Guidelines for Canadian Drinking Water Quality: Guideline technical document - Strontium
Table of Contents
Part II. Science and Technical Considerations
- 4.0 Identity, use and sources in the environment
- 4.1 Environmental fate
- 5.0 Exposure
- 5.1 Water
- 5.2 Food
- 5.3 Air
- 5.4 Consumer products
- 5.5 Soil
- 5.6 Multi-route exposure through drinking water
- 5.7 Total daily intake
- 6.0 Analytical methods
- 6.1 Recommended method
- 6.2 Other methods
- 6.3 Sample preparation
- 7.0 Treatment technology and distribution system considerations
- 7.1 Municipal scale treatment
- 7.2 Distribution system
- 7.3 Residential scale
- 8.0 Kinetics and metabolism
- 8.1 Absorption
- 8.2 Distribution
- 8.3 Metabolism
- 8.4 Excretion
- 8.5 Physiologically based pharmacokinetic models
- 9.0 Health effects
- 9.1 Effects in humans
- 9.1.4 Carcinogenicity
- 9.1.5 Developmental and reproductive toxicity
- 9.2 Effects on experimental animals
- 9.3 Mode of action
- 9.3.1 Toxic effects
- 9.3.2 Beneficial effects
- 10.0 Classification and assessment
- 10.1 Non-cancer risk assessment
- 10.2 International considerations
- 11.0 Rationale
- 12.0 References
- Appendix A: List of acronyms
Part I. Overview and Application
1.0 Guideline
The maximum acceptable concentration (MAC) for total strontium in drinking water is 7.0 mg/L.
2.0 Executive summary
Strontium is widely distributed in nature and has been identified in many different minerals. It may be present in water in the environment from natural sources (rock and soil weathering) or as a result of human activities. Although not actively mined in Canada, strontium can be released to the environment as a by-product of other mining operations or from its usage in many industries. Strontium is used in electrical applications and paint, to remove lead from zinc electrolytic solutions, in pyrotechnics and signalling devices, as well as in the manufacture of various other products (e.g., glass, ceramic permanent magnets and glazes, aluminum alloys). Strontium salts are employed in Canada for their beneficial effects on health, either as natural health products (licensed mainly to help support bone health) or in cancer therapy. Radioactive forms of strontium are used in medical applications, such as bone imaging. Strontium is naturally found in the environment as a mixture of four radioisotopes, which are considered stable. The focus of this document is limited to strontium's chemical properties. Radiological forms and/or radioactive isotopes of strontium are addressed in a separate document (Health Canada, 2009).
This guideline technical document reviews and assesses all identified health risks associated with strontium in drinking water. It assesses new studies and approaches and takes into consideration the availability of appropriate treatment technology. Based on this review, the guideline for strontium in drinking water is a maximum acceptable concentration of 7.0 mg/L.

Download the alternative format
(PDF format, 610 KB, 73 page)
Organization: Health Canada
Date published: May 24, 2019
2.1 Health effects
Although only a few studies conducted in humans have documented adverse effects of strontium on bone, many animal studies have observed adverse bone effects following ingestion of high doses of strontium. Since the highest sensitivity to adverse bone effects occurs during the first year of life, infants are considered to be the sensitive subpopulation for strontium toxicity. Consequently, the MAC of 7.0 mg/L has been established based on studies of bone effects in young rats.
2.2 Exposure
Canadians are primarily exposed to strontium through food and drinking water. Strontium concentrations in Canadian food items vary across cities and years and depend on the food item and soil conditions. Strontium levels in Canadian drinking water can vary greatly, depending on geological formations and anthropogenic activities surrounding the source water, with groundwater generally presenting higher levels than surface water. Intake of strontium from drinking water is not expected to occur through either skin contact or inhalation.
2.3 Analysis and treatment
Several analytical methods are available for the analysis of total strontium in drinking water at levels well below the MAC. Measurement should be for total strontium which includes both the dissolved and particulate forms of strontium in the water sample.
Chemical precipitation and ion exchange techniques are the two best technologies for removal of naturally occurring strontium in drinking water. At the municipal level, available technologies for the treatment of total strontium include chemical precipitation, ion exchange and reverse osmosis. Other strategies for reducing exposure to strontium include switching to a new source, blending and interconnecting with another water system.
At the residential level, treatment devices using ion exchange or reverse osmosis technologies would be effective at removing strontium from drinking water, although none are currently certified for that purpose. It is important to note that reverse osmosis systems should be installed only at the point of use, as the treated water may be corrosive to internal plumbing components.
2.4 International Considerations
The World Health Organization (WHO), European Union and Australia's National Health and Medical Research Council have not established health-based limits for chemical strontium in drinking water. The United States Environment Protection Agency has established a lifetime health advisory of 4 mg/L for strontium in drinking water.
3.0 Application of the guideline
Note: Specific guidance related to the implementation of drinking water guidelines should be obtained from the appropriate drinking water authority in the affected jurisdiction.
For drinking water supplies that occasionally experience short-term exceedances of strontium above the guideline value, it is suggested that a plan be developed and implemented to address these situations. Strategies to reduce exposure to strontium in drinking water may include source water treatment, blending, interconnecting with and/or purchasing water from another water system. For more significant, long-term exceedances that cannot be addressed through treatment, it is suggested that alternative sources of drinking water be considered.
The current analytical protocol may underestimate the concentration of total strontium in drinking water if particulate strontium is not solubilized (see Section 6.3). Utilities should consult with the responsible drinking water authority to determine sample processing requirements such as the digestion of samples.
3.1 Monitoring
3.1.1 Source characterization
Strontium occurs in both surface and groundwaters where there are celestite-rich limestone deposits. Every source water (ground and surface) should be characterized to determine if strontium is present. Frequency should take into consideration variations likely to occur:
- In surface waters, increased strontium concentrations may occur in areas with low precipitation and high evaporation rates. These conditions may lead to increased dissolved solids in the streams and thus result in proportionally more strontium in these high-salinity surface waters.
- In groundwater, strontium concentrations are less likely to fluctuate, variations have been observed between wells in dry and humid regions.
If the total strontium concentration in source water is approaching the MAC and/or the concentration is known or expected to be changing with time (e.g., anthropogenic activities are introduced), monitoring of the source water should be conducted annually. Authorities may consider reduced monitoring when it has been demonstrated that strontium is present at concentrations below the MAC in the source water and/or appropriate treatment is in place.
Utilities practising control and treatment options for addressing total strontium in source water used for drinking should assess the water quality of new sources to ensure that it does not interfere with the existing treatment processes, impact the distribution system, and cause other water quality issues.
3.1.2 Operational monitoring
Utilities that treat their water to remove strontium need to conduct frequent monitoring of the treated water in order to make the necessary process adjustments and to ensure that treatment processes continue to effectively remove strontium concentrations to below the MAC. Operational monitoring of pH is required for utilities using lime-soda ash softening. Since calcium plays an important and necessary part in the removal of strontium, sufficient calcium concentration is needed to achieve optimal strontium removal. Utilities using strong acid cation exchange resins in the sodium form should be aware that this process may introduce undesirable quantities of sodium in the treated water.
3.1.3 Compliance monitoring
When treatment is in place for strontium removal, it is recommended that compliance monitoring for total strontium be conducted annually, at a minimum, to confirm the MAC is not exceeded. Samples should be collected after treatment prior to distribution (typically at the entry point to the distribution system) and analyzed by an accredited laboratory.
3.1.4 Distribution system
Like other inorganics, strontium can accumulate in distribution systems and later be released. Consequently, monitoring should also be conducted throughout the distribution system when strontium is or was historically present in the source and/or distributed water. Monitoring programs should be designed on a system-specific basis to verify that control strategies are operating as intended and consider risk factors that contribute to the likelihood that strontium may be elevated within the drinking water system. Factors that influence strontium accumulation (e.g., iron corrosion products, manganese deposits, and calcium carbonate scale) and mobilization, such as changes to water chemistry and physical/hydraulic disturbances in the distribution system, could be used as indicators of when and where to monitor for strontium releases.
Monitoring for total strontium and other contaminants (e.g., iron, manganese, arsenic, lead) should be conducted when water quality changes or physical disruptions occur in the system. The release of strontium and other contaminants may be indicated by the presence of discoloured water or increased turbidity resulting from the release of deposits or scales present on the pipe wall. The number and location of sites for monitoring of strontium in the distribution system should take into consideration the site-specific accumulation and release risk factors. However, the absence of discoloured water should not be interpreted as the absence of a metals release.
Water utilities that have baseline data indicating that strontium is not present within the distribution system may conduct less frequent monitoring.
3.1.5 Private wells
Homeowners with private wells are encouraged to have their water tested for total strontium to ensure that the concentration in their water supply is below the MAC. In addition, homeowners with private wells using residential treatment devices should conduct routine testing on both the water entering the treatment device and the treated water to verify that the treatment device is effective. Homeowners using ion-exchange softeners should be aware that the treatment unit may introduce undesirable quantities of sodium in the treated water.
Part II. Science and Technical Considerations
4.0 Identity, use and sources in the environment
Elemental strontium (Chemical Abstracts Service [CAS] Registry No. 7440-24-6) has a molecular weight of 87.62 g/mol. It lies between calcium and barium in the alkaline earth metal group. Strontium (Sr) is only found in the +2 valence state in the environment (WHO, 2010; ANSES, 2013). Of the many isotopes of strontium that could occur, only four (84Sr, 86Sr, 87Sr, 88Sr) are found naturally; these isotopes are considered stable (MacMillan et al., 2000). Radioactive isotopes of strontium, particularly82Sr, 85Sr, 89Sr and 90Sr, are formed during nuclear fission (ATSDR, 2004) or made for medical purposes. Strontium is found naturally in the earth's crust at a concentration of 0.04% (15th most abundant element), and in seawater at a concentration of 0.0008% (8 ppm) (MacMillan et al., 2000). It readily reacts with water and oxygen and is generally found as strontium carbonate (SrCO3) and strontium sulphate (SrSO4) in minerals, but can also exist in other compounds, such as strontium phosphate [Sr3(PO4)2] and in association with sedimentary rock formations (Skoryna, 1981; ANSES, 2013). It is present in water as a hydrated cation, and can create complexes with carbonates and silicates depending on the water mineralization (Malina, 2004; WHO, 2010). Strontium has a boiling point of 375°C, a melting point of 122°C, and a vapour pressure of 0.0005 Pa (low) (U.S. EPA, 1996). Strontium salts vary in their solubility. Strontium nitrate (Sr(NO3)2), 538-790 g/L at 18°C), strontium chloride (SrCl2, 345-538 g/L at 20°C), and SrSO4 (0.14 g/L at 30ºC) are moderately soluble in water, while SrCO3 (0.01 mg/L at 25°C) has a low solubility (MacMillan et al., 2000; ATSDR, 2004). There were no data available on the Henry's law constant.
Strontium's most economically important geochemical species are SrSO4 (celestite or celestine) and SrCO3 (strontianite) (MacMillan et al., 2000; Ober, 2006). SrSO4 is the principal geochemical source of strontium and is converted into other forms for commercial sales, mainly SrCO3 and Sr(NO3)2 (Fowler, 1991). SrCO3 is used in the processes of manufacturing ceramic permanent magnets and glazes, as an alternative to lead (Fowler, 1991; ATSDR, 2004; Ober, 2014). It is also used in glass to increase strength and hardness, improve optical properties, and absorb radiation (Ober, 2006). It is used to remove lead from zinc electrolytic solutions and in the manufacture of aluminum alloys used in aerospace and automobiles. Its wide usage in electrical applications and paint is explained by physical properties allowing conduction at high temperature, resistance to corrosion, and demagnetization (Ober, 2006). Strontium nitrate and (to a lesser extent) strontium sulphate, strontium chloride, and strontium oxalate are mainly used in pyrotechnics and signalling devices to produce a bright red colour. Strontium chromate is used as a pigment in paints. Radioactive forms of strontium are used in medical applications, such as bone imaging (ATSDR, 2004).
Some strontium salts are classified as natural health products under Schedule 1, item 7 (a mineral) of the Natural Health Products Regulations (Government of Canada, 2003). Strontium citrate, strontium lactate and strontium gluconate, often in combination with other ingredients, are the main salts used in these products to help support bone health. They are also used in toothpaste to relieve tooth sensitivity.
Strontium ranelate (SrR) is a prescription drug that had been marketed in Europe for treating osteoporosis. It dissociates into two stable Sr2+ atoms and one molecule of ranelic acid in the gastrointestinal tract. Its pharmacological effect of increasing bone mineral density is attributed to the strontium moiety (EMA, 2012, 2013; Yamaguchi and Weitzmann, 2012; Health Canada, 2015a). Ranelic acid is minimally absorbed and mostly excreted. The Health Canada Drug Product Database does not currently identify any product containing strontium as the active ingredient for treating osteoporosis.
Although SrR had been approved for use for treating osteoporosis in the elderly throughout the European Union in 2004, its use was later restricted. The European Medicines Agency (EMA, 2014) considered that the benefit:risk ratio was favourable for SrR. However, the agency recommended that its use be restricted to patients who cannot use other options and those without heart or circulatory disease history, and that treatment is stopped in the case of rash, based on possible adverse effects. The marketing and distribution of SrR has been ceased by the manufacturer in 2017 (Drug and Therapeutics Bulletin, 2017). Health Canada has taken a precautionary approach in recommending the addition of warnings on labels of products containing between 4 and 682 mg of strontium aimed at those who have, or are at high risk of, heart disease, circulatory problems or blood clots. Purchasers are also advised to consult a health care practitioner for use longer than 6 months (Health Canada, 2015b). The polar ranelate moiety of SrR is poorly absorbed, not metabolized, and is rapidly excreted; hence, strontium itself is believed to be responsible for the beneficial effects observed on bone in clinical trials (Servier laboratories, 2016). It is not clear whether the toxicity mechanism of SrR is completely independent of the ranelic acid moiety. Furthermore, a single high dose of SrR might not represent environmental exposure to different strontium compounds in drinking water.
Some strontium salts (iodide, carbonate) are used in cancer therapy in Canada. Only the radiopharmaceutical strontium-89 in cancer therapy is approved for intravenous use in Canada (Canadian Cancer Society, 2017).
Significant deposits of strontium were found in Nova Scotia, mostly as SrSO4 in Cape Breton County sedimentary rocks. However, the ore is of low quality; the province's only mine started operating in 1971 and closed in 1976 (Fowler, 1991; Ober, 2006; Environment Canada, 2012; Marshall, 2013).
Only non-radiological forms of strontium will be assessed in this document, since there is already a guideline for radioactive elements (Health Canada, 2009). Strontium chromate will not be assessed, as the chromate is deemed to be responsible for the toxic effects.
4.1 Environmental fate
Strontium in the ionic form (Sr2+) represents the exchangeable fraction soluble in water (labile in soil) (Lee, 2008; Heuel-Fabianek, 2014). It mainly enters water through leaching from limestone (present in igneous and metamorphic rocks, including granites and sedimentary rocks) as hydrated Sr2+ and can move down into groundwater (Malina, 2004). Although not actively mined in Canada, strontium can be released to the environment as a by-product of other mining operations, as in the case of diamond mining in the Yukon (De Beers Canada, 2013). Air deposition from coal burning and phosphate fertilizers can also contribute smaller amounts (WHO, 2010; De Beers Canada, 2013). Strontium's mobility in soils is moderate and depends on the soil cation exchange capacity, the ion content of the cycling water, and its pH (Kaplan and Kellum, 2010). Soils with low exchangeable calcium ion content (low cation exchange capacity) or low humus favour strontium mobility (low soil-water partitioning coefficient Kd), since Sr2+ precipitates when reacting with organic matter (ATSDR, 2004; Heuel-Fabianek, 2014). A small fraction adheres to soil metal and clay particles. In fact, in mildly acidic to basic soil conditions, strontium will mainly form insoluble compounds (SrSO4 at pHs 4-8, SrCO3 above pH 8). Cycling water rich in minerals also increases strontium mobility. The majority of strontium in water exists as a hydrated ion and can react with different elements, such as nitrogen (N2), fluorine (F2), and sulphur (S) (Skoryna, 1981).
Strontium is often found in calcium minerals; however, the concentrations of the two elements are generally not directly correlated. A recent report has shown no correlation between strontium and calcium levels in groundwater in Indiana, U.S. (n = 1,832) (Najm, 2016); 95% of the water samples had strontium:calcium ratios below 0.1 (mg/mg). Rivers in the U.S. were found to have strontium:calcium ratios (strontium atoms per 1,000 calcium atoms) in the range of 0.4-16. Higher strontium:calcium ratios can be found when water cycles through SrSO4 or SrCO3 deposits, whereas lower ratios are found in water flowing through sedimentary and basalt rocks (Skougstad and Horr, 1963).
5.0 Exposure
Food and water represent the main sources of strontium exposure, but the contribution from these sources can be highly variable, with groundwater generally presenting higher levels than surface water. In the case of Canadian drinking water, strontium content can vary greatly, depending on geological formations and anthropogenic activities surrounding the source water. Allocating a 50% source contribution from drinking water is deemed appropriate, given that only two main sources of exposure have been identified (Krishnan and Carrier, 2013) and the data for these exposure sources (see Table 1 below) support the choice of allocation factor.
5.1 Water
Environment and Climate Change Canada (2017) collected freshwater quality data from over 200 federal and federal-provincial sampling sites at various locations and sampling frequencies throughout Canada's aquatic ecosystems between 2000 and 2016. Of 18,821 samples, 5 were below the detection limit (DL) of 0.005-4.0 µg/L (<0.1%), the mean level was 154.2 µg/L, the median was 114 µg/L, and the 75th percentile was 192 µg/L; 3% were above 500 µg/L and the maximum was 2,900 µg/L.
Strontium concentrations in drinking water were measured in various locations across Canada as part of the National Survey of Disinfection By-Products and Selected Emerging Contaminants in Canadian Drinking Water (n = 124; 41 samples from lakes, 48 from rivers, 35 from wells) (Health Canada, 2015c). In raw water, the mean level was 185 µg/L, the median was 115 µg/L, and the 75th percentile was 250 µg/L; 11 measurements were above 500 µg/L and the maximum was 1,600 µg/L. In treated water, the mean level was 185 µg/L, the median was 130 µg/L, and the 75th percentile was 235 µg/L; 13 measurements were above 500 µg/L and the maximum was 1,500 µg/L. Drinking water sourced from a lake had the lowest levels (median 43 µg/L), followed by river (median 150 µg/L), and well (median 210 µg/L) waters.
The Canadian Total Diet Study (TDS) is a Health Canada initiative that measures the concentrations of different chemicals in foods and uses these data to estimate dietary intakes for different age-sex groups of the Canadian population (Health Canada, 2007a). Strontium concentrations were measured in tap water in six cities between 2001 and 2007. In St John's the mean level was 10 µg/L, in Halifax it was 12 µg/L, in Montreal 150 µg/L, in Toronto 138 µg/L, in Winnipeg 36 µg/L, and in Vancouver 5-7 µg/L.
In Newfoundland and Labrador, strontium levels were measured in raw and treated water from public drinking water systems for the years 2010-2015 (Newfoundland and Labrador Department of Environment and Conservation, 2015). For the 1,184 samples of raw water analyzed, the mean level was 82 µg/L, the median was 14 µg/L, the 75th percentile was 59 µg/L and the maximum level detected was 2,990 µg/L; 204 samples (17%) had strontium levels above 100 µg/L. A total of 4,968 samples of treated water were analyzed, with a mean level of 116 µg/L, a median of 19 µg/L, a 75th percentile of 91 µg/L, and a maximum of 6,320 µg/L; (1,149 samples (23%) had strontium levels above 100 µg/L.
Nova Scotia reported municipal samples from 90 facilities between 1999 and 2014 (Nova Scotia Environment, 2016). Raw water samples (n = 191) had a mean level of 98 µg/L, a median of 17 µg/L, and a 75th percentile of 53 µg/L; 36 samples (19%) were above 100 µg/L and the maximum was 2,200 µg/L. Treated water (n = 483) had a mean level of 55 µg/L, a median of 19 µg/L, and a 75th percentile of 29 µg/L; 58 samples (12%) were above 100 µg/L and the maximum level found was 690 µg/L. There were 28 samples at or below the DL of 2-5 µg/L.
In New Brunswick, for years 2008-2016, raw water (n = 442) had a mean level of 297 µg/L, a median of 185 µg/L, and a 75th percentile of 281; 350 samples (79%) were above 100 µg/L, and the maximum detected was 3,500 µg/L (New Brunswick Department of Health, 2016). Treated distribution water (n = 523) had a mean level of 187 µg/L, a median of 82 µg/L, and a 75th percentile of 189 µg/L; 248 samples (47%) were above 100 µg/L, and the maximum observed was 2,600 µg/L.
A groundwater monitoring program in Québec reported strontium groundwater analyses across its regions between 1941 and 2012 (Ministère du Développement durable, de l'Environnement et de la Lutte contre les changements climatiques, 2016). A mean level of 857 µg/L, a median of 210 µg/L, a 75th percentile of 580 µg/L, and a maximum of 47 000 µg/L were reported; 10.5% of the levels were above 1,500 µg/L and 4% were above 4,000 µg/L (n = 1,261 for wells, DL = 2 µg/L) (Brisson, 2014). Also, the Programme de surveillance de la qualité de l'eau potable reported that three measurements were above 1,500 µg/L and one above 4,000 µg/L (DL = 0.2 µg/L) in treated groundwater from 50 installations between 2012 and 2014.
The Ambient Groundwater Geochemistry project characterizes the chemical state of groundwater for southern Ontario (area of 96 000 km2), with about 2,300 samples taken between 2007 and 2014 (Hamilton, 2015). Of 2,287 strontium samples, 31 (1.3%) were below the DL of 0.1 µg/L; the mean level was 3,528 µg/L, the median was 625 µg/L, and the 75th percentile was 2,436 µg/L; 11.2% of measurements were above 10 000 µg/L and the maximum level found was 87 832 µg/L. There was a weak correlation (0.27) between calcium and strontium concentrations in raw water.
In Manitoba, raw groundwater (n = 736) had a mean strontium level of 454 µg/L, a median of 454 µg/L, and a 75th percentile of 551 µg/L; 664 samples (90%) were above 100 µg/L, with a maximum of 7,750 µg/L observed for the years 2009-2016 (Manitoba Conservation and Water Stewardship, 2016). Treated groundwater (n = 976) had a mean level of 346 µg/L, a median of 275 µg/L, and a 75th percentile of 462 µg/L; 746 samples (76%) were above 100 µg/L, with a maximum of 7,940 µg/L. Raw surface water (n = 466) had a mean level of 124 µg/L, a median of 44 µg/L, and a 75th percentile of 214 µg/L; 170 samples (37%) were above 100 µg/L, with a maximum found of 1,010 µg/L. Treated surface water (n = 499) had a mean level of 84 µg/L, a median of 42 µg/L, and a 75th percentile of 124 µg/L; 155 samples (31%) were above 100 µg/L, and a maximum of 653 µg/L was noted. There were 24 samples at or below 1 µg/L.
In Saskatchewan, groundwater had a mean strontium level of 570 µg/L, a median of 630 µg/L, a 75th percentile of 730 µg/L and a maximum of 2,100 µg/L (n = 67); surface water had a mean level of 310 µg/L, a median of 250 µg/L, a 75th percentile of 290 µg/L and a maximum of 1,000 µg/L (n = 156) (Saskatchewan Water Security Agency, 2015). Overall, 212 samples (95%) were above 100 µg/L and 7 samples were at or below 1 µg/L).
In Alberta, strontium levels were measured in treated drinking water systems between 1999 and 2015 (Alberta Environment and Sustainable Resource Development, 2016). The mean level of dissolved strontium (filtered water, no particulates <0.45 µm) was 372 µg/L; all samples were above 100 µg/L, with a maximum of 482 µg/L (n = 6). Extractable strontium had a mean level of 308 µg/L, a median of 257 µg/L, and a 75th percentile of 385 µg/L; 919 samples (88%) were above 100 µg/L and the maximum level found was 2,820 µg/L (n = 1,042). Total strontium had a mean level of 276 µg/L, a median of 234 µg/L, and a 75th percentile of 315 µg/L; 202 samples (94%) were above 100 µg/L and the maximum level observed was 1,170 µg/L (n = 214).
5.2 Food
The average dietary intakes of strontium between 1993 and 2007 were estimated for Canadians of all age groups in seven cities (St. John's, Halifax, Montreal, Ottawa, Toronto, Winnipeg and Vancouver) as part of the TDS (Health Canada, 2007b). The averaged estimates over age categories (µg/kg body weight [bw] per day) were 75.5-83.9 for 0-6 months old, 64.9-69.6 for 6 months-4 years old, 44.7 for 5-11 years old, 28.4 for 12-19 years old, and 19.1-26.7 for 20 years old and above (Table 1). Dietary exposure to strontium can be estimated to range from 1337 to 1869 µg per day in adult Canadians (19.1-26.7 µg/kg bw per day), based on this study.
Concentrations of strontium in food items vary between countries and regions and depend on the food item and soil conditions (Chang et al., 2015). Based on the TDS conducted from 1993 to 2012, strontium concentrations found in common food items also varied between years and cities in Canada (Health Canada, 2007b). For example, strontium levels ranged from 40 to 500 µg/kg in apple juice, 200 to 11 000 µg/kg in cheese, 100 to 1,800 µg/kg in eggs, 100 to 2,500 µg/kg in tomatoes, 200 to 800 µg/kg in whole milk, 50 to 678 µg/kg in tea, and up to 100 000 µg/kg in herbs and spices.
The Maternal-Infant Research on Environmental Chemical (MIREC) Study collected biomonitoring data from infants and their mothers in 10 Canadian cities from 2008 to 2011 (Arbuckle et al., 2013). Breast milk samples (n = 845) had a mean level of 41 µg/L, a median of 37 µg/L, a 75th percentile of 48 µg/L, and a 90th percentile of 62 µg/L; 11 samples were above 100 µg/L, with a maximum of 282 µg/L (Dabeka et al., 2016). Strontium concentrations in reconstituted milk formulae (including tap water) measured in the TDS between 1993 and 2007 ranged from 247 to 844 µg/kg (Health Canada, 2007a). Values of strontium concentrations in milk formulae alone (subtracting the contribution from tap water) are used to calculate intake from food for formula-fed infants 0-6 months of age in Table 1.
5.3 Air
The Canadian National Air Pollution Surveillance (NAPS) program collects data on more than 300 ambient air pollutants across Canada (Environment Canada, 2014). Concentrations of strontium in fine (PM2.5) and coarse (PM10) particulates were reported. For fine particulates, 100 out of 453 samples were above the DL of 0.28 ng/m3 (22.1%); the mean level was 0.61 ng/m3, the median 0.39 ng/m3, and the 75th percentile 0.63 ng/m3; four samples were above 5 ng/m3, with a maximum of 15.7 ng/m3. For coarse particulates, levels in only 21 out of 1,924 samples (1.1%) were above the DL of 5 ng/m3, and the maximum observed was 17 ng/m3.
5.4 Consumer products
As described in Section 4.0, strontium compounds are part of different medicinal products available in Canada, and can be consumed in oral natural therapy formula at doses up to 680 mg per day to help support bone mineral density (Health Canada, 2015b). For individuals who have, or are at high risk for, heart disease, circulatory problems, or blood clots, warnings to consult a health care practitioner for use over 6 months are included on labels of products containing between 4 and 682 mg of strontium (Health Canada, 2015b). However, no Canadian exposure estimates from these products were found. Strontium is also a component of manufactured products such as stained glass and electrical components, but exposure and absorption from these products are considered minimal, based on the physicochemical property of the products.
5.5 Soil
Strontium levels ranged from 20 to 605 mg/kg in 162 soil samples (mean of 207 mg/kg) in Canada (Agriculture Canada, 1979). This is similar to other average strontium soil concentrations measured worldwide, reported as approximately 240 mg/kg by the World Health Organization (WHO) (WHO, 2010). Strontium concentrations in house dust, street dust and garden soil were determined in a 1992 survey including 50 residences in 10 areas of Ottawa (Rasmussen et al., 2001). The strontium concentrations found respectively for garden soil, house dust, and street dust were as follows: mean, 359, 242, and 446 mg/kg; median, 356, 249, and 445 mg/kg; 90th percentile, 401, 369, and 539 mg/kg; maximum, 437, 410, and 735 mg/kg (minimum DL: 0.1 mg/kg).
5.6 Multi-route exposure through drinking water
Exposure to strontium vapours while showering is not expected to occur, since strontium is not volatile. While the generation of mists during a shower could allow for inhalation of strontium in aerosol form, the typical multi-route assessment, which measures inhalation exposure to volatile chemicals from showering and bathing, would not accurately represent the inhalation of strontium aerosols during a shower. Moreover, dermal absorption of strontium is negligible. Ilyin et al. (1975) measured strontium absorption through intact skin in three male subjects who were exposed topically to 0.15 mL of a strontium chloride solution for 6 h. The average skin absorption of strontium was very low (0.26% of the quantity applied). Hence, the inhalation and dermal routes during showering are unlikely to contribute significantly to the total exposure.
5.7 Total daily intake
The estimated total daily intakes of strontium from drinking water, air, soil and food for age groups 0 to 6 months, 7 months to 4 years, 5 to 11 years, 12 to 19 years, and ≥20 years in the Canadian population are shown in Table 1. Daily strontium intakes from dietary supplements and other consumer products were not estimated, as there are no available data on the proportion of the general population using these products. Individual variability of strontium intakes is possible for each source. Based on the information provided in Table 1, allocating a 50% source contribution from drinking water is deemed appropriate since food and drinking water represent the two main sources of exposure to strontium.
| Age Group | Daily intake of strontium from various sources in µg/kg bw per day | ||||
|---|---|---|---|---|---|
| Drinking waterFootnote a | AirFootnote b | SoilFootnote c | FoodFootnote d | Total | |
| 0-6 mos breastfed | 0.0 (0) | <0.001 | 2.2 | 4.4 | 6.6 |
| 0-6 mos formula-fed | 115.2 (50) | <0.001 | 2.2 | 103.8 | 221.2 |
| 7 mos-4 years | 66.2 (50) | <0.001 | 1.7 | 67.3 | 135.2 |
| 5-11 years | 35.8 (40) | <0.001 | 0.6 | 44.7 | 81.1 |
| 12-19 years | 24.5 (50) | <0.001 | 0.2 | 28.4 | 53.1 |
| ≥20 years | 23.0 (50) | <0.001 | 0.1 | 22.9 | 46.0 |
|
|||||
6.0 Analytical methods
Sample processing considerations for analysis of strontium in drinking water (i.e., sample preservation, storage, digestion, etc.) can be found in the references listed below. Analysis for strontium should be carried out as directed by the responsible drinking water authority. Water utilities should discuss sample requirements with the accredited laboratory conducting the analysis to ensure that quality control procedures are met and to ensure accurate monitoring of total strontium at concentrations below the MAC.
6.1 Recommended method
The methods listed below, and recommended by United States Environmental Protection Agency (U.S. EPA), under the third Unregulated Contaminant Monitoring Rule (UCMR 3), are capable of analyzing natural strontium in drinking water. Three analytical methods: EPA Method 200.8 revision 5.4 (U.S. EPA, 1994a), SM 3125B (APHA, 2005; 2012) and ASTM 5673-10 (ASTM, 2010) for the analysis of strontium in drinking water, use an inductively coupled plasma-mass spectrometry (ICP-MS) technique. They are applicable for dissolved and total recoverable metals in drinking water. The total recoverable metal concentration is defined as the sum concentration of both the dissolved and particulate (suspended) fractions of a water sample.
The ICP-MS method is a highly sensitive detection technique: an inductively coupled plasma source is used to ionize and atomize the analyte, which is then separated by a mass spectrometer based on the mass-to-charge (m/z) ratio. Separated ions are detected by an electron multiplier or Faraday detector. Although the U.S. EPA recommends Method 200.8 and ASTM D5673-10 for monitoring strontium under the UCMR 3, strontium was not included as an analyte in these two methods and its method detection limits (MDLs) are not listed (U.S. EPA, 2012). Method SM 3125 B has an instrument detection limit (IDL) of 0.001 µg/L (APHA, 2005, 2012). The UCMR 3 stipulates that for all three methods, a minimum reporting level of 0.3 μg/L for strontium must be achieved and reported by the utilities during monitoring (U.S. EPA, 2012).
ICP-MS can be subject to a number of sources of interferences: isobaric elemental interferences, where isotopes of different elements form single- or double-charged ions of the same nominal m/z ratio and cannot be distinguished from the analyte of interest; polyatomic ion interferences, where ions with more than one atom have the same m/z ratio as the analyte of interest; and physical interferences, associated with physical processes such as transport of the sample and sample conversion processes in the plasma. Generally, the presence of high concentrations of dissolved solids in a sample may interfere with ion transmission, and physical interferences can occur when dissolved solids exceed 0.2% weight per volume (w/v) (U.S. EPA, 1994a) and 0.5% w/v (APHA, 2005; 2012).
6.2 Other methods
In addition to the recommended U.S. EPA methods, strontium can also be analyzed using the following instrumental techniques.
6.2.1 Inductively coupled plasma atomic emission spectroscopy (ICP-AES)
Both EPA Method 200.7 Rev. 4.4 (U.S. EPA, 1994b) and SM 3120B (APHA et al., 2012) are based on multi-elemental determinations by ICP-AES using sequential or simultaneous instruments. The methods have MDLs of 0.3 µg/L and 0.5 µg/L, respectively for total strontium. The instruments measure characteristic atomic-line emission spectra by optical spectrometry. After a sample solution is nebulized and the resulting aerosol is transported to a plasma torch, element-specific emission spectra are produced. The spectra are dispersed by a grating spectrometer and the intensities of the lines (or light emissions) are measured at specific wavelengths by a photosensitive device. The methods are subject to spectral interference (light emissions from spectral sources other than the element of interest) and to lesser extent to chemical interference (due to molecular compound formation, and solute vaporization and ionization effects). Physical interference may occur in EPA Method 200.7 and SM3120B when total dissolved solids are greater than 0.2% w/v or 1,500 mg/L, respectively (U.S. EPA, 1994b; APHA et al., 2012).
Similar to the methods discussed above, the USGS I-4471-97 method using ICP-AES techniques was developed for the determinations of 21 total recoverable metals in water containing undissolved particulates. A preliminary acid digestion is used to desorb and solubilize trace metals associated with the suspended sediment phase of the sample; the method has an MDL of 0.5 µg/L for strontium (USGS, 1998).
6.2.2 Flame atomic absorption spectrometry
The SM 3111B (APHA et al., 2012) and the USGS I-1800 and I-3800 methods (U.S.G.S., 1989) use atomic absorption spectrometry.
In contrast to ICP-MS and ICP-AES, the atomic absorption techniques normally conduct single-element analyses. SM 3111B is a direct air-acetylene flame atomic absorption method. The sample is aspirated into the flame and atomized. A light beam is directed through the flame, into a monochromator, and onto a detector that measures the amount of light absorbed by the atomized element in the flame. Since each metal has its own characteristic absorption wavelength, a source lamp composed of that element is used. SM 3111B has an IDL of 0.03 mg/L. No element specific interference was identified in this method (APHA et al., 2012).
The USGS methods I-1800 and I-3800 were developed for analysis of dissolved and total recoverable strontium, respectively. The methods are suitable for strontium concentrations ranging from 10 µg/L to 5,000 µg/L. Lanthanum chloride and excess potassium chloride are added to the samples to mask potential interferences from sodium, aluminum, phosphate and silica, and to control an ionization of strontium in the flame (U.S.G.S., 1989).
6.3 Sample preparation
Although the recommended methods cited above do not require hot acid digestion for total recoverable metals unless turbidity is greater than 1 nephelometric turbidity unit (NTU), research conducted on other metals such as lead and chromium has indicated that this does not accurately quantify the total metal concentration in a sample. Thus the current protocol may underestimate total strontium in drinking water when particulate strontium is present. Analytical requirements under UCMR 3 include solubilizing the acid-preserved sample by gentle heating using nitric acid, regardless of the sample turbidity or the method used (U.S. EPA, 2012). Similarly, APHA et al. (2012) recommends verifying if adequate metal recovery has occurred in different sample matrices by comparing digested and undigested results. Microwave-assisted digestion (SM 3030 K) is recommended for analysis of total recoverable metals using SM methods that are based on ICP-MS.
Influent water samples from two different lime-softening treatment plants treating a blend of groundwater and surface water have been analyzed for strontium and general water quality (pH, alkalinity, hardness, sulphate and conductivity). Since no differences were observed in strontium concentrations between the unfiltered and filtered raw water samples (pore sizes from 0.2 µm to 1.2 µm), it was concluded that strontium was present in dissolved form (<0.1 µm) in all tested samples (Najm, 2016).
Detection of both the particulate and dissolved fractions of strontium is considered a best practice for strontium determination.
7.0 Treatment technology and distribution system considerations
As an alkaline earth metal, strontium's chemistry resembles that of calcium. Strontium can exist in Sr0 or Sr2+ oxidation states; however, in the environment strontium is only found in the Sr2+ form. In water, strontium forms a hydration shell and is coordinated with six or more water molecules (U.S. EPA, 1999; Alfredo et al., 2014). There is little tendency for strontium to form complexes with organic and inorganic ligands. Dissolved strontium forms only weak aqueous complexes with carbonate, sulphate, chloride, and nitrate (U.S. EPA, 1999). Based on the assumptions that strontium exhibits similar stability with organic ligands as calcium, that strontium exists at much lower concentrations than calcium, and that it cannot compete with calcium, Stevenson and Fitch (1986) concluded that strontium would not form strong complexes with fulvic and humic acids.
Limited data exist on the removal of naturally occurring strontium in drinking water. Conventional coagulation/filtration techniques showed a low strontium removal from drinking water (up to 30%) (Gäfvert et al., 2002; Lytle et al., 2015; O'Donnell et al., 2016). In a recent review of the treatment options for naturally occurring strontium removal in drinking water, Najm (2016) concluded that both chemical precipitation and ion-exchange (IX) techniques are the two most viable technologies for strontium removal in drinking water. Early study of full-scale strontium removal indicated that both strontium and calcium removals were approximately equal and ranged from 50% to 85% at eight municipal lime-softening plants (Alexander et al., 1954). A recent survey of full-scale treatment plants using lime-soda ash precipitation and IX softening reported an effective strontium removal of 73.5% and from 89.3% to 99.9%, respectively (Lytle et al., 2015; O'Donnell et al., 2016). An evaluation of four commercially available point-of-use reverse osmosis (POU-RO) devices found that they achieved 96.6% to 99.9% strontium removal from drinking water (Lytle et al., 2015).
The majority of the existing strontium treatment studies address radioactive strontium removal from radioactive waste or from sites contaminated with radioactive waste. A variety of treatment techniques have been evaluated: lime softening (McCauley and Eliassen, 1955), sorption (Bortun et al., 1997; Bostick et al., 1997; Marinin and Brown, 2000; Kulyukhin et al., 2005; Rabideau et al., 2005; Sato et al., 2011), IX (Sivaiah et al., 2005; Marinin and Brown, 2000), nanofiltration (Gaubert et al., 1997; Hwang et al., 2002; Liang et al., 2011; Richards et al., 2011; Ding et al., 2015) and biological treatment (Achal et al., 2012; Trope et al., 2012). Many of these studies used natural strontium as a surrogate to reflect the potential effectiveness of certain treatment processes for removing radioactive strontium. In addition, natural strontium was a target in a few studies evaluating its removal by adsorption and IX from industrial waste streams.
While the chemistry of strontium removal from water is independent of its isotopes (ASTDR, 2004), Najm (2016) identified that the nature of the studies evaluating radioactive strontium removal from waste water differed from those for natural strontium. Due to the low tolerance of the organic IX resins to radiation exposure, radioactive strontium removal favours natural and/or synthetic inorganic adsorbents/ion exchange resins. The inorganic adsorbents, such as zeolites, sodium titanates, and silicotitanates, are chemically inert and stable towards ionizing radiation (Sivaiah et al., 2005; El-Kamash, 2008). In addition, since the studies have been conducted within the context of radioactive strontium removal, regeneration of the adsorbents/ion exchange resins was not considered. Despite these limitations, the studies provide valuable information on the ability and challenges facing these inorganic adsorbents/ion exchange resins for strontium removal.
7.1 Municipal scale treatment
Management strategies for strontium at the municipal-scale may include source water treatment or practices such as switching to a new source, blending and interconnecting with and/or purchasing water from another water system. Characterization of the water quality must be carried out to ensure that changes in water quality resulting from control or treatment options are assessed and that potential impacts to the distribution system are determined. Any change in water quality should not result in other compliance issues. Pilot testing of the selected treatment method or control option for strontium is also an important step to assess unintended consequences such as water quality changes.
7.1.1 Control options
Typical control options for reducing excess strontium levels in drinking water include switching to a new source, blending, and interconnecting with and/or purchasing water from another water system. Attention must be given to the water quality of a new source prior to making any changes (i.e., switching, blending, and interconnecting) to an existing supply. For example, if the new water source is more aggressive, it may cause leaching of lead or copper in the distribution system.
Switching to another source may involve drilling a new well in an aquifer containing low strontium levels, sealing off water-producing zones containing high strontium levels, or finding an uncontaminated surface water source. Switching to another source also may be limited by the availability of new sources, existing water rights, and/or costs for transporting the new source water to the treatment plant.
Blending involves diluting the strontium concentrations of a contaminated source with another source containing low or no strontium. To minimize the piping required to carry the sources to a common mixing point, it would be ideal for the sources to be close to each other. Blending usually occurs in a storage tank or a common header with the resulting strontium concentrations being below the MAC.
When interconnecting with another water system, the recipient system must consider a number of factors, including whether there is a nearby water supply that meets the strontium MAC, whether this other system is willing to interconnect or consolidate, and whether the interconnecting system can handle an increased demand resulting from additional customers. Costs are an additional consideration in the decision-making process for interconnection.
7.1.2 Conventional coagulation/filtration
Conventional coagulation/filtration is one of the most common water treatment processes used by larger water systems for removing particles and turbidity from water supplies. However, this treatment does not effectively remove strontium from drinking water (Gäfvert et al., 2002; Brown et al., 2008; Lytle et al., 2015; O'Donnell et al., 2016). The effectiveness of full-scale water treatment plants for natural strontium and calcium removal was studied in the early 1950's. Samples from 50 water treatment plants with varying natural strontium concentrations (maximum of 1.9 mg/L) were analyzed. Seven of these plants, using coagulation/filtration with either alum or ferrous sulphate as the coagulant, achieved strontium removals of 10-30% in both surface water and groundwater (Alexander et al., 1954). Jiménez and De La Montaña Rufo (2002) found that removals of strontium at 17 water treatment plants employing coagulation/flocculation did not exceed 15%. Lytle et al. (2015) and O'Donnell et al. (2016) reported similar jar-test results and confirmed the ineffectiveness of strontium removal by conventional coagulation/filtration when treating surface water spiked with 5 mg/L of strontium. Strontium removal rates were below 5.0% when using alum or ferric chloride doses up to 50 mg/L. In addition, O'Donnell et al. (2016) indicated that the coagulant dose, initial strontium concentration and pH have no impact on the strontium removal during coagulation/filtration jar tests. However, the authors found that the initial turbidity showed a very slight impact on strontium removal. Strontium removals up to 18% (alum coagulant) and 5.9% (ferric coagulant) were observed in the filtered samples when the initial turbidities were 246 NTU and 89.6 NTU, respectively. The authors concluded that coagulation/filtration treatment is not a viable strategy for strontium removal in drinking water (O'Donnell et al., 2016).
7.1.3 Chemical precipitation
Lime softening is a precipitative process that removes calcium and magnesium ions from hard water. Lime (Ca(OH)2) is added to raise the pH of the water to 9.5, causing a shift in the carbonate equilibrium resulting in calcium carbonate (CaCO3) precipitation. Soda ash (Na2CO3) is added to precipitate CaCO3 from non-carbonate calcium hardness. Ca(OH)2 and Na2 CO3may be added beyond the point of CaCO3 precipitation to then precipitate magnesium hydroxide [Mg(OH)2].
Similar to calcium, strontium can precipitate in water as a carbonate (SrCO3; solubility constant Ksp = 10-9.25), a sulphate (SrSO4; Ksp = 10-6.46) and a phosphate (Sr3(PO4)2; Ksp = 10-27.4) (Dean, 1992; Najm, 2016).
Early studies observed co-precipitation of SrCO3 with CaCO3 in the lime-softening processes. Alexander et al. (1954) compared strontium removal from source waters using a variety of treatment methods and reported that a lime-softening process was the most effective treatment for reduction of strontium with influent concentrations up to 1.9 mg/L, achieving the equivalent of 50-85% removal. Another study also observed that calcium hardness removal was essential for maximum strontium removal (McCauley and Eliassen, 1955).
Precipitation chemistry of SrCO3 and CaCO3 indicated that when both SrCO3 and CaCO3 concentrations lie on their respective saturation lines under identical operating conditions (pC-pH diagram), the strontium:calcium mass ratio (mg/mg) is 0.256:1. Thus, it is only possible to precipitate SrCO3 without also precipitating CaCO3 when the strontium:calcium mass ratio is greater than 0.256:1 (Najm, 2016). Statistical analysis of the strontium:calcium mass ratio in Indiana groundwater samples (n = 1,839) showed that less than 1.0% of the analyzed water samples had mass ratios greater than 0.256:1 and thus, in over 99.0% of the groundwater samples, SrCO3 could not be precipitated without also precipitating CaCO3. However, these analyses are related to the dataset from Indiana and may not be relevant to other water sources. For example, O'Donnell et al. (2016) observed no SrCO3 removal in the absence of calcium in a water sample with a pH as high as 11.0 containing 4.89 mg/L strontium and dissolved inorganic carbon of 85 mg/L. Despite the fact that the strontium:calcium mass ratio was greater than 0.256:1 in this sample, the results demonstrated that calcium precipitation was necessary for strontium removal (O'Donnell et al. 2016).
Based on the information discussed above, utilities practising lime softening for CaCO3removal from raw water may also be able to remove strontium using this process. Chemical precipitation is a viable treatment technology for strontium removal; however, an evaluation of the operating conditions to maximize strontium removal must be undertaken (Najm, 2016). A survey of several full-scale treatment plants included a lime-softening system, five IX softening systems, several filtration systems and two iron-based adsorption media. Located in five adjacent states surrounding the Great Lakes, these systems treated groundwaters containing from 0.25-36.3 mg/L strontium. The lime-softening plant had a raw water strontium concentration of 3.5 mg/L, a calcium concentration of 121.6 mg/L, and a magnesium concentration of 44.8 mg/L; it achieved removals of 73.5%, 82.4% and 73.2% for strontium, calcium and manganese, respectively. No operational data were available for this treatment plant (Lytle et al., 2015).
A lime softening study observed that the removal of strontium was related to the calcium removal in drinking water (Lytle et al., 2015; O'Donnell et al., 2016). This relationship was observed during jar-tests on strontium removal from three groundwaters that contained initial strontium concentrations of 4.2, 10.9 and 21.8 mg/L, calcium concentrations of 126, 112.0 and 102.0 mg/L and magnesium concentrations of 32, 32.5 and 43.7 mg/L, and had pH levels of 7.2, 7.3 and 7.3, respectively. Addition of lime ranging from 0 to 450 mg/L increased the pH levels up to pH 12.0. Na2CO3 at a concentration of 45 mg/L was used for non-carbonate hardness removal. The study observed that both strontium and calcium removals were increased with an increase in lime dose until pH levels reached10.0-11.0, at which point both strontium and calcium removals decreased with further increases in the lime dose. Magnesium reduction, on the other hand, rapidly increased at pHs greater than 11.0. For the tested groundwaters, maximum strontium removals of 78.2%, 78.5% and 77.7% and calcium removals of 86.4%, 83.2% and 82.4% were achieved at a pH of 10.7. The minimum strontium concentrations achieved in the treated waters (0.9, 2.3 and 4.8 mg/L) were dependent on strontium feed concentrations (i.e., the final strontium concentration in the treated water increased with increasing strontium concentration in the raw water). However, the maximum strontium removals, expressed as percentages, were approximately the same for these three different raw strontium concentrations. Since no strontium removal was observed in the absence of calcium in the control samples, the study suggested that calcium played a very important and necessary part in the mechanism of strontium removal. The maximum strontium removal corresponded to optimum calcium removal. The authors concluded that strontium co-precipitated with CaCO3 and is effectively removed by the lime-softening technique and that removal was related to the pH, the lime dose, and the concentrations of calcium and dissolved inorganic carbon. An analysis of the precipitates by X-ray diffraction analysis suggested that strontium is incorporated into the calcium crystal lattice (O'Donnell et al., 2016). These findings agreed with those of McCauley and Eliassen (1955) who reported that SrCO3 co-precipitated with CaCO3, forming calcite-strontianite mixed crystals (some of the calcium ions in the CaCO3 crystal are replaced by strontium ions).The authors also reported on a modified softening process (repeated precipitation process) originally intended for radioactive strontium treatment in drinking water and requiring a reduction of greater than 99.0% of strontium activity. Following the initial softening (80-90% removal), increments of calcium chloride in the presence of an excess soda ash were capable of achieving greater than 99.9% removal of radioactive strontium (McCauley and Eliassen, 1955).
An alternative to the conventional precipitative softening is a pellet-softening process. In this process, the water is injected with lime, caustic soda, or soda ash to raise the carbonate ion concentration and thus initiate the precipitation of CaCO3 and SrCO3. The water is passed through a contactor containing fluidized sand. Both CaCO3 and SrCO3 precipitate and form large pellets on the sand grains, which are then removed from the contactor. In a pilot-scale study, a pellet-softening process was operated to remove calcium in a RO concentrate stream from the treatment of a brackish groundwater. The RO concentrate contained 652.0 mg/L calcium and 5.0 mg/L strontium. Raising the pH of the water up to 10.5 with a combination of lime and caustic soda reduced the calcium concentration in the treated water to 19.0 mg/L and the strontium concentration to <0.05 mg/L. The process offers easy dewatering and transport of the formed precipitated material (Najm, 2016).
A limitation of the lime-soda ash softening process is the need to raise the water pH up to 10.6 for optimum strontium and calcium removal. The treated water may require recarbonation to reduce pH and the addition of corrosion inhibitors to protect the distribution system (to counter potentially altered corrosivity of the treated water due to the removal of hardness and alkalinity). Additionally, the large volume of sludge generated during the conventional precipitative softening process requires special handling (tank storage), treatment (mechanical dewatering) and off-site disposal.
7.1.4 Ion exchange
The U.S. EPA (2011) identified IX as a best available technology for radioactive strontium removal. Since stable and radioactive strontium isotopes behave the same chemically (ATSDR, 2004), this process is expected to be effective in removing natural strontium from drinking water (Sorg and Logsdon, 1980; El-Kamash, 2008; Sato et al., 2011).
The most common application of IX in drinking water treatment is water softening. Extensive research has been conducted on the applicability of strong-acid cation (SAC) and weak-acid cation (WAC) exchange resins for the removal of scale-forming calcium (Ca2+) and magnesium (Mg2+) cations, other alkaline earth elements including barium (Ba2+) and radium (Ra2+) and, to a lesser extent, Sr2+ in drinking water (Myers et al., 1985; Snoeyink et al., 1987; Subramonian et al., 1990; Clifford, 1999; Clifford et al., 2011). SAC exchange resins have strong acid exchange sites and can exchange ions throughout a wide pH range (2-11), while the WAC resins have a weak-acid functional group and only exchange ions in the neutral to alkaline pH range. SAC resins in the sodium (Na+) or hydrogen (H+) forms exchange Na+ or H+ cations for Ca2+, Mg2+, Ba2+ and Sr2+ ions in the water, either as carbonate hardness or noncarbonate hardness. Generally, the pH and alkalinity of the treated water from the SAC (Na+) remained relatively unchanged throughout the production run. However, using SAC resins in Na+ form may result in undesirable quantities of sodium in the treated water. SAC and WAC resins in the H+ form are alternative resins that can be used to produce sodium-free treated water. However, SAC resins in the H+ form are rarely used in water softening, due to the acidity of the treated water and the acid's inefficiency in regenerating the resins. The WAC resins in the H+ form effectively remove carbonate hardness (only) and divalent metal cations in the water. The process results in partial softening and produces treated water with a low alkalinity and low levels of total dissolved solids. The treated water requires CO2 stripping and pH adjustment (increase) to produce non-corrosive finished water (Clifford, 1999, Clifford et al., 2011).
IX resins exhibit a degree of selectivity for various ions, depending on the concentration of ions in solution and the type of resin selected (Clifford, 1999). The IX capacity and the selectivity of the resin are important considerations when selecting a resin. Clifford et al. (2011) compared separation factors of a number of cations on SAC resins (polystyrene divinylbenzene matrix with sulphonate functional group) and produced the following ion selectivity sequence (order of preference for exchange) for ten divalent cations:
Figure 1 - Strontium in Drinking Water
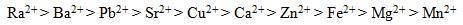
The order of preference for ion exchange is: Ra2+, Ba2+, Pb2+, Sr2+, Cu2+, Ca2+, Zn2+, Fe2+, Mg2+, Mn2+.Description Figure 1 - Strontium in Drinking Water:
The above sequence suggests that Ra2+, Ba2+ and Sr2+ cations are preferentially removed compared to Ca2+ and Mg2+ during water softening. The WAC resins exhibit the same selectivity sequence as SAC resins except that the H+ ion is the most preferred cation (Clifford et al., 2011). Due to the abundance of calcium and magnesium in natural waters and at levels that far exceed those of strontium, the presence of these ions may affect the efficiency of strontium removal (Bortun et al., 1997; Marinin and Brown, 2000; Najm, 2016) and will decrease the run length of the column to strontium breakthrough (Clifford et al., 2011).
Regenerability is another consideration when selecting a resin. In general, a resin with a high affinity for a contaminant and a long virgin run length is difficult to regenerate (Clifford, 1999). Snoeyink et al. (1987) demonstrated that barium, which has chemical and physical properties similar to strontium, accumulated onto a SAC resin with each regeneration cycle and reduced the resin's capacity during water softening.
Selective resins with a high affinity for specific contaminants have been manufactured for water treatment applications. Resins with chelating functional groups demonstrated high affinities for hardness ions and for several metals such as zinc, chromium and lead. Charizia et al. (1998) reported on the development of a selective resin for radioactive strontium removal from nuclear waste; however, Najm (2016) indicated there is no evidence that this resin or other single-use cation IX resins with a high capacity for strontium removal have yet been commercialized.
A survey evaluated the effectiveness of five full-scale IX softening systems and found that four of them had strontium removal efficiencies of 89.3-99.9% while the fifth was 33.3% (Lytle et al., 2015). The raw water strontium concentrations ranged between 0.27 and 36.3 mg/L. The highest removal (99.9%) was achieved by a system treating raw water with a strontium concentration of 36.3 mg/L. An evaluation of the IX system's effectiveness requires establishing a breakthrough curve to assist in determining the resin's bed life and the timing for regeneration. Since it was not stated at what bed volumes (BVs) the water samples were collected, the performance of these five IX systems could not be fully assessed.
A laboratory study, using strontium nitrate [Sr(NO3)2 ] as a surrogate for radioactive strontium and simulated groundwater (prepared according to the composition of a known basin underlining a nuclear site), evaluated the ability of two commercially available cation IX resins and several inorganic adsorptive materials (natural and synthetic zeolites and five new synthetic crystalline or composite materials) for radioactive strontium removal (Marinin and Brown, 2000). Based on the distribution coefficient (Kd) values obtained through the laboratory batch experiments, both commercially available cation IX resins showed the highest potential for strontium removal, with their Kd values (>200,000 and 150,000 mL/g, respectively) far exceeding those of the natural and synthetic zeolite of 680 and 1,360 mL/g, respectively. In the presence of competing ions such as calcium, magnesium and barium, the resins' Kd values for strontium have been observed to decrease. The Kd coefficient is a term used to characterize the ability of a solid phase adsorbent or resin to adsorb radioactive contaminants from contaminated liquid. The coefficient describes the volume of water that may be treated by a particular mass of adsorbent when the contaminant concentrations in the liquid and on the adsorbent reach equilibrium.
A few limitations exist when using IX treatment. Resin fouling can become a concern if the source water contains elevated levels of particulates, metals (such as iron and manganese), and/or dissolved organic matter. To preserve bed life, pretreatment may be needed to remove these inorganic and organic foulants. The use of pretreatment can add complexity to the system, increase costs, and complicate residual disposal. The application of IX treatment generates liquid waste brine that requires handling and off-site disposal. The quality of the brine, and thus its disposal limitations, will depend on the type and concentrations of its constituents. The disposal of exhausted resins can also be a limitation especially if radioactive contaminants present in raw water are also removed by IX. All of these factors need to be taken into consideration by authorities when evaluating IX as a treatment option.
7.1.5 Membrane technologies
High-pressure membrane separation processes such as RO and nanofiltration (NF) are proven technologies for removing ions from drinking water. Since strontium exists as a divalent ion (Sr2+) in water, its removal with RO or NF membranes is expected to exceed 95% under most conditions (Najm, 2016).
RO and NF processes utilize a number of mechanisms to reject inorganic constituents in drinking water (Nghiem et al., 2004). Size exclusion is an important mechanism of contaminant rejection by membranes. In an aqueous solution, the ions bind strongly with a number of water molecules by electrostatic interactions. The hydration of an ionic compound can be seen as a complexation, where water plays the role of the ligand. Complexation can significantly enhance constituent rejection, due to an increase in the ion's size (Richards et al., 2011). In addition to the size effect, an electrostatic repulsion may increase the rejection of the charged ions by the like-charged membrane. By contrast, an electrostatic attraction between oppositely charged ions and the membrane surface may decrease the rejection due to the attraction of the ions onto the membrane surface, which increases the likelihood that these ions will pass through the membrane's pores (Schaep et al., 1998; Nghiem et al., 2004; Van der Bruggen et al., 2004; Verliefde et al., 2008). Surface effects on membranes (i.e., electrostatic attraction or repulsion) have a limited duration and are rapidly diminished by fouling.
Four pilot-scale NF/RO membranes evaluated the effects of energy fluctuation and water pH levels on the rejection of inorganic contaminants, including strontium in groundwater (Richards et al. 2011). Two brackish groundwaters with strontium concentrations of 1.3 mg/L and approximately 0.5 mg/L were used in the tests. Due to the size exclusion a selected membrane achieved greater than 99.0% removal of strontium in both tested groundwaters. No impact of the operating conditions was observed (Richards et al., 2011).
A low-pressure (<1.0 MPa), spiral-wound, polyamide RO membrane was tested for the treatment of radioactive strontium and cesium (Ding et al., 2015). The test solutions were prepared by dissolving Sr(NO3)2 as the surrogate for radioactive strontium into an ultrapure water containing 1mM of several common cations (Na+, Mg2+and Ca2+) as chloride salts and 1mM of several common anions (Cl-, F-, NO3-and SO42_) as sodium salts. The technical specifications of the pilot-scale RO membrane were: molecular weight cut-off of 100 Da, salt rejection efficiency of 98%, and water recovery of 37.5%. Both permeate and reject water were recycled back to the feed reservoir to keep the strontium feed concentration in the range of 100 to 1,000 µg/L. Electrostatic interactions were found to promote strontium rejection, as evidenced by a minimum rejection rate (approximately 97.2%) that occurred at pHIEP of 5.0 (the membrane's isoelectric point). At pH values below 5, the RO membrane was positively charged, and strontium rejections greater than 99.0% were achieved due to the electrostatic repulsion. Rejection rates greater than 99.0% also were observed at pH 9.0, due to the Donnan's potential created at the boundary between the membrane surface and the solution. The RO membrane was also tested with surface water spiked with 1,000 µg/L of strontium. Greater than 97.5% strontium rejection was observed, with the flux being only slightly decreased after a 24-h operational period (Ding et al., 2015).
Two pilot-scale treatment trains were operated to determine the feasibility of reclaiming secondary effluent water from a wastewater treatment plant for potential groundwater replenishment and surface water augmentation (Liang et al., 2011). Train No. 1 consisted of an ultrafiltration unit, a RO unit, and an advanced oxidation process (AOP); train No. 2 consisted of a membrane bioreactor (MBR), a RO unit and an AOP. The ultrafiltration unit tested was a 60 gpm (3.8 L/s) continuous filtration system equipped with 12 hollow-fiber ultrafiltration membrane elements with a nominal pore size of 0.04 µm. The MBR evaluated was a 60 gpm (3.8 L/s) unit that housed two sets of ten hollow fiber membrane modules with a 0.04-µm nominal membrane pore size. The RO systems were identical: each consisted of a cartridge filter and a two-stage pressure vessel array containing a total of 21 spiral wound membrane elements. No strontium removal was observed by either the ultrafiltration or the MBR unit. Almost complete removal (99.9%) was achieved by both RO pilot units, with the median concentrations decreasing from 726 µg/L in the feed water to below 0.5 µg/L in the RO permeate. Both RO units were operated at an average flux of 12 gfd (20.4 L/m2 h) and an average recovery rate of approximately 85% (Liang et al., 2011). Similarly, in order to augment potable water supply requirements, Subramani et al. (2010) reported on the use of RO membranes for demineralizing surface water. Strontium concentrations were consistently reduced from 1.2 to 1.3 mg/L in the pretreated feed to less than the MDL of 0.01 mg/L in the permeate (Subramani et al., 2010).
Limitations of the RO process include possible membrane scaling, fouling and failure, as well as higher energy and capital costs. Calcium, barium, and silica can cause scaling and decrease membrane efficiency. Colloids and bacteria can also cause fouling. Both scaling and fouling will increase pressure drop, thus decreasing membrane life and increasing energy costs. Pretreatments such as softening and cartridge filtration and/or membrane cleaning can help obtain acceptable membrane run times. Chlorine can damage RO membranes and should be quenched using dechlorination chemicals or granular activated carbon. Since RO removes alkalinity in water, it lowers the product water pH and increases its corrosivity. Therefore, the product water pH and alkalinity must be adjusted to avoid corrosion issues in the distribution system, such as the leaching of lead and copper (Schock and Lytle, 2011).
7.1.6 Other technologies-Inorganic adsorbents/ion exchange resins
A significant number of studies have examined the use of natural and synthetic inorganic adsorbents for strontium removal from liquid waste generated by nuclear power plants or groundwater contaminated by nuclear waste. The studies addressed these inorganic materials as adsorbents, although the mechanism of strontium removal is mostly considered an IX process. Despite the observed high exchange capacity and selectivity for strontium (Bortun et al., 1997; Marinin and Brown, 2000; Kulyukhin et al., 2005; El-Kamash, 2008; Sato et al., 2011; Ivanets et al., 2014), these inorganic adsorbents/ion exchange resins appeared to be less efficient than the conventional regenerable cation exchange resins, and they are suspected to be more costly (Marinin and Brown, 2000; Najm, 2016). It should be noted that many of the inorganic adsorbents have been laboratory prepared and are not commercially available. Bench- and pilot-scale tests are recommended on the most promising adsorbents (Najm, 2016).
7.1.6.1 Zeolite and phosphate-based adsorbents
Studies have been conducted examining the use of zeolites and phosphate-based adsorbents for strontium removal in water. Zeolites (alumino-silicates e.g., clinoptilolite and mordernite) are abundant in the environment and have been used extensively as adsorbents and an IX media in wastewater treatment systems (Kulyukhin et al., 2005; El-Rahman et al., 2006; El-Kamash, 2008; Yusan and Erenturk, 2011; Ivanets et al., 2014; Araissi et al., 2016).
El-Kamash (2008) reported on the feasibility of a synthetic zeolite A (Na+ form) to adsorb strontium from aqueous solutions in batch and fixed-bed column operations. The study was another example of using a stable surrogate (SrCl2∙6H2O in distilled water) to obtain useful treatment information for radioactive isotope removal from aqueous and nuclear waste streams. Batch experiments were performed to determine the optimum pH range for removal, which occurred between 6.0 and 8.0. In the fixed-bed column experiments, the effects of the initial strontium concentration (50, 100, and 150 mg/L), bed height (3.0, 4.5, and 6.0 cm), and feed flow rate (3.12 and 5.00 mL/min) were examined. These initial strontium concentrations were much higher than concentrations encountered in water treatment plants. Overall, percentage removal of strontium ranged from 64.5% to 86.4%. The greatest removal (86.4%) was achieved for an initial strontium concentration of 100 mg/L, in a 6.0 cm deep bed, at a feed flow rate of 3.12 mL/min [equivalent to an empty bed contact time of 1.5 min]. In general, high removals were observed at lower flow rates and in deeper beds, implying longer contact times (El-Kamash, 2008). Similarly, Sato et al. (2011) and Araissi et al. (2016) indicated that synthetic zeolite "4A" was an effective IX material for the removal of strontium and barium from aqueous solutions. A test solution was prepared by dissolving SrCl2 (as a radioactive surrogate) into tap water for a target concentration of 40-50 µg/L (Sato et al., 2011). Zeolite A4 (cation exchange capacity of 5.5 meq/g) was added to the test solution at a concentration in the range of 0.001% to 1.0% (w/v) and stirred for 30 min. Strontium removal rates were found to be greater than 90% when zeolite A4 was added at a concentration of 0.01% or higher at a pH range of 5.8-8.6. Since column tests were not performed, the efficiency of this material could not be truly evaluated (Sato et al., 2011).
Kulyukhin et al. (2005) and Ivanets et al. (2014) examined the removal of strontium from water using several synthetic calcium- and magnesium-phosphate adsorbents. Ivanets et al. (2014) found that calcium hydrogen phosphate, hydroxyapatite and magnesium hydrogen phosphate were capable of adsorbing strontium up to 10.9 mg/g, 25.7 mg/g and 280.0 mg/g, respectively. The tests were conducted with a stable surrogate [Sr(NO3)2] at concentrations ranging from 10 mg/L to 5,000 mg/L. The different mechanisms of interaction between strontium ions and the studied inorganic adsorbents explained the different adsorbents' performance (Ivanets et al., 2014).
7.1.6.2 Silicotitanates
Bortun et al. (1997) extensively studied various synthetic inorganic polyvalent metal silicates and phosphates for strontium removal. In batch experiments, two sodium silicotitanates (Na2Ti2SiO7·2H2O and Na2TiSi2O7·2H2O) and sodium titanate (Na4Ti9O20) exhibited a high affinity for strontium. The experiments were conducted with a strontium concentration [as SrCl2] of 87.6 mg/L with and without the presence of competing ions (sodium and calcium). Maximum strontium uptakes of 2.0-2.5 meq Sr/g for both sodium silicotitanates, and 2.0 meq Sr/g for sodium titanate, were observed at pH levels 5.5-8.0 and 6.5-7.5, respectively. While calcium ions were the main competitive ions for selective strontium removal, sodium ions showed no effect (Bortun et al., 1997). Similarly, Marinin and Brown (2000) reported that among several inorganic adsorptive materials tested, the synthetic sodium silicotitanate and sodium titanate had higher distribution coefficients for strontium but low selectivity.
Both crystalline silicotitanates (CSTs) forms (powder and pelletized) have shown the ability to remove both natural strontium and cesium from wastewater at neutral pH levels and in the presence of other competing ions (Bostick et al., 1997; Spencer et al., 2000). In bench-scale column tests (Bostick et al., 1997) using IONSIV® IE-911(pelletized form) in hydrogen form, a strontium breakthrough of 1.0% occurred at 14,000 BVs and a breakthrough of 15.0% occurred at 120,000 BVs after 10 months of column operation. The initial concentrations were 0.1 mg/L for strontium, 45.0 mg/L for calcium, 18.0 mg/L for sodium, and 9.0 mg/L for magnesium; the pH ranged from 7.0 to 8.0. All other cations achieved a 50% breakthrough under 1,300 BVs. For comparison purposes, a tested zeolite performed less effectively for strontium removal, with 1.0% breakthrough observed at 3,000 BVs and 50.0% breakthrough at 15,000 BVs. (Bostick et al., 1997).
7.2 Distribution system
Drinking water distribution system piping is susceptible to corrosion and accumulation of scale deposits on the interior of the pipe surface. Both corrosion products and scale deposits formed in drinking water distribution systems serve as reactive sinks for such metal ions as lead, copper, arsenic, vanadium and it is reasonable to conclude that strontium ions also concentrate in the corrosion products (Gerke et al., 2013). These sinks can periodically dislodge as a result of physical/hydraulic disturbances or unstable water chemistry, allowing the contaminants to be re-mobilized into the water and creating potential health risks (Ouvrard et al., 2002; Dong et al., 2003; Schock and Holm, 2003; Schock, 2005; Schock et al., 2014; Lytle et al., 2004; Friedman et al., 2010; Gerke et al., 2013, 2014, 2016).
Maintaining stable water quality conditions is an important factor in minimizing accumulation/release of trace inorganic contaminants in the distribution system. Friedman et al. (2010) identified several key water quality conditions that should be controlled in order to maintain water stability for deposited inorganics, including pH, oxidation-reduction potential and corrosion control measures, as well as avoiding the uncontrolled blending of surface water and groundwater.
There is limited information on the accumulation of strontium in water distribution systems and the factors contributing to strontium release events. Gerke et al. (2014) evaluated strontium adsorption/desorption mechanisms to/from iron corrosion products with respect to disinfectants (chlorine and chloramine) and water flow conditions (continuous flow and maximum residence time) in model drinking water distribution systems. Corrosion products formed in both chlorine- and chloramine-disinfected water samples were composed predominately of γ-FeOOH iron oxyhydroxides, and to lesser extent of α-FeOOH and Fe3O4. The corrosion products that formed in the samples simulating continuous flow of chlorine- and chloramine-disinfected waters contained strontium concentrations of 22 mg/kg (0.002 wt%) and 47 mg/kg (0.005 wt%), respectively. Notably, in the samples simulating stagnation time in the distribution system, strontium concentrations in the corrosion products were similar and increased to 215 mg/kg (0.02 wt%) and 217 mg/kg (0.02 wt%) for the chlorine- and chloramine-disinfected water samples, respectively. Once the water flow was resumed, strontium concentrations in the scale deposits decreased to 30 mg/kg (0.003 wt%) in the chlorine-disinfected water sample and 40 mg/kg (0.004 wt%) in the chloramine-disinfected one. The concentrations and rates of strontium adsorption and desorption differed slightly based on the disinfectants used. The author indicated that iron oxyhydroxides were an important phase in the accumulation of strontium in both chlorine- and chloramine-disinfected waters (Gerke et al., 2014). Similarly, Gerke et al. (2013) reported average strontium concentrations of 3-54 mg/kg (up to 0.005 wt%) in the surface layer of iron corrosion products collected from four fully operated distribution systems (three unlined cast iron mains and one galvanized iron pipe). All treated drinking waters had strontium concentrations below 0.5 mg/L. In addition, a strontium concentration of 40.3 mg/kg (0.004 wt%) was measured in particulates collected from a polypropylene sediment filter (home installation, 4 years of operation). The detected strontium concentration was approximately the same as the average strontium concentration of 38.0 mg/kg (0.004 wt%) measured in the surface layer of the iron corrosion products in the distribution system. It was suggested that the particulate-bound strontium had detached from the pipe surface and had been transported to the residence (Gerke et al., 2013).
Studies have also found that strontium is adsorbed and/or incorporated into CaCO3 precipitates and into manganese deposits accumulated in distribution systems (Gerke et al., 2014, 2016). (Gerke et al., 2016) found that iron, chromium and strontium were accumulated within the manganese deposits that formed on lead pipes and brass connectors in two water distribution systems. The authors suggested that destabilization of manganese deposits may increase the concentration of metals at consumers' taps (Gerke et al., 2016). Scale deposits from water distribution pipe materials (polyethylene and cast iron) were collected from 16 drinking water distribution systems in Finland, as a part of a water main cleaning program (Zaheus et al. 2001). The program involved swabbing the water mains in 1996, sampling, then returning after one year to assess changes. The average strontium, iron and calcium concentrations measured in the deposit samples in 1996 were 85 mg/kg (0.008 wt%), 18% (by weight) and 3.0% (by weight), respectively. After one year, trends toward increased concentrations were observed for each of the metals. The average strontium concentration increased to 253 mg/kg (0.02 wt%), and iron and calcium concentrations increased to 31% and 5.4%, respectively, in the newly formed deposits. However, the study was originally conducted to assess microbial growth in distribution systems and no conclusion was drawn on the accumulation of inorganics in the deposits (Zaheus et al., 2001).
Based on the results of this study, the accumulation of strontium in distribution systems is not considered significant. However, the accumulation may be more significant when distributed levels are higher (e.g. near or above the MAC).
7.3 Residential scale
In cases where strontium removal is desired at the household level, for example when a household obtains its drinking water from a private well, a residential drinking water treatment device may be an option for reducing strontium concentrations. Before a treatment device is installed, the water should be tested to determine general water chemistry and verify the presence and concentrations of strontium in the source water. Periodic testing by an accredited laboratory should be conducted on both the water entering the treatment device and the finished water to verify that the treatment device is effective. Treatment devices lose their removal capacity through usage and time and need to be maintained and/or replaced. Consumers should verify the expected longevity of the components in their treatment device according to the manufacturer's recommendations and service it when required.
Health Canada does not recommend specific brands of drinking water treatment devices, but it strongly recommends that consumers use devices that have been certified by an accredited certification body as meeting the appropriate NSF International (NSF)/American National Standards Institute (ANSI) drinking water treatment unit standards. These standards have been designed to safeguard drinking water by helping to ensure the material safety and performance of products that come into contact with drinking water. Certification organizations provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada (SCC). In Canada, the SCC has accredited the following organizations to certify drinking water devices and materials as meeting NSF/ANSI standards (SCC, 2019):
- CSA Group (www.csagroup.org);
- NSF International (www.nsf.org);
- Water Quality Association (www.wqa.org);
- UL LLC (www.ul.com);
- Bureau de normalisation du Québec (www.bnq.qc.ca); and
- International Association of Plumbing & Mechanical Officials (www.iapmo.org).
An up-to-date list of accredited certification organizations can be obtained from the SCC (www.scc.ca).
Although no residential treatment devices are certified for strontium removal from drinking water, treatment devices using either RO or IX technologies would be able to remove it at the residential scale. It is recommended to use treatment devices certified as meeting NSF/ANSI Standard 58 (Reverse Osmosis Drinking Water Treatment Systems), NSF/ANSI Standard 44 (Cation Exchange Water Softeners) or NSF/ANSI Standard 53 (Drinking Water Treatment Units - Health Effects). Water that has been treated using RO may be corrosive to internal plumbing components and should be installed only at the point of use. These systems also require larger quantities of influent water to obtain the required volume of drinking water and are generally not practical for point-of-entry (POE) installation. In addition, it may be necessary to pretreat the influent water to reduce fouling and extend the service life of the RO membrane.
Through the EPA Environmental Technology Verification (ETV) Program, NSF International, an ETV Drinking Water Systems Center sponsored by the U.S. EPA for technology verifications, evaluated four commercial POU-RO devices manufactured by Watts Premier Inc. (NSF, 2006a, 2006b), EcoWater Systems, Inc. (NSF, 2005a), and Kinetico, Inc. (NSF, 2005b). The devices were challenged with individual or combined challenge contaminants, including organic and inorganic chemicals and microorganisms.
Watts Premier M-2400 POE was a skid-mounted RO system equipped with a 4 in × 40 in RO membrane cartridge [with a surface area of 82 ft2 (7.6 m2)], a pre-membrane sediment or activated carbon filter, an optional post-membrane activated carbon filter, and an optional product water tank. Natural strontium was one of the 17 challenge chemicals and microorganisms tested. Unlike the rest of the challenge chemicals and microorganisms, strontium, cesium, and cadmium were combined into one challenge with influent and permeate samples collected at the beginning and the end of the 30-min operation. Triplicate influent and effluent measurements indicated that strontium concentrations were reduced from 990 to 2 µg/L, equivalent to >99% removal (NSF, 2006a).
The Watts Premier WP-4V POU was a RO device consisting of a RO membrane, a sediment filter (for particulate matter removal), a pre-activated carbon filter (for chlorine removal), a post-activated carbon filter, and a 3-gal storage tank. For strontium and the other three inorganic chemicals, the individual challenge was conducted with only the RO membrane component in place. Again, the chemical analyses indicated almost complete removal of strontium, reducing its concentrations from 920 to 1 µg/L (NSF, 2006b).
The EcoWater Systems ERO-R450E POU was also a RO unit equipped with a RO membrane, pre- and post-activated carbon filters, and a 3.1-gal storage tank. Prior to testing, the RO membrane was service-conditioned with water for seven days. Similarly to the Watts Premier WP-4V POU device, the ERO-R450E POU device was challenged with only the RO membrane component in place and with an individual inorganic challenge chemical. Over 96% strontium removal was observed, with the concentrations reduced from an average 960 µg/L to an average of 33 µg/L (NSF, 2005a).
The Pall/Kinetico Purefecta™ POU was a RO device composed of a RO membrane, a pre- sediment filter or an activated carbon filter, a Pall bacteria/virus bio-filter, and a 3-gal pressurized bladder tank for permeate storage. After being service-conditioned for seven days, the device with only the RO membrane in place was challenged with an individual challenge chemical over a one-day period. Near-complete removal (99%) was observed; strontium concentrations were reduced from 850 to 2 µg/L (NSF, 2005b).
Several POE IX softening systems, without any description of system components or operational conditions, achieved 96.3-100% removal efficiency for treating strontium concentrations of 0.27-8.22 mg/L (O'Donnell and Lytle, 2014). Homeowners with private wells using IX softeners in sodium form should be aware that the treatment unit may introduce undesirable quantities of sodium into the treated water. The contribution of sodium to water from a water softener will vary depending on the level of hardness of the water.
8.0 Kinetics and metabolism
8.1 Absorption
When ingested, forms of strontium that are soluble or are found in food are rapidly absorbed by the stomach and small intestine, with highest rates at 1-2 h after ingestion (Skoryna, 1981; Leeuwenkamp et al., 1990). A mean oral absorption of 22-25% has been reported, varying mainly with dose levels and age of the subjects, but with no major differences between sexes and species (Kahn et al., 1969; Kostial et al., 1969; ICRP, 1993; Sips et al., 1996; Apostoaei, 2002; Li et al., 2006). At low doses, the absorption is proportional to the oral dose; however, this proportion decreases with increasing doses or when ingested with food or calcium (Skoryna, 1981; Sips et al., 1996; Nielsen, 2004).
In humans, higher absorption rates have been observed in drinking water (mean of 57%, maximum of 97%) compared with salad, milk, baby food, or calcium solutions (means of 27% to 45%; Li et al., 2006). Polysaccharides, alginate and pectin present in food were also found to lower the absorption of strontium compared to when strontium was administered in drinking water to 10 adult volunteers (Höllriegl et al., 2004). Strontium absorption is increased with low calcium intake and fasting, from 20% and 25% to 40% and 55%, respectively (ICRP, 1993). Wellman et al. (1966) showed higher strontium retention in infants than in children aged 6-9, although no details were provided on dose and duration of exposure. Variations (from -47 µg to 59 µg of strontium) in absorption (intake from breast milk minus faecal excretion) were observed in 6- to 8-day-old infants (n = 12) (Harrison et al., 1965).
Young rats absorb a higher proportion of strontium than older rats, possibly due to increased bone formation (Kshirsagar, 1985; Apostoaei, 2002). The absorption of 1 µCi of 85SrCl was higher in 14- to 18-day-old rats (95%, ±0.4, n = 31 rats) than in 22-day-old rats (74%, ±2.4, n = 5 rats), 6- to 8-week-old rats (25%, ±1.0, n = 45 rats) and 60- to 70-week-old rats (11%, ±0.8, n = 24 rats) (Taylor et al., 1962). In another study, 22-day-old rats fed different levels of calcium (0.1-2%) and a fixed level of strontium (0.04 µCi/g of food) absorbed more strontium than 200-day old rats (Palmer and Thompson, 1964). Young rats start to preferentially absorb calcium (competing with strontium) around one year of age (Kshirsagar, 1985).
When calcium levels in the plasma decrease, parathyroid hormone secretion is stimulated, which results in increased strontium absorption, bone remodelling rates, and numbers of intestinal calcium transporters (MacDonald et al., 1951; Shorr and Carter, 1952; Eisenberg and Gordan, 1961; Sips et al., 1996; Sairanen et al., 2000; Apostoaei, 2002; Llinas et al., 2006). Strontium competes with calcium for absorption in the duodenum, although strontium has a lower affinity for the intestinal transporters (Kahn et al., 1969; Schrooten et al., 1999). Rats fed lower levels of calcium (0.1%) had higher bone 90Sr retention rates than those fed higher levels of calcium (2%) (Palmer and Thompson, 1964). In ex vivo experiments with duodenal tissue, calcium decreased the absorption of strontium more than strontium reduced the absorption of calcium (Hendrix et al., 1963). Other factors such as vitamin D polymorphism, vitamin D levels and magnesium intake can also impact strontium absorption rates (Hendrix et al., 1963; Vezzoli et al., 2002).
A single oral dose of 500 mg of strontium gluconate in a human patient resulted in a rapid absorption, with peak serum concentration (Cmax) at 4 h (Skoryna, 1981). Strontium absorption was biphasic after administration of 2.5 mmol of strontium (667 mg of SrCl2) to six healthy males (Leeuwenkamp et al., 1990). The mean Cmax was 3.55 µg/mL and the area under the curve was 9,138 µg.mL/min (Leeuwenkamp et al., 1990). In another study, the maximum strontium plasma concentration was reached after 2.9 h in 16 men administered 2.5-5.0 mmol SrCl2 orally (Sips et al., 1996). The Cmax after a single oral exposure to 2 g SrR (containing 680 mg strontium) was 6 mg/L and the volume of distribution of strontium was 64 L (EMA, 2005). Patients administered 183-274 mg per day of strontium from strontium gluconate for a minimum of 3 months had a mean serum level of 5.13 mg/L (Skoryna, 1981). Maximum plasma concentration was attained 3-6 h after a single oral dose of SrR (2 g) (EMA, 2005). SrR is not found in the environment, but it dissociates into two Sr2+ atoms and an organic moiety (ranelic acid) in vivo (Meunier et al., 2004; EMA, 2013; Querido et al., 2016).
Hooded rats exposed to 0.09%, 0.19%, or 0.34% strontium chloride in drinking water had mean serum strontium levels of 1.92 mg/L, 3.8 mg/L, and 8.68 mg/L after 3 months, respectively (Skoryna, 1981). Absorption dropped after 2 years. Lactating rats were shown to absorb twice the amount of strontium as non-lactating rats (Kostial et al., 1969).
8.2 Distribution
Similarly to calcium, strontium distributes to most organs within the body and can form complexes with carbonate, phosphate, citrate, lactate, and hydroxyapatite, leading to its rapid distribution and accumulation in bones and teeth, with 99% of the body burden being in the skeleton (Storey, 1968; El and Rousselet, 1981; Humphrey et al., 2008). Following bone, the highest strontium concentrations are found in the kidney, lung, adrenal, brain, heart, muscle, and liver tissues in animals and in muscle, fat, and skin in humans (Schroeder et al., 1972; Skoryna, 1981). Hooded rats exposed to 0.09%, 0.19%, or 0.34% strontium chloride in drinking water had bone strontium:calcium ratios of 1:50, 1:27, and 1:12, respectively (Skoryna, 1981). Within cells, strontium is mainly bound to calcium-binding proteins (Ca2+ATPases, Na+-Ca+ antiport, etc.) and its highest concentrations are found in the mitochondria, sarcoplasmic reticulum, lysosomes and microsomes (Skoryna, 1981). It distributes heterogeneously in bone and replaces calcium in hydroxyapatite crystals by surface exchange or ionic substitution (Skoryna, 1981).
Bone concentration of strontium over time can vary between individuals, depending on different factors (e.g., exposure, absorption, age, diet, medication, gender, skeletal site, time of day). It is unclear whether strontium bone concentration plateaus over time in humans (Bärenholdt et al., 2009; Doublier et al., 2011; Moise et al., 2012). The strontium-90:calcium ratio in bones was found to increase most rapidly in the period between birth and 1 year, compared with older aged children and adults in the U.K. during the highly active nuclear testing period of 1959-1968 (Papworth and Vennart, 1984). The authors estimated that 10% of the dietary strontium was integrated into the bone of newborns. This proportion decreased to 4.5% at 5 years old, and then rose to 9.5% at 15 years old, before falling to a value of 4.5%, which is equivalent to the adult mean. In adults, strontium bone concentrations were found to increase with age and to be higher in trabecular tissue than in cortical bone and osteoid tissue (Bärenholdt et al., 2009; Roschger et al., 2010; Moise et al., 2012).
Amongst 32 osteoporotic females, the highest bone strontium concentrations (mean of 1.1%) were found in those receiving the SrR supplement for the longest period (7-8 years) There was a high variability in strontium bone uptake between patients (Bärenholdt et al., 2009). In femoral bones of 14 post-menopausal women, strontium tended to distribute between the bone matrixes and cement lines, and it was significantly correlated with calcium levels and the degree of mineralization (Pemmer et al., 2013). The volume of strontium distribution was 71 L over 20 days in 10 male volunteers injected with 5 mmol strontium gluconate intravenously (Moroas et al., 1991).
Placental transfer is likely, since the fetal strontium burden was found to be higher in rats injected with strontium during high ossification periods of gestation (Rönnbäck 1986). Strontium is transferred into maternal milk; after suckling, F1 rat pups had twice the strontium plasma concentration than their mothers (EMA, 2014). Indeed, SrR given at high oral doses (750 mg/kg bw per day) to rats resulted in a breast milk:plasma ratio of 73 and high plasma concentrations in the neonates (Servier laboratories, 2016).
8.3 Metabolism
Strontium is not metabolized but interacts with proteins and inorganic anions, such as carbonate and citrate (EMA, 2005).
8.4 Excretion
Elimination of strontium is principally through urine and feces in humans and animals (Harrison et al., 1965; Skoryna, 1981; Schrooten et al., 1999; Cohen-Sohal, 2002; EMA, 2005). In a study of 25 infants, strontium was mainly eliminated in the feces, and elimination increased linearly with strontium intake (Kahn et al., 1969). Whole body elimination of strontium is slow, and it has been shown to undergo renal tubular reabsorption (ATSDR, 2004). After exposure, the rapid phase of elimination is followed by a slow phase, reflecting the slower removal from the skeleton (WHO, 2010). Clearance from the bone exchangeable pools, displacement by calcium, and removal by osteoclasts are the mechanisms responsible for strontium removal from bone (Cohen-Sohal, 2002).
In 6 healthy males orally administered 667 mg of SrCl2·6H2O, plasma elimination half-lives were 5.2 h for the first phase and 47.3 h for the second phase, and the clearance was 100 mL/min/1.74 m2 (Leeuwenkamp et al., 1990). In this study, strontium was shown to be more effectively excreted in the urine than calcium. In 10 male volunteers injected with 5 mmol strontium gluconate intravenously, a total clearance of 13.5 L per day, a renal clearance of 7.8 L per day, and a half-life of 5.4 days were observed over 20 days (Moroas et al., 1991). Plasma concentrations of strontium were near the limit of detection after 20 days. In another study, the mean strontium clearance was 4 L per day (range 0.7-9.2 L) in children 4-14 years old (n = 12) and 9.7 L per day (range 7.4-12.7 L) in adults 35-63 years old administered one oral dose of 5 mg of strontium (Sutton et al., 1971). In humans exposed to radioactive strontium for decades in Russia, whole-body half-lives (clearance of strontium) of 16 and 28 years were observed for females and males, respectively (Tolstykh et al., 2014). The shorter half-life in women was hypothesized to reflect the higher rates of bone loss in post-menopausal individuals. Plasma clearance and renal clearance were estimated at 17.3 L and 10.1 L per day, respectively, and the half-life at 50-60 h, after a single oral exposure to 2 g SrR (containing 680 mg strontium) (EMA, 2005; Servier laboratories, 2016). When Sprague-Dawley rats were injected intraperitoneally with 20 µCi of SrCl2 the strontium half-life was 113.7 days (Cohn and Gusmano, 1967). In rats and monkeys, half-lives of 78 and 23 days, and volumes of distribution of 71 and 15 L were observed when 75-1150 and 9-940 mg SrR/kg bw were given orally, respectively (EMA, 2005).
8.5 Physiologically based pharmacokinetic models
The International Commission on Radiological Protection (ICRP, 1993) developed a compartmental model of the kinetics of alkaline earth elements (including radioactive strontium) in humans that is applicable to infants, children, adolescents, and adults (Shagina et al., 2015a, 2015b, 2015c); however, the model is focused on human dosimetry and age-dependent dose coefficients and does not characterize variability within age groups. The model showed variations across age groups, and it estimates that infants absorb 60% of ingested strontium, decreasing to 30% in adulthood.
A physiologically based pharmacokinetic model was developed to estimate the bone exposure of ovariectomized adult Sprague-Dawley female rats administered SrR (250 mg/kg per day) for 6 months (Pertinez et al., 2013). However, the form of strontium and the osteoporotic animal model are not representative of the environmental exposure to strontium by the general population.
9.0 Health effects
9.1 Effects in humans
In summary, strontium supplementation has been shown to be beneficial to bone; however, rickets, osteomalacia, and non-severe effects (e.g., gastrointestinal disturbances) were associated with environmental exposure. Serious side effects (e.g., cardiovascular effects) have been associated with clinical supplementation of SrR to osteoporotic participants (all patients were also supplemented with vitamin D and calcium). The significance of the health effects reported in humans is limited for the risk assessment of strontium in drinking water. The poor quality of the environmental database, the lack of consistency across trials, the trial design focus on beneficial effects on bone, the low magnitude of the side effects, and the low relevance of the drug trials to the drinking water context (because of the differences in studied subpopulations) impedes using the epidemiological database for the derivation of a point of departure. Moreover, the increase in the risk of cardiovascular effects was only observed in osteoporotic patients with a history of cardiovascular diseases. In addition, the rates of cardiovascular effects were similar to the ones in the general population, they were not observed in individual studies or in other large trials, and no mechanism of action has been suggested.
9.1.1 Beneficial effects
Strontium is likely a non-essential trace element (its role in bone turnover is not fully understood). Supplementation of strontium salts (e.g., SrR, strontium citrate) has been shown to be beneficial for the bone of animal models of osteoporosis at doses of 600 mg/kg bw per day (Bain et al., 2009; Zhao et al., 2015). It has also been shown to improve bone density in osteoporotic patients after supplementation of 680-1360 mg strontium per day from SrR in many double-blind clinical trials (Roschger et al., 2010; Doublier et al., 2011; Genuis and Bouchard, 2012; Reginster et al., 2010, 2014, 2015). The strontium concentration in drinking water has been correlated negatively with the occurrence of cavities; however, the nature of the relationship has not yet been defined (Athanassouli et al., 1983; Curzon, 1985; Li et al., 2013; Lippert and Hara, 2013).
9.1.2 Acute exposure
Very few studies have documented the acute toxicity of strontium. Single oral doses up to 7.5 g of strontium (sourced from 11 g of SrR) were well tolerated without causing symptoms in healthy young male volunteers (Servier laboratories, 2016).
9.1.3 Chronic exposure
9.1.3.1 Skeletal toxicity
Despite a large body of evidence for animal effects, only a few epidemiological studies have documented the effects of environmental exposure to strontium on humans. The ATSDR (2004) reported that effects on bone can occur when children are exposed to high levels (doses were not specified) of strontium and have vitamin D and calcium deficiencies.
In Turkey, children aged 6-60 months (n = 2,140) living in an area with high soil levels of strontium (>350 ppm) and where nutrition is based mainly on grain cereals, had an increased prevalence of having one or more symptoms of rickets (i.e., osteoid mineralization disorder in children, characterized with bulging of the wrists, bone deformities, craniotabes [softening of the skull], rachitic rosary [expansion of the anterior rib ends at the costochondral junctions], abnormal height/weight) compared with those living in areas with low levels (<350 ppm) (Ozgür et al., 1996). Although the authors did not investigate the levels of bone forming minerals in the local diet, they did observe a protective effect in infants that were breast fed.
The analysis of the bones from 100 patients on dialysis treatment from different countries found that the strontium concentration was higher in those with osteomalacia (bone mineralization disorder in adults equivalent to rickets in children) than in patients with renal osteodystrophy (D'Haese et al., 2000). However, the authors indicated the role of strontium in osteomalacia remains undefined, patients with kidney dysfunctions do not necessarily represent the general Canadian population, and bones of patients with osteomalacia also had higher concentrations of aluminum.
9.1.3.2 Cardiovascular toxicity and hypersensitivity reactions
The evidence on the risk of cardiovascular effects following environmental exposure to strontium is limited and results have shown no adverse effects. One study found that strontium levels in drinking water (0.4-37.8 mg/L) and in urine were negatively correlated with cardiovascular mortality (i.e. protective association) in people over 45 years old residing in Texas (Dawson et al., 1978), however, co-exposure to calcium, magnesium, lithium and silicone limits the interpretation of the observed association. The use of strontium at therapeutic doses (680 mg strontium/day) as SrR for the treatment of osteoporosis has been shown to be associated with adverse cardiovascular outcomes in specific patient populations such as those with a history of heart or circulatory disease; there were no data related to cardiovascular effects of strontium below therapeutic doses.
High oral doses of strontium (680-1360 mg per day from SrR) have been associated with severe side effects, such as cardiovascular effects in some osteoporosis trials. A slightly higher risk of venous thromboembolism (VTE) (relative risk [RR] = 1.6, 95% confidence interval [CI]: 1.0, 2.0) has been observed in the SrR users when combining two large European double-blind, placebo-controlled studies (the spinal osteoporosis therapeutic intervention [SOTI] study and the treatment of peripheral osteoporosis [TROPOS] study). Pooling the trials resulted in a significantly higher risk (RR = 1.6, 95% CI = 1.07; 2.38) of myocardial infarction in SrR groups compared with placebo (EMA, 2014; Servier laboratories, 2016). However, the rates were similar to the general population and not observed by the individual studies included in the RR calculation (Cianferotti et al., 2013; Reginster et al., 2014; Servier laboratories, 2016). Moreover, no mechanism of action behind these hypothetical effects has been elucidated and patients with VTE already had various risk factors (e.g., age, immobility, past medical history; Reginster et al., 2013).
No differences in biomarkers of hemostasis or cardiovascular biochemistry indices were found in osteoporotic women receiving 2 g per day SrR (n = 40) for 12 months compared with baseline values or with the placebo group (n = 40), and there were no cases of VTE (Atteritano et al., 2015). This was also observed in a study by Ulger et al. (2012) in which hemorheological parameters (erythrocyte morphology and blood viscosity) were not changed after 2 months of treatment with 2 g SrR per day in 22 osteoporotic women. A cohort study conducted in a general practice setting in England found that patients (n = 10 865, mean of 73 years old, 91% women) taking 2 g SrR per day had no increase in rates of VTE compared with other groups of patients (Osborne et al., 2010).
Another rare but serious side effect, a severe hypersensitivity reaction syndrome characterized by drug rash with eosinophilia and systemic symptoms (DRESS), has been observed in SrR post-marketing surveillance (<20 for 570 000 patient-years of exposure) and had also been previously associated with the administration of other drugs (Meunier et al., 2009; Cianferotti et al., 2013). However, a cohort study in England (n = 10 865) found no cases of DRESS in patients taking 2 g SrR per day (Osborne et al., 2010). The absence of this effect was supported by a retrospective study in postmenopausal women treated with SrR (n = 12 702) in seven European countries (Audran et al., 2013). A publication on pharmacovigilance and drug safety in France has indicated that similar health endpoints (e.g., cardiovascular, digestive system, cutaneous, etc.) have been reported by SrR users from 2006 to 2009 (Jonville-Bera and Autret-Leca, 2011). However, causality cannot be determined based on this type of report, since there was no comparison with control groups or background rates of the health endpoints (associative only, without information on a potential risk).
In three other double-blind, placebo-controlled studies (prevention of early postmenopausal bone loss by SrR [PREVOS], strontium administration for treatment of osteoporosis [STRATOS] and SrR efficacy in knee osteoarthritis trial [SEKOIA]), 0.5 to 2 g SrR per day were administered to hundreds of patients for 2 years in ±100 European centres. No differences in terms of adverse effects of therapy compared with the placebo groups were observed after 2 years of treatment, and no cases of VTE or DRESS occurred (Reginster and Meunier, 2003). Doses of up to 4 g SrR per day for 25-147 days were also well tolerated in post-menopausal women included in safety sub-groups within these trials (Servier laboratories, 2016). Although SrR had been approved for use for the treatment of osteoporosis in the elderly throughout the European Union in 2004, its use was later restricted (EMA, 2014) because randomized placebo-controlled clinical studies in approximately 7,500 postmenopausal women found an increased risk for myocardial infarctions (1.7%) compared to the controls (1.1%) (RR: 1.6, 95% CI: 1.07-2.38. The analysis also found an imbalance in the number of serious heart events (angina pectoris, coronary heart disease) in men with serious osteoporosis who took the drug compared with those given a placebo (8.7% vs. 4.6%).
No adverse effects were observed after patients from four different studies were administered supplements containing strontium from either strontium citrate (6 months to 1 year), strontium gluconate (at least 3 months), strontium lactate (3 months up to 4 years) (Shorr and Carter, 1952; Skoryna, 1981; Kaats et al., 2011; Michalek et al., 2011). No increase in the risk of cardiovascular outcomes in SrR users were reported in two Danish retrospective studies (Abrahamsen et al., 2014; Svanstrom et al., 2014) and one nested case-control study in the U.K. (Cooper et al., 2014). However, a more recent review of the clinical data from the large SOTI (1649 post-menopausal women aged 70 yrs on average) and TROPOS (5091 postmenopausal women) studies where SrR was administered to reduce the risk of vertebral fractures (SOTI) and non-vertebral fractures (TROPOS) found that SrR increases the risk of myocardial infarction and congestive heart failure (2% increase in absolute risk) (Bolland and Grey, 2016).
9.1.3.3 Gastrointestinal toxicity and other effects
The administration of SrR has been reported by a few authors as being well tolerated in many clinical trials with most adverse effects appearing to be mild and transient (Kaufman et al., 2013; Servier laboratories, 2016). However, the administration of 2 g SrR per day in combination with calcium and vitamin D to osteoporotic women over 50 years old has been associated with higher rates of nausea, diarrhoea, and headache at the beginning of therapy in large trials (SOTI and TROPOS), but no differences were maintained over time (Reginster and Meunier, 2003; Meunier et al., 2004; Roux et al., 2007; Compston, 2014; Reginster et al., 2014). In these studies creatine kinase was slightly elevated, but remained within normal values, which could be of concern for individuals with compromised renal functions since elevated levels of creatine kinase may indicate impacts on the kidneys.
9.1.4 Carcinogenicity
A few epidemiological studies have found associations between strontium exposure and cancer, including breast cancer (Chen et al., 2012), colon cancer (Kikuchi et al., 1999), and gastric cancer (Nakaji et al., 2001). However, these preliminary studies were conducted retrospectively and it is not clear how covariates were integrated into the assessment (e.g., Helicobacter pylori, diets in Asia, etc.). Given the very limited data on cancer effects in humans, the carcinogenic potential of strontium in humans cannot be determined.
9.1.5 Developmental and reproductive toxicity
A retrospective study compared strontium exposure from drinking water, crops, and soil between two regions in China: one with high prevalence of birth defects, such as limb shortening, and one with low prevalence of such defects (Yu et al., 2011). Levels of strontium in river water, well water, cropland and corn were inversely associated with birth defects (higher strontium in the area with low prevalence of birth defects). An ecological study found no association between strontium soil concentration and neural tube defects in births between 2002 and 2004 in the Luliang region of China (Huang et al., 2011). A cross-sectional study based on the U.S. National Health and Nutrition Examination Survey data from 2011-2012 of 5,107 individuals found increased odds of having low testosterone levels with increasing urine strontium concentrations (Xu et al., 2015). However, the authors stated that given the cross-sectional nature of the study, they could not assess whether strontium impacted testosterone levels concluding that further research is required to evaluate the association between long-term exposure to physiological levels of urinary strontium and low testosterone levels in adult men.
9.2 Effects on experimental animals
High doses of compounds containing Sr2+ via drinking water, food, or supplements have been associated with bone defects (rickets and osteomalacia, abnormal and reduced bone mineralization plus osteoid accumulation) in animal studies (concentrations ≥0.3-0.4% in the diet, or doses ≥525 mg/kg bw per day, with a no-observed-adverse-effect level (NOAEL) of 425 mg/kg bw per day for bone calcification). Although dosing reported in the majority of studies has been found to refer to ionic strontium, animal studies using strontium salts often do not clearly indicate whether the reported dose is reflective of strontium or a strontium complex. The dosing has therefore been reported as worded by the study authors. Where possible, authors reporting key data have been contacted to confirm that the dosing has been correctly interpreted.
9.2.1 Acute toxicity
Oral median lethal dose (LD50) values for mice are reported as 1,800 mg Sr(NO3)2/kg bw and 2,700-2,900 mg SrCl2/kg bw (ATSDR, 2004), whereas LD50 values for rats are reported as >2,000 mg SrCO3 and Sr(NO3)2/kg bw. Single doses up to 2,500 mg/kg bw SrR given orally to rodents, dogs and monkeys showed no toxicity, except for emesis in monkeys (EMA, 2005).
9.2.2 Short-term and sub-chronic exposure
9.2.2.1 Beneficial effects
Animals (i.e., adult rats, 21-day and 6-week-old mice, and 6- to 8-year-old ovariectomized goats) dosed with daily low levels of strontium (0.27-0.3% of strontium given as Sr chloride or Sr phosphate, or 68-612 mg Sr/kg bw given as SrR) in food or drinking water have shown improvements in skeletal parameters, such as increases in bone mineral density and femur and vertebrae trabecular bone formation rates after 8-104 weeks (Marie and Hott, 1986; Grynpas et al., 1996; Delannoy et al., 2002; Li et al., 2012). In particular, 28-day-old rats receiving 168 mg Sr/kg bw per day (with 77 mg calcium/kg bw per day, considered as low calcium intake) had a 17% increase in mineralized bone volume and osteoid and osteoblast surfaces compared with controls receiving no strontium supplementation after 8 weeks (Grynpas et al., 1996). The mineral apposition rate, osteoid thickness and mineralization lag time were similar between groups, supporting a lack of adverse bone effect.
9.2.2.2 Skeletal toxicity
High doses of strontium have been shown to induce growth plate defects (mainly due to the excessive enlargement of the hypertrophic zone), and inhibit calcification, leading to an overproduction of non-mineralized, osteoid bone tissue. Bone anomalies have been repeatedly observed in young laboratory animals (different strains of rats and mice) of both sexes supplemented with different forms of strontium via drinking water, food, or supplements at concentrations of ≥0.3% (equivalent to ≥525 mg/kg bw per day). For example, rickets, reduced body weight, bone growth and mineralization inhibition, humped posture, and altered bone alkaline phosphatase activity have been observed after supplementing young rats of both sexes with ≤1% strontium in the diet (containing 0.4-1.6% Ca2+) for short to intermediate periods (3 weeks to 9 months) (Storey, 1961; Johnson et al., 1968; Kshirsagar, 1976; Marie et al., 1985; Morohashi et al., 1994).
Drinking water exposure
Young male weanling SD rats (n = 8 rats per group) were supplemented with 0, 0.19%, 0.27%, 0.34%, or 0.4% of strontium (corresponding to 316 ±97.4, 425 ±149, 525 ±194, or 634 ±264 mg of strontium/kg bw per day, respectively, as calculated by the authors) as SrCl2 via drinking water and fed a semi-synthetic diet containing 0.5% calcium, Note de bas de page [1] 0.5% phosphorus, 0.16% magnesium, and 2,000 IU/kg vitamin D for 9 weeks (Marie et al., 1985). Histomorphometry of the seventh and eighth caudal vertebrae was conducted using a Zeiss 100-point integrating eye piece and a semi-automatic image analyzer. Mineral content was measured after drying and dissolving the right femur and tibia in hydrochloric acid. The endosteal calcification rate (µm per day) was measured as the distance between the two tetracycline labels divided by the duration between the two labels. An increase in osteoid tissue (volume, surface, and thickness) was observed at all strontium doses. Strontium at 0.19%, 0.27%, and 0.34% increased trabecular bone density and volume and a significant increase in the size of osteoids (volume and surface) was observed in all strontium-exposed groups. The osteoblast:osteoid ratio was only decreased at 0.4%.
The calcified bone volume was increased at 0.19 and 0.34%; however, reduced calcification rate and bone ash weight and increased osteoid thickness were observed at 0.34% and 0.4%. No differences in terms of food or water consumption, growth rate, serum vitamin D, bone calcium or phosphate levels, or tibia growth were observed between the strontium groups and the controls. Since the increase in osteoid formation was not accompanied by any reduction in the calcification or the resorption rates at doses of 0.19% and 0.27%, the authors concluded that it was associated with an increase in active bone-forming centres and those doses lower than 0.34% were not associated with an inhibition in bone mineralization rates. Bone defects observed at 0.4% (or 634 mg/kg bw per day) included reduced mineralization, decreased double-labeled osteoid surface and prolonged mineralization lag time. The significantly reduced bone mineralization rate coupled with the increase in osteoid formation at 0.34% and 0.4% are considered as bone defects. Growth rate along with water and food consumption were not different across dose groups at 9 weeks. Vitamin D in the serum was not different in the strontium group compared with the control group, while serum calcium dropped temporarily at 4 weeks in the strontium group compared with the control. Urinary phosphate at 4 weeks was significantly lower in the 0.27% dose group compared to the control group, and urinary magnesium at 9 weeks was significantly lower in the two highest dose groups compared with the control group. The most sensitive and comprehensive health point is the reduction in bone mineralization at 0.34% strontium (525 mg/kg bw per day) and can be identified as the lowest-observed-adverse-effect level [LOAEL], with a NOAEL of 0.27% (425 mg strontium/kg bw per day).
Dietary exposure
Studies with only one dose group at slightly higher concentrations (range 1.5-1.6%; doses in mg/kg not reported) of different forms of strontium in the diet (containing 0.04-1.2% Ca2+) for short-term duration (3-4 weeks) have induced similar or more severe bone symptoms in male weanling young rats (<4 weeks old) such as impairment in bone calcification and resorption with long mineralization lag time and reduced mineral apposition rate, rachitic deformities with decreased tibia density and growth, reduced body weight, replacement of trabecular bone by osteoid tissue (non-mineralized bone) with enlarged metaphyseal and epiphyseal growth plates, and disturbances in bone cell arrangements with voluminous extracellular lacunae (Storey, 1968; Matsumoto, 1976; Reinholt et al., 1984; Svensson et al., 1985, 1987; Neufeld and Boskey, 1994). None of these studies have reported dose levels in mg/kg or the amount of food intake, and most used only one dose level of strontium. The most relevant and comprehensive studies are described below.
In a study by Storey (1961), young and adult female rats (n = 3-5 rats per group, unspecified strain) ingested 1.6% calcium and strontium (sourced from SrCO3) through the diet for 20 days at concentrations of 0, 0.19%, 0.38%, 0.755%, 1% (only in young), 1.5%, and 3%. Dose levels in mg/kg and amount of food intake were not reported by the authors; however, ATSDR (2004) estimated the doses at 140 to 4,975 mg/kg bw per day. In young rats, exposures to ≥0.38% strontium resulted in cartilage plate irregularities, uncalcified zones of the metaphyseal trabeculae and diaphyses, inhibition of calcification and decreased mineral content of bone, alterations in the pattern of intercellular matrix columns, and abnormalities in the organization of the cartilage plate. The effects were more pronounced in higher doses, with larger epiphyseal plate and irregularities of the hypertrophic zone at 0.75%; reduced growth, abnormal growth of cartilage, poor calcification, and decreased ash content of bone at 1.5%; extensions of uncalcified cartilage within areas of osteoid tissue and hypertrophic zone were even more irregular at 3%. Similar changes were observed in the adult rats, but to a lesser extent. No adverse effects were observed at 0.19% strontium.
In another study, Storey (1962) exposed young and adult rats of both sexes (n = 50 total) to 1.8% strontium (sourced from SrCO3, with 1.5% calcium) in their diet for up to 9 months, with serial necropsies conducted throughout the study (dose levels in mg/kg and amount of food intake were not reported). Young rats manifested a rachitic gait, had epiphyseal cartilage plate, calcification, extracellular bone matrix irregularities, and reduced body weight from within one month of starting dosing. Bone structure abnormalities, cell arrangement disturbances, and disrupted epiphyseal plates of the tibia and femur with isolated fragments of cartilage partially replacing osteoid tissues were observed in the following months, up to 7 months. Most of these changes were also observed in the adult rats from 3 months onward, but were less severe.
Young female Wistar rats (n = 6-8 per group) were supplemented with 0.05%, 0.1%, or 0.5% (87.5, 175, or 875 µmol per day, respectively) strontium from SrCO3 in the diet for 27 days during the 36- to 63-day study period (Morohashi et al., 1994). No health effects were observed at 0.05% or 0.1%, and the body weight and length of femur endpoints were not affected at any dose. Reduction in bone resorption and formation rates and an increase in trabecular bone were observed in the femur at 0.5% strontium.
Young male weanling SD rats (n = 5 rats per group) were supplemented with 0, 0.19%, or 0.4% SrCl2 via drinking water and fed a semi-synthetic diet containing 0.5% calcium for 4 and 8 weeks (Grynpas and Marie, 1990). An increased in trabecular bone tissue (osteoid volume) was observed after 8 weeks of 0.19% supplementation. An increase in osteoid thickness with prolonged mineralization time was observed after 8 weeks of 0.4% supplementation. A trend to lower density and mineralization was observed with increasing doses. This was accompanied by a reduction of crystal length and a replacement of calcium by strontium in bone minerals.
Male weanling Wistar rats (n = 5-6 per group) were supplemented with Sr3(PO4)2 in the diet at concentrations of 0.5%, 1%, or 2% strontium (dose levels in mg/kg were not reported) for 2, 4, or 6 weeks (Kshirsagar, 1976). Growth retardation and increase in bone alkaline phosphatase activity was observed at 2%. No bone deformations were seen at 0.5% or 1% strontium, but important alterations in enzyme activities (bone alkaline phosphatase, liver, intestine, and kidney acid phosphatase) were reported at 1%.
A report by the EMA (2005) provides a synthesis of the toxicological effects of studies using the supplement SrR, although the source of the data was not specified. Osteomalacia, abnormal bone mineralization, and osteoid accumulation were induced in ovariectomized rats administered 425 mg Sr/kg bw per day from SrR in food for 41 weeks. Also, osteomalacia and long bone deformations were observed in mice exposed to 5,000 mg SrR/kg bw per day for 52 weeks or 600 mg SrR/kg bw per day for 104 weeks (no bone defects at 2,500 mg/kg bw per day for 52 weeks). Finally, ovariectomized monkeys had delayed mineralization with two doses of 625 mg SrR/kg bw per day for 52 weeks (no bone defects with 750 mg/kg bw per day for 26 weeks or with two doses of 250 mg/kg bw per day for 52 weeks).
9.2.3 Genotoxicity
Most studies have focused on the genotoxicity of radioactive strontium, and the evidence on stable strontium has generally shown a lack of genotoxicity for the element. For example, SrCl2 was shown to be non-genotoxic (no growth inhibition of the recombinant-deficient cells compared with the wild-type) in the rec-assay using two strains of the bacteria B. subtilis H17 and M45 (Kanematsu et al., 1980). The concentration of SrCl2 found to inhibit the cloning efficiency of Chinese hamster ovary cells by 50% was high compared with other compounds (87 000 µM, versus 240 µM for manganese chloride and 1.3 µM for cadmium chloride, for example) (Tan et al., 1984). Strontium (form indicated as Sr2+) did not alter the fidelity in DNA synthesis of purified DNA polymerases using synthetic polynucleotide templates (Loeb et al., 1977). SrR has been found to be negative in mutagenicity, genotoxicity, and chromosome toxicity assays on bacterial (Salmonella typhimurium and Escherichia coli) and mammalian cells (Chinese hamster fibroblast cells and human lymphocytes) in vitro and in rats in vivo (EMA, 2005; Servier laboratories, 2016). SrCl2 in drinking water (240-2,600 mg/kg bw) has induced chromosomal aberrations (gaps, breaks, nondisjunction, and polyploidy) in vivo in young (6- to 8-week-old) Swiss albino mice bone marrow cells (n = 5 per set); however, some of these concentrations exceed the Organisation for Economic Co-operation and Development (OECD) test guideline (No. 475) and the isotope of strontium was not mentioned (Ghosh et al., 1990; OECD, 1997).
9.2.4 Carcinogenicity
Few investigations of the carcinogenic effects of strontium have been performed. While radioactive forms of strontium (e.g., strontium-90) are genotoxic and cause cancers in animals, stable forms of strontium have not resulted in increases in tumour development in animals. They have not been assessed for carcinogenicity by the International Agency for Research on Cancer (IARC), the U.S. EPA or the National Toxicology Program (NTP).
The EMA (2005) reported increases in thyroid C-cell tumours in Fischer 344 male rats dosed orally with SrR at 200, 600, or 1,800 mg/kg bw per day (no details were provided on the number of animals per group). The increases were not dose-dependent (only lowest and highest doses were significant), within historical controls, and the increases in females were not significant. However, the authors indicated that the thyroid C-cell tumours could be plausible, citing a hypothetical mechanism involving long-term hypercalcemia, increased calcitonin secretion, and C-cell hypertrophy.
The EMA (2005) also reported increases in lymphocytic lymphomas, hepatocellular adenomas, ovarian adenomas, and combined lung bronchioloalveolar adenoma and carcinoma in B6C3F1 mice dosed orally with SrR at 150, 225, 300, 450, 625 or 900 mg/kg bw per day in the diet for 104 weeks; however, none were considered to be related to treatment (no study details were provided).
In conclusion, given the very limited data on cancer effects, the carcinogenic potential of strontium in animals cannot be determined.
9.2.5 Reproductive and developmental toxicity
No standard reproductive or developmental studies using environmentally relevant exposure routes have been conducted, and the sample sizes were very small in the subcutaneous exposure study. There are indications that strontium adversely affects bone organogenesis, which continues after birth, in weanling and young rodents, as described above. The WHO (2010) reported a study by NIER (2006) showing no effects on fetal development of rats (16 of each sex per dose group) from mothers gavaged with strontium sulphate (up to 2 g Sr sulphate/kg bw per day) from 2 weeks before mating until day 4 of lactation. In another study by Shibata and Yamashita (2001), as reported by the WHO (2010), an inhibition of the calcification of mandibular bone and cartilage was observed in newborn mice of mothers (n = 4) fed 2% strontium carbonate (unclear what the dose refers to) throughout pregnancy; however, there were no effects on fetal numbers or viability. Skoryna (1981) reported a study showing no toxic effects in rats supplemented with strontium over three generations (no study details were provided). High doses of SrR (1,000 mg/kg bw per day) to male and female rats did not have effects on fertility (no details provided, Servier laboratories, 2016). However, SrR was found to delay incisor eruption in the offspring of rats when given at high oral doses (750 mg SrR/kg bw per day). The delays in bone mineralization of male Wistar rats with chronic renal failure were found to be reversible after treatment with 2 g Sr/L in drinking water for 2-12 weeks was stopped (Oste et al., 2005). No effects on body weight, litter size, abnormalities or teratogenicity were observed in the progeny of female Wistar rats (n = 3 rats per group) dosed subcutaneously with 25, 50, 100, or 200 mg/kg bw Sr(NO3)2 at days 9 to 19 of pregnancy (Lansdown et al., 1972).
9.2.6 Other effects
Other effects observed in animals were respiratory difficulties, hind limb paralysis, and death in rats exposed to 0.49% strontium (unspecified form) during postnatal days 21-64 (Johnson et al., 1968). However, it is difficult to draw conclusions based on this study, since the authors indicated that some of the effects were reversible, no histological examination was conducted on the deceased animals, the form of strontium was unknown, no data or statistics on health effects were presented, and the diets were deficient in calcium.
Altered levels of acid and alkaline phosphatase activities in the liver and small intestine of young rats were observed following supplementation of the diet with 1% and 2% strontium phosphate for 2-6 weeks (Kshirsagar, 1976). The authors noted that these effects were of unclear biological significance.
Adult SPF Wistar rats (n = 6 per dose group) administered 3,000 ppm of SrCl2·6H2O in the diet (not clear what the dose referred to) and 0.85% calcium showed a small elevation in erythrocytes and white cell counts after 2 weeks, small histological changes of the thyroid, and lower pituitary weights and liver glycogen levels after 90 days in females (Kroes et al., 1977). However, there was no dose-response relationship.
9.3 Mode of action
9.3.1 Toxic effects
Although the mechanisms of toxicity remain unclear, the strontium-mediated adverse effects on bone formation are related to its similarity to calcium in terms of chemical properties, with both elements sharing metabolic pathways and interacting with similar cellular and molecular components of the organism (Skoryna, 1981; Fischer et al., 2011). Bone effects are accentuated in low-calcium diets; however, bone anomalies were also observed at normal levels of calcium (up to 1.6%). Since strontium affects bone growth, and infants have a higher intake per kilogram body weight compared to other age groups, they are particularly sensitive to the effects of strontium on bone; in animals, bone effects have been shown to be more prominent in young rats compared to adults, supporting the choice of the sensitive subpopulation (Storey, 1961, 1962; Svensson et al., 1985, 1987; Neufeld and Boskey, 1994).
The main mechanism of strontium toxicity is the direct competition with calcium for the binding of the forming hydroxyapatite crystals by physicochemical interference (Neufeld and Boskey, 1994; Boivin et al., 1996; Cabrera et al., 1999; Verberckmoes et al., 2003; Fischer et al., 2011; Hendrych et al., 2016). Strontium has been shown to lower calcium serum levels and to be concentrated at the osteoid calcification front (Schrooten et al., 1998; Shibata and Yamashita, 2001). The binding of strontium, a larger atom than calcium, and the higher carbonate content of the mineral can expand and change the structure of the apatite lattice, decrease crystal formation and growth, and increase the hydroxyapatite solubility-possibly decreasing the bone matrice stability during bone resorption processes, increasing dissolution (Skoryna, 1981; Christoffersen et al., 1997; Cabrera et al., 1999; Verberckmoes et al., 2004; Oste et al., 2005). For example, morphological studies have shown that hydroxyapatite molecules with high concentrations of strontium have a reduced crystal size and crystallinity, with increased aggregation in vitro (Querido et al., 2016). The aggregation could lead to increased interactions with the organic and cellular components of the matrices. Moreover, changes in the lattice structure were observed, affecting bond distances and angles. This potential pathway for toxicity involving a competition with calcium is supported by the rapid return to normal mineralization in rats after cessation of treatment.
Another mechanism of strontium toxicity is the interaction with different calcium-binding proteins, secondary cell messengers, and calcium-sensing G-protein receptors expressed in the bone and other cells (e.g., parathyroid gland and kidney, although effects in these organs have not been reported) (Chang et al., 1999; Pi and Quarles, 2004; Caudarella et al., 2011). These interactions can alter the morphology, arrangement and maturation of bone cells (e.g., osteoblasts), as observed in vitro and in vivo in rats fed high-strontium diets (Matsumoto, 1976; Verberckmoes et al., 2003; Oste et al., 2005; Fischer et al., 2011) and can impact bone cell functions such as collagen synthesis and matrix vesicle releases (Anderson, 1995; Kirsch et al., 2003; Oste et al., 2005). Additionally, strontium could replace calcium within the active site of the human enzyme tissue-nonspecific alkaline phosphatase (an alkaline phosphatase involved in the control of hydroxyapatite formation and bone mineralization) and modify its configuration, as shown in a crystal structure study (Llinas et al., 2006).
Furthermore, strontium can decrease serum calcium and parathyroid hormone levels via a negative loop feedback following the activation of the parathyroid gland calcium-sensing receptor, as shown in rats fed high-strontium diets (Kshirsagar, 1976; El and Rousselet, 1981; Morohashi et al., 1994; Schrooten et al., 1998; Oste et al., 2005; Fischer et al., 2011). The decrease in calcium levels is related to the inhibition of mineralization and an increase in bone resorption to increase serum levels. The thyroid histopathology observed at the high dose of the Kroes et al. (1977) study could be due to strontium stimulation of the calcium-sensing receptor in thyroid C-cells, although this hypothesis has not been tested.
The parathyroid hormone plays a key role in bone remodelling by stimulating both bone formation and resorption via the maturation of osteoblasts and osteoclasts. Strontium has been shown to reduce the absorption of calcium by indirectly inhibiting the expression of calbindin D RNA and by the renal activation of vitamin D, effects that are possibly related to the alteration in parathyroid hormone activity (El and Rousselet, 1981; Vezzoli et al., 1998).
Other mechanisms of toxicity include the delay in apoptosis of chondrocytes, leading to the overproduction of osteoids (Fischer et al., 2011), the underdevelopment of the ruffled borders of the osteoclasts, the non-calcification of collagen fibrils of fetal mice from pregnant mice receiving 2% strontium carbonate via water (Shibata and Yamashita, 2001), the increase in non-collagenous proteins (possibly altering bone mineralization) and the alteration of the metabolism of complexed acidic phospholipids leading to their accumulation in young rats fed high-strontium diets (Neufeld and Boskey, 1994). Finally, rats treated with strontium had lower serum parathyroid hormone, potentially lowering bone remodelling and decreasing renal calcium reabsorption (Oste et al., 2005).
9.3.2 Beneficial effects
Different forms of strontium have been suggested to both inhibit bone resorption and stimulate bone formation at low doses in animals (Ferraro et al., 1983; Marie and Hott, 1986; Marie et al., 1993; Grynpas et al., 1996) and humans (EMA, 2005), and the effects are likely to vary depending on the age of the individual. Strontium was shown to stimulate osteoblast proliferation and differentiation in vitro via the activation of the calcium-sensing receptor and to signal the transcription of genes associated with growth at low doses (Cabrera et al., 1999; Brennan et al., 2009; Rybchyn et al., 2011; Querido et al., 2016). In addition, improvements in the determinants of bone strength in rats and in bone microarchitecture and mineral density in osteoporotic patients have been observed after administration of SrR (Hamdy, 2009). Also, SrR has been shown to increase and reduce the expression of positive and negative regulators of bone synthesis by bone cells, respectively (Hamdy, 2009; Hurtel et al., 2009). Finally, strontium is incorporated into the bone apatite by reversible IX and calcium ionic substitution, increasing bone density (Querido et al., 2016).
10.0 Classification and assessment
Few investigations of the carcinogenic effects of strontium have been performed. In terms of cancer classification, the U.S. EPA's Integrated Risk Information System has indicated that "there is inadequate information to assess the carcinogenic potential of strontium due to the lack of adequate studies of chronic duration" (U.S. EPA, 2014). Strontium has not been assessed for carcinogenicity by the IARC or the NTP.
10.1 Non-cancer risk assessment
Strontium has been shown to be both beneficial and toxic to the animal and human skeleton. The mode of action for strontium effects on bone supports the choice of this key health endpoint as the basis to derive the health based value (HBV) in drinking water. Only a few epidemiological studies have documented adverse effects of elemental strontium on bone; a study by Özgür et al. (1996) reported a potential link between high strontium exposures and rickets in Turkish children, however, the data are not sufficient for identifying the doses associated with this effect. In animal studies, bone abnormalities (rickets, with reduced bone mineralization and osteoid accumulation) have been observed following exposure to high doses of strontium (concentrations ≥0.3-0.4% in the diet, or doses ≥525 mg/kg bw per day) whereas, exposure to lower doses have been shown to reduce bone resorption and increase bone formation with no impact on bone mineralization. Since the highest rates of bone remodelling occur during the first year of life (with much of the skeleton being replaced during this period), calcium intake is the highest in infants, along with the potential for absorbing and incorporating strontium into bone, supporting the identification of this age group as the most sensitive to strontium related bone effects(ICRP, 1993; ATSDR, 2004; Hood, 2006; Wheeless, 2011).
The study chosen to derive the HBV for strontium in drinking water is Marie et al. (1985). This study was appropriate to derive the HBV since strontium was administered via drinking water (higher absorption of strontium compared with food), multiple doses were tested, the affected bone parameters were quantified, and the study was conducted in weanling rats, which have higher strontium absorption and bone remodelling rates than adult rats. The authors provided dosing information in mg/kg bw per day, and the effects on bone were detailed based on different biomarkers and direct bone measurements. Both values on bone tissue growth and mineralization of the newly formed tissue were reported. The most sensitive and comprehensive health endpoints are the reduction in bone calcification rate with an increase in osteoid surface at the two highest doses (0.34% and 0.4% Sr chloride); based on the intakes of calcium and strontium from food and drinking water, animals in these dose groups were exposed to a ratio of approximately 3:1 calcium to strontium. A prolonged mineralization lag time was also observed at 0.4% Sr chloride. As discussed in Section 9.2, the choice of bone effects as the critical endpoint is supported by other animal studies (Storey, 1961; Johnson et al., 1968; Kshirsagar, 1976; Morohashi et al., 1994). Similar bone anomalies have also been observed in an adult rat study (Storey, 1961); however, the study is limited for risk assessment given the small sample size, short exposure duration, lack of information on animal age/strain and experimentally-determined dosing, as well as the administration of a form of strontium with low solubility (strontium carbonate) via food which may explain the reported delayed and less pronounced effects.
The amount of calcium in the diet was adequate (0.5%), but lower than in other studies on the effects of strontium on bones. A normal calcium intake for rats has been reported to range from 1.19% to 3.5% (National Research Council, 1995; Bollen and Bai, 2005; Fuchs et al., 2008), thus an intake of 0.5% calcium in this study is expected to produce a conservative HBV, since animals with a deficiency in calcium have shown increased sensitivity to strontium-induced bone defects. The calcium:strontium ratio of water concentrations can vary, depending on the types of rocks the water is sourced from (see Section 4.0); situations in which humans are exposed to drinking water containing higher levels of strontium than calcium can be of concern. Other factors have been shown to modify the rate of strontium absorption (see Section 8.1). Susceptibility may be increased in cases of vitamin D deficiency which reduces intestinal calcium absorption (Holick, 2010). Sufficient levels of calcium and vitamin D in the body can lower the amount of strontium incorporated into bones, decreasing the likelihood of adverse effects on the bones of children that have adequate calcium and vitamin D status. Hence, the co-occurrence of high levels of calcium, or even normal dietary levels as has been reported by Harrison et al. (1966) and Kostial et al. (1972) could be protective from the adverse effects of strontium on bone; however, adjusting the strontium HBV as a function of calcium intake or water concentration cannot be performed with any certainty. Calcium deficiency is rare in Canadian young children (<2.6% aged 1-3 years); however, a high proportion of Canadian adolescents have been reported with calcium deficiency (>60% aged 9-18 years) (Statistics Canada, 2004).
Benchmark Dose (BMD) modelling was conducted using the U.S. EPA software BMD Version 2.6.0.1 to estimate the 95% lower confidence limit on a decrease of one standard deviation of the BMD (BMDL) for defects in bone mineralization (continuous response) (U.S. EPA, 2015). The Exponential, Hill, Linear, Polynomial, and Power models all fit the data well (visually) generating a BMDL of 328 mg/kg bw per day (average of the models); however, the BMD approach was not retained for several reasons. Looking across endpoints, the study data are stronger for effects only at 525 mg/kg bw per day and higher; in fact, the authors stated that they documented defective bone mineralization, with strontium clearly impairing the mineralization at the highest dose (634 mg/kg per day), and that no effects were observed at 425 mg/kg per day. Secondly, the BMDL is closer to the lowest study dose which is not consistent with the observations reported by Marie et al. (1985). Finally, the BMD models had poor variance (p-value of 0.02 for adequacy of variance) which may be caused by the relatively large 95% CI of the 425 mg/kg bw per day group compared with the others, lowering the confidence in the BMDL results.
For the reasons cited above, the NOAEL approach is chosen to derive the POD, based on the most sensitive and comprehensive health endpoint, decreased bone mineralization in young rats (Marie et al., 1985).
Figure 2 - Strontium in Drinking Water
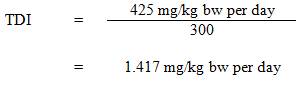
The TDI is 1.417 mg/kg bw per day. This is calculated by dividing 425 mg/kg bw per day by the uncertainty factor of 300.Description Figure 2 - Strontium in Drinking Water:
- 425 mg/kg bw per day is the NOAEL for calcification defects (Marie et al., 1985);
- 300 is the uncertainty factor (UF): × 10 for interspecies variability, × 10 for intraspecies variability (including sensitivities in pregnant women and adolescents), and × 3 for database deficiencies (absence of complete developmental toxicity studies in any species).
Figure 3 - Strontium in Drinking Water
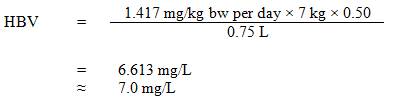
The HBV is 6.613 mg/L, rounded to 7.0 mg/L. This is calculated by multiplying 1.417 mg/kg bw per day by 7 kg, then by 0.50. This product is then divided by 0.75 L.Description Figure 3 - Strontium in Drinking Water:
- 1.417 mg/kg bw per day is the TDI, as derived above;
- 7 kg is the average body weight for infants 0-6 months of age (Health Canada, 1994);
- 0.5 is the allocation factor estimated for drinking water. Powdered infant formulas and the water used to prepare them are considered the main sources of exposure to strontium in non-breastfed infants for the first few months of life. The allocation from drinking water is assumed to be half of the total potential exposure, with the balance from the formula itself, as supported by the exposure data from Section 5. Contributions from other sources are not expected to be significant for this age group; and
- 0.75 L is the mean daily water intake for infants 0-6 months of age (Health Canada, 1994).
10.2 International considerations
This section presents drinking water guidelines, standards and/or guidance from foreign and international organizations. Variations in these values can be attributed to the age of the assessments or to differing policies and approaches, including the choice of key study and the use of different consumption rates, body weights and allocation factors.
The World Health Organization (WHO), United States Environmental Protection Agency (U.S. EPA), European Union and the Australian National Health and Medical Research Council have not established health-based regulatory limits for chemical strontium in drinking water.
The U.S. EPA has established a lifetime health advisory of 4 mg/L for strontium in drinking water based on a reference dose of 0.6 mg/kg/day (U.S. EPA, 2018). Health advisories are non-regulatory limits serving as an indicator of an acceptable level in drinking water.
11.0 Rationale
Strontium is present in many drinking water sources, both naturally and as a result of human activities. The strontium levels in Canadian drinking water will vary greatly depending on geological formations and anthropogenic activities, including mining and manufacturing operations. Strontium exists as a mixture of four naturally occurring radioisotopes considered to be stable. The focus of this document is limited to strontium's chemical properties. Based on strontium's chemical properties, exposure to strontium from drinking water would only be a concern from ingestion; exposure through either inhalation or dermal absorption is not expected to be of concern.
There is inadequate evidence to assess the carcinogenic potential of oral exposure to strontium in humans or animals, as no specific cancer studies in animals using oral exposure are available. Strontium has not been assessed for carcinogenicity by the IARC or NTP.
Bones are generally considered to be the major target for strontium toxicity in both humans and animals. Strontium has been shown to be beneficial to the animal and human skeleton, and only a few epidemiological studies have documented the adverse effects of elemental strontium on bone. However, many animal studies have observed bone abnormalities (rickets, with reduced bone mineralization and osteoid accumulation) following exposure to high doses of strontium. Since the highest rates of bone remodelling occur during the first year of life (with almost 100% of the skeleton replaced during this period), calcium intake is the highest in infants (as is the potential for strontium) supporting the identification of this age group as the most sensitive to strontium related bone effects. For these reasons, a MAC of 7.0 mg/L was derived to be protective of infants as the most sensitive population, based on decreased bone mineralization from a study in young rats.
A MAC of 7.0 mg/L is established for total strontium in drinking water. The MAC is protective of potential health effects, can be reliably measured by available analytical methods, and is achievable by municipal and residential scale treatment technologies. The guideline is based on the chemical toxicity of naturally-occurring strontium. Radiological forms and/or radioactive isotopes of strontium are addressed in a separate document (Health Canada, 2009). The toxic effects of strontium may be reduced in the presence of elevated calcium in drinking water sources, or simply through adequate levels of calcium in the diet, since calcium is more readily absorbed and incorporated into bone compared to strontium.
As part of its ongoing guideline review process, Health Canada will continue to monitor new research in this area and recommend any change to the guideline that is deemed necessary.
12.0 References
- Abrahamsen, B., Grove, E.L. and Vestergaard, P. (2014). Nationwide registry-based analysis of cardiovascular risk factors and adverse outcomes in patients treated with strontium ranelate. Osteoporos. Int., 25(2): 757-762.
- Achal, V., Pan, X. and Zhang, D. (2012). Bioremediation of strontium (Sr) contaminated aquifer quartz sand based on carbonate precipitation induced by Sr resistant halomonas sp. Chemosphere, 89: 764-768.
- Agriculture Canada (1979). Minor elements in Canadian soils. Research Branch. Land Resource Research Institute Contribution No. LRRI 27. Engineering and Statistical Research Institute Contribution No. I-71 Ottawa. Available at: http://sis.agr.gc.ca/cansis/publications/miscellaneous/1979-minor/1979-minor-elements.pdf.
- Alberta Environment and Sustainable Resource Development (2016). Personal communication with D. Reid and K. Pongar.
- Alexander, G.V., Nusbaum, R.E. and MacDonald, N.S. (1954). Strontium and calcium in municipal water supplies. J. Am. Water Works Assoc., 46(7): 643-654.
- Alfredo, K., Adams, C., Eaton, A., Roberson, A. and Stannford, B. (2014). The potential regulatory implications of strontium. American Water Works Association. Available at: www.awwa.org/Portals/0/files/legreg/ documents/2014AWWAStrontiumBriefingPaper.pdf.
- Anderson, H.C. (1995). Molecular biology of matrix vesicles. Clin. Orthop. Relat. Res. (314): 266-280.
- ANSES (2013). Avis relatif à une évaluation des risques sanitaires liés à la présence de strontium dans les eaux destinées à la consommation humaine. Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail. Saisie no 2012-SA-0262. Maisons-Alfort (ed.). France. pp. 28.
- APHA, AWWA and WEF (2005). Standard methods for the examination of water and wastewater, 21st edition. American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC.
- APHA, AWWA and WEF (2012). Standard methods for the examination of water and wastewater. 22nd edition. American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC.
- Apostoaei, A.I. (2002). Absorption of strontium from the gastrointestinal tract into plasma in healthy human adults. Health Phys., 83(1): 56-65.
- Araissi, M., Ayed, I., Elaloui, E. and Moussaoui, Y. (2016). Removal of barium and strontium from aqueous solution using zeolite 4A. Water Sci. Technol., 73(7): 1628-1636.
- Arbuckle, T.E., Fraser, W.D., Fisher, M., Davis, K., Liang, C.L., Lupien, N. et al. (2013). Cohort profile: the maternal-infant research on environmental chemicals research platform. Paediatr. Perinat. Epidemiol., 27(4): 415-425.
- ASTM (2010). ASTM D5673-10 Standard test method for elements in water by inductively coupled plasma-mass spectrometry. ASTM International, West Conshohocken, Pennsylvania.
- Athanassouli, T.M., Papastathopoulos, D.S. and Apostolopoulos, A.X. (1983). Dental caries and strontium concentration in drinking water and surface enamel. J. Dent. Res., 62(9): 989-991.
- ATSDR (2004). Toxicological profile for strontium. Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services, Atlanta, Georgia.
- Atteritano, M., Catalano, A., Santoro, D., Lasco, A. and Benvenga, S. (2015). Effects of strontium ranelate on markers of cardiovascular risk in postmenopausal osteoporotic women. Endocrine, 53(1): 305-312.
- AWWA (2014). The potential regulatory implications of strontium. American Water Works Association. Government Affairs Office, Washington, DC.
- Bain, S.D., Jerome, C., Shen, V., Dupin-Roger, I. and Ammann, P. (2009). Strontium ranelate improves bone strength in ovariectomized rat by positively influencing bone resistance determinants. Osteoporos. Int., 20(8): 1417-1428.
- Bärenholdt, O., Kolthoff, N. and Nielsen, S.P. (2009). Effect of long-term treatment with strontium ranelate on bone strontium content. Bone, 45(2): 200-206.
- Boivin, G., Deloffre, P., Perrat, B., Panczer, G., Boudeulle, M., Mauras, Y., Allain, P., Tsouderos, Y. and Meunier, P.J. (1996). Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S 12911) administration. J. Bone Miner. Res., 11(9): 1302-1311.
- Bolland, M. and Grey, A. (2016). Ten years too long: strontium ranelate, cardiac events, and the European Medicines Agency. BMJ 354:i5109. Available at: https://www.bmj.com/content/354/bmj.i5109
- Bollen, A.M. and Bai, X.Q. (2005). Effects of long-term calcium intake on body weight, body fat and bone in growing rats. Osteoporos. Int., 16(12): 1864-1870.
- Bortun, A.I., Bortun, L.N. and Clearfield, A. (1997). Evaluation of synthetic inorganic ion exchangers for cesium and strontium removal from contaminated groundwater and wastewater. Solvent Extr. Ion Exch., 15(5): 909-929.
- Bostick, D.T., DePaoli, S.M., and Guo, B. (1997). A comparative evaluation of ionsiv®ie-911 and chabazite zeolite for the removal of radiostrontium and cesium from wastewater. Chemical technology division, Oak Ridge National Laboratory, Tennessee.
- Brennan, T.C., Rybchyn, M.S., Green, W., Atwa, S., Conigrave, A.D. and Mason, R.S. (2009). Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br. J. Pharmacol., 157(7): 1291-1300.
- Brisson, I.J. (2014). Strontium stable dans l'eau potable - revue des connaissances et soutien aux directions de santé publique. Institut national de santé publique du Québec (INSPQ) (ed.). Gouvernement du Québec, pp. 7. Available at: www.inspq.qc.ca/bise/article-secondaire-strontium-stable-dans-l-eau-potable-revue-des-connaissances-et-soutien-aux-directions-de-sante-publique.
- Brown, J., Hammond, D., and Wilkins, T.Y. (2008). Handbook of assessing the impact of a radiological incident on levels of radioactivity in drinking water and risk to operatives at water treatment works: Supporting scientific report. Health Protection Agency. Center for Radiation, Chemical and Environmental Hazards. Chilton, Didcot, Oxfordshire. ISBN-978-0-85951-621-1.
- Cabrera, W.E., Schrooten, I., De Broe, M.E. and D'Haese, P.C. (1999). Strontium and bone. J. Bone Miner. Res., 14(5): 661-668.
- Canadian Cancer Society (2017). Bone metastases. Available at: www.cancer.ca/en/cancer-information/cancer-type/metastatic-cancer/bone-metastases
- Caudarella, R., Vescini, F., Buffa, A., Rizzoli, E., Ceccoli, L. and Francucci, C.M. (2011). Role of calcium-sensing receptor in bone biology. J. Endocrinol. Invest., 34(7 Suppl): 13-17.
- Chang, C.T., You, C.F., Aggarwal, S.K., Chung, C.H., Chao, H.C. and Liu, H.C. (2015). Boron and strontium isotope ratios and major/trace elements concentrations in tea leaves at four major tea growing gardens in Taiwan. Environ. Geochem. Health, 38(3): 737-748.
- Chang, W., Tu, C., Chen, T.H., Komuves, L., Oda, Y., Pratt, S.A., Miller, S. and Shoback, D. (1999). Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology, 140(12): 5883-5893.
- Chen, L.J., Tang, L.Y., He, J.R., Su, Y., Cen, Y.L., Yu, D.D., Wu, B.H., Lin, Y., Chen, W.Q., Song, E.W. and Ren, Z.F. (2012). Urinary strontium and the risk of breast cancer: a case-control study in Guangzhou, China. Environ. Res., 112: 212-217.
- Christoffersen, J., Christoffersen, M.R., Kolthoff, N. and Bärenholdt, O. (1997). Effects of strontium ions on growth and dissolution of hydroxyapatite and on bone mineral detection. Bone, 20(1): 47-54.
- Cianferotti, L., D'Asta, F. and Brandi, M.L. (2013). A review on strontium ranelate long-term antifracture efficacy in the treatment of postmenopausal osteoporosis. Ther. Adv. Musculoskelet. Dis., 5(3): 127-139.
- Clifford, D.A. (1999). Ion exchange and inorganic adsorption. Chapter 9 in: Letterman, R.D.(ed). Water quality and treatment: a handbook of community water supplies, 5th edition. American Water Works Association. Denver, Colorado. McGraw-Hill, New York, New York.
- Clifford, D., Sorg, T. and Ghurye, G. (2011). Ion exchange and adsorption of inorganic contaminants. Chapter 12 in: Water quality and treatment: a handbook of community water supplies. 6th edition. American Water Works Association, Denver, Colorado.
- Cohen-Solal, M. (2002). Strontium overload and toxicity: impact on renal osteodystrophy. Nephrol. Dial. Transplant., 17 (Suppl 2): 30-34.
- Cohn, S.H. and Gusmano, E.A. (1967). Kinetics of strontium and calcium skeletal metabolism in the rat. Exp. Biol. Med., 126(1): 79-83.
- Compston, J. (2014). Strontium ranelate lives to fight another day. Maturitas, 78(2): 75-76.
- Cooper, C., Fox, K.M. and Borer, J.S. (2014). Ischaemic cardiac events and use of strontium ranelate in postmenopausal osteoporosis: a nested case-control study in the CPRD. Osteoporos. Int., 25(2): 737-745.
- Curzon, M.E. (1985). The relation between caries prevalence and strontium concentrations in drinking water, plaque, and surface enamel. J. Dent. Res., 64(12): 1386-1388.
- Dabeka, R.W., Huang, R., Pepper, K., Gauthier, B.R., Arbuckle, T., Fraser, W. and Rawn, D.F.K. (2016). Trace element levels in human milk. MIREC Study. Unpublished data. Health Canada (available upon request).
- Dawson, E.B., Frey, M.J., Moore, T.D. and McGanity, W.J. (1978). Relationship of metal metabolism to vascular disease mortality rates in Texas. Am. J. Clin. Nutr., 31(7): 1188-1197.
- Dean, J. A. (1992). Electrolytes, electromotive force, and chemical equilibrium. Chapter 8 in: Lange's Handbook of Chemistry, 14th Ed,. McGraw-Hill, New York, New York.
- De Beers Canada (2013). Snap Lake Mine: Strontium response plan. Report No. MV2011L2-0004. File: L020. Available at: https://mvlwb.com/content/mv2011l2-0004-snap-lake-strontium-response-plan
- Delannoy, P., Bazot, D. and Marie, P.J. (2002). Long-term treatment with strontium ranelate increases vertebral bone mass without deleterious effect in mice. Metab. Clin. Exp., 51(7): 906-911.
- D'Haese, P.C., Schrooten, I., Goodman, W.G., Cabrera, W.E., Lamberts, L.V., Elseviers, M.M., Couttenye, M.M. and De Broe, M.E. (2000). Increased bone strontium levels in hemodialysis patients with osteomalacia. Kidney Int., 57(3): 1107-1114.
- Ding, S., Yang, Y., Huang, H., Liu, H. and Hou, L. (2015). Effects of feed solution chemistry on low pressure reverse osmosis filtration of cesium and strontium. J. Hazard. Mater., 294: 27.
- Dong, D., Derry, L.A. and Lion, L.W. (2003). Pb scavenging from a freshwater lake by Mn oxides in heterogeneous surface coating materials. Water Res., 37: 1662-1666.
- Doublier, A., Farlay, D., Khebbab, M.T., Jaurand, X., Meunier, P.J. and Boivin, G. (2011). Distribution of strontium and mineralization in iliac bone biopsies from osteoporotic women treated long-term with strontium ranelate. Eur. J. Endocrinol., 165(3): 469-476.
- Drug and Therapeutics Bulletin (2017). Strontium ranelate discontinued. BMJ Publishing Group Ltd. Available at: https://dtb.bmj.com/content/55/8/86.1
- Eisenberg, E. and Gordan, G.S. (1961). Skeletal dynamics in man measured by nonradioactive strontium. J. Clin. Invest., 40: 1809-1825.
- El, S.N. and Rousselet, F. (1981). Effects of stable strontium administration on calcium metabolism with particular reference to low calcium diet. In: Handbook of stable strontium. Skoryna, S.C. (ed.). Plenum Press, New York. pp. 515-544.
- El-Kamash, A.M. (2008). Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J. Hazard. Mater., 151(2-3): 432-445.
- El-Rahman, K.M., El-Kamash, A.M., El-Sourougy, M.R. and Abdel-Moniem, N.M. (2006). Thermodynamic modeling for the removal of Cs+, Sr2+, Ca2+ and Mg2+ ions from aqueous waste solutions using zeolite A. J. Radioanal. Nucl. Chem., 268(2): 221-230.
- EMA (2005). Protelos: EPAR - Scientific discussion. European Medicines Agency, unpublished, London, United Kingdom.
- EMA (2012). CHMP Type II variation assessment report. Invented name Protelos. European Medicines Agency, EMEA/H/C/000560/II/0031, London, United Kingdom.
- EMA (2013). PSUR assessment report. Strontium ranelate. European Medicines Agency, EMA/PRAC/136656/2013, London, United Kingdom.
- EMA (2014). Protelos/Osseor to remain available but with further restrictions. European Medicines Agency, EMA/235924/2014, London, United Kingdom.
- Environment Canada (2012). Summary review of performance of metal mines subject to the Metal Mining Effluent Regulations in 2012. En49-15/22F-PDF
- Environment Canada (2014). National air pollution surveillance network (NAPS) monitoring results: NAPS Quality Assured Data Products.
- Environment and Climate Change Canada (2017). National long-term water quality monitoring data.
- Ferraro, E.F., Carr, R. and Zimmerman, K. (1983). A comparison of the effects of strontium chloride and calcium chloride on alveolar bone. Calcif. Tissue Int., 35(2): 258-260.
- Fischer, D.C., Jensen, C., Rahn, A., Salewski, B., Kundt, G., D'Haese, P.C., Haffner, D. and Behets, G.J. (2011). Moderate strontium loading induces rickets in rats with mild chronic renal failure. Kidney Blood Press. Res., 34(6): 375-381.
- Fowler, J.H. (1991). Barite, celestite and fluorite in Nova Scotia. Information Circular 15. Nova Scotia Department of Natural Resources, Halifax. Available at: http://novascotia.ca/natr/meb/data/pubs/ic/ic15.pdf
- Friedman, M.J., Hill, A.S., Reiber, S.H., Valentine, R.L., Larsen, G., Young, A., Korshin, G.V. and Peng, C-Y. (2010). Assessment of inorganics accumulation in drinking water system scales and sediments. Water Research Foundation and United States Environmental Protection Agency, Denver, Colorado. Available at: www.waterrf.org/PublicReportLibrary/3118.pdf
- Fuchs, R. K., Allen, M. R., Condon, K.W., Reinwald, S.,Miller, L. M., McClenathan, D., Keck, B., Phipps, R. J. and Burr, D. B. (2008). Strontium ranelate does not stimulate bone formation in ovariectomized rats. Osteoporosis Intl, 19, 1331-1341.
- Gäfvert, T., Ellmark, C. and Holm, E. (2002). Removal of radionuclides at a waterworks. J. Environ. Radioact., 63(2): 105-115.
- Gaubert, E., Barnier, H., Maurel, A., Foos, J., Guy, A., Bardot, C. and Lemaire, M. (1997). Selective strontium removal from a sodium nitrate aqueous medium by nanofiltration - complexation. Sep. Sci. Technol., 32(1-4): 585-597.
- Genuis, S.J. and Bouchard, T.P. (2012). Combination of micronutrients for bone (COMB) study: bone density after micronutrient intervention. J. Environ. Public. Health, 2012: 354151.
- Gerke, T.L., Little, B.J., Luxton, T.P., Scheckel, K.G. and Maynard, L. (2013). Strontium concentrations in corrosion products from residential drinking water distribution systems. Environ. Sci. Technol, 47: 5171.
- Gerke, T.L., Little, B.J., Luxton, T.P., Scheckel, K.G., and Maynard, J.B. and Szabo, J. (2014). Strontium adsorption and desorption reactions in model drinking water distribution system. J. Water Supply: Res. Tech.-AQUA, 63(6): 449.
- Gerke, T.L., Little, B.J. and Barry Maynard, J. (2016). Manganese deposition in drinking water distribution systems. Sci. Total Environ., 541: 184-193.
- Ghosh, S., Talukder, G. and Sharma, A. (1990). Clastogenic activity of strontium chloride on bone marrow cells in vivo. Biol. Trace Elem. Res., 25(1): 51-56.
- Government of Canada (2003). Natural health products regulations. SOR/2003-196. Available at: http://laws-lois.justice.gc.ca/eng/regulations/SOR-2003-196/
- Grynpas, M.D. and Marie, P.J. (1990) Effects of low doses of strontium on bone quality and quantity in rats. Bone, 11(5): 313-319.
- Grynpas, M.D., Hamilton, E., Cheung, R., Tsouderos, Y., Deloffre, P., Hott, M. and Marie, P.J. (1996). Strontium increases vertebral bone volume in rats at a low dose that does not induce detectable mineralization defect. Bone, 18(3): 253-259.
- Hamdy, N.A. (2009). Strontium ranelate improves bone microarchitecture in osteoporosis. Rheumatology (Oxford), 48 Suppl 4: iv9-13.
- Hamilton, S.M. (2015). Ambient groundwater geochemistry data for southern Ontario, 2007-2014. Ontario Geological Survey, Miscellaneous Release-Data 283-Revised. Queen's Printer for Ontario. Available at: www.geologyontario.mndm.gov.on.ca/mndmfiles/pub/data/imaging/MRD283-REV//MRD283-REV.pdf .
- Harrison, G.E., Sutton, A., Shepherd, H. and Widdowson, E.M. (1965). Strontium balance in breast-fed babies. Br. J. Nutr., 19: 111-117.
- Harrison, G., Howells, G., Pollard, J., Kostial, K., and Manitaševi´c, R. (1966). Effect of dietary phosphorus supplementation on the uptake of radioactive strontium in rats. Br. J. Nutr., 20(3): 561-569.
- Health Canada (1994). Canadian Environmental Protection Act. Human health risk assessment for priority substances. Minister of Supply and Services Canada, Ottawa, Ontario. Available at: www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/contaminants/approach/approach-eng.pdf .
- Health Canada (2007a). Average dietary intakes (µg/kg bw/day) of trace elements for Canadians in different age/sex groups for Total Diet Study. Bureau of Chemical Safety, Health Products and Food Branch, Health Canada, Ottawa, Ontario. Available at: www.canada.ca/en/health-canada/services/food-nutrition/food-nutrition-surveillance/canadian-total-diet-study/dietary-intakes-contaminants-other-chemicals-different-sex-groups-canadians/average-dietary-intakes-day-trace-elements-canadians-different-sex-groups-total-diet-study-1993-1996.html .
- Health Canada (2007b). Dietary reference intakes tables. Bureau of Chemical Safety, Health Products and Food Branch, Ottawa, Ontario.
- Health Canada (2009). Guidelines for Canadian drinking water quality: guideline technical document - radiological parameters. Radiation Protection Bureau, Healthy Environments and Consumer Safety Branch, Ottawa, Ontario. Available at: www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-radiological-parameters.html
- Health Canada (2015a). Summary safety review - strontium - risk of heart and circulatory side effects. Health Products and Food Branch, Ottawa, Ontario. Available at: www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/summary-safety-review-strontium-risk-heart-circulatory.html
- Health Canada (2015b). Strontium health products: New restrictions to address possible heart and circulatory-related risks. RA-55524. Available at: http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2015/55524a-eng.php
- Health Canada (2015c). Personal communication from A.-M. Tugulea.
- Hendrix, Z.J., Alcock, N.W. and Archibald, R.M. (1963). Competition between calcium, strontium, and magnesium for absorption in the isolated rat intestine. Clin. Chem., 12: 734-744.
- Hendrych, M., Olejnickova, V. and Novakova, M. (2016). Calcium versus strontium handling by the heart muscle. Gen. Physiol. Biophys., 35(1): 13-23.
- Heuel-Fabianek, B. (2014). Partition coefficients (Kd) for the modelling of transport processes of radionuclides in groundwater. Division of Safety and Radiation Protection, Berichte des Forschungszentrums Jülich (ed.). Helmholtz Association of German Research Centres, 51 pp. Available at: www.researchgate.net/publication/303250933 _Partition_Coefficients_Kd_for_the_Modelling_of_Transport_Processes_of_Radionuclides_in_Groundwater
- Holick, M.F. (2010). The vitamin D deficiency pandemic: a forgotten hormone important for health. Public Health Rev., 32: 267-283.
- Höllriegl, V., Röhmu, M., Oeh, J., Werner, E. and Roth, P. (2004). Reduction in the absorption of dietary strontium in man by nutritional factors. GSF-National Research Center for Environment and Health, Institute of Radiation Protection, In: Proceedings of the 11th International Congress of the International Radiation Protection Association, Madrid, Spain, May 23-28, 2004). International Radiation Protection Association, Neuherberg, Germany. Available at: www.ipen.br/biblioteca/cd/irpa/2004/files/6d6.pdf
- Hood (ed.). (2006). Developmental and reproductive toxicology: A practical approach. Taylor & Francis. CRC, Florida.
- Huang, J., Wu, J., Li, T., Song, X., Zhang, B., Zhang, P. and Zheng, X. (2011). Effect of exposure to trace elements in the soil on the prevalence of neural tube defects in a high-risk area of China. Biomed. Environ. Sci., 24(2): 94-101.
- Humphrey, L.T., Dean, M.C., Jeffries, T.E. and Penn, M. (2008). Unlocking evidence of early diet from tooth enamel. Proc. Natl. Acad. Sci. U. S. A., 105(19): 6834-6839.
- Hunt, J.R., Hunt, C.D., Zito, C.A., Idso, J.P. and Johnson, L.K. (2008). Calcium requirements of growing rats based on bone mass, structure, or biomechanical strength are similar. J Nutr., 138(8): 1462-1468.
- Hurtel-Lemaire, A.S., Mentaverri, R., Caudrillier, A., Cournarie, F., Wattel, A., Kamel, S., Terwilliger, E.F., Brown, E.M. and Brazier, M. (2009). The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis. New insights into the associated signaling pathways. J. Biol. Chem., 284(1): 575-584.
- Hwang, E., Lee, K., Choo, K., Choi, S., Kim, S., Yoon, C. and Lee, C. (2002). Effect of precipitation and complexation on nanofiltration of strontium-containing nuclear wastewater. Desalination, 147(1-3): 289-294.
- ICRP (1993). Age-dependent doses to members of the public from intake of radionuclides - Part 2 ingestion dose coefficients. ICRP Publication 67. Ann. ICRP 23 (3-4). International Commission on Radiological Protection. Available at: www.icrp.org/publication.asp?id=ICRP%20Publication%2067
- Ilyin, L.A., Ivannikov, A.T., Parfenov, Y.D. and Stolyarov, V.P. (1975). Strontium absorption through damaged and undamaged human skin. Health Phys., 29(1): 75-80.
- Ivanets, A.I., Shashkova, I.L., Kitikova, N.V., Drozdova, N.V., Saprunova, N.A., Radkevich, A.V. and Kulbitskaya, L.V. (2014). Sorption of strontium ions from solutions onto calcium and magnesium phosphates. Radiochemistry, 56(1): 32-37.
- Jiménez, A. and De La Montaña Rufo, M. (2002). Effect of water purification on its radioactive content. Water Res., 36(7): 1715-1724.
- Johnson, A.R., Armstrong, W.D. and Singer, L. (1968). The incorporation and removal of large amounts of strontium by physiologic mechanisms in mineralized tissues. Calcif. Tissue Res., 2(3): 242-252.
- Jonville-Bera, A.P. and Autret-Leca, E. (2011). Adverse drug reactions of strontium ranelate (Protelos((R)) in France. Presse Med., 40(10): e453-e462.
- Kaats, G.R., Preuss, H.G., Croft, H.A., Keith, S.C. and Keith, P.L. (2011). A comparative effectiveness study of bone density changes in women over 40 following three bone health plans containing variations of the same novel plant-sourced calcium. Int. J. Med. Sci., 8(3): 180-191.
- Kahn, B., Straub, C.P., Robbins, P.J., Wellman, H.N., Seltzer, R.A. and Telles, N.C. (1969). Intake, excretion, and retention of stable strontium. Pediatrics, 43(4): 687-705.
- Kanematsu, N., Hara, M. and Kada, T. (1980). Rec assay and mutagenicity studies on metal compounds. Mutat. Res., 77(2): 109-116.
- Kaplan, L.J. and Kellum, J.A. (2010). Fluids, pH, ions and electrolytes. Curr. Opin. Crit. Care, 16(4): 323-331.
- Kaufman, J.M., Audran, M., Bianchi, G., Braga, V., Diaz-Curiel, M., Francis, R.M., Goemaere, S., Josse, R., Palacios, S., Ringe, J.D., Felsenberg, D. and Boonen, S. (2013). Efficacy and safety of strontium ranelate in the treatment of osteoporosis in men. J. Clin. Endocrinol. Metab., 98(2): 592-601.
- Kikuchi, H., Iwane, S., Munakata, A., Tamura, K., Nakaji, S. and Sugawara, K. (1999). Trace element levels in drinking water and the incidence of colorectal cancer. Tohoku J. Exp. Med., 188(3): 217-225.
- Kirsch, T., Wang, W. and Pfander, D. (2003). Functional differences between growth plate apoptotic bodies and matrix vesicles. J. Bone Miner. Res., 18(10): 1872-1881.
- Kostial, K., Gruden, N. and Durakovic, A. (1969). Intestinal absorption of calcium-47 and strontium-85 in lactating rats. Calcif. Tissue Res., 4(1): 13-19.
- Kostial, K., Gruden, N., Duraković, A., Juvancic, V. and Simonović, I. (1972). Reduction in strontium absorption in pregnant, lactating and suckling rats. Acta Radiol Ther Phys Biol. 11(3): 277-87.
- Krishnan, K. and Carrier, R. (2013). The use of exposure source allocation factor in the risk assessment of drinking-water contaminants. J. Toxicol. Environ. Health B Crit. Rev., 16(1): 39-51.
- Kroes, R., den Tonkelaar, E.M., Minderhoud, A., Speijers, G.J., Vonk-Visser, D.M., Berkvens, J.M. and van Esch, G.J. (1977). Short-term toxicity of strontium chloride in rats. Toxicology, 7(1): 11-21.
- Kshirsagar, S.G. (1976). Effect of stable strontium on the tissue alkaline and acid phosphatase activities of rat: feeding studies. J. Nutr., 106(10): 1475-1483.
- Kshirsagar, S.G. (1985). Effect of age on strontium-calcium discrimination by rat tissues. Indian. J. Exp. Biol., 23(7): 366-369.
- Kulyukhin, S.A., Krasavina, E.P., Mizina, L.V., Rumer, I.A., Tanashchuk, N.V. and Konovalova, N.A. (2005). Sorption of iodine, cesium, and strontium radionuclides on alkaline-earth hydroxyphosphates from aqueous solutions. Radiochemistry, 47(1): 84-88.
- Lansdown, A.B., Longland, R.C. and Grasso, P. (1972). Reduced foetal calcium without skeletal malformations in rats following high maternal doses of a strontium salt. Experientia, 28(5): 558-560.
- Lee, S.S. (2008). The effect of dissolved organic matter on distribution of heavy metals at the mica-water interface. ProQuest, Chicago, Illinois. 111 pp.
- Leeuwenkamp, O.R., van der Vijgh, W.J., Husken, B.C., Lips, P. and Netelenbos, J.C. (1990). Human pharmacokinetics of orally administered strontium. Calcif. Tissue Int., 47(3): 136-141.
- Li, W.B., Höllriegl, V., Roth, P. and Oeh, U. (2006). Human biokinetics of strontium. Part I: Intestinal absorption rate and its impact on the dose coefficient of 90Sr after ingestion. Radiat. Environ. Biophys., 45(2): 115-124.
- Li, Z., Peng, S., Pan, H., Tang, B., Lam, R.W. and Lu, W.W. (2012). Microarchitecture and nanomechanical properties of trabecular bone after strontium administration in osteoporotic goats. Biol. Trace Elem. Res., 145(1): 39-46.
- Li, Z., He, M., Peng, B. and Jin, Z. (2013). Strontium concentrations and isotope ratios in enamel of healthy and carious teeth in southern Shaanxi, China. Rapid Commun. Mass Spectrom., 27(17): 1919-1924.
- Liang, S., Morton, R., Tang, C., Barry, J., Knapp, T. and Smal, N. (2011). Pilot study of advanced water treatment processes to purify secondary effluent. Proceedings of the American Water Works Association Water Quality Technology Conference & Exposure. Phoenix, Arizona. Available at: www.ingentaconnect.com/content/wef/ wefproc/2011/00002011/00000014/art00046?crawler=true
- Lippert, F. and Hara, A.T. (2013). Strontium and caries: a long and complicated relationship. Caries Res., 47(1): 34-49.
- Llinas, P., Masella, M., Stigbrand, T., Menez, A., Stura, E.A. and Le Du, M.H. (2006). Structural studies of human alkaline phosphatase in complex with strontium: implication for its secondary effect in bones. Protein Sci., 15(7): 1691-1700.
- Loeb, L.A., Sirover, M.A., Weymouth, L.A., Dube, D.K., Seal, G., Agarwal, S.S. and Katz, E. (1977). Infidelity of DNA synthesis as related to mutagenesis and carcinogenesis. J. Toxicol. Environ. Health, 2(6): 1297-1304.
- Lytle, D.A., Sorg, T.J. and Frietch, C. (2004). Accumulation of arsenic in drinking water distribution systems. Environ. Sci. Technol., 38(20): 5365-5372.
- Lytle, D., Chait, H., Vu, K and O'Donnell, A. (2015). Removal of strontium from drinking water: pilot and full scale studies. Proceedings of the American Water Works Association Water Quality Technology Conference & Exposure, Salt Lake City, Utah. Available at: https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=310300
- MacDonald, N.S., Nusbaum, R.E., Stearns, R., Ezmislian, F., McArthur, C. and Spain, P. (1951). The skeletal deposition of non-radioactive strontium. J. Biol. Chem., 188(1): 137-143.
- MacMillan, J.P., Park, J.W., Gerstenberg, R., Wagner, H., Köhler, K. and Wallbrecht, P. (2000). Strontium and strontium compounds. In: Ullmann's encyclopedia of industrial chemistry. Wiley-VCH. (ed.). pp. 473-480.
- Malina, G. (2004). Ecotoxicological and environmental problems associated with the former chemical plant in Tarnowskie Gory, Poland. In: Challenges for toxicology from large contamination sites. Toxicology, 205(3): 157-172.
- Manitoba Conservation and Water Stewardship (2016). Personal communication with J. Muenster and K. Philip.
- Marie, P.J. and Hott, M. (1986). Short-term effects of fluoride and strontium on bone formation and resorption in the mouse. Metabolism, 35(6): 547-551.
- Marie, P.J., Garba, M.T., Hott, M. and Miravet, L. (1985). Effect of low doses of stable strontium on bone metabolism in rats. Miner. Electrolyte Metab., 11(1): 5-13.
- Marie, P.J., Hott, M., Modrowski, D., De Pollak, C., Guillemain, J., Deloffre, P. and Tsouderos, Y. (1993). An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J. Bone Miner. Res., 8(5): 607-615.
- Marinin, D. and Brown, G. (2000). Studies of sorbent/ion exchange material for the removal of radioactive strontium from liquid radioactive waste and high hardness groundwater. Waste Manag., 20: 545.
- Marshall, B. (2013). Facts and figures of the Canadian mining industry. The Mining Association of Canada. West Coast Editorial Associates / Wet Frog Studios. Available at: http://mining.ca/sites/default/files/documents/ FactsandFigures2013.pdf
- Matsumoto, A. (1976). Effect of strontium on the epiphyseal cartilage plate of rat tibiae-histological and radiographic studies. Jpn. J. Pharmacol., 26(6): 675-681.
- McCauley, R.F. and Eliassen, R. (1955). Radioactive-strontium removal by lime-soda softening. J. Am. Water Works Assoc., 47(5): 494-502.
- Meunier, P.J., Roux, C., Seeman, E., Ortolani, S., Badurski, J.E., Spector, T.D., Cannata, J., Balogh, A., Lemmel, E.M., Pors-Nielsen, S., Rizzoli, R., Genant, H.K. and Reginster, J.Y. (2004). The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N. Engl. J. Med., 350(5): 459-468.
- Meunier, P.J., Roux, C., Ortolani, S., Diaz-Curiel, M., Compston, J., Marquis, P., Cormier, C., Isaia, G., Badurski, J., Wark, J.D., Collette, J. and Reginster, J.Y. (2009). Effects of long-term strontium ranelate treatment on vertebral fracture risk in postmenopausal women with osteoporosis. Osteoporos. Int., 20(10): 1663-1673.
- Michalek, J.E., Preuss, H.G., Croft, H.A., Keith, P.L., Keith, S.C., Dapilmoto, M., Perricone, N.V., Leckie, R.B. and Kaats, G.R. (2011). Changes in total body bone mineral density following a common bone health plan with two versions of a unique bone health supplement: a comparative effectiveness research study. Nutr. J., 10(1): 32.
- Ministère du Développement durable, de l'Environnement et de la Lutte contre les changements climatiques (2016). Personal communication with C. Robert and A. Bolduc.
- Moise, H., Adachi, J.D., Chettle, D.R. and Pejović-Milić, A. (2012). Monitoring bone strontium levels of an osteoporotic subject due to self-administration of strontium citrate with a novel diagnostic tool, in vivo XRF: A case study. Bone, 51(1): 93-97.
- Moroas, M.E., Aronson, J.K. and Grahame-Smith, D.G. (1991). Intravenous strontium gluconate as a kinetic marker for calcium in healthy volunteers. Br. J. Clin. Pharmacol., 31(4): 423-427.
- Morohashi, T., Sano, T. and Yamada, S. (1994). Effects of strontium on calcium metabolism in rats. I. A distinction between the pharmacological and toxic doses. Jpn. J. Pharmacol., 64(3): 155-162.
- Myers, A.G., Snoeyink, V.L. and Snyder, D.W. (1985). Removing barium and radium through calcium cation exchange. J. Am. Water Works Assoc., 77(5): 60-66.
- Najm, I. (2016). Strontium in water: Critical review of its treatment options and considerations for its removal. Water Research Foundation. ISBN 978-1-60573-239-8. Available at: www.waterrf.org/Pages/Projects.aspx? PID=4604
- Nakaji, S., Fukuda, S., Sakamoto, J., Sugawara, K., Shimoyama, T., Umeda, T. and Baxter, D. (2001). Relationship between mineral and trace element concentrations in drinking water and gastric cancer mortality in Japan. Nutr. Cancer, 40(2): 99-102.
- National Research Council (U.S.) (1995). Nutrient requirements of laboratory animals. Fourth revised edition. Committee on Animal Nutrition. National Academy of Sciences, National Research Council, Washington, D.C. Available at: www.nap.edu/catalog/4758/nutrient-requirements-of-laboratory-animals-fourth-revised-edition-1995
- Neufeld, E.B. and Boskey, A.L. (1994). Strontium alters the complexed acidic phospholipid content of mineralizing tissues. Bone, 15(4): 425-430.
- New Brunswick Department of Health (2016). Personal communication with K. Gould.
- Newfoundland and Labrador Department of Environment and Conservation (2015). Personal communication with H. Khan.
- Nghiem, L. D. and Schafer, A. I. (2004). Trace contaminant removal with nanofiltration. Chapter 20 in: Nanofiltration-principals and applications. Schafer, A. I., Waite, T. D., and Fane, A. G (Eds).
- Nielsen, S.P. (2004). The biological role of strontium. Bone, 35(3): 583-588.
- NIER (2006). Combined repeated dose toxicity study with the reproduction/developmental toxicity screening test of strontium sulfate in rats (Report No. R06139), tested by Biotoxtech. Incheon, National Institute of Environmental Research [cited in OECD, 2007].
- Nova Scotia Environment (2016). Personal communication with C. Mosher.
- NSF (2005a). ETV report: Removal of chemical contaminants in drinking water - EcoWater systems incorporated ERO-R450E Drinking Water Treatment System. NSF 05/14b/EPADWCTR (EPA/600/R-05/122), U.S. Environmental Protection Agency, Washington, DC. Available at: https://cfpub.epa.gov/ si/si_public_record_Report.cfm?dirEntryId=140464
- NSF (2005b). ETV report: Removal of chemical contaminants in drinking water - Pall/Kinetico Purefecta™ Drinking Water Treatment System. NSF 05/13b/EPADWCTR, U.S. Environmental Protection Agency, Washington, DC.
- NSF (2006a). ETV report: Removal of chemical and microbial contaminants in drinking water Watts Premier, Inc. M-2400 Point-of-Entry Reverse Osmosis Drinking Water Treatment System. NSF 06/23/EPADWCTR (EPA/600/R-06/101), U.S. Environmental Protection Agency, Washington, DC.
- NSF (2006b). ETV report: removal of chemical contaminants in drinking water - Watts Premier, Inc. WP-4V Drinking Water Treatment System. EPA/600/R-06/005), U.S. Environmental Protection Agency, Washington, DC. Available at: https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=145763
- O'Donnell, A.J. and Lytle, D.A. (2014). Full scale and bench scale studies on the removal of strontium from water. Proceedings of the American Water Works Association Annual Conference & Exposure. Cincinnati, Ohio.
- O'Donnell, A.J., Lytle, D.A., Harmon, S., Vu, K., Chait, H. and Dionysiou, D. (2016). Removal of strontium from drinking water by conventional treatment and lime softening in bench-scale study. Water Res., 103:319-333.
- Ober, J.A. (2006). Strontium minerals. In: Industrial minerals & rocks: commodities, markets, and uses. Kogel, J.E. (ed.). Society for Mining, Metallurgy, and Exploration, Inc., U.S., pp. 925-934.
- Ober, J.A. (2014). Strontium. In: 2012 Minerals yearbook, volume I. Metals and minerals. U.S. Geological Survey. Strontium. Advance Release.
- OECD (1997). Test No. 475: Mammalian Bone Marrow Chromosome Aberration Test. Organisation for Economic Co-operation and Development.
- OECD (2007). SIDS initial assessment report for SIAM 24 (Paris, France, 17-20 April 2007). Paris, Organisation for Economic Co-operation and Development, Environment Directorate.
- Osborne, V., Layton, D., Perrio, M., Wilton, L. and Shakir, S.A. (2010). Incidence of venous thromboembolism in users of strontium ranelate: an analysis of data from a prescription-event monitoring study in England. Drug Saf., 33(7): 579-591.
- Oste, L., Bervoets, A.R., Behets, G.J., Dams, G., Marijnissen, R.L., Geryl, H., Lamberts, L.V., Verberckmoes, S.C., Van Hoof, V.O., De Broe, M.E. and D'Haese, P.C. (2005). Time-evolution and reversibility of strontium-induced osteomalacia in chronic renal failure rats. Kidney Int., 67(3): 920-930.
- Ouvrard, S., Simonnot, M-O. and Sardin, M. (2002). Reactive behavior of natural manganese oxides toward the adsorption of phosphate and arsenate. Ind. Eng. Chem. Res., 41, 2785-2791.
- Ozgur, S., Sumer, H. and Kocoglu, G. (1996). Rickets and soil strontium. Arch. Dis. Child., 75(6): 524-526.
- Palmer, R.F. and Thompson, R.C. (1964). Strontium-calcium interrelationships in the growing rat. Am. J. Physiol., 207: 561-566.
- Papworth, D.G. and Vennart, J. (1984). The uptake and turnover of 90Sr in the human skeleton. Phys. Med. Biol., 29(9): 1045-1061.
- Pemmer, B., Roschger, A., Wastl, A., Hofstaetter, J.G., Wobrauschek, P., Simon, R., Thaler, H.W., Roschger, P., Klaushofer, K. and Streli, C. (2013). Spatial distribution of the trace elements zinc, strontium and lead in human bone tissue. Bone, 57(1): 184-193.
- Pertinez, H., Chenel, M. and Aarons, L. (2013). A physiologically based pharmacokinetic model for strontium exposure in rat. Pharm. Res., 30(6): 1536-1552.
- Pi, M. and Quarles, L.D. (2004). A novel cation-sensing mechanism in osteoblasts is a molecular target for strontium. J. Bone Miner. Res., 19(5): 862-869.
- Querido, W., Rossi, A.L. and Farina, M. (2016). The effects of strontium on bone mineral: A review on current knowledge and microanalytical approaches. Micron, 80: 122-134.
- Rabideau, A.J., van Benschoten, J., Patel, A. and Bandilla, K. (2005). Performance assessment of a zeolite treatment wall for removing Sr-90 from groundwater. J. Contam. Hydrol., 79: 1-24.
- Rasmussen, P.E., Subramanian, K.S. and Jessiman, B.J. (2001). A multi-element profile of housedust in relation to exterior dust and soils in the city of Ottawa, Canada. Sci. Total Environ., 267(1-3): 125-140.
- Reginster, J.Y. and Meunier, P.J. (2003). Strontium ranelate phase 2 dose-ranging studies: PREVOS and STRATOS studies. Osteoporos. Int., 14 Suppl 3: S56-65.
- Reginster, J.Y., Hiligsmann, M. and Bruyere, O. (2010). Strontium ranelate: long-term efficacy against vertebral, nonvertebral and hip fractures in patients with postmenopausal osteoporosis. Ther. Adv. Musculoskelet. Dis., 2(3): 133-143.
- Reginster, J.Y., Pelousse, F. and Bruyere, O. (2013). Safety concerns with the long-term management of osteoporosis. Expert Opin. Drug Saf., 12(4): 507-522.
- Reginster, J.Y., Neuprez, A., Dardenne, N., Beaudart, C., Emonts, P. and Bruyere, O. (2014). Efficacy and safety of currently marketed anti-osteoporosis medications. Best Pract. Res. Clin. Endocrinol. Metab., 28(6): 809-834.
- Reginster, J.Y., Brandi, M.L., Cannata-Andia, J., Cooper, C., Cortet, B., Feron, J.M., Genant, H., Palacios, S., Ringe, J.D. and Rizzoli, R. (2015). The position of strontium ranelate in today's management of osteoporosis. Osteoporos. Int., 26(6): 1667-1671.
- Reinholt, F.P., Hjerpe, A., Jansson, K. and Engfeldt, B. (1984). Stereological studies on the epiphyseal growth plate in strontium-induced rickets. With special reference to the distribution of matrix vesicles. J. Bone Joint Surg. Am., 66(8): 1274-1280.
- Richards, L., Richards, B. and Schäfer, A. (2011). Salt and inorganic contaminant removal by renewable energy powered nanofiltration/reverse osmosis J. Membr. Sci., 369(1-2): 188.
- Roschger, P., Manjubala, I., Zoeger, N., Meirer, F., Simon, R., Li, C., Fratzl-Zelman, N., Misof, B.M., Paschalis, E.P., Streli, C., Fratzl, P. and Klaushofer, K. (2010). Bone material quality in transiliac bone biopsies of postmenopausal osteoporotic women after 3 years of strontium ranelate treatment. J. Bone Miner. Res., 25(4): 891-900.
- Roux, C., Fechtenbaum, J., Kolta, S., Isaia, G., Andia, J.B. and Devogelaer, J.P. (2007). Strontium ranelate reduces the risk of vertebral fracture in young postmenopausal women with severe osteoporosis. Ann. Rheum. Dis., 67(12): 1736-1738.
- Rybchyn, M.S., Slater, M., Conigrave, A.D. and Mason, R.S. (2011). An Akt-dependent increase in canonical Wnt signaling and a decrease in sclerostin protein levels are involved in strontium ranelate-induced osteogenic effects in human osteoblasts. J. Biol. Chem., 286(27): 23771-23779.
- Sairanen, S., Karkkainen, M., Tahtela, R., Laitinen, K., Makela, P., Lamberg-Allardt, C. and Valimaki, M.J. (2000). Bone mass and markers of bone and calcium metabolism in postmenopausal women treated with 1,25-dihydroxyvitamin D (Calcitriol) for four years. Calcif. Tissue Int., 67(2): 122-127.
- Saskatchewan Water Security Agency (2015). Personal communication with S. Ferris.
- Sato, I., Kudo, H. and Tsuda, S. (2011). Removal efficiency of water purifier and adsorbent for iodine, cesium, strontium, barium, and zirconium in drinking water. J. Toxicol. Sci., 36(6): 829-834.
- SCC (2015). Directory of accredited product, process and service certification bodies. Standards Council of Canada, Ottawa, Ontario. Available at: www.scc.ca/en/accreditation/product-process-and-service-certification/directory-of-accredited-clients
- Schaep, J., Van der Bruggen, B., Vandecasteele, C. and Wilms, D. (1998). Influence of ion size and charge in nanofiltration. Sep. Purif. Technol., 14(1-3): 155-162.
- Schock, M.R. (2005). Distribution systems and reservoirs and reactors for inorganic contaminants. Chapter 6 in: Distribution water quality challenges in the 21st century, 1st edition. American Water Works Association, Denver, Colorado.
- Schock, M.R. and Holm, T.R. (2003). Are we monitoring in the right places for metals and radionuclides? Journal of the New England Water Works Association, 141(2):102.
- Schock, M. and Lytle, D. (2011). Internal corrosion and deposition control. Chapter 20 in: Water quality and treatment: a handbook on drinking water. American Water Works Association, Denver, Colorado.
- Schock, M.R., Cantor, A.F., Triantafyllidou, S., Desantis, M.K. and Scheckel, K.G. (2014). Importance of pipe deposits to Lead and Copper Rule compliance. J. Am. Water Works Assoc., 106(7), E336-E349.
- Schroeder, H.A., Tipton, I.H. and Nason, A.P. (1972). Trace metals in man: strontium and barium. J. Chronic Dis., 25(9): 491-517.
- Schrooten, I., Cabrera, W., Goodman, W.G., Dauwe, S., Lamberts, L.V., Marynissen, R., Dorrine, W., De Broe, M.E. and D'Haese, P.C. (1998). Strontium causes osteomalacia in chronic renal failure rats. Kidney Int., 54(2): 448-456.
- Schrooten, I., Elseviers, M.M., Lamberts, L.V., De Broe, M.E. and D'Haese, P.C. (1999). Increased serum strontium levels in dialysis patients: an epidemiological survey. Kidney Int., 56(5): 1886-1892.
- Servier laboratories (2016). Protelos: summary of product characteristics. Available at: https://www.medicines.org.uk/emc/product/5598/smpc.
- Shagina, N.B., Fell, T.P., Tolstykh, E.I., Harrison, J.D. and Degteva, M.O. (2015a). Strontium biokinetic model for the pregnant woman and fetus: application to Techa River studies. J. Radiol. Prot., 35(3): 659-676.
- Shagina, N.B., Tolstykh, E.I., Degteva, M.O., Anspaugh, L.R. and Napier, B.A. (2015b). Age and gender specific biokinetic model for strontium in humans. J. Radiol. Prot., 35(1): 87-127.
- Shagina, N.B., Tolstykh, E.I., Fell, T.P., Smith, T.J., Harrison, J.D. and Degteva, M.O. (2015c). Strontium biokinetic model for the lactating woman and transfer to breast milk: application to Techa River studies. J. Radiol. Prot., 35(3): 677-694.
- Shibata, S. and Yamashita, Y. (2001). An ultrastructural study of osteoclasts and chondroclasts in poorly calcified mandible induced by high doses of strontium diet to fetal mice. Ann. Anat., 183(4): 357-361.
- Shorr, E. and Carter, A.C. (1952). The usefulness of strontium as an adjuvant to calcium in the remineralization of the skeleton in man. Bull. Hosp. Joint Dis., 13(1): 59-66.
- Sips, A.J., van der Vijgh, W.J., Barto, R. and Netelenbos, J.C. (1996). Intestinal absorption of strontium chloride in healthy volunteers: pharmacokinetics and reproducibility. Br. J. Clin. Pharmacol., 41(6): 543-549.
- Sivaiah, M.V., Venkatesan, K.A., Krishna, R.M., Sasidhar, P. and Murthy, G.S. (2005). Ion exchange properties of strontium on in situ precipitated polyantimonic acid in amberlite XAD-7. Sep. Purif. Technol., 44(1): 1-9.
- Skoryna, S.C. (1981). Effects of oral supplementation with stable strontium. Can. Med. Assoc. J., 125(7): 703-712.
- Skougstad, M.W. and Horr, C.A. (1963). Occurrence and distribution of strontium in natural water. Geological Survey Water-Supply Paper 1496-D. U.S. Atomic Energy Commission. U.S. Dept. of the Interior, Washington. Available at: https://pubs.usgs.gov/wsp/1496d/report.pdf
- Snoeyink, V.L., Cairns-Chambers, C. and Pfeffer, J.L. (1987). Strong-acid ion exchange for removing barium, radium, and hardness. J. Am. Water Works Assoc., 79(8): 66-72.
- Sorg, T. J. and Logsdon, G. S. (1980). Treatment technology to meet the interim primary drinking water regulations for inorganics: Part 5. J. Am. Water Works Assoc., 72 (7):411-422.
- Spencer, B.B., Wang, H. and Anderson, K.K. (2000). Thermal conductivity of IONSIV IE-911 crystalline silicotitanate and Savannah River waste simulant solutions, ORNL/TM-2000/285, Oak Ridge National Laboratory, Tennessee. Available at: https://info.ornl.gov/sites/publications/Files/Pub57520.pdf
- Statistics Canada (2004). Canadian community health survey, cycle 2.2, nutrition. Nutrient intakes from food. Provincial, regional and national summary data tables.
- Stevenson, F.J. and Fitch, A. (1986). Chemistry of complexation of metal ions with soil solution organics. Chapter 2 in: Huang P.M. and Schnitzer, M. (ed.). Interaction of soil minerals with natural organics and microbes. Soil Science Society of America. Madison, Wisconsin, pp. 29-58.
- Storey, E. (1961). Strontium "rickets": bone, calcium and strontium changes. Australas. Ann. Med., 10: 213-222.
- Storey, E. (1962). Intermittent bone changes and multiple cartilage defects in chronic strontium rickets in rats. J. Bone Joint Surg. Br., 44-B: 194-208.
- Storey, E. (1968). Calcium and strontium changes in bone associated with continuous administration of stable strontium to rats. Arch. Biochem. Biophys., 124(1): 575-581.
- Subramani, A., Liu, L., Balbis, E., Schers, G., Lehman, G. and Jacangelo, J. (2010). Application of new generation reverse osmosis membranes for treatment of surface water with high organic content. Proceedings of the American Water Works Association Water Quality Technology Conference & Exposure, Savannah, Georgia.
- Subramonian, S., Clifford, D. and Vijjeswarapu, W. (1990). Evaluating ion exchange for removing radium from groundwater. J. Am. Water Works Assoc., 82(5): 61-70.
- Sutton, A., Shepherd, H., Harrison, G.E. and Barltrop, D. (1971). Excretion and retention of stable strontium in children. Nature, 230(5293): 396-397.
- Svanstrom, H., Pasternak, B. and Hviid, A. (2014). Use of strontium ranelate and risk of acute coronary syndrome: cohort study. Ann. Rheum. Dis., 73(6): 1037-1043.
- Svensson, O., Hjerpe, A., Reinholt, F.P., Wikstrom, B. and Engfeldt, B. (1985). The effect of strontium and manganese on freshly isolated chondrocytes. Acta Pathol. Microbiol. Immunol. Scand. A., 93(3): 115-120.
- Svensson, O., Reinholt, F.P. and Engfeldt, B. (1987). The parathyroid gland in metal rickets. A stereological study. Acta Pathol. Microbiol. Immunol. Scand. A., 95(6): 309-314.
- Tan, E.L., Williams, M.W., Schenley, R.L., Perdue, S.W., Hayden, T.L., Turner, J.E. and Hsie, A.W. (1984). The toxicity of sixteen metallic compounds in Chinese hamster ovary cells. Toxicol. Appl. Pharmacol., 74(3): 330-336.
- Taylor, D.M., Bligh, P.H. and Duggan, M.H. (1962). The absorption of calcium, strontium, barium and radium from the gastrointestinal tract of the rat. Biochem. J., 83: 25-29.
- Tolstykh, E.I., Shagina, N.B. and Degteva, M.O. (2014). Increase in accumulation of strontium-90 in the maternal skeleton during pregnancy and lactation: analysis of the Techa River data. Radiat. Environ. Biophys., 53(3): 551-557.
- U.S. EPA (1994a). Method 200.8 Rev. 5.4. Determination of trace elements in waters and wastes by inductively coupled plasma - mass spectrometry. Environmental Monitoring Systems Laboratory, Office of Research and Development, U.S. Environmental Protection Agency, Cincinnati, Ohio 45268.
- U.S. EPA (1994b). Method 200.7 Rev. 4.4. Determination of metals and trace elements in water and wastes by inductively coupled plasma-atomic emission spectrometry. Environmental Monitoring Systems Laboratory, Office of Research and Development, U.S. Environmental Protection Agency, Cincinnati, Ohio 45268.
- U.S. EPA (1996). Strontium (CASRN 7440-24-6). I.A. reference dose for chronic oral exposure (RfD). U.S. Environmental Protection Agency.
- U.S. EPA (1999). Understanding variation in partition coefficient, Kd Values. Volume II: Review of geochemistry and available Kd values for cadmium, cesium, chromium, lead, plutonium, radon, strontium, thorium, titanium and uranium. Office of Air and Radiation. U.S. Environmental Protection Agency. EPA 402-R-99-004B.
- U.S. EPA (2011). Drinking water treatability database: Strontium. U.S. Environmental Protection Agency. Available at: https://iaspub.epa.gov/tdb/pages/contaminant/contaminantOverview.do?contaminantId=10600
- U.S. EPA (2012). UCMR 3 laboratory approval requirements and information document. Version 2. Technical support center. Standards and risk management division. Office of Groundwater and Drinking water, U.S. Environmental Protection Agency, Cincinnati, Ohio 45268.
- U.S. EPA (2014). Federal Register Part II, 40 CRF Part 141, Announcement of preliminary regulatory determinations for contaminants on the third drinking water contaminant candidate list; Proposed rule, 79 (202).
- U.S. EPA (2015). Benchmark dose software (BMDS) version 2.6.0.1. Software available for download from the U.S. Environmental Protection Agency. Available at: www.epa.gov/NCEA/bmds/index.html.
- U.S. EPA (2018). 2018 edition of the drinking water standards and health advisories tables. Available at: https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf
- Ulger, Z., Gurel, E.I., Halil, M., Oozen, G., Kalan, I., Seringec, N., Yavuz, B.B., Yesil, Y., Cankurtaran, M., Dikmenoglu, N. and Ariogul, S. (2012). Hemorheological changes with strontium ranelate treatment do not seem to be related to its claimed prothrombotic effects. Arch. Gerontol. Geriatr., 54(1): 218-221.
- Van der Bruggen, B., Koninckx, A. and Vandecasteele, C. (2004). Separation of monovalent and divalent ions from aqueous solution by electrodialysis and nanofiltration. Water Res., 38(5): 1347-1353.
- Verberckmoes, S.C., De Broe, M.E. and D'Haese, P.C. (2003). Dose-dependent effects of strontium on osteoblast function and mineralization. Kidney Int., 64(2): 534-543.
- Verberckmoes, S.C., Behets, G.J., Oste, L., Bervoets, A.R., Lamberts, L.V., Drakopoulos, M., Somogyi, A., Cool, P., Dorrine, W., De Broe, M.E. and D'Haese, P.C. (2004). Effects of strontium on the physicochemical characteristics of hydroxyapatite. Calcif. Tissue Int., 75(5): 405-415.
- Verliefde, A.R.D., Cornelissen, E.R., Heijman, S.G.J., Verberk, J.Q.J.C., Amy, G.L., Van Der Bruggen, B. and Van Dijk, J.C. (2008). The role of electrostatic interactions on the rejection of organic solutes in aqueous solutions with nanofiltration. J. Membr. Sci., 322(1): 52-66.
- Vezzoli, G., Baragetti, I., Zerbi, S., Caumo, A., Soldati, L., Bellinzoni, P., Centemero, A., Rubinacci, A., Moro, G. and Bianchi, G. (1998). Strontium absorption and excretion in normocalciuric subjects: relation to calcium metabolism. Clin. Chem., 44(3): 586-590.
- Vezzoli, G., Soldati, L., Proverbio, M.C., Adamo, D., Rubinacci, A., Bianchi, G. and Mora, S. (2002). Polymorphism of vitamin D receptor gene start codon in patients with calcium kidney stones. J. Nephrol., 15(2): 158-164.
- Wellman, H.N., Kahn, B., Salem, A. and Robbins, P.J. (1966). Strontium and calcium metabolism in children of various ages. International Radiation Protection Association. (IRPA 1 Rome).
- Wheeless, C.R. (ed.) (2011). Wheeless' textbook of orthopaedics. Bone remodelling.
- WHO (2010). Strontium and strontium compounds. Concise international chemical assessment document 77. International Programme on Chemical Safety, World Health Organization.
- Xu, C., Liu, Q., Liu, H., Heroux, P., Zhang, Q., Jiang, Z.Y. and Gu, A. (2015). Low serum testosterone levels are associated with elevated urinary mandelic acid, and strontium levels in adult men according to the US 2011-2012 National Health and Nutrition Examination Survey. PLoS One, 10(5): e0127451.
- Yamaguchi, M. and Weitzmann, M.N. (2012). The intact strontium ranelate complex stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-kappaB activation. Mol. Cell. Biochem., 359(1-2): 399-407.
- Yu, H.Y. and Zhang, K.L. (2011). Links between environmental geochemistry and rate of birth defects: Shanxi Province, China. Sci. Total Environ., 409(3): 447-451.
- Yusan, S. and Erenturk, S. (2011). Adsorption characterization of strontium on PAN/Zeolite composite adsorbent. World J. Nucl. Sci. Technol., 1: 6-12.
- Zhao, S., Wang, X., Li, N., Chen, Y., Su, Y. and Zhang, J. (2015). Effects of strontium ranelate on bone formation in the mid-palatal suture after rapid maxillary expansion. Drug Des. Devel. Ther., 9: 2725-2734.
Appendix A: List of acronyms
- ANSI
- American National Standards Institute
- BMD
- benchmark dose
- BMDL
- benchmark dose lower confidence limit
- BV
- bed volumes
- bw
- body weight
- Ca
- calcium
- CI
- confidence interval
- DL
- detection limit
- DNA
- deoxyribonucleic acid
- EPA
- Environmental Protection Agency (U.S.)
- HBV
- health-based value
- IARC
- International Agency for Research on Cancer
- ICP-AES
- Inductively coupled plasma atomic emission spectroscopy
- ICP-MS
- inductively coupled plasma-mass spectrometry
- IX
- ion exchange
- LOAEL
- lowest-observed-adverse-effect level
- MAC
- maximum acceptable concentration
- MDL
- method detection limit
- MIREC
- maternal-infant research on environmental chemical (study)
- NAPS
- national air pollution surveillance program
- NHANES
- national health and nutrition examination survey (U.S.)
- NOAEL
- no-observed-adverse-effect level
- NSF
- NSF International
- NTP
- National Toxicology Program (U.S.)
- NTU
- nephelometric turbidity unit
- OR
- odds ratio
- POE
- point of entry
- POU
- point of use
- RO
- reverse osmosis
- RR
- relative risk
- SAC
- strong-acid cation (exchange resins)
- SCC
- Standards Council of Canada
- SM
- Standard Methods
- Sr
- strontium
- SrR
- strontium ranelate
- TDI
- tolerable daily intake
- TDS
- total diet study
- UCMR 3
- Unregulated Contaminant Monitoring Rule 3
- WHO
- World Health Organization
Footnotes
- Footnote 1
The U.S. National Research Council has reported that 0.5% calcium was the minimum concentration to maximize bone mineralization during growth of rats, that 3.5% calcium (with calcium:phosphorus ratio of 0.9) can be sufficient for normal rat growth, and that signs of deficiency can be observed below 0.3% (National Research Council, 1995; Bollen and Bai, 2005). A recent study has shown that 0.3% calcium (strontium intake not specified) was adequate and an increase in bone structure, density, and strength parameters was observed in 4-week-old SD rats administered up to 0.3% calcium in the diet, where a plateau was reached (Hunt et al., 2008).