally dose-related and occurred over various durations and routes of exposure. Oral exposure studies indicate that for brain AChE inhibition, increasing exposure duration increases inhibition. Female rats and dogs appear to be slightly more sensitive to brain AChE inhibition, which may be related to the slower excretion leading to increased retention. When comparing no-observed-adverse-effect-levels (NOAELs) the dog appears to be slightly more sensitive to erythrocyte and brain AChE inhibition. However, since LOAELs among species are comparable, it may simply be a reflection of doses tested (Health Canada, 2005). In neonatal rats exposed to diazinon for 7 days, significant inhibition of plasma, red blood cell and brain AChE was reported. (Parker, 2003). Diazinon has also been found to inhibit AChE activity via short-term dermal and inhalation exposures(Health Canada, 2005).
Neurobehavioural effects have been noted following exposure to diazinon, however, no evidence of histopathological changes on the central nervous system were observed in subchronic and chronic studies (Health Canada, 2005).
There is no evidence of carcinogenicity in rats or mice following long-term exposure
through the diet and in vitro and in vivo mutagenicity studies indicate that overall, diazinon is not genotoxic (Health Canada, 2005).
There was no indication of teratogenicity or increased sensitivity of the fetus in unpublished developmental toxicity studies in rats and rabbits (Health Canada, 2005). In a published developmental toxicity study in rats, malformations were noted in fetuses in the presence of significant maternal toxicity (ElMazoudy et al., 2011). At maternally toxic doses in a two-generation reproductive toxicity study in rats, reduced survival and body weight gain were observed in offspring and signs of AChE inhibition (including tremors, soft stools and death), significant decreases in body-weight gain and reduced mating and fertility indices were observed in maternal animals (Health Canada, 2005). In a developmental neurotoxicity study, effects in offspring included decreased body weight, delays in sexual maturation, and effects on learning at the highest dose tested, while AChE inhibition was observed at lower dose levels. These findings in offspring were observed at dose levels associated with maternal toxicity, which included AChE inhibition and decreases in body weight gain and food consumption. There were no effects on other parameters examined in offspring, including clinical signs, auditory startle response, neuropathology and brain morphometry (Mandella, 2003). In other comparative cholinesterase studies, there was no indication of sensitivity of the fetus or nursing pups as a result of in utero or lactational exposure to diazinon via dosing of maternal animals (Mandella 2002a,b). However, acute and repeat-dose comparative cholinesterase studies demonstrated sensitivity of the young with respect to AChE inhibition when young animals were directly dosed with diazinon (Parker 2003a,b).
In a published study investigating the effects of diazinon on the hypothalamic-pituitary-testicular axis in male mice, effects on reproductive tissue weights, hormone levels, sperm parameters, as well as mating and fertility indices were observed following four weeks of gavage administration of diazinon (ElMazoudy and Attia, 2012). These findings occurred at dose levels above those associated with AChE inhibition in other studies.
2.3 Mode of action
Diazinon affects the nervous system by inhibiting AChE function in the central and peripheral nervous system. Although diazinon itself can inhibit AChE function, diazoxon is more potent and is considered the primary inhibitor of AChE function following diazinon exposure. AChE hydrolyzes and removes acetylcholine from the synapse of the pre- and postsynaptic nerve endings and in the neuromuscular junction, which terminates its action. Diazinon (or diazoxon) inhibits AChE function by forming a stable phosphorylated complex at the active site impairing its ability to remove acetylcholine. Acetylcholine accumulation causes continuous or over-stimulation of cholinergic fibers in the postganglionic parasympathetic nerve endings, neuromuscular junctions of the skeletal muscles, and cells of the central nervous system leading to hyperpolarization and receptor desensitization. The result is various muscarinic, nicotinic and central nervous system effects (ATSDR, 2008).
2.4 Selection of key study
AChE inhibition is considered as the most sensitive health endpoint following diazinon exposure (Health Canada, 2005). An unpublished 7-day AChE study with neonatal rats by Parker (2003a) was chosen by the PMRA in establishing an acceptable daily intake (ADI) for chronic dietary exposure (Health Canada, 2021c). This study is the key study for this human health risk assessment of diazinon in drinking water.
In this unpublished study, Sprague-Dawley pups (10/sex/dose) were exposed to 0, 0.03, 0.3, 3 or 30 mg/kg bw per day diazinon by gavage from post-natal day (PND) 11 to 17. Animals were observed for clinical toxicity and body weight gain throughout the study. Nine hours after the last dose animals were sacrificed with body, brain, heart, diaphragm weights recorded and AChE activity in brain, plasma and red blood cells assessed. AChE activity was determined at the time of peak effect. Signs of clinical toxicity were observed in males and females exposed to 0.3 mg/kg bw per day and above. In male and female PND 17 pups, plasma cholinesterase activity was significantly inhibited at 0.3 mg/kg bw per day and above, and brain AChE was significantly inhibited at 3 and 30 mg/kg bw per day. Erythrocyte cholinesterase activity was inhibited only at 30 mg/kg bw per day in male PND 17 pups, while a treatment-related inhibition of erythrocyte cholinesterase activity was noted starting from 3 mg/kg bw/day in PND 17 female pups. There were no significant impacts on body, heart, or diaphragm weights in pups of either sex. In post-natal day 17 males, absolute brain weights were slightly but significantly decreased in the 3 and 30 mg/kg bw per day dose groups; brain weights in female pups were not affected.
3.0 Derivation of the screening value
An ADI of 0.0016 mg/kg bw per day has been established by the PMRA to protect all populations, including infants and children, from diazinon exposure (Health Canada, 2016). The ADI is based on a benchmark dose lower confidence limit for a 20% response (BMDL20) of 0.4849 mg/kg bw/day from erythrocyte cholinesterase inhibition data derived from an unpublished 7-day AChE study with neonatal rats as described above. The ADI was derived as follows:
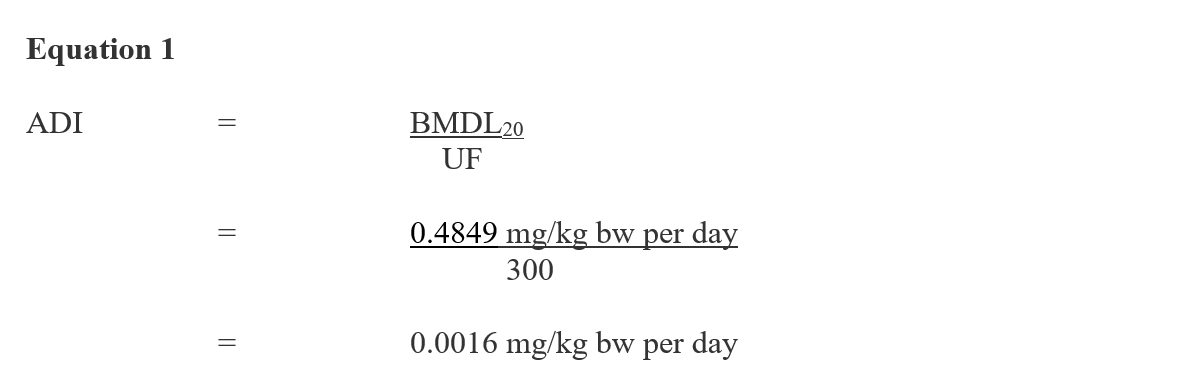
Figure 1 - Text Description
This equation calculates the acceptable daily intake for diazinon. The acceptable daily intake is calculated by dividing the benchmark dose lower confidence limit for a 20% response for diazinon (0.4849 milligrams per kilogram of body weight per day) by the uncertainty factor of 300, which equals 0.0016 milligrams per kilogram of body weight per day.
where:
BMDL20 = 0.4849 mg/kg bw per day, derived by the PMRA for erythrocyte AChE inhibition in neonatal rats from an unpublished 7-day study (Health Canada, 2021c); and
UF = uncertainty factor to account for interspecies (x10), intra-species variation (x10), PCPA hazard considerations (x1), as well as for the use of a less than lifetime study (x3). A factor of 3 for the use of a less than lifetime study is supported by benchmark dose analyses of data, which demonstrated that AChE inhibition occurred at lower dose levels in animals exposed to diazinon for longer durations. Due to the nature and level of concern for the cholinesterase endpoint, and since the BMDL20 is based on data from the sensitive subpopulation, the Pest Control Products Act factor was reduced to 1-fold (Health Canada, 2021c).
Using the ADI established by the PMRA, a drinking water screening value is derived as follows:
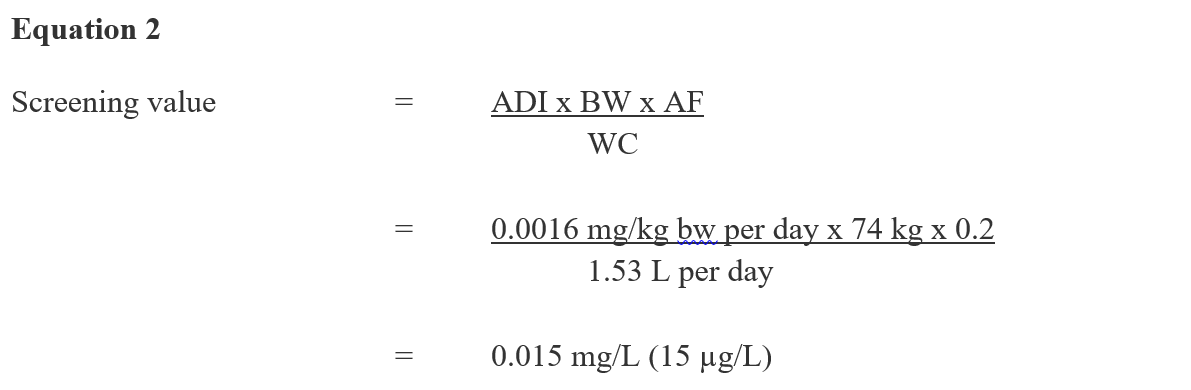
Figure 2 - Text Description
This equation calculates the drinking water screening value for diazinon. The acceptable daily intake for diazinon is multiplied by the median body weight estimated for an adult and by a source allocation factor for drinking water, and then is divided by the estimated daily volume of tap water consumed by an adult. The result is a screening value of 0.015 mg/L (15 µg/L).
where:
ADI = acceptable daily intake of 0.0016 mg/kg bw per day as derived above;
BW = the median body weight estimated for a Canadian adult is 74 kg (Health Canada, 2015).
AF = 0.2 is the allocation factor since drinking water is not a major source of exposure to diazinon and there is evidence of diazinon in other exposure sources (i.e., food) (Krishnan and Carrier, 2013).
WC = water consumption: the estimated daily volume of tap water consumed by a Canadian adult is 1.53 L (Health Canada, 2017b).
A screening value of 0.015 mg/L (15 µg/L) for diazinon is recommended by Health Canada. This screening value is considered protective for all age groups.
4.0 International considerations
Drinking water quality guidelines, standards and/or guidance established by foreign governments or international agencies may vary due to the science available at the time of assessment, as well as the utilization of different policies and approaches, such as the choice of key study, and the use of different consumption rates, body weights and allocation factors.
The United States Environmental Protection Agency (US EPA) has not established a maximum contaminant level (MCL) for diazinon in drinking water (the MCL is the equivalent of the MAC). However, the US EPA has established a non-enforceable lifetime health advisory of 0.001 mg/L in 1988 (US EPA, 2018) based on plasma cholinesterase inhibition in rats (US EPA, 1987). Health advisories serve as the informal technical guidance for unregulated drinking water contaminants in the United States.
The World Health Organization has not established a health-based guideline value for diazinon since it considers diazinon unlikely to occur in drinking water (WHO, 2017).
Australia has established a guideline value of 0.004 mg/L for diazinon in drinking water based on inhibition of plasma cholinesterase in humans (NHMRC and NRMMC, 2011).
All uses of diazinon are prohibited in the European Union (European Commission, 2007).
5.0 References
APVMA (2011). Australian Pesticides and Veterinary Medicines Authority, Chemical Review Program. Consolidated human health risk assessment for diazinon.
ATSDR (2008). Toxicological profile for diazinon. Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services, Atlanta, GA.
CFIA (2019). National Chemical Residue Monitoring Program and Chemistry Food Safety Oversight Program Annual Report 2015-2016 Canadian Food Inspection Agency.
ElMazoudy, R.H., Attia, A.A. and AbdElGawad, H.S. (2011). Evaluation of Developmental Toxicity Induced by Anticholinesterase Insecticide, Diazinon in Female Rats. Birth Defects Research (Part B), 92: 534-542. [as cited in Health Canada, 2017a].
ElMazoudy, R.H. and Attia, A.A. (2012). Endocrine-disrupting and Cytotoxic Potential of Anticholinesterase Insecticide, Diazinon in Reproductive Toxicity of Male Mice. Journal of Hazardous Materials, 209-210: 111-120. [as cited in Health Canada, 2017a].
European Commission (2007). Commission Decision of 6 June 2007 concerning the non-inclusion of diazinon in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing this substance. Official Journal of the European Union, 9.6.2007 (2007/393/EC)
Fabrizi, L., Gemma, S., Testai, E., and Vittozzi, L. (1999). Identification of the cytochrome P450 isoenzymes involved in the metabolism of diazinon in the rat liver. J Biochem Mol Toxicol 13(1): 53-61.
Health Canada (2005). Re-evaluation Note REV2005-06. Preliminary risk and value assessments for diazinon. Pest Management Regulatory Agency.
Health Canada (2009). Re-evaluation decision for diazinon RVD2009-18. Pest Management Regulatory Agency.
Health Canada (2013). Canadian total diet study. Minister of Health, Ottawa, ON.
Health Canada (2015). Food consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa 2015.
Health Canada (2016). Re-evaluation note REV2016-18. Special Review of Diazinon – Subsection 17(1) of Pest Control Products Act: Proposed Decision for Consultation, November 30, 2016.
Health Canada (2017a). Re-evaluation note REV2017-12. Special review decision: diazinon – subsection 17(1) of Pest Control Products Act. Pest Management Regulatory Agency.
Health Canada (2017b). Water consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa 2015.
Health Canada (2018). Pest Control Products Sales Report for 2018.
Health Canada (2021a). Withdrawal of Select Guidelines. Available at: link to be added upon publication
Health Canada (2021b). Maximum residue limits for diazinon.
Health Canada (2021c). Personal Communication with the Health Evaluation Directorate, Pest Management Regulatory Agency (PMRA).
Krishnan, K., Carrier, R. (2013). The use of exposure source allocation factor in the risk assessment of drinking-water contaminants. J. Toxicol. Environ. Health B Crit. Rev., 16(1):39–51.
Mandella, R.C. (2002a). Diazinon: Gestation Day 20 Cholinesterase Determinations in a Dietary Range-finding Developmental Neurotoxicity Study in Rats. Huntingdon Life Sciences, East Millstone, New Jersey. Laboratory report number: 01-4531. [as cited in Health Canada, 2017a].
Mandella, R.C. (2002b). Diazinon: A Dietary Range-finding Developmental Neurotoxicity Study in Rats. Huntingdon Life Sciences, East Millstone, New Jersey. Laboratory report number: 01-4530. [as cited in Health Canada, 2017a].
Mandella, R.C. (2003). Diazinon: A Developmental Neurotoxicity Study in Rats. Huntingdon Life Sciences, East Millstone, New Jersey. Laboratory study number: 01-4532. [as cited in Health Canada, 2017a].
NHMRC and NRMMC (2011). Australian Drinking Water Guidelines Paper 6 National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra.
Parker, S.P. (2003a). Comparative sensitivity of neonatal/juvenile and young adult CD rats after repeated exposure to diazinon. Center for Life Sciences and Toxicology, RTI International, Research Triangle Park, North Carolina. Laboratory project number: 08882.005. [as cited in Health Canada, 2017a].
Parker, S.P. (2003b). Relative Sensitivity of Neonatal/Juvenile and Young Adult CD Rats After an Acute Exposure to Diazinon. Center for Life Sciences and Toxicology, RTI International, Research Triangle Park, North Carolina, Laboratory project number: 08882.4. [as cited in Health Canada, 2017a].
Poet, T.S., Wu, H., Kousba, A.A. and Timchalk, C. (2003). In vitro rat hepatic and intestinal metabolism of the organophosphate pesticides chlorpyrifos and diazinon. Toxicol Sci 72(2):193-200.
Poklis, A., Kutz, F.W., Sperling, J.F. and Morgan, D.P. (1980). A fatal diazinon poisoning. Forensic Sci Int 15(2):135-140.
Rudzki MW, McCormick GC & Arthur AT (1991) 52-week oral toxicity study in dogs. Study no. 882014. Lab: Ciba-Geigy Corp., Research Department, Pharmaceuticals Division, Summit, New Jersey, USA. Sponsor: Ciba-Geigy Corp., Agricultural Division, Greensboro, NorthPart 2 – Toxicological Hazard Assessment 240. [as cited in: APVMA (2011)].
Carolina, USA. Study duration: 29 Aug, 1988 - 30 Aug, 1989. Report date: 14 Jun, 1991. (US GLP statement provided).
US EPA (1987). Diazinon draft health advisory. Office of Drinking Water, United States Environmental Protection Agency.
US EPA (2018). 2018 Edition of the Drinking Water Standards and Health Advisories Tables. Office of Water, United States Environmental Protection Agency.
WHO (2017). Guidelines for drinking-water quality: fourth edition incorporating the first addendum.
Geneva: World Health Organization.
Yang, R.S.H., Hodgson, E. and Dauterman, W.C. (1971). Metabolism in vitro of diazinon and diazoxon in rat
liver. J Agric Food Chem 19(1): 10-13.