Guidelines for Canadian drinking water quality: Guideline technical document – Diquat
Guideline value
The maximum acceptable concentration (MAC) for diquat in drinking water is 0.05 mg/L (50 µg/L) (measured as the cation).
Executive summary
This guideline technical document was prepared in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water and is based on assessments of diquat completed by Health Canada’s Pest Management Regulatory Agency and supporting documents.
Exposure
In Canada, diquat is an herbicide that is deliberately applied to food crops and to water sources for weed control. The general Canadian population is therefore potentially exposed to diquat through food, and to a lesser extent, drinking water. In 2018, the most recent year for which data are available, more than 500 000 kg of diquat (as active ingredient) was sold in Canada. Very low levels of diquat have been detected in foods. Data provided by provinces and territories that monitor for diquat in source and drinking water indicate that levels of diquat are below the detection limit.
Health effects
In repeat-dose animal studies, diquat primarily targeted the eyes, causing cataracts. It also affected the kidneys and liver. The MAC of 0.05 mg/L (50 µg/L) is based on cataract formation.
Analytical and treatment considerations
Currently, there is one method available for the analysis of diquat in drinking water. The method detection limit is more than an order of magnitude below the MAC.
Granular activated carbon is considered by the United States Environmental Protection Agency to be the best available technology for removing diquat from water. Membrane filtration techniques (nanofiltration and reverse osmosis or RO) and oxidation may also be effective. It is recommended that pilot- and/or bench-scale testing be conducted prior to full-scale implementation of treatment.
In cases where diquat removal is desired at a small system or household level—for example, when the drinking water supply is from a private well—a residential drinking water treatment unit may be an option. Adsorption (activated carbon) and RO represent the best potential technologies for diquat removal. When using a residential drinking water treatment unit, it is important to take samples of water entering and leaving the treatment unit and to send them to an accredited laboratory for analysis to ensure that adequate diquat removal is occurring.
Application of the guidelines
Note: Specific guidance related to the implementation of drinking water guidelines should be obtained from the appropriate drinking water authority.
The guidelines are protective against health effects from exposure to diquat in drinking water over a lifetime. Any exceedance of the MAC should be investigated and followed by the appropriate corrective actions if required. For exceedances in source water where there is no treatment in place, additional monitoring to confirm the exceedance should be conducted. If it is confirmed that source water diquat concentrations are above the MAC, then an investigation to determine the most appropriate way to reduce exposure to diquat should be conducted. This may include use of an alternate water supply or installation of treatment. Where treatment is already in place and an exceedance occurs, an investigation should be conducted to verify treatment and to determine whether adjustments are needed to lower the treated water concentration below the MAC.
Table of contents
- 1.0 Exposure considerations.
- 2.0 Health considerations.
- 3.0 Derivation of the health-based value
- 4.0 Analytical and treatment considerations
- 5.0 Management strategies
- 6.0 International considerations
- 7.0 Rationale
- 8.0 References
- Appendix A: List of abbreviations
1.0 Exposure considerations
1.1 Sources and uses
Diquat, also called 1,1ꞌ-ethylene-2,2ꞌ-bipyridinium ion or 6,7-dihydrodipyrido[1,2-a:2ꞌ,1ꞌ -c] pyrazinediium ion, is a non-selective contact herbicide and algaecide used as a pre-harvest desiccant for various terrestrial food and feed crops and for industrial oilseed and fibre crops, as a defoliant (e.g., potato haulm destruction), as a non-cropland chemical mowing agent, and as a tool for the control of aquatic weeds and algae (Health Canada, 2008). Diquat acts by generating superoxides during photosynthesis that damage cell membranes and cytoplasm (Health Canada, 2008; WHO, 2014). In Canada, diquat is sold as diquat dibromide, a highly hygroscopic salt supplied in an aqueous solution (Health Canada, 2008; Health Canada, 2010). More than 500 000 kg active ingredient of diquat was sold in Canada in 2018 (Health Canada, 2020).
Diquat and diquat dibromide have the potential to reach surface water via runoff or spray drift (Health Canada, 2008). Once in the environment, diquat dibromide completely dissociates to diquat in water (WHO, 2014). Diquat will then rapidly dissipate by adsorption to particles (e.g., suspended matter, montmorillonite clay, and phytoplankton) and sediments on which it can be retained for very long periods of time, with reported half-lives ranging from 1 to 2 days in the unsorbed state (e.g., less than 48 hours in surface waters) to more than 1 year in the sorbed state (Emmett, 2002; WHO, 2016; Magalhães et al., 2018). Diquat is not expected to leach into groundwater as it strongly adsorbs to soil particles, rendering it immobile and persistent in soils (US EPA, 1995a). Diquat does not hydrolyze and is resistant to microbial degradation under aerobic and anaerobic conditions (typically 5%–7% removal from soil per year through microbial degradation) (US EPA, 1995a; Emmett, 2002; Magalhães et al., 2018). It does undergo photodegradation, mainly to 1,2,3,4-tetrahydro-1-oxopyrido[1,2-a]-5-pyrazinium salt (TOPPS), but slowly with reported rates of 10%–20% per year (depending on the experimental conditions) (EFSA, 2015; Magalhães et al., 2018). Diquat is removed from the water column by adsorption to soil sediments, aquatic vegetation, and organic matter (US EPA, 1995a).
Owing to its very low volatility, diquat dibromide residue will occur in the air most likely as aerosols. However, its presence in the atmosphere over time is unlikely as it would normally be removed by gravitational settling, ending up in surface waters and/or in the soil where it will dissociate to the diquat ion (HSDB, 2010).
Numerous factors can affect the fate and persistence of diquat in the environment, such as water temperature, soil moisture, and the rate of microbial metabolism. Furthermore, the intensive use of fertilizers containing other cations (e.g., Ca2+, Mg2+, NH4(2+), K+) may lead to a greater desorption of diquat from sediment (Emmett, 2002).
1.2 Substance identity
Diquat (CAS RN 2764-72-9; C12H12N2) is a quaternary ammonium (divalent) cation from the bipyridylium chemical class; it has a molecular weight of 184.2 g/mol (US EPA, 1995a; Health Canada, 2008; Health Canada, 2010).
Diquat is sold as diquat dibromide (or 6,7-dihydrodipyrido(1,2-a:2ꞌ,1ꞌ-c) pyrazinediium dibromide or 1,1ꞌ -ethylene-2,2ꞌ -dipyridylium dibromide), an odourless, pale yellow crystalline solid (US EPA, 1995a; Emmett, 2002). Diquat dibromide is highly soluble in water and readily dissociates to the diquat ion (US EPA, 1995a; Emmett, 2002).
| Property | Diquat Dibromide | Interpretation |
|---|---|---|
| CAS RNFootnote a | 85-00-7 | Not applicable |
| Molecular formula | C12H12Br2N2 | Not applicable |
| Molecular weight g/mol) | 344.0 | Not applicable |
| Water solubility (g/L) | 700 at 25°C | Highly soluble in water |
| Vapour pressure (volatility) | < 0.01 mPa at 20°C (for monohydrate) | Low volatility, unlikely to be present in air |
| Henry’s law constant | 5 ×10-9Pa.m3.mol | Low volatility, unlikely to be present in air |
| Dissociation constant | Not applicable | Complete dissociation |
| n-Octanol:water partition coefficient (log Kow) |
-4.60 at 20 °C | Hydrophilic |
|
||
The synthesis of diquat dibromide may result in the formation of ethylene dibromide as a process impurity. However, test results have shown that ethylene dibromide, which is not used as a pesticide, does not persist as an impurity in diquat products as it dissipates with time (US EPA, 1995a). Furthermore, since the highly charged diquat is identified in the current assessment as the most toxicologically significant species in mammals, dose levels and water concentrations are defined, whenever possible, in terms of diquat cation, herein referred to as diquat (Health Canada, 2008; FAO and WHO, 2014).
1.3 Exposure
As an herbicide, diquat is applied to food crops and to water sources for weed control. The general Canadian population is thus exposed to diquat through food and, to a lesser extent, drinking water (Health Canada, 2008, 2010). Given its environmental fate (see Section 1.1), significant residues of the herbicide are not expected in water sources, making drinking water a minor source of exposure (US EPA, 2002; Health Canada, 2008; NHMRC and NRMMC, 2011; EFSA, 2015; OEHHA, 2016; WHO, 2016).
Data provided by the provinces and territories indicate that diquat levels are below the method reporting limit (MRL) or method detection limit (MDL) in all samples collected from a variety of water supplies in Canada, including surface water and groundwater as well as treated and distributed water where monitoring occurred (British Columbia Ministry of Health, 2019; Government of Ontario, 2019; Indigenous Services Canada, 2019; Ministère de l’Environnement et de la Lutte contre les changements climatiques du Québec, 2019; Nova Scotia Environment, 2019; Prince Edward Island Department of Communities, Land and Environment, 2019) (see Table 2).
Monitoring for diquat is not currently conducted in Manitoba, New Brunswick, Newfoundland and Labrador, Saskatchewan or Yukon (Manitoba Sustainable Development 2019; New Brunswick Department of Health, 2019; Newfoundland and Labrador Municipal Affairs and Environment, 2019; Saskatchewan Water Security Agency, 2019; Yukon Environmental Health Services, 2019).
| Jurisdiction (MDL µg/L) |
Monitoring Period | Types of Water System | Water Type: (Municipal: ground/surface – raw, treated, distributed) |
# Detects/ Samples |
|---|---|---|---|---|
| British Columbia (7) |
2013–2018 | Municipal | Surface – raw | 0/18 |
| FNIHB Ontario Region (1–50) |
2014–2018 | Public Water Systems | Ground – raw | 0/13 |
| Ground – treated | 0/190 | |||
| Ground – distribution | 0/16 | |||
| Surface – raw | 0/33 | |||
| Surface – treated | 0/308 | |||
| Surface – distribution | 0/23 | |||
| Semi Public Water Systems | Ground – raw | 0/3 | ||
| Ground – treated | 0/16 | |||
| Ground – distribution | 0/68 | |||
| Surface – raw | 0/1 | |||
| Surface – treated | 0/9 | |||
| Surface – distribution | 0/2 | |||
| Private Water Systems | Ground – treated | 0/3 | ||
| Ground – distribution | 0/50 | |||
| Surface – treated | 0/5 | |||
| FNIHB Atlantic Region (7–70) |
2014–2018 | Public Water Systems | Ground – treated | 0/4 |
| Ground – distribution | 0/4 | |||
| Surface – treated | 0/1 | |||
| FNIHB Québec Region (0.1–0.4) | 2014–2018 | - | Drinking water system | 0/4 |
| Nova Scotia (1–7) |
2007–2018 | Municipal | Ground – raw | 0/71 |
| Ground – treated | 0/35 | |||
| Surface – raw | 0/35 | |||
| Surface – treated | 0/39 | |||
| Distributed | 0/1 | |||
| Ontario (0.1) |
2008–2012 | Municipal | Ground – raw | 0/91 |
| Ground – treated | 0/25 | |||
| Unknown – raw | 0/213 | |||
| Unknown – treated | 0/223 | |||
| Unknown – distribution | 0/1 | |||
| Prince Edward Island (10) |
2007–2016 | Municipal | Ground – raw | 0/103 |
| Non-municipal | Ground – raw | 0/137 | ||
| Québec (0.1–15) |
2013–2018 | Municipal | Ground – distribution | 0/574 |
| Surface – distribution | 0/1726 | |||
| Municipal (Special Projects) Potatoes projectFootnote a [2017–2018] |
Ground – raw | 0/46 | ||
| Ground – treated | 0/17 | |||
| Ground – distribution | 0/5 | |||
| Small systemsFootnote b [2012–2018] |
Ground – raw | 0/63 | ||
| Non-municipal | - | |||
| Ground – raw | 0/43 | |||
FNIHB – First Nations and Inuit Health Branch
|
||||
In foods, diquat residues are expected only when the herbicide is applied directly to food crops (e.g., when used as a desiccant on potato plants), although residue levels are likely to be low (US EPA, 1995a; Health Canada, 2010; NHMRC and NRMMC, 2011). Results from residue trials conducted in Canada in 2015 were between 0.01 and 0.35 mg/kg (n = 24) for dry beans (including white and red kidney beans, soya beans, adzuki beans and fava beans), between 0.07 and 0.58 mg/kg (n = 9) for chickpeas, between 0.052 and 0.57 mg/kg (n = 8) for lentils, and between 0.15 and 2.1 mg/kg (n = 6) for barley (FAO, 2019). For animal commodities, testing in dairy cows fed diets containing 18, 50 and 84 ppm diquat for 30 days showed that diquat levels were below the limit of quantification (LOQ) for all milk samples (LOQ = 0.001 mg/kg) and for liver, kidney, fat and muscle samples (LOQ = 0.01 mg/kg), for all dose groups. In eggs, levels were also less than the LOQ of 0.01 mg/kg (FAO, 2019).
2.0 Health considerations
All pesticides, including diquat, are regulated by Health Canada’s Pest Management Regulatory Agency (PMRA). PMRA conducts extensive evaluations and cyclical reviews of pesticides, including unpublished and proprietary information, as well as foreign reviews by other regulatory agencies such as the United States Environmental Protection Agency (US EPA). This health assessment is based primarily on PMRA’s evaluations and supporting documentation (Health Canada, 2008; Health Canada, 2010). Additionally, any reviews and relevant literature available since PMRA’s evaluations were completed were also considered.
2.1 Kinetics
Absorption: Ingested diquat is poorly absorbed (<10%) from the gastrointestinal tract of animals, including humans. Although bioavailability values of less than 10% are usually reported, an expert committee of the European Food Safety Authority concluded that the available data support bioavailability values of 3%–4% instead (US EPA, 1995a; Emmett, 2002; FAO and WHO, 2014; EFSA, 2015; Magalhães et al., 2018). Furthermore, species differ in their absorption of diquat, with dogs exhibiting the highest absorption (Gupta and Crissman, 2013). Both the presence of food in the digestive tract and the presence of intestinal microflora that degrade diquat significantly decrease absorption (Magalhães et al., 2018).
Distribution: Once in the aqueous phase of the blood, the small amount of absorbed diquat is rapidly (i.e., within 6–18 hours) and widely distributed to several organs and tissues (e.g., liver, kidney, adrenal glands), except the brain and spinal cord (Gupta and Crissman, 2013; Magalhães et al., 2018). Despite its wide distribution, the hydrophilic and highly polar diquat does not covalently bind to macromolecules nor does it accumulate in most of the tissues, except the eye lens. Even though high diquat levels were found in the liver, the kidney, the gastrointestinal tract, and the lungs immediately after dosing, the eye lens was the primary site of significant diquat deposit up to 96 hours post-exposure (US EPA, 1995a; Emmett, 2002; FAO and WHO, 2014; EFSA, 2015; Magalhães et al., 2018).
Metabolism: Both experimental animal and human data indicate that the intracellular catabolism of free diquat is minimal and that it is predominately metabolized in the liver. Diquat metabolism, therefore, proceeds by cytochrome P450 enzymes with the formation of diquat monopyridone and diquat dipyridone as major and minor metabolites, respectively (Fuke et al., 1996; Emmett, 2002; FAO and WHO, 2014; WHO, 2014). Some data indicate that diquat biotransformation may also result in the formation of picolinic acid (or pyridine-2-carboxylic acid), presumably via picolinamide (or pyridine-2-carboxamide) as an intermediate, although this has not been clearly identified in mammals. In addition to the above-mentioned metabolites, the production of volatile compounds has been hypothesized. There is also some in vitro evidence of an alternative bacterial biotransformation of diquat occurring to a minor degree in the gastrointestinal tract with the monopyridone derivative as the major metabolite (Emmett, 2002; FAO and WHO, 2014; WHO, 2014; Magalhães et al., 2018).
Elimination: Due to its poor absorption, ingested diquat is mainly (about 90%) excreted unchanged via the feces within 24 hours with virtually no biliary excretion (<0.7% of the administered dose). In addition to the parent chemical, two other metabolites, diquat monopyridone and diquat dipyridone, are excreted in the feces (Emmett, 2002; Magalhães et al., 2018). Absorbed diquat is primarily (>90%) excreted within 48 hours in urine, mostly as the parent compound, followed by its two main metabolites and, to a lesser extent, picolinic acid (US EPA, 1995a; Fuke et al., 1996; Emmett, 2002; NHMRC and NRMMC, 2011; FAO and WHO, 2014; WHO, 2014; EFSA, 2015; Magalhães et al., 2018).
2.2 Health effects
The database for the toxicity of diquat is adequate covering several endpoints and various types of exposure. For more thorough reviews, see US EPA, 1995a, 2001; FAO/WHO, 2014; WHO, 2014; and EFSA, 2015. In general, diquat has a low acute toxicity. Repeated-dose studies in animals show that diquat may induce toxicity in multiple organs (e.g., gastrointestinal tract, kidneys and liver), with the eye being the most sensitive endpoint (FAO and WHO, 2014; WHO, 2014; EFSA, 2015).
2.3 Effects in humans
Intentional ingestion of diquat by humans may result in poisoning and even death (Magalhães et al., 2018). In general, the clinical features from acute poisoning include neurological disorders, gastrointestinal tract disturbances, renal failure, hepatic injury, and hemodynamic and cardiocirculatory complications (Valiante et al., 1992; Schmidt et al., 1999; Tanen et al., 1999; Fuke et al., 1996; Hantson et al., 2000; Jones and Vale, 2000; Emmett, 2002; Jovic-Stosic et al., 2009; WHO, 2014).
Epidemiological data specific to diquat are scarce but include reports of adverse health effects in manufacturing plant personnel (WHO, 2014).
2.4 Effects in animals
Diquat has been shown to be toxic to experimental animals with oral median lethal dose (LD50) values reported for some species as follows: 215–235 mg/kg bw in rats; 125 mg/kg bw in mice; 100–200 mg/kg bw in dogs; 100 mg/kg bw in rabbits and 100–300 mg/kg bw in monkeys (US EPA, 1995a; FAO and WHO, 2014; WHO, 2014; WHO, 2016; Magalhães et al., 2018). Diquat metabolites were found to be less toxic, with reported rat oral LD50values of >4000 mg/kg bw for diquat monopyridone and ≥ 2449 mg/kg bw for TOPPS (WHO, 2014; Magalhães et al., 2018). Adverse effects were related to the gastrointestinal tract and kidneys and potentially to the liver (US EPA, 1995a; Emmett, 2002; WHO, 2014).
Both subchronic and chronic exposure to diquat resulted in eye damage (e.g., cataracts, extralenticular lesions such as vitreous adhesions, retinal detachment and synechia) in the exposed experimental animals, including rats and dogs. Damage to the kidney, liver, adrenals, and epididymis and alterations in hematological parameters were also reported (US EPA, 2001; FAO and WHO, 2014; EFSA, 2015; WHO, 2016).
Ocular lesions (cataracts and lens opacities): Both subchronic and chronic (dietary) exposures to diquat dibromide have consistently resulted in eye damage in mouse (chronic oral toxicity study), rat (two-generation reproductive studies, subchronic oral toxicity and subchronic neurotoxicity study, chronic oral toxicity study) and dog (chronic toxicity study) studies (US EPA, 1995a; Emmett, 2002; WHO, 2014; EFSA, 2015). Eye damage was observed following chronic ingestion of diquat (at doses of up to 48.27, 19.44 and 12.5 mg/kg per day for mouse, rat and dog, respectively) and generally progressed from opacities of the lens to total opacification (i.e., cataracts); the incidence and severity of the damage were dose-related (Colley et al., 1985; Hopkins, 1990; Hodge, 1992; Emmett, 2002; WHO, 2014; EFSA, 2015). In the rat, cataracts were first observed at week 13 in the subchronic neurotoxicity study (no-observed-adverse-effect level (NOAEL) of 8 mg/kg per day) and at week 10 in the chronic toxicity study (NOAEL of 0.58 mg/kg per day) (Colley et al., 1985; Horner, 1992a). The dog was the most sensitive species, exhibiting total opacities of the eyes at week 8 in females exposed to 2.5 mg diquat/kg bw per day and at week 16 in males exposed to 12.5 mg diquat/kg bw per day. From the one-year dog study, the highest chronic NOAEL was 0.53 mg diquat/kg bw per day, based on cataracts observed in female dogs (Hopkins, 1990).
Nephrotoxicity: Diquat can induce nephrotoxicity which is generally characterized by renal tubular necrosis with subsequent decrease in the clearance of diquat and worsening of the damage (Gupta and Crissman, 2013). Diquat-induced kidney lesions were reported in mice (one chronic study), rats (two rat multigenerational studies and one lifetime study), and dogs (two chronic studies) orally exposed to up to 48, 19, and 12.5 mg diquat/kg bw per day, respectively (US EPA, 1995a; WHO, 2014; EFSA, 2015; OEHHA, 2016).
In a chronic feeding study, groups of CD-1 mice (60/sex/dose) given diets containing 0, 30, 100 or 300 ppm (equivalent to 0, 3.56, 11.96 or 37.83 mg/kg bw per day for males and 0, 4.78, 16.03, 48.27 mg/kg bw per day for females, expressed as diquat cation) for at least 104 weeks showed treatment-related effects starting at 100 ppm. Kidney effects included increased relative kidney weights in males and increased incidence of tubular hyaline droplet formation in females. Both sexes showed a slight increase in the incidence of renal tubular dilation. The no-observed-effect-level was 30 ppm (Hodge, 1992; WHO, 2014).
In a chronic/carcinogenicity study, Sprague-Dawley rats (60/sex/dose) were fed diets containing 0, 5, 15, 75 or 375 ppm (equivalent to 0, 0.19, 0.58, 2.91 or 14.99 mg/kg bw per day for males and 0, 0.24, 0.72, 3.64 or 19.44 mg/kg bw per day for females, expressed as diquat cation) for 104 weeks. Starting at 75 ppm, rats of both sex had decreased renal clearance (Colley et al., 1985; US EPA, 2001; Emmett, 2002).
In a two-generation developmental study in which pregnant Wistar-derived rats (23–24/dose) were gavaged with 9, 4, 12 or 40 mg/kg bw per day of diquat (expressed as cation) from gestation day 7 through 16, the incidence of hemorrhagic kidneys was increased in the fetuses of pregnant rats exposed to 40 mg/kg per day (Wickramaratne, 1989).
Dogs fed diquat in the diet at 12.5 mg/kg bw per day for 1 year had increased kidney weights, although no related histopathological changes were noted (Hopkins, 1990; US EPA, 2001; Emmett, 2002).
Reproductive and developmental toxicities: Experimental animal studies do not support a clear association between oral exposure to diquat and adverse reproductive and developmental outcomes (EFSA, 2015; OEHHA, 2016; WHO, 2016). No effect was observed on the reproductive function of rats fed a diet containing diquat at a dose of at least 25 mg/kg/day in two separate multigenerational reproductive toxicity studies (Fletcher, 1972; Hodge, 1990). Data from studies in which pregnant mice, rats and rabbits were exposed to diquat by gavage at doses of at least 4, 40, and 10 mg/kg per day, respectively, suggested some teratogenic effects of diquat; however, these adverse effects were generally observed at the highest dose tested, and there was no indication of an increased sensitivity of the offspring (in utero and/or postnatal) to diquat exposure (Palmer et al., 1978; Hodge, 1989; Wickramaratne, 1989). Friable and/or mottled livers were observed in the fetuses of pregnant New Zealand rabbits exposed to diquat by gavage at a dose of 10 mg/kg bw per day (Hodge, 1989; US EPA, 2001).
Neurotoxicity and other effects: There was no evidence of neurotoxicity of diquat following oral exposure. Acute and subchronic neurotoxicity studies in which rats and mice were fed diets containing up to 38.5 and 150 mg/kg bw per day of diquat, respectively, did not result in neurologically adverse effects (e.g., neuropathies, neurodegenerative effects) as evaluated by functional observation battery tests, motor activity testing and neuro-histopathological examinations (Horner, 1992a, 1992b; US EPA, 1995a, 2001; Emmett, 2002; EFSA, 2015; Minnema et al., 2016). Increased incidences of arteritis/periarteritis in blood vessels and paracortical cell hyperplasia in the lymph nodes were observed in male rats dosed with diquat at 14.88 mg/kg bw per day (Colley et al., 1985).
2.5 Genotoxicity and carcinogenicity
The current evidence indicates that diquat is neither genotoxic nor carcinogenic (US EPA, 2002; NHMRC and NRMMC, 2011; FAO and WHO, 2014; EFSA, 2015; OEHHA, 2016; WHO, 2016).
Diquat dibromide was negative in four mutagenicity assays (i.e., Ames tests, mouse bone marrow micronucleus assay, mouse dominant lethal assay, and unscheduled DNA synthesis in rat hepatocytes), but was positive in two other studies (i.e., mouse lymphoma cell assay, human blood lymphocytes, with or without metabolic activation) (US EPA, 1995a; WHO, 2016). Furthermore, there was no evidence of genotoxicity of diquat monopyridone or TOPPS (FAO and WHO, 2014).
The results of lifetime studies in mice and rats found no evidence of carcinogenicity for diquat (Colley et al., 1985; Hodge, 1992; US EPA, 1995a; NHMRC and NRMMC, 2011; FAO and WHO, 2014; EFSA, 2015; WHO, 2016). The US EPA has classified diquat dibromide as a Group E carcinogen (i.e., evidence of non-carcinogenicity for humans), while the International Agency for Research on Cancer has not reviewed the carcinogenicity of either diquat or diquat dibromide (US EPA, 1995a).
2.6 Mode of action
In mammals, the cytotoxicity of diquat was found to be associated with the parent compound. The mode of action of the cytotoxicity of the highly charged cation is generally similar to that of the other bipyridyl herbicides, such as paraquat. It involves oxidation-reduction (redox) cycling that generates reactive oxygen species and/or reactive nitrogen species and depletes the cellular pyridine nucleotides, subsequently leading to oxidative stress, cellular dysfunction, and potentially cellular necrosis (Gallagher et al., 1995; Jones and Vale, 2000; Emmett, 2002; Fussell et al., 2011; Gupta and Crissman, 2013; Gupta, 2014; Magalhães et al., 2018). In vivo redox cycling as well as in vitro lipid peroxidation of diquat have been demonstrated (Sandy et al., 1986; Circu et al., 2017).
Overall, although not conclusively demonstrated, it is anticipated that diquat redox cycling may be responsible for the specific toxicity findings, i.e., eye damage (or cataract), observed in the toxicological studies since the eyes accumulate diquat more than other tissues (Emmett, 2002; OEHHA, 2016).
2.7 Selected key study
Health Canada’s PMRA considered the eye as the most sensitive target organ across the database (Health Canada, 2008, 2010, 2019). The 1-year investigation in dogs conducted by Hopkins (1990) was identified as the key study for the human health risk assessment of diquat in drinking water. While PMRA considers a 1-year dog study to be a sub-chronic study, it is considered to be of sufficient duration for use in establishing an acceptable daily intake (ADI) and is consistent with international practice. In this specific case, the use of the 1-year dog study is supported by the similar effect and point of departure from the long-term rat study.
Groups of beagle dogs (4/sex/dose) were fed diets containing diquat at doses of 0, 0.5, 2.5, and 12.5 mg/kg bw per day in the form of diquat dibromide (equivalent 0, 0.46, 2.42 and 11.48 mg diquat/kg per day for males; 0, 0.46, 2.42, and 13.21 mg diquat/kg per day for females) for 52 weeks (Hopkins, 1990; US EPA, 1995a; US EPA, 2001; Health Canada, 2008; WHO, 2014, 2016). There were no treatment-related adverse effects on survival, clinical signs, hematology, clinical chemistry, urinalysis, or gross pathology (except the eye) at any dose level (US EPA, 2001; WHO, 2014). The major treatment-related finding from this study was eye damage and was observed starting at the 2.5 mg/kg dose level. Its incidence and severity increased with increasing dose. In the 2.5 mg/kg dose group, two out of four females exhibited unilateral cataracts (i.e., lens opacity), which first occurred during the 8th week for one female and the 40th week for the other female. In the highest dose group, three of four females and all males developed bilateral lens opacity that first occurred during the 16th week for males and 24th week for females. In addition to lens opacities, the other treatment-related alterations in the high-dose group included inflammatory changes in the gastrointestinal tract of both sexes, reproductive effects in males, and statistically significant increased kidney weights in all dogs. Furthermore, the males from the two top dose groups had decreased adrenal and epididymal weights, although these changes did not corroborate with any histopathological changes in the corresponding organs (except in the gastrointestinal tract) (US EPA, 1995a, 2001; WHO, 2014; WHO, 2016). An oral NOAEL of 0.5 mg/kg bw per day was identified in this study, based on unilateral cataracts in females and decreased adrenal and epididymal weights in males at the lowest LOAEL of 2.5 mg/kg bw per day (US EPA, 1995a; WHO, 2014).
The findings of Hopkins (1990) are supported by a 2-year combined chronic/carcinogenicity study in Sprague-Dawley rats (Colley et al., 1985). In that study male and female rats (50/sex/dose) were fed diets containing 0, 5, 15, 75 or 375 ppm diquat dibromide (equivalent to male-female: 0–0, 0.19–0.24, 0.58–0.72, 2.91–3.64, and 14.88–19.44 mg diquat/kg bw per day) for 104 weeks. An interim sacrifice (10/sex/dose) took place at 52 weeks (Colley et al., 1985; US EPA, 1995a, 2001; Health Canada, 2008; WHO, 2014, 2016). There were no treatment-related adverse effects on organ weights, urinalysis or blood biochemistry parameters (WHO, 2014). The major significant finding was the formation of cataracts. Gradual lenticular opacities occurred throughout the study, followed by total opacity (i.e., cataract) which first occurred by week 11 in a few animals from the 2 highest dose groups. Based on ophthalmologic and histopathological examinations of the eyes, the incidence of total lens opacity increased with increasing dose and time. At interim sacrifice, a few males in the 75 ppm group and up to 95% of both sexes in the 375 ppm group had cataracts. At study termination, these incidences increased to 15% in the 75 ppm group and 100% for both sexes in the 375 ppm group. Additionally, rats with severe cataracts exhibited extralenticular eye lesions (e.g., vitreous adhesions, retinal detachment, iritis, and intraocular hemorrhage) (US EPA, 2001). Although cataracts were identified in the three highest-dose groups at study termination, only one rat per sex in the 15 ppm dose group had total opacity. Based on these findings and the poor survival rate in the highest-dose groups, the data were subsequently re-evaluated and it was concluded that the incidence and severity of cataracts in the 15 ppm dose group were comparable to those of controls (Harling et al., 1997; WHO, 2014, 2016). Therefore, a systemic NOAEL of 0.58 mg diquat/kg bw per day is identified in this study, based on eye effects observed at the lowest LOAEL of 2.91 mg diquat/kg bw per day (US EPA, 1995a; WHO, 2014).
The long-term rat study by Colley et al. (1985) has a NOAEL of 0.58 mg/kg bw per day based on a LOAEL for cataracts in males and uses an assessment of the incidence and severity of this effect in comparison to the control. PMRA did not consider the distribution of effects in the lower dose group to be sufficiently different from that of the control. The US EPA and PMRA used the dog study by Hopkins (1990) as the point of departure for the ADI, which had a NOAEL of 0.5 mg/kg bw/day, and which is also based on cataracts at the LOAEL. The two studies indicate a consistent effect and point of departure across species, and Health Canada chose the slightly more conservative value.
3.0 Derivation of the health-based value
As noted above, the NOAEL of 0.5 mg/kg bw per day from the dog study by Hopkins (1990), which showed cataract formation, was selected as the basis for the current risk assessment. The NOAEL of 0.5 mg/kg bw per day is based on unilateral cataracts in females and decreased adrenal and epididymal weights in males. Also considered in the derivation of the ADI is the NOAEL of 0.58 mg/kg per day in females based on eye lesions from the study by Colley et al. (1985).
Using the NOAEL of 0.5 mg diquat/kg per day and the standard uncertainty factor of 100, as no sensitivity of the young was identified, an ADI for diquat (i.e., the bipyridyl divalent cation) was calculated as follows (Health Canada, 2008, 2019):

Text Description
The ADI for diquat is 0.005 mg/kg bw per day. This is calculated by dividing the NOAEL of 0.5 mg/kg bw per day by the uncertainty factor of 100.
where:
- 0.5 mg/kg bw per day is the NOAEL, based on cataract formation in dogs; and
- 100 is the uncertainty factor, selected to account for interspecies variation (×10) and intraspecies variation (×10).
Using the ADI of 0.005 mg/kg bw per day, a health-based value (HBV) for diquat in drinking water was derived as follows:
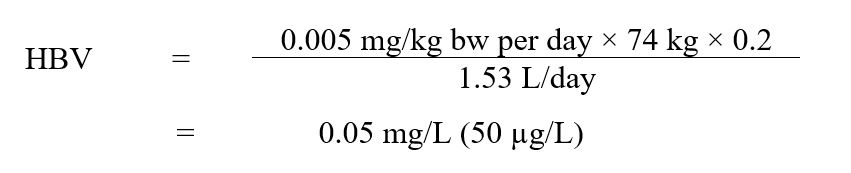
Text Description
The HBV for diquat in drinking water is 0.05 mg/L. This is calculated by multiplying the ADI for diquat (0.005 mg/kg bw per day) by the allocation factor for water (0.2), then by the average body weight for an adult (74 kg). This product is then divided by the daily volume of water consumed by an adult (1.53 L/day).
where:
- 0.005 mg/kg bw per day is the ADI derived above;
- 74 kg is the adult body weight (Health Canada, 2021);
- 0.20 is the default allocation factor for drinking water (Krishnan and Carrier, 2013);
- 1.53 L/day is the drinking water intake rate estimated for an adult (Health Canada, 2021).
4.0 Analytical and treatment considerations
4.1 Analytical methods to detect diquat
One standardized analytical method is available for the analysis of diquat in drinking water, and the MDL is summarized in Table 3. MDLs are dependent on the sample matrix, instrumentation, and selected operating conditions and will vary between individual laboratories. A number of accredited laboratories in Canada were contacted, and it was indicated that MDLs were in the same order of magnitude as that reported in Table 3. The reported MRLs ranged from 1 to 7 μg/L (ALS Environmental, 2019; Bureau Veritas Laboratories, 2019; SGS Environmental Services, 2019). The MDLs or MRLs from provincial and territorial data range from 0.1 to 70 μg/L (see Section 1.3).
Drinking water utilities should discuss sampling requirements with the accredited laboratory conducting the analysis to ensure that quality control procedures are met and that MRLs are low enough to ensure accurate monitoring at concentrations below the MAC. Sample processing considerations for the analysis of diquat in drinking water (e.g., sample preservation, storage) can be found in the reference listed in Table 3. It is important to note that quenching is critical if an oxidant is present in samples in order to prevent additional degradation of diquat prior to analysis.
| Method (Reference) |
Methodology | MDL (µg/L) |
Interferences/Comments (Operational Considerations) |
|---|---|---|---|
| US EPA Methods | |||
| EPA-NERL: 549.2 (US EPA, 1997) |
Liquid-solid extraction and high-performance liquid chromatography with ultraviolet detection | 0.72 | Matrix; Ca2+, Mg2+can cause low recovery Diquat adsorbs to surfaces, especially glass. This needs to be accounted for during sampling and analysis. |
| NERL – US EPA’s National Exposure Research Laboratory | |||
4.2 Treatment considerations
Granular activated carbon (GAC) is capable of diquat removal in larger systems (US EPA, 1995b, 1996, 2003, 2016). For small systems, the US EPA lists GAC, point-of-use GAC and powdered activated carbon as best options for meeting regulatory objectives (US EPA, 1996, 2016). The World Health Organization (WHO) (2017) states that GAC should remove diquat from water. RO, nanofiltration (NF) and oxidation are also possible technologies for diquat removal.
4.2.1 Municipal-scale treatment
The selection of an appropriate treatment process or strategy for a specific water supply will depend on many factors, including the raw water source and its characteristics, the operational conditions of the selected treatment method and the utility’s treatment goals. GAC is listed by the US EPA (1995b, 1996, 2003, 2016) as the best available technology for the removal of diquat. The US EPA defines best available technology as a treatment technology having demonstrated consistent removal of the target contaminant under field conditions (US EPA, 2003). Non-treatment management strategies may include blending to reduce diquat concentrations in treated water or switching to an alternative water source. Attention must be given to the water quality of a new source prior to making any changes to an existing supply (i.e., switching source, blending, and interconnecting). For example, if the new water source is more aggressive, it may cause leaching of lead or copper in the distribution system.
Characterization of the water quality must be carried out to ensure that changes in water quality resulting from control or treatment options are assessed and that potential impacts to the distribution system are determined. Any change in water quality should not result in other compliance issues. Pilot- or bench-scale testing of the selected treatment method or control option for diquat is important for assessing unintended consequences, such as water quality changes, and for optimizing performance.
Typical water treatment that includes conventional filtration (chemical coagulation, clarification, and rapid sand filtration) is not effective for diquat removal from drinking water (WHO, 2017). Diquat is highly adsorptive to soil, has a medium molecular weight and slowly degrades naturally in water through photolysis; these properties influence how diquat is removed from drinking water. The selection of an appropriate treatment process for a specific water supply will depend on many factors, including the raw water source and its characteristics, the operational conditions of the selected treatment method and the utility’s treatment goals. Appropriate pilot- or bench-scale testing is recommended to ensure the source water can be successfully treated.
When using oxidation or advanced oxidation processes for pesticide removal in drinking water, it is important to be aware of the potential for formation of by-products due to degradation of the target compound (Ikehata and Gamal El-Din, 2006; Beduk et al., 2012; Li et al., 2019). Removal of the target pesticide alone does not ensure that the treatment is efficient and that full mineralization (to carbon dioxide, inorganic ions and water) has been achieved. In addition, water utilities should consider the potential for the formation of disinfection by-products depending on the oxidant selected and the source water quality. Pilot-scale testing is an important step for water utilities considering oxidation and advanced oxidation treatment processes for pesticide removal in drinking water.
4.2.1.1 Activated carbon adsorption
Activated carbon adsorption is widely used to reduce the concentration of micropollutants, including pesticides, in drinking water (Haist-Gulde and Happel, 2012; van der Aa et al., 2012). Activated carbon can be applied in two ways: slurry applications using powdered activated carbon (PAC) or fixed-bed reactors with GAC (Chowdhury et al., 2013).
GAC has been recommended as the best available technology for diquat removal from drinking water (US EPA, 1995b, 2016; WHO, 2017). The US EPA (2016) states that small system compliance technologies for diquat removal are GAC, point-of-use GAC and PAC, although no references to full-, pilot- or bench-scale studies are given.
There are very few published studies of activated carbon for diquat adsorption and no studies on adsorption capacity or performance. As a result, prior to full-scale implementation, it is essential to conduct appropriate pilot- or bench-scale testing. Diquat removal from natural water using activated carbon can be negatively affected by competition from other contaminants or natural organic matter (NOM), biofilm development, temperature, influent concentration, carbon size and hydraulic loading rate (Speth and Miltner, 1998; Haist-Gulde and Happel, 2012).
Data generated through bench-scale testing to determine adsorption coefficients for pesticides are useful in predicting whether activated carbon adsorbs a particular pesticide (US EPA, 2011). In general, pesticides with an adsorption capacity constant (e.g., Freundlich coefficient) greater than 200 µg/g (L/µg)1/nare considered to be amenable to removal by carbon adsorption (Speth and Adams, 1993; Speth and Miltner, 1998; US EPA, 2011). The authors noted, however, that the capacity of activated carbon is affected by many factors, including the compound’s ionic character and the solution pH. The Freundlich coefficients in organic-free water for diquat ranged from 103 µg/g (L/µg)1/nat a pH of 3.0 to 2 910 µg/g (L/µg)1/nat a pH of 6.1 and increased to 122 000 µg/g (L/µg)1/nat a pH of 10.1 (Speth and Miltner, 1998). These results illustrate the large effect of pH on adsorption, with very little adsorption at the low pH of 3.0 to moderate adsorption at near-neutral pH and quite high adsorption at higher pH.
The use of PAC offers the advantage of providing virgin carbon when required (e.g., during the pesticide application season) (Miltner et al., 1989). The removal efficiency of PAC depends on the PAC type and dose, the contact time, the PAC characteristics (type, particle size), the adsorbability of the contaminant and the presence of NOM (Gustafson et al., 2003; Summers et al., 2010; Haist-Gulde and Happel, 2012; Chowdhury et al., 2013). The capacity of GAC to remove pesticides by adsorption depends on the filter velocity, empty bed contact time, the GAC characteristics (type, particle size, reactivation method), the adsorbability of the contaminant and the filter run time (Haist-Gulde and Happel, 2012). In addition, because GAC fixed-bed adsorbers are typically operated on a continuous basis, the GAC can become fouled (or preloaded) with NOM and may become completely or partially ineffective for pesticide removal (Knappe et al., 1999; Summers et al., 2010; Haist-Gulde and Happel, 2012; Chowdhury et al., 2013).
Several other studies investigated diquat removal from wastewater through batch experiments using GAC or various adsorbents not typically used in drinking water treatment (Dichiara et al., 2015; Hao et al., 2015; Li et al., 2017; Duman et al., 2019). The initial concentrations for these studies were much higher than typically found in drinking water, ranging from 5.43 to 80 mg/L. The removal efficiencies varied from 72.9% to 89.3%, the adsorption capacities from 36.45 to 197.53 mg/g and the adsorption rate from 0.612 to 29.1 mg/g∙min, depending on the adsorbent and the experimental conditions.
4.2.1.2 Membrane filtration
In general, NF and RO are effective pressure-driven membrane processes for the removal of pesticides from drinking water. Their effectiveness is dependent on the membrane characteristics, pesticide properties, feed water composition, operating conditions and membrane fouling (Van der Bruggen and Vandecasteele, 2003; Bellona et al., 2004; Plakas and Karabelas, 2012).
Since the main mechanism for pesticide removal using NF and RO membranes is size exclusion, the molecular weight cut-off (MWCO) of the membrane is an important characteristic. There are no studies evaluating membrane filtration for the removal of diquat, but based on its molecular weight (184.2 g/mol), membranes with an MWCO lower than this value may be effective. Because diquat is highly polar and hydrophilic—physical and chemical properties that decrease the effectiveness of membrane rejection (Plakas and Karabelas, 2012)—the removal of diquat will most likely be due to size exclusion alone.
Bellona et al. (2004) present a flow chart using the characteristics of the pesticide in water (e.g., molecular weight, log Kow, molecular diameter) and those of the membrane (e.g., MWCO, pore size) that could be used to determine the potential for removal of diquat by membrane filtration. It is important to perform appropriate testing prior to full-scale implementation with membrane and source water under the proposed operating conditions to ensure that adequate diquat removal is occurring.
4.2.1.3 Oxidation
Chemical oxidation using chlorine dioxide can be an effective treatment method for removing diquat from water depending on a variety of factors, including oxidant dose, contact time, disinfectant demand, temperature and pH.
Bench-scale testing with oxidants including chlorine dioxide, permanganate and chlorine was conducted to determine diquat degradation in distilled water (Gomaa and Faust, 1971). The initial concentrations of diquat used in this study are orders of magnitude higher than what would typically be found in source water (15–30 mg/L), and the oxidant doses are high (chlorine dioxide dose of 6.75 mg/L). It was shown that chlorine dioxide was the oxidant of choice for diquat removal, with good removal for pH greater than 8.0 and with complete reaction in less than 1 minute. The use of chlorine dioxide would require pre-adjustment of pH to slightly alkaline levels. Chlorine was found to be less effective than chlorine dioxide, but chlorine was more effective at higher pH. Potassium permanganate was also investigated and did not adequately remove diquat. Formation of by-products was not discussed in this study.
Relatively slow reaction rates for diquat have been reported for ozonation (Yao and Haag, 1991; Hu et al., 2000). Hu et al. (2000) conducted a bench-scale study evaluating oxidation rate constants of 24 pesticides using ozone. The tests were carried out using synthetic raw water at a pH of 7.5, ionic strength of 10-3M and 100 μM NaHCO3. Using an ozone dose of 1.3 mg/L, a rate constant of 67.9 M-1s-1was obtained, which was the second lowest of all pesticides examined. As a comparison, 2,4-dichlorophenoxyacetic acid (2,4-D) had a reaction rate of 298 M-1s-1and Warfarin had a reaction rate greater than 21,000 M-1s-1.
Yao and Haag (1991) investigated the oxidation reaction rate constants for 45 organic compounds using ozone (Yao and Haag, 1991). A reaction rate for diquat of 0.6 M-1s-1was obtained at a pH of 3.1, and a half-life of 15 hours was determined for pH of 7.
4.2.2 Residential-scale treatment
In cases where diquat removal is desired at the household level—for example, when a household obtains its drinking water from a private well—a residential drinking water treatment unit may be an option for decreasing diquat concentrations in drinking water. If guidance is required, consumers should contact their responsible drinking water authority. Before a treatment unit is installed, the water should be tested to determine the general water chemistry and diquat concentration in the source water. There is a lack of performance testing of treatment technologies for diquat removal; however, adsorption (activated carbon) and RO are treatment technologies that may remove diquat at the residential scale. To verify that a treatment unit is effective, water entering and leaving the treatment unit should be sampled periodically and submitted to an accredited laboratory for analysis. Units can lose removal capacity through use and time and need to be maintained and/or replaced. Consumers should verify the expected longevity of the components in the treatment unit according to the manufacturer’s recommendations and service it when required. Systems classified as residential scale may have a rated capacity to treat volumes greater than that needed for a single residence, and thus, may also be used in small systems.
Health Canada does not recommend specific brands of drinking water treatment units, but it strongly recommends that consumers use units that have been certified by an accredited certification body as meeting the appropriate NSF International/American National Standards Institute (NSF/ANSI) standards for drinking water treatment units. The purpose of the standards is to establish minimum requirements for the materials, design and construction of drinking water treatment units that can be tested by a third party. This ensures that materials in the unit do not leach contaminants into the drinking water (i.e., material safety). In addition, the standards include performance requirements that specify the level of removal that must be achieved for specific contaminants (e.g., reduction claim) that may be present in water supplies. Certification organizations (i.e., third party) provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada (SCC). Accredited organizations in Canada include:
- CSA Group
- NSF International
- Water Quality Association
- UL LLC
- Bureau de normalisation du Québec (available in French only)
- International Association of Plumbing and Mechanical Officials
- Truesdail Laboratories, Inc.
An up-to-date list of accredited certification organizations can be obtained from the SCC.
The drinking water treatment technologies that are expected to be effective for diquat removal at the residential-scale include adsorption (activated carbon) and RO. Currently, diquat is not included in the performance requirements of NSF/ANSI standards. Consumers can use a treatment unit that is certified to the standards for adsorption or for RO to ensure that the material safety has been tested.
Water that has been treated using reverse osmosis may be corrosive to internal plumbing components. Therefore, these units should be installed only at the point of use. Also, as large quantities of influent water are needed to obtain the required volume of treated water, these units are generally not practical for point-of-entry installation.
5.0 Management strategies
All water utilities should implement a risk management approach, such as the source-to-tap or water safety plan approach, to ensure water safety (CCME, 2004; WHO, 2011, 2012). These approaches require a system assessment to characterize the source water, to describe the treatment barriers that prevent or reduce contamination, to identify the conditions that can result in contamination, and to implement control measures. Operational monitoring is then established, and operational/management protocols are instituted (e.g., standard operating procedures, corrective actions and incident responses). Compliance monitoring is determined and other protocols to validate the water safety plan are implemented (e.g., record keeping, consumer satisfaction). Operator training is also required to ensure the effectiveness of the water safety plan at all times (Smeets et al., 2009).
5.1 Monitoring
Diquat can be present in groundwater and surface water in areas where it is being used depending on the type and extent of its application, environmental factors (e.g., amount of precipitation, soil type, hydrogeological setting) and environmental fate (e.g., mobility, leaching potential, degradation) in the surrounding area. Water utilities should consider the potential for diquat to enter source water (e.g., raw water supply to the drinking water system) based on site-specific considerations.
When it is determined that diquat may be present and monitoring is necessary, surface and groundwater sources should be characterized to determine the concentration of diquat. This should include monitoring of surface water sources during periods of peak use and rainfall events and/or monitoring of groundwater annually. Where baseline data indicate that diquat is not present in source water, monitoring may be reduced.
Where treatment is required to remove diquat, operational monitoring should be implemented to confirm whether the treatment process is functioning as required. The frequency of operational monitoring will depend on the water quality, fluctuations of the raw water concentrations and the treatment process. Responsible authorities should be aware of the impact of NOM on activated carbon systems, as it may impact water quality objectives for diquat removal.
Where treatment is in place for diquat removal, compliance monitoring (i.e., paired samples of source and treated water to confirm the efficacy of treatment) should be conducted at a minimum on an annual basis. When routine operational monitoring indicates the potential for contaminant breakthrough, such as with GAC, monitoring should be conducted quarterly to plan for the regeneration or replacement of the media. When a degradation process like oxidation is utilized monitoring of by-product formation should also be considered.
6.0 International considerations
This section presents drinking water guidelines, standards and/or guidance from other national and international organizations. Variations in these values can be attributed to the age of the assessments or to differing policies and approaches, including the choice of key study and the use of different consumption rates, body weights and source allocation factors.
The US EPA has set a maximum contaminant level (MCL) of 0.02 mg/L while the Australian National Health and Medical Research Council has established a guideline value of 0.007 mg/L for diquat in drinking water (NHMRC and NRMMC, 2011). The WHO has calculated a non-regulatory health-based value of 0.03 mg/L (30 µg/L) (WHO, 2016). These three values are based on cataract formation observed in a 2-year rat study conducted in 1985, but differ in their interpretation (i.e., NOAEL) and in the selection of body weights and source allocation factors (Table 4). Diquat is not approved for use in the European Union (EFSA, 2018).
| Agency (Year) |
Value (mg/L) |
Key Endpoint (Reference) |
NOAEL (mg/kg bw/d) |
UF | ADI (mg/kg bw/d) |
BW (kg) |
DW Intake (L/d) |
AF (%) |
Comments |
|---|---|---|---|---|---|---|---|---|---|
| HC - MAC (2021) |
0.05 | Cataracts in dogs (Hopkins, 1990) |
0.5 | 100 | 0.005 | 74 | 1.53 | 20 | None |
| US EPA (1992) |
0.02 | Cataracts in rats (Colley et al., 1985) |
0.22 | 100 | 0.0022 | 70 | 2 | 20 | None |
| WHO (2016) |
0.03 | Cataracts in rats (Colley et al., 1985) |
0.58 | 100 | 0.0058 | 60 | 2 | 20 | ADI established by JMPR (FAO/WHO, 2014). JMPR states the 1-year dog study by Hopkins (1990) supports their ADI. |
| Australia (2011) |
0.007 | Cataracts in rats | 0.2 | 100 | 0.002 | 70 | 2 | 10 | No reference for the cataract study is provided in NHMRC and NRMMC, 2011, although description is consistent with Colley et al., 1985. |
| EU (2018) |
N/A | Diquat is not approved for use in the European Union (EU). | |||||||
|
|||||||||
7.0 Rationale
Diquat is registered in Canada for use as a desiccant on crops and to control water-weeds and algae in still and slow-moving water. Despite its common use in Canada, data provided by provinces and territories that monitor for diquat in source and drinking water indicate that diquat levels are not significant. The eyes (cataract formation) are considered the target organ for diquat toxicity. Although no human studies have investigated the effects of diquat on eyes, animal studies conducted in several species (mice, rats, dogs) have consistently shown eye damage following repeated exposure to diquat.
A MAC of 0.05 mg/L (50 µg/L) is established for diquat in drinking water based on the following considerations:
- An HBV of 0.05 mg/L (50 µg/L) has been derived based on cataract formation in dogs.
- Diquat can be accurately measured at concentrations well below the MAC.
- Diquat can likely be removed at the municipal scale or managed through blending or an alternative water source.
The MAC is protective of potential health effects from diquat exposure, can be reliably measured by available analytical methods and is achievable by municipal and residential scale treatment technologies.
As part of its ongoing guideline review process, Health Canada will continue to monitor new research in this area, including the outcomes of PMRA’s evaluations, and will recommend any change to this guideline technical document that it deems necessary.
8.0 References
ALS Environmental (2019). Personal communication with A. Ganouri-Lumsden, Waterloo, ON.
Beduk, F., Aydin, M.E. and Ozcan, S. (2012). Degradation of malathion and parathion by ozonation, photolytic ozonation, and heterogeneous catalytic ozonation processes. Clean - Soil, Air, Water, 40(2): 179-187.
Bellona, C., Drewes, J.E., Xu, P. and Amy, G. (2004). Factors affecting the rejection of organic solutes during NF/RO treatment – a literature review. Wat. Res., 38: 2795-2809.
British Columbia Ministry of Health (2019). Personal communication with D. Fishwick, Drinking Water Manager.
Bureau Veritas Laboratories (2019). Personal communication with C. MacDermid, Mississauga, ON.
CCME (2004). From source to tap: Guidance on the multi-barrier approach to safe drinking water. Canadian Council of Ministers of the Environment, Winnipeg, Manitoba. Available at: www.ccme.ca/assets/pdf/mba_guidance_doc_e.pdf
Chowdhury, Z.K., Summers, R.S., Westerhoff, G.P., Leto, B.J., Nowack, K.O. and Corwin, C.J. (2013). Activated carbon: Solutions for improving water quality. Passantino, L. B. (ed.). American Water Works Association. Denver, Colorado.
Circu, M.L., Maloney, R.E. and Aw, T.Y. (2017). Diquat-induced cellular pyridine nucleotide redox changes and alteration of metabolic enzyme activities in colonic carcinoma cells. Chem. Biol. Interact., 264: 43-51.
Colley, J., Warren, S. and Heywood, R. (1985). Diquat dibromide: Evaluation of potential carcinogenicity and chronic toxicity by prolonged dietary administration to rats. Final Report: HRC Report No. ICI 406-83763. Unpublished study prepared by Huntington Research Centre; MRID 00145855 (as cited in US EPA, 1995a, 2001).
Dichiara, A.B., Harlander, S.F., Rogers, R.E. (2015). Fixed bed adsorption of diquat dibromide from aqueous solution using carbon nanotubes. RSC Adv., 5(76): 61508-61512.
Duman, O., Özcan, C., Gürkan Polat, T. and Tunç, S. (2019). Carbon nanotube-based magnetic and non-magnetic adsorbents for the high-efficiency removal of diquat dibromide herbicide from water: OMWCNT, OMWCNT-Fe3O4and OMWCNT-κ-carrageenan-Fe3O4nanocomposites. Environ. Pollut., 244: 723-732.
EFSA (2015). Conclusion on the peer review of the pesticide risk assessment of the active substance diquat. European Food Safety Authority. EFSA Journal, 13(11): 4308.
EFSA (2018). Final renewal report for the active substance diquat finalised in the Standing Committee on Plants, Animals, Food and Feed at its meeting on 25 May 2018 in view of the non-renewal of the approval of diquat as active substance in accordance with Regulation (EC) No 1107/2009. SANTE/10396/2016 Rev.3. European Food Safety Authority.
Emmett, K. (2002). Risk assessments for diquat dibromide - Appendix A (publication number 00-10-046). In: Final supplemental environmental impact statement for diquat dibromide. The Water Quality Program. Washington State Department of Ecology. Report Number 02-10-052.
FAO and WHO (2014). Pesticide residues in food - 2013. Report of the joint meeting of the FAO panel of experts on pesticide residues in food and the environment and the WHO core assessment group on pesticide residues. Geneva, Switzerland, from 17 to 26 September 2013. FAO plant production and protection paper 219. World Health Organization. Food and Agriculture Organization of the United Nations. Rome, Italy.
FAO (2019). Pesticides residues in food - 2018: Residues and analytical aspects. Report of the joint meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. FAO Plant Production and Protection Paper 234. Food and Agriculture Organization of the United Nations. Rome, Italy.
Fletcher, K. (1972). Diquat dibromide: Three-generation reproduction study in rats. ICI study no. HO/IH/R/334A. DPR vol. 226-005 # 916116 (as cited in OEHHA, 2016).
Fuke, C., Ameno, K., Ameno, S., Kinoshita, H. and Ijiri, I. (1996). Detection of two metabolites of diquat in urine and serum of poisoned patients after ingestion of a combined herbicide of paraquat and diquat. Arch. Toxicol., 70(8): 504-507.
Fussell, K.C., Udasin, R.G., Gray, J.P., Mishin, V., Smith, P.J., Heck, D.E. and Laskin, J.D. (2011). Redox cycling and increased oxygen utilization contribute to diquat-induced oxidative stress and cytotoxicity in Chinese hamster ovary cells overexpressing NADPH-cytochrome P450 reductase. Free Radic. Biol. Med., 50(7): 874-882.
Gallagher, E.P., Buetler, T.M., Stapleton, P.L., Wang, C., Stahl, D.L. and Eaton, D.L. (1995). The effects of diquat and ciprofibrate on mRNA expression and catalytic activities of hepatic xenobiotic metabolizing and antioxidant enzymes in rat liver. Toxicol. Appl. Pharmacol., 134(1): 81-91.
Gomaa, H.M. and Faust, S.D. (1971). Kinetics of chemical oxidation of dipyridylium quaternary salts. J. Agric. Food Chem., 19(2): 302-307.
Government of Ontario (2019). Drinking water surveillance program, available at: https://www.ontario.ca/data/drinking-water-surveillance-program.
Gupta, P.K. (2014). Herbicides and fungicides. Chapter 24 in: Biomarkers in toxicology. Gupta, R.C. (ed.). Academic Press, Boston, pp. 409-431.
Gupta, R.C. and Crissman, J.W. (2013). Agricultural chemicals. Chapter 42 in: Haschek and Rousseaux's Handbook of Toxicologic Pathology. Haschek, W.M., Rousseaux, C.G. and Wallig, M.A. (eds.). Academic Press, Boston, pp. 1349-1372.
Gustafson, D.K., Carr, K.H., Carson, D.B., Fuhrman, J.D., Hackett, A.G., Hoogheem, T.J., Snoeyink, V.L., Curry, M., Heijman, B., Chen, S., Herti, P. and van Wesenbeeck, I. (2003). Activated carbon adsorption of chloroacetanilide herbicides and their degradation products from surface water supplies. J. Water Supply Res. Technol. AQUA, 52(6): 443-454.
Haist-Gulde, B. and Happel, O. (2012). Removal of pesticides and their ionic degradates by adsorptive processes. Report no. 4022. Water Research Foundation, Denver, Colorado.
Hantson, P., Wallemacq, P. and Mahieu, P. (2000). A case of fatal diquat poisoning: Toxicokinetic data and autopsy findings. J. Toxicol. Clin. Toxicol., 38(2): 149-152.
Hao, Y., Wang, Z., Gou, J. and Wang, Z. (2015). Kinetics and thermodynamics of diquat removal from water using magnetic graphene oxide nanocomposite. Can. J. Chem. Eng., 93(10): 1713-1720.
Harling, R.J., Buist, D. and Gopinath, C. (1997). Diquat dibromide - Evaluation of potential carcinogenicity and chronic toxicity by prolonged dietary administration to rats: Addendum report 2: 2 year data. Report no. ICI 406/83763.Unpublished study prepared by Huntingdon Life Sciences, Huntingdon, Cambridgeshire, England (as cited in WHO, 2016).
Health Canada (2008). Proposed re-evaluation decision. PRVD2008-12. Diquat dibromide. Pest Management Regulatory Agency (PMRA); Health Canada, Ottawa, ON.
Health Canada (2010). Re-evaluation decision. PRVD2010-03. Diquat dibromide Pest Management Regulatory Agency (PMRA); Health Canada, Ottawa, ON.
Health Canada (2019). Personal communication from the Health Evaluation Directorate, Pest Management Regulatory Agency (PMRA).
Health Canada (2020). Pest control products sales report for 2018. PMRA, Health Canada, Ottawa, Ontario.
Health Canada (2021). Canadian exposure factors used in human health risk assessments. Fact Sheet. Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/chemical-substances/fact-sheets/canadian-exposure-factors-human-health-risk-assessments.html
Hodge, M.C.E. (1989). Diquat: Teratogenicity study in the rabbit. ICI Study no. CTL/P/2379; MRID 41198901 (as cited in US EPA, 1995a and OEHHA, 2016).
Hodge, M.C.E. (1990). Diquat: Multigeneration study in the rat. ICI Study no. CTL/P/2462. DPR vol. 226-090; MRID 41531301 (as cited in US EPA, 1995a OEHHA, 2016).
Hodge, M.C.E. (1992). Diquat: Two year feeding study in mice. ICI Study no. CTL/P/3409; MRID 42219801 (as cited in US EPA, 1995a OEHHA, 2016).
Hopkins, M.N. (1990). Diquat: 1 year feeding study in dogs. ICI Study no. CTL/P/2596. DPR vol. 226-094. Unpublished study prepared by ICI Central Toxicology Laboratory; MRID 41730301 (as cited in US EPA, 1995a and WHO, 2016).
Horner, J.M. (1992a). Diquat: Subchronic neurotoxicity study in rats. ICI study no. CTL/P/3751.Unpublished study prepared by ICI Central Toxicology Laboratory; MRID 42616101 (as cited in US EPA, 1995a).
Horner, J.M. (1992b). Diquat: Acute neurotoxicity study in rats. ICI Study no. CTL/P/3789. Unpublished study prepared by ICI Central Toxicology Laboratory; MRID 42666801 (as cited in US EPA, 1995a and WHO, 2016).
HSDB (2010). Diquat dibromide. US National Library of Medicine. National Institutes of Health, Health and Human Services, https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~9Eswj1:1:cpp.
Ikehata, K. and El-Din, M.G. (2006). Aqueous pesticide degradation by hydrogen peroxide/ultraviolet irradiation and Fenton-type advanced oxidation processes: A review. J. Environ. Eng. Sci., 5(2): 81-135.
Indigenous Services Canada (2019). Personal communication with X. Redhead.
Jones, G.M. and Vale, J.A. (2000). Mechanisms of toxicity, clinical features, and management of diquat poisoning: A review. Clin. Toxicol., 38(2): 123-128.
Jovic-Stosic, J., Babic, G. and Todorovic, V. (2009). Fatal diquat intoxication. Vojnosanit. Pregl., 66(6): 477-481.
Knappe, D.R.U., Snoeyink, V.L., Roche, P., Prados, M.J. and Bourbigot, M-M. (1999). Atrazine removal by preloaded GAC. J. Am. Water Works Assoc., 91(10): 97-109.
Krishnan, K. and Carrier, R. (2013). The use of exposure source allocation factor in the risk assessment of drinking-water contaminants. J. Toxicol. Environ. Health B Crit. Rev., 16(1): 39-51.
Li, W., Zhao, Y., Yan, X., Duan, J., Saint, C.P. and Beecham, S. (2019). Transformation pathway and toxicity assessment of malathion in aqueous solution during UV photolysis and photocatalysis. Chemosphere, 234: 204-214.
Li, Y., Zhao, R., Chao, S., Sun, B., Zhang, N., Qiu, J., Wang, C. and Li, X. (2017). A flexible magnesium silicate coated electrospun fiber adsorbent for high-efficiency removal of a toxic cationic herbicide. New J. Chem., 41(24): 15601-15611.
Magalhães, N., Carvalho, F. and Dinis-Oliveira, R.J. (2018). Human and experimental toxicology of diquat poisoning: Toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum. Exp. Toxicol., 37(11): 1131-1160.
Manitoba Sustainable Development (2019). Personal communication with D. Coulibaly, Water Quality Management Section.
Miltner, R.J., Baker, D.B., Speth, T.F., and Fronk, C.A. (1989). Treatment of seasonal pesticides in surface waters. J. Am. Water Works Assoc., 81: 43-52. Available at: https://doi.org/10.1002/j.1551-8833.1989.tb03321.x.
Ministère de l’Environnement et de la Lutte contre les changements climatiques du Québec (2019). Personal communication with P. Cantin.
Minnema, D.J., Travis, K.Z., Breckenridge, C.B., Sturgess, N.C., Butt, M., Wolf, J.C., Zadory, D., Herberth, M.T., Watson, S.L., Cook, A.R. and Botham, P.A. (2016). Dietary administration of diquat for 13 weeks does not result in a loss of dopaminergic neurons in the substantia nigra of C57BL/6J mice. Regul. Toxicol. Pharmacol., 75: 81-88.
New Brunswick Department of Environment and Local Government (2019). Personal communication with K. Gould.
Newfoundland and Labrador Municipal Affairs and Environment (2019). Personal communication with H. Khan.
NHMRC and NRMMC (2011). Australian drinking water guidelines Paper 6 national water quality management strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra. Available at: https://Nhmrc.Gov.Au/about-Us/Publications/Australian-Drinking-Water-Guidelines.
Nova Scotia Environment (2019). Personal communication with A. Polegato.
OEHHA (2016). Public health goals for carbofuran, diquat, endrin, picloram and thiobencarb in drinking water. Office of Environmental Health Hazard Assessment, California Environmental Protection Agency, US.
Palmer, A.K.; Edwards, J.A. and Woodhouse, R.N. (1978). Effect of diquat on pregnancy of the mouse. ICI Study no. 167/77642. Unpublished study prepared by Huntingdon Research Centre and submitted by Chevron Chemical Co.; MRID 00061637 (as cited in US EPA, 1995a).
PE Department of Communities, Land and Environment (2019). Personal communication with G. Somers.
Plakas, K.V. and Karabelas, A.J. (2012). Removal of pesticides from water by NF and RO membranes – A review. Desalination 287: 255-265.
Sandy, M.S., Moldeus, P., Ross, D. and Smith, M.T. (1986). Role of redox cycling and lipid peroxidation in bipyridyl herbicide cytotoxicity: Studies with a compromised isolated hepatocyte model system. Biochem. Pharmacol., 35(18): 3095-3101.
Saskatchewan Water Security Agency (2019). Personal communication with S. Ferris.
Schmidt, D.M., Neale, J. and Olson, K.R. (1999). Clinical course of a fatal ingestion of diquat. J. Toxicol. Clin. Toxicol., 37(7): 881-884.
SGS Environmental Services (2019). Personal communication with D. Griffin. Lakefield, ON.
Smeets, P.W.M.H., Medema, G.J. and van Dijk, J.C. (2009). The Dutch secret: how to provide safe drinking water without chlorine in the Netherlands. Drink. Water Eng. Sci., 2: 1-14.
Speth, T.F. and Adams J.Q. (1993). GAC and air stripping design support for the Safe Drinking Water Act. Chapter 2 in: Strategies and technologies for meeting SDWA requirements. Clark, R. and Summers, S. (eds.). Lewis Publishers, Ann Arbor, MI, pp. 47-89.
Speth, T.F. and Miltner, R.J. (1998). Technical note: adsorption capacity of GAC for synthetic organics. AWWA Journal, 90(4): 171.
Summers, R.S., Knappe, D.R.U. and Snoeyink, V.L. (2010). Adsorption of organic compounds by activated carbon. Chapter 14 in: Water quality and treatment: A handbook on drinking water. 6th edition. Edzwald, J.K. (ed.). McGraw-Hill, New York, NY, pp. 14.1-14.91.
Tanen, D.A., Curry, S.C. and Laney, R.F. (1999). Renal failure and corrosive airway and gastrointestinal injury after ingestion of diluted diquat solution. Ann. Emerg. Med., 34(4 Pt 1): 542-545.
US EPA (1995a). Reregistration Eligibility Decision (RED). Diquat dibromide. List A. Case 0288. Office of Prevention, Pesticides and Toxic Substances. Special review and reregistration division. United States Environmental Protection Agency, EPA 738-R-95-016, Washington, DC.
US EPA (1995b). National Primary Drinking Water Regulations – Diquat Factsheet. United States Environmental Protection Agency, EPA 811-F-95-003 m-T.
US EPA (1997). Method 549.2 - Determination of diquat and paraquat in drinking water by liquid-solid extraction and high performance liquid chromatography with ultraviolet detection. Rev. 1.0. Office of Research and Development, United States Environmental Protection Agency, Cincinnati, OH.
US EPA (2001). Diquat dibromide: Third HIARC report: Revisit/Re-evaluation of database by the HED hazard identification assessment review committee. United States Environmental Protection Agency, HED Doc No. 0050369, Washington, DC.
US EPA (2002). Diquat dibromide TRED facts. Office of Prevention, Pesticides and Toxic Substances. United States Environmental Protection Agency, Washington, DC.
US EPA (2003). 40 CFR Part 141, Section 141.2 National Primary Drinking Water Regulations. United States Environmental Protection Agency, Washington, DC.
US EPA (2011). Finalization of guidance on incorporation of water treatment effects on pesticide removal and transformation in drinking water exposure assessments. Office of Pesticide Programs, United States Environmental Protection Agency, Washington, DC.
US EPA (2016). Occurrence analysis for potential source waters for the third Six-Year Review of National Primary Drinking Water Regulations. United States Environmental Protection Agency, Washington, D.C. EPA 810-R-16-008.
Valiante, F., Farinati, F., Dal Santo, P., Germana, B., Di Mario, F. and Naccarato, R. (1992). Upper gastrointestinal injury caused by diquat. Gastrointest. Endosc., 38(2): 204.
van der Aa, L.T.J., Kolpa, R.J., Rietveld, L.C. and van Dijk, J.C. (2012). Improved removal of pesticides in biological granular activated carbon filters by pre-oxidation of natural organic matter. J. Water Supply Res. Technol. AQUA, 61(3): 153-163.
Van der Bruggen, B. and Vandecasteele, C. (2003). Removal of pollutants from surface water and groundwater by nanofiltration: overview of possible applications in drinking water industry. Environ. Pollut., 122: 435-445.
WHO (2004). Diquat in Drinking-water: Background document for development of WHO guidelines for drinking-water Quality. World Health Organization, Geneva, Switzerland. Available at: https://www.who.int/water_sanitation_health/dwq/chemicals/diquat.pdf.
WHO (2011). Guidelines for drinking-water quality. 4th edition. World Health Organization, Geneva, Switzerland. Available at: http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/
WHO (2012). Water safety planning for small community water supplies. World Health Organization, Geneva, Switzerland. Available at: http://www.who.int/water_sanitation_health/publications/small-commwater_supplies/en/
WHO (2014). Pesticide residues in food - 2013: Toxicological evaluations. Joint meeting of the FOA panel of experts on pesticide residues in food and the environment and the WHO core assessment group on pesticide residues. Sponsored jointly by the Food and Agriculture Organization of the United Nations and World Health Organization. World Health Organization, Geneva, Switzerland.
WHO (2016). Diquat in drinking-water. Background document for development of WHO guidelines for drinking-water quality. World Health Organization, WHO/FWC/WSH/16.50, Geneva, Switzerland.
WHO (2017). Guidelines for Drinking-Water Quality. 4th edition. Incorporating the First Addendum. World Health Organization, Geneva, Switzerland. https://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf;jsessionid=D3717A19B57F35A4E2AF8E2D7CC11C83?sequence=1
Wickramaratne, G. (1989). Diquat: Teratogenicity study in the rat. ICI Study no. CTL/P/2331; Study No. RR0399. Unpublished study prepared by ICI Central Toxicology Laboratory; MRID 41198902 (as cited in US EPA, 1995a).
Yao, C.C. and W.R. Haag (1991). Rate constant for direct reaction of ozone with several drinking water contaminants. Wat. Res., 25(7): 761-773.
Yukon Environmental Health Services (2019). Personal communication with P. Brooks.
Appendix A: List of abbreviations
- ADI
- Acceptable daily intake
- ANSI
- American National Standards Institute
- EU
- European Union
- FAO
- Food and Agriculture Organization of the United Nations
- GAC
- Granulated activated carbon
- HBV
- Health-based value
- LD50
- Median lethal dose
- LOQ
- Limit of quantification
- MAC
- Maximum acceptable concentration
- MCL
- Maximum contaminant level
- MDL
- Method detection limit
- MRL
- Method reporting limit
- MWCO
- Molecular weight cut-off
- NF
- Nanofiltration
- NHMRC
- National Health and Medical Research Council (Australia)
- NOAEL
- No-observed-adverse-effect level
- NOM
- Natural organic matter
- NRMMC
- Natural Resources Management Ministerial Council (Australia)
- NSF
- NSF International
- OEHHA
- Office of Environmental Health Hazard Assessment
- PAC
- Powdered activated carbon
- PMRA
- Pest Management Regulatory Agency
- RO
- Reverse osmosis
- SCC
- Standards Council of Canada
- TOPPS
- 1,2,3,4-tetrahydro-1-oxopyrido[1,2-a]-5-pyrazinium salt
- US EPA
- United States Environmental Protection Agency
- WHO
- World Health Organization
