Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Metribuzin
Guideline value
The maximum acceptable concentration (MAC) for metribuzin in drinking water is 0.08 mg/L (80 μg/L).
Executive summary
This guideline technical document was prepared in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water and is based on assessments of metribuzin completed by Health Canada's Pest Management Regulatory Agency and supporting documents.
Exposure
Metribuzin is a pre-and post-emergent herbicide used to control broadleaf weeds and grasses in agriculture. In 2018, the most recent year for which data are available, more than 100 000 kg of metribuzin (as an active ingredient) was sold in Canada. Metribuzin is released into the environment as surface runoff following agricultural spraying (particularly within two weeks of soil application), as tile drain effluent, from accidental discharge or as spray drift. It has the potential to leach into groundwater or move into surface water.
Data provided by provinces and territories that monitor for metribuzin indicate that metribuzin is not commonly found in source or drinking water in Canada. However, low levels of metribuzin have been found in source and treated drinking water in a few Canadian provinces during targeted monitoring programs in agricultural areas where metribuzin is applied. Although metribuzin is used on food crops, it is rarely detected in foods.

Download the entire report
(PDF format, 639 KB, 47 pages)
Organization: Health Canada
Type: Guidelines
Date published: June 2021
Related Topics
Footnotes
Health effects
In repeat-dose animal studies, metribuzin primarily targeted the liver and, to a lesser extent, the thyroid, but did not cause birth defects, reproductive effects or an increase in cancer. Of the available studies in humans, there was no relationship between exposure to metribuzin and the incidence of cancer or Parkinson's disease. The MAC of 0.08 mg/L (80 µg/L) is based on liver effects seen in a 2-year dog study.
Analytical and treatment considerations
The establishment of drinking water guidelines takes into consideration the ability to both measure the contaminant and remove it from drinking water supplies. Several analytical methods are available for measuring metribuzin in water at concentrations well below the MAC.
At the municipal level, treatment technologies that are available to effectively decrease metribuzin concentrations in drinking water include oxidation, activated carbon adsorption, and membrane filtration. These technologies are capable of achieving treated water concentrations well below the MAC. Although metribuzin may be removed by common oxidants used for disinfection (e.g., chlorine), utilities should be aware of the potential formation of degradation byproducts.
In cases where metribuzin removal is desired at a small system or household level—for example, when the drinking water supply is from a private well—a residential drinking water treatment unit may be an option. Although there are no treatment units currently certified for the removal of metribuzin from drinking water, activated carbon adsorption and reverse osmosis technologies are expected to be effective. When using a residential drinking water treatment unit, it is important to take samples of water entering and leaving the treatment unit and send them to an accredited laboratory for analysis to ensure that adequate metribuzin removal is occurring.
Application of the guidelines
Note: Specific guidance related to the implementation of drinking water guidelines should be obtained from the appropriate drinking water authority.
The guidelines are protective against health effects from exposure to metribuzin in drinking water over a lifetime. Any exceedance of the MAC should be investigated and followed by the appropriate corrective actions if required. For exceedances in source water where there is no treatment in place, additional monitoring to confirm the exceedance should be conducted. If it is confirmed that source water metribuzin concentrations are above the MAC, then an investigation to determine the most appropriate way to reduce exposure to metribuzin should be conducted. This may include use of an alternate water supply or installation of treatment. Where treatment is already in place and an exceedance occurs, an investigation should be conducted to verify treatment and to determine whether adjustments are needed to lower the treated water concentration below the MAC.
Table of contents
- 1.0 Exposure considerations
- 2.0 Health considerations
- 3.0 Derivation of the health-based value
- 4.0 Analytical and treatment considerations
- 5.0 Management strategies
- 6.0 International considerations
- 7.0 Rationale
- 8.0 References
- Appendix A: List of abbreviations
- Appendix B: Canadian water quality data.
1.0 Exposure considerations
1.1 Sources and uses
Metribuzin, or 4-amino-6-(1,1-dimethylethyl)-3-(methylthio)-1,2,4-triazin-5(4H)-one, is a pre- and post-emergent herbicide used to control broad-leaf weeds and grasses in agricultural crops by inhibiting photosynthesis (CCME, 1999; Health Canada, 2005). More than 100 000 kg active ingredient of metribuzin were sold in Canada in 2018 (Health Canada, 2020a).
Metribuzin is released into the environment as surface runoff following agricultural spraying (particularly within 2 weeks of soil application), as tile drain effluent, from accidental discharge or as spray drift, and it has the potential to leach into groundwater or move into surface water (Bastien and Madramootoo, 1992; US EPA, 1998; CCME, 1999; US EPA, 2003; Health Canada, 2005). Leaching from soil is influenced by topography, precipitation and site-specific soil characteristics (Health Canada, 2019a). Under sandy soil conditions, metribuzin is 'highly' to 'very highly' mobile and likely to leach (CCME, 1999; US EPA, 2003; EFSA, 2010). Leaching is hindered in soils with high clay and/or high organic matter content as metribuzin moderately adsorbs to soil with adsorption decreasing as soil pH increases or as organic matter content decreases (Bowman, 1991; CCME, 1999; US EPA, 2003; EFSA, 2010; Rigi et al., 2015). Microbial degradation to desamino-diketo-metribuzin (DADK), diketo-metribuzin (DK) and carbon dioxide is one of two principal routes of removal of metribuzin from soil with metribuzin being moderately persistent under aerobic conditions (half-life of 40 to 106 days) and highly persistent under anaerobic conditions (half-life of 112 to 439 days) (US EPA, 2003; EFSA, 2010). The other degradation route from soil and surface water is photodegradation (half-life of 4.3 hours to 2.5 days), which produces desamino-metribuzin (DA). However, this route is only relevant in the top 1 mm of soil exposed to direct sunlight or in shallow, clear surface water with good light penetration (US EPA, 2003; EFSA, 2010). Both DADK and DK are persistent and very mobile in soil (US EPA, 1998).
In groundwater, metribuzin mainly breaks down into the same metabolites (DADK, DK, DA) as in soil, and these metabolites are present at an order of magnitude less than the parent compound metribuzin (Lawrence et al., 1993). Metribuzin in groundwater has a half-life of 350 days in a shallow aquifer (Perry, 1990). Metribuzin resists hydrolysis with an extrapolated half-life of 1 317 days at 20 °C and pH 9 (EFSA, 2010). Based on its vapour pressure (5 to 10 mmHg at 20 °C), Henry's law constant (2.0 x 10-5 Pa m3/mol) and photo-oxidative degradation in the atmosphere (21 hours), metribuzin is not expected to volatize from either water or land surfaces or to undergo long-range airborne transport (CCME, 1999; US EPA, 2003; EFSA, 2010).
1.2 Substance identity
Metribuzin (C8H14N4OS) is a white crystalline solid belonging to the asymmetric triazinone class of chemicals (US EPA, 1998; Health Canada, 2005).
| Property | Metribuzin | Interpretation |
|---|---|---|
| CAS RN | 21087-64-9 | Not applicable |
| Molecular weight (g/mol) | 214.3 | Not applicable |
| Water solubility (g/L) | 1.2 | Highly soluble in water |
| Vapour pressure (volatility) | 5 to 10 mmHg at 20 °C | Low volatility, unlikely to contaminate air |
| Henry's law constant | 2.0 x 10-5 Pa m3/molFootnote a | Low volatilization potential |
| octanol: water partition coefficient (Log Kow) | 1.6 at pH 4-9, 20 °CFootnote a | Unlikely to bioaccumulate |
|
||
1.3 Exposure
Media-specific exposure data are limited to levels in water and food. Canadian water monitoring data for metribuzin were available from the provinces and territories (municipal and non-municipal supplies), Health Canada's Pest Management Regulatory Agency (PMRA) and Environment Canada (Environment Canada, 2011)(Appendix B), while food data were available from the United States.
Water monitoring data provided by the provinces and territories indicate that metribuzin levels are below the method reporting limit (MRL) or method detection limit (MDL) in the majority of samples. This includes samples from a variety of water supplies in Canada, including surface water and groundwater as well as treated and distributed drinking water where monitoring occurred (British Columbia Ministry of Health, 2019; Indigenous Services Canada, 2019; Manitoba Sustainable Development, 2019; Ministère de l'Environnement et de la Lutte contre les changements climatiques du Québec, 2019; Nova Scotia Environment, 2019; Government of Ontario, 2019; Prince Edward Island (P.E.I.) Department of Communities, Land and Environment, 2019).
Provinces that conducted monitoring in which all samples were reported below the MDLs are presented in Table 2. It provides information on the number and types of samples each province collected over a specified time period in which no metribuzin was detected. In addition, data provided by Indigenous Services Canada's First Nations and Inuit Health Branch for various regions in Canada (Manitoba, Ontario, Quebec and Atlantic region) indicated that metribuzin levels were below the MDLs in all samples (n = 821; MDLs 0.01 to 5 µg/L) collected between 2014 and 2018 (Indigenous Services Canada, 2019).
Water monitoring data from provinces that conducted sampling where concentrations above the detection limit were reported are summarized in Table 3. Monitoring conducted in Prince Edward Island (P.E.I.) found low concentrations ranging from 0.04 to 0.28 μg/L for municipal wells and 0.03 to 1.21 μg/L for non-municipal/private wells (P.E.I. Department of Communities, Land and Environment, 2019). Quebec reported three data sets representing samples collected from both municipal surface and groundwater supplies as well as samples collected as part of two special projects evaluating groundwater quality in potato-growing regions and small systems/private wells. Detectable concentrations were low with a concentration range in municipal systems of between 0.03 and 0.06 μg/L and a range in groundwater from the special projects monitoring of between 0.01 and 1.7 μg/L (Ministère de l'Environnement et de la Lutte contre les changements climatiques du Québec, 2019).
Monitoring for metribuzin is not currently conducted in New Brunswick, Newfoundland and Labrador, Saskatchewan or Yukon (New Brunswick Department of Health, 2019; Newfoundland and Labrador Municipal Affairs and Environment, 2019; Saskatchewan Water Security Agency, 2019; Yukon Environmental Health Services, 2019).
| Jurisdiction (MDL µg/L) |
Monitoring period | Water type (Municipal: ground/surface - raw, treated, distributed) |
# Detects/ samples |
|---|---|---|---|
| British Columbia (2.5–5) |
2013 – 2018 | Surface – raw | 0/18 |
| Manitoba (0.2) |
2015 – 2018 | Surface – ambient | 0/396 |
| Nova Scotia (0.25–7) |
2007 – 2018 | Ground – raw | 0/72 |
| Ground – treated | 0/34 | ||
| Surface – raw | 0/35 | ||
| Surface – treated | 0/40 | ||
| Distributed | 0/1 | ||
| Ontario (0.05) |
2008 – 2012 | Ground – raw | 0/214 |
| Ground – treated | 0/48 | ||
| Surface/ground – raw | 0/564 | ||
| Surface/ground – treated | 0/583 | ||
| Surface/ground – distribution | 0/1 |
| Jurisdiction (MDL µg/L) |
Monitoring period | Water type (Municipal: ground/surface - raw, treated, distribution and Non-Municipal: ground) |
# Detects/ samples | Maximum concentration (µg/L) |
|---|---|---|---|---|
| P.E.I. (0.03) |
2004 – 2017 | Ground – raw (municipal) | 12/665 | 0.28 |
| Ground – raw (non-municipal) | 27/614 | 1.21 | ||
| Quebec (0.01–0.9) |
2012 – 2018 | Ground – distribution (municipal) | 0/578 | Not available |
| Surface – distribution (municipal) | 3/1708 | 0.06 | ||
| Ground – rawFootnote a (municipal) | 1/46 | 0.06 | ||
| Ground – treatedFootnote a (municipal) | 0/17 | Not available | ||
| Ground – distributionFootnote a (municipal) | 1/5 | 0.02 | ||
| Ground – rawFootnote b (municipal) | 0/82 | Not available | ||
| Ground – rawFootnote b (non-municipal) | 14/132 | 1.70 | ||
|
||||
The Pest Management and Regulatory Agency evaluated several Canadian surface water and groundwater monitoring studies that were conducted in P.E.I., British Columbia, Alberta, Quebec, Manitoba, New Brunswick, Ontario and Nova Scotia (Health Canada, 2019a). A large amount of monitoring data was available for metribuzin in Canada, but only a few studies were from potential sources of drinking water. A total of 3 820 groundwater samples were analyzed for metribuzin. There were 259 detections (7% detection frequency), with a maximum detected concentration in groundwater of 7.5 μg/L in P.E.I. during the 1988 – 1989 sampling period. The data from the P.E.I. sites are considered to be a worst-case scenario, as metribuzin was used in the fields over a 3-year period and the wells were located downslope or within 300 metres of treated fields. A total of 10 393 surface water samples were analyzed for metribuzin. Metribuzin was detected in 849 of the samples (8% detection frequency). The two highest concentrations, 195 μg/L and 87 μg/L, were detected in P.E.I. in 2008, and one of the detections was from a sample collected during a runoff event. Since all drinking water in P.E.I. is sourced from groundwater, detections of metribuzin in P.E.I. surface water were not considered.
Overall, available monitoring data on metribuzin indicate that the maximum concentrations in Canadian drinking water sources were 7.5 µg/L in groundwater and 6.1 µg/L in surface water. The highest detections were not observed in provinces with highest metribuzin use (i.e., Saskatchewan and Ontario), but rather in areas considered to be most vulnerable to leaching and runoff due to high incidents of precipitation, sandy soil textures and shallow groundwater tables.
Using annual total precipitation by ecodistrict and the percent of total sand in agricultural areas in Canada, the following can be inferred:
- Eastern Canada receives higher precipitation than Western Canada.
- The majority of soils in the agricultural areas of Western Canada have less than 50% sand, while soils in Eastern Canada are coarser, with a higher sand content (> 69%).
- Compared to Western Canada, Eastern Canada generally has a shallower groundwater table.
- The potential to detect metribuzin in groundwater in Saskatchewan is low because of its medium- to fine-textured soils.
Based on its higher precipitation, its coarse and sandy soil texture, and its shallower groundwater table, Eastern Canada is relatively more vulnerable to leaching of metribuzin than Western Canada (Health Canada, 2019a).
Although metribuzin is applied to food crops, it is rarely detected in food samples and does not tend to accumulate in food (Bray et al., 2008). Canadian food residue data were unavailable. Monitoring data conducted by the United States Department of Agriculture's Pesticide Data Program and collated by Bray et al. (2008) did not detect any metribuzin residues in 2 586 samples of dairy products (milk, heavy cream, butter) collected from 2003 to 2005 (limit of detection ranged from 0.3 to 6.0 ppb). Metribuzin was detected in only 13 of 26 487 fruit and vegetable samples collected between 2000 and 2005. It was not detected in any barley grain, soybean or wheat flour samples (n = 4362) collected between 2002 and 2005 or in any meat samples (poultry n = 1564, pork n = 704, and beef n = 1835) (Bray et al., 2008).
2.0 Health considerations
All pesticides, including metribuzin, are regulated by PMRA. The Pest Management and Regulatory Agency conducts extensive evaluations and cyclical reviews of pesticides, including unpublished and proprietary information, as well as foreign reviews by other regulatory agencies such as the United States Environmental Protection Agency (US EPA). This health assessment is based primarily on PMRA's evaluations (Health Canada, 2005, 2006) and supporting documentation. Additionally, any reviews and relevant literature available since PMRA's evaluations were completed were also considered.
2.1 Kinetics
Absorption: Metribuzin is rapidly and almost completely absorbed (95% to 100% based on excretion data) within 36 hours following ingestion, with peak blood and tissue levels being achieved within 4 hours (Bleeke et al., 1985; Office of Environmental Health Hazard Assessment (OEHHA), 2001; EFSA, 2010). Based on a study in rats, dermal absorption of metribuzin is unlikely to occur (Löser and Kimmerle, 1972).
Distribution: Metribuzin and its metabolites are widely distributed following absorption, with the highest concentrations found in the thyroid and liver followed by kidneys and other tissues (heart, fat, ovaries, brain, muscle, plasma and testes) (OEHHA, 2001; EFSA, 2010). Thyroid tissue contained 10 times more metribuzin than liver tissue (OEHHA, 2001).
Metabolism: Based on studies in Wistar rats, metabolism of metribuzin is extensive and occurs rapidly through several pathways (deamination, hydroxylation at the t-butyl side chain, hydrolytic or aminolytic cleavage of the thioalkyl moiety, and conjugation), some of which can act in combination, producing numerous metabolites in urine, feces and tissues (Bleeke et al., 1985; Cain et al., 1987; OEHHA, 2001; EFSA, 2010; EFSA, 2018). Cytochrome P450 may be involved in the initial metabolism, yielding reactive intermediates such as metribuzin sulfoxide or deamonometribuzin sulfoxide, which then react with glutathione (GSH) (Bleeke et al., 1985; OEHHA, 2001). Conversion to mercapturic acid derivatives appears to follow conjugation with GSH (EFSA, 2018). At very high levels, or in the absence of GSH or other non-protein sulfhydrils, metabolites can bind to proteins (OEHHA, 2001). Metabolites were similar in urine and feces and include DA, DK, demethylmetribuzin (DM), tert-butylhydroxy-metribuzin, and N-acetylcysteine-metribuzin as well as many unidentified metabolites (OEHHA, 2001; EFSA, 2010; EFSA, 2018).
Elimination: Metribuzin and its metabolites are rapidly excreted in the urine and feces, with up to 96% being eliminated within 4 days (Bleeke et al., 1985; Cain et al., 1987; Mathew et al., 1998). Species, strain and sex differences in the proportions and types of metabolites eliminated in excreta were observed (OEHHA, 2001). In studies using radiolabelled (14C) metribuzin, male albino rats eliminated almost equal amounts of 14C in the urine and feces (Bleeke et al., 1985); Wistar rats excreted 55.8% to 71.5% in the feces, 27.3% to 43.4% in the urine and 0.1% in expired air (Cain et al., 1987; EFSA, 2010). Male mongrel dogs excreted twice as much 14C label in urine than in feces (Baychem, 1972). For all tissues, the elimination half-lives ranged from 18.4 to 33.6 hours and were generally shorter in males than females (OEHHA, 2001; EFSA, 2010).
2.2 Health effects
The toxicity database for metribuzin is well characterized, covering several endpoints and various types of exposure (see US EPA, 1998, 2003; OEHHA, 2001; EFSA, 2010; EFSA, 2018 for more thorough reviews). In animals, metribuzin was not acutely toxic and was well-tolerated in repeat-dose studies. It did not cause birth defects or reproductive effects. It was not carcinogenic in animal studies using rats (Wistar and Fischer 344) and mice (CD1) or in epidemiological studies. The liver and the thyroid have been identified as the target organs in animals.
2.3 Effects in humans
No human effects were discussed in Health Canada's assessments by PMRA or their supporting documents, as no human health studies were available in the published literature at that time (US EPA, 1998, 2003; Health Canada, 2005, 2006). Studies are available from the more recent literature concerning both cancer and non-cancer endpoints, and are included in this assessment.
Agricultural Health Study: The Agricultural Health Study (AHS) is a large, questionnaire-based prospective study (over 89 000 participants) investigating cancer and non-cancer endpoints in a cohort of licensed pesticide applicators and their spouses in Iowa and North Carolina. It began in 1993 with the collection of baseline information on farming practices (including pesticide use), lifestyle and health. Follow-up interviews/questionnaires (including dietary information) and DNA collection were done periodically. Cancer registries were used to assess cancer incidence. Overall, strengths of the AHS include its large size; the inclusion of a large number of women; the collection of baseline, health and lifestyle information, and genetic factors; the use of cancer registries; and the many different pesticides and diseases assessed. Its limitations include the indirect assessment of exposure (questionnaire-based), the lack of exposure refinement measurements (no induction time or latency discussion), and selection bias when controlling for multiple confounders due to the exclusion of many subjects with missing data (Sathiakumar et al., 2011).
Cancer: Several investigators have published studies based on their analysis of the AHS data. A case-control analysis by Andreotti et al. (2009) did not find an association between metribuzin and the development of pancreatic cancer after controlling for age, smoking and diabetes. Alavanja et al. (2003), examining AHS data from 1993 to 1997, found overall cancer incidence in pesticide applicators to be significantly lower than expected for 45 common pesticides, including metribuzin, when compared to incidence among males in the general populations of Iowa and North Carolina. The study also did not find an association between metribuzin use and prostate cancer incidence. Similarly, Delancey et al. (2009) did not find an association between metribuzin use and overall cancer incidence, but did suggest a potential association between metribuzin use as measured by intensity-weighted lifetime exposure days and certain lymphohematopoietic malignancies (defined as lymphomas, myelomas, and leukemias) when the low-exposure group was the referent. However, these findings were not consistent across exposure metrics or reference groups (low-exposure vs. no exposure groups) and may have resulted from the smaller sample size in the low-exposure group or from a residual confounder. A more recent non-AHS cohort study involving more than 300 000 farmers from France, Norway and the United States failed to associate metribuzin exposure with non-Hodgkin's lymphoma (NHL), leukemia, or lymphoma (Leon et al., 2019). A pooled analysis of three case-control studies from the 1980s also did not find an association between "ever" use of metribuzin by farmers and NHL (De Roos et al., 2003).
A population-based case-control study using telephone interviews was conducted as part of the Nebraska Health Study II (Lee et al., 2005). It found a significant association between metribuzin exposure and the risk of glioma among male but not female farmers. Weaknesses of the study included the low number of glioma cases (n = 9), the proportion of proxy respondents (4 of 9), recall bias, and the potential for differential misclassification (Lee et al., 2005).
Non-Cancer: Examining non-cancer endpoints, Hoppin et al. (2002) did not find an association between metribuzin use and wheeze among pesticide applicators based on AHS data collected from 1994 to 1997. However, a follow-up study using 2005 to 2010 data found an elevated, but not statistically significant, association between metribuzin use and allergic wheeze (Hoppin et al., 2017). Using AHS data up to 2003, Kamel et al. (2006) did not find a strong association between metribuzin use and Parkinson's disease. Goldner et al. (2013) did not find an association between metribuzin use by male pesticide applicators in the AHS and thyroid disease. Shrestha et al. (2018) did not find an association between metribuzin use and self-reported incidents of hypothyroidism from AHS data collected up to 2016.
2.4 Effects in animals
Repeat exposure studies in rats, mice and dogs showed metribuzin primarily affected the liver and thyroid, although other effects have also been noted (Löser and Mirea, 1974; Löser and Mohr, 1974; Hayes, 1981; Thyssen, 1981; Flucke and Hartmann, 1989; Christenson and Wahle, 1993). Metribuzin showed low acute toxicity based on oral LD50 values of 2345 mg/kg (female) and 2200 mg/kg (male) in Wistar II rats, 711 mg/kg (female) and 698 mg/kg (male) CF1 mice, > 500 mg/kg (male) in rabbits, and 250 mg/kg (male) in guinea pigs, the most sensitive species (Löser and Kimmerle, 1972). Health effects included laboured breathing and sedation (Löser and Kimmerle, 1972). Dermal LD50 values of > 20 000 mg/kg in rabbits and > 500 mg/kg in Wistar II rats and inhalation values of > 0.648 mg/L (maximum obtainable concentration was used) and > 885 mg/m3 in rats have been reported; animals showed no signs of toxicity (Crawford and Anderson, 1972; Löser and Kimmerle, 1972; Shiotsuka, 1986; Breckenridge et al., 2009).
Liver effects: The liver was the main target organ in chronic studies using dogs, mice and rats, as well as in subchronic and short-term studies using dogs, rats, and rabbits. Effects consisted of changes in liver enzymes, histopathological changes, and increased absolute and relative liver weights.
Chronic oral studies were conducted in beagles, mice and two species of rats. In a study by Löser and Mirea (1974), beagles were fed 25 to 1500 ppm of metribuzin daily for 2 years. At the highest dose, dogs had changes in liver histopathology, increased relative liver weights and increased liver enzymes, including serum glutamate-oxaloacetate transaminase (SGOT), serum glutamate-pyruvate transaminase (SGPT), alkaline phosphatase, and bromsulphthalein retention. CD1 mice fed 200 to 3200 ppm metribuzin for 2 years had increased relative liver weights, but only in the high-dose group (Hayes, 1981). Histopathological changes in the liver were seen in Wistar rats (25 to 300 ppm) and F344 rats (30 to 900 ppm) fed metribuzin for up to 2 years, but only at 300 ppm and above (Löser and Mohr, 1974; Christenson and Wahle, 1993).
Two oral subchronic studies also showed similar hepatic effects. In beagles fed ≤ 500 ppm of metribuzin for 90 days, hepatic effects including dose-dependent increases in liver weight, liver-to-body weight ratio and liver-to-brain weight ratio as well as a small decrease in SGOT and SGPT at 500 ppm males only (Chaisson and Cueto, 1970). Increased liver weights were also observed in high-dose (1500 ppm) Wistar rats fed 50 to 1500 ppm for 3 months, although liver pathology was unremarkable (Löser et al., 1969).
Short-term 21-day studies via dermal exposure (New Zealand rabbits) and inhalation (Wistar TNO/W 74 albino rats) also showed liver effects including a dose-dependent increase in cholesterol in rabbits, increased liver enzymes N-demethylase and cytochrome P450 in rabbits and rats, as well as increased O-demethylase levels and increased liver weight in rats (Thyssen, 1981; Flucke and Hartmann, 1989).
In a two-generation reproduction study using Crl:CD BR rats, liver effects (hypertrophy of the hepatocytes of the centrilobular and mid-zonal regions) were seen in high-dose males (750 ppm) and mid- and high-dose females (150 and 750 ppm, respectively) (Porter et al., 1988).
Thyroid effects: Thyroid effects included changes in thyroid weights and in levels of circulating thyroid hormones and were seen in rats, dogs and rabbits.
Increased absolute and relative thyroid weights (in males only), as well as increased thyroxine (T4) and decreased triiodothyronine (T3), were seen in a chronic study in which Fischer 344 rats were fed 30 to 900 ppm of metribuzin daily for 104 weeks (Christenson and Wahle, 1993). Similarly, thyroid weights were increased in high-dose beagles fed metribuzin (25 to 1500 ppm) daily for 2 years and in high-dose Wistar rats fed metribuzin (50 to 1500 ppm) daily for 3 months (Löser et al., 1969; Löser and Mirea, 1974).
Short-term studies showed increased absolute and relative thyroid weights at 720 mg/m3 in Wistar TNO/W 74 albino rats exposed to between 32 and 720 mg/m3 of metribuzin as an aerosol daily for 6 hours and decreased T3 levels at 1000 mg/kg bw per day in male New Zealand rabbits exposed dermally to between 40 and 1 000 mg/kg bw per day of metribuzin for 3 weeks (Thyssen, 1981; Flucke and Hartmann, 1989).
In a developmental toxicity study, mid-dose (70 ppm) and high-dose (200 ppm) pregnant CrL:CD BR rats gavaged with 25 to 200 mg/kg bw per day of metribuzin on gestation days 6 to 18 had decreased T4 levels, while high-dose rats had increased thyroid weight (Kowaski et al., 1986).
Reproductive/developmental toxicity: No fetotoxicity was observed in rats and rabbits gavaged with metribuzin (rats: 25 to 200 mg/kg bw per day; rabbits: 10 to 85 mg/kg bw per day) on gestation days 6 to 18 and rats fed metribuzin (5 to 100 mg/kg bw per day) on gestation days 6 to 15 despite maternal toxicity (decreased body weight gain and increased thyroid weights) (Machemer, 1972; Kowaski et al., 1986; Clemens and Hartnagel, 1989).
In a 3-generation reproduction study, no treatment-related effects on mating, gestation, lactation or pup development were seen in FB30 rats fed 35 to 300 ppm of metribuzin (Löser and Siegmund, 1974). No reproductive effects were seen in a 2-generation reproduction study in which Cr:CD BR rats were fed 30 to 150 ppm of metribuzin; however, high dose F0 and F1 generations consumed less food and had decreased body weight gain and hypertrophy of the liver (Porter et al., 1988).
2.5 Genotoxicity and carcinogenicity
Metribuzin was not mutagenic in a range of in vitro and in vivo tests. Negative in vitro studies (both with and without activation) included: Ames assays using Salmonella typhimurium and Escherichia coli, an SOS Chromotest using E. coli, Chinese hamster ovary (CHO)/ hypoxanthine-guanine phosphoribosyl transferase) (HGPRT mutation assay), and an unscheduled DNA synthesis assay in rat primary hepatocytes (Inukai and Iyatomi, 1977; Yang, 1986). An in vitro test using CHO cells was clastogenic but only in the presence of S9; the US EPA determined clastogenicity was not of concern as there was no evidence of mutagenicity in in vivo tests (Murli, 1990; US EPA, 1998). Negative in vivo tests included three dominant lethal mutation tests in male and female mice gavaged with metribuzin, and a cytogenetic study assessing spermatogonia in Chinese hamsters (Machemer and Lorke, 1974a, 1974b).
No increase in the incidence of tumours was seen in CD1 mice fed 200 to 3200 ppm of metribuzin for 2 years (Hayes, 1981). Both Fischer 344 rats fed 30 to 900 ppm for 52 or 104 weeks and Wister rats fed 25 to 300 ppm showed no evidence of carcinogenicity (Löser and Mohr, 1974; Christenson and Wahle, 1993).
The US EPA has classified metribuzin as Group D (not classifiable as to human carcinogenicity) based on the unavailability of human data and inadequate evidence from animal studies (US EPA, 2003), while the International Agency for Research on Cancer (IARC) has not reviewed the carcinogenicity of metribuzin.
2.6 Mode of action
An intraperitoneal study in mice and an oral study in rats found that metribuzin increases oxidative stress and alters antioxidant status causing hepatotoxicity by depleting the liver GSH content and binding to liver proteins (Bleeke et al., 1985; Chiali et al., 2013). In the liver, microsomal mixed-function oxidase metabolizes metribuzin to a reactive intermediate, likely metribuzin sulfoxide or deaminometribuzin sulfoxide, which then reacts with available thiols (especially GSH), or, in their absence, with proteins and other macromolecules (Bleeke et al., 1985).
The principal urinary metabolites of metribuzin are mercapturic acids, which arise via metribuzin sulfoxide or deaminometribuzin sulfoxide reacting with reduced glutathione (GSH) (Bleeke et al., 1985). Sulfoxidation therefore appears to activate metribuzin to an electrophilic metabolite which, in the absence of GSH, binds to tissue proteins producing hepatotoxicity (Bleeke et al., 1985).
2.7 Selected key study
Health Canada (2019a, 2019b) has identified the liver as the most sensitive target organ across the database based on a 2-year feeding study in beagle dogs (Löser and Mirea (1974). In that study, 4 beagle dogs/sex/group were administered 0, 25, 100, or 1500 ppm (0, 0.83, 3.5, or 55.6 mg/kg bw per day) of metribuzin (Bay 94 337 technical 99.5%) in the diet. Mortality rates of 75% were observed in the high-dose group (1500 ppm or 55.6 mg/kg/bw per day) in both males and females. The clinical tests performed after 12 months of metribuzin exposure suggested the presence of liver dysfunction in the dogs. Elevated activities of liver enzymes such as SGOT, SGPT, ornithine-carbamyl transferase and alkaline phosphatase, along with an increase in bromsulphthalein retention, were reported in the males beginning at 3.5 mg/kg bw per day. Increased SGPT, ornithine-carbamyl transferase and serum protein levels were observed in high-dose females. Increased mucopolysaccharide droplets in the liver and hepatic necrobiosis were observed in mid- and high-dose males and females. There were no major changes in kidney function. An increase in thyroid weight was observed in the high-dose groups of both sexes.
The 2-year dog toxicity study no-observed-effect-level (NOEL) of 0.83 mg/kg bw per day for males and females with a standard uncertainty factor of 100 was used to establish the acceptable daily intake (ADI) of 0.0083 mg/kg bw per day. At the lowest-observed-effect-level of 3.5 mg/kg bw per day, increased ornithine carboxytransferase was observed in males and increased mucopolysaccharide droplets in the liver and hepatic necrobiosis were observed in males and females.
The NOEL from the 2-year dog study was the lowest in the toxicology database and was considered protective of the effects observed in the rodent studies (Health Canada, 2020b). There is no evidence to suggest that offspring or any other subgroup would be more sensitive to metribuzin (US EPA, 2003).
3.0 Derivation of the health-based value
As noted above, the NOEL of 0.83 mg/kg bw per day for liver effects in dogs was selected as the basis for the current risk assessment. Using this NOEL, the ADI (Health Canada, 2019a) was calculated as follows:
Equation 1
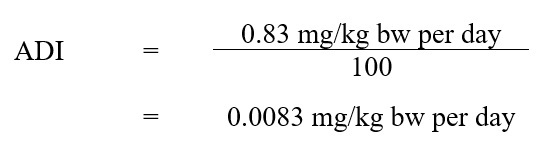
Description Equation 1:
The ADI for metribuzin is 0.0083 mg/kg bw per day. This is calculated by dividing the NOAEL of 0.83 mg/kg bw per day by the uncertainty factor of 100.
where:
- 0.83 mg/kg bw per day is the NOEL, based on liver effects in beagles; and
- 100 is the uncertainty factor, selected to account for interspecies variation (×10), intraspecies variation (×10). There are no-observed-adverse-effect levels (NOAELs) established for the rodent studies, and sensitivity of the young was not identified at the time. In addition, since an even lower NOEL was used to derive the ADI relative to the NOAELs established in the rodent studies, no additional factors were required.
Based on the ADI of 0.0083 mg/kg bw per day, a health-based value (HBV) for metribuzin in drinking water was derived as follows:
Equation 2
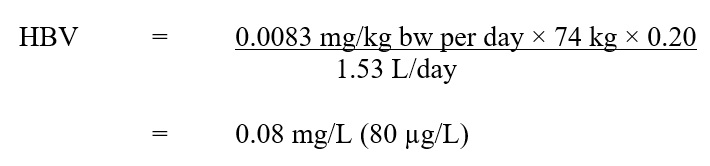
Description Equation 2:
The HBV for metribuzin in drinking water is 0.08 mg/L. This is calculated by multiplying the ADI for metribuzin (0.0083 mg/kg bw per day) by the allocation factor for water (0.2), then by the average body weight for an adult (74 kg). This product is then divided by the daily volume of water consumed by an adult (1.53 L/day).
where:
- 0.0083 mg/kg bw per day is the ADI calculated using a NOEL of 0.83 mg/kg bw per day (Health Canada, 2019a, 2019b);
- 74 kg is the adult body weight (Health Canada, 2015);
- 1.53 L per day is the daily volume of tap water consumed by an adult (Health Canada, 2017); and
- 0.20 is the default allocation factor for drinking water (Krishnan and Carrier, 2013).
4.0 Analytical and treatment considerations
4.1 Analytical methods to detect metribuzin
The standardized methods available for the analysis of metribuzin in source and drinking water and their respective MDLs are summarized in Table 4. MDLs are dependent on the sample matrix, instrumentation, and selected operating conditions and will vary between individual laboratories. The MDLs or MRLs from provincial and territorial data are in the range of 0.01 to 7 μg/L (British Columbia Ministry of Health, 2019; Manitoba Sustainable Development 2019; Ministère de l'Environnement et de la Lutte contre les changements climatiques du Quebec, 2019; Nova Scotia Environment, 2019; Indigenous Services Canada, 2019; P.E.I. Department of Communities, Land and Environment, 2019).
Additional analytical methods that are not currently standardized are available for the measurement of metribuzin in water. These methods are based on high-performance liquid chromatography with either mass spectrometry or ultraviolet detection (Flores-Garcia et al., 2011; Sinha et al., 2011; Rocha et al., 2015). These methods have reported MDLs similar to those of the standard methods listed below and are suitable for use in commercial laboratories (Haiste-Gulde and Sacher, 2019).
Drinking water utilities should discuss sampling requirements with the accredited laboratory conducting the analysis to ensure that quality control procedures are met and that MRLs are low enough to ensure accurate monitoring at concentrations below the maximum acceptable concentration (MAC). Sample processing considerations for the analysis of metribuzin in drinking water (e.g., sample preservation, storage) can be found in the references listed in Table 4. It is important to note that quenching is critical if an oxidant is present in samples in order to prevent additional degradation of metribuzin prior to analysis.
| Method (reference) |
Methodology | MDL (µg/L) |
Interferences/comments |
|---|---|---|---|
| EPA 507 Rev. 2.1 (US EPA, 1995a) |
Liquid-liquid extraction and capillary column gas chromatography (GC) with a nitrogen-phosphorus detector | 0.029 | Not available |
| EPA 508.1 Rev. 2.0 (US EPA, 1995b) |
Liquid-solid C-18 cartridge or disk extraction and gas chromatography with an electron capture detector (GC/ECD) | 0.009 | Not available |
| EPA 525.2 Rev. 2.0 (US EPA, 1995c) |
Liquid-solid C-18 cartridge or disk extraction and GC with mass spectrometry (GC/MS) | 0.062 – 0.22 | Not available |
| EPA 551.1 Rev. 1.0 (US EPA, 1995d) |
Liquid-liquid extraction and GC/ECD | 0.005 – 0.041 | Seal containers and avoid contact with plastic to avoid any potential interferences with phthalates |
4.2 Treatment considerations
Treatment technologies available to effectively decrease metribuzin concentrations in drinking water include oxidation, activated carbon adsorption, and membrane filtration. Published data on metribuzin removal in water using these technologies indicate that between 50% to 98% removal can be achieved (Miltner et al., 1989; Hofman et al., 1997; Chen et al., 2004; Chamberlain et al., 2012). At the residential scale, certified treatment devices relying on reverse osmosis or activated carbon adsorption are expected to be effective for removal of metribuzin.
4.2.1 Municipal-scale treatment
Since metribuzin concentrations are low in source water, treatment technology data reported in the literature generally have low influent concentrations (< 50 µg/L). Information on the removal efficiencies and the operational conditions from these studies are reported below as they provide an indication of the effectiveness of specific treatment technologies for metribuzin removal. The selection of an appropriate treatment process will depend on many factors, including the raw water source and its characteristics, the operational conditions of the selected treatment method and the utility's treatment goals. Bench or pilot testing is recommended to ensure the source water can be successfully treated.
4.2.1.1 Conventional treatment
Conventional filtration (chemical coagulation, clarification, and rapid sand filtration) and chlorine disinfection may reduce metribuzin concentrations through oxidation during the disinfection step (Miltner et al., 1989; Blomquist et al., 2001). Conventional clarification and filtration processes alone are not effective for the removal of metribuzin. Monitoring of metribuzin in full-scale treatment plants found no statistically significant removal of low levels of metribuzin (<5 ug/L) through clarification and filtration. Additional coagulation/flocculation jar tests confirmed no removal of metribuzin (initial concentration of 45 µg/L) from river water using an aluminum sulphate dose of 30 mg/L (Miltner et al., 1989).
4.2.1.2 Oxidation
Chemical oxidation of metribuzin using chlorine, chlorine dioxide (ClO2), permanganate (MnO4-) and ozone (O3) can be an effective treatment method for removing metribuzin from water depending on a variety of factors including oxidant dose, contact time, disinfectant demand, temperature and pH. Data for several oxidants that are effective are reported in Table 5.
Miltner et al. (1989) conducted monitoring at full-scale treatment plants and reported 94% removal of low levels of metribuzin in the post-filtration chlorination step. These results are supported by bench-scale studies that provide further evidence that metribuzin can be effectively removed using chlorine oxidation (Miltner et al., 1989; Chamberlain et al., 2012; Hu et al., 2017). Hu et al. (2017) studied the reaction of chlorine and metribuzin (initial concentration of 1000 µg/L) under varying conditions, including chlorine dose, pH, and bromide and ammonium concentrations. The authors noted that degradation rates increased with increasing temperature, decreasing pH, increasing bromide concentration, and decreasing ammonium concentration.
Bench-scale testing conducted with other oxidants including ClO2, MnO4- and O3 has reported moderate to high removal of low levels of metribuzin (Miltner et al., 1989; Chamberlain et al., 2012). Chamberlain et al. (2012) reported greater than 50% removal of 1.5 to 3 µg/L of metribuzin using a ClO2 dose and contact time that are typical for drinking water disinfection. MnO4- and O3 removals ranged from 20% to greater than 50% depending on the pH. The same study reported that oxidation with monochloramine and ultraviolet light was not effective for removal of metribuzin.
Overall, these studies demonstrate that depending on the influent metribuzin concentration and other water quality parameters, drinking water treatment plants using chlorination and other oxidants (e.g., ClO2, O3, MnO4-) are capable of lowering metribuzin concentrations below the MAC during typical treatment plant operations (Miltner et al., 1989; Chamberlain et al., 2012).
When using oxidation processes for pesticide removal in drinking water, it is important to be aware of the potential formation of by-products due to degradation of the target compound (Ikehata and Gamal El-Din, 2006; Chamberlain et al., 2012). Removal of the target pesticide alone does not ensure that the treatment is efficient and that full mineralization (to carbon dioxide, inorganic ions and water) has been achieved. In addition, water utilities should consider the potential formation of disinfection by-products based on the oxidant selected and the source water quality. Several authors have reported the presence of potential degradation products from the oxidation of metribuzin. Miltner et al. (1989) reported the presence of additional chromatographic peaks following chlorine oxidation of metribuzin (degradation by-products were not identified). Hu et al. (2017) reported that chloroform, 1,1,1-trichloroacetone, and dichloroacetonitrile were formed (less than 1% molar yield) following chlorination of metribuzin. To examine whether the target pesticides were degraded and mineralized, several authors have monitored the decline of organic carbon content (either total or dissolved) and/or chemical oxygen demand (COD) (Ikehata and Gamal El-Din, 2006; Beduk et al., 2012; Li et al., 2019). Pilot-scale testing is an important step for water utilities considering oxidation processes for pesticide removal in drinking water.
| Oxidant | Influent (µg/L) |
Oxidant Dose (mg/L) |
Removal (%) |
Process Description | Reference |
|---|---|---|---|---|---|
| Chlorine | 2.41 | 2.95 | 94 | Full-scale: Conventional surface water treatment. Chlorine applied post filtration (Chlorine dose: 2.95 mg/L; residual: 2.23 mg/L). | Miltner et al. (1989)Footnote a |
| 60.1 | 3.1 | 96 | Bench-scale: Chlorine residual: 1.8 mg/L following 6 hr reaction time. | ||
| 1.5–3 | 2–5 | 20–50 | Bench-scale: Experiments conducted under typical surface water disinfection conditions: CT: 107–173 mg·min/L; pH 6.6 and 8.6 | Chamberlain et al. (2012)Footnote b | |
| Chlorine dioxide | 60.1 | 5.9 | 100 | Bench-scale: Chlorine residual: 3.8 mg/L following 6 hr reaction time. | Miltner et al. (1989) |
| 34.4 | 2.0 | 60 | Bench-scale: Chlorine residual: 0.4 mg/L following 2 hr reaction time. | ||
| 1.5–3 | 2–3 | > 50 | Bench-scale: CT: 38–73 mg·min/L; pH 8.6 | Chamberlain et al. (2012)Footnote b | |
| Ozone | 1.5–3 | 1–2 | > 50 | Bench-scale: CT: 0.2–0.3 mg·min/L; pH 6.6 | Chamberlain et al. (2012)Footnote b |
| Permanganate | 1.5–3 | 3–5 | 20–50 | Bench-scale: CT: 134–64 mg·min/L; pH 8.6 | Chamberlain et al. (2012)Footnote b |
|
|||||
4.2.1.3 Activated carbon adsorption
Activated carbon adsorption is widely used to reduce the concentration of micropollutants, including pesticides, in drinking water (Haist-Gulde and Happel, 2012; van der Aa et al., 2012). Activated carbon can be applied in two ways: slurry applications using powdered activated carbon or fixed bed reactors with granular activated carbon (Chowdhury et al., 2013).
Although there is limited full-scale data published regarding the use of activated carbon specifically for metribuzin adsorption, it is expected to be effective for metribuzin removal based on its physical-chemical properties (e.g., solubility, molecular size, polarity) and published research on adsorption capacity. Data generated through bench-scale testing to determine adsorption coefficients for pesticides are useful in predicting whether activated carbon adsorbs a particular pesticide (US EPA, 2011). In general, pesticides with an adsorption capacity constant (e.g., Freundlich coefficient) greater than 200 µg/g (L/µg)1/n are considered to be amenable to removal by carbon adsorption (Speth and Miltner, 1989; Speth and Adams, 1993; US EPA, 2011). However, it is important to note that the presence of natural organic matter (NOM) adds complexity to activated carbon treatment because NOM competes directly for adsorption sites or fouls the carbon by blocking pores (Chowdhury et al., 2013). Since the adsorption capacity of activated carbon can be affected by many factors, including the compound's ionic character and the solution pH, appropriate testing (e.g., jar tests, rapid small scale column tests) should be conducted to confirm removal.
Powdered activated carbon
Many pesticides have been found to strongly adsorb to powdered activated carbon (PAC) (Chowdhury et al., 2013). The use of PAC offers the advantage of providing virgin carbon when required (e.g., during the pesticide application season) (Miltner et al., 1989). The removal efficiency of PAC depends on the PAC type and dose, the contact time, the PAC characteristics (type, particle size), the adsorbability of the contaminant, and the presence of NOM (Gustafson et al., 2003; Summers et al., 2010; Haist-Gulde and Happel, 2012; Chowdhury et al., 2013).
Miltner et al. (1989) compiled data from a full-scale PAC application and jar tests to estimate PAC capacity versus pesticide concentration and calculated a Freundlich adsorption coefficient of 61.7 mg/g(L/mg)1/n (61 700 µg/g(L/µg)1/n) in a natural river water. In this study, higher or similar adsorption of metribuzin compared with similar pesticides such as atrazine was observed. The authors concluded that PAC applied at dosages typically used for taste and odour control would also be sufficient for removal of several pesticides (including metribuzin) if moderate percent removal is required. Similarly, Frank et al. (1991) reported that a seasonal PAC dose of 5 mg/L completely removed metribuzin (mean influent concentration of 1.7 µg/L) from a surface water supply.
Granular activated carbon
The use of granular activated carbon (GAC) is an effective approach for treating organic contaminants that are regularly found in source water at concentrations of concern (Chowdhury et al., 2013). The capacity of GAC to remove pesticides by adsorption depends on the filter velocity, empty bed contact time (EBCT), the GAC characteristics (type, particle size, reactivation method), the adsorbability of the contaminant, and the filter run time (Haist-Gulde and Happel, 2012). In addition, because GAC fixed-bed adsorbers are typically operated on a continuous basis, the GAC can become fouled (or preloaded) with NOM and may be partially ineffective for metribuzin removal (Knappe et al., 1999; Summers et al., 2010; Haist-Gulde and Happel, 2012; Chowdhury et al., 2013). Miltner et al. (1989) evaluated metribuzin removal in a full-scale sand-replacement GAC bed. The GAC bed was 18 inches deep, 30 months old and had an EBCT of 2.81 minutes at a loading rate of 4 gpm/ft2 (9.8 m/hr). The mean influent metribuzin concentration was 0.89 µg/L and the mean removal was 57% over a period of 11 sample days. This removal efficiency was similar to those observed for other triazine herbicides, including atrazine, cyanazine and simazine.
4.2.1.4 Membrane filtration
In general, nanofiltration (NF) and reverse osmosis (RO) are effective pressure-driven membrane processes for the removal of pesticides from drinking water (Van der Bruggen and Vandecasteele, 2003; US EPA, 2011). The effectiveness of RO and NF for pesticide removal is dependent on the membrane characteristics, pesticide properties, feed water composition, operating conditions and membrane fouling (Van der Bruggen and Vandecasteele, 2003; Plakas and Karabelas, 2012).
Since the main mechanism for pesticide removal using NF and RO membranes is size exclusion, the molecular weight cut-off (MWCO) of the membrane is an important characteristic. Bellona et al., (2004) present a flowchart using the parameters of the pesticide in water (e.g., molecular weight, log Kow, molecular diameter) and those of the membrane (e.g., MWCO, pore size) to determine the potential for removal. This chart could aid in the choice of a possible membrane for metribuzin removal. Based on the molecular weight of metribuzin (217 Da), membranes with a MWCO of between 200 and 400 Da is considered appropriate for metribuzin. In addition to the sieving effect, retention of small pesticide molecules by larger pore-size membranes can be influenced by the physicochemical interactions between the pesticide and the membrane surface (Plakas and Karabelas, 2012).
| Influent (µg/L) | Rejection | Membrane Type | Process Description | Reference |
|---|---|---|---|---|
| 10 | 87% – 97% | Polyamide NF | Bench-scale: groundwater (total alkalinity 120 mg/L as CaCO3). Membrane MWCO of 300 Da. Test conditions: Spiral wound configuration, 15 or 50% recovery; flux 10 or 15 gsfd. |
Chen et al. (2004) |
| 4.5 | 85% | Cellulose-acetate RO | Bench-scale: surface water pre-treated with coagulation, sedimentation, filtration, and ultra-filtration. Test conditions: All membranes in 4x40 spiral wound configuration, 9% recovery. At 80% recovery, initial concentration of 1.4 µg/L and composite polyamide RO: 93% rejection estimated from modelling data. |
Hofman et al. (1997)Footnote a |
| 98% | Composite polyamide RO | |||
| 99% | Ultra-low pressure RO | |||
| 2.53 | 59% | Cellulose -acetate RO | Bench-scale: surface water pre-treated with coagulation, sedimentation, filtration. Test conditions: Pressure: 150–200 psi, 9% – 13% recovery, permeate flux: 7.58 – 23 gal/min. |
Fronk and Baker (1990)Footnote a |
| 76% | Polyamide RO | |||
| 100% | Thin film composite RO | |||
|
||||
Bench-scale studies have shown that RO and NF are effective for the removal of metribuzin from drinking water (Fronk and Baker, 1990; Hofman et al., 1997; Chen et al., 2004). Studies using a variety of membrane types and operating conditions for metribuzin removal are reported in Table 6. These data demonstrate that rejections of metribuzin ranging from 59% to 99% can be achieved. In general, thin-film composite and ultra-low pressure RO membranes achieved the highest rejection (99% to 100%) of metribuzin (Fronk and Baker, 1990; Hofman et al., 1997). Chen et al. (2004) found that rejection of metribuzin using a spiral-wound polyamide NF membrane was also effective (up to 97%) under optimum conditions (e.g., high flux and low recovery).
4.2.2 Residential-scale treatment
In cases where metribuzin removal is desired at the household level–for example, when a household obtains its drinking water from a private well–a residential drinking water treatment unit may be an option for decreasing metribuzin concentrations in drinking water. Before a treatment unit is installed, the water should be tested to determine the general water chemistry and metribuzin concentration in the source water. To verify that a treatment unit is effective, water entering and leaving the treatment unit should be sampled periodically and submitted to an accredited laboratory for analysis. Units can lose removal capacity through use and time and need to be maintained and/or replaced. Consumers should verify the expected longevity of the components in the treatment unit according to the manufacturer's recommendations and service it when required.
Health Canada does not recommend specific brands of drinking water treatment units, but strongly recommends that consumers use units that have been certified by an accredited certification body as meeting the appropriate NSF International/American National Standards Institute (NSF/ANSI) standards for drinking water treatment units. The purpose of these standards is to establish minimum requirements for the materials, design and construction of drinking water treatment units. This ensures that materials in the unit do not leach contaminants into the drinking water (i.e., material safety). In addition, the standards include performance requirements that specify the removal that must be achieved for specific contaminants (i.e., reduction claim) that may be present in water. Certification organizations provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada (SCC). Accredited organizations in Canada (SCC, 2019) include:
- CSA Group
- NSF International
- Water Quality Association
- UL LLC
- Bureau de normalisation du Québec (available in French only)
- International Association of Plumbing and Mechanical Officials
- Truesdail Laboratories, Inc.
An up-to-date list of accredited certification organizations can be obtained from the SCC.
The drinking water treatment technologies that are expected to be effective for metribuzin removal at the residential-scale include:
- reverse osmosis (RO)
- adsorption
Currently, metribuzin is not included in the performance requirements of NSF/ANSI standards. However, consumers can use a treatment unit that is certified to the standards for reverse osmosis or adsorption to ensure that the material safety has been tested. These standards are NSF/ANSI Standard 58 - Reverse Osmosis Drinking Water Treatment Systems and NSF/ANSI Standard 53 - Drinking Water Treatment Units - Health Effects (NSF/ANSI, 2018a, 2018b). In addition, units that have been certified for the removal of a similar pesticide, such as atrazine, are more likely to be effective for the removal of metribuzin.
Water that has been treated using reverse osmosis may be corrosive to internal plumbing components. Therefore, these units should be installed only at the point of use. Also, as large quantities of influent water are needed to obtain the required volume of treated water, these units are generally not practical for point-of-entry installation.
5.0 Management strategies
All water utilities should implement a risk management approach, such as the source-to-tap or water safety plan approach, to ensure drinking water safety (CCME, 2004; WHO, 2011, 2012). These approaches require a system assessment to characterize the source water, describe the treatment barriers that prevent or reduce contamination, identify the conditions that can result in contamination, and implement control measures. Operational monitoring is then established, and operational/management protocols are instituted (e.g., standard operating procedures, corrective actions and incident responses). Compliance monitoring is determined and other protocols to validate the water safety plan are implemented (e.g., record keeping, consumer satisfaction). Operator training is also required to ensure the effectiveness of the water safety plan at all times (Smeets et al., 2009).
5.1 Monitoring
Metribuzin can be present in groundwater and surface water in areas where it is being used depending on the type and extent of its application, environmental factors (e.g., amount of precipitation, soil type, hydrogeological setting) and environmental fate (e.g., mobility, leaching potential, degradation) in the surrounding area. Water utilities should consider the potential for metribuzin to enter source water (e.g., raw water supply to the drinking water system) based on site-specific considerations.
When it is determined that metribuzin may be present and monitoring is necessary, surface and groundwater sources should be characterized to determine the concentration of metribuzin. This should include monitoring of surface water sources during periods of peak use and rainfall events and/or monitoring of groundwater annually. Where baseline data indicate that metribuzin is not present in source water, monitoring may be reduced.
Where treatment is required to remove metribuzin, operational monitoring should be implemented to confirm whether the treatment process is functioning as required. The frequency of operational monitoring will depend on the water quality, fluctuations of the raw water concentrations and the treatment process. Responsible authorities should be aware of the impact of natural organic matter on activated carbon systems, as it may impact water quality objectives for metribuzin removal.
Where treatment is in place for metribuzin removal, compliance monitoring (i.e., paired samples of source and treated water to confirm the efficacy of treatment) should be conducted at a minimum on an annual basis. When routine operational monitoring indicates the potential for contaminant breakthrough, such as with GAC, monitoring should be conducted quarterly. When a degradation process, like oxidation, is utilized, monitoring of by-product formation should also be considered.
6.0 International considerations
This section presents drinking water guidelines, standards and/or guidance from other national and international organizations. Variations in these values can be attributed to the age of the assessments or to differing policies and approaches, including the choice of key study and the use of different consumption rates, body weights and source allocation factors (Table 7).
Australia's National Health and Medical Research Council has set a guideline value of 0.07 mg/L for metribuzin in drinking water based on a NOEL of 2 mg/kg bw per day for decreased heart weights from a 2-year rat study (NHMRC, NRMMC, 2011). The US EPA does not have a maximum contaminant level (MCL) for metribuzin in drinking water (the MCL is the equivalent of the MAC). The US EPA states that it concluded that regulation of metribuzin in drinking water would have little impact on human health risk reduction as its occurrence in public water systems and the number of people potentially exposed through drinking water are low (US EPA, 2003). The US EPA has established a non-enforceable health advisory of 0.07 mg/L. Health advisories serve as the informal technical guidance for unregulated drinking water contaminants in the United States (US EPA, 1987). The World Health Organization (WHO) has not set a specific guideline value for metribuzin.
The European Union (EU) does not have a specific chemical parametric value for individual pesticides. Instead, it has established a value of 0.1 µg/L for any individual (single) pesticide and a value of 0.5 µg/L for total pesticides found in drinking water. In establishing these values, the EU notes that it did not consider the science related to each pesticide, such as health effects. Rather, the values are based on a policy decision to keep pesticides out of drinking water (EU, 1998).
| Agency (Year) Reference |
Value (mg/L) | Key Endpoint (Reference) | NOAEL (mg/kg bw/d) | UF | ADI (mg/kg bw per day) | BW (kg) | DW Intake(L/d) | AF (%) | Comments |
|---|---|---|---|---|---|---|---|---|---|
| HC – MAC (2020) | 0.08 | Liver effects in dogs (Löser and Mirea, 1974) |
0.83 | 100 | 0.0083 | 74 | 1.53 | 20 | None |
| US EPA (1987) | 0.07 (non-regulatory lifetime HA) |
Kidney effects and changes in clinical chemistry in dogs (Löser and Mirea,1974) | 2.5 | 100 | 0.025 | 70 | 2 | 20 | Health advisories are not legally enforced standards |
| Australia (2011) | 0.07 | Decreased heart weights in rats from a 2-year study | 2 (NOEL) | 100 | 0.02 | 70 | 2 | 10 | No reference is given for the key study |
| EU (1998) | 0.1 µg/L | The EU has a value of 0.1 µg/L for any individual (single) pesticide, and a value of 0.5 µg/L for total pesticides found in drinking water. In establishing these values, the EU did not consider the science related to each pesticide, including health effects. Instead, the values are based on a policy decision to keep pesticides out of drinking water. | |||||||
|
ADI = acceptable daily intake; AF = allocation factor; BW = body weight; DW = drinking water; HA = health advisory; NOAEL = no-observed-adverse-effect level; NOEL = no-observed-effect-level; UF = uncertainty factor | |||||||||
7.0 Rationale
Metribuzin is registered in Canada to control broadleaf weeds and grasses in agriculture. Despite its common use in Canada, data provided by provinces and territories that monitor for metribuzin in source and drinking water indicate that when detected, metribuzin levels are well below the MAC. The liver is considered the target organ for metribuzin toxicity. Although no human studies have investigated the effects of metribuzin on the liver, animal studies conducted in several species (rats, mice, rabbits and dogs) have consistently shown liver toxicity following metribuzin exposure.
Health Canada, in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water, is reaffirming a MAC of 0.08 mg/L (80 µg/L) based on the following considerations:
- An HBV of 0.08 mg/L (80 µg/L) based on liver effects in beagle dogs;
- Metribuzin can be accurately measured at concentrations well below the MAC; and
- Drinking water treatment technologies are available to remove metribuzin to below the MAC.
The MAC is protective of potential health effects from metribuzin exposure. As part of its ongoing guideline review process, Health Canada will continue to monitor new research in this area, including the outcomes of PMRA's evaluations, and will recommend any change to this guideline technical document that it deems necessary.
8.0 References
- Alavanja, M.C.R., Saminic, C., Dosemeci, M.., Lubin, J., Tarone, R., Lynch, C.F., Knott, C., Thomas, K., Hoppin, J.A., Barker, J., Coble, J., Sandler, D.P. and Blair, A. (2003). Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. Am. J. Epidemiol., 157(9): 800-814.
- Andreotti, G., Beane Freeman, L.E., Hou, L., Coble, J. Rusiecky, J., Hoppin, J.A., Silverman, D.T., Alavanja, M.C.R. (2009). Agricultural pesticide use and pancreatic cancer risk in the Agricultural Health Study cohort. Int. J. Cancer, 124: 2495-2500.
- Bastien, C., and Madramootoo, C.A. (1992). Presence of pesticides in agricultural runoff from two potato fields in Québec. Can. Water Resour. J., 17(3): 200-212.
- Baychem (1972). The metabolic fate of carbonyl C-14 SENCOR in dogs. Chemagro Division of Baychem Corp. Report No. 33361 (as cited in OEHHA, 2001).
- Beduk, F., Aydin,M.E. and Ozcan, S. (2012). Degradation of malathion and parathion by ozonation, photolytic ozonation, and heterogeneous catalytic ozonation processes. Clean - Soil, Air, Water, 40(2): 179-87.
- Bellona, C., Drewes, J.E., Xu, P. and Amy, G. (2004). Factors affecting the rejection of organic solutes during NF/RO treatment - a literature review. Water Res., 19(3): 2795-2809.
- Bleeke, M.S., Smith, M.T. and Casida, J.E. (1985). Metabolism and toxicity of metribuzin in mouse liver. Pestic. Biochem. Physiol., 23: 123-130.
- Blomquist, J., Denis, J., Cowles, J., Hetrik, J., Jones, D. and Birchfield, N. (2001). Pesticides in selected water supply reservoirs and finished drinking water, 1999-2000. Summary of results from a pilot monitoring program. US Geological Survey open file report: 01-456.
- Bowman, B.T. (1991). Mobility and dissipation studies of metribuzin, atrazine and their metabolites in plainfield sand using field lysimeters. Environ. Toxicol. Chem., 10: 573-579.
- Bray, L.D., Szarka, A.Z., Heard, N.E., Hackett, D.S. and Kahrs, R.A. (2008). Chapter 27 - Dietary exposure assessment of the triazine herbicides. In: Lebaron, H.M., McFarland, J.E. and Burnside, O.C. (Eds.). The triazine herbicides. Amsterdam: Elsevier, 2008. pp. 413-423.
- Breckenridge, C.B., Werner, C., Stevens, J.T. and Sumner, D.D. (2008). Chapter 25 - Hazard assessment for selected symmetrical and asymmetrical triazine herbicides. In: Lebaron, H.M., McFarland, J.E. and Burnside, O.C. (Eds.). The triazine herbicides. Amsterdam: Elsevier, 2008. pp. 387-398.
- British Columbia Ministry of Health (2019). Personal communication with D. Fishwick.
- Cain, K., Hanlon, C. and Lane, J. (1987). The excretion and metabolism of Sencor by rats: Lab Project ID: Mobay No. 94605. Unpublished study prepared by Mobay Corp. 62 p. (as cited in US EPA, 1998).
- CCME (1999). Canadian water quality guidelines for the protection of aquatic life: Metribuzin. In: Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, Winnipeg, Manitoba.
- CCME (2004). From source to tap: Guidance on the multi-barrier approach to safe drinking water. Canadian Council of Ministers of the Environment, Winnipeg, Manitoba.
- Chaisson, C.F. and Cueto, C. (1970). 90-day subacute oral toxicity study of BAY 94337 in beagle dogs. Submitted by Mobay Chemical Corp. Kansas City, MO; MRID 00106162 (as cited in US EPA, 2003).
- Chamberlain, E., Shi, H., Wang, T., Ma, Y., Fulmer, A. and Adams, C. (2012). Comprehensive screening study of pesticide degradation via oxidation and hydrolysis. J. Agric. Food Chem., 60(1): 354-363.
- Chen, S., Taylor, J.S., Mulford, L.A. and Norris, C.D. (2004). Influences of molecular weight, molecular size, flux, and recovery for aromatic pesticide removal by nanofiltration membranes. Desalination 160: 103-111.
- Chiali, F.Z., Merzouk, H., Merzouk, S.A., Medjdoub, A. and Narce, M. (2013). Chronic low level metribuzin exposure induces metabolic alterations in rats. Pestic. Biochem. Physiol., 106(1-2): 38-44.
- Chowdhury, Z., Traviglia, A., Carter, J., Brown, T., Summers, R.S., Corwin, C.J., Zearley, T., Thurman, M., Ferrara, I., Olson, J., Thacker, R. and Barron, P. (2010). Cost-effective regulatory compliance with GAC biofilters. Water Research Foundation, Denver, Colorado.
- Chowdhury, Z.K., Summers, R.S., Westerhoff, G.P., Leto, B.J., Nowack, K.O. and Corwin, C.J. (2013). Activated carbon: Solutions for improving water quality. Passantino, L. B.(ed.). American Water Works Association. Denver, Colorado.
- Clemens, G. and Hartnagel, R. (1989). Teratology study in the rabbit with Sencor Technical (Metribuzin): Project ID 99654. Unpublished study prepared by Miles, Inc. 131 p. (as cited in US EPA, 1998, 2003).
- Crawford, C. and Anderson, R. (1972). The acute dermal toxicity of Sencor Technical and Sencor 50% Wettable Powder to rats and rabbits. Report No. 33123. Unpublished study submitted by Mobay Chemical Corp., Kansas City, MO (as cited in US EPA, 1998).
- Christenson, W. and Wahle, B. (1993) Technical grade metribuzin (Sencor): A combined chronic
toxicity/oncogenicity feeding toxicity study in the rat: Lab project number: 88-271-BM: 103970.
Unpublished study prepared by Miles Inc. 4593 p. (as cited in US EPA, 1998). - DeLancey, J.O.L, Alavanja, M.C.R., Coble, J., Blair, A., Hoppin, J.A., Austin, H.D. and Beane Freeman, L.E. (2009). Occupational exposure to metribuzin and the incidence of cancer in the Agricultural Health Study. Ann. Epidemiol., 19(6): 388-395.
- De Roos, A.J., Zahm, S.H., Cantor, K.P.., Weisenburger, D.D., Holmes, F.F., Burmeister, L.F. and Blair, A. (2003). Integrative assessment of multiple pesticides as risk factors for non-Hodgkin's lymphoma among men. Occup. Environ. Med., 60(9): e11. Available at http://www.occenvmed.com/cgi/content/full/60/9/e11.
- EFSA (2010). Conclusion regarding the peer review of the pesticide risk assessment of the active substance - metribuzin. EFSA Scientific Report 88:1-74. Available at www.efsa.europa.eu.
- EFSA (2018). Renewal assessment report under Regulation (EC) 1107/2009. Metribuzin. Volume 3 - B.6 (AS): Toxicology and metabolism data. Prepared by Rapporteur Member State Estonia and Co-rapporteur Member State Germany.
- Environment Canada (2011). Presence and levels of priority pesticides in selected Canadian aquatic ecosystems. En14-40/2011E-PDF. Water Science and Technology Directorate, Environment Canada, Ottawa, Ontario.
- EU (1998). European Union Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Official Journal of the European Communities, L 330, 5/12/1998: 32-54.
- Flores-García, M.E., Molina-Morales, Y., Balza-Quintero, A., Benítez-Díaz, P.R. and Miranda-Contreras, L. (2011). Pesticide residues in drinking water of an agricultural community in the state of Mérida, Venezuela. Invest. Clin., 52(4): 295-311.
- Flucke, W. and Hartmann, E. (1989). DIC 1468. Technical grade: (Common name: Metribuzin): Subacute dermal toxicity study in rabbits. Unpublished study. Prepared by Bayer. MRID 43970701 (as cited in US EPA1998, 2003).
Frank, R., Clegg, B.S., Sherman, C., and Chapman, N.D. (1990). Contamination of triazine and chloroacetamide herbicides in Sydenham river water and municipal drinking water, Dresden, Ontario, Canada, 1981-1987. Arch. Environ. Contam. Toxicol., 19(3): 319-324. - Frank, R., Logan, L. and Clegg, B.S. (1991). Pesticide and polychlorinated biphenyl residues in waters at the mouth of the Grand, Saugeen, and Thames rivers, Ontario, Canada, 1986-1990. Arch. Environ. Contam. Toxicol., 21: 585-595.
- Fronk, C.A. and Baker, D. (1990). Pesticide removal by membrane processes. In: Proceedings of the American Water Works Association Water Annual Conference Cincinnati, Ohio. American Water Works Association, Denver, Colorado.
- Goldner, W.S., Sandler, D.P.., Yu, F., Shostrom, V., Hoppin, J.A., Kamel, F. and LeVan, T.D. (2013). Hypothyroidism and pesticide use among male private pesticide applicators in the Agricultural Health Study. J. Occup. Environ. Med., 55(10): 1171-1178.
- Government of Ontario (2019). Drinking Water Surveillance Program, available at https://www.ontario.ca/data/drinking-water-surveillance-program.
- Gustafson, D.K., Carr, K.H., Carson, D.B., Fuhrman, J.D., Hackett, A.G., Hoogheem, T.J., Snoeyink, V.L., Curry, M., Heijman, B., Chen, S., Herti, P. and van Wesenbeeck, I. (2003). Activated carbon adsorption of chloroacetanilide herbicides and their degradation products from surface water supplies. J. Water Supply Res. Technol. AQUA, 52(6): 443-454.
- Haist-Gulde, B. and Happel, O. (2012). Removal of pesticides and their ionic degradates by adsorptive processes. Water Research Foundation, Denver, Colorado.
- Haiste-Gulde, B. and Sacher, F. (2019). Personal communication, TZW German Water Centre, Karlsruhe, Germany.
- Hayes, R.H. (1981). Metribuzin (Sencor®) oncogenicity study in mice. Unpublished study. Submitted by Mobay Chemical Corp., Kansas City, MO; MRID 00087795 (as cited in US EPA, 1998, 2003).
- Health Canada (2005). Proposed Re-evaluation Decision. PACR2005-07. Re-evaluation of metribuzin. Pest Management Regulatory Agency (PMRA), Health Canada, Ottawa, Ontario.
- Health Canada (2006). Re-evaluation Decision Document. RRD2006-15. Metribuzin. Pest Management Regulatory Agency (PMRA); Health Canada, Ottawa, Ontario.
- Health Canada (2015). Food consumption table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa 2015.
- Health Canada (2017). Water consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa 2015.
- Health Canada (2019a). Personal communication from Health Evaluation Directorate, Pest Management Regulatory Agency (PMRA).
- Health Canada (2019b). Personal communication from the Health Evaluation Directorate, Pest Management Regulatory Agency (PMRA).
- Health Canada (2020a). Pest control products sales report for 2018. PMRA, Health Canada, Ottawa, Ontario.
Health Canada (2020b). Personal communication from Health Evaluation Directorate, Pest Management Regulatory Agency (PMRA). - Hofman, J.A.M.H., Beerendonk, E.F., Folmer, H.C. and Kruithoff, J.C. (1997). Removal of pesticides and other micropollutants with cellulose-acetate, polyamide and ultra-low pressure reverse osmosis membranes. Desalination, 113: 209-214.
- Hoppin, J.A., Umbach, D.M., London, S.J., Alavanja, M.C.R. and Sandler, D.P. (2002). Chemical predictors of wheeze among farmer pesticide applicators in the Agricultural Health Study. Am. J. Respir. Crit. Care Med., 165(5): 683-689.
- Hoppin, J.A., Umbach, D.M., Long, S., London, S.J., Henneberger, P.K., Blair, A., Alavanja, M.C.R., Beane Freeman, L.E. and Sandler, D.P. (2017). Pesticides are associated with allergic and non-allergic wheeze among male farmers. Environ. Health Perspect., 125(4): 535-543.
- Hu, C., Li, A., Lin, Y., Ling, X. and Cheng, M. (2017). Degradation kinetics and DBP formation during chlorination of metribuzin. J. Taiwan Inst. Chem. Eng., 80: 1-7.
- Ikehata, K. and Gamal El-Din, M.G. (2006). Aqueous pesticide degradation by hydrogen peroxide/ultraviolet irradiation and fenton-type advanced oxidation processes: a review. J. Environ. Eng. Sc., 5 (2): 81-135.
- Indigenous Services Canada (2019). Personal communication with X. Redhead.
- Inukai, H. and Iyatomi, A. (1977). Bay 94337: Mutagenicity test on bacterial systems: Report No. 67; 54127. Unpublished study received Nov 3, 1981 under 3125-270; prepared by Nitokuno Agricultural Chemicals Institute, Japan, submitted by Mobay Chemical Corp., Kansas City, MO; CDL:246226-F (as cited in US EPA, 1998).
- Kamel, F., Tanner, C.M., Umbach, D.M., Hoppin, J.A., Alavanja, M.C.R., Blair, A., Comyns, K., Goldman, S.M., Korell, M., Langston, J.W., Ross, G.W. and Sandler, D.P. (2006). Pesticide exposure and self-reported Parkinson's disease in the Agricultural Health Study. Am. J. Epidemiol., 165(4): 364-374.
- Knappe, D.R.U., Snoeyink, V.L., Roche, P., Prados, M.J. and Bourbigot, M-M. (1999). Atrazine removal by preloaded GAC. J. Am. Water Works Assoc., 91(10): 97-109.
- Kowaski, R.L., Clemens, G.R., Bare, J. J. and Hartnagel, Jr., R.E. (1986). A teratology study with Sencor Technical (Metribuzin) in the rat: 91330. Unpublished report to the EPA by Miles Laboratories, Inc. MRID 00163802 (as cited in US EPA, 2003).
- Krishnan, K. and Carrier, R. (2013). The use of exposure source allocation factor in the risk assessment of drinking-water contaminants. J. Toxicol. Environ. Health B, 16(1): 39-51.
- Lawrence, J.R., Eldan, M. and Sonzogni, W.C. (1993). Metribuzin and metabolites in Wisconsin (U.S.A.) well water. Wat. Res., 27(8): 1263-1268.
- Lee, W.J., Colt, J.S., Heineman, E.F., McComb, R., Weisenburger, D.D., Lijinsky, W. and Ward, M.H. (2005). Agricultural pesticide use and risk of glioma in Nebraska, United States. Occup. Environ. Med., 62: 786-792.
- Leon, M.E., Schinasi, L.H., Lebailly, P., Beane Freeman, L.E., Nordby, K.-C., Ferro, G., Monnereau, A., Brouwer, M., Tual, S., Baldi, I., Kjaerheim, K., Hofmann, J.N., Kristensen, P., Koutros, S., Straif, K., Kromhout, H. and Schüz, J. (2019). Pesticide use and risk of non-Hodgkin lymphoid malignancies in agricultural cohorts from France, Norway and the USA: a pooled analysis from the AGRICOH consortium. Int. J. Epidemiol., dyz017. Available at https://doi.org/10.1093/ije/dyz017.
- Li, W., Zhao, Y., Yan, X., Duan, J., Saint, C.P. and Beechan, S. (2019). Transformation pathway and toxicity assessment of malathion in aqueous solution during UV photolysis and photocatalysis. Chemosphere, 234: 204-214.
- Löser, E., Mawdesley-Thomas, L.E. and Lorke, D. (1969). Bay 94337. Subchronic toxicological studies on rats (three-month feeding experiment). Submitted by Mobay Chemical Corp., Kansas City, MO (MRID 00106161) (as cited in US EPA, 2003).
- Löser, E. and Kimmerle, G. (1972). Acute and subchronic toxicity of®Sencor active ingredient. Pflanzenschutz-Nachrichten, 25(2): 186-209.
- Löser, E. and Mirea, D. (1974). Bay 94337: Chronic toxicity studies on dogs (two-year feeding experiment): Report No. 4887; Report No. 41814. Unpublished study submitted by Mobay Chemical Corp., Kansas City, MO. (MRID 00061260) (as cited in US EPA, 1998, 2003; Health Canada, 2019).
- Löser, E. and Mohr, U. (1974). Bay 94337: Chronic toxicity studies on rats (two-year feeding experiment): Report No. 4888; Report No. 41816. (Unpublished study received on unknown date under 5F1559; prepared by Bayer, AG and Medizinische Hoschule Hannover, submitted by Mobay Chemical Corp., Kansas City, MO; CDL:094259-A) (as cited in US EPA, 1998).
- Löser, E. and Siegmund, F. (1974). Bay 94337. Multigeneration study on rats: Report no. 4889; Report No. 41818. Unpublished study. (MRID 00061262) (as cited in US EPA, 2003).
- Machemer, L. (1972). Sencor (Bay 94 337) Studies for possible embryotoxic and teratogenic effects on rats after oral administration (Bay Report No. 3678). Submitted by Mobay Chemical Corp., Kansas City, MO (MRID 00061257) (as cited in US EPA, 2003).
- Machemer, L. and Lorke, D. (1974a). Evaluation of®Sencor for mutagenic effects on the mouse: Report No. 4942; 43068. (Unpublished study received Nov 3, 1981 under 3125-270; prepared by Bayer AG, West Germany, submitted by Mobay Chemical Corp., Kansas City, MO; CDL:246226-B) (as cited in US EPA, 1998).
- Machemer, L. and Lorke, D. (1974b). Evaluation of the mutagenic potential of®Sencor in an in vivo cytogenetic study on spermatogonia of Chinese hamster: Report No. 4961; 43067. (Unpublished study received Nov 3, 1981 under 3125-270; prepared by Bayer AG, West Germany, submitted by Mobay Chemical Corp., Kansas City, MO; CDL:246226-A) (as cited in US EPA, 1998).
- Manitoba Sustainable Development (2019). Water Quality Management Section, Winnipeg, Manitoba. Personal communication with K. Philip, Office of Drinking Water.
- Mathew, R., Kacew, S. and Khan, S.U. (1998). Bioavailability in rats of bound pesticide residues from tolerant or susceptible varieties of soybean and canola treated with metribuzin or atrazine. Chemosphere, 36(3): 589-596.
- Miltner, R.J., Baker, D.B., Speth, T.F. and Fronk, C.A. (1989). Treatment of seasonal pesticides in surface waters. J. Am. Water Works Assoc., 81: 43-52.
- Ministère du Développement durable, de l'Environnement et de la Lutte contre les changements climatiques du Quebec (2019). Personal communication with P. Cantin, Direction de l'eau potable et des eaux souterraines.
- Murli, H. (1990). Mutagenicity test on Sencor Technical in an in vitro cytogenetic assay measuring chromosomal aberration frequencies in Chinese hamster ovary (CHO) cells: Lab project number: 10857-0-437. Unpublished study prepared by Hazleton Labs America, Inc. 55 p. (as cited in US EPA, 1998 and 2003).
- New Brunswick Department of Environment and Local Government (2019). Personal communication with K. Gould, Healthy Environments Branch.
- Newfoundland and Labrador Department of Municipal Affairs and Environment (2019). Personal communication with H. Khan, Water Resources Management Division.
- NHMRC, NRMMC (2011). Australian Drinking Water Guidelines Paper 6 National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra. Available at https://Www.Nhmrc.Gov.Au/about-Us/Publications/Australian-Drinking-Water-Guidelines.
- Nova Scotia Environment (2019). Personal communication with A. Polegato, Drinking Water Management Unit.
NSF/ANSI (2018a). NSF International/American National Standards Institute Standard 53: Drinking water treatment units-health effects. NSF International, Ann Arbor, Michigan. - NSF/ANSI (2018b). NSF International/American National Standards Institute Standard 58: Reverse osmosis drinking water treatment systems. NSF International, Ann Arbor, Michigan.
- OEHHA (2001). Evidence on the developmental and reproductive toxicity of metribuzin. Draft, October, 2001. California Environmental Protection Agency, Office of Environmental Health Hazard Assessment, Reproductive and Cancer Hazard Assessment Section. Available at https://oehha.ca.gov/proposition-65/chemicals/metribuzin.
- P.E.I. Department of Communities, Land and Environment (2019). Personal communication with G. Somers.
- Perry, C.A. (1990). Source, extent, and degradation of herbicides in a shallow aquifer near Hesston, Kansas. US Geological Survey. Water-Resources Investigations Report 90-4019. Available at https://pubs.usgs.gov/wri/1990/4019/report.pdf.
- Plakas, K.V. and Karabelas, A.J (2012). Removal of pesticides from water by NF and RO membranes - a review. Desalination, 287:255-265.
- Porter, M.; Jasty, V. and Hartnagel, R. (1988). A two-generation reproduction study in rats with Sencor Technical (Metribuzin): Report No. 98295: MTD0080. Unpublished study prepared by Miles, Inc. 1025 p. (as cited in US EPA, 1998, 2003).
- Rigi, R., Farahbakhsh, M. and Rezaei, K. (2015). Adsorption and desorption behaviour of herbicide metribuzin in different soils of Iran. J. Agr. Sci. Tech., 17: 777-787.
- Rocha, A.A., Monteiro, S.H., Andrade, G. C. R. M., Vilcab, F. Z. and Tornisielo, V. L.(2015). Monitoring of pesticide residues in surface and subsurface waters, sediments, and fish in center-pivot irrigation areas. J. Braz. Chem. Soc. 26(11): 2269-2278.
- Saskatchewan Water Security Agency (2019). Personal communication with S. Ferris.
- Sathiakumar, N., MacLennan, P.A., Mandel, J. and Delzell, E. (2011). A review of epidemiologic studies of triazine herbicides and cancer. Crit. Rev. Toxicol., 41(sup.1): 1-34. SCC (2019). Directory of accredited product, process and service certification bodies. Standards Council of Canada, Ottawa, Ontario. Available at www.scc.ca/en/accreditation/product-process-and-service-certification/directory-of-accredited-clients.
- Shiotsuka, R. (1986). Acute inhalation toxicity study with metribuzin (Sencor) in rats. Study No. 85-041-18. Mobay Report No. 91754. Unpublished study prepared by Mobay Chemical Corp., Kansas City, MO (as cited in US EPA, 1998).
- Shrestha, S., Parks, C.G., Goldner, W.S., Kamel, F., Umbach, D.M., Ward, M.H., Lerro, C.C., Koutros, S., Hofmann, J.N., Beane Freeman, L.E., Sandler, D.P. (2018). Pesticide use and incident hypothyroidism in pesticide applicators in the Agricultural Health Study. Environ. Health Perspect., 26(9):97008.
- Sinha, S.N., Vasudev, K., Vishnu Vardhana Rao, M. and Odetokun, M. (2011). Quantification of organophosphate insecticides in drinking water in urban areas using lyophilization and high-performance liquid chromatography-electrospray ionization-mass spectrometry techniques. Int. J. Mass Spectrom. 300: 12-20 (2011).
- Smeets, P.W.M.H., Medema, G.J. and van Dijk, J.C. (2009). The Dutch secret: how to provide safe drinking water without chlorine in the Netherlands. Drink. Water Eng. Sci., 2: 1-14.
- Speth, T.F. and Adams, J.Q. (1993). GAC and air stripping design support for the Safe Drinking Water Act, strategies and technologies for meeting SDWA requirements. Clark, R. and S. Summers, Eds., Lewis Publishers, Ann Arbor, MI, pp. 47-89.
Speth, T.F. and Milnter, R.J. (1998). Technical note: adsorption capacity of GAC for synthetic organics. J. Am. Water Works Assoc., 90(4): 171-174. - Summers, R.S., Knappe, D.R.U. and Snoeyink, V.L. (2010). Chapter 14: Adsorption of organic compounds by activated carbon. In: Edzwald, J.K. (ed.), Water quality and treatment: A handbook on drinking water. 6th edition. McGraw-Hill, New York, NY.
- Thyssen J. (1981). DIC 1468: (Sencor Active Ingredient): Subacute inhalation studies with rats: Report No. 9679. Unpublished study. Prepared by Bayer AG, Institute of Toxicology, pp.126; MRID 00153706 (as cited in US EPA, 1998, 2003).
- US EPA (1987). Metribuzin: Health Advisory. US Environmental Protection Agency, Office of Drinking Water. Available at https://nepis.epa.gov/Exe/ZyPDF.cgi/2000SP78.PDF?Dockey=2000SP78.PDF.
- US EPA (1993). Metribuzin; CASRN 21087-64-9. Integrated Risk Information System (IRIS). Chemical Assessment Summary. US Environmental Protection Agency, National Center for Environmental Assessment. Available at https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?&substance_nmbr=75.
- US EPA(1995a). Method 507 revision 2.1. Determination of nitrogen - and phosphorus containing pesticides in water by gas chromatography with a nitrogen-phosphorus detector. National Exposure Research Laboratory. Office of Research and Development, Cincinnati, Ohio.
- US EPA(1995b). Method 508.1 revision 2.0. Determination of chlorinated pesticides, herbicides, and organohalides by liquid-solid extraction and electron capture gas chromatography. National Exposure Research Laboratory. Office of Research and Development, Cincinnati, Ohio.
- US EPA(1995c). Method 525.2 revision 2.0. Determination of organic compounds in drinking water by liquid-solid extraction and capillary column gas chromatography/mass spectrometry. National Exposure Research Laboratory. Office of Research and Development, Cincinnati, Ohio.
- US EPA(1995d). Method 551.1 revision 1.0. Determination of chlorination disinfection byproducts, chlorinated solvents, and halogenated pesticides/herbicides in drinking water by liquid-liquid extraction and gas chromatography with electron-capture detection. National Exposure Research Laboratory. Office of Research and Development, Cincinnati, Ohio.
- US EPA (1998). Reregistration Eligibility Decision (RED). Metribuzin. EPA 738-R-97-006. United States Environmental Protection Agency, Office of Pesticide Programs. Washington, D.C.
- US EPA (2003). Health Effects Support Document for Metribuzin. EPA 822-R03-004. US Environmental Protection Agency, Office of Water (430T), Health and Ecology Criteria Division. Washington, DC. Available at www.epa.gov/safewater/.
- US EPA (2011). Finalization of guidance on incorporation of water treatment effects on pesticide removal and transformation in drinking water exposure assessments. Office of Pesticide Programs, Environmental Fate and Effects Division. Washington, DC. Available at https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/finalization-guidance-incorporation-water-treatment.
- Van der Bruggen, B. and Vandecasteele, C. (2003). Removal of pollutants from surface water and groundwater by nanofiltration: overview of possible applications in drinking water industry. Environ. Pollut., 122: 435-445.
- WHO (2011). Guidelines for drinking-water quality. Fourth edition. World Health Organization, Geneva, Switzerland. Available at http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/.
- WHO (2012). Water safety planning for small community water supplies. World Health Organization, Geneva, Switzerland. Available at http://www.who.int/water_sanitation_health/publications/small-commwater_supplies/en/
- Yang, L. (1986). CHO/HGPRT mutation assay in the presence and absence of exogenous metabolic activation: test article: Sencor Technical (Metribuzin): Final report: MA study No. T4485.332.
- Unpublished Mobay report No. 91760 prepared by Microbiological Associates, Inc. 17 p. (as cited in US EPA, 1998).
- Yukon Environmental Health Services (2019). Personal communication with P. Brooks.
Appendix A: List of abbreviations
- ADI
- Acceptable daily intake
- AHS
- Agricultural Health Study
- ANSI
- American National Standards Institute
- CAS RN
- Chemical Abstracts Service Registry Number
- CHO
- Chinese hamster ovary
- COD
- Chemical oxygen demand
- DA
- Desamino-metribuzin
- DADK
- Desamino-diketo-metribuzin
- DK
- Diketo-metribuzin
- ECD
- Electron capture detector
- EU
- European Union
- GAC
- Granular activated carbon
- GC
- Gas chromatography
- GSH
- Glutathione
- HA
- Health advisory
- HBV
- Health-based value
- HGPRT
- Hypoxanthine-guanine phosphoribosyl transferase
- IARC
- International Agency for Research on Cancer
- LD50
- Median lethal dose
- MAC
- Maximum acceptable concentration
- MDL
- Method detection limit
- MRL
- Method reporting limit
- MS
- Mass spectrometry
- MWCO
- Molecular weight cut-off
- NF
- Nanofiltration
- NOEL
- No-observed-effect level
- NOM
- Natural organic matter
- NSF
- International
- PAC
- Powdered activated carbon
- P.E.I.
- Prince Edward Island
- PMRA
- Pest Management Regulatory Agency
- SCC
- Standards Council of Canada
- RO
- Reverse osmosis
- SGOT
- Serum glutamate-oxaloacetate transaminase
- SGPT
- Serum glutamate-pyruvate transaminase
- T3
- Triiodothyronine
- T4
- Thyroxine
- US EPA
- United States Environmental Protection Agency
- WHO
- World Health Organization
Appendix B: Canadian water quality data
| Jurisdiction (year sampled) | No. detects/ samples | MDL (ng/L) | Range (ng/L) | 25th percentile (ng/L) | Median (ng/L) | 75th percentile (ng/L) | |
|---|---|---|---|---|---|---|---|
| Min | Max | ||||||
| Tap Water | |||||||
| AB, SK, MB - rural communities (2004 – 2005)Footnote a | ?/29 | Not available | 8.11 | < 20.70 | Not available | Not available | Not available |
| Surface Water | |||||||
| BC - Lower Fraser Valley and Okanagan Basin (2003 – 2005) | 22/93 | 0.015 | < 0.015 | 2.74 | Not available | Not available | Not available |
| ON (2003) | 17/161 | 20.7 | 21.0 | 1230 | Not available | Not available | Not available |
| ON (2004) | 29/224 | 20.7 | 23.0 | 668 | Not available | Not available | Not available |
| ON (2005) | 14/183 | 20.7 | 23.1 | 1210 | Not available | Not available | Not available |
| ON - 10 isolated lakes (2003 – 2005) | 49/163 | 0.001 | < 0.001 | 23.1 | Not available | Not available | Not available |
| QC (2003) | 1/50 | 20 | < 20 | 20 | Not available | Not available | Not available |
| QC (2004) | 0/69 | 6-20 | Not available | Not available | Not available | Not available | Not available |
| QC (2005) | 0/62 | 20 | Not available | Not available | Not available | Not available | Not available |
| NB (2003 – 2005) | 10/57 | 30-300 | Not available | Not available | Not available | Not available | Not available |
| P.E.I. (2003 – 2005) | 2/82 | 50-80 | Not available | Not available | Not available | Not available | Not available |
| NS (2003 – 2005) | 0/48 | 30 | Not available | Not available | Not available | Not available | Not available |
| Rivers | |||||||
| AB, SK, MB - 8 sites (2003) | 0/63 | 20.7 | Not available | Not available | Not available | Not available | Not available |
| Reservoir Water | |||||||
| AB, SK, MB - 15 sites (2003 – 2004) | 1/198 | 20.7 | < 20.7 | 185 | Not available | Not available | Not available |
| Groundwater | |||||||
| P.E.I. (2004) | 2/122 | Not available | 100 | 180 | Not available | Not available | Not available |
| P.E.I. (2005) | 3/112 | Not available | Not available | 190 | Not available | Not available | Not available |
| Air | |||||||
| ON - 4 sites (2004 – 2005) | 9/12 | 0.000 | < 0.001 | 0.039 | 0.007 | 0.012 | 0.030 |
| ON - 4 sites (2004 – 2005)Footnote b | 8/12 | 0.001 | < 0.001 | 0.225 | < 0.001 | 0.022 | 0.060 |
|
|||||||