Drinking water screening value for diuron
A drinking water screening value of 0.03 mg/L (30 µg/L) is established for diuron.
Screening values
Health Canada's screening values identify limits for contaminants in water that could be used as a source of drinking water. A lifetime of exposure to these contaminants up to the screening value, both by drinking the water or by using it for showering or bathing, is not expected to increase health risks for any Canadian, including children.
Screening values are established for contaminants that are not commonly found in Canadian drinking water (either source or treated) and therefore Guidelines for Canadian Drinking Water Quality are not established. Health Canada establishes screening values for contaminants at the request of federal departments, provinces and territories (jurisdictions). These requests are usually made when there is a concern for human health because the presence of a contaminant is suspected or detected in local source water and that contaminant does not have an established limit in drinking water. Since 2020, the technical summaries for screening values are typically published online when Health Canada expects that screening values may be needed by more than one stakeholder or jurisdiction.
Screening values do not replace or supersede existing regulations. However, screening values may help jurisdictions and the public understand the potential health effects of a contaminant.
Screening values are based on a review of scientific research and international regulatory information available at the time of their development. . For pesticides, screening values align with the evaluations done by the Pest Management Regulatory Agency (PMRA), the lead authority for the safety of pesticide use in Canada, to ensure consistency.
Health Canada is committed to keeping pace with new science, including the potential health risks from contaminants that are not typically found in drinking water and do not have Guidelines for Canadian Drinking Water Quality. To this end, Health Canada includes contaminants with screening values in its cyclical prioritization of contaminants for full guideline development.
Table of Contents
- Exposure considerations
- Health considerations
- Derivation of the screening value
- International considerations
- References
Exposure considerations
Identity and sources
Diuron (N’(3,4-dichlorophenyl)-N,N-dimethylurea; C9H10Cl2N2O) is a herbicide registered for use in Canada to control annual and perennial broad leaved and grassy weeds in food crops (grapes and asparagus) and in non-crop areas (i.e., railroad, pipeline rights-of-way, petroleum tank farms, lumberyards, storage areas, industrial plant sites and drainage/irrigation ditches). Its previous use for controlling algae in ponds and dugouts has been withdrawn (Health Canada, 2006). Between 5000 and 10 000 kg of diuron active ingredient were sold in Canada in 2018 (Health Canada, 2018).
As indicated in Table 1, diuron is moderately soluble in water with negligible volatility from moist surfaces or water.
|
Property |
Value |
Interpretation |
|---|---|---|
|
CAS# |
330-54-1 |
N/A |
|
Molecular weight (g/mol) |
233.1 |
N/A |
|
Water solubility (mg/L) at 25°C |
42 |
Moderate |
|
Vapour pressure at 25°C (mPa) |
1.1 x 10-3 |
Negligible |
|
Henry’s Law constant (Pa∙m3 mol-1) |
7.0 x 10-6 |
Negligible |
|
Octanol: water partition coefficient at 25°C (Log Kow) |
2.85 |
Low |
|
||
In water, diuron is not expected to significantly adsorb to suspended solids and sediment given its low experimental log Koc value of 2.4. Volatilization from water surfaces is expected to be negligible, therefore, if released to water, diuron is expected to partition largely to water (>99%) with minimal adsorption to sediment (<1%) (Environment Canada and Health Canada, 2011). The main route of dissipation in water is microbial degradation with photolysis and hydrolysis representing minor routes of dissipation (US EPA, 2003b; Environment Canada and Health Canada, 2011). Experimental data suggest that the half-life of diuron in water is likely greater than 182 days and thus it is considered as persistent (Environment Canada and Health Canada, 2011). Diuron is mobile in soil and has the potential to reach groundwater and surface waters (US EPA, 2003b).
Exposure
Seven Canadian provinces provided drinking water monitoring data for diuron in support of the withdrawal of the Guideline for Canadian Drinking Water Quality in 2021. Monitoring data from Alberta, Manitoba, New Brunswick, Nova Scotia, Ontario, Québec and Saskatchewan from 2007 to 2015 indicate that diuron is rarely detected in Canadian drinking water supplies. Water samples taken from raw, treated and distribution systems showed that only 8 out of 5691 samples (0.1%) had detections of diuron. The maximum detected level was 1.0 µg/L (Health Canada, 2021a).
Canadian environmental monitoring data is limited for diuron. In Québec, Giroux (1998) measured diuron in surface waters at levels ranging from 0.08 to 1.8 µg/L over the period of 1996-1997. A later study detected diuron at levels ranging from 0.24 to 0.9 µg/L in Québec surface waters between the period of 1992 to 2004 (Giroux et al., 2006). An investigation of the occurrence of pesticides in groundwater of the Chateauguay River watershed over the period of August to October 2005 revealed one detection (0.47 µg/L) out of 57 rural domestic well samples reported (Giroux et al., 2010). In the Chibouet, des Hurons, Saint-Régis and Saint-Zéphirin rivers, over the period of 1992 to 2010, diuron was detected at an average frequency of zero to 2.5% (Giroux and Pelletier, 2012). In Alberta, Anderson (2005) detected diuron in 24/3052 surface water samples largely within the Lethbridge-Calgary-Edmonton corridor over the period of 1995 to 2002; a median measurable level of 0.3 µg/L and a maximum detection of 2.8 µg/L were reported.
The maximum residue limits (MRL) established for diuron in foods range from 1 ppm to 7 ppm (Health Canada, 2021b).
The National Chemical Residue Monitoring Program and the Food Safety Oversight Program are conducted by the Canadian Food Inspection Agency to verify compliance for chemical residues and contaminants in foods (domestic and imported) with Canadian standards and guidelines. From April 1, 2015 to March 31, 2016, diuron was only detected in 1/242 samples of imported oranges at 0.002 ppm and in 1/53 samples of imported pineapples at 0.013 ppm (CFIA, 2019).
Health considerations
Kinetics
In rats, diuron is readily absorbed following single and repeated oral dosing. In the first 24 hours, the majority of diuron is excreted as metabolites with 94% to 100% of the dose excreted within 96 hours. Excretion is mainly through urine (80% to 91%), with the remainder occurring in the feces (8% to 15%). Metabolites in urine and feces do not change with dosing regime or sex (APVMA, 2011).
Studies in rats and dogs exposed to diuron at 25 to 2500 ppm through the diet for 9 months to 2 years showed that the predominant metabolite generated is N-(3,4-dichlorophenyl)-urea (DCPU) with N-(3,4-dichlorophenyl)-methylurea (DCPMU), 3,4-DCA, and 3,4-dichlorophenol being less predominant (Hodge et al. 1967). Rats exposed to 14C-labelled diuron showed extensive metabolism with only 2% of the parent compound recovered in the feces. Wu (1996) demonstrated that diuron is biotransformed by N-demethylation, ring hydroxylation and conjugation resulting in the same predominant metabolites. Diuron and its metabolites have not been shown to accumulate (Pauluhn and Eben, 1986; Weber and Abbink, 1988; Wu, 1996).
Health effects
The Pest Management Regulatory Agency (PMRA) regulates all pesticides, including diuron. PMRA conducts extensive evaluations and cyclical reviews of pesticides, including unpublished and proprietary information, as well as foreign reviews by other regulatory agencies such as the United States Environmental Protection Agency (US EPA). The PMRA based its proposed re-evaluation decision on the US EPA’s assessment described in its Re-registration Eligibility Decision (RED) document for diuron (US EPA, 2003b; Health Canada, 2006).
Acute toxicity is considered low via the oral, dermal and inhalation routes. The hematopoietic system, urinary bladder and kidney are the primary target organs for subchronic and chronic toxicity following exposure to diuron (US EPA, 2001; US EPA, 2003a; US EPA, 2003b). Erythrocyte damage and compensatory hematopoiesis have been reported in rats following exposure to as low as 1 mg/kg bw per day. Urinary bladder wall thickening and swelling, together with epithelial focal hyperplasia (in the urinary tract) have been observed in rats and mice exposed to 17 to 867 mg/kg bw per day of diuron. The US EPA (2003a; 2003b) identified a chronic dietary lowest-observed-adverse-effect-level (LOAEL) of 1.0 mg/kg bw per day of diuron based on evidence of hemolytic anemia and compensatory hematopoiesis reported in a combined chronic toxicity/carcinogenicity study in rats.
No evidence of developmental toxicity was observed in rabbit fetuses exposed to diuron in utero (Dearlove, 1986a). Impacts on developing rat fetuses (litter resorption, reduced fetal body weight and delayed ossification) were only observed at doses of diuron above those inducing maternal toxicity (400 mg/kg bw per day) (Dearlove, 1986b). No effects on reproductive indices were observed in a two generation reproductive toxicity study following exposures up to 101/131 mg/kg bw per day (highest dose tested) of diuron for male and female rats, respectively (Cook, 1990). Effects on offspring (decreased body weight) were observed only at a dose level at which parental toxicity was also observed (highest dose tested). In a published rat reproductive toxicity study, no significant differences in testosterone levels, sperm counts or sperm morphology were reported in males exposed to up to 250 mg/kg bw per day. However, when high-dose (250 mg/kg bw per day) males were mated with females, there were reductions in the uterine weights of females containing fetuses, and in the number of fetuses compared to females mated with control males (Fernandes et al., 2007).
Increased incidence of urinary bladder carcinoma in male and female Wistar rats has been observed following chronic dietary exposure to 111/203 mg/kg bw per day (highest dose tested) of diuron for males and females, respectively. A non-statistically significant increased incidence of kidney carcinoma and of urinary bladder papillomas (considered treatment-related) was also observed in male rats exposed to 111 mg/kg bw per day of diuron (Schmidt, 1985). In a 24-month feeding study performed in NMRI mice, mammary gland adenocarcinomas were more prevalent in female mice exposed to the highest dose (867 mg/kg bw per day of diuron) than in controls (Eiben, 1983).
Mode of action
No information was found on the mode of action of diuron in humans or animals.
Selection of key study
Schmidt (1985) exposed Wistar rats (60/sex/group) to 0, 25, 250, or 2500 ppm (0, 1.0, 10, or 111 mg/kg bw per day for males, and 0, 1.7, 17, or 203 mg/kg bw per day for females) for 24 months. At 12 months, 10 animals/sex/group were sacrificed for an interim analysis. Animal survival was unaffected over the study duration. Discoloured (red/bloody) urine was observed in some high-dose males and body weights and weight gain were significantly (P < 0.01) decreased in high-dose males (12% to 15%) and females (6% to 14%). In the mid-dose males, body weight gain was decreased (4% to 6%; P < 0.05), but not considered biologically significant by the author. The author also reported decreased food efficiency in high-dose males and females.
The author observed hemolytic anaemia with compensatory hematopoiesis in low-dose females as well as mid- and high-dose males/females. These effects were characterised as significantly decreased red blood cell counts, hemoglobin levels, and hematocrit, along with increased mean cell volume, mean cell hemoglobin, abnormal red blood cell form, reticulocyte counts and leukocyte counts. High-dose rats had increased plasma bilirubin levels (39% to 50%) and mid- and high-dose males/females had an increased incidence of swollen, dark and discoloured spleens. All dose group animals showed a dose-related increase in relative spleen weight (18% to 220%) at both 12 and 24 months with an increased severity observed in females. Spleen fibrosis was observed at an increased incidence in high-dose males and females. Increased hematopoietic bone marrow in mid- and high-dose rats at 12 and 24 months was noted along with a decrease in fat marrow at 12 months indicating bone marrow activation.
Impacts observed on the urinary tract included bladder wall thickening in low- and high-dose males and in high-dose females. Epithelial focal hyperplasia in the urinary tract and the renal pelvis increased in incidence in high-dose males at 12 months, high-dose females at 12 and/or 24 months, and mid-dose females at 24 months. An increased incidence of urinary bladder carcinoma was observed in the high-dose males and females. A non-statistically significant increased incidence of kidney carcinoma and urinary bladder papillomas (considered treatment-related) was also seen in the high-dose males. Liver changes in females included increased weight and hyperaemia at all doses, and increased swelling, discolouration, vacuolar cell degeneration, and round cell infiltration in the high dose group. In males, there was a lack of a dose-response relationship for the liver findings, which made it difficult to clearly attribute them to treatment.
The US EPA (2003b) identified a LOAEL of 1.0 mg/kg bw per day based on evidence of hemolytic anemia and compensatory hematopoiesis (decreased erythrocyte count, increased reticulocyte counts, increased spleen weight and bone marrow activation).
Derivation of the screening value
The US EPA (2003b) established a chronic oral reference dose (RfD) of 0.003 mg/kg bw per day to protect all age groups from exposure to diuron. The RfD was derived as follows:
Equation 1
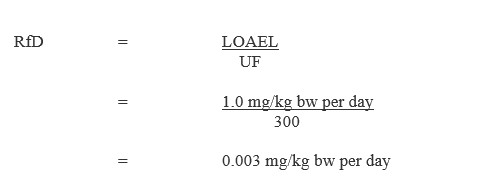
Equation 1 - Text Description
This equation calculates the chronic oral reference dose for diuron. The chronic oral reference dose is calculated by dividing the lowest observed adverse effect level for diuron (1.0 milligrams per kilogram of body weight per day) by the uncertainty factor of 300, which equals 0.003 milligrams per kilogram of body weight per day.
where:
LOAEL = 1.0 mg/kg bw per day, identified by the US EPA (2003b) for hemolytic anemia and compensatory hematopoiesis observed in rats (Schmidt, 1985); and
UF = uncertainty factor to account for interspecies (x10), intra-species variation (x10) and the use of a LOAEL (x3).
Using the RfD established by the US EPA, a drinking water screening value is derived as follows:
Equation 2
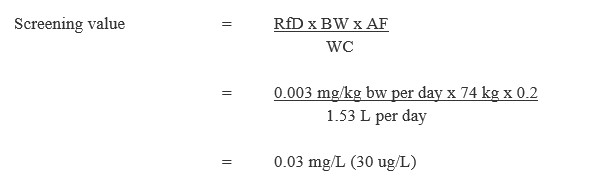
Equation 2 - Text Description
This equation calculates the drinking water screening value for diuron. The chronic oral reference dose for diuron is multiplied by the median body weight estimated for an adult and by a source allocation factor for drinking water, and then is divided by the estimated daily volume of tap water consumed by an adult. The result is a screening value of 0.03 mg/L (30 µg/L).
where:
RfD = chronic oral reference dose of 0.003 mg/kg bw per day as derived above.
BW = the median body weight estimated for a Canadian adult is 74 kg (Health Canada, 2021b).
AF = 0.2 is the default allocation factor since drinking water is not a major source of exposure to diuron and there is evidence of diuron in other exposure sources (i.e., food) (Krishnan and Carrier, 2013).
WC = water consumption: the estimated daily volume of tap water consumed by a Canadian adult is 1.53 L (Health Canada, 2021b).
A screening value of 0.03 mg/L (30 µg/L) for diuron is recommended by Health Canada. This screening value is considered protective for all age groups.
International considerations
Drinking water quality guidelines, standards and/or guidance established by foreign governments or international agencies may vary due to the science available at the time of assessment, as well as the utilization of different policies and approaches, such as the choice of key study, and the use of different consumption rates, body weights and allocation factors.
The US EPA has not established a maximum contaminant level (MCL) for diuron in drinking water (the MCL is the equivalent of the MAC). The US EPA has not established a non-enforceable lifetime health advisory, however, one- and ten- day health advisories (for a 10 kg child) were established at 1 mg/L in 1998 (US EPA, 2018) based on fetotoxicity in rats (US EPA, 1987). Health advisories serve as the informal technical guidance for unregulated drinking water contaminants in the United States.
The World Health Organization has not established a health-based guideline value for diuron in drinking water (WHO, 2017).
Australia has established a guideline value of 0.02 mg/L for diuron in drinking water based on reduced hemoglobin concentrations and increased reticulocytes in rats (NHMRC and NRMMC, 2011).
The European Union (EU) does not have a specific parametric value for individual pesticides. Instead, the EU has a value of 0.1 µg/L for any individual (single) pesticide, and a value of 0.5 µg/L for total pesticides found in drinking water. In establishing these values, the EU did not consider the science related to each pesticide, including health effects. Instead, the values are based on a policy decision to keep pesticides out of drinking water.
References
Anderson, A.M. (2005). Overview of pesticide data in Alberta surface waters since 1995. Edmonton (AB): Alberta Environment, Environmental Monitoring and Evaluation Branch. 190 p. Available from: http://environment.gov.ab.ca/info/library/7614.pdf
APVMA (2011). Australian Pesticides and Veterinary Medicines Authority, Chemical Review Program. Consolidated human health risk assessment for diuron. Available at: https://apvma.gov.au/node/12511#:~:text=The%20APVMA%20began%20a%20review,impurities%20of%20diuron%20active%20constituents)
CFIA (2019). National Chemical Residue Monitoring Program and Chemistry Food Safety Oversight Program Annual Report 2015-2016 Canadian Food Inspection Agency. Available upon request at: https://www.inspection.gc.ca/food-safety-for-industry/food-chemistry-and-microbiology/food-safety-testing-bulletin-and-reports/annual-report-2015-2016/eng/1554904884535/1554905013036
Cook, J. (1990). Reproductive and fertility effects with diuron (IN 14740): Multigeneration reproductive study in rats: Laboratory Project No. 8670-001: 560-90. Unpublished study prepared by E.I. DuPont de Nemours and Co. 1080 p. [as cited in US EPA, 2003b].
Dearlove, G. (1986a). Developmental Toxicity Study of H-16035 (Diuron) Administered by Gavage to New Zealand White Rabbits: Haskell Laboratory Report No. HLO 332-86. Unpublished study prepared by Argus Research Laboratories, Inc. 242 p. 41957301). [as cited in US EPA, 2003b].
Dearlove, G. (1986b) Developmental Toxicity Study of H-16035 (Diuron) Administered by Gavage to Rats: Haskell Laboratory Report No. HLO 410-86. Unpublished study prepared by Argus Research Laboratories, Inc. 240 p. [as cited in US EPA, 2003b].
Eiben, R. (1983). Diuron: Study for chronic toxicity and carcinogenicity with NMRI mice (administration in diet for 24 months): (Trans.) lab project number: T4010922: DIUR/TOX9. Unpublished study prepared by Bayer AG. (Wuppertal). 1532 p. [as cited in US EPA, 2003b].
Environment Canada and Health Canada (2011). Screening Assessment for the Challenge Urea, N'-(3,4-dichlorophenyl)-N,N-dimethyl- (Diuron). Available at: https://www.ec.gc.ca/ese-ees/6BC4E5D3-6E96-4EB2-938F-1BF7F2CC5701/batch10_330-54-1_en.pdf
Fernandes, G.S.A., Arena, A.C., Fernandez, C.D.B., Mercadante, A., Barbisan, L.F. and Kempinas, W.G. (2007). Reproductive effects in male rats exposed to diuron. Reprod Toxicol 23:106–112.
Giroux, I. (1998). Impact de l’utilisation des pesticides sur la qualité de l’eau des bassins versants des rivières Yamaska, L’Assomption, Chaudière et Boyer. Document rédigé par le ministère de l’Environnement et de la Faune, Direction des écosystèmes aquatiques, dans le contexte de Saint-Laurent-Vision 2000. 48 p. Available at: http://www.caaaq.gouv.qc.ca/userfiles/File/MDDEP12.PDF
Giroux, I., Robert, C. and Dassylva, N. (2006). Présence de pesticides dans l’eau au Québec : bilan dans des cours d’eau de zones en cultures de maïs et de soya en 2002, 2003 et 2004 et dans les réseaux de distribution d’eau potable, Québec, Ministère du Développement durable, de l’Environnement et des Parcs, Direction du suivi de l’état de l’environnement, Direction des politiques de l’eau et Centre d’expertise en analyse environnementale du Québec, ISBN 2-550-46504-0, 57 p., 5 annexes.
Giroux, I., Roy, N and Lamontagne, C. (2010) Présence de Pesticides dans l’Eau Souterraine en Milieu Agricole : Étude Pilote du Bassin Versant de la Rivière Châteauguay, Canadian Water Resources Journal, 35:4, 527-542, DOI: 10.4296/cwrj3504527. Available at : https://www.tandfonline.com/doi/abs/10.4296/cwrj3504527
Giroux, I. and Pelletier, L. (2012). Présence de pesticides dans l’eau au Québec : bilan dans quatre cours d’eau de zones en culture de maïs et de soya en 2008, 2009 et 2010, Québec, ministère du Développement durable, de l’Environnement et des Parcs, Direction du suivi de l’état de l’environnement, ISBN 978-2-550-64159-9 (PDF), 46 p. et 3 annexes. Available at: https://www.environnement.gouv.qc.ca/pesticides/mais_soya/bilan-4coursdeau-2008-2009-2010.pdf
Health Canada (2006). Proposed acceptability for continuing registration PACR2006-07. Re-evaluation of diuron. Pest Management Regulatory Agency. Available upon request at: https://health.canada.ca/en/health-canada/corporate/request-publication-form.html?title=PMRA%20(PACR2006-07)%20Re-evaluation%20of%20Diuron
Health Canada (2013). Canadian total diet study. Minister of Health, Ottawa, ON. Available at: https://www.canada.ca/en/health-canada/services/food-nutrition/food-nutrition-surveillance/canadian-total-diet-study/concentration-contaminants-other-chemicals-food-composites.html
Health Canada (2018). Pest Control Products Sales Report for 2018. Available at: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/corporate-plans-reports/pest-control-products-sales-report.html
Health Canada (2021a). Withdrawal of Select Guidelines. Available at: (to be added once published)
Heath Canada (2021b). Canadian exposure factors used in human health risk assessments. Fact Sheet. Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/chemical-substances/fact-sheets/canadian-exposure-factors-human-health-risk-assessments.html
Hodge, H.C., Downs, W.L., Panner, B.S., Smith, D.W., Maynard, E.A., Clayton, J.W. and Rhodes, R.C. (1967). Oral toxicity and metabolism of diuron (N-3,4-dichlorophenyl)-N’,N’-dimethylurea) in rats and dogs. Food Cosmet Toxicol 5:513–531. [as cited in Environment Canada and Health Canada, 2011].
Krishnan, K., Carrier, R. (2013). The use of exposure source allocation factor in the risk assessment of drinking-water contaminants. J. Toxicol. Environ. Health B Crit. Rev., 16(1):39–51.
NHMRC and NRMMC (2011). Australian Drinking Water Guidelines Paper 6 National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra. Available at https://Www.Nhmrc.Gov.Au/about-us/Publications/Australian-Drinking-Water-Guidelines.
Pauluhn, J. and Eben, D.-C. A. (1986). The concentration of diuron and its representative metabolites in the urine of male and female rats during a subacute inhalation study over eight weeks. Lab: Bayer AG, Fachbereich Toxikologie (Toxicology Unit) Report No. 14754. [as cited in AVPMA, 2011].
Schmidt, W. (1985). Diuron: Study for chronic toxicity and carcinogenicity with Wistar rats (administration in diet for up to 2 years): Project ID: T/801067; DuPont Report No. D/Tox17. Unpublished study prepared by Bayer AG. 1473 p. [as cited in US EPA, 2003b].
US EPA (1987). Diuron Health Advisory. Office of Drinking Water, United States Environmental Protection Agency. Available at: https://nepis.epa.gov/Exe/ZyPDF.cgi/9100I9M9.PDF?Dockey=9100I9M9.PDF
US EPA (2003a). The REVISED HED Chapter of the Reregistration Eligibility Decision Document (RED) for diuron. Available at: https://www.regulations.gov/document/EPA-HQ-OPP-2003-0349-0003
US EPA (2003b). Reregistration eligibility decision (RED) for diuron. Office of Prevention, Pesticides and Toxic Substances, United States Environmental Protection Agency. Available at: https://archive.epa.gov/pesticides/reregistration/web/pdf/diuron_red-2.pdf
US EPA (2018). 2018 Edition of the Drinking Water Standards and Health Advisories Tables. Office of Water, United States Environmental Protection Agency. Available at: https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf
Weber, H. and Abbink, J. (1988). [Phenyl-UL14C]-diuron: Investigation of the biokinetic behaviour in the rat. Lab: Institute for Metabolism Research, Metabolism Animal, PF-Zentrum Monheim, Building 6670, 5090 Leverkusen-Bayerwerk. Sponsor: Bayer AG/Sector 5, Business Group Agrochemicals, Research CE, Institute for Metabolism Research, Leverkusen / Monheim. Study No: M181068-4 & M 181069-5. Guidelines: US EPA 85-1 [as cited in AVPMA, 2011].
WHO (2017). Guidelines for drinking-water quality: fourth edition incorporating the first addendum. Geneva: World Health Organization. Available at: https://www.who.int/publications/i/item/9789241549950
Wu, D. (1996). Absorption, distribution, metabolism, and elimination of (carbon 14)-diuron in rats. Laboratory Project No. AMR 3145-94: XBL94161: RPT00247. Unpublished study prepared by XenoBiotic Labs, Inc. and E.I. du Pont de Nemours and Co. 303 p. [as cited in AVPMA, 2011].
