Residential indoor air quality guidelines for Xylenes
Download in PDF format
(1.98 MB, 80 pages)
Organization: Health Canada
Date published: October 15, 2022
Preamble
Health Canada assesses the health risks posed by specific indoor pollutants in residential environments and provides recommendations on how to reduce those risks. Residential Indoor Air Quality Guidelines (RIAQG) summarize the known health effects, pollutant sources, and exposure levels in Canadian homes and characterize the risks to health, based on the best scientific data available. Recommended exposure limits (also referred to as guideline values) for short- and/or long-term exposure to the pollutant are developed, representing indoor air concentrations below which health effects are unlikely to occur. The recommended exposure limits take into account the reference concentrations (RfC) for the pollutant and the feasibility of achieving such levels through control of indoor sources. The RIAQG also include recommendations for controlling sources or other actions to reduce exposure to the pollutant.
The RIAQG and guidance documents serve as a scientific basis for activities to evaluate and reduce the risk from indoor air pollutants, including, but not limited to:
- assessments by public health officials of health risks from indoor air pollutants in residential or similar environments;
- performance standards that may be applied to pollutant-emitting materials, products, and devices, so that their normal use does not lead to air concentrations of pollutants exceeding the recommended exposure limits; and
- communication products informing Canadians of actions they can take to reduce their exposure to indoor air pollutants and to help protect their health.
The RIAQG and guidance documents replace a series of exposure limit values for indoor air pollutants from a report entitled Exposure Guidelines for Residential Indoor Air Quality (Health Canada 1987). In addition to updates for the substances included in the 1987 report, guidelines or guidance documents will be developed for other substances that are identified as having the potential to affect human health in the indoor environment.
Table of contents
- List of tables
- List of figures
- Executive summary
- 1.0 Physical and chemical characteristics
- 2.0 sources in the air
- 3.0 Concentrations in indoor and outdoor air
- 4.0 Toxicokinetics
- 5.0 Health effects
- 6.0 Derivation of short- and long-term reference concentrations
- 7.0 Guidelines
- 8.0 References
- Appendix A: List of acronyms and abbreviations
- Appendix B: Human exposure studies
- Appendix C: Toxicological studies
- Appendix D: Other guidelines
List of tables
- Table 1. Physical and chemical properties of xylenes
- Table 2. Concentrations (µ g/m3) of xylenes in indoor and outdoor air in Canada
- Table 3. Recommended exposure limits for xylenes for indoor environments
- Table B1. Single exposure studies in human volunteers
- Table B2. Long-term exposure studies in humans
- Table C1. Acute exposure studies in experimental animals
- Table C2. Repeat exposure studies in experimental animals
- Table D1. Other short-term exposure guidelines
- Table D2. Other exposure guidelines for non-neoplastic chronic effects
List of figures
- Figure 1. Distribution of concentrations of xylenes in indoor air by season across studies
- Figure 2. Distribution of I/O ratios by season across studies conducted by Health Canada
- Figure 3. Proposed pathway for the metabolism of p-xylene (adapted from US EPA 2003; ATSDR 2007)
Executive summary
| Recommended exposure limit | Concentration | Critical effect(s) | |
|---|---|---|---|
| µg/m3 | ppb | ||
| Short-term (1 h) |
7 200 | 1 700 | Neurological symptoms (headache, fatigue); irritation of eyes, nose and throat; respiratory effects |
| Long-term (24 h) |
150 | 36 | Impaired motor coordination |
The recommended short-term (1-hour) exposure limit for xylenes is 7 200 µ g/m3 and the recommended long-term exposure limit is 150 µ g/m3 (based on a 24-hour average). The recommended exposure limits apply to all three xylene isomers (p-xylene, m-xylene, and o-xylene) in any combination.
Levels of xylenes in Canadian homes are likely below the short-term exposure limit; however, some homes may have levels that are above the long-term exposure limit, and accordingly, may pose a health risk. It is therefore recommended that exposure to xylenes be reduced by ensuring adequate ventilation and controlling indoor sources.
Background
Xylene (dimethylbenzene) is an aromatic hydrocarbon with three isomers (p-xylene, m-xylene, and o-xylene), which differ in the positions of the two methyl groups around the benzene ring. Indoor concentrations of xylenes are generally higher than outdoor concentrations. The terms xylene and xylenes can be used interchangeably.
In 1993, Health Canada derived a provisional tolerable concentration for xylenes of 180 µ g/m3. Health Canada subsequently established an Indoor Air Reference Level (IARL) of 100 µ g/m3 for xylenes in 2017, based on evidence of neurotoxicity in rats from an assessment by the United States Environmental Protection Agency (US EPA). IARLs represent concentrations of a specific volatile organic compound that are associated with acceptable levels of risk after long-term exposure, as determined by the organization or jurisdiction that performed the risk assessment. As levels of xylenes in some Canadian homes may exceed the recommended IARL, this substance was prioritized for a full health risk assessment and development of Residential Indoor Air Quality Guidelines (RIAQG).
This guideline document reviews the epidemiological, toxicological, and exposure research on xylenes as well as the conclusions from several comprehensive reviews conducted by internationally recognized health and environmental organizations. The RIAQG propose short- and long-term indoor air exposure limits for xylenes, which would minimize risks to human health, and to support the development of actions to limit xylenes emissions indoors. The guideline document also recommends various risk mitigation measures to reduce exposure to xylenes.
Sources and exposure
Xylenes occur naturally in petroleum and coal tar, and have been measured during forest fires, to a small extent. However, most ambient sources of xylenes are from human activity including industrial sources such as petroleum refineries and chemical plants and combustion of fuels in motor vehicles, including on-road mobile sources such as cars and trucks, as well as off-road mobile sources such as lawn mowers, snowmobiles and heavy construction vehicles.
In Canadian homes the indoor xylene concentrations are at least 3-fold greater than outdoor concentrations, indicating a predominance of indoor sources. Evaporative emissions from items stored in a garage, including cars, gas-powered equipment, and gasoline containers are an important source of xylenes indoors. Some building and renovation products, such as caulking, coatings and stains, as well as smoking in the home can also contribute to indoor xylene concentrations. Xylenes have been identified internationally in several consumer products (including air fresheners); however, there is no information on the possible contribution of these products to indoor xylene concentrations in Canada.
In Health Canada studies conducted in multiple cities during the winter and summer from 2005 to 2014, the median indoor xylene levels measured ranged from 2.0 to 11.1 µ g/m3. The 95th percentiles ranged from 15.6 to 212.7 µ g/m3. Preliminary data from a Health Canada study suggest that xylene levels are variable but may be higher in recently built homes.
Health effects
In humans, xylene exposure has been shown to cause eye, nose, and throat irritation, as well as some nervous system symptoms including headaches, dizziness, and nausea. In some studies, effects were also observed in tests of memory or reaction times, colour vision, and the central auditory nervous system. In laboratory animals, the most sensitive effect of inhaled xylene is neurological impairment (deficits in tests of motor coordination, pain sensitivity, spontaneous movement, and learning). At higher concentrations, other effects including hearing deficits, body weight decreases, adaptive liver changes, respiratory irritation, lung inflammation, and decreased litter size were sometimes also observed. There is insufficient data to determine whether xylenes might be carcinogenic, but they are generally not considered mutagenic or genotoxic.
In general, there is no clear difference in toxicity among the three xylene isomers: the m-, p- and o-isomers are expected to behave similarly in humans.
There are insufficient data to identify populations that could be more susceptible to the effects of xylene inhalation. A number of factors can contribute to the differences in sensitivity between individuals, including age, body weight, sex, diet and alcohol consumption, exercise, and disease states. In general, children may receive a greater internal dose of many inhaled toxicants than adults at the same exposure concentrations. However, no xylene-specific information is available on internal dose for different age groups.
Recommended short-term residential indoor air quality exposure limit
For short-term exposure to xylenes, the most sensitive endpoints were mild neurological symptoms and eye and respiratory irritation in acute exposure studies with healthy volunteers. A lowest observed adverse effect level (LOAEL) of 50 ppm (217 m g/m3) from a 2-hour study was selected as the point of departure, and the following uncertainty factors were applied: 10 to account for sensitivity in the human population and 3 to account for the use of a LOAEL. Therefore, the short-term reference concentration (RfC) is 7 200 µ g/m3 (1 700 ppb).
The Health Canada residential indoor air exposure studies provide a 24-hour sample of xylene measurements, which does not represent acute or peak exposure. These 24-hour measurements show that the short-term RfC is higher than the range of median indoor air concentrations. As this RfC is achievable in Canadian homes, the recommended short-term exposure limit for xylenes (sum of isomers) is 7 200 µ g/m3 (7.2 m g/m3). It is recommended that the short-term exposure limit be compared to a 1-hour air sample.
Short-term or acute reference exposure levels for xylenes have been derived by the California Environmental Protection Agency (CalEPA 1999; 22 m g/m3); the European Commission (2005; 20 m g/m3); the Agency for Toxic Substances and Disease Registry (ATSDR 2007; 8.7 m g/m3); and the US National Research Council (NRC 2010; 560 m g/m3). Differences in the reference exposure levels established by various jurisdictions result from the key study or endpoint that is selected, and the uncertainty factors that are applied.
Recommended long-term residential indoor air quality exposure limit
For long-term exposure to xylenes, the most sensitive endpoint was decreased motor coordination in rats. A no observed adverse effect level (NOAEL) of 50 ppm (217 m g/m3) was selected as the point of departure, and this concentration was adjusted for continuous exposure, resulting in an adjusted NOAEL of 9 ppm (39 m g/m3). The following uncertainty factors were applied: 1 for toxicokinetic differences and 2.5 for toxicodynamic differences between rats and humans, 10 for sensitivity in the human population, and 10 for database deficiencies and use of a subchronic study. Thus, the long-term RfC is 150 µ g/m3 (36 ppb).
Health Canada data indicate that there may be Canadian homes in which the long-term RfC is exceeded. However, the RfC was derived using the most recent and relevant scientific information and is consistent with the Health Canada IARL and values from other jurisdictions. In addition, reduction of xylene levels in the home through ventilation and source control is considered possible. Therefore, the recommended long-term exposure limit for xylenes (sum of isomers) is 150 µ g/m3.
When a measured xylene concentration is compared against the long-term exposure limit, the sampling time that is used should be at least 24 hours.
Long-term or chronic inhalation reference exposure levels for xylenes have been derived by the CalEPA (2000; 700 µ g/m3); the National Institute for Public health and the Environment in the Netherlands (2001; 870 µ g/m3); the US EPA (2003; 100 µ g/m3); the European Commission (2005; 200 µ g/m3); and the ATSDR (2007; 220 µ g/m3). Differences in reference exposure levels established by various jurisdictions result from the key study or endpoint that is selected, and the uncertainty factors that are applied.
Risk management recommendations
Exposure to xylenes in indoor air should be limited by ensuring adequate ventilation and controlling for indoor sources, using the strategies outlined below. Furthermore, many of these measures will also contribute to reducing the concentrations of other indoor air contaminants, generally improving indoor air quality.
- Increase ventilation, especially when using renovation or building products such as caulking, coatings, and stains:
- By opening windows when possible (check the outdoor air quality conditions in your region before opening windows: Air Quality Health Index).
- By employing mechanical ventilation strategies.
- For more information, refer to the Factsheet: Ventilation and the indoor environment (Health Canada 2018).
- If possible, do not store gasoline and other chemicals in the home or garage; if these products need to be stored, they should be well sealed.
- If you have an attached garage:
- Consider installing a garage exhaust fan.
- Make sure the interface between the attached garage and the home is properly sealed.
- Avoid idling your car, snowblower, lawnmower, or any gas-powered equipment in the garage, even with the garage door open.
- Do not smoke inside the home.
- Choose low-emission products when possible.
- Limit use of scented products and air fresheners.
1.0 Physical and chemical characteristics
The physical and chemical properties of xylenes are summarized in Table 1 (US EPA 2003; NLM 2020). There are three isomers of xylene: p-xylene, m-xylene, and o-xylene, which differ in the positions of the two methyl groups around the benzene ring. m-Xylene is commonly the predominant component in commercial mixed xylenes (40%–77%), with the other isomers each comprising up to 20% of the mass (US EPA 2003). Technical grade xylene could also contain ethylbenzene, toluene, and C9 aromatics (CalEPA 2000). Many studies of xylenes in air report the m- and p-xylene isomers together, as they are not easily separated. The terms xylene and xylenes can be used interchangeably.
| Property | Value | Chemical structure |
|---|---|---|
| Molecular formula | C8H10 | 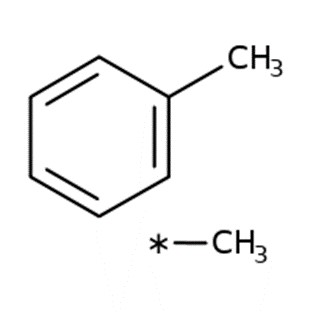 |
| Molecular weight | 107.17 g/mol | |
| CAS registry numbers | 1330-20-7 (mixed isomers); 95-47-6 (o-xylene); 108-38-3 (m-xylene); 106-42-3 (p-xylene) | |
| Density | 0.864 g/cm3 (mixed isomers) | |
| Vapour pressure | 1.065 kPa at 25 °C (mixed isomers) | |
| Water solubility | 106 mg/L (mixed isomers) | |
| Boiling point | 138.5 °C (mixed isomers) | |
| Octanol/water partition coefficient (log Kow) | 3.16 (mixed isomers) | |
| Common synonyms | Dimethylbenzene, methyltoluene
p-xylene = 1,4-dimethylbenzene m-xylene = 1,3-dimethylbenzene o-xylene = 1,2-dimethylbenzene |
|
| Conversion factors | 1 ppm = 4.34 mg/m3 at 25 °C 1 mg/m3 = 0.23 ppm |
2.0 Sources in the air
This section focuses on sources of xylenes in outdoor and indoor air. While exposure to xylenes can result from sources that contribute to media other than air (such as water, food, and soil), these are beyond the scope of this document.
2.1 Outdoor sources
Xylenes are found throughout the ambient environment and are emitted from both natural and anthropogenic sources. Xylenes occur naturally in petroleum and coal tar and have been measured during forest fires, to a small extent (ATSDR 2007). However, most ambient sources of xylenes are from human activity including industrial sources such as petroleum refineries and chemical plants (Environment Canada and Health Canada 1993; VCCEP 2005; ATSDR 2007). Xylenes are used as solvents in a variety of products including paints, varnishes, paint thinners, lacquers, coatings, adhesives, and sealants, and may also be used in the production of plastics and synthetic fibres (Environment Canada and Health Canada 1993; VCCEP 2005; ATSDR 2007). Canada’s National Pollutant Release Inventory (NPRI) indicated that in the years 2015 to 2017, the annual on-site releases of xylenes from all industrial facilities totalled over 3 200 tonnes (NPRI 2021). The majority of xylene releases (97%–99%) were to the air, with the remainder going to water and land.
Combustion represents another major anthropogenic source of xylenes, particularly the combustion of fuels in motor vehicles, including on-road mobile sources such as cars and trucks, as well as off-road mobile sources such as lawn mowers, snowmobiles, and heavy construction vehicles (Environment Canada and Health Canada 1993; VCCEP 2005; ATSDR 2007). Xylenes are also released during biomass burning (VCCEP 2005).
2.2 Indoor sources
Levels of xylenes in indoor air generally exceed those in outdoor air (Environment Canada and Health Canada 1993; VCCEP 2005; Stocco et al. 2008). In Canadian homes the indoor xylene concentrations are at least 3-fold greater than outdoor concentrations (see section 3). In a Health Canada study conducted in Windsor (Stocco et al. 2008), although numerous industrial sources of volatile organic compounds (VOCs) were identified in the area, the distance to specific outdoor sources or the number of point sources within a certain radius were not significant predictors of personal exposure. Similarly, the outdoor concentration did not strongly predict personal exposure, whereas the indoor level of m-, p-xylene was a good predictor of personal exposure. These data indicate a predominance of indoor sources of xylenes compared to outdoor sources.
2.2.1 Garages
An important source of exposure to xylenes indoors is the presence of an attached garage (Batterman et al. 2007; Héroux et al. 2008; Stocco et al. 2008; Wheeler et al. 2013; Mallach et al. 2017; Cakmak et al. 2020). Canadian homeowners generally use their garage to park vehicles and store items such as automotive products, gas-powered equipment, and solvents (Mallach et al. 2017). Many of these items have been shown to release xylenes in chamber tests, even when properly sealed and when not in operation (Won et al. 2015).
In an analysis of Health Canada indoor air data from multiple cities, having a garage attached to the house with a door was associated with a significantly higher mean xylene concentration (by 86% in Edmonton in summer, and by 255% and 134% in Regina in summer and winter, respectively). Significantly higher xylene concentrations were also associated with homes that stored gas-operated tools or gasoline in the garage (by 139%–215% in Edmonton and Halifax), or parked two or more cars in the garage (by over 750% in Halifax) (Health Canada 2021a). Similarly, in a Health Canada study of homes in Windsor Stocco et al. (2008) identified having an attached garage as a significant predictor of indoor m-, p-xylene concentration (o-xylene was measured separately), and Héroux et al. (2008) found that having an attached garage was associated with higher xylene concentrations in Quebec City homes. Based on data from the Canadian Health Measures Survey (CHMS), Wheeler et al. (2013) identified “garage on the property” as a predictor of higher levels of xylenes and found that mean levels of xylenes in homes with an attached garage were approximately double those in homes with a detached garage. Cakmak et al. (2020) also found that homes with a door connecting to an attached garage had higher concentrations of xylenes than those without.
A Health Canada study of 33 homes in Ottawa with attached garages found that the median garage to outdoor (G/O) ratios of xylenes were 80.9 and 75.8 for m-, p-xylene and o-xylene respectively, indicating the presence of garage sources (Mallach et al. 2017) (m- and p-xylene isomers were reported together, and o-xylene was treated separately). The G/O ratios were significantly decreased (p<0.05) when an exhaust fan was operating in the garage (12.9 and 10.9 for m, p-xylene and o-xylene respectively). Operation of a fan in the garage also significantly reduced the median indoor-to-outdoor (I/O) ratios of m-, p-xylene and o-xylene (from 16.09 to 10.3, and from 14.7 to 9.9, respectively); and reduced the concentrations of m, p-xylene and o-xylene indoors by 45% and 43%, respectively. This shows that garage sources are a major contributor to indoor xylene concentrations, and that this contribution can be decreased by use of a garage fan.
The median garage-to-indoor (G/I) ratios for m-, p-xylene and o-xylene in the Ottawa study were 2.4 and 4.0, respectively (Mallach et al. 2017), whereas a study conducted in 15 homes in Michigan, United States (US) reported G/I ratios exceeding 10 (Batterman et al. 2007). The lower G/I ratios in the Health Canada study compared to the US study were attributed to increased stack and wind forces during winter sampling that promoted the transfer of air from the attached garage into the home, in comparison to the spring-summer sampling done in Batterman et al. (2007).
A Canadian study on chamber tests of VOC emissions from evaporative sources in residential garages found that gasoline related products were high emitters of BTEX (benzene, toluene, ethylbenzene, and xylenes) species (Won et al. 2015). The tested gas-powered products that emitted high levels of xylenes included snow blowers, lawn mowers, lawn trimmers, and chain saws. Equipment was run for 30 minutes before being wiped off and placed in the chamber. The older products (snow blower, lawn mower, and lawn trimmer purchased in 2002 or 2003) had xylene emission factors that were 10 to 20 times higher than the same type of equipment purchased in 2014 (e.g., the older snowblower had an m, p-xylene emission factor of 4 451 µg/h compared to 218 µg/h for the newer machine). Another high emitter of xylenes was a 5-L regular grade gasoline container (with the cap closed, the m, p-xylene emission factor was 2 634 µg/h). Other xylene emitters commonly found in residential garages included paint products such as paint thinner, oil-based primer/sealant, and aerosol paint (cap closed m, p-xylene emission factors of 9, 3, and 0.4 µg/h, respectively). A cap/cover tightness test showed that paint primer and paint thinner with a cap opening had higher emissions compared to those with the cap or cover closed tightly. Low emission factors were obtained for an adhesive product, a degreaser, automotive products, wax or polishes, lubricant, roof, lawn and plant care products, and driveway products (m-, p-xylene emission factors of 2.5 µg/h or less). Emission factors for o-xylene were generally about 2 to 4 times lower than those for m-, p-xylene.
2.2.2 Infiltration from traffic
The presence of xylenes indoors could be partially explained by infiltration of outdoor vehicle combustion sources, considering that xylenes are also traffic-related VOCs (Stocco et al. 2008; Bari et al. 2015). In one study in Greece and another in China, indoor levels of xylenes were found to be influenced by location of residents, with levels being higher in urban than suburban locations (Alexopoulos et al. 2006; Du et al. 2014). In the Greek study, proximity to busy roads and to gas stations was also found to influence indoor concentrations of xylenes (Alexopoulos et al. 2006). A study in Australia also showed that xylenes were significantly elevated in homes near a major road compared to homes farther away (Cheng et al. 2016). In a study in four schools in Ottawa, MacNeill et al. (2016) showed that the indoor concentration of m-, p-xylene decreased by 25%–42% when the timing of ventilation was altered so that high ventilation periods did not correspond to rush hour traffic. However, as described above, outdoor concentration is not a good predictor of exposure to xylenes. Overall, infiltration from traffic is not expected to be an important source of xylenes in Canadian homes.
2.2.3 Building materials and consumer products
A Canadian database of chamber test emissions from building materials commonly used in Canadian homes found that xylenes were detected in 72%–84% of dry materials (including flooring products, wood-based materials, ceiling tile, and insulation materials), and 93%–100% of wet materials (including coating, adhesive, caulking, paint, wood stains, and foam sealant) (Won et al. 2013). For dry materials, 24-hour emission factors were generally low (<10 mg/m2/hour) except for acoustical ceiling tile which had the highest emissions among all dry materials (285.2 mg/m2/hour for m-, p-xylene). Wet materials generally had higher emission factors than dry materials. The highest emission factors for xylenes were seen with an oil-based removable caulking sample (over 1 500 000 mg/m2/hour for m-, p-xylene). Other caulking samples had relatively high emissions as well. Emission factors for oil- and water-based polyurethane coatings and oil-based wood stains ranged from 236.6 to 1 370.7 mg/m2/hour for m-, p-xylene. Emissions from oil-based foam sealant, as well as from adhesives and paints, were low (<15 mg/m2/hour). These results are consistent with another emissions study, in which xylenes were only detected in 14% of all tested materials (paint, wood, and insulation) (Won et al. 2014). The highest 24-hour emission factor was identified for a latex-based foam sealant (127 mg/m2/hour for m-, p-xylene). Emission factors for o-xylene were about 2 to 7 times lower than those for m-, p-xylene in these studies.
Based on results of emission tests along with material usage scenarios and parameters such as house volume, indoor air concentrations of VOCs were predicted over a 10-day period (Won et al. 2013, 2014, 2015). These simulations found that dominant sources of xylenes, such as oil-based wood stain, varnishes, and foam caulking, decay fastest at the beginning of the 10-day period, and their contributions to indoor air concentrations decrease over time until they plateau. Other sources that are not initially dominant such as thermal insulation, doors, and flooring (subflooring and solid wood) become dominant later (i.e., they have a higher contribution to air concentrations than they did in the beginning). Also, after the decay of the dominant sources (wet materials) in the first 10 days, infiltration from the garage becomes the dominant source of xylenes; the major garage sources were gasoline containers and gas-powered equipment (Won et al. 2015).
Chamber emission tests were conducted on new building products collected from the construction sites of two new homes or matched materials from retail distributors (if necessary due to the unavailability of clean samples). Products tested included flooring materials (white oak veneer, red oak laminate, plywood underlay, polyester carpet, oriented strandboard subflooring, and ceramic tile assembly with adhesive and plywood underlay), new cabinetry, painted medium density fibreboard baseboard trim, laminate countertop, structural I-joists and paint-drywall assembly. m-, p-Xylene was detected in 71% of products, and the highest predicted 14-day emission factor was for latex caulk used to install baseboard trim (detection frequency and predicted emission factors were lower for o-xylene) (Health Canada and National Research Council Canada 2019).
Using the emission factors for the building products along with the amount of each product used and the installation schedule, the concentration of xylenes in each home was modelled, and the predicted concentrations were compared to the measured concentrations. The modelled concentrations of xylenes and other hydrocarbons were severely under-predicted, which is expected since composite wood products are not a significant source of this type of contaminant. Therefore, additional modelling was conducted using the added scenario of an application of oil-based finish to a small area of the home 24 hours before sampling. Although the new results represented an improvement, the predicted concentrations were still only 10%–50% of the measured values, indicating the presence of additional xylene sources in the new homes (Health Canada and National Research Council Canada 2019).
Canadian studies have found increased levels of xylenes in homes with major renovations within the past month (Wheeler et al. 2013; Cakmak et al. 2020). The o-xylene and m, p-xylene increases were 35% and 43%, respectively (Wheeler et al. 2013). These studies were based on indoor air measurements and activity surveys for the CHMS. An association between renovations and xylene levels was also seen in a study conducted in China, where renovations included redecorating and/or refurnishing (Du et al. 2014). In a US study (Dodson et al. 2017), the mean xylene level was significantly higher in 10 homes sampled immediately after renovation (pre-occupancy) compared to the level in 27 homes sampled 1–9 months later (post-occupancy).
Cakmak et al. (2020) also found that hobbies done within the past three months were associated with higher concentrations of xylenes in Canadian homes. This includes activities such as arts using paints, pottery and ceramics using a kiln, model making using glues, solders, paints or metals, making fishing sinkers or weights, welding or soldering, auto repairs, electronics assembly or repairs, plumbing, refinishing furniture, and woodworking. Wheeler et al. (2013) also identified use of fragrance in the previous 24 hours and use of paint remover in the previous week as predictors of higher xylene concentrations (increases of 22% and 86%–88%, respectively).
Xylenes have been identified internationally in a number of consumer products (permanent pen, shoe polish, leather cleaner, laundry detergent, and air fresheners) (European Commission 2021; Shrubsole et al. 2019; Steinemann, 2017; Lim et al. 2014); however, there is no information on the possible contribution of these products to indoor xylene concentrations in Canada.
2.2.4 Smoking
Tobacco smoke can also be a source of xylenes in the home (ATSDR 2007; Wheeler et al. 2013; Niaz et al. 2015). Xylenes are present in both mainstream tobacco smoke inhaled directly by the smoker and sidestream tobacco smoke released into the environment from the other end of the cigarette (VCCEP 2005). Xylene emission factors for commercial cigarettes range from 85–470 µg/cigarette for m-, p-xylene and 40–98 µg/cigarette for o-xylene (Charles et al. 2007). In the CHMS, higher mean levels of m-/p-xylene were observed in smoking homes compared to non-smoking homes in 2012–2013 (cycle 3) (Li et al. 2019). In the CHMS in 2009–2011 (cycle 2), Wheeler et al. (2013) also identified regular smoking in the home as a predictor of higher m-, p-xylene concentrations (by 20%).
3.0 Concentrations in indoor and outdoor air
3.1 Outdoor concentrations
The National Air Pollution Surveillance (NAPS) program indicated that in 2019, across 32 monitoring stations that reported valid values, the national average concentration of xylenes was 0.91 μg/m3. This includes a rural average of 0.08 μ g/m3 (3 stations), an urban average of 0.75 μ g/m3 (17 stations), and a point source average of 1.34 μ g/m3 (12 stations including traffic stations) (Environment and Climate Change Canada 2021).
In Health Canada studies conducted in multiple cities, median outdoor concentrations of xylenes (sum of isomers) ranged from 0.3 to 2.1 µ g/m3; the 95th percentiles ranged from 1.0 to 9.6 µ g/m3 (Health Canada 2021b; 2013; 2012; 2010a; 2010b; Mallach et al. 2017; Goldberg et al. 2015; Weichenthal et al. 2013). Data from these studies are shown in Table 2.
3.2 Indoor concentrations
In Health Canada studies in a number of cities (Edmonton, Regina, Halifax, Windsor, Ottawa, Montreal) and a First Nations reserve in Manitoba (Swan Lake), median indoor concentrations of xylenes (sum of isomers)Footnote 1 ranged from 2.1 to 11.1 µ g/m3; the 95th percentiles ranged from 15.6 to 212.7 µ g/m3. The highest levels were found in Windsor. Personal monitoring was also conducted in Windsor; median concentrations of xylenes (sum of isomers)1 ranged from 4.7 to 9.0 µ g/m3; the 95th percentiles ranged from 42.2 to 119.5 µ g/m3 (Health Canada 2021b; 2013; 2012; 2010a; 2010b; Mallach et al. 2017; Goldberg et al. 2015; Weichenthal et al. 2013). These studies all used passivated canisters for air sampling.
Similar xylene concentrations were observed in other Canadian studies, including homes in Quebec City, Sioux Lookout, Nunavik, and Ottawa, as well as in the CHMS. These studies used different sampling and analysis methods, and were therefore not included in the ranges given above (National Research Council Canada 2021; INSPQ 2021; Health Canada 2021b; Li et al. 2019; Héroux et al. 2008; Zhu et al. 2005).
A Canadian pilot study looking at VOCs in two new homes found that in one house, concentrations of m-, p-xylene and o-xylene were 154.9 and 63 m g/m3, respectively (24-hour active sampling). In a second home, concentrations of m-, p-xylene and o-xylene were 27.8 and 10.1 m g/m3, respectively (Health Canada and National Research Council Canada 2019).
Data from these studies are shown in Table 2.
Preliminary data from an in-progress Health Canada study suggest that xylene levels may be higher in recently built homes.
| Location | Sampling period | Sampling methodFootnote a | Season | No. of homes | Smoking status | No. of samplesFootnote b | Concentration (μ g/m3)Footnote c | Study reference | |
|---|---|---|---|---|---|---|---|---|---|
| Median | 95th %ile | ||||||||
| Indoor | |||||||||
| Edmonton, Alberta | 2010 | Passivated canisters (7 days × 24 hours) |
Summer Winter |
50 50 |
Non-smokers | 328 337 |
3.2 6.2 |
39.6 82.4 |
Health Canada (2013) |
| Halifax, Nova Scotia | 2009 | Passivated canisters (7 days × 24 hours) |
Summer Winter |
50 50 |
Non-smokers | 331 312 |
3.1 4.0 |
105.8 49.7 |
Health Canada (2012) |
| Regina, Saskatchewan | 2007 | Passivated canisters (24 hours) |
Summer Winter |
111 106 |
Non-smokers Smokers Non-smokers Smokers |
91 13 84 21 |
6.9 5.2 4.3 3.3 |
79.9 55.8 25.2 22.7 |
Health Canada (2010a) |
| Windsor, Ontario | 2006 | Passivated canisters (5 days × 24 hours) |
Summer Winter |
46 47 |
Non-smokers | 211 224 |
10.2 4.1 |
212.7 43.5 |
Health Canada (2010b) |
| Windsor, Ontario | 2005 | Passivated canisters (5 days × 24 hours) |
Summer Winter |
45 48 |
Non-smokers | 217 232 |
11.1 4.2 |
159.2 45.7 |
Health Canada (2010b) |
| Ottawa, Ontario | 2014 | Passivated canisters (48 hours) | winter | 33 (all with attached garage) |
Non-smokers | 62 (garage fan off) 61 (garage fan on) |
4.3 3.4 |
62.9
21.1 |
Mallach et al. 2017 |
| Montreal, Quebec | 2008-2011 | Passivated canisters (24 hr) | all | 55 | - | 285 | 8.2 | 130.9 | Goldberg et al. 2015 |
| Swan Lake, Manitoba | 2011 | Passivated canisters (7 day) | winter | 20 | - | 53 | 2.1 | 15.6 | Weichenthal et al. 2013 |
| Quebec City, Quebec | 2008-2011 | TD Tubes (6-8 days) | Winter summer |
82 | Smokers and non-smokers | 317 158 |
17.3 15.8 |
151.7 143.1 |
NRC Canada 2021 |
| Sioux Lookout, Ontario | 2017-19 | TD tubes (5 days) | winter | 98 | Smokers and non-smokers | 98 | 3.12 | 29.08 | Health Canada 2021b |
| Nunavik | 2018 | TD Tubes (7 days) | Winter – “pre” Winter- “post”d |
54 | Smokers and non-smokers | 52 54 |
9.8 4.8 |
107.2 35.5 |
INSPQ 2021 |
| Quebec City, Quebec | 2005 | 3 M organic vapor monitors (7 days) | Winter | 96 | Smokers and non-smokers | 96 | m, p: 9.2 o: 3.0 |
m, p: 77.1 (max) o: 26.4 (max) |
Héroux et al. 2008 |
| Ottawa, Ontario | 2002-2003 | TD tubes (100 min) | Winter | 75 | Smokers and non-smokers | 75 | m, p: 3.59 o: 1.22 |
m, p: 16.35 (90th) o: 6.48 (90th) |
Zhu et al. 2005 |
| Across Canada | 2012-13 | TD tube (7 days) |
all | 3524 | Smokers and non-smokers | 3524 | m, p: 3.54 o: 1.13 |
m, p: 36.2 o: 10.5 |
Li et al. 2019 |
| Ottawa, Ontario | 2016 | TD tube (24 hours) |
fall | 2 | - | 2 | m, p: 27.8 O: 10.1 (House 1) |
m, p: 154.9 o: 63 (House 2) |
Health Canada and NRC Canada 2019 |
| Outdoor | |||||||||
| Edmonton, Alberta | 2010 | Passivated canisters (7 days × 24 hours) |
Summer Winter |
50 50 |
— | 324 332 |
1.1 1.2 |
3.4 9.6 |
Health Canada 2013 |
| Halifax, Nova Scotia | 2009 | Passivated canisters (7 days × 24 hours) |
Summer Winter |
50 50 |
— | 324 287 |
0.6 0.3 |
2.2 1.4 |
Health Canada 2012 |
| Regina, Saskatchewan | 2007 | Passivated canisters (24 hours) |
Summer Winter |
111 106 |
— | 108 95 |
0.7 0.7 |
2.2 4.5 |
Health Canada 2010a |
| Windsor, Ontario | 2006 | Passivated canisters (5 days × 24 hours) |
Summer Winter |
46 47 |
— | 214 214 |
2.1 1.1 |
7.1 3.3 |
Health Canada 2010b |
| Windsor, Ontario | 2005 | Passivated canisters (5 days × 24 hours) |
Summer Winter |
45 48 |
— | 216 201 |
2.0 1.1 |
7.0 3.2 |
Health Canada 2010b |
| Ottawa, Ontario | 2014 | Passivated canisters (48 hours) | winter | 33 (all with attached garage) |
Non-smokers | 127 | 0.4 | 1.0 | Mallach et al. 2017 |
| Montreal, Quebec | 2008-2011 | Passivated canisters (24 hr) | all | 55 | - | 200 | 1.5 | 6.1 | Goldberg et al. 2015 |
| Overall range from 6 studies | - | - | - | - | - | - | 0.3–2.1 | 1.0–9.6 | |
| Personal | |||||||||
| Windsor, Ontario | 2005 | Passivated canisters (5 days × 24 hours) |
Summer Winter |
45 48 |
— | 207 225 |
9.0 4.7 |
119.5 42.2 |
Health Canada 2010Footnote d |
The distribution of indoor xylene concentrations in studies conducted by Health Canada in four cities is presented in Figure 1. It should be noted that for the studies in Edmonton, Halifax, and Windsor, multiple measurements were made at each home and these values have been averaged to present one value per home, while for the Regina study a single measurement was made at each home. Strong seasonal differences were not observed.
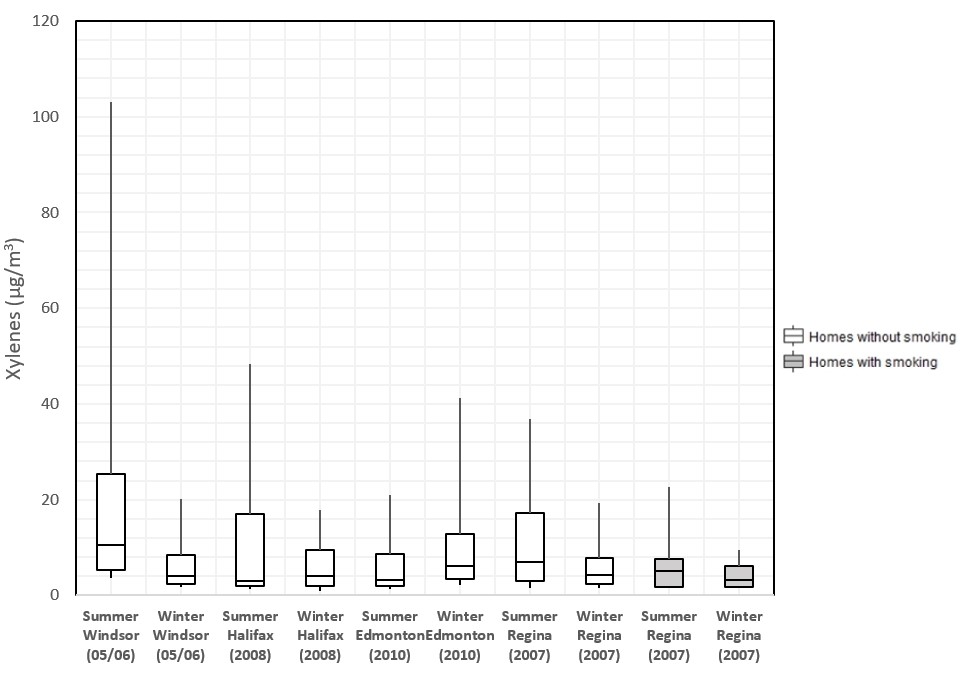
Figure 1 - Text description
Figure 1. The 75th, 50th, and 25th percentiles are represented by the top, middle, and bottom of the boxes. The whiskers represent the 90th and 10th percentiles. The concentration is shown as the sum of m-, p-, and o-xylene. Study data reports and publications show data for m- and p-xylene together, and o-xylene separately. Additional analysis was done to generate summary statistics for the sum of all three isomers (Health Canada 2021b).
3.3 Indoor/outdoor (I/O) ratios
An I/O ratio compares levels of xylenes measured inside a given home to levels measured directly outside the same home. The distribution of I/O ratios for homes in four Health Canada studies is presented in Figure 2. Median I/O ratios of xylenes in these four Canadian cities range from 2.6 to 12.3, indicating a predominance of indoor sources. In a Health Canada study conducted in 33 homes in Ottawa with attached garages, the median I/O ratios for m-, p-xylene in homes with the garage fan off and the garage fan on were 16.09 and 10.3, respectively (similar ratios were obtained for o-xylene) (Mallach et al. 2017).

Figure 2 - Text description
Figure 2. The 75th, 50th, and 25th percentiles are represented by the top, middle, and bottom of the boxes. The whiskers represent the 90th and 10th percentiles. The concentration is shown as the sum of m-, p-, and o-xylene. Study data reports and publications show data for m- and p-xylene together, and o-xylene separately. Additional analysis was done to generate ratios for the sum of all three isomers. (Health Canada 2021b)
3.4 Impact of ventilation
In an analysis of Health Canada indoor air data from three cities, a higher daily mean indoor air exchange rate was associated with significantly lower mean xylene concentrations in Edmonton in summer, and in Halifax in summer and winter (29%–73% decrease with an air exchange rate increase of 1/hour) (Health Canada 2021a). Also, as described in section 2.2.1, in a study of 33 homes in Ottawa, the operation of an exhaust fan in the attached garage significantly reduced the concentrations of m-, p-xylene and o-xylene in the home, by 45% and 43%, respectively. In a study in 54 homes in Nunavik, optimization of the ventilation system reduced m-, p-xylene and o-xylene by 52% and 53%, respectively, compared to levels prior to the optimization (INSPQ 2021). In contrast, in a study in Quebec City, although there was an increase in the mean ventilation rate in homes after a ventilation intervention (installation or optimization of a ventilation system) compared to control homes, xylene levels were similar in the two sets of homes (Lajoie et al 2015; National Research Council Canada 2021).
4.0 Toxicokinetics
4.1 Absorption, distribution, metabolism, and excretion
The characteristics of xylene pharmacokinetics are well understood, and are described in detail in US EPA (2003) and ATSDR (2007). In rodents and humans, following inhalation exposure, over 60% of the inhaled dose of xylene is rapidly absorbed into the blood and distributed around the body. Due to its lipophilicity (high Kow), xylene partitions primarily into lipid-rich tissues such as adipose and brain. The blood concentration of xylene declines rapidly as soon as exposure ends. The movement of xylene out of the blood follows first-order kinetics with a biphasic pattern, the first phase having a half-life of 0.5 to 1 hour, and the second phase a half-life of 20 to 30 hours.
Xylene metabolism occurs rapidly in the liver, and the primary route starts with oxidation of one of the methyl groups by microsomal CYP2E1, followed by conjugation with glycine and excretion in urine as methylhippuric acid (MHA) derivatives. Urinary MHAs account for over 95% of the absorbed xylene, and levels increase rapidly during the first 2 hours of exposure, reaching a peak during or immediately following the cessation of exposure, at which time the amount declines significantly but remains detectable for several days. Minor metabolites include methylbenzyl alcohols, glucuronic conjugates, and xylenols. A small fraction (less than 5%) of the absorbed xylene is rapidly eliminated unchanged in expired air, and trace amounts of unchanged xylene are eliminated in urine. The metabolic pathway of p-xylene is shown in Figure 3.

Figure 3 - Text description
Figure 3: Proposed pathway for the metabolism of p-xylene (adapted from US EPA 2003; ATSDR 2007). p-Xylene is shown as representative of all three xylene isomers. O- and m-xylenes are expected to follow the same pathway. Glucuronidation may only occur under conditions of high dose administration.
The three xylene isomers have similar chemical properties, such as log Kow, and exhibit similar patterns of absorption and distribution. In addition, the tissue/air partition coefficients (liver, fat, and muscle) and blood/air partition coefficients are similar (Adams et al. 2005; ATSDR 2007). Metabolism of the three isomers occurs via the same enzymatic pathway, resulting in urinary excretion of the corresponding MHA isomer as the predominant metabolite (See Figure 3). However, Adams et al. (2005) did note a slightly higher blood clearance of m‑xylene, which was consistent with earlier findings of higher urinary clearance of that isomer. p-Xylene and o-xylene readily cross the placenta and are distributed in amniotic fluid and embryonic and fetal tissues in mice (ATSDR 2007). Ungváry et al. (1980) showed the presence of o-xylene in fetal blood and amniotic fluid of rats.
Xylene metabolism is a saturable process, but saturation is only expected to occur at high concentrations. For example, a study in rats suggested that saturation occurs at a concentration above 225 ppm; and PBPK (physiologically based pharmacokinetic) modelling in humans suggests a linear relationship up to a concentration of 500 ppm (ATSDR 2007). As shown in section 3, levels in Canadian homes are well below this (highest 95th percentile of 213 µ g/m3 or less than 0.05 ppm).
Other VOCs including benzene, toluene, and ethylbenzene inhibit xylene metabolism by competing for the same liver enzymes (i.e., CYP2E1). However, data in humans as well as PBPK modelling in rats and humans suggest that xylene metabolism is unlikely to be inhibited at VOC concentrations to which the general population is exposed (ATSDR 2007; Health Canada 2014; Valcke and Haddad 2015; Marchand et al. 2016). Ethanol is also a substrate of CYP2E1; in addition to competing for binding sites, it induces enzyme activity. Therefore, alcohol consumption may affect xylene metabolism in individuals (MacDonald et al. 2002).
Several polymorphisms have been identified that influence CYP2E1 gene expression or enzyme activity (reviewed in Wang et al. 2020); however, there are no available studies that examine CYP2E1 polymorphism and xylene metabolism specifically. In a study of 17 human subjects, Ernstgård et al. (2003) found that despite a greater than 10-fold difference in CYP2E1 activity between individuals, there was no correlation with any toxicokinetic parameters of m-xylene. As only one subject had a different CYP2E1 genotype, no comparison could be made between CYP2E1 genotype and xylene metabolism. Despite the lower expression and activity of cytochromes including CYP2E1 in newborns and young children, PBPK modelling suggests that there is not significant variability in terms of the level of xylene metabolism in children compared to adults (Pelekis et al. 2001; Valcke and Krishnan 2011; Valcke and Haddad 2015).
4.2 Physiologically based pharmacokinetic modelling
Several PBPK models for m-xylene inhalation in rats have been described in US EPA (2003) and ATSDR (2007). These models use inhaled and exhaled xylene concentrations and blood/gas and tissue/gas partition coefficients to predict blood and tissue concentrations, assuming saturable metabolism in the liver. Some of the models were also applied to mixtures (i.e., xylene and other aromatic compounds such as toluene, benzene, and ethylbenzene). US EPA (2003) and ATSDR (2007) also reported validation of some models in rats and humans.
The US EPA (2003) used the rat model to predict the blood concentration at the duration-adjusted NOAEL of 39 mg/m3 from the critical study selected for their RfC derivation (Korsak et al. 1994). The resulting blood concentration of 144 μg/L was used in the human model to predict a corresponding human exposure concentration (HEC) of 41 mg/m3. The US EPA (2003) also used the rat model to predict the arterial blood concentration in rats during the same 13-week study. Exposure in the study was intermittent (6 hours per day, 5 days per week), and the model showed a rapid rise and fall in blood concentration corresponding to each exposure period. The US EPA then estimated the equivalent HEC using various approaches (overall time-weighted average [TWA] blood concentration averaged over 1-hour intervals for 13 weeks, the maximum blood concentration for any given exposure, and the midpoint between the maximum and minimum on any given day of exposure). They noted that the approach using the TWA likely gives the most realistic estimate of exposure in the rat study; this approach resulted in a HEC of 46 mg/m3.
ATSDR (2007) noted that PBPK modelling suggests that the urinary excretion of m-methylhippuric acid following m-xylene exposure in humans is linear at exposure concentrations up to 500 ppm (2 170 mg/m3) and may be slower in individuals with a greater percentage of body fat. Some of the other parameters that influence within-species variability of tissue and blood doses following inhalation exposures include body weight, ventilation rate, fraction of cardiac output flowing to the liver, blood/air partition coefficient, and hepatic extraction ratio (ATSDR 2007). Using individual measured data and PBPK modelling, Adams et al. (2005) suggested that the actual body burden of xylene could vary significantly, even among equivalently exposed subjects. Differences in diet, alcohol consumption, and stress could affect variation in respiratory and blood physiological parameters, accounting for some of this variability (ATSDR 2007).
4.3 Biomonitoring
Xylene in blood has been measured in the general population as an indicator of recent exposure. In the 5th cycle of the CHMS, VOCs were measured in blood of over 2 500 subjects, aged 12 to 79 (Health Canada 2019). The median and 95th percentiles of m-, p-xylene in blood were 0.065 and 0.39 µg/L, respectively; the median and 95th percentiles of o- xylene in blood were 0.020 and 0.10 µg/L, respectively. US national biomonitoring data from the National Health and Nutrition Examination Survey (NHANES) are also available; median blood levels of m-, p-xylene and o-xylene were reported as 0.19 and 0.11 µg/L, respectively in a sample from 1988–1994, and similar levels were reported in more recent general population studies in the US (US CDC 2017). In several other countries including Mexico, Italy, and Germany, blood xylene levels of 0.05 to 0.7 µg/L have been reported in non-occupationally exposed subjects (ATSDR 2007).
Smokers can have blood o- and m-, p-xylene levels that are each about twice as high as those for non-smokers (US CDC 2017). Kirman et al. (2012) used NHANES data for 314 smokers and 876 non-smokers, and noted that the geometric mean (GM) of blood concentrations of total xylene for smokers was 0.26 µg/L, whereas non-smokers had a GM of 0.16 µg/L. Similarly, Faure et al. (2020) calculated blood concentrations of xylene in 402 smokers and 1 967 non-smokers from the CHMS and reported that the GM and 95th percentiles were considerably higher for smokers (0.17 µg/L and 0.44 µg/L) than for non-smokers (0.066 µg/L and 0.26 µg/L).
Aylward et al. (2010) used PBPK modelling to estimate that the steady-state blood xylene concentration at the US EPA RfC of 100 µg/m3 would be 0.3 µg/L. Blood concentrations above this biomonitoring equivalent (BE) could indicate that the population is exposed to xylene concentrations above the US EPA RfC. The data presented by Faure et al. (2020) indicate that the GM blood concentration of xylene in the Canadian general population is below the BE. Although the 95th percentile for smokers does exceed the BE, the GM is generally a more appropriate measure of long-term population biomarker concentration (Aylward et al. 2013).
The main urine metabolite of xylene, MHA, is generally only measured in occupational settings, and reflects recent exposure. Methods for measuring MHA in urine may not be sensitive enough to use in investigations of xylene exposure for the general population (ATSDR 2007).
5.0 Health effects
Relevant studies on the health effects of xylene (including studies on individual isomers as well as mixed isomers and technical grade xylene) published up to April 2020 were reviewed. Although xylene is a component of tobacco smoke, studies of tobacco smoke were excluded as tobacco smoke is a complex mixture that contains many known toxins and carcinogens, and its health effects are not addressed in this document. Routes of exposure other than inhalation (i.e., ingestion and dermal) were not considered physiologically relevant. Health Canada evaluated the original studies identified as key in the derivation of the recommended exposure limits for xylene (see section 6). Other relevant information was drawn from previous authoritative reviews of the health effects of xylenes: (a) ATSDR’s (2007) Toxicological Profile for Xylene; (b) US EPA’s(2003) Toxicological Review of Xylenes; (c) CalEPA’s (2000) Xylenes Reference Exposure Levels; (d) CalEPA’s (2012) Evidence on the Developmental and Reproductive Toxicity of Xylene and (e) Environment Canada and Health Canada’s (1993) Priority Substances List Assessment Report: Xylenes.
Details of the key human exposure and toxicological studies presented below can also be found in appendices B and C.
5.1 Effects in humans
The limited available information on the toxicity of xylene to humans comes from single exposure volunteer studies, case reports of accidental exposure to very high concentrations, and studies of occupational exposures in which subjects were exposed to a mixture of solvents.
Single controlled exposure studies in volunteers and a case study of accidental high concentration exposure reported eye, nose and throat irritation, as well as some neurological symptoms including headaches, dizziness, and nausea. Mixed results were observed in tests of memory or reaction times following acute exposure of healthy volunteers.
Occupational studies showed similar effects such as irritation (eye irritation, sore throat), and central nervous system (CNS) symptoms including a floating sensation, headache and confusion, as well as effects in some neurological endpoints including tests of memory, colour vision, and the central auditory nervous system. These studies were limited by poorly defined exposure concentrations and durations and co-exposure to multiple chemicals, as well as by the timing of the symptom survey or testing, and in some cases, the subjective nature of self-reporting of symptoms.
The few available epidemiological studies on non-occupationally exposed groups were not of sufficient quality to draw conclusions about the effects of xylene exposure; however, they lend support to the overall weight of evidence showing that xylene has neurological effects.
5.1.1 Acute exposure
A minimal increase in subjective symptoms (headache, dizziness, feeling of intoxication) was observed during exposure in a study of 56 healthy volunteers (28 male, 28 female) exposed for 2 hours to 50 ppm m-xylene, compared to exposure to clean air or 150 ppm isopropanol (each subject experienced the three exposures, with an interval of 2 weeks in between) (Ernstgård et al. 2002). Slight respiratory effects were also reported by subjects during exposure (increased discomfort in throat and airways in women and breathing difficulty in both sexes). No mention was made of whether the subjective symptoms continued after exposure was terminated, although the study protocol included conducting a questionnaire 20 and 230 minutes post-exposure. In addition to symptom rating, the study included pulmonary function measurements and assessments of nasal swelling, nasal inflammation, and colour vision before, immediately after, and 3 hours after exposure. The only difference was a small reduction in forced vital capacity (FVC) in women only, 3 hours after (but not immediately after) xylene exposure ended. The difference was expressed as percent change compared to before xylene exposure and was significant relative to clean air exposure. The study authors proposed that factors contributing to the difference between women and men could include estrogen or smaller body size (and therefore smaller airway size and greater sensitivity). Additional studies are needed to explore these sex-based differences. ATSDR (2007) determined a minimal LOAEL of 50 ppm (217 mg/m3) m-xylene from this study, and used this value to derive an acute duration minimal risk level (MRL).
The US EPA (2003) cited several studies of volunteer exposure to xylenes at concentrations of 100 to 400 ppm for up to 4 hours, which suggest CNS effects including mild nausea, headache, reversible effects on balance and reaction times, and impaired performance on tests of memory and reaction times. However, in other studies using similar concentrations and exposure durations, effects were not observed; for example, there was no impairment of performance in tests of simple reaction time, short-term memory, or choice reaction time, and no changes in visually evoked brain potentials or electroencephalogram (EEG) patterns in several studies at 200 ppm (US EPA 2003). Carpenter et al. (1975) noted that at 230 ppm, 1 of the 6 volunteers felt dizzy during the last 2 minutes of the 15-minute exposure; none reported dizziness at 110 ppm.
Symptoms of irritation (e.g., watering eyes and sore throat) were reported in studies of volunteers exposed to 200–400 ppm xylenes for 15 minutes to 4 hours (US EPA 2003). Nose and throat irritation were also reported following exposure to mixed xylenes at an estimated concentration of 200 ppm but not 100 ppm for 3–5 minutes (Nelson et al. 1943). No increase in reports of nose and throat irritation was noted, and no change in eye blinks per minute or breaths per minute was observed in a study of 50 healthy male subjects exposed to mixed xylenes at concentrations of up to 400 ppm for 30 minutes; in the same study, eye irritation was reported by 90% of the subjects at 400 ppm, 70% at 200 ppm, and 60% at 100 ppm, compared to 56% of control subjects (Hastings et al. 1984 cited in NRC 2010; CalEPA 1999).
The US EPA (2003) noted that “concentrations around 100–200 ppm are close to the threshold level for short-term reversible neurological and irritation effects from xylenes.” CalEPA (1999) and the European Commission (2005) derived acute (1-hour) RfCs based on a NOAEL of 100 ppm (434 mg/m3) for eye, nose and throat irritation in the studies by Hastings et al. (1984), Nelson et al. (1943), and Carpenter et al. (1975).
In a case study of 15 workers accidentally exposed to xylene vapours at an estimated concentration of up to 700 ppm for up to an hour, each worker experienced at least two symptoms, including headache, nausea, vomiting, dizziness or vertigo, eye irritation, and nose or throat irritation (US EPA 2003).
5.1.2 Long-term exposure
Neurological effects
One occupational study was identified in which xylene was described as the primary exposure (Uchida et al. 1993), although other solvents were present also. Workers were selected based on a single day personal exposure measurement in which at least 70% of the solvent detected was xylene, and exposure was assumed to be the same for the entire 7-year study period. The mean xylene concentration (TWA) of personal exposure measurement) was 14 ppm. The day after exposure was measured, a health survey was done. The prevalence of subjective symptoms during the work shift and in the previous 3-month period was significantly higher in exposed workers compared to that for non-exposed workers. When the exposed individuals were divided according to xylene concentration (1–20 ppm or >21 ppm), eye irritation, sore throat, and a floating sensation showed a concentration-related increase for symptoms reported during the work shift, whereas poor appetite was the only concentration-dependent symptom reported for the previous 3 months. ATSDR (2007), CalEPA (2000), and the EU (2005) considered the mean TWA concentration of 14 ppm (61 mg/m3) to be a LOAEL and used it as the basis for their exposure limits. The US EPA (2003) did not use this study to derive their RfC, and described many study limitations, including a lack of reporting on the duration of exposure, co-exposure to other chemicals, no clear demonstration of relationships between response and dose or duration, and the inherent bias associated with self-reporting of symptoms.
More recent occupational exposure studies also suggest neurotoxicity but have limitations like those of the Uchida study. In a study of shipyard painters (Lee et al. 2005), although co-exposure to multiple solvents was identified, xylene accounted for the highest concentration in air. The exposed group of painters had significantly different results in a test of memory (SD, symbol digit substitution test) and in finger tapping speed (FT) compared to unexposed controls. Increased duration of exposure (<10 years vs. >20 years) also showed differences in these test results. Simple reaction time was not impacted in any of the analyses. The authors did not look at lead or other inorganic contaminants that may have been present in the workplace. A later study on shipyard painters also reported that, among the solvents identified, xylene had the highest concentration in air (mean concentration of 10 ppm), and therefore the urine metabolite MHA was measured in exposed workers and controls. The MHA concentration in urine was significantly correlated with results on the colour confusion index test, which the authors note can be an important indicator of the preliminary stages of nervous system disorders in workers exposed to multiple solvents (Lee et al. 2013) These studies are of limited value because of the multiple co-exposures, including toluene, ethylbenzene, and methyl isobutyl ketone.
A case study of 5 women laboratory workers exposed to xylene for 1.5 to 18 years reported CNS effects including headache and confusion (US EPA 2003). Subjects also reported chest pain, electrocardiogram abnormalities, dyspnea, cyanosis of the hands, and impaired lung function.
Two recent studies in non-occupationally exposed populations were identified that examined associations between xylene exposure and neurological symptoms. Werder et al. (2019) measured levels of xylene and other chemicals in the blood of 690 non-occupationally exposed adults and conducted a survey on the frequency of neurological symptoms during the 30 days prior. Blood xylene concentrations were 0.06, 0.13, and 0.25 ng/mL (µg/L) at the 25th, 50th, and 75th percentiles, respectively. The study authors stated that for xylene, most associations were confounded by co-exposures. However, in models restricted to non-smokers and adjusted for confounders (including blood concentrations of benzene, toluene, and ethylbenzene), there was an association between multiple CNS symptoms and the second, third, and fourth quartiles of blood xylene concentration, as well as an exposure-response trend. The CNS symptoms surveyed were dizziness, headache, nausea, sweating, and palpitations. Norback et al. (2017) measured indoor concentrations of multiple pollutants in 32 classrooms at 8 schools in Malaysia. The median xylene concentration measured by passive sampling for 1 week was 78.4 µg/m3 (0.02 ppm). Higher xylene levels were associated with self-reported fatigue in the past 3 months in the 462 students surveyed; however, there was no adjustment for co-exposure to other contaminants. No association was observed between xylene concentration and reported headache or mucosal symptoms. These studies were not of sufficient quality to draw conclusions about the effects of higher xylene exposure in the general population; however, they lend support to the overall weight of evidence for xylene exposure and neurological effects.
Auditory effects
Several studies have identified occupational exposure to solvents and noise as a potential risk factor for hearing loss. However, in most of these studies, workers were exposed to multiple solvents with or without co-exposure to excessive noise; therefore, no conclusions could be drawn about the effect of xylene specifically on hearing loss.
One study (Fuente et al. 2013) was identified that looked specifically at the effect of occupational exposure to xylene (without co-exposure to other solvents) on the auditory systems. The exposed group of 30 subjects were medical laboratory workers for whom xylene is likely to be the primary exposure due to its use as a tissue sample processing agent. None of the workers were exposed to excessive noise. The subjects described their work history in order to estimate the exposure duration, the concentration of xylene in air was measured on a single day, and urine was sampled after the last day of a work week to measure MHA. The mean xylene concentration measured in air was 36.5 mg/m3 (range 8–217 mg/m3), and the mean duration of exposure was 11.8 years (range 2–29 years). Tests of both peripheral and central auditory function were conducted on all exposed and control subjects. The xylene-exposed participants showed significantly worse results in some tests including pure-tone thresholds, pitch pattern sequence test, dichotic digit test, speech recognition in noise test, and auditory brainstem response. An estimate of cumulative dose was also obtained using the reported exposure duration and the urine MHA concentration; participants with a higher cumulative dose had poorer test results than those with a low cumulative dose. The study authors suggested that xylene is associated with adverse effects on the central auditory nervous system and sound detection abilities in humans, and proposed that the effect of xylene on the auditory system at least partially relates to neurotoxicity at the brainstem level. The authors acknowledged the study limitations, including the timing of the exposure measurements and testing.
A case study (Fuente et al. 2012) reported that the suspected cause of hearing loss in a histology laboratory worker was exposure to xylene for 20 years.
Reproductive/Developmental effects
The US EPA (2003) described some occupational exposure studies looking at rates of spontaneous abortions in workers; however, exposure was to multiple chemicals, and the number of cases was small. Therefore, these studies are not useful for characterizing xylene toxicity. Similar limitations were noted for some more recent studies. Cho et al. (2001) found increased prevalence of oligomenorrhea (menstrual cycle longer than 35 days) in exposed petrochemical workers compared to controls, but all the workers exposed to xylene were also exposed to toluene and benzene, and some were also exposed to styrene. Similarly, Desrosiers et al. (2015) looked at the birth weight of babies born to mothers who were occupationally exposed to solvents; however, they could not estimate the effect of exposure to xylene due to the small number of xylene-exposed women and co-exposure to other chemicals. Lehman et al. (2002, cited in CalEPA 2012) found that levels of m-, p-xylene in the air of infants’ homes were associated with cytokine-producing cord blood T-cells in unadjusted analyses but not after adjusting for maternal smoking during pregnancy, family atopy history, and gender.
Respiratory effects
Multiple studies looking at associations between air pollutant concentrations (including xylene) and respiratory effects such as lung function, asthma, rhinitis, or wheezing were identified. Although some associations were observed, no conclusions could be drawn regarding the effect of xylene exposure, primarily due to multiple pollutant exposures.
A few studies that included biomonitoring were identified; however, these studies were also confounded by multiple pollutant exposures. Elliott et al. (2006) measured pulmonary function associated with blood concentrations of xylene and other VOCs in the general population of the US. No association was found between xylene and any of the pulmonary function tests. Kwon et al. (2018) studied 34 subjects before and after moving to a new rehabilitation facility, where they spent 8 hours a day or more. The urinary levels of o-MHA, m-MHA, and p-MHA increased significantly after the move, indicating an increase in xylene exposure. No changes were observed in the levels of the other VOC metabolites. No significant correlations were observed between changes in urinary levels of xylene metabolites and lung function tests before and after the move. Yoon et al. (2010) studied 154 Koreans and compared urinary metabolites of BTEX with lung function. The levels of MHA were negatively associated with FEV1 and FEV1/FVC. No association between air xylene and urine MHA was observed, making it even more difficult to draw conclusions.
5.1.3 Carcinogenicity
The International Agency for Research on Cancer (IARC) (1999) classifies xylenes as Group 3 “not classifiable as to their carcinogenicity in humans” and concludes that there is inadequate evidence in humans. IARC reviewed several studies investigating the carcinogenicity of xylenes in humans but concluded that “In view of the multiple exposure circumstances in most studies, the multiple inference context of these studies, and the weak consistency of the findings, these results are not strong enough to establish whether there is an association with xylene exposure.”
Similarly, the US EPA (2003) states that under the Draft Revised Guidelines for Carcinogen Risk Assessment (1999), data are inadequate for an assessment of the carcinogenic potential of xylenes. Although some occupational studies have suggested possible carcinogenic effects of xylene exposure, they were not considered adequate to determine the carcinogenicity of xylenes, due to their small sample size (including a small number of cases), uncertainty related to exposure level, multiple solvent exposure, and short latency period (US EPA 2003),
More recent studies looking at associations between occupational exposures and risk of lung cancer, prostate cancer, leukemia, lymphoma, or multiple myeloma did not provide additional information on the carcinogenic potential of xylene, as the exposures were to multiple solvents including benzene and toluene (Miligi et al. 2006; Costantini et al. 2008; Cocco et al. 2010; Blanc-Lapierre et al. 2018; DeRoos et al. 2018; Warden et al. 2018).
5.1.4 Genotoxicity
No increases in the frequency of sister chromatid exchanges or chromosomal aberrations were observed in peripheral lymphocytes in individuals exposed to xylenes by inhalation in an occupational or experimental setting (US EPA 2003; ATSDR 2007).
More recent studies were inconclusive regarding a possible association between xylene exposure and deoxyribonucleic acid (DNA) damage. In a study of pathology laboratory technicians, where xylene was the main solvent used, De Aquino et al. (2016) found a higher frequency of DNA strand breaks in blood cells (Comet assay) in technicians than in controls, as well as in the technicians’ blood at the end of the work week compared to the start of the week (Friday vs. Monday). There were no differences in micronucleus frequency in buccal cells. Bagryantseva et al. (2010) showed that garage workers had a higher frequency of DNA strand breaks in peripheral lymphocytes compared to controls; however, in addition to elevated xylene, workers were exposed to higher levels of other solvents including benzene and polycyclic aromatic hydrocarbons.
Mixed results were obtained in studies looking at xylene exposure and markers of oxidative stress and oxidative DNA damage. Moro et al. (2010) reported a correlation between urine MHA with MDA (a lipid peroxidation biomarker) and ALA-D (an oxidative stress biomarker) in occupationally exposed painters, and Yoon et al. (2010) reported an association between urine MHA with MDA and 8-OHdG (a marker of oxidative DNA damage) in non-occupationally exposed subjects. However, several other recent studies reported no associations between levels of urinary metabolites of xylene and oxidative stress biomarkers in occupationally and non-occupationally exposed subjects (Bagryantseva et al. 2010; Kim et al. 2011; Kwon et al. 2018).
Overall, the limited available evidence does not indicate that xylenes are genotoxic in humans.
5.2 Toxicological studies
5.2.1 Neurological effects
General neurotoxicity (movement, behaviour, and learning tests)
Several studies of repeated exposure (1–6 months) in rats identified an effect level of 100 ppm in tests of motor coordination, pain sensitivity, spontaneous movement, and learning. In addition, single exposure studies in rats and mice showed deficits in neurotoxicity tests following as little as 30 minutes of exposure to xylenes at concentrations of 1 400 ppm and up.
In the key study for the US EPA Integrated Risk Information System RfC (US EPA 2003), Korsak et al. (1994) exposed rats by inhalation to 0, 50 or 100 ppm m-xylene for 6 hours per day, 5 days per week, for 3 months. The rotarod test for motor coordination was conducted after 1, 2 and 3 months of exposure, and the hot plate test for pain sensitivity was conducted only at the end of the 3 months. Both tests were conducted 24 hours following the termination of the exposure period. In the rotarod test, the animals are placed on a rotating rod and their ability to remain on the rod for 2 minutes is evaluated. The rats exposed to m-xylene showed decreased performance at all 3 time points, with an 8% failure rate for the 50 ppm exposure group and a 33% failure rate for the 100 ppm group, compared to 0% for the controls (statistically significant for the 100 ppm group). The failure rates were the same after 1, 2 and 3 months of exposure. In the hot plate test, the time between when the animal is placed on a hot plate and when it licks its paws is measured. The rats exposed to m-xylene had a statistically significant increase in pain sensitivity (12.2, 8.6, and 8.7 second response time for controls, 50 ppm and 100 ppm, respectively). The US EPA (2003) determined that 100 ppm (434 mg/m3) is the LOAEL, and 50 ppm (217 mg/m3) is the NOAEL for the study, based on the rotarod test results. The hot plate test results were not considered as reliable, because the opposite effect (decreased pain sensitivity) was observed in another study (Gralewicz and Wiaderna 2001).
In a related study, Korsak et al. (1992) exposed rats to m-xylene at a concentration of 100 ppm for 6 months or 1 000 ppm for 3 months (6 hours per day, 5 days per week). The failure rate in the rotarod test was approximately 35% 24 hours after the 6-month 100 ppm exposure, and 60% 24 hours after the 3-month 1 000 ppm exposure (0% in the controls; both exposed groups were statistically different from the controls). Spontaneous movement also decreased significantly with exposure, from about 800 per hour in controls to 400 per hour, after 6 months of exposure to 100 ppm m-xylene. The results for the 3-month 1 000 ppm exposure are not shown, but the authors state that there was a decrease in all exposed groups. The LOAEL for this study is the lowest test concentration of 100 ppm (no NOAEL could be determined).
In a study to test learning, a radial maze test was conducted in control rats and rats exposed to 100 or 1 000 ppm m-xylene for 3 months. The test was done 70–83 days after the end of the exposure period. Unlike the controls, exposed rats (at both concentrations) did not exhibit a shortening of the time needed to complete a trial or a decrease in omission errors with successive daily trials. The authors suggest that these results indicate a learning deficit in the exposed rats (Gralewicz et al. 1995). In a follow-up study, Gralewicz and Wiaderna (2001) exposed rats to 100 ppm m-xylene for 4 weeks, and did not find a difference in radial maze performance 14–18 days after the end of exposure. However, they did observe a significantly shorter step-down time in exposed rats compared to control rats in a passive avoidance test conducted 39–48 days after cessation of exposure (in the last of 6 trials only), and a significant increase in latency to paw-lick in the hot plate test conducted 50–51 days post-exposure (in the last of 3 trials only). The authors suggest that these changes indicate a decreased ability to inhibit locomotor response in a fear-inducing environment. Both of these were supporting studies for the US EPA RfC derivation (US EPA 2003).
Armenta-Resendiz et al. (2019) exposed male rats to m-xylene at concentrations of 0, 500, 1 000, 2 000, 4 000 or 8 000 ppm for 30 minutes. After a 3-minute break, behavioural tests were done (defensive burying behaviour task, step-through avoidance learning task, hot plate test, shock threshold test, social interaction and rotarod test). Results for the 500 and 1 000 ppm groups were not shown. At 2 000 ppm, a clear and significant concentration-dependent decrease in latency was observed in the passive avoidance test. At 4 000 ppm and up, there was a significant increase in latency to paw-lick in the hot plate test. All of the remaining tests showed significant changes only at the highest test concentration (8 000 ppm).
Korsak et al. (1990, cited in US EPA 2003) exposed rats to a single isomer of xylene at approximately 3 000 ppm for 6 hours and reported that the number of rats that failed the rotarod test were 19/20, 6/20 and 1/20 for the o-, m-, and p- isomers, respectively. A 4-hour exposure to xylene in rats resulted in decreased activity at concentrations above 1 900 ppm (US EPA 2003). In a study in which mice were exposed to individual xylene isomers for 30 minutes, minimally effective concentrations of 1 400–3 000 ppm were determined for disruptions in two neurological tests (lever-pressing behaviour and inverted screen test) (US EPA 2003).
Neurological effects in developmental studies
Several developmental toxicity studies conducted in rats examined neurotoxicity endpoints.
In a study of prenatal exposure on neurological effects, Hass and Jacobsen (1993) exposed pregnant rats to 200 ppm technical grade xylene on gestational days (GD) 6–20. The exact composition of the test material was not given but the authors noted that technical grade xylene is a mixture of the three isomers and could include up to 35% ethylbenzene. No maternal toxicity was observed in the exposed dams. Statistically significant decreases in rotarod performance were observed in female pups on postnatal day (PND) 22 and PND 23 (but not PND 24), and in male pups on PND 23 (but not PND 22 or 24). However, the authors indicated that testers were not blinded to the exposure status of the animals, and the animals were not tested on the same day. No differences were observed in the time of development of the surface righting reflex, cliff avoidance reflex, or auditory startle reflex. No LOAEL or NOAEL for developmental toxicity was identified by the study authors or the US EPA; however, RIVM (2001) derived their tolerable concentration in air (TCA) based on a LOAEL of 200 ppm (868 mg/m3) from Hass and Jacobsen (1993), for “behavioral impairment of offspring.”
In a later study, Hass et al. (1995) exposed pregnant rats to 500 ppm mixed xylenes (19% o-xylene, 45% m-xylene, 20% p-xylene, and 15% ethylbenzene) on GD 7–20. No maternal toxicity was observed in the exposed dams, and there were no statistically significant differences in birth weight, pup weight, or organ weights, although mean birth weight per litter and mean brain weights at day 28 were slightly decreased. In the rotarod test (conducted at age 24–26 days), the percentage of animals unable to stay on the rod for the full 30 seconds was higher in exposed animals on all 3 test days, with the greatest difference for females on day 3. The mean time was also shorter for exposed animals in each trial. However, none of the differences were statistically significant. There was no difference between control and exposed pups in developmental milestones including surface righting reflex or auditory startle; however, the air righting reflex was delayed by 1 day in the exposed group. No differences were observed in an open field test of activity (at age 27–34 days). In the Morris water maze test of learning and memory (3 months of age), there was no difference in the time to find the platform hidden in the pool, between control pups and exposed pups that were housed in an “enriched” environment (i.e., with toys). However, in exposed pups that had been in standard housing, there was a trend (not statistically significant) towards increased time to find the platform during the learning phase of the test. In a subsequent test following relocation of the platform, the difference in time was significant for exposed females kept in standard housing relative to controls. The standard housed females of the exposed group also showed a significant increase in swimming duration but no difference in swimming speed compared to controls. Significant differences between exposed and control female offspring in the time to find the platform in the Morris water maze were observed at ages 16 and 28 weeks, but not at age 55 weeks (Hass et al. 1997). The authors suggested that this could be due to partial reversal of the effect or compensation due to practice. A LOAEL of 500 ppm (2 170 mg/m3) was identified for the water maze effects (Hass et al. 1995). The US EPA noted that the effect was minimally adverse and possibly reversible.
No effects on acoustic startle response or the figure-8 maze test were observed between control rat pups and those born to dams who had been exposed to 800 or 1 600 ppm p-xylene on gestation days 7–16 (Rosen et al. 1986).
5.2.2 Auditory effects
In studies on rats exposed to xylenes for 3 to 13 weeks, LOAELs of 250–1 800 ppm (1 085–7 812 mg/m3) were identified for hearing deficits, increased auditory response thresholds, or hair cell loss.
Gagnaire et al. (2001) exposed male rats to individual xylene isomers at 0, 450, 900 or 1 800 ppm for 13 weeks (6 hours/day, 5 days/week), and conducted audiometric tests and cochleograms during and after exposure. Significant reductions in brainstem auditory-evoked potential and number of cochlear hair cells were observed at every time point, including after an 8-week recovery period, in the group exposed to 1 800 ppm p-xylene. Moderate outer hair cell (OHC) loss was seen in the 900 ppm p-xylene group, with no impact on auditory thresholds. No effects were observed with o- or m-xylene at any concentration.
Gagnaire et al. (2007a) exposed male rats for 13 weeks to 0, 250, 500, 1 000 or 2 000 ppm mixed xylenes of two different compositions (6 hours per day, 6 days per week, 14 animals per group). Auditory thresholds were tested at the end of the 4th, 8th and 13th weeks of exposure and at the end of an 8-week recovery period. No shift in audiometric thresholds was observed in the controls or in the groups exposed to 250 and 500 ppm of both test mixtures. Threshold shifts in groups exposed to higher concentrations were observed and increased slightly throughout the exposure. No recovery was observed 8 weeks after the end of exposure. Following the 8-week recovery period, a histological examination was done to determine the amount and location of hair cell loss in the cochlea. For the mixed xylenes composed of 20% o-xylene, 20% p-xylene, 40% m-xylene, and 20% ethylbenzene, one animal in the 250 ppm group and one in the 500 ppm group had significant OHC losses in the organ of Corti. Animals in the 1 000 ppm group had significant OHC loss also, and the 2 000 ppm group had almost complete loss of OHCs and some loss of inner hair cells. The damage was less extensive in the animals exposed to mixed xylenes with more o-xylene (30%) and m-xylene (50%) and less p-xylene (10%) and ethylbenzene (10%). The LOAEL for the more sensitive endpoint (hair cell loss) is 250 ppm (no NOAEL), whereas the LOAEL and NOAEL for irreversible hearing loss are 1 000 and 500 ppm, respectively. As both test mixtures contained ethylbenzene, which also has ototoxic properties (Gagnaire and Langlais 2005), the effects cannot necessarily be attributed to xylenes; however, the authors note that the role of p-xylene in the mixtures should not be ruled out completely.
Maguin et al. (2006) also conducted otological tests in rats, 4 weeks following exposure to o-, p-, or m-xylene at 0 or 1 800 ppm for 3 weeks (6 hours per day, 5 days per week). A permanent shift in auditory threshold and severe OHC losses in the cochlea were observed in the group exposed to p-xylene, but not in controls or in animals exposed to o- or m-xylene. The LOAEL for p-xylene is 1 800 ppm (no NOAEL).
Hearing loss measured by both behavioural assessment of auditory thresholds and brainstem auditory-evoked response was observed in rats exposed to mixed xylenes (10% p-xylene, 80% m-xylene, 10% o-xylene) at 800 ppm for 6 weeks. A decrease in auditory sensitivity (brainstem auditory response) was noted in rats following 9 weeks of exposure to mixed xylenes at 1 000 ppm (US EPA 2003).
Wathier et al. (2019, 2016) examined the middle ear reflex (MER) and cochlear histology of anesthetized and tracheotomized rats exposed to 3 000 ppm xylene isomers twice for 15 minutes with a break of 20 minutes between exposures. There was a transient increase in the MER amplitude during and after exposure, which was most pronounced for p-xylene, and less pronounced but still significant for m-xylene. No difference was observed in the MER of animals exposed to o-xylene, or in the morphology of the cochlea for any isomer, and there was no correlation between MER change and brain xylene concentration.
5.2.3 Reproductive and developmental effects
Developmental toxicity studies including neurotoxicity test endpoints are described in section 5.2.1. A developmental LOAEL of 500 ppm (2 170 mg/m3) in rats was determined based on minimal and reversible decreases in performance in the Morris water maze (Hass et al. 1995, 1997).
In the study by Hass and Jacobsen (1993) (described in section 5.2.1), no maternal toxicity was observed in pregnant rats exposed to 200 ppm technical grade xylene on GD 6–20. Fetuses from exposed dams had an increased incidence of delayed ossification of os maxcillare in the skull, with 18 out of 26 exposed litters affected compared to 2 out of 22 control litters. The study authors also noted higher pup weight (significant in males at birth, and in both males and females at day 28 but not day 14) and significantly earlier eye opening and ear unfolding. No NOAEL or LOAEL was identified, in part due to study limitations (see section 5.2.1).
Additional reproductive and developmental toxicity studies are summarized in the following paragraphs. In several of these studies, rat maternal body weight decreases were observed at and above 500 ppm. In one study, severe maternal toxicity in rabbits was observed at 230 ppm but not at 115 ppm. No reproductive toxicity was observed at up to 500 ppm in a one-generation study in rats, and no male fertility effects were seen at concentrations of up to 1 000 ppm in another rat study. Fetal body weights were decreased in rats at concentrations of 350 ppm and above in several studies, and in some studies an increase in dead or resorbed fetuses or decreased litter size was observed at concentrations of 700 ppm and above.
In a one-generation study (Bio/dynamics 1983, as cited in US EPA 2003), no maternal or developmental toxicity was observed in rats exposed to up to 500 ppm technical grade xylene before mating, during gestation and during lactation (6 hours per day, 5 days per week). There were also no reproductive effects in the parental generation. A NOAEL of 500 ppm was identified for reproductive and developmental toxicity (US EPA 2003).
Nylén et al. 1989 exposed male rats to 0 or 1 000 ppm xylene (isomer/composition not stated) for 61 days (18 hours per day, 7 days per week). Two weeks or 10 weeks after the end of exposure, there was no change in the percentage of intact spermatozoa, the percentage of spermatozoa with normal heads and tails, or testis weight. There was also no change in ventral prostate weight, noradrenaline concentration in vas deferens or plasma testosterone 2 weeks after the end of exposure (these parameters were not examined at the 10-week point). In addition, 3 exposed rats were reported to have been fertile when tested 14 months after cessation of exposure. The US EPA (2003) reported a NOAEL of 1 000 ppm xylene for testicular and fertility effects in male rats from this study.
No effect on litter size, pup weight or growth rate were observed between control rat pups and those born to dams who had been exposed to 800 or 1 600 ppm p-xylene on gestation days 7–16 (Rosen et al. 1986). Maternal body weight gain was decreased at 1 600 ppm but not 800 ppm, compared to controls. US EPA considered 800 ppm a NOAEL for maternal toxicity (LOAEL of 1 600 ppm), and 1 600 ppm a NOAEL for developmental toxicity.
Ungváry et al. (1980) exposed pregnant rats to individual xylene isomers for 24 hours per day on gestation days 7–14. The US EPA (2003) identified maternal NOAELs of 350 ppm and LOAELs of 700 ppm for each isomer, based on body weight decrease. Developmental NOAELs of 350 ppm for m-xylene (decreased fetal weight at 700 ppm) and p-xylene (decreased fetal body weight, decreased litter size, and post-implantation loss at 700 ppm), and 35 ppm for o-xylene (decreased fetal body weight at 350 ppm) were also identified (US EPA 2003). The US EPA (2003) also identified NOAELs of 230 ppm for maternal and developmental toxicity in rats exposed to technical grade xylene for 24 hours per day on gestation days 9–14 (230 ppm was the only test concentration in the study) (Hudak and Ungváry 1978). Similarly, no maternal or developmental toxicity was observed at up to 400 ppm mixed xylenes in rats exposed for 6 hours per day on gestation days 6–15 (Litton 1978 as cited in US EPA 2003). However, in rats exposed to mixed xylenes for 24 hours per day on gestation days 7–14, a NOAEL of 440 ppm was identified based on an increase in dead or resorbed fetuses at 780 ppm (Ungváry and Tátrai 1985).
Only one study in rabbits was identified (Ungváry and Tátrai 1985). Pregnant rabbits were exposed to mixed xylenes or a single isomer on gestation days 7–20 (24 hours per day). At a concentration of 230 ppm, severe maternal toxicity and no live fetuses were observed, but at a concentration of 115 ppm, there was no indication of maternal or developmental toxicity (based on fetal survival, weight, malformations or variations). This study was also the only mouse developmental toxicity study identified: no increase in fetal malformations or variations was observed in mouse pups up to the highest tested concentrations for mixed xylenes (230 ppm) and for individual isomers (115 ppm).
According to the US EPA (2003), the studies by Ungváry (Hudak and Ungváry 1978; Ungváry et al. 1980; Ungváry and Tátrai 1985) are limited by a lack of detailed reporting. The US EPA commented that “interpretation of statistically significant findings for increased incidence of fetuses with retarded skeletal ossification is difficult, given the inability to adjust for possible litter size covariation and the relatively small magnitude of the increased incidences.” They also stated that Ungváry and Tátrai (1985) did not report maternal body weight in a way that allowed for determination of maternal NOAELs and LOAELs for rats and mice (US EPA 2003).
In a more recent developmental toxicity study on xylenes, Saillenfait et al. (2003) exposed pregnant rats to o-, m-, or p-xylene or technical grade xylene at 0, 100, 500, 1 000 or 2 000 ppm on gestation days 6–20 (6 hours per day). No significant changes were observed in the average numbers of implantations and live fetuses, in the incidences of non-live implants and resorptions, or in fetal sex ratio; and no malformations in pups were reported. There were, however, decreases in maternal body weight gain at 1 000 ppm, decreases in fetal body weights at 500 or 1 000 ppm, and some indications of delayed ossification or increased skeletal variations at 2 000 ppm. The LOAEL for maternal toxicity based on decreased corrected body weight gain was 1 000 ppm for all three isomers (NOAEL of 500 ppm). Fetal body weights were decreased at exposure concentrations of 500 ppm and up for o-xylene and technical grade xylene (NOAELs of 100 ppm); and at 1 000 ppm and up for p- and m-xylene (NOAELs of 500 ppm).
5.2.4 Other effects
The US EPA (2003) described several subchronic and chronic gavage studies in rats and mice in which complete histopathology was conducted. The only effects observed in a chronic study in rats were decreased body weight and decreased survival in high dose males. Effects in subchronic studies in rats were limited to decreased body weights as well as increased liver weight and enzyme changes without histopathology (indicating an adaptive response), and increased kidney weight and some nephropathy in one study. In mice, decreased body weight and transient signs of nervous system depression were observed in a subchronic study, and hyperactivity was the only effect observed in a chronic study. The US EPA (2003) concluded that the liver and kidneys are not sensitive target tissues in animals.
No effects on body weight, organ weights, hematology, clinical chemistry or histology were observed in rats or dogs exposed by inhalation to mixed xylenes at up to 810 ppm for 13 weeks (Carpenter et al. 1975). Korsak et al. (1994) also noted no changes in body or organ weights or in clinical biochemistry parameters in rats exposed to m-xylene at 50 or 100 ppm for 3 months. However, in some laboratory animal studies, general toxicity such as body weight decreases and adaptive liver changes have been observed following repeated exposure to xylene by inhalation. In acute inhalation studies, respiratory irritation (indicated by a decreased respiration rate) in mice and decreased lung microsomal enzyme activities in rats were observed. Lung inflammation was also observed in a 6-week rat study and in a study on sensitized mice. Changes in kidney enzymes have been observed in some inhalation repeat exposure studies. All of these effects occurred at test concentrations higher than those at which the most sensitive endpoint (neurotoxicity) occurred.
After a 6-week exposure to 3 500 ppm o-xylene, male rats exhibited statistically significantly decreased body weights and changes in the liver (including increased absolute and relative weights, hypertrophy, decreased glycogen, and increased peroxisomes); the authors stated that liver effects were adaptive rather than adverse (US EPA 2003). Decreased body weight gains were also reported in male rats exposed for 6 or 12 months to 1 090 ppm o-xylene; and reversible liver effects (increased relative weight, hypertrophy, and enzyme changes) were observed in rats exposed to mixed xylenes for 6 months at 920 ppm but not 350 ppm (US EPA 2003). It was not clear whether any parameters other than body weight and liver effects were examined in these studies. In a more recent study of effects of xylene on the liver, Kum et al. (2007a) reported a decrease in body weight and absolute liver weight in female rats exposed to 300 ppm technical grade xylene, from GD 1 for 3 weeks, or from PND 1 for 6 weeks; inconsistent changes in liver enzymes were noted. Hepatic P450 enzyme activities were increased in rats following a 6-hour exposure to 300 ppm m-xylene; activity returned to control values 5 days post-exposure (Foy and Schatz 2004).
In contrast to the liver, microsomal enzyme activities were decreased in the nasal mucosa and lungs of rats following acute exposure to xylene (Foy and Schatz 2004; ATSDR 2007). Acute exposure (1–6 minutes) to concentrations of over 1 300 ppm xylene produced a 50% decrease in respiratory rate in mice in several studies, indicating respiratory irritation (Carpenter et al. 1975; ATSDR 2007). A significant increase in the number of apoptotic cells was observed in the lungs of young (4-week-old) and adult (12-week-old) rats exposed to technical grade xylene for 6 weeks. The authors suggested that the increase was due to inflammation (Sandikci et al. 2009). Increased lung inflammation and increased airway hyperresponsiveness were also seen in mice that were challenged intranasally with xylene following sensitization by intraperitoneal injection (Hong et al. 2019).
Increased renal enzyme activity and cytochrome P450 content were observed in some repeat exposure studies (ATSDR 2007). In a study investigating kidney effects, statistically significant increases in serum urea, and kidney glutathione and malondialdehyde were seen in rats following exposure to 300 ppm technical grade xylene for 6 weeks (Kum et al. 2007b). No difference was seen in kidney superoxide dismutase, catalase, glutathione peroxidase, or serum total protein, albumin, or creatinine.
No effect on organ weights and no histopathological changes in the lungs or kidneys were observed in several repeat exposure studies of xylene inhalation (Carpenter et al. 1975; Korsak 1994; ATSDR 2007).
5.2.5 Carcinogenicity
No carcinogenicity studies in animals exposed by inhalation to xylenes are available. The US EPA (2003) noted that the available animal data are inconclusive as to the ability of xylenes to cause a carcinogenic response. Similarly, IARC (1999) stated that there is inadequate evidence in experimental animals for the carcinogenicity of xylenes. IARC (1999) reviewed several oral gavage studies and found that there was no increase in the incidence of tumours in either mice or rats following administration of a technical grade xylene. No more recent studies were identified.
5.2.6 Genotoxicity
Overall, xylenes are not considered genotoxic. Xylenes are not mutagenic in bacterial test systems or in cultured mammalian cells, and do not induce chromosomal aberrations or sister chromatid exchanges in cultured mammalian cells (US EPA 2003). In vivo, xylenes did not induce chromosomal aberrations or micronuclei in rodent bone marrow, nor did they induce sperm head abnormalities in rats; however, technical grade xylenes were found to be weakly mutagenic in Drosophila recessive lethal tests (US EPA 2003).
Some studies have shown DNA fragmentation in the skin of rats following dermal application of m-xylene; however, this was likely related to cell death (ATSDR 2007). One more recent study showed that DNA damage (single- and double-strand breaks) was induced by m-, o-, and p-xylene in cultured human lymphocytes, an effect that was mitigated when cells were treated with free radical scavengers, suggesting that the xylene-induced damage could be due to free radical generation (Chen et al. 2008).
5.3 Summary of health effects and mode of action
5.3.1 Summary of health effects
Single exposure studies in human volunteers and a case study of accidental high concentration exposure reported eye, nose and throat irritation, as well as some CNS symptoms including headaches, dizziness, and nausea. Mixed results were observed in tests of memory or reaction times following acute exposure of healthy volunteers. Occupational studies showed similar effects including irritation (eye irritation, sore throat) and CNS symptoms such as a floating sensation, headache and confusion, as well as effects on some neurological endpoints including tests of memory, colour vision, and the central auditory nervous system. These studies were limited by poorly defined exposure concentrations and durations and co-exposure to multiple chemicals, as well as by the timing of the symptom survey or testing, and in some cases, the subjective nature of self-reporting of symptoms.
Results from studies in laboratory animals clearly show that the most sensitive effect of inhaled xylene is neurological impairment. Repeated exposure of rats to concentrations of 100 ppm (434 mg/m3) and up (generally 6 hours per day, 5 days per week, for 1–6 months) caused deficits in tests of motor coordination, spontaneous movement, and learning. Single exposure studies in rats and mice also showed deficits in neurotoxicity tests following as little as 30 minutes of exposure at concentrations of 1 400 ppm (6 076 mg/m3) and up.
Developmental neurotoxicity was not consistently observed in studies of gestational exposure in rats. In one study, minimal and reversible decreases in performance in a test of memory and learning, slight non-significant decreases in mean birth weight and pup brain weight, and a non-significant decrease in the number of pups able to complete the rotarod test for motor coordination were observed, at the only test concentration of 500 ppm (2 170 mg/m3). In another study, decreased rotarod test performance was observed at some time points only, in rat pups from dams exposed to 200 ppm (868 mg/m3); this study had significant limitations.
In other studies of gestational exposure in rats, mice and rabbits, LOAELs were based on decreases in maternal and fetal body weights. Increases in dead or resorbed fetuses or decreased litter size were sometimes also observed at higher test concentrations. Fetal body weight decreases and some indications of delayed ossification or increased skeletal variations were generally seen only at concentrations above those that caused decreases in maternal weight gain. However, o-xylene resulted in decreased fetal body weight at a lower concentration than the concentration that caused maternal body weight decrease in two studies (fetal LOAELs of 350 ppm and 500 ppm). All these effects were seen only at concentrations above those at which neurobehavioural effects were seen in adult rats.
No reproductive toxicity was observed in a one-generation study in rats, and in another rat study no male fertility effects were seen up to the highest test concentrations. Studies of hearing loss showed hearing deficits, increased auditory response thresholds, and hair cell loss in rats 3 to 13 weeks old at a xylene concentration of 250 ppm (1 085 mg/m3) and above. Other effects in laboratory animals, such as body weight decreases, adaptive liver changes, respiratory irritation, and lung inflammation were seen in some studies, but only at concentrations above those at which neurobehavioral effects occurred. While there are insufficient data to determine whether xylenes could be carcinogenic, they are generally not considered mutagenic or genotoxic.
In general, there is no clear difference in toxicity among the three xylene isomers apart from the greater potency of p-xylene in rat ototoxicity, as described below. Most of the studies on neurotoxicity in rats were conducted using m-xylene; few studies were identified in which the three isomers were tested individually. In one, rats were exposed to each xylene isomer for 6 hours; the number of rats that failed the rotarod test for motor coordination was greatest for o-xylene and least for p-xylene. In another study, mice were exposed to a single isomer for 30 minutes. p-Xylene was the most potent isomer in the inverted screen test of motor performance and o-xylene was most potent in the lever pressing test of operant behaviour; the differences were small (US EPA 2003). Most developmental toxicity tests were done with mixed isomers, although in two studies, o-xylene had a lower LOAEL for decreased fetal body weight compared to m- and p-xylene. Overall, differences in potency are inconsistent and appear to be endpoint- and study-specific.
5.3.2 Mode of action
The irritant effects of xylene on the eyes, nose and throat, as well as the acute neurological effects, are thought to be due to its solubility in lipids which enables it to interact directly with tissue membranes, causing changes in membrane structure and geometry (de Groot 2017). In rats and guinea pigs, the concentration of p-xylene in the brain dropped immediately following cessation of exposure (Gagnaire et al. 2007b). In addition, the US EPA (2003) conducted PBPK modelling of the data from the studies by Korsak et al (1994) and showed that the blood concentration of xylene would be essentially zero at the time of neurotoxicity testing (24 hours post-exposure). This would account for the reversibility of the acute effects following cessation of exposure. However, prolonged or high concentration exposure may cause lasting central nervous system toxicity. This could be due to a disturbance of proteins involved in normal functioning of the neuronal membrane (US EPA 2003).
Effects of organic solvents on the nervous system appear to be due to interactions with specific targets such as neurotransmitter receptors (de Groot 2017). Some studies have shown changes in levels of various neurotransmitters and lipid composition in the brain following exposure to xylene (US EPA 2003). Ito et al. (2002) saw changes in t-butylbicyclophosphorothionate (TBPS) binding (ligand for Gamma-aminobutyric acid type A [GABAa] receptor) in rat cerebellum and proposed that xylene could increase GABA release and/or enhance receptor function. As the GABAa receptor is a possible target for xylene-induced nervous system toxicity, and as this receptor may behave differently during brain development, exposure to xylene during the period of brain growth could be critical (de Groot 2017).
Oxidative stress may also play a role in xylene toxicity. For example, Savolainen et al. (1979, cited in US EPA 2003) showed an increase in microsomal superoxide dismutase activity in the rat brain. However, studies on biomarkers of oxidative damage in humans have produced mixed results (Bagryantseva et al. 2010; Moro et al. 2010; Yoon et al. 2010; Kim et al. 2011; Kwon et al. 2018).
Some animal studies have shown that the irreversible xylene-induced hearing loss is related to hair cell loss in the cochlea (Gagnaire et al. 2001; Maguin et al. 2006; Gagnaire et al. 2007a). However, Wathier et al. (2019, 2016) observed a transient increase in the MER amplitude in the absence of morphological changes in the cochlea. As the three isomers are similar in terms of lipophilicity but have varying potency, auditory toxicity is likely not due to interaction with cell membranes, but may be due to direct interaction of xylene with neurons (ATSDR 2007). p-Xylene is the most potent isomer, suggesting that the presence of methyl groups at the m- or o- position provides steric hindrance, preventing effects from these isomers. In humans, based on the results of behavioural auditory processing and speech perception tests, as well as auditory brainstem response, Fuente et al. (2013) proposed that xylene affects the central auditory nervous system and that auditory toxicity is therefore at least partially related to neurotoxicity. Overall, the relationship between neurotoxicity and auditory toxicity is unclear.
5.4 Susceptible populations
Insufficient data are available to identify populations that may be more susceptible to the effects of xylene inhalation. There are multiple factors that can contribute to the differences in sensitivity to xylene between individuals, including age, body weight, sex, diet, and alcohol consumption, exercise, and disease states (MacDonald et al. 2002; ATSDR 2007). As described in section 4.1, polymorphisms of the primary xylene metabolizing enzyme (CYP2E1) have been identified; however, based on the limited available data and on modelling results, these polymorphisms do not seem to have an impact on variability in xylene metabolism (Ernstgård et al. 2003; Valcke and Krishnan 2011).
In general, children can receive a greater internal dose of many inhaled toxicants than adults at the same exposure concentrations. In rats, the developing fetus does not appear to be more sensitive to the effects of xylene than adults; however, studies in which newborn or young animals are exposed to xylene have not been conducted. Despite the lower metabolic enzyme activity in newborns and young children, modelling suggests there is no significant variability in the level of xylene metabolism in children compared to adults (Pelekis et al. 2001; Valcke and Krishnan 2011; Valcke and Haddad 2015).
6.0 Derivation of short- and long-term reference concentrations
6.1 Short-term reference concentration
The most sensitive adverse endpoints for acute exposure to xylene are mild neurological and irritative symptoms reported by human subjects in the study by Ernstgård et al. (2002). The LOAEL of 50 ppm (217 mg/m3) from this study is based on a slight increase in reporting of subjective symptoms during the 2-hour exposure period (headache, dizziness, feeling of intoxication, discomfort in throat and airways, and breathing difficulty) as well as a small but significant decrease in FVC in women 3 hours after exposure. The study did not identify a NOAEL as only one test concentration was used. Other studies in human volunteers only tested higher exposure concentrations, and also reported irritative and neurological effects. For example, irritation to the nose, throat, and eyes, as well as mild nausea, headache, reversible effects on balance and reaction times, and impaired performance on tests of memory and reaction times were reported following exposures to concentrations of 100 to 400 ppm (434–1 736 mg/m3) (Nelson et al. 1943; US EPA 2003). However, these effects were not observed in other studies at similar test concentrations (i.e., 100 to 400 ppm) (Carpenter 1975; Hastings et al. 1984 cited in NRC 2010 and CalEPA 1999; US EPA 2003).
The US EPA (2003) noted that “concentrations around 100–200 ppm (434–868 mg/m3) are close to the threshold level for short-term reversible neurological and irritation effects from xylenes.” The Ernstgård et al. (2002) study was not included in the US EPA assessment, and it was the only study identified that used a test concentration lower than 100 ppm (434 mg/m3). It was also the only study identified in which objective measures of respiratory system toxicity were examined in human volunteers. The authors reported only a minimal effect in one objective parameter, in one sex only, and at one time point only; the significance of this observation is unclear. The results of the subjective questionnaires in Ernstgård et al. (2002) are consistent with other studies using higher concentrations that showed some evidence of mild irritation and neurological symptoms induced by acute xylene exposure. However, none of the available studies (including Ernstgård et al. 2002) provide evidence of dose-response, and there is uncertainty around the exact concentrations at which these short-term effects occur. It should also be noted that the odour threshold for xylene is well below the test concentrations in all volunteer studies; therefore, it is likely that subjects were aware of the smell, which may have influenced their responses. Ernstgård et al. (2002) had a larger number of volunteers of both sexes compared to most older studies which only tested a small number of males; however, the volunteers were all of similar age and there was likely some selection bias as the authors described difficulty recruiting subjects who met their criteria.
In acute animal toxicity studies, the effects observed (respiratory irritation and neurotoxicity) qualitatively support the human studies, but these studies were conducted using higher test concentrations (i.e., LOELs greater than 1 000 ppm [4 340 mg/m3]). In repeat-dose animal studies, a NOAEL of 50 ppm (217 mg/m3) for neurotoxicity was identified based on effects at 100 ppm (434 mg/m3); lung inflammation was observed in one study at 300 ppm (1 300 mg/m3), but no other clinical or histopathological effects on the respiratory system were observed in several studies at concentrations ranging from 50 ppm to 810 ppm (217–3 515 mg/m3) (see section 5 for details).
Considering the available data, the study by Ernstgård et al. (2002) was selected as the key study because it was a recent study with a larger number of volunteers of both sexes and a lower test concentration than earlier human studies, objective endpoints were measured, and the effects observed are supported by other human studies and animal data. Therefore, the 2-hour LOAEL of 50 ppm (217 mg/m3) for mild respiratory irritation, neurological symptoms, and a slightly decreased FVC was selected as the critical point of departure for derivation of a short-term RfC.
No correction is needed for exposure duration (2 hours) in the key study because no time-response relationship was demonstrated (some symptoms were significant after 1 hour and average subjective ratings did not increase during the second hour of exposure). No interspecies uncertainty factor is needed as the study involved human volunteers. The default intraspecies uncertainty factor of 10 was applied, as all the women and men tested were healthy and of similar ages. An uncertainty factor of 3 rather than the default of 10 for the use of a LOAEL with minimal effects was applied. Although a slight difference in one objective measure (decreased FVC) was observed in women at a single time point, the significance of this observation is unclear.
Therefore, the short-term RfC is 7 200 µg/m3 (1 700 ppb).
In general, there is no clear difference in toxicity among the three xylene isomers; although the critical study was conducted using m-xylene, the p- and o-isomers are expected to behave similarly. Therefore, the RfC would apply to all three xylene isomers in any combination.
6.2 Long-term reference concentration
No human study was considered adequate as the basis for deriving a long-term RfC. Although some agencies selected the occupational study by Uchida et al. (1993) as the key study, it had numerous limitations including a lack of reporting on the duration of exposure, co-exposure to other chemicals, no clear demonstration of relationships between response and dose or duration, and the inherent bias presented by self-reporting of symptoms. More recent studies of occupational exposure, such as Lee et al. (2005, 2013) and Fuente et al. (2013), and general population studies such as Werder et al. (2019) and Norback et al. (2017), are similarly limited. However, these studies lend support to the evidence that xylene is a neurotoxicant.
The most sensitive effect observed in experimental animals was a statistically significant decrease in motor coordination in rats following 1 to 3 months of exposure to m-xylene at 100 ppm (434 mg/m3) (6 hours per day, 5 days per week) (Korsak et al. 1994). In this study, there was also a statistically significant increase in pain sensitivity at 50 ppm (217 mg/m3); however, this effect was not concentration-dependent, and an opposing effect was observed in another study at 100 ppm (Gralewicz and Wiaderna 2001). Therefore this endpoint is considered less reliable. Support for neurotoxicity as the critical endpoint comes from Korsak et al. (1992), who also observed a decrease in motor coordination in rats after 6 months of exposure to 100 ppm xylene; Gralewicz et al. (1995) who found learning deficits in rats exposed to 100 ppm xylene for 3 months; and Gralewicz and Wiaderna (2001) who found rats exposed to 100 ppm xylene for 4 weeks had decreased locomotor responses in a fear-inducing environment. As 100 ppm was the only or lowest test concentration in these studies, no NOAELs were determined. Although a lower NOAEL of 35 ppm (152 mg/m3) for o-xylene was identified in a rat developmental toxicity study (based on decreased fetal body weight at 350 ppm [1 520 mg/m3] Ungváry et al. 1980), it was not considered suitable as the basis for deriving the long-term RfC because of its higher LOAEL and significant study limitations (see section 5.2.3). Similarly, the higher NOAEL of 100 ppm for decreased fetal body weight at 500 ppm (Saillenfait et al. 2003) was not selected. Therefore, Korsak et al. (1994) was selected as the critical study. As insufficient data were available for benchmark concentration modelling, the study NOAEL of 50 ppm (217 mg/m3) was selected as the point of departure for deriving the long-term RfC.
The NOAEL of 50 ppm from Korsak et al. (1994) was adjusted from the animal study exposure (6 hours per day, 5 days per week) to continuous exposure (24 hours per day, 7 days per week), resulting in an adjusted NOAEL of 9 ppm (39 mg/m3).
The default uncertainty factor of 10 for intraspecies variation ([UFh], accounting for sensitivity in the human population) was applied, because no xylene-specific information was available on variation of pharmacokinetics or response in humans. In general, children have been shown to receive a dose of many inhaled toxicants that is 2 to 3 times greater than that of adults at the same exposure concentration (US EPA 2012); this variation is incorporated into the default UFh. Studies exploring potential age-related kinetic differences in xylenes (Pelekis et al. 2001) or the CYP2E1 metabolic pathway (Valcke and Krishnan 2011) indicate that the pharmacokinetic component of the default UFh is sufficient to account for differences between children and adults. Although in rats the developing fetus does not appear to be more sensitive to the effects of xylene than adults, no studies have been conducted in which newborn or young animals are exposed to xylene. In addition, multiple factors can contribute to the differences in sensitivity between individuals, including age, sex, diet, exercise, and disease states (US EPA 2003; Adams et al. 2005).
Similarly, in the absence of chemical-specific data, the default uncertainty factor of 2.5 for toxicodynamic differences between rats and humans was applied. In order to account for toxicokinetic differences between rats and humans, the blood/gas partition coefficients (Hb/g) were compared. This approach is considered appropriate for VOCs with systemic effects (US EPA 2012). The US EPA (2003) used an (Hb/g)H of 26.4 for m-xylene in humans, which was taken from Tardif et al. (1995, as cited in US EPA 2003), and an (Hb/g)A of 46.0 for m-xylene in rats, taken from the same group (Tardif et al. 1993, as cited in US EPA 2003), in order to derive a rat: human ratio of 1.7. In other studies that estimated the Hb/g in rats and humans, the rat value was consistently greater than the value in humans (Adams et al. 2005; ATSDR 2007; McNally et al. 2012). In cases where the (Hb/g)A:(Hb/g)H is greater than 1, the US EPA (2012) recommends using the more conservative default of 1. This approach is supported by analysis of PBPK models, which predict human exposure concentrations that are very similar to the duration-adjusted rat NOAEL (details in section 4.2). Accordingly, the default uncertainty factor of 1 for interspecies toxicokinetic differences was applied.
Finally, a database uncertainty factor of 10 was applied, which includes consideration of the use of a subchronic study as well as database deficiencies. Although the critical study was subchronic in duration, no increase in frequency or severity of effects from 1 month to 3 months was observed in the critical study (Korsak et al. 1994) or in a related 6-month study (Korsak et al. 1992). The animal toxicity database identifies neurotoxicity as the most sensitive effect; however, the few developmental neurotoxicity studies available have significant limitations. Although a LOAEL for mild, reversible effects was identified in one study (Hass et al. 1995), no NOAEL was identified as only one concentration was tested (500 ppm). Uncertainty therefore exists regarding the lowest dose (or potentially sensitive age) at which learning or memory impairment would be observed, and whether neuropathology is affected. No reproductive toxicity was observed in a one-generation study; no two-generation studies are available.
A detailed discussion and justification of default uncertainty factors for the long-term RfC can be found in Ritter et al. (2007).
The total uncertainty factor to be applied to the point of departure (duration adjusted NOAEL) is 250 (UFh = 10, UFa = 2.5, UFd = 10). Therefore, the long-term RfC is 150 µg/m3 (36 ppb).
In general, there is no clear difference in toxicity among the three xylene isomers; although the critical and supporting studies on neurotoxicity in rats were conducted using m-xylene, the p- and o-isomers are expected to behave similarly. Therefore, the RfC would apply to all three xylene isomers in any combination. The RfC is expected to be protective of potential developmental effects.
As support for this RfC based on the rat NOAEL, an RfC was also derived based on the LOAEL of 14 ppm (61 mg/m3) identified in the occupational study by Uchida et al. (1993). The LOAEL was adjusted from occupational exposure (8 hours per day, 5 days per week) to continuous exposure (24 hours per day, 7 days per week), and default uncertainty factors of 10 each for sensitive individuals and use of a LOAEL were applied, to give a potential RfC of 150 µg/m3.
6.3 Exposure in canadian homes in relation to reference concentration and determination of recommended exposure limits
In recent years, Health Canada has conducted exposure studies in multiple Canadian cities including Edmonton, Halifax, Windsor, Regina, Ottawa, Montreal, and a First Nations reserve in Manitoba (Swan Lake) (Health Canada 2010a, 2010b, 2012, 2013; Mallach et al. 2017; Goldberg et al. 2015; Weichenthal et al. 2013) (see section 3.2). These studies are considered to provide the most recent and most representative data available for quantifying long-term levels of exposure in Canadian homes. Other Canadian studies using different sampling and analysis methods support these data (National Research Council Canada 2021; INSPQ 2021; Health Canada 2021b; Li et al. 2019). Preliminary data suggest that xylene levels may be higher in recently built homes (Health Canada and National Research Council Canada 2019; Health Canada 2021b).
Short- and long-term RfCs are based on the characterization of the concentration-response relationship and the application of uncertainty factors to account for variability and data gaps. The context in which these RfCs are to be applied, technical feasibility, and the availability of risk mitigation measures are not considered as part of their determination. However, these issues are relevant to the determination of short- and long-term exposure limits.
In order to determine the recommended exposure limits, the short- and long-term RfCs are first compared to available exposure data from Canadian homes. The feasibility of achieving the RfC through the control of indoor sources is then evaluated. If the RfC is considered achievable, this value is set as the recommended exposure limit. If not, a higher concentration may be selected, while still targeting an exposure limit that is protective of health based on current evidence.
In the present assessment, the criteria guiding the determination of the value for both the recommended short- and long-term exposure limits for xylene are:
- a value that is potentially achievable in Canadian homes in the absence of significant sources of indoor xylene; and
- a value that is not associated with appreciable health effects, considering the derived reference exposure levels and currently available evidence.
6.3.1 Short-term reference concentration and recommended exposure limit
The literature database provided sufficient information on the effects in humans for the development of a short-term RfC, which was determined to be 7 200 µg/m3 for xylene. The range of median indoor air xylene concentrations measured in Canadian homes in the Health Canada residential indoor air exposure studies conducted in multiple cities was 2.1 to 11.1 µg/m3, with the 95th percentile ranging from 15.6 to 212.7 µg/m3 (see Table 2) (Health Canada 2021b). The 24-hour samples collected in these studies do not represent acute or peak exposures. Short-term xylene peaks may occur with behaviours such as smoking or the use of products such as caulking, coatings, or stains. Based on the 24-hour sampling data and the expected sources present, the short-term RfC should be achievable in Canadian homes. Therefore, the recommended short-term exposure limit for xylenes is 7 200 µg/m3, for all three isomers in any combination.
6.3.2 Long-term reference concentration and recommended exposure limit
From the literature database, a long-term RfC of 150 µg/m3 was derived based on decreased motor coordination. The Health Canada residential indoor air exposure studies conducted in several cities using a passivated canister sampling method provide the best measure of chronic exposure in Canadian homes. The median indoor air xylene concentrations measured in Canadian homes in these Health Canada residential indoor air exposure studies for a 24-hour averaging period ranged from 2.1 to 11.1 µg/m3, with the 95th percentile ranging from 15.6 to 212.7 µg/m3 (see Table 2) (Health Canada 2021b). Similar xylene concentrations were observed in other Canadian studies using different sampling and analysis methods (see section 3.2). The data indicate that there may be some Canadian homes in which the RfC is exceeded. However, the RfC was derived using the most recent scientific information and is in line with the Health Canada IARL of 100 µg/m3 and reference values from other jurisdictions (see Appendix D). Important sources of xylenes in Canadian homes include gas-powered items stored in the garage; building and renovation products such as caulking, coatings, and stains; and smoking. Reduction of xylene levels in the home through ventilation and source control is considered possible. Therefore, the recommended long-term exposure limit for xylenes is 150 µg/m3, for all three isomers in any combination.
7.0 Guidelines
7.1 Recommended exposure limits
| Exposure limit | Concentration | Critical effect(s) | |
|---|---|---|---|
| µg/m3 | ppb | ||
| Short-term (1 h) |
7 200 | 1 700 | Neurological symptoms (headache, fatigue); irritation of eyes, nose and throat; respiratory effects |
| Long-term (24 h) |
150 | 36 | Impaired motor coordination |
The recommended exposure limits apply to all three xylene isomers in any combination.
It is recommended that the short-term exposure limit be compared to a 1-hour air sample.
When comparing a measured xylene concentration with the long-term exposure limit, the sampling time should be at least 24 hours, under normal conditions. Moreover, averaging the results of repeated samples taken at different times of the year will provide a more representative estimate of long-term exposure.
7.2 Risk management recommendations
Some homes in Canada may have levels of xylene above the long-term reference exposure limit derived for protection against impaired motor coordination. Exposure to xylene in indoor air should be limited by ensuring adequate ventilation and controlling for indoor sources.
The presence of an attached garage and storage of cars, gas- operated tools, or gasoline in the garage are associated with higher levels of xylene in the home. Some building products can emit high levels of xylenes, and xylene levels in the home may increase during and after home renovations. Homes where there is regular smoking also tend to have higher levels of xylenes. Other consumer products including air fresheners may emit xylenes but the contribution of these products to indoor xylene concentrations is unknown. Increased ventilation and use of an exhaust fan in the garage have been shown to reduce indoor xylene concentrations.
Exposure to xylenes in indoor air can be reduced using the strategies outlined below. Furthermore, many of these measures will also contribute to reducing the concentrations of other indoor air contaminants, generally improving indoor air quality.
- Increase ventilation, especially when using renovation or building products such as caulking, coatings and stains:
- By opening windows when possible (check the outdoor air quality conditions in your region before opening windows: Air Quality Health Index).
- By employing mechanical ventilation strategies.
- For more information, refer to the Factsheet: Ventilation and the indoor environment (Health Canada 2018).
- If possible, do not store gasoline and other chemicals in the home or garage; if these products need to be stored, they should be well sealed.
- If you have an attached garage:
- Consider installing a garage exhaust fan.
- Make sure the interface between the attached garage and the home is properly sealed.
- Avoid idling your car, snowblower, lawnmower, or any gas-powered equipment in the garage.
- Do not smoke inside the home.
- Choose low-emission products when possible.
- Limit use of scented products and air fresheners.
8.0 References
Adams J.C., Dills R.L., Morgan M.S., Kalman D.A. and Pierce C.H. (2005) A physiologically based toxicokinetic model of inhalation exposure to xylenes in Caucasian men. Regulatory Toxicology and Pharmacology, 43(2): 203–214. http://dx.doi.org/10.1016/j.yrtph.2005.07.005
Alexopoulos E.C., Chatzis C. and Linos A. (2006) An analysis of factors that influence personal exposure to toluene and xylene in residents of Athens, Greece. BMC Public Health, 6: 50. http://dx.doi.org/10.1186/1471-2458-6-50
ANSES (2020) Valeurs toxicologiques de référence : Les xylènes. Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail. https://www.anses.fr/fr/system/files/VSR2018SA0152Ra.pdf
Armenta-Reséndiz M., Ríos-Leal E., Rivera-García M.T., López-Rubalcava C. and Cruz S.L. (2019) Structure-activity study of acute neurobehavioral effects of cyclohexane, benzene, m-xylene, and toluene in rats. Toxicology and Applied Pharmacology, 376: 38–45. http://dx.doi.org/10.1016/j.taap.2019.05.016
ATSDR (2007) Toxicological Profile for Xylene. Agency for Toxic Substances and Disease Registry, Public Health Service, US Department of Health and Human Services, Atlanta, GA. http://www.atsdr.cdc.gov/toxprofiles/tp71.pdf
Aylward L.L., Kirman C.R., Blount, B.C. and Hays, S.M. (2010) Chemical-specific screening criteria for interpretation of biomonitoring data for volatile organic compounds (VOCs)–Application of steady-state PBPK model solutions. Regulatory Toxicology and Pharmacology, 58(1): 33–44.10.1016/j.yrtph.2010.05.011
Aylward L.L., Kirman C.R., Schoeny R., Portier C.J. and Hays S.M. (2013) Evaluation of biomonitoring data from the CDC National Exposure Report in a risk assessment context: Perspectives across chemicals. Environmental Health Perspectives, 121(3): 287–294. http://dx.doi.org/10.1289/ehp.1205740
Bagryantseva Y., Novotna B., Rossner P., Chvatalova I., Milcova A., Svecova V., Lnenickova Z., Solansky I. and Sram R.J. (2010) Oxidative damage to biological macromolecules in Prague bus drivers and garagemen: Impact of air pollution and genetic polymorphisms. Toxicology Letters, 199(1): 60–68. http://dx.doi.org/10.1016/j.toxlet.2010.08.007
Bari M.A., Kindzierski W.B., Wheeler A.J. Héroux, M.-E. and Wallace L.A. (2015) Source apportionment of indoor and outdoor volatile organic compounds at homes in Edmonton, Canada. Building and Environment, 90: 114–124. 10.1016/j.buildenv.2015.03.023
Batterman S., Jia C. and Hatzivasilis G. (2007) Migration of volatile organic compounds from attached garages to residences: a major exposure source. Environmental Research, 104(2): 224–240.10.1016/j.envres.2007.01.008
Bio/dynamics Inc. (1983) Parental and fetal reproduction toxicity study in rats with mixed xylene. EPA/OTS public files. Bio/dynamics Inc., East Millstone, NJ; Document # FYI-AX-0983-0209. [cited in US EPA 2003]
Blanc-Lapierre A., Sauvé J.F. and Parent M.E. (2018) Occupational exposure to benzene, toluene, xylene and styrene and risk of prostate cancer in a population-based study. Occupational and Environmental Medicine, 75(8):562–572
Cakmak S., Kauri L.M., Andrade J. and Dales R. (2020) Factors influencing volatile organic compounds in Canadian homes. Indoor and Built Environment: 1420326X20926229.
CalEPA (1999) Appendix D.2 Acute RELs and toxicity summaries using the previous version of the Hot Spots Risk Assessment guidelines. California Environmental Protection Agency, Office of Environmental Health Hazard Assessment. Available at: https://oehha.ca.gov/media/downloads/crnr/appendixd2final.pdf
CalEPA (2000) Chronic Toxicity Summary: Xylenes. Appendix D.3 Determination of Noncancer Chronic Reference Exposure Levels. Office of Environmental Health Hazard Assessment, California Environmental Protection Agency, Sacramento, CA. http://www.oehha.ca.gov/air/hot_spots/2008/AppendixD3_final.pdf#page=602
CalEPA (2012) Evidence for the Developmental and Reproductive Toxicity of Xylene. California Environmental Protection Agency, Sacramento, CA.
Carpenter C.P., Kinkead E.R., Geary D.L. Jr., Sullivan L.J. and King J. M. (1975) Petroleum hydrocarbon toxicity studies. V. Animal and human response to vapors of mixed xylenes. Toxicology and Applied Pharmacology, 33(3): 543–558.
Charles S.M., Batterman S. and Jia C. (2007) Composition and emissions of VOCs in main-and side-stream smoke of research cigarettes. Atmospheric Environment, 41(26): 5371–5384.
Chen C.S., Hseu Y.C., Liang S.H., Kuo J. and Chen S.C. (2008) Assessment of genotoxicity of methyl-tert-butyl ether, benzene, toluene, ethylbenzene, and xylene to human lymphocytes using comet assay. Journal of Hazardous Materials, 153(1-2): 351–356.
Cheng M., Galbally I.E., Molloy S.B., Selleck P.W., Keywood M.D., Lawson S.J., Powell J.C., Gillett R.W. and Dunne E. (2016) Factors controlling volatile organic compounds in dwellings in Melbourne, Australia. Indoor Air, 26: 219–230.
Cho S., Damokosh A.I., Ryan L.M., Chen D., Hu Y.A. Smith T.J., Christiani D.C. and Xu X. (2001) Effects of exposure to organic solvents on menstrual cycle length. Journal of Occupational and Environmental Medicine, 43(6): 567–575.
Cocco P., t'Mannetje A., Fadda D., Melis M., Becker N., de Sanjosé S, Foretova L., Mareckova J., Staines A., Kleefeld S., Maynadié M., Nieters A., Brennan P. and Boffetta P. (2010) Occupational exposure to solvents and risk of lymphoma subtypes: Results from the Epilymph case-control study. Occupational and Environmental Medicine, 67(5): 341–347. http://dx.doi.org/10.1136/oem.2009.046839
Costantini A.S., Benvenuti A., Vineis P., Kriebel D., Tumino R., Ramazzotti V., Rodella S., Stagnaro E., Crosignani P., Amadori D., Mirabelli D., Sommani L., Belletti I., Troschel L., Romeo L., Miceli G., Tozzi G.A., Mendico I., Maltoni S.A. and Miligi L. (2008) Risk of leukemia and multiple myeloma associated with exposure to benzene and other organic solvents: Evidence from the Italian multicenter case-control study. American Journal of Industrial Medicine, 51(11): 803–811. http://dx.doi.org/10.1002/ajim.20592
De Aquino T., Zenkner F.F., Ellwanger J.H., Prá D. and Rieger A. (2016) DNA damage and cytotoxicity in pathology laboratory technicians exposed to organic solvents. Anais Da Academia Brasileira De Ciêencias, 88(1): 227–236. http://dx.doi.org/10.1590/0001-3765201620150194
de Groot, D.M.G. (2017). Scientific review on the link between the narcotic effects of solvents and (developmental) neurotoxicity. Final report. Prepared for ECHA. Contract number ECA.6677-E3. Netherlands.
De Roos A.J., Spinelli J., Brown E.B., Atanackovic D., Baris D., Bernstein L., Bhatti P., Camp N.J., Chiu B.C., Clavel J., Cozen W., de Sanjosé, S., Dosman J.A., Hofmann J.N., McLaughlin J.R., Miligi L., Monnereau A., Orsi L., Purdue M.P., Schinasi L.H., Tricot G.J., Wang S.S., Zhang Y., Birmann B.M. and Cocco P. (2018) Pooled study of occupational exposure to aromatic hydrocarbon solvents and risk of multiple myeloma. Occupational and Environmental Medicine, 75(11): 798–806. http://dx.doi.org/10.1136/oemed-2018-105154
Desrosiers T.A., Lawson C.C., Meyer R.E., Stewart P.A., Waters M.A., Correa A. and Olshan A.F. (2015) Assessed occupational exposure to chlorinated, aromatic and Stoddard solvents during pregnancy and risk of fetal growth restriction. Occupational and Environmental Medicine, 72(8): 587–593. http://dx.doi.org/10.1136/oemed-2015-102835
Dodson R.E., Udesky J.O., Colton M.D., McCauley M., Camann D.E., Yau A.Y., Adamkiewicz G. and Rudel R.A. (2017) Chemical exposures in recently renovated low-income housing: Influence of building materials and occupant activities. Environment International.109:114–127. https://doi.org/10.1016/j.envint.2017.07.007
Du Z., Mo J., Zhang Y. and Xu Q. (2014) Benzene, toluene and xylenes in newly renovated homes and associated health risk in Guangzhou, China. Building and Environment, 72: 75–81.
Elliott L., Longnecker M.P., Kissling G.E. and London S.J. (2006) Volatile organic compounds and pulmonary function in the Third National Health and Nutrition Examination Survey, 1988-1994. Environmental Health Perspectives, 114(8): 1210–1214.10.1289/ehp.9019
Environment and Climate Change Canada 2021. National Air Pollution Surveillance (NAPS) Program Data Portal. https://open.canada.ca/data/en/dataset/1b36a356-defd-4813-acea-47bc3abd859b [updated December 2020; accessed March 2021]
Environment Canada and Health Canada (1993) Canadian Environmental Protection Act. Priority Substances List Assessment Report: Xylenes. En40-215/22E, Minister of Supply and Services Canada, Ottawa, ON.
Ernstgård L., Gullstrand E., Lof A. and Johanson G. (2002) Are women more sensitive than men to 2-propanol and m-xylene vapours? Occupational and Environmental Medicine, 59(11): 759–767.10.1136/oem.59.11.759 [doi]
Ernstgård L., Sjögren B., Warholm M. and Johanson G. (2003) Sex differences in the toxicokinetics of inhaled solvent vapors in humans 1. m-Xylene. Toxicology and Applied Pharmacology, 193(2): 147–157.
European Commission (2005) Final Report – The INDEX project: Critical Appraisal of the Setting and Implementation of Indoor Exposure Limits in the EU. European Commission - Directorate-General Joint Research Centre - Institute for Health and Consumer Protection - Physical and Chemical Exposure Unit. Ispra, Italy. EUR 21590 EN
European Commission (2021). Final opinion on toxicological reference values for certain organic chemicals emitted from squishy toys with regard to adopting limit values under the Toy Safety Directive 2009/48/EC ‘Chemicals in squishy toys’. Scientific Committee on Health, Environmental and Emerging Risks (SCHEER).
Faure S., Noisel N., Werry K., Karthikeyan S., Aylward L.L. and St-Amand A. (2020) Evaluation of human biomonitoring data in a health risk based context: An updated analysis of population level data from the Canadian Health Measures Survey. International Journal of Hygiene and Environmental Health, 223(1): 267–280.
Foy J.W. D. and Schatz R.A. (2004) Inhibition of rat respiratory-tract cytochrome p-450 activity after acute low-level m-xylene inhalation: role in 1-nitronaphthalene toxicity. Inhalation Toxicology, 16(3): 125–132.
Fuente A., McPherson B. and Cardemil F. (2013) Xylene-induced auditory dysfunction in humans. Ear and Hearing, 34(5): 651–660. http://dx.doi.org/10.1097/AUD.0b013e31828d27d7
Fuente A., McPherson B. and Hood L.J. (2012) Hearing loss associated with xylene exposure in a laboratory worker. Journal of the American Academy of Audiology, 23(10): 824–830. http://dx.doi.org/10.3766/jaaa.23.10.7
Gagnaire F. and Langlais C. (2005) Relative ototoxicity of 21 aromatic solvents. Archives of Toxicology, 79(6): 346–354.
Gagnaire F., Langlais C., Grossmann S. and Wild P. (2007a) Ototoxicity in rats exposed to ethylbenzene and to two technical xylene vapours for 13 weeks. Archives of Toxicology, 81(2): 127–143. http://dx.doi.org/10.1007/s00204-006-0124-y
Gagnaire F., Marignac B., Blachere V., Grossmann S. and Langlais C. (2007b) The role of toxicokinetics in xylene-induced ototoxicity in the rat and guinea pig. Toxicology, 231(2-3): 147–158.
Gagnaire F., Marignac B., Langlais C. and Bonnet P. (2001) Ototoxicity in Rats Exposed to
Ortho‐, Meta‐and Para‐Xylene Vapours for 13 Weeks. Pharmacology & Toxicology, 89(1): 6–14.
Goldberg M., Wheeler A., Burnett R., Mayo N., Valois M., Brophy M. and Giannetti N. (2015) Physiological and perceived health effects from daily changes in air pollution and weather among persons with heart failure: A panel study. Journal of Exposure Science and Environmental Epidemiology, 25: 187–199. https://doi.org/10.1038/jes.2014.43
Gralewicz S. and Wiaderna D. (2001) Behavioral effects following subacute inhalation exposure to m-xylene or trimethylbenzene in the rat: a comparative study. Neurotoxicology, 22(1): 79–89.
Gralewicz S., Wiaderna D. and Tomas T. (1995) Development of spontaneous, age-related nonconvulsive seizure electrocortical activity and radial-maze learning after exposure to m-xylene in rats. International Journal of Occupational Medicine and Environmental Health, 8(4): 347–360.
Hass U. and Jakobsen B.M. (1993) Prenatal toxicity of xylene inhalation in the rat: a teratogenicity and postnatal study. Pharmacology & Toxicology, 73(1): 20–23.
Hass U., Lund S.P. and Simonsen L. (1997) Long-lasting neurobehavioral effects of prenatal exposure to xylene in rats. Neurotoxicology, 18(2): 547–551.
Hass U., Lund S.P., Simonsen L. and Fries A.S. (1995) Effects of prenatal exposure to xylene on postnatal development and behavior in rats. Neurotoxicology and Teratology, 17(3): 341–349.
Health Canada. (1996). Canadian Environmental Protection Act. Priority Substances List. Supporting Documentation: Health-Based Tolerable Daily Intakes/Concentrations and Tumourigenic Doses/Concentrations for Priority Substances.
Health Canada. (2010a) Regina Indoor Air Quality Study 2007: VOC Sampling Data Summary. Health Canada, Ottawa, Ontario K1A 0K9
Health Canada. (2010b) Windsor Ontario Exposure Assessment Study: VOC Sampling Data Summary (2005, 2006). Health Canada, Ottawa, Ontario K1A 0K9
Health Canada. (2012) Halifax Indoor Air Quality Study (2009): Volatile Organic Compounds (VOC) Data Summary. Health Canada, Ottawa, Ontario K1A 0K9
Health Canada. (2013b) Edmonton Exposure Assessment Study: VOC Sampling Data Summary (2010).
Health Canada (2014) Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Toluene, Ethylbenzene and the Xylenes. https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-toluene-ethylbenzene-xylenes.html
Health Canada (2018) Ventilation and the indoor environment. Government of Canada, Ottawa. Cat.: H144-54/1-2018E-PDF.
Health Canada (2019) Fifth Report on Human Biomonitoring of Environmental Chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 5 (2016-2017) Available from: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/environmental-contaminants/fifth-report-human-biomonitoring.html [Accessed March 2021]
Health Canada (2021a) Xylene source analysis from data in Canadian homes. Available on request.
Health Canada (2021b) Data on xylene concentrations in Canadian homes. Available on request.
Héroux M.-E., Gauvin D., Gilbert N.L., Guay M., Dupuis G., Legris M. and Levesque B. (2008) Housing characteristics and indoor concentrations of selected volatile organic compounds (VOCs) in Quebec City, Canada. Indoor and Built Environment, 17(2): 128–137. http://dx.doi.org/10.1177/1420326X07089005
Hong S.-G., Hwang Y.-H., Mun S.-K., Kim S.-J., Jang H.-Y., Kim H., Paik M.-J. and Yee S.-T. (2019) Role of Th2 cytokines on the onset of asthma induced by meta-xylene in mice. Environmental Toxicology. http://dx.doi.org/10.1002/tox.22814
Hudak A. and Ungváry G. (1978) Embryotoxic effects of benzene and its methyl derivatives: toluene, xylene. Toxicology, 11: 55–63.
IARC (1999) Re-evaluation of some Organic Chemicals, Hydrazine and Hydrogen Peroxide. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, 71: 1189.
INSPQ (2021) Amélioration de la qualité de l’air intérieur par l’optimisation de la ventilation dans des logements du Nunavik. Available at: https://www.inspq.qc.ca/sites/default/files/publications/2769-amelioration-qualite-air-interieur-logements-nunavik.pdf
Ito T., Yoshitome K., Horike T. and Kira S. (2002) Distribution of inhaled m-xylene in rat brain and its effect on GABAa receptor binding. Journal of Occupational Health, 44(2): 69–75.
Kim J.H., Moon J.Y., Park E.-Y., Lee K.-H. and Hong Y.-C. (2011) Changes in oxidative stress biomarker and gene expression levels in workers exposed to volatile organic compounds. Industrial Health, 49(1): 8–14. http://dx.doi.org/10.2486/indhealth.MS1112
Kirman C.R., Aylward L.L., Blount B.C., Pyatt D.W. and Hays S.M. (2012) Evaluation of NHANES biomonitoring data for volatile organic chemicals in blood: Application of chemical-specific screening criteria. Journal of Exposure Science & Environmental Epidemiology, 22(1): 24–34.
Korsak Z., Sokal J.A. and Gorny R. (1992) Toxic effects of combined exposure to toluene and m-xylene in animals. III. Subchronic inhalation study. International Journal of Occupational Medicine and Environmental Health, 5(1): 27–33.
Korsak Z., Sokal J.A., Wasiela T. and Swiercz R. (1990) Toxic effects of acute exposure to particular xylene isomers in animals. Polish Journal of Occupational Medicine, 3(2): 221–226.
Korsak Z., Wisniewska-Knypl J. and Swiercz R. (1994) Toxic effects of subchronic combined exposure to n-butyl alcohol and m-xylene in rats. International Journal of Occupational Medicine and Environmental Health, 7(2): 155–166.
Kum C., Kiral F., Sekkin S., Seyrek K. and Boyacioglu M. (2007a) Effects of xylene and formaldehyde inhalations on oxidative stress in adult and developing rats livers. Experimental Animals, 56(1): 35–42. http://dx.doi.org/10.1538/expanim.56.35
Kum C., Sekkin S., Kiral F. and Akar F. (2007b) Effects of xylene and formaldehyde inhalations on renal oxidative stress and some serum biochemical parameters in rats. Toxicology and Industrial Health, 23(2): 115–120. http://dx.doi.org/10.1177/0748233707078218
Kwon J.-W., Park H.-W., Kim W.J., Kim M.-G. and Lee S.-J. (2018) Exposure to volatile organic compounds and airway inflammation. Environmental Health, 17(1): 65. http://dx.doi.org/10.1186/s12940-018-0410-1
Lajoie P., Aubin D., Gingras V., Daigneault P., Ducharme F., Gauvin D., Fugler D., Leclerc J., Won D., Courteau M., Gingras S., Héroux M.E., Yang W. and Schleibinger, H. (2015) The IVAIRE project–a randomized controlled study of the impact of ventilation on indoor air quality and the respiratory symptoms of asthmatic children in single family homes. Indoor Air, 25(6): 582–597.
Lee C.R., Jeong K.S., Kim Y., Yoo C.I., Lee J.H. and Choi Y.H. (2005) Neurobehavioral changes of shipyard painters exposed to mixed organic solvents. Industrial Health, 43(2): 320–326. http://dx.doi.org/10.2486/indhealth.43.320
Lee E.-H., Paek D., Kho Y.L., Choi K. and Chae H.J. (2013) Color vision impairments among shipyard workers exposed to mixed organic solvents, especially xylene. Neurotoxicology and Teratology, 37: 39–43. http://dx.doi.org/10.1016/j.ntt.2013.02.005
Lehmann I., Thoelke A., Rehwagen M., Rolle‐Kampczyk U.E., Schlink U., Schulz R., Borte M., Diez U. and Herbarth O. (2002) The influence of maternal exposure to volatile organic compounds on the cytokine secretion profile of neonatal T cells. Environmental Toxicology, 17(3): 203–210.
Li Y., Cakmak S. and Zhu J. (2019) Profiles and monthly variations of selected volatile organic compounds in indoor air in Canadian homes: Results of Canadian national indoor air survey 2012–2013. Environment International, 126: 134–144.
Lim S.K., Shin H.S., Yoon K.S., Kwack S.J., Um Y.M., Hyeon J.H., Kwak H.M., Kim J.Y., Kim T.H., Kim Y.J. and Roh T.H., 2014. Risk assessment of volatile organic compounds benzene, toluene, ethylbenzene, and xylene (BTEX) in consumer products. Journal of Toxicology and Environmental Health, Part A. 77(22−24): 1502−1521.
Litton Bionetics. (1978a) Teratology study in rats - xylene. Final report EPA/OTS Public Files. Litton Bionetics, Kensington, MD. Document 878210350. [cited in US EPA 2003]
MacDonald A., Rostami-Hodjegan A., Tucker G. and Linkens D. (2002) Analysis of solvent central nervous system toxicity and ethanol interactions using a human population physiologically based kinetic and dynamic model. Regulatory Toxicology and Pharmacology, 35(2): 165–176.
MacNeill M., Dobbin N., St‐Jean M., Wallace L., Marro L., Shin T., You H., Kulka R., Allen R.W. and Wheeler A.J. (2016) Can changing the timing of outdoor air intake reduce indoor concentrations of traffic‐related pollutants in schools? Indoor Air, 26(5): 687–701.
Maguin K., Lataye R., Campo P., Cossec B., Burgart M. and Waniusiow D. (2006) Ototoxicity of the three xylene isomers in the rat. Neurotoxicology and Teratology, 28(6): 648–656. http://dx.doi.org/10.1016/j.ntt.2006.08.007
Mallach G., St-Jean M., MacNeill M., Aubin D., Wallace L., Shin T., Van R.K., Kulka R., You H., Fugler D., Lavigne E. and Wheeler A.J. (2017) Exhaust ventilation in attached garages improves residential indoor air quality. Indoor Air, 27(2): 487–499. http://dx.doi.org/10.1111/ina.12321
Marchand A., Aranda-Rodriguez R., Tardif R., Nong A. and Haddad S. (2016) Evaluation and modeling of the impact of coexposures to VOC mixtures on urinary biomarkers. Inhalation Toxicology, 28(6): 260–273. http://dx.doi.org/10.3109/08958378.2016.1162232
Miligi L., Costantini A.S., Benvenuti A., Kriebel D., Bolejack V., Tumino R., Ramazzotti V., Rodella S., Stagnaro E., Crosignani P., Amadori D., Mirabelli D., Sommani L., Belletti I., Troschel L., Romeo L., Miceli G., Tozzi G.A., Mendico I. and Vineis P. (2006) Occupational exposure to solvents and the risk of lymphomas. Epidemiology, 17(5): 552–561. http://dx.doi.org/10.1097/01.ede.0000231279.30988.4d
Moro A.M., Charao M., Brucker N., Bulcao R., Freitas F., Guerreiro G., Baierle M., Nascimento S., Waechter F., Hirakata V., Linden R., Thiesen F.V. and Garcia S.C. (2010) Effects of low-level exposure to xenobiotics present in paints on oxidative stress in workers. Science of the Total Environment, 408(20): 4461–4467. http://dx.doi.org/10.1016/j.scitotenv.2010.06.058
National Research Council Canada (2021) Data on xylene concentrations in Canadian homes. Available on request.
Nelson K.F., Ege J.F. Jr., Ross M., Woodman L.E. and Silverman L. (1943) Sensory Response to Certain Industrial Solvent Vapors. Journal of Industrial Hygiene and Toxicology, 25(7): 282–285.
Niaz K., Bahadar H., Maqbool F. and Abdollahi M. (2015) A review of environmental and occupational exposure to xylene and its health concerns. EXCLI Journal, 14: 1167–1186. http://dx.doi.org/10.17179/excli2015-623
[NLM] National Library of Medicine (US) (2020). ChemIDPlus (Internet database). Available at: https://chem.nlm.nih.gov/chemidplus/name/Xylene [accessed September 2020].
Norback D., Hashim J.H., Hashim Z. and Ali F. (2017) Volatile organic compounds (VOC), formaldehyde and nitrogen dioxide (NO2) in schools in Johor Bahru, Malaysia: Associations with rhinitis, ocular, throat and dermal symptoms, headache and fatigue. Science of the Total Environment, 592: 153–160. http://dx.doi.org/10.1016/j.scitotenv.2017.02.215
Nylén P., Ebendal T., Eriksdotter-Nilsson M., Hansson T., Henschen A., Johnson A., Kronevi T., Kvist U., Sjöstrand N.O., Höglund G. and Olson L. (1989) Testicular atrophy and loss of nerve growth factor-immunoreactive germ cell line in rats exposed to n-hexane and a protective effect of simultaneous exposure to toluene or xylene. Archives of Toxicology, 63(4): 296–307.
Pelekis M., Gephart L. and Lerman S. (2001) Physiological-model-based derivation of the adult and child pharmacokinetic intraspecies uncertainty factors for volatile organic compounds. Regulatory Toxicology and Pharmacology, 33(1): 12–20.
Ritter L., Totman C., Krishnan K., Carrier R., Vézina A. and Morisset V. (2007) Deriving uncertainty factors for threshold chemical contaminants in drinking water. Journal of Toxicology and Environmental Health, Part B, 10(7): 527–557.
RIVM (2001) Rijksinstituut Voor Volksgezondheid En Milieu National Institute of Public Health and the Environment. RIVM Report 711701025. Baars, AJ et al. 2001. Re-evaluation of human-toxicological maximum permissible risk levels. Bilthoven, Netherlands.
Rosen M.B., Crofton K.M. and Chernoff N. (1986) Postnatal evaluation of prenatal exposure to p-xylene in the rat. Toxicology Letters, 34(2-3): 223–229.
Saillenfait A., Gallissot F., Morel G. and Bonnet P. (2003) Developmental toxicities of ethylbenzene, ortho-, meta-, para-xylene and technical xylene in rats following inhalation exposure. Food and Chemical Toxicology, 41(3): 415–429.
Sandikci M., Seyrek K., Aksit H. and Kose H. (2009) Inhalation of formaldehyde and xylene induces apoptotic cell death in the lung tissue. Toxicology and Industrial Health, 25(7): 455–461. http://dx.doi.org/10.1177/0748233709106824
Savolainen K. and Linnavuo M. (1979) Effects of m‐xylene on human equilibrium measured with a quantitative method. Acta Pharmacologica et Toxicologica, 44(4): 315–318. [cited in US EPA 2003]
Shrubsole C., Dimitroulopoulou S., Foxall K., Gadeberg B. and Doutsi A. (2019) IAQ guidelines for selected volatile organic compounds (VOCs) in the UK. Building and Environment, 165: 106382.
Steinemann A. (2017) Ten questions concerning air fresheners and indoor built environments. Building and Environment, 111: 279–284.
Stocco C., MacNeill M., Wang D., Xu X., Guay M., Brook J. and Wheeler A.J. (2008) Predicting personal exposure of Windsor, Ontario residents to volatile organic compounds using indoor measurements and survey data. Atmospheric Environment, 42(23): 5905–5912.10.1016/j.atmosenv.2008.03.024
Tardif R., Laparé S., Charest‐Tardif G., Brodeur J. and Krishnan K. (1995) Physiologically‐based pharmacokinetic modeling of a mixture of toluene and xylene in humans. Risk Analysis, 15(3): 335–342. [cited in US EPA 2003]
Tardif, R., Laparé S., Krishnan K. and Brodeur J. (1993) Physiologically based modeling of the toxicokinetic interaction between toluene and m-xylene in the rat. Toxicology and Applied Pharmacology, 120(2): 266–273. [cited in US EPA 2003]
Uchida Y., Nakatsuka H., Ukai H., Watanabe T., Liu Y., Huang M., Wang Y., Zhu F., Yin H. and Ikeda M. (1993) Symptoms and signs in workers exposed predominantly to xylenes. International Archives of Occupational and Environmental Health, 64(8): 597–605.
Ungváry G. and Tátrai E. (1985) On the embryotoxic effects of benzene and its alkyl derivatives in mice, rats and rabbits. In: Chambers P.L., Cholnoky E., Chambers C.M. (eds) Receptors and Other Targets for Toxic Substances. Archives of Toxicology (Supplement), vol 8. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-69928-3_95.
Ungváry G., Tátrai E., Hudák A., Barcza G. and Lõrincz M. (1980) Studies on the embryotoxic effects of ortho-, meta-and para-xylene. Toxicology, 18(1): 61–74.
US CDC (2017) Centers for Disease Control and Prevention. National Biomonitoring Program: Biomonitoring Summary – Xylenes. Available at: https://www.cdc.gov/biomonitoring/Xylenes_BiomonitoringSummary.html [accessed March 2021].
US EPA (2003) Toxicological Review of Xylenes (CAS No. 1330-20-7). In Support of Summary Information on the Integrated Risk Information System (IRIS). EPA/635/R-03/001, United States Environmental Protection Agency, Washington, D.C. http://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0270tr.pdf
US EPA (2012). Advances in Inhalation Gas Dosimetry for Derivation of a Reference Concentration (RfC) and Use in Risk Assessment. EPA/600/R-12/044. United States Environmental Protection Agency, Washington, D.C.
Valcke M. and Haddad S. (2015) Assessing Human Variability in Kinetics for Exposures to Multiple Environmental Chemicals: A Physiologically Based Pharmacokinetic Modeling Case Study with Dichloromethane, Benzene, Toluene, Ethylbenzene, and m-Xylene. Journal of Toxicology and Environmental Health - Part A: Current Issues, 78(7): 409–431. http://dx.doi.org/10.1080/15287394.2014.971477
Valcke M. and Krishnan K. (2011) An assessment of the impact of physico-chemical and biochemical characteristics on the human kinetic adjustment factor for systemic toxicants. Toxicology, 286(1-3): 36–47.
VCCEP (2005) Xylenes Category: m-xylene (CAS No. 108-38-3), o-xylene (CAS No. 95-47-6), p-xylene (CAS No. 106-42-3), mixed xylenes (CAS No. 1330-20-7). Voluntary Children’s Chemical Evaluation Program (VCCEP) Tier 1 Pilot Submission. Docket Number OPPTS - 00274D. American Chemistry Council. Benzene, Toluene and Xylenes VCCEP Consortium. http://www.tera.org/peer/VCCEP/xylenes/Xylenes%20VCCEP%20Submission%2010-6-05.pdf
Wang T., Du, H., Ma J., Shen L., Wei M., Zhao X., Chen L., Li M., Li G. and Xing Q. (2020) Functional characterization of the chlorzoxazone 6-hydroxylation activity of human cytochrome P450 2E1 allelic variants in Han Chinese. PeerJ, 8: e9628.
Warden H., Richardson H., Richardson L., Siemiatycki J. and Ho V. (2018) Associations between occupational exposure to benzene, toluene and xylene and risk of lung cancer in Montreal. Occupational and Environmental Medicine, 75(10): 696–702. http://dx.doi.org/10.1136/oemed-2017-104987
Wathier L., Venet T., Bonfanti E., Nunge H., Cosnier F., Parietti-Winkler C., Campo P. and Pouyatos B. (2019) Measuring the middle-ear reflex: A quantitative method to assess effects of industrial solvents on central auditory pathways. Neurotoxicology, 74: 58–66. http://dx.doi.org/10.1016/j.neuro.2019.05.007
Wathier L., Venet T., Thomas A., Nunge H., Bonfanti E., Cosnier F., Parietti-Winkler C., Campo P., Tsan P., Bouguet-Bonnet S. and Gansmüller A. (2016) Membrane fluidity does not explain how solvents act on the middle-ear reflex. Neurotoxicology, 57: 13–21. http://dx.doi.org/10.1016/j.neuro.2016.08.001
Weichenthal S., Mallach, G., Kulka, R., Black A., Wheeler A., You H., St-Jean M., Kwiatkowski R. and Sharp D. (2013) A randomized double-blind crossover study of indoor air filtration and acute changes in cardiorespiratory health in a First Nations community. Indoor Air, 23: 175–184. https://doi.org/10.1111/ina.12019
Werder E.J., Engel L.S., Blair A., Kwok R.K., McGrath J.A. and Sandler D.P. (2019) Blood BTEX levels and neurologic symptoms in Gulf states residents. Environmental Research, 175: 100–107. http://dx.doi.org/10.1016/j.envres.2019.05.004
Wheeler A.J., Wong S.L., Khouri C. and Zhu J. (2013) Predictors of indoor BTEX concentrations in Canadian residences. Health Reports, 24(5): 11–17.
Won D., Nong G., Yang W. and Collins P. (2014). Material emissions testing: VOCs from wood, paint, and insulation materials. National Research Council, Ottawa, ON.
Won D., Nong G., Yang W. and Lusztyk E. (2015). VOC emissions from evaporative sources in residential garages. National Research Council, Ottawa, ON.
Won D., Nong G., Yang W. and Schleibinger H. (2013). Material emissions data: 52 building materials tested for 124 compounds. National Research Council, Ottawa, ON.
Yoon H.I., Hong Y.-C., Cho S.-H., Kim H., Kim Y.H., Sohn J.R., Kwon M., Park S.-H., Cho M.-H. and Cheong H.-K. (2010) Exposure to volatile organic compounds and loss of pulmonary function in the elderly. European Respiratory Journal, 36(6): 1270–1276. http://dx.doi.org/10.1183/09031936.00153509
Zhu J., Newhook R., Marro L. and Chan C.C. (2005). Selected volatile organic compounds in residential air in the city of Ottawa, Canada. Environmental Science and Technology, 39: 3964–3971. https://doi.org/10.1021/es050173u
Appendix A: List of acronyms and abbreviations
- AEGL
- Acute exposure guideline limit
- ANSES
- Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (France)
- ATSDR
- Agency for Toxic Substances and Disease Registry
- BE
- Biomonitoring equivalent
- BTEX
- Benzene, toluene, ethylbenzene, and xylenes
- CalEPA
- California Environmental Protection Agency
- CDC
- Centers for Disease Control and Prevention
- CHMS
- Canadian Health Measures Survey
- CNS
- Central nervous system
- DNA
- Deoxyribonucleic acid
- EEG
- Electroencephalogram
- FT
- Finger tapping speed test
- FVC
- Forced vital capacity
- G/I
- Garage to indoor
- GD
- Gestational day
- GM
- Geometric mean
- GSH
- Glutathione
- HEC
- Human equivalent concentration
- I/O
- Indoor/outdoor
- IARC
- International Agency for Research on Cancer
- IARL
- Indoor air reference level
- INSPQ
- Institut national de santé publique du Québec
- LOAEL
- Lowest observed adverse effect level
- MER
- Middle ear reflex
- MDA
- Malondialdehyde
- MHA
- Methylhippuric acid
- MRL
- Minimal risk level
- NAPS
- National Air Pollution Surveillance
- NHANES
- National Health and Nutrition Examination Survey
- NLM
- National Library of Medicine
- NOAEL
- No observed adverse effects level
- NPRI
- National Pollutant Release Inventory
- NRC
- National Research Council
- OHC
- Outer hair cell
- PBPK
- Physiologically based pharmacokinetic
- PND
- Postnatal day
- Ppb
- Parts per billion
- Ppm
- Parts per million
- RfC
- Reference concentration
- RIAQG
- Residential Indoor Air Quality Guidelines
- RIVM
- National Institute for Public Health and the Environment (the Netherlands)
- SD
- Symbol digit substitution test
- TCA
- Tolerable concentration in air
- TWA
- Time weighted average
- UF
- Uncertainty factor
- US
- United States
- US EPA
- United States Environmental Protection Agency
- VCCEP
- Voluntary Children’s Chemical Evaluation Program
- VOC
- Volatile organic compound
Appendix B: Human exposure studies
| Study | Participants | Exposure | Results | NOAEL/LOAEL |
|---|---|---|---|---|
| Ernstgård et al. 2002 | 56 healthy volunteers (28 men, 28 women) | 50 ppm m-xylene for 2 hours (or clean air, or 150 ppm isopropanol -each subject experienced the 3 exposures with an interval of 2 weeks in between) | Compared to clean air: An increase in subjective symptoms (headache, dizziness, feeling of intoxication) during xylene exposure Slight respiratory effects (reduced FVC in women 3 hours after (but not immediately after) exposure ended, increased discomfort in throat and airways in women during exposure (self-reported), and breathing difficulty during exposure in both sexes (also self-reported) |
LOAEL 50 ppm (no NOAEL) ATSDR used this as the POD for its MRL |
| Carpenter et al. 1975 | 6 volunteers | 0, 110, 230, 460, 690 ppm mixed xylenes for 15 minutes | At 230 ppm, 1 of the 6 volunteers felt dizzy during the last 2 minutes of the 15 minute exposure; none reported dizziness at 110 ppm. One volunteer reported mild throat discomfort in the first minute of exposure to 110 and 230 ppm. Eye and nasal irritation reported at 230 ppm and up (transient) |
Not determined |
| Nelson et al. 1943 | 10 volunteers (sex not stated) | mixed xylene for 3–5 minutes (concentrations not specified) | Eye, nose, and throat irritation for the majority of volunteers at 200 ppm. Estimated that 100 ppm would be the highest concentration to which the majority of subjects could be exposed for 8 hours without irritation. | Not determined |
| Hastings et al. 1984 (cited in NRC 2010; CalEPA 1999) | 50 healthy male volunteers | mixed xylene for 30 minutes; 0, 100, 200, 400 ppm | No reports of nose and throat irritation. The percent of subjects reporting eye irritation was 56 for controls (clean air), 60 at 100 ppm, 70 at 200 ppm, and 90 at 400 ppm. | CalEPA (1999) considered 100 ppm a NOAEL. NRC (2010) considered 400 ppm a LOAEL. |
| Study | Participants | Exposure | Results | NOAEL/LOAEL |
|---|---|---|---|---|
| Uchida et al. 1993 | 175 xylene-exposed workers (107 men, 68 women) for whom the sum of the three xylene isomers accounted for 70% or more of the total solvent exposure. Controls were 241 non-exposed workers from the same factories or from factories in the same region. |
A single day personal exposure measurement; exposure was assumed to be the same for the entire 7-year study period. The mean xylene concentration (TWA) was 14 ppm (61 mg/m3). | A health survey was done the day after exposure was measured. The prevalence of subjective symptoms during the work shift and in the previous 3 month period was significantly higher in exposed workers when compared with that of non-exposed workers. When the exposed individuals were subdivided according to xylene concentration (1–20 ppm or >21 ppm), eye irritation, sore throat, and a floating sensation followed a concentration related increase for symptoms reported during the work shift, whereas poor appetite was the only concentration-dependent symptom reported for the previous 3 months. | ATSDR (2007), CalEPA (2000), and the EU (2005) considered the GM TWA concentration of 14 ppm (61 mg/m3) to be a LOAEL, and used it as the basis for their exposure limits. |
| Lee et al. 2005 | 180 shipyard painters exposed to mixed organic solvents; controls were 60 reference workers without organic solvent exposure | Air sampling of cumulative exposure for 61 of 180 painters, 3 different days each. Xylene had the highest levels. Cumulative exposure index calculated (multiple chemicals). Xylene concentration: the geometric mean (geometric standard deviation) was 22.70 ppm (4.10 ppm); range 1.6–591.2 ppm |
"Exposed" group (painters) had significant difference in SD (symbol digit substitution, tests incidental memory) and FT (finger tapping speed) of dominant hand, and FT of non-dominant hand. (confounding factors age and education considered). No difference in any test observed when actual exposure index was used for comparison (duration of work x total solvent exposure) but Increased duration of exposure (based on survey, <10 yr vs. >20 yr) showed difference in SD (p<0.05) and FT of dominant hand (p=0.052). Simple reaction time was not impacted in any of the analyses. | Not determined |
| Lee et al. 2013 | 63 shipyard painters exposed to organic solvents, especially xylene Controls: 122 non-exposed office workers in the same industry and 185 subjects from the general population |
Xylene had the highest concentration in air (mean concentration 10 ppm = 46 mg/m3) The urine metabolite MHA was also measured in exposed workers and controls. |
MHA concentration in urine was significantly correlated with results on the colour confusion index test. Corrected for age and years of education but not for other solvents. | Not determined |
| Fuente et al. 2013 | 30 medical laboratory workers | Xylene is expected to be the primary exposure due to its use as a tissue sample processing agent. Mean xylene concentration was 36.5 mg/m3, range 8–217 mg/m3 (measured on a single day) Urine MHA was measured after the last work day of the week. Exposure duration was estimated based on workers’ description of their work history. The mean duration of exposure was 11.8 years (range 2–29 years) |
The xylene-exposed participants showed significantly worse results in some tests including pure-tone thresholds, pitch pattern sequence test, dichotic digit test, speech recognition in noise test, and auditory brainstem response. An estimate of cumulative dose was also done using the reported exposure duration and the urine MHA concentration; participants with higher cumulative dose had poorer test results than those with low cumulative dose. The study authors suggest that xylene is associated with adverse effects on the central auditory nervous system and sound detection abilities in humans, and proposed that the effect of xylene on the auditory system at least partially relates to neurotoxicity at the brainstem level. | Not determined |
| Fuente et al. 2012 | 1 histology laboratory worker (case study) | Estimated exposure duration 20 years. mean xylene airborne concentration was 60 mg/m3 |
Hearing loss was proposed to be due to long-term occupational exposure to xylene. | Not determined |
| Werder et al. 2019 | 690 non-occupationally exposed adults (USA) | Xylene and other chemicals were measured in blood. Blood xylene concentrations were 0.06, 0.13, and 0.25 ng/mL at the 25th, 50th, and 75th percentiles, respectively. | A survey was conducted on the frequency of neurological symptoms during the 30 days prior. For xylene, most associations were confounded by co-exposures. However, in models restricted to non-smokers and adjusted for confounders (including blood concentrations of benzene, toluene, and ethylbenzene), there was an association between multiple CNS symptoms and the second, third, and fourth quartiles of blood xylene concentration, as well as an exposure-response trend. CNS symptoms surveyed were dizziness, headache, nausea, sweating, and palpitations. | Not determined |
| Norback et al. 2017 | 462 students at 8 schools (Malaysia) | Median xylene concentration in air for 1 week was 78 µg/m3 | Higher xylene levels were associated with self-reported fatigue in the past 3 months (no adjustment for co-exposure to other contaminants.) No association was observed between xylene concentration and reported headache or mucosal symptoms. | Not determined |
| Cho et al. 2001 | 1 408 petrochemical workers. 284 women exposed to xylene and other solvent(s). 968 controls were workers unexposed to any solvent at the same company. |
Workers were exposed to multiple solvents; none were exposed to xylene alone | Compared to unexposed group, the exposed group had increased prevalence of oligomenorrhea (menstrual cycles exceeding 35 days, determined by questionnaire) | Not determined |
| Desrosiers et al. 2015 | A population-based sample of 2 861 mother-infant pairs from the US | Occupational exposure determined by interview/ job description and databases | A modest but imprecise increase in the odds of SGA among infants whose mothers were exposed to aromatic solvents. But all of the women exposed to xylene were also exposed to toluene. | Not determined |
| Elliott et al. 2006 | General US population | Measured blood concentration of xylene | No association with any of the pulmonary function tests and xylene blood concentration | Not determined |
| Kwon et al. 2018 | 34 subjects before and after moving to a new rehabilitation facility, where they spent 8 hours a day or more. | Measured urine concentration of xylene metabolites | No significant correlations were observed between changes in urinary levels of xylene metabolites and lung function tests before and after the move. | Not determined |
| Yoon et al. 2010 | 154 Koreans | Measured urine concentration of xylene metabolites | The levels of MHA were negatively associated with FEV1 and FEV1/FVC. No association between air xylene and urine MHA was observed | Not determined |
Appendix C: Toxicological studies
| Study | Participants | Exposure | Results | NOAEL/LOAEL |
|---|---|---|---|---|
| Armenta-Resendiz et al. 2019 | Male Wistar rats, 10 per group | 0, 500, 1 000, 2 000, 4 000, or 8 000 ppm m-xylene for 30 minutes | Tests conducted 3 minutes after exposure was ended. Results were not shown or discussed for 500 or 1 000 ppm groups. At 2000 ppm, a clear and significant concentration-dependent decrease in latency was observed in the passive avoidance test. At 4 000 ppm and up, there was a significant increase in latency to paw-lick in the hot plate test. In all other tests (defensive burying behaviour task, shock threshold test, social interaction and rotarod test), significant changes only at 8 000 ppm |
Not determined |
| Korsak et al. 1990 (cited in US EPA 2003) | Male Wistar rats, 10 per group | Individual xylene isomers at 3 000 ppm for 6 hours | Rotarod test: number of failures per number of tested animals were 19/20 for o-xylene, 6/20 for m-xylene, and 1/20 for p-xylene | LOEL 3 000 ppm (lowest test concentration) No NOEL |
| Wathier et al. 2019, 2016 | Male Brown Norway rat, anesthetized and tracheotomized, n=3–5 per group | Individual xylene isomers at 3 000 ppm for 2 x 15 minutes, with 20 minutes in between | transient increase in the middle ear reflex (MER) amplitude during and after exposure, which was most pronounced for p-xylene, and less but still significant for m-xylene. No difference was observed in MER of animals exposed to o-xylene, or in morphology of the cochlea for any isomer | Not determined |
| Study | Species, Sex and Number | Exposure | Results | NOAEL/LOAEL |
|---|---|---|---|---|
| Korsak et al. 1994 | Male Wistar rat, 12 per exposed group and 24 controls | 0, 50, or 100 ppm m-xylene for 3 months (6 hours per day, 5 days per week) | Exposed rats showed decreased performance in the rotarod test for motor coordination: Failure rate of 8% for the 50 ppm group and 33% for the 100 ppm group, compared to 0% for controls (statistically significant for the 100 ppm group). The failure rates were the same after 1, 2 and 3 months of exposure (test was conducted 24 hours following the last exposure) Exposed rats also had a statistically significant increase in pain sensitivity in the hot plate test. |
LOAEL (neurotoxicity) 100 ppm NOAEL 50 ppm Selected as the critical study for the US EPA RfC derivation |
| Korsak et al. 1992 | Male Wistar rat, 12 per group | 0 or 100 ppm m-xylene for 6 months or 1 000 ppm for 3 months (6 hours per day, 5 days per week) | The failure rate in the rotarod test was approximately 35% after the 6 month 100 ppm exposure, and 60% after the 3 month 1 000 ppm exposure (0% in controls, both exposed groups were statistically different from controls). Significant decrease in spontaneous movement in both exposed groups compared to controls |
LOAEL (neurotoxicity) 100 ppm (lowest test concentration) No NOAEL |
| Gralewicz et al. 1995 | Male rat, 20 total | 0, 100 or 1 000 ppm m-xylene for 3 months (6 hours per day, 5 days per week) | In a radial maze test conducted 70–83 days after the last exposure, exposed rats did not exhibit a shortening of the time needed to complete a trial, or a decrease in omission errors with successive daily trials | LOAEL (neurotoxicity) 100 ppm (lowest test concentration) No NOAEL |
| Gralewicz and Wiaderna 2001 | Male Wistar rat, 11 per group | 0 or 100 ppm m-xylene for 4 weeks (6 hours per day, 5 days per week) | Significant difference between exposed and control rats in a passive avoidance test conducted 39–48 days after cessation of exposure (the difference was only in the last of 6 trials), and in an active avoidance test conducted 50–51 days post-exposure (the difference was only in the last of 3 trials). No difference in radial maze performance when tested 14–18 days after the end of exposure. |
LOAEL (neurotoxicity) 100 ppm (lowest test concentration) No NOAEL |
| Gagnaire et al. 2001 | Male Sprague Dawley rats, 16 per group | Individual xylene isomers at 0, 450, 900 or 1 800 ppm for 13 weeks (6 hr/day, 5 days/week) | Significant reduction in brainstem auditory-evoked potential and cochlear hair cells at every time point, including after an 8 week recovery period were observed in the group exposed to 1 800 ppm p-xylene. Moderate OHC loss was seen in the 900 ppm p-xylene group, without impact on auditory thresholds. No effects were observed with o- or m-xylene at any concentration. | LOAEL (hair cell loss) 900 ppm NOAEL 450 ppm |
| Gagnaire et al. 2007a | Male Sprague Dawley rats, 14 per group | 0, 250, 500, 1 000 or 2 000 ppm mixed xylenes for 13 weeks (6 hr per day, 6 days per week) mixture A: 20% o-xylene, 20% p-xylene, 40% m-xylene, and 20% ethylbenzene. mixture B: 30% o-xylene, 10% p-xylene, 50% m-xylene, and 10% ethylbenzene. |
No shift in audiometric thresholds was observed in the controls and in the groups exposed to 250 and 500 ppm. Threshold shifts in groups exposed to higher concentrations were observed and increased slightly throughout the exposure. No recovery was observed 8 weeks after the end of exposure. For mixture A, following an 8‑week recovery period one animal in the 250 ppm group and one in the 500 ppm group had significant OHC losses in the organ of Corti. Animals in the 1 000 ppm group had significant OHC loss also, and the 2 000 ppm group had almost complete loss of OHC and some loss of inner hair cells. Less extensive damage for mixture B groups. |
LOAEL (hair cell loss) 250 ppm (lowest test concentration) No NOAEL (hair cell loss) LOAEL (irreversible hearing loss) 1 000 ppm NOAEL (irreversible hearing loss) 500 ppm *Note that the test material contained ethylbenzene, which is known to be ototoxic |
| Maguin et al. 2006 | Male Long-Evans rats, 8 per group | 0 or 1 800 ppm o-, p-, or m-xylene for 3 weeks (6 hr per day, 5 days per week) | 4 weeks following exposure, a permanent shift in auditory threshold and severe outer hair cell losses in the cochlea were observed in the group exposed to p-xylene, but not in controls or animals exposed to o- or p-xylene. | LOAEL (hearing loss) 1 800 ppm (lowest test concentration) No NOAEL |
| Nylén et al. 1989 | Male Sprague Dawley rats, 72 total | 0 or 1 000 ppm xylene (isomer/ composition not stated) for 61 days (18 hours per day, 7 days per week) | No change in percentage of intact spermatozoa, percentage of spermatozoa with normal heads and tails, testis weight (2 or 10 weeks after exposure ended), plasma testosterone, ventral prostate weight, and noradrenaline concentration in vas deferens (2 weeks after exposure ended). Three exposed rats exposed were reported to have been fertile when tested 14 months after cessation of exposure | NOAEL (testicular, male fertility) 1000 ppm (highest test concentration) (US EPA 2003) |
| Bio/dynamics Inc. 1983 (as cited in US EPA 2003) | Male and female rats | 500 ppm technical grade xylene in rats exposed before mating, during gestation and lactation | No parental, reproductive, or developmental toxicity | NOAEL (parental, reproductive, developmental) 500 ppm (highest test concentration) |
| Hass and Jacobsen 1993 | 72 pregnant Wistar rats, 36 per group | 0 or 200 ppm technical grade xylene (composition not provided), 6 hr per day on gestation days 6–20 | No maternal toxicity was observed in the exposed dams. In pups, increased incidence of delayed ossification of os maxillare in the skull. Higher pup weight (significant in males at birth, and in both males and females at day 28 but not day 14). Exposed pups had significantly earlier eye opening and ear unfolding. Statistically significantly decreased rotarod performance was observed in female pups on PND 22 and 23 (but not PND 24), and in male pups on PND 23 (but not PND 22 or 24). No differences were observed in the time of development of the surface righting reflex, cliff avoidance reflex, or auditory startle reflex. | NOAEL (maternal toxicity) 200 ppm (highest test concentration) No LOAEL or NOAEL for developmental toxicity was identified (by the study authors or the US EPA) However, RIVM (2001) derived a tolerable concentration in air (TCA) based on a LOAEL of 200 ppm from this study |
| Hass et al. 1995; Hass et al. 1997 | 28 pregnant rats, 15 exposed and 13 controls | 0 or 500 ppm mixed xylenes (19% o-xylene, 45% m-xylene, 20% p-xylene, and 15% ethylbenzene), 6 hr per day on GD 7–20 | No effect on maternal clinical signs, body weight gain, or food consumption, gestation period, number of pups per litter, and sex distribution per litter. Exposed litters had a slight decrease in mean birth weight (5%) and a trend toward lower body weight postnatally (not significant). Significantly decreased absolute brain weight of male and female pups combined (PND 28). In the rotarod test (age 24–26 days), the % of animals not able to stay on the rod was higher in exposed animals on all 3 test days - greatest difference for females on day 3 (not statistically significant.) No difference between control and exposed pups in surface righting reflex or auditory startle; air righting was delayed by one day in the exposed group. No differences in an open field test of activity (27–34 days old). In the Morris water maze test (3 months of age) - no difference in time to find the platform between control pups and exposed pups that were housed in an “enriched” environment (i.e., with toys). However, in exposed pups that had been in standard housing, there was a trend (non-statistically significant) towards increased time to find the platform during the learning phase of the test. In a test following relocation of the platform, the difference in time and increase in swimming length were significant for exposed females from standard housing compared to controls. No difference in swimming speed. Significant differences at ages 16 and 28 weeks but not at age 55 weeks (reversible effect) |
NOAEL (maternal toxicity) 500 ppm (highest test concentration) LOAEL (developmental toxicity – reversible, water maze) 500 ppm (lowest test concentration) No NOAEL |
| Rosen et al. 1986 | pregnant rats, 18–21 per group | 0, 800 or 1 600 ppm p-xylene on GD 7–16 | Decreased maternal body weight gain at 1 600 ppm but not 800 ppm, compared to controls. No effect on litter size, pup birthweight, pup weight on PND 3, growth rate of the pups. No differences were observed in the acoustic startle response (days 13, 17, 21 and 63) or the figure-8 maze test (days 22 and 65) | LOAEL 1 600 ppm (maternal) NOAEL 800 ppm (maternal) NOAEL (developmental) 1 600 ppm (highest test concentration) |
| Saillenfait et al. 2003 | Pregnant Sprague Dawley rats, groups of 20–26 | 0, 100, 500, 1 000 or 2 000 ppm o-, m-, or x-xylene or technical grade xylene on GD 6–20 (6 hours per day) | Decreases in maternal body weight gain at 1 000 ppm and fetal body weights at 500 ppm (o- or technical grade xylene) or 1 000 ppm (m-, p-xylene). No significant changes in the average numbers of implantations and live fetuses, in the incidences of non-live implants and resorptions, or in fetal sex ratio. No malformations in pups, but some indications of delayed ossification or increased skeletal variations at 2 000 ppm. |
LOAEL (maternal) 1 000 ppm NOAEL 500 ppm LOAEL (developmental) 500 ppm NOAEL 100 ppm |
| Litton 1978 (as cited in US EPA 2003) | Pregnant rats | 400 ppm mixed xylenes on GD 6–15 | No maternal or developmental toxicity | NOAEL (maternal, developmental) 400 ppm (highest test concentration) |
| Hudak and Ungváry 1978 | Pregnant CFY rats, 20 exposed and 28 controls | 230 ppm mixed xylenes (10% o-xylene, 50% m-xylene, 20% p-xylene, 20% ethylbenzene) on GD 9–14 (24 hours per day) | No maternal or developmental toxicity | NOAEL (maternal, developmental) 230 ppm (highest test concentration) (US EPA 2003) |
| Ungváry et al.1980 | Pregnant CFY rats, 60–70 per group (60 controls in total) | 0, 35, 350, or 700 ppm o-, m-, or p-xylene 24hr/day GD 7–14 (24 hr/day) | Decreased maternal body weight at 700 ppm o-xylene, p-xylene, or m-xylene 700 ppm m-xylene - small but statistically significant decreased number of mean implantations per dam (dams in this group also died) 700 ppm p-xylene - marked post-implantation loss and a corresponding decreased mean litter size Mean fetal body weight decreases at 350 and 700 ppm o-xylene, 700 ppm m- or p-xylene. |
LOAEL (maternal) 700 ppm NOAEL 350 ppm LOAEL (developmental) 350 ppm NOAEL 35 ppm (US EPA 2003) |
| Ungváry and Tátrai 1985 | Pregnant CFY rats, 20–23 per group | 0, 60, 440, or 780 ppm mixed xylenes (composition not specified) on GD 7–15 (24 hr/day) |
At 780 ppm, significantly increased percentage of dead or resorbed fetuses | LOAEL (developmental) 780 ppm NOAEL 440 ppm |
| Ungváry and Tátrai 1985 | Pregnant New Zealand rabbits, 9–10 per group (60 controls) | 0, 115 or 230 ppm mixed xylenes (composition not specified) or individual isomers on GD 7–20 (24 hr/day) | At 230 ppm, severe maternal toxicity and no live fetuses No evidence of maternal or developmental toxicity at 115 ppm |
LOAEL (maternal, developmental) 230 ppm NOAEL 115 ppm |
| Ungváry and Tátrai 1985 | Pregnant CFLP mice, 15–18 per group (115 controls) | 0, 115, or 230 ppm mixed xylenes (composition not specified) or individual isomers on GD 6–15 (3x4 hr/day) | No evidence of maternal or developmental toxicity | NOAEL (maternal, developmental) 230 ppm (highest test concentration) |
Appendix D: Other guidelines
D1. Short-term exposure guidelines
In the Environment Canada and Health Canada’s Priority Substances List Assessment Report: Xylene, no guideline for short-term exposure to xylene was derived (Environment Canada and Health Canada 1993).
The CalEPA (1999) derived an acute (1 hour) reference exposure level of 22 mg/m3 based on a NOAEL of 100 ppm (430 mg/m3) for eye, nose, and throat irritation in volunteers exposed to xylenes for 30 minutes (Nelson et al 1943; Hastings et al. 1984; Carpenter et al. 1975). The NOAEL was adjusted for exposure duration (30 min to 1 hour), and an uncertainty factor of 10 for intraspecies variability was applied.
The European Commission (2005) selected the same endpoint, duration adjustment, and uncertainty factors as the CalEPA (1999) assessment, but because of a difference in rounding, derived a short-term exposure limit of 20 mg/m3.
The US NRC (2010) derived an acute exposure guideline limit (AEGL-1) of 130 ppm (560 mg/m3) for non-disabling effects for timeframes of 10 minutes to 8 hours, based on eye irritation at 400 ppm in humans exposed to xylene for 30 minutes (Hastings et al. 1984). An uncertainty factor of 3 was applied to account for intraspecies variability.
ATSDR (2007) derived an acute (14 days or less) minimal risk level of 2 ppm (8.7 mg/m3), based on a minimal LOAEL of 50 ppm for respiratory and neurological effects (irritation of eyes, nose and throat; reduced FVC and difficulty breathing; nausea, headache, fatigue and dizziness), in a study of 56 volunteers exposed to xylene for 2 hours (Ernstgård et al. 2002). Uncertainty factors of 3 for the use of a minimal LOAEL and 10 for intraspecies variation were applied, giving a total UF of 30. ANSES (2020) has adopted the ATSDR MRL as its acute toxicological reference value.
| Organization | Exposure guideline | Health effect |
|---|---|---|
| CalEPA (2000) | 22 mg/m3 | Eye, nose, and throat irritation |
| EU (2005) | 20 mg/m3 | Eye, nose, and throat irritation |
| US NRC (2010) | 560 mg/m3 | Eye irritation |
| ATSDR (2007), ANSES (2020) | 8.7 mg/m3 (2 ppm) | Neurological and respiratory effects |
D2. Exposure guidelines for non-neoplastic chronic effects
Previous assessments have developed proposed exposure limits for chronic or long-term xylene exposure based on neurotoxicity symptoms (anxiety, forgetfulness, floating sensation) and irritation of eyes, nose, and throat in occupationally exposed humans or on developmental toxicity or neurotoxicity in rats.
ATSDR (2007) derived a chronic minimal risk level of 220 µg/m3 based on the LOAEL of 14 ppm (61 mg/m3) in occupationally exposed humans from Uchida et al (1993). Uncertainty factors of 10 for sensitive human populations, 10 for use of a LOAEL, and 3 for a lack of supporting studies on chronic neurotoxicity were applied, giving a total UF of 300.
The European Commission (2005) derived a chronic exposure limit of 200 µg/m3 based on the LOAEL of 14 ppm (61 mg/m3) in occupationally exposed humans from Uchida et al. (1993). The LOAEL was adjusted for exposure time (8-hour work shift) and uncertainty factors of 10 for sensitive human populations and 10 for use of a LOAEL were applied, giving a total UF of 100.
The US EPA (2003) derived an inhalation RfC of 100 µg/m3, based on a NOAEL of 50 ppm (217 mg/m3) from a 13-week rat study (Korsak et al. 1994). The NOAEL was adjusted for continuous exposure (6 hours/24 hours and 5 days/7 days). Uncertainty factors of 3 for interspecies differences, 10 for sensitive human populations, 3 to account for the use of a subchronic study, and 3 for database deficiencies were applied, giving a total UF of 300. ANSES (2020) has adopted the US EPA RfC as its chronic toxicological reference value.
RIVM (2001) derived a tolerable concentration in air (TCA) of 870 µg/m3, based on a LOAEL of 200 ppm (870 mg/m3) in a rat developmental toxicity study (Hass and Jakobsen 1993). Uncertainty factors of 10 for interspecies extrapolation, 10 for sensitive human populations, and 10 for the use of a LOAEL were applied, giving a total UF of 1 000.
The CalEPA (2000) derived a chronic reference exposure level of 700 µg/m3, based on the LOAEL of 14 ppm (61 mg/m3) in occupationally exposed humans from Uchida et al (1993), adjusted for exposure time (8-hour work shift). Uncertainty factors of 10 for sensitive human populations and 3 for use of a LOAEL were applied, giving a total UF of 30.
The Government of Canada (Health Canada 1996) derived a provisional tolerable concentration of 180 µg/m3, based on a LOEL of 58 ppm (250 mg/m3) from a rat developmental study (Ungváry and Tátrai 1985), which was adjusted for differences in inhalation volume and body weights between rats and humans. Uncertainty factors of 10 for interspecies extrapolation, 10 for sensitive human populations, and 10 for the use of a LOEL and study limitations were applied, giving a total UF of 1 000.
| Organization | Exposure guideline | Health effect |
|---|---|---|
| ATSDR 2007 | 220 µg/m3 | Neurotoxicity symptoms (anxiety, forgetfulness, floating sensation) and irritation of eyes, nose, and throat |
| European Commission 2005 | 200 µg/m3 | Eye irritation, sore throat, floating sensation, and poor appetite |
| US EPA 2003; ANSES 2020 | 100 µg/m3 | Impaired motor coordination |
| RIVM 2001 | 870 µg/m3 | Developmental neurotoxicity |
| CalEPA 2000 | 700 µg/m3 | Neurotoxicity symptoms (anxiety, forgetfulness, floating sensation) and irritation of eyes, nose, and throat |
| Health Canada and Environment Canada 1993 | 180 µ g/m3 | Developmental toxicity |
